Abstract
Background
Clinical egg allergy is a common food allergy. Current management relies upon strict allergen avoidance. Oral immunotherapy might be an optional treatment, through desensitization to egg allergen.
Objectives
To determine the efficacy and safety of oral and sublingual immunotherapy in children and adults with immunoglobulin E (IgE)‐mediated egg allergy as compared to a placebo treatment or an avoidance strategy.
Search methods
We searched 13 databases for journal articles, conference proceedings, theses and trials registers using a combination of subject headings and text words (last search 31 March 2017).
Selection criteria
We included randomized controlled trials (RCTs) comparing oral immunotherapy or sublingual immunotherapy administered by any protocol with placebo or an elimination diet. Participants were children or adults with clinical egg allergy.
Data collection and analysis
We retrieved 97 studies from the electronic searches. We selected studies, extracted data and assessed the methodological quality. We attempted to contact the study investigators to obtain the unpublished data, wherever possible. We used the I² statistic to assess statistical heterogeneity. We estimated a pooled risk ratio (RR) with 95% confidence interval (CI) for each outcome using a Mantel‐Haenzel fixed‐effect model if statistical heterogeneity was low (I² value less than 50%). We rated the quality of evidence for all outcomes using GRADE.
Main results
We included 10 RCTs that met our inclusion criteria, that involved a total of 439 children (oral immunotherapy 249; control intervention 190), aged 1 year to 18 years. Each study used a different oral immunotherapy protocol; none used sublingual immunotherapy. Three studies used placebo and seven used an egg avoidance diet as the control. Primary outcomes were: an increased amount of egg that can be ingested and tolerated without adverse events while receiving allergen‐specific oral immunotherapy or sublingual immunotherapy, compared to control; and a complete recovery from egg allergy after completion of oral immunotherapy or sublingual immunotherapy, compared to control. Most children (82%) in the oral immunotherapy group could ingest a partial serving of egg (1 g to 7.5 g) compared to 10% of control group children (RR 7.48, 95% CI 4.91 to 11.38; RD 0.73, 95% CI 0.67 to 0.80). Fewer than half (45%) of children receiving oral immunotherapy were able to tolerate a full serving of egg compared to 10% of the control group (RR 4.25, 95% CI 2.77 to 6.53; RD 0.35, 95% CI 0.28 to 0.43). All 10 trials reported numbers of children with serious adverse events (SAEs) and numbers of children with mild‐to‐severe adverse events. SAEs requiring epinephrine/adrenaline presented in 21/249 (8.4%) of children in the oral immunotherapy group, and none in the control group. Mild‐to‐severe adverse events were frequent; 75% of children presented mild‐to‐severe adverse events during oral immunotherapy treatment versus 6.8% of the control group (RR 8.35, 95% CI 5.31 to 13.12). Of note, seven studies used an egg avoidance diet as the control. Adverse events occurred in 4.2% of children, which may relate to accidental ingestion of egg‐containing food. Three studies used a placebo control with adverse events present in 2.6% of children. Overall, there was inconsistent methodological rigour in the trials. All studies enrolled small numbers of children and used different methods to provide oral immunotherapy. Eight included studies were judged to be at high risk of bias in at least one domain. Furthermore, the quality of evidence was judged to be low due to small numbers of participants and events, and possible biases.
Authors' conclusions
Frequent and increasing exposure to egg over one to two years in people who are allergic to egg builds tolerance, with almost everyone becoming more tolerant compared with a minority in the control group and almost half of people being totally tolerant of egg by the end of treatment compared with 1 in 10 people who avoid egg. However, nearly all who received treatment experienced adverse events, mainly allergy‐related. We found that 1 in 12 children had serious allergic reactions requiring adrenaline, and some people gave up oral immunotherapy. It appears that oral immunotherapy for egg allergy is effective, but confidence in the trade‐off between benefits and harms is low; because there was a small number of trials with few participants, and methodological problems with some trials.
Plain language summary
Does giving daily small, steadily increasing amounts of egg protein help people with egg allergy?
Background
Until recently, the only practical option for people with food allergies was strict avoidance of foods to which they are allergic. It is difficult to avoid egg because it is found in many foods. Even with avoidance, the fear of accidentally eating foods with egg because of mislabelling or cross‐contamination is an ever‐present fear for even the most careful of people with food allergy. Accidentally eating egg‐containing foods can cause life‐threatening events.
Oral immunotherapy is a new type of treatment for egg allergy, which is also known as oral desensitization or vaccination. Treatment involves consuming a small amount of egg protein daily, which is gradually increased over time until a full serving is reached. This method could alter the allergic response to the egg protein by the body’s immune system, increasing the amount of egg that can be eaten without inducing adverse reactions.
Search date
We searched up to March 2017 for this update.
Study characteristics
We included 10 randomized controlled trials (studies that allocate people randomly by chance to receive treatment) that compared oral immunotherapy to placebo (a fake treatment not containing egg) or an egg‐avoidance diet for people with egg allergy. The 10 studies included a total of 439 children (249 in the oral immunotherapy group (treatment containing egg) and 190 in the control group (no egg)) who were aged from 1 year to 18 years.
Key results
The evidence showed that treating egg allergy by giving a small, increasing amount of egg may help most children with egg allergy to tolerate a partial serving of egg, so long as they continued to consume a daily amount of egg protein. Side effects were frequent during oral immunotherapy treatment, but were usually mild‐to‐moderate. Nevertheless, 21 of 249 children treated with oral immunotherapy for egg allergy required medicine because of a serious reaction. The studies did not report information about quality of life of children and their families during oral immunotherapy treatment.
Quality of the evidence
The trials involved small numbers and there were problems with the way they were done, therefore further research is needed.
Summary of findings
Summary of findings for the main comparison. Oral and sublingual immunotherapy versus no therapy for egg allergy.
| Oral and sublingual immunotherapy versus no therapy for egg allergy | ||||||
|
Population: children with egg allergy Setting: hospitals Intervention: oral and sublingual immunotherapy for egg allergy. (Each study used a different oral immunotherapy protocol) Comparison: placebo or egg‐free diet. (Three studies used placebo and seven studies used an egg avoidance diet as controls) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral immunotherapy versus no therapy for egg allergy | |||||
| Increase in the amount of egg that could be tolerated | Study population | RR 7.48 (4.91 to 11.38) | 410 (9 studies) | ⊕⊕⊝⊝ low1,2 | 82% of children in the oral immunotherapy group could ingest a partial serving of egg (1 g to 7.5 g) compared to 10% of the control group. | |
| 102 per 1000 | 763 per 1000 (501 to 1000) | |||||
| Complete recovery | Study population | RR 4.25 (2.77 to 6.53) | 439 (10 studies) | ⊕⊕⊝⊝ low1,2 | 45% of children receiving oral immunotherapy were able to tolerate a full serving of egg compared to 10% of the control group. | |
| 100 per 1000 | 425 per 1000 (277 to 653) | |||||
| Numbers of children with serious adverse events | See comment | See comment | Not estimable | 439 (10 studies) | ⊕⊕⊝⊝ low1,2 | All 10 trials reported numbers of children with serious adverse events (SAEs): SAEs requiring epinephrine/adrenaline occurred in 21/249 (8.4%) of children in the oral immunotherapy group and none in the control group. Because adverse events were classified differently among the studies, it is difficult to comment on whether they were under‐ or over‐estimated. Surprisingly, only one study showed a high level of SAEs (Vazquez‐Ortiz 2014): 13 children required epinephrine/adrenaline administration 18 times, and one grade 5 reaction occurred. |
| Number of children with mild‐to‐severe adverse events | Study population | RR 8.35 (5.31 to 13.12) | 439 (10 studies) | ⊕⊕⊝⊝ low1,2 | Mild‐to‐severe adverse events were frequent; 75% of children presented mild‐to‐severe adverse events during oral immunotherapy treatment versus 6.8% of children in the control group. | |
| 68 per 1000 | 568 per 1000 (361 to 892) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
The assumed risk is the risk of the control arm
1 Downgraded one level because of risk of bias: all studies were assessed at high or unclear risk of bias in at least one domain. 2 Downgraded one level because of imprecision: few events.
Background
This is an update of the 2014 version of this review. Please see the 'Differences between protocol and review' section for changes in the review since the protocol or the last version of the review.
Description of the condition
Clinical egg allergy is one of the most common food allergies in western countries, affecting up to approximately 2% of young children (Savage 2007). Osborne 2011 showed an 8.9% prevalence of challenge‐proven egg allergy in 12‐month old infants in Australia. Natural tolerance, that is, developing tolerance to egg over time, is common but some children will remain allergic until adulthood. Egg‐allergic reactions may persist throughout adulthood although symptoms may be milder. It has been shown that 75% to 85% of young children with egg allergy could tolerate heat‐denatured (baked) egg products, showing mild or no symptoms (Leonard 2012; Osborne 2011); most outgrow the allergy.
Despite this trend, recent data have shown that milk and egg allergies are becoming more persistent and that children may not be outgrowing these allergies until adolescence rather than during the first five to six years of life as previously thought (Savage 2007). It appears that the longer the egg allergy persists the less likely it is that tolerance will develop (Wood 2003b). Thus, it has become imperative to understand individualized prognoses of egg allergy and to develop clinical management that may improve the quality of life of egg‐allergic children and, ideally, promote earlier development of tolerance. It is likely that most children who tolerate baked‐egg products will develop tolerance after undergoing oral immunotherapy, and perhaps, this is achieved more rapidly if treated with oral immunotherapy (Leonard 2012). Those children who do not tolerate heat‐denatured egg are likely to be more difficult to treat (Leonard 2012). Consequently, an adequate placebo group is needed to investigate the ability of oral immunotherapy to induce long‐term tolerance.
An immunoglobulin E (IgE)‐antibody mediated allergic reaction to egg is based on circulating levels of soluble IgE, which is produced by B cells and binds to the surface of mast cells and basophils. Mast cells are found in the skin, gut, and respiratory tract and are situated adjacent to nerves and blood vessels. Among the most important of their immune functions is the propensity to bind IgE, utilizing the high‐affinity IgE receptor FcåR1. When egg allergen is re‐encountered and recognized by cell‐bound IgE, adjacent FcåR1‐IgE complexes move closer together and bring their signalling machinery into close proximity, which sets off a cascade of phosphorylation ultimately resulting in calcium influx. When calcium enters the cell, the activated mast cell undergoes degranulation and the contents of these granules are released into the extracellular space. The immediate liberation of preformed powerful vaso‐active compounds such as histamine, platelet activating factor, tryptase, carboxypeptidase, chymase, and heparin, elicit the acute symptoms of type 1 hypersensitivity reactions in the skin, gut, respiratory, and cardiovascular systems (Galli 2010). These symptoms include urticaria, angioedema, flushing, nausea, vomiting, abdominal pain, diarrhoea, wheezing, coughing and bronchospasm, rhinorrhoea, and hypotension or syncope, which can occur alone or in combination and typically begin within minutes and up to two hours after food ingestion (Burks 2008; Simons 2011).
There is no interventional therapy for egg allergy currently approved by the USA Food and Drug Administration (FDA), nor by the European Medicines Agency (EMA). Current management relies upon strict allergen avoidance, including the small quantities present in a variety of foods, and obvious sources such as desserts (Muraro 2014). In the USA, European Union, Australia, Japan, and Singapore, food‐labelling laws require food manufacturers to label packaging in plain language, listing the presence of common allergens and products (Burks 2012a). However, similar laws are not in place in many other countries, and in these settings, care is required to identify hidden forms of egg allergens, such as ovalbumin or ovomucoid (Burks 2012a). Studies that evaluated growth measurements against dietary records have suggested that food allergy puts children at risk of inadequate nutrition (Christie 2002). Specifically, where children's food allergy is concerned, it is advisable to involve a dietitian in formulating a nutritionally adequate, allergen‐free diet. The quality of life of people with food allergies and their caregivers is reduced due to the fear of incorrect labelling or accidentally ingesting egg‐containing food, and the ever‐present threat of anaphylaxis (Cummings 2010). The management of clinical egg allergy consists of teaching people with allergy and their carers to recognize the symptoms and signs of severe reaction, prompt use of epinephrine auto‐injection, and activation of emergency medicine assistance (Simons 2009). Therapeutic interventions that provide lifelong protection against potential egg ingestion are therefore required.
Description of the intervention
Traditional subcutaneous immunotherapy (also known as allergy shots) was studied since the early twentieth century and was able to induce desensitization (Ring 2011). However, subcutaneous immunotherapy treatment is not feasible due to the high rate of systemic reactions (Nelson 1997; Oppenheimer 1992). Given the safety issues associated with subcutaneous immunotherapy, oral immunotherapy and sublingual immunotherapy have been studied as optional treatments.
Although there have been reports in the literature over the last 100 years on the use of oral immunotherapy for food allergy, most research on this topic began with Patriarca 1984, who demonstrated the successful treatment of allergies to cow’s milk, egg, fish, and fruits with standardized oral immunotherapy protocols. In a pilot study of oral immunotherapy for egg allergy in children, Buchanan demonstrated the safety of a 24‐month egg oral immunotherapy protocol involving modified rush desensitization, build‐up, and maintenance phases (Buchanan 2007).
The initial aim of oral immunotherapy is to provide clinical desensitization, that is, to achieve a state in which effector cells involved in a specific immune response develop reduced reactivity or become non‐reactive upon increased introduction of an allergen. In a desensitized state, a person may be non‐reactive while regularly receiving the allergen. However, when regular administration ends the previous level of reactivity returns. The aim of treatment is initially to desensitize people to egg allergen by increasing the threshold dose of exposure, and therefore, reduce the risk of anaphylaxis. The longer‐term goal is to induce a state of tolerance to an allergen where the non‐reactive state remains present permanently through down‐regulation of the TH2 response to egg, and which will endure irrespective of whether a previously clinically reactive person continues to consume egg products or not following the completion of the treatment (Land 2011).
There are different types of oral immunotherapy protocols. Oral immunotherapy involves the regular administration of small amounts of allergen by the oral route to rapidly induce desensitization, then over time, induce tolerance to the allergen.
Studies have considered immunotherapy that is ingested and immunotherapy administered sublingually. In this review, oral immunotherapy specifically refers to ingested immunotherapy and sublingual immunotherapy refers to immunotherapy administered under the tongue.
People undergoing oral immunotherapy generally ingest a mixture of protein powder in a vehicle food (e.g. apple sauce). People undergoing sublingual immunotherapy generally receive a small amount of liquid extract under the tongue. Both treatments are typically initiated in a controlled setting where gradually increased doses of allergen are given up to a targeted dose. In standard protocols most dosing is subsequently done at home.
How the intervention might work
Oral immunotherapy involves the administration of initially very small doses (usually micrograms or milligrams) of food allergen to food‐allergic people in a controlled clinical setting. The dose of the administered food allergen is then systematically increased until a maximum tolerated dose is achieved (Jones 2009). Regular dosing with this maximal dose is then maintained at home by the person with the allergy (or their carer). Of note, it has been demonstrated that underlying the clinical benefits of oral immunotherapy were changes to multiple aspects of the immune system, leading to a dampened allergic response (Jones 2009). Changes included decrease in allergen‐specific IgE and increase in allergen‐specific IgG4 (Buchanan 2007; Itoh 2010; Patriarca 2003; Staden 2007), as well as suppression of mast cells and basophils, an increase in T regulatory cells (TRegs), and a change in cytokine profile. Additionally, micro‐array analysis of T cells has revealed changes in several apoptotic pathways, although the significance of this result remains unknown (Jones 2009). Cytokine analysis demonstrated a clear decrease in TH2 cytokines without a concomitant increase in TH1 cytokines, causing a shift in TH1 and TH2 skewing and arguing against oral immunotherapy. Rather, a decrease in IL‐2 was noted, possibly suggesting clonal anergy or deletion as a possible mechanism of oral immunotherapy (Land 2011). However, caution needs to be applied when interpreting these results; both studies had small cohorts of participants and the selection criteria for the immunological studies were not sufficiently clear (Jones 2009; Land 2011).
Successful desensitization is thought to induce immunological tolerance by generating allergen specific IL‐10 secreting Tr1 or TGF‐secreting TH3 regulatory T cells, or both (Sicherer 2010).
Why it is important to do this review
Food allergy is an IgE‐mediated immediate‐type hypersensitivity that is thought to be the result of a breakdown in the normal process of oral tolerance. Although the prevalence of food allergy continues to rise, avoidance remains the standard of care because no disease‐modifying treatments are readily available. Although questions regarding the safety of treatments, and the potential for development of long‐term immunological tolerance remain, oral immunotherapy and sublingual immunotherapy offer potential hope for the future treatment of egg allergy.
There is a need to systematically identify, critically appraise, and summarize the available evidence on the benefits and risks associated with oral immunotherapy and sublingual immunotherapy for the management of people with egg allergy.
Objectives
To determine the efficacy and safety of oral and sublingual immunotherapy in children and adults with immunoglobulin E (IgE)‐mediated egg allergy as compared to a placebo treatment or an avoidance strategy.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), quasi‐RCTs, and controlled clinical trials (CCTs) were eligible for inclusion.
Types of participants
Children and adults diagnosed with an immediate‐type egg allergy were included. Egg allergy was defined as a history of systemic clinical reaction within minutes to hours after the ingestion of egg in people with objective evidence of sensitization to egg. Objective evidence was:
a positive skin prick test (SPT) or the presence of specific IgE; and
a positive open or double‐blind, placebo‐controlled food challenge. The positive reaction to the challenge should be the immediate onset of symptoms suggestive of IgE‐mediated mechanisms such as urticaria, angioedema, vomiting, diarrhoea, abdominal pain, and any alteration in the level of consciousness.
Types of interventions
Egg oral immunotherapy or sublingual immunotherapy administered by any protocol compared with a placebo group, or treatment with a continued avoidance diet.
Types of outcome measures
Primary outcomes
Successful desensitization or achieved tolerance defined as:
an increase in the amount of egg that can be ingested and tolerated without adverse reaction (i.e. evidence of desensitization);
a complete recovery from egg allergy whether or not egg is eaten (i.e. induction of immunologic tolerance);
numbers of participants with serious adverse events; and
numbers of participants with mild‐to‐severe adverse events.
Secondary outcomes
Medication used due to adverse events.
Immunological changes suggestive of the induction of tolerance (e.g. decreased wheal diameter on SPT testing with egg, decreased egg‐specific IgE levels, increased egg‐specific IgG4 levels.
Change in quality of life.
Cost‐effectiveness of treatment.
Search methods for identification of studies
We conducted systematic searches for RCTs, quasi‐RCTs, and CCTs regardless of language, geographical area, or publication status to 31 March 2017.
Electronic searches
We searched: the Cochrane Library, MEDLINE, Embase, PubMed, ISI Web of Science, Google Scholar, AMED, BIOSIS, CAB, CINAHL, Global Health, TRIP, and WHO Global Health Library. The MEDLINE search strategies are detailed in Appendix 1 and the search strategies used for other databases are presented in Appendix 2. We combined subject search strategies with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and CCTs as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b (Higgins 2011).
Searching other resources
We identified unpublished studies, ongoing work and research in progress by searching key trials registers ( ISRCTN registry (www.isrctn.com/).; clinicaltrials.gov). We also searched the proceedings of conferences important to allergy and immunology and not included in the electronic databases. We used key articles retrieved from the electronic database searches (including seminal research studies and review articles) to conduct citation searches in Web of Science and Scopus. We searched for grey literature via Google Scholar (scholar.google.com) and contacted experts in the field.
Data collection and analysis
Selection of studies
Two review authors (OR, MAT) independently screened the retrieved titles and abstracts of records and excluded irrelevant ones. We retrieved the full text of reports of potentially relevant studies. Multiple reports from the same study were identified and grouped under a single study identifier. Two review authors (OR, MGC) independently undertook the examination of studies and selection based on the eligibility criteria. If required, study investigators were contacted to clarify eligibility. If the two review authors did not agree on including a study, even after discussion, a third author acted as an arbiter (SZ).
Data extraction and management
We created a data extraction form that included the following items: trial characteristics (setting, oral immunotherapy regimen, eligibility criteria); methodological quality (randomization, blinding, selective reporting); participant characteristics; results and outcomes. The form was piloted using two sample studies. Two review authors (OR, MGC) working independently, extracted data. We contacted study investigators when information was not available from study reports. Disagreements between review authors were resolved by discussion and, if required, arbitration by a third review author (SZ).
Assessment of risk of bias in included studies
Two review authors (SZ, MGC) worked independently using Cochrane’s risk of bias tool to evaluate each included study. We considered the following types of potential bias: selection bias (adequacy of randomization and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); and attrition bias (loss to follow‐up). Any disagreements were discussed and a third review author acted as arbiter if necessary. Assessment results were summarized in 'Risk of bias' tables. We performed sensitivity analyses to determine the influence of studies with high risk of bias on meta‐analysis results.
Measures of treatment effect
Categorical data were extracted for each intervention group, and risk ratios (RRs) and absolute risk differences (RDs) calculated. Mean and standard deviation were obtained for continuous data and analyses performed using the mean differences (MDs). For each measure of effect the 95% confidence intervals (CI) were given. The number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) were presented when RDs were statistically significant.
Unit of analysis issues
We did not anticipate any unit of analysis issues for this intervention. For example, cross‐over trials would not be a rational approach to assess immunotherapy because the goal of treatment is to induce tolerance, and a participant would not be able to serve as their own control if their immune response had been altered. We did not expect large numbers of egg‐allergic people from various cohorts, so cluster‐randomization was unlikely to be encountered.
Dealing with missing data
We attempted to contact study investigators to request missing data. Data were analysed on an intention‐to‐treat (ITT) basis whenever possible.
Assessment of heterogeneity
We anticipated clinical heterogeneity in the studies that were reviewed, including the ages of the study population and differences in the immunotherapy protocol. However, despite these potential differences we believe that we were able to analyse the studies together to assess the efficacy of oral immunotherapy. We assessed statistical heterogeneity using the I² statistic (Higgins 2011); an I² value greater than 50% implies substantial heterogeneity.
Assessment of reporting biases
We planned to assess reporting and publication bias by examining the degree of asymmetry of a funnel plot in RevMan 5.3 provided that a sufficient number of studies (n > 10) were available (RevMan 2014). However, this was not feasible.
Data synthesis
We used a Mantel‐Haenszel fixed‐effect model for meta‐analysis, and summarized evidence in a 'Summary of findings' table. All analyses were conducted using Review Manager (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
Depending on the data available, we planned to undertake subgroup analyses for: presence of asthma, other food allergies, history of previous anaphylaxis, the oral immunotherapy or sublingual immunotherapy regimen, duration of treatment, time since completion of treatment, and RCT versus non‐RCT. However, there were insufficient data for subgroup analysis to be performed.
Sensitivity analysis
A sensitivity analysis was performed for reviewed studies that were deemed at high risk of bias mainly in two domains: detection and performance biases.
'Summary of findings' table
Following standard Cochrane methodology, we created a 'Summary of findings' table for all of our outcomes. Also following standard Cochrane methodology, we assessed the quality of evidence for each outcome using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias (GRADEpro GDT)).
Results
Description of studies
Results of the search
The electronic searches from the previous version of the review and this update retrieved 96 records, of which 85 remained after removing duplicates. Two review authors screened the records based on title and abstract and excluded 59 records. We obtained the full‐text articles for 26 records. Of these, we excluded 11 trials that did not meet our inclusion criteria. Five studies are awaiting classification, and two were ongoing studies. We included 10 studies from 11 records in this review update (Figure 1).
1.
Study flow diagram.
Included studies
We included 10 published studies that met our inclusion criteria; of these, six studies (including 272 children) were added for this update (see Characteristics of included studies). The studies recruited a total of 439 children (249 intervention/190 control) who were aged from 1 to 18 years.
Three studies used placebo control (Burks 2012; Caminiti 2015; Giavi 2016), and seven studies used an avoidance diet as the control (Akashi 2017; Dello Iacono 2013; Escudero 2015; Fuentes‐Aparicio 2013; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014).
Five studies excluded children with history of anaphylaxis (Akashi 2017; Burks 2012; Escudero 2015;Giavi 2016; Pérez‐Rangel 2017), but two studies included children with history of anaphylaxis (Dello Iacono 2013; Fuentes‐Aparicio 2013). Anaphylaxis history was not clearly defined in the inclusion criteria of three studies (Caminiti 2015;Meglio 2013;Vazquez‐Ortiz 2014).
Four studies excluded children with severe or uncontrolled asthma (Dello Iacono 2013; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014). One study excluded children with severe atopic dermatitis (Vazquez‐Ortiz 2014). Two studies excluded children with egg non IgE mediated adverse events, eosinophilic oesophagitis, immune system diseases or malignant diseases, any baseline disease contra‐indicating the use of epinephrine and/or allergy to any component of the placebo (Escudero 2015;Pérez‐Rangel 2017). Children were excluded based on: diagnosis of cardiovascular disease (Vazquez‐Ortiz 2014), neuropsychiatric impairment (Vazquez‐Ortiz 2014), receiving any other immunotherapy (Meglio 2013), significant pre‐ and post‐natal diseases (although significance was not defined) (Giavi 2016), use of systemic drugs (such as antihistamines, beta‐agonists, ACE‐inhibitors) (Giavi 2016), congenital illness or malformation that may affect normal growth (Giavi 2016), and participation in another clinical trial (Giavi 2016). Two studies excluded children with parental history of unreliable management of complications and treatment (Dello Iacono 2013; Giavi 2016).
Two studies administered antihistamine treatment as a co‐intervention (Meglio 2013; Pérez‐Rangel 2017). Oral immunotherapy was used in combination with antihistamines if recurrent allergic symptoms developed in Akashi 2017. Children with asthma on preventive treatment continued therapy during oral immunotherapy in Vazquez‐Ortiz 2014. Two studies did not report exclusion criteria (Caminiti 2015; Fuentes‐Aparicio 2013).
Oral immunotherapy protocols
Egg oral immunotherapy protocols differed. Nine studies included a build‐up phase in a hospital, day treatment centre, or research centre followed by gradual up‐dosing in hospital or at home, and a maintenance phase at home (Burks 2012; Caminiti 2015; Dello Iacono 2013; Escudero 2015; Fuentes‐Aparicio 2013; Giavi 2016; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014). Akashi 2017 reported that the oral immunotherapy protocol was carried out at home only. Burks 2012 was characterized by an increasing dose in research settings followed by an approximate doubling every 30 minutes, up to 50 mg. The maximum tolerated single dose of egg was given in the clinic on the following day and provided the starting dose for the build‐up phase. Attainment of a minimum dose of 3 mg of egg white powder was required to continue dosing. The children then continued a build‐up dosing at home. For children who did not achieve a dose of 50 mg on the first day, doses were doubled every two weeks up to 50 mg. Therefore, the dose was increased to 75 mg with following increases by 25% up to 2 g. The maximum time allowed for the build‐up phase was 10 months; the dose achieved at 10 months was considered to be the maintenance dose. After reaching their highest build‐up dose (maximum 2 g), children continued this dose daily for at least two months before the month 10 oral food challenge; children in the egg oral immunotherapy group continued maintenance dosing through to 22 months. The Fuentes‐Aparicio 2013 protocol included in‐hospital administration of 1 mg egg and was continued by tripling the dose every 30 minutes, up to 30 mg. On the second day, 30 mg was administered in one single dose, with treatment continuing at home at the same dose. Subsequently, weekly increases were made in the clinic until 10 g of powdered egg, the equivalent of one egg, was reached. In Dello Iacono 2013 children started with one drop of undiluted raw hen's egg emulsion (0.015 mL) in a day hospital then continued at home with gradually increasing doses. The doses were increased at six further day hospital visits (on days 8, 29, 64, 92, 134, 176). Dose increases were customized based on the frequency and severity of side effects and in cases of intercurrent illness or worsening asthma. The oral immunotherapy protocol in Meglio 2013 started with 0.27 mg of hen's egg protein in hospital and thereafter children doubled the dose at home every eight days until day 80. Subsequently, the doses were doubled every 16 days to achieve a total daily intake of 25 mL at six months. In the Vazquez‐Ortiz 2014 oral immunotherapy protocol, pasteurized liquid egg white containing 8.3 g of protein per 100 mL was used, in which allergenicity was equivalent to raw egg white. The induction phase involved 16 weeks and started with a 2‐day in‐hospital rush phase, in which doses were increased hourly, starting with 0.083 mg egg white protein. Children were then discharged home and continued receiving the last tolerated dose once daily throughout the week. Weekly increases were given at the outpatient clinic until the equivalent of one raw egg (60 g of fresh product) could be given. The Caminiti 2015 oral immunotherapy procedure consisted of weekly administration, at the hospital clinic, of increasing doses of dehydrated egg white, diluted with sterile saline, starting with 0.1 mg. The dose was doubled every week until week 16, to achieve a cumulative dose of 4 g in approximately four months. When the dose of 4 g was tolerated, the oral immunotherapy protocol was continued with cooked or boiled egg at home. The Escudero 2015 protocol followed the procedure described in Staden 2007, which consisted of an initial dose escalation phase in hospital starting with 0.08 mg of egg white and subsequently increasing every 20 minutes up to 140 mg of egg white protein (cumulative dose was 280 mg). This was followed by a build‐up phase with a weekly increase of the last maximum tolerated dose in hospital up to 2.8 g of egg white protein and a maintenance phase, which consisted in consumption of at least one undercooked egg (defined by the authors as fried egg, scrambled or undercooked omelet) every 48 hours. Children were permitted to freely consume any other egg‐containing food. The Giavi 2016 oral immunotherapy protocol was characterized by an escalation phase in hospital increasing the dose of hydrolysed egg every 20 to 30 minutes to reach 9 ± 1 g of hydrolysed egg. In the absence of adverse events the child received the full dose of 9 ± 1 g of hydrolysed egg, and in the absence of adverse events occurring, the child received the full dose of hydrolysed egg daily for six months. The Pérez‐Rangel 2017 oral immunotherapy protocol involved a consecutive five day build‐up phase starting with a highest tolerated single dose at the baseline egg double‐blinded placebo‐controlled food challenge test (DBPCFC). This consisted in an escalation dose of egg white starting from 4 mg and finishing with 1.8 g of egg white every 20 minutes until objective IgE‐mediated manifestations or subjective mild‐to‐moderate symptoms occurred. The regimen was conducted in an outpatient clinic. The build‐up target was 3.6 g of dehydrated egg white with a cumulative dose of 5.4 g administered on the last day. The maintenance phase consisted in eating one undercooked egg every 48 hours. Moreover, children were permitted to freely consume any other egg‐containing food. In Akashi 2017, oral immunotherapy was carried out at home with a starting dose of 0.1 mg of dried powdered egg and this was increased up to 4 g with three to four days interval. The escalation protocol was based on the method used in Patriarca 2003. Children were permitted to continue oral immunotherapy in combination with antihistamines in case of recurrent allergic symptoms.
Each study used a different egg preparation: Burks 2012 used raw white egg powder. Fuentes‐Aparicio 2013 used powdered pasteurized egg. Dello Iacono 2013 and Meglio 2013 used raw egg emulsion. Vazquez‐Ortiz 2014 used pasteurized liquid egg white. Caminiti 2015, Escudero 2015 and Pérez‐Rangel 2017 used dehydrated egg white. Giavi 2016 used hydrolysed egg. Akashi 2017 used dried powdered egg.
There were also differences among included studies regarding the duration of oral immunotherapy to achieve full desensitization, defined as the ability to digest a small egg serving without adverse events. Burks 2012 reported desensitization in 75% of children after 22 months of oral immunotherapy; the target maintenance dose was 10 g egg white powder. Desensitization was evaluated by oral food challenge test. The duration of oral immunotherapy in Fuentes‐Aparicio 2013 varied between four and 28 weeks (average 10 weeks) targeted to achieve a tolerance of 10 g of whole egg. The Dello Iacono 2013 oral immunotherapy protocol duration, to achieve "maximum tolerance" by digesting 40 mL or raw egg emulsion without adverse events, was six months. Desensitization was evaluated by a DBPCFC test. The planned duration of oral immunotherapy for children to be able to tolerate 25 mL of raw egg in Meglio 2013 was 181 days. The actual duration of the oral immunotherapy was longer, with a mean duration of 215 days (range 178 to 287 days). None of the oral food challenge tests were performed for the intervention group at the end of the study; only children in the control group underwent DBPCFC at six months after enrolment. The duration of oral immunotherapy in Vazquez‐Ortiz 2014 was 12 months and desensitization was evaluated by oral food challenge to one raw egg, meaning 3.8 g of raw egg white or if not reached, the highest tolerated dose. The oral immunotherapy protocol in Caminiti 2015 aimed to achieve a tolerance a 4 g of dehydrated egg white in approximately four months and DBPCFC performed by the end of the study. Desensitized children received an egg‐containing diet with two to three eggs per week for six months, following a three‐month egg avoidance diet, after which a repeat DBPCFC was performed (Caminiti 2015). In Escudero 2015, the primary study outcome was to assess the induction of sustained unresponsiveness after three months of egg oral immunotherapy and an egg avoidance diet for one month, defined as the ability to consume 2808 mg of egg white protein without symptoms in a DBPCFC at four months. The oral immunotherapy duration in Giavi 2016 was six months with the target to tolerate 43.2 g of boiled egg on oral food challenge. The Pérez‐Rangel 2017 study was randomized for the first five months, during which an intervention group of children received egg oral immunotherapy shortly after randomization and control group children were on an egg avoidance diet; thereafter the control group was stopped and control group children who failed DBPCFC at five months began an oral immunotherapy treatment. The results reported here were extracted from the first five months of the randomized study period. No food challenge test was performed on children in the oral immunotherapy group at five months. Akashi 2017 used an oral immunotherapy protocol that lasted on average 6.5 months (range 5 to 8) aiming to achieve tolerance of 4 g dry egg powder on oral food challenge by the end of the oral immunotherapy protocol.
Clinical history of allergic reactions after egg digestion was an inclusion criterion for all studies. In two studies, positive clinical history was defined as development of symptoms within two hours after ingestion of egg (Burks 2012;Escudero 2015), and in one study the definition was as an immediate (< 1 h) reaction following egg ingestion (Giavi 2016).
Nine studies reported as one of the inclusion criteria a positive skin prick test (SPT) (Burks 2012; Caminiti 2015; Dello Iacono 2013; Escudero 2015; Fuentes‐Aparicio 2013; Giavi 2016; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014). Positive egg‐specific IgE (sIgE) levels was an inclusion criteria in all ten studies although the definition, if it was specified, varied among the studies. In Burks 2012 more than 5 kU/L for participants six years of age or older, or 12 kU/L or more for those five years old; in Giavi 2016 and in Giavi 2016 it was considered positive level of 0.35 kU/L or higher; Akashi 2017, Escudero 2015, Pérez‐Rangel 2017 defined IgE positivity if the levels were of 0.7 kU/L or higher. In seven studies DBPCFC was performed at baseline (Akashi 2017Caminiti 2015;Dello Iacono 2013;Escudero 2015;Meglio 2013;Pérez‐Rangel 2017Vazquez‐Ortiz 2014). Two studies required oral food challenge at baseline (Giavi 2016 and Fuentes‐Aparicio 2013); oral food challenge was not performed at baseline in Burks 2012.
In seven studies a DBPCFC test was performed on all children in the control group at the end of the study (Akashi 2017; Caminiti 2015; Dello Iacono 2013; Escudero 2015; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014). In three of these seven studies, DBPCFC was also performed for oral immunotherapy group participants (Akashi 2017; Dello Iacono 2013; Escudero 2015). An open oral food challenge was performed in oral immunotherapy group participants at the end of the study in five trials (Burks 2012; Caminiti 2015; Fuentes‐Aparicio 2013; Giavi 2016; Vazquez‐Ortiz 2014) and in two trials it was performed in control group participants (Burks 2012; Giavi 2016).
The follow‐up period varied for each study, and included: 36 months (Escudero 2015), 24 months (Burks 2012), 13 months (Caminiti 2015), 12 months (Vazquez‐Ortiz 2014), six months (Akashi 2017; Dello Iacono 2013; Fuentes‐Aparicio 2013, Giavi 2016; Meglio 2013) and five months (Pérez‐Rangel 2017). All children were receiving daily maintenance at the time of follow‐up.
Study funding sources
Two included RCTs did not receive financial support (Caminiti 2015; Dello Iacono 2013). Two studies were supported by national research grants (Burks 2012; Vazquez‐Ortiz 2014). Pérez‐Rangel 2017 was supported by a Merck Sereno grant and by a grant from the ALK‐Abelló pharmaceutical company. Escudero 2015 was not funded, but the English language publication was financially supported by Stallergenes pharmaceutical company. Some authors of the Giavi 2016 study are or were employees for Nestec Ltd. Akashi 2017 declared that the study was partially supported by a grant from the Ministry of Health, Labour and Welfare Japan, and funds from a Research Center of Taiho Pharmaceutical Company. Study funding sources were not reported for two studies (Fuentes‐Aparicio 2013; Meglio 2013).
Withdrawal rate
We calculated the withdrawal rate for each study. The main reason for withdrawal was the occurrence of adverse events. Interestingly, Giavi 2016 reported one dropout due to anxiety. In Burks 2012 the dropout rate was 15% for children in the oral immunotherapy group and 13% for placebo group children; the overall withdrawal rate for both study groups was 14.5%. Fuentes‐Aparicio 2013 reported a 4% overall dropout rate (7.5% for the oral immunotherapy group and 0% for the placebo group). Meglio 2013 reported an overall 5% dropout rate, with 10% in the oral immunotherapy group and 0% in the control group. There were no dropouts in Dello Iacono 2013. Vazquez‐Ortiz 2014 reported 18% withdrawal in the oral immunotherapy group and none in the control group, resulting in an overall withdrawal of 11%. Caminiti 2015 reported an overall dropout rate of 13% (6% for the oral immunotherapy group and 21% for the placebo group). Escudero 2015 had a 7% dropout rate in the oral immunotherapy group and none in the control group, the overall withdrawal rate being 3%. Giavi 2016 reported a 27% withdrawal rate in the oral immunotherapy group and none in the control group; overall withdrawal: 14%. In Pérez‐Rangel 2017, the overall withdrawal rate was 9%: 10.5% in the oral immunotherapy group and 7% in the placebo group. Akashi 2017 had an overall withdrawal rate of 17%: 22% in the oral immunotherapy group and 11% in the placebo group.
Excluded studies
We excluded 11 studies that did not meet our inclusion criteria. The reasons for exclusion were: no previous history of exposure to egg (one study); comparison group not placebo or a continued avoidance diet (seven studies); results were for combined egg and milk‐allergic participants (three studies). See Characteristics of excluded studies.
Studies awaiting classification
Our rsearch revealed abstracts for five studies with insufficient information to assess eligibility. See Characteristics of studies awaiting classification.
Ongoing studies
Searches of clinical trials registers identified two potentially relevant trials (NCT02083471; NCT01846208; Characteristics of ongoing studies). These will be assessed for inclusion in a future review update following completion of the studies.
Risk of bias in included studies
We analysed seven domains of potential risk of bias for the included studies. The risk of bias decisions are shown in Characteristics of included studies, and summarized in Figure 2 and Figure 3.
2.
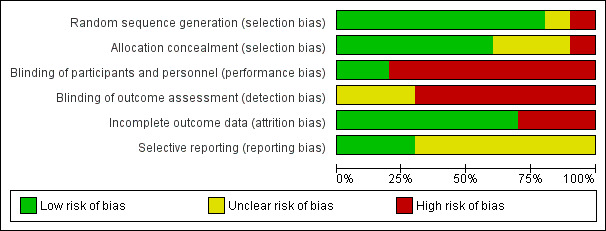
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
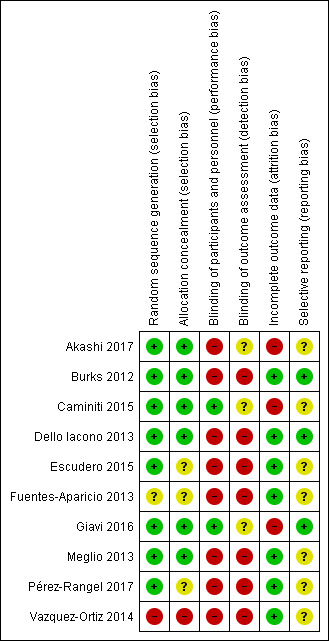
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eight included studies (Akashi 2017; Burks 2012; Caminiti 2015; Dello Iacono 2013; Escudero 2015; Giavi 2016; Meglio 2013; Pérez‐Rangel 2017) provided a description of an adequately generated random sequence and were judged to be at low risk of bias. We judged one study (Fuentes‐Aparicio 2013) to be at unclear risk of bias due to insufficient information. One study was assessed at high risk of bias because participants were divided into two groups (active or control group) based on parent's choice (Vazquez‐Ortiz 2014).
Six included studies provided a description of an adequately concealed allocation and were judged to be at low risk of bias (Akashi 2017; Burks 2012; Caminiti 2015; Dello Iacono 2013; Giavi 2016; Meglio 2013). We judged three studies to be at an unclear risk of bias due to insufficient information (Escudero 2015; Fuentes‐Aparicio 2013; Pérez‐Rangel 2017). One study was assessed at high risk of bias because participants were divided into two groups (active or control group) based on parent's choice (Vazquez‐Ortiz 2014).
Blinding
No studies were assessed at low risk of detection bias. Two studies were at low risk of bias for participant and personnel blinding (Caminiti 2015; Giavi 2016). Eight studies were assessed at high risk of bias for this domain (Akashi 2017; Burks 2012; Dello Iacono 2013; Escudero 2015; Fuentes‐Aparicio 2013; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014), but seven used egg avoidance as a control, making blinding impossible. No studies were assessed at low risk of bias for blinding outcome assessment (detection bias), but was at high risk in seven studies (Burks 2012; Dello Iacono 2013; Escudero 2015; Fuentes‐Aparicio 2013; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014). Three studies were assessed at unclear risk of bias for this domain (Akashi 2017; Caminiti 2015; Giavi 2016).
Incomplete outcome data
Three studies were assessed at high risk of attrition bias because there was an imbalance in numbers for missing data across intervention groups (Akashi 2017; Caminiti 2015; Giavi 2016). Seven studies were assessed at low risk of attrition bias because of low levels of attrition or because attrition was equal between groups (Burks 2012; Dello Iacono 2013; Escudero 2015; Fuentes‐Aparicio 2013; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014) .
Selective reporting
Protocols were available for three studies and were assessed at low risk of bias (Burks 2012; Dello Iacono 2013; Giavi 2016). Seven studies did not provide clear pre‐specified outcomes and were assessed at unclear risk of bias (Akashi 2017; Caminiti 2015; Fuentes‐Aparicio 2013; Escudero 2015; Meglio 2013; Pérez‐Rangel 2017; Vazquez‐Ortiz 2014).
Other potential sources of bias
The included studies appeared free of other sources of bias.
Effects of interventions
See: Table 1
Increase in the amount of egg that could be tolerated (Analysis 1.1)
We pooled nine studies (410 children: 234 intervention/176 control). Most children (82%) in the oral immunotherapy group could ingest a partial serving of egg (1 g to 7.5 g) compared to 10% of the control group (RR 7.48, 95% CI 4.91 to 11.38; RD 0.73, 95% CI 0.67 to 0.80; children = 410; studies = 9; I² = 27% for RR and I² = 73% for RD). Children who did not reach complete desensitization at the end of oral immunotherapy tolerated a higher quantity of egg powder compared to baseline: 4000 mg versus 600 mg (Akashi 2017); 100.8 mg versus 481.3 mg (Escudero 2015). Four children (8%) in Vazquez‐Ortiz 2014 achieved partial desensitization, defined as the ability to digest 0.8 g to1.7 g of raw egg white protein. Partial tolerance (< 40 mL but > 10 mL raw egg emulsion) was achieved in 90% of children in the oral immunotherapy group in Dello Iacono 2013. Although 80% of children in Meglio 2013 achieved intake of 25 mL of raw egg, only 30% who reached the full target dose of 25 mL raw egg without any symptoms and 50% of children presented some symptoms with duration < 2 h and spontaneous resolution. The tolerated egg quantity was not reported in four studies (Burks 2012; Caminiti 2015, Fuentes‐Aparicio 2013; Pérez‐Rangel 2017). Our GRADE assessment judged the quality of evidence to be low.
Complete recovery (Analysis 1.2)
All 10 included trials (N = 439; 249 intervention/190 control) reported this outcome. Burks 2012 reported desensitization in 75% of children after 22 months of oral immunotherapy; the target maintenance dose was 10 g egg white powder. Of note, the data from long‐term follow‐up of the Burks 2012 RCT participants who continued to receive oral immunotherapy daily demonstrated that 45% (18/40 oral immunotherapy‐treated participants) achieved sustained unresponsiveness by year 3, and 50% (20/40 oral immunotherapy‐treated participants) by year 4 (Jones 2016). Fuentes‐Aparicio 2013 reported that 92.5% of children achieved the desired tolerance of 10 g of whole egg. However, only 54% of children who completed the protocol had a good tolerance on the oral food challenge test with raw egg six months after finishing oral immunotherapy, and were allowed a diet containing egg without any restrictions. Dello Iacono 2013 used a six month oral immunotherapy protocol duration to achieve "maximum tolerance" by digesting 40 mL or raw egg emulsion without adverse events. None of the children could tolerate 40 mL of raw egg emulsion by the end of the oral immunotherapy protocol. In Meglio 2013 at the end of the oral immunotherapy protocol 80% of children were able to tolerate 25 mL of raw egg. Of note, just 30% of children (N = 3) tolerated a full raw egg dose without symptoms; 50% of children continued to have some symptoms shortly after digesting the target amount of raw egg. Complete desensitization in Vazquez‐Ortiz 2014 (which was evaluated by oral food challenge to one raw egg, meaning 3.8 g raw egg white) was achieved in 54% of children (N = 28). The oral immunotherapy protocol in Caminiti 2015 aimed to achieve a tolerance a 4 g of dehydrated egg white and 95% of children were successfully desensitized. Desensitized children were placed on an egg‐containing diet with two to three eggs per week for six months, following a three‐month egg avoidance diet. DBPCFC after this time period showed that only 31% of children (5/16 children) maintained the achieved tolerance; the remaining 69% (11/16) lost their desensitization and reacted to egg (Caminiti 2015).
In Escudero 2015, sustained unresponsiveness, defined as the ability to consume 2808 mg of egg white protein without symptoms in a DBPCFC by the end of the oral immunotherapy protocol was achieved in 37% of oral immunotherapy children. These children were allowed to regularly consume food containing egg and at least two undercooked eggs per week. Giavi 2016 aimed to tolerate 43.2 g of boiled egg by the end of the oral immunotherapy protocol and 36% of the children had a negative egg oral food challenge test.
The Pérez‐Rangel 2017 study reported complete desensitization, defined as ability to consume one undercooked egg (fried, scrambled, or omelet) without or with only mild adverse events every 48 hours, was achieved in 89.5% of oral immunotherapy group children by the end of the oral immunotherapy. Akashi 2017 used an oral immunotherapy protocol that was aiming to achieve tolerance of 4 g dry egg powder on oral food challenge: 57% of oral immunotherapy children reached complete desensitization.
A total of 439 children were analyzed (249 intervention/190 control). Fewer than half (45%) of children receiving oral immunotherapy were able to tolerate a full serving of egg compared to 10% of the control group (RR 4.25, 95% CI 2.77 to 6.53; RD 0.35, 95% CI 0.28 to 0.43; children = 439; studies = 10; I² = 22% for RR and I² = 86% for RD). Spontaneous tolerance, defined as the spontaneous resolution of egg allergy without applying specific treatments, was achieved in a total of 19 children: in Fuentes‐Aparicio 2013 (7 children); Vazquez‐Ortiz 2014 (5 children); Giavi 2016 (3 children); Meglio 2013 (2 children); Caminiti 2015 (1 child) and Escudero 2015 (1 child). Our GRADE assessment judged the quality of evidence to be low.
Adverse effects
Number of participants with serious adverse events (SAEs) (Analysis 1.3)
All 10 included trials (N = 439) reported occurrence of serious adverse events. A total of 21 children required epinephrine/adrenaline treatment (Vazquez‐Ortiz 2014, N = 13; Fuentes‐Aparicio 2013, N = 5; 1 each in Caminiti 2015; Escudero 2015 and Akashi 2017). In total, 21 (8.4%) of the 249 children receiving oral immunotherapy required epinephrine. No children in the control group required epinephrine. Our GRADE assessment judged the quality of evidence to be low.
Number of participants with mild‐to‐severe adverse events (Analysis 1.4)
All included trials (N = 439) reported occurrence of mild‐to‐severe adverse events (RR 8.35, 95% CI 5.31 to 13.12; RD 0.67, 95% CI 0.61 to 0.73; children = 439; studies = 10; I² = 29% for RR and I² = 82% for RD). Although all studies provided detailed reports of adverse events, there was significant heterogeneity in methods of reporting. Adverse events occurred most frequently either in the oral immunotherapy escalation phase or in association with oral immunotherapy dosing. Seventy‐five per cent of children presented with mild‐to‐severe adverse events during oral immunotherapy treatment versus 6.8% of control group children. Based on available data, we could not comment on whether over‐ or under‐reporting of adverse events is a concern, although this is a possibility. Our GRADE assessment judged the quality of evidence to be low.
In Burks 2012 (N = 55) adverse events rates were highest during the first 10 months of oral immunotherapy. However, no serious adverse events or need for epinephrine were reported. Adverse events, most of which were oral or pharyngeal, were associated with 25% of 11,860 doses of oral immunotherapy with egg and 3.9% of 4018 doses of placebo. In the oral immunotherapy group 78% of children had oral or pharyngeal adverse events compared with 20% in the placebo group (P < 0.001). After 10 months, the rate of symptoms in the oral immunotherapy group decreased to 8.3% (15,815 doses). In addition to dosing‐related symptoms, 437 other adverse events were reported; 96% were considered to be unrelated to dosing on the basis of the timing and types of symptoms.
Fuentes‐Aparicio 2013 (N = 72) described 21 children (52.5%) who had symptoms at some stage of oral immunotherapy treatment. In eight children (20%) symptoms were very mild, and moderate‐to‐severe in 13 children. Eight children required treatment with antihistamines and corticosteroids and five children required epinephrine treatment.
Dello Iacono 2013 (N = 20) reported that all children who received oral immunotherapy experienced adverse events (n = 53 events) although none resulted from accidental exposure to egg. According to Sampson’s classification, where grade 1 is the mildest anaphylactic reaction and grade 5 is severe anaphylactic reaction with loss of consciousness (Sampson 2003), three (5.6%) were grade 1, 10 (18.9%) were grade 2, 35 (66%) were grade 3, and five (9%) were grade 4. No children in the oral immunotherapy group had a grade 5 reaction, needed oral or intramuscular steroids or epinephrine, or required access emergency services. Eighty‐one per cent of adverse events occurred during administration of the first 6 mL, and 100% occurred with first administration of 20 mL hen's egg, which means that all children experienced some adverse events while increasing the oral immunotherapy dosage. The children in the oral immunotherapy group had relative risk of 4.96 (95% CI 3.30 to 7.45) for incurring an adverse event, but there were no significant differences in severity of reactions. No children discontinued treatment because of adverse events. Three children (30%) in the control group had a total of five adverse events because of accidental ingestion of trace amounts of egg. The severity grade was 3 or 4; no children required intramuscular epinephrine.
In Meglio 2013 (N = 20) 70% of children presented with mild‐to‐severe adverse events. No serious adverse events were reported.
In Vazquez‐Ortiz 2014 (N = 82), 45 children (90%) had dose‐related events including 13 who required epinephrine treatment. There were 1024 adverse events detected, corresponding to 7.6% of oral immunotherapy doses. The highest incidence of events occurred during the induction phase. The symptom distribution of adverse events were: vomiting (19.7%); lower respiratory tract involvement (18.8%); abdominal pain (15.7%); mouth itchiness (13.7%); urticaria (13.1%); rhinoconjunctivitis (7.7%); angioedema (6.7%); hoarseness (2.1%); diarrhoea (1.5%) atopic dermatitis flare (0.7%); and dysphagia (0.5%). Delayed adverse events represented a total of 4.3% of all reactions (3.8% gastro‐ intestinal plus 0.5% skin symptoms). The severity distribution of adverse events (as classified by Sampson's anaphylaxis grading, Sampson 2003) was: grade 1 = 30%; grade 2 = 31.2%; grade 3 = 15.9%; and grade 4 = 22.9%. Grade 4 was experienced by 58% of children receiving oral immunotherapy, consisting of a mild cough/wheeze in 78% of occasions. One child experienced a grade 5 reaction during the induction phase, leading to discontinuation. Multisystem involvement represented a total of 11.5% of all dose‐related adverse events. Accidental adverse events occurred in five children (15.6%) in the control group, nevertheless no epinephrine or adrenaline was needed. There was a total of 1024 adverse events in the oral immunotherapy group and seven in the control group.
Caminiti 2015 (N = 31) reported that adverse events occurred in three children during the desensitization procedure, one of whom discontinued oral immunotherapy. In the egg‐containing diet arm, one child had abdominal pain with urticaria after exercise, and another had rhinitis and/or asthma during a respiratory tract infection. One participant (5.9%) required epinephrine treatment. No adverse events were observed in the control group.
Escudero 2015 (N = 61) reported 145 allergic reactions as adverse events during oral immunotherapy in 70% (N = 21) of children, presented mainly in the escalation phase (70%, N = 21), followed by the build‐up phase (53%, N = 16) and reducing to 33% (N = 10) in the maintenance phase. Ninty‐eight per cent (98%) of children experienced mild symptoms and 2% had a moderate allergic reaction. In one child (0.04%) epinephrine treatment was needed due to a systemic reaction (rhinitis, urticaria, and mild respiratory distress). The protocol was modified for this child by reducing the oral immunotherapy dose by 50%. The most common adverse events were gastro‐intestinal (82%), oropharyngeal symptoms (21.5%), rhinitis (11.4%), respiratory distress (6.3%), and generalized urticaria (3.8%). Moreover, 7% of children (N = 2) withdrew from the study for non‐severe repeated allergic reactions (abdominal pain and vomiting).
Giavi 2016 (N = 29) reported that all children, except one in the oral immunotherapy group, experienced at least one adverse event during the course of treatment. There was no significant difference between the hydrolysed egg and placebo groups with respect to type of adverse events.
In Pérez‐Rangel 2017 (N = 33), safety data during the first control randomized oral immunotherapy period, which was included in our analysis, was received from the authors following personal communication (Ibanez Sandin MD 2017 [pers comm]). Fifteen of 18 children presented with adverse events in the escalation phase (n = 40 adverse events). These included: oropharyngeal symptoms (n = 29); gastro‐intestinal symptoms (n = 17); cutaneous symptoms (n = 4); and rhinitis (n = 4). All adverse events were reported to be mild. At the beginning of the build‐up phase nine of 17 children presented with adverse events (n = 45 adverse events). All adverse events were mild, and the distribution among organ systems were similar to the build‐up phase: oropharyngeal symptoms (n = 37); gastro‐intestinal symptoms (n = 20); cutaneous symptoms (n = 10); rhinitis (n = 1); and asthma (n = 1). Five months after baseline in the maintenance phase, 45 mild adverse events were reported: oropharyngeal symptoms (n = 39); gastro‐intestinal symptoms (n = 10); cutaneous symptoms (n = 18); rhinitis (n = 4); and asthma (n = 2). Participants could present multiple symptoms in the same adverse event.
Akashi 2017 (N = 36) reported that 17 of 18 children in the oral immunotherapy group experienced adverse events: vomiting (N = 4), diarrhoea (N = 5), urticaria or angioedema (N = 6), and respiratory symptoms including coughing and wheezing (N = 3). Of the three children with respiratory symptoms, one developed persistent asthma. Eight children had oral cavity itchiness and six children had mild abdominal pain, but no children required an epinephrine injection. A total of 32 mild‐to‐severe adverse events were reported.
Medication used due to adverse events
Burks 2012 mentioned that only one child received prednisone but without specifying the route, dose, or duration of treatment. In Fuentes‐Aparicio 2013 antihistamine therapy was administrated for 10 cases, steroids in nine cases, B2‐agonists in five cases, and epinephrine treatment in five cases. The administration route, dose, and duration of these medications were not reported. As mentioned previously, all children in Meglio 2013 received cetirizine treatment orally (0.25 mg/kg/day) for the duration of the study, but no other medication was reported (Meglio 2013). In Dello Iacono 2013, information on medication used was reported for both groups, in association with oral immunotherapy dose for the experimental group and for traces of egg in the control group. Unfortunately, the number of cases in which antihistamine treatment was used was not clearly reported in the oral immunotherapy group. In the control group there were three cases requiring treatment. Nebulized epinephrine was used in two cases in the oral immunotherapy group and in two cases in the control group. There were three children in the oral immunotherapy group who needed B2‐agonists, and no cases were documented for steroid administration in the oral immunotherapy group. Additionally, there were two children in the control group who required oral steroid therapy. No information was provided for dose or duration. In Vazquez‐Ortiz 2014 children with asthma on preventive treatment continued therapy during oral immunotherapy. Furthermore, according to the study protocol, antihistamines were indicated for adverse events during oral immunotherapy: rhinoconjunctivitis or skin symptoms, inhaled salbutamol for mild cough or wheeze, and epinephrine for moderate or severe anaphylaxis. There were 18 epinephrine injections in 13 cases, salbutamol was given on 159 occasions and antihistamines in 475, but without specifying the route, dose, or duration of the treatment. Caminiti 2015 reported three cases that required treatment: in one case intramuscular adrenaline, steroid, cetirizine, and inhaled salbutamol was used and oral immunotherapy was discontinued. In the second case oral antihistamine was used, and in the other case inhaled bronchodilators and steroids were used. There were no data available regarding dose or duration of treatment. Escudero 2015 reported only one event where medication was needed ‐ the child required epinephrine treatment; subsequently, the oral immunotherapy protocol was reduced by 50%. No other treatments were reported. Giavi 2016 did not report requirement for medication during oral immunotherapy treatment, despite the presence of adverse events. In Pérez‐Rangel 2017 all children received 10 mg of cetirizine daily during the build‐up phase and for half of the maintenance phase. The study authors did not report whether any medications were used to treat adverse events during the blinded phase of oral immunotherapy, but did report the use of antihistamines, corticosteroids, inhaled short‐acting B2‐broncodilatator and intramuscular adrenaline/epinephrine during the second, open phase of the study. Akashi 2017 reported use of antihistamines for recurrent adverse events, however there were no data available on how many times such a treatment was used during oral immunotherapy. The administration route, dose, and duration of these medications were not reported.
Immunological changes
Skin prick test (SPT)
Nine studies (403 children; 231 oral immunotherapy; 172 control/placebo) reported performing SPT at baseline (Burks 2012;Caminiti 2015;Dello Iacono 2013;Escudero 2015;Fuentes‐Aparicio 2013; Giavi 2016;Meglio 2013;Pérez‐Rangel 2017; Vazquez‐Ortiz 2014) and it was performed at the end of oral immunotherapy in seven studies (total number of children = 292; oral immunotherapy = 166; control/placebo = 126) (Burks 2012;Caminiti 2015;Dello Iacono 2013;Escudero 2015; Fuentes‐Aparicio 2013Meglio 2013;Pérez‐Rangel 2017). There were non‐significant differences between children in the oral immunotherapy and control groups before starting immunotherapy. A significant difference in SPT wheal size was found at the end of oral immunotherapy in all seven studies that performed the test (Table 2). Moreover, Burks 2012 showed that a decrease in wheal size from baseline to 22 months (P = 0.009) was correlated with sustained unresponsiveness at 24 months (P = 0.005).
1. Skin prick test to egg white (wheal size, mm).
| Study name | Oral immunotherapy | Control/placebo | Oral immunotherapy versus control/placebo | |||||
| Pre‐treatment | Post‐treatment | P value | Pre‐treatment | Post‐treatment | P value | P value pre‐treatment | P value post‐treatment | |
| Burks 2012 | 10.5 (2.5 to 26.0) |
not available | not available | 13.0 (7.5 to 20.0) |
not available | not available | not significant | P = 0.02 |
| Caminiti 2015 | 11.0 (6.5 to 18) |
9.2 | P = 0.05 | 9.0 (6.0 to 16.0) |
10.0 | not available | not significant | P = 0.88 |
| Dello Iacono 2013 | 10.0 (7.0 to 15.0) |
5.0 (4.0 to 13.0) |
not available | 9.25 (5.5 to 15.0) |
10.0 (5.5 to 15.0) |
not available | P = 0.976 | P = 0.007 |
| Escudero 2015 | 6.0 (3.0 to 11.0) |
5.0 (3.0 to 8.0) |
P = 0.001 | 6.0 (3.0 to 12.0) |
5.5 (0 to 13.0) |
P = 0.45 | P = 0.2 | P = 0.16 |
| Fuentes‐Aparicio 2013 | 8.74 (4 to 16) |
not available | P < 0.001 | 9.68 (3.0 to 16.0) |
not available | not available | not available | not available |
| Meglio 2013 | 5.5 (2.5 to 7.0) |
3.5 (1.0 to 5.0) |
P < 0.01 | 4.0 (1.0 to 7.0) |
6.0 (1.0 to 8.0) |
P = NS | not available | not available |
| Pérez‐Rangel 2017 | 7.0 (5.0 to 10.0) |
not available | not available | 7.0 (4.0 to 12.0) |
not available | not available | not available | P < 0.05 |
Serologic testing
Specific IgE (sIgE) levels at baseline and at the end of the oral immunotherapy protocol were investigated by using different laboratory methods in each study. However, there were no data available for sIgE levels at the end of the oral immunotherapy protocol in Vazquez‐Ortiz 2014. For sIgE determination Burks 2012, Caminiti 2015, Escudero 2015, Giavi 2016, Pérez‐Rangel 2017, Vazquez‐Ortiz 2014 used the Thermo Immuno‐CAP system (Fisher Scientific); Dello Iacono 2013 and Fuentes‐Aparicio 2013 used the Phadia CAP System fluorescence enzyme immunoassay (FEIA) (Phadia Diagnostics); Meglio 2013 used the Realtest Reverse Enzyme Allergo Sorbent Test (REAST) (Lofarma) and an allergen micro array assay (Immuno Solid‐phase Allergen Chip ISAC; VBC Genomics Bioscience Research, Vienna, Austria) in accordance with the manufacturer’s recommendations. A panel of 104 allergens (inhalants and foods), that also contained the following five purified natural hen's egg allergens: ovomucoid (Gal d 1), ovalbumin (Gal d 2), ovotransferrin (Gal d 3), lysozyme (Gal d 4), and serum albumin (Gal d 5), was used. Akashi 2017 used CAP system for sIgE determination. Because of the differences in the upper limit of measurement it was impossible to combine data.
Nevertheless, two studies reported a statistically significantly decreased levels of egg sIgE antibody after oral immunotherapy compared to control (Burks 2012; Dello Iacono 2013). In Dello Iacono 2013 (N = 20) the difference in the reduction of IgE between pre‐ and post‐oral immunotherapy was ‐14.4 kUA/L (range ‐27.6 to 0.1) in the oral immunotherapy group and 2 kUA/L (range ‐8.9 to ‐13.8) in the control group (P < 0.001). Futhermore, the median reduction in sIgE levels from baseline to the end of oral immunotherapy protocol for egg oral immunotherapy group was not statistically significantly greater than that for control group (Akashi 2017; Burks 2012; Caminiti 2015; Escudero 2015; Fuentes‐Aparicio 2013; Giavi 2016; Pérez‐Rangel 2017). Meglio 2013 (N = 20) reported statistically significant reduction for sIgE before and after oral immunotherapy protocol for oral immunotherapy group: for ovomucoid (Gald1) by REAST (20.9 ± 45.6 versus 14.8 ± 30.7; P = 0.02), and for both ovomucoid (Gald1) (6.67 ± 8.36 versus 1.78 ± 1.74; P < 0.01) and ovalbumin (Gald2) measured by ISAC method (2.92 ± 5.05 versus 0.74 ± 1.22; P < 0.02), respectively. There were no differences in reduction of sIgE measured by REAST technique from baseline compared with the final point of the study in the control group. Interestingly, there was a statistically significant increase in ovomucoid (Gald1) measured using the ISAC method in the control group at the end of the study (5.32 ± 6.89 versus 7.81 ± 10.53; P < 0.05). In Caminiti 2015 (N = 31), the egg‐specific IgE level after oral immunotherapy was 34.2 kUA/L in the intervention group and 38.9 kUA/L in the placebo group. Of note, follow‐up of these children showed that at 10 months, formerly active oral immunotherapy children who continued with an egg‐containing diet for the following six months had significantly lower sIgE levels compared to baseline (37.6 versus 12.2 kUA/L, P = 0.02). Escudero 2015 (N = 61) reported a statistically significant decrease from baseline to the end of oral immunotherapy in both groups only for ovalbumin IgE (6.3 kUA/L (0.7 to 185) versus 2.2 kUA/L (0.3 to 192), P < 0.001) and 2.2 kUA/L (0.7 to 43.5) versus 1.3 kUA/L (0.3 to 32.3), P = 0.01); no differences were seen for ovomucoid or egg white IgE. Akashi 2017 (N = 36) reported a statistically significant decrease in sIgE levels from baseline to the end of the study in the control group (median 36.4 versus 30.6 kUA/mL; P < 0.05). It was not possible to extrapolate data for sIgE antibodies in Burks 2012, Fuentes‐Aparicio 2013, or Pérez‐Rangel 2017. In Giavi 2016 (N = 29) no statistically significant difference was observed regarding sIgE levels (change over time expressed as median (min‐max) ‐3.230 kUA/L (‐8.90 to 0.42); P = 0.29).
Eight studies (total number of children = 337; oral immunotherapy = 189; control/placebo = 148) evaluated changes in egg‐specific IgG4 antibodies and all eight showed a statistically significant increase in the level of these antibodies after oral immunotherapy (Akashi 2017; Burks 2012; Caminiti 2015; Escudero 2015; Fuentes‐Aparicio 2013; Giavi 2016; Meglio 2013; Pérez‐Rangel 2017). Akashi 2017 (N = 36) demonstrated a statistically significant increase of egg white‐specific IgG4 in the oral immunotherapy group after completing the oral immunotherapy protocol (median, 13 U/mL at baseline to 94 U/mL at the end of oral immunotherapy; P < 0.01), whereas such a change was absent in the control group (median, 13 U/mL at baseline to 13 U/mL; P = 1.00). In Burks 2012 (N = 55) the median reduction of egg‐specific IgG4 by the end of oral immunotherapy for the oral immunotherapy group was significantly higher (median, 48.5 kU/L range ‐0.1 to 162.1; P < 0.01) compared to the placebo group (there were no numerical data available for the placebo group). Caminiti 2015 (N = 31) reported a significant increase of the mean egg‐specific IgG4 levels by the end of oral immunotherapy in intervention group children (29.2 µg/mL versus 0.9 µg/mL, P = 0.001). In Escudero 2015 (N = 61) there was an increase in median egg‐specific IgG4 observed from baseline to the end of oral immunotherapy in the oral immunotherapy group (0.4 mg/L (0.1 to 6.2) versus 4.4 mg/L (0.1 to 30), P = 0.0001). Giavi 2016 (N = 29) reported a significant increase over time from baseline to the end of oral immunotherapy in specific IgG4 in the active group (expressed as change over time, median (range): for egg yolk IgG4 (0.10 mg/L, 0.00 to 1.71, P = 0.01) and for ovalbumin IgG4 (0.11 mg/L, ‐0.00 to 3.59, P = 0.04); there were no differences observed in the median change of IgG4 levels for egg white (0.07 mg/L, ‐0.31 to 2.54, P = 0.07) and for ovomucoid (0.00 mg/L, ‐0.56 to 1.04, P = 0.14). Meglio 2013 (N = 20) found a statistically significant increase in the level of IgG4 only for ovalbumin (Gald2) at the end of oral immunotherapy (average, ± SD 2.6 mg/L (± 0.4) versus 2.95 mg/L (± 0.4); P = 0.02), but not for ovomucoid (Gald1) (average, ± SD 0.9 mg/L (± 0.7) versus q.8 mg/L (± 1.5). Data for the egg‐specific IgG4 levels were presented graphically in two studies and could not be extracted for analysis (Fuentes‐Aparicio 2013; Pérez‐Rangel 2017).
In addition, six studies (281 children; oral immunotherapy = 161; control/placebo = 120) showed a statistically significant increase in IgG4 compared to the control group (Burks 2012; Caminiti 2015; Escudero 2015; Fuentes‐Aparicio 2013; Giavi 2016; Pérez‐Rangel 2017). In Caminiti 2015 (N = 31) egg‐specific IgG4 levels after oral immunotherapy were statistically significantly higher in the intervention group (29.2 µg/mL) than in the placebo group (1.5 µg/mL, P = 0.001). Follow‐up of these children showed that at 10 months, formerly active oral immunotherapy children who continued with egg‐containing diet for the following six months had significantly higher sIgG4 levels compared to baseline (32.4 versus 0.9 µg/mL, P = 0.001). Median sIgG4 levels in Escudero 2015 (N = 61) were higher in the oral immunotherapy group by the end of oral immunotherapy than in the control group (4.4 mg/L (0.1 to 30) versus 0.4 mg/L (0.1 to 7.5); P = 0.001). Giavi 2016 (N = 29) reported that a greater increase of egg‐specific IgG4 occurred in the oral immunotherapy group compared to placebo by the end of oral immunotherapy, however these data were not reported. It was not possible to extrapolate precise data for sIgG4 from Burks 2012, Fuentes‐Aparicio 2013 and Pérez‐Rangel 2017 because data were reported graphically.
Two studies reported a significant reduction in basophil activation test in the oral immunotherapy group compared to placebo at the end of treatment (Burks 2012; Giavi 2016) (84 children; oral immunotherapy group = 55; placebo group = 29): in Burks 2012 the differences in CD63 activation were statistically significant (0.01 μg/mL, P = 0.002; 0.1 μg/mL, P = 0.001). In Giavi 2016 a significant decrease in percentage of CD203c+ cells (P = 0.04) occurred and there was a trend for a lower percentage of CD63+ cells (P = 0.07).
Meglio 2013 (N = 20) performed cytokines (IL‐4, IL‐5, IL‐6, IL‐10, IL‐12, IL‐13), interferon‐gamma and tumour necrosis factor‐alfa analysis and found a statistically significant increase of IL‐5 levels in the oral immunotherapy group at the end of treatment (mean, 19.00 ± 9.4 pg/mL; range = 5.00 to 32.00 versus 28.13 ± 14.7 pg/mL; range, 12.00 to 56.00; P = 0.03).
Quality of life and cost‐effectiveness
There were no data available regarding quality of life or cost‐effectiveness.
Discussion
Summary of main results
Egg oral immunotherapy appears to be an effective treatment option to induce desensitization in children with immunoglobulin E (IgE)‐mediated egg allergy, despite a difference in immunotherapy protocols. Nevertheless, oral immunotherapy is an alternative to the current therapeutic mainstay of an avoidance diet. Oral immunotherapy may have a positive effect on overall quality of life, including positive nutritional consequences and the potential avoidance of allergy related emergencies.
Nearly half (45%) of children receiving oral immunotherapy were able to tolerate a full serving of egg compared to 10% of the control group (RR 4.25, 95% CI 2.77 to 6.53; RD 0.35, 95% CI 0.28 to 0.43; children = 439; studies = 10; I² = 22% for RR and I² = 86% for RD). Our GRADE assessment judged the quality of evidence to be low (Table 1). We found that 82% of children receiving oral immunotherapy increased the amount of egg they could tolerate during the treatment period (RR 7.48, 95% CI 4.91 to 11.38; RD 0.73, 95% CI 0.67 to 0.80; children = 410; studies = 9; I² = 27% for RR and I² = 73% for RD). Our GRADE assessment judged the quality of evidence to be low (Table 1). There were differences in the levels required for partial desensitization (defined as an increase in the threshold dose, not reaching one complete raw egg) achieved. Such differences may be due to different egg oral immunotherapy protocols used in the included trials. Moreover, it seems that regular consumption of uncooked egg and other egg‐containing food after stopping oral immunotherapy may be helpful to achieve sustained unresponsiveness to egg allergy. It was reported that 16 children who received oral immunotherapy achieved desensitization after six months of an egg‐containing diet, but after three months of an egg‐avoidance diet, only five children maintained tolerance to re‐introduction of eggs to their diet (Caminiti 2015). Of note, long‐term follow up of Burks 2012 reported that 50% of the children who were treated with egg oral immunotherapy in the original cohort achieved a sustained unresponsiveness by the fourth year of oral immunotherapy.
There is a possibility that oral immunotherapy may modulate immunological response (Jones 2016; Wright 2016). Seven studies reported a significant reduction in wheal diameter on skin prick test after completion of oral immunotherapy (Burks 2012;Caminiti 2015;Dello Iacono 2013;Escudero 2015;Fuentes‐Aparicio 2013; Meglio 2013;Pérez‐Rangel 2017). Moreover, two studies showed a reduction in egg‐specific immunoglobulin E (sIgE) levels versus control (Burks 2012; Dello Iacono 2013). Two further studies showed a reduction in egg sIgE levels versus baseline in children who received oral immunotherapy (Escudero 2015; Meglio 2013). Of note, all eight studies that evaluated egg sIgG4 levels showed an increase in egg sIgG4 levels after oral immunotherapy (Akashi 2017; Burks 2012;Caminiti 2015;Escudero 2015;Fuentes‐Aparicio 2013; Giavi 2016;Meglio 2013;Pérez‐Rangel 2017). Furthermore, two studies demonstrated a significant reduction in basophil activation in the oral immunotherapy group compared to placebo at the end of treatment (Burks 2012;Giavi 2016). However, none of the immune parameters tested in the included studies were consistently predictive of the oral immunotherapy treatment outcomes (Akashi 2017; Burks 2012;Caminiti 2015;Dello Iacono 2013;Escudero 2015;Fuentes‐Aparicio 2013; Giavi 2016;Meglio 2013;Pérez‐Rangel 2017; Vazquez‐Ortiz 2014). More data are needed to better understand the underlying mechanism that might be induced by oral immunotherapy.
It is important to note that 75% of children in the intervention groups experienced at least one adverse event during oral immunotherapy (RR 8.35, 95% CI 5.31 to 13.12; RD 0.67, 95% CI 0.61 to 0.73; children = 439; studies = 10; I² = 29% for RR and I² = 82% for RD). Our GRADE assessment judged the quality of evidence to be low (Table 1). Most symptoms were related to the build‐up phase in association with oral immunotherapy dosing. However, most symptoms were mild and self‐limiting. We found that 8.4% of children had a serious adverse event, requiring epinephrine/adrenaline administration (effect not estimable, our GRADE assessment judged the quality of evidence to be low, Table 1). Because adverse events were defined differently among the included studies, it is difficult to comment on whether these were under‐ or over‐estimated. Caminiti 2015 reported 10 adverse events, but Vazquez‐Ortiz 2014 reported 1024 adverse events. Surprisingly, only one study showed a high level of serious adverse events (Vazquez‐Ortiz 2014): 13 children required epinephrine/adrenaline administration 18 times, and one grade 5 reaction occurred (according to the Sampson 2003 classification scheme). Vazquez‐Ortiz 2014 reported that available treatments were insufficiently effective. The risks associated with administering oral immunotherapy need to be evaluated against the 'real life' risks of standard care, namely an avoidance diet, although it is difficult to accurately estimate the true rate of accidental allergic reactions.
We found that reporting on different potential medications was poor. Although four included studies provided detailed information about antihistamine, steroid, epinephrine/adrenaline, and B2‐agonists administration in association with oral immunotherapy dose, and symptoms that occurred on those specific occasions, information on administration route, dose and duration of use was absent (Caminiti 2015; Dello Iacono 2013; Fuentes‐Aparicio 2013; Vazquez‐Ortiz 2014). Only Meglio 2013 and Pérez‐Rangel 2017 administrated antihistamine treatment as a co‐intervention with oral immunotherapy. It was therefore difficult to assess whether oral immunotherapy‐induced adverse allergic reactions could be avoided. Likewise, because Akashi 2017, Burks 2012 and Giavi 2016 did not provide specific information regarding medications used for adverse events (antihistamines, steroids, epinephrine/adrenaline, B2‐agonists), it was difficult to assess the extent of oral immunotherapy‐induced adverse events. No study reported on drug administration route, dose and duration of such therapy.
Sclar 2014 reported that anaphylaxis is often underestimated. In a study of food‐related allergic reactions in children, the most common reason given for lack of epinephrine/adrenaline administration to children experiencing a severe adverse event was that the event was not recognized as severe (47.7% of cases in which epinephrine/adrenaline was not used) (Fleischer 2012).
Overall, the dropout rate, mainly due to adverse events, has been reported at 15% to 20% in oral immunotherapy trials (Khoriaty 2013).The dropout rate data from Akashi 2017, Burks 2012, Caminiti 2015, Giavi 2016, Vazquez‐Ortiz 2014 agrees with the information reported previously in Khoriaty 2013. However, the withdrawal rate in the other five studies (Dello Iacono 2013; Escudero 2015; Fuentes‐Aparicio 2013; Meglio 2013; Pérez‐Rangel 2017) was surprisingly low, with Dello Iacono 2013 having no dropouts: it may be that participant collaboration is easier in a smaller study, perhaps because there is more medical attention. Dello Iacono 2013 did not discuss their high adherence rate within the study. Meglio 2013 discussed the high adherence rate in their study, giving as the possible explanation a longer intervention period; the protocol took longer than the expected period (planned 181 days) with a mean of 219 days (range 178 to 287 days). The very gradual protocol that was adopted may encourage more safety and more parental collaboration as the percentage increment between doses was the same and there were no dose 'jumps' in the daily egg administrations for the entire period of desensitization. Moreover, co‐intervention with antihistamine was used in their study. More studies are needed to confirm or to disprove such a hypothesis.
Overall completeness and applicability of evidence
All included studies addressed the primary outcome of complete tolerance of one egg serving. The 10 included studies included only children and recruitment selection bias was possible. No included studies reported on adults. The study population characteristics suggest that the included studies may be generalizable to a larger population group. Oral immunotherapy appears to be effective both in young children and in later childhood. However, children aged less than three years were included in only Giavi 2016, but the sample size was too small to draw any conclusions. More studies are needed in this age group. Nevertheless, one randomized controlled trial is registered in clinical.trials.gov that will also include adults (NCT02083471). Another factor that may influence the development of desensitization is the presence of anaphylaxis. Of the 10 included studies, two included children with a history of anaphylaxis (Dello Iacono 2013; Fuentes‐Aparicio 2013). Oral immunotherapy appeared to be effective regardless of anaphylaxis history.
Oral immunotherapy both with and without an antihistamine co‐intervention was included in the review; this did not seem to affect the treatment effect.
Of note, 45% of children achieved full tolerance after oral immunotherapy and could ingest a full serving of egg without adverse effect; these children were able to continue diets that included egg. However, concerns about compliance and interruption due to sickness should be addressed.
We found that 82% of children could tolerate a partial serving of egg, reducing dietary limitations, and risk of serious adverse event. Adverse events are likely to be the most important factors to be considered when applying evidence to practice. The difference in reporting methods for symptoms during oral immunotherapy makes those symptoms complicated to quantify. As previously mentioned, there is a risk of both under‐ or over‐estimation. The need for parenteral epinephrine treatment could be a reflection of serious adverse events. Five studies reported the data of children in the oral immunotherapy group who needed epinephrine/adrenaline treatment (Akashi 2017; Caminiti 2015; Escudero 2015; Fuentes‐Aparicio 2013; Vazquez‐Ortiz 2014). Requirement was as high as 26% of the oral immunotherapy group population (Vazquez‐Ortiz 2014). On the other hand, frequent, milder symptoms during oral immunotherapy might influence participants’ quality of life and compliance. Inquiring about the study participants' acceptance of this therapy would be insightful. If applied to practice, physicians would have to cautiously consider, and discuss with the participant at length, whether the risks are acceptable; and to ensure good compliance of the children's parents or guardians.
With one exception, all children in the included studies started oral immunotherapy during hospital admission, as day patients, or in a research centre. Akashi 2017 started oral immunotherapy treatment at the child's home, but with medical personnel available to intervene if allergic reactions occurred. During the build‐up phase children were closely monitored during visits to the clinic or research centre, where close follow‐up and individualized guidance was provided. Whether these time and personnel commitments are feasible in practice could be a factor, and are dependent on the institution providing the oral immunotherapy.
There was no information available regarding children's and their families quality of life during oral immunotherapy. It could be speculated that a high rate of even mild adverse events may negatively influence the everyday life of participants, and in the case of children, their caregivers.
Quality of the evidence
According to the GRADE approach described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the overall judgement of the quality of the body of evidence contributing to the results of the review is low. The reasons for downgrading evidence was the high likelihood of bias and imprecision of results (few events and a small number of participants). High likelihood of bias is suspected because of the methodological limitations in the studies, resulting in possible selection, performance, and detection bias. The confidence intervals were wide because of small sample sizes and variability in the estimated treatment effect. Four studies did not perform a sample size calculation and this could be a source of random error risk (Akashi 2017; Fuentes‐Aparicio 2013; Meglio 2013; Pérez‐Rangel 2017). The random error is closely related to imprecision. The results of smaller studies are subject to greater sampling variation and hence are less precise. Imprecision is reflected in the confidence interval around the intervention effect estimate from each study and in the weight given to the results of each study in the meta‐analysis (Higgins 2011). However, there was a consistently positive treatment effect of oral immunotherapy despite clinical heterogeneity, although a low I² value reflects non‐substantial statistical heterogeneity, and differences in oral immunotherapy protocols among studies.
We did not include a sufficient number of studies to create a funnel plot to assess publication bias. Given few included studies, most of which detected statistically significant results, we could not rule out the presence of reporting bias. The strength of the evidence was that there was a consistently positive treatment effect, despite clinical heterogeneity in participant characteristics and oral immunotherapy protocols. Furthermore, we could not rule out the possibility of under‐or over‐reporting of adverse events during oral immunotherapy.
Potential biases in the review process
A strength of this review was the thorough search strategy, including several databases, conference papers, and trial registries, to minimize the possibility of missing studies. A possible source of bias relates to the exclusion of potentially relevant studies when requests to the authors for additional information went unanswered. These studies are listed in the Characteristics of studies awaiting classification tables. Moreover, some studies that fulfilled our criteria were excluded because they combined results for immunotherapy for two or more foods. For example, Staden 2007, fulfilled the eligibility criteria but results for both egg and milk immunotherapy were combined in the published article and we were unable to split the data to focus only on egg allergy. It is possible that the inclusion of such data could have altered the results of the meta‐analysis. The ongoing studies (NCT02083471; NCT01846208) emphasize the need for frequent updates of this review as oral immunotherapy for egg allergy continues to be a topic of active research. Ongoing and future studies could potentially affect our conclusions.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the only systematic review on immunotherapy specifically for egg allergy. The findings of our review are in agreement with a systematic review and meta‐analysis on food allergies that included evaluation of egg oral immunotherapy as a subgroup (Nurmatov 2017).
Nurmatov 2017 included nine studies in the egg oral immunotherapy subgroup (Burks 2012;Caminiti 2015;Dello Iacono 2013;Escudero 2015;Fuentes‐Aparicio 2013Meglio 2013;Morisset 2007;Patriarca 1998;Staden 2007). Nurmatov 2017 excluded Vazquez‐Ortiz 2014, and did not include three studies included in this review (Akashi 2017; Giavi 2016;Pérez‐Rangel 2017). This was probably due to the last search date in Nurmatov 2017, which was 31 March 2016. We did not include Morisset 2007, Patriarca 1998 and Staden 2007 because these studies combined results for egg and milk allergy.
Nurmatov 2017 found that oral immunotherapy substantially reduced the risk for egg allergy (RR hen's egg = 0.22, 95% CI 0.11 to 0.45). Of note, safety data remain a challenge. Nurmatov 2017 pooled safety data for three food allergies (cow's milk, egg, and peanut allergies), making comparisons difficult.
We included 10 studies that reported significant numbers of adverse events during oral immunotherapy (Vazquez‐Ortiz 2014), which was not included in Nurmatov 2017. Another difference regarding safety between our review and Nurmatov 2017 was documentation of serious adverse events of oral immunotherapy treatment, requiring epinephrine/adrenaline administration. This was not reported in Nurmatov 2017. Moreover, medication used for the treatment of adverse events was not reported in Nurmatov 2017.
An outcome in both our review and in Nurmatov 2017 was assessment of changes in disease‐specific quality of life using a validated instrument. Results were similar in both reviews: both reported lack of quality of life data.
We agree with conclusions made by the authors of Nurmatov 2017 that oral immunotherapy may be effective in increasing desensitization in participants with IgE‐mediated egg allergy, and that oral immunotherapy is associated with a modest increased risk in serious systemic adverse events and a substantial increase in mild‐to‐severe adverse events.
Authors' conclusions
Implications for practice.
Studies to date involved few participants and evidence quality is low. Despite that, most children in the 10 included studies reached partial tolerance of egg. Moreover, some children were able to achieve full tolerance, and therefore, improved quality of life and nutritional requirements.
Oral immunotherapy is very labour intensive and induces adverse allergic reactions in virtually all participants who are treated (although with mostly mild symptoms). Eosinophilic oesophagitis occurred in 10% to 15% of people treated with immunotherapy. A major difficulty of oral immunotherapy is the frequency of adverse events although most seem to be mild‐to‐moderate and self‐limiting. The use of parenteral epinephrine/adrenaline, as suggested by the guidelines in the case of a life‐threatening adverse event (Muraro 2014), is variable and may depend on oral immunotherapy protocol. Thus, physicians and their patients need to acknowledge and accept the risks of oral immunotherapy. Because there are no standardized protocols, guidelines based on high quality clinical trials would be required before incorporating desensitization into clinical practice.
Implications for research.
Evidence quality was limited by small sample sizes and lack of methodological rigour. A larger randomized controlled trial that aims to eliminate selection, performance, and detection bias, would provide a more precise estimate of the treatment effect. Investigators should be encouraged to use a standard double‐blinded placebo‐controlled food challenge (DBPCFC) protocol, such as described by Sampson 2012, to enable better comparison of clinical trial outcomes and facilitate transition into practice.
To date, there are few data available on oral immunotherapy in toddlers and no data on oral immunotherapy in adults. Given that some proportion of young egg‐allergic children tolerate heat‐denatured (baked) egg products, and the addition of these products to their diet appears to accelerate the development of tolerance with minimal adverse events, it may be safer and easier to identify these children and simply add baked‐egg products to their diets (more studies are needed in this area).
All 10 included studies investigated oral immunotherapy. There is a gap in knowledge regarding sublingual immunotherapy which should be explored in future studies.
In this review, adverse effects were difficult to quantify because of variability in reporting. Future studies should attempt to standardize reporting of adverse effects and to develop a consensus severity scale of adverse events could facilitate reporting.
Safety of oral immunotherapy should be further rigorously and transparently investigated before oral immunotherapy could be applied in clinical practice. Futhermore, studies with longer follow‐up are needed to provide data relating to long‐term safety of oral immunotherapy.
Further investigation of the mechanisms that lead to sustained unresponsiveness is also needed. Co‐interventions to decrease the rate of adverse effects may be key and need to be explored. The quality of life of people with allergies and their caregivers should be investigated and reported in a standardized manner. A cost‐effectiveness analysis is also needed.
What's new
| Date | Event | Description |
|---|---|---|
| 15 November 2017 | New citation required but conclusions have not changed | Conclusions unchanged |
| 26 May 2017 | New search has been performed | In the 2018 review update, six additional trials were included, resulting in a total of 439 participants. |
Acknowledgements
We thank Dr Rita Banzi for her precious advice.
We thank Lindsay Stead for her guidance in the search for trials.
Appendices
Appendix 1. MEDLINE search strategy
Search term
1. "Egg Hypersensitivity"[Mesh]
2. "Immunotherapy"[Mesh]
3. 1 AND 2
4. "administration"[All Fields]
5. "sublingual"[All Fields]
6. 4 AND 5
7. "mouth"[MeSH Terms]
8. "mouth"[All Fields]
9. "oral"[All Fields]
10. "administration, sublingual"[MeSH Terms]
11. "sublingual administration"[All Fields]
12. "sublingual"[All Fields]
13. OR/6‐12
14. Clinical Trial[ptyp]
15. 3 AND 13 AND 14
Appendix 2. Other databases search strategies
1. randomz.ti,ab.
2. factorial.ti,ab.
3. (cross over or crossover or cross‐over).ti,ab.
4. placebo.ti,ab.
5. (double adj blind).ti,ab.
6. (single adj blind).ti,ab.
7. assign.ti,ab.
8. allocat.ti,ab.
9. volunteer.ti,ab.
10. CROSSOVER PROCEDURE.sh.
11. DOUBLE‐BLIND PROCEDURE.sh.
12. RANDOMIZED CONTROLLED TRIAL.sh.
13. SINGLE‐BLIND PROCEDURE.sh.
14. or/1‐13
15. egg allergy/
16. egg hypersensitivity.mp.
17. (egg and hypersensitivity).mp.
18. immunotherapy/ or oral immunotherapy/ or sublingual immunotherapy/
19. immunotherapy.mp.
20. 15 or 16 or 17
21. 18 or 19
22. 20 and 21
23. 14 and 22
Data and analyses
Comparison 1. Oral and sublingual immunotherapy versus no therapy for egg allergy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Increase in the amount of egg that can be tolerated | 9 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.48 [4.91, 11.38] |
| 2 Complete recovery | 10 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [2.77, 6.53] |
| 3 Number of participants with serious adverse events | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Number of participants with mild‐to‐severe adverse events | 10 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.35 [5.31, 13.12] |
1.1. Analysis.
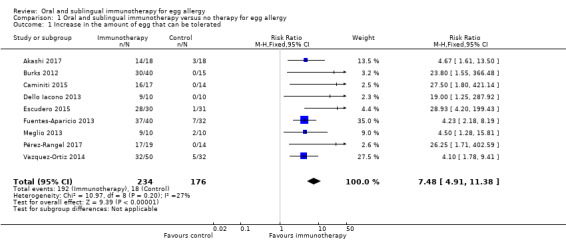
Comparison 1 Oral and sublingual immunotherapy versus no therapy for egg allergy, Outcome 1 Increase in the amount of egg that can be tolerated.
1.2. Analysis.
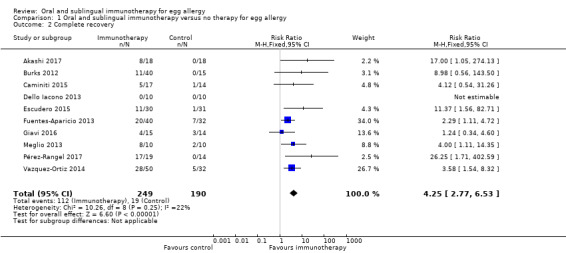
Comparison 1 Oral and sublingual immunotherapy versus no therapy for egg allergy, Outcome 2 Complete recovery.
1.3. Analysis.
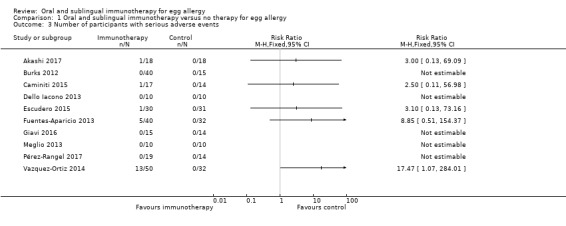
Comparison 1 Oral and sublingual immunotherapy versus no therapy for egg allergy, Outcome 3 Number of participants with serious adverse events.
1.4. Analysis.
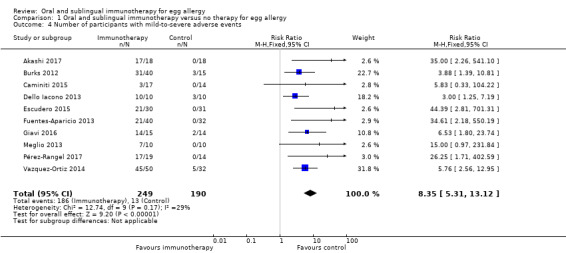
Comparison 1 Oral and sublingual immunotherapy versus no therapy for egg allergy, Outcome 4 Number of participants with mild‐to‐severe adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akashi 2017.
| Methods | Randomized controlled trial | |
| Participants | 36 participants, age 3 years to 15 years, inclusion criteria as defined by authors: (i) complete elimination of egg from the diet; (ii) egg‐specific IgE ≥ 0.7 UA/mL; (iii) positive immediate allergic reaction on double‐blind, placebo‐controlled food challenge (DBPCFC) for egg performed after admission to hospital; and (iv) participant and caregiver desire to join this study. | |
| Interventions | Participants in the oral immunotherapy group ingested the dry powder every day, while the control participants followed their usual egg avoidance diet. The starting dose of oral immunotherapy was 0.1 mg dried powdered egg, and this was increased to 0.2, 0.3, 0.6, 1, 1.5, 2, 3, 4, 6, 10, 15, 30, 50, 70, 100, 150, 200, 300, 500, 700 mg, 1, 1.2, 1.5, 2, 3, and 4 g, at 3 to 4 day intervals. Once a dose of 4 g, equivalent to the total dose in the oral food challenge, was achieved, participants continued on that dose daily until the second oral food challenge. Control group: egg avoidance diet. |
|
| Outcomes | The primary outcome was the percentage of participants who were able to tolerate 4 g powdered egg without allergic symptoms in the second oral food challenge. Secondary outcomes were comparison of cumulative tolerated dose in the first oral food challenge with that in the second oral food challenge, and the changes in egg white‐specific IgE and IgG4. |
|
| Vested interest | The authors declared no conflicts of interest. | |
| Notes | The study was approved by the institutional ethics committee, and registered with the UMIN Clinical trials registry (Clinical trial registry number, UMIN000001268). The study was partially supported by a grant from the Ministry of Health, Labour and Welfare Japan and funds from a Research Center of Taiho Pharmaceutical Company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned by means of a computerized system. |
| Allocation concealment (selection bias) | Low risk | Randomization was performed using a computerized system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All outcomes accounted for but imbalance in numbers for missing data across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
Burks 2012.
| Methods | RCT, double‐blind Placebo‐controlled |
|
| Participants | 55 participants, aged 5 to 11 years, a convincing clinical history of egg allergy (shown by the development of allergic symptoms within minutes to two hours after ingesting egg) and a serum egg‐specific IgE antibody level of more than 5 kU per litre for children 6 years of age or older, or 12 kU per litre or more for those 5 years old. | |
| Interventions | The protocol for oral immunotherapy consisted of three phases: an initial‐day dose escalation of median 18.5 mg (range 6 mg to 50 mg) a build‐up phase of median 9 mg (range 3 mg to 50 mg) a maintenance phase during which participants ingested up to 2 g of egg white powder per day Control group: placebo |
|
| Outcomes | The primary endpoint of the study was the induction of sustained unresponsiveness after 22 months The secondary endpoints were:
|
|
| Vested interest | Comment: this information was not available. | |
| Notes | Supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID) (U19AI066738 and U01AI066560) and the National Institutes of Health–National Center for Research Resources Clinical Translational Science Awards and Clinical Research Centers (RR‐024128, to Duke University Medical Center; RR‐025005, to Johns Hopkins School of Medicine; RR‐025780, to National Jewish Health; RR‐029887, to Mount Sinai School of Medicine; and RR‐029884, to the University of Arkansas for Medical Sciences). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned by means of a centralized computer algorithm |
| Allocation concealment (selection bias) | Low risk | Randomization by means of a centralized computer algorithm |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was blinded only for the first 10 months: the primary endpoint was evaluated after 22 months |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was blinded only for the first 10 months: the primary endpoint was evaluated after 22 months |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes accounted for and balance in numbers for missing data across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the protocol (NCT00461097, ClinicalTrials.gov number) were reported |
Caminiti 2015.
| Methods | Double‐blinded randomized controlled trial | |
| Participants | 31 children, age 4 to11 years, clinical history of egg allergy, positive skin prick test (SPT), specific IgE positive assay for egg, and positive double‐blind placebo‐controlled food challenge (DBPCFC) at a starting dose of 0.12 g egg white. According to clinical history, none of the children had previously consumed baked eggs. | |
| Interventions | The oral immunotherapy procedure consisted of weekly administration, at the hospital clinic, of increasing dosages of dehydrated egg white, diluted in sterile saline, starting with 0.1 mg. The dose was doubled every week until week 16, to achieve a cumulative dose of 4 g in approximately 4 months. The placebo (corn flour, indistinguishable from active) was administered following the same protocol. The doses were prepared separately by a trained nurse according to the randomization list, so that the physicians remained blinded to the treatment. All doses were administered at the hospital. Control group: placebo |
|
| Outcomes | The primary endpoint was the achievement of desensitization after the 4‐month randomized period of oral immunotherapy with dehydrated egg white. Secondary endpoints were the maintenance of tolerance to hen's egg after the 3‐month period of withdrawal, together with selected immunological parameters (IgE, IgG4, wheal diameter). | |
| Vested interest | The authors declare that they have no relevant conflicts. | |
| Notes | No funding was received for this work. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned by means of a computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Randomization by means of a computer‐generated list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The doses were prepared separately by a trained nurse according to the randomization list, so that the physicians remained blinded to the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All outcomes accounted for but imbalance in numbers for missing data across intervention group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and therefore selective outcome reporting could not be assessed |
Dello Iacono 2013.
| Methods | Randomized controlled trial | |
| Participants | 20 children, aged 5 years to 11 years, with severe IgE‐mediated hen's egg allergy, were recruited. Inclusion criteria were as follows: (i) at least 1 anaphylactic reaction (grade 3, 4 or 5 according to Sampson’s grading) after accidental exposure to trace amounts of hen's egg or egg‐derived products, within 12 months of pre‐enrolment. (ii) previous SPT/IgE positive for hen's egg (iii) a positive double‐blind placebo‐controlled food challenge (DBPCFC) at a dose of 0.9 mL of raw hen's egg emulsion |
|
| Interventions | Oral immunotherapy protocol consisted of starting with one drop of undiluted raw hen's egg emulsion (0.015 mL) flavoured with vanilla and cacao in day hospital then continued at home with gradually increasing doses mixed by the parents in the child’s breakfast (cow’s milk, soy milk, fruit juice or other) and five doubling doses in day hospital. Dose increases were customized based on the frequency and severity of side effects, and in the case of intercurrent illness or asthma worsening. Control group: egg avoidance diet |
|
| Outcomes |
|
|
| Vested interest | The authors declared no conflicts of interest | |
| Notes | There was no funding source | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer scientist not involved in the study was responsible for the computerized randomization of the children to each group. |
| Allocation concealment (selection bias) | Low risk | Computerized randomization of the children to each group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Healthcare staff and parents were aware of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Healthcare staff and parents were aware of the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the protocol (NCT01379651, ClinicalTrials.gov number) were reported |
Escudero 2015.
| Methods | Controlled randomized clinical trial | |
| Participants | 61 egg‐allergic children, aged 5 years to 17 years, with a history of symptoms of allergic reaction immediately or within two hours following egg ingestion, positive skin prick test (SPT) (≥ 3 mm) and specific IgE (sIgE) levels ≥ 0.7 kU/L for egg white, ovalbumin (OVA) and/or ovomucoid (OVM), and a positive double‐blind placebo‐controlled food challenge (DBPCFC) to dehydrated egg white at enrolment. | |
| Interventions | Children received egg oral immunotherapy for three months followed by egg avoidance diet for one month. The allergen source used in the initial‐day dose escalation and build‐up phases of the egg oral immunotherapy was dehydrated egg white. The egg oral immunotherapy protocol consisted in three phases: i. initial day dose escalation phase, which consisted in the administration in 1 day of 0.08, 0.2, 0.3, 0.5, 1, 2, 5, 9, 17, 35, 70 and 140 mg of egg white protein (cumulative dose 280 mg) at intervals of 20 minutes, ii. BP, which consisted of increases in the dose of 0.02, 0.3, 3, 14, 68, 188, 352, 1404 mg and 2808 mg of egg white protein on a weekly basis, and iii. maintenance phase (MP), which consisted of eating at least one undercooked egg (fried egg, scrambled or undercooked omelette) compulsory every 48 hours. Moreover, during this phase, the subject could freely take any other foodstuffs containing raw, cooked or heated egg (i.e. candies, sauces and ice cream). Control group: Children continued egg avoidance diet during four months |
|
| Outcomes | The primary study outcome was to assess the induction of sustained unresponsiveness (the ability to consume 2808 mg egg white protein without symptoms in a DBPCFC at four months, after oral immunotherapy for three months and egg avoidance diet throughout the entire fourth month after three months of oral immunotherapy with egg). Secondary objectives were to assess: (i) the induction of desensitization, defined by the participant’s ability to eat at least one undercooked egg (fried egg, scrambled or undercooked omelette) compulsory every 48 h during MP without adverse events; (ii) safety (number and severity of adverse events); (iii) individual potential predictors to develop sustained unresponsiveness (variables considered: baseline age, gender, history of asthma, history of previous anaphylaxis to egg ingestion, frequency and severity of adverse events during oral immunotherapy, dose triggering symptoms in DBPCFC at baseline and egg white‐, OVA‐ and OVM‐sIgE at three months); (iv) changes in the DBPCFC threshold; and (v) immunological changes throughout the trial. |
|
| Vested interest | The authors declared no conflicts of interest. | |
| Notes | The study was not funded itself, but the publication of the English version was financially supported by Stallergenes pharmaceutical company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to groups in a 1:1 ratio using a computer‐generation randomization table. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes accounted for and low level of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
Fuentes‐Aparicio 2013.
| Methods | Randomized controlled trial | |
| Participants | 72 children, aged 4 years to 15 years, with persistent egg allergy with positive to cutaneous tests and specific IgE tests to egg and its fractions (white, yolk, OVA, and OVM). | |
| Interventions | On the first day, fractionated doses were administered until reaching 31 mg of egg, beginning with 1 mg and continuing with 3, 9, and 18 mg at 30 minute intervals. On the second day, 30 mg in one single dose was administered, with the treatment continuing at home at this same dosage. Subsequently, weekly increases were made in the clinic until 10 g of powdered egg, the equivalent of one egg, was reached. Finally, tolerance of the natural food was checked. Control group: egg avoidance diet |
|
| Outcomes | The main objective ‐ to induce clinical tolerance in children with persistent allergy using an oral desensitization protocol with powdered pasteurized egg. When total tolerance was not attained, the authors aimed to raise the tolerance threshold in order to avoid potentially serious adverse events from accidental intake. | |
| Vested interest | The authors declared no conflicts of interest. | |
| Notes | Study blinding not clear. Funding information was not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes accounted for and low level of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and therefore selective outcome reporting could not be assessed |
Giavi 2016.
| Methods | Double‐blind placebo controlled randomized pilot study | |
| Participants | 29 egg‐allergic children, 1 year to 5.5 years old, diagnosed with an IgE‐mediated egg allergy based on a positive skin prick test (SPT) to egg white within the last three months as well as a positive oral challenge or a convincing history, defined as an immediate (< 1 h) reaction following isolated ingestion of egg and positive sIgE (> 0.35 kU/L) for at least one of the following: egg, egg white, ovalbumin or ovomucoid, within the last 12 months. | |
| Interventions | On day 1, tolerance of the randomized product was assessed by an oral food challenge. 91 g of product was diluted in liquid (1/5 orange juice and 4/5 carrot juice) to a final volume of 50 mL. Every 20 to 30 min the final product was administered with increasing doses as follows: Step 1, 1.5 mL; Step 2, 3.5 mL; Step 3, 10 mL; Step 4, 15 mL; and Step 5, 20 mL. Both objective and subjective symptoms were recorded. The participant passed the tolerance assessment test if no symptoms occurred after having consumed all doses. In case no adverse reaction occurred, the subject was administered 1 day later with a full dose (91 g) at once incorporated in a solid meal and again adverse events were recorded. In case of no adverse events, a sachet containing 9 ± 1 g hydrolysed egg or placebo was administered daily for 6 months. Controlled group: placebo |
|
| Outcomes | The primary outcome was a positive or negative result of the challenge test, and the cumulative dose ingested without reaction (maximum cumulative dose tolerated). The severity of the allergic reactions at the final oral food challenge was evaluated. An overall score was calculated by multiplying two scores: maximum dose tolerated (0 g: grade 3, < 1 g: grade 2, > 1 g: grade 1, equal to maximum dose tolerated: grade 0) and severity of symptoms (no reaction: grade 0, mild: grade 1, moderate: grade 2, severe: grade 3). Egg‐specific IgE and IgG4 antibodies (anti‐egg white, anti‐egg yolk, anti‐ovomucoid (OVO), anti‐ovalbumin(OVA)) and Basophil activation were assessed at the start (V0) and end (V5) of the study. | |
| Vested interest | Quote: "Several authors (YMV, AM, SN, AW) are or were employees of Nestec Ltd." | |
| Notes | No funding information was available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization at 1:1 hydrolysed egg:placebo ratio was applied by using the software TrialSys (developed at Nestle, Lau‐sanne). Stratification was performed by gender (male or female), centre (Greece, Italy or Switzerland) and age (12 months to 35 months or 36 to 66 months). |
| Allocation concealment (selection bias) | Low risk | Study products were packaged, labelled and stored at Eurofins, France. A study box containing 110 sachets of the randomized product was sent to the study site (and provided to the parents) directly after randomization of the child and after 2.5 months. Each centre received at the start of the study ‘SPT kits’ containing each a sachet of hydrolysed egg and a sachet of placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study products were packaged, labelled and stored at Eurofins, France. A study box containing 110 sachets of the randomized product was sent to the study site (and provided to the parents) directly after randomization of the child and after 2.5 months. Each centre received at the start of the study ‘SPT kits’ containing each a sachet of hydrolysed egg and a sachet of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All outcomes accounted for and imbalance in numbers for missing data across intervention groups |
| Selective reporting (reporting bias) | Low risk | The trial was registered at ClinialTrials.gov (registration identifier NCT01526863) |
Meglio 2013.
| Methods | Randomized controlled trial, controlled open study | |
| Participants | 20 children, aged 4 years to 14 years, with mild‐to‐severe IgE‐mediated HEA, according to Clark’s severity classification, were admitted. All fulfilled the inclusion criteria of being more than four yrs of age and having an IgE‐mediated HEA: positive hen's egg skin prick tests (SPTs) or hen's egg IgE > 0.35 kUA/L confirmed by means of double‐blind, placebo‐controlled food challenge (DBPCFC) or a convincing history | |
| Interventions | The schedule consisted of administering increasing amounts of raw hen's egg starting from one drop (mixed egg white and yolk) diluted 1:100 with water, corresponding to 0.27 mg of hen's egg proteins. The hen's egg doses were doubled every 8 days until day 80. Subsequently, the hen's egg doses were doubled every 16 days to achieve a total daily intake of 25 mL, in 6 months.
The protocol was suspended when the child reached 25 mL/day or the maximum tolerated dose, that is to say the dose that did not induce any symptom and that was established after the third attempt to continue with the desensitization protocol (the parents tried to administer the same dose, which caused symptoms three times, so the previous tolerated dose was considered the maximum tolerated dose). Control: hen egg‐free diet. |
|
| Outcomes |
|
|
| Vested interest | This information was not available | |
| Notes | Funding information was not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By means of a computerized randomization schedule, the participants were randomly assigned to either intervention or control |
| Allocation concealment (selection bias) | Low risk | Computerized randomization schedule |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and therefore selective outcome reporting could not be assessed |
Pérez‐Rangel 2017.
| Methods | Randomized clinical trial | |
| Participants | 33 persistent egg‐allergic children, 5 years to 18 years old, with egg allergy diagnosis based on the presence of IgE‐ mediated symptoms, positive skin prick test (SPT) (≥ 3 mm) and/or specific IgE (sIgE) levels ≥ 0.7 kU/L or higher for whole egg, egg white, ovalbumin (OVA) and/or ovomucoid (OVM), and a positive double‐blind placebo‐controlled food challenge (DBPCFC) to dehydrated egg white at enrolment. | |
| Interventions | A consecutive 5‐day build‐up phase was designed, starting at the highest tolerated single dose in the baseline egg DBPCFC. The build‐up phase target dose was 3,600 mg of dehydrated egg white (approximately 2808 mg of egg white protein). A cumulative dose of 5400 mg of dehydrated egg white (approximately 4212 mg protein) was administered on the last day. The maintenance phase consisted of eating 1 undercooked egg (undercooked fried egg, scrambled egg, or omelet) every 48 hours. In addition, the participants could freely take any other egg‐containing foodstuffs. Control group: egg avoidance diet during 5 months |
|
| Outcomes | The primary study outcome was the efficacy to induce desensitization to egg after 5 months of treatment. Desensitization was defined as the participant's ability to eat 1 undercooked egg with no or mild adverse events. Secondary objectives were:
|
|
| Vested interest | Quote: "Authors have nothing to disclose." | |
| Notes | The study was part of a research project supported in part by the Research Prize Merck Serono 2012 from the Salud 2000 Foundation and by a grant from ALK‐Abelló Laboratories. Additional data was received from the study authors (Ibanez Sandin MD 2017 [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to groups using a computer‐generated randomization table. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes accounted for and low level of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
Vazquez‐Ortiz 2014.
| Methods | Non‐randomized, controlled, parallel group intervention study | |
| Participants | 82, aged 5 years to 18 years, with IgE‐mediated egg allergy confirmed by double‐blind placebo‐controlled challenge (DBPCFC) and sIgE or skin prick test (SPT) to egg white, ovalbumin (OVA), or ovomucoid (OVM) above 0.35 kU/L and 3 mm, respectively, were included | |
| Interventions | Pasteurized liquid egg white containing 8.3 g of protein per 100 mL was used, whose allergenicity is equivalent to raw egg white. Induction phase involved 16 weeks and started with a 2‐day in‐hospital rush phase, in which increasing doses were given hourly. Participants were then discharged home and continued having the last tolerated dose once daily throughout the week. Weekly increases were given at the outpatient clinic, as follows: 0.5 mL, 0.7 mL, 1 mL, 1.3 mL, 2 mL, 2.5 mL, 3.2 mL, 4 mL, 5 mL, 8 mL, 11 mL, 15 mL, 22 mL, 30 mL, and finally, one raw egg (60 g of the fresh product). In maintenance phase, children continued having one raw egg (or their maximal tolerated dose) twice weekly uninterruptedly Control: egg avoidance diet |
|
| Outcomes | Efficacy: open challenge to one raw egg (3.8 g raw egg white protein or, if not reached, to the highest tolerated dose) at 12 months after oral immunotherapy start. Complete desensitization was defined as clinical unresponsiveness to one raw egg, whereas partial desensitization as increase in reaction threshold not reaching one raw egg. Safety: all allergic reactions occurring within 2 h after oral immunotherapy doses, as well as delayed gastrointestinal or skin symptoms suggesting a non‐IgE‐mediated mechanism, were recorded. Severity was assessed according to Sampson’s Grading |
|
| Vested interest | Quote: "The authors declare no other conflict of interests." | |
| Notes | This study was part of a research project supported by the Research Grant 2010 from the Spanish Society of Paediatric Allergy and Clinical Immunology (SEICAP) and the Research Prize 2011 from the Institute for Studies on Egg (Spain). Funding from the mentioned institutions was used for immunological studies not presented in this manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were divided into two groups (active or control group), based on parents’ choice |
| Allocation concealment (selection bias) | High risk | Participants were divided into two groups, based on parents’ choice. No computerized randomization schedule |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and therefore selective outcome reporting could not be assessed |
DBPCFC ‐ double‐blind placebo‐controlled food challenge; OVA ‐ ovalbumin; OVM ‐ ovomucoid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Buchanan 2007 | No control group |
| García Rodríguez 2011 | No control group |
| Itoh 2010 | No control group |
| Meglio 2011 | Not control group |
| Morisset 2007 | Meets inclusion criteria, but results combined egg and milk‐allergic participants |
| Palmer 2013 | No history of immediate egg‐induced allergy |
| Patriarca 1998 | Meets inclusion criteria, but results combined egg and milk‐, fish‐, apple‐ allergic participants |
| Patriarca 2007 | No control group |
| Ruiz Garcia 2012 | No control group |
| Staden 2007 | Meets inclusion criteria, but results combined egg and milk‐allergic participants |
| Vickery 2010 | Open‐label study, no control group |
Characteristics of studies awaiting assessment [ordered by study ID]
Hirajama 2016.
| Methods | Randomized controlled trial |
| Participants | 45 children with IgE‐mediated hen's egg allergy and 32 with cow's milk allergy confirmed by double‐blind placebo‐controlled food challenge (DBPCFC) test |
| Interventions | Rush oral immunotherapy in active group, egg avoidance diet in control group |
| Outcomes | Threshold dose of DBPCFC at 3 months |
| Notes | Long‐term follow‐up; evaluation at the third year of oral immunotherapy We contacted the authors for further information, but received no response. |
Itoh‐Nagato 2013.
| Methods | Randomized controlled trial |
| Participants | 45 children, aged 5 to 15 years with severe IgE allergy confirmed by DBPCFC test |
| Interventions | Active group: oral immunotherapy that consisted of two phases: rush build‐up phase to achieve the ingestion of one medium‐sized lightly cooked egg (60 g) and maintenance phase, during which children continued to consume the same amount of egg every day. Control group: egg avoidance diet |
| Outcomes | Desensitization; complete tolerance; specific IgE, IgG, IgG4, IgA targets were measured; adverse events |
| Notes | We contacted the authors for further information, but received no response. |
Pajno 2012.
| Methods | Randomized controlled trial |
| Participants | 28 children, aged 5 years to 18 years with positive allergy on food challenge test |
| Interventions | Active group: dried egg powder; control group: talcum containing powder; DBPCFC performed at the beginning of the study |
| Outcomes | Full tolerance to egg (200 mg); partial tolerance (50 g); IgE and IgG4 levels |
| Notes | We contacted the authors for further information, but received no response. |
Takahashi 2013.
| Methods | Unclear randomization |
| Participants | Children, median age 6.2 years (range 2.1 years to 14.3 years) |
| Interventions | One group received rushed oral immunotherapy in children > 5 years using less than 1 g of the threshold dose of hen's egg. One group received slow oral immunotherapy. |
| Outcomes | Ability to ingest a target dose of 50 g of hen's egg |
| Notes | We contacted the authors for further information, but received no response. |
Yanagida 2013.
| Methods | Randomized controlled trial |
| Participants | 46 participants, aged > 3 years (average 5.5 ± 1.2 years) with positive systemic reactions to heated 1/4 whole egg at open food challenge |
| Interventions | Active group: escalation phase over 3 days at hospital consisted of eating 1.5 g to 3 g scrambled egg twice daily and oral antihistamines; maintenance phase at home involved eating 1.5 g to 3 g scrambled egg every morning. This oral immunotherapy protocol did not have build‐up phase. After 1 year a new oral food challenge was performed |
| Outcomes | Achieved tolerance to eat one cooked whole egg without adverse events and achieved by escalation and maintenance phase |
| Notes | We contacted the authors for further information, but received no response. |
DBPCFC ‐ double‐blind placebo‐controlled food challenge
Characteristics of ongoing studies [ordered by study ID]
NCT01846208.
| Trial name or title | Oral desensitization to egg with subsequent induction of sustained unresponsiveness for egg‐allergic children using baked egg or egg oral immunotherapy |
| Methods | Open‐label, parallel group, RCT Quote: "The purpose of this study is to compare baked foods with egg versus egg oral immunotherapy. The intent of the study is to investigate if participants will be able to consume egg after taking baked foods with egg or egg oral immunotherapy for a period of time and then stopping for a certain period. This is referred to as tolerance or sustained unresponsiveness. This study will evaluate the effectiveness of the egg oral immunotherapy versus baked egg by having each participant ingest egg white solid or baked foods with egg. This will be done over 2 years. This study will last 2 years. All eligible subjects will receive a baked egg oral food challenge who pass the baked egg oral food challenge will then have a 2 g egg oral food challenge. Those who react to the egg oral food challenge will be randomized to baked egg or egg oral immunotherapy. Individuals who do not pass the initial baked egg oral food challenge will be assigned to egg oral immunotherapy. Those who pass the egg oral food challenge will not be eligible for the study and will be followed per site standard of care. All eligible and enrolled participants will have a 1‐year and a 2‐year oral food challenge. At selected visits, blood and urine collection, physical examination, prick skin tests, and atopic dermatitis and asthma evaluations will occur" |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Egg oral immunotherapy in the form of egg white solid with up to four oral food challenges as directed by protocol. Baked egg in the form of home‐baked goods and 'safe' commercial products with up to four oral food challenges. Commercially available egg white solid dispensed by the central manufacturer. Study product will be dispensed in vials for low doses, capsules for mid‐range doses, and bulk powder with dosing scoops for the higher doses |
| Outcomes | Primary outcome: the development of sustained unresponsiveness to egg consumption at 2 years Secondary outcome: incidence of all serious adverse events during the study; changes in egg‐specific mechanistic measures and prick skin test results |
| Starting date | July 2013 |
| Contact information | Icahn School of Medicine at Mount Sinai, Jaffe Food Allergy Institute, USA |
| Notes | www.cofargroup.org |
NCT02083471.
| Trial name or title | Cow's milk and hen's egg hyposensitization in adults |
| Methods | Allocation: randomized Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | 40 participants, aged 16 to 65 years. Inclusion criteria: allergy to egg or cow's milk verified by challenge history and specific IgE. Exclusion criteria: cardiovascular disease, active immunological disease |
| Interventions | Active comparator: specific oral tolerance induction (SOTI) with egg or cow's milk No intervention: food allergy follow‐up |
| Outcomes | Primary outcome measures: number of participants with negative results (time frame: 12 months after commencement) |
| Starting date | April 2015 |
| Contact information | Turku University Hospital, Finland, Prof J Savolainen, johannes.savolainen@tyks.fi |
| Notes |
Differences between protocol and review
We relocated two outcomes from secondary to primary outcomes because adverse events are an important consideration during oral immunotherapy. These outcomes are: number of participants with serious adverse events and number of participants with mild‐to‐severe adverse events.
Furthermore, a new secondary outcome was added: "medication used due to adverse events". This is a relevant outcome due to numbers of adverse events present during oral immunotherapy.
The "satisfaction" outcome was removed because of unclear definition.
The review objectives were rephrased to make them more clear.
Contributions of authors
Protocol draft: OR, MGC
Develop a search strategy: OR, MGC
Search for trials: OR, MAT
Obtain copies of trials: OR, MAT
Select which trials to include: OR, MGC
Extract data from trials: OR, MGC
Enter data into RevMan: OR, MGC, SZ
Carry out the analysis: OR, MGC, SZ
Interpret the analysis: OR, MGC, SZ
Draft the final review: OR, MAT, MGC, SZ
Update the review: OR, MGC, SZ, MAT
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden.
to OR
Istituto Giannina Gaslini, Genoa, Italy.
External sources
No sources of support supplied
Declarations of interest
Olga Romantsik declares she has no competing conflicts of interest.
Maria Grazia Calevo declares she has no competing conflicts of interest.
Maria Angela Tosca declares she has no competing conflicts of interest.
Simona Zappettini declares she has no competing conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Akashi 2017 {published data only}
- Akashi M, Yasudo H, Narita M, Nomura I, Akasawa A, Ebisawa M, et al. Randomized controlled trial of oral immunotherapy for egg allergy in Japanese patients. Pediatrics International 2017;59(5):534‐9. [DOI] [PubMed] [Google Scholar]
Burks 2012 {published data only}
- Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Consortium of Food Allergy Research (CoFAR). Oral immunotherapy for treatment of egg allergy in children. New England Journal of Medicine 2012;367(3):233‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA, et al. Long‐term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. Journal of Allergy and Clinical Immunology 2016;137(4):1117‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caminiti 2015 {published data only}
- Caminiti L, Pajno GB, Crisafulli G, Chiera F, Collura M, Panasci G, et al. Oral immunotherapy for egg allergy: a double‐blind placebo‐controlled study, with postdesensitization follow‐up. Journal of Allergy and Clinical Immunology: In Practice 2015;3(4):532‐9. [DOI] [PubMed] [Google Scholar]
Dello Iacono 2013 {published data only}
- Dello Iacono I, Tripodi S, Calvani M, Panetta V, Verga MC, Miceli Sopo S. Specific oral tolerance induction with raw hen’s egg in children with very severe egg allergy: A randomized controlled trial. Pediatric Allergy and Immunology 2013;24(1):66‐74. [DOI] [PubMed] [Google Scholar]
Escudero 2015 {published data only}
- Escudero C, Rodrıguez del Rıo P, Sánchez‐Garcia S, Pérez‐Rangel I, Pérez‐Farinós N, Garcia‐Fernández C, et al. Early sustained unresponsiveness after short‐course egg oral immunotherapy: a randomized controlled study in egg‐allergic children. Clinical and Experimental Allergy 2015;45(12):1833‐43. [DOI] [PubMed] [Google Scholar]
Fuentes‐Aparicio 2013 {published data only}
- Fuentes‐Aparicio V, Alvarez‐Perea A, Infante S, Zapatero L, D’Oleo A, Alonso‐Lebrero E. Specific oral tolerance induction in paediatric patients with persistent egg allergy. Allergologia et Immunopathologia 2013;41(3):143‐50. [DOI] [PubMed] [Google Scholar]
Giavi 2016 {published data only}
- Giavi S, Vissers YM, Muraro A, Lauener R, Konstantinopoulos AP, Mercenier A. Oral immunotherapy with low allergenic hydrolysed egg in egg allergic children. European Journal of Allergy and Clinical Immunology 2016;71(11):1575‐84. [DOI] [PubMed] [Google Scholar]
Meglio 2013 {published data only}
- Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE‐mediated hen’s egg allergy:a new protocol with raw hen’s egg. Pediatric Allergy and Immunology 2013;24(1):75‐83. [DOI] [PubMed] [Google Scholar]
Pérez‐Rangel 2017 {published data only}
- Pérez‐Rangel I, Rodríguez Del Río P, Escudero C, Sánchez‐García S, Sánchez‐Hernández JJ, Ibáñez MD. Efficacy and safety of high‐dose rush oral immunotherapy in persistent egg allergic children: A randomized clinical trial. Annals of Allergy, Asthma & Immunology 2017;118(3):356‐64. [DOI] [PubMed] [Google Scholar]
Vazquez‐Ortiz 2014 {published data only}
- Vazquez‐Ortiz M, Alvaro M, Piquer M, Dominguez O, Machinena A, Martın‐Mateos MA, et al. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg‐allergic children. Clinical and Experimental Allergy 2014;44(1):130‐41. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Buchanan 2007 {published data only}
- Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in non anaphylactic children with egg allergy. Journal of Allergy and Clinical Immunology 2007;119(1):199‐205. [DOI] [PubMed] [Google Scholar]
García Rodríguez 2011 {published data only}
- García Rodríguez R, Urra JM, Feo‐Brito F, Galindo PA, Borja J, Gómez E, et al. Oral rush desensitization to egg: efficacy and safety. Clinical and Experimental Allergy 2011;41(9):1289‐96. [DOI] [PubMed] [Google Scholar]
Itoh 2010 {published data only}
- Itoh N, Itagaki Y, Kurihara K. Rush specific oral tolerance induction in school‐age children with severe egg allergy: One year follow up. Allergology International 2010;59(1):43‐51. [DOI] [PubMed] [Google Scholar]
Meglio 2011 {published data only}
- Meglio P, Giampietro PG, Gabriele I, Carello R, Avitabile S, Galli E. Oral desensitisation with food is food‐specific and protein‐specific. International Journal of Immunopathology and Pharmacology 2011;24(3):803‐11. [DOI] [PubMed] [Google Scholar]
Morisset 2007 {published data only}
- Morisset M, Moneret‐Vautrin DA, Guenard L, Cuny JM, Frentz P, Hatahet R, et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow's milk allergy and 90 children with egg allergy. European Annals of Allergy and Clinical Immunology 2007;39(1):12‐9. [PubMed] [Google Scholar]
Palmer 2013 {published data only}
- Palmer DJ, Metcalfe J, Makrides M, Gold MS, Quinn P, West CE, et al. Early regular egg exposure in infants with eczema: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2013;132(2):387‐92.e1. [DOI] [PubMed] [Google Scholar]
Patriarca 1998 {published data only}
- Patriarca G, Schiavino D, Nucera E, Schinco G, Milani A, Gasbarrini GB. Food allergy in children: results of a standardized protocol for oral desensitization. Hepatogastroenterology 1998;45(19):52‐8. [PubMed] [Google Scholar]
Patriarca 2007 {published data only}
- Patriarca G, Nucera E, Pollastrini E, Roncallo C, Pasquale T, Lombardo C, et al. Oral specific desensitization in food‐allergic children. Digestive Diseases and Sciences 2007;52(7):1662‐72. [DOI] [PubMed] [Google Scholar]
Ruiz Garcia 2012 {published data only}
- Ruiz Garcia M, Haroun E, Landivar ME, Torres Hernandez JA, Sastre J. Commercial dehydrated egg white for specific oral tolerance induction (SOTI): an easier treatment for egg allergy. Journal of Investigational Allergology and Clinical Immunology 2012;22(7):529‐31. [PubMed] [Google Scholar]
Staden 2007 {published data only}
- Staden U, Rolinck‐Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy 2007;62(11):1261‐9. [DOI] [PubMed] [Google Scholar]
Vickery 2010 {published data only}
- Vickery BP, Pons L, Kulis M, Steele P, Jones SM, Burks AW. Individualized IgE‐based dosing of egg oral immunotherapy and the development of tolerance. Annals of Allergy, Asthma & Immunology 2010;105(6):444‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hirajama 2016 {published data only}
- Hirayama J, Nagao M, Fujisawa T, Itoh‐Nagato N, Shimojo N, Iwata T. The long‐term prognosis of oral immunotherapy for eggs and cow's milk allergy. European Journal of Allergy and Clinical Immunology. Conference: 35th Annual Congress of the European Academy of Allergy and Clinical Immunology, EAACI 2016. Blackwell Publishing Ltd, August 2016; Vol. 71:501.
Itoh‐Nagato 2013 {published data only}
- Itoh‐Nagato N, Fujisawa T, Shimojo N, Nagao M, Iwata T. High rate of desensitisation and tolerance induction by rush oral immunotherapy for anaphylactic children with egg allergy: a randomised controlled trial. Allergy 2013;68(Suppl 97):337‐497. [Google Scholar]
Pajno 2012 {published data only}
- Pajno GB, Caminiti L, Vita D, Crisafulli G. Oral immunotherapy for egg allergy. Clinical and immunologic results. Journal of Allergy and Clinical Immunology 2012;129(Suppl 2):AB51. [DOI] [PubMed] [Google Scholar]
Takahashi 2013 {published data only}
- Takahashi M, Taniuchi S, Soejima K, Hatano Y, Nakano K, Kaneko K. The effectiveness and safety of specific oral immunotherapy in children with food allergy at stratification. Allergy 2013;68(Suppl 97):189‐336. [Google Scholar]
Yanagida 2013 {published data only}
- Yanagida N, Minoura T, Ebisawa M. Oral immunotherapy at fixed low dose for mild to moderate hen's egg allergy. Journal of Allergy and Clinical Immunology 2013;31(Suppl 2):AB84. [Google Scholar]
References to ongoing studies
NCT01846208 {published data only}
- Oral desensitization to egg with subsequent induction of sustained unresponsiveness for egg‐allergic children using baked egg or egg oral immunotherapy. Ongoing study July 2013.
NCT02083471 {published data only}
- Cow's milk and hen's egg hyposensitization in adults. Ongoing study April 2015.
Additional references
Burks 2008
- Burks AW. Peanut allergy. Lancet 2008;371(9623):1538‐46. [DOI] [PubMed] [Google Scholar]
Burks 2012a
- Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. Journal of Allergy and Clinical Immunology 2012;129(4):906‐20. [DOI] [PubMed] [Google Scholar]
Christie 2002
- Christie L, Hine RJ, Parker JG, Burks W. Food allergies in children affect nutrient intake and growth. Journal of the American Dietetic Association 2002;102(11):1648‐51. [DOI] [PubMed] [Google Scholar]
Cummings 2010
- Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 2010;65(8):933‐45. [DOI] [PubMed] [Google Scholar]
Fleischer 2012
- Fleischer DM, Perry TT, Atkins D, Wood RA, Burks AW, Jones SM, et al. Allergic reactions to foods in preschool‐aged children in a prospective observational food allergy study. Pediatrics 2012;130:e25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galli 2010
- Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. European Journal of Immunology 2010;40(7):1843‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Ibanez Sandin MD 2017 [pers comm]
- Ibanez Sandin MD. Safety data regarding first five month of ROIT1 [personal communication]. Email to: O Romantsik 12 February 2017.
Jones 2009
- Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. Journal of Allergy and Clinical Immunology 2009;124(2):292‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2016
- Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA, et al. Long‐term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. Journal of Allergy and Clinical Immunology 2016;137(4):1117‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khoriaty 2013
- Khoriaty E, Umetsu DT. Oral immunotherapy for food allergy: towards a new horizon. Allergy, Asthma & Immunology Research 2013;5(1):3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Land 2011
- Land MH, Kim EH, Burks AW. Oral desensitization for food hypersensitivity. Immunology and Allergy Clinics of North America 2011;31(2):367‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leonard 2012
- Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, et al. Dietary baked egg accelerates resolution of egg allergy in children. Journal of Allergy and Clinical Immunology 2012;130(2):473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muraro 2014
- Muraro A, Werfel T, Hoffman‐Sommergruber K, Roberts G, Beyer K, Bindslev‐Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy Asthma Proceedings 2014;69(8):1008‐25. [DOI] [PubMed] [Google Scholar]
Nelson 1997
- Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. Journal of Allergy and Clinical Immunology 1997;99(6 Pt 1):744‐51. [DOI] [PubMed] [Google Scholar]
Nurmatov 2017
- Nurmatov U, Dhami S, Arasi S, Pajno GB, Fernandez‐Rivas M, Muraro A, et al. Allergen immunotherapy for IgE‐mediated food allergy: a systematic review and meta‐analysis. Allergy 2017;72(8):1133‐47. [DOI: 10.1111/all.13124] [DOI] [PubMed] [Google Scholar]
Oppenheimer 1992
- Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. Journal of Allergy and Clinical Immunology 1992;90(2):256‐62. [DOI] [PubMed] [Google Scholar]
Osborne 2011
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. HealthNuts Investigators. Prevalence of challenge‐proven IgE‐mediated food allergy using population‐based sampling and predetermined challenge criteria in infants. Journal of Allergy and Clinical Immunology 2011;127(3):668‐76. [DOI] [PubMed] [Google Scholar]
Patriarca 1984
- Patriarca C, Romano A, Venuti A, Schiavino D, Rienzo V, Nucera E, et al. Oral specific hyposensitization in the management of patients allergic to food. Allergologia et Immunopathologia 1984;12(4):275‐81. [PubMed] [Google Scholar]
Patriarca 2003
- Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, Pasquale T, et al. Oral desensitizing treatment in food allergy: clinical and immunological results. Alimentary Pharmacology and Therapeutics 2003;17(3):459‐65. [Erratum in: Alimentary Pharmacology and Therapeutics. 2003 May 1;17(9):1205] [DOI] [PubMed] [Google Scholar]
Ring 2011
- Rin J, Gutermuth J. 100 years of hyposensitization: history of allergen‐specific immunotherapy (ASIT). Allergy 2011;66(6):713‐24. [DOI] [PubMed] [Google Scholar]
Sampson 2003
- Sampson HA. Anaphylaxis and emergency treatment. Pediatrics 2003;111:1601‐8. [PubMed] [Google Scholar]
Sampson 2012
- Sampson HA, Gerth van Wijk R, Bindslev‐Jensen C, Sicherer S, Teuber SS, Burks AW, et al. Standardizing double‐blind, placebo‐controlled oral food challenges: American Academy of Allergy, Asthma & Immunology‐European Academy of Allergy and Clinical Immunology PRACTALL consensus report. Journal of Allergy and Clinical Immunology 2012;130(6):1260‐74. [DOI] [PubMed] [Google Scholar]
Savage 2007
- Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. Journal of Allergy and Clinical Immunology 2007;120(6):1413‐7. [DOI] [PubMed] [Google Scholar]
Sclar 2014
- Sclar DA, Lieberman PL. Anaphylaxis: underdiagnosed, underreported, and undertreated. American Journal of Medicine 2014;127(1 Suppl):S1‐5. [DOI] [PubMed] [Google Scholar]
Sicherer 2010
- Sicherer SH, Wood RA, Stablein D, Burks AW, Liu AH, Jones SM, et al. Immunologic features of infants with milk or egg allergy enrolled in an observational study (Consortium of Food Allergy Research) of food allergy. Journal of Allergy and Clinical Immunology 2010;125(5):1077‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simons 2009
- Simons FE. Anaphylaxis: Recent advances in assessment and treatment. Allergy and Clinical Immunology 2009;124(4):625‐36. [DOI] [PubMed] [Google Scholar]
Simons 2011
- Simons FE. Anaphylaxis pathogenesis and treatment. Allergy 2011;66(Suppl 95):31‐4. [DOI] [PubMed] [Google Scholar]
Wood 2003b
- Wood RA. The natural history of food allergy. Pediatrics 2003;111(6 Pt 3):1631‐7. [PubMed] [Google Scholar]
Wright 2016
- Wright BL, Kulis M, Orgel KA, Burks AW, Dawson P, Henning AK, et al. Component‐resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy 2016;71(11):1552‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Romantsik 2013
- Romantsik O, Bruschettini M, Tosca MA, Della Casa Alberighi O, Calevo MGrazia. Oral and sublingual immunotherapy for egg allergy. Cochrane Database of Systematic Reviews 2013. [DOI: 10.1002/14651858.CD010638] [DOI] [PubMed] [Google Scholar]
Romantsik 2014
- Romantsik O, Bruschettini M, Tosca MA, Zappettini S, Della Casa Alberighi O, Calevo MG. Oral and sublingual immunotherapy for egg allergy. Cochrane Database of Systematic Reviews 2014. [DOI: 10.1002/14651858.CD010638.pub2] [DOI] [PubMed] [Google Scholar]


