Abstract
Background
Heavy menstrual bleeding (HMB) is an important physical and social problem for women. Oral treatment for HMB includes antifibrinolytic drugs, which are designed to reduce bleeding by inhibiting clot‐dissolving enzymes in the endometrium.
Historically, there has been some concern that using the antifibrinolytic tranexamic acid (TXA) for HMB may increase the risk of venous thromboembolic disease. This is an umbrella term for deep venous thrombosis (blood clots in the blood vessels in the legs) and pulmonary emboli (blood clots in the blood vessels in the lungs).
Objectives
To determine the effectiveness and safety of antifibrinolytic medications as a treatment for heavy menstrual bleeding.
Search methods
We searched the Cochrane Gynaecology and Fertility (CGF) Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO and two trials registers in November 2017, together with reference checking and contact with study authors and experts in the field.
Selection criteria
We included randomized controlled trials (RCTs) comparing antifibrinolytic agents versus placebo, no treatment or other medical treatment in women of reproductive age with HMB. Twelve studies utilised TXA and one utilised a prodrug of TXA (Kabi).
Data collection and analysis
We used standard methodological procedures expected by Cochrane. The primary review outcomes were menstrual blood loss (MBL), improvement in HMB, and thromboembolic events.
Main results
We included 13 RCTs (1312 participants analysed). The evidence was very low to moderate quality: the main limitations were risk of bias (associated with lack of blinding, and poor reporting of study methods), imprecision and inconsistency.
Antifibrinolytics (TXA or Kabi) versus no treatment or placebo
When compared with a placebo, antifibrinolytics were associated with reduced mean blood loss (MD −53.20 mL per cycle, 95% CI −62.70 to −43.70; I² = 8%; 4 RCTs, participants = 565; moderate‐quality evidence) and higher rates of improvement (RR 3.34, 95% CI 1.84 to 6.09; 3 RCTS, participants = 271; moderate‐quality evidence). This suggests that if 11% of women improve without treatment, 43% to 63% of women taking antifibrinolytics will do so. There was no clear evidence of a difference between the groups in adverse events (RR 1.05, 95% CI 0.93 to 1.18; 1 RCT, participants = 297; low‐quality evidence). Only one thromboembolic event occurred in the two studies that reported this outcome.
TXA versus progestogens
There was no clear evidence of a difference between the groups in mean blood loss measured using the Pictorial Blood Assessment Chart (PBAC) (MD −12.22 points per cycle, 95% CI −30.8 to 6.36; I² = 0%; 3 RCTs, participants = 312; very low quality evidence), but TXA was associated with a higher likelihood of improvement (RR 1.54, 95% CI 1.31 to 1.80; I² = 32%; 5 RCTs, participants = 422; low‐quality evidence). This suggests that if 46% of women improve with progestogens, 61% to 83% of women will do so with TXA.
Adverse events were less common in the TXA group (RR 0.66, 95% CI 0.46 to 0.94; I² = 28%; 4 RCTs, participants = 349; low‐quality evidence). No thromboembolic events were reported in any group.
TXA versus non‐steroidal anti‐inflammatory drugs (NSAIDs)
TXA was associated with reduced mean blood loss (MD −73.00 mL per cycle, 95% CI −123.35 to −22.65; 1 RCT, participants = 49; low‐quality evidence) and higher likelihood of improvement (RR 1.43, 95% CI 1.18 to 1.74; 12 = 0%; 2 RCTs, participants = 161; low‐quality evidence). This suggests that if 61% of women improve with NSAIDs, 71% to 100% of women will do so with TXA. Adverse events were uncommon and no comparative data were available. No thromboembolic events were reported.
TXA versus ethamsylate
TXA was associated with reduced mean blood loss (MD 100 mL per cycle, 95% CI −141.82 to −58.18; 1 RCT, participants = 53; low‐quality evidence), but there was insufficient evidence to determine whether the groups differed in rates of improvement (RR 1.56, 95% CI 0.95 to 2.55; 1 RCT, participants = 53; very low quality evidence) or withdrawal due to adverse events (RR 0.78, 95% CI 0.19 to 3.15; 1 RCT, participants = 53; very low quality evidence).
TXA versus herbal medicines (Safoof Habis and Punica granatum)
TXA was associated with a reduced mean PBAC score after three months' treatment (MD −23.90 pts per cycle, 95% CI −31.92 to −15.88; I² = 0%; 2 RCTs, participants = 121; low‐quality evidence). No data were available for rates of improvement. TXA was associated with a reduced mean PBAC score three months after the end of the treatment phase (MD −10.40 points per cycle, 95% CI −19.20 to −1.60; I² not applicable; 1 RCT, participants = 84; very low quality evidence). There was insufficient evidence to determine whether the groups differed in rates of adverse events (RR 2.25, 95% CI 0.74 to 6.80; 1 RCT, participants = 94; very low quality evidence). No thromboembolic events were reported.
TXA versus levonorgestrel intrauterine system (LIUS)
TXA was associated with a higher median PBAC score than LIUS (median difference 125.5 points; 1 RCT, participants = 42; very low quality evidence) and a lower likelihood of improvement (RR 0.43, 95% CI 0.24 to 0.77; 1 RCT, participants = 42; very low quality evidence). This suggests that if 85% of women improve with LIUS, 20% to 65% of women will do so with TXA. There was insufficient evidence to determine whether the groups differed in rates of adverse events (RR 0.83, 95% CI 0.25 to 2.80; 1 RCT, participants = 42; very low quality evidence). No thromboembolic events were reported.
Authors' conclusions
Antifibrinolytic treatment (such as TXA) appears effective for treating HMB compared to placebo, NSAIDs, oral luteal progestogens, ethamsylate, or herbal remedies, but may be less effective than LIUS. There were too few data for most comparisons to determine whether antifibrinolytics were associated with increased risk of adverse events, and most studies did not specifically include thromboembolism as an outcome.
Plain language summary
Antifibrinolytics (such as tranexamic acid) for treatment of heavy menstrual bleeding
Review question
Antifibrinolytic agents are designed to reduce bleeding by inhibiting endometrial clot‐dissolving enzymes (in the uterine lining); Cochrane researchers reviewed the evidence about the effect of these medications (such as tranexamic acid, TXA) versus placebo and other medical therapies in women with heavy menstrual bleeding (HMB: defined as more than 80 millilitres (> 80 mL) of blood loss per menstrual cycle).
Background
Antifibrinolytic agents (such as tranexamic acid, TXA) are commonly used to manage HMB. However, historically there has been concern that they may cause dangerous blood clots in the legs or lungs. There are a variety of other medications that can be used to treat HMB. We compared the benefits and risks of the treatments.
Study characteristics
We found 13 randomized controlled trials (RCTs) comparing an antifibrinolytic medication with a different medical therapy, in a total of 1312 women with heavy menstrual bleeding. The evidence is current to November 2017.
Key results
Antifibrinolytic medication may improve HMB in women aged 15 to 50 years old, without substantially increasing the rate of adverse events. Evidence suggests there is a 40% to 50% reduction in the amount of menstrual blood lost per menstrual cycle for participants taking TXA. Antifibrinolytic treatment was better at improving HMB loss than other medical treatments, except for the levonorgestrel intrauterine system (LIUS), a plastic device placed in the uterus which releases hormone to prevent conception.
The evidence suggests that if 10.9% of women taking placebo report an improvement in HMB, 36.3% of women taking TXA will do so.
TXA probably improves quality of life for women with HMB.
We did not find any evidence that side effects (including life‐threatening blood clots) were increased in women taking antifibrinolytic treatment compared to placebo or other treatments for HMB. Two studies measured venous thromboembolic events: unfortunately these studies did not have enough participants to distinguish a real effect of a certain size from pure luck.
Quality of the evidence
The evidence was of very low to moderate quality. The main limitations were: risk of bias, due to participants/investigators being aware of which medication they were receiving (known as lack of blinding), or the study's methods not being reported very clearly; imprecision (i.e. repeated measurements being far apart from each other), and inconsistency (i.e. as the sample size increases, the sampling distribution becomes increasingly wide around the true parameter value).
Summary of findings
Background
Description of the condition
‘Normal’ menstrual blood loss (MBL) has been defined as 30 mL to 40 mL per menstrual cycle, whilst heavy menstrual bleeding has traditionally been defined as greater than 80 mL blood loss per cycle (Duckitt 2012). Whilst this objective cut‐off has been broadly utilized in clinical trials, such measurement (involving extracting haemoglobin from sanitary wear) is impractical outside research settings. Also, this objectively measured cut‐off of 'heavy' menstrual bleeding may not reflect the woman's experience, nor the impact of heavy menstrual bleeding (HMB) on her quality of life (QoL) (Warner 2004). International guidelines (such as that produced by the United Kingdom's National Institute for Health and Care Excellence (NICE)) base the diagnosis of HMB on women’s and clinicians’ subjective perceptions of MBL, and its resultant impact (e.g. iron‐deficiency anaemia, days off work).
HMB is an important cause of ill health in women: prevalence estimates range from 4% to 51%. This wide range is due to these studies being undertaken in different countries and clinical settings (NICE 2007). It has been estimated that HMB accounts for 5% of general practitioner consultations by women aged 30 to 49 years old (Turner 2000), whilst HMB accounts for up to one‐third of all gynaecological consultations (El‐Hemaidi 2007).
The most widely used classification system for causes of abnormal uterine bleeding in reproductive‐aged women is that of the International Federation of Gynecology and Obstetrics, called the PALM‐COEIN system. As outlined in Munro 2011, the basic classification system breaks aetiologies down into: Polyp; Adenomyosis; Leiomyoma (submucosal/other); Malignancy and hyperplasia; Coagulopathy; Ovulatory dysfunction; Endometrial; Iatrogenic; and Not yet classified. In general, the PALM aetiologies are structural, whilst the COEIN categories are non‐structural.
Around 80% of women treated for heavy menstrual bleeding have no underlying uterine abnormality (i.e. would fall into the COEIN categories listed above), yet up to 60% of women referred to a gynaecologist for HMB undergo a hysterectomy within five years of referral (Edlund 2003; Qiu 2014). HMB is the primary indication for approximately 50% of all hysterectomies (emergency plus elective) in the UK (Turner 2000), and 38% of all elective hysterectomies (Butt 2012).
Hence medical therapy, with the avoidance of potential complications of surgical management, is an attractive alternative. A wide variety of medications are available to reduce heavy menstrual bleeding, but there is considerable variation in practice, and some uncertainty about the most appropriate first line therapy: a universally applicable step‐wise approach is sorely lacking (Fox 2012; Marret 2010).
Description of the intervention
Trans‐4‐aminomethylcyclohexanocarboxylic acid (or tranexamic acid and its precursors) is an antifibrinolytic medication. Tranexamic acid (TXA) has been used to treat HMB for over four decades in many European countries; in the UK, TXA is prescribed as first‐ or second‐line medical management of HMB for over 64% of women not requiring contraception (Turner 2000). In the US, TXA was not approved for the treatment of menorrhagia until 2009 (Kaunitz 2010).
How the intervention might work
Women with HMB have been found to have increased fibrinolytic activity in their menstrual fluid (Edlund 2003).
TXA exerts its antifibrinolytic effect by reversibly blocking lysine‐binding sites on plasminogen, thus preventing plasmin from interacting with lysine residues on the fibrin polymer. By preventing plasmin and lysine residues from interacting, TXA thus slows subsequent fibrin degradation, thereby slowing the dissolution of clots. Antifibrinolytic agents have, therefore, been promoted as a treatment for heavy menstrual bleeding.
Why it is important to do this review
There is growing evidence of the utility of the levonorgestrel intrauterine system (LIUS) in managing heavy menstrual bleeding. Qiu et al published a systematic analysis comparing the levonorgestrel intrauterine device to medical management of HMB, and included Gupta 2013, a randomized controlled trial (RCT) which compared LIUS to medical management of HMB (Gupta 2013; Qiu 2014). The authors concluded that LIUS is more effective for the treatment of HMB compared with oral medical treatment. However, for women for whom an intrauterine device is contra‐indicated or who wish to avoid an LIUS, antifibrinolytic treatment still plays an important role.
Although TXA has been credited with reducing MBL by up to 60% (Leminen 2012), one study based in Somerset (UK) found that less than 15% of women presenting to their general practitioner complaining of HMB were offered antifibrinolytic treatment (Grant 2000). Recent data indicate that prescribing patterns may be changing: a randomized controlled trial carried out in general practices in East Anglia (in the UK) indicated rates of antifibrinolytic use among women with heavy menstrual bleeding were 57% among those practices given a specific evidence‐based education package, and 35% among control practices (Fender 1999).
As these medications slow the breakdown of clots, there has been anecdotal concern that antifibrinolytic agents may be associated with an increased risk of thromboembolic disease (such as deep venous thrombosis). However, venous thromboembolic events have not been reported in treatment studies, and (to date) data from population‐based studies do not support an increased incidence of venous thromboembolism with antifibrinolytic use (Leminen 2012). Long‐term studies in Sweden have shown that the incidence of thrombosis in women treated with TXA is comparable to that of women not being treated with TXA (Berntorp 2001).
Hence, it is important to do this review to assess antifibrinolytic treatment's efficacy in managing heavy menstrual bleeding, which will be particularly relevant for women who are unable/unwilling to have a LIUS inserted.
Objectives
To determine the effectiveness and safety of antifibrinolytics as a treatment for heavy menstrual bleeding
Methods
Criteria for considering studies for this review
Types of studies
We accepted as eligible for inclusion published and unpublished RCTs of antifibrinolytic therapy versus placebo, no treatment or any other medical (non‐surgical) therapy when used to reduce heavy menstrual bleeding. We excluded non‐randomized studies (e.g. studies with evidence of inadequate sequence generation such as alternate days, patient numbers) as they are associated with a high risk of bias.
Cross‐over trials were only eligible for inclusion if they reported first‐phase data, in order to minimise the chance of cross‐over bias. This is a change from the original protocol criteria for inclusion, where cross‐over trials could be included regardless of whether data were provided for the first phase of the trial. Where cross‐over trials only report findings at the end of the study the likelihood of significant bias is increased, because no adjustment is made for cross‐over effects.
Types of participants
Women of reproductive age, who are having regular heavy periods (measured either objectively or subjectively), undertake at least two months' follow‐up whilst on treatment, and who are recruited from primary care, family planning, or a specialist clinic setting were eligible for inclusion.
Exclusion criteria included: post‐menopausal bleeding; irregular menses, inter‐menstrual bleeding or both; pathological causes of HMB (e.g. a coagulopathy); and iatrogenic causes of HMB (e.g. intrauterine device/system, or anti‐coagulant medication).
Types of interventions
We included trials comparing antifibrinolytic agents (e.g. tranexamic acid and its precursors) versus no treatment, placebo, or any other medical (non‐surgical) therapy. We excluded studies that used combined treatments (e.g. a LIUS with concurrent oral TXA).
Types of outcome measures
Primary outcomes
1. Menstrual blood loss (MBL), measured by either or both of the following.
a) Objective assessment of mean blood loss in mL (using alkaline haematin method or similar, Hallberg 1964), using either change scores or end scores: where studies reported both, we used end scores.
b) Subjective assessment of blood loss using continuous measures such as Pictorial Blood Assessment Chart (PBAC) scores, using either change scores or end scores: where studies reported both, we used end scores. PBAC scores range from 0 to more than 500, and rely on women scoring each tampon and sanitary towel they use during the course of a period, in terms of how heavily blood‐stained they are. Blood clots and episodes of flooding are also recorded (Higham 1990). Studies have confirmed a significant correlation between PBAC score and the alkaline haematin method, with a PBAC score of more than 100 being indicative of HMB (Zakherah 2011).
2. Improvement in HMB: binary measures (improved/not improved) as reported by the study, giving priority to subjective measures if studies reported both.
3. Thromboembolic events.
Secondary outcomes
4. Quality of life: participant's perceived change in quality of life from baseline provided this has been recorded in a reproducible and validated format (e.g. Menorrhagia Impact Questionnaire (MIQ), SF‐36, WHOQOL‐BREF).
5. Adverse events (other than thromboembolic events), including but not limited to: any adverse event; gastrointestinal side effects; abdominal discomfort; headaches; dizziness; breast tenderness; dysmenorrhoea; changes in weight; and changes in mood.
Search methods for identification of studies
We searched for all published and unpublished randomized controlled trials of antifibrinolytic therapy for the treatment of heavy menstrual bleeding, without language restriction and in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched the following electronic databases for relevant trials.
1. The Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials (PROCITE platform) (searched 7 November 2017) (Appendix 1).
2. The Cochrane Central Register of Controlled Trials in the Cochrane Library, via the Cochrane Register of Studies Online (CRSO Web platform) (searched 7 November 2017) (Appendix 2).
3. MEDLINE (OVID platform) (searched from 1946 to 7 November 2017) (Appendix 3).
4. Embase (OVID platform) (searched from 1980 to 7 November 2017) (Appendix 4).
5. PsycINFO (OVID platform) (searched from 1806 to 7 November 2017) (Appendix 5).
The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.1.0 chapter 6, 6.4.11). The Embase, PsycINFO and CINAHL searches are combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/assets/search‐filters‐randomised‐controlled‐trials.docx).
Other electronic sources of trials included:
6. trial registers for ongoing and registered trials –
clinicaltrials.gov (a service of the US National Institutes of Health)
apps.who.int/trialsearch/Default.aspx (The World Health Organization International Trials Registry Platform search portal)
7. LILACS (Latin American and Caribbean Health Science Information database (from 1982 ongoing) and other Spanish and Portuguese language databases, found in the Virtual Health Library Regional Portal (VHL): lilacs.bvsalud.org 8. PubMed and Google Scholar (for recent trials not yet indexed in the major databases)
These databases were searched using the following subject headings and keywords: menorrhagia, dysfunctional uterine bleeding, heavy menstrual bleeding, antifibrinolytic, tranexamic acid, trans‐4‐aminomethylcyclohexanocarboxylic acid, KABI. Please see the Appendices for details.
Searching other resources
We handsearched reference lists of relevant trials and systematic reviews retrieved by the search and contact experts in the field to obtain additional data. We also handsearched relevant journals and conference abstracts that are not covered in the CGF register, in liaison with the Information Specialist. Marian Showell (Information Specialist for the Cochrane Gynaecology and Fertility Group (CGF)) performed the initial search, whilst AB‐S searched other electronic sources and resources.
Data collection and analysis
Selection of studies
The initial search was conducted by Marian Showell. After an initial screen of titles and abstracts retrieved by the search, conducted by AB‐S and AL, we retrieved the full texts of all potentially eligible studies. Two review authors (AB‐S and AL) independently examined these full text articles for compliance with the inclusion criteria and selected eligible studies. We corresponded with study investigators as required, to clarify study eligibility. Disagreements were resolved by discussion. If any reports required translation, we described the process used for data collection. We documented the selection process with a PRISMA flow chart (Figure 1).
1.
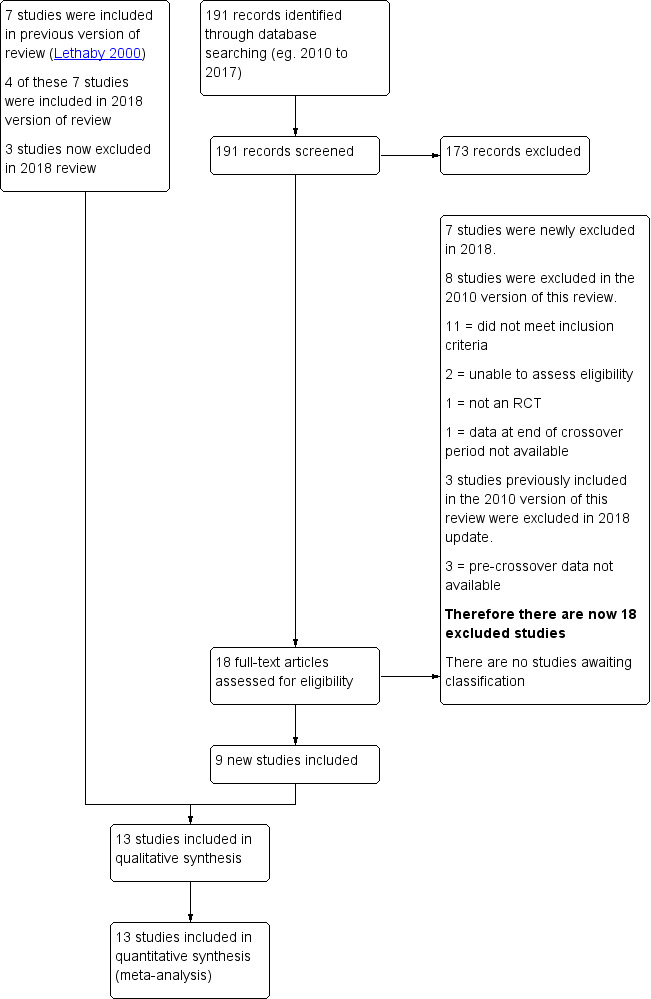
Study flow diagram (PRISMA chart).
Data extraction and management
Two review authors (AB‐S and AL) independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the authors. We resolved any disagreements by discussion; or if deadlock persisted, by involving a third review author as arbitrator. Data extracted included study characteristics and outcome data (see Data collection and analysis section for details). Where studies had multiple publications, we used the main trial report as the reference and derived additional details from secondary papers.
We corresponded with study investigators for further data on methods or results (or both), as required.
Assessment of risk of bias in included studies
Two review authors (AB‐S and AL) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias (Higgins 2011). We assigned judgement as recommended in the Cochrane Handbook for Systematic Reviews of Interventions Section 8.5 (Higgins 2011). We resolved disagreements by discussion; or if deadlock persisted, by involving a third review author as arbitrator. We described all judgements fully and present the conclusions in the 'Risk of bias' table, which we incorporated into the interpretation of review findings by means of sensitivity analyses (see below).
With respect to within‐trial selective reporting, where identified studies fail to report the primary outcome of live birth, but do report interim outcomes such as pregnancy, we assessed whether the interim values are similar to those reported in studies that also report live birth.
Measures of treatment effect
For dichotomous data (e.g. adverse event rates), we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs). For continuous data (e.g. MBL in mL), if all studies report exactly the same outcomes we calculated mean difference (MDs) between treatment groups. If similar outcomes are reported on different scales we calculated the standardized mean difference (SMD). We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We treated ordinal data (e.g. quality of life scores) as continuous data. We presented 95% confidence intervals for all outcomes. Where data to calculate ORs or MDs are not available, we utilized the most detailed numerical data available that facilitated similar analyses of included studies (e.g. test statistics, P values). We assessed whether the estimates calculated in the review for individual studies are compatible in each case with the estimates reported in the study publications.
We included either end score or change score data for measuring MBL. Where studies report both, we used end scores. For the primary outcome, we considered whether the data underlying the published result were likely to be skewed by examining the ratio of each group mean to its standard deviation. Where end scores were reported, a ratio considerably less than two indicates positive skewness, due to the fact that 'menstrual bleeding' cannot take values less than zero. Where this was deemed to be the case, we reported the results in an additional table, as they could not be pooled in the meta‐analysis without access to the raw underlying data. Where no standard deviations were reported, we took a corresponding value reported in another similar study.
Where studies reported standard deviations that were implausibly small, we assumed that these were in fact standard errors, and converted them to standard deviations, using standard methods (Higgins 2011).
Unit of analysis issues
The primary analysis was per woman randomized. Only first‐phase data from cross‐over trials was included.
Dealing with missing data
We analyzed the data on an intention‐to‐treat basis as far as possible (i.e. including all randomized participants in analysis, in the groups to which they were randomized). Attempts were made to obtain missing data from the original trialists. Where these are unobtainable, we undertook imputation of individual values for primary outcomes only. For other outcomes, we analyzed only the available data. Any imputation undertaken was subjected to sensitivity analysis (see below).
If studies reported sufficient detail to calculate mean differences but no information on associated standard deviation (SD), we assumed the outcome to have a standard deviation equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I² statistic. An I² measurement greater than 50% was taken to indicate substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies, and by being alert for duplication of data. If there were 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If the studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons.
Antifibrinolytics versus placebo or no treatment.
Antifibrinolytics versus any other medical (non‐surgical) treatment.
Any increase in the odds of a particular outcome, either beneficial (e.g. decreased MBL) or detrimental (e.g. adverse effects), were displayed graphically in the meta‐analyses to the right of the centre line, and a decrease in the odds of an outcome to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
Where data are available, we conducted subgroup analyses to determine the separate evidence within the following subgroups.
Tranexamic acid dose (< 3 g/day versus ≥ 3 g/day)
Different methods of measuring MBL: objectively, by the alkaline haematin method and subjectively by the PBAC
Differences between the control interventions (e.g. luteal phase norethisterone (NET) or medroxyprogesterone (MPA))
If we detected substantial heterogeneity, we explored possible explanations in sensitivity analyses. We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions are robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility were restricted to studies without high risk of bias;
a random‐effects model had been adopted;
alternative imputation strategies had been implemented;
the summary effect measure was relative risk rather than odds ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro and Cochrane methods. This table evaluates the overall quality of the body of evidence for the main review outcomes (MBL, adverse events) for the main review comparison (antifibrinolytic agent versus placebo or other medical therapy). Additional 'Summary of findings' tables were also prepared for the main review outcomes for other important comparisons (antifibrinolytic agent versus progestogens, antifibrinolytic agent versus ethamsylate etc.). We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias). Two review authors working independently made judgements about evidence quality (high, moderate, low or very low), with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome.
We extracted study data, formatted our comparisons in data tables, and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
The 2010 version of this review included seven studies. Our new search retrieved 191 articles. Eighteen studies not included in the previous version of this review were potentially eligible and were retrieved in full text. Nine of these new studies met our inclusion criteria. We excluded three studies from the 2010 version of this review, and there are no studies awaiting classification; (see study tables: Characteristics of included studies, Characteristics of excluded studies).
See Figure 1 for the relevant PRISMA flow chart.
Included studies
Study design and setting
Thirteen studies are included in the current update of the review, twelve with a parallel group design and one cross‐over trial.
Seven of the studies were single‐centre; the country settings included Ireland, UK, India, Turkey, and Iran. The remaining studies drew subjects from various sites across individual countries (Sweden, USA, Thailand, Iran and China).
Participants
The studies (1312 participants) included 582 women in the control (non‐antifibrinolytic) groups and 778 in the intervention (i.e. tranexamic acid) groups. Their age ranged across studies from 15 to 50 years. Of note: Kriplani 2006 included women less than 18 years old without confirming that their HMB was ovulatory, whilst Bonnar 1996 and Callender 1970 only included women more than 32 years old.
Goshtasebi 2013 was the only study to use BMI as an inclusion criterion (19 to 29 kg/m2).
Several studies used serum haemoglobin as an inclusion criterion: Fathima 2012, Freeman 2011 and Lukes 2010 required women to have a serum haemoglobin of more than 8 g/dL; Kiseli 2016 used a cut‐off of more than 10 g/dL; whilst Goshtasebi 2013 and Goshtasebi 2015 required women to have a serum haemoglobin of more than 10.5 g/dL
Three studies excluded women with a self‐reported history of irregular menstrual bleeding (Bonnar 1996; Freeman 2011; Lukes 2010); whilst Jaisamrarn 2006; and Preston 1995 tested mid‐luteal progesterone to confirm that HMB was ovulatory.
All studies except Zhang 2008 mentioned excluding women with an underlying pelvic aetiology of their HMB, although several did not elucidate how they excluded pelvic pathology (i.e. by history, examination, ultrasound, hysteroscopy or endometrial biopsy, or a combination of these).
Fathima 2012 included women with leiomyomata. Goshtasebi 2013 and Kriplani 2006 excluded women found to have uterine leiomyomata, whilst Freeman 2011 and Lukes 2010 only excluded women with fibroids thought to warrant surgical management. Goshtasebi 2015 excluded women with fibroids greater than 3 cm in diameter, and Kiseli 2016 excluded women with fibroids that were greater than 2 cm or indented the uterine cavity on ultrasound.
Several studies mentioned adenomyosis: Fathima 2012 included women with adenomyosis, whilst Kriplani 2006 specifically excluded women thought to have adenomyosis.
Bonnar 1996, Edlund 1995, Freeman 2011, Lukes 2010 and Preston 1995 required a negative Pap smear within 0 to 12 months of trial entry. Kiseli 2016 excluded women with malignant cervicovaginal pathology.
The following studies excluded women who were taking medications that might affect their menstrual pattern (such as anticoagulants, aspirin, or NSAIDs/COX‐2 inhibitors during the menstrual phase of their cycle): Edlund 1995; Freeman 2011; Goshtasebi 2013; Goshtasebi 2015; Jaisamrarn 2006; Lukes 2010; Preston 1995; Zhang 2008. Several studies listed hormonal contraception as an exclusion criterion (Edlund 1995; Fathima 2012; Freeman 2011; Goshtasebi 2013; Goshtasebi 2015; Jaisamrarn 2006; Kriplani 2006; Lukes 2010; Preston 1995; Zhang 2008). Kiseli 2016 excluded women with a history of having taken medications for menorrhagia previously.
With regard to non‐gynaecological and non‐haematological co‐morbidities: Bonnar 1996, Edlund 1995, Jaisamrarn 2006 and Zhang 2008 excluded women with renal/hepatic dysfunction, and Preston 1995 those with renal dysfunction. Bonnar 1996, Edlund 1995, Fathima 2012, Freeman 2011, Jaisamrarn 2006, Lukes 2010 and Zhang 2008 mentioned excluding women with either a history of, or definitive proof of, a coagulopathy or fibrinolytic disorder. Kiseli 2016 measured participants' coagulation profile.
The following studies excluded women who reported a history of venous thromboembolism: Bonnar 1996; Edlund 1995; Freeman 2011; Goshtasebi 2013; Goshtasebi 2015; Jaisamrarn 2006; Lukes 2010 and Zhang 2008. Freeman 2011 also excluded women with a history of arterial thrombosis (i.e. ischaemic heart disease, acute myocardial infarction, stroke/cerebrovascular accident, transient ischaemic attack); Kiseli 2016 excluded women with coronary artery disease. The following studies excluded women with a history of coagulopathy/fibrinolytic disorder: Bonnar 1996; Edlund 1995; Fathima 2012; Freeman 2011; Goshtasebi 2015; and Lukes 2010.
Fathima 2012, Freeman 2011, Kiseli 2016, and Kriplani 2006 excluded women with thyroid disease, whilst Freeman 2011 also excluded women with hyperprolactinaemia.
There were several co‐morbidities that were used as an exclusion criterion by only one study: Bonnar 1996 excluded women with inflammatory bowel disease, peptic/intestinal ulceration; Fathima 2012 excluded women with a history of diabetes, hypertension, tuberculosis, "malignancies" or hypothalamic pituitary dysfunction; Kiseli 2016 excluded women with hypertension or diabetes; Lukes 2010 excluded women with a history of sub‐arachnoid haemorrhage, "endocrinopathy" or ocular disease.
Goshtasebi 2013 simply reports excluding women with any "history of chronic diseases".
Interventions
The thirteen studies (twelve parallel group, and one cross‐over trial) used various antifibrinolytic formulations and dosage regimens, as detailed below.
Tranexamic acid, used by 12 of the 13 studies, is a synthetic analogue of the amino acid lysine. Kabi (used in Edlund 1995) is a pro‐drug of TXA.
Dosage
The majority of studies used regular dose TXA (ranging from 3 g/day to 4 g/day) (Bonnar 1996; Callender 1970; Fathima 2012; Jaisamrarn 2006; Kiseli 2016; Lukes 2010; Preston 1995; Zhang 2008).
Four other studies used low‐dose TXA (ranging from 2 g/day to 2.4 g/day) (Edlund 1995; Goshtasebi 2013; Goshtasebi 2015; Kriplani 2006).
Freeman 2011 compared low‐dose (1.95 g/day) to regular‐dose TXA (3.9 g/day) to placebo.
Treatments
Four studies compared antifibrinolytic treatment to placebo: Callender 1970 (a cross‐over trial); Edlund 1995; Freeman 2011; and Lukes 2010 (all parallel group studies). No studies compared antifibrinolytic treatment to no treatment.
Nine studies compared antifibrinolytic treatment to other medical therapies: Bonnar 1996; Fathima 2012; Goshtasebi 2013; Goshtasebi 2015; Jaisamrarn 2006; Kiseli 2016; Kriplani 2006; Preston 1995; and Zhang 2008. Of note: some studies compared antifibrinolytic treatment to two different alternative medical options.
6 assessed progestogens (short or long course) (Goshtasebi 2013; Jaisamrarn 2006; Kiseli 2016; Kriplani 2006; Preston 1995; Zhang 2008)
2 assessed NSAIDs (Bonnar 1996; Jaisamrarn 2006)
1 assessed ethamsylate (Bonnar 1996)
2 assessed herbal remedies (one assessed Safoof Habis, and another pomegranate flower) (Fathima 2012; Goshtasebi 2015)
1 assessed levonorgestrel intrauterine system (LIUS)
Of the six studies that compared TXA to progestogens, four — Jaisamrarn 2006, Kiseli 2016, Preston 1995 and Zhang 2008 — compared TXA to short‐course progestogen (e.g. days 19 to 26 of the menstrual cycle only), whilst two — Goshtasebi 2013 and Kriplani 2006 — compared TXA to long‐course progestogens (e.g. from days 5 to 26).
One study compared antifibrinolytic treatment to a Unani formulation called Safoof Habis (Fathima 2012). Unani is a type of traditional medicine widely practised in South‐East Asia. Safoof Habis is made up of: Teen Ahmer (silicate of alumina and iron oxide); Sange Jarahat (hydrated magnesium silicate); and Raal Sufaid (Vateria indica Linn, which is a species of plant in the Disterocarpaceae family, endemic to India). In Fathima 2012 the treatment arm (Safoof Habis) was made up of equal parts of all three components, and given in 5 g doses twice per day, from days 1 to 5.
One study compared TXA (500 mg four times per day on days 1 to 5) to Punica granatum Linn (pomegranate flower) (500 mg four times per day on days 1 to 5) (Goshtasebi 2015).
Outcomes
Objective assessment of women's menstrual blood loss
Five parallel group studies confirmed the participants' HMB with objective testing such as alkaline haematin testing (Bonnar 1996; Edlund 1995; Freeman 2011; Lukes 2010; Preston 1995). The alkaline haematin method of quantifying MBL was developed by Hallberg and Nilsson in the 1960s, and involves women collecting their menstrual pads, then sending them to the lab for analysis (for extraction and measurement of the amount of blood) (Hallberg 1964).
The one cross‐over study used the Oxford total body counter as a way of quantifying participants' MBL (Callender 1970).
Subjective assessment of women's menstrual blood loss
Seven studies — Fathima 2012, Goshtasebi 2013, Goshtasebi 2015, Jaisamrarn 2006, Kiseli 2016, Kriplani 2006 and Zhang 2008 — assessed women’s menstrual bleeding by Pictorial Blood Assessment Chart (PBAC) (Higham 1990).
The PBAC involves women recording the number of pads/tampons used, and documenting the degree of soiling. The PBAC has its limitations: for instance it is binary in nature and there is no international consensus on the cut‐off level for the definition of HMB. Some authors consider 100 points in the PBAC to be equivalent to 80 mL of MBL (i.e. HMB); some 150 points. Also, the PBAC does not allow for a volumetric correlation between the patient's PBAC score, and the volume of MBL.
Self‐reported improvement in HMB
The gold standard for diagnosing HMB is patient's self‐reported assessments of their own menstrual loss. Six studies asked women to self‐report heavy menstrual bleeding, using a questionnaire (Bonnar 1996; Callender 1970; Edlund 1995; Freeman 2011; Goshtasebi 2013; Goshtasebi 2015).
Quality of life measures
Eight studies reported quality of life measures. Freeman 2011, Goshtasebi 2013, Goshtasebi 2015 and Lukes 2010 used the Menorrhagia Impact Questionnaire (with lower scores representing better quality of life). In addition, Goshtasebi 2013 and Goshtasebi 2015 also used the SF‐36 quality of life questionnaire (with higher scores representing better quality of life). Jaisamrarn 2006 used a questionnaire which included six questions relating to the impact of HMB on impairment of social life, work performance, tiredness, productivity, appetite and depression; (these data could not be used because measures of variation were not reported). Kiseli 2016 used the World Health Organization's Quality of Life‐Short Form, Turkish version (WHOQOL‐BREF TR), in which patients report limitations in physical health, psychological status, social support, and limitations relating to their environment. Preston 1995 used a 5‐point scale for quality‐of‐life assessments, which evaluated general health, flooding and leakage, abdominal pain, limitation on social activities, and effect on sex life. Zhang 2008 used a 6‐item questionnaire, but it was not clear whether it was validated, or what specific items were assessed.
Adverse events
Eleven studies reported adverse events (other than thromboembolic events): Bonnar 1996; Callender 1970; Edlund 1995; Freeman 2011; Goshtasebi 2013; Goshtasebi 2015; Kiseli 2016; Kriplani 2006; Lukes 2010; Preston 1995; and Zhang 2008. The adverse events reported included: abdominal pain, allergic reaction, anxiety, back pain, bloating, breast tenderness, depression, chest pain, diarrhoea, dizziness, dysmenorrhoea, dyspepsia, excess hair growth, headache, intermenstrual bleeding, menstrual cramps, mood changes, myalgia, nausea, ocular events (lenticular opacities, blurred vision), rash, vaginal dryness, vertigo, vomiting, weight gain.
Excluded studies
Nine studies were excluded from this version of the review, for the following reasons.
1/9 was not a RCT (Muse 2010)
1/9 only included women who had a proven coagulopathy (Kouides 2009)
1/9 compared women taking tranexamic acid to women taking a combination of tranexamic and mefenamic acids (Najam 2010)
4/9 were cross‐over trials that did not provide data at the end of the first phase of the study, before participants were crossed over (Andersch 1988; Nilsson 1967; NCT01428713; Vermylen 1968)
2/9 were excluded when no response was received from the authors after several attempts to contact them (Moghtadaei 2012; Tabatabaei 2013)
Risk of bias in included studies
Please see Figure 2 and Figure 3 for summaries of the risk of bias for the included studies.
2.
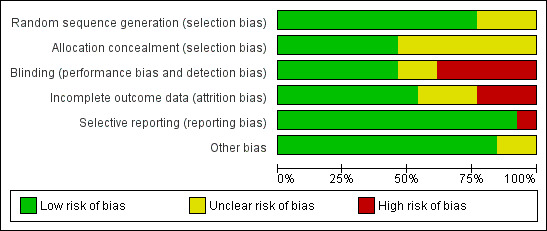
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
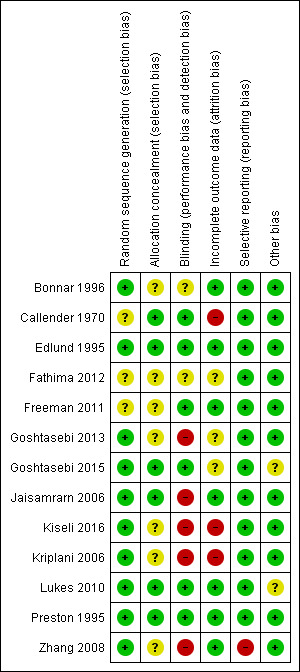
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Nine studies were rated as being at low risk of selection bias related to sequence generation, as they used computer randomization or a random numbers table. The other four studies did not describe the method used and were rated at unclear risk of this bias.
Six studies were at low risk of selection bias related to allocation concealment. Seven studies did not describe the method used, and were at unclear risk of allocation bias.
Blinding
We did not consider that blinding was likely to influence findings for the primary review outcome (MBL), where this was measured by the objective alkaline haematin method; however, where women used the PBAC, knowledge of their treatment may have influenced their assessment of blood loss. In addition, for adverse effects and subjective secondary outcomes (such as quality of life), blinding status could also potentially affect findings.
Six studies were deemed to be at low risk of this bias. Two studies did not describe the method used, so were deemed to be at unclear risk of detection bias. Five studies were deemed to be at high risk of this bias.
Incomplete outcome data
Seven studies analyzed all or most (> 95%) of the women randomized and we judged them to be at low risk of attrition bias. Three studies were at unclear risk of attrition bias. Three studies were at high risk of attrition bias.
Selective reporting
We rated most studies as at low risk of selective reporting bias. However, the abstract of Zhang 2008 only reported results where significant differences were seen between experimental and control groups, potentially leading to overly‐optimistic conclusions; this trial was reported as having a high risk of reporting bias.
Other potential sources of bias
We found no potential sources of within‐study bias in 11 studies. Two studies were thought to be at unclear risk of within‐study bias: Goshtasebi 2015, as baseline factors were only reported for women who completed the study (therefore, baseline comparability is unknown); Lukes 2010, due to imbalances between the two groups at baseline.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings for the main comparison. Antifibrinolytics compared to no treatment or placebo for heavy menstrual bleeding.
| Antifibrinolytics compared to no treatment or placebo for heavy menstrual bleeding | ||||||
| Population: women with heavy menstrual bleeding Setting: gynaecology outpatient departments; one study simply said ''clinical sites'' Intervention: antifibrinolytics Comparison: no treatment or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment or placebo | Risk with antifibrinolytics | |||||
| Menstrual blood loss: mean loss Assessed with: alkaline haematin method Follow‐up: range 3 months to 6 months | The mean menstrual blood loss: mean loss ranged from 206 mL to 252 mL | MD 53.2 mL lower (62.7 lower to 43.7 lower) | ‐ | 565 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Menstrual blood loss: improvement rates Assessed with a variety of methods Follow‐up: range 3 months to 6 months | 109 per 1000 | 363 per 1000 (200 to 662) | RR 3.34 (1.84 to 6.09) | 271 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | |
| Adverse events (any) | 836 per 1000 | 990 per 1000 | RR 1.05 (0.93 to 1.18) | 297 (1 RCT) | ⊕⊕⊝⊝ LOW3 |
Most of these adverse events were mild to moderate in severity. |
| Thromboembolic events | Only one thromboembolic event occurred in the two studies that reported this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate — the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited — the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate — the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Quality downgraded 1 level due to risk of bias (2 of 4 studies with unclear randomization and 1 with unclear allocation concealment and 1 study with high risk of attrition bias)
2 Quality downgraded 1 level due to risk of bias (1 very small study with substantial risk of attrition bias)
3 Quality downgraded 2 levels due to risk of bias (study had unclear allocation concealment and randomization method) and because of imprecision (single study).
Summary of findings 2. Antifibrinolytics compared to progestogens for heavy menstrual bleeding.
| Antifibrinolytics compared to progestogens for heavy menstrual bleeding | ||||||
| Population: women with heavy menstrual bleeding Setting: any Intervention: antifibrinolytics Comparison: progestogens | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with progestogens | Risk with antifibrinolytics | |||||
| Menstrual blood loss: mean loss (overall) Assessed with: PBAC11 Follow‐up: range 2 months to 3 months | The mean PBAC score ranged from 114 to 209 pts | MD 12.22 pts lower (30.8 lower to 6.36 higher) | ‐ | 312 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Two additional trials (at low risk of bias) had skewed data and are displayed in Additional Table 1. They both found a significant benefit for TXA. |
| Menstrual blood loss: improvement rates (overall) Assessed with: patient assessment Follow‐up: range 2 months to 3 months | 463 per 1000 | 701 per 1000 (607 to 833) | RR 1.54 (1.31 to 1.80) | 422 (5 RCTs) | ⊕⊕⊝⊝ LOW 6 | Overall effect combining short‐ and long‐course progestogens. |
| Adverse events (any) | 319 per 1000 | 210 per 1000 Need to add CI here |
RR 0.66 (0.46 to 0.94) | 349 (4 RCTs) | ⊕⊕⊝⊝ LOW10 |
|
| Thromboembolic events | No thromboembolic events were diagnosed in either group | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate — the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited — the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate — the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Quality downgraded 1 level due to inconsistency between the five trials, possibly linked to different measurement tools
2 Quality downgraded further 2 levels due to risk of bias (3 of 5 studies with unclear allocation concealment, 2 of 5 studies had high risk of detection bias and 1 study had a high risk of attrition bias)
3 Quality downgraded 2 levels due to risk of bias(high risk of detection and reporting bias)
4 Quality downgraded a further level due to imprecision (the study was a single small trial)
5 Quality downgraded 2 levels due to risk of bias (both trials at high risk of performance bias and 1 trial at high risk of attrition bias)
6 Quality downgraded 2 levels due to risk of bias (3 of 4 studies with high risk of detection bias, 1 study with risk of attrition bias and 1 study with risk of reporting bias)
7 Quality downgraded 2 levels due to risk of bias (2 of 3 studies with high risk of detection bias, 1 study at risk of reporting bias)
8 Quality downgraded 2 levels due to risk of bias (one study with high risk of performance bias and attrition bias)
9 Quality downgraded a further level because of imprecision (single small trial)
10 Quality downgraded 2 levels due to risk of bias (2 of 3 studies with unclear allocation concealment, 1 study with unclear attrition bias, 1 study with high risk of selective outcome reporting and 2 studies with unclear 'other' bias)
11 Some authors consider 100 points in the Pictorial Blood Assessment Chart (PBAC) equivalent to 80 mL of menstrual blood loss (i.e. heavy menstrual bleeding); some 150 points.
Summary of findings 3. Antifibrinolytics compared to NSAIDs for heavy menstrual bleeding.
| Antifibrinolytics compared to NSAIDs for heavy menstrual bleeding | ||||||
| Population: women with heavy menstrual bleeding Setting: all Intervention: antifibrinolytics Comparison: NSAIDs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with NSAIDs | Risk with antifibrinolytics | |||||
| Menstrual blood loss: mean loss Assessed with: alkaline haematin method Follow‐up: mean 3 months | The mean menstrual blood loss: mean loss was 148 mL | MD 73 mL lower (123.35 lower to 22.65 lower) | ‐ | 49 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Menstrual blood loss: improvement rates Assessed with: patient questionnaire Follow‐up: range 2 months to 3 months | 608 per 1000 | 869 per 1000 (717 to 1000) | RR 1.43 (1.18 to 1.74) | 161 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | |
| Adverse events (any) | The total number of adverse events per group were not measured. Individual adverse events were uncommon. | |||||
| Thromboembolic events | These were not measured in the study. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate — the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited — the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate — the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Quality downgraded 1 level for study limitations (I study with unclear risk of selection bias)
2 Quality downgraded a further level because of imprecision (single very small study)
3 Quality downgraded 2 levels because of study limitations (both studies at high risk of performance bias as women assessed this outcome and knowledge of treatment may have influenced the findings)
Summary of findings 4. Antifibrinolytics compared to ethamsylate for heavy menstrual bleeding.
| Antifibrinolytics compared to ethamsylate for heavy menstrual bleeding | ||||||
| Population: women with heavy menstrual bleeding Setting: all Intervention: antifibrinolytics Comparison: ethamsylate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with ethamsylate | Risk with antifibrinolytics | |||||
| Menstrual blood loss: mean loss Assessed with: alkaline haematin method Follow‐up: mean 3 months | The mean menstrual blood loss: mean loss was 175 mL | MD 100 mL lower (141.82 lower to 58.18 lower) | ‐ | 53 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Menstrual blood loss: improvement rates Assessed with: patient assessment Follow‐up: mean 3 months | 444 per 1000 | 693 per 1000 (422 to 1000) | RR 1.56 (0.95 to 2.55) | 53 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 | |
| Withdrawal from treatment because of adverse events | 148 per 1000 | 115 per 1000 | RR 0.78 (0.19 to 3.15) | 53 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 | |
| Thromboembolic events | Thromboembolic events were not measured in the study. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate — the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited — the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate — the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Quality downgraded 1 level due to risk of bias (single study with unclear allocation concealment)
2 Quality downgraded 1 level due to risk of bias (single small study)
3 Quality downgraded 2 levels due to risk of bias (single study with unclear allocation concealment and high risk of performance bias)
Summary of findings 5. Antifibrinolytics compared to herbal medicines for heavy menstrual bleeding.
| Antifibrinolytics compared to herbal medicines for heavy menstrual bleeding | ||||||
| Patient or population: heavy menstrual bleeding Setting: all Intervention: antifibrinolytics Comparison: herbal medicines | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with herbal medicines | Risk with antifibrinolytics | |||||
| Menstrual blood loss: mean loss ‐ after 3 months Rx Assessed with: PBAC4 | The mean PBAC score ranged from 51 to 143 pts | MD 23.90 pts lower (31.2 lower to 15.88 lower) | 121 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | One trial assessed a 'unani' formulation vs TXA and the other a pomegranate extract vs TXA | |
| Menstrual blood loss: mean loss ‐ after 3 months' follow‐up from end of Rx Assessed with: PBAC4 Follow‐up: mean 3 months | The mean PBAC score was 71.3 pts | MD 10.4 pts lower (19.2 lower to 1.6 lower) | ‐ | 45 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 | PBAC scores evaluated 3 months after Rx was completed in both groups. |
| Rates of improvement | This outcome was not reported. | |||||
| Adverse events (any) | 85 per 1000 | 191 per 1000 | RR 2.25 (0.74 to 6.80) | 94 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 2 3 | |
| Thromboembolic events | The study did not measure any thromboembolic events. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate — the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited — the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate — the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Quality downgraded 2 levels due to risk of bias (one trial had unclear selection, performance, and attrition bias; the other had unclear attrition and other bias)
2 Quality downgraded 1 level due to risk of bias(trial had unclear selection, performance and attrition bias)
3 Quality downgraded 1 level because of imprecision (single small study)
4Some authors consider 100 points in the Pictorial Blood Assessment Chart (PBAC) equivalent to 80 mL of menstrual blood loss (i.e. heavy menstrual bleeding); some 150 points.
Summary of findings 6. Antifibrinolytics compared to levonorgestrel intrauterine system.
| Antifibrinolytics compared with levonorgestrel for heavy menstrual bleeding | ||||||
|
Patient or population: heavy menstrual bleeding Settings: all Intervention: antifibrinolytics Comparison: levonorgestrel intrauterine system (IUS) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with levonorgestrel IUS | Risk with TXA | |||||
| Menstrual blood loss: median difference in PBAC score ‐ after 6 months Rx Assessed with: PBAC4 Follow‐up: outcomes measured at end of treatment |
−252.0 (IQR 124.5) 1 | −126.5 (IQR 104.5) | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2 3 | ||
| Menstrual blood loss: improvement in mean blood loss (PBAC score < 100) Assessed with: PBAC4 Follow‐up: outcomes measured at end of treatment |
850 per 1000 | 364 per 1000 (204 to 655) | RR 0.43 (0.24 to 0.77) | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2 3 | |
| Adverse events (any) | 500 per 1000 | 455 per 1000 | RR 0.83 (0.25 to 2.80) | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2 3 | |
| Thromboembolic events | The study did not measure any thromboembolic events. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IQR: Interquartile Range; OR: Odds Ratio; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Unable to calculate 'X per 1000 women', as the data are not in a normal distribution, but are skewed.
2 Quality downgraded 2 levels due to the one trial being at high risk of: performance and detection bias; and attrition bias.
3 Quality downgraded a further level because of imprecision (single small trial).
4Some authors consider 100 points in the Pictorial Blood Assessment Chart (PBAC) equivalent to 80 mL of menstrual blood loss (i.e. heavy menstrual bleeding); some 150 points.
1. Antifibrinolytics versus placebo or no treatment
There were no trials of antifibrinolytic therapy versus no treatment as the control group. Four studies compared antifibrinolytic treatment with placebo: Callender 1970; Edlund 1995; Freeman 2011; and Lukes 2010 (565 participants). Please see Table 1.
Primary outcomes
1.1 Menstrual blood loss
Four trials with 565 participants reported this outcome, using measurements of menstrual blood loss (MBL). There is evidence that tranexamic acid was associated with less MBL, compared to placebo (MD −53.20 mL per cycle, 95% CI −62.70 to −43.70; P < 0.00001, I² = 8%; 4 RCTs, participants = 565; low‐quality evidence; Analysis 1.1).
1.1. Analysis.
Comparison 1 Antifibrinolytic agent versus placebo, Outcome 1 Menstrual blood loss: mean loss.
1.2 Improvement in HMB
Three RCTs reported rates of improvement, measured either subjectively (Edlund 1995) or objectively (Callender 1970; Lukes 2010). Rates of improvement were higher in the antifibrinolytic treatment group (RR 3.34, 95% CI 1.84 to 6.09; P < 0.0001, I² = 32%; 3 RCTs, participants = 271; moderate‐quality evidence; Analysis 1.2).
1.2. Analysis.
Comparison 1 Antifibrinolytic agent versus placebo, Outcome 2 Menstrual blood loss: improvement rates.
1.3 Thromboembolic events
Only two of the studies reported this outcome. Freeman 2011 reported that there were no thrombotic or thromboembolic adverse effects in either group. One participant from the placebo group in Lukes 2010 had a deep venous thrombosis during the trial; no thrombotic events were reported in their antifibrinolytic treatment group. Unfortunately neither study was powered for this outcome.
Secondary outcomes
1.4 Quality of life
Two RCTs reported this outcome, using the Menorrhagia Impact Questionnaire. Tranexamic acid was associated with an improvement in quality of life with regard to: social/leisure activities (MD 0.52 points per cycle, 95% CI 0.31 to 0.74; P < 0.00001, I² = 0%; 2 RCTs, participants = 365; moderate‐quality evidence); physical activities (MD 0.55 points per cycle, 95% CI 0.34 to 0.77; P < 0.00001, I² = 0%; 2 RCTs, participants = 365; moderate‐quality evidence); and work in or outside the home (MD 0.55 points per cycle, 95% CI 0.30 to 0.80; P < 0.0001; 1 RCT, participants = 187; moderate‐quality evidence; Analysis 1.3). We calculated SDs for one of the studies from what appear to be SEs (Lukes 2010), as noted in the Methods section.
1.3. Analysis.
Comparison 1 Antifibrinolytic agent versus placebo, Outcome 3 Quality of life scores (change from baseline).
1.5 Adverse events (other than thromboembolic events)
There was insufficient evidence to determine whether there is a difference between the groups in the overall rate of adverse events (RR 1.05, 95% CI 0.93 to 1.18; P = 0.46; 1 RCT, participants = 297; low‐quality evidence; Analysis 1.4).
1.4. Analysis.
Comparison 1 Antifibrinolytic agent versus placebo, Outcome 4 Adverse events.
Nor was there any clear evidence of a difference in rates of any specific adverse event, though confidence intervals were wide. Events reported in the included studies were as follows: gastrointestinal adverse effects, headache, uterine cancer, vaginal dryness, dysmenorrhoea, viral upper respiratory tract infection, arthralgia, myalgia, nasal congestion, sinusitis, multiple allergies, throat irritation, anaemia, abdominal discomfort, cough, insomnia, dyspepsia, migraine.
2. Antifibrinolytics versus progestogens
Four RCTs compared antifibrinolytic treatment to oral luteal phase progestogens (during the second half of the menstrual cycle) (Jaisamrarn 2006; Kiseli 2016; Preston 1995; Zhang 2008); and two RCTs compared antifibrinolytic treatment to oral long‐course (i.e. from day 5 of the menstrual cycle) progestogens (Goshtasebi 2013; Kriplani 2006).
Primary outcomes
2.1 Menstrual blood loss
Six RCTs reported this outcome. One used an objective measure (Preston 1995, alkaline haematin method). Five used subjective measures (Goshtasebi 2013; Jaisamrarn 2006; Kiseli 2016; Kriplani 2006; Zhang 2008, PBAC).
2.1.1 Objective assessment of mean blood loss
Preston 1995 compared antifibrinolytic treatment versus luteal phase NET, assessed using the alkaline haematin method. Antifibrinolytic treatment was associated with less mean blood loss than NET: mean value (SD) after treatment with antifibrinolytic treatment was 97 (SD 89); mean value after treatment with NET was 208 (SD 135; Table 7).
1. Antifibrinolytic agent vs control: menstrual blood loss.
| Study | Comparison | Outcome | Intervention group | Control group | Finding (e.g. P value for difference between groups, as reported in primary study) | ||||
| Intervention | n | Result (mean (SD)) | Intervention | n | Result (mean (SD)) | ||||
| Jaisamrarn 2006 | TXA vs progestogens or NSAIDs | PBAC score | TXA 3 g daily | 56 | 204.4 (SD 255.7) | Norethisterone 10 mg daily (luteal phase) | 56 | 298.7 (SD 141.3) | P < 0.0001 |
| Mefenamic acid 1.5 mg daily | 56 | 278.3 (SD 164.2) | P < 0.001 | ||||||
| Preston 1995 | TXA vs luteal phase MPA | Mean loss by alkaline haematin method (end score) | TXA 4 g daily | 25 | 97 (SD 89) | Norethisterone (21 participants) 5 mg taken twice daily on days 19 to 26 of cycle. | 21 | 208 (SD 135) | P = 0.001 |
2.1.2 Subjective assessment of blood loss
Five studies measured blood loss by the PBAC tool, two of which measured long‐course progestogens (for 20 to 25 days of the menstrual cycle, Goshtasebi 2013; Kriplani 2006); the others measured luteal phase progestogen (Jaisamrarn 2006; Kiseli 2016; Zhang 2008). Data from Jaisamrarn 2006 could not be pooled in the forest plot because it was skewed; the author reported that there is evidence of less blood loss with antifibrinolytic treatment, compared to NET (mean PBAC with TXA was 204.4 (SD 255.7); mean PBAC with NET was 298.7 (SD 141.3); participants = 112; Table 7).
For the remaining trials, antifibrinolytic treatment was not associated with an improvement in MBL compared to either luteal phase progestogen or long‐course progestogen (MD −12.22 points per cycle, 95% CI −30.80 to 6.36; very low quality evidence; Analysis 2.1).
2.1. Analysis.
Comparison 2 Antifibrinolytic agent versus progestogens, Outcome 1 Menstrual blood loss: mean PBAC score.
2.2 Improvement in HMB
Four studies measuring luteal‐phase progestogens reported this outcome (Jaisamrarn 2006; Kiseli 2016; Preston 1995; Zhang 2008). Overall, antifibrinolytic treatment was associated with higher rates of improvement (RR 1.66, 95% CI 1.34 to 2.05; P < 0.00001, I² = 0%; 4 RCTs, participants = 328; low‐quality evidence; Analysis 2.4).
2.4. Analysis.
Comparison 2 Antifibrinolytic agent versus progestogens, Outcome 4 Menstrual blood loss: improvement rates.
Kriplani 2006, which measured long course progestogens (commencing on day 5 of the menstrual cycle), also found higher rates of improvement compared to baseline (RR 1.32, 95% CI 1.08 to 1.61; P = 0.006; 1 RCT, participants = 94; low‐quality evidence; Analysis 2.4).
2.3 Thromboembolic events
No studies reported this outcome.
Secondary outcomes
2.4 Quality of life
Four studies reported this outcome in a usable form (Goshtasebi 2013; Kiseli 2016; Preston 1995; Zhang 2008). Jaisamrarn 2006 also assessed quality of life, using a 6‐item questionnaire, but the data were not in a usable form.
There was no evidence of any differences in the quality of life domains measured in Preston 1995 between antifibrinolytic treatment and NET (see Analysis 2.5); these domains included general health, abdominal pain, limitation of social activities and sex life.
2.5. Analysis.
Comparison 2 Antifibrinolytic agent versus progestogens, Outcome 5 Quality of life ‐ improvement (luteal phase MPA).
Neither Goshtasebi 2013 nor Kiseli 2016 found any evidence of differences in quality of life measures between antifibrinolytic treatment and long‐course progestogens (days 5 to 26 of the menstrual cycle), using the SF‐36 or WHOQOL‐BREF TR respectively. See Analysis 2.6.
2.6. Analysis.
Comparison 2 Antifibrinolytic agent versus progestogens, Outcome 6 Quality of life ‐ SF36 (long course MPA).
Two trials assessed a more general, HMB‐specific quality of life measure (Goshtasebi 2013; Zhang 2008). These studies were pooled using a standardized mean difference analysis. There was insufficient evidence to determine whether there is a difference in the summary effect measure (standard mean difference −0.06, 95% CI −0.32 to 0.21, P = 0.67, 2 RCTs, participants = 218, low‐quality evidence). See Analysis 2.7.
2.7. Analysis.
Comparison 2 Antifibrinolytic agent versus progestogens, Outcome 7 Quality of life ‐ HMB score.
2.5 Adverse events (other than thromboembolic events)
Four studies reported overall adverse event rates (Goshtasebi 2013; Kiseli 2016; Kriplani 2006; Zhang 2008). The evidence suggested that antifibrinolytic treatment is associated with a lower rate of adverse events than progestogens (RR 0.66, 95% CI 0.46 to 0.94; P = 0.02, I² = 28%; 4 RCTs, participants = 349; low‐quality evidence; Analysis 2.8). The most common events in the antifibrinolytic treatment groups were gastrointestinal effects, vertigo and headache, whilst the most common in the progestogen groups were bleeding, headaches, breast tenderness, and gastrointestinal effects.
2.8. Analysis.
Comparison 2 Antifibrinolytic agent versus progestogens, Outcome 8 Adverse events (short and long course progestogens).
Two participants withdrew from Zhang 2008 due to side effects: one from the antifibrinolytic treatment group because of headaches, and one from the NET group due to an elevation in alanine transaminase.
Six participants in Kiseli 2016's NET group withdrew due to side effects: three due to headache, two due to bloating, and one due to weight gain. Ten participants in this study's antifibrinolytic treatment group withdrew due to side effects: five due to headache; three, nausea; one, weight gain; and one, a rash.
There was no clear evidence of a difference in rates of any specific adverse event, though confidence intervals were wide. Events reported in the included studies were as follows: gastrointestinal effects, headache, dysmenorrhoea, weight gain, allergic reaction, giddiness, intermenstrual bleeding, breast tenderness, mood changes, rash, muscle pain, bloating, nausea, spotting, excess hair growth, and depression. See Analysis 2.8.
3. Comparison of antifibrinolytic therapy versus other medical (non‐surgical) treatments: non‐steroidal anti‐inflammatory drugs (NSAIDs)
Two trials compared antifibrinolytic treatment to NSAIDS: Bonnar 1996 compared 4 g TXA/day to mefenamic acid (MFA) 500 mg TDS on days 1 to 5, while Jaisamrarn 2006 compared 3 g TXA/day to 1.5 g MFA/day on days 1 to 5.
Primary outcomes
3.1 Menstrual blood loss
Antifibrinolytic treatment was associated with less MBL, compared to NSAIDs (MD −73.00 mL per cycle, 95% CI −123.35 to −22.65; P = 0.004; 1 RCT, participants = 49; low‐quality evidence; Analysis 3.1).
3.1. Analysis.
Comparison 3 Antifibrinolytic agent versus NSAIDs, Outcome 1 Menstrual blood loss: mean loss.
3.2 Improvement in HMB
There was evidence of a difference between the groups when antifibrinolytic treatment was compared to NSAIDs (RR 1.43, 95% CI 1.18 to 1.74; P = 0.0003; 2 RCTs, participants = 161; low‐quality evidence; Analysis 3.2).
3.2. Analysis.
Comparison 3 Antifibrinolytic agent versus NSAIDs, Outcome 2 Menstrual blood loss: improvement rates.
3.3 Thromboembolic events
No studies reported this outcome.
Secondary outcomes
3.4 Quality of life
Whilst Jaisamrarn 2006 assessed quality of life using the Menorrhagia Impact Questionnaire, no quantitative data were provided for analysis.
3.5 Adverse events (other than thromboembolic events)
One trial measured adverse events (Jaisamrarn 2006). The authors did not find any evidence of a difference in the occurrence of headache and dizziness, muscle pain, or dysmenorrhoea between the antifibrinolytic treatment and NSAID groups (see Analysis 3.3).
3.3. Analysis.
Comparison 3 Antifibrinolytic agent versus NSAIDs, Outcome 3 Any adverse events.
4. Comparison of antifibrinolytic therapy versus other medical (non‐surgical) treatments: ethamsylate
There was only one study that compared antifibrinolytic treatment to ethamsylate: Bonnar 1996 compared 4 g TXA/day to ethamsylate 500 mg four times daily on days 1 to 5.
Primary outcomes
4.1 Menstrual blood loss
Bonnar 1996 used the alkaline haematin method to quantify MBL. Antifibrinolytic treatment was associated with less MBL, when compared to ethamsylate (MD −100.00 mL per cycle, 95% CI −141.82 to −58.18; P < 0.00001; 1 RCT, participants = 53; low‐quality evidence; Analysis 4.1).
4.1. Analysis.
Comparison 4 Antifibrinolytic agent versus ethamsylate, Outcome 1 Menstrual blood loss: mean loss.
4.2 Improvement in HMB
Bonnar 1996 asked women whether or not their MBL during treatment was less, the same, or more. There was no evidence of a difference between the groups in the rates of women reporting less bleeding when antifibrinolytic treatment was compared to ethamsylate (RR 1.56, 95% CI 0.95 to 2.55; P = 0.08; 1 RCT, participants = 53; very low quality evidence; Analysis 4.2).
4.2. Analysis.
Comparison 4 Antifibrinolytic agent versus ethamsylate, Outcome 2 Menstrual blood loss: improvement rates.
4.3 Thromboembolic events
The study did not report this outcome.
Secondary outcomes
4.4 Quality of life
The study did not report this outcome.
4.5 Adverse events (other than thromboembolic events)
There was no evidence of a difference in the number of women who withdrew from the antifibrinolytic treatment or ethamsylate groups between the groups when antifibrinolytic treatment was compared to ethamsylate (RR 0.78, 95% CI 0.19 to 3.15; P = 0.73; 1 RCT, participants = 53; very low quality evidence; Analysis 4.3).
4.3. Analysis.
Comparison 4 Antifibrinolytic agent versus ethamsylate, Outcome 3 Withdrawal from treatment because of adverse events.
5. Comparison of antifibrinolytic therapy versus other medical (non‐surgical) treatments: herbal remedies (Safoof Habis and Punica granatum)
One study compared antifibrinolytic treatment to Safoof Habis: Fathima 2012 compared 3 g TXA/day to Safoof Habis 5 g powder BD, on days 1 to 5. As noted previously, Safoof Habis is a Unani formulation made up of: Teen Ahmer (silicate of alumina and iron oxide); Sange Jarahat (hydrated magnesium silicate); and Raal Sufaid (Vateria indica Linn, which is a species of plant in the Disterocarpaceae family, endemic to India), in equal parts.
One trial compared antifibrinolytic treatment to Punica granatum (commonly known as pomegranate flower) (Goshtasebi 2015).
Primary outcomes
5.1 Menstrual blood loss
Fathima 2012 used a menstrual pictogram (a modified PBAC) to ascertain a subjective assessment of MBL, while Goshtasebi 2015 used PBAC.
There was evidence that antifibrinolytic treatment was associated with less MBL after three months' treatment, compared to these herbal remedies (MD −23.90 points per cycle, 95% CI −31.92 to −15.88; P < 0.00001; 2 RCTs, participants = 121; low‐quality evidence; Analysis 5.1).
5.1. Analysis.
Comparison 5 Antifibrinolytic agent versus herbal medicines, Outcome 1 Menstrual blood loss: mean loss.
The baseline values of MBL in Fathima 2012 appeared different between randomized groups (without reaching statistical significance (P = 0.107)). Hence, the mean reduction (difference between baseline and later measurement) is considered a more reliable way to compare menstrual bleeding in participants, as it adjusts for different baseline levels.
Antifibrinolytic treatment was associated with less blood loss after 3 months' treatment (based on the PBAC) than Safoof Habis (MD −24.00 points per cycle, 95% CI −32.15 to −15.85; 1 RCT, participants = 45; low‐quality evidence). Antifibrinolytic treatment is associated with sustained decreased MBL three months after the end of the treatment period, compared to Safoof Habis (MD −10.40 points per cycle, 95% CI −19.20 to −1.60; P = 0.02; 1 RCT, participants = 45; low‐quality evidence; Analysis 5.1).
Goshtasebi 2015 utilized PBAC to compare mean blood loss between the tranexamic acid and pomegranate flower groups, and their baseline (pre‐treatment) mean blood loss. Both groups showed evidence of a reduction in mean blood loss from baseline, compared to three months of treatment: the antifibrinolytic treatment group's mean reduction in blood loss was 161.31 (PBAC score) and the pomegranate flower group's mean reduction in blood loss was 140.31 (PBAC score). However, there was no evidence of a difference between the groups.
5.2 Improvement in HMB
No studies reported this outcome.
5.3 Thromboembolic events
No studies reported this outcome.
Secondary outcomes
5.4 Quality of life
Goshtasebi 2015 used an Iranian version of both the Menorrhagia Impact Questionnaire (MIQ) and the SF‐36 when assessing quality of life. The authors found no evidence of a difference between the antifibrinolytic treatment and progestogen groups when using the MIQ. Of the eight domains of the SF‐36, the only finding was of more improvement in general health (from before to after treatment) amongst the antifibrinolytic treatment group (compared to the progestogen group) (MD 10.30 points per cycle, 95% CI 2.41 to 18.19; P = 0.01; 1 RCT, participants = 76; low‐quality evidence; Analysis 5.2). Of note: there was no difference between post‐treatment SF‐36 scores in the antifibrinolytic treatment and progestogen groups.
5.2. Analysis.
Comparison 5 Antifibrinolytic agent versus herbal medicines, Outcome 2 Quality of life scores.
5.5 Adverse events (other than thromboembolic events)
No studies reported this outcome.
6. Antifibrinolytics versus levonorgestrel intrauterine system (LIUS)
One study compared antifibrinolytic treatment levonorgestrel intrauterine system (LIUS): Kiseli 2016 (62 participants). Please see Table 6.
Primary outcomes
6.1 Menstrual blood loss
The authors used median difference in PBAC scores (from baseline) to measure MBL. There is evidence that LIUS was associated with less MBL, compared to TXA (median difference in PBAC score for LIUS −252, compared to −126.5 for TXA group; P = 0.002; 1 RCT, participants = 42; very low quality evidence; Analysis 6.1).
6.1. Analysis.
Comparison 6 Antifibrinolytic agent versus levonorgestrel intrauterine system, Outcome 1 Median difference in PBAC score.
| Median difference in PBAC score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Comparison | N (TXA) | Median (TXA) | IQR (TXA) | N (LIUS) | Median (LIUS) | IQR (LIUS) | P value |
| After 6 months Rx | ||||||||
| Kiseli 2016 | TXA vs. LIUS | 22 | ‐126.5 | 104.5 | 20 | ‐252 | 124.5 | 0.002 |
Measuring the percentage difference in PBAC scores from baseline to six months of treatment revealed that the TXA group had an average percentage difference of −60.8 PBAC score (IQR 34.9, very low quality evidence), whilst the LIUS group had a −85.8 percentage difference in PBAC score after six months' treatment (IQR 20.3, very low quality evidence).
This means that if 850 per 1000 women taking LIUS improved their PBAC score to be less than 100, 364 of 1000 taking TXA did (RR 0.43, 95% CI 0.24 to 0.77; very low quality evidence).
6.2 Improvement in HMB
The authors used percentage difference in PBAC scores (from baseline to three months after treatment) to judge the improvement in menstrual bleeding. Rates of improvement were higher in the LIUS group (median PBAC score in LIUS group −85.8, compared to −60.8 for TXA group; 1 RCT, participants = 42; very low quality evidence; Analysis 6.2).
6.2. Analysis.
Comparison 6 Antifibrinolytic agent versus levonorgestrel intrauterine system, Outcome 2 Percentage difference in PBAC scores.
| Percentage difference in PBAC scores | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Comparison | N (TXA) | Median (TXA) | IQR (TXA) | N (LIUS) | Median (LIUS) | IQR (LIUS) | P value |
| After 6 months Rx | ||||||||
| Kiseli 2016 | TXA vs. LIUS | 22 | ‐60.8 | 34.9 | 20 | ‐85.8 | 20.3 | |
The authors also used the number of patients in each group reporting a PBAC score of less than 100 after three months' treatment as a measurement of their improvement in menstrual bleeding. Rates of improvement were higher in the LIUS group (RR 0.43, 95% CI 0.24 to 0.77; P = 0.004; 1 RCT, participants = 42; very low quality evidence; Analysis 6.3).
6.3. Analysis.
Comparison 6 Antifibrinolytic agent versus levonorgestrel intrauterine system, Outcome 3 Improvement in mean blood loss (PBAC score < 100).
6.3 Thromboembolic events
This study did not report thromboembolic events.
Secondary outcomes
6.4 Quality of life
Kiseli 2016 reported this outcome using the World Health Organization's Quality of Life‐Short Form, Turkish version (WHOQOL‐BREF TR). As can be seen in Analysis 6.4, there was no evidence of differences in quality‐of‐life parameters in any domain measured.
6.4. Analysis.
Comparison 6 Antifibrinolytic agent versus levonorgestrel intrauterine system, Outcome 4 Quality of life scores.
6.5 Adverse events (other than thromboembolic events)
There was no evidence of a difference between the groups in the overall rate of adverse events (OR 0.83, 95% CI 0.25 to 2.80; P = 0.77; 1 RCT, participants = 42; very low quality evidence). Nor was there any clear evidence of a difference in rates of any specific adverse event, though confidence intervals were wide. Events reported in the included studies were as follows: headache, bloating, nausea, weight gain, rash, spotting, excess hair growth, breast tenderness, and depression. See Analysis 6.5.
6.5. Analysis.
Comparison 6 Antifibrinolytic agent versus levonorgestrel intrauterine system, Outcome 5 Adverse events.
Other analyses
Sensitivity analysis using a random‐effects effect model did not substantially influence any of our findings.
There were insufficient studies to construct a funnel plot.
Discussion
Summary of main results
This Cochrane review evaluated the effectiveness and safety of antifibrinolytic medications (tranexamic acid and its alternatives, such as pro‐drug Kabi) in the medical management of heavy menstrual bleeding. Four studies compared TXA or Kabi to placebo, and six compared TXA to progestogens. There were few studies for the other comparisons: two compared TXA to NSAIDs (mefenamic acid), one compared TXA to ethamsylate, two to different herbal remedies, and one to levonorgestrel IUS (LIUS). Hence, there is insufficient evidence for these latter comparisons.
No studies were found that compared antifibrinolytic treatment to the combined oral contraceptive pill (COCP).
On the available evidence, antifibrinolytics appear to be more effective than placebo, short courses of luteal phase oral progestogens, NSAIDs, ethamsylate or herbal remedies in reducing HMB . There is a 40% to 50% reduction from baseline in MBL for participants treated with tranexamic acid. There is a 25% to 50% reduction from baseline in measured MBL for participants treated with tranexamic acid when compared to other medical therapies.
If response to treatment is defined by reduction of MBL to less than 80 mL per cycle, 56% (14/25) of women had their menstrual cycles reduced to below 80 mL per cycle in the only study that provided this information (Preston 1995).
Antifibrinolytic treatment is more effective than NET (when NET was given during the luteal phase only), but was of similar efficacy when the NET was given throughout the menstrual cycle (i.e. from day 5). There was no evidence of a reduction in MBL with antifibrinolytic treatment, compared to long‐course MPA (when judged by PBAC), but there was when it was compared to three months' treatment with Safoof Habis.
Antifibrinolytic treatment reduces HMB in studies where blood loss has been measured, and in studies using self‐reported HMB compared to oral luteal phase progestogens. Antifibrinolytic treatment was not more effective in reducing HMB as reported by the patient compared to placebo, NSAIDs or ethamsylate, although results almost reached statistical significance. There is a strong trend in favour of tranexamic acid and, with additional trials, a difference may be reported.
Antifibrinolytic therapy was not associated with reduced MBL compared to luteal phase progestogens. However, antifibrinolytic therapy was associated with a greater improvement in HMB (as perceived by the participants), compared to luteal phase progestogens. The reasons behind this apparent discrepancy could be that women's own perception of their MBL is not particularly well correlated with the actual objective volume of blood lost. NICE 2016 recognises that, in practical terms, it is women's subjective perception of their MBL that takes precedence over objective, laboratory‐based measures such as the alkaline haematin test.
LIUS showed a reduction in mean blood loss compared to antifibrinolytic treatment.
The impact of antifibrinolytic treatment on quality of life has been compared with placebo and oral progestogen therapy. Compared to placebo, antifibrinolytic treatment was associated with an improvement in quality of life.
Antifibrinolytic treatment improved quality of life in women with HMB more effectively than oral luteal phase progestogens: antifibrinolytic treatment was more effective in reducing flooding/leakage, and improving sex life. There were no differences between antifibrinolytic treatment and oral luteal phase progestogens in general health, abdominal pain, or social activities in women with HMB. Goshtasebi 2013 was the only trial to use the SF‐36 tool to assess quality of life: there was no difference between the antifibrinolytic treatment and MPA groups in regard to quality of life.
Apart from four larger studies — Freeman 2011, Jaisamrarn 2006, Lukes 2010 and Zhang 2008 — the number of women in the other eight trials was small (< 100 participants). There are some limitations to this evidence: the results are based on very small numbers of participants for all comparisons, and the data for some comparisons are heavily skewed.
Women using antifibrinolytics for HMB are likely to be using this treatment long term; any adverse events affecting adherence or safety are particularly important. With respect to the safety of tranexamic acid and alternative medical management of HMB, only five of the included studies reported adverse effects in both treatment groups. There was no evidence of a difference between the groups, but data were too scanty to reach any reliable conclusions about safety.
Freeman 2011 did not find any thrombotic or thromboembolic adverse effects in either the placebo or antifibrinolytic treatment group. One participant from the placebo group in Lukes 2010 had a deep venous thrombosis during the trial; no thrombotic events were reported in their antifibrinolytic treatment group. Neither of these trials was powered to detect this outcome.
It was outside the scope of this review's search strategy to collate cohort and case‐controlled studies which might have clarified this point. A noticeable limitation of this review is the short period of treatment in the included trials (mostly three months).
Overall completeness and applicability of evidence
This Cochrane review included 13 studies with data that were relevant to this review question.
The participants in these studies were all women who reported heavy menstrual bleeding. This was objectively confirmed using the alkaline haematin method in five studies, and the PBAC in seven studies. In clinical practice, the patient‐perceived HMB may be more practicable in judging which women have troublesome HMB requiring treatment than either the alkaline haematin method or PBAC.
Participants in the studies included women aged 15 to 50 years old, with measured or self‐reported ovulatory heavy menstrual bleeding. Hence, the conclusions drawn from these studies may not be representative of women with anovulatory HMB (i.e. during adolescence or around the menopause; or HMB due to conditions associated with anovulation, such as polycystic ovarian syndrome). Therefore, the results of this Cochrane review should be viewed with caution when attempting to extrapolate its conclusions to such patient groups.
Where MBL was measured, most studies only included women with MBL of more than 80 mL per cycle. However, 50% of women who self‐report HMB do not have a measured MBL of more than 80 mL. Hence it is unclear whether studies that only included women with MBL of more than 80 mL are relevant for the management of women in clinical practice which relies on self‐reported HMB. Of the studies that used self‐reported MBL, Callender 1970 reported a 37% decrease in MBL with antifibrinolytic treatment compared to placebo; Bonnar 1996 reported that antifibrinolytic treatment reduced MBL by 54% compared to no treatment; Edlund 1995 reported a 33% reduction in MBL with Kabi QDS, and 41% reduction with Kabi BD, both compared to placebo; Fathima 2012 reported that antifibrinolytic treatment and Safoof Habis were equally effective in reducing MBL — the PBAC score in the antifibrinolytic treatment group improved from 162.37 at baseline to 75.3 over the course of three cycles; and Preston 1995 reported that antifibrinolytic treatment reduced MBL by 45%.
Quality of the evidence
The methodological quality of the evidence varied from very low to moderate. The main limitations were risk of bias (associated with lack of blinding, and poor reporting of study methods), imprecision and inconsistency. See Summary of main results, Figure 2 and Figure 3 for more details.
Classic or modified intention‐to‐treat analysis was performed by only three studies, whilst three performed a priori power calculations (Freeman 2011; Goshtasebi 2015; Lukes 2010).
For a number of outcomes, only one trial reported data; lack of power together with major study limitations meant that the quality of the evidence for many of these outcomes was very low. In addition, where studies were not blinded and PBAC was used to measure the primary bleeding outcome or adverse events were experienced, the participants' knowledge of their treatment was likely to have influenced their assessment of their bleeding and adverse events. Hence, these findings should be considered tentative. No outcome results were supported by high‐quality evidence. A few had moderate‐quality evidence, but most had either low‐quality or very low quality evidence.
Potential biases in the review process
We attempted to identify and include all relevant studies through the standardized method of identifying studies, but it is possible we may have missed some studies. Not all trial authors responded to our requests for more information; we are unsure whether our requests always reached the author and so imputation was used for some data.
The authors attempted to minimise potential biases in the review process by undertaking duplicate selection of studies, duplicate data extraction, and duplicate assessment of risk of bias. Where there were disagreements, the authors resolved these through discussion and reaching consensus, if necessary with the help of a third author.
Agreements and disagreements with other studies or reviews
Current practice in managing HMB varies widely, both between and within countries. The United Kingdom's National Institute for Health and Clinical Excellence (NICE) guideline regarding management of HMB recommends the following medical managements, in order of preference, for women with no suspected structural/histological abnormalities underlying their HMB: levonorgestrel intrauterine system (LIUS); TXA, NSAIDs or COCP; NET (days 5 to 26 of the menstrual cycle); or injected long‐acting progestogens (NICE 2016).
The Society of Obstetricians and Gynaecologists of Canada (SCOG) outlines the following medical treatments for HMB: non‐hormonal (NSAIDs, antifibrinolytic treatment); and hormonal (COCP, levonorgestrel intrauterine system, oral progestins, Depot‐MPA, danazol, and GnRH‐agonists). Unfortunately they do not provide any clear guidance as to which is preferable, other than broad statements recommending that patients' contraceptive needs and medical co‐morbidities should be taken into account (Singh 2013). Hence our findings support current recommendations from both NICE and SCOG regarding the efficacy of antifibrinolytic treatment in managing HMB.
Lumsden 2011 is a succinct review of the use of TXA in HMB in clinical practice. The authors conclude that TXA is particularly useful for the management of HMB in women who either desire pregnancy immediately or for whom hormonal treatment is inappropriate. They describe TXA as being a well‐tolerated and cost‐effective drug that reduces MBL by 34% to 59%, whilst improving health‐related quality of life in women with HMB.
A previous systematic review investigated the efficacy of tranexamic acid in treating heavy menstrual bleeding (Naoulou 2012). In line with this current review, the authors concluded that tranexamic acid is effective and safe in management HMB: their results showed a 34% to 54% reduction in either self‐reported or measured MBL with antifibrinolytic treatment.
Another systematic review compared RCTs of non‐surgical management of HMB (Matteson 2013), which was presumed to be secondary to endometrial dysfunction and anovulation. The non‐surgical methods in the comparison included: the LIUS, combined OCP, progestins, NSAIDs, and anti‐fibrinolytics. The authors concluded that these management options' efficacies (listed from most to least efficacious) were: LIUS (71% to 95% reduction in menstrual bleeding); COCP (35% to 69% reduction); extended cycle oral progestogens (87% reduction); TXA (26% to 54% reduction); NSAIDs (10% to 52% reduction). Of note: progestins used only during the luteal phase (e.g. days 15 to 26) showed a 20% increase to 67% reduction in menstrual bleeding. Our review has found antifibrinolytic treatment's efficacy to be at the higher end of their range.
Gupta 2013 performed a pragmatic randomized trial, comparing LIUS with 'usual medical treatment', including tranexamic acid, mefenamic acid, COCP and progesterone‐only pills. Whilst all groups showed evidence of a reduction in MBL, the improvement in self‐reported MBL was greater in the LIUS group.
Authors' conclusions
Implications for practice.
Evidence suggests that antifibrinolytic treatment (such as TXA) is effective treatment with women of reproductive age with heavy menstrual bleeding: it is associated with a reduction in measured heavy menstrual bleeding when compared with placebo, NSAIDS, oral luteal progestogens, ethamsylate, or herbal remedies. On the other hand, LIUS showed a reduction in median PBAC scores compared to TXA. For some of these comparisons, differences between treatments were not always perceived by the women in the trials. These findings are based on small trials with variable quality.
Oral administration of antifibrinolytic therapy (such as TXA) does not seem to be associated with any increase in major adverse events. Most studies did not include VTE as an end‐point; studies that did measure VTE have not shown any increase in risk with antifibrinolytic treatment, but were underpowered for this outcome. Hence, there was insufficient evidence to assess thromboembolism risk with antifibrinolytic treatment.
Implications for research.
More information is needed on the comparative efficacy of antifibrinolytic treatment, compared to other medical therapies such as the combined oral contraceptive pill, LIUS, and progestogens taken for 21 days (or more) of the menstrual cycle. Future trial design needs to include such outcomes as participant satisfaction, cost‐effectiveness data, quality‐of‐life measures, and a longer duration of treatment (at least six months) to assess adverse events. Trials powered to detect any increased risk in VTE would be very helpful, but may require a prohibitively large number of patients to be enrolled. More information is needed on the efficacy of antifibrinolytic treatment in the treatment of self‐reported HMB.
Feedback
Antifibrinolytics for heavy menstrual bleeding
Summary
1. The title is accurate, but would be more informative if it mentioned tranexamic acid. One of the included trials tested another fibrinolytic, and that was a pro‐drug of tranexamic acid.
2. I would like to know more about the adverse effects reported in the trials. The review suggests that they were insignificant, but I suspect that this means statistically insignificant in the individual trials. Whether any of them were clinically significant is not clear, except that they did not apparently lead any woman to stop using the drug. But did they lead to a reduction in dose? How many women reported nausea, diarrhoea or vomiting, the three side‐effects mentioned in the British National Formulary? Were these separated in the trial reports, or were they always lumped together as gastrointestinal effects, as in the review?
3. The objectives excluded "iatrogenic menorrhagia" eg induced by an intra‐uterine device. I suggest that these be included in the next revision of the review, because the problem is a closely related one that does not seem to deserve a separate review on its own."
Reply
1. The systematic review looked at the effects of antifibrinolytic agents on menorrhagia since there is a common mode of action with these agents (see background). It would not be appropriate to limit the review to tranexamic acid alone even though this is the drug invariably used. By leaving the title broad enough, this leaves open the chance to include other agents with a similar mode of action that may be developed in the future.
2. I have included the other types of side effects in this update of the review. Even though there were no statistically significant differences, I acknowledge that there still could be clinically significant differences which are of relevance. However, none of the trials looked at these outcomes so no comment can be made. There were no differences in withdrawal rates from the trials.
3. The review excluded trials of women with intra‐uterine devices who reported heavy menstrual bleeding for 2 reasons. Firstly, women with IUSs fitted who find their bleeding intolerable will usually be advised to try another means of contraception. Secondly, an IUS, the levonorgestrel releasing IUS, has recently been developed which acts both as a contraceptive device and is also recommended as an effective treatment for heavy menstrual bleeding.
Contributors
Andrew Herxheimer, February 1999
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2018 | Review declared as stable | This is not an active area of research and any new evidence is unlikely to change the conclusions of this review. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 6 June 2018 | Amended | Correction of detail in Abstract and PLS |
| 19 April 2018 | Amended | Correction to title of plain language summary |
| 19 April 2018 | Amended | Correction of author address |
| 7 November 2017 | New search has been performed | Methods section updated to current Cochrane standards. |
| 7 November 2017 | New citation required but conclusions have not changed | Six new studies were added (Fathima 2012, Freeman 2011, Goshtasebi 2013, Goshtasebi 2015, Kiseli 2016, and Zhang 2008); and 3 previously excluded studies were included (Jaisamrarn 2006,Kriplani 2006, and Lukes 2010). Three previously included studies were found not to meet inclusion criteria and have been excluded (Andersch 1988; Nilsson 1967; Vermylen 1968). With the addition of new studies and exclusion of others, the conclusions of this review have not changed. |
| 6 November 2008 | Amended | Converted to new review format. |
| 30 August 2000 | New citation required and conclusions have changed | Substantive amendment |
Notes
A new lead reviewer was appointed to update the review. A substantive amendment of this review was performed in August 2000 and November 2017.
We excluded three cross‐over studies (included in a previous edition of this review) as they did not provide first‐phase data. (This was a change from the original protocol criterion for inclusion.)
We identified nine new trials from an updated search; and we made major structural changes to the Methods and Results sections.
Acknowledgements
We are grateful to the Cochrane Gynaecology and Fertility Group for its support, especially Marian Showell (Information Specialist), Jane Marjoribanks (Assistant Managing Editor), and Helen Nagels (Managing Editor).
We thank those pharmaceutical companies (Pharmacia, Upjohn and Lorex Synthelabo) who gave extra unpublished data; and those authors who also provided unpublished data and answered queries (Bonnar 1996; Edlund 1995; Jaisamrarn 2006; Kiseli 2016; Kouides 2009; Lukes 2010; Preston 1995; NCT01428713).
Appendices
Appendix 1. Cochrane Gynaecology and Fertility specialised register search
Searched 7 November 2017
PROCITE platform
Keywords CONTAINS "menorrhagia" or "menorrhagia‐outcome" or "Menorrhagia‐Symptoms" or "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional uterine bleeding" or Title CONTAINS "menorrhagia" or "menorrhagia‐outcome" or "Menorrhagia‐Symptoms" or "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional uterine bleeding" "
AND
Keywords CONTAINS "antifibrinolytics" or "tranexamic acid" or "KABI 2161" or Title CONTAINS "antifibrinolytics" or "tranexamic acid" or "KABI 2161"
(58 hits)
Appendix 2. CENTRAL CRSO search strategy
Searched 7 November 2017
CRS Online web platform
#1MESH DESCRIPTOR Menorrhagia EXPLODE ALL TREES 277 #2Menorrhagia:TI,AB,KY 630 #3hypermenorrhea:TI,AB,KY 17 #4(heavy menstru*):TI,AB,KY 181 #5(heavy period*):TI,AB,KY 5 #6(dysfunctional uter* bleeding):TI,AB,KY 124 #7#1 OR #2 OR #3 OR #4 OR #5 OR #6 787 #8MESH DESCRIPTOR Antifibrinolytic Agents EXPLODE ALL TREES 1116 #9MESH DESCRIPTOR Tranexamic Acid EXPLODE ALL TREES 495 #10antifibrinolytic*:TI,AB,KY 804 #11(tranexamic acid):TI,AB,KY 1330 #12kabi:TI,AB,KY 151 #13#8 OR #9 OR #10 OR #11 OR #12 2211 #14#7 AND #13 78
Appendix 3. MEDLINE search strategy
Searched from 1946 to 7 November 2017
OVID platform
1 exp Menorrhagia/ (4251) 2 hypermenorrhea.tw. (240) 3 Menorrhagia.tw. (3158) 4 heavy menstru$.tw. (812) 5 heavy period$.tw. (97) 6 dysfunctional uter$ bleeding.tw. (856) 7 or/1‐6 (6839) 8 exp antifibrinolytic agents/ or exp tranexamic acid/ (26464) 9 antifibrinolytic$.tw. (2674) 10 tranexamic acid.tw. (3450) 11 trans 4 aminomethylcyclohexanocarboxylic acid.tw. (0) 12 trans‐4‐aminomethylcyclohexanocarboxylic acid.tw. (0) 13 kabi$.tw. (641) 14 or/8‐13 (29376) 15 7 and 14 (363) 16 randomized controlled trial.pt. (498494) 17 controlled clinical trial.pt. (99301) 18 randomized.ab. (435294) 19 placebo.tw. (208626) 20 clinical trials as topic.sh. (195850) 21 randomly.ab. (299905) 22 trial.ti. (196557) 23 (crossover or cross‐over or cross over).tw. (81052) 24 or/16‐23 (1243260) 25 exp animals/ not humans.sh. (4685295) 26 24 not 25 (1145772) 27 15 and 26 (83)
Appendix 4. Embase search strategy
Searched from 1980 to 7 November 2017
OVID platform
1 exp Menorrhagia/ (8681) 2 hypermenorrhea.tw. (287) 3 Menorrhagia.tw. (4693) 4 heavy menstru$.tw. (1304) 5 heavy period$.tw. (146) 6 dysfunctional uter$ bleeding.tw. (1074) 7 or/1‐6 (10824) 8 exp antifibrinolytic agents/ or exp tranexamic acid/ (27349) 9 antifibrinolytic$.tw. (3151) 10 tranexamic acid.tw. (4758) 11 trans 4 aminomethylcyclohexanocarboxylic acid.tw. (0) 12 trans‐4‐aminomethylcyclohexanocarboxylic acid.tw. (0) 13 kabi$.tw. (3013) 14 or/8‐13 (31221) 15 7 and 14 (979) 16 Clinical Trial/ (956884) 17 Randomized Controlled Trial/ (477722) 18 exp randomization/ (76318) 19 Single Blind Procedure/ (30101) 20 Double Blind Procedure/ (142031) 21 Crossover Procedure/ (53857) 22 Placebo/ (302896) 23 Randomi?ed controlled trial$.tw. (170823) 24 Rct.tw. (26275) 25 random allocation.tw. (1713) 26 randomly allocated.tw. (28714) 27 allocated randomly.tw. (2280) 28 (allocated adj2 random).tw. (788) 29 Single blind$.tw. (20075) 30 Double blind$.tw. (177147) 31 ((treble or triple) adj blind$).tw. (730) 32 placebo$.tw. (258758) 33 prospective study/ (414653) 34 or/16‐33 (1833790) 35 case study/ (50918) 36 case report.tw. (342376) 37 abstract report/ or letter/ (1016722) 38 or/35‐37 (1401761) 39 34 not 38 (1787292) 40 15 and 39 (252)
Appendix 5. PsycINFO search strategy
From 1806 to 7 November 2017
OVID platform
1 exp Menstrual Disorders/ (1162) 2 hypermenorrhea.tw. (1) 3 Menorrhagia.tw. (79) 4 heavy menstru$.tw. (20) 5 heavy period$.tw. (10) 6 dysfunctional uter$ bleeding.tw. (25) 7 or/1‐6 (1239) 8 exp Drugs/ (287045) 9 tranexamic acid.tw. (15) 10 antifibrinolytic$.tw. (7) 11 tranexamic acid.tw. (15) 12 (trans 4 adj3 acid).tw. (3) 13 kabi$.tw. (37) 14 or/8‐13 (287097) 15 7 and 14 (249) 16 random.tw. (51306) 17 control.tw. (396719) 18 double‐blind.tw. (21066) 19 clinical trials/ (10639) 20 placebo/ (5001) 21 exp Treatment/ (698017) 22 or/16‐21 (1083414) 23 15 and 22 (184)
Data and analyses
Comparison 1. Antifibrinolytic agent versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss: mean loss | 4 | 565 | Mean Difference (IV, Fixed, 95% CI) | ‐53.20 [‐62.70, ‐43.70] |
| 1.1 Objective assessment of mean loss | 4 | 565 | Mean Difference (IV, Fixed, 95% CI) | ‐53.20 [‐62.70, ‐43.70] |
| 2 Menstrual blood loss: improvement rates | 3 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [1.84, 6.09] |
| 3 Quality of life scores (change from baseline) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Limitations in social/leisure activities | 2 | 365 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.31, 0.74] |
| 3.2 Limitations in physical activities | 2 | 365 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.34, 0.77] |
| 3.3 Limitations in work in or outside the home | 1 | 187 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.30, 0.80] |
| 4 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Any adverse event | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.18] |
| 4.2 Gastrointestinal side effects | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.55, 2.07] |
| 4.3 Headache | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.82, 1.45] |
| 4.4 Uterine cancer | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.06, 32.36] |
| 4.5 Vaginal dryness | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 3.60] |
| 4.6 Dysmenorrhoea | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.90, 1.55] |
| 4.7 Viral URTI | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.58, 2.47] |
| 4.8 Fatigue | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.68, 3.97] |
| 4.9 Musculoskeletal pain | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.82, 2.39] |
| 4.10 Arthralgia | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.72, 4.41] |
| 4.11 Myalgia | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.78, 8.16] |
| 4.12 Nasal congestion | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.77 [0.40, 113.42] |
| 4.13 Sinusitis | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.84, 9.51] |
| 4.14 Multiple allergies | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.68, 4.65] |
| 4.15 Throat irritation | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.22, 4.79] |
| 4.16 Anaemia | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.72, 4.99] |
| 4.17 Abdominal discomfort | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.30, 2.27] |
| 4.18 Cough | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.28, 2.61] |
| 4.19 Insomnia | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.21, 1.84] |
| 4.20 Dyspepsia | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.06, 0.84] |
| 4.21 Migraine | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.46, 10.09] |
Comparison 2. Antifibrinolytic agent versus progestogens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss: mean PBAC score | 3 | 312 | Mean Difference (IV, Fixed, 95% CI) | ‐12.22 [‐30.80, 6.36] |
| 1.1 Luteal phase NET | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐25.0 [‐65.54, 15.54] |
| 1.2 Long course (20‐25 days) MPA | 2 | 184 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐29.73, 12.09] |
| 2 Median difference in PBAC score | Other data | No numeric data | ||
| 2.1 After 6 months Rx | Other data | No numeric data | ||
| 3 Percentage difference in PBAC scores | Other data | No numeric data | ||
| 3.1 After 6 months Rx | Other data | No numeric data | ||
| 4 Menstrual blood loss: improvement rates | 5 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.31, 1.80] |
| 4.1 Luteal phase | 4 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.34, 2.05] |
| 4.2 Long course (20 to 25 days) | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.08, 1.61] |
| 5 Quality of life ‐ improvement (luteal phase MPA) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 General health | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.76, 3.64] |
| 5.2 Abdominal pain | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.68, 5.19] |
| 5.3 Limitation of social activities | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.85, 2.60] |
| 5.4 Sex life | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.99, 9.46] |
| 6 Quality of life ‐ SF36 (long course MPA) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Physical functioning | 2 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [‐0.67, 2.25] |
| 6.2 Role physical | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐5.63, 13.63] |
| 6.3 Bodily pain | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐11.61, 6.01] |
| 6.4 General health | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐2.49, 12.49] |
| 6.5 Vitality | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐6.62, 9.42] |
| 6.6 Social functioning | 2 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.76, 2.22] |
| 6.7 Role emotional | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐12.47, 11.47] |
| 6.8 Mental health | 2 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐1.07, 1.79] |
| 6.9 Environmental domain | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.17, 2.57] |
| 6.10 Environmental domain ‐ TR | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.39, 2.19] |
| 7 Quality of life ‐ HMB score | 2 | 218 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.32, 0.21] |
| 7.1 Luteal phase progestogen | 1 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.35, 0.35] |
| 7.2 Long course progestogen | 1 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.56, 0.27] |
| 8 Adverse events (short and long course progestogens) | 6 | 1905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.52, 0.86] |
| 8.1 Any adverse event | 4 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.94] |
| 8.2 Gastrointestinal events | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.22, 1.50] |
| 8.3 Headache | 4 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.40] |
| 8.4 Dysmenorrhoea | 2 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.29, 1.19] |
| 8.5 Weight gain | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.31, 13.46] |
| 8.6 Allergic reaction | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.12, 66.07] |
| 8.7 Intermenstrual bleeding | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.47] |
| 8.8 Giddiness | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.84] |
| 8.9 Breast tenderness | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.73] |
| 8.10 Mood changes | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.34] |
| 8.11 Muscle pain | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.10] |
| 8.12 Bloating | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.59] |
| 8.13 Nausea | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.31, 24.14] |
| 8.14 Rash | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.12, 63.63] |
| 8.15 Spotting | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.16 Excess hair growth | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.17 Depression | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.2. Analysis.
Comparison 2 Antifibrinolytic agent versus progestogens, Outcome 2 Median difference in PBAC score.
| Median difference in PBAC score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Comparison | N (TXA) | Median (TXA) | IQR (TXA) | N (NET) | Median (NET) | IQR (NET) | P value |
| After 6 months Rx | ||||||||
| Kiseli 2016 | TXA vs. NET | 22 | ‐125.5 | 124.5 | 20 | ‐160 | 90.0 | 0.002 |
2.3. Analysis.
Comparison 2 Antifibrinolytic agent versus progestogens, Outcome 3 Percentage difference in PBAC scores.
| Percentage difference in PBAC scores | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Comparison | N (TXA) | median (TXA) | IQR (TXA) | N (NET) | median (NET) | IQR(NET) | P value |
| After 6 months Rx | ||||||||
| Kiseli 2016 | TXA vs. NET | 22 | ‐60.8 | 20.3 | 20 | ‐53.1 | 34.8 | <0.001 |
Comparison 3. Antifibrinolytic agent versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss: mean loss | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alkaline haematin assessment | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐73.0 [‐123.35, ‐22.65] |
| 2 Menstrual blood loss: improvement rates | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.18, 1.74] |
| 3 Any adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Headache and dizziness | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 3.2 Muscle pain | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.12, 70.82] |
| 3.3 Dysmenorrhea | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Antifibrinolytic agent versus ethamsylate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss: mean loss | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐100.0 [‐141.82, ‐58.18] |
| 2 Menstrual blood loss: improvement rates | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.95, 2.55] |
| 3 Withdrawal from treatment because of adverse events | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.19, 3.15] |
Comparison 5. Antifibrinolytic agent versus herbal medicines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss: mean loss | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mean PBAC score (after 3 months Rx) | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐23.90 [‐31.92, ‐15.88] |
| 1.2 Mean PBAC score (3 months after end of Rx) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐10.40 [‐19.20, ‐1.60] |
| 2 Quality of life scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Change in dysmenorrhoea scores | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.25, 0.65] |
| 2.2 MIQ score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐1.26, 9.66] |
| 2.3 SF36: physical function | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐1.73, 8.93] |
| 2.4 SF36: role physical | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐9.87, 12.47] |
| 2.5 SF36: bodily pain | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐15.34, 3.34] |
| 2.6 SF36: general health | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 10.30 [2.41, 18.19] |
| 2.7 SF36: vitality | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐4.88, 9.88] |
| 2.8 SF36: social functioning | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐8.54, 3.14] |
| 2.9 SF36: role emotional | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐15.05, 8.05] |
| 2.10 SF36: mental health | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐3.78, 11.18] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any adverse event | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.74, 6.80] |
5.3. Analysis.
Comparison 5 Antifibrinolytic agent versus herbal medicines, Outcome 3 Adverse events.
Comparison 6. Antifibrinolytic agent versus levonorgestrel intrauterine system.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median difference in PBAC score | Other data | No numeric data | ||
| 1.1 After 6 months Rx | Other data | No numeric data | ||
| 2 Percentage difference in PBAC scores | Other data | No numeric data | ||
| 2.1 After 6 months Rx | Other data | No numeric data | ||
| 3 Improvement in mean blood loss (PBAC score < 100) | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.24, 0.77] |
| 4 Quality of life scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Physical domain | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.78, 2.38] |
| 4.2 Psychological domain | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.85, 1.05] |
| 4.3 Social domain | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.55, 1.95] |
| 4.4 Environmental domain | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.06, 2.06] |
| 4.5 Environmental domain TR | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.29, 1.89] |
| 5 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Any adverse event | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.80] |
| 5.2 Headache | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.34, 8.10] |
| 5.3 Bloating | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Nausea | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.36 [0.36, 151.91] |
| 5.5 Weight gain | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.11, 74.31] |
| 5.6 Rash | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.11, 74.31] |
| 5.7 Spotting | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.30] |
| 5.8 Breast tenderness | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.51] |
| 5.9 Excess hair growth | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.51] |
| 5.10 Depression | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonnar 1996.
| Methods | RCT: computer‐generated randomization list to 1 of 3 groups No blinding mentioned | |
| Participants | Country: UK No: 76 Age: 35 to 46 years Inclusion criteria: women reporting HMB confirmed to have > 80 mL per cycle loss, normal cervical smear 3 to 12 months before commencing the study Exclusion criteria: organic causes of menorrhagia found at hysteroscopy or endometrial biopsy, previous renal or hepatic impairment, VTE, inflammatory bowel disease, peptic or intestinal ulceration, coagulation or fibrinolytic disorders |
|
| Interventions | Ethamsylate 500 mg 4 times daily for days 1 to 5 of cycle (27 participants) TXA 1 g 4 times daily for days 1 to 5 of cycle (26 participants) MFA 500 mg 3 times daily for days 1 to 5 of cycle (23 participants) Duration: 3 placebo cycles and 3 treatment cycles |
|
| Outcomes | MBL: objective measurement (alkaline haematin method), duration of blood loss (days), participant's estimate of blood loss, number of sanitary towels used (end scores and change scores) Dysmenorrhoea Side effects |
|
| Notes | Clarification of data sought from authors and reply received Funded by the Health Research Board of Ireland and Pharmacia (a drug company) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized "by a computer generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details; blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrew after randomization (2 in MFA group, 2 in ethamsylate group, 1 in TXA group) and lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐determined outcomes were reported and were relevant |
| Other bias | Low risk | Groups appeared comparable at baseline |
Callender 1970.
| Methods | Cross‐over trial, with 3 control cycles, followed by 3 cycles of either intervention or placebo Thereafter, women were 'crossed over' to the other arm, for a further 3 cycles Both participants and carers were blind to treatment allocation |
|
| Participants | Country: UK No: 20 (16 were analyzed) Age: 33 to 48 years Inclusion criteria: HMB either as described by the participant or participants presenting with iron deficiency anaemia presumed to be due to HMB Exclusion criteria: significant clinical abnormality (from gynaecological examination) or significant histological abnormality (from dilatation and curettage) |
|
| Interventions | TXA 1 g 4 times daily on days 1 to 4 of cycle versus placebo 3 cycles of either TXA or placebo, followed by 3 cycles of the other |
|
| Outcomes | MBL by total body counter: 2 μg to 4 μg of Cu ⁵⁹Fe given intravenously and total body count measured at 2‐weekly intervals throughout the study Blood loss estimated from loss of radioactivity multiplied by the total blood volume (end scores and change scores) Duration of bleeding in days and number of pads used Side effects reported |
|
| Notes | Several women were able to detect the active treatment Side effects reported, but not systematically Source of funding was not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Remote allocation: the tablets were either TXA 0.5 g per tablet or a placebo of similar size and appearance The order of treatments was randomized, and neither the participants nor those conducting the study knew the identity of tablets A and B |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both participants and carers blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 withdrawals after allocation (all apparently in the placebo group (40%)): these were not included in the final analysis |
| Selective reporting (reporting bias) | Low risk | A priori outcomes reported and were relevant |
| Other bias | Low risk | Groups appeared comparable at baseline |
Edlund 1995.
| Methods | RCT; randomization performed using computer‐generated list and sealed envelopes 2:2:1 randomization with greater numbers in the active groups Double dummy technique of administration of tables |
|
| Participants | Country: Sweden Age: > 18 years Number: 91 randomized, but results complete for only 68 women Inclusion criteria: > 80 mL per cycle blood loss, regular cycles, normal‐sized uterus on clinical examination Exclusion criteria: renal or hepatic impairment, clinical pelvic pathology or cervical intra‐epithelial neoplasia, concomitant disease or medication affecting menstruation, VTE, haematological or coagulation disorders, dilatation and curette within the previous 2 months, inability to comply with the protocol |
|
| Interventions | Kabi 2161 1200 mg twice daily for days 1 to 5 of cycle (26 participants) Kabi 2161 600 mg 4 times daily for days 1 to 5 of cycle (28 participants) Placebo 2 tablets 4 times daily for days 1 to 5 of cycle (14 participants) Duration: 3 cycles |
|
| Outcomes | MBL: objective (alkaline haematin method) as absolute measurement and relative change from baseline, duration of loss (days), number of sanitary towels used; and participant's subjective assessment (end scores and change scores) Side effects reported |
|
| Notes | Authors contacted for clarification of data and additional data received Source of funding not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list 2:2:1 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, with double‐dummy technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 exclusions post randomization; 19 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported and were relevant |
| Other bias | Low risk | Groups appeared comparable at baseline |
Fathima 2012.
| Methods | RCT: randomization with lottery method Only participants were blinded Duration of trial: 1 control cycle; 3 consecutive treatment cycles; followed up for 3 cycles post‐treatment |
|
| Participants | Country: India Number of participants: 52 (35 in intervention arm; 17 in control arm) Women aged 18 to 45 years old Inclusion criteria: 'history of menorrhagia' (no details given); Hb > 8 g%; presence of pelvic pathology (e.g. endometriosis, pelvic inflammatory disease, uterine fibroids, polyps/adenomyosis) acceptable Exclusion criteria: use of COCP, IUS, diabetes, hypertension, tuberculosis, severe anaemia (not defined), ischaemic heart disease, thyroid dysfunction, blood dyscrasias, and malignancy |
|
| Interventions | Intervention arm: oral Safoof Habis, a Unani formulation (a type of traditional medicine widely practised in South‐East Asia), which was made up of Teen Ahmer (silicate of alumina and iron oxide), Sange Jarahat (hydrated magnesium silicate) and Raal Sufaid (Vateria indica Linn, which is a species of plant in the Disterocarpaceae family, endemic to India) Safoof Habis was made up of equal parts of all 3 components, and given in 5 g doses BD, from days 1 to 5 versus Control arm: oral TXA 500 mg thrice daily, from days 1 to 5 |
|
| Outcomes | MBL: menstrual pictogram (modified PBAC technique); duration of menstruation; subjective assessment of menstrual flow (end scores and change scores) Dysmenorrhoea: visual analogue scale Hb concentration Side effects/adverse drug effects: recorded with participant's diary; 'evaluated with biochemical tests' |
|
| Notes | Power calculations not performed The authors deny receiving any funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Lottery method" |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether allocation was concealed; participants only were blinded |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear; participants only blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13% drop‐out rate; reasons not provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | Groups appeared comparable at baseline |
Freeman 2011.
| Methods | Multi‐centre, randomized, double‐blind, placebo‐controlled, parallel‐group study Randomization: method of randomization not stated Blinding: participants, investigators and assessors all blinded Duration of trial: 2 control cycles pre‐treatment, followed by 3 cycles of treatment |
|
| Participants | Country: USA Number of participants: 304 randomized, 272 analyzed (106 in low‐dose TXA arm; 103 in high‐dose in control arm; 63 in placebo arm) Age: 18 to 49 years old Inclusion criteria: 'history of cyclic HMB', confirmed by alkaline haematin method during the 2 pre‐treatment cycles; normal pelvic examination and Pap smear; normal transvaginal ultrasound Exclusion criteria: 'clinically significant disease', anovulatory dysfunctional uterine bleeding, metrorrhagia, menometrorrhagia, polymenorrhoea, endometrial polyps, endometrial hyperplasia, endometrial carcinoma, cervical carcinoma, myocardial infarction, ischaemic disease, stroke, transient ischaemic attack, VTE, or coagulopathy; abnormality on electrocardiography; serum prolactin > 30 μg/L; uncontrolled hypothyroidism; severe anaemia (Hb < 8 g/dL); history of bilateral oophorectomy or hysterectomy; women who were pregnant, breast‐feeding, planning to become pregnant during the study, or became pregnant during the study; women currently taking anticoagulants, aspirin, dong quai, aminocaproic acid, hydroxychloroquine, or hormonal contraceptives Note: women with fibroids were only excluded if they were thought to require surgical management |
|
| Interventions | First intervention arm: oral TXA 0.65 g 3 times daily for up to 5 consecutive days during menstruation versus Second intervention arm: oral TXA 1.3 g 3 times daily for up to 5 consecutive days during menstruation versus Control arm: placebo tablets, 3 times daily, for up to 5 consecutive days during menstruation |
|
| Outcomes | MBL: alkaline haematin method (change scores) Subjective improvements in MBL: MIQ (i.e. QoL) Side effects/adverse drug effects: "adverse events monitoring" (conducted at each study visit); physical examination; electrocardiograph; vital signs; and laboratory evaluation |
|
| Notes | Funded by Ferring Pharmaceuticals (a drug company) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of method of randomization not provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, investigators and assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal drop‐outs. 294/304 randomized women included in safety analysis, 272/304 (90%) in efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | Groups appeared comparable at baseline |
Goshtasebi 2013.
| Methods | RCT, with parallel‐group technique Block randomization technique Participants unable to be blinded, given the differences between the regimens |
|
| Participants | Country: Iran Number of participants: 90, 19 of whom withdrew by the end of the follow‐up period Age: 20 to 45 years old Inclusion criteria: reported regular HMB; BMI 19 to 29 Exclusion criteria: “organic cause of HMB”, iron‐deficiency anaemia, previous VTE, history of chronic diseases, history of diseases known to interfere with menstrual bleeding (e.g. fibroids, anticoagulant use, COCP or other hormonal drug use), IUS in situ |
|
| Interventions | TXA 500 mg 4 times daily for days 1 to 5 of menses MPA 5 mg twice daily, for days 5 to 26 of the menstrual cycle |
|
| Outcomes | Subjective assessment of MBL, using a modified PBAC (end scores) Serum Hb and ferritin SF‐36 for QoL (Farsi version) HMB questionnaire (Farsi version) Side effects |
|
| Notes | Funded by Tarbiat Modares University | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unable to blind participants, due to different regimens It is unclear whether or not personnel were blinded Potential knowledge of treatment may have influenced the primary outcome of MBL which was measured by PBAC |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Significant loss to follow‐up, but similar numbers and reasons for each group 71/90 randomized women (79%) included in analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Groups appeared comparable at baseline |
Goshtasebi 2015.
| Methods | RCT with parallel group design Method of randomization: equal allocation ratio in blocks of 6 Double blinding with participants and nurses both blinded |
|
| Participants | Country: Iran Age: 20 to 49 years Number of participants: 94 (76 completed the trial) Inclusion criteria: self‐reported HMB, Hb ≥ 10.5 g/dL, BMI of 10 kg/m² to 29 kg/m² Exclusion criteria: history of VTE, chronic illnesses, other diseases known to interfere with menstrual bleeding (such as fibroids > 3 cm), history of iron supplementation, anticoagulant agents, COCP or other hormonal drug use, IUS in situ |
|
| Interventions | (1) TXA 500 mg 4 times daily from days 1 to 5 of cycle (2) Extract of Punica granatum Linn (from pomegranate) 4 times daily from days 1 to 5 of cycle Duration of trial: 3 consecutive menstrual cycles |
|
| Outcomes | PBAC (end scores) QoL scores (MIQ, SF36) |
|
| Notes | Funded by Tarbiat Modares University research office | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Equal allocation ratio, blocked randomization |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered and sealed opaque envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding (participants and nurses blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Significant drop‐out 76/94 (85%) randomized women included in analysis, similar drop‐out rates in each group |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Unclear risk | Baseline characteristics only reported for women who completed the study |
Jaisamrarn 2006.
| Methods | Multi‐centre RCT Central computer‐generated randomization schedule used for allocation Described as open label trial |
|
| Participants | Country: Thailand (4 sites) Mean age: 34.6 years Number of participants: 169 women were randomized and 167 completed the trial Inclusion criteria: aged between 18 and 45 years, regular menstrual cycle (21 to 35 days), serum progesterone during 5 to 9 days before menstruation of ≥ 5.0 ng/mL, PBAC score > 130 during run‐in phase, no contraindication to treatment drugs, normal renal and liver function, normal pelvic examination Exclusion criteria: concomitant diseases, organic disease, VTE, haemorrhagic or fibrinolytic disorder, hormone therapy during last 3 months, taking any medication that might affect MBL, need or desire for contraception, need for iron supplementation, inability to comply and no consent |
|
| Interventions | (1) TXA 3 g daily on days 1 to 5 of cycle (2) MFA 1.5 mg daily on days 1 to 5 of cycle (3) NET 10 mg daily on days 19 to 26 of cycle, for 2 consecutive cycles |
|
| Outcomes | MBL using PBAC (end scores) Cure rate (success rate) (defined as PBAC ≤ 130) Adverse events QoL using a 'standardized questionnaire' Acceptability of treatment Hb Duration of menstruation |
|
| Notes | Unpublished copy of trial sighted, also conference abstract Source of funding not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralised randomization scheme separate from study investigators |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open label" Potential knowledge of treatment may have influenced the primary outcome of MBL as this was measured by PBAC |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal drop‐outs |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared comparable at baseline |
Kiseli 2016.
| Methods | Single‐centre RCT Central computer‐generated randomization schedule used for allocation Participants unable to be blinded, given the differences between the regimens Duration of trial: 2 control cycles pre‐treatment; 6 cycles on treatment |
|
| Participants | Country: Turkey Mean age: 42.1 years Number of participants: 84 women were randomized and 62 completed the trial Inclusion criteria: aged between 18 and 45 years, PBAC score > 100 during 2‐month run‐in phase Exclusion criteria: abnormal pelvic ultrasound or endometrial biopsy; Hb < 10 g/dL; abnormal Pap smear result; thyroid disease; hypertension, diabetes, or coronary artery disease; history of previously taking medications for HMB; contra‐indication to current therapy |
|
| Interventions | TXA 1 g 4 times daily from day 1 for 4 days versus NET 5 mg thrice daily from day 14 to 23 of the cycle versus levonorgestrel IUS (20 μg/day) inserted during the first few days of menses Treatment for 6 cycles |
|
| Outcomes | PBAC score and associated percentage reduction in blood loss (end scores) World Health Organization QoL‐Short Form (Turkish version), in which women report limitations in physical health, psychological status, social support, and "relating to their environment" |
|
| Notes | The authors deny receiving any funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | ''Neither patients nor researchers were blinded to treatment'' Different dosage schedules make blinding impossible Potential knowledge of treatment may have influenced the primary outcome of MBL which was measured by PBAC |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant loss to follow‐up, but similar numbers for each group 62/84 randomized women (74%) included in analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared comparable at baseline and no other potential bias was identified |
Kriplani 2006.
| Methods | Single‐centre, prospective RCT Randomization by "computerised randomization table" Blinding not possible, given the different dosage schedules of the 2 arms of the study |
|
| Participants | Country: India Age: 15 to 50 years old Number of participants: 94 women Inclusion criteria: women presenting with HMB and PBAC score > 100 Excusion: fibroids, adenomyosis, endometriosis, atypia on endometrial histopathology, thyroid disease, history of hormone therapy in previous 3 months, and unwilling to trial medical management |
|
| Interventions | TXA 500 mg 4 times daily from day 1 for 5 days versus MPA 10 mg twice daily from day 5 to 25 of the cycle Treatment for 3 cycles, then subjects were followed up for 3 months after treatment had been stopped |
|
| Outcomes | PBAC score and associated percentage reduction in blood loss (end scores) Recurrence of HMB Further surgery Participant satisfaction Duration of bleeding Hb level Side effects |
|
| Notes | Funded by the Indian council of Medical Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization via "computerised randomization table" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding; different dosage schedules make blinding impossible Potential knowledge of treatment may have influenced the primary outcome of MBL which was measured by PBAC |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors stated that there were minimal drop‐outs but at the end of treatment, only 66% of MPA group but 94% of TXA group were analyzed No reasons for withdrawal were given, but response levels were reported for all participants |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Groups appeared comparable at baseline |
Lukes 2010.
| Methods | Multi‐centre (40 clinical sites), double‐blind, parallel‐group study Randomization via permuted block randomization scheme, with a block size of 8 (5 allocated to intervention arm, and 3 to placebo arm) Blinding: participants, investigators, sponsor, statisticians, clinical data management staff, and clinical monitors were all blinded Duration of trial: 2 control cycles pre‐treatment; 6 cycles of treatment |
|
| Participants | Country: USA Age: 18 to 49 years old Number of participants: 196 women randomized Inclusion criteria: history of at least 3 days of HMB over at least 4 of their last 6 cycles; confirmed during 2 cycles before treatment phase commenced; normal pelvic examination and Pap smear; no "clinically important" findings on transvaginal ultrasound; willingness to use non‐hormonal contraception during the trial Exclusion criteria: history of significant medical problem; severe anaemia (Hb < 8 g/dL); pregnant/lactating; endometrial abnormalities; cervical carcinoma; anovulatory dysfunctional uterine bleeding; glaucoma; ocular hypertension; use of anticoagulants; aspirin; dong quai; aminocaproic acid; hydroxychloroquine Note: uterine fibroids were only an exclusion criteria if thought to require surgical management |
|
| Interventions | TXA 1.3 g 3 times daily (123 participants), commenced with the onset of HMB, for up to 5 days versus placebo (73 participants) 3 times daily, commenced with the onset of HMB, for up to 5 days | |
| Outcomes | Objective measurement of MBL: alkaline haematin method (change scores) Subjective improvements in MBL: MIQ; occurrence of large blood stains Hb and ferritin concentrations Side effects |
|
| Notes | 11 of 12 authors report receiving funding from 1 (or more) drug company/companies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomization schedule |
| Allocation concealment (selection bias) | Low risk | Allocation managed by an independent group ("Fisher Clinical Services") |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, investigators and assessors were all blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28% participants (55/196) withdrew, but proportions were similar in each study arm, and 95% were included in a modified intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Unclear risk | Imbalances between groups in baseline MBL |
Preston 1995.
| Methods | Double‐blind, placebo‐controlled RCT Randomization by computer‐generated numbers, which were placed in sealed envelopes Participant and carer were blind to the treatment allocation Months 1 and 2 were placebo for all women In months 3 and 4, women took tablets on days 1 to 4 and days 19 to 26 of each cycle but only 1 of these treatments was active |
|
| Participants | Country: UK
Age: > 18 years old Number of participants: 103 recruited and underwent 2 cycles of placebo to screen for eligibility, of whom 46 were randomized to the treatment phase of the trial Inclusion criteria: cycle length 28 ± 7 days; average menstrual loss over 2 cycles > 80 mL per cycle; no hormone therapy within 3 months; no medication which may affect MBL; confirmed to be ovulating; and had complied with the protocol during the 2 months of placebo treatment Exclusion criteria: abnormal renal function; abnormal pelvic examination; abnormal cervical smear; anovulatory cycles; lack of compliance during the placebo cycles |
|
| Interventions | 2 months of placebo (to assess eligibility) followed by 2 months of either TXA (25 participants) 1 g taken 4 times daily on days 1 to 4 or NET (21 participants) 5 mg taken twice daily on days 19 to 26 of cycle | |
| Outcomes | Objective MBL: measured via the alkaline haematin method (end scores) QoL assessed using a questionnaire (at end of cycle 2 and cycle 4) using 5‐point scale for general health, amount of flooding and leakage experienced, abdominal pain, limitation to social life, effect on sex life Diary of days bleeding, number of sanitary towels used and side effects recorded by participants |
|
| Notes | Originally 103 women were recruited but 57 were excluded during the placebo cycles because of lack of objective menorrhagia (> 80 mL per cycle), anovulation or lack of compliance with protocol Additional data were provided by the author Funded by Pharmacia (a drug company) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was by computer generated numbers" |
| Allocation concealment (selection bias) | Low risk | Adequate: "opaque sealed, consecutively numbered envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Authors stated that the other group took "placebo of identical appearance to the active drug" and the treatment regimen was also the same for each group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women did not complete the trial, but all were included in the results |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Groups appeared comparable at baseline |
Zhang 2008.
| Methods | Multi‐centre, open label RCT: method of randomization used computer software Blinding was highly unlikely, given the differences in the treatment regimens (1 group took tablets twice a day, the other 3 times a day) Given treatment for 2 cycles, then followed‐up for 1 further cycle |
|
| Participants | Country: China Age: mean age 35 years old Number of participants: 106 women Inclusion criteria: "proven ovulatory menorrhagia", attending gynaecological clinics, PBAC score > 130 Exclusion criteria: heart, kidney, liver or haematological disease; having had any hormonal treatments in the 3 months prior, including an IUS; previous thrombo‐embolus |
|
| Interventions | TXA 1 g 3 times daily during days 1 to 5 (69 participants) versus NET 5 mg twice daily on days 19 to 26 (59 participants) Administered for 2 consecutive cycles |
|
| Outcomes | MBL (PBAC) Length of menstrual period 6‐item QoL questionnaire collected in the second week before, during and after each treatment cycle and a third (follow‐up) cycle |
|
| Notes | Funded by Daiichi Sankyo Co Ltd (a Japanese drug company) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer software to allocate participants randomly |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Treatment and control groups were given their pills on different days of the menstrual cycle |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that there is minimal drop‐out |
| Selective reporting (reporting bias) | High risk | The authors only report significant results in the abstract, which appears to favour the experimental treatment (TXA) For example, QoL scores were significantly improved after 1 cycle of treatment in the TXA group, but after 2 cycles of treatment and at follow‐up the scores were similar These latter findings were not reported, which suggests that the conclusions are influenced by lack of reporting |
| Other bias | Low risk | Groups of participants appeared similar at baseline |
Abbreviations and acronyms used in the above tables:
BMI = body mass index
COCP = combined oral contraceptive pill
Hb = haemoglobin
HMB = heavy menstrual bleeding
IUD = intrauterine device
IUS = intrauterine system
MBL = menstrual blood loss
MFA = mefenamic acid
MPA = medroxyprogesterone acetate
MIQ = Menorrhagia Impact Questionnaire
NET = norethisterone
PBAC = Pictorial Blood Assessment Chart
RCT = randomized controlled trial
QoL = quality of life
TXA = tranexamic acid
VTE = venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersch 1988 | Cross‐over trial which did not report data from the first phase of the study. |
| Chamberlain 1991 | Treatment group received ethamsylate. |
| Drosdal 1993 | Participants included women with IUD in situ. |
| Harrison 1976 | Treatment group received ethamsylate. |
| Kasonde 1975 | Participants had intrauterine coil devices in situ. |
| Kouides 2009 | Participants had a proven coagulopathy. |
| Moghtadaei 2012 | Author was contacted for further information about their study, to judge whether it should be included/excluded. No reply was received. |
| Muse 2010 | There was no control group. |
| Najam 2010 | Compared women taking tranexamic acid to those taking a combination of tranexamic acid and mefenamic acid. |
| NCT01190150 | Dose‐finding RCT of tranexamic acid in girls aged 12 to 16 years. |
| NCT01428713 | Data not provided for the end of the first phase of the cross‐over study. |
| NiIsson 1965 | Treatment group received ethamsylate. |
| Nilsson 1967 | Cross‐over trial which did not report data from the first phase of the study. |
| Petersen 1983 | Treatment was given for only 1 month. Criterion specified in our protocol was that we would include only studies using at least 2 months of treatment. |
| Tabatabaei 2013 | Author was contacted for further information about their study, to judge whether it should be included/excluded. No reply was received. |
| Vermylen 1968 | Cross‐over trial which did not report data from the first phase of the study. |
| Westrom 1970 | Participants had intrauterine coil devices in situ. |
| Ylikorkala 1983 | Participants had intrauterine coil devices in situ. |
Differences between protocol and review
Cross‐over trials were only eligible for inclusion if they reported first phase data, in order to minimise the chance of cross‐over bias. This is a change from the original protocol criterion for inclusion, where cross‐over trials could be included regardless of whether data were provided for the first phase of the trial. Where cross‐over trials only report findings at the end of the study, the likelihood of significant bias is increased, because no adjustment is made for cross‐over effects.
We refined the outcome measures of MBL, to avoid reporting both end scores and change scores from the same study. Where studies reported both, we reported the end scores.
In order to focus the review on the most relevant clinical outcomes, we reduced the number of outcomes and omitted indirect measures of blood loss (duration of loss, number of sanitary pads), as well as resource cost and mortality.
We increased the focus on adverse events, by making thromboembolic events a primary outcome, and reporting all other adverse events as secondary outcomes.
Contributions of authors
Alison Bryant‐Smith performed searches, selected trials for inclusion, contacted authors, extracted and entered data, and wrote the review.
Anne Lethaby helped select trials for inclusion, extracted and entered data, commented on the draft protocol, and wrote the review.
Martha Hickey reviewed and modified the review.
Sources of support
Internal sources
None, UK.
External sources
None, Other.
Declarations of interest
AB‐S, AL and CF have no conflicts of interest to declare. MH's institution has received funding from Bayer Schering Pharma for research into the mechanisms of abnormal uterine bleeding with fibroids.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bonnar 1996 {published and unpublished data}
- Bonnar J, Sheppard B. Treatment of menorrhagia during menstruation: randomized controlled trial of ethamsylate, mefenamic acid and tranexamic acid. BMJ 1996;313(7057):579‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Callender 1970 {published data only}
- Callender ST, Warner GT, Cope E. Treatment of menorrhagia with tranexamic acid. A double blind trial. British Medical Journal 1970;4(5729):214‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edlund 1995 {published and unpublished data}
- Edlund M, Andersson K, Rybo G, Lindoff CC, Astedt B, Schoult BV. Reduction of menstrual blood loss in women suffering from idiopathic menorrhagia with a novel antifibrinolytic drug (Kabi2161). British Journal of Obstetrics and Gynaecology 1995;102(11):913‐7. [DOI] [PubMed] [Google Scholar]
Fathima 2012 {published data only}
- Fathima A, Sultana A. Clinical efficacy of a Unani formulation ‘Safoof Habis’ in menorrhagia: a randomized controlled trial. European Journal of Integrative Medicine 2012;4(3):e315‐22. [Google Scholar]
Freeman 2011 {published data only}
- Freeman EW, Lukes A, VanDrie D, Mabey RG, Gersten J, Adomako TL. A dose‐response study of a novel, oral tranexamic formulation for heavy menstrual bleeding. American Journal of Obstetrics and Gynecology 2011;205(4):319.e1‐319.e7. [DOI] [PubMed] [Google Scholar]
Goshtasebi 2013 {published data only}
- Goshtasebi A, Moukhah S, Gandevani SB. Treatment of heavy menstrual bleeding of endometrial origin: randomized controlled trial of medroxyprogesterone acetate and tranexamic acid. Archives of Gynecology and Obstetrics 2013;288(5):1055‐60. [DOI] [PubMed] [Google Scholar]
Goshtasebi 2015 {published data only}
- Goshtasebi A, Mazari Z, Behboudi Gandevani S, Naseri M. Anti‐hemorrhagic activity of Punica granatum L. flower (Persian Golnar) against heavy menstrual bleeding of endometrial origin: a double‐blind, radomized controlled trial. Medical Journal of the Islamic Republic of Iran 2015;29:199. [PMC free article] [PubMed] [Google Scholar]
Jaisamrarn 2006 {published data only}
- Jaisamrarn U, Sitavarin S, Suwikrom S, Kamolpornwijit W. Randomized trial of medical therapies for menorrhagia: clinical efficacy and effect on quality of life. XVIII FIGO World Congress of Gynecology and Obstetrics; 2006 Nov 5‐10; Kuala Lumpur (Malaysia). 2006; Vol. 3:83.
Kiseli 2016 {published data only}
- Kiseli M, Kayikcioglu F, Evliyaoglu O, Haberal A. Comparison of therapeutic efficacies of norethisterone, tranexamic acid and levonogestrel‐releasing intrauterine system for the treatment of heavy menstrual bleeding: a randomized controlled study. Gynecologic and Obstetric Investigation 2016;81(5):447‐53. [DOI] [PubMed] [Google Scholar]
Kriplani 2006 {published data only}
- Kriplani A, Kulshrestha V, Agarwal N, Diwakar S. Role of tranexamic acid in management of dysfunctional uterine bleeding in comparison with medroxyprogesterone acetate. Journal of Obstetrics and Gynaecology 2006;26(7):673‐8. [DOI] [PubMed] [Google Scholar]
Lukes 2010 {published data only}
- Lukes AS, Moore KA, Muse KN, Gersten JK, Hecht BR, Edlund M, et al. Tranexamic acid treatment for heavy menstrual bleeding. Obstetrics and Gynecology 2010;116(4):865‐75. [DOI] [PubMed] [Google Scholar]
Preston 1995 {published data only}
- Preston JT, Cameron IT, Adams EJ, Smith SK. Comparative study of tranexamic acid and norethisterone in the treatment of ovulatory menorrhagia. British Journal of Obstetrics and Gynaecology 1995;102(5):401‐6. [DOI] [PubMed] [Google Scholar]
Zhang 2008 {published data only}
- Zhang Y, He F, Sun Z, LI S, Bi S, Huang X, et al. A multicenter, prospective, randomized, open comparator study on the treatment of ovulatory menorrhagia with tranexamic acid and norethisterone in China. Chinese Journal of Obstetrics and Gynaecology 2008;43(4):247‐50. [PubMed] [Google Scholar]
References to studies excluded from this review
Andersch 1988 {published data only}
- Andersch B, Milsom I, Rybo G. An objective evaluation of flurbiprofen and tranexamic acid in the treatment of idiopathic menorrhagia. Acta Obstetricia et Gynecologica Scandinavica 1988;67(7):645‐8. [DOI] [PubMed] [Google Scholar]
Chamberlain 1991 {published data only}
- Chamberlain G, Freeman R, Price F, Kennedy A, Green D, Eve L. A comparative study of ethamsylate and mefenamic acid in dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology 1991;98(7):707‐11. [DOI] [PubMed] [Google Scholar]
Drosdal 1993 {published and unpublished data}
- Drosdal H. Treatment of menorrhagia with tranexamic acid. A clinical trial of an underestimated problem in general practice. Tidsskrift for Den Norske Laegeforening 1993;113(23):2907‐10. [PubMed] [Google Scholar]
Harrison 1976 {published data only}
- Harrison RF, Campbell S. A double‐blind trial of ethamsylate in the treatment of primary and intrauterine device menorrhagia. Lancet 1976;2(7980):283‐5. [DOI] [PubMed] [Google Scholar]
Kasonde 1975 {published data only}
- Kasonde JM, Bonnar J. Effect of ethamsylate and aminocaproic acid on menstrual blood loss in women using intrauterine devices. British Medical Journal 1975;4(5987):21‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kouides 2009 {published data only}
- Kouides PA, Byams VR, Phillip CS, Stein SF, Heit JA, Lukes AS, et al. Multisite management study of menorrhagia with abnormal haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. British Journal of Haematology 2009;145(2):212‐20. [DOI] [PubMed] [Google Scholar]
Moghtadaei 2012 {published data only (unpublished sought but not used)}
- Moghtadaei P. Different medication [sic] for heavy menstrual bleeding. 3rd International and 18th National Congress of Iranian Society for Reproductive Medicine; 2012. 2012:42.
Muse 2010 {published data only}
- Muse K, Gersten J, Waldbaum A, Mabey RG, Lukes A, Moore K. Long‐term quality of life and safety of a new tranexamic acid menorrhagia treatment. Fertility and Sterility 2010;94(4):S13. [Google Scholar]
Najam 2010 {published data only}
- Najam R, Agarwal D, Tyagi R, Singh S. Comparison of tranexamic acid with a combination of tranexamic acid and mefenamic acid in reducing menstrual blood loss in ovulatory dysfunctional uterine bleeding (DUB). Journal of Clinical and Diagnostic Research 2010;4(5):3020‐5. [Google Scholar]
NCT01190150 {unpublished data only}
- NCT01190150. Lysteda Pediatric Research Equity Act (PREA) pharmacokinetic study in adolescent females with heavy menstrual bleeding. clinicaltrials.gov/ct2/show/NCT01190150 (first received 27 August 2010).
NCT01428713 {unpublished data only}
- NCT01428713. Tranexamic acid (TA) vs combined oral contraceptive (COCP) pilot study. clinicaltrials.gov/ct2/show/NCT01428713 (first received 5 September 2011).
NiIsson 1965 {published data only}
- Nilsson L, Rybo G. Treatment of menorrhagia with epsilon aminocaproic acid. A double blind investigation. Acta Obstetricia et Gynecologica Scandinavica 1965;44(3):467‐73. [DOI] [PubMed] [Google Scholar]
Nilsson 1967 {published data only}
- Nilsson L, Rybo G. Treatment of menorrhagia with an antifibrinolytic agent, tranexamic acid (AMCA). Acta Obstetricia et Gynecologica Scandinavica 1967;46:572‐80. [Google Scholar]
Petersen 1983 {published data only}
- Petersen K, Jelert H, Diernaes E, Detlefsen GU. Treatment of hypermenorrhea with tranexamic acid [Behandling af hypermenore med traneksamsyre]. Ugeskrift for Laeger 1983;145(36):2759‐60. [PubMed] [Google Scholar]
Tabatabaei 2013 {published data only (unpublished sought but not used)}
- Tabatabaei A. A clinical randomized single blind trial of medical therapies for menorrhagia using ibuprofen and tranexamic acid. International Journal of Fertility and Sterility 2013;7:120. [Google Scholar]
Vermylen 1968 {published data only}
- Vermylen J, Verhaegen‐Declercq ML, Verstraete M, Fierens F. A double blind study of the effect of tranexamic acid in essential menorrhagia. Bulletin de la Societe Royale Belge de Gynecologie et d'Obstetrique 1968;38:385‐90. [PubMed] [Google Scholar]
- Vermylen J, Verhaegen‐Declercq ML, Verstraete M, Fierens F. A double blind study of the effect of tranexamic acid in essential menorrhagia. Thrombosis et Diathesis Haemorrhagica 1968;20:583‐7. [PubMed] [Google Scholar]
Westrom 1970 {published data only}
- Bengtsson LP, Westrom L. Treatment by tranexamic acid in menorhagia in women using intrauterine devices. Lakartidningen 1971;68(33):3685‐91. [PubMed] [Google Scholar]
- Westrom L, Bengtsson LP. Effect of tranexamic acid (AMCA) in menorrhagia with intrauterine contraceptive devices. Journal of Reproductive Medicine 1970;5:154‐61. [PubMed] [Google Scholar]
Ylikorkala 1983 {published data only}
- Ylikorkala O, Viinikka L. Comparison between antifibrinolytic and antiprostaglandin treatment in the reduction of increased menstrual blood loss in women with intrauterine contraceptive devices. British Journal of Obstetrics & Gynaecology 1981;90:78‐83. [DOI] [PubMed] [Google Scholar]
Additional references
Berntorp 2001
- Berntorp E, Follrud C, Lethagen S. No increased risk of venous thrombosis in women taking tranexamic acid. Thrombosis and Haemostasis 2001;86(2):714‐5. [PubMed] [Google Scholar]
Butt 2012
- Butt JL, Jeffery ST, Spuy ZM. An audit of indications and complications associated with elective hysterectomy at a public service hospital in South Africa. International Journal of Gynaecology and Obstetrics 2012;116(2):112‐6. [DOI] [PubMed] [Google Scholar]
Duckitt 2012
- Duckitt K, Collins S. Menorrhagia. BMJ Clinical Evidence 2012;01:805. [PMC free article] [PubMed] [Google Scholar]
Edlund 2003
- Edland M, Blomback M, He S. On the correlation between local fibrinolytic activity in menstrual fluid and total blood loss during menstruation and effects of desmopressin. Blood Coagulation & Fibrinolysis 2003;14(6):593‐8. [DOI] [PubMed] [Google Scholar]
El‐Hemaidi 2007
- El‐Hemaidi I, Gharaibeh A, Shehata H. Menorrhagia and bleeding disorders. Current Opinion in Obstetrics & Gynecology 2007;19(6):513‐20. [DOI] [PubMed] [Google Scholar]
Fender 1999
- Fender GRK, Prentice A, Gorst T, Nixon RM, Duffy SW, Day NE, et al. Randomised controlled trial of educational package on management of menorrhagia in primary care: the Anglia menorrhagia education study. BMJ 1999;318(7193):1246‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2012
- Fox K. Management of heavy menstrual bleeding in general practice. Current Medical Research and Opinion 2012;28(9):1517‐25. [DOI] [PubMed] [Google Scholar]
Grant 2000
- Grant C, Gallier L, Fahey T, Pearson N, Sarangi J. Management of menorrhagia in primary care ‐ impact on referral and hysterectomy: data from the Somerset Morbidity Project. Journal of Epidemiology and Community Health 2000;54(9):709‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2013
- Gupta J, Kai J, Middleton L, Pattison H, Gray R, Daniels J. Levonogestrel intrauterine system versus medical therapy for menorrhagia. New England Journal of Medicine 2013;368(2):128‐37. [DOI] [PubMed] [Google Scholar]
Hallberg 1964
- Hallberg L, Nilsson L. Determination of menstrual blood loss. Scandinavian Journal of Clinical and Laboratory Investigation 1964;16:244‐8. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
Higham 1990
- Higham J, O'Brien P, Shaw R. Assessment of menstrual blood loss using a pictorial chart. British Journal of Obstetrics and Gynaecology 1990;97:734‐9. [DOI] [PubMed] [Google Scholar]
Kaunitz 2010
- Kaunitz A. A new treatment for menorrhagia enters the US market. OBG Management 2010;22(6):34‐5. [Google Scholar]
Leminen 2012
- Leminen H, Hurskainen R. Tranexamic acid for the treatment of heavy menstrual bleeding: efficacy and safety. International Journal of Women's Health 2012;4:413‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumsden 2011
- Lumsden MA, Wedisinghe L. Tranexamic acid for heavy menstrual bleeding. Expert Opinion on Pharmacotherapy 2011;12(13):2089‐95. [DOI] [PubMed] [Google Scholar]
Marret 2010
- Marret H, Fauconnier A, Chabbert‐Buffet N, Cravello L, Golfier F, Gondry J, et al. Clinical practice guidelines on menorrhagia: management of abnormal uterine bleeding before menopause. European Journal of Obstetrics & Gynecology and Reproductive Biology 2010;152(2):133‐7. [DOI] [PubMed] [Google Scholar]
Matteson 2013
- Matteson K, Rahn D, Wheeler T, Casiano E, Siddiqui N, Harvie H, et al. Nonsurgical management of heavy menstrual bleeding. Obstetrics and Gynecology 2013;121(3):632‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munro 2011
- Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM‐COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynecology and Obstetrics 2011;113(1):3‐13. [DOI] [PubMed] [Google Scholar]
Naoulou 2012
- Naoulou B, Tsai M. Efficacy of tranexamic acid in the treatment of idiopathic and non‐functional heavy menstrual bleeding: a systematic review. Acta Obstetricia et Gynecologica Scandinavica 2012;91(5):529‐37. [DOI] [PubMed] [Google Scholar]
NICE 2007
- Royal College of Obstetricians and Gynaecologists. Heavy menstrual bleeding. National Institute for Health and Clinical Excellence; 2007 January.
NICE 2016
- Royal College of Obstetricians and Gynaecologists. Heavy menstrual bleeding. National Institute for Health and Clinical Excellence; 2016 August.
Qiu 2014
- Qiu J, Cheng J, Wang Q, Hua J. Levonogestrel‐releasing intrauterine system versus medical therapy for menorrhagia: a systematic review and meta‐analysis. Medical Science Monitor 2014;20:1700‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2013
- Singh S, Best C, Dunn S, Lyeland N, Wolfman WL. Medical treatment. Journal of Obstetrics and Gynaecology Canada 2013;35(5):S12‐7. [DOI] [PubMed] [Google Scholar]
Turner 2000
- Turner E, Bowie P, Kellock C. First‐line management of menorrhagia: findings from a survey of general practitioners in Forth Valley. British Journal of Family Planning 2000;26(4):227‐8. [DOI] [PubMed] [Google Scholar]
Warner 2004
- Warner PE, Critchley HOD, Lumsden MA, Campbell‐Brown M, Douglas A, Murray GD. Menorrhagia II: Is the 80mL blood loss criterion useful in management of complaint of menorrhagia?. American Journal of Obstetrics and Gynecology 2004;190(5):1224‐9. [DOI] [PubMed] [Google Scholar]
Zakherah 2011
- Zakherah M, Sayed G, El‐Nashar A, Shaaban M. Pictorial blood loss assessment chart in the evaluation of heavy menstrual bleeding: diagnostic accuracy compared to alkaline hematin. Gynecologic and Obstetric Investigation 2011;71(4):281‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lethaby 2000
- Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000249] [DOI] [PubMed] [Google Scholar]


