Abstract
Background
Depression is common in the postnatal period and can lead to adverse effects on the infant and wider family, in addition to the morbidity for the mother. It is not clear whether antidepressants are effective for the prevention of postnatal depression and little is known about possible adverse effects for the mother and infant, particularly during breastfeeding. This is an update of a Cochrane Review last published in 2005.
Objectives
To assess the effectiveness of antidepressant medication for the prevention of postnatal depression, in comparison with any other treatment, placebo or standard care.
Search methods
We searched the Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR ‒ both Studies and References), CENTRAL (Wiley), MEDLINE (OVID), Embase (OVID), PsycINFO (OVID), on 13 February 2018. We also searched the World Health Organization (WHO) trials portal (ICTRP) and ClinicalTrials.gov on 13 February 2018 to identify any additional unpublished or ongoing studies.
Selection criteria
Randomised controlled trials (RCTs) of initiation of antidepressants (alone or in combination with another treatment), compared with any other treatment, placebo or standard care for the prevention of postnatal depression among women who were either pregnant or had given birth in the previous six weeks and were not currently depressed at baseline.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We requested missing information from investigators wherever possible and sought data to allow intention‐to‐treat analyses.
Main results
Two trials including a total of 81 participants fulfilled the inclusion criteria for this review. All participants in both studies had a history of postnatal depression and were not taking antidepressant medication at baseline. Both trials were conducted by the same research group. Risk of bias was low or unclear in most domains for both studies. We were unable to perform a meta‐analysis due to the small number of studies.
One study compared nortriptyline with placebo and did not find any evidence that nortriptyline was effective in preventing postnatal depression. In this study, 23% (6/26) of women who took nortriptyline and 24% (6/25) of women who took placebo experienced postnatal depression (RR 0.96, 95% CI 0.36 to 2.59, very low quality evidence) in the first 17 weeks postpartum. One woman taking nortriptyline developed mania; and one side effect, constipation, was more common among women taking nortriptyline than those taking placebo.
The second study compared sertraline with placebo. In this study, 7% (1/14) of women who took sertraline developed postnatal depression in the first 17 weeks postpartum compared with 50% (4/8) of women who took placebo. It is uncertain whether sertraline reduces the risk of postnatal depression (RR 0.14, 95% CI 0.02 to 1.07, very low quality evidence). One woman taking sertraline had a hypomanic episode. Two side effects (dizziness and drowsiness) were more common among women taking sertraline than women taking placebo.
Conclusions are limited by the small number of studies, small sample sizes and incomplete outcome data due to study drop‐out which may have led to bias in the results. We have assessed the certainty of the evidence as very low, based on the GRADE system. No data were available on secondary outcomes of interest including child development, the mother‒infant relationship, breastfeeding, maternal daily functioning, family relationships or maternal satisfaction.
Authors' conclusions
Due to the limitations of the current evidence base, such as the low statistical power of the included studies, it is not possible to draw any clear conclusions about the effectiveness of antidepressants for the prevention of postnatal depression. It is striking that no new eligible trials have been completed in the period of over a decade since the last published version of this review. Larger trials are needed which include comparisons of antidepressant drugs with other prophylactic treatments (e.g. psychological interventions), and examine adverse effects for the fetus or infant. Future reviews in this area may benefit from broadening their focus to examine the effectiveness of antidepressants for the prevention of perinatal (i.e. antenatal or postnatal) depression, which could include studies comparing antidepressant discontinuation with continuation for the prevention of relapse of depression during pregnancy and the postnatal period.
Plain language summary
Antidepressant medication for preventing postnatal depression
Review question
We examined the evidence to see whether antidepressants can prevent women from experiencing depression in the postnatal period, when compared with any other treatment, sham treatment (placebo), or standard clinical care. The studies we identified included only women who had previously experienced postnatal depression, and had a higher risk of experiencing postnatal depression again.
Background
Postnatal depression is a common condition. Approximately 10 to 15 of every 100 women experience elevated symptoms of depression in the period after giving birth, and 5 in every 100 women will experience a depressive disorder. Symptoms of depression include low mood, loss of pleasure, and feelings of guilt or worthlessness. Postnatal depression has an impact on the mother, and may have a negative impact on the well‐being of the infant and wider family.
Women with a history of depression — and particularly women who have previously experienced postnatal depression — have a higher risk of postnatal depression. Pregnant women who are not depressed, but are at high risk of developing postnatal depression, may want to consider taking measures to try to prevent depression developing in the postnatal period.
We examined whether taking antidepressants during pregnancy or after giving birth can prevent women from developing postnatal depression.
Study characteristics
We identified two small, relevant trials. All the women in these trials had a history of postnatal depression, but were not depressed or using antidepressants at the beginning of the studies. Both studies compared antidepressant medicine with placebo. Women started taking the medicine or placebo on the first day after giving birth.
In the larger study (56 women), the antidepressant given to women was nortriptyline, which is a tricyclic antidepressant. In the other study (25 women), the antidepressant used was sertraline which is a selective serotonin reuptake inhibitor (SSRI); these types of antidepressants work in different ways. The women and the researchers assessing the outcomes in both studies did not know which women were taking antidepressants and which placebo (i.e. both studies were 'double‐blind'). Both studies were funded by the National Institute of Mental Health (NIMH), a US government organisation.
Key results
There was no evidence that nortriptyline prevented postnatal depression. During the 17‐week treatment period, 6 of the 26 women taking nortriptyline experienced postnatal depression compared with 6 of the 25 women taking placebo. One woman taking nortriptyline developed mania (a state of abnormally high arousal and energy level), and constipation was more common among women taking nortriptyline, but other unwanted, or harmful, effects did not differ between groups.
In the sertraline study, 1 of the 14 women taking sertraline developed postnatal depression compared with 4 of the 8 women taking placebo (during the 17‐week treatment period). This study was very small, so we can't be sure whether the difference between sertraline and placebo is due to chance, or whether sertraline does prevent postnatal depression among women with a history of postnatal depression. One woman taking sertraline experienced a hypomanic episode (a state like mania but less severe); and dizziness and drowsiness were more common among women taking sertraline than women taking placebo.
Quality of the evidence
This evidence is current to February 2018.
We could only identify two relevant studies, which had small numbers of participants and inconsistent findings, and were conducted by the same research group. Therefore we consider the quality of evidence in this review to be very low. Further studies with larger samples are needed before we can know whether antidepressants can prevent postnatal depression.
It is worth noting that no new relevant trials have been completed in the 10 years since we last examined this evidence. It may be useful for future medical studies to investigate whether antidepressants can prevent depression during pregnancy as well as during the postnatal period; and whether women who continue to take antidepressants during pregnancy (compared with stopping medication) are less likely to have a relapse of depression at this time.
We also need studies which have longer follow‐ups periods; examine outcomes and side effects for both the mother and fetus or breastfeeding infant; and compare antidepressants with other preventative interventions (such as psychological therapies).
Summary of findings
Background
Description of the condition
Many women experience depression after they give birth, which is known as postnatal or postpartum depression (O'Hara 1996; Howard 2014). Depressive episodes are characterised by a variety of symptoms, such as low mood, loss of enjoyment, feelings of guilt or worthlessness, loss of energy and possibly suicidal ideation. Standard diagnostic criteria for depression are used to diagnose depression in the postnatal period (e.g. the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) or the Tenth Revision of the International Classification of Disease (ICD‐10) (World Health Organization 2004; American Psychiatric Association 2013). As well as the impact on the mother, postnatal depression can have negative implications for the infant and the wider family (Cox 1982; Stein 2014). These outcomes may include adverse effects on mother‒infant attachment, and the emotional or behavioural development of the child (Stein 2014).
It is estimated that approximately 10% to 15% of women experience elevated symptoms of depression in the period after giving birth, and approximately 5% of women have an episode of major depression at this time (Howard 2014). Women who have had one or more previous episodes of depression are at increased risk of developing postnatal depression, and this is particularly the case for women with a previous episode of postnatal depression (Howard 2014). The period of time in which episodes of depression are classified as 'postnatal depression' varies between studies, but often refers to onset of depression in the first three months (e.g. Yonkers 2008) or six months (e.g. Sharp 2010) after delivery. However, it is important to remember that many women presenting with depression in the postnatal period may have developed depression before delivery, either before conception or during pregnancy (Wisner 2013).
Description of the intervention
Antidepressants are medications to treat the symptoms of depression, and meta‐analyses have shown that their effectiveness increases with the severity of depression (Fournier 2010). In general, medication exposure in pregnancy should be minimised when possible, and non‐pharmacological interventions can be effective for the prevention and treatment of mild to moderate depression (Dennis 2007; Morrell 2016). However, antidepressant medications are clinically indicated for women with more severe mental disorders during pregnancy or the postnatal period when there are substantial risks to the mother, the pregnancy, and the fetus or infant (Howard 2014). Current NICE guidelines recommend the use of antidepressants for the treatment of moderate to severe depression and also for the prevention of depressive episodes in those with a high risk of relapse or a history of recurrent depression (NICE 2009). This review investigates the initiation of antidepressants during pregnancy or the early postpartum period for the prevention of postnatal depression. Antidepressants can be classified into the following types.
Selective serotonin reuptake inhibitors (SSRIs): e.g. fluoxetine, paroxetine, sertraline, citalopram
Tricyclic antidepressants (TCAs): e.g. amitriptyline, nortriptyline, doxepin
Heterocyclic antidepressants: e.g. mianserin
Monoamine oxidase inhibitors (MAOIs): e.g. phenelzine, brofaromine
Noradrenaline re‐uptake inhibitors (NARIs): e.g. reboxetine
Noradrenaline‐dopamine re‐uptake inhibitors (NDRIs): e.g. bupropion
Serotonin‐noradrenaline re‐uptake inhibitors (SNRIs): e.g. venlafaxine, duloxetine
Noradrenergic and specific serotonergic antidepressants (NASSAs): e.g. mirtazapine
Serotonin antagonist and re‐uptake inhibitors (SARIs): e.g. trazodone
Other unclassified antidepressants: e.g. vilazodone
The fetus and the infant may be affected by exposure to psychotropic drugs across the placenta or through breastfeeding, although exposure to antidepressants in breastfed infants is five to ten times lower than exposure in utero (Howard 2014). Breastfeeding women have been advised to avoid doxepin (a TCA) as its main metabolite has been found in breast milk in higher concentrations than other antidepressants (Eberhard‐Gran 2006). More broadly, there are limited data on the safety of antidepressants during breastfeeding (particularly for longer‐term child outcomes) but the findings to date suggest that the benefits of taking antidepressants may outweigh the risks in breastfeeding women who need treatment for depression (Howard 2014). The risk‒benefit ratio is less clear for women offered antidepressants as a preventative intervention in the postpartum period, but it is important to note that the women who are offered antidepressants as a preventative intervention at this time often have a history of severe episodes of postnatal depression and are therefore at higher risk of experiencing another episode of postnatal depression.
How the intervention might work
The function of antidepressants is not fully understood. There is evidence from studies of both patients and healthy controls that antidepressants affect activity in areas of the medial prefrontal cortex and limbic system that are associated with emotion processing (e.g. the anterior cingulate, amygdala and thalamus), leading to increased activity in response to positive emotions and decreased activity in response to negative emotions (see systematic review and meta‐analysis by Ma 2014). Most antidepressants appear to inhibit uptake of monoamine neurotransmitters (e.g. serotonin or noradrenaline (norepinephrine)) into neurons, which increases the concentrations of these neurotransmitters at synapses (Berton 2006). The majority of evidence on the effects of antidepressants comes from treatment studies, but there is also evidence that antidepressants can reduce the risk of relapse of depression compared with placebo (Geddes 2003). Women at higher risk of postnatal depression (such as those with a history of postnatal depression) may be more likely to benefit from preventative interventions and also more motivated to accept them. This is particularly relevant when considering antidepressant prevention of postnatal depression, given the possible adverse effects of antidepressant use on the breastfeeding infant (or on the fetus, if started during pregnancy).
Why it is important to do this review
This is an update of a Cochrane Review published in 2005, and previously assessed as up to date in 2007. Research into postnatal depression is important due to its high prevalence and potential negative impact on both the mother and child, as well as other family members (Cox 1982; Stein 2014). Antidepressants have been shown to be more effective than placebo in treating postnatal depression (Molyneaux 2014); but it is also important to consider the prevention of postnatal depression, particularly for high‐risk women. Other interventions have been found to be effective for preventing postnatal depression, for example psychological and psychosocial interventions (Morrell 2016). However, these psychological interventions are not always available and some women may be recommended antidepressants as a preventative intervention, particularly those with a history of severe postnatal depression and those who have previously responded well to antidepressants. It is therefore important to determine whether antidepressants are effective for the prevention of postnatal depression. It is also important to examine antidepressant prevention of postnatal depression owing to the additional issues around the safety for the fetus (if preventative interventions begin during pregnancy) or the breastfeeding infant.
Objectives
To assess the effectiveness of antidepressant medication for the prevention of postnatal depression, in comparison with any other treatment, placebo or standard care.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials (RCTs), including cluster and cross‐over trials.
Types of participants
Participant characteristics
Women who are pregnant or have given birth in the last six weeks, who are not depressed at the start of the trial.
Diagnosis
Women must not be depressed at the beginning of eligible trials, as the focus of the review is the prevention of depression in the postnatal period. Trials may include women with a history of depression or postnatal depression only, or offer the intervention to all at‐risk women during pregnancy or the early postpartum period.
Co‐morbidities
We placed no restrictions on studies involving participants with co‐morbid medical or psychological disease or disorders, provided the co‐morbidities were not the primary focus of the study.
Setting
We placed no restrictions on the setting of the studies.
Types of interventions
Experimental intervention
Any antidepressant medication at any dose, alone or in combination with another treatment, initiated in at least one arm of a trial. For the purposes of this review antidepressant medications were classified as follows.
Selective serotonin reuptake inhibitors (SSRIs): e.g. fluoxetine, paroxetine, sertraline, citalopram
Tricyclic antidepressants (TCAs): e.g. amitriptyline, nortriptyline, doxepin
Heterocyclic antidepressants: e.g. mianserin
Monoamine oxidase inhibitors (MAOIs): e.g. phenelzine, brofaromine
Noradrenaline re‐uptake inhibitors (NARIs): e.g. reboxetine
Noradrenaline‐dopamine re‐uptake inhibitors (NDRIs): e.g. bupropion
Serotonin‐noradrenaline re‐uptake inhibitors (SNRIs): e.g. venlafaxine, duloxetine
Noradrenergic and specific serotonergic antidepressants (NASSAs): e.g. mirtazapine
Serotonin antagonist and re‐uptake inhibitors (SARIs): e.g. trazodone
Other unclassified antidepressants: e.g. vilazodone
Comparator intervention
Placebo, standard clinical care, or any other treatment, such as psychological interventions (e.g. CBT) or psychosocial interventions (e.g. peer support).
Types of outcome measures
We included studies that meet the above inclusion criteria regardless of whether they report on the following outcomes.
Primary outcomes
1. Onset of postnatal depression. Depression was defined as measured in each trial using any of the following: clinical diagnostic interview; standard observer‐rated depression symptom scales based on a recognised diagnostic scheme (e.g. the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) or the International Classification of Disease (ICD‐10); other standardised criteria (e.g. the Research Diagnostic Criteria (RDC) (Spitzer 1978)); or screening instruments (e.g. the Edinburgh Postnatal Depression Scale (EPDS) (Cox 1987)). Studies examining onset of depression up to six months postpartum were eligible. Time to onset was reported if examined in individual studies.
2. Adverse events experienced by the mother, or the fetus/breastfed infant, or both.
Secondary outcomes
3. Acceptability of treatment assessed directly through questionnaires for trial participants or indirectly through drop‐out rates.
4. Cognitive and emotional development of the infant (e.g. assessment of the mental and psychomotor development of infants using the Mental Development Index (MDI) and Psychomotor Development Index (PDI) of the Bayley Scales of Infant Development (Bayley 2006); parental report of child development using the Parent Report of Children's Abilities‐Revised (PARCA‐R) (Johnson 2008).
5. Overall maternal satisfaction (e.g. self‐reported general satisfaction, satisfaction with self/baby/partner using the Mackay Childbirth Satisfaction Rating Scale (Goodman 2004); self‐reported beliefs, values and perceived skills regarding motherhood using the Parenting Sense of Competence Scale (Gidaud‐Wallston 1978).
6. Improvement in the maternal relationship with the baby (e.g. improved mother‒infant interactions measured using the CARE‐Index (Crittenden 1988).
7. Improvement in the mother's ability to carry out daily activities and social functioning (e.g. improved score on the Global Assessment of Functioning Scale (Endicott 1976); increased social network, measured using the Social Network Index (Cohen 1997).
8. The establishment or continuation of breastfeeding (e.g. rates of establishment, continuation or discontinuation).
9. Prevention of neglect or abuse of the baby (e.g. using the Parent‐Report Multidimensional Neglectful Behavior Scale (Kaufman Kantor 2004).
10. The effect on marital and family relationships (e.g. using the Quality of Marriage Index (Norton 1983).
Timing of outcome assessment
Zero to eight weeks: immediate effects.
Nine to 16 weeks: short‐term effects.
17 to 24 weeks: intermediate effects.
More than 24 weeks: long‐term effects.
Hierarchy of outcome measures
If multiple measures had been used for one of these outcomes, we intended to select measures which have been validated among women with postnatal depression above measures which have been validated in other populations. If possible, we intended to report one outcome from each of the above time periods.
Search methods for identification of studies
Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR)
The Cochrane Common Mental Disorders Group (CCMD) maintains two archived clinical trials registers at its editorial base in York, UK: a references register and a studies‐based register. The CCMDCTR‐References Register contains over 40,000 reports of RCTs in depression, anxiety and neurosis. Approximately 50% of these references have been tagged to individual, coded trials. The coded trials are held in the CCMDCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary; (please contact the CCMD Information Specialists for further details). Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 to 2016), Embase (1974 to 2016) and PsycINFO (1967 to 2016); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trial registers via the World Health Organization's trials portal (the International Clinical Trials Registry Platform (ICTRP)), pharmaceutical companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCMD's generic search strategies (used to identify RCTs) can be found on the Group's website. The Group’s Specialised Register had fallen out of date with the Editorial Group’s move from Bristol to York in the summer of 2016.
Electronic searches
1. CCMDCTR We conducted an update search of the CCMDCTR‐Studies and References registers on 13 February 2018 using the terms listed in Appendix 1.
2. International trial registries We also searched the WHO trials portal (ICTRP) and ClinicalTrials.gov on 13 February 2018 to identify any additional unpublished or ongoing studies using the following terms.
ICTRP: depress* and prevent* and postpartum or depress* and prevent* and postnatal or depress* and prophyla* and postpartum or depress* and prophyla* and postnatal CT.gov: (prevention or prevent or prophylaxis) and (depression or depressive) and (postnatal or post‐natal or postpartum or post‐partum)
Records were filtered (for pharmacological interventions) using Excel.
3. Web of Science (citation search) A cited reference search of the Web of Science was also conducted (4 May 2016), for reports citing the first version of this review, a companion publication published in PLOS Medicine (Howard 2006) and all of the included studies to date.
Earlier searches conducted for the first version of this review (to June 2007) can be found in Appendix 2.
4. In keeping with MECIR conduct standard c37, a final pre‐publication search was conducted on 13 February 2018 on the following databases (2016 to date).
Cochrane Common Mental Disorders Specialised Register;
Ovid MEDLINE;
Ovid Embase;
Ovid PsycINFO;
CENTRAL;
Trial Registries.
This was necessary as the Group’s Specialised Register had fallen out of date with the Editorial Group’s move from Bristol to York in the summer of 2016. The details of this search can be found in Appendix 3
Searching other resources
Grey literature
We did not search grey literature for this update of the review as relevant unpublished studies should be identified through searches of clinical trials registries.
Reference lists
We checked reference lists and conducted forward citation tracking for all included studies to identify additional studies missed from the original electronic searches (for example unpublished or in‐press citations).
Correspondence
We contacted trialists for information on unpublished or ongoing studies, or to request additional trial data when required.
Data collection and analysis
Selection of studies
Titles and abstracts of studies identified in the above search were examined by two review authors (LT and EM) independently. We removed duplicate records; and obtained the full‐text article for any publication which was potentially relevant. LT and EM then independently assessed full‐text articles to examine whether they fulfilled the inclusion criteria. Where the authors disagreed, the matter was discussed until agreement was reached; or we sought guidance and arbitration from a third review author (LH) when necessary.
We collated multiple reports related to the same study so that data from each trial were included only once even if the trial, or aspects of it, had been reported in multiple published papers. When multiple reports of a single trial were identified, we included the report with the largest sample size. We recorded the selection process in detail and produced a PRISMA flow diagram and ‘Characteristics of excluded studies’ table.
Data extraction and management
Two review authors (LT and EM) independently extracted data from the trial reports. We developed a data extraction form and piloted it for this review. We requested missing information from investigators wherever possible; and we extracted the following study characteristics.
Methods: study design, study setting, number and location of study centres, study date and duration, details of blinding/allocation concealment.
Participants: total number of participants and number in each group, inclusion and exclusion criteria, key socio‐demographic information (e.g. age), number of withdrawals.
Interventions: number of intervention groups, type of interventions and comparisons, duration of intervention and key details (e.g. dosage), details of any 'run‐in' period.
Outcomes: primary and secondary outcomes specified and reported, time points reported, adverse events, and details of measures used to assess outcomes.
Analysis: statistical techniques used, subgroup analyses, number of participants included in each analysis, study results.
Notes: trial funding and conflicts of interest of trial authors.
Where possible, we resolved any inconsistencies in the information extracted by discussion between review authors (LT and EM). When this was not possible, we sought further information from the trial investigator(s). One author (EM) entered data into Review Manager 5 software (RevMan 5); and all entries were checked by the other author (LT). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. LT spot‐checked study characteristics for accuracy against the trial report.
Main comparisons
The main planned comparison was antidepressant medication versus placebo. We had also planned to compare antidepressants with standard clinical care and with psychological or psychosocial interventions but no included studies provided data for these comparisons. Comparisons are presented separately for individual antidepressants.
Assessment of risk of bias in included studies
Two review authors (LT and EM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion, including a third review author (LH) when necessary. We assessed risk of bias in the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias (adherence to medication)
We judged each potential source of bias as high, low or unclear and provided a supporting quotation from the study report together with a justification for our judgment in the 'Risk of bias' table. We considered blinding separately for different key outcomes where necessary. We assessed selective outcome reporting by obtaining trial protocols (where possible) and comparing these with the final trial report. Where information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
Measures of treatment effect
We presented the primary outcome (postnatal depression) using risk ratios (RR) for all studies. If the RR was not presented in the original trial report, we calculated it for this review. We summarised other outcomes using the data as quoted in the original papers (e.g. odds ratio (OR), hazard ratio (HR), RR, mean difference (MD)). If there had been sufficient data for meta‐analyses to be performed on any outcomes (i.e. three or more comparable studies), we would have calculated the RR for dichotomous outcomes, the HR for time‐to‐event outcomes, and the MD or standardised mean difference (SMD) for continuous outcomes.
Dichotomous data
We calculated the RR and its 95% confidence interval (CI) for dichotomous outcome data. It has been shown that RRs are more intuitive than ORs and that ORs tend to be interpreted as RRs by clinicians (Bland 2000). This misinterpretation then leads to an overestimation of the effect. If necessary for studies included in future updates of this review, we will attempt to convert continuous primary outcome measures to dichotomous data using cut‐off points on rating scales to identify those who did and did not fulfil the criteria for depression.
Time‐to‐event data
We presented the HR and 95% CI for time‐to‐event outcome data, when applicable. In future updates of this review, if time‐to‐event data is given in an original paper but not presented as an HR then the HR will be estimated using the methods described by Tierney 2007. If there are sufficient studies presenting time‐to‐event data to conduct a meta‐analysis in future updates of this review, we will pool data following the guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Continuous data
We did not conduct any meta‐analyses for this review. However, if a meta‐analysis of continuous data is performed in future updates of the review, we will calculate the mean difference between groups (if studies used the same outcome measure) or the standardised mean difference (if studies used different measures to assess the same outcome). If future updates of this review include reports which present standard errors instead of standard deviations, we will convert these to standard deviations. If standard deviations are not reported and cannot be calculated from the available data, we will request the necessary data from trial authors. In the absence of data from authors, we will use the mean standard deviation from other comparable studies.
Unit of analysis issues
Cluster‐randomised trials
Cluster RCTs must be analysed taking into account the clustered nature of the data. No cluster RCTs meeting inclusion criteria for this review were identified, but we will use the following methods to address any cluster RCTs included in future updates of this review: we will extract the intra‐cluster correlation coefficient (ICC) for each trial or request it from study authors if not reported. If the ICC cannot be obtained, estimates of the ICC from similar studies will be used to 'correct' for clustering, in line with guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The generic inverse variance method will be used to meta‐analyse results from cluster RCTs (Higgins 2011).
Cross‐over trials
The results of cross‐over trials may be influenced by the carry‐over effect, whereby a treatment in the first randomised treatment period of the trial has an effect which carries over to the second randomised treatment period. Cross‐over trials are also not appropriate if the condition of interest is unstable (Elbourne 2002). Both of these issues apply to trials of postnatal depression prevention. We identified no cross‐over trials for inclusion in this review, but if we identify any in future updates of the review we will include only data from the first randomised treatment period.
Studies with multiple treatment groups
Trials with more than two relevant treatment groups can lead to issues with pairwise meta‐analysis. We identified no studies with more than two groups for inclusion in this review but if we identify any in future updates the following methods will be used, in line with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For dichotomous outcomes, we will combine the active treatment groups into a single arm for comparison against the control group; or we will split the control group equally for separate comparisons with the active treatment groups (based on sample size). For continuous outcomes, we will pool the means and standard deviation in each treatment group (as a function of the number of participants in each group) for comparison with the control group.
Dealing with missing data
We contacted study investigators to verify key study characteristics and obtain missing numerical outcome data where necessary. When possible, we presented data on a 'once randomised, always analyse' basis, assuming an intention‐to‐treat (ITT) analysis. When studies had not performed ITT analyses, we calculated the RR based on ITT assuming a negative outcome (i.e. onset of postnatal depression) for all women lost to follow‐up. If this was done, a sensitivity analysis was also performed assuming a positive outcome (i.e. no postnatal depression) for all women lost to follow‐up. We anticipated that some studies would have used the method of last observation carried forward (LOCF) to do an ITT analysis. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results: therefore we have indicated where we have reported LOCF data in this review. We presented ITT analysis for all primary outcomes. Where ITT analyses were not available for secondary outcomes, we reported this in the relevant section of the results.
Assessment of heterogeneity
There were insufficient studies to conduct a meta‐analysis. However, if future updates of this review include sufficient comparable studies to conduct a meta‐analysis, we will assess statistical heterogeneity visually by studying the degree of overlap of the confidence intervals for individual studies in a forest plot. We will also conduct more formal assessments of heterogeneity using a Chi² test with the P value set at 0.1 (taking into account the fact that this test has low statistical power when there are few studies). We will use the I² statistic, and will interpret its results based on the following overlapping bands, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: represents considerable heterogeneity
Assessment of reporting biases
There were insufficient studies to conduct a meta‐analysis. However, if a future update of this review includes a meta‐analysis pooling data from 10 or more studies, we will generate a funnel plot and visually inspect it for asymmetry, which can indicate publication bias. We will also consider other possible causes of funnel plot asymmetry (e.g. poor methodological quality of small studies).
Data synthesis
We identified insufficient studies in this review to conduct a meta‐analysis. However, if a future update of this review includes three of more studies using the same class of antidepressants and the same comparison group (e.g. placebo), we will perform a meta‐analysis. Due to the anticipation of clinical heterogeneity between studies, for example in the participant groups included, we will conduct a random‐effects meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We were unable to include sufficient studies in this review to conduct subgroup analyses or further investigations of heterogeneity. However, if we identify sufficient data in future updates of this review we will conduct the following subgroup analyses to investigate heterogeneity between trials and assess the effectiveness of the intervention in the following groups.
1. Studies in which women started antidepressants during pregnancy versus studies in which women started antidepressants in the postpartum period, to examine whether the timing of antidepressant use influences its effectiveness in preventing postnatal depression.
2. Studies in which all women had a history of postnatal depression versus studies in which women had a history of non‐postnatal depression, to examine whether this influences the effectiveness of antidepressant prevention of postnatal depression.
Sensitivity analysis
We were unable to include sufficient studies in this review to conduct sensitivity analyses. However, if future updates of this review include sufficient data, we will conduct a priori sensitivity analyses to examine the robustness of pooled estimates to decisions made in the methods of this systematic review. We will assess the effects of excluding studies with the following characteristics.
1. Study quality: excluding studies that had a high risk of bias in any domain. 2. Blinding: excluding antidepressant versus placebo trials that were unblinded. 3. Attrition: excluding studies with more than 20% drop‐out. 4. Validation: excluding outcomes based on non‐validated assessments.
We also planned additional sensitivity analyses to examine missing data (described in Dealing with missing data). If studies have not performed ITT analyses, we will calculate the RR assuming a negative outcome (i.e. onset of postnatal depression) for all women lost to follow‐up and a sensitivity analysis assuming a positive outcome (i.e. no postnatal depression) for all women lost to follow‐up.
'Summary of findings' tables
We have produced 'Summary of findings' tables for the key findings of the review for all main comparisons in the time frame of intermediate effects (12 to 24 weeks) (Table 1; Table 2). The tables present findings for primary and secondary outcomes (see Types of outcome measures) including the standardised effect size estimates (with 95% confidence intervals), number of studies and participants, and the quality of evidence based on standards of the GRADE working group (see Balshem 2011). The secondary outcomes included in the 'Summary of findings' tables are: acceptability of treatment, overall maternal satisfaction, improvement in the maternal relationship with the baby, and establishment or continuation of breastfeeding.
Summary of findings for the main comparison. Nortripyline for the prevention of postnatal depression.
| Nortripyline for the prevention of postnatal depression | ||||||
|
Patient or population: women with a history of postnatal depression
Intervention: nortripyline Control: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tricyclic antidepressants | |||||
| Postnatal depression (17 weeks) | 240 per 10001 | 230 per 1000 (86 to 622) | RR 0.96 (0.36 to 2.59) | 51 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| Adverse effects experienced by mother and/or foetus or nursing baby | 51 (1 study) | ⊕⊝⊝⊝ very low2,3 | 1 woman assigned to nortriptyline developed mania within the first week. Constipation was reported more frequently in the nortriptyline than placebo group (78% among women taking nortriptyline and 22% among women taking placebo; Fischer's exact test P < 0.001). This was the only side effect that was more common among women taking nortriptyline than placebo, but the other side effects assessed and the proportion of women experiencing these side effects was not reported. |
|||
| Acceptability of treatment (17 weeks) | 51 (1 study) | ⊕⊝⊝⊝ very low2,3 | Acceptability of treatment was not assessed directly but 1 woman was lost to follow‐up from the nortriptyline arm (and 1 women in the nortriptyline arm withdrew after developing mania in the first postpartum week) and 3 withdrew from the placebo arm (owing to side effects, personal reasons and pregnancy). 4 participants declined to take the study drug after randomisation. |
|||
| Overall maternal satisfaction (17 weeks) | No data available | |||||
| Improvement in the maternal relationship with the baby (17 weeks) | No data available | |||||
| Establishment or continuation of breastfeeding (17 weeks) | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Assumed risk calculated as the proportion of women on placebo with the outcome (postnatal depression) multiplied by 1000
2Downgraded due to high risk of bias in 1 domain (incomplete outcome data) 3Downgraded twice due to imprecision (only 1 small study available for this comparison)
Summary of findings 2. Sertraline for the prevention of postnatal depression.
| Sertraline for the prevention of postnatal depression | ||||||
|
Patient or population: women with a history of postnatal depression
Intervention: sertraline Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Selective serotonin reuptake inhibitors | |||||
| Postnatal depression (17 weeks) | 500 per 1000 | 70 per 1000 (10 to 535) | RR 0.14 (0.02 to 1.07) | 22 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| Adverse effects experienced by mother and/or fetus or nursing baby | 22 (1 study) | ⊕⊝⊝⊝ very low2,3 | 1 woman taking sertraline had a hypomanic episode. 2 side effects (dizziness and drowsiness) were more common among women taking sertraline than women taking placebo. |
|||
| Acceptability of treatment (17 weeks) | 22 (1 study) | ⊕⊝⊝⊝ very low2,3 | Acceptability of treatment was not assessed directly but no difference was found between the antidepressant and placebo groups in the number of women withdrawing from the study (P = 0.35, Fisher’s exact test). | |||
| Overall maternal satisfaction (17 weeks) | No data available | |||||
| Improvement in the maternal relationship with the baby (17 weeks) | No data available | |||||
| Establishment or continuation of breastfeeding (17 weeks) | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Assumed risk calculated as the proportion of women on placebo with the outcome (postnatal depression) multiplied by 1000
2Downgraded due to high risk of bias in 1 domain (incomplete outcome data) 3Downgraded twice due to imprecision (only 1 small study available for this comparison)
Results
Description of studies
Results of the search
The searches were carried out to 13 February 2018. They identified 396 references, of which 320 remained after de‐duplication. Two review authors (LT and EM) independently screened the titles and abstracts of these records and excluded 303 which did not meet the inclusion criteria. We retrieved and inspected the full‐text papers (or trial protocols) for the remaining 17 study reports. We excluded a total of six of these for not meeting our inclusion criteria: one because the trial terminated due to low recruitment (NCT02235064); and the remaining five reports (four studies) as these assessed treatment rather than prevention of postnatal depression or continuation with discontinuation of antidepressants during pregnancy for the prevention of perinatal (i.e. antenatal or postnatal) depression rather than examining initiation of antidepressants for the prevention of postnatal depression. There are two primary study reports with an additional eight secondary publications for the two trials which meet the inclusion criteria and are included in the review. In addition, there is one completed but unpublished study (NCT00276900) (see Characteristics of studies awaiting classification). We contacted the trial investigator for additional information but unfortunately it was not possible for her to respond to our request due to the period of time that has elapsed since the included trials were conducted. The study selection process is detailed in our PRISMA flow diagram (Figure 1).
1.
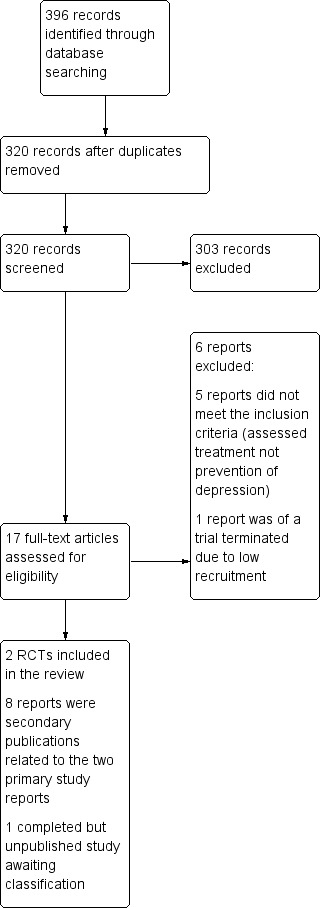
Study flow diagram.
Included studies
We included two studies in this review (Wisner 2001 and Wisner 2004), with characteristics as follows (see also Characteristics of included studies).
Design
Both studies were double‐blind, randomised controlled trials comparing an antidepressant medication with a placebo. We found no eligible cluster‐randomised or cross‐over trials.
Sample sizes
Wisner 2001 had a sample size of 56 participants at baseline, of whom 51 were included in their analyses (26 participants taking nortriptyline and 25 participants taking placebo). Wisner 2004 had a sample size of 25 women at baseline with 22 included in the analyses (14 women in the sertraline arm and 8 in the placebo arm, recruited in a 2:1 intervention to placebo ratio). In both studies, women were excluded from the analyses if they refused to take the study drug after randomisation. Three women randomised to sertraline who never took the medication were excluded from Wisner 2004, and four women were excluded from Wisner 2001 for refusing to take the study drug (treatment allocation unknown). Wisner 2001 also excluded one participant (randomised to nortriptyline) who developed mania in the first week postpartum from the analyses.
Setting
Both studies recruited pregnant women (up to 35 weeks' gestation) who were not depressed in the index pregnancy; and both began medication in the immediate postpartum period. In both trials, the first dose of the antidepressant or placebo medication was provided for women in the hospital following delivery. For both studies, participants had to be aged 21 to 45 years, and not be depressed in the index pregnancy but have at least one previous episode of postnatal depression within five years prior to enrolment. Wisner 2001 stated that to be considered to be postnatal depression, symptom onset must have been within three months of a live birth. Both studies excluded women with previous episodes of psychosis and bipolar disorder, other current Axis I diagnoses (except for generalised anxiety and panic disorder) and antisocial or borderline personality disorders. Women receiving psychotherapy or who used psychotropic medications after the first trimester of the index pregnancy were also excluded from both studies. Wisner 2004 also excluded women with an incomplete blood count or abnormal results from thyroid tests.
Interventions
Wisner 2001 examined nortriptyline (a tricyclic antidepressant) and Wisner 2004 examined sertraline (a selective serotonin reuptake inhibitor (SSRI)). In both studies the antidepressant was taken as soon as possible after delivery, ideally within 24 hours.
In Wisner 2001, the dose of nortriptyline was increased from 20 mg/day to 70 mg/day over the first week. A dose of 75 mg/day was then maintained to day 21, after which the dose was calculated individually for each participant, based on their serum level measured on day 14. The dose was then adjusted over time based on further serum levels and side effects data, in order to maintain the nortriptyline serum level between 50 mg/day and 150 mg/mL with the optimum set at 80 mg/day to 120 mg/mL. The dose was then tapered through weeks 17 to 20 at a rate of 33% per week. Doses (three capsules of either nortriptyline or placebo) were taken once daily at bedtime.
In Wisner 2004, the dose of sertraline was originally started at 50 mg/day. However, a dose reduction was recommended by the non‐blind side effects monitoring team after the first two study participants withdrew after experiencing severe headaches. Therefore, for all remaining participants the dose began with 25 mg/day increased to 50 mg/day over the first four weeks of treatment and up to 75 mg/day over weeks 5 to 17. From week 17, the dose of sertraline was tapered and treatment was discontinued at week 20.
Outcome
Primary outcome assessment
In both studies, onset of postnatal depression was determined through screening with the Hamilton Rating Scale for Depression (HAM‐D) followed by a diagnostic interview. In Wisner 2001, the HAM‐D was administered to all women weekly. If a participant scored 15 or more on the HAM‐D they were evaluated a second time within seven days. If they scored 15 or more on the HAM‐D again and met research diagnostic criteria for major depression on both occasions, the principal investigator and an independent board‐certified psychiatrist evaluated the participant. Both psychiatrists were blinded to participant allocation (i.e. antidepressants or placebo). Women were classified as having depression if both psychiatrists reached a diagnosis of major depressive disorder.
A similar but simpler approach was used in Wisner 2004. If a woman scored 15 or more on the HAM‐D on two occasions, one week apart, she was assessed by a single psychiatrist who was associated with the study but blinded to participant allocation. In both studies, the treatment period lasted 17 weeks and primary outcomes were assessed at the end of the treatment period (i.e. 17 weeks post randomisation), prior to the medication being tapered.
Both studies assessed symptoms of mania weekly and assessed side effects with the Asberg Side Effects rating scale. Adverse events occurring during both studies were also reported.
Secondary outcome assessment There was no direct assessment of the acceptability of treatment in either study: we used the number of participants withdrawing from each treatment group as an indirect measure of acceptability. Neither Wisner 2001 nor Wisner 2004 provided information about the cognitive and emotional development of the infant, overall maternal satisfaction, maternal relationship with the infant, the mother’s ability to carry out daily activities, breastfeeding, neglect or abuse of the infant or the effect on marital and family relationships.
Excluded studies
We excluded two records during full‐text screening, as they evaluated the treatment rather than prevention of postnatal depression. For full details of these studies see Characteristics of excluded studies.
New studies found at this update
No new studies have been included in the review in this update.
Studies awaiting classification
No studies are awaiting classification.
Ongoing studies
Two RCTs are currently ongoing. Both studies are investigating the use of sertraline to prevent postnatal depression compared with a placebo control. One study has completed recruitment, but results of the trial are not yet available (NCT00276900). The second RCT is currently recruiting (NCT02235064). Future updates of this review should consider these studies for inclusion. See Characteristics of ongoing studies for further details.
Risk of bias in included studies
The Characteristics of included studies section provides full details of risk of bias judgements for each study. Graphical representations of the overall risk of bias in included studies are presented in Figure 2 and Figure 3.
2.
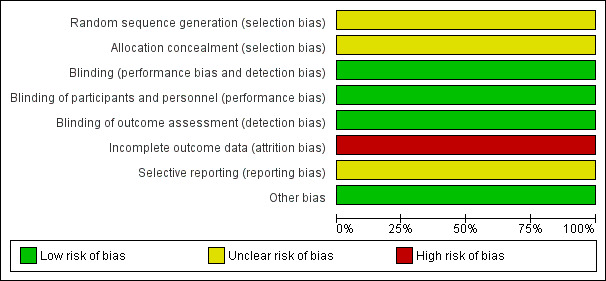
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
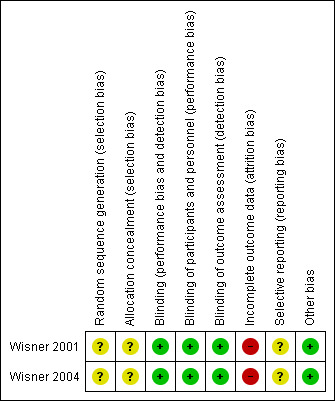
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random Sequence Generation
Neither study report gave details of the method of randomisation.
Allocation Concealment
Neither study provided information about allocation concealment.
Blinding
Blinding of participants and personnel
Both studies were conducted with a double‐blind design (participants and study personnel were blinded to treatment allocation). Blinding was facilitated in both cases by providing placebo and antidepressant medication in identical forms. Blinded study staff were questioned to assess the integrity of blinding in both studies. In Wisner 2001, staff did not successfully identify drug assignment more often than chance, other than the nurse monitoring side effects. In Wisner 2004, no staff or participants were more successful than chance at identifying allocation. Both studies were rated as low risk of bias for blinding of participants and personnel.
Blinding of outcome assessment
Both studies were rated as having low risk of bias for the blinding of outcome assessment, as mood symptom raters and the psychiatrists assessing outcomes were blind to participant group allocation. Outcome assessors were included in the assessment of blinding described above and did not successfully identify drug assignment more often than chance.
Incomplete outcome data
In Wisner 2001, four participants declined to take the study drug after randomisation and one participant developed mania in the first week postpartum: none of these five were included in the analysis. This means that analyses were not performed on a 'once randomised, always analyse' basis. In addition, one other participant randomised to nortriptyline was lost to follow‐up from the study and three participants withdrew from the placebo arm (due to side effects, personal reasons and pregnancy). These participants were included in the analyses (but censored at the week they withdrew from the study).
In Wisner 2004, three participants assigned to sertraline who did not take any medication were not included in the analyses (also therefore not performed on a 'once randomised, always analyse' basis). Of those who took sertraline, two withdrew due to side effects and one due to an episode of hypomania, but these participants were included in the analyses. It is not explicitly stated how many participants withdrew from the placebo arm but it is recorded that there was no significant difference between groups for the number of participants withdrawing from the study. The risk of bias related to incomplete outcome data in both studies was therefore rated as high owing to the exclusion of participants from the analyses and relatively high proportions of drop‐outs for the small sample sizes.
Selective reporting
Protocols were not available for either study. The risk of bias related to selective reporting was therefore unclear for both studies.
Other potential sources of bias
Both studies assessed adherence to medication with frequent serum level tests. In the Wisner 2001 study, five participants randomised to nortriptyline were considered non‐adherent (in addition to the four who withdrew without taking the study drug). Sensitivity analyses showed that excluding these non‐adherent participants did not significantly alter the results. In the Wisner 2004 study, serum levels indicated that all participants randomised to sertraline and included in the analyses were adherent. The risk of bias related to adherence to medication therefore appears to be low in both studies.
Effects of interventions
Comparison 1: nortriptyline versus placebo
One study contributed data to this comparison (Wisner 2001). Fifty‐six participants were randomised in this study and 51 participants were included in their analyses (26 assigned to nortriptyline and 25 assigned to placebo). See also Table 1. It was not possible to obtain the necessary data to analyse this study on a 'once randomised, always analyse' basis.
Primary outcomes
1.1 Number of participants who developed depression during the first six months postpartum
Wisner 2001 reported that nortriptyline was not more effective than placebo in preventing postnatal major depressive disorder (RR 0.96, 95% CI 0.36 to 2.59, P = 0.9381) after a 17‐week treatment period (see Figure 4). Six out of 26 women in the nortriptyline group developed postnatal depression compared with six out of 25 women in the placebo group. Data from participants who withdrew from the study were censored at the week they withdrew. Wisner 2001 also reported that time to onset of depression did not differ between the nortriptyline and placebo groups (exact log‐rank ≤ 0.00, exact P = 0.83). From the serum level tests, the trial authors determined that five women randomised to nortriptyline were non‐adherent; censoring these participants at the point of non‐adherence did not change the findings of the study.
4.

Forest plot of comparison: 1 Nortriptyline versus placebo, outcome: 1.1 Recurrence of postpartum major depressive disorder.
1.2 Adverse effects experienced by mother and/or foetus or nursing baby
One woman assigned to nortriptyline developed mania within the first week, but no other specific adverse events were reported. The only side effect reported more frequently in the nortriptyline than placebo group was constipation (78% among women taking nortriptyline and 22% among women taking placebo; Fischer's exact test P < 0.001). The proportion of women experiencing other side effects was not reported.
Secondary outcomes
1.3 Acceptability of treatment
Acceptability of treatment was not assessed directly. However, information regarding the number of participants dropping out or being lost to follow‐up may provide some indication of acceptability. In this study one woman was lost to follow‐up from the nortriptyline arm (and one women in the nortriptyline arm withdrew after developing mania in the first postpartum week) and three withdrew from the placebo arm (owing to side effects, personal reasons and pregnancy). Four participants declined to take the study drug after randomisation.
1.4 Cognitive and emotional development of the infant/child
No data available.
1.5 Overall maternal satisfaction
No data available.
1.6 Improvement in the maternal relationship with the baby
No data available.
1.7 Improvement in the ability of the mother to carry out daily activities and in her social functioning
No data available.
1.8 Establishment or continuation of breastfeeding
No data available.
1.9 Prevention of neglect or abuse of the baby
No data available.
1.10 Effect on marital and family relationships
No data available.
Comparison 2: sertraline versus placebo
One study contributed data for this comparison (Wisner 2004). Twenty‐five participants were randomised into this study and 22 were included in their analyses (14 assigned to sertraline and 8 assigned to placebo). See also Table 2. Three women assigned to sertraline were excluded from the analyses because they did not take the study medication. Additional analyses were conducted on a 'once randomised, always analyse' basis for this review.
Primary outcomes
2.1 Number of participants who developed depression during the first six months postpartum
Wisner 2004 reported that sertraline was more effective than placebo in preventing postnatal major depressive disorder after a 17‐week treatment period (see Figure 5). One (7%) of the 14 women who took sertraline developed depression compared with four (50%) of the eight women taking placebo (P = 0.04, Fisher exact test). The difference in rates of postnatal depression was 0.43 (95% exact CI = −0.01 to 0.84). The RR was calculated for this review and provided evidence of a trend but was below the cut‐off for statistical significance (RR 0.14, 95% CI 0.02 to 1.07, P = 0.058). For this review, we also recalculated the RR on a 'once randomised, always analyse' basis including an additional three participants who were randomised to sertraline but excluded from the trial analyses for never taking the medication. Including these three participants and assuming a negative outcome (i.e. onset of postnatal depression) for those not included in the original analyses, we calculated the RR to be 0.47 (95% CI 0.16 to 1.42, P = 0.18). Assuming a positive outcome for these three participants (i.e. no onset of postnatal depression), we calculated the RR to be 0.12 (95% CI 0.02 to 0.89, P = 0.038). Wisner 2004 also reported that the time to recurrence differed between the sertraline and placebo groups (exact Gehan‐Wilcoxon P = 0.02). The observed hazard ratio for time to recurrence of depression was 0.11 (95% exact CI = 0.02 to 1.02).
5.

Forest plot of comparison: 2 Sertraline versus placebo, outcome: 2.1 Recurrence of postpartum major depressive disorde.
2.2 Adverse effects experienced by mother and/or foetus or nursing baby
One woman taking sertraline experienced a hypomanic episode. The first two participants experienced severe headaches causing them to withdraw from the study which led to the original dosage of sertraline being reduced from 50 mg to 25 mg (see Description of studies). Two side effects were found to be significantly more frequent in the group taking sertraline: eight (57%) of the women taking sertraline and one (13%) of the women taking placebo experienced dizziness (P = 0.05 Freeman‐Halton extension of Fisher’s exact test); and drowsiness was also found to be more frequent in the sertraline arm, in which it was experienced by 14 women (100%), compared with 4 women (50%) in the placebo arm (P = 0.02; Freeman‐Halton extension of Fisher’s exact test).
Secondary outcomes
2.3 Acceptability of treatment
Wisner 2004 did not report a direct measure of acceptability; however no difference was found between the antidepressant and placebo groups in the number of women withdrawing from the study (P = 0.35, Fisher’s exact test).
2.4 Cognitive and emotional development of the infant/child
No data available.
2.5 Overall maternal satisfaction
No data available.
2.6 Improvement in the maternal relationship with the baby
No data available.
2.7 Improvement in the ability of the mother to carry out daily activities and in her social functioning
No data available.
2.8 Establishment or continuation of breastfeeding
No data available.
2.9 Prevention of neglect or abuse of the baby
No data available.
2.10 Effect on marital and family relationships
No data available.
Subgroup analyses
We were unable to conduct the planned subgroup analyses due to the small number of included studies.
Sensitivity analyses
We were unable to conduct the planned sensitivity analyses due to the small number of included studies.
Reporting bias
There were insufficient studies to assess reporting bias.
Discussion
Summary of main results
We included two RCTs examining the effectiveness of antidepressants for the prevention of postnatal depression in this review. All participants in both studies had a history of postnatal depression and were not taking antidepressant medication at baseline. There were insufficient studies to conduct a meta‐analysis. The first study provided no evidence that nortriptyline was more effective than placebo in preventing postnatal depression (see Table 1). The second study found some evidence that sertraline may be effective in preventing postnatal depression (see Table 2) but the sample size was extremely small and the risk ratio, calculated for this review, was just below statistical significance. The low statistical power of this study means that we cannot exclude the possibility that sertraline is no different from placebo in its effectiveness for preventing postnatal depression, but we also cannot exclude the possibility that sertraline has a substantial preventative effect. It is not possible to draw conclusions about the effectiveness of antidepressants for the prevention of postnatal depression until more studies are conducted including greater number of participants.
Overall completeness and applicability of evidence
This review includes two trials comparing antidepressants with placebo but we identified no studies comparing antidepressants with other preventative interventions or with standard care. Both trials examined a 17‐week treatment period with primary outcomes assessed immediately post treatment, meaning that it is not possible to draw conclusions about the optimal duration of antidepressant treatment for the prevention of postnatal depression. In addition, neither study addressed any of the secondary outcomes of this review regarding the effect of antidepressants on the infant or other family members, or secondary outcomes for the mother such as social functioning. In addition, neither study reported on the severity or course of depressive episodes, which means it is not possible to examine whether antidepressant use was associated with a milder symptom profile and course among women who did develop postnatal depression.
Recruitment rates were low and, where reported in Wisner 2001, under half of the women eligible for the study took part. This reflects many women's reluctance to take antidepressants in the perinatal period, observed in other studies and systematic reviews (Turner 2008; Molyneaux 2014), which may be particularly the case for prevention rather than treatment of depression. This issue was also highlighted by one trial identified through the searches of ClinicalTrials.gov which would have been eligible for this review but was terminated due to slow recruitment (NCT02235064).
In both of the studies that we included in this review, women were only eligible if they had a history of postnatal depression. This is unlikely to be a major limitation to the applicability of this evidence to clinical practice, as prophylactic antidepressant use in the postnatal period is most likely to be considered for this group of women. No studies were identified for this review in which antidepressants were started during pregnancy as a preventative intervention. Many women will be very unwilling to start antidepressants in pregnancy if they are not currently depressed; however, some women with a recent history of major depressive episodes may stay on their antidepressants throughout pregnancy and the postnatal period to prevent relapse.
This update of the review identified no new studies, despite the last published version of this review being completed over a decade ago. This suggests significant barriers to the completion of research in this area which may, for example, be related to the difficulties in recruiting pregnant women to randomised trials and the reluctance of many women to take antidepressants during pregnancy and breastfeeding. In addition, the lack of recent trials in this area is likely to reflect the fact that research and clinical practice is moving away from a specific focus on the prevention of postnatal depression to also consider the prevention and treatment of antenatal depression, including the continuation of antidepressants throughout pregnancy to prevent relapse of depression during pregnancy or the postnatal period. For example, one ongoing study is comparing continuation of antidepressants throughout pregnancy with preventative cognitive therapy and gradual discontinuation of antidepressants (Molenaar 2016). This pragmatic study would not meet the inclusion criteria for this systematic review, but should provide important data on whether continuation of antidepressants throughout pregnancy prevents relapse of depression. To maximise the clinical utility of future systematic reviews in this area, we recommend that reviews should examine the effectiveness of antidepressants for the prevention of perinatal depression more broadly (i.e. antenatal as well as postnatal), and should include trials investigating the effectiveness of the continuation of antidepressant use for the prevention of perinatal depression. Future studies should also consider the severity of women’s previous depression episodes, potentially stratifying by this in their randomisation and analyses. As described in Agreements and disagreements with other studies or reviews, evidence suggests that recurrence of depression after discontinuation of antidepressants may be more likely for women with a history of severe, or recent, episodes of depression.
Quality of the evidence
The studies included in this review do not allow a robust examination of the effectiveness of antidepressants in preventing postnatal depression. Only two eligible studies were identified, which included 73 participants in total in their main analyses. Both studies were generally well conducted, with effective double‐blinding for example; however neither trial was analysed on a 'once randomised, always analyse' basis as women who dropped out of the study after randomisation before taking any medication were excluded from the analyses. There was also drop‐out from both studies and, although the numbers dropping out were not high, this may have influenced results owing to the small overall sample sizes. Wisner 2001 stated that their results (that nortriptyline was not effective in preventing postnatal depression) were inconsistent with a pilot trial they had conducted on this topic that was unblinded and not randomised, which highlights the importance of high‐quality study methodology in this field. There was also inconsistency between the findings in Wisner 2001 and in Wisner 2004. Finally, both studies were conducted by the same research team, which is a limitation of the evidence base to date. Overall, the results of the studies included in this review do not allow conclusions to be drawn about the effectiveness of antidepressants for postnatal depression, but do highlight that it may be important to consider the effectiveness of different antidepressants separately.
Potential biases in the review process
We minimised potential bias in the selection of studies by employing consistent and predefined eligibility criteria. In order to reduce potential biases we also predefined our methods for extracting data and conducting risk of bias assessment; and conducted them in accordance with guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For example study screening, data extraction and risk of bias assessment were performed by two review authors independently. Publication bias may have influenced the findings of this review, as we did not search the grey literature and studies may be more likely to be published if they find that antidepressants are effective in preventing postnatal depression. However, we did search clinical trials' registries, which enabled us to identify relevant unpublished RCTs; this should limit any impact of publication bias. Overall, the review process is unlikely to have been biased, but it was limited by the extremely small number of relevant studies identified.
Agreements and disagreements with other studies or reviews
Previous systematic reviews of RCTs have shown that psychological and psychosocial interventions can be effective in preventing postnatal depression (Morrell 2016); and that antidepressants can reduce the risk of relapse among non‐pregnant adults with recent episodes of depression (Geddes 2003). There is also evidence from a Cochrane Review that antidepressants can be effective for treating postnatal depression (Molyneaux 2014). However, there have been very few trials of antidepressant prevention of postnatal depression and only two eligible RCTs were identified for this review. One of these trials suggested that antidepressants may be effective in preventing postnatal depression whereas the other trial found no evidence for this.
Related observational studies examining relapse following discontinuation of antidepressants during pregnancy have also had inconsistent findings. One prospective cohort study found a significantly higher rate of relapse among women who discontinued antidepressant medication during pregnancy than women who continued antidepressants (hazard ratio (HR) 5.0, 95% CI 2.8 to 9.1) (Cohen 2006). In contrast, another prospective cohort study found no association between antidepressant discontinuation and rate of relapse of depression during pregnancy or the first two months after delivery (adjusted HR 0.88, 95% CI 0.51 to 1.50) (Yonkers 2011). The difference in findings between these two studies may reflect differing severity of depression among participants. Yonkers 2011 recruited from obstetricians and general hospital settings, and found no difference in the rate of relapse related to antidepressant discontinuation. In contrast, Cohen 2006 recruited from specialist mental health centres and participants are therefore likely to have had more severe disorders (and were more likely to relapse following discontinuation of antidepressants). The importance of ensuring that treatment needs are met for women with severe depression or other mental disorders during pregnancy and the postnatal period was also highlighted in a recent study using UK data from women in contact with psychiatric services, which found that suicides in the perinatal period are more likely to occur among women not receiving any treatment at the time of death (Khalifeh 2016).
This Cochrane Review is an update and did not include any additional studies beyond the previous version of this review, published in 2005. The overall conclusion of the original version of this review remains: it is not possible to conclude whether or not antidepressants are effective in preventing postnatal depression.
Authors' conclusions
Implications for practice.
This systematic review only identified two completed studies of antidepressant prevention of postnatal depression. All participants in both studies had a history of postnatal depression, and were therefore at higher risk of experiencing postnatal depression again. It is not possible from these two studies to draw conclusions about the effectiveness of antidepressants in preventing postnatal depression. Decisions around the use of antidepressants for preventing postnatal depression need to be made through individual risk‐benefit analyses which take into account the preferences of the woman as well as severity and recency of previous episodes of depression.
Implications for research.
RCTs with larger sample sizes and longer‐term follow‐up are needed. It is critical that future trials assess side effects of antidepressant use for the fetus or infant, as well as for the mother. Both of the trials eligible for this review compared antidepressants with placebo, and studies comparing the effectiveness of antidepressants with evidence‐based psychological or psychosocial preventative interventions are also needed (such as cognitive behavioural therapy or interventions based on a person‐centred approach; Morrell 2016). No new studies were eligible for this update of the review, despite the last published version being completed over a decade ago. Future reviews in this area could broaden their scope to examine the effectiveness of antidepressants for the prevention of perinatal depression (i.e. antenatal as well as postnatal depression), and include trials investigating the effectiveness of the continuation of antidepressant use for the prevention of relapse of depression during the perinatal period.
What's new
| Date | Event | Description |
|---|---|---|
| 13 February 2018 | New search has been performed | The searches were updated on 13 February 2018 |
| 15 September 2016 | New citation required but conclusions have not changed | Review updated |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
| 31 January 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank the Cochrane Common Mental Disorders Group for their advice with this review.
CRG Funding Acknowledgement
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CCDANCTR update search ‐ Feb 2018
#1. antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* #2. Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Bupropion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 #3. Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or “St John*”) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (“Lu AA21004” or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* #4. Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone #5. (#1 or #2 or #3 or #4 or #5) #6. (depress* or dysthymi* or "adjustment disorder*" or "mood disorder*" or "affective disorder*" or "affective symptoms") AND (postpartum or "post partum" or post‐partum or postnatal or "post natal" or post‐natal or puerper*) #7. (#5 and #6)
Appendix 2. Earlier searches conducted to June 2007
Earlier searches, conducted for the first version of this review used the following search strategies (to 11‐June‐2007):
1. TheCCDANCTR‐Studies Register was searched using the following controlled search terms:
Diagnosis = Depression, Postpartum AND Intervention = (Antidepress* or "Monoamine Oxidase Inhibitors" or "Selective Serotonin Reuptake Inhibitors" or "Tricyclic Drugs" or Acetylcarnitine or Alaproclate or Amersergide or Amiflamine or Amineptine or Amitriptyline or Amoxapine or Befloxatone or Benactyzine or Brofaromine or Bupropion or Butriptyline or Caroxazone or Chlorpoxiten or Cilosamine or Cimoxatone or Citalopram or Clomipramine or Clorgyline or Clorimipramine or Clovoxamine or Deanol or Demexiptiline or Deprenyl or Desipramine or Dibenzipin or Diclofensine or Dothiepin or Doxepin or Duloxetine or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluparoxan or Fluvoxamine or Idazoxan or Imipramine or Iprindole or Iproniazid or isocarboxazid or Litoxetine or Lofepramine or Maprotiline or Medifoxamine or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nomifensine or Nortriptyline or Noxiptiline or Opipramol or Oxaflozane or Oxaprotiline or Pargyline or Paroxetine or Phenelzine or Piribedil or Pirlindole or Pivagabine or Prosulpride or Protriptyline or Quinupramine or Reboxetine or Rolipram or Sertraline or Setiptiline or SSRI* or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptine or Toloxatone or Tomoxetine or Tranylcypromine or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Viqualine or Zimeldine)
2. The CCDANCTR‐References Register was searched using a more sensitive set of free‐text terms to identify additional untagged/uncoded reports of RCTs:
Keyword = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder*" or "Affective Symptoms" AND Free‐text = Postpartum or "post partum" or post‐partum or Postnatal or "post natal" or post‐natal AND Free‐text = Antidepress* or "Monoamine Oxidase Inhibitors" or "Selective Serotonin Reuptake Inhibitors" or SSRI* or "Tricyclic Drugs" or Acetylcarnitin* or Alaproclat* or Amersergid* or Amiflamin* or Amineptin* or Amitriptylin* or Amoxapin* or Befloxaton* or Benactyzin* or Brofaromin* or Bupropion or Butriptylin* or Caroxazon* or Chlorpoxiten or Cilosamin* or Cimoxaton* or Citalopram or Clomipramin* or Clorgylin* or Clorimipramin* or Clovoxamin* or Deanol or Demexiptilin* or Deprenyl or Desipramin* or Dibenzipin or Diclofensin* or Dothiepin or Doxepin or Duloxetin* or Escitalopram or Etoperidon* or Femoxetin* or Fluotracen or Fluoxetin* or Fluparoxan or Fluvoxamin* or Idazoxan or Imipramin* or Iprindol* or Iproniazid or isocarboxazid or Litoxetin* or Lofepramin* or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemid* or Nefazodon* or Nialamid* or Nomifensin* or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozan* or Oxaprotilin* or Pargylin* or Paroxetin* or Phenelzin* or Piribedil or Pirlindol* or Pivagabin* or Prosulprid* or Protriptylin* or Quinupramin* or Reboxetin* or Rolipram or Sertralin* or Setiptilin* or Teniloxin* or Tetrindol* or Thiazesim or Thozalinon* or Tianeptin* or Toloxaton* or Tomoxetin* or Tranylcypromin* or Trazodon* or Trimipramin* or Venlafaxin* or Viloxazin* or Viqualin* or Zimeldin*
3. The Cochrane Central Register of Controlled Trials (CENTRAL) and the specialised register of the Cochrane Pregnancy and Childbirth Review Group were searched with the following terms: Postnatal or postpartum or puerper*
4. Searches for grey literature included:
The HSRProj in the National Library of Medicine, Washington D.C was checked for unpublished trials.
References from MIDIRS Midwifery Database was searched.
Current Controlled Trials web site: http://www.controlled trials.com/terms2.cfm was examined for ongoing studies.
The Science Citation Index was checked for references to all included studies.
Conference proceedings were examined where possible (details available from authors on request).
5. Reference lists The reference lists of all included studies were checked to identify additional studies missed from the original electronic searches (for example unpublished or in‐press citations). Relevant book chapters and their bibliographies were searched (details available from authors on request).
6. Correspondence Pharmaceutical companies were contacted directly for any relevant unpublished data. Contact was made with authors of identified trials and with experts in the field including a search for non‐English material (Professor L Appleby, Professor P Boyce, Dr T Brugha, Dr L Cohen, Dr N Glangeaud, Dr S Glasser, Dr M Marks, Dr A Wieck, Professor K Wisner). Contact with the Marcé Society through the newsletter was made. Self help groups were contacted (the National Childbirth Trust (NCT), The Association for Postnatal Illness, Postnatal Distress Association of Ireland, Postpartum Support International (PSI) and PaNDA).
Appendix 3. Update searches conducted to February 2018
Database: CENTRAL
Host: Wiley
Data Parameters: Issue 1 of 12, January 2018
Date searched: Tuesday, February 13th 2018
Searched by: Chris Cooper
Hits: 18
Search strategy:
ID Search Hits
#1 (postpartum or post partum or postnatal or post natal or puerper*) 9779
#2 MeSH descriptor: [Antidepressive Agents] explode all trees 5750
#3 (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or "re‐uptake")) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*) .mp. 1080
#4 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Amfebutamone or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or "St John*" or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or "LY2216684" or Edivoxetine or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone) 22191
#5 #2 or #3 or #4 24723
#6 #1 and #5 252
Notes: CENTRAL only = 18 records in date range 2016‐current
File: GS51 CENTRAL18.txt
Databases: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Host: OVID
Data Parameters: 1946 to Present
Date Searched: Tuesday, February 13th 2018
Searched by: Chris
Hits: 28
Search strategy:
Search Strategy:
| # | Searches | Results |
| 1 | exp Antidepressive Agents/ | 134025 |
| 2 | exp Neurotransmitter Uptake Inhibitors/ | 133352 |
| 3 | exp Monoamine Oxidase Inhibitors/ | 20964 |
| 4 | (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or "re‐uptake")) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. | 228950 |
| 5 | (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Amfebutamone or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or "St John*" or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or "LY2216684" or Edivoxetine or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).ti,ab,kw. | 76654 |
| 6 | 1 or 2 or 3 or 4 or 5 | 374868 |
| 7 | Depression, Postpartum/ | 4532 |
| 8 | ((postpartum or post partum or postnatal or post natal or puerper*) adj3 (depress* or dysthymi* or "adjustment disorder*" or "mood disorder*" or "affective disorder*" or "affective symptom*")).ti,ab,kw. | 5566 |
| 9 | 7 or 8 | 6988 |
| 10 | randomized controlled trial.pt. | 453387 |
| 11 | controlled clinical trial.pt. | 92150 |
| 12 | (randomized or randomised).ti,ab. | 517074 |
| 13 | (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kw. | 433913 |
| 14 | placebo.ab. | 186351 |
| 15 | ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy)).ti,ab,kw. | 156034 |
| 16 | (control* adj3 (trial or study or group*)).ti,ab,kw. | 692411 |
| 17 | (group* adj3 (allocat* or assign* or control* or divide* or division or distribut* or treat*)).ti,ab,kw. | 730860 |
| 18 | trial.ti,ab. | 491130 |
| 19 | 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 | 1726074 |
| 20 | 6 and 9 and 19 | 133 |
| 21 | (2016* or 2017* or 2018*).yr,dt,ed,ep. | 3328698 |
| 22 | 20 and 21 | 28 |
Notes: N/A
File: GS51MEDLINE28.txt
Database: Embase
Host: OVID
Data Parameters: 1974 to 2018 February 12
Date searched: Tuesday, February 13th 2018
Searched by: Chris Cooper
Hits: 50
Search strategy:
Search Strategy:
| # | Searches | Results |
| 1 | exp Antidepressant Agent/ | 401718 |
| 2 | Neurotransmitter Uptake Inhibitors/ | 155 |
| 3 | exp Monoamine Oxidase Inhibitor/ | 45668 |
| 4 | (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or "re‐uptake")) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. | 319952 |
| 5 | Psychopharmacology/ | 26480 |
| 6 | Psychotropic Agent/ | 27346 |
| 7 | Serotonin Receptor Affecting Agent/ or Serotonin Uptake Inhibitor/ or Serotonin Noradrenalin Reuptake Inhibitor/ or Triple Reuptake inhibitor/ | 46515 |
| 8 | Dopamine Receptor Affecting Agent/ or Dopamine Uptake Inhibitor/ | 1482 |
| 9 | Adrenergic Receptor Affecting Agent/ or Noradrenalin Uptake Inhibitor/ | 3576 |
| 10 | (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Amfebutamone or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or "St John*" or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or "LY2216684" or Edivoxetine or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).ti,ab,kw. | 100164 |
| 11 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 | 572354 |
| 12 | postnatal depression/ | 644 |
| 13 | ((postpartum or post partum or postnatal or post natal or puerper*) adj3 (depress* or dysthymi* or "adjustment disorder*" or "mood disorder*" or "affective disorder*" or "affective symptom*")).ti,ab,kw. | 7804 |
| 14 | 12 or 13 | 8082 |
| 15 | randomized controlled trial.de. | 486416 |
| 16 | randomization.de. | 76792 |
| 17 | placebo.de. | 318178 |
| 18 | placebo?.ti,ab. | 266278 |
| 19 | (randomized or randomised).ti,ab. | 725053 |
| 20 | double blind procedure/ | 146068 |
| 21 | (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kw. | 586635 |
| 22 | trial.ti. | 241798 |
| 23 | ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy)).ti,ab. | 211307 |
| 24 | (control* adj3 (trial or study or group*)).ti,ab. | 954393 |
| 25 | (group* adj3 (allocat* or assign* or control* or divide* or division or distribut* or treat*)).ti,ab. | 1039132 |
| 26 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 | 2251280 |
| 27 | 11 and 14 and 26 | 260 |
| 28 | (2016* or 2017* or 2018*).yr,dc. | 3762342 |
| 29 | 27 and 28 | 50 |
Notes: N/A
File: GS51 Embase50.txt
Database: PscyINFO
Host: OVID
Data Parameters: 1806 to February Week 1 2018
Date searched: Tuesday, February 13th 2018
Searched by: Chris Cooper
Hits: 44
Search strategy:
| # | Searches | Results |
| 1 | exp antidepressant drugs/ | 36266 |
| 2 | exp Neurotransmitter Uptake Inhibitors/ | 13404 |
| 3 | monoamine oxidase inhibitors/ | 1391 |
| 4 | (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or "re‐uptake")) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. | 65272 |
| 5 | (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Amfebutamone or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or "St John*" or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or "LY2216684" or Edivoxetine or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).ti,ab. | 32880 |
| 6 | 1 or 2 or 3 or 4 or 5 | 83585 |
| 7 | exp Postpartum Depression/ | 4151 |
| 8 | ((postpartum or post partum or postnatal or post natal or puerper*) adj3 (depress* or dysthymi* or "adjustment disorder*" or "mood disorder*" or "affective disorder*" or "affective symptom*")).ti,ab. | 4586 |
| 9 | 7 or 8 | 5597 |
| 10 | 6 and 9 | 370 |
| 11 | (2016* or 2017* or 2018*).yr,dc,mo. | 377668 |
| 12 | 10 and 11 | 44 |
Notes: N/A
File: GS51 PsycINFO44.txt
Data and analyses
Comparison 1. Nortriptyline versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of postpartum major depressive disorder | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.1. Analysis.
Comparison 1 Nortriptyline versus placebo, Outcome 1 Recurrence of postpartum major depressive disorder.
Comparison 2. Sertraline versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of postpartum major depressive disorde | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
2.1. Analysis.
Comparison 2 Sertraline versus placebo, Outcome 1 Recurrence of postpartum major depressive disorde.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Wisner 2001.
| Methods | Study design: randomized controlled trial. Method of randomisation not described. | |
| Participants |
Inclusion criteria: ≤ 35 weeks gestation, aged 21 to 45 years, history of at least 1 episode of postpartum major depressive disorder with onset of symptoms within 3 months of a live birth (beginning within 5 years prior to study enrolment).
Exclusion criteria: depression during the index pregnancy (i.e. not meeting the research diagnostic criteria for major depression); antidepressant use after the first trimester of the index pregnancy; meeting criteria for another axis 1 diagnosis (except generalised anxiety disorder or panic disorder); meeting criteria for antisocial or borderline personality disorder; history of bipolar disorder or psychosis; continuing use of psychotherapy or other psychotropic medications. 121 women were eligible and 56 women participated. 5 withdrew after randomisation (1 developed mania in 1st week postpartum and was withdrawn, 4 refused medication). 51 women were included in the main analyses (26 randomised to nortriptyline, 25 to placebo). |
|
| Interventions | Participants were randomly assigned to either: (1) Nortriptyline Treatment protocol: bedtime dose of 3 capsules given as soon as possible after birth, ideally within 24 hours, increase daily for 1 week postpartum, 20, 30, 40, 50, 60, 70 mg/day and 75 mg to day 21. Serum level from day 14 determined dose from then on to achieve 50 mg/ml to 150 mg/ml serum level. Dose tapered from week 17 at 33% per week and discontinued at week 20 postpartum. (2) Placebo Duration: same protocol for placebo as nortriptyline to help maintain blinding |
|
| Outcomes |
Timepoints for assessment: primary outcomes assessed post‐treatment (17 weeks after randomisation) Primary outcome: postnatal depression (recurrence and time to recurrence) |
|
| Notes |
Funding source: National Institute of Mental Health (NIMH) Declarations of interest among the primary researchers: Dr Wisner has received honoraria from Pfizer and Solvay. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "the primary study staff (nurse, mood symptom rater, coordinator, and principal investigator) were blind to medication assignment. The medication monitoring function (nurse) was separate from (and blind to the mood‐symptom monitoring." "We monitored the adequacy of the blind. Except for the nurse who monitored side effects, none of the other personnel were more successful than chance at identifying the drug assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind conditions" "The remainder of the protocol ... was identical in the nortriptyline and placebo groups." "The primary study staff (nurse, mood symptom rater, coordinator, and principal investigator) were blind to medication assignment. The medication monitoring function (nurse) was separate from (and blind to the mood‐symptom monitoring." "We monitored the adequacy of the blind. Except for the nurse who monitored side effects, none of the other personnel were more successful than chance at identifying the drug assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the primary study staff (nurse, mood symptom rater, coordinator, and principal investigator) were blind to medication assignment. The medication monitoring function (nurse) was separate from (and blind to) the mood‐symptom monitoring." "We monitored the adequacy of the blind. Except for the nurse who monitored side effects, none of the other personnel were more successful than chance at identifying the drug assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "of the 56 subjects, 4 declined to take the study drug after randomization. One subject (randomly assigned to nortriptyline) developed mania in the first postpartum week. Data for the clinical trial are derived from 51 subjects: 26 who received nortriptyline and 25 who received placebo. Four subjects withdrew from the protocol. Three subjects assigned to placebo withdrew because of side‐effects, personal reasons and pregnancy at 4, 10 and 14 weeks, respectively. One subject assigned to nortriptyline was lost to follow‐up at week 14. There was no difference in time to withdrawal of subjects in the nortriptyline or placebo groups (exact Savage test = 0.005, P = 0.91, df = 1)." |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting for important trial outcomes. |
| Other bias | Low risk | Quote: "five subjects who took nortriptyline were defined as noncompliant (serum nortriptyline level < 50ng/mL). Censoring of these subjects at the time of noncompliance did not change the results of the recurrence analysis (exact log‐rank < 0.00, P = 0.83)." |
Wisner 2004.
| Methods | Study design: randomized controlled trial. Method of randomisation: randomised in a 2:1 ratio for sertraline vs placebo; other details not given. | |
| Participants |
Inclusion criteria: pregnant women age 21 to 45, healthy with normal thyroid studies and blood count, < 35 weeks gestation at recruitment, at least 1 episode of postpartum major depression fulfilling DSM‐IV criteria within 5 years of study enrolment.
Exclusion criteria: depression during index pregnancy, use of psychotherapy or psychotropic medication after 1st trimester, other Axis 1 diagnoses (except general anxiety or panic disorder), antisocial or borderline personality disorder, or had psychosis or bipolar disorder. 38 eligible women screened, 25 consented to participate, 3 women randomised to sertraline never took the medication and were excluded from the analysis. 22 were participants included in the main analysis (14 randomised to sertraline and 8 randomised to placebo). |
|
| Interventions | Sertraline ‐ single post‐breakfast dose in 2 identical opaque gelatin capsules. Same protocol for placebo to help maintain blinding | |
| Outcomes |
Timepoints for Assessment: primary outcomes assessed post‐treatment (17 weeks after randomisation) Primary outcome: postnatal depression (recurrence and time to recurrence) |
|
| Notes |
Funding source: National Institute of Mental Health (NIMH) Declarations of interest among the primary researchers: Dr Wisner is on the speaker's bureau for Pfizer Inc, and the sertraline used in this study was donated by Pfizer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "the blind was continued until all subjects completed the protocol" "Sertraline or placebo was given as a single post‐breakfast dose in two identical opaque gelatin capsules" "None of the personnel or subjects was more successful than chance at identifying the drug assignment. The rates of agreement with the assigned condition ranged from 38% (for the blinded mood assessors) to 71% (for the side effects monitors), which did not differ significantly from the expected 44%–57% (Fisher’s exact test, P = 0.14–0.53)." "If the subject had a Hamilton depression scale score of 15 or higher on two occasions 1 week apart, she was evaluated by a blinded psychiatrist" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the blind was continued until all subjects completed the protocol" "Sertraline or placebo was given as a single post‐breakfast dose in two identical opaque gelatin capsules" "None of the personnel or subjects was more successful than chance at identifying the drug assignment. The rates of agreement with the assigned condition ranged from 38% (for the blinded mood assessors) to 71% (for the side effects monitors), which did not differ significantly from the expected 44%–57% (Fisher’s exact test, P = 0.14–0.53)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "if the subject had a Hamilton depression scale score of 15 or higher on two occasions 1 week apart, she was evaluated by a blinded psychiatrist" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "there was no difference in the number of women who withdrew from the study (P = 0.35, Fisher’s exact test) or in the time to withdrawal (P = 0.85, Freeman‐Halton extension of Fisher’s exact test) between subjects in the sertraline and placebo groups... In the 14 women assigned to sertraline, the one recurrence became manifest at week 17. Two women withdrew rapidly because of headaches, which resulted in the dose change already described. One subject was removed because of hypomania, and nine women completed the randomized clinical trial without recurrence." |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting for important trial outcomes. |
| Other bias | Low risk | Quote: "the women in the study were compliant, as evidenced by ranges of maternal serum sertraline and N‐desmethylsertraline levels of 23–48 and 36–66 ng/ml, respectively, across samples from the 8 weeks of collection." |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Appleby 1997 | Treatment study |
| Epperson 1996 | Treatment study |
| Heath 2000 | Treatment study |
| NCT02235064 | Terminated early due to slow recruitment; no available data |
| Sharp 2004 | Treatment study |
Characteristics of ongoing studies [ordered by study ID]
NCT00276900.
| Trial name or title | |
| Methods | Double‐blind, placebo‐controlled randomised controlled trial |
| Participants | Estimated enrolment: 300 |
| Interventions | Sertraline or placebo |
| Outcomes | Primary outcome: depressive symptoms (measured at 24 and 52 weeks postpartum). Secondary outcome: functioning (measured at 24 and 52 weeks postpartum) |
| Starting date | |
| Contact information | |
| Notes | This study is led by Katherine Wisner in the USA. The ClinicalTrials.gov record states that data collection is completed but it was not possible to obtain additional information about this study from the lead researcher. |
Differences between protocol and review
Protocol methods were updated from the previous version of this review to reflect current guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), for example to replace the CCDAN quality rating scale with the Cochrane 'Risk of bias' tool. Additional detail was also added to the original methods for clarity.
Contributions of authors
This is an update of a previous Cochrane Review. EM and LT undertook the selection of studies, data extraction, quality appraisal and interpretation of the data. All review authors contributed to the final draft of the review.
Sources of support
Internal sources
Health Services Research Dept, Institute of Psychiatry, UK.
External sources
No sources of support supplied
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Wisner 2001 {published data only}
- Gracious BL, Hanusa BH, Wisner KL, Peindl KS, Perel JM. Weight changes in postpartum women with remitted depression. Journal of Clinical Psychiatry 2005;66(3):291‐3. [DOI] [PubMed] [Google Scholar]
- Peindl KS. The use of nortriptyline for prevention of postpartum depression in a high‐risk group of women. 152nd Annual Meeting of the American Psychiatric Association, 1999 May 15‐20, Washington, DC. 1999.
- Peindl KS, Wisner KL. Successful recruitment strategies for women in postpartum mental health trials. Journal of Psychiatric Research 2003;37(2):117‐25. [DOI] [PubMed] [Google Scholar]
- Peindl KS, Wisner KL, Hanusa BH, Peindl KS. Identifying depression in the first postpartum year: Guidelines for office‐based screening and referral. Journal of Affective Disorders 2004;80(1):37‐44. [DOI] [PubMed] [Google Scholar]
- Wisner KL. Prevention and treatment of postpartum mood disorders. 152nd Annual Meeting of the American Psychiatric Association, 1999 May 15‐20, Washington, DC. 1999.
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Findling RL, Rapport D. Prevention of recurrent postpartum depression: a randomized clinical trial. Journal of Clinical Psychiatry 2001;62(2):82‐6. [DOI] [PubMed] [Google Scholar]
Wisner 2004 {published data only}
- Gracious BL, Hanusa BH, Wisner KL, Peindl KS, Perel JM. Weight changes in postpartum women with remitted depression. Journal of Clinical Psychiatry 2005;66(3):291‐3. [DOI] [PubMed] [Google Scholar]
- Sunder KR, Wisner KL, Hanusa BH, Perel JM. Postpartum depression recurrence versus discontinuation syndrome: observations from a randomized controlled trial. Journal of Clinical Psychiatry 2004;65(9):1266‐8. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Peindl KS, Perel JM, Hanusa BH, Plontek CM, Findling RL. Sertraline prevents postpartum depression. 156th Annual Meeting of the American Psychiatric Association, May 17‐22, San Francisco CA. 2003.
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of postpartum depression: a pilot randomized clinical trial. American Journal of Psychiatry 2004;161(7):1290‐2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Appleby 1997 {published data only}
- Appleby L. A controlled study of fluoxetine and cognitive ‐behavioural counselling in the treatment of postnatal depression. European Psychiatry 1998;13 Suppl 4:178s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive‐behavioural counselling in the treatment of postnatal depression. BMJ 1997;314(7085):932‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epperson 1996 {published data only}
- Epperson CN, McDougle CJ, Ward‐O'Brien D, Price LH. A controlled study of antidepressant treatment of postpartum depression. 149th Annual Meeting of the American Psychiatric Association; 1996 May 4‐9; New York, NY. 1996.
Heath 2000 {published data only}
- Heath AC, Yonkers KA, Rush AJ. Paroxetine in the treatment of postpartum depression. 153rd Annual Meeting of the American Psychiatric Association, 2000 May 13‐18, Chicago, (IL). 2000.
NCT02235064 {published data only}
- Fischer RL, Simpkins GK. Prophylactic Use of Postpartum Sertraline to Prevent Postpartum Depression. clinicaltrials.gov/ct2/show/NCT02235064 (accessed 30 October 2017). [NCT02235064]
Sharp 2004 {published data only}
References to ongoing studies
NCT00276900 {unpublished data only}
- Wisner K. Sertraline for the Prevention of Recurrent Postpartum Depression [Prevention of Recurrent Postpartum Depression]. clinicaltrials.gov/ct2/show/NCT00276900 2006.
Additional references
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatry Association, 2013. [Google Scholar]
Bland 2000
- Bland JM, Altman DG. The odds ratio. BMJ 2000;320(7247):1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2006
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of Major Depression During Pregnancy in Women Who Maintain or Discontinue Antidepressant Treatment. JAMA 2006;295(5):499. [DOI] [PubMed] [Google Scholar]
Cox 1982
- Cox J, Connor Y, Kendell RE. Prospective study of the psychiatric disorders of childbirth. British Journal of Psychiatry 1982;140:111‐7. [DOI] [PubMed] [Google Scholar]
Cox 1987
- Cox JL, Holden J, Sagovsky R. Detection of postnatal depression: development of the 10‐item Edinburgh Postnatal Depression Scale (EPDS). British Journal of Psychiatry 1987;150:782‐6. [DOI] [PubMed] [Google Scholar]
Dennis 2007
- Dennis C‐L, Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database of Systematic Reviews 2007, Issue 10. [DOI: 10.1002/14651858.CD006116.pub2] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fournier 2010
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant Drug Effects and Depression Severity. A Patient‐Level Meta‐analysis. JAMA 2010;303(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geddes 2003
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003;361(9358):653‐61. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howard 2006
- Howard LM, Boath E, Henshaw C. Antidepressant Prevention of Postnatal Depression. PLOS Medicine 2006;3(10):e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2014
- Howard LM, Molyneaux E, Dennis C‐L, Rochat T, Stein A, Milgrom J. Non‐psychotic mental disorders in the perinatal period. Lancet 2014;384(9956):1775–88. [DOI] [PubMed] [Google Scholar]
Khalifeh 2016
- Khalifeh H, Hunt IM, Appleby L, Howard LM. Suicide in perinatal and non‐perinatal women in contact with psychiatric services: 15 year findings from a UK national inquiry. Lancet Psychiatry 2016;3(3):233‐42. [DOI] [PubMed] [Google Scholar]
Molenaar 2016
- Molenaar NM, Brouwer ME, Bockting CL, Bonsel GJ, Veere CN, Torij HW, et al. Stop or go? Preventive cognitive therapy with guided tapering of antidepressants during pregnancy: study protocol of a pragmatic multicentre non‐inferiority randomized controlled trial. BMC Psychiatry 2016;16:72. [DOI: 10.1186/s12888-016-0752-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molyneaux 2014
- Molyneaux E, Howard LM, McGeown HR, Karia AM, Trevillion K. Antidepressant treatment for postnatal depression. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD002018.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrell 2016
- Morrell CJ, Sutcliffe P, Booth A, Stevens J, Scope A, Stevenson M, et al. A systematic review, evidence synthesis and meta‐analysis of quantitative and qualitative studies evaluating the clinical effectiveness, the cost‐effectiveness, safety and acceptability of interventions to prevent postnatal depression. Health Technology Assessment (Winchester, England) 2016;20(37):1‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2009
- NICE. Depression: the Treatment and Management of Depression in Adults (Update). NICE clinical guideline 90, 2009. [Google Scholar]
Sharp 2010
- Sharp DJ, Chew‐Graham C, Tylee A, Lewis G, Howard L, Anderson I, et al. A pragmatic randomised controlled trial to compare antidepressants with a community‐based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technology Assessments 2010;14(43):1‐153. [DOI] [PubMed] [Google Scholar]
Spitzer 1978
- Spitzer R, Endicott J, Robins E. Research Diagnostic Criteria; rationale and reliability. Archives of General Psychiatry 1978;35(6):773‐82. [DOI] [PubMed] [Google Scholar]
Stein 2014
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014;384(9956):1800‐19. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2008
- Turner KM, Sharp D, Folkes L, Chew‐Graham C. Women's views and experiences of antidepressants as a treatment for postnatal depression: a qualitative study. Family Practice 2008;25(6):450‐5. [DOI] [PubMed] [Google Scholar]
Wisner 2013
- Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, et al. Onset timing, thoughts of self‐harm, and diagnoses in postpartum women with screen‐positive depression findings. JAMA Psychiatry 2013;70(4):490‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Health Organization 2004
- World Health Organization. The ICD‐10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guideline. Geneva, World Health Organization, 2004. [Google Scholar]
Yonkers 2008
- Yonkers KA, Lin H, Howell HB, Heath AC, Cohen LS. Pharmacologic treatment of postpartum women with new‐onset major depressive disorder: a randomized controlled trial with paroxetine. Journal of Clinical Psychiatry 2008;69(4):659‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yonkers 2011
- Yonkers KA, Gotman N, Smith MV, Forray A, Belanger K, Brunetto WL, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy?. Epidemiology 2011;22(6):848‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]


