Abstract
Background
Necrotizing soft tissue infections (NSTIs) are severe and rapidly spreading soft tissue infections of the subcutaneous tissue, fascia, or muscle, which are mostly caused by bacteria. Associated rates of mortality and morbidity are high, with the former estimated at around 23%, and disability, sequelae, and limb loss occurring in 15% of patients. Standard management includes intravenous empiric antimicrobial therapy, early surgical debridement of necrotic tissues, intensive care support, and adjuvant therapies such as intravenous immunoglobulin (IVIG).
Objectives
To assess the effects of medical and surgical treatments for necrotizing soft tissue infections (NSTIs) in adults in hospital settings.
Search methods
We searched the following databases up to April 2018: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched five trials registers, pharmaceutical company trial results databases, and the US Food and Drug Administration and the European Medicines Agency websites. We checked the reference lists of included studies and reviews for further references to relevant randomised controlled trials (RCTs).
Selection criteria
RCTs conducted in hospital settings, that evaluated any medical or surgical treatment for adults with NSTI were eligible for inclusion. Eligible medical treatments included 1) comparisons between different antimicrobials or with placebo; 2) adjuvant therapies such as intravenous immunoglobulin (IGIV) therapy compared with placebo; no treatment; or other adjuvant therapies. Eligible surgical treatments included surgical debridement compared with amputation, immediate versus delayed intervention, or comparisons of number of interventions.
RCTs of hyperbaric oxygen (HBO) therapy for NSTI were ineligible because HBO is the focus of another Cochrane Review.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. The primary outcome measures were 1) mortality within 30 days, and 2) proportion of participants who experience a serious adverse event. Secondary outcomes were 1) survival time, and 2) assessment of long‐term morbidity. We used GRADE to assess the quality of the evidence for each outcome.
Main results
We included three trials randomising 197 participants (62% men) who had a mean age of 55 years. One trial compared two antibiotic treatments, and two trials compared adjuvant therapies with placebo. In all trials, participants concomitantly received standard interventions, such as intravenous empiric antimicrobial therapy, surgical debridement of necrotic tissues, intensive care support, and adjuvant therapies. All trials were at risk of attrition bias and one trial was not blinded.
Moxifloxacin versus amoxicillin‐clavulanate
One trial included 54 participants who had a NSTI; it compared a third‐generation quinolone, moxifloxacin, at a dose of 400 mg given once daily, against a penicillin, amoxicillin‐clavulanate, at a dose of 3 g given three times daily for at least three days, followed by 1.5 g three times daily. Duration of treatment varied from 7 to 21 days. We are uncertain of the effects of these treatments on mortality within 30 days (risk ratio (RR) 3.00, 95% confidence interval (CI) 0.39 to 23.07) and serious adverse events at 28 days (RR 0.63, 95% CI 0.30 to 1.31) because the quality of the evidence is very low.
AB103 versus placebo
One trial of 43 randomised participants compared two doses, 0.5 mg/kg and 0.25 mg/kg, of an adjuvant drug, a CD28 antagonist receptor (AB103), with placebo. Treatment was given via infusion pump for 10 minutes before, after, or during surgery within six hours after the diagnosis of NSTI. We are uncertain of the effects of AB103 on mortality rate within 30 days (RR of 0.34, 95% CI 0.05 to 2.16) and serious adverse events measured at 28 days (RR 1.49, 95% CI 0.52 to 4.27) because the quality of the evidence is very low.
Intravenous immunoglobulin (IVIG) versus placebo
One trial of 100 randomised participants assessed IVIG as an adjuvant drug, given at a dose of 25 g/day, compared with placebo, given for three consecutive days. There may be no clear difference between IVIG and placebo in terms of mortality within 30 days (RR 1.17, 95% CI 0.42 to 3.23) (low‐certainty evidence), nor serious adverse events experienced in the intensive care unit (ICU) (RR 0.73 CI 95% 0.32 to 1.65) (low‐certainty evidence).
Serious adverse events were only described in one RCT (the IVIG versus placebo trial) and included acute kidney injury, allergic reactions, aseptic meningitis syndrome, haemolytic anaemia, thrombi, and transmissible agents.
Only one trial reported assessment of long‐term morbidity, but the outcome was not defined in the way we prespecified in our protocol. The trial used the Short Form Health Survey (SF36). Data on survival time were provided upon request for the trials comparing amoxicillin‐clavulanate versus moxifloxacin and IVIG versus placebo. However, even with data provided, it was not possible to perform survival analysis.
Authors' conclusions
We found very little evidence on the effects of medical and surgical treatments for NSTI. We cannot draw conclusions regarding the relative effects of any of the interventions on 30‐day mortality or serious adverse events due to the very low quality of the evidence.
The quality of the evidence is limited by the very small number of trials, the small sample sizes, and the risks of bias in the included trials. It is important for future trials to clearly define their inclusion criteria, which will help with the applicability of future trial results to a real‐life population.
Management of NSTI participants (critically‐ill participants) is complex, involving multiple interventions; thus, observational studies and prospective registries might be a better foundation for future research, which should assess empiric antimicrobial therapy, as well as surgical debridement, along with the placebo‐controlled comparison of adjuvant therapy. Key outcomes to assess include mortality (in the acute phase of the condition) and long‐term functional outcomes, e.g. quality of life (in the chronic phase).
Plain language summary
Treatments for necrotizing (i.e. destructive) soft tissue infections in adults
What is the aim of this Cochrane Review?
We wanted to find out which medicines and surgical treatments are effective and safe for treating necrotizing soft tissue infections (NSTI). NSTI are serious infections of the tissues underneath the skin, mostly caused by bacteria.
Key messages
The available evidence from three studies is not strong enough to enable us to draw definite conclusions about the effectiveness and safety of the different treatments for NSTI assessed in this review. All studies assessed number of deaths and risk of serious side effects.
Factors affecting our confidence in the results included the following:
‐ the small number of trials and participants; ‐ weaknesses in the trial methodologies which affect the reliability of results; and ‐ poor definition of the participants’ condition.
We found no evidence that assessed antimicrobial therapy (which targets a wide range of disease‐causing bacteria and fungi) or surgical removal of damaged tissue.
In future studies, risk of death should be a key outcome in the short term (i.e. within 30 days) phase of the condition, and outcomes such as loss of work and quality of life should be assessed in the long‐term phase (after 30 days).
What was studied in the review?
We included people with NSTI. These types of infections are rare, but can become life‐threatening if left untreated, or result in amputation. NSTIs need emergency treatment, usually with antibiotics and surgical removal of the infected tissue.
We searched for studies that assessed treatments for diagnosed NSTI in hospitalised adults. This included:
‐ surgical treatments: surgical removal of damaged tissue compared with amputation, immediate versus delayed treatment, or comparison of a number of treatments; ‐ antimicrobial medicines ‐ which kill bacteria and fungi ‐ compared with placebo (i.e. an identical but inactive treatment), or each other; ‐ medicines given as add‐on therapies in addition to the primary treatment (adjuvant therapies) compared with placebo, no treatment, or other adjuvant therapies.
Our main outcomes of interest were death within 30 days, and any serious treatment side effects.
What are the main results of the review?
We found three studies, which enrolled 197 adults (117 men, average age = 55). The trials were conducted worldwide, funded by pharmaceutical companies; they assessed antimicrobial therapy or treatments that control the immune system.
One study compared two antibiotics: moxifloxacin and amoxicillin‐clavulanate, administered directly into a vein for seven to 21 days. It found no clear difference between the treatment groups in terms of number of deaths within 30 days, but we are uncertain about this result because it is based on very low‐certainty evidence.
One study compared placebo with a new type of treatment that controls immune response (called AB103) given in a single dose (of either 0.5 mg/kg or 0.25 mg/kg), administered directly into a vein. Participants also received standard treatment for NSTI based on antibiotics and surgical treatment, so AB103 was given as an adjuvant therapy. There was no clear difference between the treatment groups in terms of number of deaths within 30 days, but we are uncertain about this conclusion because it is based on very low‐certainty evidence.
One study compared injections of immunoglobulin (an antibody, part of the body’s immune system) with placebo. Both treatments were given for three consecutive days. Participants also received standard treatment for NSTI based on antibiotics and surgical treatment, thus immunoglobulin was given as an adjuvant therapy. There was no clear difference between the treatment groups in terms of the number of deaths within 30 days (low‐certainty evidence).
No study showed any clear difference between treatments in terms of serious side effects, but the evidence is not strong enough to confirm this. The immunoglobulin study listed the side effects encountered, which included kidney injury, allergic reactions, meningitis, blood clots, and infectious agents (low‐certainty evidence).
Only one trial reported assessment of long‐term illness but it was not defined as we had required in our in the protocol (the trial used another scale: the Short Form Health Survey (SF36). Survival time was reported in two trials (but not enough data were provided to analyse these results).
How up‐to‐date is this review?
We searched for studies published up to April 2018.
Summary of findings
Background
Please see an explanation of the terms we have used in Table 4.
1. Glossary of terms used.
| Term used | Explanation |
| Adjuvant treatment | Treatment that is given in addition to the primary or initial therapy to improve its effectiveness |
| Empiric antimicrobial therapy | Antimicrobial therapy given before the specific bacteria causing an infection is known |
| Aseptic meningitis | Serious inflammation of the linings of the brain not caused by pyogenic bacteria |
| Empiric antibiotic therapy | Antibiotics that acts against a wide range of bacteria |
| Bullae | Blisters on the skin usually more than 5 mm in diameters |
| Cirrhosis | Advanced liver disease |
| Crepitus | Clinical signs characterised by a peculiar sound under the skin |
| Debridement | Surgery excision of necrotic tissues (medical removal of dead, damaged, or infected tissue) |
| Endotoxin | A toxin contained in bacteria that is released only when the bacteria are broken down |
| Exotoxin | A toxin that is secreted by bacteria into the surrounding medium |
| Fascia | A fibrous connective tissue that surrounds muscle and other soft tissue. Fasciae are classified according to their distinct layers and their anatomical location: superficial fascia and deep (muscle) fascia |
| Fulminant inflammatory response | Systemic inflammatory response |
| Gram‐negative bacteria | Class of bacteria gram‐negative staining |
| Haemolytic anaemia | Decrease in the total amount of red blood cells due to the abnormal breakdown of red blood cells |
| Hyperbaric oxygen therapy | Medical use of oxygen at a level higher than atmospheric pressure. This helps fight bacteria and infection |
| Hypoxia | Insufficient levels of oxygen in blood or tissue |
| Intravenous immunoglobulin (IVIG) | Administration of antibodies through the veins |
| Motricity | Strength in upper and lower extremities after disease |
| Morbidity | Disability or degree that the health condition affects the patient |
| Mortality | Death rate |
| MRSA | Methicillin‐resistant Staphylococcus aureus |
| Myonecrosis | The destruction or death of muscle tissue |
| Necrosis | Death of body tissue |
| Obliterating endarteritis | Severe proliferating endarteritis (inflammation of the inner lining of an artery) that results in an occlusion of the lumen (the space inside a tubular structure) of the smaller vessels |
| Person‐years | Unit of measurement used to estimate rate of a disease during a defined period of observation |
| Polymicrobial | Polymicrobial infection is caused by several species of micro‐organisms |
| Subcutaneous tissue | Layer of tissue below the epidermis and the dermis of the skin. It is also called the hypodermis |
| Synergistic combination | Additive effects of bacterial agents |
| Synergistic gangrenes | Necrotizing soft tissue infection caused by a mix of bacteria (usually a mix of anaerobic and aerobic micro‐organisms) |
| Systemic | Affecting the entire body |
| Third‐generation quinolones | The quinolones are a family of synthetic broad‐spectrum antibiotic drugs |
| Thrombi | A blood clot inside a blood vessel |
| Transmissible agents | Infectious pathogens that can be transmitted |
| Vasopressors | Any medication that induces vasoconstriction of blood vessels to raise reduced blood pressure |
| Vimentin | A protein, the expression of which is increased after skeletal muscle injury |
Description of the condition
Definition
Necrotizing soft tissue infections (NSTIs) are severe life‐threatening, rapidly‐spreading, soft tissue infections of the subcutaneous tissue, fascia, or muscle mostly caused by bacteria (Anaya 2007; May 2009; Stevens 2014). Multiple descriptions of NSTIs have been published, which have used a wide range of different and confusing terms, regardless of anatomical location, microbiological characteristics, or depth of infection (Dellinger 1981). For example, necrotizing fasciitis occurring in the scrotum has been specifically labelled Fournier's gangrene (Fournier 1883). The term 'fasciitis' introduced in 1952 by Wilson gave the misleading impression that the muscular fascia is involved (Wilson 1952), whereas the fascia most commonly concerned is the superficial fascia, which comprises all the tissues between the skin and the underlying muscles (i.e. subcutaneous tissue) (Stevens 2005). Although different types of necrotizing infections have been described, they share the same principles for diagnosis and treatment strategies needing emergency surgery. To encompass all necrotizing infections (necrotizing fasciitis, Fournier's gangrene, synergistic gangrenes, gas gangrene, necrotizing cellulitis, myonecrosis), the term 'necrotizing soft tissue infections' has been proposed (Anaya 2007; Sartelli 2014; Stevens 2014).
Incidence
Data regarding the incidence of NSTI are scarce (Table 5). The reported incidence of NSTI during a five‐year period, estimated using an insurance database in the USA, was four cases per 100,000 person‐years (Ellis Simonsen 2006). The Centers for Disease Control and Prevention (CDC) reported an incidence of 500 to 1500 cases yearly (CDC 2012). More data are available for NSTI caused by group A Streptococcus (GAS). For instance, a population‐based survey of GAS‐NSTI in Canada showed an incidence ranging from 0.1 to 0.4 per 100,000 person‐years. The incidence of invasive GAS infections ranged from 2.4 to 3.1 per 100,000 person‐years, and this varied according to the period of the year and the countries studied (Kaul 1997; Lamagni 2008; Lepoutre 2011; O'Grady 2007).
2. Incidence of necrotizing fasciitis.
| Incidence of necrotizing fasciitis | ||||
| Authors | Period of study | Country | Pathology | Incidence |
| Kaul R et al (Kaul 1997) | 1991 | Canada | GAS NF | 0.085 per 100,000 p‐y |
| 1995 | 0.4 per 100,000 p‐y | |||
| Ellis Simonsen et al (Ellis Simonsen 2006) | January 1997 to December 2002 | United States | NF | 0.04 per 1000 p‐y |
| O'Grady et al (O'Grady 2007) | March 2002 to August 2004 | Australia | IGAS | 2.7 per 100,000 p‐y (10.9% of NF) |
| Lamagni et al (Lamagni 2008) | January 2003 to December 2004 | Europe | IGAS | 2.37 per 100,000 p‐y (8% of NF) |
| Lepoutre et al (Lepoutre 2011) | November 2006 to November 2007 | France | IGAS | 3.1 per 100,000 p‐y (18% of NF) |
GAS NF:group A streptococcal necrotizing fasciitis; IGAS: invasive group A streptococcal disease; NF: necrotizing fasciitis; p‐y: person‐years
Clinical features
Necrotizing soft tissue infections can affect any part of the body, but the extremities are most commonly involved (73%), followed by the trunk (13%) and the perineum (12.6%) (Goh 2014).
Presentation of the condition varies widely, ranging from limited to extensive skin and subcutaneous necrosis, possibly associated with life‐threatening sepsis. When clinical "hard signs" of NSTI are present (e.g. crepitus, skin necrosis, bullae, gas in the tissue, skin anaesthesia, and symptoms of sepsis), then establishing a diagnosis is not difficult (Stevens 2014). On the contrary, in some cases, distinction between non‐necrotizing infections and NSTIs can be difficult because of only non‐specific (e.g. pain, oedema, erythema) symptoms. A study in 2009 estimated that 50% of people with NSTIs were misdiagnosed upon hospital admission (May 2009).
A severe pain, disproportionate to skin symptoms, should raise suspicion for the diagnosis of NSTI. Futhermore, an apparent non‐necrotizing infection that does not respond to appropriate antibiotic therapy should raise suspicions of NSTI, especially in diabetic patients (Anaya 2007; Chosidow 2001).
Surgical exploration is considered as the gold standard for confirming diagnosis of NSTI. Surgeons identify intraoperative macroscopic findings consistent with NSTI including: 'gray' necrotic tissue, lack of bleeding, thrombosed vessels, 'dishwater' pus, non contracting muscle, and a positive 'finger test' result, which is characterised by lack of resistance to finger dissection in normally adherent tissues (Stevens 2014).
Pathophysiology and microbiology
Necrotizing soft tissue infections primarily involve the superficial fascia with extensive deterioration of the surrounding tissue. Most NSTI are caused by bacteria, but some cases of NSTI are caused by fungi (zygomycetes, Candida) have also been reported, mostly in immunocompromised patients (Lamb 2015). Endotoxins and exotoxins produced by bacteria induce a fulminant inflammatory response, which leads to obliterating endarteritis; thrombosis; tissue necrosis; and often, systemic illness with sepsis and multisystem organ failure (Johansson 2010). Polymicrobial forms involve a synergistic combination of aerobic and anaerobic bacterial species and are more frequently encountered than monomicrobial forms, which occur in 25% to 45% of NSTI (Elliott 2000; McHenry 1995; Sarani 2009; Wong 2003). Two recent retrospective studies have described the microbiology of polymicrobial infections (Anaya 2005; Anaya 2007). Micro‐organisms commonly retrieved in polymicrobial infections are Staphylococcus aureus (16% to 22%); Streptococcus species (17% to 19%); gram‐negative bacteria, including Escherichia coli (E. coli) and Klebsiella species (17% to 18%); and anaerobic bacteria (7% to 18%) (Anaya 2005; Anaya 2007; Sarani 2009). In a retrospective study of 182 patients with NSTI, an average of 4.4 species per patient was found (Elliott 2000). Diabetes, peripheral vascular disease, gastrointestinal perforation, or gastro‐urinary tract infections are common risk factors for polymicrobial NSTI (Stevens 2014). In monomicrobial forms, the most frequent pathogens are gram‐positive cocci:Streptococcus pyogenes andStaphylococcus aureus (Anaya 2007; Sarani 2009). However, cases of monomicrobial infections caused by gram‐negative bacteria, e.g. Aeromonas spp,Vibrio spp,E. coli, andPseudomonas aeruginosa have frequently been reported. Cirrhosis, seawater, and freshwater exposure are risk factors of NSTI caused by Aeromonas spp and Vibrio spp. (Hsiao 2008; Park 2009).
Causes and risk factors
In 80% of cases, infections develop from an initial skin lesion (traumatic wounds, insect bite, injection site, surgical wounds, perianal sources) (Stevens 2014), but 20% of patients have no visible skin lesion. In infections caused by group A Streptococcus (GAS), nearly 50% of patients have no defined portal of entry, and the infection process usually begins at the site of a prior blunt skin trauma, such as a muscle strain (Adams 1985; Stevens 1989; Stevens 2014). Bryant 2006 suggested that vimentin expression by muscle cells was increased after blunt trauma and that this then mediated focal adhesion of GAS, thereby, facilitating infection. A study of 257 patients showed blunt trauma to be significantly associated with the development of GAS necrotizing fasciitis (odds ratio (OR) 5.97, 95% confidence interval (CI) 1.04 to 34.16) (Nuwayhid 2007). General risk factors for NSTI include diabetes mellitus, peripheral vascular disease, chronic renal failure, intravenous drug use, alcoholism, immunosuppression, and obesity (Elliott 1996; Hasham 2005; Phan 2010). Diabetes was the most common pre‐existing condition found in 44.5% of patients with NSTI (Goh 2014).
The impact of necrotizing soft tissue infections
Systemic toxicity, which may progress rapidly to multiple organ failure and death, often accompanies fulminant tissue necrosis associated with NSTI (Sarani 2009). The overall mortality rate of NSTI was 23.5% in a review of 67 studies from 1980 to 2008, which included 3302 patients (May 2009). There was only a slight downward trend of mortality, from 27.8% to 21.7% for studies published between 1980 and 1999 and studies between 1999 and 2008 (May 2009).
Patients who survive require prolonged hospitalisation (38.5 ± 16 days) and additional surgery for reconstruction (87%) (Pham 2009). A meta‐analysis of eight studies reported the amputation rate as 16% (Goh 2014). A study performed to evaluate functional outcomes of surviving patients reported a mild to severe functional limitation in 30% of patients, according to the grading scale established by the American Medical Association Guides to the Evaluation of Permanent Impairment (Pham 2009).
Multiple factors associated with mortality have been demonstrated, including advanced age, comorbidities, shock on admission, acute renal failure, and microbiological characteristics. However, the only potentially modifiable reported risk factor associated with mortality is time to surgical debridement (Anaya 2005; Childers 2002; Elliott 2000; McHenry 1995). Indeed, several studies have established the impact of delayed surgical treatment of NSTI on mortality and morbidity (Bilton 1998; Boyer 2009; Elliott 2000; Kobayashi 2011; Wong 2003).
Description of the intervention
Current guidelines recommend early surgical debridement with excision of all necrotic and infected tissue until healthy tissue is exposed (Stevens 2014). Patients should be taken back to the operating room 24 hours after the first debridement to ensure that the spread of infection has been stopped and to assess whether further debridement is required (May 2009). The mean number of surgical debridements reported in a review of six retrospective studies was three per patient (Goh 2014).
Intravenous empiric antimicrobial therapy and intensive care support is generally started at the same time as surgery (Stevens 2014). The guidelines of the French Society of Dermatology (SFD) and the French Infectious Diseases Society (SPILF) for community‐acquired NSTIs of the limbs recommend a combination of penicillin (2 to 4 million units every four to six hours) plus clindamycin (600 mg every four to six hours) plus aminoglycoside (25 mg/kg/day), and for community‐acquired mixed infections, a combination of piperacillin‐tazobactam (4 g every six to eight hours) plus aminoglycoside plus clindamycin (SFD‐SPILF 2000). For empiric treatment of community‐acquired mixed infections, the guidelines of the Infectious Diseases Society of America (IDSA) recommend using agents effective against both aerobes, including methicillin‐resistantStaphylococcus aureus (MRSA), and anaerobic organisms, such as a combination of piperacillin‐tazobactam (3.37 g every six to eight hours) plus vancomycin (30 mg/kg/day) (Stevens 2014). Antibiotics are targeted at the commonly involved pathogens, such as those recommended in the aforementioned guidelines, until repeated operative procedures are no longer needed and clinical improvement has been obtained for 48 to 72 hours (Stevens 2014). The mean time duration of antimicrobial therapy is between 14 to 21 days. The use of clindamycin for NSTI due to GAS to block exotoxin production and to overcome high bacterial inocula is also recommended based on observational studies (Stevens 2014).
Reconstructive surgery by skin graft or tissue transfer may be considered only after removal of all necrotic tissues and the infection is controlled (Sarani 2009).
Adjunctive therapies, such as hyperbaric oxygen (HBO) therapy and intravenous immunoglobulin (IVIG), have also been proposed (Linner 2014; Norrby‐Teglund 2005; Wilkinson 2004). Clinical data underpinning a role for HBO therapy are very poor quality and are based on uncontrolled observational case series. A recent Cochrane Review failed to locate relevant clinical evidence to support the effectiveness of HBO therapy in the management of necrotizing fasciitis (Levett 2015). The use of IVIG has been evaluated in GAS toxic shock syndromes in observational studies with controversial results (Linner 2014; Norrby‐Teglund 2005). Therefore, their application (IVIG, HBO) as adjunctive therapies is not recommended (Stevens 2014).
How the intervention might work
Early operative surgery provides complete debridement of necrotic and infected tissues. Antibiotics prevent and treat the systemic diffusion of the infection, but tissue hypoxia and necrosis limit their efficacy. Indeed, previous studies have shown that antibiotic therapy is only an adjunct therapy to surgical treatment, which is necessary in patients with NTSI (Anaya 2005; Lamb 2015; Stevens 2005). Intensive care support, including fluid resuscitation, vasopressors, and mechanical ventilation, helps to restore intravascular volume and maintain adequate organ perfusion. The use of IVIG in cases of NSTI caused by group A streptococci could neutralise the circulating streptococcal exotoxins and thus reduce the toxin‐induced tissue necrosis (Norrby‐Teglund 1998). The rationale for using HBO would be to increase the tissue oxygen tension of infected hypoxic areas and prevent further extension of infection (Korhonen 2000).
Why it is important to do this review
Necrotizing soft tissue infection is a rare but life‐threatening infectious disease, which needs to be managed by a multidisciplinary team (dermatologist, plastic surgeon, intensive care physician, infectious disease specialist). In view of both the morbidity and mortality associated with NSTI, it is important to evaluate the evidence for medical and surgical interventions. We hope our review has highlighted gaps in the evidence and any need for future research in this area.
The plans for this review were published as a protocol 'Interventions for necrotizing soft tissue infections in adults' (Hua 2015).
Objectives
To assess the effects of medical and surgical treatments for necrotizing soft tissue infections (NSTI) in adults in hospital settings.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials with randomisation at the individual level. Cluster‐randomised trials were eligible for inclusion. We excluded cross‐over studies.
Types of participants
We included adults aged 18 years and over hospitalised with a diagnosis of necrotizing soft tissue infections (NSTI) that is defined as a soft‐tissue infection characterised by rapidly spreading inflammation and subsequent necrosis of the muscle, fascia, or subcutaneous tissue (Chelsom 1994). We placed no restrictions based on the number of participants for study selection.
Studies using a broader diagnostic category, such as 'skin and soft tissue infections' or 'complicated skin and soft tissue infections' (cSSTI), were included if a specific subgroup with NSTIs with separated results were available.
We excluded neutropenic participants with a blood neutrophil count < 500/mm³, as well as participants with cervicofacial necrotizing infections, because both need to be managed using specific therapeutic strategies.
Types of interventions
This review focused on any treatment given for NSTI during hospitalisation including the following.
Medical treatments
Antibiotics compared with placebo or compared with each other (different drug(s), different duration of therapy).
Adjuvant therapies, such as intravenous immunoglobulin (I) therapy, compared with placebo, no treatment, or other adjuvant therapies.
Surgical treatments
Surgical debridement compared with amputation or immediate versus delayed intervention or comparison of number of interventions.
We did not include hyperbaric oxygen (HBO) therapy because a review on adjunctive hyperbaric oxygen therapy for necrotizing fasciitis has already been published (Levett 2015).
Types of outcome measures
Primary outcomes
Mortality within 30 days.
Proportion of participants who experience a serious adverse event.
We defined a serious adverse event according to the US Food and Drug Administration (FDA)'s definition (Guidance FDA 2012).
Secondary outcomes
Survival time.
Assessment of long‐term morbidity: alteration of 25% of Functional Impairment Scale (binary outcome: yes or no). We planned to assess a quantitative physical limitation after NSTI according to the American Medical Association (AMA) Guides to the Evaluation of Permanent Impairment (6th edition) (Colledge 2009; Pham 2009). The AMA guides provide a method to quantify impairment expressed as a percentage of whole‐person impairment. There are five columns corresponding to impairment classes 0 to 4, for each part of the lower limb (foot or ankle, knee, hip), the upper limb (digit, wrist, elbow, shoulder), and the pelvis. Class 0 has a 0% rating attached, and all other classes have a discrete range of potential rating values as follow: Class 1 (1% to 13%), class 2 (14% to 25%), class 3 (26% to 49%), class 4 (50% to 100%) (Rondinelli 2009).
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress), or date of publication.
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 19 April 2018:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL); 2018, issue 3, in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
Embase via Ovid (from 1974) using the strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
Trials registers
We searched the following trials registers up to 19 April 2018 using the search strategies in Appendix 6:
the ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (www.clinicaltrials.gov);
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
the World Health Organization International Clinical Trials Registry platform (ICTRP) (www.who.int/trialsearch); and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
Searching other resources
Searching reference lists and other reviews
We checked bibliographies of included studies and review papers for further references to relevant trials.
Unpublished literature
We searched the trials results databases of the following pharmaceutical companies in order to identify ongoing and unpublished trials. We searched the following up to 19 April 2018 (see Appendix 6 for search terms):
We also searched relevant trials submitted to the FDA and the European Medicines Agency (EMA) for drug registration (respectively, www.accessdata.fda.gov/scripts/cder/drugsatfda/ and www.ema.europa.eu/ema/).
Adverse effects
We did not perform a separate search for adverse effects of the target intervention. However, we did examine data on adverse effects from the included studies.
Data collection and analysis
We included three 'Summary of findings' tables (Table 1; Table 2; Table 3). In these, we summarised the primary outcomes and secondary outcomes for the most important comparisons. We used the five Grading of Recommendations Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence (Guyatt 2011;GRADEpro). We used this assessment, which two review authors (CH, LLC) conducted, to inform the main text of the discussion section. A third review author (ES) resolved any disagreements between the two other authors.
Summary of findings for the main comparison. Moxifloxacin compared to amoxicillin‐clavulanate for NSTI.
| Moxifloxacin compared to amoxicillin‐clavulanate for NSTI | ||||||
| Patient or population: NSTI Setting: hospital Intervention: moxifloxacin Comparison: amoxicillin‐clavulanate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality/certainty of the evidence (GRADE) | Comments | |
| Risk with Amoxicillin‐clavulanate | Risk with Moxifloxacin | |||||
| Mortality follow‐up: 30 days | Study population | RR 3.00 (0.39 to 23.07) | 54 (1 RCT) | ⊕⊝⊝⊝ Very lowa | Data from a larger trial including several types of soft tissue infections; total number of included patients N = 804 | |
| 6 per 100 | 17 per 100 (2 to 100) | |||||
| Serious adverse events (SAE) follow‐up: 28 days | Study population | RR 0.63 (0.30 to 1.31) | 54 (1 RCT) | ⊕⊝⊝⊝ Very lowa | Description of nature of serious adverse events was not available | |
| 44 per 100 | 28 per 100 (13 to 58) | |||||
| Survival time | — | — | — | 54 (1 RCT) | ⊕⊝⊝⊝ Very lowa | The median time of death after start of antibiotic treatment was shorter in the moxifloxacin group than in the amoxicillin‐clavulanate group (10.5 days versus 42 days) (not possible to calculate hazard ratio with the data provided) |
| Assessment of long‐term morbidity | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Assumed risk for mortality was based on data of the literature (Audureau 2017; May 2009). For serious adverse effects it was based on the results of the trial. CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality/certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality/certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality/certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality/certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by five levels to very low certainty of evidence. We downgraded two levels because of high risk of bias regarding blinding (open label trial) and high risk for attrition bias because of a high rate of withdrawal (20%). We downgraded one level for serious imprecision because of small sample size (and CI of RR included 1, where reported). We downgraded a further two levels because no clear criteria for clinical diagnosis of necrotizing fasciitis were provided and because antibiotic used as comparator is not relevant (indirectness)
Summary of findings 2. AB103 compared to placebo for NSTI.
| AB103 compared to Placebo for NSTI | ||||||
| Patient or population: NSTI Setting: hospital Intervention: AB103 Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality/certainty of the evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with AB103 | |||||
| Mortality follow‐up: 30 days | Study population | RR 0.34 (0.05 to 2.16) | 43 (1 RCT) | ⊕⊝⊝⊝ Very lowa | — | |
| 23 per 100* | 6 per 100 (1 to 39) | |||||
| Serious adverse events (SAE) follow‐up: 28 days | Study population | RR 1.49 (0.52 to 4.27) | 43 (1 RCT) | ⊕⊝⊝⊝ Very lowa | There were no data about the nature of serious adverse events reported | |
| 27 per 100 | 41 per 100 (14 to 100) | |||||
| Survival time | — | — | — | — | — | Not reported |
| Assessment of long‐term morbidity | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Assumed risk for mortality was based on data of the literature (Audureau 2017; May 2009). For serious adverse effects it was based on the results of the trial. CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality/certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality/certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality/certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality/certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by three levels: one level for high risk of attrition bias, one level for no clear clinical definition of criteria for necrotizing fasciitis diagnosis at inclusion (indirectness), and one level for serious imprecision because of small sample size and CI included no difference
Summary of findings 3. Intravenous immunoglobulin compared to placebo for NSTI.
| Intravenous immunoglobulin compared to placebo for NSTI | ||||||
| Patient or population: NSTI Setting: intensive care unit Intervention: intravenous immunoglobulin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality/Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Intravenous immunoglobulin | |||||
| Mortality follow‐up: 30 days | Study population | RR 1.17 (0.42 to 3.23) | 100 (1 RCT) | ⊕⊕⊝⊝ Lowa | — | |
| 0 per 100 | 0 per 100 (0 to 0) | |||||
| Moderate | ||||||
| 23 per* 100 | 21 per 100 (8 to 58) | |||||
| Serious adverse events (SAE) follow‐up: unclear | Study population | RR 0.73 (0.32 to 1.65) | 100 (1 RCT) | ⊕⊕⊝⊝ Lowa | Serious adverse reactions included acute kidney injury, allergic reactions, aseptic meningitis syndrome, haemolytic anaemia, thrombi, and transmissible agents | |
| 22 per 100 | 16 per 100 (7 to 36) | |||||
| Survival time | — | — | — | 100 (1 RCT) | ⊕⊕⊝⊝ Lowa | The median time of death was shorter in the IVIG group than in the placebo group (25 days versus 49 days) (not possible to calculate hazard ratio with the data provided) |
| Assessment of long‐term morbidity | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Assumed risk for mortality was based on data of the literature (Audureau 2017; May 2009). For serious adverse effects it was based on the results of the trial. CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality/certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality/certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality/certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality/certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by two levels: one level for high risk of attrition bias (38% lost of follow‐up); other bias: imbalance at baseline for one dose 25 IVIG received before randomisation (40% in placebo group vs 16% IVIG group). One level for indirectness as a minority of patients have an infection linked to bacteria producing toxins
Some parts of the methods section of this review used text that was originally published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and other Cochrane protocols co‐authored by LLC (predominantly Le Cleach 2014).
Selection of studies
Two review authors (CH and RB) independently examined each title and abstract to exclude obviously irrelevant reports. The two review authors independently examined full‐text articles to determine eligibility. We contacted authors of selected studies for clarification when necessary (Table 6). We discussed disagreements to reach consensus. We listed excluded studies and documented the primary reason for exclusion.
3. Details of contacting authors.
| Study | Contact | Requested information | Contacted | Reply (last check 23 April 2017) |
| Darenberg 2003 (awaiting classification study) | Dr Norrby‐Teglund | Outcomes in the specific subgroup of patients with NSTI: ‐Mortality at day 30, ‐Proportion of patients with serious adverse events ‐Survival time ‐Patients with alteration of 25% of Functional Impairment Scale (%) |
July 28, 2015 September 07, 2015 |
No response |
| Tally 1986 (awaiting classification study) | Dr Kellum | Outcomes in the specific subgroup of patients with NSTI: ‐Mortality at day 30, ‐Proportion of patients with serious adverse events ‐Survival time ‐Patients with alteration of 25% of Functional Impairment Scale (%) |
July 24, 2015 September 07, 2015 |
No response |
| Vick‐Fragoso 2009 (included study) | Dr Bogner, Dr Petri | Outcomes in the specific subgroup of patients with NSTI: ‐Mortality at day 30, ‐Proportion of patients with serious adverse events ‐Survival time ‐Patients with alteration of 25% of Functional Impairment Scale (%) |
September 07, 2015 | Additional data to the publication provided for mortality, proportion of patients with serious adverse events and survival time. Outcome data for assessment of long term morbidity not provide |
| Bulger 2014 (included study) | Dr Bulger | Outcomes: ‐Survival time ‐Patients with alteration of 25% of Functional Impairment Scale (%) |
September 07, 2015 September 09, 2015 |
Outcome data not provided |
Data extraction and management
Two review authors (CH and RB) independently extracted data using a piloted data extraction form designed for this review based on the Cochrane Effective Practice and Organisation of Care (EPOC) Group data collection template. A third review author (LLC) resolved any disagreements between the two other review authors. One review author (CH) checked and enter the data into the Cochrane Review Manager computer software (RevMan 2014). We contacted the authors of the trials to provide missing data when required.
We extracted data relating to the following areas.
Study design: randomised (cluster or non‐cluster, randomisation procedure).
Setting: location (hospital or centre characteristics) and relevant dates, including period of recruitment and follow‐up.
Physician characteristics: number of physicians, physician specialties.
Participant characteristics: inclusion and exclusion criteria; number of participants screened and included; average age; comorbidities; sex; necrotizing soft tissue infections (NSTI) site; and severity, including microbiology, shock upon admission.
Interventions during hospitalisation with descriptions of their modalities and duration: type and time course of antibiotic treatment; adjuvant treatment, such as intravenous immunoglobulin therapy; delay to surgery; number and type of surgery (amputation versus surgical debridement); the level of experience of the surgeon; time of onset to time of first treatment.
Relevant outcome measures: 30‐day mortality, survival time, proportion of participants with serious adverse events, assessment of long‐term morbidity with work status, and rate of impairment.
Risk of bias: we also extracted for each study information on potential biases, as described in the following section.
Assessment of risk of bias in included studies
For randomised controlled trials, we used Cochrane's 'Risk of bias' tool to assess the risk of bias (Higgins 2011). We used the following seven parameters: random sequence generation and allocation concealment, blinding of patients, caregivers and outcomes assessors, incomplete outcome data, selective outcome reporting and other bias. We graded each parameter as follows: low risk of bias, unclear risk of bias, or high risk of bias. Two review authors (CH and RB) independently assessed each included study. If necessary, referral to and consensus with a third author (LLC) resolved any discrepancies between the authors. Studies were classified as having low risk of bias if none of the domains above were rated as high risk of bias and three or less were rated as unclear risk; moderate if one was rated as high risk of bias or none were rated as high risk of bias but four or more were rated as unclear risk, and all other cases were assumed to pertain to high risk of bias.
Measures of treatment effect
We classified and analysed studies by type of intervention and type of outcome.
For each pair‐wise comparison and each dichotomous outcome at each time point, we used risk ratios (RR) with 95% confidence intervals (CI) as a measure of treatment effect. For survival time evaluation, we planned to use hazard ratios (HR) with 95% CI.
Unit of analysis issues
The primary unit of analysis is the participant. We planned for clustered‐randomised trials, to check for unit of analysis errors, and if necessary, to adjust their standard errors using an estimate of the intra cluster correlation coefficient (ICC) as described in Section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In cases of multi‐dose trials, we grouped together all the different dose groups as a single arm and compared them collectively with the control group.
Dealing with missing data
When required, we requested missing data (numbers of events and numbers of participants for important dichotomous clinical outcomes) from study authors or sponsors by e‐mail (Table 6). For the main analysis, we assumed that any participant with missing outcome data did not experience clearance, whatever the group. We planned to synthesise data as analysed in each study (complete cases).
Assessment of heterogeneity
We planned to assess statistical heterogeneity by visual inspection of the forest plots and by calculating I² statistics (Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)). We planned to use the following thresholds of I² statistic to interpret heterogeneity: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity. We planned to undertake meta‐analyses only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar (Section 9.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
Where statistical heterogeneity is > 75%, we planned to not pool the data (Deeks 2011).
Assessment of reporting biases
We planned to assess reporting bias for primary end points using funnel plots. We planned to use funnel plot asymmetry tests provided validity conditions were met (low heterogeneity, 10 or more studies including at least one with significant results, and a ratio of the maximal to minimal variance across studies greater than 4) (Ioannidis 2007). In cases of evidence of small‐trial effects, we planned to perform sensitivity analyses according to a regression‐based adjustment model.
Data synthesis
We planned to pool the results of similar groups of trials (participants, interventions, and outcomes), and perform a meta‐analysis using Review Manager software (RevMan 2014, Higgins 2011). We planned to use the summary estimate based on the random‐effects model (DerSimonian–Laird method) (DerSimonian 1986). In case of a high level of heterogeneity with an I² statistic greater than 75%, we planned to not perform a meta‐analysis, and to summarise data from individual studies.
Subgroup analysis and investigation of heterogeneity
The planned subgroup analyses were:
location of NSTI (limb versus perineal versus trunk);
comorbidities (we would consider diabetes, renal failure, chronic disease, peripheral vascular disease, intravenous drug misuse, obesity, non‐steroidal anti‐inflammatory drugs (NSAID) exposure);
immune depression (as defined by diagnosis of human immunodeficiency virus, corticosteroid use or chronic immunosuppressive treatments, and active malignancy); and
microbiology of NSTI (monomicrobial versus multimicrobial forms)
Sensitivity analysis
To evaluate the strength of the associations and identify potential causes of statistical heterogeneity, we planned to perform sensitivity analysis by excluding high 'Risk of bias' studies.
Results
Description of studies
We reported the characteristics of studies in the 'Characteristics of included studies'; 'Characteristics of excluded studies', 'Characteristics of studies awaiting classification', and 'Characteristics of ongoing studies' tables.
Results of the search
The searches of the five electronic databases retrieved 204 records. The searches of the other resources identified 58 studies. No reports of studies were identified by searching the FDA and EMA reviews nor pharmaceuticals databases. We therefore had a total of 262 records.
After duplicates records were removed, we had 245 records. Following exclusion of 226 records on the basis of title and abstract, we screened 19 full‐text reports for eligibility. We excluded 13 reports that did not meet our inclusion criteria (See Characteristics of excluded studies). Two trials were classified as studies awaiting classification because data regarding outcomes in the subgroup of patients with a necrotizing soft tissue infection (NSTI) were lacking (Darenberg 2003; Tally 1986). We contacted the study authors but have not received a reply (See Characteristics of studies awaiting classification).
Three trials fulfilled the criteria for inclusion (Bulger 2014; Madsen 2017; Vick‐Fragoso 2009). All three studies, (Bulger 2014; Madsen 2017; Vick‐Fragoso 2009), declared pharmaceutical company funding (See Characteristics of included studies). One trial (Vick‐Fragoso 2009) provided both unpublished data (provided by authors) and published results. We used both the published and unpublished data since these were complementary. One study is ongoing (NCT02469857, see Characteristics of ongoing studies).
For a further description of our screening process, see the study flow diagram Figure 1.
1.
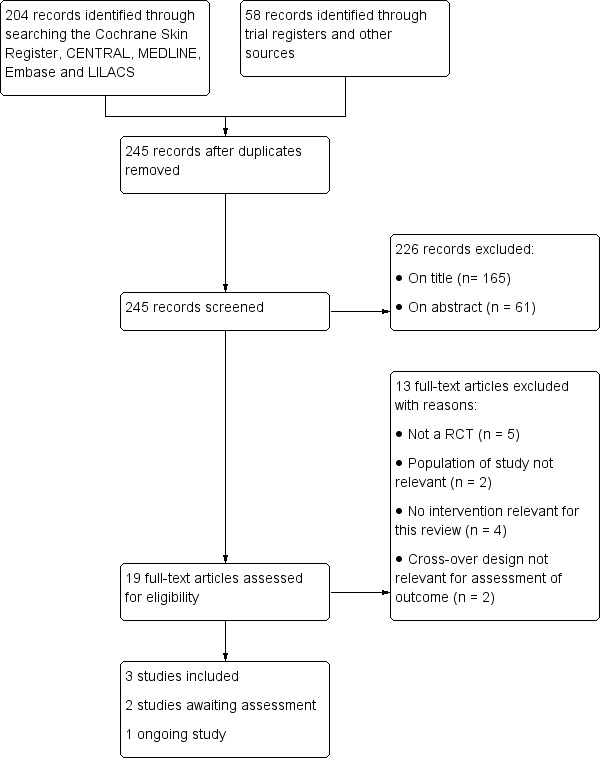
Flow diagram.
Included studies
The characteristics of the three included trials (Bulger 2014; Madsen 2017; Vick‐Fragoso 2009) are described in the Characteristics of included studies and summarised below.
Trial design
All included trials were randomised at a patient level, used a parallel‐group design, and were in a hospital setting. One trial was a non‐inferiority trial (Vick‐Fragoso 2009), and the others were placebo‐controlled (Bulger 2014; Madsen 2017). One trial was monocentric, performed in Denmark (Madsen 2017), and two were multicentred: one performed worldwide in 74 centres (Vick‐Fragoso 2009), and one performed in six centres all located in the USA (Bulger 2014).
Participants
The included trials randomised in total 197 adults with a diagnosis of NSTI: 54 in Vick‐Fragoso 2009, 43 in Bulger 2014 and 100 in Madsen 2017. The overall mean age of included patients was over 50 in the three trials (52.2 years (standard deviation: 13) for the group of patients with a diagnosis of NSTI in Vick‐Fragoso 2009; 50.7 years (range 25 to 88) in Bulger 2014; and 60 years (range 50 to 71) in Madsen 2017. There was a male predominance in the three trials, with 31 males/54 participants (57.4%) in Vick‐Fragoso 2009; 26/40 (65%) in Bulger 2014; and 62/100 (62%) in Madsen 2017. The mean Sequential Organ Failure Assessment (SOFA) score at admission was 7 in Madsen 2017 and 3 in Bulger 2014. In Madsen 2017, all patients, were hospitalised in intensive care unit, and 40% were in septic shock. In Vick‐Fragoso 2009, comorbidities were described for the global population, however there was no separate data available for the subgroup of participants with NSTI. Diabetes mellitus was diagnosed in 17/40 participants (42.5%) in one trial (Bulger 2014), and in 27/100 patients (27%) in the other (Madsen 2017). In two trials (Bulger 2014; Vick‐Fragoso 2009), bacteriological results were not available for the participants with NSTI. In Madsen 2017, infections were polymicrobial in 62/100 participants and Group A streptococcus was involved in 17/100 participants. In Madsen 2017, the involved areas were the extremities and head and neck in 52/100 participants.
Concerning diagnosis criteria for inclusion, in one trial, Vick‐Fragoso 2009, criteria for inclusion were complicated skin and soft skin infection (cSSSI): diabetic foot infection, post‐surgical wound infection, complicated cellulitis, complicated erysipelas, major abscess of the skin, infection of traumatic lesion, infected ischaemic ulcer, and necrotizing fasciitis. Patients had to have only one affected site and one of the following systemic symptoms: fever, high blood white cells count, tachycardia, increased respiratory rate, elevated C‐reactive protein and two or more local signs in the affected areas: pain, tenderness, anaesthesia or hypoaesthesia, swelling, purulent, serosanguinous, or foul‐smelling discharge, gas formation under the skin, and changes in the appearance (discolouration of the skin, necrotic areas, haemorrhagic bullae, blue‐grey patches). This study, which included mixed populations, does not provide a definition for individual conditions and specifically for the subgroup of patient with necrotizing fasciitis.
In Bulger 2014, patients were included if they had a clinical diagnosis of NSTI due to bacterial infection (e.g. necrotizing fasciitis, group A Streptococcus toxic shock, Fournier gangrene, clostridial gangrene or myonecrosis, and synergistic necrotizing cellulitis) and a need to perform urgent surgical exploration and debridement. Diagnosis of NSTI was confirmed during surgery. There was no clinical definition of NSTI in the inclusion criteria.
Madsen 2017 included patients with confirmed NSTI at surgical exploration. The diagnosis of NSTI was confirmed by the surgeon doing the initial operation on the basis of macroscopic findings such as tissue necrosis, deliquescent tissue and ‘dishwater’ fluid.
Intervention
Antimicrobial therapy
Moxifloxacin versus amoxicillin‐clavulanate
One trial compared two anti‐microbial therapies head to head in NSTI (Vick‐Fragoso 2009). The trial compared penicillin (amoxicillin‐clavulanate) with a third‐generation quinolone (moxifloxacin). Surgery could be carried out either before or after the antibiotic therapy at the discretion of the investigator. The daily dose in the amoxicillin‐clavulanate group was 3 g three times daily for at least three days, followed by 1.5 g three times daily versus 400 mg once daily in the moxifloxacin group. Total duration of treatment could vary from seven to 21 days according to clinical response. In both groups, the treatment was given intravenously for at least three days, and the decision to switch from intravenous to oral therapy was made by the investigator based on clinical response.
Adjuvant therapy versus placebo
CD28 antagonist peptide
One trial assessed AB103 as an adjuvant drug in NSTI management (Bulger 2014). AB103 (mimetic octapeptide) is an antagonist that prevents the binding of superantigen exotoxins to the CD28 receptor on T‐helper1 lymphocytes. There were three groups: high‐dose treatment 0.5 mg/kg, low‐dose treatment 0.25 mg/kg, and placebo. Single intravenous dose of AB103 via infusion pump for 10 minutes or placebo before, after, or during surgery were performed within six hours after the diagnosis of NSTI. All patients were treated with standardised empiric antimicrobial therapy and underwent surgical debridement of necrotic tissue. One patient received hyperbaric oxygen (HBO) treatment and another received intravenous immunoglobulin (IVIG); however, we did not know in which group these two patients were included.
Intravenous immunoglobulin (IVIG)
One trial compared IVIG as an adjuvant drug in the NSTI management versus placebo (Madsen 2017). Patients received IVIG, 25 g/day for three consecutive days. The first dose of trial medicine was given immediately after arrival to the intensive care unit (ICU) or in the operating room before admission to ICU. All patients were treated in accordance with the protocol centre at the discretion of the treating clinicians with standardised empiric antimicrobial therapy (meropenem, clindamycin and ciprofloxacin), repeated surgical revisions, three sessions of hyperbaric oxygenation, sepsis, and supportive intensive care.
Outcomes
In the trial of Vick‐Fragoso 2009, mortality within the 30 days, proportion of serious adverse events and survival time in patients with necrotizing fasciitis were unpublished data provided by the authors. Vick‐Fragoso 2009 did not report assessment of long‐term morbidity. Bulger 2014 reported mortality within the 30 days and the proportion of serious adverse events; however, the authors did not report any of our secondary outcomes (i.e. assessment of long‐term morbidity and survival time). In the trial of Madsen 2017, mortality within the 30 days and survival time in patients with NSTI were unpublished data provided by the authors. Assessment of long‐term morbidity was reported, but not as it was defined in the protocol for this review using another scale the Short Form Health Survey (SF36).
In the trial of Bulger 2014, other outcomes included the change in the Sequential Organ Failure Assessment (SOFA) score from baseline within 28 days, hospital length of stay, ICU–free, vasopressor‐free and ventilator‐free days, number of debridements through day seven. In the trial of Vick‐Fragoso 2009, efficacy was assessed by clinical response (CR) between days 14 to 28, defined as: cure (total resolution or marked improvement of all complicated skin and skin structure infections (cSSSI) signs and symptoms; no additional or alternative antimicrobial treatment necessary). In the trial of Madsen 2017, other outcomes included time to resolution of shock, amputation, and SOFA score.
Excluded studies
We excluded 13 trials at full‐text stage and provided reasons for exclusion in Characteristics of excluded studies.
Studies awaiting classification
Two trials including NSTI among participants with serious surgical infections (Tally 1986), and streptococcal toxic shock syndrome (Darenberg 2003), were identified, but outcome data for those with a NSTI were not reported separately. We contacted authors of these trials in order to obtain the required information however we did not receive an answer (Characteristics of studies awaiting classification).
Ongoing studies
Our search identified one ongoing trial on trial registries. This trial is summarised in the Characteristics of ongoing studies. NCT02469857 is a phase III blinded, randomised, placebo‐controlled trial in patients with NSTI, which assessed the efficacy of AB103, a CD28 antagonist peptide as an adjuvant drug in NSTI management.
Risk of bias in included studies
Please see Figure 2 and Figure 3 for a visual representation of our assessment of the risk of bias
2.
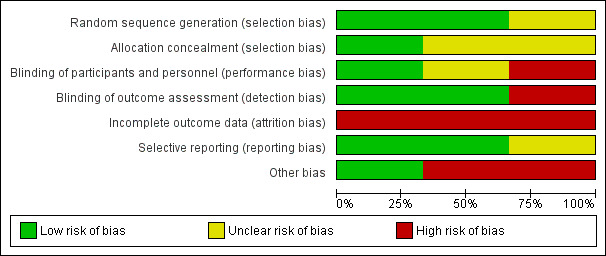
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
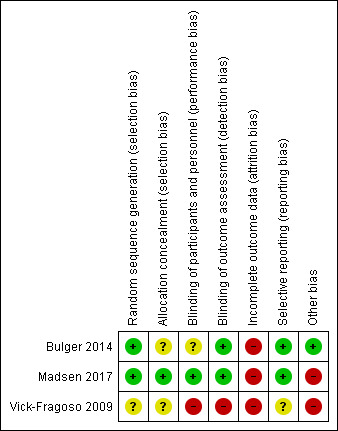
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
We considered two trials at overall high risk of bias (Madsen 2017; Vick‐Fragoso 2009), and we considered one at overall moderate risk of bias (Bulger 2014). Details of our evaluation are available in Characteristics of included studies.
Allocation
We deemed Vick‐Fragoso 2009 as being at unclear risk of selection bias as there was no statement of the method of sequence generation and concealment. We deemed Bulger 2014 to be at low risk of bias for random sequence generation and at unclear risk of bias for allocation concealment. Indeed, the randomisation sequence for the study was computer‐generated but there was no description of the method used to guarantee allocation concealment. We considered Madsen 2017 as being at low risk of bias as the randomisation sequence for the study was computer‐generated and there were sequentially numbered, opaque and sealed envelopes by a dedicated personnel who was not directly associated with the trial to ensure allocation concealment.
Blinding
The trial of Vick‐Fragoso 2009 was open‐labelled, thus participants and personnel were not blinded, we therefore considered the risk as high for performance and detection.
We considered Bulger 2014 at unclear risk of bias for performance bias. The trial was double ‐blinded, placebo‐controlled and authors reported a similar adverse effect profile. However, 2/43 (4.6%) patients randomised had received co‐treatment IVIG and HBO and there was no information on the group receiving these interventions. We considered there was a low risk of detection bias for investigator‐reported outcomes.
We judged Madsen 2017 to be at low risk of bias for performance and detection bias as the method used to guarantee blinding of participants, personnel and outcome assessor were adequate and well described.
Incomplete outcome data
We considered all studies to be at high risk of attrition bias (Bulger 2014; Madsen 2017; Vick‐Fragoso 2009).
In Vick‐Fragoso 2009, one third of participants 19/54 (35%) were lost to follow‐up in the subgroup of patients with necrotizing fasciitis. The details of withdrawal in the subgroup of patients with necrotizing fasciitis were not available. There was no information available about the method of analysis for missing data.
In Bulger 2014, the authors defined a modified intention‐to‐treat (ITT) population for efficacy analysis. They excluded three patients from the ITT population: 1/11 (9%) in the placebo group who did not meet the clinical diagnosis of NSTI and 2/15 (13.3%) in the high‐dose group.
In Madsen 2017, the primary analysis was not ITT, with missing data for 13 patients (13/100, 13%), which were not included in the analysis for the primary outcome.
Selective reporting
We judged the risk of reporting bias as unclear for the study of Vick‐Fragoso 2009, since no protocol was available on the trials register. We judged the risk of bias for the studies Bulger 2014 and Madsen 2017 as low as outcomes listed in www.clinical trials.gov were similar to those reported in the results section.
Other potential sources of bias
There were no other potential sources of bias in the trial of Bulger 2014.
There were baseline imbalances in the trial of Vick‐Fragoso 2009 and Madsen 2017. In the trial of Vick‐Fragoso 2009, the number of patients in the NSTI subgroup was two‐fold higher in the moxifloxacin treatment group (36/54) than in the amoxicillin‐clavulanate treatment group (18/54). In the trial of Madsen 2017, nearly half of patients in the placebo group (20,40%) had received one dose of IVIG (25 g) before randomisation versus eight (16%) in the IVIG group.
Effects of interventions
See: Table 1; Table 2; Table 3
Antimicrobial therapy versus antimicrobial therapy
Moxifloxacin versus amoxicillin‐clavulanate
This comparison included (Vick‐Fragoso 2009) one trial. There were 54 participants in total (36 in the moxifloxacin group and 18 in the amoxicillin‐clavulanate group).
1 Primary outcomes
1.1 Mortality within 30 days
In the moxifloxacin group, there were 6/36 (17%) deaths within 30 days and 1/18 (6%) deaths within the 30 days in the amoxicillin‐clavulanate group. According to our calculations, there were no clear differences between the two groups due to the very wide confidence intervals (risk ratio (RR) 3.00, 95% confidence interval (CI) 0.39 to 23.0) and the certainty of evidence was rated as very low with underpowered analysis (Analysis 1.1; Table 1).
1.1. Analysis.
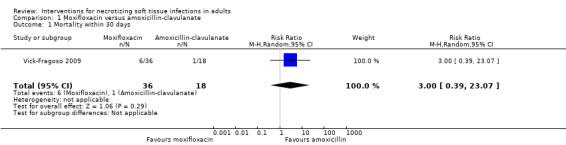
Comparison 1 Moxifloxacin versus amoxicillin‐clavulanate, Outcome 1 Mortality within 30 days.
1.2 Proportion of patients who experienced serious adverse events
In the specific subgroup of NSTI patients, at 28 days, 10/36 (28%) patients receiving moxifloxacin and 8/18 (44%) patients receiving amoxicillin‐clavulanate experienced serious adverse events. According to our calculations, there were no clear differences between the two groups (RR 0.63, 95% CI (0.30 to 1.31; Analysis 1.2) as the 95% confidence interval included 1. The certainty of evidence was rated as very low (Table 1). There were no data about the nature of serious adverse events reported.
1.2. Analysis.
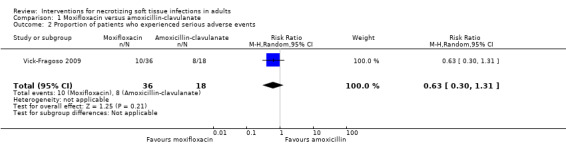
Comparison 1 Moxifloxacin versus amoxicillin‐clavulanate, Outcome 2 Proportion of patients who experienced serious adverse events.
2 Secondary outcomes
2.1 Survival time
The median time of death after start of antibiotic treatment was shorter in the moxifloxacin group than in the amoxicillin‐clavulanate group (10.5 days versus 42 days). No statistical analysis was possible with the data provided.
2.2 Assessment of long‐term morbidity
This outcome was not reported.
Adjuvant therapy versus placebo
AB103 versus placebo
One trial (Bulger 2014), included this comparison. There were 43 participants in total (32 in the AB103 group and 11 in the placebo group).
1. Primary Outcomes
1.1 Mortality within 30 days
Four deaths occurred within 30 days, 1/17 (6%) in the high‐dose arm, 1/15 (7%) in the low‐dose arm and (2/11) (18%) in the placebo group. According to our calculations, there were no clear differences for mortality rate within 30 days between groups receiving adjuvant drug versus the placebo group (RR 0.34, 95% CI 0.05 to 2.16; Analysis 2.1), and the certainty of evidence was rated as very low with underpowered analysis (Table 2).
2.1. Analysis.
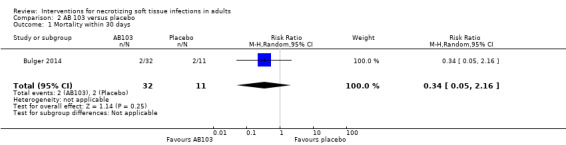
Comparison 2 AB 103 versus placebo, Outcome 1 Mortality within 30 days.
1.2 Proportion of patients who experienced serious adverse events
After drug administration, 5/17 (29%) of patients in the high‐dose arm, 8/15 (53%) in the low‐dose arm and 3/11 (27%) in the placebo group, underwent one serious adverse event at 28 days. There were no data about the nature of serious adverse events reported. According to our calculations, there were no clear differences in serious adverse events rates between groups receiving adjuvant drug versus the placebo group (RR 1.49, 95% CI 0.52 to 4.27; Analysis 2.2), and the certainty of evidence was rated as very low with underpowered analysis (Table 2).
2.2. Analysis.
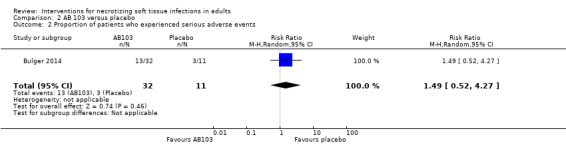
Comparison 2 AB 103 versus placebo, Outcome 2 Proportion of patients who experienced serious adverse events.
2. Secondary Outcomes
2.1 Survival time
This outcome was not reported in the study.
2.2 Assessment of long‐term morbidity
This outcome was not assessed in the study.
Intravenous immunoglobulin versus placebo
One trial (Madsen 2017) included this comparison. There were 100 participants in total (50 in the IVIG group and 50 in the placebo group).
1 Primary outcomes
1.1 Mortality within 30 days
In the IVIG group, there were 7/50 (14%) deaths within the 30 days and 6/50 (12%) in the placebo group. According to our calculations, there were no clear differences between the two groups (RR 1.17, 95% CI 0.42 to 3.23; Analysis 3.1), and the rate certainty of evidence was low (Table 3).
3.1. Analysis.
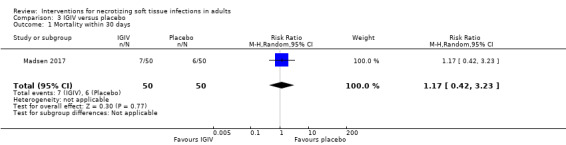
Comparison 3 IGIV versus placebo, Outcome 1 Mortality within 30 days.
1.2 Proportion of patients who experienced serious adverse events
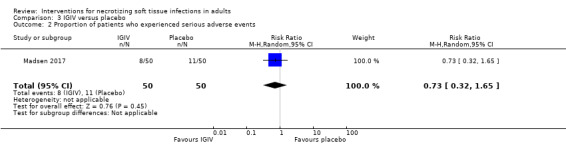
After drug administration, 8/50 (16%) of patients in IVIG group versus 11/50 (22%) in the placebo group underwent serious adverse event. Serious adverse reactions included acute kidney injury, allergic reactions, aseptic meningitis syndrome, haemolytic anaemia, thrombi, and transmissible agents. According to our calculations, there were no clear differences for serious adverse events rates between groups receiving IVIG versus the placebo group (RR 0.73, CI 95% 0.32 to 1.65; Analysis 3.2) and the rate certainty of evidence was low (Table 3). The time point of assessment was unclear.
3.2. Analysis.
Comparison 3 IGIV versus placebo, Outcome 2 Proportion of patients who experienced serious adverse events.
2 Secondary outcomes
2.1 Survival time
The median time of death was shorter in the IVIG group than in the placebo group (25 days versus 49 days) (no statistical analysis was possible with the data provided).
2.2 Assessment of long‐term morbidity
Assessment of long‐term morbidity was reported, but not as it was defined in the protocol for this review.They used a patient‐reported score: the physical component summary (PCS) of the 36‐item short form health survey to assess long‐term morbidity. There was no clear difference of median PCS scores between groups reported (mean adjusted difference 1, 95% CI 7 to 10, P = 0.81).
Discussion
Summary of main results
We identified three randomised controlled trials assessing interventions for necrotizing soft tissue infections (NSTI). These three trials included in total 197 adult participants with a diagnosis of NSTI in a hospital setting.
We could not pool findings from these three trials since the interventions compared in the trials were distinct (Table 1; Table 2; Table 3).
One trial compared amoxicillin‐clavulanate to moxifloxacin (Vick‐Fragoso 2009). One trial compared two doses of AB103, a new adjuvant drug in the management of NSTI to placebo (Bulger 2014). the third trial compared intravenous immunoglobulin (IVIG) as an adjuvant therapy in the management of NSTI (Madsen 2017).
In all trials, participants concomitantly received standard interventions, such as intravenous empiric antimicrobial therapy, surgical debridement of necrotic tissues, intensive care support, and adjuvant therapies.
Concerning our prespecified primary outcomes, there was no clear difference in terms of rate of mortality at 30 days (Analysis 1.1; Analysis 2.1; Analysis 3.1) nor in terms of rate of participants with serious adverse events (Analysis 1.2; Analysis 2.2; Analysis 3.2) in any of the three trials. The quality of evidence was very low for the comparisons of moxifloxacin compared to amoxicillin‐clavulanate, and AB103 compared to placebo, showing we are uncertain about these results. For the comparison of IVIG versus placebo, the quality of evidence was low.
There was no description of the types of serious adverse events in the moxifloxacin versus amoxicillin‐clavulanate trial or the AB103 versus placebo trial, but in the IVIG versus placebo trial, serious adverse reactions included acute kidney injury, allergic reactions, aseptic meningitis syndrome, haemolytic anaemia, thrombi, and transmissible agents.
Regarding our secondary outcomes, we obtained median survival time for two trials; however, it was not possible to perform statistical analysis with the data provided, and none of our included studies reported assessment of long‐term morbidity in the way that was required in our protocol (the trial used the Short Form Health Survey (SF36)).
There remains a lack of evidence for the use of other treatments such as empiric antimicrobial therapy as well as surgical debridement, along with poor‐quality evidence for adjuvant therapy compared against placebo. Better quality evidence is needed for the outcomes of mortality and adverse effects.
Overall completeness and applicability of evidence
Globally, the evidence concerning efficacy and safety of interventions in NSTI is very poor and was insufficient to address all of the objectives of the review or offer much external validity. We found only three trials involving 197 participants. Evidence was lacking for a number of treatments considered for NSTl; empiric antimicrobial therapy and surgical interventions were types of treatment not assessed by any included study. We found only two trials that assessed adjuvant therapy.
The only trial assessing antibiotic treatment used amoxicillin‐clavulanate, which was an inappropriate choice of control treatment in a trial of NSTI. Indeed, NSTI are mostly polymicrobial, and the emergence of resistance requires an empiric antimicrobial therapy broader than a monotherapy by amoxicillin‐clavulanate. No other trial comparing antibiotic regimen strategies was found. The efficacy and safety of hyperbaric oxygen (HBO) therapy was not in the scope of this review as it was assessed in another Cochrane Review.
Participants included were mainly men, aged over 50 years. Lack of clear definition of NSTI in two trials prevented us from determining the applicability of the trial results (Bulger 2014; Vick‐Fragoso 2009), by not providing a definition for the condition of NSTI. Some patients included in the trial of Vick‐Fragoso 2009 with a diagnosis of NSTI (13/54 (24%)) did not undergo surgery, although it is considered the gold standard intervention both for diagnosis and treatment of NSTI in the setting of a high clinical suspicion. Subgroup analysis to see if efficacy or safety could be different according to involved site, type of micro‐organism, mono‐ or ultramicrobial infection and comorbidities were not possible because of too low a number of included patients. Furthermore, except for one trial (Madsen 2017), information on comorbidities, site involved and micro‐organisms identified were not available.
Regarding outcomes, rates of mortality at 30 days and serious adverse effects (our primary outcomes) were reported by the included studies, but the results were based on low‐ or very low‐certainty evidence. Long‐term assessment of morbidity in order to assess sequelae was only reported in one trial (Madsen 2017), but it was not defined in the way we required as specified in our protocol. Data provided for survival time by authors did not allow us to perform survival analysis.
Quality of the evidence
Globally, the quality/certainty of evidence was low or very low for the efficacy and safety of interventions in NSTI; we downgraded the evidence for the three included trials.
We assessed the level of evidence for the comparison of moxifloxacin versus amoxicillin‐clavulanate as very low for all outcomes because the only trial assessing this comparison (Vick‐Fragoso 2009), was at high risk of bias due to attrition bias, imbalance in the inclusion between groups, and performance and detection bias as participants and assessors were not blinded. We also downgraded this trial because of indirectness as there was no clear definition of necrotizing fasciitis, a significant proportion of patients (10/36 (28%) in the moxifloxacin group and 3/18 (17%) in the amoxicillin‐clavulanate group) did not undergo surgery, which casts doubts on the types of infections these participants had. We also downgraded this trial for imprecision as it included only 58 participants with necrotizing fasciitis (Table 1).
We also considered that the quality of evidence was very low for the efficacy and safety of the CD28 antagonist receptor (AB103) adjuvant treatment. The only trial assessing this treatment for NSTI was at moderate risk of attrition bias (Bulger 2014); we also downgraded because of indirectness due to the absence of a clinical definition of NSTI and due to imprecision: only 43 participants were included, which is not large enough to identify or exclude significant benefit (Table 2).
We considered that the level of evidence was low for the efficacy and safety of IVIG for NSTI. The only trial assessing IVIG was at a high risk of bias for imbalance and attrition bias (Madsen 2017). Nearly half of the patients (40%) in the placebo group had received one dose of IVIG (25 g) before randomisation versus three doses (75 g) in the IVIG group, which challenges the design placebo‐controlled of this trial (indirectness) (Table 3).
The applicability of the evidence was also limited due to the small sample sizes and underpowered analysis.
Potential biases in the review process
The review considered as much evidence as it was possible to obtain, including trials that were not published. The search was not limited to necrotizing fasciitis and considered all trials concerning necrotizing soft tissue infections. There are a number of clinical trials that included a mixed population of people with a wide range of skin and skin structure infections, including NSTI, using terms such as "complicated skin and skin structure infections" (cSSSI). However, when data were not available for the very specific subgroup of NSTI, these studies were not included. The wide variety of terms to design NSTI might have led us to miss some studies who included heterogeneous populations of patients with skin and soft tissue infections.
Furthermore, the inconsistency of definitions and the variability in the definitions used for NSTI among studies might, on the other hand, have led us to include by mistake patients with cellulitis, foot ulceration in diabetic patients, dermal abscesses and ischaemic gangrene. Indeed, some patients in the included trials had a diagnosis of NSTI but did not undergo surgery, which is mandatory to confirm diagnosis of NSTI (Vick‐Fragoso 2009), suggesting these patients might have suffered from other types of skin and soft tissue infections.
Agreements and disagreements with other studies or reviews
There is, to our knowledge, no other systematic review on this topic.
Recommendations relating to interventions for NSTIs are based on expert consensus (SFD‐SPILF 2000; Stevens 2014). Guidelines recommend using an intravenous empiric antimicrobial therapy to cover gram‐positive, gram‐negative, and anaerobic organisms (Sartelli 2014; Stevens 2014). Many trials have been performed to assess the efficacy of antimicrobial agents in the treatment of complicated skin and soft tissue infections (cSSTI) (McClaine 2010). However, most of these trials excluded NSTIs. To date, there is no randomised controlled trial comparing antimicrobial therapy including only patients with NSTI. The benefit of clindamycin for NSTI due to group A Streptococcus (GAS) is only based on observational studies and has not been assessed by a trial (Carapetis 2014; Stevens 2014).
There are no randomised controlled trials assessing surgery in NSTI.
Concerning the use of IVIG, the results of the trial of Madsen 2017 is in line with a recent observational study (propensity score‐matched analysis) including 4127 patients with NSTI and shock over a period of four years in 130 centres in the USA (Kadri 2017). This study did not demonstrate any differences for hospital mortality between patients who received IVIG and those who did not (adjusted odds ratio (OR) 1.00, 95% confidence interval (CI) 0.55 to 1.83, P = 0.99), nor in specific subgroup of patients with NSTI caused by GAS or Staphylococcus aureus (SA) (Kadri 2017).
Authors' conclusions
Implications for practice.
Management of necrotizing soft tissue infections (NSTI) is complex, involving multiples interventions: antimicrobial therapy, surgical debridement, management of organ failures, and eventually adjuvant therapies.
We found only three trials that compared the following interventions:
moxifloxacin versus amoxicillin‐clavulanate;
a CD28 antagonist receptor (AB103) versus placebo;
intravenous immunoglobulin versus placebo.
We cannot make conclusions about the use of AB103, moxifloxacin, or amoxicillin‐clavulanate because results for their use were based on very low‐certainty evidence.
With regard to intravenous immunoglobulin (IVIG) compared to placebo, there may be no clear difference between the treatment groups in terms of rate of mortality at 30 days or serious adverse events.
One trial assessed long‐term morbidity but not in the way we stated that we prespecified in the protocol. Survival time was reported in two trials (but not enough data were provided to analyse these results).
We found no trials assessing other interventions, notably surgical treatment or recommended empiric intravenous antibiotic treatment.
Implications for research.
This review highlights the lack of evidence for the treatment of NSTI.
Several reasons can account for the lack of comparative effectiveness research in this field.
Management of NSTI is complex, involving multiple interventions, including antimicrobial therapy, surgical debridement, management of organ failures, and adjuvant therapies.
After a clinical hypothesis of necrotizing fasciitis, patients underwent a urgent surgical debridement. Definite diagnosis can only be obtained during the surgical debridement phase. This mandatory and very urgent surgery may hamper the inclusion of patients in clinical trials aimed at assessing therapeutic interventions for NSTI.
Placebo‐controlled trials in this life‐threatening disease are only feasible, from an ethical point of view, for assessing the effect of adjuvant therapies, since, in spite of the lack of evidence, neither surgical debridement nor antibiotic treatment could ethically be compared to placebo.
NSTIs are rare and greater co‐operation between centres to obtain sufficient power for meaningful studies is needed.
We list below methodological key points following a PICO (participants, interventions, comparator and outcomes) framework that could be considered in future trials.
Participants
NSTI needs to be clearly defined in the inclusion criteria. As diagnosis of NSTI is only confirmed during surgery, it is mandatory to state how to handle included patients eventually not diagnosed as having NSTI. All patients without restriction of age or gender need to be included in order to be more representative of the affected population. Patient comorbidities, involved site and micro‐organisms are mandatory information that have to be reported
Types of study
Because designing randomised controlled trials in NSTI is challenging, observational studies and prospective registries might be valuable alternatives to evaluate the effectiveness and safety of treatments. However, their methodology should be rigorous, as they potentially rely on propensity scores analyses (Anglemyer 2014). This type of research could allow us to evaluate long‐term outcomes, such as assessment of long‐term morbidity, and deduce applicability to a large proportion of the patient population undergoing procedures in real‐world settings across the world. The three trials in this review were at risk of attrition bias; future studies should ensure they take the necessary actions to reduce dropout and loss to follow‐up.
Interventions
A separate assessment of interventions involved in the management of NSTI (i.e. antimicrobial therapy, surgical treatment, and adjuvant treatment) would certainly not reflect the effect of combined interventions in a "therapeutic bundle". Thus, the use of integrated complex interventions to optimise the treatment of NSTI is needed (Delaney 2008). Examples of such complex interventions include management in referral centres with a multidisciplinary approach and an early goal‐directed therapy for surgical referral.
Antimicrobial therapy
There was no empiric antimicrobial therapy validated by a trial. Several issues concerning antimicrobial therapy in NSTI should be considered: the duration of treatment after surgery, the antimicrobial management strategies in patients with or without comorbidities and in patients with risk factors for infections with MRSA, (methicillin‐resistant Staphylococcus aureus) and the adjustment of the treatment after microbiological results to reduce the risk of resistance. A future trial in NSTI could examine the non‐inferiority of new antimicrobials agents, compared against standard accepted treatment.
Surgical treatment
Several issues should be considered: the extent of surgical debridement, and the number of surgical debridements required (including the "second look" surgery strategy). Study interventions and treatments tested would have to be clearly defined with a standardised protocol of care. As required in trials assessing surgical procedures, a detailed description of the different components of surgical treatment, anaesthesia management, as well as peri‐operative and postoperative care should be provided (Blencowe 2015; Boutron 2008).
Adjuvant treatment
Other adjuvant therapies using immunomodulator treatments (e.g. AB103) should be assessed in placebo‐controlled trials. The control group should receive current practice management based on guidelines and observational studies (Delaney 2008).
Outcomes
In the absence of consensual core outcome measures:
in the acute phase, mortality at a disease time point seems to be the most appropriate outcome criteria for this critical care disease (Delaney 2008); and
in the chronic phase, outcomes such as evaluation of loss of work, duration of unemployment, and level of distress needs to be evaluated. Development of validated functional assessment tools is required to assess long‐term functional outcomes, reintegration into society, and quality of life in survivors of NSTI.
Acknowledgements
We thank the Information Specialist, Elizabeth Doney, for her help during the preparation of this review.
We acknowledge Judith Mathore for her help identifying the types of outcomes that we should consider for this review.
Cochrane Skin wishes to thank Urbà González, who was the Cochrane Dermatology Editor for this review; Ben Carter, who was the Statistical Editor; Esther van Zuuren, who was the Methods Editor; the clinical referees, Dennis L Stevens and Lucy Lamb; the consumer referee, Peter Smart; and the review's copy‐editor, Heather Maxwell.
Appendices
Appendix 1. Cochrane Skin Group Specialised Register (CRS)
#1 (MeSH DESCRIPTOR Fasciitis, Necrotizing) AND (INREGISTER) [REFERENCE] [STANDARD] #2 (MeSH DESCRIPTOR Fournier Gangrene) AND (INREGISTER) [REFERENCE] [STANDARD] #3 (fournier gangrene or (synergistic and (necrotizing or necrotising) and cellulitis) or progressive synergistic bacterial gangrene or suppurative fasciiti* or (flesh‐eating and (disease or syndrome)) or necrotizing fasciitis or necrotizing fascitides or necrotising fasciitis or necrotising fascitides or necrotizing fasciitis or necrotising fasciitis or ((necrotizing or necrotising) and soft tissue infection*)) AND (INREGISTER) [REFERENCE] [STANDARD] #4 #1 OR #2 OR #3 [REFERENCE] [STANDARD]
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Fournier Gangrene] explode all trees #2 MeSH descriptor: [Fasciitis, Necrotizing] explode all trees #3 ((necrotizing or necrotising) and soft tissue infection*):ti,ab,kw #4 fournier gangrene:ti,ab,kw #5 (synergistic and (necrotizing or necrotising) and cellulitis):ti,ab,kw #6 (progressive synergistic bacterial gangrene):ti,ab,kw #7 suppurative fasciiti*:ti,ab,kw #8 flesh‐eating disease:ti,ab,kw #9 flesh‐eating bacteria syndrome:ti,ab,kw #10 necrotizing fasciitis:ti,ab,kw #11 necrotizing fascitides:ti,ab,kw #12 necrotising fasciitis:ti,ab,kw #13 necrotising fascitides:ti,ab,kw #14 necrotizing fascitis:ti,ab,kw #15 necrotising fascitis:ti,ab,kw #16 {or #1‐#15}
Appendix 3. MEDLINE (Ovid) search strategy
1. Fournier Gangrene/ 2. fournier gangrene.ti,ab. 3. (synergistic and (necrotizing or necrotising) and cellulitis).ti,ab. 4. progressive synergistic bacterial gangrene.ti,ab. 5. suppurative fasciiti$.ti,ab. 6. flesh‐eating disease.ti,ab. 7. flesh‐eating bacteria syndrome.ti,ab. 8. necrotizing fasciitis.ti,ab. 9. necrotizing fascitides.ti,ab. 10. necrotising fasciitis.ti,ab. 11. necrotising fascitides.ti,ab. 12. necrotizing fascitis.ti,ab. 13. necrotising fascitis.ti,ab. 14. Fasciitis, Necrotizing/ 15. ((necrotizing or necrotising) and soft tissue infection$).ti,ab. 16. or/1‐15 17. randomized controlled trial.pt. 18. controlled clinical trial.pt. 19. randomized.ab. 20. placebo.ab. 21. clinical trials as topic.sh. 22. randomly.ab. 23. trial.ti. 24. 17 or 18 or 19 or 20 or 21 or 22 or 23 25. exp animals/ not humans.sh. 26. 24 not 25 27. 16 and 26
[Lines 17‐26: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. Fournier Gangrene/ 2. fournier gangrene.ti,ab. 3. (synergistic and (necrotizing or necrotising) and cellulitis).ti,ab. 4. progressive synergistic bacterial gangrene.ti,ab. 5. suppurative fasciiti$.ti,ab. 6. flesh‐eating disease.ti,ab. 7. flesh‐eating bacteria syndrome.ti,ab. 8. necrotizing fasciitis.ti,ab. 9. necrotizing fascitides.ti,ab. 10. necrotising fasciitis.ti,ab. 11. necrotising fascitides.ti,ab. 12. necrotizing fascitis.ti,ab. 13. necrotising fascitis.ti,ab. 14. ((necrotizing or necrotising) and soft tissue infection$).ti,ab. 15. necrotizing fasciitis/ 16. or/1‐15 17. crossover procedure.sh. 18. double‐blind procedure.sh. 19. single‐blind procedure.sh. 20. (crossover$ or cross over$).tw. 21. placebo$.tw. 22. (doubl$ adj blind$).tw. 23. allocat$.tw. 24. trial.ti. 25. randomized controlled trial.sh. 26. random$.tw. 27. or/17‐26 28. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 29. human/ or normal human/ 30. 28 and 29 31. 28 not 30 32. 27 not 31 33. 16 and 32
Appendix 5. LILACS search strategy
((necrotizing or necrotising) and (fasciitis or fascitis)) or Fournier gangrene
We searched using the above terms and the Controlled clinical trials topic‐specific query filter within LILACS.
Appendix 6. Search strategies for trials registers & pharmaceutical databases
| The ISRCTN registry: "necrotizing fasciitis" or "necrotising fasciitis" or" necrotizing soft tissue infection" or "necrotising soft tissue infection" |
|
ClinicalTrials.gov: "necrotizing fasciitis" or "necrotising fasciitis" or" necrotizing soft tissue infection" or "necrotising soft tissue infection" |
|
The Australian New Zealand Clinical Trials Registry (ANZCTR): "necrotizing fasciitis" or "necrotising fasciitis" or" necrotizing soft tissue infection" or "necrotising soft tissue infection" |
|
WHO International Clinical Trials Registry Platform (ICTRP): "necrotizing fasciitis" or "necrotising fasciitis" or" necrotizing soft tissue infection" or "necrotising soft tissue infection" |
|
EU Clinical Trials Register: "necrotizing fasciitis" or "necrotising fasciitis" or" necrotizing soft tissue infection" or "necrotising soft tissue infection" |
|
Pharmaceuticals databases: "necrotizing fasciitis" or "necrotising fasciitis" or" necrotizing soft tissue infection" or "necrotising soft tissue infection" |
Data and analyses
Comparison 1. Moxifloxacin versus amoxicillin‐clavulanate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality within 30 days | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.39, 23.07] |
| 2 Proportion of patients who experienced serious adverse events | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.30, 1.31] |
Comparison 2. AB 103 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality within 30 days | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.05, 2.16] |
| 2 Proportion of patients who experienced serious adverse events | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.52, 4.27] |
Comparison 3. IGIV versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality within 30 days | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.42, 3.23] |
| 2 Proportion of patients who experienced serious adverse events | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.32, 1.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bulger 2014.
| Methods | Prospective, Phase IIA, randomised, parallel‐group, placebo‐controlled, double‐blinded study Multicentric (6 centres) in the USA Period of Inclusion: December 2011 to August 2012 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline characteristics N = 43, mean age of 50.7 years and 65% of men |
|
| Interventions | Each participant was treated with standardised empiric antibiotic therapy, and underwent surgical debridement of necrotic tissue Intervention 1 (n = 17) AB103, one dose intravenous (IV), 0.5 mg/kg, < 6 hours after diagnosis (infusion before, during or after the surgery) Intervention 2 (n = 15) AB103, one dose IV 0.25 mg/kg, < 6 hours after diagnosis (infusion before, during or after the surgery) Intervention 3 (n = 11) Placebo IV, < 6 hours after diagnosis (infusion before during or after the surgery) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Financial support and the drug for this trial was provided by the manufacturer Atox Bio Ltd. Authors declared conflict of interest for Bayer HealthCare, BayerSchering Pharma, Novartis, GlaxoSmith Kline and Wyeth. * outcomes reported that were of interest in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: “Patients were randomised by a computer‐generated system to receive placebo or a low or high dose of AB103 in a 10:15:15 ratio.” Comment: computer‐generated random sequence is adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote “double blinded” Comment: placebo‐controlled with similar adverse‐effect profile reported. However, concerning the risk of performance bias, 2/43 (4.6%) patients randomised had received co‐treatment IVIG and HBO and the group of these two patients was not indicated. Authors did not specify the method of blinding of physicians and participants but they assess that all investigators and care providers remained masked throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote “double blinded" "investigators remained blinded throughout the study" Comment: there was no clear description of the process used to guarantee blinding of the assessor but it was a placebo‐controlled study with similar adverse‐effect profile reported and assessment of outcome was objective: death. Then we considered there was a low risk of detection bias for investigator‐reported outcomes for the same reasons. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote “Because drug administration may have started before definitive surgical diagnosis of NSTI, a modified ITT population was defined as patients in the ITT analysis who were properly randomised, treated (received AB103 or placebo), and assessable (definitive surgical diagnosis of NSTI).” Comment: three patients were excluded from the ITT population; 1/11 (9%) in the placebo group who did not meet the clinical diagnosis of NSTI and 2/15 (13.3%) in the high‐dose group. |
| Selective reporting (reporting bias) | Low risk | Comment: in accordance with the protocol available in clinical trials.gov (NCT01417780) |
| Other bias | Low risk | No other bias identified |
Madsen 2017.
| Methods | Randomised, blinded, parallel‐group, placebo‐controlled trial Monocentric at Copenhagen University Hospital, where the management of patients with NSTI in Denmark is centralised Period of inclusion: 7 April 2014 to 1 March 2016 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline characteristics N = 100, Mean age of 60 years and 60% of men |
|
| Interventions | Each patient was treated in accordance with the clinical protocols in place at Copenhagen University Hospital with standardised empiric antibiotic therapy (meropenem, clindamycin and ciprofloxacin), repeated surgical revisions, three sessions of hyperbaric oxygenation; sepsis, and supportive intensive care. Intervention 1 (N = 50) IVIG (Privigen, CSL Behring, Bern,Switzerland), 25 g/day for three consecutive days. The first dose of trial medicine was given immediately after arrival to the ICU or in the operating room before admission to ICU. Intervention 2 (N = 50) Equivalent amount of intravenous 0.9% saline for three consecutive days (placebo) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | * outcomes reported that were of interest in the review Authors declared conflict of interest for CSL Behring. Financial support from Fresenius Kabi, Germany, and Ferring Pharmaceuticals, Denmark, was declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were randomised 1:1 to IVIG or placebo. Two allocation lists with variable block sizes of 2, 4 and 6 were computer generated." Comment: computer‐generated random sequence is adequate |
| Allocation concealment (selection bias) | Low risk |
Quote: "Two allocation lists with variable block sizes of 2, 4 and 6 were computer generated to form two separate boxes that contained sequentially numbered, opaque and sealed envelopes" "Patients were randomised by dedicated personnel who drew the next envelope from the box according to the site of NSTI. The randomisation note was handed to an ICU nurse not otherwise involved in the care of the patient who placed both IVIG and 0.9% saline in a black, opaque plastic bag, inserted an orange‐coloured infusion set into the allocated intervention (IVIG or saline) and sealed the bag with a plastic strip (more details are given in the Electronic Supplementary Material (ESM)." Comment: the method used to guarantee allocation concealment seems to be adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "The randomisation note was handed to an ICU nurse not otherwise involved in the care of the patient who placed both IVIG and 0.9% saline in a black, opaque plastic bag, inserted an orange‐coloured infusion set into the allocated intervention (IVIG or saline) and sealed the bag with a plastic strip." "Patients, clinical staff caring for the patients, research staff, the statistician and the authors when writing the first draft for the abstract (supplementary results in the ESM) were all blinded to the intervention." Comment: the method used to guarantee blinding of participants and personnel was adequate and well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Patients, clinical staff caring for the patients, research staff, the statistician and the authors when writing the first draft for the abstract (supplementary results in the ESM) were all blinded to the intervention" "The statistician (TL) did the analyses while still blinded to the intervention according to the statistical analysis plan." Comment: the method used to guarantee blinding of outcome assessment was well described and primary outcome is a patient‐reported outcome. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "129 patients were screened, of whom 100 were enrolled; 50 patients were assigned to the IVIG group and 50 patients to the placebo group" "Of the 100 patients randomised, 87 were included in the intention‐to‐treat analysis." Comment: 100 patients were randomised but the SF‐36 could not be obtained from 13 patients, thus analysis of primary outcome was based on 87patients. For the primary outcome, there were no data available for 38% of patients in each group with 25% of death and 13% of missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: in accordance with the protocol available in clinical trials.gov NCT02111161 |
| Other bias | High risk | Comment: There was baseline imbalance: number of patients who had received IVIG before randomisation was higher in placebo group 20 (40%) than in IVIG group 8 (16%) and number of patients with acute kidney injury was higher in IVIG group 5 (10%) than in placebo group 1 (2%). This is a placebo‐controlled trial, however nearly half of patients in placebo group had received one dose of IVIG before randomisation. |
Vick‐Fragoso 2009.
| Methods | Prospective, non‐inferiority randomised controlled, open‐label, parallel‐group trial Multicentric, 74 centres, worldwide Period of inclusion: April 2001 to April 2002 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Baseline characteristics ITT population Subgroup of NSTI (n = 54): 57.4% of male; mean age 52.2 years (provided upon a request to the authors) |
|
| Interventions |
Intervention 1(Per protocol (PP), n = 315); abscess n = 98; necrotizing fasciitis, n = 22; surgical wound infection n = 9; diabetic foot n = 49; complicated erysipelas n = 101; infected traumatic lesion n = 21; infected ischaemic ulcers n = 6; complicated cellulitis n = 9. moxifloxacin 400 mg per day intravenous (IV), for at least 3 days, followed by moxifloxacin 400 mg orally, once daily, for 7–21 days Intervention 2(PP, n = 317): abscess n = 93, necrotizing fasciitis, n = 13, surgical wound infection n = 13, diabetic foot n = 63, complicated erysipelas n = 95, infected traumatic lesion n = 19, infected ischaemic ulcers n = 4, complicated cellulitis n = 17 amoxicillin‐clavulanate, IV 1000 mg/200 mg three times daily for at least 3 days, followed by amoxicillin‐clavulanate 500 mg/125 mg orally, three times daily, for 7–21 days |
|
| Outcomes |
Primary outcome
CR at the TOC, days 14‐28, visit was defined as: cure (total resolution or marked improvement of all cSSSI signs and symptoms; no additional or alternative antimicrobial treatment necessary). Evaluations (both the visual description of the lesion and the assessment of clinical outcome) were performed by investigators. Secondary outcomes
|
|
| Notes | This study was sponsored by Bayer HealthCare AG. Authors declared conflict of interest for Atox Bio Ltd and Biomedical Statistical Consulting, Wynnewood, Pennsylvania. * outcomes reported that were of interest in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: “randomised into two groups in a 1:1 ratio” Comment: the method used to generate the allocation sequence was not reported |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "Patients were randomised into two groups in a 1:1 ratio to receive either moxifloxacin or the control regimen." Comment: there was no mention of how allocation concealment was guaranteed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: “open label” Comment: both participants and study personnel were aware of the assigned treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: “open‐label”, “Clinical outcomes were assessed bimodally as cure or failure, such that patients with only limited improvement in clinical status and partial resolution of symptoms would conservatively be considered as failures." Comment: assessment was performed by investigators who were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: number of randomly assigned participants: 804 Number of analysed participants for the main outcome per protocol: 632 Patients withdrawn: 172 The main reasons for withdrawal after randomisation (moxifloxacin vs amoxicillin‐clavulanate) were adverse events (6.2% vs 3.8%), insufficient therapeutic effect (4.2% vs 5.3%), patient lost to follow‐up (3.9% vs 5.3%), and protocol violation (3.4% vs 2.8%) (P > 0.1 in all cases). There was a high rate of withdrawal mainly due to serious adverse events and insufficient therapeutic effect. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available, however, the outcomes predefined in the methods were well reported in the results section. |
| Other bias | High risk | Comment: there was baseline imbalance; number of patients in the NSTI subgroup was two‐fold higher in the moxifloxacin group (36/54) treatment than in the amoxicillin‐clavulanate group (18/54) treatment |
AIDS: Acquired Immune Deficiency Syndrome; cSSSI: complicated skin and skin structure infections; cSSTI: complicated skin and soft tissue infection;CR: clinical response; ESM: electronic supplementary material; HBO: hyperbaric oxygen therapy;ICU: intensive care unit; IVIG: intravenous immunoglobulin; ITT: intention‐to‐treat;IV: intravenous; NSTI: necrotizing soft tissue infections; PCS: physical component summary; PP: per protocol; RBC: red blood cells; RRT: renal replacement therapy; SOFA: Sequential Organ Failure Assessment score; SF‐36: the Short Form (36) Health Survey; TOC: test of cure; WBC: white blood cells.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbar 2014 | The study was not a randomised controlled trial. |
| Danino 2010 | The study compared wound care dressings after surgery and not an intervention to treat NSTI. |
| Fass 1989 | The study included patients with skin and soft tissue infection with no clear definition; we were unable to contact the main author (deceased). |
| Huang 2006 | The study compared wound care dressings after surgery and not an intervention to treat NSTI. |
| Linner 2014 | The study was not a randomised controlled trial. |
| Matejec 2013 | The study's cross‐over design was not relevant for assessment of outcomes. |
| Morelli 2008 | The study's cross‐over design was not relevant for assessment of outcomes. |
| Norrby‐Teglund 2009 | The study was not a randomised controlled trial. |
| Solomkin 1986 | The definition of necrotizing soft tissue infection was inaccurate and included diabetic foot infection and Ischaemic gangrene. |
| Souyri 2008 | The study was not a randomised controlled trial. |
| Subrahmanyam 2004 | The study compared wound care dressings after surgery and not an intervention to treat NSTI. |
| Wong 2004 | The study was not a randomised controlled trial. |
| Xu 2015 | The study compared wound care dressings after surgery. |
NSTI: necrotizing soft tissue infection
Characteristics of studies awaiting assessment [ordered by study ID]
Darenberg 2003.
| Methods | This was a randomised controlled trial |
| Participants | Participants with a streptococcal toxic shock syndrome with or without NSTI |
| Interventions | Intravenous immunoglobulin G therapy versus placebo |
| Outcomes | Mortality at 28 days Time to resolution of shock Time to no further progression of the tissue infection Survival on day 180 |
| Notes | Outcomes in the subgroup of patient with NSTI are not available. Email to the study authors without answer |
Tally 1986.
| Methods | This was a randomised controlled trial |
| Participants | Participants with serious surgical infections, including intraabdominal, pelvic, biliary tract, and necrotizing soft‐tissue infections suspected of containing B.fragilis |
| Interventions | Moxalactam versus Mefoxitin, with or without tTobramycin |
| Outcomes | Cure rates |
| Notes | Outcomes in the subgroup of patient with NSTI are not available. Email to the study authors without answer |
NSTI: necrotizing soft tissue infection
Characteristics of ongoing studies [ordered by study ID]
NCT02469857.
| Trial name or title | Phase III efficacy and safety study of AB103 in the treatment of patients with necrotizing soft tissue infections (ACCUTE) |
| Methods | This was a randomised, double‐blind controlled trial |
| Participants | Participants with a necrotizing soft tissue infection |
| Interventions | AB103 0.5 mg/kg vs placebo |
| Outcomes | Clinical composite success end point (Time frame: 28 days) Safety measures: adverse events Safety measures: clinical safety laboratory Safety measures: secondary infections Recovery from acute kidney injury Time to resolution of SOFA score to ≤ 1 Critical care and hospital stay parameters: ICU days Critical care and hospital stay parameters: ventilator days Critical care and hospital stay parameters: hospital length of stay |
| Starting date | September 2015 |
| Contact information | Eileen M Bulger, MD Harborview Injury Prevention and Research Center |
| Notes |
ICU: intensive care unit; SOFA: Sequential Organ Failure Assessment score
Differences between protocol and review
Methods > Data Collection and analysis: we added the initials of the people who did the GRADE assessments (CH, LLC) and also mention how disagreements were resolved (ES).
Objectives: we expanded the objectives for clarity.
Methods >Types of participants: we clarified that we included all adults aged 18 years and over hospitalised with a diagnosis of NSTI. This was cited in the protocol but only under Types of interventions, notTypes of participants.
Methods >Type of interventions >Surgical treatments: We revised surgical treatments as type of surgery (surgical debridement, amputation). We re‐phrased "Surgical delay less than 24 hours versus surgical delay longer than 24 hours" to "immediate versus delayed intervention".
Methods >Type of interventions >Medical treatments: we clarified our comparators.
Methods > Types of studies: we added in the Methods section that cross‐over designs were excluded because trials assessed an acute life‐threatening disease (which cannot be appropriately assessed in a cross‐over study).
Methods > Types of outcomes > Secondary outcomes: we expanded the description and assessment of 'Assessment of long‐term morbidity'. However, no study in this review measured this outcome in the way we required.
Methods >Data collection and analysis: we included all outcomes in our 'Summary of findings' tables to summarise all key information, although we only planned to include our primary outcomes.
Methods >Data extraction and management: we changed the third review author who resolved any disagreements between the two other review authors to LLC because ES was not available at the time.
Methods >Assessment of risk of bias in included studies: we changed the third review author who resolved any disagreements between the two other review authors to LLC because ES was not available at the time. We added performance bias and re‐worded the domains so they reflected the Cochrane 'Risk of bias' tool. We added the following sentence for clarification: "Studies were classified as having low risk of bias if none of the domains above were rated as high risk of bias and three or less were rated as unclear risk; moderate if one was rated as high risk of bias or none were rated as high risk of bias but four or more were rated as unclear risk, and all other cases will be assumed to pertain to high risk of bias".
Methods >Dealing with missing data: we added this sentence, "we planned to synthesise data as analysed in each study (complete cases)", but could not undertake this due to lack of studies in the review.
Methods >Assessment of reporting biases: we did not perform sensitivity analyses according to a regression‐based adjustment model because no meta‐analysis was possible since the interventions compared in the trials were not similar.
Methods >Measures of treatment effects: for survival time evaluation, we planned to use hazard ratios (HR) with 95% CI; however, the required data on survival time were not available in the three included studies.
Methods >Units of analysis issues: we planned to include cluster‐randomised trials in the meta‐analyses using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); however, no clustered‐randomised trials were included in this review.
Methods >Unit of analysis issue: in cases of multi‐dose trials, we grouped together all the different dose groups as a single arm and compared them collectively with the control group.
Methods >Assessment of heterogeneity: we did not assess heterogeneity because the three included trials in this review compared different interventions. If a future update includes any new studies, heterogeneity can be assessed through forest plots and I² statistics as planned in the protocol.
Methods >Assessment of reporting bias: we planned to assess reporting bias for primary end points using funnel plots. However, the validity conditions (low heterogeneity, 10 or more studies including at least one with significant results, and a ratio of the maximal to minimal variance across studies greater than 4) for funnel plot asymmetry tests provided were not met (Ioannidis 2007). We planned to perform sensitivity analyses according to a regression‐based adjustment model; however, only three studies were included in this review, so we could not undertake any sensitivity analyses.
Methods >Data synthesis: we did not perform data synthesis because the three included trials in this review compared different interventions. Thus, we deleted all reference to pooling using a fixed‐effect model. If this review is updated, we will use the random‐effects model to pool studies in a meta‐analysis.
Methods >Subgroup analysis and investigation of heterogeneity: the planned subgroup analyses were not performed because of the low number of studies included in the review.
Methods >Sensitivity analysis: the number of included studies was insufficient to perform sensitivity analyses.
Contributions of authors
CH was the contact person with the editorial base. CH and LLC co‐ordinated contributions from the co‐authors and wrote the final draft of the review. CH, RB, and LLC screened papers against eligibility criteria. CH obtained data on ongoing and unpublished studies. CH, RB, and LLC appraised the quality of papers. CH and RB extracted data for the review and sought additional information about papers. CH entered data into RevMan. CH, RB, ES, and LLC analysed and interpreted data. LLC, ES, and CH worked on the methods sections. CH, ES, LLC, RB, NDP, PJ, and OC drafted the clinical sections of the background and responded to the clinical comments of the referees. LLC and ES responded to the methodology and statistics comments of the referees. CH was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. CH is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
Hôpital Henri Mondor, France.
Salary support
External sources
aRED (Association of Dermatology Recommendations) commission of the French Society of Dermatology, France.
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Camille Hua: nothing to declare. Romain Bosc: nothing to declare. Emilie Sbidian: nothing to declare. Nicolas De Prost: nothing to declare. Patricia Jabre: nothing to declare. Olivier Chosidow: "I have received consulting fees from Cardiome Pharma Corporation for a board meeting on dalbavancin; my institution (Hôpital Henri Mondor, Department of Microbiology) has received a research grant from the French Society of Dermatology for a metagenomic study in NSTIs (masters degree)." Laurence Le Cleach: nothing to declare. Carolyn Hughes: nothing to declare
New
References
References to studies included in this review
Bulger 2014 {published data only}
- Bulger EM, Maier RV, Sperry J, Joshi M, Henry S, Moore FA, et al. A novel drug for treatment of necrotizing soft‐tissue infections: A randomized clinical trial. JAMA Surgery 2014;149(6):528‐36. [CENTRAL: CN‐00995333; PUBMED: 24740134] [DOI] [PubMed] [Google Scholar]
Madsen 2017 {published data only}
- Madsen MB, Hjortrup PB, Hansen MB, Lange T, Norrby‐Teglund A, Hyldegaard O, et al. Immunoglobulin G for patients with necrotising soft tissue infection (INSTINCT): a randomised, blinded, placebo‐controlled trial. Intensive Care Medicine 2017;43(11):1585‐93. [CENTRAL: CN‐01365445; DOI: 10.1007/s00134-017-4786-0; PUBMED: 28421246] [DOI] [PubMed] [Google Scholar]
Vick‐Fragoso 2009 {published data only}
- Vick‐Fragoso RG, Hernández‐Oliva J, Cruz‐Alcázar CF, Amábile‐Cuevas P, Arvis P, Reimnitz JR, et al. Efficacy and safety of sequential intravenous/oral moxifloxacin vs intravenous/oral amoxicillin/clavulanate for complicated skin and skin structure infections. Infection 2009;37(5):407‐17. [CENTRAL: CN‐00752976; PUBMED: 19768381] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbar 2014 {published data only}
- Abbar M, Janane A, Dakkak Y, Ghadouane M, Ameur A. Combination of hyperbaric oxygen therapy and surgical debridement in management of fournier's gangrene. 34th Congress of the Societe Internationale d'Urologie, SIU 2014 Glasgow United Kingdom. Urology 2014;84(4 Suppl 1):S236‐7. [EMBASE: 71654403] [Google Scholar]
Danino 2010 {published data only}
- Danino AM, Guberman S, Mondie JM, Jebrane A, Servant JM. Calcium polyuronate dressing supplemented with zinc and manganese (trionic) in necrotizing dermohypodermitis of the extremities: a randomized multicentre study. Annals of Burns & Fire Disasters 2010;23(2):95‐101. [PUBMED: 21991205] [PMC free article] [PubMed] [Google Scholar]
Fass 1989 {published data only}
- Fass RJ, Plouffe JF, Russell JA. Intravenous/oral ciprofloxacin versus ceftazidime in the treatment of serious infections. American Journal of Medicine 1989;87(5A):164S‐8S. [PUBMED: 2589361] [DOI] [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang WS, Hsieh SC, Hsieh CS, Schoung JY, Huang T. Use of vacuum‐assisted wound closure to manage limb wounds in patients suffering from acute necrotizing fasciitis. Asian Journal of Surgery 2006;29(3):135‐9. [PUBMED: 16877210] [DOI] [PubMed] [Google Scholar]
Linner 2014 {published data only}
- Linner A, Darenberg J, Sjolin J, Henriques‐Normark B, Norrby‐Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: A comparative observational study. Clinical Infectious Diseases 2014;59(6):851‐7. [PUBMED: 24928291] [DOI] [PubMed] [Google Scholar]
Matejec 2013 {published data only}
- Matejec R, Kayser F, Schmal F, Uhle F, Bodeker RH, Maxeiner H, et al. Effects of corticotropin‐releasing hormone on proopiomelanocortin derivatives and monocytic HLA‐DR expression in patients with septic shock. Peptides 2013;47:133‐41. [PUBMED: 23891702] [DOI] [PubMed] [Google Scholar]
Morelli 2008 {published data only}
- Morelli A, Lange M, Ertmer C, Dunser M, Rehberg S, Bachetoni A, et al. Short‐term effects of phenylephrine on systemic and regional hemodynamics in patients with septic shock: a crossover pilot study. Shock 2008;29(4):446‐51. [PUBMED: 17885646] [DOI] [PubMed] [Google Scholar]
Norrby‐Teglund 2009 {published data only}
- Norrby‐Teglund A. Managing group A streptococcal infection: the role of IVIg. 19th European Congress of Clinical Microbiology and Infectious Diseases, Helsinki Finland. Clinical Microbiology and Infection 2009;15:S50. [EMBASE: 70070333] [Google Scholar]
Solomkin 1986 {published data only}
- Solomkin JS, Cocchetto DM. Ceftazidime versus tobramycin plus ticarcillin in the treatment of soft‐tissue infections. Clinical Therapeutics 1986;9(1):123‐34. [PUBMED: 3545476] [PubMed] [Google Scholar]
Souyri 2008 {published data only}
- Souyri C, Olivier P, Grolleau S, Lapeyre‐Mestre M, French Network of Pharmacovigilance Centres. Severe necrotizing soft‐tissue infections and nonsteroidal anti‐inflammatory drugs. Clinical & Experimental Dermatology 2008;33(3):249‐55. [PUBMED: 18261144] [DOI] [PubMed] [Google Scholar]
Subrahmanyam 2004 {published data only}
- Subrahmanyam M, Ugane SP. Honey dressing beneficial in treatment of Fournier's gangrene. Indian Journal of Surgery 2004;66(2):75‐7. [CENTRAL: CN‐00645330] [Google Scholar]
Wong 2004 {published data only}
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Critical Care Medicine 2004;32(7):1535‐41. [PUBMED: 15241098] [DOI] [PubMed] [Google Scholar]
Xu 2015 {published data only}
- Xu X‐P, Cai X‐H, Zhang L, Li X‐H, Liu Z‐H, Pan Y, et al. Clinical efficacy of vacuum sealing drainage in treatment of fournier's gangrene. World Chinese Journal of Digestology 2015;23(2):348‐52. [Google Scholar]
References to studies awaiting assessment
Darenberg 2003 {published data only}
- Darenberg J, Ihendyane N, Sjolin J, Aufwerber E, Haidl S, Follin P, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double‐blind, placebo‐controlled trial. Clinical Infectious Diseases 2003;37(3):333‐40. [PUBMED: 12884156] [DOI] [PubMed] [Google Scholar]
Tally 1986 {published data only}
- Tally FP, Kellum JM, Ho JL, O'Donnell TF, Barza M, Gorbach SL. Randomized prospective study comparing moxalactam and cefoxitin with or without tobramycin for the treatment of serious surgical infections. Antimicrobial Agents and Chemotherapy 1986;29(2):244‐9. [PUBMED: 3717931] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02469857 {unpublished data only}
- NCT02469857. Phase III efficacy and safety study of AB103 in the treatment of patients with necrotizing soft tissue infections (ACCUTE) [Phase III, randomized, double‐blind, placebo‐controlled, parallel group, study of AB103 as compared to placebo in patients with necrotizing soft tissue infections. ACCUTE (AB103 clinical composite endpoint study in necrotizing soft tissue infections)]. clinicaltrials.gov/ct2/show/NCT02469857 (First received: 6 June 2015).
Additional references
Adams 1985
- Adams EM, Gudmundsson S, Yocum DE, Haselby RC, Craig WA, Sundstrom WR. Streptococcal myositis. Archives of Internal Medicine 1985;145(6):1020‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Anaya 2005
- Anaya DA, McMahon K, Nathens AB, Sullivan SR, Foy H, Bulger E. Predictors of mortality and limb loss in necrotizing soft tissue infections. Archives of Surgery 2005;140(2):151‐7; discussion 158. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anaya 2007
- Anaya DA, Dellinger EP. Necrotizing soft‐tissue infection: diagnosis and management. Clinical Infectious Diseases 2007;44(5):705‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anglemyer 2014
- Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database of Systematic Reviews 2014;4:MR000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Audureau 2017
- Audureau E, Hua C, Prost N, Hemery F, Decousser JW, Bosc R, et al. Mortality of necrotising fasciitis: relative influence of individual and hospital‐level factors, a nationwide multilevel study, France, 2007‐2012. British Journal of Dermatology 2017;177(6):1575‐82. [DOI: 10.1111/bjd.15615; PUBMED: 28452064] [DOI] [PubMed] [Google Scholar]
Bilton 1998
- Bilton BD, Zibari GB, McMillan RW, Aultman DF, Dunn G, McDonald JC. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. American S 1998;64(5):397‐400; discussion 400‐1. [PUBMED: 9585771] [PubMed] [Google Scholar]
Blencowe 2015
- Blencowe NS, Brown JM, Cook JA, Metcalfe C, Morton DG, Nicholl J, et al. Interventions in randomised controlled trials in surgery: issues to consider during trial design. Trials 2015;16:392. [PUBMED: 26337522] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295‐309. [PUBMED: 18283207] [DOI] [PubMed] [Google Scholar]
Boyer 2009
- Boyer A, Vargas F, Coste F, Saubusse E, Castaing Y, Gbikpi‐Benissan G, et al. Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Medicine 2009;35(5):847‐53. [PUBMED: 19099288] [DOI] [PubMed] [Google Scholar]
Bryant 2006
- Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal‐muscle injury mediates the binding of Streptococcus pyogenes. Journal of Infectious Diseases 2006;193(12):1685‐92. [PUBMED: 16703512] [DOI] [PubMed] [Google Scholar]
Carapetis 2014
- Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2014;59(3):358‐65. [PUBMED: 24785239] [DOI] [PubMed] [Google Scholar]
CDC 2012
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Group A Streptococcus, 2010. www.cdc.gov/abcs/reports‐findings/survreports/gas10.html (accessed 29 April 2015).
Chelsom 1994
- Chelsom J, Halstensen A, Haga T, Hoiby EA. Necrotising fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet 1994;344(8930):1111‐5. [PUBMED: 7934492] [DOI] [PubMed] [Google Scholar]
Childers 2002
- Childers BJ, Potyondy LD, Nachreiner R, Rogers FR, Childers ER, Oberg KC, et al. Necrotizing fasciitis: a fourteen‐year retrospective study of 163 consecutive patients. American Surgeon 2002;68(2):109‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Chosidow 2001
- Chosidow O. Subacute forms of necrotizing fasciitis and necrotizing cellulitis: diagnosis criteria and surgical decision‐making. Annales de Dermatologie et de Vénérologie 2001;128(3 Pt 2):390‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Colledge 2009
- Colledge A, Hunter B, Bunkall LD, Holmes EB. Impairment rating ambiguity in the United States: the Utah impairment guides for calculating workers' compensation Impairments. Journal of Korean Medical Science 2009;24(Suppl 2):S232‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Delaney 2008
- Delaney A, Angus DC, Bellomo R, Cameron P, Cooper DJ, Finfer S, et al. Bench‐to‐bedside review: the evaluation of complex interventions in critical care. Critical Care 2008;12(2):210. [PUBMED: 18439321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dellinger 1981
- Dellinger EP. Severe necrotizing soft‐tissue infections. Multiple disease entities requiring a common approach. JAMA 1981;246(15):1717‐21. [MEDLINE: ] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elliott 1996
- Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Annals of Surgery 1996;224(5):672‐83. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 2000
- Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. American Journal of Surgery 2000;179(5):361‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ellis Simonsen 2006
- Ellis Simonsen SM, Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, et al. Cellulitis incidence in a defined population. Epidemiology & Infection 2006;134(2):293‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fournier 1883
- Fournier AJ. Devastating gangrene of the penis [Gangrene Foudroyante de la verge]. Seminars in Medicine 1883;3:345. [Google Scholar]
Goh 2014
- Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. British Journal of Surgery 2014;101(1):e119‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 11 October 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guidance FDA 2012
- Food, Drug Administration. Guidances for industry and investigators on safety reporting requirements for investigational new drug applications and bioavailability/bioequivalence studies, and a small entity compliance guide. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM332846.pdf (accessed 11 October 2017).
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583 ] [DOI] [PubMed] [Google Scholar]
Hasham 2005
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005;330(7495):830‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hsiao 2008
- Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. American Journal of Emergency Medicine 2008;26(2):170‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ioannidis 2007
- Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ : Canadian Medical Association Journal 2007;176(8):1091‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansson 2010
- Johansson L, Thulin P, Low DE, Norrby‐Teglund A. Getting under the skin: the immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clinical Infectious Diseases 2010;51(1):58‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kadri 2017
- Kadri SS, Swihart BJ, Bonne SL, Hohmann SF, Hennessy LV, Louras P, et al. Impact of intravenous immunoglobulin on survival in necrotizing fasciitis with vasopressor‐dependent shock: a propensity score‐matched analysis from 130 US hospitals. Clinical Infectious Diseases 2017;64(7):877‐85. [PUBMED: 28034881] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaul 1997
- Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population‐based surveillance for group A streptococcal necrotizing fasciitis: Clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study. American Journal of Medicine 1997;103(1):18‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kobayashi 2011
- Kobayashi L, Konstantinidis A, Shackelford S, Chan LS, Talving P, Inaba K, et al. Necrotizing soft tissue infections: delayed surgical treatment is associated with increased number of surgical debridements and morbidity. Journal of Trauma‐Injury Infection & Critical Care 2011;71(5):1400‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Korhonen 2000
- Korhonen K. Hyperbaric oxygen therapy in acute necrotizing infections with a special reference to the effects on tissue gas tensions. Annales Chirurgiae et Gynaecologiae 2000;89(214):7‐36. [MEDLINE: ] [PubMed] [Google Scholar]
Lamagni 2008
- Lamagni TL, Darenberg J, Luca‐Harari B, Siljander T, Efstratiou A, Henriques‐Normark B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. Journal of Clinical Microbiology 2008;46(7):2359‐67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamb 2015
- Lamb LE, Sriskandan S, Tan LK. Bromine, bear‐claw scratch fasciotomies, and the Eagle effect: management of group A streptococcal necrotising fasciitis and its association with trauma. Lancet Infectious Diseases 2015;15(1):109‐21. [PUBMED: 25541175] [DOI] [PubMed] [Google Scholar]
Le Cleach 2014
- Cleach L, Trinquart L, Do G, Maruani A, Lebrun‐Vignes B, Ravaud P, et al. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD009036.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lepoutre 2011
- Lepoutre A, Doloy A, Bidet P, Leblond A, Perrocheau A, Bingen E, et al. Epidemiology of invasive Streptococcus pyogenes infections in France in 2007. Journal of Clinical Microbiology 2011;49(12):4094‐100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levett 2015
- Levett D, Bennett MH, Millar I. Adjunctive hyperbaric oxygen for necrotizing fasciitis. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD007937.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
May 2009
- May AK, Stafford RE, Bulger EM, Heffernan D, Guillamondegui O, Bochicchio G, et al. Treatment of complicated skin and soft tissue infections. Surgical Infections 2009;10(5):467‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McClaine 2010
- McClaine RJ, Husted TL, Hebbeler‐Clark RS, Solomkin JS. Meta‐analysis of trials evaluating parenteral antimicrobial therapy for skin and soft tissue infections. Clinical Infectious Diseases 2010;50(8):1120‐6. [PUBMED: 20210644] [DOI] [PubMed] [Google Scholar]
McHenry 1995
- McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft‐tissue infections. Annals of Surgery 1995;221(5):558‐63; discussion 563‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norrby‐Teglund 1998
- Norrby‐Teglund A, Stevens DL. Novel therapies in streptococcal toxic shock syndrome: attenuation of virulence factor expression and modulation of the host response. Current Opinion in Infectious Diseases 1998;11(3):285‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Norrby‐Teglund 2005
- Norrby‐Teglund A, Muller MP, Mcgeer A, Gan BS, Guru V, Bohnen J, et al. Successful management of severe group A streptococcal soft tissue infections using an aggressive medical regimen including intravenous poly specific immunoglobulin together with a conservative surgical approach. Scandinavian Journal of Infectious Diseases 2005;37(3):166‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nuwayhid 2007
- Nuwayhid ZB, Aronoff DM, Mulla ZD. Blunt trauma as a risk factor for group A streptococcal necrotizing fasciitis. Annals of Epidemiology 2007;17(11):878‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Grady 2007
- O'Grady KA, Kelpie L, Andrews RM, Curtis N, Nolan TM, Selvaraj G, et al. The epidemiology of invasive group A streptococcal disease in Victoria, Australia. Medical Journal of Australia 2007;186(11):565‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Park 2009
- Park KH, Jung SI, Jung YS, Shin JH, Hwang JH. Marine bacteria as a leading cause of necrotizing fasciitis in coastal areas of South Korea. American Journal of Tropical Medicine & Hygiene 2009;80(4):646‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Pham 2009
- Pham TN, Moore ML, Costa BA, Cuschieri J, Klein MB. Assessment of functional limitation after necrotizing soft tissue infection. Journal of Burn Care & Research 2009;30(2):301‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phan 2010
- Phan HH, Cocanour CS. Necrotizing soft tissue infections in the intensive care unit. Critical Care Medicine 2010;38(9 Suppl):S460‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rondinelli 2009
- Rondinelli RD. Changes for the new AMA Guides to impairment ratings, 6th Edition: implications and applications for physician disability evaluations. PM & R : the Journal of Injury, Function, and Rehabilitation 2009;1(7):643‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sarani 2009
- Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. Journal of the American College of Surgeons 2009;208(2):279‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sartelli 2014
- Sartelli M, Malangoni MA, May AK, Viale P, Kao LS, Catena F, et al. World Society of Emergency Surgery (WSES) guidelines for management of skin and soft tissue infections. World Journal of Emergency Surgery 2014;9(1):57. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SFD‐SPILF 2000
- The French Society of Dermatology (SFD) and The French Society of infectious Disease (SPILF). Management of erysipelas and necrotizing fasciitis (long text) [Erysipèle et fasciite Nécrosante : Prise en charge]. Annales de Dermatologie et de Vénérologie 2000;127(12):1118‐37. [MEDLINE: ] [PubMed] [Google Scholar]
Stevens 1989
- Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, et al. Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A. New England Journal of Medicine 1989;321(1):1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stevens 2005
- Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clinical Infectious Diseases 2005;41(10):1373‐406. [PUBMED: 16231249] [DOI] [PubMed] [Google Scholar]
Stevens 2014
- Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2014;59(2):e10‐52. [PUBMED: 24973422] [DOI] [PubMed] [Google Scholar]
Wilkinson 2004
- Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Archives of Surgery 2004;139(12):1339‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilson 1952
- Wilson B. Necrotizing fasciitis. American Surgeon 1952;18(4):416‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Wong 2003
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. Journal of Bone & Joint Surgery ‐ American volume 2003;85‐A(8):1454‐60. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Hua 2015
- Hua C, Bosc R, Sbidian E, Prost N, Jabre P, Chosidow O, et al. Interventions for necrotizing soft tissue infections in adults. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011680] [DOI] [PMC free article] [PubMed] [Google Scholar]


