Abstract
Background
In preterm newborns, the ductus arteriosus frequently fails to close and the infants require medical or surgical closure of the patent ductus arteriosus (PDA). A PDA can be treated surgically; or medically with one of two prostaglandin inhibitors, indomethacin or ibuprofen. Case reports suggest that paracetamol may be an alternative for the closure of a PDA. An association between prenatal or postnatal exposure to paracetamol and later development of autism or autism spectrum disorder has been reported.
Objectives
To determine the effectiveness and safety of intravenous or oral paracetamol compared with placebo or no intervention, intravenous indomethacin, intravenous or oral ibuprofen, or with other cyclo‐oxygenase inhibitors for treatment of an echocardiographically diagnosed PDA in preterm or low birth weight infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 10), MEDLINE via PubMed (1966 to 6 November 2017), Embase (1980 to 6 November 2017), and CINAHL (1982 to 6 November 2017). We searched clinical trial databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials (RCT) and quasi‐randomised trials.
Selection criteria
We included RCTs in which paracetamol was compared to no intervention, placebo or other agents used for closure of PDA irrespective of dose, duration and mode of administration in preterm (≤ 34 weeks' postmenstrual age) infants. We both reviewed the search results and made a final selection of potentially eligible articles by discussion. We included studies of both prophylactic and therapeutic use of paracetamol.
Data collection and analysis
We performed data collection and analyses in accordance with the methods of the Cochrane Neonatal Review Group. We used the GRADE approach to assess the quality of evidence for the following outcomes when data were available: failure of ductal closure after the first course of treatment; neurodevelopmental impairment; all‐cause mortality during initial hospital stay (death); gastrointestinal bleed or stools positive for occult blood; and serum levels of creatinine after treatment (µmol/L).
Main results
We included eight studies that reported on 916 infants. One of these studies compared paracetamol to both ibuprofen and indomethacin. Five studies compared treatment of PDA with paracetamol versus ibuprofen and enrolled 559 infants. There was no significant difference between paracetamol and ibuprofen for failure of ductal closure after the first course of drug administration (typical risk ratio (RR) 0.95, 95% confidence interval (CI) 0.75 to 1.21; typical risk difference (RD) −0.02, 95% CI −0.09 to 0.09); I² = 0% for RR and RD; moderate quality of evidence. Four studies (n = 537) reported on gastrointestinal bleed which was lower in the paracetamol group versus the ibuprofen group (typical RR 0.28, 95% CI 0.12 to 0.69; typical RD −0.06, 95% CI −0.09 to −0.02); I² = 0% for RR and RD; number needed to treat for an additional beneficial outcome (NNTB) 17 (95% CI 11 to 50); moderate quality of evidence. The serum levels of creatinine were lower in the paracetamol group compared with the ibuprofen group in four studies (moderate quality of evidence), as were serum bilirubin levels following treatment in two studies (n = 290). Platelet counts and daily urine output were higher in the paracetamol group compared with the ibuprofen group. One study reported on long‐term follow‐up to 18 to 24 months of age following treatment with paracetamol versus ibuprofen. There were no significant differences in the neurological outcomes at 18 to 24 months (n = 61); (low quality of evidence).
Two studies compared prophylactic administration of paracetamol for a PDA with placebo or no intervention in 80 infants. Paracetamol resulted in a lower rate of failure of ductal closure after 4 to 5 days of treatment compared to placebo or no intervention which was of borderline significance for typical RR 0.49 (95% CI 0.24 to 1.00; P = 0.05); but significant for typical RD −0.21 (95% CI −0.41 to −0.02); I² = 0 % for RR and RD; NNTB 5 (95% CI 2 to 50); (low quality of evidence).
Two studies (n = 277) compared paracetamol with indomethacin. There was no significant difference in the failure to close a PDA (typical RR 0.96, 95% CI 0.55 to 1.65; I² = 11%; typical RD −0.01, 95% CI −0.09 to 0.08; I² = 17%) (low quality of evidence). Serum creatinine levels were significantly lower in the paracetamol group compared with the indomethacin group and platelet counts and daily urine output were significantly higher in the paracetamol group.
Authors' conclusions
Moderate‐quality evidence according to GRADE suggests that paracetamol is as effective as ibuprofen; low‐quality evidence suggests paracetamol to be more effective than placebo or no intervention; and low‐quality evidence suggests paracetamol as effective as indomethacin in closing a PDA. There was no difference in neurodevelopmental outcome in children exposed to paracetamol compared to ibuprofen; however the quality of evidence is low and comes from only one study. In view of concerns raised regarding neurodevelopmental outcomes following prenatal and postnatal exposure to paracetamol, long‐term follow‐up to at least 18 to 24 months' postnatal age must be incorporated in any studies of paracetamol in the newborn population. At least 19 ongoing trials have been registered. Such trials are required before any recommendations for the possible routine use of paracetamol in the newborn population can be made.
Keywords: Humans; Infant, Newborn; Acetaminophen; Acetaminophen/administration & dosage; Acetaminophen/adverse effects; Administration, Oral; Ductus Arteriosus, Patent; Ductus Arteriosus, Patent/drug therapy; Ibuprofen; Ibuprofen/administration & dosage; Ibuprofen/adverse effects; Indomethacin; Indomethacin/administration & dosage; Infant, Low Birth Weight; Infant, Premature; Injections, Intravenous; Oxygen Inhalation Therapy; Oxygen Inhalation Therapy/utilization; Randomized Controlled Trials as Topic; Treatment Outcome
Paracetamol (acetaminophen) for patent ductus arteriosus (a blood vessel necessary for fetal survival) in preterm and low birth weight infants
Review question: How effective and safe are paracetamol, which has weak anti‐inflammatory properties, compared with placebo (a substance with no active therapeutic effect), or no intervention, or nonsteroidal anti‐inflammatory drugs (indomethacin and ibuprofen), for closure of a PDA in preterm/low birth weight infants?
Background: A common complication for preterm (premature) or small babies is a patent ductus arteriosus (PDA). Blood circulation to the (as yet) non‐functioning lungs is unnecessary before birth (the fetal blood supply is oxygenated via the placenta). The PDA is a temporary fetal blood vessel that connects the pulmonary artery (the vessel that, after birth, takes blood depleted of oxygen from the heart to the lungs) to the aorta (the vessel that takes freshly oxygenated blood, returned from the lungs to the heart by the pulmonary vein, away from the heart and on the beginning of its journey round the body). In other words the PDA ‘short‐circuits’ the fetal circulation of blood through the lungs.. It is necessary to sustain life in the womb, but it should close after birth. Sometimes it remains open because of the baby's immature stage of development. A PDA can lead to life‐threatening complications. The usual treatment for PDA has been indomethacin or ibuprofen which inhibit the production of prostaglandins and promotes the closure of the PDA. Recently paracetamol (acetaminophen), a commonly used drug to treat fever or pain in infants, children and adults, has been suggested as an alternative to ibuprofen, with potentially fewer side effects. A number of case reports and case series have suggested that paracetamol may be an alternative for the closure of a PDA. Exactly how paracetamol works to close the PDA is not known, but probably involves inhibition of prostaglandin synthesis. Prostaglandins are chemical compounds which are made throughout the body (i.e. not in any one particular organ), particularly wherever soft tissues are damaged, and their production (synthesis) plays a key role in healing processes. They are known to play an important role in keeping the ductus arteriosus open (patent), so lowering their production would encourage closure of the ductus arteriosus.
Study characteristics: We identified a total of eight studies that enrolled 916 preterm infants and compared the effectiveness and safety of paracetamol versus ibuprofen, indomethacin or placebo in the treatment of a PDA in early life.
Key results: When the results of the included studies were combined, the success rate for paracetamol to close a PDA was higher than that of placebo and similar to that of ibuprofen and indomethacin. Paracetamol appears to have fewer adverse effects on kidney and liver functions. In one small study that followed children to 18 to 24 months of age there was no difference in neurodevelopmental impairment. The evidence is up to date as of November 2017.
Conclusions: Paracetamol appears to be a promising alternative to indomethacin and ibuprofen for the closure of a PDA with possibly fewer adverse effects.
Additional studies testing this intervention and including longer‐term follow‐up are needed before paracetamol can be recommended as standard treatment for a PDA in preterm infants. Several studies are ongoing that will eventually provide additional information. Because of reports of a possible association between prenatal paracetamol and the development of autism or autism spectrum disorder in childhood and language delay in girls, long‐term follow‐up to at least 18 to 24 months' postnatal age must be incorporated in any studies of paracetamol in the newborn population.
Quality of evidence: Although the healthcare providers were not always 'blinded' (unaware of which drug the infants received) we judged the quality of the evidence to be moderate.
Summary of findings
Summary of findings for the main comparison.
Paracetamol compared to ibuprofen for patent ductus arteriosus in preterm or low birth weight infants
| Paracetamol (oral or IV) compared to ibuprofen (oral or IV) for patent ductus arteriosus in preterm or low birth weight infants | ||||||
| Patient or population: preterm or low birth weight infants with patent ductus arteriosus Settings: hospitals in China (2 studies), Egypt, Jordan, and Turkey Intervention: paracetamol Comparison: ibuprofen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral ibuprofen | Oral paracetamol | |||||
| Failure of ductal closure after the first course of treatment Echocardiogram | High risk study population | RR 0.95 (0.75 to 1.21) | 559 (5 studies) | ⊕⊕⊕⊝ moderate | Bias: we had no concerns for random sequence generation in the 5 included trials but the allocation concealment was unclear in 1 of the studies. We did not downgrade the quality of evidence on this item. There were concerns about blinding of personnel and of blinding of outcome assessments. We downgraded the quality of the evidence by 1 step. Heterogeneity/Consistency: we noted no heterogeneity (I² = 0% for both RR and RD). Directness of evidence: studies were conducted in the target population. Precision: because of the relatively large sample size (559 infants), the point estimate was precise with a narrow 95% CI. Presence of publication bias: Although only 5 studies were included, the funnel plot we constructed was symmetrical. |
|
| 329 per 1000 | 312 per 1000 (200 to 438) | |||||
| All‐cause mortality during initial hospital stay Clinical assessment, no risk of bias | High risk study population | RR 0.96 (0.55 to 1.67) | 272 (3 studies) | ⊕⊕⊕⊝ moderate | Bias: we had no concerns regarding the assessment of mortality. Heterogeneity/Consistency: we noted no heterogeneity (I² = 0% for both RR and RD). Directness of evidence: studies were conducted in the target population. Precision: because of the relatively small sample size (272 infants), the point estimate was not precise with a wide 95% CI. We downgraded the quality of the evidence by 1 step. Presence of publication bias: as only 3 studies were included we did not perform a funnel plot. |
|
| 152 per 1000 | 152 per 1000 (125 to 231) | |||||
|
Neurodevelopmental impairment Clinical assessments by assessors blinded to group assignment, no risk of bias |
High risk study population | RR 0.93 (0.44 to 1.96) | 61 (1 study) | ⊕⊕⊝⊝ low | Bias: of the 75 infants eligible for follow‐up at 18 to 24 months' corrected age 61 infants were evaluated (81%).The assessor was blinded to the previous assignment to paracetamol or ibuprofen groups. Heterogeneity/Consistency: as only 1 study was included in the analysis, tests for heterogeneity were not applicable. Precision: because of the small sample size of the only included study (61 infants), the point estimate was not precise with a wide 95% CI. We downgraded the quality of the evidence by 2 steps. Presence of publication bias: as only 1 study was included we did not perform a funnel plot. |
|
| 323 per 1000 | 300 per 1000 (300 ‒ 1 study no range) | |||||
| Gastrointestinal bleed or stools positive for occult blood | High risk study population | RR 0.28 (0.12 to 0.69) | 537 (4 studies) | ⊕⊕⊕⊝ moderate | Bias: we had no concerns for random sequence generation in the 4 included trials but the allocation concealment was unclear in 1 of the studies. We did not downgrade the quality of evidence on this item. There were concerns about blinding of personnel and of blinding of outcome assessments. We downgraded the quality of the evidence by 1 step. Heterogeneity/Consistency: we noted no heterogeneity (I² = 0% for both RR and RD). Directness of evidence: studies were conducted in the target population. Precision: because of the relatively large sample size (537 infants), the point estimate was precise with a narrow 95% CI. Presence of publication bias: as only 5 studies were included we did not construct a funnel plot. |
|
| 78 per 1000 | 22 per 1000 (12 to 135) | |||||
| Serum levels of creatinine after treatment (µmol/L) Serum samples | The weighted mean difference (WMD) for serum levels of creatinine after treatment mmol/L in the intervention (paracetamol) group was 8.92 µmol/L lower (−6.55 to −11.28 lower) than in the ibuprofen group | 537 (4 studies) | ⊕⊕⊕⊝ moderate | Bias: we had no concerns for random sequence generation in the 4 included trials but the allocation concealment was unclear in 1 of the studies. We did not downgrade the quality of evidence on this item. There were concerns about blinding of personnel and of blinding of outcome assessments. However, for an objective outcome of serum creatinine level we have not downgraded the evidence. Heterogeneity/Consistency: there was high heterogeneity (I² = 84%) for WMD. We downgraded the quality of the evidence by 1 step. Directness of evidence: studies were conducted in the target population. Precision: because of the relatively large sample size (537 infants), the point estimate was precise with a narrow 95% CI. Presence of publication bias: As only 4 studies were included we did not construct a funnel plot. |
||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
Summary of findings 2.
Prophylactic administration of paracetamol versus placebo or no intervention for patent ductus arteriosus
| Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention for patent ductus arteriosus | ||||||
|
Patient or population: preterm infants with patent ductus arteriosus Settings: Neonatal intensive care units Intervention: paracetamol Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Paracetamol | |||||
|
Failure of ductal closure after 4 to 5 days of treatment PDA diagnosed by ECHO |
High risk population | RR 0.49 (0.24 to1.00) | 80 (2) | ⊕⊕⊝⊝ low | Bias: the study by Härkin 2016 was of the highest quality and with no concerns about bias. For the study by Asbagh 2015 allocation concealment was unclear as was the blinding of personnel and outcome assessments and possible reporting bias. We downgraded the quality by 1 step. Heterogeneity/Consistency: we noted no heterogeneity (I² = 0% for both RR and RD). Directness of evidence: studies were conducted in the target population. Precision: because of the small sample size (80 infants), the point estimate although not statistically significant for RR was significant for RD. The confidence interval was wide. We downgraded the quality by 1 step. Presence of publication bias: as only 2 studies were included we did not construct a funnel plot. |
|
| 415 per 1000 | 205 per 1000 (174 to 250) | |||||
|
Death Clinical assessment |
High risk population | RR 0.35 (0.04 to 3.20) | 80 (2) | ⊕⊕⊕⊝ moderate | Bias: we had no concerns regarding the assessment of mortality. Heterogeneity/Consistency: we noted no heterogeneity (I² = 0% for both RR and RD). Directness of evidence: studies were conducted in the target population. Precision: because of the small sample size (80 infants), the point estimate was not precise with a wide 95% CI. We downgraded the quality of the evidence by 1 step. Presence of publication bias: as only 3 studies were included we did not perform a funnel plot. |
|
| 49 per 1000 | 0 per 1000 0 to ) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
Summary of findings 3.
Paracetamol compared with indomethacin for patent ductus arteriosus
| Paracetamol (oral or IV) compared with indomethacin (IV) for patent ductus arteriosus | ||||||
|
Patient or population: preterm infants with patent ductus arteriosus Settings: Neonatal intensive care unit Intervention: paracetamol Comparison: indomethacin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Indomethacin | Paracetamol | |||||
|
Failure to close a PDA Assessed by ECHO |
High‐risk population | RR 0.96 (0.55 to 1.65) | 273 (2) | ⊕⊕⊕⊝ moderate | Bias: we had no concerns for random sequence generation or allocation concealment in the 2 included studies. However we did raise concerns regarding blinding of personnel and outcome assessments and for reporting bias our judgement was unclear. We downgraded the quality of evidence on this item by 1 step. Heterogeneity/Consistency: we noted no heterogeneity (I² = 11% for RR and 17% for RD) (none). Directness of evidence: studies were conducted in the target population. Precision: because of the relatively large sample size (237 infants), the point estimate was quite precise with a narrow 95% CI. Presence of publication bias: although only 5 studies were included the funnel plot was symmetrical. |
|
| 153 per 1000 | 147 per 1000 (0 to 200) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
Background
Description of the condition
The ductus arteriosus connects the pulmonary artery to the descending aorta (Clyman 2000). Normal fetal circulation is dependent on the placenta and the patency of the ductus arteriosus (PDA) (Mathew 1998). During fetal life it diverts most of the combined ventricular output away from the lungs (Clyman 2000). Following birth, and with the separation of the placenta and initiation of breathing, the circulation changes and the ductus closes (Mathew 1998). In full‐term newborns this happens within 24 to 48 hours after birth (Clyman 2000). In preterm newborns the ductus frequently fails to close. As a result, 70% of infants born before 28 weeks' postmenstrual age (PMA) require medical or surgical closure of the PDA (Clyman 2000). The failure of the ductus arteriosus to constrict after birth is due to lower intrinsic tone, less ductal muscle fibre and fewer subendothelial cushions in preterm as compared to term infants (Hammerman 1995). The immature ductus arteriosus has higher sensitivity to the vasodilating effects of prostaglandins and nitric oxide (Hammerman 1995). This is aggravated by haemodynamic derangements due to respiratory distress syndrome and surfactant therapy (Hammerman 1995). The clinical consequences of a PDA are related to the degree of left to right shunting through the ductus. Despite the ability of the left ventricle, in preterm infants, to increase its output in the face of a left to right shunt, blood flow distribution to vital organs is altered due to a drop in diastolic pressure and localized vasoconstriction (Clyman 2000). The presence of a PDA is associated with reduced middle cerebral artery blood flow velocity (Weir 1999). The haemodynamic instability caused by the left to right shunt has been associated with gastrointestinal, cerebral and renal effects including spontaneous intestinal perforation and necrotizing enterocolitis (NEC), intraventricular haemorrhage (IVH), decreased kidney function and bronchopulmonary dysplasia (BPD) and, if not managed, may lead to death.
In the two Cochrane Reviews of prophylactic use of ibuprofen and indomethacin to close a PDA in preterm infants, the spontaneous closure rate in the control group was 58% and 57% respectively (Fowlie 2010; Ohlsson 2011).
Description of the intervention
A PDA can be treated surgically; or medically with one of two prostaglandin inhibitors, indomethacin or ibuprofen. Surgical closure of a symptomatic PDA improves haemodynamics and lung compliance (Naulty 1978). However, medical treatment is still considered the treatment of choice because of the risks related to the surgery. In a large Canadian cohort (n = 3779) of very low birth weight infants, 28% received treatment for a PDA; 75% were treated with indomethacin alone, 8% with surgical ligation alone, and 17% received both indomethacin and surgical ligation (Lee 2000). Infants with lower birth weights were more likely to be treated surgically (Lee 2000). Prostaglandins play a significant role in keeping the ductus arteriosus patent (Mathew 1998). Inhibiting prostaglandin synthesis with non‐selective blockers of both cyclo‐oxygenase (COX) 1 and 2 is effective for the non‐surgical closure of PDA (Clyman 2000). However, indomethacin use is associated with transient or permanent derangement of renal function, NEC, gastrointestinal haemorrhage or perforation, alteration of platelet function and impairment of cerebral blood flow or cerebral blood flow velocity (Edwards 1990; Ohlsson 1993; Seyberth 1983; Wolf 1989).
Ibuprofen, a propionic acid derivative and non‐selective COX inhibitor, is as effective as indomethacin in closing a PDA and reduces the risk of NEC (Ohlsson 2015a). There is less evidence of transient renal insufficiency following treatment with ibuprofen compared to indomethacin (Ohlsson 2015a).
Another non‐steroidal anti‐inflammatory drug, mefenamic acid, has been reported to close a PDA (Sakhalkar 1992), but no randomised controlled trials have been reported (Ohlsson 2011; Ohlsson 2015a).
In the sheep fetus, Peterson showed that acetaminophen has potent activity on the ductus arteriosus and produces a constriction in therapeutic analgesic quantities (Peterson 1985). In humans, Simbi 2002 reported on a pregnant woman near term who took nimesulide 400 mg and acetaminophen 500 mg twice daily for three days as a medication for pain. The women noticed diminished fetal movements and one day later ultrasound confirmed lack of fetal movements and breathing. A constricted ductus arteriosus was confirmed by fetal echocardiography. Following cesarean section the male infant presented with severe mixed acidosis. An echocardiogram showed an almost completely constricted ductus arteriosus. Following intensive care the infant improved and was discharged home on day 12 after birth. At three months' follow‐up the infant was doing well. Either nimesulide or acetaminophen, or both, could be responsible for ductal closure in this case.
The complications associated with the use of indomethacin and possibly ibuprofen have encouraged the search for an alternative drug to treat a PDA. In 2011 paracetamol was suggested as an alternative (Hammerman 2011). Hammerman and colleagues reported on five preterm infants (PMA 26 to 32 weeks at birth and postnatal age of 3 to 35 days) with large, haemodynamically significant PDAs (Hammerman 2011). The infants had failed or had contraindications for treatment with ibuprofen. All infants were treated with oral paracetamol 15 mg/kg per dose every 6 hours. The treatment resulted in ductal closure in all infants within three days. No side effects were observed. The authors suggested that paracetamol could offer important therapeutic advantages over non‐steroidal anti‐inflammatory drugs (NSAIDs) (indomethacin and ibuprofen) as paracetamol has no peripheral vasoconstrictive effect, can be given to infants with clinical contraindications to NSAIDs, and appears to be effective after ibuprofen treatment failure (Hammerman 2011).
Unconjugated hyperbilirubinaemia impacts upon clearance of paracetamol (Palmer 2008). Acetaminophen‐induced hepatic failure with encephalopathy has been described in a term newborn who received oral acetaminophen every four hours by the parents following circumcision (Walls 2007).
Paracetamol can be given as prophylaxis for a PDA within 24 hours after birth or as treatment for a PDA diagnosed by echocardiography (ECHO). We include both approaches in this review.
How the intervention might work
Paracetamol is an analgesic, antipyretic derivative of acetanilide with weak anti‐inflammatory properties and is used as a common analgesic in all age groups, but may cause liver, blood cell and kidney damage (NLM 2012). In low concentrations paracetamol stimulates, and in high concentrations inhibits, the synthesis of prostaglandins. In vivo (in adults) 500 mg of paracetamol causes a pronounced reduction of prostacyclin synthesis but has no effect on thromboxane synthesis (Grèen 1989). Because in vitro paracetamol is a weak inhibitor of both COX 1 and COX 2, the possibility exists that it inhibits a so far unidentified form of COX, perhaps a COX 3 (Botting 2000). In a murine model paracetamol was found to be less potent than indomethacin for construction of the mouse ductus arteriosus in vitro (El‐Khuffash 2014).
Since the report in 2011 by Hammerman and co‐workers there have been many case series of treatment of a PDA with paracetamol in preterm infants (Hammerman 2011). In five case series, a total of 38 infants with different contraindications for the use of ibuprofen or indomethacin were included (Kessel 2014; Nadir 2014; Sinah 2013; Terrin 2014; Yurttutan 2013). Paracetamol was administered orally, intravenously or via nasogastric tube and the dose and duration of treatment varied: orally 15 mg/kg 8 hourly for 48 hours (Sinah 2013); 15 mg/kg 6 hourly for 3 days (Yurttutan 2013); 15 mg/kg 6 hourly for up to 7 days (Nadir 2014); via nasogastric tube 15 mg/kg 6 hourly for 3 to 7 days (Kessel 2014); or intravenously 7.5 to 15 mg/kg every 4 to 6 hours, with a maximum daily dose of 60 mg/kg (duration of treatment 3 days in 5 of 7 cases) (Terrin 2014). In these case reports the PDA closed in 33 of the 38 cases treated with paracetamol (86%). Kessel and co‐workers measured plasma paracetamol concentrations before the fifth dose and ninth dose and 24 hours after the last dose (Kessel 2014). Most measured paracetamol blood concentrations were comparable to those recommended for pain and fever control (10 to 20 mg/mL) (Arana 2001).
In another published case series, El‐Khuffash 2014 retrospectively evaluated the clinical effectiveness of paracetamol on the closure of a PDA, and prospectively examined its effect on the in vitro term and preterm murine ductus arteriosus. A total of 21 infants were included in the study from the Mount Sinai Hospital, Toronto, Ontario, Canada and the Rotunda Maternity Hospital, Dublin, Ireland. At the Canadian site paracetamol was either given orally as a short course (15 mg/kg 6 hourly for 48 hours) or a long course of 15 mg/kg 6 hourly for 7 days. At the Irish site paracetamol was given intravenously, 15 mg/kg 6 hourly for a minimum of 48 hours until PDA closure was confirmed on echocardiography or up to a maximum of 6 days. In both centres, the decision to administer paracetamol treatment to neonates with a haemodynamically significant PDA was after failure of two courses of either ibuprofen or indomethacin or if there were contraindications to medical treatments (El‐Khuffash 2014). No changes in PDA haemodynamics were seen in the five infants treated with a short course of paracetamol. In six of the seven infants treated with a long course the PDA closed. In eight of the nine infants treated with intravenous paracetamol the PDA closed (El‐Khuffash 2014). Paracetamol drug levels were not ascertained. The authors concluded that the effectiveness of paracetamol on PDA closure may depend on the duration of treatment and the mode of administration (El‐Khuffash 2014). The inhibitory effect of paracetamol on prostaglandin E₂ (PGE₂) may not be present at lower gestational ages (El‐Khuffash 2014).
Recently there have been concerns raised that prenatal or neonatal exposure, or both, to paracetamol could have adverse effects on brain development. Viberg and co‐workers examined whether neonatal paracetamol exposure in mice could affect the development of the brain (Viberg 2014), manifested as adult behaviour and cognitive deficits, as well as changes in the response to paracetamol. They concluded that exposure to and presence of paracetamol during a critical period of brain development in mice can induce long‐lasting effects on cognitive function and alter the adult response to paracetamol (Viberg 2014).
In an ecological study conducted in humans and using country‐level data for the period 1984 to 2005, prenatal use of paracetamol was correlated with autism or autism spectrum disorder (ASD) (Bauer 2013). To explore the relationship of early neonatal paracetamol exposure to autism and ASD, population‐weighted average male autism prevalence rates for all available countries and US states were compared to male circumcision rates, a procedure for which paracetamol has been widely prescribed since the mid‐1990s. For studies including boys born after 1995, there was a strong correlation between country‐level autism and ASD prevalence in males and a country's circumcision rate (r = 0.98) (Bauer 2013). In a Spanish birth cohort study prenatal acetaminophen exposure was associated with a greater number of autism‐spectrum symptoms in males and showed adverse effects on attention‐related outcomes for both genders (Avella‐Garcia 2016). Ystrom 2017 in a study based on the Norwegian Mother and Child Cohort study, including 2246 children with ADHD, found that long‐term maternal use of paracetamol during pregnancy was substantially associated with ADHD in offspring.
In a study from Sweden, Bornehag 2017 reported on a possible association of prenatal exposure to acetaminophen and language delay in girls at 30 months of age. The same group reviewed nine prospective cohort studies that reported on associations between prenatal use of paracetamol and neurodevelopmental outcomes in the offspring (Bauer 2018). All included studies suggested an association between prenatal paracetamol exposure and the neurodevelopmental outcomes of attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), or lower IQ. Longer duration of paracetamol use was associated with increased risk. Associations were strongest for hyperactivity and attention‐related outcomes (Bauer 2018).
It is therefore of extreme importance that infants enrolled in trials of paracetamol either for pain relief or for closure of a PDA be followed long‐term with conventional developmental tests and tests to diagnose autism and ASD (APA 2013).
Why it is important to do this review
Currently there are at least 19 ongoing trials on this topic (see Ongoing studies). It is likely that several trials will be conducted in the near future and, with regular updates, this review will track the progress of the research in a timely fashion. It is expected that paracetamol (oral or intravenous) will be compared with oral or intravenous ibuprofen, with placebo, with no intervention or with intravenous indomethacin for the effectiveness of closing a PDA. In view of recent findings in mice of adverse effects on brain development following neonatal exposure to paracetamol, and in humans of an association of neonatal exposure to paracetamol and autism or ASD and language delay, it is important that long‐term follow‐up is included in individual studies and in this systematic review.
Objectives
To determine the effectiveness and safety of intravenous or oral paracetamol compared with placebo or no intervention, intravenous indomethacin, intravenous or oral ibuprofen, or with other cyclo‐oxygenase inhibitors for closure of a PDA in preterm or low birth weight infants.
Primary objectives
To determine the effectiveness and safety of intravenous or oral paracetamol compared with placebo or no intervention for closure of a PDA in preterm or low birth weight infants.
To determine the effectiveness and safety of intravenous or oral paracetamol compared with intravenous indomethacin for closure of a PDA in preterm or low birth weight infants.
To determine the effectiveness and safety of intravenous or oral paracetamol compared with intravenous ibuprofen for closure of a PDA in preterm or low birth weight infants.
To determine the effectiveness and safety of intravenous or oral paracetamol compared with oral ibuprofen for closure of a PDA in preterm or low birth weight infants.
To determine the effectiveness and safety of intravenous or oral paracetamol compared with other cyclo‐oxygenase inhibitors (separate analyses for different cyclo‐oxygenase inhibitors) for closure of a PDA in preterm or low birth weight infants.
To determine the effectiveness and safety of prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention within 24 hours after birth for PDA.
Secondary objectives
To determine in subgroup analyses the effectiveness and safety of paracetamol for closure of a PDA in relation to postnatal ages of less than 7 days, 7 to 14 days and more than 14 days at the time of administration of the first dose of paracetamol.
-
To determine in subgroup analyses the effectiveness and safety of paracetamol for closure of a PDA in relation to:
gestational age (< 28 weeks, 28 to 32 weeks, 33 to 36 weeks);
birth weight (< 1000 g, 1000 to 1500 g, 1501 to 2500 g).
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised and quasi‐randomised controlled trials for inclusion.
Types of participants
We included infants born preterm (< 37 weeks' PMA) or with low birth weight (< 2500 g at birth) who had an echocardiographic diagnosis of a PDA regardless of their postnatal age. In the Cochrane Review of ibuprofen for the treatment of a PDA all 20 included studies made the diagnoses of a PDA by echocardiography (Ohlsson 2015a), and it is likely that would be the case in studies of the effectiveness of paracetamol in closing a PDA. For prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention within 24 hours after birth for PDA, ECHO confirmation of a PDA was not required.
Types of interventions
We included paracetamol (given via any route for the purpose of closure of PDA) in any dose versus placebo or no intervention or versus another prostaglandin inhibitor. If the intention for administration of paracetamol was not closure of PDA, we would exclude the study. We included studies that used any therapeutic regimen of paracetamol.
Types of outcome measures
Primary outcomes
Failure of PDA closure after the first course of paracetamol treatment (closure and failure of closure confirmed by echocardiographic criteria).
Neurodevelopmental outcome (neurodevelopmental outcome assessed by a standardized and validated assessment tool or a child developmental specialist, or both) at any age reported (outcome data grouped at 12, 18 and 24 months, if available).
Death or disability (outcome data grouped at 12, 18 and 24 months, if available).
Secondary outcomes
All‐cause mortality during initial hospital stay.
Neonatal mortality (death during the first 28 days of life).
Infant mortality (death during the first year of life).
Re‐opening of the ductus arteriosus (defined as echocardiographic evidence of closure followed by re‐opening of PDA at later stage).
Surgical closure of the PDA.
Treatment with indomethacin, ibuprofen or other prostaglandin inhibitor to close the PDA following treatment failure.
Duration of ventilator support (days).
Duration of need for supplementary oxygen (O₂) (days).
Pulmonary haemorrhage (blood‐stained liquid flowing from the trachea of the infant).
Pulmonary hypertension (defined as an increased mean pulmonary arterial pressure of 25 mmHg at rest) (Van Loon 2011).
Bronchopulmonary dysplasia (BPD) at 28 days (defined as O₂ requirement at 28 days postnatal age in addition to compatible clinical and roentgenographic findings).
BPD at 36 weeks' PMA (defined as O₂ requirement at 36 weeks' PMA in addition to compatible clinical and roentgenographic findings).
BPD defined according to the new criteria: mild BPD defined as a need for supplemental O₂ for ≥ 28 days but not at 36 weeks' PMA or discharge, moderate BPD as O₂ for ≥ 28 days plus treatment with < 30% O₂ at 36 weeks' PMA, and severe BPD as O₂ for ≥ 28 days plus ≥ 30% O₂ or positive pressure, or both, at 36 weeks' PMA (Ehrenkranz 2005).
Intraventricular haemorrhage (IVH) (Grade I to IV) (Papile 1978).
Severe IVH (Grade III to IV).
Periventricular leukomalacia (PVL).
Necrotizing enterocolitis (NEC) (any stage; defined as per authors) (Bell 1978).
Intestinal perforation.
Gastrointestinal bleed.
Retinopathy of prematurity (ROP) (according to the international classification of ROP); any stage and stage ≥ 3 (ICCROP 2005).
Decreased urine output (defined as < 1 mL/kg/h) during treatment.
Sepsis (clinical symptoms and signs of sepsis and a positive blood bacterial culture); this outcome was added at the full review stage.
Serum or plasma levels of creatinine (mmol/L) after treatment.
Serum or plasma levels of aspartate transaminase (AST) (IU/L) following treatment.
Serum or plasma levels of alanine transaminase (ALT) (IU/L) following treatment.
Number of infants with AST or ALT levels > 100 IU/mL.
Serum bilirubin (mmol/L) following treatment.
Hyperbilirubinaemia (serum bilirubin level higher than the exchange level according to the postnatal age and body weight).
Incidence of liver failure; evidence of acute liver injury combined with either severe coagulopathy (International Normalized Ratio (INR) > 2.0 or prothrombin time (PT) > 20 seconds) or encephalopathy with moderate coagulopathy (INR ≥ 1.5 or PT ≥ 15 seconds) (Sundaram 2011).
Duration of hospitalisation (total length of hospitalisation from birth to discharge home or death) (days).
Autism or autism spectrum disorder (ASD) in childhood (APA 2013); this outcome was added at the full review stage.
Language delay (added as an outcome in 2017).
Other side effects reported by the authors (not pre‐specified).
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 10) in the Cochrane Library; MEDLINE via PubMed (1966 to 6 November 2017); Embase (1980 to 6 November 2017); and CINAHL (1982 to 6 November 2017) using the following search terms: (Acetaminophen[Mesh] OR paracetamol OR acetaminophen) AND (Ductus Arteriosus, Patent[Mesh] OR patent ductus arteriosus or PDA), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. Conference proceedings were not specifically searched but we identified some by the other searches.
We searched clinical trial registries for ongoing or recently completed trials (ClinicalTrials.gov; the World Health Organization’s International Trials Registry and Platform, and the ISRCTN Registry).
See Appendix 2 for the search methodology of the 2015 review.
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review in order to identify additional relevant articles.
Data collection and analysis
We used standard methods recommended by Cochrane and its Neonatal Review Group.
Selection of studies
Two review authors (AO, PS) independently assessed study eligibility for inclusion in this review according to the pre‐specified selection criteria.
Data extraction and management
Two review authors (AO, PS) independently extracted data from the full‐text articles using a specifically designed spread sheet and customized form to manage information. We used these forms to decide trial inclusion and exclusion, extract data from eligible trials, and for requesting additional published information from authors of the original report. We entered and cross‐checked data using Review Manager 5 (RevMan 5) software (Review Manager 2014). We compared the extracted data for any differences. If noted, we resolved differences by mutual discussion and consensus. We contacted the authors of three identified trials and we obtained unpublished data from the Oncel group (Oncel 2014), the Dang group (Dang 2013), and Dash group (Dash 2015).
Assessment of risk of bias in included studies
Two review authors (AO, PS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2017).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements by discussion or by involving a third assessor to reach consensus. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analysed treatment effects in the individual trials using RevMan 5 (Review Manager 2014).
Dichotomous data
We reported dichotomous data using risk ratio (RR) and risk difference (RD) with respective 95% confidence intervals (CI). For those outcomes with a statistically significant RD for the pooled estimate from the meta‐analysis, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) and respective 95% CI.
Continuous data
We reported continuous data using mean difference (MD) with 95% CI.
Unit of analysis issues
The unit of randomisation was the individual infant. We did not include cross‐over or cluster‐randomised trials as those trial designs are unlikely for the intervention studied in this review — indeed, no cross‐over or cluster‐randomised trials were identified. We only considered an infant once, even though the infant might have been randomised twice by investigators. We planned to contact the authors in order to provide data resulting from the first randomisation. If we could not separate data from the first randomisation, we planned to exclude the study.
Dealing with missing data
We requested additional data from the authors of each included trial when data on important outcomes were missing or needed clarification. We did receive clarifying information from the authors of the following included trials: Dang 2013; Dash 2015; and Oncel 2014. The authors clarified that all the analyses that were published or they provided us with were intention‐to‐treat analyses.
Assessment of heterogeneity
We used RevMan 5 software to assess the heterogeneity of treatment effects between trials (Review Manager 2014). We used the two formal statistics described below.
The Chi² test, to assess whether observed variability in effect sizes between studies was greater than would be expected by chance. Since this test has low power when the number of studies included in the meta‐analysis is small, we set the alpha probability at the 10% level of significance.
The I² statistic to ensure that pooling of data was valid. We graded the degree of heterogeneity as: none, low, moderate, and high for values of < 25%, ≥ 25% to 49%, 50% to 74%, and ≥ 75% respectively (Higgins 2003).
Assessment of reporting biases
We attempted to identify the study protocols for the trials we selected for inclusion (see the table 'Characteristics of included studies'). Two studies were registered in retrospect (Al‐Lawama 2017; Dash 2015). For three trials the study protocol was not available to us (Asbagh 2015; El Mashad 2017; Yang 2016). For three studies the protocol was registered before patient recruitment started (Dang 2013; Härkin 2016; Oncel 2014). We planned to assess reporting and publication bias by examining the degree of asymmetry of a funnel plot in RevMan 5 provided that a sufficient number of studies (n = 10) were available (Review Manager 2014). However, this was not feasible as only five trials were included in any one meta‐analysis.
Data synthesis
We performed statistical analyses according to the recommendations of the Cochrane Neonatal Review Group (http://neonatal.cochrane.org/resources‐review‐authors). We analysed all infants randomised on an intention‐to‐treat basis. We analysed treatment effects in the individual trials. We used a fixed‐effect model in the meta‐analysis to combine the data. Where substantial heterogeneity existed, the potential cause of heterogeneity would have been examined in subgroup and sensitivity analyses. When we judged meta‐analysis to be inappropriate, we planned to analyse and interpret individual trials separately. For estimates of typical RR and RD, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. We would have used the standardized mean difference (SMD) to combine trials that measured the same outcome but used different scales.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the five key outcomes below for the comparisons of 'paracetamol versus ibuprofen', 'paracetamol versus placebo or no intervention' and 'paracetamol versus indomethacin'. Not all outcomes were included for all comparisons as there were too few trials/infants included in the analyses.
Failure of ductal closure after the first course of treatment.
Neurodevelopmental impairment.
All‐cause mortality during initial hospital stay (death).
Gastrointestinal bleed or stools positive for occult blood.
Serum levels of creatinine after treatment (µmol/L).
Two authors (AO, PS) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create ‘Summary of findings’ tables to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were pre‐specified.
Gestational age (< 28 weeks, 28 to 32 weeks, 33 to 36 weeks).
Birth weight (< 1000 g, 1000 to 1500 g, 1501 to 2500 g).
We planned to conduct subgroup analyses to determine the effectiveness and safety of paracetamol for closure of a PDA in relation to postnatal ages of less than 7 days, 7 to 14 days and more than 14 days at the time of administration of the first dose of paracetamol. However, the data from the included studies were not suitable for subgroup analyses according to the pre‐specified categories.
Sensitivity analysis
We planned to perform a sensitivity analysis to determine if the findings were affected by including only studies of adequate methodology, defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and less than 10% losses to follow‐up. The eight studies were of similar (moderate) quality.
Results
Description of studies
Results of the search
The literature searches in November 2017 identified six additional studies (Al‐Lawama 2017; Asbagh 2015; Dash 2015; El Mashad 2017; Härkin 2016; Yang 2016), in addition to the two studies previously included, Dang 2013 and Oncel 2014. The previously ongoing study listed as Zarkesh 2013 has now been published as Asbagh 2015. In addition to the three previously included ongoing studies (NCT01938261; NCT01291654; NCT02002741), an additional 16 studies have been entered into trials registries (ACTRN12613000289718; ACTRN12616001517460; ChiCTR‐TRC‐13003912; CTRI/2016/09/007261; CTRI/2017/10/009989; CTRI/2017/10/010012; EUCTR2015‐003177‐14‐ES; EUCTR2013‐003883‐30‐IT; IRCT2016081729404N1; Kumar 2017; NCT02056223; NCT02422966; NCT02819414; NCT03008876; NCT03103022; NCT03265782). Two studies are awaiting classification (Bagheri 2016; Kluckow 2016). For details see Figure 1.
Figure 1.
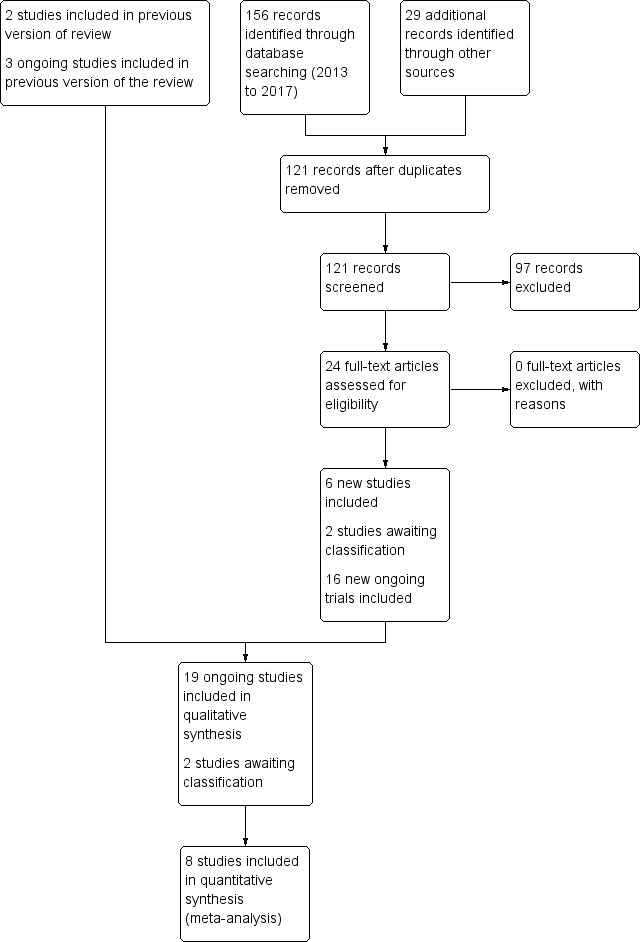
Study flow diagram: review update
Included studies
For details see the table 'Characteristics of included studies'.
Al‐Lawama 2017 was a single‐centre study conducted in the Neonatal Intensive Care Unit of Jordan University Hospital, Amman, Jordan.
Objective: to evaluate the effectiveness and safety profiles of oral paracetamol versus oral ibuprofen for PDA closure in preterm infants.
Population: preterm infants with a gestational age of ≤ 32 weeks or birth weight of ≤ 1500 g and a haemodynamically significant PDA. Exclusion criteria: Ductal‐dependent congenital heart diseases, major congenital malformation, grade 3 to 4 intraventricular haemorrhage, renal impairment (defined as a creatinine concentration of > 1.5 mg/dl), pulmonary haemorrhage, thrombocytopenia of < 60,000 / mm³ , and an elevated alanine transaminase concentration.
Intervention or contrast: the oral paracetamol group (n = 13) received 10 mg/kg/dose followed by 1 to 2 mL of 0.9% saline every 6 hours for 3 days. (10 mg/kg every 6 h for 3 days).The oral ibuprofen group received 10 mg/kg/dose followed by 1 to 2 mL of 0.9% saline once daily for 3 days. (10 mg/kg/day for 3 days).
-
Outcomes assessed:
Primary outcomes: mortality, primary PDA closure.
Secondary outcomes: secondary PDA closure, pulmonary haemorrhage, BPD, Sepsis, NEC, ROP, IVH Grade 1 to 2, IVH Grade 3 to 4, PVL.
Notes: we contacted the authors in January 2014 to obtain unpublished information regarding outcomes, and we received information in April 2014.
Asbagh 2015 was a single‐centre study conducted in Tehran, Iran.
Objective: to determine the effectiveness of prophylactic treatment with oral paracetamol for PDA in preterm infants.
Population: preterm infants with PMA ≤ 32 weeks and BW ≤ 1500 g and postnatal age < 24 hours.
Intervention or contrast: the paracetamol group received 15 mg/kg of paracetamol orally every 6 hours for 48 hours; the control group received no intervention and no placebo.
-
Outcomes assessed:
Primary outcome: failure to close a PDA by 4 to 5 days.
Secondary outcomes death, treatment with ibuprofen.
Notes: we contacted the corresponding author Dr. Zarkesh on 30 November 2017 to get clarifying information, but by 6 January 2018 we had not received a response.
Dang 2013 was a single‐centre study conducted in Changchun, China.
Objective: to evaluate the effectiveness and safety profiles of oral paracetamol to those of standard ibuprofen for PDA closure in preterm infants.
Population: preterm infants with PMA ≤ 34 weeks with echocardiographically confirmed PDA; postnatal age ≤ 14 days.
Intervention or contrast: the paracetamol group received 15 mg/kg of paracetamol orally every 6 hours for 3 days; the ibuprofen group received oral ibuprofen at an initial dose of 10 mg/kg followed by 5 mg/kg after 24 and 48 hours.
-
Outcomes assessed:
Primary outcome: rates of ductal closure after treatment confirmed by daily cardiography during treatment.
Secondary outcomes: oliguria (urine output < 1 mLkg/h), IVH, tendency to bleed, NEC, hyperbilirubinaemia, serum creatinine, death, BPD, PVL, NEC, ROP, sepsis.
Notes: we contacted the authors in January 2014 to obtain unpublished information regarding outcomes, and we received information in April 2014.
Dash 2015 was a single‐centre study conducted in Mumbai, India.
Objective: to compare the effectiveness of enteral paracetamol and IV indomethacin for closure of PDA in preterm neonates.
Population: preterm infants with birth weight ≤ 1500 g and echocardiography performed within the first 48 hours of life demonstrating PDA size ≥ 1.5 mm at the narrowest diameter, left to right shunt across the duct and ratio of the diameter of the left atrium to that of the aortic root (LA:AO) > 1.5:1.
Intervention or contrast: the paracetamol group (n = 38) received paracetamol drops through an infant feeding tube at a dose of 15 mg/kg/dose four times daily for 7 days (28 doses). The indomethacin group (n = 39) received IV indomethacin at a dose of 0.2 mg/kg/dose, diluted with normal saline to make 5 mL solution and infused over 20 minutes by syringe pump once daily for three days. As per study protocol, two additional extra doses of indomethacin were allowed in the indomethacin group, if clinical evaluation after three doses showed persistence of PDA as demonstrated by clinical signs and symptoms such as tachycardia, wide pulse pressure and persistent murmur.
-
Outcomes assessed:
Primary outcome: failure to close the PDA.
Secondary outcomes: surgical ligation, ROP, GI bleeding, NEC, pulmonary haemorrhage, IVH, sepsis, daily urine output, serum creatinine, serum bilirubin, and platelet count.
Notes: Dr. Kabra provided additional information about this trial in December 2017.
El Mashad 2017 was a single‐centre study from the Neonatal Intensive Care Unit (NICU) of Tanta University Hospital Pediatric Department, Tanta, Egypt.
Objective: to compare the effectiveness and side effects of paracetamol, indomethacin and ibuprofen in the closure of haemodynamically significant PDA (hs‐PDA) in preterm neonates mainly on renal and liver function, platelet count, and haemoglobin level.
Population: preterm infants with PMA < 28 weeks and BW < 1500 g in the first two weeks of life with hs‐PDA diagnosed with ECHO and clinical examination.
Intervention or contrast: the paracetamol group: 100 neonates received 15 mg/kg IV infusion paracetamol over 30 min followed by 15 mg/kg/6 h IV infusion for 3 days. The ibuprofen group: 100 neonates received 10 mg/kg IV infusion ibuprofen followed by 5 mg/kg/ day for 2 days. The indomethacin group: 100 neonates received 0.2 mg/kg indomethacin IV infusion over 30 min for three doses 12 h apart.
-
Outcomes assessed
Primary outcome: failure to close the PDA.
Secondary outcomes: surgical ligation, ROP, GI bleeding, NEC, pulmonary haemorrhage, IVH, sepsis, daily urine output, serum creatinine, serum bilirubin, platelet count.
Notes: none.
Härkin 2016 was a single‐centre study conducted in the Neonatal Intensive Care Unit (NICU) of Oulu University Hospital, Oulu, Finland.
Objective: to study the biologic effect of paracetamol, an inhibitor of prostaglandin synthesis, on early closure of PDA, and to evaluate possible adverse effects associated with the drug.
Population: preterm infants with PMA < 32 weeks requiring intensive care and who were < 24 hours old.
Intervention or contrast: the paracetamol group received 20 mg/kg of paracetamol IV within 24 hours of birth, followed by 7.5 mg every 6 hours for 4 days hours for 48 hours; the control group received placebo (0.45% NaCl).
-
Outcomes assessed
Primary outcomes: decrease in ductal calibre without side effects and failure to close a PDA by 4 to 5 days.
Secondary outcomes: persistent PDA treated, oliguria (< 1 mL/kg/h), polyuria (> 5 mL/kg/h), hypernatremia (> 150 mmol/L), sepsis, supplemental oxygen at 28 days, supplemental oxygen at 36 weeks' PMA, ROP treated, IVH grades 1 to 2, IVH grades 3 to 4, NEC stage 3, death, days of supplemental oxygen, highest serum bilirubin (µmol/L).
Notes: none.
Oncel 2014 was a single‐centre study conducted in Ankara, Turkey.
Objective: to compare the effectiveness and safety of oral paracetamol and oral ibuprofen for the pharmacological closure of PDA in preterm infants.
Population: preterm infants PMA ≤ 30 weeks, birth weight ≤ 1250 g with echocardiographically confirmed significant PDA; postnatal age 48 to 96 hours.
Intervention or contrast: The paracetamol group received 15 mg/kg of paracetamol orally every 6 hours for 3 days; the ibuprofen group received oral ibuprofen at an initial dose of 10 mg/kg followed by 5 mg/kg after 24 and 48 hours.
-
Outcomes assessed:
Primary outcome: rates of ductal closure after treatment by echocardiography performed by a cardiologist who was blinded to the treatment group.
Secondary outcomes: all‐cause mortality during initial hospital stay, neonatal mortality (first 28 days of life), infant mortality, re‐opening of the ductus arteriosus, surgical closure of the PDA, duration of ventilatory support, duration of need for supplementary oxygen, pulmonary haemorrhage, pulmonary hypertension, BPD (at 28 days' and at 36 weeks' PMA, severe BPD at 36 weeks' PMA), IVH (all grades and Grade III to IV), PVL, NEC, intestinal perforation, gastrointestinal bleeding, ROP (any stage, stage ≥ 3, ROP requiring laser treatment), oliguria (urine output < 1 mL/kg/h), serum levels after treatment of creatinine, bilirubin, aspartate transaminase, alanine transaminase, liver failure, duration of hospital stay, sepsis.
In 2017 the authors published neurodevelopmental outcomes of the infants enrolled in this trial; they reported on 30 children in the paracetamol group and 31 children in the ibuprofen group. They reported on neurodevelopmental impairment, MDI < 70, PDI < 70, moderate to severe cerebral palsy, blindness, deafness and MDI and PDI at 18 to 24 months corrected age.
Notes: we contacted the authors and in January 2014 received unpublished information regarding several of the outcomes listed above. The published report includes 80 patients who actually received the intervention whereas from the authors we received information on all outcomes for all 90 enrolled patients.
Yang 2016 was a single centre study conducted in Xuzhou, Jiangsu, China.
Objective: to understand the effect of paracetamol treatment on preterm infants with a significant PDA, aiming to utilize and develop plasma and urinary PGE₂ levels as indicators of progress of PDA closure in a non‐invasive manner.
Population: preterm infants with PMA < 37 weeks and admitted to hospital within 24 hours after birth. A significant PDA diagnosis was made between 15 h to 10 days after birth and confirmed through ECHO to be a significant PDA. Diagnostic criteria of echocardiography were: i) left atrial:aortic root diameter ratio, (LA:Ao) > 1.4; ii) pulmonary artery diastolic back flow (reflux); and iii) PDA vessel diameter > 1.4 mm
Intervention or contrast: the paracetamol group received 15 mg/kg acetaminophen administered orally once every 6 hours for three days. The ibuprofen group received 10 mg/kg ibuprofen administered orally as the initial dose, followed by 5 mg/kg during the first 24 hours and 48 hours later.
-
Outcomes assessed
Primary outcome: failure of primary ductal closure.
Secondary outcomes: oliguria (< 1 mL/kg/h, stools positive for occult blood, IVH (grade not stated), NEC, BPD (PMA not stated), plasma PGE₂ (ng/L), urine PGE₂ (ng/L), platelet count (x10⁹/L), serum Cr (µmol/L), glutamic‐pyruvic transaminase (U/L).
Notes: none.
Excluded studies
No randomised controlled study was excluded. In addition to three previously included ongoing studies (NCT01938261; NCT01291654; NCT02002741), an additional 16 studies were identified from trials registries (ACTRN12613000289718; ACTRN12616001517460; ChiCTR‐TRC‐13003912; CTRI/2016/09/007261; CTRI/2017/10/009989; CTRI/2017/10/010012; EUCTR2015‐003177‐14‐ES; EUCTR2013‐003883‐30‐IT; IRCT2016081729404N1; Kumar 2017; NCT02056223; NCT02422966; NCT02819414; NCT03008876; NCT03103022; NCT03265782).
Risk of bias in included studies
For details see Figure 2 ('Risk of bias' graph) and Figure 3 ('Risk of bias' summary).
Figure 2.
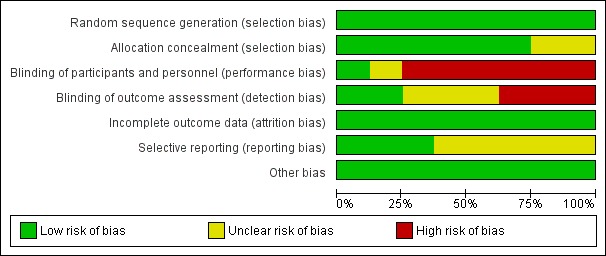
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
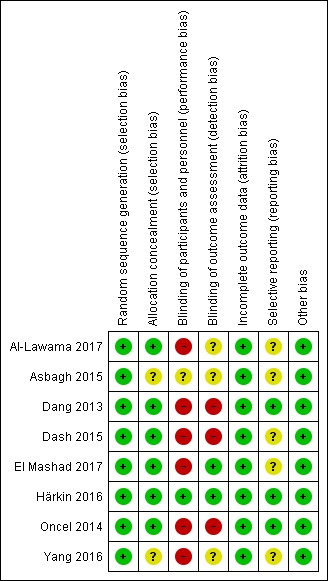
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The randomisation sequence was adequate in all studies.
Allocation
Six studies used sequentially numbered, sealed opaque envelopes for the allocation to the two treatment groups and the risk of bias was unclear in two studies.
Blinding
In the studies of paracetamol versus ibuprofen the two study drugs were administered at different time points after the initial dose (Al‐Lawama 2017; Dang 2013; El Mashad 2017; Oncel 2014; Yang 2016). Healthcare providers and researchers were not blinded to group allocation of the infants. Dang 2013 states "doctors and nurses were not blind". Oncel 2014 reports "....the intervention was not completely blinded because of the different number of doses per day of the drugs. However, the most important outcome—PDA closure—was made by a cardiologist, who was blinded to the treatment groups". In the two studies of prophylactic use of paracetamol the risk of bias was low for the study by Härkin 2016. The researchers used a placebo but in the study by Asbagh 2015 no intervention or placebo was given in the control group. In the two studies comparing paracetamol with indomethacin the study drugs were given at different time points (Dash 2015) (El Mashad 2017). Thus in all studies the caregivers and researchers would not have been blinded to the group allocation.
Incomplete outcome data
Outcome data reported for all pre‐set outcomes and for all enrolled infants in all studies.
Selective reporting
The protocols for three studies were available to us as the trials were registered (Dang 2013; Härkin 2016; Oncel 2014). The studies by Al‐Lawama 2017 and Dash 2015 were registered in retrospect and the researchers may have made changes after the original study design. The studies by El Mashad 2017; Yang 2016 were not registered and we did not have access to the protocol for the study by Asbagh 2015. For the studies by Dang 2013, Härkin 2016 and Oncel 2014 there do not seem to be any deviations from the protocols.
For three trials the study protocol was not available to us (Asbagh 2015; El Mashad 2017; Yang 2016). For three studies the protocol was registered before patient recruitment started (Dang 2013; Härkin 2016; Oncel 2014). We planned to assess reporting and publication bias by examining the degree of asymmetry of a funnel plot in RevMan 5 provided that a sufficient number of studies (n = 10) were available (Review Manager 2014). However, this was not feasible as we included only five trials in any one meta‐analysis.
Other potential sources of bias
There were no other sources of bias identified.
We considered the overall risk of bias in the eight studies to vary from low to moderate.
Effects of interventions
See: Table 1; Table 2; Table 3
Paracetamol (oral or IV) versus ibuprofen (oral or IV) (Comparison 1)
Primary outcomes
Failure of PDA closure after the first course of paracetamol treatment (closure and failure of closure confirmed by echocardiographic criteria) (Outcome 1.1)
See Analysis 1.1. Figure 4
Analysis 1.1.
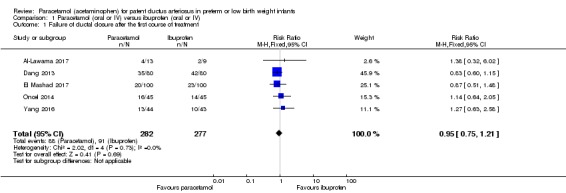
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 1 Failure of ductal closure after the first course of treatment.
Figure 4.
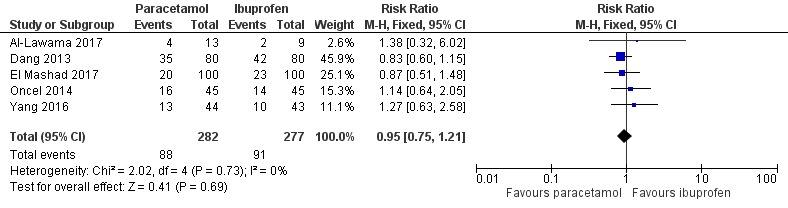
Forest plot of comparison: 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), outcome: 1.1 Failure of ductal closure after the first course of treatment.
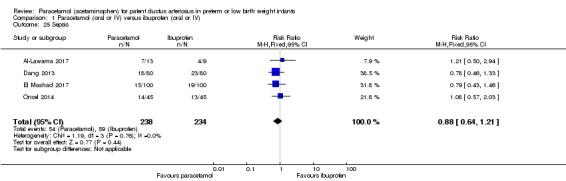
Five studies (n = 559 infants) reported on this outcome (Al‐Lawama 2017; Dang 2013; El Mashad 2017; Oncel 2014; Yang 2016). There was no significant difference between the paracetamol and the ibuprofen groups in failure of PDA closure (typical RR 0.95, 95% 0.75 to 1.21; typical RD −0.02, 95% CI −0.09 to 0.06; I² = 0% for RR and for RD). The quality of evidence according to GRADE was moderate.
Neurodevelopmental impairment at 18 to 24 months corrected age (Outcome 1.2)
See Analysis 1.2.
Analysis 1.2.
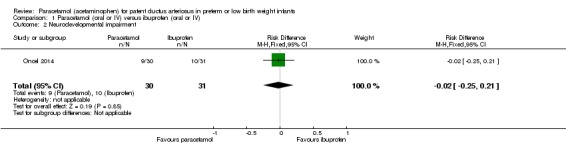
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 2 Neurodevelopmental impairment.
One study — Oncel 2014 — has reported on this outcome in 61 children. There was no significant difference between the paracetamol and the ibuprofen groups in the incidence of neurodevelopmental impairment (RR 0.93, 95% CI 0.44 to 1.96; RD −0.02, 95% CI −0.25 to 0.21). Tests for heterogeneity were not applicable. The quality of evidence according to GRADE was low.
There were no statistically significant differences between the groups for the following outcomes in the follow‐up report by Oncel 2014 (for details see the analyses): MDI < 70 Analysis 1.33; PDI < 70 Analysis 1.34; Moderate to severe cerebral palsy Analysis 1.35; Deafness Analysis 1.36; Blindness Analysis 1.37; MDI Analysis 1.38 or PDI Analysis 1.39. As there was only one study included in these analyses, tests for heterogeneity were not applicable.
Analysis 1.33.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 33 MDI < 70.
Analysis 1.34.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 34 PDI < 70.
Analysis 1.35.
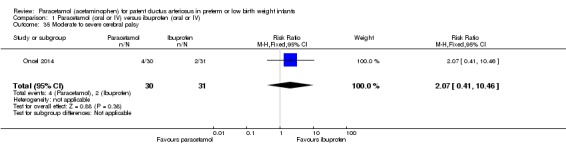
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 35 Moderate to severe cerebral palsy.
Analysis 1.36.
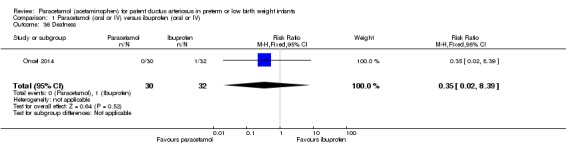
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 36 Deafness.
Analysis 1.37.
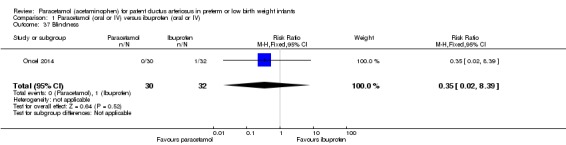
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 37 Blindness.
Analysis 1.38.
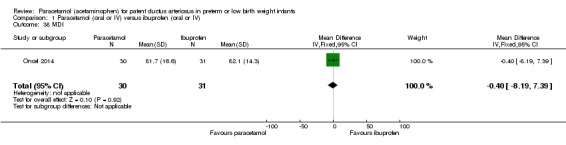
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 38 MDI.
Analysis 1.39.
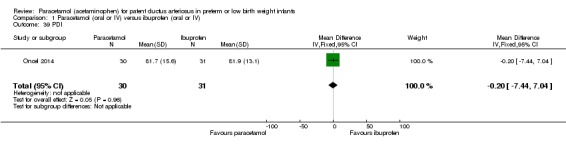
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 39 PDI.
Death or disability at 18 to 24 months
No studies reported on this combined outcome (including the follow‐up study of Oncel 2014).
Secondary outcomes
All‐cause mortality during initial hospital stay (Outcome 1.3)
See Analysis 1.3
Analysis 1.3.
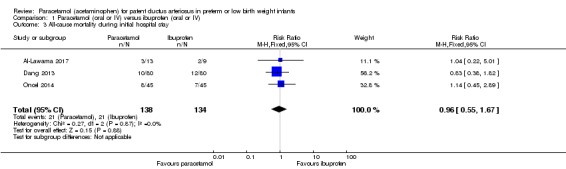
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 3 All‐cause mortality during initial hospital stay.
Three studies (n = 272 infants) reported on this outcome (Al‐Lawama 2017; Dang 2013; Oncel 2014). There was no significant difference between the paracetamol and the ibuprofen groups in all‐cause mortality during the initial hospital stay (typical RR 0.96, 95% CI 0.55 to 1.67; typical RD −0.01, 95% CI −0.09 to 0.08; I² = 0% (none) for both RR and RD). The quality of evidence according to GRADE was moderate.
Re‐opening of the ductus arteriosus (defined as echocardiographic evidence of closure followed by re‐opening of PDA at later stage) (Outcome 1.6)
See Analysis 1.6
Analysis 1.6.
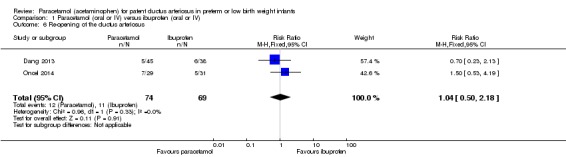
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 6 Re‐opening of the ductus arteriosus.
Two studies (n = 143 infants) reported on this outcome (Dang 2013; Oncel 2014). There was no significant difference between the paracetamol and the ibuprofen groups in the risk of re‐opening of the ductus arteriosus (typical RR 1.04, 95% CI 0.50 to 2.18; typical RD 0.01, 95% CI −0.11 to 0.13; I² = 0% (none) for RR and I² = 1% (none) for RD).
Surgical closure of the PDA following treatment failure with paracetamol or placebo (Outcome 1.7)
See Analysis 1.7
Analysis 1.7.
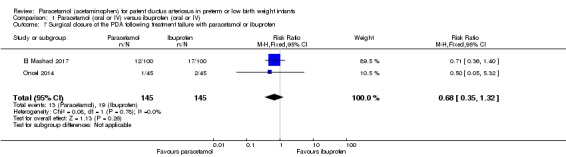
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 7 Surgical closure of the PDA following treatment failure with paracetamol or ibuprofen.
Two studies (n = 290 infants) reported on this outcome (El Mashad 2017; Oncel 2014). There was no significant difference between the paracetamol and the ibuprofen groups in surgical closure of the PDA following treatment failure (RR 0.68, 95% CI 0.35 to 1.32; RD −0.04, 95% CI −0.11 to 0.03); I² = 0% (none) for both RR and RD.
Pulmonary haemorrhage (blood‐stained liquid flowing from the trachea of the infant) (Outcome 1.9)
See Analysis 1.9
Analysis 1.9.
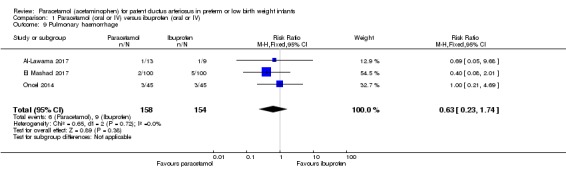
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 9 Pulmonary haemorrhage.
Three studies (n = 312 infants) reported on this outcome (Al‐Lawama 2017; El Mashad 2017; Oncel 2014). There was no significant difference between the paracetamol and the ibuprofen groups in the risk of pulmonary haemorrhage (RR 0.63, 95% CI 0.23 to 1.74; RD −0.02, 95% CI −0.07 to 0.03; I² = 0% (none) for both RR and RD).
Duration of need for supplementary oxygen (days) (Outcome 1.11)
See Analysis 1.11, Figure 4
Analysis 1.11.
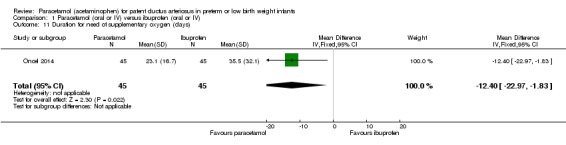
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 11 Duration for need of supplementary oxygen (days).
One study (n = 90 infants) reported on this outcome (Oncel 2014). There was a significant difference between the paracetamol and the ibuprofen groups in the duration of need of supplementary oxygen (O₂) favouring the paracetamol group (MD −12.40 days, 95% CI −22.97 to −1.83). The test for heterogeneity was not applicable.
Intraventricular haemorrhage (IVH) (Grade I to IV) (Outcome 1.16)
See Analysis 1.16
Analysis 1.16.
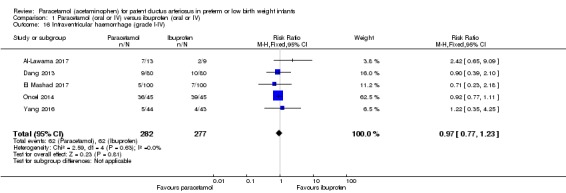
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 16 Intraventricular haemorrhage (grade I‐IV).
Five studies reported on this outcome, in 559 infants (Al‐Lawama 2017; Dang 2013; El Mashad 2017; Oncel 2014; Yang 2016). There was no significant difference between the paracetamol and the ibuprofen groups in the risk of IVH (typical RR 0.97, 95% CI 0.77 to 1.23; typical RD −0.01, 95% CI −0.06 to 0.04; I² = 0% (none) for RR and for RD).
Severe IVH (Grade III to IV) (Outcome 1.17)
See Analysis 1.17
Analysis 1.17.
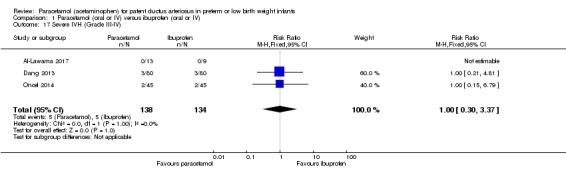
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 17 Severe IVH (Grade III‐IV).
Three studies reported on this outcome, in 272 infants (Al‐Lawama 2017; Dang 2013; Oncel 2014). There was no significant difference between the paracetamol and the ibuprofen groups in the risk of severe IVH (typical RR 1.00, 95% CI 0.30 to 3.37; typical RD 0.00, 95% CI −0.05 to 0.05; I² = 0% (none) for RR and for RD).
Periventricular leukomalacia (PVL) (Outcome 1.18)
See Analysis 1.18
Analysis 1.18.
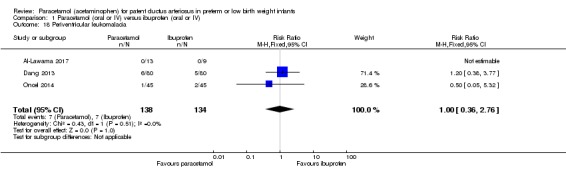
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 18 Periventricular leukomalacia.
Three studies reported on this outcome, in 272 infants (Al‐Lawama 2017; Dang 2013; Oncel 2014). There was no significant difference between the paracetamol and the ibuprofen groups in the risk of PVL (typical RR 1.00, 95% CI 0.36 to 2.76; typical RD −0.00, 95% CI −0.05 to 0.05; I² = 0% (none) for RR and for RD).
Necrotizing enterocolitis (NEC) (any stage) (Outcome 1.19)
See Analysis 1.19
Analysis 1.19.
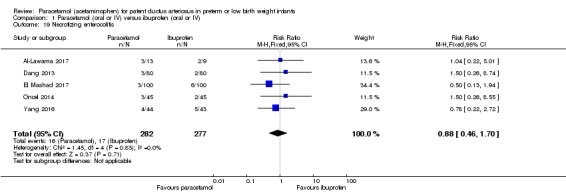
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 19 Necrotizing enterocolitis.
Five studies (n = 559 infants) reported on this outcome (Al‐Lawama 2017; Dang 2013; El Mashad 2017; Oncel 2014; Yang 2016). There was no significant difference between the paracetamol and the ibuprofen groups in the risk of NEC (typical RR 0.88, 95% CI 0.46 to 1.70; typical RD −0.01, 95% CI −0.05 to 0.03; I² = 0% (none) for RR and for RD).
Gastrointestinal bleed or stools positive for occult blood (Outcome 1.21)
See Analysis 1.21
Analysis 1.21.
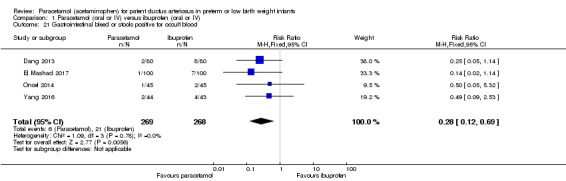
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 21 Gastrointestinal bleed or stools positive for occult blood.
Four studies (n = 537 infants) reported on gastrointestinal bleeding (Dang 2013; El Mashad 2017; Oncel 2014; Yang 2016). There was a significant difference between the paracetamol and the ibuprofen groups in the typical RR (typical RR 0.28, 95% CI 0.12 to 0.69); and significant difference in the typical RD (RD −0.06, 95% CI −0.09 to −0.02) favouring paracetamol over ibuprofen (NNTB 17, 95% CI 11 to 50; I² = 0% (none) for RR and for RD). The quality of evidence according to GRADE was moderate.
Retinopathy of prematurity (ROP) any stage (according to the international classification of ROP) (Outcome 1.22)
See Analysis 1.22
Analysis 1.22.
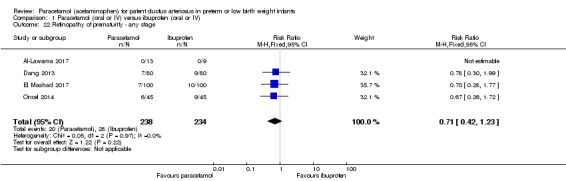
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 22 Retinopathy of prematurity ‐ any stage.
Four studies (n = 472 infants) reported on this outcome (Al‐Lawama 2017; Dang 2013; El Mashad 2017; Oncel 2014). There was no significant difference between the paracetamol and the ibuprofen groups in the risk of developing ROP (typical RR 0.71, 95% CI 0.42 to 1.23; typical RD −0.03, 95% CI −0.09 to 0.02; I² = 0% (none) for RR and for RD).
Sepsis (clinical symptoms and signs of sepsis and a positive blood bacterial culture) (Outcome 1.25)
See Analysis 1.25
Analysis 1.25.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 25 Sepsis.
Four studies (n = 472 infants) reported on this outcome (Al‐Lawama 2017; Dang 2013; El Mashad 2017; Oncel 2014). There was no significant difference between the paracetamol and the ibuprofen groups in the risk of sepsis (typical RR 0.88, 95% CI 0.64 to 1.21; typical RD −0.03, 95% CI −0.11 to 0.05; I² = 0% (none) for RR and for RD).
Oliguria (decreased urine output (defined as < 1 mL/kg/h) during treatment) (Outcome 1.26)
See Analysis 1.26
Analysis 1.26.
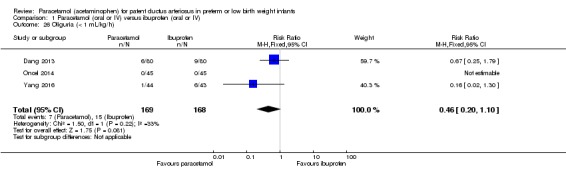
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 26 Oliguria (< 1 mL/kg/h).
Three studies (n = 337 infants) reported on this outcome (Dang 2013; Oncel 2014; Yang 2016). Oliguria did not occur in any infant in the study by Oncel 2014. There was no significant difference between the paracetamol and the ibuprofen groups in risk of oliguria (typical RR 0.46, 95% CI 0.20 to 1.10; typical RD −0.05, 95% CI −0.10 to 0.01; I² test for RR was 33% (low) and for RD 69% (moderate)).
Serum or plasma levels of creatinine (mmol/L) after treatment (Outcome 1.27)
See Analysis 1.27
Analysis 1.27.
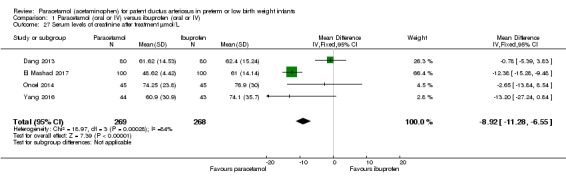
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 27 Serum levels of creatinine after treatment µmol/L.
Four studies (n = 537 infants) reported on this outcome (Dang 2013; El Mashad 2017; Oncel 2014; Yang 2016). There was a significant difference between the paracetamol and the ibuprofen groups in creatinine levels (typical weighted mean difference (WMD) −8.92 mmol/L, 95% CI −11.28 to −6.55; I² = 84% (high)). The quality of evidence according to GRADE was moderate.
Serum bilirubin (mmol/L) following treatment (Outcome 1.30)
See Analysis 1.30
Analysis 1.30.
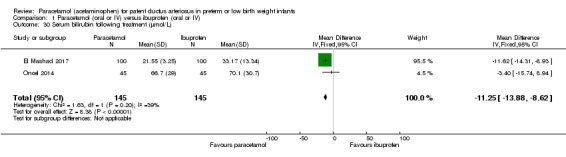
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 30 Serum bilirubin following treatment (µmol/L).
Two studies reported on this outcome, in 290 infants (El Mashad 2017; Oncel 2014). There was a significant difference between the paracetamol and the ibuprofen groups in serum bilirubin (mmol/L) following treatment, with lower serum bilirubin levels in the paracetamol group (WMD −11.25 µmol/L, 95% CI −13.88 to −8.62; I² = 39% (low)).
Hyperbilirubinaemia (serum bilirubin level higher than the exchange level according to the postnatal age and body weight) (Outcome 1.31)
See Analysis 1.31, Figure 5
Analysis 1.31.

Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 31 Hyperbilirubinaemia (serum bilirubin level higher than the exchange level according to the postnatal age and BW).
One study reported on this outcome, in 160 infants (Dang 2013). There was a significant difference in hyperbilirubinaemia favouring the paracetamol groups (RR 0.57, 95% CI 0.34 to 0.97; RD −0.15, −0.29 to −0.01; NNTB 7, 95% CI 3 to 100).
BPD (age not stated) (Outcome 1.40)
Analysis 1.40.
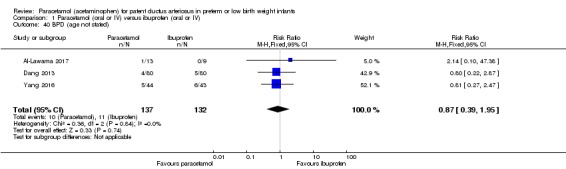
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 40 BPD (age not stated).
Three studies reported on this outcome in 269 infants (Al‐Lawama 2017; Dang 2013; Yang 2016). There was no significant difference between the paracetamol and the ibuprofen groups in BPD (age not stated) (typical RR 0.87, 95% CI 0.39 to 1.95; typical RD −0.01, 95% CI −0.07 to 0.06; I² = 0% for both RR and RD).
Plasma PGE₂ (ng/L) (Outcome 1.41)
Analysis 1.41.
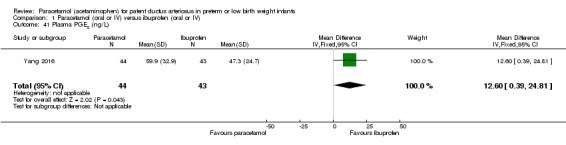
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 41 Plasma PGE2 (ng/L).
One study reported on this outcome in 87 infants (Yang 2016). The plasma PGE₂ was significantly higher in the paracetamol group compared with the ibuprofen group (MD 12.60 ng/L, 95% CI 0.39 to 24.81). Tests for heterogeneity were not applicable.
Urine PGE₂ (ng/L) (Outcome 1.42)
Analysis 1.42.
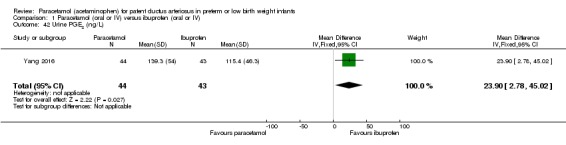
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 42 Urine PGE2 (ng/L).
One study reported on this outcome in 87 infants (Yang 2016). The urine PGE₂ was significantly higher in the paracetamol group compared with the ibuprofen group (MD 23.90 ng/L, 95% CI 2.78 to 45.02). Tests for heterogeneity were not applicable.
Platelet count following treatment (Outcome 1.43)
Analysis 1.43.
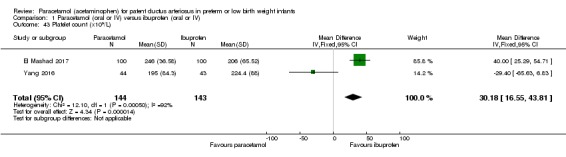
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 43 Platelet count (x109/L).
Two studies reported on this outcome in 287 infants (El Mashad 2017; Yang 2016). The platelet count was significantly higher in the paracetamol group compared with the ibuprofen group (WMD 30.18 (×10⁹/L), 95% CI 16.55 to 43.81). There was high heterogeneity for this outcome (I² = 92%).
Failure of PDA closure after the 2nd course of IV paracetamol versus IV ibuprofen (Outcome 1.45)
Analysis 1.45.
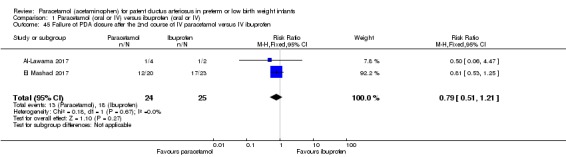
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 45 Failure of PDA closure after the 2nd course of IV paracetamol versus IV ibuprofen.
Two studies reported on this outcome in 49 infants (El Mashad 2017; Yang 2016). There was no significant difference in failure of PDA closure after the 2nd course of IV paracetamol versus IV ibuprofen (typical RR 0.79, 95% CI 0.51 to 1.21; typical RD −0.15, 95% CI −0.42 to 0.11; I² = 0% for both RR and RD.
Daily urine output (mL/kg/hour) (Outcome 1.46)
Analysis 1.46.
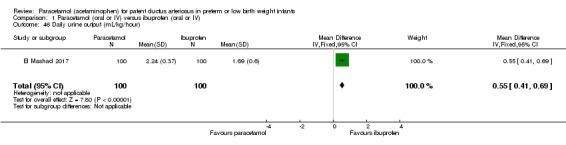
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 46 Daily urine output (mL/kg/hour).
One study reported on this outcome in 200 infants (El Mashad 2017). There was a significantly higher urine output in the paracetamol group compared with the ibuprofen group (MD 0.55 mL/kg/h, 95% CI 0.41 to 0.69). Test for heterogeneity not applicable.
Other outcomes
For the following outcomes the included studies reported separately on samples ranging from 87 to 160 infants. As only one study was included in each analysis, tests for heterogeneity were not applicable. There were no significant differences between the paracetamol (oral or IV) group compared to the ibuprofen group (IV) for the following outcomes (for details see the analyses).
Neonatal mortality (death during the first 28 days of life) Analysis 1.4; Infant mortality (death during the first year of life) Analysis 1.5; Duration of ventilator support (days) Analysis 1.8; Pulmonary hypertension (defined as an increased mean pulmonary arterial pressure of 25 mmHg at rest) Analysis 1.10; Bronchopulmonary dysplasia (BPD) at 28 days (defined as O₂ requirement at 28 days postnatal age in addition to compatible clinical and roentgenographic findings) Analysis 1.12; BPD at 36 weeks' PMA (defined as O₂ requirement at 36 weeks' PMA in addition to compatible clinical and roentgenographic findings) Analysis 1.13; Moderate to severe BPD according to the new criteria: moderate BPD defined as O₂ for ≥ 28 days plus treatment with < 30% O₂ at 36 weeks' PMA; and severe BPD as O₂ for ≥ 28 days plus ≥ 30% O₂ or positive pressure at 36 weeks' PMA, or both Analysis 1.14; Severe BPD defined according to the new criteria: severe BPD defined as O₂ for ≥ 28 days plus ≥ 30% O₂ or positive pressure at 36 weeks' PMA, or both Analysis 1.15; Intestinal perforation Analysis 1.20; ROP stage ≥ 3 (according to the international classification of ROP) Analysis 1.23; ROP requiring laser therapy Analysis 1.24; Serum or plasma levels of aspartate transaminase (AST) (IU/L) following treatment Analysis 1.28; Serum or plasma levels of ALT (IU/L) following treatment Analysis 1.29; Duration of hospitalisation (total length of hospitalisation from birth to discharge home or death, in days) Analysis 1.32; Glutamic‐pyruvic transaminase (U/L) Analysis 1.44.
Analysis 1.4.
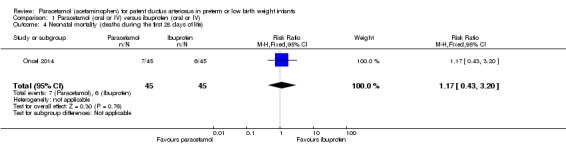
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 4 Neonatal mortality (deaths during the first 28 days of life).
Analysis 1.5.
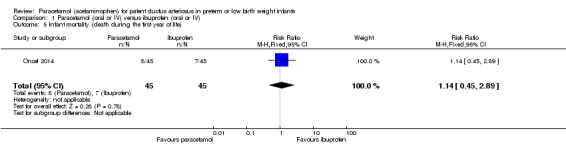
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 5 Infant mortality (death during the first year of life).
Analysis 1.8.
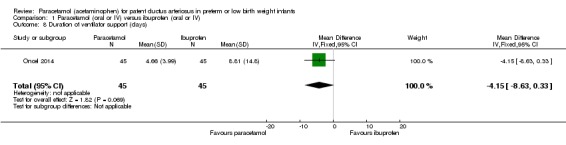
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 8 Duration of ventilator support (days).
Analysis 1.10.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 10 Pulmonary hypertension.
Analysis 1.12.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 12 BPD at 28 days.
Analysis 1.13.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 13 BPD at 36 weeks' PMA.
Analysis 1.14.
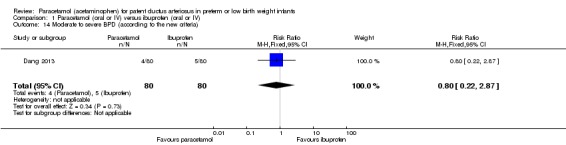
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 14 Moderate to severe BPD (according to the new criteria).
Analysis 1.15.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 15 Severe BPD (according to the new criteria).
Analysis 1.20.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 20 Intestinal perforation.
Analysis 1.23.
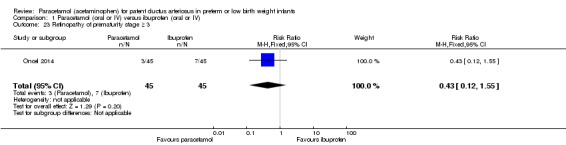
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 23 Retinopathy of prematurity stage ≥ 3.
Analysis 1.24.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 24 Retinopathy of prematurity requiring laser therapy.
Analysis 1.28.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 28 Serum levels of aspartate transaminase (AST) IU/L.
Analysis 1.29.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 29 Serum levels of alanine aminotransferase (ALT) (IU/L).
Analysis 1.32.
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 32 Duration of hospitalisation (days).
Analysis 1.44.
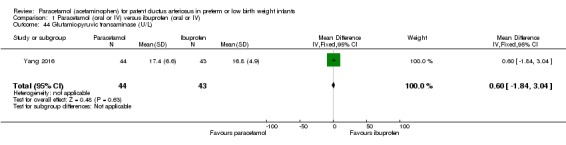
Comparison 1 Paracetamol (oral or IV) versus ibuprofen (oral or IV), Outcome 44 Glutamic‐pyruvic transaminase (U/L).
Incidence of liver failure; evidence of acute liver injury combined with either severe coagulopathy (International Normalized Ratio (INR) > 2.0 or prothrombin time (PT) > 20 seconds) or encephalopathy with moderate coagulopathy (INR ≥ 1.5 or PT ≥ 15 seconds)
One study (n = 90 infants) reported on this outcome. Liver failure did not occur in any infant enrolled in the study.
Other side effects reported by the authors (not pre‐specified)
Oncel 2014 reported that no other side effects were noted.
Autism or autism spectrum disorder (ASD) in childhood
As defined by the American Psychiatric Association (APA 2013).
No study has reported on this outcome, but Oncel 2014 indicates in his follow‐up study that the authors plan to re‐evaluate their cohort in terms of autism spectrum disorders in the future.
Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention (Comparison 2)
Failure of ductal closure after 4 to 5 days of treatment (Outcome 2.1)
Analysis 2.1.
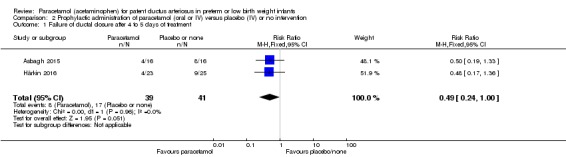
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 1 Failure of ductal closure after 4 to 5 days of treatment.
Two studies reported on this outcome in 80 infants (Asbagh 2015; Härkin 2016). Paracetamol resulted in a lower rate of failure of ductal closure after 4 to 5 days of treatment compared to placebo or no intervention of borderline significance for typical RR 0.49, 95% CI 0.24 to 1.00, (P = 0.05); but significant typical RD −0.21, 95% CI −0.41 to −0.02; NNTB 5 (95% CI 2 to 50). There was no heterogeneity for this outcome; I² = 0% for both RR and RD). The quality of evidence according to GRADE was low.
Death (Outcome 2.2)
Analysis 2.2.
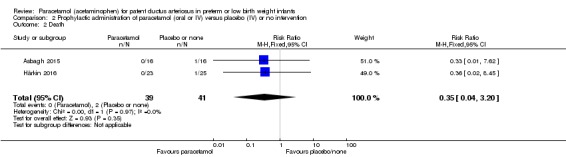
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 2 Death.
Two studies reported on this outcome in 80 infants (Asbagh 2015; Härkin 2016). There was no significant difference between the paracetamol group compared to the placebo or no intervention group (typical RR 0.35, 95% CI 0.04 to 3.20; typical RD −0.05, 95% CI −0.14 to 0.05). There was no heterogeneity for this outcome; I² = 0 % for both RR and RD. The quality of evidence according to GRADE was moderate.
For the following outcomes one study reported on 48 infants; and as only one study was included in the different analyses, tests for heterogeneity were not applicable. There were no significant differences between the paracetamol versus the placebo group for the following outcomes (for details see the analyses): Oliguria Analysis 2.3; Polyuria Analysis 2.4; Hypernatraemia Analysis 2.5; Sepsis Analysis 2.6; Supplemental oxygen at 28 days Analysis 2.7; Supplemental oxygen at 36 weeks' PMA Analysis 2.8; ROP (treated) Analysis 2.9; IVH grades 1 to 2 Analysis 2.10; IVH grades 3 to 4 Analysis 2.11; NEC stage 3 Analysis 2.12; Days of supplemental oxygen Analysis 2.13; Highest serum bilirubin (µmol/L) Analysis 2.14.
Analysis 2.3.
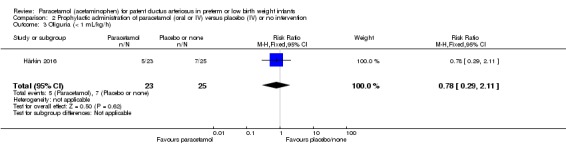
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 3 Oliguria (< 1 mL/kg/h).
Analysis 2.4.
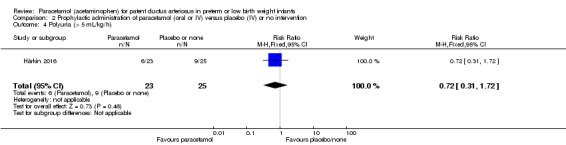
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 4 Polyuria (> 5 mL/kg/h).
Analysis 2.5.
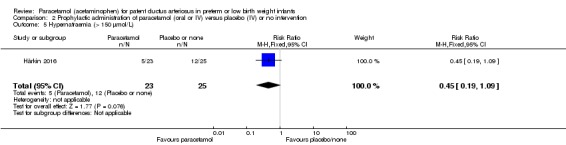
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 5 Hypernatraemia (> 150 µmol/L).
Analysis 2.6.
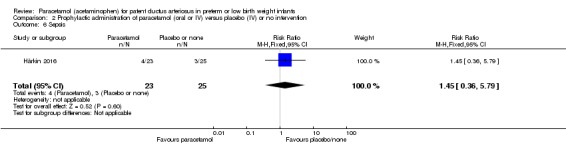
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 6 Sepsis.
Analysis 2.7.
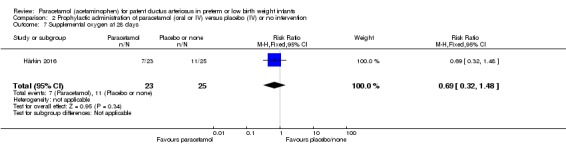
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 7 Supplemental oxygen at 28 days.
Analysis 2.8.
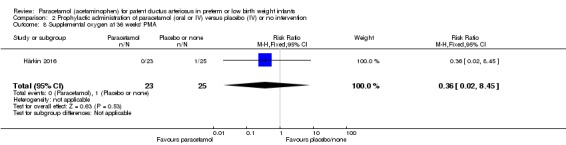
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 8 Supplemental oxygen at 36 weeks' PMA.
Analysis 2.9.
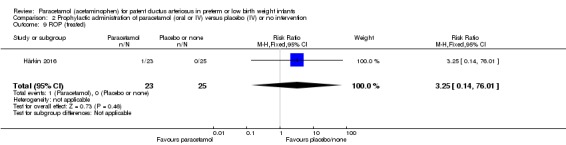
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 9 ROP (treated).
Analysis 2.10.
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 10 IVH grades 1 to 2.
Analysis 2.11.
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 11 IVH grades 3 to 4.
Analysis 2.12.
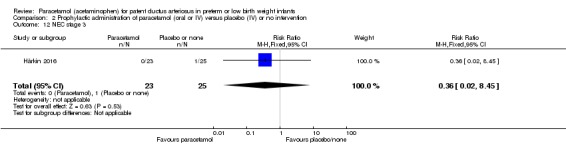
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 12 NEC stage 3.
Analysis 2.13.
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 13 Days of supplemental oxygen.
Analysis 2.14.
Comparison 2 Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention, Outcome 14 Highest serum bilirubin µmol/L.
Paracetamol (oral or IV) versus indomethacin (IV) (Comparison 3)
Failure to close a PDA (Outcome 3.1)
Analysis 3.1.
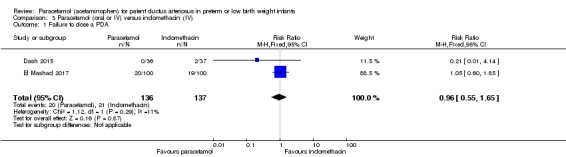
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 1 Failure to close a PDA.
Two studies reported on this outcome in 273 infants (Dash 2015; El Mashad 2017). There was no significant difference between the paracetamol group compared with the indomethacin group (typical RR 0.96, 95% CI 0.55 to 1.65; typical RD −0.01, 95% CI −0.09 to 0.08; I² = 11% for RR and 17% for RD). The quality of evidence according to GRADE was moderate.
Gastrointestinal bleed (Outcome 3.3)
Analysis 3.3.
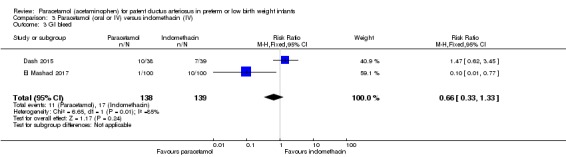
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 3 GI bleed.
Two studies reported on this outcome in 277 infants (Dash 2015; El Mashad 2017). There was no significant difference between the paracetamol group compared with the indomethacin group (typical RR 0.66, 95% CI 0.33 to 1.33; typical RD −0.04, 95% CI −0.11 to 0.03) There was high heterogeneity for these analyses (I² = 85% for RR and 76% for RD).
NEC (all grades) (Outcome 3.4)
Analysis 3.4.
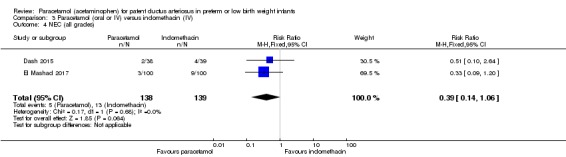
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 4 NEC (all grades).
Two studies reported on this outcome in 277 infants (Dash 2015; El Mashad 2017). There was no significant difference between the paracetamol group compared with the indomethacin group (typical RR 0.39, 95% CI 0.14 to 1.06; typical RD −0.06, 95% CI −0.11 to 0.00; I² = 0% for both RR and RD.
Sepsis (Outcome 3.5)
Analysis 3.5.
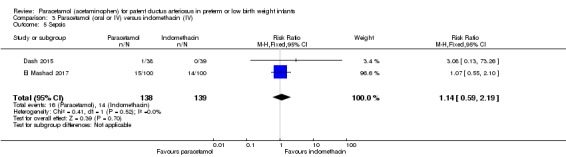
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 5 Sepsis.
Two studies reported on this outcome in 277 infants (Dash 2015; El Mashad 2017). There was no significant difference between the paracetamol group compared with the indomethacin group (typical RR 1.14, 95% CI 0.59 to 2.19; typical RD 0.01, 95% CI −0.06 to 0.09; I² = 0% for both RR and RD).
Pulmonary haemorrhage (Outcome 3.6)
Analysis 3.6.
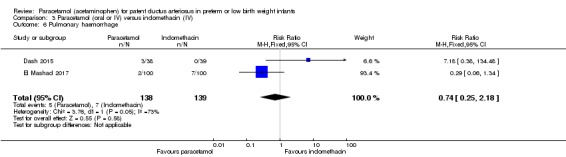
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 6 Pulmonary haemorrhage.
Two studies reported on this outcome in 277 infants (Dash 2015; El Mashad 2017). There was no significant difference between the paracetamol group compared with the indomethacin group (typical RR 0.74, 95% CI 0.25 to 2.18; typical RD −0.01, 95% CI −0.06 to 0.04; I² = 73% for RR (moderate) and 80% for RD (high)).
ROP (all grades) (Outcome 3.7)
Analysis 3.7.
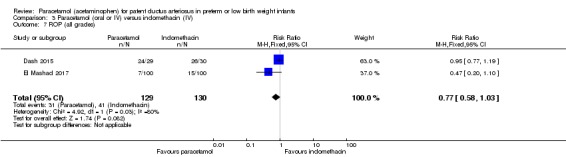
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 7 ROP (all grades).
Two studies reported on this outcome in 259 infants (Dash 2015; El Mashad 2017). There was no significant difference between the paracetamol group compared with the indomethacin group (typical RR 0.77, 95% CI 0.58 to 1.03; typical RD −0.07, 95% CI −0.15 to 0.01; I² = 80% for RR (high) and 0% for RD (none)).
IVH (all grades) (Outcome 3.9)
Analysis 3.9.
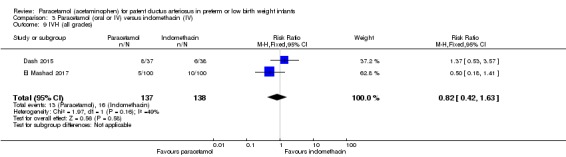
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 9 IVH (all grades).
Two studies reported on this outcome in 275 infants (Dash 2015; El Mashad 2017). There was no significant difference between the paracetamol group compared with the indomethacin group (typical RR 0.82, 95% CI 0.42 to 1.63; typical RD −0.02, 95% CI −0.09 to 0.05; I² = 49% for RR (low) and 29% for RD (low)).
Serum creatinine (µmol/L) (Outcome 3.17)
Analysis 3.15.
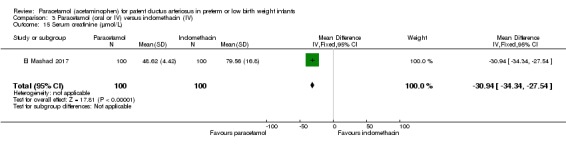
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 15 Serum creatinine (µmol/L).
One study reported on this outcome in 200 infants (El Mashad 2017). The serum creatinine was significantly lower in the paracetamol group compared with the indomethacin group (MD −30.94 µmol/L, 95% CI −34.34 to −27.54). Tests for heterogeneity were not applicable.
Serum bilirubin (µmol/L) (Outcome 3.18)
Analysis 3.18.
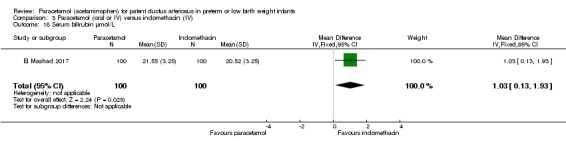
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 18 Serum bilirubin µmol/L.
One study reported on this outcome in 200 infants (El Mashad 2017). The serum bilirubin was statistically significantly higher in the paracetamol group compared with the indomethacin group (MD 1.03 µmol/L, 95% CI 0.13 to 1.93). Tests for heterogeneity were not applicable. The increase in serum bilirubin is not clinically significant.
Platelet count (x10⁹/L) (Outcome 3.19)
Analysis 3.19.

Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 19 Platelet count (x109/L).
One study reported on this outcome in 200 infants (El Mashad 2017). The platelet count was significantly higher in the paracetamol group compared with the indomethacin group (MD 112.00 (x10⁹/L), 95% CI 103.02 to 120.98). Tests for heterogeneity were not applicable.
Daily urine output (mL/kg/h) (Outcome 3.20)
Analysis 3.20.

Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 20 Daily urine output (mL/kg/h).
One study reported on this outcome in 200 infants (El Mashad 2017). The daily urine output was significantly higher in the paracetamol group compared with the indomethacin group (MD 1.14 (mL/kg/h), 95% CI 1.04 to 1.24). Tests for heterogeneity were not applicable.
Other outcomes
For the following outcomes the two included studies, Dash 2015 and El Mashad 2017, reported separately on samples of 39 to 200 infants for each outcome. As only one study was included in each analysis, tests for heterogeneity were not applicable. There were no significant differences between the paracetamol (oral or IV) group compared to the indomethacin group (IV) for the following outcomes (for details see the analyses): Renal impairment Analysis 3.2; Severe ROP needing treatment Analysis 3.8; IVH (grades III to IV) Analysis 3.10; Periventricular leukomalacia Analysis 3.11; Oxygen requirement at 28 days of age Analysis 3.12; Oxygen requirement at ≥ 36 weeks' PMA Analysis 3.13; Death Analysis 3.14; Failure to close a PDA after a 2nd course of IV paracetamol versus IV indomethacin Analysis 3.16; and Surgical ligation of PDA Analysis 3.17.
Analysis 3.2.
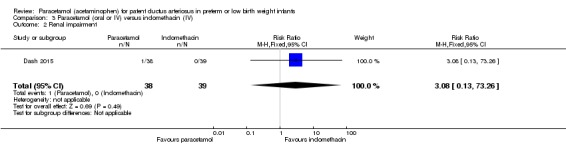
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 2 Renal impairment.
Analysis 3.8.
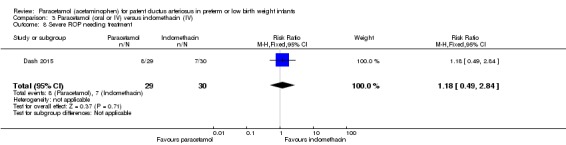
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 8 Severe ROP needing treatment.
Analysis 3.10.
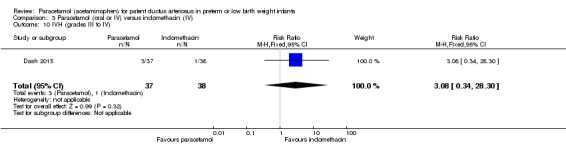
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 10 IVH (grades III to IV).
Analysis 3.11.
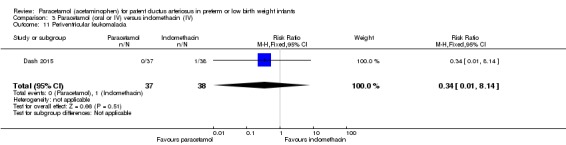
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 11 Periventricular leukomalacia.
Analysis 3.12.
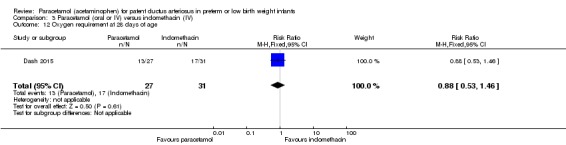
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 12 Oxygen requirement at 28 days of age.
Analysis 3.13.
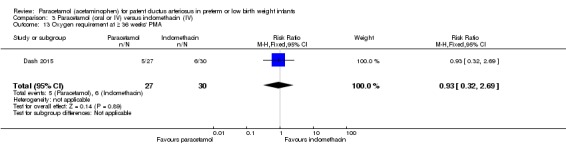
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 13 Oxygen requirement at ≥ 36 weeks' PMA.
Analysis 3.14.
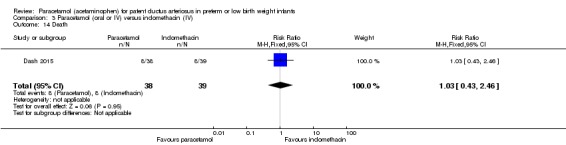
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 14 Death.
Analysis 3.16.
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 16 Failure to close a PDA after a 2nd course of IV paracetamol versus IV indomethacin.
Analysis 3.17.
Comparison 3 Paracetamol (oral or IV) versus indomethacin (IV), Outcome 17 Surgical ligation of PDA.
Discussion
Summary of main results
The eight studies completed to date have compared paracetamol to ibuprofen (five studies) (Al‐Lawama 2017; Dang 2013; El Mashad 2017; Oncel 2014; Yang 2016); paracetamol to placebo or no intervention (two studies) (Asbagh 2015; Härkin 2016); and paracetamol to indomethacin (two studies) (Dash 2015; El Mashad 2017). One study (El Mashad 2017), compared paracetamol to either ibuprofen or indomethacin. A total of 916 infants have been enrolled in these trials.
Paracetamol appears to be as effective as ibuprofen or indomethacin in closing a PDA after the first course (closure and failure of closure confirmed by echocardiographic criteria), although the trends favoured paracetamol over ibuprofen or indomethacin, and paracetamol over placebo or no intervention. Adverse effects were less common in the paracetamol group compared with the ibuprofen or the indomethacin groups. Gastrointestinal bleed or stools positive for occult blood was significantly less likely to occur in the paracetamol group versus the ibuprofen group. Serum or plasma levels of creatinine were significantly lower in the paracetamol group versus the ibuprofen group and versus the indomethacin group. Urine output was significantly higher in the paracetamol group versus the ibuprofen group and versus the indomethacin group. The platelet counts were significantly higher in the paracetamol group versus the ibuprofen and the indomethacin groups after treatment.
Overall completeness and applicability of evidence
To date, 916 infants have been enrolled in trials comparing the effectiveness and safety of paracetamol compared to ibuprofen for PDA closure in preterm infants. Larger trials are required to confirm the current promising evidence. Viber and co‐workers reported that paracetamol administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice (Viberg 2014). The dose of paracetamol used in mice was similar to that used in the two studies included in this review. In view of the possible negative impact of paracetamol on the developing brain reported in mice (Viberg 2014), the long‐term effects of paracetamol used for PDA closure or prevention and treatment of pain need to be studied carefully. In addition, in an ecological study conducted in humans and using country‐level data for the period 1984 to 2005, postnatal use of paracetamol was associated with autism or autism spectrum disorder (ASD) (Bauer 2013). In 2017, Bornehag 2017 reported in a study from Sweden of a possible association between maternal prenatal exposure to paracetamol and language delay in 30‐month‐old girls (parental report of use of fewer than 50 words).
There are at least 19 ongoing trials that would provide additional evidence regarding this topic. The researchers should be encouraged to include pharmacokinetic data to determine optimal dosing regimen, duration of treatment and mode of administration (El‐Khuffash 2014). Long‐term follow‐up should be planned to at least 18 to 24 months and preferably to school age.
Applicability of current evidence regarding similar effectiveness of paracetamol should be appropriately evaluated considering local context. Cost of oral paracetamol is very low compared to intravenous indomethacin or intravenous ibuprofen. Oral paracetamol has a favourable side‐effect profile and thus a unit may decide to use paracetamol as primary agent; however, we strongly recommend that neonates should be followed for neurodevelopmental assessment and further information gained about its impact.
Quality of the evidence
Although healthcare providers and researchers were aware of group assignment in most studies, we considered the included trials to be of good quality as the random sequence was computer generated and allocation to the two study groups was in most studies by opaque, sequentially numbered and sealed envelopes. In addition, we were able to obtain unpublished data from several studies (Dang 2013; Dash 2015; Oncel 2014), enabling us to report more accurately on outcomes. The quality of the evidence, using GRADE, was moderate for the primary outcome 'failure of ductal closure after the first course of treatment' in the comparisons of paracetamol versus ibuprofen and versus indomethacin, and moderate for other important outcomes — all cause mortality, GI bleeds, serum creatinine — but low GRADE for neurodevelopmental impairment. The quality of the evidence, using GRADE, was low for 'failure of ductal closure after the first course of treatment' but moderate for death. For the 'failure of ductal closure after the first course of treatment' for paracetamol versus indomethacin, the evidence was moderate according to GRADE.
Potential biases in the review process
We are not aware of any biases in the review process. Neither of the authors is involved in any of the trials included in the review.
Agreements and disagreements with other studies or reviews
We are aware of three systematic reviews with meta‐analyses on the topic (Das 2014; Huang 2017; Terrin 2016). In the previous version of this review (Ohlsson 2015b), we included two trials (Dang 2013; Oncel 2014). Das 2014 included the same two trials as did Terrin 2016. Huang 2017 included five trials of paracetamol versus ibuprofen with 677 neonates enrolled (Bagheri 2016; Dang 2013; El Mashad 2017; Oncel 2014; Yang 2016). We did not include Bagheri 2016 as the study is awaiting classification and to date we have not got a response from the authors. Huang 2017 concluded that "paracetamol may confer comparable treatment efficacy for the closure of PDA as ibuprofen, although paracetamol is associated with lower risk of adverse event". Mitra 2016 have published a protocol for a systematic review and network meta‐analysis of the effectiveness and safety of treatments used for the management of PDA in preterm infants. In the study by Bagheri 2016 the numbers do not add up in Figure 1 and denominators are not presented in Tables 1 and 2. We contacted the authors on two occasions — on 10 January 2017 and again on 18 November 2017 — but did not receive any response. We therefore listed the study as awaiting classification.
Authors' conclusions
Moderate quality of evidence suggests that paracetamol is as effective in closing a PDA as ibuprofen or indomethacin and is associated with fewer renal and gastrointestinal side effects. Low‐quality evidence indicates no difference in neurodevelopmental outcomes of those treated with paracetamol compared to those treated with ibuprofen. Clinicians may prefer to use paracetamol as a primary agent for closure of PDA; however, they need to be aware that concerns have been raised regarding effect of prenatal and postnatal use of paracetamol and developmental disorders and are advised to follow these infants carefully. Further research regarding the effectiveness and safety of paracetamol to close a PDA is needed before the evidence is established or rejected.
Additional larger trials are required to increase the precision of the point estimates for the primary and secondary outcomes included in this review. In view of a report in mice of adverse effects on the developing brain from paracetamol (Viberg 2014), the association between postnatal use of paracetamol and autism and autism spectrum disorder (ASD) (Bauer 2013), and prenatal exposure to paracetamol and language delay in girls at 30 months Bornehag 2017, the long‐term follow‐up to 18 to 24 months' postnatal age and preferably to school‐age should be incorporated in any studies of paracetamol to close a PDA or to prevent or treat pain.
Acknowledgements
We are grateful to Ms Jennifer Spano, Trials Search Co‐ordinator, Cochrane Neonatal Review Group, who conducted the literature searches in 2017. We are thankful to Dr Mehmet Yekta Oncel, who provided us with unpublished data from their study (Oncel 2014). We are thankful to Dr Hui Wu, who provided us with unpublished information form their study (Dang 2013). Dr Hamid Hakak translated the article by Asbagh 2015 from Farsi to English. Dr Kabra provided us with clarifying information regarding the trial by Dash 2015.
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: ((exp infant) OR (infan* OR newborn or neonat* OR premature or very low birth weight or low birth weight or VLBW or LBW).mp AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp
CINAHL: (infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Previous Search Methodology
For the 2015 review, we used the standard search strategy of the Cochrane Neonatal Review Group as outlined in the Cochrane Library. This included electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library), MEDLINE (1966 to December 2013), EMBASE (1980 to December 2013) and CINAHL (1982 to December 2013). Ms Colleen Ovelman, Trials Search Co‐ordinator, Cochrane Neonatal Review Group, conducted the searches modified as needed for the different databases. For MEDLINE the following search string was used: (paracetamol OR acetaminophen) AND (patent ductus arteriosus or PDA) AND ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])). Relevant reviews related to the topic were identified. No language restrictions were applied.
We conducted electronic searches of abstracts from the meetings of the Pediatric Academic Societies 2000 to 2013 and the Perinatal Society of Australia and New Zealand 2000 to 2013.
We searched the following clinical trials registries for ongoing or recently completed trials: clinicaltrials.gov; controlled‐trials.com; anzctr.org.au; who.int/ictrp in December 2013. We searched the Web of Science for articles quoting identified RCTs in December 2013.
We searched the first 200 hits on Google ScholarTM to identify grey literature. We limited the Google ScholarTM to the first 200 hits as in our experience the yield is poor after 200 hits.
We repeated the search of MEDLINE in August 2014 and did not identify any new trials.
Appendix 3. 'Risk of bias' tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1.
Paracetamol (oral or IV) versus ibuprofen (oral or IV)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of ductal closure after the first course of treatment | 5 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.21] |
| 2 Neurodevelopmental impairment | 1 | 61 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.25, 0.21] |
| 3 All‐cause mortality during initial hospital stay | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.67] |
| 4 Neonatal mortality (deaths during the first 28 days of life) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.43, 3.20] |
| 5 Infant mortality (death during the first year of life) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.45, 2.89] |
| 6 Re‐opening of the ductus arteriosus | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.50, 2.18] |
| 7 Surgical closure of the PDA following treatment failure with paracetamol or ibuprofen | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.35, 1.32] |
| 8 Duration of ventilator support (days) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.15 [‐8.63, 0.33] |
| 9 Pulmonary haemorrhage | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.23, 1.74] |
| 10 Pulmonary hypertension | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
| 11 Duration for need of supplementary oxygen (days) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐12.40 [‐22.97, ‐1.83] |
| 12 BPD at 28 days | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.35] |
| 13 BPD at 36 weeks' PMA | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.38, 1.30] |
| 14 Moderate to severe BPD (according to the new criteria) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.87] |
| 15 Severe BPD (according to the new criteria) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.32, 1.23] |
| 16 Intraventricular haemorrhage (grade I‐IV) | 5 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| 17 Severe IVH (Grade III‐IV) | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.37] |
| 18 Periventricular leukomalacia | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.76] |
| 19 Necrotizing enterocolitis | 5 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.70] |
| 20 Intestinal perforation | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Gastrointestinal bleed or stools positive for occult blood | 4 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.12, 0.69] |
| 22 Retinopathy of prematurity ‐ any stage | 4 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.23] |
| 23 Retinopathy of prematurity stage ≥ 3 | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.55] |
| 24 Retinopathy of prematurity requiring laser therapy | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.55] |
| 25 Sepsis | 4 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 26 Oliguria (< 1 mL/kg/h) | 3 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.20, 1.10] |
| 27 Serum levels of creatinine after treatment µmol/L | 4 | 537 | Mean Difference (IV, Fixed, 95% CI) | ‐8.92 [‐11.28, ‐6.55] |
| 28 Serum levels of aspartate transaminase (AST) IU/L | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐1.83, 10.23] |
| 29 Serum levels of alanine aminotransferase (ALT) (IU/L) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐3.58, 11.58] |
| 30 Serum bilirubin following treatment (µmol/L) | 2 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐11.25 [‐13.88, ‐8.62] |
| 31 Hyperbilirubinaemia (serum bilirubin level higher than the exchange level according to the postnatal age and BW) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.34, 0.97] |
| 32 Duration of hospitalisation (days) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐6.5 [‐21.42, 8.42] |
| 33 MDI < 70 | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.41, 2.59] |
| 34 PDI < 70 | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.33, 3.21] |
| 35 Moderate to severe cerebral palsy | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.41, 10.46] |
| 36 Deafness | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.39] |
| 37 Blindness | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.39] |
| 38 MDI | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐8.19, 7.39] |
| 39 PDI | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐7.44, 7.04] |
| 40 BPD (age not stated) | 3 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.39, 1.95] |
| 41 Plasma PGE2 (ng/L) | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 12.60 [0.39, 24.81] |
| 42 Urine PGE2 (ng/L) | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 23.90 [2.78, 45.02] |
| 43 Platelet count (x109/L) | 2 | 287 | Mean Difference (IV, Fixed, 95% CI) | 30.18 [16.55, 43.81] |
| 44 Glutamic‐pyruvic transaminase (U/L) | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.84, 3.04] |
| 45 Failure of PDA closure after the 2nd course of IV paracetamol versus IV ibuprofen | 2 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.21] |
| 46 Daily urine output (mL/kg/hour) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [0.41, 0.69] |
Comparison 2.
Prophylactic administration of paracetamol (oral or IV) versus placebo (IV) or no intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of ductal closure after 4 to 5 days of treatment | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.24, 1.00] |
| 2 Death | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.20] |
| 3 Oliguria (< 1 mL/kg/h) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.29, 2.11] |
| 4 Polyuria (> 5 mL/kg/h) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.31, 1.72] |
| 5 Hypernatraemia (> 150 µmol/L) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.19, 1.09] |
| 6 Sepsis | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.36, 5.79] |
| 7 Supplemental oxygen at 28 days | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.32, 1.48] |
| 8 Supplemental oxygen at 36 weeks' PMA | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.45] |
| 9 ROP (treated) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.14, 76.01] |
| 10 IVH grades 1 to 2 | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.24, 1.54] |
| 11 IVH grades 3 to 4 | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 16.39] |
| 12 NEC stage 3 | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.45] |
| 13 Days of supplemental oxygen | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐16.41, 11.61] |
| 14 Highest serum bilirubin µmol/L | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐10.35, 12.35] |
Comparison 3.
Paracetamol (oral or IV) versus indomethacin (IV)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a PDA | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.65] |
| 2 Renal impairment | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 73.26] |
| 3 GI bleed | 2 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.33, 1.33] |
| 4 NEC (all grades) | 2 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.14, 1.06] |
| 5 Sepsis | 2 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.59, 2.19] |
| 6 Pulmonary haemorrhage | 2 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.25, 2.18] |
| 7 ROP (all grades) | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.03] |
| 8 Severe ROP needing treatment | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.49, 2.84] |
| 9 IVH (all grades) | 2 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.42, 1.63] |
| 10 IVH (grades III to IV) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.34, 28.30] |
| 11 Periventricular leukomalacia | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.14] |
| 12 Oxygen requirement at 28 days of age | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.46] |
| 13 Oxygen requirement at ≥ 36 weeks' PMA | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.32, 2.69] |
| 14 Death | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.43, 2.46] |
| 15 Serum creatinine (µmol/L) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐30.94 [‐34.34, ‐27.54] |
| 16 Failure to close a PDA after a 2nd course of IV paracetamol versus IV indomethacin | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.40] |
| 17 Surgical ligation of PDA | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.44, 1.92] |
| 18 Serum bilirubin µmol/L | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [0.13, 1.93] |
| 19 Platelet count (x109/L) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 112.0 [103.02, 120.98] |
| 20 Daily urine output (mL/kg/h) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.14 [1.04, 1.24] |
What's new
Last assessed as up‐to‐date: 6 November 2017.
| Date | Event | Description |
|---|---|---|
| 17 January 2018 | New citation required and conclusions have changed | Paracetamol has now been compared to placebo/no intervention and to indomethacin. Paracetamol appears more effective in closing a PDA than placebo/no intervention and has a similar effectiveness as ibuprofen or indomethacin with fewer side effects. Only one small study has reported on long‐term follow‐up. |
| 17 January 2018 | New search has been performed | The literature was searched in November 2017. Six new published trials and 16 new ongoing trials were identified. |
Differences between protocol and review
We made some minor wording changes to the primary outcome. We changed from 'Failure of PDA closure within a week of administration of the first dose of paracetamol (closure and failure of closure confirmed by echocardiographic criteria)' to 'Failure of PDA closure after the first course of paracetamol (closure and failure of closure confirmed by echocardiographic criteria)'. We have added a few outcomes that the authors of the included studies reported on but that we had not anticipated. We have indicated this for the specific outcomes that were not pre‐determined.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled study conducted in the Neonatal Intensive Care Unit of Jordan University Hospital, Amman, Jordan. Study period: from March 2015 to October 2016 | |
| Participants | Inclusion criteria: preterm infants with a gestational age of ≤ 32 weeks or birth weight of ≤ 1500 g and a haemodynamically significant PDA diagnosed by ECHO. Exclusion criteria: ductal‐dependent congenital heart diseases, major congenital malformation, grade 3 to 4 intraventricular haemorrhage, renal impairment (defined as a creatinine concentration of > 1.5 mg/dL), pulmonary haemorrhage, thrombocytopenia of < 60,000/mm³ , and an elevated alanine transaminase concentration |
|
| Interventions | The oral paracetamol group (n = 13) received 10 mg/kg/dose followed by 1 to 2 mL of 0.9% saline every 6 h for 3 days. (10 mg/kg every 6 h for 3 days). The oral ibuprofen group (n = 9) received 10 mg/kg/dose followed by 1 to 2 mL of 0.9% saline once daily for 3 days. (10 mg/kg/day for 3 days). The researchers used the same dose for the 3‐day course to minimize errors |
|
| Outcomes | Primary outcome: mortality, primary PDA closure. Secondary outcomes: secondary PDA closure, pulmonary haemorrhage, BPD, Sepsis, NEC, ROP, IVH Grade 1 to 2, IVH Grade 3 to 4, PVL |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer to receive either oral paracetamol or oral ibuprofen |
| Allocation concealment (selection bias) | Low risk | Randomisation numbers were placed inside sequentially numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There were different scheduling regimens for paracetamol and ibuprofen, so staff were not blinded to the drugs administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the person conducting ECHO cardiography was blinded to the treatment or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised infants are accounted for |
| Selective reporting (reporting bias) | Unclear risk | The study was registered: ISRCTN12302923 DOI 10.1186. The study was registered in retrospect when it was completed, so we cannot judge if there were any deviations from the original protocol |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised controlled trial conducted in the Neonatal intensive care unit (NICU) of Vali‐Asr Hospital, Tehran, Iran. Study period March 2012 to March 2013 | |
| Participants | Inclusion criteria: infants ≤ to 32 weeks' PMA and birth weight ≤ 1500 g and < 24 h old Exclusion criteria: infants with congenital heart disease that required a PDA to survive, major congenital malformations, history of NSAID use in the mother, hydrops fetalis, PPHN, Apgar score < 5 at 5 min, symptomatic PDA requiring treatment with ibuprofen, vomiting or hematemesis in the first 3 days of life, G6PD positive |
|
| Interventions | The prophylaxis group (n = 16) received oral paracetamol for a period of 2 days starting during the first 24 h of life. Infants received acetaminophen drops 15 mg/kg every 6 h for 48 h (8 doses). The control group (n = 16) received no intervention or placebo. ECHOs were performed 24 to 36 h after the last given dose in the prophylaxis group and on the 4th and 5th day in the control group | |
| Outcomes | Primary outcome: failure to close a PDA. PDA was diagnosed by ECHO: internal diameter > 1.5 mm, LA/AO ratio > 1.3. Secondary outcomes: death; need for treatment with ibuprofen |
|
| Notes | In the following situations, infants were removed from the study: if the infant died, if the infant was discharged, if the infant needed ibuprofen or if the infant developed PPHN. We contacted the corresponding author Dr. Zarkesh on 30 November 2017 to get clarifying information, but by 26 March 2018 we had not received a response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used |
| Allocation concealment (selection bias) | Unclear risk | No information presented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information presented |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The ECHOs were performed by a cardiologist but no information provided if the assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all enrolled infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we cannot judge if there were any deviations or not |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised controlled trial conducted in the First Hospital of Jilin University, China. Study period 21 May 2012 to 30 March 2013 | |
| Participants | Inclusion criteria: PMA ≤ 34 weeks; postnatal age < 14 days; echocardiographic diagnosis of haemodynamically significant PDA Exclusion criteria: congenital heart disease which required PDA to maintain blood flow; life‐threatening infection; recent (within the previous 24 h) intraventricular haemorrhage, Grade 3–4; urine output < 1 mL/kg/h during the preceding 8 h; serum creatinine > 88.4 mmol/L; platelet count of < 50 × 10⁹/L; hyperbilirubinaemia requiring exchange transfusion; active necrotizing NEC and/or intestinal perforation; liver dysfunction |
|
| Interventions | Eighty infants received oral paracetamol at the dose of 15 mg/kg every 6 h for 3 days, and 80 infants received oral ibuprofen at the initial dose of 10 mg/kg followed by 5 mg/kg after 24 and 48 h. Between doses of oral ibuprofen, infants of the ibuprofen group received the same volume of dextrose 5% in water (D5W) as that given for drug administration in the paracetamol group. Whether a subject received a second course of treatment depended on echocardiography evaluation after the first course. If only minor ductal shunting was present after 2 courses without the need for respiratory support, no further treatment was given. | |
| Outcomes | Failure of PDA closure, all‐cause mortality, re‐opening of the ductus arteriosus, BPD (according to NICHD criteria: Jobe 2001), IVH (Grade I to IV; Grade I to II, Grade III to IV), PVL (diagnosed by cranial MRI), NEC (Bell staging criteria ‒ Grade IIa and above), gastrointestinal bleed, ROP (any stage), oliguria (< 1 mL/kg/h), sepsis (positive blood culture), hyperbilirubinaemia according to Maisels 2003 ‒ a serum bilirubin level higher than the exchange transfusion level according to the postnatal age and body weight), serum creatinine (µmol/L) following treatment. | |
| Notes | In the report, although references were provided for BPD and hyperbilirubinaemia, it was not possible to ascertain exactly what criteria the authors applied. Sepsis was not defined. We wrote to the authors requesting clarification. We received a response and their definitions are included for the outcomes listed above. Funded by Jilin Department of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table (according to the published protocol) |
| Allocation concealment (selection bias) | Low risk | Quote: "The participants were randomly assigned at a 1:1 ratio between oral paracetamol and ibuprofen groups by using cards in sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...doctors and nurses were not blind" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "...doctors and nurses were not blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported on an intention‐to‐treat basis which included patients who did not receive the complete course of treatment |
| Selective reporting (reporting bias) | Low risk | The study was entered in the Chinese Clinical Trial Register (http://www.chictr.org/cn). Registration number ChiCTR‐TRC‐12002177 and approved by the Hospital Ethics Committee of the First Hospital of Jilin University. There do not appear to be any deviations in study conduct between the study protocol and the publication |
| Other bias | Low risk | Appears free of other sources of bias |
| Methods | Open‐label randomised controlled trial conducted in a level III neonatal intensive care unit (NICU) of a private hospital in Mumbai, India. Study period: March 2012 to September 2013 | |
| Participants | Inclusion criteria: preterm infants with birth weight ≤1500 g and echocardiography performed within the first 48 h of life demonstrating PDA size ≥1.5 mm at the narrowest diameter, left to right shunt across the duct and ratio of the diameter of the left atrium to that of the aortic root (LA:AO) > 1.5:1. Exclusion criteria: inability to administer the study drug within 48 h of birth, structural duct‐dependent congenital heart disease, renal disease (such as multicystic dysplastic kidney and polycystic disease of kidney), dysmorphic features or congenital anomalies likely to affect life expectancy or neurologic development, maternal tocolytic therapy with indomethacin or another prostaglandin inhibitor within 72 h prior to giving birth, overt clinical bleeding at more than 1 site, platelet count < 50 × 10⁹/L, hydrops fetalis, and infant not considered viable. |
|
| Interventions | The paracetamol group (n = 38) received paracetamol drops through an infant feeding tube at a dose of 15 mg/kg/dose 4 times daily for 7 days (28 doses). The indomethacin group (n = 39) received IV indomethacin at a dose of 0.2 mg/kg/dose, diluted with normal saline to make 5 mL solution and infused over 20 mins by syringe pump once daily for 3 days. As per study protocol, 2 additional extra doses of indomethacin were allowed in the indomethacin group, if clinical evaluation after 3 doses showed persistence of PDA as demonstrated by clinical signs and symptoms such as tachycardia, wide pulse pressure and persistent murmur. |
|
| Outcomes | Primary outcome: PDA closure; (the PDA was considered to be closed if there was no evidence of any flow in the ductus arteriosus on echocardiographic and Doppler flow assessment). Secondary outcomes: death, renal impairment defined as presence of either oliguria (urine output of < 0.5 mL/kg/h) over a 6 h period or serum creatinine levels more than twice the age appropriate norms, gastro‐intestinal bleeding defined as the presence of blood‐stained or coffee ground brown gastric aspirates, necrotising enterocolitis (NEC) diagnosed as per modified Bell’s staging; pulmonary haemorrhage was diagnosed if a blood tinged tracheal aspirate was obtained; early‐ and late‐onset sepsis screen was defined as positive C‐reactive protein (CRP) before and after first 72 h of life (CRP > 6 mg/L), respectively, early‐onset sepsis defined as isolation of pathogenic organism from a blood culture collected in first 72 h of life, late‐onset sepsis defined as isolation of pathogenic organism from a blood culture collected after first 72 h of life, retinopathy of prematurity (ROP) classified as per the International classification of retinopathy — ROP needing either laser or anti‐VEGF (Avastin) therapy was labelled as severe ROP. Grading of intraventricular haemorrhage (IVH) was performed according to the Papile grading system, and features of periventricular leukomalacia (PVL) were assessed, as was requirement of supplemental oxygen at 28 days of postnatal age. Bronchopulmonary dysplasia (BPD)/chronic lung disease (CLD) was defined by the need for supplemental oxygen at 36 weeks' PMA. |
|
| Notes | We requested additional information from Dr. Kabra on 30 November 2017 and obtained information about IVH, PVL and NEC. Dr. Kabra confirmed that an abstract at a meeting in Vienna was a publication from the same study. We received the responses on 3 December 2017. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation software was used by a statistician, who was not part of the study |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was open label and study drugs were given over different length of time. Paracetamol was given through a feeding tube and indomethacin was given IV. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were not assessed blinded to treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported on all randomised infants. 2 deaths occurred in each group prior to follow‐up ECHO on day 7 |
| Selective reporting (reporting bias) | Unclear risk | The study was registered — Trial Registration No: CTRI/2012/12/003163 — but this was a retrospective registration so we cannot ascertain if there were deviations from the original protocol or not. |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised controlled trial in the neonatal intensive care unit (NICU) of Tanta University Hospital Pediatric Department, Tanta, Egypt Study period: January 2012 to December 2015 |
|
| Participants | Preterm neonates with PMA < 28 weeks or birth weight < 1500 g in the first 2 weeks of life with haemodynamically significant PDA (hs‐PDA) diagnosed with ECHO and clinical examination | |
| Interventions | Experimental intervention: Group I (paracetamol group): 100 neonates received 15 mg/kg IV infusion paracetamol over 30 min followed by 15 mg/kg/6 h IV infusion for 3 days. Dilution of paracetamol was required in neonates that weighed less than 1000 g using glucose 5% or sodium chloride 9% to achieve concentration of 2 mg/mL Group II (Ibuprofen group): 100 neonates received 10 mg/kg IV infusion ibuprofen followed by 5 mg/kg/ day for 2 days Group III (Indomethacin group): 100 neonates received 0.2 mg/kg indomethacin IV infusion over 30 min for 3 doses 12 h apart |
|
| Outcomes | Primary outcome: failure to close the PDA Secondary outcome: surgical ligation, ROP, GI bleeding, NEC, pulmonary haemorrhage, IVH, sepsis, daily urine output, serum creatinine, serum bilirubin, and platelet count |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation software was used for random sequence generation. The random number list was generated by QuickCalc GraphPad Software Inc |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done by sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The neonate was enrolled into the respective group by the doctor on duty who was not blinded and not a part of the study. All other treating staff and outcome assessors were blinded to the treatment group. This doctor might have spoken to the staff about the allocation. Drugs were given at different times and duration, thus precluding blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Echocardiography was done by a paediatric cardiologist who was blinded – so low risk for PDA assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered in a trials registry so we cannot judge if there were any deviations from a protocol |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised controlled trial conducted in the neonatal intensive care unit of Oulu University Hospital, Oulu, Finland. Study period: 18 September 2013 to 2 January 2015 | |
| Participants | Very low gestational age (< 32 weeks) infants requiring intensive care (n = 48). All infants had a PDA diagnosed by ECHO before the study drug was given and then an ECHO was performed once a day until 1 day after the study medication period | |
| Interventions | The paracetamol group (n = 23) received an IV loading dose of 20 mg/kg, given within 24 h of birth, followed by a maintenance dose of 7.5 mg/kg every 6 h for 4 days, given as 15‐min infusions The placebo group (n= 25) received 0.45% NaCl IV |
|
| Outcomes | Primary outcomes: decrease in ductal calibre without side effects; and failure to close a PDA by 4 to 5 days Secondary outcomes: persistent PDA treated, oliguria (< 1 mL/kg/h), polyuria (> 5 mL/kg/h), hypernatraemia (> 150 mmol/L), sepsis, supplemental oxygen at 28 days, supplemental oxygen at 36 weeks' PMA, ROP treated, IVH grades 1 to 2, IVH grades 3 to 4, NEC stage 3, death, days of supplemental oxygen, highest serum bilirubin ( µmol/L) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computed randomisation was performed using a 4‐block design |
| Allocation concealment (selection bias) | Low risk | The treatment allocation codes were sealed in sequentially labelled opaque envelopes. Both paracetamol and saline solutions appeared equally transparent in the syringe |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All nurses and doctors involved in the treatment and study of the infants were blinded to the study medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A separate team of nurses prepared the study drug in a study pharmacy outside NICU. The drug was given to the study patient’s nurse in a syringe |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Low risk | The study was registered in ClinicalTrials.gov: NCT01938261; European Clinical Trials Database: EudraCT 2013‐008142‐33. No deviations from the protocols were noted |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised controlled trial conducted in the neonatal intensive care unit of Zekai Tahir Burak Maternity Teaching Hospital, Ankara,Turkey. Study period February to December 2012 | |
| Participants | 90 infants with a gestational age ≤ 30 weeks, birth weight ≤ 1250 g, postnatal age 48 to 96 h, and 1 of the following echocardiographic criteria: a duct size > 1.5 mm, a left atrium‐to‐aorta ratio > 1.5, end diastolic reversal of blood flow in the aorta, or poor cardiac function in addition to clinical signs of a PDA Exclusion criteria were: the presence of major congenital abnormalities, right‐to‐left ductal shunting, life‐threatening infection, Grade III or Grade IV IVH, urine output of less than 1 mL/kg/h during the preceding 8 h, serum creatinine level > 1.6 mg/dL, platelet count < 60,000/mm³, liver failure, hyperbilirubinaemia requiring exchange transfusion, and persistent pulmonary hypertension |
|
| Interventions | 45 infants received oral paracetamol at a dose of 15 mg/kg every 6 h for 3 days and 45 infants received oral ibuprofen at an initial dose of 10 mg/kg followed by 5 mg/kg at 24 and 48 h. Both paracetamol and ibuprofen were administered via an orogastric tube, which was flushed with 1 to 2 mL of sterile water to ensure delivery of the drug | |
| Outcomes | Failure of PDA closure, all‐cause mortality, surgical closure of the PDA, duration of ventilator support, pulmonary haemorrhage, increase in grade of IVH, NEC, gastrointestinal bleed, ROP (requiring laser treatment), oliguria (not defined), sepsis (clinical symptoms and signs of sepsis and a positive blood bacterial culture), serum creatinine, bilirubin, AST and ALT, duration of hospitalisation. In 2017 the authors published neurodevelopmental outcomes of the infants enrolled in this trial; they reported on 30 children in the paracetamol group and 31 children in the ibuprofen group. They reported on neurodevelopmental impairment, MDI < 70, PDI < 70, moderate to severe cerebral palsy, blindness, deafness and MDI and PDI at 18 to 24 months corrected age. |
|
| Notes | We contacted Dr Oncel and he provided us with data for additional outcomes not reported in the published paper. In addition he provided outcome data for all 90 randomised infants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential numbers were generated at the computer centre of the NICU (information provided by the authors) |
| Allocation concealment (selection bias) | Low risk | The patients were randomly assigned to a treatment group by cards in sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Paracetamol and ibuprofen were given according to different schedules and therefore it is likely that healthcare providers were not blinded to the drug the infant was given. The authors write: "....the intervention was not completely blinded because of the different number of doses per day of the drugs. However, the most important outcome—PDA closure—was made by a cardiologist who was blinded to the treatment groups. Second, safety outcomes should have been defined more clearly before the study started to prevent overestimation in evaluation" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Paracetamol and ibuprofen were given according to different schedules and therefore it is likely that health care providers were not blinded to the drug the infant was given. The authors write: "....the intervention was not completely blinded because of the different number of doses per day of the drugs. However, the most important outcome—PDA closure—was made by a cardiologist who was blinded to the treatment groups. Second, safety outcomes should have been defined more clearly before the study started to prevent overestimation in evaluation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 infants were randomised, and we received outcome data for the 10 infants (5 in ibuprofen group and 5 in paracetamol group) who died before the treatment was completed. Thus we received outcome data on an intention‐to‐treat basis for all 90 randomised infants |
| Selective reporting (reporting bias) | Low risk | The trial was registered at ClinicalTrials.gov — NCT01536158 — and there does not seem to be any deviations between the protocol and the full publication |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised controlled trial conducted at the Neonatal Ward of the Affiliated Xuzhou Hospital of Medical College of Southeast University, Xuzhou, Jiangsu, China Study period from October 2012 to June 2015 |
|
| Participants | Preterm infants with PMA < 37 weeks and admitted to hospital within 24 h after birth. A significant PDA diagnosis was made between 15 h to 10 days after birth and confirmed through ECHO to be a significant PDA. Diagnostic criteria of echocardiography were: i) left atrial: aortic root diameter ratio, (LA:Ao) > 1.4; ii) pulmonary artery diastolic back flow (reflux); and iii) PDA vessel diameter > 1.4 mm | |
| Interventions | The paracetamol group (n = 44) received 15 mg/kg acetaminophen administered orally once every 6 h for 3 days The ibuprofen group (n = 43) received 10 mg/kg ibuprofen administered orally as the initial dose, followed by 5 mg/kg during the first 24 h and 48 h later |
|
| Outcomes | Primary outcome: failure of primary ductal closure Secondary outcomes: oliguria (< 1 mL/kg/h, stools positive for occult blood, IVH (grade not stated), NEC, BPD (PMA not stated), plasma PGE₂ (ng/L), urine PGE₂ (ng/L), platelet count (x10⁹/L), serum Cr (µmol/L), glutamic‐pyruvic transaminase (U/L) |
|
| Notes | Patients were excluded from the study if: i) patients presented with any of the following medication contraindications such as thrombocytopenia (blood platelet count < 50 × 10⁹/L), haemorrhagic disease, oliguria (urine volume per 8 h < 8 mL/kg), necrotizing colitis, intestinal perforation, high serum creatinine (> 159.1 μmol/L), and alanine aminotransferase (> 40 U/L) levels; ii) patients had congenital heart diseases such as ventricular septal defect, complex heart disease; iii) patients had incomplete treatment or wished to depart from the study due to personal reasons. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number table was used |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The dosing/timing schedule differed between the 2 drugs, so staff must have known which drug was given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated that the ECHOs were performed by a person blind to the treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The authors do not indicate that the study was registered in a trials registry at the protocol stage so we cannot judge if there were any deviations from the protocol or not |
| Other bias | Low risk | Appears free of other bias |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | Sixty‐nine neonates with PMA < 34 weeks and postnatal age < 14 days with significant PDA (confirmed through echocardiography), who had contraindications for ibuprofen and indomethacin were recruited |
| Interventions | The paracetamol group (n = 36) received oral paracetamol at a dose of 15 mg/kg/dose every 6 hours for 72 hours. The control group (n = 33) did not receive any intervention. After 72 hours, both groups were re‐evaluated by echocardiography. In case of failed closure of the PDA, the second course of treatment with paracetamol was administrated |
| Outcomes | Primary outcome: the rate of closure of PDA Secondary outcomes: the side effects of acetaminophen |
| Notes | This study was published on February 28, 2018, after our review had been submitted to the Cochrane Neonatal Editorial Office. The results will be included in the next update of the review |
| Methods | Randomised controlled trial |
| Participants | Preterm infants with PMA < 37 weeks, postnatal age ≤ 14 days and with echocardiographically diagnosed PDA with a ductus size of > 1.5 mm and a left atrium to aorta ratio of > 1.2 |
| Interventions | The paracetamol group received oral paracetamol 15 mg/kg every 6 h for 3 days. The ibuprofen group received oral ibuprofen 20 mg/kg as an initial dose followed by 10 mg/kg after 24 and 48 h |
| Outcomes | Primary outcome: rates of ductal closure on echocardiography after the complete course of both drugs Secondary outcomes: the safety of the drugs and adverse events (e.g. oliguria, IVH, tendency of bleeding, NEC, death). 160 infants were enrolled in the study, but outcomes are reported on 129 infants — 31 infants were excluded. Outcomes reported on 67 infants in the acetaminophen group and 62 in the ibuprofen group |
| Notes | We contacted the corresponding author on 10 January 2017 to obtain clarifying information at fasabzvari@gmail.com and again on 18 November 2017. We have not received a response, and therefore we have excluded the trial from the update of the review, as we need more clarifying information. It is not stated what are the denominators for the Clinical Characteristics in Table 1 |
| Methods | Randomised blinded placebo controlled trial |
| Participants | Preterm infants born at < 33 weeks' PMA with a haemodynamically significant PDA (diameter > 1.5 mm with clinical symptoms). were treated with a 5‐day course of oral paracetamol or placebo |
| Interventions | Infants were treated with a 5‐day course of oral paracetamol (n = 27) or placebo (n = 28) |
| Outcomes | Primary outcome: ductal closure by 48 h after treatment completion and decrease in size > 25% was a secondary outcome |
| Notes | By early January 2018 the study has only been reported in abstract form, with insufficient information to allow us to include the study in our update of the review. |
BPD = Bronchopulmonary dysplasia
CLD = Chronic lung disease
CRP = C‐reactive protein
ECHO = Echocardiogram
IVH = Intraventricular haemorrhage
LA/AO ratio = Left atrium/aorta ratio
MDI = Mental Developmental Index
MRI = Magnetic resonance imaging
NEC = Necrotizing enterocolitis
NICHD = National Institute of Child Health and Development
NSID = Nonsteroidal anti‐inflammatory drugs
PDA = Patent ductus arteriosus
PDI = Psychomotor Developmental Index
PMA = Post Menstrual Age
PPHN = Persistent Pulmonary Hypertension of the Newborn
PVL = Periventricular leukomalacia
ROP = Retinopathy of Prematurity
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Paracetamol for patent ductus arteriosus treatment: comparison between oral and intravenous administration |
| Methods | Randomised controlled trial. Blinded (masking used). Researchers assessing ductal closure by ECHO will be unaware for study aims and group assignment |
| Participants | Neonates with PMA < 32 weeks and birth weight < 1500 g and with ECHO evidence of PDA and contraindication to or failure of conventional medical therapy with COX inhibitors (ibuprofen or indomethacin) Age 1 to 5 days |
| Interventions | Intravenous paracetamol at a loading dose of 20 mg/kg, followed by 7.5 mg/kg every 6 h for 7 days. If ductal closure evaluated by colour‐Doppler ultrasound occurs before the 7th day of treatment, therapy will be discontinued Paracetamol at 15 mg/kg by oral route, every 6 h for 7 days. If ductal closure evaluated by colour‐Doppler ultrasound occurs before the 7th day of treatment, therapy will be discontinued |
| Outcomes | Ductal closure, assessed by colour‐Doppler ultrasound at 7 days after allocation to the intervention Mortality by 42 weeks' PMA |
| Starting date | April 2013 |
| Contact information | Prof Gianluca Terrin, Department of Gynecology‐Obstetrics and Perinatal Medicine, Sapienza University of Rome, Italy. (gianluca.terrin@uniroma1.it) |
| Notes |
| Trial name or title | Early PARacetamol (EPAR) to promote early closure of the ductus arteriosus in preterm infants |
| Methods | Not clearly stated |
| Participants | Preterm infants < 6 h old. Born at < 29 weeks' PMA with PDA (ductus arteriosus characteristics ‒ patent > 1 mm ‒ < 30% right to left shunt) |
| Interventions | "Early treatment of patent ductus arteriosus with paracetamol and to examine the safety and efficacy profile of paracetamol during the early postnatal period. We hypothesise that early treatment with paracetamol will reduce the number of infants requiring intervention for PDA and that the use of paracetamol in preterm infants with a patent ductus arteriosus will result in a higher rate of ductal closure compared with placebo. We also aim to show that paracetamol can be used safely in preterm infants during the early postnatal period". |
| Outcomes | Closure of PDA (not clearly defined) |
| Starting date | November 2016 |
| Contact information | DR. Timothy Schindler, Department of Newborn Care Royal Hospital for Women Barker St Randwick NSW 2031, Australia (tschindl@med.usyd.edu.au) |
| Notes |
| Trial name or title | Comparison of oral paracetamol versus ibuprofen in premature infants < 1500g with patent ductus arteriosus: A randomised controlled trial |
| Methods | Randomised parallel controlled trial |
| Participants | 1. Birth weight < 1500 g; 2. postnatal age within 14 days; 3. Continuous positive airway pressure with FiO₂ more than 25%; 4. Echocardiographic criteria for PDA included an increased left atrial diameter compared with the aortic root (left atrium to aortic root ratio higher than 1.4), OR visualization of the ductus (more than 1.5 mm), AND evidence of left to right blood flow through the open duct |
| Interventions | Oral paracetamol versus oral ibuprofen |
| Outcomes | Primary: the rate of ductal closure; side effects |
| Starting date | The study was executed between 21 October 2013 and 21 October 2015 |
| Contact information | Wu Hui, Department of Neonatology, The First Hospital of Jilin University, Changchun, China (wuhui97@126.com) |
| Notes | Retrospective registration. To our knowledge the study has not been published. |
| Trial name or title | Comparison of oral paracetamol versus ibuprofen for PDA closure in preterms – a randomised controlled single blinded study |
| Methods | Randomised, parallel group trial |
| Participants | Preterm neonates with 1. Congestive cardiac failure 2. Mechanical ventilation 3. LA/Aortic root ratio > 1.5. 4. Postnatal age 0 to 28 days. |
| Interventions | Paracetamol 15 mg/kg/dose 6‐hourly for 2 days by oral/orogastric route Ibuprofen 10 mg/kg initially followed by 5 mg/kg once daily for 2 days by oral/orogastric route |
| Outcomes | Echocardiographic closure of PDA 24 h after completion of course Secondary outcomes: cardio/respiratory morbidity and mortality by 28 days, growth and neurodevelopment by 1 year of age |
| Starting date | September 2014 |
| Contact information | Dr. B Bharathi, Department of Neonatology, Pondicherry, India |
| Notes | Trial registered retrospectively |
| Trial name or title | Efficacy and safety of oral paracetamol versus oral ibuprofen in management of patent ductus arteriosus in preterm neonates less than or equal to 34 weeks or less than or equal to 1800 g: A randomised control trial |
| Methods | Randomised open label controlled trial |
| Participants | Preterm neonates born at ≤ 34 weeks or birth weight ≤ 1800 g with haemodynamically significant PDA. Postnatal age 0 to 28 days. |
| Interventions | Paracetamol will be given at 15 mg/kg 8‐hourly for 3 days 3 doses ibuprofen will be given at 24 h interval at a dose of 10, 5, 5 mg/kg for neonates younger than 70 h; 14, 7, 7 mg/kg for neonates between 70 and 108 h; and 18, 9, 9 mg/kg for neonates more than 108 h |
| Outcomes | All‐cause mortality, duration of hospitalisation, duration of mechanical ventilation or nasal continuous positive airway pressure (nCPAP) use or duration of need for supplementary oxygen, IVH, PVL, NEC, intestinal perforation, gastrointestinal bleed, oliguria (urine output 1 mL/kg/h), acute renal failure, acute liver injury, hyperbilirubinaemia, sepsis, BPD, ROP, pulmonary haemorrhage, pulmonary hypertension. |
| Starting date | Not indicated |
| Contact information | Vivek Kumar Athwani, Room No. 47, Doctor hostel, SPS Hospitals, Sherpur Chowk, GT Road Ludhiana, Punjab, India (vathwani@gmail.com) |
| Notes |
| Trial name or title | Randomised controlled trial of 2 different doses of intravenous paracetamol for PDA closure in preterm infants less than 30 weeks |
| Methods | Randomised controlled trial (computer‐generated randomisation; sequentially numbered, sealed, opaque envelopes; participant, investigator and outcome assessor blinded) |
| Participants | Preterm infants with a) PMA < 28 weeks or b) between 28 and 30 weeks on invasive mechanical ventilation or on CPAP with FiO₂ requirements more than 35% AND having 2D echocardiographic evidence of haemodynamically significant PDA (duct size more than 1.5 mm narrowest internal diameter, left atrium/aorta ratio more than 1.5, left to right flow across shunt, reversal of flow in distal aorta diagnosed at 18 to 24 h of life. Postnatal age 0 to 3 days |
| Interventions | Higher dose paracetamol group: this group will receive intravenous paracetamol in dosage of 15 mg/kg/dose 4 times a day for 5 days Lower dose paracetamol group: This group will receive intravenous paracetamol in dosage of 10 mg/kg/dose 4 times a day for 3 days |
| Outcomes | Primary outcome: PDA closure rate in low dose and high dose paracetamol group. PDA closure is defined as absence of flow through the ductus Secondary outcomes: all‐cause mortality, BPD, duct reopening rate, duration of hospital stay, IVH, NEC, days on assisted ventilation and oxygen therapy, PVL, rate of adverse effects (increased serum creatinine (more than 1 mg%), oliguria (urine output less than 0.5 mL/kg/h for 6 h), increased transaminases level (more than 2 × ULN), thrombocytopenia (platelet count less than 100,000/mm³), gastrointestinal haemorrhage), requirement of ibuprofen/indomethacin, requirement of multiple courses/higher doses, ROP requiring treatment (injection Avastin or laser) and surgical ligation rate. |
| Starting date | December 2017 |
| Contact information | Dr Vaibhav Jain, Department of Neonatology, Surya Childrens Medicare, Mumbai (Suburban), Maharashtra, India (vaibhavjain100989@gmail.com) |
| Notes | Paracetamol will be given for different lengths of time — 5 days and 3 days. How will staff be blinded to the 2 treatment regimens? |
| Trial name or title | Efficacy and safety of paracetamol in comparison to ibuprofen for patent ductus arteriosus treatment in preterm infants. A randomised, open label, comparator‐controlled, prospective study |
| Methods | Randomised open label controlled trial of paracetamol versus ibuprofen |
| Participants | Preterm newborn infants (< 37 weeks' PMA) |
| Interventions | Paracetamol and ibuprofen |
| Outcomes | Primary end‐point: success rate in closing PDA using paracetamol in comparison to ibuprofen after the first 3 days of treatment. Secondary endpoints: number of re‐openings at 30 days; success rate in closing PDA after the second treatment course of ibuprofen as rescue medication; success rate of closing PDA after the first day and the second day of the first treatment course; incidence of surgical ligation at 30 days; incidence of renal failure, liver failure, gastrointestinal complications (including isolated intestinal perforation) at 30 days; incidence of death at 30 days and at 40 weeks' post conception; incidence of sepsis at 30 days; hospital‐stay duration in Neonatal Intensive Care Unit; occurrence of adverse events at 30 days |
| Starting date | November 2013 |
| Contact information | Name of Sponsor: Aziende Chimiche Riunite Angelini Francesco ACRAF S.p.A, Italy |
| Notes |
| Trial name or title | Paracetamol versus ibuprofen in preterm infants with a haemodynamically significant patent ductus arteriosus: a randomised clinical trial |
| Methods | Randomised double‐blind trial |
| Participants | Preterm infants with PMA < 30 weeks and postnatal age < 2 weeks with significant PDA diagnosed by ultrasound |
| Interventions | Paracetamol (IV) versus ibuprofen (IV) |
| Outcomes | Main objective of the trial: to compare the efficacy of the standard treatment of PDA with ibuprofen versus paracetamol in closing the patent ductus arteriosus, to determine its non‐inferiority to ibuprofen. Secondary objectives of the trial: 1. To compare the safety of both treatments by the rate of early and late complications. 2. Set the pharmacokinetics and pharmacodynamics of paracetamol in the neonatal period in infants with persistent ductus. 3. Study of biomarkers and polymorphisms in urine |
| Starting date | 2015 |
| Contact information | Not provided |
| Notes |
| Trial name or title | Safety and efficacy of venous paracetamol with venous ibuprofen in treatment of patent ductus arteriosus (PDA) among premature neonates hospitalised in NICU, Zanjan Ayatollah Musavi Hospital in 2016 to 2017 |
| Methods | Randomised non‐blinded trial |
| Participants | Neonate ≤ 34 weeks with PDA diagnosis by echocardiography |
| Interventions | 15 infants received paracetamol 15 mg/kg every 6 h for 3 days 15 infants received ibuprofen with a dose of 10 mg/kg in the first day and 5 mg/kg in the following second and third days |
| Outcomes | PDA size before and after treatment measured with echocardiography by paediatric cardiologist |
| Starting date | March 2017 |
| Contact information | Dr. Abolfazl Ebadi, Musavi hospital , Gavazang, Zanjan, Iran |
| Notes | Recruitment complete |
| Trial name or title | Oral paracetamol versus oral ibuprofen for closure of haemodynamically significant patent ductus arteriosus in preterm neonates (< 32 weeks): a blinded, randomised, active‐controlled, non‐inferiority trial |
| Methods | Multi‐site, randomised, active‐controlled, blinded, non‐inferiority trial |
| Participants | Preterm neonates of < 32 weeks’ PMA with presence of a haemodynamically significant PDA |
| Interventions | Paracetamol oral suspension administered through an orogastric tube in a dose of 15 mg/kg/dose at 6‐hourly intervals for 3 consecutive days Ibuprofen oral suspension (Ibugesic, Cipla India) would be administered through orogastric tubes in a dose of 10 mg/kg/dose followed by 5 mg/kg/dose after 24 and 48 h from the first dose. |
| Outcomes | Primary outcome: closure of PDA by the end of the last dose of the study drug or earlier, irrespective of the course of the drug Secondary outcomes: closure of PDA following a single course of study drug, closure of PDA following surgical ligation, death (due to any cause) before discharge from the hospital, reopening of PDA following initial closure, ECHO‐proven pulmonary artery hypertension, azotaemia, oliguria, hepatitis with deranged liver transaminases, deranged coagulogram, IVH (any grade of severity), severe IVH (grade 3 and intraparenchymal extension), PVL, NEC (all stages), NEC (definite and advanced stage as per modified Bell’s staging), feed intolerance, BPD and ROP |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | Paracetamol and patent ductus arteriosus (PDA) |
| Methods | Randomised controlled trial |
| Participants | Preterm infants with a haemodynamically significant PDA |
| Interventions | Group 1: paracetamol orally at a dose of 15 mg/kg every 6 h × 3 days. Group 2: indomethacin intravenously 0.2 mg/kg/dose for 3 doses |
| Outcomes | Primary outcome: closure of the ductus within 3 days. Secondary outcomes: absence of peripheral vasoconstriction, Doppler flow velocity in the anterior cerebral artery, superior mesenteric artery and renal artery before and after pharmacological treatment, absence of hepatotoxicity |
| Starting date | 6 February 2011 |
| Contact information | Cathy Hammerman, Shaare Zedek Medical centre, Israel. cathy@cc.huji.ac.il |
| Notes | ClinicalTrials.gov identifier: NCT01291654 |
| Trial name or title | The preterm infants' paracetamol study (PreParaS) |
| Methods | Randomised controlled, double‐blind trial |
| Participants | Preterm infants < 32 weeks' PMA |
| Interventions | Paracetamol infusion solution 10 mg/mL (Perfalgan®) or placebo, 0.45% saline solution. The loading dose is 20 mg/kg, and the maintenance dose 7.5 mg/kg every 6 h for 4 days |
| Outcomes | Primary outcome: ductus diameter mm/kg at postnatal age 5 days. Cumulative dose of morphine at postnatal age 5 days. Secondary outcomes: number of patients who received any treatment for PDA prescribed by an attending clinician, postnatal age at closure of PDA, left atrium to aorta ratio, number of apneic periods/day, cumulative NIAPAS screening score/day up to 5 days' postnatal age, duration of mechanical ventilation, long‐term morbidity diagnoses, deaths, paracetamol side effects, paracetamol serum concentrations (up to 5 days' postnatal age) |
| Starting date | 22 August 2013 |
| Contact information | Outi Aikio, University of Oulu, Finland; outi.aikio@ppshp.fi |
| Notes | ClinicalTrials.gov identifier: NCT01938261 |
| Trial name or title | Adding paracetamol to ibuprofen for treatment of patent ductus arteriosus in preterm infants |
| Methods | Randomised double‐blind controlled trial |
| Participants | Preterm infants born at 24 to 37 weeks' PMA, diagnosis of haemodynamically significant PDA, medical staff decided to treat with ibuprofen |
| Interventions | Group 1: ibuprofen + paracetamol (Ibuprofen 10 mg/kg once, then 5 mg/kg twice, every 24 h for a total of 3 doses and intravenous paracetamol loading dose 20 mg/kg then 10 mg/kg every 6 h for a total of 12 doses) Group 2: ibuprofen + placebo (ibuprofen 10 mg/kg once, then 5 mg/kg twice, every 24 h for a total of 3 doses and placebo (NaCl 0.9%), intravenous, at equal volume to the paracetamol in the paracetamol arm, total of 12 doses given every 6 h) |
| Outcomes | Primary outcome: the incidence of patent ductus arteriosus closure 3 to 21 days after first dose of ibuprofen by echocardiography. The need for surgical ligation of PDA. Secondary outcomes: adverse effects until discharge home ‒ renal and liver function, gastrointestinal complications |
| Starting date | February 2014 |
| Contact information | o_hochwald@rambam.health.gov.il |
| Notes | ClinicalTrials.gov identifier: NCT02002741 |
| Trial name or title | Paracetamol versus ibuprofen for PDA closure in preterm infants. (PARIDA) |
| Methods | A prospective, randomised, controlled, double‐blind, multicenter clinical trial. |
| Participants | Preterm neonates ≤ 31 + 6 days weeks' PMA with HsPDA |
| Interventions | Group A: experimental boluses of paracetamol at 15 mg/kg 4 times a day for 3 consecutive days. Group B: standard boluses of ibuprofen at 10 ‐ 5 ‐ 5 ‐ mg/kg/dose once a day for 3 consecutive days. |
| Outcomes | The rate of ductal closure after the first and second course of pharmacological treatment. (PDA diagnosed by ECHO criteria) in paracetamol versus ibuprofen group. Oliguria, IVH, NEC |
| Starting date | 9 January 2017 |
| Contact information | Contact: Paola Lago, MD; Sabrina Salvadori, MD |
| Notes |
| Trial name or title | Efficacy and safety of paracetamol in comparison to ibuprofen for Patent Ductus Arteriosus treatment in preterm infants: A randomised, open label, comparator‐controlled, prospective study. |
| Methods | Randomised open label controlled trial with parallel assignment |
| Participants |
|
| Interventions | Paracetamol IV solution 15 mg/kg (corresponding to 1.5 mL/kg) per dose every 6 h for 3 days, for a total amount of 12 doses Active Comparator: ibuprofen IV solution at an initial dose of 10 mg/kg, followed by 5 mg/kg at 24 and 5 mg/kg at 48 h intervention |
| Outcomes | Success rate in closing PDA using paracetamol in comparison to ibuprofen. Incidence of surgical ligation, death, sepsis, renal failure, liver failure, gastrointestinal complications, length of stay in the NICU |
| Starting date | December 2015 |
| Contact information | Paola Lipone (p.lipone@angelini.it) and Alessandra Del Vecchio (a.delvecchio@angelini.it) |
| Notes |
| Trial name or title | Time to re‐evaluate the kinder gentler approach to patent ductus arteriosus (PDA) in the preterm neonate |
| Methods | Randomised quadruple‐masked trial (participant, care provider, investigator, outcomes assessor) |
| Participants | Preterm neonates < 30 weeks' PMA with a PDA of borderline significance |
| Interventions | Paracetamol drops 15 mg/kg/dose × 4/day diluted 1:15 yielding dose of 2.25 mL/kg/dose to be given for 3 days |
| Outcomes | Primary outcomes: to demonstrate a decrease in the composite outcome of death or severe morbidity chronic lung disease (CLD), as shown by decreased time on supplemental oxygen and assisted ventilation. Secondary outcomes: to demonstrate a decrease in subsequently diagnosed haemodynamically significant PDA, including decrease in the need for subsequent therapy for PDA closure, decrease in surgical PDA ligations; to demonstrate a decrease in necrotizing enterocolitis (NEC) and/or ROP with treatment and to demonstrate no adverse effect on blood flow in anterior cerebral, superior mesenteric and renal arteries. |
| Starting date | June 2016 |
| Contact information | Cathy Hammerman, Shaare Zedek Medical Center, Jerusalem, Israel |
| Notes |
| Trial name or title | The efficacy of IV acetaminophen on patent ductus arteriosus closure in preterm infants |
| Methods | Randomised open label controlled trial |
| Participants | Preterm infants with 23 to 30 weeks' PMA and a PDA requiring treatment |
| Interventions | IV acetaminophen versus IV ibuprofen |
| Outcomes | Rate of PDA closure (time frame: 3 days) |
| Starting date | January 2017 |
| Contact information | Kate Tauber MD, Albany Medical College, USA (tauberk@mail.amc.edu) |
| Notes |
| Trial name or title | Combination of acetaminophen and ibuprofen in the management of patent ductus arteriosus in premature infants: A pilot study |
| Methods | Randomised open label controlled trial |
| Participants |
|
| Interventions |
|
| Outcomes | Ductal closing rate (time frame: within 24 to 48 h after completion of treatment). To determine the ductal closure rate on echocardiography after completion of a first treatment course. |
| Starting date | 12 June 2017 |
| Contact information | Dr. Sanket D Shah (sanket.shah@jax.ufl.edu) |
| Notes |
| Trial name or title | Comparison between the effect of oral paracetamol versus oral ibuprofen in the treatment of patent ductus arteriosus in preterm and low birth weight infants |
| Methods | Randomised open label trial with parallel assignment |
| Participants | PMA ≤ 35 weeks, age 2 to 7 days with colour Doppler echocardiographic evidence of PDA, urine output more than 1 mL/kg/h and creatinine concentration level less than 1.8 mg/dl |
| Interventions | Ibuprofen administered with a loading dose of 10 mg/kg/day followed by 5 mg/kg/day in 2 doses with 24 h apart for 3 days Paracetamol drug administered for 3 consecutive days in a dose of 15 mg/kg/dose every 6 h |
| Outcomes | Echo‐confirmed closure of PDA (time frame: 6 days) |
| Starting date | June 2015 |
| Contact information | Rania Ali El‐Farrash, Ain Shams University, Cairo, Egypt |
| Notes |
Contributions of authors
Both authors (AO, PS) contributed to all sections of this review.
Sources of support
Internal sources
Department of Pediatrics, Mount Sinai Hospital, Toronto, Ontario, Canada, Other.
External sources
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health.
Declarations of interest
Arne Ohlsson ‒ no conflict of interest to declare.
Prakeshkumar Shah ‒ no conflict of interest to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Al‐Lawama M, Alammori I, Abdelghani T, Badran E. Oral paracetamol versus oral ibuprofen for treatment of patent ductus arteriosus. Journal of International Medical Research 2017;46(2):811‐8. [DOI: 10.1177/0300060517722698; PUBMED: 29239259] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbagh PA, Zarkesh MR, Nili F, Sadat Nayeri FS, Naeem AT. Prophylactic treatment with oral paracetamol for patent ductus arteriosus in preterm infants: a randomized clinical trial. Tehran University Medical Journal 2015;73(2):86‐92. [http://tumj.tums.ac.ir/article‐1‐6603‐en.html] [Google Scholar]
- Dang D, Wang D, Zhang C, Zhou W, Zhou Q, Wu H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLos ONE 2013;8(11):e77888. [DOI: 10.1371/journal.pone.0077888; PUBMED: 24223740] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash SK, Kabra NS, Avasthi BS, Sharma SR, Padhi P, Ahmed J. Enteral paracetamol or intravenous indomethacin for closure of patent ductus arteriosus in preterm neonates: a randomized controlled trial. Indian Pediatrics 2015;52(7):573‐8. [PUBMED: 26244949] [DOI] [PubMed] [Google Scholar]; Kabra NS, Dash SK. Comparison of enteral paracetamol and intravenous indomethacin in closure of patent ductus arteriosus (PDA) in preterm newborns: A randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2014 07 17‐18; Vienna, Austria. 2014. [Google Scholar]
- El‐Mashad AE, El‐Mahdy H, Amrousy DE, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. European Journal of Pediatrics 2017;176(2):233‐40. [DOI: 10.1007/s00431-016-2830-7; PUBMED: 28004188] [DOI] [PubMed] [Google Scholar]
- Härkin P, Härmä A, Aikio O, Valkama M, Leskinen M, Saarela T, et al. Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. Journal of Pediatrics 2016;177:72‐7. [DOI: 10.1016/j.jpeds.2016.04.066; PUBMED: 27215779] [DOI] [PubMed] [Google Scholar]
- Oncel MY, Eras Z, Uras N, Canpolat FC, Erdeve O, Oguz SS. Neurodevelopmental outcomes of preterm infants treated with oral paracetamol versus ibuprofen for patent ductus arteriosus. American Journal of Perinatology 2017;34(12):1185–9. [DOI] [PubMed] [Google Scholar]; Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: A randomized controlled trial. Journal of Pediatrics 2014;164(3):510‐4.e1. [PUBMED: 24359938] [DOI] [PubMed] [Google Scholar]
- Yang B, Gao X, Ren Y, Wang Y, Zhang Q. Oral paracetamol vs. oral ibuprofen in the treatment of symptomatic patent ductus arteriosus in premature infants: A randomized controlled trial. Experimental and Therapeutic Medicine 2016;12(4):2531‐6. [PUBMED: 27698754] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Babaei H, Nemati R, Daryoshi H. Closure of patent ductus arteriosus with oral acetaminophen in preterm neonates: A randomized trial. Biomedical Research and Therapy 2018;5(2):2034‐44. [Google Scholar]
- Bagheri MM, Niknafs P, Sabsevari F, Torabi MH, Bijari BB, Noroozi E, et al. Comparison of oral acetaminophen versus ibuprofen in premature infants with patent ductus arteriosus. Iranian Journal of Pediatrics 2016;26(4):e3975. [DOI: 10.5812/ijp.3975; PUBMED: 27713809] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluckow MR, Carlisle H, Broom M, Woods P, Jeffery M, Desai D, et al. A randomised blinded placebo controlled trial of paracetamol to treat later PDA. Journal of Paediatrics and Child Health 2016;52(Suppl 2):100. [Google Scholar]
References to ongoing studies
- ACTRN12613000289718. Paracetamol for patent ductus arteriosus treatment: comparison between oral and intravenous administration. Australian New Zealand Clinical Trials Registry (first received 8 March 2013).
- ACTRN12616001517460. Early paracetamol (EPAR) to promote early closure of the ductus arteriosus in preterm infants. Australian New Zealand Clinical Trials Registry (first received 1 November 2016).
- ChiCTR‐TRC‐13003912. Comparison of oral paracetamol versus ibuprofen in premature infants<1500g with patent ductus arteriosus: A randomized controlled trial. Chinese Clinical Trial Register (ChiCTR) (first received 7 December 2013). [DOI] [PMC free article] [PubMed]
- CTRI/2016/09/007261. Oral ibuprofen versus paracetamol on ductus arteriosus [Comparison of oral paracetamol versus ibuprofen for PDA closure in preterms ‐ a randomized controlled single blinded study ‐ IPOD]. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2016/09/007261 (first received 8 September 2016).
- CTRI/2017/10/009989. Paracetamol versus ibuprofen for closure of patent ductus arteriosus [Efficacy and safety of oral paracetamol versus oral ibuprofen in management of patent ductus arteriosus in preterm neonates less than or equal to 34 Weeks or less than or equal to 1800 gms: a randomized control trial ‐ BAP trial]. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/10/009989 (first received 10 October 2017).
- CTRI/2017/10/010012. A clinical trial comparing low dose versus standard dose of intravenous paracetamol for PDA closure in very premature babies [Randomized controlled trial of two different doses of intravenous paracetamol for PDA closure in preterm infants less than 30 weeks]. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/10/010012 (first received 05 October 2017).
- EUCTR2013‐003883‐30‐IT. Efficacy and safety of paracetamol in the treatment of patent ductus arteriosus in preterm infants [Efficacy and safety of paracetamol in comparison to ibuprofen for patent ductus arteriosus treatment in preterm infants. A randomized, open label, comparator‐controlled, prospective study. ‐ Paracetamol in Patent Ductus Arteriosus]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐003883‐30‐IT (first received 12 November 2013).
- EUCTR2015‐003177‐14‐ES. Paracetamol versus ibuprofen in preterm infants with a hemodynamically significant patent ductus arteriosus: a randomized clinical trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐003177‐14‐ES (first received 20 April 2016).
- IRCT2016081729404N1. Safety and efficacy of venous paracetamol with venous ibubrofen in treatment of patent ductus arteriosus (PDA) among premature neonates hospitalized in NICU, Zanjan Ayatollah Musavi Hospital in 2016‐ 2017. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016081729404N1 (first received 9 May 2017).
- Kumar A, Sundaram V, Yadav R, Oleti TP, Murki S, Krishna A. Oral paracetamol versus oral ibuprofen for closure of haemodynamically significant patent ductus arteriosus in preterm neonates (<32 weeks): a blinded, randomised, active‐controlled, non‐inferiority trial. BMJ Paediatrics Open 2017;1(1):e000143. [DOI: 10.1136/bmjpo-2017-000143; CTRI/2014/08/004805] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01291654. Paracetamol and patent ductus arteriosus (PDA) [Paracetamol in the treatment of patent ductus arteriosus in the premature neonate]. Clinicaltrials.gov/show/NCT01291654 (first received 6 February 2011).
- NCT01938261. The preterm infants' paracetamol study (PreParaS). Clinicaltrials.gov/show/NCT01938261 (first received 10 September 2013).
- NCT02002741. Adding paracetamol to ibuprofen for treatment of patent ductus arteriosus in preterm infants [Adding paracetamol to ibuprofen for treatment of patent ductus arteriosus in preterm infants: a pilot, double blind, randomized, placebo‐control trial]. ClinicalTrials.gov/show/NCT02002741 (first received 6 December 2013). [DOI] [PubMed]
- NCT02056223. Paracetamol vs ibuprofen for PDA closure in preterm infants. (PARIDA) [Paracetamol versus ibuprofen for patent ductus arteriosus closure in preterm infants. A prospective, randomized, controlled, double blind, multicenter clinical trial]. clinicaltrials.gov/show/NCT02056223 (first received 10 August 2005).
- NCT02422966. Paracetamol in Patent Ductus Arteriosus [Efficacy and safety of paracetamol in comparison to ibuprofen for Patent Ductus Arteriosus treatment in preterm infants: A randomized, open label, comparator‐controlled, prospective study]. clinicaltrials.gov/show/NCT02422966 (first received 22 April 2015).
- NCT02819414. Paracetamol treatment of the borderline significant PDA [Time to re‐evaluate the kinder gentler approach to patent ductus arteriosus (PDA) in the preterm neonate]. clinicaltrials.gov/show/NCT02819414 (first received 30 June 2016).
- NCT03008876. IV acetaminophen and patent ductus arteriosus [The efficacy of IV acetaminophen on patent ductus arteriosus closure in preterm infants]. clinicaltrials.gov/show/NCT03008876 (first received 4 January 2017).
- NCT03103022. Combination of acetaminophen and ibuprofen in the management of patent ductus arteriosus in premature infants: a pilot study. clinicaltrials.gov/show/NCT03103022 (first received 06 April 2017).
- NCT03265782. Paracetamol versus ibuprofen for PDA closure [Comparison between the effect of oral paracetamol versus oral ibuprofen in the treatment of patent ductus arteriosus in preterm and low birth weight infants]. clinicaltrials.gov/show/NCT03265782 (first received 29 August 2017).
Additional references
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- Arana A, Morton NS, Hansen TG. Treatment with paracetamol in infants. Acta Anaesthesiologica Scandinavica 2001;45(1):20‐9. [PUBMED: 11152028] [DOI] [PubMed] [Google Scholar]
- Avella‐Garcia CB, Julvez J, Fortuny J, Rebordosa C, García‐Esteban R, Galán IR, et al. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. International Journal of Epidemiology 2016;45(6):1987‐96. [DOI: 10.1093/ije/dyw115; PUBMED: 27353198] [DOI] [PubMed] [Google Scholar]
- Bauer AZ, Kriebel D. Prenatal and perinatal analgesic exposure and autism: an ecological link. Environmental Health 2013;12(41):1‐13. [DOI: 10.1186/1476-069X-12-41; PUBMED: 23656698] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AZ, Kriebel D, Herbert MR, Bornehag CG, Swan SH. Prenatal paracetamol exposure and child neurodevelopment: A review. Hormones and Behavior 2018;S0018‐506X(17):30454‐3. [DOI: 10.1016/j.yhbeh.2018.01.003; PUBMED: 29341895] [DOI] [PubMed] [Google Scholar]
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Reichenberg A, Hallerback MU, Wikstrom S, Koch HM, Jonsson BA, et al. Prenatal exposure to acetaminophen and children's language development at 30 months. European Psychiatry 2018;S0924‐9338(17):32989‐9. [DOI: 10.1016/j.eurpsy.2017.10.007; PUBMED: 29331486] [DOI] [PubMed] [Google Scholar]
- Botting RM. Mechanism of action of acetaminophen: is there a cyclooxgygenase 3?. Clinical Infectious Diseases 2000;31(Suppl 5):S202‐10. [DOI: 10.1086/317520; PUBMED: 11113024 ] [DOI] [PubMed] [Google Scholar]
- Clyman R. Ibuprofen and patent ductus arteriosus. New England Journal of Medicine 2000;343(10):728‐30. [DOI: 10.1056/NEJM200009073431009; PUBMED: 10974138] [DOI] [PubMed] [Google Scholar]
- Das RR, Arora K, Naik SS. Efficacy and safety of paracetamol versus ibuprofen for treating patent ductus arteriosus in preterm infants: A meta‑analysis. Journal of Clinical Neonatology 2014;3(4):183‐90. [Google Scholar]
- Edwards AD, Wyatt JS, Richardson C, Potter A, Cope M, Delpy DT, et al. Effects of indomethacin on cerebral haemodynamics in very preterm infants. Lancet 1990;335(8704):1491‐5. [PUBMED: 1972434] [DOI] [PubMed] [Google Scholar]
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353‐60. [DOI: 10.1542/peds.2005-0249; PUBMED: 16322158] [DOI] [PubMed] [Google Scholar]
- El‐Khuffash A, Jain A, Corcoran D, Shah PS, Hooper CW, Brown N, et al. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatric Research 2014;76(3):238‐44. [DOI: 10.1038/pr.2014.82; PUBMED: 24941212] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000174.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 21 March 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
- Grèen K, Drvota V, Vesterqvist O. Pronounced reduction of in vivo prostacyclin synthesis in humans by acetaminophen (paracetamol). Prostaglandins 1989;37(3):311‐5. [PUBMED: 2664901] [DOI] [PubMed] [Google Scholar]
- Hammerman C. Patent ductus arteriosus. Clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment. Clinics in Perinatology 1995;22(2):457‐79. [PUBMED: 7671547] [PubMed] [Google Scholar]
- Hammerman C, Bin‐Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics 2011;128(6):e1618‐21. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
- Huang X, Wang F, Wang K. Paracetamol versus ibuprofen for the treatment of patent ductus arteriosus in preterm neonates: a meta‐analysis of randomized controlled trials. Journal of Maternal‐fetal & Neonatal Medicine 2017 Jul 18 [Epub ahead of print]. [DOI: 10.1080/14767058.2017.1338263; PUBMED: 28720053] [DOI] [PubMed]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [DOI: 10.1001/archopht.123.7.991; PUBMED: 16009843] [DOI] [PubMed] [Google Scholar]
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [DOI: 10.1164/ajrccm.163.7.2011060; PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
- Kessel I, Waisman D, Lavie‐Nevo K, Golzman M, Lorber A, Rotschild A. Paracetamol effectiveness, safety and blood level monitoring during patent ductus arteriosus closure: a case series. Journal of Maternal‐fetal & Neonatal Medicine 2014;27(16):1719‐21. [DOI: 10.3109/14767058.2013.871630; PUBMED: 24460433] [DOI] [PubMed] [Google Scholar]
- Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU network 1996‐1997. Pediatrics 2000;106(5):1070‐9. [PUBMED: 11061777] [DOI] [PubMed] [Google Scholar]
- Maisels MJ, Watchko JF. Treatment of jaundice in low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(6):F459‐63. [PUBMED: 14602690] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. Development of the pulmonary circulation: metabolic aspects. In: Polin RA, Fox WW editor(s). Fetal and Neonatal Physiology. Vol. 1, Philadelphia: W.B. Saunders, 1998:924‐9. [Google Scholar]
- Mitra S, Florez ID, Tamayo ME, Aune D, Mbuagbaw L, Veroniki AA, et al. Effectiveness and safety of treatments used for the management of patent ductus arteriosus (PDA) in preterm infants: a protocol for a systematic review and network meta‐analysis. BMJ Open 2016;6(7):e011271. [DOI: 10.1136/bmjopen-2016-011271; PUBMED: 27456327] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadir E, Kassem E, Foldi S, Hochberg A, Feldman M. Paracetamol treatment of patent ductus arteriosus in preterm infants. Journal of Perinatology 2014;34(10):748‐9. [DOI: 10.1038/jp.2014.96; PUBMED: 24854626] [DOI] [PubMed] [Google Scholar]
- Naulty CM, Horn S, Conry J, Avery GB. Improved lung compliance after ligation of patent ductus arteriosus in hyaline membrane disease. Journal of Pediatrics 1978;93(4):682‐4. [PUBMED: 702251] [DOI] [PubMed] [Google Scholar]
- U.S. National Library of Medicine. Drug Information Portal. druginfo.nlm.nih.gov/drugportal (accessed 21 March 2018).
- Ohlsson A, Bottu J, Govan J, Ryan ML, Fong K, Myhr T. Effect of indomethacin on cerebral blood flow velocities in very low birth weight neonates with patent ductus arteriosus. Developmental Pharmacology and Therapeutics 1993;20(1‐2):100‐6. [PUBMED: 7924757] [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD004213.pub3] [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003481.pub6; PUBMED: 23633310] [DOI] [PubMed] [Google Scholar]
- Palmer GM, Atkins M, Anderson BJ, Smith KR, Culnane TJ, McNally CM, et al. I.V. acetaminophen pharmacokinetics in neonates after multiple doses. British Journal of Anaesthesia 2008;101(4):523‐30. [DOI: 10.1093/bja/aen208; PUBMED: 18628265] [DOI] [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
- Peterson RG. Consequences associated with nonnarcotic analgesics in the fetus and newborn. Federation Proceedings 1985;44(7):2309‐13. [PUBMED: 3884385] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Sakhalkar VS, Merchant RH. Therapy of symptomatic patent ductus arteriosus in preterms with mefenemic acid and indomethacin. Indian Pediatrics 1992;29(3):313‐8. [PUBMED: 1612672] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
- Seyberth HW, Rascher W, Hackenthal R, Wille L. Effect of prolonged indomethacin therapy on renal function and selected vasoactive hormones in very‐low‐birth‐weight infants with symptomatic patent ductus arteriosus. Journal of Pediatrics 1993;103(6):979‐84. [PUBMED: 6358443] [DOI] [PubMed] [Google Scholar]
- Simbi KA, Secchieri S, Rinaldo M, Demi M, Zanardo V. In utero ductal closure following near‐term maternal self‐medication with nimesulide and acetaminophen. Journal of Obstetrics and Gynaecology 2002;22(4):440‐1. [DOI: 10.1080/01443610220141489; PUBMED: 12521476] [DOI] [PubMed] [Google Scholar]
- Sinah R, Negi V, Dalal SS. An interesting observation of PDA closure with oral paracetamol in preterm neonates. Journal of Clinical Neonatology 2013;2(1):30‐2. [DOI: 10.4103/2249-4847.109245; PUBMED: 24027742] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram SS, Alonso EM, Narkewicz MR, Zhang S, Squires RH, Pediatric Acute Liver Failure Study Group. Characterization and outcomes of young infants with acute liver failure. Journal of Pediatrics 2011;159(5):813‐8.e1. [DOI: 10.1016/j.jpeds.2011.04.016; PUBMED: 21621221] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrin G, Conte F, Scipione A, Bacchio E, Conti MG, Ferro R. Efficacy of paracetamol for the treatment of patent ductus arteriosus in preterm neonates. Italian Journal of Pediatrics 2014;40(1):21. [DOI: 10.1186/1824-7288-40-21; PUBMED: 24555510] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrin G, Conte F, Oncel MY, Scipione A, McNamara PJ, Simons S, et al. Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: a systematic review and meta‐analysis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(2):F127‐36. [DOI: 10.1136/archdischild-2014-307312; PUBMED: 26283668] [DOI] [PubMed] [Google Scholar]
- Loon RL, Roofthooft MT, Hillege HL, Harkel AD, Osch‐Gevers M, Delhaas T, et al. Pediatric pulmonary hypertension in the Netherlands, Epidemiology and characterization during the period 1991 to 2005. Circulation 2011;124(16):1755‐64. [DOI: 10.1161/CIRCULATIONAHA.110.969584; PUBMED: 21947294] [DOI] [PubMed] [Google Scholar]
- Viberg H, Eriksson P, Gordh T, Fredriksson A. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicological Sciences 2014;138(1):139‐47. [DOI: 10.1093/toxsci/kft329; PUBMED: 24361869] [DOI] [PubMed] [Google Scholar]
- Walls L, Baker CF, Sarkar S. Acetaminophen‐induced hepatic failure with encephalopathy in a newborn. Journal of Perinatology 2007;27(2):133‐5. [DOI: 10.1038/sj.jp.7211641; PUBMED: 17262050] [DOI] [PubMed] [Google Scholar]
- Weir FJ, Ohlsson A, Myhr TL, Fong K, Ryan ML. A patent ductus arteriosus is associated with reduced middle cerebral artery blood flow velocity. European Journal of Pediatrics 1999;158(6):484‐7. [PUBMED: 10378397] [DOI] [PubMed] [Google Scholar]
- Wolf WM, Snover DC, Leonard AS. Localized intestinal perforation following intravenous indomethacin in premature infants. Journal of Pediatric Surgery 1989;24(4):409‐10. [PUBMED: 2732888] [DOI] [PubMed] [Google Scholar]
- Ystrom E, Gustavson K, Brandlistuen RE, Knudsen GP, Magnus P, Susser E. Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics 2017;140(5):e20163840. [DOI: 10.1542/peds.2016-3840; PUBMED: 29084830] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurttutan S, Oncel MY, Arayici S, Uras N, Altug N, Erdeve O, et al. A different first‐choice drug in the medical management of patent ductus arteriosus: oral paracetamol. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(8):825‐7. [DOI: 10.3109/14767058.2012.755162; PUBMED: 23205872] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Ohlsson A, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low‐birth‐weight infants. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010061.pub2] [DOI] [PubMed] [Google Scholar]


