Abstract
Background
Dental caries remains a major public health problem in most industrialised countries, affecting 60% to 90% of schoolchildren and the vast majority of adults. Milk may provide a relatively cost‐effective vehicle for fluoride delivery in the prevention of dental caries. This is an update of a Cochrane Review first published in 2005.
Objectives
To assess the effects of milk fluoridation for preventing dental caries at a community level.
Search methods
We searched the Cochrane Oral Health Group Trials Register (inception to November 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2014, Issue 10), MEDLINE via OVID (1946 to November 2014) and EMBASE via OVID (1980 to November 2014). We also searched the U.S. National Institutes of Health Trials Register (https://clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch) for ongoing trials. We did not place any restrictions on the language or date of publication when searching the electronic databases.
Selection criteria
Randomised controlled trials (RCTs), with an intervention and follow‐up period of at least two years, comparing fluoridated milk with non‐fluoridated milk.
Data collection and analysis
Two authors independently assessed trial risk of bias and extracted data. We used standard methodological procedures expected by Cochrane.
Main results
We included one unpublished RCT, randomising 180 children aged three years at study commencement. The setting was nursery schools in an area with high prevalence of dental caries and a low level of fluoride in drinking water. Data from 166 participants were available for analysis. The study carried a high risk of bias. After three years, there was a reduction of caries in permanent teeth (mean difference (MD) −0.13, 95% confidence interval (CI) −0.24 to −0.02) and in primary teeth (MD −1.14, 95% CI −1.86 to −0.42), as measured by the decayed, missing and filled teeth index (DMFT for permanent teeth and dmft for primary teeth). For primary teeth, this is a substantial reduction, equivalent to a prevented fraction of 31%. For permanent teeth, the disease level was very low in the study, resulting in a small absolute effect size. The included study did not report any other outcomes of interest for this review (adverse events, dental pain, antibiotic use or requirement for general anaesthesia due to dental procedures).
Authors' conclusions
There is low‐quality evidence to suggest fluoridated milk may be beneficial to schoolchildren, contributing to a substantial reduction in dental caries in primary teeth. Due to the low quality of the evidence, further research is likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. There was only one relatively small study, which had important methodological limitations on the data for the effectiveness in reducing caries. Furthermore, there was no information about the potential harms of the intervention. Additional RCTs of high quality are needed before we can draw definitive conclusions about the benefits of milk fluoridation.
Keywords: Animals; Child; Child, Preschool; Humans; Fluoridation; Milk; Cariostatic Agents; Cariostatic Agents/therapeutic use; Dental Caries; Dental Caries/prevention & control; Fluorides; Fluorides/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Fluoridated milk for preventing tooth decay
Review question
We compared the evidence on the effects of fluoridated milk versus non‐fluoridated milk for the prevention of tooth decay.
Background
Tooth decay remains a major public health problem in most industrialised countries, affecting 60% to 90% of schoolchildren and the vast majority of adults. It is the primary cause of oral pain and tooth loss. The prevalence of tooth decay varies both between and within different countries, but generally, people in lower socioeconomic groups (measured by income, education and employment) are more affected.
Fluoride is a mineral that prevents tooth decay and can be added to drinking water, salt or milk as a public health measure to promote oral health. Fluoridated milk is often available to children alongside non‐fluoridated milk through school milk schemes or national nutritional programmes. The use of such distribution systems can provide a convenient and cost‐efficient means of targeted fluoride supplementation for children whose parents wish to participate in the programme.
Study characteristics
Authors from Cochrane Oral Health reviewed existing studies to find all available evidence up to November 2014. We searched scientific databases for clinical trials testing the effects of fluoridated milk compared with non‐fluoridated milk. Treatment had to be used and monitored for a minimum of two years.
Key results
We found one unpublished study that included 180 three‐year olds who were given either fluoridated or non‐fluoridated milk at nursery schools in an area with high prevalence of dental cavities and a low level of fluoride in drinking water. After three years, 92% of the children were available for analysis. The evidence suggests fluoridated milk may be beneficial to schoolchildren, substantially reducing the formation of cavities in baby teeth. There was no information available about any possible adverse events.
Quality of the evidence
The evidence was considered to be low quality due to the lack of relevant studies, the risk of bias in the identified study and concerns over the applicability of the results to different settings and populations. Additional studies of high quality are needed before we can draw definitive conclusions about the benefits of milk fluoridation.
Summary of findings
Summary of findings for the main comparison. Fluoridated milk compared to non‐fluoridated milk for preventing dental caries.
| Fluoridated milk compared to non‐fluoridated milk for preventing dental caries | |||||
| Patient or population: general population Settings: community Intervention: fluoridated milk Comparison: non‐fluoridated milk | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Non‐fluoridated milk | Fluoridated milk | ||||
| Caries in permanent teeth: DMFT (3 years) | The mean caries in permanent teeth: DMFT (3 years) in the control group was 0.17 | The mean caries in permanent teeth: DMFT (3 years) in the intervention group was 0.13 lower (0.24 lower to 0.02 lower) | 166 (1 study) | ⊕⊕⊝⊝ lowa,b | Disease level very low; small absolute effect size |
| Caries in primary teeth: dmft (3 years) | The mean caries in primary teeth: dmft (3 years) in the control group was 3.64 | The mean caries in primary teeth: dmft (3 years) in the intervention group was 1.14 lower (1.86 lower to 0.42 lower) | 166 (1 study) | ⊕⊕⊝⊝ lowa,b | Substantial effect size equivalent to a 31% prevented fractionc |
| Adverse effects: dental fluorosis | No evidence found | ||||
| Dental pain due to decay | No evidence found | ||||
| Antibiotics due to dental infections | No evidence found | ||||
| Requirement for general anaesthesia due to dental procedures for caries | No evidence found | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; dmft: decayed, missing and filled primary teeth; DMFT: decay, missing and filled permanent teeth. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded for risk of bias: sequence generation method unclear, and participants were not blinded.
bDowngraded for indirectness: applicability of evidence to different settings and populations unclear; there was not much baseline information about the population in the study.
cPrevented fraction (PF), expressed as percentages = (mean increment in control group − mean increment in intervention group)/mean increment in control group) x 100%. PF values between 1% to 10% are considered to be a small effect; between 10% to 20%, a moderate effect; and above 20%, a large or substantial effect.
Background
Description of the condition
Dental caries is a disease of the hard tissues of the teeth. Over time, it forms through a complex interaction between acid‐producing bacteria, fermentable carbohydrates, and numerous host factors related to teeth and saliva. It is the primary cause of oral pain and tooth loss. In its early stages, it can be arrested and potentially reversed, but without proper care, it can progress until the tooth is destroyed, causing severe pain and suffering, especially in children (Selwitz 2007).
According to the World Oral Health Report 2003, dental caries remains a major public health problem in most industrialised countries, affecting 60% to 90% of schoolchildren and the vast majority of adults (Petersen 2003). It is also the most prevalent oral disease in several Asian and Latin American countries. Although for the moment it appears to be less common and less severe in most of Africa, the report anticipates that changing living conditions and dietary habits (particularly growing sugar consumption and low exposure to fluorides) will contribute to increasing the incidence of dental caries in many African countries.
There are profound inequalities in caries status both between and within countries, and the distribution of disease within communities fluctuates (Pitts 2011). However, a recent systematic review showed that low socioeconomic position is associated with a higher risk of having caries lesions or caries experience; this association might be stronger in developed countries (Schwendicke 2015).
Description of the intervention
The use of milk as a vehicle for providing additional fluoride in a dental public health programme is attractive for several reasons. First of all, milk is already an important part of children's diets and has long been used as a nutritional supplement for vulnerable groups. As early as 1980, a randomised controlled trial (RCT) showed a small but statistically significant benefit to growth in deprived children following the provision of free school milk over two years (Baker 1980), and the NHS Centre for Reviews and Dissemination included the provision of free school milk in a list of nine evidence‐based interventions to reduce health inequalities (Smith 1997). Another RCT confirmed that increased milk consumption significantly enhanced bone mineral acquisition and attainment of peak bone mass in adolescent girls (Cadogan 1997).
Moreover, fluoridated milk can be produced in a variety of liquid forms (pasteurised, ultra‐high temperature pasteurised (UHT) and sterilised) and in powder, each containing different fluoridating compounds. Compounds used to fluoridate milk in early clinical trials and laboratory tests included sodium fluoride, calcium fluoride, disodium monofluorophosphate (MFP) and disodium silicofluoride. However, the vast majority of current fluoridated milk schemes worldwide use sodium fluoride. The exception is the caries prevention programme in rural areas of Chile, where the powdered milk and milk derivatives provided to the participating subjects are fluoridated using MFP (Villa 2009).
The choice of fluoridation method depends on many factors, including the nature of food supplement programme itself and the availability of human resources and training. Likewise, the concentration of fluoride required will depend on the age of the children, the concentration of fluoride in the local water supply and the volume of fluoridated milk ingested daily, among other considerations (Mariño 2011).
The feasibility and sustainability of a milk fluoridation scheme depends largely on the existence and type of nutritional supplement programmes. Because any kind of milk can be fluoridated, any system that provides a regular supply of milk to children could potentially provide a vehicle for fluoride delivery. Clearly, an existing milk distribution scheme or national nutrition strategy would simplify the implementation of a milk fluoridation programme (Mariño 2011).
How the intervention might work
Elevating the concentration of the fluoride ion at the plaque‐enamel interface results in a reduction in the rate of demineralisation, an increase in the rate of remineralisation, and a reduction in the rate of acid production in plaque, all of which help to prevent caries.
The use of milk as a vehicle for fluoride delivery has raised questions concerning possible chemical reactions between milk and fluoride ions, the bioavailability of systematically administered fluoride in milk, and potential interactions involving fluoride in the oral cavity (enamel, saliva, plaque and caries). The results of basic studies on milk fluoridation have been published in more than 100 peer‐reviewed papers, with increasing frequency in the past 20 years. Based on these studies, it appears that most of the fluoride added to milk forms a soluble complex with the protein fraction of milk, from which the fluoride can be liberated in ionic (and bioavailable) form. The absorption of fluorides with simultaneous food intake is slower than for fluoride without food, and the proportion absorbed depends on the calcium content of the diet. Different types of milk are consumed around the world: whole or low‐fat; fresh, pasteurised or sterilised; liquid or powdered. The bioavailability of added fluoride has been shown to be satisfactory in all of these, both on the day of milk processing and after several days' storage (Bánóczy 2013).
The fluoride ions available from the consumption of fluoridated milk are incorporated into dental enamel, which inhibits demineralisation and promotes remineralisation. In addition, 30 to 60 minutes after ingestion of fluoridated milk, the levels of fluoride in both whole saliva and dental plaque increase as a consequence of the presence of fluoridated milk in the mouth and increased concentrations of fluoride in salivary secretions following the absorption of ingested fluoride. Thus, fluoride in milk acts both systematically and topically, in the same way as fluoride in water (Bánóczy 2013).
Why it is important to do this review
Milk fluoridation, as a possible dental caries prevention medium, was first proposed by a Swiss paediatrician in the 1950s (Ziegler 1953). Since then, the caries‐inhibiting characteristics of fluoridated milk have been investigated with a view to using it in community‐based caries prevention programmes (Bánóczy 2009). Economic evaluations have demonstrated that milk provides a relatively cost‐effective vehicle for fluoride in the prevention of dental caries (Calvert 1998; Mariño 2007; Mariño 2011).
This is an update of the Cochrane review first published in 2005 (Yeung 2005). The original review found that there were insufficient studies with good quality evidence examining the effects (benefits and harms) of fluoridated milk in preventing dental caries. The included studies, however, suggested that fluoridated milk was beneficial to schoolchildren, especially for their permanent dentition.
Objectives
To assess the effects of milk fluoridation for preventing dental caries at a community level.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel RCTs, including cluster‐randomised trials (e.g. those than randomised at the level of school or class in children) and excluded quasi‐randomised trials.
We also included non‐blinded studies.
We excluded studies with an intervention or follow‐up period of less than two years. For trials designed in school settings, we included studies lasting an equivalent of two school years, even if intervention or follow‐up period fell short of 24 months.
Types of participants
General population, irrespective of age or level of risk for dental caries.
Types of interventions
Active intervention: fluoridated milk (all concentrations/dosage were considered)
Control: non‐fluoridated milk
The milk was provided directly to the children or their family. Any payment for milk should have been equivalent in the fluoridated and non‐fluoridated groups.
Types of outcome measures
Primary outcomes
Changes in caries experience or caries increment, as measured by changes in decayed, missing and filled figures on permanent teeth or surfaces (DMFT or DMFS) or primary teeth or surfaces (dmft or dmfs). Caries was assessed clinically. However, if a combined clinical and radiographic assessment was used, then this was recorded and noted
Adverse effects: dental fluorosis
Secondary outcomes
Dental pain due to decay
Antibiotics due to dental infections
Requirement for general anaesthesia due to dental procedures for caries
Search methods for identification of studies
Electronic searches
To identify potential studies for inclusion in this review, we developed detailed search strategies for each database used. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was linked with the sensitivity maximising version (2008 revision) of the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying RCTs in MEDLINE, as referenced in Section 6.4.11.1 and detailed in Box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The EMBASE search was linked to the Cochrane Oral Health Group filter for identifying RCTs.
We searched the following electronic databases.
The Cochrane Oral Health Group's Trials Register (to November 2014) (see Appendix 1).
CENTRAL (The Cochrane Library, 2014, Issue 10) (see Appendix 2).
MEDLINE via OVID (1946 to November 2014) (see Appendix 3).
EMBASE via OVID (1980 to November 2014) (see Appendix 4).
We did not place any restrictions on the language or date of publication when searching the electronic databases.
Searching other resources
We searched the following databases for ongoing trials (see Appendix 5).
U.S. National Institutes of Health Trials Register (https://clinicaltrials.gov) (to November 2014);
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch) (to November 2014).
Grey literature
In an attempt to identify unpublished or ongoing studies, we contacted the Borrow Foundation to obtain and screen the references in their database of milk fluoridation research (version 6.7, dispatched 10 December 2013) (www.borrowfoundation.org/research).
Handsearching
We handsearched the Journal of Public Health Dentistry (January 1997 to December 2003) for the original review (Yeung 2005). For the updated review, we only included handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL (see the Cochrane Master List for details of journal issues searched to date).
Reference lists
The reference lists of all included studies and relevant reviews were checked manually to identify any additional studies.
Data collection and analysis
Selection of studies
Two authors independently scanned the titles and available abstracts of all reports identified through the electronic searches.
We obtained the full text of studies that appeared to meet the inclusion criteria or for which there were insufficient data in the title and abstract to make a clear decision. Two authors then independently assessed the full reports obtained from all searches, electronic or otherwise, to establish whether the trials met the inclusion criteria or not, discussing disagreements to reach a consensus. When the two authors could not come to an agreement, we consulted a third author. We recorded all rejected studies and our reasons for excluding them in the Characteristics of excluded studies table. For all studies meeting the inclusion criteria, we extracted data and assessed the risk of bias.
Data extraction and management
Two authors independently extracted data using specially designed data extraction forms that we had previously piloted on several papers and modified as needed. We discussed disagreements, consulting a third author when necessary. We contacted study authors to clarify details or obtain missing information when necessary. We excluded data until further clarification was available if we could not reach an agreement.
For each trial, the following data were recorded.
Citation details, including year of publication, country of origin, setting and source of funding.
Details of participants, including demographic characteristics and criteria for inclusion.
Details of intervention, including type and duration of intervention, duration of follow‐up and method of administration.
Details of outcomes reported, including method of assessment and time intervals.
The following information was also noted.
Compliance (level of supervision of participants while they drank the milk they were given).
Comparability of control and treatment groups at entry.
We anticipated that some studies would report data at more than one time point. To minimise issues related to multiplicity of analysis, we planned to only extract and analyse the longest available data for each of the included studies.
Assessment of risk of bias in included studies
Two authors independently assessed the risk of bias of the included trial as part of the data extraction process.
We used the Cochrane Collaboration 'Risk of bias' assessment tool (Higgins 2011) available in Review Manager (RevMan). The domains we assessed included:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcomes assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
The review authors judged the risk of bias for each domain as 'high', 'low' or 'unclear' based on the criteria listed in Section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions, which focuses on the importance of the risk (i.e. whether the presence of the risk could have an important impact the result or the conclusion of the trial) rather than its mere presence (Higgins 2011).
If insufficient detail was reported on what happened in the study, the risk of bias would be ‘unclear' unless authors had other reasons to judge it as 'high' or 'low'. An ‘unclear’ judgement was also made if what happened in the study was adequately described, but the risk of bias was unknown or difficult to judge.
Measures of treatment effect
Prevented fraction (PF) was the measure of treatment effect presented for caries increment. PF was calculated as the mean increment in the control group minus the mean increment in the intervention group, divided by the mean increment in the control group. For an outcome such as caries increment (where discrete counts are considered to approximate to a continuous scale and are treated as continuous outcomes), this measure was considered more appropriate than the mean difference or standardised mean difference since it allowed a combination of different ways of measuring caries increment and a meaningful investigation of heterogeneity between trials. It is also simple to interpret.
For dichotomous outcomes (where the outcome of interest was either present or absent), we planned to express the estimate of treatment effect of an intervention as risk ratios (RRs) together with 95% confidence intervals (CIs) or as hazard ratios if these were available as time‐to‐event data. Where appropriate, we also planned to present the corresponding absolute reductions with risks, either as numbers needed to treat or absolute risk reduction per 1000 people. For continuous outcomes, we planned to report mean differences (MDs) and standard deviations, except for outcomes which had used difference scales, in which case we would have pooled them using the standardised mean difference.
Unit of analysis issues
Had we found cluster RCTs, we would have estimated the design effect with the appropriate methods as detailed in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
Where data were missing from the published report of a trial, we contacted the author(s) to obtain the data and clarify any uncertainty. In case of missing data, we aimed to base the review on an available case analysis, if possible followed by a sensitivity analysis if the missing data posed a high risk of bias.
For continuous data, we planned to use the methods for estimating missing standard deviations in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Otherwise we would not have undertaken any imputations or used any statistical methods to impute missing data.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by examining the type of participants, interventions and outcomes in each study.
Had we found more than one study, we would have assessed statistical heterogeneity by inspecting the point estimates and CIs on the forest plots. We planned to assess and quantify the variation in treatment effects by means of Cochran's test for heterogeneity and the I2 statistic. We considered heterogeneity to be statistically significant if the P value was less than 0.1.
A rough guide to interpreting the values obtained from the I2 statistic, provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), is as follows:
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
The importance of the observed value of the I2 statistic depends on (i) the magnitude and direction of effects and (ii) the strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or a CI for the I2 statistic).
Assessment of reporting biases
Reporting bias can be assessed between studies or within studies.
If there had been sufficient numbers of trials (more than 10) in any meta‐analysis, we would have assessed publication bias according to the recommendations on testing for funnel plot asymmetry (Egger 1997) as described in Section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have examined possible causes of any asymmetry identified or assessed it using a table to list the outcomes reported by each included study, to determine whether any studies did not report outcomes that had been reported by most studies.
We assessed within‐study reporting bias by comparing the outcomes presented in the published report against the original study protocol, whenever this could be obtained. If no protocol was available, we compared outcomes listed in the methods section against the results reported. If the study mentioned but did not adequately report non‐significant results, we considered it possible that bias in a meta‐analysis could occur, and we would have sought further information from the study authors. Otherwise, we would simply have noted a 'high' risk of bias. If there was insufficient information to judge the risk of bias, this was rated as 'unclear'.
Data synthesis
Outcomes may be assessed and reported at more than one time point in included studies. We planned to perform separate analyses for three different lengths of follow‐up periods: short term (two to three years of follow‐up), medium term (four to six years of follow‐up) or long term (at least seven years of follow‐up). We focused our main analysis on short‐term outcomes reported, as longer‐term data is not as reliable due to drop‐outs or the natural loss of primary teeth in the case of children.
We would have considered performing meta‐analyses had there been studies of similar comparisons reporting the same outcome measures. We would have combined RRs for dichotomous data and MDs for continuous data, using a random‐effects model if more than one study was found.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses to compare the following possible variations in population and intervention, if data permitted.
Indicators of background exposure to fluoride (e.g. socioeconomic status; presence of fluoride in drinking water, toothpaste, etc).
Concentration/dosage of fluoride (low vs high).
Frequency of consumption.
Method of drinking (cup/straw).
Compliance.
Sensitivity analysis
We planned to perform sensitivity analyses to examine the effect of randomisation, allocation concealment and blind outcome assessment on the overall estimates of effect. Had the data allowed it, we would have also examined the effect of including unpublished literature on the review's findings.
Summary of findings table
We used the GRADE approach to assess the quality of evidence related to each of the main outcomes. We used the GRADEprofiler to import data from RevMan to create the 'Summary of Findings' tables. To assess the overall quality of evidence for each outcome, we downgraded the evidence from 'high quality' by one level for 'serious' (or by two for 'very serious') concerns related to risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
In the 2015 review update, we revised our protocol as well as the search strategies. To take into account the changes in our protocol, we also examined earlier records to ensure no relevant studies were excluded.
The updated search identified 242 records through the electronic databases and an additional 56 records through searches of other sources. After an initial screening of the titles and abstracts, we identified 24 records requiring further examination. We obtained and assessed the full text of these where available. One unpublished trial (Maslak 2004) and two ongoing studies met our inclusion criteria. See Figure 1 for a summary of the study selection process.
1.
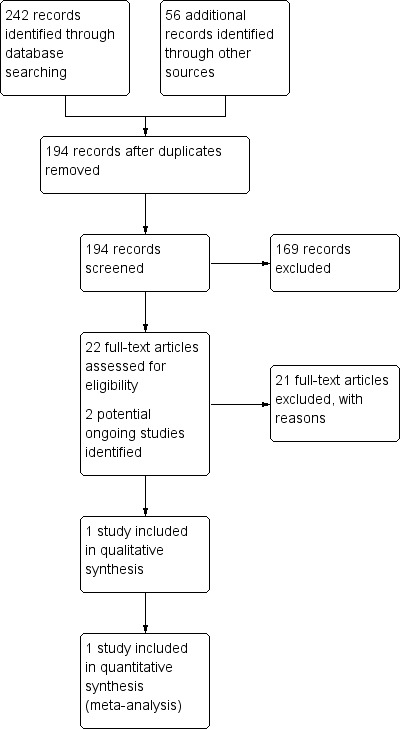
Study flow diagram.
Included studies
See Characteristics of included studies table.
One RCT was included in the review (Maslak 2004). The study was carried out in Russia and published as an abstract only. However, the investigators provided unpublished trial data.
Participants
A total of 180 children aged three years old were randomised, and 166 children were available for analysis.
Intervention
The children consumed the fluoridated milk (2.5 mg per litre) using a cup.
Comparison
The children in the comparator group received milk without added fluoride.
Outcomes
Changes in caries experience, measured by the dmft and DMFT, were reported yearly (Maslak 2004).
Excluded studies
See Characteristics of excluded studies table.
We excluded 21 studies, mainly because they were not RCTs. Others did not include relevant interventions or comparison groups. We also excluded a quasi‐randomised study (Stephen 1984) included in the previous version of this review (Yeung 2005) due to lack of adequate randomisation.
Ongoing studies
See Characteristics of ongoing studies table.
We identified two RCTs that are potentially relevant to this review (Stecksén‐Blicks; Svensäter). We tried to contact the investigators to obtain further information, but at the time of writing, we had not received any response.
Risk of bias in included studies
See the 'Risk of bias' table in the Characteristics of included studies section.
Allocation
The published abstract did not contain a description of randomisation, but the author provided more information when contacted. From correspondence with Maslak 2004, we confirmed that randomisation was carried out at an individual level (i.e. it was not a cluster‐randomised trial). Trial investigators noted that "the investigators did not participate in the selection process. The district for the project was determined by the Volgograd Administration. The kindergartens [i.e. nursery schools] were selected by the District Administration. The children were recruited by the kindergarten teachers." The author also used stratification during randomisation of these children, who regularly attended the nursery school, were aged three years, were caries free, lived in one city district, and had parents who had given written consent. However, the generation of the randomisation sequence was still unclear. It was also unclear if the sequence had been concealed prior to the randomisation or recruitment of children to investigators or teachers (who were involved in recruitment). Therefore, both allocation concealment and sequence generation were considered to carry an unclear risk of bias.
Blinding
The parents knew what type of milk was given to their children in the trial.
Trialists reported blinding of outcome assessors and the statistician involved in the analyses.
Incomplete outcome data
Maslak 2004 initially randomised 180 children, of whom 14 withdrew (5 in the test group and 9 in the control group). Seventy‐five (94%) test children and 91 (91%) control children were available for follow‐up examination at the third year and included in the analysis. Some withdrawals were due to absence during the annual examination, whilst others withdrew because of their parents' decision. Correspondence with the author suggested that an intention‐to‐treat analysis had been carried out.
Selective reporting
The protocol was not available, and only an abstract was published. There was insufficient information to judge the risk of bias ('unclear' risk of bias).
Other potential sources of bias
Compliance
The included study did not report the level of compliance (Maslak 2004).
Overall risk of bias
We present the results of the risk of bias assessments graphically in Figure 2.
2.
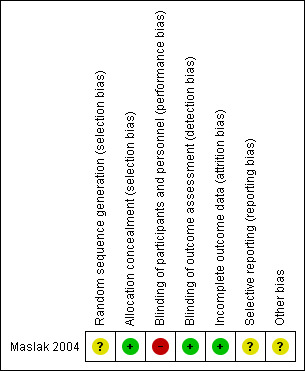
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Primary outcomes
Changes in caries experience, caries increment
Permanent teeth
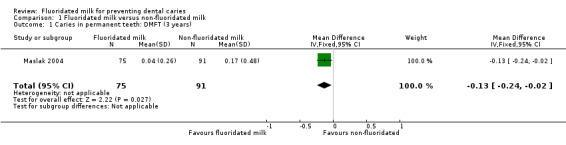
After drinking fluoridated milk for three years, there was a reduction in the DMFT between the test and control groups (MD −0.13, 95% CI −0.24 to −0.02) (Maslak 2004). The disease level was very low in the study, resulting in a small absolute effect size.
Primary teeth
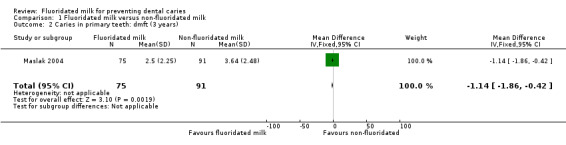
After drinking fluoridated milk for three years, there was a substantial reduction in the dmft between the test and control groups (MD −1.14. 95% CI −1.86 to −0.42) (Maslak 2004), equivalent to a PF of 31%.
Adverse effects: Dental fluorosis
Correspondence with the author (Maslak 2004) confirmed that no adverse effects were reported.
Secondary outcomes
Dental pain due to decay
No information was reported.
Antibiotics use due to dental infections
No information was reported.
Requirement for general anaesthesia due to dental procedures for caries
No information was reported.
Discussion
Summary of main results
This review looked for evidence on the effectiveness of fluoridated milk as a means of preventing dental caries in people of all ages.
Overall completeness and applicability of evidence
We confined this review to studies which were designed as RCTs. The only study we found was conducted in young children (Maslak 2004).
The included study (Maslak 2004) has a high risk of bias, and its external validity should be viewed with caution. The preventive programme, however, was appropriate, as caries prevalence was high and fluoride in drinking water was low. The applicability of the findings of this study in other settings, where the baseline level of caries and exposure to fluoride through other sources (such as drinking water and toothpaste) may differ, needs to be considered on a case‐by‐case basis.
Quality of the evidence
The quality of evidence for the main outcomes was low. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Factors that affected our confidence in effect sizes of the analysis included limitations in the study design (high risk from selection and attrition bias) and other issues in data analysis, such as not taking into account clustering effects. Although randomisation was carried out on an individual basis, analyses of programmes targeted through schools may need to take account of clustering, as children within a school may influence each other and therefore cannot be regarded as completely independent (Bland 1997). Failing to adjust for unit of analysis could lead to spurious positive findings (Altman 1997).
Intention‐to‐treat analysis is favoured in assessment of clinical effectiveness, as it mirrors the non‐compliance and treatment changes that are likely to occur when the intervention is used in practice, reducing the possibility of overestimating effectiveness due to attrition bias (when participants are excluded from the analysis) (Hollis 1999). The author of the included study reported in personal communication that intention‐to‐treat analysis had been carried out (Maslak 2004). However, this information could not be substantiated from the abstract.
Potential biases in the review process
Without a full publication of the study, it is not always possible to comprehensively assess the risk of bias in the reviews or ensure that all relevant information has been obtained. We also excluded a study that was quasi‐randomised (Stephen 1984), as this type of study design carries a high risk of selection bias.
Most analyses planned in the protocol, such as meta‐analysis, subgroup analysis, sensitivity analysis, and assessment for publication bias, could not be conducted in this review because of the lack of RCTs published on fluoridated milk.
Agreements and disagreements with other studies or reviews
Studies on the clinical effectiveness of fluoridated milk in caries prevention have been carried out in several countries using different research methods. These studies also varied with regard to the delivery of fluoridated milk, in particular, the concentration of fluoride. Reductions in caries incidence have varied from no effect (Ketley 2003; Stephen 1984) to 70% (Pakhomov 1993) in the primary dentition, and from no effect (Ketley 2003; Lopes 1984) to 97% (Pakhomov 1993) in the permanent dentition. The lack of effect shown in Ketley 2003 may be due to the lack of statistical power. On the other hand, the duration of intervention in the study by Lopes 1984 may be too short (16 months) to evaluate the effectiveness of fluoridated milk as a caries prevention method.
The findings of this updated Cochrane Review do not differ from those of the original review, which was first published in 2005 (Yeung 2005). Other systematic reviews also concluded that there is a low level of quality evidence that milk fluoridation is beneficial in preventing dental caries (Cagetti 2013; National Health and Medical Research Council 2007).
Cagetti 2013 carried out a systematic review on the caries‐prevention effect of fluoridated food. Two studies on fluoridated milk (Bian 2003; Stecksén‐Blicks 2009) fulfilled their inclusion criteria. However, as the study period for both was only 21 months, they were excluded from our review.
The systematic review by National Health and Medical Research Council 2007 identified one systematic review (Yeung 2005), no additional RCTs, no relevant cohort studies or case‐control studies. Two cross‐sectional studies (Mariño 2004; Riley 2005) met the inclusion criteria. These two studies assessed two different populations (one exposed to fluoridated milk and one not exposed to fluoridated milk) and were measured at multiple time points. As neither study was an RCT, both were excluded from the Cochrane Review.
Authors' conclusions
Implications for practice.
There was only one small RCT examining the effects of fluoridated milk in preventing dental caries, and it had serious methodological limitations. The included study suggested that fluoridated milk may be beneficial to schoolchildren in reducing the level of caries, with a substantial effect size for primary teeth. However, there was no information about the potential harms. Moreover, the study was conducted in a setting where the baseline level of caries was high and the level of fluoride in drinking water was low. Therefore, the potential to replicate the benefits observed in this study in other settings should be considered on a case‐by‐case basis. The data need to be supplemented by further RCTs to provide a high level of evidence for practice.
Implications for research.
Further trials should be well‐designed RCTs (adequate sequence generation and allocation concealment methods, blinding of participants and outcome assessors) and reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement (www.consort‐statement.org). In particular, appropriate control groups should be used, and trials should be designed with adequate power in view of a potential high drop‐out rate (> 40%) with a follow‐up period of at least two years. If a cluster‐randomised trial design is used, these should be taken into account in the analysis and reporting. An intention‐to‐treat analysis (analysing patients according to the group randomised), which is more conservative in its effect size estimation, is probably more useful in reflecting the effectiveness of the intervention. We do acknowledge that such trials will be expensive and difficult to conduct, but they are the only ones able to provide a reliable answer on the relative benefits and harms of the intervention.
Following an international consensus workshop on caries clinical trials, Pitts 2004 proposed that in new caries trials, the efficacy variables should include a measure of further demineralisation or stimulation of remineralisation in lesions. Despite this consensus statement, however, we are unaware of any other published core outcomes on the assessment of caries and impact of caries. If available (e.g. through the COMET initiative (www.comet‐initiative.org)), these should be used.
The main desirable features for a future trial are summarised below using the EPICOT structure (Brown 2006).
Evidence: There is a lack of evidence (low quality, low number of participants and studies) assessing the effectiveness and safety of fluoridated milk in the prevention of dental caries.
Population: Any age group. It will be important to stratify or report key modifiers of effects observed, including baseline caries level and fluoride exposure.
Intervention: Fluoridated milk.
Comparison: Non‐fluoridated milk or placebo.
Outcomes: Change in caries experience (measured by DMFT and dmft), adverse effects (especially dental fluorosis). Other measures of impact of caries include dental pain, antibiotics use for dental infections and use of general anaesthesia for dental treatment.
Time: Treatment and follow‐up should be at least two years.
In order to draw firm conclusions on the effectiveness of fluoridated milk, additional studies should explore other variables such as fluoride dose, number of days per year, background caries experience, time of consumption and method of drinking fluoridated milk (Bánóczy 2009).
What's new
| Date | Event | Description |
|---|---|---|
| 24 May 2018 | Review declared as stable | This review will not be updated until a substantial body of evidence on the topic becomes available. If trials are conducted and found eligible for inclusion in the future, the review would then be updated accordingly. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 1 September 2015 | Amended | Minor edit to personal communication reference. |
| 31 August 2015 | New citation required but conclusions have not changed | Change to study selection criteria: removed quasi‐randomised controlled trials; reduced the intervention and follow‐up period from a minimum of three years to two years. New author team. |
| 27 November 2014 | New search has been performed | New search performed. No new studies identified. |
| 8 August 2008 | Amended | Converted to new review format. |
Notes
This review will not be updated until a substantial body of evidence on the topic becomes available. If trials are conducted and found eligible for inclusion in the future, the review would then be updated accordingly.
Acknowledgements
We would like to acknowledge the contributions of Joseph Hitchings, Tatiana Macfarlane, Anthony Threlfall and Martin Tickle as the authors of the original review (Yeung 2005) published in 2005.
We would also like to thank the following investigators who provided additional information about their trials: Jin‐You Bian, Michael Lennon, Rodrigo Mariño, Elena Maslak and Ken Stephen.
For help with the translations of foreign papers, our thanks go to Janet Lear, Evgeny Kushnerev (Russian), Giovanni Lodi (Italian), Tatiana Macfarlane (Russian), Valeria Marinho (Portuguese) and Regina Mitezki (German).
For the 2015 review update, we would like to thank Anne Littlewood, Jo Weldon, Helen Worthington, Zipporah Iheozor‐Ejiofor and Helen Wakeford at Cochrane Oral Health. We also thank external referees Deborah Moore, Chris Neurath and Domenick Zero.
We would like to acknowledge the Borrow Foundation for providing access to additional scientific literature.
Appendices
Appendix 1. Cochrane Oral Health Group Trials Register Search Strategy
#1 (fluorid*:ti,ab) AND (INREGISTER) #2 (milk*:ti,ab) AND (INREGISTER) #3 (#1 and #2) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) Search Strategy
#1 [mh Fluorides] #2 [mh ^Fluoridation] #3 fluorid* #4 #1 or #2 or #3 #5 [mh Milk] #6 milk* #7 #5 or #6 #8 #4 and #7
Appendix 3. MEDLINE (OVID) Search Strategy
1. exp Fluorides/ 2. Fluoridation/ 3. fluorid$.mp. 4. or/1‐3 5. exp Milk/ 6. milk$.mp. 7. 5 or 6 8. 4 and 7
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Section 6.4.11.1 and detailed in Box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. EMBASE (OVID) Search Strategy
1. Fluoride/ 2. Fluoridation/ 3. fluorid$.mp. 4. or/1‐3 5. Milk/ 6. milk$.mp. 7. 5 or 6 8. 4 and 7
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. US National Institutes of Health Trials Register (ClinicalTrials.gov) and the WHO International Clinical Trials Register Platform Search Strategy
fluoride and milk
Data and analyses
Comparison 1. Fluoridated milk versus non‐fluoridated milk.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries in permanent teeth: DMFT (3 years) | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.24, ‐0.02] |
| 2 Caries in primary teeth: dmft (3 years) | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐1.86, ‐0.42] |
1.1. Analysis.
Comparison 1 Fluoridated milk versus non‐fluoridated milk, Outcome 1 Caries in permanent teeth: DMFT (3 years).
1.2. Analysis.
Comparison 1 Fluoridated milk versus non‐fluoridated milk, Outcome 2 Caries in primary teeth: dmft (3 years).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Maslak 2004.
| Methods | Parallel group randomised trial. Intervention and follow‐up for 3 years | |
| Participants |
Setting: Participants recruited from nursery schools in Volgograd, Russia. Study started in October 1998 Number randomised: 180 children; (80 in intervention, 100 in control group) Numbers available (for analysis) at 3 years: 166 (75 in intervention, 91 in control group) Age: 3 years Inclusion criteria: 3 year‐olds who were caries free Level of fluoride in drinking water: 0.18 to 0.20 mg F/l No details of other possible sources of fluoride exposure such as toothpaste |
|
| Interventions | Group 1: fluoridated milk with the fluoride level set at 2.5 mg/l Group 2: non‐fluoridated milk All children regularly consumed 180‐200 ml milk per day using a 200 g cup | |
| Outcomes | Changes in caries experience as measured by dmft and DMFT measured once yearly. 3‐year data used | |
| DOI | No information available | |
| Funding | The Borrow Foundation, UK | |
| Notes | Unpublished data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from correspondence): "A centralised randomisation scheme was used. Used stratification during randomisation of these children who were regularly attending the kindergarten [nursery school], aged 3 years, caries free, living in one city district, and whose parents have given written consent". Comment: Method of sequence generation is unclear. Moreover, the initial number of participants is 25% higher in the control group (100 vs 80). |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "The investigators did not participate in the selection process. The district for the project was determined by the Volgograd Administration. The kindergartens were selected by the District Administration. The children were recruited by the kindergarten teachers." "A centralised randomisation scheme was used. Used stratification during randomisation of these children who were regularly attending the kindergarten, aged 3 years, caries free, living in one city district, and whose parents have given written consent." Comment: This is probably low risk since randomisation was performed centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (from correspondence): "Parents knew what type of milk was used by their children." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All children were annually examined during 4 years by trained and calibrated examiners (the study was blind). Caries was diagnosed visually by probing; d3,4mft and D3,4MFT were determined." Comment: Method of blinding was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from correspondence): "Initially randomised 180 children. 5 test and 9 control children withdrew subsequently. Some withdrawals were due to absence during annual examination, whilst others withdrew because of parents' decision. Intention‐to‐treat analysis had been carried out". Comment: 166/180 (92.2%) patients randomised available for analysis; no important differential drop‐out (5/80 in intervention group, 9/100 in control group). |
| Selective reporting (reporting bias) | Unclear risk | Comment: Protocol not available. Insufficient information to judge. |
| Other bias | Unclear risk | Comment: Insufficient information to judge. Only an abstract was published, but author provided further trial information. |
dmft: decay, filled and missing primary teeth; DMFT: decayed, missing and filled permanent teeth.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bian 2003 | Not RCT. Control children had normal diet. Study duration of 21 months. |
| Bánóczy 1985 | Not RCT. Test children used collectively a particular fluoridated toothpaste, but control group used different fluoridated and non‐fluoridated toothpastes alternatively. |
| Gyurkovics 1992 | Not RCT. Study duration of 12 years, including 2‐year interruption of supply of fluoridated milk. |
| Ketley 2003 | Not RCT |
| Kouzmina 1999 | Not RCT |
| Legett 1987 | Not RCT. Study direction of 3 years including 10‐month lapse of delivery of fluoridated milk. |
| Light 1958 | Not RCT |
| Lopes 1984 | Cluster RCT. Insufficient study duration (16 months) (published in Portuguese). |
| Mariño 2001 | Not RCT |
| Mariño 2007 | The cost‐effectiveness paper of an excluded non‐RCT (Mariño 2001). |
| Pakhomov 1993 | Not RCT |
| Pakhomov 1995 | Not RCT |
| Pakhomov 2005 | Not RCT (published in Russian). |
| Pakhomov 2011 | Not RCT (published in Russian). |
| Petersson 2011 | RCT. Milk with probiotic bacteria. Insufficient study duration (15 months). |
| Riley 2005 | Not RCT |
| Rusoff 1962 | Not RCT |
| Stecksén‐Blicks 2009 | Cluster RCT. Milk with probiotic bacteria. Study duration of 21 months. |
| Stephen 1984 | Quasi‐randomised RCT. Correspondence with the author suggested that allocation was undertaken as per children's alternate alphabetical order within each subgroup. |
| Weitz 2007 | Not RCT. Positive control group had ongoing APF‐gel programme. |
| Ziegler 1964 | Not RCT (published in German). Participation was on an optional basis. |
APF: acidulated phosphate fluoride; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Stecksén‐Blicks.
| Trial name or title | Stecksén‐Blicks |
| Methods | Double‐blinded, randomised controlled trial with three parallel arms |
| Participants | 2 to 4 years old |
| Interventions | Fluoridated milk |
| Outcomes | Caries prevention |
| Starting date | |
| Contact information | Professor Christina Stecksén‐Blicks Department of Odontology Paediatric Dentistry Umeå University SE‐901 85 Umeå Sweden Email: christina.stecksen‐blicks@odont.umu.se |
| Funding | The Borrow Foundation, UK |
| Notes | Awaiting further details from the investigators |
Svensäter.
| Trial name or title | Bacterial acid tolerance ‐ a new target for fluoridated milk in caries prevention |
| Methods | RCT in general dental clinics within the Public Dental Health Service in 4 counties in Southern Sweden (Halland, Blekinge, Kronoberg and Kalmar) |
| Participants | Schoolchildren aged 12–13 years with at least one permanent first molar in approximal contact with a permanent first or second premolar |
| Interventions | Intervention: 5 mg/L NaF in 100 ml of cow's milk on a daily basis Control: 100 ml of the same milk with the same volume of sterile water as for fluoride |
| Outcomes | Change in caries experience, acid tolerance in plaque samples |
| Starting date | November 2012 |
| Contact information | Professor Gunnel Svensäter Professor and Head of Oral Biology Faculty of Odontology Malmö University S‐20506, Malmö Sweden Email: Gunnel.Svensater@mah.se |
| Funding | The Borrow Foundation, UK |
| Notes | First interim report is available at: www.mah.se/PageFiles/2990664/KOF‐SYD%20Bacterial%20acid%20tolerance.pdf |
Differences between protocol and review
The original review (Yeung 2005) was based on a published protocol (Yeung 2002).
In the 2015 review update, we have removed quasi‐randomised controlled trials from the Types of studies section. The intervention and follow‐up period was reduced from a minimum of three years to two years. We also revised our search strategies.
Moreover, slight modifications were made to the protocol (Yeung 2002) to further define the outcomes and time points measured.
Contributions of authors
Conceiving the review (CAY) Designing the review (CAY, LYC, AMG) Co‐ordinating the review (CAY) Developing search strategy (CAY) Undertaking searches (CAY, LYC) Screening search results (CAY, LYC, AMG) Organising retrieval of papers (CAY, LYC, AMG) Screening retrieved papers against inclusion criteria (CAY, LYC, AMG) Appraising quality of papers (CAY, LYC, AMG) Extracting data from papers (CAY, LYC, AMG) Writing to authors of papers for additional information (CAY) Obtaining and screening data on unpublished data (CAY, LYC) Entering data into RevMan (CAY, LYC) Analysis of data and interpretation of data (CAY, LYC, AMG) Writing the review (CAY, LYC, AMG)
Sources of support
Internal sources
Division of Dentistry, The University of Manchester, UK.
-
MAHSC, UK.
Cochrane Oral Health is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Maslak 2004 {published and unpublished data}
- Maslak EE, Afonina IV, Kchmizova TG, Litovkina LS, Luneva NA. The effect of a milk fluoridation project in Volgograd. Caries Research 2004;38(4):377. Abstract no. 60. [Google Scholar]
- Yeung CA (The University of Manchester, Manchester, UK). [personal communication]. Letter to: EE Maslak (Volgograd State Medical University, Volgograd, Russia) 30 October 2004.
References to studies excluded from this review
Bánóczy 1985 {published data only}
- Bánóczy J, Zimmermann P, Hadas É, Pintér A, Bruszt V. Caries preventive effect of milk fluoridation on children within a clinical study after 5 years [Ergebnisse mit Milchfluoridierung im klinischen Experiment an Heimkindern, nach fünf Jahren]. Oral‐prophylaxe 1985;7:12‐7. [Google Scholar]
- Bánóczy J, Zimmermann P, Hadas É, Pintér A, Bruszt V. Effect of fluoridated milk on caries: 5 year results. Journal of the Royal Society of Health 1985;105(3):99‐103. [DOI] [PubMed] [Google Scholar]
- Bánóczy J, Zimmermann P, Hadas É, Pintér A, Bruszt V. Effect of fluoridated milk on caries; 4‐ and 5‐year result. Caries Research 1985;19(2):156. Abstract no. 9. [Google Scholar]
- Bánóczy J, Zimmermann P, Pintér A, Hadas É, Bruszt V. Effect of fluoridated milk on caries: 3‐year results. Community Dentistry and Oral Epidemiology 1983;11(2):81‐5. [DOI] [PubMed] [Google Scholar]
Bian 2003 {published and unpublished data}
- Bian JY, Li RY, Wang WJ. Feasibility of milk fluoridation and trends in dental caries of children in China. Advances in Dental Research 1995;9(2):112‐5. [DOI] [PubMed] [Google Scholar]
- Bian JY, Li RY, Wang WJ. Progress of implementing the milk fluoridation project for caries prevention in children in Beijing. International Dental Journal 1995;45(5):291. Abstract no. FC‐4 #35. [Google Scholar]
- Bian JY, Wang WH, Wang WJ, Rong WS, Lo EC. Effect of fluoridated milk on caries in primary teeth: 21‐month results. Community Dentistry and Oral Epidemiology 2003;31(4):241‐5. [DOI] [PubMed] [Google Scholar]
Gyurkovics 1992 {published data only}
- Bánóczy J. Outcomes of fluoridated milk projects conducted in Hungary. Advances in Dental Research 1995;9(2):154. Abstract no. S2. [Google Scholar]
- Gyurkovics C, Hadas É, Zimmermann P, Bánóczy J. Effect of fluoridated milk on caries: 12 years results. Journal of Dental Research 1992;71(Special issue):703. Abstract no. 1501. [Google Scholar]
- Gyurkovics C, Hadas É, Zimmermann P, Bánóczy J. The effect of fluoridated milk on caries reduction over 12 years [Fluordúsított tej cariesreduktív hatásának vizsgálata 12 év után]. Fogorvosi Szemle 1992;85(7):195‐202. [PubMed] [Google Scholar]
- Gyurkovics C, Zimmermann P, Hadas É, Bánóczy J. Effect of fluoridated milk on caries: 10‐year results. Journal of Clinical Dentistry 1992;3(4):121‐4. [PubMed] [Google Scholar]
Ketley 2003 {published and unpublished data}
- Ketley CE, West JL, Lennon MA. The use of school milk as a vehicle for fluoride in Knowsley, UK; an evaluation of effectiveness. Community Dental Health 2003;20(2):83‐8. [PubMed] [Google Scholar]
Kouzmina 1999 {published data only}
- Kouzmina E, Kolesnik A, Smirnova T, Zimina V. Results of a three year implementation of milk fluoridation in the Russian Federation. Journal of Dental Research 1999;78(1 Suppl):366. Abstract no. 2086. [Google Scholar]
- Kouzmina EM, Kolesnik AG, Zimina VI, Nabatova T. Caries reduction and F‐intake in Russian children receiving F‐milk. Journal of Dental Research 1998;77(2 Suppl):712. Abstract no. 645. [Google Scholar]
Legett 1987 {published data only}
- Legett BJ Jr, Garbee WH, Gardiner JF, Lancaster DM. The effect of fluoridated chocolate‐flavored milk on caries incidence in elementary school children: two and three‐year studies. Journal of Dentistry for Children 1987;54(1):18‐21. [PubMed] [Google Scholar]
Light 1958 {published data only}
- Light AE, Smith FA, Gardner E, Hodge HC. Effect of fluoridated milk on deciduous teeth. Journal of the American Dental Association 1958;56(2):249‐50. [PubMed] [Google Scholar]
Lopes 1984 {published data only}
- Lopes ES, Bastos JR, Zaniratto JE. Dental caries prevention using fluoridation of the milk given to school children of Agudos ‐ SP, during 16 months [Prevenção da cárie dentária através da fluoretação do leite servido a escolares de Agudos ‐ SP, durante 16 meses]. Revista da Associação Paulista de Cirurgiões Dentistas 1984;38(6):419‐26. [Google Scholar]
Mariño 2001 {published and unpublished data}
- Mariño R, Villa A, Guerrero S. A community trial of fluoridated powdered milk in Chile. Community Dentistry and Oral Epidemiology 2001;29(6):435‐42. [DOI] [PubMed] [Google Scholar]
- Mariño R, Villa A, Guerrero S. The milk fluoridation program in Codegua, Chile: an evaluation after 3 years [Programa de fluoración de la leche en Codegua, Chile: evaluación al tercer año]. Pan American Journal of Public Health 1999;6(2):117‐21. [DOI] [PubMed] [Google Scholar]
- Mariño R, Villa A, Weitz A, Guerrero S. Prevalence of fluorosis in children aged 6‐9 years‐old who participated in a milk fluoridation programme in Codegua, Chile. Community Dental Health 2004;21(2):143‐8. [PubMed] [Google Scholar]
Mariño 2007 {published data only}
- Mariño R, Morgan M, Weitz A, Villa A. The cost‐effectiveness of adding fluorides to milk‐products distributed by the National Food Supplement Programme (PNAC) in rural areas of Chile. Community Dental Health 2007;24(2):75‐81. [PubMed] [Google Scholar]
Pakhomov 1993 {published data only}
- Pakhomov G, Ivanova K, Moller I, Vrabcheva M. The caries reducing effect of a community‐based milk fluoridation project in Bulgaria. Journal of Dental Research 1993;72(1 Suppl):109. Abstract no. 45. [Google Scholar]
Pakhomov 1995 {published data only}
- Ivanova K, Pakhomov GN, Moller IJ, Vrabcheva M. Caries reduction by milk fluoridation in Bulgaria. Advances in Dental Research 1995;9(2):120‐1. [DOI] [PubMed] [Google Scholar]
- Pakhomov GN, Ivanova K, Moller IJ, Vrabcheva M. Dental caries‐reducing effects of a milk fluoridation project in Bulgaria. Journal of Public Health Dentistry 1995;55(4):234‐7. [DOI] [PubMed] [Google Scholar]
Pakhomov 2005 {published data only}
- Pakhomov GN, Kolesnik AG, Shamsheva AA, Kuzmina EM, Stepanova IA. Milk fluoridization efficacy in a controlled study and dental caries experience dynamics in conditions of wide availability of local F‐containing means [Эффективность фторирования молока в контролируемом исследовании и динамика распространенности кариеса зубов в условиях широкой доступности средств местного применения фторида]. Stomatologiia 2005;84(4):37‐42. [PubMed] [Google Scholar]
Pakhomov 2011 {published data only}
- Pakhomov GN, Khutyz MKh, Zapadaeva SV, Avraamova OG, Grechka MF. Long‐term results of prevention of dental caries among children using fluoridated milk in city Maikop [Отдаленные результаты профилактики кариеса с использованием фторированного молока у детей в Майкоп]. Stomatologiia 2011;90(6):66‐70. [PubMed] [Google Scholar]
Petersson 2011 {published data only}
- Petersson LG, Magnusson K, Hakestam U, Baigi A, Twetman S. Reversal of primary root caries lesions after daily intake of milk supplemented with fluoride and probiotic lactobacilli in older adults. Acta Odontologica Scandinavica 2011;69(6):321‐7. [DOI] [PubMed] [Google Scholar]
Riley 2005 {published data only}
- Riley J, Klause BK, Manning C, Davies G, Graham J, Worthington H. The effectiveness of a school based milk fluoridation programme. Journal of Dental Research 2004;83(Special issue B):Abstract no. 0002. [Google Scholar]
- Riley JC, Klause BK, Manning CJ, Davies GM, Graham J, Worthington HV. Milk fluoridation: a comparison of dental health in two school communities in England. Community Dental Health 2005;22(3):141‐5. [PubMed] [Google Scholar]
Rusoff 1962 {published data only}
- Rusoff LL, Konikoff BS, Frye JB Jr, Johnson JE, Frye WW. Fluoride addition to milk and its effect on dental caries in school children. American Journal of Clinical Nutrition 1962;11:94‐101. [DOI] [PubMed] [Google Scholar]
Stecksén‐Blicks 2009 {published data only}
- Stecksén‐Blicks C, Sjöström I, Twetman S. Effect of long‐term consumption of milk supplemented with probiotic lactobacilli and fluoride on dental caries and general health in preschool children: a cluster‐randomized study. Caries Research 2009;43(5):374‐81. [DOI] [PubMed] [Google Scholar]
Stephen 1984 {published and unpublished data}
- Stephen KW, Boyle IT, Campbell D, McNee S, Boyle P. Five‐year double‐blind fluoridated milk study in Scotland. Community Dentistry and Oral Epidemiology 1984;12(4):223‐9. [DOI] [PubMed] [Google Scholar]
- Stephen KW, Boyle IT, Campbell D, McNee S, Fyffe JA, Jenkins AS, et al. A 4‐year double‐blind fluoridated school milk study in a vitamin‐D deficient area. British Dental Journal 1981;151(9):287‐92. [DOI] [PubMed] [Google Scholar]
- Stephen KW, Campbell D. A 4‐year double‐blind caries study with fluoridated school milk. Caries Research 1981;15(2):185. Abstract no. 17. [Google Scholar]
Weitz 2007 {published data only}
- Weitz A, Mariñanco M I, Villa A. Reduction of caries in rural school‐children exposed to fluoride through a milk‐fluoridation programme in Araucania, Chile. Community Dental Health 2007;24(3):186‐91. [PubMed] [Google Scholar]
- Weitz A, Villa A. Caries reduction in rural school‐children exposed to fluorides through a Milk‐Fluoridation Program in Araucania, Chile. Journal of Developmental Origins of Health and Disease 2009;1(Suppl 1):S287. Abstract no. P‐8C‐3‐383. [Google Scholar]
- Weitz A, Villa A. Caries reduction in rural schoolchildren exposed to fluorides through a milk fluoridation program in Araucania, Chile. Caries Research 2004;38(4):396‐7. Abstract no. 118. [Google Scholar]
- Weitz A, Villa A. Reduction of caries in rural school‐children exposed to fluorides through a a milk fluoridation program in Araucania, Chile. Journal of Dental Research 2005;84(Special issue B):Abstract No: 0289 (Latin American Federation). [Google Scholar]
Ziegler 1964 {published data only}
- Wirz R. Results of the large‐scale milk fluoridation experiment in Winterthur from 1958 to 1964 [Ergebnisse des Grossversuches mit fluoridierter Milch in Winterthur von 1958 bis 1964]. SSO. Schweizerische Monatsschrift für Zahnheilkunde. Revue mensuelle suisse d'odonto‐stomatologia. Rivista mensile svizzera di odontologia e stomatologia 1964;74:767‐84. [Google Scholar]
- Ziegler E. Report on the large‐scale experiment of adding fluorine to household milk in Winterthur [Bericht über den Winterthurer Großversuch mit Fluorzugabe zur Haushaltmilch]. Helvetica Paediatrica Acta 1964;19:343‐54. [PubMed] [Google Scholar]
References to ongoing studies
Stecksén‐Blicks {published data only}
- Stecksen‐Blicks C. Stecksen‐Blicks trial. Ongoing study.
Svensäter {published data only}
- Svensäter G. Bacterial acid tolerance ‐ a new target for fluoridated milk in caries prevention. Ongoing study.
Additional references
Altman 1997
- Altman DG, Bland JM. Statistics notes. Units of analysis. BMJ 1997;314(7098):1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 1980
- Baker IA, Elwood PC, Hughes J, Jones M, Moore F, Sweetnam PM. A randomised controlled trial of the effect of the provision of free school milk on the growth of children. Journal of Epidemiology and Community Health 1980;34(1):31‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315(7108):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2006
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al. How to formulate research recommendations. BMJ 2006;333(7572):804‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bánóczy 2009
- Bánóczy J, Rugg‐Gunn AJ. Clinical studies. In: Bánóczy J, Petersen PE, Rugg‐Gunn AJ editor(s). Milk fluoridation for the prevention of dental caries. Geneva: World Health Organization, 2009:19‐65. [Google Scholar]
Bánóczy 2013
- Bánóczy J, Rugg‐Gunn A, Woodward M. Milk fluoridation for the prevention of dental caries. Acta Medica Academica 2013;42(2):156‐67. [DOI] [PubMed] [Google Scholar]
Cadogan 1997
- Cadogan J, Eastell R, Jones N, Barker ME. Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ 1997;315(7118):1255‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cagetti 2013
- Cagetti MG, Campus G, Milia E, Lingström P. A systematic review on fluoridated food in caries prevention. Acta Odontologica Scandinavica 2013;71(3‐4):381‐7. [DOI] [PubMed] [Google Scholar]
Calvert 1998
- Calvert NW, Thomas N. The use of fluoridated school milk in the prevention of dental caries. Sheffield: Trent Institute for Health Services Research, Universities of Leicester, Nottingham & Sheffield, 1998. Guidance Note for Purchasers: 98/11. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mariño 2004
- Mariño RJ, Villa AE, Weitz A, Guerrero S. Caries prevalence in a rural Chilean community after cessation of a powdered milk fluoridation program. Journal of Public Health Dentistry 2004;64(2):101‐5. [DOI] [PubMed] [Google Scholar]
Mariño 2011
- Mariño R, Fajardo J, Morgan M. Economic evaluation of dental caries prevention programs using milk and its products as the vehicle for fluorides: cost versus benefits. In: Watson RR, Gerald JK, Preedy VR editor(s). Nutrients, Dietary Supplements, and Nutriceuticals: Cost Analysis Versus Clinical Benefits. New York: Humana Press, 2011:143‐60. [Google Scholar]
National Health and Medical Research Council 2007
- National Health and Medical Research Council. A Systematic Review of the Efficacy and Safety of Fluoridation. A Systematic Review of the Efficacy and Safety of Fluoridation. Canberra: Australian Government, 2007. [Google Scholar]
Petersen 2003
Pitts 2004
- Pitts NB, Stamm JW. International Consensus Workshop on Caries Clinical Trials (ICW‐CCT) — final consensus statements: agreeing where the evidence leads. Journal of Dental Research 2004;84(Special issue C):C125‐8. [DOI] [PubMed] [Google Scholar]
Pitts 2011
- Pitts N, Amaechi B, Niederman R, Acevedo A‐M, Vianna R, Ganss C, et al. Global oral health inequalities: dental caries task group — research agenda. Advances in Dental Research 2011;23(2):211‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schwendicke 2015
Selwitz 2007
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. The Lancet 2007;369(9555):51‐9. [DOI] [PubMed] [Google Scholar]
Smith 1997
- Smith R. Doctors can reduce the harmful effects of poverty. BMJ 1997;314(7082):698. [Google Scholar]
Villa 2009
- Villa AE. The addition of fluoride to milk. In: Bánóczy J, Petersen PE, Rugg‐Gunn AJ editor(s). Milk fluoridation for the prevention of dental caries. Geneva: World Health Organization, 2009:93‐105. [Google Scholar]
Ziegler 1953
- Ziegler E. Milk fluorination in prophylaxis of dental caries [Cariesprophylaxe durch Fluorierung der Milch]. Schweizerische Medizinische Wochenschrift 1953;83(31):723‐4. [PubMed] [Google Scholar]
References to other published versions of this review
Yeung 2002
- Yeung CA, Tickle M. Fluoridated milk for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003876] [DOI] [PubMed] [Google Scholar]
Yeung 2005
- Yeung A, Hitchings JL, Macfarlane TV, Threlfall AG, Tickle M, Glenny AM. Fluoridated milk for preventing dental caries. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003876.pub2] [DOI] [PubMed] [Google Scholar]


