Abstract
Background
Biliary atresia is a life‐threatening disease characterised by progressive destruction of both intra‐ and extra‐hepatic biliary ducts. The mainstay of treatment is Kasai portoenterostomy, as soon as the disease has been confirmed. Glucocorticosteroids are steroid hormones which act on the glucocorticoid receptor and have a range of metabolic and immunomodulatory effects. Glucocorticosteroids are used to improve the postoperative outcomes in infants who have undergone Kasai portoenterostomy.
Objectives
To assess the beneficial and harmful effects of glucocorticosteroid administration versus placebo or no intervention following Kasai portoenterostomy in infants with biliary atresia.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE Ovid, Embase Ovid, Science Citation Index Expanded (Web of Science), and online trial registries (last search: 20 December 2017) for randomised controlled trials.
Selection criteria
We included randomised clinical trials which assessed glucocorticosteroids for infants who have undergone Kasai portoenterostomy. For harm, we also considered quasi‐randomised studies, observational studies, and case‐control studies that were identified amongst the search results.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We assessed the risk of bias for each trial according to prespecified domains. We analysed data using both random‐effects and fixed‐effect models. We performed the analyses using Review Manager 5.3 and Trial Sequental Analysis software. We considered a P value of 0.025 or less, two‐tailed, as statistically significant. We planned to calculate risk ratios (RRs) for dichotomous outcomes, and the mean difference (MD) for continuous outcomes. For all association measures, we planned to use 95% confidence intervals (CIs) as well as Trial Sequential Analysis‐adjusted CIs. We used Trial Sequential Analyisis to control the risks of random errors; however, we were often unable to implement this beyond calculating the required information size as there were few trials and data. We assessed the certainty of the evidence using GRADE.
Main results
We found two randomised controlled trials fulfilling the inclusion criteria of our review. The trials provided data for meta‐analysis. We judged the two trials as trials at low risk of bias. The two trials randomised a total of 213 infants to glucocorticosteroids versus placebo. In our Trial Sequential Analysis, the required information size (that is, the meta‐analytic sample size) was not reached for any outcome. Trials were funded by charities, public organisations, and received support from private sector companies, none of which seemed to have an interest in the outcome of the respective trials. The effect of glucocorticosteroids after Kasai portoenterostomy on all‐cause mortality is uncertain; the confidence interval is consistent with appreciable benefit and harm (RR 1.00; 95% CI 0.14 to 6.90; low‐certainty evidence). The results showed little or no difference in adverse effects between the use of glucocorticosteroids or placebo after Kasai portoenterostomy, however this analysis was based on a single trial and we have low certainty in the result (RR 1.02; 95% CI 0.87 to 1.20;). Available data suggest that the proportions of infants who do not clear their jaundice at six months is similar between the two groups (RR 0.89; 95% CI 0.67 to 1.17; low‐certainty evidence). All‐cause mortality or liver transplantation did not differ at two years between the two groups (RR 1.00; 95% CI 0.72 to 1.39; low‐certainty evidence). There were no data regarding health‐related quality of life.
Our searches also yielded 19 observational studies, some of them containing limited information on harmful effects of glucocorticosteroid treatment. We presented the extracted information narratively. We identified one further ongoing trial with no currently available results.
Authors' conclusions
The two meta‐analysed randomised clinical trials present insufficient evidence to determine the effects of using glucocorticosteroids versus placebo after Kasai portoenterostomy in infants with biliary atresia on any of the primary or secondary review outcomes. There is insufficient evidence to support glucocorticosteroid use in the postoperative management of infants with biliary atresia for long‐term outcomes of all‐cause mortality or liver transplantation. It is also unclear if glucocorticosteroids are able to reduce the numbers of infants who did not clear their jaundice by six months. Further randomised, placebo‐controlled trials are required to be able to determine if glucocorticosteroids may be of benefit in the postoperative management of infants with biliary atresia treated with Kasai portoenterostomy. Such trials need to be conducted as multicentre trials.
Plain language summary
Glucocorticosteroids administered after Kasai surgical procedure for infants with blocked or damaged bile duct
Medications used postoperatively (immediately after surgery) for infants with blocked or damaged bile duct (that is, biliary atresia)
Review question Do medications, called glucocorticosteroids (steroids), have beneficial or harmful effects in the health of infants with biliary atresia operated by the Kasai surgical procedure (that is, portoenterostomy)? We reviewed if there was any difference in death, need for a liver transplant, postoperative jaundice (yellowish or greenish pigmentation of the skin and whites of the eyes), and harmful effects.
Background Biliary atresia is a rare condition that may occur once in 30,000 births. In biliary atresia, the common bile duct is blocked or damaged; as the bile cannot leave the liver, the liver becomes damaged. An operation called 'Kasai portoenterostomy' is used to replace the damaged bile ducts with a piece of the infant's intestine. This allows the bile to drain directly from the small bile ducts at the edge of the infant's liver, straight into the intestine. Medications called glucocorticosteroids have historically been used in the treatment of biliary atresia after surgery. Two benefits of glucocorticosteroids may be that they are anti‐inflammatory, and they increase bile flow. Several studies have been carried out comparing infants taking glucocorticosteroids postoperatively to those who have been given a placebo (an inactive substance that can be made to resemble an active medication or therapy). These studies try to identify if there is any measurable difference in the clearance of jaundice, survival, and need for transplantation. To organise randomised clinical trials large enough to be able to detect differences is, however, challenging.
Study characteristics We performed a search which included studies up to 20 December 2017. We identified two randomised clinical trials (where participants are divided by chance into the trial groups) which met the requirements for our review and followed‐up the participants for at least two years. We identified 19 further observational studies from which we were able to report some findings on harms in a narrative form. The randomised trials included 107 infants who were given glucocorticosteroids and 104 who were given placebo. Trials were funded by charities, public organisations, and received support from private sector companies, all of which did not seem to have any interest in the outcome of the respective trials.
Funding The included trials outlined their sources of funding, and the review authors deemed that there were no conflicts of interest. Review authors did not receive funding to carry out this review.
Key results We did not find any differences between the groups of infants treated with glucocorticosteroids compared with placebo in terms of mortality, adverse events, ability to clear jaundice, or need for a liver transplant.
Quality of the evidence We assessed the two trials as having low risk of bias (we had no concerns that their design and reporting may deviate from the truth), but they were at high risk of imprecision (inexact evaluations of outcomes). They used different categories for adverse events, and we were unable to combine the data from the trials. We could not include enough infants in our analyses (only two published trials) in order to detect small differences between the two intervention groups. The certainty of the evidence was low for mortality, adverse events, ability to clear jaundice, or need for a liver transplant outcomes. One further ongoing trial was identified, with no currently available results.
Future steps We need further randomised clinical trials that compare glucocorticosteroids with placebo in order to find out if glucocorticosteroids are of benefit in the postoperative management of infants with biliary atresia. Such trials need to be conducted at different clinical centres.
Summary of findings
for the main comparison.
| Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy | ||||||
|
Patient or population: infants with biliary atresia Settings: hospitals Intervention: glucocorticosteroids Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Risk Ratio (95% CI) | Number (no) of infants (no of RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Glucocorticosteroids | |||||
|
All‐cause mortality six months after Kasai portoenterostomy |
19 per 1000 | 19 per 1000 (3 to 131) |
1.00 (0.14 to 6.90) |
104 placebo 107 treatment (2 RCTs, Bezerra 2014; Davenport 2007) |
⊕⊕⊝⊝ low1 | |
|
Serious adverse event, two years follow‐up |
800 per 1000 | 814 per 1000 (708 to 977) |
1.02 (0.87 to 1.20) |
70 placebo 70 treatment (1 RCT, Bezerra 2014) |
⊕⊕⊝⊝ low2 | A significantly higher proportion of the treatment group had their first serious adverse event in the first 30 days after their Kasai portoenterostomy. |
| Health‐related quality of life | There are no data for this outcome in the included trials. | |||||
| Infants who did not clear jaundice at six months | 514 per 1000 | 452 per 1000 (303 to 529) |
0.89 (0.67 to 1.17) |
107 placebo 104 treatment (2 RCTs, Bezerra 2014; Davenport 2007) |
⊕⊕⊝⊝ low1 | The required information size for significance for the Trial Sequential Analysis was 540. The number of infants included in this meta‐analysis was 211, corresponding to 39.1% of the required information size. |
| All‐cause mortality or liver transplantation at two years | 402 per 1000 | 404 per 1000 (291 to 562) | 1.00 (0.72 to 1.39) | 107 placebo 104 treatment (2 RCTs, Bezerra 2014; Davenport 2007) |
⊕⊕⊝⊝ low1 | The required information size for significance for the Trial Sequential Analysis was 1774. The number of infants included in this meta‐analysis was 211, corresponding to 11.9% of the required information size. |
| Subgroup analysis of infants operated on at less than 70 days of age who did not clear their jaundice by six months after Kasai portoenterostomy | 516 per 1000 | 381 per 1000 (210 to 423) | 0.75 (0.55 to 1.11) | 64 placebo 63 treatment (2 RCTs, Bezerra 2014; Davenport 2007) |
⊕⊕⊝⊝ low1 | The required information size for significance for the Trial Sequential Analysis was 538. The number of infants included in this meta‐analysis was 127, corresponding to 23.6% of the required information size. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. RCT: randomised clinical trial. | ||||||
GRADE Working Group grades of evidence
| ||||||
1 Downgraded two levels due to imprecision of the evidence: Trial Sequential Analysis determined that the sample size was insufficient to detect a difference between the two groups.
2 Downgraded one level due to imprecision of the evidence and another level due to inconsistency of the evidence: there was heterogeneity between the trials and there were inconsistent assessments of what constituted a significant adverse event. Trial Sequential Analysis determined that the sample size was insufficient to detect a difference between the two groups.
Background
Description of the condition
Biliary atresia is a life‐threatening disease characterised by progressive destruction of both intra‐ and extra‐hepatic biliary ducts, affecting about 1 in 17,000 live births in the UK (McKiernan 2000). Aetiology is not known, although in some cases it may represent a true embryonic defect in bile duct development (Davenport 2006). In others, some studies suggest that it is a perinatal inflammatory process, possibly initiated by viral infection (Narayanaswamy 2007; Zani 2015). Either way, biliary atresia presents during the first few weeks of life with prolonged jaundice, pale stools, and dark urine (Hartley 2009).
The initial management is surgical and involves an attempt to restore biliary drainage and preserve the native liver. The timing of surgery has been shown to be important for the success of the operation, with those being operated on sooner having superior outcomes (Davenport 2006; Davenport 2008). Less than 70 days of life has been used as a cut‐off in the literature (Davenport 2008). The surgery used is Kasai portoenterostomy, which involves a radical resection of the biliary tree to the level of the porta hepatitis, exposing microscopic biliary channels presumed to retain a connection to residual intrahepatic biliary ductules. A Roux loop of the small intestine completes the reconstruction (Kasai 1968). Postoperatively, restored bile flow is variable but defines the success of the procedure; in the UK, about 80% of infants will have a significant degree of bile flow, which in about 50% will completely clear their jaundice by six months (Davenport 2004; Davenport 2011). Failure to re‐establish bile flow, however, results in the need for liver transplantation, typically within the first two years of life.
Glucocorticosteroids have been used as an adjunct to the Kasai procedure since it was first described in 1959. Kasai used glucocorticosteroids in infants who developed cholangitis postoperatively (Kasai 1978), and up until the mid 1980s the role of glucocorticosteroids was limited to this use alone. It was noted that glucocorticosteroids increased the volume of bile produced by the liver (Karrer 1985). This observation led to interest in the role of glucocorticosteroids for all infants undergoing Kasai portoenterostomy. Currently there is variability in the use of glucocorticosteroids after Kasai portoenterostomy. One study in the United States found that 46% of infants received perioperative glucocorticosteroids (Lao 2010), compared to over 90% of infants in Japan (Muraji 2004).
Description of the intervention
Administration of glucocorticosteroids postoperatively to infants who have undergone Kasai portoenterostomy is used by many to improve the postoperative outcomes. Glucocorticosteroids are steroid hormones which act on the glucocorticoid receptor and have a range of metabolic and immunomodulatory effects. The mechanism of their effect in people with biliary atresia is not completely understood, but there is some evidence to support that they have a choleretic effect, aiding bile flow postoperatively (Karrer 1985), and an anti‐inflammatory effect, decreasing inflammation within the liver or at the site of anastomosis (Hill 2015).
A range of steroids either in intravenous or oral administration have been used, including dexamethasone, hydrocortisone, prednisolone, and methylprednisolone. The most commonly used glucocorticosteroid remains prednisolone, and the doses used differ; low‐dose regimens are generally considered to be less than 4 mg/kg, and high‐dose 4 mg/kg or more (Tyraskis 2016).
How the intervention might work
If there is a significant perioperative intrahepatic inflammatory process, then anti‐inflammatory agents such as glucocorticosteroids may counteract this and improve postoperative bile flow. Alternatively, glucocorticosteroids are known to have a choleretic effect, thereby increasing restored bile flow by pharmacological rather than anti‐inflammatory means. In either case, successful restitution of bile flow will limit ongoing liver damage and increase the chances of the infant keeping their own liver.
Why it is important to do this review
Glucocorticosteroids are widely used for infants after Kasai portoenterostomy; however, the available literature is largely that of retrospective case‐series that offer conflicting opinions. We aim to evaluate the current available data from randomised clinical trials to draw conclusions on their benefits and harms.
Objectives
To assess the beneficial and harmful effects of glucocorticosteroid administration versus placebo or no intervention following Kasai portoenterostomy in infants with biliary atresia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials that report outcomes of infants who have undergone Kasai portoenterostomy. For harm, we also considered quasi‐randomised studies, observational studies, and case‐control studies that were identified amongst the search results. By not including all observational studies, we are aware that this review may be biased towards benefits and may overlook harms.
Types of participants
Infants who have undergone the Kasai portoenterostomy for biliary atresia.
Types of interventions
Glucocorticosteroids of any type, at any dose or administration regime, plus standard therapy (which may include ursodeoxycholic acid, antibiotics, and vitamin K). We considered the following comparisons.
Glucocorticosteroids versus placebo or no intervention.
Glucocorticosteroids in addition to standard therapy versus standard therapy alone.
We allowed comparisons of glucocorticosteroids with cointerventions administered equally to allocation groups, e.g. glucocorticosteroids plus supportive therapy versus supportive therapy.
Types of outcome measures
Primary outcomes
All‐cause mortality
Serious adverse events, defined as any untoward medical occurrence that was life threatening, resulted in death, or was persistent or led to significant disability, or any medical event that had jeopardised the patient, or required intervention to prevent it (ICH‐GCP 1997). All other adverse events (that is, any medical occurrence not necessarily having a causal relationship with the treatment, but that did, however, cause a dose reduction or discontinuation of the treatment) were considered as non‐serious.
Health‐related quality of life
Secondary outcomes
The percentage of infants who did not clear their jaundice six months, one year, two years, or at latest follow‐up after the Kasai portoenterostomy
All‐cause mortality or liver transplantation at one year, two years, or at latest follow‐up after the Kasai portoenterostomy
Non‐serious adverse events: for definition, see Primary outcomes
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2017), Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE Ovid, Embase Ovid, and Science Citation Index Expanded (Web of Science) (Royle 2003). See Appendix 1 for search strategies, with the time spans for the searches. The last search was conducted on 20 December 2017.
We also searched online trial registries such as ClinicalTrials.gov (clinicaltrials.gov/), European Medicines Agency (EMA) (www.ema.europa.eu/ema/), and the World Health Organization International Clinical Trial Registry Platform (www.who.int/ictrp), for any ongoing studies.
Searching other resources
We searched the reference lists of relevant identified papers for additional publications of interest.
Data collection and analysis
We performed the review following the recommendations of Cochrane (Higgins 2011), and the Cochrane Hepato‐Biliary Group module (Gluud 2017).
Selection of studies
Two review authors (AT, MD) performed the searches and merged results, deleting duplicates. Titles and abstracts were examined and irrelevant studies removed. We retrieved the full texts of studies which were potentially relevant. We linked together multiple reports of the same study, ensuring there were no duplicate data included. The two review authors independently examined the full‐text reports for compliance with the review's eligibility criteria. We chose to correspond with investigators for clarification on the study prior if needed to determine eligibility.
Data extraction and management
Two review authors (AT, MD) independently extracted data. We extracted relevant data from non‐English language publications with the help of people in command of that language. Where more than one publication of a study existed, we grouped reports together, identifying the primary reference; and we used the data carefully, inspecting them for discrepancies. Any further information required from the original author was requested by written correspondence, and any relevant information obtained in this manner was included in the review. We resolved disagreements by consultation with all review authors.
From each trial, we extracted the following information: first author, country of origin, trial design, inclusion and exclusion criteria, number of infants, infants' characteristics, trial drugs (dose, administration), additional medical therapy, follow‐up period, primary and secondary outcomes, other adverse events, and infants lost to follow‐up or withdrawn from the study.
Assessment of risk of bias in included studies
Where possible, we utilised the 'Risk of bias' assessment tool to assess the risk of bias in included studies (see below). For non‐randomised studies, we made a statement of likely bias and confounding factors and their potential effect on validity. We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the Cochrane Hepato‐Biliary Group module (Gluud 2017). Based on empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017), we assessed the following 'Risk of bias' domains in each trial.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not randomised or only quasi‐randomised. We only used these studies for the assessments of harms and not for benefits.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants. We only used these studies for the assessments of harms and not for benefits.
Blinding of participants and personnel
Low risk of bias: it was mentioned that both participants and personnel providing the interventions were blinded, and the method of blinding was described, so that knowledge of allocation was prevented during the trial.
Unclear risk of bias: it was not mentioned if the trial was blinded, or the trial was described as blinded, but the method or extent of blinding was not described, so that knowledge of allocation was possible during the trial.
High risk of bias: the trial was not blinded, so that the allocation was known during the trial.
Blinded outcome assessment
Low risk of bias: it was mentioned that personnel assessing the outcomes were blinded, and the method of blinding was described, so that knowledge of allocation was prevented during the outcome assessment.
Unclear risk of bias: it was not mentioned if the outcome assessment was blinded, or it was described as blinded, but the method or extent of blinding was not described, so that knowledge of allocation was possible during the outcome assessment.
High risk of bias: the outcome assessment was not blinded, so that the allocation was known during the outcome assessment.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, were employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: if the original trial protocol was available, the outcomes should be those stated in that protocol. If not, then the trial should have reported the following predefined outcomes: all‐cause mortality, serious adverse events, need for liver transplantation at long‐term follow‐up, and clearance of jaundice after Kasai portoenterostomy. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes will not be considered to be reliable.
Unclear risk of bias: not all predefined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined outcomes were not reported.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that may manipulate the trial design, conduct, or analyses of results of the trial.
Unclear risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received another type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other bias factors (e.g. academic bias or authors have conducted trials on the same topic) that could put it at risk of bias.
Unclear risk of bias: the trial may or may not have been free of other factors that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. academic bias or authors have conducted trials on the same topic).
We classified each trial as having low or high risk of bias overall by combining our bias assessments for all domains; we categorised trials as having low risk of bias if none of the domains were classed as high or unclear risk of bias. We considered trials as having high risk of bias if one or more domain was assessed as unclear or high risk of bias.
Measures of treatment effect
Some of the outcome data constituted a comparison of continuous data sets from groups either receiving glucocorticosteroids or not, and data were analysed accordingly. Other outcome data were time‐to‐event data and we planned to analyse them as such.
Dichotomous outcomes
We used risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes.
Continuous outcomes
We used mean differences (MDs) with 95% CIs for continuous outcomes. We used standardised mean differences (SMDs) with 95% CIs for continuous outcomes only if the included studies use different scales for quality of life.
Unit of analysis issues
We identified and documented any unit of analysis issues in the included trials. Specifically, we sought to clarify the methodology of randomisation to the respective treatment arms (i.e. as individuals or groups), identify if infants underwent more than one intervention as part of the trial, or if there were multiple observations for the same outcome. We excluded any trial in which a combination of interventions fell outside of the study design defined in Types of interventions. We also abstained from using multiple observations for a particular outcome when performing the analysis.
Dealing with missing data
We addressed missing data in the following ways.
We contacted the original investigators to request missing data.
We analysed the missing data assuming that data are missing at random.
-
Regarding our primary outcomes, we performed sensitivity analyses to assess how sensitive results are to reasonable changes in the assumptions that are made.
Extreme‐case analysis favouring the experimental intervention ('best‐worse' case scenario): none of the dropouts/participants lost from the experimental group, but all of the dropouts/participants lost from the control group experienced the outcome; we included all randomised participants in the denominator.
Extreme‐case analysis favouring the control ('worst‐best' case scenario): all drop‐outs/participants lost from the experimental group, but none from the control group experienced the outcome; we included all randomised participants in the denominator.
We addressed the potential impact of missing data on the findings of the review in the Discussion.
Assessment of heterogeneity
We analysed heterogeneity using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). We used I² values of 25%, 50%, and 75% as corresponding to low, medium, and high levels of heterogeneity.
Assessment of reporting biases
We aimed to use a funnel plot to explore bias (Egger 1997; Macaskill 2001). Asymmetry in funnel plot of trial size was used to assess this bias. We aimed to perform linear regression to determine the funnel plot asymmetry (Egger 1997). However, due to the paucity of trials such analyses could not be conducted.
Data synthesis
We performed the analyses using Review Manager 5 (RevMan 2014) and Trial Sequential Analysis version 0.9.5.10 Beta (TSA) (Thorlund 2011; TSA 2011).
Meta‐analysis
We analysed the data with a random‐effects model and a fixed‐effect model. In case of significant discrepancy between the two models, we reported both results. If there were statistically significant discrepancies in the results (e.g. one giving a significant intervention effect and the other no significant intervention effect), we reported the more conservative point estimate of the two (Jakobsen 2014). The more conservative point estimate is the estimate closest to zero effect. If the two point estimates were equal, we used the estimate with the widest CI as our main result of the two analyses. We considered a P value of 0.025 or less, two‐tailed, as statistically significant if the required information size was reached due to our three primary outcomes (Jakobsen 2014). We used the eight‐step procedure to assess if the thresholds for significance are crossed (Jakobsen 2014). For dichotomous outcomes, we calculated RRs, and for continuous outcomes the MD. For all association measures, we used 95% CIs and if possible Trial Sequential Analysis‐adjusted CI (see below). We performed the analyses using the intention‐to‐treat principle, including all randomised participants irrespective of completeness of data. Participants with missing data were to be included in the analyses using a carry forward of the last observed response. Accordingly, participants who had been lost to follow‐up were counted as being alive. In our primary analyses, we stratified trials based on the type of control intervention (placebo/no intervention, conventional therapy, or combination of therapies).
Trial Sequential Analysis
We applied Trial Sequential Analysis as cumulative meta‐analyses are at risk of producing random errors due to sparse data and repetitive testing of the accumulating data (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010; Wetterslev 2017). To minimise random errors, we calculated the required sample size (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Wetterslev 2008). The required sample size calculation accounted for the diversity present in the meta‐analysis where possible (Wetterslev 2008; Wetterslev 2009). In our meta‐analysis, the required sample size was based on the event proportion in the control group; assumption of a plausible relative risk reduction of 20% on the relative risk reduction observed in the included trials at low risk of bias; a risk of type I error of 2.5% for both primary and secondary outcomes due to the three outcomes (Jakobsen 2014); a risk of type II error of 10% (power to 90%); and the observed diversity of the meta‐analysis (Thorlund 2011). The underlying assumption of Trial Sequential Analysis is that testing for significance may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication. On the basis of the required sample size, trial sequential monitoring boundaries were constructed (Wetterslev 2008; Thorlund 2011). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size; if the trial sequential monitoring boundary is crossed before the required information size is reached, firm evidence may perhaps have been established and further trials may be superfluous. On the other hand, if the boundaries are not surpassed, it will probably be necessary to continue conducting trials in order to detect or reject a certain intervention effect. That is determined by assessing if the cumulative Z‐curve crosses the trial sequential boundaries for futility. We also used the program to calculate Trial Sequential Analysis‐adjusted CI. The CI demonstrate more clearly the insecurity with which the intervention effect is estimated before reaching the required sample size. Trial Sequential Analysis was performed in the Trial Sequential Analysis software version 0.9.5.10 Beta (Thorlund 2011).
Subgroup analysis and investigation of heterogeneity
We aimed to perform subgroup analyses for:
trials at low risk of bias compared to trials at high risk of bias;
type of drug administration;
age less than 70 days at Kasai portoenterostomy compared to age above 69 days;
glucocorticosteroid drug dosage.
Subgroup analysis was possible in the comparison of those aged less than 70 days compared to those above 69 days for the percentage of infants who did not clear their jaundice by six months after Kasai portoenterostomy. The remaining subgroups were not tested due to lack of data. We aimed to perform a test of interaction to evaluate the differences between the estimates (Altman 2003)
Sensitivity analysis
We aimed to perform sensitivity analyses as deemed necessary after the review process.
Zero‐event trials
If we identified trials with zero events in all intervention groups, we performed additional analyses. It seems unjustified and unreasonable to exclude zero‐event trials (Sweeting 2004) as this may have created a risk of inflating the magnitude of the pooled treatment effects. Therefore, we also aimed to perform a random‐effects meta‐analysis with empirical continuity correction of 0.01 in zero‐event trials using the Trial Sequential Analysis software (Thorlund 2011).
'Summary of findings' tables
We evaluated the quality of the evidence for all outcomes reported in the review using GRADE criteria (community.cochrane.org/tools/review‐production‐tools/gradepro‐gdt). We assessed five factors referring to limitations in the study design and implementation of included studies that suggest the quality of the evidence: risk of bias; indirectness of evidence (population, intervention, control, outcomes); unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of results (wide CIs and as evaluated with our Trial Sequential Analyses) (Jakobsen 2014); and a high probability of publication bias. We defined the quality of evidence as 'high', 'moderate', 'low', or 'very low'. These grades are defined as follows.
High certainty: this research provides a very good indication of the likely effect; the likelihood that the effect will be substantially different is low.
Moderate certainty: this research provides a good indication of the likely effect; the likelihood that the effect will be substantially different is moderate.
Low certainty: this research provides some indication of the likely effect; however, the likelihood that it will be substantially different is high.
Very low certainty: this research does not provide a reliable indication of the likely effect; the likelihood that the effect will be substantially different is very high.
Results
Description of studies
Results of the search
Our searches, conducted up to 20 December 2017, identified a total of 152 records. Of these, 20 were duplicates and 109 were clearly irrelevant. Thus, 23 articles were read in full: one of these 23 articles did not fulfil the inclusion criteria and another one was an ongoing trial without current published data (Zeng 2014). This trial is listed under Characteristics of ongoing studies. The remaining 21 articles were included for the qualitative analysis on adverse events, but 19 of those were excluded from the quantitative analysis. Four of the 19 studies included data on non‐serious adverse events whereas the remaining studies either declared no adverse events or did not comment on adverse events of glucocorticosteroids specifically. We have listed 19 studies in the Characteristics of excluded studies table with reasons for exclusion. We included two randomised clinical trials in our quantitative analysis (Davenport 2007;Bezerra 2014). A PRISMA flow diagram shows the study screening process (Figure 1).
1.
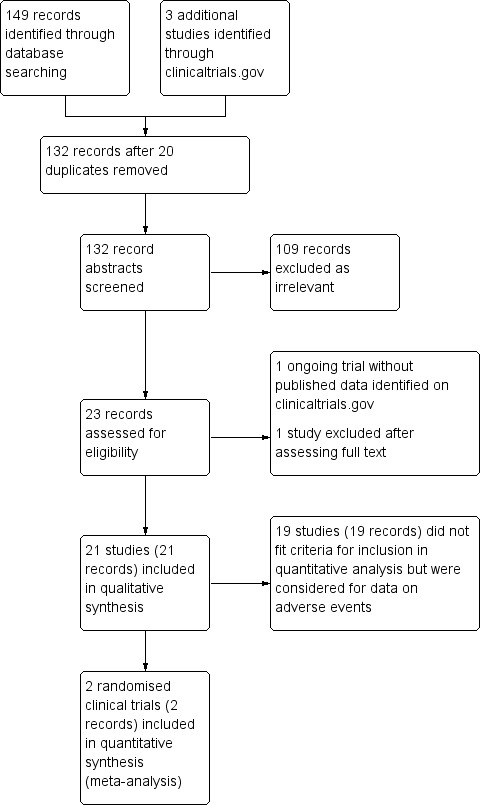
Study flow diagram
Included studies
The Characteristics of included studies table provides the details of the included studies.
The two included trials were randomised, blinded, placebo‐controlled, clinical trials; one of the trials was carried out in two centres in the UK (Davenport 2007), and the other was a multicenter trial carried out in the USA (Bezerra 2014). Both trials compared glucocorticosteroids versus placebo.
Treatment regimens in the two trials differed. In the START trial (Bezerra 2014), intravenous methylprednisolone (4 mg/kg/day) or oral prednisolone (4 mg/kg/day) was used for two weeks from the first day after the Kasai portoenterostomy, followed by oral prednisolone (2 mg/kg/day) for two weeks, followed by a tapering dose for nine weeks. The other trial used oral prednisolone 2 mg/kg for two weeks, starting seven days after the Kasai portoenterostomy, followed by 1 mg/kg/day for seven days (Davenport 2007).
The two trials randomised a total of 213 infants (73 in Davenport 2007, and 140 in Bezerra 2014). Forty‐eight per cent in the latter trial were boys, and the mean age at enrolment was 70 days in both groups (Bezerra 2014). The other trial reported a median age of 60 days in the treatment group and 54 days in the placebo group (Davenport 2007).
One trial excluded infants with biliary atresia and splenic malformation, severe cardiac anomalies, close contact with or clinical evidence of an ongoing infection, macroscopic cirrhosis, and type 1 or 2 biliary atresia (Davenport 2007). The other trial excluded infants older than 180 days, younger than 36 weeks post‐conception, or with weight of less than 2 kg (Bezerra 2014).
Excluded studies
We presented the excluded studies with reasons for exclusion in the Characteristics of excluded studies.
Risk of bias in included studies
We deemed both trials to be at low risk of bias, using the methods outlined in our protocol (Figure 2; Figure 3).
2.
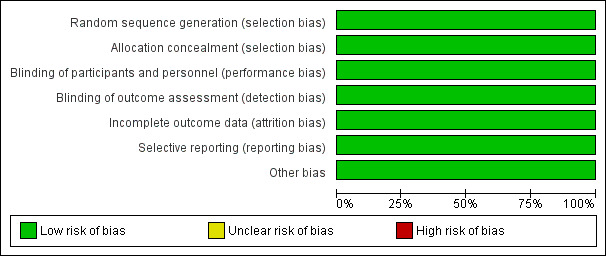
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was performed using methods of equal probability to be allocated to either group in both included trials. In Bezerra 2014, the co‐ordinating centre generated treatment randomisation codes with permutated block sizes of four (stratified by site). When we contacted the investigators of Davenport 2007, they explained that random sequence generation was performed by a third party in the pharmacy of the respective sites. Thus, we deemed the study to have low risk of bias.
We deemed allocation concealment to be at low risk of bias in both trials.
Blinding
Both trials were placebo blinded. After being contacted, the investigators of both studies confirmed that outcome assessors were blinded at the time of analysis (Bezerra 2014; Davenport 2007).
Incomplete outcome data
In Davenport 2007, attrition was two out of 73 infants and was not sufficient to conceivably affect outcomes. Twelve out of 140 infants withdrew or were lost to follow‐up in Bezerra 2014; however, their data were still included in the primary analysis, and they used imputation for long‐term follow‐up data.
Selective reporting
The registered trial protocol was available for Bezerra 2014; this study reported the predefined outcomes. Outcomes have been reported in multiple publications; however, only the one included had data relevant to our review. The trial protocol was made available after we contacted the authors of Davenport 2007. This was registered with the local research and development authority as well as the ethics authority prior to commencement of the trial, and all stated outcomes were reported on.
Other potential sources of bias
For‐profit bias
Both trials outlined their sources of funding, and we deemed that there were no conflicts of interest or potential for profit for the researchers, and so deemed the studies to be at low risk of for‐profit bias. One study, Davenport 2007, was supported by the Children's Liver Disease Foundation (UK) as well as the Wellchild trust (UK) which are both registered charities. The other study, Bezerra 2014, was supported by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) which is a part of the US National Institutes of Health. Several private companies gave support in the form of formula or medication to Bezerra 2014; however, their support had no economic or other interest in the outcome of the study (GlaxoSmithKline supplied ranitidine, Axcan Parma US supplied fat‐soluble vitamins, tocopherol polyethylene glycol succinate and ursodiol, and Mead Johnson Nutrition supplied Pregestimil).
We identified no other sources of bias in the two included trials.
Effects of interventions
See: Table 1
Comparisons
For all outcomes below, and depending on the availability of data glucocorticosteroids were compared to placebo for infants with biliary atresia following Kasai portoenterostomy.
Primary outcomes
All‐cause mortality
The START trial reported all‐cause mortality at the time point of six months after the Kasai portoenterostomy (Bezerra 2014). There were two deaths in each of the treatment and placebo groups, yielding a risk ratio (RR) of 1.00 (95% confidence interval (CI) 0.14 to 6.90; participants = 140). There was no difference between random‐ and fixed‐effects models. There was no mortality in the UK trial at six months in either treatment group (Analysis 1.1). Given that only one trial had any events and no difference between the two groups, we did not perform Trial Sequential Analysis due to too few trials and events.
1.1. Analysis.
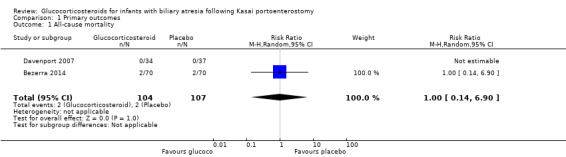
Comparison 1 Primary outcomes, Outcome 1 All‐cause mortality.
Serious adverse events
In Davenport 2007, there were no serious adverse events to report. The START trial used a broader definition of serious adverse events and their data included many non‐life‐threatening adverse events (Bezerra 2014). It was not possible to differentiate between those which were serious or not according to our definition. Nevertheless, we have provided the following discussion of the available data from the START trial. They found similar incidence in the two intervention groups: 57/70 infants (81.4%) in the glucocorticosteroid treatment group suffered a serious adverse event compared with 56/70 infants (80.0%) in the placebo group, yielding a RR of 1.02 (95% CI 0.87 to 1.20, Analysis 1.2), with no difference between random‐ and fixed‐effects models (Bezerra 2014). The study authors found that a significantly higher proportion of infants in the treatment group had their first serious adverse event during the first 30 days after the Kasai portoenterostomy: 37.2%, compared with 19.0% of the infants in the placebo group. We did not perform Trial Sequential Analysis as we were not able to extract serious adverse events according to our original definition for analysis.
1.2. Analysis.
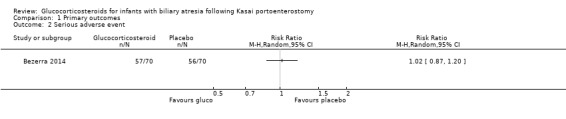
Comparison 1 Primary outcomes, Outcome 2 Serious adverse event.
The randomised data showed that there was no difference in the time to occurrence of cholangitis between the treatment and placebo groups, and that there were similar proportions of infants surviving with their native livers without cholangitis episodes at 24 months (Bezerra 2014; exact values not stated).
Health‐related quality of life
Long‐term quality of life outcomes were not systematically investigated by any trial or other studies included in the qualitative analysis; however, several studies commented on outcomes which are directly relevant to health‐related quality of life. One randomised clinical trial mentioned that there was a non‐significant difference in the proportion of infants who had an inadequate response to routine childhood immunisations: 51.5% of the infants in the treatment group compared to 38.5% of the infants in the placebo group (reported P value = 0.43; Bezerra 2014). One further study followed up growth by assessing height and weight centiles for two years after surgery and found that a similar proportion of trial participants in steroid and no‐steroid groups were above the 25th centile, which they defined as a 'good outcome' (Escobar 2006).
Secondary outcomes
The percentage of infants who did not clear their jaundice six months, one year, two years, or at latest follow‐up after Kasai‐portoenterostomy
Six months was the only time point that had data reported from both trials included in our quantitative analysis. We found that there was no difference between the glucocorticosteroid group and the placebo group in the proportion of infants who did not clear their jaundice at six months (RR of 0.88 using a fixed‐effect model and 0.89 in random‐effects model, 95% CI 0.67 to 1.17; I2 = 0; participants = 211; studies = 2; Analysis 2.1; Bezerra 2014; Davenport 2007).
2.1. Analysis.
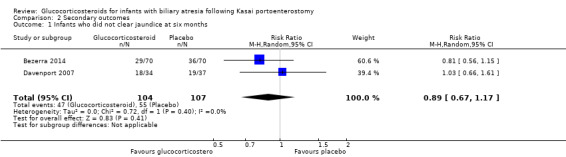
Comparison 2 Secondary outcomes, Outcome 1 Infants who did not clear jaundice at six months.
We performed Trial Sequential Analysis based on 51.4% of infants who did not clear their jaundice at six months in the placebo group; a relative risk reduction of 20%; a risk of type I error of 2.5%; and a power of 90%. There was no diversity adjustment (D2 = 0). The resulting required sample size was 1168 participants (Figure 4). The number of infants included in this meta‐analysis was 211, corresponding to 18.1% of the required sample size. The relative risk and Trial Sequential Analysis‐adjusted CI were 0.89 (0.28 to 2.78).
4.
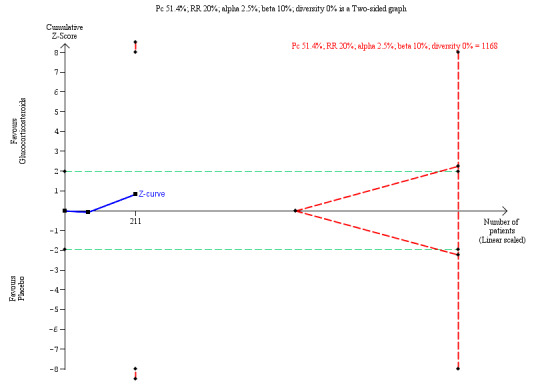
Trial Sequential Analysis of no clearance of jaundice by six months post Kasai portoenterostomy
All‐cause mortality or liver transplantation at one year, two years, or at latest follow‐up after Kasai‐portoenterostomy
The latest common follow‐up of the two included trials was two years. We found that there was no difference in the proportion of infants who either required transplantation or died at two years (RR 1.00 in both random‐effects and fixed‐effect models, 95% CI 0.72 to 1.39; I2 = 0; participants = 211; studies = 2; Analysis 2.2).
2.2. Analysis.
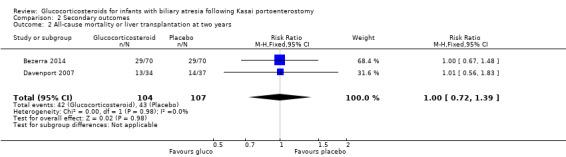
Comparison 2 Secondary outcomes, Outcome 2 All‐cause mortality or liver transplantation at two years.
We performed Trial Sequential Analysis based on 40.2% of infants requiring a liver transplant or dying in the placebo group; a relative risk reduction of 20%; a risk of type I error of 2.5%; and a power of 90%. There was no diversity adjustment (D2 = 0). The resulting required sample size was 1774 participants. The number of infants included in this meta‐analysis was 211, corresponding to 11.9% of the required sample size (information size) (Figure 5). The relative risk and Trial Sequential Analysis‐adjusted CI were 1.00 (0.26 to 3.83).
5.
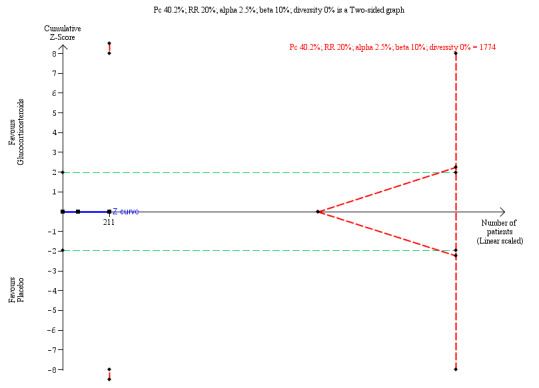
Trial Sequentil Analysis for all‐cause mortality or liver transplantation at two years
Non‐serious adverse events
For definition, see Primary outcomes.
The joint serious and non‐serious adverse events from the START trial are reported in the primary outcomes. No infants were noted to have hyperglycaemia and no fractures were seen in the two groups in the UK trial (Davenport 2007), and in the START trial 'orthopedic' adverse events were noted in three infants in the glucocorticosteroid group and two in the placebo group (Bezerra 2014).
From the 19 observational studies which we included for the qualitative analysis, there was limited information on adverse events. The majority of the studies did not have a comparison group, and the association of an adverse event with the treatment is not clear. Nevertheless, several further adverse events were noted. In Meyers 2003, which did have a 'standard therapy' control group, it was noted that beyond fluid retention and increased appetite, there were no other differences in the incidence of adverse events. Another study did clearly outline complications observed without identifying any complication that was particularly higher in the control group (Escobar 2006). Two studies which compared two different steroid dose groups found no significant differences between groups (Foroutan 2007; Japanese Biliary Atresia Society 2013).
Subgroup analysis of infants operated on at age of less than 70 days who did not clear their jaundice by six months after the Kasai portoenterostomy
Data were available from both eligible trials on the proportion of infants who did not clear their jaundice by six months in the subgroup of infants who were less than 70 days old at the time of Kasai portoenterostomy; and data on those more than 69 days old at time of Kasai portoenterostomy was deduced from the difference of the total group and those who were less than 70 days old. In the group who underwent Kasai portoenterostomy at less than 70 days of life, 38% did not clear their jaundice in the glucocorticosteroid group, compared to 52% in the placebo group (RR 0.77, 95% CI 0.46 to 1.29; I2 = 43%; participants = 127; studies = 2; Analysis 2.4). This compares to those operated on at more than 69 days of life, in which 56% did not clear their jaundice in the glucocorticosteroid group and 51% did not in the placebo group (RR 1.09, 95% CI 0.74 to 1.62, I2 = 0%; participants = 84; studies = 2; Analysis 2.5). Results of random‐effects model are reported in both groups (less than 70 days old and more than 69 days old).
2.4. Analysis.
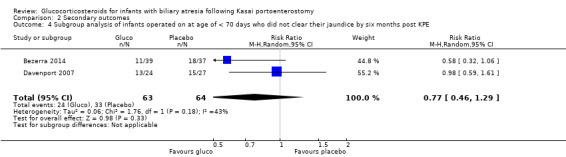
Comparison 2 Secondary outcomes, Outcome 4 Subgroup analysis of infants operated on at age of < 70 days who did not clear their jaundice by six months post KPE.
2.5. Analysis.
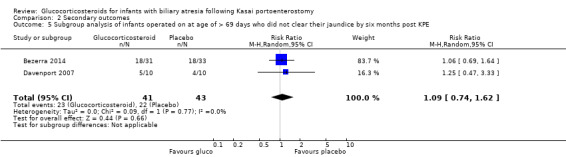
Comparison 2 Secondary outcomes, Outcome 5 Subgroup analysis of infants operated on at age of > 69 days who did not clear their jaundice by six months post KPE.
We performed Trial Sequential Analysis based on 51.4% of infants not clearing their jaundice by six months in the group that was less than 70 days old and who had taken placebo; a relative risk reduction of 20%; a risk of type I error of 2.5%; and a power of 90%. There was diversity adjustment (D2 = 45%) due to heterogeneity (Figure 6). The resulting required information size was 2108 participants. The number of infants included in this meta‐analysis was 127, corresponding to 6.0% of the information size. The relative risk and Trial Sequential Analysis‐adjusted CI were 0.77 (0.09 to 6.34).
6.
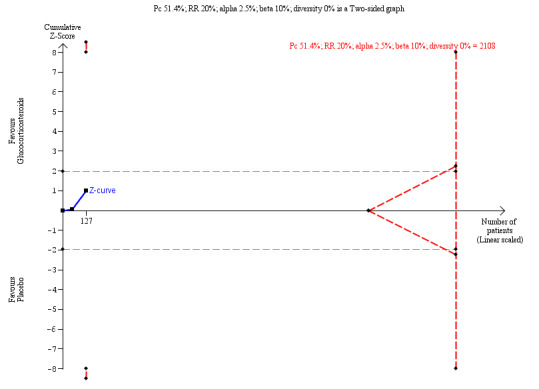
Trial Sequential Analysis of no clearance of jaundice at six months in the subgroup of infants who were less than 70 days old at the time of Kasai portoenterostomy
Certainty of the evidence
We found that the certainty of the evidence was low for all outcomes. We downgraded the certainty of the evidence by two levels for imprecision for all outcomes except adverse events. For adverse events, we downgraded one level for imprecision, and there was also inconsistency due to different definitions of what constitutes an adverse event between the two trials.
Discussion
Summary of main results
Our meta‐analysis did not identify any significant differences in our primary or secondary outcomes for infants with biliary atresia treated with glucocorticosteroids versus placebo post receiving the Kasai portoenterostomy. For the outcomes of all‐cause mortality (RR = 1.00), adverse events (RR = 1.02), or all‐cause mortality or liver transplantation (RR = 1.00), the evidence suggested that outcomes were equal between both groups. However, for infants who did not clear their jaundice by six months, the RR was lower (favouring glucocorticosteroids) in the subgroup of infants who were under 70 days old at the time of Kasai‐portoenterostomy (RR = 0.77), whereas for the whole group, RR was 0.88; neither comparison was statistically significant Absence of evidence should not be interpreted as absence of effects and we observe low certainty in our estimates.
Overall completeness and applicability of evidence
The rarity of biliary atresia makes the design and execution of large randomised clinical trial particularly difficult. For this reason, it is not surprising that the Tiral Sequential Analysis we performed suggested that far higher test groups sizes would be required to definitively answer whether a significant difference exists between the two groups. Furthoremore, the two trials reported adverse events in a different manner, which did not allow for a direct comparison. For this important outcome, further studies are needed to confirm if there is indeed no overall difference between the two groups, and to replicate the finding that adverse events occur earlier in those infants taking glucocorticosteroids.
The current evidence does not support that any difference in the two groups for their ability to clear jaundice by six months that this translates to a difference in the mortality and transplant rates in these infants. However, the duration of follow‐up of the data included in this trial was only two years, which is arguably too short for an intervention which — when successful — aims at lasting a lifetime. Overall, further trials are needed to determine if glucocorticosteroids do have any significant effect on our outcomes.
We found two further prospective studies which compared glucocorticosteroid treatment to a non‐steroid control; they found non‐significant differences in all‐cause mortality (Petersen 2008;Davenport 2013), but they did not meet the inclusion criteria for our meta‐analysis. One study reported all‐cause mortality at two years follow‐up: 3/20 infants (15%) in the group who received glucocorticosteroid with conventional therapy (fat soluble vitamins, antibiotics, ursodeoxycholic acid) died, compared to 2/29 infants (7%) in the control group who received conventional therapy alone (Petersen 2008). These mortality rates were higher than other larger prospective studies, and no clear justification for this was provided. Another study which compared no steroids (91 infants) to low‐dose glucocorticosteroids (18 infants) and high‐dose glucocorticosteroids (44 infants) found all‐cause mortality rates of 4%, 6%, and 5% respectively, at four years after Kasai portoenterostomy (Davenport 2013).
Cholangitis, which frequently occurs after Kasai portoenterostomy, is the most often reported serious adverse event in the literature, and data from both randomised clinical trials and non‐randomised studies do not show there being any increased incidence in infants treated with glucocorticosteroids.
Health‐related quality of life was also assessed from the studies included in the qualitative analysis. From non‐randomised data, there were various comments, among which a study noted 'no infant has had significant growth retardation' in a 3.8 year follow‐up of 28 infants who received either glucocorticosteroid or 'standard therapy' (Meyers 2003). Seven infants, who all received glucocorticosteroids after their Kasai portoenterostomy and were part of a 71 patient study, had 'routine psychometric testing' at primary‐school age and were found to have 'full‐scale IQ composite scores in the normal rage' (Stringer 2007). One study which performed a limited retrospective analysis of a database of 43 children's hospitals found that infants treated with glucocorticosteroids after their Kasai portoenterostomy had an average length of stay of 9.7 days which was 3.5‐day shorter length of stay (95% confidence interval (CI) 0.03 to 6.97; Lao 2010).
Our ability to gather data on quality of life was mainly limited by the length of follow‐up for the majority of studies and was never a predefined outcome in any study.
Quality of the evidence
Data included in this meta‐analysis were from only two randomised clinical trials comparing glucocorticosteroids versus placebo in infants with biliary atresia. Both of these trials were at low risk of bias and their outcomes did not contradict each other. The certainty of the evidence was low for all of the outcomes according to GRADE criteria. We downgraded our assessments of the quality of evidence for all outcomes due to imprecision as there were small sample sizes. We also downgraded our assessment of the quality of evidence on adverse events due to heterogeneity caused by the studies having different definitions of adverse events. There were no data from the included trials on health‐related quality of life.
Potential biases in the review process
No potential biases were identified in the review process.
Agreements and disagreements with other studies or reviews
A recent meta‐analysis with less strict entry criteria, which included non‐randomised studies, found a significant difference in the clearance of jaundice at six months for the subgroup of infants operated on at age less than 70 days (Chen 2015). In their analysis, 97/160 (61%) of infants with glucocorticosteroids had clearance of jaundice at six months versus 99/198 (50%) of infants who were not given glucocorticosteroids. They calculated the odds ratio for clearing jaundice at six months to be 1.59 (95% CI 1.03 to 2.45, P = 0.04), in infants treated with high‐dose steroids when aged less than 70 days at surgery. Their study introduces a higher degree of bias than ours due to also including studies which were not randomised and not placebo controlled.
Dosage is thought to impact the success of glucocorticosteroids as a treatment. The two trials we included in this meta‐analysis used different doses and regimens of glucocorticosteroids. In Davenport 2007, a regimen was used which is currently considered 'low' dose; and evidence since its publication suggests that it is less effective than the higher dose glucocorticosteroids used by Bezerra 2014. The Japanese Biliary Atresia Society conducted a randomised trial which compared two different dose regimens, one "high" dose starting at 4 mg/kg/day of prednisolone and the other "low" dose starting with 2 mg/kg/day, but without a placebo control. They found that the higher‐dose steroids correlated with a lower bilirubin level (37 μmol/L versus 58 μmol/L, P = 0.03) at two months after the operation. However, they did not report longer‐term follow‐up results (Japanese Biliary Atresia Society 2013). This difference in biochemical markers is also noted in Davenport 2013, which prospectively compared high‐dose steroids (5 mg/kg/day of prednisolone) to a low‐dose group (2 mg/kg/day) and the placebo group from their previous randomised trial and contemporaneous controls. They also found that the bilirubin levels were significantly lower in the high‐dose group when compared to the placebo/control group (58 μmol/L compared to 91 μmol/L, P = 0.002). Furthermore, they found that aspartate aminotransferase (AST) was lower in the high‐dose steroid group (118 IU/L, compared to 155 IU/L, P = 0.002), and the AST‐to‐platelet ratio index was also lower in the high‐dose steroid group (0.49 compared to 0.82, P = 0.005). The steroid effect was more pronounced in younger infants (aged less than 70 days at the point of Kasai portoenterostomy), with a reduced bilirubin level at one month (64 μmol/L compared to 117 μmol/L, P = 0.01).
There is increasing evidence that the age at which the Kasai portoenterostomy is performed can have an effect on the clearance of jaundice at six months. Our study showed that the difference was greater in those infants aged less than 70 days. However, the difference was not enough to be statistically significant. As previously mentioned, one meta‐analysis found that in the subgroup of those aged less than 70 days at the Kasai portoenterostomy, there was a significant difference between their treatment and control groups (Chen 2015).
In addition to this, one study reported that within the subgroup of infants who did receive high‐dose steroids, there was a significant difference in the clearance of jaundice in an age cohort analysis: 100% of 11 infants operated on at less than 30 days of age cleared their jaundice, compared to 66% of those operated on between 61 and 70 days of age, P = 0.05 (Tyraskis 2016). Furthermore, they found that there was a significant native liver survival benefit for those operated on at less than 45 days of age (five‐year NLS estimate 69% compared to 46%, P = 0.05). This finding is the first of its kind to show a long‐term clinical benefit, however, there was no control group who was operated on at less than 45 days of age in order to compare the degree of the potential benefit from the younger age compared to the potential benefit from the high‐dose steroids.
Authors' conclusions
Implications for practice.
The two randomised clinical trials included in our meta‐analysis present insufficient evidence to determine the effects of using glucocorticosteroids versus placebo after Kasai portoenterostomy in infants with biliary atresia, on any of the primary or secondary review outcomes. There is insufficient evidence to support glucocorticosteroid use in the postoperative management of infants with biliary atresia for long‐term outcomes of all‐cause mortality or liver transplantation. It is also unclear if glucocorticosteroids are able to lower the numbers of infants who do not clear their jaundice by six months.
Implications for research.
Further randomised, placebo‐controlled trials are required to be able to determine if glucocorticosteroids may be of benefit in the postoperative management of infants with biliary atresia. Such trials need to be conducted as multicentre trials or even multinational trials. Moreover, they need to be designed according to the SPIRIT guidelines (SPIRIT 2013a; SPIRIT 2013b), and reported according to the CONSORT guidelines (www.consort‐statement.org).
Acknowledgements
Peer reviewers: A Floreani, Italy; Masato Shinkai, Japan. Contact editor: Luit Penninga, Denmark. Sign‐off editor: Christian Gluud, Denmark.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: The views and opinions expressed in this protocol are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | December 2017 | (glucocortico* or steroid* dexamethason* or prednison* or hydrocortison* or corticosteroid* cortiso* or budesonid* or beclomethason* or (adrenal next cortex or hormone*)) and ((biliary or bile duct) and atresia) and (portoenterostom* or kasai) and (infant* or pediatr* or child* or bab* or newborn*) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | Issue 12, 2017 | #1 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees #2 glucocortico* or steroid* dexamethason* or prednison* or hydrocortison* or corticosteroid* cortiso* or budesonid* or beclomethason* or (adrenal next cortex or hormone*) #3 #1 or #2 #4 MeSH descriptor: [Biliary Atresia] explode all trees #5 (biliary or bile duct) and atresia #6 #4 or #5 #7 MeSH descriptor: [Portoenterostomy, Hepatic] explode all trees #8 portoenterostom* or kasai #9 #7 or #8 #10 MeSH descriptor: [Infant] explode all trees #11 MeSH descriptor: [Child] explode all trees #12 MeSH descriptor: [Pediatrics] explode all trees #13 infant* or pediatr* or child* or bab* or newborn* #14 #10 or #11 or #12 or #13 #15 #3 and #6 and #9 and #14 |
| MEDLINE Ovid | 1950 to December 2017 | 1. exp Adrenal Cortex Hormones/ 2. (glucocortico* or steroid* dexamethason* or prednison* or hydrocortison* or corticosteroid* cortiso* or budesonid* or beclomethason* or (adrenal next cortex or hormone*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 3. 1 or 2 4. exp Biliary Atresia/ 5. ((biliary or bile duct) and atresia).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 6. 4 or 5 7. exp Portoenterostomy, Hepatic/ 8. (portoenterostom* or kasai).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 9. 7 or 8 10. exp Infant/ 11. exp Child/ 12. exp Pediatrics/ 13. (infant* or pediatr* or child* or bab* or newborn).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 14. 10 or 11 or 12 or 13 15. 3 and 6 and 9 and 14 |
| Embase Ovid | 1980 to December 2017 | 1. exp corticosteroid/ 2. (glucocortico* or steroid* dexamethason* or prednison* or hydrocortison* or corticosteroid* cortiso* or budesonid* or beclomethason* or (adrenal next cortex or hormone*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp bile duct atresia/ 5. ((biliary or bile duct*) and atresia).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. exp portoenterostomy/ 8. (portoenterostom* or kasai).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 7 or 8 10. exp child/ 11. exp pediatrics/ 12. (infant* or pediatr* or child* or bab* or newborn).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 13. 10 or 11 or 12 14. 3 and 6 and 9 and 13 |
| Science Citation Index Expanded (Web of Science) | 1900 to December 2017 | #5 #4 AND #3 AND #2 AND #1 #4 TS=(infant* or pediatr* or child* or bab* or newborn) #3 TS=(portoenterostom* or kasai) #2 TS=((biliary or bile duct) and atresia) #1 TS=(glucocortico* or steroid* dexamethason* or prednison* or hydrocortison* or corticosteroid* cortiso* or budesonid* or beclomethason* or (adrenal next cortex or hormone*)) |
Data and analyses
Comparison 1. Primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 6.90] |
| 2 Serious adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infants who did not clear jaundice at six months | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.67, 1.17] |
| 2 All‐cause mortality or liver transplantation at two years | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 3 Non‐serious adverse events | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Subgroup analysis of infants operated on at age of < 70 days who did not clear their jaundice by six months post KPE | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.46, 1.29] |
| 5 Subgroup analysis of infants operated on at age of > 69 days who did not clear their jaundice by six months post KPE | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.74, 1.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bezerra 2014.
| Methods | Multicentre, double‐blind trial comparing glucocorticosteroid administration versus placebo | |
| Participants | 140 infants (70 in the treatment group and 70 in the placebo group) from multiple US centres Age in months at surgery, mean (standard deviation) Treatment: 2.3 (0.9) Placebo: 2.3 (0.8) Percentage of infants with biliary atresia splenic malformation syndrome Treatment: 3% Placebo: 4% Bilirubin, mean (standard deviation (SD)) [original units] Treatment: 128µmol/L (44) [7.5 mg/dl (2.6)] Placebo: 135µmol/L (48) [7.9 mg/dl (2.8)] Other biochemical indicators of liver injury and synthetic function were balanced between the 2 groups (exact values not stated). |
|
| Interventions | Starting the first day after Kasai portoenterostomy, trial participants received either intravenous methylprednisolone (4 mg/kg/day) or oral prednisolone (4 mg/kg/day) for the first 2 weeks, then oral prednisolone (2 mg/kg/day) for 2 weeks, followed by a tapering protocol for 9 weeks (n = 70) or placebo (n = 70) initiated within 72 hours of Kasai portoenterostomy. | |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Subgroup of infants operated on at age of less than 70 days was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomized with equal probability to a 13‐week course of steroid therapy or matching placebo... The data coordinating center generated treatment randomization codes with permutated block sizes of 4 (stratified by site) and provided the central pharmacy with a list of assignments for each study site." |
| Allocation concealment (selection bias) | Low risk | Quote: "participants were randomized with equal probability to a 13‐week course of steroid therapy or matching placebo." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study medications were labelled and put into a kit by the central pharmacy and distributed to study site research pharmacists who were instructed to dispense the kits to participants enrolled sequentially." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the authors confirmed that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Overall 5 infants were lost to follow‐up in the treatment group (2 withdrew and 3 were lost for other reasons) and 8 in the placebo group (4 withdrew and 4 were lost for other reasons). Imputation was used to account for missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: authors reported on all outcomes in accordance with their methods, and a study protocol was available. Some of the outcomes from the study protocol were reported in other publications but these were not of interest to our review. |
| Other bias | Low risk | None identified |
Davenport 2007.
| Methods | Double‐blind trial comparing glucocorticosteroid administration versus placebo, in two UK centres | |
| Participants | 73 infants from 2 UK centres 34 male, 39 female infants were included. Age in days at surgery, median (interquartile range) Treatment: 60 (50 to 71) Placebo: 54 (45 to 70) Preoperative bilirubin (μmol/L), median (interquartile range (IQR)) Treatment: 132 (112 to 166) Placebo: 158 (125 to 183) Preoperative AST (IU/L), median (IQR) Treatment: 163 (118 to 202) Placebo: 54 (99 to 220) Preoperative ΓGT (IU/L), median (IQR) Treatment: 420 (275 to 898) Placebo: 54 (304 to 992) |
|
| Interventions | Participants received either oral prednisolone 2 mg/kg/day on days 7 to 21 following Kasai portoenterostomy, then 1 mg/kg/day on days 22 to 28 following Kasai portoenterostomy (n = 34) or placebo (n = 37). | |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Two‐year native liver survival was reported in a graph and the exact values were confirmed with the author. Subgroup of infants who were operated on by day 70 of life was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participating infants were randomized to receive either oral prednisolone or placebo" Comment: sequence generation was performed by a centralised agency within the pharmacy and was performed independently of the study investigators. |
| Allocation concealment (selection bias) | Low risk | Comment: medications or placebo were prepared and concealed but the respective pharmacies of the hospitals included in the study. Investigators were unable to identify if glucocorticosteroid or placebo was being given to any particular patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blinded" Comment: methodology was clarified upon contacting author and investigators and clinical personnel were blinded as the administered medication was not identifiable as placebo or glucocorticosteroids by clinical staff or investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the authors confirmed that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no significant incomplete reporting was identified. Two infants excluded from the trial due to the finding that they had factors which excluded them from eligibility after already being enrolled. |
| Selective reporting (reporting bias) | Low risk | Comment: all predefined outcomes from the protocol were reported on. |
| Other bias | Low risk | None identified |
AST: aspartate aminotransferase IU/L: international units per litre ΓGT: γ‐glutamyl‐transpeptidase
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alberti 2011 | Non‐randomised trial comparing groups of different glucocorticosteroid doses |
| Chen 2015 | Meta‐analysis with no data from any new infants |
| Chung 2008 | Non‐randomised trial |
| Davenport 2013 | Non‐randomised trial |
| DeRusso 2003 | Review article |
| Dong 2013 | Non‐randomised trial comparing groups of different glucocorticosteroid doses |
| Escobar 2006 | Non‐randomised trial |
| Foroutan 2007 | Non‐randomised trial comparing groups of different glucocorticosteroid doses |
| Japanese Biliary Atresia Society 2013 | Randomised trial comparing groups of different glucocorticosteroid doses with no placebo control group |
| Kobayashi 2005 | Non‐randomised trial |
| Lao 2010 | Non‐randomised trial |
| Meyers 2003 | Non‐randomised trial |
| Petersen 2008 | Non‐randomised trial |
| Shimadera 2007 | Non‐randomised trial |
| Shneider 2012 | Groups not separated into glucocorticosteroid and placebo but were pooled together for outcome reporting. |
| Shneider 2016 | Groups not separated into glucocorticosteroid and placebo but were pooled together for outcome reporting. |
| Sokol 2007 | Review article |
| Stringer 2007 | Non‐randomised trial |
| Tyraskis 2016 | Review article with some prospective data |
Characteristics of ongoing studies [ordered by study ID]
Zeng 2014.
| Trial name or title | The effect of adjuvant steroid therapy post‐Kasai portoenterostomy in biliary atresia |
| Methods | Single‐centre open label randomised parallel controlled trial |
| Participants | Aims to recruit 100 infants in each group (glucocorticosteroid and control) |
| Interventions | Methylprednisolone, unspecified dose, duration or weaning regimen |
| Outcomes | Study ongoing, none reported |
| Starting date | 1 January 2015 |
| Contact information | Clinical lead: Sah Zheng Address: 399 Wanyuan Rd, Shanghai, China, 201102 |
| Notes |
Differences between protocol and review
The protocol part of this review is a major update. The original protocol was first published in 2010 (Parsons 2010). The changes have been made to focus on the study outcomes as well as the methods of executing the study and analysing the data. Specifically, the primary and secondary outcomes have been rearranged with a focus on harm. Furthermore, we included significantly more detail regarding the statistical analysis and the methodology of Trial Sequential Analysis. New lead review protocol author.
Contributions of authors
AT prepared the updated protocol part of the review and the review. PC prepared the updated protocol part of the review and the review. MD prepared the updated protocol part of the review and the review. All authors approved the final review version.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Athanasios Tyraskis has nothing to declare. Christopher Parsons has nothing to declare. Mark Davenport is the author of a randomised controlled trial on the use of prednisolone after the Kasai portoenterostomy (Davenport 2007).
New
References
References to studies included in this review
Bezerra 2014 {published and unpublished data}
- Bezerra JA, Spino C, Magee JC, Shneider BL, Rosenthal P, Wang KS, et al. Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA 2014;311(7):1750‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davenport 2007 {published and unpublished data}
- Davenport M, Stringer MD, Tizzard SA, McClean P, Mieli‐Vergani G, Hadzic N. Randomized, double‐blind, placebo‐controlled trial of corticosteroids after Kasai portoenterostomy for biliary atresia. Hepatology (Baltimore, Md.) 2007;46(6):1821‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alberti 2011 {published data only}
- Alberti D, Colusso M, Cheli M, Stroppa P, Bravi M, Casotti V, et al. Efficacy of high versus low dose adjuvant corticosteroid treatment in children with biliary atresia: a single‐centre, controlled study. Journal of Pediatric Gastroenterology and Nutrition 2011;52(Suppl 1):54. [Google Scholar]
Chen 2015 {published data only}
- Chen Y, Nah SA, Chiang L, Krishnaswamy G, Low Y. Postoperative steroid therapy for biliary atresia: systematic review and meta‐analysis. Journal of Pediatric Surgery 2015;50(9):1590‐4. [DOI] [PubMed] [Google Scholar]
Chung 2008 {published data only}
- Chung HY, Kak Yuen Wong K, Cheun Leung Lan L, Kwong Hang Tam P. Evaluation of a standardized protocol in the use of steroids after Kasai operation. Pediatric Surgery International 2008;24(9):1001‐4. [DOI] [PubMed] [Google Scholar]
Davenport 2013 {published data only}
- Davenport M, Parsons C, Tizzard S, Hadzic N. Steroids in biliary atresia: single surgeon, single centre, prospective study. Journal of Hepatology 2013;59(5):1054‐8. [DOI] [PubMed] [Google Scholar]
DeRusso 2003 {published data only}
- DeRusso PA. Adjuvant medical therapies after portoenterostomy for biliary atresia: time for a randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2003;35(5):634‐5. [DOI] [PubMed] [Google Scholar]
Dong 2013 {published data only}
- Dong R, Song Z, Chen G, Zheng S, Xiao XM. Improved outcome of biliary atresia with postoperative high‐dose steroid. Gastroenterology Research and Practice 2013;2013:902431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Escobar 2006 {published data only}
- Escobar MA, Jay CL, Brooks RM, West KW, Rescorla FJ, Molleston JP, et al. Effect of corticosteroid therapy on outcomes in biliary atresia after Kasai portoenterostomy. Journal of Pediatric Surgery 2006;41:99‐103. [DOI] [PubMed] [Google Scholar]
Foroutan 2007 {published data only}
- Foroutan HR, Hosseini AH, Dehghani SM, Banani SA, Bahador A, Haghighat M, et al. Peri‐operative high‐dose v post‐operative low dose steroid therapy in the management of biliary atresia: a preliminary report. Iranian Journal of Medical Sciences 2008;33(2):79‐83. [Google Scholar]
Japanese Biliary Atresia Society 2013 {published data only}
- Japanese Biliary Atresia Society, Nio M, Muraji T. Multicenter randomized trial of postoperative corticosteroid therapy for biliary atresia. Pediatric Surgery International 2013;29(11):1091‐5. [DOI] [PubMed] [Google Scholar]
Kobayashi 2005 {published data only}
- Kobayashi H, Yamataka A, Koga H, Okazaki T, Tamura T, Urao M, et al. Optimum prednisolone usage in patients with biliary atresia postportoenterostomy. Journal of Pediatric Surgery 2005;40(2):327‐30. [DOI] [PubMed] [Google Scholar]
Lao 2010 {published data only}
- Lao OB, Larison C, Garrison M, Healey PJ, Goldin AB. Steroid use after the kasai procedure for biliary atresia. American Journal of Surgery 2010;199(5):680‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyers 2003 {published data only}
- Meyers RL, Book LS, O'Gorman MA, Jackson WD, Black RE, Johnson DG, et al. High‐dose steroids, ursodeoxycholic acid, and chronic intravenous antibiotics improve bile flow after Kasai procedure in infants with biliary atresia. Journal of Pediatric Surgery 2003;38:406‐11. [DOI] [PubMed] [Google Scholar]
Petersen 2008 {published data only}
- Petersen C, Harder D, Melter M, Becker T, Wasielewski RV, Leonhardt J, et al. Postoperative high‐dose steroids do not improve mid‐term survival with native liver in biliary atresia. American Journal of Gastroenterology 2008;103(3):712‐9. [DOI] [PubMed] [Google Scholar]
Shimadera 2007 {published data only}
- Shimadera S, Iwai N, Deguchi E, Kimura O, Fumino S, Ono S. The significance of steroid therapy after hepatoportoenterostomy in infants with biliary atresia. European Journal of Pediatric Surgery 2007;17(2):100‐3. [DOI] [PubMed] [Google Scholar]
Shneider 2012 {published data only}
- Shneider BL, Magee JC, Bezerra JA, Haber B, Karpen SJ, Raghunathan T, et al. the Childhood Liver Disease Research Education Network (ChiLDREN). Efficacy of fat‐soluble vitamin supplementation in infants with biliary atresia. Pediatrics 2012;130(3):e607‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shneider 2016 {published data only}
- Shneider BL, Magee JC, Karpen SJ, Rand EB, Narkewicz MR, Bass LM, et al. Childhood Liver Disease Research Network (ChiLDReN). Total serum bilirubin within 3 months of hepatoportoenterostomy predicts short‐term outcomes in biliary atresia. Journal of Pediatrics 2016;170:211‐7. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sokol 2007 {published data only}
- Sokol RJ. Corticosteroid treatment in biliary atresia: tonic or toast?. Hepatology 2007;46(6):1675‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stringer 2007 {published data only}
- Stringer MD, Davison SM, Rajwal SR, McClean P. Kasai portoenterostomy: 12‐year experience with a novel adjuvant therapy regimen. Journal of Pediatric Surgery 2007;42(8):1324‐8. [DOI] [PubMed] [Google Scholar]
Tyraskis 2016 {published data only}
- Tyraskis A, Davenport M. Steroids after the Kasai procedure for biliary atresia: the effect of age at Kasai portoenterostomy. Pediatric Surgery International 2016;32(3):193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Zeng 2014 {unpublished data only}
- Zhen S, Lu X. The effect of adjuvant steroid therapy post‐Kasai portoenterostomy in biliary atresia. www.chictr.org.cn/showprojen.aspx?proj=10065 (accessed 18 December 2017).
Additional references
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ (Clinical Research Ed.) 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive — Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Davenport 2004
- Davenport M, Ville de Goyet J, Stringer MD, Mieli‐Vergani G, Kelly DA, McClean P, et al. Seamless management of biliary atresia in England and Wales (1999‐2002). Lancet 2004;363:1354‐7. [DOI] [PubMed] [Google Scholar]
Davenport 2006
- Davenport M, Tizzard SA, Underhill J, Mieli‐Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28‐year single‐center retrospective study. Journal of Pediatrics 2006;149:393‐400. [DOI] [PubMed] [Google Scholar]
Davenport 2008
- Davnport M, Caponcelli E, Livesey E, Hadzic N, Howard E. Surgical outcome in biliary atresia: etiology affects the influence of age at surgery. Annals of Surgery 2008;247(4):694‐8. [DOI] [PubMed] [Google Scholar]
Davenport 2011
- Davenport M, Ong E, Sharif K, Alizai N, McClean P, Hadzic N, et al. Biliary atresia in England and Wales: results of centralization and new benchmark. Journal of Pediatric Surgery 2011;46(9):1689‐94. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Sneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Rearch Ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2017
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2017, Issue 11. Art. No.: LIVER.
Hartley 2009
- Hartley JL, Davnport M, Kelly DA. Biliary atresia. Lancet 2009;374(9702):1704‐13. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2015
- Hill R, Quaglia A, Hussain M, Hadzic N, Mieli‐Vergani G, Vergani D, et al. Th‐17 cells infiltrate the liver in human biliary atresia and are related to surgical outcome. Journal of Pediatric Surgery 2015;50(8):1297‐303. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Jakobsen 2014
- Jakobsen J, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karrer 1985
- Karrer FM, Lilly JR. Corticosteroid therapy in biliary atresia. Journal of Pediatric Surgery 1985;20(6):693‐5. [DOI] [PubMed] [Google Scholar]
Kasai 1968
- Kasai M, Kimura S, Asakura Y, Suzuk H, Taira Y, Ohashi E. Surgical treatment of biliary atresia. Journal of Pediatric Surgery 1968;3(6):665‐75. [Google Scholar]
Kasai 1978
- Kasai M, Suzuki H, Ohashi E, Ohi R, Chiba T, Okamoto A. Technique and results of operative management of biliary atresia. World Journal of Surgery 1978;2(5):571‐9. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20:641‐54. [DOI] [PubMed] [Google Scholar]
McKiernan 2000
- McKiernan PJ, Baker PJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet 2000;355:25‐9. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Muraji 2004
- Muraji T, Nio M, Ohhama Y, Hashimoto T, Iwanaka T, Takamatsu H, et al. Postoperative corticosteroid therapy for bile drainage in biliary atresia‐‐a nationwide survey. Journal of Pediatric Surgery 2004;39(12):1803‐5. [DOI] [PubMed] [Google Scholar]
Narayanaswamy 2007
- Narayanaswamy B, Gonde C, Tredger JM, Hussain M, Vergani D, Davenport M. Serial circulating markers of inflammation in biliary atresia ‐ evolution of the post‐operative inflammatory process. Hepatology (Baltimore, Md.) 2007;46:180‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
SPIRIT 2013a
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
SPIRIT 2013b
- Chan A‐W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin J, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols or clinical trials. BMJ (Clinical Research Ed.) 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 17 June 2016).
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zani 2015
- Zani A, Quaglia A, Hadzic N, Zuckerman M, Davenport M. Cytomegalovirus‐associated biliary atresia: an aetiological and prognostic subgroup. Journal of Pediatric Surgery 2015;50(10):1739‐45. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Parsons 2010
- Parsons C, Davenport M. Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008735] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thyraskis 2016
- Tyraskis A, Parsons C, Davenport M. Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD008735.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


