Abstract
Background
Cerebral palsy occurs in up to 2.1 of every 1000 live births and encompasses a range of motor problems and movement disorders. One commonly occurring movement disorder amongst those with cerebral palsy is dystonia: sustained or intermittent involuntary muscle spasms and contractions that cause twisting, repetitive movements and abnormal postures. The involuntary contractions are often very painful and distressing and cause significant limitations to activity and participation.
Oral medications are often the first line of medical treatment for dystonia. Trihexyphenidyl is one such medication that clinicians often use to treat dystonia in people with cerebral palsy.
Objectives
To assess the effects of trihexyphenidyl in people with dystonic cerebral palsy, according to the World Health Organization's (WHO) International Classification of Functioning, Disability and Health (ICF) domains of impairment, activity and participation. We also assessed the type and incidence of adverse effects in people taking the drug.
Search methods
We searched CENTRAL, MEDLINE, Embase, eight other databases and two trials registers in May 2017, and we checked reference lists and citations to identify additional studies.
Selection criteria
We included randomised controlled trials comparing oral trihexyphenidyl versus placebo for dystonia in cerebral palsy. We included studies in children and adults of any age with dystonic cerebral palsy, either in isolation or with the associated movement disorders of spasticity, ataxia, chorea, athetosis and/or hypotonia. We included studies regardless of whether or not the study authors specified the method used to diagnose dystonia in their study population. Primary outcomes were change in dystonia and adverse effects. Secondary outcomes were: activity, including mobility and upper limb function; participation in activities of daily living; pain; and quality of life.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We identified one study, which was set in Australia, that met the inclusion criteria. This was a randomised, double‐blind, placebo‐controlled, cross‐over trial in 16 children (10 boys and 6 girls) with predominant dystonic cerebral palsy and a mean age of 9 years (standard deviation 4.3 years, range 2 to 17 years). We considered the trial to be at low risk of selection, performance, detection, attrition, reporting and other sources of bias. We rated the GRADE quality of the evidence as low.
We found no difference in mean follow‐up scores for change in dystonia as measured by the Barry Albright Dystonia Scale (BADS), which assesses eight body regions for dystonia on a 5‐point scale (0 = none to 4 = severe), resulting in a total score of 0 to 32. The BADS score was 2.67 points higher (95% confidence interval (CI) −2.55 to 7.90; low‐quality evidence), that is, worse dystonia, in the treated group. Trihexyphenidyl may be associated with an increased risk of adverse effects (risk ratio 2.54, 95% CI 1.38 to 4.67; low‐quality evidence).
There was no difference in mean follow‐up scores for upper limb function as measured by the Quality of Upper Extremity Skills Test, which has four domains that collectively assess 36 items (each scored 1 or 2) and produces a total score of 0 to 100. The score in the treated group was 4.62 points lower (95% CI −10.98 to 20.22; low‐quality evidence), corresponding to worse function, than in the control group. We found low‐quality evidence for improved participation (as represented by higher scores) in the treated group in activities of daily living, as measured by three tools: 18.86 points higher (95% CI 5.68 to 32.03) for the Goal Attainment Scale (up to five functional goals scored on 5‐point scale (−2 = much less than expected to +2 = much more than expected)), 2.91 points higher (95% CI 1.01 to 4.82) for the satisfaction subscale of the Canadian Occupational Performance Measure (COPM; satisfaction with performance in up to five problem areas scored on a 10‐point scale (1 = not satisfied at all to 10 = extremely satisfied)), and 2.24 points higher (95% CI 0.64 to 3.84) for performance subscale of the COPM (performance in up to five problem areas scored on a 10‐point scale (1 = not able to do to; 10 = able to do extremely well)).
The study did not report on pain or quality of life.
Authors' conclusions
At present, there is insufficient evidence regarding the effectiveness of trihexyphenidyl for people with cerebral palsy for the outcomes of: change in dystonia, adverse effects, increased upper limb function and improved participation in activities of daily living. The study did not measure pain or quality of life. There is a need for larger randomised, controlled, multicentre trials that also examine the effect on pain and quality of life in order to determine the effectiveness of trihexyphenidyl for people with cerebral palsy.
Plain language summary
Trihexyphenidyl for dystonia in cerebral palsy
Review question
Is trihexyphenidyl a helpful treatment for people with cerebral palsy who have a movement problem called dystonia?
Background
Cerebral palsy is a common condition that covers a range of movement problems. One common movement problem is dystonia, which makes it difficult for people with cerebral palsy to control their movements. They have unwanted – and often painful and distressing – muscle contractions that they cannot control. The contractions reduce people's ability to move, perform self‐care activities, speak and participate in everyday activities.
Doctors often use medications to treat this difficult condition, including trihexyphenidyl. However, all the benefits and harms of prescribing trihexyphenidyl for individuals with cerebral palsy and dystonia are still unknown.
Study characteristics
In May 2017 we searched for all clinical trials that investigated the effectiveness of trihexyphenidyl for people with dystonic cerebral palsy. We included one Australian trial that involved 16 children (10 boys, 6 girls) with cerebral palsy and dystonia. They had an average age of nine years.
The children were divided into two different groups. Both groups took 12 weeks of trihexyphenidyl and 12 weeks of a placebo (something that looks the same as trihexyphenidyl but with no active ingredient), with a 4‐week break in between during which they received neither. The only difference between the groups was that one group started with trihexyphenidyl and then had placebo, and the other group started with placebo and then had trihexyphenidyl.
Key results
We found no evidence that trihexyphenidyl was effective for reducing dystonia or improving upper arm function in children with cerebral palsy and dystonia. Trihexyphenidyl may be associated with an increased risk of side effects (agitation, constipation, dry mouth and poor sleep). There was some evidence that trihexyphenidyl may improve individual goals set by the child and family around improved participation in activities of daily living. The study did not measure pain or quality of life.
Quality of the evidence
We rated the quality of the evidence as low because the one study included a small number of children and there are no other studies to support the findings. Therefore, we are uncertain about the effectiveness of trihexyphenidyl in reducing dystonia or improving arm function and participation in everyday activities of people with cerebral palsy and dystonia.
Summary of findings
Summary of findings for the main comparison. Summary of findings: Trihexyphenidyl compared with placebo for dystonia in cerebral palsy.
| Trihexyphenidyl compared with placebo for dystonia in cerebral palsy | |||||
|
Patient or population: children with dystonic cerebral palsy Settings: one tertiary care hospital Intervention: trihexyphenidyl Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Trihexyphenidyl | ||||
|
Change in dystonia from baseline Measured by: BADS (eight body regions assessed for dystonia on a five‐point scale (0 = none to 4 = severe), minimum score 0 to maximum score 32; higher score = greater severity of dystonia) Follow‐up: 12 weeks |
The mean follow‐up score in the control group was 15.50 points | The mean follow‐up score in the intervention group was 2.67 points higher (2.55 lower to 7.90 higher) | — | 16 1 (RCT) | ⊕⊕⊝⊝ Lowa |
|
Adverse effectsb (mood disturbance, irritability, behavioural change, constipation) Measured by: counts of number and type Follow‐up: various (includes data assessed at both 12 and 28 weeks) |
375 per 1000 | 1000 per 1000 | RR 2.54 (1.38 to 4.67) | 16 1 (RCT) | ⊕⊕⊝⊝ Lowa |
|
Participation in activities of daily living:individual goal setting Measured by: GAS (up to 5 functional goals scored on a 5‐point scale (−2 = much less than expected to +2 = much more than expected); higher score = better than expected outcome) Follow‐up: 12 weeks |
The mean follow‐up score in the control group was 27.63 points | The mean follow‐up score in the intervention group was 18.86 points higher (5.68 higher to 32.03 higher) | — | 16 1 (RCT) | ⊕⊕⊝⊝ Lowa |
|
Participation in activities of daily living:satisfaction with individual goals Measured by: satisfaction subscale of the COPM (satisfaction with performance in up to 5 problem areas scored on a 10‐point scale (1 = not satisfied at all to 10 = extremely satisfied); higher score = greater satisfaction) Follow‐up: 12 weeks |
The mean follow‐up score in the control group was 2.96 points | The mean follow‐up score in the intervention group was 2.91 points higher (1.01 higher to 4.82 higher) | — | 16 1 (RCT) | ⊕⊕⊝⊝ Lowa |
|
Participation in activities of daily living:performance of individual goals Measured by: performance subscale of the COPM (up to five problem areas scored on a 10‐point scale (1 = not able to do to 10 = able to do extremely well; higher score = better performance) Follow‐up: 12 weeks |
The mean follow‐up score in the control group was 3.14 points | The mean follow‐up score in the intervention group was2.24 points higher (0.64 higher to 3.84 higher) | — | 16 1 (RCT) | ⊕⊕⊝⊝ Lowa |
| Quality of life | Not measured | ||||
| *The basis for the assumed risk is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) BADS: Barry Albright Dystonia Scale; CI: Confidence interval; COPM: Canadian Occupational Performance Measure; GAS: Goal Attainment Scale; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded two levels due to imprecision; small sample size from one study only. bAll side effects were counted as adverse effects, as defined in our protocol (Baker 2017). Adverse effect data was from both phases. It was not possible to obtain adverse effect data from the first phase only. All children were reported to have adverse effects in the treatment phase. Six out of 16 participants were reported to have adverse effects in the placebo phase; however, the timing of these is not clear. The estimated number of adverse effects in the placebo phase may therefore be lower and we may be underestimating the relative risk.
Background
Description of the condition
Cerebral palsy has been described as "a group of permanent disorders of the development of movement and posture, causing activity limitation ... [attributable] to non‐progressive disturbances that occurred in the developing fetal or infant brain" (Rosenbaum 2007). Disturbances of sensation, perception, cognition, communication and behaviour, epilepsy and secondary musculoskeletal problems often accompany the motor disorders of cerebral palsy.
Cerebral palsy is a common childhood condition, occurring in approximately 2.11 of every 1000 live births (Oskoui 2013). It encompasses motor problems such as spasticity (increased muscle tone); hyperkinetic disorders, such as dystonia and choreoathetosis where there is unwanted excessive movement; and, less commonly, ataxia (uncoordinated movement). However, many children present with a mixed movement disorder.
Dystonia frequently occurs in children with cerebral palsy and is "characterised by involuntary sustained or intermittent muscle contractions that cause twisting and repetitive movements, abnormal postures, or both” (Sanger 2003). Of the methods used to diagnose dystonia, the one with the greatest credibility is the Hypertonia Assessment Tool (HAT; Jethwa 2010). The HAT is a seven‐item, standardised clinical assessment tool for children over four years of age, which is used to reliably differentiate the various types of paediatric hypertonia, namely spasticity, dystonia and rigidity (Jethwa 2010). Dystonia can result from an abnormality in the basal‐ganglia cortical pathway and can be measured clinically using a variety of scales. It may affect one or more limbs, or be present throughout the child’s whole body, including the mouth, eyes and neck. The involuntary contractions are often very painful and distressing. Dystonia can reduce activity and participation across many of the health‐related domains described in the World Health Organization's (WHO) International Classification of Functioning, Disability and Health (ICF) model (WHO ICF 2003).
The percentage of children with cerebral palsy identified as having dominant dyskinetic cerebral palsy (including dystonia and choreoathetosis) is substantial, ranging from 6% in Australia to 15% in Europe (ACPR 2016; Bax 2006; Himmelmann 2007). Children who are accurately identified as spasticity‐dominant can also have dyskinesia, which can add to their disability and discomfort. Traditionally, identifying dystonia and differentiating it from spasticity relied primarily on clinical examination and observation, with uncertain reliability and validity. The accurate identification of dyskinesia in children with cerebral palsy has been facilitated by the development of the HAT, which reliably differentiates the subtypes of hypertonia (Jethwa 2010).
Dystonia can have devastating effects on function, including impairment of communication and swallowing, and it can create difficulties in completing self‐care tasks and being comfortably seated in a wheelchair. Children also get into cycles of dystonia causing musculoskeletal pain, which in turn exacerbates their dystonia. The worst example of this is the dystonic crisis, which can require muscle paralysis/sedation (Allen 2014). These difficulties can negatively affect the quality of life of the individual and their caregivers.
Description of the intervention
Dystonia is a difficult movement disorder with limited management options available for treatment. Oral medications are often the first‐line of treatment for this challenging condition.
Trihexyphenidyl (also known as benzhexol) is a selective muscarinic acetylcholine receptor antagonist, blocking cholinergic activity centrally and peripherally (NIH 2005). It is also thought to increase the availability of dopamine, a brain chemical that is critical in the initiation and smooth control of voluntary muscle movement. Trihexyphenidyl is available in liquid form, as a tablet, or as an extended‐release (long‐acting) capsule. The onset of action of this medication occurs within an hour of oral administration. It has a peak effect 2 to 3 hours after administration, and the duration of action can last from 6 to 12 hours.
How the intervention might work
The pathophysiology of dystonia involves a dysfunction in the basal ganglia. The pathways between the basal ganglia and the cortex are influenced by neurotransmitters that act on various receptors in feedback loops causing positive or negative effects. The mechanism of action of trihexyphenidyl in reducing dystonia is believed to be in the basal ganglia where it reduces acetylcholine and increases dopamine (Carranza del Rio 2011). By treating dystonia and its associated impairments, clinicians hope to improve activity and decrease associated pain and discomfort.
Why it is important to do this review
Medication is frequently used for dystonia but little information is available as to whether it is effective, and side effects are common. Most of the published studies assessing medications are small and descriptive, limiting the ability to draw conclusions on its effects. Prospective case series, such as Sanger 2007, along with a few retrospective case series, like Ben‐Pazi 2011, and Hoon 2001, have reported inconsistent results for trihexyphenidyl in cerebral palsy; however, there is a need to examine evidence from existing randomised trials. This review will bring together and evaluate existing trials to clarify the benefits and risks of trihexyphenidyl for dystonia in cerebral palsy.
Objectives
To assess the effects of trihexyphenidyl in people with dystonic cerebral palsy, according to the World Health Organization's (WHO) International Classification of Functioning, Disability and Health (ICF) domains of impairment, activity and participation. We also assessed the type and incidence of adverse effects in people taking the drug.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Children and adults with dystonic cerebral palsy, either in isolation or with the associated movement disorders of spasticity, ataxia, chorea, athetosis and/or hypotonia were eligible. Although we applied no age restrictions, we found no studies reporting outcomes for adults.
We included studies regardless of whether or not the study authors specified the method used to diagnose dystonia in their study population. We did this in order to include all studies with relevant findings. We do concede, however, that studies using the HAT may have greater credibility than those using other or unspecific methods of diagnosis (Jethwa 2010).
Types of interventions
Oral trihexyphenidyl, regardless of dosage or frequency of administration, used to treat dystonia in cerebral palsy.
The control group had to be a placebo group. Trials with more than one comparison group were eligible, as long as one group received trihexyphenidyl and one group received placebo.
Types of outcome measures
Primary outcomes
*Change in dystonia from baseline, as assessed using the Barry‐Albright Dystonia scale (Barry 1999), Dyskinesia Impairment Scale (Monbaliu 2012), or electrophysiological measures of dystonia
*Adverse effects (mood disturbance, irritability, behavioural change, constipation)
Secondary outcomes
Activity, including mobility and upper limb function, as measured by validated scales such as the Canadian Occupational Performance Measure (Law 2005), Global Assessment Scale (Endicott 1976), Quality of Upper Extremity Skills Test (QUEST 1992), Melbourne Assessment of Unilateral Upper Limb Function (Randall 1999), Gross Motor Function Measure (Hanna 2008), Three Dimensional Gait Analysis (Ferber 2016), Timed Up and Go Test (TUG 2005), Functional Independence Measure (WeeFIM 1994), and High‐level Mobility Assessment Tool (Williams 2006)
*Participation in activities of daily living at home, school, in the community and in the workforce, as measured by validated scales such as the Children's Assessment of Participation and Enjoyment (King 2004), the Canadian Occupational Performance Measure (Law 2005), and the Global Assessment Scale (Endicott 1976)
Pain, as measured by validated scales such as the Wong‐Baker FACES Pain Rating Scale (Wong‐Baker FACES Pain Rating Scale 2001)
*Quality of life, as measured by validated scales such as the Pediatric Quality of Life Inventory (PedsQL 1999), the Caregiver Priorities and Child Health Index of Life with Disabilities (Narayanan 2007), or other validated scale
We placed no restrictions on length of outcome follow‐up.
We used those outcomes marked with an asterisk (*) to populate the 'Summary of findings' table.
Search methods for identification of studies
This review is based on a published protocol (Baker 2017).
Electronic searches
We searched the electronic databases and trials registers listed below up to May 2017.
Cochrane Register of Studies Online, which includes the Cochrane Developmental, Psychosocial and Learning Problems Group Specialised Register (crso.cochrane.org; searched 25 May 2017).
MEDLINE Ovid (1946 to May week 3 2017).
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (searched 25 May 2017).
MEDLINE Epub Ahead of Print Ovid (searched 25 May 2017).
Embase Ovid (1974 to 2017 week 21).
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 25 May 2017).
Science Citation Index Web of Science (SCI; 1990 to 24 May 2017).
Conference Proceedings Citation Index – Science Web of Science (CPCI‐S; 1990 to 24 May 2017).
Cochrane Database of Systematic Reviews (CDSR; 2017, Issue 5) part of the Cochrane Library (searched 26 May 2017).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2. Final Issue) part of the Cochrane Library (searched 26 May 2017).
LILACS (lilacs.bvsalud.org/en; searched 26 May 2017).
ClinicalTrials.gov (clinicaltrials.gov; searched 26 May 2017).
WHO International Clinical Trials Registry Platform (ICTRP) (who.int/ictrp/en; searched 26 May 2017).
We developed a search strategy in MEDLINE, which we adapted for each of the sources listed above (Appendix 1) . We did not apply any date or language restrictions.
Searching other resources
We checked the reference lists of included reports and relevant reviews. In addition, we contacted experts to identify any additional studies not retrieved by our electronic searches.
Data collection and analysis
We were not able to use many of our planned methods because only one study met the criteria for inclusion in the review (Criteria for considering studies for this review). Details of unused methods can be found in Baker 2017 and Table 2. What follows next is a description of methods that we did use.
1. Unused methods.
| Method | Unused methods |
| Measures of treatment effect |
Continuous data For continuous outcomes we will calculate the MD and corresponding 95% CI if studies use the same rating scales. We will calculate the SMD with 95% CIs if studies use different scales to measure the same outcomes. As recommended in section 9.4.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017), we will focus on final values unless some of the studies use change scores. We will combine studies that report final values with studies that report only change scores in the same meta‐analysis, provided that the studies use the same rating scale. We will conduct the analysis according to age, as children and adults respond differently to medication. We will combine the data from all groups in studies that have trihexyphenidyl in more than one group (i.e. different frequencies) and then separate these when performing the subgroup analysis to see how the different frequencies influence the results (see item four in the Subgroup analysis and investigation of heterogeneity section). |
|
Multiple outcomes If studies provide multiple, interchangeable measures of the same construct at the same point in time, we will calculate the average SMD across the outcomes and the average estimated variances, as recommended in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). | |
| Unit of analysis issues |
Cluster‐RCTs If included trials use cluster randomisation, we will extract an ICC and use this to reanalyse the data. Where no ICC is given and a unit of analysis error appears to exist, we will contact the trial authors and ask them to provide either an ICC or the raw data to enable calculation of an ICC. Where no ICC is made available, we will search for similar studies from which we can impute an ICC, or seek statistical advice to obtain an estimate of the ICC. |
| Dealing with missing data | We will contact trial investigators to request missing data. If the trialists provide missing data, we will conduct a meta‐analysis according to intention‐to‐treat principles using all data and keeping participants in the treatment group to which they were originally randomised, regardless of the treatment they actually received, as recommended in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). If missing data are not provided, we will analyse only the available data. If there is concern regarding a high level of missing data, such that data could not be included in a meta‐analysis, we will include a qualitative summary in the text of the review. We will document missing data and attrition in the 'Risk of bias' tables, and we will explore how missing data might affect the interpretation of the results by conducting a sensitivity analysis. |
| Assessment of heterogeneity | We will assess clinical heterogeneity by comparing the between‐trials distribution of participant characteristics (e.g. children versus adults) and intervention characteristics (e.g. treatment type and dose), and assess methodological heterogeneity by comparing trial characteristics (e.g. cross‐over versus parallel design). We will evaluate statistical heterogeneity using the I2 statistic and the Chi2 test of heterogeneity, with statistical significance set at P value < 0.10. As recommended in section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017), we will consider I2 values as follows.
We will report Tau2 as an estimate of the between‐study variance when reporting the results from the random‐effects model. |
| Assessment of reporting biases | If we identify 10 or more studies, we will use funnel plots to investigate the relationship between intervention effect and study size. We will explore possible reasons for any asymmetry found. We will analyse the funnel plot of the data to ascertain asymmetry. Asymmetry of a funnel plot may indicate, among other things, publication bias or poor methodological quality (Egger 1997). |
| Data synthesis | We will synthesise results in a meta‐analysis using a fixed‐effect model when studies are similar enough with regard to the intervention, population and methods, to assume that the same treatment effect is estimated. We will synthesise results in a meta‐analysis using a random‐effects model when statistical heterogeneity is found or when studies differ enough with regard to the intervention, population, and methods, to assume that different yet related treatment effects are estimated, and when it is deemed to be clinically relevant, as recommended in section 9.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). |
| Subgroup analysis and investigation of heterogeneity | We will conduct the subgroup analyses listed below.
We will also look at the number of participants per study to determine if this is sufficient to perform a subgroup analysis. |
| Sensitivity analysis | We will conduct sensitivity analyses to investigate the effect on the overall results of excluding trials that meet the criteria described below.
We will also conduct a sensitivity analysis for studies with very low risk of bias. In addition, we will conduct a sensitivity analysis using a range of ICCs to assess the impact on treatment effect. |
CI: confidence intervals; ICC: intraclass correlation coefficient; MD: mean difference; SMD: standardised mean difference.
Selection of studies
Two review authors, ARH and AS, independently screened the titles and abstracts of the citations identified from the search, discarding those that were clearly irrelevant. They then obtained the full texts of those studies that met, or seemed to meet, the inclusion criteria (Criteria for considering studies for this review), and they assessed them for relevance. KW acted as arbiter in the event of dispute. We recorded our decisions in a PRISMA flow diagram (Moher 2009).
Data extraction and management
Two review authors (ARH and AS) independently extracted data from the included study using a data extraction form designed and piloted for this review. They extracted the following information.
Study methods and setting: study type (type of RCT), study site, country of publication, language of publication, publication type and study duration.
Participant details: age, sex, diagnosis and diagnosis tool.
Intervention details: intervention type, including dosage, mode of delivery, frequency and duration; placebo type, including dosage, mode of delivery, frequency and duration.
Outcomes: all primary and secondary outcomes (see Types of outcome measures).
We resolved disagreements through consultation with KW.
ARH entered data into Review Manager 5 (RevMan 5), which KW checked for accuracy (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (ARH and AS) independently assessed the risk of bias in the included study using the tool described in section 8.5.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). For the study, they judged the risk of bias to be either low, high or unclear, for each of the seven domains described in Appendix 2. They resolved disagreements by discussion with KW.
Measures of treatment effect
Dichotomous data
We had planned to analyse dichotomous outcomes by calculating the odds ratio (OR) and corresponding 95% confidence intervals (CI). However, because adverse effects (the only dichotomous outcome in this review) were frequent in the one included study, we calculated the risk ratio (RR) and corresponding 95% CI to provide a more interpretable statistic.
Continuous data
As recommended in section 9.4.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017), we performed an ANCOVA to estimate the change score due to the one included study using change scores. A potential problem of including change scores is that the standard deviation of changes may not be reported in the original study (Higgins 2011a).
See also Baker 2017 and Table 2.
Unit of analysis issues
The included study was a cross‐over trial, and we used the data from the first period of the cross‐over. We did not include any cluster‐randomised trials (see Baker 2017; Table 2).
Dealing with missing data
We assessed missing data and dropouts for the included study. We reported the number of participants included in the final analysis as a proportion of all participants in the study, reporting reasons for missing data (dropouts) according to their randomised groups (trihexyphenidyl and placebo) as provided in the study. We examined the differences in these rates to determine the treatment effect bias, as differences in rates of missing data between the groups are indicative of data not missing at random. This would indicate a high risk of bias for incomplete outcome reporting in the included study. This is reported in the risk of bias.
Assessment of heterogeneity
We were unable to assess heterogeneity, as we only included one study in the review (see Baker 2017; Table 2).
Assessment of reporting biases
We were unable to assess reporting biases, as we only included one study in the review (see Baker 2017; Table 2).
Data synthesis
We were unable to conduct a meta‐analysis as we only included one study in the review (see Baker 2017; Table 2).
Subgroup analysis and investigation of heterogeneity
We were unable to conduct a subgroup analysis or investigate heterogeneity, as we only included one study in the review (see Baker 2017; Table 2).
Sensitivity analysis
We were unable to conduct a sensitivity analysis, as we only included one study in the review (see Baker 2017; Table 2).
Summary of findings table
Following the recommendations in section 11.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017), and using GRADEprofiler: Guideline Development Tool (GRADEpro GDT 2015), we created a 'Summary of findings' table for the comparison, trihexyphenidyl versus placebo for dystonia in cerebral palsy. We reported the following outcomes in the table: change in dystonia; adverse effects; participation in activities of daily living; and quality of life. We reported all outcomes at 12 weeks follow‐up except for adverse effects, whose analysis contains data assessed at both 12 and 28 weeks because we were unable to separate the data.
Two review authors (AH and KW) independently assessed the quality of the evidence for each outcome contributing data using the GRADE tool (GRADE 2004), looking at study limitations, consistency of effect, imprecision, indirectness and publication bias.
Results
Description of studies
A description of the included study is in the Characteristics of included studies table.
Results of the search
We conducted a literature search up to 26 May 2017, identifying 128 original records. After screening of titles and abstracts, we retrieved three full‐text reports. We excluded one study, Reddihough 1990, because it was not an RCT (see Excluded studies; Characteristics of excluded studies table). We identified two reports of one original trial for inclusion (Rice 2009). Figure 1 shows a study flow diagram.
1.
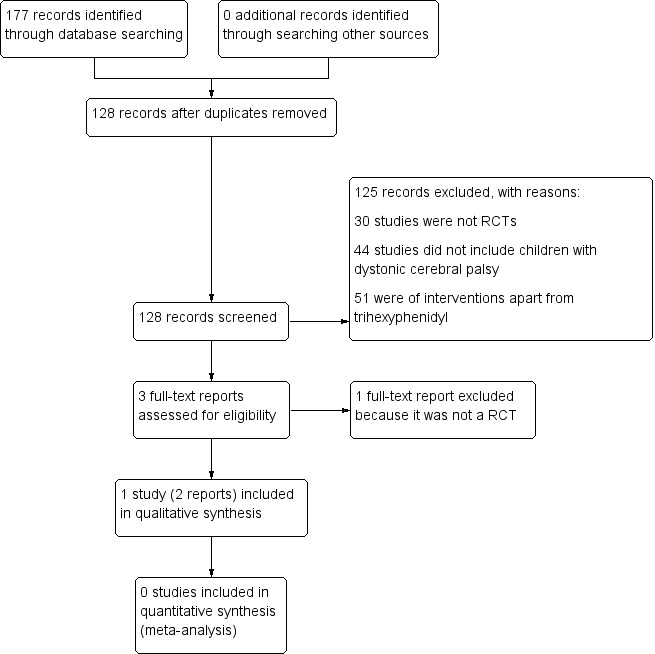
Study flow diagram.
Included studies
Study design
Rice 2009 was a randomised, double‐blind, placebo‐controlled, cross‐over trial.
Participants
The study recruited 16 children with predominant dystonic cerebral palsy and a mean age of nine years (SD 4.3 years, range 2 to 17 years). There were 10 boys and 6 girls.
Diagnosis
One of the study physicians verified the diagnosis of dystonia, but authors did not report the method used.
Randomisation
Children were randomised into either trihexyphenidyl (dose starting at 0.2 mg/kg/d in three divided doses and increased over six weeks up to a maximum 2.5 mg/kg/d in three divided doses) or placebo (liquid delivered orally at 2.5 mg/kg/day of liquid in three divided doses (equals same volume as intervention)) for 12 weeks. Parents administered the trihexyphenidyl and the placebo. There was a washout period of four weeks prior to cross‐over.
Outcomes
The primary outcomes reported were change in dystonia from baseline, assessed using the Barry Albright Dystonia Scale (BADS) and adverse effects. Authors counted all side effects as adverse effects. Secondary outcomes reported were upper limb function, assessed using the Quality of Upper Extremity Skills Test (QUEST) and participation in activities of daily living at home, school and in the community, assessed using the Canadian Occupational Performance Measure (COPM) and the Goal Attainment Scale (GAS). Trialists did not measure pain or quality of life. Children were assessed at baseline, 12 weeks and 28 weeks after commencement. We only included data from the first phase (12 weeks) in this review, except for adverse effects where it was not possible to separate first‐ and second‐phase data.
Excluded studies
We excluded one study, Reddihough 1990, because it was not an RCT. See the Characteristics of excluded studies table.
Risk of bias in included studies
Figure 2 presents a summary of the 'Risk of bias' assessment for the included trial, Rice 2009. We judged the trial as being at low risk of bias for all categories.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered Rice 2009 to be at low risk of selection bias due to both random sequence generation and allocation concealment because the trial pharmacy generated a randomisation table and kept codes concealed until after data collection was complete.
Blinding
We judged Rice 2009 to be at low risk of performance and detection bias because assessors, the patient and the family were all blinded to the intervention.
Incomplete outcome data
We judged Rice 2009 to be at low risk of attrition bias because, although two of the eight children who received the active treatment in the first phase dropped out, one child withdrew due to a family crisis unrelated to the medication.
Selective reporting
We judged Rice 2009 to be at low risk of reporting bias because it reported on all pre‐specified outcomes.
Other potential sources of bias
We identified no other potential sources of bias for Rice 2009.
Effects of interventions
See: Table 1
In order to obtain the first‐phase data only, we contacted the study authors and they provided the requested information. The follow‐up time point for the first phase was 12 weeks.
Primary outcomes
Change in dystonia from baseline
Our analysis of first‐phase data only showed no evidence of a difference in mean follow‐up scores, with an estimate that the BADS score (eight body regions assessed for dystonia on a five‐point scale (0 = none to 4 = severe), for a total score of 0 to 32) was 2.67 points higher (95% CI −2.55 to 7.90; low‐quality evidence; Figure 3), that is, worse dystonia, in the treated group.
3.
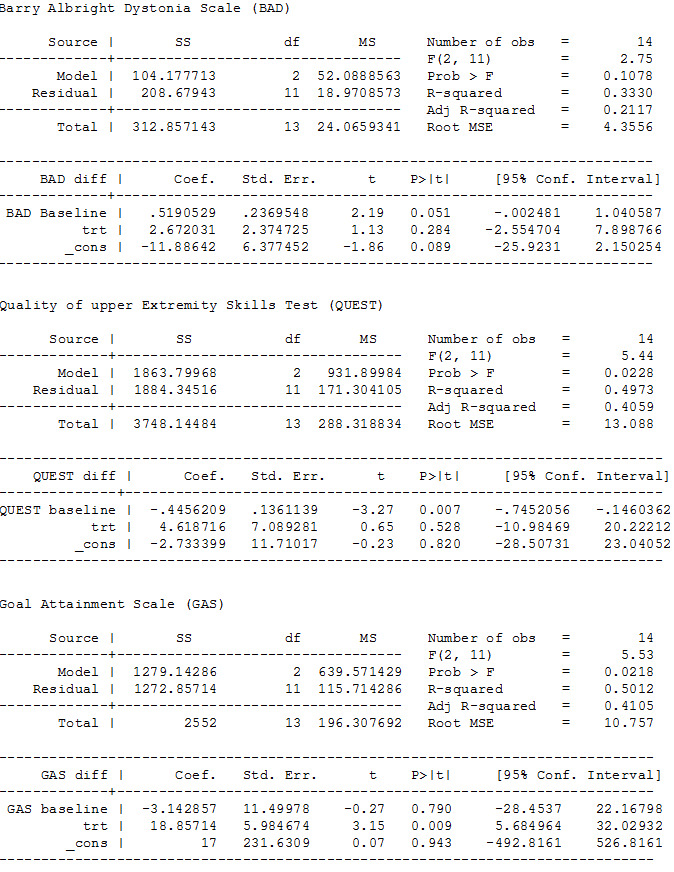
ANCOVA analyses, page 1
The results reported by the study authors from both phases using the general linear model analysis showed no evidence that trihexyphenidyl had any treatment (F(1,12) = 0.2, P = 0.67), carry‐over (F(1,12) = 1.7, P = 0.22) or order (F(1,12) = 0.3, P = 0.57) effects.
Adverse effects
The study authors reported that trihexyphenidyl increased the risk of adverse effects by 154% using data from both the first and second phases of the study. All children had reported adverse effects in the treatment phase (16/16), so all children receiving treatment in the first phase experienced adverse effects (8/8). Reported adverse effects included agitation, constipation, dry mouth and poor sleep. Authors reported that 6 out of 16 participants had adverse effects in the placebo phase; however, the timing of these effects is not clear. It is conceivable that children who received the placebo in the second phase may have experienced ongoing adverse effects related to the treatment phase.Therefore, the estimate of the number of adverse effects with placebo in the first phase may be lower than three out of eight, and we may be underestimating the increased risk with treatment.
Secondary outcomes
Activity
Our analysis of first‐phase data only showed no evidence of a difference in mean follow‐up scores as measured by the QUEST (36 items across four domains, scored one or two, producing a score of 0 to 100), with an estimated score of 4.62 points lower (95% CI −10.98 to 20.22; low‐quality evidence), that is worse upper limb function, in the treated group.
The results reported by the study authors from both phases using general linear model analysis showed no evidence that trihexyphenidyl had any treatment (F(1,12) = 0.9, P = 0.37), carry‐over (F(1,12) = 1.4, P = 0.25) or order (F(1,12) = 0.2, P = 0.90) effects.
Participation in activities of daily living
Our analysis of first‐phase data only showed a difference in mean follow‐up scores, as measured by three tools, in the treated group, with an estimate of: 18.86 points higher (95% CI 5.68 to 32.03; low‐quality evidence) for the GAS (up to five functional goals scored on 5‐point scale (−2 = much less than expected to +2 = much more than expected)), 2.91 points higher (95% CI 1.01 to 4.82; low‐quality evidence) for the satisfaction subscale of the COPM (satisfaction with performance in up to five problem areas scored on a 10‐point scale (1 = not satisfied at all to 10 = extremely satisfied)), and 2.24 points higher (CI 95% 0.64 to 3.84; low‐quality evidence; Figure 4) for the performance subscale of the COPM (performance in up to five problem areas scored on a 10‐point scale (1 = not able to do to 10 = able to do extremely well)). Higher scores represent improved participation.
4.
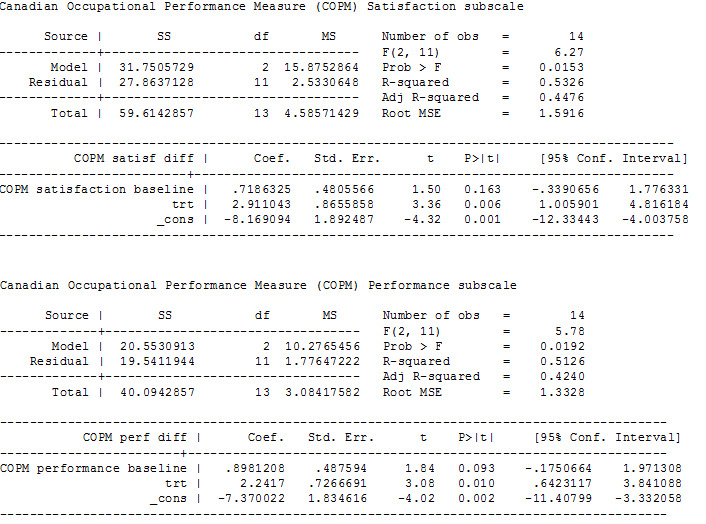
ANCOVA analyses, page 2
The results reported by the study authors from both phases using general linear model analysis showed no evidence that trihexyphenidyl had any treatment or carry‐over effects for any of the three participation measures but had statistically significant order effects on the GAS (F(1,11) = 10.2, P = 0.009) and borderline significant order effects on the performance component of the COPM (F(1,12) = 4.7, P = 0.05).
Pain and quality of life
Rice 2009 did not report on these outcomes.
Quality of the evidence
Although we judged Rice 2009 to be at low risk of bias in all categories, we considered the quality of the evidence for all reported outcomes to be low after downgrading two levels due to imprecision resulting from the inclusion of a single small study.
Discussion
Summary of main results
For our primary outcomes, the results of our analysis using phase‐one data only from the one included cross‐over trial did not generate evidence that trihexyphenidyl reduces dystonia but did identify that the risk of adverse effects is high. For the secondary outcomes, there was no evidence that trihexyphenidyl increases upper limb function, but there was improved goal attainment relating to improved participation in activities of daily living. There was a lack of internal consistency with the expectation that improvement in goal attainment and participation would be mediated by reduced dystonia. The study did not measure pain or quality of life.
The risk for adverse effects needs to be interpreted with caution, as we were unable to separate out first‐ and second‐phase data. Consequently, we may have underestimated the risk for adverse effects with treatment.
There were slight differences in the results from our analyses using phase‐one data only compared with the published authors' analyses of combined phases. Using the phase‐one data, we observed differences between the treatment and placebo scores that were in the same direction as the published authors’ analyses, but greater in magnitude. In addition, our results found a difference in COPM performance and satisfaction scales compared to the study authors. This suggests that the magnitude of the treatment effect was smaller in the second phase than in the first phase of the trial. It is not possible to determine whether this difference between analyses is due to small carry‐over effects, to chance or to the small numbers of participants in the trial.
Overall completeness and applicability of evidence
The evidence base for trihexyphenidyl in managing dystonia in people with cerebral palsy is incomplete. The only RCT we could find assessed the outcomes of dystonia reduction, adverse effects, upper limb function and goal attainment related to improved function and participation in a small sample of children and young people aged 2 to 17 years, who either walk with assistive devices or are non‐ambulant. No RCTs have evaluated pain, quality of life, or outcomes for adults with dystonic cerebral palsy or higher gross motor function.
Quality of the evidence
Using the GRADE criteria (GRADE 2004; Schünemann 2017), we rated the quality of the evidence for all outcomes as low, due to the small sample size and lack of any other RCT, leading to imprecision and inconsistency. Consequently, our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Potential biases in the review process
We conducted an extensive search of all available literature, including grey literature. We included trials regardless of publication date or language. Therefore, we are confident that this review includes all published evidence from RCTs on this topic to date. By including only RCTs, we excluded a small number of non‐randomised studies that examine trihexyphenidyl in cerebral palsy; however, these studies do not add to the quality of evidence for this intervention. One author of this review (DSR) is a co‐author of a published study that we excluded from the review, and three review authors (AH, AS and DSR) collaborate with authors of the included published trial. Independent review authors assessed the eligibility, risk of bias and quality of the evidence for this study.
Agreements and disagreements with other studies or reviews
There is little literature with which to compare the results of this review. Our results are consistent with a recent systematic review of the efficacy of oral pharmacological treatments in dyskinetic cerebral palsy. That review also included the study by Rice 2009, which met our eligibility criteria (Criteria for considering studies for this review), concluding that "trihexyphenidyl needs further investigation, primarily in dystonic patients with predominant involvement of upper limb" (Masson 2017).
Other non‐randomised studies have reported inconsistent results from studies examining trihexyphenidyl in cerebral palsy. In contrast to our review, a prospective, open‐label, multicentre pilot trial of high‐dose trihexyphenidyl in 23 children aged 4 to 15 years with cerebral palsy and dystonia impairing function in the dominant upper extremity found some improvement in upper limb function (Sanger 2007). However post hoc analyses indicated that a subgroup of children with hyperkinetic dystonia worsened over this period. Similar to our review, Sanger 2007 reported no effect for reducing dystonia or improving gross motor function, quality of life, or care and comfort. Retrospective chart reviews have reported results inconsistent with ours: Carranza del Rio 2011 reported reduction of dystonia, and Ben‐Pazi 2011 and Hoon 2001, improvements in motor function; however, methodological issues and inconsistent outcome reporting lower the quality of these results. Also, higher positive outcomes can be expected when using retrospective data. Although these non‐randomised studies add to the body of literature for trihexyphenidyl in dystonia in cerebral palsy, their results need to be interpreted with caution due to the low quality of the evidence.
Consistent with our review, all studies reported a high incidence of adverse effects.
Authors' conclusions
Implications for practice.
At present, there is insufficient evidence to know whether or not trihexyphenidyl is an effective treatment for dystonia for people with cerebral palsy. We found no evidence to suggest that trihexyphenidyl reduces dystonia or improves upper limb function. We found evidence of a high risk of adverse effects, which is consistent with non‐trial evidence and use for other indications. Trihexyphenidyl may improve individual goals set by the child and family with regards to participation in activities of daily living. RCTs have not examined the effect of trihexyphenidyl on reducing pain or improving quality of life. Clinicians and consumers should be aware of the lack of evidence about effectiveness and the high risk of adverse effects before prescribing or taking this medication for dystonia in cerebral palsy. This would not preclude cautious use for individual indications with careful monitoring and slow dose escalation so that clinicians can assess risks and benefits for each individual.
Implications for research.
The current evidence for trihexyphenidyl in people with cerebral palsy consists of only one RCT with a small sample size. There is an urgent need for larger RCTs of longer duration that also examine the effect on pain and quality of life in order to ascertain the effectiveness of trihexyphenidyl for dystonia in people with cerebral palsy. Researchers might also consider using lower‐dosing regimens to reduce the possible adverse effects of the medication, as well as using more sensitive outcome measures for dystonia, such as the Dyskinesia Impairment Scale (Monbaliu 2012). Although dystonia is a movement disorder seen frequently in children with cerebral palsy, it is not as common as spasticity. Therefore, multicentre trials are required in order to conduct adequately powered RCTs. In addition, taking into account the considerable heterogeneity in clinical presentation and management strategies seen in children with cerebral palsy, single‐agent RCTs may not be the most appropriate trial design. Other innovative trial designs may address clinical and research questions more appropriately. As neuroimaging advances our understanding of the types of brain injury an individual with dystonic cerebral palsy has, trial developers should consider brain injury type as a potential effect modifier and plan their methods accordingly.
Acknowledgements
The review team acknowledge the invaluable advice and support of the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG), including Geraldine Macdonald (Cochrane Co‐ordinating Editor), Joanne Duffield (Managing Editor), and the statistician and external peer reviewers. We also thank Margaret Anderson, the Information Specialist with CDPLPG for her assistance with designing the search strategy and conducting the search.
In addition, we acknowledge the contributions of Sarah Arnup who assisted with data analysis and interpretation, and Dr Kristine Egberts, who contributed significantly to the development of the protocol (Baker 2017).
Appendices
Appendix 1. Search strategies
1. Cochrane Register of Studies Online, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register
Searched 25 May 2017 (6 records)
#1MESH DESCRIPTOR Cerebral Palsy #2(cerebral pals*):TI,AB,KY #3(Little* disease):TI,AB,KY #4CP:TI,AB,KY #5#1 OR #2 OR #3 OR #4 #6(Antimuscarinic* or Anti‐muscarinic*):TI,AB,KY #7MESH DESCRIPTOR Antiparkinson Agents #8((antiparkinsonian or anti‐parkinsonian)):TI,AB,KY #9((Apotrihex or Apo‐Trihex)):TI,AB,KY #10(Artane):TI,AB,KY #11benzhexol*:TI,AB,KY #12hipokinon*:TI,AB,KY #13MESH DESCRIPTOR Muscarinic Antagonists #14Parkinane:TI,AB,KY #15Parkopan:TI,AB,KY #16trihexan*:TI,AB,KY #17MESH DESCRIPTOR Trihexyphenidyl #18Trihexyphenidyl*:TI,AB,KY #19((trihexidyl* or tri‐hexidyl*)):TI,AB,KY #20#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 #21#5 AND #20
2. MEDLINE Ovid
Searched 25 May 2017 (45 records)
1 Cerebral Palsy/ 2 cerebral pals$.tw,kf. 3 Little$ disease.tw,kf. 4 CP.tw. 5 or/1‐4 6 (Antimuscarinic$ or Anti‐muscarinic$).mp. 7 Antiparkinson Agents/ 8 (antiparkinsonian or anti‐parkinsonian).mp. 9 (Apotrihex or Apo‐Trihex).mp. 10 Artane.mp. 11 benzhexol$.mp. 12 hipokinon$.mp. 13 Muscarinic Antagonists/ 14 Parkinane.mp. 15 Parkopan.mp. 16 trihexan$.mp. 17 Trihexyphenidyl/ 18 Trihexyphenidyl$.mp. 19 (trihexidyl$ or tri‐hexidyl$).mp. 20 or/6‐19 21 5 and 20 22 randomized controlled trial.pt. 23 controlled clinical trial.pt. 24 randomi#ed.ab. 25 placebo$.ab. 26 drug therapy.fs. 27 randomly.ab. 28 trial.ab. 29 groups.ab. 30 or/22‐29 31 exp animals/ not humans.sh. 32 30 not 31 33 21 and 32
3. MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
Searched 25 May 2017 (2 records)
1 cerebral pals$.af. 2 Little$ disease.af. 3 CP.af. 4 or/1‐3 5 (Antimuscarinic$ or Anti‐muscarinic$).af. 6 (antiparkinsonian or anti‐parkinsonian).af. 7 (Apotrihex or Apo‐Trihex).af. 8 Artane.af. 9 benzhexol$.af. 10 hipokinon$.af. 11 Parkinane.af. 12 Parkopan.af. 13 trihexan$.af 14 Trihexyphenidyl$.af. 15 (trihexidyl$ or tri‐hexidyl$).af. 16 or/5‐15 17 4 and 16
4. MEDLINE Epub Ahead of Print Ovid
Searched 25 May 2017 (0 records)
1 cerebral pals$.af. 2 Little$ disease.af. 3 CP.af. 4 or/1‐3 5 (Antimuscarinic$ or Anti‐muscarinic$).af. 6 (antiparkinsonian or anti‐parkinsonian).af. 7 (Apotrihex or Apo‐Trihex).af. 8 Artane.af. 9 benzhexol$.af. 10 hipokinon$.af. 11 Parkinane.af. 12 Parkopan.af. 13 trihexan$.af. 14 Trihexyphenidyl$.af. 15 (trihexidyl$ or tri‐hexidyl$).af. 16 or/5‐15 17 4 and 16
5. Embase Ovid
Searched 25 May 2017 (39 records)
1 cerebral palsy/ 2 Littles disease.tw,kw. 3 cerebral pals$.tw,kw. 4 CP.tw,kw. 5 1 or 2 or 3 or 4 6 (Antimuscarinic$ or Anti‐muscarinic$).mp. 7 antiparkinson agent/ 8 (antiparkinsonian or anti‐parkinsonian).mp. 9 (Apotrihex or Apo‐Trihex).mp. 10 Artane.mp. 11 benzhexol$.mp. 12 hipokinon$.mp. 13 muscarinic receptor blocking agent/ 14 Parkinane.mp. 15 Parkopan.mp. 16 trihexan$.mp. 17 trihexyphenidyl/ 18 Trihexyphenidyl$.mp. 19 (trihexidyl$ or tri‐hexidyl$).mp. 20 or/6‐19 21 5 and 20 22 Randomized controlled trial/ 23 controlled clinical trial/ 24 Single blind procedure/ 25 Double blind procedure/ 26 triple blind procedure/ 27 Crossover procedure/ 28 (crossover or cross‐over).tw. 29 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 30 Placebo/ 31 placebo.tw. 32 prospective.tw. 33 factorial$.tw. 34 random$.tw. 35 assign$.ab. 36 allocat$.tw. 37 volunteer$.ab. 38 or/22‐37 39 21 and 38
6. CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
Searched 25 May 2017 (12 records)
S20S5 AND S19 S19S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 S18(trihexidyl* or tri‐hexidyl*) S17Trihexyphenidyl* S16trihexan* S15Parkopan S14Parkinane S13(MH "Muscarinic Antagonists") S12hipokinon* S11benzhexol* S10Artane S9(Apotrihex or Apo‐Trihex) S8(antiparkinsonian or anti‐parkinsonian) S7(MH "Antiparkinson Agents") S6(Antimuscarinic* or Anti‐muscarinic*) S5S1 OR S2 OR S3 OR S4 S4TI (CP) or AB(CP) S3Littles disease S2cerebral pals* S1(MH "Cerebral Palsy")
7. Science Citation Index Web of Science
Searched 26 May 2017 (37 records)
# 8 #7 AND #4 Indexes=SCI‐EXPANDED Timespan=1970‐2017 # 7 #6 OR #5 Indexes=SCI‐EXPANDED Timespan=1970‐2017 # 6 TS= (antiparkinsonian or "anti‐parkinsonian" or Antimuscarinic* or "Anti‐muscarinic*") Indexes=SCI‐EXPANDED Timespan=1970‐2017 # 5 TS=(Apotrihex or "Apo‐Trihex" or Artane or benzhexol* or hipokinon* or Parkinane or Parkopan or trihexan* or Trihexyphenidyl* or trihexidyl* or tri‐hexidyl*) Indexes=SCI‐EXPANDED Timespan=1970‐2017 # 4 #3 OR #2 OR #1 Indexes=SCI‐EXPANDED Timespan=1970‐2017 # 3 TS=(CP ) Indexes=SCI‐EXPANDED Timespan=1970‐2017 # 2 TS=(Little* disease) Indexes=SCI‐EXPANDED Timespan=1970‐2017 # 1 TS=(cerebral pals*) Indexes=SCI‐EXPANDED Timespan=1970‐2017
8. Conference Proceedings Citation Index – Science Web of Science
Searched 26 May 2017 (0 records)
# 8 #7 AND #4 Indexes=CPCI‐S Timespan=1990‐2017 # 7 #6 OR #5 Indexes=CPCI‐S Timespan=1990‐2017 # 6 TS= (antiparkinsonian or "anti‐parkinsonian" or Antimuscarinic* or "Anti‐muscarinic*") Indexes=CPCI‐S Timespan=1990‐2017 # 5 TS=(Apotrihex or "Apo‐Trihex" or Artane or benzhexol* or hipokinon* or Parkinane or Parkopan or trihexan* or Trihexyphenidyl* or trihexidyl* or tri‐hexidyl*) Indexes=CPCI‐S Timespan=1990‐2017 # 4 #3 OR #2 OR #1 Indexes=CPCI‐S Timespan=1990‐2017 # 3 TS=(CP) Indexes=CPCI‐S Timespan=1990‐2017 # 2 TS=(Little* disease) Indexes=CPCI‐S Timespan=1990‐2017 # 1 TS=(cerebral pals*) Indexes=CPCI‐S Timespan=1990‐2017
9. Cochrane Database of Systematic Reviews (CDSR) part of the Cochrane Library
Searched 26 May 2017 (1 record)
#1[mh "Cerebral palsy"] #2("cerebral pals*" or "Little* disease"):ti,ab #3#1 or #2 #4[mh Trihexyphenidyl] #5[mh "Muscarinic Antagonists"] #6[mh ^"Antiparkinson Agents"] #7Trihexyphenidyl*:ti,ab #8benzhexol*:ti,ab #9hipokinon*:ti,ab #10trihexan*:ti,ab #11Artane:ti,ab 10 #12(Parkopan or Parkinane):ti,ab #13(Apotrihex or Apo next Trihex):ti,ab #14(trihexidyl* or tri next hexidyl*):ti,ab #15(Antimuscarinic* or Anti next muscarinic*):ti,ab #16(antiparkinson* or anti next parkinson*):ti,ab #17{or #4‐#16} #18#3 and #17
10. Database of Abstracts of Reviews of Effects (DARE) part of the Cochrane Library
Searched 26 May 2017 (0 records)
#1[mh "Cerebral palsy"] #2("cerebral pals*" or "Little* disease") #3#1 or #2 #4[mh Trihexyphenidyl] #5[mh "Muscarinic Antagonists"] #6[mh ^"Antiparkinson Agents"] #7Trihexyphenidyl* #8benzhexol* #9hipokinon* #10trihexan* #11Artane #12(Parkopan or Parkinane) #13(Apotrihex or Apo next Trihex) #14(trihexidyl* or tri next hexidyl*) #15(Antimuscarinic* or Anti next muscarinic*) #16(antiparkinson* or anti next parkinson*) #17{or #4‐#16} #18#3 and #17
11. LILACS (lilacs.bvsalud.org/en)
Searched 26 May 2017 (3 records)
tw:((tw:(cerebral pals*)) AND (tw:(apotrihex OR "Apo‐Trihex" OR artane OR benzhexol* OR hipokinon* OR parkinane OR parkopan OR trihexan* OR trihexyphenidyl* OR trihexidyl* OR tri‐hexidyl* OR antiparkinsonian OR "anti‐parkinsonian" OR antimuscarinic* OR "Anti‐muscarinic*" ))) AND (instance:"regional") AND ( db:("LILACS"))
12. ClinicalTrials.gov
Searched 26 May 2017 (15 records*)
Basic search screen cerebral palsy AND dystonia (15 records) or Advanced search Condition| cerebral palsy AND Intervention| trihexyphenidyl (1 record)
*15 records after one duplicate removed
13. World Health Organisation International Clinical Trials Registry Platform (WHO ICTRP; who.int/ictrp/en)
Searched 26 May 2017 (17 records)
Basic search cerebral palsy AND dyston* (17) ADVANCED SEARCH CONDITON| cerebral palsy AND INTERVENTION| Trihexyphenidyl (0)
Appendix 2. Criteria for assigning risks of bias
Sequence generation
Low risk of bias: if a random component was used in the sequence generation process such as coin tossing, computer‐generated random numbers, or a table of random numbers
High risk of bias: if a non‐random component was used in the sequence generation process
Unclear risk of bias: if the sequence generation process was not described
Allocation concealment
Low risk of bias: if participants and trial investigators had no foreknowledge (i.e. prior to eligibility decisions being made avn informed consent being obtained) of intervention assignment through the use of, for example, central allocation or sequentially numbered envelopes that were opaque and sealed
High risk of bias: if participants and trial investigators had foreknowledge of intervention assignment
Unclear risk of bias: if the method of allocation concealment was not described
Blinding of participants and personnel
Low risk of bias: if there was no blinding or incomplete blinding but review authors judge the outcome is unlikely to have been influenced by the lack of blinding, or if blinding of study participants and personnel was ensured and it is unlikely that blinding could have been broken
High risk of bias: if there was no blinding or incomplete blinding and the outcome was likely influenced by the lack of blinding, or if blinding of study participants and personnel was attempted but it is likely that blinding could have been broken and the outcome influenced by the lack of blinding
Unclear risk of bias: if lack of information prohibits judgement of either low or high risk of bias, or if the study did not address this risk of bias
Blinding of outcome assessment
Low risk of bias: if there was no blinding of outcome assessment but review authors judge that the outcome measurement is unlikely to have been influenced by lack of blinding, or if blinding of outcome assessment was ensured and it is unlikely that blinding could have been broken
High risk of bias: if there was no blinding of outcome assessment and the outcome measurement was likely influenced by a lack of blinding, or if there was blinding of outcome assessment but it is likely that blinding could have been broken and the outcome measurement influenced by lack of blinding
Unclear risk of bias: if lack of information prohibits judgement of either low or high risk of bias, or if the study did not address this risk of bias
Incomplete outcome data
Low risk of bias: if no missing data were reported, or if appropriate methods were used to impute missing data, or if the reason for missing data is unlikely to be related to the true outcome
High risk of bias: if missing data were reported and no appropriate methods were used to impute missing data, or if the reason for missing data is likely to be related to the true outcome
Unclear risk of bias: if lack of information prohibits judgement of either low or high risk of bias, or if the study did not address this risk of bias
Selective reporting
Low risk of bias: if a study has a protocol and all prespecified outcomes have been reported in the prespecified manner, or if a study has no protocol but all expected outcomes have been reported
High risk of bias: if a study has a protocol and one or more prespecified outcomes have not been reported or have been reported in a manner that was not prespecified, or if a study has no protocol and all expected outcomes have not been reported
Unclear risk of bias: if lack of information prohibits judgement of either low or high risk of bias
Other sources of bias
Low risk of bias: if no other sources of bias (e.g. contamination or recruitment bias) appear to exist
High risk of bias: if other sources of bias exist
Unclear risk of bias: if lack of information permits judgement of whether other sources of bias exist
Data and analyses
Comparison 1. Trihexphenidyl versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
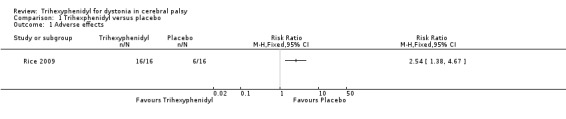
Comparison 1 Trihexphenidyl versus placebo, Outcome 1 Adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Rice 2009.
| Methods |
Study type: randomised, double‐blind, placebo‐controlled cross‐over trial Study start and end dates: not stated Study duration: 28 weeks |
|
| Participants |
Country: Australia Study site: the rehabilitation department of the Children's Hospital at Westmead, Australia Sample size: 16 Withdrawals/dropouts: 2 Age: mean 9 years (SD 4.3 years, range 2‐17 years, median 7.9 years) Sex: 10 boys, 6 girls Diagnosis: predominant dystonic cerebral palsy, with or without associated spasticity Diagnostic tool: physician clinical assessment ‐ no specific tool used Inclusion criteria: children aged 2‐18 years with predominant dystonic cerebral palsy; not treated with trihexyphenidyl or another anticholinergic medication in the previous 3 months; and use of other treatments, such as oral baclofen or intrathecal baclofen, at a stable dose for 3 months and unlikely to be altered Exclusion criteria: planned change in therapy programme over the duration of the study; surgical or medical interventions, such as orthopaedic surgery or botulinum toxin injections, scheduled during the study or in the 6 months prior to study entry |
|
| Interventions |
Intervention: trihexyphenidyl for 12 weeks. Started at 0.2 mg/kg/d in 3 divided doses and increased over 6 weeks up to a maximum of 2.5 mg/kg/d in 3 divided doses. Delivered orally in liquid format 10 mg/mL Placebo: placebo liquid delivered orally at 2.5 mg/kg/d of liquid in 3 divided doses (equals same volume as intervention) for 12 weeks Washout period: 4 weeks |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Assessment time points: baseline, 12 weeks and 28 weeks after commencement |
|
| Notes |
Funding: not stated Declarations/conflicts of interest: none *The study reported on side effects, which we considered as adverse effects. We counted all side effects as adverse effects as defined in our protocol (Baker 2017). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used a randomisation table |
| Allocation concealment (selection bias) | Low risk | Comment: the trial site pharmacy generated the randomisation table and kept codes concealed until after data collection was complete |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: assessors, patient and family all blinded to intervention and placebo. Breaking the code in the event of severe adverse effects was possible by accessing the on‐call pharmacist. However, there is no mention of whether the code needed to be broken, even for the 2 participants who withdrew |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessors were blinded as to whether child had received intervention or placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 of 8 children who both received the active treatment in the first phase did not complete the study |
| Selective reporting (reporting bias) | Low risk | Comment: reported all pre‐specified outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Reddihough 1990 | Not a randomised controlled trial |
Differences between protocol and review
-
General
Throughout the review, we have reworded the outcomes of ‘increased activity’, ‘ improved participation in activities of daily living’, ‘reduced pain’ and ‘improved quality of life’ to the following, more neutral formulations, to reflect that we are assessing the variable rather than improvement in the variable: activity, participation, pain and quality of life.
We are using the most recent chapters of the Cochrane Handbook for Systematic Reviews of Interventions.
-
Selection of studies
The protocol, Baker 2017, stated that LBB and ARH would independently screen the titles and abstracts of the citations identified from the search and obtain the full texts of those studies that met, or seemed likely to have met, the inclusion criteria and assess them for relevance. However, ARH and AS did this.
The protocol stated that the other members of the review team (KJE, DSR, AS, KW) would act as arbiters in the event of dispute; however, KW was the arbiter.
-
Data extraction and management
The protocol stated that LBB and ARH would independently extract data from the included studies using a data extraction form designed and piloted for this review, and that disagreements would be resolved through consultation with the other authors (KJE, DSR, AS, KW). ARH and AS performed the data extraction, resolving disagreements in consultation with KW.
The protocol stated that LBB would enter data into RevMan 2014 and that ARH or KJE would check it for accuracy. ARH performed data entry, and KW checked it for accuracy.
-
Asessment of risk of bias in included studies
The protocol stated that LBB and ARH would independently assess the risk of bias in the included studies and that they would resolve disagreements by consulting with the other review authors (KJE, DSR, AS, KW). ARH and AS performed the 'Risk of bias' assessment, with KW resolving disagreements.
-
Measures of treatment effect
The protocol stated that we would use an odds ratio (OR) for dichotomous data. However, adverse effects (the only dichotomous outcome in this review) were frequent in the included trial, so the risk ratio and odds ratio differ markedly. We reported the risk ratio (RR), as we believe it to be the more interpretable statistic.
The protocol stated that for continuous data we would use final values unless some of the studies used change scores, and that we would combine studies that reported final values with studies that reported only change scores in the same meta‐analysis, provided that the studies used the same rating scale. However, due to the one included study using change scores, we performed an ANCOVA to estimate the change score, as recommended in section 9.4.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
-
Summary of findings
There was inconsistency within the Methods section of the protocol around which outcomes we would use to populate the 'Summary of findings' table. The 'Types of outcome measures' section stated that we would use the outcomes marked with an asterisk, which was inconsistent with the 'Summary of findings' section. We have chosen to adhere to what is stated in the Types of outcome measures section, as this was our intention.
-
Search methods
In addition to Ovid MEDLINE, which is updated weekly, we searched Ovid MEDLINE In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE Epub Ahead of Print, which are updated daily.
Contributions of authors
Adrienne R Harvey informed the protocol design, applied eligibility criteria, assessed studies, extracted data, and led the review write‐up.
Louise B Baker conceived and designed the review protocol and assisted with the review write‐up.
Dinagh Susan Reddihough informed the protocol design and assisted with the review write‐up.
Adam Scheinberg informed the protocol design, applied eligibility criteria, assessed studies, extracted data and assisted with the review write‐up.
Katrina Williams informed the protocol design, acted as the third reviewer and assisted with the review write‐up.
Sources of support
Internal sources
-
Department of Paediatrics, The University of Melbourne, Australia.
Work on this review was completed by AH during office hours whilst employed by The University of Melbourne
External sources
None, Other.
Declarations of interest
Adrienne R Harvey is an Editor with the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG). She is funded through a Melbourne Children's Campus Career Development Award.
Louise B Baker was supported by the Lorenzo and Pamela Galli Charitable Trust during the early stages of this Cochrane Review, which was during the early stages of her Fellowship.
Dinah Susan Reddihough has received a grant from the Flack Trust, a charitable trust, for previous pilot work on the use of medications (benzhexol hydrochloride) for dystonia. The Flack Trust have no interest in the review’s findings that might lead to a real or perceived conflict of interest.
Adam Scheinberg ‐ none known.
Katrina Williams (KW) is an Editor with CDPLPG. KW declares that her position as APEX Australia Chair of Developmental Medicine is funded jointly by AFRID (APEX Foundation for Research into Intellectual Disability) and the Royal Children’s Hospital Foundation.
None of the authors were involved in the editorial processes associated with the publication of this review.
New
References
References to studies included in this review
Rice 2009 {published data only}
- Rice J, Waugh M‐C. Pilot study of trihexyphenidyl in the treatment of dystonia in children with cerebral palsy. Journal of Child Neurology 2009;24(2):176‐82. [DOI: 10.1177/0883073808322668; PUBMED: 19182155] [DOI] [PubMed] [Google Scholar]
- Rice J, Waugh M‐C. Trihexyphenidyl in the treatment of dystonic cerebral palsy: a pilot randomized controlled trial. Developmental Medicine & Child Neurology 2006;48(106 Suppl):12‐13. [onlinelibrary.wiley.com/doi/epdf/10.1111/j.1469‐8749.2006.tb12591.x] [Google Scholar]
References to studies excluded from this review
Reddihough 1990 {published data only}
- Reddihough D, Johnson H, Staples M, Hudson I, Exarchos H. Use of benzhexol hydrochloride to control drooling of children with cerebral palsy. Developmental Medicine & Child Neurology 1990;32(11):985‐9. [DOI: 10.1111/j.1469-8749.1990.tb08121.x; PUBMED: 2269408] [DOI] [PubMed] [Google Scholar]
Additional references
ACPR 2016
- Australian Cerebral Palsy Register Group. Australia and the Australian Cerebral Palsy Register for the birth cohort 1993 to 2006. Developmental Medicine & Child Neurology 2016;58(S2):3‐4. [DOI: 10.1111/dmcn.13002; PUBMED: 26806361] [DOI] [PubMed] [Google Scholar]
Allen 2014
- Allen NM, Lin J‐P, Lynch T, King MD. Status dystonicus: a practice guide. Developmental Medicine & Child Neurology 2014;56(2):105–12. [DOI: 10.1111/dmcn.12339; PUBMED: 24304390] [DOI] [PubMed] [Google Scholar]
Barry 1999
- Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry‐Albright Dystonia Scale. Developmental Medicine & Child Neurology 1999;41(6):404‐11. [DOI: 10.1111/j.1469-8749.1999.tb00626.x; PUBMED: 10400175] [DOI] [PubMed] [Google Scholar]
Bax 2006
- Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA 2006;296(13):1602‐8. [DOI: 10.1001/jama.296.13.1602; PUBMED: 17018805] [DOI] [PubMed] [Google Scholar]
Ben‐Pazi 2011
- Ben‐Pazi H. Trihexyphenidyl improves motor function in children with dystonic cerebral palsy: a retrospective analysis. Journal of Child Neurology 2011;26(7):810‐6. [DOI: 10.1177/0883073810392582; PUBMED: 21498790] [DOI] [PubMed] [Google Scholar]
Carranza del Rio 2011
- Carranza‐del Rio J, Clegg NJ, Moore A, Delgado MR. Use of trihexyphenidyl in children with cerebral palsy. Pediatric Neurology 2011;44(3):202‐6. [DOI: 10.1016/j.pediatrneurol.2010.09.008; PUBMED: 21310336] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MC, editor(s). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI: 10.1136/bmj.315.7109.629; PMC2127453; PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endicott 1976
- Endicott J, Spitzer RL, Fleiss, JL, Cohen J. The Global Assessment Scale. A procedure for measuring overall severity psychiatric disturbance. Archives of General Psychiatry 1976;33(6):766‐71. [DOI: 10.1001/archpsyc.1976.01770060086012; PUBMED: 938196] [DOI] [PubMed] [Google Scholar]
Ferber 2016
- Ferber R. 3‐D Gait. www.3dgaitanalysis.com/3d‐gait/ (accessed 3 March 2016).
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐8. [DOI: 10.1136/bmj.328.7454.1490; PMC428525; PUBMED: 15205295] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version (accessed prior to 19 April 2018). Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hanna 2008
- Hanna SE, Bartlett DJ, Rivard LM, Russell DJ. Reference curves for the Gross Motor Function Measure: percentiles for clinical description and tracking over time among children with cerebral palsy. Physical Therapy 2008;88(5):596‐607. [DOI: 10.2522/ptj.20070314; PMC2390723; PUBMED: 18339799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
Himmelmann 2007
- Himmelmann K, Hagberg G, Wiklund LM, Eek MN, Uvebrant P. Dyskinetic cerebral palsy: a population‐based study of children born between 1991 and 1998. Development Medicine & Child Neurology 2007;49(4):246‐51. [DOI: 10.1111/j.1469-8749.2007.00246.x; PUBMED: 17376133 ] [DOI] [PubMed] [Google Scholar]
Hoon 2001
- Hoon AH Jr, Freese PO, Reinhardt EM, Wilson MA, Lawrie WT Jr, Harryman SE, et al. Age‐dependent effects of trihexyphenidyl in extrapyramidal cerebral palsy. Pediatric Neurology 2001;25(1):55‐58. [PUBMED: 11483397] [DOI] [PubMed] [Google Scholar]
Jethwa 2010
- Jethwa A, Mink J, MacArthur C, Knights S, Fehlings T, Fehlings D. Development of the Hypertonia Assessment Tool (HAT): a discriminative tool for hypertonia in children. Developmental Medicine & Child Neurology 2010;52(5):e83‐7. [PUBMED: 20540176] [DOI] [PubMed] [Google Scholar]
King 2004
- King G, Law M, King S, Hurley P, Hanna S, Kertoy M, et al. Children's Assessment of Participation and Enjoyment (CAPE) and Preferences for Activities of Children (PAC). San Antonio (TX): Harcourt Assessment, Inc, 2004. [Google Scholar]
Law 2005
- Law MC, Baptise S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian Occupational Performance Measure. 4th Edition. Ottawa (ON): CAOT Publications Ace, 2005. [Google Scholar]
Masson 2017
- Masson R, Pagliano E, Baranello G. Efficacy of oral pharmacological treatments in dyskinetic cerebral palsy: a systematic review. Developmental Medicine & Child Neurology 2017;59(12):1237‐48. [DOI: 10.1111/dmcn.13532; PUBMED: 28872668] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monbaliu 2012
- Monbalui E, Orthibus E, Cat J, Dan B, Heyrman L, Prinzie P, et al. The Dyskinesia Impairment Scale: a new instrument to measure dystonia and choreoathetosis in dyskinetic cerebral palsy. Developmental Medicine & Child Neurology 2012;54(3):278–83. [PUBMED: 22428172] [DOI] [PubMed] [Google Scholar]
Narayanan 2007
- Narayanan UG, Weir S, Fehlings DL. Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD©) Questionnaire: manual and interpretation guide. www.sickkids.ca/pdfs/Research/CPChild/6573‐CPCHILD_manual.pdf. (accessed prior to 23 April).
NIH 2005
- National Institutes of Health (NIH). Trihexyphenidyl. pubchem.ncbi.nlm.nih.gov/compound/trihexyphenidyl (accessed prior to 19 April 2018).
Oskoui 2013
- Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta‐analysis. Developmental Medicine & Child Neurology 2013;55(6):509‐19. [DOI: 10.1111/dmcn.12080; PUBMED: 23346889] [DOI] [PubMed] [Google Scholar]
PedsQL 1999
- Varni JW, Seid M, Rode CA. The PedsQLTM: measurement model for the pediatric quality of life inventory. Medical Care 1999;37(2):126‐39. [www.jstor.org/stable/3767218; PUBMED: 10024117] [DOI] [PubMed] [Google Scholar]
QUEST 1992
- DeMatteo C, Law M, Russell D, Pollock N, Rosenbaum P, Walter S. QUEST: Quality of Upper Extremity Skills Test. Hamilton (ON): McMaster University, CanChild Centre for Childhood Disability Research, 1992. [Google Scholar]
Randall 1999
- Randall MJ, Johnson LM, Reddihough DS. The Melbourne Assessment of Unilateral Upper Limb Function: Test Administration Manual. Melbourne (AU): Royal Children's Hospital, 1999. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagan: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenbaum 2007
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine & Child Neurology 2007;49(109 Suppl):8‐14. [DOI: 10.1111/j.1469-8749.2007.tb12610.x; PUBMED: 17370477] [DOI] [PubMed] [Google Scholar]
Sanger 2003
- Sanger TD, Delgado MR, Gaebler‐Spira D, Hallett M, Mink JW, Task Force on Childhood Motor Disorders. Classification and definition of disorders causing hypertonia in childhood. Pediatrics 2003;111(1):e89‐97. [DOI: 10.1542/peds.111.1.e89; PUBMED: 12509602] [DOI] [PubMed] [Google Scholar]
Sanger 2007
- Sanger TD, Bastian A, Brunstrom J, Damiano D, Delgado M, Dure, L, et al. Prospective open‐label clinical trial of trihexyphenidyl in children with secondary dystonia due to cerebral palsy. Journal of Child Neurology 2007;22(5):530‐7. [DOI: 10.1177/0883073807302601; PUBMED: 17690057] [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing 'Summary of findings' tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston M, editor(s). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
TUG 2005
- Ng SS, Hui‐Chan CW. The Timed Up & Go Test: its reliability and association with lower‐limb impairments and locomotor capacities in people with chronic stroke. Archives of Physical Medicine and Rehabilitation 2005;86(8):1641–7. [DOI: 10.1016/j.apmr.2005.01.011; PUBMED: 16084820 ] [DOI] [PubMed] [Google Scholar]
WeeFIM 1994
- Msall ME, DiGaudio K, Rogers BT, LaForest S, Catanzaro NL, Campbell J, et al. The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clinical Pediatrics 1994;33(7):421‐30. [DOI: 10.1177/000992289403300708; PUBMED: 7525140] [DOI] [PubMed] [Google Scholar]
WHO ICF 2003
- World Health Organisation (WHO). International Classification of Functioning, Disability and Health (ICF). www.who.int/classifications/icf/en/ (accessed 3 March 2016).
Williams 2006
- Williams G. Introduction to the High Level Mobility Assessment Tool. www.tbims.org/combi/himat/ (accessed 3 March 2016).
Wong‐Baker FACES Pain Rating Scale 2001
- The Wong‐Baker FACES Foundation. Wong‐Baker FACES® Pain Rating Scale. wongbakerfaces.org/ (accessed 3 March 2016).
References to other published versions of this review
Baker 2017
- Baker LB, Harvey AR, Egberts KJ, Reddihough DS, Scheinberg A, Williams K. Trihexyphenidyl for dystonia in cerebral palsy. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD012430] [DOI] [PMC free article] [PubMed] [Google Scholar]


