Abstract
Background
The management of gallbladder stones (lithiasis) concomitant with bile duct stones is controversial. The more frequent approach is a two‐stage procedure, with endoscopic sphincterotomy and stone removal from the bile duct followed by laparoscopic cholecystectomy. The laparoscopic‐endoscopic rendezvous combines the two techniques in a single‐stage operation.
Objectives
To compare the benefits and harms of endoscopic sphincterotomy and stone removal followed by laparoscopic cholecystectomy (the single‐stage rendezvous technique) versus preoperative endoscopic sphincterotomy followed by laparoscopic cholecystectomy (two stages) in people with gallbladder and common bile duct stones.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE Ovid, Embase Ovid, Science Citation Index Expanded Web of Science, and two trials registers (February 2017).
Selection criteria
We included randomised clinical trials that enrolled people with concomitant gallbladder and common bile duct stones, regardless of clinical status or diagnostic work‐up, and compared laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy procedures in people undergoing laparoscopic cholecystectomy. We excluded other endoscopic or surgical methods of intraoperative clearance of the bile duct, e.g. non‐aided intraoperative endoscopic retrograde cholangiopancreatography or laparoscopic choledocholithotomy (surgical incision of the common bile duct for removal of bile duct stones).
Data collection and analysis
We used standard methodological procedures recommended by Cochrane.
Main results
We included five randomised clinical trials with 517 participants (257 underwent a laparoscopic‐endoscopic rendezvous technique versus 260 underwent a sequential approach), which fulfilled our inclusion criteria and provided data for analysis. Trial participants were scheduled for laparoscopic cholecystectomy because of suspected cholecysto‐choledocholithiasis. Male/female ratio was 0.7; age of men and women ranged from 21 years to 87 years. The run‐in and follow‐up periods of the trials ranged from 32 months to 84 months. Overall, the five trials were judged at high risk of bias. Athough all trials measured mortality, there was just one death reported in one trial, in the laparoscopic‐endoscopic rendezvous group (low‐quality evidence). The overall morbidity (surgical morbidity plus general morbidity) may be lower with laparoscopic rendezvous (RR 0.59, 95% CI 0.29 to 1.20; participants = 434, trials = 4; I² = 28%; low‐quality evidence); the effect was a little more certain when a fixed‐effect model was used (RR 0.56, 95% CI 0.32 to 0.99). There was insufficient evidence to determine the effects of the two approaches on the failure of primary clearance of the bile duct (RR 0.55, 95% CI 0.22 to 1.38; participants = 517; trials = 5; I² = 58%; very low‐quality evidence). The effects of either approach on clinical post‐operative pancreatitis were unclear (RR 0.29, 95% CI 0.07 to 1.12; participants = 517, trials = 5; I² = 24%; low‐quality evidence). Hospital stay appeared to be lower in the laparoscopic‐endoscopic rendezvous group by about three days (95% CI 3.51 to 2.50 days shorter; 515 participants in five trials; low‐quality evidence). There was very low‐quality evidence that suggested longer operative time with laparoscopic‐endoscopic rendezvous (MD 34.07 minutes, 95% CI 11.41 to 56.74; participants = 313; trials = 3; I² = 93%). The Trial Sequential Analyses of operating time and the length of hospital stay indicated that all the trials crossed the conventional boundaries, suggesting that the sample sizes were adequate, with a low risk of random error.
Authors' conclusions
There was insufficient evidence to determine the effects of the laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy techniques in people undergoing laparoscopic cholecystectomy on mortality and morbidity. The laparoscopic‐endoscopic rendezvous procedure may lead to longer operating times, but it may reduce the length of the hospital stay when compared with preoperative endoscopic sphincterotomy followed by laparoscopic cholecystectomy. However, no firm conclusions could be drawn because the quality of evidence was low or very low. If confirmed by future trials, these data might re‐design the scenario of treatment of this condition, albeit requiring greater organisational effort. Future trials should also address issues such as quality of life and cost analysis.
Plain language summary
Laparoscopic‐endoscopic rendezvous or preoperative endoscopic sphincterotomy before removing the gallbladder for gall stones or bile duct stones
Background
Only one out of every five to ten people who experiences colicky abdominal pain has stones in the gallbladder or the common bile duct. These biliary stones may lead to cholecystitis (inflammation of the gallbladder), cholangitis (infection of the bile duct), hepatic abscess (abscess in the liver), or acute pancreatitis (infection of the pancreas).
There are different techniques used to remove the stones; standard laparotomy (incision in the abdomen), laparoscopic surgery, and endoscopic surgery. Laparoscopic surgery, also called minimally invasive surgery, is a modern surgical technique, in which abdominal operations are performed through long, rigid instruments, inserted through small incisions (usually 0.5 to 1.2 cm) in the abdominal wall. Endoscopy is a more general term, which describes a technique that enables a physician to examine the inside of a hollow organ, by inserting an instrument, generally flexible, through natural body openings. For biliary stones, endoscopy is performed by passing a scope, with a light, through the mouth and down the digestive tract, The physician can see where the biliary tract (liver, bile duct, and pancreas) meets the duodenum (beginning of the small intestine), which makes it easier to pass a tube, through which stones can be removed. The injection of radiologic contrast medium highlights the biliary ducts and their content. This procedure is called endoscopic retrograde cholangiopancreatography (ERCP).
A laparotomy is used if laparoscopic surgery is contraindicated. Otherwise, the procedure involves two stages: first, endoscopic removal of stones from the bile duct, followed by laparoscopic cholecystectomy (removal of gallbladder). A combined endoscopic and laparoscopic procedure, called a laparoscopic‐endoscopic rendezvous technique, has been associated with fewer adverse effects, less patient discomfort, and shorter hospital stay.
Study characteristics
This review compared the benefits and harms of laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy (cutting the muscle between the bile and pancreatic ducts) procedures followed by laparoscopic cholecystectomy to remove stones from the gallbladder and bile duct. By searching scientific databases and trials registers, we found five randomised clinical trials that compared the two approaches, and involved a total of 516 participants. The majority of the participants were females and the age of both men and women ranged from 21 years to 87 years.
Funding
Only one trial stated they had not received industry sponsorship or other for‐profit support. None of the other trials disclosed information about funding. Three trials stated the investigators had no competing interest; the other two trials did not provide information on competing interests.
Key results
The laparoscopic‐endoscopic rendezvous approach could be associated with a lower rate of overall morbidity and clinical post‐operative pancreatitis, and a shorter hospital stay. We found no clear differences in overall mortality between the two techniques. Total operative time was longer with the rendezvous approach.
We were unable to draw firm conclusions because of the lack of data. Further research is needed to confirm whether the single‐stage approach is safer and more efficacious than the two‐stage approach, and to address other important issues, such as quality of life and cost analysis.
Quality of the evidence
The quality of the evidence was low or very low, because of small numbers of participants, high risk of bias, and inconsistent and imprecise results across trials. The evidence is current to February 2017.
Summary of findings
Summary of findings for the main comparison. Summary of findings in the analysed outcomes.
| Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy in people undergoing laparoscopic cholecystectomy for stones in the gallbladder and common bile duct | ||||||
|
Population: patients with stones in the gallbladder and common bile duct undergoing laparoscopic cholecystectomy
Settings: inpatients
Intervention: laparoscopic‐endoscopic rendezvous (LERV) Control: preoperative endoscopic sphincterotomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with preoperative endoscopic sphincterotomy | Risk with laparoscopic‐endoscopic rendezvous | |||||
| Overall mortality (30‐day postoperative; procedure‐ and non‐procedure related) |
Study population | 516 (5 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | Only 1 death in 1 trial reported, in the LERV group | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Overall morbidity (30‐day postoperative; procedure‐ and non‐procedure related) |
Study population | RR 0.59 (0.29 to 1.20) | 433 (4 RCTs) | ⊕⊕⊝⊝ LOW 1,3 | No trials defined overall morbidity in the methods section. | |
| 142 per 1000 | 84 per 1000 (41 to 169) | |||||
| Moderate | ||||||
| 128 per 1000 | 75 per 1000 (37 to 152) | |||||
| Failure of primary clearance | Study population | RR 0.55 (0.22 to 1.38) | 516 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,3,4 | ||
| 131 per 1000 | 72 per 1000 (29 to 181) | |||||
| Moderate | ||||||
| 102 per 1000 | 56 per 1000 (22 to 141) | |||||
| Clinical postoperative pancreatitis | Study population | RR 0.31 (0.09 to 1.14) | 516 (5 RCTs) | ⊕⊕⊝⊝ LOW 1,3 | ||
| 73 per 1000 | 23 per 1000 (7 to 84) | |||||
| Moderate | ||||||
| 100 per 1000 | 31 per 1000 (9 to 114) | |||||
| Operative time | The mean operative time in the control groups was 88.6 minutes | The mean operative time in the LEVR groups was 34.07 minutes higher (11.41 to 56.74 higher) | MD: 34.07 (11.41 to 56.74) | 313 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,3,5 | TSA: 23.07 (15.32 to ‐30.81) |
| Length of hospital stay | The mean length of hospital stay in the control groups was 7.5 days | The mean length of hospital stay in the LEVR groups was 3.01 days shorter (3.51 to 2.5 days shorter) | MD: ‐3.01 (‐3.51 to ‐2.50) | 515 (5 RCTs) | ⊕⊕⊝⊝ LOW 1,3 | TSA: ‐2.87 (3.66 to ‐2.07) |
| *The risk in the intervention (LEVR) group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; RCT: randomised clinical trial; TSA: Trial Sequential Analysis | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to risk of bias: high risk of performance and detection bias in all the trials, unclear risk of selection bias in two trials, unclear risk of selective reporting in three trials and high risk in one trial, unclear risk of for‐profit bias in four trials 2Downgraded one level due to imprecision: very low event rate 3Downgraded one level due to imprecision: few trials with few participants 4Downgraded one level due to inconsistency: high heterogeneity among trials ( I² = 58%) 5Downgraded one level due to inconsistency: very high heterogeneity among trials ( I² = 93%)
Background
Description of the condition
Cholecysto‐choledocolithiasis involves the concomitant presence of stones in both the gallbladder and the common bile duct. The majority of people affected by gallbladder stones (cholelithiasis) are unaware of their presence, and over a 10‐year period of follow‐up, only up to 25% of initially asymptomatic individuals will develop biliary colic (Menezes 2000; Videhult 2009; Borzellino 2010). However, the onset of pain heralds the beginning of recurrent symptoms in the majority of patients, and identifies those at risk of more serious complications. These include pancreatitis, cholecystitis, and biliary obstruction. Over a 10‐year period, such complications can be expected to occur in 2% to 3% of patients with initially silent gallbladder stones (Davidson 1988; Neoptolemos 1989). On the contrary, little is known about the natural history of common bile duct stones, but it is estimated that about half of asymptomatic common bile duct stones, discovered accidentally at intraoperative cholangiography, will spontaneously pass the papilla of Vater within six weeks (Collins 2004). Nevertheless, because retained stones may lead to pain, partial or complete biliary obstruction, cholangitis, hepatic abscess, and pancreatitis, their removal is warranted.
Description of the intervention
Before the advent of laparoscopy, open cholecystectomy and intraoperative cholangiography, followed by open common bile duct exploration, were the standard of care for common bile duct stones removal during cholecystectomy for cholelithiasis (Neoptolemos 1989). With the increasing use of laparoscopy since the early 1990s, the surgical management of these participants now encompasses a variety of strategies (Saccomani 2005). The ideal management is still a matter of debate (Martin 2006). The most used procedure is represented by preoperative endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic sphincterotomy followed by cholecystectomy (sequential two‐stage intervention; (EAES 1998; Williams 2008)).
A Cochrane review found that ERCP is the most common approach to unblocking the bile duct in general, and not only in individuals with gallstones requiring surgery (Dasari 2013). ERCP is performed by means of a duodenoscope with lateral vision that is introduced trans‐orally until the second part of the duodenum. Here the papilla of Vater is cannulated, and a sphincterotomy is performed by means of a monopolar electrode that applies tension on the common bile duct and the papilla of Vater. Biliary endoscopic sphincterotomy followed by stone extraction using a basket or balloon catheter represents standard endoscopic therapy for common bile duct stones. Successful endoscopic treatment is possible in the majority of patients, and in skilled hands, duct clearance can be achieved in over 90% of patients (Urbach 2001; Poulose 2006; Kharbutli 2008; Topal 2010). The following cholecystectomy is generally performed laparoscopically, if not contraindicated. The optimal timing of surgery is controversial (Schiphorst 2008). In the case of incomplete clearing of the common bile duct, a common bile duct exploration may be performed during the same laparascopic intervention.
The laparoscopic‐endoscopic rendezvous (LERV) technique was developed to facilitate bile duct cannulation during endoscopic sphincterotomy, and reduce the risk of failed endoscopic common bile duct clearance, and clinical post‐operative pancreatitis due to inadvertent pancreatic duct cannulation. The technique consists of an anterograde transcystic cannulation of the bile duct during laparoscopic cholecystectomy, with a guidewire that can be retrieved with a duodenoscope, thus facilitating retrograde bile duct cannulation. An over‐the‐wire sphincterotome is then inserted and standard manoeuvres of endoscopic common bile duct stones clearance are performed. The procedure is then completed by cholecystectomy in one procedure.
How the intervention might work
The feasibility of LERV has been demonstrated in several retrospective and prospective patient series (Cavina 1998; Park 2000; Tricarico 2002; Saccomani 2005). It has been associated with lower occurrence of acute pancreatitis, shorter hospital stay, and reduced costs when compared with pre‐operative endoscopic sphincterotomy in randomised clinical trials (Lella 2006; Morino 2006; Rabago 2006). Moreover, the LERV technique may have higher therapeutic success rates than preoperative endoscopic sphincterotomy and laparoscopic bile duct exploration (Filauro 2000; Saccomani 2005;La Greca 2008). The majority of endoscopists consider it easier to do than standard ERCP (La Greca 2008). Despite its advantages, several limitations need to be mentioned. People with a history of total or partial gastric resection are unlikely to be suitable for either a LERV procedure or for standard ERCP (Shimatani 2014). Other limitations are giant impacted stones, Mirizzi syndrome (a rare syndrome in which a gallstone becomes impacted in the cystic duct or neck of the gallbladder, and from here causes compression of the common bile duct), and preampullary diverticula (Williams 2002; Lella 2006; Morino 2006). Despite selective bile duct cannulation, morbidity rates of up to 19% have been reported, including post‐sphincterectomy bleeding, cystic duct leak, and pancreatitis (La Greca 2009). The procedure requires a specialised ERCP team, and takes about 60 minutes longer than laparoscopic cholecystectomy to perform (Saccomani 2005).
Why it is important to do this review
Although the sequential two‐step approach remains the predominant management, several studies have concluded that LERV affords the advantage of a single‐stage treatment for common bile duct stones in participants with symptomatic gallstones disease (Lella 2006;Morino 2006;Rabago 2006).
Despite results favouring the single‐stage approach over the two‐stage approach, preoperative ERCP is routinely used to treat common bile duct stones in participants scheduled for laparoscopic cholecystectomy (EAES 1998; Williams 2008), while laparoscopic common bile duct exploration is still considered a highly demanding procedure requiring advanced laparoscopic expertise (Poulose 2006).
A systematic review can evaluate the potential advantages of the LERV technique versus pre‐operative endoscopic sphincterotomy, as shown in previous studies (Lella 2006; Morino 2006; Rabago 2006). Two recent systematic reviews compared the one‐stage versus the two‐stage approach to cholecysto‐choledocholithiasis, but the experimental groups were treated with different techniques (LERV technique, intraoperative 'non‐aided' endoscopic retrograde cholangiography, or laparoscopic clearance of the common bile duct (Gurusamy 2011; Alexakis 2012)). Recognizing that a focused review of a single‐stage technique is important in order to ascertain its potential advantages over the more common two‐stage approach to the problem, we selected only randomised clinical trials that compared the LERV technique and the sequential two‐staged approach (preoperative endoscopic sphincterotomy and subsequent laparoscopic cholecystectomy) for the present systematic review.
Objectives
To compare the benefits and harms of endoscopic sphincterotomy and stone removal followed by laparoscopic cholecystectomy (the single‐stage rendezvous technique) versus preoperative endoscopic sphincterotomy followed by laparoscopic cholecystectomy (two stages) in people with gallbladder and common bile duct stones.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised clinical trials were eligible regardless of language or publication status (full article, thesis, or abstract). We considered non‐randomised clinical and other observational studies retrieved through literature search for additional data on harm. By choosing these approaches, we are aware of the risks of putting more emphasis on benefits than on harms.
Types of participants
We only considered randomised clinical trials enrolling participants with both cholelithiasis and choledocholithiasis, regardless of inflammatory status (cholecystitis, cholangitis, pancreatitis) and grade of biliary obstruction (overt or subclinical jaundice). We did not consider the type of diagnostic workup for common bile duct stones (i.e. intraoperative cholangiography, magnetic resonance imaging, computed tomography, ultrasonography, laboratory tests) as a limitation. Clinical signs of gallbladder and bile duct stones were:
Liver function tests elevated above the normal limits (aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transpeptidase, alkaline phosphatase, and total bilirubin).
Cholecystitis, cholangitis, and pancreatitis, alone or a combination.
Abdominal ultrasonography showing possible common bile duct stones or a dilated common bile duct (diameter above 8 mm).
Magnetic resonance cholangiopancreatography showing common bile duct stones.
Types of interventions
The LERV technique (as described by Cavina 1998) versus preoperative endoscopic sphincterotomy followed by laparoscopic cholecystectomy. We looked for trials in which contemporaneous laparoscopic cholecystectomy was performed in the control group without restrictions on the timing of the subsequent operation.
We excluded trials in which anterograde sphincterotomy or non‐aided intraoperative retrograde cholangiopancreatography interventions were performed. We did not consider trials involving participants treated only with postoperative ERCP, or with intraoperative common bile duct exploration, either laparoscopic or open.
Types of outcome measures
Primary outcomes
Overall mortality rate (procedure‐related and non‐procedure‐related), assessed at the latest follow‐up;
Overall morbidity rate. We assessed surgical morbidity (i.e. pancreatitis, bleeding, intestinal perforations) and general morbidity (i.e. pneumonia, wound infection, cardiac complications, deep venous thrombosis, etc.), assessed at the latest follow‐up;.
Failure of primary clearance (duct clearance as determined by cholangiogram, number of successful common bile duct cannulations).
Secondary outcomes
Clinical postoperative pancreatitis, whenever stated by the authors of the trials (defined as pancreatic‐like pain, persisting for at least 24 hours after ERCP, associated with a significant increase in serum amylase levels);
Health‐related quality of life assessment;
Length of operative time;
Length of hospital stay.
Search methods for identification of studies
Electronic searches
To identify ongoing and recently completed trials, we searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2017; 14 February 2017), the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library (14 February 2017), MEDLINE Ovid (1946 to 14 February 2017), Embase Ovid (1974 to 14 February 2017), and Science Citation Index Expanded Web of Science (1900 to 14 February 2017; Royle 2003), as well as Clinicaltrials.gov (clinicaltrials.gov) and the The World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/search/en). Because LERV was first standardised in 1998 (Cavina 1998), we limited database searches to reports published after 1995. We had no limitation on language of publication. We have provided the search strategies and relative time spans of the searches in Appendix 1.
Searching other resources
We screened the reference lists of potentially relevant articles for other potentially relevant citations. We handsearched the international meeting proceedings of the American Hepato‐Pancreatico‐Biliary Association from 2007 through 2016 (www.ahpba.org/archives).
Data collection and analysis
Selection of studies
Two review authors (NV and FF) independently screened the titles and abstracts of retrieved references for potentially relevant studies. After this initial assessment, we retrieved the full text of all potentially relevant studies. NV and FF independently checked the full papers for eligibility. They resolved disagreements by discussion, and requested the input of another review author (AA) if needed. The review authors recorded all reasons for exclusion.
Data extraction and management
We extracted the details of eligible studies and summarised them on a data extraction sheet. Two review authors (NV and FF) independently extracted the data, and resolved disagreements by discussion. We contacted the study authors to obtain missing information; in effect, we send an email to all contact authors (August 2016 and April 2017). We extracted the following data from the identified publications:
Year and language of publication;
Country;
Inclusion and exclusion criteria;
Other co‐interventions;
Outcomes (mentioned above);
Risk of bias (described below);
Duration of follow‐up;
Funding and conflict of interest.
Assessment of risk of bias in included studies
Two review authors (NV and FF) independently assessed the risk of bias of each trial, according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the Cochrane Hepato‐Biliary Group Module (Gluud 2017), and methodological studies (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savovic 2012; Savovic 2012a; Lundh 2017). We used the following definitions to assess risk of bias.
Random sequence generation
Low risk of bias: sequence generation using computer‐generated random numbers or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: method of sequence generation not specified.
High risk of bias: the investigators described a non‐random component in the sequence generation process, such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention.
Allocation concealment
Low risk of bias: investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based randomisation); sequentially numbered, opaque, sealed envelopes.
Unclear risk of bias: the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: investigators enrolling participants could possibly have foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed, nonopaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Blinding of participants and personnel
Low risk of bias: no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding. Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: insufficient information to permit judgement of low or high risk.
High risk of bias: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinding of outcome assessor
Low risk of bias: no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding. Blinding of outcome assessment ensured and unlikely that the blinding could have been broken.
Unclear risk of bias: insufficient information to permit judgement of low or high risk.
High risk of bias: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values (few dropouts, balance between groups, reason for dropout reported and unrelated to the intervention or the outcomes). Sufficient methods, such as multiple imputation, were employed to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data, in combination with the method used to handle missing data, were likely to induce bias on the results.
High risk of bias: reason for missing outcome data likely to be related to true outcome, with imbalance in either numbers or reasons for missing data across intervention groups.
Selective outcome reporting
Low risk: the trial reported the following pre‐defined outcomes: overall mortality, overall morbidity (surgical and general), failure of primary clearance. If the original trial protocol was available, the outcomes had to be the ones outlined in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought had to be those enumerated in the original protocol, if the trial protocol was registered before or at the time that the trial began. If the trial protocol was registered after the trial began, those outcomes were not considered reliable.
Unclear risk: not all data on pre‐defined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk: data from one or more predefined outcomes were not reported.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other kind of for‐profit support that may manipulate the trial design, conduct, or results of the trial.
Unclear risk of bias: the trial may or may not be free of for‐profit bias, as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry, or received other kinds of for‐profit support.
Other biases
Low risk of bias: the trial appeared to be free of other sources of bias.
Unclear risk of bias: there was insufficient information to assess whether other sources of bias were present.
High risk of bias: it was likely that potential sources of bias, related to the specific trial design used or other risks, were present.
We judged trials at low risk of bias in all domains, as trials at low risk of bias. In all other cases, we considered the trials at high risk of bias.
Measures of treatment effect
We calculated the risk ratio (RR) with 95% confidence intervals (CI) for dichotomous variables. When analysing continuous variables, we calculated the mean difference (MD) with 95% CI (for outcomes such as total hospital stay), or the standardised mean difference (SMD) with 95% CI (for outcomes such as pain, whenever assessed by different pain scales). Generally, in the analyses of continuous variables, means with their corresponding standard deviations (SDs) are needed to calculate weights or standardised mean differences with 95% Cl. However, some variables, i.e. length of hospital stay or length of operative time, tend to have non‐Gaussian distribution. Therefore, authors understandably use non‐parametric statistics and give their data as medians with ranges. We had planned to present mean and median data separately, but we only used the mean data for the meta‐analyses if a trial failed to report SDs for an outcome measure,
Dealing with missing data
We performed an intention‐to‐treat analysis when possible (Newell 1992). Otherwise, we performed an available‐case analysis (Higgins 2011). For continuous variables, if the mean value was missing from the report and author communications (either as numbers or graphs), we used the average from other studies. In addition, we tried to contact authors of included trials.
Assessment of heterogeneity
We considered both clinical and statistical heterogeneity. Where appropriate, we analysed data using meta‐analyses, i.e. where trials appeared similar for participants, type of intervention, duration of the trial, and outcomes. We assessed statistical heterogeneity using the I² statistic, which examines the percentage of total variation across trials due to heterogeneity, rather than chance (Higgins 2002).
We classified heterogeneity using the following I² values:
0% to 25%: low heterogeneity;
25% to 50%: moderate heterogeneity;
50% to 100%: high heterogeneity.
Assessment of reporting biases
We initially planned to use a funnel plot to explore bias, in the presence of at least 10 trials for our primary outcome (Egger 1997; Macaskill 2001). Asymmetry in the funnel plot of trial size against treatment effect was used to assess this bias. We initially planned to perform the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry, in the presence of at least 10 trials for the outcome. But because only five trials were included in this review, we could not use funnel plots.
Data synthesis
Meta‐analyses
We performed meta‐analyses according to the recommendations of Cochrane, and the Cochrane Hepato‐Biliary Group module (Higgins 2011; Gluud 2017). We used the fixed‐effect model and the random‐effects model. Because substantial heterogeneity between trials was expected, we reported only the random‐effects model. We reported the results for both models if there were differences (e.g. significant result with the fixed‐effects model, but not significant with the random‐effects model). If we detected substantial statistical heterogeneity (i.e. a high I² value), we meta‐analysed the results of the trials, provided that the majority of the individual trial results were consistent with the direction of the effect (i.e. the RR and CI largely fall on one side of the null line) upon visual examination of the forest plot. We conducted meta‐analyses using Review Manager 5 (RevMan 2014).
Trial Sequential Analysis
Trial Sequential Analysis is a tool for quantifying the statistical reliability of the data in a cumulative meta‐analysis (TSA 2017; Copenhagen Trial Unit 2017; Wetterslev 2017), adjusting alpha and beta values for sparse data, and repetitive testing on accumulating data (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; Copenhagen Trial Unit 2017; Wetterslev 2017). Trial Sequential Analysis combines a required information size calculation (cumulated sample size of a single trial, appropriately sized trials) with the threshold of statistical significance. In order to control for the risks of random errors due to sparse data and multiplicity, we performed Trial Sequential Analyses for both dichotomous and continuous outcomes (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; Copenhagen Trial Unit 2017; Wetterslev 2017). We based our calculations of the required information size on the proportion of participants with the outcome in the conventional group, a relative risk reduction of 20%, an alpha (type I error) of 5%, a beta (type II error) of 20%, and the diversity of the meta‐analysis (Wetterslev 2009).
Subgroup analysis and investigation of heterogeneity
We initially planned to perform the following subgroup analyses.
Trials with low risk of bias compared to trials with high risk of bias.
Participants with and without pancreatitis at debut.
Sensitivity analysis
We initially planned to conduct sensitivity analysis. However, all of the included trials were at high risk of bias, so we were unable to perform this analysis.
Summary of findings table
We created Table 1 using GRADEpro GDT software. The GRADE approach appraises the quality of a body of evidence, based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed (GRADEpro GDT). The quality of a body of evidence considers within‐study risk of bias, indirectness of the evidence, inconsistency of results between trials, imprecision of effect estimates, and risk of publication bias (Balshem 2011; Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2013; Mustafa 2013; Guyatt 2013a; Guyatt 2013b; Guyatt 2017).
Results
Description of studies
Results of the search
The search strategy identified a total of 214 references. Review of the citations and the international meeting proceedings yielded two additional references. After removing 51 duplicate results, we were left with 165 references for title and abstract review; we excluded 152 references because they clearly did not meet the criteria, and we selected 13 references for full‐text examination. Of these, we excluded six controlled non‐randomised clinical studies. Of the remaining six randomised clinical trials, one did not meet the inclusion criteria because a non‐aided intraoperative retrograde cholangiopancreatography was used in the LERV group (El Geidie 2011). Finally, five trials, in six reports, fulfilled the inclusion criteria and were suitable for the meta‐analysis (Lella 2006; Morino 2006; Rabago 2006; Tzovaras 2012; Sahoo 2014). Figure 1 illustrates the study flow diagram.
1.
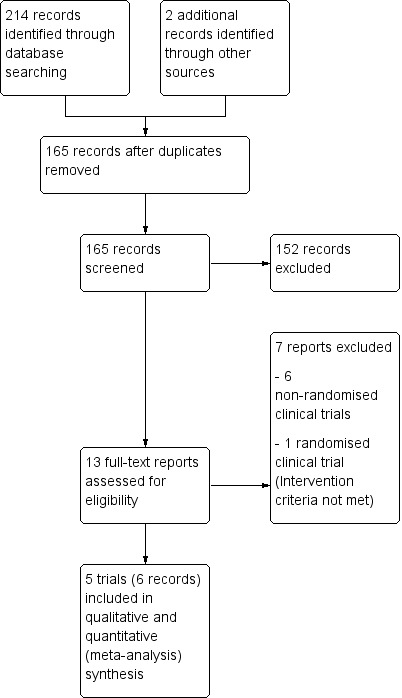
Study flow diagram
Included studies
Design
All five included trials were parallel randomised clinical trials, published in English. All were reported as full papers (Lella 2006; Morino 2006; Rabago 2006; Tzovaras 2012; Sahoo 2014). One trial was an interim analysis of an ongoing trial (Tzovaras 2012).
Sample sizes
The number of participants in each trial ranged from 83 in Sahoo 2014 to 123 in Rabago 2006. In Lella 2006, one trial participant assigned to the laparoendoscopic rendezvous technique (LERV) group was excluded from the analysis because of technical problems during the LERV procedure (loss of the wire in the intestinal loops), but he/she was included in the present meta‐analysis according to the intention‐to‐treat principle. Similarly, one trial participant in Tzovaras 2012 withdrew consent and quit the trial after completing the endoscopic retrograde cholangiopancreatography (ERCP) stage of the two‐stage approach, but he/she was included in the present meta‐analysis according to the intention‐to‐treat principle. This generated a total of 517 participants for meta‐analysis: 257 underwent LERV versus 260 underwent preoperative endoscopic sphincterotomy before cholecystectomy.
Setting
The duration of the trials ranged from 32 months in Tzovaras 2012 to 84 months in Sahoo 2014. The trials were conducted over a period of 13 years, from 1999 (Rabago 2006) to 2012 (Sahoo 2014). Four trials were European single‐centre trials conducted in Italy (Lella 2006; Morino 2006), Spain (Rabago 2006), and Greece (Tzovaras 2012); one was carried out in India (Sahoo 2014). Participants were recruited at university hospitals in three trials (Morino 2006; Tzovaras 2012; Sahoo 2014), and a non‐tertiary care centre in one trial (Rabago 2006). The trial site was not specified in one trial (Lella 2006).
Participants
Participant demographics were not detailed in Rabago 2006. In the remaining four trials, 58.5% of participants were female (male:female ratio 163:230). The age of the participants ranged from 21 years to 87 years. The American Society of Anesthesiologists (ASA) status distribution was detailed in only one trial as ASA I: 51.5%, II: 37.5%, III: 11% (Tzovaras 2012). In two other trials, ASA status was simply described as being similar between the groups (Morino 2006; Rabago 2006); ASA status was not mentioned in Lella 2006 or Sahoo 2014. Mean body‐mass index (BMI) of participants was given only in Tzovaras 2012 (mean BMI: 27).
All five trials involved adult participants with suspected cholecysto‐choledocholithiasis, scheduled for laparoscopic cholecystectomy. Three trials included only participants with common bile duct stones, confirmed on magnetic resonance cholangiopancreatography (MRCP) (Lella 2006; Morino 2006; Sahoo 2014). In Tzovaras 2012, a positive MRCP was an inclusion criterion only for participants at intermediate risk of having common bile duct stones, but it was not routinely performed in those at high risk for choledocholithiasis. Finally, MRCP was rarely used in Rabago 2006, because of low availability. Acute cholangitis, obstructive jaundice, and evidence of common bile duct stones on ultrasound were inclusion criteria in two trials (Rabago 2006; Tzovaras 2012). In Rabago 2006, participants were also included when two minor inclusion criteria were present (a recent episode of acute pancreatitis, cholecystitis, or jaundice; liver function tests elevated above normal limits; or a dilated common bile duct on ultrasound). In Lella 2006, after being screened for cholecysto‐choledocholithiasis, only participants at high risk for post‐ERCP pancreatitis were included in the trial (age < 60 years, female sex, history of relapsing pancreatitis, or a bile duct diameter < 8 mm).
Morino 2006 excluded those with acute cholangitis. Other main exclusion criteria were: age < 18 years (Lella 2006; Morino 2006; Rabago 2006; Tzovaras 2012), or > 80 years (Rabago 2006); history of previous upper abdominal surgery (Rabago 2006; Tzovaras 2012); total or partial gastric resection (Morino 2006), or choledochoduodenal anastomosis (Lella 2006); ASA status IV and V (Morino 2006; Tzovaras 2012); chronic (Lella 2006; Rabago 2006), or necrotising pancreatitis (Morino 2006); suspected pancreatobiliary malignancy (Morino 2006; Rabago 2006); pregnancy (Lella 2006; Tzovaras 2012); common bile duct stones > 12 mm (Sahoo 2014); previous sphincterotomy (Lella 2006), or ERCP attempt (Tzovaras 2012). Only Tzovaras 2012 considered a BMI higher than 35 kg/m² to be an exclusion criterion.
A baseline imbalance between groups was found in only one trial, with significantly higher mean total bilirubin and gammaglutamyl transferase levels in the preoperative endoscopic sphincterotomy group (Rabago 2006).
Overall reporting on follow‐up duration was generally poor. Only two out of five trials clearly reported follow‐up times; Rabago 2006 gave a pre‐specified length of follow‐up of 24 months, while Morino 2006 reported mean follow‐up periods of 20 months for the intervention group and 19 months for the control group.
Interventions
Preoperative ERCP and LERV were performed on either an inpatient or outpatient basis in one trial (Rabago 2006); the procedures were performed on an inpatient basis in the other four trials. Scheduling for laparoscopic cholecystectomy in the control group ranged from within eight weeks after preoperative ERCP in Rabago 2006, to within 24 to 72 hours in Morino 2006. The time interval between preoperative ERCP and laparoscopic cholecystectomy was not stated in Sahoo 2014. LERV in the intervention group in Rabago 2006 was scheduled within eight weeks of randomisation. In Lella 2006 and Morino 2006, all ERCPs in both groups of participants were performed by a single endoscopist. None of the trials reported the level of experience of those who performed the LERV procedure. Common bile duct clearance was routinely attempted using a Fogarty balloon or a Dormia basket catheter in two trials (Lella 2006; Rabago 2006), while Tzovaras 2012 only used a Fogarty balloon, and two trials did not clearly specify a device (Morino 2006; Sahoo 2014). Finally, only two trials clearly stated that prophylactic use of somatostatin was not administered before the procedure in either group of participants (Lella 2006; Tzovaras 2012).
Primary outcomes
The primary outcome used for sample size calculation varied considerably between trials: clinical post‐procedure pancreatitis (Lella 2006), treatment failure (Morino 2006), morbidity (Rabago 2006), and total hospital stay (Tzovaras 2012).
All trials included mortality as an outcome measure, but only Morino 2006 and Sahoo 2014 prespecified it as 60‐day and 30‐day mortality. Mortality data were not available in Sahoo 2014, though it was included as an outcome in the methods section of the trial publication. Because of the low‐event rate, we considered it inappropriate to include double‐zero event trials in the meta‐analysis.
All trials but one included general morbidity as an outcome (Sahoo 2014), but none of them defined it in the methods section. All trials provided data about surgical morbidity. Post‐procedure serum amylase values were reported in four of five trials (Lella 2006; Morino 2006; Tzovaras 2012; Sahoo 2014), two of which specified the time point of sampling: 12 hours after the endoscopic procedure in Tzovaras 2012, and 24 hours in Lella 2006. Only Lella 2006 defined post‐ERCP pancreatitis in the methods section. All trials reported data on failure of primary clearance. Quality of life assessment was measured in Sahoo 2014.
At the time of discharge, a questionnaire was distributed to the participants to determine their satisfaction with the surgical procedure, which was graded as high, moderate, or low. At the end of every procedure, a questionnaire was distributed to the endoscopic surgeons to determine his satisfaction with the endoscopic procedure. The questionnaire elicited an opinion of the endoscopic difficulty of the one‐stage LERV versus the classic two‐stage procedure, which was graded as simpler, comparable, or more difficult when compared to other procedure.
Secondary and additional outcomes
Four trials reported the duration of the endoscopic and surgical procedures (Lella 2006; Morino 2006; Rabago 2006; Tzovaras 2012). Lella 2006 gave the median time for the combined procedure in the preoperative endoscopic sphincterectomy group, whereas Morino 2006 and Tzovaras 2012 reported separate results for the endoscopic and surgical procedures. In Rabago 2006, it was unclear whether the data on the duration of procedures in the control group were combined or not. All trials included hospital stay as an outcome measure. In Tzovaras 2012, length of stay was in both groups calculated from randomisation until discharge. Rabago 2006 indicated length of stay from the date of the first ERCP in the preoperative ERCP group, and from the date of laparoscopic cholecystectomy in the intraoperative ERCP group. Sahoo 2014 reported this outcome without a standard deviation (SD), so we could not include data in our meta‐analysis. Finally, only two trials performed a cost analysis (Morino 2006; Rabago 2006).
Excluded studies
Seven reports did not meet the inclusion criteria. Six were excluded because they were not randomised clinical trials (Miscusi 1997; Cavina 1998; Filauro 2000; La Greca 2008; Tekin 2008; Ding 2013). The authors of one randomised trial found the LERV technique as described by Cavina technically difficult, so a non‐aided intraoperative retrograde cholangiopancreatography was used in the LERV group in most cases (El Geidie 2011).
Risk of bias in included studies
For details on risk of bias of included trials, see the 'Characteristics of included studies' tables. For an overview of the review authors' judgments about each risk of bias domain for individual trials, and across all trials, see Figure 2 and Figure 3. We judged all trials at high risk of bias.
2.
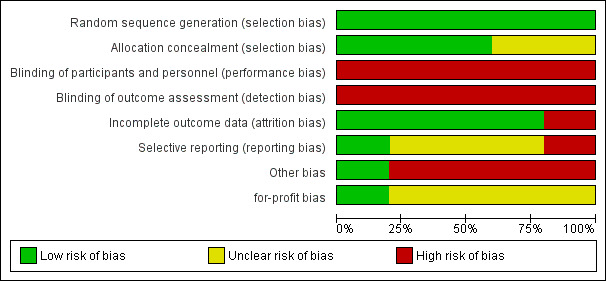
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
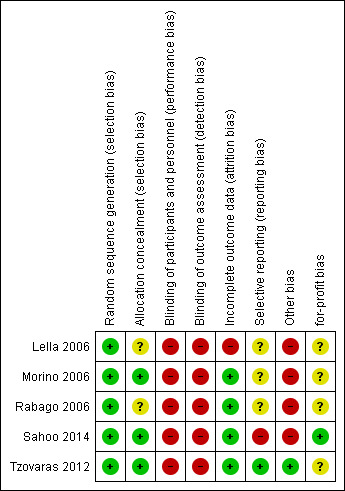
Risk of bias graph: review authors' judgements about each risk of bias item presented for each trial
Allocation
Random sequence generation: we rated all five trials at low risk of bias, since a computer‐generated list was used to randomise participants.
Allocation concealment: we rated three trials at low risk of bias, as they reported details on allocation concealment (sealed opaque envelopes) (Morino 2006; Tzovaras 2012; Sahoo 2014); we rated the other two as having unclear risk of bias because they reported no information about allocation concealment.
Blinding
In none of the trials were participants or providers blinded; the outcome assessors were not blinded in one trial (Lella 2006), and no information about outcome assessor blinding was provided in the remaining trials. We judged all trials at high risk of performance and detection bias.
Incomplete outcome data
Except for one trial, the trials accounted for all participants in the analysis; we rated them at low risk of bias. Lella 2006 excluded four patients (6.6%) from the experimental arm and one (1.6%) from the control arm from the analysis; we rated this trial at high risk of attrition bias.
Selective reporting
The trial protocol was not available for four trials; we rated three of them at unclear risk of bias. In one trial, mortality was not reported in the results, though it was listed as an outcome in the methods section, so we judged this trial at high risk of bias (Sahoo 2014). Only Tzovaras 2012 was registered at one of the available official sites for clinical trials registration and reported all the stated outcomes; we rated it at low risk of bias.
Other potential sources of bias
Only one trial declared that it was free of industry sponsorship or other for‐profit support, and we rated it at low risk of for‐profit bias (Sahoo 2014). We rated the four other trials at unclear risk of bias.
Effects of interventions
See: Table 1
We calculated results with both the random‐effects and fixed‐effect models, but we only show results from using the random‐effects model, unless there was a difference between the two.
Primary outcomes
Overall mortality (30 days postoperative)
All five trials (517 participants) measured overall mortality, but only Tzovaras 2012 reported one perioperative mortality in a 78‐year‐old, ASA III patient in the LERV group who had an uneventful LERV procedure and was discharged on the second postoperative day. He was re‐admitted on day seven with sepsis due to intra‐abdominal abscess requiring drainage; he was admitted to the intensive care unit and died of multiple organ failure on postoperative day 18. We judged the quality of evidence as low (Table 1).
Overall morbidity (30 days postoperative)
Four trials reported a lower overall morbidity in the LERV group than in the two‐stage intervention group (RR 0.59, 95% CI 0.29 to 1.20; Analysis 1.1); the effect was a little more certain when we used the fixed‐effect model (RR 0.56, 95% CI 0.32 to 0.99; participants = 434; trials = 4; I² = 28%; low‐quality evidence; Analysis 1.2). The random‐effects model might be more trustworthy as data came from different trials, and we did not feel that their homogeneity, especially the precise definition of complications, was reliable. Overall morbidity was a composite outcome, and the events that occurred in each trial were very heterogeneous (Table 2). We judged the quality of evidence as low (Table 1).
1.1. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 1 Overall morbidity (30 days postoperative).
1.2. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 2 Overall morbidity (30 days postoperative).
1. Events of the composite outcome 'overall morbidity'.
| Author | Lella 2006 | Morino 2006 | Rabago 2006 | Tzovaras 2012 | Sahoo 2014 |
| Hemobilia | NR | X | NR | X | NR |
| Acute respiratory failure with admission to intensive care unit |
NR | X | NR | no | NR |
| Early incisional hernia | NR | X | NR | no | NR |
| Bile leak | NR | no | NR | X | NR |
| Cholangitis | NR | no | NR | X | NR |
| Bleeding from sphincterotomy | NR | no | NR | X | NR |
| Bleeding form drain site | NR | no | NR | X | NR |
| Collection/biloma | NR | no | NR | X | NR |
| Wound infection | NR | no | NR | X | NR |
| Urinary retention (UTI) | NR | no | NR | X | NR |
| Duodenal perforation | X | no | NR | no | X |
NR: the authors did not report the type of post‐operative complications X: the authors reported the type of post‐operative complications no: the authors did not report the type of post‐operative complications
Failure of primary clearance
Five trials (517 participants) provided very low‐quality evidence of no clear difference between the two interventions on failure of primary clearance of stones (RR 0.55, 95% CI 0.22 to 1.38; Analysis 1.3); this result was associated with high heterogeneity (I² = 58%), generated mainly by Rabago 2006. The result with the fixed‐effect model was similar (Analysis 1.4). We judged the quality of evidence as very low (Table 1).
1.3. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 3 Failure of primary clearance.
1.4. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 4 Failure of primary clearance.
Secondary outcomes
Clinical postoperative pancreatitis
Five trials (517 participants) reported a lower incidence of acute pancreatitis in the LERV group compared with the two‐stage intervention group (RR 0.31, 95% CI 0.09 to 1.14; Analysis 1.5); the effect was a little more certain when we used the fixed‐effect model (RR 0.28, 95% CI 0.11 to 0.69; Analysis 1.6). As the definition of acute pancreatitis was not concisely described in the trials, there was moderate heterogeneity between trials (I² = 22%). Trial Sequential Analysis was not feasible due to insufficient data. We judged the quality of evidence as low (Table 1).
1.5. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 5 Clinical postoperative pancreatitis.
1.6. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 6 Clinical postoperative pancreatitis.
Quality of life assessment
Only Sahoo 2014 reported on quality of life assessment. Participant satisfaction was higher in the LERV group, in which 55% reported high satisfaction versus 7% in the two‐stage intervention group, and 41.5% versus 36% reported moderate satisfaction. Only 9.5% of the LERV group reported low satisfaction, compared to 40% in the two‐stage group. Trial Sequential Analysis was not feasible due to insufficient data. We judged the quality of evidence as low (Table 1).
Length of operative time
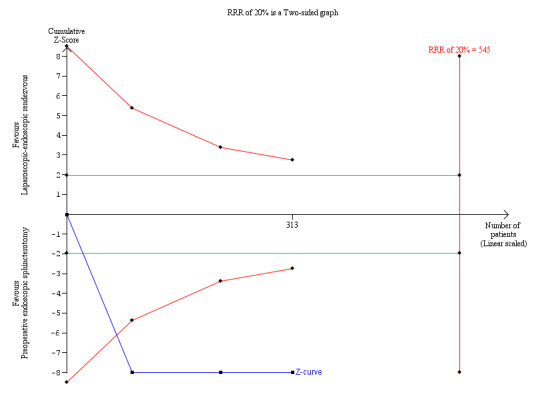
The operative time, as assessed in three of the five randomised clinical trials (313 participants), was longer in the LERV group (MD 34.07 min, 95% CI 11.41 to 56.74), although with high heterogeneity (I² = 93.0%; Analysis 1.7). The result with the fixed‐effect model was similar (Analysis 1.8). Trial Sequential Analysis demonstrated that all five trials crossed the conventional boundaries, suggesting that the sample sizes were adequate, with a low risk of random error (Figure 4). We judged the quality of evidence as very low (Table 1).
1.7. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 7 Operative time.
1.8. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 8 Operative time.
4.
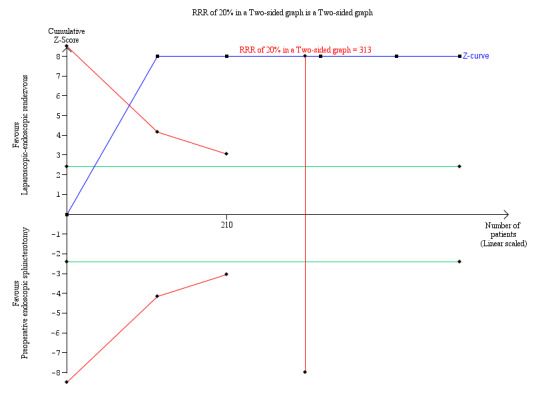
Trial Sequential Analysis of operating time. DARIS = Pc 49.52%; RRR 20%; alpha 1.6%; beta 20 %; diversity 94%.
The cumulative Z‐curve (blue line) immediately crosses the conventional boundary line. This suggests that there was a difference in the operating time between laparoscopic‐endoscopic rendezvous and preoperative endoscopic sphincterotomy, with a low risk of random error. The horizontal green lines illustrate the conventional level of statistical significance, which was intersected from the first trial. With 313 patients randomised, we had sufficient evidence to accept that preoperative endoscopic sphincterotomy took less operative time than laparoscopic‐endoscopic rendezvous. We used Trial Sequential Analysis software to conduct the analysis and to generate the figure.
Legend: square symbol: Z‐score for single study; diamond symbol: trial sequential monitoring boundary for benefit score for single study.
Abbreviations: DARIS: diversity‐adjusted required information size; Pc: control group proportion observed in the trials; RRR = a relative risk reduction.
Length of hospital stay
Five trials (515 participants) reported a shorter length of hospital stay in the LERV group (MD ‐3.01 days, 95% CI ‐3.51 to ‐2.50; I² = 12%; Analysis 1.9). The result with the fixed‐effect model was similar (Analysis 1.10). Trial Sequential Analysis revealed that all five trials crossed the conventional boundaries, suggesting that the sample sizes were adequate, with a low risk of random error (Figure 5). We judged the quality of evidence as low (Table 1).
1.9. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 9 Length of hospital stay.
1.10. Analysis.
Comparison 1 Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy, Outcome 10 Length of hospital stay.
5.
Trial Sequential Analysis of length of hospital stay. DARIS = Pc 50.29%; RRR 20%; alpha 1.6%; beta 20 %; diversity 49%.
The horizontal green lines illustrate the conventional level of statistical significance, which was intersected from the first trial. In the analysis with 515 patients randomised, we had sufficient evidence to accept that laparoscopic‐endoscopic rendezvous resulted in a shorter hospital stay than preoperative endoscopic sphincterotomy. We used Trial Sequential Analysis software to conduct the analysis and to generate the figure.
Legend: square symbol: Z‐score for single study; diamond symbol: trial sequential monitoring boundary for benefit score for single study.
Abbreviations: DARIS: diversity‐adjusted required information size; Pc: control group proportion observed in the trials; RRR = a relative risk reduction.
Subgroup analyses
We could not perform any of the two preplanned subgroup analyses on low risk of bias trials compared to trials with high risk of bias, and trial participants with and without pancreatitis at debut.
Discussion
Summary of main results
Of the 216 records identified through the database searches and other sources, we included five randomised clinical trials (517 participants) in the qualitative and quantitative synthesis. Clinical heterogeneity across the five trials was low. There was insufficient evidence to determine the effects of overall mortality(low‐quality evidence), but in reality, there was only one case out of the 517 participants included in the analysis. We found low‐quality evidence that the overall morbidity appeared to be slightly lower after laparoendoscopic rendezvous (LERV) than after the sequential two‐stage technique. We found that LERV may be associated with a slightly lower incidence of clinical post‐operative pancreatitis (low‐quality evidence). The differences were significant when using the fixed‐effect model but not with the random‐effects model. We found low‐quality evidence that length of hospital stay may have been slightly decreased, by about three days, after LERV. However, we found very low‐quality evidence that the surgical procedure may have been slightly prolonged in the LERV group. Finally, there was very low‐quality evidence that failure in primary clearance of the common bile duct did not significantly differ between the two techniques.
Overall completeness and applicability of evidence
The present meta‐analysis raises several concerns. First, clinical postoperative pancreatitis was not defined by objective criteria, such as amylase levels (which rise consistently after these procedures, even without radiological signs or symptoms), or precise clinical data (pain, fever, imaging), although the criteria were obviously the same between the two groups in each trial. Second, none of the randomised clinical trials included subgroup analyses of the results.
Alongside the apparent moderate advantages of LERV are its limitations. Intraoperative endoscopic sphincterotomy during LERV is challenging, because it requires two different specialist teams, surgeons and endoscopists. Their concomitant presence places additional organizational demands that smaller or community hospitals may not be able to handle. Moreover, each team has its own preferences for patient positioning on the operating table. Endoluminal insufflation to achieve an endoscopic view might make performing the laparoscopic procedure problematic. Also, LERV is not indicated in all participants, i.e. those with a history of total or partial gastric resection are not ideal candidates because of the technical difficulties with both the endoscopic procedure (owing to the need to reach the papilla through a reverse view) and standard ERCP, and the surgical procedure (due to supramesocolic adhesions). Other described limitations include voluminous impacted stones, biliary stenoses (e.g. Mirizzi syndrome), and peri‐ampullary diverticulum. In such situations, performing the sequential technique or attempting transcystic clearance might be a better option. For the remaining patients, the evidence was not robust regarding preference of either the LERV technique or the two‐stage technique.
All five trials reported the primary and secondary outcomes of interest, but only Sahoo 2014 reported a sort of quality of life assessment, which is of primary importance, by describing better patients compliance for the one technique over the other.
Quality of the evidence
The quality of evidence was evaluated with the GRADE approach, and in our analysis, we assessed it as low or very low for all outcomes (Table 1).
We downgraded all trials for trial limitations, or risk of bias. Only two trials reported information about allocation concealment. No trial could blind participants and personnel for the types of the intervention, and no trial reported information about blinding of the outcome assessor.
We did not believe that indirectness was present because population, interventions, and outcomes were those under consideration in our review. We downgraded for imprecision because the low number of trials and participants limited the strength of our findings. We downgraded for inconsistency of results for failure of primary clearance and operative time outcomes.
Potential biases in the review process
We conducted comprehensive searches to identify relevant trials and avoid publication bias.
The intention‐to‐treat principle was respected in all trials except for the Lella 2006 trial, in which one participant in the LERV‐treated group was excluded from the analysis because of technical problems during the LERV procedure (loss of the wire in the intestinal loops). We included data from the participant in our meta‐analysis, according to the intention‐to‐treat principle.
The primary outcome used for sample size calculation varied across trials, and the number of participants ranged from 83 to 123. We kept all the different primary outcomes used in the trials as our outcomes of interest. Four out of five trials reported on postoperative mortality, and all reported about early overall morbidity.
It might be argued that a limitation of our review was the statistical heterogeneity of the primary outcomes. This heterogeneity was most probably generated by the various components included in morbidity, and the lack of, or difference in definition of outcomes, such as pancreatitis. Even though we considered the heterogeneity to be high for some outcomes, we considered it was appropriate to conduct the meta‐analyses, mitigating the risk of bias by reporting only the random‐effects model, using other statistical methods, and interpreting the results with caution.
Furthermore, it was clearly reasonable to suspect bias in randomised clinical trials with unblinded patients. However, the average degree of bias was not known, nor its range, variation, or likely dependence on the type of outcome.
In future trials, we suggest that participants should be blinded whenever possible, therefore, it may be necessary to devote considerable resources to developing and assessing participant‐blinding procedures, especially in trials with participant‐reported outcomes.
Agreements and disagreements with other studies or reviews
No previous systematic review on this topic has been published, and no disagreement with any of the previous reports was noted.
Authors' conclusions
Implications for practice.
There was insufficient evidence to determine the effects of the laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy techniques in people undergoing laparoscopic cholecystectomy on mortality and morbidity. The laparoscopic‐endoscopic rendezvous procedure may lead to longer operating times, but it may reduce the length of the hospital stay when compared with preoperative endoscopic sphincterotomy followed by laparoscopic cholecystectomy. However, no firm conclusions could be drawn because the quality of evidence was low or very low.
Implications for research.
Few randomised clinical trials have been published to date. The published reports reflected the experience of 'pioneering’ surgical centres, which might be biased by better success rates than standard centres, and make It difficult to assess how these results could be applied to clinical practice. Future randomised clinical trials are needed to verify the possible advantage of the LERV technique over two‐stage treatment in terms of perioperative complications, especially post‐ERCP pancreatitis, while addressing other important issues, such as quality of life and cost assessments.
Acknowledgements
The Cochrane Hepato‐Biliary Group, especially Dimitrinka Nikolova, for assistance with editing and Sarah Louise Klingenberg for the search strategy, Saboor Khan and Luit Penninga for the review protocol.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of The Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors, and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Peer reviewers: Ahmet Tekin, Turkey; Riccardo Colombo, Italy. Contact editor: Saboor A Khan, UK; Emil Eik Nielsen, Denmark. Deputy Co‐ordinating Editor: Luit Penninga, Denmark. Sign‐off Editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | February 2017 | (endoscopic sphincterotom* OR EST) AND rendezvous AND (cholelithiasis OR gallstone* OR gallbladder stone) AND ((common bile duct OR choledoch*) AND (stone* OR calcul*)) |
| The Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 2017, Issue 1 | #1 MeSH descriptor: [Sphincterotomy, Endoscopic] explode all trees #2 endoscopic sphincterotom* or EST #3 #1 or #2 #4 MeSH descriptor: [Cholecystectomy, Laparoscopic] explode all trees #5 rendezvous #6 #4 or #5 #7 MeSH descriptor: [Cholelithiasis] explode all trees #8 cholelithiasis or gallstone* or gallbladder stone #9 #7 or #8 #10 MeSH descriptor: [Gallstones] explode all trees #11 (common bile duct or choledoch*) and (stone* or calcul*) #12 #10 or #11 #13 #3 and #6 and #9 and #12 |
| MEDLINE Ovid | 1946 to February 2017 | 1. exp Sphincterotomy, Endoscopic/ 2. (endoscopic sphincterotom* or EST).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 3. 1 or 2 4. exp Cholecystectomy, Laparoscopic/ 5. rendezvous.mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 6. 4 or 5 7. exp Cholelithiasis/ 8. (cholelithiasis or gallstone* or gallbladder stone).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 9. 7 or 8 10. exp Gallstones/ 11. ((common bile duct or choledoch*) and (stone* or calcul*)).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 12. 10 or 11 13. 3 and 6 and 9 and 12 14. (random* or blind* or placebo* or meta‐analysis).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 15. 13 and 14 |
| Embase Ovid SP | 1974 to February 2017 | 1. exp endoscopic sphincterotomy/ 2. (endoscopic sphincterotom* or EST).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 3. 1 or 2 4. exp CHOLECYSTECTOMY/ 5. rendezvous.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 6. 4 or 5 7. exp CHOLELITHIASIS/ 8. (cholelithiasis or gallstone* or gallbladder stone).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 9. 7 or 8 10. exp gallstone/ 11. ((common bile duct or choledoch*) and (stone* or calcul*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 12. 10 or 11 13. 3 and 6 and 9 and 12 14. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 15. 13 and 14 |
| Science Citation Index EXPANDED Web of Science | 1900 to February 2017 | #5 #4 AND #3 AND #2 AND #1 #4 TS=((common bile duct or choledoch*) and (stone* or calcul*)) #3 TS=(cholelithiasis or gallstone* or gallbladder stone) #2 TS=(rendezvous) #1 TS=(endoscopic sphincterotom* or EST) |
Data and analyses
Comparison 1. Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall morbidity (30 days postoperative) | 4 | 434 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.20] |
| 2 Overall morbidity (30 days postoperative) | 4 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.99] |
| 3 Failure of primary clearance | 5 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.22, 1.38] |
| 4 Failure of primary clearance | 5 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.37, 1.01] |
| 5 Clinical postoperative pancreatitis | 5 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.09, 1.14] |
| 6 Clinical postoperative pancreatitis | 5 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.69] |
| 7 Operative time | 3 | 313 | Mean Difference (IV, Random, 95% CI) | 34.07 [11.41, 56.74] |
| 8 Operative time | 3 | 313 | Mean Difference (IV, Fixed, 95% CI) | 34.85 [29.34, 40.37] |
| 9 Length of hospital stay | 5 | 515 | Mean Difference (IV, Random, 95% CI) | ‐3.01 [‐3.51, ‐2.50] |
| 10 Length of hospital stay | 5 | 515 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐3.37, ‐2.64] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lella 2006.
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: superiority design |
|
| Participants | 120 patients with cholecysto‐choledocholithiasis detected by transabdominal ultrasound and magnetic resonance cholangiopancreatography (MRCP); mean age 54.2 years, male 43%; history of relapsing pancreatitis: 30%; bile duct diameter < 8 mm: 12.5% Inclusion criteria: gallbladder and main bile duct stones and one or more of the following patient‐related risk factors for post‐ERCP pancreatitis: age < 60 years;history of relapsing pancreatitis; bile duct diameter < 8 mm Exclusion criteria: chronic pancreatitis and previous sphincterotomy Diagnostic criteria: gallbladder and main bile duct stones detected by both transabdominal ultrasound and MRCP |
|
| Interventions | Number of study centres: one Treatment before study: not reported Type of interventions: 60 participants treated in a single step with videolaparoscopic cholecystectomy, intraoperative cholangiography, and endoscopic sphincterotomy during the surgical procedure with the rendezvous technique versus 60 treated with preoperative ERCP and endoscopic sphincterotomy using a traditional method of bile duct cannulation |
|
| Outcomes | Rate of acute pancreatitis, level of amylasemia | |
| Notes | Run‐in period: from January 2002 to September 2004 Study terminated before regular end (for benefit or because of adverse events): no Follow‐up: not reported Funding sources: no information reported Declaration of interest: no information reported Country: Italy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation into two groups |
| Allocation concealment (selection bias) | Unclear risk | No report on concealment of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | in the LERV group "in one patient, the guidewire did not pass through the papilla, so it was necessary to make a precut. In two participants, conversion to open surgery with choledochotomy was needed: in one case due to prepapillary giant impacted stones and in the other case due to a technical problem (loss of the wire in the intestinal loops). The latter patient did not undergo the endoscopic procedure and was therefore excluded from the statistical analysis". In the other group (preoperative ERCP and endoscopic sphincterotomy performed using a traditional method of bile duct cannulation), the precut technique was needed in one patient. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was not available. |
| Other bias | High risk | The learning curve was not reported. |
| for‐profit bias | Unclear risk | Information about sponsorship or trial support not reported |
Morino 2006.
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: superiority design |
|
| Participants | 91 elective patients with cholelithiasis and common bile duct stones diagnosed at MRCP; mean age 59.5 years; male 38.4%; normal value of total bilirubin: 72.5%; normal value of gamma GT: 92%; normal value of AST: 80.15%; normal value of amylase: 26%; common bile duct diameter ⋝10 mm: 62.6% Inclusion criteria: people with gallbladder and main bile duct stones Exclusion criteria: acute cholangitis, necrotizing pancreatitis, age < 18 years, ASA status IV and V Diagnostic criteria: gallbladder and main bile duct stones were detected by transabdominal ultrasound and MRCP |
|
| Interventions | Number of study centres: one Treatment before study: not reported Type of interventions: 46 participants treated in a single step with videolaparoscopic cholecystectomy, intraoperative cholangiography, and endoscopic sphincterotomy during the surgical procedure with the rendezvous technique, and 45 treated with preoperative ERCP and endoscopic sphincterotomy using a traditional method of bile duct cannulation. |
|
| Outcomes | Morbidity, clinical pancreatitis, hyperamylasaemia, failure rate, mean hospital stay (days) | |
| Notes | Run‐in period: from May 2001 to August 2005 Study terminated before regular end (for benefit or because of adverse events): no Follow‐up: 19 to 20 months Funding sources: no information reported Declaration of interest: no information reported Country: Italy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not clearly stated whether the outcome assessors were blinded to the treatments or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. |
| Other bias | High risk | The learning curve was not reported. |
| for‐profit bias | Unclear risk | Information about sponsorship or trial support not reported |
Rabago 2006.
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: superiority design |
|
| Participants | 123 patients referred for laparoscopic cholecystectomy; mean age not reported; sex not reported; total bilirubin: intraoperative ERCP: 3.1 mg/dl (SD 2.9), pre‐operative ERCP: 2.0 mg/dl (SD 2.0); GGT: intraoperative ERCP 441 IU (SD 326 IU), pre‐operative ERCP: 334 IU (SD 281 IU) Inclusion criteria: people with intermediate risk of choledocholithiasis; one of the following major screening criteria: recent episode of cholangitis; bilirubin level > 3.5 mg/dl, or ultrasound evidence of a shadowing object within the bile duct; or at least two of the following minor screening criteria: recent episode of acute pancreatitis, cholecystitis or jaundice; elevated liver function tests above the normal limits; or a dilated common bile duct > 8 mm on ultrasound Exclusion criteria: age > 18 years to < 80 years Diagnostic criteria: gallbladder and main bile duct stones were detected by transabdominal ultrasound. Computed tomography or MRCP were optional, and rarely used in either study group. |
|
| Interventions | Number of study centres: one Treatment before study: not reported Type of interventions: 59 participants treated in a single step with videolaparoscopic cholecystectomy, intraoperative cholangiography, and endoscopic sphincterotomy during the surgical procedure with the rendezvous technique versus 64 treated with preoperative ERCP and endoscopic sphincterotomy performed using a traditional method of bile duct cannulation. |
|
| Outcomes | Success rate (on an intention‐to‐treat basis), total morbidity (mild to moderate morbidity, severe morbidity), post‐ERCP morbidity (mild to moderate morbidity, severe morbidity), post‐ERCP acute pancreatitis, post‐ERCP cholecystitis, post‐ERCP cholangitis, post‐ERCP papillar bleeding, morbidity of cholecystectomy | |
| Notes | Run‐in period: from June 1999 to June 2003 Study terminated before regular end (for benefit or because of adverse events): no Follow‐up: 24 months Funding sources: no information reported Declaration of interest: none declared Country: Spain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not clearly stated whether the outcomes assessors were blinded to the treatments or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. |
| Other bias | High risk | The learning curve was not reported. |
| for‐profit bias | Unclear risk | Information about sponsorship or trial support not reported |
Sahoo 2014.
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: superiority design |
|
| Participants | 83 patients with a diagnosis of cholecysto‐choledocholithiasis; mean age 47.95 years; male 36.1%; mean total serum bilirubin: 7.2 mg/dl; mean serum alkaline phosphatase: 619 IU/L; mean common bile duct diameter: 12.6 mm Inclusion criteria: people with diagnosis of cholelithiasis and choledocholithiasis Exclusion criteria: persons with stones in CBD > 12 mm, after undergoing laparoscopic CBD exploration Diagnostic criteria: abdominal ultrasound and MRCP |
|
| Interventions | Number of study centres: one Treatment before study: not reported Type of interventions: 42 participants treated in a single step with video laparoscopic cholecystectomy, intraoperative cholangiography, and endoscopic sphincterotomy during the surgical procedure with the rendezvous technique versus 41 treated with preoperative ERCP and endoscopic sphincterotomy. |
|
| Outcomes | Success rate of CBD clearance, incidence of multiple endoscopic procedures within 30 days of the procedure, incidence of hyperamylasaemia within 48 hours post‐ERCP, incidence of severe pancreatitis within 48 hours post‐ERCP, post‐operative hospital stay, number of deaths within 30 days of intervention, patient satisfaction concerning the surgical procedure carried out, endoscopic surgeon's satisfaction with the endoscopic procedure | |
| Notes | Run‐in period: from 2005 to 2012 Study terminated before regular end (for benefit or because of adverse events): no Follow‐up: not reported Funding sources: none Declaration of interest: none declared Country: India |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not clearly stated whether the outcomes assessors were blinded to the treatments or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | High risk | The study protocol was not available. Mortality not reported in the results, though it was declared as an outcome in the method section. |
| Other bias | High risk | The learning curve was not reported. |
| for‐profit bias | Low risk | Free of industry sponsorship or other for‐profit support |
Tzovaras 2012.
| Methods | Randomised controlled clinical trial Randomisation ratio: superiority design Interim analysis of the first 100 randomised patients |
|
| Participants | 100 patients with cholecysto‐choledocholithiasis; one patients from the control group withdrew consent after randomisation; mean age: 67.5 years; male: 46.5%; median common bile duct diameter: 9 mm; mean BMI: 27; ASA I: 51.5%, II: 37.5%, III: 11% Inclusion criteria: people with stones in gallbladder and CBD Exclusion criteria: age < 18 years, ASA status IV and V, BMI > 35, previous ERCP attempt, history of upper abdominal surgery, and pregnancy Diagnostic criteria: gallbladder and main bile duct stones were detected by both transabdominal ultrasound and MRCP |
|
| Interventions | Number of study centres: one Treatment before study: not reported Type of interventions: 50 patients treated in a single step with videolaparoscopic cholecystectomy, intraoperative cholangiography, and endoscopic sphincterotomy during the surgical procedure with the rendezvous technique versus 49 treated with preoperative ERCP and endoscopic sphincterotomy using a traditional method of bile duct cannulation |
|
| Outcomes | Mortality, morbidity, conversions, clinical pancreatitis, serum amylase, failure rate, hospital stay (days) | |
| Notes | Run‐in period: from September 2006 to April 2009 Study terminated before regular end (for benefit or because of adverse events): no Follow‐up: not reported Funding sources: no information reported Declaration of interest: none declared Country: Greece |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization created by a computer‐generated list in blocks of 20 patients. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not clearly stated whether the outcomes assessors were blinded to the treatments or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient from the control group withdrew consent after randomisation. |
| Selective reporting (reporting bias) | Low risk | The trial was registered at one of the available official sites for clinical trials registration (ClinicalTrials.gov ID: NCT00416234). |
| Other bias | Low risk | Interim analysis planned after completion of the first 100 patients |
| for‐profit bias | Unclear risk | Information about sponsorship or trial support not reported |
ERCP = endoscopic retrograde cholangiopancreatography; LERV = laparoscopic‐endoscopic rendezvous; CBD = common bile duct; MRCP = magnetic resonance cholangiopancreatography; ASA = American Society of Anesthesiologists; BMI = body mass index; AST = aspartate aminotransferase
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cavina 1998 | A controlled non‐randomised clinical study with three equally numbered study groups: 16 people with cholelithiasis underwent LERV; 16 people with common bile duct stones underwent endoscopic sphincterotomy before laparoscopic cholecystectomy, and 16 people with papillitis underwent open cholecystectomy and transduodenal sphincterotomy. |
| Ding 2013 | A controlled non‐randomised clinical study with two groups of persons with cholelithiasis and common bile duct stones or papillitis: 70 underwent LERV and 80 underwent endoscopic sphincterotomy before laparoscopic cholecystectomy. |
| El Geidie 2011 | A controlled non‐randomised clinical study with two groups of participants with cholelithiasis and common bile duct stones or papillitis: 21 underwent laparoscopic cholecystectomy combined with intraoperative endoscopic sphincterotomy and 17 had endoscopic sphincterotomy before laparoscopic cholecystectomy. In the early patients, the investigators tried to pass a guided wire through the cystic duct into the CBD to facilitate bile duct cannulation at subsequent endoscopy (the LERV technique described by Cavina and colleagues), but they found it technically difficult, and also experienced difficulties during laparoscopic cholecystectomy (due to bowel insufflation), so this step was omitted in most cases. |
| Filauro 2000 | A controlled non‐randomised clinical trial of two groups of participants with cholelithiasis and common bile duct stones or papillitis: 21 underwent LERV and 17 had endoscopic sphincterotomy before laparoscopic cholecystectomy. |
| La Greca 2008 | A controlled non‐randomised clinical trial: 21 underwent LERV and 17 had endoscopic sphincterotomy before laparoscopic cholecystectomy. |
| Miscusi 1997 | A controlled non‐randomised clinical trial with three groups of participants with cholelithiasis and common bile duct stones or papillitis: 8 underwent LERV, 73 had endoscopic sphincterotomy before laparoscopic cholecystectomy, and 16 combined laparoscopic cholecystectomy and CBD exploration (LCBDE). |
| Tekin 2008 | A controlled non‐randomised clinical trial with two groups of participants with cholelithiasis and common bile duct stones or papillitis: 35 underwent LERV and 41 had endoscopic sphincterotomy before laparoscopic cholecystectomy. |
Differences between protocol and review
The review title was changed from 'Single‐stage laparoendoscopic rendezvous and cholecystectomy versus sequential preoperative endoscopic sphincterotomy and laparoscopic cholecystectomy for the treatment of gallbladder and common bile duct stones' into "Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy in people undergoing laparoscopic cholecystectomy for stones in the gallbladder and bile duct" to better clarity the entire procedure of removal of duct stones and laparoscopic cholecystectomy in the treatment of cholecystocholedocolithiasis.
Data synthesis: The following sentence, "We will report results using the random‐effects model when heterogeneity between the trials is substantial (I² > 50%), or use the fixed‐effect model when heterogeneity between the trials is unimportant or moderate (I² < 50%). We will report both results when differences in statistical significance exist when applying the two models." was substituted with the following: "We used the fixed‐effect model and the random‐effects model. Because substantial heterogeneity between trials was expected, we reported only the random‐effects model. We reported the results for both models if there were differences (e.g. significant result with the fixed‐effects model, but not significant with the random‐effects model)".
Types of outcome measures: we moved "quality of life assessment" from primary to secondary outcomes, due to editorial reviews. Also we distinguished "clinical pancreatitis" as an independent secondary outcome measure because of the importance given in the trials to this outcome, i.e. we did not include it in surgical morbidity.
Measures of treatment effects: In case of missing SDs for an outcome measure in the trials, differently from what previously stated ("...we will assume that the SD is equal to the mean value itself.."), we excluded trials from the pooled analysis in order to reduce the confounding variables.
Assessment of heterogeneity: differently from the protocol, we simplified the four stages classification of heterogeneity into a three grades classification (low‐moderate and high).
Contributions of authors
Alberto Arezzo, Nereo Vettoretto, and Federico Famiglietti generated the idea for the review. Lorenzo Moja defined the methodology, Roberto Cirocchi helped in the review process, and Mario Morino defined the review strategy, and was in control of the review process.
Sources of support
Internal sources
University of Milan, Italy.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Lella 2006 {published data only}
- Lella F, Bagnolo F, Rebuffat C, Scalambra M, Bonassi U, Colombo E. Use of the laparoscopic‐endoscopic approach, the so‐called "rendezvous" technique, in cholecystocholedocholithiasis: a valid method in cases with patient‐related risk factors for post‐ERCP pancreatitis. Surgical Endoscopy 2006 [Epub 2006 Jan 19];20(3):419‐23. [DOI: 10.1007/s00464-005-0356-6] [DOI] [PubMed] [Google Scholar]
Morino 2006 {published data only}
- Morino M, Baracchi F, Miglietta C, Furlan N, Ragona R, Garbarini A. Preoperative endoscopic sphincterotomy versus laparoendoscopic rendezvous in patients with gallbladder and bile duct stones. Annals of Surgery 2006; Vol. 244, issue 6:889‐93; discussion 893‐6. [0003‐4932: (Print)] [DOI] [PMC free article] [PubMed]
Rabago 2006 {published data only}
- Rábago LR, Vicente C, Soler F, Delgado M, Moral I, Guerra I, et al. Two‐stage treatment with preoperative endoscopic retrograde cholangiopancreatography (ERCP) compared with single‐stage treatment with intraoperative ERCP for patients with symptomatic cholelithiasis with possible choledocholithiasis. Endoscopy 2006; Vol. 38, issue 8:779‐86. [0013‐726X: (Print)] [DOI] [PubMed]
Sahoo 2014 {published data only}
- Sahoo M, Pattnaik A, Kumar A. Randomized study on single stage laparo‐endoscopic rendezvous (intraoperative ERCP) procedure versus two stage approach (preoperative ERCP followed by laparoscopic cholecystectomy) for the management of cholelithiasis with choledocholithiasis. 11th World Congress of the International Hepato‐Pancreato‐Biliary Association; 2014 March 22‐27; Seoul, South Korea. HPB ‐ The Official Journal of The International Hepato Pancreato Biliary Association. 2014; Vol. 16 Supplement 2:64.
- Sahoo MR, Kumar AT, Patnaik A. Randomised study on single stage laparo‐endoscopic rendezvous (intra‐operative ERCP) procedure versus two stage approach (pre‐operative ERCP followed by laparoscopic cholecystectomy) for the management of cholelithiasis with choledocholithiasis. Journal of Minimal Access Surgery 2014;10:139‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzovaras 2012 {published data only}
- Tzovaras G, Baloyiannis I, Zachari E, Symeonidis D, Zacharoulis D, Kapsoritakis A, et al. Laparoendoscopic rendezvous versus preoperative ERCP and laparoscopic cholecystectomy for the management of cholecysto‐choledocholithiasis: interim analysis of a controlled randomized trial. Annals of Surgery 2012;255(3):435‐9. [ClinicalTrials.gov ID: NCT00416234] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cavina 1998 {published data only}
- Cavina E, Franceschi M, Sidoti F, Goletti O, Buccianti P, Chiarugi M. Laparo‐endoscopic "rendezvous": a new technique in the choledocholithiasis treatment. Hepato‐gastroenterology 1998;45(23):1430‐5. [0172‐6390: (print)] [PubMed] [Google Scholar]
Ding 2013 {published data only}
- Ding YB, Deng B, Liu XN, Wu J, Xiao WM, Wang YZ, et al. Synchronous vs sequential laparoscopic cholecystectomy for cholecystocholedocholithiasis. World Journal of Gastroenterology 2013;19(13):2080‐6. [2219‐2840: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
El Geidie 2011 {published data only}
- Geidie AA, Ebidy GK, Naeem YM. Preoperative versus intraoperative endoscopic sphincterotomy for management of common bile duct stones. Surgical Endoscopy 2011;25:1230‐7. [0930‐2794: (Eletronic)] [DOI] [PubMed] [Google Scholar]
Filauro 2000 {published data only}
- Filauro M, Comes P, Conca V, Coccia G, Prandi M, Bagarolo C, et al. Combined laparoendoscopic approach for biliary lithiasis treatment. Hepato‐gastroenterology 2000;47(34):922‐6. [0172‐6390: (print)] [PubMed] [Google Scholar]
La Greca 2008 {published data only}
- Greca G, Barbagallo F, Di Blasi M, Chisari A, Lombardo R, Bonaccorso R, et al. Laparo‐endoscopic "rendezvous" to treat cholecysto‐choledocolithiasis: effective, safe and simplifies the endoscopist's work. World Journal of Gastroenterology 2008; Vol. 14, issue 18:2844‐50. [1007‐9327: (Print)] [DOI] [PMC free article] [PubMed]
Miscusi 1997 {published data only}
- Miscusi G, Gasparrini M, Petruzziello L, Taglienti D, Onorato M, Otti M, et al. Endolaparoscopic "rendez‐vous" in the treatment of cholecysto‐choledochal calculosis. Il Giornale di Chirurgia 1997;18(10):655‐7. [0391‐9005: (print)] [PubMed] [Google Scholar]
Tekin 2008 {published data only}
- Tekin A, Ogetman Z, Altunel E. Laparoendoscopic "rendezvous" versus laparoscopic antegrade sphincterotomy for choledocholithiasis. Surgery 2008;144(3):442‐7. [0039‐6060: (print)] [DOI] [PubMed] [Google Scholar]
Additional references
Alexakis 2012
- Alexakis N, Connor S. Meta‐analysis of one‐ vs. two‐stage laparoscopic/endoscopic management of common bile duct stones. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2012; Vol. 14, issue 4:254‐9. [1477‐2574: (Electronic)] [DOI] [PMC free article] [PubMed]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Borzellino 2010
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Collins 2004
- Collins C, Maguire D, Ireland A, Fitzgerald E, O'Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Annals of Surgery 2004; Vol. 239, issue 1:28‐33. [0003‐4932: (Print)] [DOI] [PMC free article] [PubMed]
Copenhagen Trial Unit 2017
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C, The Copenhagen Trial Unit. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf (accessed 30 January 2017).
Dasari 2013
- Dasari BV, Tan CJ, Gurusamy KS, Martin DJ, Kirk G, McKie L, et al. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD003327.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 1988
- Davidson BR, Neoptolemos JP, Carr‐Locke DL. Endoscopic sphincterotomy for common bile duct calculi in patients with gall bladder in situ considered unfit for surgery. Gut 1988;29(1):114‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
EAES 1998
- Scientific Committee of the European Association for Endoscopic Surgery (E.A.E.S.). Diagnosis and treatment of common bile duct stones (CBDS). Results of a consensus development conference. Surgical Endoscopy 1998; Vol. 12, issue 6:856‐64. [0930‐2794: (Print)] [PubMed]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997; Vol. 315, issue 7109:629‐34. [0959‐8138: (Print)] [DOI] [PMC free article] [PubMed]
Gluud 2017
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2017, Issue 7. Art. No.: LIVER.
GRADEpro GDT
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org.
Gurusamy 2011
- Gurusamy K, Sahay SJ, Burroughs AK, Davidson BR. Systematic review and meta‐analysis of intraoperative versus preoperative endoscopic sphincterotomy in patients with gallbladder and suspected common bile duct stones. British Journal of Surgery 2011; Vol. 98, issue 7:908‐16. [1365‐2168: (Electronic)] [DOI] [PubMed]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso‐Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14‐22. [PUBMED: 28529188] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kharbutli 2008
Kjaergard 2001
La Greca 2009
- Greca G, Barbagallo F, Sofia M, Latteri S, Russello D. Simultaneous laparoendoscopic rendezvous for the treatment of cholecystocholedocholithiasis. Surgical Endoscopy 2009;24(4):769‐80. [DOI] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
Martin 2006
- Martin DJ, Vernon D, Toouli J. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003327.pub2] [DOI] [PubMed] [Google Scholar]
Menezes 2000
Moher 1998
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Neoptolemos 1989
- Neoptolemos JP, Shaw DE, Carr‐Locke DL. A multivariate analysis of preoperative risk factors in patients with common bile duct stones. Implications for treatment. Annals of Surgery 1989; Vol. 209, issue 2:157‐61. [0003‐4932: (Print)] [DOI] [PMC free article] [PubMed]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Park 2000
- Park AE, Mastrangelo MJ Jr. Endoscopic retrograde cholangiopancreatography in the management of choledocholithiasis. Surgical Endoscopy 2000;14:219‐26. [DOI] [PubMed] [Google Scholar]
Poulose 2006
- Poulose BK, Arbogast PG, Holzman MD. National analysis of in‐hospital resource utilization in choledocholithiasis management using propensity scores. Surgical Endoscopy 2006;20(2):186‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Saccomani 2005
Savovic 2012
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savovic 2012a
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schiphorst 2008
- Schiphorst AH, Besselink MG, Boerma D, Timmer R, Wiezer MJ, Erpecum KJ, et al. Timing of cholecystectomy after endoscopic sphincterotomy for common bile duct stones. Surgical Endoscopy 2008;22(9):2046‐50. [DOI] [PubMed] [Google Scholar]
Schulz 1995
Shimatani 2014
- Shimatani M, Takaoka M, Matsushita M, Okazaki K. Endoscopic approaches for pancreatobiliary diseases in patients with altered gastrointestinal anatomy. Digestive Endoscopy 2014;26 Suppl(1):70‐8. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Topal 2010
Tricarico 2002
- Tricarico A, Cione G, Sozio M, Palo P, Bottino V, Martino A, et al. Endolaparoscopic rendezvous treatment: a satisfying therapeutic choice for cholecystocholedocolithiasis. Surgical Endoscopy 2002;16:711‐3. [DOI] [PubMed] [Google Scholar]
TSA 2017 [Computer program]
- Copenhagen Trial Unit. Trial Sequential Analysis (TSA) software. Version version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2017.
Urbach 2001
- Urbach DR, Khajanchee YS, Jobe BA, Standage BA, Hansen PD, Swanstrom LL. Cost‐effective management of common bile duct stones: a decision analysis of the use of endoscopic retrograde cholangiopancreatography (ERCP), intraoperative cholangiography, and laparoscopic bile duct exploration. Surgical Endoscopy 2001; Vol. 15, issue 1:4‐13. [0930‐2794: (Print)] [DOI] [PubMed]
Videhult 2009
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2002
- Williams GL, Vellacott KD. Selective operative cholangiography and perioperative endoscopic retrograde cholangiopancreatography (ERCP) during laparoscopic cholecystectomy: a viable option for choledocholithiasis. Surgical Endoscopy 2002;16(3):465‐7. [1432‐2218: (Electronic)] [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams EJ, Green J, Beckingham I, Parks R, Martin D, Lombard M, British Society of Gastroenterology. Guidelines on the management of common bile duct stones (CBDS). Gut 2008;57(7):1004‐21. [1468‐3288: (Electronic)] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Vettoretto 2013
- Vettoretto N, Arezzo A, Famiglietti F, Cirocchi R, Moja L, Morino M. Laparoscopic‐endoscopic rendezvous versus preoperative endoscopic sphincterotomy for common bile duct stones in patients undergoing laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010507] [DOI] [PMC free article] [PubMed] [Google Scholar]


