Abstract
Background
Attention deficit hyperactivity disorder (ADHD) is a common neurodevelopmental disorder in childhood. The psychostimulant methylphenidate is the most frequently used medication to treat it. Several studies have investigated the benefits of methylphenidate, showing possible favourable effects on ADHD symptoms, but the true magnitude of the effect is unknown. Concerning adverse events associated with the treatment, our systematic review of randomised clinical trials (RCTs) demonstrated no increase in serious adverse events, but a high proportion of participants suffered a range of non‐serious adverse events.
Objectives
To assess the adverse events associated with methylphenidate treatment for children and adolescents with ADHD in non‐randomised studies.
Search methods
In January 2016, we searched CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, 12 other databases and two trials registers. We also checked reference lists and contacted authors and pharmaceutical companies to identify additional studies.
Selection criteria
We included non‐randomised study designs. These comprised comparative and non‐comparative cohort studies, patient‐control studies, patient reports/series and cross‐sectional studies of methylphenidate administered at any dosage or formulation. We also included methylphenidate groups from RCTs assessing methylphenidate versus other interventions for ADHD as well as data from follow‐up periods in RCTs. Participants had to have an ADHD diagnosis (from the 3rd to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders or the 9th or 10th edition of theInternational Classification of Diseases, with or without comorbid diagnoses. We required that at least 75% of participants had a normal intellectual capacity (intelligence quotient of more than 70 points) and were aged below 20 years. We excluded studies that used another ADHD drug as a co‐intervention.
Data collection and analysis
Fourteen review authors selected studies independently. Two review authors assessed risk of bias independently using the ROBINS‐I tool for assessing risk of bias in non‐randomised studies of interventions. All review authors extracted data. We defined serious adverse events according to the International Committee of Harmonization as any lethal, life‐threatening or life‐changing event. We considered all other adverse events to be non‐serious adverse events and conducted meta‐analyses of data from comparative studies. We calculated meta‐analytic estimates of prevalence from non‐comparative cohorts studies and synthesised data from patient reports/series qualitatively. We investigated heterogeneity by conducting subgroup analyses, and we also conducted sensitivity analyses.
Main results
We included a total of 260 studies: 7 comparative cohort studies, 6 of which compared 968 patients who were exposed to methylphenidate to 166 controls, and 1 which assessed 1224 patients that were exposed or not exposed to methylphenidate during different time periods; 4 patient‐control studies (53,192 exposed to methylphenidate and 19,906 controls); 177 non‐comparative cohort studies (2,207,751 participants); 2 cross‐sectional studies (96 participants) and 70 patient reports/series (206 participants). Participants' ages ranged from 3 years to 20 years. Risk of bias in the included comparative studies ranged from moderate to critical, with most studies showing critical risk of bias. We evaluated all non‐comparative studies at critical risk of bias. The GRADE quality rating of the evidence was very low.
Primary outcomes
In the comparative studies, methylphenidate increased the risk ratio (RR) of serious adverse events (RR 1.36, 95% confidence interval (CI) 1.17 to 1.57; 2 studies, 72,005 participants); any psychotic disorder (RR 1.36, 95% CI 1.17 to 1.57; 1 study, 71,771 participants); and arrhythmia (RR 1.61, 95% CI 1.48 to 1.74; 1 study, 1224 participants) compared to no intervention.
In the non‐comparative cohort studies, the proportion of participants on methylphenidate experiencing any serious adverse event was 1.20% (95% CI 0.70% to 2.00%; 50 studies, 162,422 participants). Withdrawal from methylphenidate due to any serious adverse events occurred in 1.20% (95% CI 0.60% to 2.30%; 7 studies, 1173 participants) and adverse events of unknown severity led to withdrawal in 7.30% of participants (95% CI 5.30% to 10.0%; 22 studies, 3708 participants).
Secondary outcomes
In the comparative studies, methylphenidate, compared to no intervention, increased the RR of insomnia and sleep problems (RR 2.58, 95% CI 1.24 to 5.34; 3 studies, 425 participants) and decreased appetite (RR 15.06, 95% CI 2.12 to 106.83; 1 study, 335 participants).
With non‐comparative cohort studies, the proportion of participants on methylphenidate with any non‐serious adverse events was 51.2% (95% CI 41.2% to 61.1%; 49 studies, 13,978 participants). These included difficulty falling asleep, 17.9% (95% CI 14.7% to 21.6%; 82 studies, 11,507 participants); headache, 14.4% (95% CI 11.3% to 18.3%; 90 studies, 13,469 participants); abdominal pain, 10.7% (95% CI 8.60% to 13.3%; 79 studies, 11,750 participants); and decreased appetite, 31.1% (95% CI 26.5% to 36.2%; 84 studies, 11,594 participants). Withdrawal of methylphenidate due to non‐serious adverse events occurred in 6.20% (95% CI 4.80% to 7.90%; 37 studies, 7142 participants), and 16.2% were withdrawn for unknown reasons (95% CI 13.0% to 19.9%; 57 studies, 8340 participants).
Authors' conclusions
Our findings suggest that methylphenidate may be associated with a number of serious adverse events as well as a large number of non‐serious adverse events in children and adolescents, which often lead to withdrawal of methylphenidate. Our certainty in the evidence is very low, and accordingly, it is not possible to accurately estimate the actual risk of adverse events. It might be higher than reported here.
Given the possible association between methylphenidate and the adverse events identified, it may be important to identify people who are most susceptible to adverse events. To do this we must undertake large‐scale, high‐quality RCTs, along with studies aimed at identifying responders and non‐responders.
Plain language summary
Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents ‐ assessment of harmful effects
Review question
Is methylphenidate administration associated with harmful effects in children and adolescents with attention deficit hyperactivity disorder (ADHD)?
Background
ADHD is one of the most common neurodevelopmental disorders in childhood and is associated with impaired functioning and negative outcomes for development. Individuals diagnosed with ADHD are often hyperactive and impulsive. Methylphenidate, a psychostimulant, is the drug most often prescribed for children and adolescents with ADHD.
Study characteristics
We searched for available research up to January 2016 and found 260 studies with different designs. We included a number of non‐randomised designs (where investigators did not assign participants to a certain treatment):
– 7 comparative cohort studies (a group of people followed over time; six studies compared 968 patients who were taking methylphenidate to 166 controls who were not taking methylphenidate; and 1 study included 1224 patients that were taking or not taking methylphenidate during different time periods); – 4 patient‐control studies (comparing two groups of people: 53,192 were taking methylphenidate, and 19,906 were not); – 177 non‐comparative cohort studies (2,207,751 participants) with no control group (i.e. who were not taking methylphenidate); – 2 cross‐sectional studies (96 participants were taking methylphenidate at a single time point); and – 70 patient reports/series (206 participants were taking methylphenidate).
We also included methylphenidate groups from randomised clinical trials (RCTs; experiments in which participants are randomly put into independent groups that compare different treatments). All RCTs assessed methylphenidate versus other interventions for ADHD and follow‐up periods from RCTs. We only used the data from the intervention arm with methylphenidate. In all the included non‐comparative cohort studies, 2,207,751 participants were taking methylphenidate. Participants' ages ranged from 3 years to 20 years.
Key results
The findings suggest that methylphenidate administration might lead to serious adverse (harmful) events, including death, cardiac problems, and psychotic disorders. About 1 in 100 patients treated with methylphenidate seemed to suffer a serious adverse event. Withdrawal from methylphenidate due to serious adverse events occurred in about 1.2 out of 100 patients treated with methylphenidate. Withdrawal from methylphenidate due to any adverse events occurred in about 7.3 out of 100 patients treated with methylphenidate. We also noted a large proportion of non‐serious adverse events. More than half the patients exposed to methylphenidate seemed to suffer one or more adverse events. Withdrawal from methylphenidate due to non‐serious adverse events occurred in about 6.2 out of 100 patients exposed to methylphenidate. Withdrawal of methylphenidate for unknown reasons was 16.2 out of 100 patients exposed to methylphenidate.
Quality of the evidence
The quality of the evidence and hence the certainty or reliability of the evidence for the comparative studies is very low. The reliability of the evidence for the non‐comparative studies is low due to weaknesses in study design. Accordingly, it is not possible to accurately estimate the risks of adverse events in children and adolescents prescribed methylphenidate.
Conclusions
Methyphenidiate might be associated with a number of serious adverse events. Methylphenidate produces a large number of other non‐serious harmful effects in children and adolescents with ADHD. We suggest that clinicians and parents are alert to the importance of monitoring adverse events in a systematic, meticulous manner. If methylphenidate is to continue to have a place in ADHD treatment in the future, we need to identify subgroups of patients in whom the benefits of methylphenidate outweigh the harms. Just as we need to be able to identify who is likely to benefit from treatment, we also need to be able to identify those who are most at risk of experiencing adverse events. In order to do this, we need to undertake large‐scale, high‐quality RCTs along with other studies aimed at identifying those who respond and those who do not respond to treatment.
Summary of findings
Summary of findings for the main comparison. Methylphenidate for children and adolescents aged 18 years and under with attention deficit hyperactivity disorder (ADHD): adverse events.
| Methylphenidate for children and adolescents aged 18 and under with attention deficit hyperactivity disorder (ADHD): adverse events | |||||
|
Patient or population: children and adolescents aged 18 years and under diagnosed with ADHD Settings: outpatient clinic, inpatient hospital ward and register data Intervention: methylphenidate Comparision: control or no control | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) at follow‐up | Quality of the evidence (GRADE) | |
| Risk with control or no control | Risk difference with Methylphenidate | ||||
| Comparative studies | |||||
|
Serious adverse events Measured by: proportion of serious adverse events (total) Average study duration (range): not stated |
12 per 1000 | 4 more per 1000 (2 more to 7 more) |
RR 1.36 (1.17 to 1.57) | 72,005 (2 studies) | ⊕⊝⊝⊝ Very lowa |
| Non‐comparative studies | |||||
|
Serious adverse events Measured by: proportion of any serious adverse events Average study duration (range): 4.7 months (14 days to 21 months) |
1.20% (0.70% to 2.00%) | — | 162,422 (51 studies) | ⊕⊝⊝⊝ Very lowb | |
|
Withdrawal of methylphenidate due to serious adverse events (non‐comparative cohort studies) Measured by: proportion of participants withdrawn from treatment Average study duration (range): 7.9 months (1 month to 37 months) |
1.20% (0.60% to 2.30%) | — | 1173 (7 studies) | ⊕⊝⊝⊝ Very lowb | |
|
Withdrawal of methylphenidate due to adverse events of unknown severity Measured by: proportion of participants withdrawn from treatment Average study duration (range): 4.6 months (21 days to 36 months) |
7.30% (5.30% to 10.0%) | — | 3708 (22 studies) | ⊕⊝⊝⊝ Very lowb | |
|
Non‐serious adverse events Measured by: proportion of any non‐serious adverse events Average study duration (range): 5.99 months (1 day to 41 months) |
51.2% (41.2% to 61.1%) | — | 13,978 (49 studies) | ⊕⊝⊝⊝ Very lowb | |
|
Withdrawal of methylphenidate due to non‐serious adverse events Measured by: proportion of participants withdrawn from treatment Average study duration (range): 5.60 months (0.6 months to 41 months) |
6.20% (4.80% to 7.90%) | — | 7142 (37 studies) | ⊕⊝⊝⊝ Very lowb | |
|
Withdrawal of methylphenidate for unknown reasons Measure by: proportion of participants withdrawn from treatment Average study duration (range): 6.13 months (1 day to 36 months) |
16.2% (13.0% to 19.9%) | — | 8340 (57 studies) | ⊕⊝⊝⊝ Very lowb | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ROBINS‐I: Risk Of Bias In Non‐randomised Studies ‐ of Interventions; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
aOutcome assessed at critical risk of bias using the ROBINS‐I. Consequently, we downgraded the quality of the evidence by 3 levels due to study limitations. bOutcome not critically assessed with ROBINS‐I due to lack of control group. However, due to the nature of the studies and the risk of confounding, we considered the studies to be at critical risk of bias. Consequently, we downgraded the quality of the evidence by 3 levels due to study limitations.
Background
Description of the condition
Attention deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed and treated childhood neurodevelopmental disorders (Scahill 2000). The estimated prevalence in children and adolescents is between 3% to 8% (Polanczyk 2007a; Thomas 2015; Willcut 2012), depending on the classification system used, with boys two to four times more likely to be diagnosed than girls (Schmidt 2009). Prevalences have remained stable over the past 30 years and do not appear to vary between countries (Polanczyk 2014). Individuals with ADHD may show difficulties in attention and cognitive functions like problem‐solving, planning, orienting, flexibility, response inhibition and working memory, as well as impulsivity and hyperactivity (Pasini 2007; Sergeant 2003). Furthermore, children and adolescents have difficulties handling affective components such as motivational delay and mood dysregulation (Castellanos 2006; Nigg 2005; Schmidt 2009). The aetiology of ADHD involves genetic, environmental and social factors but is not yet completely understood. Family and twin studies have shown a high heritability of around 70% to 80% and with a substantial overlap between the two dimensions of hyperactivity/impulsivity and inattention and with no sex differences of heritability (Franke 2012; Neale 2010). Furthermore, genetic factors may be involved in determining the persistence of ADHD into adulthood (Faraone 2000; Franke 2012). Although family studies have shown high heritability, and there are many candidate genes that may be involved in the disorder (Neale 2010), genome‐wide studies have yet to find any clear associations.
Several studies have examined environmental risk factors for ADHD; however, researchers have not found any specific predictor for elevated risk. At the population level, poverty (families living under the poverty level) is more likely to be a feature among American children and adolescents diagnosed with ADHD (CDC 2015). In a Swedish cohort of 811,803 individuals, low family income in early childhood was highly associated with ADHD (Larsson 2014). Other potential risk factors for ADHD development include low birthweight (Indredavik 2004; Van Lieshout 2015), prematurity (Bhutta 2002; Burnett 2014; Elgen 2015), maternal exposure to tobacco (Kovess 2015; Obel 2016), and exposure to chemical components like manganese and lead (Hong 2014; Hong 2015).
The diagnosis of ADHD is purely clinical, requiring recognition of excessive inattention, hyperactivity and impulsivity that interfere with or reduce the quality of social, academic, or occupational functioning (APA 2013; WHO 1992). There are 18 core symptoms of ADHD according to the principal diagnostic classification systems: International Classification of Diseases ‐ 10th Revision (ICD‐10; WHO 1992) and the Diagnostic and Statistical Manual of Mental Disorders (DSM) ‐ 4th Edition (DSM‐IV; APA 1994), ‐ 4th Edition ‐ Text Revision (DSM‐IV‐TR; APA 2000), and‐ 5th Edition (DSM‐5; APA 2013).
Both the DSM‐5 and ICD‐10 criteria require excessive inattention, hyperactivity, and impulsivity to be inconsistent with the developmental level and to be pervasive. The symptoms must be present in two or more settings and appear before the age of 6 years according to the ICD‐10 (WHO 1992), or 12 years according to the DSM‐5 (APA 2013), and they should also persist for at least six months. The DSM‐5 modified the criteria for adolescents and adults older than 17 years of age, requiring fewer perceived symptoms and providing further descriptions to better identify typical ADHD symptoms in adolescents. Earlier versions of the DSM and the ICD‐10 required that there be clear evidence of clinically significant impairment in social, academic, and occupational functioning (APA 1994; APA 2000; APA 2013; WHO 1992), but the DSM‐5 only requires that symptoms interfere with or reduce the quality of these domains (APA 2013). Furthermore the ICD‐10 and the DSM‐IV differ from the newer DSM‐5 in excluding people with autism spectrum disorder; DSM‐5 only excludes people during the course of schizophrenia or another psychotic or mental disorder.
The diagnostic criteria describe three different subtypes or 'presentations' in the DSM‐5, according to the predominant symptoms: 'predominantly inattentive type', 'predominantly hyperactive‐impulsive type', and 'combined type' – a combination of both hyperactive‐impulsive and inattentive symptoms. The DSM‐5 acknowledges the absence of validity for these subtypes by renaming them predominantly inattentive, predominantly hyperactive‐impulsive, and combined presentations (APA 2013; Willcut 2012).
Children, adolescents, and adults with ADHD are at increased risk of a broad spectrum of co‐occurring conditions, which frequently result in negative outcomes later in life (Newcorn 2008; Schmidt 2009). The Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) trial identified one or more comorbid disorders in almost 40% of the participants (MTA 1999). These included oppositional defiant disorder, conduct disorder, depression, anxiety, tics, learning disorders, and verbal and cognitive difficulties (Jensen 2001; Kadesjö 2001). ADHD has also been shown to co‐occur with bipolar disorder (Perroud 2014). More recently, studies have confirmed such comorbidity (Czamara 2013; Yoshimasu 2012), with some authors noting that excess weight and obesity are found in children with ADHD (Cortese 2016). In a study with 1480 twin pairs from Sweden, researchers found that persistent hyperactivity or impulsivity symptoms of ADHD are associated with both early‐onset tobacco and alcohol use (Chang 2012). Similarly, ADHD comorbidity with conduct disorder can lead to adverse outcomes in academic achievement, failure to complete high school, criminality, substance use disorder, and unemployment (Erskine 2016).
In addition, ADHD is associated with several harmful consequences. A cohort of participants with ADHD who were followed up to the age of 40 years demonstrated that these individuals have an elevated risk of criminality and a high risk of death before 40 years of age (Koisaari 2015). Similarly, studies from health insurance plans demonstrated not only elevated risk of injury, but also higher indirect costs of those with an ADHD diagnosis compared to diagnosis of depression (Hodgkins 2011). Recently, ADHD has been linked to increased premature mortality higher than 50%, compared to non‐ADHD patients, in a 24.9 million person‐years Danish cohort study (Dalsgaard 2015b).
To ensure high standards in assessment, diagnosis and therapeutic practice, professional and national bodies have developed guidelines (AAP 2011; CADDRA 2011; NCCMH 2009; Pliszka 2007a; Scottish Intercollegiate Guidelines Network (SIGN)). Psychosocial interventions are recommended initially for younger children and for mild to moderate symptoms (AAP 2011; NCCMH 2009; Pliszka 2007a). For more severe ADHD symptoms, stimulants, either alone or in combination with psychosocial interventions, may be necessary (AAP 2011; CADDRA 2011; NCCMH 2009).
Description of the intervention
Stimulant medication, notably methylphenidate and dexamphetamine (or dextroamphetamine), together with the non‐stimulants atomoxetine (a non‐stimulant selective noradrenaline reuptake inhibitor) and guanfacine (an alpha 2A agonist), are considered the treatments of choice along with psychosocial treatments for children and adolescents with ADHD (Greenhill 2006; NICE 2008; Pliszka 2007a). Globally, methylphenidate is the most commonly used drug prescribed for ADHD; it has been used in practice for more than 50 years (Kadesjö 2002; NCCMH 2009). Methylphenidate is used because it appears to have a favourable effect on reducing the core symptoms of excessive hyperactivity, impulsivity, and inattention in children and adolescents with ADHD. It is licensed for use in children aged six years and older. Rates of prescription of methylphenidate are high and increasing, standing at approximately 8% of children and adolescents under 15 years of age in the USA (Akinbami 2011), and around 3% to 5% in Europe (Bachmann 2017; Hodgkins 2013; Schubert 2010; Trecenõ 2012; Zoëga 2016). ADHD medication appears to be discontinued in 13% to 64% of patients from all age groups (Adler 2010), but information of the continuity of these treatments from childhood or adolescence into adulthood is still lacking. Dexamphetamine is licensed for use in children aged three years and older. It is also available for use as mixed‐amphetamine salts (levoamphetamine plus dextroamphetamine) and as a pro‐drug of dexamphetamine, lisdexamphetamine. These have a longer duration of action than dextroamphetamine. Clinical choice of preparation by clinicians and families is based on a range of factors, including the presence of co‐occurring conditions, adverse events associated with the drug, issues regarding compliance, and the preference of the child and parents.
Methylphenidate dose varies from patient to patient. The dose needs to be titrated individually in order to maximise benefits and minimise potential adverse events (Stevenson 1989). The daily therapeutic range of methylphenidate dosages varies from 5 mg to 60 mg, administered one to three times daily, depending on the release system (immediate, sustained, or extended release) and mode of administration (oral or transdermal) (Pliszka 2007a; Storebø 2015). The British National Formulary suggests that initial doses in children aged four to six years should be 2.5 mg twice daily. Where necessary, it should be increased at weekly intervals by 2.5 mg daily, to a maximum of 1.4 mg/kg daily (divided into two to three doses per day) (BNF 2018). In children aged 6 years to 18 years, the initial dose may be 5 mg once or twice daily, increased, where necessary, at weekly intervals by 5 mg to 10 mg daily in two to three divided doses. Although methylphenidate is licensed to a maximum dose of 60 mg daily, it may be increased by 2.1 mg/kg daily in two to three divided doses (maximum 90 mg daily) under specialist supervision. The bioavailability of oral methylphenidate is 11% to 52%, with an approximate duration of action of 2 to 4 hours for immediate‐release methylphenidate, 3 to 8 hours for sustained‐release methylphenidate, and 8 to 12 hours for extended‐release methylphenidate (Kimko 1999).
How the intervention might work
The pharmacodynamics of methylphenidate are still not entirely clear. Methylphenidate has both dopamine and noradrenaline transporter‐binding affinity and binds to and blocks both transporters, leading to increased availability of noradrenaline and dopamine within the synaptic cleft (Heal 2006; Volkow 1998; Volkow 2004; Volkow 2012). This is thought to increase the general firing rate via increased neurotransmission of dopamine and noradrenaline, which, in turn, has an effect on the prefrontal cortex – responsible for executive function – and is linked to sub‐performance of dopamine and noradrenaline functions associated with ADHD (Arnsten 2005). As a result, patients can improve function (through symptom control) and experience several benefits such as improved attention and reduced hyperactivity‐impulsivity (Barkley 1977; Barkley 1981; Barkley 1989; Connor 2002; Engert 2008; Schulz 2012; Shaw 2012; Solanto 1998), which may improve classroom functioning and academic learning (Biederman 2003; Cox 2004; Evans 2001; Swanson 2004).
Methylphenidate has also been correlated with a reduction of several harmful outcomes. In an extensive cohort of 710,120 individuals, including 4557 individuals diagnosed with ADHD before age 10 years, the use of methylphenidate was found to reduce emergency department visits by 46% and injuries by 44% (Dalsgaard 2015a). However, given the lack of sufficiently powered, well‐conducted randomised clinical trials (RCTs), it is not clear if these are genuine benefits or statistical artefacts (Garattini 2016; Storebø 2015). In a Swedish national register composed of 25,656 participants, Lichteinstein 2012 demonstrated a 32% and 41% reduction in criminality among men and woman respectively, when treated with medications for ADHD. In another study, the researchers found that medication was associated with a 58% risk reduction in serious transport accidents, and estimated that 41% to 49% of the accidents could have been avoided if ADHD male patients were in drug treatment (Chang 2014a). There have also been more recent reports of reductions in motor vehicle crashes in patients on methylphenidate (Chang 2017). Similarly, ADHD drugs decreased injuries among 5‐ to 10‐year‐old children from 32% to 44%, when compared to ADHD children without treatment (Dalsgaard 2015a). It is also a general concern that ADHD medication treatment can lead to substance abuse. Contrary to this, the prescription of ADHD stimulants was associated with a 31% decrease in substance abuse (Chang 2014b). A similar concern was the association of ADHD treatment and suicide, but again, the treatment was correlated with a protective effect (Chen 2014).
Why it is important to do this review
The most commonly reported adverse events associated with methylphenidate are headache, sleep problems, fatigue, and decreased appetite. Studies have indicated that methylphenidate also impairs both children's height and increases in weight (Schachar 1997; Swanson 2004; Swanson 2009).
Serious adverse events, such as psychosis and mood disorders, are reported to affect approximately 3% of children treated with methylphenidate (Block 1998; Cherland 1999; MTA 1999; NICE 2009; Pliszka 1998). An observational study supports an association between the use of stimulants and sudden unexplained death among children and adolescents (Gould 2009). The study showed an odds ratio (OR) of 7.4 (95% confidence interval (CI) 1.4 to 74.9) for use of stimulants, specifically methylphenidate, in children and adolescents with sudden death compared to aged‐matched, motor vehicle accident deaths (Gould 2009). Further research is needed to determine whether these deaths are related to methylphenidate (US FDA 2011).
Reports of sudden death in adults taking methylphenidate are also a concern (Jackson 2016). In an update (US FDA 2011), the FDA found no evidence for increased risk of serious cardiovascular events in adults treated with ADHD medications, based on two epidemiological studies (Cooper 2011; Habel 2011).
Recent reviews of methylphenidate treatment have focused on its benefits only, as opposed to its harmful effects (Charach 2013; Faraone 2002; Faraone 2006; Faraone 2010; Hanwella 2011; Maia 2017).
Relatively few randomised clinical trials included in our Cochrane Review assessing methylphenidate versus placebo or no intervention reported adverse events (Storebø 2015). Given the worldwide increase in methylphenidate prescriptions to children and adolescents, the need for an evidence‐based risk profile for serious and non‐serious adverse events remains (Bushe 2013; Cairns 2014).
To expand our understanding of adverse events, particularly where these are rare or take time to become apparent, it is necessary to bolster the limited data from RCTs by including data from non‐randomised studies (Storebø 2015).
Non‐randomised studies have a number of advantages; they are often larger (allowing for detection of rare events), have a broader range of participants (reflecting 'real‐life'), longer follow‐up times, and lower costs than RCTs (Benson 2000; Hannan 2008; Silverman 2009). Non‐randomised studies may detect adverse events due to long‐term drug exposure, which would not be detected in relatively short RCTs (Storebø 2015). Some adverse events may be too uncommon to be detected in RCTs (Loke 2011), and as a result, cohort studies, patient‐control studies, and even patient reports/series may be of value (Reeves 2011). In fact, non‐randomised studies can estimate the adverse events of treatment as well as, and maybe even more comprehensively than, RCTs (Vandenbroucke 2006).
RCTs and non‐randomised studies investigating adverse events rarely find differences in risk estimates (Golder 2011). In their review, Pitrou 2009 found that some RCTs provided no information on adverse events, and severity was often poorly defined. Only 13% of studies noted the reasons for patient withdrawal due to adverse events. Another study reported that only 18% of all paediatric RCTs published between 2006 and 2009 documented harms adequately according to CONSORT guidelines (De Vries 2010). Our findings were similar (Storebø 2015), with only 17/185 RCTs (9.20%) reporting serious adverse events and approximately 60/185 RCTs (32.0%) reporting non‐serious adverse events.
The main disadvantage, however, is that causality cannot be established in observational studies because the observed adverse event may be related to other factors. Nonetheless, in view of the poor reporting of adverse events in RCTs, non‐randomised studies may provide important data that would otherwise remain undetected (Loke 2011; Vandenbroucke 2006). Such data from non‐randomised studies may help children, adolescents, families, clinicians, and policymakers understand the relative risks and benefits, leading to better informed choices regarding methylphenidate treatment. When we deal with serious adverse events, troublesome non‐serious adverse events, and/or prevalent non‐serious adverse events, we should remember the recommendations from regulatory authorities stating that P values are of very limited value as substantial differences (expressed as relative risk or risk differences) require careful assessment and will, in addition, raise concern, depending on seriousness, severity or outcome, irrespective of the P value observed (EMA 2017). A non‐significant difference between treatments will not allow for a conclusion on the absence of a difference in safety. In other words, in line with general principles, a non‐significant test result should not be confused with the demonstration of equivalence (EMA 2017).
Objectives
To assess the adverse events associated with methylphenidate treatment for children and adolescents with ADHD in non‐randomised studies.
Methods
Criteria for considering studies for this review
Types of studies
We only included the following non‐randomised study designs (Higgins 2011).
Comparative cohort studies. Here, the experimental group was children or adolescents with ADHD exposed to methylphenidate and the control group was comparable patients not exposed to methylphenidate.
Patient‐control studies. Here, the patient ('case') group was children or adolescents with ADHD exposed to methylphenidate, and the control group was comparable patients not exposed to methylphenidate.
Non‐comparative cohort studies. In this study type, children or adolescents with ADHD were exposed to methylphenidate with no control group. We also included the methylphenidate‐treated group from randomised clinical trials (RCTs) comparing methylphenidate versus other interventions for ADHD, as these trials were not included in our previous review of RCTs assessing methylphenidate versus placebo or no intervention (Storebø 2015). In this way, we were able to expand our evidence base on adverse events during methylphenidate administration (as such groups are comparable to classic cohort studies), without double‐counting from RCTs assessing methylphenidate versus placebo or no intervention (Storebø 2015). For assessment of adverse events in RCTs assessing methylphenidate versus placebo or no intervention, the reader is referred to our systematic review on that topic (Storebø 2015).
Patient reports/series. This study type was formerly known and also described in our protocol as 'case‐studies' (Storebø 2016).
Cross‐sectional studies.
For a description of cohort, cross‐sectional, and patient‐control studies, see Table 2.
1. Study design.
| Study design | Description | |
| Cohort study | An observational study in which a defined group with ≥ 1 samples of people (the cohort) is followed over time. The outcomes of people in subsets of this cohort might be compared, to examine people who were exposed or not exposed (or exposed at different levels) to a particular intervention or other factor of interest. A prospective cohort study assembles participants and follows them into the future. A retrospective (or historical) cohort study identifies participants from past records and follows them from the time of those records to the present. Because participants are not allocated by the investigator to different interventions or other exposures, adjusted analysis is usually required to minimise the influence of other factors (confounders). | |
| Patient‐control study | A study that compares people with a specific disease or outcome of interest (cases) to people from the same population without that disease or outcome (controls), and which seeks to find associations between the outcome and prior exposure to particular risk factors. This design is particularly useful when the outcome is rare and when past exposure can be reliably measured. Patient‐control studies are usually but not always retrospective. | |
| Cross‐sectional study | Studies in which the presence or absence of disease or other health‐related variables are determined for each member of the study population or in a representative sample at one particular time. This contrasts with cohort studies, which are followed over a period of time. |
Taken from the Cochrane Glossary.
Types of participants
Children and adolescents with ADHD, with or without comorbid conditions. At least 75% of study participants were required to be aged 18 years or younger, and the mean age of the trial population had to be 18 years of age or younger. We also required that at least 75% of participants had a normal intellectual quotient (IQ > 70 points). See Differences between protocol and review.
We accepted the following diagnoses of ADHD.
A diagnosis of ADHD made according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) ‐ 3rd Edition (DSM‐III; APA 1980); 3rd Edition ‐ Revised (DSM‐III‐R; APA 1987); 4th Edition (DSM‐IV; APA 1994); 4th Edition ‐ Text Revision (DSM‐IV‐TR; APA 2000), or5th Edition (DSM‐5; APA 2013).
A diagnosis of hyperkinetic disorders made in accordance with the International Classification of Diseases ‐ 9th Revision (ICD‐9; WHO 1977) and‐10th Revision (ICD‐10; WHO 1992).
Types of interventions
Methylphenidate administered at any dosage or formulation as part of any medical treatment regimen.
In the comparative cohort studies and patient‐control studies, we included studies that compared cointerventions as long as the compared intervention groups received the same cointerventions.
In the non‐comparative cohort studies, we allowed some types of comedication but not cointervention with another type of ADHD medication.
Types of outcome measures
We defined the term 'adverse events' as any harm, adverse effect, or adverse drug reaction associated with methylphenidate. We defined 'withdrawals' as any participants withdrawn from methylphenidate medication.
Primary outcomes
Serious adverse events. A serious adverse event is defined as any event that is fatal; life‐threatening; requires inpatient hospitalisation or prolongation of existing hospitalisation; or results in persistent or significant disability, or any event that requires intervention to prevent any of these outcomes in accordance with the Guideline for Good Clinical Practice E6(R1) (ICH 1996).
Withdrawal of methylphenidate due to serious adverse events.
Withdrawal of methylphenidate due to adverse events of unknown severity.
Secondary outcomes
Non‐serious adverse events. All other adverse events, including but not confined to, the following common types of adverse events: cardiovascular, neurological, gastrointestinal, difficulty with sleep, and growth retardation in accordance with the Guideline for Good Clinical Practice E6(R1) (ICH 1996).
Withdrawal of methylphenidate due to non‐serious adverse events.
Withdrawal of methylphenidate for unknown reasons.
Adverse events were measured during treatment, at the end of treatment and at the longest follow‐up, using rating scales, spontaneous reports recorded by the investigators at regular interviews or visits, and physical examinations or para‐clinical examinations.
We reported the primary and the secondary outcomes in Table 1.
Search methods for identification of studies
Electronic searches
In February 2015, we simultaneously conducted the literature searches for this and another review by including a separate search strategy of adverse events, as described in the previous review (Storebø 2015). We predicted that reports on efficacy as well as adverse events would both describe the adverse events of methylphenidate, which is why it made good sense to combine the two search strategies and thus maximise the retrieval of relevant publications. In order to overcome poor indexing and abstracting, we listed individual brand names in the search strategies. The search strategies, as executed in both reviews, are shown in Appendix 1.
On 8 January 2016, we ran an updated version of the adverse events search strategy that included new brand names and study designs. We also searched a number of additional sources to identify grey literature.
We searched the electronic databases and trial registers listed below, in order to identify relevant studies.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1), in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 8 January 2016).
MEDLINE Ovid (1948 to January week 3 2016).
Embase Ovid (1980 to January week 3 2016).
PsycINFO Ovid (1806 to January week 3 2016).
CINAHL EBSCOhost (Cumulative Index to Nursing & Allied Health Literature; 1980 to 8 January 2016).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 14 November 2017).
Conference Proceedings Citation Index ‐ Social Science & Humanities Web of Science (CPCI‐SS&H; 1990 to 14 November 2017).
NDLTD Theses Resources (ndltd.org/resources; all available years; searched 11 January 2016).
Trove ‐ National Library of Australia (trove.nla.gov.au; all available years; searched 11 January 2016).
British Library E‐Theses Online Service (EThOS; ethos.bl.uk; all available years; searched 11 January 2016).
Deutsche Nationalbibliothek Dissertations (www.dnb.de/EN/Home/home_node.html; all available years; searched 11 January 2016).
Open Access Theses and Dissertations (OATD; oatd.org; all available years; searched 11 January 2016).
Theses Collection Wales ‐ The National Library of Wales (www.llgc.org.uk/en/discover/nlw‐resources/theses‐collection‐wales; all available years; searched 11 January 2016).
DART‐Europe E‐Theses Portal (www.dart‐europe.eu/basic‐search.php; all available years; searched 11 January 2016).
Bielefeld Academic Search Engine (BASE; www.base‐search.net/about/en; all available years; searched 11 January 2016).
Theses France (www.theses.fr/en; all available years; searched 11 January 2016).
ProQuest Open Access Dissertations (PQDT; pqdtopen.proquest.com/search.html; all available years; searched 11 January 2016).
ClinicalTrials.gov (clinicaltrials.gov; all available years; searched 11 January 2016).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; who.int/ictrp/en; all available years; searched 11 January 2016).
We did not limit the searches by language, year of publication, or type of publication. We translated relevant sections of non‐English language reports when needed.
Searching other resources
In order to find additional relevant studies not identified by the electronic searches listed above, we scrutinised the bibliographic references of identified review articles and meta‐analyses. Furthermore, we sent requests for published as well as unpublished data to pharmaceutical companies manufacturing methylphenidate, including Shire (www.shire.com), Medice (represented in Denmark by HB Pharma: www.hbpharma.dk), Janssen‐Cilag (www.janssen.com), and Novartis (www.novartis.com) (see supplementary file Letter to pharmaceutical companies). We also requested unpublished studies from several hundred authors (Figure 1).
1.
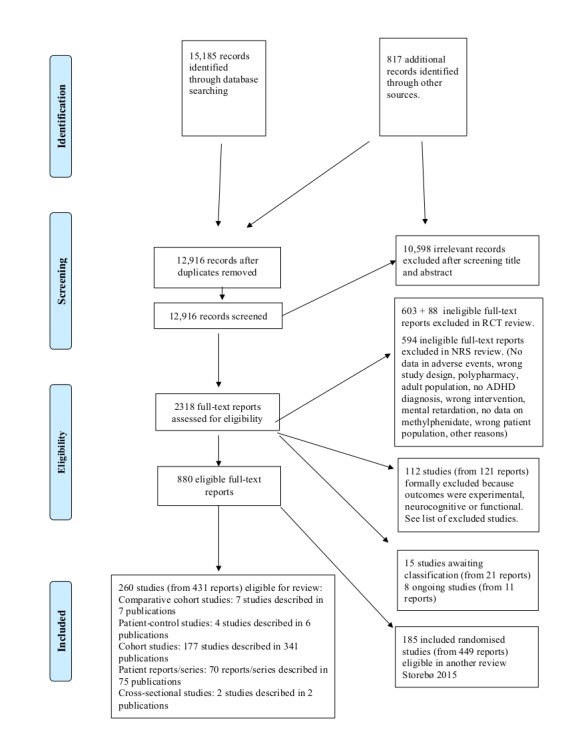
Study flow diagram
In addition, we searched the websites of the US Food and Drug Administration (FDA; www.fda.gov) and the European Medicines Agency (EMA; www.ema.europa.eu/ema).
Data collection and analysis
We conducted the review in accordance with guidance in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the PRISMA guidelines (Liberati 2009; Moher 2015), and the Cochrane ROBINS‐I tool for for assessing risk of bias in non‐randomised studies of interventions (formerly named ACROBAT) (Sterne 2014; Sterne 2016).
We performed the analysis using Cochrane's software, Review Manager 5 (RevMan 5) (Review Manager 2014).
Selection of studies
Fourteen review authors (CRMM, ER, FLM, HBK, KBR, LA, MH, MS, NP, OJS, SJH, SR, TB, and TG) worked together in groups of two and independently screened the titles and abstracts of all records retrieved by the searches; we resolved uncertainty or disagreement by consensus. We obtained the full texts of all potentially relevant reports and assessed each one against our inclusion criteria (Criteria for considering studies for this review). We discussed disagreements and consulted OJS and CG when agreement could not be reached. We have listed relevant non‐randomised studies that do not fulfil the inclusion criteria with reasons for exclusion in the Characteristics of excluded studies table. We recorded our selection process in a study flow diagram (Moher 2009).
Data extraction and management
We developed data extraction forms to facilitate standardisation of this process. We extracted data on participants, study design and methods, interventions, adverse events, and relevant data for 'Risk of bias' assessments.
All review authors extracted data. The authors worked together in groups of two, and each pair completed the data collection form independently to ensure accuracy. We resolved disagreements by discussion or used an arbiter if required. Six review authors (CRMM, FLM, HBK, MH, NP, and OJS) entered data into RevMan 5 (Review Manager 2014). In cases of insufficient data, or where data in the published study reports were unclear, we contacted the study authors requesting them to clarify the missing information (see Dealing with missing data).
Assessment of risk of bias in included studies
For each included study, two review authors (LA, SJH) used the ROBINS‐I tool and independently assessed the risk of bias of comparative cohort studies and patient‐control studies across the following seven domains (Sterne 2014; Sterne 2016).
1. Possible bias due to confounding factors
We assessed risk of bias due to:
comorbidity;
age;
sex;
subtypes of ADHD;
socioeconomic factors;
switch between ADHD medications;
adjustment of medication; and
any other confounding factor in the study.
2. Possible bias due to selection of participants
We assessed risk of bias due to:
inclusion of patients;
time from diagnosis to inclusion in study; and
naïve to previous methylphenidate exposure compared to non‐naïve patients.
For patient‐control studies, we assessed risk of bias due to selection of controls.
3. Possible bias due to measurement of interventions
We assessed risk of bias due to:
measurement of intervention status at start of follow‐up; and
self‐reporting of intervention status.
4. Possible bias due to departures from intended interventions
We assessed risk of bias due to:
compliance with assigned medication;
practitioner administration;
characteristics of the healthcare setting, for instance, public outpatient compared to hospital outpatient;
adverse events; and
lack of efficacy of treatment.
5. Possible bias due to missing data
We assessed risk of bias due to loss to follow‐up.
For patient‐control studies, we assessed risk of bias due to differences in follow‐up between exposed and non‐exposed patients.
6. Possible bias in measurement of outcomes
We assessed risk of bias due to:
self‐reporting of adverse events; and
error in instruments measuring adverse events.
7. Possible bias in selection of reported results
We assessed risk of bias due to:
type of analysis; and
selection of results.
Review authors judged each domain to be at low risk of bias, moderate risk of bias, serious risk of bias, critical risk of bias, or no information, as follows.
Low risk of bias: the study is comparable to a well‐performed RCT with regards to this domain.
Moderate risk of bias: the study is sound for a non‐randomised study with regards to this domain but cannot be considered comparable to a well‐performed RCT.
Serious risk of bias: the study has some important problems in this domain.
Critical risk of bias: the study is too problematic in this domain to provide any useful evidence on the effects of the intervention.
No information: there is no information on which to base a judgment about risk of bias for this domain.
We assigned studies an overall rating of low risk of bias when we judged them to be at low risk of bias in all domains; moderate risk of bias when we judged them to be at moderate risk of bias in at least one domain, but not at serious or critical risk of bias in any domain; serious risk of bias when we judged them to be at serious risk of bias in at least one domain, but not at critical risk of bias in any domains; critical risk of bias when we judged them to be at critical risk of bias in at least one domain; and no information when there was no clear indication that the study is at serious or critical risk of bias and there was a lack of information in one or more key domains of bias.
We resolved any disagreements by discussion. It was not possible to assess non‐comparative studies for risk of bias using ROBINS‐I because it is a prerequisite in this tool that there is a comparative study. The non‐comparative studies are at critical risk of bias mostly due to confounding factors, so we considered all these studies to be at critical risk of bias as described in the ROBINS‐I manual: "a study is too problematic to provide any useful evidence on the effects of intervention" (Sterne 2014; Sterne 2016).
There is reason to believe that other factors result in an under‐reporting of adverse events, such as a general reluctance to report them (Ioannidis 1998; Ioannidis 2009), inadequate monitoring (Loke 2011), and exclusion of patients with risk factors for adverse events (Pagsberg 2017).
Measures of treatment effect
Dichotomous data
We summarised dichotomous data as risk ratios (RR) with 95% confidence intervals (CI). We present pooled proportion data from non‐comparative studies using the Comprehensive Meta‐Analysis Software (CMA; Comprehensive Meta Analysis). See Differences between protocol and review. For numbers below 10, we gave two decimals. For numbers at 10 or above, we gave one decimal.
Continuous data
For continuous data, we calculated the mean difference (MD) between the two groups and present it with 95% CI. We used the overall MD, where possible, to compare outcome measures from studies. We estimated the standardised MD (SMD) where studies used different measures to assess the same outcome. If studies did not report means and standard deviations (SD) but reported other values, such as t‐tests and P values, we calculated the SD using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For numbers below 10, we gave two decimals. For numbers at 10 or above, we gave one decimal.
Unit of analysis issues
We included in this review a number of cross‐over trials that met our inclusion criteria (Criteria for considering studies for this review). Cross‐over trials are more prone to bias from carry‐over effects, period effects, and unit‐of analysis issues (Curtin 2002). However, as we only used the data from the methylphenidate groups from the first period of these trials, we believe that these biases do not influence the proportions reported.
Dealing with missing data
We tried to obtain missing data by contacting the authors of the studies. We wrote letters to 174 authors twice and received replies from 108. Many authors supplied us with missing sociodemographic data and missing information about methodology, and some supplied us with missing statistics. If data remained unavailable, we tried to estimate the missing data using the available information (e.g. if the SD was missing, we estimated it from the standard error, if reported).
Assessment of heterogeneity
We assessed the following types of heterogeneity: clinical (variability in participants, interventions, or settings); methodological (variation in study designs); and statistical heterogeneity (variation in intervention effects). We assessed heterogeneity between studies by visual inspection of the forest plot for overlapping CIs; using the Chi2 test for homogeneity with a significance level of α (alpha) = 0.10, and the I2 statistic for quantifying inconsistency (estimating the percentage of variation in effect estimates due to heterogeneity rather than sampling error). We judged I2 values of 0% to 40% to indicate little heterogeneity; 30% to 60%, moderate heterogeneity; 50% to 90%, substantial heterogeneity; and 75% to 100%, considerable heterogeneity (Higgins 2011). We abstained from conducting a meta‐analysis if there was a very high level of heterogeneity and the studies seemed to address different questions; Section 9.5.3.2 in theCochrane Handbook for Systematic Reviews of Interventions recommends that if "there is considerable variation in results, and particularly if there is inconsistency in the direction of effect, it may be misleading to quote an average value for the intervention effect" (Higgins 2011). Where it was not possible to undertake a meta‐analysis, we provided a narrative description of the prevalence estimate.
Not all studies used the same outcome measures. For example, for height, some studies used centimetres while other studies provided age and sex‐adjusted scores, and we could not analyse these studies.
Study characteristics that may have been important to assess included the following.
Number of confounders included in the models.
Analysis technique used.
Assessment of reporting biases
Reporting bias and missing studies are more complex issues for non‐randomised studies than for RCTs. Registration and publication of protocols for non‐randomised studies are not as common as for RCTs (Skoog 2015). We aimed to include a wide range of studies by using a broad search strategy, and we handled different forms of reporting bias, especially publication bias and outcome reporting bias, according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not draw funnel plots (estimated differences in treatment effects against their standard error) due to too few studies, nor did we perform Egger's statistical test for small‐study effects (Egger 1997). Asymmetry could be due to publication bias but also to genuine heterogeneity between small and large trials (Higgins 2011).
Data synthesis
We analysed and presented the pooled estimates of the different adverse events according to the following study designs:
comparative studies (cohort studies and patient‐control studies);
non‐comparative studies (cohort studies without a control group, including the methylphenidate‐treated group from randomised clinical trials (RCTs) comparing methylphenidate versus other interventions for ADHD excluded from our previous review (Storebø 2015), and cross sectional studies); and
patient reports/series, which we used to identify less common (rare) adverse events, defined according to the brand leader's Summary of Product Characteristics (SPC; Aagaard 2009).
As prespecified in our protocol (Storebø 2016), we tried to be as pragmatic as possible by further grouping the reported adverse events according to the main body systems affected, namely: central nervous system; cardiovascular and respiratory systems; gastrointestinal system; musculoskeletal system; immune system; urinogenital system; and other body systems. We then conducted meta‐analyses of the proportion of different adverse events under each system.
If there were adequate data, we pooled the data from comparative studies and conducted a meta‐analysis of the estimates. If there were inadequate data, we reported the results qualitatively.
We synthesised data qualitatively across some cohort studies, cross sectional studies and patient report/series when data could not be used in meta‐analyses.
Some studies have combined designs (for example, non‐comparative cohort and patient‐control study design). In those cases we synthesised the comparative and non‐comparative data separately.
Our analyses and conclusions of the results differ between comparative and non‐comparative studies. We use data from comparative cohort studies and patient‐control studies to evaluate the RR of harms. For the comparative studies, we performed a meta‐analysis according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
If clinical heterogeneity was not excessive (for example, there was not too much variability in participants' characteristics), we performed a meta‐analysis of the results using the inverse‐variance method. This method gives more weight to larger studies, reducing imprecision in the pooled estimate of effect. We used the random‐effects model in all meta‐analyses and the fixed‐effect model in sensitivity analyses (see Sensitivity analysis). The random‐effects model is best suited to our data due to the relatively high heterogeneity. In most analyses there were no significant differences between the two statistical models. In cases where there was a statistical difference, the fixed‐effect model mostly showed a higher proportion of adverse events. The decision to report the random‐effects model was therefore a conservative one.
Subgroup analysis and investigation of heterogeneity
Because of high levels of clinical and statistical heterogeneity, we conducted subgroup analyses to identify whether there was an increased risk associated with children who: had received concurrent medication; had a comorbid condition; had received methylphenidate for longer periods; were younger; or had received a higher dose of methylphenidate. Study characteristics that may have been associated with differences in risk included higher quality and independent funding.
We conducted the following subgroup analyses.
Studies with concurrent medication versus studies without any concurrent medication (children and adolescents may be more susceptible to adverse events if they are also receiving other medications).
Studies with ADHD as the only disease versus studies with ADHD and comorbidity (children and adolescents may be more susceptible to adverse events if they have other behavioural, neurological or psychological comorbidities).
Studies with a treatment duration shorter than six months versus studies with a treatment duration of six months or longer (there may be cumulative effects of methylphenidate over time).
Studies with participants with a mean age younger than 10 years versus studies with participants with a mean age of 10 years or older (younger children are more susceptible to the adverse events of methylphenidate because of their smaller size and differences in metabolism).
Studies with a low dosage of methylphenidate (below 20 mg/day) versus studies with a high dosage of methylphenidate (20 mg/day and above) (adverse events are more likely with high‐dose methylphenidate).
Cohort studies on methylphenidate originating from RCTs comparing methylphenidate to other ADHD interventions versus studies with a classic cohort design (there may be differences in estimates in higher‐quality studies (RCTs) relative to lower‐quality studies (cohorts and other observational designs)) (Garattini 2016).
Studies funded by industry versus studies not funded by industry (studies sponsored by drug companies have been shown to be less likely to report risks) (Lundh 2017).
Sensitivity analysis
As we used a random‐effects model for all analyses, we conducted a sensitivity analysis to test the robustness of our findings when using the alternative, fixed‐effect model for the outcomes of non‐serious adverse events (there were inadequate data available to test this for serious adverse events). In addition, we tested the robustness of findings when lower‐quality patient‐control studies were removed from the analysis.
See Differences between protocol and review.
'Summary of findings' table
We constructed a 'Summary of findings' table for all outcomes, using GRADEpro GDT 2015 software. We assessed the quality of the body of evidence for each outcome using the GRADE approach based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Considerations are due to: within‐study risk of bias; the directness of the evidence; heterogeneity of the data; precision of effect estimates; and risk of publication bias (Andrews 2013a; Andrews 2013b; Balshem 2011; Brunetti 2013; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Mustafa 2013). We reported all three primary (serious adverse events, withdrawal of methylphenidate due to serious adverse events, withdrawal of methylphenidate due to adverse events of unknown severity) and secondary outcomes (non‐serious adverse events, withdrawal of methylphenidate due to non‐serious adverse events, withdrawal of methylphenidate due to unknown reasons) in Table 1.
Results
Description of studies
For more information, please see Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
Results of the search
We carried out the first set of electronic searches in November 2012 (11,329 records), and ran top‐up searches in March 2014 (1274 records), February 2015 (1460 records) and January 2016 (1102 records). Conference Proceedings Citation Indexes were not available to us in 2016, but we were able to complete our searches of these two databases in November 2017 (20 records). We included additional sources to the January 2016 search in order to identify new theses but did not find any. Our electronic searches yielded a total of 15,185 records. We also identified an additional 817 publications by reading the reference lists of included articles and reviews, and from correspondence with authors and with pharmaceutical companies and other sources. We contacted the authors of 174 studies and received replies from 109.
After eliminating duplicate records, we screened 12,916 records and excluded 10,598 clearly irrelevant reports on the basis of title and abstract. We retrieved the full texts of the remaining 2318 reports, which we assessed for eligibility. We excluded 691 full‐text reports from our systematic review on RCTs assessing methylphenidate versus placebo or no intervention (Storebø 2015). We excluded a further 715 ineligible reports; see Excluded studies and Characteristics of excluded studies tables. We identified 15 studies (from 21 reports) as awaiting classification (see Characteristics of studies awaiting classification) and 8 ongoing studies (from 11 reports, see Characteristics of ongoing studies).
We included 880 reports, of which 449 described 185 RCTs and 431 reports described 260 non‐randomised studies (of which 49 originated from 49 head‐to head RCTs comparing methylphenidate versus other interventions aimed at treating ADHD). The data from the 185 RCTs in which methylphenidate was compared with placebo or no intervention are published in Storebø 2015. The present review focuses on non‐randomised studies only and thus includes 260 studies (Figure 1), which includes methylphenidate groups from the 49 RCTs comparing methylphenidate versus another medication aimed at treating ADHD, excluded in the Storebø 2015 review. Accordingly, we assessed a total of 260 non‐randomised studies described in 431 publications. For more information of these studies, please see Characteristics of included studies.
Included studies
Comparative studies
We included 11 comparative studies in this review: seven cohort studies (Cockcroft 2009; Hemmer 2001; Langevin 2012; Shin 2016; Stein 2002; Tzang 2012; Verret 2010), plus four patient‐control studies (Ayaz 2014; Dubnov‐Raz 2011; Shyu 2015; Zhang 2010). One study had both comparative and non‐comparative cohort data (Shin 2016).
Study duration
We were unable to find any information about study duration in two studies (Hemmer 2001; Stein 2002). The other studies ranged in duration from one day in Cockcroft 2009 up to 11 years in Ayaz 2014, Dubnov‐Raz 2011, Shin 2016, Shyu 2015, Tzang 2012, Langevin 2012, Verret 2010, and Zhang 2010.
Location
Two studies took place in Canada (Langevin 2012; Verret 2010), two in China (Tzang 2012; Zhang 2010), two in Israel, (Dubnov‐Raz 2011; Stein 2002), and one each in South Africa (Cockcroft 2009), South Korea (Shin 2016), Taiwan (Shyu 2015), Turkey (Ayaz 2014), and the USA (Hemmer 2001).
Settings
All were outpatient studies (Ayaz 2014; Cockcroft 2009; Dubnov‐Raz 2011; Hemmer 2001; Langevin 2012; Shin 2016; Shyu 2015; Stein 2002; Tzang 2012; Verret 2010; Zhang 2010), two of which were based on register data (Shin 2016; Shyu 2015).
Participants
There was considerable heterogeneity amongst the studies in terms of diagnostic criteria used, presence of comorbidity, numbers exposed to methylphenidate and simultaneous exposure to other medication. The mean age of children varied from 7.4 years in Zhang 2010 to 13.0 years in Stein 2002, with an age range of 3 years to 20 years. All studies involved predominantly male participants ranging from 69% in Cockcroft 2009 to 100% in both Stein 2002 and Verret 2010.
Six comparative cohort studies involved 1134 participants, 968 of whom received methylphenidate and 166 of whom did not (Cockcroft 2009; Hemmer 2001; Langevin 2012; Stein 2002; Tzang 2012; Verret 2010). A seventh study, Shin 2016, involved 1224 participants, all of whom were exposed to methylphenidate for a specific treatment period. This study only included patients with an adverse cardiovascular event. The four patient‐control studies comprised 73,098 participants: 53,192 cases who received methylphenidate and 19,906 controls who did not. All participants were aged 6 years to 19 years. The male‐to‐female ratio was approximately 80:20.
Three studies made DSM‐IV diagnoses (Ayaz 2014; Dubnov‐Raz 2011; Zhang 2010); and two made ICD‐9 and ICD‐10 diagnoses (Shin 2016; Shyu 2015). Ayaz 2014 required that ADHD medication be administered to patients at least 12 months prior to the study. Shin 2016 only included patients with an adverse cardiovascular event.
Interventions
There was considerable variation in dosage and methylphenidate preparation used as well as mean duration of exposure. The control groups not exposed to methylphenidate received atomoxetine in two studies (Ayaz 2014; Dubnov‐Raz 2011), while in eight studies the control was no treatment (Cockcroft 2009; Hemmer 2001; Langevin 2012; Shyu 2015; Stein 2002; Tzang 2012; Verret 2010; Zhang 2010). Participants in Shin 2016 received methylphenidate or no treatment at different times.
Non‐comparative studies
In the following section, we describe non‐comparative studies according to the design of the individual study, namely cohort studies, cross‐sectional studies, and patient reports/series.
Cohort studies
We included 177 cohort studies, reported in 341 publications. Of these, 49 cohorts were the methylphenidate group of the RCTs assessing this drug versus other medications for ADHD. Many of the cohort studies included in this review originally had a control group of healthy participants, but these groups did not fulfil our inclusion criteria (Criteria for considering studies for this review); we only included groups with participants diagnosed with ADHD (e.g. Sahin 2014). One study had both comparative and non‐comparative cohort data (Shin 2016).
Study duration
The cohort studies lasted from one day in Balázs 2011, Congologlu 2009, Delignieres 2011, Ilgenli 2007, and Lyon 2010 to 9.70 years in Haubold 2010. Nine studies lasted 28 days or less (Dirksen 2002; Döpfner 2011b; Galland 2010; Gau 2008; Kemner 2005 (FOCUS); Lee 2007; Park 2013; Schulz 2010; Sudarmadji 2009). Twenty‐five studies were performed over 42 days, and 15 studies over 28 days (Akhondzadeh 2003; Arnold 2010; Ashkenasi 2011; Efron 1997; Gau 2006; Hulvershorn 2012; Jung 2007; Kim 2011; Lamberti 2015; Lee 2012; Maayan 2009; McCracken 2016; Pierce 2010; Wilens 2006; Williams 2008). Sixty‐six studies lasted 42 to 180 days. Forty‐four studies lasted 180 days or more. Twelve studies did not report any study duration.
Location
Forty‐eight studies were carried out in the USA; 18 studies each in Iran, South Korea, and in Germany; 17 studies in Turkey; 7 studies in Taiwan (Chou 2012a; Chou 2012b; Gau 2006; Gau 2008; Shang 2015; Wang 2011; Yang 2004); 6 studies each in Australia (Dupuy 2008; Efron 1997; Hazell 2003; Poulton 2003; Poulton 2012; Williams 2008) and Spain (Durá‐Travé 2012; Larrañaga‐Fragoso 2015; Montañés‐Rada 2012; Tomás Vila 2010a; Valdizán Usón 2004; Valdizán Usón 2013); 5 studies each in Brazil (Chazan 2011; Guerreiro 1996; Maia 2008; Polanczyk 2007; Zeni 2007) and China (Li 2011; Su 2015; Yang 2012; Zheng 2011; Zheng 2015); 4 studies each in Israel (Golubchik 2011; Green 2011; Lahat 2000; Zelnik 2015) and Italy (Atzori 2009; Cortese 2015; Germinario 2013; Lamberti 2015); 3 studies each in Canada (Cherland 1999; Steele 2006; Weiss 2007) and the UK (Abbas 2006; McCarthy 2009; Santosh 2006); 2 studies each in Egypt (El‐Fiky 2014; Yang 2012), France (Delignieres 2011; Peyre 2012a), and Indonesia (Sudarmadji 2009; Wiguna 2012); 1 study each in India (Garg 2014), Ireland (Johnson 2013), Japan (Yatsuga 2014), New Zealand (Galland 2010), Serbia (Lakic 2012), Sri Lanka (Perera 2010), Thailand (Moungnoi 2011), the Netherlands (Van der Oord 2007), and Venezuela (Montiel‐Nava 2002).
Seven studies (3.9%) were multicentre studies, carried out in more than one country: Altin 2013 (China, Egypt, Lebanon, Russia, Taiwan, and the United Arab Emirates); Goez 2012 (Canada and Israel); Remschmidt 2005 (Germany and the UK); Wang 2007 (China, Korea, and Mexico); and Jensen 1999 (MTA), Klein 2004 and Kratochvil 2002 (Canada and the USA).
The distribution of studies among continents is: 72 studies (40.7%) in Asia, 51 studies (28.8%) in North America, 6 studies (3.40%) in South America (Chazan 2011; Guerreiro 1996; Maia 2008; Polanczyk 2007; Zeni 2007; Montiel‐Nava 2002), 38 studies (21.5%) in Europe, 8 studies (4.50%) in Oceania (Dupuy 2008; Efron 1997; Galland 2010; Hazell 2003; Poulton 2003; Poulton 2012; Williams 2008; Montiel‐Nava 2002), and 2 studies (1.1%) in Africa (El‐Fiky 2014; Yang 2012).
Setting
Most studies took place in outpatient settings, with very few in‐hospital settings (Ardic 2014; Kim 2010; Larrañaga‐Fragoso 2015; Yalcin 2014).
Participants
The 177 cohort studies included 2,207,751 participants. Not all studies provided information on age and sex. The mean age was 9.71 years; 34,753 participants were male, and 9537 were female. One large study with 2,150,362 participants did not provide information on sex (Kraut 2013).
Fifty‐two studies (29.4%) included methylphenidate‐naïve participants, 29 (16.4%) included no methylphenidate‐naïve participants, and 26 (14.9%) included a combination of naïve and non‐naïve participants. The remaining 70 studies (39.5%) did not report any information regarding drug naïvety.
Thirty studies (16.9%) included participants with ADHD diagnoses alone, while 81 studies (45.8%) included patients with one or more comorbid psychiatric conditions. In 66 studies (37.3%), it was not possible to retrieve information regarding comorbidity. Twenty‐one studies (11.9%) included participants using concurrent medications, whereas 57 studies (32.2%) excluded participants using non‐methylphenidate medication during follow‐up. Reported concurrent medication included other ADHD medications, antidepressants, antipsychotics, pain relievers, antiepileptics, anticonvulsants, antiasthmatic drugs, allergy medications, and anxiolytics. Ninety‐nine studies (55.9%) did not provide data regarding concurrent use of medication.
Interventions
The 177 studies used a range of formulations of methylphenidate. Sixty‐three (35.6%) studies did not specify the type of methylphenidate.
Fifty‐two studies (29.4%) used extended‐release methylphenidate only: 4, dexmethylphenidate (Arnold 2004; Lyon 2010; McCracken 2016; Silva 2004); 7, methylphenidate‐spheroidal oral drug absorption system (Haertling 2015; Maayan 2009; Maia 2008; Peyre 2012a; Schulz 2010; Wiguna 2012; Witt 2008); 7, methylphenidate‐transdermal patch (Arnold 2010; Ashkenasi 2011; Faraone 2007a; Findling 2009; Findling 2010; Warshaw 2010; Wilens 2008); and 34, methylphenidate‐osmotic‐release oral system. Thirteen studies did not specify which type of extended‐release methylphenidate was used in the study (Buchmann 2007; Chazan 2011; Dirksen 2002; Döpfner 2011a, OBSEER; Döpfner 2011c; Haubold 2010; Hong 2012; Kim 2015a; Mohammadi 2009; Montañés‐Rada 2012; Tomás Vila 2010b; Weiss 2007; Wigal 2015).
Twenty‐nine studies (16.4%) used immediate‐release methylphenidate only. Twenty‐two studies (12.4%) used combinations of methylphenidate‐immediate release and methylphenidate‐extended release, or combinations with placebo (Abbasi 2011; Ghanizadeh 2013). Fifteen studies (8.50%) did not specify dosage.
Sixty‐three studies (35.6%) reported methylphenidate dosage in mg/kg/day, ranging from 0.30 mg/kg/day in Efron 1997 to 1.52 mg/kg/day in Wilens 2005. Twelve studies (6.80% of the total sample) used a dosage of 0.60 mg/kg/day or less (Arman 2013; Atzori 2009; Chazan 2011; Congologlu 2009; Dittmann 2014; Efron 1997; Garg 2014; Greenberg 1987; Johnson 2013; Moungnoi 2011; Wang 2007; Zeni 2007).
One hundred and six (59.9%) studies reported methylphenidate dosage in mg/day, 64 studies (36.2%) reported dosage as a range, from 2.50 mg/day in Mayes 1994 and Perera 2010 to 120 mg/day in Döpfner 2011a, OBSEER.
Ninety‐seven studies (54.8%) reported mean dosage. The mean daily dose of methylphenidate‐immediate release was 0.67 mg/kg/day (13 studies) and 24.7 mg/day (19 studies). Mean daily dose ranged from 0.42 mg/kg/day in Atzori 2009 to 1 mg/kg/day in Schertz 1996 and Spencer 1992), and from 10 mg/day in Lahat 2000 to 36.9 mg/day in Klein 2004. For methylphenidate‐extended release, the daily dose was, on average, 0.95 mg/kg/day (18 studies) and 26.8 mg/day (26 studies), ranging from 0.48 mg/kg/day (Chazan 2011) to 1.18 mg/kg/day (Chou 2012a), and from 7.50 mg/day (Lyon 2010) to 60 mg/day (Döpfner 2011a, OBSEER).
Cross‐sectional studies
We included two cross‐sectional studies described in two publications (Stevens 2010; Thorell 2009).
Location
Thorell 2009 took place in Sweden and Stevens 2010 in the USA.
Setting
Stevens 2010 was carried out in both inpatient and outpatient clinics, whereas Thorell 2009 was an outpatient study.
Participants
The two cross‐sectional studies included a total of 96 participants, all of whom were aged between 9 and 20 years. In one study, participants were eligible for inclusion if diagnosed with ADHD and treated with higher than FDA‐approved doses of methylphenidate (> 72 mg/d) between December 2006 and August 2007 (Stevens 2010). Seventeen participants were included in the analysis. The most common comorbid disorder with ADHD was mood disorders followed by pervasive development disorders and oppositional disorders. The other study included 79 children between the ages of 9 and 17 years receiving stimulant medication (Thorell 2009). The included participants had the following comorbid diagnoses: Asperger's syndrome (n = 6), Tourette syndrome (n = 11), obsessive‐compulsive disorder (n = 2), mild mental retardation (n = 2), and oppositional defiant disorder or conduct disorder (n = 2).
Interventions
In Stevens 2010, all participants were treated with osmotic‐release oral‐system (OROS) methylphenidate. The mean total daily dose was 169 mg/day (SD 31 mg/day; range 126 mg/day to 270 mg/day) or 2.97 mg/day (SD 0.76 mg/kg/day; range 1.13 mg/kg/day to 4.21 mg/kg/day). All patients received concomitant psychotropic medication during the evaluation period. Bupropion, selective serotonin re‐uptake inhibitors, and lithium were the most commonly medications coadministered with OROS methylphenidate.
Nearly all (96.0%) the children in Thorell 2009 took methylphenidate, whereas 4.0% took amphetamine. The authors did not report methylphenidate type or dosage.
Patient reports/series
We included 70 patient reports/series described in 75 publications (Figure 1). Nineteen reports were from the USA; 16 from Turkey; five each from Israel (Artul 2014; Cohen 1992; Confino‐Cohen 2005; Gross‐Tsur 2004; Halevy 2009) and the UK (Adrian 2001; Corrigall 1996; Hollis 2007; Shibib 2009; Woolley 2003); four each from Iran (Ghanizadeh 2008a; Ghanizadeh 2008b; Ghanizadeh 2008c; Ghanizadeh 2009) and Spain (Aguilera‐Albesa 2010; Fernández‐Fernández 2010; Fernández‐Fernández 2011; Tomás Vila 2010a); three from Italy (Niederhofer 2009; Niederhofer 2011; Porfirio 2011); two each from Germany (Bernhard 2009; Holtkamp 2002), India (Agarwal 2008; Arun 2014), and Norway (Nymark 2008; Tølløfsrud 2006); and one each from Canada (Hechtman 2011), Chile (Saieh 2004), the Czech Republic (Goetz 2011), Denmark (Munk 2015), France (Coignoux 2009), Japan (Mino 1999), Sweden (Strandell 2007), and Taiwan (Tang 2010). The 70 patient reports/series included a total of 206 participants. A single report from a pharmacovigilance programme accounted for 116 of these (Strandell 2007).
All participants were aged between 4 and 20 years, with a mean age of 10 years. Seventy participants were male, 20 were female, and the sex for the remaining 116 patients was not stated. The participants were treated with both immediate‐release and extended‐release formulations of methylphenidate, and the doses ranged from 10 mg/day to 108 mg/day. The duration of treatment ranged from one day in Machado 2010 to 17 years in Ramasamy 2014.
Outcome measures: serious and non‐serious adverse events
Adverse events were measured by rating scales, including questionnaires and checklists, by spontaneous reports and/or were recorded by investigators at regular interviews or visits.
Some studies included specific measurements such as physical examinations, para‐clinical examinations, or both, including blood testing, electrocardiogram (ECG), blood pressure reading, measurement of heart rate and assessment of weight and height.
Serious adverse events were recorded in accordance with the International Conference of Harmonization (ICH) classification (ICH 1996).
Some studies combined some or all of the above modes of measurement; others used a single measure such as spontaneous reports or rating scales.
Investigators used the Barkley Side Effects Rating Scale most frequently (Barkley 1990). Other scales used included the Safety Monitoring Uniform Report Form (Greenhill 2004), the Pittsburgh Side Effects Rating Scale (Pelham 1993; Pelham 2005), and the Clinical Assessment of Side Effects.
For measuring specific adverse events, some studies used rating scales such as the Children's Depression inventory (Kovacs 1992), the Children's Yale‐Brown Obsessive‐Compulsive Scale (Scahill 1997), the Revised Children's Manifest Anxiety Scale (Reynolds 1985), the Yale Global Tic Severity Scale (Leckman 1989), the Sleep Disturbances Scale for Children (Bruni 1996), the Children's Sleep Habits Questionnaire (Owens 2000), and the Paediatric Sleep Questionnaire (Chervin 2000).
Comparative cohort studies
In the seven comparative studies that we included, four studies employed a rating scale (Cockcroft 2009; Langevin 2012; Stein 2002; Tzang 2012), two used a specific measurement (Hemmer 2001; Verret 2010), and one used diagnostic assessment (Shin 2016). Cockcroft 2009 also used a subjective pictorial scale designed specifically for the study.
Patient‐control studies
In the four included patient‐control studies, two used spontaneous reporting (Ayaz 2014; Dubnov‐Raz 2011), two used a specific measurement (Dubnov‐Raz 2011; Zhang 2010), and one used diagnostic assessment (Shyu 2015). Accordingly, one study used both spontaneous reporting and a specific measurement (Dubnov‐Raz 2011).
Cohort studies
Of the 177 included cohort studies, 84 employed a checklist, questionnaire, or rating scale. Sixty‐nine studies used a specific physiological measurement, such as heart rate or blood pressure, 26 used spontaneous reporting, and 44 used some other method to assess adverse events. Several studies used more than one mode of measurement.
Cross‐sectional studies
Of the two included cross‐sectional studies, Stevens 2010 used spontaneous reports, and Thorell 2009 used a questionnaire to measure adverse events.
Patient reports/series
Of the 70 included patient reports/series, 68 had spontaneous reporting of adverse events. One study used both spontaneous reporting and a rating scale (Coşkun 2011), one study used only a rating scale (Rapport 1996), and one study used ophthalmic examination (Lewis 2012).
Funding
Pharmaceutical companies funded 53 out of the 177 non‐comparative cohort studies, and 11 out of the 177 had authors with connections to pharmaceutical advisory boards. Forty‐five out of the 177 did not report a source of funding, and 68 out of 177 were not funded by or affiliated with pharmaceutical industries.
We performed two subgroup analyses investigating the differences in the proportion of adverse events in studies funded by industry and studies not funded by industry, and we found large differences, with many more adverse events (both serious and non‐serious) in the studies not funded by industry (see Subgroup analyses, under Effects of interventions).
Excluded studies
We took an inclusive approach by assessing 2318 full‐text reports for eligibility against our inclusion criteria (Criteria for considering studies for this review). We subsequently excluded 1285 reports as ineligible: 691 reported on RCTs (those containing new data will be included in an update of our systematic review; Storebø 2015), and 594 others either did not report data on adverse events; had ineligible study designs; involved polypharmacy; lacked an ADHD diagnosis; had ineligible interventions; had no data; focused on adults, patients with intellectual disability, or other ineligible patient populations; or otherwise failed to meet the inclusion criteria.
In addition, we formally excluded 112 studies (from 121 reports) because they did not report anything about the presence or absence of adverse events. Furthermore, most of these studies had outcomes outside of the focus of this review such as visual attention, reaction time, memory skills, and functional abnormalities. Please see the Characteristics of excluded studies tables. See Differences between protocol and review.
Studies awaiting classification
We included 15 studies awaiting classification (Arnold‐Von 2000; Dalsgaard 2011; Flapper 1989; Husár 2006; Ince 2015; Ishizaki 2001; Laezer 2015; Mulas 2014; Ptáček 2008; Radziuk 2015; Socanski 2015; Sugama 2009; TOSCA 2011; Waldon 2016; Yusufoglu 2014). Among these are five patient studies (Arnold‐Von 2000; Flapper 1989; Husár 2006; Sugama 2009; Yusufoglu 2014), one parallel‐group RCT (TOSCA 2011), one open‐label non‐controlled trial (Radziuk 2015), one review of parents reports (Mulas 2014), one observational study (Ishizaki 2001), one patient‐control study (Ptáček 2008), and two non‐comparative cohort studies (Dalsgaard 2011; Socanski 2015). The designs used in Ince 2015 and Waldon 2016 were unclear, and we could not retrieve Laezer 2015.
Ongoing studies
We included eight ongoing studies (Beau 2009; Bottelier 2014; Dahlgren 2012; Díez‐Suárez 2015; Djurkovic‐Lazic 2009; Gau 2010; Houmann 2011; Yook 2012).
Among these are one study that examines safety signal profiles in the UK Yellow Card database (Beau 2009), one placebo‐controlled trial (Bottelier 2014), one study that retrospectively investigated records of children (Dahlgren 2012), one cohort study (Djurkovic‐Lazic 2009), one longitudinal naturalistic follow‐up study (Díez‐Suárez 2015), one database study (Gau 2010), a multicenter study on CES1 genotype response in ADHD children (Houmann 2011), and one open‐label trial (Yook 2012).
Risk of bias in included studies
Risk of bias in comparative studies
Possible bias due to confounding factors
We judged the risk of bias on this domain as moderate in two studies (Stein 2002; Zhang 2010), serious in four studies (Ayaz 2014; Cockcroft 2009; Shin 2016; Tzang 2012), and critical in five studies (Dubnov‐Raz 2011; Hemmer 2001; Langevin 2012; Shyu 2015; Verret 2010).
Possible bias due to selection of participants
We judged the risk of bias on this domain as moderate in five studies (Ayaz 2014; Shyu 2015; Stein 2002; Tzang 2012; Zhang 2010), serious in five studies (Cockcroft 2009; Dubnov‐Raz 2011; Langevin 2012; Shin 2016; Verret 2010), and critical in one study (Hemmer 2001).
Possible bias due to measurement of interventions
We judged the risk of bias on this domain as moderate in eight studies (Ayaz 2014; Cockcroft 2009; Dubnov‐Raz 2011; Langevin 2012; Shyu 2015; Stein 2002; Tzang 2012; Zhang 2010), and serious in three studies (Hemmer 2001; Shin 2016; Verret 2010).
Possible bias due to departures from intended interventions
We judged the risk of bias on this domain as moderate in five studies (Ayaz 2014; Langevin 2012; Tzang 2012; Verret 2010; Zhang 2010), serious in three studies (Cockcroft 2009; Dubnov‐Raz 2011; Stein 2002), and critical in three studies (Hemmer 2001; Shyu 2015; Shin 2016).
Possible bias due to missing data
We judged the risk of bias on this domain as moderate in eight studies (Ayaz 2014; Cockcroft 2009; Dubnov‐Raz 2011; Langevin 2012; Stein 2002; Tzang 2012; Verret 2010; Zhang 2010), serious in one study (Hemmer 2001), and critical in two studies (Shyu 2015; Shin 2016).
Possible bias in measurement of outcomes
We judged the risk of bias on this domain as low in one study (Ayaz 2014), moderate in five studies (Hemmer 2001; Langevin 2012; Stein 2002; Tzang 2012; Verret 2010), and serious in five studies (Cockcroft 2009; Dubnov‐Raz 2011; Shin 2016; Shyu 2015; Zhang 2010).
Possible bias in selection of reported results
We judged the risk of bias on this domain as moderate in seven studies (Ayaz 2014; Dubnov‐Raz 2011; Langevin 2012; Stein 2002; Tzang 2012; Verret 2010; Zhang 2010), and critical in one study (Shin 2016). For three studies, no information was available regarding this domain (Cockcroft 2009; Hemmer 2001; Shyu 2015).
Overall risk of bias
Overall, we found five studies to be at serious risk of bias (Ayaz 2014; Cockcroft 2009; Stein 2002; Tzang 2012; Zhang 2010), and six studies to be at critical risk of bias (Dubnov‐Raz 2011; Hemmer 2001; Langevin 2012; Shin 2016; Shyu 2015; Verret 2010).
A table depicting the results of our 'Risk of bias' assessment for all individual studies can be seen at at zenodo.org. See supplementary file ROBINS‐I Risk of Bias Table.
Risk of bias in non‐comparative studies
In our protocol, Storebø 2016, we decided to use the ROBINS‐I tool to rate the risk of bias in the included non‐randomised studies (Sterne 2014; Sterne 2016). For studies without a control group, we initially set out to use baseline data as control information, comparing them as before‐after exposure to methylphenidate. However, a number of time‐dependent biases, such as time‐lag bias, may limit the validity of this approach (Sterne 2014; Sterne 2016). Therefore, we avoided baseline control information. Realising that the ROBINS‐I tool is designed for studies with control groups, we chose not to assess risk of bias for studies without a valid control group. Some of the included non‐randomised studies had control groups with healthy participants, but designs with healthy participants were not eligible for inclusion (see Criteria for considering studies for this review). Cochrane does not recommend any tools other than ROBINS‐I for rating risk of bias in non‐randomised studies. Thus, we deemed all studies without valid or eligible control groups to be at critical risk of bias due to many factors, with the most important being potential confounding factors.
Effects of interventions
See: Table 1
Below, we present our findings for our primary (serious adverse events) and secondary (non‐serious adverse events) outcomes, organised by comparative studies, non‐comparative studies, and patient reports/series.
A summary of the findings can be found in Table 1.
Forest plots for all meta‐analyses are available as supplementary files at zenodo.org
1. Comparative studies
1.1 Primary outcomes: serious adverse events
1.1.1 Frequency of any serious adverse events
See Analysis 1.1. Of the 11 comparative studies, only three provided data on serious adverse events. We included two studies of these studies in a meta‐analysis (Hemmer 2001; Shyu 2015); we excluded the third because it did not report on total serious adverse events (Shin 2016). Compared to no intervention, methylphenidate increased the number of patients with any serious adverse event (RR 1.36, 95% CI 1.17 to 1.57; 72,005 participants). A meta‐analysis that included the double‐zero event studies did not change this result.
1.1. Analysis.
Comparison 1 Comparative studies: number of serious adverse events, Outcome 1 Any serious adverse events.
The results of a sensitivity analysis, with the patient‐control study removed (Hemmer 2001), gives an RR of 1.36 (95% CI 1.17 to 1.57) and is thus comparable.
1.1.2 Central nervous system
See Analysis 1.2. Two studies reported on different adverse events of the central nervous system (Hemmer 2001; Shyu 2015). Compared to no intervention, methylphenidate did not increase the number of patients with seizures (RR 1.31, 95% CI 0.07 to 23.74; 1 study, 234 participants; Hemmer 2001), but it did increase the number of patients with psychotic disorder (RR 1.36, 95% CI 1.17 to 1.57; 1 study, 71,771 participants; Shyu 2015).
1.2. Analysis.
Comparison 1 Comparative studies: number of serious adverse events, Outcome 2 Central nervous system.
1.1.3 Cardiovascular and respiratory system
See Analysis 1.3. One study with 1224 participants, Shin 2016, reported on cardiovascular and respiratory adverse events. Compared to no intervention, methylphenidate increased the number of patients with arrhythmias (RR 1.61, 95% CI 1.48 to 1.74), but it did not change the number of patients with:
1.3. Analysis.
Comparison 1 Comparative studies: number of serious adverse events, Outcome 3 Cardiovascular and respiratory system.
| Cardiovascular and respiratory system | |
|---|---|
| Study | |
| Arrhythmias | |
| Shin 2016 | RR 1.61 (95% CI 1.48 to 1.74) |
| Hypertension | |
| Shin 2016 | RR 1.07 (95% CI 0.94 to 1.22) |
| Myocardial infarction | |
| Shin 2016 | RR 1.33 (95% CI 0.90 to 1.98) |
| Ischaemic stroke | |
| Shin 2016 | RR 0.70 (95% CI 0.49 to 1.01) |
| Heart failure | |
| Shin 2016 | RR 0.54 (95% CI 0.30 to 0.96) |
hypertension (RR 1.07, 95% CI 0.94 to 1.22);
myocardial infarction (RR 1.33, 95% CI 0.90 to 1.98); or
ischaemic stroke (RR 0.70, 95% CI 0.49 to 1.01).
Methylphenidate also decreased the number of patients with heart failure (RR 0.54, 95% CI 0.30 to 0.96).
No respiratory outcomes were reported.
No comparative studies reported adverse events related to the gastrointestinal, musculoskeletal, immune, urogenital or other body systems, and no comparative studies reported withdrawal of treatment due to serious adverse events or adverse events of unknown severity.
1.2 Secondary outcomes: non‐serious adverse events
Seven studies reported data on this outcome. We included five of these studies in meta‐analyses (Cockcroft 2009; Stein 2002; Tzang 2012; Verret 2010; Zhang 2010). We could not use data from two further studies in our meta‐analyses because the authors reported other types of outcomes on adverse events with single study results (Dubnov‐Raz 2011; Langevin 2012).
1.2.1 Frequency of any non‐serious adverse events
None of the seven studies reported data on the frequency of any non‐serious adverse events.
1.2.2 Central nervous system
Sleep‐related adverse events
See Analysis 2.1. Three studies with 425 participants reported data on sleep disturbance (Cockcroft 2009; Stein 2002; Tzang 2012). We conducted a meta‐analysis and found that, compared to no intervention, methylphenidate increased the number of patients with insomnia and sleep problems (RR 2.58, 95% CI 1.24 to 5.34; 3 studies, 425 participants).
2.1. Analysis.
Comparison 2 Comparative studies: number of participants with non‐serious adverse events, Outcome 1 Central nervous system: sleep‐related adverse events.
One study with 23 participants, Cockcroft 2009, compared methylphenidate to no intervention and found that methylphenidate did not increase the number of patients with:
nightmares (RR 1.15, 95% CI 0.41 to 3.21);
snoring (RR 4.62, 95% CI 0.25 to 86.7);
non‐breathing or gasping while sleeping (RR 2.77, 95% CI 0.12 to 61.7);
sleepwalking (RR 2.75, 95% CI 0.33 to 22.7);
various sleep positions (RR 2.77, 95% CI 0.12 to 61.7);
enuresis (RR 4.62, 95% CI 0.25 to 86.7); or
talking in sleep (RR 0.46, 95% CI 0.05 to 4.38).
Other specific sleep‐related adverse effects
See Analysis 2.2. Langevin 2012 (10 participants) compared methylphenidate to no intervention and found that methylphenidate did not decrease:
2.2. Analysis.
Comparison 2 Comparative studies: number of participants with non‐serious adverse events, Outcome 2 Central nervous system: other specific sleep‐related adverse events.
the number of hours of sleep (MD −0.65 hours, 95% CI −1.32 to 0.02);
the number of nocturnal movements (MD −27.84 movements, 95% CI −57.9 to 2.22); or
sleep quality (MD −0.20, 95% CI −0.74 to 0.34), as measured on a five‐point sleep quality scale (ranging from −2 = very poor to 2 = very good) from “The Morpheus Network Wake and Sleep Calendar.”
Other specific adverse events
See Analysis 2.3. One study with, Tzang 2012, reported on different adverse events of the central nervous system. Compared to no intervention, methylphenidate did not increase the number of patients with:
2.3. Analysis.
Comparison 2 Comparative studies: number of participants with non‐serious adverse events, Outcome 3 Central nervous system: other specific adverse events.
headache (RR 8.13, 95% CI 0.48 to 137.8; 235 participants); or
dizziness (RR 4.00, 95% CI 0.23 to 69.3; 335 participants).
1.2.3 Cardiovascular and respiratory system
See analysis Analysis 2.4
2.4. Analysis.
Comparison 2 Comparative studies: number of participants with non‐serious adverse events, Outcome 4 Cardiovascular and respiratory system.
Systolic blood pressure
Only one study with 43 participants reported data on this outcome (Verret 2010). Compared to no intervention, methylphenidate did not affect systolic blood pressure (MD 0.10 mmHg, 95% CI −6.27 to 6.47).
Diastolic blood pressure
Only one study with 43 participants reported data on this outcome (Verret 2010). Compared to no intervention, methylphenidate did not affect diastolic blood pressure (MD 3.50 mmHg, 95% CI −1.42 to 8.42).
Pulse rate
Only one study with 43 participants reported data on this outcome (Verret 2010). Compared to no intervention, methylphenidate did not seem to affect pulse rate (MD 0.60 beats per minute, 95% CI −7.95 to 9.15).
1.2.4 Gastrointestinal system
See Analysis 2.5. One study with 335 participants reported on gastrointestinal adverse events (Tzang 2012). Compared to no intervention, methylphenidate did not increase the number of patients with:
2.5. Analysis.
Comparison 2 Comparative studies: number of participants with non‐serious adverse events, Outcome 5 Gastrointestinal system.
nausea (RR 2.64, 95% CI 0.34 to 20.3); or
abdominal pain (RR 0.79, 95% CI 0.16 to 3.84).
However, methylphenidate did increase the number with decreased appetite (RR 15.06, 95% CI 2.12 to 106.8).
1.2.5 Musculoskeletal system
See Analysis 2.6.
2.6. Analysis.
Comparison 2 Comparative studies: number of participants with non‐serious adverse events, Outcome 6 Musculoskeletal system.
Height
Two studies reported data on height (Verret 2010; Zhang 2010). We pooled data from both studies (93 participants) in a meta‐analysis and found that, compared to no intervention, methylphenidate did not increase the number of patients with reduced height (SMD −0.93, 95% CI −2.61 to 0.75).
Weight
Four studies reported data on weight (Dubnov‐Raz 2011; Jensen 1999 (MTA); Verret 2010; Zhang 2010). We pooled data from two studies (93 participants) in a meta‐analysis (Verret 2010; Zhang 2010). Compared to no intervention, methylphenidate did not decrease weight (SMD −0.27, 95% CI −1.16 to 0.62).
Body mass index (BMI)
See Analysis 2.7. Compared to no intervention, methylphenidate decreased BMI (MD −1.60 kg/m2, 95% CI −2.96 to −0.24) in one study with 43 participants (Verret 2010).
2.7. Analysis.
Comparison 2 Comparative studies: number of participants with non‐serious adverse events, Outcome 7 Musculoskeletal system: body mass index (BMI).
Z scores
See Analysis 2.8.
2.8. Analysis.
Comparison 2 Comparative studies: number of participants with non‐serious adverse events, Outcome 8 Musculoskeletal system: z scores.
Height
Compared to no intervention, methylphenidate did not decrease the height z score (MD −0.19, 95% CI −0.43 to 0.05) in one study with 275 participants (Dubnov‐Raz 2011).
Weight
Compared to no intervention, methylphenidate did not decrease the weight z score (MD −0.07, 95% CI −0.30 to 0.16) in one study with 275 participants (Dubnov‐Raz 2011).
Dubnov‐Raz 2011 also observed no change in weight related to methylphenidate (P = 0.05) when comparing participants receiving methylphenidate (39.7 (SD 17.70) kg; n = 135) to untreated participants (35.30 (SD 13.40) kg; n = 140).
In Jensen 1999 (MTA), weight gain was apparently lower in the methylphenidate group (n = 222) at 14 months (2.36 (SD 3.00) kg) and at 24 months (3.81 (SD 2.84) kg), compared to the untreated group (14 months: 5.14 (SD 3.53) kg; 24 months: 4.83 (SD 3.10) kg; n = 106).
BMI
One study with 275 participants, Dubnov‐Raz 2011, found no difference between those in the methylphenidate group and those in the no intervention group for BMI z score (MD 0.01, 95% CI −0.22 to 0.24).
No comparative studies reported non‐serious adverse events related to the immune, urogenital, or other body systems.
1.2.6 Withdrawal of treatment
Due to non‐serious adverse events
No comparative studies reported withdrawal of treatment due to non‐serious adverse events.
For unknown reasons
See Analysis 3.1. One study with 146 participants, Zhang 2010, compared methylphenidate to no intervention, finding that it increased the proportion of participants withdrawn from methylphenidate for unknown reasons (RR 0.72 95% CI 0.62 to 0.84).
3.1. Analysis.
Comparison 3 Comparative studies: number of participants withdrawn from methylphenidate treatment, Outcome 1 Proportion of participants withdrawn from methylphenidate for unknown reasons.
2. Non‐comparative studies
2.1 Primary outcomes: serious adverse events
We included a total of 54 studies in meta‐analyses on this outcome.
2.1.1. Frequency of any serious adverse events
See Analysis 4.1. We included 50 studies (162,422 participants) that reported data on this outcome in a meta‐analysis. The proportion of participants on methylphenidate experiencing one or more serious adverse events was 1.20% (95% CI 0.70% to 2.00%; Figure 2).
4.1. Analysis.
Comparison 4 Non‐comparative studies: proportion of participants with serious adverse events, Outcome 1 Any serious adverse event.
| Any serious adverse event | ||
|---|---|---|
| Study | Symptoms | Adverse events/total number of participants |
| Altin 2013 | No patients experienced any serious adverse events | 0/221 |
| Arabgol 2015 | Severe anorexia | 1/18 |
| Ardic 2014 | Serious adverse events | 0/101 |
| Arnold 2004 | Serious adverse events | 3/89 |
| Arnold 2010 | Acute depression and suicide attempt | 1/150 |
| Ashkenasi 2011 | Hallucinations | 1/26 |
| Berek 2011 | Serious adverse events | 4/822 |
| Cherland 1999 | Serious adverse events | 13/98 |
| Chou 2012b | Serious adverse events | 0/230 |
| Cortese 2015 | Serious adverse events | 64/1426 |
| Davari‐Ashtiani 2010 | Serious adverse events | 0/16 |
| Dittmann 2014 | Serious adverse events | 0/247 |
| Döpfner 2011a, OBSEER | Serious adverse events | 21/777 |
| Döpfner 2011b | Serious adverse events | 38/822 |
| Findling 2009 | Serious adverse events | 3/157 |
| Germinario 2013 | Serious adverse events | 7/1098 |
| Gerwe 2009 | Serious adverse events | 2/263 |
| Haertling 2015 | Serious adverse events | 3/239 |
| Hammerness 2009 | Participants affected or at risk | 5/57 |
| Hammerness 2012 | Serious adverse events | 0/10 |
| Hulvershorn 2012 | Serious adverse events | 0/25 |
| Jafarinia 2012 | Serious adverse events | 0/20 |
| Jensen 1999 (MTA) | Serious adverse events | 1/245 |
| Kemner 2005 (FOCUS) | Sudden death | 0/850 |
| Kim 2011 | Serious adverse events | 0/97 |
| Lakic 2012 | Strong depressive symptoms | 1/68 |
| Lee 2014 | Serious adverse events | 542/4055 |
| Li 2011 | Serious adverse events | 0/36 |
| Maayan 2009 | Serious adverse events | 0/8 |
| McCarthy 2009 | Sudden death | 5/5351 |
| McCracken 2016 | Serious adverse events | 0/61 |
| Mohammadi 2004 | Serious adverse events | 3/116 |
| Pierce 2010 | Serious adverse events | 0/71 |
| Remschmidt 2005 | Serious adverse events | 4/89 |
| Schmidt 2002 | Generalized tonic‐clonic seizure | 1/124 |
| Schulz 2010 | Serious adverse events | 0/145 |
| Shin 2016 | Serious adverse events | 1224/123,636 |
| Sobanski 2013 | Serious adverse events | 8/347 |
| Steele 2006 | Serious adverse events | 0/73 |
| Su 2015 | Serious adverse events | 0/239 |
| Valdizán Usón 2013 | Serious adverse events | 0/689 |
| Warshaw 2010 | Serious adverse events | 0/260 |
| Weiss 2007 | Serious adverse events | 0/79 |
| Wigal 2011 | Serious adverse events | 0/31 |
| Wigal 2013 | Serious adverse events | 0/39 |
| Wigal 2015 | Serious adverse events | 1/200 |
| Wilens 2006 | Serious adverse events | 1/87 |
| Wilens 2008 | Serious adverse events | 0/127 |
| Winterstein 2009 | Serious adverse events | 171/18,238 |
| Zheng 2015 | Serious adverse events | 0/149 |
2.
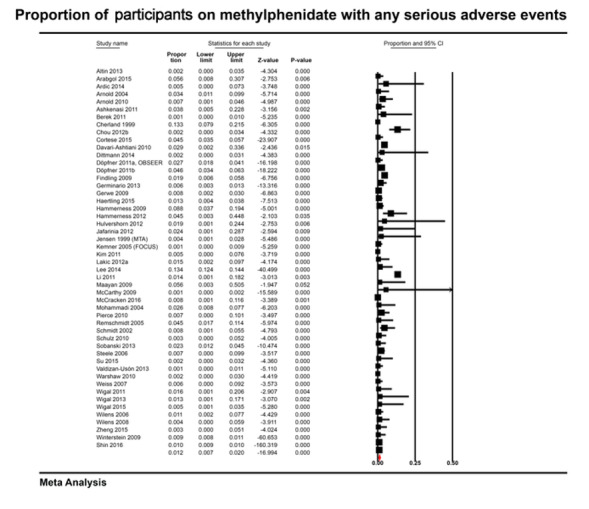
Proportion of participants on methylphenidate with any serious adverse events
2.1.2 Central nervous system
See Analysis 4.2. Twenty‐two studies reported data on serious central nervous system adverse events (Arabgol 2015; Arnold 2004; Arnold 2010; Cherland 1999; Cortese 2015; Findling 2009; Germinario 2013; Gerwe 2009; Green 2011; Jensen 1999 (MTA); Kemner 2005 (FOCUS); Lakic 2012; Lee 2014; McCarthy 2009; Mohammadi 2004; Na 2013; Remschmidt 2005; Schmidt 2002; Shyu 2015; Su 2015; Wilens 2005; Wilens 2006).
4.2. Analysis.
Comparison 4 Non‐comparative studies: proportion of participants with serious adverse events, Outcome 2 Central nervous system.
| Central nervous system | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Sudden death | ||
| Kemner 2005 (FOCUS) | Sudden death | 0/850 |
| Lee 2014 | Sudden death | 7/100 |
| McCarthy 2009 | Sudden death | 1/5351 |
| Suicide | ||
| Jensen 1999 (MTA) | Suicide | 1/245 |
| McCarthy 2009 | Suicide | 4/5351 |
| Suicide attempt | ||
| Arnold 2010 | Acute depression and suicide attempt | 1/150 |
| Lee 2014 | Suicide attempt | 6/100 |
| Remschmidt 2005 | Suicide attempt | 1/89 |
| Suicide thoughts | ||
| Remschmidt 2005 | Depressive, suicidal thoughts | 1/89 |
| Wilens 2006 | Serious adverse events were reported in only 1 participant during the open‐label, dose‐titration phase of the study | 1/87 |
| Psychotic symptoms | ||
| Cherland 1999 | Psychotic symptoms | 7/96 |
| Cortese 2015 | Hallucinations | 2/1426 |
| Findling 2009 | Paranoid behavior disorder | 3/157 |
| Green 2011 | Hallucinations | 0/22 |
| Mohammadi 2004 | Hallucinations | 0/11 |
| Na 2013 | Hallucinations | 0/55 |
| Remschmidt 2005 | Delusions | 1/89 |
| Shyu 2015 | Psychotic disorders | 856/52,646 |
| Su 2015 | Hallucinations | 1/239 |
| Wilens 2005 | Hallucinations | 0/369 |
| Seizure | ||
| Cortese 2015 | Seizures | 3/1426 |
| Schmidt 2002 | Generalized tonic‐clonic seizure | 1/124 |
| Syncope | ||
| Findling 2009 | Syncope | 1/327 |
| Germinario 2013 | Vasovagal syncope | 1/1098 |
| Tremor | ||
| Arnold 2004 | Tremor | 1/89 |
| Gerwe 2009 | Tremor | 1/306 |
| Psychiatric problems | ||
| Arnold 2004 | Rambling speech | 1/89 |
| Cortese 2015 | Psychiatric disorder | 5/1426 |
| Germinario 2013 | Psychiatric problems | 5/1098 |
| Severe depression | ||
| Cherland 1999 | Severe depression | 1/98 |
| Lakic 2012 | Strong depressive symptoms | 1/68 |
| Overdose | ||
| Lee 2014 | Overdose | 6/100 |
| Eating disorder | ||
| Cortese 2015 | Eating disorder | 1/1426 |
| Aphasia | ||
| Cortese 2015 | Aphasia | 1/1426 |
| Headache | ||
| Cortese 2015 | Headache | 3/1426 |
| Neurological disorder | ||
| Cortese 2015 | Neurologicaol disorder | 2/1426 |
| Sleep disorder | ||
| Cortese 2015 | Sleep disorder | 1/1426 |
| Mood disorder | ||
| Cortese 2015 | Mood disorder | 1/1426 |
| Loss of consciousness | ||
| Gerwe 2009 | Short loss of consciousness | 1/306 |
| Family stress | ||
| Gerwe 2009 | Family stress | 1/306 |
| Impotence | ||
| Germinario 2013 | Impotence | 1/1098 |
| Severe aggression | ||
| Remschmidt 2005 | Severe aggression | 1/89 |
| Severe anorexia | ||
| Arabgol 2015 | Severe anorexia | 1/18 |
| Amphetamine intoxication | ||
| Cherland 1999 | Amphetamine intoxication | 3/98 |
We included data from 19 of these studies in 10 meta‐analyses (Arnold 2004; Arnold 2010; Cherland 1999; Cortese 2015; Findling 2009; Germinario 2013; Gerwe 2009; Jensen 1999 (MTA); Kemner 2005 (FOCUS); Lakic 2012; Lee 2014; McCarthy 2009; Mohammadi 2004; Na 2013; Remschmidt 2005; Schmidt 2002; Shyu 2015; Su 2015; Wilens 2006). The proportion of participants on methylphenidate with:
sudden death was 0.20% (95% CI 0.00% to 15.9%; 3 studies, 6301 participants; Kemner 2005 (FOCUS); Lee 2014; McCarthy 2009; see supplementary file S1);
suicide was 0.10% (95% CI 0.0% to 0.30%; 2 studies, 5596 participants; Jensen 1999 (MTA); McCarthy 2009; see supplementary file S2);
suicide attempt was 2.10% (95% CI 0.40% to 9.00%; 3 studies, 339 participants; Arnold 2010; Lee 2014; Remschmidt 2005; see supplementary file S3);
suicidal thoughts was 1.10% (95% CI 0.30% to 4.40%; 2 studies, 176 participants; Remschmidt 2005; Wilens 2006; see supplementary file S4);
psychotic symptoms was 1.20% (95% CI 0.50% to 2.70%; 10 studies, 55,110 participants; Cherland 1999; Cortese 2015; Findling 2009; Green 2011; Mohammadi 2004; Na 2013; Remschmidt 2005; Shyu 2015; Su 2015; Wilens 2006; see supplementary file S5);
seizure was 0.30% (95% CI 0.10% to 1.10%; 2 studies, 1550 participants; Cortese 2015; Schmidt 2002; see supplementary file S6);
syncope was 0.20% (95% CI 0.00% to 0.70%; 2 studies, 1425 participants; Findling 2009; Germinario 2013; see supplementary file S7);
tremor was 0.60% (95% CI 0.20% to 2.40%; 2 studies, 395 participants; Arnold 2004; Gerwe 2009; see supplementary file S8);
psychiatric problems was 0.40% (95% CI 0.20% to 0.80%; 3 studies, 2613 participants; Arnold 2004; Cortese 2015; Germinario 2013; see supplementary file S9); and
severe depression was 1.20% (95% CI 0.30% to 4.80%; 2 studies, 166 participants; Cherland 1999; Lakic 2012; see supplementary file S10).
Seven studies reported on other adverse events related to the central nervous system.
In Lee 2014 (100 participants), the proportion of participants on methylphenidate with overdose was 6.0% (95% CI 3.0% to 12.0%).
-
In Cortese 2015 (1426 participants), the proportion of participants on methylphenidate with:
eating disorder was 0.07% (95% CI 0.01% to 0.40%);
aphasia was 0.07% (95% CI 0.01% to 0.40%);
headache was 0.02% (95% CI 0.01% to 0.06%);
neurological disorder was 0.14% (CI 0.04% to 0.50%);
sleep disorder was 0.07% (95% CI 0.01% to 0.40%); and
mood disorder was 0.07% (95% CI 0.01% to 0.40%).
-
In Gerwe 2009 (306 participants), the proportion of participants on methylphenidate with:
loss of consciousness was 0.33% (95% CI 0.06% to 2.00%); and
family stress was 0.33% (95% CI 0.06% to 2.00%).
In Germinario 2013 (1098 participants), the proportion of participants on methylphenidate with impotence was 0.09% (95% CI 0.02% to 0.50%).
In Remschmidt 2005 (89 participants), the proportion of participants on methylphenidate with severe aggression was 1.12% (95% CI 0.20% to 6.00%).
In Arabgol 2015 (18 participants), the proportion of participants on methylphenidate with severe anorexia was 5.56% (95% CI 0.10% to 25.0%).
In Cherland 1999 (98 participants), the proportion of participants on methylphenidate with amphetamine intoxication was 3.06% (95% CI 1.00% to 8.00%).
2.1.3 Cardiovascular and respiratory system
See Analysis 4.3. Five studies reported data on serious cardiovascular and respiratory adverse events (Arman 2013; Cortese 2015; Germinario 2013; Hammerness 2009; Winterstein 2009). We included these five studies across three meta‐analyses and found that the proportion of participants on methylphenidate with:
4.3. Analysis.
Comparison 4 Non‐comparative studies: proportion of participants with serious adverse events, Outcome 3 Cardiovascular and respiratory system.
| Cardiovascular and respiratory system | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Respiratory, thoracic and mediastinal disorders | ||
| Germinario 2013 | Aortic embolism | 1/1098 |
| Hammerness 2009 | Spontaneous pneumothorax | 1/57 |
| Cardiac problems | ||
| Arman 2013 | Cardiac rhythm abnormalities | 0/15 |
| Germinario 2013 | Cardiovascular problems | 2/1098 |
| Winterstein 2009 | Serious cardiac problems | 171/18,238 |
| Tachycardia | ||
| Cortese 2015 | Tachycardia | 2/1426 |
| Germinario 2013 | Tachycardia | 2/1098 |
| Severe hypertension | ||
| Cortese 2015 | Severe hypertension | 2/1426 |
| Epistaxsis | ||
| Cortese 2015 | Epistaxsis | 1/1426 |
| Viral illness | ||
| Hammerness 2009 | Participants affected by viral illness | 1/57 |
respiratory, thoracic and mediastinal disorders was 0.40% (95% CI 0.00% to 6.90%; 2 studies, 1155 participants; Germinario 2013; Hammerness 2009; see supplementary file S11);
cardiac problems was 0.70% (95% CI 0.20% to 2.40%; 3 studies, 19,351 participants; Arman 2013; Germinario 2013; Winterstein 2009; see supplementary file S12); and
tachycardia was 0.20% (95% CI 0.10% to 0.40%; 2 studies, 2524 participants; Cortese 2015; Germinario 2013; see supplementary file S13).
Two of these studies also reported on other cardiovascular and respiratory adverse events (Cortese 2015; Hammerness 2009).
-
In Cortese 2015 (1426 participants), the proportion of participants on methylphenidate with:
severe hypertension was 0.14% (95% CI 0.00% to 0.05%); and
epistaxis was 0.07% (95% CI 0.00% to 0.40%).
In Hammerness 2009 (57 participants), the proportion of participants on methylphenidate with viral illness was 1.75% (95% CI 0.03% to 9.28%).
2.1.4 Gastrointestinal system
See Analysis 4.4. One study with 1426 participants reported data on gastrointestinal adverse events (Cortese 2015). In this study, the proportion of participants on methylphenidate with:
4.4. Analysis.
Comparison 4 Non‐comparative studies: proportion of participants with serious adverse events, Outcome 4 Gastrointestinal system.
| Gastrointestinal system | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Gastrointestinal disease | ||
| Cortese 2015 | Gastrointestinal disease | 6/1426 |
| Liver disease | ||
| Cortese 2015 | Hyperbilrubinemia | 1/1426 |
gastrointestinal disease was 0.42% (95% CI 0.19% to 0.91%); and
liver disease was 0.07% (95% CI 0.00% to 0.40%).
2.1.5 Musculoskeletal system
See Analysis 4.5. We included two studies (384 participants) providing data on musculoskeletal adverse events in a meta‐analysis (Findling 2009; Hammerness 2009). The proportion of participants on methylphenidate with fractures was 1.20% (95% CI 0.10% to 11.5%; see supplementary file S14).
4.5. Analysis.
Comparison 4 Non‐comparative studies: proportion of participants with serious adverse events, Outcome 5 Musculoskeletal system: fractures.
| Musculoskeletal system: fractures | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Findling 2009 | Ankle fracture | 1/327 |
| Hammerness 2009 | Leg fracture | 2/57 |
2.1.6 Immune system
See Analysis 4.6. Only one study with 1426 participants reported data on adverse events of the immune system (Cortese 2015). In this study, the proportion of participants on methylphenidate with autoimmune disease was 0.07% (95% CI 0.00% to 0.40%).
4.6. Analysis.
Comparison 4 Non‐comparative studies: proportion of participants with serious adverse events, Outcome 6 Immune system: autoimmune disease.
| Immune system: autoimmune disease | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Cortese 2015 | Autoimmune disease | 1/1426 |
No comparative studies reported adverse events related to the urogenital or other body systems.
2.1.9 Withdrawal of treatment
Due to serious adverse events
See Analysis 5.1. Seven studies with 1173 participants reported data on the number of participants withdrawn from methylphenidate treatment due to serious adverse events (Arabgol 2015; Arnold 2010; Ashkenasi 2011; Faraone 2007a; Findling 2010; Na 2013; Wilens 2005). We conducted a meta‐analysis of data from these seven studies and found that the proportion of participants withdrawn from methylphenidate treatment due to serious adverse events was 1.20% (95% CI 0.60% to 2.30%; Figure 3).
5.1. Analysis.
Comparison 5 Non‐comparative studies: proportion of participants withdrawn from methylphenidate treatment due to serious adverse events, Outcome 1 Proportion of participants withdrawn from methylphenidate treatment due to serious adverse events.
| Proportion of participants withdrawn from methylphenidate treatment due to serious adverse events | |
|---|---|
| Study | |
| Serious adverse events | |
| Arabgol 2015 | 1/18 |
| Arnold 2010 | 1/171 |
| Ashkenasi 2011 | 1/26 |
| Faraone 2007a | 3/268 |
| Findling 2010 | 2/162 |
| Na 2013 | 1/121 |
| Wilens 2005 | 1/407 |
| Hallucinations | |
| Ashkenasi 2011 | 1/26 |
| Na 2013 | 1/121 |
| Wilens 2005 | 1/407 |
| Severe anorexia | |
| Arabgol 2015 | 1/18 |
| Suicide attempt | |
| Arnold 2010 | 1/171 |
3.
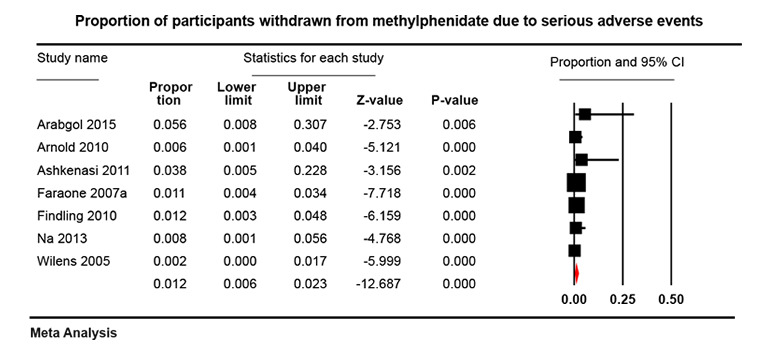
Proportion of participants withdrawn from methylphenidate due to serious adverse events
Three of the studies also reported on the proportion participants withdrawn from methylphenidate treatment due to specific serious adverse events (Arabgol 2015; Arnold 2010; Ashkenasi 2011).
All three studies reported the proportion of participants withdrawn from methylphenidate treatment due to hallucinations (0.90%, 95% Cl 0.2% to 4.4%; 554 participants; See supplementary file S15).
Arabgol 2015 (18 participants) also reported the proportion of participants withdrawn from methylphenidate treatment due to severe anorexia as 5.56% (95% Cl 1.00% to 25.8%).
Arnold 2010 (171 participants) also reported the proportion of participants withdrawn from methylphenidate treatment due to suicide attempt as 0.58% (95% Cl 0.10% to 3.23%).
Due to adverse events of unknown severity
See Analysis 5.2. We conducted a meta‐analysis of data from 22 studies (3708 participants) that reported the number of patients withdrawn from methylphenidate treatment due to adverse events of unknown severity. The proportion of participants withdrawn from methylphenidate treatment due to adverse events of unknown severity was 7.3% (95% CI 5.3% to 10.0%; Figure 4).
5.2. Analysis.
Comparison 5 Non‐comparative studies: proportion of participants withdrawn from methylphenidate treatment due to serious adverse events, Outcome 2 Proportion of participants withdrawn from methylphenidate treatment due to adverse events of unknown severity.
| Proportion of participants withdrawn from methylphenidate treatment due to adverse events of unknown severity | |
|---|---|
| Study | |
| Altin 2013 | 4/204 |
| Arnold 2004 | 4/89 |
| Arnold 2010 | 4/171 |
| Atzori 2009 | 17/189 |
| Chou 2012b | 15/296 |
| Dodangi 2011 | 2/15 |
| Döpfner 2011a, OBSEER | 26/822 |
| Findling 2009 | 51/326 |
| Kim 2015b | 8/143 |
| Kordon 2011 | 53/598 |
| Kratochvil 2002 | 5/44 |
| Lee 2009 | 28/144 |
| Lee 2012 | 1/93 |
| Maia 2008 | 3/39 |
| Mayes 1994 | 2/69 |
| McCracken 2016 | 1/69 |
| Mohammadi 2009 | 6/30 |
| Remschmidt 2005 | 16/89 |
| Steele 2006 | 6/73 |
| Yang 2004 | 2/25 |
| Yang 2012 | 15/130 |
| Yildiz 2010 | 3/50 |
4.
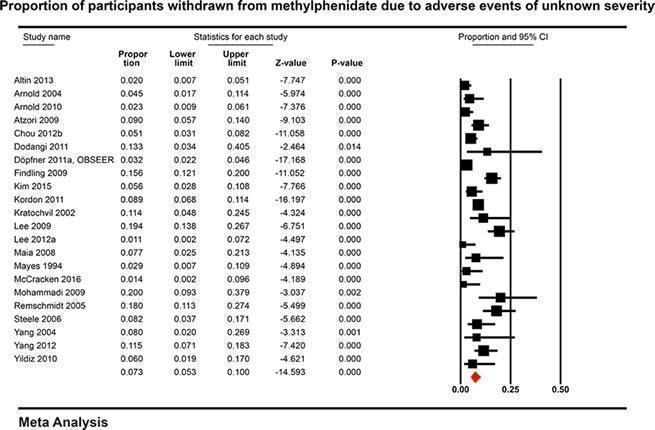
Proportion of participants withdrawn from methylphenidate due to adverse events of unknown severity
2.2 Secondary outcomes: non‐serious adverse events
All but 11 studies contributed data to our meta‐analyses (see details below). For the 11 studies which contributed data that we could not combine, we reported the results qualitatively (Cho 2012;Dubnov‐Raz 2011;Durá‐Travé 2012;Ilgenli 2007;Lahat 2000;Lamberti 2015;Özcan 2004; Poulton 2012;Schertz 1996;Spencer 1992;Vincent 1990).
Proportion of participants with adverse events of unknown severity
See Analysis 6.1. One study with 251 participants also reported data on the frequency of adverse events of unknown severity (Barbaresi 2006). In this study, the proportion of participants on methylphenidate with adverse events of unknown severity was 25.1% (95% CI 20.0% to 31.0%).
6.1. Analysis.
Comparison 6 Non‐comparative studies: proportion of participants with adverse events of unknown severity, Outcome 1 Proportion of participants with adverse events of unknown severity.
| Proportion of participants with adverse events of unknown severity | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Barbaresi 2006 | Adverse events of unknown seriousness | 63/251 |
2.2.1 Frequency of any non‐serious adverse events
See Analysis 7.1. The proportion of participants on methylphenidate with non‐serious adverse events was 51.2% (41.2% to 61.1%; 49 studies; 13,978 participants; Figure 5).
7.1. Analysis.
Comparison 7 Non‐comparative studies: proportion of participants with non‐serious adverse events, Outcome 1 Non‐serious adverse events.
| Non‐serious adverse events | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Altin 2013 | Total adverse events | 21/142 |
| Ardic 2014 | Any adverse event (with OROS (osmotic controlled release oral delivery system) methylphenidate) | 52/122 |
| Arnold 2004 | Any adverse event | 76/76 |
| Atzori 2009 | Total number of patients who experience adverse events | 54/134 |
| Barrickman 1995 | Total adverse events | 5/15 |
| Berek 2011 | Total adverse events | 195/762 |
| Chou 2012b | Total adverse events | 95/230 |
| Cortese 2015 | Total adverse events | 369/1426 |
| Dirksen 2002 | Total adverse events | 78/287 |
| Dittmann 2014 | Total adverse events | 25/195 |
| Döpfner 2011a, OBSEER | Total adverse events | 209/822 |
| Döpfner 2011b | Total adverse events | 40/106 |
| Findling 2010 | Total adverse events | 68/88 |
| Garg 2014 | Total adverse events | 18/27 |
| Gau 2008 | Total adverse events | 180/607 |
| Gerwe 2009 | Total adverse events | 160/263 |
| Goez 2012 | Total adverse events | 7/28 |
| Green 2011 | Total adverse events | 14/14 |
| Greenberg 1987 | Total adverse events | 8/49 |
| Greenhill 1983 | Total adverse events | 5/7 |
| Gucuyener 2003 | Total adverse events | 26/119 |
| Haertling 2015 | Total adverse events | 27/239 |
| Hammerness 2009 | Total adverse events | 45/57 |
| Hammerness 2012 | Total adverse events | 0/27 |
| Haubold 2010 | Total adverse events | 32/103 |
| Jensen 1999 (MTA) | Total adverse events | 150/245 |
| Karabekiroglu 2008 | Total adverse events | 28/90 |
| Khajehpiri 2014 | At least one adverse drug reaction | 71/71 |
| Kim 2015b | Total adverse events | 8/116 |
| Kordon 2011 | Total adverse events | 172/541 |
| Lee 2014 | Total adverse events | 3513/4055 |
| Lyon 2010 | Total adverse events | 7/10 |
| Mayes 1994 | Total adverse events | 35/63 |
| McCracken 2016 | Total adverse events | 58/60 |
| Na 2013 | Total number of patients who experienced adverse events | 98/103 |
| Remschmidt 2005 | Total adverse events | 76/89 |
| Schulz 2010 | Total adverse events | 36/145 |
| Sobanski 2013 | Total adverse events | 26/347 |
| Song 2012 | Total adverse events | 116/116 |
| Su 2015 | Total adverse events | 101/205 |
| Tasdelen 2015 | Total adverse events | 12/17 |
| Valdizán Usón 2013 | Total adverse events | 177/689 |
| Warshaw 2010 | Total adverse events | 242/260 |
| Weiss 2007 | Total adverse events | 64/79 |
| Wigal 2013 | Total adverse events | 42/45 |
| Wilens 2005 | Total adverse events | 344/407 |
| Wilens 2006 | Total adverse events | 96/135 |
| Yatsuga 2014 | Total adverse events | 7/50 |
| Zheng 2015 | Total adverse events | 24/123 |
5.
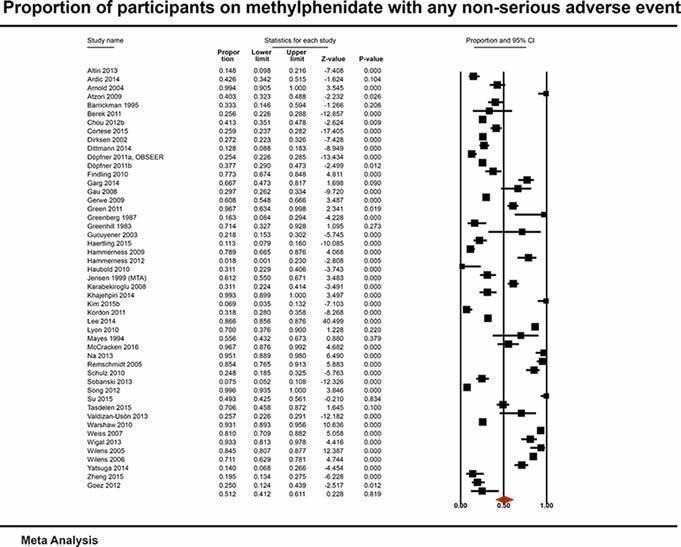
Proportion of participants on methylphenidate with any non‐serious adverse event
2.2.2 Central nervous system
See Analysis 7.2. We included 109 studies in 37 meta‐analyses.
7.2. Analysis.
Comparison 7 Non‐comparative studies: proportion of participants with non‐serious adverse events, Outcome 2 Central nervous system.
| Central nervous system | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Affect lability | ||
| Sahin 2014 | Moodiness | 10/30 |
| Wigal 2013 | Affect lability | 12/44 |
| Aggression | ||
| Barrickman 1995 | Anger | 1/15 |
| Berek 2011 | Aggressive reaction | 13/762 |
| Davari‐Ashtiani 2010 | Aggression | 1/16 |
| Dittmann 2014 | Aggression | 0/195 |
| Döpfner 2011a, OBSEER | Aggression | 31/777 |
| Döpfner 2011b | Aggression | 2/113 |
| Haertling 2015 | Aggression | 3/239 |
| Kemner 2005 (FOCUS) | Aggression | 10/850 |
| Kordon 2011 | Aggression | 10/541 |
| Lee 2014 | Aggression | 7/100 |
| Mayes 1994 | Aggression | 1/69 |
| Na 2013 | Anger | 6/103 |
| Remschmidt 2005 | Aggression | 1/105 |
| Wilens 2005 | Hostility | 8/407 |
| Anorexia | ||
| Arabgol 2015 | Anorexia | 8/15 |
| Arnold 2004 | Anorexia | 16/76 |
| Arnold 2010 | Anorexia | 6/150 |
| Berek 2011 | Anorexia | 35/762 |
| Cortese 2015 | Anorexia | 9/1426 |
| Döpfner 2011a, OBSEER | Anorexia | 9/777 |
| Döpfner 2011b | Anorexia | 0/113 |
| Gerwe 2009 | Anorexia | 20/263 |
| Greenhill 1983 | Anorexia | 5/7 |
| Haertling 2015 | Anorexia | 11/239 |
| Khajehpiri 2014 | Anorexia | 45/71 |
| Kordon 2011 | Anorexia | 23/541 |
| Kratochvil 2002 | Anorexia | 6/25 |
| Lee 2007 | Anorexia | 29/110 |
| Na 2013 | Anorexia | 66/103 |
| Song 2012 | Anorexia | 104/116 |
| Wang 2007 | Anorexia | 42/166 |
| Warshaw 2010 | Anorexia | 9/260 |
| Weiss 2007 | Anorexia | 16/79 |
| Wilens 2006 | Anorexia | 22/220 |
| Yildiz 2011 | Anorexia | 9/11 |
| Zelnik 2015 | Anorexia | 36/128 |
| Çetin 2015 | Anorexia | 12/61 |
| Anxiety | ||
| Abbasi 2011 | Anxiety | 9/20 |
| Akhondzadeh 2003 | Anxiety | 3/10 |
| Akhondzadeh 2004 | Anxiety | 3/20 |
| Amiri 2008 | Anxiety, nervousness | 4/27 |
| Barrickman 1995 | Anxiety | 1/15 |
| Blader 2010 | Anxiety or nervousness | 12/65 |
| Davari‐Ashtiani 2010 | Phobia & anxiety | 2/16 |
| Efron 1997 | Anxiousness | 76/123 |
| El‐Fiky 2014 | Anxious | 2/31 |
| Gau 2006 | Anxious | 10/32 |
| Ghanizadeh 2012 | Anxiety | 1/12 |
| Green 2011 | Anxiousness | 4/14 |
| Hammerness 2009 | Anxiety | 2/57 |
| Hazell 2003 | Anxious | 6/10 |
| Jafarinia 2012 | Anxiety | 5/20 |
| Maia 2008 | Anxious | 12/31 |
| Mohammadi 2004 | Anxiety | 0/11 |
| Mohammadi 2010 | Anxiety | 9/19 |
| Na 2013 | Anxiety | 9/103 |
| Song 2012 | Anxiety | 12/116 |
| Wilens 2005 | Anxiety | 9/407 |
| Zelnik 2015 | Anxiety | 21/128 |
| Fingernail biting | ||
| Blader 2010 | Bites fingernails | 9/65 |
| Efron 1997 | Biting fingernails | 56/123 |
| El‐Fiky 2014 | Bites fingers | 0/31 |
| Gau 2006 | Biting fingernails | 8/32 |
| Green 2011 | Biting fingernails | 3/14 |
| Hazell 2003 | Biting fingernails | 2/10 |
| Maia 2008 | Bites fingernails | 3/31 |
| Sahin 2014 | Nail biting | 3/30 |
| Shang 2015 | Nail biting | 1/66 |
| Daydreams | ||
| Efron 1997 | Daydreams | 77/123 |
| Gau 2006 | Daydreams | 10/32 |
| Green 2011 | Daydreams | 3/14 |
| Sahin 2014 | Daydreams | 7/30 |
| Difficulty falling asleep | ||
| Akhondzadeh 2003 | Difficulty falling asleep | 4/10 |
| Akhondzadeh 2004 | Difficulty falling asleep | 6/20 |
| Altin 2013 | Insomnia | 5/71 |
| Amiri 2008 | Difficulty falling asleep | 8/30 |
| Ardic 2014 | Insomnia | 19/101 |
| Arnold 2004 | Insomnia | 16/76 |
| Arnold 2010 | Insomnia | 9/171 |
| Atzori 2009 | Insomnia | 3/129 |
| Barrickman 1995 | Insomnia | 1/15 |
| Berek 2011 | Insomnia | 59/762 |
| Blader 2010 | Insomnia | 21/65 |
| Chou 2012b | Insomnia | 25/230 |
| Cortese 2015 | Insomnia | 31/1426 |
| Coşkun 2010 | Sleep problems | 4/7 |
| Davari‐Ashtiani 2010 | Sleep problems | 7/16 |
| Döpfner 2011a, OBSEER | Initial insomnia | 11/777 |
| Döpfner 2011b | Initial insomnia | 3/113 |
| Efron 1997 | Trouble sleeping | 79/123 |
| El‐Fiky 2014 | Insomnia | 2/31 |
| Famularo 1987 | Later sleep onset | 1/10 |
| Garg 2014 | Insomnia | 1/27 |
| Gau 2006 | Insomnia or trouble sleeping | 15/32 |
| Germinario 2013 | Insomnia | 1/9 |
| Gerwe 2009 | Difficulty falling asleep | 73/263 |
| Grcevich 2001 | Sleep problems | 7/75 |
| Green 2011 | Trouble sleeping | 7/34 |
| Greenhill 1983 | Insomnia | 2/7 |
| Gucuyener 2003 | Difficulty falling asleep | 19/119 |
| Haertling 2015 | Insomnia | 3/239 |
| Hazell 2003 | Sleep problems | 7/10 |
| Işeri 2007 | Sleep problems | 5/20 |
| Jafarinia 2012 | Insomnia | 10/20 |
| Jensen 1999 (MTA) | Insomnia | 12/245 |
| Johnson 2013 | Insomnia (new onset) | 12/45 |
| Jung 2007 | Insomnia | 16/83 |
| Kemner 2005 (FOCUS) | Insomnia | 53/850 |
| Khajehpiri 2014 | Insomnia | 44/71 |
| Kim 2010 | Insomnia | 6/24 |
| Kordon 2011 | Insomnia | 46/541 |
| Kratochvil 2002 | Insomnia | 7/25 |
| Lee 2007 | Insomnia | 24110 |
| Li 2011 | Difficulty falling asleep | 9/34 |
| Maayan 2009 | Trouble sleeping | 3/8 |
| Maia 2008 | Insomnia | 5/31 |
| Mayes 1994 | Insomnia | 9/63 |
| McCracken 2016 | Insomnia | 20/61 |
| Miller‐Horn 2008 | Insomnia | 2/23 |
| Mohammadi 2004 | Difficulty falling asleep | 4/11 |
| Mohammadi 2010 | Trouble sleeping | 9/19 |
| Mohammadi 2012a | Difficulty falling asleep | 8/23 |
| Mohammadi 2012b | Insomnia | 9/20 |
| Montañés‐Rada 2012 | Insomnia | 5/40 |
| Na 2013 | Trouble sleeping | 37/103 |
| Peyre 2012a | Insomnia | 21/121 |
| Remschmidt 2005 | Insomnia | 3/89 |
| Sahin 2014 | Difficulty falling asleep | 16/30 |
| Sangal 2006 | Insomnia | 21/79 |
| Shang 2015 | Insomnia | 12/66 |
| Song 2012 | Insomnia | 44/116 |
| Steele 2006 | Insomnia | 12/60 |
| Su 2015 | Insomnia | 20/205 |
| Tasdelen 2015 | Insomnia | 1/17 |
| Thorell 2009 | Difficulties falling asleep | 15/79 |
| Tomás Vila 2010b | Difficulty falling asleep | 45/114 |
| Valdizán Usón 2004 | Initial insomnia | 11/155 |
| Wang 2007 | Insomnia | 9/166 |
| Warshaw 2010 | Difficulty falling asleep | 25/260 |
| Weiss 2007 | Insomnia | 13/79 |
| Wigal 2013 | Insomnia | 9/39 |
| Wigal 2015 | Insomnia | 24/200 |
| Wilens 2005 | Insomnia | 81/229 |
| Wilens 2006 | Insomnia | 16/220 |
| Wilens 2008 | Insomnia | 25/127 |
| Yatsuga 2014 | Insomnia | 4/50 |
| Yildiz 2010 | Difficulty sleeping | 15/47 |
| Yildiz 2011 | Insomnia | 7/11 |
| Zarinara 2010 | Insomnia | 10/18 |
| Zelnik 2015 | Insomnia | 31/128 |
| Zeni 2007 | Insomnia | 21/106 |
| Zheng 2011 | Insomnia | 145/1154 |
| Zheng 2015 | Insomnia | 3/123 |
| Çetin 2015 | Insomnia | 5/61 |
| Depression | ||
| Atzori 2009 | Vegetative symptoms | 4/134 |
| Cherland 1999 | Mood symptoms or mood‐congruent psychotic symptoms | 11/98 |
| Döpfner 2011a, OBSEER | Depressed mood | 13/777 |
| Grcevich 2001 | Depression | 1/75 |
| Haertling 2015 | Depression | 1/262 |
| Kratochvil 2002 | Depression | 2/25 |
| Na 2013 | Depression | 9/103 |
| Song 2012 | Depression | 5/116 |
| Valdizán Usón 2013 | Depressive symptoms | 7/689 |
| Weiss 2007 | Depression | 5/79 |
| Wilens 2005 | Depression | 6/407 |
| Yildiz 2010 | Depressive symptoms | 4/47 |
| Yildiz 2011 | Depression | 3/11 |
| Disturbed sleep | ||
| Abbas 2006 | Sleeping problems | 4/90 |
| Abbasi 2011 | Trouble sleeping | 10/20 |
| Arabgol 2015 | Disturbed sleep | 4/15 |
| Arnold 2010 | Insomnia | 9/150 |
| Blader 2010 | Early waking | 6/65 |
| Efron 1997 | Trouble sleeping | 79/123 |
| Findling 2009 | Insomnia | 29/157 |
| Galland 2010 | Restless sleep | 14/27 |
| Gau 2008 | Poor sleep quality | 37/607 |
| Haertling 2015 | Sleep disorder | 1/239 |
| Hammerness 2009 | Insomnia | 7/57 |
| Hazell 2003 | Nightmares | 8/10 |
| Işeri 2007 | Sleep problems | 5/20 |
| Kim 2010 | Insomnia | 6/27 |
| Kim 2011 | Poor sleep | 8/97 |
| Mohammadi 2004 | Disturbed sleep | 4/11 |
| Montañés‐Rada 2012 | Insomnia | 5/40 |
| Perera 2010 | Poor sleep | 13/102 |
| Remschmidt 2005 | Delayed sleep | 1/105 |
| Steele 2006 | Sleep disorder | 3/60 |
| Su 2015 | Poor quality sleep | 1/205 |
| Valdizán Usón 2013 | Sleep disorder | 33/689 |
| Yildiz 2011 | Insomnia | 7/11 |
| Zheng 2015 | Poor sleep quality | 3/149 |
| Dizziness | ||
| Abbasi 2011 | Dizziness | 8/20 |
| Blader 2010 | Dizzy | 1/65 |
| Chou 2012b | Dizziness | 9/230 |
| Cortese 2015 | Dizziness | 5/1426 |
| Davari‐Ashtiani 2010 | Dizziness | 0/16 |
| Döpfner 2011b | Dizziness | 1/113 |
| Efron 1997 | Dizziness | 15/123 |
| El‐Fiky 2014 | Dizziness | 0/31 |
| Green 2011 | Dizziness | 3/14 |
| Haertling 2015 | Dizziness | 1/239 |
| Hammerness 2009 | Dizziness | 4/57 |
| Hazell 2003 | Dizziness | 7/9 |
| Jafarinia 2012 | Dizziness | 1/20 |
| Kemner 2005 (FOCUS) | Dizziness | 7/850 |
| Kim 2010 | Dizziness | 2/27 |
| Lee 2007 | Dizziness | 5/110 |
| Li 2011 | Dizziness | 4/34 |
| Maia 2008 | Dizziness | 3/39 |
| McCracken 2016 | Dizziness | 6/61 |
| Mohammadi 2004 | Dizziness | 2/11 |
| Mohammadi 2012b | Dizziness | 2/20 |
| Na 2013 | Dizziness | 11/103 |
| Pierce 2010 | Dizziness | 2/71 |
| Sahin 2014 | Dizziness | 6/30 |
| Shang 2015 | Dizziness | 1/66 |
| Song 2012 | Dizziness | 15/116 |
| Wang 2007 | Dizziness | 12/166 |
| Wilens 2005 | Dizziness | 7/407 |
| Yildiz 2010 | Dizziness | 2/47 |
| Drowsiness | ||
| Barrickman 1995 | Drowsiness | 1/15 |
| Efron 1997 | Drowsiness | 22/123 |
| El‐Fiky 2014 | Drowsiness | 2/31 |
| Garg 2014 | Drowsiness | 1/27 |
| Gau 2006 | Drowsiness | 6/32 |
| Germinario 2013 | Drowsiness | 1/9 |
| Ghanizadeh 2012 | Drowsiness | 2/12 |
| Green 2011 | Drowsiness | 2/14 |
| Hazell 2003 | Drowsiness | 7/10 |
| Kratochvil 2002 | Somnolens | 0/40 |
| Lyon 2010 | Drowsiness | 2/10 |
| Maia 2008 | Drowsiness | 1/39 |
| Sahin 2014 | Drowsiness | 8/30 |
| Song 2012 | Somnolence | 6/116 |
| Wilens 2005 | Somnolence | 10/407 |
| Wilens 2006 | Somnolence | 3/220 |
| Yildiz 2011 | Somnolence | 1/11 |
| Dysthymia | ||
| Haertling 2015 | Dysphemia | 1/239 |
| Mohammadi 2004 | Dysphemia | 0/16 |
| Emotional lability | ||
| Ardic 2014 | Emotional change | 22/101 |
| Davari‐Ashtiani 2010 | Emotional lability | 0/16 |
| Findling 2009 | Emotional lability | 5/157 |
| Hammerness 2009 | Moody | 2/57 |
| Kemner 2005 (FOCUS) | Emotional disturbance | 5/850 |
| Kordon 2011 | Emotional lability | 8/598 |
| Kratochvil 2002 | Emotional lability | 2/25 |
| Maayan 2009 | Emotional lability | 2/8 |
| McCracken 2016 | Affect lability | 14/61 |
| Silva 2004 | Emotional lability | 4/22 |
| Steele 2006 | Emotional lability | 9/60 |
| Warshaw 2010 | Affect lability | 18/260 |
| Weiss 2007 | Emotional lability | 3/79 |
| Wigal 2013 | Affect lability | 10/39 |
| Wilens 2005 | Emotional lability | 1/229 |
| Yildiz 2010 | Emotional lability | 12/47 |
| Euphoria/hypomania | ||
| Blader 2010 | Unusually happy | 12/65 |
| El‐Fiky 2014 | Euphoria | 0/31 |
| Gau 2006 | Euphoric | 3/32 |
| Grcevich 2001 | Agitation | 2/75 |
| Green 2011 | Unusually happy | 0/14 |
| Kim 2010 | Euphoria | 1/27 |
| Maia 2008 | Euphoric | 2/39 |
| Mohammadi 2004 | Euphoria or hypomania | 0/16 |
| Sahin 2014 | Ecstasy | 8/30 |
| Asthenia and fatigue | ||
| Abbasi 2011 | Fatigue | 13/20 |
| Arnold 2004 | Asthenia | 7/76 |
| Blader 2010 | Tired + low energy | 4/65 |
| Davari‐Ashtiani 2010 | Tiredness | 0/16 |
| Dittmann 2014 | Fatigue | 0/195 |
| Döpfner 2011b | Fatigue | 0/113 |
| Galland 2010 | Daytime sleepiness | 2/27 |
| Garg 2014 | Fatigue | 2/27 |
| Haertling 2015 | Asthenia | 1/239 |
| Kemner 2005 (FOCUS) | Fatigue | 3/850 |
| Lee 2007 | Fatigue | 5/110 |
| McCracken 2016 | Fatigue | 5/61 |
| Mohammadi 2010 | Fatigue | 4/19 |
| Na 2013 | Fatigue | 13/103 |
| Pierce 2010 | Fatigue | 3/71 |
| Sangal 2006 | Fatigue | 3/79 |
| Steele 2006 | Fatigue | 7/60 |
| Wang 2007 | Fatigue | 5/166 |
| Wigal 2015 | Fatigue | 10/200 |
| Headache | ||
| Abbas 2006 | Headache | 2/90 |
| Abbasi 2011 | Headache | 12/20 |
| Akhondzadeh 2003 | Headache | 6/10 |
| Akhondzadeh 2004 | Headache | 9/20 |
| Altin 2013 | Headache | 3/25 |
| Amiri 2008 | Headache | 7/27 |
| Ardic 2014 | Headache | 16/101 |
| Arnold 2004 | Headache | 24/76 |
| Arnold 2010 | Headache | 12/150 |
| Atzori 2009 | Headache | 6/129 |
| Barrickman 1995 | Headache | 1/15 |
| Berek 2011 | Headache | 15/762 |
| Blader 2010 | Headache | 1/65 |
| Chou 2012b | Headache | 7/230 |
| Cortese 2015 | Headache | 62/1426 |
| Dirksen 2002 | Headache | 17/287 |
| Dittmann 2014 | Headache | 1/195 |
| Döpfner 2011a, OBSEER | Headache | 9/777 |
| Döpfner 2011b | Headache | 8/113 |
| Efron 1997 | Headache | 30/123 |
| El‐Fiky 2014 | Headache | 4/31 |
| Findling 2009 | Headache | 54/157 |
| Galland 2010 | Morning headache | 3/27 |
| Garg 2014 | Headache | 4/27 |
| Gau 2006 | Headache | 11/32 |
| Gau 2008 | Headache | 21/607 |
| Gerwe 2009 | Headache | 15/263 |
| Goez 2012 | Headache | 4/28 |
| Golubchik 2011 | Headache | 2/9 |
| Green 2011 | Headache | 8/14 |
| Greenberg 1987 | Headache | 1/49 |
| Greenhill 1983 | Headache | 2/7 |
| Gucuyener 2003 | Headache | 14/119 |
| Guerreiro 1996 | Headache | 2/22 |
| Haertling 2015 | Headache | 2/239 |
| Hammerness 2009 | Headache | 14/57 |
| Hazell 2003 | Headache | 8/10 |
| Hulvershorn 2012 | Headache | 1/25 |
| Işeri 2007 | Headache | 5/20 |
| Jafarinia 2012 | Headache | 10/20 |
| Jung 2007 | Headache | 8/83 |
| Kemner 2005 (FOCUS) | Headache | 33/850 |
| Khajehpiri 2014 | Headache | 7/71 |
| Kim 2010 | Headache | 3/27 |
| Kordon 2011 | Headache | 14/541 |
| Kratochvil 2002 | Headache | 13/25 |
| Lee 2007 | Headache | 16/110 |
| Lee 2014 | Headache | 6/100 |
| Li 2011 | Headache | 3/34 |
| Maayan 2009 | Headache | 1/11 |
| Maia 2008 | Headaches | 4/39 |
| Mayes 1994 | Headache | 3/63 |
| McCracken 2016 | Headache | 23/61 |
| Miller‐Horn 2008 | Headache | 1/23 |
| Mohammadi 2004 | Headache | 7/11 |
| Mohammadi 2010 | Headache | 15/19 |
| Mohammadi 2012a | Headache | 4/23 |
| Mohammadi 2012b | Headache | 9/20 |
| Na 2013 | Headache | 32/103 |
| Perera 2010 | Headache | 13/102 |
| Peyre 2012a | Headache | 43/121 |
| Pierce 2010 | Headache | 6/71 |
| Remschmidt 2005 | Headache | 2/105 |
| Sahin 2014 | Headache | 14/30 |
| Sangal 2006 | Headache | 12/79 |
| Schulz 2010 | Headache | 9/145 |
| Shang 2015 | Headache | 14/66 |
| Silva 2004 | Headache | 4/22 |
| Song 2012 | Headache | 30/116 |
| Steele 2006 | Headache | 14/60 |
| Su 2015 | Headache | 4/205 |
| Tasdelen 2015 | Headache | 1/17 |
| Thorell 2009 | Headache | 6/79 |
| Valdizán Usón 2004 | Headache | 2/155 |
| Valdizán Usón 2013 | Headache | 11/689 |
| Wang 2007 | Headache | 16/166 |
| Warshaw 2010 | Headache | 23/260 |
| Weiss 2007 | Headache | 10/79 |
| Wigal 2013 | Headache | 7/39 |
| Wigal 2015 | Headache | 35/200 |
| Wilens 2005 | Headache | 123/229 |
| Wilens 2006 | Headache | 25/220 |
| Wilens 2008 | Headache | 27/127 |
| Yatsuga 2014 | Headache | 7/50 |
| Yildiz 2010 | Headache | 27/47 |
| Yildiz 2011 | Headache | 3/11 |
| Zarinara 2010 | Headache | 11/18 |
| Zelnik 2015 | Headache | 107/128 |
| Zheng 2011 | Headache | 143/1154 |
| Çetin 2015 | Headache | 2/61 |
| Increased need to sleep | ||
| Blader 2010 | Trouble waking | 1/65 |
| Galland 2010 | Hard to wake + difficult to wake | 14/27 |
| Mohammadi 2004 | Increased need to sleep | 5/11 |
| Mohammadi 2012a | Sleepiness | 4/23 |
| Na 2013 | Somnolence | 8/103 |
| Pierce 2010 | Somnolence | 2/71 |
| Wang 2007 | Somnolence | 6/166 |
| Zarinara 2010 | Somnolence | 3/19 |
| Involuntary movements | ||
| Balázs 2011 | Involuntary movements after methylphenidate treatment | 9/34 |
| Berek 2011 | Muscle contractions, involuntary | 34/762 |
| Blader 2010 | Shaking + tremor | 4/65 |
| Galland 2010 | Restless legs | 2/27 |
| Kordon 2011 | Muscle contraction, involuntary | 13/541 |
| Kratochvil 2002 | Hyperkinesia | 2/25 |
| Lee 2014 | Convulsion | 4/100 |
| Irritability | ||
| Abbasi 2011 | Irritability | 18/20 |
| Amiri 2008 | Irritability | 6/27 |
| Ardic 2014 | Irritability | 30/101 |
| Atzori 2009 | Irritability | 9/129 |
| Barrickman 1995 | Irritability | 1/15 |
| Blader 2010 | Irritability | 16/65 |
| Cortese 2015 | Irritabillity | 67/1426 |
| Efron 1997 | Irritable | 100/123 |
| El‐Fiky 2014 | Irritable | 6/31 |
| Findling 2009 | Irritability | 20/157 |
| Garg 2014 | Irritability | 2/27 |
| Gau 2006 | Irritable | 7/32 |
| Green 2011 | Irritability | 2/14 |
| Greenhill 1983 | Irritability | 4/7 |
| Hazell 2003 | Irritability | 3/10 |
| Işeri 2007 | Irritability | 3/20 |
| Jensen 1999 (MTA) | Irritability | 10/245 |
| Johnson 2013 | Irritability | 23/45 |
| Kemner 2005 (FOCUS) | Irritability | 7/850 |
| Khajehpiri 2014 | Irritability | 25/71 |
| Maayan 2009 | Irritability | 1/8 |
| Maia 2008 | Irritability | 10/39 |
| Mayes 1994 | Irritability | 18/63 |
| McCracken 2016 | Irritability | 12/61 |
| Mohammadi 2012a | Irritability | 10/23 |
| Na 2013 | Irritable | 16/121 |
| Sangal 2006 | Irritability | 12/79 |
| Silva 2004 | Irritability | 3/22 |
| Song 2012 | Irritability | 9/116 |
| Tasdelen 2015 | Irritability | 5/17 |
| Valdizán Usón 2004 | Irritability | 4/155 |
| Wang 2007 | Irritability | 10/166 |
| Warshaw 2010 | Irritability | 15/260 |
| Wigal 2015 | Irritability | 11/200 |
| Yildiz 2010 | Irritability | 16/47 |
| Nervousness | ||
| Arabgol 2015 | Nervousness | 5/15 |
| Berek 2011 | Nervousness | 17/762 |
| Jafarinia 2012 | Nervousness | 5/20 |
| Kordon 2011 | Nervousness | 13/541 |
| Kratochvil 2002 | Nervousness | 4/25 |
| Lee 2007 | Nervousness | 2/110 |
| Mohammadi 2010 | Nervousness | 14/19 |
| Na 2013 | Nervousness | 7/103 |
| Song 2012 | Nervousness | 9/116 |
| Steele 2006 | Nervousness | 9/60 |
| Weiss 2007 | Nervousness | 14/79 |
| Wilens 2006 | Nervousness | 6/220 |
| Yildiz 2011 | Nervousness | 9/11 |
| Çetin 2015 | Nervousness | 2/61 |
| Nightmares | ||
| Blader 2010 | Nightmares | 2/65 |
| El‐Fiky 2014 | Nightmares | 1/31 |
| Gau 2006 | Nightmares | 8/32 |
| Green 2011 | Nightmares | 1/14 |
| Khajehpiri 2014 | Nightmares | 3/71 |
| Kim 2010 | Nightmares | 1/27 |
| Maia 2008 | Nightmares | 1/31 |
| Sahin 2014 | Nightmares | 4/30 |
| Silva 2004 | Nightmares | 1/22 |
| Song 2012 | Nightmares | 10/116 |
| Tomás Vila 2010b | Nightmares | 12/114 |
| Restlessness and agitation | ||
| Blader 2010 | Restlessness | 3/65 |
| Davari‐Ashtiani 2010 | Restlessness | 4/16 |
| Dittmann 2014 | Agitation | 1/195 |
| Döpfner 2011b | Restlessness | 1/113 |
| Grcevich 2001 | Agitation | 2/75 |
| Haertling 2015 | Agitation | 2/239 |
| Khajehpiri 2014 | Agitation | 16/71 |
| Lee 2014 | Agitation | 6/100 |
| Mohammadi 2010 | Restlessness | 8/19 |
| Perera 2010 | Restlessness | 5/102 |
| Sahin 2014 | Restlessness | 9/30 |
| Steele 2006 | Agitation | 8/60 |
| Valdizán Usón 2013 | Physical activity modifications | 16/689 |
| Zarinara 2010 | Restlessness | 5/19 |
| Sadness | ||
| Abbasi 2011 | Sadness | 9/20 |
| Amiri 2008 | Sadness | 6/27 |
| Barrickman 1995 | Low mood | 1/15 |
| Blader 2010 | Crying + sadness | 17/65 |
| Davari‐Ashtiani 2010 | Sadness | 3/16 |
| Efron 1997 | Sadness or unhappiness | 69/123 |
| El‐Fiky 2014 | Sad | 3/31 |
| Garg 2014 | Sadness | 0/27 |
| Gau 2006 | Sadness | 14/32 |
| Green 2011 | Sadness | 7/14 |
| Hammerness 2009 | Sad | 1/57 |
| Hazell 2003 | Sad | 5/10 |
| Işeri 2007 | Sadness | 2/20 |
| Jensen 1999 (MTA) | Tearfulness | 6/245 |
| Johnson 2013 | Sadness | 18/45 |
| Kemner 2005 (FOCUS) | Crying | 13/850 |
| Maia 2008 | Sad | 4/31 |
| Mohammadi 2010 | Sadness | 4/19 |
| Mohammadi 2012a | Sadness | 2/23 |
| Perera 2010 | Sadness | 13/102 |
| Sahin 2014 | Sadness | 9/30 |
| Stares | ||
| Blader 2010 | Stares into space | 3/65 |
| El‐Fiky 2014 | Stares a lot | 8/31 |
| Hazell 2003 | Stares | 6/10 |
| Maia 2008 | Stares a lot or daydreams | 7/31 |
| Excessive talking | ||
| Blader 2010 | Overly talkative | 8/65 |
| Hazell 2003 | Talks too much | 5/10 |
| Khajehpiri 2014 | Speaking more | 6/71 |
| Taciturnity ('talking too little') | ||
| Blader 2010 | Less talkative | 4/65 |
| El‐Fiky 2014 | Talk less | 2/31 |
| Green 2011 | Talking little | 6/14 |
| Maia 2008 | Talk less | 9/31 |
| Tics | ||
| Ardic 2014 | Tics | 3/101 |
| Atzori 2009 | Tics | 2/129 |
| Blader 2010 | Tics | 3/65 |
| Cortese 2015 | Tics | 27/1426 |
| Davari‐Ashtiani 2010 | Tics | 1/16 |
| Döpfner 2011a, OBSEER | Tics | 100/777 |
| Döpfner 2011b | Tics | 1/113 |
| Efron 1997 | Tics | 35/123 |
| El‐Fiky 2014 | Tics or involuntary movements | 0/31 |
| Findling 2009 | Tics | 0/157 |
| Gau 2006 | Tics | 6/32 |
| Germinario 2013 | Tics | 1/9 |
| Golubchik 2011 | Motor tics exacerbation | 1/9 |
| Grcevich 2001 | Motor tics | 1/75 |
| Green 2011 | Tics | 0/14 |
| Gucuyener 2003 | Motor tics | 2/119 |
| Işeri 2007 | Tics | 3/20 |
| Johnson 2013 | Tics (new onset) | 1/45 |
| Khajehpiri 2014 | Tics | 18/71 |
| Kim 2010 | Tics | 3/27 |
| Kim 2011 | Tics | 19/97 |
| Lakic 2012 | Tics | 3/61 |
| Lee 2007 | Tics | 6/110 |
| Miller‐Horn 2008 | Tics | 2/23 |
| Mohammadi 2004 | Tics | 0/11 |
| Mohammadi 2012a | Tics | 1/23 |
| Mohammadi 2012b | Tics | 4/20 |
| Remschmidt 2005 | Tics | 7/89 |
| Sahin 2014 | Tics | 2/30 |
| Shang 2015 | Tics | 1/66 |
| Song 2012 | Tics | 7/116 |
| Su 2015 | Tourette disorder | 6/205 |
| Tasdelen 2015 | Tics | 1/17 |
| Valdizán Usón 2004 | Tics | 1/155 |
| Varley 2001 | Tics | 31/374 |
| Wilens 2005 | Twitching | 40/229 |
| Yildiz 2010 | Tics | 1/47 |
| Yildiz 2011 | Tics | 2/11 |
| Çetin 2015 | Tics | 1/16 |
| Isolation and lack of interest in others | ||
| Blader 2010 | Lack of interest | 6/65 |
| Efron 1997 | Uninterested in others | 39/123 |
| El‐Fiky 2014 | Uninterested in others | 2/31 |
| Green 2011 | Uninterested | 4/14 |
| Hazell 2003 | Uninterested | 6/10 |
| Khajehpiri 2014 | Isolation | 6/71 |
| Maia 2008 | Uninterested others | 6/31 |
| Perera 2010 | Social withdrawal | 6/102 |
| Sahin 2014 | Being in own world | 5/30 |
| Yildiz 2010 | Introversion (maybe isolation) | 5/47 |
| 'Zombie like' demeanour | ||
| Davari‐Ashtiani 2010 | Zombie like | 3/16 |
| Jensen 1999 (MTA) | Zombie like | 18/245 |
| Wilens 2005 | Apathy | 4/407 |
| Somnolence | ||
| Chou 2012b | Somnolence | 1/230 |
| Khajehpiri 2014 | Somnolence | 5/71 |
| McCracken 2016 | Somnolence | 3/61 |
| Na 2013 | Somnolence | 8/103 |
| Shang 2015 | Somnolence | 1/66 |
| Wang 2007 | Somnolence | 6/166 |
| Weiss 2007 | Somnolence | 7/79 |
| Wilens 2005 | Somnolence | 1/229 |
| Yildiz 2011 | Somnolence | 1/11 |
| Obsession | ||
| Cortese 2015 | Obsessive behaviour | 6/1426 |
| Khajehpiri 2014 | Obsession | 1/71 |
| Çetin 2015 | Obsession | 2/32 |
| Mood disorder | ||
| Cortese 2015 | Mood disorder | 49/1426 |
| Haertling 2015 | Emotional disorder of childhood | 1/239 |
| Confusional state | ||
| Haertling 2015 | Confusional state | 1/262 |
| Zelnik 2015 | Sense of dopiness | 13/128 |
| Sleep disorder | ||
| Dittmann 2014 | Sleep disorder | 6/195 |
| Döpfner 2011a, OBSEER | Sleep disorder | 9/777 |
| Su 2015 | Sleep disorder | 26/239 |
| Propensity to cry | ||
| El‐Fiky 2014 | Prone to cry | 4/31 |
| Sahin 2014 | Sudden crying | 12/30 |
| Sedation | ||
| McCracken 2016 | Sedation | 4/61 |
| Mohammadi 2010 | Sedation | 4/19 |
| Daytime sleepiness | ||
| Galland 2010 | Daytime sleepiness | 2/27 |
| Tomás Vila 2010b | Datime sleepiness | 2/114 |
| Dysphoria | ||
| Atzori 2009 | Dysphoria | 2/134 |
| Logorrhoea | ||
| Atzori 2009 | Loggorrhoea | 1/129 |
| Impaired concentration | ||
| Berek 2011 | Impaired concentration | 14/822 |
| Difficulty waking up | ||
| Galland 2010 | Difficult to wake up | 7/28 |
| Urinary incontinence | ||
| Garg 2014 | Urinary incontinence | 1/32 |
| Paralysis | ||
| Haertling 2015 | Paralysis | 1/239 |
| Affective disorder | ||
| Haertling 2015 | Affective disorder | 1/239 |
| Jumbled thoughts | ||
| Hammerness 2009 | Jumbled thoughts | 1/154 |
| Bulimia | ||
| Khajehpiri 2014 | Bulimia | 5/71 |
| Sleep late | ||
| Khajehpiri 2014 | Sleeping late | 22/71 |
| Tooth grinding | ||
| Khajehpiri 2014 | Tooth grinding | 3/71 |
| Tremor | ||
| Khajehpiri 2014 | Tremor | 2/71 |
| Stuttering | ||
| Khajehpiri 2014 | Stuttering | 4/71 |
| Flushing | ||
| Khajehpiri 2014 | Flushing | 9/71 |
| Apathy | ||
| Kordon 2011 | Apathy | 6/541 |
| Abnormal behaviour | ||
| Lee 2014 | Abnormal behaviour | 5/100 |
| Stereotypies | ||
| Mohammadi 2004 | Stereotypies | 0/16 |
| Quietness | ||
| Sahin 2014 | Quietness | 6/30 |
| EEG changes | ||
| Schmidt 2002 | Pathologic | 25/124 |
| Did not like themselves | ||
| Thorell 2009 | Does not like him‐ or herself | 10/79 |
| Sleep walking | ||
| Tomás Vila 2010b | Sleep walking | 2/114 |
| Personality and behaviour disorders | ||
| Valdizán Usón 2013 | Personality and behavior disorders | 14/633 |
| Vertigo | ||
| Yildiz 2011 | Vertigo | 3/11 |
The proportion of participants on methylphenidate with:
affect lability was 29.8% (95% CI 20.5% to 41.2%; 2 studies, 74 participants; Sahin 2014; Wigal 2013; see supplementary file S16);
aggression was 2.40% (95% CI 1.60% to 3.60%; 14 studies, 4292 participants; see supplementary file S17);
anorexia was 16.5% (95% CI 9.10% to 28.2%; 23 studies, 5739 participants; see supplementary file S18);
anxiety was 18.4% (95% CI 11.3% to 28.7%; 22 studies, 1287 participants; see supplementary file S19);
fingernail biting was 14.3% (95% CI 6.90% to 27.5%; 9 studies, 402 participants; Blader 2010; Efron 1997; El‐Fiky 2014; Gau 2006; Green 2011; Hazell 2003; Maia 2008; Sahin 2014; Shang 2015; see supplementary file S20);
daydreams was 35.1% (95% CI 16.2% to 60.1%; 4 studies, 199 participants; Efron 1997; Gau 2006; Green 2011; Sahin 2014; see supplementary S21);
difficulty falling asleep was 17.9% (95% CI 14.7% to 21.6%; 82 studies, 11,507 participants; see supplementary file S22);
depression was 4.00% (95% CI 2.20% to 7.10%; 13 studies, 2823 participants; see supplementary file S23);
disturbed sleep was 13.2% (95% CI 7.70% to 21.8%; 24 studies, 3076 participants; see supplementary file S24);
dizziness was 6.00% (95% CI 3.7% to 9.4%; 29 studies, 4521 participants; see supplementary file S25);
drowsiness was 9.50% (95% CI 5.20% to 16.6%; 17 studies, 1146 participants; see supplementary file S26);
dysthymia was 0.90% (95% CI 0.10% to 5.30%; 2 studies, 255 participants; Haertling 2015; Mohammadi 2004; see supplementary file S27);
emotional lability was 7.20% (95% CI 3.80% to 13.3%; 16 studies, 2609 participants; see supplementary file S28);
euphoria/hypomania was 7.90% (95% CI 3.90% to 15.4%; 9 studies, 329 participants; Blader 2010; El‐Fiky 2014; Gau 2006; Grcevich 2001; Green 2011; Kim 2010; Maia 2008; Mohammadi 2004; Sahin 2014; see supplementary file S29);
asthenia and fatigue was 5.30% (95% CI 3.00% to 9.40%; 19 studies, 2497 participants; see supplementary file S30);
headache was 14.4% (95% CI 11.3% to 18.3%; 90 studies, 13,469 participants; see supplementary file S31);
increased need to sleep was 11.7% (95% CI 4.40% to 27.5%; 8 studies, 485 participants; Blader 2010; Galland 2010; Mohammadi 2004; Mohammadi 2012a; Na 2013; Pierce 2010; Wang 2007; Zarinara 2010; supplementary file S32);
involuntary movements was 6.30% (95% CI 3.20% to 11.8%; 7 studies, 1554 participants; Balázs 2011; Berek 2011; Blader 2010; Galland 2010; Kordon 2011; Kratochvil 2002; Lee 2014; see supplementary file S33);
irritability was 17.2% (95% CI 11.5% to 25.0%; 35 studies, 4792 participants; see supplementary file S34);
nervousness was 11.7% (95% CI 5.80% to 22.3%; 14 studies, 2142 participants; see supplementary file S35);
nightmares was 8.10% (95% CI 5.20% to 12.5%; 11 studies, 553 participants; see supplementary file S36);
restlessness and agitation was 7.40% (95% CI 3.60% to 14.5%; 14 studies, 1793 participants; see supplementary file S37);
sadness was 16.8% (95% CI 9.40% to 28.3%; 21 studies, 1802 participants; see supplementary file S38);
stares was 22.5% (95% CI 8.50% to 47.5%; 4 studies, 137 participants; Blader 2010; El‐Fiky 2014; Hazell 2003; Maia 2008; see supplementary file S39);
excessive talking was 17.6% (95% CI 6.10% to 41.3%; 3 studies, 146 participants; Blader 2010; Hazell 2003; Khajehpiri 2014; supplementary file S40);
taciturnity ('talking too little') was 16.9% (95% CI 5.90% to 39.8%; 4 studies, 141 participants; Blader 2010; El‐Fiky 2014; Green 2011; Maia 2008; see supplementary file S41);
tics was 6.40% (95% CI 4.50% to 8.90%; 39 studies, 1980 participants; see supplementary file S42);
isolation and lack of interest in others was 15.9% (95% CI 9.40% to 25.8%; 10 studies, 524 participants; Blader 2010; Efron 1997; El‐Fiky 2014; Green 2011; Hazell 2003; Maia 2008; Sahin 2014; Yildiz 2010; Khajehpiri 2014; Perera 2010 see supplementary file S43);
'zombie‐like' demeanor was 5.20% (95% CI 1.20% to 20.6%; 3 studies, 668 participants; Davari‐Ashtiani 2010; Jensen 1999 (MTA); Wilens 2005; see supplementary file S44);
somnolence was 4.10% (95% CI 2.30% to 7.30%; 9 studies, 1016 participants; Chou 2012b; Khajehpiri 2014; McCracken 2016; Na 2013; Shang 2015; Wang 2007; Weiss 2007; Wilens 2005; Yildiz 2011; see supplementary file S45);
obsession was 1.50% (95% CI 0.20% to 8.60%; 3 studies, 1529 participants; Çetin 2015; Cortese 2015; Khajehpiri 2014; see supplementary file S46);
mood disorder was 1.50% (95% CI 0.20% to 10.6%; 2 studies, 1665 participants; Cortese 2015; Haertling 2015; see supplementary file S47);
confusional state was 2.30% (95% CI 0.10% to 39.4%; 2 studies, 390 participants; Haertling 2015; Zelnik 2015; see supplementary file S48);
sleep disorder was 3.50% (95% CI 0.80% to 14.4%; 3 studies, 1211 participants; Dittmann 2014; Döpfner 2011a, OBSEER; Su 2015; see supplementary file S49);
propensity to cry was 24.8% (95% CI 7.00% to 59.0%; 2 studies, 61 participants; El‐Fiky 2014; Sahin 2014; see supplementary file S50);
sedation was 11.8% (95% CI 3.50% to 33.2%; 2 studies, 80 participants; McCracken 2016; Mohammadi 2010; see supplementary file S51);
daytime sleepiness was 3.60% (95% CI 0.90% to 14.0%; 2 studies, 141 participants; Galland 2010; Tomás Vila 2010b; see supplementary file S52).
Sixteen studies reported data on different adverse events related to the central nervous system (Atzori 2009; Berek 2011; Garg 2014; Galland 2010; Haertling 2015; Hammerness 2009; Khajehpiri 2014; Kordon 2011; Lee 2014; Mohammadi 2004; Sahin 2014; Schmidt 2002; Thorell 2009; Tomás Vila 2010b; Valdizán Usón 2013; Yildiz 2011).
-
In Atzori 2009, the proportion of participants on methylphenidate with:
dysphoria was 1.49% (95% CI 0.40% to 5.27%, 134 participants); and
logorrhoea (that is, excessive, uncontrollable or incoherent talkativeness) was 0.78% (95% CI 0.14% to 4.27%; 129 participants).
In Berek 2011 (822 participants), the proportion of participants on methylphenidate with impaired concentration was 1.70% (95% CI 1.01% to 2.83%).
In Galland 2010 (28 participants), the proportion of participants on methylphenidate with difficulty waking up was 25.0% (95% CI 12.7% to 43.4%).
In Garg 2014 (32 participants), the proportion of participants on methylphenidate with urinary incontinence was 3.13% (95% CI 0.56% to 15.8%).
In Haertling 2015 (239 participants), the proportion of participants on methylphenidate with paralysis was 0.42% (95% CI 0.07% to 2.33%); and affective disorder was 0.42% (95% CI 0.07% to 2.33%).
In Hammerness 2009 (154 participants), the proportion of participants on methylphenidate with jumbled thoughts was 0.65% (95% CI 0.03% to 0.90%).
-
In Khajehpiri 2014 (71 participants), the proportion of participants on methylphenidate with:
bulimia was 7.04% (95% CI 3.00% to 15.5%);
sleeping late was 30.9% (95% CI 21.4% to 42.5%);
tooth grinding was 4.23% (95% CI 1.40% to 11.7%);
tremor was 2.82% (95% CI 0.08% to 9.71%);
stuttering was 5.63% (95% CI 2.21% to 13.6%); and
flushing 12.7% (95% CI 6.82% to 22.4%).
In Kordon 2011 (541 participants), the proportion of participants on methylphenidate with apathy was 1.10% (95% CI 0.50% to 2.40%).
In Lee 2014 (100 participants), the proportion of participants on methylphenidate with abnormal behaviour was 5.00% (95% CI 2.20% to 11.2%).
In Mohammadi 2004 (16 participants), the proportion of participants on methylphenidate with stereotypies was 0.00% (95% Cl 0.00% to 19.4%).
In Sahin 2014 (30 participants), the proportion of participants on methylphenidate with quietness was 20.0% (95% CI 9.50% to 37.3%).
In Schmidt 2002 (124 participants), the proportion of participants on methylphenidate with EEG changes was 20.2% (95% CI 14.0% to 28.0%).
In Thorell 2009 (79 participants), the proportion of participants on methylphenidate who did not like themselves was 12.7% (95% CI 6.50% to 21.8%).
In Tomás Vila 2010b (114 participants), the proportion of participants on methylphenidate with sleep walking was 1.75% (95% CI 0.50% to 6.20%).
In Valdizán Usón 2013 (633 participants), the proportion of participants on methylphenidate with personality and behaviour disorders was 2.20% (95% CI 1.30% to 3.70%).
In Yildiz 2011 (11 participants), the proportion of participants on methylphenidate with vertigo was 27.3% (95% CI 9.70% to 56.6%).
2.2.3 Cardiovascular and respiratory system
See Analysis 7.3. Thirty‐nine studies reported data on this outcome.
7.3. Analysis.
Comparison 7 Non‐comparative studies: proportion of participants with non‐serious adverse events, Outcome 3 Cardiovascular and respiratory system.
| Cardiovascular and respiratory system | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Cough | ||
| Arnold 2004 | Cough increased | 7/76 |
| Arnold 2010 | Cough | 5/150 |
| Findling 2009 | Cough | 38/157 |
| Kratochvil 2002 | Cough increased | 2/25 |
| Pierce 2010 | Cough | 2/71 |
| Sangal 2006 | Cough | 7/79 |
| Wang 2007 | Cough | 10/166 |
| Pharyngolaryngeal pain | ||
| Arnold 2010 | Pharyngolaryngeal pain | 5/150 |
| Findling 2009 | Pharyngolaryngeal pain | 19/326 |
| Pierce 2010 | Pharyngolaryngeal pain | 3/71 |
| Upper respiratory tract infection | ||
| Findling 2009 | Upper respiratory tract infection | 40/157 |
| Pierce 2010 | Upper respiratory tract infection | 2/71 |
| Wang 2007 | Upper respiratory tract infection | 11/166 |
| Wigal 2015 | Upper respiratory tract infection | 13/200 |
| Wiguna 2012 | Upper respiratory tract infection | 5/20 |
| Tachycardia | ||
| Blader 2010 | Heart races | 2/65 |
| Cortese 2015 | Tachycardia | 24/1426 |
| Findling 2009 | Tachycardia | 3/326 |
| Jafarinia 2012 | Tachycardia | 1/20 |
| Kratochvil 2002 | Tachycardia | 2/25 |
| Su 2015 | Tachycardia | 8/239 |
| Abnormal ECG | ||
| Findling 2009 | Abnormal ECG | 1/326 |
| Findling 2010 | Abnormal ECG | 30/162 |
| Nasal congestion | ||
| Findling 2009 | Nasal congestion | 18/157 |
| Maayan 2009 | Nasal congestion | 1/11 |
| Sangal 2006 | Nasal congestion | 10/79 |
| Palpitation | ||
| Mohammadi 2004 | Palpation | 3/11 |
| Na 2013 | Palpitations | 8/103 |
| Shang 2015 | Palpation | 1/66 |
| Systolic blood pressure | ||
| Cho 2012 | No difference | Non‐significant increase following methylphenidate |
| Döpfner 2011b | No difference | ‐ |
| Findling 2009 | Increased | The proportion of participants with a systolic blood pressure above the upper limit of normal (> 123 mmHg) ranged from 1% to 10%, whereas 1.5% had an above normal systolic blood pressure at baseline |
| Gadow 1995 | Increased | ‐ |
| Germinario 2013 | No difference | 100.6 mmHg (SD = 12.3) |
| Gerwe 2009 | No difference | Mean change from baseline: 0.1 mmHg (SD = 10.8) |
| Hazell 2003 | Decreased | Drop in systolic blood pressure in 8/10 participants |
| Kim 2010 | Decreased | 113.8 mmHg (SD = 10.2) |
| Kratochvil 2002 | Increased | 102.2 mmHg (SD = 9.89) |
| Lamberti 2015 | Increased | 105.4 mmHg (SD = 10.3) |
| Maayan 2009 | No difference | 96.2 mmHg (SD = 14.0) |
| McCracken 2016 | Increased | ‐ |
| Na 2013 | Increased | ‐ |
| Song 2012 | Increased | 105.3 mmHg (SD = 12.6) |
| Wilens 2005 | Increased | 104.7 mmHg (SD = 8.10) |
| Winsberg 1982 | Increased | 103.1 mmHg (SD = no information) |
| Yildiz 2011 | Decreased | 97 mmHg (SD = 10.8) |
| Zheng 2011 | No difference | 96.6 mmHg (SD = 10.6) |
| Zheng 2015 | No difference | No changes |
| Diastolic blood pressure | ||
| Cho 2012 | No difference | Non‐significant, marginal increase after methylphenidate |
| Döpfner 2011b | Increased | 69 mmHg (SD = 1.00) |
| Findling 2009 | Decreased | ‐ |
| Germinario 2013 | Decreased | 63.2 mmHg (SD = 10.9) |
| Gerwe 2009 | ‐ | ‐ |
| Hazell 2003 | Decreased | Drop in diastolic blood pressure in 9/10 participants |
| Kim 2010 | Decreased | 75.2 mmHg (SD = 12.0) |
| Kratochvil 2002 | Increased | 63.5 mmHg (SD = 7.90) |
| Lamberti 2015 | Increased | 59.2 mmHg (SD = 7.10) |
| Maayan 2009 | No difference | 61.4 mmHg (SD = 13.7) |
| McCracken 2016 | Increased | ‐ |
| Na 2013 | Increased | ‐ |
| Song 2012 | Increased | 66.9 mmHg (SD = 10.1) |
| Yildiz 2011 | Decreased | 63.5 mmHg (SD = 12.0) |
| Zheng 2011 | Increased | 64.6 mmHg (SD = 7.51) |
| Zheng 2015 | No change | ‐ |
| Pulse rate | ||
| Cho 2012 | Increased | ‐ |
| Döpfner 2011b | No difference | 79 beats/min (SD = 1.00) |
| Germinario 2013 | Decreased | 79.9 beats/min (SD = 12.9) |
| Ilgenli 2007 | No difference | 88 beats/min (SD = 13.80) |
| Kim 2010 | No difference | 87.5 beats/min (SD = 6.10) |
| Kim 2015a | Increased | 86.4 beats/min (SD = 11.2) |
| Kratochvil 2002 | Increased | 80.4 beats/min (SD = 9.70) |
| Lamberti 2015 | Increased | 80.5 beats/min (SD = 15.5) |
| Maayan 2009 | Decreased | 95.5 beats/min (SD = 16.0) |
| McCracken 2016 | Increased | ‐ |
| Na 2013 | Increased | 75.57 beats/min (SD = 7.33) |
| Yildiz 2011 | Increased | 80.8 beats/min (SD = 9.80) |
| Zheng 2011 | No difference | 82.5 beats/min (SD = 7.81) |
| Zheng 2015 | No difference | 82.5 beats/min (SD = 7.81) |
| Özcan 2004 | Decreased | 148.2 beats/min (SD = 29.70) |
| ECG‐QT | ||
| Cho 2012 | Non‐significant change in QT and QRS | ‐ |
| Germinario 2013 | ‐ | After 24 months, out of 77 patients treated with methylphenidate: 8 (10.4%) with altered ECG (electrocardiogram) at 24 months 11 (29.0%) with sinus bradycardia 12 (31.6%) with sinus tachycardia 6 (15.8%) with lengthened QTc |
| Ilgenli 2007 | Minimum QT of 317.00 (23.3) Maximum QT of 373.7 (21.8) |
After 2 hours: Minimum QT of 322.3 (21.6) Maximum QT of 361.8 (29.0) |
| Lamberti 2015 | QTc of 407.6 (12.4) | After 2 hours: QTc of 409.8 (12.0) |
| Cold fingers | ||
| Khajehpiri 2014 | Cold fingers | 13/71 |
| Sweating | ||
| Khajehpiri 2014 | Sweating | 6/71 |
| Hypertension | ||
| Cortese 2015 | Hypertension | 8/1426 |
| Hypotension | ||
| Cortese 2015 | Hypotension | 3/1426 |
| Respiratory, thoracic and mediastinal disorders | ||
| Haertling 2015 | Respiratory, thoracic, and mediastinal disorders | 1/239 |
| Proportion of patients with micro‐nucleated (Mn) peripheral lymphocytes | ||
| Walitza 2009 | (MN‐Cells/1000 Cells mean) | Baseline: 4.90 (SD = 4.00) 6 months: 4.50 (SD = 3.50) |
We included 18 studies in seven meta‐analyses (Arnold 2004; Arnold 2010; Blader 2010; Cortese 2015; Findling 2009; Findling 2010; Jafarinia 2012; Kratochvil 2002; Maayan 2009; Mohammadi 2004; Na 2013; Pierce 2010; Sangal 2006; Shang 2015; Su 2015; Wang 2007; Wigal 2015; Wiguna 2012). The proportion of participants on methylphenidate with:
cough was 7.7% (95% CI 3.80% to 15.2%; 7 studies, 724 participants; Arnold 2004; Arnold 2010; Findling 2009; Kratochvil 2002; Pierce 2010; Sangal 2006; Wang 2007; see supplementary file S53);
pharyngolaryngeal pain was 5.10% (95% CI 3.50% to 7.30%; 3 studies, 547 participants; Arnold 2010; Findling 2009; Pierce 2010; see supplementary file S54);
upper respiratory tract infection was 10.5% (95% CI 4.40% to 22.9%; 5 studies, 614 participants; Findling 2009; Pierce 2010; Wang 2007; Wigal 2015; Wiguna 2012; see supplementary file S55);
tachycardia was 2.50% (95% CI 1.40% to 4.30%; 6 studies, 2101 participants; Blader 2010; Cortese 2015; Findling 2009; Jafarinia 2012; Kratochvil 2002; Su 2015; see supplementary file S56);
abnormal ECG was 2.90% (95% CI 0.00% to 66.6%; 2 studies, 503 participants; Findling 2009; Findling 2010; see supplementary file S57);
nasal congestion was 11.8% (95% CI 8.30% to 16.4%; 3 studies, 247 participants; Findling 2009; Maayan 2009; Sangal 2006; see supplementary file S58); and
palpitation was 8.30% (95% CI 2.10% to 27.5%; 3 studies, 180 participants; Mohammadi 2004; Na 2013; Shang 2015; see supplementary file S59).
Systolic blood pressure
Nineteen studies provided data on this outcome (Cho 2012:Döpfner 2011b; Findling 2009; Gadow 1995; Germinario 2013; Gerwe 2009; Hazell 2003; Kim 2010; Kratochvil 2002; Lamberti 2015; Maayan 2009; McCracken 2016; Na 2013; Song 2012; Wilens 2005; Winsberg 1982; Yildiz 2011; Zheng 2011; Zheng 2015).
Nine studies reported an increase in systolic blood pressure as follows.
Cho 2012: marginal increase following methylphenidate treatment, but this difference was not significant.
Gadow 1995 (27 participants): increase of 6 mmHg at 24 months.
Kratochvil 2002 (44 participants): significant (P = 0.03) increase from 102.2 (SD 9.89) mmHg at baseline to 105.6 (SD 10.7) mmHg at follow‐up.
Lamberti 2015 (54 participants): short‐term increase from 105.4 (SD 10.3) mmHg at baseline to 109.6 (SD 11.5) mmHg two hours postmethylphenidate.
McCracken 2016 (61 participants): increase of 7.30 (SD 11.5) mmHg.
Na 2013 (121 participants): increase from baseline of 5.26 (SD 18.5) mmHg.
Song 2012 (143 participants): increase from 105.3 (SD 12.6) mmHg to 108.4 (SD 70.9) mmHg.
Winsberg 1982 (25 participants): increase from 103.1 mmHg to 110.5 mmHg at week five.
Wilens 2005 (407 participants) increase from 104.7 (SD 8.10) mmHg to 108.1 (SD 8.70) mmHg at end of treatment.
Six studies reported either no change or no apparent changes in systolic blood pressure: Döpfner 2011b (baseline: 106.0 (SD 1.00) mmHg; follow‐up at week three: 106.0 (SD 1.00) mmHg); Gerwe 2009 (mean change in systolic blood pressure between visit one and four: 0.1 (SD 10.8) mmHg); Maayan 2009 (baseline: 96.2 (SD 14.0) mmHg; follow‐up at week four: 96.09 (SD 10.1) mmHg); Zheng 2011 (baseline: 96.6 (SD 10.6) mmHg, N = 1247; follow‐up at week six: 97.5 (SD 10.3) mmHg, N = 959); Germinario 2013 (baseline: 100.6 (SD 12.3) mmHg; follow‐up at month 24: 99.6 (SD 13.0) mmHg); and Zheng 2015 (reporting no changes in blood pressure).
Four studies reported a drop in systolic blood pressure. Hazell 2003 reported a drop in 8/10 participants. There were also apparent decreases in systolic blood pressure in Kim 2010 (baseline: 113.8 (SD 10.2) mmHg; follow‐up: 110.0 (SD 10.6) mmHg), and Yildiz 2011 (baseline: 97.0 (SD 10.8) mmHg; week 12: 92.7 (SD 10.1) mmHg). One study, Findling 2009, reported that across dose levels, the proportion of participants with systolic blood pressure above the upper limit of normal (> 123 mmHg) ranged from 1% to 10%, whereas 1.5% had an above‐normal systolic blood pressure at baseline.
Diastolic blood pressure
Sixteen studies reported data on this outcome (Cho 2012; Döpfner 2011b; Findling 2009; Germinario 2013; Gerwe 2009; Hazell 2003; Kim 2010; Kratochvil 2002; Lamberti 2015; Maayan 2009; McCracken 2016; Na 2013; Song 2012; Zheng 2011; Yildiz 2011; Zheng 2015).
Six studies reported apparent increases in diastolic blood pressure in participants receiving methylphenidate: Lamberti 2015 (baseline: 59.2 (SD 7.10) mmHg; follow‐up after two hours: 63.1 (SD 7.80) mmHg); Döpfner 2011b (baseline: 69.0 (SD 1.00) mmHg; follow‐up after three weeks: 86.0 (SD 1.00) mmHg); McCracken 2016 (mean change: 5.80 (SD 10.4) mmHg); Na 2013 (mean change: 4.83 (SD 12.3) mmHg); Kratochvil 2002 (baseline: 63.5 (SD 7.9) mmHg; follow‐up: 66.46 (SD 7.41) mmHg, P = 0.04), and Song 2012 (baseline: 66.9 (SD 10.1) mmHg; follow‐up: 70.9 (SD 10.5) mm Hg). One study, Cho 2012, reported a marginal increase that was not significant.
Four studies reported minimal changes in diastolic blood pressure: Gerwe 2009 (mean change: 0.70 (SD 9.70) mmHg), Maayan 2009 (baseline: 61.4 (SD 13.7) mmHg; follow‐up at week 4: 60.9 (SD 14.5) mmHg), Zheng 2011 (baseline: 64.6 (SD 7.51) mmHg, N = 1247; follow‐up at week six: 65.6 (SD 7.32) mmHg, N = 959), and Yildiz 2011 (baseline: 63.5 (SD 12.0) mmHg; follow‐up at week 12: 62.5 (SD 9.70) mmHg). One study, Zheng 2015, reported no changes in blood pressure.
Two studies reported a drop in diastolic blood pressure: Hazell 2003 (diastolic blood pressure decreased in 9/10 participants) and Kim 2010 (baseline: 75.2 (SD 12.0) mmHg; follow‐up 72.0: (SD 8.40) mmHg). One study, Germinario 2013, reported an apparent decrease after 24 months of methylphenidate (baseline: 63.2 (SD 10.9) mmHg; follow‐up: 59.28 (SD 9.66) mmHg).
One study, Findling 2009, reported that no participants had an above‐normal diastolic blood pressure (> 90 mmHg) at baseline; however, one participant in each methylphenidate‐dose group had an above‐normal diastolic blood pressure at some point in the study.
Pulse rate
Fifteen studies reported data on this outcome (Cho 2012; Döpfner 2011b; Germinario 2013; Ilgenli 2007; Kim 2010; Kim 2015a; Kratochvil 2002; Lamberti 2015; Maayan 2009; McCracken 2016; Na 2013; Özcan 2004; Yildiz 2011; Zheng 2011; Zheng 2015).
Pulse rate was significantly higher in participants receiving methylphenidate in: Cho 2012 (mean increase 3.83 (SD 10.5) beats per min (bpm), P = 0.001) and Kratochvil 2002 (baseline: 80.4 (SD 9.70) bpm; follow‐up: 86.1 (SD 12.4) bpm, P = 0.009). There was an apparent increase in Lamberti 2015 after two hours of methylphenidate (baseline: 80.5 (SD 15.5) bpm; follow‐up: 87.7 (SD 18.4) bpm), and at week 12 in Kim 2015a (baseline: 86.4 (SD 11.2) bpm; follow‐up: 93.5 (SD 15.6) bpm). Yildiz 2011 reported increased pulse rate (baseline: 80.8 (SD 9.80) bpm; follow‐up: 84.5 (SD 13.5) bpm). Na 2013 also reported an increase from baseline of 9.65 (SD 14.6) bpm and McCracken 2016 an increase of 3.60 (SD 15.7) bpm.
There was no apparent difference in pulse rate in participants after two hours of methylphenidate in Ilgenli 2007 (baseline: 88.0 (SD 13.8) bpm; two hours: 88.4 (SD 9.70) bpm), after three weeks in Döpfner 2011b (baseline: 79 (SD 1) bpm; week three: 80 (SD 1) bpm), six weeks in Kim 2010 (baseline: 87.5 (SD 6.10) bpm; week six: 87.8 (SD 6.00) bpm), or 12 weeks in Zheng 2011 (baseline: 82.5 (SD 7.8) bpm, N = 1254; 12 weeks: 82.8 (SD 7.40) bpm, N = 942). Zheng 2015 reported no changes in pulse rate.
Two studies indicated a decrease in pulse rate: Maayan 2009 observed a decrease after four weeks (baseline: 95.5 (SD 16.0) bpm; follow‐up: 91.8 (12.2) bpm), and Germinario 2013 after 24 months (baseline: 79.9 (SD 12.9) bpm; follow‐up: 76.1 (SD 11.2) bpm). In one study, Özcan 2004, mean heart rate also seemed to decrease (baseline: 148.2 (SD 29.7) bpm, N = 42; follow‐up: 139.9 (SD 34.0) bpm, N = 42).
ECG‐QT
Four studies reported data on different ECG‐QT adverse events.
Cho 2012 (101 participants) reported no significant change in QT and QRS scores after treatment.
Germinario 2013 (840 participants) reported that 8 out of 77 (10.4%) participants receiving methylphenidate had an altered ECG at 24 months, and that out of 38 participants, 11 (29.0%) reported sinus bradycardia, 12 (31.6%) reported sinus tachycardia, and 6 (15.8%) reported lengthened corrected QT interval (QTc).
Ilgenli 2007 (25 participants) reported a minimum QT of 317.00 (SD 23.3) at baseline and 322.3 (SD 21.6) after two hours, and a maximum QT of 373.7 (SD 21.8) at baseline and 361.8 (SD 29.0) after two hours.
Lamberti 2015 (54 participants) reported at baseline a corrected QT (QTc) of 407.6 (SD 12.4) ms, which was 409.8 (SD 12.0) ms at two hours postmethylphenidate.
Other specific adverse events
Four studies reported on other adverse events.
-
In Khajehpiri 2014 (71 participants), the proportion of participants on methylphenidate with:
cold fingers was 18.3% (95% CI 11.0% to 28.9%); and
sweating was 8.45% (95% CI 3.93% to 17.2%).
-
In Cortese 2015 (1426 participants), the proportion of participants on methylphenidate with:
hypertension was 0.56% (95% CI 0.28% to 1.10%); and
hypotension was 0.21% (95% CI 0.05% to 0.62%).
In Haertling 2015 (239 participants), the proportion of participants on methylphenidate with respiratory, thoracic, and mediastinal disorders was 0.42% (95% 0.01% to 2.33%).
Walitza 2009 (26 participants) assessed the proportion of participants on methylphenidate with micro‐nucleated (Mn) peripheral lymphocytes and found no alteration in the number of Mn cells in the group of chronically treated (> 12 months) children compared to the ADHD group before treatment initiation. They also reported no elevation after initiation of methylphenidate treatment at three and six months after treatment initiation.
2.2.4 Gastrointestinal system
See Analysis 7.4. We were able to use data from 102 studies in 12 meta‐analyses. The proportion of participants on methylphenidate with:
7.4. Analysis.
Comparison 7 Non‐comparative studies: proportion of participants with non‐serious adverse events, Outcome 4 Gastrointestinal system.
| Gastrointestinal system | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Abdominal pain | ||
| Abbasi 2011 | Abdominal pain | 8/20 |
| Akhondzadeh 2003 | Abdominal pain | 3/10 |
| Akhondzadeh 2004 | Abdominal pain | 4/20 |
| Amiri 2008 | Abdominal pain | 7/27 |
| Ardic 2014 | Stomach ache | 16/101 |
| Arnold 2004 | Abdominal pain | 15/89 |
| Arnold 2010 | Abdominal pain | 7/150 |
| Atzori 2009 | Stomach pain | 3/129 |
| Barrickman 1995 | Stomach ache | 1/15 |
| Berek 2011 | Abdominal pain | 12/762 |
| Blader 2010 | Abdominal pain | 6/65 |
| Chou 2012b | Abdominal pain | 12/230 |
| Cortese 2015 | Abdominal pain | 22/1426 |
| Davari‐Ashtiani 2010 | Abdominal pain | 1/16 |
| Dirksen 2002 | Stomach ache | 14/287 |
| Dittmann 2014 | Upper abdominal pain | 4/195 |
| Döpfner 2011a, OBSEER | Gastrointestinal pain | 9/777 |
| Döpfner 2011b | Gastrointestinal pain | 6/113 |
| Efron 1997 | Stomach aches | 40/123 |
| El‐Fiky 2014 | Stomach aches | 9/31 |
| Findling 2009 | Abdominal pain | 27/157 |
| Garg 2014 | Abdominal pain | 3/27 |
| Gau 2006 | Stomach aches | 8/32 |
| Gerwe 2009 | Abdominal pain | 10/306 |
| Golubchik 2011 | Abdominal pain | 1/9 |
| Grcevich 2001 | Stomach ache | 2/75 |
| Green 2011 | Stomach aches | 9/34 |
| Greenhill 1983 | Stomach aches | 4/7 |
| Gucuyener 2003 | Stomach ache | 14/119 |
| Haertling 2015 | Abdominal pain | 4/239 |
| Hammerness 2009 | Stomach ache | 2/57 |
| Hazell 2003 | Stomach pain | 6/10 |
| Işeri 2007 | Abdominal pain | 3/20 |
| Jafarinia 2012 | Abdominal pain | 6/20 |
| Kemner 2005 (FOCUS) | Abdominal pain | 33/850 |
| Khajehpiri 2014 | Abdominal pain | 9/71 |
| Kim 2010 | Abdominal pain | 1/27 |
| Kordon 2011 | Abdominal pain | 11/541 |
| Kratochvil 2002 | Abdominal pain | 7/25 |
| Lee 2007 | Abdominal pain | 6/110 |
| Li 2011 | Abdominal pain | 12/34 |
| Lyon 2010 | Stomach discomfort | 2/10 |
| Maayan 2009 | Gastrointestinal pain | 2/8 |
| Maia 2008 | Stomach ache | 2/31 |
| McCracken 2016 | Abdominal pain | 28/61 |
| Mohammadi 2004 | Abdominal pain | 0/11 |
| Mohammadi 2010 | Abdominal pain | 8/19 |
| Mohammadi 2012a | Stomach ache | 5/23 |
| Mohammadi 2012b | Abdominal pain | 5/20 |
| Na 2013 | Stomach ache (abdominal pain) | 19/103 |
| Perera 2010 | Abdominal pain | 15/102 |
| Peyre 2012a | Abdominal pain | 7/121 |
| Pierce 2010 | Abdominal pain | 3/71 |
| Remschmidt 2005 | Abdominal pain | 3/89 |
| Sahin 2014 | Abdominal pain | 7/30 |
| Sangal 2006 | Abdominal pain | 4/79 |
| Schulz 2010 | Abdominal pain | 9/145 |
| Shang 2015 | Abdominal pain | 4/66 |
| Silva 2004 | Stomach ache | 3/22 |
| Song 2012 | Abdominal pain | 20/116 |
| Steele 2006 | Abdominal pain | 10/72 |
| Su 2015 | Abdominal pain | 10/205 |
| Tasdelen 2015 | Abdominal pain | 4/17 |
| Thorell 2009 | Stomach aches | 2/79 |
| Valdizán Usón 2004 | Abdominal pain | 1/155 |
| Wang 2007 | Abdominal pain | 15/166 |
| Warshaw 2010 | Abdominal pain | 9/260 |
| Weiss 2007 | Abdominal pain | 5/79 |
| Wigal 2013 | Abdominal pain | 17/39 |
| Wigal 2015 | Abdominal pain | 29/200 |
| Wilens 2005 | Abdominal pain | 45/229 |
| Wilens 2006 | Abdominal pain | 9/220 |
| Wilens 2008 | Abdominal pain | 15/127 |
| Yildiz 2010 | Stomach ache | 6/47 |
| Yildiz 2011 | Abdominal pain | 3/11 |
| Zarinara 2010 | Abdominal pain | 3/18 |
| Zelnik 2015 | Abdominal pain | 36/128 |
| Zheng 2011 | Stomach ache | 140/1154 |
| Çetin 2015 | Stomach ache | 3/61 |
| Constipation | ||
| Ardic 2014 | Constipation | 10/101 |
| Khajehpiri 2014 | Constipation | 4/71 |
| Mohammadi 2004 | Constipation | 2/11 |
| Shang 2015 | Constipation | 1/66 |
| Decreased appetite | ||
| Abbas 2006 | Loss of appetite | 2/90 |
| Abbasi 2011 | Loss of appetite | 12/20 |
| Akhondzadeh 2003 | Decreased appetite | 5/10 |
| Akhondzadeh 2004 | Decreased appetite | 8/20 |
| Altin 2013 | Decreased appetite | 27/221 |
| Amiri 2008 | Decreased appetite | 26/27 |
| Ardic 2014 | Decreased appetite | 47/101 |
| Arnold 2010 | Loss of appetite | 11/150 |
| Atzori 2009 | Decreased appetite | 27/129 |
| Blader 2010 | Decreased appetite | 13/65 |
| Chazan 2011 | Decreased appetite | 118/205 |
| Chou 2012b | Decreased appetite | 95/230 |
| Cortese 2015 | Decreased appetite | 213/1426 |
| Coşkun 2010 | Decreased appetite | 3/7 |
| Davari‐Ashtiani 2010 | Loss of appetite | 11/16 |
| Dirksen 2002 | Decreased appetite | 8/287 |
| Dittmann 2014 | Lack of appetite | 5/195 |
| Döpfner 2011a, OBSEER | Decreased appetite | 8/777 |
| Döpfner 2011b | Decreased appetite | 3/113 |
| Efron 1997 | Poor appetite | 69/123 |
| El‐Fiky 2014 | Decreased appetite | 12/31 |
| Famularo 1987 | Decreased appetite | 1/10 |
| Findling 2009 | Loss of appetite | 81/157 |
| Garg 2014 | Decreased appetite | 14/27 |
| Gau 2006 | Decreased appetite | 19/32 |
| Gau 2008 | Loss of appetite | 128/607 |
| Germinario 2013 | Decreased appetite | 5/9 |
| Gerwe 2009 | Loss of appetite | 83/263 |
| Ghanizadeh 2013 | Decreased appetite | 1/26 |
| Golubchik 2011 | Decrease in appetite | 4/9 |
| Grcevich 2001 | Appetite suppression | 5/75 |
| Green 2011 | Poor appetite | 13/14 |
| Gucuyener 2003 | Loss of appetite | 23/119 |
| Hammerness 2009 | Decreased appetite | 19/57 |
| Hazell 2003 | Appetite suppression | 7/10 |
| Hulvershorn 2012 | Decreased appetite | 6/25 |
| Işeri 2007 | Loss of appetite | 3/20 |
| Jafarinia 2012 | Decreased appetite | 11/20 |
| Johnson 2013 | Reduced appetite | 26/45 |
| Jung 2007 | Appetite loss | 16/83 |
| Kemner 2005 (FOCUS) | Decreased appetite | 49/850 |
| Kim 2010 | Decreased appetite | 9/27 |
| Kim 2011 | Poor appetite | 22/97 |
| Kratochvil 2002 | Anorexia | 6/25 |
| Lakic 2012 | Loss of appetite | 68/68 |
| Lee 2007 | Decreased appetite | 31/110 |
| Li 2011 | Decreased appetite | 13/34 |
| Maayan 2009 | Decreased appetite | 7/11 |
| Maia 2008 | Barkley decreased appetite | 19/31 |
| Mayes 1994 | Decreased appetite | 14/63 |
| McCracken 2016 | Decreased appetite | 31/61 |
| Miller‐Horn 2008 | Decreased appetite | 2/23 |
| Mohammadi 2004 | Loss of appetite | 4/11 |
| Mohammadi 2010 | Decreased appetite | 17/19 |
| Mohammadi 2012a | Loss of appetite | 11/23 |
| Mohammadi 2012b | Decreased appetite | 9/20 |
| Na 2013 | Decreased appetite | 66/103 |
| Perera 2010 | Loss of appetite | 51/102 |
| Peyre 2012a | Reduced appetite | 9/121 |
| Pierce 2010 | Decreased appetite | 7/71 |
| Poulton 2003 | Decreased appetite | 12/13 |
| Sahin 2014 | Loss of appetite | 21/30 |
| Sangal 2006 | Decreased appetite | 19/79 |
| Shang 2015 | Decreased appetite | 36/66 |
| Silva 2004 | Decreased appetite | 7/22 |
| Steele 2006 | Decreased appetite | 17/60 |
| Su 2015 | Decreased appetite | 72/205 |
| Tasdelen 2015 | Loss of appetite | 9/17 |
| Thorell 2009 | Loss of appetite | 21/79 |
| Valdizán Usón 2004 | Loss of appetite | 9/155 |
| Valdizán Usón 2013 | Appetite and nutrition disorders | 68/689 |
| Wang 2007 | Decreased appetite | 32/166 |
| Warshaw 2010 | Decreased appetite | 77/260 |
| Wigal 2013 | Decreased appetite | 21/39 |
| Wigal 2015 | Decreased appetite | 38/200 |
| Wilens 2005 | Appetite suppression | 55/229 |
| Wilens 2008 | Decreased appetite | 35/127 |
| Yalcin 2014 | Decreased appetite | 28/40 |
| Yatsuga 2014 | Decreased appetite | 3/50 |
| Yildiz 2010 | Loss of appetite | 27/47 |
| Zarinara 2010 | Decreased appetite | 7/18 |
| Zeni 2007 | Decreased appetite | 53/106 |
| Zheng 2011 | Loss of appetite | 552/1154 |
| Zheng 2015 | Loss of appetite | 16/123 |
| Decreased weight | ||
| Abbas 2006 | Weight loss | 2/90 |
| Abbasi 2011 | Weight loss | 6/20 |
| Amiri 2008 | Weight loss | 7/27 |
| Ardic 2014 | Weight loss | 25/101 |
| Arnold 2004 | Weight loss | 3/76 |
| Arnold 2010 | Decreased weight | 5/171 |
| Berek 2011 | Weight decrease | 10/762 |
| Cortese 2015 | Weight loss | 37/1426 |
| Davari‐Ashtiani 2010 | Weight loss | 4/16 |
| Dittmann 2014 | Weight decrease | 3/195 |
| Döpfner 2011b | Weight loss | 0/113 |
| Findling 2009 | Decreased weight | 33/157 |
| Grcevich 2001 | Weight loss | 3/75 |
| Haertling 2015 | Weight decreased | 3/239 |
| Işeri 2007 | Weight loss | 2/20 |
| Johnson 2013 | Weight loss | 9/45 |
| Kordon 2011 | Weight decrease | 8/541 |
| Kratochvil 2002 | Weight loss | 2/25 |
| Lakic 2012 | Moderate loss of body weight | 68/68 |
| Mohammadi 2012a | Weight loss | 9/23 |
| Remschmidt 2005 | Weight decrease | 1/105 |
| Sahin 2014 | Weight loss | 20/30 |
| Song 2012 | Weight loss | 4/116 |
| Warshaw 2010 | Decreased weight | 9/260 |
| Wilens 2005 | Weight loss | 8/407 |
| Yildiz 2010 | Weight loss | 12/47 |
| Yildiz 2011 | Weight loss | 5/11 |
| Çetin 2015 | Weight loss | 1/16 |
| Diarrhoea | ||
| Abbasi 2011 | Diarrhoea | 5/20 |
| Blader 2010 | Diarrhoea | 2/65 |
| Hammerness 2009 | Diarrhoea | 3/57 |
| Khajehpiri 2014 | Diarrhoea | 3/71 |
| Kordon 2011 | Diarrhoea | 7/541 |
| Kratochvil 2002 | Diarrhoea | 1/25 |
| Mohammadi 2004 | Diarrhoea | 0/11 |
| Mohammadi 2010 | Diarrhoea | 1/19 |
| Dry mouth | ||
| Abbasi 2011 | Dry mouth | 8/20 |
| Amiri 2008 | Dry mouth | 10/27 |
| Blader 2010 | Dry mouth | 2/65 |
| Davari‐Ashtiani 2010 | Dry mouth | 0/16 |
| Işeri 2007 | Dry mouth | 1/20 |
| Mohammadi 2004 | Dry mouth | 3/11 |
| Mohammadi 2010 | Dry mouth | 6/19 |
| Mohammadi 2012a | Dry mouth | 3/23 |
| Mohammadi 2012b | Dry mouth | 3/20 |
| Gastrointestinal adverse events | ||
| Dittmann 2014 | Gastrointestinal disorders | 6/247 |
| Gau 2008 | Gastrointestinal upset | 36/607 |
| Valdizán Usón 2013 | Gastrointestinal symptomatology | 9/633 |
| Nausea | ||
| Abbasi 2011 | Nausea | 5/20 |
| Akhondzadeh 2003 | Nausea | 2/14 |
| Akhondzadeh 2004 | Nausea | 3/20 |
| Amiri 2008 | Nausea | 4/27 |
| Ardic 2014 | Nausea | 14/101 |
| Arnold 2004 | Nausea | 7/76 |
| Barrickman 1995 | Nausea | 1/18 |
| Chou 2012b | Nausea | 15/230 |
| Dittmann 2014 | Nausea | 2/195 |
| Döpfner 2011a, OBSEER | Nausea | 9/777 |
| Döpfner 2011b | Nausea | 1/113 |
| Findling 2009 | Nausea | 20/157 |
| Garg 2014 | Nausea | 1/27 |
| Ghanizadeh 2013 | Nausea + vomiting | 1/26 |
| Gucuyener 2003 | Nausea | 11/119 |
| Guerreiro 1996 | Nausea | 1/22 |
| Haertling 2015 | Nausea | 4/239 |
| Hammerness 2009 | Nausea | 4/57 |
| Işeri 2007 | Nausea | 1/20 |
| Kemner 2005 (FOCUS) | Nausea | 9/850 |
| Khajehpiri 2014 | Nausea | 4/71 |
| Kim 2010 | Nausea | 1/27 |
| Kordon 2011 | Nausea | 10/541 |
| Kratochvil 2002 | Vomiting + nausea + dyspepsia | 4/25 |
| Lee 2007 | Nausea | 5/110 |
| Lee 2014 | Nausea | 5/100 |
| Li 2011 | Nausea | 16/34 |
| Mohammadi 2004 | Nausea | 0/11 |
| Mohammadi 2010 | Nausea | 4/19 |
| Na 2013 | Nausea | 32/103 |
| Pierce 2010 | Nausea | 3/71 |
| Schulz 2010 | Nausea | 8/145 |
| Song 2012 | Nausea | 9/116 |
| Su 2015 | Nausea | 11/205 |
| Tasdelen 2015 | Nausea | 1/17 |
| Wang 2007 | Nausea | 17/166 |
| Warshaw 2010 | Nausea | 13/260 |
| Wilens 2005 | Nausea | 7/407 |
| Yildiz 2010 | Nausea | 9/47 |
| Yildiz 2011 | Nausea | 5/11 |
| Zarinara 2010 | Nausea | 5/18 |
| Upset stomach | ||
| Arnold 2004 | Dyspepsia | 6/89 |
| Hulvershorn 2012 | Upset stomach | 4/25 |
| Na 2013 | Dyspepsia | 13/103 |
| Vomiting | ||
| Arnold 2004 | Vomiting | 3/76 |
| Dittmann 2014 | Vomiting | 1/195 |
| Findling 2009 | Vomiting | 23/157 |
| Jafarinia 2012 | Vomiting | 3/20 |
| Kemner 2005 (FOCUS) | Vomiting | 11/850 |
| Lee 2007 | Vomiting | 8/110 |
| Lee 2014 | Vomiting | 5/100 |
| Maayan 2009 | Vomiting | 1/8 |
| Mohammadi 2010 | Vomiting | 2/19 |
| Mohammadi 2012a | Vomiting | 3/23 |
| Mohammadi 2012b | Vomiting | 2/20 |
| Na 2013 | Vomiting | 7/103 |
| Shang 2015 | Vomiting | 5/66 |
| Silva 2004 | Vomiting | 6/22 |
| Su 2015 | Vomiting | 9/205 |
| Valdizán Usón 2004 | Vomiting | 2/155 |
| Wang 2007 | Vomiting | 6/166 |
| Wilens 2005 | Vomiting | 6/407 |
| Yildiz 2011 | Vomiting | 1/11 |
| Zarinara 2010 | Vomiting | 4/18 |
| Dyspepsia | ||
| Arnold 2004 | Dyspepsia | 6/76 |
| Cortese 2015 | Dyspepsia | 9/1426 |
| Gastroenteritis | ||
| Arnold 2004 | Gastroenteritis | 0/76 |
| Wigal 2015 | Gastroenteritis | 4/200 |
| Increased appetite | ||
| Blader 2010 | Increased appetite | 1/65 |
| Xerostomia | ||
| Khajehpiri 2014 | Xerostomia | 12/71 |
abdominal pain was 10.7% (95% CI 8.60% to 13.3%; 79 studies, 11,750 participants; see supplementary file S60);
constipation was 7.60% (95% CI 3.70% to 15.1%; 4 studies, 249 participants; Ardic 2014; Khajehpiri 2014; Mohammadi 2004; Shang 2015; see supplementary file S61);
decreased appetite was 31.1% (95% CI 26.5% to 36.2%; 84 studies, 11,594 participants; see supplementary file S62);
decreased weight was 8.60% (95% CI 4.90% to 14.7%; 28 studies, 5182 participants; see supplementary file S63);
diarrhoea was 4.70% (95% CI 2.00% to 10.9%; 8 studies, 809 participants; Abbasi 2011; Blader 2010; Hammerness 2009; Khajehpiri 2014; Kordon 2011; Kratochvil 2002; Mohammadi 2004; Mohammadi 2010; see supplementary file S64);
dry mouth was 17.8% (95% CI 9.60% to 30.6%; 9 studies, 221 participants; Abbasi 2011; Amiri 2008; Blader 2010; Davari‐Ashtiani 2010; Işeri 2007; Mohammadi 2004; Mohammadi 2010; Mohammadi 2012a; Mohammadi 2012b; see supplementary file S65);
gastrointestinal adverse events was 2.80% (95% CI 1.10% to 7.30%; 3 studies, 1487 participants; Dittmann 2014; Gau 2008; Valdizán Usón 2013; see supplementary file S66);
nausea was 7.60% (95% CI 5.30% to 10.6%; 41 studies, 5612 participants; see supplementary file S67);
upset stomach was 11.0% (95% CI 7.00% to 17.0%; 3 studies, 217 participants; Arnold 2004; Hulvershorn 2012; Na 2013; see supplementary file S68);
vomiting was 7.30% (95% CI 3.70% to 13.9%; 20 studies, 2731 participants; see supplementary file S69);
dyspepsia was 2.30% (95% CI 0.20% to 22.8%; 2 studies, 1502 participants; Arnold 2004; Cortese 2015; see supplementary file S70); and
gastroenteritis was 1.80% (95% CI 0.70% to 4.40%; 2 studies, 276 participants; Arnold 2004; Wigal 2015; see supplementary file S71).
Two studies also provided data on different gastrointestinal adverse events (Blader 2010; Khajehpiri 2014).
In Blader 2010 (65 participants), the proportion of participants on methylphenidate with increased appetite was 1.54% (95% CI 0.27% to 8.22%).
In Khajehpiri 2014 (71 participants), the proportion of participants on methylphenidate with xerostomia was 16.9% (95% CI 9.9% to 27.2%).
2.2.5 Musculoskeletal system
See Analysis 7.5. We were able to use data from 23 studies in 4 meta‐analyses.
7.5. Analysis.
Comparison 7 Non‐comparative studies: proportion of participants with non‐serious adverse events, Outcome 5 Musculoskeletal system.
| Musculoskeletal system | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Chest pain | ||
| Arnold 2004 | Chest pain | 0/76 |
| Hammerness 2009 | Chest pain | 2/57 |
| Shang 2015 | Chest pain | 1/66 |
| Height | ||
| Jensen 1999 (MTA) | No difference | ‐ |
| Kim 2010 | Increased from baseline | 128.7 cm (SD = 9.10) |
| Mohammadi 2012a | Increased from baseline | 133.8 cm (SD = 14.81) |
| Vincent 1990 | No difference | 158.7 cm (SD = 10.2) |
| Wiguna 2012 | Increased from baseline | 0.55 cm (SD = no information) |
| Yalcin 2014 | Increased from baseline | 134.6 cm (SD = 12.32) |
| Weight | ||
| Davari‐Ashtiani 2010 | Increased from baseline | Weight gain in 1/16 participants |
| Germinario 2013 | Increased from baseline | 38.4 kg (SD = 11.3) |
| Kim 2010 | No difference | 28.5 kg (SD = 6.40) |
| Kratochvil 2002 | No difference | 40.7 kg (SD = 17.2) |
| Mohammadi 2012a | Increased from baseline | 32.7 kg (SD = 14.7) |
| Vincent 1990 | No difference | Observed weight: 47.9 kg (SD = 10.3) Expected weight: 48.1 kg (SD = 10.6) |
| Wiguna 2012 | Decreased from baseline | 36.2 kg (SD = 11.9) |
| Yalcin 2014 | Increased from baseline | Weight gain in 15/33 participants |
| BMI | ||
| Gerwe 2009 | No difference | Mean change from baseline: −0.3 kg/m2 (SD = 0.70) |
| Mohammadi 2012a | No difference | 18.2 kg/m2 (SD = 4.74) |
| Yalcin 2014 | No difference | Methylphenidate group: 18.1 kg/m2 (SD = 2.76) Untreated group: 17.8k kg/m2 (SD = 2.71) |
| Height z score | ||
| Bereket 2005 | Decreased from baseline | ‐ |
| Germinario 2013 | Decreased from baseline | Z score = 0.36 (SD = 1.21) |
| Moungnoi 2011 | Decreased from baseline | Z score = 0.09 (SD = no information) |
| Poulton 2003 | Decreased from baseline | Z score = 0.26 (SD = 0.82) |
| Poulton 2012 | Decreased from baseline | Z score = 0.34 (SD = 0.81) |
| Schertz 1996 | Decreased from baseline | Z score = ‐0.1(SD = no information) |
| Spencer 1992 | Decreased from baseline | Z score = ‐0.23 (SD = 0.42) |
| Weight z score | ||
| Bereket 2005 | Decreased from baseline | 0.2 (SD = 1.04) |
| Gerwe 2009 | Decreased from baseline | 0.26 kg (SD = 1.40) |
| Moungnoi 2011 | Decreased from baseline | Z score = 0.01 (SD = no information) |
| Poulton 2003 | Decreased from baseline | Z score = 0.44 (SD = 0.97) |
| Schertz 1996 | Decreased from baseline | ‐ |
| BMI z score | ||
| Bereket 2005 | No difference | After 14 months: Age‐adjusted SD = 0.71 Sex‐adjusted SD = 0.65 |
| Dubnov‐Raz 2011 | No difference | ‐ |
| Poulton 2012 | No difference | ‐ |
| Increased mobility | ||
| Ardic 2014 | Increased mobility | 22/122 |
| Bone mineral density | ||
| Lahat 2000 | Quote: "There was no significant difference between serum calcium, phosphorus or bone‐specific alkaline phosphatase, urinary deoxypyridinoline, or bone mineral density in the two groups of children. No child deviated from his height percentile during the treatment period." | NA |
Chest pain
We combined the data from three studies (189 participants) in a meta‐analysis (Arnold 2004; Hammerness 2009; Shang 2015), and found that the proportion of participants on methylphenidate with chest pain was 2.20% (95% CI 0.80% to 6.00%; see supplementary file S72).
Height
Six studies provided data on this outcome (Jensen 1999 (MTA); Kim 2010; Mohammadi 2012a; Vincent 1990; Wiguna 2012; Yalcin 2014).
Four studies reported increases in height in cohorts prescribed methylphenidate: Kim 2010 (baseline: 128.7 (SD 9.10) cm; follow‐up: 129.4 (SD 9.10) cm), Mohammadi 2012a (baseline: 133.8 (SD 14.81) cm; follow‐up: 135.9 (SD 15.6) cm), Wiguna 2012 (MD 0.55 cm, P = 0.06), and Yalcin 2014 (baseline: 134.6 (SD 12.32) cm; follow‐up: 136.0 (SD 12.5) cm).
Vincent 1990 reported no difference in expected (158.7 (SD 10.2) cm) and observed (158.7 (SD 9.80) cm) height.
Jensen 1999 (MTA) reported no difference after 14 months in those who received methylphenidate (mean change: 5.88 (SD 1.80), n = 222), and those who did not (mean change: 6.93 (SD 2.21), n = 106), nor at 24 months (methylphenidate: mean 4.53 (SD 1.61), n = 106; no methylphenidate: mean 5.40 (SD 2.18)).
Weight
Eight studies provided data on this outcome (Davari‐Ashtiani 2010; Germinario 2013; Kim 2010; Kratochvil 2002; Mohammadi 2012a; Vincent 1990; Wiguna 2012; Yalcin 2014).
Three studies reported an increase in weight in participants receiving methylphenidate. Yalcin 2014 reported weight gain in 15/33 participants while Davari‐Ashtiani 2010 reported weight gain in 1/16. Germinario 2013 reported an increase in weight from 38.4 kg (SD 11.3) at baseline to 46.8 kg (SD 13.4) at 24 months.
Five studies reported no apparent increase or decrease in weight: Vincent 1990 (observed weight = 47.90 (SD 10.30) kg; expected weight = 48.1 (SD 10.6) kg); Kim 2010 (baseline: 28.5 (SD 6.40) kg; follow‐up: 27.6 (SD 5.90) kg, n = 285); Kratochvil 2002 (baseline: 40.7 (SD 17.2) kg; follow‐up: 40.6 (SD 18.1) kg, P = 0.20); Mohammadi 2012a (baseline: 32.7 (SD 14.7) kg; follow‐up: 33.6 (SD 17.1) kg); and Wiguna 2012 (baseline: 36.20 (SD 11.9) kg; follow‐up: 35.79 (SD 12.0) kg, P = 0.51).
BMI
Three studies provided data on this outcome (Gerwe 2009; Mohammadi 2012a; Yalcin 2014).
There was no apparent change from baseline BMI in all three studies: Gerwe 2009 (mean change from baseline: −0.30 (SD 0.70) kg/m2); Mohammadi 2012a (baseline: 18.2 (SD 4.74) kg/m2; follow‐up: 18.4 (SD 4.85) kg/m2), or Yalcin 2014 (baseline: 18.1 (SD 2.76) kg/m2; follow‐up: 17.8 (SD 2.71) kg/m2).
Z scores
Height
Seven studies reported that age‐ and sex‐adjusted scores were lower after follow‐up: mean 11.2 months in Moungnoi 2011 (baseline: z score = 0.09; one year: z score = 0.05, n = 83); after 14 months in Bereket 2005 (z score = −0.31 (SD 1.02)); after 18 months in Poulton 2003 (baseline: z score = 0.26 (SD 0.82), n = 19; 18 months: z score = −0.50 (SD 0.77), n = 13); 24 months in Germinario 2013 (baseline: z score = 0.36 (SD 1.21); 24 months: z score = 0.18 (SD 1.12)); 36 months in Poulton 2012 (baseline: z score = 0.34 (SD 0.81), n = 21; 36 months: z score = −0.13 (SD 0.76), n = 17). The difference in z score between baseline and follow‐up was also lower in Spencer 1992 (z score difference = −0.23, SD 0.42) and marginally lower in Schertz 1996 (z score difference = −0.1).
Weight
Five studies reported decreases in weight from baseline: −0.26 kg (SD 1.40) in Gerwe 2009; 0.04 z score at 14 months in Bereket 2005; 0.4 z score decrease at follow‐up (mean follow‐up 11.2 months) in Schertz 1996; a mean weight‐for‐age z score of 0.01 at baseline to −0.06 at one‐year follow‐up in Moungnoi 2011 (n = 83); from a z score of 0.44 (SD 0.97; n = 19) at baseline, to −0.15 (SD 0.98; n = 16) at 18 months, and a mean difference of −0.23 (SD 0.96; n = 17) at 36 months in Poulton 2003.
BMI
In three studies, there was no difference in the BMI of participants receiving methylphenidate over 14 months (Bereket 2005: z scores = 0.706 (SD = 0.65)); after 24 to 36 months (Poulton 2012: z score = 0.12 (SD = 0.43); N = 24), and over 48 months (Durá‐Travé 2012: z score = −0.20 (SD = 0.99); N = 160).
Other specific adverse events
Two studies reported on the proportion of participants on methylphenidate with different musculoskeletal adverse events (Ardic 2014; Lahat 2000).
In Ardic 2014 (122 participants), the proportion of participants on methylphenidate with increased mobility was 18.03% (95% CI 12% to 25%).
Lahat 2000 (18 participants) reported on bone mineral density as follows: "...no significant difference between serum calcium, phosphorus or bone‐specific alkaline phosphatase, urinary deoxypyridinoline, or bone mineral density in the two groups of children. No child deviated from his height percentile during the treatment period."
2.2.6 Immune system
See Analysis 7.6. Fourteen studies reported data on this outcome.
7.6. Analysis.
Comparison 7 Non‐comparative studies: proportion of participants with non‐serious adverse events, Outcome 6 Immune system.
| Immune system | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Nasopharyngitis | ||
| Arnold 2004 | Rhinitis | 5/76 |
| Arnold 2010 | Rhinitis | 6/150 |
| Döpfner 2011b | Rhinitis | 2/113 |
| Findling 2009 | Rhinitis | 24/157 |
| Kratochvil 2002 | Rhinitis | 8/25 |
| Pyrexia | ||
| Arnold 2004 | Fever | 7/89 |
| Findling 2009 | Fever | 33/157 |
| Wang 2007 | Pyrexia | 17/166 |
| Allergies | ||
| Hammerness 2009 | Allergies | 3/154 |
| Pierce 2010 | Pruritis | 2/71 |
| Cold symptoms | ||
| Hammerness 2009 | Cold symptoms | 2/57 |
| Kratochvil 2002 | Fever + flu syndrome | 8/25 |
| Steele 2006 | Flu‐like symptoms | 7/72 |
| Pharyngitis | ||
| Arnold 2004 | Pharyngitis | 10/76 |
| Döpfner 2011b | Pharyngitis | 2/113 |
| Kordon 2011 | Pharyngitis | 6/541 |
| Kratochvil 2002 | Pharyngitis | 3/25 |
| Sangal 2006 | Pharyngitis | 7/79 |
| Viral infection | ||
| Arnold 2004 | Viral infection | 18/76 |
| Kordon 2011 | Viral infection | 8/598 |
| Rash | ||
| Arnold 2004 | Rash | 6/76 |
| Garg 2014 | Rash | 1/27 |
| Lee 2007 | Rash | 4/110 |
| Rhinitis | ||
| Arnold 2004 | Rhinitis | 5/76 |
| Kordon 2011 | Rhinitis | 8/541 |
| Ear disorders | ||
| Hammerness 2009 | Ear pain | 3/57 |
| Fever | ||
| Kordon 2011 | Fever | 9/541 |
| Infections | ||
| Valdizán Usón 2013 | Nonspecific pathogen | 13/689 |
We conducted eight meta‐analyses using data from 13 studies (Arnold 2004; Arnold 2010; Döpfner 2011b; Findling 2009; Garg 2014; Hammerness 2009; Kordon 2011; Kratochvil 2002; Lee 2007; Pierce 2010; Sangal 2006; Steele 2006; Wang 2007). We found the proportion of participants on methylphenidate with:
nasopharyngitis to be 8.50% (95% CI 3.50% to 19.4%; 5 studies, 521 participants; Arnold 2004; Arnold 2010; Döpfner 2011b; Findling 2009; Kratochvil 2002; see supplementary file S73);
pyrexia to be 12.6% (95% CI 6.60% to 22.7%; 3 studies, 412 participants; Arnold 2004; Findling 2009; Wang 2007; see supplementary file S74);
allergies to be 2.30% (95% CI 0.90% to 5.30%; 2 studies, 225 participants; Hammerness 2009; Pierce 2010; see supplementary file S75);
cold symptoms to be 11.8% (95% CI 3.40% to 33.9%; 3 studies, 154 participants; Hammerness 2009; Kratochvil 2002; Steele 2006; see supplementary file S76);
pharyngitis to be 5.10% (95% CI 1.70% to 14.1%; 5 studies, 834 participants; Arnold 2004; Döpfner 2011b; Kordon 2011; Kratochvil 2002; Sangal 2006; see supplementary file S77);
viral infection to be 6.10% (95% CI 0.30% to 58.4%; 2 studies, 674 participants; Arnold 2004; Kordon 2011; see supplementary file S78);
rash to be 5.50% (95% CI 3.10% to 9.70%; 3 studies, 213 participants; Arnold 2004; Garg 2014; Lee 2007; see supplementary file S79); and
rhinitis to be 3.10% (95% CI 0.70% to 12.6%; 2 studies, 617 participants; Arnold 2004; Kordon 2011; see supplementary file S80).
Three studies provided data on different adverse events related to the immune system (Hammerness 2009; Kordon 2011; Valdizán Usón 2013).
In Hammerness 2009 (57 participants), the proportion of participants on methylphenidate with ear disorders was 5.30% (95% Cl 1.80% to 14.4%).
In Kordon 2011 (541 participants), the proportion of participants on methylphenidate with fever was 1.70% (95% CI 0.90% to 3.30%).
In Valdizán Usón 2013 (689 participants), the proportion of participants on methylphenidate with infections was 1.90% (95% CI 1.10% to 3.20%)
2.2.7 Urogenital system
See Analysis 7.7. We included three studies (141 participants) in this meta‐analysis (Blader 2010; Galland 2010; Tomás Vila 2010b). The proportion of participants on methylphenidate with enuresis was 9.30% (95% CI 4.10% to 19.9%; see supplementary file S81).
7.7. Analysis.
Comparison 7 Non‐comparative studies: proportion of participants with non‐serious adverse events, Outcome 7 Urogenital system: enuresis.
| Urogenital system: enuresis | ||
|---|---|---|
| Study | Symptom | Adverse events/total number of participants |
| Blader 2010 | Enuresis | 2/65 |
| Galland 2010 | Enuresis | 3/27 |
| Tomás Vila 2010b | Enuresis | 16/114 |
2.2.8 Other body systems
See Analysis 7.8. Ten studies reported data on this outcome (Abbas 2006; Arnold 2004; Congologlu 2009; Garg 2014; Ghanizadeh 2013; Haertling 2015; Khajehpiri 2014; Pierce 2010; Shang 2015; Witt 2008).
7.8. Analysis.
Comparison 7 Non‐comparative studies: proportion of participants with non‐serious adverse events, Outcome 8 Other body systems.
| Other body systems | ||
|---|---|---|
| Study | Description | Adverse events/total number of participants |
| Hair loss | ||
| Abbas 2006 | Hair loss | 1/90 |
| Khajehpiri 2014 | Hair loss | 3/71 |
| Skin problems | ||
| Haertling 2015 | Skin disorder | 1/239 |
| Khajehpiri 2014 | Acne | 2/71 |
| Pierce 2010 | Application site erythema | 2/71 |
| Shang 2015 | Skin eruption | 1/66 |
| Accidental injury | ||
| Arnold 2004 | Accidental injury | 10/76 |
| Voice frequency | ||
| Congologlu 2009 | In conclusion, this clinical trial is the only study that has examined the effects of stimulant medication on vocal acoustic parameters in children with ADHD (attention deficit hyperactivity disorder), and evaluated a small sample on and off their clinical doses of methylphenidate. We suggest that methylphenidate decreases fundamental frequency in children with ADHD, but our findings should be replicated under blind drug administration and by supporting other vocal analyses | NA |
| Hypersalivation | ||
| Garg 2014 | Hypersalivation | 1/27 |
| Nasal bleeding | ||
| Ghanizadeh 2013 | Nasal bleeding | 1/26 |
| Chromosomal aberration | ||
| Witt 2008 | % of cells with chromosomal abberations | Before: 0.48% (0.09). After: 0.46% (0.09) Study population: 25 |
Using data from across five studies (Abbas 2006; Haertling 2015; Khajehpiri 2014; Pierce 2010; Shang 2015), we conducted two meta‐analyses, in which we found the proportion of participants on methylphenidate with:
hair loss to be 2.80% (95% CI 0.00% to 9.00%; 2 studies, 161 participants; Abbas 2006; Khajehpiri 2014; see supplementary file S82); and
skin problems to be 1.80% (95% CI 0.80% to 4.00%; 4 studies, 447 participants; Haertling 2015; Khajehpiri 2014; Pierce 2010; Shang 2015; see supplementary file S83).
Five studies reported data on patients on methylphenidate with other non‐serious adverse events (Arnold 2004; Congologlu 2009; Garg 2014; Ghanizadeh 2013; Witt 2008).
Arnold 2004 (76 participants) reported the proportion of participants on methylphenidate with accidental injury as 13.2% (95% CI 7.30% to 22.6%).
Congologlu 2009 suggested that "methylphenidate decreases fundamental [voice] frequency in children with ADHD, but our findings should be replicated under blind drug administration and by supporting other vocal analyses."
Garg 2014 (27 participants) reported the proportion of participants on methylphenidate with hypersalivation as 3.70% (95% CI 0.70% to 18.3%).
Ghanizadeh 2013 (26 participants) reported the proportion of participants on methylphenidate with nasal bleeding as 3.90% (95% CI 0.70% to 2.20%).
Witt 2008 conducted a study of chromosomal aberration and "found no evidence of changes in any of the three cytogenetic endpoints examined in the lymphocytes of children treated with methylphenidate or mixed mixed amphetamine salts (MAS) based products continuously for three months".
2.2.9 Withdrawal of treatment
Due to non‐serious adverse events
See Analysis 8.1. We conducted a meta‐analysis of data from 37 studies (7142 participants) that reported on the number of patients withdrawn from methylphenidate due to non‐serious adverse events. The proportion of participants withdrawn from methylphenidate treatment due to non‐serious adverse events was 6.20% (95% CI 4.80% to 7.90%; Figure 6).
8.1. Analysis.
Comparison 8 Non‐comparative studies: proportion of participants withdrawn from methylphenidate treatment due to non‐serious adverse events, Outcome 1 Proportion of participants withdrawn from methylphenidate treatment due to non‐serious adverse events.
| Proportion of participants withdrawn from methylphenidate treatment due to non‐serious adverse events | |
|---|---|
| Study | |
| Arabgol 2015 | 2/18 |
| Arnold 2010 | 1/171 |
| Berek 2011 | 60/822 |
| Chou 2012a | 26/521 |
| Dirksen 2002 | 10/310 |
| Döpfner 2011b | 4/113 |
| Efron 1997 | 1/125 |
| Faraone 2007a | 45/268 |
| Findling 2010 | 13/162 |
| Garg 2014 | 7/33 |
| Gerwe 2009 | 43/306 |
| Greenberg 1987 | 1/50 |
| Guerreiro 1996 | 1/24 |
| Hammerness 2009 | 19/152 |
| Hong 2012 | 3/112 |
| Karabekiroglu 2008 | 8/90 |
| Khodadust 2012 | 1/15 |
| Kim 2014b | 3/57 |
| Lee 2007 | 2/119 |
| Maayan 2009 | 3/11 |
| Na 2013 | 7/121 |
| Shang 2015 | 3/80 |
| Sobanski 2013 | 5/381 |
| Song 2012 | 8/143 |
| Su 2015 | 16/239 |
| Tasdelen 2015 | 3/22 |
| Wang 2007 | 6/166 |
| Wang 2011 | 3/50 |
| Warshaw 2010 | 21/305 |
| Weber 2003 | 4/57 |
| Weiss 2007 | 4/90 |
| Wigal 2013 | 2/45 |
| Wilens 2005 | 38/407 |
| Winsberg 1982 | 5/25 |
| Yildiz 2011 | 1/12 |
| Zheng 2011 | 34/1447 |
| Çetin 2015 | 5/73 |
6.
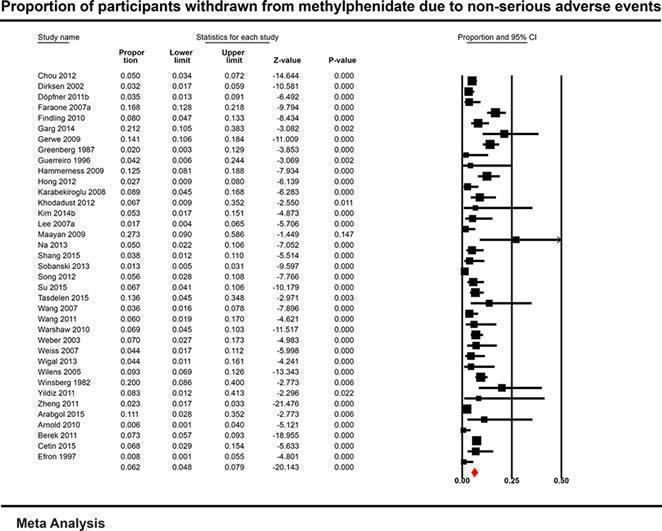
Proportion of participants withdrawn from methylphenidate due to non‐serious adverse events
For unknown reasons
See Analysis 8.2. We conducted a meta‐analysis of data from 57 studies (8340 participants) that reported on the number of patients withdrawn from methylphenidate for unknown reasons. The proportion of participants withdrawn from methylphenidate for unknown reasons was 16.2% (95% CI 13.0% to 19.9%; Figure 7).
8.2. Analysis.
Comparison 8 Non‐comparative studies: proportion of participants withdrawn from methylphenidate treatment due to non‐serious adverse events, Outcome 2 Proportion of participants withdrawn from methylphenidate for unknown reasons.
| Proportion of participants withdrawn from methylphenidate for unknown reasons | |
|---|---|
| Study | |
| Akhondzadeh 2003 | 4/14 |
| Akhondzadeh 2004 | 2/22 |
| Altin 2013 | 30/204 |
| Amiri 2008 | 3/30 |
| Ardic 2014 | 21/122 |
| Balázs 2011 | 3/37 |
| Barbaresi 2006 | 84/379 |
| Bereket 2005 | 58/72 |
| Chou 2012b | 34/296 |
| Dirksen 2002 | 13/310 |
| Dittmann 2014 | 52/247 |
| El‐Zein 2005 | 6/18 |
| Findling 2009 | 109/326 |
| Findling 2010 | 54/162 |
| Gadow 1995 | 7/34 |
| Galland 2010 | 2/30 |
| Gau 2008 | 13/137 |
| Germinario 2013 | 161/351 |
| Guerreiro 1996 | 2/24 |
| Haertling 2015 | 16/262 |
| Hammerness 2009 | 97/152 |
| Hong 2012 | 5/112 |
| Jensen 1999 (MTA) | 29/144 |
| Kim 2011 | 5/102 |
| Kim 2014b | 12/75 |
| Kim 2015a | 49/86 |
| Klein 2004 | 9/129 |
| Lee 2009 | 6/144 |
| Lee 2012 | 22/93 |
| Li 2011 | 2/36 |
| McCracken 2016 | 6/69 |
| Mohammadi 2004 | 5/16 |
| Mohammadi 2012a | 8/32 |
| Mohammadi 2012b | 3/23 |
| Peyre 2012a | 37/173 |
| Poulton 2003 | 6/19 |
| Remschmidt 2005 | 26/89 |
| Shang 2015 | 11/80 |
| Sobanski 2013 | 29/381 |
| Steele 2006 | 4/73 |
| Tasdelen 2015 | 2/22 |
| Walitza 2009 | 15/26 |
| Wang 2007 | 7/166 |
| Wang 2011 | 17/50 |
| Warshaw 2010 | 28/305 |
| Weber 2003 | 8/57 |
| Weiss 2007 | 3/90 |
| Wigal 2013 | 3/45 |
| Wilens 2005 | 140/407 |
| Wilens 2006 | 36/171 |
| Witt 2008 | 9/34 |
| Yalcin 2014 | 2/40 |
| Yang 2012 | 12/130 |
| Zarinara 2010 | 1/19 |
| Zheng 2011 | 202/1447 |
| Zheng 2015 | 25/153 |
| Çetin 2015 | 7/73 |
7.
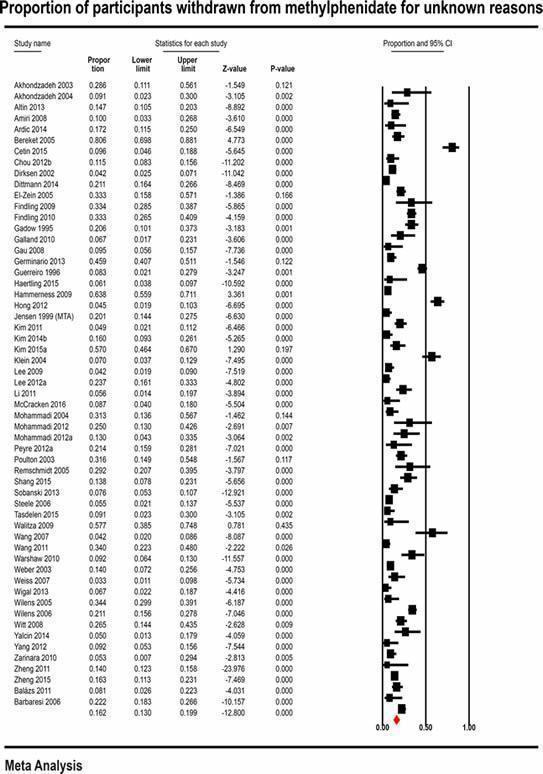
Proportion of participants withdrawn from methylphenidate for unknown reasons
3. Patient reports/series
3.1. Primary outcomes: serious adverse events
3.1.1 Frequency of any serious averse events
We found 26 patient reports/series of serious adverse events and these studies reported 149 cases of serious adverse events.
3.1.2 Central nervous system
See Analysis 9.1. We found 20 patient reports/series of serious adverse events related to the central nervous system.
9.1. Analysis.
Comparison 9 Patient reports/series: number of participants with serious adverse events, Outcome 1 Central nervous system.
| Central nervous system | |
|---|---|
| Study | |
| Psychotic conditions | |
| Abali 2007 | A 14‐year‐old girl with diagnoses of ADHD (attention deficit hyperactivity disorder), major depression and conduct disorder. During her treatment with methylphenidate of 20 mg/day and fluoxetine of 20 mg/day, she developed visual and auditory hallucinations. It may be concluded that it is possible that methylphenidate may cause hallucinations in patients treated simultaneously with fluoxetine |
| Aguilera‐Albesa 2010 | 2 case reports of the appearance of hallucinations a few hours after methylphenidate ingestion in an 8‐year‐old boy (extended‐release methylphenidate of 18 mg/day, given once daily for 2 days) and a 6‐year‐old girl (extended‐release methylphenidate of 10 mg/day for 3 days and 20 mg/day for 1 day, given once a day). Both diagnosed with ADHD according to criteria in the DSM‐IV (Diagnostic and Statistical Manual, Fourth Edition) and with IQs (intelligence quotients) > 85. These case reports suggest an individual susceptibility to psychotic symptoms after taking methylphenidate. This adverse event is considered idiosyncratic, extraordinary and unpredictable |
| Coşkun 2008 | A paediatric patient who developed tactile and visual hallucinations with the combination of osmotic release oral system (OROS) methylphenidate and fluoxetine. Comments from the study authors: In conclusion, we think that the causative agent was the combination of both medications rather than either medication alone. However, in either situation, it is important to note that this distressing side effect may occur even in the absence of underlying psychotic or substance‐related disorders, and clinicians' awareness is important in this issue, particularly in cases where polypharmacy is considered |
| Goetz 2011 | A case report of nocturnal visual hallucinations during methylphenidate treatment. A girl with ADHD and ODD (oppositional defiant disorder) experienced a 3‐hour episode of nocturnal complex bizarre visual hallucinations when treated with 18 mg of OROS methylphenidate. Nocturnal polysomnography performed 2 weeks later revealed REM (rapid eye movement) sleep reduction (17%) and fragmentation. 2 episodes of confusional arousals were recorded. This finding is typical of parasomnia associated with NREM sleep – disorder of arousal. It is hypothesized that this pre‐existing sleep impairment represents a factor of vulnerability to methylphenidate sleep side effects |
| Gross‐Tsur 2004 | 3 children with ADHD, who were treated with low doses of methylphenidate and who developed complex visual and haptic hallucinations Comments from the study authors: The causal role of methylphenidate in the development of hallucinations was based on their appearance after ingestion of the drug, resolving after its withdrawal, and the absence of psychiatric comorbidity that could explain such phenomena. In 1 patient, the hallucinations reappeared after an inadvertent re‐challenge. Because methylphenidate is a widely used, well‐studied, and safe pharmacologic agent, physicians who prescribe methylphenidate should be aware of even rare adverse manifestations occurring at therapeutic doses |
| Halevy 2009 | Several days after initiation of treatment: visual hallucinations of rats, accompanied by some tactile hallucinations. Only present during the time the patient was under the influence of methylphenidate and disappeared thereafter; immediate complete resolution upon discontinuation of the drug. Reintroduction of metyhlphenidate treatment after 2 days resulted in same complex visual hallucinations, with immediate complete resolution upon discontinuation of the drug Key conclusions of the study authors: In our case, the occurrence of hallucinations after a very low dose of methylphenidate on 2 occasions may suggest an idiosyncratic reaction. The phenomenon might also be explained by a drug‐induced dysfunction of the monoamine transmitters. Given the wide use of methylphenidate, clinicians should be aware of this possible side effect |
| Irmak 2014 | A case report of phobias and visual hallucinations during methylphenidate treatment in a 9‐year‐old boy. OROS methylphenidate gradually titrated up to 1 mg/kg. Methylphenidate prescribed at initial ADHD diagnosis and then withdrawn following the onset of phobias and visual hallucinations, as well as lack of improvement in attention problems |
| Mino 1999 | A case report on methylphenidate‐induced psychosis in an adolescent with hyperkinetic disorder; use of methylphenidate for 1 month. Three weeks after starting methylphenidate (10 mg/day), the mother reported by telephone that the patient seemed depressed. Dose of methylphenidate was reduced to 5 mg/day. A week later the patient visited the clinic. The therapist diagnosed her condition as a depressive state and discontinued methylphenidate. Six weeks after discontinuation of methylphenidate she was diagnosed with a schizophrenic‐like psychotic state, due to symptoms of delusions of reference and persecution, delusional mood, silly smile and thought block. There was no evident hallucination. The patient took antipsychotic medication for 2 months and her psychotic symptoms disappeared |
| Porfirio 2011 | 3 years after start of methylphenidate treatment: An episode of complex visual hallucinations (dramatic scenes appearing before going to sleep, sometimes during the day after ingestion of methylphenidate |
| Rashid 2007 | A case report of intensified somatic hallucinations during dose increase of methylphenidate treatment in a 10‐year‐old boy with a chronic pattern of somatization, which evolved into overt somatic hallucinations with an increase in methylphenidate dosage. This pattern of somatization was retrospectively recognized as partial somatic hallucinations |
| Shibib 2009 | A report of four cases of psychosis during methylphenidate treatment Key conclusion of the study authors: Psychosis is an important, unpredictable side effect of stimulant medication. Symptoms resolve with discontinuation of treatment. Reemergence of ADHD symptoms are rapid and re‐challenge is often indicated. It would be advisable for all professionals involved in the care and treatment of patients with ADHD to receive mental health training to aid the early recognition and appropriate management of such side effects |
| Tomás Vila 2010a | After two weeks of 50% immediate‐release and 50% extended‐release methylphenidate: visual hallucinations (insects on hands, feet, abdomen and thorax), with associated itching, initiated two hours after ingestion and ceased five hours after. Discontinuation of methylphenidate and initiation of risperidone resulted in no visual hallucinations. No re‐administration of methylphenidate due to ethical reasons Key conclusions of the study authors: This is the first case report of visual hallucinations caused by 50% immediate‐release and 50% extended‐release methylphenidate, which is not surprising considering the relative recent appearance of this preparation on the market |
| Seizures | |
| Feeney 1997 | First‐reported seizure in a patient being treated with methylphenidate and sertraline combined |
| Hemmer 2001 | Reported seizures in a boy aged six, and two girls aged six and seven, receiving 0.3 to 1 mg/kg/day of methylphenidate for six weeks, 10 and three months, respectively |
| Cerebral arteritis | |
| Trugman 1988 | Key conclusions of the study authors: Cerebral arteritis and infarction were caused by chronic use of oral methylphenidate. The Cerebrospinal fluid (CSF) profile and angiogram support the diagnosis of inflammatory arteritis, yet laboratory evaluation revealed no identifiable cause. In the six years since the stroke, while not on methylphenidate, there has been no evidence of active central nervous system or systemic vasculitis |
| Self‐harm and suicidal behaviour | |
| Arun 2014 | 2 case reports with 2 children having suicidal ideation |
| Gökce 2015 | Reported a 12‐year‐old boy who made a suicide attempt after switching from 27 mg to 36 mg of OROS methylphenidate: The patient reported irritable mood when he took the first dose of 36 mg of long‐acting methylphenidate. This might be the cause of the suicide attempt. He had a full recovery after withdrawal from methylphenidate |
| Strandell 2007 | Reported concerns about suicidal behaviour, including suicide, based on information retrieved from the WHO Collaborating Centre for International Drug Monitoring (Uppsala, Sweden). A total of 116 reports of methylphenidate related to "suicide attempts", although this term was not explicitly defined. The data included reports of methylphenidate and atomoxetine |
| Death | |
| Tølløfsrud 2006 | A case report of death caused by heart failure (acute dilated cardiomyopathy) during methylphenidate treatment |
| Dyskinesia | |
| Yilmaz 2013 | Involuntary movements started about 5 hours after taking MPH. Lip‐licking, lip‐smacking and tongue‐rolling movements. Dyskinetic tongue movements inside and outside the mouth and involuntary bilateral arm swinging while sitting and standing. Opening and closing his fingers without complete extension. Occasional repetitive movements of the feet, such as beating them against each other while sitting. About 15 hours after MPH intake both hand‐mouth movements and excessive mobility had significantly resolved. Dyskinetic symptoms had completely disappeared on the second day of hospitalization, and the patient was discharged Key conclusions of the study authors: This case is reported to emphasize the potential side effects of methylphenidate, individual differences in drug sensitivities, and drug‐receptor interactions via different mechanisms |
Psychotic conditions
Twelve patient reports/series described psychosis or psychotic symptoms related to methylphenidate.
Abali 2007 reported on a 14‐year‐old girl with ADHD, major depression, and conduct disorder. She was treated with methylphenidate (20 mg/day) and fluoxetine (20 mg/day) and developed visual and auditory hallucinations a few hours after treatment initiation.
Aguilera‐Albesa 2010 reported on an 8‐year‐old boy and 6‐year‐old girl, both with ADHD, who developed psychosis on modified‐release methylphenidate 18 mg/day (0.51 mg/kg/day) after two days, and modified‐release methylphenidate 10 mg for three days then 20 mg/day on the fourth day (0.45 mg/kg/day).
Coşkun 2008 also reported on the development of psychosis in a 10‐year‐old boy with ADHD, oppositional defiant disorder, and generalised and separation anxiety, who was also treated with a combination of methylphenidate and fluoxetine. He was taking OROS methylphenidate (18 mg/day) and fluoxetine (10 mg/day) and experienced a relatively acute‐onset episode of intense visual and tactile hallucinations three hours after taking the medication. In these cases, it was thought that the combination of methylphenidate and fluoxetine might have precipitated the psychoses.
Goetz 2011 described a girl with ADHD and oppositional defiant disorder who experienced a three‐hour episode of bizarre, complex, nocturnal visual hallucinations whilst taking OROS methylphenidate (18 mg/day). Authors reported a reduction of REM (rapid eye movement) sleep and two episodes of confusional arousals, hypothesising that her pre‐existing sleep impairment made her vulnerable to methylphenidate‐induced, adverse sleep effects.
Gross‐Tsur 2004 described three children with ADHD treated with low‐dose methylphenidate, who developed complex visual and tactile hallucinations that appeared soon after ingestion of methylphenidate and resolved after its withdrawal. In one patient, the hallucinations reappeared after an inadvertent challenge.
Halevy 2009 reported on a 15‐year‐old boy with ADHD who presented with complex visual hallucinations of rats running around and touching and smelling him soon after receiving a first low dose of methylphenidate. The hallucinations resolved upon discontinuation of the drug. Reintroduction of the drug seven years later at an even lower dose had the same effect. The authors suggested that the occurrence of hallucinations after a very low dose of methylphenidate on two occasions may suggest an idiosyncratic reaction. It could also be explained by a drug‐induced dysfunction of the monoamine transmitters. Given the wide use of methylphenidate, clinicians should be aware of this possible adverse event.
Irmak 2014 reported a nine‐year‐old boy who developed phobias and visual hallucinations during OROS methylphenidate titrated to 1 mg/kg/day.
Mino 1999 described a 16‐year‐old girl with hyperkinetic disorder who developed a psychosis after initially becoming depressed following three weeks of 10 mg/day of methylphenidate. Methylphenidate was stopped, but six weeks later she developed a psychosis with delusions of reference, thought block, and other psychotic features. These resolved on antipsychotic medication after two months. The authors highlighted the risk of methylphenidate precipitating psychosis. However, there had been a history of diagnostic uncertainty beginning with behavioural difficulties from the start of school and then treatment with antipsychotics for mania when she was 12 years old. This might suggest that methylphenidate treatment in a patient with possible additional vulnerability could precipitate psychosis.
Porfirio 2011 reported an episode of complex visual hallucinations three years after initiation of methylphenidate treatment (30 mg/day (0.5 mg/kg)).
Rashid 2007 reported a 10‐year‐old boy with ADHD and chronic somatisation who developed intensified somatic hallucinations with a change in methylphenidate dosage from 36 mg OROS methylphenidate a day to 15 mg immediate‐release methylphenidate twice daily.
Shibib 2009 describes four patients (a 14‐year‐old girl, a 14‐year‐old boy, an 8‐year‐old boy and a 10‐year‐old boy), all of whom were treated with methylphenidate in normal therapeutic doses and all of whom developed psychoses that resolved on cessation of methylphenidate treatment. Three of the four patients were taking modified‐release methylphenidate.
Tomás Vila 2010a described the development of psychosis in a 10‐year‐old patient with ADHD who was treated with 30 mg of methylphenidate (50% immediate release, 50% modified release). The patient experienced visual hallucinations (insects on hands, feet, abdomen and thorax) with associated itching. It resolved on stopping methylphenidate and treatment with risperidone.
Seizures
Two patient reports/series described seizures related to methylphenidate.
Feeney 1997 reported on a 13‐year‐old boy with ADHD and depressive disorder (not otherwise specified) who developed seizures a week after sertraline, 50 mg/day, was added to his 80 mg/day dose of methylphenidate (1.8 mg/kg/day). He had been established on 80 mg/day of methylphenidate for seven months; initially, this had been gradually titrated upwards to achieve symptom control, without significant adverse events. He was treated with cognitive behavioural therapy and family interventions for depression, but when this deteriorated after eight months of treatment, sertraline 25 mg/day was prescribed for a week and then increased to 50 mg/day. A week later he experienced a tonic‐clonic event witnessed by his father, which lasted a few seconds. All investigations were normal apart from a serum pH value of 7.20. The sertraline was discontinued and methylphenidate treatment continued unchanged without seizure recurrence.
Hemmer 2001 reported seizures in a boy aged six, and two girls aged six and seven, receiving 0.30 mg/kg/day to 1 mg/kg/day of methylphenidate for six weeks, 10 months and 3 months, respectively.
Cerebral arteritis
One patient report/series described cerebral arteritis related to methylphenidate (Trugman 1988). This study reported on cerebral arteritis and infarction thought to have been caused by chronic oral methylphenidate use in a 12‐year‐old boy. The cerebrospinal fluid profile and angiogram at the time supported the diagnosis of inflammatory arteritis, yet laboratory evaluation revealed no identifiable cause. Magnetic resonance imaging (MRI) six years on was compatible with an old infarction. In the six years since the stroke, while not on methylphenidate, there was no evidence of active, central nervous system or systemic vasculitis.
Self‐harm and suicidal behaviour
Three patient reports/series described self‐harm and suicidal behaviours related to methylphenidate.
Arun 2014 reported on two children with ADHD who developed suicidal ideation, which abated after discontinuation of methylphenidate. Neither child had depressive symptoms, and the suicidal ideation could not be explained on the basis of impulsivity.
Gökce 2015 reported on a 12‐year‐old boy who made a suicide attempt after switching from 27 mg to 36 mg of OROS methylphenidate: the patient reported irritable mood when he took the first dose of 36 mg of long‐acting methylphenidate. This might be the cause of the suicide attempt. He had a full recovery after withdrawal from methylphenidate.
Strandell 2007 reported concerns about suicidal behaviour, including suicide, based on information retrieved from the WHO Collaborating Centre for International Drug Monitoring (Uppsala, Sweden). A total of 116 reports of methylphenidate related to 'suicide attempts', although this term is not explicitly defined. The data included reports of methylphenidate and atomoxetine. They summarised reports on methylphenidate as: suicide (n = 7), suicide attempt (n = 25), depression, suicidal (n =1), intentional overdose (n = 0), intentional self‐injury (n = 8), non‐accidental overdose (n = 8), suicidal tendency (n = 21), thoughts of self‐harm (n = 0).
Death
Tølløfsrud 2006 reported a 17‐year‐old boy who died of heart failure (acute dilated cardiomyopathy) during methylphenidate treatment.
Dyskinesia
We found one patient report/series on dyskinesia related to methylphenidate (Yilmaz 2013). This study reported a patient having involuntary movements that started about five hours after methylphenidate ingestion, including lip‐licking, lip‐smacking and tongue‐rolling movements, as well as dyskinetic tongue movements inside and outside the mouth, and involuntary bilateral arm swinging while sitting and standing. Furthermore, he opened and closed his fingers without complete extension and made occasional repetitive movements of the feet, such as beating them against each other while sitting. About 15 hours after methylphenidate ingestion, both hand‐mouth movements and excessive mobility had significantly resolved. Dyskinetic symptoms had completely disappeared on the second day of hospitalisation, and the patient was discharged.
3.1.3 Cardiovascular and respiratory system
See Analysis 9.2. We found three patient reports/series on adverse events related to the cardiovascular and respiratory system (Munk 2015; Nymark 2008; Saieh 2004).
9.2. Analysis.
Comparison 9 Patient reports/series: number of participants with serious adverse events, Outcome 2 Cardiovascular and respiratory system.
| Cardiovascular and respiratory system | |
|---|---|
| Study | |
| Cardiovascular events | |
| Munk 2015 | The present case demonstrates that myocardial infarction can occur due to methylphenidate exposure in a healthy 11‐year‐old boy, without other known cardiovascular risk factors. 54 mg methylphenidate/day. Treatment duration was 2 years. Cardiac arrest followed exercise without prior complaints of chest pain/discomfort or shortness of breath. A week before the event, he had a short episode of tachycardia. Clinical examination showed that the myocardial infarct was not acute but had occurred weeks prior to the cardiac arrest. It was thought to be due to thinning of the myocardium and an adversely remodelled left ventricle. A pacemaker was inserted and methylphenidate treatment was discontinued. The only apparent cause, after extensive assessment, appeared to be the high‐dose methylphenidate treatment (maximum recommended) over an extended period |
| Nymark 2008 | A case report of serious cardiomyopathy during MPH treatment. Serious cardiovascular adverse effects (hypoxia and dyspnoea requiring hospitalisation) after 11 months of MPH treatment. Clinical examination showed signs of liver failure, renal failure and heart failure Comments of the study authors (Nymark et al): With a BMI of 40 our patient had extreme obesity. The hyperdynamic circulation, with increased cardiac output, was thought to be a compensatory adaptation to increased adipose tissue. This may have led to non‐ischaemic dilated cardiomyopathy at the expense of left ventricular hypertrophy and remodeling as may occur in severely obese subjects (McGavock et al 2006). This is, however, unlikely as the only cause in our young patient. Human obesity is also characterized by sympathetic nervous system activation (Eikelis and Esler 2005). A possible obesity‐linked susceptibility to the toxic effect of methylphenidate could therefore play a role in the development of DCM in our patient, especially with regards to the short treatment time of one year |
| Hypertension | |
| Saieh 2004 | A case report of hospitalisation due to hypertension during MPH treatment Serious adverse events 72‐hour hospitalisation, emergency unit Abdominal pain for four days prior to hospitalisation, intermittent accentuation (hours), no other symptoms Secondary hypertension: Persistent hypertension (158/88‐170/105, pulse: 78‐98 bpm). Normal physical examination. Normal eye fundus and normal cardiological examination Discontinuation of MPH: Hypertensive treatment only necessary for 24 hours Normal blood pressure after one week |
Cardiovascular events
Two patient reports/series described cardiovascular events related to methylphenidate.
Munk 2015 reported a case of myocardial infarction related to methylphenidate exposure in a cardiac‐healthy, 11‐year‐old boy without any cardiovascular risk factors, who was being treated with methylphenidate 54 mg/day. Treatment duration was two years. The boy experienced a cardiac arrest following exercise, without any prior complaints about chest discomfort or shortness of breath. A week before the event, he had a short episode of tachycardia. The examination showed that the myocardial infarction was of an older date (more than weeks), due to thinning of the myocardium and an adversely remodelled left ventricle. A pacemaker was inserted and methylphenidate treatment was discontinued. The boy was very thoroughly examined and the only thing that stands out is the methylphenidate treatment.
Nymark 2008 reported a case of serious cardiomyopathy during methylphenidate treatment; a serious adverse event (hospitalisation) relating to hypoxia and dyspnoea following 11 months of methylphenidate treatment. On investigation, signs of liver, renal, and heart failure were found.
Hypertension
One patient report/series, Saieh 2004, described hypertension related to methylphenidate. Saieh 2004 described a patient who was hospitalised due to hypertension during methylphenidate treatment. Abdominal pain with intermittent accentuation for hours was present for four days prior to hospitalisation. Secondary hypertension: persistent hypertension (158/88 mmHg to 170/105 mmHg; pulse: 78 bpm to 98 bpm). The study authors reported normal physical examination, normal eye fundus, and normal cardiological examination. After discontinuation of methylphenidate, hypertensive treatment was only necessary for 24 hours. There was also normal blood pressure after one week.
3.1.4 Gastrointestinal system
Hepatoxicity
See Analysis 9.3. We found one patient report/series on hepatoxicity related to methylphenidate (Bernhard 2009). The study authors reported a hepatotoxic reaction; liver enzymes were elevated in a four‐year‐old boy prior to therapy. Vomiting started five weeks after onset of methylphenidate therapy and increased over three days. Abdominal pain increased in intensity. Liver transaminases were elevated over 30 times the normal level, and creatine kinase (CK) level also increased (Bernhard 2009).
9.3. Analysis.
Comparison 9 Patient reports/series: number of participants with serious adverse events, Outcome 3 Gastrointestinal system: hepatoxicity.
| Gastrointestinal system: hepatoxicity | |
|---|---|
| Study | |
| Bernhard 2009 | A hepatotoxic reaction Liver enzymes were elevated prior to therapy. Vomiting starting 5 weeks after onset of methylphenidate therapy and increased within the next 3 days. Abdominal pain increased in intensity. Liver transaminases elevated over 30 times of the normal level, CK level also increased |
3.1.5 Urogenital system
Priapism
See Analysis 9.4. We found two patient reports/series on priapism related to methylphenidate.
9.4. Analysis.
Comparison 9 Patient reports/series: number of participants with serious adverse events, Outcome 4 Urogenital system: priapism.
| Urogenital system: priapism | |
|---|---|
| Study | |
| Cakin‐Memik 2010 | A case report of a 14‐year‐old male with attention deficit hyperactivity disorder (ADHD) who presented with priapism after administration of immediate‐release methylphenidate. When the usage of immediate‐release methylphenidate was terminated, priapism spontaneously disappeared |
| Schwartz 2004 | A case report of a 15‐year‐old white male with ADHD (inattention subtype) who experienced painful erections two weeks after beginning sustained release (OROS) MPH and repeated painful erections that increased with increased doses (during withdrawal). MPH use for more than 2 months. It is likely that withdrawal from OROS methylphenidate was the cause of this adolescent boy's priapism. He was embarrassed to disclose the problem to an adult until it became quite severe. It is possible that this adverse event is under‐reported due to sensitivity and embarrassment of young people |
In Cakin‐Memik 2010, a 14‐year‐old boy with ADHD reported three to four episodes of priapism per day, lasting 40 to 45 minutes for three days after receiving 20 mg/day of immediate‐release methylphenidate.
In Schwartz 2004, a 15‐year‐old boy with ADHD also experienced intermittent painful erections two weeks after initiating OROS‐methylphenidate, which recently had been increased to 36 mg/day. The symptoms had not reoccurred two weeks or six months following cessation of methylphenidate.
No [patient reports/series] reported adverse outcomes related to the musculoskeletal, immune or other body systems,or on withdrawal of treatment due to serious adverse events, or adverse events of unknown severity.
3.2 Secondary outcomes: non‐serious adverse events
3.2.1 Frequency of any non‐serious adverse events
We found 45 patient reports/series of non‐serious adverse events and these studies reported 55 cases of non‐serious adverse events.
3.2.2. Central nervous system
See Analysis 10.5. We found 23 patient reports/series of non‐serious adverse events related to the central nervous system.
10.5. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 5 Central nervous system: other specific adverse events.
| Central nervous system: other specific adverse events | |
|---|---|
| Study | |
| Anorexia | |
| Findling 1996 | A case report about an 11‐year‐old girl who experienced transient side effects after receiving methylphenidate: mild and transient anorexia, transient weight loss of 2 kg observed in the initiation of methylphenidate therapy |
| Obsessive compulsive disorder (OCD) symptoms | |
| Coşkun 2011 | A case report of OCD symptoms during methylphenidate treatment. Decreased appetite and initial headache with 18 mg/day of osmotic‐controlled release oral delivery system (OROS) methylphenidate, and obsessive‐compulsive symptoms, decreased appetite, facial grimace, and nail picking/biting with 27 mg/day of OROS methylphenidate. Gradual disappearance of symptoms, although still subsyndromal obsessive‐compulsive symptoms, with discontinuation of medication. Mild facial grimace and similar obsessive‐compulsive symptoms with re‐administration of 27 mg/day of OROS methylphenidate. After three weeks, total score of 21 on Children's Yale‐Brown Obsessive‐Compulsive Scale (CY‐BOCS) |
| Woolley 2003 | Obsessions and compulsions with anxiety. DSM‐IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edtion) diagnosis of OCD. Washing hands excessively, which was accompanied by checking rituals, reassurance seeking, and emetophobia. When methylphenidate was withdrawn, OCD symptoms decreased rapidly but increased when methylphenidate was reintroduced |
| Headache | |
| Mize 2004 | A case report of headache and mild depression during methylphenidate treatment |
| EEG changes | |
| Dupuy 2008 | A controlled before‐and‐after study investigating the effects of stimulants on EEG (electroencephaolography) coherence in 9 girls with ADHD (attention deficit hyperactivity disorder); intrahemispheric and interhemispheric coherences in girls with ADHD who were treated with stimulant medication. Girls had elevated frontal coherence in all frequency bands |
Movement‐related outcomes
See Analysis 10.1
10.1. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 1 Central nervous system: movement‐related outcomes.
| Central nervous system: movement‐related outcomes | |
|---|---|
| Study | |
| Tics | |
| Adrian 2001 | A case report of a boy aged 10 years referred to a tertiary neurodevelopmental assessment clinic for a second opinion on the management of his ADHD. He started with 20 mg/day of methylphenidate at 7 years of age and gradually increased to 40 mg/day, for 2 to 3 years. Both motor and vocal tics started at 40 mg/day of methylphenidate and subsided spontaneously after a year of treatment Authors' conclusion: The clinically explosive outbursts of tics were coincident with a period of treatment with methylphenidate for the treatment of ADHD (attention deficit hyperactivity disorder) and should not be mistaken for a symptom of the disorder |
| Chandler 1989 | Reported non‐serious adverse events. After a month, the child's mother reported motor and phonic tics again. He was treated first with methylphenidate and then with nortriptyline. With both drugs he manifested severe facial grimacing and phonic tics; apparently, the tics had only occurred in the past when the child was treated with methylphenidate. It is, however, very difficult to attribute the tics to methylphenidate with any certainty because of the common co‐occurrence of tics in children and young people with ADHD Key conclusions of the study authors: Side effects, such as hypomania, hyperreflexia and diaphoresis, have been reported in patients being treated with a combination of a monoamine oxidase inhibitor and tryptophan. By itself, L‐tryptophan may be mildly sedating. In light of such a favourable side‐effect profile, combined with no evidence for adverse interaction with stimulants, and at least some rationale from preclinical research, it should be investigated further as a means of alleviating some of the harsh consequences of psychostimulant treatment in ADHD |
| Involuntary movements or dyskinesia | |
| Hollis 2007 | A case report of acute and transient dyskinesia occurring within hours of taking modified‐release methylphenidate (Concerta XL) in a stimulant‐naïve 7‐year‐old boy who had recently stopped taking risperidone Key conclusions of the study authors: This report is the first of a dyskinesia occurring after a single first dose of methylphenidate in a previously stimulant‐naïve patient after neuroleptic withdrawal. It is also the first to link this effect with modified‐release methylphenidate. The dyskinesia described here consisted of both brief tic‐like movements |
| Machado 2010 | Choreoathetoid movements of orofacial muscles, arms and legs, with transient dystonic postures of the right arm induced by a single dose of extended‐release methylphenidate. Author believes that the immediate response ensuing chlorpromazine prescription argues in favour of a specific role for dopamine receptor antagonists in methylphenidate‐induced chorea |
| McLaren 2010 | The patient experienced spasmodic muscular contractions of his jaw. The staff noticed a forceful jaw closure, contraction and tension, and the patient had difficulties opening his mouth. 50 mg of diphenhydramine was administered intramuscularly and the patient could open his mouth within 30 minutes and with no further dystonia |
Tics
Two patient reports/series described tics related to methylphenidate.
Adrian 2001 showed the presence of clinically explosive outbursts of tics in a 10‐year‐old boy boy with ADHD receiving 20 mg/day of methylphenidate at seven years, which over a two‐to‐three‐year period was increased to 40 mg/day.
Chandler 1989 described two ADHD patients: a boy aged 12 years receiving 0.2 mg/kg of methylphenidate twice a day who reported motor and vocal tics one month after treatment, and a 9‐year‐old boy treated with the same dose of methylphenidate and subsequent nortriptyline, who reported severe facial grimacing and vocal tics.
Involuntary movements or dyskinesia
Three patient reports/series described involuntary movements or dyskinesia related to methylphenidate.
Machado 2010 reported choreoathetoid movements of orofacial muscles, arms and legs, and dystonic postures of the right arm in a 6‐year‐old girl with ADHD after an initial dose of 18 mg of extended‐release methylphenidate.
McLaren 2010 showed spasmodic muscular contractions in the jaw of a boy aged 11 years old with ADHD after receiving a dose of 108 mg of OROS methylphenidate.
Hollis 2007 showed acute and transient dyskinesia within hours after treatment with 36 mg of modified‐released methylphenidate in a 7‐year‐old, stimulant‐naïve boy who had recently stopped taking risperidone.
Mood disorders
See Analysis 10.2
10.2. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 2 Central nervous system: mood disorders.
| Central nervous system: mood disorders | |
|---|---|
| Study | |
| Depression and disturbed mood | |
| Hechtman 2011 | A case report about a 5‐year‐old boy. MPH use for around a year. Reported non‐serious adverse events. In late afternoon and early evening, he became very emotional, with frequent crying, marked irritability and many tantrums. The emotional side effects subsided after he discontinued methylphenidate treatment |
| Mino 1999 | 3 weeks after starting on 10 mg/day of methylphenidate, the mother reported by telephone that the patient seemed depressed. The dose of methylphenidate was reduced to 5 mg/day. A week later the patient visited the clinic. The therapist diagnosed her condition as a depressive state and discontinued methylphenidate. 6 weeks after discontinuation of methylphenidate, she was diagnosed with a schizophrenic‐like psychotic state, due to symptoms of delusions of reference and persecution, delusional mood, silly smile and thought block. There was no evident hallucination. The patient took antipsychotic medication for 2 months and her psychotic symptoms disappeared |
| Niederhofer 2009 | An 11‐year‐old boy received 20 mg/day of methylphenidate for 2 months, which led to depressed mood and appetite loss |
| Irritability | |
| Hechtman 2011 | A case report about a boy, who became very emotional, with frequent crying episodes, marked irritability and many tantrums when treated with methylphenidate; methylphenidate was used for around a year |
| Sabuncuoglu 2007 | 3 case reports of hyperactivity and irritability during switch from risperidone to methylphenidate Case 1: Methylphenidate introduced 2 days after abrupt discontinuation of 1 mg/day of risperidone (8 months), and produced agitation, irritability, vigilance, and violent behavior. No dyskinetic movements. Methylphenidate was discontinued and the adverse events disappeared. After a 6‐week long, drug‐free period, methylphenidate was reintroduced at 15 mg/day. No adverse events were observed Case 2: Methylphenidate introduced after discontinuation of 1 mg/day of risperidone (2 years), and produced a near manic state, irritability, agitation, racing thoughts, and distractibility. No dyskinetic movements. Methylphenidate was discontinued and the adverse events disappeared. After a couple of months, methylphenidate was reintroduced at 15 mg/day and was well‐tolerated Case 3: Methylphenidate introduced after a 1‐week medication‐free interval after treatment with 1 mg of risperidone, and produced irritability, agitation and discomfort. Methylphenidate was discontinued and the adverse events disappeared. The patient did not retry the medication Key conclusions of the study authors: This report of 3 patients draws attention to a unique condition that arises in switching from an atypical antipsychotic to stimulant medication. A drug‐free interval is recommended in switching from risperidone to methylphenidate |
| Aggression | |
| Coignoux 2009 | A case report of a 14‐year‐old boy receiving methylphenidate for 2 years who developed gestural stereotypies and verbal aggressiveness towards his parents whilst on methylphenidate treatment |
| Niederhofer 2011 | A case series describing adverse effects (xerostomia, aggression and emotional instability) among children taking methylphenidate |
| Mania/euphoria | |
| Corrigall 1996 | A case report of methylphenidate euphoria in an 11‐year‐old boy diagnosed with hyperkinetic disorder Key conclusions of the study authors: Methylphenidate euphoria can occur in young prepubertal children and this may lead to abuse of medication |
| Ghanizadeh 2009 | Mother and nursery teacher complained about increased hyper‐talkativity starting about 45 minutes after taking medication. The increased hyper‐talkativity continued for about 3–4 hours. They scored the hyper‐talking as 7–9 on a 1–10 visual analogue scale. Re‐challenge was conducted many times (more than 20 times) and hyper‐talking reoccurred after every time he took the medication |
Depression and disturbed mood
Three patient reports/series described depression and disturbed mood related to methylphenidate.
Hechtman 2011 gave an account of a 5‐year‐old boy with ADHD receiving 35 mg of OROS methylphenidate titrated to 54 mg over one to two months who was exhibiting frequent crying, marked irritability and many tantrums, which subsided after methylphenidate treatment was discontinued.
Mino 1999 reported depressed mood three weeks after initiating methylphenidate treatment (10 mg/day) in a 16‐year‐old girl with ADHD.
Niederhofer 2009 found depressed mood and appetite loss in a boy aged 11 years after two months of daily dosages of 20 mg of methylphenidate.
Irritability
Two patient reports/series described irritability related to methylphenidate.
In Hechtman 2011, emotional mood, frequent crying, marked irritability and multiple tantrums were observed in a patient report/series of a 5‐year‐old boy with ADHD receiving 54 mg of OROS methylphenidate once a day for one to two months.
In Sabuncuoglu 2007, another three reports of irritability and agitation were reported in one boy and two girls aged 5, 6 and 15 years (all diagnosed with ADHD), upon switching from 1 mg/day of risperidone to 15 mg/day of methylphenidate.
Aggression
Two patient reports/series described aggression related to methylphenidate.
In Coignoux 2009, a 14‐year‐old boy receiving 54 mg/day of extended‐release methylphenidate for two years developed gestural stereotypes and verbal aggression towards his parents.
In Niederhofer 2011, two boys aged 10 and 11 years, who were receiving 20 mg/day of methylphenidate for two months, exhibited aggression and emotional instability.
Mania/euphoria
Two patient reports/series described mania/euphoria related to methylphenidate.
Corrigall 1996 reported euphoria in a 11‐year‐old boy with ADHD and hyperkinetic disorder, who received 10 mg/day of methylphenidate for four weeks, which subsequently increased to 15 mg/day for five days.
In Ghanizadeh 2009, hypertalkativity (scaled 7 to 9 on a 10‐point visual analogue scale) was found 45 minutes after taking 10 mg/day of methylphenidate in a 5‐year old boy, continuing for three to four hours. Re‐challenge was attempted more than 20 times, with hypertalkativity reoccurring on every consecutive attempt.
Sleep problems
See Analysis 10.3
10.3. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 3 Central nervous system: sleep problems.
| Central nervous system: sleep problems | |
|---|---|
| Study | |
| Disturbed sleep | |
| Langevin 2012 | A controlled before‐and‐after study. Methylphenidate used to treat ADHD (attention deficit hyperactivity disorder), from December (time 1) to June (time 2) Key conclusions of the study authors: The principal results support the study's hypothesis and show a significant baseline difference (P = 0.008) between the nocturnal movements of children with ADHD and those of children in the control group |
| Other sleep problems | |
| Arun 2014 | A case report of a child whose sleep was impaired for 1 night after receiving methylphenidate |
| Coşkun 2010 | A case series of 7 children who experience side effects during methylphenidate treatment: Sleep problems: n = 4 Worsening of pre‐existing sleep problems: n = 2 No worsening of pre‐existing sleep problems: n = 1 |
Disturbed sleep
One patient report/series described disturbed sleep related to methylphenidate (Langevin 2012). This controlled before‐and‐after study showed a significant baseline difference (P = 0.008) between the number of nocturnal movements in a group of five ADHD children receiving an unspecified methylphenidate dosage and five ADHD children not on stimulant treatment.
Other sleep problems
Two patient reports/series described sleep problems related to methylphenidate. Arun 2014 observed impaired sleep onset in an eight‐year‐old boy with ADHD who was receiving 5 mg/day of immediate‐release methylphenidate for two days. In Coşkun 2010, the following problems were reported in a patient series of seven children with ADHD who were receiving 10 mg to 54 mg or immediate‐release/OROS methylphenidate for 4 to 30 months: sleep problems (n = 4), worsening of pre‐existing sleep problems (n = 2), and no worsening of such problems (n = 1).
Voice and speech disorders
See Analysis 10.4
10.4. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 4 Central nervous system: voice and speech disorders.
| Central nervous system: voice and speech disorders | |
|---|---|
| Study | |
| Voice problems | |
| Yalcin 2012 | Teacher and family observed disturbance of voice quality, hoarseness, bifurcation‐strain and over vibration of voice. The symptoms began on the first day of treatment with methylphenidate. Hoarseness and reduction in the voice amplitude was evident during the first visit to a psychiatric out‐hospital clinic. No symptoms were observed by parents or during the psychiatric control on drug‐free days. Experienced hoarseness when methylphenidate administered in the clinic. Voice quality returned to normal three hours after ingestion. Laryngitis or other organic conditions causing hoarseness were not observed in physical and endoscopic examination. There were no important side effects with discontinuation of methylphenidate and administration of atomoxetine |
| Stuttering | |
| Alpaslan 2015 | A case report of a 7‐year‐old boy who developed stuttering associated with the use of methylphenidate; received 10 mg/daily of short‐acting methylphenidate. 10 days after beginning treatment with short‐acting methylphenidate, the client began to stutter. Treatment was stopped. 1 week later, the patient's speech was back to normal. 4 weeks later atomoxetine treatment was started with no recurrence of stuttering |
Voice problems
One patient report/series described voice problems related to methylphenidate (Yalcin 2012). In this study, a 10‐year‐old boy with ADHD who was receiving 5 mg of immediate‐release methylphenidate, exhibited disturbances of voice quality, hoarseness, bifurcation‐strain and voice over‐vibrations beginning on the first day of treatment, which was scheduled to last for two weeks.
Stuttering
One patient report/series described stuttering related to methylphenidate (Alpaslan 2015). This study found stuttering in a 7‐year‐old boy with ADHD 10 days after receiving 10 mg/daily of immediate‐release methylphenidate. The treatment was ended immediately, and one week later the stuttering ended.
Other specific adverse events
See Analysis 10.5
Anorexia
One patient report/series described anorexia related to methylphenidate (Findling 1996). In this study, an 11‐year‐old girl with ADHD received 5 mg of methylphenidate twice a day for three months and experienced mild and transient anorexia, resulting in a weight loss of 2 kg after treatment.
Obsessive compulsive disorder (OCD) symptoms
Two patient reports/series described OCD related to methylphenidate.
Coşkun 2011 found symptoms of OCD, facial grimaces, nail picking and biting in a 10‐year‐old girl with ADHD who was receiving 27 mg/day of OROS methylphenidate for six weeks. The symptoms gradually disappeared upon discontinuation of methylphenidate and reoccurred when re‐administering the same dosage at a later stage.
Woolley 2003 found obsessions and compulsions with anxiety, such as excessive handwashing, ritual checking and emetophobia, in an 11‐year‐old boy with ADHD and comorbid OCD upon receiving 40 mg/day of immediate‐release methylphenidate for more than 15 months. These symptoms decreased rapidly upon methylphenidate withdrawal and increased when it was later reintroduced.
Headache
One patient report/series described headache related to methylphenidate (Mize 2004). This study reported headache and mild depression in a 12‐year‐old boy after a methylphenidate dosage of 26 mg.
EEG changes
One patient report/series described EEG changes related to methylphenidate (Dupuy 2008). In this study, elevated frontal coherence in all frequency bands was shown on the EEG‐measurements of nine girls with ADHD who were administered methylphenidate over six months with unreported dosages.
3.2.3 Cardiovascular and respiratory system
See Analysis 10.6. We found four patient reports/series of non‐serious adverse events related to the circulatory and respiratory system (Grossman 1985; Karaman 2010; Yu 2010; Gracious 1999).
10.6. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 6 Cardiovascular and respiratory system.
| Cardiovascular and respiratory system | |
|---|---|
| Study | |
| Vasculopathy | |
| Yu 2010 | These case reports raise the concern that adverse effects in the peripheral vascular system of children and adolescents may be associated with psychostimulant treatment Patient 1: Tachycardia at age 11 while taking Concerta, 54 mg/d. Vasculopathy at age 16. His hands and feet were a continuous blue colour on his hands and fingers, which increased in frequency in cold weather. He was diagnosed with "decreased circulation but not Raynaud's syndrome". Occured when the Concerta dose was increased from 54 mg/d to 90 mg/d in 3 months with the same Focalin (dexmethylphenidate) dose (10mg/d) Patient 2: Vasculopathy. First diagnosed with Raynaud's syndrome with finger pain and colour changes during a neurology consultation at age 10 years. At age 12 years had diffuse erythema of both earlobes, the fingers of both hands, and the toes of his left foot. Tics at age 10 years but not clear if these were present prior to treatment for ADHD Patient 4: Vasculopathy. After taking Focalin for a year, he developed persistent curling of the toes of both feet. In addition, he had reddish and purple colour changes in his hands and feet with cold exposure that lasted for 20 minutes. No associated pain and only rare paraesthesia. At age 10 years, toes 2, 3 and 4 of both feet were held in a flexed position ("curled toes"), and there was skin discolouration and excoriation |
| Cardiovascular problems | |
| Karaman 2010 | A case report of a 15‐year old boy with ADHD who developed pulmonary arterial hypertension (PAH) during OROS MPH treatment Fourth day of treatment: The patient began to experience occasional episodes of slightly shortness of breath. Continued over several months. Not associated with either exercise or anxiety At 18th month of treatment: Fainting. Normal weight. No sign of allergy, hypersensitivity, or sleep apnoea. Mean pulmonary arterial pressure of 40 mmHg at rest, otherwise no pathological findings from extensive testing (Examination: clear lungs, no murmurs, rubs, or gallops, and otherwise unremarkable. Laboratory tests, including C‐reactive protein, thyroid and liver function tests, electrolytes. Blood gases, antinuclear antibodies, D‐dimers, chest X‐ray, ventilation–perfusion scintigraphy, electrocardiography, respiratory function tests. Echography. Transthoracic echocardiogram: normal except for the mean pulmonary arterial pressure). No use of any other drug. No history of alcohol and substance use and no symptoms or signs of MPH misuse (intravenous injection). The personal and familial histories were also negative for pulmonary or cardiovascular diseases Discontinuation of OROS‐MPH, one month: free of symptoms After the fourth week of discontinuation: Mean pulmonary arterial pressure of 28 mmHg |
| Bleeding | |
| Grossman 1985 | A case report of a 7‐year‐old girl on methylphenidate treatment who developed idiopathic trombocytopenic purpura (ITP). Physical examination at the paediatric outpatient department found countless petechiae over the entire dermal surface. Numerous areas of purpura were noted, especially over her buttocks and extremities. Multiple areas of buccal mucosal and gingival bleeding with a large hematoma presented on the left lateral surface of her tongue. Clotted blood was seen in her nostrils and ear canals bilaterally. Bone marrow aspiration showed normal to increased megakaryocytes with normal red and white cell precursors. MPH was stopped and the patient was admitted. After one week of treatment for the condition, her petechia had begun to fade. She did not start on MPH again. There has been no recurrence of petechiae or bruising one year later. MPH use for seven months prior to presentation of ITP |
| Tachycardia | |
| Gracious 1999 | A case report of atrioventricular (AV) nodal re‐entrant tachycardia during stimulant treatment Key conclusions of the study authors: Stimulant medication may evoke onset of AV nodal tachyarrhythmias in patients who have the potential to develop them, possibly in combination with a selective serotonergic reuptake inhibitor Comments from the study authors: The cardiologist consulted believed this patient had a structural vulnerability of genetic etiology for the arrhythmia which was then precipitated by the stimulant |
Vasculopathy
One patient report/series described vasculopathy related to methylphenidate (Yu 2010). This study observed signs of vasculopathy in three male patients. First, a 16‐year‐old boy was diagnosed with "decreased circulation but not Raynaud's syndrome", occurring when his methylphenidate (Concerta) dose was increased from 54 mg/day to 90 mg/day for three months while on a constant dose of dexmethylphenidate (Focalin) (10 mg/day). Second, Raynaud's syndrome with finger pain and colour changes was observed in a 10‐year‐old boy on 30 mg/day of methylphenidate hydrochloride (Ritalin LA) and 2.5 mg/day of dexmethylphenidate for five years. Third, reddish and purple colour changes in the hands and feet of an 11‐year‐old boy were observed whilst on a daily dose of 20 mg of dexmethylphenidate hydrochloride (extended‐release capsules) and 10 mg of immediate‐release dexmethylphenidate for one year.
Cardiovascular problems
One patient report/series described cardiovascular problems related to methylphenidate (Karaman 2010). In this study, a 15‐year‐old with ADHD developed pulmonary arterial hypertension (PAH) after 18 months of treatment with 54 mg/day of OROS‐methylphenidate. One month following a decision to discontinue OROS methylphenidate, the symptoms disappeared.
Bleeding
One patient report/series described bleeding related to methylphenidate (Grossman 1985). This study reported on a 7‐year‐old girl with ADHD receiving 10 mg of methylphenidate three times a day for seven months, who experienced idiopathic thrombocytopenic purpura (ITP). One week following a decision to end methylphenidate treatment, the petechia began to fade, and one year later there had been no reoccurrence of petechiae or bruising.
Tachycardia
One patient report/series described tachycardia related to methylphenidate (Gracious 1999). In this study, a 13‐year‐old girl with ADHD, who was administered 20 mg of methylphenidate twice daily for three months and subsequently 20 mg of sustained‐release methylphenidate for 11 months once daily, developed atrioventricular nodal re‐entrant tachycardia during treatment.
3.2.4 Gastrointestinal system
See Analysis 10.7. We found five patient reports/series of non‐serious, gastrointestinal adverse events (Agarwal 2008; Coşkun 2009a; Ghanizadeh 2008a; Rappaport 2004; Yalcin 2012).
10.7. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 7 Gastrointestinal system.
| Gastrointestinal system | |
|---|---|
| Study | |
| Loss of appetite | |
| Agarwal 2008 | A case report of the combination of atomoxetine and methylphenidate treatment of ADHD. Non‐serious adverse events: decreased appetite and delayed onset in sleep |
| Coşkun 2009a | IR MPH, 10–20 mg/day, co‐medication: valproate 400 mg/day. Decreased appetite, no weight decrease |
| Ghanizadeh 2008a | A case report of decrease in appetite during treatment with MPH |
| Rappaport 2004 | A case report of side effects during MPH treatment in a 14‐year‐old boy with ADHD and a number of other co‐morbid conditions (whilst taking immediate‐release MPH) |
| Yalcin 2012 | Significant appetite loss, drowsiness, disturbance of voice and hoarseness Disturbance of voice and hoarseness occurred on everyday since the first day of the medication. The symptoms started short after the single morning dose and decreased gradually to dinner time and disappeared before bedtime. Drug‐free days: no symptoms. Otolaryngology consultation: no organic pathology was detected with respect to clinical and endoscopic observation. Discontinuation of MPH and prescription of atomoxetine: no symptom of hoarseness and disturbance of voice quality |
| Nausea and vomiting | |
| Rappaport 2004 | A case report of side effects during MPH treatment in a 14‐year‐old boy with ADHD and a number of other co‐morbid conditions (whilst taking extended‐release MPH: Concerta) |
Loss of appetite
All five patients reports described loss of appetite related to methylphenidate.
Agarwal 2008 found decreased appetite in an 8‐year‐old boy with ADHD on 50 mg/day of immediate‐release methylphenidate and atomoxetine for six months.
Coşkun 2009a found loss of appetite but no weight decrease in an 6‐year‐old boy receiving 10 mg/day to 20 mg/day of immediate‐release methylphenidate and 400 mg/day valproate for two months.
Ghanizadeh 2008a reported significant decreases in appetite in a 8.5‐year‐old boy with ADHD and oppositional defiant disorder on 20 mg/day of methylphenidate for one month.
Rappaport 2004 found loss of appetite whilst on an unspecified dose of immediate‐release methylphenidate in a 14‐year‐old boy with ADHD and various comorbidities, who was comedicated with olanzapine.
Yalcin 2012 observed significant appetite loss in a 11‐year‐old boy with ADHD and comorbid specific learning disorder and social anxiety, who was receiving 18 mg/day of OROS methylphenidate for two weeks. The symptoms disappeared on drug‐free days.
Nausea and vomiting
One patient report/series also described nausea and vomiting related to methylphenidate (Rappaport 2004). This study found nausea and vomiting in the same 14‐year‐old whilst on an unspecified dose of extended‐release methylphenidate.
3.2.5 Musculoskeletal system
Growth
We found one patient report/series on reduced growth related to methylphenidate (Holtkamp 2002). This study found growth impairment in a 7‐year‐old boy with ADHD on 20 mg/day to 25 mg/day of methylphenidate for 19 months (Analysis 10.8).
10.8. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 8 Musculoskeletal system: growth.
| Musculoskeletal system: growth | |
|---|---|
| Study | |
| Holtkamp 2002 | A case report of growth impairment during MPH treatment Key conclusions of the study authors: One may conclude that some children are at risk of serious growth decrement when treated with MPH. The growth of children should thus be monitored carefully, even if there are no alarming gastrointestinal side effects from MPH. We found that the determination of growth velocity was a sensitive marker for the evaluation of growth impairment in our patient |
3.2.6 Immune system
Allergic reactions
See Analysis 10.9. We found two patient reports/series on allergic reactions related to methylphenidate.
10.9. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 9 Immune system: allergic reactions.
| Immune system: allergic reactions | |
|---|---|
| Study | |
| Confino‐Cohen 2005 | A case report of pruritic maculopapular skin rash developing during MPH treatment. The rash improved with antihistamines and re‐challenge at a lower dose caused the reappearance, though less severe, of the rash. Desensitisation through graded exposure of MPH in incremental doses prevented further rash development |
| Vashi 2011 | After 8 months of using an MPH patch the patient presented with pruritic dermatitis. She had itchy, burning, red lesions. Symptoms began on her hip at the area of patch placement, then progressively spread to her arms, legs, abdomen, and back. MPH discontinued: Symptoms lasted for 2 months. First and second patch test. Re‐tested with MPH patch: Nine days after the first test the patient presented with recall reaction, characterized by a return of the original pruritic dermatitis to her entire back, similar to the eruption that had occurred months previously after therapeutical use of MPH patch. Avoidance of MPH patches: symptom‐free |
Confino‐Cohen 2005 reported pruritic maculopapular skin rash during 10 mg/day methylphenidate treatment for one week in an 8‐year‐old girl with ADHD. The rash improved with antihistamines and, while re‐challenge at a lower dose caused reappearances of rash, the symptoms were less severe the second time around.
In Vashi 2011, a 9‐year‐old girl with ADHD receiving an unknown dosage of methylphenidate patches for eight months presented with pruritic dermatitis, with itchy, burning, red lesions on her arms, legs, abdomen and back, lasting two months following methylphenidate discontinuation. Similar rashes later reoccurred upon re‐testing methylphenidate treatment.
3.2.7 Urogenital system
See Analysis 10.10. We found five patient reports/series related to the urogenital system (Ramasamy 2014; Coşkun 2009b; Tang 2010; Ghanizadeh 2008b; Williamson 2011).
10.10. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 10 Urogenitial system.
| Urogenitial system | |
|---|---|
| Study | |
| Testicular failure | |
| Ramasamy 2014 | A case report of testicular failure possibly associated with chronic use of Methylphendiate. The patient was 20 years old with treatment duration of approximately 17 years with voluntary cessation a few years ago. Dosage varied with age. The patient's complaint was initially delayed puberty. He complained of high‐pitched voice, lack of libido, low energy level, chronic fatigue and poor erectile function. The patient was advised to begin testosterone supplementation. The patient described in the case study seemed to exhibit characteristics related to the effects of chronic use of methylphenidate on development of human reproductive function. The unknown effects of methylphenidate are currently being studied, but as can be seen, one should exercise caution and patients should be followed closely when prescribing methylphenidate. However, because the developmental changes that occurred in the human participant occurred over a number of years of treatment with methylphenidate, there is very little information about the patient's condition and how it developed during that period of time |
| Sexual adverse events | |
| Coşkun 2009b | Case 1: Treated with 10‐30 mg/day of immediate‐release methylphenidate and experienced initial headache and nausea. Treated with 18 mg/day osmotic‐controlled release oral delivery system (OROS) methylphenidate and experienced emotional side effects (sense of nervousness in the chest, occasional emotional numbing), multiple daily erections (unrelated to sexual stimuli, painless and without ejaculations), occurring a few hours after ingesting methylphenidate and lasting 5‐10 minutes. No erections unrelated to sexual stimuli during a 1‐week medication‐free period. With re‐administration of 35 mg/day OROS methylphenidate for 3 weeks, experienced re‐emergence of erections of a longer duration compared with 18 mg/day of OROS methylphenidate, headache, nausea, and the same emotional side effects. No erections on drug‐free days, and no erections with discontinuation of medication. Case 2: Treated with 18 mg/day of OROS methylphenidate and experienced loss of appetite, headache, abdominal pain, sleep problems, and conjunctival injection. Headache and abdominal pain almost disappeared after 1 week. Also experienced hypersexual behaviours, with morning erections lasting 1 hour before ingestion of methylphenidate, and a weight loss of 1.5 kg within 2 months. On drug‐free days had almost no hypersexual behaviour during the day and no erection the following morning. When treated with 10‐20 mg/day of immediate‐release methylphenidate, morning erections and hypersexual behaviours decreased dramatically, sleep and appetite problems improved mildly, and there was no conjunctival injection (after three weeks). There were, however, possible withdrawal symptoms (increased irritability and hyperactivity, getting tearful easily), several hours after each dosage. Had no morning erections or hypersexual behaviours with discontinuation of OROS methylphenidate |
| Incontinence | |
| Tang 2010 | Side effects reported by the mother: IR‐MPH, 10 mg, no side effects. OROS‐MPH, 18 mg/d, no side effects. OROS‐MPH, 36 mg/d, double incontinence; stool incontinence (every day, frequent during daytime) and urinary incontinence (almost every day). The double incontinence completely resolved rapidly after the discontinuation of OROS methylphenidate 36 mg |
| Enuresis | |
| Ghanizadeh 2008b | A case report of nocturnal enuresis during MPH treatment Non‐serious adverse events MPH, titrated to 20 mg/day: Nocturnal enuresis Discontinuation of MPH: enuresis stopped immediately MPH, when titrated to 20 mg/day: Immediate re‐occurence of nocturnal enuresis Discontinuation of MPH: enuresis stopped immediately MPH, when titrated to 20 mg/day: Immediate re‐occurence of nocturnal enuresis Discontinuation of MPH: enuresis stopped immediately |
| Williamson 2011 | Two case reports showing resolution of enuresis when treated with MPH |
Testicular failure
We found one patient report/series on testicular failure related to methylphenidate (Ramasamy 2014). In this study, a 20‐year‐old male patient with ADHD, treated with methylphenidate for approximately 17 years at various doses, reported testicular failure and poor erectile function.
Sexual adverse events
One patient report/series described serious adverse events related to methylphenidate (Coşkun 2009b). This study reported multiple, daily, painless erections unrelated to any sexual stimuli and without ejaculation in a 15‐year‐old boy with ADHD administered 10 mg/day to 30 mg/day of immediate‐release methylphenidate for one month, 18 mg/day of OROS methylphenidate for one month and 36 mg/day of OROS methylphenidate for three weeks. They also found hypersexual behaviour and morning erections prior to ingesting methylphenidate, with no such behaviour on drug‐free days, on 18 mg/day of OROS‐methylphenidate, which were dramatically reduced on 10 mg/day to 20 mg/day of immediate‐release methylphenidate, only to return in fluctuations on 18 mg/day of OROS methylphenidate (with no hypersexual behaviour on drug‐free days).
Incontinence
One patient report/series described incontinence related to methylphenidate (Tang 2010). In this study, almost daily urinary incontinence on 36 mg/daily of OROS methylphenidate for two weeks was observed in an 8‐year‐old boy with ADHD, which was rapidly and completely resolved after discontinuation of the treatment dose.
Enuresis
Two patient reports/series described enuresis related to methylphenidate.
In Ghanizadeh 2008b, 20 mg/day of methylphenidate was titrated and discontinued three times for an 11‐year‐old boy with nocturnal enuresis occurring and ceasing each consecutive time.
Williamson 2011 also found resolution of daily enuresis in a 9‐year‐old boy with ADHD on 54 mg/day of extended‐release methylphenidate and an 11‐year‐old girl with ADHD on 10 mg/daily of extended‐release dexmethylphenidate for a short‐term period.
3.2.8 Other body systems
See Analysis 10.11. We found five patient reports/series of other, non‐serious adverse events (Cohen 1992; Coşkun 2009a; Ghanizadeh 2008c; Lewis 2012; Ririe 1997).
10.11. Analysis.
Comparison 10 Patient reports/series: number of participants with non‐serious adverse events, Outcome 11 Other body systems.
| Other body systems | |
|---|---|
| Study | |
| Skin reactions | |
| Cohen 1992 | Case 1: 5 days of MPH treatment: hospitalisation due to two days of severe swelling and redness of the scrotum The skin eruption resolved spontaneously four days after MPH was discontinued. Two weeks later, 18 hour after MPH retrial, the same skin eruption of the scrotum was documented. Discontinued once again, and followed by a complete resolution of the rash in four days Case 2: 7 days of MPH treatment: Six hours of severe swelling and redness of the scrotum. Discontinuation of MPH was followed by a complete resolution of rash in three days.Rechallenge with MPH two months later was followed by the same skin eruption of the scrotum within two days. Complete resolution was seen after drug withdrawal |
| Coşkun 2009a | OROS‐MPH 18 mg/day, co‐medication: valproate 400 mg/day. Maculopapular pruritic skin eruptions on the patient's neck, arms, and legs, one week after starting OROS MPH treatment Re‐administering of OROS‐MPH 18 mg/day, comedication: gabapentine 300 mg/day. Same skin eruptions with same severity, nine days after starting OROS‐MPH treatment again Discontinuation of medication: skin lesions abated within the next several weeks Restart of IR‐MPH, 10–20 mg/day, co‐medication gabapentine 300 mg/day, four months: no skin eruptions |
| Problems with sedation | |
| Ririe 1997 | A case report (6‐year‐old boy) on unexpected interaction of methylphenidate (Ritalin) with anaesthetic agents: There could be a potential risk of unwanted interactions between methylphenidate and anaesthetic agents. Based on the observations from the case report, a more detailed and systematic investigation of methylphenidate in patients undergoing anaesthesia or sedation is warranted |
| Eye problems | |
| Ghanizadeh 2008c | Photophobia, occurring a few days after initiation of MPH treatment Discontinuation of MPH at least three times: no symptoms of photophobia Re‐administration of MPH: immediate reoccurrence of photophobia |
| Lewis 2012 | This report concluded stimulant medication (MPH) should not be withheld in patients with glaucoma as long as intraocular pressure (IOP) remains well controlled |
Skin reactions
Two patient reports/series described skin reactions related to methylphenidate.
Cohen 1992 found severe swelling and redness of the scrotum in an 8‐year‐old boy with ADD, which spontaneously resolved four days after the methylphenidate dose of 10 mg/day was discontinued. Eighteen hours after methylphenidate retesting, the same skin eruption was documented, which again was resolved after another discontinuation of treatment. A 10‐year‐old boy from the same study, who was given the same methylphenidate dose, reported six hours of severe swelling and skin eruption of the scrotum within two days following treatment. The reactions were resolved upon drug withdrawal.
Coşkun 2009a found maculopapular pruritic skin eruption in an 8‐year‐old boy with ADHD following treatment with 18 mg/day of OROS methylphenidate and 400 mg/day of valproate. When the medication was discontinued, the skin lesion abated within weeks, and did not reoccur upon when restarting 10 mg/day to 20 mg/day of immediate‐release methylphenidate and 300 mg/day of gabapentine.
Problems with sedation
One patient report/series described sedation problems related to methylphenidate (Ririe 1997). This study reported difficulty with conscious sedation in a 6‐year‐old boy with ADHD on 10 mg/day of immediate‐release methylphenidate, who had a comorbid history of William's syndrome and supravalvar aortic stenosis. He remained alert and unable to lie still despite being given oral doses of sedatives, pointing to a potential interaction between methylphenidate and the anaesthetic agents.
Eye problems
Two patient reports/series described eye problems related to methylphenidate.
In Ghanizadeh 2008c, photophobia occurred a few days after initiation of 35 mg/day of methylphenidate treatment in a 7‐year‐old boy. The symptoms ceased and reoccurred upon discontinuation and readministration of methylphenidate.
In Lewis 2012, during 18 mg/day of extended‐release methylphenidate, which was titrated to 54 mg/day over six months, a 10‐year‐old girl with ADHD showed intraocular pressure in the 16 mmHg to 19 mmHg range bilaterally in one study.
No [patient reports/series] reported on withdrawal of treatment due to non‐serious adverse events or for unknown reasons.
Subgroup analyses
We conducted the subgroup analyses below using the Comprehensive Meta Analysis software (analyses not shown).
Duration of methylphenidate treatment
We found no significant difference (P = 0.83) between the proportion of participants on methylphenidate with any serious adverse events in short‐term (less than six months: 1.20%, 95% CI 0.7% to 2.1%; 41 studies, 159,407 participants) versus long‐term (six months or more: 1.1%, 95% CI 0.3% to 3.4%; 10 studies, 3015 participants) non‐comparative studies.
We found no significant difference (P = 0.74) between the proportion of participants on methylphenidate with any non‐serious adverse events in short‐term (less than six months: 52.3%, 95% CI 52.3% to 39.6%; 35 studies, 11,411 participants) versus long‐term (six months or more: 48.9%, 95% CI 33.7% to 64.3%; 14 studies, 2567 participants) non‐comparative studies.
Comorbidity
We found no significant difference (P = 0.21) in the proportion of participants experiencing non‐serious adverse events between those with ADHD and any comorbidity (54.2%, 95% CI 42.7% to 65.5%; 40 trials, 13,039 participants) versus those with ADHD and no comorbidities (40.4%, 95% CI 24.5% to 58.7%; 9 trials, 939 participants) in non‐comparative cohort studies of methylphenidate.
We could not carry out the subgroup analysis on serious adverse events due to insufficient data on comorbidity.
Concurrent medication‐intervention
We found a significant difference (P = 0.011) in the proportion of participants with ADHD experiencing any non‐serious adverse events between those taking methylphenidate and no other medication (53.6%, 95% CI 42.9% to 64.0%; 43 trials, 12547 participants) versus those also taking other medications (27.1%, 95% CI 14.8% to 44.3%; 6 trials, 1431 participants) in non‐comparative cohort studies.
We could not carry out the subgroup analysis on serious adverse events due to insufficient data on concurrent medication.
Dose of methylphenidate
We found no significant difference (P = 0.062) in the proportion of participants experiencing any non‐serious adverse events between those on low‐dose (less than 20 mg/day: 45.3%, 95% CI 33.7% to 57.5%; 35 studies, 159,278 participants) versus high‐dose (20 mg/day and above: 64.4%, 95% CI 48.4% to 77.7%; 14 studies, 3144 participants) methylphenidate in non‐comparative studies.
We could not carry out the subgroup analysis on serious adverse events due to insufficient data on dose of methylphenidate.
Age of participants
We found no significant difference (P = 0.23) in the proportion of participants on methylphenidate with any non‐serious adverse events between non‐comparative studies where the mean participant ages are 10 years or more (53.8%, 95% CI 37.2% to 69.5%; 22 studies, 159,278 participants) versus those where the mean participant ages are less than 10 years (47.1%, 95% CI 36.0% to 57.8%; 27 studies, 3144 participants) in non‐comparative studies.
We could not carry out the subgroup analysis on serious adverse events due to insufficient data on age of participants.
Study design
We found a significant difference (P = 0.01) between the proportion of participants on methylphenidate with any serious adverse events in the non‐comparative, classic cohort studies (1.10%, 95% CI 0.60% to 2.00%; 32 studies, 159,761 participants) versus those in cohort studies from RCTs assessing methylphenidate versus other intervention for ADHD (1.60%, 95% CI 1.00% to 2.30%; 18 studies, 2661 participants)
We found no significant difference (P = 0.17) between the proportion of participants on methylphenidate with any non‐serious adverse events in the non‐comparative, classic cohort studies (47.10%, 95% CI 35.6% to 58.9%; 36 studies, 13,035 participants), compared to those in cohort studies from RCTs assessing methylphenidate versus other intervention for ADHD (62.1%, 95% CI 44.4% to 77.1%; 13 studies, 943 participants)
Study funding
We found no significant difference (P = 0.22) between the proportion of participants on methylphenidate with any serious adverse events in non‐comparative cohort studies funded by industry (1.0%, 95% CI 0.6% to 1.6%; 30 studies, 137,152 participants) versus those not funded by industry (1.80%, 95% CI 0.80% to 4.10%; 20 studies, 25,270 participants); however, when looking only at the point estimate there seems to be 0.8% more serious adverse events in the group of studies not funded by industry.
On the other hand, in non‐comparative cohort studies we did find a significant difference (P = 0.02) in the proportion of participants on methylphenidate with any non‐serious adverse events between studies funded (41.6%, 95% CI 32.8% to 51.0%; 27 studies, 7981 participants) versus those not funded (64.2%, 95% CI 46.9% to 78.5%; 22 studies, 5997 participants) by industry.
Sensitivity analyses
We conducted a sensitivity analysis to test the robustness of our findings using the alternative, fixed‐effect model, for the outcome of non‐serious adverse events. In most analyses, there were no significant differences between the two statistical models. In cases where there was a statistical difference, the fixed‐effect model mostly showed a higher proportion of adverse events. Therefore, the decision to report the results of the random‐effects model was a conservative one.
We also conducted a sensitivity analysis to test the robustness of our findings to the removal of patient‐control studies from the analysis, for the outcome of any serious adverse events; we found no statistical difference (Analysis 1.1).
Discussion
Summary of main results
Our search found 260 relevant non‐randomized studies from 431 reports consisting of: 7 comparative cohort studies, 4 patient‐control studies, 177 non‐comparative cohort studies, 70 patient reports/series, and 2 cross‐sectional studies. We reported adverse events data from 2,283,509 participants worldwide, of both genders, aged between 3 and 20 years. The risk of bias in the comparative studies ranged from moderate to critical, whereas all non‐comparative studies were at critical risk of bias. The quality of evidence for all outcomes was very low according to GRADE standards. We found that the occurrence of serious adverse events in the comparative cohort and patient‐control studies was 1.36 times more frequent in participants that used methylphenidate when compared to controls. In cohort studies without a control group, we found a low proportion (1.20%) of any serious adverse events. Out of these, 22 studies had adverse events data for the central nervous system, 5 studies for the cardiovascular and respiratory system, 2 studies for the musculoskeletal system, and 1 study apiece for the gastrointestinal and immune systems. The largest proportion (51.2%) of non‐serious adverse events due to methylphenidate use were found in cohort studies without a control group. However, this can be interpreted as an imprecise finding due to the large CI. In non‐comparative cohort studies, the proportion of participants withdrawn from methylphenidate treatment due to: serious adverse events was 1.20%; adverse events of unknown severity, 7.30%, non‐serious adverse events, 6.20%; and unknown reasons, 16.2%.
Subgroup analyses
We conducted subgroup analyses for studies that included children who:
received concurrent medication versus no concurrent medication;
had comorbidities versus those who had none;
were younger versus older than 10 years of age;
received methylphenidate for six months or more versus less than six months; and
received a higher dosage of methylphenidate (20 mg/day and above) versus a lower dosage.
We also conducted a subgroup analysis in which we compared studies with higher‐quality designs (i.e. RCTs) to those of lower quality (cohorts). We concluded that adverse events reported in observational studies do not seem to depend on comorbidity or age of participants, dose or duration of methylphenidate treatment, or study design (non‐serious adverse events). However, we observed fewer adverse events in concurrent‐medication users. These subgroup analyses suggest that the most common confounding factors do not affect the proportions of adverse events. We discovered differences when comparing the proportion of participants on methylphenidate with any serious adverse events in the non‐comparative classic cohort studies to the proportion of participants on methylphenidate with any serious adverse events in the cohort studies from the RCTs, and thus reported these separately.
Finally, we conducted subgroup analyses investigating whether there were differences in the proportions of adverse events in the studies funded versus not funded by pharmaceutical companies. We found that studies funded by pharmaceutical companies had a much lower proportion of both serious and non‐serious adverse events. This is worrying, as it shows that adverse events are underestimated in industry‐sponsored studies, confirming findings from previous systematic reviews (Lundh 2012; Lundh 2017). This underpins our statements in this review that the proportions of adverse events are likely underestimated and that the proportions would likely have been higher if all studies had been conducted without industry funding.
Overall completeness and applicability of evidence
To our knowledge, this is the first systematic review that assesses adverse events of methylphenidate in children and adolescents from non‐randomised studies. The included studies investigated adverse event profiles of children in different countries, settings, time periods and clinical practice. Studies were conducted over approximately 10 years with a great deal of inconsistency in reporting and classifying adverse events. In addition, there are limited data on comorbidities and medicine use.
Surprisingly, we found few comparative non‐randomised studies. Many of the comparative studies had healthy controls and therefore could not be included as comparative studies. From these studies, however, we included those receiving methylphenidate as an observational cohort study. Accordingly, most of the data in this review come from non‐comparative cohort studies (including methylphenidate groups from RCTs assessing methylphenidate versus other interventions for ADHD, and follow‐up periods from RCTs), comprising 2,207,751 participants. These data may therefore be biased due to the absence of a control group and through uncontrolled confounding factors. Thus, readers should consider the very low quality of the included studies when interpreting the findings of this review.
Cortese 2015 reported headache and sleep disorders as serious adverse events. Whilst these may usually be considered non‐serious adverse events, it is in keeping with their definition of adverse events: "Serious adverse events (AEs) were classified as severe if their occurrence was followed by active notification by clinical centers to the Italian Medicines Agency; otherwise, they were labelled as mild. The Italian Medicines Agency requires active notification when an AE [adverse event] results in death, is life‐threatening, requires hospitalisation or prolongation of existing inpatients’ hospitalisation, results in persistent or significant disability or incapacity, or leads to a congenital anomaly or birth defect." This definition is 100% in accordance with the ICH‐GCP definition of serious adverse events (ICH 1996).
To expand our understanding of adverse events, particularly where these are rare or take time to become apparent, it is necessary to bolster the limited data from RCTs by including data from non‐randomised studies (Storebø 2015). There are a number of advantages to non‐randomised studies.
Despite the fact that these non‐randomised studies may have a higher risk of bias as regards adverse events, they are the only data available and should still be considered in the evaluation of methylphenidate as a treatment. One may debate whether controlling for confounders is necessary when determining adverse events, given that they are less likely to occur when an outcome is unintended, as opposed to an intended exposure effect (Golder 2011).
The findings from this review underscore the fact that information on long‐term adverse events in the use of methylphenidate is very limited. The increased use of methylphenidate, therefore, raises concerns about possible harms, particularly regarding unknown long‐term risks.
We found new data on unexpected adverse events, and this information is important with regard to identifying unrecognised harmful effects. Most reported adverse events were non‐serious. This information is now available to regulators so they may update the SPC with respect to frequency estimates and completeness of adverse event information.
Few scales were used to define and/or evaluate adverse events. Assessment of causality of adverse events was also poorly reported.
Quality of the evidence
Due to the presence of a large number of cohort studies without a comparator, it was only possible to evaluate the quality of the evidence for a small number of studies. These evaluations confirmed that non‐randomised studies monitoring adverse events are at critical risk of bias. The high degree of under‐reporting in these studies, particularly within cohort studies, is of great concern.
High risk of bias in RCTs has been shown to overestimate benefits and underestimate harms, and this is especially common in trials funded by industry (Lundh 2017). Thus, one can presume that the proportions we found are at the lower side of the estimate spectrum (Gluud 2008;Kjaergard 2001;Lundh 2017; Moher 1998;Savović 2012;Schulz 1995;Wood 2008); pharmaceutical companies funded 53 out of the 177 non‐comparative cohort studies included in our review. Furthermore, 11 other studies had authors with connections to pharmaceutical advisory boards, whilst 45 of 177 studies did not report source of funding.
In non‐randomised studies, a number of biases can indeed affect the estimation of the efficacy outcome. However, adverse events are not usually influenced by these sources given their unpredictable nature (Loke 2011).
Furthermore, the use of rating scales to more reliably assess adverse events was uncommon, so we believe that the proportions reported here are an underestimation of the true value (Pagsberg 2017).
The findings of this review should be interpreted whilst bearing in mind the very low quality of the included studies and their methodological limitations. Many of the uncontrolled confounding factors that might limit the value of this review, for example, duration of methylphenidate treatment, dosage of methylphenidate, comorbidity, age of participants, or study design (non‐serious adverse events), do not seem to affect the estimates when investigated in subgroup analyses. It is possible and even likely that the adverse event profile of methylphenidate could be worse than what we report here (Aagaard 2011).
Adverse events in non‐randomised studies are often the only data available on such outcomes. This is why they are still used to evaluate adverse events (Golder 2011). Moreover, the duration of most RCTs is seldom long enough to detect adverse events that may only appear after a lengthy duration (Ioannidis 2009). Schroll 2016 showed that only 3% to 33% of the total number of investigator‐reported adverse events from RCTs were reported in the clinical study reports. This further underlines the need to include non‐randomised studies when assessing harms.
Our aggregation and assessment of patient reports/series revealed a number of serious adverse events occurring during treatment with methylphenidate. We included patient reports/series of serious adverse events that developed over several years of treatment. These reports indicate a need for more comprehensive monitoring in order to allow early detection of possible serious adverse events.
Additionally, most study authors call for increased screening before commencing methylphenidate treatment. Examples of such procedures could be obtaining an ECG or an extensive family history of somatic and psychiatric illness. These measures cannot prevent all patients from developing serious adverse events as these can still occur in apparently healthy, screened patients. We therefore highlight the 'best practice' of both thorough pre‐treatment screening and continuous monitoring of the individual patient during treatment.
It is important to acknowledge the limitations of patient reports/series. Again, we must stress the fact that patient reports/series are not representative and do not necessarily reflect a cause‐effect relationship. As such, we cannot generalise data based on our included patient reports/series. The quality of our included patient reports/series also varied, and they rarely followed the standards set by CARE (CAse REport) Guidelines (Gagnier 2013). This is a common issue relating to patient reporting that has repercussions in terms of their utility as a source of evidence to inform clinical practice. It is crucial to expand both the knowledge and use of reporting guidelines to enhance the quality of published patient reports/series in the future.
One of the merits of patient reports/series is their ability to stimulate the generation of hypotheses. Based on the results of this review, one could hypothesise that the unknown severity of adverse events (which is likely to be higher than is currently recognised) and their heterogeneous characteristics, needs to be weighed up against the possible benefits of methylphenidate.
It is also central to good practice that clinicians and researchers improve their formal reporting of adverse events in order to assess more accurately the extent of adverse events experienced. Such reporting, combined with more methodologically sound research, could ensure informed, clinical decision‐making by health professionals, patients, and the public.
Not all clinical studies get published, leading to publication bias (Papanikolaou 2004). This is especially so in older studies with negative results for efficacy and safety (Ioannidis 1998). As methylphenidate was licensed in the 1960s, publication bias may well be significant, especially as many of the included studies were funded by pharmaceutical companies (Lundh 2017).
Potential biases in the review process
We excluded 112 studies (121 reports) because outcomes were outside the focus of this review (e.g. visual attention test, reaction time, memory skills, and functional abnormalities, see Excluded studies). This raises the issue of bias in our review process as we did not write to these authors asking whether they collected data on other outcomes. This potential bias, however, is not likely to change our conclusions.
With respect to identifying unpublished studies, we contacted relevant pharmaceutical companies, but none of them responded to our requests. The letter sent to pharmaceutical companies can be seen at Zenodo.org (see supplementary file 'Letter to pharmaceutical companies').
We excluded methylphenidate treatment groups from placebo‐controlled trials because these are published in our systematic review comparing methylphenidate versus placebo or no intervention (Storebø 2015). While this was done to avoid duplicate reporting (Storebø 2015), it potentially introduces bias by reducing the amount of data available for analysis. It is noteworthy that the RR for the harms outcomes from placebo‐controlled trials indicate that methylphenidate carries similar risks of serious and non‐serious adverse events as placebo (Storebø 2015). We found an increased likelihood of rare and serious harms in the present study, which differs from our previous work. There are two possible explanations for this. The first is that it could be due to confounders. The second is that the average exposure to methylphenidate in these studies is longer, given that they are observational studies rather than the relatively short durations of the RCTs. It is possible that longer average exposure to methylphenidate could have given rise to this increased likelihood of rare and serious harms (or both).
We decided to combine different types of study designs in the cohort study group; classic cohort studies, treatment groups from RCTs (similar to classic cohort studies), and cross‐sectional studies. The differences between these study designs have been investigated in subgroup analyses and reported separately when necessary.
We conducted systematic literature searches over a long period and continuously revised our search strategy in order to detect all possible relevant studies. We excluded many studies due to a lack of information about adverse events despite efforts to contact the authors for these data. We therefore assume that the current systematic review is the most up‐to‐date and comprehensive one in this area of research.
We included a few new brand names in the January 2016 search. We only searched for the new terms in the revised adverse event strategy from 2016, so there is a small risk that we might have missed a few studies.
One limitation of our review is that we were not able to assess the quality of all of the non‐comparative cohort studies using ROBINS‐I. We have, however, assessed all of these studies as being at critical risk of bias. Another limitation arises from the lack of some sensitivity analyses. However, we performed many subgroup analyses to investigate various possible sources of heterogeneity.
In many countries there has been a large increase in the abuse of methylphenidate. It is therefore important to warn the scientific community about possible methylphenidate abuse, especially in individuals with drug dependence (Frauger 2016). In Iceland, intravenous methylphenidate abuse is very common in patients with substance use disorder. This suggests that methylphenidate has high abuse potential (Bjarnadottir 2015). It is a limitation that we did not collect data on methylphenidate abuse in this review.
We did not identify any other potential biases in the review process.
Agreements and disagreements with other studies or reviews
In this study, we reported on a small number of serious adverse events affecting a substantial proportion of participants, which is concerning.
Methylphenidate exposure in children and adolescents with a diagnosis of ADHD is associated with arrhythmia and potentially with myocardial infarction during periods of use. It may also be associated with psychotic disorder (Ramstad 2018).
The high proportion of withdrawals due to adverse events of unknown severity affected almost 10% of participants. This contrasts with the findings in our systematic review of 185 RCTs, in which fewer than 10% reported serious adverse events and fewer than a third reported adverse events at all (Storebø 2015). We observed many of the previously described adverse events, including abdominal pain, decreased appetite, tics, and headache in our present review. These findings are consistent with the results of our Cochrane Review investigating the short‐term effects of methylphenidate use in RCTs (Storebø 2015).
We found evidence for previously reported serious adverse events, including psychosis, arrhythmia, myocardial infarction, seizures, and hypertension. The large cohort study by Hennessy 2010 reported no risk of serious adverse events, including cardiac problems. We excluded this large cohort study because it included children who were not diagnosed with ADHD. As an industry‐sponsored cohort study it may well be biased (Lundh 2017). Furthermore, the median follow‐up for the control group was 611 days versus only 91 days for methylphenidate users. This could increase the potential for under‐reporting of adverse events in the methylphenidate group. The Shin 2016 study suggests that the relative risk of myocardial infarction and arrhythmias was increased in the early period after initiation of methylphenidate treatment. Their findings should be interpreted with caution, according to the authors themselves, due to the potential inaccuracies in coding and incompleteness of records. The outcome measures were limited to patients with diagnoses of cardiovascular adverse events, and they could have missed such outcomes where cardiovascular events were present but not diagnosed. A great strength of this study, however, is that it included the entire South Korean population and used the national health insurance claims database for all patients with ADHD. A large cohort study including 1.8 million pregnancies found a small increase in the risk of cardiac malformations (adjusted relative risk 1.28, 95% CI 0.94 to 1.74) associated with intrauterine exposure to methylphenidate (Huybrechts 2018).
In our systematic review of RCTs, we found the proportion of participants with one or more serious adverse events to be 2.80% (95% CI 1.70% to 4.80%; 9 trials, 613 participants) in the control group, and only 1.9% (95% CI 1.10% to 3.20%; 9 trials, 919 participants) in the methylphenidate group (Storebø 2015). In the present review, we found that the proportion of serious adverse events was 1.20% (95% CI 0.70% to 2.00%; 50 studies, 162,422 participants) for non‐comparative studies.
One of the excluded studies suggested an association between the use of stimulants and sudden unexplained death among children and adolescents (Gould 2009). This was a patient‐control study using a control group of healthy participants, so it did not meet our inclusion criteria (see Criteria for considering studies for this review). However, it is an important study regarding sudden death among children using methylphenidate, supporting our findings regarding this risk.
To get an idea of the clinical relevance of the proportion of non‐serious adverse events in the uncontrolled cohort studies, we compared the data with the methylphenidate and placebo groups in our Cochrane Review of RCTs (Storebø 2015). We also compared our results with the official SPC from Denmark (Danish Health and Medicines Authority 2017), the UK (MHRA 2017), and the USA (US Food and Drug Administration (FDA) 2017) (Table 3). It is striking that there is no information about total non‐serious adverse events in any of these countries. This is worrying and comparable to the findings in our published Cochrane Review, where only a small number of the included RCTs reported on non‐serious adverse events. Some would say that this is due to the fact that there are very few non‐serious adverse events, but we are concerned that we simply do not know this because the data to determine it are lacking.
2. Data on adverse events from published systematic review on methylphenidate versus placebo or no intervention (Storebø 2015), National Summary of Product Characteristics, and from the present review.
| Adverse event | Randomised clinical trials: methylphenidate group (from Storebø 2015) | Randomised clinical trials: placebo or no intervention group (from Storebø 2015) | National Summary of Product Characteristics (UK, USA, DK) | Non‐comparative cohort studies and cohort studies from randomised trials (present review) | Non‐comparative cohort studies (present review) | Non‐comparative cohort studies from randomised trials (present review) |
| Serious adverse events | 1.90% (95% CI 1.10% to 3.20%; 9 studies, 919 participants) | 2.80% (95% CI 1.70% to 4.80%; 9 studies, 613 participants) | No information | 1.20% (95% CI 0.70% to 2.00%; 51 studies, 162,434 participants) | 1.10% (95% CI 0.60% to 2.00%; 32 studies, 159,761 participants) | 1.60% (95% CI 1.00% to 2.30%; 18 studies, 2661 participants) |
| Non‐serious adverse events | 51.4% (95% CI 41.9% to 60.9%; 21 studies, 1861 participants) | 38.3% (95% CI 30.3% to 47.0%; 21 studies, 1271 participants) | No information | 51.2% (95% CI 41.2% to 61.1%; 49 studies, 13,978 participants) | 47.1% (95% CI 35.6% to 58.9%; 36 studies, 13,035 participants) | 62.1% (95% CI 44.4% to 77.1%; 13 studies, 943 participants) |
| Headache | 11.6% (95% CI 8.80% to 13.3%; 17 studies, 1642 participants) | 9.40% (95% CI 7.10% to 12.4%; 17 studies, 1082 participants) | 1% to 10% | 14.4% (95% CI 11.3% to 18.3%; 90 studies, 13,469 participants)a | 9.90% (95% CI 7.00% to 13.9%; 57 studies, 10,929 participants | 24.3% (95% CI 18.0% to 32.1%; 33 studies, 2540 participants) |
| Anxiety | 6.50% (95% CI 1.20% to 29.2%; 3 studies, 356 participants) | 12.4% (95% CI 8.30% to 18.0%; 3 studies, 240 participants) | 1% to 10% (UK and DK); no information (USA) | 18.4% (95% CI 11.3% to 28.7%; 22 studies, 1287 participants)a | 10.2% (95% CI 5.30% to 18.9%; 8 studies, 938 participants | 27.9% (95% CI 17.8% to 40.8%; 14 studies, 349 participants |
| Sleep difficulty | 8.00% (95% CI 5.80% to 11.1%; 13 studies, 1417 participants) | 8.30% (95% CI 6.40% to 10.7%; 13 studies, 999 participants) | 1% to 10% | 17.9% (95% CI 14.7% to 21.6%; 82 studies, 11,507 participants)a | 14.3% (95% CI 11.2% to 18.2%; 51 studies, 9073 participants | 25.4% (95% CI 18.2% to 34.4%, 31 studies, 2434 participants) |
| Irritability | 6.40% (95% CI 3.70% to 10.8%; 11 studies, 1038 participants) | 3.50% (95% CI 1.40% to 8.60%; 11 studies, 778 participants) | 1% to 10% | 17.2% (95% CI 11.5% to 25%; 35 studies, 4792 participants)a | 15.5% (95% CI 10.2% to 22.7%; 21 studies, 3298 participants | 20.6% (95% CI 7.90% to 44.1%; 14 studies, 1494 participants) |
| Tics | 2.30% (95% CI 1.00% to 5.20%; 7 studies, 684 participants) | 5.50% (95% CI 3.70% to 8.10%; 7 studies, 476 participants) | No information | 6.40% (95% CI 4.50% to 8.90%; 39 studies, 1980 participants)a | 5.60% (95% CI 3.80% to 8.10%; 29 studies, 1601 participants) | 10.6% (95% CI 5.30% to 19.9%; 10 studies, 379 participants) |
| Drowsiness | 7.30% (95% CI 2.40% to 20.2%; 4 studies, 510 participants) | 6.70% (95% CI 2.60% to 16.2%; 4 studies, 310 participants) | 1% to 10% | 9.50% (95% CI 5.20% to 16.6%; 17 studies, 1146 participants)a | 7.50% (95% CI 3.10% to 17.2%; 7 studies, 644 participants) | 11.3% (95% CI 5.00% to 23.3%; 10 studies, 502 participants) |
| Sadness | 5.70% (95% CI 1.30% to 21.9%; 4 studies, 382 participants) | 4.20% (95% CI 0.90% to 16.9%; 4 studies, 318 participants) | No information | 16.8% (95% CI 9.40% to 28.3%; 21 studies, 1802 participants)a | 13.1% (95% CI 6.60% to 24.1%; 9 studies, 626 participants) | 20.6% (95% CI 8.10% to 43.1%; 12 studies, 1176 participants) |
| Fatigue | 4.80% (95% CI 2.30% to 9.90%; 6 studies, 471 participants) | 6.50% (95% CI 4.30% to 9.60%; 6 studies, 387 participants) | 1% to 10% | 5.70% (95% CI 3.00% to 10.4%; 17 studies, 2182 participants) | 5.60% (95% CI 2.80% to 10.9%; 5 studies, 673 participants) | 7.80% (95% CI 5.80% to 10.5%; 12 studies, 1509 participants) |
| Abdominal pain | 11.5% (95% CI 7.70% to 16.8%; 13 studies, 1406 participants) | 7.60% (95% CI 5.00% to 11.5%; 13 studies, 935 participants) | 0% to 10% | 10.7% (95% CI 8.60% to 13.3%; 79 studies, 11,750 participants)a | 7.60% (95% CI 5.70% to 10.0%; 46 studies, 9229 participants) | 16.3% (95% CI 11.6% to 22.4%; 33 studies, 2521 participants) |
| Decreased appetite | 17.3 (95% CI 12.3% to 24.2%; 16 studies, 1751 participants) | 4.30% (95% CI 2.40% to 7.40%; 16 studies, 1211 participants) | 1% to 10% | 31.1% (95% CI 26.5% to 36.2%; 84 studies, 11,594 participants)a | 28.8% (95% CI 23.0% to 33.5%; 57 studies, 9662 participants) | 39.7% (95% CI 27.0% to 54.0%; 27 studies, 1967 participants) |
| Vomiting | 5.70% (95% CI 4.00% to 8.00%; 11 studies, 1140 participants) | 5.10% (95% CI 3.5% to 7.4%; 11 studies, 776 participants) | 1% to 10% | 7.30% (95% CI 3.70% to 13.4%; 20 studies, 2731 participants)a | 7.10% (95% CI 2.80% to 17.0%; 11 studies, 1528 participants) | 7.20% (95% CI 3.30% to 15.1%; 9 studies, 1203 participants) |
| Nausea | 7.50% (95% CI 6.10% to 9.30%; 11 studies, 1174 participants) | 5.20% (95% CI 3.80% to 7.10%; 11 studies, 821 participants) | > 10% | 7.60% (95% CI 5.30% to 10.6%; 41 studies, 5612 participants)a | 8.00% (95% CI 7.00% to 9.10%; 22 studies, 3921 participants) | 10.4% (95% CI 5.80% to 17.9%; 19 studies, 1691 participants) |
| Decreased weight | 6.30% (95% CI 3.80% to 10.3%; 6 studies, 472 participants) | 2.40% (95% CI 1.00% to 5.70%; 6 studies, 387 participants) | 1% to 10% | 8.70% (95% CI 4.80% to 15.3%; 26 studies, 5182 participants)a | 6.60% (95% CI 3.10% to 13.3%; 17 studies, 4855 participants) | 16.6% (95% CI 8.70% to 30.6%; 9 studies, 327 participants) |
CI: confidence interval; DK: Denmark; RCT: randomised clinical trials. aWe found substantially larger proportions of several adverse events in the present review compared to both the proportions of adverse events in the placebo group in the RCTs included in our 2015 review (Storebø 2015), and the National Summary of Product Characteristics from the UK, USA, and Denmark.
We included in our present review the methylphenidate‐treated groups from 49 RCTs that compared methylphenidate with other ADHD medications in randomised head‐to‐head trials. We excluded these RCTs from our first review assessing methylphenidate versus placebo or no intervention (Storebø 2015). When one assesses the proportions of serious adverse events in the methylphenidate groups, originating from the two groups of RCTs and from the non‐randomised studies, the proportions appear comparable. However, when one assesses the proportions of non‐serious adverse events as well as individual non‐serious adverse events in Table 3, we see an emerging picture. Either the proportions of patients with non‐serious adverse events are the same or raised in the cohort studies (where investigators knew that the patient received methylphenidate) compared to the methylphenidate groups of the placebo‐ or no intervention‐controlled RCT (where investigators were not supposed to break the blinding, but where we previously have declared that we think the blind was broken (Storebø 2015)). However, any non‐serious adverse events and individual non‐serious adverse events (headache; anxiety; sleep difficulties; irritability; tics; drowsiness; sadness; abdominal pain; decreased appetite; and decreased weight) were about two to three times higher in the methylphenidate‐exposed group from the 49 RCTs that compared this drug versus other interventions also aimed at treating ADHD. The investigators were probably less prone to breaking the blind in the latter trials. Moreover, these proportions are likely closer to the real‐life, adverse events of methylphenidate. Therefore, authors, editors, regulators, and others have not been aware of the potential harms of this drug.
In summary, we found higher proportions of adverse events during methylphenidate treatment than have been reported previously. It would be appropriate for our findings to be incorporated into treatment guidelines so as to encourage better awareness of the risk of adverse events during methylphenidate treatment. It is sometimes not possible to distinguish between an adverse event and symptoms that are part and parcel of the underlying ADHD. For example, tics or sleep difficulties are commonly found in children with ADHD, and we do not know whether there is a causative effect or a chance association when such symptoms appear during methylphenidate treatment. We are aware that for many of the proportions of adverse events we describe, there was no comparator group or other data for comparison. Another possibility is that both ADHD and methylphenidate could act as 'shared partners'; a synergistic effect on the emergence of different symptoms.
A qualitative review investigated the occurrence of harmful effects from methylphenidate use in 16 empirical studies using both randomised and non‐randomised study designs (Aagaard 2011). Its findings, with respect to the type and share of serious adverse events and quality of included studies, were in agreement with the observations made in our present systematic review.
The clinical expert opinion of Graham and colleagues, on behalf of the Guideline Group for the European Network for Hyperkinetic Disorders (EUNETHYDIS), concluded that, "some of the effects examined appeared to be minimal in impact or difficult to distinguish from risk to untreated populations", whilst acknowledging that "several areas require further study to allow a more precise understanding of the risks" (Graham 2011).
The research evidence, however, is at best uncertain, as the studies we found were of very low quality, and there were many dropouts for unexplained reasons. Some of these may well have occurred due to serious adverse events. We therefore believe that better‐quality research evidence should be presented before such reassuring conclusions are accepted. We are also aware that conscious or unconscious bias may have arisen through pharmaceutical‐industry sponsorship linked to many of these authors (Lundh 2017).
Authors' conclusions
Implications for practice.
Our findings suggest that methylphenidate may be associated with a number of serious adverse events. In addition, a large number of non‐serious adverse events are reported in children and adolescents, often leading to withdrawal of treatment. Although our results suggest adverse events may be more common than previously reported, included studies were of very low quality. Therefore, we cannot be confident that the size of the effects are significantly higher compared with other treatments. Given the high rates of reported adverse events, clinicians need to carefully balance the potential risks to the potential benefits for each individual patient when considering methylphenidate for the treatment of ADHD.
In view of the possible relationship between adverse events and discontinuation of treatment, it may be necessary to record the presence or absence of sleep difficulties, appetite suppression, cardiovascular problems, psychosis and tics prior to commencement of medication, with active monitoring after medication has been prescribed. Recording family history of neuro‐developmental disorders and other physical health problems may also help to inform decisions to commence and persist with treatment.
Adverse events reported in observational studies do not seem to depend on dose or duration of methylphenidate treatment, comorbidity, age of participants, or use of cointerventions. It remains unclear how medications used just prior to methylphenidate intervention may influence the development of adverse events.
Studies funded by pharmaceutical companies reported a much lower proportion of both serious and non‐serious adverse events, confirming previous studies and systematic reviews on industry bias.
Implications for research.
Methyphenidiate may be associated with a number of serious adverse events as well as a large number of other adverse events in children and adolescents with ADHD. The increasing use of methylphenidate in childhood and adolescence, often continuing into adulthood, also supports the need for more certainty about the true amount of serious adverse events (Bachmann 2017). In order to investigate this, we need to undertake large‐scale, high‐quality RCTs along with studies aimed at identifying responders and non‐responders.
Better designed non‐randomised studies are needed to assess possible rare and unexpected adverse events occurring from methylphenidate use in the paediatric population.
Future observational studies must include a comparator group of untreated participants more often; otherwise it will not be possible to distinguish between harms related to the drug studied and harms related to the patients' disease.
The study duration ranged from 1 day to 11 years, yet most included studies were less than six months. Therefore, we need to conduct large, long‐term observational studies to be able to detect adverse events across paediatric populations and differences in prescribing practice and patterns. The ICH Guideline (E17) on the general principles for planning and design of multi‐regional clinical trials should be consulted when planning new studies in this field.
Future studies should publish depersonalised, individual participant data and report all outcomes, including adverse events (Skoog 2015). This will enable researchers conducting systematic reviews to assess differences between intervention effects according to sex, age, type of AHDH, presence of comorbidities, and use of comedications (especially antipsychotics and antidepressants). Future studies should also screen for potential adverse events. Such screening has been recently shown to increase the reporting of adverse events in adolescents on antipsychotic medication from about 50% to 100% (Pagsberg 2017).
Adverse events should be monitored through the use of structured tools in order to ensure consistency of practice amongst clinicians.
Systematic analyses of adverse events reported to national databases are also necessary, as these databases constitute an underestimated source of important data, particularly information about serious and unexpected reactions from medicine use (Aagaard 2010a; Aagaard 2010b).
What's new
| Date | Event | Description |
|---|---|---|
| 25 June 2018 | Amended | Correcting formatting issue in Table 1 |
Acknowledgements
We thank Janus Christian Jacobsen, MD, PhD, at the Copenhagen Trial Unit, for conceiving this review. We thank Erlend Glasø Faltinsen, BSc, at the Psychiatric Research Unit, Region Zealand, Denmark, for helping to check data and meta‐analyses. We thank Signe Joost, BSc, at the Psychiatric Research Unit, Region Zealand, Denmark, for helping to check data. We thank Adnan Todorovac BSc, at the Psychiatric Research Unit, Region Zealand, Denmark, for helping to check data. We thank Trine Lacoppidan Kæstel, Research Librarian, at the Psychiatric Research Unit, Region Zealand, Denmark, for helping with searches and descriptions of measurement scales. We thank Jesper Pedersen, MD, PhD, Department of Children and Youth Psychiatry, Region Zealand, Denmark, for supporting this project. We thank Jacob Riis, User Experience Lead, and Rasmus Moustgaard, Senior Systems Architect, at the Nordic Cochrane Centre, Copenhagen, Denmark, for helping with issues related to RevMan 5 (Review Manager 2014). Thanks also to the Psychiatric Research Unit, Region Zealand Psychiatry, Roskilde, Denmark; Region Zealand Research Foundation, Denmark; and the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen University Hospital, Copenhagen, Denmark, for funding and enabling the review. We thank Julian Higgins, Professor of Evidence Synthesis at the University of Bristol, for his advice on the ROBINS‐I (formerly ACROBAT‐NRSI) for assessing the risk of bias in non‐randomised studies (Sterne 2014; Sterne 2016). We also warmly thank Geraldine Macdonald (Co‐ordinating Editor), Joanne Duffield (Managing Editor), Gemma O'Loughlin (former Assistant Managing Editor) and Margaret Anderson (Information Specialist) of the Cochrane Developmental, Psychosocial and Learning Problems Review Group for providing help and support. We are grateful for the advice and support received from Toby Lasserson (Senior Editor), and David Tovey (Editor‐in‐Chief) from Cochrane's Central Editorial Unit.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (Central) in the Cochrane Library
#1 MeSH descriptor: [Methylphenidate] explode all trees #2 Methylphenidate #3 Attenta #4 Biphentin #5 Calocain #6 Centedrin* #7 Concerta #8 Daytrana #9 Dexmethylphenidat* #10 Equasym #11 Focalin #12 Medikinet #13 Meridil #14 Metadate #15 Methyl phenidat* #16 Methyl phenidylacetat* #17 Methylfenid* #18 Methylin #19 Methylofenidan #20 Methylphenid* #21 Methyl phenidyl acetat* #22 Methypatch #23 Metilfenidato #24 Motiron #25 MPH #26 Penid #27 Phenidyl hydrochlorid* #28 Phenidylat* #29 Plimasin* #30 PMS‐Methylphenid* #31 Richter Works #32 Riphenidat* #33 Ritalin* #34 Rubifen #35 Stimdat* #36 Tsentedrin* #37 MeSH descriptor: [Child] explode all trees #38 MeSH descriptor: [Adolescent] explode all trees #39 MeSH descriptor: [Infant] explode all trees #40 (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or youth*) #41 elmifiten or medikid or Omozin or Quazym or Tifinidat or Tranquilyn #42 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #41 #43 #37 or #38 or #39 or #40 #44 #42 and #43 #45 MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] explode all trees #46 ADHD:ti,ab,kw #47 ADDH:ti,ab,kw #48 (ADHS):ti,ab,kw #49 ("AD/HD"):ti,ab,kw #50 ((attention* or behav*) near/3 (defic* or dysfunc* or disorder*)):ti,ab,kw #51 (impulsiv* or inattentiv* or inattention*):ti,ab,kw #52 ((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)):ti,ab,kw #53 MeSH descriptor: [Hyperkinesis] explode all trees #54 (hyperkine*):ti,ab,kw #55 (minimal near/3 brain near/3 (disorder* or dysfunct* or damage*)):ti,ab,kw #56 (hyperactiv*):ti,ab,kw #57 #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 #58 #44 and #57
MEDLINE Ovid
Efficacy search
Block A: ADHD
1. exp "Attention Deficit and Disruptive Behavior Disorders"/ 2. adhd.ti,ab. 3. addh.ti,ab. 4. adhs.ti,ab. 5. (ad adj hd).ti,ab. 6. ((attention$ or behav$) adj3 (defic$ or dysfunc$ or disorder$)).ti,ab. 7. ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).ti,ab. 8. (impulsiv$ or inattentiv$ or inattention$).ti,ab. 9. hyperactiv$.ti,ab. 10. hyperkinesis$.ti,ab. 11. exp Hyperkinesis/ 12. (minimal adj brain adj3 disorder$).ti,ab. 13. (minimal adj brain adj3 dysfunction$).ti,ab. 14. (minimal adj brain adj3 damage$).ti,ab. 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
Block B: Study design
16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomized controlled trials.mp. 19. random allocation.mp. 20. double blind method.mp. 21. single blind method.mp. 22. clinical trial.pt. 23. (clin$ adj25 trial$).ti,ab. 24. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$ or dummy$)).mp. 25. exp clinical trial/ 26. placebos.mp. 27. placebo$.ti,ab. 28. random$.ti,ab. 29. comparative study.mp. 30. evaluation studies as topic/ 31. exp clinical trials as topic/ 32. follow up studies.mp. 33. prospective studies.mp. 34. (control$ or prospectiv$ or volunteer$).ti,ab. 35. longitudinal.tw. 36. retrospective.tw. 37. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 38. exp Clinical Trials as Topic/ 39. exp Cohort Studies/ 40. exp Case‐Control Studies/ 41. "case report*".pt. 42. case control.tw. 43. (cohort adj (study or studies)).tw. 44. "cohort analy*".tw. 45. (observational adj (study or studies)).tw. 46. cross sectional.tw. 47. (follow up adj (study or studies)).tw. 48. (case* adj report*).tw. 49. (case* adj stud*).tw. 50. case reports.pt. 51. Cross‐Sectional Studies/ 52. 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 53. 37 or 52 54. 15 and 53 [Block A AND Block B]
Block C: Methylphenidate
55. Methylphenidate.mp. or Methylphenidate/ 56. Attenta.mp. 57. Biphentin.mp. 58. Calocain.mp. 59. Centedrin*.mp. 60. Concerta.mp. 61. Daytrana.mp. 62. Dexmethylphenidat*.mp. 63. Equasym.mp. 64. Elmifiten.mp. 65. Focalin.mp. 66. Medikid.mp. 67. Medikinet.mp. 68. Meridil.mp. 69. Metadate.mp. 70. Methyl phenidat*.mp. 71. Methyl phenidylacetat*.mp. 72. Methylfenid*.mp. 73. Methylin.mp. 74. Methylofenidan.mp. 75. Methylphenid*.mp. 76. Methyl phenidyl acetat*.mp. 77. Methypatch.mp. 78. Metilfenidato.mp. 79. Motiron.mp. 80. MPH.mp. 81. Omozin.mp. 82. Penid.mp. 83. Phenidyl hydrochlorid*.mp. 84. Phenidylat*.mp. 85. Plimasin*.mp. 86. PMS‐Methylphenid*.mp. 87. Quazym.mp. 88. Richter Works.mp. 89. Riphenidat*.mp. 90. Ritalin*.mp. 91. Rubifen.mp. 92. Stimdat*.mp. 93. Tifinidat.mp. 94. Tranquilyn.mp. 95. Tsentedrin*.mp. 96. 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 97. 54 and 96 [Block A AND Block B AND Block C]
Block D Children
98. exp child/ 99. exp adolescent/ 100. exp infant/ 101. (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or youth*).mp. 102. 98 or 99 or 100 or 101 103. 97 and 102 104. 103 and 97 and 102 [Block A AND Block B AND Block C AND Block D]
Adverse events search
Block 1: Methylphenidate
105. Methylphenidate.mp. or Methylphenidate/ 106. Attenta.mp. 107. Biphentin.mp. 108. Calocain.mp. 109. Centedrin*.mp. 110. Concerta.mp. 111. Daytrana.mp. 112. Dexmethylphenidat*.mp. 113. Elmifiten.mp. 114. Equasym.mp. 115. Focalin.mp. 116. Medikid.mp. 117. Medikinet.mp. 118. Meridil.mp. 119. Metadate.mp. 120. Methyl phenidat*.mp. 121. Methyl phenidylacetat*.mp. 122. Methylfenid*.mp. 123. Methylin.mp. 124. Methylofenidan.mp. 125. Methylphenid*.mp. 126. Methyl phenidyl acetat*.mp. 127. Methypatch.mp. 128. Metilfenidato.mp. 129. Motiron.mp. 130. MPH.mp. 131. Omozin.mp. 132. Penid.mp. 133. Phenidyl hydrochlorid*.mp. 134. Phenidylat*.mp. 135. Plimasin*.mp. 136. PMS‐Methylphenid*.mp. 137. Richter Works.mp. 138. Quazym.mp. 139. Riphenidat*.mp. 140. Ritalin*.mp. 141. Rubifen.mp. 142. Stimdat*.mp. 143. Tifinidat.mp. 144. Tranquilyn.mp. 145. Tsentedrin*.mp. 146. 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 or 127 or 128 or 129 or 130 or 131 or 132 or 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145
Block 2: Adverse events
147. (ae or co or de).fs. 148. (safe or safety or (side adj1 effect*) or (undesirable adj1 effect*) or (treatment adj1 emergent) or tolerability or tolerance or tolerate or toxicity or toxic or adrs or adr or harm or harms or harmful or complication* or risk or risks or (unintended adj1 event*) or (unintended adj1 effect*)).ti,ab. 149. (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).ti,ab. 150. 147 or 148 or 149
Block 3: Children
151. exp child/ 152. exp adolescent/ 153. exp infant/ 154. (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or youth*).mp. 155. 151 or 152 or 153 or 154 156. exp mood disorders/ 157. (depression or depressive).ti,ab. 158. exp psychotic disorders/ 159. (psychosis or (psychotic adj4 symptom*)).ti,ab. 160. exp Body Weight/ or anorexia/ 161. ((loss or lose or losing or reduc*) adj3 (weight or appetite)).ti,ab. 162. ((reduc* or retard* or inhibit* or deficit*) adj4 growth).ti,ab. 163. exp Hypertension/ 164. Heart Rate/ 165. exp tachycardia/ 166. (increas* adj4 (heart rate or pulse or blood pressure)).ti,ab. 167. exp Death, Sudden/ 168. death.ti,ab. 169. exp Infertility/ 170. (((loss or reduc*) adj4 fertility) or infertility).ti,ab. 171. exp Carcinogens/ 172. exp neoplasms/ 173. ((risk adj2 cancer) or (cytogenetic adj2 effect*)).ti,ab. 174. 156 or 157 or 158 or 159 or 160 or 161 or 162 or 163 or 164 or 165 or 166 or 167 or 168 or 169 or 170 or 171 or 172 or 173 175. 150 or 174 [Block 2 Adverse events] 176. 146 and 155 and 175 177. Methylphenidate/ae, po, to [Adverse Effects, Poisoning, Toxicity] 178. 155 and 177 179. 176 or 178 [Methylphenidate AND Children AND Adverse events] 180. 104 or 179 [Efficacy OR Adverse events]
Embase Ovid
Embase efficacy search
Block A : ADHD
1. exp "Attention Deficit and Disruptive Behavior Disorders"/ 2. adhd.ti,ab. 3. addh.ti,ab. 4. adhs.ti,ab. 5. (ad adj hd).ti,ab. 6. ((attention$ or behav$) adj3 (defic$ or dysfunc$ or disorder$)).ti,ab. 7. ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).ti,ab. 8. (impulsiv$ or inattentiv$ or inattention$).ti,ab. 9. hyperactiv$.ti,ab. 10. hyperkinesis$.ti,ab. 11. exp Hyperkinesis/ 12. (minimal adj brain adj3 disorder$).ti,ab. 13. (minimal adj brain adj3 dysfunction$).ti,ab. 14. (minimal adj brain adj3 damage$).ti,ab. 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
Block B: Study design
16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomized controlled trials.mp. 19. random allocation.mp. 20. double blind method.mp. 21. single blind method.mp. 22. clinical trial.pt. 23. (clin$ adj25 trial$).ti,ab. 24. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$ or dummy$)).mp. 25. exp clinical trial/ 26. placebos.mp. 27. placebo$.ti,ab. 28. random$.ti,ab. 29. comparative study.mp. 30. evaluation studies as topic/ 31. exp clinical trials as topic/ 32. follow up studies.mp. 33. prospective studies.mp. 34. (control$ or prospectiv$ or volunteer$).ti,ab. 35. longitudinal.tw. 36. retrospective.tw. 37. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 38. exp Clinical Trials as Topic/ 39. exp Cohort Studies/ 40. exp Case‐Control Studies/ 41. "case report*".pt. 42. case control.tw. 43. (cohort adj (study or studies)).tw. 44. "cohort analy*".tw. 45. (observational adj (study or studies)).tw. 46. cross sectional.tw. 47. (follow up adj (study or studies)).tw. 48. (case* adj report*).tw. 49. (case* adj stud*).tw. 50. case reports.pt. 51. Cross‐Sectional Studies/ 52. 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 53. 37 or 52 54. 15 and 53 [Block A AND Block B]
Block C: Methylphenidate
55. Methylphenidate.mp. or Methylphenidate/ 56. Attenta.mp. 57. Biphentin.mp. 58. Calocain.mp. 59. Centedrin*.mp. 60. Concerta.mp. 61. Daytrana.mp. 62. Dexmethylphenidat*.mp. 63. Equasym.mp. 64. Elmifiten.mp. 65. Focalin.mp. 66. Medikid.mp. 67. Medikinet.mp. 68. Meridil.mp. 69. Metadate.mp. 70. Methyl phenidat*.mp. 71. Methyl phenidylacetat*.mp. 72. Methylfenid*.mp. 73. Methylin.mp. 74. Methylofenidan.mp. 75. Methylphenid*.mp. 76. Methyl phenidyl acetat*.mp. 77. Methypatch.mp. 78. Metilfenidato.mp. 79. Motiron.mp. 80. MPH.mp. 81. Omozin.mp. 82. Penid.mp. 83. Phenidyl hydrochlorid*.mp. 84. Phenidylat*.mp. 85. Plimasin*.mp. 86. PMS‐Methylphenid*.mp. 87. Quazym.mp. 88. Richter Works.mp. 89. Riphenidat*.mp. 90. Ritalin*.mp. 91. Rubifen.mp. 92. Stimdat*.mp. 93. Tifinidat.mp. 94. Tranquilyn.mp. 95. Tsentedrin*.mp. 96. 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 97. 54 and 96 [Block A AND Block B AND Block C]
Block D: children
98. exp child/ 99. exp adolescent/ 100. exp infant/ 101. (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or youth*).mp. 102. 98 or 99 or 100 or 101 103. 97 and 102 [Block A AND Block B AND Block C AND Block D] 104. 103 and 97 and 102
Adverse events search
105. Methylphenidate.mp. or Methylphenidate/ 106. Attenta.mp. 107. Biphentin.mp. 108. Calocain.mp. 109. Centedrin*.mp. 110. Concerta.mp. 111. Daytrana.mp. 112. Dexmethylphenidat*.mp. 113. Elmifiten.mp. 114. Equasym.mp. 115. Focalin.mp. 116. Medikid.mp. 117. Medikinet.mp. 118. Meridil.mp. 119. Metadate.mp. 120. Methyl phenidat*.mp. 121. Methyl phenidylacetat*.mp. 122. Methylfenid*.mp. 123. Methylin.mp. 124. Methylofenidan.mp. 125. Methylphenid*.mp. 126. Methyl phenidyl acetat*.mp. 127. Methypatch.mp. 128. Metilfenidato.mp. 129. Motiron.mp. 130. MPH.mp. 131. Omozin.mp. 132. Penid.mp. 133. Phenidyl hydrochlorid*.mp. 134. Phenidylat*.mp. 135. Plimasin*.mp. 136. PMS‐Methylphenid*.mp. 137. Richter Works.mp. 138. Quazym.mp. 139. Riphenidat*.mp. 140. Ritalin*.mp. 141. Rubifen.mp. 142. Stimdat*.mp. 143. Tifinidat.mp. 144. Tranquilyn.mp. 145. Tsentedrin*.mp. 146. 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 or 127 or 128 or 129 or 130 or 131 or 132 or 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145 147. (ae or co or de).fs. 148. (safe or safety or (side adj1 effect*) or (undesirable adj1 effect*) or (treatment adj1 emergent) or tolerability or tolerance or tolerate or toxicity or toxic or adrs or adr or harm or harms or harmful or complication* or risk or risks or (unintended adj1 event*) or (unintended adj1 effect*)).ti,ab. 149. (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).ti,ab. 150. 147 or 148 or 149 151. exp child/ 152. exp adolescent/ 153. exp infant/ 154. (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or youth*).mp. 155. 151 or 152 or 153 or 154 156. exp mood disorders/ 157. (depression or depressive).ti,ab. 158. exp psychotic disorders/ 159. (psychosis or (psychotic adj4 symptom*)).ti,ab. 160. exp Body Weight/ or anorexia/ 161. ((loss or lose or losing or reduc*) adj3 (weight or appetite)).ti,ab. 162. ((reduc* or retard* or inhibit* or deficit*) adj4 growth).ti,ab. 163. exp Hypertension/ 164. Heart Rate/ 165. exp tachycardia/ 166. (increas* adj4 (heart rate or pulse or blood pressure)).ti,ab. 167. exp Death, Sudden/ 168. death.ti,ab. 169. exp Infertility/ 170. (((loss or reduc*) adj4 fertility) or infertility).ti,ab. 171. exp Carcinogens/ 172. exp neoplasms/ 173. ((risk adj2 cancer) or (cytogenetic adj2 effect*)).ti,ab. 174. 156 or 157 or 158 or 159 or 160 or 161 or 162 or 163 or 164 or 165 or 166 or 167 or 168 or 169 or 170 or 171 or 172 or 173 175. 150 or 174 176. 146 and 155 and 175 177. Methylphenidate/ae, po, to [Adverse Effects, Poisoning, Toxicity] 178. 155 and 177 179. 176 or 178 180. 104 or 179
PsycINFO Ovid
Efficacy search
1. exp attention deficit disorder/ 2. adhd.ti,ab. 3. addh.ti,ab. 4. ADHS.ti,ab. 5. (AD adj HD).ti,ab. 6. ((attention$ or behav$) adj3 (defic$ or dysfunc$ or disorder$)).ti,ab. 7. ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).ti,ab. 8. (impulsiv$ or inattentiv$ or inattention$).ti,ab. 9. hyperactiv$.ti,ab. 10. hyperkinesis$.ti,ab. 11. exp Hyperkinesis/ 12. (minimal adj brain adj3 disorder$).ti,ab. 13. (minimal adj brain adj3 dysfunction$).ti,ab. 14. (minimal adj brain adj3 damage$).ti,ab. 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 [Block A] 16. random$.mp. 17. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or dummy or mask$)).mp. 18. placebo$.mp. 19. crossover.mp. 20. assign$.mp. 21. allocat$.mp. 22. ((clin$ or control$ or compar$ or evaluat$ or prospectiv$) adj25 (trial$ or studi$ or study)).mp. 23. exp placebo/ 24. exp treatment effectiveness evaluation/ 25. exp mental health program evaluation/ 26. exp experimental design/ 27. versus.id. 28. vs.id. 29. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. ((case* adj5 control*) or (case adj3 comparison*) or (case‐comparison or control group*)).ab,id,ti. 31. (cohort or longitudinal or prospective or retrospective).ab,id,ti. 32. (cross section* or "prevalence study").ab,id,ti. 33. clinical case study.md. 34. prospective study.md. 35. longitudinal study.md. 36. exp Followup Studies/ 37. followup study.md. 38. 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 39. 29 or 38 [Block B] 40. 15 and 39 [Block A and Block B] 41. Methylphenidate.mp. or Methylphenidate/ 42. Attenta.mp. 43. Biphentin.mp. 44. Calocain.mp. 45. Centedrin*.mp. 46. Concerta.mp. 47. Daytrana.mp. 48. Dexmethylphenidat*.mp. 49. Elmifiten.mp. 50. Equasym.mp. 51. Focalin.mp. 52. Medikid.mp. 53. Medikinet.mp. 54. Meridil.mp. 55. Metadate.mp. 56. Methyl phenidat*.mp. 57. Methyl phenidylacetat*.mp. 58. Methylfenid*.mp. 59. Methylin.mp. 60. Methylofenidan.mp. 61. Methylphenid*.mp. 62. Methyl phenidyl acetat*.mp. 63. Methypatch.mp. 64. Metilfenidato.mp. 65. Motiron.mp. 66. MPH.mp. 67. Omozin.mp. 68. Penid.mp. 69. Phenidyl hydrochlorid*.mp. 70. Phenidylat*.mp. 71. Plimasin*.mp. 72. PMS‐Methylphenid*.mp. 73. Quazym.mp. 74. Richter Works.mp. 75. Riphenidat*.mp. 76. Ritalin*.mp. 77. Rubifen.mp. 78. Stimdat*.mp. 79. Tifinidat.mp. 80. Tranquilyn.mp. 81. Tsentedrin*.mp. 82. 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 [Block C] 83. 40 and 82 [Block A AND Block B AND Block C] 84. (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or youth*).mp. 85. 83 and 84 [Block A AND Block B AND Block C AND Block D]
Adverse events search
86. Methylphenidate.mp. or Methylphenidate/ 87. Attenta.mp. 88. Biphentin.mp. 89. Calocain.mp. 90. Centedrin*.mp. 91. Concerta.mp. 92. Daytrana.mp. 93. Dexmethylphenidat*.mp. 94. Elmifiten.mp. 95. Equasym.mp. 96. Focalin.mp. 97. Medikid.mp. 98. Medikinet.mp. 99. Meridil.mp. 100. Metadate.mp. 101. Methyl phenidat*.mp. 102. Methyl phenidylacetat*.mp. 103. Methylfenid*.mp. 104. Methylin.mp. 105. Methylofenidan.mp. 106. Methylphenid*.mp. 107. Methyl phenidyl acetat*.mp. 108. Methypatch.mp. 109. Metilfenidato.mp. 110. Motiron.mp. 111. MPH.mp. 112. Omozin.mp. 113. Penid.mp. 114. Phenidyl hydrochlorid*.mp. 115. Phenidylat*.mp. 116. Plimasin*.mp. 117. PMS‐Methylphenid*.mp. 118. Quazym.mp. 119. Richter Works.mp. 120. Riphenidat*.mp. 121. Ritalin*.mp. 122. Rubifen.mp. 123. Stimdat*.mp. 124. Tifinidat.mp. 125. Tranquilyn.mp. 126. Tsentedrin*.mp. 127. 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 [Block 1] 128. "Side effects (Drug)"/ 129. (safe or safety or (side adj1 effect*) or (undesirable adj1 effect*) or (treatment adj1 emergent) or tolerability or tolerance or tolerate or toxicity or toxic or adrs or adr or harm or harms or harmful or complication* or risk or risks or (unintended adj1 event*) or (unintended adj1 effect*)).ti,ab. 130. (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).ti,ab. 131. 128 or 129 or 130 132. exp major depression/ or affective disorders/ 133. (depression or depressive).ti,ab. 134. exp psychosis/ 135. (psychosis or (psychotic adj4 symptom*)).ti,ab. 136. exp body weight/ 137. exp appetite depressing drugs/ 138. Appetite/ 139. ((loss or lose or losing or decreas* or reduc*) adj3 (weight or appetite)).ti,ab. 140. ((reduc* or retard* or inhibit* or deficit*) adj4 growth).ti,ab. 141. exp appetite depressing drugs/ 142. Appetite/ 143. exp cardiovascular disorders/ 144. exp heart rate affecting drugs/ 145. Heart rate/ 146. (increas* adj4 (heart rate or pulse or blood pressure)).ti,ab. 147. "death and dying"/ 148. death.ti,ab. 149. exp infertility/ 150. (((loss or reduc*) adj4 fertility) or infertility).ti,ab. 151. exp neoplasms/ 152. Carcinogens/ 153. ((risk adj2 cancer) or (cytogenetic adj2 effect*)).ti,ab. 154. 132 or 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145 or 146 or 147 or 148 or 149 or 150 or 151 or 152 or 153 155. 131 or 154 [Block 2] 156. 127 and 155 [Block 1 AND Block 2] 157. (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or youth*).mp.[Block 3] 158. 156 and 157 [Block 1 AND Block 2 AND Block 3] 159. 85 or 158 [Efficacy OR Adverse events]
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
#1 MeSH descriptor: [Methylphenidate] explode all trees #2 Methylphenidate #3 Attenta #4 Biphentin #5 Calocain #6 Centedrin* #7 Concerta #8 Daytrana #9 Dexmethylphenidat* #10 Equasym #11 Focalin #12 Medikinet #13 Meridil #14 Metadate #15 Methyl phenidat* #16 Methyl phenidylacetat* #17 Methylfenid* #18 Methylin #19 Methylofenidan #20 Methylphenid* #21 Methyl phenidyl acetat* #22 Methypatch #23 Metilfenidato #24 Motiron #25 MPH #26 Penid #27 Phenidyl hydrochlorid* #28 Phenidylat* #29 Plimasin* #30 PMS‐Methylphenid* #31 Richter Works #32 Riphenidat* #33 Ritalin* #34 Rubifen #35 Stimdat* #36 Tsentedrin* #37 MeSH descriptor: [Child] explode all trees #38 MeSH descriptor: [Adolescent] explode all trees #39 MeSH descriptor: [Infant] explode all trees #40 (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or youth*) #41 elmifiten or medikid or Omozin or Quazym or Tifinidat or Tranquilyn #42 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #41 #43 #37 or #38 or #39 or #40 #44 #42 and #43 #45 MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] explode all trees #46 ADHD:ti,ab,kw #47 ADDH:ti,ab,kw #48 (ADHS):ti,ab,kw #49 ("AD/HD"):ti,ab,kw #50 ((attention* or behav*) near/3 (defic* or dysfunc* or disorder*)):ti,ab,kw #51 (impulsiv* or inattentiv* or inattention*):ti,ab,kw #52 ((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)):ti,ab,kw #53 MeSH descriptor: [Hyperkinesis] explode all trees #54 (hyperkine*):ti,ab,kw #55 (minimal near/3 brain near/3 (disorder* or dysfunct* or damage*)):ti,ab,kw #56 (hyperactiv*):ti,ab,kw #57 #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 #58 #44 and #57
Conference Proceedings Citation Index ‐ Science (CPCI‐S); Conference Proceedings Citation Index ‐ Social Science & Humanities (CPCI‐SSH) Web of Science
#3 #2 AND #1 Databases=CPCI‐S, CPCI‐SSH
#2 TS=(child* or boy* or girl* or adolescen* or teen* orpreschool* or "pre school*" or infant* or baby or babies or toddler*or "school child*" or schoolchild* or youth*) Databases=CPCI‐S, CPCI‐SSH
#1 TS=(Methylphenidate or Attenta or Biphentin or Calocain orCentedrin* or Concerta or Daytrana or Dexmethylphenidat* orElmifiten or Equasym or Focalin or Medikid or Medikinet or Meridilor Metadate or "Methyl phenidat*" or "Methyl phenidylacetat" or Methylfenid or Methylin or Methylofenidan or Methylphenid* or "Methyl phenidyl acetat*" or Methypatch or Metilfenidato or Motironor MPH or Penid or Omozin or Quazym or "Phenidyl hydrochlorid*" orPhenidylat* or Plimasin* or "PMS‐Methylphenid* " or "Richter Works"or Riphenidat* or Ritalin* or Rubifen or Stimdat* or Tifinidat orTranquilyn or Tsentedrin*) Databases=CPCI‐S, CPCI‐SSH
NDLTD Theses Resources (ndltd.org/resources)
methylphenidate AND child* AND (attention deficit or hyperactiv*)
TROVE ‐ National Library of Australia (trove.nla.gov.au)
methylphenidate AND child* AND (attention OR hyperactiv*)
British Library E‐Theses Online Service (EThOS; ethos.bl.uk)
methylphenidate AND children AND attention deficit / methylphenidate AND child AND hyperactivity
Deutsche Nationalbibliothek Dissertations (www.dnb.de/EN/Home/home_node.html)
methylphenidate
Open Access Theses and Dissertations (OATD; oatd.org)
methylphenidate AND child* AND (attention deficit OR hyperactiv*)
Theses Collection Wales ‐ The National Library of Wales (www.llgc.org.uk/en/discover/nlw‐resources/theses‐collection‐wales)
methylphenidate AND child* AND (attention deficit OR hyperactiv*)
DART‐Europe E‐Theses Portal (www.dart‐europe.eu/basic‐search.php)
methylphenidate AND child*
Bielefeld Academic Search Engine (BASE; www.base‐search.net/about/en)
methylphenidate AND adhd/attention deficit/hyperactive*
Theses France (www.theses.fr/en)
methylphenidate
ProQuest Open Access Dissertations (PQDT Open; pqdtopen.proquest.com/search.html)
methylphenidate AND children AND adhd
ClinicalTrials.gov (clinicaltrials.gov)
attention deficit or adhd or hyperactivity [Conditions] | methylphenidate [Interventions] | Child
World Health Organization International Clinical Trials Registry (WHO ICTRP; who.int/ictrp/en)
Methylphenidate AND adhd OR attention OR hyperactiv*
Data and analyses
Comparison 1. Comparative studies: number of serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any serious adverse events | 2 | 72005 | Risk Ratio (IV, Random, 95% CI) | 1.36 [1.17, 1.57] |
| 2 Central nervous system | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Seizures | 1 | 234 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.07, 23.74] |
| 2.2 Psychotic disorder | 1 | 71771 | Risk Ratio (IV, Random, 95% CI) | 1.36 [1.17, 1.57] |
| 3 Cardiovascular and respiratory system | Other data | No numeric data | ||
| 3.1 Arrhythmias | Other data | No numeric data | ||
| 3.2 Hypertension | Other data | No numeric data | ||
| 3.3 Myocardial infarction | Other data | No numeric data | ||
| 3.4 Ischaemic stroke | Other data | No numeric data | ||
| 3.5 Heart failure | Other data | No numeric data |
Comparison 2. Comparative studies: number of participants with non‐serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Central nervous system: sleep‐related adverse events | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Insomnia and sleep problems | 3 | 425 | Risk Ratio (IV, Random, 95% CI) | 2.58 [1.24, 5.34] |
| 1.2 Nightmares | 1 | 23 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.41, 3.21] |
| 1.3 Snoring | 1 | 23 | Risk Ratio (IV, Random, 95% CI) | 4.62 [0.25, 86.72] |
| 1.4 Non‐breathing or gasping while sleeping | 1 | 23 | Risk Ratio (IV, Random, 95% CI) | 2.77 [0.12, 61.65] |
| 1.5 Sleepwalking | 1 | 23 | Risk Ratio (IV, Random, 95% CI) | 2.75 [0.33, 22.69] |
| 1.6 Various sleep positions | 1 | 23 | Risk Ratio (IV, Random, 95% CI) | 2.77 [0.12, 61.65] |
| 1.7 Enuresis | 1 | 23 | Risk Ratio (IV, Random, 95% CI) | 4.62 [0.25, 86.72] |
| 1.8 Talking in sleep | 1 | 23 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.05, 4.38] |
| 2 Central nervous system: other specific sleep‐related adverse events | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Number of hours sleep | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.32, 0.02] |
| 2.2 Number of nocturnal movements | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐27.84 [‐57.90, 2.22] |
| 2.3 Sleep quality | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.74, 0.34] |
| 3 Central nervous system: other specific adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Headache | 1 | 235 | Risk Ratio (IV, Random, 95% CI) | 8.13 [0.48, 137.74] |
| 3.2 Dizziness | 1 | 335 | Risk Ratio (IV, Random, 95% CI) | 4.00 [0.23, 69.27] |
| 4 Cardiovascular and respiratory system | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Systolic blood pressure | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐6.27, 6.47] |
| 4.2 Diastolic blood pressure | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 3.50 [‐1.42, 8.42] |
| 4.3 Pulse rate | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐7.95, 9.15] |
| 5 Gastrointestinal system | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Nausea | 1 | 335 | Risk Ratio (IV, Random, 95% CI) | 2.64 [0.34, 20.29] |
| 5.2 Abdominal pain | 1 | 335 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.16, 3.84] |
| 5.3 Decreased appetite | 1 | 335 | Risk Ratio (IV, Random, 95% CI) | 15.06 [2.12, 106.83] |
| 6 Musculoskeletal system | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Height | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐2.61, 0.75] |
| 6.2 Weight | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.16, 0.62] |
| 7 Musculoskeletal system: body mass index (BMI) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.96, ‐0.24] |
| 8 Musculoskeletal system: z scores | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Height z score | 1 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.43, 0.05] |
| 8.2 Weight z score | 1 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.30, 0.16] |
| 8.3 BMI z score | 1 | 275 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.22, 0.24] |
Comparison 3. Comparative studies: number of participants withdrawn from methylphenidate treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants withdrawn from methylphenidate for unknown reasons | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Non‐comparative studies: proportion of participants with serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any serious adverse event | Other data | No numeric data | ||
| 2 Central nervous system | Other data | No numeric data | ||
| 2.1 Sudden death | Other data | No numeric data | ||
| 2.2 Suicide | Other data | No numeric data | ||
| 2.3 Suicide attempt | Other data | No numeric data | ||
| 2.4 Suicide thoughts | Other data | No numeric data | ||
| 2.5 Psychotic symptoms | Other data | No numeric data | ||
| 2.6 Seizure | Other data | No numeric data | ||
| 2.7 Syncope | Other data | No numeric data | ||
| 2.8 Tremor | Other data | No numeric data | ||
| 2.9 Psychiatric problems | Other data | No numeric data | ||
| 2.10 Severe depression | Other data | No numeric data | ||
| 2.11 Overdose | Other data | No numeric data | ||
| 2.12 Eating disorder | Other data | No numeric data | ||
| 2.13 Aphasia | Other data | No numeric data | ||
| 2.14 Headache | Other data | No numeric data | ||
| 2.15 Neurological disorder | Other data | No numeric data | ||
| 2.16 Sleep disorder | Other data | No numeric data | ||
| 2.17 Mood disorder | Other data | No numeric data | ||
| 2.18 Loss of consciousness | Other data | No numeric data | ||
| 2.19 Family stress | Other data | No numeric data | ||
| 2.20 Impotence | Other data | No numeric data | ||
| 2.21 Severe aggression | Other data | No numeric data | ||
| 2.22 Severe anorexia | Other data | No numeric data | ||
| 2.23 Amphetamine intoxication | Other data | No numeric data | ||
| 3 Cardiovascular and respiratory system | Other data | No numeric data | ||
| 3.1 Respiratory, thoracic and mediastinal disorders | Other data | No numeric data | ||
| 3.2 Cardiac problems | Other data | No numeric data | ||
| 3.3 Tachycardia | Other data | No numeric data | ||
| 3.4 Severe hypertension | Other data | No numeric data | ||
| 3.5 Epistaxsis | Other data | No numeric data | ||
| 3.6 Viral illness | Other data | No numeric data | ||
| 4 Gastrointestinal system | Other data | No numeric data | ||
| 4.1 Gastrointestinal disease | Other data | No numeric data | ||
| 4.2 Liver disease | Other data | No numeric data | ||
| 5 Musculoskeletal system: fractures | Other data | No numeric data | ||
| 6 Immune system: autoimmune disease | Other data | No numeric data |
Comparison 5. Non‐comparative studies: proportion of participants withdrawn from methylphenidate treatment due to serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants withdrawn from methylphenidate treatment due to serious adverse events | Other data | No numeric data | ||
| 1.1 Serious adverse events | Other data | No numeric data | ||
| 1.2 Hallucinations | Other data | No numeric data | ||
| 1.3 Severe anorexia | Other data | No numeric data | ||
| 1.4 Suicide attempt | Other data | No numeric data | ||
| 2 Proportion of participants withdrawn from methylphenidate treatment due to adverse events of unknown severity | Other data | No numeric data |
Comparison 6. Non‐comparative studies: proportion of participants with adverse events of unknown severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with adverse events of unknown severity | Other data | No numeric data |
Comparison 7. Non‐comparative studies: proportion of participants with non‐serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐serious adverse events | Other data | No numeric data | ||
| 2 Central nervous system | Other data | No numeric data | ||
| 2.1 Affect lability | Other data | No numeric data | ||
| 2.2 Aggression | Other data | No numeric data | ||
| 2.3 Anorexia | Other data | No numeric data | ||
| 2.4 Anxiety | Other data | No numeric data | ||
| 2.5 Fingernail biting | Other data | No numeric data | ||
| 2.6 Daydreams | Other data | No numeric data | ||
| 2.7 Difficulty falling asleep | Other data | No numeric data | ||
| 2.8 Depression | Other data | No numeric data | ||
| 2.9 Disturbed sleep | Other data | No numeric data | ||
| 2.10 Dizziness | Other data | No numeric data | ||
| 2.11 Drowsiness | Other data | No numeric data | ||
| 2.12 Dysthymia | Other data | No numeric data | ||
| 2.13 Emotional lability | Other data | No numeric data | ||
| 2.14 Euphoria/hypomania | Other data | No numeric data | ||
| 2.15 Asthenia and fatigue | Other data | No numeric data | ||
| 2.16 Headache | Other data | No numeric data | ||
| 2.17 Increased need to sleep | Other data | No numeric data | ||
| 2.18 Involuntary movements | Other data | No numeric data | ||
| 2.19 Irritability | Other data | No numeric data | ||
| 2.20 Nervousness | Other data | No numeric data | ||
| 2.21 Nightmares | Other data | No numeric data | ||
| 2.22 Restlessness and agitation | Other data | No numeric data | ||
| 2.23 Sadness | Other data | No numeric data | ||
| 2.24 Stares | Other data | No numeric data | ||
| 2.25 Excessive talking | Other data | No numeric data | ||
| 2.26 Taciturnity ('talking too little') | Other data | No numeric data | ||
| 2.27 Tics | Other data | No numeric data | ||
| 2.28 Isolation and lack of interest in others | Other data | No numeric data | ||
| 2.29 'Zombie like' demeanour | Other data | No numeric data | ||
| 2.30 Somnolence | Other data | No numeric data | ||
| 2.31 Obsession | Other data | No numeric data | ||
| 2.32 Mood disorder | Other data | No numeric data | ||
| 2.33 Confusional state | Other data | No numeric data | ||
| 2.34 Sleep disorder | Other data | No numeric data | ||
| 2.35 Propensity to cry | Other data | No numeric data | ||
| 2.36 Sedation | Other data | No numeric data | ||
| 2.37 Daytime sleepiness | Other data | No numeric data | ||
| 2.38 Dysphoria | Other data | No numeric data | ||
| 2.39 Logorrhoea | Other data | No numeric data | ||
| 2.40 Impaired concentration | Other data | No numeric data | ||
| 2.41 Difficulty waking up | Other data | No numeric data | ||
| 2.42 Urinary incontinence | Other data | No numeric data | ||
| 2.43 Paralysis | Other data | No numeric data | ||
| 2.44 Affective disorder | Other data | No numeric data | ||
| 2.45 Jumbled thoughts | Other data | No numeric data | ||
| 2.46 Bulimia | Other data | No numeric data | ||
| 2.47 Sleep late | Other data | No numeric data | ||
| 2.48 Tooth grinding | Other data | No numeric data | ||
| 2.49 Tremor | Other data | No numeric data | ||
| 2.50 Stuttering | Other data | No numeric data | ||
| 2.51 Flushing | Other data | No numeric data | ||
| 2.52 Apathy | Other data | No numeric data | ||
| 2.53 Abnormal behaviour | Other data | No numeric data | ||
| 2.54 Stereotypies | Other data | No numeric data | ||
| 2.55 Quietness | Other data | No numeric data | ||
| 2.56 EEG changes | Other data | No numeric data | ||
| 2.57 Did not like themselves | Other data | No numeric data | ||
| 2.58 Sleep walking | Other data | No numeric data | ||
| 2.59 Personality and behaviour disorders | Other data | No numeric data | ||
| 2.60 Vertigo | Other data | No numeric data | ||
| 3 Cardiovascular and respiratory system | Other data | No numeric data | ||
| 3.1 Cough | Other data | No numeric data | ||
| 3.2 Pharyngolaryngeal pain | Other data | No numeric data | ||
| 3.3 Upper respiratory tract infection | Other data | No numeric data | ||
| 3.4 Tachycardia | Other data | No numeric data | ||
| 3.5 Abnormal ECG | Other data | No numeric data | ||
| 3.6 Nasal congestion | Other data | No numeric data | ||
| 3.7 Palpitation | Other data | No numeric data | ||
| 3.8 Systolic blood pressure | Other data | No numeric data | ||
| 3.9 Diastolic blood pressure | Other data | No numeric data | ||
| 3.10 Pulse rate | Other data | No numeric data | ||
| 3.11 ECG‐QT | Other data | No numeric data | ||
| 3.12 Cold fingers | Other data | No numeric data | ||
| 3.13 Sweating | Other data | No numeric data | ||
| 3.14 Hypertension | Other data | No numeric data | ||
| 3.15 Hypotension | Other data | No numeric data | ||
| 3.16 Respiratory, thoracic and mediastinal disorders | Other data | No numeric data | ||
| 3.17 Proportion of patients with micro‐nucleated (Mn) peripheral lymphocytes | Other data | No numeric data | ||
| 4 Gastrointestinal system | Other data | No numeric data | ||
| 4.1 Abdominal pain | Other data | No numeric data | ||
| 4.2 Constipation | Other data | No numeric data | ||
| 4.3 Decreased appetite | Other data | No numeric data | ||
| 4.4 Decreased weight | Other data | No numeric data | ||
| 4.5 Diarrhoea | Other data | No numeric data | ||
| 4.6 Dry mouth | Other data | No numeric data | ||
| 4.7 Gastrointestinal adverse events | Other data | No numeric data | ||
| 4.8 Nausea | Other data | No numeric data | ||
| 4.9 Upset stomach | Other data | No numeric data | ||
| 4.10 Vomiting | Other data | No numeric data | ||
| 4.11 Dyspepsia | Other data | No numeric data | ||
| 4.12 Gastroenteritis | Other data | No numeric data | ||
| 4.13 Increased appetite | Other data | No numeric data | ||
| 4.14 Xerostomia | Other data | No numeric data | ||
| 5 Musculoskeletal system | Other data | No numeric data | ||
| 5.1 Chest pain | Other data | No numeric data | ||
| 5.2 Height | Other data | No numeric data | ||
| 5.3 Weight | Other data | No numeric data | ||
| 5.4 BMI | Other data | No numeric data | ||
| 5.5 Height z score | Other data | No numeric data | ||
| 5.6 Weight z score | Other data | No numeric data | ||
| 5.7 BMI z score | Other data | No numeric data | ||
| 5.8 Increased mobility | Other data | No numeric data | ||
| 5.9 Bone mineral density | Other data | No numeric data | ||
| 6 Immune system | Other data | No numeric data | ||
| 6.1 Nasopharyngitis | Other data | No numeric data | ||
| 6.2 Pyrexia | Other data | No numeric data | ||
| 6.3 Allergies | Other data | No numeric data | ||
| 6.4 Cold symptoms | Other data | No numeric data | ||
| 6.5 Pharyngitis | Other data | No numeric data | ||
| 6.6 Viral infection | Other data | No numeric data | ||
| 6.7 Rash | Other data | No numeric data | ||
| 6.8 Rhinitis | Other data | No numeric data | ||
| 6.9 Ear disorders | Other data | No numeric data | ||
| 6.10 Fever | Other data | No numeric data | ||
| 6.11 Infections | Other data | No numeric data | ||
| 7 Urogenital system: enuresis | Other data | No numeric data | ||
| 8 Other body systems | Other data | No numeric data | ||
| 8.1 Hair loss | Other data | No numeric data | ||
| 8.2 Skin problems | Other data | No numeric data | ||
| 8.3 Accidental injury | Other data | No numeric data | ||
| 8.4 Voice frequency | Other data | No numeric data | ||
| 8.5 Hypersalivation | Other data | No numeric data | ||
| 8.6 Nasal bleeding | Other data | No numeric data | ||
| 8.7 Chromosomal aberration | Other data | No numeric data |
Comparison 8. Non‐comparative studies: proportion of participants withdrawn from methylphenidate treatment due to non‐serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants withdrawn from methylphenidate treatment due to non‐serious adverse events | Other data | No numeric data | ||
| 2 Proportion of participants withdrawn from methylphenidate for unknown reasons | Other data | No numeric data |
Comparison 9. Patient reports/series: number of participants with serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Central nervous system | Other data | No numeric data | ||
| 1.1 Psychotic conditions | Other data | No numeric data | ||
| 1.2 Seizures | Other data | No numeric data | ||
| 1.3 Cerebral arteritis | Other data | No numeric data | ||
| 1.4 Self‐harm and suicidal behaviour | Other data | No numeric data | ||
| 1.5 Death | Other data | No numeric data | ||
| 1.6 Dyskinesia | Other data | No numeric data | ||
| 2 Cardiovascular and respiratory system | Other data | No numeric data | ||
| 2.1 Cardiovascular events | Other data | No numeric data | ||
| 2.2 Hypertension | Other data | No numeric data | ||
| 3 Gastrointestinal system: hepatoxicity | Other data | No numeric data | ||
| 4 Urogenital system: priapism | Other data | No numeric data |
Comparison 10. Patient reports/series: number of participants with non‐serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Central nervous system: movement‐related outcomes | Other data | No numeric data | ||
| 1.1 Tics | Other data | No numeric data | ||
| 1.2 Involuntary movements or dyskinesia | Other data | No numeric data | ||
| 2 Central nervous system: mood disorders | Other data | No numeric data | ||
| 2.1 Depression and disturbed mood | Other data | No numeric data | ||
| 2.2 Irritability | Other data | No numeric data | ||
| 2.3 Aggression | Other data | No numeric data | ||
| 2.4 Mania/euphoria | Other data | No numeric data | ||
| 3 Central nervous system: sleep problems | Other data | No numeric data | ||
| 3.1 Disturbed sleep | Other data | No numeric data | ||
| 3.2 Other sleep problems | Other data | No numeric data | ||
| 4 Central nervous system: voice and speech disorders | Other data | No numeric data | ||
| 4.1 Voice problems | Other data | No numeric data | ||
| 4.2 Stuttering | Other data | No numeric data | ||
| 5 Central nervous system: other specific adverse events | Other data | No numeric data | ||
| 5.1 Anorexia | Other data | No numeric data | ||
| 5.2 Obsessive compulsive disorder (OCD) symptoms | Other data | No numeric data | ||
| 5.3 Headache | Other data | No numeric data | ||
| 5.4 EEG changes | Other data | No numeric data | ||
| 6 Cardiovascular and respiratory system | Other data | No numeric data | ||
| 6.1 Vasculopathy | Other data | No numeric data | ||
| 6.2 Cardiovascular problems | Other data | No numeric data | ||
| 6.3 Bleeding | Other data | No numeric data | ||
| 6.4 Tachycardia | Other data | No numeric data | ||
| 7 Gastrointestinal system | Other data | No numeric data | ||
| 7.1 Loss of appetite | Other data | No numeric data | ||
| 7.2 Nausea and vomiting | Other data | No numeric data | ||
| 8 Musculoskeletal system: growth | Other data | No numeric data | ||
| 9 Immune system: allergic reactions | Other data | No numeric data | ||
| 10 Urogenitial system | Other data | No numeric data | ||
| 10.1 Testicular failure | Other data | No numeric data | ||
| 10.2 Sexual adverse events | Other data | No numeric data | ||
| 10.3 Incontinence | Other data | No numeric data | ||
| 10.4 Enuresis | Other data | No numeric data | ||
| 11 Other body systems | Other data | No numeric data | ||
| 11.1 Skin reactions | Other data | No numeric data | ||
| 11.2 Problems with sedation | Other data | No numeric data | ||
| 11.3 Eye problems | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abali 2007.
| Methods | A patient report of hallucinations during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 14 years old IQ: 99 Sex: female Methylphenidate naïve: not stated Ethnicity: not stated Country: Turkey Comorbidity: depression, behavioural disorder Comedication: fluoxetine (20 mg/day) Sociodemographics: not stated |
|
| Interventions | Methylphenidate dosage: 20 mg/day Administration schedule: 10 mg in the morning and 10 mg in the afternoon Duration of treatment: 15 days Treatment compliance: not stated |
|
| Outcomes | Serious adverse events: Methylphenidate for 15 days: audio‐visual hallucinations. Occurrence 30 minutes after ingestion. Duration: 30 minutes Cessation of methylphenidate: no hallucinations Further trials with methylphenidate caused hallucinations, which immediately disappeared after discontinuation |
|
| Notes |
Key conclusions of the study authors: here, we report a 14‐year old girl with a diagnosis of ADHD, major depression and conduct disorder. During her treatment with methylphenidate 20 mg/day and fluoxetine 20 mg/day, she developed visual and auditory hallucinations. It may be concluded that methylphenidate in some cases may cause hallucinations in patients Supplemental information regarding IQ received through personal email correspondence with the authors in November 2013 (Abali 2013 [pers comm]) |
|
Abbas 2006.
| Methods | A cohort study with 2 study samples (N = 60 and N = 30) where participants were part of a 6‐month audit regarding the use of methylphenidate and to determine if the NICE guidelines were followed | |
| Participants | Number of participants screened: 420 Number of participants included: 90 Number of participants followed up: not stated Number of withdrawals: not stated Sex: 85 males, 5 females Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 10 years old IQ: none with intellectual disability Methylphenidate‐naïve: not stated Ethnicity: not stated Country: UK Setting: not stated Comorbidity: 28.8% general learning difficulties, 21% sleeping problems, 28.8% mental health problems Comedication: not stated Sociodemographics: not stated Inclusion criteria:
|
|
| Interventions | Methylphenidate dosage: most were treated with Ritalin Administration schedule: not stated Duration of intervention: 6 months Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events: 12% reported side effects on methylphenidate, most were transient In 3 cases, medication was stopped due to side effects |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: for 3 patients the side effects resulted in medication stop. 2 patients stopped because of headaches and 1 because of hair loss Ethics approval: not stated Funding: none Vested interest/authors' affiliations: not stated Key conclusions of the study authors: the guidelines were followed, but not fully. However this was improved significantly in the second part due to increased professional awareness about ADHD because we held many seminars on this subject. The mental health team worked more closely than before with community paediatricians via joint ADHD clinics with child and adolescent psychiatrists. Families were getting better support via the ADHD family project which was jointly funded by health, education, and social services. The audits lead to a better provision of services for children with ADHD via specialist ADHD clinics Comments from the review authors: the reference was a poster, and no full text article has been published on the subject Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding study information received through personal email correspondence with the authors in December 2013 (DeSoysa 2013 [pers comm]) |
|
Abbasi 2011.
| Methods | A 6‐week, parallel group, RCT with 2 arms
|
|
| Participants | Number of participants screened: 68 Number of participants included: 40 Number of participants randomised: ALC + MPH: 20, P + MPH: 20 Number of participants followed‐up: MPH + P: 19 Number of withdrawals: MPH + P: 1 Methylphenidate + placebo Diagnosis of ADHD: DSM‐IV‐TR (combined type: 100%) Age: mean 8.36 (1.53), range: 7‐13 years old Sex: 25 males, 5 females Methylphenidate‐naïve: 100% Ethnicity: Persian Country: Iran Comorbidity: not stated Comedication: not stated IQ: > 70 Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to MPH and ACL or MPH and placebo Methylphenidate type: not stated Methylphenidate dosage: 20‐30 mg/day depending on weight (20 mg/day for < 30 kg and 30 mg/day for > 30 kg) Administration schedule: morning and midday Duration of intervention: 6 weeks Titration period: 3 weeks after randomisation Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events:
|
|
| Notes | Sample calculation: yes Any withdrawals due to adverse events: no Ethics approval: yes Funding: this study was supported by a grant from Tehran University of Medical Sciences Key conclusions of the study authors: the principal measure of outcome was the Teacher and Parent attention deficit/hyperactivity disorder Rating Scale‐ IV. No difference was observed between the 2 groups. Side effects consisting of headache and irritability were observed more frequently in the methylphenidate plus placebo group. The results of this study do not support the application of ALC as an adjunctive therapy to methylphenidate in children and adolescents with ADHD. Comments from the review authors: patients are randomised to receive MPH plus acetyl‐L‐carnitine (ALC) or MPH plus placebo: no outcome measures regarding effect are relevant, because the study does not include a placebo or no‐intervention group. The co‐intervention (ALC/placebo) is not the same in the 2 groups, and therefore we can only use AE data regarding group 2 (MPH+P group). Supplemental data has not been possible to receive from the authors through email correspondence in August and October 2013. No reply |
|
Adrian 2001.
| Methods | A patient report of a 10‐year‐old boy referred to a tertiary neurodevelopmental assessment clinic for a second opinion on the management of his ADHD, with particular concern being expressed about aggressive outbursts and poor tolerance of methylphenidate | |
| Participants | Diagnosis of ADHD: ICD‐10 (subtype: unknown) Age: 10 years old IQ: average intelligence Sex: male Methylphenidate naïve: not stated Ethnicity: not stated Country: UK Comorbidity: not stated Comedication: not stated Sociodemographics: Uneventful pregnancy |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: started 20 mg/day at 7 years and gradually increased to 40 mg/day Duration of treatment: 2‐3 years Treatment compliance: treatment was administered until 9 to 10 years of age when parents discontinued treatment due to obsessive compulsive symptoms |
|
| Outcomes | Non‐serious adverse events:
The other adverse events were not found at the assessment 10 months after he stopped methylphenidate treatment |
|
| Notes |
Key conclusions of the study authors: clinically explosive outbursts can be induced by the pharmacological treatment of ADHD and should not be mistaken for a symptom of the disorder Comments from the study authors: explosive episodes were coincident with a period of treatment with methylphenidate Funding/vested interest: not stated Authors' affiliations: Great Ormond Street Hospital London Supplemental information regarding diagnostic criteria and treatment duration obtained through personal email correspondence with the authors in October 2013 (Adrian 2013 [pers comm]) |
|
Agarwal 2008.
| Methods | A patient report of the combination of atomoxetine and methylphenidate in the treatment of ADHD | |
| Participants | Diagnosis of ADHD: DSM‐III‐R/DSM‐I (subtype: combined) Age: 8 years old IQ: normal (attends school) Sex: male Methylphenidate naïve: no Ethnicity: not stated Country: India Comorbidity: not stated Comedication: not stated. No atomoxetine (in the period relevant for this review) Sociodemographics: not stated |
|
| Interventions | Immediate release methylphenidate gradually increased to 50 mg/day Administration schedule: 3‐4 divided dosages Duration of intervention: 6 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Decreased appetite and delayed sleep onset |
|
| Notes | Key conclusions of the study authors: the combination of atomoxetine and methylphenidate may be used more often in patients who are not able to tolerate high doses of methylphenidate or develop tolerance to it | |
Aguilera‐Albesa 2010.
| Methods | 2 patient reports of the appearance of hallucinations few hours after methylphenidate ingestion | |
| Participants |
Case 1 Diagnosis of ADHD: DSM‐IV (subtype: inattentive) Age: 8 years old IQ: > 85 Sex: male Methylphenidate naïve: not stated Ethnicity: white Country: Spain Comorbidity: procedural learning disorder Comedication: not stated Sociodemographics: not stated Case 2 Diagnosis of ADHD: DSM‐IV (subtype: inattentive) Age: 6 years old IQ: > 85 Sex: female Ethnicity: white Country: Spain Comorbidity: none Comedication: none Sociodemographics: not stated |
|
| Interventions |
Case 1 Extended release methylphenidate 18 mg/day (0.51 mg/kg/day) Administration schedule: once daily Duration of intervention: 2 days Treatment compliance: not stated Case 2 50% extended release, and 50% immediate release methylphenidate 10 mg/day (0.45 mg/kg/day) for 3 days and 20 mg/day (0.9 mg/kg/day) for 1 day Administration schedule: once daily Duration of intervention: 4 days Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events:
Case 1 After the first dose: irritability, emotional lability, motor restlessness and facial motor tics After the second dose: added auditory hallucinations (noise and unintelligible verbal expressions), and visual hallucinations (shadows approaching and receding) 24 hours after discontinuation: the symptoms remitted Case 2 After the first dose and the following days: intermittent visual hallucinations (insects, especially flies, flying around her). The symptoms improved at night After increased dose (20 mg) on day 4: visual hallucinations were associated with dread of going outside and cries of panic 1 day after discontinuation: the symptoms remitted |
|
| Notes |
Key conclusions of the study authors: these patient reports suggest an individual susceptibility to psychotic symptoms after taking methylphenidate. This side effect is considered idiosyncratic, extraordinary and unpredictable. Case 2 suggests the existence of a dose‐effect relationship Supplemental information regarding diagnostic criteria and IQ received through personal email correspondence with the authors in July 2013 (Aguilera‐Albesa 2013 [pers comm]) |
|
Akhondzadeh 2003.
| Methods | A 4‐week randomised, double‐blind, clinical trial, parallel‐design where children with ADHD are randomised to methylphenidate or selegiline | |
| Participants | Number of participants screened: not stated Number of participants included: 28 Number of participants randomised: selegiline: 14; methylphenidate: 14 Number participants followed‐up in each arm: selegiline: 13; methylphenidate: 10 Number of withdrawals in each arm: selegiline: 1; methylphenidate: 4 Methylphenidate group Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: mean 7.37 years old (SD 1.59) IQ: above 70 Sex: 10 males, 4 females Methylphenidate‐naïve: 100% Ethnicity: Persian Country: Iran Setting: outpatient clinic Comorbidity: not stated Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to receive treatment using either selegiline 5 mg/day (under 5 years of age) and 10 mg/day (over 5 years of age) or methylphenidate 1 mg/kg/day for a 4‐week double‐blind clinical trial Methylphenidate type: not stated Administration schedule: not stated Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events:
|
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: not stated Ethics approval: not explicitly stated but inferred: informed consent (parent and children) was received before the administration of any study procedure or dispensing of study medication in accordance with the ethical standards of the investigative site's institutional review board and with the Helsinki Declaration of 1975, as revised in 2000 Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: the results of the study must be considered preliminary, but they do suggest that selegiline may be beneficial in the treatment of ADHD. In addition, a tolerable side effect profile may be considered as one of the advantages of selegiline in the treatment of ADHD Supplemental information requested from the study authors twice in June 2013 with no answer |
|
Akhondzadeh 2004.
| Methods | A 6‐week double‐blind, placebo‐controlled, parallel group trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 22 Number of participants followed up: 20 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (subtype: combined (100%)) Age: mean: 7.73 (1.63), range: 5‐11 IQ: > 70 Sex: 12 males, 10 females Methylphenidate‐naïve: 100% Ethnicity: Persian Country: Iran Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 1 mg/kg/day (+ placebo: sucrose, 55 mg) Administration schedule: twice daily, 7 am and 3 pm Duration of each medication condition: 6 weeks Treatment compliance: not stated |
|
| Outcomes | Systematically recorded throughout the study and were assessed using a checklist administered by a resident of psychiatry on days 7, 14, 21, 28 and 42 Non‐serious adverse events:
|
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: none Ethics approval: not explicitly stated but inferred: informed consent (parent and children) was received before the administration of any study procedure or dispensing of study medication in accordance with the ethical standards of the investigative site's institutional review board and with the Helsinki Declaration of 1975, as revised in 2000 Funding/vested interests/authors' affiliations: not stated Key conclusions from study authors: this double‐blind, placebo‐controlled study demonstrated that zinc as a supplementary medication might be beneficial in the treat‐ ment of children with ADHD. However, further investigations and different doses of zinc are required to replicate these findings in children with ADHD. Comments from the study authors: the limitations of the present study, including lack of a full placebo group, using only a moderate dose of methylphenidate, the small number of participants, short period of follow‐up and lack of plasma zinc concentration should be considered so further research in this area is needed Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Alpaslan 2015.
| Methods | A patient report of stuttering associated with the use of methylphenidate | |
| Participants | Diagnosis of ADHD: DSM‐5 (subtype: predominantly hyperactive‐impulsive) Age: 7 years old IQ: 94 Sex: male Methylphenidate naïve: yes Ethnicity: white/Turkish Country: Turkey Comorbidity: none Comedication: none Sociodemographics: both parents had advanced no further than elementary school. The parents' history was unremarkable |
|
| Interventions | Short‐acting methylphenidate 10 mg/daily Administration schedule: not stated Duration of treatment: 4 weeks Treatment compliance: good according to regular visits record |
|
| Outcomes |
Non‐serious adverse events: 10 days after beginning short‐acting methylphenidate treatment, the client began to stutter. Treatment was stopped. 1 week later, the patient's speech was back to normal. 4 weeks later atomoxetine treatment was started with no reoccurrence of stuttering |
|
| Notes |
Key conclusions of the study authors: evidence for stuttering associated with the use of short‐acting methylphenidate is presented. Clinicians should be aware that an additional adverse effect of methylphenidate may be a beginning of a stutter Funding/vested interest: the authors received no financial support for the research and/or authorship of this article. The authors report no conflicts of interest Supplemental information regarding IQ, ethnicity and treatment compliance received through personal email correspondence with the authors in April 2016 (Alpaslan 2016 [pers comm]) |
|
Altin 2013.
| Methods | A observational, prospective and non‐interventional study of methylphenidate, atomoxetine and nootropic medication use for 12 months | |
| Participants | Number of participants screened: not stated Number of participants included: 546 Number of participants randomised: methylphenidate: 221. Atomoxetine: 234. Nootropic medicine: 91 Number followed‐up in each arm: methylphenidate: 133 (60.2%). Atomoxetine: 146 (62.4%). Nootropic med: 77 (84.6%) Number of withdrawals in each arm: methylphenidate: 52. Atomoxetine: 59. Nootropic med: 16 Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean: 9.6 (2.8) + 9.9 (2.7) + 9.4 (2.5), range: 6‐17 years old IQ: not stated Sex: male: 206 (88.0%) 180 (81.4%) 70 (76.9%) Methylphenidate‐naïve: not stated Ethnicity: white 129 (55.1%) + 93 (42.1%) + 91 (100.0%); Asian: 102 (43.6%) + 128 (57.9%) + 0 (0.0%); Black or African American: 2 (0.9%) + 0 (0.0%) + 0 (0.0%); other: 1 (0.4%) + 0 (0.0%) + 0 (0.0%). Country: Russian Federation, China, Taiwan, Egypt, United Arab Emirates, and Lebanon Setting: outpatient clinic Comorbidity: yes Comedication: yes Sociodemographics: not stated Inclusion criteria:
Exclusion criteria: Discontinuation of the original prescribed monotherapy |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: 12 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: 3 patients discontinued treatment in the methylphenidate group due to headache, anxiety and depressed mood |
|
| Notes | Sample calculation: yes Ethics approval: yes Funding/vested interest: sponsored by Eli Lilly Authors' affiliations: Eli Lilly Neuroscience, Eli Lilly & Company Turkey; Eli Lilly Egypt; Eli Lilly Canada; Lilly Research Laboratories, Indianapolis; Eli Lilly and Company, Hungary; Dept. of Neurology, Neurosurgery and Medical Genetics of Pediatric Faculty, Moscow, Russian Federation; Dept of Psychological Medicine, Children's Hospital of Fudan University, Shanghai, China Key conclusions of the study authors: after 12 months of treatment, clinical and functional outcomes were improved in children and adolescents from non‐Western countries who initiated and remained on their prescribed pharmacological monotherapy Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested from the study authors twice in June 2016 with no answer |
|
Amiri 2008.
| Methods | A 6‐week, parallel group, randomised controlled trial with 2 arms:
|
|
| Participants | Number of patients screened: not stated Number included: 60 Number randomised to methylphenidate: 30 and (modafinil): 30 Number followed‐up: MPH: 27 Number of withdrawals: MPH: 3 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (100%)) Age: mean 8.96 years old IQ: > 70 Sex: 24 males, 6 females Methylphenidate‐naïve: not stated Ethnicity: Persian Country: Iran Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to methylphenidate or modafinil Metylphenidate type: not stated Methylphenidate dosage: 20‐30 mg/day depending on weight (20 mg/day for < 30 kg and 30 mg/day for > 30 kg) Administration schedule: not stated Duration of intervention: 6 weeks Titration period: 3 weeks, initiated after randomisation Treatment compliance: not stated |
|
| Outcomes | Side effect checklist (20 side effects) administered by a child psychiatrist on days 7, 21 and 42 Haematology tests, baseline and weeks 2, 4 and 6 serum chemistry and urinalysis, baseline and week 6 Body weight and vital signs, baseline and weeks 1, 2, 4, and 6 12‐lead ECG and physical examinations, baseline and week 6 Non‐serious adverse events: Abdominal pain, anxiety/nervousness, decreased appetite, sadness, difficulty falling asleep, weight loss, nausea, dry mouth, irritability, headaches |
|
| Notes | Sample calculation: yes Ethics approval: yes Funding/vested interest: this study was supported by a grant (grant number: 3317) from Tehran University of Medical Sciences Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: no significant differences were observed between the 2 groups (modafinil vs methylphenidate) on the Parent and Teacher Rating Scale scores. Side effects of decreased appetite and difficulty falling asleep were observed more in the methylphenidate group. The results of this study indicate that modafinil significantly improved symptoms of ADHD and was well tolerated and it is beneficial in the treatment of children with ADHD Comments from the study authors: the limitations of the present study, including lack of a placebo group, using only ADHD Rating Scale for measuring outcome and the small number of participants should be considered so further research in this area is needed Comments from the review authors: checklist with 20 possible side effects administered, but only the 10 observed side effects are reported Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental adverse event data has not been possible to receive through personal email correspondence with the authors in June‐August 2013 |
|
Arabgol 2015.
| Methods | A 6‐week double‐blind clinical trial comparing methylphenidate and risperidone | |
| Participants | Number of participants screened: 38 Number of participants included: 33 Number of participants randomised: methylphenidate: 18; risperidone: 20 Number of participants followed‐up in each arm: methylphenidate: 15; risperidone: 18 Number of withdrawals in each arm: methylphenidate: 3; risperidone: 2 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (57.57%), hyperactive‐impulsive (33.33%), inattentive (9.09%)) Age: mean for the methylphenidate group: 4.73 (SD 0.77) years old (range 3‐6) IQ: > 70 Sex (in the methylphenidate group): 12 males, 3 females Methylphenidate‐naïve: not stated, but all were with no drug history since 2 weeks ago Ethnicity: not stated Country: Iran Setting: outpatient clinic Comorbidity: not stated Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Metylphenidate was started at dose 2.5 mg/day and gradually (every week) increased based on the therapeutic response and the patient's tolerance Methylphenidate type: not stated Mean methylphenidate dosage: 12.83 (SD 0.56) mg/day Administration schedule: optimal dose was 20 mg/day in 2 divided doses Duration of intervention: 6 weeks Washout prior to study initiation: 2 weeks Treatment compliance: not stated |
|
| Outcomes | Patients were assessed by a child and adolescent psychiatrist at baseline (T0); T1, 2 weeks; T2, 4 weeks and T3, 6 weeks after the intervention started. Side effects were measured using the Methylphenidate Side Effects Form Non‐serious adverse events: One 4.5‐year old girl discontinued due to severe anorexia in the second week. One 5‐year old boy discontinued because of crying and sadness in the second week. One 5‐year old boy discontinued due to increased symptoms and aggression More common side effects were anorexia (55.55%), nervousness (33.33%) and disturbed sleep (27.77%) |
|
| Notes | Sample calculation: no Any withdrawals due to adverse events: 3 Ethics approval: yes, the Hospital's Institutial Review Board and Iranian Randomized Clinical Trial Funding/vested interest: none. Funded by Behavioral Sciences Research Center affiliated with Shahid Beheshti Medical University Authors' affiliations: not stated Key conclusions of the study authors: this double‐blind, randomised and controlled study suggests risperidone is effective in alleviating total symptoms of ADHD and related co‐morbid symptoms in preschoolers and that there is no statistically significant difference between the 2 groups in alleviating ADHD symptoms Comments from the study authors: our study assessed a relatively small sample of patients in a short‐term intervention. ADHD is a generally chronic neuropsychiatric condition that may last many years, we do not know whether therapeutic effects of risperidone last for a long term or not. Also further investigations are needed to assess long‐term safety when risperidone is prescribed for very young children, regarding the possibility of adverse effects on the developing brain. Also trials are needed to evaluate eventual metabolic and cardiovascular adverse effects of risperidone in very young children. We recommend further future studies to assess the therapeutic efficacy of risperidone in preschooler ADHD with different co‐morbidities Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Ardic 2014.
| Methods | A retrospective chart review of osmotic release oral system methylphenidate and immediate release methylphenidate use for 8 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 122 (68 in the OROS‐MPH group and 54 in the IR‐MPH group) Diagnosis of ADHD: DSM‐IV (subtype: combined (89.00%), hyperactive‐impulsive (0%), inattentive (11%)) Age: mean 9.1 (1.7), range: 7‐15 years old IQ: OROS‐MPH: 98.2 (SD 16.5). IR‐MPH: 99.4 (SD 12.8) Sex: 98 males, 24 females (OROS‐MPH: 58 males, 10 females; IR‐MPH: 40 males, 14 females) Methylphenidate naïve: not stated Ethnicity: not stated Country: Turkey Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) and immediate release Mean methylphenidate dosage: OROS‐MPH 30.8 (SD 11.5) mg/day; IR‐MPH 27.5 (SD 6.1) mg/day Administration schedule: OROS‐MPH once daily, IR‐MPH twice daily Duration of intervention: 8 weeks Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: No severe or life‐threatening adverse effects were reported in either group Non‐serious adverse events: ≥ 1 adverse effect in 76% of the OROS‐MPH group and in 79.6% of the IR‐MPH group. 88% adverse events disappeared/decreased over time. Emotional changes were more frequent in IR‐MPH than OROS‐MPH group (51.9% and 32.4%, respectively; P = 0.03) |
|
| Notes | Sample calculation: not stated
Ethics approval: local approval was obtained from hospital administration for using the data on the Hospital Information System retrospectively
Funding/vested interest/authors' affiliations: while making the study no support has been taken. Eyup Sabri Ercan is in charge in the advisory board of Lilly and Janssen‐Cilag, and the other authors declare no competing financial interests
Key conclusions of the study authors: OROS‐MPH was effective and safe for Turkish children and adolescents, compared to MPH‐IR Comments from the study authors: an important limitation of our study is our inability to assess treatment adherence in both medication groups. 88% of the adverse effects in the OROS‐MPH group and 86% of those in the IR‐MPH group decreased or disappeared over time Comments from the review authors: adverse events rating scale was created by study authors, but it seems to be very similar to Barkley rating scale. According to the Methods section, heart rate, blood pressure, and weight were measured but are not reported in the paper Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not explicitly stated, but it seems that only methylphenidate‐responders were included Supplemental information regarding heart rate, blood pressure and weight has not been possible to receive from the authors. We have written twice in June 2016 without reply |
|
Arman 2013.
| Methods | A cohort study measuring heart rate and cardiac abnormalities at the second to fourth week of treatment with methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 15 Number of participants followed‐up: 15 Number of withdrawals: 0 Diagnosis of ADHD: not stated (subtype: combined (53%), inattentive (47%)) Age mean: 9.08 years (range: 7‐13 years) IQ: not stated Sex: 11 males, 4 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Turkey Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria: None stated Exclusion criteria:
|
|
| Interventions | Methylphenidate dosage: 0.25‐1 mg/kg/day Mean methylphenidate dosage: 0.6 mg/kg/day Administration schedule: not stated Duration of intervention: not stated, but the outcome measures were monitored from week 2 to 4 Treatment compliance: not stated |
|
| Outcomes | Serious adverse events: Cardiac rhythm abnormalities monitored by 12‐lead‐surface electrocardiogram and 24‐hour‐ambulatory Holter monitorisation Non‐serious adverse events: Heart rate variability (HRV) monitored by 12‐lead‐surface electrocardiogram and 24‐hour‐ambulatory Holter monitorisation |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interest/authors' affiliations: not stated Any withdrawals due to adverse events: not stated Key conclusions of the study authors: our study showed a significantly increased heart rate and decreased heart rate variability due to methylphenidate treatment in children with ADHD, suggesting an increased sympathetic tonus especially at the daytime. Risk of sudden cardiac death and serious arrhythmia has not been demonstrated Comments from the review authors: no full text available, only an abstract. No information on ADHD diagnosis so we are only able to use data on serious adverse events Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested through personal email correspondence with the authors in August 2014. They were not able to provide further information |
|
Arnold 2004.
| Methods | A 7‐centre US study consisting of a 6‐week, open‐label, dose‐titration phase (Part A) and a 2‐week, double‐blind, randomised, parallel, placebo‐controlled withdrawal study (Part B) with 2 arms:
|
|
| Participants |
Part A Number of participants screened: 116 Number of participants included: 89 Number of participants followed up: 76 Number of withdrawals: 13 Diagnosis of ADHD: DSM‐IV (subtype: combined (80%)) Age: range 6‐16 years old Sex: 72 males, 17 females IQ: not stated Methylphenidate‐naïve: 71,9% Ethnicity: not stated Country: USA Comobidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Part A Methylphenidate type: dexmethylphenidate Methylphenidate dosage: 2.5 to 10 mg twice daily depending on individual participants' prior medication experience Children who had received dl‐MPH began with half their total daily dl‐MPH dose administered as d‐MPH, but not more than 20 mg/day; those who had not previously received dl‐MPH started d‐MPH at 2.5 mg twice daily Duration of intervention: 6 week Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Part A and B: monitoring AEs and changes from baseline in vital signs (pulse and blood pressure), physical examination, and clinical laboratory parameters throughout the study |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding: not stated Vested interests/authors' affiliations: Key conclusions of the study authors: d‐MPH is safe, tolerable, and effective, with a 6‐hour duration of effect suggested by the significant difference from placebo at 6 hours on a double‐blind discontinuation Comments from the study authors: limitations: study design (withdrawal): treatment effects in such trials may be larger than those seen in unselected populations, because randomised, withdrawal phase preselected responders to the drug from the open‐label titration phase. Another possible limitation is the duration of the discontinuation (2 weeks) Supplemental information received through email correspondence with the authors in October 2013 (Arnold 2013b [pers comm]) However, authors advise us to contact the sponsoring drug company for additional information. We contacted them in November 2013 (Jones 2013 [pers comm]), but did not succeed in getting further information. |
|
Arnold 2010.
| Methods | A multisite cohort study of transdermal methylphenidate following abrupt withdrawal of extended release methylphenidate with follow‐up of 4 weeks | |
| Participants | Number of participants screened: 194 Number of participants included: 171 Number followed‐up: 150 but 164 in ITT analysis Number of withdrawals: 21 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (77%), hyperactive‐impulsive (2%), inattentive (21%)) Age: mean 9.4 years IQ: normal Sex: 117 males, 47 females Methylphenidate‐naïve: none Ethnicity: white (79%), African American (12%), Asian (0.6%), others (9%) Country: USA Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Transdermal methylphenidate following abrupt withdrawal of extended release methylphenidate Week 1: predefined dose‐transition schedule based on previous daily dose of oral extended release methylphenidate Week 2 + 3: transdermal methylphenidate titration Week 4: stable dose transdermal methylphenidate Mean final transdermal methylphenidate dosage: 26.63 mg/day Administration schedule: patches were applied to alternating hips once daily in the morning and were worn for up to 9 hours per day Duration of intervention: 28 days Treatment compliance: compliance with the treatment regimen was measured by patch counts weekly. Data not stated |
|
| Outcomes | Participant and/or parent reporting of adverse events, application site reactions (assessed on a scale ranging from 0 (no irritation) to 7 (strong reaction)), physical examinations, vital signs and ECGs. Adverse events were recorded throughout the study and for 30 days after the last dose of study drug Serious adverse events: No deaths were reported No reports of sudden death, stroke, or other serious cardiovascular events
Non‐serious adverse events:
|
|
| Notes | Sample calculation: yes Ethics approval: yes Funding: Shire Development Inc, USA Vested interest/authors' affiliations: multiple authors are employees or have received research support from pharmaceutical companies, including Shire, Novartis, Ortho‐McNeil Pharmaceuticals and Neuropharm Key conclusions of the study authors: abrupt conversion from a stable dose of oral ER‐MPH to transdermal MPH was accomplished using a predefined dose‐transition schedule without loss of symptom control; however, careful titration to optimal dose is recommended. Most AEs were mild to moderate and, with the exception of application site reactions, were similar to AEs typically observed with oral MPH This study demonstrates that MTS, when carefully titrated to optimal dose, may further improve child and family HRQL, as well as behavioural, medication worry, and economic impact item scores in participants switching to MTS from a stable dose of routinely prescribed oral ER‐MPH after a short treatment period. Furthermore, following the abrupt conversion from oral ER‐MPH to MTS, the majority of caregivers reported being highly satisfied with MTS as a treatment option for their children with ADHD. (Bukstein 2009) Comments from the study authors: study limitations: open‐label, with no control group, non‐randomised design. Relatively short duration. Spontaneous AE reporting. These results may not be generalisable to participants who are not on a stable dose of oral methylphenidate or who have a poor response Supplemental information regarding adverse outcome data received through personal email correspondence with the authors in September 2013 (Arnold 2013 [pers comm]) |
|
Artul 2014.
| Methods | A patient report of severe recurrent pancreatitis during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM (version and subtype: not stated) Age: 10 years old IQ: > 70 Sex: male Ethnicity: Arabic Country: Israel Comorbidity: none Comedication: none Sociodemographics: good socioeconomic status |
|
| Interventions | Methylphenidate dosage: 30 mg daily Administration schedule: not stated Duration of treatment: 3 weeks Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: After 3 weeks of treatment at 30 mg/daily: severe relapsing pancreatitis, 3 times in 2 months within 3 weeks after starting treatment with methylphenidate. Discontinuation: free of symptoms |
|
| Notes |
Key conclusions of the study authors: acute pancreatitis in paediatric age could be due to the use of methylphenidate Funding/vested interests: the authors have no conflicts of interest to disclose Authors' affiliations: Department of Radiology, Nazareth Hospital, EMMS, Faculty of Medicine, Bar‐Ilan University, Israel. Faculty of Medicine in the Galilee, Bar‐Ilan University, Safed, Israel Department of Nuclear Medicine, Meir Hospital, 44410 Betah Tekva, Israel. Department of Internal Medicine, EMMS Hospital, 16100 Nazareth, Israel. Pediatric Department, Nazareth Hospital, Israel Supplemental information regarding IQ, ethnicity, comorbidity and comedication received through personal email correspondence with the authors in August 2016. No information regarding exact IQ level was available, but authors state that it was above 70 (Artul 2016 [pers comm]) |
|
Arun 2014.
| Methods | 2 patient reports of suicidal ideation during methylphenidate treatment | |
| Participants |
Case 1 Diagnosis of ADHD: DSM‐IV TR (subtype: combined) Age: 8 years old IQ: > 70 Sex: male Ethnicity: Hindu Country: India Comorbidity: none Comedication: none Sociodemographics: living in joint family Case 2 Diagnosis of ADHD: DSM‐IV TR (subtype: combined) Age: 7 years old IQ: average Sex: male Ethnicity: Hindu Country: India Comorbidity: none Comedication: none Sociodemographics: living in nuclear family |
|
| Interventions |
Case 1 Immediate release methylphenidate dosage: 5 mg/day Administration schedule: once daily in the morning Duration of treatment: 2 days Treatment compliance: not stated Case 2: Methylphenidate type: not stated Methylphenidate dosage: 10 mg/day Administration schedule: once daily Duration of treatment: 12 days Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events Suicidal ideation Non‐serious adverse events Impairment in sleep onset for 1 night |
|
| Notes |
Key conclusions of the study authors: depressed mood or affective symptoms may occur as an adverse effect during Methylphenidate treatment, and impulsivity may result in attempted suicide even in ADHD children without depression. In view of the current findings and existing literature, clinicians need to be alert to the adverse effects of methylphenidate during examination of every case. Equally important is ensuring that the patients' parents and teachers of the patients are appropriately educated regarding potential adverse effects of methylphenidate Comments from the study authors: there was no depressive symptoms reported along with it, and the ideation could not be explained on the basis of impulsivity either Funding/vested interest: nil, none otherwise declared Authors' affiliations: Department of Psychiatry, Government Medical College and Hospital, Chandigarh, India Supplemental information received through personal email correspondence with the authors in September 2014 (Arun 2014 [pers comm]) |
|
Ashkenasi 2011.
| Methods | A single‐centre, open‐label, randomised cross‐over study of transdermal methylphenidate effect on sleep disturbance, where participants were randomised to 1 of 4 groups with different sequences of patch wear time (9,10,11,12 hours a day)
Phases:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 26 Number of participants followed up: 24 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (subtype: combined (77%)) Age: mean 9.3 years, range 6‐12 years old IQ: > 70 Sex: 19 males, 7 females Methylphenidate‐naïve: not stated Ethnicity: not stated Comorbidity: sleep disturbances Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to different drug condition orders Methylphenidate type: methylphenidate transdermal system Mean methylphenidate dosage: 20 mg Administration schedule: once daily in the morning, switching between 9, 10, 11 and 12 hour alternating wear time across 4 consecutive weeks. Patch wear times were maintained Monday through Thursday of each week. Patients followed the standard 9‐hour wear time schedule on weekends (i.e. Friday through Sunday) Duration of intervention: 4 weeks Washout before study initiation: 1 week before titration period to optimal dose. And a minimum of 12 hours between 2 consecutive days during the trial Titration period: before randomisation Treatment compliance: 1 participant discontinued because of non‐compliance. No information about the others |
|
| Outcomes |
Serious adverse events: Hallucinosis: 1 child "withdrew after completing the randomization phase because of a significant medication side effect that emerged later in the study (i.e., hallucinosis)". No hospitalisation, symptoms withdrew after medication was discontinued Non‐serious adverse events: Skin reactions were coded on a 3‐point scale, where 0 ¼ no change, 1 ¼ slight pink/pink, and 2 ¼ slight red/ red. Rated by caregivers Sleep was assessed according to a daily sleep diary, with entries for time of patch application and removal, sleep timing (e.g. bedtime, time to fall asleep, number of awakenings, time awake at night, and final wake time), and ratings of sleep quality (on a 5‐point scale). Entries were recorded at baseline, after determination of the optimal patch dose, daily during wear time titration, and at endpoint. The sleep diary was documented by caregivers Monday through Sunday of every week throughout the wear time titration |
|
| Notes | Funding: investigator‐sponsored grants from Shire Pharmaceuticals, Inc. and Noven Pharmaceuticals, Inc.
Vested interest/authors' affiliations: Shire Pharmaceuticals, Noven Pharmaceuticals. "..these companies were not involved in the conduct of the study or the preparation of the manuscript" Key conclusions of the study authors: patch wear time exerted no significant effect on sleep latency or total sleep time, although a trend toward improved sleep quality was evident (P ¼ 0.059) with longer patch wear times. Sleep parameters were not adversely affected by longer methylphenidate transdermal system patch wear times Comments from the study authors: limitations of the study: small sample size, for instance resulting in a weaker randomisation to patch wear time sequence that was not completely effective in balancing baseline covariates Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes. Children with previous intolerance or adverse events on methylphenidate were excluded Supplemental information received through personal email correspondence with the authors in September 2013. Not possible to get the necessary supplemental information on non‐serious adverse events (Ashkenasi 2013 [pers comm]) |
|
Atzori 2009.
| Methods | A cohort study of methylphenidate use for 36 months and factors influencing compliance and persistent use | |
| Participants | Number of participants screened:187 Number of participants included: 134 Number of participants followed up: 134 Number of withdrawals: 72 (were followed up but had stopped treatment at 36 months follow‐up) Diagnosis of ADHD: DSM‐IV (subtype: combined (83.6%), hyperactive‐impulsive (4.5%), inattentive (11.9%)) Age: mean 9 (SD 2), range 4‐16 IQ: 83 (SD 16), range 51‐114; 88 (SD 19), range 54‐129; 84 (SD 18), range 41‐124 Sex: 122 males, 12 females Methylphenidate‐naïve: none Ethnicity: not stated Country: Italy Comorbidity: 80.6% Comedication: not stated Sociodemographics: double parents: 74.6%. Urban residence: 56% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: immediate release methylphenidate Methylpenidate dosage: mean 0.42 mg/kg/per dose, range 0.3‐0.5 Administration schedule: 2‐3 daily doses of maximum 1 mg/kg per dose Duration of intervention: 36 months (15.5 months in those suspended for functional remission, 14.7 months in those suspended for other reasons) Treatment compliance: 14.9% non‐adherent (non‐adherence as taking less than 80% of medication for ≥ 8 months per year) |
|
| Outcomes | Clinician‐designed questionnaire asking for the adverse events reported in the Switzerland SPC, self‐reported, unclear 53 children did not start chronic treatment due to side effects |
|
| Notes | Sample calculation: none Ethics approval: not stated Funding/vested interests: supported in part by Sardinian Public Health Secretariat and by the Agenzia Italiana del Farmaco Authors' affiliations: Dr Zuddas has received research grants from Eli Lilly and Shire Laboratories and has been a speaker for Eli Lilly and Sanofi‐Synthelabo and has an advisory or consulting relationship with Eli Lilly, Shire Laboratories, UCB, and Astra Zeneca Key conclusions of the study authors: clinical outcome of ADHD treatment is heterogeneous. Specific clinical and social predictive parameters for long‐term methylphenidate use and compliance can be identified. An accurate tailoring of clinical intervention to the individual child appears crucial for good outcome Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes |
|
Ayaz 2014.
| Methods | A case‐control study of methylphenidate use for 12 months | |
| Participants | Number of participants screened: 2171 Number of participants included: 1348 Number of participants followed up: 877 (methylphenidate only: 788) Number of withdrawals: 195 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean continuing: 9.01 (SD 2.36); mean discontinuing: 9.52 (SD 2.37). Range: 6‐18 years old IQ: > 70 Sex: 687 males, 132 female Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Turkey Comorbidity: ODD, CD, learning disorders, mood disorders, anxiety disorders, tic, elimination disorders Comedication: atomoxetine Sociodemographics: low educational level of mother: children continuing: 67.9%; children discontinuing: 73.4%, low educational level of father: children continuing: 54.3%; children discontinuing: 56.9% Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate and extended release Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: 12 months Treatment compliance: 265 continued treatment 12 months after the initiation of the prescription |
|
| Outcomes | Since data includes atomoxetine no outcomes are usable | |
| Notes | Sample calculation: no Any withdrawals due to adverse events: yes Ethics approval: yes Funding/vested interest/authors' affiliations: none stated Key conclusions of the study authors: medication persistence, in Turkish children, can be influenced by younger age, higher hyperactivity/impulsivity scores, use of long acting MPH, addition of another ADHD medication, and addition of other psychotropic medications, absence of adverse events, efficacy level perceived by the parents Comments from the study authors: limitations due to retrospective approach of the study, the determination of medication persistence, lack of sufficient data about the treatment efficacy and side effects after each medication was switched, and information obtained on the side effects spontaneously by parental reports. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, methylphenidate responders only Supplemental information requested from the study authors in April 2016. No answer |
|
Balázs 2011.
| Methods | A cohort study of dyskinesia in children routinely using methylphenidate after a single dose of methylphenidate compared to community controls | |
| Participants | Number of participants screened: 94 Number of participants included in the ADHD group: 37 Number of cases followed up: 34 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (subtype: combined (67.6%), hyperactive‐impulsive (10.8%), inattentive (21.6%)) Age: mean 10.8 years, range 7‐17 years old IQ: normal Sex: 32 males, 5 females Methylphenidate‐naïve: 0%. Median prior exposure to methylphenidate: 1074 days Ethnicity: not stated Country: Hungary Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dose: not stated Administration schedule: single dose, the same dose which they had been prescribed for their regular treatment Duration of treatment: 1 day Treatment compliance: 3 children left the study after baseline measurements were taken |
|
| Outcomes |
Non‐serious adverse events: Dyskinesia measured before and 90‐120 minutes after administration of a single dose of methylphenidate by using the Abnormal Involuntary Movement Scale (AIMS) (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe) |
|
| Notes | Sample calculation: not stated Ethics approval: yes, the study was approved by the Regional Ethics Committee Funding: none reported Vested interest/authors' affiliations: the authors report no conflict of interest Key conclusions of the study authors: methylphenidate‐treated children with ADHD had more dyskinesia than children in the control group. Dyskinesia did not worsen after a single dose of methylphenidate Higher dyskinesia scores in the methylphenidate‐treated younger age group warrant caution in the methylphenidate treatment of ADHD; however, further studies are needed to clarify the possible causal relationship between dyskinesia and methylphenidate treatment and/or age and/or the disease itself Comments from the study authors: we found no relationship between prior methylphenidate exposure of the children with ADHD and the total severity of dyskinesia. We believe that this lack of association is because most of our patients had been receiving long‐term methylphenidate treatment before enrolment in the study. Limitations: no inclusion of treatment‐naïve children with ADHD Comments from the review authors: none of the participants were methylphenidate‐naïve, and the results from the measurements before methylphenidate administration (baseline) are therefore in fact reflecting long‐term use (median prior exposure to methylphenidate: 1074 days). This needs to be taken into account when assessing the results from the measurements after methylphenidate administration in the study (short‐term challenge). |
|
Barbaresi 2006.
| Methods | A study of stimulant use for ADHD in a birth cohort born between 1976 and 1982 | |
| Participants | Number of participants screened: 5718 Number of participants included: 379 Number of participants followed up: 295 using stimulants which included 251 receiving methylphenidate Number of withdrawals: 84 Diagnosis of ADHD: DSM‐IV (subtype: combined (67.5% stimulants, 72.1% MPH), hyperactive‐impulsive (7.4%, 4.8%), inattentive (24.6%, 21.1%)) Mean age at onset stimulant treatment: 10.4 (SD 3.6). Age at last follow‐up: 17.6 years IQ: not stated Sex: 284 males, 95 females (stimulant treatment). 198 males, 53 females (MPH) Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: yes, 2 stimulants Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: the dose of each stimulant was initially converted to methylphenidate equivalent units (MEUs) and then each dose was weighted by the duration of use. 24.4 (11.1) mg/day Administration schedule: not stated Duration of intervention: 33.8 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: 63 (22.3%) of the 283 children treated with stimulants had ≥ 1 side effect Dextroamphetamine were significantly more likely to be associated with a side effect compared to methylphenidate (10.0% vs 6.1%, OR 1.8, 95% CI 1.1 to 3.0; P = 0.034) 6.1% of 717 MPH treatment episodes (i.e. period of time during which participant was treated with MPH at a specific dose) were associated with a side effect |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interest: the project was supported by research grants from the Public Health Service, National Institutes of Health and by an investigator‐initiated research grant from McNeil Consumer and Specialty Pharmaceuticals Authors' affiliations: Department of Pediatric and Adolescent Medicine, Division of Developmental and Behavioral Pediatrics, Mayo Clinic College of Medicine. Department of Health Sciences Research, Division of Clinical Epidemiology, Mayo Clinic College of Medicine. Department of Psychiatry and Psychology, Mayo Clinic College of Medicine. Department of Health Sciences Research, Division of Biostatistics, Mayo Clinic College of Medicine Key conclusions of the study authors: clinicians made appropriate treatment decisions; there are disparities in the rates ADHD treatment for boys/girls; efficacy was comparable to clinical trials Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
Barrickman 1995.
| Methods | Double‐blind, cross‐over study comparing methylphenidate and bupropion | |
| Participants | Number of participants screened: not stated Number of participants included: 18 patients Number of participants followed up: 15 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III‐R (subtype: not stated) Age: 11.8 (SD 3.3), range 7‐16 IQ: full scale WISC‐R score 106 (SD 10), range 84‐123 Sex: 12 males, 3 females Methylphenidate‐naïve: 5 (33.33%) Ethnicity: white Country: USA Comorbidity: conduct disorder (n = 2), oppositional defiant disorder (n = 2), developmental learning disorder (n = 5) Comedication: none. Before washout methylphenidate (n = 9), methylphenidate + imipramine (n = 1) Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders, methylphenidate or bupropion. Mean methylphenidate dosage: 31 (n = 11) mg/day (20‐60 mg/day) or 0.7 (n = 0.2) mg/kg/day (0.4‐1.3 mg/kg/day) Administration schedule: morning, noon, and 4 pm if needed Duration of intervention: 6 weeks Washout prior to study initiation: 14 days Medication‐free period between intervention: 14 days Titration period: methylphenidate was administered in a dose of 0.4 mg/kg per day during the first week and the to the maximum effective dose during the next 2 weeks. Dosage was fixed for the final 3 weeks Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events: Adverse effects checklist monitored by a physician by the end of each week. Physical examination Children's Depression Inventory (CDI), rated each week by physician. Revised Children's Manifest Anxiety Scale (R‐CMAS), rated each week by physician | |
| Notes | Sample calculation: yes Ethics approval: not stated Funding: not stated Vested interest/authors' affiliations: not stated Key conclusions of the study authors: bupropion and methylphenidate were both effective and did not differ in their overall efficacy as treatment for ADHD. Thus, results of nearly all of the rating scales trended in favour of methylphenidate. There is a possibility that this insignificant difference might be a function of the relatively small dose of bupropion used in this study Supplemental information requested through personal email correspondence with the authors in June 2014. No reply | |
Berek 2011.
| Methods | A multicentre, prospective, open‐label, single‐arm, non‐interventional study of methylphenidate use for 3 months. 5 visits: baseline, 1, 3 and 6 weeks of treatment, as well as after 3 months or upon premature termination | |
| Participants | Number of participants screened: not stated Number of participants included: 822 Number of participants followed up: 74% completed the 12‐week trial Number of withdrawals: total number of dropouts was not reported, 60 dropped out due to AEs Diagnosis of ADHD: ICD‐10 (subtype: F90.0: n = 519 (63.14%). F90.1: n = 316 (38.44%). F90.8: n = 14 (1.70%). F90.9: n = 21 (2.55%). Others: n = 65 (7.91%) Age: mean 11.1, range 6‐18 (6‐9 years old (n = 251, 30.54%), 10‐12 (n = 332, 40.39%), 13‐15 (n = 197, 23.97%), 16‐18 (n = 42, 5.11%)) IQ: not stated Sex: 698 (84.91%) males, 124 (15.09%) females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Germany Comorbidity: F91.X: n = 187 (22.75%). F91 incl. F91.3: n = 140 (17.03%). F41: n = 32 (3.89%). F42: n = 15 (1.82%). F1X: n = 6 (0.73%). None: n = 496 (60.34%). Other: n = 73 (8.88%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: OROS methylphenidate Methylphenidate dosage: starting dose 31.53 (SD 13.52) mg/day. Min: 18, med: 36, max: 108. Final dose: 35.47 (SD 14.04) mg/day Administration schedule: once daily Duration of intervention: 84.10 (SD 29.49) days Treatment compliance: not stated |
|
| Outcomes | Blood pressure and heart rate measured at baseline and all visits (week 1, 3, 6 and 12 or last visit), weight measured at first and last visit Adverse events were coded according to WHO Adverse Reaction Terminology (WHOART) Sleep quality and appetite were assessed at each visit, using a 5‐point scale with categories: very good, good, satisfactory, sufficient and insufficient |
|
| Notes | Sample calculation: not stated Ethics approval: independent ethics committee, Freiburg, Germany Funding: the study was funded by Janssen‐Cilag GmbH, Neuss Germany Vested interest/authors' affiliations: KR is a consultant working for GEM, Meerbusch, Germany, who was hired by Janssen‐Cilag to carry out the statistical analyses. LS and BS are employees of Janssen‐Cilag; at the time the study was conducted, MG was also an employee of Janssen‐Cilag. MG is currently employed by Ipsen Pharma. AL has, in the past 3 years, been a speaker for Shire and Novartis. He is not an employee or a shareholder of any of these companies and has no other financial or material support, including expert testimony, patents or royalties Key conclusions of the study authors: our study suggest a clinically meaningful increase in efficacy upon transitioning onto OROS methylphenidate in the treatment of children/adolescents with ADHD who had insufficient response to and/or poor tolerability with extended release methylphenidate or atomoxetine Comments from the review authors: the data extraction is based on Berek (2011), rather than an independent synthesis of the many articles published on this study Supplemental information requested through personal email correspondence with the authors in August 2014. No reply |
|
Bereket 2005.
| Methods | A longitudinally study of 16 months of methylphenidate treatment in newly diagnosed and untreated children | |
| Participants | Number of participants screened: not stated Number of participants included: 72 Number of participants followed up: 14 Number of withdrawals: 58 Diagnosis of ADHD: DSM‐IV (subtypes: not stated) Age: mean 8.12 years, range 6.47‐10.32 IQ: none with mental retardation Sex: 10 males, 4 females Methylphenidate‐naïve: 100% Ethnicity: Turkish Country: Turkey Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 0.75 mg/kg Administration schedule: not stated Duration of intervention: 16 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Height measured with a Harpenden stadiometer. Performed every 4 months Weight measured "with minimal clothing". Performed every 4 months Height and weight measurements were transformed to SDs before the statistical analyses using normative data for Turkish children. BMI values were transformed to SDs according to data from Cole 2007 |
|
| Notes | Sample calculation: no Ethics approval: approved by local ethics committee Funding: supported by a TUBITAK grant Vested interest/authors' affiliations: not stated Key conclusions of the study authors: prepubertal children with ADHD had normal height, weight, BMI, serum IGF‐I and IGFBP‐3 and thyroid functions. Methylphenidate treatment had no sustained effects on growth parameters, IGF‐I and IGFBP‐3 during the follow‐up period of this study. However, it caused a mild decrease in total and free T4, which may warrant further monitoring Supplemental information received through personal email correspondence with the authors in January 2014 (Bereket 2014 [pers comm]) |
|
Bernhard 2009.
| Methods | A patient report of a 4‐year‐old boy with T‐cell acute lymphoblastic leukemia and in maintenance therapy with purinethol and methotrexate experiencing a hepatotoxic reaction after onset of methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: hyperactive‐impulsive) Age: 4 years and 6 months old IQ: unknown Sex: male Ethnicity: not stated Country: Germany Setting: outpatient clinic Comorbidity: T‐ALL; severe damage to white matter induced by chemo‐ and radiotherapy Comedication: purinethol and methotrexate Sociodemographics: unknown |
|
| Interventions | Methylphenidate type: short acting Ritalin Methylphenidate dosage: 10 mg, 0.6 mg/kg Administration schedule: once daily in the morning Duration of treatment: 3 weeks titration. 6 weeks treatment in total. Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: A hepatotoxic reaction: liver enzymes were elevated prior to therapy. Vomiting starting 5 weeks after onset of methylphenidate therapy and increased within the next 3 days. Abdominal pain increased in intensity. Liver transaminases elevated over 30 times of the normal level, CK level also increased |
|
| Notes |
Key conclusions of the study authors: hepatotoxicity of methylphenidate can be considered minimal. However, methylphenidate therapy in children with prior or possible hepatic damage should be monitored clinically and by laboratory testing in short intervals Comments from the study authors: liver enzymes elevated prior to treatment Funding/vested interests: none Authors' affiliations: Hospital for Children and Adolescents, University of Leipzig, Leipzig, Germany |
|
Blader 2010.
| Methods | A non‐randomised trial with no placebo group of methylphenidate use and the factors associated with aggression that is responsive versus refractory to individualised optimisation of stimulant monotherapy | |
| Participants | Number of participants screened: 304 Number of participants included: 68 Number of participants followed up: 65 Number of withdrawals: 3 Diagnosis of ADHD: not stated (subtype: not stated) Age: mean: 8.95, range: 6‐13 years old IQ: > 70 Sex: 50 males, 15 females Methylphenidate‐naïve: none Ethnicity: white: 72%, African American: 12%, Hispanic: 8%, others: 8% Country: USA Setting: outpatient clinic Comorbidity: ODD: 94%; CD: 6%; anxiety disorder: 29%; depressive disorder: 65% Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: triphasic‐release methylphenidate Methylphenidate dosage: 64 mg (refractory) and 52 mg (non‐refractory) Administration schedule: weekly dosage adjustments from 18 mg of normally 18 mg increments to reach best tolerated dosage associated with greatest overall improvement in ADHD symptoms and aggression to a maximum dosage of 90 mg/day; once daily after waking but no later than 8.30 am Duration of intervention: average 63.26 days (SD 23.98) Treatment compliance: 3 withdrawals due to low adherence |
|
| Outcomes |
Non‐serious adverse events: Weight, observer, weekly Height, observer, weekly Blood pressure, observer, weekly Heart rate, observer, weekly Barkley Behaviour and Adverse Events Questionnaire Modified, parent, weekly A BBAEQ‐M item was considered present when the parent rated it at least moderate |
|
| Notes | Sample calculation: no Ethics approval: no, approved by institutional review boards of 2 clinical sites Funding/vested interests: National Institutes of Health Grants K23MH064975 and M01RR10710, a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression and a grant for investigator‐initiated research from Abbot Laboratories Authors' affiliations:all authors have received research support in the past from pharmaceutical companies and/or consulting and/or speaking fees. Key conclusions of the study authors: among children whose aggressive behaviour develops in the context of ADHD and of oppositional defiant disorder or conduct disorder, and who had insufficient response to previous stimulant treatment in routine clinical care, systematic, well‐monitored titration of stimulant monotherapy often culminates in reduced aggression that averts the need for additional agents Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, according to exclusion criteria no. 2 |
|
Buchmann 2007.
| Methods | A cohort study of methylphenidate use for 10‐14 days | |
| Participants | Number of participants screened: not stated Number of participants included: 18 Number of participants followed up: 18 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean: 11 years, SD: 1.91 years old IQ: > 85 Sex: 15 males, 3 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Germany Comorbidity: no psychiatric comorbidity Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: extended release methylphenidate Mean methylphenidate dosage: 0.78 (SD 0.26) mg/kg/day Administration schedule: once a day in the morning Duration of intervention: 10‐14 days Treatment compliance: children were inpatients for the duration of the study and received supervision when taking their medication |
|
| Outcomes | Data on adverse events were not systematically collected, so we could not use this data | |
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: not stated Ethics approval: not stated Funding/vested interest: not stated Authors' affiliations: Department of Child and Adolescence Psychiatry and Neurology University of Rostock, Rostock, Germany. Key conclusions of the study authors: the methylphenidate group had the short interval cortical inhibition, intracortical facilitation and long interval cortical inhibition changes restored by the medication ‐ this was a very similar finding, compared to control group Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: all participants were methylphenidate‐naïve Supplemental information regarding methods received through personal email correspondence with the authors in June 2014 (Buchmann 2014 [pers comm]) |
|
Cakin‐Memik 2010.
| Methods | A patient report of a 14‐year‐old male with ADHD who presented with priapism after administration of immediate‐release methylphenidate. When the usage of immediate‐release methylphenidate was terminated, priapism spontaneously disappeared | |
| Participants | Diagnosis of ADHD: not stated (subtype: not stated) Age: 14 years old IQ: not stated Sex: male Ethnicity: not stated Country: Turkey Comorbidity: no Comedication: no Sociodemographics: not stated |
|
| Interventions | Immediate‐release methylphenidate started at 10 mg/day and after 2 months increased to 20 mg/day Administration schedule: not stated Duration of treatment: 2 months and 3 days Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Priapism: up to 3‐4 episodes per day lasting 40 to 45 minutes beginning 3 days after the dose was increased to 20 mg/day |
|
| Notes | Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: in the case of immediate‐release methylphenidate prescription to adolescent male patients, the probability of the development of priapism should not be ignored The lack of inquiry and reporting of this side effect can lead to potentially irreversible impotence. Thus, it is important that the clinician be aware of this side effect and counsel the children/adolescents and their families about its occurrence in order to improve the adaptation of methylphenidate treatment Comments from the study authors: it is important to note that, as in this case, adolescent males may be too embarrassed to report this side effect particularly when they do not know it may be linked to methylphenidate |
|
Chandler 1989.
| Methods | A patient report of 2 boys on methylphenidate treatment. 1 with preexisting tics that were aggravated by stimulants and 1 with stimulant induced tics | |
| Participants |
Case 1 Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: 12 years old IQ: > 70 Sex: male Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Case 2 Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: 9 years old IQ: > 70 Sex: male Ethnicity: not stated Country: USA Comorbidity: none Comedication: not stated Sociodemographics: not stated |
|
| Interventions |
Case 1 Methylphenidate type: not stated Methylphenidate dosage: 0.2 mg/kg Administration schedule: twice daily Duration of treatment: 1 month Treatment compliance: not stated Case 2 Methylphenidate type: not stated Methylphenidate dosage: 0.2 mg/kg Administration schedule: twice daily Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Case 1: after a month, the child's mother reported motor and phonic tics again Case 2: he was treated first with methylphenidate, and then with nortriptyline, with both drugs he manifested severe facial grimacing and phonic tics. Apparently, the tics had only occurred in the past when the child was treated with methylphenidate |
|
| Notes | Funding/vested interests/authors affiliations: not stated Key conclusions of the study authors: we report 2 cases of AD/HD children, 1 with pre‐existing tics that were aggravated by stimulants and 1 with stimulant induced tics. With combined stimulant/L‐tryptophan treatment there was sustained improvement in AD/HD symptoms and no motor or phonic tics. Side effects such as hypomania, hyperreflexia, and diaphoresis have been reported in patients on the combination of a monoamine oxidase inhibitor and tryptophan. By itself, L‐tryptophan may be mildly sedating. In light of such a favorable side effect profile, and no evidence for adverse interaction with stimulants, and at least some rationale from preclinical research, it should be investigated further as a means of alleviating some of the harsh consequences of psychostimulant treatment in AD/HD Supplemental information regarding diagnosis and IQ received through personal email correspondence with the authors in September 2013 (Gualtieri 2013 [pers comm]) |
|
Chazan 2011.
| Methods | An open clinical trial of methylphenidate use for 6 months | |
| Participants | Number of participants screened: 189 Number of participants included: 130 Number of participants followed up: 125 Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (subtype: combined (63.1%), hyperactive‐impulsive (7.7%), inattentive (23.1%)) Age: mean 9.8 (2.8) years (range 5‐17) IQ: mean 92.34 (12.61) Sex: 96 males, 34 females Methylphenidate‐naïve: 86.9% Ethnicity: white: 77.7% Country: Brazil Comorbidity: ODD (46.9%); CD (13.1%); any mood disorder (13.1%); any anxiety disorder (40.8%) Comedication: yes Sociodemographics: classes: A + B (55%); C + D (45%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release and extended release Mean methylphenidate dosage: 0.48 mg/kg/day (SD 0.22) Administration schedule: not stated Duration of intervention: 6 months Treatment compliance: 76.4% adherence |
|
| Outcomes | Barkley side effects rating scale | |
| Notes | Sample calculation: no Ethics approval: yes Funding: this work was supported by research grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil; Edital MCT/CNPq 02/2006‐Universal, 478202/2006‐7) and Hospital de Clínicas de Porto Alegre Vested interests/authors' affiliations: Prof Rohde has served as a speaker and/or consultant for Eli‐Lilly, Janssen‐Cilag, and Novartis for the last 3 years. Currently, his only industry‐related activity is taking part in the advisory board/speakers' bureau for Eli Lilly, Novartis, and Shire (USD 10,000 per year and reflecting 5% of his gross income per year). The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by him received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Abbott, Bristol‐Myers Squibb, Eli‐Lilly, Janssen‐Cilag, Novartis, and Shire. Prof Polanczyk has served as a speaker for Novartis. Drs Chazan, Borowski, Pianca, and Ludwig report no conflict of interest Key conclusions of the study authors: our results suggest that ADHD combined subtype, maternal ADHD symptoms, and social adversities are independent negative predictors of methylphenidate response in children and adolescents with ADHD. Our study provides evidence for the involvement of clinical characteristics, maternal psychopathology, and environmental stressors in the response to methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
Cherland 1999.
| Methods | A retrospective cohort study conducted as a chart review | |
| Participants | Number of participants screened: 192 Number of participants included: 98 Number of participants followed up: 98 Number of withdrawals: not stated Diagnosis of ADD and ADHD: DSM‐III‐R and DSM‐IV (subtype: not stated) Age: range 4‐17 IQ: not stated Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Canada Comorbidity: not stated Comedication: 2% Sociodemographics: not stated Inclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: range 5‐80 mg per day Administration schedule: not stated Duration of treatment: mean 1 year and 9 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: The reviewers rated whether the symptoms suggesting psychosis were side effects of the medication or part of the child's psychopathology. DSM‐IV criteria for definitions for psychotic and mood‐congruent psychotic symptoms were used 98 children treated with stimulant medication
|
|
| Notes | Sample calculation: no Any withdrawals due to adverse events: methylphenidate was withdrawn from all children and adolescents experiencing psychotic symptoms Ethics approval: not stated Funding/vested interests: not stated Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: awareness of the potential for psychotic side effects from stimulant medications is important when prescribing for children. A large prospective study would be useful to predict the frequency and classification of the side effects in children Comments from the study authors: most of the children and adolescents improved upon withdrawal of the methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding IQ, sex distribution, comedication and safety data were requested through personal email correspondence with the authors in February 2014. No reply |
|
Cho 2012.
| Methods | A 12 week prospective, exploratory, open‐labeled cohort study to achieve symptomatic remission by OROS‐methylphenidate. The purpose of the study was to investigate a possible association between norepinephrine genes and cardiovascular side effects of OROS‐methylphenidate in Korean children with ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 101 Number of participants followed up: 101 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (67.6%), hyperactive‐impulsive (5.8%), inattentive (26.4%)) Age: 8.73 (SD 1.78) years, range 6‐12 IQ: 102.3 (SD 11.9) Sex: 81 males, 20 females Methylphenidate‐naïve: 100% Ethnicity: Asian Country: South Korea Comorbidity: anxiety disorders (8.8%), ODD (6.8%), transient tic disorder (6,8%), enuresis (5.9%) Comedication: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate: osmotic release oral system Mean methylphenidate dosage: 0.98 (SD 0.52) mg/kg Administration schedule: not stated Duration of intervention: 12 weeks Titration: initial dosage was determined by child's body weight: if ≥ 30 kg, 27 mg was administered; if < 30 kg, 18 mg was administered. The dosages were increased every 2 weeks for 9 weeks until they were sufficient to achieve a therapeutic effect, on the basis of the investigators and parents' assessments of symptoms and side effects, and then these doses were maintained until the 12th week. Treatment compliance: not stated |
|
| Outcomes | Electrocardiographic (ECG) parameters, including QT interval. Resting heart rate, seated pulse, blood pressure. All measures collected at 12 weeks after treatment. These measurements were obtained 60‐120 minutes after a dose was given. The cardiovascular parameters for all participants were measured manually | |
| Notes | Sample calculation: yes Ethics approval: approved by the institutional review board (IRB) for human subjects at the Seoul National University Hospital and at 5 other hospitals in South Korea. Parents/guardians provided written informed consent, and the children or adolescents provided verbal assent regarding participation in this study. Funding: BN Kim was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) and by the Korean Janssen Pharmaceutical Company Vested interest/authors' affiliations: Janssen Korea Key conclusions of the study authors: the overall cardiovascular effects of OROS‐methylphenidate were modest. However, our findings show a positive association between norepinephrine‐related gene polymorphisms and cardiovascular response induced by methylphenidate in Korean children with ADHD. Consideration must be given to such children or adults with specific norepinephrine‐related genotypes, especially if they show significant changes in heart rate or diastolic blood pressure after OROS‐methylphenidate administration Comments from the study authors: monitoring only occurred at baseline and after 12 weeks of methylphenidate treatment. 9% of the study participants with ADHD had co‐morbid anxiety disorders which may have affected cardiovascular measures. As this study was exploratory we did not apply multiple comparison corrections for the 3 cardiovascular outcomes; future studies may strengthen and perhaps extend the current findings by applying such corrections in larger samples. According to the inclusion and exclusion criteria, no children enrolled in this study had clinical histories of hypertension, hypotension or cardiovascular disease. Therefore, no conclusions can be made about the use of methylphenidate in children with significant cardiovascular dysfunction or risk factors Supplemental information regarding outcome measures and protocol requested twice in January 2014 with no answer |
|
Chou 2012a.
| Methods | A 10‐week non‐comparative observational study in 6 outpatient clinics identifying the optimal dose of OROS‐methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 521 Number of participants followed up: 439 Number of withdrawals: 82 Diagnosis of ADHD: DSM‐IV (subtype: combined (65.6%), hyperactive‐impulsive (3.1%), inattentive (31.3%)) Age: mean 10.4 years (range 7‐17) IQ: normal Sex: 461 males, 60 females Methylphenidate‐naïve: none Ethnicity: Asian Country: Taiwan Comorbidity: ODD (4.4%), anxiety disorder (0.2%), other disorders (3.5%) Comedication: clonidine (0.6%), antipsychotics (0.4%) Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system Methylphenidate dosage: participants receiving an immediate release methylphenidate dosage < 15 mg, 15‐30 mg, and > 30 mg day were converted to 18, 36, and 54 mg once daily Administration schedule: morning Duration of intervention: 10 weeks Treatment compliance: not stated |
|
| Outcomes | Parents or caregivers completed the Chinese version of the Barkley Side Effect Scale at each visit. An adverse event was any undesirable sign, symptom, or medical condition occurring after starting the therapy and was reported by investigators. Pre‐existing medical conditions/diseases were also considered adverse events if they worsened during treatment | |
| Notes | Sample calculation: no Any withdrawals due to adverse events: not stated Ethics approval: approved by the Joint Institute Review Board, Taipei, Taiwan Funding: the study was supported by Janssen‐Cilag, Taipei, Taiwan Vested interests/authors' affiliations: Janssen‐Cilag. 5 authors had conducted clinical trials on behalf of Eli & Lilly Co and 1 on behalf of Janssen‐Cilag. 9 were speakers and consultants for Janssen‐Cila Key conclusions of the study authors: the findings suggest remission as a treatment goal for ADHD therapy by providing an optimal dosage of medication for children and adolescents with ADHD through using an effective and tolerable forced‐titration scheme Comments from the review authors: data are not pertinent to our review. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information received through personal email correspondence with the authors in February 2014 (Chou 2014 [pers comm]) |
|
Chou 2012b.
| Methods | A prospective, single‐arm, open‐label, 8‐week, multicentre cohort study of methylphenidate use for 8 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 296 Number of participants followed up: 230 Number of withdrawals: 66 Diagnosis of ADHD: DSM‐IV (subtype: combined (67.9%), hyperactive‐impulsive (1%), inattentive (30.4%)) Age: mean 9.5 (SD 2.4), range 6.0‐17.0 years old IQ: not stated Sex: 247 males, 49 females Methylphenidate‐naïve: none Ethnicity: not stated Country: Taiwan Comorbidity: participants with serious or unstable medical illness, or who have clinically significant gastrointestinal problems, glaucoma, ongoing seizure disorders, or a psychotic disorder are excluded Comedication: participants who are taking concomitant medication that is likely to interfere with safe administration of methylphenidate are excluded. Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: 18 mg, 36 mg or 54 mg. Dose was adjusted for each participant based on clinical responses and/or side effects Administration schedule: once daily Duration of intervention: 8 weeks Treatment compliance: not stated |
|
| Outcomes | Adverse events (AEs) data were reported for each visit as total data for AEs; not analysed. In addition to the AEs reported in the below table, a category of AEs titled 'Other' was reported, as no dictionary was used and events under this category were not further specified. Total no. affected by other AEs is minimum number of participants affected | |
| Notes | Sample calculation: not stated Ethics approval: not stated Funding: sponsored by Johnson & Johnson Taiwan Ltd Vested interests/authors' affiliations: principal investigators are not employed by the organisation sponsoring the study. There is an agreement between principal investigators and the sponsor (or its agents) that restricts the PI's rights to discuss or publish trial results after the trial is completed.The only disclosure restriction on the PI is that the sponsor can review results communications prior to public release and can embargo communications regarding trial results for a period that is less than or equal to 60 days. The sponsor cannot require changes to the communication and cannot extend the embargo Key conclusions of the study authors: comparing with the baseline, OROS‐methylphenidate had been demonstrated that it could significantly improve participants' ADHD behavioural symptoms and their social adjustment at school and at home Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: participants hypersensitive to methylphenidate were excluded Supplemental information regarding IQ requested through personal email correspondence with the authors in August 2014 with no reply |
|
Cockcroft 2009.
| Methods | A comparative cohort study of sleepiness in ADHD children treated with methylphenidate compared to methylphenidate‐naïve | |
| Participants | Number of patients screened: not stated Number of participants included: 30 Number included as cases: methylphenidate (n = 12), methylphenidate naïve (n = 11) Number followed up in each arm: methylphenidate: 12 and methylphenidate naïve: 11 Number of withdrawals: 7 in the medication group were excluded because they were taking another medication, or matches with unmedicated children could be not be made Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age range: 6.4‐12.7 years old IQ: normal Sex: 16 male, 7 female Methylphenidate‐naïve: 11 Ethnicity: not stated Country: South Africa Comorbidity: not stated Comedication: no Sociodemographics: not stated Inclusion criteria: Children in grades 1 through 6 from 3 South African Gauteng Department of Education (GDE) remedial primary schools ADHD diagnosis according to DSM‐IV‐TR Exclusion criteria: Children taking medications other than methylphenidate |
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: not known Administration schedule: twice daily, morning and lunchtime Duration of intervention: 1 day Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Parental questionnaire: formulated by the authors and rated at 2 time points during the day, between 1:00 pm and 3:00 pm and between 5:00 pm and 7:00 pm. Included the child's sleep habits, sleep patterns, total sleep time, sleep latency, night time arousals, daytime naps and comments regarding daytime sleepiness in the children from appropriate adults, as well as symptoms and signs of the common sleep disorders as delineated in the DSM‐IV. It also included a visual analogue scale anchored at 'not at all sleepy' to 'very sleepy' on which the parents were requested to rate their children's general levels of sleepiness Wits Faces Sleepiness: a subjective, pictorial scale designed specifically for children which consists of 5 cartoon faces depicting increasing levels of sleepiness. Rated at 8:30 am and 1:00 pm |
|
| Notes | Sample calculation: not stated Ethics approval: approved by the Committee for Research on Human Subjects of the University of Witwatersrand Vested interest/funding/authors' affiliations: not stated Key conclusions of the study authors: in a group of children with ADHD taking methylphenidate, there was a significant increase in sleepiness a few hours after taking the medication, which may then have a significant impact on their learning. The data also imply that part of the mechanism of action of methylphenidate effects in these children may be by reduction of daytime sleepiness Comments from the study authors: it is highly recommended that school‐aged children diagnosed with ADHD be routinely screened for daytime sleepiness. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding IQ, duration of study and methylphenidate dosage received through personal email correspondence with the authors in October 2013 and January 2014 (Cockcroft 2014 [pers comm]) |
|
Cohen 1992.
| Methods | 2 patient reports on fixed drug eruption of the scrotum due to methylphenidate treatment | |
| Participants | Number of participants: 2 Diagnosis of ADD: DSM Age: 8 and 10 years old IQ: > 80 Sex: male Ethnicity: unknown Country: Israel Comorbidity: unknown Comedication: none Sociodemographics: unknown |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10 mg Administration schedule: once daily Duration of intervention: case 1: 5 days. Case 2: 7 days Treatment compliance: unknown |
|
| Outcomes |
Non‐serious adverse effects: Fixed drug eruption (FXD) (a term used to describe a sharply localised dermatitis that characteristically recurs at the same site each time the offending drug is administered) Case 1 5 days of methylphenidate treatment: hospitalisation due to 2 days of severe swelling and redness of the scrotum. The skin eruption resolved spontaneously 4 days after methylphenidate was discontinued. 2 weeks later, 18 hour after methylphenidate retrial, the same skin eruption of the scrotum was documented. Discontinued once again, and followed by a complete resolution of the rash after 4 days Case 2 7 days of methylphenidate treatment: 6 hours of severe swelling and redness of the scrotum. Discontinuation of methylphenidate was followed by a complete resolution of rash after 3 days. Re‐challenge with methylphenidate 2 months later was followed by the same skin eruption of the scrotum within 2 days. Complete resolution was seen after drug withdrawal |
|
| Notes |
Key conclusions of the study authors: fixed drug rash induced by methylphenidate is a possible but rare phenomenon. Because this drug is prescribed so often, it is important for physicians to be familiar with this phenomenon Supplemental information regarding ADHD diagnosis and IQ received through personal email correspondence with first author in July 2013 (Cohen 2013 [pers comm]) |
|
Coignoux 2009.
| Methods | A patient report of ADHD and schizophrenia during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: hyperactive‐impulsive) Age: 14 years old IQ: 135 Sex: male Ethnicity: white Country: France Comorbidity: schizotypal personality disorder, infantile psychosis with schizophrenia, OD, CDD Comedication: risperidone Sociodemographics: high cultural level |
|
| Interventions | Methylphenidate type: extended release Methylphenidate dosage: 54 mg/day Administration schedule: once daily, morning Duration of treatment: 2 years Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Development of gestural stereotypes and verbal aggressiveness towards his parents on methylphenidate treatment. After 6 months 4 mg risperidone is introduced. He improves, gets a less vindictive attitude but there remains some ritualised obsessive, defensive behaviour. His IQ has dropped 20 points |
|
| Notes | Funding/vested interest: none Authors' affiliations: none Key conclusions of the study authors: this study revealed the limits of category‐based approach and international classifications as they do not express clinical singularities or possible neurobiological continuums. It also seems to confirm the possible risk of emergence of a psychosis for schizotypal participants, due to pharmaceutical induction by psychostimulants (methylphenidate). This emergence could be stopped by a combined antipsychotic treatment Comments from the study authors: in our case, the cognitive exploration was not precise enough to conclude, but emphasize that the negative development of IQ in the patient illustrates quite well the assumption of risk of change in adolescents with a significant and sharp decline in IQ to a psychotic disorder in adulthood Comments from the review authors: the authors state that methylphenidate might have induced the psychosis ‐ and therefore the patient report is included despite polypharmacy |
|
Confino‐Cohen 2005.
| Methods | A patient report of pruritic maculopapular skin rash developing during methylphenidate treatment. The rash improved with antihistamines, and re‐challenge at a lower dose caused the reappearance of the rash, though less severe. Desensitisation through graded exposure of methylphenidate in incremental doses prevented further rash development | |
| Participants | Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: 8 years old IQ: normal IQ Sex: female Ethnicity: Israeli Country: Israel Comorbidity: not stated Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: Ritalin Methylphenidate dosage: 10 mg Administration schedule: once daily Duration of treatment: 1 week Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: After a week on 10 mg Ritalin the patient developed pruritic maculopapular skin rash over the back of her hands, which spread to face, chest, abdomen, and legs within the next 2 days. Ritalin was discontinued and antihistamine treatment was initiated, and the symptoms disappeared after a week. Re‐challenge with 5 mg Ritalin was attempted. 2 days later the same itchy rash appeared on her face and chest. Ritalin was once again discontinued and the symptoms disappeared after 2 days. A desensitisation protocol was follow over 10 days with 10 mg RItalin on the 10th day. No further adverse events were noticed |
|
| Notes | Funding/vested interest: not stated Authors' affiliations: not stated Key conclusions of the study authors: allergy to methylphenidate is rare. Whenever feasible, an alternate drug should be used if a reaction to the drug occurs. When an alternative is not available or the offending drug is still the best available choice, desensitisation should be considered (for mild rather than life‐threatening conditions/reactions) Comments from the study authors: from 4 months onwards, the patient had not taken medication at weekends (1‐2 days) or holidays with no adverse reaction on readministration of the drug Supplemental information regarding IQ and diagnostic criteria received through personal email correspondence with the authors in November 2013 (Confino‐Cohen 2013 [pers comm]) |
|
Congologlu 2009.
| Methods | A cohort study of voice recordings before and after methylphenidate use | |
| Participants | Number of patients screened: not stated Number included: 22 Number followed up: 22 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: combined 100%) Age: mean 9.05 (SD 1.43) years (range 7‐12) IQ: not stated Sex: 22 males Methylpenidate‐naïve: none Ethnicity: Turkish Country: Turkey Comorbidity: no Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 0.5 mg/kg 1 hour before recording voice sample Mean MPH dosage: not stated Duration of intervention: single voice measurement after single dose of methylphenidate Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Speech samples using Multi Dimensional Voice Program (MDVP) Model 5105 Version 2.3 of the Computerized Speech, developed by Kay Elemetrics Speech recordings for acoustic analysis were made from the participants in 2 sessions held before noon: no‐medication baseline session and the medication session after methylphenidate administration of 0.5 mg/kg (approximately 60 minutes later) We have not used these data |
|
| Notes | Ethics approval: the study was approved by the Human Subject Review Committee at the Gülhane Military Medical Academy Funding/vested interest/authors' affiliations: not stated Any withdrawals due to adverse events: no Key conclusions of the study authors: this clinical trial is the only study that examined the effects of stimulant medication on vocal acoustic parameters in children with ADHD and evaluated a small sample on and off their clinical doses of methylphenidate. We suggest that methylphenidate decreases fundamental frequency in children with ADHD, but our findings should be replicated under blind drug administration and by supporting other vocal analyses Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Corrigall 1996.
| Methods | A patient report of euphoria induced by methylphenidate in an 11‐year‐old boy diagnosed with hyperkinetic disorder | |
| Participants | Diagnosis of ADHD: ICD‐10 hyperkinetic disorder Age: 11 years old IQ: no intellectual disability Sex: male Ethnicity: not stated Country: UK Comorbidity: not stated Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate 10 mg/day for 4 weeks and then increased to 15 mg/day for 5 days Administration schedule: not stated Duration of treatment: 6 weeks Treatment compliance: abuse |
|
| Outcomes |
Non‐serious adverse events: Methylphenidate induced euphoria on 15 mg/day. Symptoms discontinued after methylphenidate was withdrawn |
|
| Notes | Ethics approval: not stated Funding/vested interests: not stated Authors' affiliations: Maudsley Hospital, London Key conclusions of the study authors: euphoria induced by methylphenidate can occur in young prepubertal children and this may lead to abuse of medication Supplemental information regarding the boys' intellectual functioning received through personal email correspondence with the authors in October 2013 (Corrigall 2013 [pers comm]) |
|
Cortese 2015.
| Methods | A cohort study using the Italian national ADHD registry of methylphenidate use from June 2007 to December 2012 | |
| Participants | Number of patients screened: 2411 Number included in methylphenidate group: 1426 Number followed up in methylphenidate group: 1414 Number of withdrawals in methylphenidate group: 12 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (86.0%), hyperactive‐impulsive (2.5%), inattentive (11.6%)) Age: mean: 10.55 (SD: 2.75) years old (range: 6‐18) IQ: not known Sex: 1247 (87.4%) males, 179 (12.6%) females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Italy Comorbidity: oppositional defiant disorder (34.4%), conduct disorder (4.2%), depression (3.6%), anxiety (11.1%), learning disorder (35.9%) Comedication: not stated Sociodemographics: not stated Inclusion criteria: Participants were children/adolescents (aged 6‐18 years) included in the Italian National ADHD Registry from June 2007 to December 2012.The diagnosis of ADHD was based on DSM‐IV‐TR Exclusion criteria: Given the naturalistic design, no a priori exclusion criteria were applied |
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: 0.3‐0.6 mg/kg/dose/day Mean methylphenidate dosage: 18.3 mg/day Administration schedule: 2‐3 times a day Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Adverse events were classified as severe if their occurrence was followed by active notification by clinical centres to the Italian Medicines Agency; otherwise, they were labelled as mild. The Italian Medicines Agency requires active notification when an adverse event results in death, is life‐threatening, requires hospitalisation or prolongation of existing inpatients' hospitalisation, results in persistent or significant disability or incapacity, or leads to a congenital anomaly or birth defect Non‐serious adverse events: Data regarding adverse events are collected via a structured form, located in a restricted area of the website of the Italian ADHD registry (available upon request), which allows standardisation of the procedure across centres. Information about the following adverse events is collected via the aforementioned structured form: cardiovascular risk, hepatic toxicity, any neurological disorder, any psychiatric symptomatology, acute diseases of the skin, and any clinically relevant gastrointestinal events |
|
| Notes | Sample calculation: no Ethics approval: yes Funding/vested interest/authors' affiliations: Prof Curatolo has received honoraria from Shire for participation in Advisory Board Meetings. Drs. Cortese, Panei, Arcieri, Germinaro, Capuano, Margari, and Chiarotti declare no competing interests Key conclusions of the study authors: our naturalistic postmarketing phase IV pharmacovigilance observational study showed that while mild and severe adverse events were observed in children treated with methylphenidate and in those treated with atomoxetine, those who received atomoxetine were significantly more likely to experience adverse events Comments from the study authors; the results should be considered in light of the study limitations. This was not a randomised study, and data on adverse events were not available for all participants at follow‐up visits following the baseline assessment and treatment assignment. Our study could not be informative with regard to adverse events occurring with extended‐release formulations of methylphenidate or with other class of ADHD drugs. Average dose of methylphenidate were rather low for usual standards of treatment, the naturalistic design did not allow assessment of whether children were adequately titrated for either medications. Data on validity and reliability of measures across centres are not available, and the study did not include a control group of healthy participants Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding IQ received through personal email correspondence with the authors in June 2016 (Panei 2016 [pers comm]) |
|
Coşkun 2008.
| Methods | A patient report of tactile and visual hallucinations with the combination of OROS methylphenidate and fluoxetine | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 10 years old IQ: intellectual capacity within normal range Sex: male Ethnicity: Turkish Country: Turkey Comorbidity: oppositional defiant disorder, generalised and separation anxiety disorders Comedication: fluoxetine 10 mg/day MPH‐naïve: yes Sociodemographics: not stated |
|
| Interventions | OROS MPH 18 mg/day, comedication: fluoxetine, 10 mg/day OROS MPH 18 mg/day monotherapy, 2 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Tactile and visual hallucinations. Worsening of previous sleep disturbance and decreased appetite |
|
| Notes | Funding/vested interests: the authors have no conflict of interest with any commercial or other associations, and no financial ties to disclose in connection with the submitted article Key conclusions of the study authors: here we report a paediatric patient who developed tactile and visual hallucinations with the combination of OROS methylphenidate and fluoxetine Comments from the study authors: in conclusion, we think that the causative agent was the combination of both medications rather than either medication alone. However, in either situation, it is important to note that this distressing side effect may occur even in the absence of underlying psychotic or substance‐related disorders, and clinicians' awareness is important in this issue, particularly in cases where polypharmacy is considered Supplemental information regarding ADHD diagnostic criteria, subtype of ADHD and ethnicity received through personal email correspondence with the authors in August 2013 (Coşkun 2013 [pers comm]) |
|
Coşkun 2009a.
| Methods | A patient report of decreased appetite during immediate‐release methylphenidate treatment and maculopapular pruritic skin eruptions during OROS‐methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 8 years old IQ: > 70 Sex: male Ethnicity: not stated Country: Turkey Comorbidity: EEG abnormalities Comedication: valproate, gabapentin Sociodemographics: not stated |
|
| Interventions | Immediate release methylphenidate 10‐20 mg/day for 2 months Treatment compliance: reported forgetting to take his medication sometimes OROS methylphenidate 18 mg/day for 1 week and 9 days Treament compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Immediate release methylphenidate 10‐20 mg/day and valproate 400 mg/day: decreased appetite, no weight decrease OROS‐methylphenidate 18 mg/day and valproate 400 mg/day: maculopapular pruritic skin eruptions on the patient's neck, arms, and legs, 1 week after starting OROS methylphenidate treatment OROS‐methylphenidate free period for 5 weeks: skin lesions were almost healed Re‐administering of OROS‐methylphenidate 18 mg/day and gabapentin 300 mg/day: same skin eruptions with same severity, 9 days after starting OROS‐methylphenidate treatment again Discontinuation of medication: skin lesions abated within the next several weeks Restart of immediate release methylphenidate 10‐20 mg/day and gabapentin 300 mg/day, 4 months: no skin eruptions |
|
| Notes | Funding/vested interests: the authors report no conflicts of interest or ties Key conclusions of study authors: the patient reported AEs with OROS MPH on 2 different occasions, but no AE with IR MPH at 2 different trials. Emergence of AE with OROS MPH and disappearance with discontinuation at both trials may suggest enough causality between AE and OROS MPH treatment Comments from the review authors: unclear whether the authors believe methylphenidate caused the adverse events |
|
Coşkun 2009b.
| Methods | 2 patient reports of sexual side effects during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: 50% combined, 50% unknown) Age: 15 and 8 years old IQ: > 70 Sex: 2 males Ethnicity: Turkish Country: Turkey Comorbidity: borderline mental capacity: 50% Comedication: unknown Sociodemographics: unknown |
|
| Interventions |
Case 1 Immediate release methylphenidate: 10‐30 mg/day in a divided dosage, 1 month. Treatment compliance: low Osmotic release oral system methylphenidate: 18 mg/day, 1 month. Treatment compliance: unknown Osmotic release oral system methylphenidate: 36 mg/day, 3 weeks. Treatment compliance: unknown Case 2 Osmotic release oral system methylphenidate: 18 mg/day, 10 days. Treatment compliance: unknown Immediate release methylphenidate: 10‐20 mg/day, 3 weeks. Treatment compliance: unknown Osmotic release oral system methylphenidate: 18 mg/day. Treatment compliance: unknown |
|
| Outcomes |
Non‐serious adverse events: Case 1 Immediate release methylphenidate, 10‐30 mg/day: initial headache and nausea Osmotic release oral system methylphenidate, 18 mg/day: emotional side effects (sense of nervousness in the chest, occasional emotional numbing), multiple daily erections (unrelated to sexual stimuli, painless, without ejaculations, onset: a few hours after ingestion of methylphenidate, duration: 5‐10 minutes) Medication‐free period, 1 week: no erections unrelated to sexual stimuli Re‐administering of osmotic release oral system methylphenidate, 36 mg/day, 3 weeks: reemerging of erections, longer duration compared with osmotic release oral system‐methylphenidate 18 mg/day. Headache. Nausea. Same emotional side effects. Drug‐free days: no erections Discontinuation of medication: no erections Case 2 Osmotic release oral system methylphenidate, 18 mg/day: loss of appetite, headache, abdominal pain, sleep problems, and conjunctival injection. Headache and abdominal pain almost disappeared after 1 week. Hypersexual behaviours. Morning erections before ingestion of methylphenidate, duration: 1 hour. Weight loss: 1.5 kg within 2 months. Drug‐free days: almost no hypersexual behaviour during the day and no erection the following morning Immediate release methylphenidate, 10‐20 mg/day: morning erections and hypersexual behaviours decreased dramatically. Sleep and appetite problems improved mildly. No conjunctival injection (after 3 weeks). Possible withdrawal symptoms (increased irritability and hyperactivity, getting tearful easily), several hours after each dosage Osmotic release oral system methylphenidate, 18 mg/day: next‐morning erections, hypersexual behaviour during the day. Drug‐free days: almost no hypersexual behaviour Osmotic release oral system methylphenidate, 18 mg/day, 6:00 am: no morning erections, but still hypersexual behaviour. Sleep and appetite problems. Conjunctival injections. Sense of pins and needles in his legs after 10 days Discontinuation of osmotic release oral system methylphenidate: no morning erections or hypersexual behaviours |
|
| Notes | Funding: no financial ties or conflicts of interest Supplemental information was received through personal email correspondence with the authors in August 2013 (Coşkun 2013b [pers comm]) |
|
Coşkun 2010.
| Methods | Cohort study of methylphenidate treatment in participants with ADHD and comorbid anxiety‐ or depressive disorders | |
| Participants | Number of participants screened: not stated Number of participants included: 7 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: combined (57%), inattentive (43%)) Age: mean 11.85 (SD 2.91), range: 8‐16 years old IQ: normal Sex: 4 males, 3 females Methylphenidate‐naïve: none Ethnicity: Turkish Country: Turkey Comorbidity: generalised anxiety (86%), social anxiety (86%), panic (29%), separation anxiety (29%), obsessive‐compulsive (29%) and major depressive disorder (14%), special phobia (29%) and agoraphobia (14%) Comedication: SSRIs (57%) Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type and dosage: immediate release (15‐20 mg/day), osmotic release oral system (18‐54 mg/day), IR/OROS (10‐18 mg) Administration schedule: not stated Duration of intervention: 4‐30 months, mean 14.28 (SD 9.41) Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events:
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: the authors reported no conflict of interest related to this article Key conclusions of the study authors: young participants with diagnosis of ADHD and comorbid anxiety and depressive disorders may benefit from mirtazepine addition particularly in the presence of methylphenidate‐ or SSRI‐related sleep and/or appetite problems Comments from the review authors: the adverse events reported here are from before the start of the mirtazepine treatment which means the participants are under no treatment but methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information received through personal email correspondence with the authors in August 2013 (Coşkun 2013c [pers comm]) |
|
Coşkun 2011.
| Methods | A patient report of obsessive‐compulsive symptoms during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: unknown (subtype: combined) Age: 10 years old IQ: normal Sex: female Ethnicity: Turkish Country: Turkey Comorbidity: subsyndromal social and generalised anxiety disorders Comedication: unknown Sociodemographics: unknown |
|
| Interventions | Osmotic release oral system methylphenidate, 18 mg/day, 1 year Treatment compliance: unknown Osmotic release oral system methylphenidate, 27 mg/day, 3 + 3 weeks Treatment compliance: unknown |
|
| Outcomes |
Non‐serious adverse events: Osmotic release oral system methylphenidate, 18 mg/day: decreased appetite and initial headache Osmotic release oral system methylphenidate, 27 mg/day: obsessive‐compulsive symptoms, decreased appetite, facial grimace, nail picking/biting Discontinuation of medication: gradually disappearance of symptoms, still subsyndromal obsessive‐compulsive symptoms Re‐administering of osmotic release oral system methylphenidate, 27 mg/day: mild facial grimace, similar obsessive‐compulsive symptoms. After 3 weeks: Children's Yale‐Brown Obsessive‐Compulsive Scale (CY‐BOCS), total score = 21 |
|
| Notes |
Key conclusions of the study authors: clinicians treating children should be familiar with the emergence and management of these unusual side effects Supplemental information was received through personal email correspondence with the authors in August 2013 (Coşkun 2013d [pers comm]) |
|
Davari‐Ashtiani 2010.
| Methods | A 6‐week randomised, parallel, double‐blind clinical trial with 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 34 Number of participants randomised to methylphenidate: 16 Number of participants followed up: 16 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (100%)) Age: mean 8.62, range 6‐12 years old IQ: normal Sex: not stated Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Iran Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: initiated at 0.5 mg/kg/day and adjusted to the optimal effect. Maximum dose: 60 mg/day. Range: 0.3‐1 mg/kg/day Administration schedule: not stated Duration of intervention: 6 weeks Duration of titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: No serious adverse drug effects were observed during the trial Non‐serious adverse events Possible side effects were systematically recorded throughout the study and assessed using a checklist administered by a child psychiatrist on weeks 2, 4, 6 Decreased appetite (n = 11), decreased sleep (n = 7) |
|
| Notes | Sample calculation: statistical power calculated on the basis of the projected group size, a response rate of 30% and an alpha level of 0.05. Ethics approval: yes, approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Tehran, Iran). Funding/vested interests: this work was supported in part by the grant from the Behavioral Sciences Research Center of Shahid Beheshti University of Medical Sciences (Tehran, Iran) Key conclusions of the study authors: no significant differences were observed between the 2 protocols on the total scores of parent and teacher ADHD Rating Scale, but methylphenidate was superior to buspirone in decreasing the symptoms of inattention. The side effects of buspirone were mild and rare in comparison with methylphenidate Comments from the study authors:main limitations: small sample size, minimal dosage necessary for response and duration of treatment. The effect of buspirone on different DSM‐IV subtypes of the disorder was not explored Comments from the review authors: the article in Farsi presents the preliminary results, and the article in English presents the final results. Therefore, the data reported in the Farsi article are not used in our review Supplemental information received through personal email correspondence with the study authors in January 2014 (Davari‐Ashtiani 2014 [pers comm]) |
|
Delignieres 2011.
| Methods | A before‐after study comparing 21 children with epilepsy and ADHD with 21 ADHD‐only children using a short 'attentional capture test' after being given methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 21 epilepsy + ADHD and 21 ADHD only Number of participants followed up: 21 in each group Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: range: 6‐13 years old IQ: not stated Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: France Comorbidity: epilepsy Comedication: anti‐epileptic drugs Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate given to children in a before‐after trial, comparing 2 groups and evaluated by an attentional capture paradigm Methylphenidate type: not stated Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Safety issue reported in abstract |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: not stated Ethics approval: not stated Funding/vested interest: not stated Authors' affiliations: not stated Key conclusions of the study authors: methylphenidate is safe and effective in children with epilepsy and ADHD Comments from the review authors: the authors state that methylphenidate is safe and effective in children with epilepsy and ADHD, but this seems to be a general statement rather than a conclusion from their findings. No data are presented in the abstract to support either conclusions on the safety or on the efficacy of methylphenidate in epilepsy and/ or ADHD Supplemental information and full text article requested through email correspondence with the authors in June and November 2013. No reply |
|
Dirksen 2002.
| Methods | A multicentre, open‐label, cohort study of the effectiveness and tolerability of an extended release methylphenidate in treated and untreated children and adolescents with ADHD over 3 weeks | |
| Participants | Number of participants screened: 332 Number of participants included: 310 Number of participants initiating treatment: 308 Number of participants followed up: 287 Number of withdrawals: 23 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 11.0, range 6‐17 years old IQ: > 80 Sex: 222 males (72.1%), 86 females (27.9%) Methylphenidate‐naïve: 41% Ethnicity: white (82.1%), African American (10.4%), others: (7.5%) Country: USA Comorbidity: none Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: extended release methylphenidate hydrochloride (Metadate CD) Methylphenidate dosage: 20‐60 mg Administration schedule: once daily, in the morning before breakfast Duration of intervention: 3 weeks Treatment compliance: not stated Dosage was titrated on a weekly basis according to clinical judgement |
|
| Outcomes | Safety and tolerability were assessed by laboratory tests, vital signs and adverse events collected through spontaneous reports by parents, general questioning of the child, and investigators' observations. Assessed at 3 study visits with 7 days apart Adverse event data were collected as pre‐study events (to assess the presence of pre‐existing symptoms and events) |
|
| Notes | Sample calculation: not stated Ethics approval: approved by the Institutional Review Boards at each study centre Funding: Celltech Americas Inc. Vested interest/authors' affiliations: 3 of the 4 authors were employed by Celltech Americas Inc. Key conclusions of the study authors: methylphenidate hydrochloride is effective and well‐tolerated for clinical use in ADHD Comments from the study authors: the absence of a placebo control group makes it difficult to determine whether there was bias in evaluating effectiveness or relatedness of adverse events Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental data requested through personal email correspondence with the study authors with in March 2014. No reply |
|
Dittmann 2014.
| Methods | An observational prospective cohort study of methylphenidate use for 12 months | |
| Participants | Number of participants screened: not stated Number of participants included: 247 in stimulant group (short‐ or long‐acting methylphenidate) Number of participants followed up: 191 Number of withdrawals: 56. Diagnosis of ADHD: ICD‐10 (subtype: F90.0 (74.9%), F90.8 (0.8%), F98.8 (8.9%)). DSM‐IV (subtype: combined (8.1%), inattentive (4.9%), hyperactive/impulsive (2.0%), combined and ODD (0.4%)) Age: mean: 9.3 (SD 2.4) years (range 6‐16) IQ: low IQ (70‐84) 19 (7.7%), normal IQ (85‐114) 197 (79.8%), high IQ (115‐129) 29 (11.7%), very high IQ (> 129) 2 (0.8%) Sex: 179 males, 68 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Germany Comorbidity: conduct disorder (14.6%), ODD (13%), anxiety disorder (5.7%), depression (4.9%), tic disorder/Tourette (4.1%), other (10.1%)) Comedication: 239 (96.8%) received no concomitant medication Sociodemographics: nuclear family (66.8%), 1 biological parent, 1 step‐parent (13.8%), single mother (13.8%), foster parents (0.4%), adoptive parents (1.2%), single father (0.8%), supervised living arrangement (0.8%), family members other than parents (1.2%), unknown (1.2%) Inclusion criteria
|
|
| Interventions | Methylphenidate type: any Mean methylphenidate dosage: 0.37 (SD 0.23) mg/kg Administration schedule: not stated Duration of intervention: 12 months Treatment compliance: 178 (74.2%) had a PCSR (Pediatric Compliance Self‐Rating instrument) score ≥ 5 at every visit |
|
| Outcomes | Adverse events were analysed for patients on initial monotherapy only Adverse effects were rated at baseline, end of week 1, 2, months 1, 3, 6, 9, and 12 | |
| Notes | Sample calculation: not stated Ethics approval: the study was approved by the responsible Ethics Committee (ERBat Medical Faculty Mannheim, University of Heidelberg, Germany) Funding: research was funded by Lilly DeutschlandGmbH, Bad Homburg, Germany, and by Eli Lilly & Co., Indianapolis, USA Vested interests/authors' affiliations: not stated Key conclusions of the study authors: all outcome parameters (effectiveness) considered in this open‐label, non‐interventional trial with respect to ADHD core symptoms, ADHD‐related difficulties, and emotional expression significantly improved over time in children and adolescents with ADHD who were treated with pharmacotherapy (i.e. atomoxetine or psychostimulants) in a naturalistic setting with regard to their degree and time course, which corresponds with the well‐established findings from double‐blind controlled clinical trials Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Dodangi 2011.
| Methods | A 6 ‐week parallel trial with 2 arms:
|
|
| Participants | Number of participants screened: 34 Number of participants included: 34 Number of participants randomised to methylphenidate: not stated Number of participants followed up: 15 in the methylphenidate group Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: range 11‐18 years old IQ: not stated Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Iran Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria Not stated |
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: 6 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Children Depressive Inventory (CDI) measured at the end of the trial Revised Children's Manifest Anxiety Scale (RCMAS) at the end of the trial Drug side effects evaluated each 2 weeks during the study |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: yes, 3 gastrointestinal symptoms and 1 mania Ethics approval: not stated Funding/vested interests: not stated Key conclusions of the study authors: our study showed that duloxetine may be as effective as methylphenidate in treatment of ADHD in adolescents. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information regarding side effects received from the author in November 2013 (Dodangi 2013 [pers comm]) |
|
Dubnov‐Raz 2011.
| Methods | A case‐control study of 17 months of methylphenidate treatment using a chart review of computerised medical records | |
| Participants | Number of participants screened: 529 Number of participants included: 275 Number included as cases: 135 (methylphenidate treated) and controls: 140 (untreated) Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean 10.4 years old, range 6‐16 IQ: > 70 Sex: 200 males, 75 females Methylphenidate‐naïve: cases (none), controls (100%) Ethnicity: multiethnic Country: Israel Comorbidity: none Comedication: none Sociodemographics: a variety of different family patterns and socioeconomic status among the groups. Those who were already methylphenidate treated were 7 months older, on average, than the methylphenidate‐naïve patients, and they had a higher proportion of males. Weight, height, and body mass index z scores, which inherently correct for age and sex, did not differ significantly between these 2 subgroups. Rates of overweight and obesity were also comparable Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: regular (n = 52), slow‐release/long‐acting (n = 61), or osmotic release oral system (n = 22) Baseline methylphenidate mean dose: 0.43 mg/kg (SD 0.22), range: 0.1‐1.0 mg/kg (each 4.5 mg of osmotic release was regarded as 1 mg methylphenidate) Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes | Height and weight measured by a certified nurse at baseline and follow‐up visits Body mass index |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interests: the authors received no financial support for the research and/or authorship of this article Key conclusions of the study authors: physicians should be aware of the possibility of height and weight abnormalities in children with ADHD, with or without treatment Comments from the review authors: only the data on the methylphenidate‐treated and untreated participants with ADHD are used in this review Supplemental information requested from the authors in July 2013, but they did not have the time to find the relevant information |
|
Dupuy 2008.
| Methods | A controlled before‐after study investigating the effects of stimulants on EEG coherence in girls with ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 9 Number of participants followed up: 9 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: combined or Inattentive) Age: range 7‐12 years old IQ: > 85 Sex: female Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Australia Comorbidity: none Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: 6 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: If participants experienced any problems with their medication, parent(s) were asked to contact their doctor, their medication was changed, and they were removed from the study; this did not occur with these participants |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: none Ethics approval: Illawarra area health/University of Wollongong Human Research Ethics Committee Funding/vested interest: not stated Authors' affiliations: Brain & Behaviour Research Institute and School of Psychology, University of Wollongong, Australia Sydney Developmental Clinic, Australia Key conclusions of the study authors: intrahemispheric and interhemispheric coherences in ADHD stimulant medicated girls. Girls had elevated frontal coherence across all frequency bands Comments from the review authors: only children who had a positive response on stimulants were included in the study. We can only use this study qualitatively and not in the analyses. Exclusion of MPH non‐responders/children who have previously experienced adverse events on MPH: no |
|
Durá‐Travé 2012.
| Methods | A 4‐year cohort study examining OROS‐methylphenidate effects on growth conducted as a review of random medical records | |
| Participants | Number of participants screened: not stated Number of participants included: 187 Number of participants followed up: 160 Number of withdrawals: 27 Diagnosis of ADHD: DSM‐IV‐R (subtype: combined (84.5%), inattentive (15.5%)) Age: mean at time of diagnosis: 8.14, range: 6‐10 years old IQ: not stated Sex: 129 males, 58 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Spain Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: sustained release, osmotic release oral system Methylphenidate dose: at baseline 0.89 mg/kg/day, gradually increased to 1.31 mg/kg/day at 48 months Administration schedule: once daily Duration of intervention: 48 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Height, weight, and BMI measured at baseline (time of ADHD diagnosis and start of methylphenidate treatment), 6, 12, 18, 24, 30, 36, 42, and 48 months. Weight and height measurements were taken with patients wearing only underwear and no shoes, precision of 100 g and 0.1 cm. The growth charts and data tables of the Centro Andrea Prader (Zaragoza, Spain, 2002) were used as standard references |
|
| Notes | Sample calculation: no Any withdrawals due to adverse events: not stated Ethics approval: the study was approved by the Ethics Committee of the Navarra Hospital Complex, Pamplona, Spain Funding: the authors received no financial support for the research, authorship, and/or publication of this article Vested interests/authors' affiliations: the authors declared no potential conflicts of interest Key conclusions of the study authors: at the time the participants were diagnosed with ADHD, 1 out of every 3 patients was in a deficient nutritional situation (subnutrition or malnutrition). Continued treatment with OROS‐methylphenidate for 30 months had a negative influence on height and weight. However, we observed a recovery of anthropometric variables from the 30th to the 48th month of OROS‐methylphenidate treatment (growth‐rebound); this means that the effects of stimulant drugs, and specifically methylphenidate, on the growth curve would be a transitory condition that attenuates as time passes. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding IQ requested through personal email correspondence with the study authors with no reply |
|
Döpfner 2011a, OBSEER.
| Methods | The OBSEER study (Observation of Safety and Effectiveness of Equsym XL in Routine Care). A non‐interventional, non‐controlled, multicentre, prospective, observational, postmarketing surveillance study | |
| Participants | Number of participants screened: 852 Number of participants included: 822 Number of participants followed up: 777 (completed all 3 planned visits) Number of withdrawals: 45 Diagnosis of ADHD: ICD‐10; F90.0 430 (55.41%), F90.1 282 (36.34%), F90.8 64 (8.25%) Age: mean 10.04 (SD 2.47) years (range 6‐17) IQ: above 70 Sex: 663 males (81.25%), 153 females (18.75%) Methylphenidate‐naïve: 30.17% Ethnicity: not stated Country: Germany Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: once‐daily modified‐release methylphenidate, Equasym XL (30% immediate‐release‐ and 70% modified‐release methylphenidate) Methylphenidate dosage: 10 mg ‐ 120 mg, maximum recommended daily dose (60 mg/day) exceeded in 6 patients Administration schedule: once daily Duration of intervention: 5 days to 12 months (mean 2.26 months) Treatment compliance: not stated |
|
| Outcomes | Adverse events (AEs) were evaluated by the treating physician at each study visit, and coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 11.1 and classified into AEs and serious AEs (death, life‐threatening conditions, hospitalisation or prolongation of hospitalisation, persistent injury/disability, incapacity for work, medically significant conditions and congenital abnormalities/birth defects) DAYAS (The Daily Profile of ADHD Symptoms) completed by teacher and parents at each visit |
|
| Notes | Sample calculation: yes Ethics approval: no approval was needed for this study Funding/vested interests/authors' affiliations: the study was funded by UCB and the article (Döpfner 2011) is part of a supplement sponsored Shire Development Inc. Key conclusions from study authors: this open‐label, observational, post‐marketing surveillance study investigates the effectiveness and safety of Equasym XL which is a combination of 30% immediate‐release MPH and 70% modified‐release MPH, in the treatment of ADHD in daily clinical practice. The effectiveness of Equasym XL was rated better than prior or no therapy and generally well tolerated Comments from study authors: limitations on the study: open‐label, no control group: therefore, physicians and parents were not blinded to the study treatment or dose Some of the participants were previously treated with stimulants, therefore the results from this group can only be generalised to a population in which a switch to Equasym XL is planned due to suboptimal effects of the prior medication. This was an open‐label study with no control group, only data on adverse events are therefore extracted Supplemental information regarding data were attempted to retrieve from the authors by email. Sent twice, no answer |
|
Döpfner 2011b.
| Methods | A randomised, controlled, double‐blind, multicentre clinical cross‐over trial with 2 modified release methylphenidate interventions:
|
|
| Participants | Number of participants screened: 122 Number of participants included: 113 Number of participants followed up: 91 Number of withdrawals: 22 Diagnosis of ADHD: ICD‐10 diagnosis of hyperkinetic disorder. 61% had a subtype corresponding to DSM‐IV ADHD combined subtype Age: regarding the 113 included: mean: 10.2, range 6.0‐17.11 years IQ: 99.2 (SD 9.7) Sex: 86 males, 27 females Methylphenidate‐naïve: 0% Ethnicity: not stated Country: Germany Comorbidity: oppositional defiant disorder or conduct disorder (36%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: Medikinet (equal proportions of immediate‐ and slow‐release methylphenidate) and Concerta (immediate‐release and OROS‐methylphenidate) Methylphenidate dosage: participants were randomly assigned to 1 of 6 possible sequence combinations Each patient received the following treatments in a sequence: Lower doses: Medikinet Retard 20 mg (10 mg immediate release) or 10 mg (5 mg immediate release) Lower doses: Concerta: 18 mg (4 mg immediate release) High dose: Medikinet Retard: 30 mg (15 mg immediate release) or 20 mg (10 mg immediate release) High dose: Concerta 36 mg (8 mg immediate release) Administration schedule: once daily Duration of each medication condition: 1 week per treatment, 3 weeks in total Treatment compliance: the compliance was more than 99% |
|
| Outcomes |
Non‐serious adverse events: Both doctors, teacher and parents documented side effects weekly The DAY profile of ADHD Symptoms (DAYAS): potential adverse effects of ADHD medication were assessed by parents with 11 items for the whole week. Additionally, potential adverse effects of ADHD medication are assessed by teachers with 9 items for the whole week. Here the ratings from teachers and parents were obtained at the end of the week (Thursdays or Fridays) to provide a comprehensive assessment of the week and to avoid the possible impact of carry‐over treatment effects Furthermore, from the final report from Medice: all AE were coded according to MedDRA. The onset of the event was significant for assignment of the AE to 1 of the treatment groups (dose group/pharmaceutical form). If an event lasted over several treatment groups it was only evaluated for the treatment group in which it first appeared Blood pressure, rated weekly by investigator Heart rate, rated weekly by investigator |
|
| Notes | Sample calculation: yes Ethics approval: yes Funding/vested interests/authors' affiliations: Manfred Döpfner is currently a consultant for Medice, Shire Pharmaceutical, and Eli Lilly; has in the last 3 years received grant funding from UCB‐Pharma, Medice, and Eli Lilly; has recently served on the advisory boards of Medice, Shire Pharmaceutical, and Eli Lilly; and has spoken at events sponsored by Medice, Shire Pharmaceutical, Eli Lilly, and Janssen Cilag. Claudia Ose is CEO at Center for Clinical Studies at University Hospital Essen Germany. R. Fischer is a full‐time employee of Medice. R. Ammer is Chief Executive Officer of Medice. Andre Scherag currently serves as trial statistician on a data safety and monitoring board for Boehringer Ingelheim KG. The study is funded by Medice Company. Any withdrawals due to adverse events: 4 Key conclusions from study authors: in summary, based on the efficacy and safety data from this trial it can be claimed that Medikinet retard with a higher IR component than Concerta and an equivalent daily dose is superior to Concerta in the morning and that children and adolescents may also be treated with a lower daily dose of Medikinet retard without resulting in a clinically relevant worse effect during school time as assessed by SKAMP‐D. "Since there is no placebo group or no 'no‐intervention' group in the trial, we can only use the data on adverse events. We extract data from this study as if it was an observational study Comments from the study authors: in this study, carry‐over effects are expected to be negligible because of the short half‐life of methylphenidate. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, only participants who responded well to the medication were included Supplemental information regarding final report and protocol received through personal email correspondence with the medical director from Medice in December 2013 (Roland 2013 [pers comm]) |
|
Döpfner 2011c.
| Methods | A cohort study of methylphenidate use for 4‐6 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 467 Number of participants followed up: 447 Number of withdrawals: 20 (excluded in intention‐to‐treat analysis, but included in the tolerability evaluation) Diagnosis of ADHD: 48% ICD‐10 diagnosis of ADHD (subtype: not stated), 42% "hyperkinetic disorders which affected their social behavior" (subtype: not stated) Age: mean: 10.7 (2.5) years (range: 6‐17) IQ: not stated, but half of the children were in primary school Sex: 361 males, 106 females Methylphenidate‐naïve: 14% Ethnicity: not stated Country: Germany Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: Medikinet retard (extended release) Mean methylphenidate dosage: 22.6 mg (range: not stated) Administration schedule: once daily Duration of intervention: 4‐6 weeks Treatment compliance: was assessed by prescribing physician. In 57% of cases there was improved compliance after the medication switch; no change was observed in 39%, and compliance deteriorated in 4% |
|
| Outcomes |
Non‐serious adverse events: 11 items in the paediatric diagnosis system KIDS assesses potential adverse events of the medication In 79 patients, adverse events were recorded by the physician, and these events were described as severe in 13 cases. The most frequent adverse events were appetite disorders, head‐ and stomachache and sleep disorders |
|
| Notes | Sample calculation: no Ethics approval: non‐interventional study pursuant to Section 4 sentence 3 and Section 67 AMG (German Medicines Act) Funding/vested interest/authors' affiliations: Prof Döpfner is employed as a consultant to the following companies and receives research funding from these companies: Janssen‐Cilag, Lilly, Medice, Novartis, Shire, UCB. Dr Fischer is an employee of Medice. Ms Ose is participating in studies which are supported by Medice and UCB Any withdrawals due to adverse events: 10 Key conclusions of the study authors: the overall evidence showed that Medikinet retard was well tolerated in routine clinical practice, can be used effectively to treat symptoms of ADHD, can improve compliance to medication and can contribute to a further improvement in symptoms in previously suboptimally treated patients Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: by implication, since treatment would not be indicated for non‐responders Supplemental information regarding data requested from the authors by email in June 2014. No answer received |
|
Efron 1997.
| Methods | A 4‐week double‐blind, cross‐over study with 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 125 participants were randomly assigned to 1 of 2 possible drug condition orders (methylphenidate or dexamphetamine) Number of participants followed up: not stated Number of withdrawals: 4 due to severe adverse events (2 on methylphenidate, 2 on dexamphetamine) Diagnosis of ADHD: DSM‐IV (subtype: combined (81.8%), hyperactive‐impulsive (1.6%), inattentive (17.6%)) Age: mean 104.8 (SD 27.6) months (range 60‐179 months) IQ: mean 98.9 (SD 13.8) Sex: 114 males, 11 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Australia Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate dosage: 0.3 mg/kg/dose, rounded off to the nearest capsule size Administration schedule: twice a day, after breakfast and after lunch Duration of intervention: 2 weeks. 24‐hour washout period between interventions Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Barkley Side Effects Rating Scale (SERS): adverse events rated on a scale from 0 (absent) to 9 (severe). The list of side effects were compiled from those commonly reported anecdotally in the literature Parent rated at baseline and the end of each 14 day period |
|
| Notes | Ethics approval: yes. The study protocol was approved by the Ethics in Human Research Committee of the Royal Children's Hospital, Melbourne, Australia, and written informed consent was obtained from parents Funding: clinical research scholarship from the Royal Children's Hospital Research Foundation Vested interests/authors' affiliations: not stated Key conclusions of the study authors: nightmares, stomachaches and anxiousness decreased in frequency on methylphenidate, whereas poor appetite increased on methylphenidate. Most children with ADHD improve significantly on both methylphenidate and dexamphetamine. There was a slight advantage to methylphenidate on most measures. Many symptoms commonly attributed to stimulant medication are actually preexisting characteristics of children with ADHD and improve with stimulant treatment Comments from the study authors: a significantly greater number of side effects were reported as present by parents at baseline than during the methylphenidate condition. Parents motivated to have their child diagnosed with ADHD may have over‐reported symptoms at baseline, including adverse events Supplemental information regarding adverse events received through personal email correspondence with the authors in October and December 2013 (Barker 2013 [pers comm]) |
|
El‐Fiky 2014.
| Methods | A cohort study of methylphenidate use for 6 weeks | |
| Participants | Number of participants screened: 31 Number of participants included: 31 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐5 (subtype: combined (70.9%), hyperactive‐impulsive (6.5%), inattentive (22.6%)) Age: females: mean 8.2 (SD 3), range 5‐14 years old. Males: mean 8.4 (SD 2.2), range 6‐14 years IQ: mean: females: 100.8, males: 106.5 Sex: 21 males, 10 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Egypt Comorbidity: oppositional defiant and conduct disorders: 11 (35.4%); specific phobic disorders: 3 (9.6%); childhood depression: 2 (6.5%), multiple comorbid problems (of conduct, agoraphobia, social and specific phobias): 2 (6.5%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: male: 0.75 mg/kg (SD 0.12); female: 0.76 mg/kg (SD 0.16) Administration schedule: not stated Time points: not stated Duration of intervention: 6 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Measure method: Stimulant Drug Side Effects Rating Scale of Barkley |
|
| Notes | Sample calculation: not clear, but authors stated: "A pilot study involving 10 subjects (8 males, 2 females) was conducted to determine size and selection methods, interrater reliability and applicability of tools, and dose ranges of methylphenidate (0.4‐1 mg/kg)." Ethics approval: not stated Funding/vested interests/authors' affiliations: not stated Any withdrawals due to adverse events: not clearly stated, but no mention of dropouts Key conclusions of the study authors: no statistically significant gender differences; females recorded less improvement and showed more 'talk less' and 'less interest' as side effects. Conclusive evidence for the role of gender in ADHD require bypassing methodological limitations as well as confounding factors. Comments from the study authors: since the above mentioned gender differences were less apparent than expected in the absence of gender‐by‐ADHD interaction, they can be attributed to many confounding factors than modification of ADHD effect by gender Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information attempted to retrieve, but no contact information for the authors found |
|
El‐Zein 2005.
| Methods | A 3 months cohort study following 12 children taking methylphenidate | |
| Participants | Number of patients screened: 35 Number included: 18 Number followed up: 12 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐IV (subtypes: not stated) Age: 8.5 (SD 3.5) years old, range: not stated IQ: not stated Sex: 10 males, 2 females Methylphenidate‐naïve: 100% Ethnicity: white: 50%, African American: 33%, Hispanic: 16% Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: not stated (clinician's given choice as to what they prescribed) Methylphenidate dosage: 20 to 54 mg/day Administration schedule: not stated Duration of intervention: 3 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: A 10 ml blood sample was taken before starting methylphenidate, and a second blood sample was collected after 3 months of treatment with this drug. Type of effect: 3 cytogenetic endpoints (chromosome aberrations, sister chromatid exchanges and micronuclei frequencies) measure method (peripheral blood lymphocytes obtained pre and post treatment) |
|
| Notes | Sample calculation: not stated Ethics approval: after being informed of the study, a parent or guardian signed an informed consent that was approved by the Institutional Review Board of UTMB, the child also assented, and the study participant was then enrolled Funding/vested interest/authors' affiliations: not stated Key conclusions of the study authors: in all participants, treatment induced a significant 3, 4.3 and 2.4‐fold increase in chromosome aberrations, sister chromatid exchanges and micronuclei frequencies, respectively (PZ0.000 in all cases). These findings warrant further investigations of the possible health effects of methylphenidate in humans, especially in view of the well‐documented relationship between elevated frequencies of chromosome aberrations and increased cancer risk Comments from the review authors: the critique of the study by Preston, Kollins et al needs to be read alongside this study as it highlights considerable methodological concerns in this small cohort/patient series |
|
Famularo 1987.
| Methods | A naturalistic observational study, where the effectiveness of drug treatment (methylphenidate) were tested by a drug treatment during a grading period and no drug treatment for the next grading period | |
| Participants | Number of patients screened: not stated Number of participants included: 13 Number of participants followed up: 10 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III diagnosis of ADD (subtype: inattentive) Age: mean: 9.4, range 7‐12 years old IQ: above 90 Sex: 4 males, 6 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: not stated, but see exclusion criteria Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 0.4 to 1.2 mg/kg/day Administration schedule: twice daily Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: 1 child reported decreased appetite without weight loss Another child's sleep onset was 15‐20 minutes later than usual after initiation of pharmacological treatment There were minimal and clinically insignificant increases in pulse |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: no Ethics approval: not stated Funding/vested interest/authors' affiliations: not stated Key conclusions of the study authors: the results of this study provide tentative support for 2 conclusions: ADD without hyperactivity appears to be a potentially stimulant‐treatable condition, even though hyperactivity is specifically excluded from its symptomatology; and school grades in children with ADD without hyperactivity may be influenced by the use of stimulants Comments from the study authors: the fact that significant results were obtained with such a small sample reflects the consistency with which the effects of the medication were exhibited across participants. However, it cannot be argued that these 10 participants adequately represent all ADD without hyperactivity children. In addition, the results may have been affected by factors such as teacher bias, student motivation, and parental encouragement Supplemental information requested through personal email correspondence with the authors in August 2013 and January 2014. No reply |
|
Faraone 2007a.
| Methods | A long term, open‐label, longitudinal study of methylphenidate use for up to 37 months | |
| Participants | Number of participants screened: 268 Number of participants included: 191 Number of participants followed up: 127 Number of withdrawals: 64 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: range 6‐12 years old IQ: not stated Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: transdermal system Methylphenidate dosage: 6.25 cm2 (0.5 mg/hour) to 50 cm2 (3.6 mg/hour) Administration schedule: 12 hour wear time Duration of intervention: 37 months Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events: Weight, height and BMI, values were converted to age‐corrected standard scores (z scores) and percentiles using the normative growth charts and transformations provided by the Centers for Disease Control and Prevention | |
| Notes | Sample calculation: not stated Ethics approval: the study was approved by each site's local institutional review board or by a central IRB Funding/vested interests: this study was supported in part by a grant from Shire Pharmaceutical Development to SF Authors' affiliations: Dr Faraone receives research support from McNeil Consumer & Specialty Pharmaceuticals, Shire US, and Eli Lilly; is on the speakers' bureaus of Eli Lilly, McNeil Consumer & Specialty Pharmaceuticals, Shire US, and Cephalon; and has had an advisory or consulting relationship with the McNeil Consumer & Specialty Pharmaceuticals, Noven Pharmaceuticals, Shire US, Cephalon, and Eli Lilly. Mr Giefer has no financial relationships to disclose Key conclusions of the study authors: methylphenidate transdermal system treatment is associated with growth deficits in both weight, height, and BMI, but growth deficits attenuate over time. Baseline growth influences the effect of MTS treatment on both height, weight, and BMI, whereas prior stimulant therapy, dose, and total time treated influences only weight and BMI Comments from the study authors: prior stimulant therapy predicted smaller weight and BMI (but not height) deficits during the course of treatment with MTS. Growth velocity analyses showed that the rates of weight and BMI increases were negative in the first year of the study but positive at later time points. These findings suggest that any adverse effects of MTS on weight or BMI occur near the commencement of treatment and show some attenuation over time Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated | |
Feeney 1997.
| Methods | A patient report of af 13‐year old boy developing a tonic‐clonic ictal event on sertraline and methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: 13 years old IQ: > 70 Sex: male Ethnicity: unknown Country: USA Comorbidity: depression Comedication: sertraline Sociodemographics: unknown |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 80 mg/day (1.8 mg/kg/day) Administration schedule: not stated Duration of treatment: 10 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: No significant side effects for approximately 10 months |
|
| Notes | Funding/vested interests: not stated Authors' affiliations: Wright State University, School of Medicine, Dayton, Ohio Key conclusions of the study authors: first reported seizure in a patient with combined methylphenidate and sertraline treatment Comments from the study authors: more research is needed Supplemental information regarding ADHD diagnosis and IQ received through personal email correspondence with the authors in September 2013 (Klykylo 2013 [pers comm]) |
|
Fernández‐Fernández 2010.
| Methods | A patient series of cardiac adverse effects of methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV‐R (subtype: combined) Age: 7, 8, and 10 years old IQ: > 85 Sex: 3 males Ethnicity: not stated Country: Spain Comorbidity: no cardiovascular diseases Comedication: not stated Sociodemographics: not stated |
|
| Interventions |
Case 1 Extended release osmotic release oral system methylphenidate, 36 mg/day for 18 months. Treatment compliance: not stated Extended release osmotic release oral system methylphenidate, 18 mg/day. Treatment compliance: not stated Case 2 Extended release osmotic release oral system methylphenidate, 18 mg/day, > 9 months. Treatment compliance: not stated Case 3 Extended release osmotic release oral system methylphenidate, 18 mg/day. Treatment period: not stated. Treatment compliance: not stated Discontinuation Re‐administration of extended release osmotic release oral system methylphenidate, 18 mg/day. Treatment period: not stated. Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Case 3 Re‐administration of extended release osmotic release oral system methylphenidate, 18 mg/day: recurrence of episodes of tachycardia. Emergency department: supraventricular tachycardia treated with adenosine Non‐serious adverse events: Case 1 Extended release osmotic release oral system methylphenidate, 36 mg/day, 12‐18 months: elevated arterial blood pressure with 2 SDs. No other intercurrent factors or symptoms Extended release osmotic release oral system methylphenidate, 18 mg/day: normalisation of blood pressure Case 2 Extended release osmotic release oral system methylphenidate, 18 mg/day, 6 months: repetitive episodes of self‐limiting tachycardia. Duration: a few minutes. ECG: self‐limiting sinus tachycardia, 150 beats/minute. Maintaining methylphenidate treatment for > 3 months: some episodes of tachycardia Case 3 Extended release osmotic release oral system methylphenidate, 18 mg/day: several episodes of tachycardia |
|
| Notes | Funding/vested interests: not stated Authors' affiliations: Unidad de Neurologiá Pediátrica. Hospital Universitario Infantil Virgen del Rocío. Sevilla, España Key conclusions of the study authors: these cases should make us be cautious and investigate the possible existence of family or personal cardiovascular pathology Comments from the study authors: to date, our patient is the youngest one diagnosed with supraventricular tachycardia secondary to methylphenidate treatment Supplemental information regarding ADHD diagnostic criteria and IQ received through personal email correspondence with the authors in August 2013 (Fernández‐Fernández 2013 [pers comm]) |
|
Fernández‐Fernández 2011.
| Methods | A patient report of infantile psychosis during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV‐R (subtype: combined) Age: 10 years old IQ: > 70 Sex: male Ethnicity: not stated Country: Spain Comorbidity: not stated Comedication: not stated Sociodemographics: adopted |
|
| Interventions | 2 years of extended release methylphenidate, dosage: 0.7/kg/day 1 week of extended release methylphenidate, dosage: 1.2 mg/kg/day Administration schedule: once daily Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Infantile psychosis. Significant behavioural changes with emotional lability, mood changes, disruptive behaviour and aggressive behaviour towards his mother and relatives. Presence of inner voices Discontinuation of methylphenidate treatment: no disruptive behaviour, aggressive attitude, nor inner voices |
|
| Notes | Funding/vested interests: not stated Authors' affiliations: Unidad de Neurologiá Pediátrica. Hospital Universitario Infantil Virgen del Rocío. Sevilla, España Key conclusions of the study authors: caregivers of children treated with psychostimulants should be informed of possible side effects in order to ensure proper treatment in case of appearance. Professionals must be familiar with the adverse reactions that may occur with methylphenidate treatment. Paediatric neurologists and child psychiatrists must be agile in handling these reactions when it comes to withdrawal of the medication, treatment of the adverse reactions and prevention of misdiagnosis or the establishment of a chronic antipsychotic or stimulant treatment Supplemental information regarding ADHD diagnostic criteria and IQ received through personal email correspondence with the authors in April 2013 (Fernández‐Fernámdez 2013b [pers comm]) |
|
Findling 1996.
| Methods | A patient report of an 11‐year old girl who experienced transient side effects after receiving MPH | |
| Participants | Diagnosis of ADHD: DSM‐III‐R (subtype: combined) Age: 11 years old IQ: > 70 Sex: female Ethnicity: not stated Country: USA Comorbidity: major depression (single episode, marked severity) Comedication: sertraline 25 mg, twice daily Sociodemographics: not stated |
|
| Interventions | Methylphenidate dosage: 5 mg twice daily Duration of intervention: the patient was still taking methylphenidate at the follow‐up at 3 months. Adverse effects occurred in the initiation of the treatment Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Mild and transient anorexia, transient weight loss 2 kg observed at the initiation of methylphenidate therapy |
|
| Notes | Ethics approval: not stated Funding/vested interests/authors' affiliations: not stated Key conclusions of study authors: these cases support previous suggestions that adjunctive treatment with psychostimulants might be a safe and effective intervention for children treated with fluoxetine or sertraline who have persistent ADHD symptoms and suggest that such combined treatment might be suitable for adults as well Supplemental information: email sent twice to author in order to get data on BP and HR. No reply |
|
Findling 2009.
| Methods | A multicentre, prospective, 12‐month, open‐label, flexible‐dose, phase III extension of 4 previous trials. This trial consisted of 3 phases: a dose‐optimisation period (4 weekly visits), a dose‐maintenance period (11 monthly visits), and a 30‐day follow‐up period |
|
| Participants | Number of participants screened: not stated Number of participants included: 327 Number of participants followed up at 12 months: 157 Number of withdrawals: 170. Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (82%), hyperactive‐impulsive (2.1%), inattentive (15.9%)) Age: mean 9.2 (SD 1.9), range 6‐12 years old IQ: normal Sex: 212 males, 115 females Methylphenidate‐naïve: 47.4%, before participation in the antecedent study Ethnicity: white (73.7%), African American (14.7%), Asian (1.5%), others (10.1%) Country: USA Comorbidity: not stated Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | The patients either continued their current optimised methylphenidate transdermal system dose (from the previous study) for 12 months or entered a 4‐week stepwise dose titration phase to an optimal methylphenidate transdermal system dose, followed by an 11‐month dose maintenance phase Methylphenidate transdermal system dose: 10 mg, 15 mg, 20 mg, or 30 mg Administration schedule: 7 am, patch removed after 9 hours Duration of intervention: 12 months, the median duration of exposure to methylphenidate transdermal system during the extension study was 335 (range 7‐392) days Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: There were no serious cardiovascular events (e.g. sudden death, myocardial infarction or stroke) over the 12 months of the study. 3 serious AEs were reported in 3 participants. None of these considered related to treatment Non‐serious adverse events: Physical examinations were performed at baseline, 6 months, and the final visit Height was measured at baseline, week 4, and months 2, 4, 6, 8, 10, and 12 Weight was measured at baseline, weeks 1 through 4, and months 6, 8, 10, and 12 Participants removed their clothing and shoes and wore a gown to ensure consistency between measurements. Height was measured using a calibrated stadiometer with the participant standing on a flat surface, shoes off, and the chin parallel to the floor. Weight was measured using the same calibrated scale at each visit: Vital signs (resting systolic and diastolic blood pressure (SBP and DBP), heart rate) were measured at baseline and all subsequent visits Blood pressure and heart rate were measured in the same arm using the same cuff type in each participant after 5 minutes' rest in a seated position ‐ Clinical laboratory tests and a 12‐lead electrocardiogram (ECG) were performed at baseline, week 4, months 3 and 6, and the end of the study, and analysed by unblinded paediatric cardiologists AEs were monitored at each study visit, and coded and defined using the Medical Dictionary for Regulatory Activities version 7.0. The severity and relationship of AEs to study treatment were assessed by the study investigators. Severity was rated as mild (easily tolerated, does not interfere with usual activities), moderate (interferes with daily activities, but participant is able to function), or severe (incapacitating, participant is unable to work or complete usual activities) Current and previous patch application sites were examined and rated for skin reactions and discomfort by trained, unblinded investigators at baseline; weeks 1 through 4; months 2 through 6, 8, and 10; and the end of the study. Dermal response scale 0‐7 (no reaction ‐ strong reaction beyond site). Dermal discomfort scale: 0‐3 (no discomfort ‐ severe, intolerable discomfort) Sleep behaviour investigated by using Children's Sleep Habits Questionnaire (CSHQ, 33 items), completed by parents/caregivers at baseline; weeks 1 through 4; months 2 through 6, 8, and 10; and at the end of the study. Item scores range from 1 (rarely occurring, 0‐1 times/wk) to 3 (usually occurring, 5‐7 times/wk), with total scores ranging from 33‐99 1 month after the last dose of study drug, participants' parents or legally authorised caregivers were contacted by telephone regarding ongoing or new AEs. 2 participants who had received MTS in the earlier studies had 1 AE each (tachycardia (154 beats/min) and worsening weight loss (6.6 kg)) that began before receipt of MTS in the present study. These AEs persisted and led to the participants' withdrawal after ˜1 and 4 months of treatment, respectively: blood pressure and pulse The highest mean increase from baseline in SBP (5.8 mm Hg) across dose levels was recorded with the 30‐mg dose at month 10. The highest mean increase in DBP (5.5 mm Hg) was observed with the 20‐mg dose at week 2 Across dose levels, the proportion of participants with an SBP above the upper limit of normal (> 123 mmHg) ranged from 1% to 10%, whereas 1.5% had an above normal SBP at baseline. No participants had an above normal DBP (> 90 mmHg) at baseline; however, 1 participant in each MTS dose group had an above normal DBP at some time in the study The highest mean increase in heart rate (5.6 beats/min) occurred with MTS 15 mg at month 3 ECG. There were no apparent trends toward changes in ECG indices at any MTS dose. ECG abnormalities were considered clinically significant in 4 participants and were recorded as 4 AEs. 3 of these AEs were tachycardia (123, 136, and 122 beats/min) and 1 was prolongation of the corrected QT (QTc) interval. QTc. Another participant had a QTcB interval of 544 ms at 3 months (QTcF, 476 msec; heart rate, 133 beats/min). A repeat ECG performed ˜2 weeks later recorded a QTcB of 419 ms (QTcF, 380 ms; heart rate, 107 beats/min). Although the results of the ECG at 3 months were deemed abnormal because of the prolonged QTc interval, they were not considered clinically significant. Laboratory analyses. No clinically significant changes from baseline or in the pattern of abnormal values on haematology, clinical chemistry, or urinalysis tests Dermal AEs. 33 dermal AEs were reported by 28 participants. AEs occurring more than once were rash (n = 6), urticaria (n = 6), allergic dermatitis (n = 2), contact dermatitis (n = 2), eczema (n = 2), generalised pruritus (n = 2), and skin hyperpigmentation (n = 2). 1 participant receiving MTS 10 mg had a pruritic rash possibly related to study treatment. Another participant receiving MTS 10 mg discontinued due to allergic dermatitis on the waist and knees, also possibly related to MTS treatment Mean (SD) dermal response scores at current and previous patch application sites were 1.2 (1.1) and 0.8 (1.0), respectively, across study visits, indicating minimal erythema 15 participants had 30 instances of a dermal response score of 5 at the current patch site, 4 participants had 6 instances of a dermal response score of 6, and 2 had 2 instances of a dermal response score of 7 Sleep behaviour. No apparent overall effect on sleep behaviour. Regarding insomnia, see below Weight. 32.9 kg SD 9.9, range: (16.3‐90.6 kg), height: 1.37 (SD 0.13), range: 1.08‐1.72 m. Weight loss in 33 participants (also in table 2), poor weight gain in 1, and increased weight in 2. Mean (SD) changes in the z scores for weight, height, and BMI at the end of the study were –0.2 (0.43), –0.0 (0.44), and –0.3 (0.72), respectively. The 95% CIs for weight change from baseline to end point were 0.70 to 2.99 for participants aged 6 to 9 years and 6.40 to 10.27 for participants aged ≥10 years At visit 16, mean (SD) weight changes from baseline in participants receiving MTS 10 mg, 15 mg, 20 mg, and 30 mg were 2.73 (2.53), 1.62 (3.44), 2.49 (3.44), and 1.61 (3.41) kg, respectively. At visit 16, mean (SD) weight changes from baseline in participants receiving MTS 10, 15, 20, and 30 mg were 2.73 (2.53), 1.62 (3.44), 2.49 (3.44), and 1.61 (3.41) kg, respectively |
|
| Notes | Sample calculation: estimated enrollment 450 Ethics approval: the study protocol was approved by the appropriate institutional review board or independent ethics committee for each site Funding/vested interests: Shire Development Inc. provided study medication and support for study‐related care, and the company was involved in the study design, conduct, and data analysis Authors' affiliations: all but 1 of the authors receives or have received research support from, acted as a consultant for, and/or served on a speaker's bureau for several pharmaceutical companies Key conclusions of the study authors: long‐term efficacy in paediatric patients was demonstrated by improvements in the ADHD‐RS‐IV, CGI‐I, and PGA assessments. Most adverse events (98.3%) were mild to moderate. With the exception of application‐site reactions associated with transdermal delivery of methylphenidate, adverse events were generally typical of those associated with methylphenidate Comments from the review authors: if < 7 days had passed between the completion of the previous study and entry into the present one, baseline assessments were used from the previous study Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information and adverse event data have not been possible to receive through personal email correspondence with the authors in October‐November 2013 |
|
Findling 2010.
| Methods | This was a Phase IIIB, randomised, double‐blind, parallel‐group, placebo‐controlled, multicentre, dose‐optimisation study evaluating the efficacy and safety of
The study consisted of 4 experimental periods:
The follow‐up was an open‐label extension study for evaluating the safety and efficacy of methylphenidate transdermal system (10‐, 15‐, 20‐ or 30 mg/9‐hour patches) for participants who completed all required study visits and consisted of 3 experimental periods |
|
| Participants |
For the open‐label extension: Number of participants screened: not stated Number of participants included: 163 (110 previously on MPH and 53 on PL) Number of participants followed up: 162 Number of withdrawals 1 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 14.5 (SD 1.24) years (range 13‐17) IQ: ≥80 Sex: 121 males, 41 females Methylphenidate‐naïve: 56% (122) Ethnicity: white (78%), African American (17%), Asian (0.6%), others (4.4%) Country: USA Comorbidity: none Comedication: not stated Sociodemographics; not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: transdermal system Mean methylphenidate dosage at month 6: 10 mg (5.6.%), 15 mg (7.9%), 20 mg (32.6%), and 30 mg (53.9%); median exposure time: 168.0 days (range, 3‐200 days) Administration schedule: single patch in the morning, once daily for 9 hours Duration of intervention: 6 months Titration period: 5 weeks of the 6 months Treatment compliance: 88 fulfilled the protocol |
|
| Outcomes | Regarding the 6‐month open‐label study: Dose optimisation (5 weekly visits) versus dose maintenance (5 monthly visits): Adverse events were monitored at each study visit and assessed by an open‐ended inquiry along with specific dermatological questions asked by an investigator or qualified evaluator. AEs were considered treatment emergent if they began or worsened on or after application of the first patch and occurred before or at the same time as application of the patch. AEs coded and defined using the MedDRA, version 7.03) a 7‐day post‐treatment follow‐up. Height: measured at month 6 visit by the investigator Weight: recorded at all 5 visits by the investigator Vital signs (systolic blood pressure, diastolic blood pressure pulse), measured at all study visits, by the investigator. 12‐lead ECG performed at entry, week 4, month 3, month 6, by the investigator Blood and urine samples collected at entry, week 4, month 4, and month 6 Dermal skin reaction: measured by DRS at each study visit Sleep: measured by the non‐validated Post‐Sleep Questionnaire. Measured at the 6 month visit Any changes noted between evaluation data at study entry and data obtained at schedule visits deemed to be clinically significant by the investigator were considered an adverse event |
|
| Notes | Sample calculation: yes Ethics approval: yes Funding: this study was funded by Shire Development Inc., which was involved in the study design, conduct, and data analysis Vested interests/authors' affiliations: Dr Findling has received research support, acted as a consultant, and/or served on speakers' bureaus for Abbott, Addrenex, As‐ traZeneca, Biovail, Bristol‐Myers Squibb, Forest, GlaxoSmith Kline, KemPharm, Johnson & Johnson, Eli Lilly, Lundbeck, Neuropharm, Novartis, Organon, Otsuka, Pfizer, Sanofi‐Aventis, Sepracor, Schering‐Plough, Shire, Solvay, Supernus Pharmaceuticals, Validus, and Wyeth. Dr Katic has received research support, acted as a consultant, and/or served on speakers' bureaus for Forest, GlaxoSmithKline, Lundbeck, Merck, Novartis, Sanofi‐Aventis, Sepracor, Shire, Somerset, and Wyeth. Dr Rubin has received research support, acted as a consultant, and/or served on speakers' bureaus for Abbott, Addrenex, Cephalon, Eli Lilly, Johnson, Johnson, McNeal, Novartis, Shire, and UCB Celltech. Dr Moon received research support, acted as a consultant, and/or served on speakers' bureaus for Abbott, AstraZeneca, CNS Response, Eli Lilly, Johnson & Johnson, McNeil, Novartis, Pfizer, Sanofi‐ Aventis, Shire, Synosia Therapeutics, Takeda, and UCB Inc. Drs Civil and Li are employees of Shire Development Inc. Dr Civil is an employee of Shire Development, Inc. At the time of this study, Dr Li was an employee of Shire Development, Inc. Key conclusions of the study authors: regarding the 6 months open‐label study: methylphenidate transdermal system was generally well tolerated, and AEs were generally typical of those associated with oral methylphenidate, with the exception of application‐site reactions associated with transdermal delivery of methylphenidate Comments from the study authors: it is important to note that participants who failed to respond to psychostimulants in the past and those with conduct disorder and other psychiatric comorbidities were excluded from the study. Regarding the 6‐month study: no clinically significant findings between laboratory evaluation parameters obtained postentry relative to screening values obtained at the antecedent study Comments from the review authors: as already stated, people who earlier had failed to respond to psychostimulants were not included in the study. Therefore results can only be generalised to responders. Regarding the 6‐month open‐label study: participants who had not reached an acceptable response by the end of week 5 were withdrawn from the study Supplemental information requested from the study authors and Noven Pharmaceuticals with no reply |
|
Gadow 1995.
| Methods | A randomised, placebo‐controlled, double‐blind, cross‐over trial with 2 interventions:
Phases:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 34. Participants were randomly assigned to different orders of the 3 dosages Number of participants followed up: 12‐months follow‐up: 30; 18 months follow‐up: 26; 24 months follow‐up: 26 Number of withdrawals: 8 Diagnosis of ADHD: DSM‐III‐R (subtype: not stated) Age: mean: 8 years and 10 months, range: 6.1‐11.9 years old IQ: mean: 105.9 (SD 13.7) Sex: 31 males, 3 females Methylphenidate‐naïve: 71% Ethnicity: white: 85%, African American: 3%, Asian: 3%, Hispanic: 9% Country: USA Comorbidity (only data from 21 children from Gadow 1995): tics: 100%, anxiety and/or depressive disorder: 8/21 (38%), obsessive‐compulsive disorder: 3/21 (14%), most of the children furthermore had either oppositional defiant disorder or conduct disorder and academic problems Comedication: not during the RCT. During the follow‐up 4 children were treated with an anti‐tic medication in combination with methylphenidate (neuroleptic n = 3, clonidine, n = 1) Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate dosage: participants were randomly assigned to 1 of 4 possible drug condition orders of doses: 0.1 mg/kg (mean: 4.4 mg), 0.3 mg/kg (mean: 9.0 mg) and 0.5 mg/kg (mean: 14.0 mg) methylphenidate and placebo. Upper dosages limit was 20 mg. When the 0.5 mg/kg dose was not preceded by a low dose condition, the child was gradually build up to the moderate dose. The build‐up days occasionally fall on scheduled school observation days.The observer were unaware of these days, and observations were conducted as usual, but these data were excluded from the analyses Administration schedule: 2‐3 times daily at morning and noon, approximately 3.5 hours apart, 7 days a week Duration of each medication condition: 2 weeks Washout prior to study initiation: methylphenidate: 1 week, antipsychotic: 3 weeks, clonidine: 2 weeks Medication‐free period between intervention: none Titration period: none Treatment compliance: parents and nurses were asked to return unused medication envelopes, which allowed the researchers to assess compliance. However, no further description in the paper 24 month follow‐up: total daily dose of methylphenidate, MED (minimal effective dose ‐ after RCT): (mean 16.5 mg, range 5‐40 mg); second visit (mean 28.5 mg, range 15‐60 mg); third visit (mean 29.2 mg, range 10‐90 mg); and fourth visit (mean 34.5 mg, range 15‐92 mg) |
|
| Outcomes |
Non‐serious adverse events During the 8‐week RCT Side Effect Checklist (SSEC), 13 items, rated by parents on Saturday and Sunday and rated by teacher twice a week Global Tic Rating Scale (GTRS), rated by parents on Saturday and Sunday and rated by teacher twice a week Motor and vocal tic category was, were observers coded presence or absence of tics in the classroom, lunchroom or playground, 4 times for each medication condition Physician evaluations Yale Global Tic Severity Scale (YGTSS), rated every second week Tourette Syndrome Unidentified Rating Scale, rated every second week Global Tic Rating Scale (GTRS) (only assessed in 22 patients), rated every second week Shapiro Tourette Syndrome Severity Scale (OBS! Only assessed in 22 patients), rated every second week Motor tic frequency tics; rated in 180 5‐second intervals in a simulated classroom, and tics were coded as either present or not present in each interval, rated every second week Weight, assessed every second week Heart rate, assessed every second week Blood pressure, assessed every second week During the 24‐months follow‐up Physician evaluations All rated at MED (right after RCT) 6 months, 12 months, 18 months, and 24 months Yale Global Tic Severity Scale (YGTSS) Shapiro Tourette Syndrome Severity Scale 3 subscales from the Tourette Syndrome Unified Rating scale Total number of tics Number of tics observed in 2 minutes of quiet conversation with the physician The LeWitt Disability Scale which assesses tics and the symptoms of comorbidities The Global Tic Rating Scale (GTRS) Blood pressure Heart rate Pulse Weight Parents' ratings Based on the last 2 weeks and rated at MED (right after RCT) 6 months, 12 months, 18 months, and 24 months Stimulant Side Effect Checklist (SSEC) GTRS |
|
| Notes | Sample calculation: yes Any withdrawals due to adverse events: no Ethics approval: no information Funding: supported in part by a research grant from the Tourette Syndrome Association Inc and a Public Health Service Grant from the National Institute of Mental Health Vested interests/authors' affiliations: not stated Key conclusions from study authors: during the course of this short‐term drug evaluation, physician, teacher, and parent ratings were in uniform agreement that methylphenidate did not lead to a worsening in the severity of the children's tic disorder. Furthermore methylphenidate is an effective drug for the treatment of ADHD and oppositional and aggressive behaviour. Furthermore the follow‐up study showed that long‐term treatment with methylphenidate seems to be safe and effective for the management of ADHD behaviours in many (but not necessarily all) children with mild to moderate tic disorder. Nevertheless, careful clinical monitoring is mandatory to rule out the possibility of drug‐induced tic exacerbation in individual patients Comments from the study authors: the magnitude of clinical improvement associated with the 0.3 mg/kg dosage compared with 0.5 mg/kg dosage was generally trivial for many children. The 0.5 mg/kg dosage was associated with more side effects, but fortunately they were generally of limited clinical significance.The generalisability of the findings from this study are subject to several qualifications. First, our data pertain to observed treatment effects over an 8‐week period and therefore cannot address the issue of tic exacerbation as a function of long‐term drug exposure. Furthermore the findings pertain only to children with ADHD and with tics that are of mild to moderate severity and that occur frequently enough to be observed during 15‐minute intervals Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no, children were not excluded from participation in the study if they had prior experience with stimulant drug therapy or if such a therapy purportedly had exacerbated their tics. Comments from the review authors: 26 of the children received stimulant medication throughout the follow‐up interval, and of these children, 1 was switched to dextroamphetamine. However, we have chosen still to use the results for the 26 in our analyses. All of the included articles are a mix of different protocols, so the total number of included participants differ from article to article Supplemental information regarding cross‐over data requested through personal email correspondence with the authors in April 2013 (Gadow 2013 [pers comm]). No data regarding the interventions were available |
|
Galland 2010.
| Methods | A cross‐over trial with 2 interventions:
Phases: 2 Part of a case‐control study: ADHD group randomly assigned to 2 nights on and 2 nights off of methylphenidate versus non‐medicated non‐ADHD control group |
|
| Participants | Number of patients screened: not stated Number included: 30. Participants were randomly assigned to methylphenidate or no treatment Number followed up: 27 for cross‐over RCT, 28 for observational data Number of withdrawals: 3 for RCT, 2 for observational data RCT Diagnosis of ADHD: DSM‐IV (subtype: combined (78%/21), inattentive (22%/6)) Age: mean: 10 years 6 months, range: 6 years 7 months to 12 years 4 months IQ: above 70 Sex: 21 males, 6 females Methylphenidate‐naïve: 0% Ethnicity: white: 23, Maori: 1, other: 3 Country: New Zealand Comorbidity: oppositional defiant disorder: 9 (33%), specific phobia: 1 (4%) Comedication: no Sociodemographics: mean deprivation index: 5.5 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to methylphenidate or no medication. Methylphenidate type: Ritalin Mean methylphenidate dosage: not stated Administration schedule: 7 children had standard methylphenidate formulation once daily, 9 twice daily and 2‐3 times daily; 4 children had slow release alone, 4 in combination with once daily standard and 1 in combination with twice daily standard formulation Duration of intervention: 2 nights Washout prior to study initiation: none Medication‐free period between interventions: not clear, there was 5 to 7 days between medication and non‐medication phases Titration period: none, participants had been treated with methylphenidate for a median of 3 years 9 months (range 3 months to 6 years 8 months). ADHD children were randomised to either the first night as a medication‐ and caffeine‐free night for 48 hours prior to each study night, or a medication night where they maintained their methylphenidate dosage regimen 48 hours prior to each study night, but remained caffeine‐free Treatment compliance: urinary methylphenidate was reported as detected/or not detected. No control children returned a positive test on either night |
|
| Outcomes |
Non‐serious adverse events: Standard polysomnographic (PSG) recordings on the second night of 48 hours on or off MPH Sleep questionnaire (28‐item), completed by the parent (predominantly mother) over that week General adverse events, parent rated, observational data |
|
| Notes | Sample calculation: not reported Ethics approval: yes, Otago Ethics Committee approved the study Funding/vested interests/authors affiliations: the project was supported by a grant from the Health Research Council of New Zealand Key conclusions from study authors: findings suggest that methylphenidate reduces sleep quantity but does not alter sleep architecture in children diagnosed with ADHD Comments from the study authors: 1. It is possible that the longer sleep duration and shorter sleep latency on the off‐ compared to on‐medication night could be interpreted as purely rebound, or that the on‐medication night reflects a truer baseline and the off‐medication night reflects a medication withdrawal effect 2. The dosages and formulations of methylphenidate were not standardised 3. Only 1 PSG recording was conducted for each condition, which means the information collected could be subject to a first‐night effect Comments from the review authors: we considered this a RCT study even though the authors describe it as a case‐control study, because the ADHD children were randomly assigned to on‐ or off‐methylphenidate. Only observational data on adverse events is used here Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information received through personal email correspondence with the authors in August 2014 (Galland 2014 [pers comm]) |
|
Garg 2014.
| Methods | An open‐label randomised parallel group clinical trial of methylphenidate and atomoxetine use for 8 weeks | |
| Participants |
Methylphenidate group Number of participants screened: not stated Number of participants included: 33 Number of participants followed up: 26 Number of withdrawals: 7 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (66.7%), hyperactive‐impulsive (6.1%), inattentive (27.3%)) Age: mean: 8.47 (SD 2.22), range: 6‐14 years old IQ: > 70 Sex: 27 males (81.1%), 6 females (18.2%) Methylphenidate‐naïve: 100% Ethnicity: not stated Country: India Comorbidity: oppositional defiant disorder (15/45.5%), conduct disorder (1/3%) Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release Mean methylphenidate dosage: 11.59 (2.83) mg/day, at conclusion of the study: 17.35 (7.52) mg/day (or 0.62 mg/kg/day) Administration schedule: once or twice daily Duration of intervention: 8 weeks Treatment compliance: not stated |
|
| Outcomes | The various side effects were noted on each assessment on the Adverse Events Checklist prepared for the study. It was a semi‐structured check list enlisting all the common side effects of methylphenidate and atomoxetine. The parents were asked to rate the severity of each side effects produced in their children as mild, moderate and severe. Those who reported mild side effects were continued on the same dose. For those who developed moderate severity of side effects, dose was reduced. Those who rated any adverse effect to be severe were taken out of the study after stopping the medication Non‐serious adverse events: 18 (55%) patients developed side effects during the course of the study The commonest reported adverse effect was reduced appetite |
|
| Notes | Sample calculation: not stated Ethics approval: clearance was obtained from the ethics committee of the Government Medical College and Hospital, Chandigarh Funding/vested interest: none Key conclusions of the study authors: methylphenidate and atomoxetine are efficacious in Indian children with ADHD at lesser doses than previously used. Their efficacy and tolerability are comparable Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, exclusion of non‐responders Supplemental information received through personal email correspondence with author in July 2016 (Arneja 2016 [pers comm]) |
|
Gau 2006.
| Methods | An open, randomised, parallel, active‐controlled equivalent 28 day trial with
|
|
| Participants | Number of participants screened: not stated Number of participants included: 64 Number randomised to immediate release methylphenidate: 32, and to OROS methylphenidate: 32 Number followed up in each arm: immediate release methylphenidate: 32, OROS methylphenidate: 32 Number of withdrawals in each arm: 0 Diagnosis of ADHD: DSM‐IV/ICD‐10 (combined 50 (78.1%), hyperactive‐impulsive 2 (3.1%), inattentive 12 (18.9%)) Age: mean: 10.5, range: 6‐15 years old IQ: > 80 Sex: 58 males, 6 females Methylphenidate‐naïve: 0 Ethnicity: Asian 100% Country: Taiwan Comorbidity: no Comedication: no Sociodemographics: parents education: college: 55%, high school: 30%, junior high school: 10%, other: 5% Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release and osmotic release oral system methylphenidate Mean methylphenidate dosage: OROS: 27.7 mg/day (SD 13.5); immediate release: 26.7 mg/day (SD 7.6) Administration schedule: OROS: once daily; immediate release: 3 times daily Duration of intervention: 28 days Treatment compliance: parents were required to record the time of drug administration on an adherence sheet. Pill counting was performed for each participant. Days forgetting to take medication: immediate release group 8.6 (SD 5.7); OROS group 2.0 (SD 3.9) |
|
| Outcomes |
Non‐serious adverse events: Barkley's Side Effects Questionnaire at baseline, on day 14 and day 28 Vital signs. At baseline, on day 14 and day 28 |
|
| Notes | Sample calculation: no Ethics approval: IRB of National Taiwan University Hospital Funding/vested interest/authors' affiliations: the study was supported by Janssen‐Cilag, Taiwan. Drs. Susan S.F. Gau and Wei‐Tsuen Soong have conducted clinical trials on behalf of Janssen‐Cilag, Taiwan and Eli Lilly and Company, Taiwan. They have also been speakers for Janssen‐Cilag, Taiwan and Eli Lilly and Company, Taiwan Any withdrawals due to adverse events: no Key conclusions of the study authors: OROS methylphenidate has similar efficacy to immediate release methylphenidate, with less severity of decreased appetite. OROS methylphenidate is superior over immediate release methylphenidate in treating ADHD children and adolescents in the context of Chinese culture Comments from the study authors: the short study period limits our understanding regarding long‐term efficacy of methylphenidate and the possible side effects on appetite, cardiovascular functioning, and so on in the Chinese population Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information regarding IQ and diagnostic criteria received through personal email correspondence with the authors in October 2013 (Gau 2013 [pers comm]) |
|
Gau 2008.
| Methods | An observational national survey where all the children with bad adherence were switched to other medications among those OROS methylphenidate | |
| Participants |
Cross‐sectional study, phase 1: Number of participants screened: not stated Number of participants included: 607 Diagnosis of ADHD: DSM‐IV (subtype: combined (59.6%), hyperactive‐impulsive (11.2%), inattentive (29.2%)) Age: mean 9.5 (SD 2.4), range 5‐16 years old IQ: 89.5% had an IQ > 70 Sex: 504 males, 103 females Ethnicity: not stated Country: Taiwan Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
Cohort study, phase 2: (Patients from phase 1 with bad adherence) Number of participants screened: 604 Number of participants included: 137 Number followed up: 124 Number of withdrawals: 13 The following data areon all patients from phase 2, n = 240. Diagnosis of ADHD: DSM‐IV (combined (60.8%), hyperactive‐impulsive (8.3%), inattentive (30.8%)). Age: mean: 10.9 (SD 2.8), range: 5‐16 years old IQ: 95% > 70% Sex: 198 males, 42 females Methylphenidate‐naïve: none |
|
| Interventions |
Phase 1: Methylphenidate type: immediate release Methylphenidate dosage: 18 mg Administration schedule: 3 times daily (n = 83), twice daily (n = 308), once daily (n = 162) Duration of intervention: st least 3 of the preceding 6 months Treatment compliance: poor adherence was defined as missing ≥ 1 doses on a school day on ≥ 2 days per week for 4 weeks. 240 patients defined as poor adherents Phase 2 Methylphenidate type: osmotic release oral system Methylphenidate dosage: 24.9 mg Administration schedule: once daily Duration of intervention: 3 weeks or more Treatment compliance: not stated Patients who took IR‐MPH 5 mg once, twice or thrice daily and IR‐MPH 10 mg once, twice or thrice daily were switched to OROS‐MPH 18 mg and 36 mg per day, respectively |
|
| Outcomes | Safety measures assessed by the investigators were decreased appetite, dizziness/headache, gastrointestinal disturbance, poor sleep quality, and other side effects Non‐serious adverse events: 18.8% had poor adherence due to side effects |
|
| Notes | Sample calculation: no Ethics approval: approved by the Joint Institute Review Board, Taiwan, and the institutional review boards of each study site Funding/vested interest/authors' affiliations: the national health research institute.The work was supported by Janssen‐Cilag, Taipai, Taiwan Key conclusions of the study authors: poor adherence to medication may be an important reason for suboptimal outcome in ADHD treatment, physicians should ensure adherence with therapy before adjusting dosage or switching medication Comments from the study authors: the investigator's judgement with respect to adherence was based on patient and parent reports of missed doses without pill count, therefore, overestimate of adherence is very likely. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, excluded children with previous experience of adverse events |
|
Germinario 2013.
| Methods | A cohort study of 6‐ to 17‐year‐olds registered on the Italian ADHD National Registry from 2007 to June 2010 before beginning treatment with methylphenidate or atomoxetine (ATX) with follow‐up to 24 months | |
| Participants |
Patients with outcomes on cardiovascular measures Number of participants screened: 840 Number of participants included: 351 Number of participants followed up: 214 at 6 months, 190 at 12 months, 77 at 24 months Nnumber of withdrawals: 137 at 6 months, 161 at 12 months, 274 at 24 months Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 10.41, range 6‐17 years old IQ: not stated Sex: 305 males, 346 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Italy Comorbidity: not stated for methylphenidate subgroup Comedication: not stated Sociodemographics: not stated Patients with outcomes on weight Number of participants screened: 840 Number of participants included: 840 Number of participants followed up: 296 at 6 months, 184 at 12 months, 55 at 24 months Nnumber of withdrawals: 137 at 6 months, 161 at 12 months, 274 at 24 months Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 10.41, range 6‐17 years old IQ: not stated Sex: 305 males, 346 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Italy Comorbidity: not stated for methylphenidate subgroup Comedication: not stated Sociodemographics: not stated Number of patients screened: 840, number included: 840 followed up: 296 at 6 months, 184 at 12 months, 55 at 24 months number of withdrawals: 544 at 6 months, 656 at 12 months, 785 at 24 months The rest of the demographic data regarding the sample were described together with the atomoxetine group, and therefore this data are not extracted Patients with outcomes on height Number of participants screened: 840 Number included: 840, number followed up: 288 at 6 months, 167 at 12 months, 55 at 24 months, number of withdrawals: 552 at 6 months, 673 at 12 months, 785 at 24 months The rest of the demographic data regarding the sample were described together with the atomoxetine group, and therefore this data are not extracted. All met DSM‐IV diagnostic criteria for ADHD Patients from the Campania Region Number of patients screened: 8, number included: 8, number followed up: 5, number of withdrawals:3 The rest of the demographic data regarding the sample were described together with the atomoxetine group, and therefore this data are not extracted Patients from the Lombardy Region The national ADHD Registry contained data on 1733 patients treated with MPH or atomoxetine between June 2007 and May 2010. Number included: 229 were enrolled from 15 regional centres, 130 of these were drug‐naïve. Regarding the 130 drug naïve, 34 of these received MPH Number included: 34 number followed up: 34, number of withdrawals: 10 DSM‐4 diagnosis of ADHD: combined (79.4%), hyperactive‐impulsive (8.8%), inattentive (11.8%). Age (mean, 10.7 years). IQ: 2 participants had mental retardation. Sex (m: 28, f: 6), MPH‐naïve (100%). Ethnicity: not stated. Country: Italy. Comorbidity (type: % learning disorders 14, ODD 14, language disorder 5, mental retardation 2), comedication: 7 patients, sociodemographics: 2 were adopted, 11 only child Article on adverse events Number of patients screened: not stated, number included: 1098 followed up: 931 number of withdrawals: 167 Subtype: (combined, n = 941, inattentive = 124, hyperactivity, n = 32) Inclusion criteria: Patients aged 6 to 17 years with ADHD treated with atomoxetine or MPH who were registered with the Italian ADHD National Registry from 2007 onwards To be diagnosed with ADHD, participants had to present with significant functional impairment and symptoms had to be present before 7 years of age, persist for ≥ 6 months, be present in more than one setting Exclusion criteria: Other mental or spectrum disorder; altered baseline ECG or no ECG assessment; or no information on drug therapy, only 1 follow‐up, or follow‐up of < 6 months |
|
| Interventions | 902/1758 were treated with oral MPH chlorohydrate (Ritalin) 10 mg tablet at a dose of 0.3‐0.6 mg/kg/dose/day. The total daily dose (mean 18.4 mg) could be administered in 2‐3 doses per day at the discretion of the child's neuropsychiatrist. Duration of intervention: see under 'Description of participants'. A methylphenidate test dose of 0.3 mg/kg was administered first and the dosage increased up to 0.6 mg/kg/dose depending on clinical response and tolerability Treatment compliance: adherence to treatment was not evaluated. But participants with compliance problems were excluded Regarding the sample from the Lombardy Region MPH dosage: mean MPH dosage: 39.9 mg. Administration schedule: both stated. Duration of intervention: treatment compliance: daily dose of MPH ranged from 10 mg to 75 mg. All participants were drug‐naïve |
|
| Outcomes |
Serious adverse events: 11 experienced serious adverse reactions Arrhythmia Non‐serious adverse events: BP and HR were assessed monthly ECG assessed at baseline and every 6 months. Prolongation of QTc interval was defined as any prolongation with respect to detected value at the screening before the first administration of the drug. ECG with alterations or pathological aspects were read by paediatric cardiologist Liver status assessed every sixth month at the follow‐up Suicidal thoughts assessed every sixth month at the follow‐up Convulsion assessed every sixth month at the follow‐up BMI assessed every sixth month at the follow‐up Height and weight and BMI: monitoring was recommended monthly. The mean number of height measures per participant was 6.11, ranging from 1 to 33, whereas the mean number of weight measures per participant was 6.14 ranging from 1 to 33 Any events occurring for the first time as well as a worsening of the disorder while on the study drug were defined as adverse events. Parents were requested in advance to report any adverse events during follow‐up visits Sample from the Campania region Participants were monitored for adverse events periodically by clinicians at the reference prescription centre at 1 week, 1 month, and every 3 months for the first year, and every 6 months thereafter, monitored by trained monitors. The median follow‐up period was 289 days |
|
| Notes |
Sample calculation: yes Ethics approval: approved by the Ethical Committee of the ISS Funding/vested interest: this study was supported by an independent grant n. FARM5AJL82_001 Italian Medicine Agency (AIFA) Any withdrawals due to adverse events: 30 patients dropped out due to adverse events Authors' affiliations: authors declare no financial interests Key conclusions of the study authors: regular monitoring of cardiovascular parameters (anamnestic history and BP and HR measurements) is recommended for all patients, but should be considered mandatory, perhaps at more frequent intervals, for participants at high risk. Furthermore; ADHD drugs show a negative effect on linear growth in children in middle term. Such effect appears more evident for ATX than for MPH. The study also suggests that ATX is more likely to be reported as causing harm than methylphenidate Comments from the study authors: Limitations: it is not clear whether the observed slowdown in growth is a transient effect or a permanent potential reduction for individual growth with respect to the final height. As our observation time was only 24 months of follow‐up, we were not able to evaluate if the negative effect on growth persisted after 24 months treatment. Second we could not assess if the negative effect observed on height would persist after permanent discontinuation of drugs. Third approximately 60% of participants could not be included in the analyses Comments from Ruggiero 2012: Among all the ATX‐ or MPH‐base, most occurred in patients from Campania, probably because of our intensive monitoring program. During our study period, we introduced monitors who periodically and systematically interviewed clinicians at reference prescription centres. This was an active method to enhance the identification of adverse drug reactions (ADRs) by clinicians and to solicit them to report ADRs Comments from the review authors: In the description of participants, we have chosen to divide them up according to outcome. Many of the participants will be found in several of the descriptions according to outcomes Exclusion of MPH non‐responders/or children who have previously experienced adverse events on MPH: no Supplemental information regarding additional data and additional publications received through personal email correspondence with the authors in December 2013 and January 2014. Asked authors how they distinguished between serious adverse events and serious adverse reactions. Answer from authors: "We have catalogued the events reported compulsorily Italian Drug Agency as Serious adverse events. The serious adverse reactions are severe events for which reporting was not mandatory. I acknowledge that is a classification unorthodox [sic]" (Panei 2014 [pers comm]) |
|
Gerwe 2009.
| Methods | An 8‐week, multicentre, prospective, open‐label, single‐arm, non‐interventional trial with 2 groups:
|
|
| Participants | Number of participants screened: 313 Number of participants included: 306 Number of participants followed up: 263 Number of withdrawals: 43 (14.1%) discontinued prematurely due to adverse events (n = 37, 12.1%), and/or lack of efficacy (n = 23, 7.5%), lost to follow‐up (n = 2), non‐compliant (n = 2), gave other reasons (n = 8) Diagnosis of ADHD: ICD‐10 (F90.0 (72.5%), F90.1 (34.3%), F90.8 (1.6%), F90.9 (3.3%), others (9.2%)). Age: 10.2 (SD 2.3), range 6‐14 years old IQ: not stated Sex: 246 males (80.4%), 60 females (19.6%) Methylphenidate‐naïve: 24.5% Ethnicity: not stated Country: Germany Setting: outclinic Comorbidity: 38.6%. Conduct disorder 25.5%, conduct disorder with ODD 24.2%, anxiety disorder 5.6%, OCD 1.3% Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system Methylphenidate dosage, whole sample: 36 mg/day (median), 29.5 (SD 12.7) mg/day starting dose, 32.8 (SD 13.2) mg/day final dose, range: 18‐72 mg/day. Methylphenidate dosage, switch treatment subgroup: 31.6 (SD 12.7) mg/day starting dose, 34.4 (SD 13.5) mg/day final dose Methylphenidate dosage, initial treatment subgroup: 22.8 (SD 10.0) mg/day starting dose, 27.8 (SD 10.8) mg/day final dose Administration schedule: once daily Duration of intervention: 55 (SD 17.1) days, range 6‐113 days Treatment compliance: 2 patients discontinued due to lack of compliance |
|
| Outcomes | Documentation of adverse events at week 1, 2 and 8 or premature termination. Measurement of blood pressure and pulse frequency and ratings of quality of sleep and appetite on Likert scales from 1 (very good) to 5 (very bad) at baseline, week 1, 2 and 8 or premature termination. Measurement of height, weight and documentation of tics at baseline and week 8 or premature termination. All ratings were performed by the treating physician. Measuring instruments for the documentation of adverse effects and tics are not stated Non‐serious adverse events: A total of 319 adverse events were reported by 160 (52.3%) of 306 patients. For 161 of 319 adverse events (50.5%) in 95 patients (31%) a causal relationship between the administration of OROS methylphenidate and the event was assessed as at least possible by the investigator Serious adverse events: 4 serious adverse events were reported in 2 patients |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: 37 Ethics approval: the International Ethics Committee of the University of Freiburg, Germany Funding: this study was supported by Janssen‐Cilag GmbH, Germany Authors' affiliations: authors are employees of Janssen‐Cilag or have received consulting fees Key conclusions of the study authors: transitioning from immediate release methylphenidate or no methylphenidate‐treatment to OROS‐methylphenidate in patients aged 6‐14 years with a diagnosis of ADHD was associated with significant improvements in daily functioning in several areas of life, severity of disease and quality of life Comments from the study authors: no further information about dosage or dosing regimen of a prior MPH treatment. Neither standardised diagnostic procedures nor a pre‐defined titration scheme were performed. No specification for transition period from MPH‐IR to OROS‐MPH was made. Methylphenidate‐naïve patients experienced somewhat more adverse effects, especially insomnia and anorexia, which probably also led to the slightly larger mean decreases in weight and tendencies to impaired quality of appetite and sleep, compared to the switch treatment group Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Ghanizadeh 2008a.
| Methods | A patient report of decrease in appetite during treatment with methylphenidate | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 8.5 years old IQ: about 100 to 110 Sex: male Ethnicity: not stated Country: Iran Comorbidity: oppositional defiant disorder Comedication: not stated Sociodemographics: not stated |
|
| Interventions | MPH dosage: 20 mg daily Administration schedule: not stated Duration of intervention: 1 month Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Significant decrease in appetite |
|
| Notes | Ethics approval: informed consent was obtained Funding/vested interests: not stated Key conclusions of study authors: this report is about a child with ADHD who experienced insomnia, night terrors, and depression associated with the long‐term use of clonidine. He revealed resolution of insomnia and night terror when clonidine was removed Comments from the review authors: the adverse effects (decrease in appetite) occurred while the patient was only getting methylphenidate Supplemental information regarding IQ was retrieved through personal email communication with the author in July 2013 (Ghanizadeh 2013 [pers comm]) |
|
Ghanizadeh 2008b.
| Methods | A patient report of nocturnal enuresis during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 11 years old IQ > 80 Sex: male Ethnicity: Persian Country: Iran Comorbidity: no Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate dose: 20 mg/day, 2 months. Discontinuation of methylphenidate for 1,5 months. Re‐administering of methylphenidate, 20 mg/day, 3 months Discontinuation of methylphenidate, some months. Re‐administering of methylphenidate, 20 mg/day, 2 months. Discontinuation of methylphenidate
Type of methylphenidate: not known Administration schedule: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Methylphenidate titrated to 20 mg/day: nocturnal enuresis Discontinuation of methylphenidate: enuresis stopped immediately Methylphenidate, when titrated to 20 mg/day: immediate re‐occurence of nocturnal enuresis Discontinuation of MPH: enuresis stopped immediately Methylphenidate, when titrated to 20 mg/day: immediate re‐occurence of nocturnal enuresis Discontinuation of MPH: enuresis stopped immediately |
|
| Notes | Ethics approval: not stated Funding/vested interests: not stated Key conclusions of study authors: clinicians should be aware of this potential side effect of methylphenidate Comments from the study author: although both the boy and his mother were interviewed to obtain precise information, it should be noted that the boy may be suffering from a non‐standard type of enuresis or the parents may be biased to see a connection between drug and enuresis that does not exist. Future researches are necessary to study if there is any possible association Supplemental information was received through personal email correspondence with the author in July 2013 (Ghanizadeh 2013 [pers comm]) |
|
Ghanizadeh 2008c.
| Methods | A patient report of bilateral photophobia during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (K‐SADS‐PL) (subtype: combined) Age: 7 years old IQ > 80 Sex: male Ethnicity: Persian Country: Iran Comorbidity: no (no history of general medical condition such as albinos or migraine headache, neither in the child nor in the parents. No positive history of head trauma, and the patient never wore contact lenses. No pathological findings on physical examination by an ophthalmologist) Comedication: no Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not known Methylphenidate dosage: 35 mg/day Administration schedule: not stated Duration of intervention: 1 year with discontinuation ≥ 3 times of 3 months each Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Photophobia, occurring a few days after initiation of methylphenidate treatment Discontinuation of methylphenidate ≥ 3 times with no symptoms of photophobia Re‐administration of methylphenidate: immediate reoccurrence of photophobia |
|
| Notes | Ethics approval: not stated Funding/vested interests: not stated Key conclusions of study authors: to the authors' knowledge, no report of methylphenidate‐related photophobia has been found. This is the first report of methylphenidate‐associated photophobia. Although the underlying pathophysiology cannot be defined in a patient report, this possible side effect should be considered in patients on methylphenidate Supplemental information was received through personal email correspondence with the author in July 2013 (Ghanizadeh 2013 [pers comm]) |
|
Ghanizadeh 2009.
| Methods | A patient report of excessive talking during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 5 years old IQ: > 70 Sex: male Ethnicity: not stated Country: Iran Comorbidity: no Comedication: no Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10 mg/day Administration schedule: not stated Duration of treatment: about 7 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Mother and nursery teacher complained about increased hypertalkativity starting about 45 minutes after taking medication. The increased hyper‐talkativity continued for about 3‐4 hours. They scored hypertalkativity as 7‐9 on a 1‐10 visual analogue scale (10 is maximum) Re‐challenge was conducted more than 20 times and hyper‐talking reoccurred every time he took the medication |
|
| Notes | Ethics approval: not stated Funding/vested interests: not stated Key conclusions of study authors: this is a report of excessive talking after taking methylphenidate in a child with ADHD. Further research is necessary to study if there is any possible association |
|
Ghanizadeh 2012.
| Methods | Double‐blind, placebo controlled parallel trial
Investigating the efficacy, tolerability, and adverse effects of nortriptyline for treating enuresis in children with ADHD. No control/no‐intervention group |
|
| Participants | Number of patients screened: 45 Number included in trial: 43 Number included in placebo group: 16 First follow‐up in placebo group: 13 Number of withdrawals in placebo group: 3 Second follow‐up in placebo group: 13 Lost to final evaluation in placebo group: 1 Final evaluation in placebo group: 12 Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean: 8.9, range: 5‐14 years old IQ: above 70 Sex: 34 males, 9 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Iran Comorbidity: primary enuresis (100%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 20 mg/day (< 30 kg), 30 mg/day (> 30 kg) Administration schedule: not stated Duration of intervention: 45 days Treatment compliance: not stated |
|
| Outcomes | Spontaneously reported adverse events Systematically reported using a checklist, at baseline and 2 weeks and 4 weeks after onset of interventions, and 2 weeks after stopping the interventions Bedwetting, parent rated, daily |
|
| Notes | Sample calculation: not stated Ethics approval: yes, Shiraz University of Medical Sciences Funding: the author received a grant Vested interest/authors' affiliations: not stated Key conclusions from study authors: nortriptyline decreases the frequency of enuresis in the children with ADHD and its effect disappears after the discontinuation of treatment Comments from the study authors: the sample size was relatively small and may result in type II errors. In addition, all children were diagnosed with ADHD and formed a clinical sample. Therefore, the results of this study cannot be generalised to other settings, such as a community sample. There was a predominantly higher number of boys than girls, and also it is not clear whether these results can be generalised to other age groups. In the literature there are contradictory reports about the relationship between enuresis and sociodemographic factors. Therefore, it is questionable whether the findings of this study can be applied to other cultures. This current trial was short‐term, and the children had not received an enuresis alarm or desmopressin. Therefore, they were not therapy‐resistant children. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information received through personal email correspondence with the authors in June 2014 (Ghanizadeh 2014 [pers comm]) |
|
Ghanizadeh 2013.
| Methods | An 8‐week parallel trial with 2 arms:
|
|
| Participants | Number of patients screened: not stated Number included: 49 Number randomised to methylphenidate + placebo: 26 Number randomised to methylphenidate + folic acid: 23 Number followed up in methylphenidate + placebo: 13 Number of withdrawals in methylphenidate + placebo: 13 Methylphenidate + placebo group Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 9.9, range 5‐16 years old IQ: no estimated mental retardation Sex: 23 males, 3 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Iran Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Mean MPH dosage: 10 mg/day (< 25 kg) and 20 mg/day (> 24 kg) Administration schedule: twice daily Duration of intervention: 2 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Self‐reported measures upon dropout from study |
|
| Notes | Sample calculation: yes Ethics approval: approved by the Shiraz University of Medical Sciences Ethics Committee Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: considering the marked limitations of this trial, this report suggests that methylphenidate may improve ADHD symptoms and the quality of life of children with ADHD. Current evidence does not support that folic acid as an adjuvant is effective for treating ADHD symptoms or aggression, or improving quality of life of children with ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Goetz 2011.
| Methods | A patient report of nocturnal visual hallucinations during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 7 years old IQ: no mental retardation Sex: female Ethnicity: not stated Country: Czech Republic Comorbidity: oppositional defiant disorder Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: 18 mg/day Administration schedule: once daily Duration of treatment: 2.5 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Complex nocturnal visual hallucinations that persisted for 3 hours. No recurrence of hallucinations occurred after methylphenidate was withdrawn Non‐serious adverse events: Mild abdominal pain and decreased appetite |
|
| Notes | Ethics approval: not stated Funding/vested interest: supported by Charles University Grant GAUK 383/2010 Authors' affiliations: Dr Goetz received research support, travel support and was speaker for Janssen and Lilly (stated in a 2012 paper) Key conclusions of study authors: careful sleep history should be taken before treatment, and reevaluated in the course of therapy, especially when the dose is increased, or switched to long acting formulas |
|
Goez 2012.
| Methods | A randomised, double‐blind, cross‐over study design where participants were randomly assigned to receive a single dose of either
Follow‐up 90 minutes |
|
| Participants | Number of participants screened: not stated Number of participants included: 28 Number of participants followed up: 28 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (subtype; combined (100%)) Age: mean 10.1 years, range 6‐15 years old IQ: > 70 Sex: 26 males, 2 females Country: Canada and Israel Methylphenidate‐naïve: not stated Ethnicity: not stated Comorbidity: no major psychiatric conditions Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10 mg Administration schedule: single dose Duration of treatment: single dose Wash‐out: 2 weeks for those who received modafinil first Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Patients and their families were subsequently contacted and requested to report any adverse effects that might have occurred 7 participants (25%) reported adverse events, including abdominal pain, diarrhoea, hyposomnia, and headaches. All adverse events were minimal and resolved spontaneously All appear to have been rated up to 90 minutes following administration of methylphenidate |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding: the authors received no financial support for the research, authorship, and/or publication of this article Vested interests/authors' affiliations: the authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Key conclusions of the study authors: Modifinil may serve as an effective alternative treatment for ADHD in paediatric patients who do not respond well to methylphenidate or other stimulants Comments from the study authors: adverse events for both agents were mild and self‐limited. The study do not provide any information on long‐term drug effects and long‐term adverse effects. Furthermore, small number of participants and even smaller number of participants for whom numerical scores could be obtained. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: participants who have had an hypersensitivity reaction to either drug were excluded Supplemental information regarding the protocol and side effects requested from the study authors in October 2013 with no reply |
|
Golubchik 2011.
| Methods | A cohort study assessing methylphenidate use for 12 weeks on symptoms of ADHD and comorbid trichotillomania | |
| Participants | Number of patients screened: not stated Number included: 9 Number followed up: 9 Number of withdrawals: none mentioned Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean 11.6, range 6‐18 years old IQ: no mental retardation Sex: 3 males, 6 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Israel Comorbidity: trichotillomania (100%), tic disorder (n = 1) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 0.5‐0.7 mg/kg Administration schedule: not stated Duration of intervention: 12 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Self‐reported by patient. Not clear if it was throughout 12‐week period or at specific time points Loss of appetite, headache, excessive preoccupation with one's hair, abdominal pain, motor tic exacerbation |
|
| Notes | Sample calculation: no Any withdrawals due to adverse events: no Ethics approval: yes, approved by Mental Health Center institutional review board Funding/vested interest/authors' affiliations: not stated Key conclusions of the study authors: some efficacy of methylphenidate treatment was shown in trichotillomania patients with low rate of stressful life events. A large scale study is mandatory to evaluate the efficacy of methylphenidate for trichotillomania in ADHD/trichotillomania patients Comments from the study authors: the main limitations of this study are the open‐label design, the small sample size (N = 9), and the relatively short treatment duration (12 weeks) Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, exclusion of those with history of moderate or severe adverse events to methylphenidate |
|
Gracious 1999.
| Methods | A patient report of atrioventricular nodal re‐entrant tachycardia during stimulant treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 13 years old IQ: > 70 Sex: female Ethnicity: African American Country: USA Comorbidity: obsessive compulsive disorder and dysthymia Comedication: 50 mg/day sertraline Sociodemographics: not stated |
|
| Interventions |
First prescription Methylphenidate type: not stated Methylphenidate dosage: 30 mg Administration schedule: twice daily: morning and noon Duration of treatment: 3 months Treatment compliance: noncompliant with the afternoon dose Second prescription Methylphenidate type: sustained release methylphenidate Methylphenidate dosage: 20 mg Administration schedule: once daily: morning Duration of treatment: 11 months Treatment compliance: intermittently compliant |
|
| Outcomes |
First prescription No serious or non‐serious adverse events reported Second prescription Serious adverse events: After 5 months of treatment; 2 15‐minute episodes of chest pain, associated with tingling in her fingers and shortness of breath. Heart rate: 96. Blood pressure: 155/79. 1 month later chest pain, shortness of breath, sweating, and palpitations beginning during urination. Heart rate: 100 No cardiac complaints in the following 5 months |
|
| Notes | Funding/vested interests: none Authors' affiliations: Division of Child Psychiatry, Case Western Reserve University, University Hospitals of Cleveland, Cleveland, Ohio Key conclusions of the study authors: stimulant medication may evoke onset of atrioventricular nodal tachyarrhythmias in patients who have the potential to develop them, possibly in combination with a selective serotonergic reuptake inhibitor Comments from the study authors: the cardiologist consulted believed this patient had a structural vulnerability of genetic etiology for the arrhythmia which was then precipitated by the stimulant Supplemental information regarding ADHD diagnostic criteria and IQ received through personal email correspondence with the authors in October 2013 (Gracious 2013 [pers comm]) |
|
Grcevich 2001.
| Methods | This retrospective review of medical charts compares the efficacy, safety, dosing frequency, and medication switch rates of Adderall with methylphenidate in children and adolescents with ADHD treated in a private, outpatient psychiatric clinic over 1992 to 1998. Of the evaluable patients, 54 received Adderall, and 75 received methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: not stated Number of participants followed up: 75 Diagnosis of ADHD: DSM‐IIIR or DSM‐IV (without hyperactivity: 12, with hyperactivity: 62) Age: mean 10.2 years old IQ: not stated Sex: 58 males, 16 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: disruptive behaviour: 9, depressive disorder: 7, anxiety disorder: 1, communication disorder: 4, impulse control disorder: 1, Asperger's: 1, other disorders: 7 Comedication: no additional ADHD medications Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: 27 mg/day Administration schedule: not stated Duration of treatment: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Different types of non‐serious adverse events |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: funded by Shire Redwood Inc. Key conclusions of the study authors: Adderall and methylphenidate provided comparable efficacy and safety in children and adolescents with ADHD Comments from the study authors: the population examined may be poorer responders to ADHD treatment than the general population. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information regarding IQ received October 2013 (Grcevich 2013 [pers comm]) |
|
Green 2011.
| Methods | A 6‐month follow‐up study of participants continuing methylphenidate treatment following a 1‐day, randomised parallel trial with 2 arms:
|
|
| Participants | Number of participants screened: 34 Number of participants included: 16 Number of participants followed up: 15 Number of withdrawals: 1 Patients participating in the 1‐day RCT Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (33.3%), inattentive (50%), not otherwise specified (17.7%)) Age: mean 11.1 (SD 3.7) years old, range: 5‐20 years old IQ: mean 81.4 Sex: 20 males, 14 females Methylphenidate‐naïve: 61.8% Ethnicity: not stated Country: Israel Comorbidity: 8 (53.3%) had a psychiatric co‐morbidity, including specific phobia (n = 4), ODD (n = 4), generalised anxiety disorder (n = 3), and eating disorder not otherwise specified (n = 1). Comedication: none of the participants was on any other psychotropic medication during the study period. Sociodemographics: not stated Inclusion criteria: not stated |
|
| Interventions | RCT: participants were randomly assigned to MPH or placebo. Mean MPH dosage: 15.7 (SD 5.6) mg (0.5 mg/kg). Administration schedule: once. Duration of intervention: 1 day. Titration period: no mention. Washout prior to study initiation: 3 days. Treatment compliance: not stated Follow‐up: not stated Methylphenidate type: not stated Mean methylphenidate dosage: not stated Administration schedule: not stated Duration of treatment: 6 months Treatment compliance: 1 withdrew due to poor compliance. |
|
| Outcomes |
Non‐serious adverse events: Barkley Side Effects Rating Scale (modified Hebrew version) parent rated at 24 hours after methylphenidate administration and at 6‐month follow‐up The most common side effects after 6 months of treatment included poor appetite (93.7%), headache (66.6%), and stomachache (56.2%) None of the patients exhibited psychotic symptoms or manic, hypomanic exacerbation during the 6‐month study period |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: no Ethics approval: the study protocol was approved by the Institutional Review Board of Rabin Medical Center Funding/vested interests: no institutional or corporate/commercial relationships for the past 36 months that might pose a conflict of interest Key conclusions of the study authors: the use of methylphenidate in children with velocardiofacial syndrome (VCFS) appears to be effective and relatively safe. A comprehensive cardiovascular evaluation for children with VCFS before and during stimulant treatment is recommended Comments from the study authors: "we found that all participants (100%) with VCFS treated with methylphenidate exhibited ≥ 1 side effect". "The rate of all side effects immediately observed following initiation of treatment remained similarly high after 6 months of treatment". "Thus, according to our findings, it seems that in children with VCFS, tolerance did not develop to methylphenidate side effects". However, none of the children withdrew due to side effects Exclusion of MPH non‐responders/or children who have previously experienced adverse events on MPH: no Supplemental information regarding ADHD diagnostic criteria and safety data (from the study sample excluding participants over 18 years of age or with an IQ below 70, or both), were received through personal email correspondence with the authors in November 2013 (Green 2013 [pers comm]) |
|
Greenberg 1987.
| Methods | A 6 week non‐randomised controlled before‐after study | |
| Participants | Number of participants screened: not stated Number of participants included: 50 Number of participants followed up: 49 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: mean 9.6, range 6‐15 years old IQ: > 70 Sex: 32 males, 18 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: the children were predominantly from intact, middle class suburban families Inclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: regarding 41 responders: 0.33 mg/kg/dose to 0.5 mg/kg/dose (mean 0.4 mg/kg) Administration schedule: twice daily Duration of intervention: 6 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Of the 50 participants, 8 experienced minor, transient side effects (appetite or sleep problems) and medication was terminated for 1 participant who experienced headaches associated with increased blood pressure and tachycardia |
|
| Notes | Sample calculation: no Ethics approval: not stated Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: not relevant Supplemental information has not been possible to retrieve due to lack of contact information |
|
Greenhill 1983.
| Methods | A 2‐night polysomnographic study of children with ADHD before and after 6 months of methylphenidate treatment | |
| Participants | Number of participants screened: not stated Number of participants included: 9 Number of participants followed up: 7 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: mean 8.6 years (range 6.7‐10.7) IQ: > 70 Sex: 9 males Methylphenidate‐naïve: none Ethnicity: white: 3, Black: 4, Hispanic: 0 Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: all children were living at home with one or more parents Inclusion criteria
Exclusion criteria
|
|
| Interventions | Mean methylphenidate dosage: 1.37 mg/kg/day by the end of 6 months Administration schedule: 3 times daily Duration of intervention: 6 months 2 weeks washout period off methylphenidate before entering study. Ongoing titration until satisfied dose was obtained |
|
| Outcomes |
Non‐serious adverse events: Side effect questionnaire ‐ rated monthly by a physician Polysomnographic tests: total sleep time, sleep period time, sleep latency, sleep REM time, awake time, mean REM period length, mean REM period cycle, sleep effects All sleep parameters were recorded during a 48‐hour stay in a sleep unit. The sleep parameters were recorded pre‐drug (run 1) and on drug (run 2) |
|
| Notes | Sample calculation: no Ethics approval: not stated Funding: National Institute of Mental Health Key conclusions of the study authors: across and within (pre‐post) group comparisons showed that methylphenidate therapy was associated with delayed sleep onset, lengthened sleep, and changes in certain REM sleep variables Comment from the study authors: certain methodological problems limit the interpretation of these data, e.g. the ADHD sample was small and completely male. Most of the children did not have to be awakened since they were up before the catheter placement, but if some of the children were awakened, the total sleep for these participants is not correct. Furthermore, methylphenidate‐nonresponders and partially responders are excluded Supplemental information requested twice through personal email correspondence with the authors in September 2013. No reply |
|
Gross‐Tsur 2004.
| Methods | A patient series of 3 children with ADHD who manifested hallucinations during methylphenidate treatment at low therapeutic doses | |
| Participants | Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (33.3%), not stated (66.6%)) Age: 7, 12, 7½ years old IQ: > 70 Sex: male Ethnicity: not stated Country: Israel Comorbidity: ODD (33%), cerebral palsy (33%), mild learning disabilities (33%) Comedication: not stated Sociodemographics: adopted (33%) |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 0.25‐0.3 mg/kg, 7.5‐10 mg Administration schedule: once daily Duration of treatment: 1 for a short period, 2 for several months/1 year Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Complex visual and haptic hallucinations Case 1 Methylphenidate, 1 year: visual and haptic hallucinations starting around 1 hour after drug ingestion Placebo substitution of methylphenidate: immediate cessation of hallucinations > 2 years follow‐up: no psychiatric symptoms reappeared Case 2 Methylphenidate, short period: visual and haptic hallucinations starting 2 hours after drug ingestion, continuing for almost 2 hours Discontinuation of methylphenidate: no recurrence Re‐challenge with methylphenidate: immediate recurrence of hallucinations 3‐year follow‐up: uneventful Case 3 Methylphenidate, several months: visual and haptic hallucinations Discontinuation of methylphenidate: hallucinations ceased 2‐year follow‐up: no recurrence |
|
| Notes | Funding/vested interests: the authors received a 1‐year research grant in excess of USD 10,000 from Novartis in 1997 Authors' affiliations: no other affiliations to pharmaceutical companies stated Key conclusions of the study authors: we describe 3 children with ADHD who were treated with low doses of methylphenidate and developed complex visual and haptic hallucinations Comments from the study authors: the causal role of methylphenidate in the development of hallucinations was based on their appearance after ingestion of the drug, resolving after its withdrawal, and the absence of psychiatric comorbidity that could explain such phenomena. In 1 patient, the hallucinations reappeared after an inadvertent re‐challenge. Because methylphenidate is a widely used, well‐studied, and safe pharmacologic agent, physicians who prescribe methylphenidate should be aware of even rare adverse manifestations occurring at therapeutic doses Supplemental information regarding IQ and ADHD diagnostic criteria received through personal email correspondence with the authors in October 2013 (Shalev 2013 [pers comm]) |
|
Grossman 1985.
| Methods | A patient report on methylphenidate and idiopathic thrombocytopenic purpura (ITP) | |
| Participants | ICD‐9 diagnosis of ADHD (subtype: not known) Age: 7 years old IQ: > 70 Sex: female Ethnicity: white Country: USA Comorbidity: none Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10 mg Administration schedule: twice daily Duration of intervention: 7 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Idiopathic thrombocytopenic purpura: physical examination at the Pediatric Outpatient Department found countless petechiae over the entire dermal surface, most concentrated over her buttocks. Numerous areas of purpura were noted, especially over her buttocks and extremities. Multiple areas of buccal mucosal and gingival bleeding with a large haematoma presented on the left lateral surface of her tongue. Clotted blood was seen in her nostrils and ear canals bilaterally. Bone marrow aspiration showed normal to increased megakaryocytes with normal red and white cell precursors. Methylphenidate was stopped and the patient was admitted. After 1 week of treatment for the condition her petechia had begun to fade. She did not start on methylphenidate again. There has been no recurrence of petechiae or bruising 1 year later |
|
| Notes | Ethics approval: not stated Funding/vested interests: not stated Key conclusions of study authors: it is hoped that the report of this case will stimulate others to report their experience, or lack thereof, regarding the association of methylphenidate and idiopathic thrombocytopenic purpura. Because of the importance of this drug to the management of certain children with attention deficit disorder and its widespread use in thousands of children, it seems important to justify with data the current precaution that "periodic CBC, differential and platelet counts are advised during prolonged therapy" or eliminate the precaution as a recommendation to clinicians Comments from the study authors: the authors describe, that the patient had a mild upper respiratory tract infection 2 weeks prior to the symptoms of ITP. They describe, it is highly possible that in the case just presented the aetiology of the patient's idiopathic thrombocytopenic purpura may well have been her preceding upper respiratory tract infection rather than the methylphenidate, which she had taken without difficulty for 7 months prior to the onset of her haematologic disorder Supplemental information regarding ADHD diagnosis received through personal correspondence with the authors in July 2013 (Grossman 2013 [pers comm]) |
|
Gucuyener 2003.
| Methods | A cohort study assessing methylphenidate use amongst patients with ADHD and concomitant active seizures or EEG abnormalities | |
| Participants | Number of participants screened: not stated Number of participants included: 119 (57 with epilepsy, 62 with abnormal EEG but no seizure activity) Number of participants followed up: 119 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 9.3, range 6‐16 years old IQ: mean 92.3, range 79‐112 Sex: 98 males, 21 females Methylpenidate‐naïve: not stated Ethnicity: not stated Country: Turkey Comorbidity: epilepsy (47.9%) Comedication: antiepilepsy medication for those with epilepsy Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate dosage: 0.3‐1 mg/kg Administration schedule: initially once daily in the morning before school and titrated to twice a day (drug free on holidays) Duration of intervention: 12 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Seizure frequency, observer rated at 3 months intervals EEG findings, observer rated at 3 months intervals No seizures were observed in any of the patients with ADHD and EEG abnormalities In the ADHD with seizures group, 1 patient's seizure type, generalised tonic clonic, changed to a complex partial seizure, but the number of seizures did not increase Only 5 patients had an increased seizure frequency in the ADHD with seizures group and none of the patients in the ADHD with EEG abnormalities group The number of abnormal EEGs with nonepileptic activity was decreased significantly at the end of the study in both groups (P = 0.05) Adverse events: Drug‐related side effects |
|
| Notes | Sample calculation: no Ethics approval: not stated Funding/vested interests: not stated Key conclusions of the study authors: methylphenidate had a beneficial effect on EEG. Seizure frequency did not change from baseline. The side effects of methylphenidate were mild and transient. Methylphenidate is safe and effective in children with ADHD and concomitant active seizures or EEG abnormalities.The present data indicate that methylphenidate is a safe and effective agent in children with ADHD and active seizures of EEG abnormalities. Coadministration of methylphenidate and antiepilepsy drugs improves attention without any adverse effects on seizure threshold or EEG findings. We suggest that physicians give the combined medication to patients with ADHD and active seizures or abnormal EEG findings with close monitoring Comments from the study authors: EEG findings did not deteriorate when patients were on methylphenidate. There was a beneficial effect of methylphenidate of both EEG and seizure frequency. We observed these patients for only 1 year, and 57 epileptic patients were having active seizures. Therefore, the improvement in EEG and seizure frequency might not be completely attributable to maturation Comments from the review authors: all participants were on concomitant antiepilepsy medication Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
Guerreiro 1996.
| Methods | A cohort study of methylphenidate treatment | |
| Participants | Number of participants screened: not stated Number of participants included: 24 Number of participants followed up: 22 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐III diagnosis of ADHD (subtype: combined (around 80%), inattentive (around 20%)) Age: mean 9.1 years (range: 6.5‐13) IQ: normal, learning appropriately in regular schools Sex: 20 males, 4 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Brazil Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Initiation of immediate‐release methylphenidate treatment: 5 mg once daily. Titrated if needed to a total maximum of 10 mg daily Administration schedule: once or twice daily. Treatment pause during weekends and holidays Duration of intervention: mean 12.6 months, range: 1 month ‐ 3 years Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: The occurrence of adverse events were assessed by the family and teachers |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: not stated Authors' affiliations: the study was conducted at the Discipline of Child Neurology, Department of Neurology, Faculty of Medical Sciences (FCM), State University of Campinas (UNICAMP) Key conclusions of the study authors: the satisfactory and partial responses of methylphenidate treatment of ADHD observed in this study (79.1%) are in accordance with the literature, revealing therapeutic success in approximately 75% of the cases. We observed nausea and headache in 1 child, and only headache in another. Nausea can be controlled by lowering the dose; on the contrary, headache can be severe enough to result in cessation of treatment. Growth retardation is one of the possible side effects, which is of greater concern. Fortunately we did not observe this undesirable effect in our patients. Comments from the study authors: we might not have observed growth retardation in our study due to the use of low doses of methylphenidate and the recommendation of drug holidays Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding ADHD subtype, IQ, and type of MPH received through personal email correspondence with the authors in December 2013 (Guerreiro 2013 [pers comm]) |
|
Gökce 2015.
| Methods | Case study | |
| Participants | Number of participants screened: no data Number of participants included: no data Number of participants followed up: no data Number of withdrawals: no data Diagnosis of ADHD: combined type ADHD Age: 12 IQ: no data Sex: male Methylphenidate‐naïve: no data Ethnicity: no data Country: no data Comorbidity: no data Comedication: no data Sociodemographics: no data Inclusion criteria: no data Exclusion criteria: no data |
|
| Interventions | Methylphenidate type: extended release MPH Methylphenidate dosage: 27 mg from 10 years of age. Suicide attempt: 10 tablets of 36 mg ER‐MPH Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Physical examination: tachycardia and mildly increased blood pressure, respiration and heart rate had been determined Psychiatric examination: his mood was depressed, and tendency to sleep Laboratory tests, blood glucose level, blood urea nitrogen, creatinine, potassium (K), sodium (Na) and chloride (CL) were revealed normal The electrocardiography (ECG) parameters such as QRS duration, QT interval, R wave and PR interval were normal; however, the heart rate was elevated There was no central nervous system finding except irritability and agitation |
|
| Notes | No data | |
Haertling 2015.
| Methods | A prospective, multicentre, observational cohort study of long‐acting methylphenidate use for 12 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 262 Number of participants followed up: not stated Number of withdrawals: 23 Diagnosis of ADHD: ICD‐10 (subtype: combined (58%), hyperactive‐impulsive (34%), inattentive (8%)) Age: mean 10.9 (SD 2.5) years old (range: 11‐18) IQ: not stated Sex: 197 males, 63 females, 2 unknown Methylphenidate‐naïve: 19.1% Ethnicity: not stated Country: Germany Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: long‐acting methylphenidate Methylphenidate dosage: start with 20 mg once daily and to adjust in weekly 10 mg increments to a maximum of 60 mg/day Administration schedule: once in the morning Duration of intervention: 12 weeks Treatment compliance: not stated |
|
| Outcomes | Measure method/instrument: AEs according to the Medical Dictionary for Regulatory Activities (MedDRA), version 13
A total of 63 AEs were reported in 36 (13.7%) patients. Severity was mild in 30.2%, moderate in 39.7%, and severe in 22.2% of AEs; 7.9% of data were missing. 28 patients had AEs believed to be treatment related (10.7%). By the end of the study, more than half of the AEs had resolved completely The most frequent AEs were loss of appetite, abdominal pain, and nausea. The most frequently affected System Organ Classes were metabolism and nutritional as well as psychiatric and nervous system disorders The number of AE per patient sums up to 4 adverse reactions in 1 patient throughout the whole treatment course (50.79% of all AEs) the outcome was 'resolved'. In 20 AE‐cases (31.75%) the outcome was 'not yet resolved'. In 1 patient the outcome of the AE was not known Serious adverse events 3 (hospitalisation) (0.4% of patients) Non‐serious adverse events: 60 |
|
| Notes | Sample calculation: not stated Ethics approval: ethics committee approval Funding/vested interest: this study was initiated and sponsored by Novartis Pharma Germany. B Mueller is a full‐time employee of Novartis Pharma GmbH, the market authorisation holder of Ritalin LA. F Haertling received honorariums during the participation of this study. O Bilke‐Hentsch received honorariums as principle investigator of this study Authors' affiliations: Novartis Pharma Key conclusions of the study authors: Ritalin LA improved CGI and quality of life in children with ADHD under routine practice conditions Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated; however, children with poor response to previous treatment plans are included Supplemental information regarding IQ, comedication, comorbidity and additional AE tables requested through personal email correspondence with the authors in June 2016 (Mueller 2016 [pers comm]). The authors were not able to supply the information |
|
Halevy 2009.
| Methods | A patient report of complex visual hallucinations during methylphenidate treatment | |
| Participants | DSM‐IV‐R diagnosis of ADHD, combined type Age: 15 years old IQ: normal intelligence Sex: male Ethnicity: not stated Country: Israel Comorbidity: no (no past experience with drugs, smoking habit, or alcohol consumption. Normal physical and neurological examinations. Normal visual acuity. Electroencephalography (EEG) during a symptomatic state revealed no abnormalities and no evidence of epileptic activity) Comedication: not stated Sociodemographics: not stated |
|
| Interventions |
At 8 years of age: Methylphenidate type: Ritalin Methylphenidate dose: 10 mg daily (0.3 mg/kg) Administration schedule: not stated Treatment compliance: not stated Discontinuation Reintroduction of methylphenidate 7years later Methylphenidate type: not stated Methylphenidate dose: 0.15 mg/kg daily Administration schedule: not stated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Hallucinations Several days after initiation of treatment: visual hallucinations of rats, accompanied by some tactile hallucinations. Only present during the time the patient was under the influence of methylphenidate and disappeared thereafter. Discontinuation: immediate complete resolution Reintroduction of methylphenidate treatment. After 2 days: same complex visual hallucinations. Discontinuation: immediate complete resolution |
|
| Notes | Funding/vested interest: the authors have no conflicts of interest to disclose with regard to this article Key conclusions of the study authors: the occurrence of hallucinations after a very low dose of methylphenidate on 2 occasions may suggest an idiosyncratic reaction. The phenomenon might also be explained by a drug‐induced dysfunction of the monoamine transmitters Comments from the study authors: given the wide use of methylphenidate, clinicians should be aware of this possible side effect Supplemental information regarding IQ and diagnostic criteria received through personal email correspondence with the authors in August 2013 (Halevy 2013 [pers comm]) |
|
Hammerness 2009.
| Methods | An open‐label, prospective, long‐term study of osmotic release oral system methylphenidate for smoking prevention in adolescents with ADHD for 24 months | |
| Participants | Number of participants screened: 203 Number of participants included: 154 Number of participants followed up: 30 Number of withdrawals: 124 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 15.3 years (range 12‐18) IQ: > 75 Sex: 114 males, 40 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: none Comedication: not stated Sociodemographics: not stated Supplementary data from a 6 months period during the main study Number of participants screened: 152 Number of participants included: 114 Number of participants followed up: 57 Number of withdrawals: 57 Age: mean 14.1 years (range 12‐18) Sex: 83 males, 31 females Inclusion criteria
Exclusion criteria
Participants were dropped from the study if in the investigator's opinion there was lack of efficacy, intolerable adverse events, and/or clinically significant laboratory values, pregnancy, clinical worsening, or noncompliance with the study protocol |
|
| Interventions | Methylphenidate type: osmotic release oral system Mean methylphenidate dosage: 63.1 mg at week 6 and 67.2 mg after 6 months Administration schedule: morning Duration of intervention: 24 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Adverse events were systematically recorded at each visit. Adverse events were assessed according to a general query by the treating physician, monitoring emergent and/or ongoing subjective complaints. Adverse effects were followed to resolution There were no serious adverse events or serious cardiovascular adverse events (AEs) in the first 6 months 10 of 114 participants reported ≥ 1 cardiovascular complaint Vital signs were collected as a single, first reading, typically 7 to 10 h after morning administration of medication, during after school office visits Participants with systolic blood pressure (SBP) or diastolic BP (DBP) readings (or both) at or above the 95th percentile for sex, age and height on ≥ 3 consecutive appointments were defined as hypertensive Participants with SBP and/or DBP readings above the 90th percentile for sex, age, and height on ≥ 3 consecutive appointments were defines as prehypertensive During OROS methylphenidate treatment 8% (n = 9/114) of the sample met our defined criteria for prehypertension and 6% (n = 7/114) of the sample met criteria for hypertension It seems the denominator being used for calculation of adverse event proportions below 154 came from the 2‐year study on smoking. Incorporates 50 more participants than those studied in the present work Non‐serious adverse events Different types of adverse outcomes |
|
| Notes | Sample calculation: no Ethics approval: approved by the Massachusetts General Hospital Institutional Review board. Funding: not stated Vested interests/authors' affiliations: the authors have several affiliations to the medical industry both as researchers, speakers, consultants, etc. Key conclusions of the study authors: treatment with relatively high doses of OROS methylphenidate was associated with small but statistically significant mean increases in BP and HR, primarily during the first 6 weeks of treatment, without clinically meaningful changes in ECG. These observations are consistent with previous reports using lower doses Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes |
|
Hammerness 2012.
| Methods | A longitudinal treatment study, receiving daily doses of osmotic release oral system for 6 weeks | |
| Participants | Number of participants screened: 27 Number of participants included: 20 Number of participants followed up: 10 Number of withdrawals: 10 Diagnosis of ADHD: DSM‐IV‐TR Age: mean 14.2, range 12‐17 years old IQ: > 75 Sex: not stated (majority males) Methylphenidate‐naïve: 10% Ethnicity: not stated Country: USA Comorbidity: medically healthy Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system Mean methylphenidate dosage: 54 mg/day (0.90 mg/kg/day). Doses were clinically adjusted up to a maximal dose of 1.5 mg/kg/day, according to tolerability and symptoms, until the participant achieved an ADHD‐specific Clinical Global Impression Scale‐Improvement score of 1‐2 Administration schedule: daily Duration of intervention: 6 weeks Treatment compliance: not stated Wash out period prior to study: prior medication for ADHD participants was discontinued 1‐4 weeks prior to the initial study scan |
|
| Outcomes |
Non‐serious adverse events: Adverse events were systematically collected |
|
| Notes | Sample calculation: no Ethics approval: yes, approved by the Massachusetts General Hospital and McLean Hospital Institutional Review boards Funding: this work was supported in part by the Pediatric Psychopharmacology Council Fund and by McNeil Pharmaceuticals Vested interest/authors' affiliations: the authors have several affiliations to the medical industry both as researchers, speakers, consultants, etc. Key conclusions of the study authors: these preliminary findings suggest the presence of glutamatergic abnormalities in adolescents with ADHD, which may normalise with methylphenidate treatment. Documented clinical improvement and endpoint symptomatology by and ADHD‐specific rating scale Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes |
|
Haubold 2010.
| Methods | A retrospective cohort study of medical treatment of ADHD in a child and adolescent psychiatric practice, Germany, 1997‐2007 | |
| Participants | NB: the following characteristics include patients treated with methylphenidate, atomoxetine and amphetamine Number of participants screened: 152 Number of participants included: 103 Number of participants followed up: 103 Number of withdrawals: 0 Diagnosis of ADHD: ICD‐10 (aubtype: predominantly inattentive (31.1%), predominantly hyperactive (68.9%)) Age: mean: 9.5 years (range: 4‐18) IQ: not stated, however only 4 patients had comorbid mental retardation Sex: 92 males (89.3%), 11 females (10.7%) Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Germany Comorbidity: disruptive behaviour disorder: 68%, learning disorders: 29.1%, motor developmental disorder: 4.9%, autism spectrum disorders: 20.4%, adjustment disorder: 18.3%, emotional disorder: 6.8%, tics: 6.8%, anxiety disorder: 2.9%, Tourette syndrome: 2.9%, OCD: 1%, enuresis (plus partial encopresis): 6.8%, mental retardation: 3.9%, sleeping disorder: 1.9%, epilepsy: 1.9%, attachment disorder with disinhibition: 1.0%, adiposity: 1.0%, stutter: 1.0%, nail biting: 1.0%, harmful alcohol use: 1.0%, articulation disorder: 1%, no comorbidities: 9.7% Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate‐release methylphenidate (Ritalin, Medikinet, Equasym), extended‐release methylphenidate (Ritalin SR and Ritalin LA) and IR‐MPH + ER‐MPH. A change of type of medication took place when adverse events occurred or the effect on the core symptoms of ADHD was not satisfactory Mean of medication changes: 4 (SD 2.9) times (range: 0‐13) Methylphenidate dosage per day: 0.6 mg/kg (IR‐MPH), ER‐MPH (0.8 mg/kg), IR‐MPH + ER‐MPH (0.2 mg/kg + 0.7 mg/kg) Administration schedule: not stated Mean duration of intervention: 3.4 SD 2.10 years (range 2‐9.9) Drug holidays were permitted, and the cumulative dose of drug over the entire treatment duration of the children was calculated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Not stated Non‐serious adverse events: It was assumed that during an office visit, the doctor paid attention to all of the adverse events listed below and, if present, these have been logged. Timing, severity or duration of the occurrence of adverse events have not been described in the medical records with sufficient consistency and could therefore not be included in the evaluation No/any adverse event: trouble falling asleep or sleeping problems, loss of appetite, tic disorder, headache, abdominal pain, anxiety disorder, OCD, nausea BMI: BMI values of children in this study were compared with age and gender reference values (Appendix, Table 2 and Table 3) based on a sample of over 34,000 German children and adolescents of all ages and sexes |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: not stated Ethics approval: not stated Funding/vested interests: not stated Authors' affiliations: Faculty of Medicine, Eberhard Karls University, Tübingen, Germany. Other affiliations not stated Key conclusions of the study authors: the study shows that immediate‐release methylphenidate and the combination of immediate‐ with extended‐release methylphenidate should be first choice for ADHD drug treatment. For extended‐release methylphenidate as single treatment regimen and for atomoxetine and immediate‐release methylphenidate as combination treatment regimens, the benefit/risk ratio should be thoroughly weighed for each individual patient Exclusion of MPH non‐responders/children who have previously experienced adverse events on MPH: no |
|
Hazell 2003.
| Methods | A 6‐week, double‐blind, randomised, parallel study comparing clonidine vs placebo in methylphenidate‐treated children diagnosed with ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 29 Number of participants followed up: 25 Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 125.4 months (range: 6‐14 years old) IQ: > 70 Sex: 25 males, 4 females Methylphenidate‐naïve: none Ethnicity: white Country: Australia Comorbidity: borderline intellectual functioning: 12%, anxiety: 6%, pervasive developmental disorder: 1.5% Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Mean methylphenidate dosage: 0.67 mg/kg/day Administration schedule: not stated Duration of intervention: 6 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Parent and self‐report side effects checklists (Barkley) Pulse rate, and lying and standing blood pressure. Obtained at baseline and at weekly intervals for 6 weeks Height and weight assessed at baseline and at week 6 |
|
| Notes | Sample calculation: yes Any withdrawals due to adverse events: no Ethics approval: approved by the Hunter Area Health Research Ethics Committee and the University of Newcastle Human Research Ethics Committee Funding/vested interests: supported by the Australian Rotary Health Research Fund Authors' affiliations: none declared Key conclusions of the study authors: the findings support the continued use of clonidine in combination with psychostimulant medication to reduce conduct symptoms associated with attention‐deficit/hyperactivity disorder. Treatment is well tolerated and unwanted effects are transient Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no. But patients had to have been treated with methylphenidate for a minimum of 3 months |
|
Hechtman 2011.
| Methods | A patient report about a boy, who gets very emotional, with frequent crying, marked irritability and many tantrums when treated with methylphenidate. MPH use for around a year | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 5 years old IQ: > 70 Sex: male Ethnicity: not stated Country: Canada Comorbidity: oppositional defiant disorder and substantial learning disability in expressive and receptive language Comedication: not stated Sociodemographics: not stated |
|
| Interventions | OROS MPH dosage: 54 mg (the dosage was titrated from 36 mg to 54 mg) Administration schedule: once daily Duration of intervention: 1‐2 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: In late afternoon and early evening he became very emotional, with frequent crying, marked irritability and many tantrums. The emotional side effects subsided after he discontinued methylphenidate treatment |
|
| Notes | Funding/vested interests: Dr Hechtman declares having sat on the advisory boards/been a consultant for Eli Lilly, GlaxoSmithKline, Ortho Janssen, Purdue Pharma, and Shire Canada Comments from the study authors: children with ADHD often have other comorbid conditions that need to be addressed and treated, as stimulant medication is not likely to correct everything It is still unclear what predicts preferential response to 1 or the other stimulant. This preferential response should be kept in mind, so when children don't respond well to methylphenidate, the first change in medication should be to amphetamines Supplemental information regarding diagnostic criteria, type of ADHD and intervention period received through personal correspondence with the author in August 2013 (Hechtman 2013 [pers comm]) |
|
Hemmer 2001.
| Methods | Comparative cohort study on seizure development during MPH treatment | |
| Participants | Number of participants screened: not stated Number of participants included: 234 (stimulant: 205, control: 29) Number followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III, DSM‐III‐R or DSM‐IV (subtype: not stated) Age: range: 3‐20 years old IQ: not stated Sex: 179 males, 55 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate dosage: 0.3‐1 mg/kg/day Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Patient 1: first seizure occurred 6 weeks after initiation of MPH treatment. She experienced 2 episodes several hours apart characterised by unresponsiveness, chewing movements, and tonic eye deviation. Stimulants were not reintroduced. She had an initial normal EEG before MPH treatment Patient 2: she was treated uneventfully with MPH for 12 months, and experienced an unprovoked 4‐minute generalised tonic‐clonic seizure 2 months after discontinuation of methylphenidate. Her EEG prior to stimulant treatment revealed generalised epileptiform discharge Patient 3: experienced a 2‐minute generalised tonic‐clonic seizure with focal onset (tonic rightward head deviation) 3 years after initiation of MPH. Carbamazepine was initiated, and later replaced by phenytoin. MPH was reinitiated and there were no further seizures for the subsequent 14 months. EEG prior to stimulant treatment revealed focal epileptiform discharge Patient 4: episode 10 months after MPH initiation. He was heard to fall and was found unresponsive with upward eye deviation for approximately 2 minutes. EEG prior to stimulant treatment revealed focal epileptiform discharge |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interest: supported by the Crown Family Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: our data suggest that a normal EEG can be used to assign children to a category of 'minimal risk' for seizure. In contrast, an epileptiform EEG in neurologically normal children with ADHD predicts considerable risk for the eventual occurrence of seizure. The risk, however, is not necessarily attributable to stimulant use |
|
Hollis 2007.
| Methods | A patient report of acute and transient dyskinesia occurring within hours of taking modified release methylphenidate | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined/hyperactive‐impulsive/inattentive) Age: 7 years old IQ: Wechsler Abbreviated Scale of Intelligence language skills in the average/high‐average range, and nonverbal skills in the high‐average/superior range Sex: male Ethnicity: white British Country: UK Comorbidity: conduct disorder (with predominant aggression) Comedication: on both occasions, treatment with methylphenidate was temporally associated with a recent withdrawal of risperidone. Sociodemographics: parents separated before he was born. He has an older brother and 3 older half‐sisters, none of whom have had behaviour problems. His mother has fibromyalgia. There is no history of mental or movement disorders in the family |
|
| Interventions | Methylphenidate type: modified release methylphenidate (Concerta XL) Trial 1: 36 mg, 12 hours after abruptly stopping daily risperidone (1.5 mg) Trial 2: 18 mg, after a reducing course of risperidone over 4 weeks, with 6 risperidone‐free days before treatment |
|
| Outcomes |
Non‐serious adverse events: Trial 1: dyskinesia, marked overactivity, distress, headache, fatigue, and vomiting within 8 hours of treatment Trial 2: dyskinesia, increased aggression, difficulty sleeping within 8 hours of treatment |
|
| Notes |
Key conclusions of the study authors: This report is the first of a dyskinesia occurring after a single first dose of methylphenidate in a previously stimulant‐naïve patient after neuroleptic withdrawal. It is also the first to link this effect with modified‐release methylphenidate. The dyskinesia described here consisted of both brief tic‐like movements and more sustained dystonic movements and posturing Supplemental information regarding diagnostic criteria received through personal email correspondence with the authors in October 2013 (Hollis 2013 [pers comm]) |
|
Holtkamp 2002.
| Methods | A patient report of growth impairment during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 7.6 years old IQ: normal Sex: male Ethnicity: not stated Country: Germany Comorbidity: bronchial asthma Comedication: inhaled corticosteroids Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 0.75‐0.8 mg/kg/day Administration schedule: morning and noon Duration of treatment: 19 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Appetite loss (only in the first weeks of medicine) Growth impairment |
|
| Notes | Funding/vested interests: not stated Key conclusions of the study authors: one may conclude that some children are at risk of serious growth decrement when treated with methylphenidate. The growth of children should thus be monitored carefully, even if there are no alarming gastrointestinal side effects from methylphenidate. We found that the determination of growth velocity was a sensitive marker for the evaluation of growth impairment in our patient |
|
Hong 2012.
| Methods | An 8‐week open‐label trial to investigate the independent and interaction effects of DAT1, DRD4,ADRA2A and NET1 on treatment response to methylphenidate in ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 112 Number of participants followed up: 103 Number of withdrawals: 9 Diagnosis of ADHD: DSM‐IV (subtype: combined (69, 61.6%), hyperactive‐impulsive (6, 5.4%), inattentive (29, 25.9%), not otherwise specified (8, 7.1%)) Age: mean 9.1 (SD 2.1) years IQ: 107.4 SD 13.7 Sex: not stated Methylphenidate‐naïve: 100% Ethnicity: Asian Country: South Korea Comorbidity: ODD (15, 13.4%), anxiety disorder (12, 10.7%), enuresis (5, 4.5%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release and extended release Mean methylphenidate dosage: 19.8 SD 8.2 mg/day (initial dose) and 29.1 SD 11.6 mg/day (final dose) Administration schedule: not stated Duration of intervention: 8 weeks Treatment compliance: 1 participant's parents were anxious about their child taking psychiatric medication, this participant discontinued prematurely |
|
| Outcomes | ADHD‐RS (ADHD Rating Scale‐IV) which consists of 18 items, each item is rated from 0 (never or rarely) to 3 (very often). Rated by parents before and after 8 weeks of treatment
The study design did not include collection of side‐effect profiles, so possible reasons for dropping out were not systematically assessed Non‐serious adverse events: 2 of the 9 dropouts experienced loss of appetite after methylphenidate medication, and 1 also complained about insufficient effect. Another participant was documented to experience insomnia, and described to be hyperactive at the last visit before dropping out. The remaining dropouts are not accounted for |
|
| Notes | Sample calculation: none
Ethics approval: institutional review board for human subjects at the Seoul National University Hospital Funding: Supported by the Jun Sang‐Bae Child and Adolescent Psychiatry Research Fund of the Korean Neuropsychiatric Association in 2009 Vested interests/authors' affiliations: no conflicts of interest or financial ties Key conclusions of the study authors: genetic determinants of methylphenidate response consist of both the dopaminergic and noradrenergic gene polymorphisms, and efforts to predict response to methylphenidate should cover these 2 catecholaminergic systems and their multifaceted aspects of their interactions as well Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: all participants were methylphenidate‐naïve |
|
Hulvershorn 2012.
| Methods | A 4‐week before‐after study | |
| Participants | Number of participants screened: not stated Number of participants included: 25 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: range 10‐14 years old IQ: above 80 Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system Methylphenidate dosage: titrated to a therapeutic dose over 4 weeks Administration schedule: morning Duration of intervention: 4 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Intermittent 'hot' feeling: n = 1 Decreased appetite: n = 6 Upset stomach: n = 4 Felt jittery: n = 1 Intermittent headaches: n = 1 Headache: n = 1 |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding: Klingenstein Third Generation Foundation, Brain Behavior Research Trust (NARSAD), Indiana University Health Values Fund Authors' affiliations: not stated Key conclusions of the study authors: we present preliminary data on corticolimbic functional connectivity (FC) abnormalities seen in highly irritable youth with ADHD. Open‐label methylphenidate was well tolerated and resulted in parent‐rated improvements in ER. Methylphenidate appears to impact FC between the amygdala, visual/insular cortices and pallidum Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information regarding ADHD diagnostic criteria, IQ and adverse effect data received through personal email correspondence with the authors in August 2013 (Hulvershorn 2013 [pers comm]) |
|
Ilgenli 2007.
| Methods | A 4 hours before‐after study of methylphenidate acute effect on QT intervals | |
| Participants | Number of participants screened: not stated Number of participants included: 25 Number of participants followed up: 22 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 9.4, range 7‐15 years old IQ: no intellectual disability Sex: 11 males, 11 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Turkey Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage:10 mg Administration schedule: not stated Duration of intervention: 2 hours Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: ECG were taken 2 hours before and 2 hours after administration of methylphenidate QT dispersion was measured as the difference between maximum and minimum QT intervals QTc was calculated with the use of Bazett's formula |
|
| Notes | Sample calculation: not stated Ethics approval: yes, approved by the institutional ethical committee Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: this study reveals that methylphenidate reduces QT dispersion during the acute period, shortly after its administration. Data support the reliability of this drug in terms of early arrhythmia Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information received through personal email correspondence with the authors in November 2013 (Ilgenli 2013 [pers comm]) |
|
Irmak 2014.
| Methods | A patient report of phobias and visual hallucinations during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 9 years old IQ: WİSC‐R: performance IQ: 78, verbal IQ: 87 Sex: male Ethnicity: not stated Country: Turkey Comorbidity: Moebius syndrome Comedication: none Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: gradually titrated up to 1 mg/kg Administration schedule: not stated Duration of treatment: not stated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Methylphenidate prescribed at initial ADHD diagnosis, then withdrawn following the onset of phobias and visual hallucinations as well as lack of improvement in attention problems |
|
| Notes |
Key conclusions of the study authors: dramatic occurrence of adverse effects in our patients suggests that there is an increased vulnerability to adverse effects of methylphenidate in patients with syndromes when compared to other ADHD patients Supplemental information regarding ADHD diagnosis, IQ and comedication received through personal email correspondence with the authors in June 2016 (Irmak 2016 [pers comm]) |
|
Işeri 2007.
| Methods | 30‐day prospective cohort study of methylphenidate | |
| Participants | Number of participants screened: 428 Number of participants included: 20 Number of participants followed up: 20 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: combined 100%) Age: mean 9.27 (SD 1.59), range: 6‐12 years old IQ: normal Sex: 20 males Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Turkey Comorbidity: ODD 35% Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: short‐acting Methylphenidate dosage: 0.6 mg/kg/day Administration schedule: not stated Duration of intervention: 30 days Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Severity (0: not a problem, 1: mild, 2: moderate, 3: severe) of 13 possible side effects rated by mothers Appetite status (0: not a problem, 1: mild, 2: moderate, 3: severe) rated by mothers before and after medication Height and weight were measured before and after treatment and BMI was calculated. The patients were instructed not to change diet or physical activity during the study |
|
| Notes | Sample calculation: not stated Ethics approval: approved by the institutional ethical committee Funding: Gazi University Scientific Project Fund Key conclusions of the study authors: short‐acting methylphenidate does not affect leptin appetite at 0.6 mg/kg/day dose. Did not show significant difference between pre‐ and postleptin levels Comments from the study authors: limitations of the study: small sample size, short duration of the study, only 1 dose of methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: all participants were methylphenidate‐naïve Supplemental data regarding the vomiting and skin eruptions have not been possible to receive from the authors. We have tried to get in contact with them twice without success |
|
Jafarinia 2012.
| Methods | A 6‐week randomised, double‐blind, parallel group study with 2 arms:
|
|
| Participants | Number of participants screened: 55 Number of participants included: 44 Number of participants randomised to methylphenidate: 22 Number of participants followed up: 19 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean 9.7 (1.9) years (range 6‐17) Sex: 14 males, 6 females Methylphenidate‐naïve: 100% Ethnicity: 100% Persian Comorbidity: none Comedication: not stated IQ: above 70 Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to methylphenidate or bupropion Methylphenidate dosage range: 20‐30 mg/day depending on weight (20 mg/day for 30 kg and below and 30 mg/day for above 30 kg) Methylphenidate mean dosage at week 6: 25.5 mg/day Administration schedule: not stated Duration of intervention: 6 weeks Titration period: 3 weeks initiated after randomisation Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Side effects measured in both groups. 11 side effects were recorded during the course of the study |
|
| Notes | Sample calculation: yes Any withdrawals due to adverse events: not stated Ethics approval: yes Funding/vested interests: public funding Key conclusions of study authors: bupropion has a comparable safety and efficacy profile with methylphenidate in children and adolescents with ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: all participants were methylphenidate‐naïve Supplemental information requested from the authors twice in August 2013 with no answer |
|
Jensen 1999 (MTA).
| Methods | The Multimodal Treatment Study of Children With ADHD (MTA) is a 14‐month multicentre, randomised, parallel clinical trial with 4 arms
Phases: for the 2 groups receiving medication there was initially a 28‐day titration period. This titration phase was carried out as a randomised, double‐blind, placebo‐controlled, cross‐over trial with daily switching of MPH doses (placebo, low, middle, and high). Once the delivery of randomly assigned treatments by MTA staff stopped at 14 months, the MTA became an observational study in which participants and families were free to choose their own treatment but in the context of availability and barriers to care existing in their communities. The following follow‐up assessments took place after the RCT 10 months follow‐up (24 months after randomisation), 3‐year follow‐up, 8‐year follow‐up, 10‐year follow‐up In our review we will compare combined treatment with behavioural treatment (RCT) according to our protocol, and look at the medication treatment group as a cohort (observational) |
|
| Participants |
Titration period (Greenhill 2001) The 28 day RCT (COMB + MGT group) N = 289 (MGT: n = 144, COMB: n = 145), N completed = 256 N not finishing titration = 33. Out of the completers, 198 were assigned to an individually best dose of MPH for the 14‐month trial, the rest on other medication or no medication Main study (Jensen 1999): Number of patients screened: 4541, N included = 579, N randomised to MPH + behavioural treatment (COMB) = 145, MPH: 144, behavioural treatment (BEH): 144, N followed up in each arm: combined treatment (COMB): 142; MPH: 136; behavioural treatment (BEH): 141, number of withdrawals/dropouts in each arm: combined treatment: 3; MPH: 8; behavioural treatment: 3 All the demographic data below is for the COMB, BEH, and MGT group: DSM‐IV diagnosis of ADHD (combined (100%)) Age: mean: 8.4 years (range: 7‐9.9). IQ: mean 100.4 Sex: m: 346, f: 87 MPH‐naïve (177/2) Ethnicity: white: 60.3%, African American: 20.6%, Hispanic: 8.8%, others: 10.4% Country: USA and Canada Comorbidity: anxiety disorder 35.1%, conduct disorder 14.1%, oppositional‐defiant disorder 38.8%, affective disorder 3.5%, tic disorder 10.2%, mania/hypomania 3%) Comedication (not stated) Sociodemographics (130 families on welfare. The population range widely in SES). There were no significant differences in baseline demographics between the 3 groups 10‐month follow‐up (MTA Group 2004) Number of patients followed up in each arm: MGT: 128, COMB: 138, BEH: 139 Age: mean: 8.4 years Sex: m: 322, f: 83 There were no difference from the demographic characteristics of the originally randomised 579 MTA participants and the participants who were assessed in the 10‐month follow‐up. The only statistically significant difference among treatment groups was a trivial difference in age (MHT was 0.3 years older that BEH) 3‐year follow‐up (Jensen 2007) Number of patients followed up in each arm: MPH: 115, combined: 127 Age: mean: 11.7 years (range: 10‐13) Sex: m: 291, f: 78 There were no significant differences in baseline characteristics between participants in the 36‐month assessment and those who were unable to follow 8‐year follow‐up (Molina 2008) Number of patients followed up in each arm: MGT: 101, COMB: 119. 32.5% of the MTA sample were medicated over 50% if days in the past year Age: mean: 16.8 years (range: 13‐16). At the 8‐year assessment, 55 MTA participants had turned 18 years. Only 30% of the sample still fulfilled an ADHD diagnosis. Participants lost to the 8‐year follow‐up, compared with those retained, were more often male, had younger mothers, had less educated parents, had lower parent income, and were more likely to have been on welfare at baseline 10‐year follow‐up, blood pressure (Vitiello 2012) Number of participants followed up in each arm: MGT: 77, COMB: 93. A comparison of patients who were retained through year 10 (n = 346) and those who were not (n = 233) showed a lower proportion of males in the retained group. Furthermore the participants were divided into groups based on the following criteria: never medicated, currently medicated, previously medicated. For the currently medicated group, the number of participants were respectively 184, 184, 108, 50, and 12 for 24 months, 36 months, 72 months, 96 months and 120 months follow‐up. Medication use during the previous 30 days was the criterion for positive medication status) Growth studies: at 24 months assessment (Jensen 2004) For both growth outcomes on the originally assigned, randomised, treatment groups were collected, and furthermore naturalistic subgroups had been made. The naturalistic subgroups consisted of those who had been medicated at all the assessment points up to 24 months (medication use during the previous 30 days was the criterion for positive medication status) and those who had not been medicated at the assessment points. Patients with consistent use of medication (med/med): 255, patients with no use of medication (NoMed/NoMed): 139 COMB: n = 135 mGT, n = 120 Growth studies: up to 36‐month assessment (Swanson 2007) Naturalistic subgroups were established based on the patterns of treatment with stimulant medication. If medication was used within a 30‐day period before the assessment medication status was positive (med) and otherwise negative (no med). If an individual's medication status changed at any assessment point, then they were placed in the inconsistently medicated group. Patients with Med, n = 70. At the baseline at 14‐, 24‐, 36‐month assessment points, respectively, the percentages of medicated children taking methylphenidate were the following: 85.4%, 79.7%, 76.8%, 73.5%. The naturalistic subgroups did not differ on initial size at birth (birth weight), age, parent or teacher ratings of ADHD symptoms, sex, expected adult size (mid‐parent size), welfare status, or maternal smoking Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Titration period (Greenhill 2001) Mean MPH dose during titration period: COMB: 32.1 mg/d MGT: 28.9 mg/d. Medication management started with a 4‐day single‐blind, safety lead‐in period, during which participants were exposed to 3 progressively higher daily MPH doses given 3 times daily. This was followed by a 28‐day, double‐blind, daily, switch titration of methylphenidate hydrochloride, using 5 randomly ordered repeats each of placebo, 5 mg, 10 mg, and 15 mg or 20 mg. Cross‐site teams of experienced clinicians blindly reviewed graphs portraying parent and teacher ratings of responses to each of the 4 doses and by consensus selected each child's best dose Compliance: not stated. 29 of the 32 placebo responders had to go back to taking MPH during the maintenance period Main study (Jensen 1999) Participants were randomly assigned to MGT, COMB, or BEH. Mean MPH dosage during the main study: COMB 31.2 mg/d, MGT: 37.8 mg/d. Administration schedule: 3 times a day; breakfast, lunch and in the afternoon. Duration of intervention: 14 months. Treatment compliance: monthly pill counts, intermittent saliva measurements to monitor taking methylphenidate, and encouragement of families to make up missed visits. "the study achieved a high degree of adherence to protocol." NB: for participants not obtaining an adequate response to MPH during titration, alternate medications were titrated openly in the following order until a satisfactory one was found: dextroamphetamine, pemoline, imipramine, and if necessary, others approved by a cross‐site panel. Thus 256 participants successfully completed titration; of these, 198 of 289 participants were assigned to an individually titrated best dose of MPH. 26 were titrated to dextroamphetamine, 32 given no medication because of a robust placebo response 10 months follow‐up (MTA Group 2004) Duration of intervention: 24 months (10 months follow‐up from the 14 months RCT) Treatment compliance: not stated. From end of treatment to the first follow‐up, the percentage of participants on medication decreased for COMB (87% vs 70%) and MPH (93% vs 72%) but increased for behavioural (23% to 38%) 3‐year follow‐up (Jensen 2007) Duration of intervention: 3 years (36 months follow‐up from the 14 months RCT). Treatment compliance: not stated. At 36 months the percentage of participants on medication were 70% for the combined group, 72% for the MPH group and 45% for the behavioural intervention group 8‐year follow‐up (Molina 2009) Duration of intervention: 8 years. Mean MPH dose: 44.93 mg 10‐year follow‐up (Vitello 2012) Duration of intervention: 10 years. Treatment compliance: not stated. Mean MPH dosage: 54.3 mg |
|
| Outcomes |
Titration period (Greenhill 2001)
Main study (Jensen 1999)
Serious adverse events: Main study (Jensen 1999)
Non‐serious adverse events Main study (Jensen 1999) Participants had up to 8 additional sessions provided when needed to address clinical emergencies or instances of possible study attrition
Titration period (Greenhill 2001)
3‐year follow‐up (Molina 2007)
8‐year follow‐up (Molina 2009)
8‐year follow‐up, substance abuse (Molina 2009) The substance use outcomes were measured at all interviews beginning with the 24‐month assessment
For the analysis of stimulant treatment duration in relation to substance use at the 8‐year follow‐up, the primary outcome was number of substances used in the past 6 months, to ensure that most stimulant treatment received would have preceded substance use. Component variables included the following: 'drunk' once or more or drank alcohol 3 to 4 times or more, ≥ 1 cigarettes/day in the past month (time frame exception specific to tobacco); marijuana ≥ 2 times, and any other illicit drug use or prescription medication misuse. Secondary analyses explored each class of substances separately. Substance Abuse or Dependence (SUD). For the analysis of stimulant treatment exposure over time in relation to substance use at the 8‐year follow‐up, the primary outcome variable was SUD in the past year for any substance (excluding tobacco). Secondary analyses explored alcohol and marijuana/other drug use disorders separately 10‐year follow‐up (Vitello 2012)
Growth (Jensen 2004, Swanson 2007)
|
|
| Notes |
Sample calculation: yes power analysis, 576 participants were required Ethics approval: yes, approved by both local institutional review boards and the National Institutes of Health Office for Protection From Research Risk Exclusion of MPH non‐responders/children who have previously experienced adverse events on MPH: no Any dropouts due to adverse events: 4 patients were removed during the lead‐in (titration period) because of prohibitive side effects. 1 child with buccal movements, another with skin picking; a third with depression, crying, sleep delay, and appetite loss, and a fourth who was anorexic, listless, and emotionally constricted Comments from the study authors: Main Study (Jensen 1999) Recruitment, screening, and selection procedures aimed to collect a carefully diagnosed sample of impaired children with ADHD and a wide range of comorbid conditions and demographics characteristics representative of patients seen in clinical practice. The design did not include a no‐treatment or placebo group. More than 3/4 of participants given behavioural treatment were successfully maintained without medication throughout the study. Consequently, it should not be concluded that behavioural treatment interventions did not work. Combined treatment and medication management treatments were clinically and statistically superior to behavioural treatment and community care in reducing children's ADHD symptoms. For other areas of function (oppositional/aggressive behaviours, internalising symptoms, social skills, parent‐child relations, and academic achievement), few differences among our treatments were noted, and when found, were generally of smaller magnitude. The significantly lower total daily dose of methylphenidate in the combined treatment arm are noteworthy but not unforeseen. The importance of this finding is unclear, and a rigorous test of the question would likely require a different design Titration period (Greenhill 2001) Its short 28‐day duration also limited its generalisability to long‐term treatment. Rates of response to MPH ran between 70% to 80% within the expected range.The MTA titration study showed a steeper dose‐response curve for younger and lighter ADHD children. When ratings were collected under placebo‐controlled, double‐blind conditions, parents reported more adverse events than did teachers. For this reason, clinicians would be wise to collect MPH side effect ratings from parents in the afternoon and evenings 10‐month follow‐up (Group 2004) At follow‐up, MGT participants' dose levels (in methylphenidate equivalents) were significantly higher than COMB participants. There interesting results suggest the possibility that early COMB interventions might allow reducing overall medication requirements during later periods, consistent with findings that other have reported 3‐year follow‐up (Jensen 2007) By 36 months, none of the randomly assigned treatment groups differed significantly on any of the 5 clinical and functional outcomes. However, despite no significant group differences at the 36‐month assessment, substantial improvement was manifested by all of the groups. Because there were no untreated control groups and because all of the treatment groups were improved in terms of relevant symptomatology at 36 months compared to baseline, it is possible that all of the treatments worked, but at different rates of different time periods. It is interesting that both medication and educational services for 24 to 36 months were markers for poorer outcome at 36 months, suggesting that those who are doing poorly get more treatment yet still do not do as well as those for whom treatment is not considered essential Vitiello 2001 Comorbid anxiety, oppositional defiant disorder, or conduct disorder was not associated with statistically significant differences in MPH dose at end of titration or maintenance, number of medication changes, or time to first change. A short‐term response to placebo occurred in 32 children (of 256 who completed the double‐blind titration) but was maintained in only 3 patients in the long term. Conners 2001 It is clear that the treatment effect in the study depends on the choice of endpoint measure. The results highlight the fact that there is no 'one true outcome' for a randomised clinical trial because different measures may be sensitive to different forms of treatment 8‐year follow‐up (Molina 2008) Across time (to the 8‐year follow‐up) 17.2% of the children were medicated at every assessment beginning with 14‐months reports 10‐year follow‐up, (Vitiello 2012) This clinical trial was not specifically designed to evaluate cardiovascular function. The blood pressure and heart measurements were not conducted under double‐blind conditions, and the measurement methods varied across the clinical sites. Abnormal blood pressure values were not systematically confirmed over 3 separate assessments as required for a diagnosis of prehypertension or hypertension. The time of the day when measurements were made was variable Implications and applications for primary care providers (Jensen 2001) Behavioural treatments may help families actively cope with their child's disorder and make the necessary life accommodations to optimise family functioning, even when such treatments are not as effective as medication in reducing children's ADHD symptoms. Findings suggest that high quality treatments may have considerable impact on restoring AHDH children to normal or near‐normal functioning at home and in the classroom. Because essentially none of the ADHD children met the normal criteria that were met by 88% of comparison children drawn from the same classrooms at the study outset, the notion that ADHD is just normal behaviour labelled by uninformed parents or overwhelmed teachers appears not only implausible, but preposterous Pelham 1999 The 2 major treatment modalities ‐ behavioural and pharmacological ‐ were assessed at different time points relative to the intensive phase of treatment. Specifically, the effects of the pharmacological treatments were assessed at post‐treatment while participants were actively medicated; in contrast, the effects of BEH were assessed following fading of therapist involvement. The intensive period of BEH ended in late December or early January, and endpoint measures were typically taken 4‐6 months later ‐ usually several months after the last planned, face‐to‐face therapeutic contact. This design aspect has numerous implications for interpretation of the findings. For example, we cannot state that the medication (MPH for the vast majority) had long‐term effects. Rather, the results simply demonstrate that effects of MPH given steadily for 14 months are the same at the end of the time as the beginning (indeed the correlations between drug effects at these 2 points of the study are very high). When differences in outcome between these groups (e.g. BEH and MGT) are analysed, it is likely that combined treatment for children whose parents and teachers continued the behavioural interventions they had been taught will have an outcome superior to MGT, while combined treatment, for those whose parents and teachers did not continue BEH will be equivalent to MGT alone (which would not be surprising, as functionally that would be what they were receiving) Growth 24‐month outcomes (Jensen 2004) The growth suppression effects could be related to a medication effect, with the continuously treated subgroup having slower growth than the untreated subgroup. Alternatively, the 'continuously treated subgroup', defined by unknown self‐selection factors, could have had a slower growth rate before the start of the study, which continued during the treatment and follow‐up phases on the MTA. Our data cannot make a determination of the validity of these alternative interpretations. At this first follow‐up, our observations were of the children in the MTA when they were between the ages of 9 and 11 years, which is before expected phase of accelerated growth in adolescence and before the expected age when growth slows and final height is approximated. The rate of growth as well as the length of the growth phase together determines ultimate (adult) height, and it is possible that the consistent treatment with medication may reduce the rate but lengthen the duration of growth, so final height would be delayed but not reduced. It is possible that the never‐medicated group was pared down to good responders and the medicated groups enriched with poor behavioural responders. In the analysis of 14‐ to 24‐month change scores, the 'Medication status' was significant for both height (Chi2 = 16.16, P < 0.001) and weight (Chi2 = 13.32, P < 0.004) Growth 36 months assessment (Swanson 2007) We did not document a decrease in relative size in the group of participants with a history of treatment before entry into the MTA protocol during subsequent treatment with stimulant medication over 3 years. However, this group (the consistently medicated naturalistic subgroup) was smaller than the stimulant‐naïve group (the newly medicated naturalistic subgroup at entry, suggesting that early treatment of children (before the ages of 7‐9 years) with stimulant medication may have produced a reduction of growth rate before entry into the MTA protocol Key conclusions of the study authors: Main study (Jensen 1999) For ADHD symptoms, our carefully crafted medication management was superior to behavioural treatment and to routine community care that included medication. Our combined treatment did not yield significantly greater benefits than medications management for core ADHD symptoms but may have provided modest advantages for non‐ADHD symptom and positive functioning outcomes Vitiello 2001 For most children, initial titration found a dose of MPH in the general range of the effective maintenance dose but did not prevent the need for subsequent maintenance adjustments. For optimal pharmacological treatment of ADHD, both careful initial titration and ongoing medication management are needed Titration period (Greenhill 2001) The MTA titration protocol validated the efficacy of weekend MPH dosing and established a total daily dose limit of 35 mg of MPH for children weighing less than 25 kg. It replicated previously reported MPH response rates (77%), distribution of best doses (10‐50 mg/day) across participants, effect sizes on impairment and deportment, as well as dose‐related adverse events. With 3 times daily dosing, the MTA titration trial showed that significant stimulant medication effects on ADHD symptom reduction and drug‐related adverse events could be detected by parents and teachers using daily ratings under controlled conditions 10‐month follow‐up (MTA Group 2004) The benefits of intensive medical intervention for ADHD extend 10 months beyond the intensive treatment phase only in symptom domains and diminish over time. 3‐year follow‐up (Jensen 2007) By 36 months the earlier advantage of having had 14 months of the medication algorithm was no longer apparent, possibly due to age‐related decline in ADHD symptoms, changes in medication management intensity, starting or stopping medications altogether, or other factors not yet evaluated. 24‐ and 36‐month assessment of delinquency and substance abuse (Molina 2007) Cause‐and‐effect relationships between medication treatment and delinquency are unclear; the absence of associations between medication treatment and substance use need to be re‐evaluated at older ages. Findings underscore the need for continuous monitoring of these outcomes as children with attention deficit/hyperactivity disorder enter adolescence. There were no statistically significant effects at the P < 0.05 level of randomly assigned treatment on individuals rate of change in delinquency between baseline and 36 months. Result suggests the possibility that increasing delinquency between 24 and 36 months was associated with an increase in substance use in the same time period. We did not find evidence of protective or adverse effects of medication treatment for ADHD in either study. 8‐year follow‐up (Molina 2009) Type or intensity of 14 months of treatment for ADHD in childhood (at age 7‐9.9 years) does not predict functioning 6 to 8 years later. Rather, early ADHD symptom trajectory regardless of treatment type is prognostic. This finding implies that children with behavioural and sociodemographic advantage, with the best response to any treatment, will have the best long‐term prognosis. As a group, however, despite initial symptom improvement during treatment that is largely maintained after treatment, children with combined‐type ADHD exhibit significant impairment in adolescence. Innovative treatment approaches targeting specific areas of adolescent impairment are needed 10‐year follow‐up blood pressure (Vitiello 2012) Stimulant treatment did not increase the risk for prehypertension or hypertension over the 10‐year period of observation. However, stimulant had a persistent adrenergic effect on heart rate during treatment Growth studies, 24‐month follow‐up (Jensen 2004) In the MTA follow‐up, exploratory naturalistic analyses suggest that consistent use of stimulant medication was associated with maintenance of effectiveness but continued mild growth suppression Growth studies, 3‐year follow‐up (Swanson 2007) Children with combined type attention‐deficit/hyperactivity disorder were, as a group, larger than expected from norms before treatment but showed stimulant‐related decreases in growth rates after initiation of treatment, which appeared symptomatic within 3 years without evidence of growth rebound Pelham 1999
Comorbidity (Jensen 2001) Our findings suggest that ADHD children with and without ODD/CD and ANX differed on many baseline characteristics, outcomes, and response to treatment. Children with ANX tended to be more treatment‐responsive than ADHD + ODD/CD and even ADHD‐only participants Anxiety (March 2000) Contravening earlier studies, no adverse effects of anxiety on medication response for core ADHD or other outcomes in anxious or non‐anxious ADHD children was demonstrated. When treating ADHD, it is important to search for comorbid anxiety and negative affectivity and to adjust treatment strategies accordingly Swanson 2007 Long‐term benefits from consistent treatment were not documented; selection bias was not shown to account for the loss of relative superiority of medication over time; there was no evidence for 'catch‐up' growth; early treatment with medication did not protect against later adverse outcomes. We expect that these challenges to the field's views will contribute to future controversies about the long‐term outcomes in the MTA Substance use (Molina 2012) Our findings did not provide any evidence that ADHD medication protects from, or increases risk for, adolescent substance use or SUD. This finding held for recent medication and for days cumulatively treated with stimulants. Unmeasured confounders may have been operating because of the naturalistic follow‐up study design and we did not statistically control for psychopathology and functioning at the follow‐up assessment. The observed lack of associations between stimulant exposure over time and adolescent substance use/SUD do not discount the possibility that brain‐based changes in neural mechanisms underlying addiction vulnerability are occurring as a function of prolonged stimulant treatment. The substance use/SUD outcomes for the MTA should be considered in the context of several unique study features and limitations. All of the children in the MTA were diagnosed with the combined type of DSM‐IV ADHD, and generalisation of study results should generally not extend beyond this subtype. Our follow‐up assessments, which relied on self‐report and often with 2‐year windows, may have missed episodes of substance use, and rates may be underestimated Pelham 2000 75% of the children in the behavioural treatment group were maintained without medication for 14 months, and 64% did not meet diagnostic criteria for ADHD at 14 months based on the DISC interview (MTA Cooperative Group, 1999a, 1999b). Such findings highlight the fact that intensive behavioural treatments are a viable alternative to medication in treatment of ADHD Comments from the review authors: The authors from the MTA study have written more than 70 articles describing different outcomes and challenges of the study. We have only included those found in our comprehensive literature search or others we found relevant to include looking through the articles reference lists. We have discussed whether to include the MTA study or not since not all of the patients randomised to medication (COMB + MGT group) received MPH. Those who did not have an adequate response to MPH were given other medication (e.g. dextroamphetamine, pemoline, imipramine, or no medication). Furthermore, some of the participants in the BEH group were also medicated during the 14‐month randomisation phase. For all other studies in the review, we have only included those with pure MPH receivers. Furthermore lots of the participants did not have an ADHD diagnosis at the follow‐up assessment. At 8‐year follow‐up only 30% of the remaining participants still had a diagnosis of ADHD. However, we have chosen to use the data from MTA since it is such a large and well‐known study. All of the MTA analyses will be part of the review as sensitivity analyses Regarding Molina 2012 (substance use): we have included/asked for additional data for this article, even though that the group that were medicated in the more group were only medicated for mean 2071.10 (SD 728.87) days out of the 8 years the follow‐up took place. The following articles from the MTA study have only been assessed by one review author: Pelham 2000, Carey 2000, Swanson and Hinshaw 2007, Galanter 2003, Hinshaw 1999, Molina 2013 We sent several emails to the MTA group in order to get additional information and furthermore spoke orally to some of the authors. We did not get additional data |
|
Johnson 2013.
| Methods | A cohort stud of methylphenidate use for 6 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 96 Number of participants followed up: 77 Number of withdrawals: 9 Diagnosis of ADHD: DSM‐IV (subtype: combined (82%), hyperactive‐impulsive (9%), inattentive (9%)) Age: mean 8 (SD 2.6) years (range 4‐15) IQ: mean 92.7 (SD 15.9) Sex: 66 males, 11 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Ireland Comorbidity: oppositional defiant disorder: 60%, conduct disorder: 16% Comedication: paracetamol, salbutamol and steroid inhalers for asthma and ampicillin antibiotics Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: 45 participants took short‐acting methylphenidate. Otherwise not stated Methylphenidate dosage: mean 0.57 mg/kg/day (SD 0.19) Administration schedule: not stated Time points: not stated Duration of intervention: 1 year, however only data up to 6 weeks were reported here Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Barkley's Side Effect Rating Scale at 6 weeks, parent rated ‐ included decreased appetite, weight loss, headache, abdominal pain, irritability, sadness, insomnia, and tics |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: not stated Ethics approval: granted by 8 local research ethics committees Funding/vested interests: this study was funded by the Irish Health Research Board, Dublin Authors stated that there were no competing financial interests Key conclusions of the study authors: the study found an association between 2 CES1 SNP markers and sadness as a side effect of short‐acting methylphenidate Comments from the study authors: limitations: not cross‐over design; serum measures of methylphenidate were not obtained; study did not consider environmental factors impacting on CES1A1 function Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Jung 2007.
| Methods | A open‐label cohort study of osmotic release oral system (OROS) methylphenidate use for 4 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 91 Number of participants followed up: 83 Number of withdrawals: 8 Diagnosis of ADHD: DSM‐IV (subtype: 100% combined) Age: mean 8.46, range: 6‐12 years old IQ: mean 96 (SD 15.17). All above 70 Sex: 75 males, (90.4%), 8 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Korea Comorbidity: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (extended release) Methylphenidate dosage: start dosage was 18 or 36 mg based on the clinician's judgment and dosage was adjusted at each visit if necessary Administration schedule: once per day in the morning before 8:30 Duration of intervention: 4 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: 79.5% showed ≥ 1 harmful effects of medication with most being mild. Not mentioned how these were measured |
|
| Notes | Sample calculation: no Ethics approval: yes Funding/vested interests: Janssen Korea Pharmaceuticals LTD Key conclusions of the study authors: OROS methylphenidate improves performance on common tests of cognitive function. A further long‐term follow‐up study of these effects in ADHD is warranted Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Karabekiroglu 2008.
| Methods | A retrospective cohort of methylphenidate use for 6 months | |
| Participants | Number of participants screened: not stated Number of participants included: 90 Diagnosis of ADHD: DSM‐IV diagnosis (subtype: not stated) Age: mean: 9.0, range: 5‐16 years old) IQ: above 70 Sex: 59 males, 14 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Turkey Comorbidity: conduct disorder (16.7%); tics (23.3%); Tourette syndrome (10.5%); obsessive compulsive disorder (16.3%); pervasive developmental disorder (8.1%); learning disorder (26.7%); depression (9.2%) Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: 10‐30 mg/day Mean methylphenidate dosage: 17.6 (SD 4.95) mg/day Administration schedule: 2‐3 times/day Duration of intervention: 6 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Parents completed the Barkley Stimulant Side Effects Rating Scale (BSSERS) at baseline and on the 3rd, 7th, and 15th days of the medication |
|
| Notes | Sample calculation: not stated
Ethics approval: not stated
Funding/vested interest: "This research had a naturalistic design. Therefore, a limited financial support, which was supplied by the authors, was needed."
Key conclusions of the study authors: authors found significant decrease in appetite, and they suppose that some of the Barkley SES may represent both ADHD symptoms and methylphenidate adverse events Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: all participants were methylphenidate‐naïve Supplemental information regarding the IQ of the participants received through personal email correspondence with the authors in June 2016 (Karabekiroglu 2016 [pers comm]) |
|
Karaman 2010.
| Methods | A patient report of a 15‐year old boy with ADHD who developed pulmonary arterial hypertension (PAH) during OROS methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 15 years old IQ: intellectual capacity within normal limits Sex: male Ethnicity: not stated Country: Turkey Comorbidity: no Comedication: none MPH‐naïve: yes Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: 54 mg/day Administration schedule: once daily, morning Duration of intervention: 18 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: On the 4th day of treatment: the patient began to experience occasional episodes of slight shortness of breath. Continued over several months. Not associated with either exercise or anxiety At the 18th month of treatment: fainting. Normal weight. No sign of allergy, hypersensitivity, or sleep apnea. Mean pulmonary arterial pressure of 40 mmHg at rest, otherwise no pathological findings from extensive testing (Examination: clear lungs, no murmurs, rubs, or gallops, and otherwise unremarkable. Laboratory tests, including C‐reactive protein, thyroid and liver function tests, electrolytes. Blood gases, antinuclear antibodies, D‐dimers, chest X‐ray, ventilation‐perfusion scintigraphy, electrocardiography, respiratory function tests. Echography. Transthoracic echocardiogram: normal except for the mean pulmonary arterial pressure) No use of any other drug. No history of alcohol and substance use and no symptoms or signs of methylphenidate misuse (intravenous (IV) injection). The personal and family histories were also negative for pulmonary or cardiovascular diseases Discontinuation of OROS‐methylphenidate, 1 month: free of symptoms. Mean pulmonary arterial pressure of 28 mmHg |
|
| Notes | Funding/vested interests/affiliations: no conflict of interests or financial ties to disclose. Key conclusions of study authors: this case shows that pulmonary arterial hypertension should be considered in patients who present with dyspnoea and a reduced exertion tolerance and who are known to use methylphenidate Comments from the study authors: using the Naranjo algorithm, the likelihood that OROS methylphenidate was responsible for precipitating pulmonary arterial hypertension in our patient was judged probable |
|
Karapinar 2014.
| Methods | A patient report of sudden hearing loss associated with methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 8 years old IQ: > 70 Sex: female Ethnicity: not stated Country: Turkey Comorbidity: none Comedication: none Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: Ritalin Methylphenidate dosage: 10 mg/day Administration schedule: 5 mg twice daily Duration of treatment: 4‐5 days Treatment compliance: yes |
|
| Outcomes |
Serious adverse events: About 24 hours after taking methylphenidate the patient began feeling loss of balance, nausea, vomiting. She was kept on the medication. After third dose, her symptoms became more severe. Clinical findings suggested sensorineural hearing loss associated with drug ototoxicity in the left ear. Methylphenidate treatment was discontinued, and the hearing loss treated. At the end of the 2‐month follow‐up period, her hearing in the affected ear showed no significant improvement. |
|
| Notes | Key conclusions of the study authors: sensorineural hearing loss that occurred after the introduction of methylphenidate is a serious complication of the treatment, without resolution after discontinuation of the drug and administration of several treatments. The possibility of the development of irreversible sudden hearing loss should be borne in mind when patients are undergoing medical treatment of ADHD with methylphenidate | |
Kazanci 2015.
| Methods | 2 patient reports of dyskinesia following a single dose of methylphenidate | |
| Participants |
Case 1 Diagnosis of ADHD: DSM 5 Age: 8 years old IQ: normal Sex: male Ethnicity: not stated Country: Turkey Comorbidity: none Comedication: none Sociodemographics: uneventful pregnancy and delivery history. Born from healthy, non‐consanguineous parents without any history of any psychiatric diseases, movement or muscle disorders Case 2 Diagnosis of ADHD: DSM 5 Age: 6 years old IQ: normal Sex: male Ethnicity: not stated Country: Turkey Comorbidity: none Comedication: none Sociodemographics: uneventful pregnancy and delivery history. Born from healthy, non‐consanguineous parents without any history of any psychiatric diseases, movement or muscle disorders |
|
| Interventions |
Case 1 Methylphenidate type and dosage: methylphenidate IR 10 mg/day Methylphenidate dosage: 5 mg twice daily Duration of treatment: 3 hours Treatment compliance: yes Case 2 Methylphenidate type and dosage: OROS methylphenidate 18 mg/day Administration schedule: not stated Duration of treatment: 2 hours Treatment compliance: yes |
|
| Outcomes |
Case 1 Non‐serious adverse events: 3 hours after first dose of methylphenidate IR 5 mg: repetitive facial contortions and abnormal neck movement. The abnormal movements decreased during the following hours. Continuation of 5 mg methylphenidate immediate release twice daily. After 2 months further increase to 10 mg twice a day and no repetition of dyskinesia Case 2 Non‐serious adverse events: 2 hours after a single dose of OROS‐methylphenidate 18 mg: repetitive facial grimaces, lip smacking and protrusion of tongue. 2 hours later all symptoms have resolved. No treatment required. Continuation of 18 mg OROS‐methylphenidate. After 3 months, further increase to 27 mg OROS‐methylphenidate and no repetition of dyskinesia in the 8‐month follow‐up period |
|
| Notes | Funding/vested interest: the authors report no conflicts of interest related to this article Key conclusions of the study authors: these side effects are assumed to occur due to individual drug sensitivities. Continuation of the methylphenidate treatment, despite dyskinetic side effects, may not cause any recurrent dyskinetic movements |
|
Kemner 2005 (FOCUS).
| Methods | A 3‐week, prospective, open‐label, community‐based, multicentre, randomised, parallel study with 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 1323 Number of participants randomised to OROS‐MPH: 850 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (72%%), hyperactive‐impulsive (13%), inattentive (15%)) Regarding the OROS‐MPH group: Age: mean 8.77, range 6‐12 years old IQ: above 70 Sex: 630 males, 219 females Methylphenidate‐naïve: 57.97% Ethnicity: white: 75.12%, African American: 14.74%, Asian: 0.71%, Hispanic: 6.72%, American Indian: 0.24%, others: 2.48% Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system Washout: more than 3 days or 5 half‐lives Investigators were allowed to select starting doses Mean OROS‐MPH starting dose: 21.57 mg Titration: 2 weeks, after randomisation Mean OROS‐MPH dose at week 3: 32.7 mg (12.1) / 1.01 mg/kg (0.45) Mean OROS‐MPH dose at week 3 for the African American patients: 32.8 mg (10.9) Mean OROS‐MPH dose at week 3 for the non‐African American patients: 32.7 mg (12.2) Administration schedule: once daily, in the morning Duration of intervention: 3 weeks Treatment compliance: 92% or higher |
|
| Outcomes |
Non‐serious adverse events: Blood pressure, heart rate, height and weight were recorded at baseline, week 2 and 3 Spontaneously reported adverse effects by patients, parents or investigators |
|
| Notes | The FOCUS study: the Formal Observation of Concerta versUs Strattera, Phase IV study Sample calculation: no Ethics approval: yes Funding/vested interests: supported by funding from McNeil Consumer & Specialty Pharmaceuticals Key conclusions of the study authors: these results suggest greater ADHD symptom improvement with osmotic release oral system methylphenidate compared with atomoxetine Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, known methylphenidate non‐responders and methylphenidate‐optimal‐responders are excluded Supplemental information requested from the authors and YODA‐team in March and June 2014. No reply |
|
Khajehpiri 2014.
| Methods | A cohort study of methylphenidate use for 6 months | |
| Participants | Number of participants screened: not stated Number of participants included: 71 Number of participants followed up: 71 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 8.23, range: 4‐15 years old IQ: not stated Sex: 46 males (64.8%), 25 females (35.2%) Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Iran Comorbidity: epilepsy 11.2%, neonatal jaundice 25.4%, seizure 4.2%, respiratory disease 2.82%, Down syndrome 2.82%, urinary tract infection 1.41% Comedication: methylphenidate plus risperidone: 23 (32.4%), methylphenidate plus other medications: 8 (11.3%) Sociodemographics: not stated Inclusion criteria All children under methylphenidate treatment alone or with other agents attending a university‐affiliated psychology clinic were screened regarding all subjective and objective adverse drug reactions (ADRs) of methylphenidate No specific inclusion‐exclusion criteria regarding duration of methylphenidate treatment course, methylphenidate dose, and probable co‐administered medications for management of ADHD were used for patient selection |
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage/day: 20.5 (SD 9.6) mg (range 5‐40) Administration schedule: not stated Duration of intervention: < 1 month (2.8%) to ≥ 6 months (63.1%) Treatment compliance: 63.1% received methylphenidate for ≥ 6 months; 2.8% less than a month |
|
| Outcomes | Type of adverse event/reaction/effect: ADRs Measure method/instrument: face‐to‐face interview with patients or his/her parents at regular follow‐up office visits through a checklist of methylphenidate adverse reactions in relevant scientific literature and reviewing their brief office charts Serious adverse events: The WHO definition of a serious adverse reaction was used i.e. any adverse reaction resulting in death, life‐threatening situation, persistent or significant disability/incapacity, hospital admission, or prolonged hospital stay |
|
| Notes | Sample calculation: no Ethics approval: Tehran University Medical Ethics committee Funding/vested interest: not stated Key conclusions of the study authors: our data suggested that although methylphenidate related adverse reactions were common in children with ADHD, they were mainly mild and non‐serious Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Khodadust 2012.
| Methods | A 6‐week randomised, double‐blind, parallel group study with 2 arms:
No control/no‐intervention group |
|
| Participants | Number of participants screened: not stated Number of participants included: 30 Number of participants randomised to Ritalin: 15 and Stimdate: 15 Number followed up in each arm: Ritalin: 14 and Stimdate: 14 Number of withdrawals in each arm: Ritalin: 1 and Stimdate: 1 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined 100%) Age: Ritalin: 9.2 (0.5); Stimdate: 8.33 (0.5) IQ: above 70 Sex: Ritalin: 12 males, 3 females; Stimdate: 15 females Methylphenidate‐naïve: none Ethnicity: 100% Persian Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 methylphenidate preparations: Ritalin or Stimdate Final mean methylphenidate dose: Ritalin (29.2 (SD 9.1) mg), Stimdate (31.4 (SD 8.6) mg) Administration schedule: morning and noon Duration of intervention: 6 weeks Titration period: 4 weeks initiated after randomisation Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Nonserious adverse events checklist and telephone interviews asking about drug side effects were performed |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: none declared Key conclusions of the study authors: we recommend clinicians choose Ritalin or Stimdate according to the patient's preferences, sustained accessibility, primary response to treatment, and possible side effects encountered in course of treatment. This means that neither of these drugs has been proven to be superior to the other one Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes. See exclusion criteria 4 Supplemental information requested twice through email correspondence with the authors in June 2013. No reply |
|
Kim 2010.
| Methods | Single‐site, 6‐week, prospective, open‐label, flexible‐dose trial with osmotic release oral system methylphenidate (Concerta) monotherapy to examine OROS‐MPH effect on sleep in children with ADHD. Examination by physician at baseline, participants were seen at the first, second, fourth, and sixth weeks for repeated clinical evaluation and dosage titration | |
| Participants | Number of participants screened: not stated Number of participants included: 27 Number of participants followed up: 24 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (subtype: combined (66.7%), hyperactive‐impulsive (4.2%), inattentive (29.2%)) Age: mean 8.2 (SD 1.4) years IQ: mean 105 (SD 11.5) Sex: 22 males, 2 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: South Korea Comorbidity: ODD: 2 (9.1%) Comedication: no medications that might influence clinical status or sleep characteristics were permitted Sociodemographics: family history of ADHD: 4, 16.7% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: the mean daily dose at week 6 was 29 (SD 6.8, range 18‐45) mg or 1.08 (SD 0.24) mg/kg Administration schedule: once daily Duration of intervention: 6 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Children's Depression Inventory, rated by parents State Trait Anxiety Inventory (STAI), rated by parents Yale Global Tic Severity Scale, rated by parents Barkley Side Effect Rating Scale, rated at 1st, 2nd, 4th and 6th week by parents Questions to parents about adverse events at each follow‐up visit (1st, 2nd, 4th and 6th week) Children's Sleep Habits Questionnaire (CSHQ), measured at week 6 Height measured at week 1st, 2nd, 4th and 6th week Weight measured at week 1st, 2nd, 4th and 6th week Blood pressure measured at week 1st, 2nd, 4th and 6th week Heart rate measured at week 1st, 2nd, 4th and 6th week EKG measured at week 6 Overnight polysomnography measured at week 6 Apnea and hypopnea, scored according to the recommended criteria of the American Academy of Sleep Medicine at week 6. Apnea was defined as an absence of oronasal airflow with minimal interval of 2 respiratory cycles. Hypopnea was defined as 50% air flow reduction or more for ≥ 2 respiratory cycles resulting in EEG arousals or oxygen desaturation (Z3%) |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interests: sponsored by Janssen Korea Key conclusions of the study authors: these results suggest that OROS MPH in open‐label treatment does not appear to impair sleep on either qualitative measures of sleep or sleep architecture and may improve some aspects (including sleep quality) Comments from the study authors: no significant effects were found on any laboratory tests and EKG. Another important finding indicated that children who experienced subjective sleep difficulties showed increased sleep onset latency, sleep onset delay, and bedtime resistance compared with those without sleep complaints during treatment Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no. All participants were methylphenidate‐naïve Supplemental information regarding outcome measures requested in February 2014. No reply |
|
Kim 2011.
| Methods | A 4‐week multicentre, open‐label before‐after study | |
| Participants | Number of participants screened: not stated Number of participants included: 102 Number of participants followed up: 97 Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (subtype: combined (71.3%), hyperactive‐impulsive (5.9%), inattentive (23.8%)) Age: mean 9.4 years (range 6‐12) IQ: above 75 Sex: 94 males, 8 females Methylphenidate‐naïve: none Ethnicity: not stated Country: Korea Comorbidity: oppositional defiant disorder (17.6%), anxiety disorder (17.6%), depression (9.8%), conduct disorder (2.9%), learning disorder (17.6%), others (5.9%) Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Prior to the study stabilised on immediate release methylphenidate (IR‐MPH) with a mean dose of 25 mg daily Then osmotic release oral system methylphenidate (OROS‐MPH) dosage: 18 mg, 36 mg, or 54 mg The participants were assigned to 1 of 3 OROS‐MPH doses based on their pre‐study dose of IR‐MPH. Participants receiving 5 mg of MPH‐IR 2‐3 times daily were assigned to the 18 mg once daily group; participants receiving 10 mg of MPH‐IR 2‐3 times daily were assigned to the 36 mg once daily group; and participants receiving 15 mg of MPH‐IR 2‐3 times daily or a total daily dose > 45‐60 mg were assigned to the OROS‐MPH 54 mg once daily group. Doses could be adjusted among these 3 levels at study visit 1 and 2 on days 7 and 14 The dose of methylphenidate before entering the study was 10‐60 mg/day (mean 25.26 mg, the mean final doses of OROS‐MPH was 27.67 mg/day Administration schedule: once, morning Duration of intervention: 28 days Treatment compliance: 94.3% completed the 28 day study |
|
| Outcomes |
Non‐serious adverse events: All participants received a screening physical examination at baseline and on day 28. The participants' height and weight were also measured Adverse events were documented at each visit by recording spontaneous reports of adverse events and asking parents/caregivers about the quality of their child's sleep, their children's appetite during the past week, and whether their children had experienced tics during the past week No serious adverse events were reported during the study |
|
| Notes | Sample calculation: no Ethics approval: approved by the Institutional Review Board for human participants at Seoul National University Hospital Funding: supported by Korean Jassen Pharmaceutical Company Vested interest/authors' affiliations: none Key conclusions of the study authors: the results of this study indicated that an IR‐MPH regimen can be successfully changed to a once‐daily OROS‐MPH regimen without any serious adverse effects. The changes in parent/caregiver IOWA Conners ratings suggested that OROS‐MPH improved the control of symptoms after school, a finding that is consistent with the 12‐h duration of action of this medication. Because the therapeutic effect of OROS‐MPH is sufficiently longer than that of a twice daily dose of IR‐MPH, OROS‐MPH had significant positive effects on oppositional/defiant behaviour in addition to its effects on the core symptoms of ADHD. No significant differences between the 2 drugs were noted related to appetite loss and sleep disturbances. Tic symptoms significantly decreased after switching from IR‐MPH to OROS‐MPH Comments from the study authors: the investigators and participants were not blind to the treatment conditions Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes. The study excludes all patients who are hypersensitive to methylphenidate Supplemental information requested from the study authors in January 2014. No reply |
|
Kim 2014a.
| Methods | A review of the medical records of children and adolescents receiving MPH for ADHD between 2004 and 2011 | |
| Participants | Number of participants screened: not stated Number of participants included: 157 Number of participants followed up: 157 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: combined (71.3%), hyperactive‐impulsive (0.6%), inattentive (28.0%)) Age: mean 8.9, range 5‐14 years old IQ: 104.5 (SD 15.3), range 71‐143 Sex: 134 males, 23 females Methylphenidate‐naïve: none Ethnicity: not stated Country: Korea Comorbidity: ODD 30.6%, tic 17.8%, anxiety 11.5%, depressive disorder:4.5%, elimination disorder 2.5% Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: OROS‐MPH 52.2%, ER‐MPH 26.1%, combination 27.1% Mean methylphenidate dosage: 35.0 (SD 12.4 mg); 0.98 (SD 0.27) mg/kg/day Administration schedule: not stated Duration of intervention: ≥ 1 year; mean 28.8 months (SD 16.1); range 12‐88 months Treatment compliance: ≥ 80% |
|
| Outcomes |
Non‐serious adverse events: Weight and height Z score measured during the first and second year of MPH treatment |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: funded by the Korean Ministry of Education, Science and Technology Key conclusions of the study authors: these results suggest that methylphenidate could be related to weight and height deficit in Korean children and adolescents, although the effects were minor, and disappeared after the 1st year Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Kim 2014b.
| Methods | A naturalistic cohort study of methylphenidate use for 8 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 75 Number of participants followed up: 57 Number of withdrawals: 18 Diagnosis of ADHD: DSM‐IV (subtype: combined (48%), hyperactive‐impulsive (12%), inattentive (24%), not specified (16%)) Age: mean 9.7 (SD 2.8), range 6‐16 years old IQ: not stated Sex: 61 males, 14 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Korea Comorbidity: 4 had oppositional defiant disorder, 5 had conduct disorder, 6 had anxiety disorder, 6 had learning disorder, 10 had depressive disorder, and 10 had mild to moderate intellectual disabilities Comedication: not stated Sociodemographics: yearly household income > USD 4000 = 23 (30.7%), USD 2000‐4000 = 20 (26.7%),< USD 2000 = 12 (16.0%), unknown = 20 (26.7%) Inclusion criteria: Children and adolescents aged 6‐16 with a DSM‐IV diagnosis of ADHD, with no baseline physical or laboratory abnormalities; were able to comply with the study visitation schedule and express a voluntary wish to withdraw from the trial |
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Mean methylphenidate dosage: 36.3 (SD 15.5) mg/day Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Children who withdrew from the study because of adverse events |
|
| Notes | Sample calculation: no Ethics approval: not stated Funding/vested interests: the authors declare that they have no conflict of interest, and the organisation that sponsored the research had no part in the writing of the manuscript Key conclusions of the study authors: methylphenidate treatment for ADHD was associated with both symptom alleviation in children with ADHD and improvement in parental depressive mood and quality of life, suggesting that the effects of treatment could go beyond symptom improvement in ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Kim 2015a.
| Methods | A cohort study of methylphenidate use for 12 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 86 Number of participants followed up: 37 Number of withdrawals: 49 Diagnosis of ADHD: DSM‐IV (subtype: inattentive, hyperactive‐impulsive or both) Age: mean 8 IQ: > 70 Sex: 34 males, 3 females Methylphenidate‐naïve: 2‐month washout period prior to study Ethnicity: not stated Country: Korea Comorbidity: none Comedication: none Sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release or extended release Mean methylphenidate dosage: 22.23 (SD 8.93) mg/0.70 (SD 0.20) mg/kg Administration schedule: not stated Duration of intervention: 12 weeks Treatment compliance: 43% completed 12 week programme |
|
| Outcomes |
Non‐serious adverse events: All participants were evaluated for adverse events during each visit, and an interview with their care providers was conducted |
|
| Notes | Sample calculation: no Ethics approval: study approved by Institutional Review Board of Guro Hospital, Korea University Medical Center Funding/vested interests: financial support from Jun Sang‐Bae Child and Adolescent Psychiatry Research Grant from the Korean Foundation of Neuropsychiatric Research Key conclusions of the study authors: HF and RMSSD suggested that parasympathetic dominance in ADHD can be changed by methylphenidate treatment Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested twice from the study authors in May and June 2016 with no reply |
|
Kim 2015b.
| Methods | A prospective, multicentre, open‐label cohort study of 116 children with ADHD treated with OROS methylphenidate for 12 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 143 Number of participants followed up: 116 Number of withdrawals: 27 Diagnosis of ADHD: DSM‐IV (subtype: combined (36.2%), hyperactive‐impulsive (5.2%), inattentive (33.6%), NOS (25%)) Age: mean 9.4, range: 6‐18 years old IQ: mean 108.9 (SD 16.0) Sex: 100 males, 16 females Methylphenidate‐naïve: 89.7% Ethnicity: not stated Country: Korea Comorbidity: depression (5.2%), anxiety (7.7%), tic disorder (7.7%), ODD (10.3%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Mean methylphenidate dosage: average daily dose increased from 19.20 (SD 3.11) mg/d (0.64 (SD 0.18) mg/kg per day) at week 1 to 34.13 (SD 13.80) mg/d (1.03 (SD 0.3318) mg/kg per day) at the end of 12 weeks of dosing Administration schedule: not stated Duration of intervention: 12 weeks Treatment compliance: 12 patients dropped out because of medication non‐compliance |
|
| Outcomes | 8 participants dropped out because of adverse events (29.6%) | |
| Notes | Sample calculation: no Ethics approval: yes Funding: this study has been sponsored by Janssen, Korea Vested interests/authors' affiliations: the authors have no conflicts of interest to declare. Key conclusions of the study authors: treatment with OROS‐MPH was associated with symptomatic functional changes that were moderately correlated; therefore, symptomatic functional outcomes appear to be partially overlapped but distinct domains. Consequently, functional measures should be incorporated as important outcome measures in future treatment studies; the importance of treatments targeting functional improvement should be emphasised in the treatment of children with ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding adverse events requested through personal email correspondence with the authors in June 2016 with no reply |
|
Klein 2004.
| Methods | Randomised controlled trial parallel study comparing:
|
|
| Participants | Number of participants screened: 332 Number of participants included: 129 Number of participants randomised to each arm: MPH = 34; MPH + MPT = 34; MPH + ACT = 35 Number of withdrawals in each arm: MPH: 10; MPH + MPT: 6; MPH + ACT: 6 Diagnosis of ADHD: DSM‐III‐R Age: mean 8.2 (SD 0.8) years (range: 7.0‐9.9) IQ: mean WISC IQs were full scale, 109.5 (SD 14.5); verbal, 108.5 (SD 4.0); and performance, 108.7 (SD 15.0) Sex: 93% males, 7% females Methylphenidate‐naïve: 79.6% Ethnicity: 84% white, 13% African American, 2% Hispanic, and 1% other Country: USA and Canada Comorbidity: 55 (53.4%) ODD, 31 (30%) had 1‐2 symptoms of CD. 17 (16.5%) had an anxiety disorder (simple phobia, overanxious disorder, separation anxiety disorder), 4 (3.9%) had major depression Comedication: not stated Sociodemographics: 84 (81.2%) children lived with both parents, 13 (12.6%) with 1 parent (in all but one instance, the mother), and 6 (5.8%) with their mother and stepfather. Mean socioeconomic status was 2.5 SD 0.9 (range 1‐5) (Myers 1968). There were no significant difference in baseline demographics between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to methylphenidate (MPH), methylphenidate and multimodal psychosocial treatment (MPH + MPT) or methylphenidate and attention control psychosocial treatment (MPH + ACT). Participants were balanced for ethnicity, sex, IQ, and oppositional defiant disorder. Assignment was done in blocks of 4 to enable group treatment components Mean methylphenidate dosage: year 1: MPH = 35.8 (SD 8.5) mg, MPH + MPT = 35.6 (SD 9.4) mg, MPH + ACT = 38.4 (SD 8.5) mg. Year 2: MPH = 38.0 (SD 12.6) mg, MPH + MPT = 41.0 (SD 11.2) mg, MPH + ACT = 38.4 (SD 8.3) mg Administration schedule: 8 am, noon, 4 pm Duration of intervention: 2 years Titration period: 5 weeks duration before randomisation Treatment compliance: the percentage of positive Ritalin acid assays was 87%, without differences between groups. Medication compliance was addressed by counting returned pills |
|
| Outcomes |
Non‐serious adverse events: Children's Depression Inventory (CDI) (Kovacs 1992) Piers‐Harris Children's Self‐Concept Scale (Amato 1984) Before every visit, teachers were called to obtain information about the child's school performance. The sessions were used to assess vital signs (weight, height, pulse, blood pressure), side effects (reported by parent and child), and the child's overall condition |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding: supported by NIMH grants RO1 MH44848 (H.A.) and RO1 MH44842 (L.H.). Vested interests: Dr Klein is a member of the ADHD Advisory Board of Shire Pharmaceutical Co. Key conclusions of the study authors: in stimulant‐responsive young children with ADHD without learning and conduct disorders, there is no support for academic assistance and psychotherapy to enhance academic achievement or emotional adjustment. Significant short‐term improvements were maintained over 2 years Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes |
|
Kordon 2011.
| Methods | A prospective, open‐label, single arm, non‐interventional cohort study of osmotic release oral system (OROS) methylphenidate use for 12 weeks after abrupt switching from immediate release (IR) methylphenidate | |
| Participants | Number of participants screened: 616 Number of participants included: 598 (ITT population) Number of participants followed up: 579 Number of withdrawals: 110 (18.4%) Diagnosis of ADHD: ICD‐10 (subtype: F90.0 (63.5%), F90.1 (37%), F90.8 (2%), F90.9 (2.8%), F98.8 (9.9%)). Age: mean 10.9, range 6‐17 years old IQ: not stated Sex: 507 males, 91 females Methylphenidate‐naïve: 8.8% Ethnicity: not stated Country: Germany Comorbidity: 33.3% (subtype: conduct disorder (23.4%), oppositional defiant disorder (16.6%), anxiety disorder (3.8%), obsessive compulsive disorder (1.5%), substance abuse (0.8%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate starting dose: 29.5 SD 12 mg/day (range: 18‐108 mg/day, median 36 mg/day) Methylphenidate final dose: 33.5 SD 13.2 mg/day (range: 18‐108 mg/day, median 36 mg/day) Administration schedule: once daily Duration of intervention: 12 weeks Treatment compliance: 0.8% of ITT non compliant |
|
| Outcomes | 3 visits in clinic: baseline, after 1 month of treatment, after 3 months of treatment, or on premature termination Adverse events: World Health Organization ‐ Adverse Reactions Terminology (WHO‐ART). Rated throughout the study. Spontaneous reporting. ITT safety population included only those who had ≥ 1 post‐baseline efficacy measurement Sleep quality and appetite: rated at each visit using a 5‐point scale (1 = very good, 5 = very bad) Vital signs: blood pressure and heart rate. Measured at every visit Body weight: measured at baseline and after 3 months of treatment |
|
| Notes | Sample calculation: no Ethics approval: independent ethics committee (Freiburg, Germany) Funding/vested interests: editorial assistance funded by Janssen‐Cilag Authors' affiliations: BS is employed by Janssen‐Cilag, KR is a consultant working for GEM, and paid by Janssen‐Cilag Key conclusions of the study authors: in this naturalistic setting, transitioning from IR‐MPH to OROS‐MPH, in patients who showed previously insufficient response and/or poor tolerability, was successful. OROS methylphenidate was generally safe and well tolerated. Children/adolescents with ADHD who were switched from IR methylphenidate to OROS methylphenidate experienced clinically relevant improvements in symptoms, HRQoL and social functioning Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: unclear Supplemental information requested from the study authors twice in July 2014 with no reply |
|
Kratochvil 2002.
| Methods | A randomised, open‐label, parallel, cohort study of
|
|
| Participants | Number of participants screened: 319 Number of participants included: 228 Number randomised to methylphenidate: 44 Number followed up: 25 Number of withdrawals: 19 Diagnosis of ADHD: DSM‐IV (subtype: combined (77.3%), inattentive (22.7%)) Age: mean 10.4 (SD 2.1) years old IQ: > 70 Sex: 44 males Methylphenidate‐naïve: not stated Ethnicity: white: 81.8%, others: 18.2% Country: USA and Canada Comorbidity: ODD 59.1%, major depressive disorder 13.6%, elimination disorder 11.4% Comedication: not stated, but other psychoactive medication not permitted Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: initial dose 5 mg 1‐3 times daily with an ascending dose titration based on the investigator's assessment of clinical response and tolerability Mean methylphenidate dosage: 0.85 SD 0.53 mg/kg pr day, or 31.3 SD 18.7 mg/day Median dose: 0.74 mg/kg/day or 27.5 mg/day. Total daily dose was not to exceed 60 mg Administration schedule: 1‐3 times daily, based on clinical response and tolerability Duration of intervention: 10 weeks Treatment compliance: not stated Washout period prior to treatment ‐ duration not specified |
|
| Outcomes |
Non‐serious adverse events At weekly visits: ECG, liver function, blood count, urinalysis, open‐ended questions |
|
| Notes | Sample calculation: yes, but sample size and power computations were performed to answer questions specific to the relapse‐prevention portion of the study which followed the open‐label period described in this paper Ethics approval: yes Funding/vested interest: study funded by Eli Lilly and Co Authors' affiliations: the authors are either employees or paid consultants and/or investigators of Eli Lilly. The employees are also shareholders Key conclusions of the study authors: the results of this study provide preliminary evidence that the magnitude and profile of symptom reduction associated with atomoxetine administration and its tolerability are comparable to that observed with methylphenidate Comments from the study authors: in our trial, investigators had latitude in the frequency and timing of methylphenidate dosing, and methylphenidate outcomes might have been different had all methylphenidate‐treated patients been required to receive fixed dosing on a thrice‐daily basis. A relatively large proportion of patients in both groups withdrew from the study early. No single reason appears to have accounted for this, but this could limit the interpretability of the results. The groups were not well matched for gender. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information regarding IQ received through personal email correspondence with the authors in June 2014 (Kratochvil 2014 [pers comm]) |
|
Kraut 2013.
| Methods | A database study of methylphenidate use | |
| Participants | Number of participants screened: not stated Number of participants included: 2,150,362 Diagnosis of ADHD: ICD‐10 German Modification (subtype: not stated) Mean age: not stated, range: 3‐17 IQ: not stated Sex: not stated Ethnicity: not stated Country: Germany Comorbidity: yes Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes | 8 participants dropped out due to adverse events | |
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interests/authors' affiliations: AAK received funding from Sanofi Pasteur MSD for the annual conference of the German Society of Epidemiology in 2010. The present work is unrelated to the funding mentioned. TB served in an advisory or consultancy role for Bristol Myers‐Sqibb, Develco Pharma, Lilly, Medice, Novartis, Shire, Viforpharma; YES‐Pharma. He received conference attendance support and conference support or received speaker's fee by Lilly, Janssen McNeil,Medice, Novartis and Shire. He is/has been involved in clinical trials conducted by Lilly and Shire. The present work is unrelated to the above grants and relationships. RTM received research funding from Sanofi PasteurMSD and Bayer‐Pharma. The mentioned funding is unrelated to the present work. EG is running a department that occasionally performs studies for pharmaceutical industries with the full freedom to publish. The companies include Mundipharma, Bayer‐Pharma, Stada, Sanofi‐Aventis, Sanofi‐Pasteur, Novartis, Celgene and GSK. In the past, EG has been consultant to Bayer‐Schering, Nycomed, Teva and Novartis. The present work is unrelated to the stated relationships. IL, CL, UP, and FP declare no competing interests Key conclusions of the study authors: children starting methylphenidate treatment had a high prevalence of pre‐existing psychiatric comorbidities which may affect methylphenidate prescribing. Cardiovascular and other comorbidities were generally rare, but require attention as they were more common among those who received methylphenidate than in the control group Supplemental information requested through personal email correspondence with the authors in June 2016. Data were not available |
|
Lahat 2000.
| Methods | A cohort study of bone density in children with ADHD treated with methylphenidate for a mean of 13 (SD 4) months with a mean daily dose of 10 mg (SD 2.5) mg | |
| Participants | Number of participants screened: 20 Number of participants included in the methylphenidate group: 10 Number of participants followed up: 9 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 8.9 (SD 1.6) years old IQ: not stated Sex: 20 males Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Israel Comorbidity: not stated Comedication: none Sociodemographics: not stated There were no significant difference in baseline demographics between the 2 groups except for the use of methylphenidate Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: 0.5 mg/kg Mean methylphenidate dosage: 10 (SD 2.5) mg, range 7.5‐12.5 mg Administration schedule: not stated Duration of intervention: 12‐24 months, mean 13 (SD 4) months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Abnormal levels of:
Weight and height measured |
|
| Notes | Sample calculation: not stated Ethics approval: approved by the ethics committee of our medical centre. Funding/vested interests/authors' affiliations: not stated. On a more recent publication by Lahat, he was listed as working for Teva Pharmaceutical Industries Ltd., Teva Israel, Netanya, Israel Key conclusions of the study authors: in conclusion, our data do not support a significant effect of methylphenidate on bone mineral density turnover in children when used for 1‐2 years. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information from the study authors requested twice with no reply |
|
Lakic 2012.
| Methods | A cohort study of the influence of methylphenidate treatment on the occurrence of tics and exacerbation of pre‐existing tics | |
| Participants | Number of participants screened: not stated Number of participants included: 68 Diagnosis of ADHD: DSM‐TR‐IV (subtype: not stated) Age: range 7‐15 years old IQ: > 70 Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Serbia Comorbidity: tic disorder 9.7% Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: sustained‐release Methylphenidate dose range: 18‐36 mg/day (individualised dose) Administration schedule: not stated Duration of intervention: > 6 weeks, up to 6 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Assessment of the occurrence of tics at the time of diagnosis, start of therapy and during methylphenidate treatment. Furthermore, monitoring (type, intensity, duration) of pre‐existing tics during methylphenidate treatment |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding: no funding Authors' affiliations: not stated Key conclusions of the study authors: tics as a side effect of sustained‐release methylphenidate treatment in our patients was predominantly motor, of mild intensity and transient in nature, and did not require cessation of therapy Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental outcome data and information about IQ and funding were received through personal email correspondence with the authors in October 2013 (Lakic 2013 [pers comm]) |
|
Lamberti 2015.
| Methods | An observational prospective study of immediate‐release methylphenidate and cardiovascular effects | |
| Participants | Number of participants screened: not stated Number of participants included: 54 Number of participants followed up: 54 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐5 (subtype: not stated) Age: mean 12.14 (SD 2.6) years (range 6‐19) IQ: not stated Sex: 51 males, 3 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Italy Comorbidity: no cardiovascular, pulmonary, or endocrine disorders Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate: immediate release (IR‐MPH) Methylphenidate dosage: 10‐60 mg according to the participant's weight Mean methylphenidate dosage: 18.5 mg/day Administration schedule: each treatment condition was administered 7 days, 2‐3 times daily, at breakfast (approximately 7:30 am), at lunch (approximately 12:30 pm), and in some cases, early afternoon (approximately 3:30 pm) Duration of intervention: 4 weeks Treatment compliance: 100% |
|
| Outcomes | For each patient, 2 standard 12‐lead ECGs were obtained at a paper speed of 25 mm/second with the same instrument (Cardioline delta 3 plus), on the same day and under similar conditions. The first (predose) ECG examination was performed before the administration of the first daily dose of IR‐MPH; the second (postdose) ECG was executed 2 hours after drug intake, simultaneously with the serum peak of methylphenidate Non‐serious adverse events Treatment with immediate‐release methylphenidate was associated with a slight increase of systolic and diastolic BP |
|
| Notes | Sample calculation: not stated Ethics approval: the study was approved by the local ethics committee Funding/vested interests: none Key conclusions of the study authors: this study underlines the relative cardiac safety of IR‐MPH in childhood, even if stimulants may exert a cardiovascular effect on BP and HR. However, particular caution should be exercised by physicians in prescribing these drugs to patients with a genetic predisposition to arrhythmias. It might be useful to carry out an ECG examination in all patients starting methylphenidate therapy Comments from the study authors: the most important limitation of this study includes the lack of a long‐term follow‐up. More studies are needed to confirm the cardiovascular safety during long‐term therapy Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Langevin 2012.
| Methods | A controlled before‐after study of methylphenidate use for ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 10 Number of participants included as cases: 5 and controls: 5 Number of participants followed up: 10 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV TM (subtype: combined (80%); inattentive (20%)) Age: mean 8.13 years (range 7‐9) IQ: normal Sex: 8 males, 2 females Methylphenidate‐naïve: not stated Ethnicity: white (90%), African‐Canadian (10%) Country: Canada Comorbidity: 20% ODD, 10% seasonal affective disorder Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: immediate‐release or moderate‐release Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: 6 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Sleep quality and numbers of hours of sleep |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests/authors' affiliations: this study was supported by a grant from the University of Alberta (Killam Research Fund) Key conclusions of the study authors: the principal results support the study's hypothesis and show a significant baseline difference (P = 0.008) between the nocturnal movements of the ADHD children and those of the control children Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Larrañaga‐Fragoso 2015.
| Methods | A cohort study of methylphenidate use for 9 months | |
| Participants | Number of participants screened: not stated Number of participants included: 14 Number of participants followed up: 14 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean 11 (SD 2.79) years (range: 7‐17) IQ: not stated Sex: 6 males, 8 females Methylphenidate‐naïve: 100% Ethnicity: 100% white Country: Spain Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: unknown Methylphenidate dosage: unknown Administration schedule: unknown Duration of intervention: 9 months Treatment compliance: unknown |
|
| Outcomes | Ocular examination before and after cycloplegia was performed at each visit, including Pentacam imaging of the anterior chamber Visual acuity, sphere, spherical equivalent refraction, intraocular pressure, and cup:disk ratio | |
| Notes | Sample calculation: not stated Ethics approval: clinical research ethics committee Funding/vested interests: not stated Key conclusions of the study authors: methylphenidate does not seem to affect refraction in most children with ADHD. After 9 months of treatment, however, there was a reduction in the anterior chamber depth, which has been described as a powerful predictor of angle closure glaucoma. Further investigation of the potential ocular side effects of methylphenidate is warranted Comments from the study authors: this study is limited by the small sample size and short follow‐up period. Further studies are warranted, because the decrease in ACD observed in this study may be a risk factor for the development of angle closure glaucoma Supplemental information regarding comorbidity received through personal email correspondence with the authors in June 2016 (Larrañaga‐Fragoso 2016 [pers comm]). The authors were not able to supply us with information regarding IQ, ADHD subtype, methylphenidate dosage and type |
|
Lee 2007.
| Methods | A cohort of methylphenidate use for 3 weeks plus 1 week baseline | |
| Participants | Number of participants screened: not stated Number of participants included: 119 Number of participants followed up: 110 Number of withdrawals: 9 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 8.5 (SD1.6) years (range 6‐13) IQ: > 70 Sex: 107 males, 12 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: South Korea Comorbidity: oppositional behaviours (14.8%), conduct behaviours (2.3%), obsessive‐compulsive behaviours (0.8%), generalised anxiety symptoms (7.8%), depressive symptoms (12.5%), learning problems (18.8%) Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral sytem (OROS) Mean methylphenidate dosage: 0.87 mg/kg (SD 0.33) Administration schedule: daily Duration of intervention: 3 weeks Treatment compliance: 2 participants discontinued trial due to protocol non‐compliance |
|
| Outcomes |
Non‐serious adverse events
1 respondent withdrew from trial during first week of trial on 18 mg OROS because of decreased appetite of moderate severity while another participant withdrew in the same period because of insomnia of mild severity |
|
| Notes | Sample calculation: yes Ethics approval: not stated Funding/vested interests: funded by Janssen Korea Key conclusions of the study authors: these data provide support for the benefit of the once daily methylphenidate preparation Concerta, in the treatment of Korean children with ADHD. Children were initiated safely in this short‐term trial, and its effectiveness was evident in the behavioural, as well as neuropsychological measurements Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
Lee 2009.
| Methods | A prospective 12‐week, open‐label, multicentre study to examine optimal dosage of osmotic release oral system methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 144 Number of participants followed up: 88 Number of withdrawals: 56 Diagnosis of ADHD: not stated (subtype: not stated) Age: mean 9.43, range 6‐18 years old IQ: 109.7 (SD 16.1) Sex: 77 males, 11 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Korea Comorbidity: oppositional defiant disorder (n = 12), tic disorder (n = 9), depressive disorder (n = 3), and anxiety disorder (n = 6) Comedication: not stated Sociodemographics: not stated Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) extended release Methylphenidate dosage: 18 mg or 27 mg (depending on clinical judgment) Mean methylphenidate dosage: 0.99 mg/kg (SD 0.29) at 12 weeks (n = 88) Administration schedule: not stated Duration of intervention: 12 weeks plus 9‐week initial titration Treatment compliance: not stated |
|
| Outcomes | Of the 144 participants enrolled in the study, 8 dropped out due to adverse events (28.6%) during the titration phase | |
| Notes | Sample calculation: not stated Ethics approval: approved by the Institutional Review Boards of all 7 sites Funding/vested interests: this study was supported by Janssen Korea Ltd. The authors have no financial conflicts of interest Key conclusions of the study authors: high response time (RT) among Korean children with ADHD on a computerised continuous performance attention test RT variability may predict poor response to MPH treatment in children with ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information requested twice from the study authors regarding supplemental data on adverse events with no answer |
|
Lee 2012.
| Methods | A cohort study of methylphenidate use in children with ADHD for 4 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 93 Number of participants followed up: 63 Number of withdrawals: 30 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (84.1%), hyperactive‐impulsive (not stated), inattentive (not stated)) Age: mean 8.58 (SD 1.61) years IQ: mean 96.39 (SD 17.01) Sex: 56 males, 7 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Korea Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) or Metadate‐CD Mean methylphenidate dosage: 1.0 (SD 0.25) mg/kg. Initial doses for OROS‐MPH 18 mg and Metadate‐CD 10 mg; dose range and maximum dose for OROS‐MPH was 0.4‐1.8 mg/kg and 72 mg and for Metadate‐CD was 0.6‐1.5 mg/kg and 60 mg) Administration schedule: daily Duration of intervention: 4 weeks Treatment compliance: the children and caregivers were visited weekly to review whether medication was taken as prescribed |
|
| Outcomes |
Non‐serious adverse events
|
|
| Notes | Sample calculation: irrelevant
Ethics approval: the study protocols were reviewed and approved by each site's Institutional Review Board
Funding/vested interests: Yeungnam University research grants in 2009. No conflicts of interest statement Key conclusions of the study authors: methylphenidate had negative impacts on sleep among young ADHD children, but different preparations and doses did not affect the result Comments from the study authors: data yielded by a sleep diary, which relies on the validity of parents' observations, may not be an accurate representation of reality. Used a flexible titration method and did not divide the participants into parallel groups from the beginning. Lack of blinding. More than 30% did not complete the procedure. Comments from the review authors: participants were dropped from the study if they violated the study protocol by failing to take the prescribed medicine more than twice a week, or if they presented with serious adverse effects such as seizures or hallucinations Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested from the study authors in August 2014. Email sent twice but no answer received |
|
Lee 2014.
| Methods | A cohort study of medication‐related adverse events in children and adolescents reported to the US Food and Drugs Administration (FDA) from 2007 to 2012 | |
| Participants | Number of patients screened: not stated Number of participants included: 4055 Diagnosis of ADHD: not stated Age: mean 10.2 years (range: 1‐17) IQ: not stated Sex: not stated Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria: All patient reports submitted to the FDA Adverse Event Reporting System (FAERS) between 1 January 2007 and 27 August 2012 for children (1‐11 years) and adolescents (12‐17 yrs) Exclusion criteria: none stated |
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes | Type of adverse event: all adverse events and adverse events with serious outcomes. The description of the adverse events is coded based on a 'preferred term' from the Medical Dictionary for Regulatory Activities (MedDRA) terminology | |
| Notes | Sample calculation: no Ethics approval: not reported Funding/vested interests/authors' affiliations: no Key conclusions of the study authors: our findings highlight the high‐risk medications and the corresponding adverse events commonly reported in children and adolescents. Information from this analysis can be used to prioritise drugs and adverse events that might be investigated in future studies of drug safety in children Comments from the study authors: our findings are consistent with other studies that have used data from spontaneous reporting systems to examine the adverse events in children Supplemental information regarding data and IQ requested through personal email correspondence with the authors in June 2016 (Schumock 2016 [pers comm]). The authors were not able to retrieve the information |
|
Lewis 2012.
| Methods | A patient report of a paediatric patient with glaucoma receiving methylphenidate treatment for 8 years | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 10 years old IQ: within normal limits Sex: female Country: USA Comorbidity: primary open angle glaucoma. Physical examination within normal limits Comedication: latanoprost 0.005% eye drops twice daily Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: extended release Methylphenidate dosage: 18 mg/day titrated to 54 mg/day over 6 months Administration schedule: once daily Duration of treatment: 8 years Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events No exacerbation of glaucoma: Before methylphenidate treatment: after treatment for glaucoma, the patient's intraocular pressure was brought to a stable range between 16 mmHg and 19 mmHg in both eyes During methylphenidate treatment: ophthalmic examination every 6 months showed intraocular pressure consistently in the 16‐19 mmHg range bilaterally |
|
| Notes |
Key conclusions of the study authors: this report concluded stimulant medication (methylphenidate) should not be withheld in patients with glaucoma as long as intraocular pressure (IOP) remains well controlled Comments from the study authors: the actual risk associated with adrenergic medications in patients with open angle glaucoma is negligible, and there are no studies proving adverse effects on IOP in normal or open angle eyes Supplemental information regarding IQ received through personal email correspondence with the authors in March 2014 (Lewis 2014 [pers comm]) |
|
Li 2011.
| Methods | An 8‐week, randomised, double‐blind, parallel study with 2 interventions:
|
|
| Participants | Number of participants screened: 136 Number of participants included: 72 Number of participants randomised to methylphenidate: 36 Number followed up: 34 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 9.2, range 3‐13 years old IQ: almost all above 70 Sex: 23 males, 13 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: China Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: 1 mg/kg/day Administration schedule: not stated Duration of intervention: 8 weeks Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events:
Non‐serious adverse events:
|
|
| Notes | Sample calculation: no Ethics approval: all research procedures were permitted by the medical ethics committee of Provincial Hospital Affiliated to Shandong University Funding/vested interests: grants from the Chinese Medicine Administration Bureau of Shandong Province Authors' affiliations: not stated Key conclusions of the study authors: compared to methylphenidate, Ningdong granule is effective and safe for ADHD children in the short term, increases the homovanillic acid concentration in sera to regulate dopamine metabolism, and promises to be an alternative medication, safely and effectively Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information received through personal email correspondence with the study authors in April 2013 (Li 2013 [pers comm]) |
|
Lyon 2010.
| Methods | A cross‐over trial with 2 interventions:
10 children with ADHD and TD were given dexmethylphenidate on 1 visit and no medication on another (day 2 and 3), using a random cross‐over design |
|
| Participants | Number of participants screened: 51 Number of participants included: 13 Number of participants followed up: 10 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (50%), inattentive (50%)) Age: mean 12.7 years old, range 8‐16 years old IQ: mean 104, range 85‐118 Sex: 9 males, 1 female Methylphenidate‐naïve: not stated Ethnicity: white 70%, African American 0%, Asian 0%, Hispanic 30%, others 0% Country: USA Comorbidity: Tourette's 100% Comedication: 70% Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of (0.15 mg/kg) methylphenidate and placebo Mean methylphenidate dosage: 7.5 mg/day Administration schedule: once a day Duration of each medication condition: 1 day Washout prior to study initiation: not stated Medication‐free period between intervention: not stated Titration period: none Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events The Safety Monitoring Uniform Report Form (SMURF) (Greenhill et al. 2004) was administered by one of the investigators on day 1, and after TSP procedures on days 2 and 3 Adverse events were generally mild. 7 (70%) participants experienced ≥ 1 minor adverse event during the study. The most common adverse events possibly related to study drug were drowsiness or sedation (20%) and stomach discomfort (20%) |
|
| Notes | Sample calculation: not stated Ethics approval: all study procedures were approved by the institutional review boards (IRB) at the University of Wisconsin‐Milwaukee and New York University Langone Medical Center Key conclusions from study authors: preliminary results suggest that dexmethylphenidate does not appear to enhance tic suppressibility in children with ADHD and TD. First, there was a clear tic‐reduction effect, and not exacerbation, with a 1‐time dose of dexmethylphenidate compared to no medication in these children. Second, youths with TD and ADHD appear to be able to suppress their tics with a behavioural reward comparable to youths with TD without ADHD Comments from the study authors: some limitations of the study design need to be taken into account. First, our sample size was small and participants were recruited from a specialised clinic. Thus, findings may not generalise to non‐specialty settings. Second, 70% of participants were receiving medication for TD, anxiety, OCD, or asthma, and our results might have differed if the participants were not receiving concomitant medication. Third, investigators, parents, and participants were not blind to medication status, because medication was administered openly Comments from the review authors: ADHD outcome data are only available for 7 participants ('data from 3 participants were unusable as a result of computer malfunction') Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Maayan 2009.
| Methods | A cohort study of long‐acting methylphenidate use for 4 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 11 Number of participants followed up: 8 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (91%), hyperactive‐impulsive (9%)) Age: mean 5.1, range: 4‐5 years old IQ: above 70 Sex: 9 males, 2 females Methylphenidate‐naïve: 100% Ethnicity: white 27%, Hispanic 73% Country: USA Comorbidity: ODD 9% Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: long acting Methylphenidate dosage: 10‐30 mg/day, mean 17.73 mg/day Administration schedule: morning Duration of intervention: 4 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Decreased appetite was experienced by 7 participants (64%) following treatment initiation. 5 out of these 7 participants continued to report decreased appetite for the duration of the study Difficulty sleeping occurred in 3 participants (27%) Emotional lability and gastrointestinal pain were reported by 2 participants (18%) These adverse events were resolved by the end of the study. 1 participant who terminated the study early experienced moderate levels of insomnia, vomiting, decreased appetite, and stomach pain, and another who also terminated early experienced moderate irritability |
|
| Notes | Sample calculation: no Ethics approval: the study was approved by the New York State Psychiatric Institute Columbia University Institutional Review Board (IRB) and was conducted in accordance with the ethical standards of the 1975 Declarations of Helsinki as revised in 2000 (World Medical Association). Funding/vested interests/authors' affiliations: Novartis Pharmaceuticals Corporation provided the study medication. No financial support was received from Novartis. Dr Maayan receives grant support from Eli Lilly and Pfizer. Dr Greenhill received support from Novartis and has an consultant arrangement with Pfizer. He has been awarded research contract to study Risperidon by Johnson and Johnson and has been awarded an investigator‐initiated grant to study aripiprazole by Otsuka Key conclusions of the study authors: long‐acting methylphenidate was safe and effective for the treatment of ADHD in the 4‐ and 5‐year‐olds participating in this study. Rates of adverse events were higher than previously reported in methylphenidate trials of school‐aged children. 10‐mg/day doses failed to achieve response in the 5 children who could not tolerate higher doses Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes |
|
MacDonald 2005.
| Methods | A cross‐over study of 5 children, 4 boys, 1 girl all aged 10‐14 years old Volunteers participated in 13 sessions |
|
| Participants | Number of participants screened: 14 Number of participants included: 5 Number of participants followed up: 5 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 12, range 10‐14 IQ: not stated Sex: 4 males, 1 female Methylphenidate‐naïve: none Ethnicity: not stated Comorbidity: not stated Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: individual maintenance dose as taken by participant prior to study enrolment, range 10 mg twice/day to 30 mg thee times/day Administration schedule: morning Duration of intervention: ≥ 1 year Treatment compliance: not stated |
|
| Outcomes | No relevant outcomes | |
| Notes | Sample calculation: no Ethics approval: Human Subjects Institutional Review Board at Western Michigan University, Kalamazoo, MI Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: 3 of 5 participants reliably chose methylphenidate more often than placebo. Differences between the number of methylphenidate, placebo, and neither choices across participants were significant (P < 0.01) Comments from the study authors: relevant clinical effects were not observed under methylphenidate compared to placebo conditions. Although the questionnaires used in this study have been used to measure subjective effects in children, the psychometric integrity of these instruments has not been determined in these populations. In addition, the reading level of the children may have affected the manner in which the subjective effects were evaluated, such that the participants may not have fully understood the items on the questionnaires Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
Machado 2010.
| Methods | A patient report of a 6‐year old girl developing acute choreoathetoid movements induced by methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 6 years old IQ: above 70 Sex: female Ethnicity: not stated Country: USA Comorbidity: psychomotor developmental delay, discrete macrocephalus, congenital mild ataxia, and hyperactivity. Otherwise healthy Comedication: none Sociodemographics: her parents were first‐degree relatives. No family history of neurological disease |
|
| Interventions | Methylphenidate type: extended release Methylphenidate dosage: 18 mg/day Administration schedule: once daily in the morning Duration of treatment: single dose |
|
| Outcomes |
Serious adverse events: After single dose extended‐release methylphenidate 18 mg: choreoathetoid movements of orofacial muscles, arms, and legs, with transient dystonic postures of the right arm |
|
| Notes |
Key conclusions of the study authors: we believe that the immediate response ensuing chlorpromazine prescription argues in favor of a specific role for dopamine receptor antagonists in methylphenidate‐induced chorea Comments from the review authors: the author is not sure that the girl fulfilled criteria E of the ADHD diagnosis (DSM‐IV). The author only saw the girl once in the emergency unit Supplemental information regarding diagnosis and IQ received through personal email correspondence with the authors in October 2013 (Machado 2013 [pers comm]) |
|
Maia 2008.
| Methods | 8‐week open clinical trial | |
| Participants | Number of participants screened:159 Number of participants included: 120 Number of participants followed up: 39 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐IV (subtype: combined (67.7%)) Age: mean 12.17 (SD 2.67) IQ: mean 89.11 (SD 16.07) Sex: 36 males, 3 females Methylphenidate‐naïve: none Ethnicity: European‐Brazilian (31), other (8) Country: Brazil Comorbidity: oppositional defiant disorder (48.4%), anxiety disorder (29%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Patients switched from equivalent dose of immediate release methylphenidate to methylphenidate SODAS. The mean methylphenidate‐IR dose at baseline was 0.68 (SD 0.24) mg/kg/day and the mean methylphenidate‐SODAS dose prescribed at baseline was 0.7 (SD 0.25) mg/kg/day Methylphenidate type: spheroidal oral drug absortion system (SODAS) Methylphenidate dosage: 20‐40 mg Mean methylphenidate dosage: 0.7 (SD 0.25) mg/kg/day Administration schedule: once daily Duration of intervention: 8 weeks Treatment compliance: the treatment adherence was checked by direct inquiring patients on compliance at week 4 and 8 |
|
| Outcomes |
Serious adverse events:
Barkley Side Effect Rating Scale (SERS)
Non‐serious adverse events:
Barkley Side Effect Rating Scale (SERS) SNAP‐IV Efficacy and side events were rechecked at week 4 and 8 using respectively the total, inattentive, hyperactive/impulsive scores of the SNAP‐IV and the total score of the SERS |
|
| Notes | Sample calculation: no Ethics approval: yes Funding: Novartis Vested interest/authors' affiliations: none Key conclusions of the study authors: results suggested that switching from immediate release methylphenidate to methylphenidate SODAS did not affect stabilisation of ADHD symptoms in most patients. Methylphenidate prescription in patients with previous cardiovascular conditions must be extremely careful Comments from the review authors: the additional data received from the authors do not provide the numbers of adverse events in the paediatric population Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no | |
Man 2015.
| Methods | A cohort study of methylphenidate and the risk of trauma | |
| Participants | Number of participants screened: 17,381 Number of participants included: 4934 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: ICD‐9‐CM (subtype: not stated) Age: mean age at commencement of observation 6.9 years. Range 6‐19 IQ: not stated Sex: 4309 males (87%), 625 females (13%) Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Hong Kong Comorbidity: acute reaction to stress (1.46%), adjustment disorder (1.42%), anxiety disorder (1.64%), autism spectrum disorder (12.7%), disturbance of conduct not elsewhere classified (2.39%), disturbance of emotions specific to childhood and adolescence (10.9%), specific delays in development (12.2%), other psychiatric comorbidities (8.84%) Comedication: no atomoxetine ‐ not otherwise stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: all formulations, standard and extended release Methylphenidate dosage: median daily dosage: 20 mg, IQR of daily dosage: 20 mg (all), 20 mg(male), 15 mg (female) Administration schedule: not stated Duration of intervention: median length of prescription 70 days Treatment compliance: not stated |
|
| Outcomes | Trauma‐related ED admission Non‐trauma related ED admission |
|
| Notes | Sample calculation: with reference to the equation developed by Musonda 2006, an IRR of 0.9 with 80% power (2‐sided 95% CI) could be detected with a minimum of 4062 trauma‐related ED admissions Ethics approval: not stated Funding: funding received from the Research Grants Council (RGC, Hong Kong) under grant agreement number 781913 for the study (Effects of Attention Deficit Hyperactivity Disorder pharmacotherapy on hospital accident and emergency admission due to injury‐related events (ATHAN)) Vested interests: Dr Chan reports grants from Janssen (a division of Johnson & Johnson), BMS, Pfizer, The Research Grant Council (RGC, Hong Kong), received for other work. Dr Coghill reports grants and personal fees from Shire and Vifor; personal fees from Janssen‐Cilag, Lilly, Novartis, Flynn Pharma, Medice, and Oxford University Press, received for other work. Dr Douglas reports personal fees from GSK and Gilead, received for other work. Prof ICK Wong reports grants from the Research Grants Council (RGC, Hong Kong) (during the study) and grants from Shire, Janssen‐Cilag, Eli Lily, the European Union FP7 programme, received for other work. The other authors have indicated they have no financial relationships relevant to this article to disclose Authors' affiliations: Dr Coghill reports grants and/or personal fees from Shire, Janssen‐Cilag, Novartis, Flynn Pharma, and Medice; and Prof I.C.K. Wong was a member of the National Institute for Health and Clinical Excellence ADHD Guideline Group and the British Association for Psychopharmacology ADHD Guideline Group and acted as an advisor to Shire. The other authors have indicated they have no potential conflicts of interest to disclose Key conclusions of the study authors: our study findings support the hypothesis that methylphenidate is associated with a reduced risk of trauma‐related ED admission in children and adolescents of both genders Comments from the study authors: this outcome has important clinical, resource utilisation, and public health implications. Trauma prevention should be considered in the broader clinical assessment of methylphenidate risks and benefits aside from the traditional consideration of improving academic performance |
|
Mayes 1994.
| Methods | A cohort study of 69 children with attention deficit hyperactivity disorder (ADHD) who underwent blind methylphenidate trials | |
| Participants | Number of participants screened: not stated Number of participants included: not stated Number of participants followed up: 69 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IIIR (subtype: hyperactive‐impulsive (100%)) Age: mean 7.1 years old, range 22 months‐13 years IQ: mean 86, range 23‐136 Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: 36 had ADHD alone (with or without a learning disability) and 33 had additional neurodevelopmental disorders Comedication: not stated Sociodemographics: not stated Exclusion criteria
|
|
| Interventions | MPH was prescribed three times daily (8 am, noon, 4 pm) with a starting dose of 0.3 mg/kg rounded to the nearest 2.5 mg. MPH was trialled using an ABA design (A = no medication, B = MPH). Days per MPH dosage was a minimum 3 days (mean of 6 days) and no medication minimum of 6 days (mean 11 days). MPH was increased by 2.5 mg or 5 mg per dose until a response was achieved. The total mean days per MPH dosage was 8 days, mean days no medication 9 days. Doses for responders 50/69 ranged from a dosage of 2.5 mg to 10 mg. The dosage given to the 19/69 who did not respond or did not complete the trial (6/69) were not reported | |
| Outcomes |
Non‐serious adverse events An adverse event was defined as being reported on ≥ 2 methylphenidate days (i.e. at the response dosage for responders or at the highest dosage for non‐responders) and not reported on ≥ 2 methylphenidate‐free days |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: not stated Key conclusions of the study authors: the results confirm and add to the research literature indicating that ADHD children who are of preschool age and/or who have co‐existing neurological disorders may benefit from methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information has not been able to retrieve from authors due to lack of email address |
|
McCarthy 2009.
| Methods | A retrospective cohort study of methylphenidate, dexamphetamine and atomoxetine use | |
| Participants | Number of participants screened: approximately 3 million Number of participants included: 5351 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: not stated (subtype: not stated) Age: mean not stated, range: 2‐21 years old IQ: not stated Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: UK Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: 5 deaths:
|
|
| Notes | Sample calculation: not stated
Ethics approval: ethics approval for the study was granted by the Independent Scientific Advisory Commitee for the Medicines and Healthcare products Regulatory Agency (MHRA) database research
Funding/vested interests: the School of Pharmacy has received an education grant from Janssen Cilag for professional continued development courses. No specific funding was obtained for the conduct of this study. Ian Wong was funded by a UK Department of Health Public Health Career Scientist Award to investigate the safety of psychotropic drugs in children. Eric Taylor and CK Wong were members of the NICE guideline committee for the diagnosis and management of ADHD in children, young people and adults. The other authors have no conflict of interests relevant to the content of this study Key conclusions of the study authors: in this study, it was not possible to demonstrate an increase in the risk of sudden death associated with methylphenidate, dexamphetamine or atomoxetine. Although it was not an initial aim of this study, an increase in the risk of suicide was observed, particularly in the younger teenager category Comments from the study authors: clinicians should identify patients at increased risk for cardiovascular events and those patients at increased risk for suicide, particularly males with co‐morbid conditions, and monitor them appropriately Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
McCracken 2016.
| Methods | An 8‐week double‐blind randomised controlled trial | |
| Participants | Number of participants screened: 323 Number of participants included: 69 Number of participants followed up: 61 Number of withdrawals: 8 Diagnosis of ADHD: DSM‐IV (subtype: combined (46%), hyperactive‐impulsive (3%), inattentive (48%)) Age: mean 10.1 (SD 2.0) years (range: 7‐14) IQ: mean 101.5 (SD 13.3) Sex: 46 males (66.7%), 23 females (33.3%) Methylphenidate‐naïve: not stated. The participants received placebo only the first 4 weeks of the trial before starting methylphenidate treatment Ethnicity: white 73.9%, Black 14.5%, Asian, Pacific Islander 5.8%, Hispanic 14.5%, others 5.8% Country: USA Comorbidity: oppositional defiant disorder 24 (35%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: dexmethylphenidate Mean methylphenidate dosage: 16.0 (SD 3.9) mg (range 5‐20) Administration schedule: once daily Duration of intervention: 4 weeks Treatment compliance: not stated |
|
| Outcomes | Side effects were measured via a structured instrument, using a modification of the Physical Symptom Checklist and open‐ended clinician inquiry | |
| Notes | Sample calculation: not stated Ethics approval: yes Funding: supported by National Institute of Mental Health (NIMH) grants Vested interests/authors' affiliations: Dr Bilder has received consulting income or honoraria from EnVivo Pharmaceuticals, Forum Pharmaceuticals, Lumos Labs, Maven Research, Neurocog Trials Inc., OMDUSA, LLC, Snapchat, Takeda‐Lundbeck, and ThinkNow Inc. He has received research support from the National Institute of Mental Health, the John Templeton Foundation, and Johnson and Johnson. Dr Piacentini has received grant or research support from the National Institute of Mental Health, Pfizer Pharmaceuticals through the Duke Clinical Research Institute CAPTN Network, Psyadon Pharmaceuticals, and the Tourette Association of America. He has received financial support from the Petit Family Foundation and the Tourette Syndrome Association Center of Excellence Gift Fund. He is a co‐author of the Child OCD Impact Scale‐Revised (COIS‐R), the Child Anxiety Impact Scale (CAIS), the Parent Tic Questionnaire (PTQ), and the Premonitory Urge for Tics Scale (PUTS) assessment tools, all of which are in the public domain therefore no royalties are received. He has received royalties from Guilford Press and Oxford University Press. He has served on the speakers' bureau of the Tourette Association of America, the International Obsessive Compulsive Disorder Foundation, and the Trichotillomania Learning Center. Dr McGough has received consultant honoraria from Neurovance; research support from Purdue; material research support for investigator initiated studies from NeuroSigma and Shire; book royalties from Oxford University Press; and DSMB honoraria from Sunovion. He has provided expert testimony for Shire. Dr McCracken has received consultant honoraria from Dart Neuroscience and Think Now, Inc. Drs. Loo, Sturm, Cowen, Walshaw, Levitt, Del'Homme, and Mr Cho report no biomedical financial interests or potential conflicts of interest Key conclusions of the study authors: combination medication treatment showed consistent evidence for clinical benefits over monotherapies, possibly reflecting advantages of greater combined dopaminergic and alpha2A agonists. Adverse events were generally mild to moderate, and combination treatment showed no differences in safety or tolerability Comments from the study authors: this design allows us to compare the medication conditions by comparing all participants and time‐points for which that condition occurred, after adjusting for overall drift. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
McLaren 2010.
| Methods | A patient report of acute dystonia following withdrawal of methylphenidate treatment in the context of multiple high dose drug treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 11 years old IQ: no mental retardation Sex: male Ethnicity: not stated Country: USA Comorbidity: bipolar disorder Comedication: aripiprazole, 15 mg, twice daily. Lithium carbonate, 600 mg and 300 mg at night. Clonidine, 0.2 mg, twice daily Sociodemographics: not stated |
|
| Interventions | Extended‐release OROS methylphenidate dosage: 108 mg daily Administration schedule: not stated Duration of treatment: 4 years prior to withdrawal in hospital. The last 6 months the dose had not been changed. Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Acute dystonia after discontinuation of OROS methylphenidate The patient experienced spasmodic muscular contractions of his jaw. The staff noticed a forceful jaw closure, contraction, and tension, and the patient had difficulties opening his mouth. Intramuscular diphenhydramine at 50 mg was administered intramuscularly, and the patient could open his mouth within 30 minutes and with no further dystonia |
|
| Notes | Funding/vested interest/authors' affiliations: none declared Key conclusions of the study authors: need for vigilance regarding development of acute dystonic reactions when discontinuing methylphenidate whilst using concomitant antipsychotic drugs Comments from the review authors: the patient was on extremely high doses, not normally given to patients of this age, of all of the medication that he was on i.e. extended‐release OROS methylphenidate 108 mg (twice the recommended maximum dose), aripiprazole 15 mg twice daily (also in excess of double the normal maximum dose), lithium 900 mg daily (very high for a child of 11 years) and clonidine 0.2 mg (around double the maximum recommended dose) Supplemental information regarding patients diagnostic criteria and IQ received through personal email correspondence with the authors in October 2013 (McLaren 2013 [pers comm]) |
|
Miller‐Horn 2008.
| Methods | A retrospective cohort study of patients with ADHD attending a clinic over a 2‐year period taking methylphenidate | |
| Participants | Number of participants screened: 516 Number of participants included: 150 Number of participants followed up: 137 Number of withdrawals:13 63 took MPH, of which 40 (29.2% of whole sample) took extended release methylphenidate and 23 (16.8%) took immediate release methylphenidate. Diagnosis of ADHD: DSM‐IV (subtype: combined 121 (88.3%), hyperactive‐impulsive 4 (2.9%), inattentive 12 (8.8%)) Age: males: mean 9.9 (SD 3), range 4‐19 years old; females: 10.9 (SD 3.4), range 4‐17 years old IQ: not stated Sex: 109 males, 28 females Methylphenidate‐naïve: 65% Ethnicity: not stated Country: USA Comorbidity: 87 (64%) (type: oppositional defiant disorder/conduct disorder (16%), seizures (15%), learning disabilities (14%), PDD 13%, sleep disturbances (9%), mental retardation/cerebral palsy (7%), chronic headaches (6%), Tourette's (6%), obsessive compulsive disorder/depression (6%)) Comedication: all patients were on monotherapy for medications used in the treatment of ADHD; however, some of the patients were currently being treated for comorbid conditions Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) and immediate release OROS‐methylphenidate dosage: mean 34.2 (SD 13.6) mg/day (range 18‐54) IR‐methylphenidate dosage: mean 23.3 (SD 14.8) mg/day (range 5‐60) Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes | Retrospective database analysis. The database contained information on side effects. No other information regarding measurement available | |
| Notes | Sample calculation: not stated Ethics approval: approved by the Institutional Review Board at St. Christopher's Hospital for Children in Philadelphia Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: atomoxetine showed a significantly lower incidence of headaches than amphetamine/dextroamphetamine XR, amphetamine/dextroamphetamine or OROS‐methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested twice in June 2014 with no reply |
|
Mino 1999.
| Methods | A patient report on methylphenidate‐induced psychosis in an adolescent with hyperkinetic disorder | |
| Participants | Diagnosis of ADHD: DSM‐III‐R and later ICD‐10 (subtype: not stated) Age: 16 years old IQ: around 70 Sex: female Ethnicity: not stated Country: Japan Comorbidity: conduct disorder and secondary neurotic symptoms Comedication: not at the time when the patient took methylphenidate Sociodemographics: lives with parents and stepsister |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10 mg for 3 weeks, reduced to 5 mg for 1 week Administration schedule: once daily, morning Duration of intervention: 1 month Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: 3 weeks after starting on methylphenidate (10 mg/day), the mother reported by telephone that the patient seemed depressed. Dose of methylphenidate was reduced to 5 mg/day. A week later the patient visited the clinic. The therapist diagnosed her condition as a depressive state and discontinued methylphenidate. 6 weeks after discontinuation of methylphenidate she was diagnosed with schizophrenic‐like psychotic state, due to symptoms of delusions of reference and persecution, delusional mood, silly smile and thought block. There was no evident hallucination. The patient took antipsychotic medication for 2 months, and her psychotic symptoms disappeared. |
|
| Notes |
Key conclusions of the study authors: we discuss this case as an example of methylphenidate‐induced psychosis Comments from the study authors: we suggest that there are 2 types of methylphenidate psychosis: the first being hallucination dominant type and the second, delusion dominant type. This patient report address the second one Comments from the review authors: another article written in Japanese by the same authors found in our search. The abstracts of the 2 articles were identical, and the Japanese full‐text has therefore not been translated Funding/vested interests/authors' affiliations: not stated |
|
Mize 2004.
| Methods | A patient report of headache and mild depression during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 12 years and 3 months old IQ: 124 Sex: male Ethnicity: not stated Country: USA Comorbidity: none stated Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: Ritalin slow release Methylphenidate dosage: 20 mg/daily Administration schedule: not stated Duration of treatment: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Headache and symptoms of mild depression |
|
| Notes |
Key conclusions of the study authors: 10 sessions of haemoencephalograph appear to have produced significant change in attention Comments from the review authors: the study shortly comments that the boy has headache and symptoms of mild depression when taking methylphenidate (20 mg/daily) Funding/vested interest/authors' affiliations: not stated |
|
Mohammadi 2004.
| Methods | A 6‐week, parallel group, randomised trial with 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 32 Number of participants randomised to methylphenidate: 16 Number of participants followed up: 11 Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (subtype: combined (100%)) Age: mean 8.87 years (range 6‐14) IQ: > 70 Sex: 11 males, 5 females Methylphenidate‐naïve: 100% Ethnicity: 100% Persian Country: Iran Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylpenidate dosage: 1 mg/kg/day Administration schedule: not stated Duration of intervention: 6 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Headaches were observed more often in the methylphenidate group |
|
| Notes | Sample calculation: yes Ethics approval: not stated Funding: the study was supported by a grant from Tehran University of Medical Sciences Vested interests/authors' affiliations: not stated Key conclusions of the study authors: the results suggest that theophylline may be a useful for the treatment of ADHD. In addition, a tolerable side‐effect profile is one of the advantages of theophylline in the treatment of ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no. All participants were newly diagnosed |
|
Mohammadi 2009.
| Methods | 42 days double‐blind, parallel, randomised clinical trial with 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 60 Number of participants randomised to Ritalin: 30; Stimdate: 30 Number of participants followed up in each arm: Ritalin: 24; Stimdate: 30 Number of withdrawals in each arm: Ritalin: 6; Stimdate: 0 Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age (Ritalin): mean 9.21 years (range 6‐15) Age (Stimdate): mean 9.29 years (range 6‐15) Sex (Ritalin): 23 males, 7 females Sex (Stimdate): 22 males, 8 females Methylphenidate‐naïve: not stated Ethnicity: 100% Persian Comorbidity: not stated Comedication: not stated IQ: > 70 Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to Ritalin or Stimdate Methylphenidate type: extended release (Ritalin) or Extended release (Stimdate) Mean methylphenidate dosage: 25 mg/day. Titrated to the highest dose level; 1 mg/kg/day Ritalin or Stimdate, or a maximum of 40 mg/day Administration schedule: orally twice daily 7.30‐8.00 am and 12:00 to 1:00 pm Duration: 42 days Treatment compliance: not stated |
|
| Outcomes | No usable data | |
| Notes | Sample calculation: no Ethics approval: not stated Funding/vested interest: not stated Key conclusions of the study authors: based on the results of this study, no significant difference was observed between the 2 medications, and it seems both drugs behave in a similar way. In addition, Stimdate appears to be effective and well tolerated for ADHD in children and adolescents in Iran Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental data regarding side effects requested from the study authors twice in November 2013 with no reply |
|
Mohammadi 2010.
| Methods | A 6‐week, parallel group, double‐blind, randomised clinical trial with 2 arms:
|
|
| Participants | Number of participants screened: 65 Number of participants included: 40 Number of participants randomised to methylphenidate: 20 Number of participants followed up: 19 Number of withdrawals: 1. Diagnosis of ADHD: DSM‐IV (subtype: combined (100%)) Age: mean 9.25, range 6‐14 years old IQ: > 70 Sex: 13 males, 7 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Iran Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: Ritalin Methylphenidate dosage: 20‐30 mg/day depending on weight (20 mg/day for < 30 kg and 30 mg/day for > 30 kg) Mean methylphenidate dosage. At week 6: 25.50 mg (SD 5.10) Titration period: 3 weeks after randomisation Duration of intervention: 6 weeks Treatment compliance: not stated |
|
| Outcomes | Side effects checklist that comprises 20 side effects including psychic, neurologic, autonomic and other side effects, administered by a child psychiatrist on days 7, 21 and 42 Body weight and vital signs were measured at baseline and weeks 1, 2, 4 and 6 12‐lead ECG and physical examinations were evaluated at baseline and week 6 Haematology tests were collected at baseline and weeks 2, 4 and 6; serum chemistry and urinalysis were evaluated at baseline and week 6 |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding: the study was supported by a grant from Tehran University of Medical Sciences. Vested interests/authors' affiliations: not stated Key conclusions of the study authors: the results of this study indicate that amantadine significantly improved symptoms of ADHD and was well tolerated and it may be beneficial in the treatment of children with ADHD. Nevertheless, the present results do not constitute proof of efficacy Supplemental information requested from the study authors twice in July and August 2013 with no reply |
|
Mohammadi 2012a.
| Methods | An 8‐week double‐blind parallel‐group randomised controlled trial with 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 60 Number randomised to methylphenidate + placebo: 32 and methylphenidate + melatonin: 28 Number followed up in the methylphenidate + placebo group: 24 Number of withdrawals in the methylphenidate + placebo group: 8 Methylphenidate + placebo group Diagnosis of ADHD: ICD‐10 (subtype: combined (100%)) Age: mean 8.83 years old (range 7‐12) IQ: above 70 Sex: 17 males, 7 females Methylphenidate‐naïve: 100% were newly diagnosed Ethnicity: not stated Country: Iran Comorbidity: no Comedication: no Sociodemographics: level of family income: low (58.8%), average (23.6%), high (17.6%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: Ritalin Methylphenidate dosage: 1 mg/kg Administration schedule: not stated Duration of intervention: 8 weeks Treatment compliance: not stated |
|
| Outcomes | Sleep Disturbance Scale for Children (SDSC) sleep questionnaires and appetite questionnaires were completed by mothers at baseline, week 2, 4, and 8 Height, weight were measured by a dietitian at baseline and week 8. Weight was measured with minimal clothing and height without shoes in standard position 3‐day food records were completed by mothers at baseline and week 8 Stimulant drug side effects questionnaires were completed by mothers at week 8 (methylphenidate + placebo, n = 18) |
|
| Notes | Sample calculation: not stated Any withdrawals due to adverse events: not stated Ethics approval: yes Funding/vested interests: the study was financially supported by Research Deputy of Tehran University of Medical Sciences Authors' affiliations: not stated Key conclusions of study authors: melatonin with methylphenidate can partially improve symptoms of sleep disturbance by circadian cycle modification. However, it did not seem to reduce the attention deficiency and hyperactivity behaviour of ADHD children Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental data received through personal email correspondence with the authors in July 2013 (Mohammadi 2013 [pers comm]) |
|
Mohammadi 2012b.
| Methods | A a single‐centre randomised, double‐blind, parallel‐group clinical trial of methylphenidate use for 6 weeks | |
| Participants | Number of patients screened: 53 Number included: 46 Number randomised to methylphenidate: 23, buspirone: 23 Number followed up in each arm: methylphenidate: 20 and buspirone: 20 Number of withdrawals in each arm: methylphenidate: 3 and buspirone: 3 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (100%)) Age: mean: 9.70 (SD 3.18), range: 6‐14 years old IQ: above 70 Sex: 13 males, 7 females Methlphenidate‐naïve: 100% (n = 20) Ethnicity: not stated Country: Iran Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: immediate release (Ritalin) Mean methylphenidate dosage: 20‐30 mg/day depending on weight (20 mg/day for < 30 kg and 30 mg/day for > 30 kg) Administration schedule: not stated Duration of intervention: 6 weeks Treatment compliance: not stated |
|
| Outcomes | Adverse effects were systematically recorded at each visit (baseline, 3 weeks, and 6 weeks) using a checklist (not otherwise specified) that comprised 20 side effects | |
| Notes | Sample calculation: not stated Ethics approval: the study was approved by the Institutional Review Board (IRB) of Tehran University of Medical Sciences (grant No: 8643) Funding/vested interests: this study was supported by a grant from Tehran University of Medical Sciences (grant no: 8643) Any withdrawals due to adverse events: no Authors' affiliations: not stated Key conclusions of the study authors: the results of this study suggest that administration of buspirone has no comparable efficacy in comparison with methylphenidate in the treatment of ADHD. Nevertheless, in our study, those in the buspirone group experienced fewer adverse events than the methylphenidate group in particular regarding decreased appetite, headache and insomnia Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information requested from the authors twice through email correspondence in June 2016. No reply |
|
Montañés‐Rada 2012.
| Methods | An 8‐week prospective, open‐label cohort study of participants receiving 8 hours extended release methylphenidate | |
| Participants | Number of patients screened: 60 Number included: 40 Number followed up: 40 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean: 13.6 years old (range: 10‐16) IQ: above 100 Sex: 30 males, 10 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Spain Comorbidity: ODD: 75%, anxiety disorder: 10%, Gilles de la Tourette syndrome: 2.5%, no comorbidity: 20% Comedication: not stated Sociodemographics: low socioeconomic status: 37.5%, medium socioeconomic status: 50%, high socioeconomic status: 12.5% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: Medikinet (50% extended release and 50% immediate release methylphenidate) Methylphenidate dosage: 40‐50 mg/daily Administration schedule: twice daily. A fixed dose of 30 mg at breakfast (8:30 am) and either 10 mg (n = 34) or 20 mg (n = 6) in the afternoon (3:00 pm) Duration of intervention: 8 weeks (1 month of titration) Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Insomnia, spontaneous reported |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: HB Pharma Key conclusions of the study authors: at week 8, 63% of the participants reached complete remission, and 27.5% reached partial remission. Scores in all subscales of Eyberg, Conners (parents and teachers) were reduced till the level of normal population (except for the conduct subscale of the teacher rated) in a statistical significant level. Due to insomnia, 2 patients reduced the afternoon dose of 50/50‐ER/IR‐MPH from 20 mg to 10 mg and 3 patients changed the 20 mg 50/50‐ER/IR‐MPH afternoon dose to 10 mg IR‐MPH Comments from the study authors: our sample is representative of patients with moderate and severe symptomatology Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: see inclusion criteria 4‐6 Supplemental information regarding ADHD diagnostic criteria and IQ received through personal email correspondence with the authors in November 2013 (Montañes‐Rada 2013 [pers comm]) The publications on this study were received from the pharmaceutical company HB Pharma in June 2013 (Fischer 2013 [pers comm]) |
|
Montiel‐Nava 2002.
| Methods | A 6‐week randomised parallel trial with 2 arms:
No placebo or no‐intervention group |
|
| Participants | Number of participants screened: not stated Number of participants included: 24 Number randomised to methylphenidate: 12 and parent training: 12 Number followed up in methylphenidate‐arm: not stated Number of withdrawals in methylphenidate‐arm: not stated Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 7.16 years old (range: 6‐10) IQ: mean: 92.25 (SD 15.64). Sex: total sample: 16 males, 8 females (not stated for the methylphenidate group) Methylphenidate‐naïve: 100% Ethnicity: Marabinos (from Northwestern Venezuela) Country: Venezuela Comorbidity: academic, oppositional and various behavioural problems Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10 mg/daily at the start of the titration period Administration schedule: morning and noon Duration of intervention: 6 weeks, including 4 weeks of titration (after randomisation) Treatment compliance: not stated |
|
| Outcomes | A checklist of adverse effects were used, telephone interview with parent, once weekly during the 4‐week titration period | |
| Notes | Sample calculation: no Ethics approval: not stated Funding/vested interests: not stated Authors' affiliations: not stated Key conclusions of the study authors: to our knowledge this is the first article describing the effectiveness of methylphenidate and parent training in Venezuelan children diagnosed with ADHD. It should be considered as a preliminary study, that supports the thesis of positive effects of psychosocial and psychopharmacological interventions in children with ADHD. There was no difference in the effectiveness of the 2 types of treatment Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no (only inclusion of methylphenidate‐naïve children) Supplemental information regarding ethics approval, funding, type of methylphenidate, number of males and females in the methylphenidate group, and adverse event data were not possible to receive through personal email correspondence with the authors. Emails sent twice without reply |
|
Moungnoi 2011.
| Methods | A retrospective, descriptive cohort study of methylphenidate use for long‐term administration and its impact on growth at 6 months, 1 years, 2 years, 3 years, 4 years, and 5 years' follow‐up | |
| Participants | Number of participants screened: not stated Number of participants included: 96 Number of participants followed up: 6 participants followed up to 5 years Number of withdrawals: more than 50% by 3rd year of follow‐up Diagnosis of ADHD: DSM‐IV (subtype: combined, 100%) Age: mean 8.62 years old (SD 1.70) IQ: not stated Sex: 75 males, 21 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Thailand Comorbidity: not stated Comedication: not stated, but children who received other medication continuing more than 3 months were excluded Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: short acting methylphenidate Mean methylphenidate dosage: 6 months= 0.44 mg/kg/day, 1 year = 0.48 mg/kg/day, 2 years = 0.48 mg/kg/day, 3 years = 0.49 mg/kg/day, 4 years = 0.41 mg/kg/day and 5 years = 0.42mg/kg/day Administration schedule: not stated Duration of intervention: ≥ 1 year Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Height and weight, observer, beginning of short‐acting methylphenidate medication, 6 months, 1‐, 2‐, 3‐, 4‐, 5‐year follow‐up |
|
| Notes | Sample calculation: yes. Margin of error 5%, estimated prevalence of ADHD 3.5‐5%, CI 95% = sample size 51‐73 Ethics approval: yes, Ethics Committee of the Queen Sirikit National Institute of Child Health Funding/vested interests: none Authors' affiliations: none Key conclusions of the study authors: prolonged medication with short‐acting methylphenidate showed minimal impact on growth only at the first 6 months; however, growth could catch up in the adolescent period Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
Munk 2015.
| Methods | A patient report of cardiac arrest following myocardial infarction during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: ICD‐10 Age: 11 years old IQ: unknown Sex: male Ethnicity: not stated Country: Denmark Comorbidity: Tourette syndrome Comedication: none Sociodemographics: not stated |
|
| Interventions | Methylphenidate type and dosage: 54 mg/day Administration schedule: not stated Duration of treatment: approximately 2 years Treatment compliance: yes |
|
| Outcomes |
Serious adverse events: Cardiac arrest following exercise without any prior complaints about chest discomfort or shortness of breath. A week before the event, he had a short episode of tachycardia. The examination showed that the myocardial infarction was of an older date (more than weeks) due to thinning of the myocardium and an adversely remodeled left ventricle. A pacemaker was inserted and methylphenidate treatment was discontinued |
|
| Notes |
Key conclusions of the study authors: the present case demonstrates that myocardial infarction can happen due to methylphenidate exposure in a cardiac healthy child, without any other cardiovascular risk factors
Comments from the study authors: the boy has been very thoroughly examined and the only thing that stands out is the methylphenidate treatment Funding/vested interest/authors affiliations: the authors declare that there is no conflict of interests regarding the publication of this paper Supplemental information regarding IQ and comedication received through personal email correspondence with the authors in April 2016 (Munk 2016 [pers comm]). Not possible to retrieve information regarding ADHD subtype |
|
Na 2013.
| Methods | A 12‐week, open‐label, multicentre, phase 4 study of osmotic release oral system (OROS) methylphenidate use | |
| Participants | Number of patients screened: not stated Number of participants included: 121 Number of participants followed up: 103 Number of withdrawals: 18 Diagnosis of ADHD: DSM‐IV (subtype: combined (17.4%), hyperactive‐impulsive (0.8%), inattentive (70.2%)) Age: mean: 13.8 years old (range: 12‐18) IQ: above 70 Sex: 93 males, 28 females Methlphenidate‐naïve: not stated Ethnicity: Korean Country: Korea Comorbidity: oppositional defiant disorder: 24%, conduct disorder: 5%, tic disorder: 4.1%, depressive disorder: 4.1%, social phobia: 1.7%, generalised anxiety disorder: 2.5%, enuresis: 1.7%, encopresis: 1.7%) Comedication: no Sociodemographics not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic‐release oral system (OROS) Methylphenidate dosage: dose titration was allowed for up to 6 weeks (starting doses were 18 mg/day (if body weight < 30 kg) and 27 mg/day (if body weight equal to or higher than 30 kg), maximum allowed daily dose was 72 mg or 1.4 mg/kg) Mean methylphenidate dose at endpoint: 54.53 (SD 12.33, range 27‐72) mg/day or 0.99 (SD 0.21) mg/kg/day Administration schedule: once a day, between 6:30 and 9:00 am Duration of intervention: 12 weeks Washout before study initiation: ≥ 6 months Treatment compliance: not stated, but participants were required to take ≥ 70% of the study medication during the study period |
|
| Outcomes |
Non‐serious adverse events: Vital signs, body weight, and adverse events (both by general inquiry to patients and parents and by clinician rated checklist) were measured at every visit (day 0 (baseline) and at weeks 1, 3, 6, and 12) The study‐specific adverse events checklist consisted of 61 methylphenidate‐specific questions (Items from the Symptom Rating Scale (Barkley 1990) and the Pittsburg Side‐Effects Rating Scale (Pelham 1993) were included) Chemistry tests were performed at screening and at week 12 |
|
| Notes | Sample calculation: not stated Ethics approval: yes, the study was approved by the institutional review board of each study site Funding: this study was supported by grants from the Johnson & Johnson family of companies. The Johnson & Johnson family of companies was involved in the study design and approved the report Vested interests: disclosure statement "No competing financial interests exist". Key conclusions of the study authors: OROS methylphenidate was effective in enhancing learning skills in adolescents with ADHD. Furthermore, clinicians should supplement the subjective report on adverse events from patients or their parents with a more drug‐specific checklist to obtain drug side effects more effectively. As there are some differences in the patterns of adverse events reported by patients and their parents, it is generally recommended that clinicians obtain information from both parties when possible Comments from the review authors: safety data comparing patients, parents and clinicians as raters from Lee 2013 concerns only 47 participants, whereas efficacy data and supplemental safety data from Na 2013 concerns 121 participants Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, see exclusion criteria no. 1 (hypersensitivity to methylphenidate HCL) Supplemental information regarding safety data received through personal email correspondence with the authors in August 2014 (Lee 2014 [pers comm]) |
|
Niederhofer 2009.
| Methods | A patient report of dosage reduction of methylphenidate treatment by addition of atomoxetine. Adverse effects of methylphenidate are reported | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 11 years old IQ: 97 Sex: male Ethnicity: not stated Country: Italy Comorbidity: not stated Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 20 mg/day Administration schedule: not stated Duration of intervention: 2 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Methylphenidate 20 mg/day: depressed mood and appetite loss Dosage‐reduction to 10 mg/day: improved the depressed mood |
|
| Notes |
Key conclusions of study authors: the reported case mainly shows that the combination between atomoxetine and methylphenidate helped reducing the dose of methylphenidate and thus, probably the side effects while showing beneficial clinical effects Funding/vested interests/authors' affiliations: not stated Supplemental information regarding ADHD type received through personal email correspondence with author in July 2013 (Niederhofer 2013 [pers comm]) |
|
Niederhofer 2011.
| Methods | A patient series describing adverse effects (xerostomia, aggression and emotional instability) among children taking methylphenidate | |
| Participants |
Case with xerostomia, n = 1 Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 11 years old IQ: 97 Sex: male Ethnicity: not stated Country: Italy Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Cases with aggression (n = 1) and emotional instability (n = 1) Diagnosis of ADHD: DSM‐IV (subtype: combined) Ages: 10 and 11 years old IQ: above 70 Sex: 2 males Ethnicity: not stated Country: Italy Comorbidity: no Comedication: not stated Sociodemographics: not stated |
|
| Interventions |
Case with xerostomia Methylphenidate type: not stated Mehylphenidate dosage: 40 mg/day Administration schedule: not stated Duration of intervention: 1 week Treatment compliance: not stated Cases with aggression and emotional instability Methylphenidate type: not stated Methylphenidate dosage: 20 mg Administration schedule: not stated Duration of intervention: 2 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Xerostomia, aggression and emotional instability |
|
| Notes |
Key conclusions of study author: methylphenidate is first‐line agent for ADHD, if monotherapy is sufficient Funding/vested interests/authors' affiliations: none declared Supplemental information regarding ADHD subtype and duration of intervention received through personal email correspondence with author in July 2013 (Niederhofer 2013b [pers comm]) |
|
Nymark 2008.
| Methods | A patient report of serious cardiomyopathy during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: ICD‐10 (subtype: not stated) Age: 18 years old IQ: above 70 Sex: male Ethnicity: not stated Country: Norway Comorbidity: obesity (BMI = 40) Comedication: quetiapine fumarate (900 mg/day) for 17 months Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: extended release (Concerta) Methylphenidate dosage: 54 mg/day Administration schedule: once daily Duration of intervention: 11 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: 11 months of MPH treatment: hypoxia and dyspnoea. On investigation, signs of liver‐, renal‐, and heart‐failure were found. Noradrenalin infusion was started. Echocardiography showed dilated left ventricle and an ejection fraction (EF) of 25%. Liver function improved, noradrenalin and dobutamine were tapered, but 3 days after admission a new echocardiography showed an EF of 10%. Intensified treatment including intra‐aortic balloon pump (IABP) was instituted. Cardiac function improved, and 3 weeks later the IABP was disconnected. EF at this point was 15%. The patient was denied heart transplantation due to various cofactors. 7 months later: his clinical status was improved, function class II (New York Heart Association) with an EF estimated by echocardiography to 30%‐35% |
|
| Notes | Funding/vested interests/authors' affiliations: not stated Key conclusions of study authors:the investigation concluded with a probable relationship between the patient's cardiomyopathy and the use of methylphenidate |
|
Park 2013.
| Methods | A cohort study of methylphenidate use for 2 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 96 Number of participants followed up: 96 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (62.5%), hyperactive‐impulsive (5.2%), inattentive (27.1%), NOS (5.2%)) Age: mean: 8.70 (SD 1.41) years old (range: 6‐12) IQ: not stated Sex: 79 males, 17 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Korea Comorbidity: tic disorder: 10.4%, anxiety disorder: 8.3%, oppositional defiant disorder: 9.4%, enuresis: 6.3%, depression: 1.0% Co‐medication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) methylphenidate Methylphenidate dosage: children weighing less than 30 kg were treated with 18 mg per day, and children weighing more than 30 kg were treated with 27 mg/day Administration schedule: not stated Duration of intervention: 2 weeks Treatment compliance: not stated |
|
| Outcomes | Barkley Symptom Rating Scale: a 17‐item list of methylphenidate‐related adverse event symptoms, rated from 0 (nothing) to 9 (severe) for symptoms during the past 2 weeks. Any negative sign, symptom, syndrome, or new illness that appeared or worsened after treatment began was counted as a treatment‐emergent adverse event | |
| Notes | Sample calculation: no Ethics approval: approved by the institutional review board (IRB) for human subjects at the Seoul National University Hospital and other hospitals Funding/vested interests: the authors declare that there are no conflict of interest. The study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111523) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Republic of Korea (20110023888) Authors' affiliations: Seoul National University College of Medicine Key conclusions of the study authors: ADHD participants with the A/a genotype at the NTF3 rs6332 polymorphism showed the highest 'proneness to crying' and 'nail biting' item scores, followed by those with the G/a genotype and those with the G/G genotype (P = 0.047 and P = 0.017, respectively). These data provide preliminary evidence that genetic variation in the NTF3 gene is related to susceptibility to emotional side effects in response to methylphenidate treatment in Korean children with ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Perera 2010.
| Methods | This study prospectively analysed an outpatient treatment programme for children with ADHD. Methylpenidate use for 6 months | |
| Participants | Number of participants included: 102 Diagnosis of ADHD: DSM‐IV (subtype: combined (100%)) Age: mean 6.92 years old (range 4‐12) IQ: above 90 Sex: 90 males,12 females Ethnicity: not stated Country: Sri Lanka Comorbidity: oppositional defiant disorder, conduct disorder, specific learning disorder and tic disorder (numbers not stated) Comedication: 19 (18.3%) children were also taking other medication on a regular basis. These were anticonvulsants in 15 (14.4%) and anti‐asthmatic drugs in 4 (3.9%) Sociodemographics: not stated Inclusion criteria: Data used for this article is confined to those children who consistently attended clinics and completed 6 months of treatment/intervention Exclusion criteria:
|
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: 2.5‐40 mg daily Administration schedule: not stated Duration of intervention: 6 months Treatment compliance: data used for this article is confined to those children who consistently attended clinics and completed 6 months of treatment |
|
| Outcomes |
Non‐serious adverse events: A 7 item checklist of known side effects of methylphenidate. Parents responded by indicating 'present' or 'absent', which was recorded on a weekly basis, in the first 4 weeks. Loss of appetite, social withdrawal, restlessness, abdominal pain, headache, poor sleep, and sadness |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interest: no Authors' affiliations: none Key conclusions of the study authors: close involvement of parents in monitoring outcome of treatment of ADHD helps to focus on aspects of care relevant to them Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, children with ADHD found to be unsuitable for treatment with methylphenidate were excluded Supplemental information regarding IQ, comorbidity and methylphenidate dosage received through personal email correspondence with the authors in June 2016 (Perera 2016 [pers comm]) |
|
Peyre 2012a.
| Methods | A non‐randomised longitudinal study of methylphenidate use for up to 3 months | |
| Participants | Number of participants screened: not stated Number of participants included: 173 Number of participants followed up: 136 Number of withdrawals: 37 Diagnosis of ADHD: DSM‐IV (subtype: combined (69.12%), hyperactive‐impulsive (8.09%), inattentive (22.79%)) Age: mean 10.74 (SD 2.74) IQ: > 70 Sex: 90% males, 10% females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: France Comorbidity: conduct disorder (7.35%); ODD (50%); anxiety (32.35%); major depressive (22.06%); bipolar (1.47%); learning (33.09%) Comedication: none Sociodemographics: not stated Inclusion criteria Children and adolescents younger than 18 years who were eligible for MPH treatment for DSM‐IV ADHD and had parents able to speak and understand French. A subsample with complete data were re‐evaluated after optimal adjustment of MPH Exclusion criteria Mental retardation (IQ < 70) and chronic neurological disease |
|
| Interventions | Methylphenidate type: multiple‐dose immediate release or prolonged‐action once‐daily formulations (Ritaline LAR or ConcertaR Mean methylphenidate dosage: 0.82 mg/kg/day (SD 0.22) Administration schedule: not stated Duration of intervention: 15 days to 3 months Treatment compliance: not stated |
|
| Outcomes | Adverse effects were elicited through systematic questions (first about adverse events in general and then with a list of frequent adverse events) and medical chart review | |
| Notes | Sample calculation: no Ethics approval: local ethics committee (Comite de Protection des Personnes Pitie‐Salpetriere) Funding: supported by grants from a Programme Hospitalier de Recherche Clinique (PHRC AOR 03006) and the Institut National de la Sante et de la Recherche Medicale (INSERM AAP‐RRC‐09). Vested interests: the author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of the article Key conclusions of the study authors: Child Behavior Checklist‐Dysregulation Profile (CBCL‐DP) was associated neither with poorer response to methylphenidate nor with more side effects. There were no differences in cognitive performance between participants with and without CBCL‐DP |
|
Pierce 2010.
| Methods | An open‐label, randomised, multicentre study evaluating the pharmacokinetic properties of dl‐methylphenidate after single, multiple fixed, and escalating doses of MTS (methylphenidate transdermal system) and osmotic release oral sytem (OROS) methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 71 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Children Number randomised to OROS: 11 Number randomised to MTS: 24 Number of withdrawals: 0 Age: mean 9.5 years (range 6‐12) IQ: not stated Sex: 19 males, 16 females Methylphenidate‐naïve: not stated Ethnicity: white (25.7%), African American (74.3%) Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Adolescents Number randomised to OROS: 11 Number randomised to MTS: 25 Number of withdrawals: 0 Age: mean 14.1 years (range 13‐17) IQ: not stated Sex: 19 males, 17 females Methylphenidate‐naïve: not stated Ethnicity: white (47.2%), African American (52.8%) Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate transdermal system dosage: 10 mg in single dose phase and 15 mg, 20 mg, or 30 mg in the multiple escalating phase Osmotic release oral system methylphenidate dosage: 18 mg in single dose phase and 27 mg, 36 mg, and 54 mg in the multiple escalating phase Administration schedule: once daily Duration of intervention: 4 weeks for the whole trial, with the 4 different phases having varying duration Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events
|
|
| Notes | Sample calculation: no Ethics approval: approved by the institutional review board Funding: Shire Vested interests/authors' affiliations: Dr Pierce is an employee of Shire Pharmaceutical Development Ltd. Dr Katic receives or has received research support, acted as a consultant, and/or served on a speakers' bureau for Forest, GlaxoSmithKline, Lundbeck, Merck, Novartis, Sanofi‐Aventis, Sepracor, Shire, Somerset, and Wyeth. Ms Buckwalter is an employee of Shire Pharmaceuticals Inc. Mr Webster at the time of the study was an employee of Aptuit Ltd, a contract research organisation subcontracted to conduct work on behalf of Shire Pharmaceutical Development Ltd. Key conclusions of the study authors: overall, after single and multiple fixed or escalating doses of MTS or OROS methylphenidate, systemic exposure to the predominantly active d‐methylphenidate was greater in children compared with adolescents. As a result of drug accumulation on repeated dosing, systemic exposure to d‐methylphenidate in children after multiple escalating doses was 1.4‐ to 1.6‐fold higher for MTS compared with OROS; however, in adolescents, systemic exposure was similar for both formulations. Plasma concentrations of l‐MPH were approximately half those of d‐MPH across both age groups after single and multiple doses of MTS. In contrast, plasma concentrations of l‐MPH were negligible after single and multiple doses of OROS MPH. Reported AEs included those that are typical for oral methylphenidate treatment, with the exception of generally mild application site irritations associated with transdermal delivery of methylphenidate Comments from the study authors: no participants exhibited a DRS score higher than 2 (definite erythema, readily visible minimal oedema, or minimal popular response) immediately before the removal of the patch after 1 or 28 days. No participant had an experience of discomfort and pruritus scale score higher than 1 (mild discomfort) Comments from the review authors: it was the investigator who decided whether an adverse events was clinically significant or not Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes |
|
Polanczyk 2007.
| Methods | A pharmacogenomic study of children and adolescents consecutively evaluated during 2 years in the ADHD outpatient clinic | |
| Participants | Number of participants screened: 457 Number of participants included: 137 Number of participants followed up: 106 Number of withdrawals: 31 Diagnosis of ADHD: DSM‐IV (subtype: combined (58.5%), hyperactive‐impulsive (5.7%), inattentive (26.4%)) Age: mean 10.3 years old (range: not stated) IQ: mean: 100.4 Sex: 82 males, 24 females Methylphenidate‐naïve: 100% Ethnicity: European‐Brazilian (100%) Country: Brazil Comorbidity: conduct disorder (16.0%), oppositional defiant disorder (51.9%), mood disorder (9.4%), anxiety disorder (23.6%) Comedication: yes (9.4%) Sociodemographics: not stated Inclusion criteria:
Exclusion criteria: Not stated |
|
| Interventions | Methylphenidate type: immediate release Mean methylphenidate dosage: 0.5 mg/kg (baseline) and 0.65 mg/kg at 1 month Administration schedule: 8 am and 12 pm, with extra dose at 5‐6 pm for those needing evening coverage. Dosages of short‐acting methylphenidate were augmented until no further clinical improvement was detected or until there were limited adverse effects Duration of intervention: 3 months (1 month for 106 participants, 3 months for 89) Treatment compliance: data were excluded from 2 children because of irregular use of methylphenidate |
|
| Outcomes | Barkley Side Effect Rating Scale measured by child psychiatrists at baseline,1 and 3 months. Data not reported | |
| Notes | Sample calculation: no
Ethics approval: yes
Funding/vested interests: the ADHD program received research support from the pharmaceutical companies: Bristol Myers‐Squibb, Eli Lilly and Company, Janssen‐Cilag, and Novartis Pharmaceuticals. The study was supported by grant 471761/03‐6 from Conselho Nacional de Desenvolvimento Cientıfico e Tecnologico, Programa de Apoio a Núcleos de Excelência, and Hospital de Clínicas de Porto Alegre Key conclusions of the study authors: the study documented the effect of the G allele at the ADRA2A ‐1291 C > G polymorphism on the improvement of inattentive symptoms with methylphenidate treatment in children and adolescents with ADHD. Our findings provide clinical evidence for the involvement of the noradrenergic system in the modulation of methylphenidate action Comments from the study authors: regarding adverse events, a mixed‐effects model analysis demonstrated effects of treatment over time on the Barkley Side Effect Rating Scale scores, as expected (n = 106; F2,201.2=5.4; P=.005). However, neither an effect for the presence of the G allele (n = 106; F1,107.6=0.15; P=.69) nor an interaction effect between the presence of the G allele and treatment over time (n = 106; F2,201.2=0.71; P=.49) on the Barkley Side Effect Rating Scale scores was found during the 3 months of methylphenidate use Comments from the review authors: no data presented on side effects apart from the paragraph above Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
Porfirio 2011.
| Methods | A patient report of visual hallucinations during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 7 years at diagnosis, 11 years at occurrence of hallucinations IQ: above 70 Sex: male Ethnicity: not stated Country: Italy Comorbidity: K‐SADS‐PL excluded any psychiatric comorbidities (at age 11) Comedication: not stated Sociodemographics: mother presented with affective seasonal disorder and binge eating disorder |
|
| Interventions | Methylphenidate type: oral immediate‐release Methylphenidate dosage: 0.5 mg/kg twice daily (30 mg/day) Duration of treatment: 3 years with discontinuation of medication during summer time each year Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: 3 years after start of MPH treatment: an episode of complex visual hallucinations (dramatic scenes appearing before going to sleep, sometimes during the day after methylphenidate‐ingestion). Normal physical and neurological examinations. Standard laboratory work‐up and drug screening were within normal limits. Normal visual acuity. Sleep electroencephalography (EEG) revealed no abnormalities and no evidence of epileptic activity. K‐SADSPL excluded any psychiatric comorbidities Discontinuation: complete resolution of hallucinations 24 months follow‐up period: no further hallucinations occurred |
|
| Notes |
Key conclusions of the study authors: although the pathogenetic mechanism is unclear, the occurrence of hallucinations may be explained by a chronic increase in synaptic dopamine. Clinicians should be aware of this possible rare adverse manifestation occurring at therapeutic doses Comments from the study authors: because methylphenidate is a widely used, well studied and safe pharmacological agent, clinicians who prescribe methylphenidate should be aware of this possible rare adverse manifestation occurring at therapeutic doses Funding/vested interest/authors' affiliations: not stated Supplemental information regarding IQ received through personal email correspondence with the authors in October 2013 (Curatolo 2013 [pers comm]) |
|
Poulton 2003.
| Methods | A retrospective cohort study of growth data | |
| Participants | Number of participants screened: not stated Number of participants included: 19 Number of participants followed up: 6 months: 19, 18 months: 13, 30 months: 6, 42 months: 4 Number of withdrawals: 6 months: 0, 18 months: 6, 30 months: 13, 42 months: 15 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: range 3.1‐11.4 years old IQ: > 70 Sex: 17 males, 2 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Australia Comorbidity: not stated Comedication: clonidine Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10‐40 mg/day Mean methylphenidate dosage: 1.0 (SD 0.24) mg/kg per day, median 27.5 mg Administration schedule: not stated Duration of intervention: 6‐42 months Treatment compliance: many patients were taking less than the prescribed dose of stimulant medication, ceasing or reducing medication at weekends or during school holidays, but precise details were not available |
|
| Outcomes |
Non‐serious adverse events:
Both measures without shoes and outdoor clothing |
|
| Notes | Sample calculation: no
Ethics approval: no
Funding/vested interest: none Key conclusions of the study authors: stimulant medication is associated with a decrease in height and weight SD scores during the first 6‐30 months with a characteristic pattern on the growth chart Comments from the study authors: all children were started on dexamphetamine initially but were changed on to methylphenidate if the response was suboptimal or they experienced adverse effects. When weight loss occurred the parents were asked to reduce or omit the medication whenever possible and to encourage the child to eat more Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding data received through personal email correspondence with the authors in November 2013 (Poulton 2013 [pers comm]) |
|
Poulton 2012.
| Methods | A clinic‐based, prospective, cohort study with up to 3 years of follow‐up | |
| Participants | Number of participants screened: not stated Number of participants included: 21 Number of participants followed up: 19 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: combined (76%), inattentive (19%), hyperactive (5%)) Age: mean 7.54, range 4.99‐9.04 years old IQ: > 70 Sex: 18 males, 3 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Australia Comorbidity. not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: 24.3 mg/day (6.2 mg/day) at 6 months Administration schedule: not stated Duration of intervention: up to 36 months Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events: Growth: height, weight, and BMI z scores measured at baseline, 6 months, 18 months, 30 months. All measurement were made without shoes or outdoor clothing Height was measured to the nearest 1 mm using a wall mounted stadiometer Weight was measured to the nearest 0.1 kg using electronic scales BMI were corrected for age and sex by conversion to z scores based on Centers for Disease Control and Prevention (CDC) reference data | |
| Notes | Sample calculation: yes
Ethics approval: yes
Funding: the research was supported by the Australian Woman and Children's research Foundation (OZWAC) and by the Nepean Medical Research Foundation
Vested interests/authors' affiliations: the authors declare that they have no competing interests
Key conclusions of the study authors: stimulant medication was associated with early fat loss and reduced bone turnover. Lean tissue including bone increased more slowly over 3 years of continuous treatment than would be expected for growth in height. There was long‐term improvement in the proportion of central fat for height. This study shows that relatively minor reductions in weight on stimulant medication can be associated with long‐term changes in body composition. Further study is required to determine the effects of these changes on adults
Comments from the review authors: the study describe children both on dexamphetamine treatment and methylphenidate treatment. We have received separate data on the methylphenidate group from the study authors Exclusion of MPH non‐responders/children who have previously experienced adverse events on MPH: no Supplemental information regarding data received through personal email correspondence with the authors in October 2013 (Poulton 2013b [pers comm]) |
|
Ramasamy 2014.
| Methods | A patient report of testicular failure possibly associated with chronic use of methylphenidate | |
| Participants | Diagnosis of ADHD: DSM III‐R Age: 20 years old IQ: an exact number cannot be discerned but it is likely that the participant exhibits a normal IQ without any sort of disability Sex: male Ethnicity: Latino Country: USA Comorbidity: none Comedication: none Sociodemographics: family history was unremarkable |
|
| Interventions | Methylpenidate type: not stated Methylphenidate dosage: varied with age Administration schedule: not stated Duration of treatment: approximately 17 years with voluntary cessation a few years ago Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: The patient's complaint was initially delayed puberty. He complained of high‐pitched voice, lack of libido, low energy level, chronic fatigue and poor erectile function. The results of laboratory tests were consistent with the patient's idiopathic testicular failure and warranted further exploration of the link between chronic methylphenidate use and effects on reproductive parameters |
|
| Notes |
Key conclusions of the study authors: the patient described in our case study seemed to exhibit characteristics related to the effects of chronic use of methylphenidate on development of human reproductive function. The unknown effects of methylphenidate are currently being studied, but as can be seen, one should exercise caution and patients should be followed closely when prescribing methylphenidate Comments from the study authors: because the developmental changes that occurred in the participant occurred over a number of years of treatment with methylphenidate, there is very little information about the patient's condition and how it developed during that period of time Comments from the review authors: this case has been included although the patient is 20 years old since he had been taking methylphenidate for approximately 17 years Funding/vested interest/authors' affiliations: no competing interests were disclosed Grant information: Ranjith Ramasamy is an NIH K12 Scholar supported by a Male Reproductive Health Research Career (MHRH) Development Physician‐Scientist Award (HD073917‐01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program Supplemental information regarding ADHD diagnosis and type, comorbidity and comedication received through personal email correspondence with the authors in April 2016 (Pranav 2016 [pers comm]) |
|
Rappaport 2004.
| Methods | A patient report of side effects during methylphenidate treatment in a 14‐year‐old boy with ADHD and a number of other co‐morbid conditions | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 14 years old IQ: 80‐85 Sex: male Ethnicity: Latino Country: USA Comorbidity: PTSD, bipolar disorder, attachment disorder, language‐based learning disorder Comedication: olanzapine Sociodemographics: working single mother, sporadic contact with father. Patient and his siblings were intermittently exposed to domestic violence |
|
| Interventions | Both IR and ER MPH tried dosage: not stated Administration schedule: not stated time points: not stated Duration of treatment: not stated Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events: While taking immediate release methylphenidate (Ritalin): loss of appetite While taking extended release methylphenidate (Concerta): nausea and vomiting | |
| Notes | Key conclusions of the study authors: the patient's course and symptoms are illustrative of the maladaptive sequelae of untreated ADHD, complicated by a language‐based learning disorder. Funding/vested interest/authors' affiliations: not stated Supplemental information regarding ADHD diagnosis and IQ received through personal email correspondence with the authors in November 2013 (Rappaport 2013 [pers comm]) | |
Rapport 1996.
| Methods | Patient report of 2 6‐year‐old dizygotic twins with ADHD and ODD treated with methylphenidate | |
| Participants | ADHD diagnosis: DSM‐III‐R at time of examination. Authors state that both would have achieved the then new DSM‐IV diagnoses of ADHD combined type at the time of publication. Age: 6 years old IQ: 100 and 93 Sex: 2 girls, dizygotic twins Ethnicity: mixed white/Japanese Country: USA Comorbidity: ODD (100%) Comedication: not stated Sociodemographics: low socioeconomic status |
|
| Interventions | Methylphenidate type: immediate release methylphenidate Methylphenidate dosage: 4 doses: 5 mg (0.29 mg/kg), 10 mg (0.58 m/kg), 15 mg (0.87 mg/kg), 20 mg (1.16 mg/kg), placebo. Both children received each of the 4 methylphenidate doses Administration schedule: administration "was intentionally counterbalanced to allow for direct contrasts between no‐dose and moderate dose conditions (placebo vs 10 mg), low and high dose conditions (5 mg vs 15 mg) and identical high dose conditions (20 mg) between the 2 children Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes | None of the active treatment conditions (methylphenidate and attentional training) resulted in higher frequency or greater severity of complaints compared to baseline Non‐serious adverse events: Measures: Side Effects Rating Scale (SERS)
|
|
| Notes | Funding/vested interest: not stated Key conclusions of the study authors: brief mention should be made of the relative absence of emergent symptoms found in the present study. Although side effects are frequently reported in children undergoing psychostimulant trials (often as a function of increasing dosage), severity is typically mild, and it is advisable to gauge both frequency and severity against a placebo control owing to the high base rate of physical complaints reported by children with ADHD |
|
Rashid 2007.
| Methods | A patient report of intensified somatic hallucinations during dose increase of methylphenidate treatment at the hospital | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: unknown) Age: 10 years old IQ: > 70 Sex: male Ethnicity: unknown Country: USA Comorbidity: chronic pattern of somatisation Comedication: unknown Methylphenidate‐naïve: no. OROS methylphenidate, 36 mg/day, ≥ 2 years Sociodemographics: history of several foster placements, now adopted |
|
| Interventions | Methylphenidate dose: regular‐release methylphenidate, 10 mg, twice daily, then titrated to regular‐release methylphenidate, 15 mg, twice daily, 2 days Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Hallucinations Regular‐release methylphenidate, 10 mg, twice daily Regular‐release methylphenidate, 15 mg, twice daily, 2 days: somatic hallucinations Discontinuation of methylphenidate: complete resolution of the psychotic phenomena within 2 days Follow‐up 1 year later: no unexplained somatic complaints |
|
| Notes | Funding/vested interest: no financial relationships to disclose Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: here we describe a case of a 10‐year‐old boy with ADHD with a chronic pattern of somatisation, which evolved into overt somatic hallucinations with an increase in the methylphenidate dose. This pattern of somatisation was retrospectively recognised as partial somatic hallucinations Supplemental information regarding ADHD diagnostic criteria and IQ received through personal email correspondence with the authors in October 2013 (Rashid 2013 [pers comm]) |
|
Remschmidt 2005.
| Methods | A 21‐day multicentre, open‐label study of osmotic release oral system (OROS) methylphenidate in children and adolescents with ADHD switched from immediate release (IR) methylphenidate followed by a 12‐month follow‐up | |
| Participants | Number of participants screened: not stated Number of participants included in the main study: 105 Number of participants followed up: 101 Number of withdrawals: 4 Number of participants included in the follow‐up: 89 Number of participants followed up: 56 Number of withdrawals: 33 Diagnosis of ADHD: DSM‐IV (subtype: combined (69.5%), hyperactive‐impulsive (7.6%), inattentive (22.8%)) Age: range 6‐16 years old IQ: not stated Sex: 90 males, 15 females Methylphenidate‐naïve: none Ethnicity: not stated Country: UK and Germany Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria:
|
|
| Interventions | Methylpenidate type: osmotic release oral system Methylphenidate dosage: 18 mg, 36 mg, or 54 mg. 11.9% received 18 mg; 54.4% received 36 mg, and 33.7% received 54 mg at the end of the 21‐day main study Administration schedule: once daily, morning Duration of intervention: main study: 21 days, follow‐up: 12 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Reports of any adverse events, sleep and appetite patterns, and tics were reported by parents/caregivers up to day 21, and at months 2, 4, 6, 8, 10 and 12 |
|
| Notes | Sample calculation: not stated Ethics approval: independent Ethics Committees in each country reviewed the study protocol Funding/vested interest: this company‐initiated study was supported by a grant from Janssen‐Cilag GmbH Key conclusions of the study authors: children and adolescents can effectively and safely be switched from IR‐methylphenidate to OROS‐methylphenidate with improved symptom control and compliance Comments from the study authors: current international guidelines recommend the use of long‐acting stimulant preparations over short‐acting stimulants for the management of ADHD. The results of this study provide further support for this recommendation. These data cannot necessarily be generalised to unselected children and adolescents in clinical practice who may not be responsive to methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information requested from the review authors in June 2014 with no reply |
|
Ririe 1997.
| Methods | A patient report on unexpected interaction of methylphenidate (Ritalin) with anaesthetic agents | |
| Participants | Diagnosis of ADD/ADHD: DSM‐IV (subtype: not stated) Age: 6 years old IQ: > 70 Sex: male Ethnicity: not stated Country: USA Comorbidity: history of William's syndrome and supravalvar aortic stenosis Comedication: only during anaesthesia: midazolam 20 mg + 10 mg, ketamine 60 mg, Glycopyrrolat IV 0,1 mg, midazolam IV 5 mg Sociodemographics: living at home with parents |
|
| Interventions | Immediate release methylphenidate (Ritalin) dosage: 10 mg/day Administration schedule: twice daily Duration of treatment: 2 months Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events: Difficulty with conscious sedation. No previous problems with sedation. Despite additional oral doses of sedatives the child remained alert and unable to lie still, until intravenous sedative drugs were given. After discharge, the child developed nausea, vomiting, lethargy and dehydration, which led to hospitalisation | |
| Notes | Funding/vested interest/authors' affiliations: Novartis Key conclusions of the study authors: there could be a potential risk of unwanted interactions between methylphenidate and anaesthetic agents. Based on the observations from the patient report, a more detailed and systematic investigation of methylphenidate in patients undergoing anaesthesia or sedation is warranted Comments from the study authors: no previous problems with anaesthesia. The child had no apparent limitations of activity and no cardiac symptoms. We believe that the stimulant effect of methylphenidate may have played a major role in antagonising the sedative effect of the oral midazolam in the clinically recommended doses. The stimulant effect of methylphenidate makes it potentially very difficult to attain a desirable level of sedation without requiring large and potentially unsafe doses of sedative drugs or administration of general anaesthesia. The gastrointestinal side effects of methylphenidate, particularly decreased appetite and nausea and vomiting, may be aggravated by many of the drugs used for sedation or general anaesthesia Supplemental information regarding intellectual function and diagnostic criteria received through personal email correspondence with the authors in October 2013 (Ririe 2013 [pers comm]) |
|
Sabuncuoglu 2007.
| Methods | 3 patient reports of hyperactivity and irritability during switch from risperidone to methylphenidate | |
| Participants |
Case 1 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 5 years old IQ: no developmental delay Sex: male Ethnicity: not stated Country: Turkey Comorbidity: ODD Comedication: none Sociodemographics: not stated Case 2 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 6 years old IQ: 70‐90 Sex: female Ethnicity: not stated Country: Turkey Comorbidity: borderline intellectual functioning Comedication: none Sociodemographics: not stated Case 3 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 15 years old IQ: > 90 Sex: female Ethnicity: not stated Country: Turkey Comorbidity: ODD and learning disorder Comedication: none Sociodemographics: not stated |
|
| Interventions |
Case 1: immediate release methylphenidate dosage: 15 mg/day. Administration schedule: not stated. Duration of treatment: not stated. Treatment compliance: not stated Case 2: immediate release methylphenidate dosage: 15 mg/day. Administration schedule: not stated. Duration of treatment: not stated. Treatment compliance: not stated Case 3: immediate release methylphenidate dosage: 18 mg/day. Administration schedule: once, morning. Duration of treatment: not stated. Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Case 1: methylphenidate introduced 2 days after abrupt discontinuation of risperidone 1 mg/day (8 months) and produced agitation, irritability, vigilance, and violent behaviour. No dyskinetic movements. Methylphenidate was discontinued and the adverse events disappeared. After a 6‐week long drug‐free period, methylphenidate was reintroduced at 15 mg/day. No adverse events were observed Case 1: methylphenidate introduced after discontinuation of risperidone 1 mg/day (2 years) and produced a near manic state, irritability, agitation, racing thoughts, and distractibility. No dyskinetic movements. Methylphenidate was discontinued and the adverse events disappeared. After a couple of months methylphenidate was reintroduced at 15 mg/day and was well‐tolerated Case 1: methylphenidate introduced after a 1‐week medication free interval after treatment with risperidone 1 mg, and produced irritability, agitation and discomfort. Methylphenidate was discontinued and the adverse events disappeared. The patient did not retry the medication |
|
| Notes | Funding/vested interest/authors' affiliations: not stated Key conclusions of the study authors: this report of 3 patients draws attention to a unique condition that arises in switching from atypical antipsychotic to stimulant medication. A drug‐free interval is recommended in switching from risperidone to methylphenidate Comments from the study authors: the severity of adverse events correlated well with methylphenidate administration, ceased upon discontinuation of treatment and, interestingly, readministration of methylphenidate after an interval was associated with no adverse events in the first and second patient Supplemental information regarding IQ received through personal email correspondence with the authors in October 2013 (Sabuncuoglu 2013 [pers comm]) |
|
Sahin 2014.
| Methods | A study of methylphenidate use for 2 months | |
| Participants | Number of patients screened: not stated Number of participants included: 30 Number of participants followed up: 30 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: combined (46.7%), hyperactive‐impulsive (10%), inattentive (43.3%)) Age: mean 9.54 (SD 2.83), range 6‐18 years old IQ: > 70 Sex: 24 males, 6 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Turkey Comorbidity: disruptive behaviour disorder (DBD): 17, anxiety disorder: 5, learning disability: 3, enuresis/encopresis: 3, tic disorder: 2 Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Mean methylphenidate dosage: 0.70 (SD 0.20) mg/kg/day (0.64 (SD 0.16) mg/kg/day in the 1st month and 0.76 (SD 0.25) mg/kg/day in the 2nd month) Administration schedule: not stated Duration of intervention: 2 months Treatment compliance: 100% |
|
| Outcomes |
Non‐serious adverse events:
|
|
| Notes | Sample calculation: no Ethics approval: yes Funding: sponsored by Ondokuz Mayis University project number PYO.TIP.1904.12.013. Vested interests/authors' affiliations: none of the authors report conflicts of interest Key conclusions of the study authors: leptin and brain‐derived neurotrophic factor (BDNF) were not associated with poor appetite and/or weight loss due to methylphenidate treatment. However, ghrelin and adiponectin might be biomolecules that play a role in underlying neurobiological mechanisms of methylphenidate‐related appetite or weight loss. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Saieh 2004.
| Methods | A patient report of hospitalisation due to hypertension during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 8 years old IQ: normal Sex: male Ethnicity: not stated Country: Chile Comorbidity: not stated Comedication: not stated Sociodemographics: maternal family history of hypertension |
|
| Interventions | Methylphenidate type: Ritalin Methylphenidate dosage: 0.32 mg/kg Administration schedule: not stated Duration of treatment: 6 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: 72‐hour hospitalisation, emergency unit: Abdominal pain for 4 days prior to hospitalisation, intermittent accentuation (hours), no other symptoms Secondary hypertension: persistent hypertension (158/88 ‐ 170/105, pulse: 78‐98 bpm). Normal physical examination. Normal eye fundus and normal cardiological examination. Discontinuation of methylphenidate: hypertensive treatment only necessary for 24 hours. Normal blood pressure after 1 week |
|
| Notes |
Key conclusions of the study authors: we want to draw attention to our experience of a patient who had hypertension, which was clearly related to the administration of MPH, and which disappeared when MPH was discontinued Comments from the study authors: unfortunately, only a few relevant publications and no national studies on the subject exist, so we do not know exactly the extent of this problem, although from anecdotal experience of verbal communication the incidence of tachycardia and hypertension appear to be low. Prospective studies should be performed to find the true incidence of complications with the use of methylphenidate and thus further establish careful and appropriate control of MPH‐treated patients to avoid these complications Funding/vested interest/authors affiliations: no affiliations to pharmaceutical companies stated Supplemental information regarding ADHD diagnostic criteria, IQ, type and dose of MPH, and duration of hospitalisation received through personal email correspondence with the authors in October and December 2013 (Saieh 2013 [pers comm]) |
|
Sangal 2006.
| Methods | A 7‐week randomised, double‐blind, cross‐over trial of:
|
|
| Participants | Number of participants screened: 107 Number of participants included: 85 Number of participants followed up: 75 Number of withdrawals: 10 Diagnosis of ADHD: DSM‐IV (subtype: combined (67.9%), hyperactive‐impulsive (2.4%), inattentive (29.8%)) Age: mean 10.1 (SD 2.0), range 6 to 14 years old IQ: not stated Sex: 64 (males, 21 females) Methylphenidate‐naïve: 43.5% Ethnicity: white: 72.9%, others: 27.1% Country: USA Comorbidity: ODD (48.2%); conduct disorder (3.5%); anxiety/agoraphobia (1.2%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: 42.29 mg/day (range 15 to 60), or 1.12 mg/kg per day Administration schedule: 3 time points Duration of treatment: 7 weeks Medication‐free period between intervention: 10‐20 days Treatment compliance: measured by pill counts at visits in 7‐12 day intervals, ranging from 87.5% to 100% |
|
| Outcomes |
Non‐serious adverse events
|
|
| Notes | Sample calculation: yes Ethics approval: yes Funding: this research was funded by Eli Lilly and Company, which markets atomoxetine. Some patients received doses that were off‐label Vested interests/authors' affiliations: this was an industry supported study sponsored by Eli Lilly. The data were analysed by statisticians at Eli Lilly, including Dr Sutton, one of the authors. The manuscript was written as a combined effort of all authors. Dr Sangal has received research support from Eli Lilly, Merck, Organon, Cephalon, and Novartis. Dr Owens has received research support from Cephalon, Sanofi‐ Aventis, Johnson & Johnson, Sepracor, and Eli Lilly; is a member of the speakers' bureau for Eli Lilly; is a consultant for Cephalon; and is an advisory board member for Cephalon, Pfizer, and Eli Lilly. Drs. Sutton, Allen, Schuh, and Kelsey are employees of Eli Lilly Key conclusions from study authors: patients receiving atomoxetine twice daily had shorter sleep‐onset latencies, relative to methylphenidate 3 times daily, based on objective actigraphy and polysomnography data Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information requested from the study authors twice in January/February 2014. No reply received |
|
Santosh 2006.
| Methods | 2 separate retrospective cohort studies (only study 2 is used here due to polypharmacy) | |
| Participants | Number of participants screened: not stated Number of participants included: 52 (ADHD: 25, ADHD + ASD: 27) Number of participants followed up: 52 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 11.08 years old IQ: mean 89.56 Sex: ADHD group 80% males, 20% females. ADHD + ASD group 88.89% males, 11.11% females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: UK Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: range of follow‐up was between 1 and 6 months, mean: 87 days Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events Pre‐ and post‐treatment: routine ratings using the side effects module of the Treatment Outcome Rating Scale | |
| Notes | Sample calculation: no Ethics approval: not stated Funding/vested interests: both the pilot study and current development and deployment of the DENEM™ system have been supported by an award from Guys' and St Thomas' Charity Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: both studies presented here support previous findings from smaller studies that show children with autism and ADHD can respond as well to stimulants as children with ADHD alone. Although randomised controlled trials remain the gold standard for efficacy studies, systems like this that allow clinicians to continue rigorous and consistent monitoring for many years have a valuable role to play. Furthermore, such monitoring systems which now exist electronically can easily accumulate large data sets and reveal details about long‐term effectiveness and long‐term side effects of medication that are unlikely to be discovered in short‐term trials Comments from the review authors: the article describes 2 studies. Some of the participants in the first study are not receiving MPH but dexamphetamine instead, and for this reason the first study is excluded from our review. The second described study is included Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information and data have not been possible to retrieve through personal email correspondence with the authors in February‐June 2014 |
|
Schertz 1996.
| Methods | A retrospective study examining predictors of weight loss in children with ADHD treated with methylphenidate or dextroamphetamine sulfate | |
| Participants | Number of participants screened: not stated Number of participants included: 60 (total) Number of participants included in methylphenidate group: 32 Number followed up: 60 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype: not stated) Age: mean 7.5 years old (range: 3.6‐15.5) IQ: no mental retardation Sex: 29 males, 3 females Methylphenidate‐naïve: 0 Ethnicity: white 77%, African American 10%, Asian: 3%, Hispanic: 10% Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: 25.5 mg or 1 mg/kg/day Administration schedule: not stated Duration of intervention: mean duration 11.2 months (range 6‐20 months) Treatment compliance: not assessed |
|
| Outcomes | Weight and body mass index (BMI) were the 2 measures of adiposity used. Weight was expressed in terms of z scores derived from US normative data | |
| Notes | Sample calculation: no Ethics approval: not stated Funding: not stated Vested interests/authors' affiliations: not stated Key conclusions of the study authors: pretreatment weight, adjusted for age, gender and height is a significant predictor of weight loss in children with ADHD treated with either methylphenidate or dextroamphetamine sulphate. In contrast, pretreatment age, duration of treatment, and weight‐adjusted dose were not found to be significant predictors Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information regarding height and weight for the methylphenidate group requested from the authors by email correspondence in January 2014. No reply |
|
Schmidt 2002.
| Methods | A retrospective cohort study analysing the EEGs of 124 children and adolescents treated with methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 124 Number of participants followed up: 124 Number of withdrawals: 0 Diagnosis of ADHD: ICD‐10 (subtype: F90.0, predominantly inattentive type (64,5%), F90.1, predominantly hyperactive type (35,5%)) Age: mean 10.3: years old (range 6.1‐17.1) IQ: 4% were intellectually disabled Sex: 113 males, 11 females Methylphenidate‐naïve: both methylphenidate naïve and not methylphenidate naïve participants included in the study Ethnicity: not stated Country: Germany Comorbidity: nocturnal enuresis (4%), mild intellectual disability (4%), mixed disorders of conduct and emotions (3.2%), absence epilepsy (0.8%), focal epilepsy (1.6%), developmental anomaly or developmental delay (0%), no comorbidity (64.5%) Comedication: 94.4% of the sample did not use another type of medicine apart from methylphenidate. 2.4% used anticonvulsant, and 0.8% were treated with different medicines Sociodemographics: not stated Inclusion criteria:
Exclusion criteria: None stated |
|
| Interventions | Methylphenidate type: Ritalin Mean methylphenidate dosage: 0.5‐1.0 mg/kg Administration schedule: not stated Mean duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: EEG (according to the German guidelines) measures by medical staff and under supervision from one of the authors (a doctor). A total of 4 categories were formed to evaluate the EEG:
|
|
| Notes | Sample calculation: no Ethics approval: yes Funding/vested interest: no funding Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: whether or not methylphenidate medication influences the occurrence of epileptic seizures remains unsettled. Given the data from this study, we would conclude that an EEG during therapy with methylphenidate is not necessary. Before commencing a planned methylphenidate therapy, however, an EEG should be performed. Comments from the review authors: there were several parameters from the EEG assessment in the article. However since we in the review have chosen to analyse it according to EEG changes yes/no we have only used the 4 categories that the authors formed Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding funding, ethics approval received through personal email correspondence with the authors in December 2013 (Sinzig 2013 [pers comm]). Not able to get separate data on the participants without intellectual disability |
|
Schulz 2010.
| Methods | A multicentre, randomised, cross‐over trial with 2 interventions:
Phases: 2 phases of 1 week each. All participants were treated with Ritalin LA |
|
| Participants | Number of participants screened: 159 Number of participants included: 150 Number of participants followed up: 145 Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (subtype: combined (67.3%), hyperactive‐impulsive (6%), inattentive (26.7%)) Age: mean 9.7 (SD 1.6) years old (range 6‐12) IQ: not stated, but patients not meeting minimum intelligence requirements were excluded Sex: 112 males, 38 females Methylphenidate‐naïve: 0% Ethnicity: white: 95.3%, African American: 1.3%, Asian: 2%, others: 1.3% Country: Germany Comorbidity: not clear, see exclusion criteria 1 Comedication: not clear, see exclusion criteria 8 Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: Ritalin LA (extended release) Mean methylphenidate dosage: 20 mg or 40 mg Administration schedule: not stated Duration of intervention: 14 days Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: No deaths or serious adverse events occurred in the study Non‐serious adverse events: A total number of 36 patients (24%) experienced a total of 64 adverse events, 33 of which were considered to be related to study medication No mentioning of how the adverse events were measured |
|
| Notes | Sample calculation: no Ethics approval: yes, Central Ethics Committee University Freiburg Funding/vested interest: sponsored by Novartis Pharma GmbH, Germany Authors' affiliations: several authors have received funding from medical companies Key conclusions of the study authors: all of the clinical rating scales showed consistently no difference between the 2 breakfast conditions. The clinical efficacy of Ritalin LA is not influenced by breakfast and works independently of food intake Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes |
|
Schwartz 2004.
| Methods | A patient report of stuttering priapism associated with withdrawal from sustained‐release methylphenidate | |
| Participants | Diagnosis of ADHD: DSM‐ IV‐TR (subtype: inattentive) Age: 15 years old IQ: not stated Sex: male Methylphenidate naïve: yes Ethnicity: white Country: USA Comorbidity: not stated Comedication: no Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: osmotic release oral system, OROS (Concerta) Methylphenidate dosage: begun at 27 mg/day Monday through Saturday with drug holiday on Sunday, then increased to 36 mg/day, followed by 7 days where medication was not taken, then a dose increase to 54 mg/day Administration schedule: once daily, in the morning Duration of treatment: 2 months Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Intermittent painful priapism beginning on the second drug holiday (the Sunday after the dosage was increased to 36 mg/day). 5‐10 episodes lasting up to an hour were experienced for at least half of the week with a pain score of 5/10. With the increase dose of 54 mg/kg the duration and pain associated with episodes increased When the patient was weaned off methylphenidate, the symptoms resolved and had not re‐occurred at either 2 weeks or 6 months follow‐up |
|
| Notes | Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: it is likely that withdrawal from OROS methylphenidate was the cause of this adolescent boy's priapism Comments from the study authors: it is possible that this adverse event is underreported due to embarrassment about discussing the problem Supplemental information regarding IQ and ADHD diagnosis received through personal correspondence with the authors in September 2013(Schwartz 2013 [pers comm]) |
|
Shang 2015.
| Methods | A cohort study of methylphenidate use for 24 weeks | |
| Participants | Number of participants screened: 174 Number of participants included: 160 Number included in the methylphenidate group: 80 Number of participants followed up: 66 Number of withdrawals: 14 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean: 9.64 (SD 2.42) years old (range: 7‐16) IQ: mean: 106 Sex: 70 males, 10 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Taiwan Comorbidity: not stated Comedication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Mean methylphenidate dosage: 27.83 mg/day (SD 12.44) Administration schedule: once daily Duration of intervention: 24 weeks Treatment compliance: assessed by pill count and interview, results not reported |
|
| Outcomes |
Non‐serious adverse events: Safety measures, including decreased appetite, vomiting, insomnia, somnolence, dizziness, stomachaches, headaches, palpitations, and dry mouth, were assessed at each visit by open‐ended questions during a clinical interview first Structured interview listing all the potential adverse effects at each visit Vital signs and body weight monitored at each visit |
|
| Notes | Ethics approval: yes. The Research Ethics Commitee of National Taiwan University Hospital approved this study prior to implementation Funding/vested interest: no Authors' affiliations: SSG, CYS and HYL were on the speakers' bureau for Janssen‐Cilag and Eli‐Lilly & Co., Taiwan Key conclusions of the study authors: both drugs are associated with significant improvement in symptoms while also being safe Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding comorbidity and treatment compliance requested through personal email correspondence with the authors in June 2016. No reply |
|
Shibib 2009.
| Methods | A report of 4 cases of psychosis during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined 100%) Age: 14, 8, 10, and 14 years old IQ: above 70 Sex: 1 female, 3 males Ethnicity: not stated Country: UK Comorbidity: conduct disorder (Case 4) Comedication: no, and no use of illicit substances Sociodemographics: not stated |
|
| Interventions |
Case 1 Methylphenidate type: extended release (Equasym XL) Methylphenidate dosage: gradually increased to 30 mg over 4 weeks Administration schedule: once daily Duration of treatment: 1 month titration, hereafter 4 months treatment Treatment compliance: not stated Case 2 Methylphenidate type: immediate release (Ritalin) Methylphenidate dosage: gradually increased from 5 mg once daily to 10 mg twice daily Administration schedule: once/twice daily Duration of treatment: 7 days Treatment compliance: not stated Case 3 Methylphenidate type: immediate release and extended release (Concerta XL) Methylphenidate dosage: immediate release: gradually increased to 15 mg daily. Extended release: 36 mg, later increased to 54 mg daily Administration schedule: immediate release: not stated. Extended release: once daily Duration of treatment: immediate release: not stated. Extended release: 3 weeks Treatment compliance: because compliance became an issue treatment with immediate release methylphenidate was stopped, and extended release methylphenidate was initiated. No information about compliance during treatment with extended release methylphenidate Case 4 Methylphenidate type: extended release (Concerta XL) Methylphenidate dosage: 18 mg Administration schedule: not stated Duration of treatment: 24 hours Treatment compliance: because compliance became an issue treatment with immediate release methylphenidate was stopped, and extended release methylphenidate was initiated. No information about compliance during treatment with extended release methylphenidate |
|
| Outcomes |
Serious adverse events: Case 1 Psychosis. Visual hallucinations. Paranoid Discontinuation: symptoms resolved spontaneously Case 2 Psychosis. Auditory and visual hallucinations. Suspicious Discontinuation: symptoms resolved spontaneously Case 3 Psychosis. Suspicious. Auditory hallucinations Discontinuation: symptoms resolved spontaneously Readministration of extended release methylphenidate (low dose) as an adjunct to atomoxetine, 5 weeks: "completely uncharacteristic behaviour", throwing furniture, making "silly" noises, and irritability. "Being unable to feel himself" Discontinuation: this behaviour resolved spontaneously within 72 hours Readministration of immediate release methylphenidate (15 mg, twice daily): no psychotic symptoms reported Case 4 Psychosis. Suspicious. Paranoid ideation. Denied any hallucinatory experiences although he was observed to be responding to possible auditory hallucinations Discontinuation: symptoms resolved spontaneously Re‐challenged with immediate release methylphenidate (10 mg 3 times daily): well tolerated Non‐serious adverse events Case 1 Extended release methylphenidate gradually increased to 30 mg over 4 weeks: loss of appetite Case 3 Immediate release methylphenidate gradually increased to 15 mg daily: poor appetite |
|
| Notes |
Key conclusions of the study authors: psychosis is an important, unpredictable side effect of stimulant medication. Symptoms resolve with discontinuation of treatment. Reemergence of ADHD symptoms are rapid and re‐challenge is often indicated Comments from the study authors: it would be advisable for all professionals involved in the care and treatment of patients with ADHD to receive mental health training to aid the early recognition and appropriate management of such side effects Funding/vested interest/authors' affiliations: not stated Supplemental information regarding ADHD diagnostic criteria, ADHD subtype, and IQ received through personal email correspondence with the authors in August‐October 2013 (Shibib 2013 [pers comm]) |
|
Shin 2016.
| Methods | Self‐controlled patient series analysis of cardiovascular events associated with methylphenidate treatment in children and young people with ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 1224 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: ICD‐10 (subtype: not stated) Age: under 17 or 17 IQ: not stated Sex: 75‐80% males Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Korea Comorbidity: the prevalence of comorbidities varied across adverse events. Depressive episode was the most common comorbidity (n = 15, 29% in participants with myocardial infarction), though only 9 (13.4%) participants with ischaemic stroke had this condition. In contrast, mental retardation occurred in 12 (18%) participants with an ischaemic stroke, while only 2 (5%) with heart failure had this condition comedication: antidepressants and antipsychotics were often co‐prescribed (15‐27%) Sociodemographics: not stated Inclusion criteria:
Exclusion criteria: None stated |
|
| Interventions | Methylphenidate type: not described Mean methylphenidate dosage: not stated Mean duration of methylphenidate exposure: 0.5 years for all events except heart failure, which was 0.3 years |
|
| Outcomes | All data used were obtained from secondary electronic records for the study participants. This study had both comparative cohort data and non‐comparative cohort data Non‐serious adverse events: These were all patients aged ≤ 17 years who had at least one recorded diagnosis of ADHD (ICD‐10 code F90), had started taking methylphenidate (ATC (Anatomical Therapeutic Chemical) code N06BA04), and had an incident cardiovascular adverse event with a recorded diagnosis during the study period (1 January 2008 and 31 December 2011) |
|
| Notes | Ethics approval: this study was approved by the institutional review board of the Korea Institute of Drug Safety and Risk Management, Seoul. Obtaining informed consent from the study population was waived by the board Funding: this research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. EER was supported by an NHMRC fellowship (GNT1110139). NP was supported by an NHMRC early career fellowship (GNT1035889) Vested interests: all authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work Key conclusions of the study authors: our self‐controlled patient series study suggests an increased risk of cardiac events associated with methylphenidate use Comments from the study authors: methylphenidate exposure in children/young people with a diagnosis of ADHD is associated with arrhythmia and potentially with myocardial infarction in specific time periods of use. With the increased use of drugs for ADHD globally, the benefits of methylphenidate should be carefully weighed against the potential cardiovascular risks of these drugs in children and adolescents Inclusion of methylphenidate responders only or exclusion of children who have previously experienced adverse events on methylphenidate: no |
|
Shyu 2015.
| Methods | A case‐control study | |
| Participants | Number of patients screened: for ADHD group: 146,063. Control group: 1,000,000 Number included: 146, 098 Number included as cases (MPH): 73,049 and controls (no intervention): 73,049 Number followed up in each arm: MPH: 73,049 and C: 73,049 Number of withdrawals in each arm: 73,014 were excluded from the ADHD group. No data on control group Diagnosis of ADD: DSM‐?/ICD‐? diagnosis of ADHD (combined (%), hyperactive‐impulsive (%), inattentive (%)). Age: cases (mean 9.4 years, SD 3.3), controls (mean 9.6 years, SD 3.8) IQ: no data Sex: cases: 14,742 females, 58,302 males. Controls: 20,057 females, 52,992 males MPH‐naïve: no data Ethnicity: no data Country: Taiwan Comorbidity (type: %): cases: ODD/conduct disorder (11.1%), PDD (8.7%), tic disorders (6.6%), intellectual disability (14.2%), psychotic disorder (1.7%), and schizophrenia (0.9%) Controls: ODD/conduct disorder (0.3%), PDD (0.3%), tic disorders (0.9%), intellectual disability (0.9%), psychotic disorder (0.2%), and schizophrenia (0.1%) Comedication (no/yes) no data Sociodemographics (e.g. double or single parent family, low, middle or upper class). No data Inclusion criteria 1. Diagnosed with ADHD between January 1999 and December 2011 2. Was a part of the National Health Insurance Research Database of Taiwan (NHIRD‐TW) Exclusion criteria 1. Were diagnosed with ADHD before 31 December 1999 2. Had a diagnosis of psychotic disorders that preceded the diagnosis of ADHD 3. Born after 31 December 1999 |
|
| Interventions | Methylphenidate type: no data MPH dosage: no data Administration schedule: no data Duration of intervention: medical records followed from January 2000 to December 2011 Treatment compliance: no data |
|
| Outcomes | Outcomes were defined as having diagnoses of schizophrenia spectrum disorders, including schizophrenia, schizophreniform disorder, schizoaffective disorder (ICD‐9‐CM code 295.X), delusional disorder (ICD‐9‐CM code 297.X), brief psychotic disorder and psychotic disorder NOS (ICD‐9‐CM code 298.X). Diagnoses of schizophrenia spectrum disorders and date of diagnosis were identified based on the insurance status, and outpatient and hospitalisation claims databases. Having a diagnosis of any psychotic disorder (ICD‐9‐CM code 295.X, 297.X or 298.X) and schizophrenia (ICD‐9‐CM code 295.X) were set as 2 different outcomes and were analysed separately Serious adverse events: "Compared to ADHD patients who were never exposed to MPH, MPH use among ADHD patients significantly increased the risk of developing any psychotic disorder (aHR 1.20, 95% CI 1.04 to 1.40). However, MPH use did not significantly increase the specific risk of developing schizophrenia (aHR 1.16, 95% CI 0.94 to 1.42) among patients with ADHD" |
|
| Notes | Sample calculation: no data Ethics approval: no data Funding: supported by the Chang Gung Memorial Hospital Research Project Vested interests/authors' affiliations: the authors declare no conflicts of interest Key conclusions of the study authors: compared to ADHD patients who were never exposed to MPH, MPH use among ADHD patients significantly increased the risk of developing any psychotic disorder; however, it did not significantly increase the specific risk of developing schizophrenia |
|
Silva 2004.
| Methods | Open‐label cohort study to determine efficacy of a single daily dose of dexmethylphenidate (d‐MPH) in 22 children with ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 22 Number of participants followed up: 20 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 8.7 years old (range 6‐12) IQ: not stated Sex: 17 males, 5 females Methylphenidate‐naïve: n = 18 Ethnicity: white 54%, African American 23%, Hispanic 23% Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria
|
|
| Interventions | Methylphenidate type: dexmethylphenidate (d‐MPH) Mean methylphenidate dosage: the starting dose was 2.5 mg/day and titrated to up to a maximum 30 mg/day based on clinical effect and tolerability over 8 weeks. The mean daily dose was 16.0 mg/day Administration schedule: once daily in the morning Duration of treatment: not stated Treatment compliance: was confirmed by tablet and bottle counts |
|
| Outcomes |
Non‐serious adverse events Participants were seen at least weekly and monitored for adverse events. Apart from nightmares, data were only recorded for adverse events when reported by ≥ 2 participants |
|
| Notes | Sample calculation: not stated Ethics approval: approved by Institutional Review Board Funding/vested interest: supported by Celgene Corporation and the NIH Authors' affiliations: New York University, 4 Rivers Clinical Research and Celgene Corporation (4/8 authors) Key conclusions of the study authors: a single daily dose of d‐MPH is safe and effective in controlling ADHD symptoms alleviating the need for midday dosing. Patients who failed prior therapy including dl‐MPH may respond to d‐MPH. A controlled study to confirm these data are warranted Comments from the study authors: in controlled clinical trials, d‐MPH was at least as efficacious and safe as dl‐MPH, at half the dose. In contrast to dl‐MPH it was effective on objective measures 6 hours post‐dose Comments from the review authors: although the only outcome which was measured into the extension period after the 8‐week trial was adverse events, only data up to the end of the 8‐week trial are reported. This study examines the efficacy of d‐MPH, which is not identical to standard methylphenidate (i.e. d‐ and l‐ isomers) Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, see exclusion criteria 1 Supplemental information regarding data requested from the authors in April 2014. No reply received |
|
Sobanski 2013.
| Methods | A cohort study of methylphenidate use for 6‐12 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 381 Number of participants followed up: 347 Number of withdrawals: 34 Diagnosis of ADHD: ICD‐10 (subtype: combined (64.8%), hyperactive‐impulsive (32.3%), inattentive (2.6%)) Age: mean 14.0 years old (range: 12‐17) IQ: not stated Sex: 307 males, 74 females Methylphenidate‐naïve: 7.3% Ethnicity: not stated Country: Germany Comorbidity: speech and language disorders and learning disabilities (8.9%), depressive disorders (3.1%) and anxiety disorders (2.4%). Asperger's syndrome (1%) tic disorders (1.3%) Comedication: yes, 42 patients (11%) (type: not stated) Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: Medikinet retard (50% extended‐release component) Mean methylphenidate dosage: 35.7 (SD 15.1, range 5‐120) mg/d, and at end point 0.7 (SD 0.3, range 0.2‐2.8) mg/kg of the body weight Administration schedule: all patients received Medikinet retard in the morning, 16% received a second dose at lunchtime and 4% on a pro re nata basis Duration of intervention: the median observational period for the treatment with Medikinet retard was 70 days Treatment compliance: not stated |
|
| Outcomes | Adverse events were assessed by spontaneous report during the clinical interview at each visit (at T1 and T2) | |
| Notes | Sample calculation: no Ethics approval: yes Funding/vested interest/authors affiliations: the study was funded by Medice. Esther Sobanski has received consulting income and research support from Eli Lilly, Medice, Novartis and Shire and research support from the German Research Foundation, German Ministry of Education and Research. She receives royalties from books by Medizinisch Wissenschaftliche Verlagsgesellschaft and Dansk Psykologisk Forlag. Manfred Dopfner received consulting income and research support from Lilly, Medice, Shire and Vifor and research support from the German Research Foundation, German Ministry of Education and Research. He receives royalties from books and psychological tests published by Hogrefe, Beltz and Huber. Claudia Ose has received an unrestricted educational grant for statistical and administrative support from Medice. Roland Fischer is the medical director of Medice Key conclusions of the study authors: the findings suggest that pharmacologically treated adolescents with ADHD and insufficient symptom reduction and/or treatment adherence benefit from switching to Medikinet retard and that it is well tolerated when given in clinical routine care Comments from the study authors: adverse events were assessed by spontaneous reports but not by the use of structured measures possibly resulting in underreporting. Most patients that were included in the study had an indication for a medication switch. Thus, the study does not allow for conclusions about general effectiveness or superiority of Medikinet retard compared to alternative medications Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information on adverse events requested through personal email correspondence with the authors in July 2014 (Sobanski 2014 [pers comm]). Authors not able to provide further information |
|
Song 2012.
| Methods | A 12‐week, multicentre, open‐label trial of osmotic release oral system (OROS) methylphenidate in Korean children with ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 143 Number of participants followed up: 116 Number of withdrawals: 27 Diagnosis of ADHD: DSM‐IV (subtype: combined 35.4%, hyperactive‐impulsive 6.3%, inattentive 34.3%, not specified 24.5%) Age: mean 9.36 years old (range 6‐18) IQ: mean 108 Sex: 121 males, 22 females Methylphenidate‐naïve: 89.5% Ethnicity: 100% Asian Country: Korea Comorbidity: tic disorder 7%, anxiety disorder 3.9%, depression 4.1%, oppositional defiant disorder/conduct disorder 27.3% Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Mean methylphenidate dosage: 30.05 mg/day Administration schedule: once daily, in the morning Duration of intervention: 12 weeks Treatment compliance: not stated |
|
| Outcomes | Barkley Side Effects Rating Scale | |
| Notes | Sample calculation: not stated Ethics approval: yes. Approved by the institutional review boards for all participating centres Funding/vested interest: supported by Janssen Korea Key conclusions of the study authors: optimal mean dose of OROS‐methylphenidate was significantly different by age groups. Higher doses was needed in older aged groups than younger groups. Effectiveness and tolerability of OROS‐methylphenidate in symptoms of ADHD sustained for up to 12 weeks Comments from the study authors: positive bias may have resulted from enrolment of only participants who could tolerate OROS‐methylphenidate and experienced efficacy during the dose titration period. Our findings may not be generalised to long‐term effects of methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested through email correspondence with the authors in May 2014. No reply |
|
Spencer 1992.
| Methods | A cohort study of methylphenidate use for 14.2 (SD 10.7) months | |
| Participants | Number of participants screened: not stated Number of participants included: 29 Number followed up: 29 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R (subtype: not stated) Age: mean 7.8 SD 2.4 IQ: not stated Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria: 1. DSM‐III‐R diagnosis of ADHD |
|
| Interventions | Methylphenidate type: immediate release Mean methylphenidate dosage: 31.4 (SD 17.6) mg (1 mg/kg SD 0.5) Administration schedule: not stated Duration of intervention: 14.2 (SD 10.7) months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Assessment of growth deficits: simple growth deficit, percent deficit, change in cumulative frequency percentiles, percent change, standardised height deficit, deficits in growth velocity Malnutrition index |
|
| Notes | Sample calculation: not stated
Ethics approval: not stated
Funding/vested interest: not stated
Authors' affiliations: not stated Key conclusions of the study authors: although there were statistically significant weight deficits in children treated with both desipramine and methylphenidate compared with normal controls, only those treated with methylphenidate sustained height deficits that attained statistical significance Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested through personal email correspondence with the authors in September and October 2013. No reply |
|
Steele 2006.
| Methods | An open‐label, 8‐week, multicentre, randomised, parallel trial with 2 arms:
|
|
| Participants | Number of participants screened: 187 Number of participants included: 147 Number randomised to OROS‐methylphenidate: 73 and IR‐methylphenidate: 74 Number of participants followed up: OROS‐methylphenidate: 61; IR‐methylphenidate: 62 Number of withdrawals: OROS‐methylphenidate: 12; IR‐methylphenidate: 12 Diagnosis of ADHD: DSM‐IV (subtype: combined (79.3%), hyperactive‐impulsive (2.1%), inattentive (18.6%) Age: mean: not stated (range: 6‐12) IQ: above 70 Sex: 121 males, 24 females Methylphenidate‐naïve: not stated Ethnicity: white: 86.9%, African American: 3.4%, Asian: 0.7%, others: 9.0% Country: Canada Comorbidity: oppositional defiant disorder (40.7%), conduct disorder (0.7%), anxiety (4.1%) Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system and immediate release methylphenidate (patients were randomly assigned) Mean daily dose of OROS‐methylphenidate: 37.8 (SD 11.9) mg (1.17 (SD 0.52) mg/kg; range 18‐54 mg) Mean daily dose of IR‐methylphenidate: 33.3 (SD 13.2) mg (1.03 (SD 0.46) mg/kg; range 10‐70 mg) Administration schedule: OROS‐methylphenidate once daily in the morning and IR‐methylphenidate 2‐3 times daily Duration of intervention: 8 weeks Titration period: 4 weeks initiated after randomisation Washout: at study entry, patients on stimulant or non‐stimulant medication to treat ADHD underwent a minimum 3‐day washout Treatment compliance: the percentage of participants who missed any dose during the trial: IR‐methylphenidate (84%) and OROS‐methylphenidate (56%) |
|
| Outcomes |
Non‐serious adverse events: Adverse events, physical examination, vital signs, and body weight, height |
|
| Notes | Sample calculation: yes (130 participants) Ethics approval: yes Funding/vested interest: this research was supported by Janssen‐Ortho Inc., Canada Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: once‐daily OROS‐methylphenidate is significantly more effective than usual care with IR‐methylphenidate based on multiple outcome measures including remission rate Exclusion of methylphenidate non‐responders: yes, known non‐responders excluded (see exclusion criteria 1) Supplemental information requested through email correspondence with the authors in September 2013. No reply |
|
Stein 2002.
| Methods | A comparative cohort study of 32 non‐medicated ADHD male adolescents, 35 ADHD male adolescents receiving methylphenidate, and 77 controls (no ADHD) | |
| Participants | Number of participants screened: 150 Number of participants included: 95 (non‐medicated: 32; medicated: 35; controls: 77) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: mean: non‐medicated: 13.06 years; medicated: 13.26 years old (range not stated) IQ: not stated Sex: 144 males Methylphenidate‐naïve: non‐medicated group had not been receiving any medication for the treatment of ADHD for ≥ 6 months prior to the study Ethnicity: Jewish‐Ashkenazi (European descent) 46%, Jewish‐Sepharadi (Middle Eastern descent) 54% Country: Israel Comorbidity: not stated Comedication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
Controls:
|
|
| Interventions | Methylphenidate type: immediate release Mean methylphenidate dosage: 18 mg/day Administration schedule: once or twice/day (morning/noon) Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Questionnaires on sleep |
|
| Notes | Sample calculation: no Ethics approval: the Israel Ministry of Education and the principals of the 2 participating schools approved the study. Written informed consent was obtained Funding/vested interests: not stated Key conclusions of the study authors: the study did not find a greater severity of sleep disturbances among non‐medicated male adolescents diagnosed with ADHD in childhood compared to control participants. Sleep disturbance among male students receiving methylphenidate treatment was significantly greater compared with the non‐medicated group Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested through personal email correspondence with the authors in April 2014. No reply |
|
Stevens 2010.
| Methods | A cross‐sectional study of youths treated with high‐dose osmotic release oral system (OROS) methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 17 Diagnosis of ADHD: DSM‐IV (subtype: combined 11, inattentive 6) Age: mean 16.2 years old (range 11‐20) IQ: not stated Sex: 13 males, 4 females Methylphenidate‐naïve: no Ethnicity: white 88%, Native American 12% Country: USA Comorbidity: depressive spectrum disorders 47%, pervasive developmental disorders 41%, oppositional defiant disorder 41%, bipolar spectrum disorders 29%, fetal alcohol syndrome 12% Comedication: bupropion (n = 10), SSRIs (n = 8), lithium (n = 5), alpha‐2 agonists (n = 4), atypical antipsychotics (n = 3), lamotrigine (n = 3), trazodone (n = 2), SNRIs (n = 1), oxcarbazepine (n = 1), tricyclic antidepressants (n = 1) Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Mean methylphenidate dosage: 169 mg/day SD 31 (range: 126‐270) Administration schedule: not stated Duration of intervention: not stated, but ≥ 2 weeks stabilised on same dose Treatment compliance: not stated |
|
| Outcomes | Heart rate, systolic blood pressure, and diastolic blood pressure measured No adverse events or adverse cardiovascular outcomes were reported |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interest: the data analysis of this research was funded by institutional funds from the Pediatric Psychopharmacology Unit at Massachusetts General Hospital Authors' affiliations: George is a speaker for McNeil, Shire, Novartis and Lilly, consultant for McNeil and Shire. Wilens receives grant support from Abbott, McNeil, Lilly, Merck and Shire, is a speaker for Lilly, McNeil, Novartis and Shire, is a consultant for Abbott, McNeil, Lilly, NIH, Novartis, Merck and Shire Key conclusions of the study authors: high‐dose OROS methylphenidate used in combination with other medications, was not associated with either unusually elevated plasma methylphenidate concentrations or with clinically meaningful changes in vital signs Comments from the review authors: requirement to be stabilised on OROS methylphenidate de facto excludes patients with poor or adverse response Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: see above Supplemental information regarding data requested through email correspondence with the authors in June 2014. No reply |
|
Strandell 2007.
| Methods | A series of spontaneously reported cases of suicidal and self‐damaging behaviour from VigiBase | |
| Participants | Number of cases reported: 116 Diagnostic criteria not stated, nor subtype Age(range): 5‐17 years old IQ: not stated Sex: not stated Ethnicity: not stated Country: not stated Comorbidity: 78 were depressed, otherwise not stated Comedication: in 33 cases methylphenidate and atomoxetine were co‐reported. 17 depressed patients were on antidepressants, and 42 were on antidepressants (without depression) ‐ (not clear if they were on atomoxetine or methylphenidate) Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Depression suicidal Intentional overdose Intentional self‐injury Non‐accidental injury Suicidal tendency Suicide Suicide attempt Thoughts of self‐harm |
|
| Notes | Ethics approval: not stated Funding/vested interest/authors' affiliations: not stated Key conclusions of the study authors: the number of reports with suicidal behaviour in children and adolescents raises concern. The reports do not only imply possible suicidal behaviour: young patients actually commit suicide Comments from the review authors: this case‐series is based on a database of spontaneously reported adverse events. Therefore, some of these cases might have been reported in other case‐reports Supplemental information, specifically asking for full‐text article and additional information, requested through email correspondence with the authors in November 2013. No reply |
|
Su 2015.
| Methods | A multicentre, prospective, single‐arm, open‐label study on methylphenidate use for 8 weeks with extended observation for up to 24 weeks | |
| Participants | Number of participants screened: 248 Number of participants included: 239 Number of participants followed up: 205 Number of withdrawals: 34 Diagnosis of ADHD: DSM‐IV (subtype: combined 61.1%, predominantly inattentive type: 34.3%, predominantly hyperactive‐impulsive type: 3.8%, not specified: 0.8%) Age: mean: 9.2 (SD 2.02) years old (range 6‐16) IQ: not stated Sex: 203 male, 36 females Methylphenidate‐naïve: all the children were psychotropic drug naïve or had received anti‐ADHD drugs (including OROS‐methylphenidate, atomoxetine, monoamine oxidase inhibitors, clonidine, other α2‐adrenergic receptor agonists, tricyclic antidepressants, theophylline, and bishydroxycoumarin) for 6 months or longer before the trial with the treatment course not more than 1 month or were currently having effective immediate release methylphenidate treatment Ethnicity: Asian 94.6%, other 5.4% Country: China Comorbidity: not stated Comedication: recorded, but not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: 18 mg/day, 36 mg/day and 54 mg/day Administration schedule: for methylphenidate‐naïve dose participants: adjustment phase for 3 weeks, optimal dosage treatment for 5 weeks. For non‐naïve participants: 18 mg/day for previous 5 mg 2‐3 times a day, 36 mg/day for previous 10 mg 2‐3 times a day and 54 mg/day for previous 15 mg 2‐3 times a day or higher Duration of intervention: 8‐24 weeks Treatment compliance: most patients (> 90%) in the full analysis set had compliance between 80% and 120%, during the 8‐ and 24‐week treatment periods |
|
| Outcomes | Safety and tolerability were monitored throughout the study by the evaluation of the incidence and type of treatment emergent adverse events and changes in clinical laboratory test results, vital signs, sleep status, tics, appetite, height, and weight | |
| Notes | Sample calculation: none Ethics approval: approved the institutional review board of Peking University sixth Hospital and other independent ethics committees at all sites Funding/vested interests: sponsored by Xi'an Janssen Pharmaceutical Ltd, and supported by the Major State Basic Research Development Program of China. YW has served on advisory boards of Xi'an Janssen Pharmaceutical Ltd and Eli Lilly & Company. JZ and JQ are full‐time employees of Xi'an Janssen Pharmaceutical Ltd. Key conclusions of the study authors: most of the children experienced symptom relief with no severe adverse events. The OROS‐methylphenidate at the dosage levels of 18 mg, 36 mg, and 54 mg once daily was generally well tolerated in Chinese children with ADHD between the ages of 6‐16 years Comments from the study authors: an open‐label, non‐comparator, non‐randomised study design is a major limitation for this study. No blinded trained clinician raters collected the study data. Also, the outcome measures were mainly based on the parent reports. As the role of measurement in ADHD makes school settings critical, hence, the data of ADHD symptom expression in school settings are not presented. To further improve this situation, we recommend the incorporation of direct evaluations from teachers/instructors in the follow‐up studies in China Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested through personal email correspondence with the authors in May 2016. No reply |
|
Sudarmadji 2009.
| Methods | A double‐blind, randomised parallel trial of methylphenidate use for 2 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 84 1 group randomised to 5 mg methylphenidate daily, other group to 10 mg methylphenidate daily (number of participants in each group not stated) Number followed up in each arm: not stated Number of withdrawals in each arm: not stated Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean not stated (range: 6‐14) IQ: above 70 (attending elementary school) Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Indonesia Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria: None stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 5 mg or 10 mg Administration schedule: once daily Duration of intervention: 2 weeks Treatment compliance: not stated |
|
| Outcomes | No description of measures | |
| Notes | Sample calculation: not stated
Ethics approval: not stated
Funding/vested interests: not stated
Authors' affiliations: not stated
Key conclusions of the study authors: treatment with 5 mg methylphenidate was more effective compared with 10 mg methylphenidate to improve the attention, decrease hyperactivity and increase the cognitive function. The side effects of 5 mg/daily methylphenidate were milder compared with 10 mg/daily
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information was attempted to be retrieved through contact with the authors in July 2014. We were not able to find contact information on any of the authors |
|
Tang 2010.
| Methods | A patient report of stool and urinary incontinence during treatment with osmotic release oral system (OROS) methylphenidate | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 8 years old IQ: above 70 Sex: male Ethnicity: not stated Country: Taiwan Comorbidity: no Comedication: no Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: immediate and extended release Methylphenidate dosage: immediate release, 10 mg. OROS 18 mg/day then 36 mg/day Administration schedule: immediate release, each morning. OROS methylphenidate, once daily Duration of intervention: immediate release methylphenidate, 2 weeks. OROS methylphenidate, 2 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Side effects reported by the mother: Immediate release methylphenidate 10 mg: no side effects OROS methylphenidate 18 mg/day: no side effects OROS methylphenidate, 36 mg/day: double incontinence. Stool incontinence (every day, frequent during daytime) and urinary incontinence (almost every day). The double incontinence completely resolved after the discontinuation of OROS methylphenidate 36 mg |
|
| Notes |
Key conclusions of study authors: the causality in this case might be dose‐related, and careful monitoring is highly suggested Comments from the authors: the Adverse Drug Reaction Probability score in this patient was 7, denoting a probable adverse reaction caused by OROS methylphenidate 36 mg. Because of ethical considerations, this patient did not undergo re‐challenge with OROS methylphenidate Funding/vested interests/authors' affiliations: not stated |
|
Tasdelen 2015.
| Methods | A prospective cohort study of methylphenidate use | |
| Participants | Number of participants screened: not stated Number of participants included: 22 Number of participants followed up: 17 Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (subtype: combined (100%)) Age: mean 9.59 years old (range: 7‐12) IQ: mean: 102 (SD 10) Sex: 17 males, 5 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Turkey Comorbidity: not stated Comedication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) 36‐54 mg Mean methylphenidate dosage: 0.9 mg/kg/day Administration schedule: not stated Duration of intervention: mean 7 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Several adverse events (loss of appetite, abdominal pain, irritability, tingle, headache, nausea, insomnia and tics) were observed in 12 out of 22 patients receiving OROS methylphenidate. Not stated how these adverse events were reported or measured |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interests: none Authors' affiliations: none Key conclusions of the study authors: behaviour and cognitive functionality are recovered simultaneously using OROS‐methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no, all patients were newly diagnosed and methylphenidate naïve Supplemental information regarding IQ and specific adverse events received through personal email correspondence with the authors in June 2016 (Tasdelen 2016 [pers comm]) |
|
Tekin 2015.
| Methods | A patient report of focal dystonic reaction during MPH treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 15 years old IQ: not known Sex: female Ethnicity: not stated Country: Turkey Comorbidity: none Comedication: none Sociodemographics: uneventful pregnancy and delivery. Family members had no history of psychiatric or movement disorders |
|
| Interventions | Methylphenidate type: modified‐release Methylphenidate dosage: 27 mg/day Administration schedule: not stated Duration of treatment: 9 days Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: After 9 days of methylphenidate treatment: involuntary extensor muscular contraction of right hand and wrist, tension and severe pain. The dystonic reaction subsided after biperiden and diazepam administration. No opportunity for re‐challenge, because the patient refused to take methylphenidate again |
|
| Notes |
Key conclusions of the study authors: clinicians should consider acute dystonia as a rare adverse event that might emerge during modified release methylphenidate treatment Comments from the study authors: to our knowledge, this is the first reported case of focal dystonia after initiation of methylphenidate in a drug‐naïve otherwise healthy attention‐deficit/hyperactivity disorder (ADHD) patient. The patient had a history of similar adverse effects due to immediate‐release methylphenidate. Therefore, acute dystonic reaction was considered to be due to methylphenidate treatment. Naranjo Adverse Drug Reaction Probability Scale score was 5 Funding/vested interests/authors' affiliations: the authors have no financial relationships or conflicts of interest to disclose. There was no financial support relevant to the article Ethics approval: the patient and the patient's mother gave written informed consent for the publication of this patient report Supplemental information regarding ADHD diagnosis and any comedication received through personal email correspondence with the authors April 2016 (Soyata 2016 [pers comm]). No information regarding the patient's IQ was available |
|
Thorell 2009.
| Methods | A cross‐sectional study of children on stimulant treatment studying positive and negative effects | |
| Participants | Number of participants screened/questionnaires sent out to: 132 Number of participants included/returned questionnaires: 79 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 13.14 years old (range 9‐17) IQ: above 70, except for 2 children with mild mental retardation Sex: 62 males, 17 females Methylphenidate‐naïve: none Ethnicity: not stated Country: Sweden Comorbidity: Aspergers (7.6%), Tourette syndrome (13.9%), obsessive‐compulsive disorder (2.5%), mild mental retardation (2.5%), oppositional defiant disorder/conduct disorder (2.5%) Comedication: no Sociodemographics: not stated Inclusion criteria:
|
|
| Interventions | Methylphenidate type: 96% were taking methylphenidate and 4% amphetamine Dosage: not stated Administration schedule: not stated Duration of intervention: mean 3.12 years (range: 6 months to 12 years) Treatment compliance: most children knew how it felt to be off medication. Only 8% of the parents in the present study indicated that the child never forgot to take his or her medication |
|
| Outcomes |
Non‐serious adverse events Children's and parents questionnaire of negative events (4‐point Likert‐type scale), parent and self‐reported |
|
| Notes | Sample calculation: none reported Ethics approval: yes, the study was approved by the local ethics committee Funding: the study was supported by a grant from Majblommans Riksförbund Vested interests/authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: Swedish children treated with stimulants generally experienced positive treatment effects in many areas, especially in the school setting, and a majority wished to continue taking their medication. There was, however a small group of children who reported a relatively large number of negative effects. Few differences between parents and children were found for positive effects, although parents reported higher levels of negative effects Comments from the study authors: excluding the children with comorbid diagnoses did not make any significant changes in the results. The 4 participants not taking methylphenidate and who were mentally retarded are excluded from the data Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding adverse event data for patients taking methylphenidate without intellectual disability (n = 75) received through personal email correspondence with the authors in October 2013 (Thorell 2013 [pers comm]) |
|
Tomás Vila 2010a.
| Methods | A patient report of visual hallucinations during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 10 years old IQ: above 70 Sex: male MPH‐naïve: no Ethnicity: not stated Country: Spain Comorbidity: none stated Comedication: not stated Sociodemographics: lives with his grandmother |
|
| Interventions | Methylphenidate type: 50% immediate release and 50% extended release Methylphenidate dose: 30 mg/day (1 mg/kg/day) Administration schedule: not stated Duration of treatment: 2 weeks Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: After 2 weeks of 50% immediate release and 50% extended release methylphenidate: visual hallucinations (insects on hands, feet, abdomen and thorax) with associated itching, initiated 2 hours after ingestion and ceased 5 hours after Discontinuation of methylphenidate and initiation of risperidone: no visual hallucinations No readministration of methylphenidate due to ethical reasons |
|
| Notes |
Key conclusions of the study authors: this is the first patient report of visual hallucinations caused by 50% immediate release, 50% extended release methylphenidate, which is no wonder when you consider that the preparation has been marketed recently Funding/vested interests/authors' affiliations: not stated Supplemental information regarding diagnostic criteria and IQ received through personal email correspondence with the authors in September 2013 (Tomás 2013 [pers comm]) |
|
Tomás Vila 2010b.
| Methods | A multicentre 3‐month cohort study that investigates the impact of methylphenidate treatment on sleep | |
| Participants | Number of participants screened: not stated Number of participants included: 114 Number of participants followed up: 114 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: combined (54.5%), hyperactive‐impulsive (6.3%), inattentive (39.3%)) Age: mean 8.8 years old (range 4‐15) IQ: normal intelligence Sex: 79% males, 27% females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Spain Comorbidity: not stated Comedication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type and mean dosage: immediate release methylphenidate, mean dose: 18.5 mg (n = 42). Intermediate release‐methylphenidate, mean dose: 23.3 mg (n = 34). Extended release‐methylphenidate, mean dose: 32.9 mg (n = 38) Administration schedule: not stated Duration of intervention: minimum 3 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Spanish abbreviated (18 questions chosen) version of the Paediatric Sleep Questionnaire (PSQ) rated by neuro‐paediatricians |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/authors' affiliations: the authors declare no conflicts of interest Key conclusions of the study authors: the results of the study suggest that methylphenidate does not have a negative impact on sleep in patients with ADHD and comorbid sleep disorders. Methylphenidate improves the quality of sleep in those patients Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding ADHD diagnostic criteria and IQ received through personal email correspondence with the authors in October 2013 (Tomás 2013b [pers comm]) |
|
Trugman 1988.
| Methods | A patient report of cerebral arteritis during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: ICD‐9 (unspecified hyperkinetic syndrome) Age: 12 years old IQ: not stated Sex: male Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10 mg Administration schedule: twice daily Duration of treatment: 5‐12 years of age (7 years) Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Right hemiparesis, 7 years following daily consumption of methylphenidate Aphasia Non‐serious adverse events: Headaches, intermittently before onset of stroke |
|
| Notes |
Key conclusions of the study authors: cerebral arteritis and infarction were caused by chronic oral methylphenidate use. The CSF profile and angiogram support the diagnosis of inflammatory arteritis, yet laboratory evaluation revealed no identifiable cause. In the 6 years since the stroke, while not on methylphenidate there has been no evidence of active central nervous system or systemic vasculitis Comments from the study authors: given its pharmaceutical similarity to amphetamine, the association of methylphenidate with cerebral vasculitis is not unexpected, yet has not been previously reported. Physicians who prescribe methylphenidate for long‐term use should be aware of this potential complication and specifically question patients regarding symptoms of cerebral ischaemia, including headache Supplemental information regarding diagnostic criteria received through personal email correspondence with the authors in September 2013 (Trugman 2013 [pers comm]) |
|
Tzang 2012.
| Methods | A prospective observational study for 48 weeks. Patients categorised into 4 groups based on occurring treatment:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 757 Number of participants included in each group: IR methylphenidate: 265, OROS methylphenidate: 293, IR and OROS methylphenidate: 129, controls (no intervention): 70 Number of participants followed up in each arm: IR methylphenidate: 265, OROS methylphenidate: 293, IR and OROS methylphenidate: 129, controls: 70 Number of withdrawals in each arm: IR methylphenidate: 0, OROS methylphenidate: 0, IR and OROS methylphenidate: 0, controls: 0 Diagnosis of ADHD: DSM‐IV (combined (58.52%), hyperactive‐impulsive (4.76%), inattentive (36.72%)) Age: mean 10.05 (SD 2.65) years old (range: 6‐18 years) IQ: not stated Sex: 607 males, 150 females Methylphenidate‐naïve: not stated Ethnicity: white (0%), African American (0%), Asian (100%), Hispanic (0%), others (0%) Country: Taiwan Comorbidity: oppositional defiant disorder (21.80%), conduct disorder (1.85%), anxiety disorder (3.04%), depressive disorder (0.40%), tic disorder (2.64%), Tourette disorder (1.59%), somatisation (5.15%), others (7.40%) Comedication: 6.61% Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: immediate release (IR) and osmotic release oral system (OROS) Methylphenidate dosage: not stated Administration schedule: OROS‐methylphenidate once a day, other groups not stated Duration of intervention: 48 weeks Treatment compliance: not stated |
|
| Outcomes | The frequency of adverse effects was determined using a symptom checklist that included the following items: decreased appetite, nausea, somnolence, insomnia, headache, dizziness, abdominal pain, and stomachache at 12, 24, 36, and 48 weeks | |
| Notes | Sample calculation: no information Ethics approval: not stated Funding/vested interests/authors' affiliations: supported by Janssen‐Cilag, Taiwan Key conclusions of the study authors: OROS methylphenidate treatment at the adequate dosage can achieve higher remission and recovery rates, produce greater functional improvement, and result in better treatment adherence that IR methylphenidate treatment Supplemental information requested through email correspondence with the authors in December 2013 and January 2014. No reply |
|
Tølløfsrud 2006.
| Methods | A patient report of death caused by heart failure (acute dilated cardiomyopathy) during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: ICD‐10 (subtype: not stated) Age: 17 years old IQ: no intellectual disability Sex: male Ethnicity: not stated Country: Norway Comorbidity: Tourette's syndrome Comedication: none Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 15 mg Administration schedule: 3 times daily Duration of treatment: 10 years Treatment compliance: not stated |
|
| Outcomes | Serious adverse events: Heart failure resulting in death | |
| Notes |
Key conclusions of the study authors: heart failure leading to death, possibly caused by methylphenidate treatment Comments from the study authors: dilated cardiomyopathy can be a rare side effect of methylphenidate use Funding/vested interest/authors' affiliations: none Supplemental information regarding ADHD diagnosis and IQ received through personal email correspondence with the authors in November 2013 (Tølløfsrud 2013 [pers comm]) |
|
Valdizán Usón 2004.
| Methods | A 12‐month cohort study | |
| Participants | Number of participants screened: not stated Number of participants included: 170 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated for the total sample. Subtype for the sample with polysomnography data: combined (40%), hyperactive‐impulsive (9%), inattentive (57%)) Age: mean 8 years old (range not stated) IQ: above 70 Sex: 121 males, 27 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Spain Comorbidity: immunological diseases (33.3%) Comedication: no medication for sleep initiation Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: 10‐40 mg/day, adjusted as needed Administration schedule: morning and noon Duration of intervention: 12 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Reports of side effects Complete blood test Thyroid hormones and cortisol levels Nocturnal polysomnography (n = 46), initiated at 10 pm and disrupted at 7 am. Evaluated parameters: sleep efficiency, total registered time, latency time of sleep initiation, number of awakenings, total time of sleep and efficiency, duration of each phase and latency time of REM sleep |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interest: not stated Authors' affiliations: not stated Key conclusions of the study authors: it is more likely that the ADD subgroup continues in the adult age and, although as a minority, immunological disorders and/or epileptiform paroxysms are associated. The effect of methylphenidate may be observed by seriated recording of digitalised cortical bioelectrical activity, with synchronic course to the clinical response Comments from the review authors: EEG was conducted every 6th month, but the methylphenidate treatment was paused 24 hours before, so we cannot use these data Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding ethics approval, funding, protocol, patient demographics, and data not possible to receive through personal email correspondence with the authors. Emails sent several times in December 2013. No reply |
|
Valdizán Usón 2013.
| Methods | A cohort (observational, retrospective, non‐interventional, multicentre) study of methylphenidate use for 1 month, 3 months, 6 months, and 1 year | |
| Participants | Number of participants screened: not stated Number of participants included: 680 Number of participants followed up: 680 (safety population), 561 (4‐16 years old) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV TR (subtype: combined (64.9%), hyperactive‐impulsive (5%), inattentive (30.05%)) Age: mean 9.497 SD 2.538 (under 6 years old: mean: 5.27, range: not stated; 6‐16 years old: mean 9.77, range: 6‐16) IQ: above 80 Sex: 454 males, 105 females (under 6 years old: 31 males, 3 females; 6‐16 years old: 423 males, 102 females) Methylphenidate‐naïve: 0% Ethnicity: not stated Country: Spain Comorbidity: anxiety disorder (13.01%), oppositional defiant disorder (23.35%), learning disorder (50.09%), conduct disorder (30.30%), depressive disorder (5.35%), tics (6.95%). Other associated symptoms were substance abuse (0.36%), apathy (9.68%) and anhedonia (4.36%), developmental coordination disorder (12.66%), pervasive developmental disorder (10.52%), and generalised epilepsy (1.78%) Comedication: yes (under 6 years old: 10 (29.41%); 6‐16 years old: 114 (21.63%)) Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: immediate release Mean methylphenidate dosage: mean starting dose: 16.72 mg/day, mean dose at 1‐month follow‐up: 18.76 mg/day; mean dose at 6‐month follow‐up: 20.58 mg/day; mean dose at 1‐year follow‐up: 22 mg/day. Administration schedule: not stated Duration of intervention: medical records for the years 2002‐2006 (mean = 10.01 months) Follow‐up after 1 month, 6 months and 1 year Treatment compliance: not stated |
|
| Outcomes | A year after treatment information on adverse events was obtained Safety outcomes included adverse events and serious adverse events reported, and their recurrence, duration, and relationship with the study drug Obtained by a single questionnaire ‐ presence or absence and number of adverse events per patient |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interest: the authors report no conflicts of interest in this work Key conclusions of the study authors: good efficacy and safety results were found for immediate‐release methylphenidate in patients with ADHD Comments from the study authors: a limitation of the study is that even when favorable results were found overall in reduction of the number of symptoms and a significant global improvement, these results are only preliminary due to the limited numbers of patients included Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information received through personal email correspondence with the authors in July 2014 (Valdizán 2014 [pers comm]) |
|
Van der Oord 2007.
| Methods | A 4‐week pseudorandomised, placebo‐controlled, cross‐over study investigating the additional value of short‐term intensive multimodal behaviour therapy to optimally titrated methylphenidate Follow‐up 4.5 to 7.5 years after treatment |
|
| Participants | Number of participants screened: not stated Number of participants included in methylphenidate group: 23; included in methylphenidate + multimodal behaviour therapy: 27 Number of participants followed up in methylphenidate group: 21 Number of withdrawals in methylphenidate group: 2 Diagnosis of ADHD: DSM‐IV/I (subtype: combined (n = 31), hyperactive‐impulsive (n = 3), inattentive (n = 16)) Age: mean 9.6 years old IQ: 96.81 Sex: not stated Methylphenidate‐naïve: 100% Ethnicity: white: 89%, others: 11% Country: Netherlands Setting: Comorbidity: oppositional defiant disorder/conduct disorder 61.9%, oppositional defiant disorder 46%, conduct disorder 4% Comedication: no Sociodemographics: most parents medium to high education level Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 5 mg, 10 mg, and 20 mg in titration period Administration schedule: 7:30 am, 12:30 pm Duration of intervention: 4 week pseudo randomised cross‐over design, 1 week medication‐free, 5 weeks optimal dose Treatment compliance: high, weekly phone calls to parents and teachers |
|
| Outcomes | The MTA Side Effect Rating Scale was used to assess side effects | |
| Notes | Sample calculation: yes (power calculation) Ethics approval: not stated Funding: not stated Vested interest/authors' affiliations: not stated Key conclusions of the study authors: no evidence was found for the additive effect of multimodal behaviour therapy next to optimally titrated methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding data on adverse events received through personal email correspondence with the authors in January 2014 (Van der Oord 2014 [pers comm]) |
|
Varley 2001.
| Methods | A retrospective chart review | |
| Participants | Number of participants screened: 555 Number of participants included: 517 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 11 years old (range not stated) IQ: not stated Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: not stated Comorbidity: tics Comedication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Retrospective review of medical records. It was recorded whether the participants did or did not have a reported history of tic emergence in the course of pharmacologic treatment in that clinic. 8.3% (31 of 374) of participants treated with methylphenidate developed tics |
|
| Notes | Sample calculation: no Ethics approval: not stated Funding/vested interest: supported by a National Institutes of Health Biomedical Research Support Grant, 1991 Authors' affiliations: not stated Key conclusions of the study authors: tics was not related to dose nor treatment length, and may not be related to stimulants Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested through personal email correspondence with the authors in April 2014. No reply |
|
Vashi 2011.
| Methods | A patient report on allergic contact dermatitis caused by methylphenidate transdermal system | |
| Participants | Diagnosis of ADHD: DSM‐IV (subtype: not known) Age: 9 years old IQ: above 70 Sex: female Ethnicity: not stated Country: USA Comorbidity: none Comedication: not stated Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: transdermal system Methylphenidate dosage: not known Administration schedule: not stated Duration of intervention: 8 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Allergic contact dermatitis: after 8 months of using a methylphenidate patch the patient presented with pruritic dermatitis. She had itchy, burning, red lesions. Symptoms began on her hip at the area of patch placement, then progressively spread to her arms, legs, abdomen, and back Methylphenidate discontinued: symptoms lasted for 2 months. First and second patch test. Re‐tested with methylphenidate patch: 9 days after the first test the patient presented with recall reaction, characterised by a return of the original pruritic dermatitis to her entire back, similar to the eruption that had occurred months previously after therapeutical use of methylphenidate patch Avoidance of methylphenidate patches: symptom‐free |
|
| Notes |
Key conclusions of study authors: this is the first reported case of allergic contact dermatitis caused by methylphenidate present within the transdermal system Supplemental information regarding diagnosis and IQ received through personal email correspondence with first author in August 2013 (Vashi 2013 [pers comm]) |
|
Verret 2010.
| Methods | A comparative cohort study assessing the impact of methylphenidate on physical functioning and gross motor performance | |
| Participants | Number of participants screened: not stated Number of participants included: 70 Number included in each group: ADHD + methylphenidate: 24, ADHD no methylphenidate: 19 Number followed up in each group: ADHD + methylphenidate: 24 and ADHD no methylphenidate: 19 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (94.3%), hyperactive‐impulsive (5.7%) Age: mean not stated (range: 7‐12) IQ: not stated Sex: 70 males, 0 females Methylphenidate‐naïve: the ADHD no methylphenidate group: 19 Ethnicity: not stated Country: Canada Comorbidity: ADHD + methylphenidate group: opposition or anxiety (29%), anxiety, opposition or obsessive‐compulsive disorder (13%); ADHD no methylphenidate group: opposition or anxiety (37%) Comedication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylpheniate dosage: not stated Administration schedule: not stated Duration of intervention: average 24 months (range 5‐72 months) Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events Anthropometric and vital measures (height, weight, BMI, resting heart rate and blood pressure) | |
| Notes | Sample calculation: no Ethics approval: yes, Research Ethics Committee of the Riviere‐des‐Prairies Hospital Funding/vested interest: none stated Authors' affiliations: none stated Key conclusions of the study authors: fitness level of children with ADHD using medication or not, including body composition, flexibility and muscular endurance was similar to that of the control group. The only difference was observed for BMI which was lower in children with ADHD using medication. Both groups of children with ADHD presented significantly lower scored for locomotion skills Comments from the study authors: in this study, it was not possible to obtain precise data for dosage of medication. Because of this methodological issue, the impact of dosage and time of prescription on growth parameters could not be established Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Vincent 1990.
| Methods | A retrospective cohort study of medical records | |
| Participants | Number of participants screened: not stated Number of participants included: 31 Number of participants followed up: 31 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: mean 12.9 (SD 0.8) years old (range not stated) IQ: not stated Sex: 25 males, 6 females Methylphenidate‐naïve: 0% Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: 34 (SD 14) mg/day or 0.75 (SD 0.29) mg/kg/day Administration schedule: not stated. Duration of intervention: 15 participants received methylphenidate for 6‐12 months, 9 participants for 1‐4 years, 7 participants for 5‐7 years (range: 6 months to 6 years and 11 months) Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Weight and height: participants were measured in indoor clothing and stocking feet on a platform scale twice, 6‐12 months apart (mean: 221 SD 57 days) by treating physician. Weight to nearest 0.1 kg, height to nearest cm. Initial measurements were randomly distributed throughout the year so as to eliminate any seasonal effects. Expected height and weight obtained from National Center for Health Statistics |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interest: not stated Authors' affiliations: not stated Key conclusions of the study authors: the results of this retrospective study suggest that methylphenidate use at customary doses does not noticeably impair adolescent growth velocities over 6‐12 months treatment Comments from the study authors: analysis of age, gender, dose, and other characteristics on the basis of length of treatment was not feasible because of sample size Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested through email correspondence with the authors in September and October 2013. No reply |
|
Walitza 2007.
| Methods | A prospective study analysing genomic damage in children with ADHD (initial sample size 38 children) before and 1 (30 children), 3 (21 children), and 6 (8 children) months after initiation of methylphenidate therapy. In addition a group of 9 children receiving chronic methylphenidate treatment were investigated | |
| Participants |
Prospective group Number of participants screened: not stated Number of participants included: 38 Number of participants followed up: 1 month: 30, 3 months: 21, 6 months: 8 number of withdrawals: 1 month: 8, 3 months: 17, 6 months: 30 Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean: 10 years old (range: 4.9‐17.0) IQ: above 70 Sex: 29 males, 9 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Germany Comorbidity: not stated Comedication: no Sociodemographics: not stated Chronic group Number of participants screened: not stated Number of participants included: 9 Diagnosis of ADHD: DSM‐IV‐TR (subtype: not stated) Age: mean 11.2 years old (range 7.1‐16.0) IQ: above 70 Sex: 9 males, 0 females Methylphenidate‐naïve: 0%. All treated previously 6 months to 2 years Ethnicity: not stated Country: Germany Comorbidity: yes, 4 patients received additional medicine, but diseases not specified Comedication: yes, 4 patients; 1 received valproic acid and 3 received risperidone Sociodemographics: not stated Both groups Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: prospective group: 0.54 mg/kg/day (5‐40 mg/day) increasing to 0.74 mg/kg/day (15‐45 mg/day) by the end of the trial. Chronic methylphenidate users: 0.83 mg/kg/day Administration schedule: not stated Duration of intervention: 1‐6 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Vital signs ECG Micronucleus analysis (in peripheral lymphocytes): blood samples (7.5 mL each) were collected 1 day before, and 1, 3, and 6 months after methylphenidate treatment. The micronucleus scoring was carried out by a single scorer 6 times for each sample in blinded manner Clinical laboratory values: white and red blood cell count, electrolytes, transaminases measured The measure for genomic damage was the frequency of micronuclei, a subset of chromosomal aberrations, in peripheral lymphocytes, obtained from blood samples No abnormal parameters were observed except slightly reduced values of total iron, without signs of hypochromic microcytic anaemia, which occurred in some patients before and during the treatment, independent of micronucleus deviation |
|
| Notes | Sample calculation: not stated Ethics approval: approved by the ethics committee of the University of Würzburg (study no. 140/03) Funding/vested interests: partially funded by grants from the German Research Foundation (Deutsche Forschungsgemeinschaft; KFO 125/1‐1) and from the Interdisciplinary Center for Clinical Research (IZKF N‐5 (1)) and was performed independent of financial or other support by companies producing or selling methylphenidate Authors' affiliations: the authors declare they have no competing financial interest Key conclusions of the study authors: the concern regarding a potential increase in the risk of developing cancer later in life after long‐term methylphenidate treatment is not supported. The study did not find any alteration in the number of micronucleated cells after methylphenidate treatment at 3 follow‐up intervals (up to 6 months) Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: 2 withdrew after 1 month of treatment due to lack of effect Supplemental information regarding information on whether any of the children had intellectual disability received through personal email correspondence with the authors in October 2013 (Stopper 2013 [pers comm]). Also asked for vital signs and ECG in August 2014 (Stopper 2014 [pers comm]). The author no longer has access to this |
|
Walitza 2009.
| Methods | A prospective study (both cohort study: the drug naïve group, and cross‐sectional study: the chronically treated group) analysing genomic damage in 3 different group of children:
|
|
| Participants |
Drug‐naïve group Number of participants screened: not stated Number of participants included: 26 Number of participants followed up: 3 months: 17, 6 months: 11 Number of withdrawals: 3 months: 9, 6 months: 15 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 8.6 years old (range 5‐16) IQ: above 70 Sex: 20 males, 6 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Germany Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Chronically methylphenidate‐treated group Number of participants screened: not stated Number of participants included: 21 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean: 11.4 years old (range 9‐16) IQ: above 70 Sex: 16 males, 5 females Methylphenidate‐naïve: none. All but 1 treated more than 12 months with methylphenidate Ethnicity: not stated Country: Germany Comorbidity: yes, but not specified Comedication: 4. 1 also took atomoxetine, 1 sulthiame, and 2 risperidone Sociodemographics: not stated Both groups Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: after 3 months: 0.46 (SD 0.22) mg/kg/day. After 6 months: 0.46 (SD 0.24) mg/kg/day. Chronically treated group: 0.80 (SD 0.31) mg/kg/day Administration schedule: not stated Duration of intervention: 6 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Vital signs, blood pressure, pulse, ECG Blood test to detect haematological abnormality. Micronucleus analysis (measure of genomic damage): blood sampling and sampling of buccal mucosa cells: baseline, 3, and 12 months after initiation of treatment. Only 1 blood sample/buccal mucosa sample at a certain point of time for the chronic group |
|
| Notes | Sample calculation: not stated Ethics approval: approved by the ethics committee of the University of Würzburg (study no. 124/06) Funding/vested interest: this study (124/06) was funded by grants from the IZKF Wuerzburg (Interdisciplinary Clinical Center of the University of Wuerzburg), project N5‐1, and supported by a grant from the Deutsche Forschungsgemeinschaft (KFO 125) Authors' affiliations: during the course of this study, HS was asked to serve as an independent consultant for Novartis Pharmaceutical Company. Consulting occurred after this study was finished (all data evaluated) and did not influence any aspect of this study. The other authors declare no potential conflicts of interest Key conclusions of the study authors: in this study, we did not find any alteration in the number of micronucleated cells in the group of chronically treated (> 12 months) children compared to the ADHD group before treatment initiation. We also did not find any elevation after initiation of methylphenidate treatment at 2 follow‐up intervals at 3 and 6 months after treatment initiation. This is the result from both investigated tissues, peripheral blood lymphocytes and buccal mucosa cells. Therefore, no induction of genomic damage in ADHD patients due to methylphenidate therapy was detectable, supporting our previous study (124/06) Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: 2 withdraw after 1 month of treatment due to lack of effect Supplemental information regarding the children's intellectual function received through personal email correspondence with the authors in October 2013 (Stopper 2013 [pers comm]), and mean and SD on measure of genomic damage received in March 2014 (Stopper 2014b [pers comm]) |
|
Wang 2007.
| Methods | A multicountry, multicentre, randomised, double‐blind, 8‐week study in children and adolescents with ADHD to test the hypothesis that atomoxetine is non‐inferior to methylphenidate on conventional ADHD symptom measures | |
| Participants | Number of participants screened: 361 Number of participants included: 166 included Number of participants followed up: 152 Number of withdrawals: 14 Diagnosis of ADHD: DSM‐IV (subtype: combined (57.2%), hyperactive‐impulsive (3.6%), inattentive (39.2%)) Age: 9.9 (SD 2.3) years IQ: not stated Sex: 134 males (80.7%), 32 females (19.3%) Methylphenidate‐naïve: 74.7% Ethnicity: Asian (91.6%), Hispanic (8.4%) Country: China (n = 242), Korea (n = 60), Mexico (n = 28) Comorbidity: ODD (17.5%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: 0.2‐0.6 mg/kg/day Administration schedule: morning and lunch Duration of intervention: 8 weeks Treatment compliance: the study completion rate was high 91.6% Titration: treatment titrated from 0.2mg/kg/day to 0.4 mg/kg/day on Day 5 and either maintained or titrated upward or downward within the range 0.2‐0.6 mg/kg/day |
|
| Outcomes |
Non‐serious adverse events Tolerability measures ‐ assessment of treatment emergent adverse events via open‐ended questions Monitoring of vital signs ‐ ECG and clinical laboratory tests (chemistries, haematology and urinalysis) 6 patients (3.6%) discontinued due to:
|
|
| Notes | Sample calculation: yes, approximately 330 patients between the 2 arms Ethics approval: not stated Funding: not stated Vested interest/authors' affiliations: not stated Key conclusions of the study authors: atomoxetine was non‐inferior to methylphenidate in improving ADHD symptoms based on response rates. Treatment‐emergent adverse effects experienced significantly more frequently in the atomoxetine group compared with the methylphenidate group Supplemental information requested through personal email correspondence with the authors in January 2014 with no reply |
|
Wang 2011.
| Methods |
|
|
| Participants | Number of participants screened: not stated Number of participants included: 50 Number of participants followed up: 30 Number of withdrawals: 20 Diagnosis of ADHD: DSM‐IV diagnosis (subtype: combined (48%), hyperactive‐impulsive (22%), inattentive (30%)) Age: mean 7.56, range 6‐12 years old IQ: above 70 Sex: 40 males, 10 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Taiwan Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 5‐15 mg/day Mean methylphenidate dosage: 10.62 mg/daily at 1 month, 14.15 mg/daily at 3 months and 12.84 mg/daily at 6 months. Administration schedule: not stated Duration of intervention: 24 weeks Treatment compliance: the drug compliance at each visit was confirmed to the reports of patients' parents and the remnant drug |
|
| Outcomes |
Non‐serious adverse events No reporting of adverse events 3 patients discontinued prematurely due to decreased appetite and weight loss |
|
| Notes | Sample calculation: no Ethics approval: approved by the Institutional Review Board of Chang Gung Memorial Hospital Funding: the study was funded by the Chang‐Gung Memorial Hospital Research Project Vested interests/authors' affiliations: the authors have no conflicts of interest to declare Key conclusions of the study authors: DHEA (dehydroepiandrosterone), but not the cortisol basal level, may be a biological laboratory marker for ADHD, particularly for performance on the CPT. Both the causal relationship between DHEA and ADHD and the role of DHEA in treating ADHD require further investigation Supplemental information received through personal email correspondence with the authors in December 2013 (Wang 2013 [pers comm]) |
|
Warshaw 2010.
| Methods | Methylphenidate transdermal system use for 7 weeks. 4 weeks dose optimisation followed by 3 weeks on optimised dose. Follow‐up visit 30 days after last dose | |
| Participants | Number of participants screened: 309 Number of participants included: 305 Number of participants followed up: 260 Number of withdrawals: 45 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 9.1, range: 6‐12 years old IQ: not stated Sex: 215 males, 90 females Methylphenidate‐naïve: not stated Ethnicity: white (77.7%), African American (11.5%), Asian (0.7%), Hispanic (23.9%), others (10.2%) Country: USA Comorbidity: not stated Comedication: only medication non concomitant with central nervous system effects Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: transdermal system Methylphenidate dosage: 10, 15, 20, 30 mg Mean methylphenidate dosage: 20.1 mg Administration schedule: MTS 9 hours per day Duration of intervention: 7 weeks Treatment compliance: 260 (84.1%) completed the study |
|
| Outcomes |
Non‐serious adverse events: Safety was assessed at each clinic visit (baseline, at weeks 1 through 5, week 7, and week 11) by evaluating adverse events reported spontaneously; analysing changes in vital signs, and conducting physical examinations. Dermal reactions were classified as an adverse event when pharmacologic treatment for the reaction was required Experience of Discomfort scale, Transdermal System Adherence scale, and Dermal Response Scale |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding: the study was funded by Shire Development, Inc., Wayne, Pennsylvania Vested interests/authors' affiliations: Dr Warshaw has served as a consultant to Shire. Dr Squires is a full‐time employee of Shire and a stock shareholder in Johnson & Johnson, Pfizer, and Shire. Dr Li was a full‐time employee of Shire at the time of the study and is now an employee of Cerexa, Inc, Oakland, California. Dr Civil is a full‐time employee of Shire. Dr Paller has served as a consultant to Shire Key conclusions of the study authors: the results of this study indicate that dermal reactions with methylphenidate use were predominantly mild to moderate. Dermal reactions appeared to be of an irritant contact dermatitis form; they dissipated rapidly with time and most resolved with continued treatment. Overall, less than 1% of participants manifested sensitisation to methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental data requested through personal email correspondence with the authors in May 2016 but none were available |
|
Weber 2003.
| Methods | A retrospective cohort study investigating the side effects of methylphenidate in children | |
| Participants | Number of participants screened: 73 Number of participants included: 57 Number of participants followed up: 45 Number of withdrawals: 12 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 11.1, range 7.2‐18.0 years old IQ: range 85‐115 Sex: 42 males, 3 females Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Germany Comorbidity: anxiety, depression, disturbance in attention, antisocial behaviour and aggressive behaviour Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: 16.8 mg Administration schedule: not stated Duration of intervention: mean 2.7 years, range 0.2‐12.3 years Treatment compliance: questions regarding compliance were included in the mailed questionnaire; however, no results are reported |
|
| Outcomes | Non‐serious adverse events German version of Barkley Side Effects Rating Scale (17 items), parent rated every sixth week | |
| Notes | Sample calculation: not stated
Ethics approval: not necessary at the time the study were carried out
Funding/vested interests: no funding
Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: the application of methylphenidate in therapy of attention deficit disorder and the interpretation of side effects of methylphenidate is a multimodal task. Adverse effects are not correlated with daily doses, age, the severity of body complaints and the presence of neuroticism and extraversion. The children with more side effects showed more emotional comorbidity Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding diagnostic criteria, ethics approval, funding, ratings and drug naïvety received through personal email correspondence with the authors in December 2013 (Weber 2013 [pers comm]) |
|
Weiss 2007.
| Methods | A 5‐11 week randomised, double‐blind, cross‐over comparison of
6‐month open‐label extension period on MLR‐methylphenidate |
|
| Participants |
Cross‐over phase Number of participants screened: 110 Number of participants included: 90 Number of participants followed up: 79 Number of withdrawals: 11 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 11.0, range 6.4‐17.5 years old IQ: above 80 Sex: 74 males, 16 females Methylphenidate‐naïve: 59% Ethnicity: white: 83%, African American: 6%, Asian: 4%, others: 7%) Country: Canada Comorbidity: oppositional defiant disorder (37.5%) Comedication: none Sociodemographics: not stated Open‐label phase Number of participants included: 76 Number of participants followed up: 61 Number of withdrawals: 15 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 11.0, range 6.4‐16.8 years old IQ: above 80 Sex: 62 males, 14 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Canada Comedication: none Sociodemographics: not stated Inclusion criteria
Criteria for entering the open‐label phase: the patients must choose to continue receiving MLR‐MPH after completion of the first part of the study Exclusion criteria
|
|
| Interventions |
Cross‐over Methylphenidate type: long duration multi‐layer release methylphenidate or immediate release methylphenidate Mean methylphenidate dosage: MLR‐MPH: 32.0 (SD 8.4) mg; IR‐MPH: 32.5 (SD 8.6) mg Administration schedule: MLR‐MPH: once daily in the morning. IR‐MPH: twice daily, morning and noon Duration of each medication condition: 2 weeks on a stable dose Washout: 1 week baseline medication washout period Titration period: initial daily dose was based on body weight. Daily dose was titrated in 10‐mg increments over a period of 2‐3 weeks at weekly clinical visits. Titration was halted if the dose was not tolerated, or if the investigator‐rated CGI‐I scale was rated 1‐2. There were 2 weeks of observation on this stable dose Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Cross‐over Clinical assessment of Side Effects (CASE). The scale consist of 26 possible adverse events. Teacher and parent rated by telephone interview before next clinical visit. Side effects, including sleep quality, were assessed on daily basis Open‐label 3 follow‐up visits at 2, 4 and 6 months. CASE; teacher and parent rated by telephone interview just before next clinical visit The 4 cases that dropped out due to side effects dropped out because of the following events: Long‐duration multi‐layer release methylphenidate
Immediate release methylphenidate
|
|
| Notes | Sample calculation: yes Ethics approval: the study protocol and consent form were approved by the Research Ethics Committees at each site Funding: Purdue Pharma Vested interests/authors' affiliations: the study was sponsored by Purdue Pharma (Canada). Dr Weiss is a consultant for Eli Lilly, Janssen‐Ortho, Novartis, Purdue Pharma and Shire. Dr Hechtman is a consultant for Eli Lilly, Janssen‐Ortho, Novartis, Purdue Pharma and Shire. Drs. Hechtman, Jain, Quinn, and Weiss are on the advisory board for Purdue Pharma. Dr Turgay is a consultant for the same company. Mr. Donnelly, Mr. Reiz, Mr. Harsanyi, and Dr Darke are employees of Purdue Pharma. The other authors have no financial relationships to disclose Key conclusions of the study authors: clinical implications of the study results include that once daily, MLR‐MPH is an effective treatment for ADHD that produces equivalent improvements to twice‐daily dosing of IR‐MPH in home and school situational behaviour, while maintaining a favourable side‐effect profile and a prolonged duration of effect Comments from the study authors: a large portion of participants were previous methylphenidate responders, which may have reduced any chance of finding differences. As participants received active medication in both phases if the study, higher effect sizes could be expected because of investigators' and participants' expectation, although this would more closely approximate the clinical situation than a comparison placebo Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information regarding protocol, data for CASE, adverse events on the 4 dropouts, and data on all periods received through personal email correspondence with study funder, Purdue Pharma in August 2013 (Harsanyi 2013 [pers comm]) |
|
Wigal 2011.
| Methods | A double blind, double‐dummy randomised cross‐over study with osmotic release oral system (OROS) methylphenidate and immediate release methylphenidate given with 5 different breakfast conditions | |
| Participants | Number of participants screened: not stated Number of participants included: 32 Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: 30 Number of withdrawals: 1 Diagnosis of ADHD: DSM (subtype: not stated) Age: mean 10.1 (SD 1.5), range 7‐12 years old IQ: not stated Sex: 26 males, 5 females Methylphenidate‐naïve: none Ethnicity: white: 67.7%, African American: 1%, Hispanic: 3%, others: 6% Comorbidity: not stated Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 5 possible drug condition orders of equivalent to OROS MPH 18 mg, 36 mg and 54 mg and IR MPH 5 mg, 10 mg and 15 mg based on pre‐study established doses. Group 1
Group 2
Mean MPH dosage: not stated. Administration schedule: not stated. Duration of each medication condition: not stated. Washout prior to study initiation: not stated. Medication‐free period between intervention: not stated. Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events Vital signs measured pre‐dose, then every 1.5‐2.5 h until 11.5 h postdose |
|
| Notes | Sample calculation: not stated Ethics approval: not stated, but UC Irvine Institutional Review Board approved consent procedures Funding/vested interest/authors' affiliations: Sharon Wigal on a number of drug company advisory boards. Suneel Gupta is a full‐time employee of Impax Pharmaceuticals and previously worked for a number of drug companies. Lynne Starr and Erica Everin are employees of a drug company. The study was sponsored by ALZA corporation. Ortho‐McNeil Janssen Scientific Affairs funded an editorial on the study Key conclusions of the study authors: the results of this study demonstrate that in children with ADHD administering OROS methylphenidate with or without food produces similar PK and PD profiles Comments from the review authors: despite lack of information about ADHD diagnostic criteria, the article is included ‐ because it reports data on serious adverse events (n = 0), deaths (n = 0). This means, that only data regarding serious adverse events should be extracted. Wrong NCT‐number reported Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information received through personal email correspondence with the authors in August 2014. No answer received |
|
Wigal 2013.
| Methods | 4‐6 weeks open‐label treatment (dose optimisation), cross‐over, and 2‐week double‐blind with 2 interventions:
|
|
| Participants | Number of participants screened: 45 Number of participants included: 44 Number of participants followed up: 39 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐IV diagnosis of ADHD (subtype: combined (70.5%), hyperactive‐impulsive (2.3%), inattentive (27.3%)) Age: mean 8.8, range 6‐12 years old IQ: not stated Sex: 32 males, 12 females Methylphenidate‐naïve: none Ethnicity: white (79.5%), Black/African American (9.1%); Asian (6.8%); other (4.5%) Country: USA Comorbidity: elimination disorder (9.1%); oppositional defiant disorder (18.2%); specific phobias (4.5%) Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different sequences of methylphenidate and placebo Methylphenidate type: liquid formulation of extended release methylphenidate Mean methylphenidate dosage: 32.8 mg/day Administration schedule: 4 times daily Duration of each medication condition: 1 week Washout prior to study initiation: yes (1 day for stimulants) Medication‐free period between intervention: no Titration period: 3 weeks initiated before randomisation Treatment compliance: 2 withdrawals of assent/consent, 2 adverse events, 1 lack of efficacy, 1 lost to follow‐up |
|
| Outcomes |
ADHD symptoms ‐ SKAMP, ADHD‐RS (open‐label phase) Non‐serious adverse events 42 participants (93.3%) experienced a treatment‐emergent adverse event 3 (6.7%) participants with severe (affect lability, aggression, and initial insomnia) and 2 (4.4%) participants had to discontinue medication (affect lability and aggression) Open‐label phase: decreased appetite (55.6%), abdominal pain upper (42.2%), affect lability (26.7%), initial insomnia (22.2%), insomnia (17.8%), and headache (17.8%) Other AEs reported in ≥ 5% of the participants during the open phase: vomiting, diarrhoea, logorrhea, aggression, dizziness, irritability, fatigue, upper respiratory tract infection, cough, and flushing Double‐blind phase: 11 (24.4%) participants had a AE while receiving NWP06 and 5 (11.1%) participants had a AE while receiving placebo |
|
| Notes | Sample calculation: no Ethics approval: yes Key conclusions from study authors: NWP06 resulted in significant improvements in the SKAMP‐combined score at 4 hours post‐dose as compared with placebo in the completers. This study shows that NWP06 significantly improved ADHD symptoms in school‐aged children and was well tolerated Comments from the study authors: "This study of NWP06 allowed inclusion of patients who were either treatment naïve or had previously been treated with stimulants" "Subjects were required to have been in need of pharmacological treatment for ADHD" "Our population more closely reflects a real‐world population and provides a more rigorous test of the study drug." Comments from the review authors: laboratory school environment, and lack of the ADHD‐RS. Race/ethnicity doesn't reflect a real‐world population Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not clear Supplemental information requested from study authors in August 2014. No reply |
|
Wigal 2015.
| Methods | A multicentre study of 4 phases: screening, double‐blind (1 week placebo controlled), open‐label (11 week dose optimisation), and a 30 day safety follow‐up | |
| Participants | Number of participants screened: 280 Number of participants included: 230 Number of participants followed up: 200 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐ IV‐TR (subtype: combined (61%), inattentive (33%)) Age: mean 10.8 years (range 6‐18) IQ: > 80 Sex: 154 males, 76 females Methylphenidate‐naïve: 148 Ethnicity: white: 68.7%, African American: 23%, Asian:1.3%, others: 7% Country: USA Comorbidity: ODD 22, enuresis 14 Comedication: not stated Sociodemographics: unknown Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: extended release Methylphenidate dosage: 10‐60 mg/day Methylphenidate dosage during 11 weeks open‐label titration phase: 30 mg (27.7%), 40 mg (25.2%), 50 mg (17.8%), 20 mg (16.8%), 60 mg (8.9%), 15 mg (2.0%), 10 mg (1.5%) Administration schedule: once daily, in the morning, no later than 10 am Duration of intervention: 11‐12 weeks Treatment compliance: not stated |
|
| Outcomes | Vital signs, physical examination, ECG, clinical laboratory evaluations, C‐SSRS, and AEs were collected at each visit | |
| Notes | Sample calculation: not stated
Ethics approval: the study protocol, amendments, and informed consent were reviewed and approved by an Institutional Review Board for each study site
Funding: this research was funded by Rhodes Pharmaceuticals LP Vested interests/authors' affiliations: Dr Wigal has been an advisory board and speakers bureau member/consultant for Eli Lilly, Ironshore, Neos, NextWave, Noven, NuTec, Pfizer, Purdue, Rhodes Pharmaceuticals LP, Shionogi, Shire, and Tris and has received grant and research support from Eli Lilly, Forest, Ironshore, the National Institutes of Health, NextWave, Noven, NuTec, Purdue, Rhodes Pharmaceuticals LP, Shire, Sunovion, and Tris. Dr Nordbrock is a consultant for Rhodes Pharmaceuticals LP Dr Adjei and Dr Kupper are employees of Rhodes Pharmaceuticals LP Dr Childress has received research support from Shire, Novartis, NextWave, Lilly, Forest Research Institute, Johnson & Johnson, Sepracor, Otsuka, Sunovion, Pfizer, Shionogi, Noven, Ironshore, Rhodes, Theravance, Neurovance, Neos, Arbor, Tris, and Purdue. She has received consulting fees or honoraria from Ironshore, Pfizer, Shionogi, Rhodes, Shire, Novartis, and Neos; support for travel from Ironshore, Pfizer, Shire, Novartis, and NextWave; writing assistance on projects from Shire, Novartis, NextWave, Pfizer, Ironshore, and Arbor; and payment for lectures from Shire, Novartis, and Pfizer. Dr Greenhill has received research support from the National Institute on Drug Abuse/National Institutes of Health and Shire and is on the advisory board for BioBehavioral Diagnostics Key conclusions of the study authors: dose‐related improvements in ADHD‐RSIV scores that exceeded those of placebo were observed in patients treated with methylphenidate‐MLR. No new safety signals were noted Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Wiguna 2012.
| Methods | 12‐week open, prospective, non‐randomised trial using a pre‐test and post‐test design | |
| Participants | Number of participants screened: not stated Number of participants included: not stated Number of participants followed up: 21 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV/ICD‐10 (subtype: combined (71.4%), inattentive (28.6%)) Age: mean 8.52, 7‐10 years old IQ: mean 110.14 Sex: 17 males, 4 females Methylphenidate‐naïve: 100% Ethnicity: Indonesian Comorbidity: none Comedication: none Sociodemographics: low to average socioeconomic status Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were assessed before and after methylphenidate‐treatment Methylphenidate type: long acting Methylphenidate dosage: 20 mg Administration schedule: daily Duration of intervention: 12 weeks Treatment compliance: not stated |
|
| Outcomes | Weight, height, pulse, blood pressure and interim history: every 2 weeks | |
| Notes | Sample calculation: yes Ethics approval: yes Funding: not stated Vested interests/authors' affiliations: not stated Key conclusions of the study authors: findings of this pilot study are important in that it demonstrated, with stimulant treatment, significant neurochemical changes ‐ thought to reflect functional improvement and improved neuroplasticity ‐ in the prefrontal cortices of children with ADHD |
|
Wilens 2005.
| Methods | A multicentre, open‐label, cohort study of methylphenidate osmotic release oral system (OROS) use for 24 months | |
| Participants | Number of participants screened: 436 Number of participants included: 407 Number of participants followed up: 289 (12 months), 229 (24 months) Number of withdrawals: 118 (12 months), 178 (24 moths) Diagnosis of ADHD: DSM‐IV (subtype: combined (75.5%), hyperactive‐impulsive (5.9%), inattentive (18.4%), not determined ( 0.2%)) Age: mean 9.2, range 6‐13 years old IQ: not stated Sex: 338 males, 69 females Methylphenidate‐naïve: none Ethnicity: white: 86%, African American: 5.7%, Asian: 0.7%, Hispanic:4.4%, others: 3.2% Country: USA Comorbidity: tics (11.8%) Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: extended release (OROS) Methylphenidate dosage: 18 mg/day, 36 mg/day, or 54 mg/day Mean methylphenidate dosage: 35 mg at study entry, 41 mg at the end of 12 months, and 44.2 mg at month 21/24 Administration schedule: once daily Duration of intervention: 21 to 24 months Treatment compliance: 86.4% |
|
| Outcomes |
Non‐serious adverse events Growth (weight, height), rated objectively by observer at monthly visits for the first 12 months and then at 15, 18, 21, and 24 months Cardiac (heart rate, blood pressure), rated objectively by observer at monthly visits for the first 12 months and then at 15, 18, 21, and 24 months Sleep quality, tics, rated subjectively by parents at monthly visits for the first 12 months and then at 15, 18, 21, and 24 months Laboratory tests (urinalysis, haematology/complete blood counts, electrolytes, and liver function tests) were performed at baseline, at 6 and 12 months, and at the end of the study (21/24 months) At 12 months, 28 (6.9%) discontinued study medication prematurely because of adverse events. At 21/24 months, 38 discontinued study medication prematurely because of adverse events. From final: there was a 26% increase in mean daily dose over the study period, with most of the increase occurring during year 1. In general, treatment was well tolerated, with 31 (7.6%) participants discontinuing because of adverse events. Comparison of the baseline characteristics of participants discontinuing the study compared with those continuing with treatment for 21/24 months did not reveal any significant differences between the 2 groups for ADHD subtype, teacher and parent/caregiver IOWA Conners inattention/overactivity and oppositional/defiant baseline scores, previous stimulant exposure, presence of comorbidity, specific comorbidity, race, sex, or type of school classroom |
|
| Notes | Sample calculation: no Ethics approval: reviewed and approved by the institutional review board of participating centres prior to initiation of the study Funding: supported by McNeil Consumer & Specialty Pharmaceuticals Vested interests/authors' affiliations: all authors work for different medical companies Key conclusions of the study authors: sustained effectiveness of OROS methylphenidate was maintained for up to 24 months with minimal effects on growth, tics, vital signs, or laboratory test values, as demonstrated by stable IOWA Conners ratings and sustained improvements in peer interaction and Global Assessment Scale scores Comments from the study authors: the prospective trial was terminated by the sponsor (concomitant with US FDA approval) between 21 and 24 months of participation for administrative reasons that were not related to safety or effectiveness. Therefore, although data were available for a minority of participants at 24 months (n = 56), we refer to the final end point as 21/24 months Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes. Included children had participated in previous controlled studies and were methylphenidate responders |
|
Wilens 2006.
| Methods | A 2‐week randomised, double‐blind, 15‐centre, parallel trial with 2 arms:
Preceded by a 4 week open‐label dose titration phase and followed by a 8 week open‐label follow‐up |
|
| Participants | Number of participants screened: not stated Number of participants included: 220 in the 4 week dose titration phase Number of participants randomised to methylphenidate: 87 and placebo: 90 Number followed up in the methylphenidate group: 71 Number of withdrawals in methylphenidate group: 16 Number followed up: 171 (total) Number completed follow‐up: 135 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean: 14.6 years old (range 13‐18) IQ: not stated Sex: 142 males, 35 females Methylphenidate‐naïve: not stated, but ADHD treatment naïve (n = 24) Ethnicity: white (75.1%), African American (13.6%), others (11.3%) Country: USA Comorbidity: no Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to OROS‐methylphenidate or placebo Mean methylphenidate dosage: 0.84 mg/kg Administration schedule: once daily Duration of intervention: 2 weeks Titration period: 4 weeks initiated before randomisation. All participants initiated therapy at 18 mg/day, and clinical response was measured after 1 week. If response to treatment was inadequate, as per the a priori study definition, the dose was titrated upward (in 18 mg increments) at 1 week intervals for up to 4 weeks, with the maximum dose being 72 mg/day Treatment compliance: not stated 8‐week open‐label follow‐up on individualised dosage |
|
| Outcomes |
ADHD symptoms ADHD‐RS, clinician and parent rated, completed at baseline and weekly during double blind phase Serious adverse events Serious adverse events were reported in only 1 participant during the open‐label dose titration phase of the study. While being treated with OROS, 18 mg/daily, a 16‐year‐old female participant with a history of depression and suicidal ideation threatened suicide on the third day of medication use after an argument with her mother. A decision was made to discontinue study medication, and the symptoms resolved No serious adverse events were reported during the double‐blind phase Non‐serious adverse events Heart rate and blood pressure, recorded by a clinician weekly throughout whole study ECG at screening and at the end of the double‐blind phase of the study Spontaneous reports to the investigator of adverse events were recorded at weekly visits Safety assessments made at monthly visit and every 2 weeks between monthly visits during the follow‐up Height and weight were assessed at baseline and at weeks 4 and 8 in the follow‐up study No participants experienced clinically important effects on ECG‐indexes, heart rate or blood pressure during the study |
|
| Notes | Sample calculation: yes Ethics approval: yes, approval of the study design was obtained from the institutional review boards for all participating centres before initiation of the study Key conclusions of the study authors: in adolescents, once‐daily OROS methylphenidate significantly reduced ADHD symptoms and was well tolerated using dosages up to 72 mg/daily. Adolescents required, on average, a higher absolute dose but a lower weight‐adjusted dose (mg/kg) of OROS than was previously reported in children. The incidence of adverse events was not related to dose Comments from the study authors: participants were titrated to their individualised dosage before the double‐blind phase of the study may have biased the results toward a positive response in the double‐blind phase. The short duration of the double‐blind phase also may have decreased the likelihood of detecting potential rare adverse events. The rates of adverse events reported for OROS have been underestimated, because participants entering this study phase were already stabilised on an affective tolerated dosage of medication Supplemental information requested through email correspondence with the authors in December 2013 and January 2014. No reply |
|
Wilens 2008.
| Methods | An open‐label, 5‐week, dose optimisation period followed by a randomised, double‐blind, 8‐centre, 3‐way, 3‐week, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: 148 Number included in open‐label dose‐titration: 128 Number randomly assigned to 1 of 3 possible drug orders: 120 Number followed up for safety: 127 and for efficacy: 117 Number of withdrawals: 2 Characteristics of the 127 followed up for safety Diagnosis of ADHD: DSM‐IV (combined or hyperactive‐impulsive (92,1%)) Age: mean 8.8 (SD 1.84), range 6‐12 years old IQ: above 80 Sex: 84 males, 43 females Methylphenidate‐naïve: not stated Ethnicity: white (62.2%/63.2%), African American (not stated/15.4%), Asian (not stated) Country: USA Comorbidity: not allowed Comedication: not stated Sociodemographics: not stated Characteristics of the 117 followed up for efficacy Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 8.8 (SD 0.2), range 6‐12 years old IQ: above 80 Sex: 75 males, 42 females Methylphenidate‐naïve: not stated Ethnicity: white (63.2%), African American (15.4%), Asian (0%), others (21.4%) Country: USA Comorbidity: not allowed Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug orders of (4 and 6 hours) methylphenidate and placebo Mean methylphenidate dosage: 10 mg patch (n = 15), 15 mg patch (n = 34), 20 mg patch (n = 32), and 30 mg patch (n = 36) Administration schedule: once daily in the morning, patch worn for 9 h daily, and 4 or 6 h for the cross‐over assessments Duration of each medication condition: 1 day Washout: none Titration period: 5 weeks before randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms SKAMP, teacher, rated at randomisation (week 5) and 2 hrs after patch application end of treatment (week 6, 7, and 8) Quality of life ADHD Impact Module‐Child (AIM‐C) ‐ Child Impact Scale and Family Impact Scale, rated at baseline and randomisation (week 5) and end of study (week 8) Non‐serious adverse events Vital signs were evaluated at screening, baseline, and on weeks 1 to 8 (erythema, oedema, papules, and vesicles, discomfort, haematology, urinalysis, and electrocardiographic measures were completed at screening, baseline, and weeks 5 and 8) No clinically meaningful changes from baseline were observed in vital signs, ECG, urinalysis, and haematological results, or physical examinations Adverse events were recorded from the time informed consent was signed until 30 days (week 12) after the last drug treatment |
|
| Notes | Sample calculation: yes Ethics approval: yes, institutional review board at each site approved the study Comments from the study authors: important to note that participants who failed to respond to psychostimulants in the past and those with conduct disorder were excluded from the study. The results of this study therefore should not be extrapolated to these patient populations. From Manos: the lack of placebo comparison has the potential to confound the findings of this study. The relatively short study duration (about 2 months) may not be enough time to capture some emerging changes in HRQoL Key conclusions from study authors: all of the efficacy measures indicated that 4‐ and 6‐hour wear times improved ADHD symptoms Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes, participants with a history of failing to respond to psychostimulant treatment were also excluded Supplemental information requested from the authors twice in August 2014. No reply |
|
Williams 2008.
| Methods | A cohort study of methylphenidate use for 4 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 51 Number of participants followed up: 33 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype: combined: 58.8%, inattentive: 37.3%, hyperactive‐impulsive: 3.9%). Age: mean 13.79 (SD 2.33, range 8‐17) years old IQ: above 80 Sex: 51 males Methylphenidate‐naïve: 51% Ethnicity: not stated Country: Australia Comorbidity: not stated C‐medication: no participant was taking concurrent medications known to affect the CNS Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: immediate release Mean methylphenidate dosage: 24.1 mg/day (range 10‐60 mg/day). 26 medication naïve titrated to maximum effective dose (0.4‐1.3 mg/kg) in a week with 10 mg increments then kept on a stable dose for a week. 25 had 3 day washout then resumed optimal methylphenidate dose after baseline testing Administration schedule: methylphenidate given 60 minutes before testing Duration of intervention: 4 weeks Treatment compliance: not stated |
|
| Outcomes | No adverse events were reported | |
| Notes | Sample calculation: not stated Ethics approval: not stated Funding: an Australia Research Council (ARC) Linkage Grant with industry partner Brain Resource Company (BRC) (Grant no. LP0349079) supported this work. LMW holds a competitive, peer‐reviewed Pfizer Senior Research Fellowship. We acknowledge the support of the Brain Resource International Database (under the auspices of the Brain Resource Company) for data acquisition and processing. All scientific decisions are made independent of any BRC commercial decisions via the independently operated scientific division, BRAINnet Vested interests/authors' affiliations: DP, HK, and SC have no conflicts of interest to declare. As an industry partner on the ARC‐linkage grant that supported this research (Grant no. LP0349079), BRC contributed to the salary of DFH as research officer on this grant for 2003‐2005. MK uses BRC computerised cognitive tests in private practice. LMW and CRC hold a few private shares in BRC (1% of the company value), and CRC holds a number of share options in the BRC. EG is the chief executive officer of BRC. However, scientific decisions are made independent of the BRC operations, and access to the Brain Resource International Database for scientific purposes is coordinated via an independent scientific network BRAINnet Key conclusions of the study authors: methylphenidate normalised neural activity and produced some improvement of emotion recognition but had no impact on negative mood. Disruptions to emotional brain function may be improved with methylphenidate Supplemental information received through personal email correspondence with the authors in August 2014 (Williams 2014 [pers comm]) |
|
Williamson 2011.
| Methods | 2 patient reports of resolution of enuresis when treated with methylphenidate | |
| Participants |
Case 1 Diagnosis of ADHD: DSM‐IV (subtype: combined) Age: 9 years old IQ: normal Sex: female Ethnicity: not stated Country: USA Comorbidity: primary nocturnal enuresis Comedication: no Sociodemographics: not stated Case 2 Diagnosis of ADHD: DSM‐IV (subtype: inattentive) Age: 11 years old IQ: normal Sex: male Ethnicity: not stated Country: USA Comorbidity: primary nocturnal enuresis Comedication: no Sociodemographics: not stated |
|
| Interventions |
Case 1 Methylphenidate type: extended release (Concerta) Methylphenidate dosage: 54 mg Administration schedule: once daily Duration of intervention: not stated Treatment compliance: not stated Case 2 Methylphenidate type: extended release dexmethylphenidate (Focalin XR) Methylphenidate dosage: 10 mg Administration schedule: once daily Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Case 1 Cessation of enuresis occurred immediately after initiating the stimulant Case 2 On days taking stimulant, no enuresis occurred. On days when not taking stimulant, enuresis did occur |
|
| Notes |
Key conclusions of study authors: our patient series add to the existing literature of associations between ADHD and enuresis Comments from the study authors: information was gathered only from parents. We did not obtain collateral information from teachers or any other sources Funding/vested interests/authors' affiliations: this work was supported by the University of Alabama's Research Grants Committee. The authors report no conflicts of interest |
|
Winsberg 1982.
| Methods | A non‐randomised, double‐blind cross‐over trial with fixed doses of methylphenidate given twice a day for a week each in the following schedule:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 25 Participants were administered successive 5 1‐week treatment conditions of fixed oral dose given twice daily Number of participants followed up: 20 Number of withdrawals: 5 Diagnosis of ADHD: DSM‐III (subtype: not stated) Age: mean 9.27, range 6.7‐12.1 IQ: mean 98.2 (14.2) Sex: 25 males Methylphenidate‐naïve: not stated Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were administered successive 5 1‐week treatment conditions of fixed oral doses given twice daily according to the following order: placebo 1; 0.25 mg/kg; 0.50 mg/kg; 1.00 mg/kg; placebo 2 Methylphenidate type: not stated Duration of intervention: 5 weeks Washout: not stated Titration period: none Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Treatment‐emergent side effects, weight, blood pressure measured at the end of each treatment period (after 1 week intervention). Blood pressures were obtained 2 hours following the administration of the oral dose |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests/authors' affiliations: not stated Key conclusions from study authors: teacher and parents ratings showed increased improvement in social behaviour as a function of methylphenidate dose. No drug effects were obtained on cognitive performance. Methylpenidate plasma concentrations were significantly associated with oral dose and with measures of social behaviour. No relationship was found with cognitive behaviour. Side effects at the largest dose were severe enough to require discontinuation of treatment for 5 children, but were relatively mild for the rest of the children Supplemental information requested through personal email correspondence with authors in September 2013. Not able to get supplemental information regarding data on side effects, and therefore we cannot use the data in the meta‐analyses but only report them as part of a weighted mean (Hungund 2013 [pers comm]) |
|
Winterstein 2009.
| Methods | A 10 year retrospective cohort design evaluating the cardiac safety of methylphenidate and amphetamine salts | |
| Participants | Number of participants screened: 2,131,953 Number of participants included: 30,576 Number of participants included in methylphenidate arm: 18,238 Number of participants followed up: 18,238 Number of withdrawals: 0 Diagnosis of ADHD: ICD‐9 (subtype: not stated) Age: mean 8.5‐9.2, range: 3‐20 years old IQ: not stated Sex: 13332 males, 4906 females Methylphenidate‐naïve: 100% Ethnicity: white: 44‐48.2%, African American: 31.6‐34.7%, Hispanic: 14.4‐15.5% Country: USA Comorbidity: circulatory disease (3.5%) Comedication: bronchodilators 15.5‐16.3%, antidepressants 14‐16.4%, antipsychotics 8.2% Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: not stated Treatment compliance: not stated |
|
| Outcomes |
Serious adverse events: Data assembled from the Florida Medicaid fee‐for‐service programme Cardiac event defined as a first emergency department (ED) visit for cardiac disease or symptoms: myocardial infarction, stroke, hypertensive disease (excluding malignant causes), angina, aortic or thoracic aneurysm, arrhythmias, syncope, or tachycardia or palpitation ED visits were chosen as clinical end point because they occur more frequently than hospital admissions or cardiac death and provided the best power to detect even subtle difference between drugs |
|
| Notes | Sample calculation: no Ethics approval: not stated Funding: funded in part by the Florida Department of Health, Agency for Healthcare Administration Vested interests/authors' affiliations: Dr Gerhard was in part funded by a grant from the Agency for Healthcare Research and Quality Key conclusions of the study authors: exposure to methylphenidate and amphetamines salts showed similar risk for cardiac ED visits Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information requested through personal email correspondence with the authors in January 2014 with no reply |
|
Witt 2008.
| Methods | Open‐label parallel design study of methylphenidate or dexamphetamine use for 3 months | |
| Participants | Number of participants screened: 84 Number of participants included: 63 Number of participants randomised to methylphenidate: 34 Number of participants followed up in the methylphenidate group: 25 Number of withdrawals: 9 Diagnosis of ADHD: DSM‐IV (subtype: combined (48%), hyperactive‐impulsive (4%), inattentive (44%), not specified (4%)) Age: mean 8.9 (SD 1.9), range 6‐12 years old IQ: not stated Sex: 16 males, 9 females Methylphenidate‐naïve: 100% Ethnicity: white: 64%, African American: 32%, Hispanic: 4% Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: Concerta (n = 24), Ritalin LA (n = 1) Methylphenidate dosage: n = 3, 8 mg Concerta/day; n = 11, 27 mg Concerta /day; n = 7, 36 mg Concerta/day; n = 3, 54 mg Concerta/day; n = 1, 30 mg Ritalin LA/day Administration schedule: first 4 weeks comprised weekly dose titration assessment with clinician using standardised parent/teacher rating scales to ensure adequate dose given. Thereafter, monthly reviews of medication, with adjustment of dose, as needed time points: once daily dose Duration of intervention: 90 days Treatment compliance: 96% |
|
| Outcomes |
Serious adverse events: Cytotoxic damage to cells cultured from blood samples Non‐serious adverse events: Other adverse events have been recorded, but the data are not available. AEs recorded include insomnia, syncope, loss of appetite, and a worsening of ADHD symptoms |
|
| Notes | Sample calculation: the target enrollment of 30 participants in each group was based on the projected number of participants needed to detect a doubling of any of the cytogenetic endpoints with ≥ 90% power at a 0.05 level of significance. Posthoc power analyses with actual sample sizes confirmed that, even with less‐than‐target enrollment, the power was ≥ 90% for detecting a doubling of each of the 3 cytogenetic endpoints within each treatment group because the variability of each endpoint was smaller than anticipated
Ethics approval: this study protocol was reviewed and approved by the institutional review boards of both Duke University Medical Center and the National Institute of Environmental Health Sciences
Funding/vested interests/authors' affiliations: Dr Kollins received research support and/or honoraria/ consulting fees from the following sources during the conduct of this study: Athenagen, Eli Lilly, Psychogenics, Pfizer, New River Pharmaceuticals, Shire Pharmaceuticals, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, and Environmental Protection Agency. Dr Chrisman received honoraria and was on the speakers' bureaus of Shire Pharmaceuticals and McNeil‐PPC. The other authors report no conflicts of interest Key conclusions of the study authors: the present study found no evidence of changes in any of the 3 cytogenetic endpoints examined in the lymphocytes of children treated with methylphenidate or mixed amphetamine salts based products continuously for 3 months. Our results add to a growing body of evidence that therapeutic levels of methylphenidate do not induce chromosomal damage in humans Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no, only methylphenidate‐naïve patients included Supplemental data on adverse events requested from the study authors. Answer received in July 2014: data are not available (Witt 2014 [pers comm]) |
|
Woolley 2003.
| Methods | A patient report of obsessive‐compulsive disorder (OCD) emerging during methylphenidate treatment | |
| Participants | Diagnosis of ADHD: ICD‐10 (subtype: not stated) Age: 11 years old IQ: normal Sex: male Ethnicity: not stated Country: UK Comorbidity: obsessive‐compulsive disorder Comedication: Risperidone (1 mg/day) Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: immediate release Methylphenidate dosage: 40 mg/day Administration schedule: not stated Duration of treatment: more than 15 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Obsessions and compulsions with anxiety (DSM‐IV diagnosis of OCD). Washing his hands excessively, accompanied by checking rituals, reassurance seeking, and emetophobia. When methylphenidate was withdrawn the OCD symptoms decreased rapidly but increased when methylphenidate was reintroduced |
|
| Notes |
Key conclusions of the study authors: obsessive‐compulsive disorder (OCD) may emerge with stimulant treatment for attention deficit hyperactivity disorder (ADHD). We report a case of OCD worsening with methylphenidate treatment but not with dexamphetamine Funding/vested interest/authors affiliation: not stated Supplemental information regarding ADHD diagnosis and IQ received through personal email correspondence with the authors in October 2013 (Heyman 2013 [pers comm]) |
|
Yalcin 2012.
| Methods | 2 patient reports of methylphenidate use and development of dysphonic symptoms | |
| Participants |
Case 1 Diagnosis of ADHD: DSM‐IV Age: 10 years old IQ: normal Sex: male Country: Turkey Comorbidity: none Comedication: none Sociodemographics: living in metropolitan area with high school student mother who doesn't work and university graduate father who works as sergeant in the army, and older sister Case 2 Diagnosis of ADHD: DSM‐IV Age: 11 years old IQ: normal Sex: male Country: Turkey Comorbidity: specific learning disorder and social anxiety Comedication: not stated Sociodemographics: fourth child of high school graduate father who works as official and elementary school graduate mother |
|
| Interventions |
Case 1 Immediate release methylphenidate dosage: 5 mg Administration schedule: twice a day Duration of intervention: 2 weeks Treatment compliance: not stated Case 2 OROS methylphenidate dosage: 18 mg/day Administration schedule: morning Duration of intervention: 2 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Case 1 Disturbance of voice quality, hoarseness and bifurcation‐strain and over vibration of voice observed by teacher and family. The symptoms began on the first day of methylphenidate treatment. Hoarseness and reduction in the voice amplitude was evident during the first psychiatric control Drug‐free days: no symptoms observed by parents or during the psychiatric control. Methylphenidate administration in the clinic: hoarseness. 3 hours after ingestion: voice quality returned to normal. Physical and endoscopic examination: laryngitis or other organic conditions causing hoarseness were not observed Discontinuation of methylphenidate and administration of atomoxetine: no important side effects Case 2 Significant appetite loss, drowsiness, disturbance of voice and hoarseness. Disturbance of voice and hoarseness occurred every day since the first day on the medication. The symptoms started shortly after the single morning dose and decreased gradually until dinner time and disappeared before bedtime Drug‐free days: no symptoms. Otolaryngology consultation: no organic pathology was detected with respect to clinical and endoscopic observation Discontinuation of methylphenidate and prescription of atomoxetine: no symptom of hoarseness or disturbance of voice quality |
|
| Notes | Funding/vested interest/authors' affiliations: the authors reported no conflict of interest related to this article Key conclusions of the study authors: 2 male children developed disturbances in voice quality and hoarseness associated with MPH use, which required drug discontinuation Comments from the study authors: according to the Naranjo 1981 classification, +5 point was calculated (+5‐8 possibly related side effect) for the hoarseness and impairment of voice quality associated with methylphenidate for these patients Supplemental information regarding IQ and ADHD diagnostic criteria received through personal email correspondence with the authors in March 2013 (Yalcin 2013 [pers comm]) |
|
Yalcin 2014.
| Methods | A cohort study of osmotic release oral system (OROS) methylphenidate use for 60 days | |
| Participants | Number of participants screened: not stated Number of participants included: 40 Number of participants followed up: 33 Number of withdrawals: 7 Diagnosis of ADHD: DSM‐IV (subtype: combined (54.54%), hyperactive‐impulsive (18.18%), inattentive (27.27%) Age: mean 9.20 years (range 6‐12) IQ: not stated Sex: 33 males Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Turkey Comorbidity: oppositional defiant disorder: 9.09%; enuresis: 6.06% Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: 18 mg Administration schedule: fixed daily dose (no titration) Duration of intervention: 60 days Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Weight and height before and after methylphenidate. Mothers rated severity of methylphenidate associated adverse reactions (sleep problems, headache, tics, loss of appetite,abdominal pain, weight loss, sadness, mouth dryness, nausea, vomiting, fears, irritability, skin eruption and others): 0, not a problem; 1, mild; 2, moderate; 3, severe. Before and after medication appetite status of the children was rated by the mothers |
|
| Notes | Sample calculation: not stated Ethics approval: Gazi University Medical Faculty, Ethics Committee Funding/vested interests: this research was not supported by university funds or any drug company Key conclusions of the study authors: this is the first study which directly aims to determine methylphenidate's effect on serum active ghrelin levels. Further research with higher methylphenidate doses and/or other stimulants such as atomoxetine and amphetamine should be done as ghrelin is also associated with obesity, alcohol and drug addiction and reward system pathologies, which are also closely related to attention deficit hyperactivity disorder Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no, 100% were methylphenidate‐naïve |
|
Yang 2004.
| Methods | A 16 week, open‐label methylphenidate treatment study | |
| Participants | Number of participants screened: not stated Number of participants included: 25 Number of participants followed up: 19 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐IV (subtype: combined (100%)) Age: mean 8.7 (SD 1.7) years (range: 6 years and 4 months ‐ 11 years and 10 months) IQ: full intelligence quotients available from 10 participants (mean 100, range 86‐129). The other 9 participants were estimated to be normal Sex: 14 males, 5 females Methylphenidate‐naïve: not stated Ethnicity: 100% Asian Country: Taiwan Comorbidity: none Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 10 mg/day (starting dose, bodyweight < 30kg) or 20 mg/day (starting dose, bodyweight ≥ 30 kg) Mean methylphenidate dosage: 18.95 (SD 7.56, range 10‐35) mg/day, equivalent to 0.61 mg/kg of bodyweight (SD 0.15, range 0.38‐0.87 mg/kg) Administration schedule: twice daily, mornings before school and at 1 pm Titration period: 3 weeks Duration of intervention: 16 weeks Treatment compliance: 4 noncompliant |
|
| Outcomes | The study does not mention any measuring of adverse events. However it is reported, that 2 participants dropped out due to intolerable medication side effects | |
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: not stated Authors' affiliations: Department of Psychiatry, Kaohsiung Medical University Hospital and Department of Psychiatry, Chung Shan Medical University Hospital, Taiwan Key conclusions of the study authors: the methylphenidate treatment demonstrated improvement in domains of classroom/home behaviours and academic performance, but showed minimal change on neuropsychological functioning in Taiwanese ADHD children. The finding of academic gain was unexpected, which might be due to the greater interest in achievement and better compliance to cultural expectations by Taiwanese versus Western students, which translated into more rapid improvement in academic performance Comments from the study authors: limitations of the study: the present study was conducted in a naturalistic manner, without a placebo controlled or contrast group and without blinding. The diagnoses were made by clinicians, without the benefit of standardised diagnostic instruments administered by a blind clinician. The exclusion criterion dyslexia was not tested Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no |
|
Yang 2012.
| Methods | A single‐blind (rater blinded) randomised parallel trial comparing:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 262 Number of participants randomised to methylphenidate: 130 Number of participants followed up: 85 Number of withdrawals: 39 Diagnosis of ADHD: DSM‐IV (subtype: inattentive (42%), combined (56.5%), hyperactive/impulsive (1.2%) Age: mean 9.64 (SD 1.95), range 7‐14 years old IQ: above 70, mean 102.99 (15.01) Sex: 129 males, 23 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: China Comorbidity: oppositional defiant disorder (n = 44), conduct disorder (n = 2) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: started at 18 mg/daily and could be increased each week to 36 mg/daily and then 54 mg/daily according to the patients response Mean methylphenidate dosage: not stated Administration schedule: not stated Duration of intervention: titration + 4‐6 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: 15 dropped out of the methylphenidate group due to adverse events |
|
| Notes | Sample calculation: yes Ethics approval: yes Funding: Xian‐Janssen Pharmaceutical Ltd. Vested interests/authors' affiliations: Xian‐Janssen, Eli Lilly Key conclusions of the study authors: the results imply that both OROS‐methylphenidate and atomoxetine could improve EF in ADHD children Comments from the study authors: there are a number of methodological limitations in the current study. First, we did not include a group that received placebo; therefore, there might have been a bias in the results for the potential placebo effect when we estimated the effect of each medication. The relatively small sample size in the atomoxetine group may have underestimated its therapeutic effect. Further, the slight differential effect between OROS‐methylphenidate and atomoxetine treatment groups might also have been a type I error. We excluded youths with significant current psychiatric co‐morbidity, restricting the generalisation the findings to more co‐morbid, clinically relevant populations Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information requested from the study authors in May 2014. No reply |
|
Yatsuga 2014.
| Methods | A cohort study of methylphenidate use for 3 months | |
| Participants | Number of participants screened: not stated Number of participants included:, 50 Number of participants followed up: 50 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV TR (subtype not stated) Age: mean 9.7 years (SD 2 years 8 months) (range: 6‐16) IQ: mean 93.15 Sex: 50 males Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Japan Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: 18/27 mg (0.5‐1.2 mg/kg/day) Administration schedule: not stated Duration of intervention: 3 months Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: In all, 7 children reported some kind of adverse effect: 2 children reported headache, 3 children had appetite loss, 3 children had sleeplessness and 1 child reported appetite loss and sleeplessness |
|
| Notes | Sample calculation: not stated
Ethics approval: ethics committee of Graduate School of Medical Sciences, Kumamoto University
Funding/vested interests/authors' affiliations: Grant‐in‐Aid for Scientific Research (B) and Challenging Exploratory Sports, Science and Technology (MEXT) of Japan (KAKENHI:grant number 24300149 and 23650223 to A.T). Partially supported by Grant in Aid for Scientific Research from Japan‐U.S. Brain Research Cooperation Program (grant number 210201 to A.T) as well as the Research Grants from the University of Fukui to AT. The funding organisations had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or in preparation, review or approval of the manuscript. No authors have financial or personal relations that could pose a conflict of interest
Any withdrawals due to adverse events: none Key conclusions of the study authors: this study showed no relation between the COMT genotype and methylphenidate adverse effects Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: none indicated |
|
Yildiz 2010.
| Methods | An 8‐week, open‐label, prospective, parallel study with 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 90 Number of participants randomised: OROS‐MPH: 50 and IR‐MPH: 40 Number of participants followed up: OROS‐MPH: 47 and IR‐MPH: 36 Number of withdrawals: OROS‐MPH: 3 and IR‐MPH: 4 Diagnosis of ADHD: DSM‐IV (subtype: combined (IR‐MPH: 75.0%, OROS‐MPH: 80.9%), inattentive (IR‐MPH: 25.0%, OROS‐MPH: 19.1%)) Age: mean 9.7 SD 1.8, range 7‐15 years old (IR‐MPH: 9.3 SD 1.3 and OROS‐MPH: 10.0 SD 2.1) IQ: above 80. WISC‐R total IQ: IR‐MPH: 99.9 SD 14.2 and OROS‐MPH: 99.5 SD 10.9 Sex: IR‐MPH: 28 males, 8 females. OROS‐MPH: 41 males, 6 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Turkey Comorbidity: specific learning difficulties (IR‐MPH: 22.2%, OROS‐MPH: 23.4%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Dosage the first 4 weeks: IR‐MPH: 10 mg/day. OROS‐MPH: 18 mg/day After the fourth week: IR‐MPH: 10 mg/day (n = 16) or 20 mg/day (n = 20) and OROS‐MPH: 18 mg/day (n = 22) or 36 mg/day (n = 25) Duration of intervention: 60 days Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Based on Side Effects of Stimulant Medications for Screening Scale developed by Turgay, there are 16 side effects for psychostimulants. Parents filled out the form for each side effects based on a 4‐point Likert scale (0: none, 1: rare, 2: moderate, 3: severe) in 4th and 8th week |
|
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interests: not stated Key conclusions of the study authors: OROS‐methylphenidate was found to be as effective as IR‐methylphenidate in the treatment of behavioural symptoms in Turkish children with ADHD. These results demonstrated that both drugs were effective and well tolerated in the treatment of Turkish children with ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes |
|
Yildiz 2011.
| Methods | A 14‐week parallel, randomised, open‐label trial (first 2 weeks screening phase) with 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 12 Number of participants followed up: 11 Number of withdrawals: 1 Diagnsosis of ADHD: DSM‐IV‐TR (subtype: combined (66.7%), inattentive ( 33.3%)). Age: mean 10.16, range 8‐14 years old IQ: none with mental retardation Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Turkey Comorbidity: oppositional defiant disorder: 7, learning disorder: 6 Comedication: participants taking concomitant psychoactive medications were excluded from the study Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Mean methylphenidate dosage: 1.07 mg/kg/day Administration schedule: morning dose, once daily Duration of intervention: 12 weeks Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Weight, height, pulse, systolic, diastolic blood pressure, AST, ALT, EEG, ECG, observer, beginning and end point (12 weeks) Self‐reported medication related adverse events 18 item list, parent/children, at 4, 8 and 12 weeks 1 child discontinued the study due to chest pain and palpitations |
|
| Notes | Sample calculation: no Ethics approval: approved by the Local Independent Ethics Committee Funding/vested interests: not stated Authors' affiliations: Department of Child and Adolescent Psychiatry, Kocaeli University, Izmit, Turkey Key conclusions of the study authors: treatment responses were not significantly different between the 2 study groups. OROS‐methylphenidate led to a significantly greater reduction in teacher T‐DSM‐IV‐S scores. OROS‐methylphenidate was more effective than atomoxetine on Stoop‐5 time and number of corrections. Significant decrease in the percentage of perseverative errors on WCST in the OROS‐methylphenidate group was seen. In the OROS‐methylphenidate group, patients most frequently reported anorexia, nervousness, insomnia, headache, nausea and weight loss. When all the results are considered, although both drugs can be considered effective in ADHD treatment, more remarkable improvement is provided by OROS‐methylphenidate based on the rates across informant and neuropsychological evaluation |
|
Yilmaz 2013.
| Methods | A patient report of methylphenidate‐induced acute orofacial and extremity limb dyskinesia | |
| Participants | Diagnosis of ADHD: DSM‐IV Age: 7 years old IQ: above 70 Sex: male Ethnicity: Turkish Country: Turkey Comorbidity: epilepsy Comedication: sodium valproate Sociodemographics: not stated |
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 18 mg Administration schedule: once daily in the morning Duration of treatment: 1 dose Treatment compliance: good |
|
| Outcomes |
Serious adverse events: Involuntary movements started about 5 hours after taking methylphenidate. Lip‐licking, lip‐smacking and tongue‐rolling movements. Dyskinetic tongue movements inside and outside the mouth and involuntary bilateral arm swinging while sitting and standing. Opening and closing his fingers without complete extension. Occasional repetitive movements of the feet, such as beating them against each other while sitting. About 15 hours after methylphenidate intake both hand‐mouth movements and excessive mobility had significantly resolved. Dyskinetic symptoms had completely disappeared on the second day of hospitalisation, and the patient was discharged |
|
| Notes | Funding/vested interest/authors' affiliations: authors declare that there are no potential conflicts of interest Key conclusions of the study authors: this case is reported to emphasise the potential side effects of methylphenidate, individual differences in drug sensitivities, and drug‐receptor interactions via different mechanisms Comments from the study authors: antiepileptic therapy may increase the sensitivity to the side effects of methylphenidate Supplemental data received through personal email correspondence with the authors in December 2013 (Yilmaz 2013 [pers comm]) |
|
Yu 2010.
| Methods | A patient report of 4 boys with attention‐deficit/hyperactivity disorder who developed vasculopathy during treatment with psychostimulants. 3 received treatment with methylphenidate | |
| Participants | Diagnosis of ADHD: ICD‐9/ICD‐10 (subtype: not stated) Age: 11, 12, 16 years old (mean: 13) IQ: no mentally retarded Sex: 3 males Ethnicity: white Country: USA Comorbidity: case 2 (suspected bipolar disorder) Comedication: case 1 (Adderall); case 2 (Abilify and Lamictal, later on they were discontinued and Seroquel was added) Sociodemographics: not stated |
|
| Interventions |
Case 1 First methylphenidate attempt: Extended release methylphenidate dosage: 54 mg/daily (Concerta). Comedication: Adderall. Administration schedule: not stated. Duration of treatment: 2 years. Treatment compliance: not stated Second methylphenidate attempt: Immediate release‐/dex‐ and extended release‐methylphenidate dosage: Concerta 90 mg/daily and Focalin 20 mg/daily. Administration schedule: not stated. Duration of treatment: 9 months. Treatment compliance: not stated Total duration of psychostimulant treatment: 9 years Case 2 Immediate‐ and extended release methylphenidate dosage: long acting Ritalin: 30 mg/daily and Focalin 2.5 mg/daily Administration schedule: not stated Duration of current treatment: 4 years Total duration of psychostimulant treatment: 5 years Treatment compliance: not stated Case 4 Extended release/dex‐ and immediate release/dex methylphenidate dosage: Focalin XR 20 mg/daily, Focalin IR 10 mg/daily Administration schedule: twice daily, XR in the morning, and IR in the afternoon Duration of treatment: 1 year Total duration of psychostimulant treatment: 7 years Treatment compliance: not stated |
|
| Outcomes |
Non‐serious adverse events: Case 1 Tachycardia at age 11 while taking Concerta, 54 mg/daily Vasculopathy at age 16. Hands and feet were a continuous blue color which increased in frequency in cold weather. Diagnosed with decreased circulation but not Raynaud's syndrome. Occured when the Concerta dose was increased from 54 mg/daily to 90 mg/daily in 3 months with the same Focalin dose (10 mg/daily) Case 2 Vasculopathy. First diagnosed with Raynaud's syndrome with finger pain and colour changes during a neurology consultation at age 10. At age 12 had diffuse erythema of both earlobes, the fingers of both hands, and the toes of the left foot Tics at age 10 ‐ not clear if these were present prior to ADHD treatment Case 4 Vasculopathy. After taking Focalin for a year, he developed persistent curling of the toes of both feet. In addition, he had reddish and purple colour changes in his hands and feet with cold exposure that lasted for 20 min. No associated pain and only rare paresthesia. At age 10 toes 2, 3, and 4 of both feet were held in a flexed position ('curled toes'), and there was skin discoloration and excoriation |
|
| Notes | Funding/vested interest: none Authors' affiliations: Drs Ronald and Elizabeth Weller are co‐owners of the Children's Interview for Psychiatric Syndromes (and the parent's version) and have received annual royalties from copyright ownership of this diagnostic interview. Dr Elizabeth Weller was the principal investigator for a grant from GlaxoSmithKline to investigate the tolerability and efficacy of lamotrigine in children and adolescents diagnosed with bipolar disorder Key conclusions of the study authors: these patient reports raise the concern that adverse effects in the peripheral vascular system of children and adolescents may be associated with psychostimulant treatment Comments from the review authors: none of the patients had their psychostimulants decreased or discontinued. Due to the severe nature of their ADHD symptoms, these patient could not stop or reduce their psychostimulant treatment to determine whether their vascular symptoms would improve Supplemental information received through personal email correspondence with the authors in October 2013 (Yu 2013 [pers comm]) |
|
Zarinara 2010.
| Methods | A 6‐week double‐blind, parallel‐group randomised clinical trial with 2 arms:
No control/no‐intervention group |
|
| Participants | Number of participants screened: 60 Number of participants included: 38 Number randomised to methylphenidate: 19 Number of participants followed up in the methylphenidate group: 18 Number of withdrawals in the methylphenidate group: 1 MPH group Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (100%)) Age: mean 9.57 years old (range 6‐13) IQ: above 70 Sex: 13 males, 6 females Methylphenidate‐naïve: 100% (newly diagnosed) Ethnicity: Persian (100%) Country: Iran Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Methylphenidate dosage: 20‐30 mg/day depending on weight (20 mg/day for < 30 kg and 30 mg/day for > 30 kg) Methylphenidate titration: 3 weeks (week 1: 10 mg/day twice daily; week 2: 20 mg/day twice daily; and week 3: 30 mg/day for children > 30 kg 3 times daily) Duration of intervention: 6 weeks inclusive titration Treatment compliance: not stated |
|
| Outcomes | Adverse effects checklist (20 possible adverse events), rated by a child psychiatrist on days 7, 21, 42. Body weight and vital signs assessed at baseline, week 1, 2, 4, 6. 12‐lead ECG and physical examination at baseline and week 6 | |
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interests/authors' affiliations: this study was supported by a grant from Tehran University of Medical Sciences Key conclusions of the study authors: the results suggest that venlafaxine may be useful for the treatment of ADHD. In addition, a tolerable side‐effect profile is one of the advantages of venlafaxine in the treatment of ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding data requested through personal communication with the authors in August 2013. No reply |
|
Zelnik 2015.
| Methods | A naturalistic study of osmotic release oral system (OROS) methylphenidate use for ≥ 1 year | |
| Participants | Number of participants included: 128 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean: not stated (range 7‐17) IQ: patients with intellectual disabilities were excluded Sex: 83 males, 40 females Methylphenidate‐naïve: not stated, but all were newly diagnosed Ethnicity: not stated Country: Israel Comorbidity: anxiety 27.2‐46.8%, depression 1.2‐9.7%, all psychiatric comorbidities 48.1%‐74.5% Comedication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: group I: lower dose OROS + short acting formulation; group II: OROS Mean methylphenidate dosage: group I: 0.83 SD 0.21 mg/kg; group II: 1.06 SD 0.29 mg/kg Administration schedule: group I: not stated; group II: OROS once daily Duration of intervention: ≥ 1 year Treatment compliance: not stated |
|
| Outcomes | In the present study data were retrospectively collected of all the patients that were treated with OROS methylphenidate and characterised the clinical characteristics of those who tolerated it well in comparison with those that better tolerated lower doses of OROS methylphenidate together with shorter acting methylphenidate, offering them a more potent level during school hours while lessening the effect during the afternoon and evening hours | |
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interest: no Authors' affiliations: none Key conclusions of the study authors: while OROS methylphenidate and other long‐acting methylphenidate formulations prove beneficial for most children and adolescents with ADHD, some patients (mostly those with psychiatric comorbidities) might sometimes better tolerate a lower OROS methylphenidate dose combined with short‐acting methylphenidate formulation. Additional larger‐scale prospective studies are required to validate our preliminary observation Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated |
|
Zeni 2007.
| Methods | A cohort study of methylphenidate use for 1 month | |
| Participants | Number of participants screened: 111 Number of participants included:106 Number of participants followed up: not stated Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (subtype: combined (58.5%), hyperactive‐impulsive (7.5%), inattentive (26.4%)) Age: mean 10.26, range 4‐17 years old IQ: not stated Sex: 82 males, 24 females Methylphenidate‐naïve: 100% Ethnicity: 100% European‐Brazilian Country: Brazil Setting: outpatient clinic Comorbidity: conduct disorder (16%), oppositional defiant disorder (51.9%), mood disorders (9,4%), anxiety disorders (23.8%) Comedication: none Sociodemographics: not stated Inclusion criteria
|
|
| Interventions | Methylphenidate type: short acting Mean methylphenidate dosage: 0.5 mg/kg/day Administration schedule: not stated Duration of intervention: 1 month Treatment compliance: 2 patients excluded due to irregular use of methylphenidate |
|
| Outcomes |
Non‐serious adverse events Barkley SERS:
|
|
| Notes | Sample calculation: no Ethics approval: yes Funding/vested interest: governmental agencies: Conselho Nacional de Desenvolvimento Cientıfico e Tecnologico (CNPq, Brazil); Grant number: 471761/03‐6; Grant sponsor: Programa de Apoio a Nucleos de Excelencia (PRONEX, Brazil); Grant sponsor: Hospital de Clınicas de Porto Alegre Key conclusions of the study authors: no significant association was detected between polymorphisms of dopaminergic (DRD4, DAT1) and serotonergic genes (HTR1B, HTR2A, and 5‐HTT) on the response nor side effects to the treatment with methylphenidate Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no, 100% were methylphenidate‐naïve Supplemental information received through personal email correspondence with the authors in September 2016 (Zeni 2016 [pers comm]) |
|
Zhang 2010.
| Methods | A case‐control study of methylphenidate use for 2‐4 years | |
| Participants | Number of participants screened: not stated Number of participants included: 175 Number included as cases: methylphenidate 126; controls (no intervention) 29 Number followed up in each arm: methylphenidate 46; controls 2 Number of withdrawals in each arm: methylphenidate 98; controls 27 Diagnosis of ADHD: DSM‐IV (subtype: combined (74.65%), hyperactive‐impulsive (8.9%), inattentive (16.43%)) Age: mean 7.42 years old (range 6‐9.8) IQ: not stated Sex: 126 males, 20 females Methylphenidate‐naïve: not stated Ethnicity: not stated Country: China Comorbidity: oppositional defiant disorder (39.73%), conduct disorder (4.79%), learning disorder (8.22%), tics (4.79%) Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: immediate release Mean methylphenidate dosage: 0.27‐0.64 mg/kg Administration schedule: 2 time points (mornings and afternoons) Duration of intervention: 2‐4.8 years Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events: Height Weight BMI | |
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interest: not stated Authors' affiliations: Department of Paediatrics, First Affiliated Hospital, Sun Yat‐sen University, Guangzhou, P. R. China Key conclusions of the study authors: methylphenidate inhibits linear growth of children, but it is correlated to methylphenidate treatment length; there was no influence on weight; there was no differences on BMI Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: not stated Supplemental information requested through personal email correspondence with the authors in June 2014. Email sent twice but no answer received |
|
Zheng 2011.
| Methods | A 6‐week multicentre, prospective, naturalistic, open‐label, pilot study evaluating the effectiveness and safety of osmotic release oral system (OROS) methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 1447 Number of participants followed up: 1154 Number of withdrawals: 293 Diagnosis of ADHD: DSM‐IV (subtype: combined (56.8%), hyperactive‐impulsive (9.8%), inattentive (28.5%), unidentified type (3.4%)) Age: 9.53 (SD 2.35) years old (range 6‐16) IQ: not stated Sex: 1219 males, 228 females Methylphenidate‐naïve: not stated (no current methylphenidate treatment: 1304 (90%)) Ethnicity: Asian (97.8%), others (2.2%) Country: China Comorbidity: no Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
During the 6‐week treatment period, participants would be excluded if the following conditions occurred:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: 18, 36 or 54 mg once daily at the discretion of investigators. However, the recommended dosing strategy is: methylphenidate‐naïve: 18 mg; currently receiving 5 mg immediate release methylphenidate 2‐3 times a day: 18 mg OROS methylphenidate; currently receiving 10 mg immediate release methylphenidate 2‐3 times a day or a total daily dose of 40 mg immediate release methylphenidate: 36 mg OROS methylphenidate; and currently receiving 15 mg immediate release methylphenidate 2‐3 times a day or a total daily dose of > 45‐60 mg: 54 mg OROS methylphenidate Doses of OROS methylphenidate could be adjusted between a total daily dose range of 18 and 54 mg after 14 days of treatment by the investigator on the basis of safety and efficacy. At the end of the study 96.8% participants received an OROS methylphenidate dose of 18 mg, 3.1% 36 mg and 0.1% 54 mg Duration of intervention: 6 weeks Treatment compliance: 93.4% of the patients showed good compliance (defined as more than 80% adherence to the prescribed regimen) |
|
| Outcomes |
Non‐serious adverse events Blood pressure, pulse rate measurement, adverse event review were conducted by the investigator at baseline, week 2, 4 and 6 Parents rated the participant's sleep quality and were asked about the presence of motor and/or verbal tics at baseline, week 2, 4 and 6 |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Funding: funded by Xi'an Jassen Pharmaceutical Ltd, China Key conclusions of the study authors: this open‐label, naturalistic study provides further evidence of effectiveness and safety of OROS methylphenidate in school‐aged children under routine practice Comments from the study authors: since the patients in this study only received 6‐weeks OROS methylphenidate treatment, the long‐term adverse events such as influence on growth and weight can not be observed. This is a limitation of this study because ADHD patients need long‐term treatment Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: yes Supplemental information requested through email correspondence with the first author in September 2013. No reply |
|
Zheng 2015.
| Methods | A 12‐week, prospective, multicentre, open‐label, self‐controlled clinical study of methylphenidate use for 12 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 153 Number of participants followed up: 123 Number of withdrawals: 30 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 9.2 (SD 1.5) years old (range 6‐12) IQ: 85 or more Sex: 108 males, 20 females Methylphenidate‐naïve: 90.4% Ethnicity: Han (96.9%), others (3.1%) Country: China Comorbidity: not stated Comedication: none Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system (OROS) Methylphenidate dosage: during the optimised treatment phase (weeks 3‐12), 73.5% children received OROS methylphenidate 18 mg once daily and 26.5% received 36 mg once daily (weeks 7‐12) Administration schedule: once daily Duration of intervention: 12 weeks Treatment compliance: the mean course of OROS methylphenidate treatment was 80.1 days and total compliance was 93.6% |
|
| Outcomes | Children had a general physical and blood examination (if required) before initiating OROS methylphenidate. Blood pressure, pulse rate, concomitant medications, adverse events (AEs), and treatment review were conducted at baseline and at each visit. A total of 149 patients were included in the safety analysis set | |
| Notes | Sample calculation: not stated Ethics approval: yes Funding/vested interest: the study presented in this report was supported by Xi'an Janssen Pharmaceutical Ltd. Authors' affiliations: Ms. Jian‐Min Zhuo and Dr Sheng‐Nan Xie are full‐time salaried employees of Xi'an‐Janssen Pharmaceutical Ltd. Drs. Yi Zheng, Hong‐Yun Gao, Zhi‐Wei Yang, Fu‐Jun Jia, Fang Fang, and Rong Li have served on advisory boards of Xi'an Janssen Pharmaceutical Ltd., and Eli Lilly and Company Key conclusions of the study authors: in conclusion, this open‐label study suggests that the OROS methylphenidate improves academic and cognitive performance in Chinese school‐aged children with ADHD. The treatment was safe and generally well‐tolerated over the period of 12 weeks Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: see exclusion criteria 4 |
|
Çetin 2015.
| Methods | A randomised, open‐label parallel study of methylphenidate or atomoxetine use for 6 months | |
| Participants | Number of participants screened: not stated Number of participants included: 145 Number of participants randomised to methylphenidate: 73 Number of participants followed up on methylphenidate: 61 Number of withdrawals: 12 Diagnosis of ADHD: DSM‐IV‐TR (subtype: combined (91.8%), inattentive (8.2%)) Age mean: 9.47 (SD 2.32) years old IQ: above 90 Sex: 9 males (86.9%), 8 females (13.1%) Methylphenidate‐naïve: 100% Ethnicity: not stated Country: Turkey Comorbidity: none Comedication: not stated Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: osmotic release oral system Mean methylphenidate dosage: 0.73 (SD 0.22) mg/kg/day Administration schedule: not stated Duration of intervention: 6 months Treatment compliance: not stated |
|
| Outcomes | The adverse effects and tolerability of medications were evaluated using a questionnaire including 12 questions about anorexia, insomnia, stomachache, nervousness, headache, weight loss, rash, obsessions, sedation, epistaxis, tics and others, in addition to open ended questions The rate of adverse effects observed was 31.1% (n = 19) in the OROS‐methylphenidate group Treatment was stopped in 5 patients because of the adverse effects Non‐serious adverse events:
|
|
| Notes | Sample calculation: not stated Ethics approval: yes, the study protocol was approved by local Ethics Committee Funding/vested interests/authors' affiliations: not stated Key conclusions of the study authors: OROS‐methylphenidate and atomoxetine had similar efficacies and adverse effect profiles in the treatment of ADHD Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: all participants were methylphenidate‐naïve |
|
Özcan 2004.
| Methods | A cohort study comparing time domain heart rate variability in non‐ADHD controls and ADHD cases treated with methylphenidate for 12 weeks | |
| Participants | Number of participants screened: not stated Number of participants included: 73 Number included as cases (ADHD): 42 Number included as controls (non‐ADHD): 31 ADHD group Number of participants followed up: 42 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: mean 11.1 years old (range 6‐15) Sex: 34 males, 8 females IQ: above 70 Country: Turkey Setting: Ethnicity: not stated Methylphenidate‐naïve: not stated Comorbidity: not stated Comedication: no Sociodemographics: not stated Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Methylphenidate type: not stated Mean methylphenidate dosage: 10 mg (2 x 5 mg) Administration schedule: not stated Duration of treatment: 12 weeks Treatment compliance: not stated |
|
| Outcomes | Non‐serious adverse events: heart rate variability | |
| Notes | Sample calculation: not stated
Ethics approval: yes
Funding/vested interests: not stated
Authors' affiliations: no affiliations to pharmaceutical companies stated Key conclusions of the study authors: methylphenidate decreased the time domain HRV parameters in ADHD group. Therefore, close cardiac follow‐up is necessary for the detection of side effects of methylphenidate especially in patients who are under risk for developing cardiac arrhythmias and in patients using methylphenidate together with drugs affecting central nervous system Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events on methylphenidate: no Supplemental information regarding IQ has not been possible to receive through personal email correspondence with the authors. Emails sent twice to several of the authors |
|
ADD: attention deficit disorder; ADD‐H: attention deficit disorder with hyperactivity; ADHD: attention deficit hyperactivity disorder; AE: adverse event;bpm: beats per minute; CGI‐S: Clinical Global Impression‐Severity; CI: confidence interval; CK: cytokine; CNS: central nervous system; C‐SSRS: Columbia Suicide Severity Rating Scale; DBP: diastolic blood pressure; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition;ECG: electrocardiogram; ED: emergency department; EEG: electroencephalogram; EF: ejection fraction; EKG: electrocardiogram; FDA: Food and Drug Administration; ER: extended release; HCL: hydrochloride; HRQoL: health‐related quality of life; ICD: International Classification of Diseases; IQ: intelligence quotient; IQR: interquartile range; IR: immediate release; IRR: interrater reliability;ITT: intention‐to‐treat; LLC: limited liability company; LOCF: last observation carried forward; LTFU: loss to follow‐up; MAOI: monoamine oxidase inhibitors; MedDRA: Medical Dictionary for Regulatory Activities; MPH: methylphenidate; MPT: methylphenidate trial; MRI: magnetic resonance imaging; MTA: Multimodal Treatment Study of Children With ADHD; MTS: methylphenidate transdermal system; NICE: National Institute for health and Care Excellence; NNTB: number needed to treat for an additional beneficial outcome; OCD: obsessive compulsive disorder; ODD: oppositional defiant disorder; OROS: osmotic Release Oral System; PDD: pervasive developmental disorder; PTS: placebo transdermal system; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; SD: standard deviation; SERS: Side Effects Rating Scale; SBP: systolic blood pressure; SODAS: spheroidal oral drug absorption system; SSRI: selective serotonin reuptake inhibitors; WISC: Wechsler Intelligence Scale for Children.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bart 2010 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Beauchaine 2003 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Becker 2011 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Beery 1994 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Benamor 2014 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Biederman 1999 | Not possible to obtain the disaggregated data for the methylphenidate group |
| Biederman 2009 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Bohane 2010 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Brossard‐Racine 2012 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Byrne 1998 | No data on adverse events |
| Casat 1995 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Cavadas 2007 | No data on adverse events |
| Chambers 2012 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Chavez 1999 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Chelonis 2010 | Lack of relevant outcome measures |
| Chelonis 2011 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Clarke 2003 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Conzelmann 2014 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Cooper 2011 | Unable to obtain the disaggregated data on children receiving methylphenidate, either from the publication or through personal email correspondence with the study authors |
| De Zeeuw 2012 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| DeVito 2009 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Drtílková 1990 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Epstein 2010 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Faraone 2007b | No data on adverse events |
| Fegert 2006 | No data on adverse events |
| Foodman 1996 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Frank 1993 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Funk 1993 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Gau 2009 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Gimpel 2005 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Glenngård 2013 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Gormez 2012 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Gould 2009 | No ADHD diagnosis |
| Gualtieri 1985 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Guimarães 2009 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Gurkan 2011 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Hautmann 2013 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Hawcutt 2012 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Hawk 2003 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Heiser 2004 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Hidas 2011 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Hood 2005 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Hoza 1992 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Hwang 2012 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Jonkman 1999 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Kerdar 2007 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Kereszturi 2008 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Keulers 2007 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Klein 2002 | No relevant outcomes (experimental, neurocognitive or functional). No data on adverse events |
| Kramer 2001 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Lajoie 2005 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Lawrence 2005 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Matier 1992 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Mayes 1993 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Miller 1994 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Miller 1996 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Miranda 2006 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Monden 2012a | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Monden 2012b | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Nahshoni 2012 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Neef 2005 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Negrao 2011 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Nigg 1996 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Nikles 2005 | No relevant outcome measures (experimental, neurocognitive or functional). The paper investigates the patients' satisfaction with N‐of‐1 trials |
| Nolan 1999 | Unable to obtain the disaggregated data for the methylphenidate group |
| Ohashi 2010 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Orgill 1996 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Overcash 2005 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Ozdag 2004 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Palomino 2012 | Unable to obtain the disaggregated data for the methylphenidate group |
| Park 2012 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Pelham 1986 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Pelham 2011 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Perera 2012 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Pierce 2008 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Poulton 2013 | Unable to obtain the disaggregated data for the methylphenidate group |
| Prehn‐Kristensen 2011 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Rapport 1985 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Rhodes 2004 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Rhodes 2006 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Roman 2002 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Rubia 2009 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Rubia 2009a | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Rubio 2011 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Schecklmann 2011 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Scheres 2003 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Scheres 2006 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Schmiedeler 2009 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Shafritz 2004 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Shaywitz 1982 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Sheppard 1999 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Snyder 2008 | Unable to obtain the disaggregated data for the methylphenidate group |
| Solanto 1989 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Spivak 2001 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Stein 1999 | Unable to obtain the disaggregated data for the methylphenidate group |
| Syrigou‐Papavasiliou 1988 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Tabori‐Kraft 2007 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Tamm 2007 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Tillery 1998 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Trenque 2009 | Unable to obtain the disaggregated data for the methylphenidate group |
| Van der Meere 2009 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Van der Oord 2012 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Vance 1999 | Unable to obtain the disaggregated data for the methylphenidate group |
| Vickers 2002 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Vogt 2011 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Winterstein 2007 | Unable to obtain the disaggregated data for the methylphenidate group |
| Winterstein 2012 | Unable to obtain the disaggregated data for the methylphenidate group |
| Wong 2012 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Yeh 2012 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Zachor 2006 | Unable to obtain the disaggregated data for the methylphenidate group |
| Zalsman 2003 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
| Zang 2005 | No relevant outcome (experimental, neurocognitive or functional). No data on adverse events |
ADHD: attention deficit hyperactivity disorder.
Characteristics of studies awaiting assessment [ordered by study ID]
Arnold‐Von 2000.
| Methods | Non‐randomised study of 2 cases |
| Participants | ADHD diagnosis according to DSM‐III‐R |
| Interventions | Type of methylphenidate not stated |
| Outcomes | Not stated |
| Notes | The article is written in Dutch, and we have not yet been able to obtain a translation |
Dalsgaard 2011.
| Methods | A longitudinal, prospective cohort study of the cardiovascular safety of stimulants |
| Participants | All children born in Denmark between 1990 and 1999; children with ADHD were identified from within this cohort |
| Interventions | Type of methylphenidate not stated |
| Outcomes | Cardiovascular adverse events |
| Notes | Found too late in the review process to include |
Flapper 1989.
| Methods | Non‐randomised study of 8 cases |
| Participants | Children with a diagnosis of ADHD as well as Minimal Brain Dysfunction (MBD) according to DSM‐III |
| Interventions | Type methylphenidate not found |
| Outcomes | Effects and adverse events |
| Notes | The article is written in Dutch and we have not yet been able to obtain a translation |
Husár 2006.
| Methods | Case‐study |
| Participants | 14‐year‐old boy with priapism |
| Interventions | Olanzapine and methylphenidate (diazepam and metamisolum) |
| Outcomes | Priapism occurrence |
| Notes | The article is written in Czech and we have not yet been able to obtain a translation |
Ince 2015.
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Uncelar |
| Notes | Not able to retrieve article |
Ishizaki 2001.
| Methods | Observational study |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Serious adverse events |
| Notes | The article is written in Japanese, and we have not yet been able to obtain a translation |
Laezer 2015.
| Methods | Not retrieved |
| Participants | Not retrieved |
| Interventions | Not retrieved |
| Outcomes | Not retrieved |
| Notes | We were not able to find the paper |
Mulas 2014.
| Methods | Review of patient records |
| Participants | 23 participants, 5‐16 years' old with epilepsy |
| Interventions | Methylphenidate, type unclear |
| Outcomes | Clinical course |
| Notes | The article is written in Spanish, and we have not yet been able to obtain a translation |
Ptáček 2008.
| Methods | Case‐control study |
| Participants | Group of boys (n = 46) diagnosed with ADHD |
| Interventions | Methylphenidate, type not stated |
| Outcomes | Various unspecified anthropometric characteristics |
| Notes | The article is written in Czech, and we have not yet been able to obtain a translation |
Radziuk 2015.
| Methods | Open‐label, non‐controlled trial |
| Participants | 30 children and adolescents with epilepsy and comorbid ADHD |
| Interventions | Methylphenidate, type not stated |
| Outcomes | Tolerability to methylphenidate, quality of life, seizure frequency |
| Notes | — |
Socanski 2015.
| Methods | Prospective study on children with ADHD |
| Participants | 517 children diagnosed with ADHD (82% male), age 5‐14 years |
| Interventions | Methylphenidate, type not stated |
| Outcomes | Initial positive response to methylphenidate after 4‐6 weeks titration, and the use of methylphenidate at 1‐year follow‐up |
| Notes | Conference abstract. Could not retrieve full‐text report |
Sugama 2009.
| Methods | Cases with ADHD |
| Participants | 181 participants with ADHD |
| Interventions | Switched medication from conventional immediate‐release preparations of methylphenidate to extended‐release tablets (OROS) |
| Outcomes | Types of developmental disorders, drug dosages, efficacy, adverse events, concomitant medication, other relevant problems, and so on, prior to switching the medications |
| Notes | The article is written in Japanese, and we have not yet been able to obtain a translation |
TOSCA 2011.
| Methods | A 4‐site, phase 2, parallel‐group RCT:
|
| Participants | Sample size: 160 patients included Age range: 6‐12 years' old (inclusive) ADHD diagnosis: DSM‐IV Comorbidity: disruptive behaviour disorder (conduct disorder or oppositional defiant disorder 100%), significant aggressive behaviour Country: USA Baseline demographics: no supplemental data Inclusion criteria:
Exclusion criteria:
|
| Interventions | At baseline, participants were randomised to OROS‐methylphenidate + placebo or OROS‐methylphenidate + risperidone. OROS methylphenidate was initiated at 18 mg each morning and titrated up to a limit of 54 mg or 72 mg (depending on weight: < 25 kg and > 25 kg, respectively) by week 2. If participants did not demonstrate sufficient improvement on methylphenidate by end of week 3 (defined as normative value + 0.5 SD on the NCBRF D‐Total score and the Clinical Global Impressions Scale (CGI‐I) of 1, parent training or risperidone was added. However, if the second drug was not needed at week 3 and the child's behaviour subsequently deteriorated on the optimal stimulant dose, the prescriber was able to add the second medication through the sixth week of the study. At end of week 9, participants were classified as clinical responders (CGI‐I = 1‐2 and NCBRF D‐Total reduced by 25% relative to baseline). Responders were followed on their originally assigned conditions for 12 weeks of double‐blinded extension. Non‐responders were treated clinically, as appropriate, based on the study team's best judgment. 1‐year follow‐up assessment Treatment compliance: no information Although the stimulant of first choice in this trial was OROS methylphenidate, owing primarily to its extended duration of action, it was not mandated in the protocol so as not to exclude children who had prior poor response or who had difficulty swallowing pills. It was required that the substituted medication be a stimulant and that the dosage was matched in potency to the OROS methylphenidate |
| Outcomes | Screen and endpoint: ECG
Week 0, 1‐2, 3, 4‐8, 9, 13‐17, 21, 52: heart rate, blood pressure, height, weight, and hip‐waist ratio, extrapyramidal symptoms, appetite, sleep patterns Parent‐rated adverse event scale specific to stimulants or teacher rated stimulant adverse events Treatment‐induced motor disturbances: clinician rated Abnormal Involuntary Movement Scale (AIMS), Simpson‐Angus Rating Scale, Barnes Akathisia Scale |
| Notes | Comment: design article regarding the TOSCA study Sample calculation: yes Ethics approval: yes Funding: National Institute of Mental Health (NIMH) Adverse event data regarding the open‐label phase and the placebo + methylphenidate + PT group in the parallel phase, extension phase and follow‐up are usable according to the inclusion criteria for this review Personal correspondence with the authors in June 2013: distribution of adverse event data are not possible until after publication (Aman 2013 [pers comm]) |
Waldon 2016.
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | Not able to retrIeve full‐text report |
Yusufoglu 2014.
| Methods | A patient report of acneiform eruptions and dermatitis during methylphenidate treatment |
| Participants | 12‐year‐old boy with a diagnosis of ADHD |
| Interventions | Methylphenidate, type not stated |
| Outcomes | Non‐serious adverse events |
| Notes | Conference abstract. Not able to retrieve additional data |
ADHD: attention deficit hyperactivity disorder; DSM‐III: Diagnostic and Statistical Manual of Mental Disorders, Third Edition; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ECG: electrocardiogram; IQ: intelligence quotient; OROS: osmotic controlled‐release delivery system; RCT: randomised clinical trial.
Characteristics of ongoing studies [ordered by study ID]
Beau 2009.
| Trial name or title | Spontaneous report safety signals profile and comparative Bayesian analysis of ADHD medication in children in the UK |
| Methods | Examines safety signal profiles in the UK Yellow Card database |
| Participants | Children under 16 years' old using methylphenidate and atomoxetine |
| Interventions | Methylphenidate and atomoxetine |
| Outcomes | Psychiatric adverse events. White blood cell count, tics, alopecia, arrhythmia, suicidal ideation, depressed mood, aggression and appetite |
| Starting date | October 2008 |
| Contact information | Liam.smeeth Email: liam.smeeth@lshtm.ac.uk |
| Notes | Correspondence with authors October 2013. The paper will not be published in a foreseeable future (Smeeth 2013 [pers comm]) |
Bottelier 2014.
| Trial name or title | The effects of psychotropic drugs on developing brain (ePOD) study: methods and design |
| Methods | Double‐blind, placebo‐controlled trial |
| Participants | Boys aged 10‐12 years and adults aged 23‐40 years for methylphenidate treatment 50 children and 50 adult male patients, diagnosed with ADHD (all subtypes), and in need of pharmacological therapy |
| Interventions | Methylphenidate, type not specified |
| Outcomes | Adverse events, disruptive behaviours, functioning, depression, anxiety, among others |
| Starting date | Not stated |
| Contact information | Liesbeth Reneman Email: L.Reneman@amc.uva.nl |
| Notes | Ongoing study. Protocol published |
Dahlgren 2012.
| Trial name or title | Overweight and obese children with diagnosed attention deficit hyperactivity disorder have a favourable weight loss with methylphenidate 1‐year data |
| Methods | Retrospectively investigated records of children with ADHD |
| Participants | 100 children with ADHD, 24 of whom (14 females and 10 males, age range 5.9‐16.8 years) were overweight (BMI z score < 1.5 SD) |
| Interventions | Methylphenidate (dose range 10‐54 mg). Type not specified |
| Outcomes | BMI, height |
| Starting date | Not stated |
| Contact information | jovanna.dahlgren@vgregion.se |
| Notes | Correspondence with Jovanna Dahlgren in August 2013 revealed that a paper is in preparation (Dahlgren 2013 [pers comm]) |
Djurkovic‐Lazic 2009.
| Trial name or title | School children with ADHD ‐ effects of once‐daily OROS methylphenidate treatment ADHD symptoms included improvement academic functioning |
| Methods | An ongoing cohort study of children and adolescents aged 7 to 14 years with ADHD treated with OROS‐methylphenidate |
| Participants | Number of participants screened: not stated Number of participants included: 32 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: range 7‐14 years' old IQ: not stated Sex: not stated Methylphenidate‐naïve: not stated Ethnicity: not stated Country: Serbia Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
|
| Interventions | Methylphenidate type: OROS Methylphenidate dosage: 18‐54 mg Administration schedule: once daily Duration of intervention: not stated Treatment compliance: not stated |
| Outcomes | Adverse events, vital signs, EEG (no further data) |
| Starting date | — |
| Contact information | J Djurkovic‐Lazic, Institute for Psychophisiological and Speach Pathology, Belgrade |
| Notes | Sample calculation: not stated Ethics approval: not stated Funding/vested interests: not stated Authors' affiliations: not stated Key conclusions of the study authors: (PRELIMINARY) "Besides our expecting on reduction of ADHD symptoms with minimal effects on vital sings, values on growth, tick, laboratory test, it considers too, better academic functioning social relationship with continual therapy" Comments from the review authors: we have not been able to obtain the full‐text report. Therefore, the data are incomplete. |
Díez‐Suárez 2015.
| Trial name or title | Long‐term effects of medication for ADHD in weight and height in children and adolescents |
| Methods | A longitudinal, naturalistic follow‐up study of 497 children with ADHD |
| Participants | ADHD diagnosis, type not stated. Mean age 10.66 (SD 3.84) years. 79.9% males |
| Interventions | Methylphenidate, type not stated |
| Outcomes | Weight, height |
| Starting date | Not stated |
| Contact information | Azucena Diez‐Suarez. www.linkedin.com/in/azucena‐d%C3%ADez‐su%C3%A1rez‐10b14245 atpacientecun@unav.es |
| Notes | Personal email correspondence with the authors in June 2016: awaiting the full publication (Castro‐Manglano 2016 [pers comm]) |
Gau 2010.
| Trial name or title | The influence of using methylphenidate on the coming up of psychiatric disorders in children with ADHD |
| Methods | Database study of children with new onset ADHD between 1999 and 2003 and age‐ and sex‐matched healthy controls |
| Participants | 2109 children (aged > 18 years) with onset of ADHD. Type of ADHD not stated. Mean onset age of 7.3 years, 79.8% were males |
| Interventions | Methylphenidate, type not stated |
| Outcomes | Incidence of other comorbid psychiatric disorders (i.e. depression, bipolar disorder, dysthymia, anxiety, conduct disorder and oppositional defiant disorder) |
| Starting date | Not found |
| Contact information | Dr Churn‐Shiouh Gau Email: csgau206@cde.org.tw |
| Notes | Correspondence with authors in October 2013: no publications yet (Gau 2013b [pers comm]) |
Houmann 2011.
| Trial name or title | Individualised methylphenidate therapy based on pharmacogenetics: focus on carboxylesterase1 (CES1) |
| Methods | Multicentre study on CES1 genotype response in ADHD children |
| Participants | 200 drug‐naïve children with ADHD |
| Interventions | Methylphenidate, type not specified |
| Outcomes | CES1 genotype response, adverse reaction, discontinuation of treatment, treatment failure |
| Starting date | 2011, otherwise not specified |
| Contact information | Tine Bodil Houmann Email: Tine.Houmann@regionh.dk |
| Notes | Personal email correspondence with the first author in September 2013 revealed the study is ongoing (Houmann 2013 [pers comm]) |
Yook 2012.
| Trial name or title | Obesity is associated with non‐response to prolonged‐release methylphenidate treatment in ADHD |
| Methods | 8‐week, open‐label trial |
| Participants | 90 children and adolescents, aged 6‐15 years' old, diagnosed with ADHD (types not specified) |
| Interventions | Up to 54 mg of OROS‐methylphenidate |
| Outcomes | ADHD symptoms, functioning, Barkley Stimulant Side Effect Rating Scale |
| Starting date | Not stated |
| Contact information | Ki‐Hwan Yook Email: cha99@cha.ac.kr |
| Notes | Correspondence with first author in August 2013: no publications yet (Yook 2013 [pers comm]) |
ADHD: attention deficit hyperactivity disorder; BMI: body mass index; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition;OROS: osmotic controlled‐release delivery system; SD: standard deviation.
Differences between protocol and review
-
We decided to also include the methylphenidate‐treated group from RCTs assessing this stimulant versus other interventions as well as cross‐sectional studies. Definitions are shown in Table 2.
-
In our protocol, Storebø 2016b, we stated that study participants had to be 18 years or younger with an intellectual quotient (IQ) greater than 70. However, we would have had to exclude many studies if we had followed these criteria. Consequently, we decided during the review process that studies eligible for inclusion were those in which at least 75% of participants were aged 18 years or younger, and the mean age of the trial population was 18 years or younger. We also required that at least 75% of participants had a normal IQ (> 70). These arbitrary decisions were taken without looking at the data of the individual studies.
-
We included studies where methylphenidate administered at any dosage or formulation was a part of any medical treatment regimen. Compared to what we wrote in our protocol (Storebø 2016), we decided to accept concurrent medication with "over‐the‐counter types of drugs", but not comedication with other types of ADHD medication.
-
We recategorised some outcomes that are usually considered as "not serious adverse events", like "hypertension" and "sleep disorder", as serious adverse events, in light of the data collected by some study authors.
We decided to add withdrawal of methylphenidate due to serious adverse events and due to adverse events of unknown severity as primary outcomes, and withdrawal of methylphenidate due to non‐serious adverse events and for unknown reasons as secondary outcomes, as these represent important data. There were several reasons for this decision. First, the description of adverse events generally lacked detail. Second, 'withdrawal' means that it is likely that the balance between beneficial and harmful effects will have been disrupted, with the latter prevailing, otherwise sane physicians would not stop a medication they had prescribed themselves. We have tried to be as fair as possible, listing withdrawal due to adverse events of unknown severity as a primary outcome and withdrawal for unknown reasons as a secondary outcome. However, we know that the primary outcome, withdrawal of methylphenidate due to adverse events of unknown severity, for example, may be due to non‐serious adverse events, while the secondary outcome, withdrawal for unknown reasons, may be caused by serious adverse events and should have been a primary outcome. Nonetheless, we took these decisions without looking at the data of the individual studies, so they are not data driven. We decided to include withdrawal data also, as we believe that these are important.
-
We have divided our withdrawal data into the following categories.
Withdrawal of methylphenidate due to serious adverse events (primary outcome).
Withdrawal of methylphenidate due to adverse events of unknown severity (primary outcome).
Withdrawal of methylphenidate due to non‐serious adverse events (secondary outcome).
Withdrawal of methylphenidate for unknown reason (secondary outcome).
-
-
We widened the scope of our search to include the following databases.
Trove ‐ National Library of Australia (trove.nla.gov.au).
British Library E‐theses Online Service (EThOS; ethos.bl.uk).
Deutsche Nationalbibliothek Dissertations (www.dnb.de/EN/Home/home_node.html).
Open Access Theses and Dissertations (OATD; oatd.org).
Theses Collection Wales ‐ The National Library of Wales (www.llgc.org.uk/en/discover/nlw‐resources/theses‐collection‐wales).
DART‐Europe E‐theses portal (www.dart‐europe.eu/basic‐search.php).
Bielefeld Academic Search Engine (BASE; www.base‐search.net/about/en).
Theses France (www.theses.fr/en).
ProQuest Open Access Dissertations (PQDT; pqdtopen.proquest.com/search.html).
-
-
Selection of studies and Data extraction and management
More review authors than stated in the protocol screened titles and abstracts, entered data into RevMan 5 and conducted statistical analyses in RevMan 5 (Review Manager 2014).
-
We chose only to summarise dichotomous data as risk ratios (RR) with 95% confidence intervals (CI) and to present pooled proportion data from non‐comparative studies.
We did not calculate the risk difference and the number needed to treat for an additional harmful outcome.
-
Assessment of reporting biases.
We did not draw funnel plots (estimated differences in treatment effects against their standard error) due to there being too few studies.
We also did not perform Egger's statistical test for small‐study effects due to there being too few studies.
-
In the comparative cohort studies, we compared the intervention group to the control group and not to baseline values of the intervention group, as stated erroneously in the protocol (Storebø 2016).
-
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analyses on sex and subtype of ADHD as stipulated in our protocol (Storebø 2016), due to lack of relevant data.
-
We conducted the subgroup analyses (listed below) due to the very high heterogeneity of the data, to investigate whether these different aspects affected the proportion of adverse events. These analyses were not specifically planned in our protocol (Storebø 2016).
Studies with concurrent medication compared to studies without any concurrent medication.
Studies with ADHD as the only disease compared to studies with ADHD and comorbidity.
Studies with a treatment duration shorter than six months compared to studies with a treatment duration of six months or longer.
Studies with participants with a mean age younger than 10 years compared to studies with participants with a mean age of 10 years or older.
Studies with a low dosage of methylphenidate (below 20 mg/day) compared to studies with a high dosage of methylphenidate (20 mg/day and above).
Cohort studies on methylphenidate originating from RCTs assessing methylphenidate versus other ADHD interventions compared to studies with a classic cohort design.
Studies funded by industry compared to studies not funded by industry.
-
-
We conducted only two sensitivity analyses:
analytical technique used (for example, fixed‐effect versus random‐effects models); and
study design (patient‐control studies compared to comparative cohort studies).
We did not perform a sensitivity analysis to assess whether the findings were sensitive to decisions made during the review process.
As all studies were assessed as having overall serious or critical risk of bias, we could not perform a sensitivity analysis on the impact of bias.
We did not conduct a sensitivity analysis regarding the type of data collection (e.g. different ways to assess adverse events), due to lack of relevant data.
We also did not conduct an analysis comparing available outcome data to those following the intention‐to‐treat (ITT) approach, due to lack of relevant data.
-
-
We excluded 112 studies (121 reports) because outcomes were outside the focus of this review (e.g. visual attention test, reaction time, memory skills, and functional abnormalities). These studies did not report any adverse events.
-
Written language
In order to avoid demeaning patients to "cases", we chose to name "case‐control studies" as "patient‐control studies", and "case reports" as "patient reports/series".
Instead of naming study participants as "subjects", we used the term "participants".
Contributions of authors
All of the authors contributed to writing the protocol for this review (Storebø 2016). KBR developed the search strategy. OJS, NP, ER, HBK, CRMM, FLM, MH, TG, MS, SR, KBR, SJH, LA, and TB selected the studies. All authors contributed to data extraction and evaluation of bias. OJS, NP, ER, HBK, CRMM, and FLM entered data into RevMan 5 (Review Manager 2014). OJS, NP, ER, HBK, RK, SJH, and LA performed the statistical analysis. All authors contributed to writing the discussion and the final review.
OJS is the guarantor for the review
Sources of support
Internal sources
-
Psychiatric Research Unit, Region Zealand Psychiatry, Roskilde, Denmark.
Ole Jakob Storebø, Nadia Pedersen, Erica Ramstad, Helle B Krogh, Frederik Løgstrup Magnusson, Mathilde Holmskov, Trine Gerner, and Erik Simonsen worked on this review during office hours
-
Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen University Hospital, Denmark.
Maria Skoog and Christian Gluud worked on this review during office hours
External sources
-
Region Zealand Research Foundation, Denmark.
This Cochrane Review is supported by a grant (DKKr 532,901.00) from the Region Zealand Research Foundation
Declarations of interest
Ole Jakob Storebø is an Associate Editor with Cochrane Developmental, Psychosocial and Learning Problems. Nadia Pedersen ‐ none known. Erica Ramstad ‐ none known. Maja Lærke Kielsholm ‐ none known. Signe Sofie Nielsen ‐ none known. Helle B Krogh ‐ none known. Carlos R Moreira‐Maia (CRMM) receives financial research support from the government agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). CRMM received fees in February 2016 for the development of educational materials on excessive daytime sleepiness for Libbs. Libbs also make products for the treatment of ADHD. CRMM received travel awards from the Health Technology Assessment Institute (IATS), Universidade Federal do Rio Grande do Sul (UFRGS); and travel, accommodation, and registration support to the fourth and fifth World Congresses on ADHD from the World Federation of ADHD. Carlos is an author on Maia 2008 and declares that he was not involved in assessing the eligibility of this study, extracting data, assessing risk of bias or grading the quality of the evidence. Frederik L Magnusson ‐ none known. Mathilde Holmskov ‐ none known. Trine Gerner ‐ none known. Maria Skoog ‐ none known. Susanne Rosendal ‐ none known. Camilla Groth's (CGr) institution received funds from the Lundbeck Foundation to finance part of her PhD in the paediatric field on Tourette syndrome. CGr confirms that none of these funds were used to work on this review. Donna Gillies is an Editor with Cochrane Developmental, Psychosocial, and Learning Problems. Kirsten Buch Rasmussen ‐ none known. Dorothy Gauci ‐ none known. Morris Zwi sits on the UK Paediatric Medicines Expert Advisory Group at the Medicines and Healthcare Regulatory Agency, which considers applications regarding the licensing of paediatric medicines. Payment for MZ's attendance at this meeting goes to his NHS organisation. As a clinician working with patients who have ADHD, he prescribes methylphenidate regularly. Richard Kirubakaran is currently employed by Cochrane South Asia, funded by the Indian Council for Medical Research, India, and Effective Healthcare Research Consortium for the Department for International Development, UK. Sasja J Håkonsen ‐ none known. Lise Aagaard (LA) received travelling grants from the pharmaceutical companies Pfizer, Swedish Orphan BioVitrum and Shire. None of the travelling grants are related to this review. LA confirms that she has nothing to declare in relation to ADHD research. The Pfizer travel grants were received in 2015 and 2016 for attending the European Association of Hospital Pharmacist Annual Conferences. No activities in relation to treatment of ADHD were observed. A travel grant was received in 2015 from Swedish Orphan Biovitrum for attending the C1 Inhibitor Conference in Budapest. C1 inhibitor drugs are involved in the treatment of the rare disease hereditary angioedema. Travel grants were received in 2015 and 2016 from Shire for attending the Bradykinin Scientific Meeting in Copenhagen and Stockholm. Bradykinin mediator drugs are involved in the treatment of the rare disease hereditary angioedema. Erik Simonsen ‐ none known. Christian Gluud is Co‐ordinating Editor of Cochrane Hepato‐Biliary.
Edited (no change to conclusions)
References
References to studies included in this review
Abali 2007 {published data only}
- Abali O, Mukaddes NM. Methylphenidate induced hallucinations: case report. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2007; Vol. 17, issue 4:195‐7.
Abbas 2006 {published data only}
- Abbas E, Desoysa R. Use of methylphenidate for attention‐deficit‐hyperactivity disorder in childhood. Developmental Medicine and Child Neurology 2006;48(Suppl 107):23. [DOI: 10.1111/j.1469-8749.2006.tb12600.x] [DOI] [Google Scholar]
Abbasi 2011 {published data only}
- Abbasi SH, Heidari S, Mohammadi MR, Tabrizi M, Ghaleiha A, Akhondzadeh S. Acetyl‐L‐carnitine as an adjunctive therapy in the treatment of attention‐deficit/hyperactivity disorder in children and adolescents: a placebo‐controlled trial. Child Psychiatry and Human Development 2011;42(3):367‐75. [DOI: 10.1007/s10578-011-0220-y; PUBMED: 21336630] [DOI] [PubMed] [Google Scholar]
Adrian 2001 {published data only}
- Adrian N. Explosive outbursts associated with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(6):618‐9. [DOI: 10.1097/00004583-200106000-00002; PUBMED: 11392335] [DOI] [PubMed] [Google Scholar]
Agarwal 2008 {published data only}
- Agarwal V, Sitholey P. Combination of atomoxetine and methylphenidate in attention deficit/hyperactivity disorder: a case report. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2008;17(3):160. [PMC2527769] [PMC free article] [PubMed] [Google Scholar]
Aguilera‐Albesa 2010 {published data only}
- Aguilera‐Albesa S, Yoldi‐Petri ME, Molins‐Castiella T, Durá‐Travé T. Hallucinations caused by the introduction of methylphenidate at low doses. Revista de Neurologia 2010;51(4):254‐5. [PUBMED: 20648469] [PubMed] [Google Scholar]
Akhondzadeh 2003 {published data only}
- Akhondzadeh S, Tavakolian R, Davari‐Ashtiani R, Arabgol F, Amini H. Selegiline in the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial. Progress in Neuro Psychopharmacology and Biological Psychiatry 2003;27(5):841‐5. [DOI: 10.1016/S0278-5846(03)00117-9; PUBMED: 12921918] [DOI] [PubMed] [Google Scholar]
Akhondzadeh 2004 {published data only}
- Akhondzadeh S, Mohammadi MR, Khademi M. Zinc sulfate as an adjunct to methylphenidate for the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial. BMC Psychiatry 2004;4(9):1‐6. [DOI: 10.1186/1471-244X-4-9; PMC400741; PUBMED: 15070418] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alpaslan 2015 {published data only}
- Alpaslan AH, Coşkun KŞ, Kocak U, Gorücü Y. Stuttering associated with the use of short‐acting oral methylphenidate. Klinik Psikofarmakoloji Bülteni [Journal of Clinical Psychopharmacology] 2015;35(6):739‐41. [DOI: 10.1097/JCP.0000000000000403; PUBMED: 26436866] [DOI] [PubMed] [Google Scholar]
Altin 2013 {published data only}
- Altin M, El‐Shafei AA, Yu M, Desaiah D, Treuer T, Zavadenko N, et al. Pharmacological treatment for attention deficit hyperactivity disorder: functional outcomes in children and adolescents from non‐Western countries. Drugs in Context 2013;2013:212260. [DOI: 10.7573/dic.212260; PMC3884848] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Novick D, Treuer T, Montgomery W, Haynes VS, Wu S, et al. Predictors and consequences of adherence to the treatment of pediatric patients with attention‐deficit/hyperactivity disorder in Central Europe and East Asia. Patient Preference and Adherence 2013;30(7):987‐95. [DOI: 10.2147/PPA.S50628; PMC3794850; PUBMED: 24124351] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truer T, Feng Q, Desaiah D, Altin M, Wu S, El‐Shafei A, et al. Predictors of pharmacological treatment outcomes with atomoxetine or methylphenidate in patients with attention‐deficit/hyperactivity disorder from China, Egypt, Lebanon, Russian Federation, Taiwan, and United Arab Emirates. International Journal of Clinical Practice 2014;68(9):1152‐62. [DOI: 10.1111/ijcp.12437; PUBMED: 24703228] [DOI] [PubMed] [Google Scholar]
Amiri 2008 {published data only}
- Amiri S, Mohammadi MR, Mohammadi M, Nouroozinejad GH, Kahbazi M, Akhondzadeh S. Modafinil as a treatment for Attention‐Deficit/Hyperactivity Disorder in children and adolescents: a double blind, randomized clinical trial. Progress in Neuro Psychopharmacology & Biological Psychiatry 2008;32(1):145‐9. [DOI: 10.1016/j.pnpbp.2007.07.025; PUBMED: 17765380] [DOI] [PubMed] [Google Scholar]
Arabgol 2015 {published data only}
- Arabgol F, Panaghi L, Nikzad V. Risperidone versus methylphenidate in treatment of preschool children with attention‐deficit hyperactivity disorder. Iranian Journal of Pediatrics 2015;25(1):1‐6. [DOI: 10.5812/ijp.265; PMC4505976] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ardic 2014 {published data only}
- Ardic UA, Ercan ES, Ercan E, Yuce D, Basay BK. Osmotic release oral system methylphenidate is more effective than immediate release methylphenidate: a retrospective chart review in Turkish children with attention deficit hyperactivity disorder. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2014;24(4):342‐9. [DOI: 10.5455/bcp.20141009112739] [DOI] [Google Scholar]
- Ercan ES, Akyol‐Ardic U, Ercan E, Yuce D, Kabukcu‐Basay B. OROS‐MPH is more effective than IR‐MPH in Turkish children: a retrospective chart review. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2014;24(1):306‐7. [DOI: 10.5455/bcp.20141009112739] [DOI] [Google Scholar]
Arman 2013 {published data only}
- Arman AR, Yilmaz S, Carkaxhiu G, Soysal D, Akalin F. Risk assessment using heart rate variability due to methylphenidate treatment in children with attention deficit hyperactivity disorder. European Child and Adolescent Psychiatry. 15th International Congress of European Society for Child and Adolescent Psychiatry (ESCAP); 2013 Jul 1; Dublin (Ireland). New York: Springer, 2013; Vol. 22:S302‐S303.
Arnold 2004 {published data only}
- Arnold LE. Questions regarding your study "A double‐blind, placebo‐controlled withdrawal trial of dexmethylphenidate hydrochloride in children with attention deficit hyperactivity disorder" [personal communication]. Email to: EL Ramstad 17 October 2013. [DOI] [PubMed]
- Arnold LE, Lindsay RL, Conners CK, Wigal SB, Levine AJ, Johnson DE, et al. A double‐blind, placebo‐controlled withdrawal trial of dexmethylphenidate hydrochloride in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2004; Vol. 14, issue 4:542‐54. [DOI: 10.1089/cap.2004.14.542; PUBMED: 15662146] [DOI] [PubMed]
Arnold 2010 {published data only}
- Arnold LE, Bozzolo DR, Hodgkins P, McKay M, Beckett‐Thurman L, Greenbaum M, et al. Switching from oral extended‐release methylphenidate to the methylphenidate transdermal system: continued attention‐deficit/hyperactivity disorder symptom control and tolerability after abrupt conversion. Current Medical Research and Opinion 2010;26(1):129‐37. [DOI: 10.1185/03007990903437412; PMC3875401; PUBMED: 19916704] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukstein OG, Arnold LE, Landgraf JM, Hodgkins P. Does switching from oral extended‐release methylphenidate to the methylphenidate transdermal system affect health‐related quality‐of‐life and medication satisfaction for children with attention‐deficit/hyperactivity disorder?. Child and Adolescent Psychiatry and Mental Health 2009;3(1):39. [DOI: 10.1186/1753-2000-3-39; PMC2796990; PUBMED: 20003260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Artul 2014 {published data only}
- Artul S, Artoul F, Habib G, Nseir W, Bisharat B, Nijim Y. Severe recurrent pancreatitis in a child with ADHD after starting treatment with methylphenidate (Ritalin). Case Reports in Gastrointestinal Medicine 2014;2014:319162. [DOI: 10.1155/2014/319162; PMC3965929; PUBMED: 24711932; PUBMED: 24711932] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arun 2014 {published data only}
- Arun P, Sahni S. Methylphenidate and suicidal ideation: report of two cases. Indian Journal of Psychiatry 2014;56(1):79‐81. [DOI: 10.4103/0019-5545.124721; PMC3927251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashkenasi 2011 {published data only}
- Ashkenasi A. Effect of transdermal methylphenidate wear times on sleep in children with ADHD. Journal of Neurology 2012;259(Suppl 1):S33‐4. [DOI: 10.1007/s00415-012-6524-4] [DOI] [PubMed] [Google Scholar]
- Ashkenasi A. Effect of transdermal methylphenidate wear times on sleep in children with attention deficit hyperactivity disorder. Pediatric Neurology 2011;45(6):381‐6. [DOI: 10.1016/j.pediatrneurol.2011.09.003; PUBMED: 22115000] [DOI] [PubMed] [Google Scholar]
Atzori 2009 {published data only}
- Atzori P, Ancilletta B, Usala T, Danjou F, Zuddas A. Predictive parameters for methylphenidate use and compliance in children with attention deficit hyperactivity disorder. European Neuropsychopharmacology 2006;16(Suppl 1):S73‐4. [DOI: 10.1016/S0924-977X(06)80087-8] [DOI] [Google Scholar]
- Atzori P, Usala T, Carucci S, Danjou F, Zuddas A. Predictive factors for persistent use and compliance of immediate‐release methylphenidate: a 36‐month naturalistic study. Journal of Child and Adolescent Psychopharmacology 2009;19(6):673‐81. [DOI: 10.1089/cap.2008.0146; PUBMED: 20035585] [DOI] [PubMed] [Google Scholar]
Ayaz 2014 {published data only}
- Ayaz M, Ayaz AB, Soylu N, Yüksel S. Medication persistence in Turkish children and adolescents with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2014;24(8):442‐7. [DOI: 10.1089/cap.2014.0002; PUBMED: 25010598] [DOI] [PubMed] [Google Scholar]
Balázs 2011 {published data only}
- Balázs J, Dallos G, Keresztény A, Czobor P, Gádoros J. Methylphenidate treatment and dyskinesia in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2011;21(2):133‐8. [DOI: 10.1089/cap.2010.0030; PUBMED: 21486166] [DOI] [PubMed] [Google Scholar]
Barbaresi 2006 {published data only}
- Barbaresi WJ, Colligan RC, Weaver AL, Voigt RG, Kilian JM, Katusic SK. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics 2013;132(4):637‐44. [DOI: 10.1542/peds.2012-2354] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Pankratz VS, Weaver AL, Weber KJ, et al. How common is attention‐deficit/hyperactivity disorder? Incidence in a population‐based birth cohort in Rochester, Minn. Archives of Pediatrics and Adolescent Medicine 2002;156(3):217‐24. [PUBMED: 11876664] [DOI] [PubMed] [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long‐term stimulant medication treatment of attention‐deficit/hyperactivity disorder: results from a population‐based study. Journal of Developmental and Behavioral Pediatrics 2006;27(1):1‐10. [PUBMED: 16511362] [DOI] [PubMed] [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long‐term stimulant medication treatment of attention‐deficit/hyperactivity disorder: results from a population‐based study. Journal of Developmental and Behavioral Pediatrics 2014;27(1):448‐57. [PUBMED: 16511362] [DOI] [PubMed] [Google Scholar]
- Harris MN, Voigt RG, Barbaresi WJ, Voge GA, Kilian JM, Weaver AL, et al. ADHD and learning disabilities in former late preterm infants: a population‐based birth cohort. Pediatrics 2013;132(3):630‐6. [DOI: 10.1542/peds.2012-3588; PMC3876753; PUBMED: 23979091] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harstad EB, Weaver AL, Katusic SK, Colligan RC, Kumar S, Chan E, et al. ADHD, stimulant treatment, and growth: a longitudinal study. Pediatrics. 2014;134(4):935‐44. [DOI: 10.1542/peds.2014-0428; PUBMED: 25180281] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic SK, Barbaresi W, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Case definition in epidemiologic studies of ADHD. Annals of Epidemiology 2005;15(6):430‐7. [DOI: 10.1016/j.annepidem.2004.12.004; PUBMED: 15967390] [DOI] [PubMed] [Google Scholar]
- Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Potential influence of migration bias in birth cohort studies. Mayo Clinic Proceedings 1998;73(11):1053‐61. [DOI: 10.4065/73.11.1053] [DOI] [PubMed] [Google Scholar]
- Mellon MW, Natchev BE, Katusic SK, Colligan RC, Weaver AL, Voigt RG, et al. Incidence of enuresis and encopresis among children with attention‐deficit/hyperactivity disorder in a population‐based birth cohort. Academic Pediatrics 2013;13(4):322‐7. [DOI: 10.1016/j.acap.2013.02.008; PMC3886550; PUBMED: 23680296] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasu K, Barbaresi WJ, Colligan RC, Kilian JM, Voigt RG, Weaver AL, Katusic SK. Written‐language disorder among children with and without ADHD in a population‐based birth cohort.. Pediatrics 2011;128(3):605‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasu K, Barbaresi WJ, Colligan RC, Voigt RG, Kilian JM, Weaver AL, et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population‐based birth cohort study. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2012;53(10):1036‐43. [DOI: 10.1111/j.1469-7610.2012.02567.x; PMC3608464; PUBMED: 22647074] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrickman 1995 {published data only}
- Barrickman LL, Perry PJ, Allen AJ, Kuperman S, Arndt SV, Herrmann KJ, et al. Bupropion versus methylphenidate in the treatment of attention‐deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(5):649‐57. [DOI: 10.1097/00004583-199505000-00017; PUBMED: 7775360] [DOI] [PubMed] [Google Scholar]
Berek 2011 {published data only}
- Alfred A, Lindermueller A, Mattejat F, Dichter S, Schaeuble B. Transitioning onto OROS MPH is associated with improved functioning and quality of life in adolescents with ADHD. European Psychiatry. 18th European Congress of Psychiatry. 2010 Feb 27‐March 2; Munich (Germany) 2010;25(Suppl 1):375. [DOI: 10.1016/S0924-9338(10)70372-2] [DOI] [Google Scholar]
- Berek M, Kordon A, Hargarter L, Mattejat F, Slawik L, Rettig K, et al. Improved functionality, health related quality of life and decreased burden of disease in patients with ADHD treated with OROS® MPH: is treatment response different between children and adolescents?. Child & Adolescent Psychiatry & Mental Health 2011;5(1):26. [DOI: 10.1186/1753-2000-5-26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordon A, Hultzsch W, Mattejat F, Dichter S, Schaeuble B. Transitioning onto OROS MPH is associated with improved functioning and quality of life in children and adolescents with ADHD. European Psychiatry. 18th European Congress of Psychiatry. 2010 Feb 27‐March 2; Munich (Germany). 2010; Vol. 25, issue Suppl 1:962. [DOI: 10.1016/S0924-9338(10)70953-6] [DOI]
- Niederkirchner K, Slawik L, Wermelskirchen D, Rettig K, Schauble B. Transitioning to OROS® methylphenidate from atomoxetine is effective in children and adolescents with ADHD. Expert Review of Neurotherapeutics 2011;11(4):499‐508. [DOI: 10.1586/ern.11.18] [DOI] [PubMed] [Google Scholar]
- Schaeuble B, Mattejat F, Rettig K, Kordon A, Berek M. Changes in function and quality of life aspects in children with ADHD after transitioning onto OROS MPH. European Child and Adolescent Psychiatry.14th International Congress of European Society for Child and Adolescent Psychiatry (ESCAP); June 11‐15 2011; Helsinki (Finland). 2011; Vol. Suppl 1:S131.
- Wolff C, Alfred A, Lindermüller A, Rettig K, Mattejat F, Gerwe M, et al. Effect of transitioning from extended‐release methylphenidate onto osmotic, controlled‐release methylphenidate in children/adolescents with ADHD: results of a 3‐month non‐interventional study. Current Medical Research and Opinion 2011;27(Suppl 2):35‐44. [DOI: 10.1185/03007995.2011.601733; PUBMED: 21787126] [DOI] [PubMed] [Google Scholar]
Bereket 2005 {published data only}
- Bereket A, Turan S, Karaman MG, Haklar G, Ozbay F, Yazgan MY. Height, weight, IGF‐I, IGFBP‐3 and thyroid functions in prepubertal children with attention deficit hyperactivity disorder: effect of methylphenidate treatment. Hormone Research 2005;63(4):159‐64. [DOI: 10.1159/000084683; PUBMED: 15795512] [DOI] [PubMed] [Google Scholar]
Bernhard 2009 {published data only}
- Bernhard MK, Hugle B, Merkenschlager A. Elevated liver enzymes under therapy with methylphenidate in a boy with T‐cell leukemia. Journal of Pediatric Neurology 2009; Vol. 7, issue 3:297‐9. [DOI: 10.3233/JPN-2009-0303] [DOI]
Blader 2010 {published data only}
- Blader JC, Pliszka SR, Jensen PS, Schooler NR, Kafantaris V. Stimulant‐responsive and stimulant‐refractory aggressive behavior among children with ADHD. Pediatrics 2010;126(4):e796‐806. [DOI: 10.1542/peds.2010-0086; PMC2956067; PUBMED: 20837589] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader Joseph C. Methylphenidate dose may reduce aggressive behavior in children with ADHD. Brown University Child & Adolescent Psychopharmacology Update 2010;12(12):1‐7. [DOI: 10.1002/cpu.20129] [DOI] [Google Scholar]
Buchmann 2007 {published data only}
- Buchmann J, Gierow W, Weber S, Hoeppner J, Klauer T, Benecke R, et al. Restoration of disturbed intracortical motor inhibition and facilitation in attention deficit hyperactivity disorder children by methylphenidate. Biological Psychiatry 2007;62(9):963‐9. [DOI: 10.1016/j.biopsych.2007.05.010; PUBMED: 17719015] [DOI] [PubMed] [Google Scholar]
- Buchmann J, Gierow W, Weber S, Hoeppner J, Klauer T, Wittsock M, et al. Modulation of transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) by medication with methylphenidate (MPH). Neuroscience Letters 2006;405(1‐2):14‐8. [DOI: 10.1016/j.neulet.2006.06.026; PUBMED: 16815631] [DOI] [PubMed] [Google Scholar]
- Buchmann J, Wolters A, Haessler F, Bohne S, Nordbeck R, Kunesch E. Disturbed transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD). Clinical Neurophysiology 2003;114(11):2036‐42. [PUBMED: 14580601] [DOI] [PubMed] [Google Scholar]
Cakin‐Memik 2010 {published data only}
- Cakin‐Memik N, Yildiz O, Sişmanlar SG, Karakaya I, Ağaoğlu B. Priapism associated with methylphenidate: a case report. Turkish Journal of Pediatrics 2010;52(4):430‐4. [PUBMED: 21043394] [PubMed] [Google Scholar]
Çetin 2015 {published data only}
- Çetin FH, Işik Taner Y, Taş Torun Y, Tunca H. Atomoxetine and methylphenidate for the treatment of attention deficit hyperactivity disorder: a six‐month follow‐up study. Improved choices of psychotropic medications: better mental health outcomes. 5th International Congress on Psychopharmacology and International Symposium on Child and Adolescent Psychopharmacology (TAP‐ICP); October 30‐November 3 2013; Antalya (Turkey). 2013; Vol. 23:S80.
- Çetin FH, Taş TY, Işik Taner Y. Atomoxetine versus OROS methylphenidate in attention deficit hyperactivity disorder: a six‐month follow up study for efficacy and adverse effects. Turkiye Klinikleri Journal of Medical Sciences 2015;35(2):88‐96. [DOI: 10.5336/medsci.2015-43336] [DOI] [Google Scholar]
Chandler 1989 {published data only}
- Chandler ML, Barnhill JL, Gualtieri CT, Patterson DR. Tryptophan antagonism of stimulant‐induced tics. Journal of Clinical Psychopharmacology 1989;9(1):69‐70. [PUBMED: 2708560] [DOI] [PubMed] [Google Scholar]
Chazan 2011 {published data only}
- Bruxel EM, Salatino‐Oliveira A, Genro JP, Zeni CP, Polanczyk GV, Chazan R, et al. Association of a carboxylesterase 1 polymorphism with appetite reduction in children and adolescents with attention‐deficit/hyperactivity disorder treated with methylphenidate. Pharmacogenomics Journal 2013;13(5):476‐80. [DOI: 10.1038/tpj.2012.25; PUBMED: 22688218] [DOI] [PubMed] [Google Scholar]
- Chazan R, Borowski C, Pianca T, Ludwig H, Rohde LA, Polanczyk G. Do phenotypic characteristics, parental psychopathology, family functioning, and environmental stressors have a role in the response to methylphenidate in children with attention‐deficit/hyperactivity disorder? A naturalistic study from a developing count. Journal of Clinical Psychopharmacology 2011;31(3):309‐17. [DOI: 10.1097/JCP.0b013e318217b4df; PUBMED: 21508864] [DOI] [PubMed] [Google Scholar]
- Salatino‐Oliveira A, Genro JP, Zeni C, Polanczyk GV, Chazan R, Guimarães AP, et al. Catechol‐O‐methyltransferase valine158methionine polymorphism moderates methylphenidate effects on oppositional symptoms in boys with attention‐deficit/hyperactivity disorder. Biological Psychiatry 2011;70(3):216‐21. [DOI: 10.1016/j.biopsych.2011.03.025; PUBMED: 21550019] [DOI] [PubMed] [Google Scholar]
Cherland 1999 {published data only}
- Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5‐year review. Canadian Journal of Psychiatry 1999;44(8):811‐3. [DOI: 10.1177/070674379904400810; PUBMED: 10566114] [DOI] [PubMed] [Google Scholar]
Cho 2012 {published data only}
- Cho S‐C, Kim B‐N, Cummins TD, Kim J‐W, Bellgrove MA. Norepinephrine transporter ‐3081(A/T) and alpha‐2A‐adrenergic receptor MspI polymorphisms are associated with cardiovascular side effects of OROS‐methylphenidate treatment. Journal of Psychopharmacology 2012; Vol. 26, issue 3:380‐9. [DOI: 10.1177/0269881111405356] [DOI] [PubMed]
Chou 2012a {published data only}
- Chou WJ, Chen SJ, Chen YS, Liang HY, Lin CC, Tang CS, et al. Remission in children and adolescents diagnosed with attention‐deficit/ hyperactivity disorder via an effective and tolerable titration scheme for osmotic release oral system methylphenidate. Journal of Child and Adolescent Psychopharmacology 2012;22(3):215‐25. [DOI: 10.1089/cap.2011.0006; PMC3373222; PUBMED: 22537358] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chou 2012b {published data only}
- WJ Chou, LJ Wang. The family impact in children and adolescent with ADHD after the switch of treatment from immediate‐release MPH to OROS‐MPH. Neuropsychiatrie de l'Enfance et de l'Adolescence 2012;60(5S):S262. [DOI: 10.1016/j.neurenf.2012.04.683] [DOI] [Google Scholar]
Cockcroft 2009 {published data only}
- Cockcroft K, Ashwal J, Bentley A. Sleep and daytime sleepiness in methylphenidate medicated and un‐medicated children with attention‐deficit/hyperactivity disorder (ADHD). African Journal of Psychiatry 2009;12(4):275‐9. [PUBMED: 20033109] [DOI] [PubMed] [Google Scholar]
Cohen 1992 {published data only}
- Cohen HA, Ashkenazi A, Nussinovitch M, Gross S, Frydman M. Fixed drug eruption of the scrotum due to methylphenidate. Annals of Pharmacotherapy 1992;26(11):1378‐9. [DOI: 10.1177/106002809202601107; PUBMED: 1477441] [DOI] [PubMed] [Google Scholar]
Coignoux 2009 {published data only}
- Coignoux Y, Estingoy P, Bastard A. From hyperactivity to schizophrenia? Clinical, neurobiological and therapeutic discussion of a case [De l'hyperactivité à la schizophrénie? Discussion clinique, neurobiologique et thérapeutique, à propos d'un cas]. Annales Médico‐psychologiques, Revue Psychatrique 2009;167(1):57‐65. [DOI: 10.1016/j.amp.2008.11.006] [DOI] [Google Scholar]
Confino‐Cohen 2005 {published data only}
- Confino‐Cohen R, Goldberg A. Successful desensitization of methylphenidate‐induced rash. Journal of Child and Adolescent Psychopharmacology 2005;15(4):703‐5. [DOI: 10.1089/cap.2005.15.703; PUBMED: 16190802] [DOI] [PubMed] [Google Scholar]
Congologlu 2009 {published data only}
- Congologlu MA, Turkbay T, Ciyiltepe M, Durukan I, Dogangun B, Yuce M. Immediate effects of methylphenidate on vocal acoustic parameters in children with attention deficit hyperactivity disorder. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2009;19(4):365‐72. [Google Scholar]
Corrigall 1996 {published data only}
- Corrigall R, Ford T. Methylphenidate euphoria. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(11):1421. [DOI: 10.1097/00004583-199611000-00005; PUBMED: 8936903] [DOI] [PubMed] [Google Scholar]
Cortese 2015 {published data only}
- Cortese S, Panei P, Arcieri R, Germinario EA, Capuano A, Margari L, et al. Safety of methylphenidate and atomoxetine in children with Attention‐Deficit/Hyperactivity Disorder (ADHD): data from the Italian National ADHD Registry. CNS Drugs 2015;29(10):865‐77. [DOI: 10.1007/s40263-015-0266-7; PUBMED: 26293742] [DOI] [PubMed] [Google Scholar]
Coşkun 2008 {published data only}
- Coşkun M, Zoroglu S. Tactile and visual hallucinations in a child with methylphenidate and fluoxetine combination. Journal of Clinical Psychopharmacology 2008;28(6):723‐5. [DOI: 10.1097/JCP.0b013e31818ce752; PUBMED: 19011457] [DOI] [PubMed] [Google Scholar]
Coşkun 2009a {published data only}
Coşkun 2009b {published data only}
- Coşkun M, Zoroglu S. A report of two cases of sexual side effects with OROS methylphenidate. Journal of Child and Adolescent Psychopharmacology 2009;19(4):477‐9. [DOI: 10.1089/cap.2008.0161; PUBMED: 19702503] [DOI] [PubMed] [Google Scholar]
Coşkun 2010 {published data only}
- Coşkun M, Ahmetoglu E, Ozturk M. Mirtazapine treatment for comorbid anxiety/depressive disorders in young subjects with attention‐deficit hyperactivity disorder: case series. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2010;20(3):246‐51. [Google Scholar]
Coşkun 2011 {published data only}
- Coşkun M. Methylphenidate induced obsessive‐compulsive symptoms treated with sertraline. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2011;21(3):274. [DOI: 10.5455/bcp.20110904061403] [DOI] [Google Scholar]
Davari‐Ashtiani 2010 {published data only}
- Babaki ME, Ashtiani RD, Razjooyan K, Amini H. Comparing the effects of buspirone and methylphenidate in children with attention deficit hyperactivity disorder. Iranian Journal of Psychiatry and Clinical Psychology 2009; Vol. 15, issue 3:223‐30.
- Davari‐Ashtiani R, Shahrbabaki ME, Razjouyan K, Amini H, Mazhabdar H. Buspirone versus methylphenidate in the treatment of attention deficit hyperactivity disorder: a double‐blind and randomized trial. Child Psychiatry and Human Development 2010;41(6):641‐8. [DOI: 10.1007/s10578-010-0193-2; PUBMED: 20517641] [DOI] [PubMed] [Google Scholar]
- Shahrbabaki ME, Sabzevari L, Haghdoost A, Davari‐Ashtiani R. Buspiron versus methylphenidate in the treatment of children with ADHD. Brain, Mind and Development. 20th World IACAPAP (International Association for Child and Adolescent Psychiatry and Allied Professions) Congress; July 21‐25 2012; Paris (France). 2012; Vol. 60:Suppl 1.
Delignieres 2011 {published data only}
- Delignieres A, Querne L, Bourel‐Ponchel E, Ardoint A, Berquin P. Attention‐deficit disorders and epilepsy in childhood: use methylphenidate treatment?. European Journal of Paediatric Neurology. 9th Congress of the European Paediatric Neurology Society (EPNS); May 11‐14 2011; Cavtat (Croatia). 2011; Vol. 15:S127‐8.
Dirksen 2002 {published data only}
- Dirksen SJ, D'Imperio JM, Birdsall D, Hatch SJ. A postmarketing clinical experience study of Metadate CD. Current Medical Research and Opinion 2002;18(7):371‐80. [PUBMED: 12487502] [DOI] [PubMed] [Google Scholar]
Dittmann 2014 {published data only}
- Dittmann RW, Banaschewski T, Schacht A, Wehmeier PM. Findings from the observational COMPLY study in children and adolescents with ADHD: core symptoms, ADHD‐related difficulties, and patients' emotional expression during psychostimulant or nonstimulant ADHD treatment. Attention Deficit and Hyperactivity Disorders 2014;6(2014):291‐302. [DOI: 10.1007/s12402-014-0136-z; PUBMED: 24705867] [DOI] [PubMed] [Google Scholar]
Dodangi 2011 {published data only}
- Dodangi N, Tehrani‐Doost M, Mahmoudi‐Gharaei J, Rajabi G. Duloxetine in comparison with methylphenidate in treatment of adolescents with ADHD. European Child and Adolescent Psychiatry. 14th International Congress of ESCAP European Society for Child and Adolescent Psychiatry; June 11‐15 2011; Helsinki (Finland). 2011; Vol. 20:S116.
- Mahmoudi‐Gharaei J, Dodangi N, Tehrani‐Doost M, Faghihi T. Duloxetine in the treatment of adolescents with attention deficit/hyperactivity disorder: an open‐label study. Human Psychopharmacology 2011;26(2):155‐60. [DOI: 10.1002/hup.1188; PUBMED: 21455975] [DOI] [PubMed] [Google Scholar]
Döpfner 2011a, OBSEER {published data only}
- Becker A, Roessner V, Breuer D, Döpfner M, Rothenberger A. Relationship between quality of life and psychopathological profile: data from an observational study in children with ADHD. European Child and Adolescent Psychiatry 2011;20(Suppl 2):S267‐75. [DOI: 10.1007/s00787-011-0204-2; PMC3180591; PUBMED: 21901415] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer D, Görtz‐Dorten A, Rothenberger A, Döpfner M. Assessment of daily profiles of ADHD and ODD symptoms, and symptomatology related to ADHD medication, by parent and teacher ratings. European Child and Adolescent Psychiatry 2011;20(Suppl 2):S289‐96. [DOI: 10.1007/s00787-011-0206-0; PMC3180560; PUBMED: 21901413] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döpfner M, Breuer D, Walter D, Rothenberger A. An observational study of once‐daily modified‐release methylphenidate in ADHD: the effect of previous treatment on ADHD symptoms, other externalising symptoms and quality‐of‐life outcomes. European Child and Adolescent Psychiatry 2011;20(Suppl 2):S277‐88. [DOI: 10.1007/s00787-011-0205-1; PMC3098980; PUBMED: 21901414] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döpfner M, Görtz‐Dorten A, Breuer D, Rothenberger A. An observational study of once‐daily modified‐release methylphenidate in ADHD: effectiveness on symptoms and impairment, and safety. European Child and Adolescent Psychiatry 2011;20(Suppl 2):S243‐55. [DOI: 10.1007/s00787-011-0202-4; PMC3180616; PUBMED: 21901417] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döpfner M, Rothenberger A, Ose C. Assessment of Equasym XL under routine conditions by physicians, parents, teachers, and children. International Journal of Neuropsychopharmacology 2008;11(Suppl 1):290. [DOI: 10.1017/S1461145708009462] [DOI] [Google Scholar]
- Görtz‐Dorten A, Breuer D, Hautmann C, Rothenberger A, Döpfner M. What contributes to patient and parent satisfaction with medication in the treatment of children with ADHD? A report on the development of a new rating scale. European Child and Adolescent Psychiatry 2011; Vol. 20, issue Suppl 2:S297‐307. [DOI: 10.1007/s00787-011-0207-z; PMC3180627] [DOI] [PMC free article] [PubMed]
- Rothenberger A, Becker A, Breuer D, Döpfner M. An observational study of once‐daily modified‐release methylphenidate in ADHD: quality of life, satisfaction with treatment and adherence. European Child and Adolescent Psychiatry 2011;20(Suppl 2):257‐65. [DOI: 10.1007/s00787-011-0203-3; PMC3180635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Döpfner 2011b {published data only}
- Döpfner M, Ose C, Fischer R, Ammer R, Scherag A. Comparison of the efficacy of two different modified release methylphenidate preparations for children and adolescents with attention‐deficit/hyperactivity disorder in a natural setting: comparison of the efficacy of Medikinet® retard and Concerta® ‐ a randomized, controlled, double‐blind multicenter clinical crossover trial. Journal of Child & Adolescent Psychopharmacology 2011;21(5):445‐54. [DOI: 10.1089/cap.2010.0082; PMC3205792; PUBMED: 21790298] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döpfner M, Ose C, Fischer R, Ammer R, Scherag A. The CoMeCo‐trial: comparison of the efficacy of two methylphenidate preparations for children and adolescents with ADHD in a natural setting. European Child and Adolescent Psychiatry.14th International Congress of ESCAP European Society for Child and Adolescent Psychiatry (ESCAP); June 11‐15, 2011; Helsinki (Finland) 2011;20:S116. [Google Scholar]
Döpfner 2011c {published data only}
- Döpfner M, Breuer D, Ose C, Fischer R. Methylphenidate with modified release in routine treatment [Methylphenidat mit modifizierter Freisetzung in der Routineversorgung]. Monatsschrift Kinderheilkunde 2011;159(11):1119‐25. [DOI: 10.1007/s00112-011-2413-7] [DOI] [Google Scholar]
Dubnov‐Raz 2011 {published data only}
- Dubnov‐Raz G, Perry A, Berger I. Body mass index of children with attention‐deficit/hyperactivity disorder. Journal of Child Neurology 2011;26(3):302‐8. [DOI: 10.1177/0883073810380051; PUBMED: 20929910] [DOI] [PubMed] [Google Scholar]
- Yang R, Mao S, Zhang S, Li R, Zhao Z. Body mass index of children with attention‐deficit/hyperactivity disorder. Journal of Child Neurology 2012; Vol. 27, issue 4:545‐6. [DOI: 10.1177/0883073811435326] [DOI] [PubMed]
Dupuy 2008 {published data only}
- Dupuy FE, Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG coherence in girls with attention‐deficit/hyperactivity disorder: stimulant effects in good responders. International Journal of Psychophysiology 2008;70(3):151‐7. [DOI: 10.1016/j.ijpsycho.2008.07.012; PUBMED: 18708101] [DOI] [PubMed] [Google Scholar]
Durá‐Travé 2012 {published data only}
- Durá‐Travé T, Yoldi‐Petri ME, Gallinas‐Victoriano F, Zardoya‐Santos P. Effects of osmotic‐release methylphenidate on height and weight in children with attention‐deficit hyperactivity disorder (ADHD) following up to four years of treatment. Journal of Child Neurology 2012; Vol. 27, issue 5:604‐9. [DOI: 10.1177/0883073811422752; PUBMED: 22190507] [DOI] [PubMed]
- Durá‐Travé T, Yoldi‐Petri ME, Zardoya‐Santos P. Nutrition and attention deficit hyperactivity disorder: developmental follow‐up of the anthropometric variables of a group of patients receiving treatment with osmotic controlled‐release methylphenidate [Nutrición y trastorno por déficit de atención/hiperactividad: seguimiento evolutivo de las variables antropométricas de un grupo de pacientes en tratamiento con metilfenidato de liberación osmótica]. Revista de Neurologia 2011;53(5):257‐64. [PUBMED: 21796603] [PubMed] [Google Scholar]
Efron 1997 {published data only}
- Efron D. Methylphenidate versus dextroamphetamine in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(5):500. [PUBMED: 10230180] [DOI] [PubMed] [Google Scholar]
- Efron D, Jarman FC, Barker MJ. Child and parent perceptions of stimulant medication treatment in attention deficit hyperactivity disorder. Journal of Paediatrics and Child Health 1998;34(3):288‐92. [DOI: 10.1046/j.1440-1754.1998.00224.x] [DOI] [PubMed] [Google Scholar]
- Efron D, Jarman FC, Barker MJ. Medium‐term outcomes are comparable with short‐term outcomes in children with attention deficit hyperactivity disorder treated with stimulant medication. Journal of Paediatrics and Child Health 2000;36(5):457‐61. [PUBMED: 11036801] [DOI] [PubMed] [Google Scholar]
- Efron D, Jarman FC, Barker MJ. Methylphenidate versus dexamphetamine in children with attention deficit hyperactivity disorder: a double‐blind, crossover trial. Pediatrics 1997;100(6):E6. [PUBMED: 9382907] [DOI] [PubMed] [Google Scholar]
- Efron D, Jarman FC, Barker MJ. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double‐blind, crossover trial. Pediatrics 1997;100(4):662‐6. [PUBMED: 9310521] [DOI] [PubMed] [Google Scholar]
El‐Fiky 2014 {published data only}
- El‐Fiky MR, El‐Nahas GM, El‐Shahawy HH, Abdel Aziz MF, Ali DH, Khalifa NM. Study the gender differences in ADHD children: is the response to CNS stimulants a case of one fits both?. Indian Streams Research Journal 2014;4(5):1‐10. [Google Scholar]
El‐Zein 2005 {published data only}
- El‐Zein RA, Abdel‐Rahman SZ, Hay MJ, Lopez MS, Bondy ML, Morris DL, et al. Cytogenetic effects in children treated with methylphenidate. Cancer Letters 2005;230(2):284‐91. [DOI: 10.1016/j.canlet.2005.01.003; PUBMED: 16297714] [DOI] [PubMed] [Google Scholar]
- Preston RJ, Kollins SH, Swanson JM, Greenhill LL, Wigal T, Elliott GR, et al. Comments on 'Cytogenetic effects in children treated with methylphenidate' by El‐Zein et al. Cancer Letters 2005;230(2):292‐4. [DOI: 10.1016/j.canlet.2005.05.038; PUBMED: 16019134] [DOI] [PubMed] [Google Scholar]
Famularo 1987 {published data only}
- Famularo R, Fenton T. The effect of methylphenidate on school grades in children with attention deficit disorder without hyperactivity: a preliminary report. Journal of Clinical Psychiatry 1987;48(3):112‐4. [PUBMED: 3818551] [PubMed] [Google Scholar]
Faraone 2007a {published data only}
- Faraone SV, Giefer EE. Long‐term effects of methylphenidate transdermal delivery system treatment of ADHD on growth. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(9):1138‐47. [DOI: 10.1097/chi.0b013e31806ad1d7; PUBMED: 17712237] [DOI] [PubMed] [Google Scholar]
Feeney 1997 {published data only}
- Feeney DJ, Klykylo WM. Medication‐induced seizures. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(8):1018‐9. [DOI: 10.1097/00004583-199708000-00007; PUBMED: 9256580] [DOI] [PubMed] [Google Scholar]
Fernández‐Fernández 2010 {published data only}
- Fernández‐Fernández MA, Rufo‐Campos M, Mateos‐Checa R, Muñoz‐Cabello B, Madruga‐Garrido M, Blanco‐Martínez B. Cardiovascular side effects secondary to treatment with methylphenidate [Reacciones adversas cardiovasculares secundarias al tratamiento con metilfenidato]. Revista de Neurologia 2010;50(9):573‐4. [PUBMED: 20443179] [PubMed] [Google Scholar]
Fernández‐Fernández 2011 {published data only}
- Fernández‐Fernández MA, Rufo‐Campos M, Mateos‐Checa R, Muñoz‐Cabello B, Madruga‐Garrido M, Blanco‐Martínez B. Infantile psychosis secondary to methylphenidate. Revista de Neurologia 2011;52(7):446‐7. [PUBMED: 21425116] [PubMed] [Google Scholar]
Findling 1996 {published data only}
- Findling RL. Open‐label treatment of comorbid depression and attentional disorders with co‐administration of serotonin reuptake inhibitors and psychostimulants in children, adolescents, and adults: a case series. Journal of Child and Adolescent Psychopharmacology 1996;6(3):165‐75. [DOI: 10.1089/cap.1996.6.165; PUBMED: 9231310] [DOI] [PubMed] [Google Scholar]
Findling 2009 {published data only}
- Findling RL, Wigal SB, Bukstein OG, Boellner SW, Abikoff HB, Turnbow JM, et al. Long‐term tolerability of the methylphenidate transdermal system in pediatric attention‐deficit/hyperactivity disorder: a multicenter, prospective, 12‐month, open‐label, uncontrolled, phase III extension of four clinical trials. Clinical Therapeutics 2009;31(8):1844‐55. [DOI: 10.1016/j.clinthera.2009.08.002; PUBMED: 19808143] [DOI] [PubMed] [Google Scholar]
- Turnbow JM, Findling RL, López FA, Burnside JC, Bukstein OG. 12‐month safety and efficacy of methylphenidate transdermal system in attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology 2008;18(s4):S562‐3. [DOI: 10.1016/S0924-977X(08)70857-5] [DOI] [Google Scholar]
Findling 2010 {published data only}
- Findling RL, Katic A, Rubin R, Moon E, Civil R, Li Y. A 6‐month, open‐label, extension study of the tolerability and effectiveness of the methylphenidate transdermal system in adolescents diagnosed with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2010;20(5):366‐75. [DOI: 10.1089/cap.2009.0122; PUBMED: 20973707] [DOI] [PubMed] [Google Scholar]
- Findling RL, Turnbow J, Burnside J, Melmed R, Civil R, Li Y. A randomized, double‐blind, multicenter, parallel‐group, placebo‐controlled, dose‐optimization study of the methylphenidate transdermal system for the treatment of ADHD in adolescents. CNS Spectrums 2010;15(7):419‐30. [PUBMED: 20625364] [DOI] [PubMed] [Google Scholar]
- Keating GM. Methylphenidate transdermal system in attention‐deficit hyperactivity disorder in adolescents: profile report. Drugs in R&D. G.M. Keating, Adis, Private Bag 65901, 41 Centorian Drive, Mairangi Bay, North Shore 0754, Auckland, New Zealand. E‐mail: demail@springer.com, 2012; Vol. 12, issue 3:171‐3. [DOI: 10.2165/11207490-000000000-00000; PMC3585903; PUBMED: 22934753] [DOI] [PMC free article] [PubMed]
Gadow 1995 {published data only}
- Gadow D, Nolan E, Sprafkin J, Sverd J. School observations of children with attention‐deficit hyperactivity disorder and comorbid tic disorder: effects of methylphenidate treatment. Journal of Developmental and Behavioral Pediatrics 1995;16(3):167–76. [PUBMED: 7560119] [PubMed] [Google Scholar]
- Gadow D, Sverd J, Sprafkin J, Nolan EE, Ezor SN. Efficacy of methylphenidate for attention‐deficit hyperactivity disorder in children with tic disorder. Annual Progress in Child Psychiatry and Child Development 1995;52(6):494‐522. [PUBMED: 7771914] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE, Sverd J. Methylphenidate in hyperactive boys with comorbid tic disorder: II. Short‐term behavioral effects in school settings. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(3):462‐71. [DOI: 10.1097/00004583-199205000-00012; PUBMED: 1592778] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Schwartz J. Anxiety and depression symptoms and response to methylphenidate in children with attention‐deficit hyperactivity disorder and tic disorder. Journal of Clinical Psychopharmacology 2002;22(3):267‐74. [PUBMED: 12006897] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sverd J, Sprafkin J, Nolan EE, Grossman S. Long‐term methylphenidate therapy in children with comorbid attention‐deficit hyperactivity disorder and chronic multiple tic disorder. Archives of General Psychiatry 1999;56(4):330‐6. [PUBMED: 10197827] [DOI] [PubMed] [Google Scholar]
- Nolan EE, Gadow KD. Children with ADHD and tic disorder and their classmates: behavioral normalization with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(5):597‐604. [DOI: 10.1097/00004583-199705000-00009; PUBMED: 9136493] [DOI] [PubMed] [Google Scholar]
- Nolan EE, Gadow KD. Relation between ratings and observations of stimulant drug response in hyperactive children. Journal of Clinical Child Psychology 1994;23(1):78‐90. [DOI: 10.1207/s15374424jccp2301_10] [DOI] [Google Scholar]
- Sprafkin J, Gadow KD. Double‐blind versus open evaluations of stimulant drug response in children with attention‐deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1996;6(4):215‐28. [DOI: 10.1089/cap.1996.6.215; PUBMED: 9231315] [DOI] [PubMed] [Google Scholar]
- Sverd J, Gadow KD, Nolan EE, Sprafkin J, Ezor SN. Methylphenidate in hyperactive boys with comorbid tic disorder: I. Clinic evaluations. Advances in Neurology 1992; Vol. 58:271‐81. [PUBMED: 1414633] [PubMed]
Galland 2010 {published data only}
- Galland BC, Tripp EG, Gray A, Taylor BJ. Apnea‐hypopnea indices and snoring in children diagnosed with ADHD: a matched case‐control study. Sleep and Breathing 2011;15(3):455‐62. [DOI: 10.1007/s11325-010-0357-0; PUBMED: 20440568] [DOI] [PubMed] [Google Scholar]
- Galland BC, Tripp EG, Taylor BJ. Methylphenidate effects on sleep in children. Brown University Child and Adolescent Psychopharmacology Update 2010;12(3):5‐6. [DOI: 10.1002/cpu.20111] [DOI] [Google Scholar]
- Galland BC, Tripp EG, Taylor BJ. The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: a matched case‐control study. Journal of Sleep Research 2010;19(2):366‐73. [DOI: 10.1111/j.1365-2869.2009.00795.x; PUBMED: 20050995] [DOI] [PubMed] [Google Scholar]
Garg 2014 {published data only}
- Garg J, Arun P, Chavan BS. Comparative efficacy of methylphenidate and atomoxetine in oppositional defiant disorder comorbid with attention deficit hyperactivity disorder. International Journal of Applied and Basic Medical Research 2015;5(2):114‐8. [DOI: 10.4103/2229-516X.157162; PMC4456885] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg J, Arun P, Chavan BS. Comparative short term efficacy and tolerability of methylphenidate and atomoxetine in attention deficit hyperactivity disorder. Indian Pediatrics 2014;51(7):550‐4. [PUBMED: 25031133] [DOI] [PubMed] [Google Scholar]
Gau 2006 {published data only}
- Gau SS, Shen HY, Soong WT, Gau CS. An open‐label, randomized, active‐controlled equivalent trial of osmotic release oral system methylphenidate in children with attention‐deficit/hyperactivity disorder in Taiwan. Journal of Child and Adolescent Psychopharmacology 2006;16(4):441‐55. [DOI: 10.1089/cap.2006.16.441; PUBMED: 16958569] [DOI] [PubMed] [Google Scholar]
Gau 2008 {published data only}
- Gau SS, Chen SJ, Chou WJ, Cheng H, Tang CS, Chang HL, et al. National survey of adherence, efficacy, and side effects of methylphenidate in children with attention‐deficit/hyperactivity disorder in Taiwan. Journal of Clinical Psychiatry 2008;69(1):131‐40. [PUBMED: 18312048] [DOI] [PubMed] [Google Scholar]
Germinario 2013 {published data only}
- Arcieri R, Germinario EP, Bonati M, Masi G, Zuddas A, Vella S, et al. Cardiovascular measures in children and adolescents with attention‐deficit/hyperactivity disorder who are new users of methylphenidate and atomoxetine. Journal of Child and Adolescent Psychopharmacology 2012;22(6):423‐31. [PUBMED: 23362511] [DOI] [PubMed] [Google Scholar]
- Balia C, Anedda A, Granitzio F, Carucci S, Zuddas A. Long term therapy with methylphenidate induces modest effects on growth in ADHD children. European Neuropsychopharmacology 2013;23(Suppl 1):S80. [DOI: 10.1016/S0924-977X(13)70090-7] [DOI] [Google Scholar]
- Didoni A, Sequi M, Panei P, Bonati M, Lombardy ADHD Registry Group. One‐year prospective follow‐up of pharmacological treatment in children with attention‐deficit/hyperactivity disorder. European Journal of Clinical Pharmacology 2011;67(10):1061‐7. [DOI: 10.1007/s00228-011-1050-3; PUBMED: 21538145] [DOI] [PubMed] [Google Scholar]
- Germinario EP, Arcieri R, Bonati M, Zuddas A, Masi G, Vella S, et al. Attention‐deficit/hyperactivity disorder drugs and growth: an Italian prospective observational study. Journal of Child and Adolescent Psychopharmacology 2013;23(7):440‐7. [DOI: 10.1089/cap.2012.0086; PMC3778954] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panei P, Arcieri R. ADHD register: post‐marketing evaluation of the benefit‐risk profile of drugs and promotion of the appropriateness [Il registro dell'ADHD: valutazione post‐marketing del profilo beneficio‐rischio dei farmaci e promozione dell'appropriatezza]. Recenti Progressi in Medicina 2013;104(6):254‐61. [DOI: 10.1701/1295.14326; PUBMED: 23801229] [DOI] [PubMed] [Google Scholar]
- Panei P, Arcieri R, Bonati M, Bugarini M, Didoni A, Germinario E. Safety of psychotropic drug prescribed for attention‐deficit/hyperactivity disorder in Italy. Adverse Drug Reaction Bulletin 2010;260:999‐1002. [old.iss.it/binary/uvco/cont/Safety_of_psychotropic_drug_prescribed_for_1.pdf] [Google Scholar]
- Panei P, Arcieri R, Vella S, Bonati M, Martini N, Zuddas A. Italian attention‐deficit/hyperactivity disorder registry. Pediatrics 2004;114(2):514. [PUBMED: 15286248] [DOI] [PubMed] [Google Scholar]
- Peddis C, Esu L, Tronci MG, Carucci S, Panei P, Zuddas A. The Italian ADHD National Registry: 3 years of active pharmacovigilance in developmental neuropsychiatry. European Child and Adolescent Psychiatry 2011;20(Suppl 1):S125. [DOI: 10.1007/s00787-011-0181-5] [DOI] [Google Scholar]
- Peddis C, Sanna E, Usala T, Arcieri R, Germinario E, Bugarini M, et al. Active pharmacovigilance in developmental neuropsychiatry: the Italian ADHD National Registry. European Child and Adolescent Psychiatry 2010;19(Suppl 1):S27. [DOI: 10.1007/s00787-010-0117-5] [DOI] [Google Scholar]
- Ruggiero S, Rafaniello C, Bravaccio C, Grimaldi G, Granato R, Pascotto A, et al. Safety of attention‐deficit/hyperactivity disorder medications in children: an intensive pharmacosurveillance monitoring study. Journal of Child and Adolescent Psychopharmacology 2012;22(6):415‐22. [DOI: 10.1089/cap.2012.0003; PUBMED: 23234585] [DOI] [PubMed] [Google Scholar]
Gerwe 2009 {published data only}
- Gerwe M, Stollhoff K, Mossakowski J, Kuehle HJ, Goertz U, Schaefer C, et al. Tolerability and effects of OROS® MPH (Concerta®) on functioning, severity of disease and quality of life in children and adolescents with ADHD: results from a prospective, non‐interventional trial. Attention Deficit and Hyperactivity Disorders 2009;1(2):175‐86. [DOI: 10.1007/s12402-009-0010-6; PUBMED: 21432582] [DOI] [PubMed] [Google Scholar]
Ghanizadeh 2008a {published data only}
- Ghanizadeh A. Insomnia, night terror, and depression related to clonidine in attention‐deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology 2008;28(6):725‐6. [DOI: 10.1097/JCP.0b013e31818ce741; PUBMED: 19011458] [DOI] [PubMed] [Google Scholar]
Ghanizadeh 2008b {published data only}
- Ghanizadeh A. Methylphenidate‐associated enuresis in attention deficit hyperactivity disorder. Journal of Pediatric Urology 2008;4(4):306‐7. [DOI: 10.1016/j.jpurol.2007.10.001; PUBMED: 18644535] [DOI] [PubMed] [Google Scholar]
Ghanizadeh 2008c {published data only}
- Ghanizadeh A. Questions regarding 'Insomnia, night terror, and depression related to clonidine in attention‐deficit/hyperactivity disorder' [personal communication]. Email to: EL Ramstad 4 July 2013.
- Ghanizadeh A, Aghakhani K. Photophobia and methylphenidate. Psychopharmacology Bulletin 2008;41(1):171‐3. [PUBMED: 18362880] [PubMed] [Google Scholar]
Ghanizadeh 2009 {published data only}
- Ghanizadeh A. Excessive talking triggered by methylphenidate in a boy with ADHD. Pharmacopsychiatry 2009;42(1):35‐6. [DOI: 10.1055/s-0028-1083821; PUBMED: 19153945] [DOI] [PubMed] [Google Scholar]
Ghanizadeh 2012 {published data only}
- Ghanizadeh A, Haghighat R. Nortriptyline for treating enuresis in ADHD‐‐a randomized double‐blind controlled clinical trial. Pediatric Nephrology 2012;27(11):2091‐7. [DOI: 10.1007/s00467-012-2211-z; PUBMED: 22700161] [DOI] [PubMed] [Google Scholar]
Ghanizadeh 2013 {published data only}
- Ghanizadeh A, Sayyari Z, Mohammadi MR. Effect of methylphenidate and folic acid on ADHD symptoms and quality of life and aggression: a randomized double blind placebo controlled clinical trial. Iranian Journal of Psychiatry 2013;8(3):108‐12. [PMC3887226; PUBMED: 24454418] [PMC free article] [PubMed] [Google Scholar]
Goetz 2011 {published data only}
- Goetz M, Prihodova I, Hrdlicka M. Long lasting complex nocturnal hallucinations during Osmotic Release Oral System (OROS) methylphenidate treatment in a 7‐year old girl. Neuro Endocrinology Letters 2011;32(5):619‐22. [PUBMED: 22167138] [PubMed] [Google Scholar]
Goez 2012 {published data only}
- Goez HR, Scott O, Nevo N, Bennett‐Back O, Zelnik N. Using the test of variables of attention to determine the effectiveness of modafinil in children with attention‐deficit hyperactivity disorder (ADHD): a prospective methylphenidate‐controlled trial. Journal of Child Neurology 2012;27(12):1547‐52. [DOI: 10.1177/0883073812439101; PUBMED: 22447850] [DOI] [PubMed] [Google Scholar]
- Zelnik N, Nevo N, Goez H, Bennett‐Back O. Modafinil vs. methylphenidate for children and adolescents with ADHD. European Journal of Paediatric Neurology 2011;15(Suppl 1):S126‐7. [DOI: 10.1016/S1090-3798(11)70442-1] [DOI] [Google Scholar]
Gökce 2015 {published data only}
- Gökce S, Önal A. Suicide attempt in a 12 year old boy after switching 27 mg to 36 mg of OROS methylphenidate. European Child and Adolescent Psychiatry 2015;24:s 219. [Google Scholar]
Golubchik 2011 {published data only}
- Golubchik P, Golubchik L, Sever JM, Weizman A. The beneficial effect of methylphenidate in ADHD with comorbid separation anxiety. International Clinical Psychopharmacology 2014;29(5):274‐8. [DOI: 10.1097/YIC.0000000000000034; PUBMED: 24743562] [DOI] [PubMed] [Google Scholar]
- Golubchik P, Kodesh A, Weizman A. Attention‐deficit/hyperactivity disorder and comorbid subsyndromal depression: what is the impact of methylphenidate on mood?. Clinical Neuropharmacology 2013;36(5):141‐5. [DOI: 10.1097/WNF.0b013e31829eb204; PUBMED: 24045603] [DOI] [PubMed] [Google Scholar]
- Golubchik P, Sever J, Weizman A. Methylphenidate treatment in children with attention deficit hyperactivity disorder and comorbid social phobia. International Clinical Psychopharmacology 2014;29(4):212‐5. [DOI: 10.1097/YIC.0000000000000029; PMC4047304; PUBMED: 24448460] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubchik P, Sever J, Weizman A, Zalsman G. Methylphenidate treatment in pediatric patients with attention‐deficit/hyperactivity disorder and comorbid trichotillomania: a preliminary report. Clinical Neuropharmacology 2011;34(3):108‐10. [DOI: 10.1097/WNF.0b013e31821f4da9; PUBMED: 21586916] [DOI] [PubMed] [Google Scholar]
Gracious 1999 {published data only}
- Gracious BL. Atrioventricular nodal re‐entrant tachycardia associated with stimulant treatment. Journal of Child and Adolescent Psychopharmacology 1999;9(2):125‐8. [DOI: 10.1089/cap.1999.9.125; PUBMED: 10461823] [DOI] [PubMed] [Google Scholar]
Grcevich 2001 {published data only}
- Grcevich S, Rowane WA, Marcellino B, Sullivan‐Hurst S. Retrospective comparison of Adderall and methylphenidate in the treatment of attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2001;11(1):35‐41. [DOI: 10.1089/104454601750143401; PUBMED: 11322743] [DOI] [PubMed] [Google Scholar]
Green 2011 {published data only}
- Green T, Weinberger R, Diamond A, Berant M, Hirschfeld L, Frisch A, et al. The effect of methylphenidate on prefrontal cognitive functioning, inattention, and hyperactivity in velocardiofacial syndrome. Journal of Child and Adolescent Psychopharmacology 2011;21(6):589‐95. [DOI: 10.1089/cap.2011.0042; PUBMED: 22149470] [DOI] [PubMed] [Google Scholar]
- Green T, Weinberger R, Weizman A, Kotler M, Gothelf D. Effect of methylphenidate on neurocognitive functioning in velocardiofacial syndrome: a randomized placebo‐controlled trial. Biological Psychiatry 2009;65(Suppl 8):147 S. [Google Scholar]
Greenberg 1987 {published data only}
- Greenberg LM. An objective measure of methylphenidate response: clinical use of the MCA. Psychopharmacology Bulletin 1987;23(2):279‐82. [PUBMED: 3615775] [PubMed] [Google Scholar]
Greenhill 1983 {published data only}
- Greenhill L, Puig‐Antich J, Goetz R, Hanlon C, Davies M. Sleep architecture and REM sleep measures in prepubertal children with attention deficit disorder with hyperactivity. Sleep 1983;6(2):91‐101. [PUBMED: 6878986] [DOI] [PubMed] [Google Scholar]
Grossman 1985 {published data only}
- Grossman LK, Grossman NJ. Methylphenidate and idiopathic thrombocytopenic purpura‐‐is there an association?. Journal of Family Practice 1985;20(3):302‐4. [PUBMED: 4038730] [PubMed] [Google Scholar]
Gross‐Tsur 2004 {published data only}
- Gross‐Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology 2004;63(4):753‐4. [PUBMED: 15326264] [DOI] [PubMed] [Google Scholar]
- Panei P, Arcieri R, Vella S, Bonati M, Martini N, Zuddas A. Italian attention‐deficit/hyperactivity disorder registry. Pediatrics 2004;114(2):514. [PUBMED: 15286248] [DOI] [PubMed] [Google Scholar]
Gucuyener 2003 {published data only}
- Gucuyener K, Erdemoglu AK, Senol S, Serdaroglu A, Soysal S, Kockar AI. Use of methylphenidate for attention‐deficit hyperactivity disorder in patients with epilepsy or electroencephalographic abnormalities. Journal of Child Neurology 2003;18(2):109‐12. [DOI: 10.1177/08830738030180020601; PUBMED: 12693777] [DOI] [PubMed] [Google Scholar]
Guerreiro 1996 {published data only}
- Guerreiro MM, Montenegro MA, Piva RT, Moura‐Ribeiro MV. Attention deficit disorder: treatment with methylphenidate [Distúrbio do déficit de atenção: tratamento com metilfenidato]. Arquivos de Neuro‐Psiquiatria 1996;54(1):25‐9. [DOI: 10.1590/S0004-282X1996000100004] [DOI] [PubMed] [Google Scholar]
Haertling 2015 {published data only}
- Haertling F, Mueller B, Bilke‐Hentsch O. Effectiveness and safety of a long‐acting, once‐daily, two‐phase release formulation of methylphenidate (Ritalin® LA) in school children under daily practice conditions. Attention Deficit and Hyperactivity Disorders 2015;7(2):157‐64. [DOI: 10.1007/s12402-014-0154-x; PMC4449385; PUBMED: 25346231] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halevy 2009 {published data only}
- Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. Journal of Child Neurology 2009;24(8):1005‐7. [DOI: 10.1177/0883073808331357; PUBMED: 19502578] [DOI] [PubMed] [Google Scholar]
Hammerness 2009 {published data only}
- Effects of high‐dose OROS methylphenidate. Brown University Child and Adolescent Psychopharmacology Update 2009;11(8):7‐8. [DOI: 10.1002/cpu.20097] [DOI] [Google Scholar]
- Daniels SR. Cardiovascular effects of methylphenidate. Journal of Pediatrics 2009;155(1):A3. [DOI: 10.1016/j.jpeds.2009.05.023] [DOI] [PubMed] [Google Scholar]
- Hammerness P, Georgiopoulos A, Doyle RL, Utzinger L, Schillinger M, Martelon M, et al. An open study of adjunct OROS‐methylphenidate in children who are atomoxetine partial responders: II. Tolerability and pharmacokinetics. Journal of Child and Adolescent Psychopharmacology 2009;19(5):493‐9. [DOI: 10.1089/cap.2008.0126; PMC2861956; PUBMED: 19877973] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammerness 2012 {published data only}
- Hammerness P, Biederman J, Petty C, Henin A, Moore CM. Brain biochemical effects of methylphenidate treatment using proton magnetic spectroscopy in youth with attention‐deficit hyperactivity disorder: a controlled pilot study. CNS Neuroscience and Therapeutics. P. Hammerness, Massachusetts General Hospital, 185 Alewife Brook Parkway, Suite 2000, Cambridge, MA 02138, United States. E‐mail: phammerness@partners.org: Blackwell Publishing Ltd (9600 Garsington Road, Oxford OX4 2XG, United Kingdom), 2012; Vol. 18, issue 1:34‐40. [DOI: 10.1111/j.1755-5949.2010.00226.x; PMC4084790; PUBMED: 21143432] [DOI] [PMC free article] [PubMed]
Haubold 2010 {published data only}
- Haubold KH. Attention Deficit Hyperactivity Disorder ADHD ‐ A Retrospective Population‐based Analysis Concerning the Treatment of Children and Adolescents with Stimulant Medication in a Child and Adolescent Psychiatry Practice [PhD thesis]. Tübingen: Eberhard Karls Universität Tübingen, 2009. [Google Scholar]
Hazell 2003 {published data only}
- Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(8):886‐94. [DOI: 10.1097/01.CHI.0000046908.27264.00] [DOI] [PubMed] [Google Scholar]
Hechtman 2011 {published data only}
- Hechtman L. Treatment of ADHD in patients unresponsive to methylphenidate. Journal of Psychiatry and Neuroscience 2011;36(3):216. [DOI: 10.1503/jpn.110020; PMC3080516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemmer 2001 {published data only}
- Hemmer SA, Pasternak JF, Zecker SG, Trommer BL. Stimulant therapy and seizure risk in children with ADHD. Pediatric Neurology 2001;24(2):99‐102. [PUBMED: 11275457] [DOI] [PubMed] [Google Scholar]
Hollis 2007 {published data only}
- Hollis CP, Thompson A. Acute dyskinesia on starting methylphenidate after risperidone withdrawal. Pediatric Neurology 2007;37(4):287‐8. [DOI: 10.1016/j.pediatrneurol.2007.05.017; PUBMED: 17903675] [DOI] [PubMed] [Google Scholar]
Holtkamp 2002 {published data only}
- Holtkamp K, Peters‐Wallraf B, Wuller S, Pfäaffle R, Herpertz‐Dahlmann B. Methylphenidate‐related growth impairment. Journal of Child and Adolescent Psychopharmacology 2002;12(1):55‐61. [DOI: 10.1089/10445460252943579; PUBMED: 12014596] [DOI] [PubMed] [Google Scholar]
Hong 2012 {published data only}
- Hong SB, Kim JW, Cho SC, Shin MS, Kim BN, Yoo HJ. Dopaminergic and noradrenergic gene polymorphisms and response to methylphenidate in Korean children with attention‐deficit/Hyperactivity disorder: is there an interaction?. Journal of Child and Adolescent Psychopharmacology 2012; Vol. 22, issue 5:343‐52. [DOI: 10.1089/cap.2011.0076; PUBMED: 23083021] [DOI] [PubMed]
Hulvershorn 2012 {published data only}
- Hulvershorn L. Methylphenidate treatment of chronic irritability: symptom improvement and underlying neural mechanisms. Biological Psychiatry 2015;77(1S):444S. [Google Scholar]
- Hulvershorn LA, Hummer T, Wang Y, Loth A, Anand A. The impact of methylphenidate on corticolimbic functional connectivity in children with ADHD and chronic irritability. Biological Psychiatry 2012;71(8):193S. [Google Scholar]
Ilgenli 2007 {published data only}
- Ilgenli TF, Congologlu A, Ozturk C, Turkbay T, Akpinar O, Kilicaslan F. Acute effect of methylphenidate on QT interval duration and dispersion in children with attention deficit hyperactivity disorder. Advances in Therapy 2007;24(1):182‐8. [PUBMED: 17526476] [DOI] [PubMed] [Google Scholar]
Irmak 2014 {published data only}
- Irmak A, Ince‐Tasdelen B, Ozmen S, Oztop DB. Treatment choice in association of attention deficit‐hyperactivity disorder with Williams and Moebius Syndromes: case reports. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2014;24(Suppl 1):226. [Google Scholar]
Işeri 2007 {published data only}
- Işeri E, Kilic BG, Senol S, Karabacak NI. Effects of methylphenidate on leptin and appetite in children with attention‐deficit hyperactivity disorder: an open label trial. Methods and Findings in Experimental and Clinical Pharmacology 2007;29(1):47‐52. [DOI: 10.1358/mf.2007.29.1.1063491; PUBMED: 17344944] [DOI] [PubMed] [Google Scholar]
Jafarinia 2012 {published data only}
- Jafarinia M, Mohammadi MR, Modabbernia A, Ashrafi M, Khajavi D, Tabrizi M, et al. Bupropion versus methylphenidate in the treatment of children with attention‐deficit/hyperactivity disorder: randomized double‐blind study. Human Psychopharmacology 2012; Vol. 27, issue 4:411‐8. [DOI: 10.1002/hup.2242; PUBMED: 22806822] [DOI] [PubMed]
Jensen 1999 (MTA) {published data only}
- Abikoff H. Tailored psychosocial treatments for ADHD: the search for a good fit. Journal of Clinical Child Psychology 2001; Vol. 30, issue 1:122‐5. [DOI: 10.1207/S15374424JCCP3001_14; PUBMED: 11294070] [DOI] [PubMed]
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Archives of General Psychiatry 1997;54(9):865‐70. [PUBMED: 9294378] [DOI] [PubMed] [Google Scholar]
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott GR, Greenhill LL, et al. NIMH collaborative multimodal treatment study of children with ADHD (MTA): design, methodology, and protocol evolution. Journal of Attention Disorders 1997;2(3):141‐58. [DOI: 10.1177/108705479700200301] [DOI] [Google Scholar]
- Arnold LE, Chuang S, Davies M, Abikoff HB, Conners CK, Elliott GR, et al. Nine months of multicomponent behavioral treatment for ADHD and effectiveness of MTA fading procedures. Journal of Abnormal Child Psychology 2004;32(1):39‐51. [PUBMED: 14998110] [DOI] [PubMed] [Google Scholar]
- Arnold LE, Elliott M, Sachs L, Bird H, Kraemer HC, Wells KC, et al. Effects of ethnicity on treatment attendance, stimulant response/dose, and 14‐month outcome in ADHD. Journal of Consulting and Clinical Psychology 2003;71(4):713‐27. [PUBMED: 12924677] [DOI] [PubMed] [Google Scholar]
- Carey WB. What the multimodal treatment study of children with attention deficit/hyperactivity disorder did and did not say about the use of methylphenidate for attention deficits. Pediatrics 2000;105(4):863‐4. [PUBMED: 10742336] [DOI] [PubMed] [Google Scholar]
- Conners CK, Epstein JN, March JS, Angold A, Wells KC, Klaric J, et al. Multimodal treatment of ADHD in the MTA: an alternative outcome analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):159‐67. [DOI: 10.1097/00004583-200102000-00010; PUBMED: 11211364] [DOI] [PubMed] [Google Scholar]
- Galanter CA, Carlson GA, Jensen PS, Greenhill LL, Davies M, Li W, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. Journal of Child and Adolescent Psychopharmacology 2003;13(2):123‐36. [DOI: 10.1089/104454603322163844; PUBMED: 12880507] [DOI] [PubMed] [Google Scholar]
- Greene RW, Ablon JS. What does the MTA study tell us about effective psychosocial treatment for ADHD?. Journal of Clinical Child Psychology 2001;30(1):114‐21. [DOI: 10.1207/S15374424JCCP3001_13; PUBMED: 11294069] [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Abikoff HB, Arnold LE, Cantwell DP, Conners CK, Elliott G, et al. Medication treatment strategies in the MTA Study: relevance to clinicians and researchers. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(10):1304‐13. [DOI: 10.1097/00004583-199610000-00017; PUBMED: 8885584] [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Vitiello B, Davies M, Clevenger W, Wu M, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):180‐7. [DOI: 10.1097/00004583-200102000-00012; PUBMED: 11211366] [DOI] [PubMed] [Google Scholar]
- Hansen L, Thomsen PH. How to treat ADHD/DAMP? Is there a conclusive answer? A critical survey of the MTA trial?. Ugeskr Laeger 2005;167(48):4555‐9. [PUBMED: 16324436] [PubMed] [Google Scholar]
- Hinshaw SP. Moderators and mediators of treatment response for children with attention‐deficit/hyperactivity disorder: the Multimodal Treatment Study of children with attention‐deficit/hyperactivity disorder. Archives of General Psychiatry 1999;56(12):1088‐96. [PUBMED: 10591284] [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, March JS, Abikoff H, Arnold LE, Cantwell DP, Conners CK, et al. Comprehensive assessment of childhood Attention‐Deficit Hyperactivity Disorder in the context of a multisite, multimodal clinical trial. Journal of Attention Disorders 1997;1(4):217‐34. [DOI: 10.1177/108705479700100403] [DOI] [Google Scholar]
- Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, et al. 3‐year follow‐up of the NIMH MTA study. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):989‐1002. [DOI: 10.1097/CHI.0b013e3180686d48; PUBMED: 17667478] [DOI] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Leonora N, Newcorn JH, Abikoff HB, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry 2001; Vol. 40, issue 2:147‐58. [DOI: 10.1097/00004583-200102000-00009; PUBMED: 11211363] [DOI] [PubMed]
- Jensen PS, Hinshaw SP, Swanson JM, Greenhill LL, Conners CK, Arnold LE, et al. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. Journal of Developmental and Behavioral Pediatrics 2001;22(1):60‐73. [PUBMED: 11265923] [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14‐month randomized clinical trial of treatment strategies for attention‐deficit/hyperactivity disorder. Archives of General Psychiatry 1999;56(12):1073‐86. [DOI: 10.1001/archpsyc.56.12.1073; PUBMED: 10591283] [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health multimodal treatment study of ADHD follow‐up: 24‐month outcomes of treatment strategies for attention‐deficit/hyperactivity disorder. Pediatrics 2004;113(4):754‐61. [PUBMED: 15060224] [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health multimodal treatment study of ADHD follow‐up: changes in effectiveness and growth after the end of treatment. Pediatrics 2004;113(4):762‐9. [PUBMED: 15060225] [DOI] [PubMed] [Google Scholar]
- March JS, Swanson JM, Arnold LE, Hoza B, Conners CK, Hinshaw SP, et al. Anxiety as a predictor and outcome variable in the multimodal treatment study of children with ADHD (MTA). Journal of Abnormal Child Psychology 2000;28(6):527‐41. [PUBMED: 11104315] [DOI] [PubMed] [Google Scholar]
- Molina BS, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):1028‐40. [DOI: 10.1097/chi.0b013e3180686d96; PUBMED: 17667481] [DOI] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Eugene AL, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent substance use in the multimodal treatment study of attention‐deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. Journal of the American Academy of Child and Adolescent Psychiatry 2013;52(3):250‐63. [DOI: 10.1016/j.jaac.2012.12.014; PMC3589108; PUBMED: 23452682] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow‐up of children treated for combined‐type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(5):484‐500. [DOI: 10.1097/CHI.0b013e31819c23d0; PMC3063150] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieweg EH. Does ADHD medication stop working after 2‐3 years? On the surprising, but little known follow‐up of the MTA study [Is adhd‐medicatie na 2‐3 jaar uitgewerkt? Over de verrassende, maar weinig bekendefollow‐up van het mta‐onderzoek]. Tijdschrift voor Psychiatrie 2010;52(4):245‐54. [PUBMED: 20503165] [PubMed] [Google Scholar]
- Owens EB, Hinshaw SP, Kraemer HC, Arnold LE, Abikoff HB, Cantwell DP, et al. Which treatment for whom for ADHD? Moderators of treatment response in the MTA. Journal of Consulting and Clinical Psychology 2003;71(3):540‐52. [PUBMED: 12795577] [DOI] [PubMed] [Google Scholar]
- Pelham WE Jr. The NIMH multimodal treatment study for attention‐deficit hyperactivity disorder: just say yes to drugs alone?. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 1999;44(10):981‐90. [DOI: 10.1177/070674379904401004; PUBMED: 10637677] [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greiner AR, Hoza B, Hinshaw SP, Swanson JM, et al. Behavioral versus behavioral and pharmacological treatment in ADHD children attending a summer treatment program. Journal of Abnormal Child Psychology 2000;28(6):507‐25. [PUBMED: 11104314] [DOI] [PubMed] [Google Scholar]
- Richters JE, Arnold LE, Jensen PS, Abikoff H, Conners CK, Greenhill LL, et al. NIMH collaborative multisite multimodal treatment study of children with ADHD: I. Background and rationale. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(8):987‐1000. [DOI: 10.1097/00004583-199508000-00008; PUBMED: 7665456] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Arnold LE, Kraemer H, Hechtman L, Molina B, Hinshaw SP, et al. Evidence, interpretation, and qualification from multiple reports of long‐term outcomes in the Multimodal Treatment Study of children with ADHD (MTA): Part II: supporting details. Journal of Attention Disorders 2008;12(1):15‐43. [DOI: 10.1177/1087054708319525; PUBMED: 18573924] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Arnold LE, Kraemer H, Hechtman L, Molina B, Hinshaw SP, et al. Evidence, interpretation, and qualification from multiple reports of long‐term outcomes in the Multimodal Treatment study of Children With ADHD (MTA): part I: executive summary. Journal of Attention Disorders 2008;12(1):4‐14. [DOI: 10.1177/1087054708319345; PUBMED: 18573923] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow‐up. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):1015‐27. [DOI: 10.1097/chi.0b013e3180686d7e; PUBMED: 17667480] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Hinshaw SP, Arnold LE, Gibbons RD, Marcus S, Hur K, et al. Secondary evaluations of MTA 36‐month outcomes: propensity score and growth mixture model analyses. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):1003‐14. [DOI: 10.1097/CHI.0b013e3180686d63; PUBMED: 17667479] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):168‐79. [DOI: 10.1097/00004583-200102000-00011; PUBMED: 1121136] [DOI] [PubMed] [Google Scholar]
- Vitiello B, Elliott GR, Swanson JM, Arnold LE, Hechtman L, Abikoff H, et al. Blood pressure and heart rate over 10 years in the multimodal treatment study of children with ADHD. American Journal of Psychiatry 2012; Vol. 169, issue 2:167‐77. [DOI: 10.1176/appi.ajp.2011.10111705; PMC4132884; PUBMED: 21890793] [DOI] [PMC free article] [PubMed]
- Vitiello B, Severe JB, Greenhill LL, Arnold LE, Abikoff HB, Bukstein OG, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):188‐96. [DOI: 10.1097/00004583-200102000-00013; PUBMED: 11211367] [DOI] [PubMed] [Google Scholar]
Johnson 2013 {published data only}
- Johnson KA, Barry E, Lambert D, Fitzgerald M, McNicholas F, Kirley A, et al. Methylphenidate side effect profile is influenced by genetic variation in the attention‐deficit/hyperactivity disorder‐associated CES1 gene. Journal of Child and Adolescent Psychopharmacology 2013;23(10):655‐64. [DOI: 10.1089/cap.2013.0032; PUBMED: 24350812] [DOI] [PubMed] [Google Scholar]
Jung 2007 {published data only}
- Jung C, Choi S, Jeong S, Song CJ, Seo W, Chung US, et al. Multicenter, open‐label study to evaluate the effects of methylphenidate‐OROS (Concerta®) on cognitive functions in children with attention deficit hyperactivity disorder. Clinical Psychopharmacology and Neuroscience 2007;5(1):31‐7. [Google Scholar]
- Lee JH, Jung CH, Song CJ, Choi SY, Jeong SH, Chung US, et al. Multi‐center study for evaluation of efficacy and safety of methylphenidate‐OROS in children with ADHD. European Neuropsychopharmacology 2007;17(Suppl 4):S571‐2. [DOI: 10.1016/S0924-977X(07)70890-8] [DOI] [Google Scholar]
Karabekiroglu 2008 {published data only}
- Karabekiroglu K, Yazgan Y M, Dedeoglu C. Can we predict short‐term side effects of methylphenidate immediate‐release?. International Journal of Psychiatry in Clinical Practice 2008;12(1):48‐54. [DOI: 10.1080/13651500701435954] [DOI] [PubMed] [Google Scholar]
Karaman 2010 {published data only}
- Pulmonary arterial hypertension with methylphenidate OROS initiation. Brown University Child & Adolescent Psychopharmacology Update 2010;12(9):8‐8. [DOI: 10.1002/cpu.20123] [DOI] [Google Scholar]
- Karaman MG, Atalay F, Tufan AE, Erdogan A. Pulmonary arterial hypertension in an adolescent treated with methylphenidate. Journal of Child & Adolescent Psychopharmacology 2010;20(3):229‐31. [DOI: 10.1089/cap.2009.0095; PUBMED: 20578938] [DOI] [PubMed] [Google Scholar]
Karapinar 2014 {published data only}
- Karapinar U, Saglam O, Dursun E, Cetin B, Salman N, Sahan M. Sudden hearing loss associated with methylphenidate therapy. European Archives of Otorhinolaryngology 2014;271(1):199‐201. [DOI: 10.1007/s00405-013-2763-y; PUBMED: 24141471] [DOI] [PubMed] [Google Scholar]
Kazanci 2015 {published data only}
- Kazanci SY, Tarakcioglu M C, Bulbul L, Saglam N O. Should we continue methylphenidate treatment despite orofacial or extremity dyskinesias?. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2015;25(4):399‐402. [DOI: 10.5455/bcp.20150902042021] [DOI] [Google Scholar]
Kemner 2005 (FOCUS) {published data only}
- Kemner JE, Starr HL, Bowen DL, Ciccone PL, Lynch JM. Greater symptom improvement and response rates with OROS® MPH vs. atomoxetine in children with ADHD. International Journal of Neuropsychopharmacology 2004;7:S273‐4. [DOI: 10.1017/S1461145704004547] [DOI] [Google Scholar]
- Kemner JE, Starr HL, Ciccone PE, Hooper‐Wood CG, Crockett RS. Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. Advances in Therapy 2005;22(5):498‐512. [PUBMED: 16418159] [DOI] [PubMed] [Google Scholar]
- Kemner JE, Starr HL, Hooper‐Wood CG, Ciccone PE. Greater improvement and response rates with OROS (R) methylphenidate compared with atomoxetine in children with attention‐deficit/hyperactivity disorder. Pharmacotherapy 2004;24(10):1462‐3. [DOI: 10.1592/phco.24.14.1419.43157] [DOI] [Google Scholar]
- Starr HL, Kemner J. Multicenter, randomized, open‐label study of OROS methylphenidate versus atomoxetine: treatment outcomes in African‐American children with ADHD. Journal of the National Medical Association 2005;97(10 Suppl):11S‐6S. [PMC2640623; PUBMED: 16350601] [PMC free article] [PubMed] [Google Scholar]
Khajehpiri 2014 {published data only}
- Khajehpiri Z, Mahmoudi‐Gharaei J, Faghihi T, Karimzadeh I, Khalili H, Mohammadi M. Adverse reactions of Methylphenidate in children with attention deficit‐hyperactivity disorder: report from a referral center. Journal of Research in Pharmacy Practice 2014;3(4):130‐6. [DOI: 10.4103/2279-042X.145389; PMC4262859; PUBMED: 25535621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khodadust 2012 {published data only}
- Khodadust N, Jalali A‐H, Ahmadzad‐Asl M, Khademolreza N, Shirazi E. Comparison of two brands of methylphenidate (Stimdate® vs. Ritalin®) in children and adolescents with attention deficit hyperactivity disorder: a double‐blind, randomized clinical trial. Iranian Journal of Psychiatry and Behavioral Sciences 2012; Vol. 6, issue 1:26‐32. [PMC3939941] [PMC free article] [PubMed]
Kim 2010 {published data only}
- Chung I, Han CH, Kim HW, Cho SC. The effect of prolonged‐release methylphenidate on the sleep of children with attention‐deficit/hyperactivity disorders. European Neuropsychopharmacology 2010;20(Suppl 3):S624‐5. [Google Scholar]
- Kim HW, Yoon IY, Cho SC, Kim BN, Chung S, Lee H, et al. The effect of OROS methylphenidate on the sleep of children with attention‐deficit/hyperactivity disorder. International Clinical Psychopharmacology 2010;25(2):107‐15. [DOI: 10.1097/YIC.0b013e3283364411; PUBMED: 20093941] [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim BN, Kim YN, Cheong US, Kim JW, Hwang JW, Shin MS, et al. Switching from Methylphenidate‐Immediate Release (MPH‐IR) to Methylphenidate‐OROS (OROS‐MPH): a multi‐center, open‐label study in Korea. Clinical Psychopharmacology and Neuroscience 2011;9(1):29‐35. [DOI: 10.9758/cpn.2011.9.1.29; PMC3568652; PUBMED: 23430964] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014a {published data only}
- Kim HW, Kim SO, Shon S, Lee JS, Lee HJ, Choi JH. Effect of methylphenidate on height and weight in Korean children and adolescents with attention‐deficit/hyperactivity disorder: a retrospective chart review. Journal of Child and Adolescent Psychopharmacology 2014;24(8):448‐53. [DOI: 10.1089/cap.2014.0025; PUBMED: 25285915] [DOI] [PubMed] [Google Scholar]
Kim 2014b {published data only}
- Kim Y, Kim B, Chang JS, Kim BN, Cho SC, Hwang JW. Parental quality of life and depressive mood following methylphenidate treatment of children with attention‐deficit hyperactivity disorder. Psychiatry and Clinical Neurosciences 2014;68(7):506‐14. [DOI: 10.1111/pcn.12155; PUBMED: 24417707] [DOI] [PubMed] [Google Scholar]
Kim 2015a {published data only}
- Kim HJ, Yang J, Lee MS. Changes of heart rate variability during methylphenidate treatment in attention‐deficit hyperactivity disorder children: a 12‐week prospective study. Yonsei Medical Journal 2015;56(5):1365‐71. [DOI: 10.3349/ymj.2015.56.5.1365; PMC4541668] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015b {published data only}
- Kim E, Cheon KA, Joung YS, Kim JY, Song DH. The relationship between symptomatic and functional changes of Korean children and adolescents with attention‐deficit/hyperactivity disorder treated with osmotic‐controlled release oral delivery system‐methylphenidate. Clinical Neuropharmacology 2015; Vol. 38, issue 1:30‐5. [DOI: 10.1097/WNF.0000000000000064; PUBMED: 25580917] [DOI] [PubMed]
Klein 2004 {published data only}
- Hechtman L, Abikoff H, Klein RG, Weiss G, Respitz C, Kouri J, et al. Academic achievement and emotional status of children with ADHD treated with long‐term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(7):812‐9. [DOI: 10.1097/01.chi.0000128796.84202.eb; PUBMED: 15213582] [DOI] [PubMed] [Google Scholar]
- Klein RG, Abikoff H, Hechtman L, Weiss G. Design and rationale of controlled study of long‐term methylphenidate and multimodal psychosocial treatment in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(7):792‐801. [DOI: 10.1097/01.chi.0000128798.91601.fe; PUBMED: 15213580] [DOI] [PubMed] [Google Scholar]
Kordon 2011 {published data only}
- Kordon A, Stollhoff K, Niederkirchner K, Mattejat F, Rettig K, Schäuble B. Exploring the impact of once‐daily OROS® methylphenidate (MPH) on symptoms and quality of life in children and adolescents with ADHD transitioning from immediate‐release MPH. Postgraduate Medicine 2011;123(5):27‐38. [DOI: 10.3810/pgm.2011.09.2457; PUBMED: 21904084] [DOI] [PubMed] [Google Scholar]
Kratochvil 2002 {published data only}
- Kratochvil CJ, Heiligenstein JH, Dittmann R, Spencer TJ, Biederman J, Wernicke J, et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open‐label trial. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(7):776‐84. [DOI: 10.1097/00004583-200207000-00008; PUBMED: 12108801] [DOI] [PubMed] [Google Scholar]
Kraut 2013 {published data only}
- Kraut AA, Langner I, Lindemann C, Banaschewski T, Petermann U, Petermann F, et al. Comorbidities in ADHD children treated with methylphenidate: a database study. BMC Psychiatry 2013;13:11. [DOI: 10.1186/1471-244X-13-11; PMC3544568; PUBMED: 23294623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lahat 2000 {published data only}
- Lahat E, Weiss M, Ben‐Shlomo A, Evans S, Bistritzer T. Bone mineral density and turnover in children with attention‐deficit hyperactivity disorder receiving methylphenidate. Journal of Child Neurology 2000;15(7):436‐9. [DOI: 10.1177/088307380001500702; PUBMED: 10921512] [DOI] [PubMed] [Google Scholar]
Lakic 2012 {published data only}
- Lakic A, Kesic A, Dronjak D. Tics in children with attention‐deficit/hyperactivity disorder under treatment with the sustained‐release form of methylphenidate. European Neuropsychopharmacology 2012; Vol. 22, issue Suppl 2:S420. [DOI: 10.1016/S0924-977X(12)70658-2] [DOI]
Lamberti 2015 {published data only}
- Lamberti M, Italiano D, Guerriero L, D'Amico G, Siracusano R, Ingrassia M, et al. Evaluation of acute cardiovascular effects of immediate‐release methylphenidate in children and adolescents with attention‐deficit hyperactivity disorder. Neuropsychiatric Disease and Treatment 2015;11:1169‐74. [DOI: 10.2147/NDT.S79866; PMC4431494; PUBMED: 26056451] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langevin 2012 {published data only}
- Langevin R, Ramdé J. Attention deficit hyperactivity disorder (ADHD) in children, seasonal photoperiods, nocturnal movements and diurnal agitation. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2012;21(1):53‐8. [PMC3269251] [PMC free article] [PubMed] [Google Scholar]
Larrañaga‐Fragoso 2015 {published data only}
- Larrañaga‐Fragoso P, Noval S, Rivero JC, Boto‐de‐los‐Bueis A. The effects of methylphenidate on refraction and anterior segment parameters in children with attention deficit hyperactivity disorder. Journal of AAPOS 2015;19(4):322‐6. [DOI: 10.1016/j.jaapos.2015.04.005; PUBMED: 26235791] [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee SI, Hong SD, Kim S. Efficacy and tolerability of OROS methylphenidate in Korean children with AD/HD ‐ Reply. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2007; Vol. 31, issue 5:1151‐2. [DOI: 10.1016/j.pnpbp.2007.03.019] [DOI] [PubMed]
- Lee SI, Hong SD, Kim SY, Kim EJ, Kim JH, Kim JH, et al. Efficacy and tolerability of OROS methylphenidate in Korean children with attention‐deficit/hyperactivity disorder. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2007;31(1):210‐6. [DOI: 10.1016/j.pnpbp.2006.09.002; PUBMED: 17046131] [DOI] [PubMed] [Google Scholar]
- Pae CU. Comments on efficacy and tolerability of OROS methylphenidate in Korean children with attention‐deficit/hyperactivity disorder. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2007;31(5):1151‐2. [DOI: 10.1016/j.pnpbp.2007.03.018] [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee SH, Song DH, Kim BN, Joung YS, Ha EH, Cheon KA, et al. Variability of response time as a predictor of methylphenidate treatment response in Korean children with attention deficit hyperactivity disorder. Yonsei Medical Journal 2009;50(5):650‐5. [DOI: 10.3349/ymj.2009.50.5.650; PMC2768239; PUBMED: 19881968] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee SH, Seo WS, Sung HM, Choi TY, Kim SY, Choi SJ, et al. Effect of methylphenidate on sleep parameters in children with ADHD. Psychiatry Investigation 2012;9(4):384‐90. [DOI: 10.4306/pi.2012.9.4.384; PMC3521116] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee WJ, Lee TA, Pickard AS, Caskey RN, Schumock GT. Drugs associated with adverse events in children and adolescents. Pharmacotherapy 2014;34(9):918‐26. [DOI: 10.1002/phar.1455; PUBMED: 24990656] [DOI] [PubMed] [Google Scholar]
Lewis 2012 {published data only}
Li 2011 {published data only}
Lyon 2010 {published data only}
- Dexmethylphenidate in youth with ADHD and Tourette's. Brown University Child and Adolescent Psychopharmacology Update 2010;12(11):4‐5. [DOI: 10.1002/cpu.20127] [DOI] [Google Scholar]
- Lyon GJ, Coffey B, Castellanos XF, Woods D. Improving TIC‐related response inhibition: comparing the effects of dexmethylphenidate to placebo in children and adolescents with ADHD and chronic TIC disorders. International Journal of Neuropsychopharmacology 2008;11(Suppl 1):292. [Google Scholar]
- Lyon GJ, Coffey B, Woods D, Samar S, Conelea C, Bauer CC, et al. Improving tic‐related response inhibition: comparing the effects of dexmethylphenidate to no medication in children and adolescents with Attention Deficit Hyperactivity Disorder (ADHD) and chronic tic disorders. Journal of Child and Adolescent Psychopharmacology 2010;20(6):537‐8. [DOI: 10.1089/cap.2010.2064] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GJ, Samar SM, Conelea C, Trujillo MR, Lipinski CM, Bauer CC, et al. Testing tic suppression: comparing the effects of dexmethylphenidate to no medication in children and adolescents with attention‐deficit/hyperactivity disorder and Tourette's disorder. Journal of Child and Adolescent Psychopharmacology 2010;20(4):283‐9. [DOI: 10.1089/cap.2010.0032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maayan 2009 {published data only}
- Methylphenidate for preschool ADHD. Brown University Child and Adolescent Psychopharmacology Update 2009;11(7):4‐4. [DOI: 10.1002/cpu.20095] [DOI] [Google Scholar]
- Maayan L, Paykina N, Fried J, Strauss T, Gugga SS, Greenhill L. The open‐label treatment of attention‐deficit/hyperactivity disorder in 4‐ and 5‐year‐old children with beaded methylphenidate. Journal of Child and Adolescent Psychopharmacology 2009;19(2):147‐53. [PMC2935832; PUBMED: 19374023] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDonald 2005 {published data only}
- MacDonald Fredericks E, Kollins SH. A pilot study of methylphenidate preference assessment in children diagnosed with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2005;15(5):729‐41. [DOI: 10.1089/cap.2005.15.729; PUBMED: 16262590] [DOI] [PubMed] [Google Scholar]
Machado 2010 {published data only}
- Machado A, Cerqueira J, Rodrigues M, Soares‐Fernandes J. Is methylphenidate‐induced chorea responsive to chlorpromazine?. Journal of Neuropsychiatry and Clinical Neurosciences 2010;22(3):352.e20. [DOI: 10.1176/jnp.2010.22.3.352.e20; PUBMED: 20686158] [DOI] [PubMed] [Google Scholar]
Maia 2008 {published data only}
- Maia CR. Evalution of the Exchange of Immediate Release Methylphenidate for Prolonged Release Methylphenidate in Attention Deficit/Hyperactivity Disorder [Availação da troca do metilfenidato de liberação imediata para o metilfenidato de liberação prolongada no transtorno de déficit de atenção/hiperatividade] [Masters thesis]. Rio Grande (BZ): The Federal University of Rio Grande, 2009. [Google Scholar]
- Maia CR, Matte BC, Ludwig HT, Rohde LA. Switching from methylphenidate immediate release to MPH‐SODAS in attention‐deficit/hyperactivity disorder. European Child and Adolescent Psychiatry 2008;17(3):133‐42. [DOI: 10.1007/s00787-007-0647-7; PUBMED: 17846812] [DOI] [PubMed] [Google Scholar]
Man 2015 {published data only}
- Man KK, Chan EW, Coghill D, Douglas I, Douglas I, Ip P, et al. Methylphenidate and the risk of trauma. Pediatrics 2015;135(1):40‐8. [DOI: 10.1542/peds.2014-1738; PUBMED: 25511122] [DOI] [PubMed] [Google Scholar]
Mayes 1994 {published data only}
- Mayes SD, Crites DL, Bixler EO, Humphrey FJ 2nd, Mattison RE. Methylphenidate and ADHD: influence of age, IQ and neurodevelopmental status. Developmental Medicine and Child Neurology 1994;36(12):1099‐107. [PUBMED: 7525394] [DOI] [PubMed] [Google Scholar]
McCarthy 2009 {published data only}
- McCarthy S, Cranswick N, Potts L, Taylor E, Wong IC. Mortality associated with attention‐deficit hyperactivity disorder: a retrospective cohort study of children, adolescents and young adults using the general practice research database. Drug Safety 2009;32(11):1089‐96. [DOI: 10.2165/11317630-000000000-00000; PUBMED: 19810780] [DOI] [PubMed] [Google Scholar]
McCracken 2016 {published data only}
- Loo SK, Bilder R, Cho AL, Sturm A, Cowen J, Welker PW, et al. Effects of d‐Methylphenidate, Guanfacine, and their combination on electroencephalogram resting state spectral power in Attention‐Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(8):674‐82.e1. [DOI: 10.1016/j.jaac.2016.04.020; PMC5003618; PUBMED: 27453081] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT, McGough JJ, Loo SK, Levitt J, Del'Homme M, Cowen J, et al. Combined stimulant and Guanfacine administration in Attention‐Deficit/Hyperactivity Disorder: a controlled, comparative study. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(8):657‐66.e1. [DOI: 10.1016/j.jaac.2016.05.015; PMC4976782; PUBMED: 27453079] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLaren 2010 {published data only}
- McLaren JL, Cauble S, Barnett RJ. Aripiprazole induced acute dystonia after discontinuation of a stimulant medication. Journal of Clinical Psychopharmacology 2010;30(1):77‐8. [DOI: 10.1097/JCP.0b013e3181c92eb2; PUBMED: 20075654] [DOI] [PubMed] [Google Scholar]
Miller‐Horn 2008 {published data only}
- Miller‐Horn JW, Kaleyias J, Valencia I, Melvin JJ, Khurana DS, Hardison HH, et al. Efficacy and tolerability of ADHD medications in a clinical practice. Journal of Pediatric Neurology 2008;6(1):5‐10. [DOI: 10.1055/s-0035-1557417] [DOI] [Google Scholar]
Mino 1999 {published data only}
- Mino Y, Aoki S. Methylphenidate‐induced psychosis in an adolescent with hyperkinetic disorder. Kawasaki Medical Journal 1999;25(2):81‐5. [DOI: 10.11482/KMJ25(2)81-85.1999.pdf] [DOI] [Google Scholar]
- Mino Y, Ohara H, Nagamatsu I, Nagamatsu K. Methylphenidate psychosis in an adolescent with attention deficit disorder. Japanese Journal of Child and Adolescent Psychiatry 1986; Vol. 27, issue 3:178‐87.
Mize 2004 {published data only}
- Mize W. Hemoencephalography ‐ a new therapy for attention deficit hyperactivity disorder (ADHD): case report. Journal of Neurotherapy 2004;8(3):77‐97. [DOI: 10.1300/J184v0803_06] [DOI] [Google Scholar]
Mohammadi 2004 {published data only}
- Mohammadi MR, Kashani L, Akhondzadeh S, Izadian ES, Ohadinia S. Efficacy of theophylline compared to methylphenidate for the treatment of attention‐deficit hyperactivity disorder in children and adolescents: a pilot double‐blind randomized trial. Journal of Clinical Pharmacy and Therapeutics 2004;29(2):139‐44. [DOI: 10.1111/j.1365-2710.2004.00545.x; PUBMED: 15068402] [DOI] [PubMed] [Google Scholar]
Mohammadi 2009 {published data only}
- Mohammadi MR, Akhondzadeh S, Mehr NK, Mohammadi M, Mahintorabi S. Comparison of Stimdate with Ritalin in children and adolescents with attention deficit hyperactivity disorder. Iranian Journal of Psychiatry 2009;4(1):31‐4. [Google Scholar]
Mohammadi 2010 {published data only}
- Mohammadi MR, Kazemi MR, Zia E, Rezazadeh SA, Tabrizi M, Akhondzadeh S. Amantadine versus methylphenidate in children and adolescents with attention deficit/hyperactivity disorder: a randomized, double‐blind trial. Human Psychopharmacology 2010;25(7‐8):560‐5. [DOI: 10.1002/hup.1154; PUBMED: 21312290] [DOI] [PubMed] [Google Scholar]
Mohammadi 2012a {published data only}
- Mohammadi MR, Mostafavi SA, Keshavarz SA, Eshraghian MR, Hosseinzadeh P, Hosseinzadeh‐Attar MJ, et al. Melatonin effects in methylphenidate treated children with attention deficit hyperactivity disorder: a randomized double blind clinical trial. Iranian Journal of Psychiatry 2012;7(2):87‐92. [PMC free article] [PubMed] [Google Scholar]
- Mostafavi SA, Mohammadi MR, Hosseinzadeh P, Eshraghian MR, Akhondzadeh S, Hosseinzadeh‐Attar MJ, et al. Dietary intake, growth and development of children with ADHD in a randomized clinical trial of Ritalin and Melatonin co‐administration: through circadian cycle modification or appetite enhancement?. Iranian Journal of Psychiatry 2012;7(3):114‐9. [PMC3488866; PUBMED: 23139692] [PMC free article] [PubMed] [Google Scholar]
Mohammadi 2012b {published data only}
- Mohammadi MR, Hafezi P, Galeiha A, Hajiaghaee R, Akhondzadeh S. Buspirone versus methylphenidate in the treatment of children with attention‐ deficit/ hyperactivity disorder: randomized double‐blind study. Acta Medica Iranica 2012;50(11):723‐8. [PUBMED: 23292622] [PubMed] [Google Scholar]
Montañés‐Rada 2012 {published data only}
- Montañés Rada F, Martínez Granero MA, Vidal Formoso M, Sanchez Romero S, Andrés Prado MJ. Extended release methylphenidate (medikinet®) twice a day, a prospective open trial [Metilfenidato de liberación prolongada 50/50 (medikinet®) dos veces al día, estudio abierto prospectivo]. Revista de Psiquiatria Infanto‐Juvenil 2012;29(1):50‐8. [Google Scholar]
Montiel‐Nava 2002 {published data only}
- Montiel‐Nava C, Peña JA, Espina‐Mariñes G, Ferrer‐Hernandez ME, López‐Rubio A, Puertas‐Sánchez S, et al. A pilot study of methylphenidate and parent training in the treatment of children with attention deficit hyperactivity disorder [Estudio piloto de metilfenidato y entrenamiento a padres en el tratamiento de niños con trastorno por déficit de atención‐hiperactividad]. Revista de Neurologia 2002;35(3):201‐5. [PUBMED: 12235578] [PubMed] [Google Scholar]
Moungnoi 2011 {published data only}
- Moungnoi P, Maipang P. Long‐term effects of short‐acting methylphenidate on growth rates of children with attention deficit hyperactivity disorder at Queen Sirikit National Institute of Child Health. Journal of the Medical Association of Thailand 2011;94(Suppl 3):S158‐63. [PUBMED: 22043770] [PubMed] [Google Scholar]
Munk 2015 {published data only}
- Munk K, Gormsen L, Kim WY, Andersen NH. Cardiac arrest following a myocardial infarction in a child treated with methylphenidate. Case Reports in Pediatrics 2015;2015:905097. [DOI: 10.1155/2015/905097] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Na 2013 {published and unpublished data}
- Lee MS, Lee SI, Hong SD, Kim JH, Choi J, Joung YS. Two different solicitation methods for obtaining information on adverse events associated with methylphenidate in adolescents: a 12‐week multicenter, open‐label study. Journal of Child and Adolescent Psychopharmacology 2013;23(1):22‐7. [DOI: 10.1089/cap.2012.0018; PUBMED: 23347125] [DOI] [PubMed] [Google Scholar]
- Na KS, Lee SI, Hong SD, Kim JH, Shim SH, Choi J, et al. Effect of osmotic‐release oral system methylphenidate on learning skills in adolescents with attention‐deficit/hyperactivity disorder: an open‐label study. International Clinical Psychopharmacology 2013;28(4):184‐92. [DOI: 10.1097/YIC.0b013e3283612509; PUBMED: 23587983] [DOI] [PubMed] [Google Scholar]
Niederhofer 2009 {published data only}
- Niederhofer H. Atomoxetine may improve methylphenidates' efficacy in treatment of ADHD?. Psychiatria Danubina 2009;21(3):330. [PUBMED: 19794351] [PubMed] [Google Scholar]
- Niederhofer H. Questions regarding your study 'Combining carbamazepine, neuroleptics and acetylcholinesterase inhibitors with methylphenidate only reduces adverse side effects, but is less effective than a combination with atomoxetine' [personal communication]. Email to: HB Krogh 31 July 2013. [DOI] [PubMed]
Niederhofer 2011 {published data only}
- Niederhofer H. Combining carbamazepine, neuroleptics and acetylcholinesterase inhibitors with methylphenidate only reduces adverse side effects, but is less effective than a combination with atomoxetine. Medical Hypotheses 2011;76(5):764‐5. [DOI: 10.1016/j.mehy.2011.02.011; PUBMED: 21397406] [DOI] [PubMed] [Google Scholar]
Nymark 2008 {published data only}
- Ghanizadeh A. A commentary on "A young man with acute dilated cardiomyopathy associated with methylphenidate". Vascular Health and Risk Management 2009;5(2):525‐6. [DOI: 10.2147/VHRM.S5108] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark TB, Hovland A, Bjørnstad H, Nielsen EW. A young man with acute dilated cardiomyopathy associated with methylphenidate. Vascular Health and Risk Management 2008;4(2):477‐9. [PMC2496981; PUBMED: 18561524] [DOI] [PMC free article] [PubMed] [Google Scholar]
Özcan 2004 {published data only}
- Özcan IT, Toros F, Pekdemir H, Çiçek D, Çamsari A, Yurttaş M, et al. The effect of methylphenidate on time domain heart rate variability in the treatment of attention deficit and hyperactivity disorder [Dikkat eksikliği hiperaktivite bozukluğu tedavisinde metilfenidat kullanımının zaman bağımlı kalp hızı değişkenliği üzerine etkisi]. Turkish Journal of Child and Adolescent Mental Health 2004;11(3):117‐22. [Google Scholar]
Park 2013 {published data only}
- Park S, Kim BN, Kim JW, Shin MS, Cho SC, Kim JH, et al. Neurotrophin 3 genotype and emotional adverse effects of osmotic‐release oral system methylphenidate (OROS‐MPH) in children with attention‐deficit/hyperactivity disorder. Journal of Psychopharmacology 2014;28(3):220‐6. [DOI: 10.1177/0269881113480989; PUBMED: 23471121] [DOI] [PubMed] [Google Scholar]
Perera 2010 {published data only}
- Perera H, Jeewandara KC, Seneviratne S, Guruge C. Assessment of outcome of an ADHD treatment program using parent feedback. Sri Lanka Journal of Psychiatry 2010;1(2):51‐5. [DOI: 10.4038/sljpsyc.v1i2.2575] [DOI] [Google Scholar]
Peyre 2012a {published data only}
- Peyre H, Speranza M, Cortese S, Wohl M, Purper‐Ouakil D. Do ADHD children with and without child behavior checklist‐dysregulation profile have different clinical characteristics, cognitive features, and treatment outcomes?. Journal of Attention Disorders 2012;19(1):63‐71. [DOI: 10.1177/1087054712452135; PUBMED: 22837549] [DOI] [PubMed] [Google Scholar]
Pierce 2010 {published data only}
- Pierce D, Katic A, Buckwalter M, Webster K. Single‐ and multiple‐dose pharmacokinetics of methylphenidate administered as methylphenidate transdermal system or osmotic‐release oral system methylphenidate to children and adolescents with attention deficit hyperactivity disorder. Journal of Clinical Psychopharmacology 2010;30(5):554‐64. [DOI: 10.1097/JCP.0b013e3181f0c2f6; PUBMED: 20814325] [DOI] [PubMed] [Google Scholar]
Polanczyk 2007 {published data only}
- Polanczyk G, Zeni C, Genro JP, Guimarães AP, Roman T, Hutz MH, et al. Association of the adrenergic alpha2A receptor gene with methylphenidate improvement of inattentive symptoms in children and adolescents with attention‐deficit/hyperactivity disorder. Archives of General Psychiatry 2007;64(2):218‐24. [DOI: 10.1001/archpsyc.64.2.218; PUBMED: 17283289] [DOI] [PubMed] [Google Scholar]
Porfirio 2011 {published data only}
- Porfirio MC, Giana G, Giovinazzo S, Curatolo P. Methylphenidate‐induced visual hallucinations. Neuropediatrics 2011;42(1):30‐1. [DOI: 10.1055/s-0031-1275738; PUBMED: 21500140] [DOI] [PubMed] [Google Scholar]
Poulton 2003 {published data only}
- Poulton A, Cowell CT. Slowing of growth in height and weight on stimulants: a characteristic pattern. Journal of Paediatrics and Child Health 2003;39(3):180‐5. [PUBMED: 12654140] [DOI] [PubMed] [Google Scholar]
Poulton 2012 {published data only}
- Poulton A, Briody J, McCorquodale T, Melzer E, Herrmann M, Baur LA, et al. Weight loss on stimulant medication: how does it affect body composition and bone metabolism? ‐ A prospective longitudinal study. International Journal of Pediatric Endocrinology 2012;2012(1):30. [DOI: 10.1186/1687-9856-2012-30; PMC3549744; PUBMED: 23216890] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton A, Evans R, Melzer E, Bui Q. Stimulant medication affects growth but not bone age. Archives of Disease in Childhood 2014;99(Suppl 1):A4.A5. [DOI: 10.1136/archdischild-2014-306237.10] [DOI] [Google Scholar]
- Poulton A, Nanan R. Differential effects of dexamphetamine and methylphenidate on symptoms of inattention‐overactivity, oppositional‐defiant and weight. Archives of Disease in Childhood 2014;99(Suppl 1):A70. [DOI: 10.1136/archdischild-2014-306237.167] [DOI] [Google Scholar]
Ramasamy 2014 {published data only}
- Ramasamy R, Dadhich P, Dhingra A, Lipshultz L. Case report: testicular failure possibly associated with chronic use of methylphenidate. F1000Research 2014;3:207. [DOI: 10.12688/f1000research.5163.1; PMC4215747; PUBMED: 25383187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rappaport 2004 {published data only}
- Rappaport N, Coffey B. Psychopharmacology in the school setting: therapeutic challenges in an adolescent with attention deficit hyperactivity disorder, possible bipolar disorder, and other comorbidity. Journal of Child and Adolescent Psychopharmacology 2004;14(1):3‐7. [DOI: 10.1089/104454604773840418; PUBMED: 15142385] [DOI] [PubMed] [Google Scholar]
Rapport 1996 {published data only}
- Rapport MD, Loo S, Isaacs P, Goya S, Denney C, Scanlan S. Methylphenidate and attentional training. Comparative effects on behavior and neurocognitive performance in twin girls with attention‐deficit/hyperactivity disorder. Behavior Modification 1996;20(4):428‐50. [DOI: 10.1177/01454455960204004; PUBMED: 8875814] [DOI] [PubMed] [Google Scholar]
Rashid 2007 {published data only}
- Somatic hallucinations with methylphenidate in a 10‐year‐old boy. Brown University Child and Adolescent Psychopharmacology Update 2007;9(11):8‐8. [DOI: 10.1002/cpu.20055] [DOI] [Google Scholar]
- Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):945‐6. [DOI: 10.1097/CHI.0b013e318067fd7c; PUBMED: 17667474] [DOI] [PubMed] [Google Scholar]
Remschmidt 2005 {published data only}
- Hoare P, Remschmidt H, Medori R, Ettrich C, Rothenberger A, Santosh P, et al. 12‐month efficacy and safety of OROS MPH in children and adolescents with attention‐deficit/hyperactivity disorder switched from MPH. European Child and Adolescent Psychiatry 2005;14(6):305‐9. [DOI: 10.1007/s00787-005-0486-3; PUBMED: 16220214] [DOI] [PubMed] [Google Scholar]
- Remschmidt H, Hoare P, Ettrich C, Rothenberger A, Santosh P, Schmidt M, et al. Symptom control in children and adolescents with attention‐deficit/hyperactivity disorder on switching from immediate‐release MPH to OROS® MPH. European Child and Adolescent Psychiatry 2005;14(6):297‐304. [DOI: 10.1007/s00787-005-0467-6] [DOI] [PubMed] [Google Scholar]
Ririe 1997 {published data only}
- Ririe DG, Ririe KL, Sethna NF, Fox L. Unexpected interaction of methylphenidate (Ritalin) with anaesthetic agents. Paediatric Anaesthesia 1997;7(1):69‐72. [PUBMED: 9041578] [DOI] [PubMed] [Google Scholar]
Sabuncuoglu 2007 {published data only}
- Sabuncuoglu O. Risperidone‐to‐methylphenidate switch reaction in children: three cases. Journal of Psychopharmacology 2007;21(2):216‐9. [DOI: 10.1177/0269881107069466; PUBMED: 17329303] [DOI] [PubMed] [Google Scholar]
- Sabuncuoglu O. Severe adverse events following risperidone‐to‐methylphenidate switch. Brown University Child and Adolescent Psychopharmacology Update 2007;9(5):8‐8. [DOI: 10.1002/cpu.20043] [DOI] [Google Scholar]
Sahin 2014 {published data only}
- Sahin S, Yuce M, Alacam H, Karabekiroglu K, Say GN, Salis O. Effect of methylphenidate treatment on appetite and levels of leptin, ghrelin, adiponectin, and brain‐derived neurotrophic factor in children and adolescents with attention deficit and hyperactivity disorder. International Journal of Psychiatry in Clinical Practice 2014;18(4):280‐7. [DOI: 10.3109/13651501.2014.940054; PUBMED: 24994482] [DOI] [PubMed] [Google Scholar]
Saieh 2004 {published data only}
- Saieh AC. Methylphenidate: arterial hypertension, a side effect to take into account [Metilfenidato: hipertensión arterial, un efecto a tener en cuenta]. Revista Chilena de Pediatria 2004;75(1):80‐1. [DOI: 10.4067/S0370-41062004000100014] [DOI] [Google Scholar]
Sangal 2006 {published data only}
- Rostain AL. Sleep disturbances and ADHD medications. Current Psychiatry Reports 2007;9(5):399‐400. [PUBMED: 17915079] [DOI] [PubMed] [Google Scholar]
- Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep 2006;29(12):1573‐85. [PUBMED: 17252888] [DOI] [PubMed] [Google Scholar]
Santosh 2006 {published data only}
- Santosh PJ, Baird G, Pityaratstian N, Tavare E, Gringras P. Impact of comorbid autism spectrum disorders on stimulant response in children with attention deficit hyperactivity disorder: a retrospective and prospective effectiveness study. Child: Care, Health and Development 2006;32(5):575‐83. [DOI: 10.1111/j.1365-2214.2006.00631.x; PUBMED: 16919137] [DOI] [PubMed] [Google Scholar]
Schertz 1996 {published data only}
- Schertz M, Adesman AR, Alfieri NE, Bienkowski RS. Predictors of weight loss in children with attention deficit hyperactivity disorder treated with stimulant medication. Pediatrics 1996;98(4 Pt 1):763‐9. [PUBMED: 8885958] [PubMed] [Google Scholar]
Schmidt 2002 {published data only}
- Schmidt JK, Plück J, Gontard A. Waiver of EEG diagnostics prior to and during methylphenidate therapy: dangerous or justifiable? [Verzicht auf eine EEG‐Diagnostik vor Beginn und unter einer Therapie mit Methylphenidat: gefährlich oder gerechtfertigt?]. Zeitschrift fur Kinder‐ und Jugendpsychiatrie und Psychotherapie 2002;30(4):295‐302. [DOI: 10.1024/1422-4917.30.4.295] [DOI] [PubMed] [Google Scholar]
Schulz 2010 {published data only}
- Schulz E, Fleischhaker C, Hennighausen K, Heiser P, Haessler F, Linder M, et al. A randomized, rater‐blinded, crossover study comparing the clinical efficacy of Ritalin(®) LA (methylphenidate) treatment in children with attention‐deficit hyperactivity disorder under different breakfast conditions over 2 weeks. Attention Deficit and Hyperactivity Disorders 2010;2(3):133‐8. [DOI: 10.1007/s12402-010-0031-1; PUBMED: 21432599] [DOI] [PubMed] [Google Scholar]
Schwartz 2004 {published data only}
- Ginsberg DL. Priapism due to withdrawal from sustained‐release methylphenidate. Primary Psychiatry 2004;11(10):25‐6. [Google Scholar]
- Schwartz RH, Rushton HG. Stuttering priapism associated with withdrawal from sustained‐release methylphenidate. Journal of Pediatrics 2004;144(5):675‐6. [DOI: 10.1016/j.jpeds.2003.12.039; PUBMED: 15127013] [DOI] [PubMed] [Google Scholar]
Shang 2015 {published data only}
- Shang CY, Pan YL, Lin HY, Huang LW, Gau SS. An open‐label, randomized trial of methylphenidate and atomoxetine treatment in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2015;25(7):566‐73. [DOI: 10.1089/cap.2015.0035; PUBMED: 26222447] [DOI] [PubMed] [Google Scholar]
Shibib 2009 {published data only}
- Shibib S, Chalhoub N. Stimulant induced psychosis. Child and Adolescent Mental Health 2009;14(1):20‐3. [DOI: 10.1111/j.1475-3588.2008.00490.x] [DOI] [Google Scholar]
Shin 2016 {published data only}
- Shin JY, Roughead EE, Park BJ, Pratt NL. Cardiovascular safety of methylphenidate among children and young people with attention‐deficit/hyperactivity disorder (ADHD): nationwide self controlled case series study. BMJ 2016;353:i2550. [DOI: 10.1136/bmj.i2550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shyu 2015 {published data only}
- Lee MJ, Yang KC, Shyu YC, Yuan SS, Yang CJ, Lee SY, et al. Attention‐deficit hyperactivity disorder, its treatment with medication and the probability of developing a depressive disorder: a nationwide population‐based study in Taiwan. Journal of Affective Disorders 2016;189:110‐7. [DOI: 10.1016/j.jad.2015.09.015; PUBMED: 26433758] [DOI] [PubMed] [Google Scholar]
- Shyu YC, Yuan SS, Lee SY, Yang CJ, Yang KC, Lee TL, et al. Attention‐deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population‐based study in Taiwan. Schizophrenia Research 2015;168(1‐2):161‐7. [DOI: 10.1016/j.schres.2015.08.033; PUBMED: 26363968] [DOI] [PubMed] [Google Scholar]
Silva 2004 {published data only}
- Silva R, Tilker HA, Cecil JT, Kowalik S, Khetani V, Faleck H, et al. Open‐label study of dexmethylphenidate hydrochloride in children and adolescents with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2004;14(4):555‐63. [DOI: 10.1089/cap.2004.14.555; PUBMED: 15662147] [DOI] [PubMed] [Google Scholar]
- Tilker H, Silva R, Cecil J, Kowalik S, Faleck H, Robinson W, et al. Open label, pilot study of dexmethylphenidate in children with ADHD. Journal of Child and Adolescent Psychopharmacology 2002;12(4):295. [DOI: 10.1089/104454602762599835] [DOI] [PubMed] [Google Scholar]
Sobanski 2013 {published data only}
- Sobanski E, Döpfner M, Ose C, Fischer R. A non‐interventional study of extended‐release methylphenidate in the routine treatment of adolescents with ADHD: effectiveness, safety and adherence to treatment. Attention Deficit and Hyperactivity Disorders 2013;5(4):387‐95. [DOI: 10.1007/s12402-013-0113-y; PUBMED: 23794192] [DOI] [PubMed] [Google Scholar]
Song 2012 {published data only}
- Song DH, Choi S, Joung YS, Ha EH, Kim BN, Shin YJ, et al. Titrating optimal dose of osmotic‐controlled release oral delivery (OROS)‐methylphenidate and its efficacy and safety in Korean children with ADHD: a multisite open labeled study. Psychiatry Investigation 2012; Vol. 9, issue 3:257‐62. [DOI: 10.4306/pi.2012.9.3.257; PMC3440475] [DOI] [PMC free article] [PubMed]
- Song DH, Choi S, Joung YS, Kim BN, Ha EH, Shin YJ, et al. Titrating optimal dose of OROS‐MPH and its efficacy and safety in Korean children with ADHD: multicenter, open labeled study. International Journal of Neuropsychopharmacology 2010;13(Suppl 1):193‐4. [DOI: 10.1017/S1461145710000635] [DOI] [Google Scholar]
Spencer 1992 {published data only}
- Spencer T, Biederman J, Wright V, Danon M. Growth deficits in children treated with desipramine: a controlled study. Journal of the American Academy of Child & Adolescent Psychiatry 1992;31(2):235‐43. [DOI: 10.1097/00004583-199203000-00009; PUBMED: 1564024] [DOI] [PubMed] [Google Scholar]
Steele 2006 {published data only}
- Hechtman L. Effects of treatment on the overall functioning of children with ADHD. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2009;14(Suppl 1):10‐5. [PMC2547092] [PMC free article] [PubMed] [Google Scholar]
- Steele M, Riccardelli R, Binder C. The effectiveness of OROS® methylphenidate (Concerta®) vs. usual treatment with immediate‐release methylphenidate (IR MPH) in children aged 6‐12 years with attention deficit hyperactivity disorder (ADHD). International Journal of Neuropsychopharmacology 2004;7(Suppl 2):S442. [DOI: 10.1017/S1461145704004547] [DOI] [Google Scholar]
- Steele M, Weiss M, Swanson J, Wang J, Prinzo RS, Binder CE. A randomized, controlled effectiveness trial of OROS‐methylphenidate compared to usual care with immediate‐release methylphenidate in ADHD. Canadian Journal of Clinical Pharmacology 2006;13(1):e50‐62. [PUBMED: 16456216] [PubMed] [Google Scholar]
- Steele MM, Prinzo R, Binder C. Effectiveness of Concerta versus twice or thrice daily IR‐MPH in children with ADHD. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta (GA). 2005.
- Steele MM, Prinzo R, Binder C. Long‐term effectiveness and safety of Concerta in children with ADHD: a six‐month study. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta (GA). 2005.
Stein 2002 {published data only}
- Stein D, Pat‐Horenczyk R, Blank S, Dagan Y, Barak Y, Gumpel TP. Sleep disturbances in adolescents with symptoms of attention‐deficit/hyperactivity disorder. Journal of Learning Disabilities 2002;35(3):268‐75. [DOI: 10.1177/002221940203500308; PUBMED: 15493323] [DOI] [PubMed] [Google Scholar]
Stevens 2010 {published data only}
- Stevens JR, George RA, Fusillo S, Stern TA, Wilens TE. Plasma methylphenidate concentration in youths treated with high‐dose osmotic release oral system formulation. Journal of Child and Adolescent Psychopharmacology 2010;20(1):49‐54. [DOI: 10.1089/cap.2008.0128; PUBMED: 20166796] [DOI] [PubMed] [Google Scholar]
Strandell 2007 {published data only}
- Strandell J, Starr K. Reports of suicide related behaviour with methylphenidate and atomoxetine in children and adolescents. Drug Safety 2007;30(10):919‐90. [DOI: 10.2165/00002018-200730100-00105] [DOI] [Google Scholar]
Su 2015 {published data only}
- Su Y, Li H, Chen Y, Fang F, Xu T, Lu H, et al. Remission rate and functional outcomes during a 6‐month treatment with Osmotic‐Release Oral‐System methylphenidate in children with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology 2015;35(5):525‐34. [DOI: 10.1097/JCP.0000000000000389; PUBMED: 26267421] [DOI] [PubMed] [Google Scholar]
Sudarmadji 2009 {published data only}
- Sudarmadji SS, Meliala L, Aziz A. Improvement of cognitive function in attention deficit hyperactivity disorder (ADHD) treatment by methylphenidate (MPH) of elementary school students at Bantul District, Yogyakarta Special Regency. Journal of the Neurological Sciences 2009;285(Suppl 1):S236. [DOI: 10.1016/S0022-510X(09)70898-6] [DOI] [Google Scholar]
Tang 2010 {published data only}
- Tang CS, Chou WJ, Cheng AT. Osmotic release oral system (OROS) methylphenidate‐induced double incontinence: a case report. Primary Care Companion to the Journal of Clinical Psychiatry 2010;12(3):PCC.09l00870. [DOI: 10.4088/PCC.09l00870pur; PMC2947529] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tasdelen 2015 {published data only}
- Tasdelen BI, Karakaya E, Oztop DB. Effects of atomoxetine and osmotic release oral system‐methylphenidate on executive functions in patients with combined type attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2015;25(6):494‐500. [DOI: 10.1089/cap.2014.0155; PUBMED: 26218871] [DOI] [PubMed] [Google Scholar]
Tekin 2015 {published data only}
- Tekin U, Soyata AZ, Oflaz S. Acute focal dystonic reaction after acute methylphenidate treatment in an adolescent patient. Journal of Clinical Psychopharmacology 2015;35(2):209‐11. [DOI: 10.1097/JCP.0000000000000266; PUBMED: 25607477] [DOI] [PubMed] [Google Scholar]
Thorell 2009 {published data only}
- Thorell LB, Dahlström K. Children's self‐reports on perceived effects on taking stimulant medication for ADHD. Journal of Attention Disorders 2009;12(5):460‐8. [DOI: 10.1177/1087054708320430; PUBMED: 18685139] [DOI] [PubMed] [Google Scholar]
Tomás Vila 2010a {published data only}
- Tomás Vila M, Izquierdo Quevedo FJ, Cerdán Vera MT, Fernández A, Artás Figueres M, Revert Gomas M. Visual hallucinations caused by methylphenidate. Anales de Pediatria 2010;72(3):229‐30. [DOI: 10.1016/j.anpedi.2009.10.013; PUBMED: 20097145] [DOI] [PubMed] [Google Scholar]
Tomás Vila 2010b {published data only}
- Tomás Vila M, Aleu Pérez‐Gramunt M, Beseler Soto B, Benac Prefasi M, Pantoja Martínez J, Pitarch Castellano I. Methylphenidate and sleep: results of a multicentre study on a population of children with attention deficit hyperactivity disorder. Anales de Pediatria 2010;73(2):78‐83. [DOI: 10.1016/j.anpedi.2010.05.013; PUBMED: 20605120] [DOI] [PubMed] [Google Scholar]
Trugman 1988 {published data only}
- Trugman JM. Cerebral arteritis and oral methylphenidate. Lancet 1988;1(8585):584‐5. [PUBMED: 2894511] [DOI] [PubMed] [Google Scholar]
Tzang 2012 {published data only}
- Tzang RF, Wang YC, Yeh CB, Hsu CD, Liang HY, Yang PC, et al. Naturalistic exploration of the effect of osmotic release oral system‐methylphenidate on remission rate and functional improvement in Taiwanese children with attention‐deficit‐hyperactivity disorder. Psychiatry and Clinical Neurosciences 2012; Vol. 66, issue 1:53‐63. [DOI: 10.1111/j.1440-1819.2011.02289.x; PUBMED: 22250610] [DOI] [PubMed]
Tølløfsrud 2006 {published data only}
- Tølløfsrud C, Hoel T. A young man with acute dilated cardiomyopathy. Tidsskrift for Den Norske Laegeforening 2006;126(10):1338‐9. [PUBMED: 16715575] [PubMed] [Google Scholar]
Valdizán Usón 2004 {published data only}
- Valdizan Uson JR. Diagnostic and therapeutic electroclinical correlation with immediate action methylphenidate in ADHD [Correlación electrónica diagnóstica y terapéutica con metilfeniato de acción inmediata en TDAH]. Revista Espanola de Pediatria 2004;60(3):181‐7. [Google Scholar]
Valdizán Usón 2013 {published data only}
- Valdizán Uson JR. Methylphenidate in children and adolescents with attention‐deficit/ hyperactivity disorder: The DIHANA Study. Acta Pediatrica Espanola 2013;71(3):67‐76. [Google Scholar]
- Valdizán Uson JR, Cánovas‐Martínez A, Lucas‐Taracena MT, Díaz‐Atienza F, Eddy‐Ives LS, Fernandez‐Jaen A, et al. Response to methylphenidate by adult and pediatric patients with attention‐deficit/hyperactivity disorder: the Spanish multicenter DIHANA study. Neuropsychiatric Disease and Treatment 2013;9:211‐8. [DOI: 10.2147/NDT.S35836; PMC3573811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Oord 2007 {published data only}
- Oord S, Prins PJ, Oosterlaan J, Emmelkamp PM. Does brief, clinically based, intensive multimodal behavior therapy enhance the effects of methylphenidate in children with ADHD?. European Child and Adolescent Psychiatry 2007;16(1):48‐57. [DOI: 10.1007/s00787-006-0574-z; PUBMED: 16972117] [DOI] [PubMed] [Google Scholar]
Varley 2001 {published data only}
- Varley CK, Vincent J, Varley P, Calderon R. Emergence of tics in children with attention deficit hyperactivity disorder treated with stimulant medications. Comprehensive Psychiatry 2001;42(3):228‐33. [DOI: 10.1053/comp.2001.23145; PUBMED: 11349243] [DOI] [PubMed] [Google Scholar]
Vashi 2011 {published data only}
- Vashi NA, Souza A, Cohen N, Franklin B, Cohen DE. Allergic contact dermatitis caused by methylphenidate. Contact Dermatitis 2011;65(3):183‐85. [DOI: 10.1111/j.1600-0536.2011.01949.x; PUBMED: 21827513] [DOI] [PubMed] [Google Scholar]
Verret 2010 {published data only}
- Verret C, Gardiner P, Béliveau L. Fitness level and gross motor performance of children with attention‐deficit hyperactivity disorder. Adapted Physical Activity Quarterly 2010;27(4):337‐51. [PUBMED: 20956839] [DOI] [PubMed] [Google Scholar]
Vincent 1990 {published data only}
- Vincent J, Varley CK, Leger P. Effects of methylphenidate on early adolescent growth. American Journal of Psychiatry 1990;147(4):501‐2. [DOI: 10.1176/ajp.147.4.501; PUBMED: 2316739] [DOI] [PubMed] [Google Scholar]
Walitza 2007 {published data only}
- Walitza S, Werner B, Romanos M, Warnke A, Gerlach M, Stopper H. Does methylphenidate cause a cytogenetic effect in children with attention deficit hyperactivity disorder?. Environmental Health Perspectives 2007;115(6):936‐40. [DOI: 10.1289/ehp.9866; PMC1892117] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walitza 2009 {published data only}
- Stopper H, Kampf K, Romanos M, Warnke A, Walitza S, Gerlach M. Does methylphenidate cause a cytogenetic effect in children with attention deficit hyperactivity disorder?. International Journal of Neuropsychopharmacology 2008;11:290. [Google Scholar]
- Walitza S, Kampf K, Artamonov N, Romanos M, Gnana Oli R, Wirth S, et al. No elevated genomic damage in children and adolescents with attention deficit/hyperactivity disorder after methylphenidate therapy. Toxicology Letters 2009;184(1):38‐43. [DOI: 10.1016/j.toxlet.2008.10.011] [DOI] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang Y, Zheng Y, Du Y, Song DH, Shin YJ, Cho SC, et al. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double‐blind comparison trial. Australian and New Zealand Journal of Psychiatry 2007;41(3):222‐30. [DOI: 10.1080/00048670601057767; PUBMED: 17464703] [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang LJ, Hsiao CC, Huang YS, Chiang YL, Ree SC, Chen YC, et al. Association of salivary dehydroepiandrosterone levels and symptoms in patients with attention deficit hyperactivity disorder during six months of treatment with methylphenidate. Psychoneuroendocrinology 2011;36(8):1209‐16. [DOI: 10.1016/j.psyneuen.2011.02.014; PUBMED: 21411231] [DOI] [PubMed] [Google Scholar]
- Wang LJ, Huang YS, Chiang YL, Hsiao CC, Shang ZY, Chen CK. Clinical symptoms and performance on the Continuous Performance Test in children with attention deficit hyperactivity disorder between subtypes: a natural follow‐up study for 6 months. BMC Psychiatry 2011;11:65. [DOI: 10.1186/1471-244X-11-65; PMC3111344; PUBMED: 21504587] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Huang YS, Hsiao CC, Chiang YL, Wu CC, Shang ZY, et al. Salivary dehydroepiandrosterone, but not cortisol, is associated with attention deficit hyperactivity disorder. World Journal of Biological Psychiatry 2011;12(2):99‐109. [DOI: 10.3109/15622975.2010.512090; PUBMED: 20822373] [DOI] [PubMed] [Google Scholar]
- Wang LJ, Wu CC, Lee SY, Tsai YF. Salivary neurosteroid levels and behavioural profiles of children with attention‐deficit/hyperactivity disorder during six months of methylphenidate treatment. Journal of Child and Adolescent Psychopharmacology 2014;24(6):336‐40. [DOI: 10.1089/cap.2013.0122; PUBMED: 24956271] [DOI] [PubMed] [Google Scholar]
Warshaw 2010 {published data only}
- Warshaw EM, Squires L, Li Y, Civil R, Paller AS. Methylphenidate transdermal system: a multisite, open‐label study of dermal reactions in pediatric patients diagnosed with ADHD. Primary Care Companion to the Journal of Clinical Psychiatry 2010;12(6):e1‐e9. [DOI: 10.4088/PCC.10m00996pur; NCT00434213; PMC3067997; PUBMED: 21494336] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2003 {published data only}
- Weber P, Bubl R, Lütschg J. Side effects of methylphenidate in children. Prevalence and associated factors [Nebenwirkungen einer Methylphenidat‐Therapie bei Schulkindern]. Monatsschrift Kinderheilkunde 2003;151(4):399‐404. [DOI: 10.1007/s00112-002-0590-0] [DOI] [Google Scholar]
Weiss 2007 {published data only}
- Weiss M, Hechtman L, Turgay A, Jain U, Quinn D, Ahmed TS, et al. Once‐daily multilayer‐release methylphenidate in a double‐blind, crossover comparison to immediate‐release methylphenidate in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2007;17(5):675‐88. [DOI: 10.1089/cap.2006.0101; PUBMED: 17979587] [DOI] [PubMed] [Google Scholar]
Wigal 2011 {published data only}
- Wigal SB, Gupta S, Heverin E, Starr HL. Pharmacokinetics and therapeutic effect of OROS methylphenidate under different breakfast conditions in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2011;21(3):255‐63. [DOI: 10.1089/cap.2010.0083; PUBMED: 21663428] [DOI] [PubMed] [Google Scholar]
Wigal 2013 {published data only}
- Liquid version of methylphenidate shows efficacy in school trial. Brown University Child and Adolescent Psychopharmacology Update 2013;15(3):1‐3. [DOI: 10.1002/cpu.20183/epdf] [DOI] [Google Scholar]
- Wigal S, Childress A, Belden H, Berry S. NWP06, an extended‐release oral suspension of methylphenidate, improved attention‐deficit/hyperactivity disorder symptoms compared with placebo in a laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2013;23(1):3‐10. [DOI: 10.1089/cap.2012.0073; PMC3696913] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigal 2015 {published data only}
- Wigal SB, Nordbrock E, Adjei AL, Childress A, Kupper RJ, Greenhill L. Efficacy of methylphenidate hydrochloride extended‐release capsules (Aptensio XRTM) in children and adolescents with attention‐deficit/hyperactivity disorder: a phase III, randomized, double‐blind study. CNS Drugs 2015;29(4):331‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiguna 2012 {published data only}
- Wiguna T, Guerrero AP, Wibisono S, Sastroasmoro S. Effect of 12‐week administration of 20‐mg long‐acting methylphenidate on Glu/Cr, NAA/Cr, Cho/Cr, and mI/Cr ratios in the prefrontal cortices of school‐age children in Indonesia: a study using 1H Magnetic Resonance Spectroscopy (MRS). Clinical Neuropharmacology. T. Wiguna, Child and Adolescent Psychiatry Division, Department of Psychiatry, University of Indonesia, Jl. Salemba Raya 4, Jakarta 10430, Indonesia. E‐mail: twiga00@yahoo.com: Lippincott Williams and Wilkins (530 Walnut Street,P O Box 327, Philadelphia PA 19106‐3621, United States), 2012; Vol. 35, issue 2:81‐5. [DOI: 10.1097/WNF.0b013e3182452572; PUBMED: 22318191] [DOI] [PubMed]
Wilens 2005 {published data only}
- Biederman J. Evaluation of once‐daily OROS methylphenidate in children with attention‐deficit/hyperactivity disorder: effects on sleep, appetite, and tics. European Neuropsychopharmacology 2003;13(Suppl 4):S447‐8. [Google Scholar]
- Biederman J. Treatment of attention‐deficit/hyperactivity disorder with the once‐daily OROS formulation of methylphenidate: effect on growth in children. European Neuropsychopharmacology 2003;13(Suppl 4):S448‐9. [Google Scholar]
- Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1‐year, open‐label study of OROS methylphenidate in children with ADHD. Journal of Attention Disorders 2007;11(2):157‐66. [DOI: 10.1177/1087054706295663; PUBMED: 17494833] [DOI] [PubMed] [Google Scholar]
- Lerner MA, Spencer T. Analysis of growth in children with ADHD during long‐term therapy with once‐daily OROS® methylphenidate. Pediatric Academic Societies' Annual Meeting; 2004 May 1‐4; San Francisco (CA). 2004; Vol. 55.
- Palumbo DR, Starr HL. Once‐daily OROS methylphenidate: Impact on tics in children with attention deficit hyperactivity disorder. Annals of Neurology 2003;54(Suppl 7):S27. [DOI: 10.1002/ana.5049/epdf] [DOI] [Google Scholar]
- Spencer TJ, Faraone SV, Biederman J, Lerner M, Cooper KM, Zimmerman B, Concerta Study Group. Does prolonged therapy with a long‐acting stimulant suppress growth in children with ADHD?. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(5):527‐37. [DOI: 10.1097/01.chi.0000205710.01690.d4; PUBMED: 16670649] [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Faraone SV, Lerner M, Cooper KM, Zimmerman B. Effect of long‐term treatment with stimulant medication on growth? Drs. Spencer et al. Reply. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(3):306. [DOI: 10.1097/chi.0b013e31802ed960] [DOI] [PubMed] [Google Scholar]
- Wigal SB, Wilens TE, Wolraich M, Lerner M. Hematologic and blood biochemistry monitoring during methylphenidate treatment in children with attention‐deficit/hyperactivity disorder: 2‐year, open‐label study results. Pediatrics 2007;120(1):e120‐8. [DOI: 10.1542/peds.2006-1402] [DOI] [PubMed] [Google Scholar]
- Wilens T, Biederman J, Lerner M, Concerta Study Group. Effects of once‐daily osmotic‐release methylphenidate on blood pressure and heart rate in children with attention‐deficit/hyperactivity disorder: results from a one‐year follow‐up study. Journal of Clinical Psychopharmacology 2004;24(1):36‐41. [DOI: 10.1097/01.jcp.0000106223.36344.df; PUBMED: 14709945] [DOI] [PubMed] [Google Scholar]
- Wilens T, McBurnett K, Stein M, Lerner M, Spencer T, Wolraich M. ADHD treatment with once‐daily OROS methylphenidate: final results from a long‐term open‐label study. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(10):1015‐23. [DOI: 10.1097/01.chi.0000173291.28688.e7; PUBMED: 16175106] [DOI] [PubMed] [Google Scholar]
- Wilens T, McBurnett K, Stein M, Lerner M, Spencer T, Wolraich M. ADHD treatment with once‐daily OROS methylphenidate: final results from a long‐term open‐label study: erratum. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(5):632. [DOI: 10.1097/00004583-200605000-00020] [DOI] [PubMed] [Google Scholar]
- Wilens T, Pelham W, Stein M, Conners CK, Abikoff H, Atkins M, et al. ADHD treatment with once‐daily OROS methylphenidate: interim 12‐month results from a long‐term open‐label study. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(4):424‐33. [DOI: 10.1097/01.CHI.0000046814.95464.7D; PUBMED: 12649629] [DOI] [PubMed] [Google Scholar]
Wilens 2006 {published data only}
- Oral system methylphenidate for teen ADHD. Brown University Child and Adolescent Psychopharmacology Update 2006;8(3):4‐5. [DOI: 10.1002/cpu.20015] [DOI] [Google Scholar]
- Biederman J. Effectiveness and safety of the once‐daily OROS formulation of methylphenidate in adolescents with attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology 2003;13(Suppl 4):S448. [Google Scholar]
- Greenhill LL. Safety and efficacy of OROS MPH in adolescents with ADHD. 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco (CA). 2003.
- McGough JJ, McBurnett K, Bukstein O, Wilens TE, Greenhill L, Lerner M, et al. Once‐daily OROS methylphenidate is safe and well tolerated in adolescents with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2006;16(3):351‐6. [DOI: 10.1089/cap.2006.16.351; PUBMED: 16768642] [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Stein MA, Cooper KM. Dose‐response characteristics in adolescents with attention‐deficit/hyperactivity disorder treated with OROS methylphenidate in a 4‐week, open‐label, dose‐titration study. Journal of Child and Adolescent Psychopharmacology 2010;20(3):187‐96. [DOI: 10.1089/cap.2009.0102; PUBMED: 20578931] [DOI] [PubMed] [Google Scholar]
- Wilens TE, McBurnett K, Bukstein O, McGough J, Greenhill L, Lerner M, et al. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention‐deficit/hyperactivity disorder. Archives of Pediatrics and Adolescent Medicine 2006;160(1):82‐90. [DOI: 10.1001/archpedi.160.1.82; PUBMED: 16389216] [DOI] [PubMed] [Google Scholar]
Wilens 2008 {published data only}
- López FA, Landgraf JM, Wilens TE. Quality of life and parent satisfaction with the methylphenidate transdermal system. European Neuropsychopharmacology 2008;18(Suppl 4):S562. [DOI: 10.1016/S0924-977X(08)70856-3] [DOI] [Google Scholar]
- López FA, Wilens TE, Wigal SB, Turnbow JM. Effects of variable wear times on transdermal methylphenidate in attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology 2008;18(Suppl 4):S561‐2. [DOI: 10.1016/S0924-977X(08)70855-1] [DOI] [Google Scholar]
- Manos M, Frazier TW, Landgraf JM, Weiss M, Hodgkins P. HRQL and medication satisfaction in children with ADHD treated with the methylphenidate transdermal system. Current Medical Research and Opinion 2009;25(12):3001‐10. [DOI: ; PUBMED: 10.1185/03007990903388797] [DOI] [PubMed] [Google Scholar]
- Wilens TE, Boellner SW, López FA, Turnbow JM, Wigal SB, Childress AC, et al. Varying the wear time of the methylphenidate transdermal system in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(6):700‐8. [DOI: 10.1097/CHI.0b013e31816bffdf; PUBMED: 18434918] [DOI] [PubMed] [Google Scholar]
Williams 2008 {published data only}
- Williams LM, Hermens DF, Palmer D, Kohn M, Clarke S, Keage H, et al. Misinterpreting emotional expressions in attention‐deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects. Biological Psychiatry 2008;63(10):917‐26. [DOI: 10.1016/j.biopsych.2007.11.022; PUBMED: 18272140] [DOI] [PubMed] [Google Scholar]
Williamson 2011 {published data only}
- Resolution of enuresis with stimulant treatment of ADHD. Brown University Child and Adolescent Psychopharmacology Update 2011;13(6):8‐8. [DOI: 10.1002/cpu.20141] [DOI] [Google Scholar]
- Williamson LB, Gower M, Ulzen T. Clinical case rounds in child and adolescent psychiatry: enuresis and ADHD in older children and an adolescent treated with stimulant medication: a case series. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2011;20(1):53‐5. [PMC3024723] [PMC free article] [PubMed] [Google Scholar]
Winsberg 1982 {published data only}
- Winsberg BG, Kupietz SS, Sverg J, Hungund BL, Young NL. Methylphenidate oral dose plasma concentrations and behavioral response in children. Psychopharmacology 1982;76(4):329‐32. [PUBMED: 6812106] [DOI] [PubMed] [Google Scholar]
Winterstein 2009 {published data only}
- Winterstein AG, Gerhard T, Shuster J, Saidi A. Cardiac safety of methylphenidate versus amphetamine salts in the treatment of ADHD. Pediatrics 2009;124(1):e75‐80. [DOI: 10.1542/peds.2008-3138; PMC3856396; PUBMED: 19564272] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterstein AG, Gerhard T, Shuster J, Zito J, Johnson M, Liu H, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Annals of Pharmacotherapy 2008;42(1):24‐31. [DOI: 10.1345/aph.1K143; PUBMED: 18042808] [DOI] [PubMed] [Google Scholar]
Witt 2008 {published data only}
- Witt KL, Shelby MD, Itchon RN, Faircloth M, Kissling GE, Chrisman AK, et al. Methylphenidate and amphetamine do not induce cytogenetic damage in lymphocytes of children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(12):1375‐83. [DOI: 10.1097/CHI.0b013e3181893620; PMC2807376; PUBMED: 18978633] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolley 2003 {published data only}
- Woolley JB, Heyman I. Dexamphetamine for obsessive‐compulsive disorder. American Journal of Psychiatry 2003;160(1):183. [DOI] [PubMed] [Google Scholar]
Yalcin 2012 {published data only}
- Yalcin O, Aslan AA, Sari BA, Turkbay T. Possible methylphenidate related hoarseness and disturbances of voice quality: two pediatric cases. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2012; Vol. 22, issue 3:278‐82. [DOI: 10.5455/bcp.20120731061626] [DOI]
Yalcin 2014 {published data only}
- Yalcin O, Iseri E, Bukan N, Ercin U. Effects of long acting methylphenidate on ghrelin levels in male children with attention deficit hyperactivity disorder: an open label trial. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2014;24(2):146‐57. [DOI: 10.5455/bcp.20130708042604] [DOI] [Google Scholar]
Yang 2004 {published data only}
- Yang P, Chung LC, Chen CS, Chen CC. Rapid improvement in academic grades following methylphenidate treatment in attention‐deficit hyperactivity disorder. Psychiatry and Clinical Neurosciences 2004;58(1):37‐41. [PUBMED: 14678455] [DOI] [PubMed] [Google Scholar]
Yang 2012 {published data only}
- Yang L, Cao Q, Shuai L, Li H, Chan RC, Wang Y. Comparative study of OROS‐MPH and atomoxetine on executive function improvement in ADHD: a randomized controlled trial. International Journal of Neuropsychopharmacology 2012; Vol. 15, issue 1:15‐26. [DOI: 10.1017/S1461145711001490; PUBMED: 22017969] [DOI] [PubMed]
Yatsuga 2014 {published data only}
- Yatsuga C, Toyohisa D, Fujisawa TX, Nishitani S, Shinohara K, Matsuura N, et al. No association between catechol‐O‐methyltransferase (COMT) genotype and attention deficit hyperactivity disorder (ADHD) in Japanese children. Brain and Development 2014;36(7):620‐5. [DOI: 10.1016/j.braindev.2013.08.006; PUBMED: 24035255] [DOI] [PubMed] [Google Scholar]
Yildiz 2010 {published data only}
- Yildiz Öç Ö, Ağaoğlu B, Karakaya I, Şişmanlar ŞG, Çakin Memik N. Efficiency and tolerability of OROS‐methylphenidate in Turkish children and adolescents with attention‐deficit/hyperactivity disorder. Anadolu Psikiyatri Dergisi 2010;11(1):44‐50. [Google Scholar]
Yildiz 2011 {published data only}
- Yildiz Ö, Şişmanlar ŞG, Memik NÇ, Karakaya I, Ağaoğlu B. Atomoxetine and methylphenidate treatment in children with ADHD: the efficacy, tolerability and effects on executive functions. Child Psychiatry and Human Development 2011;42(3):257‐69. [DOI: 10.1007/s10578-010-0212-3; PUBMED: 21165694] [DOI] [PubMed] [Google Scholar]
Yilmaz 2013 {published data only}
- Methylphenidate‐induced orofacial and extremity dyskinesia. Brown University Child and Adolescent Psychopharmacology Update 2012;14(8):7‐7. [DOI: 10.1002/cpu.20169] [DOI] [PubMed] [Google Scholar]
- Yilmaz AE, Donmez A, Orun E, Tas T, Isik B, Sonmez FM. Methylphenidate‐induced acute orofacial and extremity dyskinesia. Journal of Child Neurology 2013;28(6):778‐80. [DOI: 10.1177/0883073812449905; PUBMED: 22791547] [DOI] [PubMed] [Google Scholar]
Yu 2010 {published data only}
- Yu ZJ, Parker‐Kotler C, Tran K, Weller RA, Weller EB. Peripheral vasculopathy associated with psychostimulant treatment in children with attention‐deficit/hyperactivity disorder. Current Psychiatry Reports 2010;12(2):111‐5. [DOI: 10.1007/s11920-010-0093-y; PUBMED: 20425295] [DOI] [PubMed] [Google Scholar]
Zarinara 2010 {published data only}
- Zarinara AR, Mohammadi MR, Hazrati N, Tabrizi M, Rezazadeh SA, Rezaie F, et al. Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double‐blind comparison trial. Human Psychopharmacology 2010;25(7‐8):530‐5. [DOI: 10.1002/hup.1148; PUBMED: 20860068] [DOI] [PubMed] [Google Scholar]
Zelnik 2015 {published data only}
- Zelnik N, Terkel‐Dawer R. The clinical profile of children with ADHD that require OROS‐methylphenidate combined with shorter‐acting formulations. ADHD Attention Deficit and Hyperactivity Disorders 2015;7(4):313‐8. [DOI: 10.1007/s12402-015-0168-z; PUBMED: 25838111] [DOI] [PubMed] [Google Scholar]
Zeni 2007 {published data only}
- Zeni CP, Guimarães AP, Polanczyk GV, Genro JP, Roman T, Hutz MH, et al. No significant association between response to methylphenidate and genes of the dopaminergic and serotonergic systems in a sample of Brazilian children with attention‐deficit/hyperactivity disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 2007;144B(3):391‐4. [DOI: 10.1002/ajmg.b.30474; PUBMED: 17171656] [DOI] [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang H, Du M, Zhuang S. Impact of long‐term treatment of methylphenidate on height and weight of school age children with ADHD. Neuropediatrics 2010;41(2):55‐9. [DOI: 10.1055/s-0030-1261893; PUBMED: 20799150] [DOI] [PubMed] [Google Scholar]
Zheng 2011 {published data only}
- Zheng Y, Wang YF, Qin J, Wang LW, Zou LP, Jin XM, et al. Prospective, naturalistic study of open‐label OROS methylphenidate treatment in Chinese school‐aged children with attention‐deficit/hyperactivity disorder. Chinese Medical Journal 2011;124(20):3269‐74. [PUBMED: 22088519] [PubMed] [Google Scholar]
Zheng 2015 {published data only}
- Zheng Y, Liang JM, Gao HY, Yang ZW, Jia FJ, Liang YZ, et al. An open‐label, self‐control, prospective study on cognitive function, academic performance, and tolerability of osmotic‐release oral system methylphenidate in children with attention‐deficit hyperactivity disorder. Chinese Medical Journal 2015;128(22):2988‐97. [DOI: 10.4103/0366-6999.168948; PMC4795269; PUBMED: 26608976] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bart 2010 {published data only}
- Bart O, Podoly T, Bar‐Haim Y. A preliminary study on the effect of methylphenidate on motor performance in children with comorbid DCD and ADHD. Research in Developmental Disabilities 2010;31(6):1443‐7. [DOI: 10.1016/j.ridd.2010.06.014; PUBMED: 20650602] [DOI] [PubMed] [Google Scholar]
Beauchaine 2003 {published data only}
- Beauchaine TP, Gartner Joseph. A linear growth curve analysis of inpatient treatment response by conduct‐disordered, ADHD, and comorbid preadolescents. Aggressive Behavior 2003;29(5):440‐56. [DOI: 10.1002/ab.10066] [DOI] [Google Scholar]
Becker 2011 {published data only}
- Becker A, Roessner V, Breuer D, Döpfner M, Rothenberger A. Relationship between quality of life and psychopathological profile: data from an observational study in children with ADHD. European Child and Adolescent Psychiatry 2011;20(Suppl 2):S267‐75. [DOI: 10.1007/s00787-011-0204-2; PMC3180591; PUBMED: 21901415] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beery 1994 {published data only}
- Beery SH. Behavioral Disinhibition, Anxiety, and Response to Methylphenidate and Behavior Management in Children with Attention Deficit Hyperactivity Disorder [PhD thesis]. Miami (FL): University of Miami, 1994. [Google Scholar]
Benamor 2014 {published data only}
- Benamor L. H‐Magnetic resonance spectroscopy study of stimulant medication effect on brain metabolites in French Canadian children with attention deficit hyperactivity disorder. Neuropsychiatric Disease and Treatment 2014;10:47‐54. [DOI: 10.2147/NDT.S52338; PMC3901735] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biederman 1999 {published data only}
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention‐deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104(2):e20. [PUBMED: 10429138] [DOI] [PubMed] [Google Scholar]
Biederman 2009 {published data only}
- Biederman J, Monuteaux MC, Spencer T, Wilens TE, Faraone SV. Do stimulants protect against psychiatric disorders in youth with ADHD? A 10‐year follow‐up study. Pediatrics 2009;124(1):71‐8. [DOI: 10.1542/peds.2008-3347; 10.1542/peds.2008‐3347; PUBMED: 19564285] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohane 2010 {published data only}
- Bohane L, Young M, Rowlandson P. Medication in attention deficit hyperactivity disorder and ADHD with Autistic Spectrum Disorder (ASD). Procedia ‐ Social and Behavioral Sciences 2010;5:655‐9. [DOI: 10.1016/j.sbspro.2010.07.160] [DOI] [Google Scholar]
Brossard‐Racine 2012 {published data only}
- Brossard‐Racine M, Shevell M, Snider L, Bélanger SA, Majnemer A. Motor skills of children newly diagnosed with Attention Deficit Hyperactivity Disorder prior to and following treatment with stimulant medication. Research in Developmental Disabilities 2012;33(6):2080‐7. [DOI: 10.1016/j.ridd.2012.06.003; PUBMED: 22796639] [DOI] [PubMed] [Google Scholar]
Byrne 1998 {published data only}
- Byrne JM, Bawden HN, DeWolfe NA, Beattie TL. Clinical assessment of psychopharmacological treatment of preschoolers with ADHD. Journal of Clinical and Experimental Neuropsychology 1998;20(5):613‐27. [DOI: 10.1076/jcen.20.5.613.1121; PUBMED: 10079039] [DOI] [PubMed] [Google Scholar]
Casat 1995 {published data only}
- Casat CD, Pearson DA, Davelaar MJ, Cherek DR, Van‐Davelaar MJ. Methylphenidate effects on a laboratory aggression measure in children with ADHD. Psychopharmacology Bulletin 1995;31(2):353‐6. [PUBMED: 7491391] [PubMed] [Google Scholar]
Cavadas 2007 {published data only}
- Cavadas M, Pereira LD, Mattos P. Effects of methylphenidate in auditory processing evaluation of children and adolescents with attention deficit hyperactivity disorder [Efeito do metilfenidato no processamento auditivo em crianças e adolescentes com transtorno do deficit de atenção/hiperatividade]. Arquivos de Neuro‐Psiquiatria 2007;65(1):138‐43. [DOI: 10.1590/S0004-282X2007000100028; PUBMED: 17420844] [DOI] [PubMed] [Google Scholar]
Chambers 2012 {published data only}
- Chambers NA, Pascoe E, Kaplanian S, Forsyth I. Ingestion of stimulant medications does not alter bispectral index or clinical depth of anesthesia at 1 MAC sevoflurane in children. Paediatric Anaesthesia 2012;22(4):341‐4. [DOI: 10.1111/j.1460-9592.2011.03717.x; PUBMED: 21988202] [DOI] [PubMed] [Google Scholar]
Chavez 1999 {published data only}
- Chavez H, Ozolins D, Losek JD. Hypoglycemia and propranolol in pediatric behavioral disorders. Pediatrics 1999;103(6 Pt 1):1290‐2. [PUBMED: 10353945] [DOI] [PubMed] [Google Scholar]
Chelonis 2010 {published data only}
- Chelonis JJ, Johnson TA, Ferguson SA, Kubacak B, Edwards M, Paule M. Effects of methylphenidate on motivation in children with attention‐deficit/hyperactivity disorder. Neurotoxicology and Teratology 2010;32(4):504. [DOI: 10.1016/j.ntt.2010.04.031] [DOI] [Google Scholar]
Chelonis 2011 {published data only}
- Chelonis JJ, Johnson TA, Ferguson SA, Berry KJ, Kubacak B, Edwards MC, et al. Effect of methylphenidate on motivation in children with attention‐deficit/hyperactivity disorder. Experimental and Clinical Psychopharmacology 2011;19(2):145‐53. [DOI: 10.1037/a0022794; PUBMED: 21463072] [DOI] [PubMed] [Google Scholar]
Clarke 2003 {published data only}
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Clarke DC, Croft R J. Effects of stimulant medications on children with attention‐deficit/hyperactivity disorder and excessive beta activity in their EEG. Clinical Neurophysiology 2003;114(9):1729‐37. [PUBMED: 12948803] [DOI] [PubMed] [Google Scholar]
Conzelmann 2014 {published data only}
- Conzelmann A, Gerdes AB, Mucha RF, Weyers P, Lesch KP, Bähne CG, et al. Autonomic hypoactivity in boys with attention‐deficit/hyperactivity disorder and the influence of methylphenidate. World Journal of Biological Psychiatry 2014;15(1):56‐65. [DOI: 10.3109/15622975.2013.829584; PUBMED: 24410179] [DOI] [PubMed] [Google Scholar]
Cooper 2011 {published data only}
- Cooper WO. E‐mail regarding your study 'ADHD drugs and serious cardiovascular events in children and young adults'. Email to: M Holmskov 10 September 2014.
- Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, et al. ADHD drugs and serious cardiovascular events in children and young adults. New England Journal of Medicine 2011;365(20):1896‐904. [DOI: 10.1056/NEJMoa1110212] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeVito 2009 {published data only}
- DeVito EE, Blackwell AD, Clark L, Kent L, Dezsery AM, Turner DC, et al. Methylphenidate improves response inhibition but not reflection‐impulsivity in children with attention deficit hyperactivity disorder (ADHD). Psychopharmacology 2009;202(1‐3):531‐9. [DOI: 10.1007/s00213-008-1337-y; PMC2704617; PUBMED: 18818905] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Zeeuw 2012 {published data only}
- Zeeuw P, Mandl RC, Hulshoff Pol HE, Engeland H, Durston S. Decreased frontostriatal microstructural organization in attention deficit/hyperactivity disorder. Human Brain Mapping 2012; Vol. 33, issue 8:1941‐51. [DOI: 10.1002/hbm.21335; PUBMED: 21826757] [DOI] [PMC free article] [PubMed]
Drtílková 1990 {published data only}
- Drtílková I, Misurec J, Náhunek K. The paradox effect of psychostimulants in the treatment of the child hyperkinetic syndrome. Activitas Nervosa Superior 1990;32(4):302‐3. [PUBMED: 1982036] [PubMed] [Google Scholar]
Epstein 2010 {published data only}
- Epstein JN, Langberg JM, Lichtenstein PK, Altaye M, Brinkman WB, House K, et al. Attention‐deficit/hyperactivity disorder outcomes for children treated in community‐based pediatric settings. Archives of Pediatrics & Adolescent Medicine 2010;164(2):160‐5. [DOI: 10.1001/archpediatrics.2009.263; PUBMED: 20124145] [DOI] [PubMed] [Google Scholar]
Faraone 2007b {published data only}
- Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1‐year, open‐label study of OROS methylphenidate in children with ADHD. Journal of Attention Disorders 2007;11(2):157‐66. [DOI: 10.1177/1087054706295663; PUBMED: 17494833] [DOI] [PubMed] [Google Scholar]
Fegert 2006 {published data only}
- Fegert JM, Hebebrand J, Kommission Entwicklungspsychopharmakologie der drei Fachgesellschaften. Statement to questionable cardiac risks of application of stimulants [Stellungnahme zu fraglichen kardialen Risiken der Stimulanziengabe]. Zeitschrift für Kinder‐ und Jugendpsychiatrie und Psychotherapie 2006;34(4):295‐97. [DOI: 10.1024/1422-4917.34.4.295] [DOI] [PubMed] [Google Scholar]
Foodman 1996 {published data only}
- Foodman A, McPhillips K. ADD and soft signs. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(7):841‐2. [PUBMED: 8768341] [DOI] [PubMed] [Google Scholar]
Frank 1993 {published data only}
- Frank Y. Visual event related potentials after methylphenidate and sodium valproate in children with attention deficit hyperactivity disorder. Clinical EEG 1993;24(1):19‐24. [PUBMED: 8420693] [DOI] [PubMed] [Google Scholar]
Funk 1993 {published data only}
- Funk JB, Chessare JB, Weaver MT, Exley AR. Attention deficit hyperactivity disorder, creativity, and the effects of methylphenidate. Pediatrics 1993;91(4):816‐9. [PUBMED: 8464673] [PubMed] [Google Scholar]
Gau 2009 {published data only}
- Gau SS, Chiu CD, Shang CY, Cheng AT, Soong WT. Executive function in adolescence among children with attention‐deficit/hyperactivity disorder in Taiwan. Journal of Developmental and Behavioral Pediatrics 2009;30(6):525‐34. [DOI: 10.1097/DBP.0b013e3181c21c97; PUBMED: 19884851] [DOI] [PubMed] [Google Scholar]
Gimpel 2005 {published data only}
- Gimpel GA, Collett BR, Veeder MA, Gifford JA, Sneddon P, Bushman B, et al. Effects of stimulant medication on cognitive performance of children with ADHD. Clinical Pediatrics 2005;44(5):405‐11. [DOI: 10.1177/000992280504400504; PUBMED: 15965546] [DOI] [PubMed] [Google Scholar]
Glenngård 2013 {published data only}
- Glenngård AH, Hjelmgren J, Thomsen P H, Tvedten T, Prtz C. Eliciting patient preferences for ADHD treatment using Discrete Choice Experiment in Sweden, Norway and Denmark. Journal of Mental Health Policy and Economics 2011;14(Suppl 1):S8‐9. [Google Scholar]
- Glenngård AH, Hjelmgren J, Thomsen PH, Tvedten T. Patient preferences and willingness‐to‐pay for ADHD treatment with stimulants using discrete choice experiment (DCE) in Sweden, Denmark and Norway. Nordic Journal of Psychiatry 2013;67(5):351‐9. [DOI: 10.3109/08039488.2012.748825; PUBMED: 23245636] [DOI] [PubMed] [Google Scholar]
Gormez 2012 {published data only}
- Gormez V, Avery B, Mann H. An additional mid‐afternoon dose of immediate release methylphenidate (MPH‐IR) with Concerta XL provides better symptom control in children and adolescents with attention deficit/hyperactivity disorder (ADHD). European Psychiatry 2012;27(Suppl 1):1. [DOI: 10.1016/S0924-9338(12)74460-7] [DOI] [Google Scholar]
Gould 2009 {published data only}
- Gould MS, Walsh BT, Munfakh JL, Kleinman M, Duan NH, Olfson M, et al. Sudden death and use of stimulant medications in youths. American Journal of Psychiatry 2009;166(9):992‐1001. [DOI: 10.1176/appi.ajp.2009.09040472; PUBMED: 19528194] [DOI] [PubMed] [Google Scholar]
- Gould MS, Walsh BT, Munfakh JL, et al. Study finds link between stimulants and sudden unexplained death in young people. Brown University Psychopharmacology Update 2009;20(12):1,1‐5,6. [DOI: 10.1002/pu.20105] [DOI] [Google Scholar]
- Kuehn BM. Stimulant use linked to sudden death in children without heart problems. JAMA: Journal of the American Medical Association 2009;302(6):613‐4. [DOI: 10.1001/jama.2009.1115; PUBMED: 19671894] [DOI] [PubMed] [Google Scholar]
- Uhl D. Stimulant therapy: sudden death cases under methylphenidate [Plötzliche Todesfälle unter Methylphenidat]. Deutsche Apotheker Zeitung 2009;26:46‐8. [Google Scholar]
Gualtieri 1985 {published data only}
- Gualtieri CT, Hicks RE. Neuropharmacology of methylphenidate and a neural substrate for childhood hyperactivity. Psychiatric Clinics of North America 1985;8(4):875‐92. [PUBMED: 2867534] [PubMed] [Google Scholar]
Guimarães 2009 {published data only}
- Guimarães AP, Zeni C, Polanczyk G, Genro JP, Roman T, Rohde LA, et al. MAOA is associated with methylphenidate improvement of oppositional symptoms in boys with attention deficit hyperactivity disorder. International Journal of Neuropsychopharmacology 2009;12(5):709‐14. [DOI: 10.1017/S1461145709000212; PUBMED: 19309535] [DOI] [PubMed] [Google Scholar]
Gurkan 2011 {published data only}
- Gurkan CK, Yurumez E, Akca OF, Bilgic A, Turkoglu S, Kilic BG, et al. The effect of treatment on multiple symptom domains and quality of life in children and adolescents with ADHD: A 3‐year follow‐up study. European Child and Adolescent Psychiatry 2011;20(Suppl 1):S170. [DOI: 10.1007/s00787-011-0181-5] [DOI] [Google Scholar]
Hautmann 2013 {published data only}
- Hautmann C, Rothenberger A, Döpfner M. An observational study of response heterogeneity in children with attention deficit hyperactivity disorder following treatment switch to modified‐release methylphenidate. BMC Psychiatry 2013;13:219. [DOI: 10.1186/1471-244X-13-219; PMC3846116; PUBMED: 24004962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawcutt 2012 {published data only}
- Hawcutt DB, Mainie P, Riordan A, Smyth RL, Pirmohamed M. Reported paediatric adverse drug reactions in the UK 2000‐2009. British Journal of Clinical Pharmacology 2012;73(3):437‐46. [DOI: 10.1111/j.1365-2125.2011.04113.x; PMC3370348; PUBMED: 21988288] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawk 2003 {published data only}
- Hawk LW Jr, Yartz AR, Pelham WE Jr, Lock TM. The effects of methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention‐deficit hyperactivity disorder. Psychopharmacology 2003;165(2):118‐27. [DOI: 10.1007/s00213-002-1235-7; PUBMED: 12417963] [DOI] [PubMed] [Google Scholar]
Heiser 2004 {published data only}
- Heiser P, Frey J, Smidt J, Sommerlad C, Wehmeier PM, Hebebrand J, et al. Objective measurement of hyperactivity, impulsivity, and inattention in children with hyperkinetic disorders before and after treatment with methylphenidate. European Child and Adolescent Psychiatry 2004;13(2):100‐4. [DOI: 10.1007/s00787-004-0365-3; PUBMED: 15103535] [DOI] [PubMed] [Google Scholar]
Hidas 2011 {published data only}
- Hidas A, Noy AF, Birman N, Shapira J, Matot I, Steinberg D, et al. Oral health status, salivary flow rate and salivary quality in children, adolescents and young adults with ADHD. Archives of Oral Biology 2011; Vol. 56, issue 10:1137‐41. [DOI: 10.1016/j.archoralbio.2011.03.018; PUBMED: 21514566] [DOI] [PubMed]
Hood 2005 {published data only}
- Hood J, Baird G, Rankin PM, Isaacs E. Immediate effects of methylphenidate on cognitive attention skills of children with attention‐deficit‐hyperactivity disorder. Developmental Medicine and Child Neurology 2005;47(6):408‐14. [DOI: 10.1111/j.1469-8749.2005.tb01162.x/pdf] [DOI] [PubMed] [Google Scholar]
Hoza 1992 {published data only}
- Hoza B, Pelham WE Jr, Sams SE, Carlson C. An examination of the 'dosage' effects of both behavior therapy and methylphenidate on the classroom performance of two ADHD children. Behavior Modification 1992;16(2):164‐92. [DOI: 10.1177/01454455920162002; PUBMED: 1580892] [DOI] [PubMed] [Google Scholar]
Hwang 2012 {published data only}
- Hwang J, Kim B, Kim Y, Yoo H. Methylphenidate‐osmotic release oral delivery system reduces parenting stress in parents of children and adolescents with ADHD in Korea. European Neuropsychopharmacology 2012; Vol. 22, issue Suppl 2:S415.
Jonkman 1999 {published data only}
- Jonkman LM, Kemner C, Verbaten MN, Engeland H, Camfferman G, Buitelaar JK, et al. Attentional capacity, a probe ERP study: differences between children with attention‐deficit hyperactivity disorder and normal control children and effects of methylphenidate. Psychophysiology 2000;37(3):334‐46. [PUBMED: 10860411] [PubMed] [Google Scholar]
- Jonkman LM, Kemner C, Verbaten MN, Engeland H, Kenemans JL, Camfferman G, et al. Perceptual and response interference in children with attention‐deficit hyperactivity disorder, and the effects of methylphenidate. Psychophysiology 1999;36(4):419‐29. [DOI: 10.1111/1469-8986.3640419] [DOI] [PubMed] [Google Scholar]
Kerdar 2007 {published data only}
- Kerdar MS, Scheuerpflug P, Srdinko P, Wewetzer C, Warnke A, Romanos M. Quantitative effect of treatment with methylphenidate on EEG ‐ a pilot study [EEG‐Veränderungen unter Methylphenidat ‐ eine Pilotstudie]. Z Kinder Jugendpsychiatr Psychother 2007;35(4):247‐55; quiz 255‐6. [DOI: 10.1024/1422-4917.35.4.247; PUBMED: 17970368] [DOI] [PubMed] [Google Scholar]
Kereszturi 2008 {published data only}
- Kereszturi E, Tarnok Z, Bognar E, Lakatos K, Farkas L, Gadoros J, et al. Catechol‐O‐methyltransferase Val158Met polymorphism is associated with methylphenidate response in ADHD children. American Journal of Medical Genetics 2008;147B(8):1431‐5. [DOI: 10.1002/ajmg.b.30704; PUBMED: 18214865] [DOI] [PubMed] [Google Scholar]
Keulers 2007 {published data only}
- Keulers EH, Hendriksen JG, Feron FJ, Wassenberg R, Wuisman‐Frerker MG, Jolles J, et al. Methylphenidate improves reading performance in children with attention deficit hyperactivity disorder and comorbid dyslexia: an unblinded clinical trial. European Journal of Paediatric Neurology 2007;11(1):21‐8. [DOI: 10.1016/j.ejpn.2006.10.002; PUBMED: 17169593] [DOI] [PubMed] [Google Scholar]
Klein 2002 {published data only}
- Klein C, Fischer B Jr, Fischer B, Hartnegg K. Effects of methylphenidate on saccadic responses in patients with ADHD. Experimental Brain Research 2002;145(1):121‐5. [DOI: 10.1007/s00221-002-1105-x; PUBMED: 12070751] [DOI] [PubMed] [Google Scholar]
Kramer 2001 {published data only}
- Kramer AF, Cepeda NJ, Cepeda ML. Methylphenidate effects on task‐switching performance in attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(11):1277‐84. [DOI: 10.1097/00004583-200111000-00007; PUBMED: 11699801] [DOI] [PubMed] [Google Scholar]
Lajoie 2005 {published data only}
- Lajoie G, Anderson V, Anderson P, Tucker AR, Robertson IH, Manly T. Effects of methylphenidate on attention skills in children with attention deficit/hyperactivity disorder. Brain Impairment 2005;6(1):21‐32. [DOI: 10.1375/brim.6.1.21.65479] [DOI] [Google Scholar]
Lawrence 2005 {published data only}
- Lawrence CA, Barry RJ, Clarke AR, Johnstone SJ, McCarthy R, Selikowitz M, et al. Methylphenidate effects in attention deficit/hyperactivity disorder: electrodermal and ERP measures during a continuous performance task. Psychopharmacology 2005;183(1):81‐91. [DOI: 10.1007/s00213-005-0144-y; PUBMED: 16160877] [DOI] [PubMed] [Google Scholar]
Matier 1992 {published data only}
- Matier K, Halperin JM, Sharma V, Newcorn JH, Sathaye N. Methylphenidate response in aggressive and nonaggressive ADHD children: distinctions on laboratory measures of symptoms. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(2):219‐25. [DOI: 10.1097/00004583-199203000-00007; PUBMED: 1564022] [DOI] [PubMed] [Google Scholar]
Mayes 1993 {published data only}
- Mayes SD, Bixler EO. Reliability of global impressions for assessing methylphenidate effects in children with attention‐deficit hyperactivity disorder. Perceptual and Motor Skills 1993;77(3 Pt 2):1215‐8. [DOI: 10.2466/pms.1993.77.3f.1215; PUBMED: 8170770] [DOI] [PubMed] [Google Scholar]
Miller 1994 {published data only}
- Miller LG, Kraft IA. Application of actigraphy in the clinical setting: use in children with attention‐deficit hyperactivity disorder. Pharmacotherapy 1994;14(2):219‐23. [PUBMED: 8197043] [PubMed] [Google Scholar]
Miller 1996 {published data only}
- Miller DC, Kavcic V, Leslie JE. ERP changes induced by methylphenidate in boys with attention‐deficit hyperactivity disorder. Journal of Attention Disorders 1996; Vol. 1, issue 2:95‐113. [DOI: 10.1177/108705479600100203] [DOI]
Miranda 2006 {published data only}
- Miranda A, Jarque S, Rosel J. Treatment of children with ADHD: psychopedagogical program at school versus psychostimulant medication. Psicothema 2006;18(3):335‐41. [PUBMED: 17296053] [PubMed] [Google Scholar]
Monden 2012a {published data only}
- Monden Y, Dan H, Nagashima M, Dan I, Kyutoku Y, Okamoto M, et al. Clinically‐oriented monitoring of acute effects of methylphenidate on cerebral hemodynamics in ADHD children using fNIRS. Clinical Neurophysiology 2012; Vol. 123, issue 6:1147‐57. [DOI: 10.1016/j.clinph.2011.10.006; PUBMED: 22088661] [DOI] [PubMed]
Monden 2012b {published data only}
- Monden Y, Dan H, Nagashima M, Dan I, Tsuzuki D, Kyutoku Y, et al. Right prefrontal activation as a neuro‐functional biomarker for monitoring acute effects of methylphenidate in ADHD children: an fNIRS study. NeuroImage: Clinical 2012;1(1):131‐40. [DOI: 10.1016/j.nicl.2012.10.001; PMC3757725; PUBMED: 24179746] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nahshoni 2012 {published data only}
- Nahshoni E, Golubchik P, Glazer J, Sever J, Strasberg B, Imbar S, et al. Late potentials in the signal‐averaged electrocardiogram in pre‐pubertal children with ADHD, before and after methylphenidate treatment. European Child and Adolescent Psychiatry 2012;21(2):75‐8. [DOI: 10.1007/s00787-011-0233-x; PUBMED: 22160611] [DOI] [PubMed] [Google Scholar]
Neef 2005 {published data only}
- Neef NA, Bicard DF, Endo S, Coury DL, Aman MG. Evaluation of pharmacological treatment of impulsivity in children with attention deficit hyperactivity disorder. Journal of Applied Behavior Analysis 2005;38(2):135‐46. [DOI: 10.1901/jaba.2005.116-02; PMC1226151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Negrao 2011 {published data only}
- Negrao BL, Bipath P, Westhuizen D, Viljoen M. Autonomic correlates at rest and during evoked attention in children with attention‐deficit/hyperactivity disorder and effects of methylphenidate. Neuropsychobiology 2011;63(2):82‐91. [DOI: 10.1159/000317548; PUBMED: 21178382] [DOI] [PubMed] [Google Scholar]
Nigg 1996 {published data only}
- Nigg JT, Swanson JM, Hinshaw SP. Covert visual spatial attention in boys with attention deficit hyperactivity disorder: lateral effects, methylphenidate response and results for parents. Neuropsychologia 1996;35(2):165‐76. [PUBMED: 9025120] [DOI] [PubMed] [Google Scholar]
Nikles 2005 {published data only}
- Nikles CJ, Clavarino AM, Del‐Mar CB. Using n‐of‐1 trials as a clinical tool to improve prescribing. British Journal of General Practice 2005;55(512):175‐80. [PMC1463086] [PMC free article] [PubMed] [Google Scholar]
Nolan 1999 {published data only}
- Nolan EE, Gadow KD, Sprafkin J. Stimulant medication withdrawal during long‐term therapy in children with comorbid attention‐deficit hyperactivity disorder and chronic multiple tic disorder. Pediatrics 1999;103(4 Pt 1):730‐7. [PUBMED: 10103294] [DOI] [PubMed] [Google Scholar]
Ohashi 2010 {published data only}
- Ohashi K, Vitaliano G, Polcari A, Teicher MH. Unraveling the nature of hyperactivity in children with attention‐deficit/hyperactivity disorder. Archives of General Psychiatry 2010;67(4):388‐96. [DOI: 10.1001/archgenpsychiatry.2010.28; PUBMED: 20368514] [DOI] [PubMed] [Google Scholar]
Orgill 1996 {published data only}
- Orgill A, Serfontein S. Behavioural and cognitive effects of stimulant and non stimulant drugs in childhood attention deficit disorder. European Neuropsychopharmacology 1996;6(Suppl 3):56. [DOI: 10.1016/0924-977X(96)87562-6] [DOI] [Google Scholar]
Overcash 2005 {published data only}
- Overcash SJ. The effect of ROSHI protocol and cranial electrotherapy stimulation on a nine‐year‐old anxious, dyslexic male with attention deficit disorder: a case study. Journal of Neurotherapy 2005;9(2):63‐77. [DOI: 10.1300/J184v09n02_05] [DOI] [Google Scholar]
Ozdag 2004 {published data only}
- Ozdag MF, Yorbik O, Ulas UH, Hamamcioglu K, Vural O. Effect of methylphenidate on auditory event related potential in boys with attention deficit hyperactivity disorder. International Journal of Pediatric Otorhinolaryngology 2004;68(10):1267‐72. [DOI: 10.1016/j.ijporl.2004.04.023; PUBMED: 15364497] [DOI] [PubMed] [Google Scholar]
Palomino 2012 {published data only}
- Palomino MD, Martín‐Calero MJ, Marques G. Involvement of community pharmacists on attention‐deficit/hyperactivity disorder patients. A pilot study [Implicación del farmacéutico comunitario en la dispensación a pacientes con trastorno por déficit de atención e hiperactividad. Un estudio piloto]. Pharmaceutical Care España 2012;14(5):183‐92. [Google Scholar]
Park 2012 {published data only}
- Park MH, Kim JW, Yang YH, Hong SB, Park S, Kang H, et al. Regional brain perfusion before and after treatment with methylphenidate may be associated with the G1287A polymorphism of the norepinephrine transporter gene in children with attention‐deficit/hyperactivity disorder. Neuroscience Letters 2012; Vol. 514, issue 2:159‐63. [DOI: 10.1016/j.neulet.2012.02.079; PUBMED: 22405810] [DOI] [PubMed]
Pelham 1986 {published data only}
- Pelham WE, Milich R, Walker JL. Effects of continuous and partial reinforcement and methylphenidate on learning in children with Attention Deficit Disorder. Journal of Abnormal Psychology 1986;95(4):319‐25. [PUBMED: 3805493] [DOI] [PubMed] [Google Scholar]
Pelham 2011 {published data only}
- Pelham WE Jr, Waschbusch DA, Hoza B, Gnagy EM, Greiner AR, Sams SE, et al. Music and video as distractors for boys with ADHD in the classroom: comparison with controls, individual differences, and medication effects. Journal of Abnormal Child Psychology 2011; Vol. 39, issue 8:1085‐98. [DOI: 10.1007/s10802-011-9529-z; PUBMED: 21695447] [DOI] [PubMed]
Perera 2012 {published data only}
- Perera H, Jeewandara KC, Seneviratne S, Guruge C. Combined ω3 and ω6 supplementation in children with attention‐deficit hyperactivity disorder (ADHD) refractory to methylphenidate treatment: a double‐blind, placebo‐controlled study. Journal of Child Neurology 2012; Vol. 27, issue 6:747‐53. [DOI: 10.1177/0883073811435243; PUBMED: 22596014] [DOI] [PubMed]
Pierce 2008 {published data only}
- Pierce D, Dixon CM, Wigal SB, McGough JJ. Pharmacokinetics of methylphenidate transdermal system (MTS): results from a laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2008;18(4):355‐64. [DOI: 10.1089/cap.2007.0148; PUBMED: 18759645] [DOI] [PubMed] [Google Scholar]
Poulton 2013 {published data only}
- Poulton AS, Melzer E, Tait PR, Garnett SP, Cowell Chris, Baur LA, et al. Growth and pubertal development of adolescent boys on stimulant medication for attention deficit hyperactivity disorder. Medical Journal of Australia 2013;198(1):29‐32. [PUBMED: 23330767] [DOI] [PubMed] [Google Scholar]
Prehn‐Kristensen 2011 {published data only}
- Prehn‐Kristensen A, Krauel K, Hinrichs H, Fischer J, Malecki U, Schuetze H, et al. Methylphenidate does not improve interference control during a working memory task in young patients with attention‐deficit hyperactivity disorder. Brain Research 2011;1388:56‐68. [DOI: 10.1016/j.brainres.2011.02.075; PUBMED: 21385569] [DOI] [PubMed] [Google Scholar]
Rapport 1985 {published data only}
- Rapport MD, DuPaul GJ, Smith NF. Rate‐dependency and hyperactivity: methylphenidate effects on operant responding. Pharmacology, Biochemistry, and Behavior 1985;23(1):77‐83. [PUBMED: 4034622] [DOI] [PubMed] [Google Scholar]
Rhodes 2004 {published data only}
- Rhodes SM, Coghill DR, Matthews K. Methylphenidate restores visual memory, but not working memory function in attention deficit‐hyperkinetic disorder. Psychopharmacology 2004;175(3):319‐30. [DOI: 10.1007/s00213-004-1833-7; PUBMED: 15138760] [DOI] [PubMed] [Google Scholar]
Rhodes 2006 {published data only}
- Rhodes SM, Coghill DR, Matthews K. Acute neuropsychological effects of methylphenidate in stimulant drug‐naïve boys with ADHD II‐‐broader executive and non‐executive domains. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2006;47(11):1184‐94. [DOI: 10.1111/j.1469-7610.2006.01633.x; PUBMED: 17076758] [DOI] [PubMed] [Google Scholar]
Roman 2002 {published data only}
- Roman T, Szobot C, Martins S, Biederman J, Rohde LA, Hutz MH. Dopamine transporter gene and response to methylphenidate in attention‐deficit/hyperactivity disorder. Pharmacogenetics 2002;12(6):497‐9. [PUBMED: 12172219] [DOI] [PubMed] [Google Scholar]
Rubia 2009 {published data only}
- Rubia K, Halari R, Cubillo AI, Taylor ET. Methylphenidate modulates inferior and orbitofrontal brain activation in ADHD during sustained attention and reward. European Neuropsychopharmacology 2009; Vol. 19, issue Suppl 3:S304‐5. [DOI: 10.1016/S0924-977X(09)70452-3] [DOI]
Rubia 2009a {published data only}
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication‐naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology 2009;57(7‐8):640‐52. [DOI: 10.1016/j.neuropharm.2009.08.013; PUBMED: 19715709] [DOI] [PubMed] [Google Scholar]
Rubio 2011 {published data only}
- Rubio B, Hernández S, Verche E, Martín R, González‐Pérez P. A pilot study: differential effects of methylphenidate‐OROS on working memory and attention functions in children with attention‐deficit/hyperactivity disorder with and without behavioural comorbidities. Attention Deficit and Hyperactivity Disorders 2011;3(1):13‐20. [DOI: 10.1007/s12402-010-0035-x; PUBMED: 21432614] [DOI] [PubMed] [Google Scholar]
Schecklmann 2011 {published data only}
- Schecklmann M, Schaldecker M, Aucktor S, Brast J, Kirchgässner K, Muhlberger A, et al. Effects of methylphenidate on olfaction and frontal and temporal brain oxygenation in children with ADHD. Journal of Psychiatric Research 2011;45(11):1463‐70. [DOI: 10.1016/j.jpsychires.2011.05.011; PUBMED: 21689828] [DOI] [PubMed] [Google Scholar]
Scheres 2003 {published data only}
- Scheres A, Oosterlaan J, Swanson J, Morein‐Zamir S, Meiran N, Schut H, et al. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. Journal of Abnormal Child Psychology 2003;31(1):105‐20. [PUBMED: 12597703] [DOI] [PubMed] [Google Scholar]
Scheres 2006 {published data only}
- Scheres A, Oosterlaan J, Sergeant JA. Speed of inhibition predicts teacher‐rated medication response in boys with attention deficit hyperactivity disorder. International Journal of Disability, Development and Education 2006;53(1):93‐109. [DOI: 10.1080/10349120500510107] [DOI] [Google Scholar]
Schmiedeler 2009 {published data only}
- Schmiedeler S, Schwenck C, Schneider W. The mental process of negating values in children with ADHD and effects of medical treatment [Die Verarbeitung von Negationen bei Kindern mit ADHS und der Einfluss medikamentöser Behandlung]. Kindheit und Entwicklung 2009;18(3):137‐43. [DOI: 10.1026/0942-5403.18.3.137] [DOI] [Google Scholar]
Shafritz 2004 {published data only}
- Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. American Journal of Psychiatry 2004;161(11):1990‐7. [DOI: 10.1176/appi.ajp.161.11.1990] [DOI] [PubMed] [Google Scholar]
Shaywitz 1982 {published data only}
- Shaywitz SE, Hunt RD, Jatlow P. Psychopharmacology of attention deficit disorder: pharmacokinetic, neuroendocrine, and behavioral measures following acute and chronic treatment with methylphenidate. Pediatrics 1982;69(6):688‐94. [PUBMED: 7079034] [PubMed] [Google Scholar]
Sheppard 1999 {published data only}
- Sheppard DM, Bradshaw JL, Mattingly JB, Lee P. Effects of stimulant medication on the lateralisation of line bisection judgements of children with attention deficit hyperactivity disorder. Journal of Neurology, Neurosurgery and Psychiatry 1999;66(1):57‐63. [PMC1736185; PUBMED: 9886453] [DOI] [PMC free article] [PubMed] [Google Scholar]
Snyder 2008 {published data only}
- Snyder AM, Maruff P, Pietrzak RH, Cromer JR, Snyder PJ. Effect of treatment with stimulant medication on nonverbal executive function and visuomotor speed in children with attention deficit/hyperactivity disorder (ADHD). Child Neuropsychology 2008;14(3):211‐26. [DOI: 10.1080/09297040701220005; PUBMED: 17852127] [DOI] [PubMed] [Google Scholar]
Solanto 1989 {published data only}
- Solanto MV, Wender EH. Does methylphenidate constrict cognitive functioning?. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(6):897‐902. [DOI: 10.1097/00004583-198911000-00014; PUBMED: 2808260] [DOI] [PubMed] [Google Scholar]
Spivak 2001 {published data only}
- Spivak B, Vered Y, Yoran‐Hegesh R, Graft E, Averbuch E, Vinokurow S, et al. The influence of three months of methylphenidate treatment on platelet‐poor plasma biogenic amine levels in boys with attention deficit hyperactivity disorder. Human Psychopharmacology 2001;16(4):333‐7. [DOI: 10.1002/hup.298; PUBMED: 12404569] [DOI] [PubMed] [Google Scholar]
Stein 1999 {published data only}
- Jerome L. Can methylphenidate facilitate sleep in children with attention deficit hyperactivity disorder?. Journal of Child and Adolescent Psychopharmacology 2001;11(1):109. [DOI: 10.1089/104454601750143564; PUBMED: 11322740] [DOI] [PubMed] [Google Scholar]
- Stein MA. Unravelling sleep problems in treated and untreated children with ADHD. Journal of Child and Adolescent Psychopharmacology 1999;9(3):157‐68. [DOI: 10.1089/cap.1999.9.157; PUBMED: 10521009] [DOI] [PubMed] [Google Scholar]
Syrigou‐Papavasiliou 1988 {published data only}
- Syrigou‐Papavasiliou A, Lycaki H, LeWitt PA, Verma NP, Spivak D, Chayasirisobhon S. Dose‐response effects of chronic methylphenidate administration on late event‐related potentials in attention deficit disorder. Clinical Electroencephalography 1988;19(3):129‐33. [DOI: 10.1177/155005948801900306] [DOI] [PubMed] [Google Scholar]
Tabori‐Kraft 2007 {published data only}
- Tabori‐Kraft J, Sørensen M, Kærgaard M, Dalsgaard S, Thomsen PH. Is OPTAx[TM] useful for monitoring the effect of stimulants on hyperactivity and inattention?. European Child and Adolescent Psychiatry 2007;16(5):347‐51. [DOI: 10.1007/s00787-006-0571-2] [DOI] [PubMed] [Google Scholar]
Tamm 2007 {published data only}
- Tamm L, Carlson CL. Task demands interact with the single and combined effects of medication and contingencies on children with ADHD. Journal of Attention Disorders 2007;10(4):372‐80. [DOI: 10.1177/1087054706289946; PUBMED: 17449836] [DOI] [PubMed] [Google Scholar]
Tillery 1998 {published data only}
- Tillery KL. A Double‐Blind Study of the Central Auditory Processing and Auditory Continuous Test Performances of Children with Attention Deficit Hyperactivity Disorder and Central Auditory Processing Disorder Under Ritalin and Placebo Conditions [PhD thesis]. Buffalo (NY): University of Buffalo, The State University of New York, 1998. [Google Scholar]
Trenque 2009 {published data only}
- Taam MA, Boissieu P, Dukic S, Herlem E, Trenque T. Hallucinations: a case/noncase study in the French Pharmacovigilance Database. Pharmacoepidemiology and Drug Safety 2013;22(Suppl 1):315‐6. [DOI: 10.1002/pds.3512] [DOI] [Google Scholar]
- Trenque T. Methylphenidate off‐label use. Drug Safety 2012;35(10):891. [Google Scholar]
- Trenque T, Herlem E, Germain M L. Spontaneous reporting with methylphenidate: insufficient data. Drug Safety 2009;32(10):983. [DOI: 10.2165/11316660-000000000-00000] [DOI] [Google Scholar]
- Trenque T, Maura G, Herlem E. Spontaneous reporting with methylphenidate: off‐label use in growth?. Fundamental and Clinical Pharmacology. 2012; Vol. 26:105.
Vance 1999 {published data only}
- Vance AL, Luk ES, Costin J, Tonge BJ, Pantelis C. Attention deficit hyperactivity disorder: anxiety phenomena in children treated with psychostimulant medication for 6 months or more. Australian and New Zealand Journal of Psychiatry 1999;33(3):399‐406. [DOI: 10.1046/j.1440-1614.1999.00575.x] [DOI] [PubMed] [Google Scholar]
Van der Meere 2009 {published data only}
- Meere JJ, Shalev RS, Borger N, Wiersema JR. Methylphenidate, interstimulus interval, and reaction time performance of children with attention deficit/hyperactivity disorder: a pilot study. Child Neuropsychology 2009;15(6):554‐66. [DOI: 10.1080/09297040902758803; PUBMED: 19296298] [DOI] [PubMed] [Google Scholar]
Van der Oord 2012 {published data only}
- Oord S, Geurts HM, Prins PJ, Emmelkamp PM, Oosterlaan J. Prepotent response inhibition predicts treatment outcome in attention deficit/hyperactivity disorder. Child Neuropsychology 2012;18(1):50‐61. [DOI: 10.1080/09297049.2011.559159; PUBMED: 21819279] [DOI] [PubMed] [Google Scholar]
Vickers 2002 {published data only}
- Vickers JN, Rodrigues ST, Brown LN. Gaze pursuit and arm control of adolescent males diagnosed with attention deficit hyperactivity disorder (ADHD) and normal controls: evidence of a dissociation in processing visual information of short and long duration. Journal of Sports Sciences 2002;20(3):201‐16. [DOI: 10.1080/026404102317284763] [DOI] [PubMed] [Google Scholar]
Vogt 2011 {published data only}
- Vogt C, Williams T. Early identification of stimulant treatment responders, partial responders and non‐responders using objective measures in children and adolescents with hyperkinetic disorder. Child and Adolescent Mental Health 2011; Vol. 16, issue 3:144‐9. [DOI: 10.1111/j.1475-3588.2010.00593.x] [DOI] [PubMed]
Winterstein 2007 {published data only}
- Winterstein AG, Gerhard T, Shuster J, Johnson M, Zito JM, Saidi A. Cardiac safety of central nervous system stimulants in children and adolescents with attention‐deficit/hyperactivity disorder. Pediatrics 2007;120(6):e1494‐501. [DOI: 10.1542/peds.2007-0675; PUBMED: 18055666] [DOI] [PubMed] [Google Scholar]
Winterstein 2012 {published data only}
- Winterstein AG, Gerhard T, Kubilis P, Saidi A, Linden S, Crystal S, et al. Cardiovascular safety of central nervous system stimulants in children and adolescents: population based cohort study. BMJ 2012; Vol. 345:e4627. [DOI: 10.1136/bmj.e4627] [DOI] [PMC free article] [PubMed]
Wong 2012 {published data only}
- Wong CG, Stevens MC. The effects of stimulant medication on working memory functional connectivity in attention‐deficit/hyperactivity disorder. Biological Psychiatry 2012; Vol. 71, issue 5:458‐66. [DOI: 10.1016/j.biopsych.2011.11.011; PMC4120250; PUBMED: 22209640] [DOI] [PMC free article] [PubMed]
Yeh 2012 {published data only}
- Yeh CB, Huang WS, Lo MC, Chang CJ, Ma KH, Shyu JF. The rCBF brain mapping in adolescent ADHD comorbid developmental coordination disorder and its changes after MPH challenging. European Journal of Paediatric Neurology 2012; Vol. 16, issue 6:613‐8. [DOI: 10.1016/j.ejpn.2012.02.007; PUBMED: 22417719] [DOI] [PubMed]
Zachor 2006 {published data only}
- Zachor DA, Roberts AW, Hodgens JB, Isaacs JS, Merrick J. Effects of long‐term psychostimulant medication on growth of children with ADHD. Research in Developmental Disabilities 2006;27(2):162‐74. [DOI: 10.1016/j.ridd.2004.12.004; PUBMED: 15955659] [DOI] [PubMed] [Google Scholar]
Zalsman 2003 {published data only}
- Zalsman G, Pumeranz O, Peretz G, Ben‐Dor DH, Dekel S, Horesh N, et al. Attention patterns in children with attention deficit disorder with or without hyperactivity. Scientific World Journal 2003;3:1093‐107. [DOI: 10.1100/tsw.2003.94; PUBMED: 14625396] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zang 2005 {published data only}
- Zang YF, Jin Z, Weng XC, Zhang L, Zeng YW, Yang L, et al. Functional MRI in attention‐deficit hyperactivity disorder: evidence for hypofrontality. Brain and Development 2005;27(8):544‐50. [DOI: 10.1016/j.braindev.2004.11.009; PUBMED: 15876503] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Arnold‐Von 2000 {published data only}
- Arnold‐von Saher RA, Boer F, Burggraaf J. The clinical use of a double‐blind placebo‐controlled trial with methylphenidate: advantages and limitations [Pleidooi voor een dubbelblinde proefbehandeling met methylfenidaat in de dagelijkse klinische praktijk van ADHD bij kinderen]. Tijdschrift voor Psychiatrie 2000;42(11):837‐44. [Google Scholar]
Dalsgaard 2011 {published data only}
- Dalsgaard S, Kvist AP, Leckman JF, Nielsen HS, Simonsen M. Cardiovascular safety of stimulants in children with attention‐deficit/hyperactivity disorder: a nationwide prospective cohort study. Journal of Child and Adolescent Psychopharmacology 2014;24(6):1‐9. [DOI: 10.1089/cap.2014.0020; PMC4137345; PUBMED: 24956171] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry 2015;2(8):702‐9. [DOI: 10.1016/S2215-0366(15)00271-0; PUBMED: 26249301] [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Nielsen HS, Simonsen M. Long‐term cardiac adverse effects of ADHD medication in children and adolescents: a nationwide register based follow‐up study. European Child and Adolescent Psychiatry 2011;20(Suppl 1):S107. [DOI: 10.1007/s00787-011-0181-5] [DOI] [Google Scholar]
Flapper 1989 {published data only}
- Flapper BC, Koopman HM, Heltzel A. Methylphenidate a stimulant in treatment of children with minimal brain dysfunction. Tijdschrift voor Kindergeneeskunde 1989;57(1):14‐20. [PubMed] [Google Scholar]
Husár 2006 {published data only}
- Husár M, Zerhau P. Priapism in childhood ‐ case report of 14‐year‐old boy [Priapismus v dĕtsékm veku ‐ kazuistika 14 letého chlapce]. Rozhledy V Chirurgii 2006;85(7):329‐30. [PUBMED: 17044274] [PubMed] [Google Scholar]
Ince 2015 {published data only}
- Ince E, Algedik P, Demirdogen ES, Emul M, Demir T. The relationship between acute dyskinesia with a single dose of methylphenidate and recent risperidone discontinuation in a child with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2015;25(4):378‐9. [DOI: 10.1089/cap.2014.0148; PUBMED: 25920038] [DOI] [PubMed] [Google Scholar]
Ishizaki 2001 {published data only}
- Ishizaki A, Sugama M. Methylphenidate therapy in 141 patients with hyperkinetic disorder or with pervasive developmental disorder and hyperkinesia. No To Hattatsu 2001;33(4):323‐8. [PUBMED: 11494575] [PubMed] [Google Scholar]
Laezer 2015 {published data only}
- Laezer KL. Effectiveness of psychoanalytic psychotherapy and behavioral therapy treatment in children with attention deficit hyperactivity disorder and oppositional defiant disorder. Journal of Infant, Child, and Adolescent Psychotherapy 2015, 2015;14(2):111‐28. [DOI: 10.1080/15289168.2015.1014991] [DOI] [Google Scholar]
Mulas 2014 {published data only}
- Mulas F, Roca P, Ros‐Cervera G, Gandía‐Benetó R, Ortiz‐Sánchez P. Pharmacological management of attention deficit hyperactivity disorder with methylphenidate and atomoxetine within a context of epilepsy [Manejo farmacológico del trastorno por déficit de atención/hiperactividad con metilfenidato y atomoxetina en un contexto de epilepsia]. Revista de Neurologia 2014;58(Suppl 1):S34‐9. [PUBMED: 25252667] [PubMed] [Google Scholar]
Ptáček 2008 {published data only}
- Ptáček R, Kuželová H, Paclt I, Žukov I. Effect of medication on anthropometric characteristic of ADHD children [Vliv medikace na antropometrické charakteristiky dětí s ADHD]. Ceska a Slovenska Psychiatrie 2008;104(8):415‐9. [Google Scholar]
- Ptáček R, Kuželová H, Paclt I, Žukov I, Fischer S. Developmental changes in children with ADHD and effecto [sic] of medication on growth [Vyvojove zmeny u deti s ADHD a vliv medikace na rust]. Psychiatrie 2009;13(Suppl):104‐5. [Google Scholar]
Radziuk 2015 {published data only}
- Radziuk AL, Kieling RR, Santos K, Rotert R, Bastos F, Palmini AL. Methylphenidate improves the quality of life of children and adolescents with ADHD and difficult‐to‐treat epilepsies. Epilepsy and Behavior 2015;46:215‐20. [DOI: 10.1016/j.yebeh.2015.02.019; PUBMED: 25940104] [DOI] [PubMed] [Google Scholar]
Socanski 2015 {published data only}
Sugama 2009 {published data only}
- Sugama M, Ishizaki A. Clinical assessment of the effect of switch from immediate‐release to extended‐release methylphenidate in 181 patients with isolated attention deficit/hyperactivity disorder (AD/HD) or AD/HD associated with pervasive developmental disorder. No to Hattatsu 2009;41(6):436‐41. [PUBMED: 19928542] [PubMed] [Google Scholar]
TOSCA 2011 {published data only}
- Polypharmacy in treating severe childhood aggression. Brown University Child and Adolescent Psychopharmacology Update 2012;14(1):1‐4. [DOI: 10.1002/cpu.20155] [DOI] [Google Scholar]
- Aman MG, Bukstein OG, Gadow KD, Arnold LE, Molina BS, McNamara NK, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention‐deficit/hyperactivity disorder?. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(1):47‐60. [DOI: 10.1016/j.jaac.2013.09.022; PMC3984501; PUBMED: 24342385] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer CA, Arnold LE, Bukstein OG, Findling RL, Gadow KD, Li Xi, et al. The treatment of severe child aggression (TOSCA) study: design challenges. Child and Adolescent Psychiatry and Mental Health 2011;5(1):36. [DOI: 10.1186/1753-2000-5-36; PMC3231878; PUBMED: 22074813] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waldon 2016 {published data only}
- Gendron M, Rusak B, Rajda M, Corkum PV. Assessing the impact of methylphenidate on sleep in children with ADHD using polysomnography and actigraphy. Sleep 2012;35:A374. [Google Scholar]
- Waldon J, Begum E, Gendron M, Rusak B, Andreou P, Rajda M, et al. Concordance of actigraphy with polysomnography in children with and without attention‐deficit/hyperactivity disorder. Journal of Sleep Research 2016;25(5):524‐33. [PUBMED: 27140929] [DOI] [PubMed] [Google Scholar]
Yusufoglu 2014 {published data only}
- Yusufoglu C, Sonkaya AR, Taymur I. Henoch‐Schönlein Purpura with attention deficit hyperactivity disorder. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2014;24(Suppl 1):S158‐S159. [Google Scholar]
References to ongoing studies
Beau 2009 {published data only}
- Beau R, Douglas I, Smeeth L, Evans SJ. Spontaneous report safety signals profile and comparative Bayesian analysis of ADHD medication in children in the UK. Pharmacoepidemiology and Drug Safety 2009;18(S1):S208‐9. [DOI: 10.1002/pds.1806] [DOI] [Google Scholar]
Bottelier 2014 {published data only}
- Bottelier MA, Schouw ML, Klomp A, Tamminga HG, Schrantee AG, Bouziane C, et al. The effects of Psychotropic drugs On Developing brain (ePOD) study: methods and design. BMC Psychiatry 2014;14(48):2‐12. [DOI: 10.1186/1471-244X-14-48] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahlgren 2012 {published data only}
- Dahlgren J, Wentz E. Overweight and obese children with diagnosed attention deficit hyperactivity disorder have a favourable weight loss with methylphenidate ‐ one year data. Obesity Facts 2012; Vol. 5, issue Suppl 1:208.
Díez‐Suárez 2015 {published data only}
- Castro‐Manglano P, Vallejo‐Valdivielso M, Díez‐Suárez A, Figueroa‐Quintana A. Factors that may predict a good response to pharmacological treatment. European Child and Adolescent Psychiatry 2015;24(Suppl 1):S74‐5. [DOI: 10.1007/s00787-015-0714-4] [DOI] [Google Scholar]
- Díez‐Suárez A, Vallejo‐Valdivielso M, Mendez J, Castro‐Manglano P. Long term effects of medication for ADHD in weight and height in children and adolescents. European Child and Adolescent Psychiatry 2015;24(Suppl 1):S74. [DOI: 10.1007/s00787-015-0714-4] [DOI] [Google Scholar]
- Díez‐Suárez A, Vallejo‐Valdivieso M, Marín‐Méndez J, Castro‐Manglano P, Soutullo C. Weight, height and body mass index in patients with adhd treated with methylphenidate. European Child and Adolescent Psychiatry 2015;24(Suppl 1):S175. [DOI: 10.1007/s00787-015-0714-4] [DOI] [PubMed] [Google Scholar]
- Vallejo‐Valdivielso M, Castro‐Manglano P, Díez‐Suárez A, Marín‐Méndez J, Figueroa‐Quintana A. Clinical and neurocognitive predictive factors for good response to methylphenidate treatment in a ADHD sample in Spain: a naturalistic follow‐up study. European Child and Adolescent Psychiatry 2015;24(Suppl 1):S137. [DOI: 10.1007/s00787-015-0714-4] [DOI] [Google Scholar]
Djurkovic‐Lazic 2009 {published data only}
- Djurkovic‐Lazic J, Lazic B, Milojevic N, Djuric D. School children with ADHD ‐ effects of once‐daily OROS methylphenidate treatment ADHD symptoms included improvement academic functioning. European Psychiatry 2009;24(Suppl 1):S396. [DOI: 10.1016/S0924-9338(09)70629-7] [DOI] [Google Scholar]
Gau 2010 {published data only}
- Gau CS, Chen CF, Gau SS. The influence of using methylphenidate on the coming up of psychiatric disorders in children with ADHD. Pharmacoepidemiology and Drug Safety 2010;19(Suppl 1):S152‐3. [DOI: 10.1002/pds.2019] [DOI] [Google Scholar]
Houmann 2011 {published data only}
- Houmann T, Skovgaard AM, Rasmussen HB. Individualised methylphenidate therapy based on pharmacogenomics: focus on carboxylesterase1 (CES1). European Child and Adolescent Psychiatry 2011;20(Suppl 1):S120. [DOI: 10.1007/s00787-011-0181-5] [DOI] [Google Scholar]
Yook 2012 {published data only}
- Yook KH, Kim YW, Suh HS. Obesity is associated with non‐response to prolonged‐release methylphenidate treatment in ADHD. European Neuropsychopharmacology 2012;22(Suppl 2):S415‐6. [DOI: 10.1016/S0924-977X(12)70651-X] [DOI] [Google Scholar]
Additional references
Aagaard 2009
- Aagaard L, Hansen EH. Information about ADRs explored by pharmacovigilance approaches: a qualitative review of studies on antibiotics, SSRIs and NSAIDs. BMC Clinical Pharmacology 2009;9:4. [DOI: 10.1186/1472-6904-9-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aagaard 2010a
- Aagaard L, Hansen EH. Adverse drug reactions reported for systemic antibacterials in Danish children over a decade. British Journal of Clinical Pharmacology 2010;70(5):765‐8. [DOI: 10.1111/j.1365-2125.2010.03732.x; PMC2997318] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aagaard 2010b
- Aagaard L, Christensen A, Hansen EH. Information about adverse drug reactions reported in children: a qualitative review of empirical studies. British Journal of Clinical Pharmacology 2010;70(4):481‐91. [DOI: 10.1111/j.1365-2125.2010.03682.x; PMC2950983] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aagaard 2011
- Aagaard L, Hansen EH. The occurrence of adverse drug reactions reported for attention deficit hyperactivity disorder (ADHD) medications in the pediatric population: a qualitative review of empirical studies. Neuropsychiatric Disease and Treatment 2011; Vol. 7:729‐44. [DOI] [PMC free article] [PubMed]
AAP 2011
- Subcommittee on Attention‐Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management, Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and the treatment of attention‐deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011;128(5):1077‐22. [DOI: 10.1542/peds.2011-2654; PMC4500647; PUBMED: 2200306] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abali 2013 [pers comm]
- Abali O. Possible inclusion of 'Methylphenidate induced hallucinations: case report' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 6 November 2013.
Adler 2010
- Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgraduate Medicine 2010;122(1):184‐91. [DOI: 10.3810/pgm.2010.01.2112] [DOI] [PubMed] [Google Scholar]
Adrian 2013 [pers comm]
- Adrian N. Questions regarding your article 'Explosive outbursts associated with methylphenidate' [personal communication]. Email to: HB Krogh 24 October 2013.
Aguilera‐Albesa 2013 [pers comm]
- Aguilera‐Albesa S. Inclusion of 'Alucinaciones tras la introducción de metilfenidato en dosis bajas' in a systematic review [personal communication]. Email to: EL Ramstad 1 July 2013.
Akinbami 2011
- Akinbami LJ, Liu X, Pastor PN, Reuben CA. Attention deficit hyperactivity disorder among children aged 5‐17 years in the United States, 1998–2009. NCHS Data Brief 2011; Vol. 70:1‐8. [PUBMED: 22142479] [PubMed]
Alpaslan 2016 [pers comm]
- Alpaslan AH. Supplemental data [personal communication]. Email to: MJ Kielsholm 22 April 2016.
Aman 2013 [pers comm]
- Aman M. Questions regarding the TOSCA study [personal communication]. Email to: EL Ramstad 28 June 2013.
Andrews 2013a
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719‐25. [DOI: 10.1016/j.jclinepi.2012.03.013] [DOI] [PubMed] [Google Scholar]
Andrews 2013b
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation ‐ determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726‐35. [DOI: 10.1016/j.jclinepi.2013.02.003] [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III). 3rd Edition. Washington (DC): American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM III‐R). 3rd Edition. Washington (DC): American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Text revision (DSM‐IV‐TR). 4th Edition. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th Edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Arneja 2016 [pers comm]
- Arneja J. Questions regarding your study 'Comparative short term efficacy and tolerability of methylphenidate and atomoxetine in attention deficit hyperactivity disorder' [personal communication]. Email to: SS Nielsen 2 July 2016.
Arnold 2013 [pers comm]
- Arnold LE. Questions regarding a study of yours ‐ inclusion in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 20 September 2013.
Arnold 2013b [pers comm]
Arnsten 2005
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through α2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behavioral and Brain Functions 2005;1(1):2. [DOI: ; PMC1143775; PUBMED: 15916700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Artul 2016 [pers comm]
- Artul S. Supplemental data [personal communication]. Email to: SS Nielsen 16 August 2016.
Arun 2014 [pers comm]
- Arun P. Methylphenidate and suicidal ideation IQ [personal communication]. Email to: M Holmskov 1 September 2014.
Ashkenasi 2013 [pers comm]
Bachmann 2017
- Bachmann CJ, Wiljlaars LP, Kalverdijk LJ, Burcu M, Glaeske G, Schuiling‐Veninga CCM, et al. Trends in ADHD medication use in children and adolescents in five western countries, 2005‐2012. European Neuropsychopharmacology 2017;27(5):484‐93. [DOI: 10.1016/j.euroneuro.2017.03.002; PUBMED: 28336088] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015] [DOI] [PubMed] [Google Scholar]
Barker 2013 [pers comm]
- Barker MJ. Inclusion of one of your studies in a Cochrane systematic review [personal communication]. Emails to: EL Ramstad (22 October, 6 December 2013) 6 December 2013.
Barkley 1977
- Barkley RA. A review of stimulant drug research with hyperactive children. Journal of Child Psychology and Psychiatry 1977;18(2):137‐65. [DOI: 10.1111/j.1469-7610.1977.tb00425.x] [DOI] [PubMed] [Google Scholar]
Barkley 1990
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo‐controlled evaluation. Pediatrics 1990;86(2):184‐192. [PUBMED: 2196520] [PubMed] [Google Scholar]
Benson 2000
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. New England Journal of Medicine 2000;342(25):1878‐86. [DOI: 10.1056/NEJM200006223422506] [DOI] [PubMed] [Google Scholar]
Bereket 2014 [pers comm]
- Bereket A. 'Height, weight, IGF‐I, IGFBP‐3 and thyroid functions in prepubertal children with attention deficit hyperactivity disorder' questions [personal communication]. Email to: T Nilausen 6 January 2014.
Bhutta 2002
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school‐aged children who were born preterm: a meta‐analysis. Journal of the American Medical Association 2002;288(6):728‐37. [PUBMED: 12169077] [DOI] [PubMed] [Google Scholar]
Biederman 2003
- Biederman J. Pharmacotherapy for attention‐deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow‐up of youths with and without ADHD. Journal of Clinical Psychiatry 2003;64(Suppl 11):3‐8. [PUBMED: 14529323] [PubMed] [Google Scholar]
Bjarnadottir 2015
- Bjarnadottir GD, Haraldsson HM, Rafnar BO, et al. Prevalent intravenous abuse of methylphenidate among treatment‐seeking patients with substance abuse disorders: a descriptive population‐based study. Journal of Addiction Medicine 2015;9(3):188‐94. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2018
- BNF. Methylphenidate hydrochloride: indications and dose. British National Formulary (accessed 9 February 2017). [DOI: 10.18578/BNF.995526496; www.medicinescomplete.com/mc/bnfc/2011/PHP2479‐methylphenidate‐hydrochloride.htm] [DOI]
Brunetti 2013
- Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. Journal of Clinical Epidemiology 2013;66(2):140‐50. [DOI: 10.1016/j.jclinepi.2012.04.012] [DOI] [PubMed] [Google Scholar]
Bruni 1996
- Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research 1996;5(4):251‐61. [PUBMED: 9065877] [DOI] [PubMed] [Google Scholar]
Buchmann 2014 [pers comm]
Burnett 2014
- Burnett A, Davey CG, Wood SJ, Wilson‐Ching M, Molloy C, Cheong JLY, et al. Extremely preterm birth and adolescent mental health in a geographical cohort born in the 1990s. Psychological Medicine 2014;44(7):1533‐44. [DOI: 10.1017/S0033291713002158] [DOI] [PubMed] [Google Scholar]
Bushe 2013
- Bushe CJ, Savill NC. Suicide related events and attention deficit hyperactivity disorder treatments in children and adolescents: a meta‐analysis of atomoxetine and methylphenidate comparator clinical trials. Child and Adolescent Psychiatry and Mental Health 2013;7(1):19. [DOI: 10.1186/1753-2000-7-19; PMC3691607] [DOI] [PMC free article] [PubMed] [Google Scholar]
CADDRA 2011
- Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA). Canadian ADHD Practice Guidelines. Vol. 3rd Edition, Toronto (ON): CADDRA, 2011. [Google Scholar]
Cairns 2014
- Cairns R, Daniels B, Wood DA, Brett J. ADHD medication overdose and misuse: the NSW Poisons Information Centre experience, 2004‐2014. Medical Journal of Australia 2016;204(4):154. [PUBMED: 26937669] [DOI] [PubMed] [Google Scholar]
Castellanos 2006
- Castellanos FX, Sonuga‐Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Sciences 2006;10(3):117‐23. [DOI: 10.1016/j.tics.2006.01.011] [DOI] [PubMed] [Google Scholar]
Castro‐Manglano 2016 [pers comm]
- Castro‐Manglano P. Questions regarding your study 'Long‐term effects of medication for ADHD in weight and height in children and adolescents' [personal communication]. Email to: SS Nielsen 3 June 2016.
CDC 2015
- Centers for Disease Control and Prevention (CDC). QuickStats: percentage of children aged 5‐17 years with diagnosed attention deficit/hyperactivity disorder (ADHD), by poverty status and sex – National Health Interview Survey, 2011–2014. Morbidity and Mortality Weekly Report 2015; Vol. 64, issue 40:1156. [PUBMED: 26469562] [DOI] [PubMed]
Chang 2012
- Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early‐onset substance use: a Swedish twin study. Journal of Abnormal Child Psychology 2012;40(3):425‐35. [DOI: 10.1007/s10802-011-9575-6; PUBMED: 21947618] [DOI] [PubMed] [Google Scholar]
Chang 2014a
- Chang Z, Lichtenstein P, D'Onofrio BM, Sjölander A, Larsson H. Serious transport accidents in adults with ADHD, and the effect of medication: a population based study. JAMA Psychiatry 2014;71(3):319‐25. [DOI: 10.1001/jamapsychiatry.2013.4174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2014b
- Chang Z, Lichtenstein P, Hallder L, D'Onofrio B, Serlachius E, Fazel S, Långström N, Larsson H. Stimulant ADHD medication and risk for substance abuse. Journal of Child Psychology and Psychiatry 2014;55(8):878‐85. [DOI: 10.1111/jcpp.12164] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2017
- Chang Z, Quinn PD, Hur K, Gibbons RD, Sjölander A, Larsson H, D'Onofrio BM. Associations between medication use for attention‐deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry 2017;74(6):597‐603. [DOI: 10.1001/jamapsychiatry.2017.0659] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charach 2013
- Charach A, Carson P, Fox S, Ali MU, Beckett J, Lim CG. Interventions for preschool children at high risk for ADHD: a comparative effectiveness review. Pediatrics 2013;131(5):e1584–604. [DOI: 10.1542/peds.2012-0974; PUBMED: 23545375] [DOI] [PubMed] [Google Scholar]
Chen 2014
- Chen Qi, Sjölander A, Runeson B, D'Onofrio BM, Lichtenstein P, Larsson H. Drug treatment for attention‐deficit/hyperactivity disorder and suicidal behaviour: register based study. BMJ 2014;348:g3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chervin 2000
- Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep‐disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Medicine 2000;1(1):21‐32. [PUBMED: 10733617] [DOI] [PubMed] [Google Scholar]
Chou 2014 [pers comm]
- Chou WJ. Remission in children and adolescents further information [personal communication]. Email to: T Nilausen 7 February 2014.
Cockcroft 2014 [pers comm]
- Cockcroft K. Regarding your study 'Sleep and daytime sleepiness in methylphenidate medicated and un‐medicated children with attention‐deficit/hyperactivity disorder (ADHD)' [personal communication]. Emails to: EL Ramstad (8 October 2013, 27 January 2014) 27 January 2014.
Cohen 2013 [pers comm]
- Cohen HA. Questions regarding your study 'Fixed drug eruption of the scrotum due to methylphenidate' [personal communication]. Email to: HB Krogh 12 July 2013.
Cole 2007
- Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 2007;335(7612):194‐7. [DOI: 10.1136/bmj.39238.399444.55] [DOI] [PMC free article] [PubMed] [Google Scholar]
Comprehensive Meta Analysis
- BioStat. Comprehensive Meta Analysis. www.meta‐analysis.com (accessed 9 February 2018).
Confino‐Cohen 2013 [pers comm]
- Confino‐Cohen R. Questions regarding your case report 'Successful desensitization of methylphenidate‐induced rash' [personal communication]. Email to: HB Krogh 17 November 2013.
Corrigall 2013 [pers comm]
- Corrigall R. Questions regarding your case report 'Methylphenidate euphoria' [personal communication]. Email to: HB Krogh 22 October 2013.
Cortese 2016
- Cortese S, Moreira‐Maia CR, St. Fleur D, Morcillo‐Peñalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: a systematic review and meta‐analysis. American Journal of Psychiatry 2016;173(1):34‐43. [DOI: 10.1176/appi.ajp.2015.15020266] [DOI] [PubMed] [Google Scholar]
Cox 2004
- Cox DJ, Merkel RL, Penberthy JK, Kovatchev B, Hankin CS. Impact of methylphenidate delivery profiles on driving performance of adolescents with attention‐deficit/hyperactivity disorder: a pilot study. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(3):269‐75. [PUBMED: 15076259] [DOI] [PubMed] [Google Scholar]
Coşkun 2013 [pers comm]
- Coşkun M. Questions regarding four of your case reports/series [personal communication]. Email to: EL Ramstad 2 August 2013.
Coşkun 2013b [pers comm]
- Coşkun M. Questions regarding four of your case reports/series [personal communication]. Email to: EL Ramstad 2 August 2013.
Coşkun 2013c [pers comm]
- Coşkun M. Questions regarding four of your case reports/series [personal communication]. Email to: EL Ramstad 2 August 2013.
Coşkun 2013d [pers comm]
- Coşkun M. Questions regarding four of your case reports/series [personal communication]. Email to: EL Ramstad 2 August 2013.
Curatolo 2013 [pers comm]
- Curatolo P. Possible inclusion of 'Methylphenidate‐induced visual hallucinations' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 4 October 2013.
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. III: The issue of carry over. Statistics in Medicine 2002;21(15):2161‐73. [DOI] [PubMed] [Google Scholar]
Czamara 2013
- Czamara D, Tiesler CM, Kohlböck G, Berdel D, Hoffmann B, Bauer CP, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLOS ONE 2013;8(5):e63859. [DOI: 10.1371/journal.pone.0063859] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahlgren 2013 [pers comm]
- Dahlgren J. Questions regarding 'Overweight and obese children with diagnosed attention deficit hyperactivity disorder have a favourable weight loss with methylphenidate ‐ one year data' [personal communication]. Email to: EL Ramstad 29 August 2013.
Dalsgaard 2015a
- Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry 2015;2(8):702‐9. [DOI: 10.1016/S2215-0366(15)00271-0] [DOI] [PubMed] [Google Scholar]
Dalsgaard 2015b
- Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet 2015;385(9983):2190‐6. [DOI: 10.1016/S0140-6736(14)61684-6] [DOI] [PubMed] [Google Scholar]
Danish Health and Medicines Authority 2017
- Danish Health and Medicines Authority. Produktresuméer–human. www.produktresume.dk/docushare/dsweb/View/Collection‐96 Accessed 2 May 2017.
Davari‐Ashtiani 2014 [pers comm]
- Davari‐Ashtiani R. Buspirone versus methylphenidate additional data [personal communication]. Email to: T Nilausen 1 January 2014.
De Vries 2010
- Vries TW, Roon EN. Low quality of reporting adverse drug reactions in paediatric randomised controlled trials. Archives of Disease in Childhood 2010;95(12):1023‐6. [DOI: 10.1136/adc.2009.175562; PUBMED: 20551194] [DOI] [PubMed] [Google Scholar]
DeSoysa 2013 [pers comm]
- DeSoysa R. Questions regarding your abstract 'Use of methylphenidate for attention‐deficit‐hyperactivity disorder in childhood' [personal communication]. Email to: HB Krogh 30 December 2013.
Dodangi 2013 [pers comm]
- Dodangi N. Questions regarding your abstract 'Duloxetine in comparison with methylphenidate in treatment of adolescents with ADHD' [personal communication]. Email to: TD Nilausen 2 November 2013.
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI: 10.1136/bmj.315.7109.629; PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elgen 2015
- Elgen SK, Sommerfelt K, Leversen KT, Markestad T. Minor neurodevelopmental impairments are associated with increased occurrence of ADHD symptoms in children born extremely preterm. European Child and Adolescent Psychiatry 2015;24(4):463‐70. [DOI: ] [DOI] [PubMed] [Google Scholar]
EMA 2017
- European Medicines Agency. Guideline on multiplicity issues in clinical trials. Committee for Human Medicinal products (CHMP) 2017.
Engert 2008
- Engert V, Pruessner JC. Dopaminergic and noradrenergic contributions to functionality in ADHD: the role of methylphenidate. Current Neuropharmacology 2008;6(4):322‐8. [DOI: 10.2174/157015908787386069] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erskine 2016
- Erskine HE, Norman RE, Ferrari AJ, Chan GC, Copeland WE, Whiteford HA, et al. Long‐term outcomes of attention‐deficit/hyperactivity disorder and conduct disorder: a systematic review and meta‐analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(10):841–50. [doi: 10.1016/j.jaac.2016.06.016; PUBMED: 27663939] [DOI] [PubMed] [Google Scholar]
Evans 2001
- Evans SW, Pelham WE, Smith BH, Bukstein O, Gnagy EM, Greiner AR, et al. Dose‐response effects of methylphenidate on ecologically valid measures of academic performance and classroom behavior in adolescents with ADHD. Experimental and Clinical Psychopharmacology 2001;9(2):163‐75. [PUBMED: 11518092] [DOI] [PubMed] [Google Scholar]
Faraone 2000
- Faraone SV, Biederman J, Spencer T, Wilens T, Seidman LJ, Mick E, et al. Attention‐deficit/hyperactivity disorder in adults: an overview. Biological Psychiatry 2000;48(1):9‐20. [PUBMED: 10913503] [DOI] [PubMed] [Google Scholar]
Faraone 2002
- Faraone SV, Biederman J, Roe C. Comparative efficacy of Adderall and methylphenidate in attention‐deficit/hyperactivity disorder: a meta‐analysis.. Journal of Clinical Psychopharmacology 2002;22(5):468–73. [DOI] [PubMed] [Google Scholar]
Faraone 2006
- Faraone S V, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for A DHD using meta‐analysis. Medscape General Medicine 2006;8(4):4. [PMC free article] [PubMed] [Google Scholar]
Faraone 2010
- Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention‐deficit/hyperactivity disorder using meta‐analysis of effect sizes. Journal of Clinical Psychiatry 2010;71(6):754‐63. [DOI] [PubMed] [Google Scholar]
Fernández‐Fernámdez 2013b [pers comm]
- Fernández‐Fernámdez MA. Interest in including 'Infantile psychosis secondary to methylphenidate' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 5 April 2013.
Fernández‐Fernández 2013 [pers comm]
- Fernández‐Fernández MA. Cardiovascular side effects questions on diagnosis and IQ. Email to: E Ramstad 4 August 2013.
Fischer 2013 [pers comm]
- Fischer R. AW: Cochrane review on MPH. Email to: OJ Storebø 14 June 2013.
Franke 2012
- Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CHD, Ramos‐Quiroga JA, et al. International Multicentre persistent ADHD CollaboraTion. The genetics of attention deficit/hyperactivity disorder in adults, a review. Molecular Psychiatry 2012;17(10):960‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frauger 2016
- Frauger E, Amaslidou D, Spadari M, Allaria‐Lapierre V, Braunstein D, Sciortino V, et al. Patterns of methylphenidate use and assessment of its abuse among the general population and individuals with drug dependence. European Addiction Research 2016;22(3):119‐26. [DOI: 10.1159/000439273; PUBMED: 26491869] [DOI] [PubMed] [Google Scholar]
Gadow 2013 [pers comm]
Gagnier 2013
- Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, CARE Group. The CARE Guidelines: Consensus‐based Clinical Case Reporting Guideline Development. Global Advances in Health and Medicine 2013;2(5):38‐43. [doi: 10.7453/gahmj.2013.008. PMID: 2441669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galland 2014 [pers comm]
- Galland B. Regarding your study [personal communication]. Email to: M Holmskov 14 August 2014.
Garattini 2016
- Garattini S, Jakobsen JC, Wetterslev J, Bertelé V, Banzi R, Rath A, et al. Evidence‐based clinical practice: overview of threats to the validity of evidence and how to minimise them. European Journal of Internal Medicine 2016;32:13‐21. [DOI: 10.1016/j.ejim.2016.03.020; PUBMED: 27160381] [DOI] [PubMed] [Google Scholar]
Gau 2013 [pers comm]
- Gau S. Questions regarding your study 'An open‐label, randomized, active‐ controlled equivalent trial of osmotic release oral system methylphenidate in children with attention‐deficit/hyperactivity disorder in Taiwan' [personal communication]. Email to: HB Krogh 9 October 2013.
Gau 2013b [pers comm]
- Gau CS. Possible inclusion of 'The influence of using methylphenidate on the coming up of psychiatric disorders in children with ADHD' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 22 October 2013.
Ghanizadeh 2013 [pers comm]
- Ghanizadeh A. Questions regarding 'Insomnia, night terror, and depression related to clonidine in attention‐deficit/hyperactivity disorder' [personal communication]. Email to: EL Ramstad 4 July 2013.
Ghanizadeh 2014 [pers comm]
Gluud 2008
- Gluud LL, Thorlund K, Gluud C, Woods L, Harris R, Sterne JA. Correction: reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2008;149(3):219. [DOI] [PubMed] [Google Scholar]
Golder 2011
- Golder S, Loke YK, Bland M. Meta‐analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLOS Medicine 2011;8(5):e1001026. [DOI: 10.1371/journal.pmed.1001026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goyette 1978
- Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners Parent and Teacher Rating Scales. Journal of Abnormal Child Psychology 1978;6(2):221‐36. [PUBMED: 670589] [DOI] [PubMed] [Google Scholar]
Gracious 2013 [pers comm]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.).. GRADEpro GDT. Version accessed prior to 12 March 2018. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.)., 2015.
Graham 2011
- Graham J, Banaschewski T, Buitelaar J, et al. European guidelines on managing adverse effects of medication for ADHD. European Child and Adolescent Psychiatry 2011;20(1):17‐37. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grcevich 2013 [pers comm]
Green 2013 [pers comm]
- Green T. Possible inclusion of 'Effect of methylphenidate on neurocognitive functioning in velocardiofacial syndrome: a randomized placebo‐controlled trial' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 1 November 2013.
Greenhill 2004
- Greenhill LL, Vitiello B, Fisher P, Levine J, Davies M, Abikoff H, et al. Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. Journal of the American Academy of Child and Adolescent Psychiatry 2004; Vol. 43, issue 12:1488‐96. [DOI: 10.1097/01.chi.0000142668.29191.13; PUBMED: 15564818] [DOI] [PubMed]
Greenhill 2006
- Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Efficacy and safety of immediate‐release methylphenidate treatment for preschoolers with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(11):1284‐93. [DOI: 10.1097/01.chi.0000235077.32661.61] [DOI] [PubMed] [Google Scholar]
Grossman 2013 [pers comm]
- Grossman N. Questions regarding your study 'Methylphenidate and idiopathic thrombocytopenic purpura‐‐is there an association?' [personal communication]. Email to: HB Krogh 12 July 2013.
Gualtieri 2013 [pers comm]
- Gualtieri C. Questions regarding your study 'Tryptophan antagonism of stimulant‐induced tics' [personal communication]. Email to: HB Krogh 13 September 2013.
Guerreiro 2013 [pers comm]
- Guerreiro MM. Inclusion of 'Attention deficit disorder: treatment with methylphenidate' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 4 December 2013.
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383–94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395–400. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407–15. [DOI: 10.1016/j.jclinepi.2010.07.017] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283–93. [DOI: 10.1016/j.jclinepi.2011.01.012] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294–302. [DOI: 10.1016/j.jclinepi.2011.03.017] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303–10. [DOI: 10.1016/j.jclinepi.2011.04.014] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311–6. [DOI: 10.1016/j.jclinepi.2011.06.004] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151–7. [DOI: 10.1016/j.jclinepi.2012.01.006] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158–72. [DOI: 10.1016/j.jclinepi.2012.01.012] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173–83. [DOI: 10.1016/j.jclinepi.2012.08.001] [DOI] [PubMed] [Google Scholar]
Habel 2011
- Habel LA, Cooper WO, Sox CM. ADHD medications and risk of serious cardiovascular events in young and middle‐aged adults. JAMA 2011;306(224):2673‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halevy 2013 [pers comm]
- Halevy A. Questions regarding 'Methylphenidate induction of complex visual hallucinations' [personal communication]. Email to: EL Ramstad 5 August 2013.
Halperin 1984
- Halperin JM, Gittelman R, Klein DF, Rudel RG. Reading‐disabled hyperactive children: a distinct subgroup of attention deficit disorder with hyperactivity?. Journal of Abnormal Child Psychology 1984;12(1):1‐14. [PUBMED: 6715686] [DOI] [PubMed] [Google Scholar]
Hannan 2008
- Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovascular Interventions 2008;1(3):211‐7. [DOI: 10.1016/j.jcin.2008.01.008] [DOI] [PubMed] [Google Scholar]
Hanwella 2011
- Hanwella R, Senanayake Mi, Silva V. Comparative efficacy and acceptability of methylphenidate andatomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta‐analysis. BMC Psychiatry 2011;11(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harsanyi 2013 [pers comm]
- Harsanyi Z. Questions regarding your study 'Once‐daily multilayer‐release methylphenidate in a double‐blind, crossover comparison to immediate‐release methylphenidate in children with attention‐deficit/hyperactivity disorder' [personal communication]. Email to: HB Krogh 27 August 2013.
Heal 2006
- Heal DJ, Pierce DM. Methylphenidate and its isomers: their role in the treatment of attention‐deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs 2006;20(9):713‐38. [PUBMED: 16953648] [DOI] [PubMed] [Google Scholar]
Hechtman 2013 [pers comm]
- Hechtman L. 'Treatment of ADHD in patients unresponsive to methylphenidate' [personal communication]. Email to: HB Krogh 7 August 2013.
Hennessy 2010
- Hennessy S, Schelleman H, Daniel GW, Bilker WB, Kimmel SE, Guevara J, et al. Cardiovascular safety of ADHD medications: rationale for and design of an investigator‐initiated observational study. Pharmacoepidemiology and Drug Safety 2010;19(9):934‐41. [DOI: 10.1002/pds.1992; PUBMED: 20623519] [DOI] [PubMed] [Google Scholar]
Heyman 2013 [pers comm]
- Heyman I. Questions regarding your case report 'Dexamphetamine for obsessive‐ compulsive disorder' [personal communication]. Email to: HB Krogh 27 October 2013.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodgkins 2011
- Hodgkins P, Montejano L, Sasané R, Huse D. Risk of injury associated with attention‐deficit/hyperactivity disorder in adults enrolled in employer‐sponsored health plans: a retrospective analysis. Primary Care Companion for CNS Disorders 2011;13(2):PCC.10m01031. [DOI: 10.4088/PCC.10m01031; PMC3184594; PUBMED: 21977357] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodgkins 2013
- Hodgkins P, Setyawan J, Mitra D, Davis K, Quintera J, Fridman M, et al. Management of ADHD in children across Europe: patient demographics, physician characteristics and treatment patterns. European Journal of Pediatrics 2013;172(7):895‐906. [DOI: 10.1007/s00431-013-1969-8; PMC3701791; PUBMED: 23440479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollis 2013 [pers comm]
- Hollis C. Questions regarding your case report 'Acute dyskinesia on starting methylphenidate after risperidone withdrawal' [personal communication]. Email to: HB Krogh 9 October 2013.
Hong 2014
- Hong SB, Kim JW, Choi BS, Hong YC, Park EJ, Shin MS, et al. Blood manganese levels in relation to comorbid behavioral and emotional problems in children with attention‐deficit/hyperactivity disorder. Psychiatry Research 2014;220(1‐2):418‐25. [PUBMED: 25064383] [DOI] [PubMed] [Google Scholar]
Hong 2015
- Hong SB, Im MH, Kim JW, Park EJ, Shin MS, Kim BN, et al. Environmental lead exposure and attention deficit/hyperactivity disorder symptom domains in a community sample of South Korean school‐age children. Environmental Health Perspectives 2015;123(3):271‐6. [PUBMED: 25280233] [DOI] [PMC free article] [PubMed] [Google Scholar]
Houmann 2013 [pers comm]
- Houmann T. Inclusion of INDICES in a Cochrane systematic review [personal communication] [Inklusion af INDICES i et Cochrane systematisk review]. Email to: EL Ramstad 13 September 2013.
Hulvershorn 2013 [pers comm]
- Hulvershorn LA. Methylphenidate [personal communication]. Email to: EL Ramstad 28 August 2013.
Hungund 2013 [pers comm]
- Hungund A. Questions regarding the study 'Methylphenidate oral dose plasma concentrations and behavioral response in children' [personal communication]. Email to: HB Krogh 11 September 2013.
Huybrechts 2018
- Huybrechts KF, Bröms G, Christensen LB, Einarsdóttir K, Engeland A, Furu K, et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the international pregnancy safety study consortium. JAMA Psychiatry 2018;75(2):167‐75. [DOI: 10.1001/jamapsychiatry.2017.3644; 29238795; PUBMED: PMC5838573] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH 1996
- International Council for Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice E6(R1) 1996. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (accessed 6 May 2011).
Ilgenli 2013 [pers comm]
Indredavik 2004
- Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Fayers P, Brubakk AM. Psychiatric symptoms and disorders in adolescents with low birth weight. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(5):F445‐50. [DOI: 10.1136/adc.2003.038943] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 1998
- Ioannidis JP, Contopoulos‐Ioannidis DG. Reporting of safety data from randomised trials. Lancet 1998;352(9142):1752‐3. [DOI: 10.1016/S0140-6736(05)79825-1; PUBMED: 9848355] [DOI] [PubMed] [Google Scholar]
Ioannidis 2009
- Ioannidis JP. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Archives of Internal Medicine 2009;169(19):1737‐9. [DOI: 10.1001/archinternmed.2009.313; PUBMED: 19858427] [DOI] [PubMed] [Google Scholar]
Irmak 2016 [pers comm]
- Irmak A. Supplemental data (treatment choice in association of ADHD with Williams and Moebius Syndromes) [personal communication]. Email to: MJ Kielsholm 1 June 2016.
Jackson 2016
- Jackson JW. The cardiovascular safety of methylphenidate. BMJ 2016;353:i2874. [DOI: 10.1136/bmj.i2874; PMC4887615; PUBMED: 27245078] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2001
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):147‐58. [DOI: 10.1097/00004583-200102000-00009] [DOI] [PubMed] [Google Scholar]
Jones 2013 [pers comm]
- Jones A. Inquiry to Celgene regarding dexmethylphenidate HCI [personal communication] [Henvendelse til Celgene angående dexmethylphenidat HCl]. Email to: HB Krogh 22 November 2013.
Kadesjö 2001
- Kadesjö B, Gillberg C. The comorbidity of ADHD in the general population of Swedish school‐age children. Journal of Child Psychology and Psychiatry and Allied Disciplines 2001;42(4):487‐92. [DOI: 10.1111/1469-7610.00742] [DOI] [PubMed] [Google Scholar]
Kadesjö 2002
- Kadesjö B. [ADHD hos barn och vuxna]. ADHD in Children and Adults. Stockholm: Socialstyrelsen, 2002. [Google Scholar]
Karabekiroglu 2016 [pers comm]
- Karabekiroglu K. Questions regarding your study 'Can we predict short‐term side effects of methylphenidate immediate‐release?' [personal communication]. Email to: SS Nielsen 9 June 2016.
Kimko 1999
- Kimko HC, Cross JT, Abernethy DR. Pharmacokinetics and clinical effectiveness of methylphenidate. Clinical Pharmacokinetics 1999;37(6):457–70. [PUBMED: 10628897] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Klykylo 2013 [pers comm]
- Klykylo WM. Questions regarding your study 'Medication‐induced seizures' [personal communication]. Email to: HB Krogh 16 September 2013.
Koisaari 2015
- Koisaari T, Michelsson K, Holopainen JM, Maksimainen R, Päivänsalo J, Rantala K, et al. Traffic and criminal behavior of adults with attention deficit‐hyperactivity with a prospective follow‐up from birth to the age of 40 years. Traffic Injury Prevention 2015;16(8):824‐30. [DOI: 10.1080/15389588.2015.1029068; PUBMED: 25837647] [DOI] [PubMed] [Google Scholar]
Kovacs 1992
- Kovacs M. Children's Depression Inventory. North Tonawanda (NY): Multi‐Health Systems, Inc, 1992. [Google Scholar]
Kovess 2015
- Kovess V, Keyes KM, Hamilton A, Pez O, Bitfoi A, Koç C, et al. Maternal smoking and offspring inattention and hyperactivity: results from a cross‐national European survey. European Child and Adolescent Psychiatry 2015;24(8):919‐29. [DOI: 10.1007/s00787-014-0641-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kratochvil 2014 [pers comm]
Lakic 2013 [pers comm]
- Lakic A. Answers to questions [personal communication]. Email to: EL Ramstad 24 October 2013.
Larrañaga‐Fragoso 2016 [pers comm]
Larsson 2014
- Larsson H, Sariaslan A, Långström N, D'Onofrio B, Lichtenstein P. Family income in early childhood and subsequent attention deficit/hyperactivity disorder: a quasi‐experimental study. Journal of Child Psychology and Psychiatry and Allied Disciplines 2014;55(5):428‐35. [PUBMED: 24111650] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leckman 1989
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician‐rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(4):566‐73. [DOI] [PubMed] [Google Scholar]
Lee 2014 [pers comm]
- Lee M. This is more data about BP [personal communication]. Email to: M Holmskov 20 August 2014.
Lewis 2014 [pers comm]
- Lewis J. IQ in glaucoma patient treated with methylphenidate [personal communication]. Email to: OJ Storebø 14 March 2014.
Li 2013 [pers comm]
- Li AY. Questions regarding your study [personal communication]. Email to: HB Krogh 10 April 2013.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichteinstein 2012
- Lichtenstein P, Hallder L, Zetterqvist J, Sjölander A, Serlachius E, Fazel S, et al. Medication for attention deficit‐hyperactivity disorder and criminality. Engl J Med 2012;367(21):2006‐14. [DOI: 10.1056/NEJMoa1203241] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loke 2011
- Loke YK, Price D, Herxheimer A. Chapter 14: Adverse effects. In Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbok.cochrane.org.
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Machado 2013 [pers comm]
- Machado A. Possible inclusion of 'Is methylphenidate‐induced chorea responsive to chlorpromazine?' in a Cochrane systematic review [personal communication]. Email to: A Machado 2 October 2013.
Maia 2017
McLaren 2013 [pers comm]
- McLaren JL. Questions regarding your case study 'Aripiprazole induced acute dystonia after discontinuation of a stimulant medication' [personal communication]. Email to: HB Krogh 17 October 2013.
MHRA 2017
- Medicines and Healthcare Products Regulatory Agency (MHRA). Medicines information: SPC & PILs. www.mhra.gov.uk/Safetyinformation/Medicinesinformation/SPCandPILs/ (accessed 2 May 2017).
Mohammadi 2013 [pers comm]
- Mohammadi MR. Questions regarding 'Amantadine versus methylphenidate in children and adolescents with attention deficit/hyperactivity disorder: a randomized, double‐ blind trial' [personal communication]. Email to: EL Ramstad 9 July 2013.
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2015
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Systematic Reviews 2015;4(1):1‐9. [DOI: 10.1186/2046-4053-4-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montañes‐Rada 2013 [pers comm]
- Montañes‐Rada F. Possible inclusion of '50/50 prolonged‐release methylphenidate (medikinet®) twice daily, prospective open study' in a Cochrane systematic review [personal communication] [Metilfenidato de liberación prolongada 50/50 (medikinet®) dos veces al día,estudio abierto prospectivo]. Email to: EL Ramstad 4 November 2013.
MTA 1999
- MTA Cooperation Group. A 14‐month randomized clinical trial of treatment strategies for attention‐deficit/hyperactivity disorder. The MTA Cooperative Group. Archives of General Psychiatry 1999;56(12):1073‐86. [DOI: 10.1001/archpsyc.56.12.1073] [DOI] [PubMed] [Google Scholar]
Mueller 2016 [pers comm]
- Mueller B. Supplemental information [personal communication]. Email to: ML Kielsholm 15 June 2016.
Munk 2016 [pers comm]
- Munk K. Supplementary data [personal communication] [Supplerende data]. Email to: ML Kielsholm 19 April 2016.
Musonda 2006
- Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self‐controlled case series studies. Statistics in Medicine 2006;25(15):2618‐31. [DOI: 10.1002/sim.2477] [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42.e5. [DOI: 10.1016/j.jclinepi.2013.02.004] [DOI] [PubMed] [Google Scholar]
Myers 1968
- Myers JK, Bean LL. A Decade Later: A Follow‐up of Social Class and Mental Illness. New York (NY): John Wiley & Sons, Inc, 1968. [Google Scholar]
Naranjo 1981
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clinical Pharmacology and Therapeutics 1981;30(2):239‐45. [PUBMED: 7249508] [DOI] [PubMed] [Google Scholar]
NCCMH 2009
- National Collaborating Centre for Mental Health (NCCMH) commissioned by the National Institute for Health and Clinical Excellence. Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. National Clinical Practice Guideline Number 72. www.nccmh.org.uk/downloads/ADHD/CG72FullGuideline.pdf (accessed 11 December 2015).
Neale 2010
- Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, et al. Meta‐analysis of genome‐wide association studies of attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2010;49(9):884‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newcorn 2008
- Newcorn JH. Co‐morbidity in adults with ADHD. CNS Spectrums 2008;13(Suppl 12):12‐5. [DOI: 10.1017/S1092852900003199] [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Collaborating Centre for Mental Health (NCCMH) commissioned by the National Institute for Health and Clinical Excellence. Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. National Clinical Practice Guideline Number 72. www.nice.org.uk/guidance/cg72/resources/attention‐deficit‐hyperactivity‐disorder‐diagnosis‐and‐management‐975625063621 (accessed prior to 15 May 2017).
Niederhofer 2013 [pers comm]
- Niederhofer H. Questions regarding your study 'Combining carbamazepine, neuroleptics and acetylcholinesterase inhibitors with methylphenidate only reduces adverse side effects, but is less effective than a combination with atomoxetine' [personal communication]. Email to: HB Krogh 31 July 2013. [DOI] [PubMed]
Niederhofer 2013b [pers comm]
- Niederhofer H. Questions regarding your study 'Combining carbamazepine, neuroleptics and acetylcholinesterase inhibitors with methylphenidate only reduces adverse side effects, but is less effective than a combination with atomoxetine' [personal communication]. Email to: HB Krogh 31 July 2013. [DOI] [PubMed]
Nigg 2005
- Nigg JT, Casey BJ. An integrative theory of attention‐deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology 2005;17(3):785‐806. [DOI: 10.1017/S0954579405050376] [DOI] [PubMed] [Google Scholar]
Obel 2016
- Obel C, Zhu JL, Olsen J, Breining S, Li J, Grønborg TK, et al. The risk of attention deficit hyperactivity disorder in children exposed to maternal smoking during pregnancy ‐ a reexamination using a sibling design. Journal of Child Psychology and Psychiatry 2016; Vol. 57, issue 4:532‐7. [DOI: 10.1111/jcpp.12478; PUBMED: 26511313] [DOI] [PubMed]
Owens 2000
- Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school‐aged children. Sleep 2000;23(8):1043‐51. [PubMed] [Google Scholar]
Pagsberg 2017
- Pagsberg AK, Jeppesen P, Klauber DG, Jensen KG, Rudå D, Stentebjerg‐Olesen M, et al. Quetiapine extended release versus aripiprazole in children and adolescents with first‐episode psychosis: the multicentre, double‐blind, randomised tolerability and efficacy of antipsychotics (TEA) trial. Lancet Psychiatry 2017;4(8):605‐8. [DOI] [PubMed] [Google Scholar]
Panei 2014 [pers comm]
- Panei P. Questions regarding your study 'The Italian ADHD National Registry' [personal communication]. Email to: HB Krogh 27 Janurary 2014.
Panei 2016 [pers comm]
- Panei P. Questions regarding your study 'Safety of methylphenidate and atomoxetine in children with attention‐deficit/hyperactivity disorder (ADHD): data from the Italian National ADHD Registry' [personal communication]. Email to: HB Krogh 13 June 2016.
Papanikolaou 2004
- Papanikolaou PN, Churchill R, Wahlbeck K, Ioannidis JP. Safety reporting in randomized trials of mental health interventions. American Journal of Psychiatry 2004;161(9):1692‐7. [DOI: 10.1176/appi.ajp.161.9.1692; PUBMED: 15337661] [DOI] [PubMed] [Google Scholar]
Pasini 2007
- Pasini A, Paloscia C, Alessandrelli R, Porfirio MC, Curatolo P. Attention and executive functions profile in drug naive ADHD subtypes. Brain and Development 2007;29(7):400‐8. [PUBMED: 17207595] [DOI] [PubMed] [Google Scholar]
Pelham 1993
- Pelham WE Jr. Pharmacotherapy for children with attention‐deficit hyperactivity disorder. School Psychology Review 1993;22(2):199‐227. [Google Scholar]
Pelham 2001
- Pelham WE, Gnagy EM, Burrows‐Maclean L, Williams A, Fabiano GA, Morrisey SM, et al. Once‐a‐day Concerta methylphenidate versus three‐times‐daily methylphenidate in laboratory and natural settings. Pediatrics 2001;107(6):E105. [DOI: 10.1542/peds.107.6.e105; PUBMED: 11389303] [DOI] [PubMed] [Google Scholar]
Pelham 2005
- Pelham WE, Burrows‐MacLean L, Gnagy EM, Fabiano GA, Coles EK, Tresco KE, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Experimental and Clinical Psychopharmacology 2005;13(2):111‐26. [DOI: 10.1037/1064-1297.13.2.111; PUBMED: 15943544] [DOI] [PubMed] [Google Scholar]
Perera 2016 [pers comm]
- Perera H. Questions regarding your study 'Assessment of outcome of an ADHD treatment program using parent feedback' [personal communication]. Email to: SS Nielsen 1 June 2016.
Perroud 2014
- Perroud N, Cordera P, Zimmermann J, Michalopoulos G, Bancila V, Dayer A, et al. Comorbidity between attention deficit hyperactivity disorder (ADHD) and bipolar disorder in a specialized mood disorders outpatient clinic. Journal of Affective Disorders 2014;168:161‐6. [DOI: 10.1016/j.jad.2014.06.053] [DOI] [PubMed] [Google Scholar]
Pitrou 2009
- Pitrou I, Boutron I, Ahmad N, Ravaud P. Reporting of safety results in published reports of randomized controlled trials. Archives of Internal Medicine 2009;169(19):1756‐61. [DOI: 10.1001/archinternmed.2009.306; PUBMED: 19858432] [DOI] [PubMed] [Google Scholar]
Pliszka 2007a
- Pliszka S, The AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(7):894‐921. [DOI: 10.1097/chi.0b013e318054e724; PUBMED: 17581453] [DOI] [PubMed] [Google Scholar]
Polanczyk 2007a
- Polanczyk G, Rohde LA. Epidemiology of attention‐deficit/hyperactivity disorder across the lifespan. Current Opinion in Psychiatry 2007;20(4):386‐92. [PUBMED: 17551354] [DOI] [PubMed] [Google Scholar]
Polanczyk 2014
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta‐regression analysis. International Journal of Epidemiology 2014;43(2):434‐42. [PUBMED: 24464188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poulton 2013 [pers comm]
Poulton 2013b [pers comm]
- Poulton A. Questions [personal communication]. Email to: H Krogh 18 October 2013.
Pranav 2016 [pers comm]
- Pranav D. Supplemental information [personal communication]. Email to: Kielsholm ML 22 April 2016.
Ramstad 2018
- Ramstad E, Storebø OJ, Nilausen TD, Krog HB, Moreira‐Maia CR, Magnusson FL, Holmskov M, Skoog M, Rosendal S, Groth C, Gillies D, Zwi M, Kirubakaran R, Gluud C, Simonsen E. Hallicunations and other psychotic symptoms in response to methylphenidate in children and adolescents with attention deficit hyperactivity disorder (ADHD): a Cochrane Systematic Review with Meta‐analysis and Trial Sequential Analysis.. Scandinavian Journal of Child and Adolescent Psychiatry and Psychology 2018 [Epub ahead of print];6(1):ns. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rappaport 2013 [pers comm]
- Rappaport N. Psychopharmacology in the school setting [personal communication]. Email to: Krogh HB 11 November 2013.
Rashid 2013 [pers comm]
- Rashid J. Questions regarding 'Methylphenidate and somatic hallucinations' ‐ possible inclusion in a Cochrane systematic review [personal communication]. Email to: Ramstad EL 8 October 2013.
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 14: Including non‐randomized studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 1985
- Reynolds CR, Richmond BO. Revised Children's Manifest Anxiety Scale. RCMAS Manual. Los Angeles (CA): Western Psychological Services, 1985. [Google Scholar]
Ririe 2013 [pers comm]
- Ririe D. Questions regarding your study 'Unexpected interaction of methylphenidate (Ritalin) with anaesthetic agents' [personal communication]. Email to: HB Krogh 15 October 2013.
Roland 2013 [pers comm]
- Roland F. Questions regarding your study 'Comparison of the efficacy of two different modified release methylphenidate preparations for children and adolescents with attention‐deficit/hyperactivity disorder in a natural setting' [personal communication]. Email to: HB Krogh 10 December 2013.
Sabuncuoglu 2013 [pers comm]
- Sabuncuoglu O. Severe adverse events IQ questions [personal communication]. Email to: HB Krogh 11 October 2013.
Saieh 2013 [pers comm]
- Saieh AC. Possible inclusion of 'Methylphenidate: high blood pressure, an effect to take into account' in a Cochrane systematic review [personal communication] [Metilfenidata: hipertensión arterial, un efecto a tener en cuenta]. Emails to: EL Ramstad (23 October, 2 December 2013) 2 December 2013.
Savović 2012
- Savović J, Jones H, Altman D, Harris R, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Scahill 1997
- Scahill L, Riddle MA, McSwiggin‐Hardin M, Ort SI, King RA, Goodman WK, et al. Children's Yale‐Brown Obsessive Compulsive Scale: reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):844‐52. [DOI: 10.1097/00004583-199706000-00023; PUBMED: 9183141] [DOI] [PubMed] [Google Scholar]
Scahill 2000
- Scahill L, Schwab‐Stone M. Epidemiology of ADHD in school‐age children. Child and Adolescent Psychiatric Clinics of North America 2000;9(3):541‐55. [PUBMED: 10944656] [PubMed] [Google Scholar]
Schachar 1997
- Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):754‐63. [DOI: 10.1097/00004583-199706000-00011; PUBMED: 9183129] [DOI] [PubMed] [Google Scholar]
Schmidt 2009
- Schmidt S, Petermann F. Developmental psychopathology: attention deficit hyperactivity disorder (ADHD). BMC Psychiatry 2009;9:58. [DOI: 10.1186/1471-244X-9-58] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroll 2016
- Schroll JB, Penninga EI, Gøtzsche PC. Assessment of adverse events in protocols, clinical study reports, and published papers of trials of Orlistat: a document analysis. PLOS Medicine 2016;13(8):e1002101. [DOI: 10.1371/journal.pmed.1002101; PMC4987052; PUBMED: 27529343] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schubert 2010
- Schubert I, Köster I, Lehmkuhl G. The changing prevalence of attention‐deficit/hyperactivity disorder and methylphenidate prescriptions: a study of data from a random sample of insurees of the AOK Health Insurance Company in the German state of Hesse, 2000–2007. Deutsches Ärzteblatt International 2010;107(36):615‐21. [DOI: 10.3238/arztebl.2010.0615; PMC2947846; PUBMED: 20948775] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2012
- Schulz KP, Fan J, Bédard AC, Clerkin SM, Ivanov I, Tang CY, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention‐deficit/hyperactivity disorder. Archives of General Psychiatry 2012;69(9):952‐61. [DOI: 10.1001/archgenpsychiatry.2011.2053; PUBMED: 22945622] [DOI] [PubMed] [Google Scholar]
Schumock 2016 [pers comm]
- Schumock G. Questions regarding your study 'Drugs associated with adverse events in children and adolescents' [personal communication]. Email to: ML Kielsholm 6 June 2016.
Schwartz 2013 [pers comm]
Scottish Intercollegiate Guidelines Network (SIGN)
- Scottish Intercollegiate Guidelines Network (SIGN). Management of attention deficit and hyperkinetic disorders in children and young people. A national clinical guideline. www.sign.ac.uk/pdf/sign112.pdf (accessed prior to 15 May 2017).
Sergeant 2003
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioural Reviews 2003;27(7):583‐92. [PUBMED: 14624803] [DOI] [PubMed] [Google Scholar]
Shaffer 1996
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab‐Stone ME, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC‐2.3): description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(7):865‐77. [PUBMED: 8768346] [DOI] [PubMed] [Google Scholar]
Shalev 2013 [pers comm]
- Shalev RS. Possible inclusion of 'Hallucinations during methylphenidate therapy' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 24 October 2013.
Shaw 2012
- Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, et al. A systematic review and analysis of long‐term outcomes in attention deficit hyperactivity disorder: effects of treatment and non‐treatment. BMC Medicine 2012;10:99. [DOI: 10.1186/1741-7015-10-99] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shibib 2013 [pers comm]
- Shibib S. Questions regarding 'Stimulant induced psychosis' ‐ possible inclusion in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 28 August to 25 October 2013.
Silverman 2009
- Silverman SL. From randomized controlled trials to observational studies. American Journal of Medicine 2009;122(2):114‐20. [DOI: 10.1016/j.amjmed.2008.09.030; PUBMED: 19185083] [DOI] [PubMed] [Google Scholar]
Sinzig 2013 [pers comm]
- Sinzig J. Questions regarding your study 'Waiver of diagnosis before starting and undergoing therapy with methylphenidate: dangerous or justified' [personal communication] [Verzicht auf eine diagnostik vor beginn und unter einer therapie mit methylphenidat: gefärlich oder gerechtfertigt?]. Email to: HB Krogh 19 December 2013.
Skoog 2015
- Skoog M, Saarimäki JM, Gluud C, Sheinin M, Erlendsson K, Aamdal S, et al. Transparency and registration in clinical research in the Nordic countries. NordicTrial Alliance, NordForsk 2015:1‐108.
Smeeth 2013 [pers comm]
- Smeeth L. Questions regarding your abstract 'Spontaneous report safety signals profile and comparative Bayesian analysis of ADHD medication in children in the UK' [personal communication]. Email to: HB Krogh 14 October 2013.
Sobanski 2014 [pers comm]
Solanto 1998
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention‐deficit hyperactivity disorder: a review and integration. Behavioural Brain Research 1998;94(1):127‐52. [DOI: 10.1016/S0166-4328(97)00175-7] [DOI] [PubMed] [Google Scholar]
Soyata 2016 [pers comm]
- Soyata A. Supplemental information [personal communication]. Email to: MJ Kielsholm 22 April 2016.
Sterne 2014
- Sterne JAC, Higgins JPT, Reeves BC, on behalf of the development group for ACROBAT‐NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomised Studies of Interventions (ACROBAT‐NRSI), Version 1.0.0, 24 September 2014. www.riskofbias.info (accessed 25 May 2015).
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016; Vol. 355, issue i4919. [DOI: 10.1136/bmj.i4919; PMC5062054; PUBMED: 27733354] [DOI] [PMC free article] [PubMed]
Stevenson 1989
- Stevenson RD, Wolraich ML. Stimulant medication therapy in the treatment of children with attention deficit hyperactivity disorder. Pediatric Clinics of North America 1989;36(5):1183‐97. [PUBMED: 2677938] [DOI] [PubMed] [Google Scholar]
Stopper 2013 [pers comm]
- Stopper H. Question regarding your study 'Does methylphenidate cause a cytogenetic effect in children with attention deficit hyperactivity disorder?' [personal communication]. Email to: EL Ramstad 10 October 2013.
Stopper 2014 [pers comm]
- Stopper H. Questions regarding your study 'Does methylphenidate cause a cytogenetic effect in children with attention deficit hyperactivity disorder?' for a Cochrane Systematic Review [personal communication]. Email to: HB Krogh 4 August 2014.
Stopper 2014b [pers comm]
- Stopper H. AW: Questions regarding your study. Email to: HB Krogh 21 March 2014.
Storebø 2015
- Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD009885.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swanson 2003
- Swanson JM, Gupta S, Lam A, Shoulson I, Lerner MA, Modi N, et al. Development of a new once‐a‐day formulation of methylphenidate for the treatment of attention‐deficit/hyperactivity disorder: proof‐of‐concept and proof‐of‐product studies. Archives of General Psychiatry 2003;60(2):204‐11. [PUBMED: 12578439] [DOI] [PubMed] [Google Scholar]
Swanson 2004
- Swanson JM, Wigal SB, Wigal T, Sonuga‐Barke E, Greenhill LL, Biederman J, et al. A comparison of once‐daily extended‐release methylphenidate formulations in children with attention‐deficit/hyperactivity disorder in the laboratory school (the Comacs study). Pediatrics 2004;113(3 Pt 1):e206‐16. [PUBMED: 14993578] [DOI] [PubMed] [Google Scholar]
Swanson 2009
- Swanson J. The MTA follow‐up into adolescence: implications for personalized treatment. International Society for Research in Child and Adolescent Psychopathology (ISRCAP); 2009 June 19, Seattle. 2009.
Tasdelen 2016 [pers comm]
- Tasdelen BI. Supplemental information [personal communication]. Email to: SS Nielsen 16 June 2016.
Thomas 2015
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention‐deficit/hyperactivity disorder: a systematic review and meta‐analysis. Pediatrics 2015;135(4):e994‐e1001. [DOI: 10.1542/peds.2014-3482] [DOI] [PubMed] [Google Scholar]
Thorell 2013 [pers comm]
- Thorell L. Possible inclusion of 'Children's self‐reports on perceived effects on taking stimulant medication for ADHD' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 17 October 2013.
Tomás 2013 [pers comm]
- Tomás Vila M. Questions regarding 'Visual hallucinations caused by methylphenidate' ‐ possible inclusion in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 9 September 2013.
Tomás 2013b [pers comm]
- Tomás Vila M. Possible inclusion of 'Methylphenidate and sleep: Results of a multicentre study on a population of children with attention deficit hyperactivity disorder' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 30 October 2013.
Trecenõ 2012
- Treceño C, Martín Arias LH, Sáinz M, Salado I, García Ortega P, Velasco V, et al. Trends in the consumption of attention deficit hyperactivity disorder medications in Castilla y León (Spain): changes in the consumption pattern following the introduction of extended release methylphenidate. Pharmacoepidemiology and Drug Safety 2012;21(4):435–41. [DOI: 10.1002/pds.2348; PUBMED: 22253017] [DOI] [PubMed] [Google Scholar]
Trugman 2013 [pers comm]
- Trugman J. Questions regarding your case report 'Cerebral arteritis and oral methylphenidate' [personal communication]. Email to: HB Krogh 27 September 2013.
Tølløfsrud 2013 [pers comm]
- Tølløfsrud C. About the article 'Young man with acute dilated cardiomyopathy' [personal communication] [Ung mann med akutt dilatert kardiomyapati]. Email to: HB Krogh 24 November 2013.
US FDA 2011
- US Food, Drug Administration (FDA). Communication about an ongoing safety review of stimulant medications used in children with attention‐deficit/hyperactivity disorder (ADHD). 1.usa.gov/1M5AYoq (accessed 6 May 2011).
US Food and Drug Administration (FDA) 2017
- US Food, Drug Administration (FDA). FDA approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm (accessed 14 May 2017).
Valdizán 2014 [pers comm]
- Valdizán Usón JR. Regarding your study 'Response to methylphenidate by adult and pediatric patients with attention‐deficit/hyperactivity disorder: the Spanish multicenter DIHANA study' [personal communication]. Email to: M Holmskov 10 July 2014.
Van der Oord 2014 [pers comm]
- Oord S. Regarding your study 'Does brief, clinically based, intensive multimodal behavior therapy enhance the effects of methylphenidate in children with ADHD?' [personal communication]. Email to: TD Nilausen 2 January 2014.
Van Lieshout 2015
- Lieshout RJ, Boyle MH, Saigal S, Morrison K, Schmidt LA. Mental health of extremely low birth weight survivors in their 30s. Pediatrics 2015;135(3):452‐9. [PUBMED: 25667243] [DOI] [PubMed] [Google Scholar]
Vandenbroucke 2006
- Vandenbroucke JP. What is the best evidence for determining harms of medical treatment?. Canadian Medical Association Journal 2006;174(5):645‐6. [DOI: 10.1503/cmaj.051484; PMC1389828; PUBMED: 16505461] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vashi 2013 [pers comm]
- Vashi N. Questions regarding your article 'Allergic contact dermatitis caused by methylphenidate' [personal communication]. Email to: HB Krogh 1 August 2013.
Volkow 2012
- Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, et al. Methylphenidate‐elicited dopamine increases in ventral striatum are associated with long‐term symptom improvement in adults with attention deficit hyperactivity disorder. Journal of Neuroscience 2012;32(3):841‐9. [PUBMED: 22262882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013 [pers comm]
- Wang LJ. Regarding two of your studies from 2011 [personal communication]. Email to: TD Nilausen 21 December 2013.
Weber 2013 [pers comm]
- Weber P. Questions regarding your study 'Nebenwirkungen einer Methylphenidat‐Therapie bei Schulkindern' [personal communication]. Email to: HB Krogh 4 December 2013.
WHO 1977
- World Health Organization. International Classification of Diseases, Ninth Revision. Vol. 1, Geneva: World Health Organization, 1977. [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. [Google Scholar]
Willcut 2012
- Willcutt EG. The prevalence of DSM‐IV attention‐deficit/hyperactivity disorder: a meta‐analytic review. Neurotherapeutics 2012;9(3):490‐9. [DOI: 10.1007/s13311-012-0135-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2014 [pers comm]
- Williams L. Regarding your study 'Misinterpreting emotional expressions in attention‐deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects' [personal communication]. Email to: M Holmskov 26 August 2014.
Witt 2014 [pers comm]
- Witt KL. Questions regarding your study 'Methylphenidate and amphetamine do not induce cytogenetic damage in lymphocytes of children with ADHD' [personal communication]. Email to: FL Magnusson 9 July 2014.
Wolraich 2001
- Wolraich ML, Greenhill LL, Pelham W, Swanson J, Wilens T, Palumbo D, et al. Randomized, controlled trial of OROS methylphenidate once a day in children with attention‐deficit/hyperactivity disorder. Pediatrics 2001;108(4):883‐92. [PUBMED: 11581440] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yalcin 2013 [pers comm]
- Yalcin O. Interest in including 'Possible methylphenidate related hoarseness and disturbances of voice quality: two pediatric cases' in a Cochrane systematic review [personal communication]. Email to: EL Ramstad 27 March 2013.
Yilmaz 2013 [pers comm]
- Yilmaz AE. Question regarding your study 'Methylphenidate‐induced orofacial and extremity dyskinesia' [personal communication]. Email to: T Nilausen 20 December 2013.
Yook 2013 [pers comm]
- Yook KH. Questions regarding 'Oobesity is associated with non‐response to prolonged‐release methylphenidate treatment in ADHD' [personal communication]. Email to: EL Ramstad 20 August 2013.
Yoshimasu 2012
- Yoshimasu K, Barbaresi WJ, Colligan RC, Voigt RG, Killian JM, Weaver AL, et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders: a population‐based birth cohort study. Journal of Child Psychology and Psychiatry and Allied Disciplines 2012;53(10):1036‐43. [PUBMED: 22647074] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2013 [pers comm]
- Yu Z. Questions regarding your article 'Peripheral vasculopathy associated with psychostimulant treatment in children with attention‐deficit/hyperactivity disorder' [personal communication]. Email to: HB Krogh 16 October 2013.
Zeni 2016 [pers comm]
- Zeni C. Regarding your study 'No significant association between response to methylphenidate and genes of the dopaminergic and serotonergic systems in a sample of Brazilian children with attention‐deficit/hyperactivity disorder' [personal communication]. Email to: CR Moreira Maia 14 September 2016.
Zoëga 2016
- Zoëga H, Furu M, Halldórsson M, Thomsen PH, Sourander A, Martikainen JE. Use of ADHD drugs in the Nordic countries: a population‐based comparison study. Acta Psychiatrica Scandinavica May 2011;123(5):360‐7. [DOI: 10.1111/j.1600-0447.2010.01607.x; PUBMED: 20860726] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Storebø 2016
- Storebø OJ, Pedersen N, Ramstad E, Krogh HB, Moreira‐Maia CR, Magnusson FL, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents ‐ assessment of harmful effects in non‐randomised studies. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012069] [DOI] [PMC free article] [PubMed] [Google Scholar]


