Abstract
Background
Edentulism is relatively common and is often treated with the provision of complete or partial removable dentures. Clinicians make final impressions of complete dentures (CD) and removable partial dentures (RPD) using different techniques and materials. Applying the correct impression technique and material, based on an individual's oral condition, improves the quality of the prosthesis, which may improve quality of life.
Objectives
To assess the effects of different final‐impression techniques and materials used to make complete dentures, for retention, stability, comfort, and quality of life in completely edentulous people.
To assess the effects of different final‐impression techniques and materials used to make removable partial dentures, for stability, comfort, overextension, and quality of life in partially edentulous people.
Search methods
Cochrane Oral Health’s Information Specialist searched the following databases: Cochrane Oral Health’s Trials Register (to 22 November 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Register of Studies, to 22 November 2017), MEDLINE Ovid (1946 to 22 November 2017), and Embase Ovid (21 December 2015 to 22 November 2017). The US National Institutes of Health Trials Registry (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. No restrictions were placed on language or publication status when searching the electronic databases, however the search of Embase was restricted by date due to the Cochrane Centralised Search Project to identify all clinical trials and add them to CENTRAL.
Selection criteria
We included randomised controlled trials (RCTs) comparing different final‐impression techniques and materials for treating people with complete dentures (CD) and removable partial dentures (RPD). For CD, we included trials that compared different materials or different techniques or both. In RPD for tooth‐supported conditions, we included trials comparing the same material and different techniques, or different materials and the same technique. In tooth‐ and tissue‐supported RPD, we included trials comparing the same material and different dual‐impression techniques, and different materials with different dual‐impression techniques.
Data collection and analysis
Two review authors independently, and in duplicate, screened studies for eligibility, extracted data, and assessed the risk of bias for each included trial. We expressed results as risk ratios (RR) for dichotomous outcomes, and as mean differences (MD) or standardised mean differences (SMD) for continuous outcomes, with 95% confidence intervals (CI), using the random‐effects model. We constructed 'Summary of findings' tables for the main comparisons and outcomes (participant‐reported oral health‐related quality of life, quality of the denture, and denture border adjustments).
Main results
We included nine studies in this review. Eight studies involved 485 participants with CD. We assessed six of the studies to be at high risk of bias, and two to be at low risk of bias. We judged one study on RPD with 72 randomised participants to be at high risk of bias.
Overall, the quality of the evidence for each comparison and outcome was either low or very low, therefore, results should be interpreted with caution, as future research is likely to change the findings.
Complete dentures
Two studies compared the same material and different techniques (one study contributed data to a secondary outcome only); two studies compared the same technique and different materials; and four studies compared different materials and techniques.
One study (10 participants) evaluated two stage–two step, Biofunctional Prosthetic system (BPS) using additional silicone elastomer compared to conventional methods, and found no evidence of a clear difference for oral health‐related quality of life, or quality of the dentures (denture satisfaction). The study reported that BPS required fewer adjustments. We assessed the quality of the evidence as very low.
One study (27 participants) compared selective pressure final‐impression technique using wax versus polysulfide elastomeric (rubber) material. The study did not measure quality of life or dentures, and found no evidence of a clear difference between interventions in the need for adjustments (RR 0.81, 95% CI 0.38 to 1.70). We assessed the quality of the evidence as very low.
One study compared two stage–two step final impression with alginate versus silicone elastomer. Oral health‐related quality of life measured by the OHIP‐EDENT seemed to be better with silicone (MD 7.20, 95% CI 2.71 to 11.69; 144 participants). The study found no clear differences in participant‐reported quality of the denture (comfort) after a two‐week 'confirmation' period, but reported that silicone was better for stability and chewing efficiency. We assessed the quality of the evidence as low.
Three studies compared single‐stage impressions with alginate versus two stage‐two step with elastomer (silicone, polysulfide, or polyether) impressions. There was no evidence of a clear difference in the OHIP‐EDENT at one month (MD 0.05, 95% CI ‐2.37 to 2.47; two studies, 98 participants). There was no evidence of a clear difference in participant‐rated general satisfaction with dentures at six months (MD 0.00, 95% CI ‐8.23 to 8.23; one study, 105 participants). We assessed the quality of the evidence as very low.
One study compared single‐stage alginate versus two stage‐two step using zinc‐oxide eugenol, and found no evidence of a clear difference in OHIP‐EDENT (MD 0.50, 95% CI ‐2.67 to 3.67; 39 participants), or general satisfaction (RR 3.15, 95% CI 0.14 to 72.88; 39 participants) at six months. We assessed the quality of the evidence as very low.
Removable partial dentures
One study randomised 72 participants and compared altered‐cast technique versus one‐piece cast technique. The study did not measure quality of life, but reported that most participants were satisfied with the dentures and there was no evidence of any clear difference between groups for general satisfaction at one‐year follow‐up (low‐quality evidence). There was no evidence of a clear difference in number of intaglio adjustments at one year (RR 1.43, 95% CI 0.61 to 3.34) (very low‐quality evidence).
Authors' conclusions
We conclude that there is no clear evidence that one technique or material has a substantial advantage over another for making complete dentures and removable partial dentures. Available evidence for the relative benefits of different denture fabrication techniques and final‐impression materials is limited and is of low or very low quality. More high‐quality RCTs are required.
Keywords: Humans; Dental Impression Materials; Dental Impression Technique; Denture, Partial, Removable; Dentures; Denture Design; Denture Design/methods; Denture Retention; Denture Retention/methods; Mouth, Edentulous; Mouth, Edentulous/rehabilitation; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Techniques and materials for final impressions when making complete and partial removable dentures
Review question
In this review, conducted through Cochrane Oral Health, our aim was to evaluate which technique and material should be used for the final impression when making complete and partial removable dentures, to increase the quality of the denture, and improve oral health‐related quality of life for the individual.
Background
It is common for elderly people to have lost some, or all of their teeth (edentulism). This has a significant impact on their quality of life. There are several steps to making complete and removable partial dentures. The final impression is a very important step for ensuring the quality of the denture in terms of satisfaction, comfort, stability of the denture, and chewing ability. There are a number of different techniques and materials used for making the final impression for complete dentures or removable partial dentures. There is no consensus on which are the best.
Study characteristics
The evidence in this review is current to 22 November 2017. We found eight studies with a total of 485 participants for complete dentures, and one study with 72 participants for removable partial dentures. The participants ranged from 45 to 75 years old, and had been without their teeth for 10 to 35 years. The studies compared different materials used to make the final impression for dentures (alginate, zinc‐oxide eugenol, wax, and addtional silicone, polysulfide or polyether) and different techniques for making the final impression (open‐mouth versus closed‐mouth, single‐stage versus two stage‐two step), or both.
Key results
For most comparisons and outcomes, there was no evidence of a clear difference between the techniques or materials compared.
Very low quality‐evidence from one study (10 participants) suggested that making dentures with an additional silicone elastomer biofunctional prosthetic required fewer adjustments than conventional methods.
Low‐quality evidence from another study (144 participants) suggested that complete dentures made with silicone elastomer in a two stage–two step final impression, may be better than those made with alginate, in terms of oral health‐related quality of life, stability of the denture, and chewing efficiency.
With the limited evidence available, we are unable to draw any conclusions about the best impression techniques and materials for complete and partial removable dentures. There is a need for further research in this area.
Quality of the evidence
The quality of the evidence base overall is low to very low. Only one or two studies assessed each intervention and comparison, and most of the studies were at high risk of bias. Many of the studies did not measure our key outcomes. For both complete and partial removable dentures, we conclude that we have no reliable findings.
Summary of findings
Summary of findings for the main comparison. Complete dentures: same materials, different final‐impression techniques.
| BPS versus CCD techniques for making dentures for completely edentulous people | ||||||
|
Population: completely edentulous people Setting: university department of prosthodontics Intervention: biofunctional prosthetic system (Accu‐dent System) Comparison: traditional technique | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number pf participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with selective pressure | |||||
| Participant‐reported oral health‐related quality of life (OHIP‐EDENT) Follow‐up: 3 months |
10 (1 RCT) | ⊕⊝⊝⊝ very low1 | OHIP‐EDENT median scores: BPS 34.5; CCD 35.8. No clear difference between groups2 | |||
| Participant‐reported quality of the denture ‐ denture satisfaction Follow‐up: 3 months |
10 (1 RCT) | ⊕⊝⊝⊝ very low1 | VAS median scores: BPS 86.5; CCD 88. No clear difference between groups2 | |||
| Number of border adjustments and sore spots after insertion of denture Follow‐up: 3 months |
10 (1 RCT) | ⊕⊝⊝⊝ very low1 | Median number of denture adjustments: BPS 3.5; CCD 4.5. BPS required fewer adjustments2 | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio; BPS: closed mouth two stage‐two step with addition silicone elastomer (Biofunctional Prosthetic System); CCD: open mouth two stage‐two step conventional technique using elastomer; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: We are confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of the evidence by one level for high risk of bias and two levels for sparse data
2 Data were taken directly from the published study report
Summary of findings 2. Complete dentures: same technique, different final‐impression materials.
| Wax versus polysulfide rubber for making dentures for completely edentulous people | ||||||
|
Population: completely edentulous people Setting: university dental clinic Intervention: wax Comparison: polysulfide rubber | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with rubber | Risk with wax | |||||
| Participant‐reported oral health‐related quality of life (OHIP‐EDENT) | Not measured | |||||
| Participant‐reported quality of the denture | Not measured | |||||
| Number of border adjustments and sore spots after insertion of denture Follow‐up: one year |
571 per 1000 | 463 per 1000 (217 to 971) | RR 0.81 (0.38 to 1.70) | 27 (1 RCT) | ⊕⊝⊝⊝ very low1 2 3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded for risk of bias due to the single study contributing data for this outcome being at unclear risk of bias in many domains
2 Downgraded for imprecision due to the wide 95% CI starting from 0.38 highly beneficial to 1.70 no benefit.
3 Downgraded for indirectness due to the single study with only 27 participants. Hence generalisation becomes difficult.
Summary of findings 3. Complete dentures: same technique, different final‐impression materials.
| Alginate versus silicone for making dentures for completely edentulous people | ||||||
|
Population: completely edentulous people Setting: university dental clinic Intervention: alginate Comparison: silicone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with silicone | Risk with alginate | |||||
| Participant‐reported oral health‐related quality of life (OHIP‐ EDENT) (low score better oral health) Follow‐up: 2 weeks |
Mean score 28.9 | MD 7.2 higher on average (2.71 higher to 11.69 higher) | ‐ | 144 (1 RCT) | ⊕⊕⊝⊝ low1, 2 | |
| Participant‐reported quality of the denture Follow‐up: two weeks |
The study reported that there was no difference between groups for comfort, but that more participants reported better stability and chewing efficiency with silicone. Data were not amenable to analysis | |||||
| Number of border adjustments and sore spots after insertion of denture Follow‐up: two weeks |
Not measured | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded for indirectness as it is the only study that has used alginate as final (wash) impression hence it cannot be generalised.
2 Downgraded for imprecision as the 95% CI is wide and includes the possibility of the mean difference being clinically unimportant.
Summary of findings 4. Complete dentures: different techniques, different materials.
| Single stage with alginate versus two stage‐two step elastomer for making dentures for completely edentulous people | ||||||
|
Population: completely edentulous people Setting: university dental clinic Intervention: single stage with alginate Comparison: two stage‐two step with elastomer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with elastomer | Risk with alginate | |||||
| Participant‐reported oral health‐related quality of life (OHIP‐EDENT) Follow‐up: 1 month | Mean (OHIP‐EDENT) score was 18 | MD 0.05 higher on average (2.37 lower to 2.47 higher) | ‐ | 98 (2 RCTs) | ⊕⊝⊝⊝ very low1 2 3 | |
| Participant‐reported quality of the denture ‐ general satisfaction Follow‐up: 6 months | Mean general satisfaction was 79 | MD 0 on average (8.23 lower to 8.23 higher) | ‐ | 105 (1 RCT) | ⊕⊝⊝⊝ very low2 3 4 | Satisfaction with maxillary and mandibular dentures were also assessed separately by two studies (155 participants), with no evidence of a difference in satisfaction between groups at six months. |
| Number of border adjustments and sore spots after insertion of denture | Not measured | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded for imprecision due to the wide 95% CI
2 Downgraded for indirectness due to study participants with long period of edentulousness (mean range 24 to 38 years) and poor prognostic factors for success of denture in both groups (ACP classification was at least 69% to 73% in ACP III and ACP IV)
3 Downgraded for risk of bias as allocation concealment was at unclear risk of bias and clinicians were not blinded.
4 Downgraded for imprecision due to the wide 95% CI. Single study with 105 participants
Summary of findings 5. Complete dentures: different techniques, different materials.
| Single‐stage with alginate versus two step‐two stage with ZoE for making dentures for completely edentulous people | ||||||
|
Population: completely edentulous people Setting: dental school hospital Intervention: single‐stage with alginate Comparison: two step‐two stage with ZoE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with ZoE | Risk with alginate | |||||
| Participant‐reported oral health‐related quality of life (OHIP‐EDENT) Follow‐up: 6 months | Mean OHIP‐EDENT score 5.5 |
MD 0.5 higher (2.67 lower to 3.67 higher) | ‐ | 39 (1 RCT) |
⊕⊝⊝⊝ very low1 2 | |
| Participant‐reported quality of the denture: general satisfaction Follow‐up: 6 months | 25 per 1000 | 79 per 1000 (4 to 1000) | RR 3.15 (0.14 to 72.88) | 39 (1 RCT) | ⊕⊝⊝⊝ very low1 2 | |
| Number of border adjustments and sore spots after insertion of denture | Not measured | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio; OR: odds ratio. ZoE: zinc‐oxide eugenol | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded for indirectness due to the single study with only 39 participants included in this trial. Therefore, it is difficult to generalise.
2 Downgraded twice for serious imprecision, small sample size and wide 95% CI.
Summary of findings 6. Removable partial dentures. Tooth‐tissue‐supported conditions: same material, different dual‐impression techniques.
| Altered cast compared with one‐piece cast (polyether) for final impression | ||||||
| Population: people requiring removable partial dentures Setting: university dental clinic Intervention: altered cast Comparison: one‐piece cast | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Participant‐reported oral health‐related quality of life | Not measured | |||||
| Participant‐reported quality of the denture: general satisfaction | The study reported that 50 of 57 participants were moderately to completely satisfied, with no significant difference between the groups. Data not reported separately for each group. | 57 (1) | ⊕⊕⊝⊝ low1 2 | |||
| Number of intaglio adjustments Follow up: one year |
194 per 1000 | 278 per 1000 (119 to 649) |
RR 1.43 (0.61 to 3.34) |
72 (1) | ⊕⊝⊝⊝ very low1 2 3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level as allocation concealment was unclear and there was a high risk of bias for incomplete outcome data and blinding of personnel
2 Downgraded one level as a single study contributed data for this outcome
3 Downgraded one level because 95% CI is wide
Background
Description of the condition
The increase in life expectancy in both high‐income and low‐income countries could result in the global population over 60 years of age surpassing two billion by 2050 (United Nations 2013). Edentulism is among the 50 most common diseases, affecting 2.3% of the total global population in 2010 (Vos 2012). The prevalence of partially and completely edentulous people is likely to increase, as the risk of tooth loss increases with age (Urzua 2012).
Complete edentulism is a chronic and irreversible condition, having a major impact on the oral and general health of an individual (Atwood 1971; Gift 1992). The global prevalence of complete edentulousness ranges from about 3% to 21%, and varies depending on age, sex, socioeconomic status, education, dental awareness, patient to dentist ratio, and demography (Cunha‐Cruz 2007; Peltzer 2014; Steele 2012). This condition affects diet and nutritional status (Hutton 2002; Lee 2004), and people who are edentulous may have comorbid conditions that make it difficult to adapt to complete dentures as they age (Emami 2013). The only cost‐effective, non‐implant treatment to restore dentition is complete dentures (MacEntee 1998). A complete denture is defined as "a fixed or removable dental prosthesis that replaces the entire dentition and associated structures of the maxillae or mandible" (GPT 2017).
Partial edentulousness is more prevalent than complete edentulousness (Jeyapalan 2015; Slade 2014; Tanasić 2015). Loss of teeth correlates with an increase in obesity and a decrease in nutritional status, psychological self‐image, and quality of life (Emami 2013; Friedman 2014; Gil‐Montoya 2015; Goel 2016; Hilgert 2009; Huang 2013; Hugo 2009; Kandelman 2008; Rodrigues 2012; Roohafza 2015). The partial loss of teeth may be replaced by fixed or removable treatment options based on number of teeth lost and condition of the residual ridge.
Description of the intervention
Complete dentures
A dental impression is defined as "a negative imprint or a positive digital image display of intraoral anatomy used to cast or print a 3D replica of the anatomic structure that is to be used as a permanent record or in the production of a dental restoration or prosthesis" (GPT 2017). Dental practitioners can make the impression in a single stage (abbreviated impression) or in two stages (preliminary impression made for the purposes of diagnosis, or for the construction of a tray, followed by final impression) (Trapozzano 1939). The final‐impression techniques and materials used for complete dentures date back to 1900s (Paulino 2015; Zinner 1981). They make the impression using an open‐mouth or closed‐mouth approach, in one or two steps (Boucher 1951). In the single‐step procedure, border moulding and recording the final impression are performed simultaneously, using the same material, either a resinous wax, or a monophase elastomer (Joglekar 1968; Loh 1997; Minagi 1987). The two‐step final‐impression technique begins with border moulding, followed by a final‐impression procedure (Chaffee 1999; Friedman 1957; Smith 1979). Border moulding is defined as "the shaping of impression material along the border areas of an impression tray by functional or manual manipulation of the soft tissues adjacent to the borders to duplicate the contour and size of the vestibule". It is also defined as determining the extension of a prosthesis, by using tissue function or manual manipulation of the tissues to shape the border area of an impression material (GPT 2017). It can be accomplished by using either a sectional or a single‐step technique, using different types of materials. These techniques may be further classified as operator‐manipulated, or functionally moulded, based on the condition of the ridge and operator's preference. In terms of materials, the sectional technique involves border moulding in sections using a low‐fusing impression compound (Friedman 1957). The single‐step border moulding technique uses polyether, and addition silicone of differing viscosities (Chaffee 1999; Smith 1979; Solomon 1973; Solomon 2011).
Clinicians can make the final impression (sometimes referred to as the wash impression) for complete dentures using different techniques and materials (Starcke 1975). These have evolved along with our understanding of the biology of the tissues, and advances in available impression materials. The techniques can be grouped into mucostatic, mucocompressive, selective pressure, functional, and neutral zone impression techniques (Addison 1944; Beresin 1976; Boucher 1943; Cagna 2009; Freeman 1969; Solomon 1973; (Figure 1)). The impression materials used are impression plaster, resinous wax, zinc‐oxide eugenol impression paste, alginate, polysulfide, addition silicone, and polyether (Boucher 1951; Daou 2010; Joglekar 1968; Koran 1977; Mehra 2014; Trapozzano 1939). See Figure 2.
1.
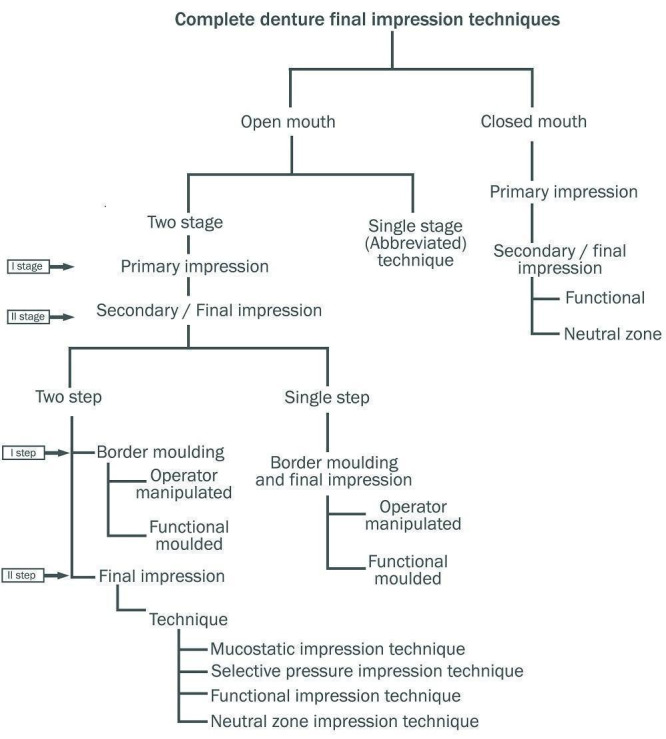
Complete denture final impression techniques (Al‐Ahmar 2008; Drago 2003; Freeman 1969; Paulino 2015; Petropoulos 2003)
2.
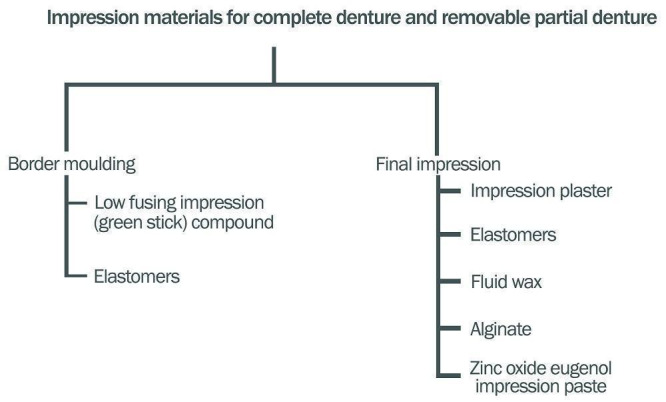
Impression materials for complete denture and removable partial denture (Freeman 1969; Phoenix 2008)
Removable partial dentures
The distribution of occlusal forces varies, based on the condition of the partially edentulous state, in removable cast partial dentures (RPD). In a tooth‐supported partial denture, the occlusal forces are mainly distributed to the abutment teeth rather than the edentulous ridge, so the final impression is used to record the tissues in their anatomic state, in order to produce an accurate master cast (Applegate 1960; Leupold 1966). The materials and techniques used for recording the final impression in tooth‐supported conditions are alginates and elastomers, with either a custom or a stock tray. In tooth‐ and tissue‐supported partial dentures, a special or dual impression procedure is indicated, due to the relative discrepancy in the degree of movement that occurs between the tooth and mucosa covering the ridge, in response to occlusal forces (Hindels 1957). The different techniques are classified into physiologic and selective pressure impression techniques (Phoenix 2008). The physiologic impression techniques are the McLean‐Hindels technique, the functional reline method, and the fluid wax impression (altered cast) techniques (see Figure 3). In the selective pressure technique, the ridge is selectively relieved to redirect forces to stress‐bearing areas during impression making (Akerly 1978; Applegate 1937; Applegate 1960; Hindels 1957; Leupold 1965; McLean 1936; Sajjan 2010; Santana‐Penin 1998).
3.
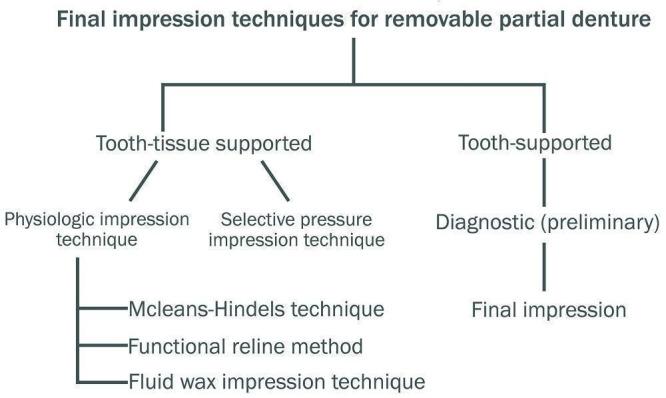
Final impression techniques for removable partial denture (Phoenix 2008)
How the intervention might work
Complete dentures
The ultimate goal of the removable prosthesis is to maintain oral health, function, aesthetics, comfort, and psychological well‐being of the patient (Bell 1968). To achieve these goals, it is essential to obtain an accurate recording of the denture base foundation within functional and physiologically tolerable limits of the tissue (Drago 2003; El‐Khodary 1985; Massad 2005). Of the five cardinal objectives of an impression, the two main factors that prevent dislodgement of the dentures and increase chewing efficiency are retention and stability (Boucher 1944, Friedman 1957; Jacobson 1983a; Jacobson 1983b). Loss of these qualities lead to a decrease in denture efficiency, thereby reducing comfort, mastication, speech, self‐esteem, and patient satisfaction (Silva 2014). Retention and stability are directly related to a patient's compliance in wearing the dentures. Denture‐related problems can be due to patient‐ or dentist‐related factors, or processing errors (Critchlow 2011). Complaints about dentures are most often due to faulty design, so are dentist‐, rather than patient‐related (Brunello 1998; Laurina 2006). The most common denture‐related problems are insufficient retention and improper jaw relations; both are directly and indirectly related to the final‐impression technique, and the material used to make the dentures (Kotkin 1985).
Removable partial dentures
Cast partial removable dental prostheses are based on the theory of broad stress distribution, and aim to preserve the remaining dentition (DeVan 1952; Steffel 1951). In a distal extension, partially edentulous situation (tooth‐ and tissue‐supported conditions), a destructive class‐I lever is created, because of the compressibility of the mucosa of the edentulous ridge relative to the remaining tooth under occlusal load, which tends to overload the abutment tooth (Holmes 1965; Leupold 1966). The various dual final‐impression techniques used to make cast partial dentures and semi‐precision attachments, help reduce the transfer of excessive stress to the abutment tooth during occlusal loading, thereby improving support, and preserving the health of the remaining oral tissues (Blatterfein 1980; Leupold 1966). Hence, the choice of final‐impression material and technique is very important.
Why it is important to do this review
Treatment with complete or partial dentures involves multiple steps, some of which are crucial for success. One such step is the final‐impression procedure. Retention, stability, support, chewing efficiency, patient comfort, and overall satisfaction depend on the correct recording of the final impression during the making of complete and partial dentures (Cunha 2013). There are no evidence‐based clinical practice guidelines for the fabrication of removable dental prostheses to inform policy makers, healthcare providers, patients, or the public (Owen 2006). There are narrative reviews, but to date, no systematic review with meta‐analysis has provided evidence to guide the selection of one material, technique, or both, over another, for edentulous people (Carlsson 2013; Daou 2010; Rao 2010; Zinner 1981).
Objectives
To assess the effects of different final‐impression techniques and materials used to make complete dentures for retention, stability, comfort, and quality of life in completely edentulous people.
To assess the effects of different final‐impression techniques and materials used to make removable partial dentures for stability, comfort, overextension, and quality of life in partially edentulous people.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and cross‐over trials in any language that dealt with impression making for both complete dentures and removable partial dentures.
Types of participants
Complete dentures
Participants who were completely edentulous, and had undergone treatment for complete dentures in both arches, regardless of age, sex, and socioeconomic status.
Participants with a complete denture in either the upper or lower jaw, if outcomes were reported for that particular arch.
We excluded participants with implant‐supported or retained prosthesis, as well as overdentures and immediate denture prosthesis.
Removable partial dentures
Participants who were partially edentulous, and required rehabilitation with permanent, removable, partial dentures for one or both arches.
We excluded participants with implant‐supported or retained dentures, with any form of intracoronal or extracoronal attachments, except Akers and bar clasp, transitional partial denture, treatment partial denture, temporary partials, overdenture, and immediate partial denture.
Types of interventions
We only considered the impression materials and prescribed technique(s) used for border moulding and final impression.
Complete dentures
Interventions that compared the following.
Same material using different techniques.
Same technique using different materials.
Different techniques using different materials.
Different techniques (comparisons 1 and 2)
single stage using one‐step final impression
two‐stage techniques using primary and final impression, either single‐step or two‐step
Different techniques (comparison 3)
different impression techniques for flabby ridge
different neutral zone techniques for resorbed ridge
any of the above techniques, and 1 and 2 done with different materials
Different final‐impression materials (comparisons 1, 2, and 3)
alginate
zinc‐oxide eugenol
elastomeric impression materials
impression plaster
green stick
fluid wax
Removable partial dentures
Interventions that compared the following.
-
Tooth‐supported conditions, using:
same material and different techniques;
same technique with different materials.
-
Tooth‐tissue‐supported conditions, using:
same material and different dual‐impression techniques;
different dual‐impression techniques with different materials.
Different types of final‐impression material
alginate
zinc‐oxide eugenol
elastomeric impression materials
green stick
fluid wax
impression plaster
Types of outcome measures
Primary outcomes
Complete dentures
Participant‐reported oral health‐related quality of life, measured with any pre‐validated questionnaire (including all domains in the Oral Health Impact Profile Questionnaire (OHIP), OHIP in Edentulous Adults (OHIP‐EDENT), OHIP‐14, OHIP‐20, OHIP‐49, GOHAI (Geriatric Oral Health Assessment Index)).
Participant‐reported quality of the denture assessment, measured by any pre‐validated questionnaire, including retention, stability, comfort, chewing ability, satisfaction, and denture dislodgement during function, for one or all of the factors.
Removable partial dentures
Participant‐reported oral health‐related quality of life, measured with any pre‐validated questionnaire (including all domains in the OHIP, OHIP‐EDENT, OHIP‐14, OHIP‐20, OHIP‐49, GOHAI.
Participant‐reported quality of the denture assessment, measured by any pre‐validated questionnaire, including stability, comfort, chewing ability, satisfaction, and denture dislodgement during function, for one or all of the factors.
Secondary outcomes
Complete dentures
Number of border adjustments or sore spots, measured up to one month after insertion of dentures.
Denture base retention (movement), stability, and overextension, assessed quantitatively or qualitatively by a calibrated operator, up to one month after insertion.
Participant‐reported preference for any technique or material.
Dislodgement of the denture during function.
Removable partial dentures
Number of border and intaglio adjustments, measured up to one month after insertion of dentures. We did not include sore spots as an outcome for removable partial dentures, as this may occur due to other components of the partial dentures, such as poor design or placement of the components during fabrication, and are not always due to overextension of the borders of the dentures.
Number of years of service after which a reline was required.
Abutment mobility, gingival health, and denture base adaptation, assessed quantitatively by operators.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials without language or publication status restrictions:
Cochrane Oral Health’s Trials Register (searched 22 November 2017) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; in the Cochrane Register of Studies, searched 22 November 2017) (Appendix 2);
MEDLINE Ovid (1946 to 22 November 2017) (Appendix 3);
Embase Ovid (21 December 2015 to 22 November 2017) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Due to the Cochrane Centralised Search Project to identify all clinical trials in the database and add them to CENTRAL, only the most recent months of the Embase database were searched. See the searching page on the Cochrane Oral Health website for more information. No other restrictions were placed on the date of publication when searching the electronic databases.
Searching other resources
We searched the following trial registries for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 22 November 2017) (Appendix 5);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 22 November 2017) (Appendix 5).
We screened the references of included studies to identify additional records, and we checked any review articles for studies not identified by the above‐mentioned search strategy. We contacted authors of published papers for more information. We translated non‐English records, with help from translators identified through Cochrane Oral Health.
We did not perform a separate search for adverse effects of interventions used; we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
We imported all the retrieved search results from the different databases to reference manager software, and removed duplicates. As a part of the data extraction process, two review authors (SJ and BPS) independently evaluated all retrieved studies by cross‐checking the title and abstract against the review inclusion and exclusion criteria. When the title or abstract did not clearly state the objectives, methods, and results, the authors retrieved the full text, along with additional information, if required. When multiple publications of one study were identified, we linked these under the same study identification. After initial screening, two review authors compared their selection of included studies and came to an agreement on ambiguous studies. When the review authors had a different opinion, the third and fourth authors (MPP and BR), and the methodologist (RK) were consulted, and arrived at a final agreement on the inclusion of the study. When one of the two screening authors was an author of a retrieved study, another author assessed its eligibility, to avoid bias. We explained the reasons for exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
We generated a customised comprehensive data extraction form with the objectives of this review. We pilot tested the data form, extracting and recording the following information for included studies.
General information: study ID, name of the review author (extractor), details of the study authors, year and date, publisher, journal and study design.
Study eligibility: inclusion and exclusion criteria, interventions and comparators, types of outcomes.
Population and setting: population description, setting, methods of recruitment of participants.
Methods: aim of study, design, unit of allocation, date of the start and end of the trial, total study duration, ethical approval obtained.
Participants: total number randomised, baseline imbalance, withdrawals and exclusion, age, sex, type of ridge, comorbidities, other treatments received after intervention.
Intervention: number of groups, impression technique and materials used in intervention and control groups, if trialists performed facebow transfer, and the occlusal registration techniques and scheme. For cross‐over trials, we recorded the period of habituation prior to cross‐over.
Outcomes: primary and secondary outcomes, and the time points at which they were assessed.
Others: funding source, conflict of interest.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies for internal validity, as per Higgins 2011, in the following domains.
Sequence generation (selection bias).
Allocation sequence concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome assessment (attrition bias).
Selective outcome reporting bias (reporting bias).
With cross‐over trials, if the design was suitable for the outcome, we did not consider the duration of washout because Hyde 2014 showed that there was no period effect or carry‐over effect in a randomised cross‐over denture trial. We accepted a minimum period of one to two weeks of habituation prior to cross‐over.
We graded the risk of bias as low, high, or unclear, based on pre‐set criteria presented in Appendix 6, conforming to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We created a 'Risk of bias' summary graph and figure, and we used our judgements to grade the overall quality of evidence for each comparison and outcome in the 'Summary of findings' tables. We contacted authors of the studies for clarification regarding the randomisation and allocation concealment domains. If the author did not respond, and the methods used were not clearly stated in the article, we assessed that study as being at unclear risk of bias. Two review authors (SJ and BPS) independently assessed the risk of bias of the included studies, and MPP checked all 'Risk of bias' assessments. We resolved any ambiguity through consensus among all authors.
Measures of treatment effect
When studies recorded outcomes as dichotomous data, we reported the risk ratio (RR) with 95% confidence interval (CI). When investigators reported outcomes as continuous data, we used the difference in means (MD) if the outcomes were measured on the same scale, and the standardised mean difference (SMD) if measured on a different scale, with 95% confidence interval (CI).
Unit of analysis issues
In parallel‐group trials, we considered the participant to be the unit for analysis. In multi‐arm trials, we had intended to combine similar arms where appropriate. For cross‐over trials, we analysed the data appropriately, taking into account the paired nature of the data (see Data synthesis section)
Dealing with missing data
We contacted some of the study authors, requesting they provide us with missing data. However, we were unsuccessful in getting the missing data. Therefore, when feasible, we estimated the missing data using results reported in the article, by following methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We analysed and investigated heterogeneity at three levels: clinical, methodological, and statistical. Heterogeneity due to clinical and methodological factors included age, trial type, outcomes measured, use of facebow, follow‐up, risk of bias, type or classification of the ridge, and other factors that arose after the analysis. For statistical heterogeneity, we checked the direction and magnitude of the effect, along with overlapping CI and point estimates.
We assessed statistical heterogeneity using the Chi² statistic, with a level of significance of 0.1 instead of 0.05. We used the I² statistic to quantify heterogeneity, according to Higgins 2011. The I² statistic indicates the degree of heterogeneity, and the value ranges from 0 to 100. A higher value indicates greater heterogeneity. A rough guide for interpretation of I² is: 0% to 40% ‐ might not be important; 30% to 60% ‐ may represent moderate heterogeneity; 50% to 90% ‐ may represent substantial heterogeneity; 75% to 100% ‐ may represent considerable (very substantial) heterogeneity. When I² was higher than 60%, we investigated heterogeneity using a random‐effects model, or explored it using a subgroup analysis. When heterogeneity was higher than 80%, we did not pool data.
Assessment of reporting biases
We did not use funnel plots to look for publication bias, as there were too few studies.
Data synthesis
We undertook data analysis using Review Manager 5 (RevMan), following the methods stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Review Manager 2014). When there was similarity across the participants, interventions, and outcomes, we performed a meta‐analysis. We combined MDs for continuous data (or SMDs for studies using different scales), and RRs for dichotomous data. Our general approach was to use a random‐effects model. With this approach, the CIs for the average intervention effect were wider than those obtained using a fixed‐effect model, leading to a more conservative interpretation.
We extracted appropriate data from the crossover trials (Elbourne 2002), and we used the generic inverse variance (GIV) method to enter log RR or mean differences and their respective standard errors into RevMan. For cross‐over studies, we intended to use the Becker‐Balagtas method (BB OR) to calculate log odds ratios (ORs), as indicated by Curtin 2002 to accommodate data pooling from cross‐over and parallel‐group studies in a single meta‐analyses, and facilitate data synthesis; however, we did not meta‐analyse any dichotomous data (see Stedman 2011).
Subgroup analysis and investigation of heterogeneity
We had planned the following subgroup analyses for complete dentures, but we were only able to conduct the analysis by trial type.
Use of a facebow transfer with semi‐adjustable articulator during complete dentures treatment.
Types of ridges (we grouped them using American College of Prosthodontics (ACP) classification I and II versus III and IV, or Atwoods classification above order III versus below order III).
Performance of intervention on a single arch or on both arches, for the primary outcomes.
Trial type.
Sensitivity analysis
As there were inadequate data, we did not perform any of the sensitivity analyses we had planned in order to evaluate the robustness of the pooled estimate.
Summarising findings
We generated 'Summary of findings' tables for comparisons 1, 2, and 3. Comparison 3 compared the most widely and commonly used technique and material in the fabrication of complete dentures. We summarised the findings for our key outcomes: participant‐reported quality of life, participant‐reported quality of the denture, and number of border adjustments and sore spots after insertion of the denture. We did not generate a 'Summary of findings' table for removable partial dentures.
We assessed the quality of the evidence as high, moderate, low, or very low, in accordance with section 11.5 of Higgins 2011, using GRADE methods and the GRADEPro software package (GRADE 2004; GRADEpro 2015). We graded the body of evidence based on the risk of bias of included studies, indirectness of the evidence, inconsistency between results, imprecision of measure of effects, and publication bias. We provided a citation and rationale for the figure we used to calculate the assumed risk.
Results
Description of studies
See the 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Results of the search
The search of electronic databases yielded a total of 1191 records, and we identified seven additional records from other sources. After we removed duplicates, we had 889 records remaining. We screened the titles and abstracts of these records, and rejected 857 records. We assessed 32 full‐text reports for eligibility. We excluded 17 reports (12 on complete dentures (CD); five on removable partial dentures (RPD)). We listed the reasons for exclusion in Characteristics of excluded studies. There is one ongoing study on CD (NCT02339194). We included nine studies in 14 reports: eight studies on CD (Firtell 1992; Hyde 2010; Hyde 2014; Jo 2015; Kawai 2005; Matsuda 2015; Nunez 2015; Regis 2013), and one study on RPD (Frank 2004). We present the study selection process in Figure 4.
4.
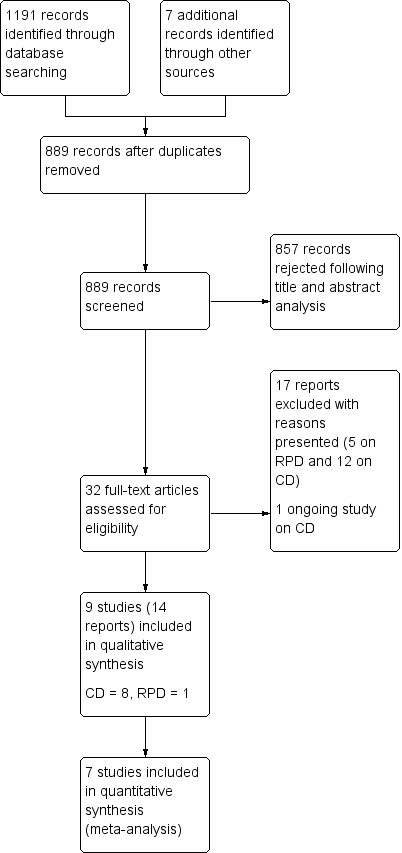
Study flow diagram
Included studies
Complete dentures
Characteristics of trial setting and design
Location
Of the eight studies on complete dentures we included in the review, two were conducted in Japan (Jo 2015; Matsuda 2015), two in Brazil (Nunez 2015; Regis 2013), two in the UK (Hyde 2010; Hyde 2014), one in Canada (Kawai 2005), and one in the USA (Firtell 1992). The setting for seven studies was a dental school, or university hospital, and one study was conducted in a general hospital in Canada.
Design
Four trials had a two‐arm, parallel‐group design (Firtell 1992; Kawai 2005; Nunez 2015; Regis 2013) and three studies had a two‐arm, cross‐over design (Hyde 2014; Jo 2015; Matsuda 2015). The final study, Hyde 2010, had a three‐arm, cross‐over design, but only one outcome (denture preference) was measured based on the randomisation of participants to the three arms. The study's other outcomes were assessed after participants had chosen which of the dentures they preferred and had worn them for three months; as these measurements were not based on a randomised comparison, we did not include these data.
Duration
Trial length varied from 1.2 to 2.9 years; one study stated only the enrolment period (0.7 years; (Regis 2013)); and one study did not state the trial duration (Firtell 1992).
Funding
Only one trial did not specify any funding source (Firtell 1992). Hyde 2010 was funded by a grant from the Dunhill Medical Trust; Hyde 2014 was funded by a NIHR‐RfPB (National Institute of Health ‐ Research for Patient Benefit grant); Jo 2015 was supported by the Japanese Society for the Promotion of Science; Kawai 2005 by the Nihon University Grant for Overseas Research and the Suzuki Memorial Grant from the Canadian Institutes of Health Research; Matsuda 2015 was supported by the research fund of Osaka University Graduate School of Dentistry, where both of the authors (K Mastudaand, Y Maeda) are remunerated instructors, who had given educational lectures at the request of the Ivoclar Vivadent company, and who conducted and supervised the study; Nunez 2015 was supported by a grant from the Brazilian National Research Council and State Foundation Research of Goias; and Regis 2013 was funded by a FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) grant.
A priori sample size calculation
Of the eight studies included, five trials reported sample size with 80% power estimates (Hyde 2014; Jo 2015; Kawai 2005; Nunez 2015; Regis 2013); in one pilot study no sample size calculation was done (Matsuda 2015); one study did not report clearly (Hyde 2010); and one study did not report sample size (Firtell 1992). The sample size of the studies varied from 10 (Matsuda 2015) to 122 (Kawai 2005).
A total of 435 participants were randomised from eight trials, with a mean of 54.3 participants per trial, and a range of 10 to 122. The participants were both male and female, who were edentulous in the upper and lower arch. The average age of the participants ranged from 45 to 75 years. Three studies reported the period of edentulousness, which ranged from 10 to 35 years (Kawai 2005; Nunez 2015; Regis 2013). Three studies reported patient classification based on American College of Prosthodontics classification for completely edentulous patients, and found about 60% to 75% of ACP‐III and ACP–IV in both groups (Jo 2015; Kawai 2005; Regis 2013). Three studies did not report demographic details of participants (Firtell 1992; Hyde 2010; Hyde 2014). The other studies reported demographic details, which were balanced at baseline. Most studies excluded people with temporomandibular disorders, psychological disorders, allergies to acrylic or silicone, dysfunction disorders of the masticatory system, debilitating systemic disease or oral mucosal disease, and decline in cognitive function.
Characteristics of the intervention
Comparison 1: same material and different techniques
We found two studies for the Comparsion 1, comparing same material and different techniques (Hyde 2010; Matsuda 2015).
One study (pilot) compared open versus closed mouth/two stage‐two step silicone elastomers (Matsuda 2015). In Group 1, Biofunctional Prosthetic System CD fabrication method (BPS) preliminary impression (Accu‐dent System I) was made using irreversible hydrocolloid as tray and syringe material. The custom trays were fabricated using Gnathometer‐M tracing and final impressions were made in 'mouth‐closed' position with both light‐ and heavy‐body vinyl polysiloxane impression material. In Group 2, the conventional CD fabrication method, the preliminary impression was made with irreversible hydrocolloid impression material with a stock metal tray. The final impression was made with a custom tray, border moulded with impression compound and made with hydrophilic vinyl polysiloxane impression material, which was same for both groups. The other differences between the two intervention arms (co‐intervention) were that jaw relation was taken as tentative along with the primary impression and second jaw relation with vertical maxillomandibular relationship and horizontal relation recorded using Gnathometer‐M intraoral gothic arch tracing device with a silicone bite registration paste. Hence the BPS method had one less step than the conventional method of denture fabrication.
One study was a cross‐over, three‐arm trial (Hyde 2010). For all groups, the preliminary impression was made with irreversible hydrocolloid using a stock tray (different two stage‐two step techniques with silicone). Final impression in the control group was done with standard technique using wax relief on a custom tray, making a relatively mucostatic impression. The second intervention was traditional technique (redistributing pressure) using tin foil of 0.6 mm on the primary cast while making a custom tray. The third intervention was selective pressure impression technique, which involves placing relief holes on the tray after removing impression material over mental foramen before final impression. All impressions were taken with medium‐bodied silicone material followed by a light‐bodied silicone final impression.
Comparison 2: same technique and different materials
We identified two trials comparing same technique with different materials (Firtell 1992; Hyde 2014).
Firtell 1992 compared two stage‐two step/wax versus polysulfide. This was a two‐arm, parallel‐group trial in which a stock metal tray with irreversible hydrocolloid was used for preliminary impression. A custom tray was made and border moulding with green stick moulding compound and relieved for open‐mouth selective pressure final impression either with fluid wax (Group 1) or polysulfide impression material (Group 2) .
In Hyde 2014, which was a two‐arm, cross‐over trial of two stage‐two step/alginate versus silicone, preliminary impression was made with alginate impression material in a stock metal tray. One group used alginate as final impression after border moulding with green stick and the other group used heavy body for upper and light body for lower border moulding and final impression was made with silicone. All other procedures were the same until the insertion of denture. Washout period was eliminated by using a novel habituation period of wearing unadjusted dentures for two weeks.
Comparison 3: different techniques and different materials
We found four studies using different materials with different techniques (Jo 2015; Kawai 2005; Nunez 2015; Regis 2013).
In Jo 2015, the only difference between the two interventions was at the impression stage: the simplified method of fabricating complete dentures used alginate (single‐stage impression with alginate versus two stage‐two step silicone) and the conventional method used border moulding with green stick and silicone final impression. All clinical and lab phases of denture fabrication were the same until denture insertion. Washout period was one month and relined old denture was used during the washout period. No co‐intervention was used.
Kawai 2005 employed a simplified procedure of denture fabrication using (single‐stage) alginate impression material with a stock metal tray. The traditional/conventional method (two stage‐two step) used preliminary alginate impression with a stock metal tray. The final impression was made with a custom tray, border moulded with impression compound and final impression with polyether rubber impression material. The traditional procedure used the co‐intervention of facebow, semi‐adjustable articulator and remount procedure than simplified procedure until denture insertion.
In Nunez 2015, a two‐arm, parallel‐group study, the simplified procedure of denture fabrication used (single‐stage) alginate impression materials with a stock metal tray. The traditional/conventional method (two stage‐two step) used preliminary alginate impression with a stock metal tray. The final impression was made with a custom tray, border moulded with impression compound and polysulfide impression material (elastomer). The traditional procedure used the co‐intervention of facebow, but both methods used average setting on semi‐adjustable articulator and no remount procedure than simplified procedure until denture insertion.
In Regis 2013, a two‐arm, parallel‐group study, the simplified procedure of denture fabrication used (single‐stage) alginate impression materials with a stock metal tray. The traditional/conventional method (two stage‐two step) used preliminary alginate impression with a stock metal tray. The final impression was made with a custom tray, border moulded with impression compound and final impression with zinc‐oxide eugenol impression paste. The traditional procedure also used the co‐intervention of facebow and two try‐in steps, but both methods used an average setting on semi‐adjustable articulator and no remount procedure compared to simplified procedure until denture insertion.
Impression materials for complete denture
One study used wax (Firtell 1992); all eight studies used elastomers as final‐impression material, either polysulfide, polyether or addition silicone (Firtell 1992; Frank 2004; Hyde 2010; Hyde 2014; Jo 2015; Kawai 2005; Matsuda 2015; Nunez 2015); and five studies used alginate as final‐impression material (Hyde 2014; Kawai 2005; Nunez 2015; Regis 2013; Jo 2015). Only one trial used zinc‐oxide eugenol (Regis 2013), and no study used impression plaster as final‐impression material.
Characteristics of outcome assessments
The primary outcomes in our review were participant‐reported oral health‐related quality of life (OHRQoL; using the OHIP, OHIP‐EDENT, OHIP 14, OHIP 20, OHIP 49, and the GOHAI questionnaires), and participant‐reported quality of the denture assessment for retention, stability, comfort, chewing and masticatory ability, satisfaction, and denture dislodgement during function.
All studies published after 2010 assessed OHRQoL using the OHIP‐EDENT questionnaire as one of the main outcomes (Hyde 2010; Hyde 2014; Jo 2015; Matsuda 2015; Nunez 2015).
Five studies evaluated participant‐rated general satisfaction with the new dentures using a 100‐mm or a 10‐point visual analogue scale (VAS) (Jo 2015; Kawai 2005; Matsuda 2015; Nunez 2015; Regis 2013).
Five studies assessed participant‐rated comfort, stability, aesthetics, ability to speak, ease of cleaning, and masticatory ability on 10‐point VAS or 5‐point Likert Scale (Hyde 2010; Hyde 2014; Kawai 2005; Nunez 2015), or qualitatively (Matsuda 2015).
Two studies reported the number of adjustments required post denture insertion (Firtell 1992; Matsuda 2015).
Three studies assessed participants' preference for dentures (Hyde 2010; Hyde 2014; Matsuda 2015).
Two trials reported operator‐rated quality of denture (Regis 2013; Kawai 2005).
Some outcomes in Firtell 1992 were measured but not reported.
From Hyde 2010, we used only the data for our secondary outcome 'denture preference' as the measurement of the other outcomes was not based on a randomised comparison.
Duration of follow‐up
Duration of follow‐up varied from 24 hours to one year. See Table 7 for details.
1. Outcomes and follow‐up times.
| Outcomes | 24 hours | One week | Two weeks | One month | Three months | Six months | One year |
| Participant‐reported oral health‐related quality of life (OHIP‐EDENT) | Hyde 2014 |
Jo 2015; Nunez 2015 |
Matsuda 2015; Hyde 2010; Regis 2013 |
Nunez 2015; Regis 2013 |
|||
| Participant‐reported quality of the denture, for satisfaction, comfort, masticatory ability and speech | Hyde 2014 | Nunez 2015 |
Matsuda 2015; Kawai 2005 |
Nunez 2015; Kawai 2005 |
|||
| Number of denture adjustments required post insertion | Firtell 1992 | Firtell 1992 | Matsuda 2015 | Firtell 1992 | |||
| Operator‐reported quality of the denture | Regis 2013 |
Regis 2013; Kawai 2005 |
|||||
| Participant preference for dentures (impression material and technique) | Hyde 2014 | Jo 2015; Hyde 2010 | Matsuda 2015 |
Removable partial dentures (RPD)
We included one parallel‐group, arm trial on RPD for tooth‐tissue‐supported conditions, which compared same material and different dual‐impression techniques (Frank 2004). It was conducted at University of Washington, Seattle, USA. Recruitment period and funding source were not mentioned in the study.
Characteristics of participants
They recruited participants from those undergoing routine prosthodontic treatment at the pre‐doctoral patient pool at the University of Washington. Patients requiring mandibular Kennedy Class‐1 RPD with at least one indirect retainer on premolar or canine were included in the study and no exclusion criteria were stated.
Characteristics of the intervention
The study compared an altered‐cast technique, in which the framework was fabricated after the impression of distal extension and made in the laboratory versus a one‐piece cast, in which the framework was fabricated and checked for fit intraorally. The preliminary impression with a stock tray, and final impression with an auto‐polymerising resin custom tray, were made with a standardised mixture of equal parts of light‐body and medium‐body polyether for both groups.
Characteristics of the outcomes
They measured under and over extension, base movement, adaptation of the denture base, gingival sulcus depth, mobility, gingival index of direct retainer abutment, resorption, tissue quality of the edentulous ridge, participant‐reported general satisfaction, most liked, and most disliked feature of the removable partial dentures.
Excluded studies
We reported the reasons for excluding studies in the 'Characteristics of excluded studies' table. We excluded 17 articles (12 on CD and five on RPD). In CD, three studies evaluated denture bases mounted on cast (Adnan 2010; Al‐Judy 2015; Birtles 2015); two studies compared the same material and technique for both groups (Heydecke 2008; Nascimento 2004); one study evaluated mandibular dentures only (McCord 2005); one study was quasi‐randomised (Sharif 2013); one study was not an RCT (Tasleem 2013); two study compared relining material (Wegner 2011; DRKS00000149); and two were ongoing studies evaluating polyamide (NCT03043456; NCT03234803). We excluded five reports on RPD: two studies compared two different types of prostheses (Au 2000; Hundal 2015); one was not randomised (Hochman 1998); one was an ongoing study evaluating polyamide and polyetheretheketone (NCT03025555) and one had removable partial against complete denture (RBR‐8fs5ww).
Ongoing studies
We identified one relevant ongoing study: NCT02339194. See the 'Characteristics of ongoing studies' table.
Risk of bias in included studies
We assessed the risk of bias using the Cochrane 'Risk of bias' tool. For CD studies, we judged six studies to be at high risk of bias (Firtell 1992; Jo 2015; Kawai 2005; Matsuda 2015; Nunez 2015; Regis 2013), and two to be at low risk of bias (Hyde 2010; Hyde 2014). For RPD, we judged the one study to be at high risk of bias (Frank 2004). See Figure 5 and Figure 6.
5.
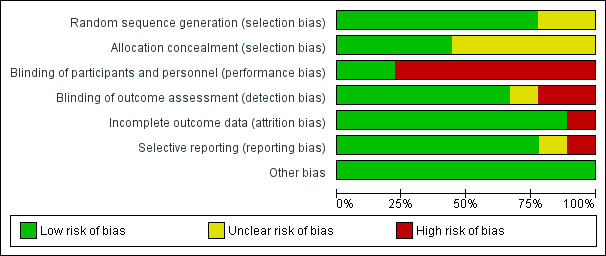
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' domain, presented as percentages across all included studies
6.
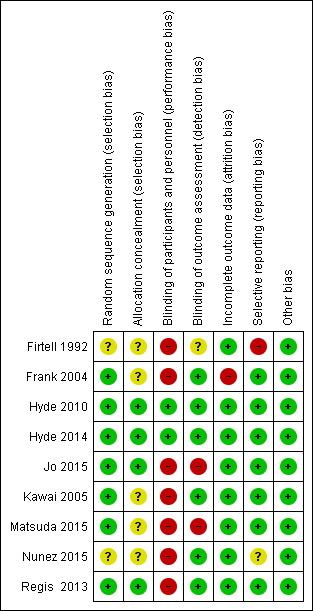
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' domain for each included study
Allocation
Complete dentures
We assessed the method for random sequence generation as low risk of bias for six studies that reported computer‐generated random sequence (Hyde 2010; Hyde 2014; Jo 2015; Kawai 2005; Matsuda 2015; Regis 2013) and as unclear for two studies (Firtell 1992; Nunez 2015). We assessed the concealment of allocation as low risk of bias for four studies (Hyde 2010; Hyde 2014; Jo 2015; Regis 2013), and as unclear for four studies (Firtell 1992; Kawai 2005; Matsuda 2015; Nunez 2015).
Removable partial dentures
We assessed Frank 2004 as low risk of bias for random sequence generation and unclear risk of bias for allocation concealment.
Blinding
Complete dentures
We assessed the blinding of participants and personnel as low risk of bias for Hyde 2010 and Hyde 2014 because randomisation was done only at the delivery of dentures, followed by adjustment of the dentures. We assessed six studies as high risk of bias as it was not possible to blind the operator in these trials (Firtell 1992; Jo 2015; Kawai 2005; Matsuda 2015; Nunez 2015; Regis 2013).
We assessed the blinding of outcome assessment as low risk of bias for five studies (Hyde 2010; Hyde 2014; Kawai 2005; Nunez 2015; Regis 2013). We assessed it as high risk of bias for two studies, one did not blind the outcome assessor (Jo 2015), and in other, the same resident dentists operated and evaluated the outcomes for each participant (Matsuda 2015). Firtell 1992 was at unclear risk of bias as it did not describe blinding.
Removable partial dentures
We assessed blinding of participants and personnel (performance bias) in Frank 2004 as high risk of bias as it is not possible to blind the operator and blinding of participants was not reported. We assessed the blinding of outcome assessment (detection bias) as low risk of bias.
Incomplete outcome data
Complete dentures
We assessed incomplete outcome data for all eight studies as low risk of bias.
Removable partial dentures
We assessed Frank 2004 as high risk of attrition bias as loss to follow‐up was 19 participants at one year (15.1%).
Selective reporting
Complete dentures
We assessed six studies as low risk of reporting bias (Hyde 2010; Hyde 2014; Jo 2015; Kawai 2005; Matsuda 2015; Regis 2013); one study as unclear risk of bias, as it did not report general satisfaction of the denture but reported maxillary and mandibular denture satisfaction separately (Nunez 2015); and one study as high risk of bias, as it reported only denture adjustment, and other outcomes were not reported (Firtell 1992).
Removable partial dentures
We assessed Frank 2004 as low risk of reporting bias.
Other potential sources of bias
No other potential sources of bias were found.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Complete dentures
Comparison 1 (same material and different techniques)
Closed mouth two stage‐two step with addition silicone elastomer (Biofunctional Prosthetic System (BPS)) versus open mouth two stage‐two step conventional technique (CCD) using elastomer
This comparison was assessed in one very small study (10 participants), which was at high risk of bias (Matsuda 2015). Although both interventions used a similar material for the final impression, they differed in impression technique, jaw registration methods, type of teeth, and occlusal scheme. Data were taken directly from the paper. See Table 1.
Primary outcomes
At three months, Matsuda 2015 measured participant‐reported oral health‐related quality of life, using the OHIP‐EDENT‐J. The median OHIP‐EDENT‐J score was 34.5 for the BPS group and 35.8 for the CCD group.
For participant‐reported denture satisfaction, assessed using a 100‐mm VAS, the median score was 86.5 for the BPS group and 88 for the CCD group.
There was very low‐quality evidence of no clear difference between groups for either outcome.
Secondary outcomes
Matsuda 2015 reported that the median number of denture adjustments was 3.5 for the BPS group and 4.5 for the CCD group at three months.
When participants were asked which denture they would prefer to use long term, nine out of 10 opted for the one made with the BPS complete denture technique.
Denture base retention and dislodgement of the denture during function were not measured.
Two stage–two step with addition silicone elastomer impression material : selective pressure technique versus traditional technique (redistributing pressure) versus a control (placebo) technique (relatively mucostatic standard impression procedure)
Hyde 2010 compared these three techniques, analysing 69 participants. No cointerventions were given to either group after the final impression.
Primary outcomes
Participant‐reported quality of life was measured but it was based on an assessment of dentures participants had chosen, therefore it was not a randomised comparison. Quality of the dentures was not assessed.
Secondary outcomes
The study assessed participant preference for dentures at four to five weeks. Participants were more likely to prefer dentures made using the selective pressure technique over dentures made in the traditional method or using a control technique (33 participants chose dentures made using the selective pressure technique, 19 chose the traditionally‐made denture and 14 chose the control denture). There was no clear preference between the traditional and control denture groups.
This study did not measure the number of adjustments, denture base retention, or dislodgement of the denture during function.
Comparison 2 (same technique and different materials)
Two stage–two step selective pressure final‐impression technique using wax versus polysulfide elastomeric impression material
One study at high risk of bias evaluated this comparison and provided very low‐quality evidence for the outcomes (Firtell 1992; 27 participants; Table 2).
Primary outcomes
Neither of the primary outcomes were reported in this study.
Secondary outcomes
There was no evidence of a clear difference in the need for denture adjustments over one year of follow‐up between the wax and polysulfide groups (RR 0.81, 95% CI 0.38 to 1.70; Analysis 1.1).
1.1. Analysis.
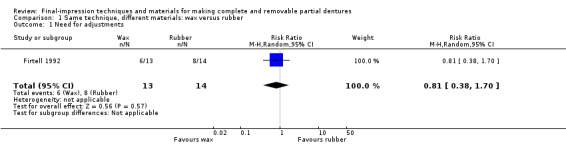
Comparison 1 Same technique, different materials: wax versus rubber, Outcome 1 Need for adjustments.
Firtell 1992 did not measure denture base retention, participant‐reported preference, or dislodgement of the denture during function.
Two stage‐two step (alginate versus silicone elastomers)
One study at low risk of bias evaluated this comparison. Hyde 2014 compared an alginate final impression after border moulding with green stick, and a light‐body silicone final impression after border moulding with heavy‐ and regular‐body silicone. This was the only study to use alginate for the final impression (wash impression). Seventy‐eight out of 85 participants completed the trial. None of the participants received any cointerventions.
Primary outcomes
Participant‐reported oral health‐related quality of life was measured at two weeks using the OHIP‐EDENT. Low‐quality evidence favoured silicone (MD 7.20, 95% CI 2.71 to 11.69; 144 participants; Analysis 2.1; Table 3). The difference between the groups was more than six units, which is the minimally clinical important difference for the OHIP‐EDENT, although the 95% confidence interval included scores under six units (John 2009).
2.1. Analysis.
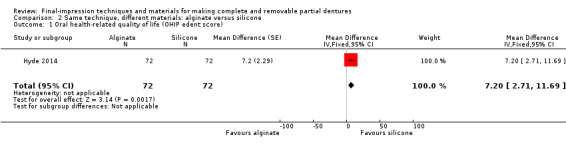
Comparison 2 Same technique, different materials: alginate versus silicone, Outcome 1 Oral health‐related quality of life (OHIP edent score).
Participant‐reported quality of the dentures was assessed using 5‐point Likert scales after two weeks of confirmation, for comfort, stability, and chewing efficiency. There was no evidence of a clear difference for comfort, but more participants favoured silicone for stability and chewing efficiency. These data were not amenable to meta‐analysis.
Secondary outcomes
There was evidence that one material was preferred over another after two weeks (confirmation period): silicone impressions 57.7% (41 participants), and alginate impressions 23.9% (17 participants). Both dentures were equally satisfactory for seven participants (9.9%) and equally unsatisfactory for six participants (8.5%) (McNemar chi‐square 12.02, 1 df, P = 0.0005).
They did not measure number of adjustments, denture base retention, or dislodgement of the denture during function.
Comparison 3 (different techniques and different materials)
Four trials (all at high risk of bias) addressed this comparison (Jo 2015; Kawai 2005; Nunez 2015; Regis 2013).
Single‐stage alginate (simplified method) versus two stage‐two step elastomer (silicone, polysulfide, or polyether) conventional method
Three studies compared simplified (alginate) and conventional methods of fabricating dentures with a silicone final impression and no cointervention (Jo 2015), a polyether cointervention of facebow transfer (Kawai 2005), and a polysulfide cointervention of facebow transfer (Nunez 2015).
Primary outcomes
Jo 2015 and Nunez 2015 measured participant‐reported oral health‐related quality of life using the OHIP‐EDENT. Two studies provided very low‐quality evidence of little or no difference between the alginate and elastomer groups at one month (MD 0.05, 95% CI ‐2.37 to 2.47; 98 participants; Analysis 3.1; Table 4).
3.1. Analysis.
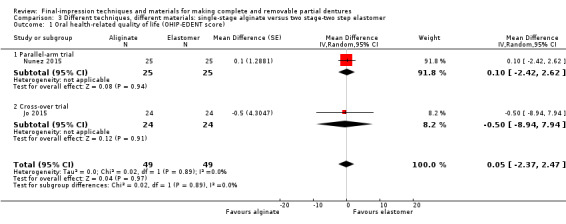
Comparison 3 Different techniques, different materials: single‐stage alginate versus two stage‐two step elastomer, Outcome 1 Oral health‐related quality of life (OHIP‐EDENT score).
Jo 2015 and Kawai 2005 measured participant‐reported quality of the denture (general satisfaction with new dentures) with a 100‐mm VAS. There was very low‐quality evidence of no clear difference between fabrication methods at one month (MD ‐6.50, 95% CI ‐20.08 to 7.08; one study, 48 participants); three months (MD 0.00, 95% CI ‐7.17 to 7.17; one study, 108 participants); or six months (MD 0.00, 95% CI ‐8.23 to 8.23; one study, 105 participants) (Analysis 3.2; Table 4). The participants had ACP II and III greater than 65% to 73% in both groups.
3.2. Analysis.
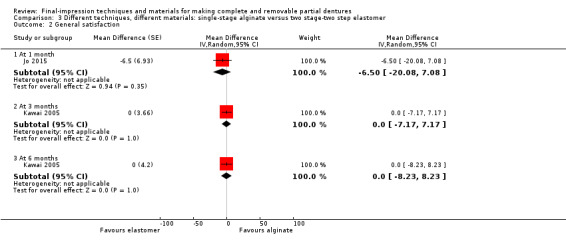
Comparison 3 Different techniques, different materials: single‐stage alginate versus two stage‐two step elastomer, Outcome 2 General satisfaction.
Nunez 2015 and Kawai 2005 measured maxillary and mandibular denture satisfaction at six months. There was very low‐quality evidence of no clear difference between the alginate and elastomer groups for maxillary denture satisfaction (SMD ‐0.02, 95% CI ‐0.34 to 0.29; two studies, 155 participants; Analysis 3.3), or mandibular denture satisfaction (SMD 0.21, 95% CI ‐0.11 to 0.52; two studies, 155 participants; Analysis 3.4; Table 4). The participants had a mean period of edentulousness ranging from 23.9 to 25.1 years in Nunez 2015 and 32 to 38 years in Kawai 2005.
3.3. Analysis.
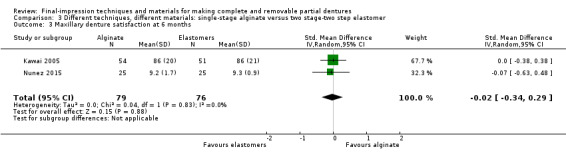
Comparison 3 Different techniques, different materials: single‐stage alginate versus two stage‐two step elastomer, Outcome 3 Maxillary denture satisfaction at 6 months.
3.4. Analysis.
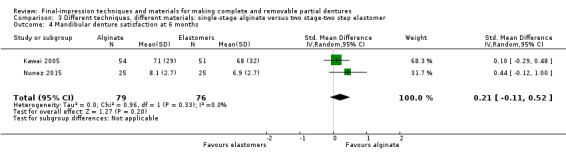
Comparison 3 Different techniques, different materials: single‐stage alginate versus two stage‐two step elastomer, Outcome 4 Mandibular denture satisfaction at 6 months.
Secondary outcomes
There was insufficient evidence of a clear difference between groups at six‐month follow‐up in operator‐assessed quality of the dentures (Kawai 2005).
None of the other secondary outcomes were assessed.
Single stage‐alginate (simplified method) versus two stage‐two step (traditional method) with zinc‐oxide eugenol impression material
In one study, the traditional group had cointerventions of facebow transfer and extra try‐in appointments (Regis 2013). A total of 42 participants were randomised; 39 completed the study. The outcomes were evaluated at baseline, three months and six months.
Primary outcomes
There was very low‐quality evidence of no clear difference between methods for participant‐reported oral health‐related quality of life (OHIP‐EDENT) at three months (MD ‐2.20, 95% CI ‐5.57 to 1.17; Analysis 4.1), or six months (MD 0.50, 95% CI ‐2.67 to 3.67; Analysis 4.1; Table 5).
4.1. Analysis.
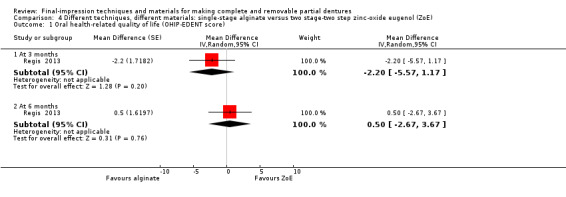
Comparison 4 Different techniques, different materials: single‐stage alginate versus two stage‐two step zinc‐oxide eugenol (ZoE), Outcome 1 Oral health‐related quality of life (OHIP‐EDENT score).
There was very low‐quality evidence of no clear difference between methods in participant‐reported quality of the dentures (general satisfaction) at three months (RR 3.15, 95% CI 0.14 to 72.88; Analysis 4.2), or six months (RR 3.15, 95% CI 0.14 to 72.88; Analysis 4.2; Table 5).
4.2. Analysis.
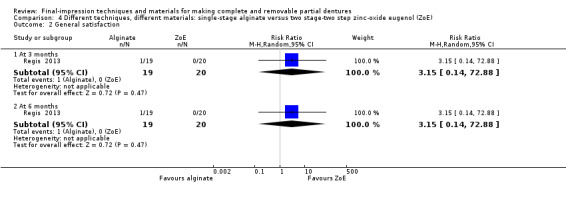
Comparison 4 Different techniques, different materials: single‐stage alginate versus two stage‐two step zinc‐oxide eugenol (ZoE), Outcome 2 General satisfaction.
There was insufficient evidence of difference for quality of the dentures in all other domains in both periods.
Secondary outcomes
There was insufficient evidence of a clear difference between groups for operator‐assessed quality of the dentures for retention and stability of dentures (Regis 2013).
None of the other secondary outcomes were assessed.
Removable partial dentures
Comparision 1: Tooth‐supported conditions
Same materials and different techniques
We did not find any trials for this comparison.
Different materials and the same technique
We did not find any trials for this comparison.
Comparison 2: Tooth‐tissue supported conditions
Same material and different dual‐impression techniques
Frank 2004 (72 participants randomised, 53 participants analysed), which was at high risk of bias, compared an altered‐cast impression (ACIP) to a one‐piece cast (OPC), made with polyether final impression for distal extension base. Multiple outcomes were evaluated. See Table 6.
Primary outcomes
There was low‐quality evidence of no clear difference between groups for general satisfaction at one year. The study reported that 50 of 57 participants were moderately to completely satisfied, with no significant difference between the groups. The data are not reported separately for the groups. Frank 2004 did not measure quality of life. See Table 6.
Secondary outcomes
There was very low‐quality evidence of no clear differences for intaglio adjustment at baseline (RR 1.43, 95% CI 0.61 to 3.34; Analysis 5.1). At one‐year follow‐up, there was very low‐quality evidence of no clear differences between the groups for operator‐assessed gingival health at right abutment (RR 1.05, 95% CI 0.56 to 1.98; Analysis 5.4), left abutment (RR 1.10, 95% CI 0.46 to 2.64; Analysis 5.4) and abutment mobility in right abutment (RR 2.90, 95% CI 0.87 to 9.61; Analysis 5.3) and left abutment (RR 1.16, 95% CI 0.40 to 3.37; Analysis 5.3).
5.1. Analysis.
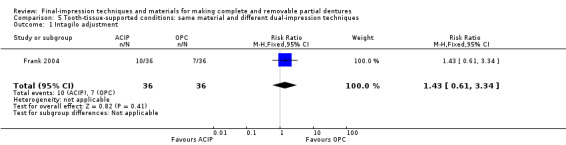
Comparison 5 Tooth‐tissue‐supported conditions: same material and different dual‐impression techniques, Outcome 1 Intagilo adjustment.
5.4. Analysis.
Comparison 5 Tooth‐tissue‐supported conditions: same material and different dual‐impression techniques, Outcome 4 Gingival index.
5.3. Analysis.
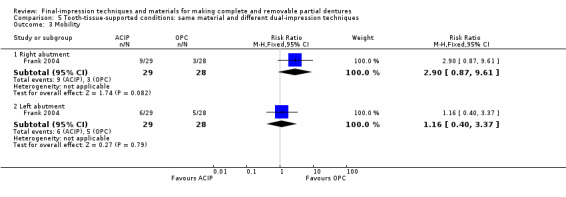
Comparison 5 Tooth‐tissue‐supported conditions: same material and different dual‐impression techniques, Outcome 3 Mobility.
Denture base adaptation was measured with silicone at the buccal shelf and ridge crest. At one‐year follow‐up, the OPC group had poor adaptation over the crest of the ridge compared to the ACIP group, with no clear difference between the groups in the buccal shelf area (MD ‐0.11, 95% CI ‐0.18 to ‐0.04; Analysis 5.2).
5.2. Analysis.
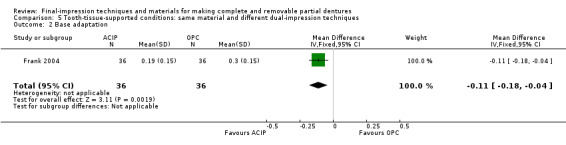
Comparison 5 Tooth‐tissue‐supported conditions: same material and different dual‐impression techniques, Outcome 2 Base adaptation.
Different dual‐impression techniques and different materials
We did not find any trials for this comparison.
Discussion
Summary of main results
This review compared different impression techniques and materials for fabricating complete dentures (CD) and removable partial dentures (RPD).
Complete dentures
We included eight studies for CD (Firtell 1992; Hyde 2010; Hyde 2014; Jo 2015; Kawai 2005; Matsuda 2015; Nunez 2015; Regis 2013). For the three main comparisons, the studies compared six different techniques and materials. We did not find clear evidence to show that one technique or material had any substantial advantage over another.
There was low‐quality evidence that silicone was a better final‐impression material for oral health‐related quality of life than alginate (Table 3).
There was very low‐quality evidence of no clear differences between the single‐stage impression alginate and the two stage‐two step elastomer groups in participant‐reported quality of life using OHIP‐EDENT (Table 4). The main reasons were that up to 69% of participants in one group were ACP classification Class III and IV, while 73% in the other group were the same classification (Jo 2015), and the mean duration of edentulousness was 23.9 (1 to 40) years for one group, and 25.1 (3 to 47) years in the other group (Nunez 2015).
There was low‐quality evidence that participants were more likely to prefer dentures made with the selective pressure technique compared to traditional and standard (relative mucostatic) methods.
For all other comparisons of different techniques and materials for complete dentures, findings were based on single studies and we rated the evidence as very low quality.
Removable partial dentures
A single study evaluated altered‐cast impression (ACIP) and one‐piece cast (OPC) for distal extension base for RPD and found no evidence of a clear difference at one‐year follow‐up; however, the quality of the evidence base is low to very low, so we cannot be sure of this finding.
Overall completeness and applicability of evidence
Complete dentures
Included studies were conducted in five countries: two in Japan (Jo 2015; Matsuda 2015), two in Brazil (Nunez 2015; Regis 2013), two in the UK (Hyde 2010; Hyde 2014), one in Canada (Kawai 2005), and one in the USA (Firtell 1992). Seven studies were conducted in a dental school at a university hospital, one was conducted in a general hospital. In contrast, most participants are treated in clinics. One study did not report demographic details of the participants. In the four studies that compared single‐stage impression alginate and the two stage‐two step elastomer, more than 65% of the participants had a poor prognosis for complete dentures, with confounding variables equally distributed among the two groups. Therefore, our results are not applicable to different demographics of participants or settings.
In the single‐stage simplified denture fabrication method, none of the studies clearly stated the technique used to make the final impression with alginate, or its viscosity. In the two‐stage method, the technique of making the primary impression was not stated. The primary impression technique could clearly affect the outcome of the final impression. In four studies, the borders of the denture for single‐stage final impression with alginate were delineated using anatomical landmarks by marking on the cast, which could cause bias when the same examiner was not used, because it cannot be standardised for all casts, leading to under‐ or over‐extension of the denture borders. Three of the four studies that compared single‐stage impression alginate with the two stage‐two step elastomer, and one of the two studies that compared closed‐mouth two stage‐two step with addition silicone elastomer and open‐mouth two stage‐two step conventional technique using elastomer had cointerventions in one arm, which could either improve or nullify the effects of the intervention. We could not do subgroup analysis to confirm the effects of cointerventions, as most outcomes and follow‐ups had only one study.
When comparing impression materials, only one study compared polysulfide, polyether, impression wax, and zinc‐oxide eugenol impression paste, which is the most widely used material in many middle‐income countries (Regis 2013). Most studies used addition silicone (polyvinyl siloxane); impression plaster was used in none.
No studies compared functional method and neutral zone techniques for recording the final impression (see Figure 1), which is one of the preferred methods for participants with poor prognostic factors, to improve retention and stability of the dentures (Porwal 2013).
In general, the studies provided low‐ to very low‐quality evidence, with poor external validity, so we have little confidence in the results.
Removable partial dentures
We only found one study for removable partial dentures, which was at high risk of bias and provided only very low‐quality evidence. There were no studies that compared the same material and different techniques; different materials and the same technique for tooth‐supported conditions; or different dual‐impression techniques for tooth‐ and tissue‐supported conditions (Figure 2; Figure 3).
Quality of the evidence
The overall body of evidence for final‐impression techniques and materials in the fabrication of complete and removable partial dentures is very low quality. Most comparisons, outcomes, and follow‐up times were evaluated in single studies, and further research is highly likely to change the results and our confidence in them.
We have limited confidence in the results from one study that compared two stage–two step selective pressure final‐impression technique using alginate with silicone elastomeric impression materials for our primary outcome of oral health‐related quality of life as it used alginate as wash impression material (Table 3).
We have very little confidence in our estimate from two studies that compared single‐stage impression alginate with the two stage‐two step elastomer for oral health‐related quality of life. We downgraded the evidence because of risk of bias and participant characteristics (Table 4).
Potential biases in the review process
We have tried to minimise bias at every stage of the review. To find relevant studies, we searched all databases, with no restriction on language. We contacted authors to clarify eligibility for inclusion, request missing data, and checked risk of bias during data extraction. We did not do funnel plot analysis as we had only one study for most comparisons.
Agreements and disagreements with other studies or reviews
There are many qualitative reviews for complete denture impressions (Bitragunta 2011; Boucher 1951; Collett 1970; Daou 2010; Freeman 1969; Glupker H 1942; Rao 2010; Starcke 1975); one narrative review on complete denture impressions (Carlsson 2013); and one systematic review addressing complete denture fabrication methods (Paulino 2015), but we did not find any systematic reviews on complete denture impression techniques and materials. The narrative review included all study designs that assessed the type of impression techniques and materials, and inferred that single‐stage impression with alginate in stock tray was equal to, or slightly better than, two stage‐two step impression techniques. The systematic review on simplified versus traditional technique also concluded that there was some advantage of the simplified method with single‐stage impression with alginate over the traditional method using two stage‐two step impression technique, for chewing efficiency and quality of life. Neither review considered that 65% to 100% of the participants in both groups had a poor prognosis for complete dentures, hence we differed in our conclusions. Both the systematic review and narrative review agree that more high‐quality RCTs are required.
For removable partial dentures, we found only qualitative reviews, but no systematic review on impressions.
Authors' conclusions
Implications for practice.
There is very limited evidence on which to base selection of the final‐impression technique and material for fabrication of complete dentures and removable partial dentures.
Implications for research.
Complete dentures
Impression techniques and jaw registration methods are critical for the success of the complete dentures, and are controlled by participant factors, and operator and fabrication technique in clinics and labs.The most pertinent factor is the participant factor, which needs to controlled for prognostic factors or use the Prosthodontic Diagnostic Index (ACP classification; (Gray 2012)), while jaw registration, operator and fabrication techniques can be controlled in both groups.
The results of this review stress the need for future research. When conducting future trials, the following important factors must be taken into consideration.
There are multiple steps to fabricate prosthodontics; two vital steps for success with complete dentures are impression stage and jaw relation registration stage. When we compare impression techniques and materials, all further steps must be the same for both groups without any cointervention, until denture insertion. Further research is required to determine the minimally clinically important difference (MCID) for all outcomes, and investigators should use existing validated tools to measure them.
Study design: we prefer parallel‐group rather than cross‐over trials to prevent participants from receiving no treatment during the washout period; however, one study showed there was no period effect or carry‐over effect in a randomised cross‐over denture trial (Hyde 2014). When reporting cross‐over trials, data from both periods must be reported separately to enable them to be used in meta‐analysis; as a minimum, separate reporting of the first period data is required. Using unadjusted data in a review will lead to a judgement that the evidence is low quality.
Participants: although participant factors cannot be controlled in a clinical setting, we think that grouping participants based on prognostic factors using ACP classification, and evaluating those with favourable prognosis (ACP I and II) and unfavourable prognosis (III and IV) separately, will reduce the effect of participant factors on the intervention. Few studies compared ACP I and II using different materials and techniques. No trial addressed severely resorbed ridges using neutral zone techniques and flabby ridges separately.
Interventions and comparisons: more trials comparing the same materials and techniques used in this review are needed, as most comparisons were evaluated in only a single study. Future trials must compare different functional impression and neutral zone techniques for different participant characteristics not addressed in this review.
Outcomes: future trials should measure participant‐reported outcomes as primary outcomes, and use both the primary and secondary outcomes stated in this review to make future studies comparable with existing studies, for qualitative and quantitative analysis. Reporting outcomes with unadjusted dentures and post adjustments may show the real difference between the interventions, but it may lead to ethical problems, hence, correlating the number of adjustments with comfort may be clinically and ethically relevant.
Follow‐period: the period of follow‐up can be from 24 hours, to two weeks, one, three, six months and one year. Any follow‐up beyond one year may be confounded by resorption of the ridge. Most existing trials did not use the same follow‐up period and were therefore not comparable.
Removable partial dentures
Study design: we prefer parallel‐group trial to cross‐over trials because participants are allowed to wear their old dentures or are advised not to wear any dentures during the washout period of a cross‐over trial.
Intervention and comparison: more studies are required that compare the same materials and different techniques; different materials and the same technique for tooth‐supported conditions; and different dual impression techniques for tooth‐tissue‐supported conditions.
Outcomes: future trials must address participant‐reported outcome as primary outcomes, and should use the primary and secondary outcomes stated in this review.
Follow‐up period: we need trials with both short‐term and long‐term follow‐up periods.
Acknowledgements
The authors would like to acknowledge the contribution of Anne Littlewood (Information Specialist), Jo Weldon and Janet Lear (Cochrane Oral Health). We thank the Co‐ordinating Editors of Cochrane Oral Health (Professor Helen V Worthington and Professor Jan E Clarkson) and Prathap Tharyan (Director, Cochrane South Asia) for their immense contribution to producing this review. We would like to thank Dr. Fahd N. Al Qahtani, Dean of Al Baha University, Saudi Arabia, for his support. We also thank editor Philip Riley (Cochrane Oral Health), referee Ali Zaid and copy editor Victoria Pennick. This review is an output of a protocol development workshop organised by Cochrane South Asia (funded by UK aid, Department of International Development), CMC Vellore, India. For input to the protocol of the review, we would also like to thank Helen Wakeford and Tanya Walsh (Cochrane Oral Health), and referees Paul Hyde and Hugh Devlin.
I (SJ) would like to thank my wife Dr Rajeswari Avudaiappan for helping me with the preparation of the manuscript.
Appendices
Appendix 1. Cochrane Oral Health Trials Register search strategy
1 (edentulous:ti,ab) AND (INREGISTER) 2 ((teeth and (missing or absent or absence)):ti,ab) AND (INREGISTER) 3 ((denture* or "dental prosthes*"):ti,ab) AND (INREGISTER) 4 (#1 or #2 or #3) AND (INREGISTER) 5 (("dental impress*" or "dental imprint*"):ti,ab) AND (INREGISTER) 6 (("negative production" and (technique* or technic*)):ti,ab) AND (INREGISTER) 7 (((denture* or "dental prosthes*") and ("single stage" or "two stage" or "1 stage" or "2 stage" or "one stage" or "one step" or "1 step" or "two step" or "2 step")):ti,ab) AND (INREGISTER) 8 ((mucostatic or "muco compress*" or "selective pressure" or "functional impression technique*" or "neutral zone impression*" or "open mouth" or "closed mouth" or "border moulding" or "hand manipulat*" or "physiologic impression technique" or "Mcleans‐Hindels technique" or "functional reline method" or "fluid wax impression technique"):ti,ab) AND (INREGISTER) 9 ((cast* and (alter* or one‐piece)):ti,ab) AND (INREGISTER) 10 ((alginate* or "zinc oxide eugenol" or elastomer* or elastomeric or plaster*):ti,ab) AND (INREGISTER) 11 ((dental and negative and material*):ti,ab) AND (INREGISTER) 12 (#5 or #6 or #7 or #8 or #9 or #10 or #11) AND (INREGISTER) 13 (#4 and #12) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh "mouth, edentulous"] #2 edentulous #3 (teeth and (missing or absent or absence)) #4 [mh dentures] #5 (denture* or "dental prosthes*") #6 {or #1‐#5} #7 [mh ^"Dental impression technique"] #8 [mh ^"Dental casting technique"] #9 (("dental impress*" or "dental imprint*") near/5 (technique* or technic*)) #10 ("negative production" near/5 (technique$ or technic$)) #11 ((denture* or "dental prosthes*") and ("single stage" or "two stage" or "1 stage" or "2 stage" or "one stage" or "one step" or "1 step" or "two step" or "2 step")) #12 (mucostatic or "muco compress*" or "selective pressure" or "functional impression technique*" or "neutral zone impression*" or "open mouth" or "closed mouth" or "border moulding" or "hand manipulat*") #13 (cast* and (alter* or one‐piece)) #14 ("physiologic impression technique" or "Mcleans‐Hindels technique" or "functional reline method" or "fluid wax impression technique") #15 {or #7‐#14} #16 [mh "Dental impression materials"] #17 (alginate* or "zinc oxide eugenol" or elastomer* or elastomeric or plaster*) #18 ("dental impress*" near/5 material*) #19 ("dental imprint*" near/5 material*) #20 (dental near/5 negative near/5 material*) #21 {or #16‐#20} #22 #15 or #21 #23 #6 and #22
Appendix 3. MEDLINE Ovid search strategy
1. exp mouth, edentulous/ 2. edentulous.mp. 3. (teeth adj5 (missing or absent or absence)).mp. 4. exp Dentures/ 5. (denture$ or "dental prosthes$").mp. 6. or/1‐5 7. Dental impression technique/ 8. Dental casting technique/ 9. (("dental impress$" or "dental imprint") adj5 (technique$ or technic$)).mp. 10. ("negative production" adj5 (technique$ or technic$)).mp. 11. ((denture$ or "dental prosthes$") and ("single stage" or "two stage" or "1 stage" or "2 stage" or "one stage" or "one step" or "1 step" or "two step" or "2 step")).mp. 12. (mucostatic or "muco compress$" or "selective pressure" or "functional impression technique$" or "neutral zone impression$" or "open mouth" or "closed mouth" or "border moulding" or "hand manipulat$").mp. 13. (cast$ and (alter$ or one‐piece)).mp. 14. ("physiologic impression technique" or "Mcleans‐Hindels technique" or "functional reline method" or "fluid wax impression technique").mp. 15. or/7‐14 16. exp Dental impression materials/ 17. (alginate$ or "zinc oxide eugenol" or elastomer$ or elastomeric or plaster$).mp. 18. ("dental impress$" adj5 material$).mp. 19. ("dental imprint$" adj5 material$).mp. 20. (dental adj5 negative adj5 material$).mp. 21. or/16‐20 22. 15 or 21 23. 6 and 22 This search was combined with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. edentulous.mp. 2. (teeth adj5 (missing or absent or absence)).mp. 3. exp Denture/ 4. (denture$ or "dental prosthes$").mp. 5. or/1‐4 6. Dental impression/ 7. (("dental impress$" or "dental imprint") adj5 (technique$ or technic$)).mp. 8. ("negative production" adj5 (technique$ or technic$)).mp. 9. ((denture$ or "dental prosthes$") and ("single stage" or "two stage" or "1 stage" or "2 stage" or "one stage" or "one step" or "1 step" or "two step" or "2 step")).mp. 10. (mucostatic or "muco compress$" or "selective pressure" or "functional impression technique$" or "neutral zone impression$" or "open mouth" or "closed mouth" or "border moulding" or "hand manipulat$").mp. 11. ("physiologic impression technique" or "Mcleans‐Hindels technique" or "functional relinemethod" or "fluid wax impression technique").mp. 12. (cast$ and (alter$ or one‐piece)).mp. 13. or/6‐12 14. (alginate$ or "zinc oxide eugenol" or elastomer$ or elastomeric or plaster$).mp. 15. ("dental impress$" adj5 material$).mp. 16. ("dental imprint$" adj5 material$).mp. 17. (dental adj5 negative adj5 material$).mp. 18. or/14‐17 19. 13 or 18 20. 5 and 19
The above subject search was linked to adapted version of the Cochrane Embase Project filter for identifying RCTs in Embase Ovid (see www.cochranelibrary.com/help/central‐creation‐details.html for information):
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and WHO International Clinical Trials Registry Platform search strategy
denture and impression
denture and imprint
denture and material
Appendix 6. Overall risk of bias
| Risk of Bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Data and analyses
Comparison 1. Same technique, different materials: wax versus rubber.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for adjustments | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.38, 1.70] |
Comparison 2. Same technique, different materials: alginate versus silicone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral health‐related quality of life (OHIP edent score) | 1 | 144 | Mean Difference (Fixed, 95% CI) | 7.2 [2.71, 11.69] |
Comparison 3. Different techniques, different materials: single‐stage alginate versus two stage‐two step elastomer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral health‐related quality of life (OHIP‐EDENT score) | 2 | 98 | Mean Difference (Random, 95% CI) | 0.05 [‐2.37, 2.47] |
| 1.1 Parallel‐arm trial | 1 | 50 | Mean Difference (Random, 95% CI) | 0.1 [‐2.42, 2.62] |
| 1.2 Cross‐over trial | 1 | 48 | Mean Difference (Random, 95% CI) | ‐0.5 [‐8.94, 7.94] |
| 2 General satisfaction | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 At 1 month | 1 | Mean Difference (Random, 95% CI) | ‐6.50 [‐20.08, 7.08] | |
| 2.2 At 3 months | 1 | Mean Difference (Random, 95% CI) | 0.0 [‐7.17, 7.17] | |
| 2.3 At 6 months | 1 | Mean Difference (Random, 95% CI) | 0.0 [‐8.23, 8.23] | |
| 3 Maxillary denture satisfaction at 6 months | 2 | 155 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.34, 0.29] |
| 4 Mandibular denture satisfaction at 6 months | 2 | 155 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.11, 0.52] |
Comparison 4. Different techniques, different materials: single‐stage alginate versus two stage‐two step zinc‐oxide eugenol (ZoE).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral health‐related quality of life (OHIP‐EDENT score) | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | Mean Difference (Random, 95% CI) | ‐2.2 [‐5.57, 1.17] | |
| 1.2 At 6 months | 1 | Mean Difference (Random, 95% CI) | 0.5 [‐2.67, 3.67] | |
| 2 General satisfaction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.14, 72.88] |
| 2.2 At 6 months | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.14, 72.88] |
Comparison 5. Tooth‐tissue‐supported conditions: same material and different dual‐impression techniques.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intagilo adjustment | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.61, 3.34] |
| 2 Base adaptation | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.18, ‐0.04] |
| 3 Mobility | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Right abutment | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.87, 9.61] |
| 3.2 Left abutment | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.40, 3.37] |
| 4 Gingival index | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Right abutment | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.98] |
| 4.2 Left abutment | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.46, 2.64] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Firtell 1992.
| Methods | Study design: parallel‐group RCT Conducted in University of California, USA Number of centres: not stated Recruitment period: not stated Funding source: not stated Study duration: not stated | |
| Participants | 30 edentulous participants who had worn dentures previously were randomly assigned into two groups of 15 | |
| Interventions | Preliminary impression was made with irreversible hydrocolloid in stock metal tray for 30 participants Group 1 ‐ final‐impression wax used. 15 randomised; 13 completed study Group 2 ‐ light body polysulfide impression. 15 randomised; 14 completed study Dentures were inserted and adjustments were recorded over one year. | |
| Outcomes | Dentures inserted were evaluated for stability, patient comfort, health of oral tissues, and need for denture adjustments, checked after 24 hours, one week, six months, and one year. | |
| Notes | Only one‐year denture adjustment results were reported; other outcomes were not reported in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fifteen patients were randomly selected for final impression materials either a light‐body polysulfide rubber or wax." Comment: method of randomisation not stated in the article |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated in the article |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not stated in the article, but blinding of personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated in the article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: three participants in the wax final‐impression group and one participant in the polysulfide group were lost to follow‐up after one year; this was not stated clearly in the text of the article. |
| Selective reporting (reporting bias) | High risk | Quote: "Complete dentures made from these impressions were checked after 24 h, one week, six months, and one year to determine denture stability, patient comfort, health of oral tissues, and need for denture adjustments." Comment: only denture adjustments were reported for one year as planned. |
| Other bias | Low risk | Comment: no other bias found |
Frank 2004.
| Methods | Study design: parallel‐group RCT Conducted in: University of Washington, Seattle, USA Number of centres: 1 Recruitment period: not mentioned Funding source: none Study duration: not stated | |
| Participants | People undergoing routine prosthodontics treatment were drawn from the pre‐doctoral patient pool at the University of Washington. The inducement for participation was that no fees would be charged for treatment or for examination, and prophylaxis provided at one‐year recall. Inclusion criteria: patients receiving a mandibular Kennedy Class‐1 RPD with at least one indirect retainer on premolar or canine Exclusion criteria: none stated 72 participants were randomly assigned into two groups. Group 1: altered‐cast technique, 26 completed Group 2: one‐piece cast, 27 completed |
|
| Interventions |
Group 1: altered‐cast technique
First appointment: preliminary impression with stock tray
Second appointment: autopolymerising resin custom tray was underextended from the anticipated extension of the base by 0.5 mm and final impression with mixture of equal parts of light body and medium body
Third appointment: framework border moulding and impression of the base Lab procedure: altered cast was made Fourth appointment: jaw relation Fifth appointment: try‐in Sixth appointment: denture insertion. Group 2: one‐piece cast First appointment: preliminary impression with stock tray Second appointment: autopolymerising resin custom tray underextended from the anticipated extension of the base by 0.5 mm and final impression with light body and medium body mixture Lab procedure: one master cast was made Third appointment: fitting of the frame work Fourth appointment: jaw relation Fifth appointment: try‐in Sixth appointment: denture insertion |
|
| Outcomes | Multiple outcomes were evaluated at one year. Underextension and overextension of the denture base Base movement Base adaptation Sulcus depth of all abutments with direct retainer Mobility of direct retainer abutment Gingival index of direct retainer abutment Resorption of the edentulous ridge Tissue quality of the edentulous ridge General satisfaction of the participant Most liked feature of the RPD Most disliked feature of the RPD | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned randomly by computer program to a treatment group after the definitive cast and framework had been made." Comment: adequate method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "an evaluator, blind to the fabrication method, assessed border extensions by visual inspection and adjusted the borders" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: all outcomes were reported, but loss to follow‐up was 19/72 participants after one year |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Low risk | Comment: no other bias found |
Hyde 2010.
| Methods | Study design: cross‐over RCT (three arms) Conducted in Leeds Dental Institute, University of Leeds, United Kingdom Number of centres: 1 Recruitment period: November 2006 to November 2008 Funding source: funded by a grant from Dunhill Medical Trust Study duration: November 2006 to June 2009 | |
| Participants |
Inclusion criteria: people who were able to attend, with edentulous lower arch; had the mental foramen apparent clinically or radiographically on the denture‐bearing area of the lower ridge Exclusion criteria: people who were allergic to acrylic or silicone rubber 102 people assessed for eligibility. 69 randomised and allocated to block randomisation. 66 completed study |
|
| Interventions |
Intervention 1: control ‐ standard technique (relative mucostatic)
The dentures were constructed on a cast made using relatively mucostatic impression. The impression procedure used a spaced acrylic custom tray taken with medium‐bodied silicone material, followed by a light‐bodied silicone final impression. Intervention 2: traditional technique (redistribution of pressure) This method used 0.6 mm metal foils as relief, placed over the area of the mental foramina, and processed the finished denture on the spaced cast. Intervention 3: selective pressure technique To relieve pressure over the mental foramen, silicone from impression was removed by cutting from the area above the mental foramen. The custom impression tray was perforated in this area and final impression was made using light‐bodied silicone. Block randomisation was used to encode the dentures. The order of randomisation was maintained by a designated research nurse who delivered the encoded denture, without the prior knowledge of the clinician, for the participant to wear for one week. The participant wore each denture for one week and collected the second and third denture to assess each denture individually. The cross‐over did not have a washout period. Participants were given all three dentures together to wear for 1 to 2 weeks each, and asked to report the preferred denture. The preferred denture was worn for three months by the participant and all outcomes were assessed. |
|
| Outcomes | Primary outcome: impact of new dentures on quality of life by using OHIP‐14 self‐assessment questionnaire at baseline and after three months Secondary outcome: participant preference for dentures after four to five weeks of usage |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order in which the dentures were assessed by the patient was determined by a block randomisation procedure." |
| Allocation concealment (selection bias) | Low risk | Quote: "The order the encoded dentures were to be worn was revealed on the day of insertion of the first denture by a designated research nurse, without the prior knowledge of the clinician." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The clinician, dental nurse and participants were blinded to the method of fabrication of the dentures. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessments were performed by participants; they were recorded by the research nurse and the dentist remained blinded to the choice. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only three participants were lost to follow‐up, and this was accounted for in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes were reported as planned. |
| Other bias | Low risk | Comment: no other bias found |
Hyde 2014.
| Methods | Study design: cross‐over RCT Conducted in Leeds Dental Institute, University of Leeds, United Kingdom Number of centres:1 Recruitment period: not reported Funding source: funded by NIHR‐RfPB grant Study duration: April 2010 to January 2013 | |
| Participants | All edentulous patients, both male and female, over 18 years of age, were recruited from primary care referrals to the Leeds Dental Institute. Inclusion criteria: edentulous patients over 18 years of age who required complete dentures, and were willing to come for follow‐up and sign the informed consent Exclusion criteria: people who had an oral tumour, required an obturator, had extreme xerostomia, had a known hypersensitivity to silicone or alginate, or would benefit from selective pressure impressions 85 participants recruited, 83 initial randomisation, 80 habituation randomisation, 78 adjustment randomisation, 72 completed follow‐up |
|
| Interventions | The only difference between the two interventions was at the final‐impression stage; both the methods used two stage‐two step final impression, they varied only in the border moulding and final‐impression material used. Intervention group: alginate final‐impression material Preliminary impression: alginate impression materials with stock metal tray. Final impression done with custom tray fabricated with autopolymerising resin, border moulding with green stick, and final impression with alginate Intervention group: silicone final‐impression material Preliminary impression made with alginate impression materials with stock metal tray. For final impression, the trays used for silicone impressions were border moulded in silicone, using heavy‐bodied for the upper and regular‐bodied for the lower, and the final impression taken with light‐bodied silicone. All other phases of denture fabrication were the same for the two interventions. Participants wore the unadjusted dentures, based on randomised blocks coded as red and blue dots, with alternate wearing for two weeks (cross‐over) starting with red denture (habituation period). Primary outcome evaluated for preference for unadjusted dentures. Washout period eliminated by using a novel habituation method for two weeks of unadjusted dentures. Following the initial habituation period, dentures were re‐coded to block randomisation for green and yellow, and administered using an automated 24‐hour telephone system. Participants then wore dentures in the assigned order for two 8‐week periods (cross‐over), during which time adjustments were performed by an independent blinded clinician (adjustment period). Finally, participants took both sets of dentures for a final two‐week period (confirmation period), at the end of which they returned for the final assessment. |
|
| Outcomes | Primary outcome
1. Participant preference for the unadjusted dentures following the two‐week 'habituation period' Secondary outcomes 1. Patient perception of denture comfort, stability, and chewing efficiency of the dentures, using 5‐point Likert scales 2. Patient preference for the adjusted dentures following the two week 'confirmation period' 3. OHIP‐EDENT questionnaires assessing patient oral health‐related quality of life following each adjustment period 4. Patient perception of comfort and taste of each impression material using a 5‐point Likert scale at the impression stage 5. Patient preference for the impression of materials at the impression stage Baseline OHIP‐EDENT questionnaires were completed by participants prior to denture construction |
|
| Notes | Use of unadjusted dentures for habituation may raise an ethical issue as it is well established that all dentures require adjustment prior to denture insertion. In this trial, geriatric participants used unadjusted dentures for two weeks. This study published a protocol and presented outcomes in two publications. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The completed unadjusted dentures were labelled by random allocation with blue and red dots. The randomisation was blocked by variable block sizes to ensure balance between groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation was blocked by variable block sizes to ensure balance between groups, and concealed in sequentially numbered envelopes created by the statistician, and securely stored in the randomisation locker at DenTCRU." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The casts were allocated a number (blind to the clinician) which allowed the later identification of the dentures. At all subsequent stages of denture construction, the clinician was blind to the impression material used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients and the clinical team were blind to these allocations. Finally, patients took both sets of dentures for a final two‐week period (‘Confirmation Period’) at the end of which they returned for the final assessment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 11 participants lost to follow‐up but equally distributed across groups (85 participants recruited, 83 initial randomisation, 80 habituation randomisation, 78 in adjustment randomisation, 72 completed follow‐up) |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as planned. |
| Other bias | Low risk | No other bias found |
Jo 2015.
| Methods | Study design: cross‐over RCT Conducted in: Japan Graduate Dental School, Tokyo Number of centres: 1 Recruitment period: started August 2013 Funding source: supported by the Japanese Society for Promotion of Science Study duration: until one month follow‐up after denture insertion. August 2013 to October 2014 | |
| Participants | Patients who were edentulous in both arches and who required a new pair of dentures ‐ 11 males (46%) and 13 females (54%), with average age of 74 ± 9.6 years. More than 50% of participants in both groups belonged to the American College of Prosthodontics (ACP) class III.
Inclusion criteria: ability to independently travel to the clinic for prosthodontics of TMDU Hospital
Faculty; adequate understanding of written and spoken Japanese; ability to understand and respond to a
questionnaire. Exclusion criteria: dementia or existing psychiatric conditions; current use of dentures 277 participants recruited by telephone, 248 declined to participate, 29 assessed for eligibility, 2 excluded, 27 randomised and allocated to two groups of 14 and 13. Total of three participants withdrew (2 in the first phase and 1 in the second phase). All participants gave informed consent, signed a letter of consent, and underwent a preliminary examination that included a panoramic radiographic survey. |
|
| Interventions | The only difference between the two intervention was at the impression stage: the simplified method of fabricating CD used single‐stage impression for mandibular impression and two stage‐two step for maxillary impression; the conventional method used two stage‐two step final‐impression method. All other steps were the same for both groups. Two dentists with 10 to 12 years' experience performed the clinical procedure. Intervention group: simplified method Preliminary impression: alginate impression materials with stock metal tray. Final impression: custom tray fabricated autopolymerising resin, border moulding with green stick, and final impression with silicone (final cast not used for processing primary cast used) Jaw relation: single maxillomandibular relation record without facebow transfer and intraoral gothic arch tracing and articulated using average value articulator Try‐in: done Denture insertion: denture inserted and adjusted four times every week Intervention group: conventional method Preliminary impression: alginate impression materials with stock metal tray Final impression: custom tray fabricated autopolymerising resin, border moulding with green stick and final impression with silicone Jaw relation: single‐sitting maxillomandibular relation record without facebow transfer and intra oral gothic arch tracing and articulated using average value articulator Try‐in: done Denture insertion: denture inserted and adjusted four times every week Washout period was one month, and relined old dentures were used during the washout period. |
|
| Outcomes | Primary outcome: general participant satisfaction with the new dentures measured using 100‐mm visual analogue scales (VAS) from 0 = completely dissatisfied to 100 = completely satisfied Secondary outcome Oral health‐related quality of life (OHRQoL) measured using OHIP‐EDENT‐J. OHIP‐EDENT‐J has been cross‐culturally adapted for the Japanese population. | |
| Notes | The major difference between the two groups was that in the simplified method group, two‐stage final mandibular impression was used as a dummy and not used for processing, but the maxillary two‐stage final impression was used for processing. The authors confirmed the same when contacted. UMINCTR Clinical Trial, unique trial number: UMIN000009875 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation to the C‐S and S‐C groups was performed based on the classification system for complete edentulism of The American College of Prosthodontists (ACP) with stratified randomisation." |
| Allocation concealment (selection bias) | Low risk | Quote: "The C‐S group had the conventional method used first, followed by the simplified method. The S‐C group went through the procedures in the reverse order. None of the clinicians or participants were told about the methods or the order of allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding of the clinicians, the laboratory workers, and the researcher who performed assessments and analysis of outcomes was impossible because they could view the master casts." Quote: "Blinding of participants was possible as they were not told the order of fabrication, which method was used first (simplified or conventional), and the number of clinical appointments was the same in the conventional and simplified method groups, due to the inclusion of the mandibular final dummy impressions in the simplified method." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Blinding of the clinicians, the laboratory workers, and the researcher who performed assessments and analysis of outcomes was impossible because they could view the master casts." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: total number of participants was 27, allocated randomly to two groups of 14 and 13. Total of 3 participants withdrew (2 in the first phase and 1 in the second phase). All were accounted for in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as planned |
| Other bias | Low risk | No other bias found |
Kawai 2005.
| Methods | Study design: single‐blinded, parallel‐group RCT Conducted in Montreal General Hospital, Canada Number of centres: 1 Recruitment period and method: between December 2000 and December 2002, large public advertisement, and advertised in retirement homes in province of Québec, Canada Funding source: supported by the Nihon university grant for overseas research and the Suzuki Memorial grant from the Canadian Institutes of Health Research Study duration: follow‐up till May 2003 | |
| Participants | Both males and females who were edentulous and aged between 45
and 75 years Inclusion criteria: significant problems with at least one of their existing dentures. Possess an adequate understanding of written and spoken French, and ability to understand and respond to a test questionnaire. Exclusion criteria: symptoms of temporomandibular disorders, xerostomia, orofacial motor disorders, severe oral manifestations of systematic disease, or psychological or psychiatric conditions that could influence response to treatment 128 potential participants recruited after applying inclusion and exclusion criteria, 122 participants included in study and randomised to two groups Group T ‐ 61 randomised; 51 completed study Group S ‐ 61 randomised; 54 completed study |
|
| Interventions | Traditional procedure and simplified procedures differed in the following ways: methods of final impression, use of a facebow, type of articulator used, and use of a remount procedure.
Two experienced prosthodontists, familiar with both procedures treated participants Intervention group: simplified procedure Single impression: alginate impression materials with stock metal tray. Technique not mentioned Jaw relation: occlusal registration (without facebow transfer) articulated using average value articulator Occlusion: bilateral balanced occlusion Try‐in: done Denture insertion: denture inserted and was adjusted four times every week. Comparator group: traditional procedure Preliminary impression: alginate impression materials with stock metal tray; technique not mentioned Final impression: final impression was taken with a custom tray, border moulded with impression compound, and final impression with polyether rubber impression. Jaw relation: occlusal registration with facebow transfer and semi‐adjustable articulator was used along Occlusion: bilateral balanced occlusion Try‐in: done Denture insertion: remounting procedure done, denture inserted and adjusted until participant and clinician satisfied All lab work carried out by one commercial lab All participants returned for follow‐up visits at 3 and 6 months post denture insertion |
|
| Outcomes | Primary outcome: participant rating on a 100‐mm VAS of overall general satisfaction with their set of dentures Secondary outcomes: participant VAS ratings of the maxillary and mandibular dentures alone for satisfaction, comfort, stability, aesthetics, ability to speak, ease of cleaning, and ability to chew soft white bread, hard cheese, raw carrot, sausage, steak, raw apple, and lettuce. Data were gathered at the 3‐ and 6‐month follow‐up visits by a blinded research assistant. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was stratified by clinical condition as a potential confounder according to diagnostic guidelines issued by The American College of Prosthodontists (Class I–Class IV). A blinded research assistant assigned subjects to the groups using computer generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no adequate information available in article |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding of the clinicians to treatment allocation was not possible; however, the subjects were not told to which group they were assigned." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The clinical assessment of denture quality at the 6‐month recall was carried out by four prosthodontists, who were blinded both to group allocation and to the purpose of the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were 15/122 participants lost to follow‐up; however, the number of dropouts was equal in both groups. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as planned |
| Other bias | Low risk | Comment: no other bias found |
Matsuda 2015.
| Methods | Study design: cross‐over RCT Conducted in Dept of Prosthodontics, Osaka University, Japan Number of centres: 1 Recruitment period: not stated Funding source: supported by Research fund of Osaka University Graduate School of Dentistry Study duration: between November 2010 and April 2012 | |
| Participants | Edentulous patients were recruited from the outpatient roster of the Department of Removable Prosthodontics at the Osaka University Dental Clinic. Participants enrolled were both male and female, with a mean age of 75 years Inclusion criteria: (a) healthy adult requiring a new set of complete dentures, (b) complete dentures worn for at least 3 years previously, and (c) mentally receptive. Exclusion criteria: (a) dysfunction disorders of the masticatory system, (b) debilitating systemic disease, or oral mucosal disease, and (c) decline in cognitive function. 10 assessed for eligibility and then randomised BPSCD Group ‐ 5 completed follow‐up evaluation after 3 months CD Group ‐ 5 completed follow‐up evaluation after 3 months |
|
| Interventions | Treating clinicians were 10 resident dentists with a mean clinical experience of 2 years. Each clinician attended one participant.
Intervention group: biofunctional prosthetic system complete denture fabrication method (BPSCD) First appointment: preliminary impression with the Accu‐dent System I using low viscosity syringe irreversible hydrocolloid material and high viscosity irreversible hydrocolloid as tray material. A tentative maxillomandibular relationship was recorded using centric tray. Second appointment: custom trays fabricated with the help of the Gnathometer M tracing. The final impressions were made with the patient in the 'mouth‐closed' position, using both light‐ and heavy‐body vinyl polysiloxane impression material. At the same appointment, the vertical maxillomandibular relationship and horizontal relation recorded using Gnathometer M intraoral gothic arch tracing device with a silicone bite registration paste. Occlusion: bilateral balanced occlusion with semi‐anatomic teeth Third appointment: try‐in was done Fourth appointment: denture inserted and adjustments were done for one week; the end of the adjustment process was when the participant was free of pain or major inconvenience. Comparator group: conventional complete denture fabrication method First appointment: preliminary impression with irreversible hydrocolloid impression materials with stock metal tray. Technique not mentioned Second appointment: final impression taken with a custom tray, border moulded with impression compound and final impression with Hydrophilic vinyl polysiloxane impression material Third appointment jaw relation: vertical maxillomandibular registration at 2 mm and horizontal jaw relation using silicone occlusal registration paste. Occlusion: bilateral balanced occlusion with semi‐anatomic teeth Fourth appointment: try‐in and any adjustment was done Fifth appointment: denture inserted and adjustments done for one week; the end of the adjustment process was when the participant was free of pain or major inconvenience. All lab work was carried out by one dental technician with 10 years experience at the dental laboratory of the Osaka dental hospital. Participants returned for follow‐up visits after 3 months, no washout period before second denture insertion, follow‐up after 3 months post‐insertion. |
|
| Outcomes | 1. Oral Health‐Related Quality of Life questionnaire, specially designed for edentulous people (Oral Health Impact Profile for edentulous subjects (OHIP‐EDENT)) 2. Number of adjustments required for each technique to deliver pain‐free fitting 3. Participants were asked to state which denture gave them superior occlusal feel, comfort, aesthetics, and retention, and which dentures they wished to keep for the long term. All outcomes were evaluated after 3 months; there was no washout period before second denture insertion; follow‐up was three months post‐insertion. |
|
| Notes | Both authors, Kenichi Matsuda and Yoshinobu Maeda, are remunerated instructors who have given educational lectures at the request of the Ivoclar Vivadent company, and conducted and supervised the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly divided into two groups (Group 1 or Group 2) within the stratified blocks of participants. The blocks were generated from a random‐number table by a specialist in statistics." Comment: adequate method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly divided into two groups (Group 1 or Group 2) within the stratified blocks of participants. The blocks were generated from a random‐number table by a specialist in statistics." Comment: not stated clearly in the article |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "To maintain the “blind” nature of the trial, patients were not informed about the type of dentures they had received or about the differences between the two types. However, due to the design of this clinical trial, blinding of the treating clinicians and dental technicians was not possible." Comment: blinding is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Each clinician attended to one patient." Quote: "All the patients attended the follow‐up appointments. The clinical outcomes were assessed after three months of comfortably wearing the set of dentures (calculated from the 1st day on which the patients felt no pain or discomfort with the new dentures). Subsequently, the prostheses were changed and a second evaluation was carried out after three months." Comment: blinding of outcome assessors was not done as each clinician attended to one participant. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up. 10 randomised ‐ 5 to BPSCD group and 5 to CD group. All 10 completed follow‐up evaluation after three months. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as planned |
| Other bias | Low risk | Comment: no other bias found |
Nunez 2015.
| Methods | Study design: parallel‐group RCT Conducted in: Federal University of Goias, Brazil Number of centres: 1 Recruitment period: not reported Funding source: supported by a grant from Brazillian National Research Council and State Foundation Research of Goias Study duration: from June 2010 and to April 2012 | |
| Participants | Patients from the Brazilian public health system referred for complete dentures at the School of Dentistry of the Federal University of Goias. Inclusion criteria: both males and females, aged between 63 to 65 years, fully edentulous, requiring new upper and lower conventional CD Exclusion criteria: unable to cooperate, or with poor general health, or conditions that could influence response to treatment, such as temporomandibular disorders, orofacial motor disorders, severe oral manifestations of systematic disease, or a psychological or psychiatric condition 50 participants allocated into two groups Traditional group: 25 randomised; 23 completed follow‐up Simplified group: 25 randomised; 22 completed follow‐up |
|
| Interventions | The traditional method and simplified method differed in the following ways: methods of impression and use of a facebow. One operator performed all clinical procedures. Intervention group: simplified method Single impression: alginate impression materials with stock metal tray. Technique not mentioned Jaw relation: occlusal registration (without facebow transfer) articulated using average setting on semi‐adjustable articulator Occlusion: bilateral balanced occlusion Try‐in: bilateral balanced articulation check during try‐in Denture insertion: denture inserted and adjustments concluded when the clinician and participants agreed to terminate the visit. Comparator group: traditional method Preliminary impression: alginate impression materials with stock metal tray. Technique not mentioned Final impression: selective‐pressure final impression taken with a custom tray, border moulded with impression compound, and final impression with polysulfide impression Jaw relation: occlusal registration with facebow transfer and articulated using average setting on semi‐adjustable articulator Occlusion: bilateral balanced occlusion Try‐in: bilateral balanced articulation check during try‐in Denture insertion: denture inserted and adjustments concluded when the clinician and the participants agreed to terminate the visit. No remount procedure was done in either group. All lab work was carried out by same dental technician, using standardised procedure for both groups. Participants returned for follow‐up visits at 30 days (1 month) and 6 months post‐insertion. |
|
| Outcomes | 1. Oral health‐related quality of life (Brazilian OHIP‐EDENT). 4 different scale domains were assessed: (i) masticatory discomfort and disability (4 items), (ii) psychological discomfort and disability (5 items), (iii) social disability (5 items), and (iv) oral pain and discomfort (5 items). 2. Satisfaction of upper and lower denture on a 10‐point VAS 3. General satisfaction with dentures was measured using the participants’ ratings of their overall satisfaction and satisfaction with comfort, stability, aesthetics, ability to speak, and ability to chew. All satisfaction items measured on a 10‐point VAS All treatment outcomes measured before insertion of new denture, at 30 days (1 month) and 6 months post‐insertion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All participants who met the inclusion criteria signed an informed consent and were randomly assigned by simple randomisation to two groups." Comment: method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All treatments were performed by the same dentist." Comment: not clearly stated in the article. Trial author did not respond to this query. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "All treatments were performed by the same dentist. Blinding of the dentist to group allocation was not possible, though participants were not aware of the treatment protocol to which they were assigned." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome assessments were performed by a dentist blinded to the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 5 participants lost to follow‐up in both groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: overall general satisfaction with dentures not reported, but maxillary and mandibular denture satisfaction were reported separately. Trial authors did not respond to this query. |
| Other bias | Low risk | Comment: no other biases found |
Regis 2013.
| Methods | Study design: parallel‐arm RCT Conducted in Ribeirao Preto Dental School, Brazil Number of centres: 1 Recruitment period: October 2010 to April 2011 Funding source: funded by FAPESP Study duration: not mentioned | |
| Participants | Participants, both male and female, aged over 45 years and edentulous for at least 1 year, requesting treatment with upper and lower complete dentures, were recruited through referral by the Brazilian public health system to the Ribeirao Preto Dental School. Inclusion criteria: mental receptiveness, good understanding of spoken Portuguese, and provision of informed consent Exclusion criteria: compromised masticatory system disorders, pathological changes of residual ridges, and debilitating systemic diseases 72 assessed, 42 randomised, 39 completed study S group ‐ 21 participants, 19 completed study at 6 month follow‐up C group ‐ 21 participants, 20 completed study at 6 month follow‐up |
|
| Interventions | The traditional method and conventional method of CD fabrication differed in the following ways: methods of impression and use of a facebow and single try‐in appointment for one group. Intervention group: simplified method Single impression: irreversible hydrocolloid impression materials with stock metal tray. Technique not mentioned Jaw relation: registration of maxillomandibular relationship (without facebow transfer) articulated using 15‐degree flat plane indicator on semi‐adjustable articulator Try‐in: done Denture insertion: denture inserted and adjustments done at 1st, 7th, and 14th day; further appointment if required, until no participants presented with discomfort or mucosal irritation Comparator group: traditional method Preliminary impression: alginate impression materials with stock metal tray. Technique not mentioned Final impression: taken with a custom tray, border moulded with impression compound and final impression with zinc‐oxide eugenol impression paste Jaw relation: maxillomandibular registration with facebow transfer and articulated using average setting on semi‐adjustable articulator of 30‐degree horizontal condylar inclination, and 15‐degree lateral condylar inclination Occlusion: anatomic teeth with 33 bilateral balanced occlusion Anterior try‐in: anterior teeth done separately Posterior try‐in: posterior teeth try‐in Denture insertion: denture inserted and adjustments done at 1st, 7th, and 14th day; further appointment if required, until no participants presented with discomfort or mucosal irritation No remount procedure done in either group All lab work carried out by same dental technician under the supervision of the operating clinician |
|
| Outcomes | Outcomes evaluated at baseline, 3 months and 6 months for: 1. Patient‐reported OHRQoL using OHIP‐EDENT questionnaire 2. Patient‐reported satisfaction of the denture 3. Operator‐assessed quality of the denture | |
| Notes | Single study published as three publications for different outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly divided into two groups and received new dentures fabricated according to a simplified (Group S) or a conventional method (Group C – comparator). Allocation to group was performed according to a sequence of computer‐generated random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "A researcher (R2) uninvolved with other trial procedure prepared and secured the codes, which were concealed by means of numbered, opaque, and sealed envelopes. For each participant, an envelope was opened after the first appointment, following the obtainment of an initial set of casts." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: one publication of the study states that blinding was done whenever applicable Comment: it was not possible to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A blinded researcher (P3) applied both instruments before treatment (baseline) and at the 3‐ and 6‐month follow‐up appointments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 39 participants completed the study. Only 3 participants lost to follow‐up, so it is unlikely the loss to follow‐up influenced the outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes stated in methods section were reported in results section |
| Other bias | Low risk | No other biases found |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adnan 2010 | This study was not randomised and it evaluated denture bases and not dentures. |
| Al‐Judy 2015 | This study was not randomised and it evaluated denture bases and not dentures. |
| Au 2000 | This study evaluated removable partial dentures made with two different types of materials. |
| Birtles 2015 | The outcomes were not patient‐reported and the study compared two neutral zone impressions with and without maxillary denture on mounted dental cast. |
| DRKS00000149 | The study assessed relining of the denture and not impression materials for complete denture fabrication. |
| Heydecke 2008 | This study compared the same impression techniques and material for both groups. |
| Hochman 1998 | This study was not randomised and it evaluated RPD frame work and not the prosthesis. |
| Hundal 2015 | The outcomes were evaluated for two different types of prosthesis, one being flexible denture. |
| McCord 2005 | Only the mandibular denture was assessed and reported in this study in completely edentulous patients. |
| Nascimento 2004 | This study compared the same impression techniques and material for both groups. |
| NCT03025555 | Outcomes were evaluated for two different types of RPD, one was a flexible denture (polyamide), and the other was polyetheretherketone. |
| NCT03043456 | Outcomes were evaluated for two different types of prostheses, one was a flexible complete denture (polyamide). |
| NCT03234803 | Outcomes were evaluated for two different types of prostheses, one was a flexible denture. |
| RBR‐8fs5ww | The study assessed removable partial denture versus complete denture. |
| Sharif 2013 | This study was quasi‐randomised. |
| Tasleem 2013 | This study was not randomised. |
| Wegner 2011 | This study compared two relining materials on dentures and not impression material. |
Characteristics of ongoing studies [ordered by study ID]
NCT02339194.
| Trial name or title | Application of a simplified method of complete denture fabrication for severely resorbed mandibular ridges |
| Methods |
Study design: single‐blinded, Phase 3 parallel RCT Conducted in University Federal do Ceara, Brazil Number of centres: 1 Recruitment period: not stated Funding source: University Federal do Ceara Study duration: 18 months |
| Participants | Participant estimate: 72 Inclusion criteria: (1) severely resorbed mandibular alveolar bones; (2) complete edentulism for at least one year; (3) desire to receive a pair of new conventional complete dentures; (4) mental receptiveness; (5) good understanding of spoken Portuguese; (6) both males and females 45 years and older Exclusion criteria: (1) disorders of the masticatory system; (2) pathological changes of residual ridges; (3) debilitating systemic diseases |
| Interventions | Procedure: denture fabrication technique Group A: experimental ‐ simplified protocol ‐ denture fabrication technique (single impression with alginate through prefabricated trays for both arches) Group B: no intervention – traditional protocol (only mandibular secondary impression made with border moulding using compound and impression rubber in custom tray) |
| Outcomes | Primary outcomes: oral health‐related quality of life (time frame 6 months) assessed using Brazilian version of Oral Health Impact Profile for edentulous patients inventory Secondary outcomes: (1) satisfaction with dentures assessed by means of two specific questionnaires (time frame 6 months); (2) denture quality by clinical examination (time frame 3 months); (3) masticatory performance assessed by colorimetric method (time frame 6 months) |
| Starting date | January 2014 |
| Contact information | Samara M Sales DDS, samarah_sales@hotmail.com, 8596523217 |
| Notes | Recruiting participants |
Differences between protocol and review
We simplified the title. The following terms were added to the search strategy ("physiologic impression technique", "Mcleans‐Hindels technique", "functional reline method", "fluid wax impression technique"). We were unable to use many of our planned methods due to lack of data.
Contributions of authors
Dr Srinivasan Jayaraman: developing the idea and creating the framework for the review, screening search records, assessing risk of bias in the included studies, writing and editing all sections of the review, providing clinical expertise Dr Balandra Singh: writing and editing the Background and Methods, screening search records, assessing risk of bias in the included studies Dr Ramanathan: writing and editing all sections of the review, providing clinical expertise Dr Murkukan Pazhaniappan Pillai: writing and editing all sections of the review, checking risk of bias assessments, providing clinical expertise L MacDonald: assisting with review writing, editing the review R Kirubakaran: extracting and analysing data, editing the review
Sources of support
Internal sources
Cochrane South Asia, Prof. BV Moses Center for Evidence‐Informed Health Care and Health Policy, Christian Medical College, Vellore, India.
-
King George's Medical University, Lucknow, India.
Balendra Pratap Singh received salary, IT, library support and travel support to attend "Protocol Development Workshop" at CMC, Vellore.
School of Dentistry, The University of Manchester, Manchester Academic Health Sciences Centre (MAHSC) and NIHR Manchester Biomedical Research Centre, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (http://oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and Swiss Society for Endodontology, Switzerland.
-
Department for International Development (DFID), UK.
Project funding for the Effective Healthcare Research Consortium; salary for Richard Kirubakaran during the review stage.
Declarations of interest
Srinivasan Jayaraman: is the principal investigator of an RCT being conducted on impression techniques in complete dentures.The author has no financial conflict of interest as the study is self‐funded and yet to start. Richard Kirubakaran: none known Balendra Pratap Singh: none known Murukan Pazhaniappan Pillai: none known Laura MacDonald: none known Balasubramanian Ramanathan: none known
Edited (no change to conclusions)
References
References to studies included in this review
Firtell 1992 {published data only}
- Firtell DN, Koumjian JH. Mandibular complete denture impressions with fluid wax or polysulfide rubber: a comparative study. Journal of Prosthetic Dentistry 1992;67(6):801‐4. [DOI] [PubMed] [Google Scholar]
Frank 2004 {published data only}
- Frank RP, Brudvik JS, Noonan CJ. Clinical outcome of the altered cast impression procedure compared with use of a one‐piece cast. Journal of Prosthetic Dentistry 2004;91(5):468‐76. [DOI] [PubMed] [Google Scholar]
Hyde 2010 {published data only}
- Hyde TP, Craddock HL, Blance A, Brunton PA. A cross‐over randomised controlled trial of selective pressure impressions for lower complete dentures. Journal of Dentistry 2010;38(11):853‐8. [DOI] [PubMed] [Google Scholar]
Hyde 2014 {published data only}
- Gray JC, Navarro‐Coy N, Pavitt SH, Hulme C, Godfrey M, Craddock HL, et al. Improvdent: improving dentures for patient benefit. A crossover randomised clinical trial comparing impression materials for complete dentures. BMC Oral Health 2012;12:37‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C, Yu G, Browne C, O'Dwyer J, Craddock H, Brown S, et al. Cost effectiveness of silicone and alginate impressions for complete dentures. Journal of Dentistry 2014;42(8):902‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TP. Regarding missing data on OHIP [personal communication]. Email to: Hyde 16 August 2017.
- Hyde TP, Craddock HL, Gray JC, Pavitt SH, Hulme C, Godfrey M, et al. A randomised controlled trial of complete denture impression materials. Journal of Dentistry 2014;42(8):895‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jo 2015 {published data only}
- Jo. Regarding missing data [personal communication ]. Email to : Manabu kanazawa 21 March 2017.
- Jo A, Kanazawa M, Sato Y, Iwaki M, Akiba N, Minakuchi S. A randomized controlled trial of the different impression methods for the complete denture fabrication: patient reported outcomes. Journal of Dentistry 2015;43(8):989‐96. [PUBMED: 26051546] [DOI] [PubMed] [Google Scholar]
Kawai 2005 {published data only}
- Kawai Y, Murakami H, Shariati B, Klemetti E, Blomfield JV, Billette L, et al. Do traditional techniques produce better conventional complete dentures than simplified techniques?. Journal of Dentistry 2005;33(8):659‐68. [PUBMED: 16139697] [DOI] [PubMed] [Google Scholar]
- Kawai Y, Murakami H, Takanashi Y, Lund JP, Feine JS. Efficient resource use in simplified complete denture fabrication. Journal of Prosthodontics 2010;19(7):512–6. [DOI] [PubMed] [Google Scholar]
Matsuda 2015 {published data only}
- Matsuda K, Kurushima Y, Maeda Y, Enoki K, Mihara Y, Ikebe K. Crossover trial for comparing the biofunctional prosthetic system with conventional procedures. European Journal of Prosthodontics 2015; Vol. 3:64‐70.
Nunez 2015 {published data only}
- Nunez. Regarding missing information [perosnal communication]. Email to : Claudio Rodrigues lesle 28 March 2017.
- Nunez MC, Silva DC, Barcelos BA, Leles CR. Patient satisfaction and oral health‐related quality of life after treatment with traditional and simplified protocols for complete denture construction. Gerodontology 2015;32(4):247‐53. [PUBMED: 24147575] [DOI] [PubMed] [Google Scholar]
Regis 2013 {published data only}
- Cunha TR, Della Vecchia MP, Regis RR, Ribeiro AB, Muglia VA, Mestriner W, et al. A randomised trial on simplified and conventional methods for complete denture fabrication: masticatory performance and ability. Journal of Dentistry 2013;41(2):133‐42. [DOI] [PubMed] [Google Scholar]
- Della Vecchia MP, Regis RR, Cunha TR, Andrade IM, Matta JCS, Souza RF. A randomized trial on simplified and conventional methods for complete denture fabrication: cost analysis. Journal of Prosthodontics 2014;23(3):182‐91. [DOI] [PubMed] [Google Scholar]
- Regis RR, Cunha TR, Della Vecchia MP, Ribeiro AB, Silva Lovoto CH, Souza RF. A randomised trial of a simplified method for complete denture fabrication: patient perception and quality. Journal of Oral Rehabilitation 2013;40:535‐45. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adnan 2010 {published data only}
- Adnan M, AlJudy HJ, Fatihallah AA. Comparative study maxillary complete denture base retention using different impression materials. Iraq Academic Scientific Journals 2010;7(1):126‐32. [Google Scholar]
Al‐Judy 2015 {published data only}
- Al‐Judy HJ. Comparison of the effect of sectional border moulding using different molding and final impression materials on the retention of maxillary complete denture bases. IOSR ‐ Journal of Dental and Medical Sciences 2015;14(7):35‐40. [Google Scholar]
Au 2000 {published data only}
- Au AR, Lechner SK, Thomas CJ, Mori T, Chung P. Titanium for removable partial dentures (III): 2‐year clinical follow‐up in an undergraduate programme. Journal of Oral Rehabilitation 2000;27(11):978‐84. [DOI] [PubMed] [Google Scholar]
Birtles 2015 {published data only}
- Birtles A, Craddock H, Kang J, Hyde TP. A randomised controlled study comparing the anterior mandibular labio‐lingual neutral zone position in edentulous subjects with and without their maxillary denture in‐situ. The European Journal of Prosthodontics and Restorative Dentistry 2015;23(2):78‐84. [PubMed] [Google Scholar]
DRKS00000149 {published data only}
- DRKS00000149. A clinical comparison of active and passive functional impression techniques in edentulous elderly patients. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00000149 (first received 30 June 2009; note: this trial was registered retrospectively).
Heydecke 2008 {published data only}
- Heydecke G, Wolkewitz M, Türp JC, Strub JR. Simplified versus comprehensive fabrication of complete dentures: patient ratings of denture satisfaction from a randomized crossover trial. Quintessence International 2008;39(2):107‐16. [PubMed] [Google Scholar]
Hochman 1998 {published data only}
- Hochman N, Yaniv O. Comparative clinical evaluation of removable partial dentures made from impressions with different materials. Compendium of Continuing Education in Dentistry 1998;19(2):200‐2, 204‐6. [PubMed] [Google Scholar]
Hundal 2015 {published data only}
- Hundal M, Madan R. Comparative clinical evaluation of removable partial dentures made of two different materials in Kennedy Applegate class II partially edentulous situation. Medical Journal Armed Forces India 2015;71(Suppl 2):S306‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCord 2005 {published data only}
- McCord JF, McNally LM, Smith PW, Grey NJA. Does the nature of the definitive impression material influence the outcome of (mandibular) complete dentures?. European Journal of Prosthodontics and Restorative Dentistry 2005;13(3):105‐8. [PubMed] [Google Scholar]
Nascimento 2004 {published data only}
- Nascimento DFF, Patto RBL, Marchini L, Cunha VPP. Double‐blind study for evaluation of complete dentures made by two techniques with and without face‐bow. Brazilian Journal of Oral Sciences 2004;3(9):439‐45. [Google Scholar]
NCT03025555 {published data only}
- NCT03025555. Patient satisfaction and dimensional accuracy of Bre‐Flex and Peek for removable partial dentures. clinicaltrials.gov/show/NCT03025555 (first received 19 January 2017).
NCT03043456 {published data only}
- NCT03043456. Evaluation of patient satisfaction and microbiological changes in injectable thermoplastic resin and conventional acrylic resin complete dentures. clinical.gov/show/NCT03043456trials (first received 6 February 2017).
NCT03234803 {published data only}
- NCT03234803. Metal reinforced flexible poly‐amide complete denture versus heat cured polymethyl methacrylate in terms of oral health related quality of life: randomized clinical trial. clinicaltrials.gov/show/NCT03234803 (first received 31 July 2017).
RBR‐8fs5ww {published data only}
- RBR‐8fs5ww. A clinical study comparing the technique of forming functional model changed and direct functional impression technique for removable partial dentures free distal end. www.ensaiosclinicos.gov.br/rg/RBR‐8fs5ww/v1/xml/ictrp (first received 26 July 2012).
Sharif 2013 {published data only}
- Sharif M, Azad AA, Ahmad S. Comparison of patients satisfaction level with complete dentures fabricated by neutral zone technique and conventional technique. Pakistan Oral and Dental Journal 2013;33(1):187‐91. [Google Scholar]
Tasleem 2013 {published data only}
- Tasleem R, Bin Saeed MH, Javed MU. Comparison of complete denture fabricated by two different border molding materials, in terms of patients' satisfaction. Journal of Ayub Medical College, Abbottabad: JAMC 2013;25(3‐4):78‐80. [PubMed] [Google Scholar]
Wegner 2011 {published data only}
- Wegner K, Zenginel M, Buchtaleck J, Rehmann P, Wostmann B. Influence of two functional complete‐denture impression techniques on patient satisfaction: dentist‐manipulated versus patient‐manipulated. International Journal of Prosthodontics 2011;24(6):540‐3. [PubMed] [Google Scholar]
References to ongoing studies
NCT02339194 {published data only}
- NCT02339194. Application of a simplified method of complete denture fabrication for severely resorbed mandibular ridges. clinicaltrials.gov/ct2/show/record/NCT02339194 (first received 15 January 2015).
Additional references
Addison 1944
- Addison PI. Mucostatic impressions. Journal of the American Dental Association 1944;31(13):941‐6. [Google Scholar]
Akerly 1978
- Akerly WB. A combination impression and occlusal registration technique for extension‐base removable partial dentures. Journal of Prosthetic Dentistry 1978;39(2):226‐9. [DOI] [PubMed] [Google Scholar]
Al‐Ahmar 2008
- Al‐Ahmar AO, Lynch CD, Locke M, Youngson CC. Quality of master impressions and related materials for fabrication of complete dentures in the UK. Journal of Oral Rehabilitation 2008;35(2):111‐15. [DOI] [PubMed] [Google Scholar]
Applegate 1937
- Applegate OC. The cast saddle partial denture. Journal of the American Dental Association 1937;24(8):1280‐91. [Google Scholar]
Applegate 1960
- Applegate OC. An evaluation of the support for the removable partial denture. Journal of Prosthetic Dentistry 1960;10(1):112‐23. [Google Scholar]
Atwood 1971
- Atwood DA. Reduction of residual ridges: a major oral disease entity. Journal of Prosthetic Dentistry 1971;26(3):266‐79. [DOI] [PubMed] [Google Scholar]
Bell 1968
- Bell DH Jr. Problems in complete denture treatment. Journal of Prosthetic Dentistry 1968;19(6):550‐60. [DOI] [PubMed] [Google Scholar]
Beresin 1976
- Beresin VE, Schiesser FJ. The neutral zone in complete dentures. Journal of Prosthetic Dentistry 1976;36(4):356‐67. [DOI] [PubMed] [Google Scholar]
Bitragunta 2011
- Bitragunta RP, SashiPurna CR, Mallikarjun M. Systematic review of complete denture impression techniques. Indian Journal of Dental Advancements 2011;3(4):673‐80. [Google Scholar]
Blatterfein 1980
- Blatterfein L, Klein IE, Miglino JC. A loading impression technique for semiprecision and precision removable partial dentures. Journal of Prosthetic Dentistry 1980;43(1):9‐14. [DOI] [PubMed] [Google Scholar]
Boucher 1943
- Boucher CO. Impressions for complete dentures. Journal of the American Dental Association 1943;30(1):14‐25. [DOI] [PubMed] [Google Scholar]
Boucher 1944
- Boucher CO. Complete denture impressions based upon the anatomy of the mouth. Journal of the American Dental Association 1944;31(17):1174‐81. [Google Scholar]
Boucher 1951
- Boucher CO. A critical analysis of mid‐century impression techniques for full dentures. Journal of Prosthetic Dentistry 1951;1(4):472‐91. [DOI] [PubMed] [Google Scholar]
Brunello 1998
- Brunello DL, Mandikos MN. Construction faults, age, gender, and relative medical health: factors associated with complaints in complete denture patients. Journal of Prosthetic Dentistry 1998;79(5):545‐54. [DOI] [PubMed] [Google Scholar]
Cagna 2009
- Cagna DR, Massad JJ, Schiesser FJ. The neutral zone revisited: from historical concepts to modern application. Journal of Prosthetic Dentistry 2009;101(6):405‐12. [DOI] [PubMed] [Google Scholar]
Carlsson 2013
- Carlsson GE, Örtorp A, Omar R. What is the evidence base for the efficacies of different complete denture impression procedures? A critical review. Journal of Dentistry 2013;41(1):17‐23. [DOI] [PubMed] [Google Scholar]
Chaffee 1999
- Chaffee NR, Cooper LF, Felton DA. A technique for border molding edentulous impressions using vinyl polysiloxane material. Journal of Prosthodontics 1999;8(2):129‐34. [DOI] [PubMed] [Google Scholar]
Collett 1970
- Collett HA. Final Impressions for complete dentures. Journal of Prosthetic Dentistry 1970;23(3):250‐64. [DOI] [PubMed] [Google Scholar]
Critchlow 2011
- Critchlow SB, Ellis JS, Field JC. Reducing the risk of failure in complete denture patients. Dental Update 2011;39(6):427‐30. [DOI] [PubMed] [Google Scholar]
Cunha 2013
- Cunha TR, Della Vecchia MP, Regis RR, Ribeiro AB, Muglia VA, Mestriner W, et al. A randomised trial on simplified and conventional methods for complete denture fabrication: masticatory performance and ability. Journal of Dentistry 2013;41(2):133‐42. [DOI] [PubMed] [Google Scholar]
Cunha‐Cruz 2007
- Cunha‐Cruz J, Hujoel PP, Nadanovsky P. Secular trends in socio‐economic disparities in edentulism: USA, 1972‐2001. Journal of Dental Research 2007;86(2):131‐6. [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in Medicine 2002;21(15):2145‐9. [DOI] [PubMed] [Google Scholar]
Daou 2010
- Daou EE. The elastomers for complete denture impression: a review of the literature. Saudi Dental Journal 2010;22(4):153‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeVan 1952
- DeVan MM. The nature of the partial denture foundation: suggestions for its preservation. Journal of Prosthetic Dentistry 1952;2(2):210‐8. [Google Scholar]
Drago 2003
- Drago CJ. A retrospective comparison of two definitive impression techniques and their associated postinsertion adjustments in complete denture prosthodontics. Journal of Prosthodontics 2003;12(3):192‐7. [DOI] [PubMed] [Google Scholar]
El‐Khodary 1985
- El‐Khodary NM, Shaaban NA, Abdel‐Hakim AM. Effect of complete denture impression technique on the oral mucosa. Journal of Prosthetic Dentistry 1985;53(4):543‐9. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Emami 2013
- Emami E, Souza RF, Kabawat M, Feine JS. The impact of edentulism on oral and general health. International Journal of Dentistry 2013;2013:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 1969
- Freeman SP. Impressions for complete dentures. Journal of the American Dental Association 1969;79(5):1173‐8. [DOI] [PubMed] [Google Scholar]
Friedman 1957
- Friedman S. Edentulous impression procedures for maximum retention and stability. Journal of Prosthetic Dentistry 1957;7(1):14‐26. [Google Scholar]
Friedman 2014
- Friedman PK, Kaufman LB, Karpas SL. Oral health disparity in older adults: dental decay and tooth loss. Dental Clinics of North America 2014;58(4):757‐70. [DOI] [PubMed] [Google Scholar]
Gift 1992
- Gift HC, Redford M. Oral health and the quality of life. Clinics in Geriatric Medicine 1992;8(3):673‐83. [PubMed] [Google Scholar]
Gil‐Montoya 2015
- Gil‐Montoya JA, Mello AL, Barrios R, Gonzalez‐Moles MA, Bravo M. Oral health in the elderly patient and its impact on general well‐being: a nonsystematic review. Clinical Interventions in Aging 2015;10:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glupker H 1942
- Glupker H. Complete denture impression materials their application and manipulation. Journal of American Dental Association 1942;29(1):2216‐20. [Google Scholar]
Goel 2016
- Goel K, Singh SV, Chand P, Rao J, Tripathi S, Kumar L, et al. Impact of different prosthodontic treatment modalities on nutritional parameters of elderly patients. Journal of Prosthodontics 2016;25(1):21‐7. [DOI] [PubMed] [Google Scholar]
GPT 2017
- Ferro KJ, Morgano SM, Driscoll CF, Freilich MA, Guckes AD, Knoernschild KL, et al. The glossary of prosthodontic terms ‐ ninth edition. Journal of Prosthetic Dentistry May 2017;117(5S):e1‐105. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 2 Dec 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gray 2012
- Gray JC, Navarro‐Coy N, Pavitt SH, Hulme C, Godfrey M, Craddock HL, et al. Improvdent: Improving dentures for patient benefit. A Cross over randomised clinical trial comparing impression materials for complete dentures. BMC Oral Health 2012;12:37‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hilgert 2009
- Hilgert JB, Hugo FN, Sousa Mda L, Bozzetti MC. Oral status and its association with obesity in Southern Brazilian older people. Gerodontology 2009;26(1):46‐52. [DOI] [PubMed] [Google Scholar]
Hindels 1957
- Hindels GW. Stress analysis in distal extension partial dentures. Journal of Prosthetic Dentistry 1957;7(2):197‐205. [Google Scholar]
Holmes 1965
- Holmes JB. Influence of impression procedures and occlusal loading on partial denture movement. Journal of Prosthetic Dentistry 1965;15(3):474‐81. [DOI] [PubMed] [Google Scholar]
Huang 2013
- Huang DL, Chan KC, Young BA. Poor oral health and quality of life in older U.S. adults with diabetes mellitus. Journal of the American Geriatrics Society 2013;61(10):1782‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hugo 2009
- Hugo FN, Hilgert JB, Luz Rosário de Sousa M, Cury JA. Oral status and its association with general quality of life in older independent‐living south‐Brazilians. Community Dentistry and Oral Epidemiology 2009;37(3):231‐40. [DOI] [PubMed] [Google Scholar]
Hutton 2002
- Hutton B, Feine J, Morais J. Is there an association between edentulism and nutritional state?. Journal of the Canadian Dental Association 2002;68(3):182‐7. [PubMed] [Google Scholar]
Jacobson 1983a
- Jacobson TE, Krol AJ. A contemporary review of the factors involved in complete denture retention, stability, and support. Part I: retention. Journal of Prosthetic Dentistry 1983;49(1):5‐15. [DOI] [PubMed] [Google Scholar]
Jacobson 1983b
- Jacobson TE, Krol AJ. A contemporary review of the factors involved in complete dentures. Part II: stability. Journal of Prosthetic Dentistry 1983;49(2):165‐72. [DOI] [PubMed] [Google Scholar]
Jeyapalan 2015
- Jeyapalan V, Krishnan CS. Partial edentulism and its correlation to age, gender, socio‐economic status and incidence of various Kennedy’s classes – a literature review. Journal of Clinical and Diagnostic Research 2015;9(6):ZE14‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joglekar 1968
- Joglekar AP, Sinkford JC. Impression procedure for problem mandibular complete dentures. Journal of the American Dental Association 1968;77(6):1303‐7. [DOI] [PubMed] [Google Scholar]
John 2009
- John MT, Reissmann DR, Szentpétery A, Steele J. An approach to define clinical significance in prosthodontics. Journal of Prosthodontics 2009;18:455‐60. [DOI] [PubMed] [Google Scholar]
Kandelman 2008
- Kandelman D, Petersen PE, Ueda H. Oral health, general health, and quality of life in older people. Special Care in Dentistry 2008;28(6):224‐36. [DOI] [PubMed] [Google Scholar]
Koran 1977
- Koran A, Powers JM, Craig RG. Apparent viscosity of materials used for making edentulous impressions. Journal of the American Dental Association 1977;95(1):75‐9. [DOI] [PubMed] [Google Scholar]
Kotkin 1985
- Kotkin H. Diagnostic significance of denture complaints. Journal of Prosthetic Dentistry 1985;53(1):73‐7. [DOI] [PubMed] [Google Scholar]
Laurina 2006
- Laurina L, Soboleva U. Construction faults associated with complete denture wearers' complaints. Stomatologija 2006;8(2):61‐4. [PubMed] [Google Scholar]
Lee 2004
- Lee JS, Weyant RJ, Corby P, Kritchevsky SB, Harris TB, Rooks R, et al. Edentulism and nutritional status in a biracial sample of well‐functioning, community‐dwelling elderly: the health, aging, and body composition study. American Journal of Clinical Nutrition 2004;79(2):295‐302. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leupold 1965
- Leupold RJ, Kratochvil FJ. An altered‐cast procedure to improve tissue support for removable partial dentures. Journal of Prosthetic Dentistry 1965;15(4):672‐8. [DOI] [PubMed] [Google Scholar]
Leupold 1966
- Leupold RJ. A comparative study of impression procedures for distal extension removable partial dentures. The Journal of Prosthetic Dentistry 1966;16(4):708‐20. [Google Scholar]
Loh 1997
- Loh PL. An alternative for making master impressions for complete dentures. Journal of the American Dental Association 1997;128(10):1436‐7. [DOI] [PubMed] [Google Scholar]
MacEntee 1998
- MacEntee MI, Walton JN. The economics of complete dentures and implant‐related services: a framework for analysis and preliminary outcomes. Journal of Prosthetic Dentistry 1998;79(1):24‐30. [DOI] [PubMed] [Google Scholar]
Massad 2005
- Massad J, Davis WJ, Lobel W, June R, Thornton J. Improving the stability of maxillary dentures: the use of polyvinyl siloxane impression materials for edentulous impressions. Dentistry Today 2005;24(2):118,120‐3; quiz 123, 140. [PubMed] [Google Scholar]
McLean 1936
- McLean DW. The partial denture as a vehicle for function. Journal of the American Dental Association 1936;23(7):1271‐8. [Google Scholar]
Mehra 2014
- Mehra M, Vahidi F, Berg RW. A complete denture impression technique survey of postdoctoral prosthodontic programs in the United States. Journal of Prosthodontics 2014;23(4):320‐7. [DOI] [PubMed] [Google Scholar]
Minagi 1987
- Minagi S, Sato Y, Akagawa Y, Tsuru H. Concept and technique for making an accurate final impression for complete dentures using a thixotropic impression material. International Journal of Prosthodontics 1987;1(2):149‐52. [PubMed] [Google Scholar]
Owen 2006
- Owen CP. Guidelines for a minimum acceptable protocol for the construction of complete dentures. International Journal of Prosthodontics 2006;19(5):151‐8. [PubMed] [Google Scholar]
Paulino 2015
- Paulino MR, Alves LR, Gurgel BC, Calderon PS. Simplified versus traditional techniques for complete denture fabrication: a systematic review. Journal of Prosthetic Dentistry 2015;113(1):12‐6. [DOI] [PubMed] [Google Scholar]
Peltzer 2014
- Peltzer K, Hewlett S, Yawson AE, Moynihan P, Preet R, Wu F, et al. Prevalence of loss of all teeth (edentulism) and associated factors in older adults in China, Ghana, India, Mexico, Russia and South Africa. International Journal of Environmental Research and Public Health 2014;11(11):11308‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petropoulos 2003
- Petropoulos VC, Rashedi B. Current concepts and techniques in complete denture final impression procedures. Journal of Prosthodontics 2003;12(4):280‐7. [DOI] [PubMed] [Google Scholar]
Phoenix 2008
- Verrett DG. Special impression procedures for tooth‐tissue‐supported removable partial dentures. In: Phoenix RD, Cagna DR, DeFreest CF editor(s). Stewart's Clinical Removable Partial Prosthodontics. 4th Edition. Chicago, IL: Quintessence Pub, 2008:351‐66. [Google Scholar]
Porwal 2013
- Porwal A, Sasaki K. Current status of the neutral zone: a literature review. Journal of Prosthetic Dentistry 2013;109(2):129‐34. [DOI] [PubMed] [Google Scholar]
Rao 2010
- Rao S, Chowdhary R, Mahoorkar S. A systematic review of impression technique for conventional complete denture. The Journal of Indian Prosthodontic Society 2010;10(2):105‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodrigues 2012
- Rodrigues SM, Oliveira AC, Vargas AM, Moreira AN, Ferreira EF. Implications of edentulism on quality of life among elderly. International Journal of Environmental Research and Public Health 2012;9(12):100‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roohafza 2015
- Roohafza H, Afghari P, Keshteli AH, Vali A, Shirani M, Adibi P, et al. The relationship between tooth loss and psychological factors. Community Dental Health 2015;32(1):16‐9. [PubMed] [Google Scholar]
Sajjan 2010
- Sajjan C. An altered cast procedure to improve tissue support for removable partial denture. Contemporary Clinical Dentistry 2010;1(2):103‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santana‐Penin 1998
- Santana‐Penin U, Lozano JG. An accurate method for occlusal registration and altered‐cast impression for removable partial dentures during the same visit as the framework try‐in. Journal of Prosthetic Dentistry 1998;80(5):615‐8. [DOI] [PubMed] [Google Scholar]
Silva 2014
- Silva JCM, Santos JFF, Marchini L. Factors influencing patients' satisfaction with complete dentures: a qualitative study. Brazilian Dental Science 2014;17(2):83‐8. [Google Scholar]
Slade 2014
- Slade GD, Akinkugbe AA, Sanders AE. Projections of U.S. edentulism prevalence following 5 decades of decline. Journal of Dental Research 2014;93(10):959‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1979
- Smith DE, Toolson LB, Bolender CL, Lord JL. One‐step border molding of complete denture impressions using a polyether impression material. Journal of Prosthetic Dentistry 1979;41(3):347‐51. [DOI] [PubMed] [Google Scholar]
Solomon 1973
- Solomon EG. Functional impression technique for complete denture construction with silicone elastomer. Journal of the Indian Dental Association 1973;45(2):29. [PubMed] [Google Scholar]
Solomon 2011
- Solomon EG. Single stage silicone border molded closed mouth impression technique—part II. Journal of Indian Prosthodontic Society 2011;11(3):183‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Starcke 1975
- Starcke EN Jr. A historical review of complete denture impression materials. Journal of the American Dental Association 1975;91(5):1037‐41. [DOI] [PubMed] [Google Scholar]
Stedman 2011
- Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2011;40(6):1732‐4. [DOI] [PubMed] [Google Scholar]
Steele 2012
- Steele JG, Treasure ET, O'Sullivan I, Morris J, Murray JJ. Adult Dental Health Survey 2009: transformations in British oral health 1968‐2009. British Dental Journal 2012;213(10):523‐7. [DOI] [PubMed] [Google Scholar]
Steffel 1951
- Steffel VL. Fundamental principles involved in partial denture design. Journal of the American Dental Association 1951;42(5):534‐44. [DOI] [PubMed] [Google Scholar]
Tanasić 2015
- Tanasić IV, Tihacek‐Sojić LĐ, Milić‐Lemić AM. Prevalence and clinical effects of certain therapy concepts among partially edentulous Serbian elderly. Journal of Prosthodontics 2015;24(8):610‐4. [doi: 10.1111/jopr.12261] [DOI] [PubMed] [Google Scholar]
Trapozzano 1939
- Trapozzano VR. Securing edentulous impressions with zinc oxide‐eugenol impression paste. Journal of the American Dental Association 1939;26(9):1527‐31. [Google Scholar]
United Nations 2013
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing Report 2013. ST/ESA/SER.A/348. Available at www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2013.pdf. (accessed on 1 April 2016).
Urzua 2012
- Urzua I, Mendoza C, Arteaga O, Rodríguez G, Cabello R, Faleiros S, et al. Dental caries prevalence and tooth loss in Chilean adult population: first National Dental Examination Survey. International Journal of Dentistry 2012;2012:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012;380(9859):2163‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zinner 1981
- Zinner ID, Sherman H. An analysis of the development of complete denture impression techniques. Journal of Prosthetic Dentistry 1981;46(3):242‐9. [DOI] [PubMed] [Google Scholar]


