Abstract
Background
Panic disorder is characterised by repeated, unexpected panic attacks, which represent a discrete period of fear or anxiety that has a rapid onset, reaches a peak within 10 minutes, and in which at least four of 13 characteristic symptoms are experienced, including racing heart, chest pain, sweating, shaking, dizziness, flushing, stomach churning, faintness and breathlessness. It is common in the general population with a lifetime prevalence of 1% to 4%. The treatment of panic disorder includes psychological and pharmacological interventions. Amongst pharmacological agents, the National Institute for Health and Care Excellence (NICE) and the British Association for Psychopharmacology consider antidepressants, mainly selective serotonin reuptake inhibitors (SSRIs), as the first‐line treatment for panic disorder, due to their more favourable adverse effect profile over monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs). Several classes of antidepressants have been studied and compared, but it is still unclear which antidepressants have a more or less favourable profile in terms of effectiveness and acceptability in the treatment of this condition.
Objectives
To assess the effects of antidepressants for panic disorder in adults, specifically:
1. to determine the efficacy of antidepressants in alleviating symptoms of panic disorder, with or without agoraphobia, in comparison to placebo;
2. to review the acceptability of antidepressants in panic disorder, with or without agoraphobia, in comparison with placebo; and
3. to investigate the adverse effects of antidepressants in panic disorder, with or without agoraphobia, including the general prevalence of adverse effects, compared to placebo.
Search methods
We searched the Cochrane Common Mental Disorders' (CCMD) Specialised Register, and CENTRAL, MEDLINE, EMBASE and PsycINFO up to May 2017. We handsearched reference lists of relevant papers and previous systematic reviews.
Selection criteria
All double‐blind, randomised, controlled trials (RCTs) allocating adults with panic disorder to antidepressants or placebo.
Data collection and analysis
Two review authors independently checked eligibility and extracted data using a standard form. We entered data into Review Manager 5 using a double‐check procedure. Information extracted included study characteristics, participant characteristics, intervention details and settings. Primary outcomes included failure to respond, measured by a range of response scales, and treatment acceptability, measured by total number of dropouts for any reason. Secondary outcomes included failure to remit, panic symptom scales, frequency of panic attacks, agoraphobia, general anxiety, depression, social functioning, quality of life and patient satisfaction, measured by various scales as defined in individual studies. We used GRADE to assess the quality of the evidence for each outcome
Main results
Forty‐one unique RCTs including 9377 participants overall, of whom we included 8252 in the 49 placebo‐controlled arms of interest (antidepressant as monotherapy and placebo alone) in this review. The majority of studies were of moderate to low quality due to inconsistency, imprecision and unclear risk of selection and performance bias.
We found low‐quality evidence that revealed a benefit for antidepressants as a group in comparison with placebo in terms of efficacy measured as failure to respond (risk ratio (RR) 0.72, 95% confidence interval (CI) 0.66 to 0.79; participants = 6500; studies = 30). The magnitude of effect corresponds to a number needed to treat for an additional beneficial outcome (NNTB) of 7 (95% CI 6 to 9): that means seven people would need to be treated with antidepressants in order for one to benefit. We observed the same finding when classes of antidepressants were compared with placebo.
Moderate‐quality evidence suggested a benefit for antidepressants compared to placebo when looking at number of dropouts due to any cause (RR 0.88, 95% CI 0.81 to 0.97; participants = 7850; studies = 30). The magnitude of effect corresponds to a NNTB of 27 (95% CI 17 to 105); treating 27 people will result in one person fewer dropping out. Considering antidepressant classes, TCAs showed a benefit over placebo, while for SSRIs and serotonin‐norepinephrine reuptake inhibitor (SNRIs) we observed no difference.
When looking at dropouts due to adverse effects, which can be considered as a measure of tolerability, we found moderate‐quality evidence showing that antidepressants as a whole are less well tolerated than placebo. In particular, TCAs and SSRIs produced more dropouts due to adverse effects in comparison with placebo, while the confidence interval for SNRI, noradrenergic reuptake inhibitors (NRI) and other antidepressants were wide and included the possibility of no difference.
Authors' conclusions
The identified studies comprehensively address the objectives of the present review.
Based on these results, antidepressants may be more effective than placebo in treating panic disorder. Efficacy can be quantified as a NNTB of 7, implying that seven people need to be treated with antidepressants in order for one to benefit. Antidepressants may also have benefit in comparison with placebo in terms of number of dropouts, but a less favourable profile in terms of dropout due to adverse effects. However, the tolerability profile varied between different classes of antidepressants.
The choice of whether antidepressants should be prescribed in clinical practice cannot be made on the basis of this review.
Limitations in results include funding of some studies by pharmaceutical companies, and only assessing short‐term outcomes.
Data from the present review will be included in a network meta‐analysis of psychopharmacological treatment in panic disorder, which will hopefully provide further useful information on this issue.
Plain language summary
Antidepressants for panic disorder in adults
Why is this review important?
Panic disorder is common in the general population. It is characterised by panic attacks, periods of fear or anxiety with a rapid onset in which other characteristic symptoms are experienced (involving bodily systems and fearful thoughts). The treatment of panic disorder includes psychological and pharmacological interventions, often used in combination. Among pharmacological interventions, the standard treatment suggested by guidelines is different classes of antidepressants. Evidence for their effectiveness and acceptability is unclear.
Who will be interested in this review?
People with panic disorder and general practitioners.
What questions does this review aim to answer?
How effective are antidepressants compared to a sham treatment (known as placebo) in treating panic disorder?
What is the acceptability of antidepressants compared to placebo in treating panic disorder?
How many unintended and untoward effects (adverse effects) do antidepressants have compared to placebo in people with panic disorder?
Which studies were included in the review?
We searched electronic databases to find all relevant studies. The medical studies included in the review compared treatment with antidepressants or placebo in adults with a diagnosis of panic disorder. The studies also had to be randomised controlled trials (RCTs), which means adults were allocated at random (by chance alone) to receive the treatment or placebo. We included 41 RCTs for a total of 9377 people in the review.
What does the evidence from the review tell us?
We found evidence showing that antidepressants are better than placebo in terms of effectiveness and number of people leaving the study early. However, our findings also showed that antidepressants are less well tolerated than placebo, producing more dropouts due to adverse effects. Results are limited in the following ways: some studies were funded by pharmaceutical companies, and only short‐term outcomes were assessed. We found almost no data on other clinically relevant outcomes, such as functioning and quality of life. The quality of the available evidence ranged from very low to high.
What should happen next?
Studies with outcomes assessed at longer‐term follow‐up visits should be carried out to establish whether the effect is transient or maintained. Trials should better report any harms experienced by participants during the trial. In addition, a further analysis with an approach called 'network meta‐analysis' will include all psychopharmacological treatments available for panic disorder, and will likely shed further light on this compelling issue, also being able to provide more information with regard to comparative efficacy of different available interventions.
Summary of findings
Background
Description of the condition
Panic disorder is characterised by repeated, unexpected panic attacks, which are discrete periods of fear or anxiety that have a rapid onset, reach a peak within 10 minutes and in which at least four of 13 characteristic symptoms are experienced. Many of these symptoms involve bodily systems, such as racing heart, chest pain, sweating, shaking, dizziness, flushing, stomach churning, faintness and breathlessness. Further recognised panic attack symptoms involve fearful cognitions, such as the fear of collapse, going mad or dying, and derealisation (APA 1994).
Panic disorder first entered diagnostic classification systems in 1980 with the publication of DSM‐III, following observations that people with panic attacks responded to treatment with the tricyclic antidepressant (TCA) imipramine (Klein 1964). To diagnose panic disorder, further conditions must be met relating to the frequency of attacks, the need for some to come on ‘out of the blue’ rather than in a predictable, externally‐triggered situation, and exclusions where attacks are attributable solely to medical causes or panic‐inducing substances, notably caffeine. DSM‐IV requires additionally that at least one attack has been followed by either a) persistent concern about having additional attacks, b) worry about the implications of the attack or its consequences or c) a significant change in behaviour related to the attacks (APA 1994).
Panic disorder is common in the general population with a lifetime prevalence of 1% to 4% (Bijl 1998; Eaton 1994). In primary care settings, panic syndromes have been reported to have a prevalence of around 10% (King 2008). Its aetiology is not fully understood and is probably heterogeneous. Biological theories incorporate the faulty triggering of an inbuilt anxiety response, possibly a suffocation alarm. Evidence for this comes from biological challenge tests (lactate and carbon dioxide are used to trigger panic in those with the disorder) and from animal experiments and neuroimaging studies in humans that show activation of fear circuits, such as that involving the periaqueductal grey matter (Gorman 2000).
Agoraphobia is anxiety about being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having a panic attack (APA 1994). Agoraphobia can occur with panic disorder (APA 1994). About 25% of people suffering from panic disorder also have agoraphobia (Kessler 2006). The presence of agoraphobia is associated with increased severity and worse outcome (Kessler 2006). There are several risk factors that predict the development of agoraphobia in people suffering from panic disorder: female gender, more severe dizziness during panic attacks, cognitive factors, dependent personality traits and social anxiety disorder (Starcevic 2009).
Panic disorder, with or without agoraphobia, is highly comorbid with other psychiatric disorders, such as drug dependence, major depression, bipolar I disorder, social phobia, specific phobia, and generalised anxiety disorder (Grant 2006). It is estimated that generalised anxiety disorder co‐occurs in 68% of people with panic disorder, whilst major depression has a prevalence of 24% to 88% among people with panic disorder (Starcevic 2009).
Description of the intervention
The treatment of panic disorder includes psychological and pharmacological interventions, often used in combination (Furukawa 2007). Historically, pharmacological interventions for panic disorder have been based on the use of mono‐amino oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) (Bruce 2003). However, MAOIs and TCAs are burdened by severe adverse effects, such as dietary restrictions (to avoid hypertensive crisis) for MAOIs, and anticholinergic, arrhythmogenic and overall poor tolerability for TCAs (Wade 1999). Benzodiazepines (BDZs), particularly high‐potency ones, have been used as a safer alternative in panic disorder (Stein 2010), although the long‐term outcome may be less good (NICE 2011). Recent guidelines (APA guideline: APA 2009; BAP guideline: BAP 2005; NICE Guideline: NICE 2011) consider antidepressants, mainly selective serotonin reuptake inhibitors (SSRIs), as the first‐line treatment for panic disorder, due to their more favourable adverse effect profile over MAOIs and TCAs. A meta‐analysis comparing SSRIs and TCAs in panic disorder showed that SSRIs are as effective as TCAs, and are better tolerated (Bakker 2002), although other studies showed a possible overestimation of the efficacy of SSRIs over older antidepressants in panic disorder (Anderson 2000; Otto 2001). It appears that TCAs can still have a role in the treatment of panic disorder.
How the intervention might work
The main classes with evidence of efficacy in panic disorder are antidepressant drugs that augment the function of the monoamines serotonin or noradrenaline, or both. Considering the serotonergic antidepressants (SSRIs such as fluoxetine, paroxetine, sertraline and citalopram), these drugs promote the transmission of the neurotransmitter serotonin across brain synapses; most notably in the dorsal raphe nucleus (Briley 1993). They prevent reuptake of serotonin into nerve terminals by inhibiting serotonin transporters, thus allowing more to be available for neurotransmission. In panic disorder, imaging studies have revealed reduced expression of the 5H1A receptor (Nash 2008), which has an inhibitory function, so the increased serotonin throughput may in part serve to overcome this deficit of inhibition. Noradrenergic antidepressants can similarly increase transmission of the catecholamine noradrenaline. Some antidepressants such as the serotonin‐norepinephrine reuptake inhibitor (SNRI) drugs (e.g. venlafaxine, duloxetine) and TCAs can enhance both serotonin and noradrenaline transmission by inhibiting both transporters.
Why it is important to do this review
Antidepressants are widely used in panic disorder. Published randomised controlled trials (RCTs) have shown some evidence of efficacy. However, no systematic study on all antidepressants in panic disorder has been conducted recently, to our knowledge. A meta‐analysis published in 2007 focused on combined psychotherapy and antidepressants in panic disorder (Furukawa 2007), and a more recent systematic review focused on psychological treatments only (Sanchez‐Meca 2010). Furukawa 2007 concluded that either combined psychotherapy and antidepressants or psychotherapy alone may be chosen as first line treatment for panic disorder. Sanchez‐Meca 2010 reported that exposure, relaxation training and breathing retraining have the most robust evidence. A network meta‐analysis was also performed to compare different psychological therapies for panic disorder (Pompoli 2016). A meta‐analysis of Bakker and colleagues included 43 studies comparing SSRIs and TCAs (Bakker 2002). The authors concluded that SSRIs and TCAs are of equal efficacy in the treatment of panic disorder, with a better tolerability profile for SSRIs. A recent Cochrane Review comparing active treatments (antidepressants and benzodiazepines) for panic disorder included 35 RCTs and failed to detect important differences between these treatments (Bighelli 2016). Both these studies were focused on active comparisons, and inform on the comparative efficacy of antidepressants with other available compounds. Antidepressants as a group were not compared with placebo, so it was not possible to estimate the effect size of antidepressant treatment in panic disorder.
To our knowledge, the most recent meta‐analysis specifically focused on antidepressants in panic disorder was published in 2013 (Andrisano 2013). This systematic review compared newer antidepressants with placebo, and included the SSRI, SNRI and NRI. The authors included 50 studies, of which only 26 were RCTs, with no requirements on blinding. Andrisano and colleagues found sertraline, paroxetine, fluoxetine and venlafaxine to be more effective than placebo on change in panic symptoms. Venlafaxine, fluoxetine, sertraline, paroxetine, citalopram and mirtazapine were found to have a lower number of dropouts compared to placebo. However, the authors only considered total dropouts and did not investigate dropouts due to adverse effects or the number of participants experiencing adverse effects. Due to all these limitations, results from this review should be cautiously interpreted and considered only as preliminary.
An up‐to‐date review is needed to help prescribers identify the effect size of active treatment compared to placebo in panic disorder, in order to be better guided in the choice of the pharmacological agent.
One other Cochrane Review in people with panic disorder, comparing benzodiazepines with placebo is ongoing, and will be of further help to identify effective treatments in panic disorder (Guaiana 2013a).
Based on these data, a Cochrane network meta‐analysis of psychopharmacological treatment in panic disorder is also in progress (Guaiana 2017).
Objectives
To assess the effects of antidepressants for panic disorder in adults, specifically:
to determine the efficacy of antidepressants in alleviating symptoms of panic disorder, with or without agoraphobia, in comparison to placebo;
to review the acceptability of antidepressants in panic disorder, with or without agoraphobia, in comparison with placebo; and
to investigate the adverse effects of antidepressants in panic disorder, with or without agoraphobia, including the general prevalence of adverse effects, compared to placebo.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, double‐blind, controlled trials using a parallel‐group design that compare antidepressants with placebo as monotherapy.
We included cross‐over trials, randomised, placebo‐controlled trials with more than two arms, and cluster‐randomised, placebo‐controlled trials.
We excluded quasi‐randomised trials, such as those allocated by using alternate days of the week.
Types of participants
Participants aged 18 years or older with a primary diagnosis of panic disorder, with or without agoraphobia, diagnosed according to any of the following criteria: Feighner criteria, Research Diagnostic Criteria, DSM‐III, DSM‐III‐R, DSM‐IV or ICD‐10. In case study eligibility focused on agoraphobia, rather than panic disorder, studies were to be included if operationally diagnosed according to the above‐named criteria and when it could be safely assumed that at least 30% of the participants were suffering from panic disorder as defined by the above criteria. There is evidence that more than 95% of people with agoraphobia seen clinically also suffer from panic disorder (Goisman 1995). However, we did not find any studies with such inclusion criteria.
We excluded participants with serious comorbid physical disorders (e.g. myocardial infarction, chronic obstructive pulmonary disorder, uncontrolled diabetes, electrolyte disturbances) as they may confound treatment effectiveness and tolerability.
We included participants with comorbid mental disorders, but we examined the effect of including these participants in sensitivity analyses.
Types of interventions
Any trial comparing antidepressants as monotherapy with placebo in the treatment of panic disorder, with or without agoraphobia.
We included only acute treatment studies treating participants for less than six months. We excluded relapse prevention studies.
We included the following antidepressants.
Tricyclic antidepressants (TCAs): amitriptyline, amoxapine, clomipramine, desipramine, dosulepin/dothiepin, doxepin, imipramine, lofepramine, maprotiline, nortriptyline, protriptyline, trimipramine
Selective serotonin reuptake inhibitors (SSRIs): fluoxetine, fluvoxamine, sertraline, citalopram, paroxetine, escitalopram
Monoamine oxidase inhibitors (MAOIs): phenelzine, isocarboxazid, tranylcypromine, moclobemide, brofaromine
Serotonin‐noradrenaline reuptake inhibitors (SNRIs): venlafaxine, desvenlafaxine, duloxetine, milnacipran
Noradrenergic and specific serotonergic antidepressants (NaSSAs): mirtazapine
Noradrenergic and dopaminergic reuptake inhibitors (NDRIs): bupropion
Noradrenergic reuptake inhibitors (NRIs): reboxetine
Others: agomelatine, trazodone, nefazodone, mianserin, maprotiline, non‐conventional herbal products (e.g. hypericum)
We included studies in which irregular (i.e. not daily) use of benzodiazepines took place. Excluding such studies would have been meaningless because it has been documented that a minority of people take benzodiazepines surreptitiously when they are prohibited by the protocol (Clark 1990). We excluded studies in which benzodiazepines were regularly administered at a constant dosage for a long time or as part of the study medication. We noted possible differences in co‐interventions (such as differential usage of benzodiazepines in antidepressant trials) and examined their influence in sensitivity analyses.
We did not apply any restriction on dose, frequency, intensity or duration.
We excluded studies administering psychological therapies targeted at panic disorder concurrently.
Types of outcome measures
When multiple measures were used, we gave preference to the ones in the order listed below for each outcome.
Primary outcomes
Failure to respond, that is, lacking substantial improvement from baseline as defined by the original investigators. Examples of improvement would be “very much or much improved” according to the Clinical Global Impressions Scale, more than 40% reduction in the Panic Disorder Severity Scale score (which is considered as equivalent to "very much or much improved" on the Clinical Global Impression of Improvement Scale (Furukawa 2009), and more than 50% reduction in the Fear Questionnaire Agoraphobia Subscale (which is considered as an appropriate rate of response according to Bandelow 2006). For this outcome we calculated the number of participants who failed to meet these improvement criteria.
Total number of dropouts for any reason as a proxy measure of treatment acceptability.
Secondary outcomes
Failure to remit, that is, lacking of satisfactory end state as defined by global judgment of the original investigators. Examples of satisfactory end‐state would be "panic free" and "no or minimal symptoms" according to the Clinical Global Impression Severity Scale. For this outcome, we calculated the number of participants who failed to meet these remission criteria.
Panic symptom scales and global judgment on a continuous scale. Examples include Panic Disorder Severity Scale total score (0 to 28), Clinical Global Impression Severity Scale (1 to 7), and Clinical Global Impression Change Scale (1 to 7). When multiple measures were used, we gave preference in the order above, preferring to use panic symptom scales where available. We indicated the actual measure entered into meta‐analyses at the top of the listings in the Characteristics of included studies.
Frequency of panic attacks, as recorded, for example, by a panic diary.
Agoraphobia, as measured, for example, by the Fear Questionnaire, Mobility Inventory, or behavioural avoidance test.
General anxiety, as measured, for example, by the Hamilton Rating Scale for Anxiety, Beck Anxiety Inventory, State‐Trait Anxiety Index, Sheehan Patient‐Rated Anxiety Scale, or Anxiety Subscale of SCL‐90‐R.
Depression, as measured, for example, by the Hamilton Depression Rating Scale, Beck Depression Inventory, or Depression Subscale of SCL‐90‐R.
Social functioning, as measured, for example, by the Sheehan Disability Scale, Global Assessment Scale, or Social Adjustment Scale‐Self Report.
Quality of life, as measured for example by SF‐36 or SF‐12.
Patient satisfaction with treatment
Economic costs
Number of dropouts due to adverse effects
Number of participants experiencing at least one adverse effect
Timing of outcome assessment
All outcomes are short term, which we define as acute‐phase treatment that would normally last two to six months.
When studies reported response rates at different time points within two to six months, we preferred the time point closest to 12 weeks.
Search methods for identification of studies
Electronic searches
1. The Cochrane Common Mental Disorders' Specialised Register (CCMDCTR)
Cochrane Common Mental Disorders (CCMD) maintains two clinical trials registers at their editorial base in York, UK: a references register and a studies‐based register. The CCMDCTR‐References Register contains over 31,500 reports of RCTs in depression, anxiety and neurosis. Approximately 65% of these references have been tagged to individual, coded trials. The coded trials are held in the CCMDCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary; please contact the CCMD Information Specialist for further details. Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 to date), Embase (1974 to date) and PsycINFO (1967 to date); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library) and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers via the World Health Organization's trials portal (the International Clinical Trials Registry Platform (ICTRP)), pharmaceutical companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCMDs' generic search strategies (used to identify RCTs) can be found on the Group's website.
We searched the CCMD registers using the following terms:
CCMDCTR‐Studies
Diagnosis = "panic disorder" and Intervention = placebo* We screened records for placebo‐controlled antidepressant trials.
CCMDCTR‐References
We searched the References Register using the free‐text term 'panic*' to identify additional untagged references. We screened abstracts for antidepressant trials and retrieved full‐text articles, where necessary, to check for placebo controls.
2. National and international trials registers
We also conducted complementary searches on the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov.
Searching other resources
Review authors checked the reference lists of all included studies, non‐Cochrane systematic reviews and major textbooks of affective disorders (written in English), for published reports and citations of unpublished research. We also conducted a citation search via the Web of Science (included studies only) to identify additional works. We contacted experts in the field.
Data collection and analysis
Selection of studies
Two review authors (MC and AC) independently selected trials for inclusion in this systematic review.
MC and AC inspected the search retrieval by reading the titles and abstracts to see if they met the inclusion criteria. We resolved possible doubts by consultation with the review co‐authors. We obtained each potentially‐relevant study located in the search as a full‐text article; the review authors independently assessed them for inclusion and, in the case of discordance, sought resolution by discussion between the two. We calculated the discordance in the selection of studies using Cohen's Kappa (k) (Cohen 1960), a more robust measure than a simple per cent agreement calculation, since it takes into account the agreement between review authors that occurs by chance. When it was not possible to evaluate the study because of language problems or missing information, we classified the study as 'awaiting assessment' until we could obtain a translation or further information.The reasons for the exclusion of potentially‐relevant trials are reported in the 'Characteristics of excluded studies' table.
We recorded all decisions made during the selection process, along with numbers of studies and references, and we presented them in a PRISMA flow diagram at the end of the review (Figure 1; Moher 2009).
1.

Study flow diagram
Data extraction and management
Two review authors used a data extraction form to independently extract the data from included studies concerning participant characteristics (age, sex, severity of panic disorder, study setting), intervention details (dosage, duration of study, sponsorship), study characteristics (blinding, allocation, etc) and outcome measures of interest. We piloted the data extraction sheet on a sample of 10% of the included studies. Again, we resolved any disagreement by consensus or by involving a third member of the review team. If necessary, we contacted authors of studies to obtain clarification.
Main comparisons
Antidepressants as a whole versus placebo
TCAs versus placebo
SSRIs versus placebo
MAOIs versus placebo
SNRIs versus placebo
NaSSAs versus placebo
NDRIs versus placebo
NRIs versus placebo
Other antidepressants versus placebo
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in included studies using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome assessment, the completeness of outcome data, selective reporting and other biases. We also considered sponsorship bias.
We assessed and categorised the risk of bias in each domain and overall as either:
low risk of bias, plausible bias unlikely to seriously alter the results;
high risk of bias, plausible bias that seriously weakens confidence in the results;
unclear risk of bias, plausible bias that raises some doubt about the results.
If the assessors disagreed, we made the final rating with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. We also reported non‐concurrence in quality assessment.
Measures of treatment effect
The main outcome result was reduction of severity of panic and agoraphobia symptoms. Improvement was usually presented as a change in a panic disorder scale(s) (mean and standard deviation) or as a dichotomous outcome (responder or non‐responder, remitted or not‐remitted), or both.
Binary or dichotomous data
For binary outcomes we calculated a standard estimation of the random‐effects model risk ratio (RR) and its 95% confidence interval (CI). It has been shown that a random‐effects model has a good generalisability and that RR is more intuitive than odds ratio (Furukawa 2002; Boissel 1999). Furthermore, odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This may lead to an overestimation of the impression of the effect (Deeks 2011). For all primary outcomes, we calculated the number needed to treat for an additional beneficial outcome or harmful outcome (NNTB or NNTH) and its 95% CI using Visual Rx (www.nntonline.net/), while taking account of the event rate in the control group.
Continuous data
Summary statistics
We used standardised mean difference (SMD) as originally planned in the protocol, when studies used an idiosyncratic scale that is seldom or never used elsewhere (e.g. Phobia Scale for Agoraphobia). However, when all the included studies used the same standard scales such as Hamilton Rating Scale for Anxiety and Hamilton Depression Rating Scale, we used mean differences (MDs).
Endpoint versus change data
Trials usually report results either using endpoint means and standard deviation of scales or using change in mean values from baseline of assessment rating scales. We prefer to use scale endpoint data, which typically cannot have negative values and are easier to interpret from a clinical point of view. If endpoint data were unavailable, we used the change data in separate analyses. Where we used MD, we pooled results based on change data and endpoint data in the same analysis.
Unit of analysis issues
Cross‐over trials
Cross‐over trials are trials in which all participants receive both the control and intervention treatment but in a different order. The major problem is a carryover effect from the first phase to the second phase of the study, especially if the condition of interest is unstable (Elbourne 2002). As this is the case with panic disorder, randomised cross‐over studies were eligible, but we planned to use only data up to the point of first cross over. However, there were no cross‐over trials included in our review.
Studies with multiple treatment groups
Where a study involved more than two treatment arms, especially two appropriate dose groups of the same drug, we pooled the different dose arms and treated them as one group; we included 10 trials of this kind. If the trial involved one placebo arm and two or more arms of different antidepressants, we compared each arm with placebo separately. In this case, a unit‐of‐analysis error can occur, because of the unaddressed differences between the estimated intervention effects from multiple comparisons (Deeks 2011), resulting in double counting. In order to avoid that, we included each pair‐wise comparison separately, according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions, section 16.5.4 (Higgins 2011b). If the variable was continuous, only the total number of participants was divided up, leaving means and standard deviations unchanged. There were seven studies to which this analysis was applicable.
Cluster‐randomised trials
In cluster‐randomised trials groups of individuals rather than individuals are randomised to different interventions. If we had identified cluster placebo‐controlled randomised trials, we planned to use the generic inverse variance technique, providing such trials had been appropriately analysed, while taking into account intraclass correlation coefficients to adjust for cluster effects. If study authors had not adjusted for the effects of clustering, we planned to do this by obtaining an intracluster correlation coefficient and then following the guidance given in chapter 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). However, there were no cluster‐randomised trials included in our review.
Dealing with missing data
We tried to contact the study authors for all relevant missing data.
Dichotomous outcomes
We calculated response or remission on treatment using an intention‐to‐treat analysis (ITT). We followed the principle 'once randomised always analysed'. Where participants left the study before the intended endpoint, we assumed that they would have experienced the negative outcome. We planned to test the validity of the above assumption by sensitivity analysis, applying worst and best case scenarios. However, this was not possible, as explained in Effects of interventions section. When dichotomous outcomes were not reported but the baseline mean and standard deviation on a panic disorder scale were reported, we calculated the number of responding or remitted participants according to a validated imputation method (Furukawa 2005). We checked the validity of the above approach by sensitivity analysis excluding studies with imputed data. If necessary, we contacted authors of studies to obtain data or clarification, or both.
Continuous outcomes
Concerning continuous data, the Cochrane Handbook of Systematic Reviews of Interventions recommends avoiding imputation of continuous data and suggests using the data as presented by the original authors (Higgins 2011b). Where ITT data were available we used them in preference to 'per‐protocol analysis'. If necessary, we contacted authors of studies to obtain data or clarification, or both.
Skewed or qualitative data
We planned to present skewed and qualitative data descriptively.
We considered several strategies for skewed data. If papers reported a mean and standard deviation and there was also an absolute minimum possible value for the outcome, we divided the mean by the standard deviation. If the value obtained was less than 2 then we concluded that some skewness was indicated. If the value obtained was less than 1 (i.e. the standard deviation was bigger than the mean) skewness was almost certain. If papers did not report the skewness and simply reported means, standard deviations and sample sizes, we used these numbers. Because these data may not have been properly analysed and can be misleading, we conducted analysis with and without these studies. If the data had been log‐transformed for analysis, and the geometric means were reported, skewness would be reduced. This is the recommended method of analysis of skewed data (Deeks 2011). If papers used non‐parametric tests and described averages using medians, they could not be formally pooled in the analysis. We followed the recommendation made in the Cochrane Handbook and the results of these studies have been reported in a table in our review, along with all other papers. This means that the data are not lost from the review and that we can consider the results when drawing conclusions, even if they cannot be formally pooled in the analyses.
Missing statistics
When only P or standard error (SE) values were reported, we calculated standard deviations (SDs) (Altman 1996). In the absence of supplementary data after we had requested them from the study authors, we calculated the SDs according to a validated imputation method (Furukawa 2006).
Assessment of heterogeneity
Following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions, we quantified heterogeneity using the I2 statistic (Higgins 2003). The Cochrane Handbook for Systematic Reviews of Interventions recommends overlapping intervals for I2 interpretation (section 9.5.2, Deeks 2011), as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
We also used the Chi2 test and its P value to determine the direction and magnitude of the treatment effects. In a meta‐analysis of few trials Chi2 will be underpowered to detect heterogeneity, if it exists. We used P = 0.10 as a threshold of statistical significance.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). A funnel plot is usually used to investigate publication bias. However, it has a limited role when there are only few studies of similar size. Secondly, asymmetry of a funnel plot does not always reflect publication bias. Visual inspection of funnel plots has been used to assess publication bias as well as the statistical test for funnel plot asymmetry proposed by Egger or Rücker (Egger 1997; Rücker 2008; Sterne 2011). However we did not use funnel plots for outcomes if there were 10 or fewer studies, or if all studies were of similar size.
Data synthesis
We used a random‐effects model to calculate the treatment effects. We preferred the random‐effects model as it takes into account differences between studies even when there is no evidence of statistical heterogeneity. It gives a more conservative estimate than the fixed‐effect model. We note that the random‐effects model gives added weight to small studies, which can either increase or decrease the effect size. We applied a fixed‐effect model, on primary outcomes only, to see whether it markedly changed the effect size.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses are often exploratory in nature and should be interpreted cautiously because they often involve multiple analyses and can lead to false positive results. While keeping in mind the above reservations, we planned to perform the following subgroup analyses:
for classes of antidepressants, such as TCAs, SSRIs, and others;
for participants with agoraphobia and for participants without agoraphobia, because the same treatment may have differential effectiveness with regard to panic and agoraphobia;
acute‐phase treatment studies that last for less than four months versus acute‐phase treatment studies that last for four months or more.
However, we were unable to conduct the first two of these subgroup analyses since we did not find relevant studies (see Effects of interventions).
Sensitivity analysis
We planned to conduct the following sensitivity analyses for the primary outcomes only in order to examine if the results changed and check for the robustness of the observed findings:
excluding trials with high risk of bias (i.e. trials with inadequate allocation concealment and blinding; with incomplete data reporting, or with high probability of selective reporting, or both);
excluding trials with dropout rates greater than 20%;
excluding studies funded by the pharmaceutical company marketing each antidepressant. This sensitivity analysis is particularly important in view of the repeated findings that funding strongly affects outcomes of research studies and because industry sponsorship and authorship of clinical trial reports have increased over the last 20 years (Als‐Nielsen 2003; Bhandari 2004; Buchkowsky 2004; Lexchin 2003);
excluding studies whose protocols do not explicitly prohibit concomitant use of BDZ. According to Clark 1990, 10% to 20% of those assigned to placebo or imipramine arms in a RCT took explicitly‐prohibited anxiolytic medication;
excluding studies whose participants clearly had significant psychiatric co‐morbidities, including primary or secondary depressive disorders;
applying best and worst case scenarios to studies where participants left the study before the endpoint;
excluding studies where number of responding participants is calculated according to an imputation method.
It was not possible to perform sensitivity analysis 6, as explained in Effects of interventions.
Our routine application of random‐effects and fixed‐effect models as well as our secondary outcomes of remission rates and continuous severity measures might be considered additional forms of sensitivity analyses.
'Summary of findings' table
We presented our results using 'Summary of findings' tables, in which we assessed the quality of the evidence applying the GRADE approach (GRADE Working Group 2004; Schünemann 2011). 'Summary of findings' tables include the primary outcomes, failure to respond and total number of dropouts, and secondary outcomes, failure to remit, panic symptom scales as further measures of efficacy and number of dropouts due to adverse effects as a measure of tolerability.
Results
Description of studies
Results of the search
The number of references identified by the searches (last update May 2017) was 1746. We excluded 1615 references after assessment of titles and abstracts. We retrieved 131 full‐text reports for full inspection, describing 76 unique RCTs. Of these, we excluded 23 studies, and placed 11 studies in the awaiting assessment group; one study is ongoing. Finally, we included 41 RCTs (49 placebo controlled study arms) including 9377 participants, 8252 for the arms of interest (described in 95 reports) in the review. In case of missing information, we contacted authors of the included studies for additional information, and two of them responded (Drs. Lavori, CNCPS 1992, and Stahl, Stahl 2003‐cit, Stahl 2003‐esc). See Figure 1 for a PRISMA flow diagram depicting the study selection process (Moher 2009).
Included studies
Forty‐one RCTs (43 'studies') were included in this review, with characteristics as follows (see also Characteristics of included studies).
Design
All 41 included studies were parallel‐group, individually randomised controlled studies. Two studies were three‐armed and included placebo comparison of two antidepressants belonging to the same class. Since this might be confusing when reading the forest plots, these studies have been labelled according to the drug used (Gentil 1993‐clo; Gentil 1993‐imi; Stahl 2003‐cit; Stahl 2003‐esc), and appear therefore twice. When reporting the number of studies that contributed for each analysis, studies contributing with more comparisons are counted only once.
Sample sizes
The sample sizes ranged between six (Mavissakalian 1989) and 445 (Sheehan 2005) participants in each arm. Twenty‐six studies included overall sample sizes over 100: Asnis 2001, (n = 188), Ballenger 1998 (n = 278), Barlow 2000 (n = 312), Bradwejn 2005 (n = 361), Caillard 1999 (n = 180), Cassano 1999 (n = 274), CNCPS 1992 (n = 1168), GSK 1994 (n= 226), Koszycki 2011 (n = 251), Lecrubier 1997 (n = 368), Liebowitz 2009 (n = 343), Londborg 1998 (n = 177), Michelson 2001 (n = 180), Nair 1996 (n = 148), Pollack 1998 (n = 176), Pollack 2007‐a (n = 653), Pollack 2007‐b (n = 663), Schweizer 1993 (n = 106), Sharp 1996 (n = 190), Sheehan 2005 (n = 889), Stahl 2003‐cit (also referred to as Stahl 2003‐esc) (n = 380), Tsutsui 1997 (n = 169), Tsutsui 2000a (n = 171), Tsutsui 2000b (n = 120), Wade 1997 (n = 475).
Setting
A total of 27 trials enrolled only outpatients, one trial enrolled only inpatients, both inpatients and outpatients were enrolled in three trials and a total of two trials enrolled patients in primary care centres or in general practice. For the remaining eight trials, the setting was unclear. Thirteen trials were conducted in the USA, three each in the Netherlands, Japan and European sites, two in Canada, and one each in Brazil, France, Germany and the UK; 10 trials were multinational, and three did not provide information about the country.
Participants
The proportion of women ranged from 47% to 92%. Mean age ranged from 30.63 to 61.24 years.
Interventions
Twenty‐six studies included two arms of interest for this review, while the remaining studies had three or more arms. Seventeen studies compared TCAs to placebo; 22, SSRIs; four, SNRIs; one, MAOIs; one, NRIs; and two included a comparison between other antidepressants (nefazodone and ritanserin) and placebo. No trials looked at NaSSAs or NDRIs.
Duration of the intervention ranged from 8 to 28 weeks.
Outcomes
Thirty studies reported data on response rates, measured with improvement on Clinical Global Impression of Improvement scale (CGI‐I), Clinical Global Impression Severity of Illness Score (CGI‐S) or Panic Disorder Severity Scale (PDSS). The number of dropouts for any reason was reported in 38 studies. Twenty‐three studies reported on remission rates, remission being defined in the studies with the criterion "patients free from full panic attacks". Twenty‐four studies reported data on panic symptoms (using Panic and Anticipatory Anxiety Scale (PAAS), PDSS, CGI‐S), 24 on frequency of panic attacks, 19 on agoraphobia (using Fear Questionnaire (FQ) and Marks‐Sheehan Phobia Scale), 28 on general anxiety (using Hamilton Anxiety Rating Scale (HAS) and Hamilton Rating Scale for Anxiety (HAMA)), 18 on depression (using Hamilton Depression Rating Scale (HDRS), Beck Depression Inventory (BDI), Montgomery–Åsberg Depression Rating Scale (MADRS)). Sixteen studies reported data on social functioning (using Sheehan Disability Scale (SDS)), 5 on quality of life. Three studies reported data on patient satisfaction; none of the studies reported on economic costs. Thirty‐one studies had data on dropouts due to adverse effects, and 15 on number of patients experiencing at least one adverse effect.
Excluded studies
See: Characteristics of excluded studies.
Twenty‐three studies, initially selected did not meet our inclusion criteria and we excluded them for the following reasons: six had an ineligible study design; two trials included participants younger than 18 years; four included participants who were not primarily diagnosed with panic disorder; two studies included participants with anxiety disorders in general, but the randomisation was not stratified by the presence of panic disorder; and nine studies had an ineligible comparison group.
Ongoing studies
See: Characteristics of ongoing studies.
Our search identified one ongoing study (Kruimel 2015), comparing escitalopram versus placebo.
Studies awaiting classification
See Characteristics of studies awaiting classification.
We classified 10 studies as awaiting classification.
Risk of bias in included studies
For details of the 'Risk of bias' judgements for each study, see Characteristics of included studies. Graphical representations of the overall risk of bias in included studies are presented in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
The majority of studies (39 RCTs) did not report the methods of random sequence generation; only two studies specified this information, and we classified them as low risk (Koszycki 2011; Pollack 1998).
Allocation concealment
Only four studies reported details on allocation concealment and we classified them as low risk (Koszycki 2011; Tsutsui 1997; Tsutsui 2000a; Tsutsui 2000b).
Blinding
All 41 included RCTs were reported to be double‐blind, mostly without providing any further detail. Sixteen RCTs reported details on strategies to ensure blinding of participants and key study personnel, and we classified them as low risk for performance bias. We classified 11 studies as low risk for detection bias.
Incomplete outcome data
We rated 10 trials as adequate in terms of addressing incomplete outcome data, while we classified 20 studies as unclear risk and 11 as high risk.
Selective reporting
The study protocol was not available for almost all studies so it is difficult to make a judgment on the possibility of outcome reporting bias. However, in 21 studies results were consistent with the outcomes pre‐specified in the methods section and clearly reported in the results, so we evaluated them as low risk. Using the same criterion, we judged 11 studies to be at high risk.
Other potential sources of bias
Twenty‐seven of the included studies were funded by the pharmaceutical industry, and they did not report details on the role of the funder in planning, conducting and writing the study; for this reason we rated them as high risk. Ten studies did not specify the source of funding. Four studies were funded by public grants or explicitly declared not to have received funding from pharmaceutical companies, so we classified them as low risk (Broocks 1998; Gentil 1993‐clo; Mavissakalian 1989; Van Vliet 1993).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Antidepressants compared to placebo for panic disorder.
| Antidepressants compared to placebo for panic disorder | ||||||
| Patient or population: adults with panic disorder Settings: in‐ and outpatients Intervention: antidepressants Comparison: placebo | ||||||
| Outcomes (2‐6 months post‐treatment) | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antidepressants | |||||
| Failure to respond | 556 per 1000 | 400 per 1000 (367 to 439) | RR 0.72 (0.66 to 0.79) | 6500 (30 studies) | ⊕⊕⊝⊝ low1,2 | A RR of 0.72 means that the treatment with antidepressants decreases the risk of nonresponse to treatment by 28% compared to placebo. |
| Total number of dropouts | 319 per 1000 | 281 per 1000 (258 to 309) | RR 0.88 (0.81 to 0.97) | 7850 (38 studies) | ⊕⊕⊕⊝ moderate1 | A RR of 0.88 means that the treatment with antidepressants decreases the risk of leaving the study early by 12% compared to placebo. |
| Failure to remit | 595 per 1000 | 494 per 1000 (464 to 524) | RR 0.83 (0.78 to 0.88) | 6164 (24 studies) | ⊕⊕⊕⊝ moderate1 | A RR of 0.83 means that the treatment with antidepressants decreases the risk of non reaching remission by 17% compared to placebo. |
|
Panic symptoms ‐ endpoint score (various scales) |
The mean endpoint score for panic symptoms in the intervention groups was 0.44 standard deviations lower (0.58 lower to 0.30 lower) | 3699 (15 studies) | ⊕⊕⊝⊝ low1,3 | We calculated SMD of endpoint scores. The results show a benefit for antidepressants compared to placebo. The size of effect can be considered between small and moderate (Cohen 1988). | ||
|
Panic symptoms ‐ mean change (various scales) |
The mean change in panic symptoms in the intervention groups was 0.53 standard deviations lower (0.72 lower to 0.33 lower) | 2010 (10 studies) | ⊕⊕⊝⊝ low1,4 | We calculated SMD of mean change scores. The results show a benefit for antidepressants compared to placebo. The size of effect can be considered moderate (Cohen 1988). | ||
| Number of dropouts due to adverse effects | 57 per 1000 | 85 per 1000 (72 to 102) | RR 1.49 (1.25 to 1.78) | 7688 (33 studies) | ⊕⊕⊕⊝ moderate1 | A RR of 1.49 means that the treatment with antidepressants increases the risk of leaving the study because of adverse effects by 49%, compared to placebo. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one point due to high dropout rates (> 30%) in many studies. Moreover, random sequence generation and allocation concealment were unclear in most of the studies. 2Downgraded one point due to substantial heterogeneity (I2 = 67%). 3Downgraded one point due to substantial heterogeneity (I2 = 68%). 4Downgraded one point due to substantial heterogeneity (I2 = 73%).
Summary of findings 2. Tricyclic antidepressants compared to placebo for panic disorder.
| Tricyclic antidepressants (TCAs) compared to placebo for panic disorder | ||||||
| Patient or population: adults with panic disorder Settings: in‐ and outpatients Intervention: TCA Comparison: placebo | ||||||
| Outcomes (2‐6 months post‐treatment) | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | TCA | |||||
| Failure to respond | 659 per 1000 | 481 per 1000 (415 to 566) | RR 0.73 (0.63 to 0.86) | 829 (9 studies) | ⊕⊕⊕⊝ moderate1 | A RR of 0.73 means that treatment with TCA decreases the risk of nonresponse to treatment by 27% compared to placebo. |
| Total number of dropouts | 408 per 1000 | 302 per 1000 (257 to 351) | RR 0.74 (0.63 to 0.86) | 1906 (17 studies) | ⊕⊕⊕⊝ moderate1 | A RR of 0.74 means that treatment with TCA decreases the risk of leaving the study early by 26% compared to placebo. |
| Failure to remit | 581 per 1000 | 476 per 1000 (401 to 575) | RR 0.82 (0.69 to 0.99) | 1294 (8 studies) | ⊕⊕⊝⊝ low1,2 | A RR of 0.82 means that treatment with TCA decreases the risk of not reaching remission by 18% compared to placebo. |
|
Panic symptoms ‐ endpoint score (various scales) |
The mean endpoint score for panic symptoms in the intervention groups was 0.50 standard deviations lower (0.62 lower to 0.39 lower) | 1247 (7 studies) | ⊕⊕⊕⊝ moderate1 | We calculated SMD of endpoint scores. The results show a benefit for TCA compared to placebo. The size of effect can be considered moderate (Cohen 1988). | ||
|
Panic symptoms ‐ mean change (various scales) |
The mean change in panic symptoms in the intervention groups was 2.09 standard deviations lower (4.07 lower to 0.12 lower) | 70 (2 studies) | ⊕⊝⊝⊝ very low1,3,4 | We calculated SMD of mean change scores. The results show a benefit for TCA compared to placebo. The size of effect can be considered large (Cohen 1988). | ||
| Number of dropouts due to adverse effects | 44 per 1000 | 87 per 1000 (58 to 128) | RR 1.97 (1.33 to 2.91) | 1641 (10 studies) | ⊕⊕⊕⊝ moderate1 | A RR of 1.97 means that treatment with TCA increases the risk of leaving the study because of adverse effects by 97% compared to placebo. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference; TCA: tricyclic antidepressant | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one point due to high dropout rates (> 30%) in many studies. Moreover, random sequence generation and allocation concealment were unclear in most of the studies. 2Downgraded one point due to substantial heterogeneity (I2 = 63%). 3Downgraded two points due to considerable heterogeneity (I2 = 89%). 4Downgraded one point due to imprecision: number of participants included in the analysis is very low.
Summary of findings 3. Selective serotonin reuptake inhibitors compared to placebo for panic disorder.
| Selective serotonin reuptake inhibitors (SSRIs) compared to placebo for panic disorder | ||||||
| Patient or population: adults with panic disorder Settings: in‐ and outpatients Intervention: SSRIs Comparison: placebo | ||||||
| Outcomes (2‐6 months post‐treatment) | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | SSRI | |||||
| Failure to respond | 545 per 1000 | 408 per 1000 (365 to 457) | RR 0.75 (0.67 to 0.84) | 4000 (21 studies) | ⊕⊕⊝⊝ low1,2 | A RR of 0.75 means that treatment with SSRI decreases the risk of to treatment by 25% compared to placebo. |
| Total number of dropouts | 292 per 1000 | 290 per 1000 (263 to 319) | RR 0.99 (0.90 to 1.09) | 4302 (23 studies) | ⊕⊕⊕⊝ moderate1 | A RR close to 1 means that the risk of leaving the study early is no different with treatment with SSRI or with placebo. |
| Failure to remit | 557 per 1000 | 451 per 1000 (418 to 490) | RR 0.81 (0.75 to 0.88) | 3339 (16 studies) | ⊕⊕⊕⊕ moderate1 | A RR of 0.81 means that treatment with SSRI decreases the risk of not reaching remission by 19% compared to placebo. |
|
Panic symptoms ‐ endpoint score (various scales) |
The mean endpoint score for panic symptoms in the intervention groups was 0.28 standard deviations lower (0.39 lower to 0.17 lower) | 1625 (6 studies) | ⊕⊕⊕⊝ moderate1 | We calculated SMD of endpoint scores. The results show a benefit for SSRI compared to placebo. The size of effect can be considered between small and moderate (Cohen 1988). | ||
|
Panic symptoms ‐ mean change (various scales) |
The mean change in panic symptoms in the intervention groups was 0.43 standard deviations lower (0.58 lower to 0.29 lower) | 1255 (7 studies) | ⊕⊕⊕⊝ moderate1 | We calculated SMD of mean change scores. The results show a benefit for SSRI compared to placebo. The size of effect can be considered between small and moderate (Cohen 1988). | ||
| Number of dropouts due to adverse effects | 67 per 1000 | 97 per 1000 (77 to 121) | RR 1.45 (1.16 to 1.81) | 4131 (22 studies) | ⊕⊕⊕⊝ moderate1 | A RR of 1.45 means that treatment with SSRI increases the risk of leaving the study because of adverse effects by 45% compared to placebo. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference; SSRI: selective serotonin reuptake inhibitor | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one point due to high dropout rates (> 30%) in many studies. Moreover, random sequence generation and allocation concealment were unclear in most of the studies. 2Downgraded one point due to substantial heterogeneity (I2 = 64%).
Summary of findings 4. Serotonin‐norepinephrine reuptake inhibitors compared to placebo for panic disorder.
| Serotonin‐norepinephrine reuptake inhibitor (SNRI) compared to placebo for panic disorder | ||||||
| Patient or population: adults with panic disorder Settings: outpatients Intervention: SNRIs Comparison: placebo | ||||||
| Outcomes (2‐6 months post‐treatment) | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | SNRI | |||||
| Failure to respond | 495 per 1000 | 302 per 1000 (203 to 451) | RR 0.61 (0.41 to 0.91) | 1531 (4 studies) | ⊕⊕⊝⊝ low1 | A RR of 0.61 means that treatment with SNRI decreases the risk of nonresponse to treatment by 39% compared to placebo. |
| Total number of dropouts | 254 per 1000 | 237 per 1000 (176 to 321) | RR 0.93 (0.69 to 1.26) | 1531 (4 studies) | ⊕⊕⊕⊝ moderate2 | A RR close to 1 means that the risk of leaving the study early is no different with treatment with SNRI or with placebo. |
| Failure to remit | 667 per 1000 | 561 per 1000 (500 to 634) | RR 0.84 (0.75 to 0.95) | 1531 (4 studies) | ⊕⊕⊕⊝ moderate3 | A RR of 0.84 means that treatment with SNRI decreases the risk of not reaching remission by 16% compared to placebo. |
|
Panic symptoms ‐ endpoint score (various scales) |
The mean endpoint score for panic symptoms in the intervention groups was 0.28 standard deviations lower (0.44 lower to 0.12 lower) | 723 (2 studies) | ⊕⊕⊕⊕ high | We calculated SMD of endpoint scores. The results show a benefit for SNRI compared to placebo. The size of effect can be considered between small and moderate (Cohen 1988). | ||
|
Panic symptoms ‐ mean change (various scales) |
The mean change in panic symptoms in the intervention groups was 0.41 standard deviations lower (0.60 lower to 0.23 lower) | 685 (2 studies) | ⊕⊕⊕⊕ high | We calculated SMD of mean change scores. The results show a benefit for SNRI compared to placebo. The size of effect can be considered between small and moderate (Cohen 1988). | ||
| Number of dropouts due to adverse effects | 43 per 1000 | 64 per 1000 (40 to 103) | RR 1.49 (0.92 to 2.40) | 1531 (4 studies) | ⊕⊕⊕⊝ moderate4 | A RR of 1.49 means that treatment with SNRI increases the risk of leaving the study because of adverse effects by 49% compared to placebo. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference; SNRI: serotonin‐norepinephrine reuptake inhibitor | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two points due to considerable heterogeneity (I2 = 89%). 2 Downgraded one point due to substantial heterogeneity (I2 = 60%). 3 Downgraded one point due to substantial heterogeneity (I2 = 57%). 4 Downgraded one point due to serious imprecision: 95% CI ranges from no difference to appreciable benefit with placebo.
All comparisons and outcomes with data are reported below. Time point for outcome assessment was short term (acute‐phase treatment, two to six months), with preference given for the time point closest to 12 weeks.
Comparison 1. Antidepressants versus placebo
Forty studies including 8220 participants provided data for at least one outcome for this comparison. See also: Table 1.
Primary outcomes
1.1 Failure to respond
We found low‐quality evidence showing a benefit for antidepressants over placebo in terms of response rates (RR 0.72, 95% CI 0.66 to 0.79; participants = 6500; studies = 30).
The effect estimate is very precise, with a small confidence interval, even if the degree of heterogeneity between studies was substantial (I2 = 67%) (Analysis 1.1). The magnitude of effect corresponds to a NNTB of 7 (95% CI 6 to 9): that means 7 people would need to be treated with antidepressants in order for one to benefit. Visual inspection of the funnel plot suggested that publication bias might have occurred, although Egger's test was not statistically significant (P = 0.058).
1.1. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 1 Failure to respond.
All classes of antidepressants showed a benefit over placebo: TCAs (RR 0.73, 95% CI 0.63 to 0.86; participants = 829; studies = 9; I2 = 37% , moderate‐quality evidence; NNTB of 6, 95% CI 5 to 11), SSRIs (RR 0.75, 95% CI 0.67 to 0.84; participants = 4000; studies = 21; I2 = 64%, low‐quality evidence; NNTB of 8, 95% CI 6 to 12), MAOIs (RR 0.55, 95% CI 0.34 to 0.88; participants = 29; studies = 1; NNTB of 3, 95% CI 2 to 25), SNRIs (RR 0.61, 95% CI 0.41 to 0.91; participants = 1531; studies = 4; I2 = 89%, low‐quality evidence; NNTB of 6, 95% CI 4 to 23), NRIs (RR 0.71, 95% CI 0.51 to 0.97; participants = 82; studies = 1; NNTB of 5, 95% CI 3 to 43), with the exception of other antidepressants (RR 0.93, 95% CI 0.75 to 1.15; participants = 29; studies = 1). No data were available for NaSSAs or NDRIs.
Test for subgroup difference did not reveal heterogeneity between classes of antidepressants (P = 0.24), I² = 25.8%).
Results of this outcome are shown in Figure 4.
4.

Forest plot of comparison 1. Antidepressants versus placebo, outcome 1.1, failure to respond
1.2 Total number of dropouts
In comparison with placebo, fewer participants receiving antidepressants dropped out due to any cause (RR 0.88, 95% CI 0.81 to 0.97; participants = 7850; studies = 38; I2 = 30%, moderate‐quality evidence) (Analysis 1.2). The magnitude of effect corresponds to a NNTB of 27 (95% CI 17 to 105); treating 27 people will result in one person fewer dropping out. Visual inspection of the funnel plot suggested that publication bias might have occurred, although Egger's test was not statistically significant (0.639).
1.2. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 2 Total number of dropouts.
When looking at classes of antidepressants, only TCAs (RR 0.74, 95% CI 0.63 to 0.86; participants = 1906; studies = 17; I2 = 11%, moderate‐quality evidence) (NNTH of 10, 95% CI 7 to 18) and NRIs (RR 0.50, 95% CI 0.28 to 0.90; participants = 82; studies = 1) (NNTH of 4, 95% CI 3 to 19) had a benefit over placebo, while moderate‐quality evidence showed no difference between SSRIs and placebo (RR 0.99, 95% CI 0.90 to 1.09; participants = 4302; studies = 23; I2 = 0%) and between SNRIs and placebo (RR 0.93, 95% CI 0.69 to 1.26; participants = 1531; studies = 4; I2 = 60%, moderate‐quality evidence).
One study on other antidepressants found no dropouts both for ritanserin and placebo arms, so it was not possible to calculate a RR.
Test for subgroup differences revealed a substantial heterogeneity between classes of antidepressants (P = 0.002, I2 = 79.7%).
No data were available for MAOIs, NaSSAs and NDRIs for this outcome.
Results of this outcome are shown in Figure 5.
5.

Forest plot of comparison 1. Antidepressants versus placebo, outcome 1.2, total number of dropouts
Secondary outcomes
1.3 Failure to remit
We found moderate‐quality evidence showing a benefit for antidepressants compared to placebo in terms of remission rates (RR 0.83, 95% CI 0.78 to 0.88; participants = 6164; studies = 24); heterogeneity was moderate (I2 = 40%) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 3 Failure to remit.
All classes of antidepressants showed a benefit over placebo on this outcome: TCAs (RR 0.82, 95% CI 0.69 to 0.99; participants = 1294; studies = 8; I2 = 63%, low‐quality evidence), SSRIs (RR 0.81, 95% CI 0.75 to 0.88; participants = 3339; studies = 16; I2 = 30%, moderate‐quality evidence) and SNRIs (RR 0.84, 95% CI 0.75 to 0.95; participants = 1531; studies = 4; I2 = 57%, moderate‐quality evidence), without any subgroup differences (P = 0.89, I2 = 0%).
No data were available for MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.4 Panic symptoms ‐ endpoint score
We found low‐quality evidence showing a benefit for antidepressants in comparison with placebo in decreasing panic symptoms, measured as a continuous outcome with endpoint scores (SMD ‐0.44, 95% CI ‐0.58 to ‐0.30; participants = 3699; studies = 15), with substantial heterogeneity between studies (I2 = 68%) (Analysis 1.4).
1.4. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 4 Panic symptoms ‐ endpoint score.
All classes of antidepressants showed a benefit over placebo: TCAs (SMD ‐0.50, 95% CI ‐0.62 to ‐0.39; participants = 1247; studies = 7; I2 = 0%, moderate‐quality evidence), SSRIs (SMD ‐0.28, 95% CI ‐0.39 to ‐0.17; participants = 1625; studies = 6; I2 = 4%, moderate‐quality evidence), MAOIs (SMD ‐3.68, 95% CI ‐4.93 to ‐2.43; participants = 29; studies = 1), SNRIs (SMD ‐0.28, 95% CI ‐0.44 to ‐0.12; participants = 723; studies = 2; I2 = 0%, high‐quality evidence) and NRIs (SMD ‐1.02, 95% CI ‐1.50 to ‐0.53; participants = 75; studies = 1).
The difference between subgroups was considerable (P < 0.001, I2 = 90.6%)
No data were available for NaSSAs, NDRIs and other antidepressants for this outcome.
1.5 Panic symptoms ‐ mean change
Low‐quality evidence showed a benefit for antidepressants in comparison with placebo in decreasing panic symptoms, measured as a continuous outcome with mean change from baseline scores (SMD ‐0.53, 95% CI ‐0.72 to ‐0.33; participants = 2010; studies = 10), with substantial heterogeneity between studies (I2 = 73%) (Analysis 1.5).
1.5. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 5 Panic symptoms ‐ mean change.
When looking at classes of antidepressants, all classes showed a benefit over placebo: TCAs (SMD ‐2.09, 95% CI ‐4.07 to ‐0.12; participants = 70; studies = 2; I2 = 89%, very low‐quality evidence), SSRIs (SMD ‐0.43, 95% CI ‐0.58 to ‐0.29; participants = 1255; studies = 7; I2 = 36%, moderate‐quality evidence), SNRIs (SMD ‐0.41, 95% CI ‐0.60 to ‐0.23; participants = 685; studies = 2; I2 = 17%, high‐quality evidence).
The subgroups did not differ significantly (P = 0.25, I² = 27.3%)
No data were available for MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.6 Frequency of panic attacks ‐ endpoint score
Antidepressants as a whole showed a benefit over placebo in terms of frequency of panic attacks measured at endpoint (SMD ‐0.43, 95% CI ‐0.66 to ‐0.20; participants = 1671; studies = 17); heterogeneity was substantial (I2 = 78%) (Analysis 1.6).
1.6. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 6 Frequency of panic attacks ‐ endpoint score.
All classes of antidepressants compared to placebo decreased the frequency of panic attacks: TCAs (SMD ‐0.83, 95% CI ‐1.38 to ‐0.28; participants = 470; studies = 8; I2 = 84%), SSRIs (SMD ‐0.17, 95% CI ‐0.32 to ‐0.02; participants = 1126; studies = 8; I2 = 25%) and NRIs (SMD ‐0.91, 95% CI ‐1.39 to ‐0.44; participants = 75; studies = 1).
The subgroup differed significantly (P < 0.002, I2 = 84.3%)
No data were available for MAOIs, SNRIs, NaSSAs, NDRIs and other antidepressants for this outcome.
1.7 Frequency of panic attacks ‐ mean change
In terms of frequency of panic attacks measured as change from baseline, antidepressants as a whole showed a benefit over placebo (SMD ‐0.43, 95% CI ‐0.72 to ‐0.14; participants = 2579; studies = 8), with a considerable heterogeneity between studies (I2 = 91%) (Analysis 1.7).
1.7. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 7 Frequency of panic attacks ‐ mean change.
When looking at classes of antidepressants, we found evidence showing no difference between TCAs and placebo (SMD ‐0.08, 95% CI ‐0.36 to 0.21; participants = 204; studies = 2; I2 = 0%), a benefit for SSRIs over placebo (SMD ‐0.16, 95% CI ‐0.30 to ‐0.03; participants = 949; studies = 5; I2 = 0%) and a benefit for SNRIs over placebo (SMD ‐0.87, 95% CI ‐1.35 to ‐0.39; participants = 1426; studies = 4; I2 = 94%).
Subgroup difference was substantial (I2 = 76%).
No data were available for MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.8 Agoraphobia ‐ endpoint score
We found evidence showing a benefit for antidepressants over placebo in terms of agoraphobia measured as a continuous outcome with endpoint scores (SMD ‐0.69, 95% CI ‐0.99 to ‐0.39; participants = 2987; studies = 12); heterogeneity was considerable (I2 = 91%) (Analysis 1.8).
1.8. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 8 Agoraphobia ‐ endpoint score.
Six studies about TCAs suggested a benefit in the direction of antidepressants over placebo (SMD ‐0.59, 95% CI ‐1.31 to 0.13; participants = 1122; I2 = 94%); SSRIs showed a benefit over placebo (SMD ‐0.50, 95% CI ‐0.71 to ‐0.29; participants = 1732; studies = 6; I2 = 67%), as well as MAOIs (SMD ‐5.38, 95% CI ‐7.03 to ‐3.72; participants = 29; studies = 1) and NRIs (SMD ‐1.56, 95% CI ‐2.09 to ‐1.04; participants = 75; studies = 1). One study found no difference between other antidepressants and placebo (SMD 0.23, 95% CI ‐0.56 to 1.02; participants = 29; studies = 1).
The was a considerable difference between classes of antidepressants (P < 0.001, I2 = 92%).
No data were available for SNRIs, NaSSAs and NDRIs for this outcome.
1.9 Agoraphobia ‐ mean change
We found evidence showing a benefit for antidepressants over placebo in terms of agoraphobia measured as a continuous outcome with mean change from baseline scores (SMD ‐0.68, 95% CI ‐1.19 to ‐0.17; participants = 1792; studies = 7), with a considerable heterogeneity (I2 = 96%) (Analysis 1.9).
1.9. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 9 Agoraphobia ‐ mean change.
TCAs showed a benefit over placebo (SMD ‐0.46, 95% CI ‐0.84 to ‐0.08; participants = 237; studies = 3; I2 = 33%), as well as SNRIs (SMD ‐0.33, 95% CI ‐0.47 to ‐0.20; participants = 1029; studies = 3; I2 = 0%), while wide CI for SSRIs includes no difference with placebo on agoraphobia.
The subgroups did not differ significantly (P = 0.51, I2 = 0%).
No data were available for MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.10 General anxiety ‐ endpoint score
We found evidence showing a benefit for antidepressants over placebo in terms of general anxiety measured as a continuous outcome with endpoint scores (SMD ‐0.46, 95% CI ‐0.63 to ‐0.29; participants = 3168; studies = 17); heterogeneity between studies was substantial (I2 = 71%) (Analysis 1.10). The estimate of effect showed a benefit for TCAs compared to placebo (SMD ‐0.35, 95% CI ‐0.48 to ‐0.21; participants = 1351; studies = 9; I2 = 12%), as well as SSRIs (SMD ‐0.42, 95% CI ‐0.58 to ‐0.26; participants = 1759; studies = 9; I2 = 42%), and MAOIs (SMD ‐7.28, 95% CI ‐9.43 to ‐5.14; participants = 29; studies = 1). One study found no difference between other antidepressants and placebo (SMD 0.08, 95% CI ‐0.70 to 0.87; participants = 29).
1.10. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 10 General anxiety ‐ endpoint score.
Subgroup difference between classes of antidepressants was considerable (P < 0.001, I2 = 92.8%).
No data were available for SNRIs, NaSSAs, NDRIs and NRIs for this outcome.
1.11 General anxiety ‐ mean change
When measuring general anxiety as a continuous outcome with mean change from baseline scores, we also found a benefit for antidepressants over placebo (SMD ‐0.33, 95% CI ‐0.47 to ‐0.20; participants = 2477; studies = 11), with a moderate heterogeneity (I2 = 57%) (Analysis 1.11). When looking at classes of antidepressants, the confidence interval for TCAs included no difference with placebo (SMD ‐0.62, 95% CI ‐1.28 to 0.04; participants = 294; studies = 4; I2 = 81%), while both SSRIs (SMD ‐0.32, 95% CI ‐0.47 to ‐0.18; participants = 1251; studies = 7; I2 = 38%) and SNRIs (SMD ‐0.26, 95% CI ‐0.44 to ‐0.07; participants = 932; studies = 3; I2 = 40%) showed a benefit over placebo.
1.11. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 11 General anxiety ‐ mean change.
There was no difference between the subgroups (P = 0.54, I² = 0%).
No data were available for MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.12 Depression ‐ endpoint score
We found evidence showing a benefit for antidepressants over placebo in terms of depression measured as a continuous outcome with endpoint scores (SMD ‐0.41, 95% CI ‐0.57 to ‐0.25; participants = 1794; studies = 12); heterogeneity was moderate (I2 = 43%) (Analysis 1.12).
1.12. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 12 Depression ‐ endpoint score.
All individual classes of antidepressants were better than placebo on this outcome: TCAs (SMD ‐0.54, 95% CI ‐0.73 to ‐0.35; participants = 779; studies = 6; I2 = 17%), SSRIs (SMD ‐0.27, 95% CI ‐0.45 to ‐0.09; participants = 957; studies = 7; I2 = 23%) and MAOIs (SMD ‐1.00, 95% CI ‐1.78 to ‐0.22; participants = 29; studies = 1).
One study showed a possible benefit for other antidepressants over placebo (SMD 0.21, 95% CI ‐0.58 to 1.00; participants = 29).
Subgroup difference between classes of antidepressants was moderate (P = 0.03, I2 = 65.7%).
No data were available for SNRIs, NaSSAs, NDRIs and NRIs for this outcome.
1.13 Depression ‐ mean change
When measuring depression as a continuous outcome with mean change from baseline scores, we also found a benefit for antidepressants over placebo (SMD ‐0.40, 95% CI ‐0.55 to ‐0.24; participants = 1052; studies = 6; I2 = 28%) (Analysis 1.13).
1.13. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 13 Depression ‐ mean change.
Both classes of TCAs (SMD ‐0.58, 95% CI ‐1.09 to ‐0.08; participants = 229; studies = 3; I2 = 56%) and SSRIs (SMD ‐0.36, 95% CI ‐0.51 to ‐0.21; participants = 823; studies = 5; I2 = 11%) were more effective than placebo on depressive symptoms.
The subgroups did not differ (P = 0.40, I² = 0%).
No data were available for MAOIs, SNRIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.14 Social functioning ‐ endpoint score
Antidepressants showed a benefit in comparison to placebo on social functioning measured as endpoint scores (SMD ‐0.29, 95% CI ‐0.40 to ‐0.18; participants = 1872; studies = 9; I2 = 11%) (Analysis 1.14). TCAs showed a benefit over placebo (SMD ‐0.29, 95% CI ‐0.42 to ‐0.16; participants = 927; studies = 4; I2 = 0%), as well as SSRIs (SMD ‐0.43, 95% CI ‐0.70 to ‐0.16; participants = 550; studies = 5; I2 = 47%), while one study on SNRIs did not show a difference from placebo on this outcome (SMD ‐0.15, 95% CI ‐0.40 to 0.10; participants = 395).
1.14. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 14 Social functioning ‐ endpoint score.
There was no difference between the subgroups (P = 0.32, I² = 13.0%).
No data were available for MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.15 Social functioning ‐ mean change
Similarly, measuring social functioning as mean change from baseline on continuous measures we found a benefit of antidepressants over placebo (SMD ‐0.29, 95% CI ‐0.42 to ‐0.16; participants = 1429; studies = 7; I2 = 27%) (Analysis 1.15). Classes of TCAs (SMD ‐0.38, 95% CI ‐0.70 to ‐0.07; participants = 199; studies = 2; I2 = 8%) and SSRIs (SMD ‐0.40, 95% CI ‐0.56 to ‐0.24; participants = 693; studies = 4; I2 = 0%) showed a benefit over placebo, while SNRIs did not (SMD ‐0.10, 95% CI ‐0.27 to 0.07; participants = 537; studies = 2; I2 = 0%).
1.15. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 15 Social functioning ‐ mean change.
Subgroup difference between classes of antidepressants was substantial (I2 = 71.3%).
No data were available for MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.16 Quality of life ‐ endpoint score
We found no difference between antidepressants and placebo on this outcome (SMD ‐0.13, 95% CI ‐0.29 to 0.03; participants = 1675; studies = 5; I2 = 59%) (Analysis 1.16). SSRIs showed a more favourable profile than placebo (SMD ‐0.28, 95% CI ‐0.51 to ‐0.04; participants = 746; studies = 4; I2 = 58%) , while SNRIs were not different from placebo (SMD 0.03, 95% CI ‐0.11 to 0.17; participants = 929; studies = 3; I2 = 0%).
1.16. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 16 Quality of life.
Subgroup difference between classes of antidepressants was substantial (P = 0.03, I2 = 79.2%).
No data were available for TCAs, MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
1.17 Patient satisfaction
We found evidence showing a benefit for antidepressants over placebo on patient satisfaction (SMD ‐0.53, 95% CI ‐1.01 to ‐0.05; participants = 521; studies = 3), with a considerable heterogeneity (I2 = 80%) (Analysis 1.17). Very few data are available, with only one study about TCAs (SMD ‐1.66, 95% CI ‐2.51 to ‐0.82; participants = 30) and two about SNRIs (SMD ‐0.27, 95% CI ‐0.45 to ‐0.09; participants = 491; studies = 2; I2 = 0%).
1.17. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 17 Patient satisfaction.
The difference between classes of antidepressants was considerable (P = 0.002, I2 = 90%).
1.18 Economic costs
No data were available for this outcome.
1.19 Number of dropouts due to adverse effects
We found moderate‐quality evidence showing a higher number of dropouts due to adverse effects occurring with antidepressants compared to placebo (RR 1.49, 95% CI 1.25 to 1.78; participants = 7688; studies = 33; I2 = 0%) (Analysis 1.19).
1.19. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 19 Number of dropouts due to adverse effects.
When looking at classes of antidepressants, TCAs (RR 1.97, 95% CI 1.33 to 2.91; participants = 1641; studies = 10; I2 = 0%, moderate‐quality evidence) and SSRI (RR 1.45, 95% CI 1.16 to 1.81; participants = 4131; studies = 22; I2 = 0%, moderate‐quality evidence) had a higher number of dropouts in comparison with placebo, while CIs for other classes were broader and included no difference: SNRIs (RR 1.49, 95% CI 0.92 to 2.40; participants = 1531; studies = 4; I2 = 0%, moderate‐quality evidence), NRIs (RR 0.48, 95% CI 0.04 to 5.05; participants = 82; studies = 1), other antidepressants (RR 0.51, 95% CI 0.18 to 1.47; participants = 303; studies = 2)
Subgroup difference between classes of antidepressants was moderate (P = 0.14, I2 = 41.8%).
No data were available for MAOIs, NaSSAs and NDRIs for this outcome.
1.20 Number of participants experiencing at least one adverse effect
Antidepressants were less well tolerated than placebo when looking at number of participants experiencing at least one adverse effect (RR 1.11, 95% CI 1.07 to 1.15; participants = 4246; studies = 15; I2 = 0%) (Analysis 1.20).
1.20. Analysis.

Comparison 1 Antidepressants versus placebo, Outcome 20 Number of participants experiencing at least one adverse effect.
All classes of antidepressants showed a higher number of participants with adverse effects in comparison to placebo: TCAs (RR 1.22, 95% CI 1.04 to 1.42; participants = 256; studies = 2; I2 = 40%), SSRIs (RR 1.10, 95% CI 1.05 to 1.16; participants = 2459; studies = 14; I2 = 9%) and SNRIs (RR 1.09, 95% CI 1.03 to 1.16; participants = 1531; studies = 4; I2 = 0%).
There was no significant difference between the antidepressant classes (P = 0.43, I² = 0%).
No data were available for MAOIs, NaSSAs, NDRIs, NRIs and other antidepressants for this outcome.
Comparison 2. TCAs versus placebo
Sixteen studies including 2551 participants provided data for at least one outcome for this comparison. See also: Table 2.
We have set out in detail below the findings for individual TCAs compared to placebo.
Primary outcomes
2.1 Failure to respond
We found evidence showing a benefit for imipramine compared to placebo (RR 0.75, 95% CI 0.60 to 0.94; participants = 282; studies = 4; I2 = 31%), and for clomipramine compared to placebo (RR 0.74, 95% CI 0.56 to 0.97; participants = 626; studies = 4; I2 = 67%). One study on desipramine did not show a difference with placebo (RR 0.60, 95% CI 0.32 to 1.14; participants = 56; studies = 1) (Analysis 2.1).
2.1. Analysis.

Comparison 2 TCAs versus placebo, Outcome 1 Failure to respond.
2.2 Total number of dropouts
We found evidence showing a benefit for imipramine compared to placebo (RR 0.74, 95% CI 0.60 to 0.91; participants = 1285; studies = 10; I2 = 19%). Clomipramine did not show a difference in number of dropouts compared to placebo (RR 0.81, 95% CI 0.62 to 1.07; participants = 719; studies = 6; I2 = 31%), as well as one study on desipramine (RR 0.18, 95% CI 0.04 to 0.75; participants = 56) (Analysis 2.2).
2.2. Analysis.

Comparison 2 TCAs versus placebo, Outcome 2 Total number of dropouts.
Secondary outcomes
2.3 Failure to remit
One study on desipramine showed a benefit over placebo (RR 0.40, 95% CI 0.18 to 0.88; participants = 56; studies = 1), while for imipramine (RR 0.83, 95% CI 0.67 to 1.02; participants = 1081; studies = 6; I2 = 63%) and clomipramine (RR 0.96, 95% CI 0.81 to 1.14; participants = 244; studies = 1) we found no difference in comparison with placebo in terms of remission rates (Analysis 2.3).
2.3. Analysis.

Comparison 2 TCAs versus placebo, Outcome 3 Failure to remit.
2.4 Panic symptoms ‐ endpoint score
We found a benefit for imipramine over placebo on this outcome (SMD ‐0.47, 95% CI ‐0.60 to ‐0.35; participants = 1056; studies = 5; I2 = 0%). Also one study on clomipramine showed a benefit over placebo (SMD ‐0.69, 95% CI ‐1.04 to ‐0.35; participants = 158), as well as one study on desipramine (SMD ‐0.62, 95% CI ‐1.16 to ‐0.08; participants = 56) (Analysis 2.4).
2.4. Analysis.

Comparison 2 TCAs versus placebo, Outcome 4 Panic symptoms ‐ endpoint score.
2.5 Panic symptoms ‐ mean change
One study on imipramine showed a benefit over placebo (SMD ‐1.14, 95% CI ‐1.81 to ‐0.46; participants = 40), as well as one study on clomipramine (SMD ‐3.16, 95% CI ‐4.27 to ‐2.04; participants = 30) (Analysis 2.5).
2.5. Analysis.

Comparison 2 TCAs versus placebo, Outcome 5 Panic symptoms ‐ mean change.
2.6 Frequency of panic attacks ‐ endpoint score
We found evidence showing a benefit for imipramine compared to placebo (SMD ‐1.07, 95% CI ‐1.87 to ‐0.26; participants = 279; studies = 6; I2 = 87%). One study on clomipramine showed a direction in favour of clomipramine over placebo (SMD ‐0.38, 95% CI ‐0.72 to ‐0.04; participants = 158), as well as one study on desipramine (SMD ‐0.23, 95% CI ‐0.76 to 0.29; participants = 56) (Analysis 2.6).
2.6. Analysis.

Comparison 2 TCAs versus placebo, Outcome 6 Frequency of panic attacks ‐ endpoint score.
2.7 Frequency of panic attacks ‐ mean change
Both imipramine (SMD ‐0.31, 95% CI ‐0.93 to 0.32; participants = 40; studies = 1) and clomipramine (SMD ‐0.02, 95% CI ‐0.28 to 0.25; participants = 222; studies = 1) (Analysis 2.7) were not different from placebo.
2.7. Analysis.

Comparison 2 TCAs versus placebo, Outcome 7 Frequency of panic attacks ‐ mean change.
2.8 Agoraphobia ‐ endpoint score
We found evidence showing a benefit for imipramine compared to placebo, although the estimate of effect was small (SMD ‐0.22, 95% CI ‐0.35 to ‐0.09; participants = 920; studies = 4; I2 = 0%). One study on clomipramine showed a benefit for the drug compared to placebo (SMD ‐2.40, 95% CI ‐2.77 to ‐2.03; participants = 194), and one study revealed a direction of the effect in favour desipramine over placebo (Analysis 2.8).
2.8. Analysis.

Comparison 2 TCAs versus placebo, Outcome 8 Agoraphobia ‐ endpoint score.
2.9 Agoraphobia ‐ mean change
One study found no difference between imipramine and placebo (SMD ‐0.08, 95% CI ‐0.70 to 0.54; participants = 40), while we found evidence showing a benefit for clomipramine over placebo (SMD ‐0.57, 95% CI ‐0.93 to ‐0.21; participants = 256; studies = 2; I2 = 19%) (Analysis 2.9).
2.9. Analysis.

Comparison 2 TCAs versus placebo, Outcome 9 Agoraphobia ‐ mean change.
2.10 General anxiety ‐ endpoint score
Imipramine (SMD ‐0.32, 95% CI ‐0.52 to ‐0.12; participants = 1014; studies = 6; I2 = 22%), clomipramine (SMD ‐0.45, 95% CI ‐0.68 to ‐0.22; participants = 352; studies = 2; I2 = 10%) and desipramine (SMD ‐0.31, 95% CI ‐0.84 to 0.21; participants = 56; studies = 1) were all more effective than placebo in reducing general anxiety measured as a continuous outcome with endpoint scores (Analysis 2.10).
2.10. Analysis.

Comparison 2 TCAs versus placebo, Outcome 10 General anxiety ‐ endpoint score.
2.11 General anxiety ‐ mean change
When measuring general anxiety as change from baseline scores, evidence did not show a difference between imipramine and placebo (SMD ‐0.20, 95% CI ‐0.63 to 0.24; participants = 82; studies = 2; I2 = 0%), and clomipramine and placebo (SMD ‐1.19, 95% CI ‐2.99 to 0.61; participants = 273; studies = 2; I2 = 93%) (Analysis 2.11).
2.11. Analysis.

Comparison 2 TCAs versus placebo, Outcome 11 General anxiety ‐ mean change.
2.12 Depression ‐ endpoint score
Both imipramine (SMD ‐0.61, 95% CI ‐0.85 to ‐0.37; participants = 656; studies = 5; I2 = 23%) and clomipramine (SMD ‐0.37, 95% CI ‐0.65 to ‐0.09; participants = 194; studies = 1) showed a benefit over placebo (Analysis 2.12).
2.12. Analysis.

Comparison 2 TCAs versus placebo, Outcome 12 Depression ‐ endpoint score.
2.13 Depression ‐ mean change
One study on imipramine did not show a difference from placebo (SMD ‐0.30, 95% CI ‐0.92 to 0.33; participants = 40), while for clomipramine we found evidence in the direction of a benefit over placebo (SMD ‐0.81, 95% CI ‐1.73 to 0.12; participants = 248; studies = 2; I2 = 76%) (Analysis 2.13).
2.13. Analysis.

Comparison 2 TCAs versus placebo, Outcome 13 Depression ‐ mean change.
2.14 Social functioning ‐ endpoint score
We found evidence showing a benefit for imipramine over placebo (SMD ‐0.29, 95% CI ‐0.42 to ‐0.16; participants = 927; studies = 4; I2 = 0%) (Analysis 2.14).
2.14. Analysis.

Comparison 2 TCAs versus placebo, Outcome 14 Social functioning ‐ endpoint score.
2.15 Social functioning ‐ mean change
One study showed no difference between imipramine and placebo (SMD ‐0.10, 95% CI ‐0.72 to 0.52; participants = 40), while a study on clomipramine showed a benefit of the drug in comparison to placebo (Analysis 2.15).
2.15. Analysis.

Comparison 2 TCAs versus placebo, Outcome 15 Social functioning ‐ mean change.
2.16 Patient satisfaction
One study found a benefit for clomipramine over placebo on this outcome (MD ‐2.50, 95% CI ‐3.55 to ‐1.45; participants = 30) (Analysis 2.16).
2.16. Analysis.

Comparison 2 TCAs versus placebo, Outcome 16 Patient satisfaction.
2.17 Number of dropouts due to adverse effects
We found evidence showing that imipramine was less well tolerated than placebo, when looking at number of dropouts due to adverse effects (RR 2.60, 95% CI 1.56 to 4.34; participants = 1138; studies = 6; I2 = 0%); the same was true for clomipramine, although the magnitude of effect was smaller (RR 1.50, 95% CI 0.93 to 2.43; participants = 658; studies = 4; I2 = 0%) (Analysis 2.17).
2.17. Analysis.

Comparison 2 TCAs versus placebo, Outcome 17 Number of dropouts due to adverse effects.
2.18 Number of participants experiencing at least one adverse effect
One study on imipramine showed no difference with placebo for this outcome (RR 1.11, 95% CI 1.00 to 1.23; participants = 98), as well as one study on clomipramine (RR 1.31, 95% CI 1.14 to 1.51; participants = 245; studies = 1) (Analysis 2.18).
2.18. Analysis.

Comparison 2 TCAs versus placebo, Outcome 18 Number of participants experiencing at least one adverse effect.
Comparison 3. SSRIs versus placebo
Twenty‐two studies including 5571 participants provided data for at least one outcome for this comparison. See also: Table 3.
We have set out in detail below the findings for individual SSRIs compared to placebo.
Primary outcomes
3.1 Failure to respond
We found evidence of a benefit over placebo for paroxetine (RR 0.70, 95% CI 0.60 to 0.82; participants = 2469; studies = 9; I2 = 47%), fluoxetine (RR 0.46, 95% CI 0.27 to 0.76; participants = 180; studies = 1), fluvoxamine (RR 0.60, 95% CI 0.36 to 1.01; participants = 430; studies = 5; I2 = 86%) and citalopram (RR 0.82, 95% CI 0.65 to 1.04; participants = 628; studies = 2; I2 = 78%). Three studies on sertraline (RR 0.87, 95% CI 0.76 to 1.00; participants = 470; studies = 3; I2 = 0%) and one on escitalopram (RR 0.84, 95% CI 0.73 to 0.96; participants = 254; studies = 1) did not reveal a difference with placebo (Analysis 3.1).
3.1. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 1 Failure to respond.
3.2 Total number of dropouts
There was no difference in number of dropouts due to any cause between most antidepressants and placebo: paroxetine (RR 1.00, 95% CI 0.88 to 1.12; participants = 2524; studies = 9; I2 = 1%), sertraline (RR 1.10, 95% CI 0.86 to 1.40; participants = 647; studies = 4; I2 = 0%), fluoxetine (RR 1.50, 95% CI 0.71 to 3.16; participants = 180; studies = 1), fluvoxamine (RR 0.98, 95% CI 0.78 to 1.24; participants = 500; studies = 6; I2 = 0%) and citalopram (RR 0.84, 95% CI 0.64 to 1.09; participants = 628; studies = 2; I2 = 0%). One study showed that escitalopram was better tolerated in comparison with placebo (RR 0.64, 95% CI 0.44 to 0.94; participants = 254; studies = 1) (Analysis 3.2).
3.2. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 2 Total number of dropouts.
Secondary outcomes
3.3 Failure to remit
Paroxetine (RR 0.82, 95% CI 0.75 to 0.90; participants = 2214; studies = 6; I2 = 28%) and sertraline (RR 0.76, 95% CI 0.61 to 0.95; participants = 353; studies = 2; I2 = 0%) showed a benefit over placebo, whereas for other antidepressants, the available evidence did not reveal a difference in comparison with placebo: fluoxetine (RR 0.80, 95% CI 0.64 to 1.00; participants = 180; studies = 1), fluvoxamine (RR 0.73, 95% CI 0.51 to 1.03; participants = 461; studies = 5; I2 = 74%), citalopram (RR 0.95, 95% CI 0.76 to 1.19; participants = 251; studies = 1) and escitalopram (RR 0.86, 95% CI 0.68 to 1.09; participants = 254; studies = 1) (Analysis 3.3).
3.3. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 3 Failure to remit.
3.4 Panic symptoms ‐ endpoint score
We found evidence showing a benefit for paroxetine in comparison with placebo in decreasing panic symptoms, measured as a continuous outcome with endpoint scores (SMD ‐0.28, 95% CI ‐0.38 to ‐0.17; participants = 1420; studies = 3; I2 = 0%). Evidence about fluvoxamine also showed a trend in this direction (SMD ‐0.26, 95% CI ‐0.62 to 0.10; participants = 308; studies = 3; I2 = 54%) (Analysis 3.4).
3.4. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 4 Panic symptoms ‐ endpoint score.
3.5 Panic symptoms ‐ mean change
All antidepressants were effective in comparison with placebo on this outcome: paroxetine (SMD ‐0.45, 95% CI ‐0.79 to ‐0.11; participants = 684; studies = 3; I2 = 79%), sertraline (SMD ‐0.34, 95% CI ‐0.64 to ‐0.04; participants = 175; studies = 1), fluoxetine (SMD ‐0.61, 95% CI ‐0.91 to ‐0.31; participants = 180; studies = 1), citalopram (SMD ‐0.33, 95% CI ‐0.59 to ‐0.07; participants = 226; studies = 1) and escitalopram (SMD ‐0.41, 95% CI ‐0.67 to ‐0.16; participants = 239; studies = 1) (Analysis 3.5).
3.5. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 5 Panic symptoms ‐ mean change.
3.6 Frequency of panic attacks ‐ endpoint score
We found evidence showing a trend in the direction of a benefit for paroxetine over placebo in decreasing the number of panic attacks (SMD ‐0.17, 95% CI ‐0.40 to 0.06; participants = 368; studies = 2; I2 = 0%), and a similar result for sertraline (SMD ‐0.20, 95% CI ‐0.54 to 0.14; participants = 477; studies = 3; I2 = 69%). Fluvoxamine was not different from placebo on this outcome (SMD 0.04, 95% CI ‐0.46 to 0.54; participants = 305; studies = 3; I2 = 75%) (Analysis 3.6).
3.6. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 6 Frequency of panic attacks ‐ endpoint score.
3.7 Frequency of panic attacks ‐ mean change
We found evidence showing a benefit for paroxetine over placebo (SMD ‐0.16, 95% CI ‐0.28 to ‐0.03; participants = 983; studies = 4; I2 = 0%), and one study showing a possible benefit with fluoxetine (SMD ‐0.22, 95% CI ‐0.51 to 0.08; participants = 180) (Analysis 3.7).
3.7. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 7 Frequency of panic attacks ‐ mean change.
3.8 Agoraphobia ‐ endpoint score
We found evidence showing a benefit for paroxetine (SMD ‐0.33, 95% CI ‐0.46 to ‐0.20; participants = 1022; studies = 2; I2 = 0%), citalopram (SMD ‐0.75, 95% CI ‐1.21 to ‐0.28; participants = 603; studies = 2; I2 = 85%) and escitalopram (SMD ‐0.58, 95% CI ‐0.83 to ‐0.32; participants = 239; studies = 1) in comparison with placebo. One study also showed a possible benefit for fluvoxamine (SMD ‐0.45, 95% CI ‐1.08 to 0.19; participants = 39) (Analysis 3.8).
3.8. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 8 Agoraphobia ‐ endpoint score.
3.9 Agoraphobia ‐ mean change
We found data that failed to reveal a difference between paroxetine and placebo on this outcome (SMD ‐1.25, 95% CI ‐3.04 to 0.55; participants = 663; studies = 3; I2 = 99%) (Analysis 3.9).
3.9. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 9 Agoraphobia ‐ mean change.
3.10 General anxiety ‐ endpoint score
All antidepressants were effective in reducing general anxiety measured as endpoint scores: (SMD ‐0.30, 95% CI ‐0.43 to ‐0.17; participants = 1023; studies = 2; I2 = 0%), fluvoxamine (SMD ‐0.62, 95% CI ‐1.00 to ‐0.24; participants = 440; studies = 6; I2 = 69%) and citalopram (SMD ‐0.40, 95% CI ‐0.63 to ‐0.16; participants = 377; studies = 1) (Analysis 3.10).
3.10. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 10 General anxiety ‐ endpoint score.
3.11 General anxiety ‐ mean change
We found evidence showing a benefit for paroxetine (SMD ‐0.43, 95% CI ‐0.58 to ‐0.28; participants = 684; studies = 3; I2 = 0%) and fluoxetine (SMD ‐0.51, 95% CI ‐0.81 to ‐0.22; participants = 180; studies = 1) over placebo. One study on citalopram (SMD ‐0.04, 95% CI ‐0.30 to 0.22; participants = 226) and on escitalopram (SMD ‐0.18, 95% CI ‐0.44 to 0.07; participants = 239) did not show a difference versus placebo (Analysis 3.11).
3.11. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 11 General anxiety ‐ mean change.
3.12 Depression ‐ endpoint score
Fluvoxamine was more effective than placebo in reducing depressive symptoms measured as endpoint scores (SMD ‐0.39, 95% CI ‐0.77 to ‐0.01; participants = 383; studies = 5; I2 = 64%), as well as citalopram (SMD ‐0.25, 95% CI ‐0.49 to ‐0.02; participants = 377; studies = 1). One study showed a possible benefit for paroxetine (SMD ‐0.23, 95% CI ‐0.51 to 0.04; participants = 278) (Analysis 3.12).
3.12. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 12 Depression ‐ endpoint score.
3.13 Depression ‐ mean change
We found evidence showing a benefit for paroxetine (SMD ‐0.47, 95% CI ‐0.75 to ‐0.19; participants = 350; studies = 2; I2 = 38%) and fluoxetine (SMD ‐0.43, 95% CI ‐0.73 to ‐0.14; participants = 180; studies = 1) over placebo. One study on citalopram (SMD ‐0.14, 95% CI ‐0.40 to 0.13; participants = 226) and on escitalopram (SMD ‐0.23, 95% CI ‐0.49 to 0.02; participants = 239) showed a trend in favour of these antidepressants compared to placebo (Analysis 3.13).
3.13. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 13 Depression ‐ mean change.
3.14 Social functioning ‐ endpoint score
We found evidence showing a benefit for fluvoxamine over placebo (SMD ‐0.51, 95% CI ‐0.74 to ‐0.28; participants = 311; studies = 4; I2 = 0%). One study on paroxetine showed no difference with placebo on this outcome (SMD ‐0.11, 95% CI ‐0.33 to 0.11; participants = 317) (Analysis 3.14).
3.14. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 14 Social functioning ‐ endpoint score.
3.15 Social functioning ‐ mean change
We found evidence showing a benefit for paroxetine (SMD ‐0.41, 95% CI ‐0.59 to ‐0.24; participants = 570; studies = 3; I2 = 0%) and fluoxetine (SMD ‐0.43, 95% CI ‐0.73 to ‐0.14; participants = 180; studies = 1) over placebo (Analysis 3.15).
3.15. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 15 Social functioning ‐ mean change.
3.16 Quality of life
We found evidence showing a benefit for sertraline (SMD ‐0.48, 95% CI ‐0.80 to ‐0.17; participants = 156; studies = 1), citalopram (SMD ‐0.27, 95% CI ‐0.53 to ‐0.01; participants = 226; studies = 1) and escitalopram (SMD ‐0.41, 95% CI ‐0.67 to ‐0.16; participants = 239; studies = 1) in comparison with placebo. One study on paroxetine did not find a difference with placebo (SMD 0.03, 95% CI ‐0.19 to 0.26; participants = 317; studies = 1) (Analysis 3.16).
3.16. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 16 Quality of life.
3.17 Number of dropouts due to adverse effects
We found evidence showing no difference compared to placebo for paroxetine (RR 1.28, 95% CI 0.87 to 1.88; participants = 2353; studies = 8; I2 = 34%), fluoxetine (RR 1.67, 95% CI 0.41 to 6.77; participants = 180; studies = 1), citalopram (RR 1.01, 95% CI 0.55 to 1.84; participants = 628; studies = 2; I2 = 0%), escitalopram (RR 0.86, 95% CI 0.34 to 2.16; participants = 254; studies = 1). Sertraline (RR 1.96, 95% CI 0.99 to 3.86; participants = 647; studies = 4; I2 = 21%) and fluvoxamine (RR 1.99, 95% CI 1.17 to 3.38; participants = 500; studies = 6; I2 = 0%) were less well tolerated than placebo, when looking at number of dropouts due to adverse effects (Analysis 3.17).
3.17. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 17 Number of dropouts due to adverse effects.
3.18 Number of participants experiencing at least one adverse effect
For all SSRI antidepressants we found evidence that ranged between no difference with placebo to a possible higher number of participants experiencing adverse effects: paroxetine (RR 1.10, 95% CI 1.01 to 1.19; participants = 1338; studies = 6; I2 = 28%), sertraline (RR 1.12, 95% CI 0.99 to 1.26; participants = 522; studies = 3; I2 = 27%), fluoxetine (RR 1.32, 95% CI 0.78 to 2.21; participants = 180; studies = 1), fluvoxamine (RR 1.11, 95% CI 0.90 to 1.36; participants = 288; studies = 2; I2 = 79%), citalopram (RR 1.13, 95% CI 1.00 to 1.28; participants = 251; studies = 1) and escitalopram (RR 1.07, 95% CI 0.94 to 1.22; participants = 254; studies = 1) (Analysis 3.18).
3.18. Analysis.

Comparison 3 SSRIs versus placebo, Outcome 18 Number of participants experiencing at least one adverse effect.
Comparison 4. MAOIs versus placebo
One study including 29 participants provided data for at least one outcome for this comparison. The study compared brofaromine to placebo.
Primary outcomes
4.1 Failure to respond
One study showed a benefit for brofaromine over placebo on response rates (RR 0.55, 95% CI 0.34 to 0.88; participants = 29) (Analysis 4.1).
4.1. Analysis.

Comparison 4 MAOIs versus placebo, Outcome 1 Failure to respond.
Secondary outcomes
4.2 Panic symptoms
One study showed a benefit for brofaromine over placebo in reducing panic symptoms (MD ‐6.20, 95% CI ‐7.38 to ‐5.02; participants = 29) (Analysis 4.2).
4.2. Analysis.

Comparison 4 MAOIs versus placebo, Outcome 2 Panic symptoms.
4.3 Agoraphobia
One study showed a benefit for brofaromine over placebo (MD ‐23.00, 95% CI ‐25.98 to ‐20.02; participants = 29) (Analysis 4.3).
4.3. Analysis.

Comparison 4 MAOIs versus placebo, Outcome 3 Agoraphobia.
4.4 General anxiety
One study showed a benefit for brofaromine over placebo (MD ‐8.50, 95% CI ‐9.30 to ‐7.70; participants = 29) (Analysis 4.4).
4.4. Analysis.

Comparison 4 MAOIs versus placebo, Outcome 4 General anxiety.
4.5 Depression
One study showed a benefit for brofaromine over placebo on depressive symptoms (MD ‐8.50, 95% CI ‐9.30 to ‐7.70; participants = 29) (Analysis 4.5).
4.5. Analysis.

Comparison 4 MAOIs versus placebo, Outcome 5 Depression.
Comparison 5. SNRIs versus placebo
Four studies including 2020 participants provided data for this comparison. See also: Table 4.
We have set out in detail below the findings for individual SNRIs compared to placebo.
Primary outcomes
5.1 Failure to respond
We found evidence showing a benefit for venlafaxine over placebo on response rates (RR 0.66, 95% CI 0.51 to 0.86; participants = 1693; studies = 4; I2 = 78%) (Analysis 5.1).
5.1. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 1 Failure to respond.
5.2 Total number of dropouts
We found no difference between venlafaxine and placebo on dropout rates (RR 0.91, 95% CI 0.66 to 1.24; participants = 1693; studies = 4; I2 = 69%) (Analysis 5.2).
5.2. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 2 Total number of dropouts.
Secondary outcomes
5.3 Failure to remit
We found evidence showing a benefit for venlafaxine over placebo in remission rates (RR 0.84, 95% CI 0.74 to 0.94; participants = 1693; studies = 4; I2 = 63%) (Analysis 5.3).
5.3. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 3 Failure to remit.
5.4 Panic symptoms ‐ endpoint score
Venlafaxine was more effective than placebo in reducing panic symptoms measured as endpoint scores (SMD ‐0.30, 95% CI ‐0.44 to ‐0.16; participants = 801; studies = 2; I2 = 0%) (Analysis 5.4).
5.4. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 4 Panic symptoms ‐ endpoint score.
5.5 Panic symptoms ‐ mean change
When looking at mean change scores of continuous measures, venlafaxine was more effective than placebo in reducing panic symptoms (SMD ‐0.43, 95% CI ‐0.62 to ‐0.25; participants = 763; studies = 2; I2 = 34%) (Analysis 5.5).
5.5. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 5 Panic symptoms ‐ mean change.
5.6 Frequency of panic attacks
Venlafaxine was more effective than placebo in reducing the frequency of panic attacks (MD ‐1.21, 95% CI ‐1.83 to ‐0.58; participants = 1582; studies = 4; I2 = 95%) (Analysis 5.6).
5.6. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 6 Frequency of panic attacks.
5.7 Agoraphobia
We found evidence showing a benefit for venlafaxine over placebo (MD ‐7.32, 95% CI ‐9.87 to ‐4.76; participants = 1107; studies = 3; I2 = 0%) (Analysis 5.7).
5.7. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 7 Agoraphobia.
5.8 General anxiety
We found evidence showing a benefit for venlafaxine over placebo (SMD ‐0.27, 95% CI ‐0.46 to ‐0.08; participants = 1010; studies = 3; I2 = 52%) (Analysis 5.8).
5.8. Analysis.
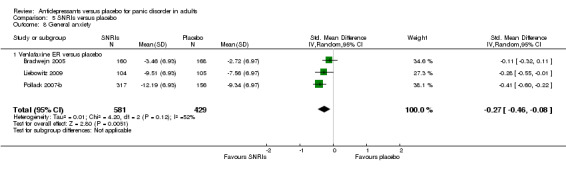
Comparison 5 SNRIs versus placebo, Outcome 8 General anxiety.
5.9 Social functioning ‐ endpoint score
One study showed a trend in favour of venlafaxine on this outcome (MD ‐0.70, 95% CI ‐1.65 to 0.25; participants = 473; studies = 1) (Analysis 5.9).
5.9. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 9 Social functioning ‐ endpoint score.
5.10 Social functioning ‐ mean change
Two studies did not show a difference between venlafaxine and placebo (MD ‐0.85, 95% CI ‐2.26 to 0.56; participants = 537; studies = 2; I2 = 0%) (Analysis 5.10).
5.10. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 10 Social functioning ‐ mean change.
5.11 Quality of life
We found evidence showing no difference between venlafaxine and placebo on this outcome (MD 0.31, 95% CI ‐0.93 to 1.55; participants = 1007; studies = 3; I2 = 0%) (Analysis 5.11).
5.11. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 11 Quality of life.
5.12 Patient satisfaction
We found evidence showing a benefit for venlafaxine over placebo (MD ‐0.40, 95% CI ‐0.66 to ‐0.14; participants = 491; studies = 2; I2 = 0%) (Analysis 5.12).
5.12. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 12 Patient satisfaction.
5.13 Number of dropouts due to adverse effects
We found evidence showing no difference between venlafaxine and placebo on this outcome (RR 1.34, 95% CI 0.88 to 2.04; participants = 1693; studies = 4; I2 = 0%) (Analysis 5.13).
5.13. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 13 Number of dropouts due to adverse effects.
5.14 Number of participants experiencing at least one adverse effect
We found evidence showing that participants taking venlafaxine have higher probability of experiencing adverse effects, in comparison to participants taking placebo (RR 1.09, 95% CI 1.03 to 1.15; participants = 1693; studies = 4; I2 = 0%) (Analysis 5.14).
5.14. Analysis.

Comparison 5 SNRIs versus placebo, Outcome 14 Number of participants experiencing at least one adverse effect.
Comparison 6. NRIs versus placebo
One study including 82 participants provided data for this comparison. The study compared reboxetine with placebo.
Primary outcomes
6.1 Failure to respond
One study showed a benefit for reboxetine over placebo in terms of response rates (RR 0.71, 95% CI 0.51 to 0.97; participants = 82) (Analysis 6.1).
6.1. Analysis.

Comparison 6 NRIs versus placebo, Outcome 1 Failure to respond.
6.2 Total number of dropouts
Reboxetine was better tolerated than placebo (RR 0.50, 95% CI 0.28 to 0.90; participants = 82; studies = 1) (Analysis 6.2).
6.2. Analysis.

Comparison 6 NRIs versus placebo, Outcome 2 Total number of dropouts.
Secondary outcomes
6.3 Panic symptoms
We found no difference between reboxetine and placebo on this outcome (Analysis 6.3).
6.3. Analysis.

Comparison 6 NRIs versus placebo, Outcome 3 Panic symptoms.
6.4 Frequency of panic attacks
One study showed a benefit for reboxetine over placebo (MD ‐4.60, 95% CI ‐6.83 to ‐2.37; participants = 75) (Analysis 6.4).
6.4. Analysis.

Comparison 6 NRIs versus placebo, Outcome 4 Frequency of panic attacks.
6.5 Agoraphobia
One study showed a benefit for reboxetine over placebo (MD ‐2.00, 95% CI ‐2.57 to ‐1.43; participants = 75) (Analysis 6.5).
6.5. Analysis.

Comparison 6 NRIs versus placebo, Outcome 5 Agoraphobia.
6.6 Number of dropouts due to adverse effects
One study did not show a difference between reboxetine and placebo on this outcome (RR 0.48, 95% CI 0.04 to 5.05; participants = 82; studies = 1) (Analysis 6.6).
6.6. Analysis.

Comparison 6 NRIs versus placebo, Outcome 6 Number of dropouts due to adverse effects.
Comparison 7. Other antidepressants versus placebo
Two studies including 333 participants provided data for this comparison. The studies compared nefazodone and ritanserin with placebo.
Primary outcomes
7.1 Failure to respond
One study on ritanserin showed no difference with placebo on response rates (RR 0.95, 95% CI 0.79 to 1.14; participants = 39) (Analysis 7.1).
7.1. Analysis.

Comparison 7 Other antidepressants versus placebo, Outcome 1 Failure to respond.
7.2 Total number of dropouts
One study on ritanserin did not find a difference with placebo on number of dropouts (RR 0.32, 95% CI 0.01 to 7.35; participants = 39) (Analysis 7.2).
7.2. Analysis.

Comparison 7 Other antidepressants versus placebo, Outcome 2 Total number of dropouts.
Secondary outcomes
7.3 Agoraphobia
One study on ritanserin did not find a difference with placebo on this outcome (MD 2.26, 95% CI ‐3.97 to 8.49; participants = 39) (Analysis 7.3).
7.3. Analysis.

Comparison 7 Other antidepressants versus placebo, Outcome 3 Agoraphobia.
7.4 General anxiety
One study on ritanserin did not find a difference with placebo on this outcome (MD 0.20, 95% CI ‐1.16 to 1.56; participants = 39; studies = 1) (Analysis 7.4).
7.4. Analysis.

Comparison 7 Other antidepressants versus placebo, Outcome 4 General anxiety.
7.5 Depression
One study on ritanserin did not find a difference with placebo on this outcome (MD 0.88, 95% CI ‐1.53 to 3.29; participants = 39; studies = 1) (Analysis 7.5).
7.5. Analysis.

Comparison 7 Other antidepressants versus placebo, Outcome 5 Depression.
7.6 Number of dropouts due to adverse effects
One study on nefazodone did not find a difference with placebo on this outcome (RR 0.51, 95% CI 0.18 to 1.47; participants = 274) (Analysis 7.6). One study on ritanserin had no dropout both for the drug and the placebo arm, so it was not possible to calculate a RR.
7.6. Analysis.

Comparison 7 Other antidepressants versus placebo, Outcome 6 Number of dropouts due to adverse effects.
Subgroup analyses
Subgroup analysis by antidepressant class is reported under Comparison 1 (Analysis 1.1 and Analysis 1.2).
None of the included studies reported the exclusion of participants with panic disorder and agoraphobia, so it was not possible to conduct subgroup analysis 2.
We found no acute‐phase treatment studies that lasted for four months or more, so it was not possible to perform subgroup analysis 3.
Sensitivity analyses
Excluding studies with high risk of bias (comparisons 8 to 11)
Antidepressants versus placebo
8.1 Failure to respond
Excluding 13 studies at high risk of bias did not substantially change the original analysis (RR 0.79, 95% CI 0.72 to 0.87; participants = 3819; studies = 17; I2 = 56%) (Analysis 8.1).
8.1. Analysis.

Comparison 8 High risk of bias excluded ‐ antidepressants versus placebo, Outcome 1 Failure to respond.
8.2 Total number of dropouts
Excluding 19 studies at high risk of bias did not substantially change the original analysis, but the CI now includes the possibility of no difference between antidepressants and placebo (RR 0.91, 95% CI 0.82 to 1.00; participants = 3983; studies = 20; I2 = 7%) (Analysis 8.2).
8.2. Analysis.

Comparison 8 High risk of bias excluded ‐ antidepressants versus placebo, Outcome 2 Total number of dropouts.
TCAs versus placebo
9.1 Failure to respond
Excluding three studies at high risk of bias did not change the results for imipramine (RR 0.76, 95% CI 0.61 to 0.96; participants = 239; studies = 3; I2 = 25%). For clomipramine, the CI now includes the possibility of no difference compared to placebo (RR 0.67, 95% CI 0.40 to 1.13; participants = 407; studies = 3; I2 = 75%), and no data are available for desipramine (Analysis 9.1).
9.1. Analysis.

Comparison 9 High risk of bias excluded ‐ TCAs versus placebo, Outcome 1 Failure to respond.
9.2 Total number of dropouts
Excluding seven studies at high risk of bias did not change the original analysis for imipramine (RR 0.72, 95% CI 0.55 to 0.95; participants = 364; studies = 5; I2 = 0%) and clomipramine (RR 0.81, 95% CI 0.63 to 1.03; participants = 500; studies = 4; I2 = 0%), while no data are now available for desipramine (Analysis 9.2).
9.2. Analysis.

Comparison 9 High risk of bias excluded ‐ TCAs versus placebo, Outcome 2 Total number of dropouts.
SSRIs versus placebo
10.1 Failure to respond
Excluding eight studies at high risk of bias CI now includes the possibility of no difference for paroxetine versus placebo (RR 0.81, 95% CI 0.65 to 1.00; participants = 1436; studies = 5; I2 = 45%), and the same for fluvoxamine (RR 0.65, 95% CI 0.21 to 2.06; participants = 170; studies = 2; I2 = 92%). Results for sertraline did not substantially change from the original analysis (RR 0.85, 95% CI 0.72 to 0.99; participants = 301; studies = 2; I2 = 0%) (Analysis 10.1). None of the studies at high risk of bias that we excluded were on fluoxetine, citalopram or escitalopram, so the results did not change from original analyses (Analysis 3.1).
10.1. Analysis.

Comparison 10 High risk of bias excluded ‐ SSRIs versus placebo, Outcome 1 Failure to respond.
10.2 Total number of dropouts
Excluding 10 studies at high risk of bias, results for paroxetine did not substantially change from the original analysis (RR 1.02, 95% CI 0.87 to 1.18; participants = 1552; studies = 5; I2 = 0%), as well as results for sertraline (RR 0.95, 95% CI 0.63 to 1.45; participants = 301; studies = 2) and fluvoxamine (RR 1.50, 95% CI 0.71 to 3.16; participants = 180; studies = 1; I2 = 0%) (Analysis 10.2). None of the studies at high risk of bias that we excluded were on fluoxetine, citalopram or escitalopram, so the results did not change from original analyses (Analysis 3.2).
10.2. Analysis.

Comparison 10 High risk of bias excluded ‐ SSRIs versus placebo, Outcome 2 Total number of dropouts.
MAOIs versus placebo
Excluding one study for high risk of bias, no studies provided data for this comparison.
SNRIs versus placebo
11.1 Failure to respond
Excluding three studies at high risk of bias CI for venlafaxine now includes no difference with placebo (RR 0.84, 95% CI 0.66 to 1.07; participants = 343; studies = 1) (Analysis 11.1).
11.1. Analysis.

Comparison 11 High risk of bias excluded ‐ SNRIs versus placebo, Outcome 1 Failure to respond.
11.2 Total number of dropouts
Excluding three studies at high risk of bias, results for venlafaxine now range from no difference to lower number of dropouts with placebo (RR 1.23, 95% CI 0.88 to 1.72; participants = 343; studies = 1) (Analysis 11.2).
11.2. Analysis.

Comparison 11 High risk of bias excluded ‐ SNRIs versus placebo, Outcome 2 Total number of dropouts.
NRI versus placebo
We did not exclude any studies, so the results did not change from original analyses (Analysis 6.1; Analysis 6.2).
Other antidepressants versus placebo
Excluding one study for high risk of bias, no studies provided data for this comparison.
Excluding studies with dropout rates greater than 20% (comparisons 12‐14)
Antidepressants versus placebo
12.1 Failure to respond
Excluding 24 studies for high dropout rates, results did not substantially change from original analysis (RR 0.55, 95% CI 0.37 to 0.80; participants = 511; studies = 6; I2 = 84%) (Analysis 12.1).
12.1. Analysis.

Comparison 12 High dropout rates excluded ‐ Antidepressants versus placebo, Outcome 1 Failure to respond.
12.2 Total number of dropouts
Excluding 31 studies for high dropout rates, results changed in the direction of no difference between antidepressants and placebo (RR 1.12, 95% CI 0.75 to 1.68; participants = 577; studies = 7; I2 = 0%) (Analysis 12.2).
12.2. Analysis.

Comparison 12 High dropout rates excluded ‐ Antidepressants versus placebo, Outcome 2 Total number of dropouts.
TCAs versus placebo
13.1 Failure to respond
Excluding seven studies with dropout rates greater than 20%, the results changed for imipramine, with a broader CI that includes the possibility of no difference with placebo (RR 0.47, 95% CI 0.17 to 1.30; participants = 37; studies = 1). Clomipramine remains more efficacious compared to placebo (RR 0.14, 95% CI 0.04 to 0.52; participants = 30; studies = 1), whereas no studies are available anymore for desipramine (Analysis 13.1).
13.1. Analysis.

Comparison 13 High dropout rates excluded ‐ TCAs versus placebo, Outcome 1 Failure to respond.
13.2 Total number of dropouts
Excluding 12 studies with dropout rates greater than 20%, imipramine (RR 1.08, 95% CI 0.48 to 2.42; participants = 132; studies = 3; I2 = 0%) and clomipramine (RR 0.11, 95% CI 0.01 to 1.90; participants = 30; studies = 1) do not show a benefit in comparison with placebo. No data are available for desipramine (Analysis 13.2).
13.2. Analysis.

Comparison 13 High dropout rates excluded ‐ TCAs versus placebo, Outcome 2 Total number of dropouts.
SSRIs versus placebo
14.1 Failure to respond
Excluding 17 studies with dropout rates greater than 20%, results on sertraline did not substantially change from original analysis (RR 0.85, 95% CI 0.72 to 1.00; participants = 176; studies = 1), while for fluvoxamine the effect in comparison with placebo increased (RR 0.26, 95% CI 0.12 to 0.57; participants = 39; studies = 1) (Analysis 14.1).
14.1. Analysis.

Comparison 14 High dropout rates excluded ‐ SSRIs versus placebo, Outcome 1 Failure to respond.
No studies were excluded for fluoxetine, so results are identical to the original analysis. No data are now available for paroxetine, citalopram and escitalopram.
14.2 Total number of dropouts
Excluding 19 studies with dropout rates greater than 20%, results on sertraline did not substantially change from original analysis (RR 1.13, 95% CI 0.60 to 2.12; participants = 176; studies = 1), while for fluvoxamine the CI became broader and very imprecise, ranging from less dropout with antidepressant and less dropout with placebo (RR 0.32, 95% CI 0.01 to 7.35; participants = 39; studies = 1) (Analysis 14.2).
14.2. Analysis.

Comparison 14 High dropout rates excluded ‐ SSRIs versus placebo, Outcome 2 Total number of dropouts.
We did not exclude any studies for fluoxetine, so the results are identical to the original analysis. No data are now available for paroxetine, citalopram and escitalopram.
MAOIs versus placebo
We did not exclude any studies, so the results have not changed from the original analyses (Analysis 4.1).
SNRI versus placebo
Excluding four studies with high dropout rates, no studies provided data for this comparison.
NRI versus placebo
Excluding one study with high dropout rates, no studies provided data for this comparison.
Other antidepressants versus placebo
We did not exclude any studies, so the results have not changed from the original analyses (Analysis 7.1; Analysis 7.2).
Excluding studies funded by the pharmaceutical company marketing each antidepressant (comparisons 15‐17)
Antidepressants versus placebo
15.1 Failure to respond
We excluded 18 studies from this analysis because they had been funded by the pharmaceutical company marketing the antidepressant, without a clarification about the role of the funder in planning, conducting and writing the study. Results did not substantially change from the original analysis (RR 0.78, 95% CI 0.66 to 0.92; participants = 1183; studies = 12; I2 = 62%) (Analysis 15.1).
15.1. Analysis.

Comparison 15 Funded excluded ‐ antidepressants versus placebo, Outcome 1 Failure to respond.
15.2 Total number of dropouts
Excluding 23 funded studies, CI now includes the possibility of no difference between antidepressants and placebo (RR 0.84, 95% CI 0.69 to 1.04; participants = 1331; studies = 15; I2 = 27%) (Analysis 15.2).
15.2. Analysis.

Comparison 15 Funded excluded ‐ antidepressants versus placebo, Outcome 2 Total number of dropouts.
TCAs versus placebo
16.1 Failure to respond
We excluded four funded studies for this outcome. Results for imipramine did not substantially change from the original analysis (RR 0.76, 95% CI 0.61 to 0.96; participants = 239; studies = 3; I2 = 25%), whereas the effect favouring clomipramine over placebo increased (RR 0.14, 95% CI 0.04 to 0.52; participants = 30; studies = 1) (Analysis 16.1). No studies were excluded for desipramine, so results are identical to original analysis.
16.1. Analysis.

Comparison 16 Funded excluded ‐ TCAs versus placebo, Outcome 1 Failure to respond.
16.2 Total number of dropouts
We excluded seven funded studies. Results for imipramine now include the possibility of no difference with placebo (RR 0.84, 95% CI 0.56 to 1.25; participants = 393; studies = 6; I2 = 36%), and the same happens for clomipramine, where the CI is now very large (RR 0.77, 95% CI 0.02 to 25.22; participants = 70; studies = 2; I2 = 80%) (Analysis 16.2). We did not exclude any studies for desipramine, so the results are identical to the original analysis.
16.2. Analysis.

Comparison 16 Funded excluded ‐ TCAs versus placebo, Outcome 2 Total number of dropouts.
SSRIs versus placebo
17.1 Failure to respond
We excluded 14 funded studies. Results for paroxetine changed showing no difference with placebo (RR 1.06, 95% CI 0.73 to 1.54; participants = 297; studies = 2; I2 = 0%); the same for fluvoxamine (RR 0.55, 95% CI 0.12 to 2.58; participants = 136; studies = 2; I2 = 94%). Results on sertraline did not substantially change from the original analysis (RR 0.93, 95% CI 0.73 to 1.19; participants = 294; studies = 2; I2 = 0%). After excluding studies, no data were available for fluoxetine, citalopram and escitalopram (Analysis 17.1).
17.1. Analysis.

Comparison 17 Funded excluded ‐ SSRIs versus placebo, Outcome 1 Failure to respond.
17.2 Total number of dropouts
After excluding 15 funded studies, results did not substantially change from the original analyses for paroxetine (RR 0.95, 95% CI 0.65 to 1.38; participants = 297; studies = 2; I2 = 0%), sertraline (RR 1.05, 95% CI 0.77 to 1.43; participants = 294; studies = 2; I2 = 0%) and fluvoxamine (RR 1.03, 95% CI 0.76 to 1.40; participants = 189; studies = 3; I2 = 0%) (Analysis 17.2). No data are available anymore for fluoxetine, citalopram and escitalopram.
17.2. Analysis.

Comparison 17 Funded excluded ‐ SSRIs versus placebo, Outcome 2 Total number of dropouts.
MAOIs versus placebo
We did not exclude any studies, so the results did not change from the original analyses (Analysis 4.1).
SNRI versus placebo
Excluding four funded studies, no studies provided data for this comparison.
NRI versus placebo
We did not exclude any studies, so the results did not change from original analyses (Analysis 6.1; Analysis 6.2).
Other antidepressants versus placebo
We did not exclude any studies, so the results did not change from original analyses (Analysis 7.1; Analysis 7.2).
Excluding studies whose protocols did not explicitly prohibit concomitant use of BDZ (comparisons 18‐21)
Antidepressants versus placebo
18.1 Failure to respond
We excluded 21 studies because concomitant use of benzodiazepines was not explicitly prohibited to enrolled participants. After excluding these studies, the results did not substantially change from the original analysis (RR 0.76, 95% CI 0.66 to 0.86; participants = 1978; studies = 9; I2 = 42%) (Analysis 18.1).
18.1. Analysis.

Comparison 18 Irregular benzodiazepine use excluded ‐ antidepressants versus placebo, Outcome 1 Failure to respond.
18.2 Total number of dropouts
Excluding 22 studies there was a very small change in results on number of dropouts, and CI now includes the possibility of no difference with placebo (RR 0.86, 95% CI 0.73 to 1.02; participants = 3109; studies = 16; I2 = 37%) (Analysis 18.2).
18.2. Analysis.

Comparison 18 Irregular benzodiazepine use excluded ‐ antidepressants versus placebo, Outcome 2 Total number of dropouts.
TCAs versus placebo
19.1 Failure to respond
After excluding seven studies, results on clomipramine did not substantially change from the original analysis (RR 0.71, 95% CI 0.58 to 0.88; participants = 158; studies = 1), while for imipramine CI now includes the possibility of no difference with placebo (RR 0.65, 95% CI 0.42 to 1.00; participants = 40; studies = 1). No data are available anymore for desipramine (Analysis 19.1).
19.1. Analysis.

Comparison 19 Irregular benzodiazepine use excluded ‐ TCAs versus placebo, Outcome 1 Failure to respond.
19.2 Total number of dropouts
After excluding nine studies, results on imipramine (RR 0.71, 95% CI 0.59 to 0.84; participants = 1003; studies = 6; I2 = 2%) and clomipramine (RR 0.69, 95% CI 0.46 to 1.02; participants = 180; studies = 1) did not substantially change from the original analysis (Analysis 19.2). No data are available for desipramine.
19.2. Analysis.

Comparison 19 Irregular benzodiazepine use excluded ‐ TCAs versus placebo, Outcome 2 Total number of dropouts.
SSRIs versus placebo
20.1 Failure to respond
After excluding 14 studies, results on paroxetine now include no difference with placebo (RR 0.86, 95% CI 0.58 to 1.26; participants = 1131; studies = 3; I2 = 63%), and the same for fluvoxamine (RR 0.54, 95% CI 0.27 to 1.08; participants = 112; studies = 2; I2 = 58%). Results on sertraline did not substantially change from the original analysis (RR 0.85, 95% CI 0.72 to 1.00; participants = 176; studies = 1). No data are now available for fluoxetine, citalopram and escitalopram (Analysis 20.1).
20.1. Analysis.

Comparison 20 Irregular benzodiazepine use excluded ‐ SSRIs versus placebo, Outcome 1 Failure to respond.
20.2 Total number of dropouts
After excluding 14 studies, results on paroxetine (RR 1.09, 95% CI 0.91 to 1.31; participants = 1186; studies = 3; I2 = 0%), sertraline (RR 1.13, 95% CI 0.60 to 2.12; participants = 176; studies = 1) and fluvoxamine (RR 0.73, 95% CI 0.43 to 1.26; participants = 212; studies = 4; I2 = 0%) did not substantially change from the original analyses (Analysis 20.2).
20.2. Analysis.

Comparison 20 Irregular benzodiazepine use excluded ‐ SSRIs versus placebo, Outcome 2 Total number of dropouts.
No data are now available for fluoxetine, citalopram and escitalopram.
MAOIs versus placebo
Excluding one study where concomitant use of benzodiazepines was not prohibited no studies provided data for this comparison.
SNRI versus placebo
21.1 Failure to respond
After excluding three studies, results changed and now include the possibility of no difference with placebo (RR 0.82, 95% CI 0.65 to 1.04; participants = 361; studies = 1) (Analysis 21.1).
21.1. Analysis.

Comparison 21 Irregular benzodiazepine use excluded ‐ SNRIs versus placebo, Outcome 1 Failure to respond.
21.2 Total number of dropouts
Excluding three studies did not substantially change the results from the original analysis (RR 1.13, 95% CI 0.80 to 1.59; participants = 361; studies = 1) (Analysis 21.2).
21.2. Analysis.

Comparison 21 Irregular benzodiazepine use excluded ‐ SNRIs versus placebo, Outcome 2 Total number of dropouts.
NRI versus placebo
After excluding one study where concomitant use of benzodiazepines was not prohibited, no studies provided data for this comparison.
Other antidepressants versus placebo
After excluding one study where concomitant use of benzodiazepines was not prohibited, no studies provided data for this comparison.
Excluding studies whose participants clearly had significant psychiatric comorbidities, including primary or secondary depressive disorders (comparisons 22‐25)
Antidepressants versus placebo
22.1 Failure to respond
Excluding seven studies including participants with psychiatric comorbidities did not substantially change results on response rates from the original analysis (RR 0.71, 95% CI 0.63 to 0.79; participants = 4921; studies = 24; I2 = 72%) (Analysis 22.1).
22.1. Analysis.

Comparison 22 Psychiatric comorbidities excluded ‐ antidepressants versus placebo, Outcome 1 Failure to respond.
22.2 Total number of dropouts
Excluding 11 studies where participants with psychiatric comorbidities were included did not substantially change results on dropouts from the original analysis (RR 0.86, 95% CI 0.77 to 0.94; participants = 5088; studies = 28; I2 = 18%) (Analysis 22.2).
22.2. Analysis.

Comparison 22 Psychiatric comorbidities excluded ‐ antidepressants versus placebo, Outcome 2 Total number of dropouts.
TCAs versus placebo
23.1 Failure to respond
Excluding one study on imipramine changed the results to include the possibility of no difference with placebo (RR 0.76, 95% CI 0.58 to 1.01; participants = 172; studies = 3; I2 = 24%) (Analysis 23.1). We did not exclude any studies on clomipramine or desipramine, so the results did not change from original analyses.
23.1. Analysis.

Comparison 23 Psychiatric comorbidities excluded ‐ TCAs versus placebo, Outcome 1 Failure to respond.
23.2 Total number of dropouts
Excluding four studies, results on imipramine did not substantially change from the original analysis (RR 0.69, 95% CI 0.49 to 0.99; participants = 303; studies = 6; I2 = 23%), while results on clomipramine changed in the direction of favouring antidepressant over placebo (RR 0.77, 95% CI 0.62 to 0.95; participants = 680; studies = 5; I2 = 0%) (Analysis 23.2). We did not exclude any studies on desipramine, so the results did not change from original analyses.
23.2. Analysis.

Comparison 23 Psychiatric comorbidities excluded ‐ TCAs versus placebo, Outcome 2 Total number of dropouts.
SSRIs versus placebo
24.1 Failure to respond
After excluding four studies, results on paroxetine (RR 0.73, 95% CI 0.59 to 0.91; participants = 1574; studies = 8; I2 = 57%) and sertraline (RR 0.98, 95% CI 0.74 to 1.29; participants = 169; studies = 1) did not substantially change from original analyses. Results on fluvoxamine now include the possibility of no difference with placebo (RR 0.56, 95% CI 0.30 to 1.02; participants = 388; studies = 4; I2 = 89%) (Analysis 24.1). We did not exclude any studies on fluoxetine, citalopram and escitalopram, so results did not change from the original analyses.
24.1. Analysis.

Comparison 24 Psychiatric comorbidities excluded ‐ SSRIs versus placebo, Outcome 1 Failure to respond.
24.2 Total number of dropouts
Excluding five studies, results on paroxetine (RR 0.93, 95% CI 0.81 to 1.08; participants = 1635; studies = 8; I2 = 0%), sertraline (RR 1.16, 95% CI 0.81 to 1.68; participants = 169; studies = 1) and fluvoxamine (RR 1.01, 95% CI 0.80 to 1.28; participants = 450; studies = 5; I2 = 0%) did not substantially change from the original analyses (Analysis 24.2). We did not exclude any studies on fluoxetine, citalopram and escitalopram, so the results did not change from the original analyses.
24.2. Analysis.

Comparison 24 Psychiatric comorbidities excluded ‐ SSRIs versus placebo, Outcome 2 Total number of dropouts.
MAOIs versus placebo
We did not exclude any studies, so the results did not change from the original analyses (Analysis 4.1).
SNRIs versus placebo
25.1 Failure to respond
Excluding one study did not substantially change the results from the original analysis (RR 0.54, 95% CI 0.34 to 0.88; participants = 1350; studies = 3; I2 = 92%) (Analysis 25.1).
25.1. Analysis.
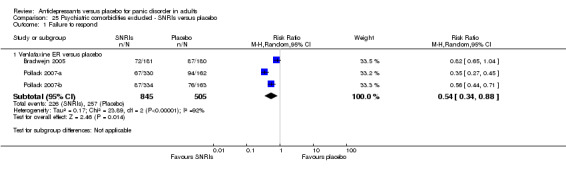
Comparison 25 Psychiatric comorbidities excluded ‐ SNRIs versus placebo, Outcome 1 Failure to respond.
25.2 Total number of dropouts
Excluding one study did not substantially change the results from the original analysis (RR 0.82, 95% CI 0.58 to 1.15; participants = 1350; studies = 3; I2 = 65%) (Analysis 25.2).
25.2. Analysis.

Comparison 25 Psychiatric comorbidities excluded ‐ SNRIs versus placebo, Outcome 2 Total number of dropouts.
NRI versus placebo
We did not exclude any studies, so the results did not change from the original analyses (Analysis 6.1; Analysis 6.2).
Other antidepressants versus placebo
We did not exclude any studies, so the results did not change from the original analyses (Analysis 7.1; Analysis 7.2).
Applying best and worst case scenarios to studies where participants left the study before the endpoint
The main analyses, according to the study protocol, considered participants who discontinued early as treatment failures (worst case scenario) as this approach was considered more conservative. A sensitivity analysis based on a best case scenario, which considers all dropouts as responders or remitters, was not feasible as the vast majority of studies carried forward, and included in the analyses, some observations on dropouts. This did not allow us to make the assumptions that all dropouts were responders.
Excluding studies where number of responding participants was calculated according to an imputation method (comparisons 26‐27)
Antidepressants versus placebo
26.1 Failure to respond
Excluding two studies that calculated number of failures to respond according to an imputation method did not substantially change the results from the original analysis (RR 0.72, 95% CI 0.65 to 0.79; participants = 6313; studies = 28; I2 = 69%) (Analysis 26.1).
26.1. Analysis.

Comparison 26 Imputation excluded ‐ antidepressants versus placebo, Outcome 1 Failure to respond.
26.2 Total number of dropouts
After excluding two studies, the results did not substantially change from the original analysis (RR 0.89, 95% CI 0.81 to 0.97; participants = 7618; studies = 36; I2 = 27%) (Analysis 26.2).
26.2. Analysis.

Comparison 26 Imputation excluded ‐ antidepressants versus placebo, Outcome 2 Total number of dropouts.
TCAs versus placebo
After excluding the only study on desipramine, no studies provided data on desipramine. We did not exclude any studies on clomipramine and imipramine, so the results did not change from the original analyses (Analysis 2.1; Analysis 2.2).
SSRIs versus placebo
27.1 Failure to respond
Excluding one study on sertraline did not substantially change the results from the original analysis (RR 0.93, 95% CI 0.73 to 1.19; participants = 294; studies = 2; I2 = 0%) (Analysis 27.1). We did not exclude any studies on paroxetine, fluoxetine, fluvoxamine, citalopram or escitalopram.
27.1. Analysis.

Comparison 27 Imputation excluded ‐ SSRIs versus placebo, Outcome 1 Failure to respond.
27.2 Total number of dropouts
Excluding one study on sertraline did not substantially change the results from the original analysis (RR 1.09, 95% CI 0.84 to 1.42; participants = 471; studies = 3; I2 = 0%) (Analysis 27.2). We did not exclude any studies on paroxetine, fluoxetine, fluvoxamine, citalopram or escitalopram.
27.2. Analysis.

Comparison 27 Imputation excluded ‐ SSRIs versus placebo, Outcome 2 Total number of dropouts.
MAOIs versus placebo
We did not exclude any studies, so the results did not change from the original analyses (Analysis 4.1).
SNRI versus placebo
We did not exclude any studies, so the results did not change from the original analyses (Analysis 5.1; Analysis 5.2).
NRI versus placebo
We did not exclude any studies, so the results did not change from the original analyses (Analysis 6.1; Analysis 6.2).
Other antidepressants versus placebo
We did not exclude any studies, so the results did not change from the original analyses (Analysis 7.1; Analysis 7.2).
Reporting Bias
We formally checked the presence of publication bias with a visual inspection of funnel plots. Regarding the primary outcome, 'Failure to respond', a visual investigation of the funnel plot suggests that some studies with a low number of participants favouring placebo against TCAs may be missing, and this may have led to an overestimation of the efficacy of TCAs compared to placebo.
For the primary outcome, 'Total dropouts', a visual investigation of the funnel plot suggests that some small studies favouring placebo against SSRIs may be missing, and this may have led to an overestimation of the acceptability of SSRIs.
In general, we can not exclude the presence of unpublished studies, and the impact of unpublished literature on the results of this review is uncertain. We expect that the analysis of published literature only would lead to overestimation of the efficacy of a given intervention.
Discussion
Summary of main results
By systematically searching for evidence on the efficacy and tolerability of antidepressants and placebo for panic disorder in adults (see Table 1; Table 2; Table 3; Table 4) we were able to include 41 studies including 9377 participants, 8252 for the arms of interest.
For all predefined outcomes, we compared antidepressants as a whole to placebo. Results of this comparison are shown in Analysis 1, which also reports subgroup analyses by classes of antidepressants: TCAs, SSRIs, MAOIs (only one study on brofaromine), SNRIs, NRIs (only one study on reboxetine) and other antidepressants (only one study on ritanserin). No studies reported data on NaSSAs or NDRIs. In Analyses 2 to 9 we displayed results by individual antidepressants included in each class. We followed this analytical approach not only to generate overall treatment estimates, but also to provide specific information on classes and individual antidepressants, which may be more useful for healthcare professionals and users.
We found low‐quality evidence suggesting a benefit for antidepressants in comparison with placebo in terms of efficacy measured as failure to respond (RR 0.72, 95% CI 0.66 to 0.79; participants = 6500; studies = 30; I2 = 67%). This outcome measured the rate of response of participants who failed to reach a substantial improvement, according to predefined improvement criteria (see Primary outcomes). We observed the same findings when we compared classes of antidepressants with placebo, with the exception of a group of antidepressants that we categorised as 'other' antidepressants. Similarly, analysis of failure to remit showed a benefit for antidepressants as a group (RR 0.83, 95% CI 0.78 to 0.88; participants = 6164; studies = 24; I2 = 40%), and also as individual classes compared to placebo. In terms of panic symptoms, frequency of panic attacks, agoraphobia and general anxiety, similar findings emerged.
In terms of depressive symptoms, we observed a benefit for antidepressants over placebofor TCAs, SSRIs and MAOIs, while results did not show a difference for other antidepressants compared with placebo. TCAs were also more effective than placebo in terms of social functioning, and SSRIs were more effective than placebo in terms of quality of life. For remaining classes of antidepressants we found no or very little evidence, that could not inform on their efficacy on these secondary outcomes.
In terms of dropouts due to any cause, we found moderate‐quality evidence showing a benefit for antidepressants compared to placebo (RR 0.88, 95% CI 0.81 to 0.97; participants = 7850; studies = 38; I2 = 30%), even though the upper limit of the confidence interval is close to no difference. Interestingly, considering antidepressant classes, while TCAs showed a benefit over placebo, for SSRIs and SNRIs no difference was observed.
We found antidepressants as a whole to be less well tolerated than placebo. In particular, TCAs and SSRIs were associated with more dropouts due to adverse effects in comparison with placebo. In agreement with this finding, antidepressants as a whole were also associated with more participants experiencing adverse effects (RR 1.11, 95% CI 1.07 to 1.15; participants = 4246; studies = 15; I2 = 0%), and this was found for all classes (TCAs, SSRIs and SNRIs).
Overall completeness and applicability of evidence
The evidence that this review was able to summarise comprehensively addresses its objectives. The majority of studies provided data for the primary outcomes specified in the protocol, allowing us to include a considerable number of studies and participants in the analyses. It was therefore possible to generate useful information on the efficacy and acceptability of antidepressants in comparison with placebo.
In terms of applicability, considering the high number of studies and participants, we can argue that this population may reflect in a satisfactory way the characteristics of people with panic disorder seen in 'real world' settings, despite the well‐known limitations of all randomised studies that should always be acknowledged. One limitation to generalisability may also be connected with the exclusion of studies in which regular use of benzodiazepines was allowed, since this practice might be common in real‐life settings.
Quality of the evidence
The overall methodological quality of the included studies was unclear. No study showed an overall low risk of bias. The majority of studies showed mixed features, with a large prevalence of unclear risk of bias in different domains; however, this may reflect a lack of exhaustive reporting rather than a clear evidence of bias. This is consistent with the findings of a general suboptimal reporting of RCTs in medical journals despite the large diffusion of instruments designed to help transparent reporting, such as the CONSORT Statement (Schultz 2010).
The GRADE methodology allows the provision of outcome‐specific information concerning the overall quality of evidence (GRADE Working Group 2004). In general, confidence in the estimates of effect ranged from 'low' to 'moderate' for most of the outcomes assessed. Study findings were generally quite precise, with small confidence intervals and a high number of participants. Reasons to downgrade the quality of the evidence were primarily due to limitations in the included studies and inconsistency (heterogeneity between studies' results). In agreement with this judgment, we argue that, for the primary outcomes, treatment estimates may be considered quite robust, and further research is unlikely to change our confidence in the estimate of effect.
Potential biases in the review process
Several possible limitations of this review should be highlighted. Some limitations are intrinsically related to the actual process of retrieving, collecting, selecting and extracting data. In order to reduce the potential bias of this complex process two review authors independently worked on each of these steps. It has been highlighted that two independent extractors are overall more reliable than extraction performed by a single author followed by verification by a second author (Buscemi 2006). We applied the same process for the 'Risk of bias' assessment. Furthermore, disagreements were always discussed with a third author. Another relevant problem concerns the 'systematic' nature of the search. We chose to include only randomised studies as they provide the strongest level of evidence available.
In this type of review there is some risk of publication bias, which means that negative studies may not have been published. Although the search was thorough, it is possible that we may not have identified some unpublished studies, considering that there are no shared procedures to perform this kind of search.
We formally checked the presence of publication bias with visual inspection of funnel plots and Egger's test (Egger 1997). Regarding the primary outcome, 'Failure to respond', a visual inspection of the funnel plot suggested that some studies with a low number of participants favouring placebo against TCAs may be missing, and this may have led to an overestimation of the efficacy of TCAs compared to placebo. However, Egger's test was only of border statistical significance (P = 0.058).
For the primary outcome, 'Total dropouts', a visual investigation of the funnel plot suggested that some small studies favouring placebo against SSRIs might be missing, and this might have led to an overestimation of the acceptability of SSRIs. For this outcome, Egger's test was not statistically significant (P = 0.639).
In general, we cannot exclude the presence of unpublished studies, and the impact of unpublished literature on the results of this review is uncertain; it is expected that the analysis of published literature only would lead to overestimation of the efficacy of a given intervention.
In addition, it is important to bear in mind that some of the included studies were funded by the pharmaceutical industry, and this may again introduce an overestimation of the efficacy of interventions.
Agreements and disagreements with other studies or reviews
Overall, the results of this systematic review are in line with previous reviews and meta‐analyses, each of which dealt with particular classes of antidepressants for panic disorder. Our review makes an important contribution to the global understanding of antidepressant therapy for this disorder as well as providing more detailed information about outcomes and comparisons.
Andrisano and colleagues, who compared newer antidepressants with placebo in panic disorder, found that SSRIs (sertraline, paroxetine, fluoxetine) and SNRI (venlafaxine) were more effective than placebo in decreasing panic symptoms (Andrisano 2013). This finding is in agreement with our results. However, the Andrisano review has some methodological limitations, as it included three nonrandomised and 21 uncontrolled studies.
These authors also concluded that individual SSRIs and SNRIs are better tolerated than placebo. This conclusion is in contrast with our findings, that showed no difference between SSRI and placebo (see below). Moreover, in the Andrisano review it is based on total dropouts only. We argue that total dropouts should be seen as a measure of acceptability, implying that participants may drop out for a number of reasons, including efficacy and tolerability. The Andrisano review did not consider more specific measures of tolerability, like the number of dropouts due to adverse effects or the number of participants experiencing adverse effects. In our review, treatment with antidepressants was associated with a higher number of dropouts due to adverse effects and with a higher number of participants who experienced adverse effects, but total dropouts favoured antidepressants. It is therefore possible to speculate that dropouts due to inefficacy or due to other reasons were in favour of antidepressants, but we did not extract this outcome.
Bakker and colleagues, who carried out a systematic review comparing TCAs and SSRIs in people with panic disorder, concluded that TCAs and SSRI were equally effective (Bakker 2002). A similar finding was reported by Bighelli and colleagues (Bighelli 2016). Even though we did not include head‐to‐head comparisons in this review, indirectly we can argue that TCAs and SSRIs are associated with similar magnitude of effect over placebo.
In this review we showed a worse tolerability profile for both TCAs and SSRIs in comparison with placebo, with a higher number of participants experiencing adverse effects and leaving the study due to adverse effects. On the contrary, in terms of total number of dropouts, we found TCAs better than placebo, while we were unable to separate SSRIs from placebo. Although based on the data from this review we can only indirectly compare classes of antidepressants, our findings seems partially in contrast with Bakker 2002, that found SSRIs to be better tolerated than TCAs. The Bighelli review also found a difference in favour of SSRIs compared to TCAs in terms of number of participants who experienced at least one adverse effect.
We plan to add the results of this systematic review to an ongoing Cochrane network meta‐analysis of drug interventions for people with panic disorder, which will likely help rank treatments in terms of efficacy and tolerability in a more accurate way.
Authors' conclusions
Implications for practice.
The findings of this review confirmed that antidepressants were found to be more effective than placebo in treating panic disorder. Efficacy can be quantified as a number needed to treat for an additional beneficial outcome of 7, implying that 7 patients need to be treated with antidepressants in order for one to benefit. Also, antidepressants as a whole showed a benefit in comparison with placebo in terms of number of dropouts, but a less favourable profile in terms of dropout due to adverse effects. However, the tolerability profile varied between different classes of antidepressant.
The choice of which antidepressant should be prescribed cannot be made on the basis of this review only, and rather it may be based on a larger body of evidence on antidepressant efficacy and tolerability, including evidence on active comparisons between antidepressants and between antidepressants and other treatments, such as benzodiazepines.
Data on long‐term tolerability issues associated with antidepressant exposure should also be carefully considered.
Implications for research.
According to the GRADE methodology, the results described in this systematic review are mainly based on evidence of moderate quality.
Therefore, at least for the primary outcomes, we are moderately confident in the effect estimate, that is likely to be close to the true effect. Further research is unlikely to substantially change our confidence in the overall estimates of effect (see Quality of the evidence). Despite possible limitations in the identified studies, it seems unlikely that future randomised studies will generate findings that may change this general conclusion. Clearly, we recognised the lack of studies focused on pragmatic outcome measures, such as quality of life and social functioning, that were seldom investigated by the studies included in this review.
On the contrary, we highlight the need for collecting and summarising evidence on head‐to‐head comparisons of antidepressants, and between antidepressants and other interventions. A Cochrane Review on this topic may hopefully shed light on the comparative effectiveness and tolerability of active treatments for panic disorder, clarifying the potential need for further studies on the treatment of this condition (Bighelli 2016).
The results of this systematic review will contribute to a Cochrane network meta‐analysis of drug treatments for people with panic disorder, which is in progress, aiming to rank drug treatments with antidepressants, benzodiazepines and azapirones for efficacy and tolerability.
Notes
This review is one of separate reviews examining the efficacy and tolerability of pharmacological and non‐pharmacological treatments for panic disorders. These individual reviews will then be combined in a multiple‐treatment meta‐analysis using multiple‐treatments model methodology (protocol to be published in the Cochrane Database of Systematic Reviews). Please note that the majority of the text in the Methods sections for the protocols is identical since the full reviews have been following the same methodology.
Acknowledgements
We would like to thank the Cochrane Common Mental Disorders' Editorial Team for their support, information and advice.
We would like to thank all study authors that answered our requests for additional data, and especially Drs. Lavori (CNCPS 1992) and Stahl (Stahl 2003‐cit; Stahl 2003‐esc).
Screening of references and double checking were performed using Rayyan (Ouzzani 2016).
CRG funding
The National Institute for Health Research (NIHR) is the largest single funder of Cochrane Common Mental Disorders.
Disclaimer
The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 31 | 6500 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.66, 0.79] |
| 1.1 TCAs versus placebo | 9 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.63, 0.86] |
| 1.2 SSRIs versus placebo | 21 | 4000 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.67, 0.84] |
| 1.3 MAOIs versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.88] |
| 1.4 SNRIs versus placebo | 4 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.41, 0.91] |
| 1.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.97] |
| 1.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Total number of dropouts | 40 | 7850 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.97] |
| 2.1 TCAs versus placebo | 17 | 1906 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.86] |
| 2.2 SSRIs versus placebo | 23 | 4302 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.09] |
| 2.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 SNRIs versus placebo | 4 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.26] |
| 2.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
| 2.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Failure to remit | 24 | 6164 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.78, 0.88] |
| 3.1 TCAs versus placebo | 8 | 1294 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.69, 0.99] |
| 3.2 SSRIs versus placebo | 16 | 3339 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.75, 0.88] |
| 3.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 SNRIs versus placebo | 4 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.75, 0.95] |
| 3.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 NRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Other antidepressants versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Panic symptoms ‐ endpoint score | 15 | 3699 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.58, ‐0.30] |
| 4.1 TCAs versus placebo | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.62, ‐0.39] |
| 4.2 SSRIs versus placebo | 6 | 1625 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.39, ‐0.17] |
| 4.3 MAOIs versus placebo | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.68 [‐4.93, ‐2.43] |
| 4.4 SNRIs versus placebo | 2 | 723 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.44, ‐0.12] |
| 4.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 NRIs versus placebo | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.50, ‐0.53] |
| 4.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Panic symptoms ‐ mean change | 10 | 2010 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.72, ‐0.33] |
| 5.1 TCAs versus placebo | 2 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐4.07, ‐0.12] |
| 5.2 SSRIs versus placebo | 7 | 1255 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.58, ‐0.29] |
| 5.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 SNRIs versus placebo | 2 | 685 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.60, ‐0.23] |
| 5.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Frequency of panic attacks ‐ endpoint score | 16 | 1671 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.66, ‐0.20] |
| 6.1 TCAs versus placebo | 8 | 470 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.38, ‐0.28] |
| 6.2 SSRIs versus placebo | 8 | 1126 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.32, ‐0.02] |
| 6.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 SNRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 NRIs versus placebo | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.39, ‐0.44] |
| 6.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Frequency of panic attacks ‐ mean change | 8 | 2579 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.72, ‐0.14] |
| 7.1 TCAs versus placebo | 2 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.36, 0.21] |
| 7.2 SSRIs versus placebo | 5 | 949 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.30, ‐0.03] |
| 7.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 SNRIs versus placebo | 4 | 1426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.35, ‐0.39] |
| 7.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Agoraphobia ‐ endpoint score | 13 | 2987 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.99, ‐0.39] |
| 8.1 TCAs versus placebo | 6 | 1122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.31, 0.13] |
| 8.2 SSRIs versus placebo | 6 | 1732 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.71, ‐0.29] |
| 8.3 MAOIs versus placebo | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.38 [‐7.03, ‐3.72] |
| 8.4 SNRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.7 NRIs versus placebo | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐2.09, ‐1.04] |
| 8.8 Other antidepressants versus placebo | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.56, 1.02] |
| 9 Agoraphobia ‐ mean change | 7 | 1792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.19, ‐0.17] |
| 9.1 TCAs versus placebo | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.84, ‐0.08] |
| 9.2 SSRIs versus placebo | 3 | 526 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.95, 0.51] |
| 9.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 SNRIs versus placebo | 3 | 1029 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.47, ‐0.20] |
| 9.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 General anxiety ‐ endpoint score | 17 | 3168 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.63, ‐0.29] |
| 10.1 TCAs versus placebo | 9 | 1351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.48, ‐0.21] |
| 10.2 SSRIs versus placebo | 9 | 1759 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.58, ‐0.26] |
| 10.3 MAOIs versus placebo | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐7.28 [‐9.43, ‐5.14] |
| 10.4 SNRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Other antidepressants versus placebo | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.70, 0.87] |
| 11 General anxiety ‐ mean change | 12 | 2477 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.47, ‐0.20] |
| 11.1 TCAs versus placebo | 4 | 294 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.28, 0.04] |
| 11.2 SSRIs versus placebo | 7 | 1251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.47, ‐0.18] |
| 11.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 SNRIs versus placebo | 3 | 932 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.44, ‐0.07] |
| 11.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Depression ‐ endpoint score | 12 | 1794 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.57, ‐0.25] |
| 12.1 TCAs versus placebo | 6 | 779 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.73, ‐0.35] |
| 12.2 SSRIs versus placebo | 7 | 957 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.45, ‐0.09] |
| 12.3 MAOIs versus placebo | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.78, ‐0.22] |
| 12.4 SNRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.8 Other antidepressants versus placebo | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.58, 1.00] |
| 13 Depression ‐ mean change | 7 | 1052 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.55, ‐0.24] |
| 13.1 TCAs versus placebo | 3 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.09, ‐0.08] |
| 13.2 SSRIs versus placebo | 5 | 823 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.51, ‐0.21] |
| 13.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 SNRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Social functioning ‐ endpoint score | 9 | 1872 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.40, ‐0.18] |
| 14.1 TCAs versus placebo | 4 | 927 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.42, ‐0.16] |
| 14.2 SSRIs versus placebo | 5 | 550 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.70, ‐0.16] |
| 14.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 SNRIs versus placebo | 1 | 395 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 14.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Social functioning ‐ mean change | 7 | 1429 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.42, ‐0.16] |
| 15.1 TCAs versus placebo | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.70, ‐0.07] |
| 15.2 SSRIs versus placebo | 4 | 693 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.56, ‐0.24] |
| 15.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 SNRIs versus placebo | 2 | 537 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 15.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Quality of life | 6 | 1675 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.29, 0.03] |
| 16.1 TCAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 SSRIs versus placebo | 4 | 746 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.51, ‐0.04] |
| 16.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 SNRIs versus placebo | 3 | 929 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.11, 0.17] |
| 16.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Patient satisfaction | 3 | 521 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.01, ‐0.05] |
| 17.1 TCAs versus placebo | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.51, ‐0.82] |
| 17.2 SSRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 SNRIs versus placebo | 2 | 491 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.45, ‐0.09] |
| 17.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Economic costs | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 TCAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 SSRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 MAOIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 SNRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.5 NaSSAs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.6 NDRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.7 NRIs versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.8 Other antidepressants versus placebo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Number of dropouts due to adverse effects | 33 | 7688 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.25, 1.78] |
| 19.1 TCAs versus placebo | 10 | 1641 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [1.33, 2.91] |
| 19.2 SSRIs versus placebo | 22 | 4131 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.16, 1.81] |
| 19.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 SNRIs versus placebo | 4 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.92, 2.40] |
| 19.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.05] |
| 19.8 Other antidepressants versus placebo | 2 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.47] |
| 20 Number of participants experiencing at least one adverse effect | 16 | 4246 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.07, 1.15] |
| 20.1 TCAs versus placebo | 2 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.04, 1.42] |
| 20.2 SSRIs versus placebo | 14 | 2459 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.05, 1.16] |
| 20.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 SNRIs versus placebo | 4 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.03, 1.16] |
| 20.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.7 NRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.8 Other antidepressants versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. TCAs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Imipramine versus placebo | 4 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.94] |
| 1.2 Clomipramine versus placebo | 4 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.56, 0.97] |
| 1.3 Desipramine versus placebo | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.32, 1.14] |
| 2 Total number of dropouts | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Imipramine versus placebo | 10 | 1285 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.60, 0.91] |
| 2.2 Clomipramine versus placebo | 6 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.07] |
| 2.3 Desipramine versus placebo | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.75] |
| 3 Failure to remit | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Imipramine versus placebo | 6 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.67, 1.02] |
| 3.2 Clomipramine versus placebo | 1 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.14] |
| 3.3 Desipramine versus placebo | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.18, 0.88] |
| 4 Panic symptoms ‐ endpoint score | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Imipramine versus placebo | 5 | 1056 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.60, ‐0.35] |
| 4.2 Clomipramine versus placebo | 1 | 158 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.04, ‐0.35] |
| 4.3 Desipramine versus placebo | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.16, ‐0.08] |
| 5 Panic symptoms ‐ mean change | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Imipramine versus placebo | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.81, ‐0.46] |
| 5.2 Clomipramine versus placebo | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.16 [‐4.27, ‐2.04] |
| 6 Frequency of panic attacks ‐ endpoint score | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Imipramine versus placebo | 6 | 279 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.87, ‐0.26] |
| 6.2 Clomipramine versus placebo | 1 | 158 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.72, ‐0.04] |
| 6.3 Desipramine versus placebo | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.76, 0.29] |
| 7 Frequency of panic attacks ‐ mean change | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Imipramine versus placebo | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.93, 0.32] |
| 7.2 Clomipramine versus placebo | 1 | 222 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.28, 0.25] |
| 8 Agoraphobia ‐ endpoint score | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Imipramine versus placebo | 4 | 920 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.35, ‐0.09] |
| 8.2 Clomipramine versus placebo | 1 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐2.77, ‐2.03] |
| 8.3 Desipramine versus placebo | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.94, 0.12] |
| 9 Agoraphobia ‐ mean change | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Imipramine versus placebo | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.70, 0.54] |
| 9.2 Clomipramine versus placebo | 2 | 256 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.93, ‐0.21] |
| 10 General anxiety ‐ endpoint score | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Imipramine versus placebo | 6 | 1014 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.52, ‐0.12] |
| 10.2 Clomipramine versus placebo | 2 | 352 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.68, ‐0.22] |
| 10.3 Desipramine versus placebo | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.84, 0.21] |
| 11 General anxiety ‐ mean change | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Imipramine versus placebo | 2 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.63, 0.24] |
| 11.2 Clomipramine versus placebo | 2 | 273 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.99, 0.61] |
| 12 Depression ‐ endpoint score | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Imipramine versus placebo | 5 | 656 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.85, ‐0.37] |
| 12.2 Clomipramine versus placebo | 1 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.65, ‐0.09] |
| 13 Depression ‐ mean change | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Imipramine versus placebo | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.92, 0.33] |
| 13.2 Clomipramine versus placebo | 2 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.73, 0.12] |
| 14 Social functioning ‐ endpoint score | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Imipramine versus placebo | 4 | 927 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.42, ‐0.16] |
| 15 Social functioning ‐ mean change | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Imipramine versus placebo | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.72, 0.52] |
| 15.2 Clomipramine versus placebo | 1 | 216 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.75, ‐0.21] |
| 16 Patient satisfaction | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Clomipramine versus placebo | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐3.55, ‐1.45] |
| 17 Number of dropouts due to adverse effects | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Imipramine versus placebo | 6 | 1138 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.56, 4.34] |
| 17.2 Clomipramine versus placebo | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.93, 2.43] |
| 18 Number of participants experiencing at least one adverse effect | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Imipramine versus placebo | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.23] |
| 18.2 Clomipramine versus placebo | 1 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.14, 1.51] |
Comparison 3. SSRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Paroxetine versus placebo | 9 | 2469 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.60, 0.82] |
| 1.2 Sertraline versus placebo | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 1.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.76] |
| 1.4 Fluvoxamine versus placebo | 5 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.01] |
| 1.5 Citalopram versus placebo | 2 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 1.6 Escitalopram versus placebo | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.96] |
| 2 Total number of dropouts | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Paroxetine versus placebo | 9 | 2524 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.12] |
| 2.2 Sertraline versus placebo | 4 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.40] |
| 2.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.71, 3.16] |
| 2.4 Fluvoxamine versus placebo | 6 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.24] |
| 2.5 Citalopram versus placebo | 2 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.09] |
| 2.6 Escitalopram versus placebo | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.44, 0.94] |
| 3 Failure to remit | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Paroxetine versus placebo | 6 | 2214 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.75, 0.90] |
| 3.2 Sertraline versus placebo | 2 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.95] |
| 3.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.64, 1.00] |
| 3.4 Fluvoxamine versus placebo | 5 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.51, 1.03] |
| 3.5 Citalopram versus placebo | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 3.6 Escitalopram versus placebo | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.68, 1.09] |
| 4 Panic symptoms ‐ endpoint score | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Paroxetine versus placebo | 3 | 1420 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.38, ‐0.17] |
| 4.2 Fluvoxamine versus placebo | 3 | 308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.62, 0.10] |
| 5 Panic symptoms ‐ mean change | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Paroxetine versus placebo | 3 | 684 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.79, ‐0.11] |
| 5.2 Sertraline versus placebo | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.64, ‐0.04] |
| 5.3 Fluoxetine versus placebo | 1 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.91, ‐0.31] |
| 5.4 Citalopram versus placebo | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 5.5 Escitalopram versus placebo | 1 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.67, ‐0.16] |
| 6 Frequency of panic attacks ‐ endpoint score | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Paroxetine versus placebo | 2 | 368 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.40, 0.06] |
| 6.2 Sertraline versus placebo | 3 | 477 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.54, 0.14] |
| 6.3 Fluvoxamine versus placebo | 3 | 305 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.46, 0.54] |
| 7 Frequency of panic attacks ‐ mean change | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Paroxetine versus placebo | 4 | 983 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.28, ‐0.03] |
| 7.2 Fluoxetine versus placebo | 1 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.51, 0.08] |
| 8 Agoraphobia ‐ endpoint score | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Paroxetine versus placebo | 2 | 1022 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.46, ‐0.20] |
| 8.2 Fluvoxamine versus placebo | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.08, 0.19] |
| 8.3 Citalopram versus placebo | 2 | 603 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.21, ‐0.28] |
| 8.4 Escitalopram versus placebo | 1 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.83, ‐0.32] |
| 9 Agoraphobia ‐ mean change | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Paroxetine versus placebo | 3 | 663 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐3.04, 0.55] |
| 10 General anxiety ‐ endpoint score | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Paroxetine versus placebo | 2 | 1023 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.43, ‐0.17] |
| 10.2 Fluvoxamine versus placebo | 6 | 440 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [1.00, ‐0.24] |
| 10.3 Citalopram versus placebo | 1 | 377 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.63, ‐0.16] |
| 11 General anxiety ‐ mean change | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Paroxetine versus placebo | 3 | 684 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.58, ‐0.28] |
| 11.2 Sertraline versus placebo | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.44, 0.16] |
| 11.3 Fluoxetine versus placebo | 1 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.81, ‐0.22] |
| 11.4 Citalopram versus placebo | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.30, 0.22] |
| 11.5 Escitalopram versus placebo | 1 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.44, 0.07] |
| 12 Depression ‐ endpoint score | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Paroxetine versus placebo | 1 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.51, 0.04] |
| 12.2 Fluvoxamine versus placebo | 5 | 383 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.77, ‐0.01] |
| 12.3 Citalopram versus placebo | 1 | 377 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.49, ‐0.02] |
| 13 Depression ‐ mean change | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Paroxetine versus placebo | 2 | 350 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.75, ‐0.19] |
| 13.2 Fluoxetine versus placebo | 1 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.73, ‐0.14] |
| 13.3 Citalopram versus placebo | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.40, 0.13] |
| 13.4 Escitalopram versus placebo | 1 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.49, 0.02] |
| 14 Social functioning ‐ endpoint score | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Paroxetine versus placebo | 1 | 317 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.33, 0.11] |
| 14.2 Fluvoxamine versus placebo | 4 | 311 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.74, ‐0.28] |
| 15 Social functioning ‐ mean change | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Paroxetine versus placebo | 3 | 570 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.59, ‐0.24] |
| 15.2 Fluoxetine versus placebo | 1 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.73, ‐0.14] |
| 16 Quality of life | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Escitalopram versus placebo | 1 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.67, ‐0.16] |
| 16.2 Citalopram versus placebo | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.53, ‐0.01] |
| 16.3 Paroxetine versus placebo | 1 | 317 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.19, 0.26] |
| 16.4 Sertraline versus placebo | 1 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.80, ‐0.17] |
| 17 Number of dropouts due to adverse effects | 22 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Paroxetine versus placebo | 8 | 2353 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.87, 1.88] |
| 17.2 Sertraline versus placebo | 4 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.99, 3.86] |
| 17.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.41, 6.77] |
| 17.4 Fluvoxamine versus placebo | 6 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.17, 3.38] |
| 17.5 Citalopram versus placebo | 2 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.55, 1.84] |
| 17.6 Escitalopram versus placebo | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.16] |
| 18 Number of participants experiencing at least one adverse effect | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Paroxetine versus placebo | 6 | 1338 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.01, 1.19] |
| 18.2 Sertraline versus placebo | 3 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.99, 1.26] |
| 18.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.78, 2.21] |
| 18.4 Fluvoxamine versus placebo | 2 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.90, 1.36] |
| 18.5 Citalopram versus placebo | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.00, 1.28] |
| 18.6 Escitalopram versus placebo | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.94, 1.22] |
Comparison 4. MAOIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Brofaromine versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.88] |
| 2 Panic symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Brofaromine versus placebo | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐6.20 [‐7.38, ‐5.02] |
| 3 Agoraphobia | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Brofaromine versus placebo | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐23.0 [‐25.98, ‐20.02] |
| 4 General anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Brofaromine versus placebo | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐8.5 [‐9.30, ‐7.70] |
| 5 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Brofaromine versus placebo | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐5.26, ‐0.94] |
Comparison 5. SNRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Venlafaxine ER versus placebo | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.86] |
| 2 Total number of dropouts | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.66, 1.24] |
| 2.1 Venlafaxine ER versus placebo | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.66, 1.24] |
| 3 Failure to remit | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.74, 0.94] |
| 3.1 Venlafaxine ER versus placebo | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.74, 0.94] |
| 4 Panic symptoms ‐ endpoint score | 2 | 801 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.44, ‐0.16] |
| 4.1 Venlafaxine ER versus placebo | 2 | 801 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.44, ‐0.16] |
| 5 Panic symptoms ‐ mean change | 2 | 763 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.62, ‐0.25] |
| 5.1 Venlafaxine ER versus placebo | 2 | 763 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.62, ‐0.25] |
| 6 Frequency of panic attacks | 4 | 1582 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.83, ‐0.58] |
| 6.1 Venlafaxine ER versus placebo | 4 | 1582 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.83, ‐0.58] |
| 7 Agoraphobia | 3 | 1107 | Mean Difference (IV, Random, 95% CI) | ‐7.32 [‐9.87, ‐4.76] |
| 7.1 Venlafaxine ER versus placebo | 3 | 1107 | Mean Difference (IV, Random, 95% CI) | ‐7.32 [‐9.87, ‐4.76] |
| 8 General anxiety | 3 | 1010 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.46, ‐0.08] |
| 8.1 Venlafaxine ER versus placebo | 3 | 1010 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.46, ‐0.08] |
| 9 Social functioning ‐ endpoint score | 1 | 473 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.65, 0.25] |
| 9.1 Venlafaxine ER versus placebo | 1 | 473 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.65, 0.25] |
| 10 Social functioning ‐ mean change | 2 | 537 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐2.26, 0.56] |
| 10.1 Venlafaxine ER versus placebo | 2 | 537 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐2.26, 0.56] |
| 11 Quality of life | 3 | 1007 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.93, 1.55] |
| 11.1 Venlafaxine ER versus placebo | 3 | 1007 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.93, 1.55] |
| 12 Patient satisfaction | 2 | 491 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.66, ‐0.14] |
| 12.1 Venlafaxine ER versus placebo | 2 | 491 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.66, ‐0.14] |
| 13 Number of dropouts due to adverse effects | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.88, 2.04] |
| 13.1 Venlafaxine ER versus placebo | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.88, 2.04] |
| 14 Number of participants experiencing at least one adverse effect | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.03, 1.15] |
| 14.1 Venlafaxine ER versus placebo | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.03, 1.15] |
Comparison 6. NRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Reboxetine versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.97] |
| 2 Total number of dropouts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Reboxetine versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
| 3 Panic symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Reboxetine versus placebo | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐1.87, ‐0.73] |
| 4 Frequency of panic attacks | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Reboxetine versus placebo | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐4.6 [‐6.83, ‐2.37] |
| 5 Agoraphobia | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Reboxetine versus placebo | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐2.57, ‐1.43] |
| 6 Number of dropouts due to adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Reboxetine versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.05] |
Comparison 7. Other antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.14] |
| 1.1 Ritanserin versus placebo | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.14] |
| 2 Total number of dropouts | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.35] |
| 2.1 Ritanserin versus placebo | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.35] |
| 3 Agoraphobia | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐3.97, 8.49] |
| 3.1 Ritanserin versus placebo | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐3.97, 8.49] |
| 4 General anxiety | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.16, 1.56] |
| 4.1 Ritanserin versus placebo | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.16, 1.56] |
| 5 Depression | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐1.53, 3.29] |
| 5.1 Ritanserin versus placebo | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐1.53, 3.29] |
| 6 Number of dropouts due to adverse effects | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.47] |
| 6.1 Nefazodone versus placebo | 1 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.47] |
| 6.2 Ritanserin versus placebo | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. High risk of bias excluded ‐ antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 18 | 3819 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.72, 0.87] |
| 1.1 TCAs versus placebo | 6 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.94] |
| 1.2 SSRIs versus placebo | 13 | 2796 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.71, 0.91] |
| 1.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 SNRIs versus placebo | 1 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.07] |
| 1.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.97] |
| 1.8 Other antidepressants versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Total number of dropouts | 21 | 3983 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.00] |
| 2.1 TCAs versus placebo | 9 | 729 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.63, 0.94] |
| 2.2 SSRIs versus placebo | 13 | 2829 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| 2.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 SNRIs versus placebo | 1 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.88, 1.72] |
| 2.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
| 2.8 Other antidepressants versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. High risk of bias excluded ‐ TCAs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Imipramine versus placebo | 3 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.96] |
| 1.2 Clomipramine versus placebo | 3 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.40, 1.13] |
| 2 Total number of dropouts | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Imipramine versus placebo | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.95] |
| 2.2 Clomipramine versus placebo | 4 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.03] |
| 2.3 Desipramine versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. High risk of bias excluded ‐ SSRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Paroxetine versus placebo | 5 | 1436 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.00] |
| 1.2 Sertraline versus placebo | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 0.99] |
| 1.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.76] |
| 1.4 Fluvoxamine versus placebo | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.21, 2.06] |
| 1.5 Citalopram versus placebo | 2 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.91] |
| 1.6 Escitalopram versus placebo | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 2 Total number of dropouts | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Paroxetine versus placebo | 5 | 1552 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.18] |
| 2.2 Sertraline versus placebo | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.63, 1.45] |
| 2.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.71, 3.16] |
| 2.4 Fluvoxamine versus placebo | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.40] |
| 2.5 Citalopram versus placebo | 2 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.09] |
| 2.6 Escitalopram versus placebo | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.44, 0.94] |
Comparison 11. High risk of bias excluded ‐ SNRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Venlafaxine ER versus placebo | 1 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.07] |
| 2 Total number of dropouts | 1 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.88, 1.72] |
| 2.1 Venlafaxine ER versus placebo | 1 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.88, 1.72] |
Comparison 12. High dropout rates excluded ‐ Antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 6 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.80] |
| 1.1 TCAs versus placebo | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.08, 0.92] |
| 1.2 SSRIs versus placebo | 3 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.25, 1.04] |
| 1.3 MAOIs versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.88] |
| 1.4 SNRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 NRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Total number of dropouts | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.75, 1.68] |
| 2.1 TCAs versus placebo | 4 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.39, 2.10] |
| 2.2 SSRIs versus placebo | 3 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.76, 1.95] |
| 2.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 SNRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 NRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. High dropout rates excluded ‐ TCAs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Imipramine versus placebo | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.17, 1.30] |
| 1.2 Clomipramine versus placebo | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.52] |
| 2 Total number of dropouts | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Imipramine versus placebo | 3 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.48, 2.42] |
| 2.2 Clomipramine versus placebo | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.90] |
| 2.3 Desipramine versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. High dropout rates excluded ‐ SSRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Paroxetine versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Sertraline versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.00] |
| 1.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.76] |
| 1.4 Fluvoxamine versus placebo | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.12, 0.57] |
| 1.5 Citalopram versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Escitalopram versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Total number of dropouts | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Paroxetine versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Sertraline versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.60, 2.12] |
| 2.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.71, 3.16] |
| 2.4 Fluvoxamine versus placebo | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.35] |
| 2.5 Citalopram versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Escitalopram versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Funded excluded ‐ antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 12 | 1183 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.66, 0.92] |
| 1.1 TCAs versus placebo | 5 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 1.2 SSRIs versus placebo | 6 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.16] |
| 1.3 MAOIs versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.88] |
| 1.4 SNRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.97] |
| 1.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Total number of dropouts | 16 | 1331 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.04] |
| 2.1 TCAs versus placebo | 9 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.51, 1.20] |
| 2.2 SSRIs versus placebo | 7 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.22] |
| 2.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 SNRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
| 2.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. Funded excluded ‐ TCAs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Imipramine versus placebo | 3 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.96] |
| 1.2 Clomipramine versus placebo | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.52] |
| 1.3 Desipramine versus placebo | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.32, 1.14] |
| 2 Total number of dropouts | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Imipramine versus placebo | 6 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.56, 1.25] |
| 2.2 Clomipramine versus placebo | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.02, 25.22] |
| 2.3 Desipramine versus placebo | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.75] |
Comparison 17. Funded excluded ‐ SSRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Paroxetine versus placebo | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.73, 1.54] |
| 1.2 Sertraline versus placebo | 2 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 1.3 Fluoxetine versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Fluvoxamine versus placebo | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.12, 2.58] |
| 1.5 Citalopram versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Escitalopram versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Total number of dropouts | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Paroxetine versus placebo | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.65, 1.38] |
| 2.2 Sertraline versus placebo | 2 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.77, 1.43] |
| 2.3 Fluoxetine versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Fluvoxamine versus placebo | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.40] |
| 2.5 Citalopram versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Escitalopram versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 18. Irregular benzodiazepine use excluded ‐ antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 9 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.66, 0.86] |
| 1.1 TCAs versus placebo | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.84] |
| 1.2 SSRIs versus placebo | 6 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.96] |
| 1.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 SNRIs versus placebo | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 1.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 NRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Other antidepressants versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Total number of dropouts | 16 | 3109 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.02] |
| 2.1 TCAs versus placebo | 7 | 1183 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.60, 0.82] |
| 2.2 SSRIs versus placebo | 8 | 1565 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.24] |
| 2.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 SNRIs versus placebo | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.59] |
| 2.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 NRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Other antidepressants versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 19. Irregular benzodiazepine use excluded ‐ TCAs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Imipramine versus placebo | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.42, 1.00] |
| 1.2 Clomipramine versus placebo | 1 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.88] |
| 2 Total number of dropouts | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Imipramine versus placebo | 6 | 1003 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.59, 0.84] |
| 2.2 Clomipramine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.02] |
| 2.3 Desipramine versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 20. Irregular benzodiazepine use excluded ‐ SSRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Paroxetine versus placebo | 3 | 1131 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.58, 1.26] |
| 1.2 Sertraline versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.00] |
| 1.3 Fluvoxamine versus placebo | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.27, 1.08] |
| 2 Total number of dropouts | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Paroxetine versus placebo | 3 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.91, 1.31] |
| 2.2 Sertraline versus placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.60, 2.12] |
| 2.3 Fluvoxamine versus placebo | 4 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.26] |
Comparison 21. Irregular benzodiazepine use excluded ‐ SNRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Venlafaxine ER versus placebo | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 2 Total number of dropouts | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.59] |
| 2.1 Venlafaxine ER versus placebo | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.59] |
Comparison 22. Psychiatric comorbidities excluded ‐ antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 25 | 4921 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.63, 0.79] |
| 1.1 TCAs versus placebo | 8 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.88] |
| 1.2 SSRIs versus placebo | 17 | 2848 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.65, 0.86] |
| 1.3 MAOIs versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.88] |
| 1.4 SNRIs versus placebo | 3 | 1188 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.33, 0.91] |
| 1.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.97] |
| 1.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Total number of dropouts | 29 | 5088 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.94] |
| 2.1 TCAs versus placebo | 12 | 904 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.88] |
| 2.2 SSRIs versus placebo | 18 | 2885 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 2.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 SNRIs versus placebo | 3 | 1188 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.19] |
| 2.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
| 2.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 23. Psychiatric comorbidities excluded ‐ TCAs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Imipramine versus placebo | 3 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.01] |
| 1.2 Clomipramine versus placebo | 4 | 565 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.96] |
| 1.3 Desipramine versus placebo | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.32, 1.14] |
| 2 Total number of dropouts | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Imipramine versus placebo | 6 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.49, 0.99] |
| 2.2 Clomipramine versus placebo | 5 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.95] |
| 2.3 Desipramine versus placebo | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.75] |
Comparison 24. Psychiatric comorbidities excluded ‐ SSRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Paroxetine versus placebo | 8 | 1574 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.59, 0.91] |
| 1.2 Sertraline versus placebo | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.29] |
| 1.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.76] |
| 1.4 Fluvoxamine versus placebo | 4 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.02] |
| 1.5 Citalopram versus placebo | 2 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.91] |
| 1.6 Escitalopram versus placebo | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 2 Total number of dropouts | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Paroxetine versus placebo | 8 | 1635 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.08] |
| 2.2 Sertraline versus placebo | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.81, 1.68] |
| 2.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.71, 3.16] |
| 2.4 Fluvoxamine versus placebo | 5 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.28] |
| 2.5 Citalopram versus placebo | 2 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.09] |
| 2.6 Escitalopram versus placebo | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.44, 0.94] |
Comparison 25. Psychiatric comorbidities excluded ‐ SNRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Venlafaxine ER versus placebo | 3 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.88] |
| 2 Total number of dropouts | 3 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.15] |
| 2.1 Venlafaxine ER versus placebo | 3 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.15] |
Comparison 26. Imputation excluded ‐ antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 29 | 6313 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.65, 0.79] |
| 1.1 TCAs versus placebo | 8 | 796 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.86] |
| 1.2 SSRIs versus placebo | 20 | 3846 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.66, 0.84] |
| 1.3 MAOIs versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.88] |
| 1.4 SNRIs versus placebo | 4 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.41, 0.91] |
| 1.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.97] |
| 1.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Total number of dropouts | 38 | 7618 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.97] |
| 2.1 TCAs versus placebo | 16 | 1850 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.65, 0.83] |
| 2.2 SSRIs versus placebo | 22 | 4126 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.09] |
| 2.3 MAOIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 SNRIs versus placebo | 4 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.26] |
| 2.5 NaSSAs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 NDRIs versus placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 NRIs versus placebo | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
| 2.8 Other antidepressants versus placebo | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 27. Imputation excluded ‐ SSRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Paroxetine versus placebo | 9 | 2408 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.84] |
| 1.2 Sertraline versus placebo | 2 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 1.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.76] |
| 1.4 Fluvoxamine versus placebo | 5 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 0.98] |
| 1.5 Citalopram versus placebo | 2 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.91] |
| 1.6 Escitalopram versus placebo | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 2 Total number of dropouts | 22 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Paroxetine versus placebo | 9 | 2524 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.12] |
| 2.2 Sertraline versus placebo | 3 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.84, 1.42] |
| 2.3 Fluoxetine versus placebo | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.71, 3.16] |
| 2.4 Fluvoxamine versus placebo | 6 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.24] |
| 2.5 Citalopram versus placebo | 2 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.09] |
| 2.6 Escitalopram versus placebo | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.44, 0.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asnis 2001.
| Methods | Study design: 8 weeks, multi‐centre, double‐blind, placebo‐controlled outpatient clinical trial, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): fluvoxamine arm mean age (years) 34.2 (SD = 10.2, range 19‐65), placebo arm mean age (years) 36.7 (SD = 9.8, range 20‐63) Sex: 64 men, 115 women Location: outpatients, 4 centres throughout the USA Co‐morbidities: excluded Rescue medication: discouraged, but allowed for night time sedation (lorazepam 1‐2 mg or chloral hydrate 1‐2 mg) |
|
| Interventions | Participants were randomly assigned to either: 1. fluvoxamine arm (randomised n = 93) Duration: 8 weeks Treatment protocol: flexible dosage; range = 100‐300 mg/day, mean 4.2 cps/day (SD = 1.4) 2. placebo arm (randomised n = 95) Duration: 8 weeks Treatment protocol: flexible dosage, mean 5.1 cps/day (SD = 1.2) |
|
| Outcomes |
Timepoints for assessment: at baseline and weekly until week 8 Outcomes
|
|
| Notes |
Date of study: not specified Funding source: unclear Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomized", however the sequence generation process is not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is defined as "double‐blind". Quote: "Treatment was started with a daily dosage of one capsule (50 mg fluvoxamine or matching placebo) [...]" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: fluvoxamine group 29/93 (31.2%), placebo group 29/95 (30.5%). There are high dropout rates in each arm. Reasons for leaving the study early are relatively balanced between the two groups (see table 1). Quote: "Conclusions were based on the last observation carried forward to the end of the study (LOCF) analyses for the intention to treat population (all patients randomized to double‐blind treatment who provided some on‐drug efficacy data)". However, the tables do not report the number of analysed participants. |
| Selective reporting (reporting bias) | High risk | The primary outcome is clearly reported in the methods, quote: "The primary efficacy measurement, the DPAI, was designed to identify panic attacks". However, the DPAI scores are not reported in the text and tables. All other measurements are reported. |
| Other bias | High risk | Quote: "The authors thank Drs. R.I.H. and A.M. who were at Solvay Duphar for their help in providing statistical assistance and a thorough review of the manuscript". A risk of sponsorship bias cannot be excluded. |
Ballenger 1998.
| Methods | Study design: 10 weeks, double‐blind, randomised (cluster randomisation), placebo‐controlled, parallel‐design, multicentre clinical trial | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder, with or without agoraphobia Method of diagnosis: not specified Age (years): placebo arm mean age 37.3 (SD = 10.4), paroxetine 10 mg arm mean age 36.1 (SD = 9.1), paroxetine 20 mg arm mean age 35.9 (SD = 10.1) and paroxetine 40 mg mean age 36.3 (SD = 10.8) Sex: 95 men, 183 women Location: outpatients Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. paroxetine 10 mg arm (randomised n = 67) Duration: 10 weeks Treatment protocol: fixed dosage 10 mg/day 2. paroxetine 20 mg arm (randomised n = 70) Duration: 10 weeks Treatment protocol: fixed dosage 20 mg/day 3. paroxetine 40 mg arm (randomised n = 72) Duration: 10 weeks Treatment protocol: fixed dosage 40 mg/day 4. placebo arm (randomised n = 69) Duration: 10 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Timepoints for assessment: at baseline, week 4 and week 10 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: sponsored by the drug company marketing the drug Declarations of interest among the primary researchers: apparently connected with the drug company marketing the drug |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomized", however the sequence generation process is not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as "double‐blind", however procedures for ensuring the blindness of participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: paroxetine 10 mg group 22/67 (32.8%), paroxetine 20 mg group 23/70 (32.8%), paroxetine 40 mg group 22/72 (30.5%), placebo group 23/69 (33.3%). The dropout rate is high in every arm and reasons for leaving the study are apparently balanced between groups as reported in table 2 in the paper). Quote: "Results for the intent‐to‐treat population were determined on the basis of the data sets for both completer analysis (observed cases) and endpoint analysis (last observation carried forward)". Outcome measures reported are consistent with an ITT analysis (as reported in table 3 in the paper). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are clearly pre‐specified in the protocol of the study and in the "measurements" paragraph of the paper. All relevant data are clearly reported in tables. |
| Other bias | Unclear risk | A "disclosure of interest" paragraph is not reported. |
Barlow 2000.
| Methods | Study design: 12 weeks and then 6 months, multicentre, randomised, double‐blind, placebo‐controlled clinical trial, parallel groups, cluster randomisation | |
| Participants |
Diagnosis: panic disorder with or without mild agoraphobia Method of diagnosis: ADIS‐R (Anxiety Disorder Interview Schedule‐Revised, diagnosis confirmed 2 weeks prior to first treatment visit) Age (years): mean 36.1 (SD = 10.7) Sex: 62.5% women Location: not specified Co‐morbidities: patients with depression were not excluded, unless suicidal Rescue medication: allowed up to 20 doses of benzodiazepines (or 10 alprazolam equivalent) |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine arm (randomised n = 83) Duration: 12 weeks Treatment protocol: flexible dosage. "the dose was titrated 10 mg every other day until 50 mg per day and then was flexible, with efforts to reach 100 mg by the end of week 3 and 200 by week 5" 2. CBT alone arm (randomised n = 77) Duration:12 weeks Treatment protocol: unclear 3. CBT plus imipramine arm (randomised n = 65) Duration:12 weeks Treatment protocol: flexible dosage; range = 10‐60 mg/day 4. CBT plus placebo arm (randomised n = 63) Duration: 12 weeks Treatment protocol: flexible dosage 5. placebo arm (randomised n = 24) Duration: 12 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Timepoints for assessment: at baseline, at week 12 and then at month 4, 5, and 6 Outcomes:
|
|
| Notes |
Date of study: May 1991‐April 1998 Funding source: the study was mostly funded by public financial support. Sponsorship bias is unlikely to have occurred. Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however details on the random sequence generation are not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trained independent evaluators were employed (see "Assessment" paragraph) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trained independent evaluators were employed (see "Assessment" paragraph) |
| Selective reporting (reporting bias) | Low risk | Primary endpoints are divided in continuous outcome measures (average item score for the PDSS) and categorical outcome measures (responders based on CGI). All relevant data are reported in tables. |
| Other bias | Unclear risk | Study authors received various financial support from pharmaceutical agencies. Quote: "Imipramine and matching placebo were provided by Teva Pharmaceuticals USA". The study was mostly funded by public financial support. |
Bergink 2005.
| Methods | Study design: 9 weeks, randomised (individual randomisation), double‐blind, parallel, placebo‐controlled clinical trial, parallel groups | |
| Participants |
Diagnosis: panic disorder with and without agoraphobia according to the DSM IV criteria Method of diagnosis: PDSS, CGI and number of panic attacks per week Age (years): the mean age was 41 for the metabotropic glutamate (LY354740), 44 for paroxetine and 45 for placebo Sex: 18 men and 27 women Location: University Medical Centre (UMC) in Utrecht, the Netherlands Co‐morbidities: excluded Rescue medication: not permitted |
|
| Interventions | Participants were randomly assigned to either: 1. LY354740 arm (randomised n = 18) Duration: 9 weeks Treatment protocol: flexible dosage; range = 100‐200 mg/day 2. paroxetine arm (randomised n = 9) Duration: 9 weeks Treatment protocol: flexible dosage; range = 10‐60 mg/day 3. placebo arm (randomised n = 0) Duration: 9 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and then at week 3, 6, 9 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: unclear Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible patients were assigned in a 1:1:1:1 ratio to one of the following four treatment groups: LY354740 100 mg/day, LY354740 200 mg/day, paroxetine, placebo". |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as "double blind" but no further details are given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details are given in the text |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number and the reasons for dropouts are specified. Data analysis was performed on the intent‐to treat population using the LOCF |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcome data are shown in a table. |
| Other bias | Unclear risk | It is unclear whether the study authors received a grant for the study. |
Black 1993.
| Methods | Study design: 8 weeks, double‐blind, placebo‐controlled trial, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder with or without agoraphobia Method of diagnosis: SCID Age (years): fluvoxamine arm mean age 35.1 (SD = 10.4), CBT arm mean age 38.7 (SD = 12.4) and placebo arm mean age 37.0 (SD = 9.9) Sex: 22 men, 53 women Location: outpatient setting, multicentre, USA Co‐morbidities: patients with a diagnosis of major depression were also included, medical comorbidities were excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. fluvoxamine arm (randomised n = 25) Duration: 8 weeks Treatment protocol: flexible dosage; range = up to 300 mg per day, mean 230 mg (4.6 cps)/day 2. CBT arm (randomised n = 25) Duration: 8 weeks Treatment protocol: psychotherapy sessions 3. placebo arm (randomised n = 25) Duration: 8 weeks Treatment protocol: flexible dosage, 5.5 cps/day |
|
| Outcomes |
Timepoints for assessment: at baseline and then at week 4 and 8 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: financed by a drug company Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to the drug study (n=50) or to the cognitive therapy (n=25) [...]". The sequence generation process is not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Investigators and subjects remained "blind" to this assignment (ie, fluvoxamine vs placebo)". However, procedures for ensuring the concealment of allocation are not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Medications [...] were administered in a double‐blind fashion". However, procedures for ensuring the blinding are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Assessments were made by the project coordinator (JG) or a psychiatrist (DWB or RW)". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate: fluvoxamine group 4/25 (16%), placebo group 7/25 (28%). The rate of dropouts in the placebo group was higher than in the fluvoxamine group, and reasons for leaving the study early are unbalanced, particularly considering dropouts for ineffectiveness. In the "statistical analysis" paragraph both "completer analysis" and ITT analysis with a "last observation carried forward" approach are mentioned, however it is not clear which one has been employed for data reported in tables, since the number of analysed participants is not reported. |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes are not clearly pre‐specified in the text. Data from all the rating scales are clearly reported in graphs, with the exception of the frequency of panic attacks. |
| Other bias | High risk | Quote: "The study was sponsored in part through a grant from Reid‐Rowell Pharmaceuticals Inc, Atlanta, Ga". The role of the funder in planning, conducting and writing the study is not discussed. |
Bradwejn 2005.
| Methods | Study design: 10 weeks, flexible dose , double‐blind, randomised (individual randomisation), parallel groups, placebo‐controlled study | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: DSM‐IV and modified Mini International Neuropsychiatric Interview Age (years): 38.9 (SD = 12.4) for the venlafaxine ER arm and 38.8 (SD = 12.1) for the placebo arm Sex: venlafaxine arm, 61 men and 99 women; placebo arm, 69 men, 99 women Location: outpatient setting, 50 sites in Canada, Europe and South Africa Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. venlafaxine ER arm (randomised n = 181) Duration: 10 weeks Treatment protocol: flexible dosage; range = 75‐225 mg/day, mean = 162.9 mg/day (SD = 60.6) at week 10 2. placebo arm (randomised n = 180) Duration: 10 weeks Treatment protocol: flexible dosage; range = 1‐3 capsules |
|
| Outcomes |
Timepoints for assessment: at baseline and then at 2, 3, 4, 6,8 and 10 weeks Outcomes
|
|
| Notes |
Date of study: not specified Funding source: the study was funded by the company marketing the drug Declarations of interest among the primary researchers: the primary researcher received a funding from drug companies for the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomised". No further info about the random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information is given about the allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as double blind but it is unclear whether the investigators were "blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study is described as double blind but it is unclear whether the investigators were "blind". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The dropout rate is over 25% and it is reported in the flow chart of the study. The study authors used ITT analysis. |
| Selective reporting (reporting bias) | Low risk | The results are clearly reported in the tables and in the text. |
| Other bias | High risk | The study was funded by the company marketing the drug. The primary researcher received funding from drug companies for the study. |
Broocks 1998.
| Methods | Study design: 10 weeks, placebo‐controlled study, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R and ICD‐10 criteria diagnosis of panic disorder and agoraphobia Method of diagnosis: SCID for DSM‐III‐R Age (years): 18‐50; exercise arm mean age 31.8 (SD = 9.5), clomipramine arm mean age 33.9 (SD = 9.2) and placebo arm mean age 34.8 (SD = 6.8) Sex: 23 men, 23 women Location: outpatient setting, Germany Co‐morbidities: excluded Rescue medication: prometazine 25‐50 mg |
|
| Interventions | Participants were randomly assigned to either: 1. clomipramine arm (randomised n = 15) Duration: 10 weeks Treatment protocol: fixed dosage; range = 37.5‐112.5 mg/day 2. aerobic exercise‐running arm (randomised n = 16) Duration: 10 weeks Treatment protocol: running schedule 3. placebo arm (randomised n = 15) Duration: 10 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and then at 10 weeks Outcomes:
|
|
| Notes |
Date of study: unclear Funding source: grant from a car factory Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The sequence generation process is not described. Moreover the randomisation procedure was divided in 2 steps, quote: "At baseline, patients were randomly assigned to the clomipramine/placebo group (n = 30) or the exercise group (n = 6). The study therapists (A.B., G.P., and A.G.) were not blind to this assignment. Patients in the drug group were further randomly assigned to receive either clomipramine (n = 15) or placebo (n = 15). The assignment was done by the hospital pharmacist; investigators and subjects remained blind to this assignment". This may have altered the balance between the 3 arms, which are however described as comparable. |
| Allocation concealment (selection bias) | Unclear risk | Selection bias is likely to have occurred. See above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: exercise group 5/16 (31.2%); clomipramine group 0/15 (0%); placebo group 4/15 (26.7%). Dropout rates are high for 2 groups, with reasons for leaving the study apparently balanced. An ITT analysis was performed and data were imputed with a LOCF approach. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes are clearly reported in tables. |
| Other bias | Low risk | Supported by a grant from a car factory so it is unlikely that a sponsorship bias might have occurred. |
Caillard 1999.
| Methods | Study design: 8 weeks, multicentre, randomised (individual randomisation), parallel groups, double‐blind, three arms, placebo‐controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder with or without agoraphobia Method of diagnosis: participants had to fulfil the DSM‐III‐R criteria for panic disorder, with a minimum score of 20 on the HAMA), and a minimum of 5 points for the 2 first items (anxious mood and tension), after the 1‐week, single‐blind period Age (years): clomipramine low‐dose arm mean age 38 (SD = 10), clomipramine high‐dose arm mean age 35.5 (SD = 11) and placebo arm mean age 37 (SD = 10) Sex: 64 men, 94 women Location: outpatient setting, multicentre (15 sites in France) Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. clomipramine low‐dose arm (randomised n = 61) Duration: 8 weeks Treatment protocol: fixed dosage; 60 mg/day 2. clomipramine high‐dose arm (randomised n = 62) Duration: 8 weeks Treatment protocol: fixed dosage; 150 mg/day 3. Placebo arm (randomised n = 57) Duration: 8 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and weekly Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the sponsor is the drug company marketing clomipramine Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however details on the sequence generation process are not provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: clomipramine "low dose" group 15/61 (25%); clomipramine "high dose" group 22/62 (37%); placebo 25/57 (45%). Dropout rates are high (more than 20%), unbalanced between groups both in number and in terms of reasons for leaving the study early. The intention‐to‐treat analysis included all 180 randomised participants and was applied only for categorical data. Instead, only participants who strictly observed the protocol were included in the explanatory analysis. However, according to Table 2, not all randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The aim of this study was to investigate the dose‐response relationship for clomipramine in patients with panic disorder [...]". However, the primary outcome measure and time‐point employed are not clearly reported. All relevant data are reported in the text and tables. |
| Other bias | High risk | Quote: "This study was supported in part by the NOVARTIS Company and by the French University Antidepressant Group". The role of the funder in planning, conducting and writing the study is not discussed. |
Cassano 1999.
| Methods | Study design: 12 weeks, multicentre, double‐blind, randomised controlled trial, parallel groups | |
| Participants |
Diagnosis: DSM‐IV diagnosis of panic disorder, with or without agoraphobia Method of diagnosis: not stated Age (years): not specified Sex: not specified Location: 23 European sites, outpatient settings Co‐morbidities: not stated Rescue medication: not stated |
|
| Interventions | Participants were randomly assigned to either: 1. nefazodone arm (randomised n = 135) Duration: 12 weeks Treatment protocol: flexible dosage; range = 100‐600 mg/day, mean = 453 mg/day (SD = not specified) 2. placebo arm (randomised n = 139) Duration: 12 weeks Treatment protocol: flexible |
|
| Outcomes |
Timepoints for assessment: endpoint at 10 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: Bristol‐Myers Squibb (declared) Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data regarding efficacy and dropouts are not fully reported. |
| Selective reporting (reporting bias) | Unclear risk | Data regarding efficacy and dropouts are not fully reported. |
| Other bias | High risk | Funding for the study was provided by the drug company marketing the drug. |
CNCPS 1992.
| Methods | Study design: 8 weeks, randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder with limited or extensive phobic avoidance (panic attacks with agoraphobia) Method of diagnosis: "patients were evaluated by Structured Clinical Interview for DSM‐III Diagnosis, Upjohn (SCID‐UP)" Age (years): mean age = 34, SD not provided Sex: 62% female, 38% male Location: in and outpatient setting, 12 centres in USA, Spain, Denmark, Germany, England, Italy, Brazil, Mexico, France, Colombia, Austria, Sweden, Canada, Belgium Co‐morbidities: patients with psychotic disorders, dementia, bipolar disorder, alcoholism or drug abuse within the last 6 months or significant medical problems were excluded. Patients with current major depression were excluded unless the depression was judged to be secondary to the anxiety disorder and did not have melancholic or psychotic features. Rescue medication: not allowed Quote "patients taking CNS drugs, including benzodiazepines, were excluded from the study. During the washout period, blood was drawn for benzodiazepines screening". |
|
| Interventions |
Participants were randomly assigned to either: 1. imipramine arm (n = 391) Duration: 8 weeks Treatment protocol: flexible dosage; range = 25‐250 mg, mean = 155, SD not provided 2. alprazolam arm (n = 386) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1‐10 mg, mean = 5.7, SD not provided 3. placebo arm (n = 391) Duration: 8 weeks |
|
| Outcomes |
Timepoints for assessment: baseline, weekly, endpoint Outcomes:
|
|
| Notes |
Date of study: data collection: 1984‐1987 Funding source: sponsored by Upjohn Company, Kalamazoo, Michigan Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned"; "alprazolam, imipramine or placebo were assigned in 12 randomization blocks of the basic three cell random‐assignment, parallel treatment‐design. [...] At each center patients were blindly and randomly assigned to alprazolam, imipramine or placebo treatment, based on a table of random numbers [...]. Patients removed from the protocol before three weeks had to be replaced; after three weeks, non‐completers were not replaced." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote "double‐blind design". No further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "double‐blind design". No further details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "of 1168 patients randomized, 1122 met criteria for ITT". |
| Selective reporting (reporting bias) | High risk | In the primary publication, data on Panic Attack scale are not reported; data on Physician's global Improvement scale are only partially reported, and without the number of participants evaluated; data on other continuous outcomes (HRSA, HRSD) are reported without number of participants evaluated. Other data are partially reported in secondary publication of this study. |
| Other bias | High risk | Sponsored by Upjohn Company, Kalamazoo, Michigan; the role of the funder in planning, conducting and writing the study is not discussed. |
Den Boer 1990.
| Methods | Study design: 8 weeks, randomised controlled trial, parallel‐group, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder Method of diagnosis: Utrecht Panic Attack Inventory Age (years): 36.8 (SD = 7.3) fluvoxamine, 37.3 (SD = 6.8) placebo Sex: 49 women, 11 men (n = 60) Location: outpatient clinic of the department of Biological Psychiatry of the University Hospital in Utrecht Co‐morbidities: excluded Rescue medication: no information provided |
|
| Interventions | Participants were randomly assigned to either: 1. fluvoxamine arm (randomised n = 20) Duration: 8 weeks Treatment protocol: fixed dosage, 150 mg/day 2. ritanserin arm (randomised n = 20) Duration: 8 weeks Treatment protocol: fixed dosage 20 mg/day 3. placebo arm (n = 19) Duration: 8 weeks Treatment protocol: 2 tablets |
|
| Outcomes |
Timepoints for assessment: at weeks 4, 6 and 8 Outcomes:
|
|
| Notes |
Date of study: no information provided Funding source: Dutch foundation of Phobic Disorders Declarations of interest among the primary researchers: none known |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of the three groups". no further details are given. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "medication was started with one tablet containing either 10 mg ritanserin or 75 mg fluvoxamine or placebo once daily for one week". it is not clear whether the capsules were identical or not. No other blinding methods are specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This is a double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "There was one drop‐out because of deterioration of symptomatology during the 1st week of treatment" After the dropout the groups are still comparable Analyses were performed on completers' data, no imputation was performed |
| Selective reporting (reporting bias) | High risk | Results are partially reported and some are only reported unclearly (by graph). |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Gentil 1993‐clo.
| Methods | Study design: 8 week, double‐blind outpatient clinical trial, parallel design, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not further specified "instruments and procedures" Age (years): 36.17 Sex: 21 men (35%), 39 women (65%) Location: outpatients setting, Brazil Co‐morbidities: people with previous history or presently meeting operational criteria for obsessive‐compulsive disorder, primary major depression or psychoses were excluded. People meeting criteria for major depression without melancholia could still be included, provided that two doctors agreed that their depression was secondary to panic disorder Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine arm (randomised n = 20) Duration: 8 weeks Treatment protocol: flexible dosage; range = 25‐200 mg/day, mean = 113.8 mg/day (SD = 9.5) 2. clomipramine arm (randomised n=20) Duration: 8 weeks Treatment protocol: flexible dosage; range = 10‐60 mg/day, mean = 50 mg/day (SD = 4.2) 3. Placebo arm (randomised n = 20) Duration: 8 weeks Treatment protocol: flexible dosage, gradually titrated up to a maximum of 8 cps/day |
|
| Outcomes |
Timepoints for assessment: at baseline and then at week 2, 4, 6, 8 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: none Declarations of interest among the primary researchers: none The study Gentil 1993, with three arms including two antidepressants of the same class, is presented separately for the imipramine and clomipramine arms in comparison to placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated [...]", however the sequence generation process is not discussed. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "double‐blind treatment"; "Capsules were in the hospital pharmacy with tablets of the commercially available TCAs or propanteline (placebo) and filled up with lactose. The dose range of propanteline was selected to give mild to moderate peripheral anticholinergic effects". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "double‐blind treatment"; "Capsules were in the hospital pharmacy with tablets of the commercially available TCAs or propanteline (placebo) and filled up with lactose. The dose range of propanteline was selected to give mild to moderate peripheral anticholinergic effects". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 participants left the trial before completing the first 4 weeks of treatment and were replaced. No information provided on incomplete outcome data management. |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes are not pre‐specified. The rating scales employed are reported, however some outcomes were apparently chosen in an arbitrary way (quote: "The sum of scores for ShAS items 32 (’severity of spontaneous attacks with more than three symptoms’), 33 (’severity of limited panic attacks or symptoms’) and 35 (’severity of situational panic attacks’) were taken as an index of the ’severity of panic’. The sum of scores on items 1‐31 and 34 of the ShAS were defined as the ’anxiety symptoms score’"). Quote: "Only the most relevant data will be presented". The number of participants allocated to each arm is not clearly reported in the text, tables or graphs, although it can ben derived. |
| Other bias | Low risk | Quote: "this study was not supported by the facturer of the drug tested". |
Gentil 1993‐imi.
| Methods | Study design: 8 week, double‐blind outpatient clinical trial, parallel design, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not further specified "instruments and procedures" Age (years): 36.17 Sex: 21 men (35%), 39 women (65%) Location: outpatients setting, Brazil Co‐morbidities: people with previous history or presently meeting operational criteria for obsessive‐compulsive disorder, primary major depression or psychoses were excluded. People meeting criteria for major depression without melancholia could still be included, provided that two doctors agreed that their depression was secondary to panic disorder Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine arm (randomised n = 20) Duration: 8 weeks Treatment protocol: flexible dosage; range = 25‐200 mg/day, mean = 113.8 mg/day (SD = 9.5) 2. clomipramine arm (randomised n = 20) Duration: 8 weeks Treatment protocol: flexible dosage; range = 10‐60 mg/day, mean = 50 mg/day (SD = 4.2) 3. placebo arm (randomised n = 20) Duration: 8 weeks Treatment protocol: flexible dosage, gradually titrated up to a maximum of 8 cps/day |
|
| Outcomes |
Timepoints for assessment: at baseline and then at week 2, 4, 6, 8 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: none Declarations of interest among the primary researchers: none The study Gentil 1993, with three arms, is presented separately for the imipramine and clomipramine arms in comparison to placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated [...]", however the sequence generation process is not discussed. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "double‐blind treatment"; "Capsules were in the hospital pharmacy with tablets of the commercially available TCAs or propanteline (placebo) and filled up with lactose. The dose range of propanteline was selected to give mild to moderate peripheral anticholinergic effects". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "double‐blind treatment"; "Capsules were in the hospital pharmacy with tablets of the commercially available TCAs or propanteline (placebo) and filled up with lactose. The dose range of propanteline was selected to give mild to moderate peripheral anticholinergic effects". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 participants left the trial before completing the first 4 weeks of treatment and were replaced. No information provided on incomplete outcome data management. |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes are not pre‐specified. The rating scales employed are reported, however some outcomes were apparently chosen in an arbitrary way (quote: "The sum of scores for ShAS items 32 (’severity of spontaneous attacks with more than three symptoms’), 33 (’severity of limited panic attacks or symptoms’) and 35 (’severity of situational panic attacks’) were taken as an index of the ’severity of panic’. The sum of scores on items 1‐31 and 34 of the ShAS were defined as the ’anxiety symptoms score’"). Quote: "Only the most relevant data will be presented". The number of participants allocated to each arm is not clearly reported in the text, tables or graphs, although it can be derived. |
| Other bias | Low risk | Quote: "this study was not supported by the facturer of the drug tested". |
GSK 1994.
| Methods | Study design: a double‐blind, multicentre, flexible‐dose study, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder Method of diagnosis: SCID Age (years): paroxetine arm mean age = 39.1 (SD = 11.1), alprazolam arm mean age = 39.5 (SD = 12.5) and placebo arm mean age = 39.0 (SD = 11.8) Sex: 146 women and 80 men Location: outpatients,16 centres in the USA Co‐morbidities: excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. paroxetine arm (randomised n = 77) Duration: 10 weeks Treatment protocol: flexible dosage; range = 10‐60 mg/day 2. alprazolam arm (randomised n = 77) Duration: 10 weeks Treatment protocol: flexible dosage; range = 1‐6 mg/day 3. Placebo arm (randomised n = 72) Duration: 10 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and week 3, 4, 10 Outcomes:
|
|
| Notes |
Date of study: November 1992‐April 1994 Funding source: the drug company marketing the drug funded this unpublished study Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All subjects in the ITT population (which included all subjects who received any double blind medication) for whom at least one valid post efficacy evaluation was available were included in the ITT efficacy analysis. All subjects randomized were included in the safety analysis". |
| Selective reporting (reporting bias) | Low risk | All the outcomes are reported in tables. |
| Other bias | High risk | This is an unpublished study funded by the drug company marketing paroxetine. |
Hoehn‐Saric 1993.
| Methods | Study design: 8 weeks, double‐blind, placebo‐controlled outpatient clinical trial, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: SCID Age (years): mean age 38.0 (SD = 9.6) Sex: 16 men, 20 women Location: outpatient department at Johns Hopkins Hospital (Baltimore, Maryland, USA) Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. fluvoxamine arm (randomised n = 25) Duration: 8 weeks Treatment protocol: flexible dosage; range = 100‐300 mg/day, mean 206.8 mg/day 2. placebo arm (randomised n = 25) Duration: 8 weeks Treatment protocol: flexible dosage, mean = 5.6 cps/day |
|
| Outcomes |
Timepoints for assessment: at baseline and then weekly until week 8 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: cps of fluvoxamine or placebo were provided by the drug company marketing the drug Declarations of interest among the primary researchers: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The sequence generation procedure is not discussed. 50 patients were randomised (25 for each group), however only those who were still eligible after the single‐blind phase took the medication. This procedure may have affected the effect of randomisation. The balance between the two arms is not discussed or reported in graphs. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: fluvoxamine group 6/25 (24%); placebo group 7/25 (28%), which are high dropout rates. However, 25 is the number originally allocated to each arm (see above, selection bias). Among the original 50 participants, some (not clear how many) were excluded after a single‐blind phase. 37 participants completed the study, however only those who had complete sets of data (36 participants) were analysed, which seems to be consistent with a 'per protocol' analysis. |
| Selective reporting (reporting bias) | High risk | Quote: " [...] we predicted that treatment with fluvoxamine would be more effective than placebo in reducing the frequency and severity of panic attacks". However, it is not clear which exactly is the primary outcome and how it was assessed. Mean scores and SDs are clearly reported for the baseline assessment (figure 1), but only graphically reported for weekly assessments. |
| Other bias | High risk | Cps of fluvoxamine or placebo were provided by Solvay Co. The role of the funder in planning and conducting the study is not discussed. |
Johnston 1995.
| Methods | Study design: 28 weeks, placebo‐controlled, double‐blind clinical trial, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III agoraphobia Method of diagnosis: Age (years): 18‐70 (mean = 37, SD = 10) Sex: women Location: unclear Co‐morbidities: excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. clomipramine arm (randomised n = 16) Duration: 28 weeks Treatment protocol: flexible/fixed dosage; range = 25‐300 mg/day, mean = 68.3 mg/day (SD = 39.7) 2. clomipramine + CBT arm (randomised n = 17) Duration: 28 weeks Treatment protocol: flexible dosage; range = 25‐300 mg/day, mean = 133.3 mg/day (SD = 58.7) 3. placebo arm (randomised n = 16) Duration: 28 weeks Treatment protocol: flexible/fixed dosage; range = 25‐300 mg/day, mean = 154.41 mg/day (SD = 51.7) 4. placebo + CBT (randomised n = 15) Duration: 28 weeks Treatment protocol: flexible/fixed dosage; range = 25‐300 mg/day, mean = 139.3 mg/day (SD = 73.7) |
|
| Outcomes |
Timepoints for assessment: at baseline, week 1, 2, 3, 4, and then at 4 weekly intervals thereafter for a total of 28 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the drug was supplied by the drug company that produces it and by Health and Welfare Canada Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about the sequence generation is provided. Quote: "random sequential assignment of patients to each of the four groups was carried out". |
| Allocation concealment (selection bias) | Unclear risk | No information is provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participants and the personnel administering the drug are described as blinded. Quote: "the study was double blind for medication status with the principal investigator, therapists and subjects being unaware of whether placebo or clomipramine was being administered to individuals" and "study medications were supplied in coded vials with sealed keys to be consulted in emergency". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors are described as blinded. Quote: "the study was double blind for medication status with the principal investigator, therapists and subjects being unaware of whether placebo or clomipramine was being administered to individuals". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of dropouts is reported and it seems that there were some significant differences between dropouts and participants. Quote: "mean scores on 45 of the 48 outcome and demographic measures were higher for the drop‐out group than for those who completed the clinical trial". |
| Selective reporting (reporting bias) | Unclear risk | Data are only graphically reported (in box and whisker plot) so their interpretation is not easy. The only table reported doesn't specify the differences between clomipramine and placebo. |
| Other bias | High risk | The study was supported by the drug company marketing clomipramine. |
Koszycki 2011.
| Methods | Study design: 12 weeks randomised (individual randomisation), parallel groups, double‐blind, placebo‐controlled, multicentre clinical trial. The "acute phase" lasted 12 weeks. Participants who showed adequate response were eligible to enter a 12‐week extension treatment. | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of diagnosis: psychiatric interview and a Structured Clinical Interview for DSM‐IV (SCID) Age (years): sertraline arm mean age 36.40 (SD = 10.0), placebo arm mean age 35.24 (SD = 9.9), sertraline + SCBT arm mean age 36.22 (SD = 10.9), placebo + SCBT arm mean age 36.80 (SD = 12.2) Sex: 90 men, 161 women Location: outpatient, 15 academic health centres in Canada Co‐morbidities: "co‐morbid depression, generalized anxiety disorder, social phobia, somatization disorder and specific phobia were allowed as long as these conditions were secondary to and not clinically more prominent than the PD with or without agoraphobia" Rescue medication: oxazepam up to 60 mg/week allowed. It was used at least once by the 55.9% of the participants and the weekly mean dose range was 24.8 mg/week (SD = 30.9) to 33.7 mg/week (SD = 18) |
|
| Interventions | Participants were randomly assigned to either: 1. sertraline arm (randomised n = 63) Duration: 12 weeks Treatment protocol: flexible dosage; range = 25‐200 mg/day, mean = 116.1 mg/day (SD = 59.6) 2. sertraline + SCBT arm (randomised n = 61) Duration: 12 weeks Treatment protocol: flexible dosage; range = 25‐200 mg/day, mean = 95.8 mg/day (SD = 57.6) 3. placebo + SCBT arm (randomised n = 65) Duration: 12 weeks Treatment protocol: flexible dosage; mean = 138.3 mg/day (SD=59.5) 4. placebo arm (randomised n = 62) Duration: 12 weeks Treatment protocol: flexible dosage, mean = 138.3 mg/day (SD = 59.5) |
|
| Outcomes |
Timepoints for assessment: at baseline at week 1, 2, 3, 4, 6, 8, 10 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was supported by the drug company marketing sertraline Declarations of interest among the primary researchers: one of the primary researchers declared a conflict of interest with several drug companies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated to one of four groups by a computer generated randomization code [...]". |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators at each site were provided with a sealed envelope that contained the identification of the study drug being administered to the patient. In a medical emergency, the investigator was authorized to break the code for that subject only". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo and sertraline were provided as matching capsules and administered double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome assessments were made by investigators who were blind to allocation of the drug and who were not told whether the patient was assigned to SCBT. Patients were instructed not to divulge their SCBT assignment to the investigators". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: placebo arm (30.6%); sertraline arm (25.4%). Dropout rates are high. Reasons for leaving the study early are apparently balanced between groups, with the exception of adverse effects (9 in placebo arms versus 5 in antidepressant arm). An ITT was performed. Quote: "The mixed model methodology, as opposed to conventional repeated‐measures ANOVA, allows all available observations on each patient to be used without having to use an imputation procedure such as last‐observation carried forward". Only those who had no postbaseline assessment were excluded from the ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Data are poorly reported |
| Other bias | High risk | The study was supported by the drug company marketing sertraline; the role of the funder in planning, conducting and writing the study is not discussed. |
Lecrubier 1997.
| Methods | Study design: 12‐week, randomised (individual randomisation), double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): paroxetine arm range = 19‐66, clomipramine arm range = 19‐57 and placebo arm range = 18‐62 Sex: approximately 60% female for each group Location: outpatient setting, 39 centres in 13 countries (Belgium, Denmark, France, Hungary, Ireland, Israel, Italy, the Netherlands, Norway, Spain, Switzerland, the UK and Yugoslavia (i.e. Serbia, Croatia, Bosnia and Herzegovina, Slovenia, Macedonia and Montenegro at the time of writing; 2018)) Co‐morbidities: excluded Rescue medication: chloral hydrate for night‐time sedation |
|
| Interventions | Participants were randomly assigned to either: 1. paroxetine arm (randomised n = 123) Treatment protocol: flexible dosage; range = 20‐60 mg/day Duration: 12 weeks 2. clomipramine arm (randomised n = 122) Treatment protocol: flexible dosage; range = 50‐150 mg/day Duration: 12 weeks 3. placebo arm (randomised n = 123) Treatment protocol: flexible dosage; range = not stated Duration: 12 weeks |
|
| Outcomes |
Timepoints for assessment: weekly during the placebo run‐in period (3 weeks), on the day of randomisation (baseline) and at the end of weeks 1, 2, 3, 4, 6, 9 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: sponsored by the drug company marketing paroxetine Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" but no further detail is given about the random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate is over 20% in all the groups (and was homogeneous between the groups, around 30% more. They use ITT analysis (defining ITT as "all patients randomised to double blind treatment who provided at least some on‐drug safety and tolerability data). |
| Selective reporting (reporting bias) | Unclear risk | Almost all the outcomes are reported in tables. |
| Other bias | High risk | Sponsored by the drug company marketing paroxetine; the role of the funder in planning, conducting and writing the study is not discussed. |
Liebowitz 2009.
| Methods | Study design: 10 weeks, randomised (individual), parallel groups, double‐blind, placebo‐controlled, multicentre clinical trial | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): venlafaxine ER arm mean age 36 (SD = 12.4) and placebo arm mean age 36.7 (SD = 12.0) Sex: 107 men, 203 women Location: outpatient setting, in 56 sites (7 in Canada and 49 in USA) Co‐morbidities: people with a secondary major depression or GAD were eligible. Any other clinically significant Axis I or Axis II disorders, or HAM‐D score ≥ 18 at baseline were excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. venlafaxine ER arm (randomised n = 175) Duration: 10 weeks Treatment protocol: flexible dosage; range = 37.5 to 225 mg/day 2. placebo arm (randomised n = 168) Duration: 10 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and then at week 1, 2, 3, 4, 6, 8 and 10 Outcomes:
|
|
| Notes |
Date of study: the study was conducted from April 2001‐December 2002 Funding source: drug company marketing the drug is likely to have sponsored the study Declarations of interest among the primary researchers: declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however the process of sequence generation is not clearly reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as double‐blind, however methods for ensuring blindness of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates: venlafaxine arm 55/175 (31.4%); placebo arm 43/168 (25.6%). Dropout rates are high in both arms and reasons for leaving the study early are apparently balanced, according with Figure 1. Quote: "The primary analysis population for efficacy variables was the intent‐to‐treat (ITT) population". However, as reported in Figure 1, the ITT population does not match with participants randomly assigned at baseline. Quote: "Patients in the ITT population were those who had a baseline PAAS evaluation and at least 1 double‐blind, on‐therapy evaluation of the primary efficacy variable during visits 3 to 10 and within 3 days of stopping the study medication before taper". This is consistent with an 'as treated' analysis. In the ITT population imputations were performed with a LOCF approach. |
| Selective reporting (reporting bias) | Low risk | The primary outcome measure is defined as "the percentage of patients free of full‐symptom panic attacks as measured with the Panic and Anticipatory Anxiety Scale (PAAS)", however the precise time point of interest is not clearly specified. All relevant data are clearly reported in the text and tables. |
| Other bias | High risk | Quote: "This clinical trial and analysis were sponsored by Wyeth Research, Collegeville, Pa". No other details on the role of funder in planning and conducting the study are provided. |
Londborg 1998.
| Methods | Study design: multisite, double‐blind, parallel and fixed‐dose design, randomised (individual randomisation) controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R diagnosis of panic disorder with or without agoraphobia Method of diagnosis: SCID (Structured Clinical interview for DSM‐III‐R) Age (years): 18.9‐74.5 (the average age of participants was 38.8 years) Sex: 53% men, 47% women Location: outpatient setting, 7 sites in USA (6 western USA and 1 in West Virginia) Co‐morbidities: participants with a secondary diagnosis of an affective disorder, anxiety states including generalised anxiety disorder, social or simple phobia, obsessive‐compulsive disorder or post‐traumatic stress disorder or personality disorder were permitted to participate Rescue medication: choral hydrate for sleep |
|
| Interventions | Participants were randomly assigned to either: 1. sertraline 50 mg arm (randomised n = 43) Duration: 12 weeks Treatment protocol: fixed dosage 50 mg/day 2. sertraline 100 mg arm (randomised n = 44) Duration: 12 weeks Treatment protocol: fixed dosage 100 mg/day 3. sertraline 200 mg arm (randomised n = 45) Duration: 12 weeks Treatment protocol: fixed dosage 200 mg/day 3. placebo arm (randomised n = 45) Duration: 12 weeks Treatment protocol: fixed dosage, number of tablets not specified |
|
| Outcomes |
Timepoints for assessment: at the end of weeks 1, 2, 3, 4, 6, 8, 10 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: drug company marketing sertraline Declarations of interest among the primary researchers: RW is a Senior Associate Medical Director at the drug company marketing sertraline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned by site, with a blocking factor of four". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the subjects were randomly assigned by site". No further details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "study medication was taken with the evening meal as a single dose of two capsules contained in a blister pack". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information is provided about investigators' blinding condition. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rate is high (> 20%). Quote: "of the 177 safety‐evaluable subjects, 63 (36%) withdrew from the study, 28 due to adverse experiences and 12 because of insufficient clinical response... The difference among the groups was not statistically significant when subjects in the placebo group were compared with pooled subjects taking sertraline (31% and 37%)" The investigators used the LOCF. Quote: "parallel analyses of efficacy parameters were performed both for end‐point with last observation carried forward". |
| Selective reporting (reporting bias) | Low risk | The data related to primary outcomes are reported in the text, in tables and graphs. |
| Other bias | High risk | The study was funded by the drug company marketing sertraline. RW is a Senior Associate Medical Director at the drug company marketing sertraline. |
Lydiard 1993.
| Methods | Study design: 12‐week, placebo‐controlled, parallel groups, individual randomisation, double‐blind study | |
| Participants |
Diagnosis: DSM‐III‐R diagnosis of panic disorder with or without agoraphobia Method of diagnosis: structured interview for DSM‐III‐R Age (years): DMI arm mean age = 38.1 SD = 6.9, placebo arm mean age = 35.1 SD = 1.3 Sex: sex distribution between the 2 arms is unclear Location: primary care setting, South Carolina (USA) Co‐morbidities: excluded Rescue medication: apparently not permitted, but this is not explicit |
|
| Interventions | Participants were randomly assigned to either: 1. desipramine arm (randomised n = 28) Duration: 12 weeks Treatment protocol: flexible dosage; range = 50‐200 mg/day, mean = 177 mg (SD = 81) 2. placebo arm (randomised n = 28) Duration: 12 weeks Treatment protocol: flexible dosage; range = 50‐200 mg/day, mean = 242 mg/day (SD = 54) |
|
| Outcomes |
Timepoints for assessment: at baseline, 8 and 12 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: unclear Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomised", but no information about the random sequence generation is provided. Quote: "the patients were randomly assigned to either DMI or placebo". |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double blind", no further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double blind", no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rate in the DMI group is around 7%, while the dropout rate in the placebo group is 39%, so it is high. Investigators used a data imputation technique. Quote: "we calculated the 12‐week outcome for all patients completing at least 8 weeks' treatment by bringing the last observed value forward, expressing these as 12‐week outcome". |
| Selective reporting (reporting bias) | Low risk | All the outcomes are reported in a table in a clear way. |
| Other bias | Unclear risk | It is unclear whether the study was funded by a drug company marketing desipramine or not. No declaration of interest is mentioned. |
Mavissakalian 1989.
| Methods | Study design: 8 weeks, randomised controlled trial, (individual randomisation), parallel groups | |
| Participants |
Diagnosis: DSM‐III diagnostic criteria for agoraphobia with panic Method of diagnosis: not stated Age (years): M = 33.5 (SD = 9) Sex: 78% female (n = 25), 22% men (n = 7) Location: outpatient setting, USA Co‐morbidities: people with no evidence of primary or current major depression Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine low‐dose (0.5 mg/kg/d) arm (randomised n = 10) Duration: 8 weeks Treatment protocol: weight‐adjusted dosage; the range is not specified 2. imipramine medium‐dose (1.5 mg/kg/d) arm (randomised = 9) Duration: 8 weeks Treatment protocol: weight‐adjusted dosage; the range is not specified 3. imipramine high‐dose (3 mg/kg/d) arm (randomised = 6) Duration: 8 weeks Treatment protocol: weight‐adjusted dosage; the range is not specified 4. placebo arm (randomised = 7) Duration: 8 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Timepoints for assessment: at pretreatment, week 0, week 8 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: the study was supported by grants MH40141 and MH42730 from the National Institute of Mental Health Declarations of interest among the primary researchers: none known |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided about the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical capsules were used. Quote: "Throughout the trial the patients were given consecutively dated vials prepared by the hospital pharmacist, each containing four identical‐looking tablets (composed of placebo or 10, 25 or 75 mg of imipramine hydrochloride)" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as a double blind trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The dropout rate is reported in the text. Quote: "of the 43 patients accepted into the experimental phase of the study following the two‐week single blind placebo phase, 11 (26%) dropped out owing to side effects: three (25%) from the 12 in the medium‐dose group and eight (57%) from the 14 in the high‐dose group. High dropout rate Data imputation was not performed and only completers were considered in the analysis. |
| Selective reporting (reporting bias) | Low risk | The data are clearly reported in a table. |
| Other bias | Low risk | The study was supported by grants MH40141 and MH42730 from the National Institute of Mental Health. |
Michelson 2001.
| Methods | Study design: 12 weeks, randomised (individual randomisation), parallel groups, double‐blind, placebo‐controlled | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of diagnosis: SCID Age (years): mean age in fluoxetine arm 36.5 (SD = 10.3), mean age in placebo arm 34.8 (SD = 9.8) Sex: in fluoxetine arm 48% (n = 43) men, 52% (n = 47) women; in placebo arm 41% (n = 37) men, 59% (n = 53) women (overall number: 80 men and 100 women) Location: outpatients, psychiatric clinics, 9 sites in Europe Co‐morbidities: excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. fluoxetine arm (randomised n = 90) Duration: 12 weeks Treatment protocol: flexible dosage; range = 20‐60 mg/day, mean = 29.8 mg/day (SD is not specified) 2. placebo arm (randomised n = 90) Duration: 12 weeks Treatment protocol: flexible dosage (the number of tablets is not specified) |
|
| Outcomes |
Time points for assessment: at baseline, 6, 12 weeks (endpoint) Outcomes:
|
|
| Notes |
Date of study: not reported in the primary publication Funding source: unclear Declarations of interest among the primary researchers: some authors are employees of the company marketing the drug, others are paid consultants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is reported as randomised, but no information is provided about the random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described just as quote: "double blind trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate is reported in the text. Quote: "among randomised patients, the number of patients reaching the final visit after 12 weeks of fluoxetine or placebo therapy was similar for both groups (fluoxetine n = 75, 83.3%); placebo n = 80 (88.8%). The total number of discontinuations due to adverse effects was similar for both groups (fluoxetine n = 5, 5.5%), (placebo n = 3, 3.3%)... other reasons for discontinuation included lack of efficacy (fluoxetine n = 5, 5.5%), (placebo n = 3, 3.3%)... patients lost to follow up... patient decision... and protocol requirement..." Despite the dropouts the groups still seem comparable. Data imputation was performed (ITT analysis). |
| Selective reporting (reporting bias) | Low risk | The data of all the outcome measures are clearly reported in tables as mean scores and mean changes from baseline. Standard deviations are specified. |
| Other bias | High risk | Sponsorship bias: some study authors are employees of the company marketing the drug, other are paid consultants. |
Nair 1996.
| Methods | Study design: 8 weeks, double‐blind, randomised (individual randomisation), parallel‐group, multicentre trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic Disorder with or without agoraphobia Method of diagnosis: DSM‐III criteria for panic disorder, Sheehan PAAS, CGI Age (years): 18‐65 (mean 34.9) Sex: male (N = 66), female (N = 66) Location: Montreal, Ottawa, Hamilton, Canada, outpatient setting Co‐morbidities: excluded Rescue medication: oxazepam up to 60 mg daily or chloral hydrate up to 2000 daily were permitted both during the placebo run‐in and the first 4 weeks of the double‐blind period. |
|
| Interventions | Participants were randomly assigned to either: 1. fluvoxamine arm (randomised n = 50) Duration: 8 weeks Treatment protocol: flexible dosage range = 50‐300 mg/day, mean = 171.4 mg/day (SD = not specified) 2. imipramine arm (randomised n = 48) Duration: 8 weeks Treatment protocol: flexible dosage; range = 50‐300 mg/day, mean = 164.7 mg/day (SD = not specified) 3. placebo arm (randomised n = 50) Duration: 8 weeks Treatment Ppotocol: flexible dosage; range = 1‐6 cps/day, mean = 4 cps/day (SD = not specified) |
|
| Outcomes |
Timepoints for assessment: at baseline,and at weeks 2, 3, 5, 8 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: grant by Ortho McNeil Ltd Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is provided about the random sequence generation, even though the study is defined as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | No information is provided about the allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " the study medication was in form of identically appearing capsules each containing either placebo, 50 mg of fluoxetine or 50 mg of imipramine". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the study medication was in the form of identically appearing capsules each containing either placebo, 50 mg of fluvoxamine or 50 mg of imipramine". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate is reported precisely. Despite the dropout the groups still seem comparable. Data imputation was performed together with an analysis of completers' data. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by Orto McNeil Ltd; the role of the funder in planning, conducting and writing the study is not discussed. |
Pohl 1989.
| Methods | Study design: 8 weeks, randomised controlled trial, individual randomisation, parallel groups | |
| Participants | Diagnosis: DSM‐III Panic disorder or agoraphobia with panic attacks Method of diagnosis: not stated Age (years): for buspirone, mean = 31.1 (SD = 2.1); for placebo, mean = 31.6 (SD = 2.2); for imipramine, M = 29.2 (SD = 2.2) Sex: for buspirone, 44% women, 56% men; for placebo 50% women, 50% men Location: outpatients, USA Co‐morbidities: excluded Rescue medication: none | |
| Interventions | Participants were randomly assigned to either:
1. buspirone arm (randomised n = 18)
Duration: 8 weeks
Treatment protocol: flexible dosage; range = 10‐60 mg, mean = 29.5 (SD = 4.0)
2. imipramine arm (randomised n = 20)
Duration: 8 weeks
Treatment protocol: flexible dosage; range = 50‐300 mg, mean = 140 (SD = 17.5) 3. placebo arm ( randomised n = 22) Duration: 8 weeks Treatment protocol: flexible |
|
| Outcomes |
Timepoints for assessment: weekly for the first 4 weeks, and biweekly for the last 4 weeks Outcomes:
|
|
| Notes | Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All eligible patients were randomized to 8 weeks of double‐blind treatment with buspirone, imipramine or placebo following an initial 4‐7 days of single blind placebo wash‐out." No further details about randomisation are provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical capsules were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of the assessors is not described even though the trial is described as "double blind". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate |
| Selective reporting (reporting bias) | High risk | The measures of primary outcome are specified in the text (in the "efficacy measures" chapter under the "methods" section) but the results are reported in graphs and not in a table or in the text as numbers |
| Other bias | Unclear risk | No information is provided about a possible sponsorship of the study |
Pollack 1998.
| Methods | Study design: 10 weeks, flexible dose, multicentre trial, random assignment (individual), parallel groups, placebo‐controlled | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder with or without agoraphobia Method of diagnosis: SCID Age (years): mean age in sertraline arm 37.8 (SD = 11.6), mean age in placebo arm 34.9 (SD = 9.6) Sex: 115 women, 63 men Location: outpatient setting, 10 sites, USA and Brazil Co‐morbidities: "patients with comorbid dystimic, personality, or other anxiety disorders could be included if the panic disorder was judged to be the principal diagnosis" Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. sertraline arm (randomised n = 88) Duration: 10 weeks Treatment protocol: flexible dose, range 25‐200 mg/day, mean 118.1 mg/day (SD = 62.9) 2. placebo arm (randomised n = 88) Duration: 10 weeks Treatment protocol: flexible dose, range unknown, mean 147.5 mg/day (SD = 55.5) |
|
| Outcomes |
Timepoints for assessment: at baseline and at weeks 1, 2, 3, 4, 6, 8 and 10 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: supported by the company marketing the drug Declarations of interest among the primary researchers: one of the primary researcher is an employee of the company marketing the drug. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence generation is explained. Quote: "patients were randomly assigned by computer‐generated numbers to 10 weeks of double blind treatment with either sertraline or placebo". |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate is less than 20%. They apparently imputed missing data. Quote: "patients who took at least one dose of double blind medication and completed any additional assessment were included in the analysis for safety and efficacy". |
| Selective reporting (reporting bias) | Low risk | Outcomes are clearly reported in tables. |
| Other bias | High risk | The study was financially supported by the drug company marketing the drug and one of the primary researchers was an employee of the company itself. |
Pollack 2007‐a.
| Methods | Study design: 12 weeks, multicentre, double‐blind, randomised (individual), parallel‐group study | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder (with or without agoraphobia) Method of diagnosis: diagnosis of panic disorder with or without agoraphobia assessed with the modified Mini‐International Neuropsychiatric Interview (MINI) Age (years): placebo arm mean age 35.1 (SD = 9.48), venlafaxine ER 75 mg arm mean age 35.8 (SD = 9.97), venlafaxine ER 225 mg age 37.1 (SD = 11.8) and paroxetine arm mean age 37.5 (SD = 11) Sex: 204 men, 420 women Location: outpatient, 39 sites (19 in Argentina, 13 in Mexico, 6 in Chile and 1 in Costa Rica) Co‐morbidities: people were excluded if they had a DSM‐IV diagnosis of major depressive disorder or generalised anxiety disorder that was considered by the investigator to be primary (i.e. causing a higher degree of distress or impairment than panic disorder) or any other clinically significant Axis I or II disorder that was predominant within 6 months of study initiation. Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. venlafaxine 75 mg arm (randomised n = 163) Duration: 12 weeks Treatment protocol: fixed dosage, 75 mg/day 2. venlafaxine 225 mg arm (randomised n = 167) Duration: 12 weeks Treatment protocol: fixed dosage, 225 mg/day 3. paroxetine 40 mg arm (randomised n = 161) Duration: 12 weeks Treatment protocol: fixed dosage, 40 mg/day 4. Placebo arm (randomised n = 162) Duration: 12 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and then at weeks 1, 2, 3, 4, 6, 8, 10 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the drug company marketing venlafaxine sponsored the trial Declarations of interest among the primary researchers: declared in acknowledgements |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were "randomly divided", however the process of sequence generation is not clearly reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...identically appearing capsules..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...identically appearing capsules..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "statistical analysis on the primary and secondary outcome measures were performed for an ITT population of patients who had at least one post randomisation visit on therapy using LOCF values". |
| Selective reporting (reporting bias) | Unclear risk | Continuous data at endpoint are reported only in graphs. |
| Other bias | High risk | Quote: "Funding for this study was provided by Wyeth Research, Collegeville, Pennsylvania". The role of funder in planning, conducting and writing the study are not provided. |
Pollack 2007‐b.
| Methods | Study design: 12 weeks, multicentre, double‐blind, randomised (individual randomisation), parallel‐group, placebo‐ and comparator‐controlled study | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of diagnosis: Mini International Neuropsychiatric Interview Age (years): venlafaxine 75 mg arm mean age 36.2 (SD = 10.7), venlafaxine 150 mg arm mean age 37.7 (SD = 11.5), paroxetine arm mean age 37.6 (SD = 11.3) Sex: 207 men, 427 women Location: outpatient setting, 71 sites around Europe Co‐morbidities: excluded if DSM‐IV diagnosis of MDD or GAD or any other clinically significant Axis I or Axis II disorder that was considered to be primary. Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. venlafaxine ER 75 mg arm (randomised n = 166) Duration: 12 weeks Treatment protocol: fixed dosage, 75 mg/day 2. venlafaxine ER 150 mg arm (randomised n = 168) Duration: 12 weeks Treatment protocol: fixed dosage; 150 mg/day 2) paroxetine arm (randomised n = 166) Duration: 12 weeks Treatment protocol: fixed dosage; 40 mg/day 3. placebo arm (randomised n = 163) Duration: 12 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and then at weeks 1, 2, 3, 4, 6, 8, 10 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was sponsored by the drug company marketing venlafaxine ER Declarations of interest among the primary researchers: declared in acknowledgements |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however the process of sequence generation is not clearly reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study medication was provided as identical‐appearing capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Study medication was provided as identical appearing capsules and was to be taken once daily with food". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "statistical analysis on the primary and secondary outcome measures were performed for an ITT population of patients who had at least one post randomisation visit on therapy using LOCF values". |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | Quote: "Contract grant sponsor: Wyeth Research". The role of funder in planning, conducting and writing the study are not provided. |
Schweizer 1993.
| Methods | Study design: 8‐week, prospective, double‐blind, flexible‐dose, placebo‐controlled comparison of alprazolam and imipramine, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III agoraphobia with panic disorder, panic disorder with limited phobic avoidance or uncomplicated panic disorder Method of diagnosis: Structured Clinical Interview for DSM‐III, a medical history and a review of systems, physical examination and laboratory assessments Age (years): 19‐57, mean 33 (SD = 7) Sex: 80 women, 26 men Location: Psychopharmacology Research unit at the University of Pennsylvania and at Pennsylvania Hospital, Philadelphia, USA Co‐morbidities: excluded Rescue medication: not permitted |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine arm (randomised n = 34) Duration: 8 weeks Treatment protocol: flexible dosage; range = 25‐250 mg/day, mean = 152 mg/day (SD = 65) 2. alprazolam arm (randomised n = 37) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1‐10 mg/day, mean = 5.4 mg/day (SD = 2.1) 3. placebo (lactose filler) arm (randomised n = 35) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1‐10 tablets, mean = 7.0 tablets (SD = 2.5) |
|
| Outcomes |
Timepoints for assessment: at weeks 1, 2, 3, 4, 5, 6, and 8 during the acute phase. Then monthly for 6 months until the completion of the 32 weeks of study treatment Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: Upjohn Co. and Public Health Service Grant MH‐08957 Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors declare that participants were "randomly assigned" to the treatments and placebo groups but no further information about the random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients were dispensed identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients were dispensed identical capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study authors clearly report the number and the reasons for dropout. They also specified: "while high attrition rate in the imipramine and the placebo treatment groups posed a problem for the statistical analysis of the various outcome measures, attrition rates themselves constituted an important and independent outcome measure." "Overall differences among the three study treatments in proportions of participants retained in the study over time was highly significant". |
| Selective reporting (reporting bias) | Low risk | All the outcomes are represented with results in tables and graphs. |
| Other bias | High risk | Sponsored by Upjohn Co; the role of the funder in planning, conducting and writing the study is not discussed. |
Sharp 1996.
| Methods | Study design: randomised (individual randomisation), parallel groups, double‐blind, fixed‐dose design, 12 weeks + 6 months of follow‐up | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): 18‐70, 36.62 in fluvoxamine arm, 42.28 in placebo arm, 37.27 in fluvoxamine + placebo arm, 38.81 in placebo + CBT arm, 33.23 in CBT arm Sex: 115 women, 32 men Location: general practice/primary care, Scotland, UK Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1) fluvoxamine arm (randomised n = 36) Treatment protocol: fixed dose, range 50‐150 mg/day, mean = 150 mg/day Duration: 12 weeks 2. placebo arm (randomised n = 37) Treatment protocol: fixed dose; range not stated Duration: 12 weeks 3. fluvoxamine + CBT ( randomised n = 38) Treatment protocol: fixed dose; 150 mg/day Duration: 12 weeks 4. placebo + CBT arm (randomised n = 36) Treatment protocol: fixed dose; range not stated Duration: 12 weeks (5) CBT arm (randomised n = 43) Treatment protocol: 30‐60‐min sessions Duration: 12 weeks |
|
| Outcomes |
Timepoints for assessment: at baseline and at weeks 1, 2, 4, 6, 8, 10 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: funded by the company marketing the drug Declarations of interest among the primary researchers: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further information about random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information about the allocation concealment is provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The active and the placebo tablets seem to be identical. Quote: "medication was supplied in 50 mg tablets, patients receiving placebo were given the equivalent number of tablets at each appointment, thus maintaining the double blind status". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was an independent assessor monitoring the data collection. Quote: "JA acted as independent monitor; data collected were monitored at monthly intervals throughout the duration of study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate is around 19% in the fluvoxamine group and around 24% in the placebo group. It is not clear whether missing data were imputed. |
| Selective reporting (reporting bias) | Unclear risk | Scores of the scales used for the treatment evaluation are poorly reported. |
| Other bias | High risk | Funded by the company marketing the drug. |
Sheehan 1990.
| Methods | Study design: 8‐week, double‐blind, placebo‐controlled study, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III panic disorder with or without agoraphobia Method of diagnosis: SCID‐UP (Structured Clinical Interview for DSM‐III) Age (years): 38.3 mean age in the buspirone group, 32.6 mean age in imipramine group and 34.5 mean age in placebo group Sex: women: 83.3% in buspirone group, 66.7% in imipramine group and 66.7 in placebo group Location: outpatients, the geographic location is unclear Co‐morbidities: excluded Rescue medication: chloral hydrate for night sedation in all the arms |
|
| Interventions | Participants were randomly assigned to either: 1. buspirone arm (randomised n = 18) Duration: 8 weeks Treatment protocol: flexible dosage; range = 10‐60 mg/day, mean = 57.2 mg/day (SD = 4.8) 2. imipramine arm (randomised n = 19) Duration: 8 weeks Treatment protocol: flexible dosage; range = 25‐300 mg/day, mean = 291.7 mg/day (SD = 14.8) 3. placebo arm (randomised n = 18) Duration: 8 weeks Treatment protocol: flexible dosage; range = 2‐12 capsules, mean = 10.9 (SD = 2.4) |
|
| Outcomes |
Timepoints for assessment: baseline (after placebo washout) and weekly for 8 weeks Outcomes:
|
|
| Notes |
Date of study: unclear Funding source: the study has been financed by the company that manufactures bupropion Declarations of interest among the primary researchers: not explicit |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is given about the generation of the allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither the participants nor the personnel could guess which treatment was given. Quote: "medication was randomized and prepared in identical‐appearing capsules containing 5 mg of buspirone, 25 mg of imipramine, or placebo. Treatment was started with one capsule twice a day and could be increased by one capsule per day every 3 or 4 days during the first 13 days". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of the assessors is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate is reported in the text. Quote: "there were two drop outs after week 4 in the buspirone group and one drop out after baseline in the imipramine group". Data imputation was performed, but they reported the completer analyses more accurately. Quote: "Completer analyses included the 52 patients who completed 8 weeks of treatment. Endpoint analyses included the 54 patients who completed at least 1 week of treatment, with the last observation carried forward for the two dropouts. Because these analyses were nearly identical, the more conservative completer analyses are presented and only brief reference is made to endpoint analyses". |
| Selective reporting (reporting bias) | Low risk | The results are clearly reported in a table (table 3) for each scale used for assessing the participants. |
| Other bias | Unclear risk | The study was financed by the company that manufactures bupropion and this can be taken in account when reading the results. Quote: "This study was supported by grant 1922 from the Bristol‐Myers Pharmaceutical Company. The results are based on authors' analysis of the data. The authors thank Mrs. Baker for valuable technical assistance in the preparation of the manuscript." |
Sheehan 2005.
| Methods | Study design: pooled analysis of 3 identical, double‐blind, placebo‐controlled, parallel‐group, individually randomised, 10‐week clinical trials | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: DSM‐IV Age (years): 18‐65, mean 37.6 (SD = 10.22) in paroxetine CR group, 37.8 (SD = 10.61) in placebo group Sex: 356 men, 543 women Location: USA and Canada, outpatient setting Co‐morbidities: inclusion of people with secondary Axis I disorders Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. paroxetine CR arm (randomised n = 444) Duration: 10 weeks Treatment protocol: flexible dosage; range = 12.5‐75 mg/day, mean = 50 mg/day (SD = not specified) 2. Placebo arm (randomised n = 445) Duration: 10 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and at weekly and bi‐weekly intervals Outcomes:
|
|
| Notes |
Date of study: November 1996‐September 1997 Funding source: the studies were sponsored by the company marketing paroxetine CR Declarations of interest among the primary researchers: the study author declares to have financial associations with many companies that produce psychoactive pharmaceutical agents |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The studies are described as "randomised", but no information about the random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The studies are described as "double blind", no other information is provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information is provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of dropouts and the reasons of withdrawals are clearly reported. The study authors use the data imputation. Quote: "Efficacy and safety analysis were carried out on the modified intention‐to‐treat (ITT) population, defined as all patients who were randomly assigned to treatment, received at least 1 dose of study medication, and had at least 1 postbaseline assessment". |
| Selective reporting (reporting bias) | Low risk | The results of the primary and secondary efficacy outcomes are reported in tables and graphs. |
| Other bias | High risk | The studies were sponsored by the company marketing paroxetine CR; the role of the funder in planning, conducting and writing the study is not discussed. |
Sheikh 2000.
| Methods | Study design: 8 weeks, randomised (individual randomisation), parallel groups, double‐blind, placebo‐controlled, flexible‐dose design | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: SCID‐UP (Structured Clinical Interview for DSM‐III‐R‐Patient Version) and MMSE (Mini mental state Exam) Age (years): 55‐73, mean = 61.24 SD = 5.27 Sex: 23 women, 2 men Location: Palo Alto Veterans Administration Hospital, Palo Alto, California Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine arm (randomised n = 10) Duration: 8 weeks Treatment protocol: flexible dosage; range = 10‐200 mg/day, mean = 77.5 mg/day (SD = 59.4) 2. alprazolam arm (randomised n = 8) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1‐6 mg/day, mean = 2.87 mg/day (SD = 1.66) 3. placebo arm (randomised n = 7) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1‐10 cps |
|
| Outcomes |
Timepoints for assessment: weeks 1, 2, 3, 4, 6, 8 (endpoint) and at withdrawal (week 10) Outcomes:
|
|
| Notes |
Date of study: 1988‐1990 Funding source: medications provided by UpJohn Company Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomised" but no further details are given about the random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "medication for this double blinding protocol were provided by Upjohn Co. in the form of identical looking capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "medication for this double blinding protocol were provided by Upjohn Co. in the form of identical looking capsules". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "this is a pilot study compromised by small sample size and six of seven of the seven subjects in the placebo groups withdrawing early in the treatment course. This limitation prevent statistical analysis between the three groups". |
| Selective reporting (reporting bias) | Low risk | Mean scores at baseline and endpoint are given for all the outcomes in the text. The actual number of participants§ evaluated at the endpoint is given only for the placebo group. |
| Other bias | High risk | Medications provided by UpJohn Company (which is the company marketing alprazolam). It is unclear whether the primary investigators have any conflict of interest. |
Stahl 2003‐cit.
| Methods | Study design: 10 weeks, randomised (individual randomisation), double‐blind, parallel‐group, flexible‐dose, placebo‐controlled, multicentre study | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of diagnosis: DSM‐IV criteria Age (years): 18‐80, M = 38.5 (SD 12.9) for placebo group, M = 37.5 (SD 11.6) for escitalopram group, M = 36.9 (SD 10.2) for citalopram group Sex: 209 women, 157 men (randomised) Location: outpatients, 36 centres, USA Co‐morbidities: excluded Rescue medication: zolpidem as needed for sleep |
|
| Interventions | Participants were randomly assigned to either: 1. escitalopram arm (randomised n = 129, safety population n = 128) Duration: 10 weeks Treatment protocol: flexible dosage; range = 5‐20 mg, mean = 10.8 mg (no SD specified) 2. citalopram arm (randomised n = 126, safety population n = 119) Duration: 10 weeks Treatment protocol: flexible dosage; range = 10‐40 mg, mean = 21.3 mg (no SD specified) 3. placebo arm Duration: 10 weeks (randomised n = 125, safety population n = 119) Treatment protocol: flexible dosage 1‐4 tablets |
|
| Outcomes |
Timepoints for assessment: at study entry (screening at days 1 and 8 of the lead‐in period), at baseline (day of randomisation) and at the end of weeks 1, 2, 4, 6, 8, and 10 of the double‐blind treatment Outcomes:
|
|
| Notes |
Date of study: 30 September 1999‐5 July 2001 Funding source: Asahi, AstraZeneca, Bayer, Boeringer‐Ingelheim, Bristol‐meyers Squibb, Cephalon, Cypress Bioscience, Oierre Fabre, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Lundbeck, Organon, Parke‐Davis, Pfizer, Pharmacia, Sanofi‐Synthelabo, Solvay, Watson, Wyeth, Yamanouchi Declarations of interest among the primary researchers: one of the study authors received grant/research support from Asahi, AstraZeneca, Bayer, Boeringer‐Ingelheim, Bristol‐meyers Squibb, Cephalon, Cypress Bioscience, Oierre Fabre, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Lundbeck, Organon, Parke‐Davis, Pfizer, Pharmacia, Sanofi‐Synthelabo, Solvay, Watson, Wyeth, Yamanouchi, while the other two are employees of Forest Laboratories The study Stahl 2003, with three arms including two antidepressants of the same class, is presented separately for the citalopram and escitalopram arms in comparison to placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is defined as randomised but no further details on sequence generation are provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The ITT set consisted of 351 patients, 125 treated with escitalopram, 112 with citalopram and 114 with placebo". Dropout rates were different between treatment groups (escitalopram = 24.2%, citalopram = 31.9%) |
| Selective reporting (reporting bias) | Low risk | The results are clearly reported in tables for each scale used for assessing the participants, both at baseline and at endpoint. |
| Other bias | High risk | Sponsorship bias: all the study authors were employed by the drug company marketing the drug. |
Stahl 2003‐esc.
| Methods | Study design: 10 weeks, randomised (individual randomisation), double‐blind, parallel‐group, flexible‐dose, placebo‐controlled, multicentre study | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of Diagnosis: DSM‐IV criteria Age (years): 18‐80 years, M = 38.5 (SD 12.9) for placebo group, M = 37.5 (SD 11.6) for escitalopram group, M = 36.9 (SD 10.2) for citalopram group Sex: 209 women, 157 men (randomised) Location: outpatients, 36 USA centres Co‐morbidities: excluded Rescue medication: zolpidem as needed for sleep |
|
| Interventions | Participants were randomly assigned to either: 1. escitalopram arm (randomised n = 129, safety population n = 128) Duration: 10 weeks Treatment protocol: flexible dosage; range = 5‐20 mg, mean = 10.8 mg (no SD specified) 2. citalopram arm (randomised n = 126, safety population n = 119) Duration: 10 weeks Treatment protocol: flexible dosage; range = 10‐40 mg, mean = 21.3 mg (no SD specified) 3. Placebo arm Duration: 10 weeks (randomised n = 125 safety, population n = 119) Treatment protocol: flexible dosage 1‐4 tablets |
|
| Outcomes |
Timepoints for assessment: at study entry (screening at days 1 and 8 of the lead‐in period), at baseline (day of randomisation) and at the end of weeks 1,2,4,6,8, and 10 of the double blind treatment Outcomes:
|
|
| Notes |
Date of study: 30 September 1999‐5 July 2001 Funding source: Asahi, AstraZeneca, Bayer, Boeringer‐Ingelheim, Bristol‐meyers Squibb, Cephalon, Cypress Bioscience, Oierre Fabre, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Lundbeck, Organon, Parke‐Davis, Pfizer, Pharmacia, Sanofi‐Synthelabo, Solvay, Watson, Wyeth, Yamanouchi Declarations of interest among the primary researchers: one of the study authors received grant/research support from Asahi, AstraZeneca, Bayer, Boeringer‐Ingelheim, Bristol‐meyers Squibb, Cephalon, Cypress Bioscience, Oierre Fabre, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Lundbeck, Organon, Parke‐Davis, Pfizer, Pharmacia, Sanofi‐Synthelabo, Solvay, Watson, Wyeth, Yamanouchi, while the other two are employees of Forest Laboratories. The study Stahl 2003, with three arms including two antidepressants of the same class, is presented separately for the citalopram and escitalopram arms in comparison to placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is defined as randomised but no further details on sequence generation are provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The ITT set consisted of 351 patients, 125 treated with escitalopram, 112 with citalopram and 114 with placebo". Dropout rates were different between treatment groups (escitalopram = 24.2%, citalopram = 31.9%). |
| Selective reporting (reporting bias) | Low risk | The results are clearly reported in tables for each scale used for assessing the participants, both at baseline and at endpoint. |
| Other bias | High risk | Sponsorship bias: all the study authors were employed by the drug company marketing the drug. |
Taylor 1990.
| Methods | Study design: 8 weeks, randomised (individual), parallel groups, double‐blind, placebo‐controlled | |
| Participants |
Diagnosis: DSM‐III panic disorder Method of diagnosis: SCID‐UP Age (years): imipramine arm mean age 34.1, alprazolam arm mean age 35.0 and placebo arm mean age 34.9 Sex: 21 men, 58 women Location: Stanford Anxiety Disorders Clinic (USA) Co‐morbidities: only people with MDD, if secondary to the diagnosis of panic disorder, were included Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine arm (randomised n = 27) Treatment protocol: flexible dosage; range = 30‐270 mg/day , mean = 147 mg/day (SD = not specified) Duration: 8 weeks 2. alprazolam arm (randomised n = 26) Treatment protocol: flexible dosage; range = 1‐8 mg/day, mean = 3.7 mg/day (SD = not specified) Duration: 8 weeks 3. placebo arm (randomised n = 26) Treatment protocol: flexible dosage; range = 1‐10 tablets, mean = 6.8 tablets (SD = not specified) Duration: 8 weeks |
|
| Outcomes |
Timepoints for assessment: baseline and weekly until week 8 Outcomes:
|
|
| Notes |
Date of study: not reported Funding source: sponsored by the company marketing alprazolam Declarations of interest among the primary researchers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised", but no further information about the random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double blind": no further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only, unequal dropout rate (alprazolam: 8%, imipramine: 19%) |
| Selective reporting (reporting bias) | High risk | Almost all the efficacy outcome measures described in the methods are reported in the results, but data are incomplete (standard deviations are not always presented). Furthermore, SAFTEE event form is not reported. |
| Other bias | High risk | Sponsored by the company marketing alprazolam |
Tsutsui 1997.
| Methods | Study design: 12 weeks, randomised (cluster randomisation), parallel design, placebo‐controlled, double‐blind | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder Method of diagnosis: not specified Age (years): some participants > 65, range unclear Sex: they show the ratio of gender, however, it is not for the randomised population, but for the population included in the analysis. Location: inpatient, multicentre trial all over Japan Co‐morbidities: excluded Rescue medication: lorazepam |
|
| Interventions | Participants were randomly assigned to either: 1. sertraline low‐dose arm (randomised n = 59) Duration: 12 weeks Treatment protocol: fixed dosage; 75 mg/day 2. sertraline high‐dose arm (randomised n = 54) Duration: 12 weeks Treatment protocol: fixed dosage; 150 mg/day 3. placebo arm randomised n = 56) Duration: 12 weeks Treatment Protocol: fixed dosage; number of tablets not specified |
|
| Outcomes |
Timepoints for assessment: baseline and at 12 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: unclear Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation. The method is not specified. |
| Allocation concealment (selection bias) | Low risk | An independent researcher randomly allocated participants. He passed identical tablets to clinician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participants and the physician were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts from analysis were over 20%, no imputation for missing data was performed. |
| Selective reporting (reporting bias) | Unclear risk | There is no protocol of this study, so we cannot decide the selective reporting. |
| Other bias | Unclear risk | Researcher conflicts of interest are unclear. |
Tsutsui 2000a.
| Methods | Study design: 8 weeks, randomised controlled trial (cluster randomisation), parallel design, double‐blind | |
| Participants |
Diagnosis: DSM‐IV panic disorder Method of diagnosis: not stated Age (years): inclusion criteria included 65 years. They showed the age range of population included into their analysis. It was from 18‐60, however, this is not the age range of population randomised. So we cannot decide if randomised population included 65 years persons = “unclear”. Sex: They show the ratio of gender, however, it is not for the randomised population, but for the population included in the analysis Location: in and outpatient setting, all over Japan. (multicentre trial) Co‐morbidities: excluded Rescue medication: lorazepam, zopiclone, brotizolam, lormetazepam, rilmazafone |
|
| Interventions | Participants were randomly assigned to either: 1. paroxetine arm (randomised n = 87) Duration: 8 weeks Treatment protocol: fixed dosage 30 mg/day 2. placebo arm (randomised n = 84) Duration: 8 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Timepoints for assessment: at baseline, at 8 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was sponsored by the company marketing the drug Declarations of interest among the primary researchers: conflict of interest among primary researchers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation trial. No further details are provided about the random sequence generation. |
| Allocation concealment (selection bias) | Low risk | An independent researcher randomly allocated participant. He passed identical tablets to clinician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participants and the personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether the researchers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts from analysis were over 20%, no imputation for missing data was performed. |
| Selective reporting (reporting bias) | Unclear risk | There is no protocol of this study, so we cannot decide the selective reporting. |
| Other bias | High risk | The study was sponsored by the company marketing the drug. Conflict of interest among primary researchers. |
Tsutsui 2000b.
| Methods | Study design: 8 weeks, randomised (cluster randomisation), parallel design, placebo‐controlled, double‐blind trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder Method of diagnosis: not stated Age (years): range 18‐72 Sex: distribution of gender in randomised population not reported Location: in and outpatient setting, all over Japan Co‐morbidities: excluded Rescue medication: lorazepam |
|
| Interventions | Participants were randomly assigned to either: 1. paroxetine low‐dose arm (randomised n = 38) Treatment protocol: fixed dosage 20 mg/day Duration: 8 weeks 2. paroxetine high‐dose arm (randomised n = 45) Treatment protocol: fixed dosage 30 mg/day Duration: 8 weeks 3. placebo arm (randomised n = 37) Treatment protocol: fixed dosage Duration: 8 weeks |
|
| Outcomes |
Timepoints for assessment: baseline and 8 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was sponsored by the company marketing the drug Declarations of interest among the primary researchers: conflict of interest among the primary researchers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation. No further details are provided about the random sequence generation. |
| Allocation concealment (selection bias) | Low risk | An independent researcher randomly allocated participants. He passed identical tablets to clinician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and the physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether the assessors were blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts from analysis were over 20%. ITT analysis was used, but the method of imputation was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | There is no protocol of this study, so we cannot decide the selective reporting. |
| Other bias | High risk | The study was sponsored by the company marketing the drug. Conflict of interest among the primary researchers. |
Uhlenhuth 1989.
| Methods | Study design: randomised (individual), parallel groups, double‐blind, placebo‐controlled | |
| Participants |
Diagnosis: DSM‐III criteria for panic disorder or agoraphobia Method of diagnosis: SCID‐UP, 1983 version Age (years): mean 31.54 years (SD = 7.12) Sex: 58% women Location: Anxiety Disorders Clinic of the University of Chicago, USA Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine arm (randomised n = 20) Duration: 8 weeks Treatment protocol: fixed dosage; range = 75‐225 mg/day, mean = 225 mg/day 2. alprazolam 2 mg arm (randomised n = 20) Duration: 8 weeks Treatment protocol: fixed dosage; range = 1‐2 mg/day, mean = 2 mg/day 3. alprazolam 6 mg arm (randomised n = 21) Duration: 8 weeks Treatment protocol: fixed dosage; range = 1‐6 mg/day, mean = 6 mg/day 3. placebo arm (randomised n = 20) Duration: 8 weeks Treatment protocol: fixed dosage, 8 cps/day |
|
| Outcomes |
Timepoints for assessment: at 1, 2, 3, 4, 6 and 8 weeks (endpoint) Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was financially supported by the company marketing alprazolam Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomised", but no further information about the random sequence generation is provided. Quote: "Eighty‐one patients were assigned at random." |
| Allocation concealment (selection bias) | Unclear risk | No information is provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all patients received two identical‐appearing capsules four times daily" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all patients received two identical‐appearing capsules four times daily" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "two sets of outcome analysis were employed; one included all 81 patients who entered treatment, and the other included only the 63 patients who completed at least 4 weeks of treatment. Both sets of analysis presented here were based on the final (last available) clinical score for each patient (endpoint analysis). Patterns of dropout by treatment were analysed by survival analysis using the actuarial life table method." |
| Selective reporting (reporting bias) | Low risk | All the outcomes are represented with data in tables both for the entire sample of participants who were randomised and for those who completed 4 weeks of treatment. |
| Other bias | High risk | The study was financially supported by the company marketing alprazolam. |
Van Vliet 1993.
| Methods | Study design: 12‐week, double‐blind, placebo‐controlled, individual randomisation, parallel groups | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: SCL‐90 Age (years): 26‐49 (mean = 32 SD = 6.4) Sex: 27 women, 3 men Location: outpatient clinic of the department of Biological Psychiatry of the University Hospital in Utrecht, Netherlands Co‐morbidities: excluded Rescue medication: oxazepam to a maximum of 30 mg daily, if required |
|
| Interventions | Participants were randomly assigned to either: 1. brofaromine arm (randomised n = 15) Duration: 12 weeks Treatment protocol: flexible dosage; range = 50‐150 mg/day, mean = not stated (SD = not stated) 2. placebo arm (randomised n = 14) Duration: 12 weeks Treatment protocol: flexible dosage; range = not stated, mean = not stated (SD = not stated) |
|
| Outcomes |
Timepoints for assessment: weekly for 12 weeks (some outcomes were evaluated at the baseline and at the endpoint only) Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: none declared Declarations of interest among the primary researchers: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of the two treatment groups". No further details are provided. The number of participants randomised per arm is unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Procedures for ensuring blinding are not described in the paper |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The only information reported about dropout is that one participant in the placebo group was withdrawn from the study at week 8 because of lack of efficacy. Other reasons for withdrawal are not discussed. Thus it is not clear whether the 2 groups are still comparable or not after the dropout. Data imputation is not clearly discussed, however apparently only completers were analysed (consistent with 'per protocol analysis'). |
| Selective reporting (reporting bias) | High risk | The measures of primary outcome are not clearly specified and mean scores of the scales are graphically reported in figures and only partially reported in the text. |
| Other bias | Low risk | It is unlikely that sponsorship bias could have influenced the results. quote: "The authors wish to thank Mrs M de Wol˜Ferdinandusse, director of the Dutch Foundation of Phobic Disorders, and the Laboratory of Biological Psychiatry of the University Hospital Utrecht, head Mr A Klompmakers" |
Versiani 2002.
| Methods | Study design: 8 weeks, multicentre, placebo‐controlled, randomised (individual) parallel‐group, double‐blind clinical trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): mean age in reboxetine arm 36.5 (SD = 10.4), mean age in placebo arm 35.1 (SD = 10.9) Sex: 50 women, 25 men Location: Brazil and Italy Co‐morbidities: excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. reboxetine arm (randomised n = 42) Duration: 8 weeks Treatment protocol: flexible dosage; range = 2‐8 mg/day 2. placebo arm (randomised n = 40) Duration: 8 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Timepoints for assessment: weekly for 8 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: not specified Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" but not further information is given about the random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts. Quote: "a last observation carried forward analysis was conducted and included all patients who received at least 3 weeks of treatment". |
| Selective reporting (reporting bias) | Unclear risk | The outcomes are reported in the graphs and in the text. For some data they don't specify the SD. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Wade 1997.
| Methods | Study design: 8 weeks, randomised (individual), double‐blind, placebo‐ and clomipramine‐controlled, multicentre, flexible dose within a fixed‐dose range, parallel‐group, comparative study | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder Method of diagnosis: CAS Age (years): 18‐65 (mean age 38) Sex: 142 men, 333 women (70% of the whole sample) Location: 22 centres in 4 countries (Finland, Sweden, Netherlands, UK) Co‐morbidities: excluded Rescue medication: oxazepam up to a maximum daily dose of 30 mg/day during the screening week, and up to a maximum dose of 20 mg/day during the first 2 weeks of the studied treatment |
|
| Interventions | Participants were randomly assigned to either: 1. citalopram 10‐15 arm (randomised n = 97) Duration: 8 weeks Treatment protocol: flexible dosage in a fixed range; range = 10‐15mg/day 2. citalopram 20‐30 arm (randomised n = 95) Duration: 8 weeks Treatment protocol: flexible dosage in a fixed range; range = 20‐30 mg/day 3. citalopram 40‐60 arm (randomised n = 89) Duration: 8 weeks Treatment protocol: flexible dosage in a fixed range; range = 40‐60 mg/day 4. clomipramine arm (randomised n = 98) Duration: 8 weeks Treatment protocol: flexible dosage in a fixed range; range = 60‐90 mg/day (5) placebo arm (randomised n = 96) Duration: 8 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Timepoints for assessment: at baseline and at week 1,2,4,6,8 Outcomes:
|
|
| Notes |
Date of study: unclear Funding source: unclear Declarations of interest among the primary researchers: 1 study author is an employee of the drug company marketing citalopram |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is defined as "randomised" but no information about the random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is defined as "double blind" but no further information is provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate is accurately reported in the text and in the table (Table 1) and after the dropouts it seems like the groups could still be comparable. Quote: "a total of 115 patients from this population failed to complete the study. There were no statistically significant differences between the total number of discontinuations in each group". Study authors use data imputation. Quote "the primary analysis of efficacy was based upon the relative number of responding patients at Week 8 for the ITT population and by use of the last observation carried forward (LOCF)" |
| Selective reporting (reporting bias) | High risk | Data are only partially reported in tables; (main outcome responders are reported in a graph, so numbers can't be clearly interpreted). |
| Other bias | Unclear risk | One of the study authors' affiliations refers to Lundbeck. |
BDI: Beck Depression Inventory; CAS: Clinical Anxiety Scale; CBT: cognitive behavioural therapy; CGI: Clinical Global Impression Scale; CGI‐I: Clinical Global Impression ‐ Improvement; CGI‐S: Clinical Global Impression Severity of Illness; CNS: central nervous system; cps: capsules; CR: controlled release; DPAI: Daily Panic Attack Inventory; DSM: Diagnostic and Statistical Manual of Mental Disorders; ER: extended release; FQ: Fear Questionnaire; GAD: Generalized Anxiety Disorder; HAMA: Hamilton Anxiety Rating Scale; HDRS: Hamilton Depression Rating Scale; ITT: intention‐to‐treat; LOCF: last observation carried forward; MADRS: Montgomery Asberg Depression Rating Scale; MDD: Major Depressive Disorder; n: number; PAAS: Panic and Anticipatory Anxiety Scale; PDSS: Panic Disorder Severity Scale; PGE: Patient Global Evaluation; PGI: Patient Global Impression; PGI‐P: Patient Rated Global Improvement Scale; Q‐LES‐Q: Quality of Life Enjoyment and Satisfaction Questionnaire; SAFTEE: Systematic Assessment for treatment emergent events; SCBT: self‐administered cognitive behavior therapy; SCID: Structured Clinical Interview for DSM; SCID‐UP: Structured Clinical Interview for DSM, update; SD: standard deviation; SDS: Sheehan Disability Scale; ShAS: Sheehan Anxiety Scales; STAI: State Trait Anxiety Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balon 1993 | Study design (panicogenic) |
| Dusseldorp 2007 | Study design (not double blind) |
| Evans 1986 | Combined therapy with psychotherapy |
| Fahy 1992 | Participants were younger than 18 years |
| GlaxoSmithKline 2002 | Regular use of benzodiazepines was allowed, so treatment with antidepressant cannot be considered as monotherapy |
| Green 1988 | Study design (not randomised) |
| Hoffart 1993 | Participants were not primarily diagnosed with panic disorder |
| Ito 1995 | Participants were younger than 18 years |
| Kahn 1987 | Participants were diagnosed with anxiety disorders including panic disorder, but the randomization was not stratified by diagnosis |
| Kamijima 2005 | Participants were not primarily diagnosed with panic disorder |
| Kawashima 2012 | Study design |
| Keller 1993 | Participants were not primarily diagnosed with panic disorder |
| Klerman 1990 | Participants were not primarily diagnosed with panic disorder |
| Modigh 1992 | Regular use of benzodiazepines was allowed, so treatment with antidepressant cannot be considered as monotherapy |
| Oehrberg 1995 | Combined therapy with CBT |
| Pfizer Sertraline Company Study 2003 | Study design (withdrawal) |
| Saxena 1992 | Wrong comparison: brofaromine versus clomipramine (not placebo‐controlled) |
| Versiani 2001 | Participants were diagnosed with anxiety disorders including panic disorder, but the randomisation was not stratified by diagnosis |
| Yang 2005 | Wrong comparison: mirtazapine versus paroxetine (not placebo‐controlled) |
| Yang 2006 | Wrong comparison: citalopram versus paroxetine (not placebo‐controlled) |
| Yeragani 1992 | Study design (panicogenic) |
| Zhao 2003 | Wrong comparison: venlafaxine versus venlafaxine + CBT (not placebo‐controlled) |
| Zitrin 1983 | Other behaviour/supportive therapy used |
CBT: cognitive behaviour therapy
Characteristics of studies awaiting assessment [ordered by study ID]
Anon 1994.
| Methods | Not specified |
| Participants | Not specified |
| Interventions | Imipramine versus diazepam versus placebo versus baclofen versus propranolol |
| Outcomes | Placebo‐controlled study of efficacy of imipramine, diazepam, baclofen and propranolol in people with panic disorder |
| Notes | No full text available |
Diukova 1991.
| Methods | Double‐blind, placebo‐controlled |
| Participants | 69 people with panic attacks |
| Interventions | Amitriptyline (13 participants) versus placebo (number of participants not specified) versus clonazepam (20 participants) |
| Outcomes | Efficacy of amitriptyline, placebo, clonazepam in the treatment of autonomic crises |
| Notes | Waiting for translation from Russian to English |
Mallick 2003.
| Methods | Multicentre, double‐blind, 12‐week, randomised |
| Participants | 577 people with DSM‐IV diagnosis of panic disorder |
| Interventions | Venlafaxine ER versus placebo |
| Outcomes | To characterize patient‐reported functionally and quality of life in panic disorder and compare treatment with venlafaxine ER, paroxetine or placebo |
| Notes | Apparently same content as Musgnung 2005 (to be confirmed by the full text), no full text available |
Musgnung 2005.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | People with DSM‐IV diagnosis of panic disorder (placebo = 157; venlafaxine ER 75 mg = 156; venlafaxine ER 225 mg = 160; paroxetine CR = 151) |
| Interventions | Venlafaxine ER versus paroxetine CR versus placebo |
| Outcomes | To evaluate the short‐term efficacy of venlafaxine ER in treating panic disorder |
| Notes | No full text available |
Papp 1996.
| Methods | Double‐blind, placebo‐controlled |
| Participants | Not specified |
| Interventions | Imipramine versus placebo and CBT |
| Outcomes | Not specified |
| Notes | No full text available |
Pfizer NCT00044772.
| Methods | Double‐blind, placebo‐controlled |
| Participants | People with panic disorder |
| Interventions | Venlafaxine versus paroxetine versus placebo |
| Outcomes | Efficacy, safety, and tolerability of venlafaxine ER capsules in the treatment of outpatients with panic disorder in comparison to those of placebo |
| Notes | No results posted (clinicaltrials.gov checked on 23 June 2017) |
Sandmann 1995.
| Methods | Double‐blind, placebo‐controlled |
| Participants | Not specified |
| Interventions | Fluvoxamine versus placebo |
| Outcomes | Effect of fluvoxamine in panic disorder |
| Notes | No full text available |
Schneier 1998.
| Methods | Double‐blind, placebo‐controlled, 8‐week clinical trial |
| Participants | 102 people with panic disorder |
| Interventions | Fluoxetine versus imipramine versus placebo |
| Outcomes | To assess the utility of application of pattern analysis to a panic disorder clinical trial with a high placebo response rate |
| Notes | No full text available |
Tsutsui 2000.
| Methods | Early phase ii study of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, in the treatment of panic disorder |
| Participants | Not specified |
| Interventions | Paroxetine hydrochloride |
| Outcomes | Not specified |
| Notes | No full text available |
Uhlenhuth 2002.
| Methods | Double‐blind, placebo‐controlled, randomised, 8‐week study |
| Participants | 452 people with DSM‐III‐R panic disorder |
| Interventions | Moclobemide75 mg versus moclobemide 150 mg versus moclobemide 300 mg versus moclobemide 600 mg versus moclobemide 900 mg versus placebo |
| Outcomes | Analysis of a third study |
| Notes | No full text of the principal publication available, merge between data of low‐ dose moclobemide and placebo arms |
Wolkow 1996.
| Methods | Double‐blind, parallel, 12‐week trial |
| Participants | 152 people with DSM‐III diagnosis of panic disorder interventions sertraline versus placebo |
| Interventions | Sertraline versus placebo |
| Outcomes | To compare the efficacy and safety of sertraline and placebo in people with a DSM‐III‐R diagnosis of panic disorder |
| Notes | No full text available |
CBT: cognitive behavioural therapy; CR: controlled release; ER: extended release
Characteristics of ongoing studies [ordered by study ID]
Kruimel 2015.
| Trial name or title | NCT01551225 |
| Methods | Randomised controlled interventional trial, parallel design, double‐blind (subject, caregiver, investigator) |
| Participants | People with irritable bowel syndrome and panic disorder, 18‐70 |
| Interventions | Escitalopram versus placebo (40 participants per arm) |
| Outcomes | Efficacy of treatment |
| Starting date | January 2012 |
| Contact information | Dr. Joanna Kruimel (j.kruimel@mumc.nl), Maastricht University Medical Center, the Netherlands |
| Notes | Estimated completion date: January 2016 (final data collection date for primary outcome measure). clinicaltrials.gov checked in June 2016 reports no results yet. |
Differences between protocol and review
During the course of this review the DSM‐5 was published. We extended the inclusion criteria for studies using DSM‐5 diagnostic criteria. However, we were unable to identify such a study in this review.
The intended primary outcome was originally defined 'rate of response' at the protocol stage. However, in order to have consistency in the direction of forest plots, we extracted data as number of participants who failed to meet improvement criteria, as defined by the authors of each study. Therefore, we renamed the primary outcome 'rate of response' as 'failure to respond'. This allowed us to present results in such a way that the area to the left of the line of no effect always indicated a favourable outcome for antidepressants. We applied the same reasoning to the outcome 'failure to remit', that was originally defined as 'rates of remission' in the protocol; we extracted data on lack of remission from the studies and presented them in forest plots in such a way that the area to the left of the line of no effect always indicated a favourable outcome for antidepressants.
In addition to the planned overall antidepressants versus placebo comparison (stratified by class), we added separate comparisons of individual classes of antidepressants versus placebo, in order also to present data about single drugs belonging to each class of antidepressant versus placebo. This level of information is the most relevant from a clinician's point of view.
We conducted sensitivity analyses only for primary outcomes. Sensitivity excluding studies in which responders were calculated according to a validated imputation method and applying best and worst case scenarios were listed in the planned sensitivity analyses, as they were mentioned in the 'dealing with missing data' section of the protocol; therefore they were also considered and discussed in the Results section.
In the protocol we planned to run meta‐regression analyses in case subgroups were found to be significantly different from one another. This did not happen, therefore it was not necessary to run meta‐regression.
In the protocol we planned to calculate agreement in the selection of studies using Cohen's Kappa (K). However, discordances were less than expected, and were resolved by reaching a consensus through discussion between review authors, so we decided not to include a formal measure of agreement.
The criterion for remission in the included studies was to have zero panic attacks. Considering that we did not find any validated threshold to impute remission from continuous measures that was consistent with this definition, we did not carry out any imputation for remission.
Contributions of authors
GG devised the idea for the review. GG, CB and AC worked on the first draft of the protocol. MK and CB provided suggestions. MC and AC collected the data; IB and CB ran the analyses; MC, AC, FG, GG, MK, GT and TF provided suggestions and input; IB and CB drafted and critically revised the manuscript; all authors reviewed and approved the final version of the review. IB is the guarantor of the review.
Declarations of interest
IB: none MC: none AC is supported by the NIHR Oxford Cognitive Health Clinical Research Facility and was expert witness for Accord Healthcare for a patent issue about quetiapine extended release. FG: none GG: none MK: none GT: none TAF has received lecture fees from Eli Lilly, Janssen, Meiji, MSD, Otsuka, Pfizer and Tanabe‐Mitsubishi, and consultancy fees from Sekisui Chemicals and Takeda Science Foundation. He has received royalties from Igaku‐Shoin and Nihon Bunka Kagaku‐sha publishers. He has received grant or research support from the Japanese Ministry of Education, Science, and Technology, the Japanese Ministry of Health, Labour and Welfare, the Japan Society for the Promotion of Science, the Japan Foundation for Neuroscience and Mental Health, Mochida and Tanabe‐Mitsubishi. He is diplomate of the Academy of Cognitive Therapy. CB: none
New
References
References to studies included in this review
Asnis 2001 {published data only}
- Asnis GM, Hameedi FA, Goddard AW, Potkin SG, Black D, Jameel M, et al. Fluvoxamine in the treatment of panic disorder: a multi‐center, double‐blind, placebo‐controlled study in outpatients. Psychiatry Research 2001;103:1‐14. [DOI] [PubMed] [Google Scholar]
Ballenger 1998 {published and unpublished data}
- Ballenger JC, Wheadon DE, Steiner M, Bushnell W, Gergel IP. Double‐blind, fixed‐dose, placebo‐controlled study of paroxetine in the treatment of panic disorder. American Journal of Psychiatry 1998;155:36‐42. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline. A double‐blind, placebo‐controlled, multicenter study of fixed doses of paroxetine (10, 20 and 40 mg) given as a single oral dose daily, in the treatment of panic disorder. GSK‐Clinical Study Register (www.gskclinicalstudyregister.com) 1994. [No.:MY1047/BRL‐029060/1/CPMS‐120]
Barlow 2000 {published data only}
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive‐behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA 2000;283(19):2529‐36. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Gorman J, Shear MK, Lewin D, Martinez J, Ray S, et al. A comparison of medication side effect reports by panic disorder patients with and without concomitant cognitive behavior therapy. American Journal of Psychiatry 2007;164(2):273‐5. [DOI] [PubMed] [Google Scholar]
- Shear MK, Houck P, Greeno C, Masters S. Emotion‐focused psychotherapy for patients with panic disorder. American Journal of Psychiatry 2001;158(12):1993‐8. [DOI] [PubMed] [Google Scholar]
Bergink 2005 {published data only}
- Bergink V, Westenberg HGM. Metabotropic glutamate II receptor agonists in panic disorder: a double‐blind clinical trial with LY354740. International Clinical Psychopharmacology 2005;20(6):291‐3. [DOI] [PubMed] [Google Scholar]
Black 1993 {published data only}
- Black DW, Wesner R, Bowers W, Gabel J. A comparison of fluvoxamine, cognitive therapy and placebo in the treatment of panic disorder. Archives of General Psychiatry 1993;50:44‐50. [DOI] [PubMed] [Google Scholar]
Bradwejn 2005 {published data only}
- Bradwejn J, Ahokas A, Stein DJ, Salinas E, Emilien G, Whitaker T. Venlafaxine extended‐release capsules in panic disorder: flexible‐dose, double‐blind, placebo‐controlled study. The British Journal of Psychiatry 2005;187:352‐9. [DOI] [PubMed] [Google Scholar]
Broocks 1998 {published data only}
- Bandelow B, Broocks A, George A, Meyer T, Pralle L, Bartmann U, et al. The use of the panic and agoraphobia scale (P & A) in a controlled clinical trial. Pharmacopsychiatry 2000;33:174‐81. [DOI] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, et al. Comparison of aerobic exercise, clomipramine and placebo in the treatment of panic disorder. American Journal of Psychiatry 1998;155:603‐9. [DOI] [PubMed] [Google Scholar]
- Broocks A, Stevinson C. Exercise may help treat panic disorder. Focus on Alternative and Complementary Therapies 1999;4(2):84‐5. [Google Scholar]
Caillard 1999 {published data only}
- Caillard V, Rouillon F, Viel JF, Markabi S, French University Antidepressants Group. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double‐blind controlled study. Acta Psychiatrica Scandinavica 1999;99:51‐8. [DOI] [PubMed] [Google Scholar]
Cassano 1999 {published data only}
- Cassano G, Benkert O, Wade A, Lepola U, Bellodi L, Skoglund L, et al. A multicenter, double‐blind comparison of nefazodone and placebo in the treatment of panic disorder. European Neuropsychopharmacology 1999;9(Suppl 5):S250. [Google Scholar]
CNCPS 1992 {published data only}
- Albus M, Lecrubier Y, Maier W, Buller R, Rosenberg R, Hippius H. Drug treatment of panic disorder: early response to treatment as a predictor of final outcome. Acta Psychiatrica Scandinavica 1990;82(5):359‐65. [DOI] [PubMed] [Google Scholar]
- Albus M, Maier W, Shera D, Bech P. Consistencies and discrepancies in self‐ and observer‐rated anxiety scales. A comparison between the self‐ and observer‐rated Marks‐Sheehan scales. European Archives of Psychiatry and Clinical Neuroscience 1990;240(2):96‐102. [DOI] [PubMed] [Google Scholar]
- Andersch S, Hanson L, Hallstrom T. Panic disorder: a five‐year follow‐up study in 52 patients. European Journal of Psychiatry 1997;11(3):145‐55. [Google Scholar]
- Andersch S, Hetta J. A 15‐year follow‐up study of patients with panic disorder. European Psychiatry 2003;18(8):401‐8. [DOI] [PubMed] [Google Scholar]
- Andersch S, Rosenberg NK, Kullingsjö H, Ottosson JO, Bech P, Bruun‐Hansen J, et al. Efficacy and safety of alprazolam, imipramine and placebo in treating panic disorder. A Scandinavian multicenter study. Acta Psychiatrica Scandinavica. Supplementum 1991;365:18‐27. [DOI] [PubMed] [Google Scholar]
- Berlanga C, Canetti A, Chavez E, Fuente R, Carmen Lara M, Leon C, et al. Pharmacologic treatment of panic disorders: comparative report on the efficacy and safety of alprazolam and imipramine in a controlled study [Tratamiento farmacologico de las crisis de angustia Reporte comparativo de la eficacia y seguridad del alprazolam y la imipramina en un estudio controlado]. Salud Mental 1991;14(1):1‐5. [Google Scholar]
- Buller R, Maier W, Goldenberg IM, Lavori PW, Benkert O. Chronology of panic and avoidance, age of onset in panic disorder, and prediction of treatment response. A report from the Cross‐National Collaborative Panic Study. European Archives of Psychiatry and Clinical Neuroscience 1991;240(3):163‐8. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Toni C, Petracca A, Deltito J, Benkert O, Curtis G, et al. Adverse effects associated with the short‐term treatment of panic disorder with imipramine, alprazolam or placebo. European Neuropsychopharmacology 1994;4(1):47‐53. [DOI] [PubMed] [Google Scholar]
- Cross National Collaborative Panic Study, second phase investigators. Drug treatment of panic disorder. Comparative efficacy of alprazolam, imipramine and placebo. British Journal of Psychiatry 1992;160:191‐202. [DOI] [PubMed] [Google Scholar]
- Curtis GC, Massana J, Udina C, Ayuso JL, Cassano GB, Perugi G. Maintenance drug therapy of panic disorder. Journal of Psychiatry Research 1993;27(Suppl 1):127‐42. [DOI] [PubMed] [Google Scholar]
- Deltito JA, Argyle N, Buller R, Nutzinger D, Ottosson JO, Brandon S, et al. The sequence of improvement of the symptoms encountered in patients with panic disorder. Comprehensive Psychiatry 1991;32(2):120‐9. [DOI] [PubMed] [Google Scholar]
- Deltito JA, Argyle N, Klerman GL. Patients with panic disorder unaccompanied by depression improve with alprazolam and imipramine treatment. Journal of Clinical Psychiatry 1991;52(3):121‐7. [PubMed] [Google Scholar]
- Green MA, Curtis GC. Personality disorders in panic patients: response to termination of antipanic medication. Journal of Personality Disorders 1988;2(4):303‐14. [Google Scholar]
- Katschnig H, Amering M, Stolk JM, Klerman GL, Ballenger JC, Briggs A, et al. Long‐term follow‐up after a drug trial for panic disorder. British Journal of Psychiatry 1995;167(4):487‐94. [DOI] [PubMed] [Google Scholar]
- Klerman GL. Depression and panic anxiety: the effect of depressive co‐morbidity on response to drug treatment of patients with panic disorder and agoraphobia. Journal of Psychiatric Research 1990;24(Suppl 2):27‐41. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Coleman JH, Purpura RP. The design and conduct of the Upjohn Cross‐National Collaborative Panic Study. Psychopharmacology Bulletin 1986;22(1):59‐64. [PubMed] [Google Scholar]
- Lavori PW, Dawson R, Shera D. A multiple imputation strategy for clinical trials with truncation of patient data. Statistics In Medicine 1995;14(17):1913‐25. [DOI] [PubMed] [Google Scholar]
- León CA, Arango MV, Arevalo W, Calvo A, Montoya A, León A. Comparison of the effect of alprazolam, imipramine and placebo in the treatment of panic disorders in Cali, Colombia [Comparacion del efecto del alprazolam, la imipramina y placebo en el tratamiento del trastorno de panico en Cali, Colombia]. Acta Psiquiatrica y Psicologica de America Latina 1990;36(1‐2):59‐72. [PubMed] [Google Scholar]
- Maier W, Albus M, Buller R, Nutzinger D, Shera D, Bech P. Self‐ and observer assessment in anxiolytic drug trials: a comparison of their validity. European Archives of Psychiatry and Clinical Neuroscience 1990;240(2):103‐8. [DOI] [PubMed] [Google Scholar]
- Maier W, Roth SM, Argyle N, Buller R, Lavori P, Brandon S, et al. Avoidance behaviour: a predictor of the efficacy of pharmacotherapy in panic disorder?. European Archives of Psychiatry and Clinical Neuroscience 1991;241(3):151‐8. [DOI] [PubMed] [Google Scholar]
- Maier WA, Roth M, Buller R, Argyle N, Rosenberg R, Sydney B, et al. Agoraphobia in panic disorder: an indicator of the severity of panic disorder or a distinct diagnostic entity?. Psychiatric Annals 1991;21(6):374‐81. [Google Scholar]
- Rifkin A. The sequence of improvement of the symptoms encountered in patients with panic disorder. Comprehensive Psychiatry 1991;32(6):559‐60. [DOI] [PubMed] [Google Scholar]
- Rosenberg NK, Mellergård M, Rosenberg R, Beck P, Ottosson JO. Characteristics of panic disorder patients responding to placebo. Acta Psychiatrica Scandinavica Supplementum 1991;365:33‐8. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Bech P, Mellergård M, Ottosson JO. Alprazolam, imipramine and placebo treatment of panic disorder: predicting therapeutic response. Acta Psychiatrica Scandinavica. Supplementum 1991;365:46‐52. [DOI] [PubMed] [Google Scholar]
- Swoboda H, Amering M, Windhaber J, Katschnig H. The long‐term course of panic disorder‐an 11 year follow‐up. Journal of Anxiety Disorders 2003;17(2):223‐32. [DOI] [PubMed] [Google Scholar]
Den Boer 1990 {published data only}
- Boer JA, Westenberg HGM. Serotonin function in panic disorder: a double blind placebo controlled study with fluvoxamine and ritanserin. Psychopharmacology 1990;102:85‐94. [DOI] [PubMed] [Google Scholar]
- Westenberg HG, Boer JA. Selective monoamine uptake inhibitors and a serotonin antagonist in the treatment of panic disorder. Psychopharmacology Bulletin 1989;25(1):119‐23. [PubMed] [Google Scholar]
Gentil 1993‐clo {published data only}
- Gentil V, Lotufo‐Neto F, Andrade L, Cordas T, Bernik M, Ramos R, et al. Clomipramine, a better reference drug for panic/agoraphobia. I. Effectiveness comparison with imipramine. Journal of Psychopharmacology 1993;7(4):316‐24. [DOI] [PubMed] [Google Scholar]
- Marcourakis T, Gorenstein C, Gentil V. Clomipramine, a better reference drug for panic/agoraphobia. II. Psychomotor and cognitive effects. Journal of Psychopharmacology 1993;7(4):325‐30. [DOI] [PubMed] [Google Scholar]
Gentil 1993‐imi {published data only}
- Gentil V, Lotufo‐Neto F, Andrade L, Cordas T, Bernik M, Ramos R, et al. Clomipramine, a better reference drug for panic/agoraphobia. I. Effectiveness comparison with imipramine. Journal of Psychopharmacology 1993;7(4):316‐24. [DOI] [PubMed] [Google Scholar]
- Marcourakis T, Gorenstein C, Gentil V. Clomipramine, a better reference drug for panic/agoraphobia. II. Psychomotor and cognitive effects. Journal of Psychopharmacology 1993;7(4):325‐30. [DOI] [PubMed] [Google Scholar]
GSK 1994 {unpublished data only}
- GlaxoSmithKline. A double‐blind, multicentered, flexible‐dose study of paroxetine, alprazolam and placebo in the treatment of panic disorder. GSK Clinical Study Register (www.gsk‐clinicalstudyregister.com) 1994/04. [Study No.:MY‐1048/BRL‐029060/1/CPMS‐223(PAR223)]
Hoehn‐Saric 1993 {published data only}
- Hoehn‐Saric R, McLeod DR, Hipsley PA. Effect of fluvoxamine on panic disorder. Journal of Clinical Psychopharmacology 1993;13(5):321‐26. [PubMed] [Google Scholar]
Johnston 1995 {published data only}
- Johnston DG, Troyer IE, Whitsett SF, Dalby T. Clomipramine treatment and behaviour therapy with agoraphobic women. Canadian Journal of Psychiatry 1995;40(4):192‐99. [PubMed] [Google Scholar]
Koszycki 2011 {published data only}
- Bradwejn J, Koszycki D, Segal ZV. Efficacy of self‐administered cognitive behavior therapy and sertraline in panic disorder. 156th Annual Meeting of the American Psychiatric Association. San Francisco CA, May 17‐22 2003. [SP No.115]
- Koszycki D, Taljaard M, Segal Z, Bradwejn J. A randomized trial of sertraline, self‐administered cognitive behavior therapy, and their combination for panic disorder. Psychological Medicine 2011;41:373‐83. [DOI] [PubMed] [Google Scholar]
Lecrubier 1997 {published data only}
- Bakker A, Dyck R, Spinhoven P, Balkom AJLM. Paroxetine, clomipramine and cognitive therapy in the treatment of panic disorder. Journal of Clinical Psychiatry 1999;60:831‐38. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline. A double‐blind placebo‐controlled comparative study of paroxetine and clomipramine in the treatment of panic disorder. GSK‐ClinicalStudy Register (www.gsk‐clinicalstudyregister.com) 1993/11/12. [Study No.: MY‐1036/BRL‐029060/1/CPMS‐187 (PAR 187)]
- Lecrubier Y, Bakker A, Dunbar G, Judge R, Collaborative Paroxetine Panic Study Investigators. A comparison of paroxetine, clomipramine and placebo in the treatment of panic disorder. Acta Psychiatrica Scandinavica 1997;95:145‐52. [DOI] [PubMed] [Google Scholar]
- Spinhoven P, Balkom AJLM. Paroxetine, clomipramine and cognitive therapy in the treatment of panic disorder. American Psychiatric Association Annual Meeting. Toronto, Ontario, Canada, 1998.
Liebowitz 2009 {published data only}
- Liebowitz MR, Asnis G, Mangano R, Tzanis E. A double‐blind, placebo‐controlled, parallel‐group, flexible‐dose study of venlafaxine extended release capsules in adult outpatients with panic disorder. Journal of Clinical Psychiatry 2009;70(4):550‐61. [DOI] [PubMed] [Google Scholar]
Londborg 1998 {published data only}
- Londborg PD, Wolkow R, Smith WT, DuBoff E, England D, Ferguson J, et al. Sertraline in the treatment of panic disorder. A multi‐site, double‐blind, placebo‐controlled, fixed‐dose investigation. The British Journal of Psychiatry 1998;173:54‐60. [DOI] [PubMed] [Google Scholar]
Lydiard 1993 {published data only}
- Lydiard RB, Morton WA, Emmanuel NP, Zealberg JJ, Laraia MT, Stuart GW, et al. Preliminary report: placebo‐controlled, double‐blind study of the clinical and metabolic effects of desipramine in panic disorder. Psychopharmacology Bulletin 1993;29(2):183‐8. [PubMed] [Google Scholar]
Mavissakalian 1989 {published data only}
- Groot CM, Mavissakalian MR. Blood pressure and heart rate response of panic disorder patients receiving imipramine in a dose‐response treatment paradigm. Journal of Clinical Psychopharmacology 1994;14(2):107‐10. [PubMed] [Google Scholar]
- Mavissakalian MR, Perel JM. Imipramine dose‐response relationship in panic disorder with agoraphobia. Archives of General Psychiatry 1989;46:127‐31. [DOI] [PubMed] [Google Scholar]
Michelson 2001 {published data only}
- Lydiard RB, Pollack MH, Judge R, Michelson D, Tamura R. Fluoxetine in panic disorder: a placebo‐controlled study. 10th European College of Neuropsychopharmacology Congress, 13th‐17th September 1997. Vienna, Austria, 1997.
- Michelson D, Allgulander C, Dantendorfer K, Knezevic A, Maierhofer D, Micev V, et al. Efficacy of usual antidepressant dosing regimens of fluoxetine in panic disorder: randomised, placebo‐controlled trial. The British Journal of Psychiatry 2001;179:514‐8. [DOI] [PubMed] [Google Scholar]
Nair 1996 {published data only}
- Bakish D, Hooper CL, Filteau MJ, Charbonneau Y, Fraser G, Lee West D, et al. A double‐blind placebo‐controlled trial comparing fluvoxamine and imipramine in the treatment of panic disorder with or without agoraphobia. Psychopharmacology Bulletin 1996;32(1):135‐41. [PubMed] [Google Scholar]
- Nair NPV, Bakish D, Saxena B, Amin M, Schwartz G, West TEG. Comparison of fluvoxamine, imipramine and placebo in the treatment of outpatients with panic disorder. Anxiety 1996;2:192‐8. [DOI] [PubMed] [Google Scholar]
Pohl 1989 {published data only}
- Pohl R, Balon R, Yeragani VK, Gershon S. Serotoninergic anxiolytics in the treatment of panic disorder: a controlled study with buspirone. Psychopathology 1989;22(Suppl 1):60‐7. [DOI] [PubMed] [Google Scholar]
Pollack 1998 {published data only}
- Baumel B, Bielski R, Carman J, Goodman W, Hegel M, Houck C, et al. Double‐blind comparison of sertraline and placebo in patients with panic disorder. Poster Presentations P‐1o‐14.
- Pohl R, Wolkow RM, Clary CM. Sertraline in the treatment of panic disorder: a double‐blind multicenter study. American Journal of Psychiatry 1998;155:1189‐95. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Otto MW, Worthington JJ, Gus Manfro G, Wolkow R. Sertraline in the treatment of panic disorder: a flexible‐dose multicenter trial. Archives of General Psychiatry 1998;55:1010‐6. [DOI] [PubMed] [Google Scholar]
- Wolkow R, Apter J, Clayton A, Coryell W, Cunningham L, McEntee W, et al. Double‐blind, flexible dose study of sertraline and placebo in patients with panic disorder. Poster Presentations P‐10‐17.
Pollack 2007‐a {published data only}
- Pollack M, Whitaker T, Mangano R, Gao B. A comparison of venlafaxine XR and paroxetine in the treatment of outpatients with panic disorder. ACNP 2004 Annual Meeting S201‐S202.
- Pollack MH, Mangano R, Entsuah R, Tzanis E, Simon NM. A randomized controlled trial of venlafaxine ER and paroxetine in the treatment of outpatients with panic disorder. Psychopharmacology 2007;194:233‐42. [DOI] [PubMed] [Google Scholar]
Pollack 2007‐b {published data only}
- Pollack MH, Lepola U, Koponen H, Simon NM, Worthington JJ, Emilien G, et al. A double‐blind study of the efficacy of venlafaxine extended‐release, paroxetine and placebo in the treatment of panic disorder. Depression and Anxiety 2007;24:1‐14. [DOI] [PubMed] [Google Scholar]
Schweizer 1993 {published data only}
- Rickels K, Schweizer E. Panic disorder: long‐term pharmacotherapy and discontinuation. Journal of Clinical Psychopharmacology 1998;18(6 Suppl 2):12‐8. [DOI] [PubMed] [Google Scholar]
- Rickels K, Schweizer E, Weiss S, Zavodnick S. Maintenance drug treatment for panic disorder. II. Short‐ and long‐term outcome after drug taper. Archives of General Psychiatry 1993;50:61‐8. [DOI] [PubMed] [Google Scholar]
- Schweizer E, Rickels K, Weiss S, Zavodnick S. Maintenance drug treatment of panic disorder I. Results of a prospective, placebo‐controlled comparison of alprazolam and imipramine. Archives of General Psychiatry 1993;50:51‐60. [DOI] [PubMed] [Google Scholar]
Sharp 1996 {published data only}
- Sharp DM, Power KG, Simpson RJ, Swanson V, Anstee JA. Global measures of outcome in a controlled comparison of pharmacological and psychological treatment of panic disorder and agoraphobia in primary care. British Journal of General Practice 1997;47:150‐5. [PMC free article] [PubMed] [Google Scholar]
- Sharp DM, Power KG, Simpson RJ, Swanson V, Moodie E, Anstee JA, et al. Fluvoxamine, placebo and cognitive behaviour therapy used alone and in combination in the treatment of panic disorder and agoraphobia. Journal of Anxiety Disorders 1996;10(4):219‐42. [Google Scholar]
Sheehan 1990 {published data only}
- Sheehan DV, Raj AB, Hartnett Sheehan K, Soto S. Buspirone effective for panic disorder?. Journal of Clinical Psychopharmacology 1990;10(1):3‐11. [PubMed] [Google Scholar]
Sheehan 2005 {published data only}
- GlaxoSmithKline. A double‐blind, placebo‐controlled, flexible‐dosing trial to evaluate the efficacy of controlled‐release paroxetine in the treatment of panic disorder. GSK‐Clinical Study Register (www.gsk‐clinicalstudyregister.com) 1997. [Study No.: 29060/497]
- GlaxoSmithKline. A double‐blind, placebo‐controlled, flexible‐dosing trial to evaluate the efficacy of controlled‐released paroxetine in the treatment of panic disorder. GSK‐Clinical Study Register (www.gsk‐clinicalstudyregister.com) 1997. [Study No.: 29060/495]
- GlaxoSmithKline. A double‐blind, placebo‐controlled, flexible‐dosing trial to evaluate the efficacy of modified‐release paroxetine in the treatment of panic disorder. GSK‐Clinical Study Register (www.gsk‐clinicalstudyregister.com) 1997. [Study No.: 29060‐494]
- Sheehan DV, Burnham DB, Iyengar MK, Perera P. Efficacy and tolerability of controlled‐release paroxetine in the treatment of panic disorder. Journal of Clinical Psychiatry 2005;66:34‐40. [PubMed] [Google Scholar]
Sheikh 2000 {published data only}
- Sheikh JI, Swales PJ. Treatment of panic disorder in older adults: a pilot study comparison of alprazolam, imipramine and placebo. International Journal of Psychiatry in Medicine 2000;29(1):107‐17. [DOI] [PubMed] [Google Scholar]
Stahl 2003‐cit {published and unpublished data}
- Bandelow B, Stein DJ, Dolberg OT, Andersen HF, Baldwin DS. Improvement of quality of life in panic disorder with escitalopram, citalopram, or placebo. Pharmacopsychiatry 2007;40:152‐6. [DOI] [PubMed] [Google Scholar]
- Stahl S, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychiatry 2003;64:1322‐7. [DOI] [PubMed] [Google Scholar]
Stahl 2003‐esc {published and unpublished data}
- Bandelow B, Stein DJ, Dolberg OT, Andersen HF, Baldwin DS. Improvement of quality of life in panic disorder with escitalopram, citalopram, or placebo. Pharmacopsychiatry 2007;40:152‐6. [DOI] [PubMed] [Google Scholar]
- Stahl S, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychiatry 2003;64:1322‐7. [DOI] [PubMed] [Google Scholar]
Taylor 1990 {published data only}
- Clark DB, Taylor B, Roth WT, Hayward C, Ehlers A, Margraf J, et al. Surreptitious drug use by patients in a panic disorder study. American Journal of Psychiatry 1990;147(4):507‐9. [DOI] [PubMed] [Google Scholar]
- Margraf J, Ehlers A, Roth WT, Clark DB, Sheikh J, Agras WS, et al. How "blind" are double‐blind studies?. Journal of Consulting and Clinical Psychology 1991;59(1):184‐7. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Hayward C, King R, Ehlers A, Margraf J, Maddock R, et al. Cardiovascular and symptomatic reduction effects of alprazolam and imipramine in patients with panic disorder: results of a double blind, placebo‐controlled trial. Journal of Clinical Psychopharmacology 1990;10(2):112‐8. [DOI] [PubMed] [Google Scholar]
Tsutsui 1997 {published data only}
- Tsutsui S, Osada H, Muranaka M, Namiki M, Katsura T, Kamijima K, et al. Clinical evaluation of sertraline hydrochloride, a selective serotonin reuptake inhibitor for panic disorder: a double‐blind, placebo‐controlled trial. Journal of Neuropsychopharmacology 1997;19:639‐57. [Google Scholar]
Tsutsui 2000a {published data only}
- Tsutsui S. Clinical evaluation of paroxetine Hcl, a selective serotonin reuptake inhibitor, in the treatment of panic disorder: late phase II double‐blind, parallel group study. Japanese Pharmacology and Therapeutics 2000;28:S271‐94. [Google Scholar]
Tsutsui 2000b {published data only}
- Tsutsui S. Clinical evaluation of paroxetine Hcl, a selective serotonin reuptake inhibitor, in the treatment of panic disorder: phase III double‐blind, parallel group study. Japanese Pharmacology and Therapeutics 2000;28:S295‐314. [Google Scholar]
Uhlenhuth 1989 {published data only}
- Uhlenhuth EH, Matuzas W, Glass RM, Easton C. Response of panic disorder to fixed doses of alprazolam or imipramine. Journal of Affective Disorders 1989;17:261‐70. [DOI] [PubMed] [Google Scholar]
Van Vliet 1993 {published data only}
- Vliet IM, Westenberg HGM, Boer JA. MAO inhibitors in panic disorder: clinical effects of treatment with brofaromine. Psychopharmacology 1993;112:483‐9. [DOI] [PubMed] [Google Scholar]
Versiani 2002 {published data only}
- Versiani M, Cassano G, Perugi G, Benedetti A, Mastalli L, Nardi A, et al. Reboxetine, a selective norepinephrine reuptake inhibitor, is an effective and well tolerated treatment for panic disorder. Journal of Clinical Psychiatry 2002;63(1):31‐7. [DOI] [PubMed] [Google Scholar]
Wade 1997 {published data only}
- Leinonen E, Lepola U, Koponen H, Turtonen J, Wade A, Lehto H. Citalopram controls phobic symptoms in patients with panic disorder: randomized controlled trial. Journal of Psychiatry & Neuroscience 2000;25(1):24‐32. [PMC free article] [PubMed] [Google Scholar]
- Lepola U, Wade AG, Leinonen EV, Koponen HJ, Frazer J, Sjoedin I, et al. A controlled, prospective, 1‐year trial of citalopram in the treatment of panic disorder. Journal of Clinical Psychiatry 1998;59:528‐34. [DOI] [PubMed] [Google Scholar]
- Wade A, Overe KF, Lemming O. Weight monitoring during two long‐term trials of citalopram. European Neuropsychopharmacology 1999;9 Suppl 5:S221. [Google Scholar]
- Wade AG, Lepola U, Koponen HJ, Pedersen V, Pedersen T. The effect of citalopram in panic disorder. The British Journal of Psychiatry 1997;170:549‐53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Balon 1993 {published data only}
- Balon R, Yeragani VK, Pohl R, Merlos B, Sherwood P. Changes in appetite and weight during the pharmacological treatment of patients with panic disorder. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 1993;38:19‐22. [DOI] [PubMed] [Google Scholar]
Dusseldorp 2007 {published data only}
- Dusseldorp E, Spinhoven P, Bakker A, Dyck R, Balkom AJLM. Which panic disorder patients benefit from which treatment: cognitive therapy or antidepressants?. Psychotherapy & Psychosomatics 2007;76(3):154‐61. [DOI] [PubMed] [Google Scholar]
Evans 1986 {published data only}
- Evans L, Kenardy J, Schneider P, Hoey H. Effect of a selective serotonin uptake inhibitor in agoraphobia with panic attacks. A double‐blind comparison ofzimeldine, imipramine and placebo. Acta Psychiatrica Scandinavica 1986;73(1):49‐53. [DOI] [PubMed] [Google Scholar]
Fahy 1992 {published data only}
- Fahy TJ, O'Rourke D, Brophy J, Schazmann W, Sciascia S. Clomipramine and lofepramine in DSM III‐R panic disorder: a placebo controlled trial. Clinical Neuropharmacology 1992;15(1 pt B):94. [DOI] [PubMed] [Google Scholar]
- Fahy TJ, O’Rourke D, Brophy J, Schazmann W, Sciascia S. The Galway Study of Panic Disorder I: clomipramine and lofepramine in DSM‐III‐R panic disorder: a placebo controlled trial. Journal of Affective Disorders 1992;25(1):63‐75. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2002 {unpublished data only}
- GlaxoSmithKline. Clinical comparison of paroxetine and placebo on the symptoms emerging during the taper phase of a chronic benzodiazepine treatment, in patients suffering from a variety of anxiety disorders. GSK‐ Clinical Study Register (www.gsk‐clinicalstudyregister.com) 2002.
Green 1988 {published data only}
- Green MA, Curtis GC. Personality disorders in panic patients: response to termination of antipanic medication. Journal of Personality Disorders 1988;2(4):303‐14. [Google Scholar]
Hoffart 1993 {published data only}
- Hoffart A, Due Madsen J, Lande B, Gude T, Bille H, Torgersen S. Clomipramine in the treatment of agoraphobic in patients resistant to behavioral therapy. Journal of Clinical Psychiatry 1993;54(12):481‐7. [PubMed] [Google Scholar]
Ito 1995 {published data only}
- Ito LM, Gorenstein C, Gentil V, Miyakawa E. Minnesota multiphasic personality inventory correlates of panic disorder with agoraphobia: changes with treatment. Brazilian Journal of Medical and Biological Research 1995;28(9):961‐5. [PubMed] [Google Scholar]
Kahn 1987 {published data only}
- Kahn RS, Westenberg HG, Verhoeven WM, Gispen de Wied CC, Kamerbeak WD. Effect of a serotonin precursor and uptake inhibitor in anxiety disorders: a double‐blind comparison of 5‐hydroxytryptophan, clomipramine and placebo. International Clinical Psychopharmacology 1987;2(1):33‐45. [DOI] [PubMed] [Google Scholar]
Kamijima 2005 {published data only}
- Kamijima K, Kuboki T, Kumano H, Burt T, Cohen G, Arano I, et al. A placebo‐controlled, randomized withdrawal study of sertraline for panic disorder in Japan. International Clinical Psychopharmacology 2005;20(5):265‐73. [DOI] [PubMed] [Google Scholar]
Kawashima 2012 {published data only}
- Kawashima S, Ichiki M, Ono S, Katayama S, Matsuki S, Iimori M. The effectiveness of hypnosis for patients with panic disorder (2): its possibilities for reduction of symptom severity. Journal of Tokyo medical University 2012;70(3):351‐9. [Google Scholar]
Keller 1993 {published data only}
- Keller MB, Lavori PW, Goldenberg IM, Baker LA, Pollack MH, Sachs GS, et al. Influence of depression on the treatment of panic disorder with imipramine, alprazolam and placebo. Journal of Affective Disorders 1993;28(1):27‐38. [DOI] [PubMed] [Google Scholar]
Klerman 1990 {published data only}
- Klerman GL. Depression and panic anxiety: the effect of depressive comorbidity on response to drug treatment of patients with panic disorder and agoraphobia. Journal of Psychiatric Research 1990;24(Suppl 2):27‐41. [DOI] [PubMed] [Google Scholar]
Modigh 1992 {published data only}
- Modigh K, Westenberg P, Eriksson E. Superiority of clomipramine over imipramine in the treatment of panic disorder: a placebo‐controlled trial. Journal of Psychopharmacology 1992;12(4):251‐61. [PubMed] [Google Scholar]
Oehrberg 1995 {published data only}
- Oehrberg S, Christiansen PE, Behnke K, Borup AL, Severin B, Soegaard J, et al. Paroxetine in the treatment of panic disorder: a randomised, double‐blind, placebo‐controlled study. British Journal of Psychiatry 1995;167(3):374‐9. [DOI] [PubMed] [Google Scholar]
Pfizer Sertraline Company Study 2003 {unpublished data only}
- Pfizer‐Sertraline‐Company‐Study. A placebo‐controlled, double‐blind, comparative study of sertraline in the treatment of subjects with panic disorder ‐ randomized withdrawal study. Pfizer‐Clinical Study Register (www.clinicalstudyresults.com) 2003.
Saxena 1992 {published data only}
- Saxena B, Bakish D, Bowen R, D’Souza J. Brofaromine and clomipramine in panic disorder: a double‐blind study. Clinical Neuropharmacology 1992;15(1 ptB):60. [Google Scholar]
Versiani 2001 {published data only}
- Versiani MV, Montgomery SA, Stahl SM, Schwartz GE. [Effect of the novel selective noradrenaline reuptake inhibitor reboxetine on anxiety]. 154th Annual Meeting of the American Psychiatry Association, 5‐10 May 2001. New Orleans, LA, 2001. [NR635]
Yang 2005 {published data only}
- Yang H, Yu C, Gao H. The control study of mirtazapine in treating patients with panic disorder. Medical Journal of Chinese People's Health 2005;17(3):133‐5. [Google Scholar]
Yang 2006 {published data only}
- Yang H, Gao H, Yu C. Effect of citalopram in the treatment of panic disorder. Shandong Archives of Psychiatry 2006;19(2):186‐7. [Google Scholar]
Yeragani 1992 {published data only}
- Yeragani VK, Pohl R, Balon R, Ramesh C, Weinberg P. Imipramine‐induced jitteriness and decreased serum iron levels. Neuropsychobiology 1992;25:8‐10. [DOI] [PubMed] [Google Scholar]
Zhao 2003 {published data only}
- Zhao C, Wang Y, Shen X. Venlafaxine plus cognitive behavior therapy in treatment of panic disorder. Shandong Archives of Psychiatry 2003;16(1):12. [Google Scholar]
Zitrin 1983 {published data only}
- Zitrin CM, Klein DF, Woerner MG, Ross DC. Treatment of phobias. Comparison of imipramine hydrochloride and placebo. Archives of General Psychiatry 1983;40(2):125‐38. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Anon 1994 {published data only}
- Anon. Placebo‐controlled study of efficacy of imipramine, diazepam, baclofen and propranolol in patients with panic disorder. Obozrenie Psikhiatrii Med Psikhologii Im V M Bekhtereva 1994:43‐50.
Diukova 1991 {published data only}
- Diukova GM, Shepeleva IP, Vorob’eva OV. Treatment of autonomic crises (attacks of panic). Zhurnal Nevropatologii i Psikhiatrii Imeni S. S. Korsakova 1991;91(5):3‐5. [PubMed] [Google Scholar]
- Diukova GM, Shepeleva IP, Vorov’eva OV. Treatment of autonomic attacks (panic attacks). Journal of Russian and East European Psychiatry 1993;26(1):22‐7. [Google Scholar]
Mallick 2003 {published data only}
- Mallick R, Gao B. Quality of life and functionality in panic disorder improve with treatment. 156th Annual Meeting of the American Psychiatric Association, 17‐22 May 2003. San Francisco, CA, 2003. [NR798]
Musgnung 2005 {published data only}
- Musgnung J, Pollack M, Whitaker T, Mangano R, Gao B. Short‐term treatment of panic disorder with venlafaxine XR or paroxetine: a placebo‐controlled trial. Psychiatriki 2005;16(Suppl 1):223. [Google Scholar]
Papp 1996 {published data only}
- Papp LA. Pharmacotherapy. Xth World Congress of Psychiatry, 23‐28 August, 1996. Madrid, Spain, 1996.
Pfizer NCT00044772 {unpublished data only}
- Pfizer. Study evaluating venlafaxine ER in patients with panic disorder. clinicaltrials.gov/ct2/show/record/NCT00044772?term=NCT00044772&rank=1 2009.
Sandmann 1995 {published data only}
- Sandmann J, Lorch B, Bandelow B, Benkert O. Effect of fluvoxamine in panic disorder. A double‐blind placebo‐controlled study. Pharmacopsychiatry 1995;28:209. [Google Scholar]
Schneier 1998 {published data only}
- Schneier FR, Fallon BA, Lin S‐H, Marshall RD, Vermes D, Sanchez‐Lacay JA, et al. Pattern analysis of a clinical trial of fluoxetine in panic disorder. 151st Annual Meeting of the American Psychiatry Association, 30 May‐4 June 1998. Toronto, Ontario, Canada, 1998.
Tsutsui 2000 {published data only}
- Tsutsui S. Early phase II study of paroxetine Hcl, a selective serotonin reuptake inhibitor, in the treatment of panic disorder. Japanese Pharmacology and Therapeutics 2000;28(Suppl 1):S253‐69. [Google Scholar]
Uhlenhuth 2002 {published data only}
- Uhlenhuth EH, Warner TD, Matuzas W. Interactive model of therapeutic response in panic disorder: moclobemide, a case in point. Journal of Clinical Psychopharmacology 2002;22(3):275‐84. [DOI] [PubMed] [Google Scholar]
Wolkow 1996 {published data only}
- Wolkow R. A double‐blind, parallel, 12‐week comparison of sertraline and placebo in patients with panic disorder. Xth World Congress of Psychiatry. Madrid, Spain, 1996.
References to ongoing studies
Kruimel 2015 {unpublished data only}
- Kruimel J, Leue C. Escitalopram trial for Irritable Bowel Syndrome (IBS) patients with panic disorder. clinicaltrials.gov May 2015. [NCT01551225]
Additional references
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. JAMA 2003;290:921‐8. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland MJ. Detecting skewness for summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2000
- Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta‐analysis of efficacy and tolerability. Journal of Affective Disorders 2000;58:19‐36. [DOI] [PubMed] [Google Scholar]
Andrisano 2013
- Andrisano C, Chiesa A, Serretti A. Newer antidepressants and panic disorder: a meta‐analysis. International Clinical Psychopharmacology 2013;28(1):33‐45. [DOI] [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. American Psychiatric Publishing, 1994. [Google Scholar]
APA 2009
- American Psychiatric Association. American Psychiatric Association Practice Guideline for the Treatment of Panic Disorder. American Psychiatric Publishing, 2009. [Google Scholar]
Bakker 2002
- Bakker A, Balkom AJ, Spinhoven P. SSRIs vs TCAs in the treatment of panic disorder: a meta‐analysis. Acta Psychiatrica Scandinavica 2002;106:163‐7. [DOI] [PubMed] [Google Scholar]
Bandelow 2006
- Bandelow B, Baldwin DS, Dolberg OT, Andersen HF, Stein DJ. What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder?. Journal of Clinical Psychiatry 2006;67:1428‐34. [DOI] [PubMed] [Google Scholar]
BAP 2005
- Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JR, et al. Evidence‐based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology 2005;19:567‐96. [DOI] [PubMed] [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170:477‐80. [PMC free article] [PubMed] [Google Scholar]
Bighelli 2016
- Bighelli I, Trespidi C, Castellazzi M, Cipriani A, Furukawa TA, Girlanda F, et al. Antidepressants and benzodiazepines for panic disorder in adults. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD011567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bijl 1998
- Bijl RV, Ravelli A, Zessen G. Prevalence of psychiatric disorder in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Social Psychiatry and Psychiatric Epidemiology 1998;33:587‐95. [DOI] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. Comparison of the indices and their use. Therapie 1999;54:405‐11. [PubMed] [Google Scholar]
Briley 1993
- Briley M, Moret C. Neurobiological mechanisms involved in antidepressant therapies. Clinical Neuropharmacology 1993;16:387‐400. [DOI] [PubMed] [Google Scholar]
Bruce 2003
- Bruce SE, Vasile RG, Goisman RM, Salzman C, Spencer M, Machan JT, et al. Are benzodiazepines still the medication of choice for patients with panic disorder with or without agoraphobia?. American Journal of Psychiatry 2003;160:1432‐8. [DOI] [PubMed] [Google Scholar]
Buchkowsky 2004
- Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Annals of Pharmacotherapy 2004;38:579‐85. [DOI] [PubMed] [Google Scholar]
Buscemi 2006
- Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. Journal of Clinical Epidemiology 2006;59(7):697‐703. [DOI] [PubMed] [Google Scholar]
Clark 1990
- Clark DB, Taylor CB, Roth WT, Hayward C, Ehlers A, Margraf J, et al. Surreptitious drug use by patients in a panic disorder study. American Journal of Psychiatry 1990;147:507‐9. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. Mahwah, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. 8th International Cochrane Colloquium; Cape Town, South Africa, 25‐28 October 2000. 2000.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Eaton 1994
- Eaton WW, Kessler RC, Wittchen H‐U, Magee WJ. Panic and panic disorder in the United States. American Journal of Psychiatry 1994;151:413‐20. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;317:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31:72‐6. [DOI] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20:49‐52. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [DOI] [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa TA, Watanabe N, Churchill R. Combined psychotherapy plus antidepressants for panic disorder with or without agoraphobia. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004364.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2009
- Furukawa TA, Katherine Shear M, Barlow DH, Gorman JM, Woods SW, Money R, et al. Evidence‐based guidelines for interpretation of the Panic Disorder Severity Scale. Depression and Anxiety 2009;26(10):922‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goisman 1995
- Goisman RM, Warshaw MG, Steketee GS, Fierman EJ, Rogers MP, Goldenberg I, et al. DSM‐IV and the disappearance of agoraphobia without a history of panic disorder: new data on a controversial diagnosis. American Journal of Psychiatry 1995;152:1438‐43. [DOI] [PubMed] [Google Scholar]
Gorman 2000
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. American Journal of Psychiatry 2000;157:493‐505. [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2006
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Goldstein RB, Smith S, et al. The epidemiology of DSM‐IV panic disorder and agoraphobia in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry 2006;67:363‐74. [DOI] [PubMed] [Google Scholar]
Guaiana 2013a
- Guaiana G, Barbui C, Chiodo D, Cipriani A, Davies SJC, Koesters M. Benzodiazepines versus placebo for panic disorder in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010677] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guaiana 2017
- Guaiana G, Barbui C, Caldwell DM, Davies SJC, Furukawa TA, Imai H, et al. Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta‐analysis. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012729] [DOI] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kessler 2006
- Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, Walters EE. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Archives of General Psychiatry 2006;63:415‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2008
- King M, Nazareth I, Levy G, Walker C, Morris R, Weich S, et al. Prevalence of common mental disorders in general practice attendees across Europe. British Journal of Psychiatry 2008;192:362‐7. [DOI] [PubMed] [Google Scholar]
Klein 1964
- Klein DF. Delineation of two drug‐responsive anxiety syndromes. Psychopharmacologia 1964;5:397‐408. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. PLOS Medicine 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nash 2008
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5‐HT1A receptor binding in people with panic disorder: positron emission tomography study. British Journal of Psychiatry 2008;1993:229‐34. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence. Generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults [CG113]. London: National Institute for Health and Care Excellence, 2011. [Google Scholar]
Otto 2001
- Otto MW, Tuby KS, Gould RA, McLean RY, Pollack MH. An effect‐size analysis of the relative efficacy and tolerability of serotonin selective reuptake inhibitors for panic disorder. American Journal of Psychiatry 2000;158:1989‐92. [DOI] [PubMed] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Systematic Reviews 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pompoli 2016
- Pompoli A, Furukawa TA, Imai H, Tajika A, Efthimiou O, Salanti G. Psychological therapies for panic disorder with or without agoraphobia in adults: a network meta‐analysis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011004.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27:746‐63. [DOI] [PubMed] [Google Scholar]
Sanchez‐Meca 2010
- Sánchez‐Meca J, Rosa‐Alcázar AI, Marín‐Martínez F, Gómez‐Conesa A. Psychological treatment of panic disorder with or without agoraphobia: a meta‐analysis. Clinical Psychology Review 2010;30:37‐50. [DOI] [PubMed] [Google Scholar]
Schultz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:698‐702. [DOI: 10.1136/bmj.c332] [DOI] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Starcevic 2009
- Starcevic V. Anxiety Disorders in Adults: A Clinical Guide. Oxford University Press, 2009. [Google Scholar]
Stein 2010
- Stein M, Steckler T, Lightfoot JD, Hay E, Goddard AW. Pharmacologic treatment of panic disorder. Current Topics in Behavioral Neurosciences 2010;2:469‐85. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Wade 1999
- Wade AG. Antidepressants in panic disorder. International Clinical Psychopharmacology 1999;14(Suppl 2):13‐17. [PubMed] [Google Scholar]
References to other published versions of this review
Guaiana 2013b
- Guaiana G, Barbui C, Chiodo D, Cipriani A, Davies SJC, Koesters M. Antidepressants versus placebo for panic disorder in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010676] [DOI] [PMC free article] [PubMed] [Google Scholar]


