Abstract
Background
Insomnia disorder is a subjective condition of unsatisfactory sleep (e.g. sleep onset, maintenance, early waking, impairment of daytime functioning). Insomnia disorder impairs quality of life and is associated with an increased risk of physical and mental health problems including anxiety, depression, drug and alcohol abuse, and increased health service use. hypnotic medications (e.g. benzodiazepines and 'Z' drugs) are licensed for sleep promotion, but can induce tolerance and dependence, although many people remain on long‐term treatment. Antidepressant use for insomnia is widespread, but none is licensed for insomnia and the evidence for their efficacy is unclear. This use of unlicensed medications may be driven by concern over longer‐term use of hypnotics and the limited availability of psychological treatments.
Objectives
To assess the effectiveness, safety and tolerability of antidepressants for insomnia in adults.
Search methods
This review incorporated the results of searches to July 2015 conducted on electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 6), MEDLINE (1950 to 2015), Embase (1980 to 2015) and PsycINFO (1806 to 2015). We updated the searches to December 2017, but these results have not yet been incorporated into the review.
Selection criteria
Randomised controlled trials (RCTs) of adults (aged 18 years or older) with a primary diagnosis of insomnia and all participant types including people with comorbidities. Any antidepressant as monotherapy at any dose whether compared with placebo, other medications for insomnia (e.g. benzodiazepines and 'Z' drugs), a different antidepressant, waiting list control or treatment as usual.
Data collection and analysis
Two review authors independently assessed trials for eligibility and extracted data using a data extraction form. A third review author resolved disagreements on inclusion or data extraction.
Main results
The search identified 23 RCTs (2806 participants).
Selective serotonin reuptake inhibitors (SSRIs) compared with placebo: three studies (135 participants) compared SSRIs with placebo. Combining results was not possible. Two paroxetine studies showed significant improvements in subjective sleep measures at six (60 participants, P = 0.03) and 12 weeks (27 participants, P < 0.001). There was no difference in the fluoxetine study (low quality evidence).
There were either no adverse events or they were not reported (very low quality evidence).
Tricyclic antidepressants (TCA) compared with placebo: six studies (812 participants) compared TCA with placebo; five used doxepin and one used trimipramine. We found no studies of amitriptyline. Four studies (518 participants) could be pooled, showing a moderate improvement in subjective sleep quality over placebo (standardised mean difference (SMD) ‐0.39, 95% confidence interval (CI) ‐0.56 to ‐0.21) (moderate quality evidence). Moderate quality evidence suggested that TCAs possibly improved sleep efficiency (mean difference (MD) 6.29 percentage points, 95% CI 3.17 to 9.41; 4 studies; 510 participants) and increased sleep time (MD 22.88 minutes, 95% CI 13.17 to 32.59; 4 studies; 510 participants). There may have been little or no impact on sleep latency (MD ‐4.27 minutes, 95% CI ‐9.01 to 0.48; 4 studies; 510 participants).
There may have been little or no difference in adverse events between TCAs and placebo (risk ratio (RR) 1.02, 95% CI 0.86 to 1.21; 6 studies; 812 participants) (low quality evidence).
'Other' antidepressants with placebo: eight studies compared other antidepressants with placebo (one used mianserin and seven used trazodone). Three studies (370 participants) of trazodone could be pooled, indicating a moderate improvement in subjective sleep outcomes over placebo (SMD ‐0.34, 95% CI ‐0.66 to ‐0.02). Two studies of trazodone measured polysomnography and found little or no difference in sleep efficiency (MD 1.38 percentage points, 95% CI ‐2.87 to 5.63; 169 participants) (low quality evidence).
There was low quality evidence from two studies of more adverse effects with trazodone than placebo (i.e. morning grogginess, increased dry mouth and thirst).
Authors' conclusions
We identified relatively few, mostly small studies with short‐term follow‐up and design limitations. The effects of SSRIs compared with placebo are uncertain with too few studies to draw clear conclusions. There may be a small improvement in sleep quality with short‐term use of low‐dose doxepin and trazodone compared with placebo. The tolerability and safety of antidepressants for insomnia is uncertain due to limited reporting of adverse events. There was no evidence for amitriptyline (despite common use in clinical practice) or for long‐term antidepressant use for insomnia. High‐quality trials of antidepressants for insomnia are needed.
Plain language summary
Antidepressants for insomnia
Why is this review important?
Insomnia (having difficulty falling or staying asleep) is common, approximately one in five people report sleep problems in the preceeding year. Insomnia can cause daytime fatigue, distress, impairment of daytime functioning and reduced quality of life. It is associated with increased mental health problems, drug and alcohol abuse, and increased healthcare use. Management depends on the duration and nature of the sleep problem. It may involve: treating coexisting medical problems; providing advice on sleep habits and lifestyle (known as sleep hygiene); medicines and psychological therapies such as cognitive behavioural therapy (CBT, which is a talking therapy).
Medicines called hypnotics (for example, temazepam and 'Z' drugs) are most commonly used to treat insomnia and are known to help sleep, but can have problems such as tolerance (needing to take more of the medicine to get the same effect) and dependence (physical or mental problems if the medicine is stopped). Guidelines recommend only short‐term use of hypnotics (two to four weeks). However, millions of people worldwide take long‐term hypnotic medicines.
Antidepressants are widely prescribed for insomnia despite not being licensed for this use, and uncertain evidence for their effectiveness. This may be because of the concerns regarding hypnotic medicines. Psychological treatments such as CBT are known to help insomnia, but availability is limited. Thus, alternative medicines, such as antidepressants (used to treat depression) and antihistamines (used to treat allergies), are sometimes tried. Assessing the evidence for the unlicensed use of these medicines is important.
Who will be interested in this review?
People with sleep problems and their doctors will be interested in this review to better understand the research evidence and enable informed decision‐making regarding using antidepressants for insomnia.
What questions did this review aim to answer?
The aim was to find out how well antidepressants work in treating insomnia in adults, how safe they are and if they have any side effects.
Which studies did we include in the review?
We included randomised controlled trials (clinical studies where people were randomly put into one of two or more treatment groups; these trials provide the most reliable and highest quality evidence) of adults with an insomnia diagnosis. People could have had other conditions (comorbidities) in addition to insomnia. We included any dose of antidepressant (but not combinations with another antidepressant) compared with placebo (pretend treatment), other medicines for insomnia (e.g. benzodiazepines or 'Z' drugs), a different antidepressant, waiting list control or 'treatment as usual.'
What did the evidence from the review tell us?
We reviewed 23 studies with 2806 people with insomnia. Overall, the quality of the evidence was low due to a small number of people in the studies, and problems with how the studies were undertaken and reported. We often could not combine the individual study results. There was low quality evidence to support short‐term (i.e. weeks rather than months) use for some antidepressants. There was no evidence for the antidepressant amitriptyline, which is commonly used in clinical practice, or to support long‐term antidepressant use for insomnia. The evidence did not support the clinical current practice of prescribing antidepressants for insomnia.
What should happen next?
High quality trials of antidepressants for insomnia are needed to provide better evidence to inform clinical practice. Additionally, health professionals and patients should be made aware of the current paucity of evidence for antidepressants commonly used for insomnia management.
Summary of findings
Background
Description of the condition
Insomnia disorder is a subjective condition of unsatisfactory sleep, in terms of sleep onset, sleep maintenance or early waking (Wilson 2010). It is a disorder that impairs daytime well‐being and subjective abilities and functioning, and so can be considered a '24‐hour' disorder. It often starts with a clear event such as unusual stress at work or is associated with illness of self or family, or bereavement. Once the triggering circumstances have diminished or have been addressed as far as possible, most people will return to normal sleep if they adhere to good sleep habits. However, the condition may go on to be a chronic complaint (i.e. symptoms persisting more than a month), and the main factor influencing this is anxiety about sleep (Morin 2003). Essential features of insomnia are heightened arousal and learned sleep‐preventing associations.
In early classification systems, insomnia was often classified into primary and secondary, where secondary insomnia referred to insomnia occurring in association with another disorder. However, most recent classification systems have moved away from this division as the distinction is now considered unhelpful (Perlis 2010). The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM‐5) recommends the use of the term 'insomnia disorder,' which we use for this review, but we are aware that past research papers have used other terms to describe insomnia (DSM‐V 2015).
Studies of the prevalence of insomnia in the general population indicate that one third of adults in Western countries experience difficulty with sleep initiation or maintenance at least once a week (LeBlanc 2009; Sateia 2004), and 6% to 15% are thought to meet the criteria for insomnia disorder in that they report sleep disturbance plus significant daytime dysfunction (LeBlanc 2009; Sivertsen 2009). There is a higher incidence of insomnia in women, and the incidence increases in both men and women as they get older.
Insomnia may be present alongside other disorders such as depression, anxiety disorders and physical problems (Baglioni 2011). Once other disorders are properly treated, insomnia may persist and need treatment. It is important to treat insomnia because the condition causes decreased quality of life (Chevalier 1999; Leger 2001; Philip 2006); is associated with impaired functioning in many areas such as memory and executive function (Altena 2008; Edinger 2008; Nissen 2011); and leads to increased risk of a new episode or relapse of depression, anxiety and possibly cardiovascular disorders (Breslau 1996; Neckelmann 2007; Vgontzas 2009).
Description of the intervention
The goal of treating insomnia disorder is to lessen suffering and improve daytime function. The two main treatment classes shown to be effective, at least in the short term, are psychological and pharmacological treatments; although evidence is limited for longer‐term effects (Riemann 2009). The type of treatment chosen should be patient‐guided, should take into account the particular pattern of the problem (i.e. sleep onset or staying asleep) and should be evidence based (Wilson 2010).
Psychological treatments
Psychological interventions designed for insomnia, usually consisting of a package of educational, behavioural and cognitive therapy, improve insomnia. Based on extensive published evidence, including nine systematic reviews or meta‐analyses, the National Institutes of Health 'Consensus and State of the Science Statement' concluded that a cognitive behavioural therapy (CBT) package is "as effective as prescription medications are for short‐term treatment of chronic insomnia. Moreover, there are indications that the beneficial effects of CBT, in contrast to those produced by medications, may last well beyond the termination of active treatment" (NIH 2005). The UK consensus on the treatment of insomnia also recommended that CBT should be used as first‐line treatment depending on patient choice, but pointed out that this therapy may not be available, or the patient may not wish to engage in it, and therefore the choice may be a drug treatment (Wilson 2010).
Drug treatments for insomnia
Most of the licensed drugs for insomnia are allosteric modulators of the GABA‐A receptor, and thus enhance gamma‐aminobutyric acid (GABA) function in the brain. The benzodiazepines and 'Z' drugs (zopiclone, eszopiclone, zolpidem and zaleplon) are in this category and these are commonly referred to as 'hypnotic' medications. These drugs are all effective in insomnia (Buscemi 2007; NIH 2005), but as well as promoting sleep they are anxiolytic, anticonvulsant and myorelaxant, and can cause ataxia and memory problems when taken other than just before a period in bed. If their effect persists after waking up in the morning, they are described as having 'hangover' effects. Therefore, differences in the duration of action of individual drugs are of particular importance, with short‐acting drugs giving rise to less risk of next‐day effects such as sedation, and impairment of skills such as driving.
A melatonin preparation is licensed for the treatment of insomnia in people aged over 55 years, and this drug does not give rise to motor or memory effects (Lemoine 2007; Wade 2007). Clinical trials have begun to measure daytime outcomes for hypnotic medications, and beneficial effects have been reported for melatonin in people over 55 years of age (Auld 2017), as well as for zolpidem, zopiclone, eszopiclone and lormetazepam (NICE 2004; Wilson 2010) These measures have not been used in studies of other drugs, so their effects on daytime function are not well documented.
Duration of prescribing
It has long been stated that hypnotic medication should not be used long term for the treatment of insomnia. This was the consensus view of the panel of a 1983 National Institute of Health Consensus Conference on the medication treatment of insomnia (NIH 1983), which became a guideline for clinical practice in the USA. Later, the UK Committee on Safety of Medicines (Committee on Safety of Medicines 1988), the Royal College of Psychiatrists, and the National Institute for Clinical Excellence (NICE) guidance (now the National Institute for Health and Care Excellence) also recommended only short‐term use (NICE 2004). While it was appreciated that benzodiazepine hypnotic agents had a favourable risk‐benefit ratio and were first‐line agents for insomnia management, these reports expressed concerns about the risks of tolerance and dependence, and recommended their use should be limited to periods of two to four weeks. This view was not based on data demonstrating an unfavourable transition in the risk‐benefit ratio after two to four weeks of treatment, but appeared to have emerged because no substantive placebo‐controlled trials of hypnotics had been carried out for longer than a few weeks. Despite the recommendation for duration of treatment with hypnotic drugs being only two to four weeks, many millions of people worldwide remain on long‐term treatment (Balter 1992; Ishigooka 1999; Mellinger 1985; Ohayon 1999; Wilson 2010). Trials of nightly dosing for up to six months' duration suggest that tolerance and withdrawal do not generally occur with some hypnotics (zolpidem: six months of 'as needed' treatment (Krystal 2008); eszopiclone: two studies of six‐months' duration (Krystal 2003; Walsh 2007); ramelteon: one six‐month study with outcome assessed with polysomnography (PSG) but not self‐report (Mayer 2009); and temazepam: one two‐month study (Morin 1999)). Other agents have not been studied for longer durations. Therefore, the available evidence does not suggest there is an unfavourable risk/benefit transition at three to four weeks for any agent. However, the recommendations remain in place, and clinicians are generally unwilling to prescribe for long periods.
Antidepressants
The use of antidepressants to treat insomnia is widespread (Everitt 2014; Morlock 2006; NHS Digital 2011; Wilson 2010), but can be considered to be 'off‐label' as none is licensed for insomnia. One consensus statement from the British Association of Psychopharmacology (BAP) highlighted that "low‐doses (sub‐therapeutic of depression) of sedating tricyclics, particularly amitriptyline, dosulepin and doxepin, have been used for decades to treat insomnia. This is particularly common practice in the UK" (Wilson 2010), and that "low doses of amitriptyline (10 mg or 25 mg) have been used for long periods in many patients with chronic illness particularly those with pain syndromes." Antidepressants are also widely prescribed 'off licence' in the USA for insomnia, with trazodone, a triazolopyridine derivative, being the most commonly prescribed at subtherapeutic antidepressant doses (Lai 2011).
How the intervention might work
Factors that have influenced the use of antidepressants for insomnia are:
low‐dose antidepressants, particularly the tricyclic antidepressant (TCA) amitriptyline, are helpful in the treatment of chronic pain and studies have reported reduction in pain‐related sleep disturbance (Saarto 2010);
some sedating antidepressants improve sleep problems in people with depression (Mayers 2005; Wilson 2005); and
there is no prescribing duration limitation on antidepressant use in insomnia, so clinicians may perceive these medications have the potential for longer‐term use.
The proposed mechanism of action for low‐dose amitriptyline is as a histamine H1 receptor antagonist, although 5‐HT2 and cholinergic muscarinic antagonism may also contribute. Trazodone, the second most frequently prescribed medication for insomnia in the USA, is an antagonist at 5‐HT1A, 5‐HT2 and alpha1 adrenergic receptors as well as a weak 5‐HT reuptake inhibitor. Trimipramine blocks alpha1 adrenergic, histamine H1, dopamine D2, 5‐HT2 and cholinergic receptors (Wilson 2010).
One meta‐analysis of drugs used in treatment of chronic insomnia described seven studies that used antidepressants (doxepin, trazodone, trimipramine) to treat insomnia at doses used in depression (Buscemi 2007). The review concluded that there was some evidence that antidepressants, particularly doxepin and trazodone, may be effective treatments for chronic insomnia, with similar adverse effects to benzodiazepines, but highlighted the paucity of evidence, as did the BAP consensus statement (Wilson 2010).
Other factors that should be considered with the use of antidepressants for insomnia are: toxicity in overdose for amitriptyline and other TCAs; tolerability and adverse effect issues such as morning 'hangover' effects; and increased restless leg syndrome, periodic limb movements in sleep and sleep bruxism with selective serotonin reuptake inhibitors (SSRIs), venlafaxine, mianserin and mirtazapine.
Why it is important to do this review
Antidepressants are widely prescribed for insomnia despite not being licensed for this indication and there being a poor evidence base for their effectiveness in insomnia. A significant factor in this widespread prescription is likely to be concern regarding the longer‐term use of hypnotic medications, and guidelines suggesting that long‐term use of hypnotics should be avoided due to potential dependency and addiction. Clinicians seek alternative treatments for insomnia that can be used longer term. There is poor availability of psychological treatments, thus alternative medications such as antidepressants and antihistamine are tried. We systematically reviewed the evidence (or lack of it) behind this practice, including the efficacy, safety and tolerability of antidepressants. Other Cochrane Reviews explored other aspects of insomnia management (i.e. new‐generation hypnotics (Rösner 2013), acupuncture (Cheuk 2012), and CBT (Aversa Lopes 2009)). Together, these reviews highlight what is known about insomnia management and what further research is needed to provide clinicians with the information they require to manage this common and troublesome condition.
Objectives
To assess the effectiveness, safety and tolerability of antidepressants for insomnia in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) including cluster and cross‐over RCTs.
Types of participants
We included adults (aged 18 or over) with a diagnosis of insomnia (to include Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV), International Classification of Sleep Disorders (ICSD), International Statistical Classification of Diseases and Health Related Problems, 10th revision (ICD‐10) (WHO 1992), and other well‐recognised classifications), We also included participants with insomnia defined on validated rating scales such as the Hamilton Rating Scale for Depression Sleep subscale (HAM‐D Insomnia).
We included all participant types (including people with comorbid depression or anxiety disorder and other comorbidities).
Types of interventions
Experimental intervention
We included any antidepressant (administered for at least three days) as monotherapy including all doses.
We organised antidepressants into classes for the purposes of this review, as follows.
SSRIs: fluvoxamine, fluoxetine, paroxetine, sertraline, citalopram, escitalopram.
TCAs: amitriptyline, imipramine, trimipramine, doxepin, desipramine, protriptyline, nortriptyline, clomipramine, dothiepin, lofepramine.
Heterocyclic antidepressants: mianserin, amoxapine, maprotiline.
-
Monoamine oxidase inhibitors (MAOI):
irreversible: phenelzine, tranylcypromine, isocarboxazid;
reversible: brofaramine, moclobemide, tyrima.
-
'Other' antidepressants:
noradrenaline reuptake inhibitors (NARIs): reboxetine, atomoxetine;
noradrenaline‐dopamine reuptake inhibitors (NDRIs): amineptine, bupropion;
serotonin‐noradrenaline reuptake inhibitors (SNRIs): venlafaxine, milnacipram, duloxetine;
noradrenergic and specific serotonergic antidepressants (NASSAs): mirtazapine;
serotonin antagonists and reuptake inhibitors (SARIs): trazodone;
unclassified:agomelatine, vilazodone.
Comparator interventions
Placebo.
Other medications for insomnia (e.g. benzodiazepines, 'Z' drugs).
A different antidepressant.
Waiting list control or treatment as usual.
Types of outcome measures
Primary outcomes
Efficacy: any subjective improvement in sleep quality or satisfaction with sleep, total sleep duration (measured in hours or minutes), sleep onset latency (measured as time taken to fall asleep), number of nocturnal awakenings or total nocturnal awakening time (measured in hours or minutes) or sleep efficiency (measured as a ratio of time asleep to time in bed).
A variety of rating scales were reported (e.g. the Pittsburgh Sleep Quality Index (PSQI) (Buysse 1989); Insomnia Severity Index (ISI) (Morin 2011); Hamilton Rating Scale for Depression ‐ Sleep disturbance factor (HRSD or HAM‐D) (Hamilton 1960); visual analogue scales (VAS)).
Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity.
Secondary outcomes
Objective measures of change in sleep (such as electroencephalogram (EEG) or PSG data).
Tolerability: reported information on tolerability (e.g. problems with daytime drowsiness, dropout rates).
Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning.
Timing of outcome assessments
Some trials had multiple sleep diary end points. We report end points consistently reported across studies rather than the protocol‐stated primary end point.
Search methods for identification of studies
Electronic searches
We searched the following electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 6), Ovid MEDLINE (1950 to July 2015), Ovid Embase (1980 to July 2015), Ovid PsycINFO (1806 to July 2015). The initial search was carried out 6 November 2013 and updated on 8 July 2015. We applied no date or language restrictions (Appendix 1).
In keeping with Cochrane Methodological Expectations of Cochrane Intervention Reviews (MECIR) conduct standard C37, we ran additional, prepublication searches (3 August 2016 and 12 December 2017), but the results were not incorporated in the review. In 2017, we added the drug term 'Esmirtazapine' to the search strategies and back‐dated the search, as appropriate.
Searching other resources
We reviewed the reference lists of included studies to identify further relevant studies. Ongoing studies were identified through searching the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), Clinical Trials.gov, and the International Federation of Pharmaceutical Manufacturers & Associations platform (IFPMA Clinical Trials Portal) (8 July 2015).
We updated the search of ClinicalTrials.gov and the ICTRP to 12 December 2017.
We contacted key researchers in the area to ask about ongoing work or unpublished studies they might know of.
Data collection and analysis
Selection of studies
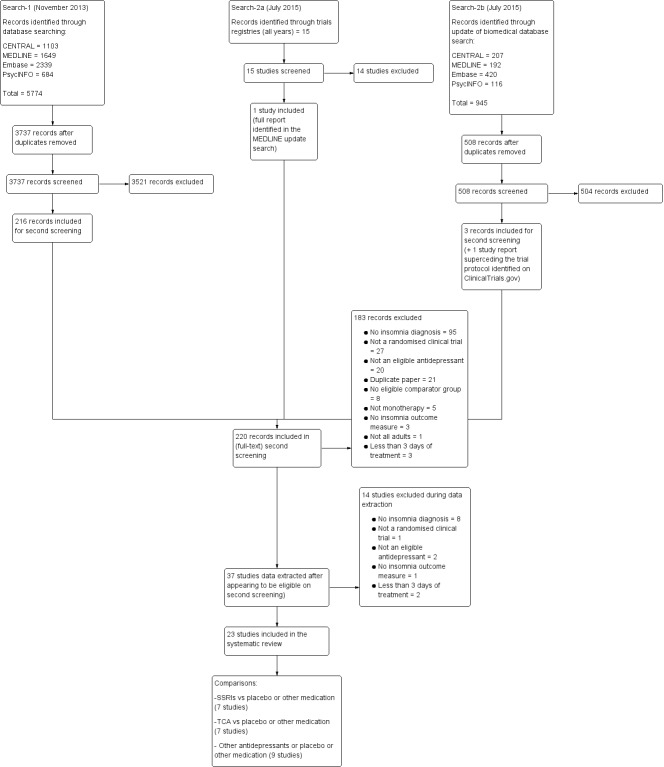
Two review authors independently identified studies using a previously prepared inclusion criteria form that had been piloted previously. A third review author resolved disagreements concerning the selection of studies. The review authors were not blinded to the names of the trial authors, institutions or journal of publication. The process of study identification and its results are outlined in Figure 1 according to the PRISMA statement (Moher 2009).
1.
Study flow diagram. SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant.
Data extraction and management
Two review authors independently extracted data using a data extraction form. The extraction form was piloted before use and in the case of discrepancy, we consulted a third review author. We collected information on participants (age, gender, diagnostic criteria, sample size, country, setting, number of participants randomised and number followed up), intervention (drug, dosage, length of treatment, any concomitant interventions, controls, placebo) and outcome measures (subjective improvement in sleep, rating scale, numbers of adverse events, objective measures of change in sleep, reported information on tolerability).
Main planned comparisons
The main comparisons were each identified antidepressant versus:
placebo;
other medications for insomnia (e.g. benzodiazepines, 'Z' drugs);
other antidepressants; and
waiting list control or treatment as usual.
These comparisons were made initially on a drug level and then were combined at a class of drug level. We only combined drugs in analyses from the same class. The 'other' antidepressants category (see Types of interventions) is presented together, but only drugs of the same class were combined to produce a pooled effect (e.g. trazodone).
Assessment of risk of bias in included studies
Two review authors independently assessed each study for bias in accordance with the Cochrane 'Risk of bias' tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Bias was assessed in terms of random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. Each type of bias was assessed as low, high or unclear risk, depending on the availability of information and the likelihood of bias. If insufficient details were provided or the risk was uncertain in the trial, the level of bias was described as 'unclear.' If the two review authors determining the level of bias disagreed, a third review author (HE) assessed the evidence and made a decision regarding the level of bias.
Measures of treatment effect
For dichotomous variables, we calculated risk ratios (RR) and risk difference (RD) with 95% confidence intervals (CI).
For continuous outcomes, we calculated mean differences (MD) where studies used the same scale, and standardized mean differences (SMD) where studies used different scales, with their 95% CIs.
Unit of analysis issues
Cross‐over trials
We identified no cross‐over trials.
If in updates of the review we include cross‐over trials where sufficient data are present, we plan to include in the analysis data from the first period only to avoid carry‐over effects.
Cluster‐randomised trials
We identified no cluster‐randomised trials.
If in updates of the review we include cluster‐randomised trials, we plan to conduct the analysis at the same level as the allocation using a summary measure from each cluster. However, if this appears to unnecessarily reduce the power of the study due to the number and size of the clusters, we will seek statistical advice to determine if an RR or MD (or SMD if different scales have been used) with CIs can be calculated accounting for the cluster design based on a 'multi‐level model' or another appropriate method (Higgins 2011).
Studies with multiple treatment groups
In studies with multiple treatment groups, we included the same group of participants only once in the meta‐analysis to avoid multiple comparisons. We combined groups to compare a single pair‐wise comparison where possible. If this was not appropriate, we chose one pair of interventions and excluded the others.
Dealing with missing data
Where data were suspected to be missing, we contacted the main author of the primary study. If this was unsuccessful, we imputed absent information for continuous data by carrying the last observation forward (Higgins 2011). A sensitivity analysis was undertaken excluding high levels of missing data. For dichotomous data, we performed an intention‐to‐treat analysis.
Assessment of heterogeneity
Before meta‐analysis, we assessed studies for clinical homogeneity with respect to type of therapy, control group and outcomes. For studies judged as clinically homogeneous, we estimated statistical heterogeneity using the I2 statistic (Higgins 2011), using the following as an approximate guide to interpretation: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity. In cases of considerable heterogeneity, we explored the data further, including by subgroup analyses, in an attempt to explain the heterogeneity.
Assessment of reporting biases
Comprehensive searching for trials helped to reduce the risk of reporting biases. There were insufficient identified trials in each group to enter into funnel graphs (which require more than 10 studies) (trial effect versus variance) in an attempt to investigate the likelihood of overt publication bias (Higgins 2011).
Data synthesis
If studies were sufficiently homogeneous for their pooling to be clinically meaningful, we performed a meta‐analysis using a random‐effects model, regardless of the I2 results. We performed the analysis using Review Manager 5 software (Review Manager 2014) and produced forest plots for all analyses.
Subgroup analysis and investigation of heterogeneity
Where sufficient data were available, we performed subgroup analysis to look at:
people with a diagnosis of depression or anxiety compared with people without a diagnosis of depression or anxiety, since a treatment effect of antidepressants on the symptoms of depression and anxiety may impact on sleep;
people with a recorded physical comorbidity (e.g. back pain), as people with physical comorbidities may have different causes for their sleep problems to people with lone insomnia, and this may impact on the effect of antidepressants in these groups and
dose as a variable, particularly at low dose as a subgroup, as some antidepressants have been widely used in lower than usual antidepressant range treatment doses for the management of sleep problems (e.g. amitriptyline 10 mg). 'Low‐dose' antidepressants are defined as lower than the usual dose range for treatment of depression.
Subgroup analyses are hypothesis forming rather than hypothesis testing, and therefore have been interpreted with caution.
Sensitivity analysis
Where sufficient studies existed, we excluded studies that were at higher risk of bias to assess if study quality affects the results. Planned sensitivity analyses included trials with:
low numbers of participants (i.e. fewer than 10 per arm);
lack of double blinding of participants;
poor concealment of group allocation and
significant levels of missing data.
'Summary of findings' tables
We prepared 'Summary of findings' tables, summarising the key findings of the systematic review in line with the standard methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included the main outcomes (subjective and objective improvement in sleep and daytime functioning), the magnitude of effect, and the amount and quality of evidence. We used the GRADE approach to assessing the quality of the body of evidence. The findings are presented by antidepressant group (SSRI, TCA, other antidepressants). There were insufficient data to pool results for SSRIs compared with placebo so these were presented as a narrative description.
Results
Description of studies
Results of the search
The initial biomedical database searches (to July 2015) identified 6719 references, 4245 of which remained after deduplication. We excluded 4025 references on assessment of title and abstract; retrieved 220 full‐text papers for full inspection; excluded 183 of these full‐text papers; extracted data for 37 studies, but at this stage excluded another 14 studies, as on further inspection they did not meet our inclusion criteria; leaving 23 studies (23 references) included in the final qualitative descriptions and quantitative analyses.
In keeping with MECIR conduct standard C37, we ran searches within 12 months of publication. The update searches (2016 and 2017) identified 1073 references and, after screening these, we identified 10 studies of interest. Eight of these were placed in 'Studies awaiting classification' (see Characteristics of studies awaiting classification table) and two were added to 'Ongoing studies' (bringing the total to three) (see Characteristics of ongoing studies table). These studies will be incorporated in an update of this review, as appropriate.
We contacted 14 authors of included papers and six key trialists in the research field for additional information or information on ongoing trials by email with at least one follow‐up request. Five of the six key trialists responded, but identified no other ongoing trials. Three of the trial authors responded, but could not locate the additional information requested (Le Bon 2003; Reynolds 2006; Ware 1989).
The PRISMA flow diagram, which includes search results to July 2015 (only) is in Figure 1.
Included studies
The review included 23 studies (Corruble 2013; Fava 2002; Finnerty 1978; Friedmann 2008; Gillin 1997; Hajak 2001; Khazaie 2013; Krystal 2010; Krystal 2011; Lankford 2012; Le Bon 2003; Palomaki 2003; Reynolds 2006; Riemann 2002; Rios Romenet 2013; Roth 2011; Rush 1998; Satterlee 1995; Shell 2012; Stein 2012; Walsh 1998; Ware 1989; Zhou 2002) (see Characteristics of included studies table).
One trial required translation from Chinese (Zhou 2002).
Design
All studies were randomised and all but one (Rios Romenet 2013) were double‐blind. Three trials followed up participants for less than four weeks, 13 followed up for four to eight weeks, four followed up for eight to 24 weeks and three followed up for more than 24 weeks.
Sample sizes
The mean number of participants per study was 125 with a minimum sample size of 16 and a maximum of 324.
Setting
Fifteen of the included trials were conducted in the USA, two in Germany, one in Finland, one in Canada, one in Belgium, one in Iran and one international trial. Twenty‐one trials recruited outpatients and two recruited inpatients (Palomaki 2003; Zhou 2002).
Participants
The studies used a range of diagnostic criteria/scores for insomnia: the Diagnostic and Statistical Manual of Mental Disorders (DSM) Criteria or ICSD Criteria (or both DSM and ICSD) (Hajak 2001; Krystal 2010; Krystal 2011; Lankford 2012; Le Bon 2003; Reynolds 2006; Riemann 2002; Roth 2011; Rush 1998; Walsh 1998); PSQI (Friedmann 2008; Stein 2012); Hamilton Rating Scale for Depression ‐ Sleep disturbance factor (HRSD or HAM‐D) (Corruble 2013; Fava 2002; Gillin 1997; Palomaki 2003; Satterlee 1995), Global Sleep Assessment Questionnaire (GSAQ) (Khazaie 2013); history of sleep disturbance (Finnerty 1978; Shell 2012; Ware 1989); minimal Scales for Outcomes in Parkinson's disease (SCOPA) sleep nocturnal subscore and six months of insomnia (Rios Romenet 2013); Chinese Classification of Mental Disorders (CCMD‐2‐R) for chronic primary insomnia (Zhou 2002).
The mean age of participants was 47.3 years. The mean age in the included studies ranged from 26 to 73 years.
Four studies specified recruiting older adults or elderly participants (aged more than 65 years: Krystal 2010; Lankford 2012; 60 to 80 years: Zhou 2002; more than 50 years: Reynolds 2006).
On average, most participants were women. The mean proportion of women was 60%.
Some studies reported other diagnoses (in addition to insomnia): seven reported a diagnosis of depression (Corruble 2013; Fava 2002; Finnerty 1978; Gillin 1997; Rush 1998; Satterlee 1995; Ware 1989); three were in people with substance abuse, two were in people with alcohol detoxification (Friedmann 2008; Le Bon 2003), one with methadone administration (Stein 2012). One study recruited inpatients with acute ischaemic stroke (Palomaki 2003). One study recruited outpatients with idiopathic Parkinson's disease (Rios Romenet 2013). One study recruited women in the third trimester of pregnancy (Khazaie 2013).
Interventions
Three studies compared SSRIs with placebo; two used paroxetine (Reynolds 2006; Zhou 2002), and one used fluoxetine (Satterlee 1995).
One study compared the SSRI paroxetine with alprazolam (Zhou 2002).
One study compared SSRIs with each other (fluoxetine, sertraline and paroxetine) (Fava 2002).
Three studies compared SSRIs with another antidepressant (agomelatine, nefazodone) (Corruble 2013; Gillin 1997; Rush 1998).
Six studies compared TCAs with placebo, five used doxepin (Hajak 2001; Krystal 2010; Krystal 2011; Lankford 2012; Rios Romenet 2013), and one used trimipramine (Riemann 2002). One study compared doxepin with lormetazepam (Riemann 2002), one doxepin with imipramine (Finnerty 1978), and one imipramine with trimipramine (Ware 1989).
Eight studies compared 'other' antidepressants with placebo (one mianserin (Palomaki 2003); seven trazodone (Friedmann 2008; Khazaie 2013; Le Bon 2003; Roth 2011; Shell 2012; Stein 2012; Walsh 1998). One study compared an 'other' antidepressant (trazodone) with another insomnia medication (zolpidem) (Walsh 1998).
Outcomes
The studies used a range of different outcome scales: PSQI (Buysse 1989), ISI (Morin 2011), HRSD or HAM‐D (Hamilton 1960), global satisfaction with sleep scores and VAS.
Excluded studies
We excluded 18 studies (from all search results to 2017). Eleven studies were excluded as part of the full analysis (searches to July 2015).
Two studies were read in full, but then excluded after discussion with Cochrane as tryptophan was excluded from the list of includable antidepressants (Adam 1979; Ferrero 1987). Tryptophan is a supplement not an antidepressant.
Seven studies did not, on careful reading, fulfil the criteria for a primary diagnosis of insomnia (Botros 1989; Boyle 2012; Chen 2002; Fairweather 1997; Kaynak 2004; Moon 1991; Stephenson 2000).
Two studies had fewer than three days/nights of intervention treatment (Roth 2007; Scharf 2008).
See the Characteristics of excluded studies table for details.
Studies awaiting classification
There are eight studies awaiting classification (Ahmed 2016; Ivgy‐May 2015a; Ivgy‐May 2015b; Krystal 2012; Merck 2008; Miljatovic 2012; Shirazi 2016; Wu 2015). See the Characteristics of studies awaiting classification table for details.
Ongoing studies
We identified three ongoing studies: Morin 2015 comparing a behavioural intervention with trazodone or zolpidem in 82 participants in the USA with 12 months' follow‐up; NCT02139098 comparing amitriptyline 50 mg, zolpidem and placebo in 150 participants in Germany; and ChiCTR‐IPR‐16009475 comparing trazodone, alprazolam, quetiapine and zolpidem. See the Characteristics of ongoing studies table for details.
Risk of bias in included studies
The risk of bias across all studies is shown in Figure 2 and Figure 3. Most studies had low or unclear risk of bias across most bias domains. Only four of the 20 studies did not meet criteria for low risk of bias related to at least one type of bias (Gillin 1997; Krystal 2010; Walsh 1998; Zhou 2002). All studies that had some level of high risk only did so for one or two bias domains, with the majority only carrying risk in one domain. Overall, the risk of bias analysis revealed that no studies were high risk, that is carried a high‐risk profile over all or most bias domains.
2.
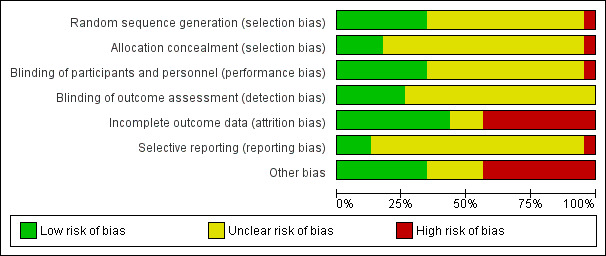
Summary of risk of bias across all included studies for each risk of bias item
3.
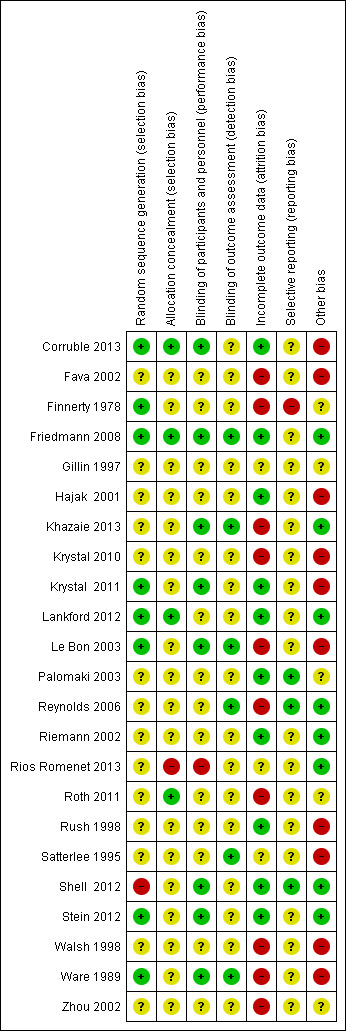
Random sequence allocation (selection bias)
One study showed high risk of bias (Shell 2012). This trial recruited a disproportionate amount of participants from certain sites because of higher enrolment at these sites, which is likely to have caused uneven randomisation. The remaining trials were at low or unclear risk of selection bias.
Allocation
Rios Romenet 2013 was at high risk of selection bias due to block randomisation. The remaining trials were at low or unclear risk of selection bias. Most studies provided no data on allocation concealment.
Blinding
In terms of successful blinding of participants and personnel, Rios Romenet 2013 did not administer placebo tablets, instead they administered red light as placebo. The use of this condition as placebo means that participants were not blinded to the type of treatment they received (high risk of bias). The remaining trials were at low or unclear risk of performance bias. All trials were at risk of low or unclear detection bias.
Incomplete outcome data
A higher proportion of studies showed incomplete outcome data that is likely to reflect high risk of bias. Five studies found an elevated proportion of participants did not complete the trial (26% to 74% of enrolled participants) (high risk of bias; Fava 2002; Finnerty 1978; Reynolds 2006; Roth 2011; Zhou 2002). Seven studies did not follow an intention‐to‐treat principle (high risk of bias; Finnerty 1978; Krystal 2010; Khazaie 2013; Le Bon 2003; Reynolds 2006; Walsh 1998; Ware 1989). Either elevated rates of incomplete data or not adhering to the intention‐to‐treat principle may result in an under‐representation of participants with severe illness or adverse effects, therefore inflating the positive results of the study. The remaining trials were at low or unclear risk of attrition bias.
Selective reporting
One study showed high risk of bias (Finnerty 1978). This study used the Finnerty‐Goldberg scale to assess sleep disturbance, but the findings were not reported in full. The majority of studies showed unclear risk of bias as the relationship between prespecified primary outcomes and the results were not always clearly defined.
Other potential sources of bias
Regarding other forms of bias that may influence the outcomes, sponsorship bias could be evaluated across all 23 studies. Ten studies were funded by pharmaceutical companies and evidenced no attempt to report the findings as independent to the interests of the company (e.g. by using an external company to control the blinding of participants or analyse the data (Corruble 2013; Fava 2002; Hajak 2001; Krystal 2010; Krystal 2011; Le Bon 2003; Rush 1998; Satterlee 1995; Walsh 1998; Ware 1989). Hence, these 10 studies reflect a high level of sponsorship bias. Three of the eight studies evidencing low sponsorship bias were funded by pharmaceutical companies, but showed independence of the results through using external companies for blinding and data analysis. Studies that showed an unclear level of sponsorship bias often included a vague disclosure statement that made reference to a pharmaceutical company, but the relationship as drug provider or sponsor of the study remained ambiguous. Only two studies included no disclosure statement (Finnerty 1978; Zhou 2002). With regard to bias unrelated to sponsorship, Friedmann 2008 reported that participants in the trazodone group believed more than participants in the placebo group that they were taking active medication. Although this bias in perception may be expected with taking medication such a trazodone, it has the potential to change the outcomes of the study. Furthermore, the adherence to medication is questionable in this study, because there is a large discrepancy between the adherence percentages reported by automatic recording (37% to 43%) in comparison with self‐report (82% to 83%).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Selective serotonin reuptake inhibitors compared with placebo for insomnia.
| SSRIs compared with placebo for insomnia | |||
| Patient or population: adults with insomnia Setting: hospital inpatients and outpatients Intervention: SSRI (paroxetine 10‐20 mg or fluoxetine 20 mg) Comparison: placebo | |||
| Outcomes | Impact | No of participants (studies) | Quality of the evidence (GRADE) |
| Subjective measures of sleep (quality, duration, sleep onset latency, nocturnal awakenings, sleep, efficiency) (HAM‐D sleep subscale or PSQI at 6 or 12 weeks) | Combining results between studies was not possible. 2 paroxetine studies showed significant improvements in subjective sleep measures at 6 weeks (n = 60, P = 0.03) and 12 weeks (n = 27, P ≤ 0.001) measured using PSQI compared to placebo. No significant difference in the fluoxetine study (n = 48), which showed a change on the HAM‐D of 2.5 in the fluoxetine arm and 1.8 in the placebo arm at 8 weeks. | 135 (3 RCTs) | ⊕⊕⊝⊝ Low1 |
| Other subjective measures of sleep (total sleep time, sleep onset latency, nocturnal awakenings and subjective sleep efficiency) (PSQI) (at 12 weeks) | Data were very limited for other subjective sleep outcomes. 1 study reported other subjective measures of sleep at 12 weeks with paroxetine compared to placebo in 27 participants. This showed significantly increased total sleep time and subjective sleep efficiency, and reduced nocturnal awakenings and sleep onset latency with paroxetine (P ≤ 0.001) for all these measures. | 27 (1 RCT) |
⊕⊝⊝⊝ Very low1,2 |
| Adverse events (at 12 weeks) | No clear data for adverse events. No adverse events reported to be found the 12‐week paroxetine study (n = 27). Adverse events were not reported in the 6‐week paroxetine study or the 8‐week fluoxetine study. | 27 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 |
| PSG sleep outcomes (at 12 weeks) | Data were very limited for PSG outcomes. Only 1 RCT of paroxetine over 6 weeks with 60 participants reported PSG data. It showed no significant difference in sleep efficiency, but did show wake after sleep onset time to be significantly reduced (P = 0.02), increased time to fall asleep (P = 0.04) and increased alertness (P = 0.008) with paroxetine compared to placebo. | 60 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 |
| HAM‐D: Hamilton Rating Scale for Depression; n: number of participants; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index; RCT: randomised controlled trial; SSRI: selective serotonin reuptake inhibitor. | |||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
1Downgraded two levels for unclear risk of bias: lack of information on randomisation, allocation concealment and blinding in included studies and low numbers.
2Downgraded one level for imprecision: lack of reporting or sparse data for relevant outcome.
Summary of findings 2. Tricyclic antidepressants compared with placebo for insomnia.
| TCA compared with placebo for insomnia | ||||||
| Patient or population: adults with insomnia Setting: hospital outpatients Intervention: TCAs (doxepin 1 mg, 3 mg, 6 mg, 10 mg or 25‐50 mg or trimipramine 25‐200 mg) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with TCA | |||||
| Subjective measure of sleep quality (ISI, PSQI) (at 4, 6 or 12 weeks) | ‐ | The mean subjective measure of sleep quality in the intervention group was 0.39 standard deviations lower (0.56 lower to 0.21 lower) | ‐ | 518 (4 RCTs) | ⊕⊕⊕⊝ Moderate1 | Results suggested TCA improved subjective measures of sleep quality with a moderate effect size when measured at 4‐12 weeks. |
| Adverse events (at 4, 6 or 12 weeks) | 383 per 1000 | 393 per 1000 (294 to 502) | RR 1.02 (0.86 to 1.21) | 812 (6 RCTs) | ⊕⊕⊝⊝ Low1,2 | Results showed no significant difference in adverse events between TCA and placebo, but the evidence was low quality. |
| PSG sleep outcomes: sleep latency (at 4 and 12 weeks) | The mean sleep latency in the placebo group ranged from 17.43 to 34.9 min | The mean sleep latency in the TCA group was 4.27 min shorter (9.01 shorter to 0.48 longer) | ‐ | 510 (4 RCTs) | ⊕⊕⊕⊝ Moderate1 | Results show no difference in PSG sleep latency. |
| PSG sleep outcomes: sleep efficiency (at 4 and 12 weeks) | The mean sleep efficiency in the placebo group ranged from 65% to 82.84% | The mean sleep efficiency in the TCA group was 6.29 percentage points higher (3.17 higher to 9.41 higher) | ‐ | 510 (4 RCTs) | ⊕⊕⊕⊝ Moderate1 | Results suggested TCA improved sleep efficiency by an amount that may have clinical relevance. |
| PSG sleep outcomes: total sleep time (at 4 and 12 weeks) | The mean total sleep time in the placebo group ranged from 343.7 min to 408.2 min | The mean total sleep time in the TCA group 22.88 min longer (13.17 longer to 32.59 longer) | ‐ | 510 (4 RCTs) | ⊕⊕⊕⊝ Moderate1 | Results suggested TCA improved total sleep time by an amount that is likely to have clinical relevance. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAM‐D: Hamilton Rating Scale for Depression; ISI: Insomnia Severity Index; min: minute; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index; RCT: randomised controlled trial; RR: risk ratio; TCA: tricyclic antidepressant. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for unclear risk of bias: lack of information on randomisation, allocation concealment and blinding in included studies.
2Downgraded one level for very wide confidence interval including both large benefit and some harm.
Summary of findings 3. 'Other' antidepressants compared with placebo for insomnia.
| 'Other' antidepressants compared with placebo for insomnia | ||||||
| Patient or population: adults with insomnia Setting: outpatients Intervention: other antidepressants (trazodone 25‐150 mg) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with "other antidepressant" | |||||
| Subjective measure of sleep quality (PSQI, visual analogue scale or subjective rating of sleep at 6 months or 2 weeks or 7 days) | ‐ | The mean subjective measure of sleep quality in the intervention group was 0.34 standard deviations lower in the intervention group (0.02 to 0.66 standard deviations lower) | ‐ | 370 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | These results show improved subjective sleep quality for other antidepressants and placebo indicating a small effect size. |
| Adverse events | ‐ | ‐ | ‐ | 217 (2 RCTs) |
⊕⊕⊝⊝ Low1,2 | Combining results was not possible. 1 paper (n = 201) reported that 2 placebo‐treated and 5 trazodone‐treated participants withdrew due to adverse events (excessive sleepiness, dizziness, headache, vomiting and mild elevation of blood pressure) and that the trazodone group (65.4%) reported significantly more adverse effects at 2 weeks than the placebo group (75%) (P = 0.003). Another paper (n = 16) reported hangovers (n = 5) and dizziness (n = 2) in the trazodone group compared to hangovers (n = 1), headache (n = 2) and skin irritation (n = 1) in the placebo group. |
| PSG sleep outcomes: sleep efficiency (at 1 week and 4 weeks | The mean sleep efficiency in the placebo group ranged from 81.7% to 85.3 % | The mean sleep efficiency in the TCA group was 1.38 percentage points higher (2.87 lower to 5.67 higher) | ‐ | 169 (2 RCTs) | ⊕⊕⊝⊝ Low1,2 | Results showed no significant difference in sleep efficiency between other antidepressants and placebo. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n: number of participants; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index; RCT: randomised controlled trial; TCA: tricyclic antidepressant | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for unclear risk of bias; lack of information on randomisation, allocation concealment and blinding in included studies.
2Downgraded one level for imprecision; very wide confidence intervals, small numbers or both.
1. Selective serotonin reuptake inhibitors
1.1. Selective serotonin reuptake inhibitors versus placebo
Three studies comprising 135 participants compared an SSRI with a placebo (Reynolds 2006; Satterlee 1995; Zhou 2002). The study results could not be pooled as Satterlee 1995 reported no standard deviations (SD)/standard errors and Zhou 2002 reported all the elements of the PSQI as separate items. However, we provide a descriptive analysis below.
Primary outcomes
1.1.1. Subjective measure of sleep quality
Satterlee 1995 examined change from baseline to eight weeks on the HAM‐D sleep subscale in participants randomised to fluoxetine or placebo treatment. The change from baseline was 2.5 points in the fluoxetine group and 1.8 points in the placebo group.
Reynolds 2006 reported the subjective sleep quality from the Pittsburgh Diary‐based Measures of participants treated with paroxetine compared to placebo. The authors observed a small difference between the groups over the six‐week study period favouring the paroxetine group (P = 0.03).
Zhou 2002 did not report sleep quality.
1.1.2. Subjective measure of total sleep duration
Zhou 2002 found significantly improved total sleep time compared to placebo at 12 weeks (P < 0.001).
1.1.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
Zhou 2002 found significantly improved total sleep onset latency compared with placebo at 12 weeks (P < 0.001).
1.1.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
Zhou 2002 found significantly fewer awakenings after sleep onset in the paroxetine group compared with placebo at 12 weeks (P < 0.001).
1.1.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed).
Zhou 2002 found significantly improved total sleep efficiency compared with placebo at 12 weeks (P < 0.001).
1.1.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
No studies reported safety data.
Secondary outcomes
1.1.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
Only Reynolds 2006 collected PSG data.
1.1.7.1. Sleep latency
Compared with the placebo group, the paroxetine group took significantly longer to fall asleep (P = 0.04).
1.1.7.2. Sleep efficiency
There was no significant difference in the sleep efficiency of the paroxetine group compared with the placebo group.
1.1.7.3. Total sleep time
The study did not report total sleep time.
1.1.7.4. Waking time after sleep onset
The waking time after sleep onset was significantly reduced in the paroxetine group compared with the placebo group (P = 0.02).
1.1.7.5. Rapid eye movement latency
The study did not report rapid eye movement (REM) latency.
1.1.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
Reynolds 2006 reported an improvement in daytime alertness in the paroxetine group compared with the placebo group (P = 0.008).
1.2. Selective serotonin reuptake inhibitors versus other insomnia medication
Only one study compared an SSRI (paroxetine) with another insomnia medication (alprazolam), with 30 participants randomised to each intervention (Zhou 2002).
Primary outcomes
1.2.1. Subjective measure of sleep quality
The study reported the sleep parameters of the PSQI at the end of 12 weeks. There was a significant difference between the paroxetine and alprazolam groups at the 5% level in favour of paroxetine.
1.2.2. Subjective measure of total sleep duration
The paroxetine group reported that the mean total sleep time increased by 3.7 hours (SD 1.1). The total sleep time increased by 1.6 hours (SD 0.6) in the alprazolam group. This difference was significant at the 1% level.
1.2.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
Mean time to falling asleep was shorted by 64 minutes (SD 28) in the paroxetine group compared with 50 minutes (SD 22) in the alprazolam group.
1.2.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
Time awake reduced by 1.6 hours (SD 0.5) in the paroxetine group and by 0.8 hours (SD 0.9) in the alprazolam group.
1.2.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed).
Sleep efficiency improved by 40 percentage points (SD 22) in the paroxetine group compared with 23 points (SD 18) in the alprazolam group.
1.2.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
There were no serious adverse effects reported in either group. Two participants dropped out of the paroxetine group and four dropped out of the alprazolam group because of adverse effects.
Secondary outcomes
1.2.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
The study did not record objective measures of change in sleep.
1.2.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
The study did not record effect on daytime symptoms/functioning.
1.3. Selective serotonin reuptake inhibitors versus other antidepressant
Three studies compared an SSRI with another antidepressant medication in 489 participants with depression and insomnia (Corruble 2013; Gillin 1997; Rush 1998).
Primary outcomes
1.3.1. Subjective measure of sleep quality
The three studies using different scales were combined to assess subjective improvement in sleep quality. Where more than one time point was reported, results were pooled for the end point of the study. Corruble 2013 reported the PSQI at 24 weeks, Gillin 1997 reported the HAM‐D at eight weeks and Rush 1998 reported the HDRS at eight weeks. There was no significant difference in measure of sleep quality between SSRIs and other antidepressants (SMD 0.04, 95% CI ‐0.42 to 0.50; I2 = 78%; Analysis 1.1), but the level of heterogeneity was high.
1.1. Analysis.
Comparison 1 Selective serotonin reuptake inhibitors (SSRI) versus other antidepressants, Outcome 1 Subjective measure of sleep quality.
1.3.2. Subjective measure of total sleep duration
None of the studies reported subjective measure of total sleep duration.
1.3.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
One study reported a subjective measure of sleep onset latency, but found no differences between escitalopram and agomelatine with respect to time taken to fall asleep at 12 and 24 weeks (Corruble 2013).
1.3.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
None of the studies reported subjective measure of number of nocturnal awakenings.
1.3.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed)
None of the studies reported subjective measures of sleep efficiency.
1.3.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
Three studies reported adverse events (Corruble 2013; Gillin 1997; Rush 1998). There was no difference in effect between SSRI treatment and other antidepressant treatment (RR 1.36, 95% CI 0.76 to 2.44; I2 = 0%; Analysis 1.2).
1.2. Analysis.
Comparison 1 Selective serotonin reuptake inhibitors (SSRI) versus other antidepressants, Outcome 2 Adverse events.
Secondary outcomes
1.3.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
Rush 1998 reported EEG data on sleep latency. The mean in the nefazodone group was 23.8 (SD 33.1) and the mean in the fluoxetine group was 31.4 (SD 37.7) at eight weeks' follow‐up.
Gillin 1997 and Rush 1998 both reported sleep efficiency percentages for comparisons of nefazodone versus fluoxetine. There was a small effect in favour of nefazodone (MD ‐7.55, 95% CI ‐10.54 to ‐4.56; I2 = 0%; Analysis 1.3).
1.3. Analysis.
Comparison 1 Selective serotonin reuptake inhibitors (SSRI) versus other antidepressants, Outcome 3 Sleep efficiency.
1.3.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
Corruble 2013 used a VAS to record daytime symptoms, "feeling good" and "daytime sleepiness." At 24 weeks, the mean change from baseline for the "feeling good" scale in the escitalopram group was 38.0 (SD 34.0) and in the agomelatine group was 40.7 (SD 31.9). For the daytime sleepiness scale, the mean change from baseline for the escitalopram group was ‐32.3 (SD 32.5) and for the agomelatine group was ‐29.5 (SD 34.2).
1.4. Selective serotonin reuptake inhibitors versus other selective serotonin reuptake inhibitors
One study compared the effectiveness of three SSRI medications, fluoxetine, paroxetine and sertraline, against one another (Fava 2002). Based on the HAM‐D score at baseline, there were 119 participants with an insomnia diagnosis (34 fluoxetine, 41 sertraline and 44 paroxetine).
Primary outcomes
1.4.1. Subjective measure of sleep quality
The change from baseline scores on the sleep disturbance scale of the HAM‐D indicated no differences in effect between the three groups. Both the fluoxetine and sertraline groups experienced a change from baseline of 3.1 points (SD 2.0). The change from baseline for the paroxetine group was 2.9 (SD 2.4).
1.4.2. Subjective measure of total sleep duration
The study did not record subjective measure of total sleep duration.
1.4.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
The study did not record subjective measure of sleep onset latency.
1.4.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
The study did not record subjective measure of number of nocturnal awakenings or total nocturnal awakening time.
1.4.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed).
The study did not record subjective measure of sleep efficiency.
1.4.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
Adverse events were not reported separately for the participants with an insomnia diagnosis.
Secondary outcomes
1.4.7. Objective measures of change in sleep (such as electroencephalogram data)
The study did not record objective measures of change in sleep.
1.4.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
The study did not record effect on daytime symptoms/functioning.
2. Tricyclic antidepressants
2.1. Tricyclic antidepressants versus placebo
Six studies (812 participants) examined the effectiveness of TCAs compared with placebo: three in primary insomnia (Hajak 2001; Krystal 2010; Krystal 2011; Lankford 2012; Riemann 2002), and one in insomnia associated with Parkinson's disease (Rios Romenet 2013).
Primary outcomes
2.1.1. Subjective measure of sleep quality
Five studies measured subjective sleep quality. Krystal 2010; Lankford 2012; and Rios Romenet 2013 reported the ISI. These were at 12 weeks in the Krystal 2010 study and at six weeks in Lankford 2012 and Rios Romenet 2013. Riemann 2002 reported the PQSI at four weeks. Hajak 2001 used a VAS to assess sleep quality at four weeks; however, this was not included as it was not possible to accurately read the figures from the graph provided. The results for the remaining four studies with 518 participants were pooled. The results indicated that sleep quality was significantly better in the TCA groups than in the placebo groups (SMD ‐0.39, 95% CI ‐0.56 to ‐0.21; I2 = 0%; Analysis 2.1).
2.1. Analysis.
Comparison 2 Tricyclic antidepressants (TCA) versus placebo, Outcome 1 Subjective measure of sleep quality.
2.1.2. Subjective measure of total sleep duration
Two studies reported subjective total sleep time at follow‐up (Krystal 2010; Lankford 2012). This was four weeks for Lankford 2012 and 12 weeks for Krystal 2010. There was no significant difference in total sleep duration between the TCA group and the placebo group (MD 31.68 minutes, 95% CI ‐12.40 to 75.77; I2 = 91%; Analysis 2.2), but there was a high level of heterogeneity. Krystal 2010 also reported total sleep time at four weeks' follow‐up. When we pooled the studies at four weeks' follow‐up rather than at the end point, the results remained unchanged with no significant difference in reported total sleep time between the TCA group and the placebo group (MD 22.98 minutes, 95% CI ‐4.98 to 50.93; I2 = 76%; Analysis 2.2).
2.2. Analysis.
Comparison 2 Tricyclic antidepressants (TCA) versus placebo, Outcome 2 Subjective total sleep time.
2.1.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
One study reported subjective measure of sleep onset latency (Krystal 2010). At week 12, the score in the placebo group was 55.5 (SD 39.5). In the doxepin groups, the score was 37.5 (SD 22.8) in the doxepin 1 mg group and 39.9 (SD 30.3) in the doxepin 3 mg group. This showed an effect in favour of doxepin compared with placebo for both groups (doxepin 1 mg: P = 0.046; doxepin 3 mg: P = 0.003).
2.1.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
One study reported a subjective measure of waking time after sleep onset (Lankford 2012). There was a difference in favour of the doxepin 6 mg group compared to the placebo group at four weeks with lower mean waking time after sleep onset in the doxepin group (mean 66.5, SD 43.9) compared with placebo (mean 78.9, SD 56.5) (P < 0.01).
2.1.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed)
None of the studies examined subjective measure of sleep efficiency.
2.1.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
Six studies reported the incidence of adverse effects and events (Hajak 2001; Krystal 2010; Krystal 2011; Lankford 2012; Riemann 2002; Rios Romenet 2013). The pooled results in showed no difference in the number of adverse effects between TCAs and placebo (RR 1.02, 95% CI 0.86 to 1.21; I2 = 34%; Analysis 2.3).
2.3. Analysis.
Comparison 2 Tricyclic antidepressants (TCA) versus placebo, Outcome 3 Adverse events.
Secondary outcomes
2.1.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
Four studies reported objective measures of change in sleep measured by EEG (Hajak 2001; Krystal 2010; Krystal 2011; Riemann 2002). They included sleep latency, sleep efficiency, total sleep time, waking time after sleep onset and REM percentage.
2.1.7.1. Sleep latency
Four studies reported EEG data on sleep latency time. The pooled analysis showed no significant difference in sleep latency time between TCA and placebo (MD ‐4.27, 95% CI ‐9.01 to 0.48; I2 = 0%; Analysis 2.4).
2.4. Analysis.
Comparison 2 Tricyclic antidepressants (TCA) versus placebo, Outcome 4 Sleep latency.
2.1.7.2. Sleep efficiency
Four studies reported EEG data on sleep efficiency. The results indicated improved sleep efficiency in the TCA group compared with placebo (MD 6.29, 95% CI 3.17 to 9.41; I2 = 0%; Analysis 2.5).
2.5. Analysis.
Comparison 2 Tricyclic antidepressants (TCA) versus placebo, Outcome 5 Sleep efficiency.
2.1.7.3. Total sleep time
Four studies reported total sleep time. The pooled analysis indicated a longer total sleep time in the TCA group compared with placebo (MD 22.88 minutes, 95% CI 13.17 to 32.59; I2 = 0%; Analysis 2.6).
2.6. Analysis.
Comparison 2 Tricyclic antidepressants (TCA) versus placebo, Outcome 6 Total sleep time.
2.1.7.4. Waking time after sleep onset
Three studies reported waking time after sleep onset (Hajak 2001; Krystal 2010; Krystal 2011). Waking time was lower in the TCA group than the placebo group (MD ‐14.63 minutes, 95% CI ‐25.99 to ‐3.27; I2 = 75%; Analysis 2.7); however, there was a high level of heterogeneity. This may be because the results in Hajak 2001 expressed wakings after sleep onset as a percentage of sleep time.
2.7. Analysis.
Comparison 2 Tricyclic antidepressants (TCA) versus placebo, Outcome 7 Waking time after sleep onset.
2.1.7.5. Rapid eye movement latency
Two studies reported REM latency (Hajak 2001; Riemann 2002). The pooled analysis indicated that the TCA group spent more time in REM latency than the placebo group (MD 26.37 minutes, 95% CI 7.94 to 44.80; I2 = 0%; Analysis 2.8).
2.8. Analysis.
Comparison 2 Tricyclic antidepressants (TCA) versus placebo, Outcome 8 Rapid eye movement latency latency.
2.1.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
One study reported changes in daytime fatigue and cognitive functioning (Rios Romenet 2013). There was a significant improvement in daytime functioning on the Krupp Fatigue Severity Score in the doxepin group compared with placebo (P = 0.02) and in cognitive functioning Montreal Cognitive Assessment (MoCA) in the doxepin group compared with placebo (P = 0.007). Riemann 2002 reported on the "feeling rested in the morning" subscale of the SF‐A scale. The placebo group had a mean score of 2.82 (SD 1.05) at four weeks compared with 3.08 (SD 0.72) in the TCA group (P = 0.02)
2.2. Tricyclic antidepressants versus other insomnia medication
One study reported the effects of a TCA compared to another insomnia medication, comparing trimipramine to lormetazepam (Riemann 2002). There were 19 participants in the trimipramine group and 18 in the lormetazepam group.
Primary outcomes
2.2.1. Subjective measure of sleep quality
The mean PSQI score at four weeks was 8.39 (SD 3.36) in the lormetazepam group and 9.39 (SD 3.35) in the trimipramine group (P = 0.13) indicating no difference in effect between the groups.
2.2.2. Subjective measure of total sleep duration
The study did not report subjective measure of total sleep duration.
2.2.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
The study did not report subjective measure of sleep onset latency.
2.2.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
The study did not report subjective measure of number of nocturnal awakenings or total nocturnal awakening time.
2.2.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed).
The study did not report subjective measure of sleep efficiency.
2.2.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
Six participants (33.3%) in the lormetazepam group reported 13 adverse events. In the trimipramine group, the rate of adverse events was significantly higher with 15 participants (78.9%) reporting 42 adverse events.
Secondary outcomes
2.2.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
The study reported PSG results at four weeks. None of the differences in these objective measures were different between groups.
2.2.7.1. Sleep latency
Sleep latency was 26.31 minutes (SD 33.61) in the lormetazepam group and 23.34 minutes (SD 24.45) in the trimipramine group (P = 0.68).
2.2.7.2. Sleep efficiency
Sleep efficiency was 86.25% (SD 8.05) in the lormetazepam group and 84.53% (SD 15.20) in the trimipramine group (P = 0.22).
2.2.7.3. Total sleep time
Total sleep time was 408.61 minutes (SD 47.29) in the in the lormetazepam group and 406.13 minutes (SD 77.25) in the trimipramine group (P = 0.11).
2.2.7.4. Waking time after sleep onset
The study did not report waking time after sleep onset.
2.2.7.5. Rapid eye movement latency
There was no difference in REM latency in the lormetazepam group, with a mean of 82.86 minutes (SD 44.14) compared with 125.21 minutes (SD 117.23) in the trimipramine group (P = 0.45).
2.2.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
There was a significant difference in "feeling rested in the morning" as measured by the SF‐A scale (P = 0.02). The mean at four weeks in the lormetazepam group was 2.92 (SD 0.87) and in the trimipramine group was 3.08 (SD 0.72).
2.3. Tricyclic antidepressants versus other antidepressant
One study compared doxepin with imipramine in depressed people with insomnia (Finnerty 1978). There were 71 participants randomised to doxepin and 68 participants randomised to imipramine.
Primary outcomes
2.3.1. Subjective measure of sleep quality
The mean score on the sleep disturbance factor of the Hamilton Depression scale at four weeks was 0.4 in both groups. The Finnerty‐Goldberg Sleep scale was also used. Although the authors presented no data, they stated there was no statistically significant difference between groups.
2.3.2. Subjective measure of total sleep duration
The study did not record subjective measure of total sleep duration.
2.3.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
The study did not record subjective measure of sleep onset latency.
2.3.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
The study did not record subjective measure of number of nocturnal awakenings or total nocturnal awakening time.
2.3.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed)
The study did not record subjective measure of sleep efficiency.
2.3.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
There were 45 doxepin‐treated participants and 44 imipramine‐treated participants who experienced adverse effects. In the doxepin group, 75% of these adverse effects were "mild to moderate" while the figure was 82% in the imipramine group.
Secondary outcomes
2.3.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
The study did not record objective measures of change in sleep.
2.3.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
The study did not record effect on daytime symptoms/functioning.
2.4. Tricyclic antidepressants versus other tricyclic antidepressants
One study compared trimipramine with imipramine in depressed people with insomnia (Ware 1989). There were 15 participants randomised to the trimipramine group and 19 participants randomised to the imipramine group.
Primary outcomes
2.4.1. Subjective measure of sleep quality
Participants reported their sleep quality on a Likert scale from 1 "not at all" to 4 "extremely." The change from baseline to 30 days was 0.4 in the trimipramine group and 0.5 in the imipramine group.
2.4.2. Subjective measure of total sleep duration
The mean change from baseline to 30 days was 1.1 hours in the trimipramine group and 0.7 hours in the imipramine group. There was no overall difference between the groups, but there was an interaction between drug and study day (P < 0.01). There was an immediate increase in hours of sleep in the trimipramine group while the improvement in the imipramine group was more gradual.
2.4.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
The mean score in the trimipramine group improved by 25 minutes in the 30 days from baseline and the score in the imipramine group improved by 7 minutes. There was a statistically significant interaction between the drug and the study day (P < 0.01). For the both groups, sleep latency improved in the first two weeks, but this was maintained only in the trimipramine group.
2.4.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
The study did not record subjective measure of number of nocturnal awakenings or total nocturnal awakening time.
2.4.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed)
The study did not record subjective measure of sleep efficiency.
2.4.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
Two imipramine‐treated participants dropped out of the study because of adverse reactions. During the first two weeks, there were significantly fewer adverse reactions in the trimipramine group (P = 0.02). The authors reported that this was not due to any one type or class of adverse reaction and that none of the adverse reactions were serious.
Secondary outcomes
2.4.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
2.4.7.1 Sleep latency
Sleep latency improved by 16 minutes from baseline in the trimipramine group, but the imipramine group reported taking an additional 7 minutes to fall asleep (P < 0.01).
2.4.7.2 Sleep efficiency
There was significant greater (P < 0.01) sleep efficiency in the trimipramine group, where the change from baseline was 0.12, compared to the imipramine group, where the change was ‐0.06.
2.4.7.3 Total sleep time
The trimipramine group increased their total sleep time by 55 minutes compared to a decrease in total sleep time of 28 minutes in the imipramine group (P < 0.01).
2.4.7.4 Waking time after sleep onset
There was a decrease of nine in the percentage to time awake after sleep onset in the trimipramine group over the 30‐day period compared to an increase of eight in the imipramine group (P < 0.01).
3.1.7.5 Rapid eye movement latency
There was a mean increase in REM sleep latency of 183 minutes in the imipramine group compared with an increase of only 3 minutes in the trimipramine group (P < 0.01).
3.1.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
The study did not report the effect on daytime symptoms/functioning.
3. 'Other' antidepressants
3.1. 'Other' antidepressants versus placebo
Three studies provided useable data on other antidepressants versus placebo; two in primary insomnia (Roth 2011; Walsh 1998), and one in opiate dependence with insomnia (Stein 2012). All looked at trazodone versus placebo in 370 participants. Shell 2012 (in insomnia), Friedmann 2008 and Le Bon 2003 (in abstinent alcoholics with insomnia), and Khazaie 2013 (in women during the third trimester of pregnancy) also examined trazodone versus placebo, but did not provide sufficient data for it to be included in the pooled results. Palomaki 2003 looked at mianserin versus placebo in people with stroke with insomnia, but did not provide extractable data for pooled analysis.
Primary outcomes
3.1.1. Subjective measure of sleep quality
Three studies provided data on a subjective measure of sleep quality that could be pooled. Stein 2012 measured the PSQI at six months. Walsh 1998 included a subjective rating of sleep quality at two weeks. Roth 2011 used VAS to measure "difficulty sleeping" at seven days. There was a slight improvement in subjective sleep quality in the trazodone group compared with placebo (SMD ‐0.34, 95% CI ‐0.66 to ‐0.02; I2 = 49%; Analysis 3.1) (Figure 4).
3.1. Analysis.
Comparison 3 Other antidepressants versus placebo, Outcome 1 Subjective measure of sleep quality.
4.
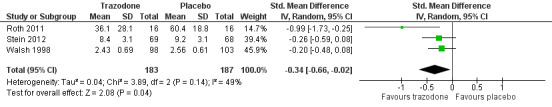
Forest plot of comparison: 3 'Other' antidepressants versus placebo, outcome: 3.1 Subjective measure of sleep quality.
Friedmann 2008 reported improved sleep quality in the trazodone group compared to placebo as measured by the PSQI at one and three months, but there was no significant difference at six months. Shell 2012 reported no significant difference between the trazodone and placebo group at 14 days' follow‐up.
Palomaki 2003 reported a significant effect on the three sleep items of the HDRS in favour of mianserin compared to placebo at two months (P = 0.02), but by six months there were no longer any differences between groups.
3.1.2. Subjective measure of total sleep duration
One study reported subjective total sleep time (Stein 2012). The total sleep time in the trazodone group was 406.1 minutes and in the placebo group was 389.4 minutes (P = 0.67).
3.1.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
One study reported subjective sleep latency (Stein 2012). The trazodone group averaged 36.6 minutes and the placebo group averaged 38.5 minutes (P = 0.69).
3.1.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
Two studies examined the total number of nocturnal awakenings (Stein 2012; Walsh 1998). There were significantly fewer awakenings in the trazodone group compared with the placebo group (MD ‐0.31, 95% CI ‐0.52 to ‐0.11; I2 = 0%; Analysis 3.2).
3.2. Analysis.
Comparison 3 Other antidepressants versus placebo, Outcome 2 Number of nocturnal awakenings.
3.1.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed)
One study reported a subjective measure of sleep efficiency (Stein 2012). In the trazodone group, the mean sleep efficiency was 84.5% while in the placebo group, it was 81.6% (P = 0.87), suggesting no difference between the groups.
3.1.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
Walsh 1998 reported that two placebo‐treated and five trazodone‐treated participants withdrew as a result of adverse events. The intervention group reported significantly more adverse effects than the placebo group.
Le Bon 2003 reported hangovers in five participants and dizziness in two participants in the trazodone group compared to hangovers in one participant, headache in two participants and skin irritation in one participant in the placebo group.
Secondary outcomes
3.1.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
3.1.7.1. Sleep latency
Roth 2011 reported no difference in sleep latency, with 26.2 minutes (SD 28.6) in the trazodone group compared with 24.5 minutes (SD 18.7) in the placebo group (P = 0.556).
Le Bon 2003 reported no difference in sleep latency, with 53 minutes in the trazodone group compared with 26 minutes in the placebo group.
3.1.7.2. Sleep efficiency
Two studies reported sleep efficiency (Roth 2011; Stein 2012. These results were pooled and there was no significant difference between trazodone and placebo (MD 1.38, 95% CI ‐2.87 to 5.63; I2 = 0%; Analysis 3.3). Le Bon 2003 also reported an improvement in sleep efficiency in the trazodone group compared to placebo (P = 0.015).
3.3. Analysis.
Comparison 3 Other antidepressants versus placebo, Outcome 3 Sleep efficiency.
Khazaie 2013 reported significantly improved sleep efficiency in the trazodone group compared to the placebo group (P < 0.0001) at six weeks, but no significant difference at two weeks.
3.1.7.3. Total sleep time
One study reported total sleep time (Stein 2012). This was 355.9 minutes in the trazodone group and 344.1 minutes in the placebo group (P = 0.62). Le Bon 2003 reported no difference in total sleep time. This was 340 minutes in the trazodone group compared to 314 minutes in the placebo group.
Khazaie 2013 reported significantly longer total sleep time in the trazodone group compared to placebo at six weeks (P < 0.0001), but no significant difference at two weeks.
3.1.7.4. Waking time after sleep onset
Roth 2011 reported no difference in waking time after sleep onset with 52.9 minutes (SD 54.9) in the trazodone group compared with 74.3 minutes (SD 61.1) in the placebo group (P = 0.401).
Le Bon 2003 reported improved wake to sleep onset in the trazodone group (3%) compared to the placebo group (12%) (P = 0.015).
3.1.7.5. Rapid eye movement latency
In Roth 2011, REM latency was 93.0 (SD 53.1) in the trazodone group compared with 84.3 (SD 40.8) in the placebo group (P = 0.385). In Le Bon 2003, REM latency was 98 minutes in the trazodone group compared with 81 minutes in the placebo group. Neither study showed a significant difference between the groups.
3.1.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
Walsh 1998 reported that daily morning ratings of sleepiness did not differ among groups at any time point, neither did ratings of disruption at work or in social and family life. Friedmann 2008 reported morning drowsiness in 20 (31.3%) participants in the trazodone group and 32 (48.5%) participants in the placebo group. The difference was borderline significant (P = 0.05). Khazaie 2013 reported daytime sleepiness in the trazodone group, but none of the participants discontinued treatment.
3.2. 'Other' antidepressants versus other insomnia medications
One study compared trazodone and zolpidem (Walsh 1998). It included 91 trazodone‐treated participants and 90 zolpidem‐treated participants. The primary comparison was between active treatments and placebo, therefore limited data were available to compare trazodone to zolpidem, but the authors did indicate where comparisons found no significant differences.
Primary outcomes
3.2.1. Subjective measure of sleep quality
The study used a subjective rating of sleep quality at two weeks and indicated no significant difference between the groups.
3.2.2. Subjective measure of total sleep duration
There were no significant differences between the trazodone and zolpidem groups with respect to subjective total sleep duration.
3.2.3. Subjective measure of sleep onset latency (measured as time taken to fall asleep)
There were no significant differences between the trazodone and zolpidem groups with respect to subjective sleep onset latency.
3.2.4. Subjective measure of number of nocturnal awakenings or total nocturnal awakening time
There were no significant differences between the trazodone and zolpidem groups with respect to total nocturnal awaking time.
3.2.5. Subjective measure of sleep efficiency (measured as a ratio of time asleep over time in bed)
The study did not report subjective measure of sleep efficiency.
3.2.6. Safety: number and type of spontaneously reported and measured adverse events, including reports of toxicity
The study reported that five zolpidem‐treated and five trazodone‐treated participants withdrew as a result of adverse events. Therefore, there were no significant differences between groups.
Secondary outcomes
3.2.7. Objective measures of change in sleep (such as electroencephalogram/polysomnography data)
The study did not report objective measures of change in sleep.
3.2.8. Effect on daytime symptoms/functioning: reported information on changes in daytime symptoms/functioning
Daily morning ratings of sleepiness did not differ among the groups at any time point, neither did ratings of disruption at work or in social and family life.
Subgroup analyses
Diagnosis of depression or anxiety
Selective serotonin reuptake inhibitors
Five studies included people with a depression or anxiety diagnosis (Corruble 2013; Fava 2002; Gillin 1997; Rush 1998; Satterlee 1995). Satterlee 1995 did not provide data that could be pooled as no SDs could be obtained. However, this study also found no statistically significant difference. Fava 2002 compared three SSRIs with one another and found no difference in effect. The remaining three studies are those pooled in Analysis 1.1.
Tricyclic antidepressants
Only one study in this category included people with a depression or anxiety diagnosis (Finnerty 1978). It found no differences between groups (see Section 2.3. Tricyclic antidepressants versus other antidepressant).
Recorded physical comorbidity (e.g. back pain)
Data regarding comorbid conditions was fairly limited. Only two studies attributed a known health condition to participants (Palomaki 2003; Rios Romenet 2013), but did not provide data that could be extracted for analysis. Therefore, it was not possible to conduct an analysis for this subgroup.
Low dose
The only studies in which low doses were employed were trials in which the intervention was doxepin and compared it to placebo. Within this subgroup, three studies used a low dose, while only one (Riemann 2002) used a higher dose. Hajak 2001 also used a higher dose, but it was not possible to extract data from the VAS for this study. The three low‐dose studies echoed the findings above for all TCA versus placebo. The results for the single higher‐dose study was in the same direction. In this small sample, there was no evidence of the effect varying by dose (test for subgroup differences: Chi2 = 0.86, degrees of freedom (df) = 1 (P = 0.35), I2 = 0%).
Sensitivity analyses
Lack of blinding
Only one study was at high risk of bias due to non‐blinded assignment (Rios Romenet 2013). Excluding this study from Analysis 2.1. changed the overall result very little from SMD ‐0.39 (95% CI ‐0.56 to ‐0.21) to SMD ‐0.38 (95% CI ‐0.57 to ‐0.19) and did not change the inferences: the subjective sleep quality was improved in the TCA group compared with the placebo group.
Poor concealment
One study was at high risk of bias due to poor concealment as no placebo tablets were given (Rios Romenet 2013). However, as stated in the sensitivity analysis above, excluding this study did not alter the inferences of the comparison between TCA and placebo.
Low numbers
The protocol defined low numbers as fewer than 10 per arm. One study had only six participants per arm (Rios Romenet 2013). Excluding this study did not alter the inferences of the comparison between TCA and placebo.
Missing data
Five studies were at high risk of bias due to a large proportion of the outcome data being missing. These were Fava 2002 (27%), Finnerty 1978 (30%), Reynolds 2006 (26%), Roth 2011 (74%) and Zhou 2002 (27%). Of these, only Roth 2011 was included in a pooled analysis. Excluding this study from the comparison of 'other antidepressants versus placebo' changes the effect from SMD ‐0.34 (95% CI ‐0.66 to ‐0.02) to SMD ‐0.22 (95% CI ‐0.44 to ‐0.01). This did not alter the overall inference of the comparison, which favoured "other antidepressants" over placebo.
Reporting bias
One study was at high risk of reporting bias and was not included in pooled data analysis (Finnerty 1978).
Discussion
Summary of main results
Searches conducted to July 2015 identified 4245 references; 220 were screened in full text and 23 studies with 2806 participants were included in the review. The included studies did not report all the outcomes that were prespecified in the protocol.
Selective serotonin reuptake inhibitors
We found very low quality evidence comparing SSRIs with placebo (Table 1). Three studies including 135 eligible participants compared SSRIs with placebo. Combining results was not possible due to reporting differences. Two paroxetine studies showed significant improvements in subjective sleep measures at six and 12 weeks. There was no difference in the fluoxetine study. One study also reported PSG results and a daytime function outcome. There was increased objectively measured sleep latency and reduced waking after sleep onset in the paroxetine group, and increased subjective daytime alertness.
One study with 60 participants and a significant risk of bias compared an SSRI with another insomnia medication. The paroxetine group showed significantly lower sleep onset latency and waking during the night, and significantly greater total sleep time and sleep efficiency, compared with alprazolam. There were no serious adverse effects reported in either group.
We found very low to moderate quality evidence comparing an SSRI with another antidepressant. Three studies compared SSRI (escitalopram or fluoxetine) with agomelatine or nefazodone, all were conducted in people with major depressive disorder who also had insomnia. Combining these studies of 489 participants, Analysis 1.1 measures of subjective sleep quality showed heterogeneity was high so it was not possible to infer a clear effect when comparing SSRIs with other antidepressants. Objective results from PSG recordings showed that sleep efficiency and sleep onset latency improved in the nefazodone groups and worsened slightly in the fluoxetine groups (Analysis 1.3). There were no differences between drug groups on measures of daytime function reported.
One study compared three different SSRIs in major depression with insomnia (fluoxetine, paroxetine and sertraline) and found no difference on sleep measured by HAM‐D sleep items.
In all the SSRI studies, adverse events were either not reported, or showed similar low rates between drug and placebo, or between different drugs (Analysis 1.2).
Tricyclic antidepressants
We found low to moderate quality evidence comparing TCAs with placebo (Table 2). Six studies (812 participants) compared a TCA with placebo (five used doxepin, one used trimipramine). We found no studies of amitriptyline. Four studies (518 participants) with moderate quality evidence could be pooled showing significant improvement in subjective sleep quality compared with placebo (Analysis 2.1). PSG measurements of objective sleep, with moderate quality evidence, showed increased sleep efficiency (Analysis 2.5), longer sleep time (Analysis 2.6), and decreased waking during the night (Analysis 2.7). Two studies reported changes in daytime function. They reported significant improvements in fatigue and cognitive function in the doxepin group compared with placebo, and an increase in feeling rested in the morning and well‐being in the evening after trimipramine.
There was no significant difference in reported adverse effects or events between TCAs and placebo, though the quality of the evidence was low.
Three studies compared a TCA with another medication. One study compared doxepin with lormetazepam, one compared doxepin with imipramine and one compared trimipramine with imipramine. None revealed significant differences on secondary outcomes, but in one study, the trimipramine group showed more improvement than the imipramine group.
'Other' antidepressants versus placebo or other insomnia medications
We found very low to moderate quality evidence comparing other antidepressants with placebo (Table 3). Eight studies compared other antidepressants with placebo (one used mianserin; seven used trazodone). Three trazodone studies (370 participants) provided extractable data of moderate quality indicating improvement in subjective sleep outcomes for trazodone over placebo (Analysis 3.1). One study of trazodone measured PSG and found a significant effect of trazodone to decrease night‐time awakenings (Analysis 3.2) and sleep efficiency (Analysis 3.3). Three trazodone studies reported on adverse events or effects in trazodone groups compared to placebo groups (i.e. 'morning grogginess,' and increased dry mouth), but quality was low and there were insufficient data to draw inferences.
Where possible, we performed subgroup analyses, but data were limited and thus the results must be treated with caution. The subgroup analyses detected no consistent influence of the degree of depression or anxiety at baseline assessment, the presence of comorbid physical ill‐health, or drug dosage (Analysis 4.1).
4.1. Analysis.
Comparison 4 Subgroup analysis ‐ low dose compared to not low dose, Outcome 1 Subjective measure of sleep quality.
Overall completeness and applicability of evidence
A concerted and repeated search of the published literature resulted in the identification of 23 eligible studies and repeated attempted contact with researchers in the field generated no additional relevant studies. The final database included seven studies with SSRIs, eight studies with TCAs, no studies with MAOIs and eight studies with 'other' antidepressants (seven used trazodone and one used mianserin). The studies displayed a broad range of methodologies, employed a wide array of subjective (questionnaires, scales, diaries) and objective (PSG or 'sleep EEG') outcome measures, at a variety of end points and came from different settings and patient groups (including comorbid drug and alcohol misuse and parkinsonism). Insomnia was not the primary inclusion criterion for participants in some of the papers, where the main focus was on another diagnosis such as depression or anxiety. The sleep outcomes were not always the primary outcome measure for the research and the sleep data were sometimes presented in a limited way that made data extraction challenging. When there were sufficient studies with broadly similar design to permit group analysis, there was sometimes significant heterogeneity between studies, so rendering the findings difficult to interpret.
Our risk of bias assessments (conducted independently by two review authors, any disagreements assessed and determined by a third review author) reveal that while most studies were not assessed to have a high risk of bias overall, quite a few had an unclear level of bias for several categories. This was particularly so for allocation concealment. Five studies were at high risk of bias for incomplete data outcomes and eight studies at high risk of bias for sponsorship.
The included trials had a variety of reporting methods and end points and it was sometimes not possible to fully extract useable data for the published paper that could be combined (e.g. when VAS were presented in the papers). In this case the authors were always contacted (often several times) to request further clarification or data. Only three authors responded and unfortunately they were unable to provide the requested additional data.
This review and meta‐analysis reveals a limited evidence base on which to make inferences about the potential value or otherwise of antidepressant drugs for managing people with primary insomnia (now known as insomnia disorder). The studies identified were typically small with design limitations or unclear assessments of bias, which make it difficult to identify reliable findings and to draw robust conclusions. What was clear was that published trials provided no evidence to support the long‐term use of an antidepressant drug in the management of people with primary insomnia. There is some evidence to support the short‐term use of some TCAs (low‐dose doxepin) or trazodone, but insufficient evidence to support the short‐term use of an SSRI. There was no evidence or amitriptyline, which is one of the most commonly prescribed antidepressants for insomnia in clinical practice.
Our review found evidence for a small, but significant advantage for TCA over placebo on subjective assessment of sleep quality (pooled data from four studies). However, it should be noted that the pooled TCA studies were all of doxepin, which is a drug used in depression at doses between 75 mg/day and 300 mg/day. At these higher doses, it is an inhibitor of reuptake of noradrenaline and serotonin, which is how it probably exerts its antidepressant action. However, in the studies included in this review doxepin was used at very low doses (1 mg, 3 mg and 6 mg), at which it blocks histamine receptors but has very little action on other receptors or reuptake. It is probable that this antihistamine action underlies its effects in the studies of insomnia included in the meta‐analysis in this review (Wilson 2010). Doxepin is now licensed in the USA (but not in Europe) for the treatment of insomnia at doses of 3 mg and 6 mg at night.
The data on tolerability and safety of antidepressants for insomnia was similarly limited, with many papers not reporting these outcomes. Where pooling of data was possible (SSRI compared with other antidepressants and TCA compared with placebo) there were no significant differences in tolerability and safety, but the overall quality of this evidence was low. In other settings, TCA are known to have significant tolerability problems and are potentially fatal if taken in overdose (Wilson 2010). As such it currently inadvisable to recommend the use of a TCA for the short‐term treatment of people with primary insomnia, even though this is an approach that seems to be commonly adopted in clinical practice.
Quality of the evidence
Overall, the quality of the evidence included in this review was very low to moderate on the GRADE evidence profile. Thus, the estimates of effect should be considered uncertain as further research could change the estimate of effects and the degree of confidence for its applicability in clinical practice.
For SSRIs, data could be pooled when compared with other antidepressants, but not when compared with placebo or other insomnia medications. Three studies contributed to the subjective measures of sleep quality pooled data. These were RCTs, but had serious risks of bias because of lack of information in the papers on randomisation, allocation concealment and blinding. Heterogeneity was also high at 78%. Thus, the quality of evidence was downgraded to low. For adverse event and sleep efficiency data there were very wide CIs and for sleep efficiency there was also small numbers of participants, but heterogeneity was not high. Thus, these outcomes had a low GRADE quality assessment.
For TCA, data could be pooled when compared with placebo, but not when compared with other insomnia medications or other antidepressants. Four studies contributed to the subjective measures of sleep quality pooled data. These were RCTs, but had serious risks of bias because of lack of information in the papers on randomisation, allocation concealment and blinding. Heterogeneity was not high. This gave a moderate GRADE quality assessment. For adverse event and sleep efficiency data there were very wide CIs and for sleep efficiency there was also small numbers of participants giving a low GRADE quality assessment.
For other antidepressants, data could be pooled when compared with placebo, but not when compared with other insomnia medications or other antidepressants. Three studies contributed to the subjective measures of sleep quality pooled data. These were all RCTs of trazodone but had serious risks of bias because of lack of information in the papers on randomisation, allocation concealment and blinding. Heterogeneity was not high. This gave moderate GRADE quality assessment. For sleep efficiency data there were wide CIs and small numbers of participants giving a low GRADE quality assessment.
Potential biases in the review process
Potential limitations of this review include identification, assessment and data extraction of eligible studies and antidepressant categorisation.
The Cochrane Common Mental Disorders group assisted us with a rigorous database search to ensure a robust search strategy and identification of as many potentially eligible studies as possible. Key trialists and authors in the research field were also contacted to ask for any additional studies they were aware of (either published or underway). However, there is still a risk of publication bias with RCTs particularly of those with negative outcomes. A significant number of studies found were published more than 15 years ago, before the advent of robust clinical trial registries and a number were funded by pharmaceutical companies. There may be unpublished studies that we have not identified. There were insufficient studies to perform a funnel plot to assess publication bias. A potential additional source of bias could be a failure to identify papers in populations with secondary insomnia, where sleep disturbance was measured as a secondary outcome, and where the sleep outcomes were reported in the full text of the paper but not clearly identified in the abstract, key words or database subject headings. Our focus was on a clear definition of insomnia at baseline rather than just reports of sleep disturbance. Our search terms were sensitive for insomnia but did not search for vaguer terms such as sleep disturbance. One way to explore if there was potentially valid additional data published would be to consider increasing the sensitivity of the search by including terms for sleep/sleep disturbance together with terms for comorbid conditions where insomnia is most prevalent.
We attempted to include as many trials as possible and included a range of definitions of insomnia and comparator treatments: placebo; medications for insomnia (such as short‐acting benzodiazepines, and so‐called 'Z‐drugs'); other antidepressants; waiting list control and treatment as usual. Antidepressant medications were included at all doses and there was no restriction of eligibility for comorbidities. Indeed, many of the included trials were undertaken in populations with a primary diagnosis of depression or anxiety and we extracted reported data on those with an defined insomnia disorder in addition. After discussion with the Cochrane Mental Health group, trials of tryptophan were excluded as it was deemed a dietary supplement rather than an antidepressant. Trials involving quetiapine were excluded as it was deemed an antipsychotic rather than an antidepressant. Trials with fewer than three days of drug treatment were also excluded as it was deemed that clinically important effects of antidepressants on insomnia (by definition a long‐term condition) required more than a one or two doses of an antidepressant to be able to determine the effect.
There were a small number of studies included in the pooled meta‐analyses. These were analysed as per the protocol, using a random‐effects model. However, with small numbers of studies, a fixed‐effect model may provide more robust estimates of effect. Future updates to this review will include a sensitivity analysis using the fixed‐effect approach.
For this review, antidepressants were grouped as described in the protocol into traditional classes such as TCAs, SSRIs and other antidepressants. This traditional antidepressant categorisation has acknowledged limitations due to the varying mechanisms of actions (i.e. reuptake inhibitors and modulators, receptor blockers and enzyme inhibitors) and there has been the been some debate in recent years regarding alternative nomenclatures and classifications. In particular, our category 'other' antidepressants included a range of disparate antidepressants and combining these in meta‐analysis could have been questioned. In the review, due to the data available, all the pooled data in the 'other' antidepressant group relate to a single antidepressant (trazodone).
Agreements and disagreements with other studies or reviews
Analyses from this study concur with other published reviews and papers highlighting the paucity of evidence for the use of antidepressants for insomnia and the need for further high‐quality trials, while acknowledging the limited evidence for the short‐term use of some antidepressants (Buscemi 2007; Wilson 2010).
Buscemi 2007 included eight RCTs of antidepressants (doxepin, trazodone, trimipramine and pivagabine) compared with placebo for insomnia duration of one night to five weeks, which the authors described as moderate quality on the Jadad scale. PSG data were the main outcome measures. They reported that these studies favoured antidepressants (weighted mean difference (WMD) PSG, sleep onset latency ‐7.0 minutes, 95% CI ‐10.7 to ‐3.3), but sleep diary results were 'fewer and non‐significant' (WMD sleep diary, sleep onset latency ‐12.2 minutes, 95% CI ‐22.3 to ‐2.2). They included three studies that reported safety and they reported a significant increased risk of harm in the antidepressant group compared with placebo (risk difference 0.09) with the most commonly reported adverse events being somnolence, headache, dizziness and nausea. Our results agree with the conclusions of this systematic review: there is some evidence for short‐term use of some antidepressants (particularly trazodone and doxepin) for insomnia, but that there is paucity of data and that further studies are needed to establish long‐term safety and efficacy and to determine if they are equivalent in efficacy to benzodiazepines and 'Z' drugs.
Mayers 2005 published a systematic review that included RCTs assessing the impact on sleep of antidepressants when compared with placebo or other antidepressants, but did not require a primary diagnosis of insomnia and did not perform any meta‐analyses. They reported that antidepressants were associated with differing effects in sleep profiles with variations within and between classes.
Krystal 2009 published a compendium of placebo‐controlled trials of the risks/benefits of pharmacological treatments for insomnia. This paper highlighted the lack of data on the efficacy and safety in antidepressants and documented only RCTs in doxepin and trazodone.
Thaler 2012 identified six head‐to‐head trials (involving 1061 participants) of the effect of second‐generation antidepressants on insomnia in people with depression, but only two of the included trials required an initial diagnosis of insomnia. They reported the strength of the evidence to be low to moderate, being weakened by inconsistency and imprecision, but concluded the evidence suggested that the SSRIs did not differ with regards to their effect on insomnia.
Authors' conclusions
Implications for practice.
This comprehensive literature search identified only a small number of studies with short‐term follow‐up on the use of antidepressants for managing primary insomnia. The findings of the included studies provide only equivocal data supporting short‐term use for some tricyclic antidepressants (doxepin in low dose), and for trazodone, but with no evidence to support long‐term use. There was no evidence for amitriptyline despite its common use in clinical practice, or to support long‐term antidepressant use for insomnia. Current research evidence does not support the widespread practice of prescribing antidepressants for insomnia. Health professionals and patients should be made aware of the current paucity of evidence for antidepressant medications commonly used for insomnia management. Increased access to, and provision of, other evidenced based ways to manage insomnia should be explored, such as increased access to psychological therapies (e.g. cognitive behavioural therapy for insomnia).
Implications for research.
There is a need for randomised placebo‐controlled trials of antidepressants within the setting of primary medical care. Previous studies have many identified risks of bias and a number of design limitations. These should be avoided in a new study by ensuring that the trial is independent, of sufficient size to permit some participant attrition, with adequate power to be able to generate reliable findings, and with prespecified primary subjective (rather than objective) outcome measures. The study design should include assessments of the balance of benefit and risk after both acute treatment (arguably at four to eight weeks) and continuation treatment (arguably at between 26 and 52 weeks) and should include robust data collection regarding adverse effects. Because of the common somatic and psychiatric comorbidity of insomnia, the study design should permit the inclusion of people in whom insomnia is comorbid with physical illness or mental disorder (or both), with sensitivity analyses to take account of the influence of coexisting medical conditions.
Acknowledgements
CRG funding acknowledgement
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
The NIHR School of Primary Care Research (SPCR) provided a small grant to part support undertaking this review, as did the Primary Care and Population Sciences (PCPS) Unit at the University of Southampton.
Ms Francesca Bolognesi for administrative support, database setup and management.
Dr Ruihua Hou for translating studies.
Dr Konstantin Poscharny for translating studies.
Jin Sun, Centre for Evidence‐Based Chinese Medicine, Beijing for translating studies.
Dr Lily Lai for facilitating translation of studies
Disclaimer
The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Database search strategies: CENTRAL, MEDLINE, Embase, PsycINFO
The Cochrane Central Register of Controlled Trials (CENTRAL) Issue 10, 2013 (n = 1103) (updated to 2017, Issue 11) [Condition] #1. MeSH descriptor: [SLEEP INITIATION AND MAINTENANCE DISORDERS] explode all trees #2. insomni* or dyssomni* #3. ("sleep impact scale" or "sleep questionnaire" or "sleep scale" or "sleep evaluation questionnaire" or "sleep quality index" or PSQI or "sleep impairment index" or "sleepiness scale" or "sleep log" or "sleep diar*"):ti,ab #4. (sleep NEAR (initiation or onset or maintenance)):ti,ab #5. (nocturnal NEXT (wake* or awake*)):ti,ab #6. sleep:ti #7. (#1 or #2 or #3 or #4 or #5 or #6) [Intervention] #8. MeSH descriptor: [ANTIDEPRESSIVE AGENTS] explode all trees #9. MeSH descriptor: [MONOAMINE OXIDASE INHIBITORS] explode all trees #10. MeSH descriptor: [NEUROTRANSMITTER UPTAKE INHIBITORS] explode all trees #11. (antidepress* or "anti depress*" or anti‐depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic):ti,ab #12. (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin* or (Bupropion or Amfebutamone) or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamin* or (CX157 or Tyrima or Tririma) or Demexiptilin* or Deprenyl or (Desipramin* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin* or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or (Lu AA21004 or Vortioxetine) or (Lu AA24530 or Tedatioxetine) or (LY2216684 or Edivoxetine) or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone) #13. (#8 or #9 or #10 or #11 or #12) #14. (#7 AND #13)
Ovid MEDLINE 1950 to 6 November 2013 (updated 8 July 2015, 3 August 2016, 12 December 2017) [Condition] 1. exp “SLEEP INITIATION AND MAINTENANCE DISORDERS”/ 2. insomni*.tw. 3. SLEEP/de [drug effects] 4. exp SLEEP STAGES/de [drug effects] 5. WAKEFULNESS/de [drug effects] 6. (sleep impact scale or sleep questionnaire or sleep scale or sleep evaluation questionnaire or sleep quality index or PSQI or sleep impairment index or sleepiness scale or sleep log or sleep diar*).tw. 7. (sleep adj3 (initiation or onset or maintenance)).tw. 8. (nocturnal adj (wake* or awake*)).tw. 9. or/1‐8 [Intervention] 10. exp ANTIDEPRESSIVE AGENTS/ 11. exp MONOAMINE OXIDASE INHIBITORS/ 12. exp NEUROTRANSMITTER UPTAKE INHIBITORS/ 13. (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic).mp. 14. (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin* or (Bupropion or Amfebutamone) or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamin* or (CX157 or Tyrima or Tririma) or Demexiptilin* or Deprenyl or (Desipramin* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin* or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or (Lu AA21004 or Vortioxetine) or (Lu AA24530 or Tedatioxetine) or (LY2216684 or Edivoxetine) or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).mp. 15. or/10‐14 [RCT Filter] 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomi#ed.ti,ab. 19. randomly.ab. 20. placebo.ab. 21. trial.ab. 22. groups.ab. 23. (control* adj3 (trial or study)).ab,ti. 24. ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy)).mp. 25. exp “SLEEP INITIATION AND MAINTENANCE DISORDERS”/dt [drug therapy] 26. (animals not (humans and animals)).sh. 27. or/16‐25 28. 27 not 26 [Condition + Intervention + RCT Filter] 29. (9 and 15 and 28)
Ovid Embase 1980 to 2013 Week 44 (updated 8 July 2015, 3 August 2016, 12 December 2017) [Condition] 1. exp *SLEEP/ 2. exp *INSOMNIA/ 3. INSOMNIA/dt [drug therapy] 4. SLEEP/dt,pd [drug therapy, pharmacology] 5. (insomni* or sleep* or dyssomni* or wake* or awake* or chrono*).ti. 6. (sleep adj3 (initiation or onset or maintenance)).tw. 7. (nocturnal adj (wake* or awake*)).tw. 8. INSOMNIA SEVERITY INDEX/ 9. PITTSBURGH SLEEP QUALITY INDEX/ 10. EPWORTH SLEEPINESS SCALE/ 11. SLEEP PARAMETERS/ or SLEEP PATTERN/ or SLEEP QUALITY/ or SLEEP TIME/ 12. (insomnia rating scale* or WHIIRS or insomnia severity index or insomnia treatment scale or sleep impact scale or sleep questionnaire or sleep scale or sleep evaluation questionnaire or sleep quality index or PSQI or sleep impairment index or sleepiness scale or sleep log or sleep diar*).mp. 13. or/1‐12 [Intervention] 14. PSYCHOPHARMACOLOGY/ 15. PSYCHOTROPIC AGENT/ 16. exp ANTIDEPRESSANT AGENT/ 17. SEROTONIN RECEPTOR AFFECTING AGENT/ or SEROTONIN UPTAKE INHIBITOR/ or SEROTONIN NORADRENALIN REUPTAKE INHIBITOR/ or TRIPLE REUPTAKE INHIBITOR/ 18. DOPAMINE RECEPTOR AFFECTING AGENT/ or DOPAMINE UPTAKE INHIBITOR/ 19. ADRENERGIC RECEPTOR AFFECTING AGENT/ or NORADRENALIN UPTAKE INHIBITOR/ 20. NEUROTRANSMITTER UPTAKE INHIBITORS/ 21. exp MONOAMINE OXIDASE INHIBITOR/ 22. (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic).mp. 23. (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin* or (Bupropion or Amfebutamone) or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamin* or (CX157 or Tyrima or Tririma) or Demexiptilin* or Deprenyl or (Desipramin* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin* or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or (Lu AA21004 or Vortioxetine) or (Lu AA24530 or Tedatioxetine) or (LY2216684 or Edivoxetine) or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).mp. 24. or/14‐23 [RCT Filter] 25. randomized controlled trial.de. 26. randomization.de. 27. placebo.de. 28. placebo.ti,ab. 29. randomi#ed.ti,ab. 30. randomly.ab. 31. SINGLE BLIND PROCEDURE/ or DOUBLE BLIND PROCEDURE/ or TRIPLE BLIND PROCEDURE/ 32. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab. 33. FACTORIAL DESIGN/ 34. factorial*.ti,ab. 35. (assign or assigned).ab. 36. allocat*.ab. 37. crossover procedure.de. 38. (crossover* or cross over*).ti,ab. 39. (control* adj3 (trial or study)).ti,ab. 40. ((animal or nonhuman) not (human and (animal or nonhuman))).de. 41. or/25‐39 42. 41 not 40 [Condition + Intervention + RCT Filter] 43. (13 and 24 and 42)
Ovid PsycINFO 1806 to October Week 5 2013 (updated 8 July 2015, 3 August 2016, 12 December 2017) 1. INSOMNIA/ 2. (insomni* or dyssomni*).ti,ab,id,tm. 3. (sleep impact or sleep questionnaire or sleep scale or sleep evaluation or sleep quality or PSQI or sleep impairment or sleepiness scale or sleep log or sleep diar*).ab,id,tm. 4. (sleep adj3 (initiation or onset or maintenance)).ti,ab,id. 5. (nocturnal adj (wake* or awake*)).ti,ab,id. 6. or/1‐5 7. exp ANTIDEPRESSANT DRUGS/ 8. exp SEROTONIN NOREPINEPHERINE REUPTAKE INHIBITORS/ or exp SEROTONIN REUPTAKE INHIBITORS/ 9. exp NEUROTRANSMITTER UPTAKE INHIBITORS/ 10. exp MONOAMINE OXIDASE INHIBITORS/ 11. exp TRICYCLIC ANTIDEPRESSANT DRUGS/ 12. NOREPINEPHRINE/ 13. SEROTONIN/ 14. (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic).ti,ab,id. 15. (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin* or (Bupropion or Amfebutamone) or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamin* or (CX157 or Tyrima or Tririma) or Demexiptilin* or Deprenyl or (Desipramin* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin* or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or (Lu AA21004 or Vortioxetine) or (Lu AA24530 or Tedatioxetine) or (LY2216684 or Edivoxetine) or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).ti,ab,id. 16. or/7‐15 17. treatment effectiveness evaluation.sh. 18. clinical trials.sh. 19. mental health program evaluation.sh. 20. placebo.sh. 21. placebo.ti,ab,id. 22. randomly.ab. 23. randomi#ed.ti,ab,id. 24. trial.ti,ab. 25. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).mp. 26. (control* adj3 (trial or study)).ti,ab,id. 27. factorial*.ti,ab,id. 28. allocat*.ab. 29. (assign or assigned).ab. 30. (crossover* or cross over*).ti,ab,id. 31. "2000".md. [Methodology: Treatment Outcome/Clinical Trial] 32. or/17‐31 33. (6 and 16 and 32)
Data and analyses
Comparison 1. Selective serotonin reuptake inhibitors (SSRI) versus other antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective measure of sleep quality | 3 | 489 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.42, 0.50] |
| 2 Adverse events | 3 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.76, 2.44] |
| 3 Sleep efficiency | 2 | 157 | Mean Difference (IV, Random, 95% CI) | ‐7.55 [‐10.54, ‐4.56] |
Comparison 2. Tricyclic antidepressants (TCA) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective measure of sleep quality | 4 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.56, ‐0.21] |
| 2 Subjective total sleep time | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 End of follow‐up | 2 | 469 | Mean Difference (IV, Random, 95% CI) | 31.68 [‐12.40, 75.77] |
| 2.2 4‐week follow‐up | 2 | 469 | Mean Difference (IV, Random, 95% CI) | 22.98 [‐4.98, 50.93] |
| 3 Adverse events | 6 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.21] |
| 4 Sleep latency | 4 | 510 | Mean Difference (IV, Random, 95% CI) | ‐4.27 [‐9.01, 0.48] |
| 5 Sleep efficiency | 4 | 510 | Mean Difference (IV, Random, 95% CI) | 6.29 [3.17, 9.41] |
| 6 Total sleep time | 4 | 510 | Mean Difference (IV, Random, 95% CI) | 22.88 [13.17, 32.59] |
| 7 Waking time after sleep onset | 3 | 473 | Mean Difference (IV, Random, 95% CI) | ‐14.63 [‐25.99, ‐3.27] |
| 8 Rapid eye movement latency latency | 2 | 77 | Mean Difference (IV, Random, 95% CI) | 26.37 [7.94, 44.80] |
Comparison 3. Other antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective measure of sleep quality | 3 | 370 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.66, ‐0.02] |
| 2 Number of nocturnal awakenings | 2 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.52, ‐0.11] |
| 3 Sleep efficiency | 2 | 169 | Mean Difference (IV, Random, 95% CI) | 1.38 [‐2.87, 5.63] |
Comparison 4. Subgroup analysis ‐ low dose compared to not low dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective measure of sleep quality | 4 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.56, ‐0.21] |
| 1.1 Not low dose | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.36, ‐0.02] |
| 1.2 Low dose | 3 | 481 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.55, ‐0.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Corruble 2013.
| Methods | Randomised, double‐blind, controlled trial (flexible dose) | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): no formal diagnosis of insomnia made; however, baseline scores of HAM‐D sleep component, VAS sleep measures and PSQI scores indicate baseline insomnia. We reported on subset of 187 participants with high levels of sleep complaints (PSQI ≥ 13 at baseline). Other diagnoses: MDD Number of participants randomised: 324 total; 187 in the insomnia subgroup which represented 57.7% of randomised participants. Agomelatine: n = 164 Escitalopram: n = 160 Number of participants: Agomelatine: n = 144 completed 12 weeks; n = 124 completed 24 weeks Escitalopram: n = 137 completed 12 weeks; n = 115 completed 24 weeks Age, mean (SD) years: Agomelatine: 43.6 (12.9) Escitalopram: 42.8 (11.8) Gender (M/F): Agomelatine: 26.8%/73.2% Escitalopram: 31.3%/68.7% Race/ethnicity: not reported Country: international study with 51 centres across Australia, Brazil, Canada, France, Russia, South Africa and the UK Setting: multiple outpatient centres Included: people with single or recurrent episode of MDD for ≥ 4 weeks with or without melancholic features based on HAM‐D 17 total score ≥ 22, CGI‐S score ≥ 4 or HADS ≥ 11. HAM‐D 17 score had to be stable between selection and inclusion (decrease < 20%), without seasonal pattern, without psychotic features and without catatonic features. Participants were required to be physically healthy or to have stabilised significant illnesses on the basis of medical history, physical examination, 12‐lead ECG and clinical laboratory tests. For insomnia subgroup: PSQI ≥ 13 Excluded: MDE with seasonal pattern or psychotic features; chronic MDE (> 2 years); bipolar I or II disorder; MDD superimposed on dysthymic disorder; current panic disorder; OCD; PTSD; acute stress disorder; schizoaffective or any other psychotic disorder; neurological disorders or severe or uncontrolled organic disorders. Exclusion criteria also included transaminases values > 2 times ULN, alkaline phosphatase > 3 ULN or total bilirubin > 34 μmol/L or positive plasma β‐hCG or a combination of these; alcohol or drug abuse or dependence within the past 12 months; any personality disorder, and risk of suicide. People were excluded if they had not responded to an appropriate dose of 2 different previous antidepressant treatments (54 weeks), if they had received insight‐oriented and structured psychotherapy (within 3 months), light‐therapy started (within 2 weeks), oral antipsychotic drugs (within 4 weeks), neuroleptics at low dose (within 2 weeks), depot neuroleptics (within 6 months), electroconvulsive therapy (within 3 months). The washout periods were as follows: antidepressants (1 week), non‐selective MAOIs and tricyclic antidepressants (2 weeks) and fluoxetine (5 weeks). Hypnotics, anxiolytics and neuroleptic agents were prohibited during the study and before inclusion depending on their half‐life. Withdrawals: Agomelatine: n = 20 at 12 weeks; n = 13 at 24 weeks Escitalopram: n = 23 at 12 weeks; n = 15 at 24 weeks Baseline imbalances: no obvious differences noted The demographic and baseline characteristics of the subgroup (187 insomnia participants) were not different from those observed in the set of randomised participants. Date study undertaken: July 2007 ‐ September 2008 |
|
| Interventions |
Intervention: agomelatine 25 mg per day taken in the evening (± 8 p.m.), increased to 50 mg per day in case of insufficient improvement. Increase started from week 2 onwards. Comparator: escitalopram 10 mg per day taken in the evening (± 8 p.m.), increased to 20 mg per day in case of insufficient improvement. Increase started from week 2 onwards. |
|
| Outcomes |
Primary outcome PSQI score, mean (SD) at baseline; 12‐week change from baseline; 24‐week change from baseline Secondary outcomes VAS daytime symptoms Dropout rates |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced (non‐adaptive) randomisation with stratification on the clinical centre was used (Pg 3 "Allocation to treatment"). |
| Allocation concealment (selection bias) | Low risk | Treatment allocation and dose increase controlled centrally using and Interactive Response System, blind for participants and investigators (Pg 3 "Allocation to treatment"). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Considering allocation and that treatments were identically labelled it seems like the blinding was convincing. No details given (Pg 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43/324 participants dropped out during the 12‐week trial and 28/267 participants dropped out of the 24‐week trial period (Pg 5, Figure 1). |
| Selective reporting (reporting bias) | Unclear risk | No details provided |
| Other bias | High risk | Authors received consultation fees, salaries and grants for this research from a pharmaceutical company. |
Fava 2002.
| Methods | Randomised, double‐blind, parallel group, controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): low (< 4) or high (≥ 4) baseline insomnia using the HAM‐D sleep disturbance factor score. Other diagnoses: MDD for ≥ 1 month using DSM‐IV criteria or atypical depressive disorder using DSM‐IV criteria. Assessed by SCID for DSM‐IV. Number of participants randomised: n = 284 Fluoxetine: n = 92 Sertraline: n = 96 Paroxetine: n = 96 Age, mean (SD) years: Fluoxetine: 42.1 (13.5) Sertraline: 44.0 (14.7) Paroxetine: 42.5 (14.7) Gender (M/F): 40.5%/59.5% Fluoxetine 44%/63% Sertraline 42.7%/57.3% Paroxetine 41.7%/58.3% Race/ethnicity: not reported Country: USA Setting: 15 psychiatric academic centres. Outpatients only Included: men and women outpatients, aged ≥ 18 years, who, for ≥ 1 month, met the DSM‐V criteria for MDD or atypical MDD using the DSM‐IV, as assessed by SCID for DSM‐IV. Participants were required to exhibit a baseline score > 16 on the first 17 items (HAM‐D‐17) of the 28‐item HAM‐D (HAM‐D‐28). Excluded: pregnant or lactating women or women of child‐bearing potential not using a medically accepted means of contraception; serious suicidal risk; serious comorbid illness that was not stabilised; presence of a seizure disorder with a seizure occurring within the past year; presence of any of the following DSM‐IV diagnoses: organic mental disorder, substance‐use disorder, schizophrenia, delusional disorder, psychotic disorders, not elsewhere classified, bipolar disorder and antisocial personality disorder; mood‐congruent or mood‐incongruent psychotic features; history of allergy to the study of drugs or history of multiple adverse drug reactions; concomitant use of any antidepressant (other than study drugs), anxiolytic, or other psychotropic medication within 7 days before study entry, with the exception of choral hydrate; use of MAOIs within 2 weeks of active therapy or anticipated need to use an MAOI within 5 weeks of discontinuing the study; hyper‐ or hypothyroidism (thyroid replacement was allowed, and people were allowed to enter if they were clinically and biochemically euthyroid); and lack of response to treatment of current major depression episode by any SSRI defined as ≥ 6 weeks of treatment with fluoxetine ≥ 40 mg/day, sertraline ≥ 150 mg/day or paroxetine ≥ 40 mg/day. Withdrawals: n = 77 Fluoxetine: n = 24 (26.1%) Sertraline: n = 26 (27.1%) Paroxetine: n = 27 (28.1%) Baseline imbalances: treatment groups were comparable at baseline with respect to age, gender and severity of illness. |
|
| Interventions |
Intervention: fluoxetine 20 mg/day for 4 weeks then increased to 40 mg or 60 mg/ day after week 4 Comparator 1: sertraline 50 mg/day for 4 weeks then increased to 100 mg, 150 mg or 200 mg/day after week 4 Comparator 2: paroxetine 20 mg/day for 4 weeks then increased to 40 mg or 60 mg/day after week 4 |
|
| Outcomes |
Primary outcomes Sleep disturbance factor score in high insomnia baseline participants (LOCF) Reduction in early insomnia severity (HAM‐D item 4) from baseline to end point in high insomnia baseline participants Reduction in middle insomnia severity (HAM‐D item 5) from baseline to end point. Reduction in late insomnia severity (HAM‐D item 6) from baseline to end point in high insomniacs at baseline Secondary outcome Number of participants withdrawing from study due to adverse events or lack of efficacy (total sample not just the high insomniac group) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The study design and partial results have been presented elsewhere and the full report is in preparation” (Pg 139, paragraph 2). |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind, mentioned in abstract, but no further detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, mentioned in abstract, but no further detail. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind, mentioned in abstract, but no further detail. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 77 participants discontinued following randomisation (Pg 143). |
| Selective reporting (reporting bias) | Unclear risk | Study was reported across several different papers. |
| Other bias | High risk | Study funded by a pharmaceutical company, no disclosure of independence of blinding or analysis. |
Finnerty 1978.
| Methods | Double‐blind, randomised study | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): no formal insomnia diagnosis mentioned, instead "Patients with sleep disturbance were considered for treatment." Other diagnoses: neurotic depression and minimum score of 7 on Raskin Scale for Depression Number of participants randomised: n = 139 Doxepin: n = 71 Imipramine: n = 68 Number of participants: n = 97 Doxepin: n = 49 Imipramine: n = 48 Age, mean (range) years: Doxepin: 38.5 (20‐67) Imipramine: 38.6 (19‐65) Gender (M/F): Doxepin: 20/29 Imipramine: 16/32 Race/ethnicity: not reported Country: Boston and Philadelphia, USA Setting: 2 outpatient treatment and research centres Included: people with primary diagnosis of neurotic depression with a minimum score of 7 on the Raskin Scale for depression and with sleep disturbance symptoms. Excluded: people with physical contraindications such as glaucoma, urinary retention, severe organic disease or the potential to become pregnant; history of alcoholism; sensitivity to tricyclic antidepressants; who received MAOIs or any other psychotropic drug in 2 weeks prior to study. Withdrawals: n = 42 Doxepin: n = 22 (failure to keep study visit n = 7: drug toxicity n = 13; intercurrent illness n = 1; violation of protocol n = 1) Imipramine: n = 20 (failure to keep study visit n = 10; drug toxicity n = 10) Baseline imbalances: fewer men than women in the imipramine group and in comparison with the doxepin group |
|
| Interventions |
Intervention: doxepin taken before bedtime; initial dose 100 mg, could be titrated to 150 mg after 1 week of treatment and up to 200 mg after 2 weeks of treatment (mean dose 112.7 mg/day) Comparator: imipramine taken before bedtime; initial dose 100 mg, could be titrated to 150 mg after 1 week of treatment and up to 200 mg after 2 weeks of treatment (mean dose 116.7 mg/day) |
|
| Outcomes |
Primary outcomes HAM‐D Finnerty‐Goldberg Sleep scale Sleep Disturbance Factor Secondary outcome Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated pattern of randomisation in groups of 4 (Pg 853, second paragraph) |
| Allocation concealment (selection bias) | Unclear risk | No details given, but reported double blind |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given, but reported double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given, but reported double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 42/139 participants did not complete the study, i.e. 30% (Pg 853, "Results"); no ITT |
| Selective reporting (reporting bias) | High risk | Finnerty‐Goldberg scale used to assess sleep disturbance, but findings not reported in full (Pg 853) |
| Other bias | Unclear risk | No disclosure of conflicts of interest |
Friedmann 2008.
| Methods | Randomised controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): sleep disturbance during previous episodes of abstinence PSQI ≥ 5 Other diagnoses: alcoholism, depression Number of participants randomised: n = 173 Trazodone: n = 88 Placebo: n = 85 Age, mean (SD) years: Trazodone: 41 (6.8) Placebo: 41 (7.7) Gender: > 90% men Race/ethnicity: > 85% white Country: USA Setting: secondary care, short‐term detoxication programme, outpatients at alcohol treatment centre Included: alcohol as the principal substance, DSM‐IV criteria for current alcohol dependence, sleep disturbance during previous of abstinence or a global score or greater on the PSQI, aged 18‐65 years, adequate contraception if female and ability to understand instructions Excluded: DSM‐IV criteria for current dependence on drugs other than nicotine, or Axis I disorder (people with "substance‐induced mood disorder" or dysthymia were not excluded); current suicidality; psychotropic, antidepressant, anxiolytic or antidipsogenic (naltrexone, disulphiram and acamprosate) medication; pro erectile, herbal or sleep medication; pregnancy/lactation, ischaemic heart disease, cardiac arrhythmias; priapism, or hypotension; history of obstructive sleep apnoea, emphysema or poorly controlled diabetes mellitus with nocturia ≥ 2 times per night; life expectancy ≤ 6 months; no address; no contact person Withdrawals: n = 28 lost to FU Trazodone: n = 6 lost to FU; n = 9 withdrew Placebo: n = 4 lost to FU; n = 9 withdrew Baseline imbalances: no significant imbalances |
|
| Interventions |
Intervention: trazodone 50‐150 mg at bedtime for 12 weeks. Participants were instructed to begin with 1 tablet 1 hr before bedtime, and titrate dosage until they reached a balance between sleep response and morning lethargy, or up to 3 tablets maximum. Then FU after stopping medication to 6 months Comparator: placebo: same tablet regimen as intervention group |
|
| Outcomes |
Primary outcomes PSQI mean change at 3 months compared to baseline PSQI mean change at 1 month PSQI mean change at 6 months (i.e. 3 months after drug withdrawal) Sleep quality equal by 6 months (i.e. once trazodone stopped sleep quality equalised) Secondary outcomes Tolerability |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised 'urn' randomisation software. It balanced for depression, gender and homelessness (Pg 1653). |
| Allocation concealment (selection bias) | Low risk | Double‐blind identical tablets |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Consort diagram. No difference in age, race, gender, education level etc. of those lost to FU (Pg 1655). 18.5% lost to FU |
| Selective reporting (reporting bias) | Unclear risk | ITT. Imputation of missing values. Same results as full information maximum likelihood |
| Other bias | Low risk | No protocol. Adherence to medication taking measured by self‐report and MEMS. 82‐83% self‐report medication taking 37‐43% on MEMS. Increased number believed they were taking active trazodone in the trazodone group 79% (49) compared with 48.8% (32) in placebo group. Sponsorship bias: low risk; no involvement of pharmaceutical companies |
Gillin 1997.
| Methods | Randomised, double‐blind, multicentre, controlled, parallel group trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): 1 of the following subjective criteria for a sleep disturbance: difficulty in falling asleep on a nightly basis, waking up during the night, inability to fall asleep again after waking during the night on HAM‐D Other diagnoses: moderate to severe nonpsychotic MDD (DSM III‐R) on the basis of a structured clinical interview (minimal score of 18 on the first 17 items of HAM‐D‐17 Number of participants randomised: n = 44, but only 43 evaluable Nefazodone: n = 23 (1 not evaluable) Fluoxetine: n = 20 Age, mean (SD) years: Nefazodone: 35.3 (1.8) Fluoxetine: 36.7 (1.9) Gender (M/F): Nefazodone: 8/16 Fluoxetine: 6/14 Race/ethnicity: 68% white; 5% black; 6% Hispanic: 1% Asian Nefazodone: 15 white; 4 black; 5 Hispanic, 0 Asian Fluoxetine: 15 white; 1 black; 1 Hispanic; 1 Asian Country: University of California, San Diego, University of Pennsylvania, USA Setting: psychiatric outpatients, 4 sites Included: minimal score of 18 on the first 17 items of the HAM‐D‐17 at end of the baseline phase of no medication. And 1 of the following subjective criteria for a sleep disturbance: difficulty in falling asleep on a nightly basis, waking up during the night, inability to fall asleep again after waking during the night Excluded: shift workers, primary sleep disorders independent of affective disturbance, current general medical conditions or history of psychoactive substance disorder use within 12 months prior to study entry. DSM III R axis disorders ‐ organic mental syndromes and disorders, bipolar disorder ‐ depressed and schizophrenia, delusional disorder or psychotic disorders. Pregnant, lactating or sexually active women not using an approved method of contraception |
|
| Interventions |
Intervention: nefazodone (days 1‐7, 200 mg/day (100 mg twice daily); days 8‐56, 400 mg/day (200 mg twice daily) If clinically indicated the dose could be increased to 500 mg/day on day 29 Comparator: fluoxetine days 20 mg/day for 56 days If clinically indicated, the dose could be increased to 40 mg/day on day 29. |
|
| Outcomes |
Primary outcome Sleep disturbance assessments ‐ items on HAM‐D and 4 items on clinician and self‐rated IDS (end point defined at last observation at or before week 8) Secondary outcomes Sleep consolidation Tolerability/adverse events Sleep architecture |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Parallel design; stated double‐blind double‐dummy dosing scheme |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis (Pg 187) |
| Other bias | Unclear risk | Declaration included, but meaning unclear |
Hajak 2001.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): ICSD and DSM‐IV criteria; primary insomnia and fulfilled criteria for ICSD psychophysiological insomnia. Diagnosis made by physician specialised in psychiatry and neurology and qualified a sleep expert by German Sleep Society Number of participants randomised: n = 47, but only 40 completed Doxepin: n = 20 Placebo: n = 20 Age, mean (SD) years: 47 (11) Doxepin: 47.6 (11.3) Placebo: 47.4 (16.8) Gender (M/F): 8/32 Doxepin: 3/17 Placebo: 5/15 Race/ethnicity: not reported Country: Germany Setting: sleep disorders centres Included: primary Insomnia; free of psychotropic medications including hypnotics for 2 weeks prior to start of study Excluded: acute, chronic and recurrent somatic and psychiatric disorders excluded by physical examination routine laboratory tests, ECG, EEG and semi‐structured interview; sleep disorder other than primary insomnia excluded by interview and PSG; urine toxicology performed for benzodiazepines and drugs of abuse Withdrawals: n = 7 Doxepin: n = 4 Placebo: n = 3 Baseline imbalances: similar CGI score at baseline (mean ± SD): 4.50 ± 0.76 in doxepin group and 4.55 ± 0.76 in placebo group |
|
| Interventions |
Intervention: doxepin 25‐50 mg for 4 weeks followed by 2 weeks placebo withdrawal. Doxepin given orally 1 hr before bedtime. 1 capsule for first week if deemed ineffective then increased to 2 capsules Comparator: placebo for 6 weeks: 1 capsule for first week if deemed ineffective then increased to 2 capsules |
|
| Outcomes |
Primary outcomes SE as measured by PSG on baseline first night 4 weeks of treatment and first and third night of withdrawal and after 2 weeks withdrawal CGI Severity of illness and Global improvement in sleep (investigator rating) Participant‐rated sleep quality and working ability (participant rating) Sleep quality rating on VAS Secondary outcomes Rebound sleep parameters on stopping Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported double blind, but no further detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported double blind, but no further detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/47 participants dropped out and these were accounted for in table 1 (Pg 456). |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | High risk | Funded by pharmaceutical company, but no proof of mitigating factors that promoted independence of results. |
Khazaie 2013.
| Methods | Randomised controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): participants underwent a structured psychiatric interview using the DSM‐IV‐TR and completed the GSAQ to screen for subjective sleep problems Other diagnoses: third trimester of pregnancy Number of participants randomised: n = 67 Number of participants: n = 54 Trazodone: n = 18 Diphenhydramine: n = 19 Placebo: n = 17 Age, mean (SD) years: Trazodone: 22.6 (5.6) Diphenhydramine: 27 (4.9) Placebo: 25.5 (4.4) Gender: all women Race/ethnicity: Persian Country: Iran Setting: Kermanshah University Medical Sciences Included: psychiatric interview performed to exclude volunteers with any other psychiatric disorder such as baseline depression, and to confirm the diagnosis of insomnia for which participants were originally referred for treatment Excluded: volunteers underwent a routine physical examination and ultrasonographic assessment. Excluded people with gestational diabetes mellitus, hypertension, pre‐eclampsia, history of chronic somatic disease, fetal disorder or drug abuse; volunteers with a history of sleep or mood disorders prior to their pregnancy and any previous antidepressant use The psychiatric interview was performed to exclude volunteers with any other psychiatric disorder such as baseline depression and to confirm the diagnosis of insomnia for which participants were originally referred for treatment Withdrawals: n = 7 Trazodone: n = 2 lost to FU Diphenhydramine: n = 2 lost to FU Placebo: n = 3 (lost to FU n = 2 and excluded as admitted with psychosis requiring antipsychotic medication n = 1) Baseline imbalances: participants matched by age. Demographics of participants similar in all treatment groups (Pg 903, table 1) Date study undertaken: October 2008 to April 2012 Funding source: supported by a grant from Department of Research, Kermanshah University of Medical Sciences (Research no. 86014) Declarations of interest by authors: none stated |
|
| Interventions |
Intervention 1: trazodone 50 mg/day self‐administered 1 hr before bedtime Intervention 2: diphenhydramine 25 mg/day self‐administered 1 hr before bedtime Comparator 1: placebo self‐administered 1 hr before bedtime |
|
| Outcomes |
Primary outcomes None Secondary outcomes Actigraphic sleep outcomes, sleep duration and SE Adverse effects |
|
| Notes | Wrist actigraphy for 3 successive days at baseline and after 2 and 6 weeks; used to monitor TST and SE objectively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blind to their treatment type throughout the study (Pg 902) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical evaluation by psychiatrist who was blind to the study design and participant treatment group (Pg 902) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/61 participants did not complete the study, however no ITT analysis; 7 excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Disclosure: not funded by a pharmaceutical company |
Krystal 2011.
| Methods | Randomised controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): DSM‐IV‐TR Other diagnoses: none Number of participants randomised: n = 229 Number of participants: n = 221 Doxepin 3 mg: n = 75 Doxepin 6 mg: n = 73 Placebo: n = 73 Age, mean (SD) years: Doxepin 3 mg: 45.5 (10.6) Doxepin 6 mg: 44.2 (11.1) Placebo: 43.6 (12.3) Gender (M/F): 27%/73% Doxepin 3 mg: 23%/77% Doxepin 6 mg: 29%/ 71% Placebo: 30%/70% Race/ethnicity: 48% white; 33% African‐American; 16% Hispanic; 3% other Doxepin 3 mg: 44% white; 35% African‐American: 20% Hispanic: 1% other Doxepin 6 mg: 53% white: 29% African‐American: 14% Hispanic: 4% other Placebo: 48% white: 34% African‐American: 15% Hispanic: 2% other Country: USA Setting: outpatients clinics Included: DSM‐IV primary insomnia; PSG criteria: LPS > 10 min on both PSG screening nights; mean wake time during sleep ≥ 60 min on both PSG screening nights, TST > 240 and ≤ 400 min on both screening nights Excluded: excessive use of alcohol, nicotine or caffeinated beverages; unintentional napping more than twice per week; having a variation in bedtime > 2 hr on 5 of 7 nights; use of a hypnotic or any other medication known to affect sleep; PSG: ≥ 10 apnoea/hypopnoea events or PLM events with arousals of sleep or periodic leg movements with arousals/hr of sleep Withdrawals: n = 18 Baseline imbalances: none |
|
| Interventions |
Intervention 1: doxepin 3 mg/day Intervention 2: doxepin 6 mg/day Comparator: placebo |
|
| Outcomes |
Primary outcomes sTST, LSO, subjective (length of) wakings after sleep onset, sNAASO and sleep quality (‐3 = extremely poor to +3 = excellent) Measures at baseline, and nights 1, 15 and 29 (and mean of 1‐29) Outcomes measured in terms of (significant) improvement from baseline (for each intervention) and in relation to placebo (at each time point); no direct comparisons were made between doxepin doses Secondary outcomes TST, LPS, (length of) wakings after sleep onset NAW, SE in last quarter and wake time after sleep Measures at baseline, and nights 1, 15 and 29 (and mean of 1‐29) Outcomes measured in terms of (significant) improvement from baseline (for each intervention) and in relation to placebo (at each time point); no direct comparisons were made between doxepin doses |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Very well described (Pg 1434) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (Pg 1434) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis; participant numbers were stable throughout the study |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | High risk | Study funded by pharmaceutical company; no evidence of independence of blinding or analysis |
Krystal 2010.
| Methods | Randomised, parallel group, controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): DSM‐IV‐TR primary diagnosis of insomnia Other diagnoses: not stated Number of participants randomised: n = 240 Doxepin 1 mg: n = 77 Doxepin 3 mg: n = 82 Number of participants: n = 214 Doxepin 1 mg: n = 70 Doxepin 3 mg: n = 74 Age, mean (SD) years: 71.4 (5.2) Doxepin 1 mg: 71.3 (5.2) Doxepin 3 mg: 71.4 (4.9) Placebo: 71.5 (5.5) Gender (M/F): 35%/65% Doxepin 1 mg: 35%/65% Doxepin 3 mg: 30%/70% Race/ethnicity: 80% white; 9% African‐American; 9% Hispanic; 2% other Doxepin 1 mg: 82% white; 6% African‐American; 10% Hispanic; 1% other Doxepin 3 mg: 77% white; 12% African‐American; 11% Hispanic; 1% other Placebo: 83% white; 7% African‐American; 5% Hispanic; 5% other Country: USA Setting: multicentre study in 31 sleep centres Included: aged > 65 years; insomnia (DSM‐IV‐TR and sleep diaries) > 3 months, PSG LPS > 10 min, wake time during sleep ≥ 60 min and TST > 240 and ≤ 390 Excluded: excessive use of alcohol, nicotine or caffeinated beverages (no measurement given); intentional napping > twice a week; variation in bedtime; use of hypnotic medication or other medication that affects sleep; ≥ 15 apnoea/hypopnoea events per hr Withdrawals: Doxepin 1 mg: 7‐9% (1% adverse event, 3% protocol violation, 4% non‐compliance, 1% other) Doxepin 3 mg: 8‐10% (4% adverse event, 2% consent withdrawn, 1% protocol violation, 2% other) Placebo: 11‐14% (4% adverse effect; 7% consent withdrawn; 2% protocol violation) Baseline imbalances: slight gender imbalances across 3 groups |
|
| Interventions |
Intervention: doxepin 1 mg and 2 mg; night‐time administration of drug 30 min prior to bed time; supervised in the laboratory on study nights or self‐administered at home. Administered for 12 weeks Comparator: placebo; night‐time administration of drug 30 min prior to bed time; supervised in the laboratory on study nights or self‐administered at home. Administered for 12 weeks. 1 week of single‐blind placebo administration to all eligible participants prior to treatment phase |
|
| Outcomes |
Primary outcomes Subjective ratings of LSO, TST and sleep quality ISI PGI scale of sleep, 5‐item rating Secondary outcomes EEG data reported for WASO, TST, SE% last quarter of the night, NAW and LPS Measures of next‐day psychomotor functioning, subjective next‐day alertness or drowsiness Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation done by an external person/group, but no details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported double blind, but no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported double blind, but no further details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26/240 participants did not complete the study (Pg 1555, "study population"). No imputation for ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Some self‐report data that were not available at baseline were imputed. |
| Other bias | High risk | Study funded by a pharmaceutical company and authors salaries were paid by the same company. |
Lankford 2012.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): DSM‐IV‐TR diagnosis of primary insomnia Other diagnoses: not known Number of participants randomised: n = 255 Doxepin: n = 130 Placebo: n = 125 Number of participants: n = 237 Doxepin: n = 124 Placebo: n = 113 Age, mean (SD) years: Doxepin: 72.4 (6.0) Placebo 72.5 (5.9) Gender (M/F) Doxepin: 32%/68% Placebo: 39%/61% Race/ethnicity: Doxepin: 88% white; 8% African‐American; 2% Hispanic 4%; other 2% Placebo: 87% white; 6% African‐American; 2% Hispanic 4%; other 4% Country: USA Setting: outpatient clinics Included: diagnosis of insomnia, men and women aged ≥ 65 years with ≥ 3 months' history of DSM‐IV diagnosis of primary insomnia In run‐in week needed to have ≥ 60 min of sWASO, ≥ 30 min LSO, and ≤ 6.5 hr of subjective TST ≥ 4 nights per week during the placebo lead‐in period, reported variation in bedtime ≤ 2 hr Excluded: excessive use of alcohol, nicotine or caffeinated beverages; intentional napping more than twice per week; having a variation in bedtime ≥ 2 hrs over the previous 3 months; or use of a hypnotic or any other medication known to affect sleep Withdrawals: n = 18 Baseline imbalances: none |
|
| Interventions |
Intervention: doxepin 6 mg night‐time single dose for 4 weeks Comparator: placebo for 4 weeks |
|
| Outcomes |
Primary outcomes sTST at week 1 LSO at week 1, sTST at weeks 2‐4, sWASO, LSO (weeks 2‐4), sNAASO and sleep quality (scale from ‐3 to 3); CGI, PGI scale, ISI Secondary outcome Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomisation with a computer‐generated randomisation scheme (Pg 134, Section 2.4 "Procedures") |
| Allocation concealment (selection bias) | Low risk | Very well described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Low risk | Funded by pharmaceutical company, but randomisation and analysis performed independently |
Le Bon 2003.
| Methods | Randomised, double‐blind, controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): alcohol‐induced sleep disorders, insomnia type (DSM‐IV) Other diagnoses: alcohol dependence with physiological dependence as defined by the DSM‐IV Number of participants randomised: n = 18 Number of participants: n = 16 Age, mean (SD) years: 43.8 (8.3) Gender (M/F): 16/1 Race/ethnicity: not stated Country: Belgium Setting: Brugmann University Hospital alcohol detoxification unit up to night 3 then weekly FUs at clinic Included: aged 18‐65 years; alcohol dependence with physiological dependence as defined by the DSM‐IV; alcohol‐induced sleep disorders, insomnia type (DSM‐IV); co‐operativeness and sufficient intellectual and emotional capacity to comply with protocol requirements Excluded: history of mood, anxiety, dementia or psychosis disorder previous to the excessive consumption of alcohol; use of street drugs or non‐prescribed tranquillisers within the 12 months prior to the preinclusion visit; psychotropic drugs within 2 weeks before the preinclusion visit (anxiolytics, hypnotics, antidepressants, neuroleptics, carbamazepine, beta‐blocking agents (except if prescribed before alcohol detoxification), clonidine, antihistamines (if necessary, loratadine or terfenadine were permitted for at most 5 consecutive days), narcotic analgesics, amphetamines and related substances; severe medical condition; laboratory tests outside the normal range and deemed clinically significant by the investigator; positive alcohol screen in breath; pregnancy risk of pregnancy or lactation; use of any investigational medication within 30 days prior to the start of study or prevision to receive any investigational medicine other than the study medication during the course of the study; previous treatment with trazodone. Withdrawals: n = 2 Baseline imbalances: at day 1, no difference in weight, height, biological values, levels and duration of diazepam treatment was observed between the 2 subgroups. Night 1 was discarded to exclude potential first‐night effects and night 2 was used as the no medication baseline. Of the sleep parameters, only the arousal index was significantly greater in the trazodone group than in the placebo group. |
|
| Interventions |
Intervention: trazodone 50 mg titrated up to 200 mg for 4 weeks double blind Comparator: placebo for 4 weeks (identical capsules equivalent to trazodone 50‐200 mg capsules) double blind |
|
| Outcomes | Measured on nights 2, 3 and 28 Primary outcomes SE including sleep onset latency (SE11) SE after sleep onset (SE12) Time in bed Sleep period time TST Sleep onset latency NAW Number of stage shifts Adverse events Secondary outcomes REM sleep REM sleep latency REM density Eye movements/hr Non‐REM sleep) Slow wave sleep Apnoea‐hypopnoea index Arousals Dropouts |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by the statistical software (Pg 378) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported that blinding was maintained until the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/18 dropped out due to relapse plus no ITT mentioned (Pg 380) |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis not mentioned |
| Other bias | High risk | Sponsored by a pharmaceutical company with no evidence of independence of results reported |
Palomaki 2003.
| Methods | Randomised, double‐blind, controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): insomnia was not diagnosed, but just rated on 3 items of the HAM‐D Other diagnoses: acute ischaemic stroke Number of participants randomised: n = 100 Mianserin: n = 51 Placebo: n = 49 Number of participants: n = 81 Mianserin: n = 42 Placebo: n = 39 Age, mean (SD) years: Mianserin: 55.7 (11.1) Placebo: 54.7 (10.1) Gender (M/F): Mianserin: 36/15 Placebo: 32/17 Race/ethnicity: not reported Country: Finland Setting: inpatients Department of Neurology, University of Helsinki Included: acute ischaemic stroke inpatients aged < 71 years admitted to Department of Neurology Excluded: older people because of a reported risk of mianserin‐related leukopenia and agranulocytosis in elderly people. People were not eligible for the study if stroke had occurred more than 30 days earlier, if CT or MRI examinations were not compatible with acute ischaemic stroke, or if informed consent was not obtained from the patient or a carer. Excluded were also those with other severe diseases than ischaemic stroke, such as severe cardiovascular, renal or liver disease, psychosis, alcoholism or dementia Withdrawals: n = 19 Mianserin: n = 9 (lack of efficacy n = 1, lack of compliance n = 1, adverse effects n = 6, death n = 1) Placebo: n = 10 (lack of efficacy n = 3, lack of compliance n = 3, adverse effects n = 3, death n = 1) Baseline imbalances: (Table 1) participants in the mianserin group had more heart disease (n = 17) than participants in the placebo group (n = 10) |
|
| Interventions |
Intervention: mianserin 30 mg for up to 10 days then increased to 60 mg/night for 12 months followed by withdrawal over 4 weeks Comparator: placebo/presumably 1 or 2 tablets/might for 12 months followed by withdrawal over 4 weeks |
|
| Outcomes |
Primary outcome Composite score from 3 HAM‐D sleep items Secondary outcome Needing for sleep‐promoting medication |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Ten patients on placebo and 9 on mianserin discontinued the treatment prematurely" (Pg 60). |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported briefly (Pg 58‐60). |
| Other bias | Unclear risk | No mention or disclosure of funding |
Reynolds 2006.
| Methods | Randomised, double‐blind, controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): DSM‐IV primary insomnia: difficulty initiating or maintaining sleep or non‐restorative sleep for ≥ 1 month; clinically significant distress or functional impairment; sleep disturbance did not occur exclusively during the course of narcolepsy, breathing‐related sleep disorder, circadian rhythm disorder or parasomnia; disturbance did not occur exclusively during the course of another mental health disorder; disturbance was not the result of the direct physiological effects of a substance or a general medical condition Clinical assessment was by a study investigator and the project co‐ordinator used SCID for DSM‐IV to determine diagnosis. Other diagnoses: adults aged > 55 years with primary insomnia Number of participants randomised: n = 27 Number of participants: n = 27 Paroxetine: n = 14 Placebo: n = 13 Age, mean (SD) years: Paroxetine: 67.4 (10.5) Placebo: 66.5 (7.4) Gender: not reported Race/ethnicity: not reported Country: USA Setting: clinical referral and media announcements night‐time monitoring was undertaken at the Western Psychiatric Institute and Clinic, Clinical Neurosciences Centre, Pittsburgh, USA Included: aged ≥ 55 years and meeting DSM‐IV primary insomnia (see above) Excluded: if PSG showed sleep‐disordered breathing or periodic limb movements; if urine toxicology showed benzodiazepine or other substances Withdrawals: n = 7 Paroxetine: n = 4 (failure to improve n = 2, rash n = 3, daytime stimulant use n = 1) Placebo: n = 3 (failure to improve n = 2, respondent burden n = 1) Baseline imbalances: reported the 2 treatment groups did not differ significantly on any demographic or clinical measures (Pg 804, first paragraph) |
|
| Interventions |
Intervention: paroxetine 10 mg + sleep hygiene (initially adjusted after 2 weeks based on presence of possible adverse effects up to maximum 20 mg/day) adjusted under double‐blind conditions Duration: 6 weeks Comparator: placebo + sleep hygiene (10 mg initially adjusted after 2 weeks based on presence of possible adverse effects up to maximum 20 mg/day) adjusted under double‐blind conditions |
|
| Outcomes |
Primary outcomes Diagnostic response status Diary‐based measures Secondary outcome PSG |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned," but nothing else (Pg 804). |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported double blind in abstract and on Pg 804, but did not say what placebo was or whether looked identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported blinded evaluator at 6 weeks (Pg 804) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numerous withdrawals (Pg 804). Not ITT |
| Selective reporting (reporting bias) | Low risk | No details given |
| Other bias | Low risk | Full disclosure, no pharmaceutical company influenced the result |
Riemann 2002.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): DSM‐III‐R criteria sleep disorders fulfilling the criteria for primary insomnia or dyssomnia not otherwise classified verified by Structured Interview for Sleep Disorders SIS‐D. Participants had insomnia for ≥ 1 month and had to have a regular bedtime at approximately 11 p.m. (± 60 min) Other diagnoses: unclear although other current and lifetime psychiatric diagnosis and serious organic disorders excluded Number of participants randomised: n = 65 Trimipramine: n = 19 Lormetazepam: n = 18 Placebo: n = 18 Number of participants: n = 46 Trimipramine: n = 16 Lormetazepam: n = 15 Placebo: n = 1 Age, mean (SD) years: Trimipramine: 47.0 (10.8) Lormetazepam: 45.3 (10.3) Placebo: 48.8 (11.6) Gender (M/F) Trimipramine: 10/9 Lormetazepam: 9/9 Placebo: 13/5 Race/ethnicity: white Country: Germany Setting: outpatients; 12 sleep disorders clinics located at psychiatry university hospitals Included: aged 18‐70 years; insomnia diagnosis according to DSM‐III‐R criteria for ≥ 1 month; bedtime 11 p.m. ± 60 min Excluded: serious organic disease excluded by urine test, blood test, EEC, ECG, medical and neurological examination; any current or lifetime psychiatric disorder excluded by psychiatric examination and MADRS < 20; pregnancy or risk of pregnancy; sleep apnoea (apnoea index > 5/hr); period leg movements during sleep (> 5/hr) Withdrawals: n = 9 Trimipramine: n = 3 (adverse events n = 2; reversal of previous diagnosis n = 1) Lormetazepam: n = 3 (lack of efficacy of drug n = 2; reversal of previous diagnosis n = 1) Placebo: n = 3 (lack of efficacy of drug n = 2; withdrawal of consent n = 1) Baseline imbalances: imbalance of M:F ratio in the placebo group. Number of males much higher in placebo group |
|
| Interventions |
Intervention 1: trimipramine; self‐administered a flexible dose of 50‐200 mg prior to bedtime. Dose titrated according to participant reported efficacy as follows: day 1‐2: 25 mg; day 3‐4: 50 mg; day 5‐6: 75 mg; day 7‐9: 100 mg; day 10: 150 mg; day 11‐28: 200 mg Dosage could be varied starting on day 4 according to the reported effectiveness of the drug. Hence, trimipramine dosage at the end of the study could vary from 50 mg to 200 mg Intervention 2: lormetazepam 1 mg at night‐time Comparator 1: placebo; self‐administered a flexible amount of tablets from 1‐4 in the evening prior to bedtime. In practise, all participants took 4 tablets Comparator 2: lormetazepam; self‐administered a flexible amount of tablets in the evening prior to bed‐time. The first tablet contained lormetazepam 1 mg. Because the wording was unclear, it was not evident whether this was 1‐4 tablets although the dosage remained the same for all participants. |
|
| Outcomes |
Primary outcomes PSQI SF‐A, subjective sleep measures SF‐A the subscales 'sleep quality,' 'feeling refreshed in the morning,' 'well‐being in the evening,' 'exhaustedness in the evening' and 'psychosomatic symptoms during sleep' were analysed. These scales range from 1 to 5, with 1 denoting impaired quality etc., whereas a score of 5 represents positive estimates Secondary outcomes EEG data reported SE and sleep latency. Other reported variables in the study include TST, number of wake periods, wake percentage of sleep period time, stages 1‐4 percentage, REM percentage and REM latency Rates of adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation details were not given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported double blind, but no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported double blind, but no further details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/55 participants did not complete the study (first line of results section). ITT based on LOCF |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Low risk | Independent company analysed the results; study funded by pharmaceutical company |
Rios Romenet 2013.
| Methods | Randomised, 3‐arm, controlled pilot study | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): minimal SCOPA‐sleep nocturnal subscore ≥ 7. Insomnia present ≥ 6 months Other diagnoses: idiopathic PD Disease duration (mean ± SD): 5.0 ± 3.3 years Number of participants randomised: n = 18 Doxepin: n = 6 CBT: n = 6 Placebo: n = 6 Age, mean (SD) years: 66.4 (12.4) Gender (M/F): 14/4 Race/ethnicity: all participants spoke English or French Country: Quebec, Canada Setting: recruited from movement disorders clinics of the McGill University health centre Included: idiopathic PD and insomnia Excluded: frequent (more than twice weekly) use of sedative medications at night (including sedative antidepressants), untreated restless legs syndrome, night shift work or other occupational causes of abnormal sleep pattern, insomnia related to suboptimal dopaminergic therapy, other reversible causes of insomnia detected on baseline interview, premenopausal women not using effective methods of contraception, dementia (defined according to PD dementia criteria), change in dopaminergic therapy over the preceding 3 months, Hoehn and Yahr > 4 (i.e. non‐ambulatory), use of non‐selective MAOI or rasagiline (due to potential doxepin contraindication), hypersensitivity to doxepin, untreated narrow angle glaucoma or severe urinary retention Withdrawals: n = 2 CBT: n = 1 (unable to follow instructions and could not complete evaluations) Placebo: n = 1 (health problems) Baseline imbalances: no significant differences between groups at baseline in age, sex, disease duration, levodopa use, disease severity, or primary or secondary sleep outcomes (table 1). Participants in the CBT group had lower baseline MoCA scores (cognitive functioning) |
|
| Interventions |
Intervention 1: doxepin 10 mg at bedtime Intervention 2: CBT, included 3 key interventions sleep hygiene training, CBT and bright light therapy. CBT and sleep hygiene instituted by the Department of Psychiatry of the Jewish General Hospital, Montreal. Group setting ‐ 6 × 90 min weekly sessions with 2 participants per group. Light therapy daily for 30 min (morning or night depending on nature of sleep problem) Comparator: "placebo" ‐ "inactive" ‐ consisted of 30‐min light therapy using red light below the threshold required to entrain light cycles No placebo capsules were given Participants were informed that some forms of light therapy were expected to be less active, but were not told what type of condition was inactive |
|
| Outcomes |
Primary insomnia outcomes ISI SCOPA ‐ night scale Adverse events Secondary outcomes: Daytime fatigue scores (FSS) Cognitive function (MoCA) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Block randomisation" (block size = 9) Because CBT is group therapy randomisation of 1 participant to CBT led to automatic assignment of subsequent 2 participants to the non‐pharmacological arm (Pg 671). |
| Allocation concealment (selection bias) | High risk | No placebo tablets were given (Pg 671). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo was not disclosed as an inactive placebo, but treatment assignment was otherwise non‐blinded (Pg 671). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on who undertook the outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear ITT |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Low risk | Full disclosure, no conflict of interest |
Roth 2011.
| Methods | Randomised controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): "primary insomnia" confirmed by overnight PSG (SE ≤ 85%) and an unstructured interview Other diagnoses: none Number of participants: n = 16 Age, mean (SD) years: 44 (11) Gender (M/F): 4 M/12 Race/ethnicity: 11 white, 3 African‐American, 1 Hispanic Country: USA Setting: recruited through media advertising and outpatient clinics at Wake Forest School of Medicine, Winston‐Salem, NC, USA Include: primary insomnia (American Psychiatric Association 1994) determined by unstructured interview with a board‐certified sleep physician who followed appropriate DSM‐IV and Research Diagnostic Criteria, a score of 0 on the Patient Health Questionnaire items 1 and 9, no psychotropic medications within 2 weeks of initial screening and either self‐reported sleep latency of ≥ 30 min or self‐reported WASO ≥ 45 min Excluded: determined from the Structured Clinical Interview for the DSM‐IV, urinalysis, 7 days of sleep diaries and physical examination were any active psychiatric disorder or therapy, uncontrolled asthma, COPD, thyroid disease or symptoms of menopause, chronic sleep disturbing pain, poorly controlled diabetes, cardiac disease, use of medication or herbal treatments known to facilitate or interfere with sleep, pregnancy or breastfeeding, self‐reported bedtime earlier than 9 p.m. or later than 1 a.m. > 2 times a week, self‐reported habitual rise time later than 9.00 a.m. > 2 times a week, BMI > 35 kg/m2, alcohol‐use disorders identification test score > 11, habitual smoking between 11 p.m. and 7 a.m. and use of illicit drugs Withdrawals: 63 participants gave informed consent; 47 did not complete the entire study. Most common reasons for exclusion: SE > 85% (19% of 47 participants), did not show up for laboratory visit (17%), tested positive for illicit drugs (15%), evidence of sleep apnoea (15%) and current MDE (9%) Date study undertaken: published 2011 FU period and main outcome measurement points: 3 weeks (day 1, day 7, week 2 drug‐ and session‐free washout period, week 3 procedures identical to those of week 1, with the converse drug administered) Funding source: National Institute of Mental Health Grant (no 082280) and Institute of Alcohol abuse and Alcoholism grant (no. 017056) Declarations of interest by authors: McCall: Speaker bureaus for MERCK and Sepracor, Scientific advisor MERCK, Sealy and Sepracor. Other authors: none Other/notes: Procedures: 2 nights PSG in sleep laboratory before drug administration weeks 4 drug study sessions: 2 trazodone, 2 placebo over the course of 3 weeks First night on medication in sleep laboratory then 5 nights (nights 2‐6) on same medication at home 30 min before bedtime, return to laboratory on day 7 for PSG. Week 2 drug free washout Week 3 identical to week 1 with the alternative drug to week 1 Order of the drugs was randomised. 9/16 participants received trazodone week 1 and placebo week 3 Ethical approval specified Main findings: trazodone associated with fewer night‐time awakenings, minutes of Stage 1 sleep and self‐reported difficulties in sleeping |
|
| Interventions |
Intervention 1: trazodone 50 mg 30 min before bedtime for 7 days for 3 weeks. Trazodone was split into 2 halves and encapsulated on a gelatine capsule and with added methylcellulose Comparator 1: placebo (methylcellulose in an identical gelatine capsule) |
|
| Outcomes |
Primary outcome VAS Secondary outcomes PSG and multiple sleep latency test: Total awakenings Slow wave sleep Sleep latency REM latency WASO Day‐time effects of the medications: Short term memory Verbal learning Equilibrium Arm muscle endurance Trazodone produced small, but significant cognitive and motor impairments in: verbal learning and short‐term memory; trazodone decreased long‐term storage significantly on Selective Reminding Test; arm muscle endurance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "randomised," but no further information on how (Pg 554) |
| Allocation concealment (selection bias) | Low risk | Quote: “Identically appearing empty gelatin capsule.” Prepared and randomised by the institution's clinical trials pharmacy (Pg 554). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Summary abstract stated double blind (Pg 552) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 63 participants consented, 47 excluded (Pg 553) |
| Selective reporting (reporting bias) | Unclear risk | Appeared to report all outcomes |
| Other bias | Unclear risk | W Vaughn McCall: Speaker's bureaus for Merck and Sepracor, Scientific Advisor for Merck, Sealy and Sepracor, but funding not mentioned. Alicia J Roth and Anthony Liguori: none. |
Rush 1998.
| Methods | Randomised, double‐blind study | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): based on the following criteria people had to report ≥ 1 of the following sleep disturbances as part of their depressive symptomatology: difficulty in falling asleep on a nightly basis; waking up during the night or inability to fall asleep again after getting out of bed (DSM criteria A for insomnia) Other diagnoses: MDD (based on DSM‐III‐R criteria) Number of participants randomised: n = 125 Nefazodone: n = 64 Fluoxetine: n = 61 Number of participants: n = 104 60 evaluable for efficacy Age, mean (SD) years: Nefazodone: 36 (8.4) Fluoxetine: 37 (9.5) Gender (M/F): Nefazodone: 26/38 Fluoxetine: 18/43 Race/ethnicity: Nefazodone: 70% white, 9% African‐American, 13% Hispanic, 0% Asian‐American Fluoxetine: 85% white, 7% African‐American, 3% Hispanic, 5% Asian‐American Country: USA Setting: 10 sites across the USA Included: aged 19‐55 years with a DSM‐III‐TR diagnosis of MDD (moderate‐severe). Minimum score of 18 on the first 17 items of the HDRS‐17. Participants had to have 1 of the following sleep disturbance: difficulty in falling asleep on a nightly basis; waking up during the night; inability to fall asleep again after getting out of bed Excluded: engaged in shift work; had independent sleep/wake disorders identified on PSG; had documented significant concurrent general medical conditions or met DSM‐III‐R criteria for psychoactive substance use disorder within the year prior to study; other major lifetime DSM‐III‐R Axis I disorder (e.g. organic mental syndromes, bipolar, any psychotic, any eating, panic or OCDs); pregnant, lactating or sexually active women not using an adequate method of contraception Withdrawals: n = 21 Nefazodone: n = 6 (adverse effects) Fluoxetine: n = 5 (adverse effects) Baseline imbalances: more women in the fluoxetine group than in the nefazodone group; sleep latency shorter in the nefazodone group at baseline; NAW greater in the nefazodone group at baseline |
|
| Interventions |
Interventions: nefazodone 100 mg administered twice daily on days 1‐7; 200 mg administered twice daily on days 8‐56. If clinically indicated, the dose was increased to 500 mg/day on day 29 or after Comparator: fluoxetine 20 mg administered in the morning on days 1‐56. If clinically indicated, the dose was increased to 40 mg/day on day 29 or after |
|
| Outcomes |
Primary outcomes HDRS sleep disturbance factor IDS‐C sleep disturbance factor IDS‐SR sleep disturbance factor Secondary outcomes EEG outcomes: sleep latency; SE; NAW; % awake and movement time; % of time spent in stages 1, 2, 3/4, REM, REM latency; reduced REM latency Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details given of double‐dummy regimen, but not whether fluoxetine group received morning and night capsules, which may have affected blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/125 participants did not complete the study. |
| Selective reporting (reporting bias) | Unclear risk | Details not given |
| Other bias | High risk | Study funded by pharmaceutical company and evidence of independence of results was presented. |
Satterlee 1995.
| Methods | Randomised, placebo‐controlled study | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): HAM‐D sleep total of ≥ 4 at baseline Other diagnoses: DSM‐III‐R major depression Number of participants randomised: n = 89 (all groups: sleep disturbed, melancholic and reduced REM latency groups) Only those with baseline sleep disturbance: HAM‐D sleep total ≥ 4 (49 participants) suitable for entry into this Cochrane Review, but 1 dropped out Number of participants: n = 48 Only the sleep disturbed group was appropriate for inclusion in the meta‐analysis. Fluoxetine: n = 23 Placebo: n = 25 Age, mean (range) years: 40.4 (18‐63) Gender (M/F): 26/63 Race/ethnicity: given for all the groups in total: 79 (88.8%) white Country: USA Setting: 6 different outpatient centres in the USA Included: DSM‐III‐R major depression for ≥ 1 month with a single or recurrent episode or bipolar disorder type II depressed phase. People with > 1 depressive episode needed to have a minimum 10‐week euthymic interval. HAM‐D score ≥ 15. Participants also had to be placebo non‐responders Excluded: pregnant or lactating; had serious medical or psychotic illness or failed to respond to ≥ 3 antidepressants at doses of imipramine 200 mg or equivalent for ≥ 3 weeks; had seizures after age 12 years, organic mental disorder, substance‐abuse disorder (including alcohol) during the past year, antisocial personality disorder, history of ≥ 3 suicide attempts with clear MDD melancholic type, multiple adverse drug reactions, allergy to fluoxetine, hypertensive treatment other than diuretic or calcium channel blocker; were taking other psychotropic medication (except chloral hydrate); had taken fluoxetine within 12 weeks prior to the PSG studies, potential to use a MAOI within 5 weeks of discontinuation of treatment, serious suicide risk, narcolepsy, sleep apnoea, periodic limb movement, increased thyroid stimulating hormone value or were taking thyroid supplements, GAD, OCD, panic disorders, phobias, PTSD, conditions or took medication that could influence REM latency or were going to ongoing psychotherapy Withdrawals: 1 participant had no postbaseline information and was. therefore. not included in the analysis. Baseline imbalances: no demographic data mentioned per stratified group |
|
| Interventions |
Intervention 1: fluoxetine 20 mg daily administered in the morning Comparator 1: placebo administered in the morning |
|
| Outcomes |
Primary outcomes: Mean change in HAM‐D sleep total: baseline to 8 weeks Worsening in HAM‐D sleep total: both baseline to 1 week and baseline to 8 weeks Improvement in HAM‐D sleep total: both baseline to 1 week and baseline to 8 weeks Change (% improved, unchanged, worsened) in HAM‐D sleep total: both baseline to 1 week and baseline to 8 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Adaptive randomisation scheme which decreased the probability that patients would be randomised to ineffective or intolerable therapy. A sequence was used that increased the probability of a participant receiving fluoxetine if they responded to the treatment" (paragraph 2). |
| Allocation concealment (selection bias) | Unclear risk | Reported double bind, but no further details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only 1 participant was excluded from the analysis (Pg 230, paragraph 1). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | High risk | Lead author worked for pharmaceutical company, no declaration and no other details |
Shell 2012.
| Methods | Randomised, double‐bind, 4‐armed, controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): history of sleep disturbance lasting > 6 weeks and defined by perceived lack of restorative sleep Other diagnoses: none Number of participants randomised: n = 111 Trazodone: n = 36 Sentra PM: n = 28 Sentra PM + trazodone: n = 22 Placebo: n = 25 Number of participants: n = 110 (the participant who did not complete the trial was carried forward as ITT). Age, range: 18‐75 years. No results on actual age of those recruited or gender. Data not available for each arm Gender: not reported Race/ethnicity: not reported Country: USA Setting: 12 independent sites around the USA Included: men and non‐pregnant and non‐lactating women aged 18‐75 years with history of sleep disturbance lasting > 6 weeks and defined by perceived lack of restorative seep were enrolled by the study physician in each site. Excluded: people currently taking tricyclic antidepressants; who had previously taken Sentra PM, trazodone or another amino acid formulation; with biochemical abnormalities that would put the person at risk or invalidate study findings Withdrawals: n = 1 (included as ITT) Baseline imbalances: uneven randomisation occurred due to higher enrolment rates at some of the clinical sites; however, this did not affect the statistical outcome of the study. Authors state that table 2 demonstrated that the 4 study groups were "statistically comparable" at baseline. |
|
| Interventions |
Intervention 1: trazodone 50 mg daily at bedtime + 2 capsules Sentra‐like placebo at bedtime Comparator 1: placebo (1 trazodone‐like placebo and 2 Sentra‐like placebo at bedtime Comparator 2: Sentra PM alone (a neurotransmitter based medical food) 2 capsule dose at bedtime + 1 trazodone‐like placebo at home Comparator 3: Sentra PM 2 capsule dose + trazodone 50 mg at bedtime |
|
| Outcomes |
Primary outcomes Quality of sleep assessed by the sleep latency using the PSQI. reported in tables 2 (baseline) and 3 (14 day). Table 3 had no CI Morning grogginess measured by the LSEQ Hours of sleep Secondary outcomes Latency Hours of sleep |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "randomised," but no further information given. Also reported uneven recruitment between sites caused uneven randomisation (Pg 67‐68). |
| Allocation concealment (selection bias) | Unclear risk | Data about participants unavailable (Pg 67). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported double blind. All participants received identical capsules at the same time. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant did not complete the trial, but data were carried forward as ITT |
| Selective reporting (reporting bias) | Low risk | Independent biostatistician analysed data. All randomised participants were included, both ITT and completed (Pg 68, statistical analysis section). |
| Other bias | Low risk | Study was funded by a pharmaceutical company, but the analysis was performed independently. |
Stein 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): PSQI ≥ 6 Other diagnoses: participants were previously opioid dependent, receiving methadone for ≥ 1 month Number of participants randomised: n = 137 Trazodone: n = 69 Placebo: n = 68 Number of participants: n = 123 Trazodone: n = 62 Placebo: n = 61 Age, mean (SD) years: 38.2 (8.6) Trazodone: 38.0 (8.8) Placebo: 38.5 (8.7) Gender (M/F): 64/73 Trazodone: 35/34 Placebo: 29/39 Race/ethnicity: Total: 117 (85.4%) white, 10 (7.3%) African‐American, 10 (7.3%) Hispanic Placebo: 61 (89.7%) white, 2 (2.9%) African‐American, 5 (7.4%) Hispanic Country: USA Setting: multicentre; 8 different methadone maintenance clinics in the Providence, Rhode Island metropolitan area Included: PSQI ≥ 6; ability to read, speak and understand English; plans to continue methadone maintenance for ≥ 6 months Excluded: symptoms suggestive of psychotic disorder, schizophrenia, gross cognitive dysfunction, current use of trazodone or psychotropic medication (last 30 days), inability or refusal to terminate pro‐erectile agents, pregnancy, lactation or inability or refusal to use contraception for women, unstable housing such as a shelter or halfway house Withdrawals: n = 14 Trazodone: n = 7 Placebo: n = 7 Baseline imbalances: relatively more African‐American participants in the trazodone group (n = 8) than the placebo group (n = 2) |
|
| Interventions |
Intervention: trazodone self‐administered 50‐150 mg at bedtime, so participants could self‐titrate to an effective dose of 50‐150 mg Comparator: placebo self‐administered at bedtime |
|
| Outcomes |
Primary outcomes PSQI Minimum sleep period, minimum TST, SE, times awakened, restfulness rating reported Mean TST, mean sleep onset latency, mean SE, mean NAW, mean restfulness rating reported Secondary outcomes Objective sleep measures: SE, sleep period time, TST, stage 1 sleep %, stage 2 sleep %, SWS %, REM %, time awake %, arousal index, apnoea index Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence without stratification (Pg 66, "Treatment" paragraph 11) |
| Allocation concealment (selection bias) | Unclear risk | Details of the sequence and blinding were provided, but not of the allocation (Pg 66, "Treatment" paragraph 11) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding maintained by a staff member outside the project. Placebo "provided in identical capsule form" (Pg 66, paragraph 12) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14/137 participants did not complete the 6 months study; no details given. |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Low risk | Full disclosure; no conflict of interest |
Walsh 1998.
| Methods | Double‐blind, placebo‐controlled, parallel group study | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): DSM‐IIIR criteria: minimum 1‐month history of disturbed sleep, characterised by a self‐reported sleep latency of ≥ 30 min and a self‐reported sleep duration of (mean ± SD) 4 ± 6 hr ≥ 3 nights per week. Additionally, complaints of significant daytime fatigue or decreased daytime functioning as a result of poor sleep must have been reported. Other diagnoses: any significant medical or psychiatric disorder as determined by clinical interview was excluded Number of participants randomised: n = 306 Trazodone: n = 100 Zolpidem: n = 102 Placebo: n = 104 Number of participants: n = 278 Trazodone: n = 90 Zolpidem: n = 91 Placebo: n = 97 Age: no specific data except that participants were aged 21‐65 years and that there were no age differences between groups Gender (M/F): 113 (37%)/193 (63%) Race/ethnicity: 253 (84%) white, 53 (16%) unspecified Country: USA Setting: 10 different US sites Included: aged 21‐65 years, meeting the DSM‐IIIR criteria and reporting during a 1‐week, single‐blind, placebo lead‐in period both of the following criteria on ≥ 3 nights: self‐report sleep latency of ≥ 30 min, and self‐report sleep duration of (mean ±) 4 ± 6 hr. Excluded: any significant medical or psychiatric disorder (as determined by clinical interview by a physician), history suggestive of sleep apnoea or PLM, smoking > 10 cigarettes per day, weight varying > 25% from desirable weight based on the Metropolitan Life Insurance Table, pregnancy or risk of becoming pregnant, and lactation. Recent history of drug addiction, alcoholism or drug abuse; history of sensitivity to CNS depressants, regular use of any medication that would interfere with the study, use of any investigational drug within 30 days of study entry and previous use of zolpidem precluded participation. Benzodiazepines or non‐prescription sleep medication had to be discontinued for (mean 177 SD) 7 ± 25 days, depending upon duration of action. Finally, a positive urine drug screen for CNS‐active drugs, participation in a weight loss programme, shift work or any other regularly changing sleep schedule, precluded study participation. Withdrawals: n = 28 Trazodone: n = 10 (adverse events n = 5) Zolpidem: n = 11 (adverse events n = 5) Placebo: n = 7 (adverse events n = 2) Baseline imbalances: none obvious, but demographic data were not well described. |
|
| Interventions |
Intervention: trazodone 50 mg taken nightly at bedtime. Comparator 1: zolpidem 10 mg taken nightly at bedtime Comparator 2: placebo taken nightly at bedtime |
|
| Outcomes |
Primary outcomes Subjective sleep latency and subject sleep duration Secondary hypnotic efficacy measures: ease of falling asleep, NAW, subjective wake time after sleep onset and sleep quality Participant global impression of effect of therapy: number and % of participants responding, sleep status, sleep improvement, time to fall asleep and sleep time Secondary outcomes Impact on ability to function Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention was made regarding the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given regarding how different capsules for zolpidem and trazodone influenced blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although only 28/306 participants dropped out (Pg 192, last paragraph of "Patients") the analysis was not ITT and the number of participants reported in the outcome data changed from the original reported sample size without adequate justification. |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | High risk | Funded by a pharmaceutical company with no evidence of independence of results reported |
Ware 1989.
| Methods | Randomised, double‐blind, controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): participants had 1 of the following: < 5 hr TST; sleep latency > 30 min; early morning wakening ≥ 60 min; or > 4 awakenings per night Other diagnoses: major unipolar depressive disorder according to DSM III criteria determined during psychiatric interview. Hamilton rating score > 20. Covi Anxiety Scores (22) > 8 Number of participants randomised: n = 34 Number of participants: n = 30 Age, mean (SD) years: Trimipramine: 39 (6.7) Imipramine: 42 (12) Gender (M/F): Trimipramine: 4/10 Imipramine: 6/10 Race/ethnicity: not reported Country: USA Setting: San Antonia Medical Centre, Texas Included: participants had 1 of the following: < 5 hr TST; sleep latency > 30 min; early morning wakening ≥ 60 min or > 4 awakenings per night A major unipolar depressive disorder according to DSM III criteria determined during psychiatric interview. Hamilton rating score of > 20. Covi Anxiety Scores (22) > 8. Excluded: people who had received electroshock therapy within 3 months of entry into study, who had used psychotropic drugs within 2 weeks of study entry (the initial interview), who were on any drug that might interfere with PSG data, who had abused drugs or alcohol within 1 year of study entry or who had any illness that might interfere with study measurements Withdrawals: n = 4 Baseline imbalances: no significant difference between groups in terms of age, weight and gender distribution. Except for the first week, the mean dose of trimipramine was significantly higher than imipramine throughout the study. Baseline sleep results were similar for both groups (shown in table). Date study undertaken: not stated. Paper published in 1989 Funding source: supported in part by a grant from Ives Laboratories (American Home Products, Inc.) Declarations of interest by authors: none stated |
|
| Interventions | Intervention 1: trimipramine days 1 and 2, 75 mg; day 3, 100 mg; days 4 and 5, 125 mg; days 6 and 7, 150 mg; days 8 and 9, 175 mg; days 10‐42, 200 mg. Participants who developed significant adverse drug reactions had their dose stabilised or reduced Intervention 2: imipramine days 1 and 2, 75 mg; day 3, 100 mg; days 4 and 5, 125 mg; days 6 and 7, 150 mg, days 8 and 9, 175 mg; days 10‐42, 200 mg. Participants who developed significant adverse drug reactions had their dose stabilised or reduced |
|
| Outcomes |
Primary outcomes Sleep latency Hours of sleep How satisfactory was your sleep? Secondary outcomes None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to receive trimipramine or imipramine in a 1:1 randomisation ratio (Pg 539, last paragraph) |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind (Pg 539, procedures section) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind (Pg 539, procedures section) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/34 participants withdrawn and excluded (Pg 540, data analysis section) |
| Selective reporting (reporting bias) | Unclear risk | No ITT analysis (Pg 540, data analysis section) |
| Other bias | High risk | Sponsored by a pharmaceutical company with no evidence of independence of results reported |
Zhou 2002.
| Methods | Randomised controlled trial | |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): met the diagnostic criteria (CCMD‐2‐R) for chronic primary insomnia Number of participants randomised: n = 90 Number of participants: n = 90 Alprazolam: n = 30 Paroxetine: n = 30 Placebo: n = 30 Age, mean (SD) years: Paroxetine: 68 (12) Alprazolam: 67 (13) Placebo: 69 (12) Gender (M/F): Paroxetine: 8/22 Alprazolam: 7/23 Placebo: 10/20 Race/ethnicity: Chinese Country: China Setting: hospital inpatients Included: people admitted to hospital aged 60‐80 years who met the diagnostic criteria (CCMD‐2‐R) for chronic primary insomnia, they also had the scores of HAM‐D < 7 and HAMA < 5. Excluded: people with severe physical and psychiatric illnesses Withdrawals: Paroxetine: n = 5 (financial difficulties n = 2, no response n = 1, adverse effects n = 2) Alprazolam: n = 9 (no response n = 5, adverse effects n = 4) Placebo: n = 10 (symptom deterioration n = 10) Baseline imbalances: statement in the paper: "There were no significant differences among these three groups in terms of gender, age, disease duration and educational levels." PSQI 7 component scores: there were no significant difference among the 3 groups in terms of the total score of PSQI and 7 component scores at baseline (P > 0.05). |
|
| Interventions |
Intervention 1: paroxetine group: initial dosage 10‐20 mg taken in the morning; dosages of paroxetine and alprazolam were adjusted according to the insomnia symptoms and adverse effects. Duration of treatment was 12 weeks Comparator 1: alprazolam 0.4‐0.8 mg 30 min before bedtime Comparator 2: placebo 2 tablets (made of starch) 30 min before bedtime |
|
| Outcomes |
Primary outcomes Sleep parameters of PSQI Clinical effects Secondary outcome Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate, only 24 completed the study |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
β‐hCG: beta human chorionic gonadotropin; BMI: body mass index; CBT: cognitive behavioural therapy; CCMD‐2‐R: Chinese Classification of Mental Disorders; CGI: Clinical Global Impression Scale; CGI‐S: Clinical Global Impression ‐ Severity; CI: confidence interval; CNS: central nervous system; COPD: chronic obstructive pulmonary disease; CT: computed tomography; DSM: Diagnostic and Statistical Manual of Mental Disorders; ECG: electrocardiogram; EEG: electroencephalogram; F: female; FSS: Fatigue Severity Scale; FU: follow‐up; GAD: generalised anxiety disorder; GSAQ: Global Sleep Assessment Questionnaire; HADS: Hospital Anxiety and Depression Scale; HAMA: Hamilton Anxiety Rating Scale; HAM‐D: Hamilton Rating Scale for Depression; HDRS: Hamilton Rating Scale for Depression; hr: hour; ICSD: International Classification of Sleep Disorders; IDS: Inventory for Depressive Symptomology; IDS‐C: Inventory for Depressive Symptomology ‐ Clinician; IDS‐SR: Inventory for Depressive Symptomology ‐ Self Report; ISI: Insomnia Severity Index; ITT: intention to treat; LOCF: last observation carried forward; LPS: latency to persistent sleep; LSEQ: Leeds Sleep Evaluation Questionnaire; LSO: latency to sleep onset; M: male; MADRS: Montgomery and Åsberg Depression Rating Scale; MAOI: monoamine oxidase inhibitor; MDD: major depressive disorder; MDE: major depressive episode; MEMS: medication event monitoring system; min: minute; MoCA: Montreal Cognitive Assessment; MRI: magnetic resonance imaging; n: number; NAW: number of awakenings after sleep onset; OCD: obsessive‐compulsive disorder; PD: Parkinson's disease; Pg: page; PGI: Patient Global Impression; PLM: periodic limb movement disorder; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index; PTSD: post‐traumatic stress disorder; REM: rapid eye movement; SCID: Structured Clinical Interview Depression; SCOPA: Scales for Outcomes in Parkinson's disease; SD: standard deviation; SE: sleep efficiency; SE%: sleep efficiency percentage; SF‐A: Schlaffragebogen A; sNAASO: subjective number of awakenings after sleep onset; SSRI: selective serotonin reuptake inhibitor; sTST: subjective total sleep time; sWASO: subjective wake after sleep onset; TST: total sleep time; ULN: upper limit of normal; VAS: visual analogue scale; WASO: wake after sleep onset.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1979 | Study of tryptophan, which is a supplement not an antidepressant |
| Botros 1989 | No clear criteria for insomnia diagnosis at baseline |
| Boyle 2012 | No clear criteria for insomnia diagnosis at baseline |
| Carney 2017 | Does not fulfil monotherapy criteria |
| Chen 2002 | No clear criteria for insomnia diagnosis at baseline |
| Fairweather 1997 | No clear criteria for insomnia diagnosis at baseline. |
| Ferrero 1987 | Study of tryptophan, which is a supplement not an antidepressant. |
| Hajak 1996 | Single dose of doxepin in the RCT, excluded as treatment ≤ 2 days |
| Herman 2009 | No clear criteria for insomnia diagnosis at baseline; only 64% of included participants had insomnia on the sleep scale. |
| Karsten 2017 | No primary insomnia diagnosis, sample of healthy men |
| Kaynak 2004 | No valid outcome, it was a cross‐over and they only had 1 subjective measure (PSQI), 1 at beginning and 1 at end of whole study so no placebo‐drug comparison possible. |
| Moon 1991 | No clear criteria for insomnia diagnosis at baseline |
| Palesh 2012 | Lack of an insomnia diagnosis at baseline |
| Roth 2007 | Only 2 nights of treatment |
| Ruwe 2016 | Only 2 nights of treatment |
| Scharf 2008 | Only 2 nights of treatment |
| Stein 2011 | Concurrent methadone administration to opioid addicts, therefore violated the monotherapy criteria for the meta‐analysis |
| Stephenson 2000 | No clear criteria for insomnia diagnosis at baseline |
PSQI: Pittsburgh Sleep Quality index; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Ahmed 2016.
| Methods | Single site, double‐blind, placebo‐controlled, 2‐period cross‐over study |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): subjective complaint of maintaining sleep ≥ 3 times per week for ≥ 1 month and the subjective sleep diary demonstrating sleep disturbance for ≥ 2 weeks prior to randomisation Other diagnoses: fibromyalgia Number of participants randomised: n = 19 Number of participants: Milnacipran then placebo: n = 8 Placebo then milnacipran: n = 7 Age, mean (range) years: 49.2 (28‐72) Gender: predominantly women Race/ethnicity: predominantly white Country: US Setting: single site; Cleveland Sleep Research Centre Included: men or women aged ≥ 18 years meeting the American College of Rheumatology (1991) criteria for fibromyalgia at screening along with clinically significant sleep disturbance, defined as subjective complaint of maintaining sleep ≥ 3 times per week for ≥1 month and the subjective sleep diary demonstrating sleep disturbance for ≥ 2 weeks prior to randomisation. Participants understood and were willing to co‐operate with the study procedures, before they signed the informed consent form. They were instructed to maintain a normal daytime awake and night‐time sleep schedule, and with a customary bedtime between 9 p.m. and midnight, and rise time 5 a.m. and 9 a.m. Excluded: people presenting with unstable uncontrolled medical conditions. People showing obstructive sleep apnoea with an apnoea‐hypopnoea index of ≥ 15 episodes per hr of sleep, or PLMAI of ≥ 15 episodes per hr during the baseline PSG, or both. However, people with a history of obstructive sleep apnoea controlled with nasal CPAP with demonstrated nightly compliance were allowed to participate in the study. People with psychiatric illnesses were accepted, but excluded if they were severely depressed or deemed to be at significant risk for suicide. People with uncontrolled glaucoma; unable to discontinue prohibited medications; women who were lactating or pregnant; history of alcohol, narcotic, benzodiazepines or other substance abuse within 1 year prior to the study; excessive caffeine use, defined as a consumption > 500 mg of caffeine or other xanthines; smoking more than one‐half pack/day or alcohol use > 14 units/week and history of allergy to milnacipran. Withdrawals: during the study, treatment was discontinued in 4 participants, with 2 discontinued because (AEs occurred during milnacipran treatment in 2, a serious AE (gallstones) unrelated to study drug occurred during placebo treatment in 1 and consent following milnacipran treatment was withdrawn in 1). 2 participants in whom treatment was discontinued had a study drug‐related AE (petechial rash and pruritus). Baseline imbalances: predominantly women and white (17 of 19 (89.5%)), with mean age 49.2 (range 28‐72) years and mean (SD) weight of 196.7 (54.0) lb. Mean (SD) duration of fibromyalgia was 9.2 (6.9) years and mean (SD) time since diagnosis of fibromyalgia was 4.2 (5.1) years. |
| Interventions |
Intervention: milnacipran 100 mg/day Comparator: milnacipran (100 mg/day) or placebo for cross‐over period 1 Comparator: placebo or milnacipran 100 mg/day for cross‐over period 1 |
| Outcomes |
Primary outcomes Medical Outcomes Study Sleep Scale Fibromyalgia Impact Questionnaire, the Brief Pain Inventory short form Fatigue Severity Scale Numeric Rating Scale of sleep quality as part of subjective sleep questionnaire (sleep diary) administered throughout the study Secondary outcomes PSG AEs |
| Notes |
Ivgy‐May 2015a.
| Methods | Double‐blind, randomised, placebo‐controlled, parallel‐group study |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): diagnosed with primary insomnia (according to the DSM‐IV) ≥ 1 month before study entry Number of participants randomised: n = 419 Number of participants: n = 366 Ezmirtazapine 3.0 mg: n = 117 Ezmirtazapine 4.5 mg: n = 124 Placebo: n = 125 Age range, years: 18‐65 Gender (of those randomised): Esmirtazapine 3.0 mg: 40/93 F Esmirtazapine 4.5 mg: 48/85 F Placebo: 45/87 F Race/ethnicity: mixed Country: US and Canada Setting: 43 outpatient research clinics Included: aged 18‐65 years and diagnosed with primary insomnia, that is, according to the DSM‐IV, ≥ 1 month before study entry. They also had to fulfil the following PSG criteria on 2 screening/baseline PSG nights: mean TST < 6.5 hr (and ≥ 3 and < 7 hr on both nights), WASO ≥ 45 min (and ≥30 min on both nights), and LPS ≥ 15 min (and ≥ 10 min on both nights). Excluded: other sleep or circadian disorders, current or recent history of depression, history of substance abuse, other conditions potentially causing sleep disturbances and drugs known to affect the sleep‐wake function (e.g. anxiolytics, sedatives, antidepressants, antipsychotics and centrally acting antihistamines) Withdrawals: data from 1 of the sites (n = 15) were not included in the efficacy analysis due to concerns about the eligibility of participants; based on audit findings, concerns were raised about the credibility of the data. Ezmirtazapine 3.0 mg: n = 22 (AEs n = 11, withdrew consent n = 3, lack of compliance n = 1, reasons unrelated to study n = 4, other n = 3) Ezmirtazapine 4.5 mg: n = 14 (AEs n = 5, withdrew consent n = 2, lack of compliance n = 3, reasons unrelated to study n = 1, other n = 3) Placebo: n = 11 (AEs n = 2, withdrew consent n = 4, lost to follow‐up n = 1, other n = 4) Baseline imbalances: overall, the treatment groups were well balanced with respect to baseline demography and clinical characteristics, although there were slightly fewer white participants in the esmirtazapine 3.0 mg group. |
| Interventions |
Intervention: esmirtazipine 3.0 mg Comparator 1: esmirtazipine 4.5 mg Comparator 2: placebo |
| Outcomes |
Primary outcomes Participant‐reported sTST, SL, number of awakenings, WASO, sleep quality and satisfaction with sleep duration (mean of weekly values and over entire 6‐week period); and ISI scores and the Investigator's Global Rating (mean at days 15 and 36). Rebound was assessed using PSG (days 43 and 44) and sleep diary data (days 43‐49) during the run‐out period. Withdrawal was assessed using the Tyrer Benzodiazepine Withdrawal Symptoms Questionnaire, completed at baseline, and on days 36 and 50. This is a 20‐item questionnaire completed by participants; each symptom was rated as absent (0), moderate (1) or severe (2), and the maximum possible score is 40. Safety and tolerability were assessed by monitoring AEs, physical examinations, vital signs, routine laboratory parameters and electrocardiograms. Residual daytime effects of treatment assessed using the Bond‐Lader rating scale; a morning alertness VAS; data on daytime functioning, energy levels and napping (recorded in an electronic questionnaire completed in the evening); and a digit symbol substitution test on the mornings following PSG assessments. Secondary outcomes PSG was conducted at the end of days 1, 15 (week 2) and 36 (week 5). Participants also completed an electronic sleep diary each morning during the treatment period. Primary end point was PSG‐measured WASO, and the key secondary end point was PSG‐measured LPS (mean at days 1, 15 and 36). Other secondary efficacy end points were: TST, number of awakenings, WASO per quarter of the night and sleep architecture (PSG‐assessed; mean of entire 6‐week period and mean at days 1, 15 and 36 for WASO per quarter). |
| Notes |
Ivgy‐May 2015b.
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): DSM‐IV Other diagnoses: "chronic insomnia" (i.e. ≥ 1 month) Number of participants randomised: n = 526 Number of participants: n = 526 Age, mean (SD) years: Esmirtazapine 1.5 mg: 44.8 (12.4) Esmirtazapine 3.0 mg: 45.6 (12.0) Esmirtazapine 4.5 mg; 44.5 (12.2) Placebo: 46.2 (11.3) Gender: shown in Table 1 (Pg 834) for all 4 treatment groups. Esmirtazapine 1.5 mg: 45/92 M/F Esmirtazapine 3.0 mg: 80/85 M/F Esmirtazapine 4.5 mg: 56/72 M/F Placebo: 45/90 M/F Race/ethnicity: shown in Table 1 (Pg 834) for all 4 treatment groups Esmirtazapine 1.5 mg: white n = 110 (80.3%); black n = 22 (16.1%) Esmirtazapine 3.0 mg: white n = 97 (77.6%); black n = 22 (17.6%) Esmirtazapine 4.5 mg: white n = 99 (77.3%); black n = 19 (14.8%) Placebo: white n = 109 (80.7%); black n = 21 (15.6%) Country: USA and Canada Setting: not stated Included: n = 526 Excluded: n = 330 Withdrawals: n = 62 Baseline imbalances: stated as being "well balanced" Date study undertaken: December 2006 to August 2008 Funding Source: Organon (pharmaceutical manufacturer; subsidiary of Merck) Declarations of interest by authors: explicit (Pg 836); several authors were current or former employees of the pharmaceutical company Merck |
| Interventions |
Intervention 1: esmirtazapine 1.5 mg every night Intervention 2: esmirtazapine 3.0 mg every night Intervention 3: esmirtazapine 4.5 mg every night Exclusion of "other medication affecting sleep" Comparator: placebo |
| Outcomes |
Primary outcomes TST (primary end point) in min SL (key secondary end point) in min Secondary outcomes WASO (min) ISI‐responder (%) IGRC‐responder (%) |
| Notes | Meets inclusion criteria. Well presented data in Figures 2 and 3 (Pg 834) |
Krystal 2012.
| Methods | Randomised, double‐blind, controlled trial |
| Participants | 538 with a diagnosis of primary insomnia were randomised 1:1:1:1 to receive esmirtazapine 0.5 mg, 1.5 mg, 3 mg or placebo |
| Interventions |
Intervention 1: esmirtazapine 0.5 mg for 16 days Intervention 1: esmirtazipine 1.5 mg for 16 days Intervention 1: esmirtazipine 3 mg for 16 days Comparator: placebo for 16 days |
| Outcomes |
Primary outcome PSG measured WASO mean over nights 1, 2, 15 and 16 Secondary outcome LPS mean over nights 1, 2, 15 and 16 |
| Notes | Conference abstract only. Unable to find further papers or contact the study author. |
Merck 2008.
| Methods | Randomised, double‐blind, controlled trial |
| Participants | 460 participants Inclusion criteria: aged 18‐64 years; signed written informed consent after the scope and nature of the investigation had been explained; had shown capability to complete the LogPad questionnaires; had difficulty falling asleep, maintaining sleep or have early morning awakening Exclusion criteria: significant medical or psychiatric illness causing sleep disturbances; history of bipolar disorder or attempted suicide or have a family (immediate family) history of suicide; sleep disorder such as sleep‐related breathing disorder, restless leg syndrome, narcolepsy; significant other medical illness such as acute or chronic pain, or heart, kidney or liver disease within the last year; currently diagnosed or meet the criteria for MDD or have been treated for MDD in the last 2 years; substance abuse, excessive use of alcohol (determined by the physician) or drug addiction within the last year; night workers or rotating shift workers or plan to travel through more than 3 time zones; routinely nap during the day; body mass index ≥ 36. |
| Interventions |
Intervention: esmirtazapine 4.5 mg once a day for 6 months Comparator: placebo tablets once a day for 6 months |
| Outcomes |
Primary outcome Change from baseline in TST over 6‐months. TST was defined as the time recorded for sleep diary question 6 "How much time did you actually spend sleeping?" as reported by participants using a LogPad (hand‐held electronic data capture device). Baseline was defined as the mean TST from the placebo run‐in period. Change from baseline was calculated as the mean of combined data from weeks 14‐26, using LOCF approach. Secondary outcomes Number of participants who experienced AEs up to 31 weeks. AE defined as any unfavourable and unintended change in the structure, function or chemistry of the body whether or not considered related to study drug. Number of participants who experienced AEs was combined for 6‐month treatment period and the 7‐day discontinuation period. Number of participants who discontinued study drug due to an AE up to 27 weeks. An AE defined as any unfavourable and unintended change in the structure, function or chemistry of the body whether or not considered related to study drug. Number of participants who discontinued study drug due to an AE was combined for the 6‐month treatment period and the 7‐day discontinuation period. Change from baseline in SL in 6‐month treatment period (baseline and mean of weeks 14‐26). SL defined as the time recorded for sleep diary question 3 "How long did it take you to fall asleep?", as reported by participants using a LogPad. Baseline was defined as the mean SL from the placebo run‐in period. Change from baseline was calculated as the mean of combined data from weeks 14‐26, using an LOCF approach. Change from baseline in WASO in 6‐month treatment period (baseline and mean of weeks 14‐26). WASO defined as the time recorded for sleep diary question 5 "How much time were you awake, after falling asleep initially?", as reported by participants using a LogPad. Baseline was defined as the mean WASO from the placebo run‐in period. Change from baseline was calculated as the mean of combined data from weeks 14‐26, using an LOCF approach. Other outcome measures Change from baseline in NAW in 6‐month treatment period (baseline and mean of weeks 14‐26). NAW defined as the number of times recorded for sleep diary question 4a "How many times did you wake up during the night?", as reported by participants using a LogPad. Baseline was defined as the mean NAW from the placebo run‐in period. Change from baseline was calculated as the mean of combined data from weeks 14‐26, using an LOCF approach. Change From baseline in sleep quality in 6‐month treatment period (baseline and the mean of weeks 14‐26). Sleep quality assessed using a VAS in response to the sleep diary question 7 "Rate the quality of your sleep last night", as reported by participants using a LogPad. Responses could range from 0 = Very poor to 100 = Excellent, with a higher score indicating greater sleep quality. Baseline was defined as the mean sleep quality score from the placebo run‐in period. Change from baseline was calculated as the mean of combined data from weeks 14‐26, using an LOCF approach. Change from baseline in satisfaction with sleep duration in 6‐month treatment period (baseline and the mean of weeks 14‐26). Satisfaction with sleep duration was assessed using a VAS in response to the sleep diary question 8 "How satisfied are you about your sleep duration of last night?", as reported by participants using a LogPad. Responses could range from 0 = Very unsatisfied to 100 = Fully satisfied, with a higher score indicating great satisfaction with sleep duration. Baseline was defined as the mean satisfaction with sleep duration score from the placebo run‐in period. Change from baseline was calculated as the mean of combined data from weeks 14‐26, using an LOCF approach. Change from baseline in 2 aggregate measures of the SF‐36 Health Survey Score in 6‐month treatment period (baseline and week 26). SF‐36 is a participant‐rated questionnaire that consists of 8 scaled scores: vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning and mental health, which are the weighted sums of the questions in their section. Each scale is directly transformed into a 0‐100 scale on the assumption that each of the 8 questions carries equal weight. The SF‐36 can be divided into 2 aggregate summary measures: the Physical Component Summary and the Mental Component Summary. Scores range from 0 to 100, with a lower score indicating more disability. Baseline was defined as the SF‐36 score assessed at randomisations. Change from baseline in IGR in 6‐month treatment period (baseline and week 26). The IGR is a clinician‐rated 7‐point scale used to assess the severity of illness. Severity is rated on a scale from 1 = Normal to 7 = Extremely severe. Baseline was defined as the last non‐missing value obtained during the placebo run‐in period. Change from baseline in IGR in 7‐day discontinuation period. |
| Notes | Data from NCT website only. Unable to find any publications or contact authors. |
Miljatovic 2012.
| Methods | Randomised, double‐blind controlled trial |
| Participants | Number of participants: n = 37 |
| Interventions |
Intervention: venlafaxine 75‐150 mg/day for 8 weeks Comparator: mirtazapine 15‐45 mg/day for 8 weeks |
| Outcomes |
Primary outcome SL, sleep efficiency and WASO Secondary outcome Objective sleep physiology |
| Notes | Conference abstract only. Unable to find further papers or contact the study author. |
Shirazi 2016.
| Methods | Randomised controlled trial |
| Participants | 60 postmenopausal women with sleep disturbances Setting: Yas Hospital in 2011‐2013 |
| Interventions |
Intervention: Melissa 600 mg for 8 weeks' follow‐up Intervention: citalopram 20 mg increased to 30 mg after 1 week for 8 weeks' follow‐up Comparator: placebo for 8 weeks for 8 weeks' follow‐up |
| Outcomes |
Primary outcome PSQI |
| Notes | Abstract only. Unable to source an English copy of the paper or contact the authors. |
Wu 2015.
| Methods | Randomised controlled trial |
| Participants |
Insomnia diagnosis criteria: PSQI score ≥ 7 Number of participants randomised: n = 78 Number of participants: n = 71 Citalopram: n = 35 Doxepin: n = 36 Age range: 45‐64 years Gender of those randomised (M/F): Citalopram: 35.9%/64.1% Doxepin: 20.5%/79.5% Race/ethnicity: not mentioned, but presumably Chinese Country: China Setting: The Jinshan Hospital of Fudan University Included: aged 45‐64 years who had not received any psychotropic drugs for ≥ 2 weeks or hormonal agents and immunomodulators in the 6 months prior to initiation of study. Excluded: severe medical conditions (cancer, cardiovascular and cerebrovascular diseases, thyroid disorders), pregnancy and lactation and mental retardation disorders. Withdrawals: 3 participants in citalopram group and 2 participants in doxepin group did not complete the study due to adverse drug reactions Baseline imbalances: 15.4% more women in doxepin group at randomisation |
| Interventions |
Intervention: citalopram 20 mg/day, taken after breakfast Comparison: doxepin 12.5 mg/day, taken 30 min before sleep at night |
| Outcomes |
Primary outcomes PSQI HAMA Headache, aggravated insomnia, blood pressure increase, hyperexcitability, nausea and vomiting, dizziness, palpitations, frequent urination, somnolence and numbness |
| Notes |
AE: adverse event; CPAP: continuous positive airway pressure; DSM: Diagnostic and Statistical Manual of Mental Disorders; F: female; HAMA: Hamilton Anxiety Rating Scale; hr: hour; IGR: Investigator Global Rating; ISI: Insomnia Severity Index; lb: pounds; LOCF: last observation carried forward; LPS: latency to persistent sleep; m: male; MDD: major depressive disorder; n: number; NAW: number of awakenings; Pg: page; PLMAI: periodic limb movements associated with arousal; SF‐36: 36‐item Short Form; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index; SD: standard deviation; SL: sleep latency; sTST: subjective total sleep time; TST: total sleep time; VAS: visual analogue score; WASO: wake after sleep onset.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IPR‐16009475.
| Trial name or title | The Research of Chronic Insomnia Clinical Evaluation and Optimisation of Treatment |
| Methods | Randomised, parallel controlled trial |
| Participants |
Number of participants randomised: n = 100 Inclusion criteria: aged 18‐65 years; primary insomnia according to the DSM‐IV‐TR criteria; having sleep difficulties on ≥ 3 nights per week for ≥ 6 months Exclusion criteria: past or current DSM‐IV‐TR diagnosis of depressive disorder, dysthymic disorder, bipolar disorder, generalised anxiety disorder, panic disorder, post‐traumatic stress disorder, psychotic disorder or substance use disorder; any significant physical illnesses; significant risk of suicide; pregnancy |
| Interventions | Intervention: alprazolam (31 participants); zolpidem (31 participants); trazodone (19 participants); quetiapine (19 participants) |
| Outcomes |
Primary outcome PQSI at week 4 |
| Starting date | 13 April 2012 |
| Contact information | Li Huafang; lhlh_5@163.com |
| Notes | No paper found |
Morin 2015.
| Trial name or title | Sequenced Therapies for Comorbid and Primary Insomnias |
| Methods | Randomised controlled trial |
| Participants |
Insomnia diagnosis criteria (method of diagnosis): ISI score > 10 indicating at least "mild" insomnia; and a score ≥ 2 on either the interference or distress item of the screening ISI, indicating the insomnia causes significant distress or impairment in social, occupational or other areas of functioning. These criteria represent those provided in the DSM‐IV‐TR87, Research Diagnostic Criteria and the International Classification of Sleep Disorders, and will ensure a sample with clinically relevant insomnia Number of participants (thus far): n = 82 Age, mean years: 49.7 Gender: 36/46 M/F Race: mixed Country: USA and Canada Inclusion criteria: complaint of persistent (i.e. > 1 month) difficulties initiating or maintaining sleep despite adequate opportunity for sleep; sleep onset latency or wake time after sleep onset > 30 min on ≥ 3 nights per week during 2 weeks sleep diary monitoring; ISI score > 10 indicating at least "mild" insomnia; and a score ≥ 2 on either the interference or distress item of the screening ISI, indicating the insomnia causes significant distress or impairment in social, occupational or other areas of functioning. These criteria represent those provided in the DSM‐IV‐TR87, Research Diagnostic Criteria and the International Classification of Sleep Disorders, and will ensure a sample with clinically relevant insomnia Exclusion criteria: untreated psychiatric disorder (e.g. major depression) as these conditions have specific treatments and it would be inappropriate not to offer those treatments; lifetime diagnosis of any psychotic or bipolar disorder as sleep restriction and medications for insomnia may precipitate mania and hallucinations; imminent risk for suicide; alcohol or drug abuse within the past year, since benzodiazepine receptor agonists are cross‐tolerant with alcohol; terminal or progressive physical illness (e.g. cancer, chronic obstructive pulmonary disease) or neurological degenerative disease (e.g. dementia); current use of medications known to cause insomnia (e.g. steroids); sleep apnoea (apnoea/hypopnoea index > 15), restless legs syndrome, periodic limb movement during sleep with arousal > 15 per hour, or a circadian rhythm sleep disorder (e.g. advanced sleep phase syndrome); habitual bedtimes later than 2:00 a.m. or rising times later than 10:00 a.m.; consuming > 2 alcoholic beverages per day on a regular basis |
| Interventions |
Intervention 1: behavioural insomnia therapy. Sleep hygiene, stimulus control and sleep restriction presented in 4 sessions. Comparator 1: zolpidem 5 mg or 10 mg Intervention 2: behavioural: cognitive therapy. Cognitive restructuring, constructive worry, behavioural experiments presented in 4 sessions. Comparator 2: trazodone 50 mg to 150 mg |
| Outcomes |
Primary outcome ISI change from baseline (remission) (at 6 and 12 weeks; 3, 6, 9 and 12 months) Secondary outcome Sleep diary and PSG sleep measures; subjective ratings of sleep and daytime function; adverse events; dropout rates and treatment acceptability |
| Starting date | June 2011 |
| Contact information | Professor Charles Morin; cmorin@psy.ulaval.ca |
| Notes | Author correspondence on 6 September 2016: "I am afraid these data have not yet been published other than in abstract form and are not available for distribution at this time." |
NCT02139098.
| Trial name or title | Phase III Study on Alternative Dosing Regimens in the Pharmacotherapy of Mild to Moderate Insomnia |
| Methods | Randomised, double‐blind, controlled trial |
| Participants |
Aged: 18‐69 years
Gender: men and women
Accepts healthy volunteers: no
Inclusion criteria: aged 18‐69 years; fluent in German language; provide written informed consent; ability to understand the explanations and instructions given by the study physician and the investigator Exclusion criteria: sleep disorders caused by medical factors (e.g. sleep apnoea, restless legs syndrome, narcolepsy, substance‐induced insomnia); contraindications to study medication intake according to the information sheet for health professionals (Summary of medicinal Product Characteristics, Fachinformation in Germany) assessed by physical examination (including ECG) and medical history; allergies to amitriptyline hydrochloride or any of its ingredients; allergies to zolpidem or any of its ingredients; acute intoxication with alcohol, analgesics, hypnotics or any other psychotropic drug; urinary retention; delirium; untreated closed‐angle glaucoma; prostatic hyperplasia; pyloric stenosis; paralytic ilius; suicidal thoughts; liver/kidney/pulmonary insufficiency; myasthenia gravis; hypokalaemia; bradycardia; coronary heart disease, cardiac arrhythmias, long QT syndrome or other clinically relevant cardiac disorders; increased risk of seizures/history of seizures; substance dependence syndrome/history of substance dependence syndrome; allergies to ingredients of placebo or novel‐tasting drink; currently pregnant (verified by urine pregnancy test) or lactating; people scoring ≥ 12 on the Epworth Sleepiness Scale; people scoring < 8 or > 21 on the ISI; people with a mental disorder as verified by the SCID (major depression, psychosis, brain injury, substance abuse or dependency syndrome during the last 6 months before V1); nicotine consumption > 10 cigarettes/day; unwillingness to refrain from alcohol consumption throughout the study; concomitant medication interfering with study medication intake due to potential interactions (all psychotropic medication including analgesics and muscle relaxants, hypericum derivatives, antihypertensives, antiarrhythmic agents, antibiotics, cisapride, antimalaria drugs, diuretics, imidazole antifungals, cumarin derivatives, antihistamines, calcium channel blockers, medications that enlarge the QT interval or may lead to hypokalaemia); change in concomitant medication regimen during the last 2 weeks prior to visit 1 or after randomisation; intake of psychotropic medication during the last 3 months; participation in any other clinical trial 3 months prior to visit 1; women of childbearing age not using 2 highly effective contraceptive methods; employee of the sponsor or the principal investigator |
| Interventions |
Intervention: amitriptyline flexible dosing 50 mg capsule before going to bed on 8 out of 17 nights/placebo Intervention: zolpidem flexible dosing 5 mg capsule before going to bed on 8 out of 17 nights/placebo Active comparator: amitriptyline fixed dosing 50 mg capsule before going to bed on 8 out of 17 nights Active comparator: zolpidem fixed dosing 5 mg capsule before going to bed on 8 out of 17 nights Active comparator: amitriptyline continuous dosing 50 mg capsule before going to bed on 13 out of 17 nights |
| Outcomes |
Primary outcomes Objective total sleep time assessed by PSG (change from baseline to day 10 after first medication intake) Objective sleep onset latency assessed by PSG (change from baseline to day 10 after first medication intake) Self‐reported total sleep time assessed by sleep diary (change from baseline to day 10 after first medication intake) assessed by sleep diary Self‐reported sleep onset latency (change from baseline to day 10 after first medication intake) assessed by sleep diary Secondary outcomes Percentage of REM sleep assessed by PSG (change from baseline to day 10 after first medication intake) REM onset latency assessed by PSG (change from baseline to day 10 after first medication intake) Objective sleep efficiency assessed by actigraphy (change from baseline to day 17 after first medication intake) Objective total sleep time assessed by actigraphy (change from baseline to day 17 after first medication intake) Self‐reported total sleep time assessed by sleep diary (change from baseline to day 18 after first medication intake) Self‐reported sleep onset latency assessed by sleep diary (change from baseline to day 18 after first medication intake) Self‐reported sleep onset latency (evaluation) assessed by sleep diary (change from baseline to day 18 after first medication intake) |
| Starting date | May 2014 |
| Contact information | Professor Winfried Rief; rief@staff.uni‐marburg.de |
| Notes | Estimate study completion date December 2017 |
DSM: Diagnostic and Statistical Manual of Mental Disorders; ECG: electrocardiogram; ISI: Insomnia Severity Index; PQSI: Pittsburgh Quality Sleep Index; PSG: polysomnography; REM: rapid eye movement; SCID: Structured Clinical Interview for Depression.
Differences between protocol and review
Trials with fewer than three days of drug treatment were excluded from the review as it was deemed that clinically important effects of antidepressants on insomnia (by definition a long‐term condition) required more than one or two doses of an antidepressant to be able to determine the effect. This three‐day minimum had not been prespecified in the protocol.
We have removed the term 'primary' from before 'insomnia diagnosis' in the 'Type of participants' section. 'Primary insomnia' is now largely outdated as terminology with the newer definition being 'Insomnia disorder.' Our use of this terminology had created confusion on peer review as some had interpreted it to mean that papers should only be included if the main focus of the paper was insomnia (i.e. insomnia was the primary diagnosis reported in the title). This is not what we, the review authors, had originally intended and would lose valuable data by excluding papers that focused on depression or anxiety, but had a clear definition of insomnia at baseline and collected and reported good sleep outcomes. Different conditions coexist and it is most often not possible to say which is more relevant to the patient's overall status. It is often a question of perspective or of emphasis in the way the paper is written. We had clearly stated a priori that participants should be included with all types of comorbidity (e.g. anxiety or depression) and insomnia and this is what we have done in this review. The key criteria for inclusion was having a clear entry criteria of a definition of insomnia at baseline.
Contributions of authors
HE, SW, DB, AMayers, AMalizia helped to develop the research question and wrote the protocol.
AMayers and HE and the Cochrane Group wrote the search strategy.
HE, DB, SW, GL, AMalizia and CM first and second screened the papers.
AMayers and HE reviewed ongoing studies.
CM managed the study databases.
BS undertook the statistical analysis.
HE, SW, GL, DB and BS wrote this review.
All review authors provided comments and suggestions on draft versions of the review and approved the current version.
Sources of support
Internal sources
The NIHR School of Primary Care Research (SPCR) provided a small grant to part support undertaking this review, as did the Primary Care and Population Sciences (PCPS) Unit at the University of Southampton., UK.
External sources
No sources of support supplied
Declarations of interest
HE: has no interests to declare.
DB: on behalf of his employer DB has held research grants from Bristol‐Myers Squibb, Cephalon, Eli Lilly Ltd, GlaxoSmithKline, H. Lundbeck A/S, Pierre Fabre, Pfizer Ltd, Roche and Vernalis Ltd. He has served on advisory boards hosted by Astra Zeneca, Bristol‐Myers Squibb, Eli Lilly Ltd, GlaxoSmithKline, Grünenthal, H. Lundbeck A/S, Pierre Fabre and Pfizer Ltd. He is a past president of Depression Alliance and current Medical Patron of Anxiety UK.
BS: has no interests to declare.
GL: has no interests to declare.
AMayers: has no interests to declare.
ALM: since medical qualification ALM has worked as Director of Clinical Pharmacology in SmithKlineBeecham and has dormant pension contributions through a third‐party pension provider. He has received research support from Wyeth, Medtronic and Organon on behalf of his employers at the time and been a speaker or accepted hospitality (or both) from Eli Lilly, Astra Zeneca, Lundbeck, Pfizer, Medtronic and Servier.
CCFM: has no interests to declare.
SW: has received honoraria for lectures from pharmaceutical companies making antidepressants, although none since 2009. SW has no financial interest in any pharmaceutical company.
New
References
References to studies included in this review
Corruble 2013 {published data only}
- Corruble E, Bodinate C, Belaidi C, Goodwin GM. Efficacy of agomelatine and escitalopram on depression, subjective sleep and emotional experiences in patients with major depressive disorder: a 24 week randomised, controlled, double blind trial. International Journal of Neuropsychopharmacology 2013;16(10):2219‐34. [DOI] [PubMed] [Google Scholar]
Fava 2002 {published data only}
- Fava M, Hoog SL, Judge RA, Kopp JB, Nisson ME, Gonzales JS. Acute efficacy of fluoxetine versus sertraline and paroxetine in major depressive disorder including effects of baseline insomnia. Journal of Clinical Psychopharmacology 2002;22(2):137‐47. [DOI] [PubMed] [Google Scholar]
Finnerty 1978 {published data only}
- Finnerty RJ, Goldberg HL, Rickels K. Doxepin versus imipramine in psychoneurotic depressed patients with sleep disturbance: a double‐blind study. Journal of Clinical Psychiatry 1978;39(12):852‐6. [PubMed] [Google Scholar]
Friedmann 2008 {published data only}
- Friedmann PD, Rose JS, Swift R. Trazodone for sleep disturbance after alcohol detoxification: a double‐blind, placebo‐controlled trial. Alcoholism Clinical Experimental Research 2008;32(9):1652‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillin 1997 {published data only}
- Gillin JC, Rapaport M, Erman MK, Winokur A, Albata BJ. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician‐rated measures of sleep in depressed patients: a double‐blind, 8‐week clinical trial. Journal of Clinical Psychiatry 1997;58(5):185‐92. [DOI] [PubMed] [Google Scholar]
Hajak 2001 {published data only}
- Hajak G, Rodenbeck A, Voderholzer U, Riemann D, Cohrs S, Hohagen F, et al. Doxepin in the treatment of primary insomnia: a placebo‐controlled, double‐blind, polysomnographic study. Journal of Clinical Psychiatry 2001;62(6):453‐63. [DOI] [PubMed] [Google Scholar]
Khazaie 2013 {published data only}
- Khazaie H, Ghadami MR, Knight DC, Emamian F, Tahmasian M. Insomnia treatment in the third trimester of pregnancy reduces postpartum depression symptoms: a randomised clinical trial. Psychiatry Research 2013;210:901‐5. [DOI] [PubMed] [Google Scholar]
Krystal 2010 {published data only}
- Krystal AD, Durrence HH, Scharf M, Jochelson P, Rogowski R, Ludington E. Efficacy and safety of doxepin 1 mg and 3 mg in a 12‐week sleep laboratory and outpatient trial of elderly subjects with chronic primary insomnia. Sleep 2010;33(11):1553‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krystal 2011 {published data only}
- Krystal AD, Lankford A, Durrence HH, Ludington E, Jochelson P, Rogowski R. Efficacy and safety of doxepin 3 and 6 mg in a 35‐day sleep laboratory trial in adults with chronic primary insomnia. Sleep 2011;34(10):1433‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lankford 2012 {published data only}
- Lankford A, Rogowski R, Essink B, Ludington E, Durrence HH, Roth T. Efficacy and safety of doxepin 6 mg in a four‐week outpatient trial of elderly adults with chronic primary insomnia. Sleep Medicine 2012;13(2):133‐8. [DOI] [PubMed] [Google Scholar]
Le Bon 2003 {published data only}
- Bon O, Murphy JR, Staner L, Hoffmann G, Kormoss N, Kentos M. Double‐blind, placebo‐controlled study of the efficacy of trazodone in alcohol post‐withdrawal syndrome: polysomnographic and clinical evaluations. Journal of Clinical Psychopharmacology 2003;23(4):377‐83. [DOI] [PubMed] [Google Scholar]
Palomaki 2003 {published data only}
- Palomaki H, Berg A, Meririnne E, Kaste M, Lönnqvis R, Lehtihalmes M, et al. Complaints of post‐stroke insomnia and its treatment with mianserin. Cerebrovascular Diseases 2003;15(1‐2):56‐62. [DOI] [PubMed] [Google Scholar]
Reynolds 2006 {published data only}
- Reynolds CF, Buysse DJ, Miller MD, Pollock BG, Hall M, Mazumdar S. Paroxetine treatment of primary insomnia in older adults. American Journal of Geriatric Psychiatry 2006;14(9):803‐7. [DOI] [PubMed] [Google Scholar]
Riemann 2002 {published data only}
- Riemann D, Voderholzer U, Cohrs S, Rodenbeck A, Hajak G, Rüther E. Trimipramine in primary insomnia: results of a polysomnographic double‐blind controlled study. Pharmacopsychiatry 2002;35(5):165‐74. [DOI] [PubMed] [Google Scholar]
Rios Romenet 2013 {published data only}
- Rios Romenets S, Creti L, Fichten C, Bailes S, Libman E, Pelletier A. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson's disease: a randomised study. Parkinsonism & Related Disorders 2013;19(7):670‐5. [DOI] [PubMed] [Google Scholar]
Roth 2011 {published data only}
- Roth AJ, McCall W, Liguori A. Cognitive, psychomotor and polysomnographic effects of trazodone in primary insomniacs. Journal of Sleep Research 2011;20(4):552‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rush 1998 {published data only}
- Rush AJ, Armitage R, Gillin JC, Yonkers KA, Winkour A, Moldofsky H. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biological Psychology 1998;44(1):3‐14. [DOI] [PubMed] [Google Scholar]
Satterlee 1995 {published data only}
- Satterlee WG, Faires D. The effects of fluoxetine on symptoms of insomnia in depressed patients. Psychopharmacological Bulletin 1995;31(2):227‐37. [PubMed] [Google Scholar]
Shell 2012 {published data only}
- Shell WE, May LA, Bullias DH, Pavlik SL, Silver DS. Sentra PM (a medical food) and trazodone in the management of sleep disorders. Journal of Central Nervous System Diseases 2012;4:65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2012 {published data only}
- Stein MD, Kurth, Sharkey KM, Anderson BJ, Corso RP, Millman RP. Trazodone for sleep disturbance during methadone maintenance: a double‐blind, placebo‐controlled trial. Drug and Alcohol Dependence 2012;120(1‐3):65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1998 {published data only}
- Walsh JK, Erman M, Erwin CW, Jamieson A, Mahowald M, Regestein Q. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII‐R primary insomnia. Human Psychopharmacology: Clinical and Experimental 1998;13(3):191‐8. [Google Scholar]
Ware 1989 {published data only}
- Ware JC, Brown FW, Moorad PJ, Pittard JT, Corbert B. Effects on sleep: a double‐blind study comparing trimipramine to imipramine in depressed insomniac patients. Sleep 1989;12(6):191‐8. [DOI] [PubMed] [Google Scholar]
Zhou 2002 {published data only}
- Zhou CL, Xie HJ, Wang LQ, Tang X. Clinical observation on effects of paroxetine and alprazolam in the treatment of elderly patients with chronic primary insomnia. Chinese Journal of Geriatrics 2002;21(3):185‐7. [Google Scholar]
References to studies excluded from this review
Adam 1979 {published data only}
- Adam K, Oswald I. One gram of L‐tryptophan fails to alter the time taken to fall asleep. Neuropharmacology 1979;18(12):1025‐7. [DOI] [PubMed] [Google Scholar]
Botros 1989 {published data only}
- Botros WA, Ankier SI, Priest RG, McManus IC, Samir ZY. Clinical assessment and performance tasks in depression: a comparison of amitriptyline and trazodone. British Journal of Psychiatry 1989;155:479‐82. [DOI] [PubMed] [Google Scholar]
Boyle 2012 {published data only}
- Boyle J, Eriksson ME, Grible L, Gouni R, Johnsen S, Coppini DV. Randomised, placebo‐controlled comparison of amitriptyline, duloxetine, and pregabaline in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care 2012;35(12):2451‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 2017 {published data only}
- Carney CE, Edinger JD, Kuchibhatla M, Lachowski AM, Bogouslavsky O, Krystal AD. Cognitive behavioral insomnia therapy for those with insomnia and depression: a randomized controlled clinical trial [NCT00620789]. Sleep 2017;40:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2002 {published data only}
- Chen X, Zheng HM, Shun XJ. Comparison of trazodone and paroxetine in treating dyssomnia of anxiety disorder. Chinese Journal of New Drugs and Clinical Remedies 2002;21(12):727‐30. [Google Scholar]
Fairweather 1997 {published data only}
- Fairweather DB, Stanley N, Hindmarch I. Fluoxetine and dothiepin in depressed patients: a comparison of efficacy and effects on cognition and subjective sleep. European Neuropsychopharmacology 1997;7(S2):148. [Google Scholar]
Ferrero 1987 {published data only}
- Ferrero F, Zahnd J. Tryptophan in the treatment of insomnia in hospitalised psychiatric patients. Encephale 1987;13(1):35‐7. [PubMed] [Google Scholar]
Hajak 1996 {published data only}
- Hajak G, Rodenbeck A, Adler L, Huether G, Bandelow B, Herrendorf G. Nocturnal melatonin secretion and sleep after doxepin administration in chronic primary insomnia. Pharmacopsychiatry 1996;29(5):187‐92. [DOI] [PubMed] [Google Scholar]
Herman 2009 {published data only (unpublished sought but not used)}
- Herman B, Mychaskiw M, Mandel F. Comparison of sleep outcomes in generalized anxiety disorder following treatment with pregabalin or venlafaxine‐XR. European Psychiatry 2009;24 (Suppl 1):S528. [Google Scholar]
- Mychaskiw MA, Alvir JM, Herman BK, Pallanti S, Joshi A. Insomnia and quality of life in generalized anxiety disorder: impact on clinical presentation and response to pregabalin and venlafaxine‐XR. European Psychiatry 2009;24 (Suppl 1):S534. [Google Scholar]
Karsten 2017 {published data only}
- Karsten J, Hagenauw La, Kamphuis J, Lancel M. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double‐blind, cross‐over, placebo‐controlled trial. Journal of Psychopharmacology 2017;31(3):327‐37. [DOI] [PubMed] [Google Scholar]
Kaynak 2004 {published data only}
- Kaynak H, Kaynak D, Gözükirmizi E, Guilleminault C. The effects of trazodone on sleep in patients treated with stimulant antidepressants. Sleep Medicine 2004;5(1):15‐20. [DOI] [PubMed] [Google Scholar]
Moon 1991 {published data only}
- Moon CAL, Pownall R. A double‐blind study of the effects of Dothiepin (75/150 mg nocte) on sleep and morning psychomotor function. British Journal of Clinical Research 1991;2:21‐31. [Google Scholar]
Palesh 2012 {published data only}
- Palesh O, Mustian KM, Roscoe JA, Morrow GR, Perlis ML, Heckler CE. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Medicine 2012;13(9):1184‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2007 {published data only}
- Roth T, Rogowski R, Hull S, Schwartz H, Koshorek G, Corser B. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia. Sleep 2007;30(11):1555‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruwe 2016 {published data only}
- Ruwe F, Ijzerman‐Boon P, Roth T, Zammit G, Ivgy‐May N. A phase 2 randomized dose‐finding study with esmirtazapine in patients with primary insomnia. Journal of Clinical Psychopharmacology 2016;36(5):457‐64. [DOI] [PubMed] [Google Scholar]
Scharf 2008 {published data only}
- Scharf M, Rogowski R, Hull S, Cohn M, Mayleben D, Feldman N. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: a randomized, double‐blind, placebo‐controlled crossover study. Journal of Clinical Psychiatry 2008;69(10):1557‐64. [DOI] [PubMed] [Google Scholar]
Stein 2011 {published data only}
- Stein DJ, Lopez AG. Effects of escitalopram on sleep problems in patients with major depression or generalized anxiety disorder. Advances in Therapy 2011;28(11):1021‐37. [DOI] [PubMed] [Google Scholar]
Stephenson 2000 {published data only}
- Stephenson DA, Harris B, Davies RH, Mullin JM. The impact of antidepressants on sleep and anxiety: a comparative study of fluoxetine and dothiepin using the Leeds Sleep Evaluation Questionnaire. Human Psychopharmacology 2000;15(7):529‐34. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ahmed 2016 {published data only}
- Ahmed M, Aamir R, Jishi Z, Scharf MB. The effects of milnacipran on sleep disturbance in fibromyalgia: a randomized, double‐blind, placebo‐controlled, two‐way crossover study. Journal of Clinical Sleep Medicine 2016;12(1):79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivgy‐May 2015a {published data only}
- Ivgy‐May N, Ruwe F, Krystal A, Roth T. Esmirtazapine in non‐elderly adult patients with primary insomnia: efficacy and safety from a randomized, 6‐week sleep laboratory trial. Sleep Medicine 2015;16:838‐44. [DOI] [PubMed] [Google Scholar]
Ivgy‐May 2015b {published data only}
- Ivgy‐May N, Roth T, Ruwe F, Walsh J. Esmirtazapine in non‐elderly adult patients with primary insomnia: efficacy and safety from a 2‐week randomized outpatient trial. Sleep Medicine 2015;16(7):831‐7. [DOI] [PubMed] [Google Scholar]
Krystal 2012 {published data only}
- Krystal AD, Roth T, Pong A, Stet L, Ivgy‐May N. Efficacy and safety of esmirtazapine in elderly patients with primary insomnia in a 2‐week sleep laboratory trial. Sleep 2012;35:A222. [DOI] [PubMed] [Google Scholar]
Merck 2008 {published data only}
- Merck Sharp, Dohme Corp. A 6‐month efficacy and safety study of org 50081 in adult patients with chronic primary insomnia (21106/P05701/MK‐8265‐002). ClinicalTrials.gov/show/NCT00631657 Date first registered: 10 March 2008.
Miljatovic 2012 {published data only}
- Miljatovic AM. Comparative effects of venlafaxine and mirtazepine on sleep physiology measures in patients with major depressive disorder and insomnia. European Psychiatry 2012;27 (Suppl 1):1. [Google Scholar]
Shirazi 2016 {published data only}
- Shirazi M, Saedi N, Shariat M, Azadi F, Tanha FD. Comparison of melissa with citalopram and placebo in treatment of sleep disorders in menopausal women: clinical trial. Tehran University Medical Journal 2016;74(8):562‐8. [Google Scholar]
Wu 2015 {published data only}
- Wu J, Chang F, Zu H. Efficacy and safety evaluation of citalopram and doxepin on sleep quality in comorbid insomnia and anxiety disorders. Experimental and Therapeutic Medicine 2015;10:1303‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐IPR‐16009475 {published data only}
- ChiCTR‐IPR‐16009475, Shanghai Mental Health Center. The research of chronic insomnia clinical evaluation and optimization of treatment. www.chictr.org.cn/showproj.aspx?proj=16136 Date first registered: 18 October 2016.
Morin 2015 {published data only (unpublished sought but not used)}
- Morin CM, Edinger JD, Krystal AD, Beaulieu‐Bonneau S, Ivers H, Guay B, et al. Sequenced therapies for comorbid and primary insomnia: preliminary findings of a randomized controlled trial. Sleep 2015;40 (Suppl 1):A127. [Google Scholar]
NCT02139098 {published data only}
- NCT02139098. Alternative dosing regimens in the pharmacotherapy of insomnia (ALPHASOM). clinicaltrials.gov/ct2/show/NCT02139098 Date first registered: 15 May 2014.
Additional references
Altena 2008
- Altena E, Werf YD, Strijers RL, Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. Journal of Sleep Research 2008;17(3):335‐43. [DOI] [PubMed] [Google Scholar]
Auld 2017
- Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. 2017;34:10‐22. [DOI] [PubMed] [Google Scholar]
Aversa Lopes 2009
- Aversa Lopes E, Silva AB, Macedo CR, Soares B, Saconato H, Atallah ÁN. Cognitive behavioural therapy for insomnia. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006814] [DOI] [Google Scholar]
Baglioni 2011
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U. Insomnia as a predictor of depression: a meta‐analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders 2011;135:1‐19. [DOI] [PubMed] [Google Scholar]
Balter 1992
- Balter MB, Uhlenhuth EH. New epidemiologic findings about insomnia and its treatment. Journal of Clinical Psychiatry 1992;53 Suppl:34‐9. [PubMed] [Google Scholar]
Breslau 1996
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biological Psychiatry 1996;39(6):411‐8. [DOI] [PubMed] [Google Scholar]
Buscemi 2007
- Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta‐analysis of RCTs. Journal of General Internal Medicine 2007;22(9):1335‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds III CF, Monk TH, Berman SB, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193‐213. [DOI] [PubMed] [Google Scholar]
Cheuk 2012
- Cheuk DK, Yeung WF, Chung KF, Wong V. Acupuncture for insomnia. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD005472.pub3] [DOI] [Google Scholar]
Chevalier 1999
- Chevalier H, Los F, Boichut D, Bianchi M, Nutt DJ, Hajak G. Evaluation of severe insomnia in the general population: results of a European multinational survey. Journal of Psychopharmacology 1999;13(4 Suppl 1):21‐4. [DOI] [PubMed] [Google Scholar]
Committee on Safety of Medicines 1988
- Committee on Safety of Medicines. Benzodiazepines, dependence and withdrawal symptoms. Medicines Control Agency 1988;21:1‐2. [Google Scholar]
DSM‐V 2015
- American Psychiatric Association. Insomnia disorder. Diagnostic and Statistical Manual of Mental Disorders, 5th edition 2015.
Edinger 2008
- Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep 2008;31(5):599‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Everitt 2014
- Everitt H, McDermott L, Leydon G, Yules H, Baldwin D, Little P. General practitioners' management strategies for patients with insomnia: a survey and qualitative interview study. British Journal of General Practice 2014;64:88‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ishigooka 1999
- Ishigooka J, Suzuki M, Isawa S, Muraoka H, Murasaki M, Okawa M. Epidemiological study on sleep habits and insomnia of new outpatients visiting general hospitals in Japan. Psychiatry and Clinical Neurosciences 1999;53(4):515‐22. [DOI] [PubMed] [Google Scholar]
Krystal 2003
- Krystal AD, Walsh JK, Laska E, Caron J, Amato DA, Wessel TC, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double‐blind, placebo‐controlled study in adults with chronic insomnia. Sleep 2003;26(7):793‐9. [DOI] [PubMed] [Google Scholar]
Krystal 2008
- Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T. Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study. Sleep 2008;31(1):79‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krystal 2009
- Krystal AD. A compendium of placebo‐controlled trials of the risks/benefits of pharmacological treatments for insomnia: the empirical basis for U.S. clinical practice. Sleep Medicine Reviews 2009;13(4):265‐74. [DOI] [PubMed] [Google Scholar]
Lai 2011
- Lai L, Tan MH, Lai YC. Prevalence and factors associated with off‐label antidepressant prescriptions for insomnia. Drug, Healthcare and Patient Safety 2011;3:27‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
LeBlanc 2009
- LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population‐based sample. Sleep 2009;32(8):1027‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leger 2001
- Leger D, Scheuermaier K, Philip P, Paillard M, Guilleminault C. SF‐36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosomatic Medicine 2001;63(1):49‐55. [DOI] [PubMed] [Google Scholar]
Lemoine 2007
- Lemoine P, Nir T, Laudon M, Zisapel N. Prolonged‐release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. Journal of Sleep Research 2007;16(4):372‐80. [DOI] [PubMed] [Google Scholar]
Mayer 2009
- Mayer G, Wang‐Weigand S, Roth‐Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6‐month nightly ramelteon administration in adults with chronic primary insomnia. Sleep 2009;32(3):351‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayers 2005
- Mayers AG, Baldwin DS. Antidepressants and their effect on sleep. Human Psychopharmacology 2005;20(8):533‐59. [DOI] [PubMed] [Google Scholar]
Mellinger 1985
- Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates. Archives of General Psychiatry 1985;42(3):225‐32. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morin 1999
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late‐life insomnia: a randomized controlled trial. Journal of the American Medical Association 1999;281(11):991‐9. [DOI] [PubMed] [Google Scholar]
Morin 2003
- Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosomatic Medicine 2003;65(2):259‐67. [DOI] [PubMed] [Google Scholar]
Morin 2011
- Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34(5):603‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morlock 2006
- Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of national ambulatory medical survey data, 1997‐2002. Clinical Therapeutics 2006;28(7):1044‐53. [DOI] [PubMed] [Google Scholar]
Neckelmann 2007
- Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep 2007;30(7):873‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS Digital 2011
- NHS Digital. Prescription Cost Analysis England 2010. digital.nhs.uk/data‐and‐information/publications/statistical/prescription‐cost‐analysis/prescription‐cost‐analysis‐england‐2010 2011 (accessed June 2011).
NICE 2004
- National Institute for Clinical Excellence (NICE). Guidance on the use of zaleplon, zolpidem and zopiclone for the short‐term management of insomnia (Technology Appraisal 77). www.nice.org.uk/guidance/ta77 (accessed 12 September 2013). London: NICE, 2004.
NIH 1983
- National institute of Health Consensus Development Programme. Drugs and insomnia: the use of medications to promote sleep. consensus.nih.gov/1983/1983insomniadrugs039html.htm 1983.
NIH 2005
- NIH. National Institutes of Health State of the Science conference statement on manifestations and management of chronic insomnia in adults. Sleep 2005;28(9):1049‐57. [DOI] [PubMed] [Google Scholar]
Nissen 2011
- Nissen C, Kloepfer C, Feige B, Piosczyk H, Spiegelhalder K, Voderholzer U. Sleep‐related memory consolidation in primary insomnia. Journal of Sleep Research 2011;20(1 Pt 2):129‐36. [DOI] [PubMed] [Google Scholar]
Ohayon 1999
- Ohayon MM, Caulet M, Arbus L, Billard M, Coquerel A, Guieu JD. Are prescribed medications effective in the treatment of insomnia complaints?. Journal of Psychosomatic Research 1999;47(4):359‐68. [DOI] [PubMed] [Google Scholar]
Perlis 2010
- Perlis M, Shaw P, Cano G, Espie C. Chapter 78: models of insomnia. In: Kryger M, Roth T, Dement W editor(s). Principles and Practice of Sleep Medicine. 5th Edition. St Louis (MO): Saunders, 2010:850‐65. [Google Scholar]
Philip 2006
- Philip P, Leger D, Taillard J, Quera‐Salva MA, Niedhammer I, Mosqueda JG. Insomniac complaints interfere with quality of life but not with absenteeism: respective role of depressive and organic comorbidity. Sleep Medicine 2006;7(7):585‐91. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riemann 2009
- Riemann D, Perlis, ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioural therapies. Sleep Medicine Reviews 2009;13:205‐14. [DOI] [PubMed] [Google Scholar]
Saarto 2010
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. Journal of Neurology, Neurosurgery and Psychiatry 2010;81(12):1372‐3. [DOI] [PubMed] [Google Scholar]
Sateia 2004
- Sateia MJ, Nowell PD. Insomnia. Lancet 2004;364(9449):1959‐73. [DOI] [PubMed] [Google Scholar]
Sivertsen 2009
- Sivertsen B, Krokstad S, Mykletun A, Overland S. Insomnia symptoms and use of health care services and medications: the HUNT‐2 study. Behavioral Sleep Medicine 2009;7(4):210‐22. [DOI] [PubMed] [Google Scholar]
Thaler 2012
- Thaler KJ, Morgan LC, Noord M, Gaynes BN, Hansen R, Lux L, et al. Comparative effectiveness of second‐generation antidepressants for accompanying anxiety insomnia and pain in depressed patients: a systematic review. Depression and Anxiety 2012;29(6):495‐505. [DOI] [PubMed] [Google Scholar]
Vgontzas 2009
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela‐Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009;32(4):491‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 2007
- Wade AG, Ford I, Crawford G, McMahon AD, Nir T, Laudon M. Efficacy of prolonged release melatonin in insomnia patients aged 55‐80 years: quality of sleep and next‐day alertness outcomes. Current Medical Research and Opinion 2007;23(10):2597‐605. [DOI] [PubMed] [Google Scholar]
Walsh 2007
- Walsh JK, Krystal AD, Amato DA, Rubens R, Caron J, Wessel TC. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep 2007;30(8):959‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. ICD‐10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. [Google Scholar]
Wilson 2005
- Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs 2005;65(7):927‐47. [DOI] [PubMed] [Google Scholar]
Wilson 2010
- Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN. British Association for Psychopharmacology consensus statement on evidence‐based treatment of insomnia, parasomnias and circadian rhythm disorders. Journal of Psychopharmacology 2010;24(11):1577‐601. [DOI] [PubMed] [Google Scholar]