Abstract
Background
Endometrial cancer is the sixth most common cancer in women worldwide and most commonly occurs after the menopause (75%) (globocan.iarc.fr). About 319,000 new cases were diagnosed worldwide in 2012. Endometrial cancer is commonly considered as a potentially 'curable cancer,' as approximately 75% of cases are diagnosed before disease has spread outside the uterus (FIGO (International Federation of Gynecology and Obstetrics) stage I). The overall five‐year survival for all stages is about 86%, and, if the cancer is confined to the uterus, the five‐year survival rate may increase to 97%. The majority of women diagnosed with endometrial cancer have early‐stage disease, leading to a good prognosis after hysterectomy and removal of the ovaries (oophorectomy), with or without radiotherapy. However, women may have early physiological and psychological postmenopausal changes, either pre‐existing or as a result of oophorectomy, depending on age and menopausal status at the time of diagnosis. Lack of oestrogen can cause hot flushes, night sweats, genital tract atrophy and longer‐term adverse effects, such as osteoporosis and cardiovascular disease. These changes may be temporarily managed by using oestrogens, in the form of hormone replacement therapy (HRT). However, there is a theoretical risk of promoting residual tumour cell growth and increasing cancer recurrence. Therefore, this is a potential survival disadvantage in a woman who has a potentially curable cancer. In premenopausal women with endometrial cancer, treatment induces early menopause and this may adversely affect overall survival. Additionally, most women with early‐stage disease will be cured of their cancer, making longer‐term quality of life (QoL) issues more pertinent. Following bilateral oophorectomy, premenopausal women may develop significant and debilitating menopausal symptoms, so there is a need for information about the risk and benefits of taking HRT, enabling women to make an informed decision, weighing the advantages and disadvantages of using HRT for their individual circumstances.
Objectives
To assess the risks and benefits of HRT (oestrogen alone or oestrogen with progestogen) for women previously treated for endometrial cancer.
Search methods
We searched the Cochrane Register of Controlled Trials (CENTRAL 2017, Issue 5), MEDLINE (1946 to April, week 4, 2017) and Embase (1980 to 2017, week 18). We also searched registers of clinical trials, abstracts of scientific meetings and reference lists of review articles.
Selection criteria
We included randomised controlled trials (RCTs), in all languages, that examined the efficacy of symptom relief and the safety of using HRT in women treated for endometrial cancer, where safety in this situation was considered as not increasing the risk of recurrence of endometrial cancer above that of women not taking HRT.
Data collection and analysis
Two review authors independently assessed whether potentially relevant studies met the inclusion criteria. We used standard methodological procedures expected by Cochrane.
Main results
We identified 2190 unique records, evaluated the full text of seven studies and included one study with 1236 participants. This study reported tumour recurrence in 2.3% of women in the oestrogen arm versus 1.9% of women receiving placebo (risk ratio (RR) 1.17, 95% confidence interval (CI) 0.54 to 2.50; very low‐certainty evidence). The study reported one woman in the HRT arm (0.16%) and three women in the placebo arm (0.49%) who developed breast cancer (new malignancy) during follow‐up (RR 0.80, 95% CI 0.32 to 2.01; 1236 participants, 1 study; very low‐certainty evidence). The study did not report on symptom relief, overall survival or progression‐free survival for HRT versus placebo. However, they did report the percentage of women alive with no evidence of disease (94.3% in the HRT group and 95.6% in the placebo group) and the percentage of women alive irrespective of disease progression (95.8% in the HRT group and 96.9% in the placebo group) at the end of the 36 months' follow‐up. The study did not report time to recurrence and it was underpowered due to closing early. The authors closed it as a result of the publication of the Women's Health Initiative (WHI) study, which, at that time, suggested that risks of exogenous hormone therapy outweighed benefits and had an impact on study recruitment. No assessment of efficacy was reported.
Authors' conclusions
Currently, there is insufficient high‐quality evidence to inform women considering HRT after treatment for endometrial cancer. The available evidence (both the single RCT and non‐randomised evidence) does not suggest significant harm, if HRT is used after surgical treatment for early‐stage endometrial cancer. There is no information available regarding use of HRT in higher‐stage endometrial cancer (FIGO stage II and above). The use of HRT after endometrial cancer treatment should be individualised, taking account of the woman's symptoms and preferences, and the uncertainty of evidence for and against HRT use.
Keywords: Female; Humans; Breast Neoplasms; Breast Neoplasms/epidemiology; Early Termination of Clinical Trials; Endometrial Neoplasms; Endometrial Neoplasms/chemically induced; Endometrial Neoplasms/epidemiology; Endometrial Neoplasms/surgery; Estrogen Replacement Therapy; Estrogen Replacement Therapy/adverse effects; Neoplasm Recurrence, Local; Neoplasm Recurrence, Local/chemically induced; Neoplasm Recurrence, Local/epidemiology; Neoplasms, Second Primary; Neoplasms, Second Primary/epidemiology; Quality of Life
Plain language summary
Hormone replacement therapy (HRT) for women previously treated for endometrial cancer
The issue Endometrial cancer develops from the lining of the womb (uterus). It is the sixth most common cancer worldwide and mainly affects women around the time of or after the menopause (the final menstrual period). At an early stage, where the cancer has not spread outside of the womb, survival rates are excellent with a five‐year survival of up to 97%. Treatment of endometrial cancer normally involves surgery to remove the womb, fallopian tubes (that connect the uterus to the ovaries) and ovaries (which produce eggs) (hysterectomy and bilateral salpingo‐oophorectomy). This may cause the onset of menopausal symptoms in women diagnosed prior to the menopause, or women may already be suffering from menopausal symptoms when they are diagnosed.
Hormone replacement therapy (HRT) is used to treat menopausal symptoms such as hot flushes, night sweats and vaginal dryness. In younger menopausal women, HRT may also help to maintain bone strength and prevent osteoporosis (weak bones). However, the safety of HRT after endometrial cancer is not known. Some types of endometrial cancer cells may be stimulated to grow by oestrogen, which is the main hormone in some types of HRT. Therefore, HRT has the potential to increase the growth of endometrial cancer cells left behind after treatment (due to microscopic undetected spread outside of the womb, fallopian tubes and ovaries), so promoting tumour recurrence (regrowth). Some doctors may not prescribe HRT after a diagnosis of endometrial cancer due to this theoretical risk. However, most women treated for early‐stage endometrial cancer will not have any residual cancer cells following surgery. Menopausal symptoms can severely affect quality of life and early menopause can affect long‐term health. HRT could potentially improve quality of life and long‐term health, and women treated for endometrial cancer need to be able to balance the risks and benefits of HRT to decide about their treatment.
The aim of the review The aim of this systematic review was to determine the effectiveness (does it improve symptoms) and safety of HRT in women who have been treated for endometrial cancer. Safety of HRT in this situation included effects on survival and the specific risk of endometrial cancer regrowing.
What were the main findings? We searched clinical trial databases to look for any evidence of effectiveness and safety of HRT use in women who had had endometrial cancer up to May 2017. We only found one study that randomly allocated women to receive either HRT or a placebo (pretend treatment). This found no difference in the likelihood of the cancer regrowth between the two groups. They showed that HRT may or may not increase the risk of recurrence of developing a new cancer. They did not provide any information on survival or symptom relief. However, the study was not completed due to poor recruitment into the clinical trial, so was not large enough to definitively say whether the use of HRT could be recommended after treatment for early endometrial cancer.
Quality of the evidence We are uncertain whether HRT increases the risk of recurrence after a diagnosis of endometrial cancer, as the certainty of the current evidence was very low. We identified only one randomised trial and this trial did not include enough women to definitely answer the question. This trial also had areas of potential bias that reduced our certainty in the results.
What were the conclusions? Limited, very‐low certainty, evidence suggests that HRT may have little or no effect on the risk of endometrial cancer returning for women who have been treated surgically for an early‐stage endometrial cancer. There were no data to say whether HRT had an effect on overall survival after hysterectomy for endometrial cancer.
Summary of findings
Summary of findings for the main comparison. Oestrogen replacement therapy compared to placebo for women previously treated for endometrial cancer.
| Oestrogen replacement therapy compared to placebo for women previously treated for endometrial cancer | ||||||
| Patient or population: women previously treated for endometrial cancer Setting: oncology follow‐up Intervention: oestrogen replacement therapy Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty (quality) of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with oestrogen replacement therapy | |||||
| Rate of symptom relief | — | — | — | — | — | — |
| Rate of tumour recurrence follow‐up: median 36 months | Study population |
RR 1.17 (0.54 to 2.50) |
1236 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | — | |
| 19 per 1000 | 14 per 1000 (9 to 40) | |||||
| Rate of appearance of a new malignancy follow‐up: median 36 months | Study population | RR 0.80 (0.32 to 2.01) | 1236 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | — | |
| 16 per 1000 | 13 per 1000 (5 to 33) | |||||
| Rate of survival: overall survival | — | — | — | — | — | The single study did not report overall survival of control and intervention groups individually, though it did report the percentage of participants alive at the end of follow‐up (median follow‐up: 35.7 months; 94.3% in the HRT group and 95.6% in the placebo group). |
| Rate of survival: progression‐free survival | — | — | — | — | — | The study did not report progression‐free survival of control and intervention groups individually, though it did report the percentage of participants alive, with no evidence of disease at the end of follow‐up (median follow‐up: 35.7 months; 95.8% in the HRT group and 96.9% in the placebo group). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level as the single RCT was closed prior to achieving its accrual goal. This was a serious departure from the study design and a serious risk of bias.
2Downgraded one level as there were insufficient data with respect to allocation concealment and description of the intervention, along with a significant risk of attrition bias.
3Downgraded one level for imprecision, as the single included study was underpowered to detect significant differences in the primary outcomes.
Background
Description of the condition
Endometrial cancer is a disease in which malignant (cancer) cells develop within the lining of the womb (uterus) (NCI 2015). Globally, endometrial cancer is the sixth most common invasive cancer in women, and the 14th most common cancer overall, with the incidence varying between continents (globocan.iarc.fr). More than 319,000 new cases were diagnosed worldwide in 2012 (Ferlay 2013). Endometrial cancer incidence rates are highest in Northern America, and lowest in South Central Asia, which partly reflects varying data quality, but also reflects differences in risk factors associated with endometrial cancer (Ferlay 2013). In the USA, modelling predictions show a likely 55% increase in endometrial cancer between 2010 and 2030 largely due to rising levels of obesity (Sheikh 2014).
Endometrial cancer is primarily a disease of postmenopausal woman, with about 25% of cases occurring in premenopausal women and 5% occurring in women younger than 40 years of age (Pecorelli 2005). Endometrial cancer is considered a potentially 'curable cancer,' as approximately 75% of cases are diagnosed before the disease has spread outside the uterus (stage I). The overall five‐year survival for all stages is about 86%, and, if the cancer is confined to the uterus, the five‐year survival rate may increase to 97% (Sonoda 2006) (see Appendix 1 for details of current FIGO (International Federation of Gynecology and Obstetrics) staging).
Despite the advances that have been made in other cancers, both the annual incidence and the death rate associated with endometrial cancer appear to be rising. The main risk factors for endometrial cancer are long‐term exposure to unopposed oestrogen (nulliparity, early menarche, late menopause or long‐term use of oestrogen only hormone replacement therapy (HRT)), diabetes, hypertension, and medicines such as the selective oestrogen receptor modulator, tamoxifen (Akhmedkhanov 2001; Siegel 2015; von Gruenigen 2005). By contrast, women who smoke and who have a history of long‐term oral contraceptive use are at decreased risk of endometrial cancer (Sonoda 2006). Data from one large randomised controlled trial (RCT) also suggested that the use of combined HRT (oestrogen with progestogen) reduced the risk of endometrial cancer (Chlebowski 2015).
Endometrial cancer can develop from a background of normal, atrophic or hyperplastic endometrium and has been divided into two types: type I and type II (Kurman 1994). Type I cancers are more common and most are well to moderately differentiated oestrogen‐dependent endometrioid adenocarcinoma. They tend to occur in younger women and are less aggressive. About 10% of endometrial cancers are type II, which are generally of a higher grade and more aggressive. Type II cancers tend to occur in older women and have a worse prognosis. They more commonly arise spontaneously, and may not be oestrogen driven (Morch 2015). Histologically, high‐grade endometrial cancers (poorly differentiated endometrioid or non‐endometrioid cancers, such as serous or clear cell carcinoma) are more likely to be associated with metastatic disease (Amant 2005; Bokhman 1983; Sonoda 2006).
The standard treatment for early‐stage endometrial cancer (stage I and stage II) is a hysterectomy, including the removal of the uterine cervix, both fallopian tubes and ovaries. Cancers with a serous histological subtype are more likely to spread transcoelomically (via the peritoneal cavity) and so an omental biopsy is recommended as part of the surgical staging procedure. The role of systematic retroperitoneal (pelvic and para‐aortic) lymph node dissection remains controversial, although there is no evidence of therapeutic benefit for cancers that are thought to be confined to the uterus at the time of surgery (Frost 2015). Laparoscopic hysterectomy (an operation performed in the abdomen or pelvis through small incisions with the aid of a camera), with or without node dissection, is now considered the standard approach for early‐stage endometrial cancer (Conrad 2015; Galaal 2012; Kyrgiou 2015).
In addition to surgery to remove the uterus, fallopian tubes and ovaries for endometrial cancer, subsequent (adjuvant) use of external‐beam radiation therapy (EBRT) or vaginal vault brachytherapy (VBT) may be used in women with intermediate‐ and high‐risk histological factors. In early‐stage endometrial cancers, EBRT or VBT reduces the risk of local recurrence, but has no demonstrable survival advantage (ASTEC/EN5 Study Group 2009; Kong 2012; Nout 2010).
Approximately 13% of women with endometrial cancer present with advanced disease. There is moderate‐quality evidence that chemotherapy after primary surgery increases survival time by approximately 25% compared with radiotherapy alone in advanced disease (Galaal 2014). More research is needed to evaluate which regimens are most effective and least toxic. The most recent RCT looking at treatment, PORTEC‐3 (Postoperative Radiation Therapy for Endometrial Carcinoma) has shown chemotherapy‐related morbidity in 25% of women receiving it, but outcome data are not yet reported (PORTEC study group 2016).
Description of the intervention
The decline in circulating oestrogen around the time of menopause may be associated with troublesome menopausal symptoms including hot flushes, night sweats, vaginal dryness and painful intercourse. The evidence is very limited, but women who experience premature menopause (under 40 years of age) or early menopause (under 45 years of age) may have more troublesome symptoms, and may also be at increased risk of osteoporosis and cardiovascular disease (CVD) (Muka 2016; Svejme 2012). HRT is an effective treatment for menopausal symptoms and may also improve quality of life in symptomatic women. In younger menopausal women, HRT may reduce the long‐term risk of osteoporotic fracture, ischaemic stroke, dementia and CVD, although, evidence for this is limited (Faubion 2015). Beyond the treatment of menopausal symptoms (vasomotor symptoms and vaginal dryness), the relative risks and benefits of HRT for women who go through natural menopause at the usual age (50 to 52 years) is controversial (Marjoribanks 2017).
Current HRT regimens contain oestrogen plus a progestogen for women who retain their uterus. The purpose of the progestogen is to protect against the risk of endometrial hyperplasia and carcinoma conferred by long‐term unopposed oestrogen treatment in women with an intact uterus (Beresford 1997; Gelfand 1989; Hammond 1979; Paterson 1980; Persson 1989; Pike 1997; Voight 1991; Whitehead 1979; Woodruff 1994). Women who have had a hysterectomy for benign reasons are not advised to take additional progestogen. HRT can be administered as tablets, patches or gel preparations and combined (oestrogen plus progestogen) HRT can be taken continuously or in a cyclical schedule (Feeley 2001). Tibolone, a synthetic steroid hormone drug with weak oestrogenic, progestinic and androgenic actions, is also effective for the treatment of menopausal symptoms (Formoso 2016).
The majority of women diagnosed with endometrial cancer have early‐stage disease, leading to an excellent prognosis after total hysterectomy (removal of the uterus and cervix) and removal of the fallopian tubes and ovaries, with or without adjuvant radiotherapy. In premenopausal and some perimenopausal women, bilateral oophorectomy will lead to menopausal symptoms and in younger women, surgical menopause potentially increases the risk of long‐term osteoporosis and CVD (Muka 2016; Svejme 2012). Because menopausal symptoms may impair quality of life after endometrial cancer and because HRT is the most effective treatment for menopausal symptoms, the safety of HRT after endometrial cancer needs to be established. Potential concerns about using HRT after endometrial cancer are based on the knowledge that oestrogen exposure increases endometrial cancer risk and most endometrial cancers are oestrogen sensitive (Akhmedkhanov 2001; Shuster 2010). The addition of progestogen to oestrogen in combined HRT provides protection for the endometrium against the potential adverse effects of oestrogen alone and reduces the risk of developing endometrial cancer (Chlebowski 2015). For this reason, clinicians may consider using combined HRT after hysterectomy for endometrial cancer. However, combined HRT confers a different risk:benefit ratio compared to oestrogen alone, including increasing the risk of breast cancer (Marjoribanks 2017). This complicates decision making about whether to use HRT after endometrial cancer and, if so, whether to use oestrogen alone or combined HRT.
How the intervention might work
Treatment for endometrial cancer may lead to menopausal symptoms. HRT has been shown to reduce menopausal symptoms including vasomotor symptoms (hot flushes and night sweats) and vaginal dryness (Sarri 2015). However, endometrial cancers may be oestrogen sensitive so use of HRT may stimulate the growth of residual cancer cells following treatment for endometrial cancer (Akhmedkhanov 2001). There is currently no consensus in the literature about how to measure efficacy with hormonal therapy or any other treatment for menopausal symptoms. However, we note that this being addressed by the COMMA Initiative (Core Outcomes in Menopause: International collaboration to measure core outcomes in menopause) and therefore this could be considered as an outcome measure in a subsequent review.
Why it is important to do this review
Most women with endometrial cancer have undergone some or all of the following therapies that may reduce oestrogen levels by removing or inactivating ovaries: hysterectomy combined with bilateral salpingo‐oophorectomy, chemotherapy and radiotherapy. This may lead to new‐onset or increased menopausal symptoms. HRT effectively relieves menopausal symptoms and may improve quality of life in symptomatic women but the safety of HRT and whether oestrogen alone or oestrogen plus progestogen should be used is not known. This is a topic that has been highlighted as important to women in a James Lind Alliance priority setting partnership (Wan 2016). If HRT is not used after endometrial cancer, there is growing evidence that several non‐hormonal options are effective (Sarri 2015). The available non‐hormonal options are outside the scope of this review, but are covered in a review of evidence (Hickey 2017).
Women with troublesome menopausal symptoms after endometrial cancer treatment who are requesting treatment need to know the risks and benefits of HRT, so they can make an informed decision. There is also a need to determine if the efficacy of HRT in women treated for endometrial cancer is similar to that in women with no history of the disease (MacLennan 2011).
Objectives
To assess the risks and benefits of HRT (oestrogen alone or oestrogen with progestogens) for women previously treated for endometrial cancer.
Note: risks in this situation were considered as not increasing the risk of recurrence of endometrial cancer above that of women not taking HRT.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs, in all languages, that examine the efficacy and safety of HRT in women previously treated for endometrial cancer. All publications, including abstracts were reviewed.
Types of participants
We included all women with treated endometrial cancer.
Types of interventions
Oestrogen alone versus placebo.
Oestrogen combined with another agent, such as progestogens versus oestrogen alone.
Oestrogen combined with another agent, such as progestogens versus placebo.
Comparisons of different formulations of hormone (dosage, route of administration, schedule, etc.).
Noretynodrel derivatives (e.g. tibolone) were not included for the purpose of this review due to their different mode of action.
Types of outcome measures
Primary outcomes
Rate of symptom relief (number of months to symptom relief; percentage of women who obtained symptom relief): hot flushes, vaginal atrophy, cardiovascular risk, osteoporosis risk, others.
Rate of tumour recurrence: number of months to tumour recurrence.
Rate of appearance of a new malignancy (breast cancer, etc.): number of months to appearance of a new malignancy.
Rate of survival: overall survival (OS) by clinical or surgical stage or both; progression‐free survival (PFS) by clinical or surgical stage.
Secondary outcomes
-
Adverse effects.
-
Short‐term:
breast tenderness
migraine headache
abdominal bloating;
nausea;
skin rashes;
increase triglycerides;
coronary artery disease;
thrombophlebitis;
stroke.
-
Long‐term:
gall stones;
coronary artery disease;
breast cancer.
-
Search methods for identification of studies
We compiled detailed search strategies in consultation with the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Information Specialists. We improved the sensitivity of the search strategies by including key words from relevant trials that were not detected by earlier searches. There were no language or publication restrictions.
Terms related to the treatment: hormone replacement therapy; oestrogen replacement therapy; HRT; ERT; oestrogen; estrogen; progesterone; progestin. Terms related to the disease: endometrial cancer; endometrial neoplasm; endometrial carcinoma; endometrial tumour or tumor; cancer of uterine corpus; EMC.
Electronic searches
We searched the following electronic bibliographic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5) (Appendix 2);
MEDLINE Ovid (1946 to April week 4 2017) (Appendix 3);
Embase Ovid (1980 to 2017, week 18) (Appendix 4).
We also searched the BIOSIS databases, Proceedings of the FIGO and the American Congress of Obstetricians and Gynecologists (ACOG) for conference proceedings.
Searching other resources
We searched for ongoing trials in the following sources:
metaRegister of Controlled Trials;
ClinicalTrials.gov;
controlled‐trials.com;
NHMRC clinical trials register;
World Health Organization International Clinical Trials Registry Platform.
Reference lists: we conducted backward and forward citation tracking for all relevant studies for further possible titles.
Grey literature search: this was limited to practice guidelines within the UK.
Unpublished literature search: we handsearched reports of conferences for 2015 to 2017:
Gynaecologic Oncology (Annual meeting of the American Society of Gynaecologic Oncologists);
Annual Meeting of the International Gynaecologic Cancer Society;
European Society of Gynaecological Oncology (ESGO) conference;
British Gynaecological Cancer Society meetings;
Menopause Society meetings.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved into the reference management database Endnote X7 and removed duplicates. Two review authors (KE and SR) independently examined abstracts and titles from the initial search to identify studies that met the inclusion criteria. We retrieved the full text of any potentially eligible studies and studies without abstracts. The third review author (MH) made the final decision on inclusion/exclusion if disagreements occurred. We excluded any studies that did not meet the inclusion criteria.
Data extraction and management
For included studies, two review authors (KE, SR) independently extracted data using the proforma: Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013 (epoc.cochrane.org/epoc‐specific‐resources‐review‐authors).
We collected the following data.
Participant characteristics (inclusion criteria, age, stage of disease at diagnosis, comorbidities, recurrence).
Number in each study arm, number lost to follow‐up.
HRT use: regimen, dose, specific hormones, adverse effects, duration of use.
Risk of bias, duration of follow‐up and outcomes and deviations from protocol.
Recurrence: time to recurrence from primary treatment and commencement of HRT.
For time to event data, we extracted the log of the hazard ratio (HR) and its standard error (SE) from the original research; if these were not reported, we estimated the log and SE (Parmar 1998).
For adverse events (dichotomous outcomes), if it was not possible to use an HR, we extracted the participants in each arm to estimate a risk ratio (RR) with 95% confidence interval (CI).
For quality of life measures (continuous outcomes), we estimated the mean difference (MD) between the intervention and control arms and its SE.
Assessment of risk of bias in included studies
Two review authors (KE, SR) independently assessed the risk of bias for each study and report it in the 'Risk of bias' table of each study according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements with a third review author (MH) if necessary. Results were summarised in Figure 1 and Figure 2.
1.
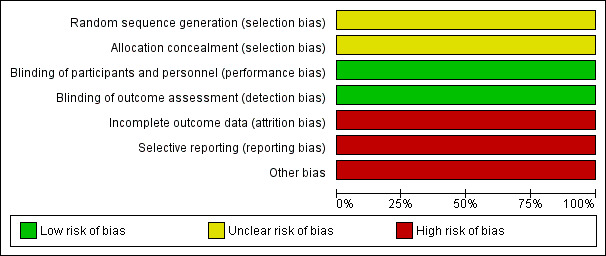
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
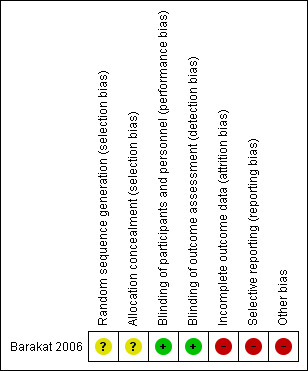
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We compared outcome measures for binary data using risk ratios (RR). For continuous data, we used MDs. If continuous data had been reported using geometric means, we combined the findings on a log scale and reported the results on the original scale. We reported medians and ranges in tables only.
Unit of analysis issues
For cluster‐randomised trials, depending on the information available, we planned to use the effective sample size or inflated SE method to conduct a meta‐analysis. We planned to employ sensitivity analyses to investigate the robustness of the conclusions.
For cross‐over trials, we planned to conduct an assessment of their suitability and particular sources of bias along with paired analysis.
For studies with more than two treatment groups, we planned to identify the relevant treatment groups and, to avoid a unit‐of‐analysis error, combine groups to create a single pair‐wise comparison.
Due to the inclusion of a single included study, there was no requirement to review unit of analysis issues but will be in future updates of this review should sufficient studies become available.
Dealing with missing data
We did not need to contact any trial authors to retrieve missing data. We did contact them for clarification regarding the method of random sequence generation for the selection bias item for the 'Risk of bias' assessment, but did not receive a response.
Assessment of heterogeneity
As there were insufficient high‐quality data in the one included study, we did not perform a meta‐analysis.
Assessment of reporting biases
We did not assess for potential bias due to only one study being included.
Data synthesis
There were not appropriate data to pool due to only one RCT being included.
Subgroup analysis and investigation of heterogeneity
We had intended to explore the following potential sources of heterogeneity using subgroup analyses:
route of hormone administration;
dosage of hormone;
regimen of hormone;
type of hormone;
age of the participant (less than or greater than 60 years);
severity of the disease (e.g. surgical stage, type, grade, invasive capacity, lymph metastasis);
HRT after surgery and HRT after radiotherapy.
Due to the inclusion of a single study, such subgroup analyses were not possible, but will be used in future updates of this review should sufficient studies become available.
Sensitivity analysis
If necessary, we planned to exclude trials with high risk of bias to examine their effect on the results. We also planned to examine the effect of assuming poor outcomes for missing values in a sensitivity analysis. However, due to the inclusion of a single included study, there was no requirement to conduct sensitivity analyses but will be carried out in a future updates if appropriate.
Summary of findings for assessing the certainty of the evidence
We used the GRADE approach to evaluate the certainty (quality) of evidence for outcomes (GRADE Working Group 2004). We downgraded or upgraded the quality level of the study depending on the presence of the following factors using GRADEpro GDT 2015.
-
Downgraded quality level for:
limitations in study design and implementation;
indirectness of evidence;
unexplained heterogeneity or inconsistency of results;
imprecision of results;
high probability of publication bias.
-
Upgraded quality level when:
there was a large magnitude of effect;
when all plausible confounding would have reduced a demonstrated effect or suggested a spurious effect when the results showed no effect;
there was a dose‐response gradient.
Results
Description of studies
Results of the search
We conducted electronic searches in May 2017, which yielded 2190 deduplicated records. Two review authors (KE, SR) excluded obviously irrelevant records after reading the titles and abstracts. Where the contents of the paper were not clear from the abstract, we obtained the full‐text papers to be rigorous in the searching. Our initial sift identified only seven potentially relevant studies for which the full texts were obtained. We excluded six studies on review of the full text. The one included RCT is discussed under the Included studies section and the excluded papers in Excluded studies (see PRISMA flow diagram; Figure 3).
3.

Study flow diagram.
Two review authors (KE, SR) independently searched the grey literature and trial registries and found no additional relevant studies.
Included studies
One trial met the inclusion criteria (Barakat 2006). See Characteristics of included studies table.
Design
Between 1997 and 2003, 1236 women were randomly assigned to receive either oestrogen replacement therapy (HRT) alone or placebo following surgery for histologically confirmed early‐stage endometrial cancer. Included participants had surgically staged FIGO IA, IB, IC (see Appendix 5 for 1988 FIGO staging system and Appendix 1 for the current 2009 staging). Randomisation was stratified across three strata (FIGO stage 1A, IB/IC and II) and median follow‐up was 35.7 months.
Inclusion criteria for randomisation included an indication for treatment with HRT due to menopausal symptoms (hot flushes, vaginal symptoms) or as prophylaxis in the presence of increased cardiovascular or osteoporotic risk. Exclusion criteria included liver dysfunction, history of thromboembolic disease or other cancer within five years, with the exception of non‐melanoma of the skin. CVD would now be considered a contraindication to HRT, but was not considered to be so when this trial started recruitment, and recruitment stopped after the publication of the Women's Health Initiative (WHI) study.
Participants
Participants were well balanced across the two arms of the study for baseline demographic and clinical characteristics. The modal age category for both groups was 51 to 60 years. There were largely equivalent numbers by tumour stage, grade and histopathological subtype. There were no significant differences in other surgico‐pathological factors between the groups including rates of lymphovascular space invasion or extent of tumour myometrial invasion.
Interventions
All participants had undergone abdominal or laparoscopically assisted vaginal hysterectomy and bilateral salpingo‐oophorectomy as a minimum, with equivalent numbers undergoing each type of surgery across both groups. Nodal sampling was at the discretion of the attending surgeon and was equivalent in each group. Women received HRT or placebo and the study was double‐blinded to participant and clinician. Slightly fewer women in the HRT group received adjuvant therapy in the form of postoperative radiotherapy (8.1% with HRT versus 11.2% with placebo). Participants who met the inclusion criteria entered into the trial within 20 weeks of their surgery.
Excluded studies
After obtaining the full text, we excluded six publications for the following reasons.
Five publications were reports of non‐randomised trials including prospective and retrospective data comparing outcomes in women who received HRT following treatment for endometrial cancer with women who did not receive HRT (Ayhan 2006; Chapman 1996; Creasman 1986; Lee 1990; Suriano 2001).
One publication was a review of trials comparing outcomes in women who received HRT following treatment for endometrial cancer with women who did not receive HRT (Shim 2014). This publication did not identify any RCT‐level evidence.
For further details on all excluded studies, see the Characteristics of excluded studies table.
Risk of bias in included studies
Allocation
Adequate information was not given with respect to the method of random sequence generation or allocation concealment in the one included study. This study had an overall unclear risk of allocation bias. We emailed the author team for clarification, but did not receive a response.
Blinding
The included study was at low risk of performance and detection bias. Blinding of both participants and physicians was reported, although further details about the methods used to ensure adequate blinding were not reported. On completion of the study, a high proportion of treating physicians correctly guessed the allocation of participants to HRT or placebo. However, the review authors concluded that the outcome assessment was unlikely to have been influenced by deficiencies in the blinding process.
Incomplete outcome data
The study was at high risk of attrition bias. Outcome data for the main outcome measures were complete. Data were analysed according to intention‐to‐treat principles and cross‐over between groups was clearly identified with all participants included in the final analysis. However, there was significant departure of the intervention received from that assigned at randomisation: 41.1% (251) women in the HRT arm were compliant for the entire treatment period compared with 50.1% (305) in the placebo arm. However, studies looking at HRT use such as the Women's Health Initiative (WHI) study also show lack of compliance in taking HRT (74.4% in WHI study).
Selective reporting
There was insufficient information regarding the prespecified study objectives to permit judgement of the risk of reporting bias.
Other potential sources of bias
It should be noted that this trial was closed before reaching its accrual goal of 2018 participants due to publication of the Women's Health Initiative (WHI) study. The results of the WHI trial, suggesting that risks of exogenous hormone therapy outweighed benefits, resulted in a continually decreasing recruitment rate, such that the investigators felt that the goal of 2018 participants would not be reached in a reasonable time.
Effects of interventions
See: Table 1
See Summary of main results for the main comparison and Table 1.
Primary outcomes
Rate of symptom relief
The study did not report on the rate of symptom relief in HRT versus placebo (Barakat 2006).
Rate of tumour recurrence: number of months to tumour recurrence
The study reported tumour recurrence in 2.3% of participants in the HRT arm versus 1.9% of participants receiving placebo (RR 1.17, 95% CI 0.54 to 2.50; 1236 participants, 1 study; very low‐certainty evidence) (Barakat 2006). The time to recurrence was not reported.
Rate of appearance of a new malignancy: number of months to appearance of a new malignancy
The study reported one participant in the HRT arm (0.16%) and three participants in the placebo arm (0.49%) developed breast cancer during follow‐up (RR 0.80, 95% CI 0.32 to 2.01; 1236 participants, 1 study; very low‐certainty evidence) (Barakat 2006). The incidence of new breast cancer in either arm was too small to enable meaningful conclusions to be drawn. The time from entry to the study to appearance of breast cancer was not reported.
Rate of survival: overall survival by clinical or surgical stage, or both; progression‐free survival by clinical/surgical stage
The study did not report on OS or PFS by clinical/surgical stage for HRT versus placebo (Barakat 2006). The percentage of participants alive with no evidence of disease at the end of the 36‐month follow‐up period was 94.3% in the HRT group and 95.6% in the placebo group. The percentage of participants alive irrespective of disease progression at the end of the 36 months' follow‐up was 95.8% in the HRT group and 96.9% in the placebo group.
Secondary outcomes
Adverse effects
The study reported that in the HRT arm three participants (0.5%) died of coronary heart disease and two participants (0.2%) died of pulmonary embolism (Barakat 2006). This compared with four deaths from coronary heart disease (0.6%) and no deaths from pulmonary embolism in the placebo group. The incidences of these outcomes were too small to enable meaningful conclusions to be drawn.
Discussion
Summary of main results
The only RCT of HRT after endometrial cancer was a study that closed before recruiting a sufficient sample size. This trial also had methodological limitations. The limited evidence from this study showed no difference in recurrence risk between HRT users versus placebo (RR 1.17, 95% CI 0.54 to 2.50). This is consistent with available non‐randomised evidence (see Agreements and disagreements with other studies or reviews). The current evidence suggested that HRT may or may not increase the risk of recurrence of developing a new cancer after treatment for early‐stage endometrial cancer. The efficacy of HRT for menopausal symptoms and the impact on quality of life or long‐term health or survival is unknown. There is no available information in the literature regarding HRT and advanced endometrial cancer that is not early stage (i.e. stage II or above).
Overall completeness and applicability of evidence
The published evidence was of very‐low certainty, as assessed by GRADE and so we remain uncertain about the safety of HRT after treatment for early‐stage endometrial cancer. The single RCT evaluated in this review used oestrogen alone of an unspecified formulation or dose. We found no randomised studies comparing oestrogen alone with combination agents (e.g. oestrogen plus progestogen), or comparing combination agents with placebo. We are not aware of any ongoing studies. No additional RCTs were identified by another review of HRT in endometrial cancer identified through our search strategy (Shim 2014).
Quality of the evidence
This single RCT was closed prior to achieving its accrual goal and was thus underpowered to detect significant differences in the primary outcome measures (disease recurrence, death), resulting in wide CIs. In addition, information was missing about allocation concealment and description of the intervention, and there was a significant risk of attrition bias. According to the GRADE guidance for the assessment of quality of evidence this constitutes a very serious risk of bias and makes the study results unreliable. The overall assessment of the quality of evidence using the GRADE assessment method was very low.
Potential biases in the review process
We undertook a comprehensive search of available evidence to identify eligible studies. Two review authors independently sifted the initial search results and identified potentially relevant studies. Those review authors then independently extracted data from the included study. It is possible that there are incomplete and unreported studies that have not been included.
Agreements and disagreements with other studies or reviews
Given the limited RCT‐level evidence, we feel that it is important to discuss available non‐randomised evidence for the use of HRT in women previously treated for endometrial cancer, which are consistent with the conclusions of the included RCT. The search identified five non‐randomised studies (Ayhan 2006; Chapman 1996; Creasman 1986; Lee 1990; Suriano 2001), and one systematic review (Shim 2014), all of which attempted to evaluate the risk of recurrence or death in women using HRT following treatment for endometrial cancer.
Ayhan 2006 prospectively compared 50 participants who were given combination oral HRT with 52 control participants who did not receive HRT matched for tumour stage, age and multiple prognostic factors. In this study, all participants in the HRT group initiated treatment within eight weeks of surgery. The study concluded that there was no increased risk of recurrence or death in women using HRT following treatment of early‐stage endometrial cancer.
Chapman 1996 reviewed the outcomes of 123 women with stage I and stage II endometrial cancer, 62 of whom received oestrogen replacement, either by oral or transdermal routes, alone or in combination with progesterone. There was no difference in disease‐free survival or recurrence in this study, although the oestrogen‐treated group had earlier stage disease and less depth of invasion suggesting that this study was at critical risk of bias.
Creasman 1986 compared the outcomes of 47 participants who received oral or vaginal oestrogen therapy following surgery with 174 participants who did not receive oestrogen. All women had stage I disease only. After controlling for known prognostic indicators, they reported longer disease‐free survival in the oestrogen group. When tumour oestrogen receptor status was included in the multivariate analysis, this protective effect was nullified. This was a non‐randomised study.
Lee 1990 reported on 44 women with low‐risk (early‐stage, low‐grade) endometrial cancer who were treated with oral HRT and compared them to to 99 women who did not receive HRT. They found that the rates of recurrence and death were higher in the non‐HRT group, although this group also included participants with high‐grade cancers, which the treatment group did not, suggesting that this study was at critical risk of bias.
These three studies were limited by their retrospective methodologies, a lack of control group and the long latency observed between primary surgical treatment and initiation of HRT. Our literature search identified two publications that attempted to address these problems (Shim 2014; Suriano 2001).
Suriano 2001 reported on 75 paired women, retrospectively matched for tumour stage and age. Half of the women in the HRT group received combination oral oestrogen and progesterone with the remainder receiving oral oestrogen alone. All women in the treatment group commenced therapy within six months of their primary surgery.
One systematic review and meta‐analysis (Shim 2014) included data from these five non‐randomised studies (Ayhan 2006; Chapman 1996; Creasman 1986; Lee 1990; Suriano 2001), in addition to that from the RCT included in this review (Barakat 2006). The meta‐analysis indicated no adverse effect on the risk of recurrence of endometrial cancer in participants using HRT. Subgroup analysis suggested outcomes that favoured HRT use, though the authors recognised the limitations and potential confounding factors in the data and concluded that the available literature did not suggest that use of HRT after endometrial cancer increased the risk of recurrence.
Authors' conclusions
Implications for practice.
We are uncertain whether hormone replacement therapy (HRT) after the treatment of early‐stage endometrial cancer increases recurrence, as the quality of the evidence is low. Limited evidence from one RCT that failed to meet its recruitment targets and several non‐randomised studies suggest that HRT does not increase the risk of recurrence of early endometrial cancer. It remains unknown whether HRT after endometrial cancer should contain a progestogen, but, in the general population of postmenopausal women, risks of combined HRT exceed those of oestrogen alone. Decision making about HRT after endometrial cancer should be individualised and based on evidence from the general population (Sarri 2015).
Implications for research.
Based on low‐quality evidence, HRT does not appear to increase the risk of recurrence of early‐stage endometrial cancer. This is an area importance to women who have been treated for endometrial cancer through the Womb Cancer Alliance priority setting partnership (Wan 2016). Survival rates from early endometrial cancer are high and quality of life issues are important. Future RCTs should aim to establish the safety of HRT after endometrial cancer (risk of recurrence, disease‐specific survival and overall survival), the efficacy of treatment and whether additional progestogen is indicated. Any trial should have an adequate follow‐up period to evaluate longer‐term benefits and risks of HRT. The available evidence only included studies with women with early‐stage endometrial cancer and there are no data available regarding women with advanced endometrial cancer.
Acknowledgements
The authors thank Jo Morrison (Co‐ordinating Editor), Clare Jess (Managing Editor) and Jo Platt (Information Specialist) from Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers. We acknowledge Jing Fu, Xue Peng and Lina Hu who wrote the original draft of the protocol.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
We would like to thank the referees for many helpful suggestions and comments, some of these include Fani Kokka, Khadra Galaal, Ben Carter and Katharine Tylko‐Hill.
Appendices
Appendix 1. 2009 FIGO staging of endometrial cancer
-
Stage I: tumour confined to the corpus uteri
IA: no or less than half myometrial invasion
IB: invasion equal to or more than half of the myometrium
Stage II: tumour invades cervical stroma, but does not extend beyond the uterus
-
Stage III: local or regional (or both) spread of the tumour
IIIA: tumour invades the serosa of the corpus uteri or adnexae (or both)
IIIB: vaginal or parametrial (or both) involvement
IIIC: metastases to pelvic or para‐aortic (or both) lymph nodes
IIIC1: positive pelvic nodes
IIIC2: positive para‐aortic lymph nodes with or without positive pelvic lymph nodes
-
Stage IV: tumour invades bladder mucosa, bowel mucosa, distant metastases, or a combination
IVA: tumour invasion of bladder or bowel (or both) mucosa
IVB: distant metastases, including intra‐abdominal metastases or inguinal nodes (or both)
Endocervical glandular involvement only should be considered as Stage I and no longer as Stage II.
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Endometrial Neoplasms explode all trees #2 endometr* near/5 (tumor* or tumour* or neoplas* or malignan* or carcinom* or cancer* or adenocarcinoma*) #3 #1 OR #2 #4 MeSH descriptor Hormone Replacement Therapy explode all trees #5 hormone replacement therap* #6 MeSH descriptor Estrogens explode all trees #7 estrogen* or oestrogen* #8 MeSH descriptor Progestins explode all trees #9 progestin* #10 #4 OR #5 OR #6 OR #7 OR #8 OR #9 #11 #3 AND #10
Appendix 3. MEDLINE Ovid search strategy
1 exp Endometrial Neoplasms/ 2 (endometr* adj5 (tumor* or tumour* or neoplas* or malignan* or carcinom* or cancer* or adenocarcinoma*)).mp. 3 1 or 2 4 exp Hormone Replacement Therapy/ 5 hormone replacement therap*.mp. 6 exp Estrogens/ 7 (estrogen* or oestrogen*).mp. 8 exp Progestins/ 9 progestin*.mp. 10 or/4‐9 11 randomized controlled trial.pt. 12 controlled clinical trial.pt. 13 randomized.ab. 14 placebo.ab. 15 clinical trials as topic.sh. 16 randomly.ab. 17 trial.ti. 18 or/11‐17 19 3 and 10 and 18 20 exp animals/not humans.sh. 21 19 not 20
key: mp = title, original title, abstract, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier sh = subject heading ti = title ab = abstract pt = publication type
Appendix 4. Embase Ovid search strategy
1 exp endometrium tumor/ 2 (endometr* adj5 (tumor* or tumour* or neoplas* or malignan* or carcinom* or cancer* or adenocarcinoma*)).mp. 3 1 or 2 4 exp hormone substitution/ 5 hormone replacement therap*.mp. 6 exp ESTROGEN/ 7 (estrogen* or oestrogen*).mp. 8 exp gestagen/ 9 progestin*.mp. 10 4 or 5 or 6 or 7 or 8 or 9 11 crossover procedure/ 12 double‐blind procedure/ 13 randomized controlled trial/ 14 single‐blind procedure/ 15 random*.mp. 16 factorial*.mp. 17 (crossover* or cross over* or cross‐over*).mp. 18 placebo*.mp. 19 (double* adj blind*).mp. 20 (singl* adj blind*).mp. 21 assign*.mp. 22 allocat*.mp. 23 volunteer*.mp. 24 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25 3 and 10 and 24
key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 5. 1988 FIGO staging of endometrial cancer
-
Stage I: tumour confined to the corpus uteri
IA: tumour limited to the endometrium
IB: tumour invasion less than half the myometrium
IC: tumour invasion more than half the myometrium
-
Stage II: cervical involvement
IIA: endocervical glandular involvement only
IIB: cervical stromal invasion
-
Stage III
IIIA: tumour invades serosa or adnexa or positive peritoneal cytology
IIIB: vaginal metastases
IIIC: metastases to the pelvic or para‐aortic lymph nodes
-
Stage IV
IVA: tumour involves bladder or bowel mucosa
IVB: distant metastases
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barakat 2006.
| Methods | Randomised controlled trial. Randomisation was stratified across 3 strata (FIGO stage 1A, IB/IC and II endometrial cancer). Median follow‐up: 35.7 months. |
|
| Participants | 1236 participants with surgically staged FIGO IA, IB, IC or occult stage II endometrial cancer. Inclusion criteria: indication for treatment with HRT due to symptomatic hypo‐oestrogenic state or as prophylaxis in the presence of increased cardiovascular or osteoporotic risk Exclusion criteria: liver dysfunction, history of thromboembolic disease or other cancer within 5 years, with the exception of non‐melanoma of the skin |
|
| Interventions | Intervention: oestrogen replacement therapy (unspecified formulation) Control: placebo |
|
| Outcomes | Tumour recurrence (2.3% of participants in the HRT arm versus 1.9% of participants in the placebo arm Time to recurrence: not reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given regarding the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information given regarding the measures undertaken to ensure allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was reported, though with minimal detail. However, outcomes were unlikely to have been significantly affected by deficiencies in the blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was reported, though with minimal detail. However, outcomes were unlikely to have been significantly affected by deficiencies in the blinding of participants and personnel. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was significant departure from the assigned treatment with very poor compliance (41.1%) among participants in the treatment arm. |
| Selective reporting (reporting bias) | High risk | The trial could not report all relevant oncological outcomes due to premature closure. |
| Other bias | High risk | The trial was closed prior to achievement of its accrual goal. |
FIGO: International Federation of Gynecology and Obstetrics; HRT: hormone replacement therapy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ayhan 2006 | Non‐randomised study |
| Chapman 1996 | Non‐randomised study |
| Creasman 1986 | Non‐randomised study |
| Lee 1990 | Non‐randomised study |
| Shim 2014 | Review |
| Suriano 2001 | Non‐randomised study |
Contributions of authors
KE was responsible for rewriting the protocol, developing the search strategy, extracting and analysing data, and developing and editing the review.
SR was responsible for data extraction and analysis and writing and editing the results and findings.
MH as an expert in menopause reviewed and edited the review as appropriate and assisted with data analysis.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
KE: none known.
SR: none known.
MH: none known.
New
References
References to studies included in this review
Barakat 2006 {published data only}
- Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS, Gynecologic Oncology Group Study. Randomized double‐blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2006;24(4):587‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ayhan 2006 {published data only}
- Ayhan A, Taskiran C, Simsek S, Sever A. Does immediate hormone replacement therapy affect the oncologic outcome in endometrial cancer survivors?. International Journal Gynecological Cancer 2006;16(2):805‐8. [DOI] [PubMed] [Google Scholar]
Chapman 1996 {published data only}
- Chapman JA, DiSaia PJ, Osann K, Roth PD, Gillotte DL, Berman ML. Estrogen replacement in surgical stage I and II endometrial cancer survivors. American Journal of Obstetrics and Gynecology 1996;175(5):1195‐200. [DOI] [PubMed] [Google Scholar]
Creasman 1986 {published data only}
- Creasman WT, Henderson D, Hinshaw W, Clarke‐Pearson DL. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstetrics and Gynecology 1986;67(3):326‐30. [PubMed] [Google Scholar]
Lee 1990 {published data only}
- Lee RB, Burke TW, Park RC. Estrogen replacement therapy following treatment for stage I endometrial carcinoma. Gynecologic Oncology 1990;36(2):189‐91. [DOI] [PubMed] [Google Scholar]
Shim 2014 {published data only}
- Shim SH, Lee SJ, Kin SN. Effect of hormone replacement therapy on the rate of recurrence in endometrial cancer survivors: a meta‐analysis. European Journal of Cancer 2014;50(9):1628‐37. [DOI] [PubMed] [Google Scholar]
Suriano 2001 {published data only}
- Suriano KA, McHale M, McLaren CE, Li KT, Re A, DiSaia PJ. Estrogen replacement therapy in endometrial cancer patients. Obstetrics and Gynecology 2001;97(4):555‐60. [DOI] [PubMed] [Google Scholar]
Additional references
Akhmedkhanov 2001
- Akhmedkhanov A, Zeleniuch‐Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer. Review of the evidence and research perspectives. Annals of the New York Academy of Sciences 2001;943:296‐315. [DOI] [PubMed] [Google Scholar]
Amant 2005
- Amant F, Moerman P, Neven P, Timmerman D, Limbergen E, Vergote I. Endometrial cancer. Lancet 2005;366(9484):491‐505. [DOI] [PubMed] [Google Scholar]
ASTEC/EN5 Study Group 2009
- ASTEC/EN5 Study Group, Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta‐analysis. Lancet 2009;373(9658):137‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beresford 1997
- Beresford SAA, Weiss NS, Voight LF, McKnight B. Risk of endometrial cancer in relation to oestrogen combined with cyclic progestagen therapy in postmenopausal women. Lancet 1997;349(9050):458‐61. [DOI] [PubMed] [Google Scholar]
Bokhman 1983
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology 1983;15(1):10‐7. [DOI] [PubMed] [Google Scholar]
Chlebowski 2015
- Chlebowski RT, Anderson GL, Sarto GE, Haque R, Runowicz CD, Aragaki AK, et al. Continuous combined estrogen plus progestin and endometrial cancer: the Women's Health Initiative Randomized Trial. Journal of the National Cancer Institute 2015;108(3):pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conrad 2015
- Conrad LB, Ramirez PT, Burke W, Naumann RW, Ring KL, Munsell MF, et al. Role of minimally invasive surgery in gynaecologic oncology: an updated survey of members of the society of gynecologic oncology. International Journal of Gynecoloical Cancer 2015;25(6):1121‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faubion 2015
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long‐term health consequences of premature or early menopause and considerations for management. Climacteric 2015;18(4):483‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feeley 2001
- Feeley KM, Wells M. Hormone replacement therapy and the endometrium. Journal of Clinical Pathology 2001;54(6):435‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2013
- Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European Journal of Cancer 2013;49(6):1374‐403. [DOI] [PubMed] [Google Scholar]
Formoso 2016
- Formoso G, Perrone E, Maltoni S, Balduzzi S, Wilkinson J, Basevi V, et al. Short‐term and long‐term effects of tibolone in postmenopausal women. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD008536.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frost 2015
- Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD007585.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galaal 2012
- Galaal K, Bryant A, Fisher AD, Al‐Khaduri M, Kew F, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006655.pub2] [DOI] [PubMed] [Google Scholar]
Galaal 2014
- Galaal K, Al Moundhri M, Bryant A, Lopes AD, Lawrie TA. Adjuvant chemotherapy for advanced endometrial cancer. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD010681.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelfand 1989
- Gelfand MM, Ferenczy A. A prospective 1‐year study of estrogen and progestin in postmenopausal women: effects on the endometrium. Obstetrics and Gynecology 1989;74(3 Pt 1):398‐402. [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;7454:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed November 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2105.
Hammond 1979
- Hammond CB, Jelovsek FR, Lee KL, Creasman WT, Parker RT. Effects of long term estrogen replacement therapy. II. Neoplasia. American Journal of Obstetrics and Gynecology 1979;133(5):537‐47. [DOI] [PubMed] [Google Scholar]
Hickey 2017
- Hickey M, Szabo RA, Hunter MS. Non‐hormonal treatments for menopausal symptoms. BMJ 2017;359:j5101. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kong 2012
- Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD003916.pub3] [DOI] [PubMed] [Google Scholar]
Kurman 1994
- Kurman RJ, Zaino RJ, Norris HJ. Endometrial carcinoma. In: Kurman RJ editor(s). Blaustein’s Pathology of the Female Genital Tract. 4th Edition. New York (NY): Springer, 1994:439‐86. [Google Scholar]
Kyrgiou 2015
- Kyrgiou M, Swart AM, Qian W, Warwick J. A comparison of outcomes following laparoscopic and open hysterectomy with or without lymphadenectomy for presumed early‐stage endometrial cancer: Results from the Medical Research Council ASTEC Trial. International Journal of Gynaecological Cancer 2015;25(8):1424‐36. [DOI] [PubMed] [Google Scholar]
MacLennan 2011
- MacLennan AH. HRT in difficult circumstances: are there any absolute contraindications?. Climacteric 2011;14(4):409‐17. [DOI] [PubMed] [Google Scholar]
Marjoribanks 2017
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long‐term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD004143.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morch 2015
- Mørch LS, Kjaer SK, Keiding N, Løkkegaard E, Lidegaard Ø. The influence of hormone therapies on type I and II endometrial cancer: a nationwide cohort study. International Journal of Cancer 2015;138(6):1506‐15. [DOI] [PubMed] [Google Scholar]
Muka 2016
- Muka T, Oliver‐Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all‐cause mortality: a systematic review and meta‐analysis. JAMA Cardiology 2016;1(7):767‐76. [DOI] [PubMed] [Google Scholar]
NCI 2015
- National Cancer Institute. Endometrial Cancer. Available from www.cancer.gov/types/uterine/hp (accessed 29 June 2016).
Nout 2010
- Nout RA, Smit VT, Putter H, Jürgenliemk‐Schulz IM, Jobsen JJ, Lutgens LC, et al. PORTEC Study Group. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high‐intermediate risk (PORTEC‐2): an open‐label, non‐inferiority, randomised trial. Lancet 2010;375(9717):816‐23. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistical Methodology 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Paterson 1980
- Paterson ME, Wade‐Evans T, Sturdee DW, Thom MH, Studd JW. Endometrial disease after treatment with oestrogens and progestogens in the climacteric. BMJ 1980;280(6217):822‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pecorelli 2005
- Pecorelli S, Pasinetti B, Angioli R, Favalli G, Odicino F. Systemic therapy for gynecological neoplasms: ovary, cervix and endometrium. Cancer Chemotherapy and Biological Response Modifiers 2005;22:515‐44. [PubMed] [Google Scholar]
Persson 1989
- Persson I, Adami HO, Bergkvist L, Lindgren A, Pettersson B, Hoover R, et al. Risk of endometrial cancer after treatment with oestrogens alone or in conjunction with progestogens: results of a prospective study. BMJ 1989;298(6667):147‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pike 1997
- Pike MC, Peters RK, Cozen W, Probst‐Hensch NM, Felix JC, Wan PC, et al. Estrogen‐progestin replacement therapy and endometrial cancer. Journal of the National Cancer Institute 1997;89(15):1110‐6. [DOI] [PubMed] [Google Scholar]
PORTEC study group 2016
- Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie‐Meder C, et al. PORTEC study group. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high‐risk endometrial cancer (PORTEC‐3): an open‐label, multicentre, randomised, phase 3 trial. Lancet Oncology 2016;17(8):1114‐26. [DOI] [PubMed] [Google Scholar]
Sarri 2015
- Sarri G, Davies M, Lumsden MA, Guideline Development Group. Diagnosis and management of menopause: summary of NICE guidance. BMJ 2015;12(351):h5746. [DOI] [PubMed] [Google Scholar]
Sheikh 2014
- Sheikh MA, Althouse AD, Freese KE, Soisson S, Edwards RP, Welburn S. USA endometrial cancer projections to 2030: should we be concerned?. Future Oncology 2014;10(16):2561‐8. [DOI] [PubMed] [Google Scholar]
Shuster 2010
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long‐term health consequences. Maturitas 2010;65(2):161‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2015
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA: a Cancer Journal for Clinicians 2015;65(1):5‐29. [DOI] [PubMed] [Google Scholar]
Sonoda 2006
- Sonoda Y, Barakat R. Screening and the prevention of gynecologic cancer: endometrial cancer. Best Practice and Research. Clinical Obstetrics and Gynaecology 2006;20(2):363‐77. [DOI] [PubMed] [Google Scholar]
Svejme 2012
- Svejme O, Ahlborg HG, Nilsson JÅ, Karlsson MK. Early menopause and risk of osteoporosis, fracture and mortality: a 34‐year prospective observational study in 390 women. British Journal of Obstetrics and Gynaecology 2012;119(7):810‐6. [DOI] [PubMed] [Google Scholar]
Voight 1991
- Voigt LF, Weiss NS, Chu J, Daling JR, McKnight B, Belle G. Progestagen supplementation of exogenous oestrogens and risk of endometrial cancer. Lancet 1991;338(8762):274‐7. [DOI] [PubMed] [Google Scholar]
von Gruenigen 2005
- Gruenigen VE, Gil KM, Frasure HE, Jenison EJ, Hopkins MP. The impact of obesity and age on quality of life in gynecologic surgery. American Journal of Obstetrics and Gynecology 2005;193(4):1369‐75. [DOI] [PubMed] [Google Scholar]
Wan 2016
- Wan YL, Beverley‐Stevenson R, Carlisle D, Clarke S, Edmonson RJ, Glover S, et al. Working together to shape the endometrial cancer research agenda: the top ten unanswered research questions. Gynecologic Oncology 2016;143(2):287‐93. [DOI] [PubMed] [Google Scholar]
Whitehead 1979
- Whitehead MI, King RJ, McQueen J, Campbell S. Endometrial histology and biochemistry in climacteric women during oestrogen and progestogen therapy. Journal of the Royal Society of Medicine 1979;72(5):322‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodruff 1994
- Woodruff JD, Pickar JH, the Menopause Study Group. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. The Menopause Study Group. American Journal of Obstetrics and Gynecology 1994;170(5 Pt 1):1213‐23. [DOI] [PubMed] [Google Scholar]


