Abstract
Background
Major depression and other depressive conditions are common in people with cancer. These conditions are not easily detectable in clinical practice, due to the overlap between medical and psychiatric symptoms, as described by diagnostic manuals such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) and International Classification of Diseases (ICD). Moreover, it is particularly challenging to distinguish between pathological and normal reactions to such a severe illness. Depressive symptoms, even in subthreshold manifestations, have been shown to have a negative impact in terms of quality of life, compliance with anti‐cancer treatment, suicide risk and likely even the mortality rate for the cancer itself. Randomised controlled trials (RCTs) on the efficacy, tolerability and acceptability of antidepressants in this population are few and often report conflicting results.
Objectives
To assess the efficacy, tolerability and acceptability of antidepressants for treating depressive symptoms in adults (aged 18 years or older) with cancer (any site and stage).
Search methods
We searched the following electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 6), MEDLINE Ovid (1946 to June week 4 2017), Embase Ovid (1980 to 2017 week 27) and PsycINFO Ovid (1987 to July week 4 2017). We additionally handsearched the trial databases of the most relevant national, international and pharmaceutical company trial registers and drug‐approving agencies for published, unpublished and ongoing controlled trials.
Selection criteria
We included RCTs comparing antidepressants versus placebo, or antidepressants versus other antidepressants, in adults (aged 18 years or above) with any primary diagnosis of cancer and depression (including major depressive disorder, adjustment disorder, dysthymic disorder or depressive symptoms in the absence of a formal diagnosis).
Data collection and analysis
Two review authors independently checked eligibility and extracted data using a form specifically designed for the aims of this review. The two authors compared the data extracted and then entered data into Review Manager 5 using a double‐entry procedure. Information extracted included study and participant characteristics, intervention details, outcome measures for each time point of interest, cost analysis and sponsorship by a drug company. We used the standard methodological procedures expected by Cochrane.
Main results
We retrieved a total of 10 studies (885 participants), seven of which contributed to the meta‐analysis for the primary outcome. Four of these compared antidepressants and placebo, two compared two antidepressants, and one three‐armed study compared two antidepressants and placebo. In this update we included one additional unpublished study. These new data contributed to the secondary analysis, while the results of the primary analysis remained unchanged.
For acute‐phase treatment response (6 to 12 weeks), we found no difference between antidepressants as a class and placebo on symptoms of depression measured both as a continuous outcome (standardised mean difference (SMD) −0.45, 95% confidence interval (CI) −1.01 to 0.11, five RCTs, 266 participants; very low certainty evidence) and as a proportion of people who had depression at the end of the study (risk ratio (RR) 0.82, 95% CI 0.62 to 1.08, five RCTs, 417 participants; very low certainty evidence). No trials reported data on follow‐up response (more than 12 weeks). In head‐to‐head comparisons we only retrieved data for selective serotonin reuptake inhibitors (SSRIs) versus tricyclic antidepressants, showing no difference between these two classes (SMD −0.08, 95% CI −0.34 to 0.18, three RCTs, 237 participants; very low certainty evidence). No clear evidence of a beneficial effect of antidepressants versus either placebo or other antidepressants emerged from our analyses of the secondary efficacy outcomes (dichotomous outcome, response at 6 to 12 weeks, very low certainty evidence). In terms of dropouts due to any cause, we found no difference between antidepressants as a class compared with placebo (RR 0.85, 95% CI 0.52 to 1.38, seven RCTs, 479 participants; very low certainty evidence), and between SSRIs and tricyclic antidepressants (RR 0.83, 95% CI 0.53 to 1.30, three RCTs, 237 participants). We downgraded the certainty (quality) of the evidence because the included studies were at an unclear or high risk of bias due to poor reporting, imprecision arising from small sample sizes and wide confidence intervals, and inconsistency due to statistical or clinical heterogeneity.
Authors' conclusions
Despite the impact of depression on people with cancer, the available studies were very few and of low quality. This review found very low certainty evidence for the effects of these drugs compared with placebo. On the basis of these results, clear implications for practice cannot be deduced. The use of antidepressants in people with cancer should be considered on an individual basis and, considering the lack of head‐to‐head data, the choice of which agent to prescribe may be based on the data on antidepressant efficacy in the general population of individuals with major depression, also taking into account that data on medically ill patients suggest a positive safety profile for the SSRIs. To better inform clinical practice, there is an urgent need for large, simple, randomised, pragmatic trials comparing commonly used antidepressants versus placebo in people with cancer who have depressive symptoms, with or without a formal diagnosis of a depressive disorder.
Plain language summary
Antidepressants for the treatment of depression in people with cancer
The issue Depressive states are frequent among people suffering from cancer. Often depressive symptoms are a normal reaction or a direct effect of such a severe and life‐threatening illness. It is therefore not easy to establish when depressive symptoms become a proper disorder and need to be treated with drugs. Current scientific literature reveals that depressive symptoms, even when mild, can have a relevant impact on the course of cancer, reducing people's overall quality of life and affecting their compliance with anti‐cancer treatment, as well as possibly increasing the likelihood of death.
The aim of the review It is important to assess the possible beneficial role of antidepressants in adults (aged 18 years or above) with cancer. The aim of this review is to assess the efficacy and acceptability of antidepressants for treating depressive symptoms in patients with cancer at any site and stage.
What are the main findings? We systematically reviewed ten studies assessing the efficacy of antidepressants, for a total of 885 participants. The evidence is current to 3 July 2017. Due to the small number of people in the studies, and issues with how the studies reported what was done, there is uncertainty over whether antidepressants were better than placebo in terms of depressive symptoms after 6 to 12 weeks of treatment. We did not have enough evidence to determine how well antidepressants were tolerated in comparison with placebo. Our results did not show whether any particular antidepressant was better than any other in terms of both beneficial and harmful effects. To better inform clinical practice, we need large studies which randomly assign people to different treatments. Currently, we cannot draw reliable conclusions about the effects of antidepressants on depression in people with cancer.
Certainty of the evidence The certainty of the evidence was very low because of a lack of information about how the studies were designed, low numbers of people in the analysis of results, and differences between the characteristics of the studies and their results.
What are the conclusions? Despite the impact of depression on people with cancer, the available studies were very few and of low quality. This review found very low certainty evidence for the effects of these drugs compared with placebo.
Summary of findings
Background
Description of the condition
The prevalence of major depression among people with cancer has been estimated to be around 15% in oncological and haematological settings, with similar rates in palliative care settings. Adding other depressive diagnoses, including dysthymia and minor depression, prevalence rates rise up to 20% in oncological and haematological settings, and up to 25% in palliative care settings (Mitchell 2011). However, a precise estimation of the prevalence of depression in cancer patients is difficult due to the influence of many variables, including site and stage of cancer, type of anti‐cancer treatment, and diagnostic tools employed (Caruso 2017).
Formulating a diagnosis of depression in patients affected by serious medical conditions is particularly challenging, as several symptoms of the medical condition may overlap with those described in the Diagnostic and Statistical Manual of Mental Disorders (DSM) (APA 1994) and the International Classification of Diseases (ICD) (WHO 1992) for depression, such as fatigue, weight loss and sleep disturbances (Thompson 2017). Furthermore, besides physical symptoms, cancer progression is associated with functional, social and relational impairment. Even recurrent thoughts of death might be a normal reaction to a limited life expectancy or to severe pain syndromes (Breitbart 2000). It has recently been reported that atypical depressive symptoms, such as anxiety, despair, fatigue, post‐traumatic stress symptoms, body image distortions, inner restlessness and social withdrawal might be more frequent in this population, and need to be taken into account when depressive symptoms are assessed (Brenne 2013; Diaz‐Frutos 2016; Ebede 2017; Yi 2017).
Cancer may increase patients' susceptibility to depression in several ways. First, a reaction to a severe diagnosis and the forthcoming deterioration of health status may constitute a risk factor for depression; second, treatment with immune response modifiers and chemotherapy regimens, and experiencing of metabolic and endocrine alterations, chronic pain and extensive surgical interventions, may represent additional contributing factors (Irwin 2013; Onitilo 2006; Sotelo 2014).
In people with cancer, depression and other psychiatric comorbidities are responsible for worsened quality of life (Arrieta 2013), lower compliance with anti‐cancer treatment (Colleoni 2000), prolonged hospitalisation (Prieto 2002), higher suicide risk (Shim 2012), and greater psychological burden on the family (Kim 2010). Furthermore, depression is likely to be an independent risk factor for cancer mortality (Lloyd‐Williams 2009; Pinquart 2010), with estimates as high as a 26% greater mortality rate among patients with depressive symptoms and a 39% higher mortality rate among those with a diagnosis of major depression (Satin 2009). The effects of depression on mortality may differ by cancer site, being higher in people with lung, gastrointestinal (in particular, pancreatic), and brain cancer, and lower in those with genitourinary and skin cancer (Onitilo 2006; Hartung 2017). However, data are sparse and conflicting on this compelling issue (Pinquart 2010). As a consequence, individuals with cancer and major depression or depressive symptoms may have radically different features compared with individuals without cancer in terms of underlying risk factors, natural history, outcome and antidepressant treatment response (Brenne 2013; Irwin 2013).
Description of the intervention
Antidepressants are the most common psychotropic drugs prescribed in people with depression. Amongst antidepressants, many different agents are available, including tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin‐noradrenaline reuptake inhibitors (SNRIs) and other newer agents, such as agomelatine, mirtazapine, reboxetine and bupropion. It has been repeatedly shown that SSRIs are not more effective than TCAs (Anderson 2000; Mottram 2009), but are better tolerated and safer in overdose than TCAs (Anderson 2000; Barbui 2001; Henry 1995).
In a narrative review covering pharmacological, psychological and psychosocial interventions, Li 2012 reported controversial findings on the effectiveness of antidepressants for the prevention and treatment of depressive symptoms in people with cancer. There were few available trials and the findings were not consistent. It has been suggested that in people with cancer, Canadian Network for Mood and Anxiety Treatments (CANMAT) level I evidence (at least two randomised controlled trials (RCTs) with adequate sample sizes, preferably placebo‐controlled, or meta‐analysis with narrow confidence intervals (CIs), or both) (Kennedy 2016) is available only for mianserin for the treatment of depressive symptoms and for paroxetine for the prevention of new episodes (Li 2012). A meta‐analysis of the efficacy of psychological and pharmacological interventions by Hart 2012 identified only four eligible trials assessing the efficacy of antidepressant drugs. A more recent meta‐analysis, carried out by Laoutidis 2013, found six placebo‐controlled trials and three head‐to‐head trials concerning the treatment of depression in people with cancer at any stage and site. Among these trials, substantial heterogeneity was found (i.e. relevant variability of participants, interventions and outcome due to different clinical, methodological and statistical approaches) (Higgins 2011). The meta‐analysis showed an improvement in depressive symptoms in patients treated with antidepressants, with an overall risk ratio of 1.56 (95% confidence interval (CI) 1.07 to 2.28). No difference in dropouts was found between groups. Subgroup analysis failed to identify differences between TCAs and SSRIs, and found that subsyndromal depressive symptoms (i.e. symptoms which do not reach the status of a formal depressive syndrome as it is described by diagnostic manuals, such as DSM or ICD) may similarly improve with antidepressant treatment (Laoutidis 2013). Similar findings have been previously shown in physically ill people in a meta‐analytic study (Rayner 2010).
A meta‐analysis by Walker 2014, which included trials carried out in people with a formal diagnosis of depression, found limited evidence in favour of the use of antidepressant drugs. However, only two placebo‐controlled trials were included, and in both of them the antidepressant was mianserin, an agent rarely used in current clinical practice. More recently, Riblet and colleagues (Riblet 2014), who systematically reviewed the evidence comparing antidepressants and placebo in individuals with any type and stage of cancer and comorbid depression of any severity, retrieved 10 trials suitable for a meta‐analysis on the efficacy of antidepressants. They concluded that fluoxetine, paroxetine and mianserin may improve cancer‐related depression. However, one quasi‐randomised trial was included and two trials included patients who were not depressed at baseline.
Rayner 2011a conducted a meta‐analytic study on the efficacy of antidepressants in people receiving palliative care (including cancer and several other life‐threatening illnesses) and suffering from depression (including major depressive disorder, adjustment disorder and dysthymic disorder based on standardised criteria, and/or according to a score above a certain cut‐off on validated tools). This review detected a beneficial effect associated with antidepressant treatment and suggested that people in palliative care with milder depressive disorders, as well as major depression, may be responsive to antidepressant treatment. These findings were incorporated into European guidelines on the management of depression in palliative cancer care (Rayner 2011b), in which use of an antidepressant is recommended, not only in major depression but also in mild depression, if symptoms persist after first‐line treatments have failed (including assessment of the quality of relationships with significant others, psychosocial support, guided self‐help programmes and brief psychological interventions). However, there is still a lack of evidence as to whether antidepressants are all similarly effective in this population.
How the intervention might work
Antidepressants are a heterogeneous class of drugs, in which a common mechanism of action is not traceable. Their therapeutic action may be related to their ability to affect serotonin, norepinephrine and dopamine neurotransmission systems, according to the broadly studied theory about monoamine dysregulation as the key neurophysiological event underlying mood disorders. However, in recent years, alternative mechanisms have been shown, making progressively clearer the complexity of interactions between several systems on which the action of these drugs rely. For instance, current research on new antidepressant drugs focuses on affecting mechanisms related to glutamate (Lapidus 2013) and melatonin transmission (Hickie 2011), neural proliferation and plasticity in limbic areas (Pilar‐Cuéllar 2013), and endocrine system activities (hypothalamic‐pituitary‐adrenal axis in particular) (Sarubin 2014), as well as antioxidant, anti‐inflammatory and immunologic pathways (Lopresti 2012).
The extent to which each of these components can contribute to the dysregulation of the brain's homeostatic system could vary extensively among different individuals and also with several biological, environmental and psychological factors (Shelton 2007). For this reason, even if the efficacy of antidepressants has been proven for some kinds of depressive conditions, we cannot assume these data to be reliable in the same way for people with cancer, for whom several further factors may be involved in the pathogenesis (including psychological, immunologic and metabolic factors, as well as pain and highly distressing treatments). Some authors have suggested a possible beneficial effect of antidepressants in cancer biology (Gil‐Ad 2008; Ahmadian 2017; Chan 2017; Zingone 2017). However, these findings are largely explorative and need to be further investigated; and it is not clear whether the effect of antidepressants may differ according to the specific cancer type or site, or both. Few systematic reviews have explored this issue, retrieving only small numbers of studies from which to draw conclusions (Carvalho 2014; Walker 2014).
In most cases antidepressant dose should be gradually titrated and it can be some weeks before the treatment takes effect. Antidepressants may require adjustment over time to ensure an appropriate dose is given. Moreover, it has been highlighted that compliance represents a relevant factor for an antidepressant's efficacy (Vergouwen 2003).
Why it is important to do this review
Providing better interventions for people with cancer and depressive symptoms is an important goal. Single pharmacological, psychological and physical interventions are not an exhaustive response for such a complex and multi‐faceted condition, which is likely to benefit from integrated, multi‐component approaches (Anwar 2017; Sharpe 2014). With this in mind, a Cochrane systematic review on the efficacy, tolerability and acceptability of antidepressants is needed in addition to existing Cochrane systematic reviews on psychotherapy (Akechi 2008; McCaughan 2017), psychosocial (Galway 2012; Semple 2013), physical (Furmaniak 2016; Shin 2016) and complementary interventions (Bradt 2015; Cramer 2017).
A systematic review by Laoutidis 2013 included participants with depressive disorder and subsyndromal depressive symptoms, identified nine randomised trials for inclusion and showed antidepressants to be superior to placebo. In their review, however, only trials in English were included, unpublished trials were not sought, and trials with depression as a secondary outcome were excluded. Further, the authors performed a meta‐analysis on dichotomous data only. Another review (Ostuzzi 2015) included people with a diagnosis of depressive disorder, subsyndromal depressive symptoms, and also people without an assessment of depressive symptoms at baseline, provided that they received antidepressant treatments for emotionally distressing cancer‐related manifestations (such as fatigue, insomnia, asthenia or cancer pain), The meta‐analysis showed a beneficial effect of antidepressants over placebo in treating depressive symptoms as a whole, and the effect remained statistically significant when considering separately participants with a formal diagnosis of major depression or depressive symptoms at baseline, and participants for whom antidepressant use was related to other distressing cancer‐related symptoms. In addition, antidepressants showed to be effective in improving quality of life.
Considering these limitations and that available systematic reviews provide contrasting findings (Hart 2012; Laoutidis 2013; Li 2012; Rodin 2007), there is still uncertainty as to the true efficacy of antidepressants (Rooney 2010; Rooney 2013; Walker 2014). Moreover, most of the previous reviews focused on elevated depressive symptoms (Hart 2012), or major depression (Iovieno 2011; Ng 2011; Walker 2014), while current findings suggest that depressive symptoms, even in subsyndromal manifestations, could represent an independent risk factor for the burden of disease (Arrieta 2013; Brenne 2013; Pinquart 2010; Satin 2009). Although the efficacy of antidepressants in minor depression, dysthymia and adjustment disorder is still not clear (Barbui 2011; Casey 2011; Silva de Lima 1999; Silva de Lima 2005), different authors suggest that antidepressants are effective in people suffering from severe medical illness (including cancer), even for subthreshold depressive symptoms (Laoutidis 2013; Rayner 2010; Rayner 2011a).
Based on this evidence we carried out a systematic review Ostuzzi 2015 (full review). In this previous version of the review we found no significant differences between antidepressants (as a class) and placebo in treating depressive symptoms, and this evidence was of very low certainty. Similarly, we found no significant differences between SSRIs and TCAs; this evidence was also of very low certainty. In this update, we have sought to include new relevant studies, or to retrieve new data from studies which were previously ongoing or awaiting classification.
Objectives
To assess the efficacy, tolerability and acceptability of antidepressants for treating depressive symptoms in adults (aged 18 years or older) with cancer (any site and stage).
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials (RCTs). We excluded trials using quasi‐random methods. We included trials published in any language.
Types of participants
We included adults (aged 18 years or older) with any primary diagnosis of cancer (confirmed with appropriate clinical and instrumental assessment) and major depressive disorder, adjustment disorder, dysthymic disorder or depressive symptoms in the absence of a formal diagnosis of major depression. We included participants receiving antidepressants for other indications (e.g. fatigue, neuropathic pain, hot flushes, etc.) only if the criterion of being affected by one of the above‐mentioned depressive conditions was met at the time of enrolment.
For trials including a diagnosis of depression, we included any standardised criteria. Most recent trials use DSM‐IV (APA 1994), or ICD‐10 (WHO 1992) criteria. Older trials use ICD‐9 (WHO 1978), DSM‐III (APA 1980) or DSM‐ III‐R (APA 1987), or other diagnostic systems. For trials including depressive symptoms in the absence of a formal diagnosis of major depression, we only included those employing standardised criteria to measure depressive symptoms and with evidence of adequate validity and reliability. Most recent trials use the Hamilton Rating Scale for Depression (HRSD) (Hamilton 1960), the Beck Depression Inventory (BDI) (Beck 1961), the Montgomery‐Åsberg Depression Rating Scale (MADRS) (Montgomery 1979), or the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983).
Types of interventions
We included the following antidepressants, reported in the Anatomical Therapeutic Chemical/Defined Daily Dose (ATC/DDD) Index (updated to December 2017) from the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology website (www.whocc.no):
non‐selective monoamine reuptake inhibitors, such as amitriptyline, desipramine, imipramine, imipramine oxide, nortriptyline, clomipramine, dosulepine, doxepin, opipramol, trimipramine, lofepramine, dibenzepin, protriptyline, iprindole, melitracen, butriptyline, amoxapine, dimetacrine, amineptine, maprotiline, quinupramine;
selective serotonin reuptake inhibitors, such as fluoxetine, fluvoxamine, citalopram, escitalopram, paroxetine, sertraline, alaproclate, etoperidone, zimelidine;
monoamine oxidase A inhibitors, such as moclobemide, toloxatone;
non‐selective monoamine oxidase inhibitors, such as isocarboxazid, nialamide, phenelzine, tranylcypromine, iproniazide, iproclozide;
any newer antidepressant and any other non‐conventional antidepressive agents, such as mianserin, trazodone, nefazodone, mirtazapine, bupropion, venlafaxine, desvenlafaxine, duloxetine, reboxetine, agomelatine, milnacipran, oxitriptan, tryptophan, nomifensine, minaprine, bifemelane, viloxazine, oxaflozane, medifoxamine, tianeptine, pivagabine, gepirone, vilazodone, Hyperici herba.
The comparison group was placebo or any other antidepressants (head‐to‐head comparisons), or both.
We excluded trials in which antidepressants were compared with another type of psychopharmacological agent, i.e. psycho‐stimulants, anxiolytics, anticonvulsants, antipsychotics or mood stabilisers.
Types of outcome measures
Primary outcomes
Efficacy as a continuous outcome
We extracted and analysed group mean scores at different time points and, if these were not available, group mean change scores, on the Hamilton Rating Scale for Depression (HRSD), Montgomery and Åsberg Depression Rating Scale (MADRS) or Clinical Global Impression Rating scale (CGI), or on any other depression rating scale with evidence of adequate validity and reliability, as follows:
early response: between one and four weeks, giving preference to the time point closest to two weeks;
acute phase treatment response: between 6 and 12 weeks, giving preference to the time point given in the original trial as the study endpoint;
follow‐up response: after 12 weeks, giving preference to the time point closest to 24 weeks.
The acute phase treatment response (between 6 and 12 weeks) was our primary outcome of interest. If the acute phase treatment response was reported, we then reported early response and follow‐up response as secondary outcomes.
Secondary outcomes
Efficacy as a dichotomous outcome
Treatment responders during the 'acute phase' (between 6 and 12 weeks): proportion of participants showing a reduction of at least 50% on the HRSD or MADRS or any other depression scale (e.g. the Beck Depression Inventory (BDI) or the Center for Epidemiologic Studies Depression Scale (CES‐D)), or who were 'much or very much improved' (score 1 or 2) on the Clinical Global Impression‐Improvement (CGI‐I) scale, or the proportion of participants who improved using any other pre‐specified criterion.
Social adjustment
Mean scores on social adjustment rating scales, e.g. Global Assessment of Functioning (GAF), as defined by each of the trials, during the 'acute phase' (between 6 and 12 weeks).
Health‐related quality of life
Mean scores on quality of life (QoL) rating scales during the 'acute phase' (between 6 and 12 weeks). We gave preference to illness‐specific QoL measures, such as the European Organisation for Research and Treatment into Cancer Quality of Life Questionnaire‐30 (EORTC QLQ‐30) (Aaronson 1993), the Functional Assessment of Cancer Therapy (FACT) scale (Cella 1993), and the Short Form (36) Health Survey (SF‐36) (Ware 1980; Ware 1992). When such tools were not employed, we used a general health‐related QoL measure with evidence of adequate validity and reliability, as defined by each of the trial
Dropouts:
number of participants who dropped out during the trial as a proportion of the total number randomised (total dropout rate, also referred as "acceptability");
number of participants who dropped out due to inefficacy during the trial as a proportion of the total number randomised (dropout rates due to inefficacy);
number of participants who dropped out due to adverse effects during the trial as a proportion of the total number randomised (dropout rates due to adverse effects, also referred as "tolerability").
We extracted dropouts at trial endpoint only.
Search methods for identification of studies
Electronic searches
We searched the following electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 6) in the Cochrane Library (searched 3 July 2017) (Appendix 1), MEDLINE Ovid (1946 to June week 4 2017) (Appendix 2), Embase Ovid (1980 to 2017 week 217) (Appendix 3) and PsycINFO Ovid (1987 to June 2017 week 4) (Appendix 4).
Searching other resources
Handsearches
We handsearched the trial databases of the following drug‐approving agencies for published, unpublished and ongoing controlled trials: the Food and Drug Administration (FDA) in the USA (http://www.fda.gov), the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom (http://www.mhra.gov.uk/), the European Medicines Agency (EMA) in the European Union (http://www.ema.europa.eu), the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan (http://www.pmda.go.jp/english/) and the Therapeutic Goods Administration (TGA) in Australia (http://www.tga.gov.au/).
We additionally searched the following trial registers: clinicaltrials.gov in the USA (http://clinicaltrials.gov/), ISRCTN and National Research Register in the United Kingdom (www.isrctn.com/), UMIN‐CTR in Japan (www.umin.ac.jp/ctr/), the ANZ‐CTR in Australia and New Zealand (www.anzctr.org.au/), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) and the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) Clinical Trials Portal (www.ifpma.org/tag/clinical‐trials/).
We also handsearched appropriate journals and conference proceedings relating to depression treatment in people with cancer. We also handsearched the web sites of the most relevant pharmaceutical companies producing antidepressants, such as GlaxoSmithKline (www.gsk‐clinicalstudyregister.com/), Sanofi (www.sanofi.com/en/science‐and‐innovation/clinical‐trials‐and‐results/), Janssen (www.janssen.com/clinical‐trials), Lundbeck (www.lundbeck.com/trials), Pfizer (www.pfizer.co.uk/clinical‐trials), Abbott (www.abbott.com/policies/clinical‐trials.html), Lilly (www.lillytrials.com/), and Merck (www.merck.com/research/discovery‐and‐development/clinical‐development/home.html) for published, unpublished and ongoing controlled trials.
We also searched reference lists of included trials and other relevant studies.
Personal communication
We searched the websites of pharmaceutical companies (list reported in the methods) and contacted the authors of the unpublished studies. Only one author provided data from one unpublished study.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (Endnote) and removed duplicates. Two review authors (GO and FM) examined the remaining references independently. We excluded those trials that clearly did not meet the inclusion criteria, and we obtained copies of the full text of potentially relevant references. Two review authors (GO and FM) independently assessed the eligibility of retrieved trials. Disagreements were resolved by discussion between the two review authors and, if necessary, with a third review author (CB). We documented reasons for exclusion. We collated multiple reports of the same trials to ensure that no data were included in the meta‐analysis more than once.
Data extraction and management
Two review authors (GO and FM), working independently and in duplicate, extracted data from the included trials using a data collection sheet (see Appendix 5), which was developed in accordance with recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; chapter 7). If the trial was a three (or more)‐armed trial involving a placebo arm, we also extracted data from the placebo arm.
Data included:
first author, year and journal;
methodological features (study design, randomisation, blinding and allocation concealment, follow‐up period);
participant characteristics (gender, age, study setting, number of participants randomised to each arm, depression diagnosis, previous history of depression, cancer site and stage, cancer treatment);
intervention details (antidepressant and other interventions employed, dosage range, mean daily dosage prescribed);
outcome measures for each time point of interest. Continuous measures encompassed mean scores of rating scales, standard deviation or standard error; dichotomous measures were endpoint response rate and dropout rate, which were calculated on a strict intention‐to‐treat (ITT) basis;
cost analysis (estimates of the cost of resources employed to perform the trial);
presence of sponsorship by a drug company.
Alongside the data which contributed to meta‐analysis, we collected characteristics of participants, settings, interventions and methodological approaches, in order to provide an overall view of the available evidence on this topic (see Description of studies), as well as to perform an accurate assessment of the risk of bias (see Risk of bias in included studies). These elements provided a crucial contribution to the discussion, with particular regards to the clinical applicability of the results of the study (see Overall completeness and applicability of evidence; Implications for practice).
Assessment of risk of bias in included studies
Two review authors (GO and FM) independently assessed the risk of bias of all included trials in accordance with Cochrane's tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which includes the following domains: random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (detection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting of outcomes (reporting bias) and other biases. To determine the risk of bias of a trial, for each criterion we evaluated the presence of sufficient information and the likelihood of potential bias. We rated each criterion as 'low risk of bias', 'high risk of bias' or 'unclear risk of bias' (indicating either lack of information or uncertainty over the potential for bias). Particular attention was given to the adequacy of the random allocation concealment and blinding of participants, personnel and outcome assessors. If inadequate details of methodological characteristics of trials were provided, we contacted the authors in order to obtain further information. If the raters disagreed, the final rating was made by consensus with the involvement (if necessary) of a third review author (CB). We summarised results in a 'Risk of bias' graph and a 'Risk of bias' summary and discussed and interpreted the results of meta‐analysis in light of the findings and with respect to the risk of bias.
Measures of treatment effect
1. Continuous data
We evaluated the efficacy of treatments as a continuous measure, namely the group mean scores on depression rating scales at the acute phase (between 6 and 12 weeks). We employed other continuous data for some secondary outcomes, namely efficacy at early response (between one and four weeks), efficacy at follow‐up response (after 12 weeks), social adjustment and health‐related quality of life.
2. Dichotomous data
We employed dichotomous data for some secondary outcomes, namely efficacy as the number of treatment responders at the acute phase (between 6 and 12 weeks), and the proportion of dropouts.
Unit of analysis issues
1. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, the participants can differ systematically from their initial state, even despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). Both effects are very likely in major depression, thus we planned to use only data from the first phase of cross‐over trials.
2. Cluster‐randomised trials
We planned to use the generic inverse variance technique to appropriately analyse cluster‐randomised trials, taking into account intra‐class correlation coefficients to adjust for cluster effects.
Dealing with missing data
At some degree of loss to follow‐up, data must lose credibility (Xia 2009). For any particular outcome, if more than 50% of data were unaccounted for, we did not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a trial were lost, but the total loss was less than 50%, we planned to mark such data with (*) to indicate that such a result may be prone to bias. When dichotomous or continuous outcomes were not reported, we asked trial authors to supply the data.
We calculated dichotomous data on a strict intention‐to‐treat (ITT) basis: dropouts were always included in this analysis. Where participants had been excluded from the trial before the endpoint, we assumed that they experienced a negative outcome by the end of the trial. For continuous variables, we applied a loose ITT analysis, whereby all the participants with at least one post‐baseline measurement were represented by their last observations carried forward (LOCF), with due consideration of potential biases, including number and timings of dropouts in each arm.
When relevant outcomes were not reported, we asked trial authors to supply the data. In the absence of data from authors, we only employed validated statistical methods to impute missing outcomes, with due consideration of the possible bias of these procedures, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and with www.missingdata.org.uk. When standard deviations (SDs) were not reported, we asked authors to supply the data. When only the standard error (SE) or t‐statistics or P values were reported, we calculated SDs according to Altman 1996. In the absence of data from the authors, we substituted SDs with those reported in other trials in the review (Furukawa 2006).
Assessment of heterogeneity
We investigated heterogeneity between trials using the I2 statistic (Higgins 2003; Ioannidis 2008) (we considered an I2 value equal to or more than 50% to indicate substantial heterogeneity) and by visual inspection of the forest plots.
Assessment of reporting biases
We had planned to use the tests for funnel plot asymmetry to investigate small‐study effects (Sterne 2000), if there were at least 10 trials included in the meta‐analysis, with cautious interpretation of the results by visual inspection (Higgins 2011). Since we were unable to conduct any analysis including at least 10 trials we did not use a funnel plot. When evidence of small‐study effects was identified, we aimed to investigate possible reasons for funnel plot asymmetry, including publication bias.
Data synthesis
If a sufficient number of clinically similar studies was available, we pooled their results in meta‐analyses.
For continuous data we pooled the mean differences (MDs) with a 95% confidence interval (CI) between the treatment arms at the time point of interest, if all trials measured the outcome using the same rating scale; otherwise we pooled standardised mean differences (SMDs). For dichotomous data, we pooled the risk ratio (RR) with a 95% CI. For the analysis of dichotomous data we employed the Mantel‐Haenszel methods. For statistically significant results, we calculated the number needed to treat to provide benefit (NNTB). We included trials that compared more than two intervention groups of the same drug (i.e. different dosages) in meta‐analysis by combining arms of the trials into a single group, for the intervention and for the control group respectively, as recommended in section 16.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If data were binary, we simply added and combined them into one group or divided the comparison arm into two (or more) as appropriate. If data were continuous, we combined the data following the formula in Chapter 7, section 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included trials that compared two or more antidepressants with placebo as independent comparisons, splitting the 'shared' group (placebo) into two or more groups with smaller sample size (Higgins 2011).
We chose a random‐effects model as heterogeneity was expected (Higgins 2011). We only considered direct comparisons for the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We aimed to perform the following subgroup analyses for the primary outcome:
psychiatric diagnosis, separating major depressive disorder, and pooling data from studies including only participants with adjustment disorder, dysthymic disorder, depressive symptoms;
previous history of depressive conditions;
antidepressant class, in particular separating SSRIs, TCAs and other antidepressants;
cancer site, separating breast cancer and other sites;
cancer stage, separating early stages (stage 0 and I) and late stages (stage II, III and IV);
gender.
We interpreted subgroup analyses with caution, as multiple analyses can lead to false positive conclusions (Oxman 1992).
Sensitivity analysis
We aimed to perform the following sensitivity analyses for the primary outcome:
excluding trials in which the randomisation process was not clearly reported;
excluding trials with unclear concealment of random allocation;
excluding trials that did not employ adequate blinding of participants, healthcare providers and outcome assessors;
excluding trials that did not employ depressive symptoms as their primary outcome;
excluding trials with imputed data.
'Summary of findings' table
We prepared 'Summary of findings' tables, summarising the key findings of the systematic review in line with the standard methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). These findings include:
-
antidepressants compared to placebo for depressive symptoms in people with cancer:
efficacy as a continuous outcome;
efficacy as a dichotomous outcome;
dropouts.
-
antidepressants compared to other antidepressants for depressive symptoms in people with cancer:
efficacy as a continuous outcome;
efficacy as a dichotomous outcome;
dropouts.
Results
Description of studies
Results of the search
See Figure 1 for an illustration of the process of study selection. The search of the electronic databases retrieved 4957 references. After eliminating the duplicates, we identified 3981 references for screening. We added 39 further references from the handsearching of articles' references and the websites of drug‐approving agencies and pharmaceutical companies. Two review authors (GO, FM) independently checked 10% of the titles. Since the degree of agreement was 'good' according to the Cochrane Handbook for Systematic Reviews of Interventions (simple kappa statistic 0.73), one review author (GO) checked the remaining titles. From the 241 titles identified, the two review authors independently checked 50% of the abstracts. The degree of agreement was 'fair' according to the Cochrane Handbook for Systematic Reviews of Interventions (simple kappa statistic 0.41). The two review authors discussed the abstracts for which there was inconsistency between them and achieved complete agreement. One review author (GO) checked the remaining abstracts. The two review authors examined the full text of all of the 98 studies identified after the abstract check in detail. Ten studies fulfilled the criteria for eligibility and were included in the review (Costa 1985; EUCTR2008‐002159‐25‐FR; Fisch 2003; Holland 1998; Musselman 2006; Navari 2008; NCT00387348; Pezzella 2001; Razavi 1996; Van Heeringen 1996). Only seven studies contributed to the meta‐analysis for the primary outcome (EUCTR2008‐002159‐25‐FR; Fisch 2003; Holland 1998; Musselman 2006; Pezzella 2001; Razavi 1996; Van Heeringen 1996). Two studies (Costa 1985; NCT00387348) contributed only to the meta‐analysis for secondary outcomes and Navari 2008 did not provide useful data for the meta‐analysis.
1.
Flow diagram.
Included studies
We included a total of ten studies: eight published studies (Costa 1985; Fisch 2003; Holland 1998; Musselman 2006; Navari 2008; Pezzella 2001; Razavi 1996; Van Heeringen 1996), and two unpublished studies (EUCTR2008‐002159‐25‐FR; NCT00387348). A total of 885 participants were involved in these studies. A detailed description of each study is reported in the section Characteristics of included studies.
Design and interventions
All the included studies were reported to be randomised and double‐blind. The participants were followed up for four weeks in one trial (Costa 1985), five weeks in one trial (Razavi 1996), six weeks in three trials (Holland 1998; Musselman 2006; Van Heeringen 1996), eight weeks in two trials (NCT00387348; Pezzella 2001), 12 weeks in one trial (EUCTR2008‐002159‐25‐FR), 24 weeks in one trial (Navari 2008) and for a mean of 15 weeks in one trial (range between 4 and 24 weeks) (Fisch 2003). Seven studies had two arms and explored the efficacy of an antidepressant versus placebo (Costa 1985; EUCTR2008‐002159‐25‐FR; NCT00387348; Fisch 2003; Navari 2008; Razavi 1996; Van Heeringen 1996). In five of these studies the antidepressant was a selective serotonin reuptake inhibitor (SSRI) (EUCTR2008‐002159‐25‐FR; NCT00387348; Fisch 2003; Navari 2008; Razavi 1996), and in two the tetracyclic antidepressant mianserin was evaluated (Costa 1985; Van Heeringen 1996). Two studies compared two antidepressants with a two‐arm, head‐to‐head study design (paroxetine versus amitriptyline and fluoxetine versus desipramine respectively) (Holland 1998; Pezzella 2001). One study used a three‐arm design, comparing paroxetine versus desipramine versus placebo (Musselman 2006). In these three studies the head‐to‐head comparisons were between a tricyclic antidepressant and a SSRI.
Sample sizes
The mean number of participants per study was approximately 88, with a minimum sample size of 24 (NCT00387348), and a maximum of 193 (Navari 2008). Only three studies had more than 100 participants (Fisch 2003; Navari 2008; Pezzella 2001).
Setting
Four trials enrolled only outpatients (Fisch 2003; Musselman 2006; Navari 2008; Van Heeringen 1996). Inpatients and outpatients were enrolled in one trial (Costa 1985). For the remaining five trials the setting was not clearly reported (EUCTR2008‐002159‐25‐FR; NCT00387348; Holland 1998; Pezzella 2001; Razavi 1996).
Participants
Two trials excluded people aged over 65 years (Holland 1998; Van Heeringen 1996), while no trials included only elderly participants. The population of participants was heterogeneous in terms of diagnosis of depression. One trial enrolled only participants with a diagnosis of major depression based on the Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM‐III) in association with a score greater than 16 on the 21‐item Hamilton Rating Scale for Depression (HRSD) (Van Heeringen 1996). One trial enrolled participants with a diagnosis of major depression according to DSM‐IV, to Endicott criteria, and with a score higher than 14 on 17‐item HRSD (NCT00387348). One trial enrolled participants with major depression according to International Classification of Diseases‐ tenth revision (ICD‐10) criteria (Pezzella 2001). Three studies enrolled both people with a diagnosis of major depression and people with adjustment disorders based on DSM‐III‐R (Holland 1998), on DSM‐III‐R in association with a score greater than 14 on the first 17 items of the 21‐item HRSD (Musselman 2006), or on DSM‐III‐R in association with a score greater than 13 on the Hospital Anxiety and Depression Scale (HADS) (Razavi 1996). However, in the Musselman 2006 trial only people with major depression took part in the study. Three studies enrolled people with depressive symptoms, but without a formal diagnosis of depression according to a cut‐off score on standardised rating scales, respectively Two‐Question Screening Survey (TQSS) greater than 2 (Fisch 2003; Navari 2008) and Hospital Anxiety and Depression Scale (HADS) greater than 11 (EUCTR2008‐002159‐25‐FR). One study (Costa 1985) used alternative criteria for defining depression (quote: "diagnosis of depression according to the criteria proposed by Stewart [Stewart 1965] for medically ill patients, with slight additional inclusion criteria suggested by Kathol and Petty [Kathol 1981] [...]") in association with a cut‐off score on standardised rating scales, Zung Self‐Rating Depression Scale (ZSRDS) greater than 41; 17‐item HRSD greater than 16.
With regards to the cancer type and stage, three studies had mixed populations (Costa 1985; Holland 1998; Razavi 1996), but the majority of participants suffered from breast cancer. In Fisch 2003, the population was quite equally distributed between breast, thoracic, genitourinary and other types of cancer. Four studies included only women with breast cancer (Musselman 2006; Navari 2008; Pezzella 2001; Van Heeringen 1996). One study (EUCTR2008‐002159‐25‐FR) included only people suffering from head and neck cancer and another (NCT00387348) included only people suffering from lung or gastro‐intestinal cancer. In two studies the cancer stage was not clearly reported (Fisch 2003; Razavi 1996). Two studies included only people with early stages ("localized" or "early locally advanced" disease) (Navari 2008; Van Heeringen 1996), while all other studies also recruited people with late‐stage disease (Costa 1985; EUCTR2008‐002159‐25‐FR; Holland 1998; Musselman 2006; Pezzella 2001). One study (NCT00387348) included only people with late locally advanced or metastasised disease.
Outcomes
For efficacy outcomes, most of the randomised controlled trials (RCTs) provided continuous data such as mean score or mean change on standardised rating scales, including those considered reliable for the aims of this review, such as HRSD (Costa 1985; Musselman 2006; NCT00387348; Van Heeringen 1996), Montgomery‐Åsberg Depression Rating Scale (MADRS) (EUCTR2008‐002159‐25‐FR; Razavi 1996), or other scales (Fisch 2003; Pezzella 2001). One study (Navari 2008) provided only dichotomous data, defining "responders" those who achieved a certain improvement in the rating scale score. This study provided these data only for the six‐month assessment and thus could not be included in the meta‐analysis.
For secondary outcomes, the majority of the studies provided complete data on total dropouts, due to inefficacy and side‐effects. Three studies provided only partial data on dropouts (Fisch 2003; Navari 2008; NCT00387348). Very few studies reported data on other secondary outcomes, such as social adjustment (Pezzella 2001) and quality of life (Fisch 2003; Pezzella 2001).
We included a total of 479 people in the efficacy analysis on a continuous outcome between 6 and 12 weeks (primary outcome) and 592 on a dichotomous outcome; 175 in the social adjustments analysis; 305 in the quality of life analysis; and 716 in the analysis of dropouts.
Excluded studies
We excluded most of the retrieved references after title and abstract screening. Of the 98 studies selected for a full‐text evaluation, we excluded 88: 74 did not meet one or more inclusion criteria (mostly a wrong diagnostic status), 12 were double reports and 2 were added to Studies awaiting classification. No ongoing studies were retrieved (Figure 1).
In particular, one study did not enrol patients with cancer, while in 35 studies participants were not depressed when enrolled or the studies enrolled a population with mixed psychiatric symptoms (e.g. both anxious and depressed patients). Nine studies were not randomised and one was actually a review of other studies. For eight studies the comparison group was not reliable because no placebo or active comparator were employed. For three studies, for which only the abstract or the protocol was available, we contacted the authors who informed us that these studies had been withdrawn or changed in their design. Details are reported in Characteristics of excluded studies.
Risk of bias in included studies
We found the overall methodological quality of the included studies to be unclear or low (see Figure 2; Figure 3). Only five studies had a low risk of bias for at least one item (EUCTR2008‐002159‐25‐FR; NCT00387348; Fisch 2003; Musselman 2006; Pezzella 2001).
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Almost all the studies had an 'unclear risk' for the selection bias domain — which includes random sequence generation and allocation concealment — because procedures for ensuring adequate concealment of allocation were not reported in the paper or in the protocol, and because information about the adequacy of the allocation sequence generation were not provided. Only one study (Fisch 2003) clearly described the procedures for randomisation and allocation of participants, which were properly performed.
Blinding
With the exception of NCT00387348, which had a 'low risk' of performance and detection bias, we considered all the included studies to have an 'unclear risk'. The studies were described as "double‐blind", however they did not report who was blinded among practitioners, outcome assessors and statisticians; neither did they describe procedures for ensuring the blinding of both participants and who administered the intervention.
Incomplete outcome data
The risk of attrition bias appeared to be a particularly relevant issue, with different reasons between studies. We considered six studies to have a 'high risk' because no imputation for missing data was performed, resulting in a 'per protocol analysis' or an 'as treated analysis' (even if the term 'intention‐to‐treat analysis' was often reported) (EUCTR2008‐002159‐25‐FR; Fisch 2003; Holland 1998; Navari 2008; Razavi 1996; Van Heeringen 1996). Furthermore, in three of these studies this issue was associated with a dropout rate higher than 20% in at least in one arm, which could possibly induce bias in the intervention effect estimate (Holland 1998; Razavi 1996; Van Heeringen 1996). For two studies we considered the risk of bias as 'unclear' since the intention‐to‐treat analysis was properly performed (Costa 1985; Musselman 2006), but the dropout rate was particularly high (40.5% in the placebo arm in Costa 1985; and 38% in the paroxetine arm, 36% in the desipramine arm and 45% in the placebo arm in Musselman 2006). For two studies (Pezzella 2001; NCT00387348) we considered the risk to be 'low' since the intention‐to‐treat analysis was properly performed and the dropout rate was not particularly relevant.
Selective reporting
The risk of reporting bias was particularly inconsistent between studies. For two studies the risk was 'high' as primary outcomes were not clearly prespecified and were poorly reported in the text (Holland 1998; Navari 2008). For four studies the risk was 'unclear' as primary outcomes were not clearly prespecified, but relevant outcomes of interest were properly reported in the results (Costa 1985; Pezzella 2001; Razavi 1996; Van Heeringen 1996). For the remaining studies all the prespecified primary outcomes were reported for the time points of interest (EUCTR2008‐002159‐25‐FR; NCT00387348; Fisch 2003; Musselman 2006).
Other potential sources of bias
With regards to the possible occurrence of other types of bias, we found no relevant baseline imbalance of the population composition. Furthermore, we systematically assessed the risk of sponsorship bias and in five studies this bias could not be ruled out since the possible conflicts of interest, as well as the role of funders in planning, conducting and writing the study were not discussed (Costa 1985; Fisch 2003; Musselman 2006; Navari 2008; Pezzella 2001). For these studies we considered the risk of bias to be 'unclear'. For three studies we considered the risk to be 'high', as the funder was a pharmaceutical company and its role in planning, conducting and writing the study was not discussed (Holland 1998; Razavi 1996; Van Heeringen 1996). In one study a pharmaceutical company funded the cost of drugs but did not play any relevant role in planning, conducting and writing the study (EUCTR2008‐002159‐25‐FR). One study was clearly funded by non‐profit institutes (NCT00387348).
Effects of interventions
Summary of findings for the main comparison. Antidepressants compared to placebo for people with cancer and depression.
| Antidepressants compared to placebo for patients with cancer and depression | ||||||
| Patient or population: adults with cancer and depression Settings: in‐ and outpatients Intervention: antidepressants Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainity (quality) of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antidepressants | |||||
| Efficacy as a continuous outcome Follow‐up: 6 to 12 weeks | The mean efficacy as a continuous outcome (SMD) in the intervention groups was 0.45 standard deviations lower (1.01 lower to 1.11 higher) | 266 (5 studies, 6 comparisons) | ⊕⊝⊝⊝ very low1,2,3,4 | |||
| Efficacy as a dichotomous outcome Follow‐up: 6 to 12 weeks | 358 per 1000 | 294 per 1000 (222 to 387) | RR 0.82 (0.62 to 1.08) | 417 (5 studies, 6 comparisons) | ⊕⊝⊝⊝ very low1,3,4,5 | |
| Dropouts due to any cause (acceptability) Follow‐up: 4 to 12 weeks | 215 per 1000 | 187 per 1000 (105 to 328) | RR 0.85 (0.52 to 1.38) | 479 (7 studies, 7 comparisons) | ⊕⊝⊝⊝ very low1,3,4,6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded as no studies described the outcome assessment as masked. This should be considered a major limitation, which is likely to result in a biased assessment of the intervention effect. 2 Downgraded due to heterogeneity ‐ I² = 77%. An I² between 50% and 75% suggests a serious risk of inconsistency (unexplained heterogeneity), which may arise from relevant differences in populations, interventions and outcomes of the studies entered into the analysis. 3 Downgraded due to very low number of participants recruited (fewer than 100 individuals in both treatment arms) and 95% CI includes both no effect and appreciable benefit or appreciable harm, which suggests the risk of very serious imprecision of the results and thus low confidence in their reliability. 4 Downgraded due to high risk of sponsorship bias. 5 Downgrade due to heterogeneity ‐ I² = 49%. See above 6 Downgrade due to heterogeneity ‐ I² = 53%. See above.
Summary of findings 2. Selective serotonin reuptake inhibitors (SSRIs) compared to tricyclic antidepressants (TCAs) for people with cancer and depression.
| SSRIs compared to TCAs for patients with cancer and depression | ||||||
| Patient or population: patients with cancer and depression Settings: in‐ and outpatients Intervention: SSRIs Comparison: TCAs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty (Quality) of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TCAs | SSRIs | |||||
| Efficacy as a continuous outcome Follow‐up: 6 to 12 weeks | The mean efficacy as a continuous outcome (SMD) in the intervention groups was 0.08 standard deviations lower (0.34 lower to 0.18 higher) | 237 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | |||
| Efficacy as a dichotomous outcome Follow‐up: 6 to 12 weeks | Study population | RR 1.10 (0.78 to 1.53 | 199 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 388 per 1000 | 454 per 1000 (256 to 799) | |||||
| Dropouts due to any cause (acceptability) Follow‐up: 4 to 12 weeks | Study population | RR 0.83 (0.53 to 1.3) | 237 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 261 per 1000 | 217 per 1000 (138 to 339) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded as no studies described the outcome assessment as masked. This should be considered a major limitation, which is likely to result in a biased assessment of the intervention effect. 2 Downgraded as very low number of participants recruited (fewer than 100 individuals in both treatment arms) and 95% CI includes both no effect and appreciable benefit or appreciable harm, which suggests the risk of very serious imprecision of the results and thus low confidence in their reliability. 3 Downgraded as one study out of three had a high risk of sponsorship bias.
Primary outcome: efficacy at 6 to 12 weeks (continuous outcome)
1.1 Antidepressants versus placebo
We found no statistically significant difference between antidepressants as a class and placebo, with a standardised mean difference (SMD) of −0.45(95% confidence interval (CI) −1.01 to 0.11, five RCTs, 266 participants; very low certainty evidence) (see Analysis 1.1; Figure 4).
1.1. Analysis.
Comparison 1 Depression: efficacy as a continuous outcome at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo.
4.

Forest plot of comparison: 1 Depression: efficacy at 6‐12 weeks (continuous outcome), outcome: 1.1 Antidepressants versus placebo.
1.2 Antidepressants versus antidepressants
We found no statistically significant difference between selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) as classes, with a SMD of −0.08 (95% CI −0.34 to 0.18, three RCTs, 237 participants) (see Analysis 1.2; Figure 5).
1.2. Analysis.
Comparison 1 Depression: efficacy as a continuous outcome at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants.
5.
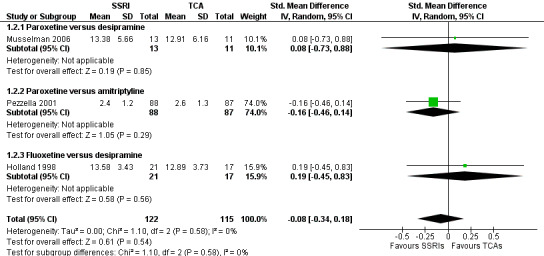
Forest plot of comparison: 1 Depression: efficacy at 6‐12 weeks (continuous outcome), outcome: 1.2 Antidepressants versus Antidepressants.
Secondary outcomes
2 Efficacy at one to four weeks (continuous outcome)
2.1 Antidepressants versus placebo
We found no statistically significant difference between antidepressants as a class and placebo, with a SMD of −0.29 (95% CI −0.72 to 0.13, five RCTs, 310 participants) (see Analysis 2.1).
2.1. Analysis.
Comparison 2 Depression: efficacy as a continuous outcome at 1 to 4 weeks, Outcome 1 Antidepressants versus placebo.
For antidepressants versus antidepressants, no studies provided data for this outcome. For efficacy after 12 weeks (continuous outcome), no studies provided data for this outcome.
3 Efficacy at 6 to 12 weeks (dichotomous outcome)
3.1 Antidepressants versus placebo
We found no statistically significant difference between antidepressants as a class and placebo in terms of response rate, with a risk ratio (RR) of 0.82 (95% CI 0.62 to 1.08, five RCTs, 417 participants; very low certainty evidence) (see Analysis 3.1).
3.1. Analysis.
Comparison 3 Depression: efficacy as a dichotomous outcome at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo.
3.2 Antidepressants versus antidepressants
We found no statistically significant difference in terms of response rate between SSRIs and TCAs as classes, with a RR of 1.10 (95% CI 0.78 to 1.53, two RCTs, 199 participants, very low certainty evidence) (see Analysis 3.2).
3.2. Analysis.
Comparison 3 Depression: efficacy as a dichotomous outcome at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants.
4 Social adjustment at 6 to 12 weeks
4.1 Antidepressants versus antidepressants
Only one study provided data for this outcome, showing no statistically significant difference between paroxetine and amitriptyline, with a mean difference (MD) of 0.10 (95% CI −0.38 to 0.58, 175 participants, negative values favour paroxetine) on the MADRS rating scale (see Analysis 4.1).
4.1. Analysis.
Comparison 4 Social adjustment at 6 to 12 weeks, Outcome 1 Antidepressants versus antidepressants.
For antidepressants versus placebo, no studies provided data for this outcome.
5 Quality of life at 6 to 12 weeks
5.1 Antidepressants versus placebo
We found no statistically significant difference between antidepressants as a class and placebo, with a SMD of 0.05 (95% CI ‐0.27 to 0.37, two RCTs, 152 participants) (see Analysis 5.1).
5.1. Analysis.
Comparison 5 Quality of life at 6 to 12 weeks, Outcome 1 Antidepressants versus placebo.
5.2 Antidepressants versus antidepressants
Only one study provided data for this outcome, showing no statistically significant difference between paroxetine and amitriptyline, with a MD of 6.50 (95% CI 0.21 to 12.79, 153 participants, negative values favour paroxetine) on the MADRS rating scale (see Analysis 5.2).
5.2. Analysis.
Comparison 5 Quality of life at 6 to 12 weeks, Outcome 2 Antidepressants versus antidepressants.
6 Dropouts due to inefficacy
6.1 Antidepressants versus placebo
We found no statistically significant difference between antidepressants as a class and placebo, with a RR of 0.41 (95% CI 0.13 to 1.32, six RCTs, 455 participants) (see Analysis 6.1).
6.1. Analysis.
Comparison 6 Dropouts due to inefficacy, Outcome 1 Antidepressants versus placebo.
6.2 Antidepressants versus antidepressants
We found no statistically significant difference between SSRIs and TCAs as classes, with a RR of 0.85 (95% CI 0.14 to 5.06, three RCTs, 237 participants) (see Analysis 6.2).
6.2. Analysis.
Comparison 6 Dropouts due to inefficacy, Outcome 2 Antidepressants versus antidepressants.
7 Dropouts due to side effects (tolerability)
7.1 Antidepressants versus placebo
We found no statistically significant difference between antidepressants as a class and placebo, with a RR of 1.19 (95% CI 0.54 to 2.62, seven RCTs, 479 participants) (see Analysis 7.1).
7.1. Analysis.
Comparison 7 Dropouts due to side effects (tolerability), Outcome 1 Antidepressants versus placebo.
7.2 Antidepressants versus antidepressants
We found no statistically significant difference between SSRIs and TCAs as classes, with a RR of 1.04 (95% CI 0.55 to 1.99, three RCTs, 237 participants) (see Analysis 7.2).
7.2. Analysis.
Comparison 7 Dropouts due to side effects (tolerability), Outcome 2 Antidepressants versus antidepressants.
8 Dropouts due to any cause (acceptability)
8.1 Antidepressants versus placebo
We found no statistically significant difference between antidepressants as a class and placebo, with a RR of 0.85 (95% CI 0.52 to 1.38, seven RCTs, 479 participants; very low certainty evidence) (see Analysis 8.1).
8.1. Analysis.
Comparison 8 Dropouts due to any cause (acceptability), Outcome 1 Antidepressants versus placebo.
8.2 Antidepressants versus antidepressants
We found no statistically significant difference between SSRIs and TCAs as classes, with a RR of 0.83 (95% CI 0.53 to 1.30, three RCTs, 237 participants; very low certainty evidence) (see Analysis 8.2).
8.2. Analysis.
Comparison 8 Dropouts due to any cause (acceptability), Outcome 2 Antidepressants versus antidepressants.
Subgroup analyses
1. Psychiatric diagnosis
Results from this subgroup analysis did not materially change the main findings for the primary outcome, which remains not statistically significant in both people with major depressive disorder and people with adjustment disorder, dysthymic disorder or depressive symptoms. This is true for both the 'antidepressant‐placebo' and the 'head‐to‐head' comparisons (see Analysis 9.1 and Analysis 9.2).
9.1. Analysis.
Comparison 9 Subgroup analysis: psychiatric diagnosis, Outcome 1 Antidepressants versus placebo.
9.2. Analysis.
Comparison 9 Subgroup analysis: psychiatric diagnosis, Outcome 2 Antidepressants versus antidepressants.
2. Previous history of depressive conditions
We did not perform this analysis since the data provided were not sufficient to measure the primary outcome in this subgroup of participants.
3. Antidepressant class
In the main analysis we pooled data separating the following classes of antidepressants: SSRIs, TCAs and other antidepressants. Considering the 'antidepressant‐placebo' comparison, we found no statistically significant effect for both SSRIs (SMD −0.21, 95% CI −0.50 to 0.08, four RCTs, 194 participants) and TCAs (MD 0.27, 95% CI ‐5.13 to 5.67, one trial, 17 participants). However, we found mianserin, the only compound in the 'other antidepressants' class, to be effective over placebo (MD −8.2, 95% CI −10.6 to −5.77, one trial, 55 participants) (see Analysis 1.1). In this analysis MDs are reported as SMDs. The difference between the subgroups was statistically significant (P value < 0.0001). The 'head‐to‐head' comparison did not show statistically significant differences between SSRIs and TCAs as classes (SMD −0.08, 95% CI −0.34 to 0.18, three studies, 237 participants) (see Analysis 1.2).
4. Cancer site
Results from this subgroup analysis did not materially change the main findings for the primary outcome. No statistically significant effect was found when pooling studies that enrolled only women with breast cancer (see Analysis 10.1 and Analysis 10.2). It was technically feasible to separate these two subgroups, however the 'other sites' subgroup could not be considered a reliable comparison with the 'breast cancer' subgroup because, even if in these studies people with different types of cancer were enrolled, the vast majority of them were actually affected by breast cancer.
10.1. Analysis.
Comparison 10 Subgroup analysis: cancer site, Outcome 1 Antidepressants versus placebo.
10.2. Analysis.
Comparison 10 Subgroup analysis: cancer site, Outcome 2 Antidepressants versus antidepressants.
5. Cancer stage
Results from this subgroup analysis did not materially change the main findings for the primary outcome (see Analysis 11.1 and Analysis 11.2). Two studies among those comparing antidepressants versus placebo enrolled only people with late‐stage disease (Costa 1985; Holland 1998), however the study by Costa 1985 did not provide data for the primary outcome (efficacy at 6 to 12 weeks) and was not included in the analysis. Other studies had a mixed population in terms of cancer stage, with the exception of Razavi 1996, in which only people in a stage 0 (carcinoma in situ, early form) were enrolled. Considering the 'head‐to‐head' comparison, only one study (Holland 1998) enrolled people with early‐stage disease, showing no statistically significant differences between SSRIs and TCAs as classes (MD 0.69, 95% CI −1.61 to 2.99, one trial, 38 participants), while other studies had a mixed population.
11.1. Analysis.
Comparison 11 Subgroup analysis: cancer stage, Outcome 1 Antidepressants versus placebo.
11.2. Analysis.
Comparison 11 Subgroup analysis: cancer stage, Outcome 2 Antidepressants versus antidepressants.
6. Gender
This analysis is encompassed in the 'cancer site' analysis, because the 'female participant' subgroup matches with the 'breast cancer' subgroup (see Analysis 10.1). A subgroup analysis for men only was not feasible, since other studies enrolled both male and female participants.
Sensitivity analyses
1. Excluding trials in which the randomisation process is not clearly reported
We did not perform this sensitivity analysis because no studies, with the exception of Fisch 2003, reported clear details on random sequence generation and concealment of random allocation.
2. Excluding trials with unclear concealment of random allocation
See above.
3. Excluding trials that did not employ adequate blinding of participants, healthcare providers and outcome assessors
We did not perform this sensitivity analysis because no studies reported clear details on the procedures for ensuring blinding.
4. Excluding trials that did not employ depressive symptoms as their primary outcome
Only one study assessed depressive symptoms as a secondary outcome (Fisch 2003), and it contributed only to the 'antidepressants versus placebo' analysis. Results from this sensitivity analysis did not materially change the main findings for the primary outcome (see Analysis 12.1).
12.1. Analysis.
Comparison 12 Sensitivity analysis: excluding trials that did not employ depressive symptoms as their primary outcome, Outcome 1 Antidepressants versus placebo.
5. Excluding trials with imputed data
Five studies did not impute missing data, applying a 'per protocol' or an 'as treated' analysis (EUCTR2008‐002159‐25‐FR; Fisch 2003; Navari 2008; Razavi 1996; Van Heeringen 1996). These studies contributed only to the 'antidepressants versus placebo' analysis. After removing trials with imputed data the meta‐analysis still did not show a statistically significant superiority of antidepressants over placebo, with a SMD of −0.64(95% CI ‐1.35 to 0.06, four trials, 231 participants) (see Analysis 13.1).
13.1. Analysis.
Comparison 13 Sensitivity analysis: excluding trials with imputed data, Outcome 1 Antidepressants versus placebo.
Discussion
Summary of main results
We included a total of ten randomised controlled trials (RCTs), involving 885 participants, in the present systematic review. The included studies did not report all the outcomes that were prespecified in the protocol. Seven of the RCTs provided continuous data, which contributed to the meta‐analysis for the primary outcome (Analysis 1.1; Analysis 1.2). Only one study (Navari 2008) did not provide data suitable for the meta‐analysis. The majority of studies provided detailed data on dropouts, while for some other secondary outcomes very few trials provided data (Analysis 4.1; Analysis 5.1; Analysis 5.2). Compared to the previous version of this systematic review, our updated electronic search and handsearch for new studies (and for new data on previously ongoing and 'awaiting classification' studies), allowed us to identify new data from one study (NCT00387348). However, this study contributed only to secondary outcomes (in particular Analysis 2.1, Analysis 7.1, Analysis 8.1) because of its relatively short follow‐up period (only four weeks). Therefore, the main data from the previous version of this systematic review and meta‐analysis remains unchanged.
Overall, we detected no evidence of a difference between antidepressants as a class and placebo in terms of efficacy (both on continuous and dichotomous outcomes), acceptability (dropouts due to any cause), and tolerability (dropouts due to adverse events). For the primary outcome ('efficacy as a continuous outcome at 6 to 12 weeks') we found only mianserin to be effective over placebo. For the primary outcome, the sensitivity analysis excluding trials with imputed data gave similar results. We cannot rule out benefit in the early response phase (one to four weeks), but this comes from an analysis with substantial statistical variation. No trials assessed follow‐up response (more than 12 weeks). In head‐to‐head comparisons, we retrieved only data for selective serotonin reuptake inhibitors (SSRIs) versus tricyclic antidepressants (TCAs) and found no difference between these two classes.
For the secondary outcome 'remission rate at 6 to 12 weeks', we found no differences for both the antidepressant‐placebo and the head‐to‐head comparisons. Very few studies contributed to the secondary outcomes 'social adjustment' and 'quality of life', and thus no relevant findings emerged. For the secondary outcome, we found only mianserin to have statistically significant lower dropouts due to inefficacy and dropouts due to any cause compared with placebo. In head‐to‐head comparisons we retrieved only data for SSRIs versus TCAs and found no difference between these two classes.
Overall completeness and applicability of evidence
The study population was quite homogeneous in terms of cancer diagnosis. The vast majority of people were affected by breast cancer. Some degree of heterogeneity was found in terms of stage of cancer, anti‐cancer treatments and psychiatric diagnosis, including different depressive conditions. The overall number of participants was very low, and thus this population could hardly reflect the complexity of people with cancer from a 'real world' setting. Furthermore, it is worth noting that no studies were conducted in older people only, despite this population representing a relevant part of the oncologic population.
The majority of studies enrolled a very small number of participants and did not provide data for all the outcomes specified in the protocol. For these reasons most of the analyses were underpowered and this relevantly limits the overall completeness of evidence. In particular, we chose to consider efficacy as a continuous outcome at 6 to 12 weeks as the primary outcome, being in our opinion a more reliable outcome for people in clinical practice. However, we had to exclude some trials from this analysis, because they did not report continuous outcomes or they performed the assessment at a different time point.
Another important issue was retrieving data from unpublished studies. Even though we found a relatively consistent number of unpublished trials in the above mentioned online registers, reliable data which we could included in the meta‐analysis were not available. Very few authors replied to our request for information or data and only one unpublished study was included. One trial was clearly ongoing and we classified four studies as 'awaiting classification', as they were eligible according to the protocol or the abstract, but did not provide any data feasible for the meta‐analysis. Considering the overall small number of studies included and the uncertainty of the meta‐analysis results, it is plausible that these studies could have made a relevant difference to our analysis.
We chose to consider only the dropout rate due to adverse events as a proxy of the tolerability of treatments because in this particular population the most common side effects of antidepressants (e.g. asthenia, sedation, headache, nausea and gastrointestinal problems) are very likely to be caused also by other anti‐cancer therapies, pain syndromes or the direct effects of cancer. We know from previous literature that antidepressants are generally well tolerated by people with medical illness (Rayner 2010), even when very complex and advanced (including people with cancer) (Rayner 2011a). However, some authors showed possible toxicities of antidepressants in this population (Stockler 2007), and recent findings raised the issue of possible cardiac effects of citalopram and escitalopram (Nosé 2016; Sarganas 2014), which may be particularly relevant for people with cancer. For this reason, further analysis may be relevant for assessing the occurrence of adverse effects likely linked to the assumption of antidepressants.
It has been suggested that the efficacy of tamoxifen, a drug broadly used for prevention and treatment of breast cancer, could be lessened by some antidepressants that act on CYP2D6 inhibitors. This would therefore worsen the prognosis of these people in a five‐year period (Kelly 2010). The most relevant effect as been shown for paroxetine, however other drugs — such as fluoxetine, bupropion and duloxetine — could theoretically have a similar effect, and should be therefore avoided in these patients (Andrade 2012). This possible effect is unlikely to have affected our analysis, since two studies used paroxetine (Musselman 2006; Pezzella 2001), and only one (Musselman 2006) included participants possibly taking tamoxifen, and the follow‐up period was relatively short to appreciate this potentially harmful effect.
Quality of the evidence
The overall methodological quality of the included studies was poor (see Figure 2; Figure 3). Only one study (NCT00387348) showed an overall low risk of bias, however this study was severely limited by the low number of included participants (only 24), and contributed only to secondary analyses. The majority of studies showed mixed features, with the large prevalence of an 'unclear risk' of bias in different domains, which seems to reflect the lack of exhaustive reporting rather than a clear evidence of bias. This is consistent with the finding of general suboptimal reporting of RCTs in medical journals despite the large diffusion of instruments designed to help transparent reporting, such as the CONSORT Statement (Turner 2012).
The GRADEpro Guideline Development Tool (GDT) is a web‐based tool for summarizing and evaluating the certainty of evidence from scientific data, including systematic reviews and meta‐analyses (Guyatt 2008). The output of this process is represented in 'Summary of findings' tables, which are the basis for developing evidence‐based healthcare guidelines according to the GRADE approach (Andrews 2013; Ostuzzi 2013). We employed the GRADEpro GDT to provide outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison and the magnitude of effect of the interventions examined. Our overall confidence in the estimate of effect was 'very low' for all of the main outcomes assessed (see Table 1; Table 2). This judgement reflects some issues in the included studies, namely the high risk of bias (due to poor methodological quality and high dropout rates), inconsistency (due to the high degree of heterogeneity between studies) and imprecision (due to the low number of participants in each trial and wide confidence intervals). In accordance with this, any estimate of effect in this review should be considered very uncertain, and further research is very likely to change the estimate of effect and thus the degree of confidence for its applicability in routine clinical practice.
Potential biases in the review process
There are several possible limitations of this review, and thus the interpretation of results should remain provisional and tentative.
Some limitations are intrinsically related to the actual process of retrieving, collecting, selecting and extracting data. In order to reduce the potential bias of this complex process, two review authors independently worked on each of these steps. With regards to the selection of relevant studies, the degree of agreement between the two authors was evaluated with the calculation of 'simple kappa statistics', which confirmed the reliability of the selection process (see Results of the search). It has been highlighted that data extraction done by two independent extractors is, overall, more reliable than the extraction performed by a single author followed by verification by a second author (Buscemi 2006). We applied the same process for the 'Risk of bias' assessment. Furthermore, disagreements were discussed with a third author, who also checked the data extracted from RCTs when the analysis was performed. Another relevant problem concerns the 'systematic' nature of the search. We chose to include only randomised trials as they provide the strongest level of evidence available. In this type of review there is some risk of publication bias, which means that negative studies may have not been published. Some authors of this review are expert in the field, thus it is unlikely that significant studies were overlooked. However, whilst the search was thorough, it is possible that there are still unpublished studies which have not been identified, considering that there are no shared procedures to perform this kind of search (Chan 2012). The impact of unpublished literature on the results of this review is uncertain, however it is expected that the analysis of only published literature would lead to overestimation of the efficacy of a given intervention (Turner 2008). Moreover, the search date is June 2017 and there are two studies classified as 'awaiting classification', the eligibility of which is yet to be determined. At the end of this process, we identified very few studies and the data of interest obtained were relatively limited.
It is important to consider that some of the included studies were funded by the pharmaceutical industry, and this may again introduce an overestimation of the efficacy of interventions.
To assess efficacy, we gave preference to rating scales administered by clinicians or expert assessors (Hamilton Rating Scale for Depression ‐ HRSD, Montgomery and Åsberg Depression Rating Scale ‐ MADRS, Clinical Global Impression Rating scale ‐ CGI). Even though they are standardised tools commonly used in antidepressant trials, they are all potentially prone to observer bias. In three studies self‐administered questionnaires were used (EUCTR2008‐002159‐25‐FR; Fisch 2003; Navari 2008). We noted some heterogeneity in terms of outcome measurement, and this might represent a limitation in interpreting the effect of interventions. For instance, in Analysis 1.1, Analysis 2.1, Analysis 6.1 and Analysis 8.1 only the study by Van Heeringen 1996 shows a clear beneficial effect of the antidepressant (in this case, mianserin) over placebo, which deeply affects the final result of the meta‐analyses. In general, the positive effect shown in the mianserin studies (Costa 1985; Van Heeringen 1996) had a relevant impact on overall results (see Analysis 2.1; Analysis 3.1). Another limitation is the use of non‐specific rating scales, designed for assessing specific psychiatric symptoms and domains, rather than mood disorders in medically ill people.
One important limitation of the included trials (and consequently of the present review) is that not all studies reported a continuous outcome for the chosen time points, underpowering the analyses and undermining the possibility of finding significant differences between comparisons.
Quality of life (QoL) and social functioning were rarely reported in the included studies. This possibly limits our interpretation of the efficacy of intervention, which should not be focused only on depression, considering that comorbid depressive symptoms deeply impact the overall burden of disease alongside QoL and functioning (Arrieta 2013). Some authors also described a relevant impact of comorbid depression on cancer mortality (Lloyd‐Williams 2009; Pinquart 2010; Satin 2009). This outcome was not described in the included studies, due to relatively short periods of follow‐up.
The dropout rate due to any cause is considered a consistent measure for the acceptability of treatment, as it encompasses not only dropouts due to adverse events, but also due to inefficacy and any other cause. However, this is only a proxy measure for this outcome since it comprises very heterogeneous reasons for leaving the study early, detailed description of which was beyond the aim of this review.
For one three‐armed study (Musselman 2006) which compared paroxetine versus desipramine versus placebo, we chose to split the 'shared' group (in this case the placebo group) into two groups with smaller sample size, in order not to report in the analysis the same subpopulation of patients. These smaller groups contributed to one comparison each (namely paroxetine versus placebo and desipramine versus placebo). In the analysis of dichotomous outcomes the number of events was also split between the two comparisons. This method, although considered reliable according to the Cochrane Handbook for Systematic Reviews of Interventions (16.5.4) (Higgins 2011), is not the most recommended since it only partially overcomes the unit of analysis error (because the resulting comparisons remain correlated). In this case, however, this approach allowed us to perform a detailed subgroup analysis for antidepressant classes. Alternatively, the two antidepressant arms should have been pooled together and compared with the placebo group. However, these two drugs have different mechanisms of action and thus are not expected to share a 'class effect', and this would have created an artificial arm, which does not exist in clinical practice.
Finally, it is very relevant to note that people suffering from different types and stages of cancer can hardly be considered as a homogeneous group, considering there are several differences in genetic, biological and immunological mechanisms, as well as in physical and psychosocial impairment. Due to the paucity of data, several subgroup analyses that would have investigated these characteristics were not feasible. We were able to perform only a few subgroup analyses, which were underpinned by poor data. We interpreted the results from these analyses cautiously, since multiple calculations may risk producing a result that is statistically significant by chance alone.
Agreements and disagreements with other studies or reviews
Analyses from this study draw a different picture with respect to previous reviews and meta‐analyses. Results from the meta‐analyses by Hart 2012 and Walker 2014 are hardly comparable to the present study, since they enrolled only patients with "elevated depressive symptoms" and a formal diagnosis of major depression, respectively. Conversely, the meta‐analysis by Laoutidis 2013 included the same studies as our review, with the only difference of two (rather small) unpublished studies (EUCTR2008‐002159‐25‐FR; NCT00387348). In Laoutidis 2013, a superiority of antidepressants versus placebo in terms of 'therapeutic response' (as a dichotomous outcome) was shown, with a risk ratio of 1.56 (95% confidence interval (CI) 1.07 to 2.28, P = 0.021). Their analysis slightly differs from the one performed in the present systematic review, where we found no statistically significant difference (see Analysis 3.1). In contrast with the meta‐analysis by Laoutidis 2013, the study carried out by Navari and colleagues (Navari 2008) was not eligible for our analysis as our focus was the 'acute phase treatment response' (between 6 and 12 weeks), while this study reported the number of responders at week 24. Other differences refer to different approaches employed in the definition of some intention‐to‐treat (ITT) populations. Moreover, in Laoutidis 2013 no analyses of continuous outcomes were performed and, similarly to our analysis, no differences between SSRIs and TCAs were found. Additionally, the review and meta‐analysis by Riblet 2014 is difficult to compare with the present one, as it included some trials that were excluded from our analysis, in particular one quasi‐randomised trial (Wang 2011), and two trials where patients were not depressed at baseline (Del Carmen 1990; Roscoe 2005).
The use of antidepressants in people with cancer has been studied in many different ways in the scientific literature, focusing not only on treating depressive symptoms or disorders, but also on preventing depression (e.g. Morrow 2003, in which antidepressants appeared effective in a population of 549 patients), or treating some cancer‐related symptoms, such as hot flushes, fatigue, insomnia, hyporexia and weigh loss, etc. For the majority of these studies people were enrolled on the basis of medical symptoms and a proper assessment of concomitant depressive conditions was not always performed. These studies were not included in the present review, however they may contribute to broaden the discussion about the clinical suitability of antidepressants in people with cancer, since it has been claimed that a continuum of depressive experiences, ranging from distressing cancer‐related symptoms to proper depressive symptoms or disorders, can be detected in this population (Brenne 2013; Mitchell 2011; Raison 2003) and can be effectively treated with antidepressants (Ostuzzi 2015).
Some non‐randomised studies were retrieved (Biglia 2005; Caldera 2009; Evans 1988; KCT0000076; NCT00234195; NCT01725048; Tondlova 1997), however for most of them only conference procedures or protocols were available. Moreover, results from the remaining studies can hardly provide a relevant contribution to the discussion, since they were performed on very small populations of patients (Biglia 2005; Evans 1988).
We did not retrieve any ongoing studies, and classified two studies as 'awaiting classification' (UMIN000008768; N0405078066 ). Data from these studies, even partial or provisional, were not available, thus their possible impact remains unclear.
Given the relevant amount of literature on this topic, the role of antidepressant drugs in this group of people seems to represent a relevant issue in routine clinical practice. However, clear indications from this heterogeneous literature cannot be easily derived.
Authors' conclusions
Implications for practice.
There is a very low number of randomised trials assessing the efficacy of antidepressants in cancer patients, despite the relevance of this issue. Moreover, evidence for the effects we have found in terms of the efficacy and acceptability of antidepressants in people with cancer is of very low quality. Data from the present review failed to reveal any statistically significant beneficial effect of these drugs over placebo, with the only exception of mianserin (see Figure 4). Although this drug was compared with placebo in two studies only, with small numbers of included participants, it showed some beneficial effects in terms of efficacy and acceptability. Mianserin is often used in oncological settings for its beneficial profile on sleep and appetite, as well as mood. Conversely, this drug is seldom used in routine clinical practice in psychiatric settings and very few data from randomised controlled trials (RCTs) are available on its efficacy in people with major depression. This compound is considered to have a similar profile to mirtazapine, the efficacy of which has been largely shown, but with a possible unfavourable tolerability profile with respect to selective serotonin reuptake inhibitors (SSRIs) (Cipriani 2009). The efficacy, tolerability and acceptability of these drugs in severely medically ill people is yet to be assessed. Thus, the clinical meaning of these results is uncertain and no clear implications for clinical practice can be drawn. Similarly, no significant differences between one drug and another emerged (see Figure 5).
Finding an appropriate treatment for depressive symptoms in people with cancer is a relevant goal in routine clinical practice, as shown by the ongoing discussion in the scientific literature. There is a growing awareness of the need for a multi‐dimensional approach, encompassing biological, social and psychological issues, as highlighted by previous reviews (Akechi 2008; Galway 2012). A proper evaluation of subthreshold depressive symptoms seems essential, also considering their potentially relevant impact on the prognosis of cancer, although it is not easy to discern when it is worthwhile to introduce an antidepressant. Very few and unspecific indications could be derived from the available guidelines (NICE 2009; Rayner 2011b). In general, based on the results of the current review, the possible role of antidepressants is still controversial and should be assessed each time by the clinician on an individual basis. The choice of which antidepressant to prescribe can hardly be made on the basis of this review; rather, it may be based on the data on antidepressant efficacy in the general population of individuals with major depression. Additionally, the data on antidepressant efficacy in medically ill people — which suggest a positive safety profile of SSRIs (Rayner 2010; Rayner 2011a) — may also be considered.
Implications for research.
The results described in this systematic review come from evidence of very low certainty according to the GRADE methodology. Moreover, in many cases studies were financially supported by pharmaceutical industries. Consequently, there is a high risk that these studies do not provide sufficient and adequate information for clinicians in real‐world settings. The present review highlights the strong need for further studies, which should be conducted to high methodological standards and with the primary intent of providing clinicians with useful practical data on the effectiveness of antidepressant drugs, firstly over placebo and subsequently in head‐to‐head comparisons. Alongside rating scales, pragmatic outcome measures, such as quality of life and social functioning, should also be considered.
Despite the high prevalence of depression in people with cancer and its substantial impact, the number of randomised trials assessing the efficacy of antidepressants in oncology is still very low. We recognise that these studies are extremely difficult to conduct, as depression is not always considered a major concern by doctors and by people with cancer, who are sometimes reluctant to admit its existence. Moreover, promoting this type of trial may be not considered as a priority for anti‐cancer research funding agencies.
Further basic research on the pathogenetic pathways of depression in medically ill people is needed. This could be helpful for identifying possible therapeutic targets, and would also allow the assessment of new, possibly effective drugs with comparative study designs. In recent years, we witnessed a growing interest in detecting possible specific mechanisms involved in pathogenesis of depressive experiences in different types of cancer (Bowinik 2014; Sotelo 2014).
Generally SSRIs are considered to have a good therapeutic index among antidepressants. However, some other antidepressants could be theoretically helpful in this particular population, being possibly effective not only for depression, but also for medical symptoms. For example, some non‐controlled studies are available on the effect of mirtazapine for insomnia and hyporexia, or duloxetine for pain perception, hot flushes and so on. In actuality no randomised trials in people with cancer are available with these compounds.
In line with the conclusions from the previous version of this review, in order to increase the evidence on the compelling issue of depressive symptoms in people with cancer, there is a need for large, simple, pragmatic RCTs comparing commonly used antidepressants (SSRIs, serotonin‐norepinephrine reuptake inhibitor (SNRIs), mirtazapine) versus placebo in individuals with cancer and depressive symptoms, with or without a formal diagnosis of a depressive disorder.
What's new
| Date | Event | Description |
|---|---|---|
| 3 July 2017 | New search has been performed | We updated the literature searches and revised the flow‐chart describing study selection according to the additional search performed. |
| 3 July 2017 | New citation required but conclusions have not changed | We identified one additional unpublished study which contributed data to some of the secondary analysis. The overall conclusions of the review did not change. |
Acknowledgements
The authors thank Robin Grant and Alasdair Rooney for their clinical expertise, Gail Quinn and Clare Jess (Managing Editors), Tracey Harrison (Assistant Managing Editor), Joanne Platt (Cochrane Information Specialist) from Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers. They also thank Dr Chin‐Kuo Chan (BPH, MS, PHD, King's College London) and Dr Irina Telegina (MD, Astrakhan State Medical Academy) for having kindly provided their help with translating from Chinese and from Russian respectively.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Neoplasms] explode all trees #2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocrcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma* ) #3 #1 or #2 #4 MeSH descriptor: [Depression] explode all trees #5 MeSH descriptor: [Depressive Disorder] explode all trees #6 MeSH descriptor: [Adjustment Disorders] explode all trees #7 (depress* or melanchol* or ((adjustment or reactive or dysthymic) near/5 disorder*)) #8 #4 or #5 or #6 or #7 #9 Any MeSH descriptor with qualifier(s): [Drug therapy ‐ DT] #10 MeSH descriptor: [Antidepressive Agents] explode all trees #11 MeSH descriptor: [Heterocyclic Compounds] explode all trees #12 MeSH descriptor: [Serotonin Uptake Inhibitors] explode all trees #13 MeSH descriptor: [Adrenergic Uptake Inhibitors] explode all trees #14 MeSH descriptor: [Monoamine Oxidase Inhibitors] explode all trees #15 (desipramine or imipramine or clomipramine or opipramol or trimipramine or lofepramine or dibenzepin or amitriptyline or nortriptyline or protriptyline or doxepin or iprindole or melitracen or butriptyline or dosulepin or amoxapine or dimetacrine or amineptine or maprotiline or quinupramine or zimeldine or fluoxetine or citalopram or paroxetine or sertraline or alaproclate or fluvoxamine or etoperidone or escitalopram or isocarboxazid or nialamide or phenelzine or tranylcypromine or iproniazide or iproclozide or moclobemide or toloxatone or oxitriptan or tryptophan or mianserin or nomifensine or trazodone or nefazodone or minaprine or bifemelane or viloxazine or oxaflozane or mirtazapine or bupropion or medifoxamine or tianeptine or pivagabine or venlafaxine or milnacipran or reboxetine or gepirone or duloxetine or agomelatine or desvenlafaxine or vilazodone or hyperici herba or hypericum perforatum or st john* wort* or saint john* wort*) #16 (anti‐depress* or antidepress* or drug therap* or pharmacotherap* or trycyclic* or TCA* or heterocyclic* or serotonin uptake or SSRI* or SNRI* or monoamine oxidase inhibitor* or MAOI*) #17 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 #3 and #8 and #17
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Neoplasms/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or lymphoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).mp. 3 1 or 2 4 Depression/ 5 exp Depressive Disorder/ 6 Adjustment Disorders/ 7 (depress* or melanchol* or ((adjustment or reactive or dysthymic) adj5 disorder*)).mp. 8 4 or 5 or 6 or 7 9 drug therapy.fs. 10 exp Antidepressive Agents/ 11 exp Heterocyclic Compounds/ 12 exp Serotonin Uptake Inhibitors/ 13 exp Adrenergic Uptake Inhibitors/ 14 exp Monoamine Oxidase Inhibitors/ 15 (anti‐depress* or antidepress* or drug therap* or pharmacotherap* or trycyclic* or TCA* or heterocyclic* or serotonin uptake or SSRI* or SNRI* or monoamine oxidase inhibitor* or MAOI*).mp. 16 (desipramine or imipramine or clomipramine or opipramol or trimipramine or lofepramine or dibenzepin or amitriptyline or nortriptyline or protriptyline or doxepin or iprindole or melitracen or butriptyline or dosulepin or amoxapine or dimetacrine or amineptine or maprotiline or quinupramine or zimeldine or fluoxetine or citalopram or paroxetine or sertraline or alaproclate or fluvoxamine or etoperidone or escitalopram or isocarboxazid or nialamide or phenelzine or tranylcypromine or iproniazide or iproclozide or moclobemide or toloxatone or oxitriptan or tryptophan or mianserin or nomifensine or trazodone or nefazodone or minaprine or bifemelane or viloxazine or oxaflozane or mirtazapine or bupropion or medifoxamine or tianeptine or pivagabine or venlafaxine or milnacipran or reboxetine or gepirone or duloxetine or agomelatine or desvenlafaxine or vilazodone or hyperici herba or hypericum perforatum or st john* wort* or saint john* wort*).mp. 17 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18 3 and 8 and 17 19 randomized controlled trial.pt. 20 controlled clinical trial.pt. 21 randomized.ab. 22 placebo.ab. 23 clinical trials as topic.sh. 24 randomly.ab. 25 trial.ti. 26 19 or 20 or 21 or 22 or 23 or 24 or 25 27 18 and 26 28 exp animals/ not humans.sh. 29 27 not 28
key:
mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier
pt = publication type ab = abstract sh = subject heading ti = title
Appendix 3. Embase (Ovid) search strategy
1 exp neoplasm/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocrcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).ti,ab. 3 1 or 2 4 exp depression/ 5 adjustment disorder/ 6 (depress* or melanchol* or ((adjustment or reactive or dysthymic) adj3 disorder*)).ti,ab. 7 4 or 5 or 6 8 exp antidepressant agent/ 9 exp heterocyclic compound/ 10 exp serotonin uptake inhibitor/ 11 exp adrenergic receptor affecting agent/ 12 exp monoamine oxidase inhibitor/ 13 (anti‐depress* or antidepress* or drug therap* or pharmacotherap* or trycyclic* or TCA* or heterocyclic* or serotonin uptake or SSRI* or SNRI* or monoamine oxidase inhibitor* or MAOI*).ti,ab. 14 (desipramine or imipramine or clomipramine or opipramol or trimipramine or lofepramine or dibenzepin or amitriptyline or nortriptyline or protriptyline or doxepin or iprindole or melitracen or butriptyline or dosulepin or amoxapine or dimetacrine or amineptine or maprotiline or quinupramine or zimeldine or fluoxetine or citalopram or paroxetine or sertraline or alaproclate or fluvoxamine or etoperidone or escitalopram or isocarboxazid or nialamide or phenelzine or tranylcypromine or iproniazide or iproclozide or moclobemide or toloxatone or oxitriptan or tryptophan or mianserin or nomifensine or trazodone or nefazodone or minaprine or bifemelane or viloxazine or oxaflozane or mirtazapine or bupropion or medifoxamine or tianeptine or pivagabine or venlafaxine or milnacipran or reboxetine or gepirone or duloxetine or agomelatine or desvenlafaxine or vilazodone or hyperici herba or hypericum perforatum or st john* wort* or saint john* wort*).ti,ab. 15 8 or 9 or 10 or 11 or 12 or 13 or 14 16 3 and 7 and 15 17 crossover procedure/ 18 double‐blind procedure/ 19 randomized controlled trial/ 20 single‐blind procedure/ 21 random*.mp. 22 factorial*.mp. 23 (crossover* or cross over* or cross‐over*).mp. 24 placebo*.mp. 25 (double* adj blind*).mp. 26 (singl* adj blind*).mp. 27 assign*.mp. 28 allocat*.mp. 29 volunteer*.mp. 30 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31 16 and 30 32 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 33 31 not 32
key: [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 4. PsycINFO search strategy
1 exp Neoplasms/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocrcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).ti,ab. 3 1 or 2 4 "depression (emotion)"/ 5 exp major depression/ 6 (depress* or melanchol* or ((adjustment or reactive or dysthymic) adj3 disorder*)).ti,ab. 7 4 or 5 or 6 8 exp antidepressant drugs/ 9 exp neurotransmitter uptake inhibitors/ 10 exp monoamine oxidase inhibitors/ 11 exp Drug Therapy/ 12 (anti‐depress* or antidepress* or drug therap* or pharmacotherap* or trycyclic* or TCA* or heterocyclic* or serotonin uptake or SSRI* or SNRI* or monoamine oxidase inhibitor* or MAOI*).ti,ab. 13 (desipramine or imipramine or clomipramine or opipramol or trimipramine or lofepramine or dibenzepin or amitriptyline or nortriptyline or protriptyline or doxepin or iprindole or melitracen or butriptyline or dosulepin or amoxapine or dimetacrine or amineptine or maprotiline or quinupramine or zimeldine or fluoxetine or citalopram or paroxetine or sertraline or alaproclate or fluvoxamine or etoperidone or escitalopram or isocarboxazid or nialamide or phenelzine or tranylcypromine or iproniazide or iproclozide or moclobemide or toloxatone or oxitriptan or tryptophan or mianserin or nomifensine or trazodone or nefazodone or minaprine or bifemelane or viloxazine or oxaflozane or mirtazapine or bupropion or medifoxamine or tianeptine or pivagabine or venlafaxine or milnacipran or reboxetine or gepirone or duloxetine or agomelatine or desvenlafaxine or vilazodone or hyperici herba or hypericum perforatum or st john* wort* or saint john* wort*).ti,ab. 14 8 or 9 or 10 or 11 or 12 or 13 15 3 and 7 and 14 16 clinical trials/ 17 (random* or trial* or group* or placebo*).ti,ab. 18 16 or 17 19 15 and 18
Appendix 5. Data collection sheet
Review author name (GO; FM; CB)
1. First author, Year and Journal ___________ 2. Comparisons: AD1 __________________________________ AD2 __________________________________ AD3 __________________________________ PLB yes [ ] no [ ] 3. Weeks of follow‐up |___||___| (insert the longest duration of randomised follow‐up) 4. Randomisation |___| 0 = unclear
1 = clearly reported authors’ statement____________ (If it is unclear please report the authors’ statement)
5. Double blinding |___| 0 = unclear 1 = yes 2 = no
6. Concealment allocation |___| 0 = unclear 1 = yes (clearly mentioned according to the Cochrane Handbook)
7. AD1 sample |___||___||___| AD2 sample |___||___||___| AD3 sample |___||___||___| PLB sample |___||___||___| (Please insert the number of patients randomised to receive each AD drug)
8. Setting |___| 0 = unclear 2 = outpatients 1 = inpatients 3 = in and outpatients
9. Type of participants |___| 0 = unclear 1 = major depressive disorder 3 = dysthymic disorder 2 = adjustment disorders 4 = depressive symptoms (rating scales) ‘depression’ definition (authors’ statement)____________ (If it is unclear please report the authors’ statement)
10. Diagnostic criteria for 'depression' or depressive symptoms |___| 0 = unclear 3 = ICD‐10, DSM‐IV 1 = DSM‐III 4 = rating scales (HRSD, BDI, etc.) 2 = DSM III‐R 5 = implicit criteria (e.g. ICD‐9) diagnostic criteria (authors’ statement)_______________ (If it is unclear please report the authors’ statement)
11. Depressive symptoms employed as |___| 0 = primary trial outcome 1 = secondary trial outcome
12. Previous history of depression |___| 0 = exclusion criteria 1 = patients included N |_________| % |_________|
13. Elderly patients |___| 0 = unclear 2 = yes, some elderly (> 65 year old) patients 1 = no 3 = yes, all are 65 years old or older
14. Gender of patients male |________________________| N |_________| % |_________| female |________________________| N |_________| % |_________|
15. Cancer site (If the study includes a population with mixed cancer diagnosis, please insert the number and/or the percentage of patients for each site. If it is unclear please report the authors’ statement) site 1 |________________________| N |_________| % |_________| site 2 |________________________| N |_________| % |_________| site 3 |________________________| N |_________| % |_________| site 4 |________________________| N |_________| % |_________| site 5 |________________________| N |_________| % |_________| cancer site (authors’ statement)____________
16. Cancer stage |______________________________|
(If the study includes a population with mixed cancer diagnosis, please insert the number and/or the percentage of patients for each stage. If it is unclear please report the authors’ statement) 0 = unclear 1 = Stage 0 (carcinoma in situ; early form) N |_________| % |_________| 2 = Stage I (localised) N |_________| % |_________| 3 = Stage II (early locally advanced) N |_________| % |_________| 4 = Stage III (late locally advanced) N |_________| % |_________| 5 = Stage IV (metastasised) N |_________| % |_________| cancer stage (authors’ statement)______________________________________
17. Cancer treatment |___| (If the study includes a population with mixed cancer diagnosis, please insert the number and/or the percentage of patients for each treatment. If it is unclear please report the authors’ statement) 0 = unclear 1 = chemotherapy N |_________| % |_________| 2 = radiotherapy N |_________| % |_________| 2 = surgery N |_________| % |_________| 3 = other treatment |__________________________| N |_________| % |_________| cancer stage (authors’ statement)_____________
18. Severe adverse events (if the type or the number of adverse events are not reported or are unclearly reported, please report the authors’ statement) 1. ______________________ N |_________| % |_________| 2. ______________________ N |_________| % |_________| 3. ______________________ N |_________| % |_________| 4. ______________________ N |_________| % |_________| adverse events (authors’ statement)______________________________________
19. Antidepressant (AD) doses AD1 dose *METHODS |___||___||___| ‐ |___||___||___| r = unclear N.B. Is this a fixed or flexible dosing schedule? Fixed Flexible * (Please consider the range of ID dose reported in the method section of the study report) **RESULTS |___||___||___| . |___||___| SD |___||___|.|___| r = unclear N.B. Is this a mean dose? Yes No ** (Please consider the average ID dose administered during the study period or, if this figure is not available, consider the average ID dose received by the majority of patients) D2 dose *METHODS |___||___||___| ‐ |___||___||___| r = unclear N.B. Is this a fixed or flexible dosing schedule? Fixed Flexible **RESULTS |___||___||___| . |___||___| SD |___||___|.|___| r = unclear N.B. Is this a mean dose? Yes No AD3 dose *METHODS |___||___||___| ‐ |___||___||___| r = unclear N.B. Is this a fixed or flexible dosing schedule? Fixed Flexible **RESULTS |___||___||___| . |___||___| SD |___||___|.|___| r = unclear N.B. Is this a mean dose? Yes No
20. Mean score AT BASELINE: r = unclear/no data available AD1 N |___||___||___| HDRS |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) * Specify the N. of items in HDRS |___||___| N |___||___||___| MADRS |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| CGI |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (quality of life) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (social adjustment) AD2 N |___||___||___| HDRS |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| MADRS |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| CGI |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (quality of life) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (social adjustment) AD3 N |___||___||___| HDRS |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| MADRS |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| CGI |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (quality of life) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (social adjustment) PLACEBO N |___||___||___| HDRS |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| MADRS |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| CGI |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (quality of life) N |___||___||___| _____ |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (social adjustment)
EFFICACY AS A CONTINUOUS OUTCOME
21. ENDPOINT RESPONSE WEEK …..……(choose the time point given in the original study as the study endpoint) Mean score: r = unclear Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (Please insert the number of evaluable subjects at follow‐up, the mean score at follow‐up at the HDRS or MADRS or CGI or any other rating scale. If the study used the LOCF, record the values based on the LOCF. If the SD is not available extract the standard error)
22. 1 to 4 weeks RESPONSE RATE WEEK ………(choose the time point closest to week 2) Mean score: r = unclear Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (Please insert the number of evaluable subjects at follow‐up, the mean score at follow‐up at the HDRS or MADRS or CGI or any other rating scale. If the study used the LOCF, record the values based on the LOCF. If the SD is not available extract the standard error)
23. 6 to 12 weeks RESPONSE RATE WEEK ………(choose the time point closest to the original study endpoint) Mean score: r = unclear Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) (Please insert the number of evaluable subjects at follow‐up, the mean score at follow‐up at the HDRS or MADRS or CGI or any other rating scale. If the study used the LOCF, record the values based on the LOCF. If the SD is not available extract the standard error)
24. 14 to 24 weeks RESPONSE RATE WEEK ………(choose the time point closest to week 24) Mean score: r = unclear Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|)
(Please insert the number of evaluable subjects at follow‐up, the mean score at follow‐up at the HDRS or MADRS or CGI or any other rating scale. If the study used the LOCF, record the values based on the LOCF. If the SD is not available extract the standard error)
EFFICACY AS A DICHOTOMOUS OUTCOME
25.ENDPOINT RESPONSE RATE (6 to 12 weeks) WEEK …..……(choose the time point closest to the original study endpoint) 50% or greater reduction on ___________________ AD1 50% reduction RESPONDERS |___||___||___| out of |___||___||___| r = unclear AD2 50% reduction RESPONDERS |___||___||___| out of |___||___||___| AD3 50% reduction RESPONDERS |___||___||___| out of |___||___||___| Placebo 50% reduction RESPONDERS |___||___||___| out of |___||___||___| (Please insert which rating scale has been used, the number of patients with a 50% or more improvement ‐ at the HAM‐D, MADRS, or any other depression scale ‐, and the number of included patients at that time point. Typically, a trial would include N patients, but include N – p – q patients in the assessment, as these p patients have never returned and are hence excluded even from the LOCF analyses and q patients drop out in the course of the treatment and their last observed values are carried forward; in this instance, if q patients are somehow accounted for at the time point in question, then, N – p would be the denominator here. In some instances, only responders among N – p – q patients are reported.)
AD1 CGI‐I RESPONDERS |___||___||___| out of |___||___||___| r = unclear AD2 CGI‐I RESPONDERS |___||___||___| out of |___||___||___| AD3 CGI‐I RESPONDERS |___||___||___| out of |___||___||___| Placebo CGI‐I RESPONDERS |___||___||___| out of |___||___||___| (Please insert the number of patients 'much or very much improved' on CGI‐Improvement, and the number of included patients at that time point.)
26. SOCIAL ADJUSTMENT (GAF and others) (6 to 12 weeks) WEEK …..…… (choose the time point closest to the original study endpoint) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) 27. HEALTH‐RELATED QUALITY OF LIFE (6 to 12 weeks) WEEK …..…… (choose the time point closest to the original study endpoint) (give preference to EORTC QLQ‐30, FACT, SF‐36 and other to illness‐specific QoL scales, where available) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Rating scale:______________________________ AD1 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD2 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) AD3 N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|) Placebo N |___||___||___| score |___||___||___|.|___||___| SD |___|.|___||___| (SE |___|.|___||___|)
DROPOUT RATE
28. DROPOUTS = patient discontinuing the study before the end of follow‐up r = unclear
| Dropouts due to: |
AD1 number |
AD2 number |
AD3 number |
PLACEBO number |
| A ‐ Inefficacy B ‐ Side effects C ‐ TOTAL* |
||||
* The total number of dropout patients might not be the sum of dropouts for inefficacy and side effects, because in some studies patients drop out from the study for other/unknown reasons
29. Cost analysis |___| 0 = unclear 1 = yes 2 = no
30. Drug company sponsored trial |___| 0 = unclear 1 = yes, sponsored by a drug company 2 = no (A trial is judged 'drug company sponsored' if it is so declared in the conflict of interest or in the acknowledgment or if some of the authors are company employees. There may be other instances, and use your common sense)
31. NOTES
Data and analyses
Comparison 1. Depression: efficacy as a continuous outcome at 6 to 12 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 5 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.01, 0.11] |
| 1.1 SSRIs | 4 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.50, 0.08] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.95, 1.04] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |
| 2 Antidepressants versus antidepressants | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.18] |
| 2.1 Paroxetine versus desipramine | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.73, 0.88] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.46, 0.14] |
| 2.3 Fluoxetine versus desipramine | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.45, 0.83] |
Comparison 2. Depression: efficacy as a continuous outcome at 1 to 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 5 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.72, 0.13] |
| 1.1 SSRIs | 3 | 182 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.27, 0.32] |
| 1.2 Other antidepressants | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.26, ‐0.16] |
Comparison 3. Depression: efficacy as a dichotomous outcome at 6 to 12 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 5 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.62, 1.08] |
| 1.1 SSRIs | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.11] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.42, 2.86] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.75] |
| 2 Antidepressants versus antidepressants | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.78, 1.53] |
| 2.1 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.79, 1.63] |
| 2.2 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.33, 2.18] |
Comparison 4. Social adjustment at 6 to 12 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus antidepressants | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.38, 0.58] |
| 1.1 Paroxetine versus amitriptyline | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.38, 0.58] |
Comparison 5. Quality of life at 6 to 12 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 2 | 152 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.27, 0.37] |
| 1.1 SSRIs | 2 | 152 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.27, 0.37] |
| 2 Antidepressants versus antidepressants | 1 | 153 | Mean Difference (IV, Random, 95% CI) | 6.5 [0.21, 12.79] |
| 2.1 Paroxetine versus amitriptyline | 1 | 153 | Mean Difference (IV, Random, 95% CI) | 6.5 [0.21, 12.79] |
Comparison 6. Dropouts due to inefficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 6 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.32] |
| 1.1 SSRIs | 4 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.10, 7.31] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 2.92 [0.16, 52.47] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.65] |
| 2 Antidepressants versus antidepressants | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.14, 5.06] |
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.14, 5.06] |
Comparison 7. Dropouts due to side effects (tolerability).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 7 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.54, 2.62] |
| 1.1 SSRIs | 5 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.71, 5.57] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.04, 7.25] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.15, 2.35] |
| 2 Antidepressants versus antidepressants | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.55, 1.99] |
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.41, 3.62] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.08] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.18, 16.25] |
Comparison 8. Dropouts due to any cause (acceptability).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 7 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.38] |
| 1.1 SSRIs | 5 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.84, 2.24] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.24, 2.23] |
| 1.3 Other antidepressants | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.25, 0.75] |
| 2 Antidepressants versus antidepressants | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.30] |
| 2.1 Fluoxetine versus desipramine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.29, 1.68] |
| 2.2 Paroxetine versus amitriptyline | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 2.3 Paroxetine versus desipramine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.37, 3.00] |
Comparison 9. Subgroup analysis: psychiatric diagnosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 4 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.23, 0.21] |
| 1.1 Patients with major depressive disorder | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.94, 0.78] |
| 1.2 Patients with adjustment disorder, dysthymic disorder, depressive symptoms | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.67, 0.10] |
| 2 Antidepressants versus antidepressants | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.1 Patients with major depressive disorder | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.2 Patients with adjustment disorder, dysthymic disorder, depressive symptoms | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Subgroup analysis: cancer site.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 5 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.01, 0.11] |
| 1.1 Patients with breast cancer | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.94, 0.78] |
| 1.2 Patients with other cancer types | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.54, 0.06] |
| 2 Antidepressants versus antidepressants | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.18] |
| 2.1 Patients with breast cancer | 2 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.2 Patients with other cancer types | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.45, 0.83] |
Comparison 11. Subgroup analysis: cancer stage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.66, 0.16] |
| 1.1 Patients with an early stage cancer | 1 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.65, 0.31] |
| 1.2 Patients with a late stage cancer | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.30, 0.33] |
| 2 Antidepressants versus antidepressants | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐1.61, 2.99] |
| 2.1 Patients with an early stage cancer | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐1.61, 2.99] |
| 2.2 Patients with a late stage cancer | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Sensitivity analysis: excluding trials that did not employ depressive symptoms as their primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 4 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.23, 0.25] |
| 1.1 SSRIs | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.58, 0.18] |
| 1.2 Tricyclic antidepressants | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.95, 1.04] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |
Comparison 13. Sensitivity analysis: excluding trials with imputed data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressants versus placebo | 4 | 231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.35, 0.06] |
| 1.1 SSRIs | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.54, 0.06] |
| 1.2 Tricyclic antidepressants | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Other antidepressants | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.40, ‐1.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Costa 1985.
| Methods | 8‐week, randomised study | |
| Participants | Female participants, age 18 years and over, affected by cancer (mixed sites, including breast, ovary, uterine cervix and others) at any stage, diagnosed with depression, according to the criteria proposed by Stewart 1965 for medically ill patients, with slight additional inclusion criteria suggested by Kathol and Petty (7): (i) low mood and loss of interest for at least 3 weeks; (ii) at least 4 of the following: difficulty in concentration or memory problems, irritability, feelings of worthlessness or hopelessness, fear of losing one's mind, lack of initiative, frequent crying or wanting to die, suicide attempt; (iii) social impairment at work, home etc; (iv) anorexia, sleep disturbance, fatigue, motor retardation. Further inclusion criteria were depression succeeding or paralleling development of cancer; Zung Self‐Rating Depression Scale (ZSRDS) score greater than 41; Hamilton Depression Rating Scale (HDRS) items 1 to 17 score greater than 16; and informed consent of the patient. Participants were mostly inpatients, but rates of in‐ and outpatients are not reported. | |
| Interventions | Mianserin: 36 participants. The dose was flexible starting from 10 mg, 1 tablet per day in the first week and 2 tablets per day from the second week (range not reported; mean dose between weeks 1 and 4 was 44.5 mg/day) Placebo: 37 participants |
|
| Outcomes | Efficacy and tolerability of mianserin versus placebo, assessed with Zung Self‐Rating Depression Scale (ZSRDS); Hamilton Depression Rating Scale (HDRS‐17); Clinical Global Impression Scale for Severity of Illness (CGI‐S); Clinical Global Impression Scale for Severity of Illness (CGI‐I); Efficacy Index (EI) and a checklist for somatic findings and side effects | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated"; no further details on the sequence generation process. However, quote: "Treatment groups were well matched for social data (education, occupation and marital status) [not reported in tables]. Treatment groups were also well matched for main cancer localizations, clinical stages of cancer, and baseline Karnofsky scores [reported in tables]." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patient compliance and physician blindness were good throughout the trial. Thus, the number of psychiatrist's correct guesses as to which treatment the patients were receiving (22, mianserin; 16, placebo) were not significantly higher than expected by chance". Procedures for ensuring the blinding of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Efficacy was evaluated using double‐blind assessment...". No further clarifications on which procedure was used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates: in the mianserin group 7/36 (19.4%), in the placebo group 15/37 (40.5%). The imbalance in total rates and possible different reason for losses between groups is not discussed. All randomised participants were included in the analysis, which is consistent with an 'intention‐to‐treat' analysis (but this term is not reported). Quote: "[...] the only treatment comparison known to be unbiased is that based on the analysis of all randomised patients". Missing data were imputed according to the LOCF, quote: "Data used in the statistical analysis of efficacy were based on the 'last assessment carried forward approach' in which missing scores for those patients who dropped out before day 21 had their last observed score assigned to the missing assessment". Even if there was a high dropout rate in the placebo group, the risk of bias was rated as 'unclear' rather than 'high', since the ITT analysis and LOCF imputation were properly performed. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly pre‐specified in the methods (quote: "[...] compare the efficacy and safety of mianserin in women with cancer [...]"). However, outcomes of interest are properly reported in the results. Scores for HDRS, ZSRDS, CGI‐S, EI and the number of participants with each side effect on the checklist were reported for every week. The number of responders is reported, but only according to the CGI‐I endpoint scores. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out since a 'financial disclosure' or possible conflicts of interest are not reported. |
EUCTR2008‐002159‐25‐FR.
| Methods | 12 weeks, randomised, double‐blind, placebo‐controlled study | |
| Participants | People with (a) cancer of the upper aerodigestive tract (buccal cavity, larynx, oropharynx, hypopharynx), solitary or multiple synchronous localisations, stage I to IVb, to be treated by surgery and/or radiotherapy and/or chemotherapy (first‐line curative treatment); (b) HADS more than 11 (excluded those with a diagnosis of major depressive episode with severity criteria and/or suicidal thoughts); (c) aged between 18 and 75 years, having signed an informed consent | |
| Interventions | Escitalopram: 20 participants Placebo: 18 participants |
|
| Outcomes | Primary outcome: subscore depression of the HADS, W12 Secondary outcomes: CES‐D; MADRS; CGI; SCL‐90‐R; health‐related quality of life (EORTC QLQC‐30, H‐N 35), alcohol or tobacco consumption (CO, CDT) |
|
| Notes | Data were partially provided by the authors before the publication of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported (unpublished study) |
| Allocation concealment (selection bias) | Unclear risk | Not reported (unpublished study) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported (unpublished study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported (unpublished study) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate: escitalopram arm 4/20 (20%); placebo arm 3/18 (16.7%). Only participants who completed the assessment at each time point were analysed and missing data were not imputed ('per protocol' analysis). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes are reported for the endpoint assessment (week 12) and for week 4. |
| Other bias | Low risk | The baseline features of the population of the study are not reported. The Gustave Roussy, which is a private non‐profit hospital, was the sponsor of the trial. Lundbeck funded only the costs of drugs and did not play any role in planning, conducting and writing the study. |
Fisch 2003.
| Methods | Randomised, placebo‐controlled, multicentre (15 centres) study | |
| Participants | Ambulatory people of either sexes with advanced cancer (mixed sites) and depressive symptoms, as assessed with a score of 2 or greater on the Two‐Question Screening Survey (TQSS), excluding people with major depression diagnosed by a psychiatrist in the past 6 months. All participants gave informed consent | |
| Interventions | Fluoxetine: 83 participants. The dose was 20 mg/day, fixed Placebo: 80 participants |
|
| Outcomes | The primary outcome was the quality of life (QoL) assessed with the Functional Assessment of Cancer Therapy–General (FACT‐G, version 3). The secondary outcome was the depressive symptoms assessed with the 11‐item BZSDS. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[...] randomly assigned in a double‐blind manner to receive either fluoxetine (20‐mg tablets) or an identical placebo tablet. The randomisation was performed centrally through a preprinted randomisation table, and the study drug was sent by overnight mail directly to the patient" and "Patients in each study arm were comparable at baseline with respect to age, sex, performance status, symptom status regarding pain and depression, disease distribution, and current treatment with chemotherapy." |
| Allocation concealment (selection bias) | Low risk | Quote: "[...] The randomisation was performed centrally through a preprinted randomisation table, and the study drug was sent by overnight mail directly to the patient." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients were then randomly assigned in a double‐blind manner to receive either fluoxetine (20‐mg tablets) or an identical placebo tablet". This should ensure patient blinding. The study is described as 'double‐blind', however procedures for ensuring the blinding of who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants who completed the assessment at each time point were analysed and missing data were not imputed ('per protocol' analysis). At the 'primary endpoint' (second visit, mean of 4.6 (fluoxetine group) versus 4.7 (placebo group) weeks from baseline) 64 versus 65 participants were assessed (over 83 versus 80 participants randomised). Only dropout rates due to side effects at the end of the study are reported, and whether there was imbalance between groups in term of reasons for leaving the study early is not discussed. |
| Selective reporting (reporting bias) | Low risk | Relevant data for the pre‐specified (methods) outcomes are reported (results). |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out since a 'financial disclosure' or possible conflicts of interest are not reported. |
Holland 1998.
| Methods | 6‐week, prospective, randomised, double‐blind, multicentric (6 investigative sites) study | |
| Participants | Women affected by cancer (mostly breast cancer at stage II, II, IV) and major depressive disorder (for at least 30 days before entering the study) or adjustment disorder with depressed mood (for at least 60 days before entering the study), according to the criteria of DSM‐III‐R and a score of more than 14 on the first 17 items of the HAM‐D. Participants gave signed informed consent. | |
| Interventions | Fluoxetine: 17 participants. The dose was 20 mg/day for the first month, thereafter the dose was flexible. However, the maximum dose allowed is not reported Desipramine: 21 participants, starting with a dose of 25 mg/day and titrated in 25 mg/week increments to a dose of 100 mg/day at week 4. Thereafter the dose was flexible to a maximum of 150 mg/day. There was not a placebo arm, but all participants received placebo + active drug (alternated during the day) in order to maintain the blindness ('double‐dummy' approach). |
|
| Outcomes | Safety and efficacy of fluoxetine versus desipramine. Depression and anxiety were assessed with the 17‐item Hamilton Rating Scale for Depression (HAM‐D‐17), the Hamilton Anxiety Rating Scale (HAM‐A), the Clinical and Patient's Global Impression (CGI and PGI) scales. Quality of life was assessed with the Functional Living Index for Cancer (FLIC), the Memorical Pain Assessment Card (MPAC), and the SF‐36 Health Survey. Adverse events were self reported and evaluated weekly through clinical assessment | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[...] a 6‐week, double‐blind (randomisation of placebo non‐responders) phase [...]. Treatment groups [...] had comparable demographics and baseline psychiatric assessment scores". No further details on the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Fluoxetine‐treated patients received 20 mg of active drug in the morning and placebo in the evening. Desipramine‐treated participants received 25 mg of active drug in the evening and placebo in the morning". The study is described as double‐blind, however procedures for ensuring the blinding of who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The assessment was performed by the clinician, whose blindness is not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate: 6 participants in the fluoxetine group (6/17, 35.3%) and 7 participants in the desipramine group (7/21, 33.3%). Number of participants and reasons for discontinuation are apparently balanced between the 2 groups. According to the text missing data were imputed, quote: "The endpoint analysis calculated changes from baseline [...] to the last observation carried forward...", however whether a proper ITT analysis was applied is unclear, since the number of analysed participants is not reported in the text or in the graphs. |
| Selective reporting (reporting bias) | High risk | Outcomes are not clearly pre‐specified (quote: "[...] our study prospectively examined the safety and efficacy of fluoxetine and desipramine in 40 depressed women [...]"). Outcomes of interest are poorly reported: neither mean scores on scales nor rates of remission are reported at any time point. The baseline‐to‐endpoint mean changes are represented in graphs, but not clearly reported in the text. |
| Other bias | High risk | Quote: "This work was sponsored by Eli Lilly and Company". The role of funders in planning, conducting and writing the study is not discussed. |
Musselman 2006.
| Methods | 6‐week, randomised, double‐blind, placebo‐controlled, multicentric (2 centres), parallel‐group study | |
| Participants | Female outpatients aged 18 to 75 years with a current diagnosis of breast carcinoma (stage I‐IV); DSM‐III‐R criteria for major depression or adjustment disorder with depressed mood for at least 2 months; score of at least 14 on the first 17 items of the 21‐items HAM‐D; last cancer treatment within the last 5 years | |
| Interventions | Paroxetine: 13 participants. The dose was flexible, starting with 20 mg/day for the first 4 weeks, thereafter it could be increased at 40 mg/day. Desipramine: 11 participants. The dose was flexible, starting with 25 mg/day and gradually titrated to 125 mg/day within the fourth week; thereafter it could be increased by 25 mg/day every 3 days up to 200 mg/day as the maximum dose. Placebo: 11 participants |
|
| Outcomes | Efficacy and tolerability of paroxetine versus desipramine versus placebo in women with breast cancer, assessed with 21‐item observer‐rated Hamilton Rating Scale for Depression (HAM‐D), 14‐item observer‐rated Hamilton Rating Scale for Anxiety (HAM‐A), Clinical Global Impression Scale for Severity of Illness (CGI‐S), routine adverse event monitoring and vital assessment for exploring tolerability. Quote: "The primary efficacy parameter was the mean change from baseline in the total score of the 21‐item HAM‐D. The secondary outcome measure was the mean change from baseline in the CGI‐S score." | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were then randomly assigned to one of the three double‐blind treatment groups"; no further details on the sequence generation process. The 3 groups were similar for demographic and clinical features (with the exception of stage, being less advanced in the placebo‐treated group, and previous chemotherapy, being less frequent in the placebo‐treated group). |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as "double‐blind", however procedures for ensuring the blinding of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates: 5/13 (38.5%) participants in paroxetine group; 4/11 (36.4%) participants in desipramine group; 5/11 (45.4%) in placebo group. Reason for leaving the study are apparently balanced between groups, however dropout rates are relevant. Moreover, a relevant portion of missing data are possibly related to the true outcome (2 versus 2 versus 0 participants dropped due to inefficacy). Missing data were imputed. Quote: "Data are presented from the intention‐to‐treat population" and "the last‐observation‐carried‐forward approach was applied for the missing data due to early dropout in the study." |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes are reported for the endpoint assessment (week 6). |
| Other bias | Unclear risk | 3 authors report having received research support from several drug companies. Sponsorship bias cannot be ruled out since the funders of the study and their role in planning, conducting and writing it are not reported. |
Navari 2008.
| Methods | 24‐week, randomised, double‐blind, placebo‐controlled study | |
| Participants | Women with early‐stage breast cancer (stages I, II) who were candidates for adjuvant hormonal therapy, local radiation and/or adjuvant chemotherapy treatment and had depressive symptoms, as indicated by a score of 2 or greater on the Two Question Screening Survey (TQSS). Participants who were "clinically depressed" were excluded. | |
| Interventions | Fluoxetine: number of participants not reported. The dose was 20 mg/day (not clearly reported if it was a fixed dose) Placebo: number of participants not reported |
|
| Outcomes | Efficacy of fluoxetine versus placebo on depressive symptoms (assessed with the 11‐item Brief Zung Self‐Rating Depression Scale ‐ BZSDS), quality of life (assessed with the Functional Assessment of Cancer Therapy–General ‐ FACT‐G, version 3) and completion of adjuvant treatment. Quote: "The primary end points of the study were depressive symptoms, quality of life, and completion of adjuvant treatment." | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients with depressive symptoms were randomised to a daily oral antidepressant or a placebo"; no further details on the sequence generation process. Quote: "The groups were comparable at baseline in terms of age, disease distribution, performance status, and level of depressive symptoms". However, only the total number of randomised participants is reported, not the number of participants in each arm. Tables report results for 90 participants per arm |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as 'double‐blind', however procedures for ensuring the blinding of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 193 people were randomly assigned, but the number of participants for each arm is not reported. 180/193 (93%) participants completed the study. Dropout rates among the 2 groups and reasons for leaving the study early are not clearly reported. Missing data were not imputed and only participants who completed the study were analysed ('per protocol' analysis). |
| Selective reporting (reporting bias) | High risk | Results are reported only for subgroups (according to the type of adjuvant therapy assumed) not pre‐specified. For relevant outcomes only results for "relevant improvement in depressive symptoms at 6 months" are reported, however how "significant improvement" is assessed is not clearly discussed. |
| Other bias | Unclear risk | The Reich Family Endowment provided financial support for this investigation (not clearly reported if it is a private funder). The role of funders in planning, conducting and writing the study is not discussed. |
NCT00387348.
| Methods | Interventional, randomised, cross‐over, 8‐week, double‐blind study. The randomisation was stratified according to stage of disease (stage IIIB with effusions vs stage IV) and current treatment (radiation vs chemotherapy vs novel agent). | |
| Participants | Patients diagnosed with advanced lung or gastrointestinal cancer and major depressive disorder (according to DSM‐IV and Endicott criteria). Age: 35 to 85 years. | |
| Interventions | The study had a cross‐over design. Patients were randomised into three arms: placebo‐escitalopram (the switch from one to the other took place after 4 weeks), escitalopram‐placebo, and placebo‐placebo. In the first phase of the trial 11 patients received escitalopram 10 mg/day and 13 patients received placebo. | |
| Outcomes | Primary outcomes: response rate, defined as a 50% reduction in the Hamilton Depression Rating Scale (HAM‐D) scores over 4 weeks; change in Hamilton Depression Rating Scale (HAM‐D) scores at week 4. Seconday outcome: side effect burden, defined as the total score of the UKU Side Effects Rating Scale. | |
| Notes | According to the protocol the study started in March 2006 and was supposed to be completed in April 2011. Results for primary and secondary outcomes for the first 4 weeks of treatment were made available at clinicaltrials.gov. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however details on the sequence generation were not provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Masking: Triple (Participant, Care Provider, Investigator)" and "[...] one placebo pill identical in appearance to the escitalopram pill [...]". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apparently an ITT analysis was performed, considering that all randomised patients were analysed in the majority of analyses, including therefore also patients who left the study early. However, the methodology employed to impute missing data is not discussed (note that only the protocol of the study is available). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were clearly prespecified in the protocol, and were reported. |
| Other bias | Low risk | The study was supported by the Massachusetts General Hospital and the National Cancer Institute (NCI). |
Pezzella 2001.
| Methods | 8‐week, multicentric (25 centres), double‐blind, parallel‐group, randomised study | |
| Participants | Women, aged 18 to 65 years (according to data reported in tables, older participants were also analysed), with a diagnosis of breast cancer (at any stage, but without cerebral metastases), with a rating of less than 2 on the World Health Organization (WHO) performance status scale and a life expectancy greater than 3 months; who had received chemotherapy and were scheduled to receive further cycles during the study period, and had received tamoxifen or paclitaxel and were scheduled to receive further treatment during the study. Participants had to be diagnosed with a mild, moderate or severe depressive episode, according to International Classification of Disease‐10 (ICD‐10) and have a score of greater than 16 on the Montgomery Åsberg Depression Rating Scale (MADRS). All participants gave written informed consent | |
| Interventions | Paroxetine: 88 participants. Flexible dose, starting with 20 mg/day for the first 3 weeks. Thereafter the dose could be increased to 30 mg/day (after week 3) and to 40 mg/day (after week 5) if clinically indicated Amitriptyline: 87 participants. Flexible dose, titrating up to 75 mg/day within the first 3 weeks. Thereafter the dose could be increased to 100 mg/day (after week 3) and to 150 mg/day (after week 5) if clinically indicated. Placebo capsules were administered in order to maintain blindness. |
|
| Outcomes | Quote: "[...] primary aim of comparing the efficacy and tolerability of paroxetine and amitriptyline in the treatment of depression in women with breast cancer". Efficacy was assessed with MADRS, CGI‐S, Functional Living Index Cancer (FLIC) and patient's global evaluation (PGE) at endpoint. Tolerability was assessed by recording adverse events and evaluating vital signs and laboratory parameters. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...a multicenter, double‐blind, parallel‐group, randomised study" and "...study participants [...] were randomly assigned in a ratio of 1:1 to 8‐weeks treatment with either paroxetine [...] or amitriptyline [...]"; no further details on the sequence generation process. However, according to the tables, clinical and demographic features are similar between the 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "...a multicenter, double‐blind, parallel‐group, randomised study" and "a double‐dummy technique was used to ensure blinding". Procedures for ensuring the blinding of who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates: 16/88 (18.2%) in the paroxetine group; 19/87 (21.8%) in the amitriptyline group. Side effects represent the most frequent reason for withdrawal (9 versus 10 participants). Other reasons are not discussed, however rates and reasons for losses are apparently balanced between groups. Imputations for missing data were performed. Quote: "Visitwise and endpoint statistical analyses were performed on the intent‐to‐treat (ITT) population (i.e. all participants who had taken at least one dose of study medication and who had at least one on‐dose efficacy assessment). Endpoint analyses were constructed from week 8 observations, where available, and on a ‘last observation carried forward' basis for participants who had discontinued study medication prematurely." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly prespecified (quote: "[...] primary aim of comparing the efficacy and tolerability of paroxetine and amitriptyline [...]"), however key outcomes are reported as mean change scale scores at different time points. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out since a 'financial disclosure' is not reported. |
Razavi 1996.
| Methods | 5‐week, double‐blind, placebo‐controlled, randomised, multicentric trial (14 centres) | |
| Participants | People (mostly females), aged over 18 years, diagnosed with an adjustment disorder (with a depressive mood or with mixed features) or from a major depressive disorder (excluding MDD with melancholic features) as defined by the DSM‐III‐R "in relation to" a cancer disease that had been diagnosed for a period of between 6 weeks and 7 years. Participants had to have a score of 13 or higher on the Hospital Anxiety and Depression Scale (HADS) before and after the 1‐week period of placebo treatment, a rating of 60 or higher on the Karnofsky Performance Scale, and had to provide written informed consent | |
| Interventions | Fluoxetine: 45 participants. The dose was 20 mg 1 tablet per day Placebo: 46 participants |
|
| Outcomes | Effectiveness and tolerance of fluoxetine versus placebo, assessed with the Hospital Anxiety and Depression Scale (HADS), Montgomery and Åsberg Depression Rating Scale (MADRS), Hamilton Anxiety Scale (HAS), Revised Symptom Checklist (SCL90‐R) and the Spitzer Quality of Life Index (SQOLI). The main assessment criterion was the success rate defined by a HADS score lower than 8 after 5 weeks of treatment. Treatment tolerance was assessed with AMDP5, weight, blood pressure, pulse, biochemical and haematological tests and spontaneous side effect reports. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a double‐blind, placebo‐controlled, randomised, multicenter trial"; no further details on the sequence generation process. "The descriptive statistics for the baseline characteristics (demographic data and clinical variables) are comparable in the two treatment arms, except for delay since diagnosis, which was longer in the PA [placebo] group than in the FA [fluoxetine] group for randomised participants (P value = 0.03)." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as "double‐blind", however procedures for ensuring the blinding of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates: 15/45 (33.3%) participants in the fluoxetine group, 7/46 (15.2%) participants in the placebo group. Relevant rate particularly for the intervention group. There is imbalance between groups, however reasons for leaving the study early are described as apparently balanced between group. Quote: "Data analyses were performed [...] on an intent‐to‐treat basis on all randomised patients for the success rate, response rate and spontaneous side‐effect reports. For evolution of assessment scales, analyses were performed on an intent‐to‐treat basis on patients who completed the study". However, only data for participants who completed the study have been analysed (according to a 'per protocol' analysis), and actually missing data were not imputed. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly pre‐specified (quote: "[...] evaluate, in a double‐blind placebo‐controlled design, the effectiveness of fluoxetine to treat and/or to control anxiety and depression [...]"). For relevant outcomes mean scores on rating scales are reported for 'visit 1' (but it is not clearly explained if it matches with the baseline point) and for 'visit 5'. |
| Other bias | High risk | Quote: "This study was supported by grants from Lilly France and Lilly Benelux". The role of funders in planning, conducting and writing the study is not discussed. |
Van Heeringen 1996.
| Methods | 6‐week, randomised, double‐blind, placebo‐controlled, single‐centre study | |
| Participants | Women over 18 years with breast cancer at stage I or II, without metastases, not qualifying for primary surgical treatment, treated with radiotherapy, and depression, diagnosed according to DSM‐III criteria, and a score of at least 16 on the 21‐item HDRS | |
| Interventions | Mianserin: 28 participants. The dose was fixed at 30 mg/day for the first week and 60 mg/day thereafter. Placebo: 27 participants |
|
| Outcomes | Efficacy and safety of mianserin versus placebo. Depression was assessed with the 21‐item HRDS after 2, 4 and 6 weeks. Tolerability was assessed with the ROSE (Record of Symptoms Emerging) and clinical evaluation of vital signs and laboratory measurements | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After baseline assessment [...] patients still satisfying entrance criteria were randomised to treatment with mianserin (M; n = 28) or placebo (P; n = 27)..." and "Both treatment groups were well matched regarding baseline characteristics...". No further details on the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "...a randomised, double‐blind, placebo‐controlled study" and "...mianserin (M; n = 28) or placebo (P; n = 27), which had been prepared as indistinguishable capsules and given as a single night‐time dose". Not reported who was blinded (clinician, statistician, outcome assessor) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates: mianserin group 6/28 (21.4%); placebo group 15/27 (55.5%); 2 versus 11 due to inefficacy, 2 versus 4 due to side effects.The imbalance in total rates and in reasons for losses between groups is not discussed. This might have introduced bias, since dropouts in the placebo group mostly referred to inefficacy, which is likely related to the true outcome. Quote: "Efficacy analyses were performed on an intention to‐treat basis, thus including the patients who received at least one dose of study medication and had at least one post‐baseline efficacy assessment. Last observation carried forward (LOCF) analysis was performed at each assessment point, substituting missing values at all subsequent assessments by the last available value". Actually not all the randomised participants were analysed, but only those who received at least one dose of medication and had at least one assessment, which is closer to an 'as treated' analysis. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly prespecified (quote: "The aim of our study was to evaluate the efficacy and safety of mianserin in patients with breast cancer [...]"). However, mean change scores on HDRS, response rates and rates of relevant adverse events are reported. |
| Other bias | High risk | Quote: "This study was supported by a grant from NV Organon, Oss, The Netherlands". The role of funders in planning, conducting and writing the study is not discussed. |
BZSDS: Brief Zung Self‐Rating Depression Scale CDT: Carbohydrate‐deficient transferrin CGI: Clinical Global Impression scale CGI‐I/CGI‐S: Clinical Global Impression Scale for Severity of Illness CO: Test for diffusing capacity for carbon monoxide DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders ‐ III ‐ Revision EI: Efficacy Index EORTC: European Organisation for Research and Treatment of Cancer HADS: Hospital Anxiety and Depression Scale HAM‐D: Hamilton Depression Rating Scale HRSD: Hamilton Rating Scale for Depression ITT: Intention‐to‐treat LOCF: Last observation carried forward MADRS: Montgomery Åsberg Depression Rating Scale MDD: major depressive disorder ZSRDS: Zung Self‐Rating Depression Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amodeo 2012 | Wrong comparison: participants in the 2 arms received the same drug at different doses |
| Biglia 2005 | Wrong design: not randomised |
| Biglia 2009 | Wrong comparison: control group without placebo |
| Boekhout 2011 | Wrong condition: participants not depressed at enrollment |
| Caldera 2009 | Wrong design: not randomised |
| Cankurtaran 2008 | Wrong condition: participants with panic disorder and generalised anxious disorder were also enrolled |
| Capriglione 2016 | Wrong condition: participants not depressed at enrollment. |
| Capuron 2002 | Wrong condition: participants not depressed at enrollment |
| Capuron 2003 | Wrong condition: participants not depressed at enrollment |
| Del Carmen 1990 | Wrong condition: participants not depressed at enrollment |
| Durand 2012 | Wrong condition: participants not depressed at enrollment |
| Ell 2010 | Wrong design. This is a review and it refers to 3 studies, none of which are eligible |
| Evans 1988 | Wrong design: not randomised |
| Heras 2013 | Wrong condition: participants not depressed at enrollment |
| Hua 2009 | Wrong comparison: control group without placebo |
| ISRCTN51232664 | Study eligible according to the protocol, however no published or unpublished data were retrieved. We contacted the authors and they stated that the study never started due to concerns around drug interactions and cancer symptoms. No further clarifications were provided |
| JPRN‐UMIN000003383 | Wrong design: not randomised |
| Kalso 1996 | Wrong condition: participants not depressed at enrollment |
| Kamath 2010 | Only the abstract of the study was available. Study eligible according to the abstract, but the author's feedback was negative: the study has been concluded due to recruitment issues |
| Kautio 2008 | Wrong condition: participants not depressed at enrollment |
| KCT0000076 | Wrong design: not randomised |
| Kimmick 2006 | Wrong condition: participants not depressed at enrollment |
| Loibl 2007 | Wrong condition: participants not depressed at enrollment |
| Lydiatt 2008 | Wrong condition: participants not depressed at enrollment |
| Marasanov 2013 | Wrong condition: participants not depressed at enrollment |
| Morrow 2003 | Wrong condition: participants not depressed at enrollment |
| Musselman 2013 | Wrong condition: participants not depressed at enrollment |
| NCT00005805 | Wrong condition: participants not depressed at enrollment |
| NCT00066859 | According to information provided by the author (Prof. EG Shaw) the study closed due to the low number of patients enrolled (only 8) |
| NCT00129467 | Wrong comparison: the experimental arm received methylphenidate plus SSRI, the control arm received placebo plus SSRI |
| NCT00234195 | Wrong design: not randomised |
| NCT00352885 | Wrong condition: participants not depressed at enrollment |
| NCT00488072 | Wrong condition: participants not depressed at enrollment |
| NCT00536172 | Wrong condition: participants not depressed at enrollment |
| NCT00740571 | Wrong comparison: no placebo or antidepressant in the control group |
| NCT00832520 | Wrong condition: participants not depressed at enrollment |
| NCT01219673 | Wrong condition: participants not depressed at enrollment |
| NCT01256008 | The study is eligible according to the protocol. We contacted the authors and they provided negative feedback; the design of the study has been changed and the antidepressant arm has been removed |
| NCT01501396 | Wrong condition: participants not depressed at enrollment |
| NCT01598584 | According to information provided by the author (Dr Yi Ba) the study was withdrawn before enrollment. |
| NCT01719861 | Wrong design: not randomised |
| NCT01725048 | Wrong design: not randomised |
| NCT02443194 | Wrong condition: participants not depressed at enrollment |
| NCT02650544 | Wrong condition: participants not depressed at enrollment |
| NCT03086148 | Wrong intervention: ketamine not included among antidepressants according to WHO/DDD |
| Ng 2014 | Wrong comparison: control group without placebo |
| Nunez 2013 | Wrong condition: participants not depressed at enrollment |
| Palesh 2012 | Wrong condition: participants not depressed at enrollment |
| Panerai 1990 | Wrong condition: not only participants affected by cancer recruited |
| Rodriguez 2011 | Wrong comparison: control group without placebo |
| Roscoe 2005 | Wrong condition: participants not depressed at enrollment |
| Stockler 2007 | Wrong condition: mixed population was enrolled, also including participants with fatigue and anxious symptoms |
| Taraz 2013 | Wrong condition: participants not affected by cancer |
| Theobald 2002 | Wrong condition: participants not depressed at enrollment |
| Tondlova 1997 | Wrong design: not randomised |
| Tondlova 2002 | Wrong condition: participants not depressed at enrollment |
| UKCCCR | Wrong condition: participants not depressed at enrollment |
| Vitolins 2013 | Wrong population: patients not depressed at enrollment. |
| Zhang 2003 | Wrong design: the study described as "randomised", but the treatment received by the comparison arm is not clearly reported |
| Zhang 2011 | Wrong comparison: control group without placebo |
| Zimmerman 2016 | Wrong population: patients not depressed at enrollment |
| Zvukova 2010 | Wrong condition: participants with thyroid cancer and benign thyroid tumours were recruited, and not only depressed participants were recruited |
SSRI: selective serotonin reuptake inhibitor
Characteristics of studies awaiting assessment [ordered by study ID]
N0405078066.
| Methods | Randomised controlled trial |
| Participants | People with lung cancer |
| Interventions | Venlafaxine versus placebo |
| Outcomes | Effects on symptom profiles after 12 weeks (not clearly specified) |
| Notes | According to the protocol the study has been completed, but no published or unpublished data have been retrieved. Not clear if the study is eligible. Authors did not reply to our request for clarification and for data. |
UMIN000008768.
| Methods | Parallel, randomised, open‐label study |
| Participants | Male and females with cancer, diagnosed with major depression; age greater than 20 years |
| Interventions | Mirtazapine versus duloxetine hydrochloride |
| Outcomes | Primary outcome: change in HAM‐D scores between pretreatment baseline and 6‐week treatment |
| Notes | The study is eligible according to the abstract, but results are not available. Authors did not reply to our request for data. |
HAM‐D: Hamilton Depression Rating Scale
Differences between protocol and review
We amended the Selection of studies paragraph to report that only the Endnote software was used.
In the paragraph Subgroup analysis and investigation of heterogeneity we clarified that the subgroup analyses were performed only for the primary outcome. We further specified which subgroups were considered.
We updated the section Description of the intervention with a brief discussion of a recent review and meta‐analysis (Riblet 2014).
In the section Objectives we replaced the term 'people' with 'adults (aged 18 years or older)'.
In the section Data extraction and management we made clear that the endpoint response rate and dropout rate were calculated on a strict intention‐to‐treat (ITT) basis.
In the section Measures of treatment effect we described which measures for the continuous and dichotomous outcomes were retrieved for the analyses. We moved the methodology for pooling these data from this section to the Data synthesis section, where we also specified the use of the Mantel‐Haenszel methods for the analysis.
We moved the discussion on multiple intervention groups from the section Unit of analysis issues to the Data synthesis section.
In the Data synthesis section we removed the list of comparisons performed, namely antidepressants versus placebo and antidepressants versus antidepressants, as it was already reported in the paragraph Types of interventions. In this section we added a more detailed description on how data were managed and entered in the analysis, including the use of a random‐effects model.
Contributions of authors
GO, CB and MH planned the study. GO and FM retrieved and selected the studies, extracted the data and performed the quality assessment. GO and CB ran the analysis. GO drafted the manuscript, which was critically revised by FM, SD, CB and MH.
Sources of support
Internal sources
-
Department of Public Health and Community Medicine, Section of Psychiatry, University of Verona, Italy.
CB receives salary support from the University of Verona. GO is a PhD student and receives salary support in the form of a public grant from the Italian Ministry of Health.
-
Department of Psychological Medicine, The Institute of Psychiatry, King's College London, UK.
MH and FM receive salary support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
-
Département Interdisciplinaire de Soins de Support, Gustave Roussy, France.
SD receives salary support from the Institute Gustave Roussy, Paris.
External sources
No sources of support supplied
Declarations of interest
Giovanni Ostuzzi ‐ nothing to declare Faith Matcham ‐ nothing to declare Sarah Dauchy ‐ nothing to declare Corrado Barbui ‐ nothing to declare Matthew Hotopf ‐ nothing to declare
Sarah Dauchy conducted a multi‐centre trial of participants with cancer and depressive symptoms that compared the efficacy of escitalopram versus placebo. This trial was supported financially by the Institut Gustave‐Roussy and Lundbeck. To prevent bias the author was not involved in assessing the eligibility of the study, or in the extraction of data and quality assessment.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Costa 1985 {published data only}
- Costa D, Mogos I, Toma T. Efficacy and safety of mianserin in the treatment of depression of women with cancer. Acta Psychiatrica Scandinavica 1985;72(Suppl 320):85‐92. [DOI] [PubMed] [Google Scholar]
EUCTR2008‐002159‐25‐FR {unpublished data only}
- Dauchy S, Saltel P, Rey A, Consoli SC, Dolbeault S, Razavi D, et al. A randomized, double‐blind, placebo‐controlled trial of escitalopram for the treatment of emotional distress during treatment for head and neck cancer [Etude randomisée, en double aveugle, contre placebo, de l’Efficacité de l’ Escitalopram dans le traitement de la détresse émotionnelle des sujets atteints de cancer ORL en cours de traitement]. International Clinical Trials Registry Platform 2014. [EUCTR2008‐002159‐25‐FR]
Fisch 2003 {published data only}
- Fisch MJ, Loehrer PJ, Kristeller J, Passik S, Jung S, Shen J, et al. Fluoxetine versus placebo in advanced cancer outpatients: a double‐blinded trial of the Hoosier Oncology Group. Journal of Clinical Oncology 2003;21(10):1937‐43. [DOI: 10.1200/JCO.2003.08.025] [DOI] [PubMed] [Google Scholar]
Holland 1998 {published data only}
- Holland JC, Romano SJ, Heiligenstein JH, Tepner RG, Wilson MG. A controlled trial of fluoxetine and desipramine in depressed women with advanced cancer. Psycho‐oncology 1998;7:291–300. [DOI] [PubMed] [Google Scholar]
Musselman 2006 {published data only}
- Musselman DL, Somerset WI, Guo Y, Manatunga AK, Porter M, Penna S, et al. A double‐blind, multicenter, parallel‐group study of paroxetine, desipramine, or placebo in breast cancer patients (stages I, II, III, and IV) with major depression. Journal of Clinical Psychiatry 2006;67(2):288‐96. [DOI] [PubMed] [Google Scholar]
Navari 2008 {published data only}
- Navari RM, Brenner MC, Wilson MN. Treatment of depressive symptoms in patients with early stage breast cancer undergoing adjuvant therapy. Breast Cancer Research and Treatment 2008;112:197–201. [DOI: 10.1007/s10549-007-9841-z] [DOI] [PubMed] [Google Scholar]
NCT00387348 {published data only}
- Pirl W. Escitalopram in treating depression in patients with advanced lung or gastrointestinal cancer. clinicaltrials.gov 2012. [NCT00387348]
Pezzella 2001 {published data only}
- Pezzella G, Moslinger‐Gehmayr R, Contu A. Treatment of depression in patients with breast cancer: a comparison between paroxetine and amitriptyline. Breast Cancer Research and Treatment 2001;70:1‐10. [DOI] [PubMed] [Google Scholar]
Razavi 1996 {published data only}
- Razavi D, Allilaire JF, Smith M, Salimpour A, Verra M, Desclaux B, et al. The effect of fluoxetine on anxiety and depression symptoms in cancer patients. Acta Psychiatrica Scandinavica 1996;94:205‐10. [DOI] [PubMed] [Google Scholar]
Van Heeringen 1996 {published data only}
- Heeringen K, Zivkov M. Pharmacological treatment of depression in cancer patients. A placebo‐controlled study of mianserin. British Journal of Psychiatry 1996;69:440‐4. [10.1192/bjp.169.4.440] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amodeo 2012 {published data only}
- Amodeo L, Castelli L, Leombruni P, Cipriani D, Biancofiore A, Torta R. Slow versus standard up‐titration of paroxetine for the treatment of depression in cancer patients: a pilot study. Support Care Cancer 2012;20:375–84. [DOI: 10.1007/s00520-011-1118-8] [DOI] [PubMed] [Google Scholar]
Biglia 2005 {published data only}
- Biglia N, Torta R, Roagna R, Maggiorotto F, Cacciari F, Ponzone R, et al. Evaluation of low‐dose venlafaxine hydrochloride for the therapy of hot flushes in breast cancer survivors. Maturitas 2005;52(1):78‐85. [PUBMED: 16143229] [DOI] [PubMed] [Google Scholar]
Biglia 2009 {published data only}
- Biglia N, Sgandurra P, Peano E, Moggio G, Spatola M, Palmisano D. Duloxetine and escitalopram for treatment of hot flushes in breast cancer survivors. Maturitas 2009;63(Suppl 1):S34. [Google Scholar]
Boekhout 2011 {published data only}
- Boekhout AH, Vincent AD, Dalesio OB, Bosch J, Foekema‐Töns JH, Adriaansz S, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Oncology 2011;29(29):3862‐8. [DOI: 10.1200/JCO.2010.33.1298] [DOI] [PubMed] [Google Scholar]
Caldera 2009 {published data only}
- Caldera PC, Amodeo L, Borio R, Ramonda E, Torta R. Algorithm‐based treatment for depression in cancer outpatients: efficacy and tolerability evaluation of newer antidepressants. Psycho‐oncology 2009;18(Suppl 2):S317. [DOI: 10.1002/pon.1594] [DOI] [Google Scholar]
Cankurtaran 2008 {published data only}
- Cankurtaran ES, Ozalp E, Soygur H, Akbiyik DI, Turhan L, Alkis N. Mirtazapine improves sleep and lowers anxiety and depression in cancer patients: superiority over imipramine. Supportive Care in Cancer 2008;16(11):1291‐8. [DOI: 10.1007/s00520-008-0425-1] [DOI] [PubMed] [Google Scholar]
Capriglione 2016 {published data only}
- Capriglione S, Plotti F, Montera R, Luvero D, Lopez S, Scaletta G, et al. Role of paroxetine in the management of hot flashes in gynecological cancer survivors: Results of the first randomized single‐center controlled trial. Gynecological Oncology 2016;143(3):584‐8. [DOI] [PubMed] [Google Scholar]
Capuron 2002 {published data only}
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon‐alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002;26(5):643‐52. [PUBMED: 11927189] [DOI] [PubMed] [Google Scholar]
Capuron 2003 {published data only}
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon‐alpha–induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biological Psychiatry 2003;54(9):906‐14. [DOI: ] [DOI] [PubMed] [Google Scholar]
Del Carmen 1990 {published data only}
- Carmen L, Plancarte R, Fuente JR. Amitriptilin as coanalgesic in cancerous patients [La amitriptilina como coanalgesico en pacientes con cancer]. Salud Mental 1990;13(4):1‐6. [Google Scholar]
Durand 2012 {published data only}
- Durand JP, Deplanque G, Montheil V, Gornet JM, Scotte F, Mir O, et al. Efficacy of venlafaxine for the prevention and relief of oxaliplatin‐induced acute neurotoxicity: results of EFFOX, a randomized, double‐blind, placebo‐controlled phase III trial. Annals of Oncology 2012;23(1):200‐5. [DOI] [PubMed] [Google Scholar]
Ell 2010 {published data only}
- Ell K, Aranda MP, Xie B, Lee PJ, Chou CP. Collaborative depression treatment in older and younger adults with physical illness: pooled comparative analysis of three randomized clinical trials. American Journal of Geriatric Psychiatry 2010;18(6):520‐30. [DOI: 10.1097/JGP.0b013e3181cc0350] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1988 {published data only}
- Evans DL, McCartney CF, Haggerty JJ. Treatment of depression in cancer patients is associated with better life adaptation: a pilot study. Psychosomatic Medicine 1988;50:72‐6. [DOI] [PubMed] [Google Scholar]
Heras 2013 {published data only}
- Heras P, Kritikos K, Hatzopoulos A, Kritikos N, Heras V, Mitsibounas D. The role of paroxetine in fatigue and depression of patients under chemotherapeutic treatment. American Journal of Therapeutics 2013;20:254–6. [DOI: 10.1097/MJT.0b013e318187de2c] [DOI] [PubMed] [Google Scholar]
Hua 2009 {published data only}
- Hua X. Investigation and intervening therapy to depression of malignant hematological diseases Baixuebing, Linbaliu. Baixuebing, Linbaliu [Journal of Leukaemia and Lymphoma] 2009;18(7):432‐6. [ETOCRN258368457] [Google Scholar]
ISRCTN51232664 {unpublished data only}
- Montgomery C (contact name). A randomized controlled trial of venlafaxine versus placebo for depression amongst persons with lymphoma or leukaemia. International Standard Randomised Controlled Trial Number Register 2003. [DOI: 10.1186/ISRCTN51232664] [DOI]
JPRN‐UMIN000003383 {unpublished data only}
- Ozaki N (principal investigator). A study in the effects of sertraline on depressive symptoms of patients with inoperable advanced pancreatic cancer. University Hospital Medical Information Network (UMIN) Center ‐ Controlled Trials Register 2010. [UMIN000003383]
Kalso 1996 {published data only}
- Kalso E, Tasmuth T, Neuvonen PJ. Amitriptyline effectively relieves neuropathic pain following treatment of breast cancer. Pain 1996;64(2):293‐302. [PUBMED: 8740607] [DOI] [PubMed] [Google Scholar]
Kamath 2010 {published data only}
- Kamath J, Banga A, Tannenbaum S, Claffey K, Zhang W, Winokur A. A randomized, double‐blind placebo‐controlled study evaluating the efficacy of Lovaza (omega‐3‐acid‐ethyl esters) compared to placebo for the treatment of depressive and anxiety symptoms in patients with breast cancer. Psycho‐oncology 2010;19(Suppl 2):s269. [DOI: 10.1002/pon.1776] [DOI] [Google Scholar]
Kautio 2008 {published data only}
- Kautio AL, Haanpää M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy‐induced neuropathic symptoms. Journal of Pain and Symptom Management 2008;35(1):31‐9. [PUBMED: 17980550] [DOI] [PubMed] [Google Scholar]
KCT0000076 {unpublished data only}
- Kim J, Kim S. Effects of bupropion and escitalopram for depression in cancer patients. International Clinical Trials Registry Platform 2011. [KCT0000076]
Kimmick 2006 {published data only}
- Kimmick GG, Lovato J, McQuellon R, Robinson E, Muss HB. Randomized, double‐blind, placebo‐controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. The Breast Journal 2006;12(2):114‐22. [DOI] [PubMed] [Google Scholar]
Loibl 2007 {published data only}
- Loibl S, Schwedler K, Minckwitz G, Strohmeier R, Mehta KM, Kaufmann M. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients—a double‐blind, randomized study. Annals of Oncology 2007;18:689–93. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lydiatt 2008 {published data only}
- Lydiatt WM, Denman D, McNeilly DP, Puumula SE, Burke WJ. A randomized, placebo‐controlled trial of citalopram for the prevention of major depression during treatment for head and neck cancer. Otolaryngology—Head and Neck Surgery 2008;134(5):528‐35. [DOI: 10.1001/archotol.134.5.528] [DOI] [PubMed] [Google Scholar]
Marasanov 2013 {published data only}
- Marasanov SB, Mokhov EM, Gordeeva OA. Pharmacological correction of emotional status in breast cancer patients in the postoperative period [ФАРМАКОЛОГИЧЕСКАЯ КОРРЕКЦИЯ пСихоэмоционАльного и СТАТУСА У БОЛЬНЫХ РАКОМ МОЛОЧНОЙ ЖЕЛЕЗЫВ ПоСЛЕИПЕРАЦИоННоМ ПЕРИОДЕ]. ВОПРОСЫ онкологии (Oncology Questions) 2013;59(2):95‐9. [PubMed] [Google Scholar]
Morrow 2003 {published data only}
- Morrow GR, Hickok JT, Roscoe JA, Raubertas RF, Andrews PL, Flynn PJ, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double‐blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Journal of Clinical Oncology 2003;21(24):4635‐41. [DOI: 10.1200/JCO.2003.04.070] [DOI] [PubMed] [Google Scholar]
Musselman 2013 {published data only}
- Musselman D, Royster EB, Wang M, Long Q, Trimble LM, Mann TK, et al. The impact of escitalopram on IL‐2‐induced neuroendocrine, immune, and behavioral changes in patients with malignant melanoma: preliminary findings. Neuropsychopharmacology 2013;38(10):1921‐8. [DOI: 10.1038/npp.2013.85] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00005805 {unpublished data only}
- Straus DJ. St. John's Wort in relieving fatigue in patients undergoing chemotherapy or hormone therapy for cancer. clinicaltrials.gov 2000.
NCT00066859 {unpublished data only}
- Miller AA, Shaw EG. Sertraline Compared With Hypericum Perforatum (St.John's Wort) in Treating Depression. clincaltrials.gov 2013.
NCT00129467 {unpublished data only}
- Ganzini LK. Methylphenidate for depressed cancer patients receiving palliative care. clinicaltrials.gov 2005.
NCT00234195 {unpublished data only}
- Mago R. Wellbutrin XL, major depressive disorder and breast cancer. clinicaltrials.gov 2005.
NCT00352885 {unpublished data only}
- Musselman DL, Lawson D, Miller A. Evaluating the effectiveness of escitalopram in preventing or reducing depressive symptoms in people receiving interleukin‐2 treatment. ClinicalTrials.gov 2006.
NCT00488072 {unpublished data only}
- Dalal S. Effects of mirtazapine on appetite in advanced cancer patients. ClinicalTrials.gov 2007.
NCT00536172 {unpublished data only}
- Burke WJ. Evaluating the effectiveness of escitalopram to prevent depression in head and neck cancer patients receiving treatment (PROTECT). ClinicalTrials.gov 2007.
NCT00740571 {published data only}
- Vissers K. Amitriptyline or pregabalin to treat neuropathic pain in incurable cancer (Off‐label). ClinicalTrials.gov 2009. [NCT00740571]
NCT00832520 {unpublished data only}
- Verschraegen C. Phase II study of Remeron for cancer patients losing more than 10% of their body weight. ClinicalTrials.gov 2009.
NCT01219673 {unpublished data only}
- Rosenthal DI. Symptom burden in head and neck cancer. ClinicalTrials.gov 2010.
NCT01256008 {published data only}
- He JC (study chair). Intervention study of depression in breast cancer patients. ClinicalTrial.gov 2010. [NCT01256008]
NCT01501396 {unpublished data only}
- Waqar S. Megestrol acetate with or without mirtazapine in treating cancer patients with weight loss or loss of appetite. ClinicalTrials.gov 2011.
NCT01598584 {unpublished data only}
- Yi Ba. Mirtazapine plus gemcitabine versus gemcitabinein metastasis pancreatic cancer. clincaltrials.gov 2012. [NCT01598584]
NCT01719861 {published data only}
- Neal JW. Phase 2a desipramine in small cell lung cancer and other high‐grade neuroendocrine tumors. clinicatrials.gov 2017. [NCT01719861] [DOI] [PMC free article] [PubMed]
NCT01725048 {unpublished data only}
- Park E. Pilot study to evaluate individualized choice of antidepressants in patients with cancer. ClinicalTrials.gov 2012.
NCT02443194 {published data only}
- Roll M. The effect of duloxetine on mood, quality of life and cognitive functioning in glioblastoma patients. clinicaltrials.gov 2015. [NCT02443194]
NCT02650544 {published data only}
- Zhang L. Efficacy and safety analyses of mirtazapine in NSCLC patients with depression. clinicaltrials.gov 2016. [NCT02650544]
NCT03086148 {published data only}
- Han R, Peng Y. Ketamine and postoperative depressive symptom. ClinicalTrials.gov 2017. [NCT03086148]
Ng 2014 {published data only}
- Ng CG, Boks MP, Roes KC, Zainal NZ, Sulaiman AH, Tan SB, et al. Rapid response to methylphenidate as an add‐on therapy to mirtazapine in the treatment of major depressive disorder in terminally ill cancer patients: a four‐week, randomized, double‐blinded, placebo‐controlled study. European Neuropsychopharmacology 2014;24:491‐8. [DOI: 10.1016/j.euroneuro.2014.01.016] [DOI] [PubMed] [Google Scholar]
Nunez 2013 {published data only}
- Nunez GR, Pinczowski H, Zanellato R, Tateyama L, Schindler F, Fonseca F, et al. Bupropion for control of hot flashes in breast cancer survivors: a prospective, double‐blind, randomized, crossover, pilot phase II trial. Journal of Pain and Symptom Management 2013;45(6):969‐79. [DOI: 10.1016/j.jpainsymman.2012.06.011] [DOI] [PubMed] [Google Scholar]
Palesh 2012 {published data only}
- Palesh OG, Mustian KM, Peppone LJ, Janelsins M, Sprod LK, Kesler S, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Medicine 2012;13(9):1184‐90. [DOI: 10.1016/j.sleep.2012.06.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Panerai 1990 {published data only}
- Panerai AE, Monza G, Movilia P, Bianchi M, Francucci BM, Tiengo M. A randomized, within‐patient, cross‐over, placebo‐controlled trial on the efficacy and tolerability of the tricyclic antidepressants chlorimipramine and nortriptyline in central pain. Acta Neurologica Scandinavica 1990;82(1):34‐8. [PUBMED: 2239134] [DOI] [PubMed] [Google Scholar]
Rodriguez 2011 {published data only}
- Rodríguez Vega B, Palao A, Torres G, Hospital A, Benito G, Pérez E, et al. Combined therapy versus usual care for the treatment of depression in oncologic patients: a randomized controlled trial. Psycho‐oncology 2011;20(9):943‐52. [DOI: 10.1002/pon.1800] [DOI] [PubMed] [Google Scholar]
Roscoe 2005 {published data only}
- Roscoe JA, Morrow GR, Hickok JT, Mustian KM, Griggs JJ, Matteson SE, et al. Effect of paroxetine hydrochloride (Paxilò) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Research and Treatment 2005;89(3):243‐9. [PUBMED: 15754122] [DOI] [PubMed] [Google Scholar]
Stockler 2007 {published data only}
- Stockler MR, O'Connell R, Nowak AK, Goldstein D, Turner J, Wilcken NR, et al. Zoloft's Effects on Symptoms and Survival Time Trial Group. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression:a placebo‐controlled double‐blind randomised trial. Lancet Oncology 2007;8(7):603‐12. [PUBMED: 17548243] [DOI] [PubMed] [Google Scholar]
Taraz 2013 {published data only}
- Taraz M, Khatami MR, Dashti‐Khavidaki S, Akhonzadeh S, Noorbala AA, Ghaeli P, et al. Sertraline decreases serum level of interleukin‐6 (IL‐6) in haemodialysis patients with depression: results of a randomized double‐blind, placebo‐controlled clinical trial. International Immunopharmacology 2013;17(3):917‐23. [DOI: 10.1016/j.intimp.2013.09.020] [DOI] [PubMed] [Google Scholar]
Theobald 2002 {published data only}
- Theobald DE, Kirsh KL, Holtsclaw E, Donaghy K, Passik SD. An open‐label, crossover trial of mirtazapine (15 and 30 mg) in cancer patients with pain and other distressing symptoms. Journal of Pain and Symptom Management 2002;23(5):442‐7. [PUBMED: 12007762] [DOI] [PubMed] [Google Scholar]
Tondlova 1997 {published data only}
- Tondlova H, Bagtecky J. Citalopram in the treatment of depression in patients suffering from simultaneous serious somatic disorders. European Neuropsychopharmacology 1997;7(2):188. [DOI: ] [Google Scholar]
Tondlova 2002 {published data only}
- Tondlová H, Baštecký J. Citalopram and dosulepine in adjuvant treatment of oncological pain [Citalopram a dosulepin v adjuv antním léčení nádorové bolesti]. Bolest 2002;4. [Google Scholar]
UKCCCR {unpublished data only}
- UK Co‐ordinating Committee on Cancer Research (UKCCCR). Neuropathic Pain Trial I: a national randomised trial of amitriptyline versus placebo. controlled‐trials.com 2007. [PA/NPS]
Vitolins 2013 {published data only}
- Vitolins MZ, Griffin L, Tomlinson WV, Vuky J, Adams PT, Moose D, et al. Randomized trial to assess the impact of venlafaxine and soy protein on hot flashes and quality of life in men with prostate cancer. Journal of Clinical Oncology 2013;31(32):4092‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2003 {published data only}
- Zhang L. Paroxetine in treatment of cancer patients with anxiety and depression. Zhongguo zin li wei sheng za zhi (Chinese Mental Health Journal) 2003;17(7):482‐3. [ETOCRN134568493] [Google Scholar]
Zhang 2011 {published data only}
- Zhang J, Fang F, Zhang C. Preliminary study of association between breast cancer and depression [乳腺癌与抑郁相关性的初步研究]. Tumor 2011;31(5):457‐9. [DOI: 10.3781/j.issn.1000-7431.2011.05.015] [DOI] [Google Scholar]
Zimmerman 2016 {published data only}
- Zimmerman C, Atherton PJ, Pachman D, Seisler D, Wagner‐Johnston N, Dakhil S, et al. MC11C4: a pilot randomized, placebo‐controlled, double‐blind study of venlafaxine to prevent oxaliplatin‐induced neuropathy. Supportive Care in Cancer 2016;24(3):1071‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zvukova 2010 {published data only}
- Zvukova EM, Mokhov EM, Marasanov SB. Pharmacological correction of psychoemotional states of patients with thyroid nodules in the preoperative period [ФАРМАКОЛОГИЧЕСКАЯ КОРРЕКЦИЯ ПСИХОЭМОЦИОНАЛЬНОГОСТАТУСА БОЛЬНЫХ С УЗЛОВЫМИ ОБРАЗОВАНИЯМИЩИТОВИДНОЙ ЖЕЛЕЗЫ В ПРЕДОПЕРАЦИОННОМ ПЕРИОДЕГоУ ВПО Тверская государственная медицинская академия]. Russian Journal of Medicine 2011;3c(6):31‐3. [Google Scholar]
References to studies awaiting assessment
N0405078066 {unpublished data only}
- Rankin E. Randomised double‐blind placebo controlled trial of venlafaxine in recently diagnosed lung cancer patients: effects on symptom profiles after 12 weeks. controlled‐trials.com 2003. [N0405078066]
UMIN000008768 {unpublished data only}
- Nishimura R, Matsushita M. An randomized comparative study of efficacy and safety between mirtazapine and duloxetine hydrochloride in patients with cancer. UMIN Clinical Trials Registry 2012. [UMIN000008768]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The EORTC QLQ‐C30: a quality of life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365‐75. [DOI] [PubMed] [Google Scholar]
Ahmadian 2017
- Ahmadian E, Eftekhari A, Babaei H, Nayebi AM, Eghbal MA. Anti‐cancer effects of citalopram on hepatocellular carcinoma cells occur via cytochrome C release and the activation of NF‐kB. Anticancer Agents in Medicinal Chemistry 2017;17(11):1570‐7. [DOI: 10.2174/1871520617666170327155930] [DOI] [PubMed] [Google Scholar]
Akechi 2008
- Akechi T, Okuyama T, Onishi J, Morita T, Furukawa TA. Psychotherapy for depression among incurable cancer patients. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005537.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2000
- Anderson IM, Nutt DJ, Deakin JF. Evidence‐based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology (Oxford, England) 2000;14(1):3‐20. [DOI] [PubMed] [Google Scholar]
Andrade 2012
- Andrade C. Breast cancer and antidepressant use. Journal of Clinical Psychiatry 2012;73(9):e1156‐7. [DOI: 10.4088/JCP.12f08054] [DOI] [PubMed] [Google Scholar]
Andrews 2013
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation‐determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726‐35. [DOI: 10.1016/j.jclinepi.2013.02.003] [DOI] [PubMed] [Google Scholar]
Anwar 2017
- Anwar N, Kuppili PP, Balhara YPS. Depression and physical non‐communicable diseases: The need for an integrated approach. WHO South‐East Asia Journal of Public Health 2017;6(1):12‐7. [DOI: 10.4103/2224-3151.206158.] [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III). 3rd Edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R). 3rd Edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
Arrieta 2013
- Arrieta O, Angulo LP, Núñez‐Valencia C, Dorantes‐Gallareta Y, Macedo EO, Martínez‐López D, et al. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non‐small cell lung cancer. Annals of Surgical Oncology 2013;20(6):1941‐8. [DOI] [PubMed] [Google Scholar]
Barbui 2001
- Barbui C, Hotopf M. Amitriptyline v. the rest: still the leading antidepressant after 40 years of randomised controlled trials. British Journal of Psychiatry 2001;178:129‐44. [DOI] [PubMed] [Google Scholar]
Barbui 2011
- Barbui C, Cipriani A, Patel V, Ayuso‐Mateos JL, Ommeren M. Efficacy of antidepressants and benzodiazepines in minor depression: systematic review and meta‐analysis. British Journal of Psychiatry 2011;198(1):11‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Bowinik 2014
- Botwinick IC, Pursell L, Yu G, Cooper T, Mann JJ, Chabot JA. A biological basis for depression in pancreatic cancer. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2014;16(8):740‐3. [DOI: 10.1111/hpb.12201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradt 2015
- Bradt J, Shim M, Goodill SW. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD007103.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Breitbart 2000
- Breitbart W, Rosenfeld B, Pessin H, Kaim M, Funesti‐Esch J, Galietta M, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA 2000;284(22):2907‐11. [PUBMED: 11147988] [DOI] [PubMed] [Google Scholar]
Brenne 2013
- Brenne E, Loge JH, Kaasa S, Heitzer E, Knudsen AK, Weston E. Depressed patients with incurable cancer: which depressive symptoms do they experience?. Palliative and Supportive Care 2013;11(6):491‐500. [DOI: ] [DOI] [PubMed] [Google Scholar]
Buscemi 2006
- Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. Journal of Clinical Epidemiology 2006;59:697‐703. [DOI] [PubMed] [Google Scholar]
Caruso 2017
- Caruso R, Nanni MG, Riba M, Sabato S, Mitchell AJ, Croce E, et al. Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncologica 2017;56(2):146‐55. [DOI: 10.1080/0284186X.2016.1266090] [DOI] [PubMed] [Google Scholar]
Carvalho 2014
- Carvalho AF, Hyphantis T, Sales PM, Soeiro‐de‐Souza MG, Macêdo DS, Cha DS, et al. Major depressive disorder in breast cancer: a critical systematic review of pharmacological and psychotherapeutic clinical trials. Cancer Treatment Reviews 40;3:349‐55. [DOI: 10.1016/j.ctrv.2013.09.009] [DOI] [PubMed] [Google Scholar]
Casey 2011
- Casey P, Bailey S. Adjustment disorders: the state of the art. World Psychiatry 2011;10(1):11‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cella 1993
- Cella D, Tulsky D, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11:570‐9. [DOI] [PubMed] [Google Scholar]
Chan 2012
- Chan AW. Out of sight but not out of mind: how to search for unpublished clinical trial evidence. BMJ 2012;3(344):d8013. [DOI: 10.1136/bmj.d8013] [DOI] [PubMed] [Google Scholar]
Chan 2017
- Chan HL, Chiu WC, Chen VC, Huang KY, Wang TN, Lee Y, et al. SSRIs associated with decreased risk of hepatocellular carcinoma: A population‐based case‐control study.. Psycho‐oncology 2018;27(1):187‐92. [DOI: 10.1002/pon.4493] [DOI] [PubMed] [Google Scholar]
Cipriani 2009
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. Lancet 2009;373:746–58. [10.1016/S0140‐ 6736(09)60046‐5] [DOI] [PubMed] [Google Scholar]
Colleoni 2000
- Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet 2000;356(9238):1326‐7. [DOI] [PubMed] [Google Scholar]
Cramer 2017
- Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health‐related quality of life, mental health and cancer‐related symptoms in women diagnosed with breast cancer. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD010802.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz‐Frutos 2016
- Diaz‐Frutos D, Baca‐Garcia E, García‐Foncillas J, López‐Castroman J. Predictors of psychological distress in advanced cancer patients under palliative treatments. European Journal of Cancer Care 2016;25(4):608‐15. [DOI: 10.1111/ecc.12521] [DOI] [PubMed] [Google Scholar]
Ebede 2017
- Ebede CC, Jang Y, Escalante CP. Cancer‐related fatigue in cancer survivorship. The Medical Clinics of North America 2017;101(6):1085‐97. [DOI: 10.1016/j.mcna.2017.06.007] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Furmaniak 2016
- Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD005001.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Galway 2012
- Galway K, Black A, Cantwell M, Cardwell CR, Mills M, Donnelly M. Psychosocial interventions to improve quality of life and emotional wellbeing for recently diagnosed cancer patients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007064.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gil‐Ad 2008
- Gil‐Ad I, Zolokov A, Lomnitski L, Taler M, Bar M, Luria D, et al. Evaluation of the potential anti‐cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer‐xenografted mice. International Journal of Oncology 2008;33(2):277‐86. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ: GRADE Working Group. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 2012
- Hart SL, Hoyt MA, Diefenbach M, Anderson DR, Kilbourn KM, Craft LL, et al. Meta‐Analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. Journal of the National Cancer Institute 2012;104(13):990‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartung 2017
- Hartung TJ, Brähler E, Faller H, Härter M, Hinz A, Johansen C, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. European Journal of Cancer 2017;72:46‐53. [DOI: 10.1016/j.ejca.2016.11.017] [DOI] [PubMed] [Google Scholar]
Henry 1995
- Henry JA, Alexander CA, Sener EK. Relative mortality from overdose of antidepressants. BMJ 1995;310(6974):221‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hickie 2011
- Hickie IB, Rogers NL. Novel melatonin‐based therapies: potential advances in the treatment of major depression. Lancet 2011;378:621‐31. [DOI: 10.1016/S0140-6736(11)60095-0] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ioannidis 2008
- Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta‐analysis. Journal of Evaluation in Clinical Practice 2008;14(5):951‐7. [DOI] [PubMed] [Google Scholar]
Iovieno 2011
- Iovieno N, Tedeschini E, Ameral VE, Rigatelli M, Papakostas GI. Antidepressants for major depressive disorder in patients with a co‐morbid axis‐III disorder: a meta‐analysis of patient characteristics and placebo response rates in randomized controlled trials. International Clinical Psychopharmacology 2011;26:69‐74. [DOI] [PubMed] [Google Scholar]
Irwin 2013
- Irwin MR. Depression and insomnia in cancer: prevalence, risk factors, and effects on cancer outcomes. Current Psychiatry Reports 2013;15(11):404. [DOI: 10.1007/s11920-013-0404-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kathol 1981
- Kathol RG, Petty F. Relationship of depression to medical illness. A critical review. Journal of Affective Disorders 1981;3:111‐21. [DOI] [PubMed] [Google Scholar]
Kelly 2010
- Kelly CM, Juurlink DN, Gomes T, Duong‐Hua M, Pritchard KI, Austin PC, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ 2010;340(c693):1‐8. [DOI: 10.1136/bmj.c693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 2016
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Müller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R, CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Canadian Journal of Psychiatry 2016;61(9):540‐60. [10.1177/0706743716659417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2010
- Kim Y, Spillers RL. Quality of life of family caregivers at 2 years after a relative's cancer diagnosis. Psycho‐oncology 2010;19:431‐40. [DOI] [PubMed] [Google Scholar]
Laoutidis 2013
- Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta‐analysis. BMC Psychiatry 2013;13(140):1‐21. [DOI: 10.1186/1471-244X-13-140] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lapidus 2013
- Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatric Disease and Treatment 2013;9:1101‐12. [DOI: 10.2147/NDT.S36689] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2012
- Li M, Fitzgerald P, Rodin G. Evidence‐based treatment of depression in patients with cancer. Journal of Clinical Oncology 2012;30(11):1187‐96. [DOI] [PubMed] [Google Scholar]
Lloyd‐Williams 2009
- Lloyd‐Williams M, Shiels C, Taylor F, Dennis M. Depression – an independent predictor of early death inpatients with advanced cancer. Journal of Affective Disorders 2009;113:127‐32. [DOI] [PubMed] [Google Scholar]
Lopresti 2012
- Lopresti AL, Hood SD, Drummond PD. Multiple antidepressant potential modes of action of curcumin: a review of its anti‐inflammatory, monoaminergic, antioxidant, immune‐modulating and neuroprotective effects. Journal of Psychopharmacology 2012;26(12):1512‐24. [DOI: 10.1177/0269881112458732] [DOI] [PubMed] [Google Scholar]
McCaughan 2017
- McCaughan E, Parahoo K, Hueter I, Northouse L, Bradbury I. Online support groups for women with breast cancer. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD011652.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2011
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncology 2011;12:160‐74. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Mottram 2009
- Mottram PG, Wilson K, Strobl JJ. Antidepressants for depressed elderly. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003491.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2011
- Ng CG, Boks MPM, Zainal NZ, Wit NJ. The prevalence and pharmacotherapy of depression in cancer patients. Journal of Affective Disorders 2011;131:1‐7. [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health & Clinical Excellence (NICE). Depression in adults with a chronic physical health problem. Treatment and management [CG91]. National Clinical Practice Guideline 2009.
Nosé 2016
- Nosè M, Bighelli I, Castellazzi M, Martinotti G, Carrà G, Lucii C, et al: STAR NETWORK GROUP. Prevalence and correlates of QTc prolongation in Italian psychiatric care: Cross‐sectional multicentre study. Epidemiology and Psychiatric Sciences 2016;25(6):532‐40. [DOI: 10.1017/S2045796015000906] [DOI] [PMC free article] [PubMed] [Google Scholar]
Onitilo 2006
- Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all‐cause mortality in adults with cancer and differential effects by cancer site. General Hospital Psychiatry 2006;28:396‐402. [DOI] [PubMed] [Google Scholar]
Ostuzzi 2013
- Ostuzzi G, Bighelli I, Carrara BV, Dusi N, Imperadore G, Lintas C, et al. Making the use of psychotropic drugs more rational through the development of GRADE recommendations in specialist mental healthcare. International Journal of Mental Health Systems 2013;7(1):14. [DOI: 10.1186/1752-4458-7-14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostuzzi 2015
- Ostuzzi G, Benda L, Costa E, Barbui C. Efficacy and acceptability of antidepressants on the continuum of depressive experiences in patients with cancer: Systematic review and meta‐analysis. Cancer Treatment Reviews 2015;41(8):714‐24. [10.1016/j.ctrv.2015.06.003] [DOI] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [DOI] [PubMed] [Google Scholar]
Pilar‐Cuéllar 2013
- Pilar‐Cuéllar F, Vidal R, Díaz A, Castro E, dos Anjos S, Pascual‐Brazo J, et al. Neural plasticity and proliferation in the generation of antidepressant effects: hippocampal implication. Neural Plasticity 2013;2013:1‐21. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinquart 2010
- Pinquart M, Duberstein PR. Depression and cancer mortality: a meta‐analysis. Psychological Medicine 2010;40(11):1797‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prieto 2002
- Prieto JM, Blanch J, Atala J, Carreras E, Rovira M, Cirera E, et al. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem‐cell transplantation. Journal of Clinical Oncology 2002;20(7):1907‐17. [DOI] [PubMed] [Google Scholar]
Raison 2003
- Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biological Psychiatry 2003;54(3):283–94. [DOI] [PubMed] [Google Scholar]
Rayner 2010
- Rayner L, Price A, Evans A, Valsraj K, Higginson IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD007503.pub2] [DOI] [PubMed] [Google Scholar]
Rayner 2011a
- Rayner L, Price A, Evans A, Valsraj K, Hotopf M, Higginson IJ. Antidepressants for the treatment of depression in palliative care: systematic review and meta‐analysis. Palliative Medicine 2011;25(1):36‐51. [DOI] [PubMed] [Google Scholar]
Rayner 2011b
- Rayner L, Price A, Hotopf M, Higginson IJ. The development of evidence‐based European guidelines on the management of depression in palliative cancer care. European Journal of Cancer 2011;47:702‐12. [DOI] [PubMed] [Google Scholar]
Riblet 2014
- Riblet N, Larson R, Watts BV, Holtzheimer P. Reevaluating the role of antidepressants in cancer‐related depression: a systematic review and meta‐analysis. General Hospital Psychiatry 2014;36:466‐73. [DOI] [PubMed] [Google Scholar]
Rodin 2007
- Rodin G, Lloyd N, Katz M, Green E, Mackay JA, Wong RK, Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence‐Based Care. The treatment of depression in cancer patients: a systematic review. Supportive Care in Cancer 2007;15(2):123‐36. [DOI] [PubMed] [Google Scholar]
Rooney 2010
- Rooney A, Grant R. Pharmacological treatment of depression in patients with a primary brain tumour. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD006932.pub2] [DOI] [PubMed] [Google Scholar]
Rooney 2013
- Rooney A, Grant R. Pharmacological treatment of depression in patients with a primary brain tumour. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD006932.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarganas 2014
- Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug‐induced long QT syndrome and Torsade de Pointes in Germany. Europace 2014;16(1):101‐8. [DOI: 10.1093/europace/eut214] [DOI] [PubMed] [Google Scholar]
Sarubin 2014
- Sarubin N, Nothdurfter C, Schmotz C, Wimmer AM, Trummer J, Lieb M, et al. Impact on cortisol and antidepressant efficacy of quetiapine and escitalopram in depression. Psychoneuroendocrinology 2014;39:141‐51. [DOI: 10.1016/j.psyneuen.2013.10.008] [DOI] [PubMed] [Google Scholar]
Satin 2009
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients. A meta‐analysis. Cancer 2009;22:5349‐61. [DOI] [PubMed] [Google Scholar]
Semple 2013
- Semple C, Parahoo K, Norman A, McCaughan E, Humphris G, Mills M. Psychosocial interventions for patients with head and neck cancer. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009441.pub2] [DOI] [PubMed] [Google Scholar]
Sharpe 2014
- Sharpe M, Walker J, Holm Hansen C, Martin P, Symeonides S, Gourley C, et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology‐2): a multicentre randomised controlled effectiveness trial. Lancet 2014;384(9948):1099‐108. [10.1016/S0140‐6736(14)61231‐9] [DOI] [PubMed] [Google Scholar]
Shelton 2007
- Shelton RC. The molecular neurobiology of depression. Psychiatric Clinics of North America 2007;30(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shim 2012
- Shim EJ, Park JH. Suicidality and its associated factors in cancer patients: results of a multi‐center study in Korea. International Journal of Psychiatry in Medicine 2012;43(4):381‐403. [DOI] [PubMed] [Google Scholar]
Shin 2016
- Shin ES, Seo KH, Lee SH, Jang JE, Jung YM, Kim MJ, Yeon JY. Massage with or without aromatherapy for symptom relief in people with cancer. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD009873] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva de Lima 1999
- Silva de Lima M, Hotoph M, Wessely S. The efficacy of drug treatments for dysthymia: a systematic review and meta‐analysis. Psychological Medicine 1999;29(6):1273‐89. [DOI] [PubMed] [Google Scholar]
Silva de Lima 2005
- Silva de Lima M, Moncrieff J, Soares B. Drugs versus placebo for dysthymia. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sotelo 2014
- Sotelo JL, Musselman D, Nemeroff C. The biology of depression in cancer and the relationship between depression and cancer progression. International Review of Psychiatry 2014;26(1):16–30. [DOI] [PubMed] [Google Scholar]
Sterne 2000
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119‐29. [DOI] [PubMed] [Google Scholar]
Stewart 1965
- Stewart MA, Drake F, Winokur G. Depression among medically ill patients. Diseases of the Nervous System 1965;26:479‐85. [PubMed] [Google Scholar]
Thompson 2017
- Thompson LMA, Bobonis Babilonia M. Distinguishing depressive symptoms from similar cancer‐related somatic symptoms: implications for assessment and management of major depression after breast cancer. Southern Medical Journal 2017;110(10):667‐72. [DOI: 10.14423/SMJ.0000000000000705] [DOI] [PubMed] [Google Scholar]
Turner 2008
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressants trials and its influence on apparent efficacy. New England Journal of Medicine 2008;358:252–60. [DOI: 10.1056/NEJMsa065779] [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.MR000030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergouwen 2003
- Vergouwen AC, Bakker A, Katon WJ, Verheij TJ, Koerselman F. Improving adherence to antidepressants: a systematic review of interventions. Journal of Clinical Psychiatry 2003;64(12):1415‐20. [PUBMED: 14728101] [DOI] [PubMed] [Google Scholar]
Walker 2014
- Walker J, Sawhney A, Holm Hansen C, Ahmed S, Martin P, Symeonides S, et al. Treatment of depression in adults with cancer: a systematic review of randomized controlled trials. Psychological Medicine 2014;44(5):897‐907. [DOI] [PubMed] [Google Scholar]
Wang 2011
- Wang DS, Li GL, Chen JH, Liu XM. Effects of psychological interventions in cancer patients undergoing radiotherapy. Chinese Journal of Clinical Psychology 2011;19(4):561‐3. [Google Scholar]
Ware 1980
- Ware JB, Brook RH, Williams KN, Stewart AL, Davies‐Avery A. Conceptualisation and measurement of health for adults in the health insurance study. Model of Health and Methodology. Santa Monica, California: Rand Corporation, 1980; Vol. 1. [R‐1987/1‐HEW]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36) 1: conceptual framework and item selection. Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
WHO 1978
- WHO. The Ninth Revision of the International Classification of Diseases and Related Health Problems (ICD‐9). Geneva: World Health Organization, 1978. [Google Scholar]
WHO 1992
- WHO. The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD‐10). Geneva: World Health Organization, 1992. [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Losing participants before the trial ends erodes credibility of findings. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Yi 2017
- Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. The Medical Clinics of North America 2017;101(6):1099‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]
Zingone 2017
- Zingone A, Brown D, Bowman ED, Vidal O, Sage J, Neal J, et al. Relationship between anti‐depressant use and lung cancer survival. Cancer Treatment and Research Communications 2017;10:33‐9. [DOI: 10.1016/j.ctarc.2017.01.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ostuzzi 2014 (protocol)
- Ostuzzi G, Matcham F, Dauchy S, Barbui C, Hotopf M. Antidepressants for the treatment of depression in patients with cancer. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostuzzi 2015 (full review)
- Ostuzzi G, Matcham F, Dauchy S, Barbui C, Hotopf M. Antidepressants for the treatment of depression in people with cancer. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011006.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


