Abstract
Background
Liver transplantation is an established treatment option for end‐stage liver failure. Now that newer, more potent immunosuppressants have been developed, glucocorticosteroids may no longer be needed and their removal may prevent adverse effects.
Objectives
To assess the benefits and harms of glucocorticosteroid avoidance (excluding intra‐operative use or treatment of acute rejection) or withdrawal versus glucocorticosteroid‐containing immunosuppression following liver transplantation.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded and Conference Proceedings Citation Index ‐ Science, Literatura Americano e do Caribe em Ciencias da Saude (LILACS), World Health Organization International Clinical Trials Registry Platform, ClinicalTrials.gov, and The Transplant Library until May 2017.
Selection criteria
Randomised clinical trials assessing glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression for liver transplanted people. Our inclusion criteria stated that participants should have received the same co‐interventions. We included trials that assessed complete glucocorticosteroid avoidance (excluding intra‐operative use or treatment of acute rejection) versus short‐term glucocorticosteroids, as well as trials that assessed short‐term glucocorticosteroids versus long‐term glucocorticosteroids.
Data collection and analysis
We used RevMan to conduct meta‐analyses, calculating risk ratio (RR) for dichotomous variables and mean difference (MD) for continuous variables, both with 95% confidence intervals (CIs). We used a random‐effects model and a fixed‐effect model and reported both results where a discrepancy existed; otherwise we reported only the results from the fixed‐effect model. We assessed the risk of systematic errors using 'Risk of bias' domains. We controlled for random errors by performing Trial Sequential Analysis. We presented our results in a 'Summary of findings' table.
Main results
We included 17 completed randomised clinical trials, but only 16 studies with 1347 participants provided data for the meta‐analyses. Ten of the 16 trials assessed complete postoperative glucocorticosteroid avoidance (excluding intra‐operative use or treatment of acute rejection) versus short‐term glucocorticosteroids (782 participants) and six trials assessed short‐term glucocorticosteroids versus long‐term glucocorticosteroids (565 participants). One additional study assessed complete post‐operative glucocorticosteroid avoidance but could only be incorporated into qualitative analysis of the results due to limited data published in an abstract. All trials were at high risk of bias. Only eight trials reported on the type of donor used. Overall, we found no statistically significant difference for mortality (RR 1.15, 95% CI 0.93 to 1.44; low‐quality evidence), graft loss including death (RR 1.15, 95% CI 0.90 to 1.46; low‐quality evidence), or infection (RR 0.88, 95% CI 0.73 to 1.05; very low‐quality evidence) when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression. Acute rejection and glucocorticosteroid‐resistant rejection were statistically significantly more frequent when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (RR 1.33, 95% CI 1.08 to 1.64; low‐quality evidence; and RR 2.14, 95% CI 1.13 to 4.02; very low‐quality evidence). Diabetes mellitus and hypertension were statistically significantly less frequent when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (RR 0.81, 95% CI 0.66 to 0.99; low‐quality evidence; and RR 0.76, 95% CI 0.65 to 0.90; low‐quality evidence). We performed Trial Sequential Analysis for all outcomes. None of the outcomes crossed the monitoring boundaries or reached the required information size. Hence, we cannot exclude random errors from the results of the conventional meta‐analyses.
Authors' conclusions
Many of the benefits and harms of glucocorticosteroid avoidance or withdrawal remain uncertain because of the limited number of published randomised clinical trials, limited numbers of participants and outcomes, and high risk of bias in the trials. Glucocorticosteroid avoidance or withdrawal appears to reduce diabetes mellitus and hypertension whilst increasing acute rejection, glucocorticosteroid‐resistant rejection, and renal impairment. We could identify no other benefits or harms of glucocorticosteroid avoidance or withdrawal. Glucocorticosteroid avoidance or withdrawal may be of benefit in selected patients, especially those at low risk of rejection and high risk of hypertension or diabetes mellitus. The optimal duration of glucocorticosteroid administration remains unclear. More randomised clinical trials assessing glucocorticosteroid avoidance or withdrawal are needed. These should be large, high‐quality trials that minimise the risk of random and systematic error.
Plain language summary
Glucocorticosteroid‐free versus glucocorticosteroid‐containing immunosuppression for liver transplanted patients
Review question
We assessed whether avoiding or withdrawing glucocorticosteroids was better or worse than continuing to use glucocorticosteroids for immunosuppression after liver transplantation.
Background
Glucocorticosteroids are used to prevent rejection of the liver after transplantation by suppressing the immune system. Some centres use glucocorticosteroids indefinitely after liver transplantation whilst others slowly reduce them, and others do not use glucocorticosteroids at all. Glucocorticosteroids have a number of important adverse effects, which may lead to illness and sometimes death in liver transplantation. These adverse effects include diabetes mellitus, high blood pressure, and infection.
With recent developments in immunosuppression, glucocorticosteroids no longer feature as the main immunosuppressant used following transplantation. The use of new immunosuppressant medication may mean that glucocorticosteroids may no longer be necessary after transplantation. Rather than helping to prevent rejection of the liver graft they might cause adverse effects. The benefits of avoiding glucocorticosteroids or withdrawing them after a short while remain unclear.
Study characteristics We searched for trials comparing glucocorticosteroid avoidance or withdrawal to continuing glucocorticosteroids. Seventeen randomised clinical trials were included, of which 16 trials involving 1347 participants provided numeric data for the meta‐analyses. All of the studies assessed adults who had received a liver transplant. Of the 16 randomised clinical trials included in the meta‐analyses, 10 trials assessed avoidance of glucocorticosteroids compared with slowly reducing glucocorticosteroids (782 participants) and six trials assessed withdrawal of glucocorticosteroids following a slow reduction compared with a longer reduction or long‐term use of glucocorticosteroids (565 participants). Only eight trials reported on the type of donor used. The evidence is current to May 2017.
Key results Rejection, severe rejection, and kidney failure may be increased by avoiding or withdrawing glucocorticosteroids compared with continuing glucocorticosteroids. Diabetes mellitus and high blood pressure may be reduced by avoiding or withdrawing glucocorticosteroids compared with continuing glucocorticosteroids. We did not find any difference in survival of the patients, survival of the liver, other adverse effects, or health‐related quality of life.
Quality of the evidence
We assessed all of the trials we included as being at high risk of bias, which means that they may overestimate the benefits and underestimate the harms of avoiding or withdrawing glucocorticosteroids. The evidence was either low quality or very low quality.
Conclusion There is still some uncertainty about the benefits and harms of avoiding or withdrawing glucocorticosteroids after transplantation. Avoiding or withdrawing glucocorticosteroids appears to increase rejection, severe rejection, and kidney failure but seems to reduce diabetes mellitus and high blood pressure. We found no other obvious benefits or harms of avoiding or withdrawing glucocorticosteroids. More randomised clinical trials are needed to assess avoidance and withdrawal of glucocorticosteroids for liver transplanted patients.
Summary of findings
Summary of findings for the main comparison. Glucocorticosteroid avoidance or withdrawal compared to glucocorticosteroid‐based immunosuppression for liver transplanted patients.
| Glucocorticosteroid avoidance or withdrawal compared to glucocorticosteroid‐based immunosuppression for liver transplanted patients | ||||||
| Patient or population: liver transplanted patients Setting: inpatient and outpatient Intervention: glucocorticosteroid avoidance or withdrawal Comparison: glucocorticosteroid‐based immunosuppression | ||||||
| Outcomes** | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Risk with glucocorticosteroid‐based immunosuppression | Risk with glucocorticosteroid avoidance or withdrawal | |||||
| All‐cause mortality | Study population | RR 1.15 (0.93 to 1.44) | 1323 (15 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | The quality of the evidence was considered low for both glucocorticosteroid avoidance and glucocorticosteroid withdrawal. Trial Sequential Analysis‐adjusted CI 0.77‐1.66. | |
| 166 per 1000 | 191 per 1000 (154 to 239) | |||||
| Moderate | ||||||
| 204 per 1000 | 234 per 1000 (189 to 293) | |||||
| Graft loss including death | Study population | RR 1.15 (0.90 to 1.46) | 1002 (11 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | The quality of the evidence was considered low for both glucocorticosteroid avoidance and glucocorticosteroid withdrawal. Trial Sequential Analysis‐adjusted CI 0.75‐2.01. | |
| 175 per 1000 | 203 per 1000 (159 to 259) | |||||
| Moderate | ||||||
| 218 per 1000 | 253 per 1000 (198 to 322) | |||||
| Acute rejection | Study population | RR 1.33 (1.08 to 1.64) | 1347 (16 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | The quality of the evidence was considered low both glucocorticosteroid avoidance and glucocorticosteroid withdrawal. Trial Sequential Analysis‐adjusted CI 0.92‐1.90. | |
| 173 per 1000 | 230 per 1000 (187 to 283) | |||||
| Moderate | ||||||
| 194 per 1000 | 257 per 1000 (209 to 317) | |||||
| Infection | Study population | RR 0.88 (0.73 to 1.05) | 778 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | The quality of the evidence was considered very low for both glucocorticosteroid avoidance and glucocorticosteroid withdrawal. Trial Sequential Analysis‐adjusted CI 0.49‐1.71. | |
| 359 per 1000 | 316 per 1000 (262 to 377) | |||||
| Moderate | ||||||
| 402 per 1000 | 354 per 1000 (293 to 422) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We assessed all outcomes at latest follow‐up (range 13 months to 108 months). CI: confidence interval; RR: risk ratio; RCT: randomised clinical trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level due to risk of bias: all trials were at high risk of bias. 2Downgraded one level due to imprecision identified in the Trial Sequential Analysis; 95% CI includes both benefit and harm. 3Downgraded one level due to significant heterogeneity identified between subgroups; avoidance versus withdrawal.
Background
Liver transplantation is an established treatment option for end‐stage liver failure in selected patients and results in improved quality and quantity of life (Pillai 2009; Dienstag 2012). Currently, liver transplant recipients have a one‐year survival of over 90% and a five‐year survival of over 75% (Perera 2009).
Description of the condition
Over 1800 liver transplantations per year (whole liver or split liver) were performed from post‐mortem and living donors in the Eurotransplant region from 2008 to 2012 (Eurotransplant 2012). However, at the end of 2011 there were 2406 people in need of liver transplantation (Eurotransplant 2012). In the UK, 784 liver transplantations were carried out in 2012 through 2013, but 494 patients remained on the waiting list as of 31 March 2013 (NHS Blood and Transplant 2013). In the USA, 6445 livers were transplanted in 2013 including 252 living donor liver transplants (OPTN 2014). In 2012, in the UK, the indications for liver transplantation from deceased donors included alcoholic liver disease (18.5%), hepatitis C virus (HCV) cirrhosis (8.1%), hepatocellular carcinoma (17.1%), primary sclerosing cholangitis (8.2%), primary biliary cirrhosis (7.6%), and metabolic diseases (8.1%). Of the deceased donor transplants, 88% were elective procedures and 12% for fulminant hepatic failure (Johnson 2014).
Description of the intervention
Liver transplant recipients have to take life‐long immunosuppressive medication in order to achieve an effective prophylaxis against allograft rejection. The most commonly used immunosuppressive agents are calcineurin inhibitors (e.g. cyclosporine, tacrolimus), antiproliferative agents (e.g. azathioprine, mycophenolate mofetil), and glucocorticosteroids (e.g. methylprednisolone). In addition, mammalian target of rapamycin inhibitors (e.g. sirolimus, everolimus) are used to prevent rejection. Induction agents are often used to prevent rejection and facilitate calcineurin inhibitor and glucocorticosteroid minimisation (Lupo 2008; Neumann 2012; Kim 2013; Herzer 2016). Glucocorticosteroids decrease interleukin 1, 2, and 6 activity and non‐specifically inhibit T‐cell activation. Adverse effects due to glucocorticosteroids such as hypertension, hyperglycaemia, hypercholesterolaemia, and obesity are well known. In some cases, hypertension is reported in over 50% of patients (Neal 2005; Llado 2006), but a glucocorticosteroid bolus is still given at the time of transplantation and tapered after a while (Fernandez 1998; Hatz 1998; Renoult 2005; Hirose 2006). Cyclosporin A and tacrolimus are both calcineurin inhibitors. Calcineurin normally activates nuclear factor of activated T cells, which leads to production of interleukin 2 and 4 that stimulate growth and differentiation of the T‐cell response. Tacrolimus is used more widely than cyclosporin due to reduced acute rejection and increased graft survival, but tacrolimus carries a higher risk of new‐onset diabetes after transplant (NODAT) (Ho 1996; Ojo 2003; Haddad 2006). Despite the favourable profile of tacrolimus compared with cyclosporine, tacrolimus still carries a significant risk of renal failure and many trials have investigated the replacement of tacrolimus with other drugs, usually sirolimus or everolimus (Penninga 2012; Sterneck 2014). Mycophenolate mofetil (MMF; also known as mycophenolic acid; MPA) inhibits inosine monophosphate dehydrogenase (IMPDH). This enzyme is responsible for de novo synthesis of guanosine nucleotides. The inhibition by MMF has cytostatic effects on T‐ and B‐lymphocytes. MMF is still preferred to azathioprine (Allison 2000; Knight 2009).
How the intervention might work
Through the use of calcineurin inhibitors, liver transplantation has become a standard procedure with good long‐term results (Haddad 2006). However, the burden of life‐long immunosuppressive treatment in liver transplant recipients causes increased morbidity and mortality. Optimal long‐term immunosuppressive treatment to reduce morbidity and mortality without leading to graft loss has become of major importance. Treatment with glucocorticosteroids induces bone loss and may lead to cardiovascular risk factors (e.g. hypertension, hyperlipidaemia, obesity, glucose intolerance) (Hatz 1998). Avoidance of glucocorticosteroids may reduce this excess morbidity without having an effect on graft loss (Knight 2011). In addition, use of glucocorticosteroids after transplantation might reduce physical and mental health‐related quality of life, and increase symptoms of anxiety (Zaydfudim 2012). Furthermore, glucocorticosteroids might increase the risk and severity of hepatitis C recurrence in patients transplanted for hepatitis C. Hence, glucocorticosteroid avoidance and reduction regimens for liver transplant recipients have been developed and studied, but it is still uncertain whether these regimens offer clear benefits (Segev 2008). These long‐term adverse events and the development of relatively new immunosuppressive medication (e.g. basiliximab) may potentially enable the reduction or withdrawal of glucocorticosteroids as an immunosuppressive treatment (Vanrenterghem 1999; Ganschow 2005; Penninga 2014).
There is some evidence that glucocorticosteroid avoidance or withdrawal could be beneficial (Adams 2001; Kato 2005; Cintorino 2006; Llado 2006; Moench 2007; Penninga 2014a), but the overall effect still remains unclear. Five reviews with meta‐analyses on glucocorticosteroid avoidance or withdrawal for liver transplanted people have been published, showing a possible advantage in cardiovascular risk factors (e.g. diabetes mellitus, hypertension), a possible benefit in cytomegalovirus (CMV) infection and a possible benefit for people transplanted for HCV‐induced liver disease (Segev 2008; Sgourakis 2009; Knight 2011; Gu 2014; Lan 2014). One Cochrane network meta‐analysis of maintenance immunosuppression for liver transplanted patients has been published showing a possible decrease in adverse events, but a possible increase in retransplantation with glucocorticosteroid avoidance or withdrawal (Rodríguez‐Perálvarez 2017).
Why it is important to do this review
It is possible that glucocorticosteroids could be withdrawn following liver transplantation or completely avoided without any negative effects whilst reducing the adverse effects associated with glucocorticosteroids. However, people may face more adverse events due to increased use of other immunosuppressants.
Objectives
To assess the benefits and harms of glucocorticosteroid avoidance (excluding intra‐operative use or treatment of acute rejection) or withdrawal versus glucocorticosteroid‐containing immunosuppression following liver transplantation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials evaluating the benefits and harms of complete glucocorticosteroid avoidance (excluding intra‐operative use or treatment of acute rejection) or withdrawal versus glucocorticosteroid‐containing immunosuppression for liver transplanted people. We did not include non‐randomised clinical trials or trials that reported per‐treatment analysis rather than intention‐to‐treat analysis. For evaluation of harms, we included quasi‐randomised clinical trials and observational trials that we identified during our searches for randomised clinical trials.
We did not apply any restrictions on date of publication, language, or publication status (published or unpublished work).
Types of participants
We included people of any age, sex, and ethnic group during and after liver transplantation, in any care setting, irrespective of diagnosis and disease stage, type of graft (live donor, cadaveric, split, whole, domino), and prescribed medication. We did not include participants with other transplanted organs or those with a previous liver transplant.
Types of interventions
We included randomised clinical trials that investigated weaning off, versus not weaning off, glucocorticosteroids, as well as trials that compared standard immunosuppression without glucocorticosteroids versus standard immunosuppression including glucocorticosteroids directly following transplantation.
We allowed co‐interventions (e.g. induction with basiliximab, co‐administration of an antiproliferative such as mycophenolate mofetil) if received equally by all intervention groups of the trial.
Types of outcome measures
Outcome measures did not form part of the eligibility criteria for including trials in this review. We assessed all outcomes at latest follow‐up.
Primary outcomes
All‐cause mortality.
Graft loss including death.
Acute rejection. This is diagnosed by the combination of abnormal liver biochemical variables (e.g. bilirubin, aspartate transaminase, alanine transaminase, alkaline phosphatases, gamma glutamyl transpeptidase), clinical signs such as fever, and liver histological changes including mononuclear portal inflammation, bile duct damage, and subendothelial inflammation of portal or terminal hepatic veins (IWP 1995; IP 2000).
Infection.
We have not included serious adverse events as an outcome as following organ transplantation the number of serious adverse events is extremely high. As a result of this, very few trials in transplantation report serious adverse events as an outcome and instead report outcomes individually (e.g. diabetes mellitus, infection, hypertension). As well as this, most transplant recipients experience one or more serious adverse events following transplantation, meaning that the number of adverse events may be 100% in both groups. This means neither complete nor consistent serious adverse event reporting can be guaranteed. Instead, we analysed selected outcomes individually.
Secondary outcomes
Other adverse events. Adverse events were defined as any untoward medical occurrence not necessarily having a causal relationship with the treatment but resulting in a dose reduction or discontinuation of treatment (ICH‐GCP 1997).
Chronic rejection. Chronic rejection was characterised by liver histological changes including the progressive loss of interlobular bile ducts and arteriopathy characterised by foam cell infiltration of the arterial intima.
Glucocorticosteroid‐resistant rejection.
Diabetes mellitus (de novo diabetes mellitus as described in the study or total number of people with diabetes mellitus).
Cytomegalovirus (CMV) infection (infection requiring treatment).
Hepatitis C virus (HCV) recurrence.
Malignancy.
Post‐transplantation lymphoproliferative disorder (PTLD).
Renal function (renal failure requiring dialysis, renal insufficiency, estimated glomerular filtration rate, and serum creatinine).
De novo autoimmune hepatitis.
Hypertension.
Hyperlipidaemia.
Cholesterol (serum cholesterol and hypercholesterolaemia).
Health‐related quality of life.
Search methods for identification of studies
We searched for eligible trials for the earliest entrance date possible until the latest search date.
We managed all references with Refworks©.
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2018; May 2017), Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5), MEDLINE Ovid (1946 to May 2017), Embase Ovid (1974 to May 2017), Science Citation Index Expanded (Web of Science; 1900 to May 2017), Conference Proceedings Citation Index ‐ Science (Web of Science; 1990 to May 2017) (Royle 2003) and LILACS (Literatura Americano e do Caribe em Ciencias da Saude; Clark 2002; 1982 to May 2017). Appendix 1 gives the search strategies with the time spans of the searches. As the review progressed, we did not need to improve the search strategies.
We also searched the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/), ClinicalTrials.gov (clinicaltrials.gov/), and The Transplant Library (Pengel 2011).
Searching other resources
We contacted experts in the field, such as scientific societies for liver transplantation, and we asked whether they have been involved in any further trials or are aware of recent or ongoing trials on the effects of glucocorticosteroids for liver transplanted patients. We tried to identify unpublished trials by contacting manufacturers of glucocorticosteroids (i.e. Ratiopharm, Astellas, Aventis, Novartis, Merck, Hexal, Pfizer, Roche).
We searched the reference lists of identified trials, non‐randomised trials, and other systematic reviews for additional publications of interest.
Data collection and analysis
Selection of studies
Four review authors (CF, EH, JP, SW) independently assessed the retrieved references for eligibility and resolved disagreements by discussion with another author (LP). The excluded studies and the reasons for their exclusion are listed in the table Characteristics of excluded studies.
Data extraction and management
We extracted data on source, inclusion and exclusion criteria, description of participants and setting, interventions and co‐interventions, outcomes, and sample size calculation using a data extraction sheet. We did not identify any cross‐over trials. We extracted data using the intention‐to‐treat principle. We translated all trials reported in non‐English language journals before assessment. Where multiple publications of a trial exist, we grouped the publications together and we extracted data from the most complete publication and any relevant outcomes that are only reported in one of the other publications. Where further information was required, we contacted the original authors requesting this.
Assessment of risk of bias in included studies
Four review authors (CF, JP, EH, SW) independently assessed the risk of bias of the trials, without masking them. We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2018). Due to the risk of biased overestimation of beneficial intervention effects (or underestimating of harmful effects) in randomised trials (Schulz 1995; Moher 1998; Kjaergard 2001;Wood 2008; Savović 2012; Savović 2012a; Lundh 2017), we assessed the following bias risk domains with definitions below. If information was not available in the published trial, we contacted the authors in order to assess the trials correctly.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: it was mentioned that both participants and personnel providing the interventions were blinded, and the method of blinding was described, so that knowledge of allocation was prevented during the trial.
Unclear risk of bias: it was not mentioned if the trial was blinded, or the trial was described as blinded, but the method or extent of blinding was not described, so that knowledge of allocation was possible during the trial.
High risk of bias: the trial was not blinded, so that the allocation was known during the trial.
Blinded outcome assessment
Low risk of bias: it was mentioned that both participants and personnel providing the interventions were blinded, and the method of blinding was described, so that knowledge of allocation was prevented during the trial.
Unclear risk of bias: it was not mentioned if the trial was blinded, or the trial was described as blinded, but the method or extent of blinding was not described, so that knowledge of allocation was possible during the trial.
High risk of bias: the trial was not blinded, so that the allocation was known during the trial.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, have been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias in the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk: the trial reported the following pre‐defined outcomes: all‐cause mortality, graft loss including death, acute rejection, and infection. If the original trial protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, we did not consider those outcomes to be reliable.
Unclear risk: not all pre‐defined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk: one or more pre‐defined outcomes were not reported.
For‐profit bias
Low risk of bias: the trial appears to be free of industry sponsorship or other type of for‐profit support that may manipulate the trial design, conduct, or results of the trial.
Uncertain risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received another type of for‐profit support.
Other risk of bias
Low risk of bias: the trial appears to be free of other components that could put it at risk of bias.
Uncertain risk of bias: the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias: there are other factors in the trial that could put it at risk of bias.
We considered trials assessed as having 'low risk of bias' in all of the specified individual domains as trials with 'low risk of bias'. We considered trials assessed as having 'uncertain risk of bias' or 'high risk of bias' in one or more of the specified individual domains as trials with 'high risk of bias'.
Measures of treatment effect
For dichotomous variables, we used risk ratio (RR) and 95% confidence intervals (CI).
For continuous variables, we used the mean difference (MD) with 95% CI. If we had been able to identify different measures for the health‐related quality of life outcome, we would have used standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
In the case of trials using multiple treatment groups, we considered only the group in which glucocorticosteroids were administered versus the group in which either placebo or no intervention was administered. If we had been able to identify trials assessing two or more groups with different glucocorticosteroid‐containing immunosuppression regimens compared to a control group, we would have included data from all the groups and ensured that participants were included only once per meta‐analysis. If we had been able to identify any cross‐over trials, we would have extracted data from the first period of treatment only.
Dealing with missing data
Where possible, we contacted the original authors of articles with missing outcomes, missing summary data, or missing individual data to request the missing data.
We included all participants irrespective of compliance or follow‐up. We analysed all available data and performed best‐worst and worst‐best case scenario analyses in the event of missing data.
Assessment of heterogeneity
We explored heterogeneity by the Chi2 test with significance set at P = 0.01, and we measured the quantity of heterogeneity with the I2 statistic (Higgins 2002).
We assessed clinical heterogeneity by examining the included trials for differences between the trials in types of participants (including age, indication for transplantation, and presence of hepatitis C infection), quantity of glucocorticosteroid used (duration of treatment and daily dose), and additional immunosuppression (use of induction agents, use of antiproliferative agents, and use of calcineurin inhibitors).
Assessment of reporting biases
We used a funnel plot to explore publication bias (Egger 1997; Macaskill 2001), as we identified more than 10 randomised trials. We used the linear regression approach described by Egger and colleagues to determine the funnel plot asymmetry (Egger 1997).
Data synthesis
We performed the meta‐analyses according to the recommendations of Cochrane (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2018). We used the software package Review Manager 5.3 to conduct meta‐analyses when there were two or more eligible trials (RevMan 2014). For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval. For continuous variables, we calculated the mean difference (MD) with 95% confidence interval. We used a random‐effects model (DerSimonian 1986), and a fixed‐effect model (DeMets 1987). In case of discrepancy between the two models, we reported both results; otherwise, we reported only the results from the fixed‐effect model. We grouped trials investigating complete avoidance of glucocorticosteroids together with trials investigating a rapid taper of glucocorticosteroids as 'glucocorticosteroid avoidance and withdrawal' (Gluc avoid) protocols. We presented both avoidance and rapid tapers as separate subtotals and where a discrepancy exists between the two protocols, we reported both results separately.
Trial Sequential Analysis
We applied Trial Sequential Analysis, as cumulative meta‐analyses are at risk of producing random errors because of sparse data and repetitive testing on accumulating data (Thorlund 2011b; TSA 2011; Wetterslev 2017). To minimise random errors, we calculated the diversity‐adjusted required information size (DARIS) (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Wetterslev 2008; Wetterslev 2009). The DARIS calculation accounts for the heterogeneity present in the meta‐analysis. In our meta‐analysis, the DARIS was based on the assumption of a plausible RR reduction of 20% (Wetterslev 2008). The underlying assumption of Trial Sequential Analysis is that significance testing may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication, and if more than one trial was published in a year, we added trials alphabetically according to the family name of the first author. On the basis of the risk for type I (5%) and type II (20%) errors, the chosen RR, the proportion with the outcome in the control group, and the observed heterogeneity, we calculated the DARIS and we constructed the trial sequential monitoring boundaries for benefits and harms (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; Wetterslev 2017). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size. If the cumulative Z‐curve crosses the trial sequential monitoring boundary for benefit or harm before the required information size is reached in a cumulative meta‐analysis, firm evidence may have been established and further trials may be superfluous. On the other hand, if the sequential monitoring boundaries are not surpassed and the trial monitoring boundaries for futility are not crossed, it is most probably necessary to continue doing trials in order to detect or reject a certain intervention effect. We used as default a type I error of 5%, type II error of 20%, and a DARIS as found in the conventional meta‐analysis unless otherwise stated (Wetterslev 2008; Thorlund 2011a).
Subgroup analysis and investigation of heterogeneity
We performed the following pre‐defined subgroup analyses.
Different immunosuppressive agents.
Co‐interventions: comparing the intervention effect of trials with one, two, or three co‐interventions.
Duration of treatment with glucocorticosteroids.
Trials before the year 2000 compared to trials in and after the year 2000 (since immunosuppression protocols have changed notably since 2000).
We were unable to perform the following pre‐defined subgroup analyses due to lack of evidence.
Trials at low risk of bias compared to trials at high risk of bias.
Paediatric compared to adult liver transplantation.
Time between transplantation and start of glucocorticosteroid administration, determined by the median time.
Different indications for transplant.
Sensitivity analysis
We determined potential sensitivity analyses when we assessed our results to examine the robustness of our findings.
Zero event trials
Review Manager 5 software is unable to handle trials with zero events in both intervention groups when meta‐analyses are performed as risk ratios or odds ratios. It seems unjustified and unreasonable to exclude zero event trials (Keus 2009), and potentially create the risk of inflating the magnitude of the pooled treatment effects. Therefore, we also performed a random‐effects meta‐analysis with empirical continuity correction of 0.01 in zero event trials (Sweeting 2004; Keus 2009), using the R software (R 2017).
'Summary of findings' tables
We constructed a 'Summary of findings' table for the comparison glucocorticoid‐free versus glucocorticoid‐containing immunosuppression following liver transplantation, presenting data on all primary outcomes and assessing the quality of the evidence based on risk of bias, imprecision, indirectness, heterogeneity, and risk of publication bias. We used the software GRADEpro© (GRADEpro 2008) to create Table 1.
Results
Description of studies
Results of the search
Our electronic searches identified 4893 references (Figure 1). Searching of bibliographies found 115 additional references. Exclusion of duplicates and irrelevant references left 17 completed randomised clinical trials published in a total of 70 publications (32 peer‐reviewed journal articles, 37 conference abstracts, and one clinical trials registry listing) (see Characteristics of included studies; Characteristics of excluded studies). One of the randomised clinical trials was published in a conference abstract and did not provide sufficient numeric data to allow incorporation of these data into our meta‐analysis (Zhong 2010). Of the 16 randomised clinical trials included in our meta‐analysis, four of the trials were published only in peer‐reviewed journals (Belli 1998; Chen 2007; Hu 2008; Ju 2012), eleven of the trials were published as both peer‐reviewed journal articles and conference abstracts (Tisone 1999; Belli 2001; Pageaux 2004; Margarit 2005; Reggiani 2005; Llado 2006; Moench 2007; Vivarelli 2007; Lerut 2008; Pelletier 2013; Ramirez 2013), and one was published only as a conference abstract (Studenik 2005).
1.
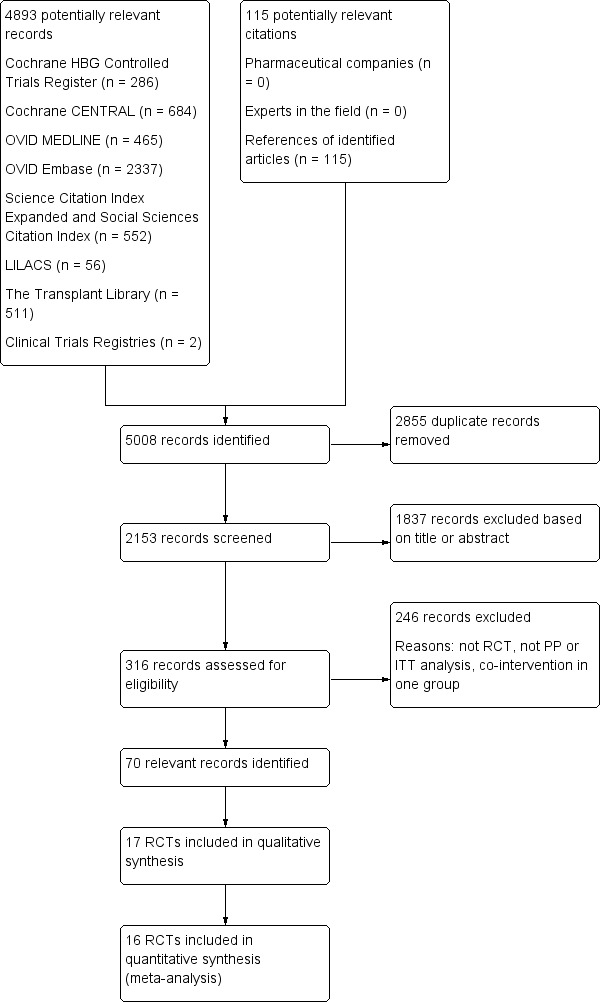
Flow chart to show studies included and excluded. RCT – randomised clinical trial; PP ‐ per protocol; ITT – intention‐to‐treat; HBG – Hepato‐Biliary Group.
Included studies
We included 16 randomised clinical trials in our meta‐analysis, of which 15 trials were two‐armed trials and one was a three‐armed trial (Belli 2001). An additional trial, published in conference abstracts, could not be included in the quantitative analysis as it did not describe the number of participants allocated to each arm of the trial (Zhong 2010). The abstract published data on 182 participants and it is not clear from the abstract if the trial had been completed at the time of the conference. The trial was anticipated to include a total of 300 participants according to the study record within the National Library of Medicine Clinical Trials Registry (Zhong 2010). It is not possible to extract accurate numeric data from the abstract. The abstract reports the percentage of participants in each group of the trial who develop each outcome, but it does not report how many participants are randomised to each arm. For this reason, for the remainder of our quantitative results, we refer to the 16 completed trials which can be incorporated into the quantitative analysis of this review.
The 16 trials included a total of 1347 participants in whom glucocorticosteroids were compared as follows: complete glucocorticosteroid avoidance (excluding intra‐operative use or treatment of acute rejection) versus short‐term glucocorticosteroids was compared in 10 trials with a total of 782 participants (Tisone 1999; Belli 2001; Margarit 2005; Reggiani 2005; Studenik 2005; Llado 2006; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013); and short‐term glucocorticosteroids versus long‐term glucocorticosteroids were compared in six trials with a total of 565 participants (Belli 1998; Pageaux 2004; Chen 2007; Moench 2007; Vivarelli 2007; Hu 2008). The additional trial compared complete glucocorticosteroid avoidance (excluding intra‐operative use or treatment of acute rejection) versus an unspecified duration of glucocorticosteroids (Zhong 2010).
As stated, complete glucocorticosteroid avoidance (excluding intra‐operative use or treatment of acute rejection) was used in the experimental group in 10 trials (Tisone 1999; Belli 2001; Margarit 2005; Reggiani 2005; Studenik 2005; Llado 2006; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013). These trials of complete post‐transplant glucocorticosteroid avoidance allowed glucocorticosteroids during the perioperative period and for treatment of acute rejection. Seven trials used no glucocorticosteroids in the perioperative period (Tisone 1999; Belli 2001; Margarit 2005; Reggiani 2005; Llado 2006; Pelletier 2013; Ramirez 2013), two trials used 500 mg glucocorticosteroids in the perioperative period (Studenik 2005; Ju 2012), and one trial used 100 mg glucocorticosteroids in the perioperative period (Lerut 2008).
For the full details of glucocorticosteroid regimens (including doses, frequencies, durations, and tapers) for each arm in all 16 trials included in the meta‐analysis and the trial included in the qualitative analysis see Characteristics of included studies.
Characteristics of the studies
Sixteen of the trials are published in English. One of the trials is published only in Mandarin (Hu 2008). Two of the trials have additional publications in languages other than English: one abstract is published in German (Moench 2007), and one article in Mandarin (Ju 2012).
Mean follow‐up time was reported in 12 trials and varied from 13 months to 108 months (Belli 1998; Tisone 1999; Belli 2001; Margarit 2005; Reggiani 2005; Studenik 2005; Moench 2007; Vivarelli 2007; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013).
Four of the 17 trials were multicentre (Pageaux 2004; Llado 2006; Vivarelli 2007; Zhong 2010), and the remaining 13 were single centre (Belli 1998; Tisone 1999; Belli 2001; Margarit 2005; Reggiani 2005; Studenik 2005; Chen 2007; Moench 2007; Hu 2008; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013).
All 17 of the trials consisted of exclusively adult populations.
Mean age of the intervention groups was reported in 14 trials (Belli 1998; Tisone 1999; Pageaux 2004; Margarit 2005; Reggiani 2005; Llado 2006; Chen 2007; Moench 2007; Vivarelli 2007; Hu 2008; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013). Mean age of the participants ranged from 42 to 58 years. Sex ratio of the participants was reported in 12 trials (Belli 1998; Tisone 1999; Pageaux 2004; Margarit 2005; Reggiani 2005; Llado 2006; Chen 2007; Moench 2007; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013). The total number of male participants in the 12 trials was 845 (73.0%) and the total number of female participants was 312 (27.0%).
All of the trials report the primary indications for transplantation. In 12 trials there were a variety of indications (Belli 1998; Tisone 1999; Pageaux 2004; Margarit 2005; Reggiani 2005; Studenik 2005; Llado 2006; Moench 2007; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013). Two trials exclusively included participants with hepatitis C virus (HCV) cirrhosis as the primary indication for transplantation, with a total of 71 participants (Belli 2001; Vivarelli 2007). Three trials exclusively included participants with hepatocellular carcinoma as the primary indication for transplantation (Chen 2007; Hu 2008; Zhong 2010). A total of 258 participants were reported as having HCV cirrhosis as the primary indication for transplantation, although there might have been more participants who had an alternative primary indication but were also HCV positive. Two trials published separate articles dealing with a cohort of HCV‐positive participants including a total of 124 participants (Llado 2006; Lerut 2008). One trial described the outcomes of HCV‐positive participants as a separate cohort within the main article, including a total of 35 participants (Margarit 2005).
Eight trials reported on the type of donor used. In six of the trials, the grafts were obtained exclusively from deceased (cadaveric) donors (Pageaux 2004; Llado 2006; Vivarelli 2007; Hu 2008; Ju 2012; Ramirez 2013). In two of the trials, the grafts were obtained from both deceased (cadaveric) and living donors (Moench 2007; Lerut 2008), but in one of these trials the deceased donors were exclusively donors after brain death (Moench 2007). The remaining trials did not report on type of donor used (Belli 1998; Tisone 1999; Belli 2001; Margarit 2005; Reggiani 2005; Studenik 2005; Chen 2007; Zhong 2010; Pelletier 2013).
Fifteen trials reported on the duration of glucocorticosteroid administration in the glucocorticosteroid‐containing arm. One trial administered glucocorticosteroids for 64 days in the glucocorticosteroid‐containing arm (Lerut 2008). Seven trials administered glucocorticosteroids for three months in the glucocorticosteroid‐containing arm (Tisone 1999; Belli 2001; Margarit 2005; Reggiani 2005; Llado 2006; Hu 2008; Ju 2012). One trial administered glucocorticosteroids for three to six months in the glucocorticosteroid‐containing arm (Pelletier 2013). Two trials administered glucocorticosteroids for six months in the glucocorticosteroid‐containing arm (Moench 2007; Ramirez 2013). One trial administered glucocorticosteroids for nine months in the glucocorticosteroid‐containing arm (Studenik 2005). One trial administered glucocorticosteroids for 25 months in the glucocorticosteroid‐containing arm (Vivarelli 2007). Two trials administered glucocorticosteroids indefinitely in the glucocorticosteroid‐containing arm (Belli 1998; Chen 2007). Two trials did not report the duration of glucocorticosteroid administration in the glucocorticosteroid‐containing arm (Pageaux 2004; Zhong 2010). For the subgroup analyses on duration of glucocorticosteroid administration, we grouped the trials together as 'less than or equal to three months', 'greater than three and up to six months', and 'greater than six months'.
Five trials were commenced before 2000 (Belli 1998; Tisone 1999; Belli 2001; Pageaux 2004; Margarit 2005), and the remaining 12 trials were commenced from 2000 onwards (Reggiani 2005; Studenik 2005; Llado 2006; Chen 2007; Moench 2007; Vivarelli 2007; Hu 2008; Lerut 2008; Zhong 2010; Ju 2012; Pelletier 2013; Ramirez 2013).
Three trials reported no missing data at latest follow‐up and provided adequate data to explain if any participants were not included in the analysis so that these participants could be included in the meta‐analysis (Moench 2007; Lerut 2008; Ramirez 2013). One of these trials reported 12/124 participants refusing biopsy at five years (Lerut 2008). Nine trials did not report the number of dropouts adequately (Tisone 1999; Belli 2001; Reggiani 2005; Studenik 2005; Llado 2006; Chen 2007; Zhong 2010; Ju 2012; Pelletier 2013). Five trials reported at least one participant lost to follow‐up, with a total of 25/642 participants in the glucocorticosteroid avoidance or withdrawal group lost to follow‐up and 21/651 participants in the glucocorticosteroid‐containing group lost to follow‐up. One trial reported two dropouts in each group (Belli 1998). One trial reported three dropouts in the glucocorticosteroid withdrawal group and four dropouts in the glucocorticosteroid‐containing group (Hu 2008). One trial reported one dropout in the glucocorticosteroid withdrawal group and no dropouts in the glucocorticosteroid‐containing group (Margarit 2005). One trial reported 19 dropouts in the glucocorticosteroid withdrawal group and 12 dropouts in the glucocorticosteroid‐containing group (Pageaux 2004). One trial reported no dropouts in the glucocorticosteroid withdrawal group and three dropouts in the glucocorticosteroid‐containing group (Vivarelli 2007). One trial excluded 16 participants from the reported acute rejection rate due to treatment failure (Belli 1998). Our protocol stated that all available data should be analysed using the intention‐to‐treat principle (Fairfield 2014). Therefore, we included the three participants in the glucocorticosteroid withdrawal group and 13 participants in the long‐term glucocorticosteroid group as 'lost to follow‐up' for the outcome 'acute rejection'.
Concomitant immunosuppression
All trials reported on concomitant immunosuppression, but this varied between trials. Of the 17 trials all used a calcineurin inhibitor with 11 using tacrolimus (Margarit 2005; Reggiani 2005; Studenik 2005; Chen 2007; Moench 2007; Vivarelli 2007; Hu 2008; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013), and six used cyclosporine A (Belli 1998; Tisone 1999; Belli 2001; Pageaux 2004; Llado 2006; Zhong 2010). One trial replaced tacrolimus with sirolimus when clinically indicated (Ju 2012). Of the 11 trials in which tacrolimus was used, five of the trials used no other concomitant immunosuppression as described in the intervention groups (Margarit 2005; Moench 2007; Vivarelli 2007; Hu 2008; Lerut 2008) (see Characteristics of included studies).
Seven of the 17 trials used an antiproliferative agent, with six trials using mycophenolate mofetil (Reggiani 2005; Studenik 2005; Chen 2007; Ju 2012; Pelletier 2013; Ramirez 2013), and one trial using azathioprine (Tisone 1999). All of the trials that used mycophenolate mofetil also used tacrolimus and the one trial that used azathioprine used cyclosporine A.
Induction therapy with a non‐glucocorticosteroid agent was used in nine of the trials. Two trials used rabbit antithymocyte globulin (RATG) (Belli 1998; Belli 2001); six trials used basiliximab (Pageaux 2004; Llado 2006; Zhong 2010; Ju 2012; Pelletier 2013; Ramirez 2013); and one trial used daclizumab (Studenik 2005).
Concomitant immunosuppression consisted of a calcineurin inhibitor used in combination with an antiproliferative agent in three trials (Tisone 1999; Reggiani 2005; Chen 2007). Concomitant immunosuppression consisted of a calcineurin inhibitor used in combination with induction therapy in five trials (Belli 1998; Belli 2001; Pageaux 2004; Llado 2006; Zhong 2010). Concomitant immunosuppression consisted of triple therapy with a calcineurin inhibitor, an antiproliferative agent, and induction therapy in four trials (Studenik 2005; Ju 2012; Pelletier 2013; Ramirez 2013).
Excluded studies
We excluded 27 trials after reading the full text of the articles. These articles mostly related to randomised clinical trials but did not assess glucocorticosteroid‐containing versus glucocorticosteroid‐free immunosuppression. We explained the reasons for their exclusion in Characteristics of excluded studies.
Risk of bias in included studies
Trial methodology was only adequately reported in two of the trials (Moench 2007; Lerut 2008) (see Figure 2; Figure 3). We considered all 17 of the trials to be at high risk of bias as we considered one or more of the bias components of each trial to be at unclear risk of bias due to inadequately reported methodology or at high risk of bias (Belli 1998; Tisone 1999; Belli 2001; Pageaux 2004; Margarit 2005; Reggiani 2005; Studenik 2005; Llado 2006; Chen 2007; Moench 2007; Vivarelli 2007; Hu 2008; Lerut 2008; Zhong 2010; Ju 2012; Pelletier 2013; Ramirez 2013).
2.
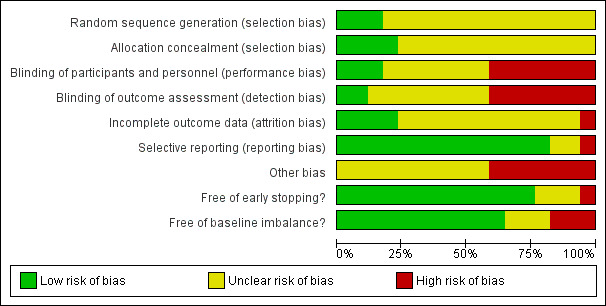
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
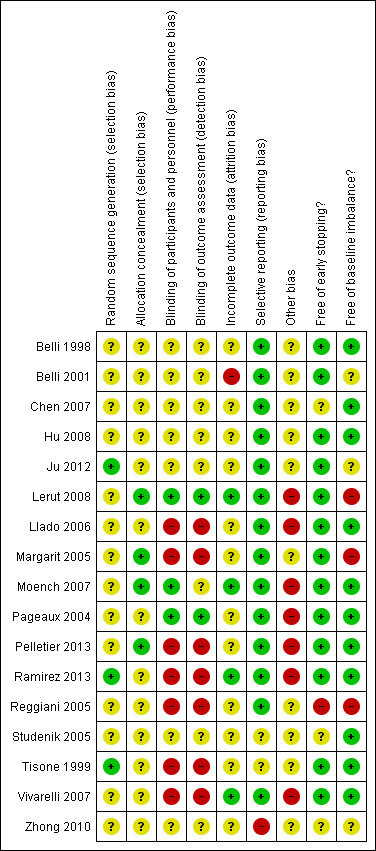
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of the allocation sequence was adequately reported in three trials (Tisone 1999; Ju 2012; Ramirez 2013), and inadequately reported in 14 trials (Belli 1998; Belli 2001; Pageaux 2004; Margarit 2005; Reggiani 2005; Studenik 2005; Llado 2006; Chen 2007; Moench 2007; Vivarelli 2007; Hu 2008; Lerut 2008; Zhong 2010; Pelletier 2013).
Allocation concealment was adequate in four trials (Margarit 2005; Moench 2007; Lerut 2008; Pelletier 2013), and inadequately reported in 13 trials (Belli 1998; Tisone 1999; Belli 2001; Pageaux 2004; Reggiani 2005; Studenik 2005; Llado 2006; Chen 2007; Vivarelli 2007; Hu 2008; Zhong 2010; Ju 2012; Ramirez 2013).
Blinding
Three trials reported accurately and applied adequate methods for blinding of participants (Pageaux 2004; Moench 2007; Lerut 2008). One of these trials blinded participants but not outcome assessors (Moench 2007). Seven trials did not report on blinding (Belli 1998; Belli 2001; Studenik 2005; Chen 2007; Hu 2008; Zhong 2010; Ju 2012), and seven trials did not perform blinding (Tisone 1999; Margarit 2005; Reggiani 2005; Llado 2006; Vivarelli 2007; Pelletier 2013; Ramirez 2013).
Incomplete outcome data
In four trials, either no data were missing or missing data were adequately reported and unlikely to have influenced outcome results (Moench 2007; Vivarelli 2007; Lerut 2008; Ramirez 2013). In the remaining 13 trials missing data were inadequately addressed (Belli 1998; Tisone 1999; Belli 2001; Pageaux 2004; Margarit 2005; Reggiani 2005; Studenik 2005; Llado 2006; Chen 2007; Hu 2008; Zhong 2010; Ju 2012; Pelletier 2013). In one trial, a participant was excluded following a re‐transplant and death 10 days later (Ramirez 2013); as this occurred after randomisation, we had to re‐enter the participant into the analysis for inclusion in the meta‐analysis. In one trial, three participants were excluded due to early death (two participants) and positive cross‐match (one participant) (Margarit 2005); as this occurred after randomisation, we had to re‐enter the participants into the analysis for inclusion in the meta‐analysis: we added one case of mortality to each group and one case of missing data to the glucocorticosteroid‐free group as well as we adjusted the totals accordingly. The trial did not make comment on any other missing data. One trial excluded nine participants due to early death (five participants) and ABO‐blood group incompatibility (four participants) (Ju 2012), reporting on the original allocated groups of the deaths but not the ABO‐blood group incompatibility; as this occurred after randomisation, we had to re‐enter the participants who suffered from early mortality into the analysis for inclusion in the meta‐analysis. One trial excluded eight participants due to early death (three participants), graft loss (two participants), change to alternative primary immunosuppressant (two participants), and de novo hepatitis B virus (HBV) infection (one participant) (Vivarelli 2007); as this occurred after randomisation, we had to re‐enter the participants into the analysis for inclusion in the meta‐analysis: we added the cases of mortality and graft loss to the intervention groups accordingly, and we counted the change in immunosuppressant and HBV infection as loss to follow‐up. As some of these participants were randomised but excluded from the analysis, they might not have been included in the demographic data except where authors had provided relevant details. In the three‐armed trial, six participants died and one developed portal vein thrombosis (Belli 2001). The participants were split between the three arms (two in the standard therapy arm; three in the glucocorticosteroid‐free arm; and two in the glucocorticosteroid‐free and ribavirin arm), but which group the participant with portal vein thrombosis was in and which groups the deaths occurred in was not reported. We could not include the outcome of mortality in this trial in the main analysis, but it was possible to include it in the best‐worst, worst‐best analysis: the number of participants suffering from mortality is either one or two in the standard therapy arm and either two or three in the glucocorticosteroid‐free arm, and we used these values in the analysis.
Missing summary data
One trial reported mean arterial pressure, serum cholesterol, and fasting blood glucose, but it did not provide a standard deviation or range (Ramirez 2013). Furthermore, in this trial, no exact P values were reported, but P values were described as "NS" (not significant) (Ramirez 2013). These results are included in this review.
Selective reporting
We had no access to the protocols for any of the trials other than the trial only included in qualitative analysis (Zhong 2010). One trial was published only in an abstract, so no comment on selective reporting could be made (Studenik 2005). Of the 15 remaining trials, 14 reported expected clinical outcome measures or outcomes as specified in the methods section of the article (Belli 1998; Belli 2001; Pageaux 2004; Margarit 2005; Reggiani 2005; Llado 2006; Chen 2007; Moench 2007; Vivarelli 2007; Hu 2008; Lerut 2008; Ju 2012; Pelletier 2013; Ramirez 2013). One trial did not report expected outcome of hypertension described in the introduction and discussion section of the article (Tisone 1999).
Other potential sources of bias
Seven trials reported part or full industry sponsorship (Pageaux 2004; Llado 2006; Moench 2007; Vivarelli 2007; Lerut 2008; Pelletier 2013; Ramirez 2013). Four trials reported sponsorship exclusively from other sources (Margarit 2005; Hu 2008; Zhong 2010; Ju 2012). The remaining six trials did not report on sponsorship (Belli 1998; Tisone 1999; Belli 2001; Reggiani 2005; Studenik 2005; Chen 2007).
Three of the 17 trials reported a required sample size calculation (Llado 2006; Moench 2007; Lerut 2008), whilst the remainder did not (Belli 1998; Tisone 1999; Belli 2001; Pageaux 2004; Margarit 2005; Reggiani 2005; Studenik 2005; Chen 2007; Vivarelli 2007; Hu 2008; Zhong 2010; Ju 2012; Pelletier 2013; Ramirez 2013).
Thirteen of the trials appeared to be free from early stopping. One of the trials was stopped early following an interim analysis. The stopping criteria were not described in the trial that was stopped early (Reggiani 2005). Two trials did not report adequately on early stopping (Studenik 2005, Chen 2007). One trial was published only as a conference abstract and reported only preliminary findings; the data are not included in this review due to inadequate reporting of participants in each intervention group (Zhong 2010).
Eleven of the 17 trials are free from baseline imbalance (Belli 1998; Tisone 1999; Pageaux 2004; Studenik 2005; Llado 2006; Chen 2007; Moench 2007; Vivarelli 2007; Hu 2008; Pelletier 2013; Ramirez 2013). Three trials reported on significant baseline imbalance (Margarit 2005; Reggiani 2005; Lerut 2008). In three of the trials, the baseline characteristics were inadequately reported to allow comparison (Belli 2001; Zhong 2010; Ju 2012).
Effects of interventions
See: Table 1
See Table 1 for the effects of glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression for liver transplanted patients.
Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression
All‐cause mortality
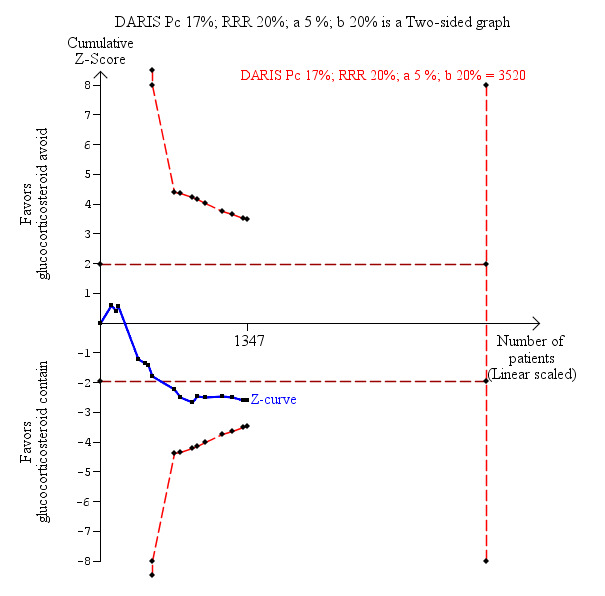
Fifteen trials with 1323 participants reported adequately on mortality, and overall we found no statistically significant difference when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (128/659 (19%) versus 110/664 (17%); risk ratio (RR) 1.15, 95% confidence interval (CI) 0.93 to 1.44; low‐quality evidence; Analysis 1.1). One trial reported the total number of deaths and a portal vein thrombosis as a composite outcome for the entire trial but did not adequately describe to which group the portal vein thrombosis and the deaths belonged (Belli 2001). As a result of this, the trial could not be included for this outcome except in the best‐worst and worst‐best analyses (Analysis 8.1; Analysis 9.1). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 3520 participants was not obtained (Figure 4).
1.1. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 1 Mortality.
8.1. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 1 Mortality.
9.1. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 1 Mortality.
4.
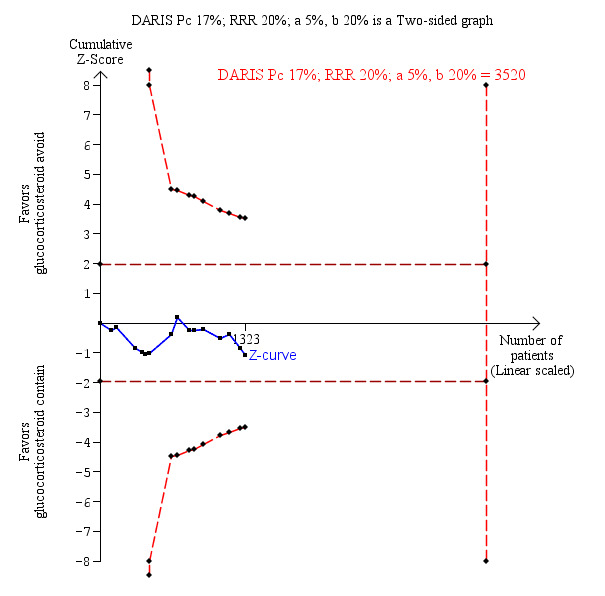
Mortality: glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid containing immunosuppression. Trial Sequential Analysis of the effect of glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression on mortality based on 15 trials with 1323 participants. The diversity‐adjusted required information size (DARIS) of 3520 participants was calculated on the basis of type I error of 5%, type II error of 20% and risk reduction of 20%, and information size was adjusted for diversity (0%). The cumulative Z‐curve does not cross trial sequential monitoring boundaries, and the required information size was not reached.
Graft loss including death
Eleven trials with 1002 participants reported on graft loss including death, and overall we found no statistically significant difference when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (118/631 (19%) versus 97/638 (15%); RR 1.15, 95% CI 0.90 to 1.46; low‐quality evidence; Analysis 1.2). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 2423 participants was not obtained (Figure 5).
1.2. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 2 Graft loss including death.
5.
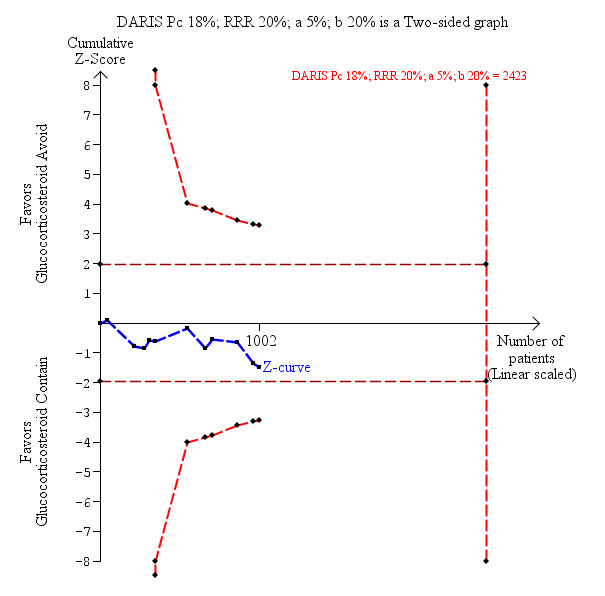
Graft loss including death: glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid containing immunosuppression. Trial Sequential Analysis of the effect of glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression on graft loss including death based on 11 trials with 1002 participants. The diversity‐adjusted required information size (DARIS) was calculated on the basis of type I error of 5%, type II error of 20% and risk reduction of 20%, and information size was adjusted for diversity (0%). The cumulative Z‐curve does not cross trial sequential monitoring boundaries, and the required information size was not reached.
Acute rejection
Acute rejection was defined as the total number of participants who experienced one or more rejection episodes. Sixteen trials with 1347 participants reported on acute rejection, and acute rejection was statistically significantly more frequent when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (150/670 (22%) versus 117/677 (17%); RR 1.33, 95% CI 1.08 to 1.64; low‐quality evidence; Analysis 1.3). However, Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 3520 participants was not obtained (Figure 6).
1.3. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 3 Acute rejection.
6.
Acute rejection: glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid containing immunosuppression. Trial Sequential Analysis of the effect of glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression on acute rejection based on 16 trials with 1347 participants. The diversity‐adjusted required information size (DARIS) was calculated on the basis of type I error of 5%, type II error of 20% and risk reduction of 20%, and information size was adjusted for diversity (0%). The cumulative Z‐curve does not cross trial sequential monitoring boundaries, and the required information size was not reached.
Infection
Eight trials with 778 participants reported adequately on infection, and overall we found no statistically significant difference when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (120/382 (31%) versus 142/396 (36%); RR 0.88, 95% CI 0.73 to 1.05; very low‐quality evidence; Analysis 1.4). Infection was defined in each of the eight trials as the number of participants who experienced one or more infection. Two other trials reported the total number of cases of infection including those with multiple episodes of infection (Margarit 2005; Lerut 2008). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 3222 participants was not obtained.
1.4. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 4 Infection.
Other adverse events
No trials reported on adverse events. A number of trials reported "deaths due to an adverse event" or separate adverse events such as the development of de novo diabetes mellitus but none of the trials reported the total number of adverse events.
Chronic rejection
Nine trials with 974 participants reported on chronic rejection, and overall we found no statistically significant difference when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid containing immunosuppression (16/482 (3%) versus 15/492 (3%); RR 1.08, 95% CI 0.56 to 2.10; very low‐quality evidence; Analysis 1.5). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 26,534 participants was not obtained.
1.5. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 5 Chronic rejection.
Glucocorticosteroid‐resistant rejection
Glucocorticosteroid‐resistant rejection was defined as the total number of participants who experienced one or more glucocorticosteroid‐resistant rejections. Ten trials with 1020 participants reported on glucocorticosteroid‐resistant rejection, and glucocorticosteroid‐resistant rejection was statistically significantly more frequent when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (27/505 (5%) versus 13/515 (3%); RR 2.14, 95% CI 1.13 to 4.02; very low‐quality evidence; Analysis 1.6). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 2190 participants was not obtained.
1.6. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 6 Glucocorticosteroid‐resistant rejection.
Diabetes mellitus
Twelve trials with 1185 participants reported on diabetes mellitus, and diabetes mellitus was not significantly different when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (125/588 (21%) versus 156/597 (26%); RR 0.82, 95% CI 0.64 to 1.07; low‐quality evidence) when we applied the random‐effects model. However, when we applied the fixed‐effect model, diabetes mellitus was statistically significantly less frequent when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (RR 0.81, 95% CI 0.66 to 0.99; low‐quality evidence; Analysis 1.7). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 3348 participants was not obtained.
1.7. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 7 Diabetes mellitus.
Cytomegalovirus (CMV) infection
CMV infection was defined as the development of CMV disease requiring treatment. Seven trials with 786 participants reported on CMV infection, and overall we found no statistically significant difference when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (28/387 (7%) versus 38/399 (10%); RR 0.74, 95% CI 0.48 to 1.16; low‐quality evidence; Analysis 1.8). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 6429 participants was not obtained.
1.8. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 8 CMV.
Hepatitis C virus (HCV) recurrence
Ten trials with 477 participants reported on HCV recurrence, and overall we found no statistically significant difference when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (159/232 (69%) versus 162/245 (66%); RR 1.03, 95% CI 0.92 to 1.15; very low‐quality evidence; Analysis 1.9). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve but the required information size of 435 participants was obtained, meaning that we can exclude a relative risk reduction of 20% or more.
1.9. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 9 HCV recurrence.
Malignancy
Three trials with 528 participants reported on de novo malignancy, and overall we found no statistically significant difference when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (3/258 (1%) versus 7/270 (3%); RR 0.52, 95% CI 0.16 to 1.74; very low‐quality evidence; Analysis 1.10). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 22,911 participants was not obtained.
1.10. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 10 Malignancy.
Post‐transplant lymphoproliferative disorder
Two trials with 330 participants reported on post‐transplant lymphoproliferative disorder, and overall we found no statistically significant difference when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (3/162 (2%) versus 1/168 (1%); RR 2.39, 95% CI 0.36 to 15.95; very low‐quality evidence; Analysis 1.11). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 70,005 participants was not obtained.
1.11. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 11 Post‐transplant lymphoproliferative disorder.
Renal function
No trials reported on renal failure requiring dialysis.
Four trials with 447 participants reported on renal insufficiency, and overall we found no statistically significant difference when glucocorticosteroid avoidance was compared with glucocorticosteroid‐containing immunosuppression (67/216 (31%) versus 77/231 (33%); RR 0.93, 95% CI 0.73 to 1.19; very low‐quality evidence; Analysis 1.12). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 3735 participants was not obtained.
1.12. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 12 Renal insufficiency.
No trials reported on estimated glomerular filtration rate.
Four trials with 309 participants reported on creatinine (mg/dL), and creatinine was not significantly different when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (MD 0.01 mg/dL, 95% CI ‐0.21 to 0.23; very low‐quality evidence) when we applied the random‐effects model. However, when we applied the fixed‐effect model, creatinine was statistically significantly raised when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (MD 0.11 mg/dL, 95% CI 0.07 to 0.16; very low‐quality evidence; Analysis 1.13).
1.13. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 13 Creatinine.
De novo autoimmune hepatitis
No trials reported on de novo autoimmune hepatitis.
Hypertension
Ten trials with 1098 participants reported on hypertension, and hypertension was statistically significantly less frequent when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (157/543 (29%) versus 210/555 (38%); RR 0.76, 95% CI 0.65 to 0.90; low‐quality evidence; Analysis 1.14). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 3409 participants was not obtained.
1.14. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 14 Hypertension.
Hyperlipidaemia
Four trials with 400 participants reported on hyperlipidaemia, and overall we found no statistically significant difference when glucocorticosteroid avoidance was compared with glucocorticosteroid‐containing immunosuppression (13/197 (7%) versus 18/203 (9%); RR 0.75, 95% CI 0.38 to 1.48; very low‐quality evidence; Analysis 1.15). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 7214 participants was not obtained.
1.15. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 15 Hyperlipidaemia.
Cholesterol
Five trials with 556 participants reported on serum cholesterol (mg/dL), and serum cholesterol was statistically significantly reduced when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (mean difference (MD) ‐18.49 mg/dL, 95% CI ‐22.02 to ‐14.96; very low‐quality evidence; Analysis 1.16).
1.16. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 16 Cholesterol.
Two trials with 266 participants reported on hypercholesterolaemia, and hypercholesterolaemia was not significantly different when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression (16/134 (12%) versus 28/132 (21%); RR 0.56, 95% CI 0.32 to 1.00; very low‐quality evidence; Analysis 1.17). Trial Sequential Analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve and the required information size of 20,334 participants was not obtained.
1.17. Analysis.
Comparison 1 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression, Outcome 17 Hypercholesterolaemia.
Health‐related quality of life
No trials reported on health‐related quality of life.
Zero event trial correction
Trials with zero events in both intervention groups were found in several of the analyses. For all of these analyses, we applied a random‐effects meta‐analysis with empirical continuity correction of 0.01 using the R software (R 2017). This correction of zero event trials resulted in none of the analyses yielding statistically significantly different results (i.e. all statistically significant differences in results between the groups remained statistically significantly different after zero event trial correction, and all non‐statistically significant differences in results between the groups remained non‐statistically significantly different after zero event trial correction).
Subgroup analyses
We were not able to perform our predefined subgroup analysis on trials at low risk of bias compared to trials at high risk of bias, as we considered none of the trials included in the review to be at low risk of bias.
We were not able to perform our predefined subgroup analysis on trials with paediatric participants compared to trials with adult participants, as all of the trials included in the review recruited exclusively adult participants.
We were not able to perform our predefined subgroup analysis on the median time between transplantation and the commencement of glucocorticosteroid administration, as none of the trials included in the review reported this in their methodology.
We performed subgroup analyses on glucocorticosteroid avoidance compared to glucocorticosteroid withdrawal (Analysis 1.1 through Analysis 1.17). Tests for subgroup differences between glucocorticosteroid avoidance and glucocorticosteroid withdrawal were not statistically significantly different for most outcomes, except for the outcomes 'Infection', 'Creatinine' and 'Hypercholesterolaemia'. We found a statistically significant interaction for infection (P = 0.04). This difference between glucocorticosteroid avoidance and glucocorticosteroid withdrawal is caused by one trial using glucocorticosteroid withdrawal that caused significantly fewer infections in the glucocorticosteroid avoidance or withdrawal group compared to trials in which glucocorticosteroid avoidance was used (RR 0.12, 95% CI 0.02 to 0.89). We found a statistically significant interaction for creatinine (P = 0.0004). This difference between glucocorticosteroid avoidance and glucocorticosteroid withdrawal is caused by two trials using glucocorticosteroid withdrawal that caused significantly lower creatinine in the glucocorticosteroid avoidance or withdrawal group compared to trials in which glucocorticosteroid avoidance was used (MD ‐0.06 mg/dL, 95% CI ‐0.16 to 0.05). We found a statistically significant interaction for hypercholesterolaemia (P = 0.008). This difference is caused by one trial reporting no statistically significant difference and one trial reporting statistically significantly lower rates of hypercholesterolaemia in the glucocorticosteroid avoidance and withdrawal arm. There are only a small number of studies reporting on infection, creatinine, and hypercholesterolaemia.The difference observed between subgroups for these outcomes may therefore be due to a factor other than glucocorticosteroid use.
We performed subgroup analyses on type of calcineurin inhibitor used (tacrolimus or cyclosporine A) (Analysis 2.1 through Analysis 2.16). Tests for subgroup differences between type of calcineurin inhibitor used as a co‐intervention were not statistically significantly different for most outcomes, except for the outcome 'Creatinine' for which we found a statistically significant interaction (P < 0.00001). This difference between type of calcineurin inhibitor used as co‐intervention is caused by one trial using the calcineurin inhibitor tacrolimus, which caused significantly higher serum creatinine levels in the glucocorticosteroid avoidance or withdrawal group compared to trials in which cyclosporine A was used (MD 0.25 mg/dL, 95% CI 0.19 to 0.31).
2.1. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 1 Mortality.
2.16. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 16 Cholesterol.
We performed subgroup analyses on type of antiproliferative agent (azathioprine or mycophenolate mofetil) compared to no antiproliferative agent (Analysis 3.1 through Analysis 3.14). Tests for subgroup differences between the type of antiproliferative agent used as a co‐intervention when compared to no antiproliferative agent were not statistically significantly different for most outcomes, except for the outcome 'Creatinine', for which we found a statistically significant interaction (P < 0.00001). This difference between the type of antiproliferative agent used as a co‐intervention is caused by one trial using the antiproliferative agent mycophenolate mofetil, which caused significantly higher serum creatinine in the glucocorticosteroid avoidance or withdrawal group compared to trials in which azathioprine or no antiproliferative agent were used (MD 0.25 mg/dL, 95% CI 0.19 to 0.31).
3.1. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 1 Mortality.
3.14. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 14 Cholesterol.
We performed subgroup analyses on type of induction agent (basiliximab, daclizumab, or rabbit antithymocyte globulin) compared to no induction agent (Analysis 4.1 through Analysis 4.16). Tests for subgroup differences between the type of induction therapy used as a co‐intervention when compared to no induction agent were not statistically significantly different for most outcomes, except for the outcomes 'Infection', 'Creatinine', 'Hypertension' and 'Cholesterol'. We found a statistically significant difference for infection (P = 0.04). This difference between the type of induction therapy used as a co‐intervention is caused by the induction agent rabbit antithymocyte globulin, which caused significantly fewer infections in the glucocorticosteroid avoidance or withdrawal group compared to trials in which basiliximab or no induction agents were used (RR 0.12, 95% CI 0.02 to 0.89). We found a statistically significant interaction for serum creatinine (P < 0.00001). This difference between the type of induction therapy used as a co‐intervention is caused by the induction agent basiliximab, which caused significantly higher serum creatinine in the glucocorticosteroid avoidance or withdrawal group compared to trials in which no induction agent was used (MD 0.25 mg/dL, 95% CI 0.19 to 0.31). We found a statistically significant interaction for hypertension (P = 0.03). This difference between the type of induction therapy used as a co‐intervention is caused by the induction agent rabbit antithymocyte globulin, which caused significantly lower rates of hypertension in the glucocorticosteroid avoidance or withdrawal group compared to trials in which basiliximab or no induction agent were used (RR 0.30, 95% CI 0.16 to 0.57). We found a statistically significant interaction for serum cholesterol (P = 0.0001). This difference between the type of induction therapy used as a co‐intervention is caused in part by the induction agent rabbit antithymocyte globulin, which caused significantly lower serum cholesterol in the glucocorticosteroid avoidance or withdrawal group compared to trials in which basiliximab was used (MD ‐70.00 mg/dL, 95% CI ‐101.17 to ‐39.83) and in part by one trial that did not use an induction agent, which caused significantly lower serum cholesterol in the glucocorticosteroid avoidance or withdrawal group compared to trials in which basiliximab was used (MD ‐146.00 mg/dL, 95% CI ‐192.16 to ‐99.84).
4.1. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 1 Mortality.
4.16. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 16 Cholesterol.
We performed subgroup analyses on the number of co‐interventions given (monotherapy, dual therapy, or triple therapy) (Analysis 5.1 through Analysis 5.16). Tests for subgroup differences between the number of co‐interventions given were not statistically significantly different for most outcomes, except for the outcomes 'Creatinine' and 'Cholesterol'. We found a statistically significant interaction for serum creatinine (P < 0.00001). This difference between the number of co‐interventions given is caused by the use of triple therapy in one trial, which caused significantly higher serum creatinine in the glucocorticosteroid avoidance or withdrawal group compared to monotherapy or triple therapy (MD 0.25 mg/dL, 95% CI 0.19 to 0.31). We found a statistically significant difference for serum cholesterol (P < 0.00001). This difference between the number of co‐interventions given is caused by the use of monotherapy in one trial, which caused significantly higher serum cholesterol in the glucocorticosteroid avoidance or withdrawal group compared to dual therapy or triple therapy (MD 35.00 mg/dL, 95% CI 12.31 to 57.69).
5.1. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 1 Mortality.
5.16. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 16 Cholesterol.
We performed subgroup analyses on the duration of glucocorticosteroid use in the longer glucocorticosteroid taper arm or the long‐term glucocorticosteroid arm (up to three months of glucocorticosteroids; greater than three months and up to six months of glucocorticosteroids; or greater than six months of glucocorticosteroids) (Analysis 6.1 through Analysis 6.13). One trial did not report on the duration of glucocorticosteroid use in the glucocorticosteroid‐containing arm and was not included in this sub‐analysis (Pageaux 2004). Tests for subgroup differences between duration of glucocorticosteroid use in the glucocorticosteroid‐containing arm were not statistically significantly different for most outcomes, except for the outcomes 'Creatinine', 'Hypertension', 'Cholesterol' and 'Hypercholesterolaemia'. We found a statistically significant difference for serum creatinine (P = 0.00001). This difference between the duration of glucocorticosteroid use is caused by one trial using three to six months of glucocorticosteroids in the glucocorticosteroid‐containing group, which caused significantly higher serum creatinine in the glucocorticosteroid avoidance or withdrawal group compared to trials using two to three months of glucocorticosteroids and more than six months of glucocorticosteroids in the glucocorticosteroid‐containing arm (MD 0.25 mg/dL, 95% CI 0.19 to 0.31). We found a statistically significant difference for hypertension (P = 0.001). This difference between duration of glucocorticosteroid use in the glucocorticosteroid‐containing arm is caused, in part, by one trial which used long‐term glucocorticosteroid in the glucocorticosteroid‐containing arm, which caused significantly lower rates of hypertension in the glucocorticosteroid avoidance or withdrawal group compared to trials using two to three months or three to six months of glucocorticosteroids in the glucocorticosteroid‐containing arm (RR 0.30, 95% CI 0.16 to 0.57). We found a statistically significant difference for cholesterol (P = 0.002). This difference between duration of glucocorticosteroid use in the glucocorticosteroid‐containing arm is caused by two trials using long‐term glucocorticosteroids in the glucocorticosteroid‐containing arm, which caused significantly lower serum cholesterol in the glucocorticosteroid avoidance or withdrawal group compared to trials using two to three months or three to six months of glucocorticosteroids in the glucocorticosteroid‐containing arm (MD ‐92.75 mg/dL, 95% CI ‐118.01 to ‐67.50). We found a statistically significant interaction for hypercholesterolaemia (P = 0.008). This difference between duration of glucocorticosteroid use in the glucocorticosteroid‐containing is due to the small number of trials reporting on hypercholesterolaemia, with one trial reporting no statistically significant difference and one trial reporting statistically significantly lower rates of hypercholesterolaemia in the glucocorticosteroid avoidance and withdrawal arm. The difference observed between subgroups for hypercholesterolaemia may therefore be due to a factor other than duration of glucocorticosteroid use.
6.1. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 1 Mortality.
6.13. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 13 Hypercholesterolaemia.
We performed subgroup analyses on trials commenced before the year 2000 and trials commenced from 2000 onwards (Analysis 7.1 through Analysis 7.16). Tests for subgroup differences between trials commenced before 2000 and trials commenced from 2000 onwards were not statistically significantly different for most outcomes, except for the outcomes 'Creatinine', 'Hypertension', and 'Cholesterol'. We found a statistically significant interaction for creatinine (P < 0.00001). This difference between trials commenced before 2000 and trials commenced from 2000 onwards is caused by one trial started after 2000, which caused significantly higher serum creatinine in the glucocorticosteroid avoidance or withdrawal group compared to a trial started before 2000 (MD 0.25 mg/dL, 95% CI 0.19 to 0.31). We found a statistically significant difference for hypertension (P = 0.03). This difference between trials commenced before 2000 and trials commenced from 2000 onwards is caused by one trial started before 2000, which caused significantly lower rates of hypertension in the glucocorticosteroid avoidance or withdrawal group compared to trials started after 2000 (RR 0.30, 95% CI 0.16 to 0.57). We found a statistically significant difference for cholesterol (P = 0.03). This difference between trials commenced before 2000 and trials commenced from 2000 onwards is caused by one trial started before 2000, which caused significantly lower serum cholesterol in the glucocorticosteroid avoidance or withdrawal group compared to trials started after 2000 (MD ‐70.00 mg/dL, 95% CI ‐101.17 to ‐39.83).
7.1. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 1 Mortality.
7.16. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 16 Cholesterol.
The statistically significant interactions in serum creatinine and serum cholesterol between many of the subgroups are unlikely to reflect actual differences between the subgroups. Instead they are likely to reflect the relatively small number of trials that report on these outcomes and the considerable heterogeneity influencing these outcomes.
Best‐worst and worst‐best analyses
We found trials with missing data in several of the analyses. For each of these analyses, we applied a best‐worst analysis and a worst‐best analysis.
Best‐worst analyses
The best‐worst analyses (best results possible for glucocorticosteroid avoidance or withdrawal) did not yield statistically significantly different results from the conventional meta‐analysis except for acute rejection, infection, glucocorticosteroid‐resistant rejection, CMV infection, malignancy, post‐transplant lymphoproliferative disorder, and hyperlipidaemia (Analysis 8.1 through Analysis 8.12). We observed no statistically significant difference in the best‐worst analyses for acute rejection (RR 1.04, 95% CI 0.85 to 1.26) or glucocorticosteroid‐resistant rejection (RR 1.00, 95% CI 0.61 to 1.65) when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression. We found statistically significant reductions in the best‐worst analyses for infection (RR 0.80, 95% CI 0.67 to 0.96), CMV infection (RR 0.57, 95% CI 0.37 to 0.87), malignancy (RR 0.21, 95% CI 0.07 to 0.61), post‐transplant lymphoproliferative disorder (RR 0.24, 95% CI 0.07 to 0.85), and hyperlipidaemia (RR 0.40, 95% CI 0.21 to 0.73) when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression. However, it is unlikely that all 12 participants lost to follow‐up in the glucocorticosteroid‐containing immunosuppression arm of Pageaux 2004 suffered from malignancy and post‐transplant lymphoproliferative disorder. We found no statistically significant differences between the best‐worst analyses and the conventional meta‐analysis for mortality, graft loss including death, chronic rejection, diabetes mellitus, or hypertension when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression.
8.12. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 12 Hyperlipidaemia.
Worst‐best analyses
The worst‐best analyses (worst results possible for glucocorticosteroid avoidance or withdrawal) did not yield statistically significantly different results from the conventional meta‐analysis except for mortality, graft loss including death, chronic rejection, diabetes mellitus, malignancy, post‐transplant lymphoproliferative disorder, hypertension, and hyperlipidaemia (Analysis 9.1 through Analysis 9.13). We observed no statistically significant difference in the worst‐best analyses for diabetes mellitus (RR 0.95, 95% CI 0.79 to 1.15) or hypertension (RR 0.87, 95% CI 0.75 to 1.02) when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression. We found statistically significant increases in the worst‐best analyses for mortality (RR 1.35, 95% CI 1.10 to 1.67), graft loss including death (RR 1.38, 95% CI 1.08 to 1.74), chronic rejection (RR 2.45, 95% CI 1.40 to 4.31), malignancy (RR 3.05, 95% CI 1.38 to 6.73), post‐transplant lymphoproliferative disorder (RR 15.64, 95% CI 3.08 to 79.56), and hyperlipidaemia (RR 1.92, 95% CI 1.12 to 3.28) when the glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression. However, it is unlikely that all 19 participants lost to follow‐up in the glucocorticosteroid withdrawal arm of Pageaux 2004 suffered from malignancy and post‐transplant lymphoproliferative disorder. We found no statistically significant differences between the best‐worst analyses and the conventional meta‐analysis for acute rejection, infection, glucocorticosteroid‐resistant rejection, CMV infection, or renal insufficiency when glucocorticosteroid avoidance or withdrawal was compared with glucocorticosteroid‐containing immunosuppression.
9.13. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 13 Hyperlipidaemia.
Adverse events reported in non‐randomised studies
Our search was primarily to identify randomised clinical trials and systematic reviews. However, the search returned multiple citations from quasi‐randomised or non‐randomised studies. In these studies, we searched for adverse events that were different to those reported in the randomised clinical studies in terms of number or type of adverse event. We were unable to find any unique adverse events in the non‐randomised studies and we found no significant discrepancy in the rates of the adverse events reported in the randomised trials of this systematic review. We did not find any unique adverse events reported in the completed study that we were not able to incorporate to the meta‐analysis (Zhong 2010).
Publication bias
We performed a linear regression test to explore funnel plot asymmetry for any outcomes reported in 10 or more trials (Egger 1997). We found no asymmetry for mortality, graft loss including death, acute rejection, glucocorticosteroid‐resistant rejection, or hepatitis C virus recurrence. We identified tendencies towards significant asymmetry for diabetes mellitus (P = 0.06) and hypertension (P = 0.07). This asymmetry may be due to heterogeneity introduced by one study (Belli 1998); when this study was removed, no asymmetry was detected.
Discussion
Summary of main results
We identified 16 completed randomised clinical trials including 1347 participants and one other trial for which limited data were available. Eleven of these completed trials compared glucocorticosteroid avoidance with short‐term glucocorticosteroids and the remaining six compared rapid glucocorticosteroid tapers with longer tapers or long‐term glucocorticosteroids. All but one trial were two‐armed parallel‐group trials with one three‐armed parallel group trial for which only the control arm and the relevant intervention arm were included in our review. We aimed to assess mortality, graft loss including death, acute rejection, infection, adverse events, chronic rejection, glucocorticosteroid‐resistant rejection, diabetes mellitus, cytomegalovirus (CMV) infection, hepatitis C virus (HCV) recurrence, malignancy, post‐transplant lymphoproliferative disorder, renal failure requiring dialysis, renal insufficiency, estimated glomerular filtration rate (eGFR), serum creatinine, de novo autoimmune hepatitis, hypertension, hyperlipidaemia, serum cholesterol, hypercholesterolaemia, and health‐related quality of life. Adverse events, renal failure requiring dialysis, eGFR, de novo autoimmune hepatitis, and health‐related quality of life were not reported in any of the trials. We assessed all other outcomes in the meta‐analysis.
Acute rejection appeared to be increased when glucocorticosteroid avoidance or withdrawal were compared with glucocorticosteroid‐containing immunosuppression. Glucocorticosteroid‐resistant rejection appeared to be increased when glucocorticosteroid avoidance or withdrawal were compared with glucocorticosteroid‐containing immunosuppression. Diabetes mellitus appeared to be increased when glucocorticosteroid avoidance or withdrawal were compared with glucocorticosteroid‐containing immunosuppression, when we applied the fixed‐effect, but not the random‐effects model. Serum creatinine appeared to be increased when glucocorticosteroid avoidance or withdrawal were compared with glucocorticosteroid‐containing immunosuppression, when we applied the fixed‐effect, but not the random‐effects model. Hypertension appeared to be reduced when glucocorticosteroid avoidance or withdrawal were compared with glucocorticosteroid‐containing immunosuppression. Serum cholesterol appeared to be reduced when glucocorticosteroid avoidance or withdrawal were compared with glucocorticosteroid‐containing immunosuppression.
We found no evidence for an increase or decrease in mortality, graft loss including death, infection, chronic rejection, CMV infection, HCV recurrence, malignancy, post‐transplant lymphoproliferative disorder, renal insufficiency, hyperlipidaemia, or hypercholesterolaemia when comparing glucocorticosteroid avoidance or withdrawal with glucocorticosteroid‐containing immunosuppression. We performed Trial Sequential Analysis for all outcomes, and for none of the outcomes were the monitoring boundaries crossed or the required information size reached. Hence, we cannot exclude random errors for the results of the conventional meta‐analyses.
We identified five trials exclusively composed of or reporting cohorts of hepatitis C virus‐infected participants, including 231 participants.
Overall completeness and applicability of evidence
We included 16 completed trials in our meta‐analysis, which compared glucocorticosteroid avoidance or withdrawal with glucocorticosteroid‐containing immunosuppression. We could not perform meta‐analyses on each of our predefined outcomes as the trials we identified did not report on all of them. We were unable to include one completed trial due to inadequate data published for this trial.
All of the trials reported on acute rejection. Almost all of the trials reported on mortality, graft loss including death, and diabetes mellitus. Most trials reported on infection, chronic rejection, glucocorticosteroid‐resistant rejection, HCV recurrence, and hypertension. Few trials report on CMV infection, malignancy, post‐transplant lymphoproliferative disorder, renal insufficiency, serum creatinine, hyperlipidaemia, serum cholesterol, and hypercholesterolaemia. None of the trials reported on adverse events, renal failure requiring dialysis, eGFR, de novo autoimmune hepatitis, or health‐related quality of life. Of the outcomes for which few trials reported results, many had conflicting results, as demonstrated by the moderate or significant level of heterogeneity identified in the analyses.
Our meta‐analyses include a variety of immunosuppressive regimens including different combinations and types of calcineurin inhibitor, antiproliferative agent, and induction agent and include the majority of the agents in common use. One induction agent in common use, alemtuzumab, was not used in any of the trials.
Follow‐up in the included trials ranged from six months to 10 years. Our review has very limited evidence for long‐term outcomes for glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression. Long‐term effects are particularly relevant for mortality, graft loss, malignancy, and post‐transplant lymphoproliferative disorder.
The participants included in each of the trials do not fully reflect the characteristics of the general liver transplant population. None of the trials included in our review included paediatric participants and only a limited number included living donors. Only eight of the trials reported on type of donor. There is, however, a variety of concomitant immunosuppressants reflecting the majority of immunosuppressants in current use as well as a variety of indications for transplantation.
Quality of the evidence
The quality of our review findings and interpretations are limited by the number of trials included in the review and the low quality of certain aspects within the trials. For several of the comparisons only a very small number of trials could be included, with limited reporting on the rarer outcomes of interest. These factors are responsible for the broad confidence intervals representing imprecision in many of our analyses.
Our review is limited by indirectness as it does not include paediatric participants or multiple organ transplant recipients. As well as this, many of the included trials listed living donors in their exclusion criteria. For this reason our results cannot be directly related to these patients.
We explored statistical heterogeneity with the Chi2 test and quantified heterogeneity using the I2 statistic (Higgins 2002). The Chi2 test is not as effective for situations where few trials with few participants are included in a meta‐analysis, such as is the case for our review. This means that many of the outcomes for which we found a statistically significant difference indicate a moderate or significant level of heterogeneity. It also means that in situations in which a non‐statistically significant result was shown, it could still have been influenced by heterogeneity. To overcome this uncertainty, we applied both fixed‐effect and random‐effects meta‐analysis models, and reported both models when we found differences. In our review, the fixed‐effect model identified several statistically significant differences, which were not identified by the random‐effects model. We considered six outcomes (infection, chronic rejection, diabetes mellitus, malignancy, renal insufficiency, and hypertension) to have moderate levels of heterogeneity. We considered three outcomes (creatinine, cholesterol, and hypercholesterolaemia) to have significant levels of heterogeneity. The outcomes with the highest levels of heterogeneity were reported in only a small number of the included trials. Two of these outcomes were also continuous outcomes and demonstrated considerable inconsistency between the small number of studies in which they were reported. The heterogeneity identified in the outcomes 'Diabetes mellitus' and 'Hypertension' is due to one trial in the glucocorticosteroid withdrawal sub‐analysis (Belli 2001). This trial, with over 100 participants, which used rabbit antithymocyte globulin, also used the highest cumulative glucocorticosteroid dose in the glucocorticosteroid‐containing group. As glucocorticosteroids are known to increase the rates of hypertension and diabetes mellitus (Hatz 1998), we believe that this comparatively high glucocorticosteroid dose may be responsible for the inconsistency in these outcomes. Following the sensitivity analyses, we found that this trial is also responsible for several of the identified subgroup differences.
We detected possible publication bias for hypertension and diabetes mellitus. This, however, may be due to the heterogeneity introduced by one study and when this study is removed from the analysis, no possibility of publication bias is detected.
Risk of bias is known to be responsible for overestimation of intervention benefits and underestimation of intervention harms in randomised trials with inadequate methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001;Wood 2008; Savović 2012; Savović 2012a; Lundh 2017). Of the 17 included trials, three trials (18%) reported adequate generation of the randomisation sequence, four (24%) reported adequate allocation concealment, three (18%) reported adequate blinding of participants, two (12%) reported adequate blinding of outcome assessors, four (24%) appear to be uninfluenced by incomplete outcome data, 14 (82%) appear to be free from selective reporting, and we could consider none to be free from 'other bias', with reasons being industry sponsorship and lack of reporting of required sample size calculation. Thirteen (76%) appear to be free from early stopping, and 11 (65%) appear to be free from baseline imbalance. We considered all trials to be at high risk of bias.
Potential biases in the review process
We performed a systematic review and meta‐analysis in accordance with the methodology described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We followed our peer‐reviewed and prepublished protocol with predefined participants, interventions, comparisons, and outcomes to avoid biases in the review preparation (Fairfield 2014). We performed a comprehensive and extensive literature search for both published and unpublished data from a variety of sources that met our predefined inclusion criteria. We extracted all available data and based our meta‐analysis on the intention‐to‐treat principle. We performed several sub‐analyses and sensitivity analyses when appropriate to assess the robustness of our data. We performed empirical continuity correction for zero event trials.
Our meta‐analysis includes larger numbers of randomised clinical trials on glucocorticosteroid avoidance or withdrawal than other meta‐analyses published on this topic (Segev 2008; Sgourakis 2009; Knight 2011; Gu 2014; Lan 2014), improving the quality and comprehensiveness, and reducing the risks of imprecision.
Although we contacted various experts in the field and pharmaceutical companies, our search might have missed unpublished data including trials with negative results. This bias remains difficult to avoid. We performed linear regression tests to identify asymmetry in funnel plots in order to identify any possible publication bias. We also contacted the authors of any trials with incomplete data to obtain any unpublished or missing data.
In addition, we conducted Trial Sequential Analyses for all outcomes (Wetterslev 2008; Thorlund 2011b; TSA 2011; Wetterslev 2017) in order to test the robustness of our results. We calculated the DARIS on the basis of type I error of 5%, type II error of 20%, and risk reduction of 20%, and adjusted the information size for diversity (Wetterslev 2009). For all the Trial Sequential Analyses, the cumulative Z‐curve did not cross trial sequential monitoring boundaries, and the DARIS was not reached; hence, we cannot exclude random errors regarding our results (play of chance). Except for the outcome HCV recurrence, trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, but the required information size of 435 participants was obtained, meaning that we can exclude a relative risk reduction of 20% or more regarding HCV recurrence.
Our search was conducted in May 2017 and it is possible that more recent studies may have been published, which are not considered in our review.
Agreements and disagreements with other studies or reviews
Five non‐Cochrane meta‐analyses on glucocorticosteroid avoidance or withdrawal for liver transplanted patients have been published (Segev 2008; Sgourakis 2009; Knight 2011; Gu 2014; Lan 2014), as well as one Cochrane network meta‐analysis assessing maintenance immunosuppression for liver transplanted patients (Rodríguez‐Perálvarez 2017). Three of these meta‐analyses also include trials in which glucocorticosteroids have been compared with another agent (Segev 2008; Sgourakis 2009; Gu 2014), but have reported these as sub‐analyses allowing for comparisons with our review. One review focuses on comparison of monotherapy with glucocorticosteroid‐containing combinations although included studies where monotherapy was compared with three immunosuppressive agents of which one was a glucocorticosteroid (Lan 2014). Our review deals more extensively with risk of bias (systematic errors) and risk of random errors (play of chance) in the randomised clinical trials we identified. We have also performed a much larger number of sub‐analyses, and performed Trial Sequential Analyses for all outcomes.
Overall, the meta‐analysis in Segev 2008 found a decrease in cholesterol, CMV infection, and HCV recurrence but an increase in acute rejection with glucocorticosteroid avoidance or withdrawal, although no difference in mortality, graft loss, hypertension, diabetes mellitus, glucocorticosteroid‐resistant rejection, or infection was observed. Segev 2008 reports statistically significantly decreased rates of acute rejection, glucocorticosteroid‐resistant rejection, and diabetes mellitus when glucocorticosteroids are replaced with an alternative immunosuppressive agent. This also means that overall the rates of acute rejection are decreased when these trials are assessed in combination with trials where glucocorticosteroids are not replaced. One possible reason behind the comparatively lower rates of diabetes mellitus when glucocorticosteroids were replaced rather than withdrawn or avoided is that the majority of the trials in the review treated acute rejection with glucocorticosteroids and the higher rates of acute rejection in the trials where glucocorticosteroids were avoided or withdrawn results in glucocorticosteroids being administered for rejection treatment. These pulses of glucocorticosteroids may have increased the rates of diabetes mellitus, masking any benefit gained from not using them (Hatz 1998). This may also explain why Segev 2008 identified statistically significant reductions in HCV recurrence with glucocorticosteroid avoidance or withdrawal whilst our review did not. This is because glucocorticosteroid pulses are known to promote HCV recurrence and the higher rates of acute rejection identified in our review resulted in higher rates of glucocorticosteroid pulses (Sheiner 1995; Singh 1996).
Overall, the meta‐analysis in Sgourakis 2009 found a decrease in diabetes mellitus, CMV infection, and cholesterol and an increase in acute rejection with glucocorticosteroid avoidance or withdrawal, although no difference in mortality, graft loss, glucocorticosteroid‐resistant rejection, chronic rejection, infection, hypertension, renal insufficiency, and mortality in HCV‐infected participants was observed. Sgourakis 2009 also found a decrease in acute rejection for trials where glucocorticosteroids were replaced by an alternative immunosuppressive agent.
Overall the meta‐analysis in Knight 2011 found a decrease in diabetes mellitus and no significant increases or decreases in any other outcomes including mortality, graft loss, hypertension, acute rejection, and cholesterol with glucocorticosteroid avoidance or withdrawal. Knight 2011 contains only seven trials and many of the analyses have significant levels of heterogeneity. A non‐significant trend was identified in many of the outcomes, but the low number of trials is likely to have caused wider confidence intervals, preventing genuine effects from being identified.
Overall, the meta‐analysis in Gu 2014 found a decrease in diabetes mellitus and CMV infection and no significant increases or decreases in any other outcomes including mortality, graft loss, acute rejection, chronic rejection, HCV recurrence, infection, and hypertension with glucocorticosteroid avoidance or withdrawal.
The meta‐analysis in Lan 2014 claims to have found a total of 14 randomised clinical trials assessing immunosuppression monotherapy (using tacrolimus, cyclosporine, or mycophenolate mofetil) versus glucocorticosteroid‐containing immunosuppression. A total of seven of the "randomised studies" included in Lan 2014 appear to relate to only three studies (Belli 1998; Moench 2007; Manousou 2009), which have been subject to duplicate publication. The authors of Lan 2014 have included the results of each report as a separate study in each relevant meta‐analysis meaning that the same participants have been included multiple times. As a consequence of the probability of duplicate publication bias in the meta‐analysis by Lan and colleagues. (Lan 2014), we have therefore decided not to make any comparisons with the results of our review. A letter has been sent to the editor of the relevant journal highlighting the inclusion of duplicate studies in Lan 2014 (Fairfield 2017).
Overall, the network meta‐analysis in Rodríguez‐Perálvarez 2017 found a reduction in adverse events but no change in mortality or graft rejection with glucocorticosteroid avoidance or withdrawal in cyclosporine‐based regimens when making direct comparison. Rodríguez‐Perálvarez 2017 found a reduction in adverse events with no change in mortality, graft rejection, or retransplantation with glucocorticosteroid avoidance or withdrawal in cyclosporine‐based regimens when making indirect comparison. Overall, the network meta‐analysis in Rodríguez‐Perálvarez 2017 found an increase in retransplantation but no change in chronic kidney disease with glucocorticosteroid avoidance or withdrawal in tacrolimus‐based regimens when making direct comparison. Rodríguez‐Perálvarez 2017 found no change in mortality, graft loss, renal impairment, or retransplantation with glucocorticosteroid avoidance or withdrawal in tacrolimus‐based monotherapy regimes when making indirect comparison. Rodríguez‐Perálvarez 2017 found an increase in retransplantation with glucocorticosteroid avoidance or withdrawal in tacrolimus‐ and mycophenolate mofetil‐based regimens when making indirect comparison. The network meta‐analysis in Rodríguez‐Perálvarez 2017 includes only three individual studies assessing glucocorticosteroid avoidance and withdrawal.
In accordance with these meta‐analyses, we found statistically significant decreases in diabetes mellitus and cholesterol as well as a statistically significant increase in acute rejection with glucocorticosteroid avoidance or withdrawal when applying conventional meta‐analyses. Similar to the other meta‐analyses, we found no statistically significant changes in mortality, graft loss, chronic rejection, and infection. We also found a statistically significant increase in glucocorticosteroid‐resistant rejection and a statistically significant decrease in hypertension with glucocorticosteroid avoidance or withdrawal.
Reduction in CMV infection, HCV recurrence and adverse events with an increase in retransplantation were not shown in our review. The differences between the findings of our review and those of other published meta‐analyses may be due to our review excluding studies comparing glucocorticosteroids with alternative immunosuppressive agents.
A similar meta‐analysis has been performed for kidney transplantation (Knight 2010). The review contained 34 trials with a total of 5637 participants and assessed the benefits and harms of glucocorticosteroid avoidance or withdrawal in kidney transplant recipients. Knight 2010 found statistically significant reductions in hypertension (risk ratio (RR) 0.90, 95% confidence interval (CI) 0.85 to 0.94), hypercholesterolaemia (RR 0.76, 95% CI 0.67 to 0.87), diabetes mellitus (RR 0.64, 95% CI 0.50 to 0.83), and creatinine clearance (weighted mean difference (WMD) ‐3.06 mL/min, 95% CI ‐4.66 to ‐1.45), as well as statistically significant increases in acute rejection (RR 1.56, 95% CI 1.31 to 1.87) and creatinine (WMD 4.24 μmol/L, 95% CI 2.08 to 6.40) with glucocorticosteroid avoidance or withdrawal. Knight 2010 observed no statistically significant differences in mortality, graft loss, or glucocorticosteroid‐resistant rejection. These findings are very similar to the findings of our review. The differences observed in Knight 2010 in creatinine in kidney transplant recipients were not found in our review for liver transplant recipients; this may be due to the small number of trials included in our review that reported serum creatinine.
Knight 2011 also reports the outcomes with glucocorticosteroid avoidance or withdrawal for heart and pancreas transplantation although only one trial was identified in each. Esmore 1989 reports statistically significant reductions in the number of antihypertensives required (0.8 ± 0.6 antihypertensives versus 1.3 ± 0.7 antihypertensives) and serum cholesterol (5.4 ± 1.2 mmol/L versus 6.2 ± 0.9 mmol/L), as well as statistically significant increases in rejection rates within the first three months from transplantation (2.3 ± 0.23 episodes per 100 patient days versus 1.5 ± 0.18 episodes per 100 patient days) and glucocorticosteroid‐resistant rejection (26.4% versus 10.2%) with glucocorticosteroid avoidance or withdrawal for heart transplant recipients. Esmore 1989 reports no statistically significant differences in mortality or graft loss with glucocorticosteroid avoidance or withdrawal. Gruessner 2001 reports a statistically significant reduction in cholesterol and triglyceride levels in simultaneous pancreas and kidney transplant recipients with glucocorticosteroid avoidance or withdrawal (rates not given). Gruessner 2001 reports no statistically significant differences in mortality or graft loss with glucocorticosteroid avoidance or withdrawal.
Possible benefits of glucocorticosteroid avoidance and withdrawal, including reductions in cardiovascular risk factors, were identified in this review. However, possible increases in acute rejection and glucocorticosteroid‐resistant rejection were also identified. These findings are similar to reviews of glucocorticosteroid avoidance and withdrawal for heart and kidney transplant recipients. Unfortunately the benefits and harms found in the conventional meta‐analysis could not be confirmed by Trial Sequential Analyses meaning that we cannot exclude random errors.
Authors' conclusions
Implications for practice.
Our review has a low to moderate quality of evidence for the effects of glucocorticosteroid avoidance or withdrawal. The effects of glucocorticosteroid avoidance or withdrawal remain uncertain. Our review showed no clear benefits or harms for mortality, graft loss including death, infection, chronic rejection, cytomegalovirus (CMV) infection, hepatitis C virus (HCV) recurrence, malignancy, post‐transplant lymphoproliferative disorder, renal insufficiency, creatinine, hyperlipidaemia, cholesterol, or hypercholesterolaemia. Hypertension and diabetes mellitus may be reduced, but acute rejection and glucocorticosteroid‐resistant rejection may be increased with glucocorticosteroid avoidance or withdrawal. Glucocorticosteroid‐free immunosuppression may provide a safe alternative for liver transplanted patients who are intolerant of glucocorticosteroids. Although we found no statistically significant difference for mortality or graft loss, these findings should be interpreted with caution.
Implications for research.
Given the results of our analysis, it appears that appropriately sized randomised clinical trials comparing glucocorticosteroid avoidance or withdrawal with glucocorticosteroid‐containing immunosuppression in liver transplant participants using contemporarily adjunctive immunosuppression are warranted. As episodes of acute rejection following liver transplantation tend to occur more frequently in the initial weeks following transplantation (Wiesner 1998), trials investigating whether short‐term glucocorticosteroids (first few weeks) reduce the rates of acute rejection without exposing liver transplant recipients to cardiovascular risk factors for long periods of time appear to be warranted. We feel it may be of benefit to construct a high‐quality three‐arm trial comparing complete postoperative glucocorticosteroid avoidance, short‐term glucocorticosteroids, and long‐term glucocorticosteroids.
Our review did not identify any statistically significant increase or decrease in HCV recurrence with glucocorticosteroid‐free immunosuppression despite reports that glucocorticosteroids increase the severity of HCV hepatitis (Sheiner 1995; Singh 1996; Segev 2008; Sgourakis 2009). One possible reason for this is the higher rate of acute rejection in the glucocorticosteroid‐free arm, which was treated with glucocorticosteroid pulses. It is possible that with the use of alternative immunosuppression strategies to prevent rejection that glucocorticosteroid‐free immunosuppression may lead to lower rates of HCV recurrence (Hibi 2015). Our review identified a number of studies published between 2009 and 2017 in which glucocorticosteroids were replaced with an alternative immunosuppressant. An updated systematic review and meta‐analysis of these studies is merited.
These trials should be conducted with low risk of systematic error (bias) and low risk of random error (play of chance), and should follow the 'SPIRIT' guidelines (SPIRIT 2013a; SPIRIT 2013b) and 'CONSORT' guidelines (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 16 November 2017 | New search has been performed | Searches performed until May 2017. One new trial added, but data could only be incorporated into qualitative analysis. |
| 8 November 2017 | New citation required but conclusions have not changed | Background updated. New references added. Searches re‐executed. Long‐term follow‐up of one previously included trial now incorporated to meta‐analysis. Three further meta‐analyses addressing same or similar review question identified and incorporated to review discussion. |
Acknowledgements
Thanks go to Gero Langer and Susanne Saal for their work on the protocol (Langer 2009). Thanks go to Sheila Fisken, Sarah Klingenberg, and Stuart Falconer for giving advice on the search strategies and other methods of identifying trials.
Peer reviewers: Andres Cardenas, Spain; Rosa M Martin‐Mateos, Spain; Marco Carbone; UK. Contact editor: Saboor A'Khan, UK. Sign‐off editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | May 2017 | ((liver OR hepat*) AND (transplant* OR graft*)) AND (glucocorticosteroid* OR corticosteroid* OR steroid* OR gluco‐corticoid* OR cortico‐steroid* OR methylpredniso* OR methyl‐predniso* OR predniso* OR dexamethaso* OR dexa‐methaso* or monotherapy*) AND (immunosuppres* or tacrolimus* or mycopheno* or MMF* or (monoclonal adj3 antibod*) or mab* or daclizumab* or basiliximab* or cyclosporin* or ciclosporin* or calcineurin inhibitor* or calcineurin antagonist* or purine inhibitor* or purine antagonist* or sirolimus* or rapamycin* or everolimus* or methotrexate* or azathioprine* or muromonab* or orthoclon* or OKT3* or anti‐CD3* or antithymocyte* or ATG* or anti‐IL2* or anti‐CD52* or campath* or FK506* or steroid‐free* or corticosteroid‐free* or glucocorticoid‐free* or glucocorticosteroid‐free* or steroid‐sparing* or corticosteroid‐sparing* or glucocorticoid‐sparing* or glucocorticosteroid‐sparing* or steroid‐avoid* or corticosteroid‐avoid* or glucocorticoid‐avoid* or glucocorticosteroid‐avoid* or steroid‐taper* or corticosteroid‐taper* or glucocorticoid‐taper* or glucocorticosteroid‐taper* or steroid‐withdraw* or corticosteroid‐withdraw* or glucocorticoid‐withdraw* or glucocorticosteroid‐withdraw* or steroid‐eliminat* or corticosteroid‐eliminat* or glucocorticoid‐eliminat* or glucocorticosteroid‐eliminat* or steroid‐minimi* or corticosteroid‐minimi* or glucocorticoid‐minimi* or glucocorticosteroid‐minimi*) |
| Cochrane Central Register of Controlled Trials (CENTRAL) | Issue 5 of 12 2017 | #1 MeSH descriptor: [Liver Transplantation] explode all trees #2 ((liver or hepat*) and (transplant* or graft*)) #3 #1 or #2 #4 MeSH descriptor: [Steroids] explode all trees #5 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees #6 MeSH descriptor: [Methylprednisolone] explode all trees #7 MeSH descriptor: [Prednisone] explode all trees #8 MeSH descriptor: [Glucocorticoids] explode all trees #9 MeSH descriptor: [Dexamethasone] explode all trees #10 glucocorticosteroid* or corticosteroid* or steroid* or gluco‐corticoid* or cortico‐steroid* or methylpredniso* or methyl‐predniso* or predniso* or dexamethaso* or dexa‐methaso* or monotherapy* #11 #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Immunosuppression] explode all trees #13 MeSH descriptor: [Immunosuppressive Agents] explode all trees #14 MeSH descriptor: [Antibodies, Monoclonal] explode all trees #15 MeSH descriptor: [Mycophenolic Acid] explode all trees #16 MeSH descriptor: [Antilymphocyte Serum] explode all trees #17 MeSH descriptor: [Tacrolimus] explode all trees #18 MeSH descriptor: [Cyclosporine] explode all trees #19 MeSH descriptor: [Sirolimus] explode all trees #20 MeSH descriptor: [Muromonab‐CD3] explode all trees #21 immunosuppres* or tacrolimus* or mycopheno* or MMF* or (monoclonal adj3 antibod*) or mab* or daclizumab* or basiliximab* or cyclosporin* or ciclosporin* or calcineurin inhibitor* or calcineurin antagonist* or purine inhibitor* or purine antagonist* or sirolimus* or rapamycin* or everolimus* or methotrexate* or azathioprine* or muromonab* or orthoclon* or OKT3* or anti‐CD3* or antithymocyte* or ATG* or anti‐IL2* or anti‐CD52* or campath* or FK506* #22 steroid‐free* or corticosteroid‐free* or glucocorticoid‐free* or glucocorticosteroid‐free* or steroid‐sparing* or corticosteroid‐sparing* or glucocorticoid‐sparing* or glucocorticosteroid‐sparing* or steroid‐avoid* or corticosteroid‐avoid* or glucocorticoid‐avoid* or glucocorticosteroid‐avoid* or steroid‐taper* or corticosteroid‐taper* or glucocorticoid‐taper* or glucocorticosteroid‐taper* or steroid‐withdraw* or corticosteroid‐withdraw* or glucocorticoid‐withdraw* or glucocorticosteroid‐withdraw* or steroid‐eliminat* or corticosteroid‐eliminat* or glucocorticoid‐eliminat* or glucocorticosteroid‐eliminat* or steroid‐minimi* or corticosteroid‐minimi* or glucocorticoid‐minimi* or glucocorticosteroid‐minimi* #23 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 #24 #3 AND #11 AND #23 |
| MEDLINE Ovid | 1946 to May 2017 | #1 exp Liver Transplantation/ #2 ((liver or hepat*) and (transplant* or graft*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] #3 1 or 2 #4 exp Steroids/ #5 exp Glucocorticoids/ #6 exp Adrenal Cortex Hormones/ #7 exp Methylprednisolone/ #8 exp Prednisone/ #9 exp Dexamethasone/ #10 (glucocorticosteroid* or corticosteroid* or steroid* or gluco‐corticoid* or cortico‐steroid* or methylpredniso* or methyl‐predniso* or predniso* or dexamethaso* or dexa‐methaso* or monotherapy*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] #11 4 or 5 or 6 or 7 or 8 or 9 or 10 #12 exp Immunosuppressive Agents/ #13 exp Antibodies, Monoclonal/ #14 exp Tacrolimus/ #15 exp Mycophenolic Acid/ #16 exp Cyclosporine/ #17 exp Sirolimus/ #18 exp Muromonab‐CD3/ #19 exp Antilymphocyte Serum/ #20 (immunosuppres* or tacrolimus* or FK506* or mycopheno* or MMF* or (monoclonal adj3 antibod*) or mab* or daclizumab* or basiliximab* or cyclosporin* or ciclosporin* or calcineurin inhibitor* or calcineurin antagonist* or purine inhibitor* or purine antagonist* or sirolimus* or rapamycin* or everolimus* or methotrexate* or azathioprine* or muromonab* or orthoclon* or OKT3* or anti‐CD3* or antithymocyte* or ATG* or anti‐IL2* or anti‐CD52* or campath*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] #21 (steroid‐free* or corticosteroid‐free* or glucocorticoid‐free* or glucocorticosteroid‐free* or steroid‐sparing* or corticosteroid‐sparing* or glucocorticoid‐sparing* or glucocorticosteroid‐sparing* or steroid‐avoid* or corticosteroid‐avoid* or glucocorticoid‐avoid* or glucocorticosteroid‐avoid* or steroid‐taper* or corticosteroid‐taper* or glucocorticoid‐taper* or glucocorticosteroid‐taper* or steroid‐withdraw* or corticosteroid‐withdraw* or glucocorticoid‐withdraw* or glucocorticosteroid‐withdraw* or steroid‐eliminat* or corticosteroid‐eliminat* or glucocorticoid‐eliminat* or glucocorticosteroid‐eliminat* or steroid‐minimi* or corticosteroid‐minimi* or glucocorticoid‐minimi* or glucocorticosteroid‐minimi* or ((steroid* or corticosteroid* or glucocorticoid* or glucocorticosteroid*) adj3 (free* or spar* or avoid* or taper* or withdraw* or eliminat* or minimi* or without*))).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] #22 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 #23 (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] #24 3 and 11 and 22 and 23 |
| Embase Ovid | 1974 to May 2017 | #1 exp Liver Transplantation/ #2 ((liver or hepat*) and (transplant* or graft*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] #3 1 or 2 #4 exp Steroids/ #5 exp Glucocorticoids/ #6 exp Adrenal Cortex Hormones/ #7 exp Methylprednisolone/ #8 exp Prednisone/ #9 exp Dexamethasone/ #10 (glucocorticosteroid* or corticosteroid* or steroid* or gluco‐corticoid* or cortico‐steroid* or methylpredniso* or methyl‐predniso* or predniso* or dexamethaso* or dexa‐methaso* or monotherapy*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] #11 4 or 5 or 6 or 7 or 8 or 9 or 10 #12 exp Immunosuppressive Agents/ #13 exp Antibodies, Monoclonal/ #14 exp Tacrolimus/ #15 exp Mycophenolic Acid/ #16 exp Cyclosporine/ #17 exp Sirolimus/ #18 exp Muromonab‐CD3/ #19 exp Antilymphocyte Serum/ #20 (immunosuppres* or tacrolimus* or FK506* or mycopheno* or MMF* or (monoclonal adj3 antibod*) or mab* or daclizumab* or basiliximab* or cyclosporin* or ciclosporin* or calcineurin inhibitor* or calcineurin antagonist* or purine inhibitor* or purine antagonist* or sirolimus* or rapamycin* or everolimus* or methotrexate* or azathioprine* or muromonab* or orthoclon* or OKT3* or anti‐CD3* or antithymocyte* or ATG* or anti‐IL2* or anti‐CD52* or campath*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] #21 (steroid‐free* or corticosteroid‐free* or glucocorticoid‐free* or glucocorticosteroid‐free* or steroid‐sparing* or corticosteroid‐sparing* or glucocorticoid‐sparing* or glucocorticosteroid‐sparing* or steroid‐avoid* or corticosteroid‐avoid* or glucocorticoid‐avoid* or glucocorticosteroid‐avoid* or steroid‐taper* or corticosteroid‐taper* or glucocorticoid‐taper* or glucocorticosteroid‐taper* or steroid‐withdraw* or corticosteroid‐withdraw* or glucocorticoid‐withdraw* or glucocorticosteroid‐withdraw* or steroid‐eliminat* or corticosteroid‐eliminat* or glucocorticoid‐eliminat* or glucocorticosteroid‐eliminat* or steroid‐minimi* or corticosteroid‐minimi* or glucocorticoid‐minimi* or glucocorticosteroid‐minimi* or ((steroid* or corticosteroid* or glucocorticoid* or glucocorticosteroid*) adj3 (free* or spar* or avoid* or taper* or withdraw* or eliminat* or minimi* or without*))).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] #22 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 #23 (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] #24 3 and 11 and 22 and 23 |
| Science Citation Index EXPANDED and Conference Proceedings Citation Index ‐ Science (Web of Science) | 1900 to May 2017 1990 to May 2017 (Conference Proceedings) |
#6 #5 AND #4 #5 TS=(random* or blind* or placebo* or meta‐analys*) #4 #3 AND #2 AND #1 #3 TS=(immunosuppres* or tacrolimus* or mycopheno* or MMF* or (monoclonal adj3 antibod*) or mab* or daclizumab* or basiliximab* or cyclosporin* or ciclosporin* or calcineurin inhibitor* or calcineurin antagonist* or purine inhibitor* or purine antagonist* or sirolimus* or rapamycin* or everolimus* or methotrexate* or azathioprine* or muromonab* or orthoclon* or OKT3* or anti‐CD3* or antithymocyte* or ATG* or anti‐IL2* or anti‐CD52* or campath*) #2 TS=(glucocorticosteroid* or corticosteroid* or steroid* or gluco‐corticoid* or cortico‐steroid* or methylpredniso* or methyl‐predniso* or predniso* or dexamethaso* or dexa‐methaso*) #1 TS=((liver or hepat*) and (transplant* or graft*)) |
| Literatura Americana e do Caribe em Ciencias de Saude (LILACS) (Bireme) | Bireme; 1982 to May 2017 | ((liver or hepat$) and (transplant$ or graft$)) [Words] and (glucocorticosteroid$ or corticosteroid$ or steroid$ or gluco‐ corticoid$ or cortico‐steroid$ or methylpredniso$ or methyl‐predniso$ or predniso$ or dexamethaso$ or dexa‐ methaso$) [Words] and (immunosuppress$ or tacrolimus$ or mycopheno$ or MMF$ or monoclonal antibod$ or mab$ or daclizumab$ or basiliximab$ or cyclosporin$ or ciclosporin$ or calcineurin inhibitor$ or calcineurin antagonist$ or purine inhibitor$ or purine antagonist$ or sirolimus$ or rapamycin$ or everolimus$ or methotrexate$ or azathioprine$ or muromonab$ or orthoclon$ or OKT3$ or anti‐CD3$ or antithymocyte$ or ATG$ or anti‐IL2$ or anti‐CD52$ or campath$) [Words] |
Data and analyses
Comparison 1. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 15 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.44] |
| 1.1 Glucocorticosteroid avoidance | 9 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.84, 1.48] |
| 1.2 Glucocorticosteroid withdrawal | 6 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.86, 1.72] |
| 2 Graft loss including death | 11 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.46] |
| 2.1 Glucocorticosteroid avoidance | 8 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.77, 1.39] |
| 2.2 Glucocorticosteroid withdrawal | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.93, 2.24] |
| 3 Acute rejection | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.08, 1.64] |
| 3.1 Glucocorticosteroid avoidance | 10 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.04, 1.81] |
| 3.2 Glucocorticosteroid withdrawal | 6 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.93, 1.76] |
| 4 Infection | 8 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.05] |
| 4.1 Glucocorticosteroid avoidance | 6 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.15] |
| 4.2 Glucocorticosteroid withdrawal | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.90] |
| 5 Chronic rejection | 9 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.10] |
| 5.1 Glucocorticosteroid avoidance | 6 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.32, 2.08] |
| 5.2 Glucocorticosteroid withdrawal | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.55, 3.78] |
| 6 Glucocorticosteroid‐resistant rejection | 10 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.13, 4.02] |
| 6.1 Glucocorticosteroid avoidance | 7 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.89, 3.98] |
| 6.2 Glucocorticosteroid withdrawal | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.86, 9.49] |
| 7 Diabetes mellitus | 12 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.99] |
| 7.1 Glucocorticosteroid avoidance | 7 | 674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.17] |
| 7.2 Glucocorticosteroid withdrawal | 5 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.94] |
| 8 CMV | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.16] |
| 8.1 Glucocorticosteroid avoidance | 5 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.39, 1.49] |
| 8.2 Glucocorticosteroid withdrawal | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.41, 1.30] |
| 9 HCV recurrence | 10 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
| 9.1 Glucocorticosteroid avoidance | 7 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.08] |
| 9.2 Glucocorticosteroid withdrawal | 3 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.96, 1.44] |
| 10 Malignancy | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.74] |
| 10.1 Glucocorticosteroid avoidance | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.13, 2.08] |
| 10.2 Glucocorticosteroid withdrawal | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.05, 5.80] |
| 11 Post‐transplant lymphoproliferative disorder | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.36, 15.95] |
| 11.1 Glucocorticosteroid avoidance | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.61] |
| 11.2 Glucocorticosteroid withdrawal | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.13, 77.77] |
| 12 Renal insufficiency | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 12.1 Glucocorticosteroid avoidance | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 13 Creatinine | 4 | 309 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 13.1 Glucocorticosteroid avoidance | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.10, 0.20] |
| 13.2 Glucocorticosteroid withdrawal | 2 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.16, 0.05] |
| 14 Hypertension | 10 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.90] |
| 14.1 Glucocorticosteroid avoidance | 6 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 1.00] |
| 14.2 Glucocorticosteroid withdrawal | 4 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.91] |
| 15 Hyperlipidaemia | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.48] |
| 15.1 Glucocorticosteroid avoidance | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.45, 2.52] |
| 15.2 Glucocorticosteroid withdrawal | 2 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.14, 1.41] |
| 16 Cholesterol | 6 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐18.49 [‐22.02, ‐14.96] |
| 16.1 Glucocorticosteroid avoidance | 3 | 343 | Mean Difference (IV, Fixed, 95% CI) | ‐18.33 [‐21.93, ‐14.72] |
| 16.2 Glucocorticosteroid withdrawal | 3 | 268 | Mean Difference (IV, Fixed, 95% CI) | ‐22.06 [‐38.94, ‐5.18] |
| 17 Hypercholesterolaemia | 2 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 1.00] |
| 17.1 Glucocorticosteroid avoidance | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.55, 2.61] |
| 17.2 Glucocorticosteroid withdrawal | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.08, 0.59] |
Comparison 2. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 15 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.44] |
| 1.1 Tacrolimus | 11 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.92, 1.51] |
| 1.2 Cyclosporine A | 4 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.69, 1.74] |
| 2 Graft loss including death | 11 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.46] |
| 2.1 Tacrolimus | 8 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.85, 1.47] |
| 2.2 Cyclosporine A | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.72, 2.09] |
| 3 Acute rejection | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.08, 1.64] |
| 3.1 Tacrolimus | 11 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.02, 1.77] |
| 3.2 Cyclosporine A | 5 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.80] |
| 4 Infection | 8 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.05] |
| 4.1 Tacrolimus | 4 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.27] |
| 4.2 Cyclosporine A | 4 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.05] |
| 5 Chronic rejection | 9 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.10] |
| 5.1 Tacrolimus | 4 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.44, 2.76] |
| 5.2 Cyclosporine A | 5 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.41, 2.76] |
| 6 Glucocorticosteroid‐resistant rejection | 10 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.13, 4.02] |
| 6.1 Tacrolimus | 7 | 603 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.01, 5.97] |
| 6.2 Cyclosporine A | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.74, 4.55] |
| 7 Diabetes mellitus | 12 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.99] |
| 7.1 Tacrolimus | 9 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.15] |
| 7.2 Cyclosporine A | 3 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.41, 0.90] |
| 8 CMV infection | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.16] |
| 8.1 Tacrolimus | 4 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.38] |
| 8.2 Cyclosporine A | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.40] |
| 9 HCV recurrence | 10 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
| 9.1 Tacrolimus | 5 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 9.2 Cyclosporine A | 5 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.23] |
| 10 Malignancy | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.74] |
| 10.1 Tacrolimus | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.49] |
| 10.2 Cyclosporine A | 2 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.22] |
| 11 Post‐transplant lymphoproliferative disorder | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.36, 15.95] |
| 11.1 Tacrolimus | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.61] |
| 11.2 Cyclosporine A | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.13, 77.77] |
| 12 Renal insufficiency | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 12.1 Tacrolimus | 3 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
| 12.2 Cyclosporine A | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.16] |
| 13 Creatinine | 4 | 309 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 13.1 Tacrolimus | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.12, 0.22] |
| 13.2 Cyclosporine A | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 14 Hypertension | 10 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.90] |
| 14.1 Tacrolimus | 7 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.65, 1.06] |
| 14.2 Cyclosporine A | 3 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.58, 0.88] |
| 15 Hyperlipidaemia | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.48] |
| 15.1 Tacrolimus | 3 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.44, 2.02] |
| 15.2 Cyclosporine A | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.07, 1.72] |
| 16 Cholesterol | 6 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐18.49 [‐22.02, ‐14.96] |
| 16.1 Tacrolimus | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐18.38 [‐22.09, ‐14.67] |
| 16.2 Cyclosporine A | 3 | 347 | Mean Difference (IV, Fixed, 95% CI) | ‐19.56 [‐31.05, ‐8.07] |
2.2. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 2 Graft loss including death.
2.3. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 3 Acute rejection.
2.4. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 4 Infection.
2.5. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 5 Chronic rejection.
2.6. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 6 Glucocorticosteroid‐resistant rejection.
2.7. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 7 Diabetes mellitus.
2.8. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 8 CMV infection.
2.9. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 9 HCV recurrence.
2.10. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 10 Malignancy.
2.11. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 11 Post‐transplant lymphoproliferative disorder.
2.12. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 12 Renal insufficiency.
2.13. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 13 Creatinine.
2.14. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 14 Hypertension.
2.15. Analysis.
Comparison 2 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (CNI subgroups), Outcome 15 Hyperlipidaemia.
Comparison 3. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 15 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.44] |
| 1.1 No antiproliferative agent | 8 | 928 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.92, 1.66] |
| 1.2 Mycophenolate mofetil | 6 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.51] |
| 1.3 Azathioprine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.40, 2.28] |
| 2 Graft loss including death | 11 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.46] |
| 2.1 No antiproliferative agent | 6 | 748 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.82, 1.52] |
| 2.2 Mycophenolate mofetil | 4 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.82, 1.96] |
| 2.3 Azathioprine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.40, 2.28] |
| 3 Acute rejection | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.08, 1.64] |
| 3.1 No antiproliferative agent | 9 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.97, 1.56] |
| 3.2 Mycophenolate mofetil | 6 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.15, 3.04] |
| 3.3 Azathioprine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.27, 3.36] |
| 4 Infection | 8 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.05] |
| 4.1 No antiproliferative agent | 3 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.02] |
| 4.2 Mycophenolate mofetil | 4 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.27] |
| 4.3 Azathioprine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.75, 1.58] |
| 5 Chronic rejection | 9 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.10] |
| 5.1 No antiproliferative agent | 7 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.66, 2.79] |
| 5.2 Mycophenolate mofetil | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
| 5.3 Azathioprine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Glucocorticosteroid‐resistant rejection | 10 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.13, 4.02] |
| 6.1 No antiproliferative agent | 6 | 748 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.13, 4.02] |
| 6.2 Mycophenolate mofetil | 3 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Azathioprine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Diabetes mellitus | 12 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.99] |
| 7.1 No antiproliferative agent | 8 | 928 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.00] |
| 7.2 Mycophenolate mofetil | 4 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.25] |
| 8 CMV infection | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.16] |
| 8.1 No antiproliferative agent | 5 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.45, 1.12] |
| 8.2 Mycophenolate mofetil | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Azathioprine | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.19, 19.63] |
| 9 HCV recurrence | 10 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
| 9.1 No antiproliferative agent | 7 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.22] |
| 9.2 Mycophenolate mofetil | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.22] |
| 9.3 Azathioprine | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.52] |
| 10 Renal insufficiency | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 10.1 No antiproliferative agent | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.16] |
| 10.2 Mycophenolate mofetil | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.30, 29.52] |
| 11 Creatinine | 4 | 309 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 11.1 No antiproliferative agent | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.47, 0.23] |
| 11.2 Mycophenolate mofetil | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.13, 0.23] |
| 11.3 Azathioprine | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 12 Hypertension | 10 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.90] |
| 12.1 No antiproliferative agent | 7 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.62, 0.88] |
| 12.2 Mycophenolate mofetil | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
| 13 Hyperlipidaemia | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.48] |
| 13.1 No antiproliferative agent | 3 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.32, 1.62] |
| 13.2 Mycophenolate mofetil | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.24, 2.85] |
| 14 Cholesterol | 6 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐18.49 [‐22.02, ‐14.96] |
| 14.1 No antiproliferative agent | 3 | 412 | Mean Difference (IV, Fixed, 95% CI) | ‐8.08 [‐18.99, 2.82] |
| 14.2 Mycophenolate mofetil | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐19.84 [‐23.60, ‐16.08] |
| 14.3 Azathioprine | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐41.10, 19.10] |
3.2. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 2 Graft loss including death.
3.3. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 3 Acute rejection.
3.4. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 4 Infection.
3.5. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 5 Chronic rejection.
3.6. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 6 Glucocorticosteroid‐resistant rejection.
3.7. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 7 Diabetes mellitus.
3.8. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 8 CMV infection.
3.9. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 9 HCV recurrence.
3.10. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 10 Renal insufficiency.
3.11. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 11 Creatinine.
3.12. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 12 Hypertension.
3.13. Analysis.
Comparison 3 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (antiproliferative subgroups), Outcome 13 Hyperlipidaemia.
Comparison 4. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 15 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.44] |
| 1.1 No induction therapy | 8 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.50] |
| 1.2 Basiliximab | 5 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.85, 1.81] |
| 1.3 Rabbit antithymocyte globulin | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.51, 2.50] |
| 1.4 Daclizumab | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.35] |
| 2 Graft loss including death | 11 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.46] |
| 2.1 No induction therapy | 6 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.39] |
| 2.2 Basiliximab | 4 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.99, 2.12] |
| 2.3 Daclizumab | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.73] |
| 3 Acute rejection | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.08, 1.64] |
| 3.1 No induction therapy | 8 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.94, 1.71] |
| 3.2 Basiliximab | 5 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.05, 2.05] |
| 3.3 Rabbit antithymocyte globulin | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.67] |
| 3.4 Daclizumab | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.67, 7.34] |
| 4 Infection | 8 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.05] |
| 4.1 No induction therapy | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.86, 1.77] |
| 4.2 Basiliximab | 5 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |
| 4.3 Rabbit antithymocyte globulin | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.89] |
| 5 Chronic rejection | 9 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.10] |
| 5.1 No induction therapy | 4 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.60, 5.78] |
| 5.2 Basiliximab | 3 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.29, 1.89] |
| 5.3 Rabbit antithymocyte globulin | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.16, 6.72] |
| 6 Glucocorticosteroid‐resistant rejection | 10 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.13, 4.02] |
| 6.1 No induction therapy | 5 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.01, 5.97] |
| 6.2 Basiliximab | 5 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.74, 4.55] |
| 7 Diabetes mellitus | 12 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.99] |
| 7.1 No induction therapy | 6 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.24] |
| 7.2 Basiliximab | 5 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.60, 1.06] |
| 7.3 Rabbit antithymocyte globulin | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.77] |
| 8 CMV infection | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.16] |
| 8.1 No induction therapy | 4 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.50, 1.44] |
| 8.2 Basiliximab | 3 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.27, 1.30] |
| 9 HCV recurrence | 10 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
| 9.1 No induction therapy | 4 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.18] |
| 9.2 Basiliximab | 4 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
| 9.3 Rabbit antithymocyte globulin | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.55, 3.11] |
| 10 Malignancy | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.74] |
| 10.1 No induction therapy | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.49] |
| 10.2 Basiliximab | 2 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.22] |
| 11 Post‐transplant lymphoproliferative disorder | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.36, 15.95] |
| 11.1 No induction therapy | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.61] |
| 11.2 Basiliximab | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.13, 77.77] |
| 12 Renal insufficiency | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 12.1 No induction therapy | 3 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
| 12.2 Basiliximab | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.16] |
| 13 Creatinine | 4 | 309 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 13.1 No induction therapy | 3 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.15, ‐0.01] |
| 13.2 Basiliximab | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.19, 0.31] |
| 14 Hypertension | 10 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.90] |
| 14.1 No induction therapy | 5 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.08] |
| 14.2 Basiliximab | 4 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| 14.3 Rabbit antithymocyte globulin | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.57] |
| 15 Hyperlipidaemia | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.48] |
| 15.1 No induction therapy | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.38, 2.72] |
| 15.2 Basiliximab | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.22, 1.49] |
| 16 Cholesterol | 6 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐18.49 [‐22.02, ‐14.96] |
| 16.1 No induction therapy | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐51.27 [‐76.48, ‐26.06] |
| 16.2 Basiliximab | 3 | 408 | Mean Difference (IV, Fixed, 95% CI) | ‐17.10 [‐20.69, ‐13.51] |
| 16.3 Rabbit antithymocyte globulin | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐70.0 [‐100.17, ‐39.83] |
4.2. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 2 Graft loss including death.
4.3. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 3 Acute rejection.
4.4. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 4 Infection.
4.5. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 5 Chronic rejection.
4.6. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 6 Glucocorticosteroid‐resistant rejection.
4.7. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 7 Diabetes mellitus.
4.8. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 8 CMV infection.
4.9. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 9 HCV recurrence.
4.10. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 10 Malignancy.
4.11. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 11 Post‐transplant lymphoproliferative disorder.
4.12. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 12 Renal insufficiency.
4.13. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 13 Creatinine.
4.14. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 14 Hypertension.
4.15. Analysis.
Comparison 4 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (induction therapy subgroups), Outcome 15 Hyperlipidaemia.
Comparison 5. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 15 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.44] |
| 1.1 Monotherapy | 5 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.90, 1.83] |
| 1.2 Dual therapy | 6 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.68, 1.42] |
| 1.3 Triple therapy | 4 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.79, 1.90] |
| 2 Graft loss including death | 11 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.46] |
| 2.1 Monotherapy | 4 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.48] |
| 2.2 Dual therapy | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.69, 1.93] |
| 2.3 Triple therapy | 3 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.86, 2.11] |
| 3 Acute rejection | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.08, 1.64] |
| 3.1 Monotherapy | 5 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.81, 1.59] |
| 3.2 Dual therapy | 7 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.07, 1.95] |
| 3.3 Triple therapy | 4 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.84, 2.88] |
| 4 Infection | 8 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.05] |
| 4.1 Dual therapy | 5 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.09] |
| 4.2 Triple therapy | 3 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.20] |
| 5 Chronic rejection | 9 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.10] |
| 5.1 Monotherapy | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.60, 5.78] |
| 5.2 Dual therapy | 5 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.41, 2.76] |
| 5.3 Triple therapy | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
| 6 Glucocorticosteroid‐resistant rejection | 10 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.13, 4.02] |
| 6.1 Monotherapy | 4 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.01, 5.97] |
| 6.2 Dual therapy | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.74, 4.55] |
| 6.3 Triple therapy | 3 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Diabetes mellitus | 12 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.99] |
| 7.1 Monotherapy | 5 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.70, 1.28] |
| 7.2 Dual therapy | 4 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.41, 0.89] |
| 7.3 Triple therapy | 3 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.31] |
| 8 CMV infection | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.16] |
| 8.1 Monotherapy | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.38] |
| 8.2 Dual therapy | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.40] |
| 8.3 Triple therapy | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 HCV recurrence | 10 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
| 9.1 Monotherapy | 3 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.25] |
| 9.2 Dual therapy | 5 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.23] |
| 9.3 Triple therapy | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.22] |
| 10 Malignancy | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.74] |
| 10.1 Monotherapy | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.49] |
| 10.2 Dual therapy | 2 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.22] |
| 11 Post‐transplant lymphoproliferative disorder | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.36, 15.95] |
| 11.1 Monotherapy | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.61] |
| 11.2 Dual therapy | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.13, 77.77] |
| 12 Renal insufficiency | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 12.1 Monotherapy | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.56] |
| 12.2 Dual therapy | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.20] |
| 13 Creatinine | 4 | 309 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 13.1 Monotherapy | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.47, 0.23] |
| 13.2 Dual therapy | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.15, ‐0.01] |
| 13.3 Triple therapy | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.19, 0.31] |
| 14 Hypertension | 10 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.90] |
| 14.1 Monotherapy | 4 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.10] |
| 14.2 Dual therapy | 4 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.57, 0.88] |
| 14.3 Triple therapy | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.32] |
| 15 Hyperlipidaemia | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.48] |
| 15.1 Monotherapy | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.38, 2.72] |
| 15.2 Dual therapy | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.07, 1.72] |
| 15.3 Triple therapy | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.24, 2.85] |
| 16 Cholesterol | 6 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐18.49 [‐22.02, ‐14.96] |
| 16.1 Monotherapy | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 35.0 [12.31, 57.69] |
| 16.2 Dual therapy | 4 | 401 | Mean Difference (IV, Fixed, 95% CI) | ‐26.94 [‐38.10, ‐15.79] |
| 16.3 Triple therapy | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐19.0 [‐22.77, ‐15.23] |
5.2. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 2 Graft loss including death.
5.3. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 3 Acute rejection.
5.4. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 4 Infection.
5.5. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 5 Chronic rejection.
5.6. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 6 Glucocorticosteroid‐resistant rejection.
5.7. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 7 Diabetes mellitus.
5.8. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 8 CMV infection.
5.9. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 9 HCV recurrence.
5.10. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 10 Malignancy.
5.11. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 11 Post‐transplant lymphoproliferative disorder.
5.12. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 12 Renal insufficiency.
5.13. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 13 Creatinine.
5.14. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 14 Hypertension.
5.15. Analysis.
Comparison 5 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (co‐interventions subgroups), Outcome 15 Hyperlipidaemia.
Comparison 6. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 14 | 1149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.87, 1.36] |
| 1.1 2 to 3 months glucocorticosteroid | 7 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.70, 1.41] |
| 1.2 > 3 to 6 months glucocorticosteroids | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.00, 2.18] |
| 1.3 > 6 months glucocorticosteroids | 4 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.55, 1.33] |
| 2 Graft loss including death | 10 | 828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.83, 1.37] |
| 2.1 2 to 3 months glucocorticosteroid | 5 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.29] |
| 2.2 > 3 to 6 months glucocorticosteroids | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.01, 2.04] |
| 2.3 > 6 months glucocorticosteroids | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.25, 1.47] |
| 3 Acute rejection | 15 | 1173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.01, 1.62] |
| 3.1 2 to 3 months glucocorticosteroid | 8 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.97, 1.75] |
| 3.2 > 3 to 6 months glucocorticosteroids | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.83, 2.15] |
| 3.3 > 6 months glucocorticosteroids | 4 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.54, 2.12] |
| 4 Infection | 7 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.08] |
| 4.1 2 to 3 months glucocorticosteroid | 4 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] |
| 4.2 > 3 to 6 months glucocorticosteroids | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.54] |
| 4.3 > 6 months glucocorticosteroids | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.89] |
| 5 Chronic rejection | 8 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.59, 2.71] |
| 5.1 2 to 3 months glucocorticosteroid | 5 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.41, 3.73] |
| 5.2 > 3 to 6 months glucocorticosteroids | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.51, 5.24] |
| 5.3 > 6 months glucocorticosteroids | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.42] |
| 6 Glucocorticosteroid‐resistant rejection | 9 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.93, 4.01] |
| 6.1 2 to 3 months glucocorticosteroid | 5 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.89, 3.98] |
| 6.2 > 3 to 6 months glucocorticosteroids | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 69.55] |
| 6.3 > 6 months glucocorticosteroids | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Diabetes mellitus | 11 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.03] |
| 7.1 2 to 3 months glucocorticosteroid | 6 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.54, 1.01] |
| 7.2 > 3 to 6 months glucocorticosteroids | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.81, 1.66] |
| 7.3 > 6 months glucocorticosteroids | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.39, 1.03] |
| 8 CMV infection | 6 | 612 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.18] |
| 8.1 2 to 3 months glucocorticosteroid | 4 | 462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.39, 1.49] |
| 8.2 > 3 to 6 months glucocorticosteroids | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.35] |
| 9 HCV recurrence | 9 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| 9.1 2 to 3 months glucocorticosteroid | 5 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 9.2 > 3 to 6 months glucocorticosteroids | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.22] |
| 9.3 > 6 months glucocorticosteroids | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.89, 2.09] |
| 10 Creatinine | 4 | 309 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 10.1 > 6 months glucocorticosteroids | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 10.2 2 to 3 months glucocorticosteroid | 2 | 210 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.18, 0.30] |
| 10.3 > 3 to 6 months glucocorticosteroids | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.16, 0.06] |
| 11 Hypertension | 9 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.63, 0.89] |
| 11.1 2 to 3 months glucocorticosteroid | 6 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.92] |
| 11.2 > 3 to 6 months glucocorticosteroids | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.80, 1.40] |
| 11.3 > 6 months glucocorticosteroids | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.57] |
| 12 Cholesterol | 6 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐18.49 [‐22.02, ‐14.96] |
| 12.1 2 to 3 months glucocorticosteroid | 2 | 243 | Mean Difference (IV, Fixed, 95% CI) | ‐11.00 [‐23.43, 1.43] |
| 12.2 > 3 to 6 months glucocorticosteroids | 2 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐17.55 [‐21.27, ‐13.83] |
| 12.3 > 6 months glucocorticosteroids | 2 | 158 | Mean Difference (IV, Fixed, 95% CI) | ‐92.75 [‐118.01, ‐67.50] |
| 13 Hypercholesterolaemia | 2 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 1.00] |
| 13.1 2 to 3 months glucocorticosteroid | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.55, 2.61] |
| 13.2 > 3 to 6 months glucocorticosteroids | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.08, 0.59] |
6.2. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 2 Graft loss including death.
6.3. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 3 Acute rejection.
6.4. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 4 Infection.
6.5. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 5 Chronic rejection.
6.6. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 6 Glucocorticosteroid‐resistant rejection.
6.7. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 7 Diabetes mellitus.
6.8. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 8 CMV infection.
6.9. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 9 HCV recurrence.
6.10. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 10 Creatinine.
6.11. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 11 Hypertension.
6.12. Analysis.
Comparison 6 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (treatment duration subgroups), Outcome 12 Cholesterol.
Comparison 7. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 15 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.44] |
| 1.1 Pre‐2000 | 4 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.90, 2.06] |
| 1.2 Post‐2000 | 11 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.83, 1.40] |
| 2 Graft loss including death | 11 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.46] |
| 2.1 Pre‐2000 | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.65, 2.40] |
| 2.2 Post‐2000 | 8 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.47] |
| 3 Acute rejection | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.08, 1.64] |
| 3.1 Pre‐2000 | 5 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.91, 1.75] |
| 3.2 Post‐2000 | 11 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.05, 1.80] |
| 4 Infection | 8 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.05] |
| 4.1 Pre‐2000 | 3 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.04] |
| 4.2 Post‐2000 | 5 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.77, 1.15] |
| 5 Chronic rejection | 9 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.10] |
| 5.1 Pre‐2000 | 5 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.25, 2.31] |
| 5.2 Post‐2000 | 4 | 564 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.57, 3.03] |
| 6 Glucocorticosteroid‐resistant rejection | 10 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.13, 4.02] |
| 6.1 Pre‐2000 | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.84, 5.57] |
| 6.2 Post‐2000 | 7 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.90, 4.96] |
| 7 Diabetes mellitus | 12 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.99] |
| 7.1 Pre‐2000 | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.88] |
| 7.2 Post‐2000 | 9 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 8 CMV infection | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.16] |
| 8.1 Pre‐2000 | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.21] |
| 8.2 Post‐2000 | 4 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.17] |
| 9 HCV recurrence | 10 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
| 9.1 Pre‐2000 | 5 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.22] |
| 9.2 Post‐2000 | 5 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.92, 1.19] |
| 10 Malignancy | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.74] |
| 10.1 Pre‐2000 | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.05, 5.80] |
| 10.2 Post‐2000 | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.13, 2.08] |
| 11 Post‐transplant lymphoproliferative disorder | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.36, 15.95] |
| 11.1 Pre‐2000 | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.13, 77.77] |
| 11.2 Post‐2000 | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.61] |
| 12 Renal insufficiency | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 12.1 Pre‐2000 | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.85, 1.96] |
| 12.2 Post‐2000 | 3 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.12] |
| 13 Creatinine | 4 | 309 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 13.1 Pre‐2000 | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 13.2 Post‐2000 | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.12, 0.22] |
| 14 Hypertension | 10 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.90] |
| 14.1 Pre‐2000 | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.40, 0.79] |
| 14.2 Post‐2000 | 7 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.03] |
| 15 Hyperlipidaemia | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.48] |
| 15.1 Pre‐2000 | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.30, 1.91] |
| 15.2 Post‐2000 | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.27, 2.01] |
| 16 Cholesterol | 6 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐18.49 [‐22.02, ‐14.96] |
| 16.1 Pre‐2000 | 2 | 149 | Mean Difference (IV, Fixed, 95% CI) | ‐40.42 [‐61.73, ‐19.11] |
| 16.2 Post‐2000 | 4 | 462 | Mean Difference (IV, Fixed, 95% CI) | ‐17.87 [‐21.45, ‐14.29] |
7.2. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 2 Graft loss including death.
7.3. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 3 Acute rejection.
7.4. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 4 Infection.
7.5. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 5 Chronic rejection.
7.6. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 6 Glucocorticosteroid‐resistant rejection.
7.7. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 7 Diabetes mellitus.
7.8. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 8 CMV infection.
7.9. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 9 HCV recurrence.
7.10. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 10 Malignancy.
7.11. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 11 Post‐transplant lymphoproliferative disorder.
7.12. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 12 Renal insufficiency.
7.13. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 13 Creatinine.
7.14. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 14 Hypertension.
7.15. Analysis.
Comparison 7 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (pre‐2000 and post‐2000 subgroups), Outcome 15 Hyperlipidaemia.
Comparison 8. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 1.1 Glucocorticosteroid avoidance | 10 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.87, 1.52] |
| 1.2 Glucocorticosteroid withdrawal | 6 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.12] |
| 2 Graft loss including death | 11 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.24] |
| 2.1 Glucocorticosteroid avoidance | 8 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.77, 1.39] |
| 2.2 Glucocorticosteroid withdrawal | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.61, 1.33] |
| 3 Acute rejection | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.85, 1.26] |
| 3.1 Glucocorticosteroid avoidance | 10 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.04, 1.81] |
| 3.2 Glucocorticosteroid withdrawal | 6 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.02] |
| 4 Infection | 8 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.67, 0.96] |
| 4.1 Glucocorticosteroid avoidance | 6 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.15] |
| 4.2 Glucocorticosteroid withdrawal | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.12, 0.50] |
| 5 Chronic rejection | 9 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.33, 1.05] |
| 5.1 Glucocorticosteroid avoidance | 6 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.32, 2.08] |
| 5.2 Glucocorticosteroid withdrawal | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 1.02] |
| 6 Glucocorticosteroid‐resistant rejection | 10 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.65] |
| 6.1 Glucocorticosteroid avoidance | 7 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.89, 3.98] |
| 6.2 Glucocorticosteroid withdrawal | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.27, 1.13] |
| 7 Diabetes mellitus | 12 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.58, 0.86] |
| 7.1 Glucocorticosteroid avoidance | 7 | 674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.17] |
| 7.2 Glucocorticosteroid withdrawal | 5 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.39, 0.70] |
| 8 CMV infection | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.87] |
| 8.1 Glucocorticosteroid avoidance | 5 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.39, 1.49] |
| 8.2 Glucocorticosteroid withdrawal | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.27, 0.81] |
| 9 Malignancy | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.61] |
| 9.1 Glucocorticosteroid avoidance | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.13, 2.08] |
| 9.2 Glucocorticosteroid withdrawal | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.57] |
| 10 Post‐transplant lymphoproliferative disorder | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.07, 0.85] |
| 10.1 Glucocorticosteroid avoidance | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.61] |
| 10.2 Glucocorticosteroid withdrawal | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 11 Hypertension | 10 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.60, 0.82] |
| 11.1 Glucocorticosteroid avoidance | 6 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 1.00] |
| 11.2 Glucocorticosteroid withdrawal | 4 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.47, 0.76] |
| 12 Hyperlipidaemia | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.21, 0.73] |
| 12.1 Glucocorticosteroid avoidance | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.45, 2.52] |
| 12.2 Glucocorticosteroid withdrawal | 2 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.45] |
8.2. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 2 Graft loss including death.
8.3. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 3 Acute rejection.
8.4. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 4 Infection.
8.5. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 5 Chronic rejection.
8.6. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 6 Glucocorticosteroid‐resistant rejection.
8.7. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 7 Diabetes mellitus.
8.8. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 8 CMV infection.
8.9. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 9 Malignancy.
8.10. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 10 Post‐transplant lymphoproliferative disorder.
8.11. Analysis.
Comparison 8 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (best‐worst analysis), Outcome 11 Hypertension.
Comparison 9. Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.67] |
| 1.1 Glucocorticosteroid avoidance | 10 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.86, 1.49] |
| 1.2 Glucocorticosteroid withdrawal | 6 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.23, 2.38] |
| 2 Graft loss including death | 11 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.08, 1.74] |
| 2.1 Glucocorticosteroid avoidance | 8 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.41] |
| 2.2 Glucocorticosteroid withdrawal | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [1.47, 3.41] |
| 3 Acute rejection | 16 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.25, 1.88] |
| 3.1 Glucocorticosteroid avoidance | 10 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.05, 1.83] |
| 3.2 Glucocorticosteroid withdrawal | 6 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.29, 2.31] |
| 4 Infection | 8 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.87, 1.23] |
| 4.1 Glucocorticosteroid avoidance | 6 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.15] |
| 4.2 Glucocorticosteroid withdrawal | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.89, 2.50] |
| 5 Chronic rejection | 9 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.40, 4.31] |
| 5.1 Glucocorticosteroid avoidance | 6 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.32, 2.08] |
| 5.2 Glucocorticosteroid withdrawal | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [2.16, 11.01] |
| 6 Glucocorticosteroid‐resistant rejection | 10 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.71 [2.07, 6.66] |
| 6.1 Glucocorticosteroid avoidance | 7 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.95, 4.17] |
| 6.2 Glucocorticosteroid withdrawal | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.63 [2.95, 25.28] |
| 7 Diabetes mellitus | 12 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.15] |
| 7.1 Glucocorticosteroid avoidance | 7 | 674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.19] |
| 7.2 Glucocorticosteroid withdrawal | 5 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.76, 1.32] |
| 8 CMV infection | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.87, 1.90] |
| 8.1 Glucocorticosteroid avoidance | 5 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.41, 1.59] |
| 8.2 Glucocorticosteroid withdrawal | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.04, 2.78] |
| 9 Malignancy | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.38, 6.73] |
| 9.1 Glucocorticosteroid avoidance | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.13, 2.08] |
| 9.2 Glucocorticosteroid withdrawal | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.71 [2.58, 44.45] |
| 10 Post‐transplant lymphoproliferative disorder | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.64 [3.08, 79.56] |
| 10.1 Glucocorticosteroid avoidance | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.61] |
| 10.2 Glucocorticosteroid withdrawal | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 43.89 [2.70, 714.49] |
| 11 Renal insufficiency | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.21] |
| 11.1 Glucocorticosteroid avoidance | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.21] |
| 12 Hypertension | 10 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.02] |
| 12.1 Glucocorticosteroid avoidance | 6 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.67, 1.01] |
| 12.2 Glucocorticosteroid withdrawal | 4 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.18] |
| 13 Hyperlipidaemia | 4 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.12, 3.28] |
| 13.1 Glucocorticosteroid avoidance | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.51, 2.73] |
| 13.2 Glucocorticosteroid withdrawal | 2 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.28, 5.44] |
9.2. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 2 Graft loss including death.
9.3. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 3 Acute rejection.
9.4. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 4 Infection.
9.5. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 5 Chronic rejection.
9.6. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 6 Glucocorticosteroid‐resistant rejection.
9.7. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 7 Diabetes mellitus.
9.8. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 8 CMV infection.
9.9. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 9 Malignancy.
9.10. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 10 Post‐transplant lymphoproliferative disorder.
9.11. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 11 Renal insufficiency.
9.12. Analysis.
Comparison 9 Glucocorticosteroid avoidance or withdrawal versus glucocorticosteroid‐containing immunosuppression (worst‐best analysis), Outcome 12 Hypertension.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belli 1998.
| Methods | Trial design: randomised, single‐centre clinical trial Mean follow‐up: total: 41 ± 16 months, range 4 to 68 months Study duration: date of randomisation to last follow‐up before 28 February 1997, or patient death or re‐transplantation Language: English Type of information: journal article Judgement on quality: unclear risk of bias |
|
| Participants | Setting: Ospedale Niguarda Ca' Granda, Milan, Italy Allocation of participants: 104 participants, 50 allocated to long‐term glucocorticosteroids, 54 allocated to short‐term glucocorticosteroids Sex ratio: total: 74 (71%) males, 30 (29%) females Intervention A: 37 (74%) males, 13 (26%) females Intervention B: 37 (68.5%) males, 17 (31.5%) females Mean age: total: not reported Intervention A: 45 ± 14 Intervention B: 42 ± 16 Indication (no. (%)): (indications reported for whole study population but not intervention groups) HCV: 42 (40.4%) HBV: 24 (23.1%) HBV and HCV: 8 (7.7%) Alcoholic cirrhosis: 9 (8.7%) Primary biliary cirrhosis: 6 (5.8%) Cryptogenic cirrhosis: 8 (7.7%) Others: 7 (6.7%) Type of donor: not reported Inclusion criteria: adult liver transplant recipients Exclusion criteria: previous liver transplant, previous other organ transplant, multiorgan transplant Other: rejection before randomisation (n (%)): Intervention A: 15 (30%) Intervention B: 22 (41%) |
|
| Interventions | Intervention A: methylprednisolone: from day 90, 20 mg per day with 5 mg reductions every 2 weeks until stopped Intervention B: methylprednisolone: from day 90, 20 mg per day with 5 mg reductions every 2 weeks until maintenance dose of 0.1 mg/kg/day continued for duration of study Concomitant immunosuppression: Rabbit antithymocyte globulins: 2 mg/kg/day for 5 to 7 days from day 0 Cyclosporine A: 200 to 300 ng/mL (from day 90 for "first months") and 150 ng/mL to 250 ng/mL thereafter Methylprednisolone: 1000 mg intraoperatively; 200 mg at day 1; 160 mg at day 2; 120 mg at day 3; 80 mg at day 4; 40 mg at day 5; 20 mg at day 6; then continued at the same dose until day 90 |
|
| Outcomes | Patient survival, acute rejection, chronic rejection, hypertension, diabetes, severe bone complications, infections, serum cholesterol, recurrent hepatitis B, recurrent hepatitis C and treatment failure | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from the publication: "One hundred four first orthotopic liver transplantations performed between May 1991 and June 1995 at the Niguarda Hospital in Milan and surviving long enough to reach the randomization time point were prospectively assigned to one of the two maintenance immunosuppressive regimens. Fifty patients were randomized to receive cyclosporine plus long‐term corticosteroids (Group I) and 54 patients were randomized to cyclosporine monotherapy (Group II)." Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of participants and medical staff not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All predefined outcomes and clinically relevant outcomes appear to be reported |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Comment: No evidence of baseline imbalance reported in "Table 3" |
Belli 2001.
| Methods | Trial design: randomised, single‐centre clinical trial Mean follow‐up: not reported Intervention A: 22 months Intervention B: 21 months Study duration: randomisation from November 1997 to November 1999, duration from randomisation not reported Language: English Type of information: journal article Judgement on quality: high risk of bias |
|
| Participants | Setting: Ospedale Niguarda Ca' Granda, Milan, Italy Allocation of participants: 24 participants, 13 allocated to glucocorticosteroids, 11 allocated to no intervention Sex ratio: total: not reported Intervention A: not reported Intervention B: not reported Mean age: total: not reported Intervention A: not reported Intervention B: not reported Indication (no. (%)): HCV cirrhosis: total: 24 (100%), Intervention A: 13 (100%), Intervention B: 11 (100%) Type of donor: not reported Inclusion criteria: adult liver transplant recipients with HCV cirrhosis Exclusion criteria: not reported |
|
| Interventions | Intervention A: no intervention Intervention B: glucocorticosteroids for 3 months, doses not reported Concomitant immunosuppression: Rabbit antithymocyte globulin: dose not reported, given for 5 days Azathioprine: dose not reported, given for 1 month Cyclosporine A: dose not reported |
|
| Outcomes | Acute rejection, chronic rejection, recurrent hepatitis C, severe cholestasis, ALT, mortality, portal vein thrombosis | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: not reported One intervention group was excluded from the meta‐analysis as differences between hepatitis C virus prophylaxis (ribavirin) were noted Although the overall data for mortality and portal vein thrombosis have been reported, the exact number of participants in each group with these outcomes is not reported, therefore these results are not included in the meta‐analysis but are included in the best‐worst worst‐best analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from the publication: "Between November 1997 and November 1999 37 patients (pts) were randomized to one of three groups: ..." Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of participants and medical staff not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment:Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) | High risk | Quote from the publication: "Of these 37 pts, only 30 were considered in the analysis. Seven pts were excluded because of early death after transplant (6 pts) or because of concurrent confounding clinical problems (1 pt with portal vein thrombosis)." Comment: Mortality and portal vein thrombosis not reported fully. As this is a three‐arm trial it is not possible to accurately record data on mortality within each group as the trial does not report which arm of the trial the portal vein thrombosis occurred in and which arms of the trial each mortality occurred in. Therefore, the outcomes have not been included in the meta‐analysis but have been included in the best‐worst worst‐best analyses. Otherwise, number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Unclear risk | Comment: Baseline characteristics not reported |
Chen 2007.
| Methods | Trial design: randomised, single‐centre clinical trial Mean follow‐up: not reported Intervention A: not reported Intervention B: not reported Study duration: not reported Language: English Type of information: journal article Judgement on quality: unclear risk of bias |
|
| Participants | Setting: Tongji Hospital, Wuha, Hubei Province, China Allocation of participants: 54 participants, 28 allocated to Intervention A, 26 allocated to Intervention B Sex ratio: total: 53 (98%) males, 1 (2%) female Intervention A: 27 (96%) males, 1 (4%) female Intervention B: 26 (100%) males, 0 (0%) female Mean age: total: not reported Intervention A: 45.7 ± 3.5 Intervention B: 47.4 ± 6.3 Indication (no. (%)): Hepatocellular carcinoma: total: 54 (100%), Intervention A: 28 (100%), Intervention B: 26 (100%) Type of donor: not reported Inclusion criteria: not reported Exclusion criteria: not reported Other: Cold ischaemia time (minutes): total: not reported, Intervention A: 486.1 ± 97.0, Intervention B: 462.1 ± 88.0 Warm ischaemia time (minutes): total: not reported. Intervention A: 51.5 ± 3.4, Intervention B: 50.8 ± 3.1 |
|
| Interventions | Intervention A: glucocorticosteroids: 3 months rapid taper to stop at 3 months, type of glucocorticosteroid and doses not reported Intervention B: glucocorticosteroids: 3 months slow taper with 10 mg/day maintenance long‐term, type of glucocorticosteroid and doses during taper not reported Concomitant immunosuppression: Methylprednisolone: 500 mg/day for 3 days Tacrolimus: aiming for trough doses of 6 to 8 micrograms/mL for 1 year and then 4 to 6 micrograms/mL thereafter Mycophenolate mofetil: 0.5 to 1 g/day for 1 year and then stopped at 1 year |
|
| Outcomes | Mortality, acute rejection, creatinine, HCC recurrence, ALT, cholesterol, fasting blood sugar | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from the publication: "Fifty‐four patients suffering from advanced‐stage hepatoma (all exceeding the Milan criterion) underwent liver transplantation between April 2003 and June 2005. There were two immunosuppressive protocols: 28 patients (group A) were given an early steroid‐withdrawal protocol and 26 patients (group B) were given a steroid‐maintenance protocol." Quote from the publication: "This randomized clinical study was focused on a particular group of recipients who suffered from advanced‐stage hepatocellular carcinoma before liver transplantation." Quote from the "Comments" section of the publication: "The present study was a randomized clinical trial of steroid withdrawal after liver transplantation in patients with advanced‐stage hepatocellular carcinoma. We have cited several articles from other investigators that report research on steroid withdrawal after liver transplantation." Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of participants and medical staff not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Unclear risk | Comment: Study does not appear to be stopped early |
| Free of baseline imbalance? | Low risk | Quote from the publication: "Factors such as age at transplantation, stage of carcinoma, Child‐Pugh score, graft cold ischemic time, anhepatic phase, operation time, and mean level of liver function before operation were noted, and these parameters were well matched in both groups (Table 1)." Comment: Study appears to be free from baseline imbalance |
Hu 2008.
| Methods | Trial design: randomised, single‐centre clinical trial Mean follow‐up: not reported Study duration: 6 months from randomisation, randomisation from September 2006 to March 2008 Language: Mandarin Type of information: journal article Judgement on quality: unclear risk of bias |
|
| Participants | Setting: Organ Transplantation Center, the First Affiliated Hospital of Sun Yat‐Sen University, Guangzhou, China Allocation of participants: 76 participants, 36 allocated to Intervention A, 40 allocated to Intervention B Sex ratio: total: not reported Intervention A: 5:1 (numbers and % not reported) Intervention B: 4:1 (numbers and % not reported) Mean age: total: not reported Intervention A: 47.6+/‐5.8 Intervention B: 45.2+/‐6.5 Indication (no. (%)): not reported Type of donor: deceased donor Inclusion criteria: first liver transplantation, hepatocellular carcinoma, aged 18 to 65, deceased donor transplantation and informed consent given Exclusion criteria: previous liver transplant, multi‐organ transplantation, living donor transplantation, ABO‐incompatible transplantation. Primary disease: primary sclerosing cholangitis or autoimmune hepatitis. Preoperative psychiatric symptoms, gastric ulcer, use of hormones, diabetes mellitus, hypertension, hyperlipidaemia or malignancy other than primary hepatocellular carcinoma. Participation in other trials |
|
| Interventions | Intervention A: no intervention Intervention B: prednisone from day 8, commencing at 48 mg reduced by 8 mg every 3 days to a maintenance dose of 4 mg by day 26, stopped after 3 months Concomitant immunosuppression: Tacrolimus: 3 mg intraoperatively then adjusted postoperatively to 8 to 12 micrograms/mL Methylprednisolone: 1000 mg intraoperatively, then 500 mg on day 1, 240 mg on day 2, 200 mg on day 3, 160 mg on day 4, 80 mg on day 5, 40 mg on day 6 and 20 mg on day 7 |
|
| Outcomes | Mortality, infection, hepatic artery thrombosis, hypertension, diabetes mellitus, hyperlipidaemia, neurotoxicity, gastrointestinal complications, other adverse events | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: National Nature foundation, China Medical Board in New York, Nature foundation of Guangzhou province |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of participants and medical staff not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Comment: Study appears to be free from baseline imbalance |
Ju 2012.
| Methods | Trial design: randomised, single‐centre clinical trial Mean follow‐up: total: not reported; Intervention A: 23 months (range: 12 to 36 months); Intervention B: 21 months (range: 12 to 36 months) Study duration: 3 years from randomisation, randomisation from September 2006 to September 2008 Language: English Type of information: journal article Judgement on quality: unclear risk of bias |
|
| Participants | Setting: Organ Transplantation Center, the First Affiliated Hospital of Sun Yat‐Sen University, Guangzhou, China Allocation of participants: 87 participants, 44 allocated to Intervention A, 43 allocated to Intervention B Sex ratio: total: 64 (78.0%) males, 18 (22.0%) females Intervention A: not reported Intervention B: not reported Mean age: total: 45.7 (range: 26 to 68) Intervention A: not reported Intervention B: not reported Indication (no. (%)): (indications reported for whole study population but not intervention groups) Hepatocellular carcinoma: total: 36 (43.9%) HBV cirrhosis: total: 33 (40.2%) HCV cirrhosis: total: 3 (3.7%) Alcoholic cirrhosis: total: 3 (3.7%) Severe hepatitis: total: 6 (7.3%) Polycystic liver: total: 1 (1.2%) Type of donor: deceased donor Inclusion criteria: adult liver transplant recipients Exclusion criteria: pretransplant infection (except HBV, HCV), marginal grafts (donors with moderate to severe NAFLD, HBV infection, age > 60, cold ischaemia > 14 hours), multiorgan transplants, retransplant, partial liver transplant including living donor, lack of consent, ABO incompatibility |
|
| Interventions | Intervention A: no intervention Intervention B: methylprednisolone at 240 mg on day 1 tapered by 10 mg/day for 8 days. Prednisone at 48 mg on day 9 with 8 mg tapered until 4 mg/day by day 26 before stopping at 3 months Concomitant immunosuppression: Methylprednisolone: 500 mg intraoperatively Basiliximab: 20 mg perioperatively Tacrolimus: commenced on day 4 at 0.04 mg/kg/day aiming for trough levels of 8 to 12 ng/mL, tapered to 6 to 10 ng/mL by 3 months and 5 to 8 ng/mL by 6 months Mycophenolate mofetil: as required Sirolimus: as required |
|
| Outcomes | Mortality, acute rejection, CMV infection, hypertension, hyperlipidaemia, hyperglycaemia, infection | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: National High Technology Research and Development Program of China, the Key Clinical Project from the Ministry of Health, National Natural Science Foundation of China, special fund for science research by Ministry of Health, the China Medical Board in New York, the Key Projects in the National Science & Technology Pillar Program during the Eleventh Five‐Year Plan Period of China and Science and Technology Planning Project of Guangdong Province |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "After screening, 91 patients were randomized to receive standard immunosuppressive protocol (SP group) or 24‐hour steroid avoidance protocol (24‐h SA group) according to random sequence generated by SPSS software (SPSS: An IBM Company, version 13.0, IBM Corporation, Armonk, New York, USA)." Comment: Randomisation achieve by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of participants and medical staff not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote from the publication: "Nine patients were excluded from the analysis owing to ABO blood type incompatibility (4/9) and perioperative death (5/9). Among the patients who died perioperatively, 3 patients in the SP group died of acute heart failure (1/5), renal failure (1/5), and massive intraperitoneal bleeding (1/5), and 2 patients in the 24‐h SA group died of renal failure (1/5) and primary allograft nonfunction (1/5)." Comment: The publication provides sufficient information to allow participants excluded due to perioperative mortality to be re‐entered into the meta‐analysis. Otherwise, the number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Unclear risk | Quote from the publication: "There was no significant difference between the groups when comparing the number of cases and duration of MMF or sirolimus use postoperatively (Table 1)." Comment: The publication reports that there is no significant difference between mycophenolate mofetil or sirolimus use between the groups both other baseline characteristics are not reported |
Lerut 2008.
| Methods | Trial design: randomised, double‐blinded, placebo‐controlled, single‐centre clinical trial Mean follow‐up: total: 48 months (range: 12 to 84 months) Study duration: 5 years from randomisation Language: English Type of information: journal article Judgement on quality: high risk of bias |
|
| Participants | Setting: Université Catholique de Louvain Cliniques, Universitaires Saint‐Luc, Brussels, Belgium Allocation of participants: 156 participants, 78 allocated to Intervention A, 78 allocated to Intervention B Sex ratio: total: 98 (62.8%) males, 58 (37.2%) females Intervention A: 50 (64.1%) males, 28 (35.9%) females Intervention B: 48 (61.5%) males, 30 (38.5%) females Mean age: total: not reported Intervention A: 52.1 ± 13.0 Intervention B: 49.0 ± 12.7 Indication (no. (%)): HCV cirrhosis: total: 35 (22.4%), Intervention A: 21 (26.9%), Intervention B: 14 (17.9%) Cholestatic disease: total: 18 (11.5%), Intervention A: 10 (12.8%), Intervention B: 8 (10.3%) Vascular disease: total: 3 (1.9%), Intervention A: 3 (3.8%), Intervention B: 0 (0%) Metabolic disease: total: 9 (5.8%), Intervention A: 2 (2.6%), Intervention B: 7 (9.0%) Benign tumour: total: 9 (5.8%), Intervention A: 4 (5.1%), Intervention B: 5 (6.4%) Hepatocellular carcinoma: total: 37 (23.7%), Intervention A: 19 (24.4%), Intervention B: 18 (23.1%) Fulminant failure: total: 22 (14.1%), Intervention A: 9 (11.5%), Intervention B: 13 (16.7%) Type of donor: living and deceased donors Inclusion criteria: adult liver transplant recipient Exclusion criteria: unfavourable oncological diagnosis, already included in another RCT Other: Ischaemia time: Intervention A: 603+/‐231 minutes, Intervention B: 682+/‐204 minutes Artificial organ support: total: 11 (7.1%), Intervention A: 10 (12.8%), Intervention B: 1 (1.3%) Right liver living liver transplantation: total: 9 (5.8%), Intervention A: 0 (0%), Intervention B: 9 (11.5%) Baseline imbalance: the intervention groups differ significantly in relation to ischaemia time, living donor liver transplantation and artificial organ support. |
|
| Interventions | Intervention A: matched placebo Intervention B: methylprednisolone started at 16 mg then tapered every 14 days by 4 mg from day 21 to stop at day 64 Concomitant immunosuppression: Tacrolimus: aiming for trough level of 5 to 8 ng/mL Hydrocortisone: 1000 mg intraoperatively |
|
| Outcomes | Mortality, graft loss, acute rejection, glucocorticosteroid‐resistant rejection, chronic rejection, infection, bacterial infection, viral infection, fungal infection, CMV infection, bilirubin, ALT, GGT, post‐transplant lymphoproliferative disorder (PTLD), renal insufficiency, diabetes mellitus, new‐onset diabetes after transplantation (NODAT), hyperuricaemia, hypercholesterolaemia, hypertension, de novo hypertension, osseo‐muscular pain or fractures, cataract, Karnofsky index, recurrent hepatitis C, intrahepatic biliary problems | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: yes Sources of funding: the Belgian FRSM, Astellas Pharma, Munchen, Germany |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from the publication: "The patients were randomized, 1:1 into our previously used IS scheme consisting of TAC‐low dose and short‐term steroids (TAC‐ST; n 78) or into TAC‐placebo (TAC‐PL; n 78)." Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The randomization was done at the end of surgery using serially numbered, sealed, and opaque envelopes." Comment: Adequate allocation concealment using sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "Steroids and placebos were administered in identical plastic containers containing a similar number of identical, opaque capsules. Their number, corresponding to a reducing dose that covered a post‐LT period of 64 days, was prepared by an independent pharmacist." Quote from the publication: "All patients, health care providers, and outcome assessor teams were blinded until the 12‐month analysis was complete." Comment: Double‐blinded trial, both participants and medical staff blinded to treatment. Adequate placebo using identical opaque capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from the publication: "All patients, health care providers, and outcome assessor teams were blinded until the 12‐month analysis was complete." Comment: All outcome assessors including pathologists blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "There were no dropouts or withdrawals in either intervention group." Quote from the 2014 publication: "Five‐year biopsy was done in 112 (89.6%) patients (Table 2). Twelve (9.6%) patients with stable, normal liver tests refused a biopsy (n = 11); once (0.8%) tissue material was insufficient for analysis." No missing outcome data, no withdrawals. Data missing from participants refusing liver biopsy in the five‐year follow‐up unlikely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | High risk | Quote from the publication: "This work was supported by a grant from the Belgian FRSM (3.4548.02). Astellas Pharma, Munchen, Germany provided the randomization envelopes and an unrestricted grant, which will be used to cover pharmacodynamic and viral (HCV) monitoring, which are not included in this report." Comment: Study is industry sponsored |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | High risk | Quote from the publication: "Technical variants were not taken into account when randomizing the patients because in our center, results of elective living LT and split LT are similar to those obtained after whole LT. The need for artificial organ support also was not considered for randomization, as the placebo group would have a lower degree of IS anyway. The randomization was also independent of the presence of positive lymphocytotoxic cross‐match and of viral HBV or HCV infection. The characteristics of the study population are summarized in Table 1. The groups differed significantly in relation to total ischemia time, frequency of living donor LT, need for artificial (renal, hepatic, and pulmonary) organ support, and mean creatinine values during the first 14 post‐LT days." Comment: Study not free from baseline imbalance |
Llado 2006.
| Methods | Trial design: randomised, multicentre, open‐label clinical trial Mean follow‐up: not reported Study duration: randomisation between April 2001 and September 2004, 6 months from randomisation (longer for HCV‐positive patients) Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: 7 transplantation centres in Spain Allocation of participants: 198 participants, 102 allocated to Intervention A, 96 allocated to Intervention B Sex ratio: total: 155 (78.3%) males, 43 (21.7%) females Intervention A: 80 (78.4%) males, 22 (21.6%) females Intervention B: 75 (78.1%) males, 21 (21.9%) females Mean age: total: not reported Intervention A: 55.4 ± 8.9 Intervention B: 52.9 ± 9.5 Indication (no. (%)): HCC: total: 63 (31.8%), Intervention A: 34 (33.3%), Intervention B: 29 (30.2%) HCV cirrhosis: total: 46 (23.2%), Intervention A: 20 (19.6%), Intervention B: 26 (27.1%) HBV cirrhosis: total: 14 (7.1%), Intervention A: 8 (7.8%), Intervention B: 6 (6.3%) Alcoholic cirrhosis: total: 55 (27.8%), Intervention A: 29 (28.4%), Intervention B: 26 (27.1%) Other: total: 20 (10.1%), Intervention A: 11 (10.8%), Intervention B: 9 (9.4%) Type of donor: deceased donor Inclusion criteria: liver transplant recipients from cadaveric donors aged > 18 Exclusion criteria: exclusion criteria: transplant for fulminant liver disease, retransplant, previous or concurrent other organ transplant, autoimmune hepatitis, primary biliary cirrhosis, HIV infection, likely poor compliance Other: Disease status: HCV‐positive recipient: total: 88 (44.4%), Intervention A: 45 (44.1%), Intervention B: 43 (44.8%) CMV‐positive recipient: total: 165 (83.3%), Intervention A: 83 (81.3%), Intervention B: 82 (85.4%) Diabetes mellitus pretransplant: total: 49 (24.7%), Intervention A: 28 (27.5%), Intervention B: 21 (21.9%) Glycated haemoglobin pretransplant: total: not reported, Intervention A: 4.9 ± 1.5, Intervention B: 4.6 ± 0.9 Hypertension pretransplant: total: 17 (8.6%), Intervention A: 11 (10.8%), Intervention B: 6 (6.3%) Serum cholesterol pretransplant: total: not reported, Intervention A: 3.8 ± 1.2, Intervention B: 4.0 ± 1.3 (%) |
|
| Interventions | Intervention A: no intervention Intervention B: hydrocortisone: 500 mg intraoperatively, then 0.5 mg/kg/day for days 1 to 5, 0.25 mg/kg/day for days 6 to 30, 0.15 mg/kg/day for days 31 to 90, no intervention from day 91 Concomitant immunosuppression: Basiliximab: 20 mg intraoperatively Cyclosporine A: started at 10 mg/kg/day aiming for trough levels of 800 ng/mL to 1200 ng/mL |
|
| Outcomes | Mortality, graft loss, acute rejection, glucocorticosteroid‐resistant rejection, chronic rejection, adverse events, infections, bacterial infection, viral infection, fungal infection, CMV infection, HSV infection, metabolic decompensations, diabetes mellitus, hypertension, recurrent hepatitis C, treatment failure, renal failure, neurological deficit, gingival hypertrophy, de novo malignancy, cholesterol, triglyceride, days until rejection | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: yes Sources of funding: Novartis Pharma, TV3 Marathon Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from the publication: "The trial was an open‐label, not‐blinded, prospective, randomized study." Comment: Blinding of participants and medical staff not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from the publication: "The trial was an open‐label, not‐blinded, prospective, randomized study." Comment: Blinding of outcome assessors not performed |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote from the publication: "Two patients were exclude from the study after randomization because of protocol violations." Comment: The protocol violations are not described and the groups to which these patients were randomised is not described. Otherwise, the number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | High risk | Quote from the publication: "The authors who have taken part in this study have declared a relationship with the manufacturers of the drugs involved and they received funding from the drug companies involved to carry out their research." Quote from the publication: "We are grateful to Infociencia Clinical Research for monitoring the study, and especially thank Cati Bonet for the statistical analysis. This research was supported by Novartis Pharma, and by the TV3 Marathon Foundation." Comment: Study is partly industry sponsored |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Quote from the publication: "Patient demographics and baseline characteristics were similar between groups (Table 1). It should be noted that 45% of patients were HCV‐positive. Main operative and initial post‐operative evolution was also similar between groups (Table 2)". Comment: Study free from baseline imbalance |
Margarit 2005.
| Methods | Trial design: randomised, single‐centre clinical trial Mean follow‐up: 44 months (range: 3 to 60) Study duration: randomisation from October 1998 to September 2000, 5 years from randomisation Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: Liver Transplantation Unit, Hospital General Vall Hebron, Barcelona, Spain Allocation of participants: 63 participants, 33 allocated to Intervention A, 30 allocated to Intervention B Sex ratio: total: 43 (71.7%) males, 17 (28.3%) females Intervention A: 25 (78.1%) males, 7 (21.9%) females Intervention B: 18 (64.3%) males, 10 (35.7%) females Mean age: total: not reported Intervention A: 56 ± 8 Intervention B: 57 ± 7 Indication (no. (%)): HCV cirrhosis: total: 35 (58.3%), Intervention A: 15 (46.9%), Intervention B: 20 (71.4%) Alcoholic cirrhosis: total: 16 (26.7%), Intervention A: 11 (34.4%), Intervention B: 5 (17.9%) HBV cirrhosis: total: 5 (8.3%), Intervention A: 2 (6.3%), Intervention B: 3 (10.7%) Cryptogenic cirrhosis: total: 2 (3.3%), Intervention A: 2 (6.3%), Intervention B: 0 (0%) Haemochromatosis: total: 2 (3.3%), Intervention A: 2 (6.3%), Intervention B: 0 (0%) Type of donor: not reported Inclusion criteria: first elective liver transplant, informed consent Exclusion criteria: renal failure, preoperative steroid consumption |
|
| Interventions | Intervention A: no intervention Intervention B: methylprednisolone: 100 mg twice daily tapered to 20 mg/day by day 6 and tapered to complete stop at 3 months if possible Concomitant immunosuppression: Tacrolimus: 0.05 mg/kg twice daily aiming for trough levels of 10 to 15 ng/mL for "a few weeks" and 8 to 12 ng/mL thereafter |
|
| Outcomes | Mortality, infection, bacterial infection, viral infection, fungal infection, toxicity, HCV recurrence, severity of recurrent hepatitis C, renal insufficiency, de novo hypertension, de novo diabetes mellitus, dyslipidaemia, neurological complications, diarrhoea | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: Fujisawa GM |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Low risk | Quote from email correspondence with author (Bilbao, I) in response to the question "How was the randomisations sequence generated?": "68 closed envelopes numbered 1 to 68 respectively, were prepared before starting the trial. The envelopes were opened consequently when the surgery started." Comment: Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Blinding of participants and medical staff not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from email correspondence with author (Bilbao, I) in response to question "Were the outcome assessors blinded to the patient's treatment?": "NO". Quote from email correspondence with author (Bilbao, I) in response to question "Were the pathologists confirming rejection blinded to the patient's treatment?": "YES". Comment: Blinding of pathologists performed; blinding of all other outcome assessors not performed |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote from the publication: "Three patients were excluded after randomization because of perioperative death (n = 2) and positive cross‐match (n = 1)." Quote from email correspondence with author (Bilbao, I) in response to question "Of the three patients who died after randomization, had any of the patients begun treatment? If this is the case which group were the two deaths part of and which was the patient with the positive cross‐match part of?": "One death belonged to Tacro group and the other to the Tacro + Prednisone. The patient with positive crossmatch belonged to Tacro group. The death in Tacro group did not begin treatment because intraoperative problems and at the end of the surgery received standard immunosuppression at that time (Tacro + steroids). The one in Tacro + steroids died at 3 rd day post‐op and began treatment. The patient with positive crossmatch began treatment for 36 hours, but then was changed to standard triple therapy" Comment: Three participants removed from analysis following randomisation following data‐dependent processes not described in exclusion criteria. It was possible to incorporate some of the data on mortality from these participants based on email correspondence with the author. Otherwise, the number of withdrawals and reasons for withdrawal were not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to have been reported |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | High risk | Quote from the publication: "No differences were found between treatment groups except for a higher incidence of graft steatosis in TACRO + ST and of HCV‐positive patients in the TACRO group. Primary graft dysfunction secondary to ischaemic–reperfusion injury that could affect tacrolimus metabolism and pharmacokinetic parameters was similar in both groups." Comment: Baseline imbalance observed in recipient HCV cirrhosis and donor graft steatosis |
Moench 2007.
| Methods | Trial design: randomised, double‐blinded, placebo‐controlled, single‐centre clinical trial Mean follow‐up: not reported as all patients followed up at 5 years, except deaths Study duration: 5 years from randomisation, randomisation from February 2000 to July 2004 Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: Johannes Gutenberg University Mainz Hospital, Langenbeckstrasse 1, Mainz, Germany Allocation of participants: 110 participants, 54 allocated to Intervention A, 56 allocated to Intervention B Sex ratio: total: 74 (67.3%) males, 36 (32.7%) females Intervention A: 36 (66.7%) males, 18 (33.3%) females Intervention B: 38 (67.9%) males, 18 (32.1%) females Mean age: total: not reported Intervention A: 53.5 ± 8.3 Intervention B: 53.6 ± 10.4 Indication (no. (%)): Hepatocellular carcinoma: total: 40 (36.4%), Intervention A: 19 (35.2%), Intervention B: 21 (37.5%) HBV cirrhosis: total: 19 (17.3%), Intervention A: 7 (13.0%), Intervention B: 12 (21.4%) HCV cirrhosis: total: 31 (28.2%), Intervention A: 15 (27.8%), Intervention B: 16 (28.6%) Alcoholic cirrhosis: total: 37 (33.6%), Intervention A: 21 (38.9%), Intervention B: 16 (28.6%) Primary biliary cirrhosis or primary sclerosing cholangitis: total: 8 (7.3%), Intervention A: 5 (9.3%), Intervention B: 3 (5.4%) Type of donor: deceased donor after brain death (DBD) or living‐related donor Inclusion criteria: orthotopic liver transplant recipients aged > 18 receiving transplant for any indication, recipients of whole or partial liver grafts from brain dead donors as well as living‐related donors, oral informed consent Exclusion criteria: previous organ transplants including liver retransplantation; initial, sequential or parallel therapy with other immunosuppressive drugs besides the study protocol; corticosteroid therapy within 6 months before transplantation; HIV infection; pregnancy and breast feeding; allergy to or intolerance of study medication; participation in another clinical study Other: Partial graft: total: 6 (5.5%), Intervention A: 3 (5.6%), Intervention B: 3 (5.4%) Deceased donor: total: 100 (90.9%), Intervention A: 50 (92.6%), Intervention B: 50 (89.3%) Living donor: total: 10 (9.1%), Intervention A: 4 (7.4%), Intervention B: 6 (10.7%) |
|
| Interventions | Intervention A: matched placebo Intervention B: methylprednisolone: 12 mg/day from day 15 to 60, 8 mg/day from day 61 to 180 then tapered to stop over 2 weeks Concomitant immunosuppression: Tacrolimus: initial dose of 0.01 mg/kg/day with target trough levels 10 to 15 ng/mL for days 0 to 42 and 5 ng/mL to 10 ng/mL thereafter Methylprednisolone: 1000 mg before reperfusion, 100 mg on day 1, 75 mg on day 2, 48 mg on day 3 and 4, 36 mg on day 5 and 6, 24 mg on day 7 and 8, 16 mg on days 9 to 13 and 12 mg on day 14 |
|
| Outcomes | Mortality, graft loss, acute rejection, time to first rejection, severity of rejection, recurrent acute rejection, glucocorticosteroid‐resistant rejection, chronic rejection, hypertension, diabetes mellitus, infection, CMV infection, post‐transplant lymphoproliferative disorder, hypercholesterolaemia, hypertriglyceridaemia, osteoporosis, cholesterol, triglyceride, creatinine, HDL cholesterol, LDL cholesterol, fasting blood glucose, neurological toxicity, abnormal liver function, abnormal renal function. | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: yes Sources of funding: Astellas Pharma Munich, Germany |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Patient care and study conduct complied with good clinical practice.1:1 randomization was performed by a blinded randomization list generated by the Biomathematical Institute prior to transplantation in eligible patients (Figure 1)." Comment: Allocation concealment not fully described, however, the publication describes the randomisation sequence as "blinded" in addition to the blinding or participants and outcome assessors described elsewhere |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "This was a 12‐month, prospective, randomized, double‐blinded, placebo‐controlled investigator driven, monocenter trial comparing early FK506 monotherapy with FK506 plus steroids." Quote from the publication: "After day 14, patients received study medication, either placebo or methylprednisolon capsules in a double‐blinded way. Study medication was manufactured, packed and blinded by the Pharmacy Department of Johannes Gutenberg University Hospital." Quote from the publication: " Patients who experienced three or more episodes of acute rejection during the follow‐up were excluded from the trial, unblinded and received individualized immunosuppressive therapy. Comment: Double‐blinded trial, both participants and medical staff blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of pathologists not described |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "The number of patients completing the follow‐up were 35 (66.7%) in the steroid group and 36 (62.5%) in the placebo group (p = 0.801, chi‐square). Reasons for withdrawal from the trial were death (n = 14, 12.7%), recurrent rejection (n = 11, 10.0%), severe adverse events (n = 10, 9.1%) and secondary refusal to informed consent (n = 1, 0.9%)." Quote from the publication: "Fourteen (12.7%) patients died during follow‐up, 6 (11.1%) in the steroid group and 8 (14.3%) in the placebo group (p = 0.617, chi‐square). At month 6, 46 patients were still in the steroid and the placebo group. Eight patients were withdrawn from the steroid group and 10 patients were withdrawn from the placebo group during the first 6 months. At month 12, 35 patients were still in the steroid group and 36 patients were still in the placebo group. Eleven patients were withdrawn from the steroid group and 10 patients were withdrawn from the placebo group during month 6 until month 12." Comment: The numbers and reasons for dropouts are adequately described |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | High risk | Quote from the publication: "The study was supported by Astellas Pharma Munich, Germany." Comment: Study is industry sponsored |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Quote from the publication: "Patientsdemographicsandbaselinecharacteristics were comparable in both groups and are demonstrated in Table 1." Comment: Study free from baseline imbalance |
Pageaux 2004.
| Methods | Trial design: randomised, multicentre, double‐blinded, placebo‐controlled clinical trial Mean follow‐up: not reported Study duration: 1 year from randomisation, randomisation from December 1999 to August 2001 Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: 7 transplantation centres in France Allocation of participants: 174 participants, 90 allocated to Intervention A, 84 allocated to Intervention B Sex ratio: total: 124 (71.3%) males, 50 (28.7%) females Intervention A: 68 (75.6%) males, 22 (24.4%) females Intervention B: 56 (66.7%) males, 28 (33.3%) females Mean age: total: not reported Intervention A: 52 ± 10.4 Intervention B: 52.7 ± 8.8 Indication (no. (%)): Alcoholic cirrhosis: total: 84 (48.3%), Intervention A: 45 (50.0%), Intervention B: 39 (46.4%) HCV cirrhosis: total: 26 (14.9%), Intervention A: 12 (13.3%), Intervention B: 14 (16.7%) HBV cirrhosis: total: 12 (6.9%), Intervention A: 8 (8.9%), Intervention B: 4 (4.8%) Primary biliary cirrhosis: total: 11 (6.3%), Intervention A: 6 (6.7%), Intervention B: 5 (6.0%) Hepatocellular carcinoma: total: 11 (6.3%), Intervention A: 5 (5.6%), Intervention B: 6 (7.1%) Primary sclerosing cholangitis: total: 4 (2.3%), Intervention A: 1 (1.1%), Intervention B: 3 (3.6%) Other: total: 26 (14.9%), Intervention A: 13 (14.4%), Intervention B: 13 (15.5%) Type of donor: deceased donor Inclusion criteria: adult liver transplant recipients undergoing first cadaveric liver transplant Exclusion criteria: primary graft dysfunction, early retransplantation (before randomisation), renal insufficiency (creatinine > 200 ìmol/L), uncontrolled infection, multiorgan failure, cardiac arrest and presence of adenocarcinoma |
|
| Interventions | Intervention A: equivalent placebo Intervention B: prednisone: started on day 8 (dose and duration not reported) Concomitant immunosuppression: Basiliximab: 20 mg on day 0 and day 4 Cyclosporine A: started within 24 hours of transplant aiming for trough levels of 200 ng/mL to 400 ng/mL from day 0 to 3 months and tapered to 150 ng/mL to 300 ng/mL Methylprednisolone: 500 mg intraoperatively, 200 mg on day 1, which was tapered to reach 20 mg on day 7 |
|
| Outcomes | Mortality, graft loss, acute rejection, diabetes mellitus, recurrent hepatitis C, multiorgan failure, sepsis, intraabdominal haemorrhage, unsatisfactory therapeutic effect, hypertrichosis, surgical complications, renal failure, adverse events, CMV infection, CMV disease, infections, de novo malignancy, neurological complications, psychiatric complications, gastrointestinal disorders | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: Novartis Pharma |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from the publication: "A prospective, 1‐year, comparative, double‐blind, placebo‐controlled study, with informed consent and institutional review board approval, was conducted in 15 French liver transplantation centers." Comment: Double‐blinded trial, both participants and medical staff blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: All outcome assessors including pathologists blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | High risk | Quote from the publication: "Supported by a grant from Novartis Pharma." Comment: Study is industry sponsored |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Quote from the publication: "There were no differences between the 2 groups concerning clinical characteristics and indications for transplantation (Table1). Independently of the diagnoses, 17 patients in each group had a positive hepatitis C virus serology. The 2 groups were similar in terms of Child‐Turcotte‐Pugh score, with a majority in stage C (40% in group 1 and 50% in group 2). After transplantation, the cyclosporine blood trough levels and the daily doses of corticosteroids / placebo were similar in the 2 groups (Table 2)." Comment: Study is free from baseline imbalance |
Pelletier 2013.
| Methods | Trial design: randomised, single‐centre, open‐label clinical trial Mean follow‐up: 2095 days ± 117 Study duration: 7 years, randomisation from June 2002 to May 2005 Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: Section of Transplant Surgery, University of Michigan, Michigan, USA Allocation of participants: 100 participants, 50 allocated to Intervention A, 50 allocated to Intervention B Sex ratio: total: 76 (76%) males, 24 (24%) females Intervention A: 38 (76%) males, 12 (24%) females Intervention B: 38 (76%) males, 12 (24%) females Mean age: total: not reported Intervention A: 54 ± 1 Intervention B: 56 ± 1 Indication (no. (%)): (some patients reported as having multiple indications) HCV cirrhosis: total: 54 (54%), Intervention A: 31 (62%), Intervention B: 23 (46%) Alcoholic cirrhosis: total: 42 (42%), Intervention A: 19 (38%), Intervention B: 23 (46%) Hepatocellular carcinoma: total: 20 (20%), Intervention A: 9 (18%), Intervention B: 11 (22%) Primary biliary cirrhosis or primary sclerosing cholangitis: total: 6 (6%), Intervention A: 1 (2%), Intervention B: 5 (10%) Cryptogenic cirrhosis: total: 15 (15%), Intervention A: 8 (16%), Intervention B: 7 (14%) Type of donor: not reported Inclusion criteria: all consecutive, consenting participants undergoing liver transplantation at the University of Michigan between June 2002 and May 2005 Exclusion criteria: participants aged < 18 years, multiple organ recipients and participants who required post‐transplant steroid therapy for an indication other than prevention of rejection, such as autoimmune hepatitis or inflammatory bowel disease Other: BMI (kg/m2): total: not reported, Intervention A: 30 ± 1, Intervention B: 29 ± 1 Pretransplant antihypertensive: total: 73 (73%), Intervention A: 36 (72%), Intervention B: 37 (74%) Pretransplant diabetes mellitus: total: 32 (32%), Intervention A: 20 (40%), Intervention B: 12 (24%) Pretransplant coronary artery disease: total: 8 (8%), Intervention A: 5 (10%), Intervention B: 3 (6%) Pretransplant haemodialysis: total: 4 (4%), Intervention A: 3 (6%), Intervention B: 1 (2%) MELD score: total: not reported, Intervention A: 16 ± 1, Intervention B: 18 ± 1 Warm ischaemia time (minutes): total: not reported, Intervention A: 64 ± 7, Intervention B: 54 ± 3 Cold ischaemia time (minutes): total: not reported, Intervention A: 518 ± 34, Intervention B: 518 ± 24 Donor age: total: Intervention A: 38 ± 3, Intervention B: 37 ± 2 Donor sex ratio: total: 68 (68%) males, 32 (32%) females; Intervention A: 31 (62%) males, 19 (38%) females; Intervention B: 37 (74%) males, 13 (26%) females Donor ethnicity: total: 80 (80%) white, 20 (20%) non‐white; Intervention A: 39 (78%) white, 11 (22%) non‐white; Intervention B: 41 (82%) white, 9 (18%) non‐white Donor death from stroke: total: 50 (50%), Intervention A: 25 (50%), Intervention B: 25 (50%) Donor CMV positive: total: 67 (67%), Intervention A: 35 (70%), Intervention B: 32 (64%) |
|
| Interventions | Intervention A: no intervention Intervention B: Dexamethasone: 50 mg intraoperatively Prednisone: 3‐ to 6‐month taper (dose not reported) Concomitant immunosuppression: Tacrolimus: started within 24 hours aiming for trough levels of 10 ng/mL to 15 ng/mL for days 0 to 30, 8 ng//mL to 12 ng/mL days 31 to 60, 4 ng/mL to 8 ng/mL from day 61 (tacrolimus withheld until day 4 in patients who received basiliximab induction) MMF: dose and timings not reported Basiliximab: intraoperatively and day 4 (dose not reported) given to 12 (24%) patients receiving Intervention A and 13 (26%) patients receiving Intervention B |
|
| Outcomes | Mortality, graft loss, acute rejection, time to first rejection, chronic rejection, recurrent hepatitis C, primary non‐function, hepatic artery thrombosis, hepatic vein or IVC stenosis, biliary complications, postoperative acute renal failure, postoperative chronic renal failure, duration of high dependency stay, reoperation for bleeding, retransplantation, infections, surgical site infection, pneumonia, urinary tract infection, septicaemia, peritonitis, BMI, cholesterol, HDL, LDL, triglycerides, creatinine, diabetes mellitus, hypertension | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: Astellas Pharma Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Enrolledcandidateswererandomizedtoeither the ‘steroids’ or ‘no‐steroids’ groups using a closed‐envelope system." Comment: Study used "closed envelope system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from the publication: "All consecutive, consenting candidates undergoing liver transplantation at the University of Michigan between June 2002 and May 2005 were enrolled into a prospective, open‐label, randomized controlled trial (RCT) to evaluate the effects of complete steroids avoidance." Comment: Blinding of participants and medical staff not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from the publication: "All consecutive, consenting candidates undergoing liver transplantation at the University of Michigan between June 2002 and May 2005 were enrolled into a prospective, open‐label, randomized controlled trial (RCT) to evaluate the effects of complete steroids avoidance." Comment: Blinding of outcome assessors not performed |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | High risk | Quote from the publication: "This study was supported by a grant from Astellas Pharma, Inc., Deerfield, IL, USA." Comment: No sample size calculation reported, study is industry sponsored |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Quote from the publication: "Donor and recipient characteristics are summarized in Table 1. The two groups of recipients were well matched with respect to gender, age, race, cause of liver failure, comorbidities, Model of End‐stage Liver Disease (MELD) score, renal function and ischaemic times." Comment: Study is free from baseline imbalance |
Ramirez 2013.
| Methods | Trial design: randomised, single‐centre, open‐label clinical trial Mean follow‐up: 64.4 months (range: 10.6 to 79.6) Study duration: randomisation from February 2006 and November 2007 Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: Division of Transplantation, Department of Surgery, Thomas Jefferson University, Philadelphia, USA Allocation of participants: 40 participants, 20 allocated to Intervention A, 20 allocated to Intervention B Sex ratio: total: 25 (62.5%) males, 15 (37.5%) females Intervention A: 12 (60%) males, 8 (40%) females Intervention B: 13 (65%) males, 7 (35%) females Mean age: total: not reported Intervention A: 48.1 ± 4.3 Intervention B: 45.5 ± 3.5 Indication (no. (%)): HCV cirrhosis: total: 25 (62.5%), Intervention A: 11 (55.0%), Intervention B: 14 (70.0%) HBV cirrhosis: total: 4 (10.0%), Intervention A: 2 (10.0%), Intervention B: 2 (10.0%) Primary sclerosing cholangitis: total: 2 (5.0%), Intervention A: 2 (10.0%), Intervention B: 0 (0%) Hepatocellular carcinoma: total: 21 (52.5%), Intervention A: 10 (50.0%), Intervention B: 11 (55.0%) Alcoholic cirrhosis: total: 9 (22.5%), Intervention A: 3 (15.0%), Intervention B: 6 (30.0%) Non‐alcoholic steatohepatitis: total: 1 (2.5%), Intervention A: 1 (5.0%), Intervention B: 0 (0%) Budd‐Chiari syndrome: total: 1 (2.5%), Intervention A: 0 (0%), Intervention B: 1 (5.0%) Cryptogenic cirrhosis: total: 3 (7.5%), Intervention A: 2 (10.0%), Intervention B: 1 (5.0%) Type of donor: deceased donors Inclusion criteria: first adult liver transplant, age 18 to 72, cold ischaemic time < 20 hours Exclusion criteria: positive pregnancy test, previous organ transplant, multiple organ transplant recipients, women of childbearing potential not using the prescribed contraceptive methods, known sensitivity to basiliximab or class of basiliximab, participants with severe medical condition(s) that in the view of the investigator prohibits participation in the study, and use of any other investigational agent within 30 days prior to enrolment Other: Pretransplant MELD: total: not reported, Intervention A: 23.2 ± 1.5, Intervention B: 24.4 ± 2.0 |
|
| Interventions | Intervention A: no intervention Intervention B: methylprednisolone: 1000 mg intraoperatively, then tapered to 50 mg 6‐hourly on day 1, 40 mg 6‐hourly on day 2, 30 mg 6‐hourly on day 3, 20 mg 6‐hourly on day 4, 20 mg 12‐hourly on day 5 and then 20 mg once daily, tapered until stop at 6 months Concomitant immunosuppression: Tacrolimus: started at 0.1 mg/kg aiming for 8 ng/mL to 12 ng/mL for 1 month and then 5 ng/mL to 8 ng/mL thereafter Mycophenolate mofetil: 1000 mg every 12 hours via nasogastric tube until tolerating oral medication after which 720 mg enteric‐coated mycophenolate sodium twice daily orally for 3 months Basiliximab: 20 mg intraoperatively and on day 4 Prophylaxis: Ganciclovir or valganciclovir: 450 mg once daily for at least 3 months Trimethoprim sulfa: 3 times per week, dose and duration not reported Nystatin swish and swallow: 3 times daily, dose and duration not reported |
|
| Outcomes | Mortality, graft loss, acute rejection, infection, CMV infection, recurrent hepatitis C, severity of HCV recurrence, diabetes mellitus, hypertension, weight, cholesterol, mean arterial pressure, fasting blood glucose, ALT, AST, bilirubin | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: Novartis Corporation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Randomization was performed by the TJUH Investigational Drug Pharmacy Service who dispensed study drug based on the computer‐generated randomization schedule and the study protocol." Comment: Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from the publication: "Between February 2006 and November 2007, 40 adult recipients of deceased donor primary OLT at TJUH were enrolled into this prospective, controlled, randomized, non‐blinded, pilot trial (Clinical Trials.gov; ID: NCT00296244)." Comment: Blinding of participants and medical staff not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from the publication: "Between February 2006 and November 2007, 40 adult recipients of deceased donor primary OLT at TJUH were enrolled into this prospective, controlled, randomized, non‐blinded, pilot trial (Clinical Trials.gov; ID: NCT00296244)." Comment: Blinding of outcome assessors not performed |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Between February 2006 and November 2007, 40 adult OLT recipients were enrolled in the study and 20 recipients were randomized to each group. One recipient in the CS‐free group required a retransplantation for hepatic artery thrombosis on post‐OLT day 16. Because he expired within 10 d after retransplantation, follow‐up data were short and untenable, and therefore, he was excluded from the study analysis (Fig. 1)." Comment: One withdrawal and reason for the withdrawal adequately described |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | High risk | Quote from the publication: "The authors would like to acknowledge Novartis Corporation for providing financial grant to conduct the clinical trial." Comment: No sample size calculation reported; study is industry sponsored |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Quote from the publication: "Donor characteristics were comparable between the two groups (Table 1). Other than a significantly higher mean recipient age and longer median hospital stay in CS‐free (23 d) compared with CS group (15 d), recipient demographics and peri‐operative data were similar between the two groups." Comment: Baseline imbalance is unlikely to significantly affect outcomes |
Reggiani 2005.
| Methods | Trial design: randomised, single‐centre, open‐label clinical trial Mean follow‐up: 31 ± 7 months Study duration: not reported Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: IRCCS Ospedale Maggiore, Milan, Italy Allocation of participants: 30 participants, 18 allocated to Intervention A, 12 allocated to Intervention B Sex ratio: total: 21 (70%) males, 9 (30%) females Intervention A: 13 (72.2%) males, 5 (27.8%) females Intervention B: 8 (66.7%) males, 4 (33.3%) females Mean age: total: not reported Intervention A: 50.4 ± 8.9 Intervention B: 49.7 ± 4.6 Indication (no. (%)): HCV or HBV cirrhosis: total: 21 (70.0%), Intervention A: 14 (77.8%), Intervention B: 7 (58.3%) Alcoholic cirrhosis: total: 3 (10.0%), Intervention A: 1 (5.6%), Intervention B: 2 (16.7%) Haemochromatosis: total: 2 (6.7%), Intervention A: 1 (5.6%), Intervention B: 1 (8.3%) Primary biliary cirrhosis: total: 1 (3.3%), Intervention A: 1 (5.6%), Intervention B: 0 (0.0%) Acute liver failure: total: 1 (3.3%), Intervention A: 1 (5.6%), Intervention B: 0 (0.0%) Cryptogenic cirrhosis: total: 1 (3.3%), Intervention A: 0 (0.0%), Intervention B: 1 (8.3%) Polycystic liver disease: total: 1 (3.3%), Intervention A: 0 (0.0%), Intervention B: 1 (8.3%) Type of donor: not reported Inclusion criteria: not reported Exclusion criteria: not reported Other: Hepatocellular carcinoma: total: 14 (46.7%), Intervention A: 12 (66.7%), Intervention B: 2 (16.7%) |
|
| Interventions | Intervention A: methylprednisolone: no intervention Intervention B: 1000 mg intraoperatively then 200 mg/day tapered to 40 mg/day at day 5, 20 mg on day 6 then tapered to stop at 3 months Concomitant immunosuppression: Tacrolimus: started at 0.1 mg/kg aiming for trough levels of 10 ng/mL to 15 ng/mL for 2 weeks then 8 ng/mL to 10 ng/mL thereafter Mycophenolate mofetil: 750 mg twice daily for 1 month, 500 mg twice daily thereafter |
|
| Outcomes | Mortality, surgical complications, tacrolimus levels, MMF levels, acute rejection, graft loss, infections, diarrhoea, "peptic symptoms", impaired renal function, leukopenia, thrombocytopenia, anaemia, neurotoxicity, diabetes mellitus, hypertension | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from the publication: "To assess the efficacy and safety or a primary immunosuppressive regimen with tacrolimus (Tac) and low‐dose mycophenolate mofetil (MMF) without steroids and to determine the exposure to mycophenolic acid (MPA) in the early post‐operative period, we performed a single‐center, randomized 1:1, open‐label, controlled study planned to be 60 liver transplantation patients randomized into 2 groups: group A, tacrolimus + MMF (750 mg orally twice a day); and group B, tacrolimus + MMF (750 mg orally twice a day) + steroids." Comment: Blinding of participants and medical staff not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from the publication: "To assess the efficacy and safety or a primary immunosuppressive regimen with tacrolimus (Tac) and low‐dose mycophenolate mofetil (MMF) without steroids and to determine the exposure to mycophenolic acid (MPA) in the early post‐operative period, we performed a single‐center, randomized 1:1, open‐label, controlled study planned to be 60 liver transplantation patients randomized into 2 groups: group A, tacrolimus + MMF (750 mg orally twice a day); and group B, tacrolimus + MMF (750 mg orally twice a day) + steroids." Comment: Blinding of outcome assessors not performed |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All specified outcomes appear to be reported |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | High risk | Quote from the publication: "Patient enrollment was stopped after an interim analysis by the Ethical Committee." Comment: Study stopped early due to data dependent process (interim analysis) |
| Free of baseline imbalance? | High risk | Quote from the publication: "Hepatocellular carcinoma was diagnosed in 2 (16.7%) patients in group A and in 12 (66.7%) patients in group B." Comment: Significantly increased rates of pretransplant hepatocellular carcinoma in Intervention B |
Studenik 2005.
| Methods | Trial design: randomised, single‐centre clinical trial Mean follow‐up: 13 months (range: 2 to 23) Study duration: not reported Language: English Type of information: abstract Judgement on quality: unclear risk |
|
| Participants | Setting: Brno, Czech Republic Allocation of participants: 39 participants, 19 allocated to Intervention A, 20 allocated to Intervention B Sex ratio: total: not reported Intervention A: not reported Intervention B: not reported Mean age: total: not reported Intervention A: not reported Intervention B: not reported Indication (no. (%)): not reported Type of donor: not reported Inclusion criteria: not reported Exclusion criteria: not reported Other: baseline characteristics reported as comparable |
|
| Interventions | Intervention A: no intervention Intervention B: 9‐month glucocorticosteroid taper (dose, duration and type of glucocorticosteroid medication not reported) Concomitant immunosuppression: Tacrolimus: dose and duration not reported Mycophenolate mofetil: dose and duration not reported Hydrocortisone: 500 mg intraoperatively Daclizumab: 1 mg/kg intraoperatively then 1 mg/kg 2 to 7 days later depending on initial dose effect on CD25 expression on peripheral T‐lymphocytes |
|
| Outcomes | Mortality, graft loss, acute rejection, hypertension, diabetes mellitus, CMV infection, leucopenia and CD25 expression on peripheral T lymphocytes | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of participants and medical staff not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study protocol not available and results only published in abstract |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Unclear risk | Comment: Study only published in abstract |
| Free of baseline imbalance? | Low risk | Quote from the publication: "Both groups were comparable in all observed indicators." Comment: Study is reported as being free from baseline imbalance |
Tisone 1999.
| Methods | Trial design: randomised, single‐centre, open‐label clinical trial Mean follow‐up: 108 ± 4 months Study duration: 10 years from randomisation Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: Ospedale S. Eugenio, Piazzale dell'Umanesimo, Rome, Italy Allocation of participants: 45 participants, 22 allocated to Intervention A, 23 allocated to Intervention B Sex ratio: total: 34 (75.6%) males, 11 (24.4%) females Intervention A: 16 (72.7%) males, 6 (26.1%) females Intervention B: 18 (72%) males, 5 (21.7%) females Mean age: total: not reported Intervention A: 49.0 ± 9.8 Intervention B: 50.5 ± 6.2 Indication (no. (%)): HCV cirrhosis: total: 15 (33.3%), Intervention A: 8 (36.4%), Intervention B: 7 (30.4%) HBV cirrhosis: total: 13 (28.9%), Intervention A: 7 (31.8%), Intervention B: 6 (26.1%) Alcoholic cirrhosis: total: 6 (13.3%), Intervention A: 2 (9.1%), Intervention B: 4 (17.4%) Cryptogenic cirrhosis and others: total: 11 (24.4%), Intervention A: 5 (22.7%), Intervention B: 6 (26.1%) Type of donor: not reported Inclusion criteria: adult liver transplant recipients (> 20 years of age and < 62), HBsAg‐positive participants were only considered for inclusion if repeatedly HBV‐DNA negative Exclusion criteria: positive HIV serology, positive for IgM anti‐cytomegalovirus, HBV‐DNA‐positive participants Other: Donor age: total: not reported, Intervention A: 38.3 ± 14, Intervention B: 35.3 ± 16 Donor sex ratio: total: 30 (66.7%) male, 15 (33.3%) female; Intervention A: 13 (59.1%) males, 9 (39.1%) females; Intervention A: 17 (73.9%) males, 6 (26.1%) females Cold ischaemia time (hours): total: not reported, Intervention A: 6.2+/‐2.8, Intervention B: 6.4+/‐1.8 Possible selective outcome reporting: hypertension is not reported in any of the relevant publications |
|
| Interventions | Intervention A: No intervention Intervention B: Methylprednisolone: 20 mg/day (duration not reported) Prednisone: (starting from withdrawal of methylprednisolone) 20 mg/day until day 30 then tapered "gradually" to 5 mg/day and stopped at 3 months Concomitant immunosuppression: Cyclosporine A: aiming for trough levels of 350 ng/mL to 450 ng/mL for "the first few months" then 250 ng/mL to 350 ng/mL thereafter Azathioprine: 1 to 1.5 mg/day (duration not reported) |
|
| Outcomes | Mortality, graft loss, acute rejection, primary non‐function, poor initial function, normal function, chronic rejection, infection, CMV infection, recurrent hepatitis C, renal failure (requiring dialysis), AST, bilirubin, alkaline phosphatase, GGT, creatinine, cyclosporine serum levels, time in intensive treatment unit, time in hospital, glucose, cholesterol | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Patients were randomly assigned, using a computer‐generated list, to receive a standard immunosuppressive therapy composed of cyclosporine microemulsion (Neoral), in doses to maintain trough whole‐blood levels (monoclonal fluorescence assay) of 350‐450 ng/ml during the first months and 250‐350 ng/ml thereafter, and azathioprine (1‐1.5 mg/day), with (group A) or without (group B) corticosteroids." Comment: Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from the publication: "To address the latter points, we conducted a prospective open‐label randomized pilot study on a consecutive series of patients undergoing orthotopic liver transplantation (OLTx) at our institution." Comment: Blinding of participants and medical staff not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from the publication: "To address the latter points, we conducted a prospective open‐label randomized pilot study on a consecutive series of patients undergoing orthotopic liver transplantation (OLTx) at our institution." Comment: Blinding of outcome assessors not performed |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study does not report hypertension despite mentioning this as a potential complication of glucocorticosteroid use in both the introduction and the discussion sections |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Quote from the publication: "As summarized in Table 1, the two groups did not differ in the demographic or clinical features of donors and recipients, or in the duration of cold ischemia. In particular, there were no differences in the UNOS status or in the hemodynamic data and the laboratory findings related to liver and kidney function between the two groups." Comment: Study free from baseline imbalance |
Vivarelli 2007.
| Methods | Trial design: randomised, multicentre, open‐label clinical trial Mean follow‐up: 841 days (range: 130 to 1376) Study duration: not reported Language: English Type of information: journal article Judgement on quality: high risk |
|
| Participants | Setting: 2 transplantation centres in Italy Allocation of participants: 47 participants, 22 allocated to Intervention A, 25 allocated to Intervention B Sex ratio: total: not reported Intervention A: not reported Intervention B: not reported Mean age: total: not reported Intervention A: 58.9 (range: 43 to 66) Intervention B: 57.2 (range: 41 to 67) Indication (no. (%)): HCV cirrhosis: total: 47 (100.0%), Intervention A: 22 (100.0%), Intervention B: 25 (100.0%) Type of donor: deceased donors Inclusion criteria: HCV positive first‐time whole liver recipients from deceased donors Exclusion criteria: HBsAg‐positive, previous transplant, partial grafts, living donors Other: HCV‐RNA titres (Meq/mL): total: not reported, Intervention A: 0.755 (range: < 0.003 to 4.3), Intervention B: 0.765 (< 0.003 to 8.04) MELD score: total: not reported, Intervention A: 16 (range: 8 to 25), Intervention B: 15 (range: 7 to 28) Pretransplant diabetes mellitus: total: 11 (23.4%), Intervention A: 5 (22.7%), Intervention B: 6 (24.0%) |
|
| Interventions | Intervention A: prednisone: tapered from 25 mg/day to 15 mg/day from days 6 to day 30, 15 mg/day on days 31 to 45, 10 mg/day on days 46 to 60, 5 mg/day on days 61 to 75, 2.5 mg/day on days 76 to 90) and stopped at day 91 Intervention B: prednisone: 25 mg/day on day 6 tapered to 15 mg/day by day 31, 15 mg/day on days 31 to 90, 10 mg/day on days 91 to day 180, 7.5 mg/day on days 181 to 270, 5 mg/day from day 271 to the end of the first postoperative year, 2.5 mg for the second postoperative year and stopped at the end of the second postoperative year Concomitant immunosuppression: Methylprednisolone: intraoperatively and on days 1 to 5 (dose not reported) Tacrolimus: aiming for trough level of 5 ng/mL to 15 ng/mL for the first 3 months and then 5 ng/mL to 10 ng/mL thereafter |
|
| Outcomes | Mortality, graft loss, acute rejection, treatment failure, recurrent hepatitis C, HCV‐RNA levels, Scheuer fibrosis, acute rejection requiring steroids, acute rejection requiring multiple steroids, need for antiviral treatment (anti‐HCV), diabetes mellitus, tacrolimus levels | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: Astellas Pharma Italia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from the publication: "The study was conducted at the Liver Transplant Centres of Bologna and Padua, Italy, in an open‐label, not‐blinded, prospective and randomized fashion." Comment: Blinding of participants and medical staff not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from the publication: "The study was conducted at the Liver Transplant Centres of Bologna and Padua, Italy, in an open‐label, not‐blinded, prospective and randomized fashion." Comment: Blinding of outcome assessors not performed |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Median follow‐up was 841 days (130‐1376); apart from those who died or lost their graft earlier, all patients had at least 2 year follow‐up." Comment: Missing data unlikely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes appear to be fully reported |
| Other bias | High risk | Quote from the publication: "The authors declare that FG is employed by Astellas Pharma Italia and he coordinated the Centres involved in the study and helped in the data collection. Astellas Pharma Italia supported the study financially and coordinated the Centres involved (EPASTER Study, investigator originated and driven)." Comment: No sample size calculation reported; study industry sponsored |
| Free of early stopping? | Low risk | Comment: Study not stopped early |
| Free of baseline imbalance? | Low risk | Comment: Baseline characteristics are listed in Table 1. All listed characteristics are similar between the groups with P values > 0.05. Study is free from baseline imbalance |
Zhong 2010.
| Methods | Trial design: randomised, multicentre, double‐blinded, placebo‐controlled clinical trial Mean follow‐up: not reported Study duration: not reported Language: English Type of information: abstract (abstract appears to present preliminary data for the first 182 participants randomised) Judgement on quality: unclear risk |
|
| Participants | Setting: Shanghai First People's Hospital Allocation of participants: target enrolment of 300 participants, current participants not adequately reported (study ongoing) Sex ratio: total: not reported Intervention A: not reported Intervention B: not reported Mean age: total: not reported Intervention A: not reported Intervention B: not reported Indication (no. (%)): (hepatocellular carcinoma primary indication for all transplants) Hepatocellular carcinoma: total: not reported (100%), Intervention A: not reported (100%), Intervention B: not reported (100%) Type of donor: not reported Inclusion criteria: liver transplant recipients with hepatocellular carcinoma Exclusion criteria: death within 3 months of transplantation, inability to provide written informed consent prior to study entry |
|
| Interventions | Intervention A: no intervention Intervention B: methylprednisolone 10 mg/kg intraoperatively and a further 10 mg/kg given over 1 week Concomitant immunosuppression: Tacrolimus/cyclosporine A: dose not reported (NOTE: published abstract reports use of cyclosporine A, register on clinicaltrials.gov reports use of tacrolimus) Basiliximab: 20 mg given twice (timings not reported) |
|
| Outcomes | Mortality, graft loss, acute rejection, infection, bacterial infection, de novo diabetes mellitus, recurrent hepatitis B, hypertension, neurological complications, tumour size, tumour differentiation, histological staging of tumour, recurrence‐free survival | |
| Notes | Cross‐over between intervention arms: no Sample size calculation: not reported Sources of funding: Shanghai Jiao Tong University School of Medicine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Generation of randomisation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of participants and medical staff not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Number of withdrawals and reasons for withdrawal not reported, estimated enrolment not achieved and results only published in abstract |
| Selective reporting (reporting bias) | High risk | Comment: Results only published in abstract, graft survival not reported |
| Other bias | Unclear risk | Comment: No sample size calculation reported |
| Free of early stopping? | Unclear risk | Comment: Study only published in abstract |
| Free of baseline imbalance? | Unclear risk | Comment: Baseline characteristics not described, presence or absence of baseline imbalance not reported |
ABO: blood group ALT: alanine aminotransferase AST: aspartate aminotransferase BMI: body mass index CMV: cytomegalovirus GGT: gamma‐glutamyl transferase HBsAg: hepatitis B surface antigen HBV: hepatitis B virus HCC: hepatocellular carcinoma HCV: hepatitis C virus HDL: high density lipoprotein HIV: human immunodeficiency virus HSV: herpes simplex virus IgM: immunoglobulin M IVC: inferior vena cava LDL: low density lipoprotein MELD: model for end‐stage liver disease MMF: mycophenolate mofetil NAFLD: non‐alcoholic fatty liver disease RCT: randomised clinical trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benitez 2010 | Randomised clinical trial comparing RATG with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Boillot 2005 | Randomised clinical trial comparing daclizumab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Cosimi 1987 | Randomised clinical trial comparing muromonab CD3 with glucocorticosteroids for treatment of acute rejection; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Cuervas‐Mons 2009 | Randomised clinical trial comparing mycophenolate mofetil with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Day 2004 | Randomised clinical trial comparing continuation of tacrolimus monotherapy with tacrolimus discontinuation and replacement with mycophenolate mofetil and glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| De Simone 2007 | Randomised clinical trial comparing basiliximab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Filipponi 2004 | Method reports study as a randomised clinical trial with ITT. Results are reported instead as a per‐treatment analysis with patients moved between arms for analysis as a result of a data‐dependent process. This does not appear to have been carried out using pre‐specified criteria. Our inclusion criteria state that we are only considering randomised clinical trials that present their data in an ITT analysis for this review. We made attempts to contact the author to request the original data so that ITT analysis could be completed. |
| Foroncewicz 2009 | Randomised clinical trial comparing daclizumab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Ganschow 2007 | Randomised clinical trial comparing high‐ and low‐dose glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Hu 2013 | Non‐randomised clinical trial comparing multiple immunosuppression regimens |
| Jonas 2001 | Randomised clinical trial comparing tacrolimus‐based dual therapy with cyclosporine A‐based quadruple therapy in which glucocorticosteroid withdrawal was assessed as an outcome; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Junge 2005 | Randomised clinical trial comparing mycophenolate mofetil with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Kato 2007 | Randomised clinical trial comparing daclizumab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Klintmalm 2011 | Randomised clinical trial comparing daclizumab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Lupo 2008 | Randomised clinical trial comparing basiliximab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Manousou 2009 | Randomised clinical trial comparing monotherapy of tacrolimus with triple therapy of tacrolimus, azathioprine and glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| McDiarmid 1995 | Randomised clinical trial comparing glucocorticosteroid continuation with glucocorticosteroid withdrawal over 1 year post‐transplant; investigation of alteration in an existing immunosuppression strategy rather than a primary immunosuppression strategy |
| Nair 2006 | Randomised clinical trial comparing RATG with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Nair 2008 | Randomised clinical trial comparing PEG interferon alpha 2b, ribavirin and amantadine with PEG interferon alpha 2b and ribavirin in 2 glucocorticosteroid‐free arms; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Neumann 2012 | Randomised clinical trial comparing daclizumab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Otero 2009 | Randomised clinical trial comparing daclizumab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Saliba 2012 | Randomised clinical trial comparing concentration‐controlled mycophenolate mofetil with fixed‐dose mycophenolate mofetil and glucocorticosteroids; differences in concomitant immunosuppression therefore no comment on glucocorticosteroid avoidance or withdrawal possible |
| Spada 2006 | Randomised clinical trial comparing basiliximab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Takada 2013 | Randomised clinical trial comparing mycophenolate mofetil with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Teisseyre 2006 | Randomised clinical trial comparing saline with methylprednisolone for prevention of ischaemia reperfusion injury; no comment on glucocorticosteroid avoidance or withdrawal for post‐transplantation immunosuppression possible |
| Turner 2006 | Randomised clinical trial comparing RATG with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
| Washburn 2001 | Randomised clinical trial comparing daclizumab with glucocorticosteroids; no comment on glucocorticosteroid avoidance or withdrawal possible |
ITT: intention‐to‐treat Muromonab CD3: muromonab cluster of differentiation 3 PEG: pegylated RATG: rabbit antithymocyte globulin
Differences between protocol and review
Cholesterol and hypercholesterolaemia added to secondary outcomes.
Renal function outcome modified.
Number of sub‐analyses reduced.
Definition of the sub‐analysis of 'co‐interventions' changed.
Per‐treatment analyses added to exclusion criteria.
Methods section modified to include "Assessment of heterogeneity", "Unit of analysis Issues" and "Measures of treatment effect"
Contributions of authors
CF prepared a draft protocol. LP and CF wrote the final version of the protocol published previously. SW, EH, and JP commented on the draft and approved of the final version of the protocol. CF ran the searches. CF contacted pharmaceutical companies and experts in the field. CF, JP, SW, and EH selected studies for inclusion. CF, JP, SW, and EH extracted data. CF contacted authors to request additional information. CF, JP, SW, and EH made assessments of bias. CF entered trial data and performed analyses. EH and CF worked on the code for empirical continuity correction for zero event trials and the linear regression test for funnel plot asymmetry. LP completed the trial sequential analyses. CF completed the results section and the discussion. CF, LP, EH, JP, and SW wrote the author's conclusions. LP, JP, EH, and SW made comments on the draft and approved the final version of the review.
Sources of support
Internal sources
University of Edinburgh, College of Medicine and Veterinary Medicine, UK.
Royal Infirmary Edinburgh, Hepatobiliary‐Pancreatic Surgical Services and Edinburgh Transplant Unit, UK.
Royal Infirmary Edinburgh, Clinical Surgery, UK.
National University Hospital Rigshospitalet, Copenhagen, Denmark.
External sources
No sources of support supplied
Declarations of interest
Cameron Fairfield: none known. Luit Penninga: none known. James Powell: none known. Ewen M Harrison: none known. Stephen J Wigmore: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Belli 1998 {published data only (unpublished sought but not used)}
- Belli LS, Alberti AB, Vangeli M, Airoldi A, Pinzello G. Tapering off steroids three months after liver transplantation is not detrimental for hepatitis C virus disease recurrence. Liver Transplantation 2003;9(2):201‐2. [DOI: 10.1053/jlts.2002.50033] [DOI] [PubMed] [Google Scholar]
- Belli LS, Carlis L, Rondinara GF, Romani F, Alberti A, Pirotta V, et al. Prospective randomized trial of steroid withdrawal in liver transplant patients: preliminary report. Transplant International 1994;7(Suppl 1):S88‐90. [DOI] [PubMed] [Google Scholar]
- Belli LS, Carlis L, Rondinara G, Alberti AB, Bellati G, Gasperi A, et al. Early cyclosporine monotherapy in liver transplantation: a 5‐year follow‐up of a prospective, randomized trial. Hepatology (Baltimore, Md.) 1998;27(6):1524‐9. [DOI: 10.1002/hep.510270609] [DOI] [PubMed] [Google Scholar]
- Carlis L, Belli LS, Colella G, Rondinara GF, Slim AO, Alberti A, et al. Serum lipid changes in liver transplantation: effect of steroids withdrawn in a prospective randomized trial under cyclosporine A therapy. Transplantation Proceedings 1999;31(1‐2):391‐3. [10.1016/S0041‐1345(98)01675‐3] [DOI] [PubMed] [Google Scholar]
- Carlis L, Belli LS, Rondinara GF, Alberti A, Sansalone CV, Colella G, et al. Early steroid withdrawal in liver transplant patients: final report of a prospective randomized trial. Transplantation Proceedings 1997;29(1‐2):539‐42. [DOI: 10.1016/S0041-1345(96)00255-2] [DOI] [PubMed] [Google Scholar]
- Romani F, Belli LS, Carlis L, Rondinara GF, Alberti A, Sansalone CV, et al. Cyclosporin monotherapy (after 3 months) in liver transplant patients: a prospective randomized trial. Transplantation Proceedings 1994;26(5):2683‐5. [PubMed] [Google Scholar]
Belli 2001 {published data only (unpublished sought but not used)}
- Belli L, Alberti A, Airoldi A, Cernuschi A, Rondinara G, Carlis L. Early ribavirin treatment and avoidance of steroids in HCV positive liver transplant candidates: preliminary report of a prospective randomized trial [abstract]. Journal of Hepatology 2000;32(Suppl 2):44. [DOI: 10.1016/S0168-8278(00)80501-2] [DOI] [Google Scholar]
- Belli LS, Alberti AB, Rondinara GF, Carlis L, Corti A, Mazza E, et al. Early ribavirin treatment and avoidance of corticosteroids in hepatitis C virus (HCV)‐positive liver transplant recipients: interim report of a prospective randomized trial. Transplantation Proceedings 2001;33(1‐2):1353‐4. [10.1016/S0041‐1345(00)02507‐0] [DOI] [PubMed] [Google Scholar]
Chen 2007 {published data only (unpublished sought but not used)}
- Chen Z‐S, He F, Zeng F‐J, Jiang J‐P, Du D‐F, Liu B. Early steroid withdrawal after liver transplantation for hepatocellular carcinoma. World Journal of Gastroenterology 2007;13(39):5273‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2008 {published data only (unpublished sought but not used)}
- Hu AB, He XS, Wu ZP, Zhu XF, Ma Y, Wang DP, et al. Evaluation of efficacy and safety on steroid withdraw at the seventh day after liver transplantation. Zhonghua Wai Ke Za Zhi 2008;46(15):1126‐8. [PubMed] [Google Scholar]
Ju 2012 {published data only (unpublished sought but not used)}
- Ju WQ, Guo ZY, Ling X, He XS, Wu LW, Tai Q, et al. Twenty‐four hour steroid avoidance immunosuppressive regimen in liver transplant recipients. Experimental and Clinical Transplantation 2012;10(3):258‐62. [DOI] [PubMed] [Google Scholar]
- Ju WQ, He XS, Tan YL, Wu LW, Tai Q, Hu AB, et al. Two‐dose steroid combined with two‐dose daclizumab and tacrolimus regimen in liver transplant recipients. Zhonghua Wai Ke Za Zhi 2009;47(14):1064‐6. [PubMed] [Google Scholar]
- Wu LW, Guo ZY, Tai Q, Ju WQ, Wang DP, Hu AB, et al. Steroid elimination within 24 hours after orthotopic liver transplantation: effectiveness and tolerability. Hepatobiliary & Pancreatic Diseases International 2012;11(2):137‐42. [DOI] [PubMed] [Google Scholar]
Lerut 2008 {published and unpublished data}
- Bonaccorsi‐Riani E, Sempoux C, Piette N, Julliard O, Kabamba B, Ciccarelli O, et al. Impact of steroid‐avoidance immunosuppression on long‐term outcome after liver transplantation for HCV cirrhosis: the need for well documented long‐term follow‐up. Acta Gastroenterologica Belgica 2012;75(4):411‐8. [PubMed] [Google Scholar]
- Lerut J, Ciccarelli O, Bonaccorsi‐Riani E, Mathis J, Verbaandert C, Talpe S, et al. Tacrolimus (tac) monotherapy in liver transplantation (lt): one‐year results of a prospective, randomized, double‐blinded placebo‐controlled study. Transplantation International 2009;22:84. [DOI] [PubMed] [Google Scholar]
- Lerut J, Mathys J, Lemaire J, Thuyne V, Talpe S, Sempoux C. Tacrolimus monotherapy (TAC‐mono) in 100 adult liver transplant (LT) recipients: one year results of a prospective, randomised, blinded, placebo‐controlled, single centre study. Transplantation 2004;78(2):173. [Google Scholar]
- Lerut J, Mathys J, Lemaire J, Thuyne V, Talpe S, Verbaandert C, et al. Tacrolimus monotherapy (tac‐mono) in 100 adult liver transplant (lt) recipients: one year results of a prospective, randomized, blinded, placebo‐controlled, investigator driven, single centre study [abstract]. 3rd International Congress on Immunosuppression. San Diego, 2004.
- Lerut J, Mathys J, Lemaire J, Thuyne V, Talpe S, Sempoux C, et al. Tacrolimus monotherapy(TAC‐MONO)in 100 adult liver transplant (LT) recipients: one year results of a prospective, randomized, blinded, placebo‐controlled, investigated driven, single centre study. Liver Transplantation 2005;11(7):C24. [Google Scholar]
- Lerut J, Mathys J, Verbaandert C, Ciecarelli O, Lemaire J, Hetsch N, et al. Tacrolimus monotherapy (TAC‐MONO) in 156 primary adult liver transplant (LT) recipients: results of a prospective, randomized, placebo‐controlled study. American Journal of Transplantation 2007;7:470. [Google Scholar]
- Lerut J, Mathys J, Verbaandert C, Lemaire J, Hetsch N, Ciccarelli O, et al. Tacrolimus monotherapy (TAC‐MONO) in adult liver transplantation (LT): results of a prospective, randomized, placebo‐controlled blinded study. Transplantation International 2007;20:31. [Google Scholar]
- Lerut J, Mathys J, Verbaandert C, Talpe S, Ciccarelli O, Lemaire J, et al. Tacrolimus monotherapy in liver transplantation: one‐year results of a prospective, randomized, double‐blind, placebo‐controlled study. Annals of Surgery 2008;248(6):956‐67. [DOI: 10.1097/SLA.0b013e31819009c9] [DOI] [PubMed] [Google Scholar]
- Lerut JP, Pinheiro RS, Lai Q, Stouffs V, Orlando G, Rico Juri JM, et al. Is minimal, [almost] steroid‐free immunosuppression a safe approach in adult liver transplantation? Long‐term outcome of a prospective, double blind, placebo‐controlled, randomized, investigator‐driven study. Annals of Surgery 2014;260(5):886‐92. [DOI: 10.1097/SLA.0000000000000969] [DOI] [PubMed] [Google Scholar]
- Pinheiro RS, Lai Q, Juri JMR, Santa EC, Ciccarelli O, D'Albuquerque LAC, et al. Is steroid (almost) free immunosuppression a safe approach in adult liver transplantation? Long‐term outcome of a prospective double blind, placebo‐controlled randomized investigator driven study. Liver Transplantation 2013;19:S90. [DOI] [PubMed] [Google Scholar]
Llado 2006 {published data only (unpublished sought but not used)}
- Llado L, Baliellas C, Fabregat J, Ramos E, Torras J, Serrano T, et al. Influence of immunosuppression without steroids in long‐term evolution of HCV‐recurrence after liver transplantation: results of a prospective randomized study. Liver Transplantation 2011;17(6):S86. [Google Scholar]
- Llado L, Fabregat J, Castellote J, Ramos E, Xiol X, Torras J, et al. Immunosuppression without steroids after liver transplantation in HCV‐infected patients: results and histological evolution from a prospective randomized study. Liver Transplantation 2008;14(7):S103‐4. [DOI] [PubMed] [Google Scholar]
- Llado L, Figueras J, Memba R, Xiol X, Garcia‐Gil A, Gonzalez‐Pinto N, et al. Immunosuppression without steroids in liver transplantation reduces infectious and metabolic complications but increases rejection rates in non‐HCV patients. Liver Transplantation 2005;11(7):C16. [Google Scholar]
- Lladó L, Fabregat J, Castellote J, Ramos E, Xiol X, Torras J, et al. Impact of immunosuppression without steroids on rejection and hepatitis C virus evolution after liver transplantation: results of a prospective randomized study. Liver Transplantation 2008;14(12):1752‐60. [DOI] [PubMed] [Google Scholar]
- Lladó L, Xiol X, Figueras J, Ramos E, Memba R, Serrano T, et al. Erratum ‐ Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: results from a prospective multicenter randomized study (vol 44, pg 710, 2006). Journal of Hepatology 2006;45(1):166. [DOI] [PubMed] [Google Scholar]
- Lladó L, Xiol X, Figueras J, Ramos E, Memba R, Serrano T, et al. Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: results from a prospective multicenter randomized study. Journal of Hepatology 2006;44(4):710‐6. [DOI: 10.1016/j.jhep.2005.12.010] [DOI] [PubMed] [Google Scholar]
- Xiol X, Castelloote J, Vazquez S, Llado L, Figueras J, Lama C. Prospective randomized trial on the safety and efficacy of steroid free immunosuppression after liver transplantation. Preliminary results [abstract]. Journal of Hepatology 2003;38(Suppl 2):51. [Google Scholar]
Margarit 2005 {published and unpublished data}
- Bilbao I, Lazaro JL, Escartin A, Bergamini S, Margarit C. Potential beneficial effect of steroid‐free immunosuppression with tacrolimus, after liver transplantation for hepatitis C viral cirrhosis. Liver Transplantation 2004;10(6):C74. [Google Scholar]
- Bilbao I, Pou L, Castells L, Charco R, Murio E, Hidalgo E, et al. Tacrolimus in monotherapy in liver transplantation comparison with dual regimen of tacrolimus with steroids. Hepatology (Baltimore, Md.) 2000;32(4 Pt 2):598A. [Google Scholar]
- Bilbao I, Pou L, Lazaro JL, Castells L, Charco R, Murio E. Tacrolimus in monotherapy in liver transplant. Comparison with a dual regimen of tacrolimus with steroids [abstract]. Journal of Hepatology 2001;34(1):61‐2. [Google Scholar]
- Charco R, Bilbao I, Chavez R, Castells LI, Hidalgo E, Margarit C. Low incidence of hypercholesterolemia among liver transplant patients under tacrolimus monotherapy immunosuppression. Transplantation Proceedings 2002;34(5):1555‐6. [DOI] [PubMed] [Google Scholar]
- Margarit C, Bilbao I, Castells L, Lopez I, Pou L, Allende E, et al. A prospective randomized trial comparing tacrolimus and steroids with tacrolimus monotherapy in liver transplantation: the impact on recurrence of hepatitis C. Transplantation International 2005;18(12):1336‐45. [DOI: 10.1111/j.1432-2277.2005.00217.x] [DOI] [PubMed] [Google Scholar]
Moench 2007 {published data only (unpublished sought but not used)}
- Everson GT. Is early steroid withdrawal after liver transplantation feasible with tacrolimus monotherapy?. Nature Clinical Practice Gastroenterology and Hepatology 2008;5(4):194‐5. [DOI] [PubMed] [Google Scholar]
- Moench C, Barreiros AP, Schuchmann M, Bittinger F, Thiesen J, Hommel G, et al. Tacrolimus monotherapy without steroids after liver transplantation‐‐a prospective randomized double‐blinded placebo‐controlled trial. American Journal of Transplantation 2007;7(6):1616‐23. [DOI] [PubMed] [Google Scholar]
- Moench C, Grebe A, Schuchmann M, Otto G. FK506 monotherapy early steroid reduction for liver transplant recipients ‐ a prospective randomised double blinded placebo controlled trial [abstract]. Liver Transplantation 2005;11(7):C25. [Google Scholar]
- Moench C, Grebe A, Schunchmann M, Bittinger F, Lohse AW, Otto G. FK506 monotherapy after early steroid reduction for liver transplant recipients ‐ prospective randomised double blinded placebo controlled trial [abstract]. 3rd International Congress on Immunosuppression. San Diego, 2004.
- Moench C, Schuchmann M, Bittinger F, Otto G. FK506 monotherapy after early steroid withdrawal for liver transplant recipients ‐ a prospective randomised double blinded placebo controlled trial [abstract]. Transplantation 2006;82:230. [Google Scholar]
- Moench C, Schuchmann M, Otto G. Remodeling of glucose after liver transplantation under steroid freedom ‐ results of a prospective randomised placebo‐controlled double‐blind study. Zeitschrift für Gastroenterologie 2007;45(1):119. [Google Scholar]
- Weiler N, Thrun I, Hoppe‐Lotichius M, Zimmermann T, Kraemer I, Otto G. Early steroid‐free immunosuppression with FK506 after liver transplantation: long‐term results of a prospectively randomized double‐blinded trial. Transplantation 2010;90(12):1562‐6. [DOI] [PubMed] [Google Scholar]
Pageaux 2004 {published data only (unpublished sought but not used)}
- Pageaux G, Boillot O, Calmus Y, Ducerf C, Vanlemmens C, Boudjema K, et al. Early steroid withdrawal after liver transplantation: a placebo controlled study. Hepatology (Baltimore, Md.) 2003;38(4):370A. [DOI] [PubMed] [Google Scholar]
- Pageaux GP, Calmus Y, Boillot O, Ducerf C, Vanlemmens C, Boudjema K, et al. Steroid withdrawal at day 14 after liver transplantation: a double‐blind, placebo‐controlled study. Liver Transplantation 2004;10(12):1454‐60. [DOI] [PubMed] [Google Scholar]
Pelletier 2013 {published data only (unpublished sought but not used)}
- Magar A, Pelletier SJ, Englesbe MJ, Welling TH, Al‐Holou WN, Fontana RJ, et al. Randomized prospective study suggesting worse outcomes for complete steroid avoidance in liver transplantation. Hepatology (Baltimore, Md.) 2006;44(4):405A. [Google Scholar]
- Pelletier SJ, Ammori JB, Englesbe MJ, Sung RS, Magee JC, Fontana RJ. A prospective, randomized trial of complete steroid avoidance in liver transplantation. Liver Transplantation 2008;14(7 Suppl 1):S224. [DOI] [PubMed] [Google Scholar]
- Pelletier SJ, Debroy MA, Sung RS, Magee JC, Merion RM, Campbell DA, et al. Randomized prospective study of complete steroid avoidance in liver transplantation. Liver Transplantation 2004;10(6):C74. [DOI] [PubMed] [Google Scholar]
- Pelletier SJ, Debroy MA, Vanderwall K, Sung RS, Magee JC, Campbell DA, et al. Analysis of early outcomes of a prospective, randomized trial of complete steroid avoidance in liver transplantation. Transplantation 2004;78(2):378. [DOI] [PubMed] [Google Scholar]
- Pelletier SJ, Englesbe MJ, Vanderwall K, Sung RS, Magee JC, Merion RM, et al. Complete steroid avoidance in a randomized controlled trial is not associated with a decreased incidence of hepatitis C virus recurrence following liver transplantation [abstract]. 3rd International Congress on Immunosuppression. San Diego, 2004.
- Pelletier SJ, Nadig SN, Lee DD, Ammori JB, Englesbe MJ, Sung RS, et al. A prospective, randomized trial of complete avoidance of steroids in liver transplantation with follow‐up of over 7 years. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2013;15(4):286‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier SJ, Vanderwall K, Debroy MA, Englesbe MJ, Sung RS, Magee JC, et al. Preliminary analysis of early outcomes of a prospective, randomized trial of complete steroid avoidance in liver transplantation. Transplantation Proceedings 2005;37(2):1214‐6. [DOI] [PubMed] [Google Scholar]
Ramirez 2013 {published data only (unpublished sought but not used)}
- Doria C, Ramirez CB, Frank A, Navarro V, Herrine S, Rossi S, et al. Complete corticosteroid (CS)‐avoidance immunosuppression (is) regimen with basiliximab (BSX) induction and tacrolimus (TAC) in adult liver transplantation (OLT) [abstract no: 1247]. Transplantation 2008;86(2S):423. [Google Scholar]
- Doria C, Ramirez CB, Frank A, Navarro V, Herrine S, Rossi S, et al. Safety and efficacy of enteric coated mycophenolate sodium (EC‐MPS) in combination with basiliximab (BSX) induction, tacrolimus (TAC) with or without corticosteroids (CS) in adult liver transplantation (OLT) [abstract no: 1246]. Transplantation 2008;86(2S):423. [Google Scholar]
- Ramirez CB, Doria C, Frank A, Navarro V, Herrine S, Rossi S, et al. Complete corticosteroid (CS)‐avoidance with basiliximab (BSX) induction, tacrolimus (TAC) and enteric coated mycophenolate sodium (EC‐MPS) is safe and effective in adult liver transplantation: preliminary results [abstract no: P166]. Transplantation International 2007;20(Suppl 2):135. [Google Scholar]
- Ramirez CB, Doria C, Frank A, Navarro V, Herrine S, Rossi S, et al. Safety and efficacy of enteric coated mycophenolate sodium (EC‐MPS) in conjunction with basiliximab (BSX) induction, tacrolimus (Tac) with or without corticosteroids (CS) in adult liver transplantation [abstract no: P164]. Transplantation International 2007;20(Suppl 2):135. [Google Scholar]
- Ramirez CB, Doria C, Frank AM, Armenti ST, Marino IR. Completely steroid‐free immunosuppression in liver transplantation: a randomized study. Clinical Transplantation 2013;27(3):463‐71. [DOI] [PubMed] [Google Scholar]
- Ramirez CB, Doria C, Frank AM, Navarro V, Herrine S, Rossi S. Safety and efficacy of steroid‐free immunosuppression using basiliximab induction, tacrolimus and enteric coated mycophenolate sodium in adult liver transplantation. Liver Transplantation 2008;14(7 Suppl 1):S109. [Google Scholar]
Reggiani 2005 {published data only (unpublished sought but not used)}
- Reggiani P, Arru M, Regazzi M, Gatti S, Molinaro MD, Caccamo L, et al. A "steroid‐free" tacrolimus and low‐dose mycophenolate mofetil primary immunosuppression does not prevent early acute rejection after liver transplantation. Transplantation Proceedings 2005;37(4):1697‐9. [DOI] [PubMed] [Google Scholar]
- Reggiani P, Regazzi M, Arru M, Gatti S, Rossi G, Molinaro MD, et al. A "steroid‐free" tacrolimus and low‐dose mycophenolate mofetil primary immunosuppression does not prevent early acute rejection after liver transplantation [abstract]. 3rd International Congress on Immunosuppression. San Diego, 2004. [DOI] [PubMed]
Studenik 2005 {published data only (unpublished sought but not used)}
- Studenik P, Mejzlik V, Stouracova M, Ondrasek J, Cerny J. Steroid free tacrolimus and mycophenolate mofetil based immunosuppression in liver transplant recipients. Open label, randomised, prospective study [abstract]. Liver Transplantation 2005;11(7):C42. [Google Scholar]
Tisone 1999 {published data only (unpublished sought but not used)}
- Tisone G, Angelico M, Palmieri G, Pisani F, Anselmo A, Baiocchi L, et al. A pilot study on the safety and effectiveness of immunosuppression without prednisone after liver transplantation. Transplantation 1999;67(10):1308‐13. [DOI] [PubMed] [Google Scholar]
- Tisone G, Angelico M, Palmieri G, Pisani F, Baiocchi L, Anselmo A, et al. Prednisone is unnecessary as routine treatment after liver transplantation (OLT) [abstract]. Journal of Hepatology 1998;28(1 Suppl 1):53. [Google Scholar]
- Tisone G, Angelico M, Palmieri G, Pisani F, Baiocchi L, Vennarecci G, et al. Immunosuppression without prednisone after liver transplantation is safe and associated with normal early graft function: preliminary results of a randomized study. Transplantation International 1998;11(Suppl 1):S267‐9. [DOI] [PubMed] [Google Scholar]
- Tisone G, Angelico M, Vennarecci G, Palmieri G, Buonomo O, Negrini S, et al. Metabolic findings after liver transplantation within a randomised trial with or without steroids. Transplantation Proceedings 1998;30(4):1447‐8. [DOI] [PubMed] [Google Scholar]
- Tisone G, Anselmo A, Tariciotti L, Monaco A, Daniele S, Luca L, et al. Long‐term results of a randomized trial on immunosuppression with or without steroids after liver transplantation [abstract no: P148]. Transplantation International 2007;20(Suppl 2):131. [Google Scholar]
- Tisone G, Tariciotti L, Daniele S, Monaco A, Luca L, Manuelli M, et al. Long‐term results of a randomized trial on immunosuppression with or without steroids after liver transplantation in human. American Journal of Transplantation 2007;7(Suppl 2):Abstract No. 1274. [Google Scholar]
Vivarelli 2007 {published data only (unpublished sought but not used)}
- Vivarelli M, Burra P, Barba G, Canova D, Senzolo M, Cucchetti A. Effect of different steroid schedules on HCV recurrence after liver transplantation: results of the Epaster trial. Liver Transplantation 2008;14(7 Suppl 1):S178. [Google Scholar]
- Vivarelli M, Burra P, Barba G, Canova D, Senzolo M, Cucchetti A, et al. Influence of steroids on HCV recurrence after liver transplantation: a prospective study. Journal of Hepatology 2007;47(6):793‐8. [DOI] [PubMed] [Google Scholar]
Zhong 2010 {published data only (unpublished sought but not used)}
- NCT01137084. Liver transplantation results in hepatocellular carcinoma patients with immunosuppression without steroids. clinicaltrials.gov/ct2/show/NCT01137084 June 4, 2010. [NCT01137084]
- Zhong L, Peng Z, Liu J, Peng G, Wang C. Liver transplantation results in hepatocellular carcinoma patients with immunosuppression without steroids from a prospective multicenter randomized study in China. Liver Transplantation 2010;16(6):S184. [Google Scholar]
References to studies excluded from this review
Benitez 2010 {published data only}
- Benitez CE, Puig‐Pey I, Lopez M, Martinez‐Llordella M, Lozano JJ, Bohne F, et al. ATG‐fresenius treatment and low‐dose tacrolimus: results of a randomized controlled trial in liver transplantation. American Journal of Transplantation 2010;10(10):2296‐304. [DOI] [PubMed] [Google Scholar]
Boillot 2005 {published data only}
- Boillot O, Mayer D, Boudjema K. Effective and safe steroid‐free immunosuppression with a tacrolimus/daclizumab regimen after liver transplantation [abstract]. American Journal of Transplantation 2003;3:324. [Google Scholar]
- Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, et al. Corticosteroid‐free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transplantation 2005;11(1):61‐7. [DOI] [PubMed] [Google Scholar]
Cosimi 1987 {published data only}
- Cosimi AB, Cho SI, Delmonico FL, Kaplan MM, Rohrer RJ, Jenkins RL. A randomized clinical trial comparing OKT3 and steroids for treatment of hepatic allograft rejection. Transplantation 1987;43(1):91‐5. [DOI] [PubMed] [Google Scholar]
Cuervas‐Mons 2009 {published data only}
- Cuervas‐Mons V, Herrero JI, Gomez MA, Gonzalez I, Serrano T, Mata M, et al. Impact of an steroid‐free immunosuppression regimen (tacrolimus+mycophenolate mofetil) versus a conventional regimen (tacrolimus+steroids) in cardiovascular risk factors after liver transplantation: preliminary results [abstract no: 477]. American Journal of Transplantation 2009;9(Suppl 2):329. [Google Scholar]
- Cuervas‐Mons V, Herrero JI, Gomez MA, Gonzalez‐Pinto I, Serrano T, Mata M, et al. Impact of tacrolimus and mycophenolate mofetil regimen vs. a conventional therapy with steroids on cardiovascular risk in liver transplant patients. Clinical Transplantation 2015;29(8):667‐77. [DOI: 10.1111/ctr.12557] [DOI] [PubMed] [Google Scholar]
Day 2004 {published data only}
- Day CP, O'Grady J, Mayer D, Simpson K, Millson CE, Solomons N, et al. A randomised controlled trial of calcineurin inhibitor (CNI) replacement with mycophenolate mofetil and steroids in liver transplant patients with renal dysfunction. Hepatology (Baltimore, Md.) 2004;40(4 Suppl 1):547A. [Google Scholar]
De Simone 2007 {published data only}
- Simone P, Carlis L, Filipponi F, Grazi GL, Cuomo O, Santaniello W, et al. Results of a multicenter, randomized, open‐label, controlled clinical trial comparing basiliximab versus steroids in hepatitis C positive liver transplant patients. Transplantation International 2007;20:33. [Google Scholar]
- Simone P, Carlis L, Grazi GL, Cuomo O, Calise F, Castagneto M, et al. Results of a multicenter, randomized, open‐label trial comparing basiliximab vs. steroids in HCV liver transplant patients. American Journal of Transplantation 2007;7:312. [Google Scholar]
Filipponi 2004 {published data only (unpublished sought but not used)}
- Filipponi F, Callea F, Salizzoni M, Grazi GL, Fassati LR, Rossi M, et al. Double‐blind comparison of hepatitis C histological recurrence rate in HCV+ Liver transplant recipients given basiliximab + steroids or basiliximab + placebo, in addition to cyclosporine and azathioprine. Transplantation 2004;78(10):1488‐95. [DOI] [PubMed] [Google Scholar]
Foroncewicz 2009 {published data only}
- Foroncewicz B, Mucha K, Ryszkowska E, Ciszek M, Ziolkowski J, Porowski D, et al. Safety and efficacy of steroid‐free immunosuppression with tacrolimus and daclizumab in liver transplant recipients: 6‐year follow‐up in a single center. Transplantation Proceedings 2009;41(8):3103‐6. [DOI] [PubMed] [Google Scholar]
- Foroncewicz B, Mucha K, Ryszkowska E, Krawczyk M, Paczek L. Efficacy and safety of steroid‐free immunosuppression with tacrolimus and daclizumab in liver transplant recipients. Six years follow‐up in a single centre. Transplantation 2010;90:642. [DOI] [PubMed] [Google Scholar]
Ganschow 2007 {published data only}
- Ganschow R, Melter M, Wallot M, Schulz A, Pfister E, Baumann U, et al. Maintained efficacy with steroid minimization after pediatric liver transplantation with basiliximab (Simulect (R)) induction therapy: a multicenter randomized 12‐month trial. Pediatric Transplantation 2005;9:87. [Google Scholar]
- Ganschow R, Melter M, Wallot M, Schulz A, Pfister E, Baumann U, et al. Maintained efficacy with steroid minimization after pediatric liver transplantation with basiliximab (Simulect) induction therapy: a multicenter randomized 12‐month trial. Clinical Immunology 2006;119:S37‐8. [Google Scholar]
- Ganschow R, Melter M, Wallot M, Schulz A, Pfister ED, Baumann U, et al. Maintained efficacy with steroid minimization after pediatric liver transplantation with basiliximab (Simulect) induction therapy: a multicenter randomized 12‐month trial [abstract]. Liver Transplantation 2005;11(7):C20. [Google Scholar]
- Ganschow R, Melter M, Wallot M, Schulz A, Pfister ED, Baumann U, et al. Maintained efficacy with steroid minimization in pediatric liver transplant recipients with basiliximab (Simulect) induction therapy: a German multicenter randomized 12‐month trial. Pediatric Transplantation 2007;11:36. [Google Scholar]
Hu 2013 {published data only}
- Hu AB, Wu LW, Tai Q, Zhu XF, He XS. Safety and efficacy of four steroid‐minimization protocols in liver transplant recipients: 3‐year follow‐up in a single center. Journal of Digestive Diseases 2013;14(1):38‐44. [DOI] [PubMed] [Google Scholar]
Jonas 2001 {published data only}
- Jonas S, Guckelberger O, Tullius SG, Steinmuller T, Muller AR, Grauhan O, et al. Corticosteroid‐free therapy after tacrolimus‐based dual immunosuppression versus cyclosporine‐based quadruple‐induction therapy. Transplantation Proceedings 2001;33(3):2232‐3. [DOI] [PubMed] [Google Scholar]
Junge 2005 {published data only}
- Junge G, Neuhaus R, Schewior L, Klupp J, Guckelberger O, Langrehr JM, et al. Withdrawal of steroids: a randomized prospective study of prednisone and tacrolimus versus mycophenolate mofetil and tacrolimus in liver transplant recipients with autoimmune hepatitis. Transplantation Proceedings 2005;37(4):1695‐6. [DOI] [PubMed] [Google Scholar]
- Junge G, Neuhaus R, Schewior LV, Klupp J, Langrehr JM, Tullius S, et al. Withdrawal of steroids: a randomized prospective study of prednisone and tacrolimus versus mycophenolate mofetil and tacrolimus in liver transplant recipients with autoimmune hepatitis [abstract]. 3rd International Congress on Immunosuppression. San Diego, 2004. [DOI] [PubMed]
- Langrehr JM, Neumann UP, Lang M, Muller AR, Jonas S, Settmacher U, et al. First results from a prospective randomized trial comparing steroid‐free induction therapy with tacrolimus and MMF versus tacrolimus and steroids in patients after liver transplantation for HCV. Transplantation Proceedings 2002;34(5):1565‐6. [DOI] [PubMed] [Google Scholar]
- Mogl MT, Neumann UP, Bahra M, Langrehr JM, Klupp J, Neuhaus P. A prospective randomized trial comparing tacrolimus and mycophenolate mofetil versus tacrolimus and steroids as immunosuppressive induction therapy in hepatitis C positive patients after OLT [abstract]. 3rd International Congress on Immunosuppression. San Diego, 2004.
- Mogl MT, Neumann UP, Langrehr JM, Neuhaus P. A prospective randomized trial comparing steroid‐free immunosuppression induction with tacrolimus and MMF versus tacrolimus and steroids in patients with HCV. American Journal of Transplantation 2004;4(Suppl 8):364. [Google Scholar]
Kato 2007 {published data only}
- Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, et al. Randomized trial of steroid‐free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation 2007;84(7):829‐35. [DOI] [PubMed] [Google Scholar]
- Kato T, Neff G, Montalbano M, Hung OM, Lavandera R, Weppler D, et al. Steroid‐free induction with tacrolimus and daclizumab in liver transplant recipients with hepatitis C ‐ a preliminary report of a prospective randomized trial. American Journal of Transplantation 2001;1(Suppl 1):179. [Google Scholar]
- Kato T, Neff GW, Montalbano M, Hung O, Lavandera R, Levi D, et al. Steroid‐free induction with daclizumab and tacrolimus in liver transplant recipients with Hepatitis C ‐ a preliminary report [abstract]. Hepatology (Baltimore, Md.) 2001;34(4):362A. [Google Scholar]
- Kato T, Yoshida H, Gaynor J, Martinez E, Nishida S, Moon J. Impact of steroid‐free induction, MMF and pre‐emptive antiviral therapy for liver transplant recipients with hepatitis C [abstract no: 1446]. American Journal of Transplantation 2005;5(Suppl 11):524. [Google Scholar]
- Kato T, Yoshida H, Hung O, Montalbano M, Neff G, Sadfar K, et al. Steroid free immunosuppression for liver transplantation recipients with hepatitis C ‐ a prospective randomized study. Gastroenterology 2004;126(4):A699. [Google Scholar]
- Kato T, Yoshida H, Hung O, Montalvano M, Neff G, Sadfar K, et al. Steroid free induction for liver transplant recipients with hepatitis C ‐ a prospective randomized study. American Journal of Transplantation 2004;4(Suppl 8):504. [Google Scholar]
- Kato T, Yoshida H, Sadfar K, Martinez E, Madariaga J, Nishida S. Steroid free induction and pre‐emptive antiviral therapy for liver transplant recipients with hepatitis C ‐ prospective randomized study. Transplantation 2004;78(2):379. [DOI] [PubMed] [Google Scholar]
- Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, et al. Steroid‐free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Transplantation Proceedings 2005;37(2):1217‐9. [DOI] [PubMed] [Google Scholar]
Klintmalm 2011 {published data only}
- Fasola C, Heffron T, Sher L, Douglas D, Brown R, Ham J, et al. Multicenter randomized hepatitis C (HCV) three trial post liver transplantation (OLT): a one‐year follow up. American Journal of Transplantation 2005;5:276. [Google Scholar]
- Fasola C, Klintmalm G, Hepatitis C Three Group. Multicenter randomized hepatitis C‐three trial post liver transplantation: one‐year interim report. Liver Transplantation 2006;12(5):C115. [Google Scholar]
- Fasola CG, Heffron TG, Sher L, Douglas DD, Brown R, Ham J, et al. Multicenter randomized hepatitis C (HCV) three trial post liver transplantation (OLT): a 90‐day report. Hepatology (Baltimore, Md.) 2004;40(4 Suppl 1):163A. [Google Scholar]
- Klintmalm G, Fasola CG, Jennings L, Heffron TG, Sher LS, Mulligan DC, et al. Hepatitis C (HCv)‐3 study: severe HCV recurrence post liver transplantation (OLT) is decreased in patients treated with mycophenolate mofetil, particularly, in the absence of steroids. Hepatology (Baltimore, Md.) 2008;48(Suppl 4):338A. [Google Scholar]
- Klintmalm G, Fasola CG, Jennings LW. Hepatitis C (HCV)‐3 study: day‐90 protocol biopsy (PB) grade is a surrogate marker for severe HCV recurrence (R), onPB, at years 1 and 2 post liver transplantation (OLT). Hepatology (Baltimore, Md.) 2009;50(4 Suppl):1028A. [Google Scholar]
- Klintmalm GB, Davis GL, Teperman L, Netto GJ, Washburn K, Rudich SM, et al. A randomized, multicenter study comparing steroid‐free immunosuppression and standard immunosuppression for liver transplant recipients with chronic hepatitis C. Liver Transplantation 2011;17(12):1394‐403. [DOI] [PubMed] [Google Scholar]
- Klintmalm GB, Fasola CG, Jennings L. Hepatitis C (HCV)‐3 study: decreased incidence of severe HCV recurrence (R) post liver transplantation (OLT) in patients (Pt) treated with mycophenolate mofetil (MMF), particularly, in the absence of steroids (Pred) [abstract no: 52]. American Journal of Transplantation 2009;9(Suppl 2):206. [Google Scholar]
- Klintmalm GB, Fasola CG, Jennings L, Heffron TG, Sher L, Mulligan D. Hepatitis C (HCV)‐3 study: benefits of a steroid‐free immunosuppression (is) regimen in HCV‐infected liver transplant recipients (OLT) may become evident only after long‐term follow up. Liver Transplantation 2008;14(7 Suppl 1):S105. [Google Scholar]
- Klintmalm GB, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, et al. Corticosteroid‐free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1‐year interim results of the HCV‐3 study. Liver Transplantation 2007;13(11):1521‐31. [DOI] [PubMed] [Google Scholar]
Lupo 2008 {published data only}
- Lupo L, Panzera P, Tandoi F, Carbotta G, Giannelli G, Santantonio T, et al. Basiliximab versus steroids in double therapy immunosuppression in liver transplantation: a prospective randomized clinical trial. Liver Transplantation 2008;86(7):925‐31. [DOI] [PubMed] [Google Scholar]
- Lupo L, Ricci P, Caputi L, Tandoi F, Aquilino F, Palma G, et al. Basiliximab vs steroids in liver transplantation immunosuppression. A prospective randomized clinical trial. Liver Transplantation 2005;11(7):C75. [Google Scholar]
Manousou 2009 {published data only}
- Cholongitas E, Samonakis D, Manousou P, Shusang V, Dhillon A, Quaglia A, et al. Randomised trial of tacrolimus monotherapy vs. tacrolimus/azathioprine/prednisolone after liver transplantation for HCV cirrhosis. Liver Transplantation 2006;12(5):C61. [Google Scholar]
- Manousou P, Cholangitas E, Samonakis D, Tsochatzis E, Corbani A, Dhillon AP, et al. Reduced fibrosis in recurrent HCV with tacrolimus, azathioprine and steroids versus tacrolimus: randomised trial long term outcomes. Gut 2014;63(6):1005‐13. [DOI: 10.1136/gutjnl-2013-305606] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manousou P, Samonakis D, Cholongitas E, Patch D, O'Beirne J, Dhillon AP, et al. Outcome of recurrent hepatitis C virus after liver transplantation in a randomized trial of tacrolimus monotherapy versus triple therapy. Liver Transplantation 2009;15(12):1783‐91. [DOI] [PubMed] [Google Scholar]
- Manousou P, Samonakis D, Corbani A, Cholongitas E, Sigalas A, Xirouchakis E, et al. Long term results of a randomized trial of tacrolimus monotherapy versus triple therapy in HCV cirrhosis liver transplant recipients. Hepatology (Baltimore, Md.) 2007;46(4):484A. [Google Scholar]
- Manousou P, Samonakis D, Tsochatzis E, Cholongitas E, Davidson J, Patch D, et al. Long term‐8 year follow up of a randomized trial of tacrolimus monotherapy versus triple therapy after liver transplantation for HCV cirrhosis. Journal of Hepatology 2013;58:S11. [Google Scholar]
- Samonakis DN, Cholongitas E, Triantos CK, Quaglia A, Senzolo M, Dhillon AP, et al. Randomised trial of tacrolimus monotherapy vs. tacrolimus/azathioprine/prednisolone after liver transplantation for HCV cirrhosis: preliminary results. Journal of Hepatology 2005;42(Suppl 2):47. [Google Scholar]
- Samonakis DN, Mela M, Quaglia A, Triantos CK, Thalheimer U, Leandro G, et al. Rejection rates in a randomised trial of tacrolimus monotherapy versus triple therapy in liver transplant recipients with hepatitis C virus cirrhosis. Transplant Infectious Disease 2006;8(1):3‐12. [DOI] [PubMed] [Google Scholar]
McDiarmid 1995 {published data only}
- McDiarmid SV, Farmer DA, Goldstein LI, Martin P, Vargas J, Tipton JR, et al. A randomized prospective trial of steroid withdrawal after liver transplantation. Transplantation 1995;60(12):1443‐50. [DOI] [PubMed] [Google Scholar]
Nair 2006 {published data only}
- Eason JD, Blazek J, Mason A, Loss GE. Steroid‐free immunosuppression through thymoglobulin induction in liver transplantation: results of a prospective randomized trial [abstract]. Hepatology (Baltimore, Md.) 2000;32(4):208A. [Google Scholar]
- Eason JD, Loss GE, Blazek J, Nair S, Mason AL. Steroid‐free liver transplantation using rabbit antithymocyte globulin induction: results of a prospective randomized trial. Liver Transplantation 2001;7(8):693‐7. [DOI] [PubMed] [Google Scholar]
- Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE Jr. Steroid‐free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation 2003;75(8):1396‐9. [DOI] [PubMed] [Google Scholar]
- Nair S, Loss G, Cohen A, Eason J. Severity of recurrent hepatitis C infection after liver transplant: a comparative study between steroid induction vs induction with rabbit anti thymocyte globulin: results of a randomized controlled study. Hepatology (Baltimore, Md.) 2004;40(4 Suppl 1):241A. [Google Scholar]
- Nair S, Loss GE, Cohen AJ, Eason JD. Induction with rabbit antithymocyte globulin versus induction with corticosteroids in liver transplantation: impact on recurrent hepatitis C virus infection. Transplantation 2006;81(4):620‐2. [DOI] [PubMed] [Google Scholar]
Nair 2008 {published data only}
- Nair S, Lipscomb J, Eason J. Efficacy of interferon based antiviral therapy for recurrent hepatitis C in patients who received steroid free immunosuppression for liver transplantation. Liver Transplantation 2008;86(3):418‐22. [DOI] [PubMed] [Google Scholar]
Neumann 2012 {published data only}
- Neumann U, Samuel D, Trunecka P, Gugenheim J, Gerunda G, Friman S. A randomized multicenter study comparing a tacrolimus‐based protocol with and without steroids in HCV‐positive liver allograft recipients. Journal of Transplantation 2012;2012:894215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otero 2009 {published data only}
- Otero A, Varo E, Ortiz de Urbina J, Martin‐Vivaldi R, Cuervas‐Mons V, Gonzalez‐Pinto I, et al. Steroid‐free maintenance regimen versus standard treatment in liver transplant recipients [abstract]. 3rd International Congress on Immunosuppression. San Diego, 2004.
- Otero A, Varo E, Urbina JO, Martin‐Vivaldi R, Cuervas‐Mons V, Gonzalez‐Pinto I, et al. A prospective randomized open study in liver transplant recipients: daclizumab, mycophenolate mofetil, and tacrolimus versus tacrolimus and steroids. Liver Transplantation 2009;15(11):1542‐52. [DOI] [PubMed] [Google Scholar]
Saliba 2012 {published data only}
- Saliba F, Durand F, Gugenheim J, Radenne S, Leroy V, Neau‐Cransac M, et al. Steroid‐free regimen and optimization of mycophenolic acid (MPA) exposure in liver transplant recipients: final results of a randomized multicenter trial. Hepatology (Baltimore, Md.) 2012;56:515A. [Google Scholar]
- Saliba F, Rostaing L, Gugenheim J, Durand F, Radenne S, Leroy V, et al. Corticosteroid‐sparing and optimization of mycophenolic acid exposure in liver transplant recipients receiving mycophenolate mofetil and tacrolimus: a randomized, multicenter study. Transplantation 2016;100(8):1705‐13. [DOI: 10.1097/TP.0000000000001228] [DOI] [PubMed] [Google Scholar]
- Saliba F, Rostaing L, Gugenheim J, Durand F, Radenne S, Neau‐Cransac M. Optimisation of mycophenolic acid (MPA) exposure for a steroid‐free regimen in liver graft recipients: interim results from the Celleste Study [abstract no: 458]. American Journal of Transplantation 2010;10(Suppl 4):174. [Google Scholar]
Spada 2006 {published data only}
- Spada M, Bertani A, Colledan M, Sonzogni A, Guizzetti M, Lucianetti A, et al. A randomized trial for tacrolimus and steroids vs. tacrolimus and basiliximab in pediatric liver transplantation. Pediatric Transplantation 2005;9:57. [Google Scholar]
- Spada M, Bertani A, Petz W, Torri E, Sonzogni A, Guizzetti M, et al. A randomized trial for tacrolimus and steroids vs tacrolimus and basiliximab in paediatric liver transplantation [abstract]. 3rd International Congress on Immunosuppression. San Diego, 2004.
- Spada M, Bertani A, Petz W, Torri E, Sonzogni A, Guizzetti M, et al. A randomized trial for tacrolimus and steroids vs. tacrolimus and basiliximab in pediatric liver transplantation. Hepatology (Baltimore, Md.) 2004;40(4 Suppl 1):473A. [Google Scholar]
- Spada M, Petz W, Bertani A, Riva S, Sonzogni A, Giovannelli M, et al. Randomized trial of basiliximab induction versus steroid therapy in pediatric liver allograft recipients under tacrolimus immunosuppression. American Journal of Transplantation 2006;6(8):1913‐21. [DOI] [PubMed] [Google Scholar]
Takada 2013 {published data only}
- Takada Y, Kaido T, Asonuma K, Sakurai H, Kubo S, Kiuchi T, et al. Randomized trial comparing tacrolimus and steroid with tacrolimus and mycophenolate mofetil among HCV‐positive recipients of living donor liver transplantation. Liver Transplantation 2012;18:S227. [DOI] [PubMed] [Google Scholar]
- Takada Y, Kaido T, Asonuma K, Sakurai H, Kubo S, Kiuchi T, et al. Randomized, multicenter trial comparing tacrolimus plus mycophenolate mofetil to tacrolimus plus steroids in hepatitis C virus‐positive recipients of living donor liver transplantation. Liver Transplantation 2013;19(8):896‐906. [DOI] [PubMed] [Google Scholar]
Teisseyre 2006 {published data only}
- Teisseyre J, Kalicinski P, Markiewicz M, Szymczak M, Ismail H, Pawlowska J, et al. A comparison of two methods of tacrolimus based immunosuppression with or without MMF for early steroid withdrawal in children after liver transplantation – preliminary results. Liver Transplantation 2006;12(5):C71. [Google Scholar]
Turner 2006 {published data only}
- Turner S, Dhamarajah S, Bosomworth M, Bellamy M, Leeds Liver Transplant Group. Effect of perioperative steroids on renal function after liver transplantation. Anaesthesia 2006;61(3):253‐9. [DOI] [PubMed] [Google Scholar]
Washburn 2001 {published data only}
- Jensen A, Maxwell P. Six‐year follow‐up of "steroid elimination 24 hours after liver transplantation using daclizumab, tacrolimus, and mycophenolate mofetil" [abstract]. American Journal of Transplantation 2006;6:210. [DOI] [PubMed] [Google Scholar]
- Washburn K, Speeg K, Esterl R. Liver transplantation using minimal steroids, prograf, cellcept and daclizumab [abstract]. Transplantation 2000;69:S166. [Google Scholar]
- Washburn K, Speeg KV, Esterl R, Cigarroa F, Pollack M, Tourtellot C, et al. Steroid elimination 24 hours after liver transplantation using daclizumab, tacrolimus,and mycophenolate mofetil. Transplantation 2001;72(10):1675‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2001
- Adams RW, Chapman RL, Smallwood GA. Steroid withdrawal in liver transplant recipients. Progress in Transplantation 2001;11(3):217‐23. [1526‐9248] [DOI] [PubMed] [Google Scholar]
Allison 2000
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000;47(2‐3):85‐118. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Cintorino 2006
- Cintorino D, Riva S, Spada M, Minervini M, Sonzogni A, Scotti Foglieni C, et al. Corticosteroid‐free immunosuppression in pediatric liver transplantation: safety and efficacy after a short‐term follow‐up. Transplantation Proceedings 2006;38(4):1099‐100. [0041‐1345] [DOI] [PubMed] [Google Scholar]
Clark 2002
- Clark OAC, Castro AA. Searching the Literatura Americana o de Caribe em Ciencias de Saude. International Journal of Epidemiology 2002;31(1):112‐4. [DOI: 10.1093/ije/31.1.112] [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dienstag 2012
- Dienstag JL, Cosimi AB. Liver transplantation ‐ a vision realized. New England Journal of Medicine 2012;367(16):1483‐5. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esmore 1989
- Esmore DS, Spratt PM, Keogh AM, Chang VP. Cyclosporine and azathioprine immunosuppression without maintenance steroids: a prospective randomized trial. Journal of Heart Transplantation 1989;8(3):194‐9. [PubMed] [Google Scholar]
Eurotransplant 2012
- Eurotransplant. Annual Report 2012. www.eurotransplant.org/cms/mediaobject.php?file=AR2012.pdf (accessed 25 March 2014).
Fairfield 2017
- Fairfield CJ, Harrison EM, Wigmore SJ. Duplicate publication bias weakens the validity of meta‐analysis of immunosuppression after transplantation. World Journal of Gastroenterology 2017;23(39):7198‐200. [DOI: 10.3748/wjg.v23.i39.7198] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez 1998
- Fernandez‐Miranda C, Guijarro C, Calle A, Loinaz C, Gonzalez‐Pinto I, Gomez‐Izquierdo T, et al. Lipid abnormalities in stable liver transplant recipients‐effects of cyclosporin, tacrolimus, and steroids. Transplant International 1998;11(2):137‐42. [0934‐0874] [DOI] [PubMed] [Google Scholar]
Ganschow 2005
- Ganschow R, Grabhorn E, Schulz A, Hugo A, Rogiers X, Burdelski M. Long‐term results of basiliximab induction immunosuppression in pediatric liver transplant recipients. Pediatric Transplantation 2005;9(6):741‐5. [1397‐3142] [DOI] [PubMed] [Google Scholar]
Gluud 2018
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2018, Issue 2. Art. No.: LIVER.
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Grade Working Group 2004‐2007, 2008.
Gruessner 2001
- Gruessner RW, Sutherland DE, Parr E, Humar A, Gruessner AC. A prospective, randomized, open‐label study of steroid withdrawal in pancreas transplantation‐a preliminary report with 6‐month follow‐up. Transplantation Proceedings 2001;33(1‐2):1663‐4. [DOI] [PubMed] [Google Scholar]
Gu 2014
- Gu J, Wu X, Lu L, Zhang S, Bai J, Wang J, et al. Role of steroid minimization in the tacrolimus‐based immunosuppressive regimen for liver transplant recipients: a systematic review and meta‐analysis of prospective randomized controlled trials. Hepatology International 2014;8:198‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haddad 2006
- Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005161.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatz 1998
- Hatz HJ. Glucocorticosteroids: immunological basis, pharmacology and therapeutic guidelines [Glucocorticoide: immunologische Grundlagen, Pharmakologie und Therapierichtlinien]. Medizinisch‐Pharmakologisches Kompendium. Vol. 12, Stuttgart: Wissenschaftliche Verlagsgesellschaft, 1998:514. [3804714862] [Google Scholar]
Herzer 2016
- Herzer K, Strassburg CP, Braun F, Engelmann C, Guba M, Lehner F. Selection and use of immunosuppresive therapies after liver transplantation: current German practice. Clinical Transplantation 2016;30(5):487‐501. [DOI: 10.1111/ctr.12708] [DOI] [PubMed] [Google Scholar]
Hibi 2015
- Hibi T, Shinoda M, Itano O, Obara H, Kitago M, Abe Yu, et al. Steroid minimization immunosuppression protocol using basiliximab in adult living donor liver transplantation for hepatitis C virus‐related cirrhosis. Hepatology Research 2015;45(12):1178‐84. [DOI: 10.1111/hepr.12486] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirose 2006
- Hirose R, Vincenti F. Immunosuppression: today, tomorrow, and withdrawal. Seminars in Liver Disease 2006;26(3):201‐10. [0272‐8087] [DOI] [PubMed] [Google Scholar]
Ho 1996
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al. The mechanism of action of cyclosporin A and FK506. Clinical Immunology and Immunopathology 1996;80(3):S40‐5. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania: Barnett International/PAREXEL, 1997. [Google Scholar]
IP 2000
- An International Panel. Update of the international Banff Schema for liver allograft rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. Hepatology (Baltimore, Md.) 2000;31:792. [DOI] [PubMed] [Google Scholar]
IWP 1995
- International Working Party. Terminology for hepatic allograft rejection. Hepatology (Baltimore, Md.) 1995;22:648. [PubMed] [Google Scholar]
Johnson 2014
- Johnson RJ, Bradbury LL, Martin K, Neuberger J. Organ donation and transplantation in the UK‐the last decade: a report from the UK national transplant registry. Transplantation 2014;97(Suppl 1):S1‐27. [DOI] [PubMed] [Google Scholar]
Kato 2005
- Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, et al. Steroid‐free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Transplantation Proceedings 2005;37(2):1217‐9. [0041‐1345] [DOI] [PubMed] [Google Scholar]
Keus 2009
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses that include zero‐event randomized trials. American Journal of Gastroenterology 2009;104:546‐51. [DOI] [PubMed] [Google Scholar]
Kim 2013
- Kim R, Stock PG, Smith JM, Heimbach JK, Skeans MA, Edwards EB, et al. OPTN/SRTR 2011 Annual Data Report: Liver. American Journal of Transplantation 2013;13(S1):73‐102. [DOI: 10.1111/ajt.12021] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Knight 2009
- Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009;87(6):785‐94. [DOI] [PubMed] [Google Scholar]
Knight 2010
- Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta‐analysis. Transplantation 2010;89(1):1‐14. [DOI] [PubMed] [Google Scholar]
Knight 2011
- Knight SR, Morris PJ. Steroid sparing protocols following nonrenal transplants; the evidence is not there. A systematic review and meta‐analysis. Transplant International 2011;24(12):1198‐207. [DOI] [PubMed] [Google Scholar]
Lan 2014
- Lan X, Liu M‐G, Chen H‐X, Liu H‐M, Zeng W, Wei D, et al. Efficacy of immunosuppression monotherapy after liver transplantation: a meta‐analysis. World Journal of Gastroenterology 2014;20(34):12330‐40. [DOI: 10.3748/wjg.v20.i34.12330] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Neal 2005
- Neal DA, Brown MJ, Wilkinson IB, Alexander GJ. Mechanisms of hypertension after liver transplantation. Transplantation 2005;79(8):935‐40. [DOI] [PubMed] [Google Scholar]
NHS Blood and Transplant 2013
- NHS Blood and Transplant. Organ Donation and Transplantation Activity Report 2012/2013. www.nhsbt.nhs.uk/ 2013 (accessed 25 March 2014).
Ojo 2003
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. New England Journal of Medicine 2003;349(10):931‐40. [DOI] [PubMed] [Google Scholar]
OPTN 2014
- Organ Procurement and Transplantation Network. Transplants by donor type. optn.transplant.hrsa.gov/latestData/rptData.asp 2014 (accessed 10 November 2015).
Pengel 2011
- Pengel L, Morris P. The Transplant Library of randomized controlled trials and systematic reviews. Transplantation 2011;92(6):613‐6. [DOI] [PubMed] [Google Scholar]
Penninga 2012
- Penninga L, Wettergren A, Chan A‐W, Steinbrüchel DA, Gluud C. Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD008852.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penninga 2014
- Penninga L, Wettergren A, Wilson CH, Chan A‐W, Steinbrüchel DA, Gluud C. Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD010253.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penninga 2014a
- Penninga L, Wettergren A, Wilson CH, Chan A‐W, Steinbrüchel DA, Gluud C. Antibody induction versus corticosteroid induction for liver transplant recipients. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD010252.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perera 2009
- Perera MT, Mirza DF, Elias E. Liver transplantation: issues for the next 20 years. Journal of Gastroenterology and Hepatology 2009;24(Suppl 3):S124‐31. [DOI] [PubMed] [Google Scholar]
Pillai 2009
- Pillai AA, Levitsky J. Overview of immunosuppression in liver transplantation. World Journal of Gastroenterology 2009;15(34):4225‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
R 2017
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing 2017. [http://www.R‐project.org/]
Renoult 2005
- Renoult E, Buteau C, Lamarre V, Turgeon N, Tapiero B. Infectious risk in pediatric organ transplant recipients: is it increased with the new immunosuppressive agents?. Pediatric Transplantation 2005;9(4):470‐9. [1397‐3142] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodríguez‐Perálvarez 2017
- Rodríguez‐Perálvarez M, Guerrero‐Misas M, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Maintenance immunosuppression for adults undergoing liver transplantation: a network meta‐analysis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD011639.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Segev 2008
- Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, et al. Steroid avoidance in liver transplantation: meta‐analysis and meta‐regression of randomized trials. Liver Transplantation 2008;14(4):512‐25. [DOI] [PubMed] [Google Scholar]
Sgourakis 2009
- Sgourakis G, Radtke A, Fouzas I, Mylona S, Goumas K, Gockel I, et al. Corticosteroid‐free immunosuppression in liver transplantation: a meta‐analysis and meta‐regression of outcomes. Transplant International 2009;22(9):892‐905. [DOI] [PubMed] [Google Scholar]
Sheiner 1995
- Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology (Baltimore, Md.) 1995;21(1):30‐4. [PubMed] [Google Scholar]
Singh 1996
- Singh N, Gayowski T, Ndimbie OK, Nedjar S, Wagener MM, Yu VL. Recurrent hepatitis C virus hepatitis in liver transplant recipients receiving tacrolimus: association with rejection and increased immunosuppression after transplantation. Surgery 1996;119(4):452‐6. [DOI] [PubMed] [Google Scholar]
SPIRIT 2013a
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
SPIRIT 2013b
- Chan A‐W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin J, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols or clinical trials. BMJ (Clinical Research Ed.) 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterneck 2014
- Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, et al. Everolimus and early calcineurin inhibitor withdrawal: 3‐year results from a randomized trial in liver transplantation. American Journal of Transplantation 2014;14(3):701‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23:1351‐75. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011a
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis ‐ a simulation study. PLoS One 2011;6(10):e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011b
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 10 November 2015).
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Vanrenterghem 1999
- Vanrenterghem Y. Strategies to reduce or replace steroid dosing. Transplantation Proceedings 1999;31(Suppl 8A):7S‐10S. [0041‐1345] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiesner 1998
- Wiesner RH, Demetris AJ, Belle SH, Seaberg EC, Lake JR, Zetterman RK, et al. Acute hepatic allograft rejection: Incidence, risk factors, and impact on outcome. Hepatology (Baltimore, Md.) 1998;28(3):638‐45. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaydfudim 2012
- Zaydfudim V, Feurer ID, Landman MP, Moore DE, Wright JK, Pinson CW. Reduction in corticosteroids is associated with better health‐related quality of life after liver transplantation. Journal of the American College of Surgeons 2012;214(2):164‐73. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fairfield 2014
- Fairfield C, Penninga L, Powell J, Harrison EM, Wigmore S. Glucocorticosteroid‐free versus glucocorticosteroid‐containing immunosuppression for liver transplanted patients. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD007606.pub2] [DOI] [Google Scholar]
Fairfield 2015
- Fairfield C, Penninga L, Powell J, Harrison EM, Wigmore SJ. Glucocorticosteroid‐free versus glucocorticosteroid‐containing immunosuppression for liver transplanted patients. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD007606.pub3] [DOI] [PubMed] [Google Scholar]
Langer 2009
- Langer G, Saal S, Großmann K, Grothues D, Wienke A. Glucocorticosteroids for liver transplanted patients. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007606] [DOI] [Google Scholar]


