Abstract
The stress-related neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) is implicated in neuromodulation of learning and memory. PACAP can alter synaptic plasticity and has direct actions on neurons in the amygdala and hippocampus that could contribute to its acute and persistent effects on the consolidation and expression of conditioned fear. We recently demonstrated that intracerebroventricular (ICV) infusion of PACAP prior to fear conditioning (FC) results in initial amnestic-like effects followed by hyper-expression of conditioned freezing with repeated testing, and analyses of immediate-early gene c-Fos expression suggested that the central nucleus of the amygdala (CeA), but not the lateral/basolateral amygdala (LA/BLA) or hippocampus, are involved in these PACAP effects. Here, we extend that work by examining the expression of the synaptic plasticity marker activity-regulated cytoskeleton-associated protein (Arc/Arg 3.1) after PACAP administration and FC. Male Sprague-Dawley rats were implanted with cannula for ICV infusion of PACAP-38 (1.5 μg) or vehicle followed by FC and tests for conditioned freezing. One hour after FC, Arc protein expression was significantly elevated in the CeA and bed nucleus of the stria terminalis (BNST), interconnected structures that are key elements of the extended amygdala, in rats that received the combination of PACAP+FC. In contrast, Arc expression within the subdivisions of the hippocampus, or the LA/BLA, were unchanged. A subpopulation of Arc-positive cells in both the CeA and BNST also express PKCdelta, an intracellular marker that has been used to identify microcircuits that gate conditioned fear in the CeA. Consistent with our previous findings, on the following day conditioned freezing behavior was reduced in rats that had been given the combination of PACAP+FC—an amnestic-like effect—and Arc expression levels had returned to baseline. Given the established role of Arc in modifying synaptic plasticity and memory formation, our findings suggest that PACAP-induced overexpression of Arc following fear conditioning may disrupt neuroplastic changes within populations of CeA and BNST neurons normally responsible for encoding fear-related cues that, in this case, results in altered fear memory consolidation. Hence, PACAP systems may represent an axis on which stress and experience-driven neurotransmission converge to alter emotional memory, and mediate pathologies that are characteristic of psychiatric illnesses such as post-traumatic stress disorder.
Keywords: PACAP, Arc, amygdala, BNST, fear conditioning, PKCdelta
1. Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) belongs to the secretin/glucagon superfamily of peptides and exists in two biologically active forms (as 38- and 27-amino acid peptides) found in peripheral tissues and brain (Vaudry et al., 2009). PACAP-38 is the predominant form in the brain and shares identical amino acid sequence homology in species including mice, rats, sheep, and humans, indicating strong evolutionary conservation (Montero et al., 2000). Although many biological and behavioral functions have been ascribed to PACAP actions in the CNS, a role for this neuropeptide as a modulator of learning and memory is emerging (Borbely et al., 2013; Zhou et al., 2002). The high density of PACAPergic afferents and PACAP-type-I receptors (PAC1) within the extended amygdala (e.g. central nucleus of the amygdala [CeA] and bed nucleus of the stria terminalis [BNST]; Alheid & Heimer, 1988; Hannibal 2002; Joo et al., 2004; Piggins et al., 1996) suggests a role in modulating neural activity related to stress, anxiety, and fear-related learning (Hammack & May, 2014; Iemolo et al., 2016; Lebow & Chen, 2016). Along these lines, we have demonstrated that PACAP influences NMDA and AMPA-dependent synaptic transmission in the CeA (Cho et al., 2012), which receives heavy PACAPergic innervation from the parabrachial nucleus (Missig et al., 2014) and may be endogenously released in response to pain.
Recently, we demonstrated that intracerebroventricular (ICV) infusion of PACAP prior to fear conditioning can produce persistent blockade of conditioned freezing measured one or seven days after training (Meloni et al., 2016). This effect is temporary, however, as freezing re-emerges with repeated testing and results in a hyper-expression of freezing one week later. PACAP also simultaneously elevates serum corticosterone (CORT) during fear conditioning, consistent with its role as a stress-related peptide and known stimulatory effects on corticotropin-releasing factor (CRF) neurons of the paraventricular nucleus of the hypothalamus (PVN; Agarwal et al., 2005; Tsukiyama et al., 2011). However, CORT levels return to normal one day later (the test day), suggesting that elevated CORT does not account for the expression of the amnestic-like effects. We also measured expression of the protein product of the immediate early gene c-Fos following PACAP administration and fear conditioning to map brain areas activated during consolidation of the fear memory to better understand brain systems involved in PACAP effects; we found significant elevations in c-Fos expression in the CeA and PVN, but reductions in other brain areas such as the lateral habenula, indicating effects at many different neural nodes.
The current study was designed to enable a deeper level of resolution of brain areas and potential mechanisms involved in PACAP effects on fear memory consolidation by examining a known molecular marker of synaptic plasticity and memory formation: activity-regulated cytoskeleton-associated protein (Arc, also known as Arg3.1; Nikolaienko et al., 2017; Steward et al., 2015; Tzingounis & Nicoll, 2006). Like c-Fos, Arc is an immediate-early gene product and is not only a regulatory transcription factor (Korb et al., 2013), but also an effector molecule directly involved in activity-dependent structural remodeling in dendritic spines, AMPA receptor trafficking, and LTP/LTD/homeostatic plasticity (Bramham et al., 2008; Li et al., 2015; Shepherd & Bear, 2011). Brain areas examined for PACAP-dependent effects on Arc expression include those known to be involved in cue and context dependent fear conditioning such as the amygdala (including CeA) and dorsal hippocampus (Huff et al., 2006; Lonergan et al., 2010; Plath et al., 2006). We also examined the BNST, which is implicated in fear learning (Goode & Maren 2017), although there are limited reports using Arc as a readout for fear-induced neuroplastic changes in this structure (e.g. Pelrine et al., 2016; Ravinder et al., 2013).
As with our previous studies (Meloni et al. 2016), our rationale for using ICV administration of PACAP rather than localized delivery (e.g. Legradi et al., 2007; Roman et al., 2014) was to investigate how PACAP actions in multiple brain areas might simultaneously interact to affect Arc expression and subsequent learning of aversive contingencies, as might be expected under circumstances that activate PACAP systems (e.g., stress). The complex interactions of multiple brain areas recruited during fear conditioning (Maren et al., 2013; Zelikowsky et al., 2014), and modified by the effects of exogenously applied PACAP, may produce a different behavioral outcome to that seen with brain-specific infusions alone (Schmidt et al., 2015). Because systemic PACAP does not cross the blood-brain barrier, ICV administered PACAP recapitulates a condition where elevated levels of endogenous PACAP—putatively increased by exposure to stress or pain—affects multiple brain areas undergoing neuroplastic changes as a consequence of exposure to trauma (i.e., the learning-inducing event). Given that preclinical fear conditioning paradigms have been useful tools to help understand the neurobiology of psychiatric conditions such as PTSD (Mahan & Ressler, 2012), where dysfunction within PACAP systems has been implicated (Ressler et al., 2011), the current study may have useful face and construct validity for studying these illnesses as they appear in humans.
2. Methods and Materials
2.1. Animals
Male Sprague-Dawley rats (250 g) were obtained from Charles River Labs; all rats used in these studies came from the same room at the same facility (Raleigh, NC). They were housed in groups of four and acclimated to the McLean Hospital vivarium for six weeks until surgery. Rats were maintained on 12/12 h light dark cycles and food and water were provided ad libitum. Experiments were performed from 10 a.m. to 4 p.m. Sample sizes were determined on the basis of our previous work using the conditioned-freezing test (Meloni et al., 2014; Meloni et al., 2016) and immunohistochemical analyses examining the effect of fear conditioning on expression of Arc protein (Lonergan et al., 2010; Tayler et al., 2011). All animal procedures were approved by McLean Hospital’s Institutional Animal Care and Use Committee (Office of Laboratory Animal Welfare Assurance number A3685–01) in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (8th Edition).
2.2. Intracerebroventricular (ICV) cannulation
Rats were anesthetized with Nembutal (65 mg/kg, IP) and placed in a Kopf stereotaxic instrument (model 900; Kopf Instruments, Tujunga, CA) with blunt ear bars. The skin was retracted, and a hole was drilled in the skull above the lateral ventricle. Stainless-steel guide cannulas (23 gauge; Plastics One, Roanoke, VA) with an internal dummy stylet extending 1.5 mm beyond the guide cannula tip were lowered into the brain using the following coordinates: −0.8 mm caudal to bregma, +1.3 mm lateral to the midline, −3.5 mm ventral to dura. Three stainless-steel screws (size 0–80; Small Parts, Miami Lakes, FL) were also placed in the skull to anchor the guide cannula and dental acrylic (Stoelting, Wood Dale, IL) was used to cement the cannula in place. Rats were kept warm during recovery, and then singly housed in plastic Nalgene cages (45 × 24 × 20 cm) with wood-shaving bedding.
2.3. ICV PACAP infusion
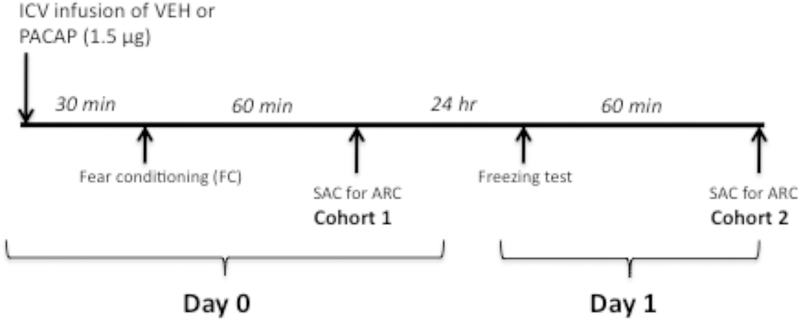
The timeline for procedures and tests are illustrated in Figure 1. Ten days after surgery, rats were transported in their home cages to a room adjacent to the fear conditioning room, placed in individual plastic cages, and their dummy stylettes were removed and replaced with infusion cannulas (30 gauge, 1.5 mm projection from the tip of the guide cannula; Plastics One) attached to Hamilton microsyringes (10 μl) by polyethylene tubing. A Harvard Apparatus infusion pump (model 22; Harvard Apparatus, Holliston, MA) was used to deliver 3.0 μl of either vehicle [artificial CSF (aCSF); Harvard Bioscience, Holliston, MA) or PACAP-38 (0.5 μg/μl; Bachem, Torrance, CA) directly into the lateral ventricle at a rate of 1.0 μl/min for 3 min. The infusion cannulas were left in place for 2 min after the infusion before the dummy stylettes were replaced. Rats were placed back in their individual home cages for 30 min followed by fear conditioning.
Figure 1.
Schematic of the experimental design used in this study. On Day 0, animals in Cohort 1 were fear conditioned 30 min after ICV infusion of vehicle (VEH) or PACAP (1.5 μg). Sixty minutes later animals were sacrificed (SAC) for immunohistochemical analysis of Arc expression. Twenty-four h later (Day 1), animals in Cohort 2 treated with VEH or PACAP on Day 0 were tested for conditioned freezing and sacrificed 60 min later for immunohistochemical analysis of Arc.
2.4. Fear-conditioning apparatus and procedure
Rats were trained and tested for fear conditioning using procedures adapted from Phillips and LeDoux (1992) as described (Meloni et al., 2016). These procedures enabled us to evaluate the expression of conditioned freezing to both context alone and cues (a tone presented within the same context) that were conditioned during elevated brain PACAP levels. Conditioning (Day 0) and testing (Day 1) were conducted using a system comprising four identical 19×9×14cm Plexiglas behavioral chambers, each contained in a sound-attenuating cubicle (Med-Associates, Georgia VT). On the conditioning day, rats were placed in chambers and after 2 min received two pairings of a 30-s, 5-kHz, 75-dB tone (CS) co-terminating with a 0.6-mA, 0.5-s footshock (US) delivered through the floorbars of the chamber. Shock reactivity (cage movement in response to shock delivery) was measured after each training trial by an accelerometer at the base of the cage. Accelerometer analog output was amplified and digitized on a scale of 0–20 units by an analog-to-digital card interfaced with a PC computer (Med-Associates). The inter-trial interval of CS-US pairings was 30 s. Control animals that did not receive fear conditioning were treated in an identical manner except that shocks were not delivered. After an additional 30 s in the chamber, animals were returned to their home cages; for animals sacrificed on Day 0, animals were left in their home cages in the testing room for 60 min before sacrifice; this time for sacrifice was chosen based on data showing maximum levels of Arc expression 60–90 minutes post-fear conditioning in rats (Lonergan et al., 2010). For other animals, twenty-four hours after training rats were returned to the testing chambers and after 2 min animals were exposed to the tone CS (5-kHz, 75-dB) for 60 s. Animals were removed from the cages and placed in their home cage in the testing room for 60 min before sacrifice. Freezing behavior video-recorded on Day 1 was scored by an experimenter blind to treatment conditions. Percent freezing was calculated as the % total time that animals remained immobile (frozen), other than breathing, during the first 2 min of re-exposure to the chamber (Context alone) and during the 60-s CS presentation (Context plus tone).
2.5. Arc immunohistochemistry
Selected areas were chosen for examination based on the known involvement of these brain regions in stress, fear conditioning and to expand on our earlier analyses of molecular markers of PACAP-induced effects (Meloni et. al., 2016).
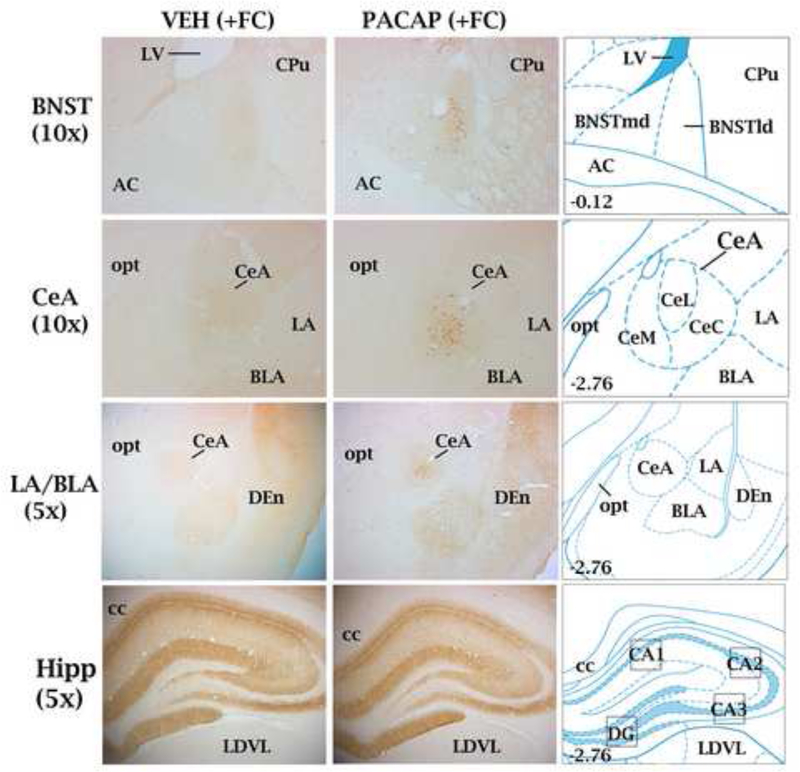
Expression of Arc protein was measured in Cohort 1 on Day 0 (60 min after fear conditioning) and in Cohort 2 on Day 1 (60 min after the freezing test). Rats were overdosed with sodium pentobarbital (115 mg/kg; IP) and upon loss of toe-pinch reflex they were perfused intracardially with 0.9% saline (200 ml) followed by 2% paraformaldehyde, 0.05% gluteraldehyde, and 0.2% picric acid in 0.1 M PBS (500 ml) pH7.4. Brains were removed, stored for 3–4 d in a 30% sucrose/0.1 M PBS solution, and then cut serially in 40 μm coronal sections, with every third section placed in a 4-ml borosilicate glass vial (16 sections/vial) for processing of A rc immunohistochemistry. All incubations were done on a rocker platform at room temperature. Sections were incubated in 0.6% hydrogen peroxide in 0.1 M PBS for 30 min followed by 3 washes (5 min) in 0.1M PBS. Sections were preincubated in antibody medium (2% normal donkey serum, 1% bovine serum albumin, 00.3% Triton-X-100 in 0.1M PBS) for 2 h followed by incubation for 24 h with a mouse monoclonal antibody against Arc (1:1600; Santa Cruz Biotechnology; #sc-17839) diluted in antibody medium. The sections were washed with PBS and incubated for 1 h with a donkey anti-mouse biotinylated secondary antibody (1:400; minimum species cross-reactivity; Jackson ImmunoResearch). The sections were washed with PBS and incubated for 30 min with the avidin-biotin complex (Vector Laboratories, Burlingame CA). The sections were then incubated for 5 min in 3,3’-diaminobenzidine/H2O2 (Sigma Fast; Sigma) as a chromogen for visualization of Arc-positive cells and label through the brain areas of interest. Sections were mounted on microscope slides and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA) and observed with a Zeiss Axioscope 2 (Zeiss,Oberkochen, Germany). Still frame images were captured with a digital camera (Axiocam, Zeiss) interfaced with a PC using image-acquisition software. To quantify the number of Arc positive cells within each brain area, a fixed region-of-interest (ROI) template was transcribed from the atlas of Paxinos and Watson (2004) for that brain area and affixed to the captured images using Adobe Photoshop software (CS6; Adobe System Incorporated, Mountain View, CA, USA) using structural landmarks for placement of the template (Meloni et al., 2016). Neurons with round/oval nuclei clearly stained brown were tagged and counted in each brain area by an observer blind to the treatment conditions using the Adobe Photoshop analysis count tool. Data represent the average of Arc counts from the left and right sides of the brain averaged across at least three coronal sections (“middle” section for each brain area shown in Figure 2 with “first” and “third” sections approximately +/− 0.120 mm rostral and caudal, respectively) for each brain area for each treatment condition.
Figure 2.
Representative coronal sections at different magnifications through brain areas examined for Arc expression in animals that received either VEH (left column) or PACAP (1.5 μg; middle column) and were fear conditioned (+FC). Right column: brain atlas plates from the atlas of Paxinos and Watson (2004) showing the location of brain areas examined for Arc expression relative to other landmarks. For the bed nucleus of the stria terminalis (dosolateral division; BNSTld), central nucleus of the amygdala (CeA) and granule layer of the dentate gyrus Arc-positive cell bodies were counted; boxed areas on atlas plates represent areas where optical density measurements were used to quantify Arc expression. Brain section levels are indicated in millimeters posterior to bregma. Central amygdaloid nucleus, lateral subdivision (CeL); central amygdaloid nucleus, medial subdivision (CeM); central amygdaloid nucleus, capsular subdivision (CeC); lateral amygdala (LA); basolateral amygdala (BLA); hippocampus (Hipp); lateral ventricle (LV); caudate-putamen (CPu); anterior commissure (ac); optic tract (opt); dorsal endopiriform nucleus (Den); corpus calllosum (cc); laterodorsal thalamic nucleus, ventrolateral (LDVL).
Arc expression encompassing dendritic processes and cells in the LA and BLA and oriens/pyramidal cell layers of the dentate gyrus (DG), CA1, CA2 and CA3 regions of the hippocampus was quantified by calculating the optical density (O.D.) of pixels using ImageJ software for Macintosh (Scion Corp, Fredrick, MD, USA); ImageJ is a public domain, JAVA-based image processing program developed by the National Institutes of Health (NIH). Image files obtained as described above from three coronal sections from each treatment group were switched to gray scale for threshold adjustments (scale 0–141 units normalized to background values of white matter from corpus callosum) and mean values of O.D. were calculated from 0.5 mm2 regions with a fixed template placed over each of the brain areas (after Figge et al., 2013).
2.6. Double-label fluorescent immunohistochemistry
Forty-μm sections from the CeA and BNST were preincubated in antibody medium for 2 h followed by incubation for 24 h at room temperature with primary antibodies: Arc: mouse monoclonal antibody (1:1600; Santa Cruz Biotechnology; #sc-17839); CRF: goat polyclonal antibody (1:1000; Santa Cruz Biotechnology; #sc-1761); PKCdelta: rabbit monoclonal antibody (1:500; Abcam; #ab182126); PACAP: rabbit polyclonal antibody (1:1000; Bachem; #T-4473). Sections were washed with 0.1M PBS and incubated for 2 h with secondary antibodies (1:200) directed against the species of the primary antibody: Alexa Fluor 488 donkey anti-mouse (Arc) or anti-rabbit (PACAP) or Alexa Fluor 594 donkey anti-goat (CRF) or anti-rabbit (PKCdelta). Sections were mounted on glass slides in water and coverslipped with ProLong Diamond Antifade mounting media (ThermoFisher Scientific).
2.7. Histology for ICV cannula placement
Sections were mounted on microscope slides and coverslipped with Permount for verification of cannula placement in the lateral ventricle; all animals used in this study were found to have accurate cannula placement.
2.8. Statistical analysis
Data are presented as means ± standard error (SEM). The effect of PACAP in non-fear conditioned (No FC) and fear conditioned (FC) animals on Arc expression in different brain areas (Day 0 and Day 1), and on freezing behavior (Day 1) was analyzed using ANOVAs with treatment group (VEH-No FC, PACAP-No FC, VEH-FC, PACAP-FC) as a between-subjects factor and brain area as a within-subjects factor. For measurements yielding significant main effects, subsequent multiple pairwise comparisons were made using post hoc Newman-Keuls tests.
3. Results
3.1. Arc localization
Figure 2 illustrates representative sections through the brain areas examined for Arc expression in animals that received either VEH or PACAP (1.5 μg) and were fear conditioned (non-fear conditioned animals that received VEH or PACAP not shown). Arc-positive cells in the CeA (primarily restricted to the lateral division; CeL) and BNST (restricted to lateral dorsal subnucleus corresponding to the oval nucleus) were plentiful in PACAP-treated animals but rarely observed in VEH-treated animals. Numerous Arc-positive cells were also seen in the granule layer of the dentate gyrus (GrDG) of the hippocampus, but the majority of Arc expression in the hippocampus and lateral (LA) and basolateral (BLA) nuclei of the amygdala was diffuse neuropil and dendritic-field (e.g. oriens layers of the CA1-CA3 of the hippocampus) labelling. Consistent with our previous finding (Meloni et al., 2016), shock reactivity did not differ between VEH and PACAP-treated rats that were fear conditioned (data not shown).
3.1. Arc quantification after fear conditioning
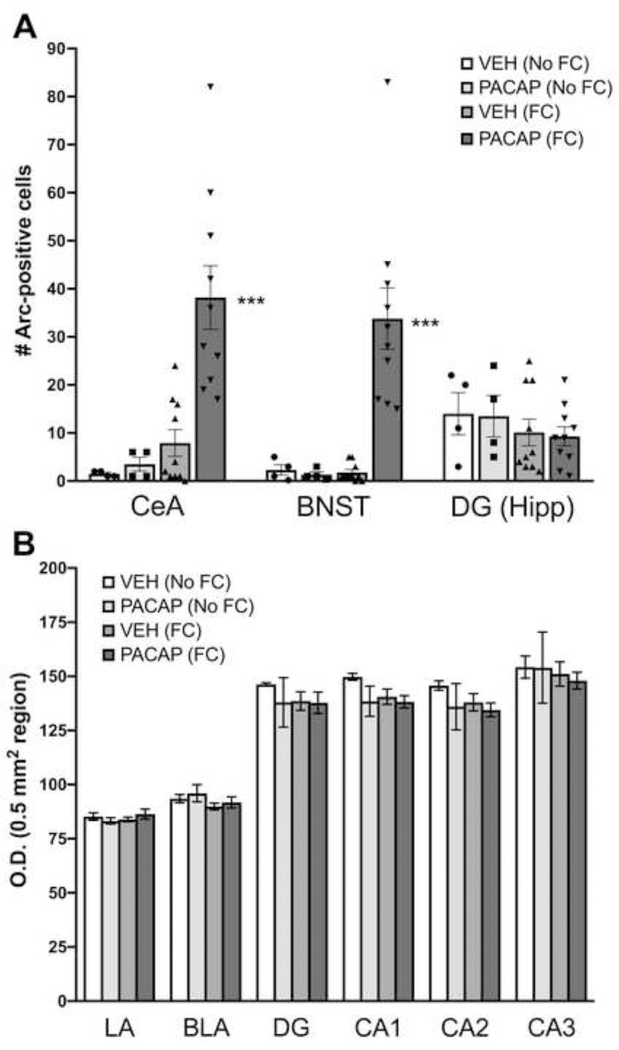
As shown in Figure 3, in rats sacrificed 60 min after fear conditioning (Cohort 1), the number of cells expressing Arc in the CeA and BNST were significantly higher in those administered ICV PACAP (1.5 μg). A two-way ANOVA of treatment group by brain area showed a main effect of treatment (F3,24=15.8, P<0.0001). Subsequent pairwise comparisons revealed significantly higher numbers of Arc-positive cells in the CeA and BNST in the PACAP-FC treatment group than all other treatment groups, including animals in the VEH-FC group (P<0.0005). There was a non-significant trend for fear conditioning itself to increase Arc expression in the CeA in VEH-treated animals (VEH-No FC vs. VEH-FC). A separate one-way ANOVA of Arc-positive cells that were observed in the GrDG of the hippocampus revealed no significant differences between treatment groups.
Figure 3.
Quantification of Arc Expression after PACAP treatment and fear conditioning. (A) Expression of Arc protein in cell bodies in the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST) or granule layer of the dentate gyrus (GrDG) of the hippocampus (Hipp) in animals that received either vehicle (VEH) or PACAP (1.5 μg) and were either fear conditioned (FC) or not (No FC). Individual data points for each treatment group are represented in scatter plots on the bar graphs (B) Expression of Arc protein measured by optical density (O.D.) in the lateral (LA), basolateral amygdala (BLA), dentate gyrus (DG) or CA1-CA3 subdivision of the dorsal hippocampus across treatment conditions. VEH (No FC), n=4; PACAP-No FC, n=4; VEH (FC), n=10; PACAP (FC), n=10. ***P<0.0005 compared to all other groups. Data are shown as mean±s.e.m.
Arc labeling in the LA, BLA, and layers of the hippocampus (DG, CA1-CA3) was quantified by optical density measurements of 0.5 mm2 regions in each of these areas. A two-way ANOVA of Arc levels in the LA and BLA across treatment groups showed a main effect of brain area (F1,24= 41.09, P < 0.0001) indicating higher levels of Arc expression in the BLA subdivision compared to the LA subdivision. However, the main effect of treatment was not significant, indicating no groups differences in Arc expression in this part of the amygdaloid complex. A two-way ANOVA of Arc levels in the DG, CA1, CA2, and CA3 divisions of the dorsal hippocampus across treatment groups showed a main effect of brain area (F3,24= 21.32, P < 0.0001) where the CA3 subdivision showed the heaviest expression of Arc compared to all other hippocampal areas. The main effect of treatment and the brain area x treatment interactions were not significant.
3.3. Arc double-labelling
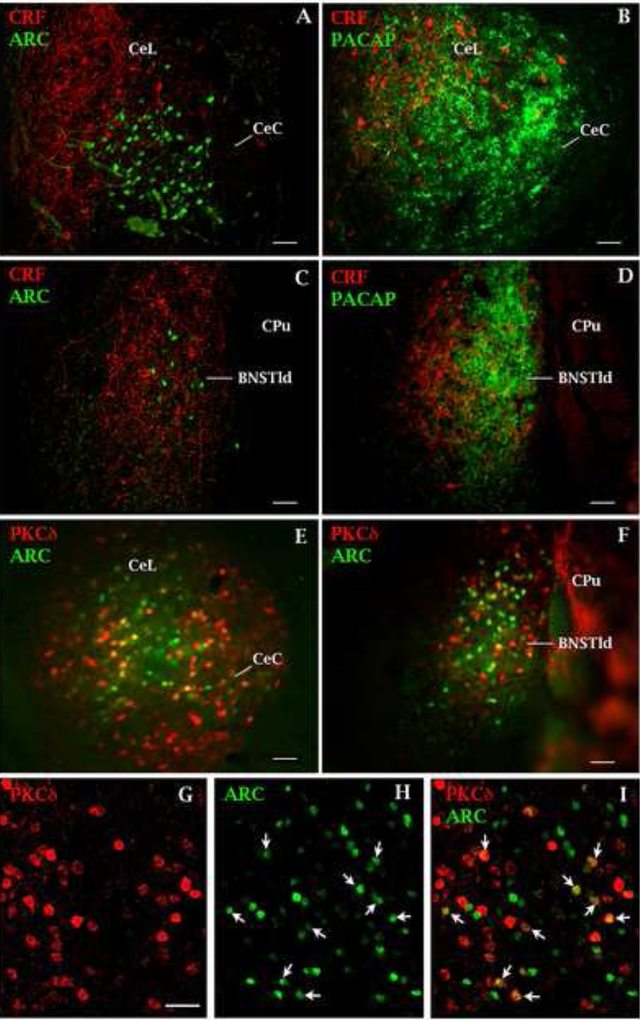
Figure 4 shows representative microscope images of Arc-positive cells in the CeA (4A) and BNST (4C) from a rat that received PACAP plus fear conditioning and was sacrificed 60 min later. In the CeA, Arc-positive cells were primarily located in the lateral subdivision of the CeA (CeL) and dorsal to the capsular division of the central nucleus of the amygdala (CeC); in the BNST, Arc-positive cells were restricted to the BNSTld subdivision. Sections were double-labeled for corticotropin releasing factor (CRF) to illustrate the anatomical relationship between CRF-positive neurons and Arc-positive neurons in the CeL (4A) and BNSTld (4B). We did not observe CRF labeling co-localized with Arc labeling in either the CeA or BNST in these representative sections. As shown in Figure 4B and 4D, these regions of Arc expression corresponds to areas that receives heavy PACAPergic afferent innervation in the CeA (4B) and BNST (4D). A comparison of Figure 4A and 4C with Figure 4B and 4D (respectively) suggests that PACAP+FC-induced Arc-expressing neurons in the CeL and BNSTld would be located within the terminal field of afferent PACAPergic innervation arising presumably from the parabrachial nucleus (Missig et al., 2014). Figure 4 also shows representative PKCdelta immunoreactivity double-labeled with Arc-positive cells in the CeA (4E, G–H) and BNST (4F); quantification of Arc-PKCdelta co-expression in the CeA and BNST is shown in Table 1 and indicates that, in both brain areas, 37% of Arc-positive cells are PKCdelta-expressing cells.
Figure 4.
Co-expression of neuronal markers with Arc protein in the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) in animals treated with PACAP and fear conditioned. Representative confocal microscope image of a coronal section through the CeA (A) and BNST (C) showing Arc expression (green label-filled cells) in neurons of the lateral division of the CeA (CeL) and BNST (BNSTld). Sections were co-labeled for corticotropin releasing factor (CRF; red label-filled cells); Arc protein was found more ventral to this population of CeL neurons – primarily in the capsular subdivision (CeC) and embedded among CRF-positive neuron in the BNSTld. Fluorescent microscope image of coronal sections through the CeA (B) and BNST (D) adjacent to those shown in A & C (respectively) showing the location of PACAPergic fibers (green label) corresponding to the areas where Arc-positive cells were found. Fluorescent microscope image of coronal sections through the CeA (E) and BNST (F) showing Arc-positive (green) and PKCdelta-positive cells (red) that co-express both markers (yellow). (G) Higher power representative confocal microscope image of PKCdelta-positive neurons in the CeL subdivision of the CeA. (H) Same section as in (G) showing Arc-positive neurons; neurons that co-express Arc and PKCdelta are labeled with arrows in (H) and (F) and appear as yellow cells from the merged image in (E). A higher magnification image and 3-D section of PKCdelta and Arc protein co-expression in the CeA can be found in Supplementary Material (Supplementary Figure 1). Scale bars = 50 um. CeA and BNST images taken from same plane and orientation as that shown in Figure 2; −2.76 and −0.12 mm posterior from bregma, respectively.
Table 1.
Quantification and co-expression of Arc- and PKCdelta-positive cells from PACAP-treated rats that were fear conditioned:
| Brain Area | #Arc+ cells | #PKCdelta+ cells | #co-expressing cells | % co-expression |
|---|---|---|---|---|
| CeA | 22 (±4) | 61 (±14) | 9 (±1) | 37 (±4) |
| BNST | 22 (±5) | 52 (±13) | 7 (±1) | 37 (±7) |
Average of left and right sides across at least three coronal brain sections for each area (n=3). Data are shown as mean ±s.e.m.
3.4. Arc quantification after behavioral testing
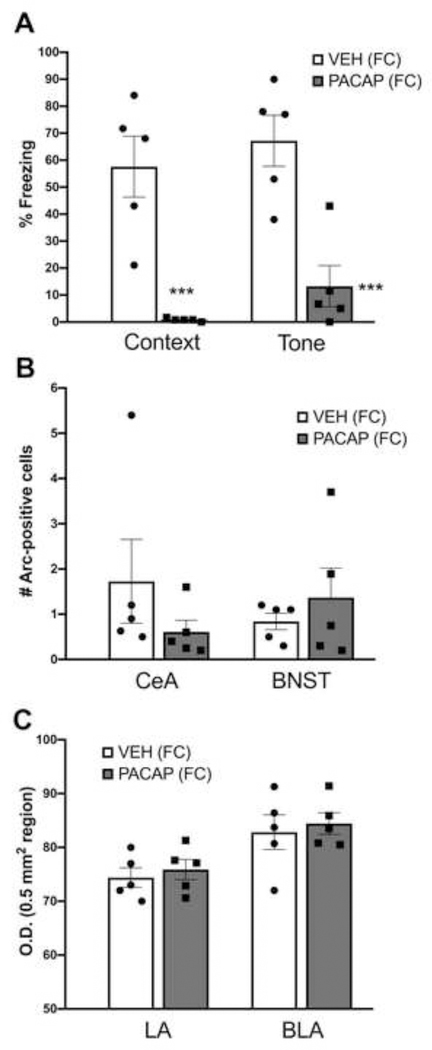
In a replication of our previous findings (Meloni et al., 2016), Figure 5A illustrates the effect of PACAP (1.5 μg) on conditioned freezing measured on Day 1: namely, conditioned freezing to both the context alone and in the presence of the tone was lower in PACAP-treated rats. A two-way ANOVA with treatment as a between-subject comparison and stimulus condition (context alone and tone) as a within-subjects comparison revealed a main effect of treatment (F1,8=40.6, P<0.0001). Individual pairwise comparisons revealed significant differences in freezing levels between VEH and PACAP-treated animals to both the context alone and the tone (P<0.0005). Despite the clear differences in the amount of conditioned freezing between VEH and PACAP-treated rats, there were no differences in the number of Arc-positive cells in the CeA or BNST (Figure 5B) between the groups at this time point. Further, the strong Arc expression in the CeA and BNST seen in PACAP+FC-treated animals seen on Day 0 (average of 30–40 Arc-positive cells) was absent, with only a few Arc-positive cells seen on the test day. A two-way ANOVA examining O.D. levels of Arc in the LA and BLA revealed a main effect of brain area (F1,8=11.8, P<0.05); this effect was carried by higher overall levels of Arc expression in the BLA compared to the LA, but there were no treatment effects or a treatment x brain area interaction.
Figure 5.
Effects of PACAP on conditioned freezing and Arc expression. (A) Percent freezing to the context alone and in the presence of the tone in animals treated with vehicle (VEH) or PACAP (1.5 μg) and fear conditioned (FC) on Day 0. Expression of Arc protein in cell bodies in the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST) (B) or measured by optical density (O.D.) in the lateral (LA) and basolateral amygdala (BLA) (C) following the freezing test. VEH (FC), n=5; PACAP (FC), n=5. ***P<0.0005 compared to VEH (FC) group. Data are shown as mean±s.e.m. Individual data points for each treatment group are represented in scatter plots on the bar graphs.
4. Discussion
We show that ICV PACAP given prior to fear conditioning results in significant elevations in Arc levels in the CeA and BNST, but not the dorsal hippocampus or LA/BLA complex. PACAP alone or fear conditioning alone were not sufficient to produce this effect; rather, the combination of PACAP plus fear conditioning (PACAP+FC) was necessary to elevate Arc in these regions. PACAP+FC-induced Arc expression was heaviest in the lateral parts of the BNST, roughly corresponding to the oval nucleus of the BNST, and in the lateral division of the CeA (CeL). Double-labeling of Arc-positive cells in the BNST and CeA with PKCdelta—an intracellular signaling molecule expressed in a sub-population of CeL neurons (so-called “CeL off” neurons; Haubensak et al., 2010)—indicated that a percentage of these cells co-express both markers and may represent a population of neurons that receive PACAPergic input and contribute to local microcircuits that gate conditioned fear (Ciocchi et al., 2010; Haubensak et al., 2010). Consistent with previous findings (Meloni et al., 2016), rats that received PACAP+FC and were tested 24 hr later (Day 1) showed a significant reduction in freezing—an amnestic-like effect—compared to vehicle-treated animals, but interestingly, Arc expression in the extended amygdala was no different from control levels on this day. Together, these results extend a growing body of evidence for a role of PACAP in modulating learning and memory and raise the possibility that transient expression of Arc—i.e., that observed one hour after fear conditioning but not the following days after behavioral testing—within interconnected circuits may interfere with consolidation and underlie the unique behavioral phenotype seen here and previously reported (Meloni et al., 2016).
The association of Arc with learning and memory is well established, although the mechanisms underlying its multiple functions as a synaptic plasticity molecule are still being elucidated (Nikolaienko et al., 2017). Here, we show that PACAP administration prior to fear conditioning, but not PACAP alone or fear conditioning alone, is required to induce robust Arc expression in the CeA and BNST. The lack of a significant effect of fear conditioning alone on Arc expression in the CeA in VEH-treated rats (No FC vs FC treatment groups) was somewhat surprising given the involvement of AMPA currents and corresponding plasticity in CeA neuronal populations during fear conditioning (Sanford et al., 2017; 10 CS-US pairings). However, our data are consistent with reports showing that neither mild (one CS-US pairing; Chau et al., 2013) nor more intense (five CS-US pairings; Ravinder et al., 2013) conditioning is sufficient to induce Arc expression in the CeA. We acknowledge that these specific outcomes may depend on the specific experimental design that we used, and that other designs may produce different outcomes. Our data provide the basis for a more comprehensive examination of parametrics in future studies.
We note that the effects of PACAP on Arc expression are similar to that seen after administration of the selective serotonin reuptake inhibitor fluoxetine followed by fear conditioning (Ravinder et al., 2013). In that report, however, fluoxetine treatment prior to fear conditioning produced a significant increase in freezing measured 24 hr later whereas we observed a significant decrease in freezing at this timepoint. Hence, although both pretreatments produce robust Arc expression within the extended amygdala (but not the LA or BLA), there is divergence in how these treatments ultimately affect the expression of conditioned fear. These differences may be related to activation of specific neuronal populations in these areas, or the recruitment of different brain areas sensitive to PACAP or fluoxetine respectively, that influences the consolidation or expression of freezing.
One possibility is that PACAP+FC effects on Arc expression may reflect amplification of intracellular cascades activated by PACAP actions at its cognate receptor PAC1 and/or VPAC½ (Gs and Gq coupled receptors with high densities in the CeA and BNST; Dickson & Finlayson, 2009; Joo et al., 2004) together with putative glutamatergic drive (i.e., induced by fear conditioning) converging on populations of CeA and BNST neurons. Consistent with this possibility, we have previously shown that PACAP potentiates NMDA receptor-dependent EPSCs in a majority of CeA neurons after electrical stimulation of afferent input from the BLA (Cho et al., 2012). Further, this LTP-like effect required insertion of AMPA receptor subunits (GluA1) into the postsynaptic cell membrane to sustain the effect. Given reports indicating that NMDA receptor-dependent LTP requires Arc synthesis during specific time windows (e.g. early-phase LTP; see Shepherd & Bear, 2011), PACAP-induced increases in Arc expression might be expected to enhance consolidation of fear-related information. However, an abundance of work has shown that Arc also plays a major role in the phenomenon of long term depression (LTD) induced by weakening of excitatory synapses through endocytosis of AMPA receptors (Waung et al., 2008; Wilkerson et al., 2017). It is proposed that this Arc-dependent mechanism regulates homeostatic plasticity (synaptic scaling) which allows neurons to adjust their synaptic strength and respond to neurotransmission in a new dynamic range (Davis, 2006; Shepherd et al., 2006). Hence the overall effect of PACAP on Arc expression and memory after fear conditioning is likely to be complex given that Arc-mediated trafficking of AMPA receptors affects both LTP and LTD and proceeds through different temporal phases of molecular effects (Bramham et al., 2008; Lonegan et al., 2010), only some of which were captured in the present study. As the source of endogenous PACAP to the CeA and BNST is primarily from the brainstem (Kozicz et al., 1998; Missig et al., 2014), and may be recruited in response to pain or somatic trauma, the interaction of this neuropeptide system with neural pathways driving experience-dependent induction of Arc may have unique consequences for the memory of a particular experience.
The Arc protein measured in the current study was found to be localized to different cellular compartments, depending on the brain areas examined. In the CeA, BNST, and GrDG of the hippocampus, Arc expression was predominately found in the cell body and nucleus, which enabled quantification of the number of Arc-positive cells and co-expression analyses. Korb et al. (2013) demonstrated that translocation of Arc protein to the nucleus leads to a reduction in AMPA receptor GluA1 subunit transcription and decreased synaptic strength. Although AMPA receptor/GluA1 subunit density was not measured in the current study, the combined effects of Arc-induced AMPA endocytosis at the cell surface (Chowdhury et al., 2006) plus a reduction of GluA1 subunit transcription in the nucleus (Korb et al., 2013) could profoundly decrease AMPA-mediated neurotransmission (Rial Verde et al., 2006) in the extended amygdala where PACAP+FC induced exceptionally high levels of cellular Arc expression. Given that AMPA receptor antagonism or knockdown in the amygdala blocks the expression of conditioned fear (Rumpel et al., 2005; Walker & Davis, 1997), our data raise the possibility that a similar mechanism may account for the reduction in the expression of freezing seen 24 hr after PACAP+FC. Indeed, mice lacking ubiquitin ligases that degrade Arc (e.g. Ube3a and Triad3A knockout mice) exhibit overaccumulation of neuronal Arc, a decrease in synaptic AMPA receptors, and significantly reduced levels of contextual and cued conditioned freezing (Baudry et al., 2012; Greer et al., 2010; Mabb et al., 2014; Tai and Schuman 2010). However, Arc knockout mice—which show impaired late-phase LTP and reduced LTD plasticity—also exhibit significantly reduced levels of both cue and context conditioned freezing (Plath et al., 2006). Hence, either overexpression or ablation of Arc can interfere with normal synaptic plasticity mechanisms and memory, which may indicate a mechanism by which Arc can differentially regulate AMPA receptor trafficking with temporal specificity (Bramham et al., 2008; Shepherd & Bear, 2011). This finding also suggests that transcription and translation of Arc must operate within a narrow range in order for normal synaptic plasticity and memory mechanism to be engaged, and that operation outside of this range may lead to paradoxical reductions in consolidation of fear-related memory. Such effects are often conceptualized as maladaptive, particularly in the context of stress-related conditions such as PTSD, although amnesia in response to stress or trauma may have adaptive elements under some circumstances.
In contrast to expression patterns observed in the CeA and BNST, Arc protein expression in the hippocampus was predominantly found in the dendritic layers of the dorsal hippocampus, where it is known to localize to activated synapses (Steward et al., 1998), and in diffuse processes of the LA/BLA complex. Here, we used optical density measurements to quantify the effects of PACAP+FC on Arc expression but found no differences between treatment groups in any of these areas. This is unexpected, considering that it has been well reported that PACAP can alter NMDA and AMPA-receptor currents, modify phosphorylation of GluA1 AMPA receptor subunits, and affect synaptic strength and metaplasticity in the hippocampus (Ciranna & Cavallaro 2003; Costa et al., 2008; Kondo et al., 1997; Macdonald et al., 2005; Roberto et al., 2001; Ster et al., 2009; Toda et al, 2015; Yang et al., 2010). These previous findings suggest that changes in Arc expression levels should occur after PACAP administration in the current study. Further, other groups have reported increases in Arc protein and mRNA in the hippocampus, LA and BLA after fear conditioning alone (Chau et al., 2013; Gouty-Colomer et al., 2016; Huff et al., 2006; Lonergan et al., 2010; Ploski et al., 2008; Tayler et al., 2011) that were not evident under our experimental conditions. As is the case here with Arc, we previously failed to observe any changes in c-Fos expression in the hippocampus and basolateral amygdala after fear conditioning or PACAP administration alone (Meloni et al., 2016), areas that usually show robust immediate early gene expression after fear conditioning (Milanovic et al., 1998). One potential explanation is that the stressful experience of the surgery to implant the ICV cannula may interact with pathways that mediate immediate-early gene (Arc, c-Fos) expression in these brain areas after fear conditioning, as others have shown that stress and the experience of the animal is important for subsequent immediate early gene expression (Molteni et al., 2010; Radulovic et al., 1998). Our quantification methods are sufficiently sensitive to detect treatment-induced changes in Arc expression in these areas, considering that we were able to detect overall differences between subdivisions of the same brain structure: indeed, BLA and CA3 showed stronger overall Arc expression than the LA and other regions of the hippocampus, respectively. Interestingly, stress itself is not sufficient to alter Arc mRNA levels in the BNST or hippocampus (Ons et al., 2004). This appears consistent with our observations after administration of PACAP—a known stress peptide that is capable of significantly increasing corticosterone levels (Agarwal et al., 2005)—which did not affect Arc levels in any brain areas when given alone (PACAP+No FC treatment condition).
We explored whether the population of Arc-expressing cells in the CeA and BNST after PACAP+FC represents a homogenous grouping of cells that could be identified through co-expression of other neuronal markers. To accomplish this, we examined co-labelling of Arc-positive neurons with the stress peptide CRF and the intracellular protein kinase PKCdelta, both of which are known to be heavily expressed in neurons in the CeL subdivision of the CeA and BNST (Day et al., 1999; Haubensak et al., 2010) where we observed heaviest Arc expression. Arc-positive cells appeared to be a separate population from CRF-containing cells in both the CeA and BNST, but did co-label with PKCdelta in both areas and to approximately the same extent (~37% of Arc-positive neurons were also PKCdelta-positive). More exhaustive future analyses may reveal that the remainder (and majority) of Arc-positive cells in the CeA and BNST co-express other neuropeptides such as enkephalin, neurotensin or somatostatin, which are also found in high densities in these areas (Day et al., 1999; Li et al., 2013). In a series of reports examining microcircuits that modulate the flow of fear-related neural input in-and-out of the CeA, separate populations of GABAergic PKCdelta-positive and PKCdelta-negative neurons in the CeL were shown to modulate freezing behavior through intra- (i.e. reciprocal) and inter-CeA subunit inhibition (Ciocchi et al., 2010; Haubensak et al., 2010; Yu et al., 2017). Inhibition of PKCdelta-positive neurons projecting from the CeL to the CeM (the main output nucleus of the CeA) enhances conditioned freezing, as the CeM becomes disinhibited and drives fear responses such as freezing through downstream activation of effector areas (e.g. the periaqueductal gray; Herry & Johansen 2014). Hence, Arc expression and subsequent changes in synaptic plasticity in a subset of this population of neurons could alter neurotransmission, and subsequently the expression of fear, through this microcircuit. However, given that the majority of Arc-positive neurons are not PKCdelta-expressing neurons (~63%), Arc-mediated changes in synaptic plasticity within this other population of CeL neurons (so-called “CeL on” neurons; Haubensak et al., 2010) would also influence the final output to the CeM, and consequently the expression of fear. Coupled with the observation that these changes are also occurring in heterogeneous populations in the BNST, and given the heavy reciprocal connections between the CeA and BNST which may influence behavioral output (Lebow & Chen 2016; Sun et al., 1991), understanding the complete neural mechanisms and pathways by which PACAP alters memory after fear conditioning will require extensive additional research.
The behavioral phenotype observed after administration of PACAP+FC may recapitulate features of peritraumatic dissociation (Candel & Merckelbach, 2004), including amnesia and alterations in memory for various components of the trauma experience. Dissociation is a complex but common symptom of exposure to trauma that involves “disruption in and fragmentation of the usually integrated functions of consciousness, memory, identity, body awareness and perception of self and the environment” (Dorahy & van der Hart, 2015; Lanius et al., 2010) and may be a major risk factor for the development of PTSD (Breh & Seidler, 2007). Based on an extensive literature describing the role of Arc as a “master regulator” of synaptic plasticity and reported deficits in memory when disrupted (Shepherd & Bear, 2011), the results of the current study suggest that memory of the conditioning event may not be accurately consolidated due to PACAP-dependent, Arc-mediated disturbances within and between integrated neural ensembles that normally code emotionally charged aspects of the aversive memory. The result is a complex behavioral phenotype that reflects amnestic-like effects early on, but that changes over time with the re-emergence and hyper-expression of freezing after repeated re-experiencing (Meloni et al., 2016). Given accumulating evidence suggesting an association between dysfunction within brain PACAP systems and PTSD (Pohlack et al 2015; Ressler et al., 2011), the current study may provide new insight on the pathogenic mechanisms involved in this disorder, including the dissociative subtype of PTSD or other disorders that involve stress and emotional trauma such as dissociative identity disorder (Sar, 2014). Future studies designed to examine the relationship between alterations in PACAP systems (e.g. peptide levels in blood or cerebrospinal fluid, genetic aberrations in PACAP or PACAP receptor-coding genes; Cooper et al., 2013; Wang et al., 2013) and dissociative disorders would be a first step that could potentially lead to identification of an objective biomarker for diagnoses and help facilitate development of more effective strategies to treat these conditions.
Supplementary Material
Supplementary Figure 1. High magnification (100x) confocal microscope 3-D image through the central nucleus of the amygdala (CeA) showing Arc-positive cells (green) co-labelled with PCKdelta (red).
Highlights.
PACAP increased activity-regulated cytoskeleton associated protein after fear conditioning
Significant increases were seen in the extended amygdala but not the hippocampus
Expression was co-localized with PKCdelta in the extended amygdala
Freezing behavior was significantly reduced in PACAP treated rats one day after fear conditioning
Acknowledgments
This research was funded by National Institutes of Health grant MH097860 (WAC).
Footnotes
Conflicts of interest:
WAC has served as a paid consultant for Psy Therapeutics within the past 2 years. The other authors report no disclosures relevant to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Agarwal A, Halvorson LM, Legradi G (2005). Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Molecular Brain Research,138, 45–57. 10.1016/j.molbrainres.2005.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience, 27, 1–39. 10.1016/0306-4522(88)90217-5 [DOI] [PubMed] [Google Scholar]
- Baudry M, Kramer E, Xu X, Zadran H, Moreno S, Lynch G, Gall C, Bi X (2012). Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiology of Disease, 47, 210–215. 10.1016/j.nbd.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely E, Scheich B, Helyes Z (2013). Neuropeptides in learning and memory. Neuropeptides, 47, 439–450. 10.1016/j.npep.2013.10.012 [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF (2008). The immediate early gene Arc/Arg3.1: regulation, mechanisms, and function. Journal of Neuroscience, 28,11760–11767. 10.1523/JNEUROSCI.3864-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breh DC, Seidler GH (2007). Is peritraumatic dissociation a risk factor for PTSD? Journal of Trauma & Dissociation, 8, 53–69. https://doi-org.ezpprod1.hul.harvard.edu/10.1300/J229v08n01_04 [DOI] [PubMed] [Google Scholar]
- Candel I, Merckelbach H (2004). Peritraumatic dissociation as a predictor of post-traumatic stress disorder: a critical review. Comprehensive Psychiatry, 45, 44–50. 10.1016/j.comppsych.2003.09.012 [DOI] [PubMed] [Google Scholar]
- Chau LS, Prakapenka A, Fleming SA, Davis AS, Galvez R (2013). Elevated Arc/Arg 3.1 protein expression in the basolateral amygdala following auditory trace-cued fear conditioning. Neurobiology of Learning and Memory, 106, 127–133. 10.1016/j.nlm.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Cho JH, Zushida K, Shumyatsky GP, Carlezon WA Jr., Meloni EG, Bolshakov VY (2012). Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. Journal of Neuroscience, 32, 14165–14177. 10.1523/JNEUROSCI.1402-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdury S, Shepher JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF (2006). Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron, 52, 445–459. 10.1016/j.neuron.2006.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature, 468, 277–282. doi: 10.1038/nature09559 [DOI] [PubMed] [Google Scholar]
- Ciranna L, Cavallaro S (2003). Opposing effects by pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide on hippocampal synaptic transmission. Experimental Neurology, 184, 778–784. 10.1016/S0014-4886(03)00300-5 [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Narasimhan S, Rickels K, Lohoff FW (2013). Genetic polymorphisms in the PACAP and PAC1 receptor genes and treatment response to venlafaxine XR in generalized anxiety disorder. Psychiatric Research, 210, 1299–1300. 10.1016/j.psychres.2013.07.038 [DOI] [PubMed] [Google Scholar]
- Costa L, Santangelo F, Li Volsi G, Ciranna L (2009). Modulation of AMPA receptormediated ion currents by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus. Hippocampus, 19, 99–109. DOI: 10.1002/hipo.20488 [DOI] [PubMed] [Google Scholar]
- Davis GW (2006). Homeostatic control of neural activity: from phenomenology to molecular design. Annual Review of Neuroscience, 29, 307–323. https://doi-org.ezpprod1.hul.harvard.edu/10.1146/annurev.neuro.28.061604.135751 [DOI] [PubMed] [Google Scholar]
- Day HEW, Curran EJ, Watson SJ, Akil H (1999). Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1β. Journal of Comparative Neurology, 413, 113–128. DOI: [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K (2009). VPAC and PAC receptors: from ligands to function. Pharmacology & Therapeutics, 121, 294–316. 10.1016/j.pharmthera.2008.11.006 [DOI] [PubMed] [Google Scholar]
- Dorahy MJ, van der Hart O (2015). DSM-5’s ‘PTSD with dissociative symptoms: challenges and future directions. Journal of Trauma & Dissociation, 16, 7–28. https://doi-org.ezp-prod1.hul.harvard.edu/10.1080/15299732.2014.908806 [DOI] [PubMed] [Google Scholar]
- Figge DA, Rahman I, Dougherty PJ, Rademacher DJ (2013). Retrieval of contextual memories increases activity-regulated cytoskeleton-associated protein in the amygdala and hippocampus. Brain Structure and Function, 218, 1177–1196. https://doi-org.ezp-prod1.hul.harvard.edu/10.1007/s00429-012-0453-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim T-K, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME (2010). The Angelman syndrome protein Ube3A regulates synapse development by ubiquitinating Arc. Cell, 140, 704–716. DOI: 10.1016/j.cell.2010.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Maren S (2017). Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learning and Memory, 24, 480–491. doi: 10.1101/lm.044206.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouty-Colomer LA, Hosseini B, Marcelo IM, Schreiber J, Slump DE, Yamaguchi S, Houweling AR, Jaarsma D, Elgersma Y, Kushner SA (2016). Arc expression identifies the lateral amygdala fear memory trace. Molecular Psychiatry, 21, 364–375. doi: 10.1038/mp.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong H-W, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ (2010). Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature, 468, 270–276. doi: 10.1038/nature09553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, May V (2014). Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biological Psychiatry, 78, 167–177. 10.1016/j.biopsych.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J (2002). Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. Journal of Comparative Neurology, 453, 389–417. doi: 10.1002/cne.10418 [DOI] [PubMed] [Google Scholar]
- Herry C, Johansen JP (2014). Encoding of fear learning and memory in distributed neuronal circuits. Nature Neuroscience, 17, 1644–1654. doi: 10.1038/nn.3869 [DOI] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW (2006). Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. Journal of Neuroscience, 26, 1616–1623. 10.1523/JNEUROSCI.4964-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Seiglie M, Blasio A, Cottone P, Sabin V (2016). Pituitary adenylate cyclaseactivating polypeptide (PACAP) in the central nucleus of the amygdala induces anxiety via melanocortin receptors. Psychopharmacology, 233, 3269–3277. doi: 10.1038/nn.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI (2004). Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. Journal of Comparative Neurology, 476, 388–413. doi: 10.1007/s00213-016-4366-y [DOI] [PubMed] [Google Scholar]
- Kondo T, Tominaga T, Ichikawa M, Iijima T (1997). Differential alteration of hippocampal synaptic strength induced by pituitary adenylate cyclase activating polypeptide-38 (PACAP-38). Neuroscience Letters, 221, 189–192. 10.1016/S0304-3940(96)13323-1 [DOI] [PubMed] [Google Scholar]
- Korb E, Wilkinson CL, Delgado RN, Lovero KL, Finkbeiner S (2013). Arc in the nucleus regulates PML-dependent GluA1 transcription and homeostatic plasticity. Nature Neuroscience, 16, 874–883. doi: 10.1038/nn.3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, Arimura A (1998). The source of origin of PACAP- and VIP-immunoreactive fibers in the laterodorsal division of the bed nucleus of the stria terminalis in the rat. Brain Research, 810, 211–219. 10.1016/S0006-8993(98)00692-1 [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D (2010). Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry, 167, 640–647. https://doi-org.ezp-prod1.hul.harvard.edu/10.1176/appi.ajp.2009.09081168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, Chen A (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21, 450–463. doi: 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM (2007). Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plasticity, Article ID 79102, 10.1155/2007/79102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B (2013). Experience-dependent modification of a central amygdala fear circuit. Nature Neuroscience, 16, 332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Pehrson AL, Waller JA, Dale E, Sanchez C, Gulinello M (2015). A critical evaluation of the activity regulated cytoskeleton-associated protein (Arc/Arg3.1)’s putative role in regulating dendritic plasticity, cognitive processes, and mood in animal models of depression. Frontiers in Neuroscience, 9, 279. doi: 10.3389/fnins.2015.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan ME, Gafford GM, Jarome TJ, Helmstetter FJ (2010) Time-dependent expression of Arc and Zif268 after acquisition of fear conditioning. Neural Plasticity, Article ID 139891. 10.1155/2010/139891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabb AM, Je HS, Wall MJ, Robinson CG, Larsen RS, Qiang Y, Correa SAL, Ehler MD (2014). Triad3A regulates synaptic strength by ubiquitination of Arc. Neuron, 82, 1299–1316. DOI: 10.1016/j.neuron.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF (2005). Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires Gαq, protein kinase C, and activation of Src. Journal of Neuroscience, 25, 11374–11384. PMID: 16339032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ (2012). Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in Neuroscience, 35, 24–35. 10.1016/j.tins.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14, 417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Gillis TE, Manoukian J, Kaufman MJ (2014). Xenon impairs reconsolidation of fear memories in a rat model of post-traumatic stress disorder (PTSD). PLoS One. 9 (8): e106189. doi: 10.1371/journal.pone.0106189. 10.1371/journal.pone.0106189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Venkataraman A, Donahue RJ, Carlezon WA (2016). Bi-directional effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on fear-related behavior and c-Fos expression after fear conditioning in rats. Psychoneuroendocrinology, 151, 359–367. 10.1016/j.psyneuen.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J (1998). Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Research, 784, 37–47. 10.1016/S0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Missig G, Roman CW, Vizzard MA, Braas KM, Hammack SE, May V (2014). Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology, 86, 38–48. 10.1016/j.neuropharm.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Chourbaji S, Brandwein C, Racagni G, Gass P, Riva MA (2010). Depression-prone mice with reduced glucocorticoid receptor expression display an altered stress-dependent regulation of brain-derived neurotrophic factor and activity-regulated cytoskeleton-associated protein. Journal of Psychopharmacology, 24, 595–603. DOI: 10.1177/0269881108099815 [DOI] [PubMed] [Google Scholar]
- Montero M, Yon L, Kikuyama S, Dufour S, Vaudry H (2000). Molecular evolution of the growth hormone-releasing hormone/pituitary adenylate cyclase-activating polypeptide gene family. Functional implication in the regulation of growth hormone secretion. Journal of Molecular Endocrinology, 25, 157–168. doi: 10.1677/jme.0.0250157. [DOI] [PubMed] [Google Scholar]
- Nikolaienko. O, Patil S, Eriksen MS, Bramham CR (2017). Arc protein: a flexible hub for synaptic plasticity and cognition. Seminars in Cell & Developmental Biology. 10.1016/j.semcdb.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Ons S, Marti O, Armario A (2004). Stress-induce activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. Journal of Neurochemistry, 89, 1111–1118. DOI: 10.1111/j.1471-4159.2004.02396.x [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, (2004) The Rat Brain in Stereotaxic Coordinates, sixth ed. New York: Academic Press. [Google Scholar]
- Pelrine E, Pasik SD, Bayat L, Goldschmiedt D, Bauer EP (2016). 5-HT2C receptors in the BNST are necessary for the enhancement of fear learning by selective serotonin reuptake inhibitors. Neurobiology of Learning and Memory, 136,189–195. 10.1016/j.nlm.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosi MR, Lipp H-P, Grant SGN, Bliss TVP, Wolfer DP, Kuhl D (2006). Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron, 52, 437–444. 10.1016/j.neuron.2006.08.024 [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106, 274–285. http://dx.doi.org.ezp-prod1.hul.harvard.edu/10.1037/0735-7044.106.2.274 [DOI] [PubMed] [Google Scholar]
- Piggins HD, Stamp JA, Burns J, Rusak B, Semba K (1996). Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. Journal of Comparative Neurology, 376, 278–294. doi: [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Park K, Monsey MS, Overeem KA, Schafe GE (2008). The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. Journal of Neuroscience, 28, 12383–12395. 10.1523/JNEUROSCI.1662-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Cacciaglia R, Winkelmann T, Schad LR, Witt SH, Rietschel M, Flor H (2015). Neural mechanism of a sex-specific risk variant for posttraumatic stress disorder in the type I receptor of the pituitary adenylate cyclase activating polypeptide. Biological Psychiatry, 78,840–847. 10.1016/j.biopsych.2014.12.018 [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J (1998). Relationship between Fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. Journal of Neuroscience,18, 7452–7461. PMID: 9736664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinder S, Burghardt NS, Brodsky R, Bauer EP, Chattarji S (2013). A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment. Translational Psychiatry, 3, e209. doi: 10.1038/tp.2012.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 470, 492–497. doi: 10.1038/nature09856. Erratum in: Nature. 2011 477, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT (2006). Increased expression of the immediate-early gene Arc/Arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron, 52, 461–474. 10.1016/j.neuron.2006.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Scuri R, Brunelli M (2001). Differential effects of PACAP-38 on synaptic responses in rat hippocampal CA1 region. Learning & Memory, 8, 265–271. 10.1101/lm.40501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Lezak KR Hartsock MJ, falls WA, Braas KM, Howard AB, Hammack SE, May V (2014). PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology, 47, 151–165. 10.1016/j.psyneuen.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R (2005). Postsynaptic receptor trafficking underlying a form of associative learning. Science, 308, 83–88. DOI: 10.1126/science.1103944 [DOI] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Palmiter RD, Clark M Zweifel LS (2017) A central amygdala CRF circuit facilitates learning about weak threats. Neuron, 93, 164178 10.1016/j.neuron.2016.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar V (2014). The many faces of dissociation: opportunities for innovative research in psychiatry. Clinical Psychopharmacology and Neuroscience, 12, 171–179. DOI: 10.9758/cpn.2014.12.3.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SD, Myskiw JC, Furini CR, Schmidt BE, Cavalcante LE, Izquierdo I (2015). PACAP modulates the consolidation and extinction of the contextual fear conditioning through NMDA receptors. Neurobiology of Learning and Memory, 118, 120–124. 10.1016/j.nlm.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF (2011). New views of Arc, a master regulator of synaptic plasticity. Nature Neuroscience, 14, 279–284. doi: 10.1038/nn.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF (2006). Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron, 52, 475–484. 10.1016/j.neuron.2006.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ster J, de Bock F, Bertaso F, Abitbol K, Daniel H, Bockaert J, Fagni L (2009). Epac mediates PACAP-dependent long-term depression in the hippocampus. Journal of Physiology, 587, 101–113. 10.1113/jphysiol.2008.157461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF (1998). Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron, 21, 741–751. DOI: 10.1016/S0896-6273(00)80591-7 [DOI] [PubMed] [Google Scholar]
- Steward O, Farris S, Pirbhoy PS, Darnell J, Van Driesche SJ, (2015). Localization and local translation of Arc/Arg3.1 mRNA at synapses: some observation and paradoxes. Frontiers in Molecular Neuroscience, 7, 1–15. doi: 10.3389/fnmol.2014.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD (1991). Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Research Bulletin, 27, 651–662. DOI: 10.1016/0361-9230(91)90041-H [DOI] [PubMed] [Google Scholar]
- Tai H-C, Schuman EM (2010). Angelman syndrome:finding the lost Arc. Cell, 140, 608–610. DOI: 10.1016/j.cell.2010.02.019 [DOI] [PubMed] [Google Scholar]
- Tayler KK, Lowry E, Tanaka K, Levy B, Reijmers L, Mayford M, Wiltgen BJ, (2011). Characterization of NMDAR-independent learning in the hippocampus. Frontiers in Behavioral Neuroscience, 5,28. doi: 10.3389/fnbeh.2011.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda AMA, Huganir RL (2015). Regulation of AMPA receptor phosphorylation by the neuropeptide PACAP38. Proceedings of the National Academy of Sciences, 112, 6712–6717. https://doi-org.ezp-prod1.hul.harvard.edu/10.1073/pnas.1507229112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, Morita Y, Tanida M, Tajiri M, Hazama K, Ogata K, Hashimoto H, Baba A (2011). PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress, 14, 368–375. DOI: 10.3109/10253890.2010.544345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis A, Nicoll R (2006). Arc/Arg3.1: Linking gene expression to synaptic plasticity and memory. Neuron, 52, 403–407. 10.1016/j.neuron.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H, (2009). Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacological Reviews, 61, 283–357. 10.1124/pr.109.001370 [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of Neuroscience, 17, 9375–9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY (2013). PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. Journal of Affective Disorders, 150, 156–159. 10.1016/j.jad.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM (2008). Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron, 59, 84–97. 10.1016/j.neuron.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JR, Albanesi JP, Huber KM (2017). Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: implications in health and disease. Seminars in Cell & Developmental Biology 10.1016/j.semcdb.2017.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Ahrens S, Zhang X, Schiff H, Ramakrishnan C, Fenno L, Deisseroth K, Zhao F, Luo M-H, Gong L, He M, Zhou P, Paninski L, Li B (2017). The central amygdala controls learning in the lateral amygdala. Nature Neuroscience, 20, 1680–1685. doi: 10.1038/s41593-017-0009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Lei G, Jackson MF, Macdonald JF, 2010. The involvement of PACAP/VIP system in the synaptic transmission in the hippocampus. Journal of Molecular Neuroscience, 42, 319–326. doi: 10.1007/s12031-010-9372-7 [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow M (2014). Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. Journal of Neuroscience, 34, 8462–8466. 10.1523/JNEUROSCI.3624-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Shioda S, Yada T, Inagaki N, Pleasure SJ, Kikuyama S (2002). PACAP and its receptors exert pleiotropic effects in the nervous system by activating multiple signaling pathways. Current Protein and Peptide Science, 3, 423–439. doi: 10.2174/1389203023380576 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. High magnification (100x) confocal microscope 3-D image through the central nucleus of the amygdala (CeA) showing Arc-positive cells (green) co-labelled with PCKdelta (red).