Abstract
Background
Identification of the causes of stillbirth is critical to the primary prevention of stillbirth and to the provision of optimal care in subsequent pregnancies. A wide variety of investigations are available, but there is currently no consensus on the optimal approach. Given their cost and potential to add further emotional burden to parents, there is a need to systematically assess the effect of these interventions on outcomes for parents, including psychosocial outcomes, economic costs, and on rates of diagnosis of the causes of stillbirth.
Objectives
To assess the effect of different tests, protocols or guidelines for investigating and identifying the causes of stillbirth on outcomes for parents, including psychosocial outcomes, economic costs, and rates of diagnosis of the causes of stillbirth.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (31 August 2017), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (15 May 2017).
Selection criteria
We planned to include randomised controlled trials (RCTs), quasi‐RCTs, and cluster‐RCTs. We planned to include studies published as abstract only, provided there was sufficient information to allow us to assess study eligibility. We planned to exclude cross‐over trials.
Participants included parents (including mothers, fathers, and partners) who had experienced a stillbirth of 20 weeks' gestation or greater.
This review focused on interventions for investigating and identifying the causes of stillbirth. Such interventions are likely to be diverse, but could include:
* review of maternal and family history, and current pregnancy and birth history; * clinical history of present illness; * maternal investigations (such as ultrasound, amniocentesis, antibody screening, etc.); * examination of the stillborn baby (including full autopsy, partial autopsy or noninvasive components, such as magnetic resonance imaging (MRI), computerised tomography (CT) scanning, and radiography); * umbilical cord examination; * placental examination including histopathology (microscopic examination of placental tissue); and * verbal autopsy (interviews with care providers and support people to ascertain causes, without examination of the baby).
We planned to include trials assessing any test, protocol or guideline (or combinations of tests/protocols/guidelines) for investigating the causes of stillbirth, compared with the absence of a test, protocol or guideline, or usual care (further details are presented in the Background, see Description of the intervention).
We also planned to include trials comparing any test, protocol or guideline (or combinations of tests/protocols/guidelines) for investigating the causes of stillbirth with another, for example, the use of a limited investigation protocol compared with a comprehensive investigation protocol.
Data collection and analysis
Two review authors assessed trial eligibility independently.
Main results
We excluded five studies that were not RCTs. There were no eligible trials for inclusion in this review.
Authors' conclusions
There is currently a lack of RCT evidence regarding the effectiveness of interventions for investigating and identifying the causes of stillbirth. Seeking to determine the causes of stillbirth is an essential component of quality maternity care, but it remains unclear what impact these interventions have on the psychosocial outcomes of parents and families, the rates of diagnosis of the causes of stillbirth, and the care and management of subsequent pregnancies following stillbirth. Due to the absence of trials, this review is unable to inform clinical practice regarding the investigation of stillbirths, and the specific investigations that would determine the causes.
Future RCTs addressing this research question would be beneficial, but the settings in which the trials take place, and their design, need to be given careful consideration. Trials need to be conducted with the utmost care and consideration for the needs, concerns, and values of parents and families. Assessment of longer‐term psychosocial variables, economic costs to health services, and effects on subsequent pregnancy care and outcomes should also be considered in any future trials.
Plain language summary
Interventions for investigating and identifying the causes of stillbirth
What is the issue?
There are many causes of stillbirth, including the mother having high blood pressure or diabetes before the pregnancy, an infection such as malaria, HIV or syphilis, congenital abnormalities in the baby, issues with how well the placenta is functioning, and pregnancy continuing past the due date. Sometimes a baby dies as a result of multiple causes. The death of a baby to stillbirth is a devastating event for parents, families, and communities. To prevent stillbirths, we need to understand more about why they occur. Understanding why a baby died may also help parents to cope with their grief, and assist them in care planning for future pregnancies.
Many different tests and investigations can be done to help find out why a baby died. These tests and investigations differ in the level of expertise required, how invasive they are, and their economic costs. Tests, procedures or guidelines for investigating and identifying the causes of stillbirth include looking at the medical history of the parents, any problems during the pregnancy, maternal investigations (such as ultrasound, amniocentesis, antibody screening), examination of the stillborn baby, examination of the umbilical cord and placenta, and interviews with care providers and support people to determine causes without examination of the baby (verbal autopsy). Currently there is no standard approach to investigating the causes of stillbirth.
Why is this important?
Searching for causes of stillbirth can be difficult emotionally for families, and financially costly to health services and sometimes to parents. Some tests and investigations may be more helpful than others in identifying the causes of stillbirth. There is a need to assess systematically which approaches are most helpful in finding causes of stillbirth, how cost‐effective the different approaches are, what the emotional and social effects on parents are, what impact the investigations have on future pregnancies, and the end result of future pregnancies.
What evidence did we find?
We searched for evidence on 15 May 2017. We did not find any trials for inclusion in this review. We excluded five trials because they were not randomised controlled trials.
What does this mean?
There is no evidence available to guide how best to investigate the causes of stillbirth. Seeking to determine the causes of a baby's death is an essential component of quality maternity care in any setting. Future trials on this topic would be helpful, but such trials would need to be designed in a way that ensures all parents in the trial still receive the minimum standard of care in their local setting. Future trials would need to be conducted with the utmost care and consideration for the needs, concerns, and values of parents and families. Assessment of longer‐term psychosocial variables, economic costs to health services, and effects on subsequent pregnancy care and outcomes should be considered in any future trials.
Background
Description of the condition
Stillbirth is associated with profound and long‐lasting adverse psychosocial outcomes for families and care providers, along with wider economic impacts on health systems and society (Heazell 2016). The global burden of stillbirth is high, with an estimated 2.6 million stillbirths (at 28 weeks' gestation or greater) occurring every year. Although many of these deaths are preventable, global reduction in stillbirth rates remains slow and has not matched declines in maternal or child mortality (Lawn 2016).
Accurate data on causes of stillbirth are limited, partly due to the difficulty in assigning causation, often owing to multifactorial circumstances (Silver 2007; Whitfield 1986). The use of various, disparate classification systems for assigning cause of death also hampers understanding of causes at global and regional levels (Flenady 2015; Flenady 2017; Leisher 2016; Wojcieszek 2016). Flenady 2016 showed wide variation in the contribution of different causes of stillbirth. For example, the proportion of stillbirths related to infection ranged from 5% to 22%, and the proportion of stillbirths related to congenital abnormalities ranged from 6% to 27%. "Unexplained" deaths were reported for up to 76% of cases, and showed particularly wide variation. In a global review of 85 national stillbirth reports across 50 countries, "unexplained" was the most commonly reported cause of death category among the 489,089 stillbirths included (Reinebrant 2017).
Difficulties ascertaining causes of death are often compounded by limited availability of clinical information. In some low‐ and middle‐income countries (LMICs), minimal or no diagnostic investigations are available (Flenady 2010; McClure 2018). Many stillbirths are not reported, especially among the large proportion of births occurring outside formal health services. Despite these limitations, it is clear that over half of stillbirths (globally) are related to intrapartum complications, and that increased access to skilled birth attendants and emergency obstetric care could eliminate most of these deaths (Lawn 2016). Infections such as malaria and syphilis and placental conditions such as poor growth of the baby (also called fetal growth restriction (FGR)), placental abruption (when the placenta breaks away from the wall of the uterus), and pre‐eclampsia (high blood‐pressure and protein in the urine) are other commonly reported causes of stillbirth. Pre‐existing hypertension and pre‐existing diabetes are also common, important risk factors for stillbirth. These risk factors are often associated with obesity and maternal age over 35 years, which are independent risk factors for stillbirth that are also increasingly common throughout the developed and developing worlds (Flenady 2011). Other major risk factors for stillbirth include smoking, primiparity (where a woman is giving birth for the first time), multiple pregnancy, previous stillbirth (Flenady 2011), and post‐term pregnancy (Flenady 2011; Lawn 2016).
Although the burden of stillbirth lies predominantly in LMICs, thousands of potentially preventable stillbirths also occur in high‐income countries (HICs) (Flenady 2016). In these settings, most stillbirths occur in the antenatal period, and are associated with placental dysfunction (Flenady 2016). Disparities in stillbirth rates are clearly evident, with the risk of stillbirth among disadvantaged women roughly double that of more advantaged women (Flenady 2016).
Description of the intervention
Accurate determination of causes and contributing factors is needed to reduce stillbirth rates. To identify causes of death, collection of data related to the stillbirth, such as demographic data, maternal risk factors and labour and birth information are required (Barfield 2011; Flenady 2018). A range of investigations, as described in further detail below, may also be used.
This review focused on interventions for investigating and identifying the causes of stillbirth. Such interventions are diverse, but may include:
review of maternal and family history, and current pregnancy and birth history;
clinical history of present illness;
maternal investigations (such as ultrasound, amniocentesis, antibody screening, etc);
examination of the stillborn baby (including full autopsy, partial autopsy or noninvasive components, such as magnetic resonance imaging (MRI), computerised tomography (CT) scanning, and radiography);
umbilical cord examination;
placental examination including histopathology (microscopic examination of placental tissue); and
verbal autopsy (interviews with care providers and support people to ascertain causes, without examination of the baby).
Among the investigations available to parents and clinicians, autopsy is considered the 'gold standard' in determining causes of death (Alderliesten 2003; Lyon 2004; Rose 2006). Autopsy can identify a wide range of causes of stillbirth, including infection, anaemia, and morphologic (structural) and metabolic (relating to metabolism) abnormalities (Silver 2007). However, there is variation in the reported yield of the procedure following a stillbirth (Gordijn 2002). Furthermore, many factors influence parents' decision regarding whether to consent to autopsy (Breeze 2012), and some parents may decline to avoid subjecting their baby to invasive examination (Lyon 2004). Cultural and religious practices, such as the requirement of prompt burial or for the baby's body to be left undisturbed, or both, may also influence decision making (Gordijn 2007). In many cases, whether to consent to an autopsy of their baby is one of the most difficult and pressing decisions facing parents immediately following stillbirth. The information and counselling parents receive at this time, and their interactions with care providers, can impact upon their grief and longer‐term psychosocial functioning (Burden 2016; Heazell 2012). Some parents later regret their decision regarding an autopsy of their baby (Heazell 2012; Rankin 2002), which may only add to parents' emotional suffering. Despite the need, there are currently no interventions to support parents' decision making around this procedure (Horey 2013). Medical personnel are often reluctant to approach parents about autopsy for various reasons, including emotional burden (Khong 2010; Rose 2006) or because they do not believe the investigation will yield any new information, or both (Lyon 2004; Rose 2006). Therefore, when full autopsy is not possible, alternative investigations such as partial autopsy, minimally invasive tissue sampling (Castillo 2015), fetal CT scan, fetal radiography (Lim 2005), and fetal MRI (Alderliesten 2003; Arthurs 2015; Thayyil 2013; Vullo 2016) may be performed.
Alongside autopsy, examination of the placenta and testing for chromosomal abnormalities have shown high value in ascertaining causes of death (Bukowski 2011; Korteweg 2008; Korteweg 2012). Other investigations that may be used in the routine evaluation of stillbirth include (but are not limited to) maternal thyroid, liver and kidney function tests, testing for gestational diabetes (Flenady 2018), toxicology screening (to detect maternal drug use), and tests for various infections and viruses (Silver 2007). Investigations to identify maternal antibodies and blood clotting disorders (thrombophilias) may be informative in some situations.
As stated earlier, the ability to undertake certain investigations may be extremely limited in some settings, due to a lack of resources and low(er) attendance at health services (Flenady 2010). Financial costs incurred by health services can be a significant barrier to performing a thorough investigation following stillbirth. As shown in a UK study (Mistry 2013), the cost of diagnostic investigations following stillbirth may as high as GBP 1804 per pregnancy, with perinatal autopsy being the most costly component. Many such sophisticated diagnostic investigations are simply unavailable in LMICs. In these settings, verbal autopsy (involving interviews with care providers and support people, without examination of the baby) may be used as an indirect method of ascertaining cause of death (Nausheen 2013). Therefore, it is also important to identify which tests are feasible in a setting where economic cost and access to equipment are barriers.
How the intervention might work
A high‐quality investigation into the causes of stillbirth will ideally place minimal emotional and financial burden on parents, staff, and health services, while maintaining high diagnostic yield (Corabian 2005; Lim 2005; Silver 2007). A number of formal protocols are currently in use to standardise investigations following stillbirth but, as shown in a systematic review (Corabian 2005), there is large variation in the investigations that may be recommended. A specific comprehensive investigation protocol has previously been shown to be cost‐effective when incorporated into routine stillbirth evaluation (Michalski 2002). Comprehensive investigation protocols may be advantageous where there are multiple, interacting causes which may not otherwise be detected (Silver 2007). Another approach to the workup of stillbirth is selective testing, where investigations are undertaken based on clinical features and presumed diagnosis (Flenady 2010; Lim 2005). A selective approach to investigations may reduce economic costs (Lim 2005), while also minimising the chance of producing incidental positive test results that cause anxiety among parents and are unhelpful in ascertaining a cause of death (Korteweg 2012; Silver 2007). Sequential testing, where tests are undertaken based on the results of other tests, has been proposed as another alternative (Flenady 2010; Korteweg 2012). This approach suggests a platform of basic tests with follow‐up investigations undertaken as indicated (Korteweg 2012). For example, while routine thrombophilia testing is not commonly recommended, it may be considered where there is evidence of placental complications, such as FGR or placental abruption (Korteweg 2010; Silver 2007).
High‐quality tests, protocols or guidelines for investigating and identifying the causes of stillbirth have the potential to reduce the number of unexplained deaths and misdiagnosed causes of death. Investigations may also have value in confirming clinical diagnoses, providing additional information that may not have been expected from basic clinical information (Gordijn 2002; Horn 2004), and excluding specific causes of death (Horn 2004; Korteweg 2012). Relying solely on death certificates to identify causes of death is problematic. Death certificates are not required for stillbirths in many LMICs, and many births occur outside formal health services, and not reported in vital statistics (Flenady 2015). Even in high‐income settings, the causes of death documented on death certificates are frequently inaccurate or incomplete (Cockerill 2012; Headley 2009; Measey 2007). Retrospective cohort studies have shown that the proportion of deaths initially classified as unexplained can be reduced by up to 65% following investigation and clarification (Headley 2009; Measey 2007).
Accurate identification of causes of stillbirth may not only aid in emotional closure for parents, it may also provide a platform for clinical management in subsequent pregnancies (Flenady 2018; Michalski 2002; Silver 2007). Indeed, the need for information to plan future pregnancies is one of the most important factors that influences parents' decision regarding whether to have an autopsy of their baby (Breeze 2012) and is highly likely to affect their satisfaction with care. The identification of a known recurrent cause of death, or an unexplained death despite thorough investigation, may prompt additional testing and surveillance in subsequent pregnancies (Mistry 2013). In contrast, the identification of a known non‐recurrent cause may reassure parents and spare them from unnecessary testing and intervention in subsequent pregnancies (Silver 2007). The Mistry 2013 study showed pregnancies subsequent to stillbirths that were unexplained or had known recurrent causes were GBP 500 more costly than pregnancies subsequent to stillbirths due to known non‐recurrent causes. The study suggested that while a comprehensive workup of stillbirth may bring a higher initial cost to health systems, the economic costs of care in subsequent pregnancies may be reduced if investigations can exclude recurrent causes and reduce unexplained deaths (Mistry 2013). Importantly, the quality of the postmortem investigation and report, regardless of the investigations performed, will affect the likelihood of yielding clinically meaningful information (Cartlidge 1995; Corabian 2005).
Why it is important to do this review
The prevention of stillbirth requires an understanding of its causes. Understanding of the causes of stillbirth is also critical to the psychosocial well‐being of bereaved parents, and to the planning of management of their subsequent pregnancies, including counselling about recurrence risks. Diagnostic investigations aim to meet these needs, but there is currently no consensus on the optimal approach to investigations, including even the most basic set of key minimal investigations recommended (Korteweg 2012; Lim 2005). Given the wide variety of investigations that are potentially available, their economic cost, and potential to add further to parents' emotional burden, there is a need to systematically assess the effect of these interventions on outcomes for families, including psychosocial outcomes, and on rates of diagnosis of the causes of stillbirth.
Objectives
To assess the effect of different tests, protocols or guidelines for investigating and identifying the causes of stillbirth on outcomes for parents, including psychosocial outcomes, economic costs, and rates of diagnosis of the causes of stillbirth.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs), quasi‐RCTs, and cluster‐RCTs. We excluded cross‐over trials. We planned to include studies published as abstract only, provided there was sufficient information to allow us to assess study eligibility.
Types of participants
We planned to include parents (including mothers, fathers, and partners) who had experienced a stillbirth of 20 weeks' gestation or greater.
Types of interventions
We planned to include trials assessing any test, protocol or guideline (or combinations of tests/protocols/guidelines) for investigating the causes of stillbirth, compared with the absence of a test, protocol or guideline, or usual care (see Description of the intervention for further details about types of tests or investigations that might be included in protocols or guidelines).
We also planned to include trials comparing any test, protocol or guideline (or combinations of tests/protocols/guidelines) for investigating the causes of stillbirth with another (for example, the use of a limited investigation protocol compared with a comprehensive investigation protocol).
Types of outcome measures
No core outcomes were identified for this review topic. We therefore selected a range of important outcomes with respect to the effects of investigations, as below.
Primary outcomes
Change in the final cause(s) of death from the presumed or clinical a priori cause of death.
Final cause(s) of death unknown.
Additional parental counselling or subsequent pregnancy care information (e.g. determination of or change in recurrence risk; exclusion of suspected causes of death; exclusion of recurrent causes of death; or combination of these).
Parental satisfaction with the process and outcomes of investigations.
Secondary outcomes
In postpartum period
Confirmation of clinical diagnosis.
Attainment of unexpected findings.
Exclusion of suspected causes of death.
Exclusion of recurrent causes of death.
Compliance with test/protocol/guideline.
Parental understanding of the cause(s) of death.
Parental regret about the investigations performed.
Parental attitudes towards process and outcomes of investigations.
Parental psychosocial outcomes including anxiety and quality of life.
Parental satisfaction with care around the time of death and at follow‐up.
Care provider satisfaction with the process and outcomes of investigations.
Frequency of investigations performed, e.g. maternal and family history; maternal investigations prior to birth; stillborn examination; umbilical cord examination; placental examination.
Costs of investigations (economic).
In subsequent pregnancy
Stillbirth.
Neonatal death.
Preterm birth.
Induction of labour.
Caesarean birth.
Low birthweight.
Parental anxiety.
Costs of the subsequent pregnancy (economic).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (31 August 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the 'Specialized Register' section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences; and
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results were screened by two review authors and the full text of all relevant trial reports identified through the searching activities described above were reviewed. Based on the intervention described, each trial report was assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and was then added to the Register. The Information Specialist searched the Register for each review using this topic number rather than keywords. This resulted in a more specific search and any search results would have been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports on 15 May 2017 using the terms in Appendix 1.
Searching other resources
We planned to search the reference lists of retrieved studies for further eligible studies. We did not apply date or language restrictions to any searches.
Data collection and analysis
No eligible studies were identified by the search for this version of the review, but for future updates, we plan to use the methods outlined in Appendix 2. These methods are based on a standard template used by Cochrane Pregnancy and Childbirth.
Results
Description of studies
Results of the search
There were no eligible trials identified from the search strategy within the Cochrane Pregnancy and Childbirth's Trials Register. We retrieved 224 records from ClinicalTrials.gov, equating to 152 trials when duplicates were removed. We retrieved 46 records from ICTRP, equating to 23 trials when duplicates were removed. All records were screened‐out or excluded based on full‐text review (see Figure 1).
1.
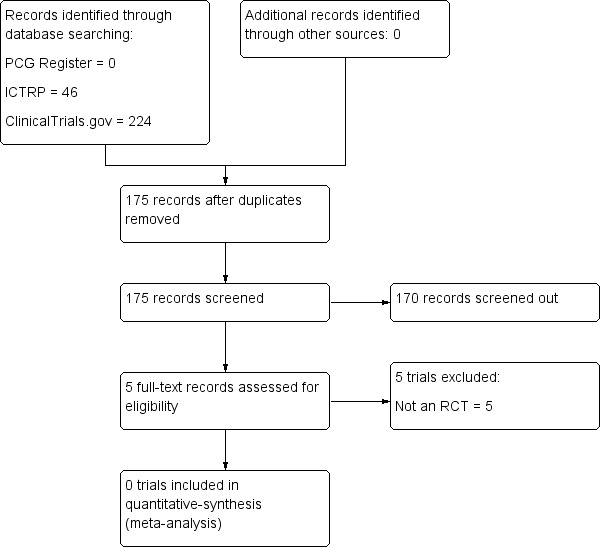
Study flow diagram.
Excluded studies
Five records underwent full‐text review and were excluded based on study design/failure to meet eligibility criteria (see Characteristics of excluded studies). One of these studies (Brasseur‐Daudruy 2014), a non‐randomised trial assessing the effectiveness of MRI as a diagnostic tool following intrauterine fetal death, has since been discontinued due to low recruitment. Another non‐randomised trial (Rüegger 2014) is currently assessing the yield of minimally invasive or 'virtual autopsy' (a combination of postmortem imaging and imaging‐guided biopsies) as compared to conventional autopsy. It is expected that this study will be completed in 2018.
Risk of bias in included studies
Not applicable.
Effects of interventions
Not applicable.
Discussion
There is currently a lack of randomised controlled trial evidence regarding the effectiveness of interventions for investigating and identifying causes of stillbirth. Seeking to understand the causes of stillbirth is fundamental to quality maternity care, but it remains unclear what effect these interventions have on certain outcomes, including psychosocial outcomes for parents and families, rates of diagnosis of the causes of stillbirth, and subsequent pregnancy care and management. This review highlights the challenges clinicians and researchers face in developing high‐quality clinical practice guidelines to guide the investigation of stillbirths.
On the basis of other literature (assessing non‐randomised studies), fundamental components of the investigation of stillbirths appear to include comprehensive maternal (medical, social, family) and pregnancy history, and external examination of the baby. Even where not diagnostic in isolation, these investigations may be highly informative in directing further testing. Perinatal autopsy is often a highly important investigation (Corabian 2005; Gordijn 2002; Headley 2009; Killeen 2004; Korteweg 2012; Measey 2007; Miller 2016; Page 2017; Rossi 2017), alongside placental examination and histopathology (Headley 2009; Heazell 2009; Korteweg 2012; Page 2017). Other investigations, including genetic analyses (Korteweg 2008; Korteweg 2012; Reddy 2012; Rosenfeld 2015) and tests for the detection of maternal‐fetal haemorrhage (the passage of fetal blood cells into the maternal circulation) (Korteweg 2012; O'Leary 2015; Page 2017; Silver 2007), have also shown high value in identifying the causes of stillbirths.
Until appropriately designed randomised controlled trials have been conducted, clinical guidelines regarding approaches to the investigation of stillbirth may continue to be based upon the information yielded from such existing non‐randomised studies, most of which are specific to high‐income countries.
Authors' conclusions
Implications for practice.
Due to the absence of randomised controlled trials, this review is unable to inform clinical practice regarding the effectiveness of interventions for investigating and identifying causes of stillbirth.
Implications for research.
High‐quality clinical trials are needed to measure the effectiveness of interventions for investigating and identifying the causes of stillbirth. However, there are difficulties in conducting randomised trials addressing this research question, due to its ethical and practical complexities. As such, the design of future trials would need to be considered carefully, ensuring that the current standard of care in a given local setting is maintained. It may be appropriate to evaluate a comprehensive investigation protocol against a selective protocol where, in the latter, investigations are limited to those that appear warranted on the basis of clinical history or the suspected diagnosis, or both. Another trial design may involve randomising to a conceal versus reveal protocol, where all pertinent investigations are carried out (and results shared with parents), but the information yielded from certain investigations is either provided or withheld from a panel of experts, depending on the intervention arm.
With either of the options outlined above, trialists would need to ensure clinical equipoise in the given approaches to investigation, both from an ethical standpoint, and to aid in the recruitment of trial participants. Although it was excluded from the current review on the basis of study design, the discontinued Brasseur‐Daudruy 2014 trial highlights the difficulties researchers face in recruiting participants for trials addressing this research question. Therefore, depending on the setting, the need for clinical equipoise may preclude certain types of intervention arms/investigation protocols, such as any that were deemed to be at risk of missing an important diagnosis. Trials evaluating a new investigation protocol against no intervention may be highly informative, but would only be feasible and appropriate in settings where no or minimal stillbirth investigations are currently offered (such as in some low‐ and middle‐income countries (LMICs)).
Notwithstanding, any such trials must be conducted with the utmost care and consideration for the needs, concerns, and values of parents, and should be designed in such a way to allow follow‐up and assessment of longer‐term psychosocial variables, economic costs to health services, and effects on subsequent pregnancy care and outcomes. Effective methods for communicating with bereaved parents about stillbirth risk factors and recommendations for subsequent pregnancy care, based upon the findings of investigations, should also be explored.
There remains a place for a series of Cochrane Diagnostic Test Accuracy reviews in the context of perinatal death, to understand precisely how well specific diagnostic investigations perform in their capacity to discriminate accurately between the true presence and absence of specific conditions (e.g. fetal‐maternal haemorrhage). For such reviews to be carried out, an appropriate reference standard would need to be identified and clearly defined. Such a reference standard might be comprehensive autopsy by a skilled perinatal pathologist coupled with placental examination and histopathology. The influence of subjective clinical criteria for diagnosing certain conditions (e.g. gestational diabetes) should also be assessed.
New software programs enabling quantitative, objective assessment of placental tissue have been reported (Kidron 2017; Ptacek 2016). The use of image analysis software may provide additional information to that received from qualitative assessment alone. Such techniques may assist with standardisation of placental histological examination, but further research into their diagnostic utility is needed (Kidron 2017). Economic costs and feasibility also need to be considered. Developing basic minimal practices for identifying causes of stillbirths in LMICs also remains a priority.
Acknowledgements
We thank the Cochrane Pregnancy and Childbirth Group for support with title registration and protocol development.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), members of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
The following search terms were applied. We ran each line separately and deduplicated manually.
stillbirth AND cause
stillbirth AND causes
miscarriage AND cause
miscarriage AND causes
abortion AND cause
abortion AND causes
pregnancy loss AND cause
pregnancy loss AND causes
Appendix 2. Methods to be used in future updates
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third review author.
We will create a study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We will design a form to extract data. For eligible studies, two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult a third review author. We will enter data into Review Manager software (Review Manager 2014) and check for accuracy. When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third assessor.
1. Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
3.1. Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
3.2. Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
5. Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by 1 to 5 above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
Assessing the quality of the evidence using the GRADE approach
We will use the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons:
change in the final cause(s) of death from the presumed or clinical a priori cause of death;
final cause(s) of death unknown;
additional parental counselling or subsequent pregnancy care information (e.g. determination of or change in recurrence risk; exclusion of suspected causes of death; exclusion of recurrent causes of death; or combination of these);
parental satisfaction with the process and outcomes of investigations;
compliance with test/protocol/guideline;
costs of investigations (economic); and
stillbirth (in the subsequent pregnancy).
We will use the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (Review Manager 2014) in order to create 'Summary of findings' tables. A summary of the intervention effect and a measure of quality for each of the above outcomes will be produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
We plan to include multi‐armed trials, ensuring analyses are independent. If multi‐armed trials are included, we will split the ‘shared’ group into two or more groups with smaller sample size, and include two or more (reasonably independent) comparisons. Alternatively, we will combine groups to create a single pair‐wise comparison.
Cross‐over trials
We will exclude cross‐over designs as these are unlikely to be a valid study design for Pregnancy and Childbirth reviews.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either the Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (Review Manager 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
We plan to consider separately those trials assessing any test/protocol or guideline (or combinations of tests/protocols/guidelines) compared with no test/protocol/guideline or usual care, and those comparing different tests/protocols/guidelines.
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses across the primary outcomes:
number and type of investigations included in the protocol/guideline (e.g. single/limited investigation(s) recommended versus multiple/comprehensive investigations recommended);
quality of the autopsy/postmortem report (e.g. low quality versus high quality, or not meeting a minimum standard versus meeting a minimum standard);
presumed cause(s) of death prior to investigations: unexplained stillbirth at time of death versus stillbirth with known/presumed cause(s);
gestational age at death: death at less than 28 weeks' gestation versus at 28 weeks' gestation or greater;
setting: low‐ and middle‐income countries versus high‐income countries; and
classification systems used.
We will assess subgroup differences by interaction tests available within RevMan (Review Manager 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
Sensitivity analyses will be conducted to explore the effects of trial quality and trial design on the outcomes. We will explore the effects of trial quality assessed by allocation concealment and random sequence generation (considering selection bias), by omitting studies rated as 'high risk of bias' (including quasi‐randomised trials) or 'unclear risk of bias' for these components.
We will investigate the effects of the randomisation unit (individual versus cluster) on the outcomes, and the impact of including studies with high levels of missing data. We will explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity, and the effects of any assumptions made such as the value of the ICC used for cluster‐randomised trials.
We will use primary outcomes in sensitivity analyses.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bellver 2010 | Non‐randomised trial; recurrent spontaneous abortion (defined as first trimester losses of 5 to 14 weeks' gestation) |
| Bloemenkamp 2011 | Non‐randomised trial |
| Brasseur‐Daudruy 2014 | Non‐randomised trial |
| Rüegger 2014 | Non‐randomised trial |
| Sugiura 2013 | Non‐randomised trial |
Differences between protocol and review
Any differences between the published protocol for this review (Wojcieszek 2017) and this full review are listed below.
Additional terms for ICTRP and ClinicalTrials.gov searches (see Appendix 1).
The secondary outcome 'costs of investigations' has been edited to 'Costs of investigations (economic)'.
Contributions of authors
Aleena M Wojcieszek led the drafting of the review with contributions from all authors, including methodological input provided by Vicki Flenady, Philippa Middleton and Emily Shepherd, and content input provided by Vicki Flenady and Katherine J Gold. Aleena M Wojcieszek and Emily Shepherd undertook screening of search records. Vicki Flenady, Philippa Middleton, and Aleena M Wojcieszek designed the protocol with contribution from all authors.
Sources of support
Internal sources
Mater Research Institute, The University of Queensland, Australia.
Robinson Research Institute, The University of Adelaide, Australia.
Women's and Children's Health Research Institute, The University of Adelaide, Australia.
External sources
National Health and Medical Research Council (NHMRC), Australia.
Declarations of interest
Aleena M Wojcieszek: is an Associate Investigator for a National Health and Medical Research Council (NHMRC) Centre of Research Excellence in stillbirth and member of the International Stillbirth Alliance Scientific Advisory Committee executive.
Emily Shepherd: none known.
Philippa Middleton: is a chief investigator for an NHMRC Centre of Research Excellence in stillbirth.
Glenn Gardener: is an associate investigator for an NHMRC Centre of Research Excellence in stillbirth and serves as an unpaid board member for the International Stillbirth Alliance. He was also PI on two NHMRC funded studies in the area of stillbirth (not eligible for inclusion in this review).
David A Ellwood: has received sitting fees from the Australian Medical Council but this work is not related to this Cochrane Review; has received payment for providing expert witness reviews for medico‐legal cases unrelated to the topic under review; is a chief investigator for an NHMRC Centre for Research Excellence in stillbirth.
Elizabeth M McClure: none known.
Katherine J Gold: serves as an unpaid board member for the International Stillbirth Alliance.
Teck Yee Khong: has received fees for expert testimony for plaintiffs and defence in cases related to cerebral palsy and stillbirth; royalties from Springer, London (publishers) in respect of book publication (Fetal and Neonatal Pathology 5th ed); expenses for attending scientific meetings of paediatric pathology societies; and holds shares in one health insurance provider (Medibank) listed in the Australian Stock Exchange.
Robert M Silver: is conducting NIH sponsored research investigating pregnancy as a window to future maternal health, human placental function and clinical obstetric trials. None of these directly address the work for this report. He is also a member of the International stillbirth Alliance Scientific Research Committee.
Jan Jaap HM Erwich: is chair of a foundation for the organisation of conferences on stillbirth and perinatal death, without a fee; received funding across 2002‐2006 to investigate the causes of stillbirth (not eligible for inclusion in this review).
Vicki Flenady: is the lead investigator of the Centre of Research Excellence in Stillbirth in Australia which received funding from the National Health and Medical Research Council in November 2016. Her salary is part‐funded by the MHMRC through a Career Development Fellowship.
New
References
References to studies excluded from this review
Bellver 2010 {published data only}
- Bellver J, Meseguer M, Muriel L, García‐Herrero S, Barreto MA, Garda AL, et al. Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Human Reproduction 2010;25(7):1713‐21. [DOI] [PubMed] [Google Scholar]
Bloemenkamp 2011 {published data only}
- Bloemenkamp KWM. Recurrent miscarriages: causes, treatment and consequences. trialregister.nl/trialreg/admin/rctview.asp?TC=3107 (first received 18 October 2011).
Brasseur‐Daudruy 2014 {published data only}
- Brasseur‐Daudruy M. Comparative evaluation of IRM and autopsy in the evaluation of intra uterine fetal death (COMPER). clinicaltrials.gov/show/NCT02148055 (first received 22 May 2014).
Rüegger 2014 {published data only}
- Rüegger CM, Bartsch C, Martinez RM, Ross S, Bolliger SA, Koller B, et al. Minimally invasive, imaging guided virtual autopsy compared to conventional autopsy in foetal, newborn and infant cases: study protocol for the paediatric virtual autopsy trial. BMC Pediatrics 2014;14:15. [DOI: 10.1186/1471-2431-14-15] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugiura 2013 {published data only}
- Sugiura M. Genetic analysis of recurrent miscarriage. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000014408 (first received 18 November 2013).
Additional references
Alderliesten 2003
- Alderliesten ME, Peringa J, Hulst VPM, Blaauwgeers HLG, Lith JMM. Perinatal mortality: clinical value of postmortem magnetic resonance imaging compared with autopsy in routine obstetric practice. BJOG: an international journal of obstetrics and gynaecology 2003;110(4):378‐82. [PubMed] [Google Scholar]
Arthurs 2015
- Arthurs OJ, Taylor AM, Sebire NJ. Indications, advantages and limitations of perinatal postmortem imaging in clinical practice. Pediatric Radiology 2015;45(4):491‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barfield 2011
- Barfield WD, Committee on Fetus and Newborn. Standard terminology for fetal, infant, and perinatal deaths. Pediatrics 2011;128(1):177‐81. [DOI] [PubMed] [Google Scholar]
Breeze 2012
- Breeze AC, Statham H, Hackett GA, Jessop FA, Lees CC. Perinatal postmortems: What is important to parents and how do they decide?. Birth 2012;39(1):57‐64. [DOI] [PubMed] [Google Scholar]
Bukowski 2011
- Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA 2011;306(22):2459‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burden 2016
- Burden C, Bradley S, Storey C, Ellis A, Heazell AE, Downe S, et al. From grief, guilt pain and stigma to hope and pride–a systematic review and meta‐analysis of mixed‐method research of the psychosocial impact of stillbirth. BMC Pregnancy and Childbirth 2016;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cartlidge 1995
- Cartlidge PH, Dawson AT, Stewart JH, Vujanic GM. Value and quality of perinatal and infant postmortem examinations: cohort analysis of 400 consecutive deaths. British Medical Journal 1995;310(6973):155‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castillo 2015
- Castillo P, Ussene E, Ismail MR, Jordao D, Lovane L, Carrilho C, et al. Pathological methods applied to the investigation of causes of death in developing countries: minimally invasive autopsy approach. PloS One 2015;10(6):e0132057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cockerill 2012
- Cockerill R, Whitworth MK, Heazell AEP. Do medical certificates of stillbirth provide accurate and useful information regarding the cause of death?. Paediatric and Perinatal Epidemiology 2012;26(2):117–23. [DOI] [PubMed] [Google Scholar]
Corabian 2005
- Corabian P, Scott A. Protocols for Stillbirth Investigation. Alberta Heritage Foundation for Medical Research, 2005. [Google Scholar]
Flenady 2010
- Flenady V, Silver R, Incerpi M, Fretts R, Pattinson R, Erwich JJ. Essential diagnostic workup of stillbirths. In: Facchinetti F, Dekker G, Baronciani D, Saade G editor(s). Stillbirth: Understanding and Management. London: Informa Healthcare, 2010. [Google Scholar]
Flenady 2011
- Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet 2011;377(9774):1331‐40. [DOI] [PubMed] [Google Scholar]
Flenady 2015
- Flenady V. Epidemiology of fetal and neonatal death. In: Khong TY, Malcomson RDG editor(s). Keeling's Fetal and Neonatal Pathology. 1st Edition. Springer International Publishing, 2015. [Google Scholar]
Flenady 2016
- Flenady V, Wojcieszek AM, Middleton P, Ellwood D, Erwich JJ, Coory M, et al. Stillbirths: recall to action in high‐income countries. Lancet 2016;387(10019):691‐702. [DOI] [PubMed] [Google Scholar]
Flenady 2017
- Flenady V, Wojcieszek AM, Ellwood E, Leisher SH, Erwich JJ, Draper E, et al. Classification of causes and associated conditions for stillbirths and neonatal deaths. Seminars in Fetal & Neonatal Medicine 2017;22(3):176‐85. [DOI] [PubMed] [Google Scholar]
Flenady 2018
- Flenady V, Oats J, Gardener G, Masson V, McCowan L, Kent A, et al. for the PSANZ Care around the time of stillbirth and neonatal death guidelines group. Clinical Practice Guideline for Care Around Stillbirth and Neonatal Death. Version 3. NHMRC Centre of Research Excellence in Stillbirth, Brisbane, Australia 2018.
Gordijn 2002
- Gordijn SJ, Erwich JJHM, Khong TY. Value of the perinatal autopsy: critique. Pediatric and Developmental Pathology 2002;5(5):480‐8. [DOI] [PubMed] [Google Scholar]
Gordijn 2007
- Gordijn SJ, Erwich JJHM, Khong TY. The perinatal autopsy: Pertinent issues in multicultural Western Europe. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2007;132(1):3‐7. [DOI] [PubMed] [Google Scholar]
Headley 2009
- Headley E, Gordon A, Jeffery H. Reclassification of unexplained stillbirths using clinical practice guidelines. Australian and New Zealand Journal of Obstetrics and Gynaecology 2009;49(3):285‐9. [DOI] [PubMed] [Google Scholar]
Heazell 2009
- Heazell AEP, Martindale EA. Can post‐mortem examination of the placenta help determine the cause of stillbirth?. Journal of Obstetrics and Gynaecology 2009;29(3):225‐8. [DOI] [PubMed] [Google Scholar]
Heazell 2012
- Heazell AEP, McLaughlin MJ, Schmidt EB, Cox P, Flenady V, Khong TY, et al. A difficult conversation? The views and experiences of parents and professionals on the consent process for perinatal postmortem after stillbirth. BJOG: an international journal of obstetrics and gynaecology 2012;119(8):987‐97. [DOI] [PubMed] [Google Scholar]
Heazell 2016
- Heazell AEP, Siassakos D, Blencowe H, Burden C, Bhutta ZA, Cacciatore J, et al. Stillbirths: economic and psychosocial consequences. Lancet 2016;387(10018):604‐16. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horey 2013
- Horey D, Flenady V, Heazell AEP, Khong TY. Interventions for supporting parents’ decisions about autopsy after stillbirth. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009932.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horn 2004
- Horn L‐C, Langner A, Stiehl P, Wittekind C, Faber R. Identification of the causes of intrauterine death during 310 consecutive autopsies. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2004;113(2):134‐8. [DOI] [PubMed] [Google Scholar]
Khong 2010
- Khong TY. Pathology investigations for stillbirths. In: Facchinetti F, Dekker G, Baronciani D, Saade G editor(s). Stillbirth: Understanding and Management. London: Informa Healthcare, 2010:91‐9. [Google Scholar]
Kidron 2017
- Kidron D, Vainer I, Fisher Y, Sharony R. Automated image analysis of placental villi and syncytial knots in histological sections. Placenta 2017;53:113‐8. [DOI] [PubMed] [Google Scholar]
Killeen 2004
- Killeen OG, Burke C, Devaney D, Clarke TA. The value of the perinatal and neonatal autopsy. Irish Medical Journal 2004;97(8):241‐4. [PubMed] [Google Scholar]
Korteweg 2008
- Korteweg FJ, Bouman K, Erwich JJ, Timmer A, Veeger NJ, Ravisé JM, et al. Cytogenetic analysis after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstetrics and Gynecology 2008;111(4):865‐74. [DOI] [PubMed] [Google Scholar]
Korteweg 2010
- Korteweg FJ, Erwich JJ, Folkeringa N, Timmer A, Veeger NJ, Ravisé JM, et al. Prevalence of parental thrombophilic defects after fetal death and relation to cause. Obstetrics and Gynecology 2010;116(2 Pt 1):355‐64. [DOI] [PubMed] [Google Scholar]
Korteweg 2012
- Korteweg FJ, Erwich JJ, Timmer A, Meer J, Ravisé JM, Veeger NJGM, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. American Journal of Obstetrics and Gynecology 2012;206(1):53.e1‐12. [DOI] [PubMed] [Google Scholar]
Lawn 2016
- Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387(10018):587‐603. [DOI] [PubMed] [Google Scholar]
Leisher 2016
- Leisher SH, Teoh Z, Reinebrant HE, Allanson E, Blencowe H, Erwich JJ, et al. Seeking order amidst chaos: A systematic review of classification systems for causes of stillbirth and neonatal death, 2009‐2014. BMC Pregnancy and Childbirth 2016;16(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2005
- Lim TL, Tan KH, Tee CS, Yeo GS. Investigating stillbirths using a simplified obstetric events‐based protocol. Singapore Medical Journal 2005;46(2):63‐8. [PubMed] [Google Scholar]
Lyon 2004
- Lyon A. Perinatal autopsy remains the "gold standard". Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(4):F284. [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2018
- McClure EM, Garces A, Saleem S, Moore JL, Bose CL, Esamai F, et al. Global Network for Women's and Children's Health Research: probable causes of stillbirth in low‐ and middle‐income countries using a prospectively defined classification system. BJOG: an international journal of obstetrics and gynaecology 2018;125(2):131‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Measey 2007
- Measey M‐A, Charles A, D’Espaignet ET, Harrison C, DeKlerk N, Douglass C. Aetiology of stillbirth: unexplored is not unexplained. Australian and New Zealand Journal of Public Health 2007;31(5):444‐9. [DOI] [PubMed] [Google Scholar]
Michalski 2002
- Michalski ST, Porter J, Pauli RM. Costs and consequences of comprehensive stillbirth assessment. American Journal of Obstetrics and Gynecology 2002;186(5):1027‐34. [DOI] [PubMed] [Google Scholar]
Miller 2016
- Miller ES, Minturn L, Linn R, Weese‐Mayer DE, Ernst LM. Stillbirth evaluation: a stepwise assessment of placental pathology and autopsy. American Journal of Obstetrics and Gynecology 2016;214(1):115.e1‐6. [DOI] [PubMed] [Google Scholar]
Mistry 2013
- Mistry H, Heazell AE, Vincent O, Roberts T. A structured review and exploration of the healthcare costs associated with stillbirth and a subsequent pregnancy in England and Wales. BMC Pregnancy and Childbirth 2013;13:236. [DOI: 10.1186/1471-2393-13-236] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nausheen 2013
- Nausheen S, Soofi SB, Sadiq K, Habib A, Turab A, Memon Z, et al. Validation of verbal autopsy tool for ascertaining the causes of stillbirth. PloS One 2013;8(10):e76933. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Leary 2015
- O'Leary BD, Walsh CA, Fitzgerald JM, Downey P, McAuliffe FM. The contribution of massive fetomaternal hemorrhage to antepartum stillbirth: a 25‐year cross‐sectional study. Acta Obstetricia et Gynecologica Scandinavica 2015;94(12):1354‐8. [DOI] [PubMed] [Google Scholar]
Page 2017
- Page JM, Christiansen‐Lindquist L, Thorsten V, Parker CB, Reddy UM, Dudley DJ, et al. Diagnostic tests for evaluation of stillbirth: results from the stillbirth collaborative research network. Obstetrics and Gynecology 2017;129(4):699‐706. [DOI] [PubMed] [Google Scholar]
Ptacek 2016
- Ptacek I, Smith A, Garrod A, Bullough S, Bradley N, Batra G, et al. Quantitative assessment of placental morphology may identify specific causes of stillbirth. BMC Clinical Pathology 2016;16(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rankin 2002
- Rankin J, Wright C, Lind T. Cross sectional survey of parents' experience and views of the postmortem examination. British Medical Journal 2002;324(7341):816‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2012
- Reddy UM, Page GP, Saade GR, Silver RM, Thorsten VR, Parker CB, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. New England Journal of Medicine 2012;367(23):2185‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinebrant 2017
- Reinebrant H, Leisher SH, Coory M, Henry S, Wojcieszek AM, Gardener G, et al. Making stillbirths visible: a systematic review of globally reported causes of stillbirth. BJOG: an international journal of obstetrics and gynaecology 2017;125(2):212‐24. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2006
- Rose C, Evans M, Tooley J. Falling rates of perinatal postmortem examination: are we to blame?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006;91(6):F465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenfeld 2015
- Rosenfeld JA, Tucker ME, Escobar LF, Neill NJ, Torchia BS, McDaniel LD, et al. Diagnostic utility of microarray testing in pregnancy loss. Ultrasound in Obstetrics & Gynecology 2015;46(4):478‐86. [DOI] [PubMed] [Google Scholar]
Rossi 2017
- Rossi AC, Prefumo F. Correlation between fetal autopsy and prenatal diagnosis by ultrasound: A systematic review. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;210:201‐6. [DOI: 10.1016/j.ejogrb.2016.12.024] [DOI] [PubMed] [Google Scholar]
Silver 2007
- Silver RM, Varner MW, Reddy U, Goldenberg R, Pinar H, Conway D, et al. Work‐up of stillbirth: a review of the evidence. American Journal of Obstetrics and Gynecology 2007;196(5):433‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thayyil 2013
- Thayyil S, Sebire NJ, Chitty LS, Wade A, Chong WK, Olsen O, et al. Post‐mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet 2013;382(9888):223‐33. [DOI] [PubMed] [Google Scholar]
Vullo 2016
- Vullo A, Panebianco V, Cannavale G, Aromatario M, Cipolloni L, Frati P, et al. Post‐mortem magnetic resonance foetal imaging: a study of morphological correlation with conventional autopsy and histopathological findings. Radiologia Medica 2016;121(11):847‐56. [DOI] [PubMed] [Google Scholar]
Whitfield 1986
- Whitfield CR, Smith NC, Cockburn F, Gibson AA. Perinatally related wastage ‐ a proposed classification of primary obstetric factors. BJOG: an international journal of obstetrics and gynaecology 1986;93(7):694. [PubMed] [Google Scholar]
Wojcieszek 2016
- Wojcieszek AM, Reinebrant HE, Leisher SH, Allanson E, Coory M, Erwich JJ, et al. Characteristics of a global classification system for perinatal deaths: a Delphi consensus study. BMC Pregnancy and Childbirth 2016;16:223. [DOI: 10.1186/s12884-016-0993-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wojcieszek 2017
- Wojcieszek AM, Shepherd E, Middleton P, Gardener G, Ellwood DA, McClure EM, et al. Interventions for investigating and identifying the causes of stillbirth. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012504] [DOI] [PMC free article] [PubMed] [Google Scholar]


