Abstract
Background
Cardiovascular disease (CVD) is the major cause of mortality worldwide. Coronary artery disease (CAD) contributes to half of mortalities caused by CVD. The mainstay of management of CAD is medical therapy and revascularisation. Revascularisation can be achieved via coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI). Peripheral arteries, such as the femoral or radial artery, provide the access to the coronary arteries to perform diagnostic or therapeutic (or both) procedures.
Objectives
To assess the benefits and harms of the transradial compared to the transfemoral approach in people with CAD undergoing diagnostic coronary angiography (CA) or PCI (or both).
Search methods
We searched the following databases for randomised controlled trials on 10 October 2017: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and Web of Science Core Collection. We also searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform in August 2017. There were no language restrictions. Reference lists were also checked and we contacted authors of included studies for further information.
Selection criteria
We included randomised controlled trials that compared transradial and transfemoral approaches in adults (18 years of age or older) undergoing diagnostic CA or PCI (or both) for CAD.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. At least two authors independently screened trials, extracted data, and assessed the risk of bias in the included studies. We contacted trial authors for missing information. We used risk ratio (RR) for dichotomous outcomes and mean difference (MD) or standardised mean difference (SMD) for continuous data, with their 95% confidence intervals (CIs). All analyses were checked by another author.
Main results
We identified 31 studies (44 reports) including 27,071 participants and two ongoing studies. The risk of bias in the studies was low or unclear for several domains. Compared to the transfemoral approach, the transradial approach reduced short‐term net adverse clinical events (NACE) (i.e. assessed during hospitalisation and up to 30 days of follow‐up) (RR 0.76, 95% CI 0.61 to 0.94; 17,133 participants; 4 studies; moderate quality evidence), cardiac death (RR 0.69, 95% CI 0.54 to 0.88; 11,170 participants; 11 studies; moderate quality evidence). However, short‐term myocardial infarction was similar between both groups (RR 0.91, 95% CI 0.81 to 1.02; 19,430 participants; 11 studies; high quality evidence). The transradial approach had a lower procedural success rate (RR 0.97, 95% CI 0.96 to 0.98; 25,920 participants; 28 studies; moderate quality evidence), but was associated with a lower risk of all‐cause mortality (RR 0.77, 95% CI 0.62 to 0.95; 18,955 participants; 10 studies; high quality evidence), bleeding (RR 0.54, 95% CI 0.40 to 0.74; 23,043 participants; 20 studies; low quality evidence), and access site complications (RR 0.36, 95% CI 0.22 to 0.59; 16,112 participants; 24 studies; low quality evidence).
Authors' conclusions
Transradial approach for diagnostic CA or PCI (or both) in CAD may reduce short‐term NACE, cardiac death, all‐cause mortality, bleeding, and access site complications. There is insufficient evidence regarding the long‐term clinical outcomes (i.e. beyond 30 days of follow‐up).
Plain language summary
Radial artery versus femoral artery approach for performing coronary catheter procedures in people with coronary artery disease
Review question
Should physicians introduce the catheter (a long, thin tube) through the femoral artery (transfemoral access via the groin) or the radial artery (transradial access via the wrist) to reach the coronary arteries (blood vessels supplying the heart) for the diagnosis or treatment of coronary artery disease?
Background
Coronary artery disease contributes to half of deaths caused by cardiovascular (heart and blood vessels) disease. Restoration of adequate blood flow through the coronary arteries can be achieved by introducing a catheter through a peripheral artery. This allows the introduction of balloons through the aorta (major artery of the heart) to dilate coronary artery narrowing or place arterial scaffolds (tubes called stents) to keep the coronary arteries open. Two main peripheral arteries can provide access; traditionally, the femoral (groin) artery, and more recently, the radial artery (one of two major arteries in the forearm). While gaining popularity, the transradial approach can be more challenging than the transfemoral approach, which may translate to longer procedural durations and technical failures. In addition, this raises concerns regarding radiation exposure to patients and physicians being higher with the transradial approach. We sought to compare the advantages and disadvantages of both approaches to help inform healthcare decisions.
Study characteristics
Our search yielded 31 eligible studies comparing the transradial approach to the transfemoral approach in people undergoing diagnostic or therapeutic (or both) coronary catheterisation procedures in different settings, whether urgent (during heart attacks (myocardial infarctions)) or elective (planned procedure). The trials were carried out in many countries and regions, including Canada, China, Europe, Japan, and USA. We also identified two ongoing studies. The evidence was current to October 2017.
Key results
Transradial access was associated with a reduction in the composite outcome (comprising two or more combined outcomes) of net adverse clinical events (NACE), including death from cardiac causes, myocardial infarction (injury of the heart muscle), stroke (insult to the brain), need to reintervene on the same site of coronary artery stenosis (narrowing), and bleeding during the first 30 days following intervention. When assessing individual outcomes, the risk of myocardial infarction and stroke was similar between groups. Transradial access reduced death from cardiac causes, death from all causes during the first 30 days following intervention, bleeding, and local complications at the access site. The transradial approach shortened the length of stay in hospital, but was associated with a higher radiation exposure and more technical failures requiring an alternate vascular access route.
Quality of the evidence
We rated the quality of the evidence for short‐term myocardial infarction and all‐cause death as high. We rated short‐term NACE, cardiac death, and success of the procedure as moderate quality evidence. Evidence for bleeding and access site complications was low quality.
Summary of findings
Summary of findings for the main comparison. Transradial compared to transfemoral approach for diagnostic coronary angiography and percutaneous coronary intervention in people with coronary artery disease.
| Transradial compared to transfemoral approach for diagnostic coronary angiography and percutaneous coronary intervention in people with coronary artery disease | ||||||
| Patient or population: people with coronary artery disease undergoing diagnostic coronary angiography and percutaneous coronary intervention Setting: inpatient Intervention: transradial Comparison: transfemoral | ||||||
| Outcomes1 | Anticipated absolute effects2 (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Short‐term NACE | 90 per 1000 | 68 per 1000 (55 to 85) | RR 0.76 (0.61 to 0.94) | 17,133 (4 RCTs) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| Short‐term cardiac death | 26 per 1000 | 18 per 1000 (14 to 23) | RR 0.69 (0.54 to 0.88) | 11,170 (11 RCTs) | ⊕⊕⊕⊝ Moderate4 | ‐ |
| Short‐term MI | 55 per 1000 | 50 per 1000 (45 to 56) | RR 0.91 (0.81 to 1.02) | 19,430 (11 studies) | ⊕⊕⊕⊕ High | Although 15 studies reported this outcome, 4 had 0 events in either group, so the meta‐analysis was based on 11 studies. |
| Success of the procedure | 979 per 1000 | 950 per 1000 (940 to 960) | RR 0.97 (0.96 to 0.98) | 25,920 (28 RCTs) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| Short‐term all‐cause mortality | 18 per 1000 | 14 per 1000 (11 to 17) | RR 0.77 (0.62 to 0.95) | 18,955 (10 RCTs) | ⊕⊕⊕⊕ High | ‐ |
| Bleeding | 98 per 1000 | 53 per 1000 (39 to 73) | RR 0.54 (0.40 to 0.74) | 23,043 (20 RCTs) | ⊕⊕⊝⊝ Low3,4 | Although 22 studies reported this outcome, 2 had 0 events in either group, so the meta‐analysis was based on 20 studies. |
| Access site complications | 50 per 1000 | 18 per 1000 (11 to 30) | RR 0.36 (0.22 to 0.59) | 16,112 (24 RCTs) | ⊕⊕⊝⊝ Low3,4 | ‐ |
| CI: confidence interval; MI: myocardial infarction; NACE: net adverse clinical events; RCTs: randomised controlled trials; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1All outcomes reported in the 'Summary of findings' table were short‐term (i.e. assessed during hospitalisation and up to 30 days of follow‐up).
2The risk in the intervention group (and its 95% confidence interval) was based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
3Substantial heterogeneity, downgraded by 1 level.
4Asymmetrical funnel plot. Publication bias detected, downgraded by 1 level.
Background
Description of the condition
People with coronary artery disease (CAD) usually present with pain or discomfort in the centre of the chest that may radiate to the arms, left shoulder, elbows, jaw, or back. In addition, the person may experience shortness of breath (WHO 2017). Revascularisation therapy for CAD is mainly indicated in people with acute coronary syndrome as well as people with stable CAD who do not respond to optimal medical therapy or in people who demonstrate marked limitation of physical activity (or both). Optimal medical therapy includes lifestyle modification and pharmacological agents, including antiplatelet agents, statins, β‐blockers, and angiotensin‐converting enzyme inhibitors (ESC‐EACTS 2010). For almost half a century, coronary artery bypass grafting has been regarded as the most effective revascularisation therapy. However, its role has been increasingly challenged since the late 1990s by the evolution of percutaneous coronary intervention (PCI); particularly with the introduction of drug‐eluting stents (Taggart 2013).
Description of the intervention
PCI, which includes coronary angiography (CA) and revascularisation procedures such as balloon angioplasty and intracoronary stenting (Wensley 2008), is used in the management of CAD. The idea behind PCI is to mechanically intervene upon a stenosed segment of a coronary vessel to improve flow. Interventional cardiologists gain access via a peripheral artery, mainly the femoral or radial arteries (Kotowycz 2012). The traditional approach for PCI, ever since its introduction in 1977, has been through the femoral artery, owing to its large calibre providing easy access (Venkitachalam 2009). The access site for the standard transfemoral approach is through the groin. Campeau was the first to introduce CA via the transradial approach in 1989 (Campeau 1989; Triantafyllou 2009), whereby the approach is through the forearm, 2.5 cm to 5 cm above the wrist joint (Hess 2014). The wire is passed through the needle after successful puncture. Then, the needle is withdrawn from the artery with the wire kept in place. This is followed by introduction of the sheath and the coronary catheters used to perform the diagnostic CA or the guiding catheters used for balloon angioplasty with or without stent placement (Almany 1999). Other access sites include the transbrachial and transulnar approaches, although rarely used in contemporary clinical practice (Kiemeneij 1997; Hsueh 2017).
How the intervention might work
The transfemoral approach for cardiac catheterisation and intervention has gained widespread acceptance. Its advantages include a long history of use (Venkitachalam 2009), coupled with technical ease, and the capacity for clinicians to use larger catheters and equipment (Triantafyllou 2009). However, it is plagued with some disadvantages that are inherent with this type of access. These include a requirement for the person to have prolonged bed rest and an association with more back pain, urinary retention, and neuropathy than the radial approach (Dal Molin 2015). Vascular complications of the transfemoral approach include pseudo‐aneurysms, arteriovenous fistulas, and significant bleeding, including retroperitoneal haematomas (Brueck 2009).
The radial approach has certain inherent advantages. Vascular complications are less frequent and the dual blood supply limits the potential for limb‐threatening ischaemia (Agostoni 2004). The approach is advantageous for people with severe occlusive aortoiliac disease or difficulty laying down (e.g. due to back pain, obesity, or congestive heart failure) (Almany 1999). Earlier ambulation contributes to people's preference to this approach (Kotowycz 2012).
However, there are potential disadvantages to the radial approach. They include the following.
The radial artery is smaller than the femoral (approximately 2 mm to 2.3 mm) (Kim 2011). Consequently, some interventions may be technically challenging via the radial route due to the size of the technology required (e.g. large bore rotational atherectomy) (Watt 2009).
Vessel spasm is more common (Kim 2011).
Guide placement is more challenging and requires learning a different technique with a steep learning curve (Hess 2014).
Why it is important to do this review
The transradial approach in PCI is an alternative to the routine transfemoral approach. A systematic review conducted by Bertrand 2012, comparing both approaches, included 76 studies (15 randomised and 61 observational) with inconclusive results from the randomised trials. We planned to include only randomised controlled trials (RCTs) in our review, consider diagnostic and therapeutic procedures, and prespecified multiple clinically relevant subgroups. The major guideline bodies have been uncertain about the benefit of one approach over the other; particularly as the presumed benefit of the radial approach was undermined by the drawback of lack of operator experience. European Society of Cardiology (ESC 2015) and National Institute for Health and Care Excellence (NICE 2013) guidelines recommend the radial over the femoral approach based on data from two major trials, while the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines/Society for Cardiovascular Angiography (AHA 2015) guideline still does not recommend one procedure over another. We aimed to pool data from all RCTs to produce high‐quality synthesised evidence that informs healthcare decisions concerning these two approaches.
Objectives
To assess the benefits and harms of the transradial compared to the transfemoral approach in people with CAD undergoing diagnostic CA or PCI (or both).
Methods
Criteria for considering studies for this review
Types of studies
We included all published, unpublished, and ongoing RCTs.
Types of participants
Adults (18 years of age or older) of either gender undergoing diagnostic CA or PCI (or both) for CAD.
Types of interventions
Transradial versus transfemoral approach for diagnostic CA or PCI (or both).
Types of outcome measures
Primary outcomes
Net adverse clinical events (NACE), defined as a composite of cardiac death, stroke, myocardial infarction (MI), target lesion revascularisation, and bleeding, or as defined by trialists.
Cardiac death.
MyocardiaI infarction (MI).
Success of the procedure, defined as completion of procedure without cross‐over to another access site, or as defined by trialists (not prespecified).
Secondary outcomes
All‐cause mortality.
Bleeding (combined major and minor).
Stroke (ischaemic or haemorrhagic), as defined by trialists.
Access site complications (e.g. haematoma, arteriovenous fistula, vasospasm, pseudoaneurysm, and perforation).
Total radiation dose.
Length of hospital stay.
Participant satisfaction, including early or reduced (or both) pain on ambulation, early hospital discharge, or as defined by trialists.
Reporting one or more of the above outcomes in the trial was not an inclusion/exclusion criterion for the review.
Timing of outcome assessment
Short‐term; assessed during hospitalisation and up to 30 days of follow‐up.
Long‐term; assessed beyond 30 days of follow‐up.
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases on 10 October 2017:
Cochrane Central Register of Controlled Trials (CENTRAL): 2017, Issue 9 (the Cochrane Library);
MEDLINE: Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE (Ovid, 1946 to 10 October 2017);
Embase (Ovid, 1980 to 2017 week 41);
Web of Science Core Collection (Thomson Reuters, 1900 to 10 October 2017).
We developed search strategies for each database based on the preliminary search strategy developed for MEDLINE but revised appropriately for each database to take account of differences in controlled vocabulary and syntax rules (Appendix 1). We applied the Cochrane sensitivity‐maximising RCT filter (Lefebvre 2011) MEDLINE (Ovid) and adaptations of it to the other databases, except CENTRAL. We did not restrict searches by language or date of publication.
Searching other resources
Ongoing trials
We searched the following databases up to August 2017 for ongoing trials (Appendix 1):
ClinicalTrials.gov (ClinicalTrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/default.aspx).
Checking reference lists
We handsearched reference lists of all included primary studies and relevant review articles for additional references.
Personal communication
We contacted the authors of identified trials where we required additional information. We requested further information relevant to the review that was not apparent in the published work. We also asked if they knew of any other published or unpublished studies relevant to the review that were not included in the references.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane (MECIR 2016).
Selection of studies
Two authors (AK and RA or MA) independently assessed all records retrieved by the searches for inclusion. We resolved any disagreements through discussion or consulting another author (AN), if required. We created a study flow diagram to map out the number of records identified, included, and excluded (Figure 1). We contacted the authors of the studies if we could not retrieve the full text. We excluded studies reported as abstracts if the authors did not reply, there was no contact information provided, and where the abstract did not provide enough information. We will reconsider those studies if relevant data becomes available. We contacted a relevant professional translator when required.
1.
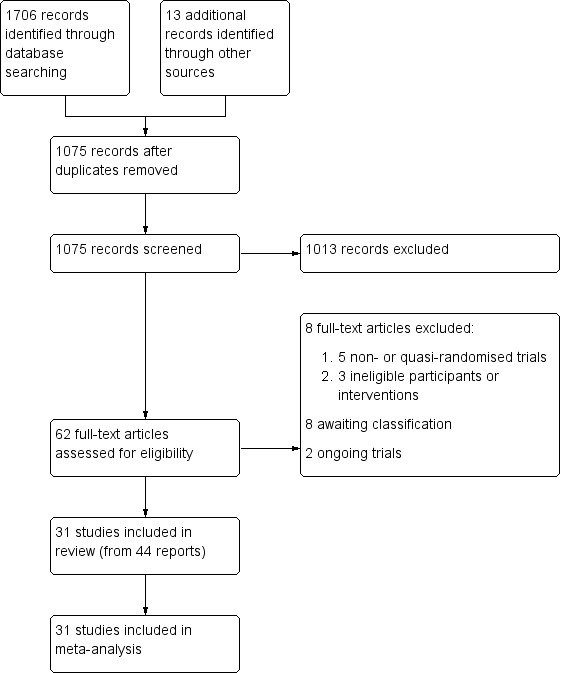
PRISMA study flow diagram.
Data extraction and management
We piloted a data extraction form. For eligible studies, two authors (AK and RA or MA) extracted the data using the agreed form. We resolved discrepancies through discussion or consulting another author (AN). Three authors (AK, AM, and MA) entered the data into Review Manager 5 Software and checked them for accuracy (RevMan 2014). When information was unclear, we contacted authors of the original reports to request further details. In case of the need for translation, we contacted a relevant professional translator and used a translated data extraction form.
Assessment of risk of bias in included studies
Three authors (AK, RA, and MA) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (Appendix 2). We resolved any disagreements either by discussion or by involving a fourth author (AN).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) for outcomes using the same scale or standardised mean difference (SMD) for outcomes using difference scales with 95% CI.
Unit of analysis issues
Cluster‐randomised trials
We did not include any cluster‐randomised trials. In any future update, we plan to adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.4 or 16.3.6) using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population.
Multi‐arm intervention trials
When trials included multi‐arm interventions, we combined arms using the same access site (e.g. right and left transradial approaches) and excluded arms that were irrelevant to the scope of our review (e.g. transbrachial approach). For details on how we dealt with individual multi‐arm intervention studies included in our review, see Description of studies and Characteristics of included studies table.
Dealing with missing data
For included studies, we noted levels of attrition. We contacted the authors to request missing data. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we included all participants randomised to each group in the analyses, and all participants analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention). The denominator for each outcome in each trial was calculated as the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We inspected forest plots visually for signs of heterogeneity and assessed statistical heterogeneity in each meta‐analysis using the T², I², and Chi² statistics. We regarded heterogeneity as substantial if T² was greater than zero, and either I² was 50% or greater, or there was a low P value (less than 0.1) in the Chi² test for heterogeneity.
Assessment of reporting biases
We investigated reporting biases using funnel plots with visual assessments for asymmetry. We performed formal evaluations using Egger's test (Egger 1997) when there was asymmetry.
Data synthesis
We carried out statistical analysis using Review Manager 5 Software (RevMan 2014). We used fixed‐effect meta‐analyses for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect (i.e. where trials were examining the same intervention, and the trials' populations and methods were sufficiently similar). If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity (I² statistic 50% or greater or P less than 0.1), we used random‐effects meta‐analyses to produce an overall summary if a mean treatment effect across trials was considered clinically meaningful. We presented the random‐effects summary as the mean range of possible treatment effects and presented random‐effects RRs with 95% CI, and the estimates of T² and I².
GRADE and 'Summary of findings' table
We used GRADEpro 2014 to import data from Review Manager 5 (RevMan 2014) to create Table 1. A summary of the intervention effect and a measure of quality for each of the outcomes was produced using the GRADE approach (Schünemann 2009). The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence was downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations in the five mentioned considerations. Two authors (MA and AN) made GRADE assessments and the decisions on downgrading. This was approved by all other authors.
Main outcomes for 'Summary of findings' table
Short‐term NACE, defined as a composite of cardiac death, stroke, MI, target lesion revascularisation, and bleeding, or as defined by trialists.
Short‐term cardiac death.
Short‐term MI.
Success of the procedure, defined as completion of procedure without cross‐over to another access site, or as defined by trialists.
Short‐term all‐cause mortality.
Bleeding (combined major and minor).
Access site complications, including haematoma, arteriovenous fistula, vasospasm, and perforation.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity (I² statistic 50% or greater or P less than 0.1), we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and, if it was, used random‐effects analyses to produce it.
We carried out the following subgroup analyses:
participants undergoing diagnostic CA versus PCI;
participants undergoing elective versus primary PCI;
participants with acute ST‐segment elevation myocardial infarction (STEMI) versus non‐ST‐segment elevation acute coronary syndrome (NSTE‐ACS);
women versus men.
We restricted the subgroup analysis to primary outcomes. We assessed subgroup differences by interaction tests available within Review Manager 5 (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effects of trial quality by omitting studies at high or unclear risk of bias when considering allocation concealment (selection bias) and incomplete outcome data (attrition bias). We carried out sensitivity analyses to explore the effects of fixed‐effect or random‐effects analyses for outcomes with substantial statistical heterogeneity. We intended to restrict this to the primary outcomes.
Results
Description of studies
We provided descriptions of studies in the Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
The searches for the review were run on 10 October 2017. Our search yielded 1706 records identified through database searches along with 13 additional reports identified through other resources. We identified 1075 records after removal of duplicates. After an initial screening, we marked 62 records for retrieval and assessment of their full text for eligibility. We excluded eight studies, identified two ongoing studies, and eight are awaiting classification. We included 44 reports of 31 studies (Figure 1).
Included studies
Thirty‐one studies, from 44 reports, fulfilled our eligibility criteria. All included studies provided quantitative data that were included in the meta‐analysis with 27,071 participants (see Characteristics of included studies table for details regarding characteristics for all included participants).
Design
All included studies were parallel group trials, except five studies that had multi‐arms (Benit 1997; Kiemeneij 1997; Louvard 2001; Reddy 2004; Santas 2009).
Setting
Five trials were conducted in China (Gan 2009; He 2012; Hou 2010; Li 2007; Wang 2012), four trials were conducted in the US (Cooper 1999; Mann 1998; Michael 2013; Reddy 2004), and five trials were multi‐centre (Jolly 2011; Louvard 2004; Rao 2014; Romagnoli 2012; Valgimigli 2015). The remaining 16 trials were conducted in Germany (Achenbach 2008; Brueck 2009; Lange 2006), France (Brasselet 2007; Louvard 2001), Japan (Saito 2003), Spain (Santas 2009), Netherlands (Kiemeneij 1997; Slagboom 2005), Turkey (Akturk 2014), Czech Republic (Bernat 2014), Poland (Koltowski 2014), Greece (Ziakas 2010), Canada (Cantor 2005), Belgium (Benit 1997), Austria (Schernthaner 2018), and Brazil (De Andrade 2017).
Participants
Age
Studies were similar in the baseline age (years) of the included participants with transradial (mean (standard deviation (SD)): 63.78 (6.16); range: 53.60 to 82.60) and transfemoral (mean (SD): 64.17 (6.06); range: 52.30 to 83.00).
Gender
Studies included men and women. One study included only men (Benit 1997), and one study included only women (Rao 2014), while two studies provided separate data for men and women (Jolly 2011; Valgimigli 2015).
Clinical characteristics
Two trials included people undergoing CA (Cooper 1999; Louvard 2001), while 13 trials involved people undergoing PCI (Benit 1997; Bernat 2014; Brasselet 2007; Gan 2009; Hou 2010; Kiemeneij 1997; Koltowski 2014; Li 2007; Mann 1998; Romagnoli 2012; Saito 2003; Slagboom 2005; Wang 2012). The remaining studies involved people undergoing CA or PCI (or both) (Achenbach 2008; Akturk 2014; Brueck 2009; Cantor 2005; De Andrade 2017; He 2012; Jolly 2011; Lange 2006; Louvard 2004; Michael 2013; Rao 2014; Reddy 2004; Santas 2009; Schernthaner 2018; Valgimigli 2015; Ziakas 2010).
Regarding urgency of the procedure, five trials included people undergoing elective procedures only (Benit 1997; Cooper 1999; Kiemeneij 1997; Lange 2006; Reddy 2004). Fifteen studies enrolled people undergoing urgent procedures (Bernat 2014; Brasselet 2007; Cantor 2005; De Andrade 2017; Gan 2009; Hou 2010; Jolly 2011; Koltowski 2014; Li 2007; Mann 1998; Romagnoli 2012; Saito 2003; Schernthaner 2018; Valgimigli 2015; Wang 2012). The remaining 11 studies included people undergoing either elective or urgent procedures (Achenbach 2008; Akturk 2014; Brueck 2009; He 2012; Louvard 2001; Louvard 2004; Michael 2013; Rao 2014; Santas 2009; Slagboom 2005; Ziakas 2010).
Regarding participant's presentation, two trials included people with stable angina (Cooper 1999; Reddy 2004). Ten trials enrolled people with STEMI (Bernat 2014; Brasselet 2007; Cantor 2005; Gan 2009; Hou 2010; Koltowski 2014; Li 2007; Romagnoli 2012; Saito 2003; Wang 2012), while one trial included people with NSTE‐ACS (De Andrade 2017). Four trials enrolled people with acute coronary syndrome whether STEMI or NSTE‐ACS (Jolly 2011; Mann 1998; Schernthaner 2018; Valgimigli 2015). Fourteen trials included people with either stable angina or unstable coronary syndromes (Achenbach 2008; Akturk 2014; Benit 1997; Brueck 2009; He 2012; Kiemeneij 1997; Lange 2006; Louvard 2001; Louvard 2004; Michael 2013; Rao 2014; Santas 2009; Slagboom 2005; Ziakas 2010).
Intervention
Two studies randomised participants to right transradial, left transradial, or transfemoral approach (Louvard 2001; Santas 2009), so we combined the right and left transradial arms. Two trials randomised participants to transfemoral, transradial, or transbrachial approach (Benit 1997; Kiemeneij 1997). We excluded the arm of participants undergoing transbrachial intervention.
One trial randomised participants to either transradial approach, transfemoral approach by 4F sheath with no closure device, or transfemoral approach by 6F sheath with closure device (Reddy 2004). We combined the transfemoral approach by 4F sheath and transfemoral approach by 6F sheath arms.
The remaining 26 studies randomised participants to either transradial or transfemoral approach (Achenbach 2008; Akturk 2014; Bernat 2014; Brasselet 2007; Brueck 2009; Cantor 2005; Cooper 1999; De Andrade 2017; Gan 2009; He 2012; Hou 2010; Jolly 2011; Koltowski 2014; Lange 2006; Li 2007; Louvard 2004; Mann 1998; Michael 2013; Rao 2014; Romagnoli 2012; Saito 2003; Schernthaner 2018; Slagboom 2005; Valgimigli 2015; Wang 2012; Ziakas 2010).
Funding source
Most included studies did not report a funding source (Akturk 2014; Benit 1997; Brasselet 2007; Brueck 2009; Cantor 2005; De Andrade 2017; Gan 2009; He 2012; Hou 2010; Lange 2006; Li 2007; Louvard 2001; Louvard 2004; Mann 1998; Reddy 2004; Saito 2003; Santas 2009; Schernthaner 2018; Wang 2012; Ziakas 2010). Two trials were investigator‐initiated and declared no external funding source (Koltowski 2014; Romagnoli 2012). The remaining studies were mostly funded by grants from different sponsors, institutes, and respective ministries of health (Achenbach 2008; Bernat 2014; Cooper 1999; Jolly 2011; Kiemeneij 1997; Michael 2013; Rao 2014; Slagboom 2005; Valgimigli 2015). Details of funding sources are outlined separately (see Characteristics of included studies table).
Excluded studies
We excluded eight studies (see Characteristics of excluded studies table): three studies were quasi‐randomised (Bhat 2017; Chodor 2009; Chodor 2011), two were non‐randomised (Kallinikou 2016; Qi 2017), two had ineligible interventions (Genereux 2011; Marti 2015), and one had ineligible participants (Scalone 2014).
Studies awaiting classification
There were eight studies awaiting classifications since only abstracts were available with no usable data (Akturk 2012; Dorniak 2009; Gavrilidis 2009; Koltowski 2012; Li 2011; Mann 1996; Skvaril 2012; Wei 2006). Authors were contacted to request full information with no response. One abstract did not have available contact information. We shall reconsider these studies in the future if further information becomes available.
Ongoing studies
We identified two ongoing studies (ARISE‐2; SAFARI‐STEMI) (see Characteristics of ongoing studies table).
Risk of bias in included studies
We provided detailed descriptions of the risk of bias in the included studies in the 'Risk of bias' tables. See Figure 2 and Figure 3 for a summary of risk of bias assessments.
2.
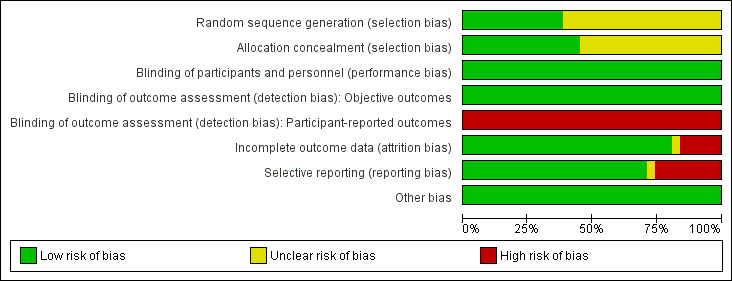
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
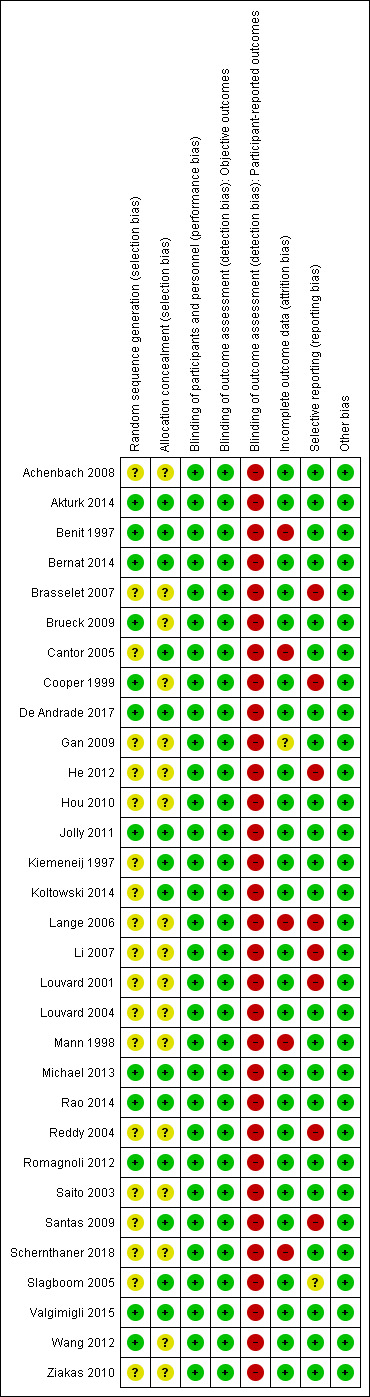
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Nine studies were at low risk in sequence generation and allocation concealment domains (Akturk 2014; Benit 1997; Bernat 2014; De Andrade 2017; Jolly 2011; Michael 2013; Rao 2014; Romagnoli 2012; Valgimigli 2015).
In three studies, the sequence generation was low risk while the allocation concealment was unclear (Brueck 2009; Cooper 1999; Wang 2012). Five studies had adequate allocation concealment with unclear sequence generation (Cantor 2005; Kiemeneij 1997; Koltowski 2014; Santas 2009; Slagboom 2005).
The remaining 14 studies were unclear regarding sequence generation and allocation concealment (Achenbach 2008; Brasselet 2007; Gan 2009; He 2012; Hou 2010; Lange 2006; Li 2007; Louvard 2001; Louvard 2004; Mann 1998; Reddy 2004; Saito 2003; Schernthaner 2018; Ziakas 2010).
Blinding
Neither the participants nor the physicians were blinded in the included trials due to the modus operandi of the interventions. Given the nature of the intervention, blinding is not feasible and we considered the risk of performance bias to be low. Regarding detection bias, we assessed blinding separately for different classes of outcomes. We judged the risk of detection bias to be low in objective outcomes, and high in participant‐reported outcomes since lack of blinding can potentially introduce bias for this class of outcomes through multiple pathways (Higgins 2011).
Incomplete outcome data
We judged the risk of attrition bias as low in 25 studies (Achenbach 2008; Akturk 2014; Bernat 2014; Brasselet 2007; Brueck 2009; Cooper 1999; De Andrade 2017; He 2012; Hou 2010; Jolly 2011; Kiemeneij 1997; Koltowski 2014; Li 2007; Louvard 2001; Louvard 2004; Michael 2013; Rao 2014; Reddy 2004; Romagnoli 2012; Saito 2003; Santas 2009; Slagboom 2005; Valgimigli 2015; Wang 2012; Ziakas 2010). Five studies were at a high risk of attrition bias (Benit 1997; Cantor 2005; Lange 2006; Mann 1998; Schernthaner 2018), while Gan 2009 had unclear risk of attrition bias.
Selective reporting
Twenty‐two studies were at low risk of reporting bias (Achenbach 2008; Akturk 2014; Benit 1997; Bernat 2014; Brueck 2009; Cantor 2005; De Andrade 2017; Gan 2009; Hou 2010; Jolly 2011; Kiemeneij 1997; Koltowski 2014; Louvard 2004; Mann 1998; Michael 2013; Rao 2014; Romagnoli 2012; Saito 2003; Schernthaner 2018; Valgimigli 2015; Wang 2012; Ziakas 2010), while Slagboom 2005 had unclear risk of reporting bias. The remaining eight studies were at high risk of reporting bias (Brasselet 2007; Cooper 1999; He 2012; Lange 2006; Li 2007; Louvard 2001; Reddy 2004; Santas 2009).
Other potential sources of bias
There were no other sources of bias.
Overall risk of bias
Based on our prespecified risk of bias assessment (Appendix 2), we judged eight trials (Akturk 2014; Bernat 2014; De Andrade 2017; Jolly 2011; Michael 2013; Rao 2014; Romagnoli 2012; Valgimigli 2015) as low risk, 12 trials (Benit 1997; Brasselet 2007; Cantor 2005; Cooper 1999; He 2012; Lange 2006; Li 2007; Louvard 2001; Mann 1998; Reddy 2004; Santas 2009; Schernthaner 2018) as high risk, and 11 trials (Achenbach 2008; Brueck 2009; Gan 2009; Hou 2010; Kiemeneij 1997; Koltowski 2014; Louvard 2004; Saito 2003; Slagboom 2005; Wang 2012; Ziakas 2010) as unclear risk of bias. Additional information can be found in the 'Risk of bias' summary (Figure 2 and Figure 3).
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
Net adverse clinical events
Four studies reported short‐term NACE (Bernat 2014; Jolly 2011; Romagnoli 2012; Valgimigli 2015). Transradial approach reduced short‐term NACE (RR (random‐effects) 0.76, 95% CI 0.61 to 0.94; 17,133 participants; 4 studies; T² = 0.03; I² = 66%; moderate quality evidence; Analysis 1.1). No trials reported long‐term NACE. In addition, the definition of NACE differed between these four trials. The heterogeneity in the included components of this endpoint between trials was an inherent problem of most composite outcomes and likely explained the observed heterogeneity.
1.1. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 1 Short‐term NACE.
Subgroup analysis
Diagnostic coronary angiography versus percutaneous coronary intervention
On subgroup comparisons, diagnostic CA and PCI had a similar risk in terms of short‐term NACE (test for subgroup differences: Chi² = 1.98, degrees of freedom (df) = 1 (P = 0.16), I² = 49.5%; Analysis 1.2).
1.2. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 2 Short‐term NACE (CA vs PCI).
ST‐segment elevation myocardial infarction versus non‐ST‐segment elevation acute coronary syndrome
There was a difference between the STEMI and NSTE‐ACS groups in terms of short‐term NACE (test for subgroup differences: Chi² = 3.11, df = 1 (P = 0.08), I² = 67.9%; Analysis 1.3). In people with STEMI, there was a reduction of short‐term NACE with the transradial approach (RR 0.67, 95% CI 0.51 to 0.87; 7676 participants; 4 studies; I² = 57%; Analysis 1.3), whereas in people with NSTE‐ACS, there was no difference in short‐term NACE between the two groups (RR 0.94, 95% CI 0.71 to 1.23; 9457 participants; 2 studies; I² = 66%; Analysis 1.3).
1.3. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 3 Short‐term NACE (STEMI vs NSTE‐ACS).
Women versus men
On subgroup comparisons, women and men had a similar risk in terms of short‐term NACE (test for subgroup differences: Chi² = 1.95, df = 1 (P = 0.16), I² = 48.7%; Analysis 1.4).
1.4. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 4 Short‐term NACE (women vs men).
Cardiac death
Eleven studies reported short‐term cardiac death (Cantor 2005; Gan 2009; Hou 2010; Koltowski 2014; Romagnoli 2012; Saito 2003; Schernthaner 2018; Slagboom 2005; Valgimigli 2015; Wang 2012; Ziakas 2010). Compared to transfemoral approach, there was a reduction in cardiac death with the transradial approach (RR 0.69, 95% CI 0.54 to 0.88; 11,170 participants; 11 studies; I² = 0%; moderate quality evidence; Analysis 1.5).
1.5. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 5 Short‐term cardiac death.
Subgroup analysis
ST‐segment elevation myocardial infarction versus non‐ST‐segment elevation acute coronary syndrome
On subgroup comparisons, STEMI and NSTE‐ACS had a similar risk in terms of short‐term cardiac death (test for subgroup differences: Chi² = 1.52, df = 1 (P = 0.22), I² = 34.2%; Analysis 1.6).
1.6. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 6 Short‐term cardiac death (STEMI vs NSTE‐ACS).
Sensitivity analysis
We visually explored publication bias by inspecting the funnel plot (Figure 4). We noted some asymmetry, suggesting possible publication bias. On formal evaluation of asymmetry, the intercept (B0) was ‐0.35855 (95% CI ‐0.76296 to 0.04586), with t = 2.00564, df = 9. The one‐tailed P value was 0.03793, and the two‐tailed P value was 0.07586. This could be attributed to the 'small‐studies effect.'
4.
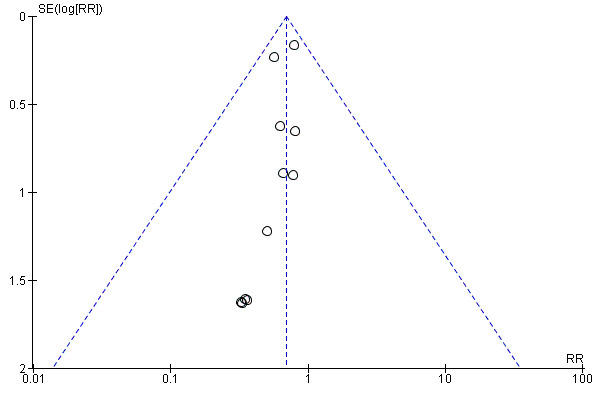
Funnel plot of comparison: 1 Transradial versus transfemoral, outcome: 1.5 Short‐term cardiac death.
Myocardial infarction
Fifteen studies reported short‐term MI (Benit 1997; Bernat 2014; Cantor 2005; Gan 2009; Hou 2010; Jolly 2011; Kiemeneij 1997; Louvard 2004; Mann 1998; Romagnoli 2012; Saito 2003; Schernthaner 2018; Slagboom 2005; Valgimigli 2015; Wang 2012), four of which had no events in either arm, so the meta‐analysis was based on 11 studies. Three studies reported long‐term MI (Gan 2009; Saito 2003; Schernthaner 2018). The risk of short‐term MI was similar with the transradial and transfemoral approaches (RR 0.91, 95% CI 0.81 to 1.02; 19,430 participants; 11 studies; I² = 0%; high quality evidence; Analysis 1.7). There was no evidence of a difference between groups in terms of long‐term MI, likely due to the limited number of events (RR 1.77, 95% CI 0.38 to 8.20; 556 participants; 3 studies; I² = 0%; Analysis 1.11).
1.7. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 7 Short‐term MI.
1.11. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 11 Long‐term MI.
Subgroup analysis
Elective versus primary percutaneous coronary intervention
On subgroup comparisons, the risk of short‐term MI was similar with elective and primary PCI (test for subgroup differences: Chi² = 0.43, df = 1 (P = 0.51); I² = 0%; Analysis 1.8).
1.8. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 8 Short‐term MI (elective vs primary PCI).
ST‐segment elevation myocardial infarction versus non‐ST‐segment elevation acute coronary syndrome
STEMI and NSTE‐ACS had a similar risk of short‐term MI (test for subgroup differences: Chi² = 0.59, df = 1 (P = 0.44), I² = 0%; Analysis 1.9).
1.9. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 9 Short‐term MI (STEMI vs NSTE‐ACS).
Sensitivity analysis
After omitting studies rated as high or unclear risk of bias for selection or attrition (or both) bias domains, risk of short‐term MI remained unchanged and did not differ between the transradial and transfemoral approaches (RR 0.92, 95% CI 0.81 to 1.05; 18377 participants; 6 studies, I² = 0%; Analysis 1.10).
1.10. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 10 Short‐term MI (sensitivity analysis).
Success of the procedure
Twenty‐eight studies reported success of the procedure, which was mainly defined as the completion of procedure without cross‐over to another access site, or as defined by trialists (Achenbach 2008; Akturk 2014; Benit 1997; Bernat 2014; Brasselet 2007; Brueck 2009; Cantor 2005; Cooper 1999; De Andrade 2017; Gan 2009; He 2012; Hou 2010; Jolly 2011; Kiemeneij 1997; Koltowski 2014; Li 2007; Louvard 2004; Mann 1998; Michael 2013; Rao 2014; Reddy 2004; Romagnoli 2012; Saito 2003; Santas 2009; Schernthaner 2018; Valgimigli 2015; Wang 2012; Ziakas 2010). There was a higher incidence of cross‐over with the transradial approach (RR (random‐effects) 0.97, 95% CI 0.96 to 0.98; 25,920 participants; 28 studies; T² = 0.00; I² = 76%; moderate quality evidence; Analysis 1.12). The definition of procedural success differed between trials, which likely explains the observed heterogeneity.
1.12. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 12 Success of the procedure.
Subgroup analysis
Diagnostic coronary angiography versus percutaneous coronary intervention
On subgroup comparisons, CA and PCI had a similar procedural success rate (test for subgroup differences: Chi² = 0.35, df = 1 (P = 0.55), I² = 0%; Analysis 1.13).
1.13. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 13 Success of the procedure (CA vs PCI).
Elective versus primary percutaneous coronary intervention
Elective and primary PCI had a similar procedural success rate (test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.97), I² = 0%; Analysis 1.14).
1.14. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 14 Success of the procedure (elective vs primary PCI).
ST‐segment elevation myocardial infarction versus non‐ST‐segment elevation acute coronary syndrome
STEMI and NTSTE‐ACS had a similar procedural success rate (test for subgroup differences: Chi² = 0.01, df = 1 (P = 0.92), I² = 0%; Analysis 1.15).
1.15. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 15 Success of the procedure (STEMI vs NSTE‐ACS).
Women versus men
Women and men had similar procedural success (test for subgroup differences: Chi² = 0.79, df = 1 (P = 0.37), I² = 0%; Analysis 1.16).
1.16. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 16 Success of the procedure (women vs men).
Sensitivity analysis
After omitting studies rated as high or unclear risk of bias for selection or attrition (or both) bias domains, the transradial approach had a lower procedural success rate compared to the transfemoral approach (RR (random‐effects) 0.95, 95% CI 0.94 to 0.96; 21,820 participants; 11 studies; T² = 0.00; I² = 60%; Analysis 1.17).
1.17. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 17 Success of the procedure (sensitivity analysis).
Secondary outcomes
All‐cause mortality
Thirteen studies reported short‐term all‐cause mortality (Akturk 2014; Benit 1997; Bernat 2014; Brasselet 2007; Brueck 2009; Jolly 2011; Kiemeneij 1997; Koltowski 2014; Louvard 2004; Mann 1998; Saito 2003; Slagboom 2005; Valgimigli 2015), three of which had no events in either arm, so the meta‐analysis was based on 10 studies. Three studies provided data on long‐term all‐cause mortality (Bernat 2014; Gan 2009; Saito 2003). The radial approach was associated with a reduction in short‐term all‐cause mortality (RR 0.77, 95% CI 0.62 to 0.95; 18,955 participants; 10 studies; I² = 0%; high quality evidence; Analysis 1.18). However, there was no difference in long‐term all‐cause mortality between the transradial and transfemoral groups (RR 0.62, 95% CI 0.29 to 1.32; 1013 participants; 3 studies; I² = 0%; Analysis 1.19).
1.18. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 18 Short‐term all‐cause mortality.
1.19. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 19 Long‐term all‐cause mortality.
Bleeding (combined major and minor)
Twenty‐two studies provided data on incidence of bleeding (Achenbach 2008; Akturk 2014; Benit 1997; Bernat 2014; Brasselet 2007; Cantor 2005; Cooper 1999; De Andrade 2017; Gan 2009; Hou 2010; Jolly 2011; Kiemeneij 1997; Koltowski 2014; Louvard 2001; Rao 2014; Romagnoli 2012; Saito 2003; Schernthaner 2018; Slagboom 2005; Valgimigli 2015; Wang 2012; Ziakas 2010), two of which reported no events in either arm, so the meta‐analysis was based on 20 studies. Transradial approach group was associated with a lower incidence of bleeding (RR (random‐effects) 0.54, 95% CI 0.40 to 0.74; 23,043 participants; 20 studies; T² =0.21; I² = 87%; low quality evidence; Analysis 1.20). The heterogeneity may be explained by the addition of the new "minimal" TIMI (Thrombolysis In Myocardial Infarction) bleeding group to the routine classification of major and minor, where the minimal was added to minor. Furthermore, some trialists used manual compression with the radial approach versus device closure with the femoral approach; contrary to the routine practice of manual only compression in the participants with femoral access and device closure in participants with radial access.
1.20. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 20 Bleeding.
Publication bias
We visually explored publication bias by inspecting the funnel plot (Figure 5). We noted some asymmetry, suggesting possible publication bias. On formal evaluation of asymmetry, the intercept (B0) was ‐1.36899 (95% CI ‐2.36344 to ‐0.37455), with t = 2.89223, df = 18. The one‐tailed P value was 0.00485, and the two‐tailed P value was 0.00971. This could be attributed to the 'small‐studies effect.'
5.
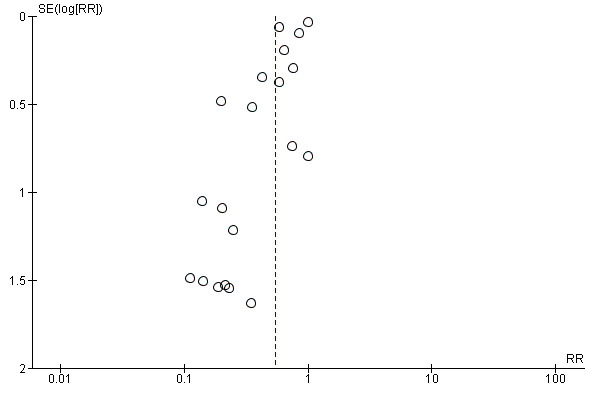
Funnel plot of comparison: 1 Transradial versus transfemoral, outcome: 1.20 Bleeding.
Stroke (ischaemic or haemorrhagic)
Ten studies reported short‐term stroke (Achenbach 2008; Bernat 2014; Brueck 2009; Cantor 2005; Cooper 1999; Jolly 2011; Koltowski 2014; Romagnoli 2012; Schernthaner 2018; Valgimigli 2015), one of which had no events in either arm, so the meta‐analysis is based on nine studies. The risk of stroke was similar between the transradial and transfemoral groups (RR 1.08, 95% CI 0.74 to 1.60; 19,017 participants; 9 studies; I² = 0%; Analysis 1.21).
1.21. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 21 Short‐term stroke.
Access site complications
Twenty‐four studies reported access site complications (Achenbach 2008; Akturk 2014; Benit 1997; Bernat 2014; Brasselet 2007; Brueck 2009; Cantor 2005; Cooper 1999; De Andrade 2017; Gan 2009; Hou 2010; Jolly 2011; Kiemeneij 1997; Li 2007; Louvard 2001; Louvard 2004; Mann 1998; Michael 2013; Rao 2014; Reddy 2004; Santas 2009; Schernthaner 2018; Wang 2012; Ziakas 2010). The transradial approach was associated with a reduced risk of access site complications (RR (random‐effects) 0.36, 95% CI 0.22 to 0.59; 16,112 participants; 24 studies; T² = 1.00; I² = 82%; low quality evidence; Analysis 1.22). Access site complications included different components in each trial, which likely explains the observed heterogeneity.
1.22. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 22 Access site complications.
Publication bias
We visually explored publication bias by inspecting the funnel plot (Figure 6). We noted some asymmetry, suggesting possible publication bias. On formal evaluation of asymmetry, the intercept (B0) was ‐1.60435 (95% CI ‐3.10584 to ‐0.10286), with t = 2.21594, df = 22. The one‐tailed P value was 0.01867, and the two‐tailed P value was 0.03735. This could be attributed to the 'small‐studies effect.'
6.
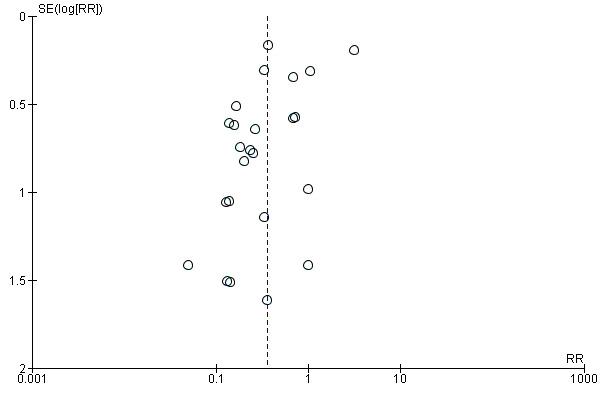
Funnel plot of comparison: 1 Transradial versus transfemoral, outcome: 1.22 Access site complications.
Total radiation dose
Four studies reported total radiation dose (Achenbach 2008;Lange 2006; Michael 2013; Schernthaner 2018). The units used in reporting (dose area product in mGy/cm² or Gy/cm² versus air kerma radiation exposure in Gy) were negligibly different among studies, so we used SMDs to pool the results, with all mGy/cm² converted to Gy/cm². Additionally, one of the studies only reported values for ad hoc PCI with no distinction between CA and PCI radiation exposure in the same participants. It also did not report the results of four participants who were randomised, but underwent PCI on subsequent days. Overall, the transradial approach was associated with a higher total radiation dose compared to the transfemoral approach (SMD 0.19, 95% CI 0.07 to 0.32; 980 participants; 4 studies; I² = 7%; Analysis 1.23).
1.23. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 23 Total radiation dose.
Subgroup analysis
Diagnostic coronary angiography versus percutaneous coronary intervention
Post hoc subgroup analysis was performed for total radiation dose, since some studies reported separate results for CA and PCI, while other studies reported mixed results on the whole randomised population, with no distinction between values of CA and PCI. The subgroups were as follows: CA only, PCI only, and CA plus PCI (i.e. mixed reporting). This heterogeneous reporting likely explains the substantial heterogeneity and lack of difference between the subgroups (test for subgroup differences: Chi² = 3.77, df = 2 (P = 0.15), I² = 47.0%). However, when individual subgroups were assessed, CA via the transradial approach was associated with a higher total radiation dose (SMD 0.28, 95% CI 0.06 to 0.50; 321 participants; 2 studies; I² = 0%; Analysis 1.23), while PCI via either approach had a similar total radiation dose (SMD ‐0.16, 95% CI ‐0.55 to 0.23; 102 participants; 1 study; Analysis 1.23). Studies that reported mixed population results (i.e. CA plus PCI) demonstrated a higher total radiation dose associated with the transradial approach (SMD 0.21, 95% CI 0.04 to 0.38; 557 participants; 2 studies; I² = 0%; Analysis 1.23).
Length of hospital stay
Ten trials reported on the length of hospital stay (Akturk 2014; Benit 1997; Brasselet 2007; De Andrade 2017; Gan 2009; Hou 2010; Kiemeneij 1997; Louvard 2001; Mann 1998; Saito 2003). In comparison to the transfemoral approach, the transradial approach was associated with a shorter hospital stay (MD (random‐effects) ‐1.06 days, 95% CI ‐1.49 to ‐0.63; 2798 participants; 10 studies; T² = 0.37; I² = 95%; Analysis 1.24).
1.24. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 24 Length of hospital stay.
Sensitivity analysis
We explored this considerable heterogeneity by carefully considering clinical and methodological factors. A sensitivity analysis that included studies with a low risk of selection and attrition bias showed no difference in the length of hospital stay between the two approaches (MD (IV, random) ‐0.15 days, 95% CI ‐0.41 to 0.11; 952 participants; 3 studies; I² = 0%; Analysis 1.25).
1.25. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 25 Length of hospital stay (sensitivity analysis).
Participant satisfaction
Only one trial reported the effect of the approach on participant satisfaction (Jolly 2011). Participants were more satisfied in the transradial group and it was the preferred approach for the subsequent procedures when compared to the transfemoral route (RR 1.58, 95% CI 1.52 to 1.63; 7021 participants; 1 study; Analysis 1.26).
1.26. Analysis.
Comparison 1 Transradial versus transfemoral approach, Outcome 26 Participant satisfaction.
Discussion
Summary of main results
We identified 31 studies that met our inclusion criteria. The RCTs included people undergoing elective or urgent CA or PCI (or both). There was a reduction in NACE (moderate quality evidence) and cardiac death (moderate quality evidence) with the transradial approach. There was no difference between the groups regarding MI (high quality evidence). Procedural success was less with the transradial approach, due to a higher rate of cross‐over to a different arterial access (moderate quality evidence). Short‐term all‐cause mortality (high quality evidence), bleeding (low quality evidence), and access site complications (low quality evidence) were less with the transradial approach. There was no difference in risk of long‐term mortality or stroke. The transradial approach was associated with a reduction in length of hospital stay and more participants preferred the transradial approach for their next procedure.
Overall completeness and applicability of evidence
The included studies were conducted in countries with different levels of income and included participants undergoing transradial or transfemoral CA or PCI (or both). The participants were aged 18 years and older with an age range of 52 to 83 years, which may restrict the applicability of the current evidence to this age group. The pooled studies provided a sufficient number of participants (31 studies, from 44 reports, with 27,071 participants). However, they did not report on long‐term NACE (i.e. beyond 30 days of follow‐up). In fact, only four trials including 17,133 participants reported NACE. Additionally, the definition of NACE differed substantially between these four trials. The heterogeneity in the included components of this endpoint between trials is an inherent problem of most composite outcomes and is a limitation to our analysis. Also, few studies assessed long‐term MI and all‐cause mortality. Regarding stroke, it was assessed as defined by the trialists as most of included studies did not classify them (i.e. ischaemic versus haemorrhagic). Trials included different definitions of bleeding, yet the definition of major bleeding was the same in different classification systems (e.g. TIMI, GUSTO (Global Utilization Of Streptokinase and TPA for Occluded arteries), and BARC (Bleeding Academic Research Consortium)), which has the impact on mortality. It is the definition of minor and minimal bleeding that is slightly different. Some subgroups were only reported by a limited number of trials, so caution should be exercised when interpreting these results. Most of the included studies excluded people with cardiogenic shock, as the radial puncture is technically more difficult and is time consuming in this situation. We still have two ongoing trials that will end in 2018 and 2019. Both studies will be included in the update of this review.
Quality of the evidence
Our systematic review included 31 studies, from 44 reports, with 27,071 participants. Accordingly, we believe the total number of participants and events was sufficient for our analysis, leaving no concern for imprecision. Most of the studies were unclear in either randomisation or allocation concealment methods, or both. Neither the participants nor the physicians were blinded, but we judged performance and detection biases as low risk, owing to the modus operandi of the interventions with most outcomes being objective and unlikely to be affected by lack of blinding. The sensitivity analyses showed results consistent with the primary comparison after exclusion of trials that had unclear allocation concealment or high risk of attrition bias. We are confident that the included studies clearly answered the review question, with no concern for indirectness in participants, interventions, comparators, or outcomes. We evaluated the quality of evidence using the GRADE approach. We noted some evidence of publication bias for short‐term cardiac death, bleeding, and access site complications. This may be attributed to the 'small‐studies effect' and was one of the main reasons for downgrading quality of evidence by one level. Another reason for downgrading was serious limitations in the study design. There was high quality evidence for short‐term MI, but moderate quality evidence for short‐term NACE, cardiac death, and procedural success. Regarding secondary outcomes, there was high quality evidence for short‐term all‐cause mortality, but low for bleeding and access site complications. It is worth noting that only 15 studies reported short‐term MI, which may introduce selective reporting bias. However, the evidence was still judged as high‐quality, since we only based our grading decisions on the studies included in the meta‐analysis.
Potential biases in the review process
The methodological rigour of Cochrane Reviews minimises bias in the process of conducting systematic reviews. We performed a comprehensive search to identify all eligible studies. We applied no restrictions by language or date of publications. Two authors independently assessed the eligibility of studies for inclusion and the risk of bias in each included study. The workflow of this rigorous screening, data extraction, and risk of bias assessment was continuously audited by an experienced Cochrane author (AN). However, any search strategy has a certain risk of missing relevant studies. Also, there is always a pragmatic restriction to the number of resources searched and an English language bias in the resources selected. Additionally, we did not receive missing primary outcome data from trialists regarding long‐term follow‐up.
Agreements and disagreements with other studies or reviews
Our review has a broad question encompassing the whole spectrum of CAD. It includes participants undergoing diagnostic CA as well as elective and primary PCI. We scrutinised multiple prespecified, clinically relevant subgroups and only include RCTs. We identified 20 systematic reviews comparing the transradial and transfemoral approaches among different populations, a few of which with similar eligibility criteria to ours.
Vorobcsuk 2009 and Jang 2012 included RCTs, case‐control studies, and cohort studies. They compared the safety and efficacy of transradial and transfemoral approaches in people with STEMI. Vorobcsuk 2009 reported a significant reduction in major bleeding, major adverse cardiac events (MACE), and length of hospital stay, but a longer fluoroscopic time and higher rate of cross‐over with the transradial approach. There was no difference in procedural duration or time to reperfusion, which are outcomes that we did not investigate. However, the longer fluoroscopic time observed with the transradial approach is in line with the higher total radiation dose that we observed in our analysis. Jang 2012 showed reduction in MACE, mortality, bleeding, and length of hospital stay with the transradial approach, which is consistent with our results.
Agostoni 2004, Jolly 2009, Liu 2015, Mitchell 2012, and Plourde 2015 compared the transradial and transfemoral approaches in people undergoing CA or PCI (or both). Similar to our analysis, they included only RCTs. Agostoni 2004 included 3224 participants from 12 RCTs and reported a significantly lower rate of access site complications with the transradial approach, while risk of MACE was similar in both approaches; likely related to the fewer number of participants and events. Jolly 2009 reported a reduction in major bleeding and a composite of death, MI, and stroke. Liu 2015 had a different search methodology, as they mainly searched Chinese databases, which was not part of our search strategy, and so they identified 27 RCTs including 8749 Chinese participants. They reported a lower success rate with the transradial approach in people undergoing CA, but similar in people undergoing PCI. We find this a particularly interesting finding which has no clear explanation. Additionally, risk of MACE was similar between the two approaches. The authors concluded and highlighted the safety and efficacy of the transradial approach in Chinese populations. We cannot make any affirmative statements in that regard. Mitchell 2012 included 14 RCTs and concluded that the transradial approach was favourable in terms of cost‐benefit value, which is outside the scope of our review, although quite unsurprising given the reduced complications as well as length of hospital stay demonstrated by our analysis. Plourde 2015 included 19,328 participants from 24 RCTs and showed that the transradial access was associated with a small but significant increase in fluoroscopic time for diagnostic CA and PCI. We did investigate this outcome, but similarly showed an increase in total radiation dose.
Bertrand 2012, Ferrante 2016, and He 2014 compared the transradial and transfemoral approaches in people undergoing PCI. Bertrand 2012 included 761,919 participants from 15 randomised and 61 observational studies. They reported reduction in bleeding and mortality with the transradial approach, although their findings were mainly derived from observational studies. Ferrante 2016 included 22,843 participants from 24 RCTs and reported significantly lower risk for all‐cause mortality, MACE, major bleeding, and major vascular complications with the transradial approach, while the rates of MI and stroke were similar in the two groups. He 2014 included 2188 participants from 11 studies, with majority favouring the transradial approach, as it was associated with a lower rate of vascular complications and major bleeding than the transfemoral approach. All these conclusions concur with ours.
Gandhi 2015 and Pancholy 2015 compared the transradial and transfemoral approaches in people with cardiogenic shock. In our review, all RCTs excluded participants with cardiogenic shock. Gandhi 2015 included 7753 participants from six observational studies and reported reduction in access site‐related and major bleeding. Pancholy 2015 included 8131 participants from eight studies and reported a reduction in short‐term MACE. Both reviews reported a reduction in mortality with the transradial approach. This could serve as an extrapolation of our findings, which may be applicable to people with cardiogenic shock.
Ando 2015, Del Furia 2016, and Ruiz‐Rodriguez 2016 compared the transradial and transfemoral approaches in people with ACS. Ando 2015 included 17,133 participants from four multi‐centre trials, Ruiz‐Rodriguez 2016 included 44,854 participants from 15 RCTs plus 17 cohort studies, and Del Furia 2016 included 12 RCTs. The three reviews showed reductions in MACE, bleeding, and mortality with the transradial approach. Ando 2015 reported a longer procedural time and higher rate of access‐site cross‐over in the transradial group. Ruiz‐Rodriguez 2016 demonstrated fewer access‐related complications with the transradial approach. Del Furia 2016 found no differences in risk of stroke and MI between approaches. All conclusions stated in the three reviews were in line with our observed findings.
Alonzo 2016 and colleagues pooled data of 777 participants in a retrospective registry from two centres comparing the transradial and transfemoral approaches with systemic closure by FemoSeal in people undergoing primary PCI. The transradial approach was associated with a lower risk of major bleeding. They found no difference in minor bleeding and MACE. Their design may have limited the number of participants and events. Additionally, we did not report on major and minor bleeding separately.
Ando 2016 and colleagues included 131,339 people undergoing invasive management for NSTE‐ACS from 11 randomised and observational studies. They found that long‐term mortality was lower with the transradial approach, which they attributed to a higher risk of major bleeding with the transfemoral approach and we do agree with their speculation. However, our review showed no difference between approaches in terms of long‐term all‐cause mortality after pooling the results of three RCTs (1013 participants). We may be limited by a fewer number of events, since we restricted our inclusion criteria only to RCTs.
Huang 2016 and colleagues investigated the gender disparity between approaches by pooling 15 RCTs and observational studies with 3,921,848 participants. They concluded that there was a significant gender disparity in terms of females having more adverse events and cross‐over rates, and stated that the transradial approach was safer and more efficacious in both genders. This was not supported by our results, which found no gender disparity. The inclusion of such large number of participants in their analysis is definitely a plus and, since we restricted our analysis only to RCTs, our numbers may be limited in terms of showing evidence of a difference in that regard.
Sirker 2016 only addressed stroke as an outcome of interest in their meta‐analysis on 112,343 participants from 36 studies, comprising RCTs, prospective cohort studies, and retrospective cohort studies, as well as cohort studies with an unclear design. Despite the differences in included study designs, they had a similar conclusion that risk of stroke is similar between the two approaches. Their key message was that stroke risk should not be a barrier to adoption of radial‐default practice, which we do agree with supported by our findings.
Shah 2017 and colleagues conducted both a standard meta‐analysis as well as a network meta‐analysis using mixed‐treatment comparison models, on 13 trials including 15,615 participants, to address the impact of operator experience on outcomes with transradial and transfemoral approaches. They concluded that survival differences reported in trials in favour of the transradial approach may be driven by a greater incidence of adverse events with the transfemoral approach, rather than a true beneficial effect of the transradial approach. They observed that the transradial approach seemed to reduce major bleeding events only if performed by radial‐experienced operators. Since our study design was different and we did not perform a network meta‐analysis or stratify outcomes by operator experience, we cannot make any judgements on the validity of these conclusions, but we believe this is an interesting area for further investigation.
Authors' conclusions
Implications for practice.
In people undergoing diagnostic CA or PCI (or both), the transradial approach may reduce the risk of net adverse clinical events (NACE), cardiac death, all‐cause mortality, bleeding, and access site complications. According to our findings, it is possible that major bleeding, including huge haematomas, retroperitoneal haematomas, and large haemoglobin drops, can influence cardiovascular and all‐cause mortality. This likely explains the better observed outcomes with the transradial approach. However, the results of this review should be put into context; for instance, the publication bias observed with reported bleeding may serve as an alternative explanation to our findings. Additionally, treating physicians should be alert to the longer learning curve and keep in mind that proficiency in the transfemoral approach should be maintained for special clinical scenarios where the transradial approach may not be readily feasible.
Implications for research.
In future randomised controlled trials comparing transradial and transfemoral approaches, investigators should:
assess long‐term outcomes (i.e. beyond 30 days of follow‐up);
attempt inclusion of participants with cardiogenic shock.
Acknowledgements
We thank the editorial team of the Cochrane Heart Group for editorial suggestions provided and Nicole Martin from the Cochrane Heart Group for developing the search strategy for this review. We also thank Wangyu Cai for his help with Chinese translation and data extraction. We appreciate the contribution of Mohamed Abdelazeem in the early stages of protocol development of this review.
Appendices
Appendix 1. Search strategy
CENTRAL
#1 MeSH descriptor: [Percutaneous Coronary Intervention] explode all trees
#2 (PCI or "percutaneous coronary intervention*")
#3 "percutaneous coronary revascularization*"
#4 (balloon near/3 angioplast*)
#5 atherectom*
#6 #1 or #2 or #3 or #4 or #5
#7 transradial
#8 MeSH descriptor: [Radial Artery] this term only
#9 #7 or #8
#10 #6 and #9
MEDLINE Ovid
1. exp Percutaneous Coronary Intervention/
2. (PCI or "percutaneous coronary intervention*").tw.
3. "percutaneous coronary revascularization*".tw.
4. (balloon adj3 angioplast*).tw.
5. atherectom*.tw.
6. or/1‐5
7. transradial.tw.
8. Radial Artery/
9. 7 or 8
10. 6 and 9
11. randomized controlled trial.pt.
12. controlled clinical trial.pt.
13. randomized.ab.
14. placebo.ab.
15. drug therapy.fs.
16. randomly.ab.
17. trial.ab.
18. groups.ab.
19. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20. exp animals/ not humans.sh.
21. 19 not 20
22. 10 and 21
Embase Ovid
1. exp percutaneous coronary intervention/
2. (PCI or "percutaneous coronary intervention*").tw.
3. "percutaneous coronary revascularization*".tw.
4. (balloon adj3 angioplast*).tw.
5. atherectom*.tw.
6. 1 or 2 or 3 or 4 or 5
7. transradial.tw.
8. radial artery/
9. 7 or 8
10. 6 and 9
11. random$.tw.
12. factorial$.tw.
13. crossover$.tw.
14. cross over$.tw.
15. cross‐over$.tw.
16. placebo$.tw.
17. (doubl$ adj blind$).tw.
18. (singl$ adj blind$).tw.
19. assign$.tw.
20. allocat$.tw.
21. volunteer$.tw.
22. crossover procedure/
23. double blind procedure/
24. randomized controlled trial/
25. single blind procedure/
26. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
27. (animal/ or nonhuman/) not human/
28. 26 not 27
29. 10 and 28
Web of Science
# 11 #10 AND #9
# 10 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)
# 9 #8 AND #5
# 8 #7 OR #6
# 7 TS="radial arter*"
# 6 TS=transradial
# 5 #4 OR #3 OR #2 OR #1
# 4 TS=atherectom*
# 3 TS=(balloon NEAR/3 angioplast*)
# 2 TS="percutaneous coronary revascularization*"
# 1 TS=(PCI or "percutaneous coronary intervention*")
Clinicaltrials.gov and ICTRP
Search terms: "coronary" and "radial"
Appendix 2. Risk of bias assessment
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk (any truly random process, e.g. random number table; computer random number generator); or
unclear risk.
There was no option to assess "random sequence generation" as high risk of bias as we had prespecified that we are not including quasi‐RCTs from the outset.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the method as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes); or
unclear risk.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
The nature of the intervention did not allow for blinding of participants. We considered that studies were at low risk of bias if we judged that the lack of blinding could not have affected the results.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received and provide any information relating to whether the intended blinding was effective.
We assessed the method as:
low risk;
high risk; or
unclear risk.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we reincluded missing data in the analyses that we undertook. We assessed methods as:
low risk;
high risk; or
unclear risk.
We categorised greater than 20% missing data as 'high' risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk (where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; the study did not include results of a key outcome that would have been expected to have been reported); or
unclear risk.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk;
high risk; or
unclear risk.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the likely magnitude and direction of bias and whether it would impact the findings.
We assessed the methods as:
low risk (low risk of bias for all key domains);
high risk (high risk of bias for one or more key domains); or
unclear risk (unclear risk of bias for one or more key domains).
We explored the impact of the level of bias through undertaking Sensitivity analysis.
Data and analyses
Comparison 1. Transradial versus transfemoral approach.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term NACE | 4 | 17133 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.94] |
| 2 Short‐term NACE (CA vs PCI) | 3 | 8729 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.98] |
| 2.1 CA | 1 | 2361 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.66, 1.44] |
| 2.2 PCI | 3 | 6368 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.48, 0.95] |
| 3 Short‐term NACE (STEMI vs NSTE‐ACS) | 4 | 17133 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.64, 0.93] |
| 3.1 STEMI | 4 | 7676 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.51, 0.87] |
| 3.2 NSTE‐ACS | 2 | 9457 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.23] |
| 4 Short‐term NACE (women vs men) | 2 | 15515 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 4.1 Women | 2 | 4093 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.62, 0.92] |
| 4.2 Men | 2 | 11422 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 5 Short‐term cardiac death | 11 | 11170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.54, 0.88] |
| 6 Short‐term cardiac death (STEMI vs NSTE‐ACS) | 1 | 8404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.04] |
| 6.1 STEMI | 1 | 4010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.29] |
| 6.2 NSTE‐ACS | 1 | 4394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 1.00] |
| 7 Short‐term MI | 11 | 19430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 8 Short‐term MI (elective vs primary PCI) | 9 | 18409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.04] |
| 8.1 Elective | 2 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.53, 2.74] |
| 8.2 Primary PCI | 7 | 17697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 9 Short‐term MI (STEMI vs NSTE‐ACS) | 1 | 8404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.05] |
| 9.1 STEMI | 1 | 4010 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.73, 1.43] |
| 9.2 NSTE‐ACS | 1 | 4394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.04] |
| 10 Short‐term MI (sensitivity analysis) | 6 | 18377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.05] |
| 11 Long‐term MI | 3 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.38, 8.20] |
| 12 Success of the procedure | 28 | 25920 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.96, 0.98] |
| 13 Success of the procedure (CA vs PCI) | 14 | 5787 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.94, 0.98] |
| 13.1 CA | 2 | 890 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.01] |
| 13.2 PCI | 13 | 4897 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.94, 0.98] |
| 14 Success of the procedure (elective vs primary PCI) | 19 | 20052 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.98] |
| 14.1 Elective | 4 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.92, 1.02] |
| 14.2 Primary PCI | 15 | 19065 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.98] |
| 15 Success of the procedure (STEMI vs NSTE‐ACS) | 11 | 3248 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 15.1 STEMI | 10 | 3008 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 15.2 NSTE‐ACS | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.93, 1.02] |
| 16 Success of the procedure (women vs men) | 2 | 1887 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.93, 0.97] |
| 16.1 Women | 1 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.93, 0.97] |
| 16.2 Men | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.00] |
| 17 Success of the procedure (sensitivity analysis) | 11 | 21820 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.94, 0.96] |
| 18 Short‐term all‐cause mortality | 10 | 18955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.62, 0.95] |
| 19 Long‐term all‐cause mortality | 3 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.29, 1.32] |
| 20 Bleeding | 20 | 23043 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.74] |
| 21 Short‐term stroke | 9 | 19017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.60] |
| 22 Access site complications | 24 | 16112 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.22, 0.59] |
| 23 Total radiation dose | 4 | 980 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.07, 0.32] |
| 23.1 CA | 2 | 321 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.06, 0.50] |
| 23.2 PCI | 1 | 102 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.55, 0.23] |
| 23.3 CA plus PCI (mixed reporting) | 2 | 557 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [0.04, 0.38] |
| 24 Length of hospital stay | 10 | 2798 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.49, ‐0.63] |
| 25 Length of hospital stay (sensitivity analysis) | 3 | 952 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.41, 0.11] |
| 26 Participant satisfaction | 1 | 7021 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.52, 1.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Achenbach 2008.
| Methods | Parallel‐group RCT comparing TRA versus TFA for CA and intervention in people aged ≥ 75 years. | |
| Participants |
307 people undergoing CA and intervention All participants referred for inpatient invasive angiography because of suspected coronary artery disease or suspected progression of known coronary disease and aged ≥ 75 years. Inclusion criteria: both the TR and TF approach needed to be clinically possible (e.g. negative Allen's test as assessed by pulse oxymetry), availability for a follow‐up visit 24 h after procedure, normal platelet count and plasmatic coagulation, Hb ≥ 9.0 g/dL, and informed consent to study participation. Exclusion criteria: cardiogenic shock, reduced renal function (creatinine > 1.5 mg/dL, because of potential future need of the radial artery to create an AV fistula) and planned simultaneous right and left heart catheterisation. |
|
| Interventions |
Group 1: TRA (n = 152) Group 2: TFA (n = 155) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Study also reported:
|
|
| Notes | Germany Funding source: grant sponsor: Deutsche Stiftung für Herzforschung, Frankfurt, Germany; Grant number: F/05/03 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted. |
Akturk 2014.
| Methods | Single‐centre parallel‐group RCT. Participants were randomised to TRA group and 437 participants were randomised to TFA group. Randomisation carried out using a concealed computer‐generated random sequence on an intention‐to‐treat basis. Randomisation list managed by nursing staff who informed interventional cardiologist of assigned approach just before procedure. Person responsible for participant registration and randomisation was not involved in treatment of participant. | |
| Participants |
836 participants with CA with suspicion of coronary artery disease Exclusion criteria: cardiogenic shock, acute STEMI, history of coronary artery bypass surgery, previous ipsilateral TRA, Raynaud's syndrome, simultaneous right heart catheterisation, necessity for a preprocedural implantation of a transient pacemaker, chronic renal insufficiency (creatinine > 2.0 mg/dL) with the potential necessity of using the radial artery as a native fistula in the future, people having haemodialysis with an AV fistula, absence of an experienced operator, participant refusal, and aged > 75 years because there may be some difficulties with the use of VAS among elderly people |
|
| Interventions |
Group 1: TRA group (n = 408) Group 2: TFA group (n = 428) |
|
| Outcomes |
|
|
| Notes | Turkey Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out using a concealed computer generated random sequence." |
| Allocation concealment (selection bias) | Low risk | "The randomisation list was managed by the nursing staff who informed the interventional cardiologist of the assigned approach just before the procedure. The person responsible for patient registration and randomisation was not in any way involved in the treatment of the patient." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes were unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention. We judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Blinding not reported; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participants' levels of pain due to being subjective and unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
Benit 1997.
| Methods | Multi‐centre multi‐arm RCT. Participants randomly assigned by telephone from a central office to either femoral, brachial, or radial approach. | |
| Participants | People scheduled to undergo coronary angioplasty as treatment of symptomatic ischaemic heart disease with an angiographic significant coronary artery stenosis (primary or restenotic) in a native artery measuring 3.0 mm (balloon diameter as reference) were eligible for the study. 150 men enrolled at 6 different sites between October 1994 and November 1995. Inclusion criteria: men, normal modified Allen's test, good pulsating radial artery, no multiple previous brachial cutdown procedures, no aortobifemoral bypass, no documented subclavian stenosis, and no contraindications to anticoagulant or antiplatelet therapy or to coronary stenting (important proximal coronary artery tortuosity, large side branch originating in lesion, lesion > 15 mm of length, angiographic evidence of thrombus) Exclusion criteria: women because of small size of radial arteries |
|
| Interventions |
Group 1: brachial approach (n = 38) Group 2: TFA (n = 56) Group 3: TRA (n = 56) Transbrachial arm excluded in our analysis |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Belgium Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned by telephone from a central office to either femoral or brachial or radial approach." |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned by telephone from a central office to either femoral or brachial or radial approach." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes are unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study performed "per‐protocol analysis." "Of these 150 patients, nine were excluded for further analysis because stent implantation did not occur using the scheduled approach." |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted. |
Bernat 2014.
| Methods | Randomised, national, multi‐centre, parallel‐group trial. Operators performed randomisation with personal password through computerised web system. | |
| Participants |
707 people with STEMI Inclusion criteria: people admitted to hospital with acute STEMI, within 12 h of symptom onset, and referred for an invasive approach with ability to use both access sites. Exclusion criteria: cardiogenic shock or inability to obtain written informed consent, prior aortobifemoral bypass, absence of bilateral radial or femoral artery pulses, participation in another ongoing clinical trial, negative Allen's test or Barbeau test type D curve, and treatment with oral anticoagulants |
|
| Interventions |
Group 1: TRA (n = 348) Group 2: TFA (n = 359) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Czech Republic Funding source: supported by Czech Republic Ministry of Health for conceptual development of research organization 00669806, Faculty Hospital in Pilsen, Czech Republic, and Charles University Research Fund (project no. P36). Data analysis supported by International Chair on Interventional Cardiology and Transradial Approach, established at Laval University, Quebec City, Quebec, Canada. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The operators performed randomisation with personal password through computerized web system." |
| Allocation concealment (selection bias) | Low risk | "The operators performed randomisation with personal password through computerized web system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all study's prespecified (primary and secondary) outcomes of interest in review were reported in the prespecified way (ClinicalTrials.gov Identifier: NCT1136187). |
| Other bias | Low risk | None noted. |
Brasselet 2007.
| Methods | Parallel‐group RCT comparing radial and femoral approaches to PCI using abciximab and 5F guiding‐catheters in AMI | |
| Participants |
114 participants consecutively and prospectively enrolled between January 2004 and September 2005 Inclusion criteria: ACS with ST‐segment elevation associated with sustained chest pain Exclusion criteria: haemodynamic instability (i.e. Killip state > 2 or cardiogenic shock), need for an intra‐aortic balloon pump or temporary pacemaker, history of CABG or intolerance to abciximab |
|
| Interventions |
Group 1: TRA (n = 57) Group 2: TFA (n = 57) |
|
| Outcomes |
|
|
| Notes | France Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to the nature of the intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but we judged risk of bias as high due to failure to report key outcomes expected to be reported for such a study (adverse cardiac events, i.e. cardiac death, stroke, MI, target lesion revascularisation) |
| Other bias | Low risk | None noted. |
Brueck 2009.
| Methods | Single‐centre, parallel‐group RCT. Eligible participants randomly assigned by computer generation (in 2 blocks in a 1:1 ratio) to TFA or TRA | |
| Participants | 1024 participants undergoing coronary catheterisation Inclusion criteria: participants referred for diagnostic or interventional cardiac catheterisation were screened for participation. Exclusion criteria: history of coronary artery bypass surgery, cardiogenic shock, known difficulties with the femoral approach (i.e. Leriche syndrome, severe peripheral artery disease, large abdominal aortic aneurysm) or radial approach (i.e. Raynaud's syndrome), simultaneous right heart catheterisation, pathological Allen's test, necessity for a preprocedural implantation of a transient pacemaker, chronic renal insufficiency (creatinine > 2.0 mg/dL) with the potential necessity of using the radial artery as a native fistula in the future, People receiving haemodialysis with an AV fistula, absence of an experienced operator, or participant refusal. |
|
| Interventions |
Group 1: TFA (n = 512) Group 2: TRA (n = 512) |
|
| Outcomes |
|
|
| Notes | Germany Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomly assigned by computer generation (in 2 blocks in a 1:1 ratio) to either transfemoral or transradial catheterisation." |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
Cantor 2005.
| Methods | Parallel‐group randomised, multi‐centre, pilot study. Randomisation performed in a concealed manner using sealed envelopes | |
| Participants |
50 people with STEMI who were referred for primary or rescue PCI at participating PCI centres were screened for eligibility. For primary PCI, participants could be enrolled within 12 h of symptom onset and for rescue PCI, participants could be enrolled within 12 h of thrombolysis. Rescue PCI was performed for suspected failed reperfusion or reocclusion based on symptoms and ECG changes Exclusion criteria: cardiogenic shock, abnormal Allen's test result, or contraindications to GP IIb/IIIa inhibitor use (active bleeding, major surgery/biopsy/significant trauma in past 6 weeks, systolic blood pressure > 200 mmHg or diastolic blood pressure > 110 mmHg, INR > 2, recent non‐compressible vascular puncture, central nervous system structural damage or stroke/transient ischaemic attack within the last 6 months, baseline platelet count < 100,000 cells/µL) |
|
| Interventions |
Group 1: TRA (n = 25) Group 2: TFA (n = 25) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
All outcomes evaluated during initial hospitalisation and at 30 days, except QoL, which was assessed at 24 h and 1 week after PCI. |
|
| Notes | Canada Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed in a concealed manner using sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding QoL due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | QoL after 1 week was assessed only for 36 participants (28% dropouts) without clearly accounting for the dropouts. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
Cooper 1999.
| Methods | Parallel‐group RCT. Participants randomly assigned by coin toss to TFA or TRA | |
| Participants |
200 participants referred for diagnostic cardiac catheterisation were screened for participation Inclusion criteria: palpable femoral and radial pulses, and normal Allen's test Exclusion criteria: known or suspected vascular disease precluding access, unstable coronary symptoms, need for additional procedures during same hospitalisation, or unable or unwilling to give informed consent Participants not excluded for age, sex, body size, or race |
|
| Interventions |
Group 1: TRA (n = 101) Group 2: TFA (n = 99) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Study also reported:
All outcomes assessed 1 day and 1 week after catheterisation, QoL assessed before catheterisation |
|
| Notes | USA Funding source: supported by a grant from Cordis Corporation. Dr Cohen was supported in part by a Clinician‐Scientist Award from the American Heart Association. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned by coin toss to either transfemoral or transradial catheterisation." |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding of assessors of participant preference and QoL; however, blinding impossible due to nature of intervention, but we judged risk of bias as high due to these outcomes being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No patient was lost to follow‐up." |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but we judged risk of bias as high due to failure to report key outcomes expected to be reported for such a study (adverse cardiac events, i.e. cardiac death, MI) |
| Other bias | Low risk | None noted |
De Andrade 2017.
| Methods | Parallel‐group, national, multi‐centre, non‐inferiority RCT comparing the radial versus femoral approach using VCD. Randomisation using a randomised sequence obtained by computer algorithms and maintained in individual, opaque, and closed envelopes to conceal the allocation process. | |
| Participants | Between July 2012 and March 2015, 240 people with NSTEMI undergoing early invasive strategy were randomised to radial or femoral approach with use of the Angio‐Seal VCD (St Jude Medical, St Paul, MN, USA). Participants had to have at least 2 of 3 high‐risk criteria: ischaemic changes in 12‐lead ECG, positive biomarkers, or age > 60 years. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group 1: TRA (n = 120) Group 2: TFA (n = 120) |
|
| Outcomes |
Primary outcome: assessed at 30 days
Secondary outcomes: assessed at 12 months
|
|
| Notes | Brazil Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients will be randomised for the radial or femoral technique with VCD using a randomised sequence obtained by computer algorithms and maintained in individual, opaque and closed envelopes to conceal the allocation process." |
| Allocation concealment (selection bias) | Low risk | "Patients will be randomised for the radial or femoral technique with VCD using a randomised sequence obtained by computer algorithms and maintained in individual, opaque and closed envelopes to conceal the allocation process." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes are unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all study's prespecified primary outcomes (30 days) of interest in review were reported in the prespecified way. **However, it must be noted that none of the study's prespecified secondary outcomes (1 year of follow‐up) were reported in this publication or in any other separate following publications. |
| Other bias | Low risk | None noted |
Gan 2009.
| Methods | Parallel‐group randomised, multi‐centre, open‐label trial, where participants were randomly divided into 2 groups: TRA (radial group) and TFA (femoral group). | |
| Participants |
195 people with AMI, within 12 h from onset of symptoms, randomly divided into 2 groups: 90 participants treated by TRA (radial group) and 105 participants by TFA (femoral group) Inclusion criteria included: typical chest pain lasting > 30 min and < 12 h, nitrate losing efficacy, with ST segment elevation > 0.1 mV in limb leads or > 0.2 mV in ≥ 2 adjacent chest leads Exclusion criteria for TRA included: negative Allen's test (these cases were switched to TFA group) |
|
| Interventions |
Group 1: TRA (n = 90) Group 2: TFA (n = 105) |
|
| Outcomes |
Outcomes assessed inhospital and at 6 months of follow‐up |
|
| Notes | China Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators not masked to treatment allocation, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessors not masked to treatment allocation; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up at 6 months: 23/190 (12%) Dropouts not accounted for |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
He 2012.
| Methods | Single‐centre parallel‐group RCT | |
| Participants |
360 participants (200 men, 160 women) single centre, aged 25‐78 years, mean (± SD) age 59.3 ± 8.9 years Inclusion criteria: people with diagnostic CA or coronary artery intervention |
|
| Interventions |
Group 1: TRA (n = 180) Group 2: TFA (n = 180) |
|
| Outcomes |
|
|
| Notes | China Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but we judged risk of bias as high due to failure to report key outcomes expected to be reported for such a study (adverse cardiac events, i.e. cardiac death, stroke, MI, target lesion revascularisation) |
| Other bias | Low risk | None noted |
Hou 2010.
| Methods | Single‐centre parallel‐group RCT where participants were randomly divided into TRA group and TFA group. | |
| Participants | From August 2005 to September 2008, 200 people with AMI were included in study which took place in Department of Cardiology, The Tenth People's Hospital, Tongji University, Shanghai, China. After written informed consent obtained, participants were randomly divided into TRA group and TFA group. Exclusion criteria were clinical indications to femoral approach due to cardiogenic shock, history of coronary bypass graft, negative Allen's test, and non‐palpable radial artery. |
|
| Interventions |
Group 1: TRA (n = 100) Group 2: TFA (n = 100) |
|
| Outcomes |
Endpoints recorded from start of procedure to 1‐month follow‐up |
|
| Notes | China Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One month follow‐up was complete in all patients." |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
Jolly 2011.
| Methods | Multi‐centre, parallel‐group RCT. Participants were randomly assigned (1:1) to radial or femoral access by a 24‐h computerised central automated voice response system located at the Population Health Research Institute in Hamilton, Canada. Randomisation in permuted blocks of variable sizes (2, 4, and 6), stratified by centre. | |
| Participants |
7021 people with ACS (with or without ST elevation) randomised to radial or femoral access for CA/intervention Inclusion criteria: eligible for RIVAL (RadIal Vs femorAL access for coronary intervention) if they presented with non‐ST‐segment elevation ACS or ST‐segment elevation ACS, they were to be managed with an invasive approach, they had intact dual circulation of the hand documented by Allen's test Exclusion criteria: aged < 18 years; active bleeding or significant increased risk of bleeding (severe hepatic insufficiency, current peptic ulceration, proliferative diabetic retinopathy); uncontrolled hypertension; cardiogenic shock; prior CABG surgery with use of > 1 internal mammary artery; documented severe peripheral vascular disease precluding a femoral approach; previously entered in study; investigational treatment (drug or device) within the previous 30 days; medical, geographic, or social factors making study participation impractical or inability to provide written informed consent and to understand the full meaning of the informed consent |
|
| Interventions |
Group 1: TRA (n = 3507) Group 2: TFA (n = 3514) |
|
| Outcomes |
Primary outcome:
Secondary outcomes at 48 h and 30 days:
Other outcomes:
Safety outcomes:
Radiation outcomes:
PCI complications:
Study also reported:
|
|
| Notes | Canada, Finland, India, Czech Republic, Poland, Spain, Israel, Brazil, France, and England Funding source: funding for the RIVAL trial was provided by Sanofi‐Aventis, Population Health Research Institute, and the Canadian Network and Center for Trials Internationally (CANNeCTIN), which was funded by the Canadian Institutes of Health Research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned (1:1) to radial or femoral access by a 24‐hour computerised central automated voice response system located at the Population Health Research Institute in Hamilton, Canada." |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned (1:1) to radial or femoral access by a 24‐hour computerised central automated voice response system located at the Population Health Research Institute in Hamilton, Canada." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were not masked to treatment allocation, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "A masked central committee adjudicated the primary outcome, components of the primary outcome, and stent thrombosis." |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding of assessors of outcomes of pain at access site and participant preference for next procedure; however, blinding impossible due to nature of intervention, but we judged risk of bias as high due to these outcomes being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all study's prespecified (primary and secondary) outcomes of interest in review were reported in prespecified way (ClinicalTrials.gov Identifier: NCT01014273). |
| Other bias | Low risk | None noted |
Kiemeneij 1997.
| Methods | Single‐centre multi‐arm RCT. Randomisation performed using sealed envelopes containing a code for TR, transbrachial, or TF angioplasty. Envelopes were ordered at random and included a registration number from 1 to 900. | |
| Participants | 900 included who underwent PTCA at the department. Inclusion criteria: stable and unstable angina selected for single or multi‐vessel PTCA of lesions in native coronary arteries and venous bypass grafts. Underwent PTCA by the TRI, transbrachial approach, or TFA. Exclusion criteria: vascular status (absence of pulse in femoral, brachial, or radial arteries; abnormal Allen's test results; failed previous arterial access); cardiac status (chronic total occlusion, acute MI, expected severe haemodynamic deterioration during balloon inflation or after PTCA failure leading to intra‐aortic pumping or right heart catheterisation for haemodynamic monitoring, expected need for a temporary pacemaker); procedural (ad hoc PTCA after TF diagnostic catheterisation, indwelling sheath from previous arterial puncture, planned primary stent implantation, planned coronary atherectomy); no consent |
|
| Interventions | Arm 1: TRA PTCA (n = 300) Arm 2: transbrachial approach PTCA (n = 300) Arm 3: TFA PTCA (n = 300) The transbrachial arm was excluded in our analysis. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Primary outcomes recorded from start of procedure to 1‐month follow‐up. |
|
| Notes | The Netherlands Funding source: study supported by a grant from Scimed Life Systems, Inc., Maple Grove, MN. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation. |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed by opening a sealed envelope containing a code for either transradial (R), transbrachial (B) or transfemoral (F) angioplasty." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted. |
Koltowski 2014.
| Methods | Parallel‐group, open‐label RCT. After informed consent acquired, participants randomised to femoral or radial access. The random sequence generated and allocation concealed using opaque envelopes containing icon‐cards. | |
| Participants |
103 people with STEMI screened between September 2010 and October 2012. Inclusion criteria: pain duration between 20 min and 24 h, ST‐segment elevation measured at J point in 2 contiguous leads ≥ 0.25 mV in men aged < 40 years, ≥ 0.2 mV in men aged > 40 years, or ≥ 0.15 mV in women in leads V2‐V3 or ≥ 0.1 mV in other leads (or both) (in the absence of left ventricular hypertrophy or LBBB) or newly emerged LBBB, aged ≥ 18 years, and person's informed consent Exclusion criteria: INR > 1.4, thrombocytopenia < 100 × 10³, previous CABG, known vascular access difficulties or complications, active bleeding, gastric or duodenal peptic ulcer, current or planned dialysis, severe liver failure (MELD > 10 points), uncontrolled hypertension (> 160/100 mmHg), cardiogenic shock, and low compliance to long‐term follow‐up. |
|
| Interventions |
Group 1: TRA (n = 52) Group 2: TFA (n = 51) |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Study also reported:
|
|
| Notes | Poland Funding source: investigator‐initiated trial funded by authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Low risk | "The random sequence was generated and the allocation was concealed using opaque envelopes containing icon‐cards." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label RCT, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Open‐label RCT; however, blinding impossible due to nature of intervention, but we judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Open‐label RCT; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding QoL due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
Lange 2006.
| Methods | Parallel‐group RCT. After informed consent was obtained, catheterisation procedure was performed randomly by TFA or TRA by same operator. | |
| Participants | After completion of procedure, only cases were included for data analysis who fulfilled the following criteria: procedure was uncomplicated and right femoral or right radial artery could be accessed without difficulties; only coronary angiograms were performed (i.e. cases with bypass graft angiography, left ventricular cineangiography, or aortography were excluded); coronary interventions were done electively on 1 single epicardial vessel. Cases with bypass graft intervention, bifurcation lesions, and chronic total occlusions were excluded. | |
| Interventions |
Group 1: TRA (n = 146) Group 2: TFA (n = 151) |
|
| Outcomes |
|
|
| Notes | Germany Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Only cases were included for data analysis who fulfilled the following criteria: the procedure was uncomplicated and the right femoral or right radial artery could be accessed without difficulties." Study performed "per‐protocol analysis." |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but we judged risk of bias as high due to failure to report key outcomes expected to be reported for such a study (adverse cardiac events (i.e. cardiac death, stroke, MI, target lesion revascularisation). |
| Other bias | Low risk | None noted |
Li 2007.
| Methods | Parallel‐group RCT of TRA versus TFA | |
| Participants | From June 2004 to June 2006, 370 consecutive participants admitted to hospital diagnosed as AMI within 12 h from onset of chest pain. Exclusion criteria: for TRA: negative Allen's test, aorto‐arteritis, cardiogenic shock, non‐palpable radial artery, severe tortuosity of radial arteries or body height < 150 cm. 244 men and 126 women randomly allocated to TRA or TFA |
|
| Interventions |
Group 1: TRA (n = 184) Group 2: TFA (n = 186) |
|
| Outcomes |
|
|
| Notes | China Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding low back pain due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but we judged risk of bias as high due to failure to report key outcomes expected to be reported for such a study (adverse cardiac events (i.e. cardiac death, stroke, MI, target lesion revascularisation) |
| Other bias | Low risk | None noted |
Louvard 2001.
| Methods | Multi‐arm RCT. Learning curve taken into account in randomisation of participants. 1 operators with experience in left radial approach randomised participants into left TRA and TFA and the other operator, with previous right radial approach experience into right TRA and TFA. Randomisation ratio was 2 participants in TRA group for 1 in TFA group to obtain 3 even groups. | |
| Participants |
210 participants randomised over 6‐month period between November 1998 and April 1999. Inclusion criteria: normal Allen's test and participant informed consent. Exclusion criteria: clinical (AMI), angiographic (previous bypass grafting), or technical (known difficulty with TFA, right heart catheterisation, simultaneous renal or aortic angiography, and absence of indication for ventricular angiogram). No other selection made as to symptoms, age, gender, weight, or height. |
|
| Interventions |
Group 1: TFA (n = 70) Group 2: right TRA (n = 70) Group 3: left TRA (n = 70) Right and left TRA groups were combined in our analysis. |
|
| Outcomes |
|
|
| Notes | France Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant comfort due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but we judged risk of bias as high due to failure to report key outcomes expected to be reported for such a study (adverse cardiac events (i.e. cardiac death, stroke, MI, target lesion revascularisation). |
| Other bias | Low risk | None noted |
Louvard 2004.
| Methods | Multi‐centre parallel‐group RCT. Randomisation carried out using blinded allocation list before clinical examination of femoral arteries or assessment of hand blood supply. | |
| Participants | Aged > 80 years presenting for CA or PCI. Inclusion criteria: informed consent. All clinical presentations were permissible (including ST‐segment elevation AMI), except cardiogenic shock. Exclusion criteria: history of bypass grafting surgery using 2 in situ internal mammary arteries, documented severe peripheral artery disease, or with previous unsuccessful approaches |
|
| Interventions |
Group 1: TRA (n = 192) Group 2: TFA (n = 185) |
|
| Outcomes |
Primary outcome: Composite vascular endpoint comprising ≥ 1 of the following:
Secondary outcomes:
Other outcomes:
|
|
| Notes | France and UK Funding source: not described We contacted the authors for missing data regarding randomisation methods, but received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted. |
Mann 1998.
| Methods | Parallel‐group RCT. Participants were randomised to either radial or femoral group access prior to the catheterisation procedure. | |
| Participants | 142 people with ACS admitted to hospital between April and July 1997. Participants within this group who underwent coronary stenting were included. | |
| Interventions |
Group 1: TRA (n = 74) Group 2: TFA (n = 68) |
|
| Outcomes |
|
|
| Notes | USA Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study performed "as treated" analysis: (during course of study, 6/74 (8%) participants randomised to TRA had a negative Allen's test or Doppler examination (or both) suggesting an incomplete palmer arch; these were included in TFA group. Of the remaining 68 participants in TRA group, 65 participants had their procedures performed TR. In 3 participants, radial artery was not successfully cannulated, and these were also included in TFA group. Thus, 77 participants who had their procedures performed from TFA included 9 radial cross‐overs). |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
Michael 2013.
| Methods | Single‐centre, parallel‐group RCT. Participants randomised to TRA or TFA in 1:1 ratio using opaque, numbered, sealed envelopes containing randomisation assignment based on a computer‐generated random sequence. | |
| Participants | 128 participants having previously undergone CABG surgery and referred for cardiac catheterisation at Dallas VA Medical Center. Inclusion criteria: previously undergone CABG surgery and referred for diagnostic or interventional cardiac catheterisation. Exclusion criteria: presenting with ST‐segment elevation AMI, abnormal Allen's test results, known difficulty obtaining vascular access via either femoral or radial artery, or aged > 90 years. |
|
| Interventions |
Group 1: TRA (n = 64) Group 2: TFA (n = 64) |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
All endpoints assessed separately for diagnostic angiography and PCI (i.e. measurements for diagnostic catheterisation ended with completion of diagnostic angiography and measurements for PCI began on PCI initiation and ended on PCI completion). Data regarding participant satisfaction collected 1 day after procedure. |
|
| Notes | USA Funding source: Dr Michael received a cardiovascular training grant from the National Institutes of Health, Award Number T32HL007360. Dr Banerjee received a research grant from Boston Scientific and an institutional research grant from Gilead. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised to TR or TF access in a 1:1 ratio using opaque, numbered, sealed envelopes containing randomisation assignment based on a computer generated random sequence." |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised to TR or TF access in a 1:1 ratio using opaque, numbered, sealed envelopes containing randomisation assignment based on a computer generated random sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant satisfaction due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all study's prespecified (primary and secondary) outcomes of interest in review were reported in prespecified way (ClinicalTrials.gov Identifier: NCT01446263). |
| Other bias | Low risk | None noted |
Rao 2014.
| Methods | Multi‐centre, parallel‐group, open‐label RCT. After informed consent obtained, but before obtaining arterial access for procedure, participants randomly assigned (1:1) to TRA or TFA. Web‐based randomisation performed using a DCRI proprietary Simple Internal Randomization Engine in block fashion within sites. Participants enrolled at sites performing ad hoc PCI randomised before diagnostic angiography. All primary endpoint events were adjudicated by an independent Clinical Events Committee. | |
| Participants |
1775 women (691 undergoing PCI) randomised at 60 sites. Inclusion criteria: women, aged ≥ 18 years, undergoing urgent or elective PCI, undergoing diagnostic angiography for ischaemic symptoms with possible PCI, and ability to provide informed consent for trial participation. Exclusion criteria: peripheral arterial disease prohibiting vascular access, bilateral abnormal Barbeau tests, haemodialysis access (AV fistula or graft) in the arm to be used for PCI in case of assignment to radial approach (the opposite arm may be used for radial access if a dialysis graft is present in 1 arm provided that the opposite arm has a normal Barbeau test result), valvular heart disease requiring valve surgery, planned right heart catheterisation, primary PCI for STEMI, presence of bilateral internal mammary artery coronary bypass grafts, participation in investigational drug or device study currently or within 30 days before enrolment, INR ≥ 1.5 while treated with oral vitamin K antagonists (i.e. warfarin), receipt of oral factor Xa or IIa inhibitors ≤ 24 h before procedure, planned staged PCI within 30 days after index procedure. |
|
| Interventions |
Group 1: TRA (n = 891) Group 2: TFA (n = 884) |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Secondary endpoints were assessed only in the subgroup of participants undergoing PCI. |
|
| Notes | England, USA, Canada Funding source: Abbott Vascular, Medtronic Vascular (grant no. A 1054367), Terumo Medical, The Medicines Company, Daiichi Sankyo/Eli Lilly and Company (grant no. H7TUS‐X014), ACIST Medical, Guerbet, FDA Office of Women's Health (grant no. HHSF223201111381P), and The Duke Clinical Research Institute funded this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to radial or femoral access. The allocation ratio for randomisation is 1:1 between treatment arms, and Web‐based randomisation was performed using a DCRI proprietary Simple Internal Randomization Engine in block fashion within sites." |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned to radial or femoral access. The allocation ratio for randomisation is 1:1 between treatment arms, and Web‐based randomisation was performed using a DCRI proprietary Simple Internal Randomization Engine in block fashion within sites." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "All primary end point events will be adjudicated by an independent Clinical Events Committee." |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participants' access site preference for their next procedure due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Follow‐up for the primary endpoints was available for 99.3% of the total randomised cohort." |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all study's prespecified (primary and secondary) outcomes of interest in review were reported in the prespecified way (ClinicalTrials.gov Identifier: NCT01406236). |
| Other bias | Low risk | None noted. |
Reddy 2004.
| Methods | Multi‐arm RCT. Participants were randomly assigned to TRA and TFA for cardiac catheterisation. | |
| Participants |
75 participants referred for diagnostic cardiac catheterisation were screened for study participation. Inclusion criteria: aged ≥ 18 years, easily palpable femoral and radial pulses, and normal Allen's test. Exclusion criteria: vascular disease of upper or lower extremities precluding access at either femoral or radial artery, prior femoral arterial graft surgery, unstable coronary syndromes, haemodynamically unstable patients with MI who require an intervention within 7 days, people for whom additional procedures were planned at the same setting or during the same hospital stay (e.g. PCI, peripheral angiography or intervention, electrophysiology study), and people unable or unwilling to provide informed consent. |
|
| Interventions |
Group 1: TFA 6F with AngioSeal closure device (n = 25) Group 2: TFA 4F without a closure device (n = 25) Group 3: TRA (n = 25) The 2 TFA groups were combined in our analysis. |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Assessed at 1 day and 1 week post procedure follow‐up. |
|
| Notes | USA Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding QoL due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data obtained for 73 participants at 1‐day and 70 participants at 1‐week follow‐up visits. |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but we judged risk of bias as high due to failure to report key outcomes expected to be reported for such a study (adverse cardiac events (i.e. cardiac death, stroke, MI, target lesion revascularisation). |
| Other bias | Low risk | None noted |
Romagnoli 2012.
| Methods | Multi‐centre, parallel‐group RCT. All enrolled participants were randomised (1:1 ratio) to TRA or TFA according to opaque, numbered, sealed envelopes with randomisation based on a computer‐generated random series and stratified by centre. | |
| Participants | People with suspected STEACS planned for early revascularisation strategy (within 24‐h of symptom onset) were eligible. Exclusion criteria: contraindication to either radial or femoral vascular access (e.g. abnormal Allen's test or known severe peripheral vascular disease), recent stroke (within 4 weeks), anticoagulant therapy assumption with an INR 2, or other severe bleeding diathesis. Cardiogenic shock or haemodynamic instability (or both) did not preclude enrolment. |
|
| Interventions |
Group 1: TRA (n = 500) Group 2: TFA (n = 501) |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Italy and The Netherlands Funding source: no extramural funding used to support the work, and authors were solely responsible for design, conduct, and final contents of study. We contacted the authors regarding long‐term outcome data. They responded that the data were unavailable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All enrolled patients were randomised (1:1 ratio) to radial or femoral access according to opaque, numbered, sealed envelopes with randomisation based on a computer generated random series and stratified by centre." |
| Allocation concealment (selection bias) | Low risk | "All enrolled patients were randomised (1:1 ratio) to radial or femoral access according to opaque, numbered, sealed envelopes with randomisation based on a computer generated random series and stratified by centre." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were not blinded to procedure, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "Endpoint adjudication was performed by a blinded central independent clinical‐event committee." |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant was lost at 30‐day follow‐up; thus, all 1001 participants were included in final intention‐to‐treat analyses. 14 of participants originally randomised to femoral arm were crossed over to radial access 47 of participants originally randomised to radial arm were crossed over to femoral access; however, intention‐to‐treat analysis performed. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all study's prespecified primary outcomes (30‐days) of interest in review were reported in prespecified way (ClinicalTrials.gov Identifier: NCT01420614). **However, it must be noted that none of the study's prespecified secondary outcomes (1‐year follow‐up) were reported in this publication or in any other separate subsequent publications. |
| Other bias | Low risk | None noted |
Saito 2003.
| Methods | Parallel‐group RCT. Participants randomised to TRI or TFI groups before arterial puncture. | |
| Participants |
149 participants with AMI Inclusion criteria: AMI within 12 h from onset of study if written informed consent obtained before emergency catheterisation, onset and location of infarction were clearly documented, participant had not received any type of thrombolytic therapies, aged > 20 years, and normal Allen's test. When a participant came into the catheterisation laboratory with shock, that participant was considered eligible for randomisation if the radial artery pulse could be felt. Exclusion criteria: radial artery pulse too weak for successful radial artery puncture, culprit vessel was previous coronary bypass graft, and operator for that particular person did not consider that both TRI and TFI would be equally feasible. |
|
| Interventions |
Group 1: TRI (n = 77) Group 2: TFI (n = 72) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | Japan Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total dropouts at 9 months' follow‐up: 10/149 (6%); TRI group: 4/77 (5%); TFI group: 6/72 (8%) |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
Santas 2009.
| Methods | Multi‐arm RCT. Randomised to undergo TFA, right radial approach (RRA), or left radial approach (LRA) using a block design to ensure that same number of participants would be assigned to each technique for arterial access. Randomisation list kept by nursing staff, who informed interventional cardiologist of assigned approach prior to procedure. | |
| Participants |
1005 consecutive participants who underwent cardiac catheterisation between January 2007 and July 2007. Allen's test was performed in all participants assigned to radial approach; TR catheterisation not attempted if results of Allen's test were clearly abnormal (in which case, it was considered that the procedure could not be completed via said approach). Exclusion criteria: none |
|
| Interventions |
Group 1: left TRA (n = 335) Group 2: right TRA (n = 335) Group 3: TFA (n = 335) Right and left TR groups were combined in our analysis. |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Spain Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Low risk | "The randomisation list was in the hands of the nursing staff, who informed the interventional cardiologist of the assigned approach prior to the procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but we judged risk of bias as high due to failure to report key outcomes expected to be reported for such a study (adverse cardiac events (i.e. cardiac death, stroke, MI, target lesion revascularisation) |
| Other bias | Low risk | None noted |
Schernthaner 2018.
| Methods | Multi‐centre parallel‐design RCT. Participants presenting with AMI were randomly assigned (1:1) to TRA or TFA group for diagnostic CA and PCI, if indicated. | |
| Participants | 250 participants included between April 2010 and November 2011 in 4 different centres in Austria. Inclusion criteria: AMI with or without ST‐segment elevation with imminent immediate invasive intervention and had given written informed consent to participate in the study protocol prior to treatment. Diagnosis of AMI based on clinical symptoms, elevated serum CK or cardiac troponin I levels (or both) and standardised ECG changes. Treating interventional cardiologist had to have expertise for both techniques and had to perform both approaches on a regular basis for diagnostic and intervention purposes (> 150 PCI per year, including at least 40% radial procedures within previous year). Dual circulation of hand assessed by Allen's test in all participants prior to study inclusion. Exclusion criteria: pathological Allen's test, cardiogenic shock with need for implantation of an intra‐aortic balloon pump, any type of vasculitis leading to ischaemia, AV fistula for haemodialysis, peripheral artery disease precluding femoral access, CABG with bilateral mammary artery grafts. |
|
| Interventions |
Group 1: TRA (n = 125) Group 2: TFA (n = 125) |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Clinical follow‐up performed at 30 days and at 1 year. |
|
| Notes | Austria Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up 20%, without clearly accounting for the dropouts: "The 1‐year follow up data were available from 199 patients." |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
Slagboom 2005.
| Methods | Parallel‐group RCT. Randomisation to TRA or TFA performed by opening a sealed envelope containing a code for TR or TF angioplasty. Envelopes ordered at random and contained an inclusion number. | |
| Participants |
Inclusion criteria: stable and unstable angina pectoris (Braunwald class 1 and 2), type A and B lesions (NHLBI criteria); type C lesions only if there was an intention and technical possibility to implant a stent; multi‐vessel disease; and multi‐vessel PTCA if not more than 1 treated vessel remained unstented. Exclusion criteria: people with an AMI, with unstable angina Braunwald class 3, type C lesion, and chronic total occlusion with anticipated difficult stenting, expected haemodynamic collapse in case of reocclusion, last remaining vessel or unprotected left main PTCA, intracoronary thrombus, any reason for using catheter equipment > 6F (i.e. for non‐balloon technique), non‐PTCA‐related reason for hospitalisation, negative Allen's test for adequate collateral blood supply of the hand, and inability or refusal to give informed consent. |
|
| Interventions |
Group 1: TRA (n = 322) Group 2: TFA (n = 322) |
|
| Outcomes |
Primary outcomes: (all within 24 h)
Secondary outcomes:
Other outcomes:
The above outcomes were reported at 24 h of follow‐up outpatient and inhospital, in addition the study reported 1‐month follow‐up of the following outcomes:
|
|
| Notes | The Netherlands Funding source: Benelux, Boston Scientific |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed by opening a sealed envelope containing a code for either transradial or transfemoral angioplasty." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low regarding the following outcomes due to being objective and unlikely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant comfort due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Study did not report secondary outcomes prespecified in the methods section (cost‐effectiveness and participant comfort). |
| Other bias | Low risk | None noted. |
Valgimigli 2015.
| Methods | Multi‐centre parallel‐design RCT. Before start of angiography, participants centrally allocated (1:1) to radial or femoral access for diagnostic angiography and PCI, if indicated, using a web‐based system to ensure adequate concealment of allocation. Randomisation sequence was computer generated, blocked and stratified by intended new or ongoing use of ticagrelor or prasugrel, type of ACS (ST‐segment elevation MI, troponin positive or negative, non‐ST‐segment elevation ACS), and anticipated use of immediate PCI. Outcome assessors masked to allocated stent, whereas participants and treating physicians were not. | |
| Participants |
8404 people with ACS who were about to undergo CA and possible PCI, if indicated. Inclusion criteria: ACS with or without ST‐segment elevation MI, about to undergo an invasive approach, and interventional cardiologist was willing to proceed with either radial or femoral access and had expertise for both, including ≥ 75 coronary interventions performed, and ≥ 50% of interventions in ACS via the radial route during the previous year. Participants presenting with non‐ST‐segment elevation ACS were eligible if they had a history consistent with new or worsening ischaemia, occurring at rest or with minimal activity within 7 days before randomisation, and fulfilled at least 2 high‐risk criteria. People with ST‐segment elevation MI were eligible if they presented within 12 h of the onset of symptoms or 12‐24 h after onset if there was evidence of continuing ischaemia or previous fibrinolytic treatment, and if they had ST‐segment elevation of ≥ 1 mm in ≥ 2 contiguous leads, new LBBB, or true posterior MI. People with cardiogenic shock, severe peripheral vascular disease, or previous CABG surgery were eligible. Exclusion criteria: use of low molecular weight heparin in previous 6 h, GP IIb/IIIa inhibitors in previous 3 days or any PCI in previous 30 days. |
|
| Interventions |
Group 1: TRA (n = 4197) Group 2: TFA (n = 4207) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
All outcomes assessed at 30 days. |
|
| Notes | Italy, the Netherlands, Spain, and Sweden Funding source: The Medicines Company and Terumo We attempted to contact the authors for raw data to be included in our subgroup analyses, but we could not obtain a valid e‐mail address. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was computer generated, blocked and stratified." |
| Allocation concealment (selection bias) | Low risk | "Before start of angiography, patients were centrally allocated (1:1) to radial or femoral access using a web‐based system to ensure adequate concealment of allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Outcome assessors were masked to the allocated stent, whereas patients and treating physicians were not." We judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "Outcome assessors were masked to the allocated stent." "An independent clinical events committee masked to treatment allocation adjudicated all suspected outcome events by reviewing relevant medical records after site monitoring by Trial Form Support (Lund, Sweden) in Italy and the Netherlands, FLS‐Research Support (Barcelona, Spain) in Spain, and Gothia Forum (Västra Götaland, Sweden) in Sweden." |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up to 30 days available in 4183 participants in TRA group and 4191 participants in TFA group Dropouts: total 0.35%, TRA 0.33%, TFA 0.38% |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all study's prespecified (primary and secondary) outcomes of interest in review were reported in prespecified way (ClinicalTrials.gov Identifier: NCT01433627). |
| Other bias | Low risk | None noted |
Wang 2012.
| Methods | Parallel‐group RCT. Eligible participants were 1:1 randomly assigned to TFA group or TRA group by computer generation (in 2 blocks in a 1:1 ratio). | |
| Participants |
119 consecutive participants undergoing STEMI who were to receive routine early PCI within 12 h after thrombolysis were enrolled from July 2008 to December 2010. Inclusion criteria: typical clinical presentation; ST elevation of > 0.2 mm in ≥ 2 adjacent precordial leads or 0.1 mm in adjacent limb leads, or new LBBB; received intravenous thrombolysis within 6 h from symptom onset in the non‐PCI hospital; and admitted to hospital within 12 h after intravenous thrombolysis. Exclusion criteria: contradictions of thrombolysis; history of CABG; cardiogenic shock; known difficulties with TFA (i.e. Leriche syndrome, severe peripheral artery disease, large abdominal aortic aneurysm) or TRA (i.e. Raynaud's syndrome); pathological Allen's test; necessity for a preprocedural implantation of a transient pacemaker or intra‐aortic balloon pump; chronic renal insufficiency (creatinine ≥ 2.0 mg/dL) with the potential necessity of using the radial artery as a native fistula in the future; people on haemodialysis with AV fistula; or refusal to participate. |
|
| Interventions |
Group 1: TRA (n = 60) Group 2: TFA (n = 59) |
|
| Outcomes |
|
|
| Notes | China Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were 1:1 randomly assigned to transfemoral (TFI group) or transradial catheterisation (TRI group) by a computer generation (in 2 blocks in a 1:1 ratio)." |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study did not report blinding, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Angiographic results evaluated by 2 independent cardiologists who were blinded to the procedures with the use of qualitative angiographic analysis. Regarding all other outcomes, study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted. |
Ziakas 2010.
| Methods | Parallel‐group RCT. Randomisation was performed by an interventional fellow before arterial puncture in a ratio of 1:1 with stratification according to age > 70 years and preprocedural use of dual antiplatelet therapy (defined as the combination of aspirin with any thienopyridine). Operators were not blinded to group assignment, because preparation requirements were needed for procedures. | |
| Participants |
56 participants receiving chronic warfarin treatment, were referred to catheterisation laboratory with a clinical indication for CA enrolled between May 2007 and October 2009. Inclusion criteria: aged > 18 years, easily palpable femoral and radial pulses, normal Allen's test, and preprocedural INR 1.8‐3.5, regardless of indication or duration of warfarin treatment Exclusion criteria: absence of pulse in femoral or radial arteries, vascular disease of upper or lower extremities precluding access at either femoral or radial artery, prior femoral arterial graft surgery, history of arterial access failure in previous catheterisation attempts, preceding thrombolysis, haemodynamically unstable patients, serum creatinine > 1.7 mg/dL, and inability to provide informed consent. |
|
| Interventions |
Group 1: TRA (n = 27) Group 2: TFA (n = 29) |
|
| Outcomes |
All participants were clinically followed up during hospitalisation, and at 1‐week post procedure if discharged earlier. |
|
| Notes | Greece Funding source: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation. Randomisation performed by an interventional fellow before arterial puncture in ratio of 1:1 with stratification according to age > 70 years and preprocedural use of dual antiplatelet therapy. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Operators not blinded to group assignment, but we judged risk of bias as low since it is an operative procedure and outcomes unlikely to be influenced by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as low due to outcomes being objective and unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Participant‐reported outcomes | High risk | Study did not report blinding; however, blinding impossible due to nature of intervention, but we judged risk of bias as high regarding participant‐reported outcomes due to being subjective and likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but we judged risk of bias as low due to reporting key outcomes expected to be reported for such a study. |
| Other bias | Low risk | None noted |
ACS: acute coronary syndrome; AMI: acute myocardial infarction; AV: arteriovenous; BARC: Bleeding Academic Research Consortium; CA: coronary angiography; CABG: coronary artery bypass graft; CK: creatine kinase; CPK: creatine phosphokinase; DAP: dose area product; ECG: electrocardiography; FFR: fractional flow reserve; GP IIb/IIIa: glycoprotein IIb/IIIa; h: hour; Hb: haemoglobin; INR: international normalized ratio; IVUS: intravascular ultrasound; LBBB: left bundle branch block; MACE: major adverse cardiac events; MELD: model for end‐stage liver disease; MI: myocardial infarction; min: minute; n: number of participants; NACE: net adverse clinical events; NHLBI: National Heart, Lung, and Blood Institute; NSTEMI: non‐ST‐elevation myocardial infarction; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; QoL: quality of life; RCT: randomised controlled trial; SD: standard deviation; STEACS: ST‐elevation acute coronary syndrome; STEMI: ST‐elevation myocardial infarction; TF: transfemoral; TFA: transfemoral approach; TFI: transfemoral intervention; TIMI: Thrombolysis In Myocardial Infarction; TR: transradial; TRA: transradial approach; TRI: transradial intervention; TVR: target vessel revascularisation; VAS: visual analogue scale; VCD: vascular closure device.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bhat 2017 | Quasi‐randomised study "with odd serials going into one arm and even into another." |
| Chodor 2009 | Quasi‐randomised study "randomisation was conducted in the admission room based on year of birth: group I included individuals born in even years and group II those born in odd years." |
| Chodor 2011 | Quasi‐randomised study "randomisation was based on year of birth (even number‐TRA; odd number‐TFA)." |
| Genereux 2011 | Participants were randomised to interventions other than those of the review (heparin + GPIIb/IIIa vs bivalirudin) and comparison of TRA vs TFA was a post‐hoc analysis. |
| Kallinikou 2016 | Non‐randomised study "allocation by the judgement of the clinician." |
| Marti 2015 | Participants were randomised to interventions other than those of interest to the review. |
| Qi 2017 | Non‐randomised study "allocation by the judgement of the clinician." |
| Scalone 2014 | Participants were receiving heart transplants undergoing routine angiogram; they did not meet inclusion criteria of review. |
GP IIb/III: glycoprotein IIb/IIIa; TFA: transfemoral approach; TRA: transradial approach.
Characteristics of studies awaiting assessment [ordered by study ID]
Akturk 2012.
| Methods | Parallel‐group RCT. Participants were randomly assigned to TFA (n = 428) or TRA (n = 408) groups. |
| Participants | 836 participants undergoing coronary catheterisation. |
| Interventions | Coronary catheterisation, including angiography and angioplasty via TFA or TRA |
| Outcomes | Pain at level of vascular access site, overall procedure time, and vascular complications |
| Notes | Only abstract available and available information did not allow data extraction and assessment of risk of bias. Authors contacted but received no response. We shall consider the study if further information becomes available. |
Dorniak 2009.
| Methods | Parallel‐group RCT. Participants randomised to either TFA (n = 107) or TRA (n = 116) |
| Participants | 223 consecutive participants with STEMI with < 12‐hour anginal pain |
| Interventions | Primary PCI for STEMI, performed via radial vs femoral arterial access. |
| Outcomes | TIMI grade flow, total procedural time, time to first balloon inflation, total cannulation time, fluoroscopy time, and volume of contrast media used |
| Notes | Only the abstract available and available information does not allow data extraction and assessment of risk of bias. Authors contacted but received no response. We shall consider the study if further information becomes available. |
Gavrilidis 2009.
| Methods | Parallel‐group RCT. Prospective comparison of cardiac catheterisation in participants receiving uninterrupted oral anticoagulation (n = 35) randomly allocated to TFA (n = 18) or TRA (n = 17). |
| Participants | 35 consecutive participants receiving warfarin therapy who were referred for a clinically indicated coronary angiography were prospectively recruited. |
| Interventions | Coronary angiography: Group 1: TFA (n = 18) Group 2: TRA (n = 17) Coronary interventions: Group 1: TFA (n = 5) Group 2: TRA (n = 5) |
| Outcomes | Procedural success, site‐access‐related complications, and inhospital adverse cardiac events, defined as death of any cause, myocardial infarction, or urgent revascularisation |
| Notes | Only the abstract available and available information does not allow data extraction and assessment of risk of bias. No author contact information available. We shall consider the study if further information becomes available. |
Koltowski 2012.
| Methods | Parallel‐group RCT. Participants were randomised to TFA (n = 48) or TRA (n = 52). |
| Participants | 100 participants with STE‐ACS admitted for PCI |
| Interventions | Group 1: TFA Group 2: TRA |
| Outcomes | HRQoL, including mobility, self‐care, and pain. An EQ‐5D, visual analogue scale (VAS) and MacNew instruments were used to assess the HRQoL. |
| Notes | Only the abstract available and available information did not allow data extraction and assessment of the risk of bias. Authors contacted but received no response. We shall consider the study if further information becomes available. |
Li 2011.
| Methods | Parallel group RCT. 1637 participants aged ≥ 60 years with diagnosed CHD or suspected CHD were selected and randomly divided into TRA (n = 909) and TFA (n = 728). |
| Participants | 1637 participants aged ≥ 60 years old with diagnosed CHD or suspected CHD |
| Interventions | PCI: Group 1: TRA (n = 909) Group 2: TFA (n = 728) |
| Outcomes | Time and success rate of puncture, time of angiography, X‐ray exposure time of angiography, success rate of PCI, complications in puncture site, incidence of vagal reflex, mean length of stay, and MACE 3 months after PCI. |
| Notes | Only the abstract available and available information did not allow data extraction and assessment of risk of bias. Publication is a journal requiring special access that could not be obtained. We shall consider the study if further information becomes available. |
Mann 1996.
| Methods | Parallel‐group RCT. 152 participants prospectively randomised to have angioplasty performed by TRA or TFA. |
| Participants | 152 participants undergoing angioplasty |
| Interventions | Group 1: angioplasty via right TRA Group 2: angioplasty via right TFA |
| Outcomes |
|
| Notes | Only the abstract available and available information does not allow data extraction and assessment of risk of bias. Authors contacted but received no response. We shall consider the study if further information becomes available. |
Skvaril 2012.
| Methods | Multi‐arm randomised trial |
| Participants | 456 participants undergoing either diagnostic coronary angiography or interventions. |
| Interventions | Diagnostic coronary angiography or interventions Group 1: TRA‐L (n = 154) Group 2: TRA‐R (n = 159) Group 3: left TFA (n = 143) |
| Outcomes |
Outcomes were evaluated separately for diagnostic procedures and interventions. |
| Notes | Only the abstract available and available information does not allow data extraction and assessment of risk of bias. Authors contacted but received no response. We shall consider the study if further information becomes available. |
Wei 2006.
| Methods | Parallel‐group RCT. |
| Participants | 216 participants with AMI within 12 h |
| Interventions | Group 1: TRA‐pPCI (n = 110) Group 2: TFA‐pPCI (n = 106) |
| Outcomes | Success of procedure, access‐site complications, time of lying in bed (procedure duration), length of hospital stay, and psychological factor scores of depression and anxiety. |
| Notes | Only the abstract available and available information did not allow data extraction and assessment of risk of bias. Publication is a journal requiring special access that could not be obtained. We shall consider the study if further information becomes available. |
CHD: coronary heart disease; HRQoL: health‐related quality of life; MACE: major adverse cardiac event; n: number of participants; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; RCT: randomised controlled trial; STEAC: ST‐elevation acute coronary; STEMI: ST‐elevation myocardial infarction; TF: transfemoral; TFA: transfemoral approach; TFA‐pPCI: transfemoral approach primary percutaneous coronary intervention; TIMI: thrombolysis in myocardial infarction; TR: transradial; TRA: transradial approach; TRA‐L: left transradial approach; TRA‐pPCI: transradial approach primary percutaneous coronary intervention; TRA‐R: right transradial approach.
Characteristics of ongoing studies [ordered by study ID]
ARISE‐2.
| Trial name or title | Vascular Closure Device versus Transradial Approach in Primary Percutaneous Coronary Intervention (ARISE‐2) |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: none (open label) Primary purpose: treatment |
| Participants | Estimated enrolment: 300 Sex/gender: either Aged: ≥ 18 years Inclusion criteria:
|
| Interventions |
|
| Outcomes | Major vascular access site complications (48 h post‐procedure) Major vascular complications related to arterial access site will be evaluated during hospitalisation by physical examination and duplex ultrasonography and include major bleeding, retroperitoneal haemorrhage, compartment syndrome, pseudoaneurysm, arteriovenous fistula, limb ischaemia, or need for vascular surgery repair. |
| Starting date | January 2016 |
| Contact information | Contact: Pedro B Andrade, PhD; +551434025561; pedroberaldo@gmail.com Contact: Robson A Barbosa, RN; +551434025555; enf.robsonbarbosa@gmail.com |
| Notes | Estimated primary completion date: January 2018 Country: Brazil |
SAFARI‐STEMI.
| Trial name or title | Femoral versus Radial Access for Primary PCI (SAFARI‐STEMI) |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: none (open label) Primary purpose: prevention |
| Participants | Estimated enrolment: 4884 Ages eligible for study: ≥ 18 years (adult, senior) Sexes eligible for study: all Accepts healthy volunteers: no Inclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome: all‐cause mortality (30 days) Secondary outcomes: death, reinfarction, or stroke (30 days and 6 months)
|
| Starting date | July 2011 |
| Contact information | Michel R Le May, MD; 613‐696‐7297; mlemay@ottawaheart.ca Melissa Blondeau; 613‐696‐7000 ext 18948; mblondeau@ottawaheart.ca |
| Notes | Estimated primary completion date: August 2019 |
ECG: electrocardiogram; PPCI: primary percutaneous coronary intervention.
Differences between protocol and review
There was no option to assess random sequence generation as high risk of bias, as we had prespecified that we were not including quasi‐RCTs in the protocol.
We revised our first primary outcome from adverse cardiac events to net adverse clinical events, since only the latter includes bleeding outcomes.
Although not prespecified, we included success of the procedure as a primary outcome; the reason being that the transradial approach is more technically demanding than the transfemoral approach, as it involves using a smaller calibre artery compared to the femoral artery that is bigger in size and more readily accessible. The definition mainly entails the completion of procedure without cross‐over to another access site.
We included the definition for participant satisfaction, including early or reduced (or both) pain on ambulation, early hospital discharge, or as defined by trialists.
One of the subgroup analyses was redefined to include NSTE‐ACS, since it encompasses NSTEMI as well as unstable angina, which were included in some studies.
We added a post‐hoc subgroup analysis for total radiation dose, since CA and PCI inherently have different relative durations and consequently different radiation exposure, so it was deemed more appropriate to report on overall outcomes as well as into subgroups.
We defined two time frames for outcome assessment; the reason being that some studies identified in our review reported both short‐ and long‐term outcomes, so we defined two time frames for an appropriate pooling of the results in our meta‐analysis.
We focused on short‐term outcomes in the 'Summary of findings' table, since access‐related procedural effect primarily influences short‐term outcomes and that was the main focus of most included studies.
Contributions of authors
AK: Screening titles, abstracts, and full‐texts for eligibility; data extraction from the included studies; assessment of risk of bias; data entry in data and analyses tables; conducting meta‐analyses; interpreting results; drafting the manuscript; revising the final draft.
RA: Screening titles, abstracts, and full‐texts for eligibility; data extraction from the included studies; assessment of risk of bias; revising the final draft.
AM: Data entry in data and analyses tables; conducting meta‐analyses; interpreting results; drafting the results, discussion, abstract, and plain language summary sections; revising the final draft.
MZ: Providing clinical content expertise and revising the final draft.
MA: Data extraction from the included studies; assessment of risk of bias; data entry in the tables of included studies; data entry in data and analyses tables; creating GRADE 'Summary of findings' table; revising the final draft.
AN: Performing handsearches and searching reference lists; revising the assessment of risk of bias and resolving disagreements; conducting meta‐analyses; creating GRADE 'Summary of findings' table; interpreting results; revising the final draft.
All authors approved the final draft of the full review.
Sources of support
Internal sources
-
Egyptian Center for Evidence Based Medicine, Egypt.
Advanced author training and methodological support.
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
AK: no conflicts of interest to disclose.
RA: no conflicts of interest to disclose.
AM: no conflicts of interest to disclose.
MZ: no conflicts of interest to disclose.
MA: no conflicts of interest to disclose.
AN: no conflicts of interest to disclose.
New
References
References to studies included in this review
Achenbach 2008 {published data only}
- Achenbach S, Ropers D, Kallert L, Turan N, Krahner R, Wolf T, et al. Transradial versus transfemoral approach for coronary angiography and intervention in patients above 75 years of age. Catheterization and Cardiovascular Interventions 2008;72:629‐35. [DOI] [PubMed] [Google Scholar]
Akturk 2014 {published data only}
- Akturk E, Kurtoglu E, Ermis N, Acikgoz N, Yagmur J, Altuntas MS, et al. Comparison of pain levels of transradial versus transfemoral coronary catheterization: a prospective and randomized study. Anadolu Kardiyoloji Dergisi [Anatolian Journal of Cardiology] 2014;14:140‐6. [DOI] [PubMed] [Google Scholar]
Benit 1997 {published data only}
- Benit E, Missault L, Eeman T, Carlier M, Muyldermans L, Materne P, et al. Brachial, radial, or femoral approach for elective Palmaz‐Schatz stent implantation: a randomized comparison. Catheterization and Cardiovascular Diagnosis 1997;41:124‐30. [DOI] [PubMed] [Google Scholar]
Bernat 2014 {published data only}
- Bernat I, Horak D, Stasek J, Mates M, Pesek J, Ostadal P, et al. ST‐segment elevation myocardial infarction treated by radial or femoral approach in a multicenter randomized clinical trial: the STEMI‐RADIAL trial. Journal of the American College of Cardiology 2014;63:964‐72. [DOI] [PubMed] [Google Scholar]
Brasselet 2007 {published data only}
- Brasselet C, Tassan S, Nazeyrollas P, Hamon M, Metz D. Randomised comparison of femoral versus radial approach for percutaneous coronary intervention using abciximab in acute myocardial infarction: results of the FARMI Trial. Heart 2007;93:1556‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brueck 2009 {published data only}
- Brueck M, Bandorski D, Kramer W, Wieczorek M, Höltgen R, Tillmanns H. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovascular Interventions 2009;2:1047‐54. [DOI] [PubMed] [Google Scholar]
Cantor 2005 {published data only}
- Cantor WJ, Puley G, Natarajan MK, Dzavik V, Madan M, Fry A, et al. Radial versus femoral access for emergent percutaneous coronary intervention with adjunct glycoprotein IIb/IIIa inhibition in acute myocardial infarction‐the RADIAL‐AMI pilot randomized trial. American Heart Journal 2005;150:543‐9. [DOI] [PubMed] [Google Scholar]
Cooper 1999 {published data only}
- Cooper CJ, El‐Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. American Heart Journal 1999;138(3):430‐6. [DOI] [PubMed] [Google Scholar]
De Andrade 2017 {published data only}
- Andrade PB, Mattos LA, Rinaldi FS, Bienert IC, Barbosa RA, Labrunie A, et al. Comparison of a vascular closure device versus the radial approach to reduce access site complications in non‐ST‐segment elevation acute coronary syndrome patients: the Angio‐seal versus the radial approach in acute coronary syndrome trial. Catheterization and Cardiovascular Interventions 2017;89:976‐82. [DOI] [PubMed] [Google Scholar]
- Andrade P, Mattos L, Tebet M, Rinaldi F, Esteves V, Nogueira E, et al. Design and rationale of the AngioSeal versus the Radial approach In acute coronary SyndromE (ARISE) trial: a randomized comparison of a vascular closure device versus the radial approach to prevent vascular access site complications in non‐ST‐segment elevation acute coronary syndrome patients. Trials 2013;14:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gan 2009 {published data only}
- Gan L, Lib Q, Liuc R, Zhaoc Y, Qiuc J, Liao Y. Effectiveness and feasibility of transradial approaches for primary percutaneous coronary intervention in patients with acute myocardial infarction. Journal of Nanjing Medical University 2009;23(4):270‐4. [Google Scholar]
He 2012 {published data only}
- He F, Zhang H, Li HM, Liu Y, Xing XC, Gu XF, et al. Clinical studies on different coronary artery interventional therapies through femoral artery or radial artery approaches. Zhonghua Liu Xing Bing Xue za Zhi 2012;33(5):534‐5. [PubMed] [Google Scholar]
Hou 2010 {published data only}
- Hou L, Wei YD, Li WM, Xu WY. Comparative study on transradial versus transfemoral approach for primary percutaneous coronary intervention in Chinese patients with acute myocardial infarction. Saudi Medical Journal 2010;31(2):158‐62. [PubMed] [Google Scholar]
Jolly 2011 {published data only}
- Jolly SS, Cairns J, Niemela K, Steg PG, Natarajan MK, Cheema AN, et al. Effect of radial versus femoral access on radiation dose and the importance of procedural volume: a substudy of the multicenter randomized RIVAL trial. JACC. Cardiovascular Interventions 2013;6:258‐66. [DOI] [PubMed] [Google Scholar]
- Jolly SS, Cairns J, Yusuf S, Niemela K, Steg PG, Worthley M, et al. Procedural volume and outcomes with radial or femoral access for coronary angiography and intervention. Journal of the American College of Cardiology 2014;63:954‐63. [DOI] [PubMed] [Google Scholar]
- Jolly SS, Niemela K, Xavier D, Widimsky P, Budaj A, Valentin V, et al. Design and rationale of the radial versus femoral access for coronary intervention (RIVAL) trial: a randomized comparison of radial versus femoral access for coronary angiography or intervention in patients with acute coronary syndromes. American Heart Journal 2011;161:254‐60. [DOI] [PubMed] [Google Scholar]
- Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011;377:1409‐20. [DOI] [PubMed] [Google Scholar]
- Mehta SR, Jolly SS, Cairns J, Niemela K, Rao SV, Cheema AN, et al. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST‐segment elevation. Journal of the American College of Cardiology 2012;60:2490‐9. [DOI] [PubMed] [Google Scholar]
- Pandie S, Mehta SR, Cantor WJ, Cheema AN, Gao P, Madan M, et al. Radial versus femoral access for coronary angiography/intervention in women with acute coronary syndromes: insights from the RIVAL trial (Radial Vs femorAL access for coronary intervention). JACC. Cardiovascular Interventions 2015;8:505‐12. [DOI] [PubMed] [Google Scholar]
Kiemeneij 1997 {published data only}
- Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. Journal of the American College of Cardiology 1997;29(6):1269‐75. [DOI] [PubMed] [Google Scholar]
Koltowski 2014 {published data only}
- Koltowski L, Filipiak KJ, Kochman J, Pietrasik A, Huczek Z, Balsam P, et al. Cost‐effectiveness of radial vs. femoral approach in primary percutaneous coronary intervention in STEMI ‐ randomized, control trial. Hellenic Journal of Cardiology 2016;57(3):198‐202. [DOI] [PubMed] [Google Scholar]
- Koltowski L, Filipiak KJ, Kochman J, Pietrasik A, Rdzanek A, Huczek Z, et al. Access for percutaneous coronary intervention in ST segment elevation myocardial infarction: radial vs. femoral ‐ a prospective, randomised clinical trial (OCEAN RACE). Kardiologia Polska 2014;72(7):604‐11. [DOI] [PubMed] [Google Scholar]
- Koltowski L, Filipiak KJ, Tomaniak M, Kochman J, Pietrasik A, Rdzanek A, et al. A prospective randomised comparison of minor bleedings in transradial vs. transfemoral access percutaneous coronary interventions for STEMI: a new FEMORAL bleeding classification. Kardiologia Polska 2014;72(9):790‐7. [DOI] [PubMed] [Google Scholar]
- Koltowski L, Koltowska‐Haggstrom M, Filipiak KJ, Kochman J, Golicki D, Pietrasik A, et al. Quality of life in patients with ST‐segment elevation myocardial infarction undergoing percutaneous coronary intervention ‐ radial versus femoral access (from the OCEAN RACE Trial). American Journal of Cardiology 2014;114:516‐21. [DOI] [PubMed] [Google Scholar]
Lange 2006 {published data only}
- Lange HW, Boetticher H. Randomized comparison of operator radiation exposure during coronary angiography and intervention by radial or femoral approach. Catheterization and Cardiovascular Interventions 2006;67:12‐6. [DOI] [PubMed] [Google Scholar]
Li 2007 {published data only}
- Li WM, Li Y, Zhao JY, Duan YN, Sheng L, Yang BF, et al. Safety and feasibility of emergent percutaneous coronary intervention with the transradial access in patients with acute myocardial infarction. Chinese Medical Journal 2007;120(7):598‐600. [PubMed] [Google Scholar]
Louvard 2001 {published data only}
- Louvard Y, Lefevre T, Allain A, Morice M. Coronary angiography through the radial or the femoral approach: the CARAFE study. Catheterization and Cardiovascular Interventions 2001;52:181‐7. [DOI] [PubMed] [Google Scholar]
Louvard 2004 {published data only}
- Louvard Y, Benamer H, Garot P, Hildick‐Smith D, Loubeyre C, Rigattieri S, et al. Comparison of transradial and transfemoral approaches for coronary angiography and angioplasty in octogenarians (the OCTOPLUS study). American Journal of Cardiology 2004;94:1177‐80. [DOI] [PubMed] [Google Scholar]
Mann 1998 {published data only}
- Mann T, Cubeddu G, Bowen J, Schneider JE, Arrowood M, Newman WN, et al. Stenting in acute coronary syndromes: a comparison of radial versus femoral access sites. Journal of the American College of Cardiology 1998;32:572‐6. [DOI] [PubMed] [Google Scholar]
Michael 2013 {published data only}
- Michael TT, Alomar M, Papayannis A, Mogabgab O, Patel VG, Rangan BV, et al. A randomized comparison of the transradial and transfemoral approaches for coronary artery bypass graft angiography and intervention: the RADIAL‐CABG Trial (RADIAL Versus Femoral Access for Coronary Artery Bypass Graft Angiography and Intervention). JACC. Cardiovascular Interventions 2013;6:1138‐44. [DOI] [PubMed] [Google Scholar]
Rao 2014 {published data only}
- Hess CN, Krucoff MW, Sheng S, Anstrom KJ, Barham WB, Gilchrist IC, et al. Comparison of quality‐of‐life measures after radial versus femoral artery access for cardiac catheterization in women: results of the Study of Access Site for Enhancement of Percutaneous Coronary Intervention for Women quality‐of‐life substudy. American Heart Journal 2015;170:371‐9. [DOI] [PubMed] [Google Scholar]
- Hess CN, Rao SV, Kong DF, Aberle LH, Anstrom KJ, Gibson CM, et al. Embedding a randomized clinical trial into an ongoing registry infrastructure: unique opportunities for efficiency in design of the Study of Access site For Enhancement of Percutaneous Coronary Intervention for Women (SAFE‐PCI for Women). American Heart Journal 2013;166(3):421‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SV, Hess CN, Barham B, Aberle LH, Anstrom KJ, Patel TB, et al. A registry‐based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE‐PCI for Women (Study of Access Site for Enhancement of PCI for Women) trial. JACC. Cardiovascular Interventions 2014;7:857‐67. [DOI] [PubMed] [Google Scholar]
Reddy 2004 {published data only}
- Reddy BK, Brewster PS, Walsh T, Burket MW, Thomas WJ, Cooper CJ. Randomized comparison of rapid ambulation using radial, 4 French femoral access, or femoral access with AngioSeal closure. Catheterization and Cardiovascular Interventions 2004;62:143‐9. [DOI] [PubMed] [Google Scholar]
Romagnoli 2012 {published data only}
- Romagnoli E, Biondi‐Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, et al. Radial versus femoral randomized investigation in ST‐segment elevation acute coronary syndrome: the RIFLE‐STEACS (Radial Versus Femoral Randomized Investigation in ST‐Elevation Acute Coronary Syndrome) study. Journal of the American College of Cardiology 2012;60(24):2481‐9. [DOI] [PubMed] [Google Scholar]
Saito 2003 {published data only}
- Saito S, Tanaka S, Hiroe Y, Miyashita Y, Takahashi S, Tanaka K, et al. Comparative study on transradial approach vs. transfemoral approach in primary stent implantation for patients with acute myocardial infarction: results of the test for myocardial infarction by prospective unicenter randomization for access sites (TEMPURA) trial. Catheterization and Cardiovascular Interventions 2003;59:26‐33. [DOI] [PubMed] [Google Scholar]
Santas 2009 {published data only}
- Santas E, Bodi V, Sanchis J, Núñez J, Mainar L, Miñana G, et al. The left radial approach in daily practice. A randomized study comparing femoral and right and left radial approaches. Revista Espanola de Cardiologia 2009;62(5):482‐90. [DOI] [PubMed] [Google Scholar]
Schernthaner 2018 {published data only}
- Schernthaner C, Hammerer M, Harb S, Heigert M, Hoellinger K, Lassnig E. Radial versus femoral access site for percutaneous coronary intervention in patients suffering acute myocardial infarction: a randomized prospective multicenter trial. Wiener Klinische Wochenschrift 2018;130(5‐6):182‐9. [DOI] [PubMed] [Google Scholar]
Slagboom 2005 {published data only}
- Slagboom T, Kiemeneij F, Laarman GJ, Wieken R. Outpatient coronary angioplasty: feasible and safe. Catheterization and Cardiovascular Interventions 2005;64:421‐7. [DOI] [PubMed] [Google Scholar]
Valgimigli 2015 {published data only}
- Ando G, Cortese B, Frigoli E, et al. Acute kidney injury after percutaneous coronary intervention: Rationale of the AKI‐MATRIX (Acute Kidney Injury‐Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of AngioX) sub‐study. Catheterization and Cardiovascular Interventions 2015;86:950‐7. [DOI] [PubMed] [Google Scholar]
- Ando G, Russo F, Gargiulo G, Russo N, Windecker S, Salomone M, et al. Acute Kidney Injury After Radial or Femoral Access for Invasive Acute Coronary Syndrome Management: AKI‐MATRIX. Journal of the American College of Cardiology 2017;69(21):2592‐603. [DOI] [PubMed] [Google Scholar]
- Sciahbasi A, Frigoli E, Sarandrea A, Rothenbühler M, Calabrò P, Lupi A, et al. Radiation Exposure and Vascular Access in Acute Coronary Syndromes: the RAD‐Matrix Trial. Journal of the American College of Cardiology 2017;69(20):2530‐7. [DOI] [PubMed] [Google Scholar]
- Sciahbasi A, Frigoli E, Sarandrea A, Rothenbühler M, Calabrò P, Lupi A, et al. Randomized comparison of operator radiation exposure comparing transradial and transfemoral approach for percutaneous coronary procedures: rationale and design of the minimizing adverse haemorrhagic events by Transradial access site and systemic implementation of angioX ‐ RAdiation Dose study (RAD‐MATRIX). Cardiovascular Revascularization Medicine 2014;15(4):209‐13. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Calabro P, Cortese B, Frigoli E, Garducci S, Rubartelli P, et al. Scientific Foundation and Possible Implications for Practice of the Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of AngioX (MATRIX) Trial. Journal of Cardiovascular Translational Research 2014;7:101‐11. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Gagnor A, Calabro P, Frigoli E, Leonardi S, Zaro T, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015;385:2465‐76. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, MATRIX investigators. Design and rationale for the Minimizing Adverse haemorrhagic events by Transradial access site and systemic Implementation of angioX program. American Heart Journal 2014;168(6):838‐45. [DOI] [PubMed] [Google Scholar]
- Vranckx P, Frigoli E, Rothenbuhler M, Tomassini F, Garducci S, Ando G, et al. Radial versus femoral access in patients with acute coronary syndromes with or without ST‐segment elevation. European Heart Journal 2017;38(14):1069‐80. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang YB, Fu XH, Wang XC, Gu XS, Zhao YJ, Hao GZ, et al. Randomized comparison of radial versus femoral approach for patients with STEMI undergoing early PCI following intravenous thrombolysis. Journal of Invasive Cardiology 2012;24(8):412‐6. [PubMed] [Google Scholar]
Ziakas 2010 {published data only}
- Ziakas AG, Koskinas KC, Gavrilidis S, Giannoglou GD, Hadjimiltiades S, Gourassas I. Radial versus femoral access for orally anticoagulated patients. Catheterization and Cardiovascular Interventions 2010;76:493‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bhat 2017 {published data only}
- Bhat FA, Changal KH, Raina H, Tramboo NA, Rather HA. Transradial versus transfemoral approach for coronary angiography and angioplasty ‐ a prospective, randomized comparison. BMC Cardiovascular Disorders 2017;17(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chodor 2009 {published data only}
- Chodor P, Krupa H, Kurek T, Sokal A, Swierad M, Was T, et al. RADIal versus femoral approach for percutaneous coronary interventions in patients with Acute Myocardial Infarction (RADIAMI): a prospective, randomized, single‐center clinical trial. Cardiology Journal 2009;16(4):332‐40. [PubMed] [Google Scholar]
Chodor 2011 {published data only}
- Chodor P, Kurek T, Kowalczuk A, Swierad M, Was T, Honisz G, et al. Radial vs femoral approach with StarClose clip placement for primary percutaneous coronary intervention in patients with ST‐elevation myocardial infarction. RADIAMI II: a prospective, randomised, single centre trial. Kardiologia Polska 2011;69(8):763‐71. [PubMed] [Google Scholar]
Genereux 2011 {published data only}
- Genereux P, Mehran R, Palmerini T, Caixeta A, Kirtane AJ, Lansky AJ, et al. Radial access in patients with ST‐segment elevation myocardial infarction undergoing primary angioplasty in acute myocardial infarction: the HORIZONS‐AMI trial. EuroIntervention 2011;7(8):905‐16. [DOI] [PubMed] [Google Scholar]
Kallinikou 2016 {published data only}
- Kallinikou Z, Puricel SG, Ryckx N, Togni M, Baeriswyl G, Stauffer JC, et al. Radiation exposure of the Operator During Coronary Interventions (from the RADIO Study). American Journal of Cardiology 2016;118(2):188‐94. [DOI] [PubMed] [Google Scholar]
Marti 2015 {published data only}
- Marti V, Brugaletta S, Garcia‐Picart J, Delgado G, Cequier A, Iniguez A, et al. Radial versus femoral access for angioplasty of ST‐segment elevation acute myocardial infarction with second‐generation drug‐eluting stents. Revista Espanola de Cardiologia 2015;68(1):47‐53. [DOI] [PubMed] [Google Scholar]
Qi 2017 {published data only}
- Qi G, Sun Q, Xia Y, Wei L. Emergency percutaneous coronary intervention through the left radial artery is associated with less vascular complications than emergency percutaneous coronary intervention through the femoral artery. Clinics (Sao Paulo, Brazil) 2017;72(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scalone 2014 {published data only}
- Scalone G, Brugaletta S, Martin‐Yuste V, Seixo F, Cotes C, Gomez‐Monterrosas O, et al. RAndomized Comparison of raDIal vs. femorAL access for routine catheterization of heart transplant patients (RADIAL‐heart transplant study). Transplantation Proceedings 2014;46(10):3262‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Akturk 2012 {published data only}
- Akturk E, Kurtoglu E, Ermis N, Acikgoz N, Yagmu J, Altuntas MS, et al. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty: which approach is suitable for which patient?. International Journal of Cardiology 2012;155:S39. [Google Scholar]
Dorniak 2009 {published data only}
- Dorniak W, Pinna GD, Klaudel J, Lajkowski Z, Pawlowski K, Krasowski W, et al. Procedural characteristics of radial versus femoral arterial access during primary percutaneous coronary intervention in STEMI patients. European Heart Journal 2009;30(Suppl):334. [Google Scholar]
Gavrilidis 2009 {published data only}
- Gavrilidis S, Giannoglou G, Gourasas I, Koskinas K, Parharidis G, Stefanidis T, et al. A randomized comparison of the radial and femoral approaches for coronary angiography and percutaneous coronary intervention during uninterrupted oral anticoagulation: preliminary results. Catheterization and Cardiovascular Interventions 2009;73:S85‐6. [Google Scholar]
Koltowski 2012 {published data only}
- Koltowski L, Filipiak K, Kochman J, Opolski G, Pietrasik A. Lower periprocedural quality of life in STEMI patients undergoing PCI with femoral access. Journal of the American College of Cardiology 2012;60(17):B119‐20. [Google Scholar]
Li 2011 {published data only}
- Li X, Chen Q, Wang Z, Ke D, Wu Q. Comparison on transradial versus transfemoral approach for coronary angiography and angioplasty in the elderly with coronary heart disease. Chinese Journal of Interventional Imaging and Therapy 2011;8(4):259‐62. [Google Scholar]
Mann 1996 {published data only}
- Mann JT 3rd, Cubeddu MG, Schneider JE, Arrowood M. Right radial access for PTCA: a prospective study demonstrates reduced complications and hospital charges. Journal of Invasive Cardiology 1996;8(Suppl D):40D‐4D. [PubMed] [Google Scholar]
Skvaril 2012 {published data only}
- Skvaril J, Cernohous M, Jarkovsky P, Kockova R, Maly M, Sedlon P. Increased radiation exposure in transradial approach. A real drawback or an obsolete problem?. Journal of the American College of Cardiology 2012;60(17):B114. [Google Scholar]
Wei 2006 {published data only}
- Wei Y, Fu X, Sun S. The comparative study on psychological influence of transradial vs transfemoral artery access for percutaneous coronary intervention in patients with acute myocardial infarction. Chinese Journal of Rehabilitation Medicine 2006;21(3):349‐52. [Google Scholar]
References to ongoing studies
ARISE‐2 {published and unpublished data}
- Vascular Closure Device versus Transradial Approach in Primary Percutaneous Coronary Intervention (ARISE‐2). Ongoing study January 2016.
SAFARI‐STEMI {published and unpublished data}
- Femoral versus Radial Access for Primary PCI (SAFARI‐STEMI). Ongoing study July 2011.
Additional references
Agostoni 2004
- Agostoni P, Biondi‐Zoccai G, Benedictis M, Rigattieri S, Turri M, Anselmi M, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta‐analysis of randomized trials. Journal of the American College of Cardiology 2004;44(2):349‐56. [DOI] [PubMed] [Google Scholar]
AHA 2015
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cereek B, et al. ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST‐elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2015:CIR.0000000000000336.
Almany 1999
- Almany SL, O'Neill WW. Radial Artery Access for Diagnostic and Interventional Procedures. Ann Arbor (MI): Accumed Systems, 1999. [Google Scholar]
Alonzo 2016
- Alonzo A, Rigattieri S, Giovannelli F, Russo C, Sciahbasi A, Berni A, et al. Transfemoral approach with systematic use of FemoSeal closure device compared to transradial approach in primary angioplasty. Catheterization and Cardiovascular Interventions 2016;87(5):849‐54. [DOI] [PubMed] [Google Scholar]
Ando 2015
- Ando G, Capodanno D. Radial versus femoral access in invasively managed patients with acute coronary syndrome: a systematic review and meta‐analysis. Annals of Internal Medicine 2015;163(12):932‐40. [DOI] [PubMed] [Google Scholar]
Ando 2016
- Ando G, Porto I, Montalescot G, Bolognese L, Trani C, Oreto G, et al. Radial access in patients with acute coronary syndrome without persistent ST‐segment elevation: systematic review, collaborative meta‐analysis, and meta‐regression. International Journal of Cardiology 2016;222:1031‐9. [DOI] [PubMed] [Google Scholar]
Bertrand 2012
- Bertrand OF, Belisle P, Joyal D, Costerousse O, Rao SV, Jolly SS, et al. Comparison of transradial and femoral approaches for percutaneous coronary interventions: a systematic review and hierarchical Bayesian meta‐analysis. American Heart Journal 2012;163(4):632‐48. [DOI] [PubMed] [Google Scholar]
Campeau 1989
- Campeau L. Percutaneous radial artery approach for coronary angiography. Catheterization and Cardiovascular Diagnosis 1989;16(1):3‐7. [DOI] [PubMed] [Google Scholar]
Dal Molin 2015
- Dal Molin A, Faggiano F, Bertoncini F, Buratti G, Busca E, Casarotto R, et al. Bed rest for preventing complications after transfemoral cardiac catheterisation: a protocol of systematic review and network meta‐analysis. Systematic Reviews 2015;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Furia 2016
- Furia F, Giustino G, Chieffo A. Targeting transradial approach: an updated systematic review and meta‐analysis. Panminerva Medica 2016;58(4):329‐40. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Christoph M. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
ESC 2015
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. European Heart Journal 2016;37(3):267‐315. [DOI] [PubMed] [Google Scholar]
ESC‐EACTS 2010
- The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Guidelines on myocardial revascularization. European Journal of Cardio‐Thoracic Surgery 2010;38(Suppl 1):S1‐S52. [DOI] [PubMed] [Google Scholar]
Ferrante 2016
- Ferrante G, Rao SV, Juni P, Costa BR, Reimers B, Condorelli G, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta‐analysis of randomized trials. JACC. Cardiovascular Interventions 2016;9(14):1419‐34. [DOI] [PubMed] [Google Scholar]
Gandhi 2015
- Gandhi S, Kakar R, Overgaard CB. Comparison of radial to femoral PCI in acute myocardial infarction and cardiogenic shock: a systematic review. Journal of Thrombosis and Thrombolysis 2015;40(1):108‐17. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 9 August 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
He 2014
- He P, Yang Y, Hu F. Transradial versus transfemoral percutaneous coronary intervention in elderly patients: a systematic overview and meta‐analysis. Chinese Medical Journal 2014;127(6):1110‐7. [PubMed] [Google Scholar]
Hess 2014
- Hess C, Peterson E, Neely M, Dai D, Hillegass W, Krucoff M, et al. The learning curve for transradial percutaneous coronary intervention among operators in the United States. Circulation 2014;129:2277‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hsueh 2017
- Hsueh SK, Cheng CI, Fang HY, Omran MM, Liu WH, Chung WJ, et al. Feasibility and safety of transulnar catheterization in ipsilateral radial artery occlusion. International Heart Journal 2017;58(3):313‐9. [DOI] [PubMed] [Google Scholar]
Huang 2016
- Huang F, Huang B, Wang P, Zhang C, Zuo Z, Liao Y, et al. Gender disparity in the safety and efficacy of radial and femoral access for coronary intervention: a systematic review and meta‐Analysis. Angiology 2016;67(9):810‐9. [DOI] [PubMed] [Google Scholar]
Jang 2012
- Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim DK, et al. The transradial versus the transfemoral approach for primary percutaneous coronary intervention in patients with acute myocardial infarction: a systematic review and meta‐analysis. EuroIntervention 2012;8(4):501‐10. [DOI] [PubMed] [Google Scholar]
Jolly 2009
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta‐analysis of randomized trials. American Heart Journal 2009;157(1):132‐40. [DOI] [PubMed] [Google Scholar]
Kim 2011
- Kim J, Yoon J. Transradial approach as a default route in coronary artery interventions. Korean Circulation Journal 2011;41(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotowycz 2012
- Kotowycz M, Džavík V. Radial artery patency after transradial catheterization. Circulation: Cardiovascular Intervention 2012;5:127‐33. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 2015
- Liu P, Gao XL, Li BF, Ding XZ, Wang ZH, Dang YP, et al. Radial versus femoral artery access for percutaneous coronary angiography and intervention: a systematic review and meta‐analysis of randomized controlled trials in Chinese population. International Journal of Clinical and Experimental Medicine 2015;8(10):17151‐66. [PMC free article] [PubMed] [Google Scholar]
MECIR 2016
- Higgins JP, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London, Version 1.05 2016.
Mitchell 2012
- Mitchell MD, Hong JA, Lee BY, Umscheid CA, Bartsch SM, Don CW. Systematic review and cost‐benefit analysis of radial artery access for coronary angiography and intervention. Circulation: Cardiovascular Quality and Outcomes 2012;5(4):454‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- NICE guidelines [CG167]. Myocardial infarction with ST‐segment elevation: acute management, 2013. www.nice.org.uk/guidance/cg167/chapter/1‐recommendations (accessed prior to 29 March 2018).
Pancholy 2015
- Pancholy SB, Palamaner Subash Shantha G, Romagnoli E, Kedev S, Bernat I, Rao SV, et al. Impact of access site choice on outcomes of patients with cardiogenic shock undergoing percutaneous coronary intervention: a systematic review and meta‐analysis. American Heart Journal 2015;170(2):353‐61. [DOI] [PubMed] [Google Scholar]
Plourde 2015
- Plourde G, Pancholy SB, Nolan J, Jolly S, Rao SV, Amhed I, et al. Radiation exposure in relation to the arterial access site used for diagnostic coronary angiography and percutaneous coronary intervention: a systematic review and meta‐analysis. Lancet 2015;386(10009):2192‐203. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruiz‐Rodriguez 2016
- Ruiz‐Rodriguez E, Asfour A, Lolay G, Ziada KM, Abdel‐Latif AK. Systematic review and meta‐analysis of major cardiovascular outcomes for radial versus femoral access in patients with acute coronary syndrome. Southern Medical Journal 2016;109(1):61‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Shah 2017
- Shah R, Askari R, Haji S, Rashid A. Mortality and operator experience with vascular access for percutaneous coronary intervention in patients with acute coronary syndromes: a pairwise and network meta‐analysis of randomized controlled trials. International Journal of Cardiology 2017;248:114‐9. [DOI] [PubMed] [Google Scholar]
Sirker 2016
- Sirker A, Kwok C, Kotronias R, Bagur R, Bertrand O, Butler R, et al. Influence of access site choice for cardiac catheterization on risk of adverse neurological events: asystematic review and meta‐analysis. American Heart Journal 2016;181:107‐19. [DOI] [PubMed] [Google Scholar]
Taggart 2013
- Taggart DP. Stents or surgery in coronary artery disease in 2013. Annals of Cardiothoracic Surgery 2013;2:431‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Triantafyllou 2009
- Triantafyllou K. Transradial percutaneous coronary intervention in acute coronary syndromes: a case report and review of the literature. Hospital Chronicles 2009; Vol. 4, issue 4:166‐71.
Venkitachalam 2009
- Venkitachalam L, Kip KE, Selzer F, Wilensky RL, Slater J, Mulukutla SR, et al. Twenty‐year evolution of percutaneous coronary intervention and its impact on clinical outcomes: a report from the National Heart, Lung, and Blood Institute‐sponsored, multicenter 1985‐1986 PTCA and 1997‐2006 dynamic registries. Circulation: Cardiovascular Interventions 2009;2(1):6‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vorobcsuk 2009
- Vorobcsuk A, Konyi A, Aradi D, Horvath IG, Ungi I, Louvard Y, et al. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction. Systematic overview and meta‐analysis. American Heart Journal 2009;158(5):814‐21. [DOI] [PubMed] [Google Scholar]
Watt 2009
- Watt J, Oldroyd KG. Radial versus femoral approach for high‐speed rotational atherectomy. Catheterization and Cardiovascular Interventions 2009;74(4):550‐4. [DOI] [PubMed] [Google Scholar]
Wensley 2008
- Wensley C, Kent B, McAleer MB, Savage SM, Stewart JT. Pain relief for the removal of femoral sheath after percutaneous coronary intervention. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006043.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization (WHO). Cardiovascular diseases (CVDs). www.who.int/mediacentre/factsheets/fs317/en/ (accessed prior to 17 April 2018).


