Abstract
Background
Endometriosis is a common gynaecological condition which affects many women of reproductive age worldwide and is a major cause of pain and infertility. The combined oral contraceptive pill (COCP) is widely used to treat pain occurring as a result of endometriosis, although the evidence for its efficacy is limited.
Objectives
To determine the effectiveness, safety and cost‐effectiveness of oral contraceptive preparations in the treatment of painful symptoms ascribed to the diagnosis of laparoscopically proven endometriosis.
Search methods
We searched the following from inception to 19 October 2017: the Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials, the Cochrane CENTRAL Register of Studies Online (CRSO), MEDLINE, Embase, PsycINFO, CINAHL (Cumulative Index to Nursing and Allied Health Literature), and the trial registers ClinicalTrials.gov and the World Health Organization Clinical Trials Registry Platform (WHO ICTRP). We also handsearched reference lists of relevant trials and systematic reviews retrieved by the search.
Selection criteria
We included randomised controlled trials (RCT) of the use of COCPs in the treatment of women of reproductive age with symptoms ascribed to the diagnosis of endometriosis that had been made visually at a surgical procedure.
Data collection and analysis
Two review authors independently assessed study quality and extracted data. One review author was an expert in the content matter. We contacted study authors for additional information. The primary outcome was self‐reported pain (dysmenorrhoea) at the end of treatment.
Main results
Five trials (612 women) met the inclusion criteria. Only three trials (404 women) provided data that were suitable for analysis.
Combined oral contraceptive pill versus placebo
Two trials compared COCP with a placebo. These studies were at high risk of bias. For GRADE outcomes (self‐reported pain (dysmenorrhoea) at the end of treatment), the quality of the evidence very low. Evidence was downgraded for imprecision as it was based on a single, small trial and for the visual analogue scale data there were wide confidence intervals (CIs). There appeared to have been substantial involvement of the pharmaceutical company funding the trials.
Treatment with the COCP was associated with an improvement in self‐reported pain at the end of treatment as evidenced by a lower score on the Dysmenorrhoea verbal rating scale (scale 0 to 3) compared with placebo (mean difference (MD) ‐1.30 points, 95% CI ‐1.84 to ‐0.76; 1 RCT, 96 women; very low quality evidence), a lower score on the Dysmenorrhoea visual analogue scale (no details of scale) compared with placebo (MD ‐23.68 points, 95% CI ‐28.75 to ‐18.62, 2 RCTs, 327 women; very low quality evidence) and a reduction in menstrual pain from baseline to the end of treatment (MD 2.10 points, 95% CI 1.38 to 2.82; 1 RCT, 169 women; very low quality evidence).
Combined oral contraceptive pill versus medical therapies
One underpowered trial compared the COCP with another medical treatment (goserelin). The study was at high risk of bias; the trial was unblinded and there was insufficient detail to judge allocation concealment and randomisation. For GRADE outcomes (self‐reported pain (dysmenorrhoea) at the end of treatment), the quality of the evidence ranged from low to very low.
At the end of treatment, the women in the goserelin group were amenorrhoeic and therefore no comparisons could be made between the groups for the primary outcome. At six months' follow‐up, there was no clear evidence of a difference between women treated with the COCP and women treated with goserelin for measures of dysmenorrhoea on a visual analogue scale (scale 1 to 10) (MD ‐0.10, 95% CI ‐1.28 to 1.08; 1 RCT, 50 women; very low quality evidence) or a verbal rating scale (scale 0 to 3) (MD ‐0.10, 95% CI ‐0.99 to 0.79; 1 RCT, 50 women; very low quality evidence). At six months' follow‐up, there was no clear evidence of a difference between the COCP and goserelin groups for reporting complete absence of pain as measured by the visual analogue scale (risk ratio (RR) 0.36, 95% CI 0.02 to 8.43; 1 RCT, 50 women; very low quality evidence) or the verbal rating scale (RR 1.00, 95% CI 0.93 to 1.08; 1 RCT, 49 women; low quality evidence).
Authors' conclusions
Based on the limited evidence from two trials at high risk of bias and limited data for the prespecified outcomes for this review, there is insufficient evidence to make a judgement on the effectiveness of the COCP compared with placebo and the findings cannot be generalised.
Based on the limited evidence from one small trial that was at high risk of bias, there is insufficient evidence to make a judgement on the effectiveness of the COCP compared with other medical treatments. Only one comparison was possible, with the medical intervention being goserelin, and the findings cannot be generalised.
Further research is needed to fully evaluate the role of COCPs in managing pain‐related symptoms associated with endometriosis. There are other formulations of the combined hormonal contraception such as the transdermal patch, vaginal ring or combined injectable contraceptives which this review did not cover but should be considered in future updates.
Keywords: Female; Humans; Contraceptives, Oral; Contraceptives, Oral/therapeutic use; Dysmenorrhea; Dysmenorrhea/drug therapy; Endometriosis; Endometriosis/complications; Endometriosis/drug therapy; Goserelin; Goserelin/therapeutic use; Pelvic Pain; Pelvic Pain/drug therapy; Pelvic Pain/etiology; Randomized Controlled Trials as Topic
Plain language summary
Modern combined oral contraceptives for treatment of pain associated with endometriosis
Review question
The combined oral contraceptive pill (COCP) is commonly used to treat pain associated with endometriosis but how well it works is unclear.
Background
Endometriosis is a common women's healthcare condition where the endometrium (lining of the womb) grows at sites outside the womb, such as the ovaries (which produce eggs). Endometriosis is commonly found in women with painful periods, pain with sexual intercourse, pelvic pain and infertility (difficulty in having a baby). Hormonal treatments, including COCPs and medicines called gonadotrophin‐releasing hormone analogues (for example, goserelin) are used to relieve the pain symptoms associated with endometriosis. However, many of the hormonal treatments have side effects which limit their acceptability and duration of use.
Study characteristics
Cochrane authors searched for clinical studies on 19 October 2017. We found five trials, including 612 women, that met the inclusion criteria. The studies took place in Egypt, the US, Japan and Italy.
Key results
Only three of the included studies provided data in a format that could be analysed in this review.
Combined oral contraceptive pill versus placebo
We found two trials including 354 women that compared the COCP with a placebo (pretend treatment). The evidence was at high risk of bias. There was very low quality evidence that treatment with the COCP was associated with an improvement in self‐reported dysmenorrhoea (period pain) at the end of treatment measure on a verbal rating scale (where the woman rates her pain as (for example) "no pain," "slight pain," "moderate pain," "severe pain" and "unbearable pain") and low quality evidence for an improvement in self‐reported dysmenorrhoea pain at the end of treatment using a visual rating scale (where the woman marks her pain visually on a line) compared with placebo. There was very low quality evidence that there was a reduction in menstrual pain from the beginning to the end of treatment in the COCP group compared with women having a placebo.
Combined oral contraceptive pill versus other medical treatment
We found one trial of 50 women that compared the COCP with another medical treatment (goserelin).
The study was at high risk of bias. At the end of treatment, the women in the goserelin group were not having a period and therefore we could not compare the groups.
Six months after the end of treatment, there was very low quality evidence that there was no clear difference between women treated with the COCP and women treated with goserelin for self‐reported dysmenorrhoea, using a visual rating scale or a verbal rating scale. Six months after the end of treatment, there was very low quality evidence that there was no clear evidence of a difference between the COCP and goserelin groups for reporting complete absence of pain, as measured by a visual rating scale and low quality evidence using a verbal rating scale.
Quality of the evidence
The quality of the evidence was very low quality. The main reasons for downgrading the evidence were because the data were based on a single small trial with wide variation in results and lack of details about how the study had been designed. There were some concerns with two of the studies that were funded by a pharmaceutical company that also had input into the design of the trial, data collection and the analysis of data. This means that we cannot be confident about the results.
Summary of findings
Summary of findings for the main comparison. Combined oral contraceptive pill compared to placebo or no treatment for pain associated with endometriosis.
| Combined oral contraceptive pill (COCP) compared to placebo/no treatment for pain associated with endometriosis | ||||||
| Patient or population: women with endometriosis Setting: Japan Intervention: COCP Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with COCP | |||||
| Self‐reported pain (dysmenorrhoea) at the end of treatment: dysmenorrhoea VRS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment: Dysmenorrhoea VRS was 3.7 | MD 1.3 lower (1.84 lower to 0.76 lower) | ‐ | 96 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | VRS ranged from 0 to 3. |
| Self‐reported pain (dysmenorrhoea) at the end of treatment: dysmenorrhoea VAS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment: Dysmenorrhoea VAS was 46.2 | MD 23.68 lower (28.75 lower to 18.62 lower) | ‐ | 327 (2 RCTs) | ⊕⊝⊝⊝ Very low2,3 | No details provided for the VAS. |
| Self‐reported pain: menstrual pain reduction from baseline to end of treatment | The mean menstrual pain (reduction from baseline to end of treatment) was 3.00 | MD 2.10 lower (1.38 lower to 2.82 lower) | ‐ | 169 (1 RCT) |
⊕⊝⊝⊝ Very low1,2 | Used a VAS from 0 to 10 where 10 was extreme pain. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COCP: combined oral contraceptive pill; MD: mean difference; RCT: randomised controlled trial; VAS: visual analogue scale; VRS: verbal rating scale. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Imprecision: evidence was based on a single small trial; downgraded one level.
2Risk of bias: trial judged to be at high risk of bias; downgraded two levels.
3Imprecision: evidence based on a single trial including 96 women; wide confidence intervals; downgraded two levels.
Summary of findings 2. Combined oral contraceptive pill compared to other medical treatment for pain associated with endometriosis.
| Combined oral contraceptive pill (COCP) compared to other medical treatment for pain associated with endometriosis | ||||||
| Patient or population: women with endometriosis Setting: Italy Intervention: COCP Comparison: other medical treatment (goserelin) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with other medical treatment | Risk with COCP | |||||
| Self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up: VAS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up was 7.5 | MD 0.1 lower (1.28 lower to 1.08 higher) | ‐ | 50 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | VAS ranged from 1 to 10. |
| Self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up: VRS | The mean self‐reported pain (dysmenorrhoea) at the end of treatment (continuous data): dysmenorrhoea at 6 months' follow‐up was 4.8 | MD 0.1 lower (0.99 lower to 0.79 higher) | ‐ | 50 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | VRS ranged from 0 to 3. |
| Self‐reported pain (dysmenorrhoea) (dichotomous data): reduction of pain to zero at 6 months' follow‐up: VAS | 38 per 1000 | 14 per 1000 (1 to 324) | RR 0.36 (0.02 to 8.43) | 50 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | VAS ranged from 1 to 10. |
| Self‐reported pain (dysmenorrhoea) (dichotomous data) ‐ reduction of pain to zero at 6 months' follow‐up: VRS | 1000 per 1000 | 1000 per 1000 (930 to 1000) | RR 1.00 (0.93 to 1.08) | 49 (1 RCT) | ⊕⊕⊝⊝ Low1,3 | VRS ranged from 0 to 3. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COCP: combined oral contraceptive pill; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale; VRS: verbal rating scale. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Risk of bias: no blinding and randomisation and allocation concealment unclear; downgraded one level.
2Imprecision: evidence from a single small trial including 50 women; wide confidence intervals crossing the line of no effect; downgraded two levels.
3Imprecision: evidence from a single small trial reporting data on 49 women; downgraded one level.
Background
Description of the condition
Endometriosis is a common gynaecological condition which affects many women of reproductive age worldwide and is a major cause of pain and infertility (Jacobson 2002). Symptomatic endometriosis most frequently causes pelvic pain. The pain may occur at the same as menstrual bleeding (dysmenorrhoea), during or after sexual intercourse (dyspareunia and post‐coital pain) or be present as other pelvic pain occurring in a cyclical or non‐cyclical pattern (Fauconnier 2005). More rarely, endometriotic lesions may occur outside the pelvic region and these may also cause cyclical pain (Lancaster 1995). The prevalence of symptomatic endometriosis in the general population is difficult to estimate. One review of observational studies suggested that the prevalence of chronic pelvic pain ranged from 5.7% to 26.6% (Ahangari 2014). Women who experience symptomatic endometriosis report significant reduction in their quality of life, with impact on many aspects of life (Jones 2004). Endometriosis can also be found in asymptomatic women; for example, during a routine surgical procedure such as sterilisation.
Endometriosis is defined histologically as the presence of endometrial glands or stroma in sites other than the uterine cavity, most commonly the peritoneum and ovaries. The main pathological processes associated with endometriosis are peritoneal inflammation and fibrosis, and the formation of adhesions and ovarian cysts. The aetiology of endometriosis remains unclear although there is now considerable evidence that it is a complex genetic trait (Barlow 2005). Retrograde menstrual flow has long been accepted to be central to the development of endometriosis. This is unlikely to be an exclusive mechanism as retrograde menstrual flow is not limited to women who develop endometriosis (Johnson 2006), and retrograde menstruation does not explain the occurrence of endometriosis at all sites. It seems likely that in affected women, a genetic or immunologically linked susceptibility combines with retrograde menstruation to create endometriotic lesions in the pelvis (Crosignani 2006).
Description of the intervention
Hormonal therapies aim to induce atrophy within the hormonally dependent ectopic endometrium. Definitive diagnosis is made by surgical inspection of the peritoneal cavity (Kennedy 2006). Treatment options include surgical removal of the abnormal tissue, symptomatic treatment, hormonal treatment or combinations of all three (Yap 2004). The aim of treatment, other than to reduce symptoms, has been to remove or decrease the activity of the deposits of ectopic endometrium that are thought to be responsible for the symptoms of endometriosis. This can be achieved surgically by destroying or removing the implants. Unfortunately, endometriosis tends to recur, both following surgery and when medication is stopped and may later develop into deep infiltrating endometriosis (endometrial deposits outside the uterus).
How the intervention might work
The clinical observation of apparent symptom resolution during pregnancy gave rise to the concept of treating women with a pseudopregnancy regimen (Kistner 1959). Combinations of high‐dose oestrogens and progestogens were first used then progestogens alone (Kistner 1958). High doses of oestrogen and progestogen are now only very rarely, if ever, prescribed and modern low‐dose combined oral contraceptive pills (COCPs) are used in clinical practice without much high level evidence of their effectiveness. The combined oral contraceptive pill (COCP) has been observed to reduce menstrual flow and cause decidualisation of endometriotic implants with decreased cell proliferation and increased apoptosis (Meresman 2002). For some time there has also been epidemiological evidence that the current use of COCPs is associated with a reduced incidence of endometriosis (Vessey 1993). Moreover, COCPs have an advantage over other hormonal treatments in that they can be taken for prolonged periods of time during reproductive life and are generally more acceptable to women than alternative hormonal treatments, which improves compliance.
Why it is important to do this review
Current guidelines suggest that empirical treatment, without first performing a diagnostic laparoscopy, with an COCP can be given to treat pain symptoms suggestive of endometriosis (Dunselman 2014). This review evaluated the evidence for COCPs in women who experience pain in association with endometriosis, but noted that response to empirical treatment did not confirm the presence or absence of disease and that such treatment might obscure the later development of deep infiltrating endometriosis (more severe disease).
Objectives
To determine the effectiveness, safety and cost‐effectiveness of oral contraceptive preparations in the treatment of painful symptoms ascribed to the diagnosis of laparoscopically proven endometriosis.
Methods
Criteria for considering studies for this review
Types of studies
All truly randomised controlled trials (RCT) using COCPs in the treatment of symptomatic endometriosis. We included trials with no treatment rather than a placebo arm and analysed them separately. We excluded quasi‐randomised controlled trials and included cross‐over studies only if pre‐cross‐over data were available.
Types of participants
Women of reproductive age who complained of symptoms ascribed to the diagnosis of endometriosis. The diagnosis must have been established during a surgical procedure performed prior to the start of treatment. Studies in both primary and secondary healthcare settings were considered.
We excluded trials where women had asymptomatic disease or infertility alone. We excluded trials where the endometrial deposits were outside the uterus.
Types of interventions
We considered the following comparisons:
COCP versus placebo/no treatment.
COCP versus other medical therapies (danazol, gonadotrophin‐releasing hormone analogues, progestogens, anti‐progestogens, levonorgestrel‐releasing intrauterine systems).
COCP versus conservative surgical treatment.
We considered:
only those treatments intended to relieve symptoms;
modern COCPs (defined as an ethinylestradiol dose 35 μg or less) taken conventionally, continuously or in a tricyclic regimen versus any other medical therapy, no treatment or placebo irrespective of dosage, route of administration or duration of treatment;
studies comparing COCP with medical therapy included the use of levonorgestrel‐releasing intrauterine systems;
studies comparing COCP with surgical excision or laser ablation of endometriotic implants or those that claimed to interrupt neural pathways (presacral neurectomy or laparoscopic uterosacral nerve ablation).
We excluded:
studies involving surgical removal of pelvic organs;
studies of alternative or complementary therapies;
studies exploring the use of hormonal treatment as an adjunct to surgery or other medical treatment for endometriosis.
All studies were included whether or not the duration of symptoms was specified.
Types of outcome measures
Primary outcomes
Self‐reported pain (dysmenorrhoea) at the end of treatment (as defined by trialists).
Secondary outcomes
Cyclical pain (non‐menstrual): recurrence, frequency and severity (pain scores, days lost off work, use of analgesics).
Lower abdominal or pelvic pain of a non‐cyclical nature: recurrence, frequency and severity (pain scores, days lost off work, use of analgesics).
Dyspareunia (pain during sexual intercourse): recurrence, frequency and severity (pain scores, days lost off work, use of analgesics).
Postcoital pain (pain following sexual intercourse): recurrence, frequency and severity (pain scores, days lost off work, use of analgesics).
Dyschezia (pain on defecation): recurrence, frequency and severity (pain scores, days lost off work, use of analgesics).
Any other pain symptom ascribed to endometriosis.
Participant satisfaction.
Withdrawal from treatment group: rates.
Adverse effects occurring during therapy (including pregnancy).
Adverse effects persisting after treatment.
Economic evaluations.
We assessed measures of subjective symptomatic relief (of any or all symptoms) using quantitative measures (such as visual analogue scales) or qualitative measures (such as the terms cured, better, same or worse). Outcome measures for each pain symptom were considered at the end of treatment and, when possible, three, six, nine, 12 and 18 months later. Symptom recurrence could occur both during treatment and after the end of treatment.
Objective evaluation of resolution of endometriotic implants at second‐look laparoscopy was also assessed, where possible, using standard scoring systems such as the revised American Society of Reproductive Medicine classification system (ASRM 1997).
Search methods for identification of studies
This is an update of the previous review that included one study (Davis 2007).
Electronic searches
We searched for all published and unpublished RCTs of any COCP in the treatment of symptomatic endometriosis, without language restrictions and in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist (from database inception to 19 October 2017).
We searched:
the Cochrane CGF Specialised Register of Controlled Trials, PROCITE platform (Appendix 1);
the Cochrane Central Register of Studies Online (CRSO Web platform) (Appendix 2);
MEDLINE (Epub Ahead of Print, In‐Process and Other Non‐Indexed Citations) Ovid (Appendix 3);
Embase Ovid (Appendix 4);
PsycINFO Ovid (Appendix 5);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) EBSCO (Appendix 6).
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying RCTs, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.1.0, Section 6.4.11) (Higgins 2011). The Embase, CINAHL and PsycINFO searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (www.sign.ac.uk/methodology/filters.html#random).
We searched the following trials registers to identify ongoing and registered clinical trials (19 October 2017):
ClinicalTrials.gov (a service of the US National Institutes of Health) (www.clinicaltrials.gov);
World Health Organization Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/Default.aspx).
We used the keywords 'endometriosis AND oral contraceptive.'
Searching other resources
We handsearched reference lists of relevant trials and systematic reviews retrieved by the search, and relevant journals and conference abstracts not covered in the CGF Specialised Register of Controlled Trials, in liaison with the Information Specialist.
Data collection and analysis
Selection of studies
In the update of this review, two review authors (JB and TC) independently screened titles and abstracts retrieved by the search in accordance with the inclusion criteria. Three review authors (JB, TC and AP) retrieved and examined for compliance full texts of all potentially eligible studies. We resolved disagreements by discussion.
Data extraction and management
Two review authors (of JB, AP and TC) independently extracted and verified study characteristics and outcome data from eligible studies using a data extraction form designed and piloted according to Cochrane guidelines. We sought additional information on trial methodology and results from the authors of three trials (Ali 2013; Fedele 2008; Kitawaki 2012), and received a reply from Kitawaki 2012.
Where studies had multiple publications, we collated the reports of the same study so that each study, rather than each report, was the unit of interest for the review, and such studies had a single identifier with multiple references.
Assessment of risk of bias in included studies
Two review authors (of JB, AP and TC) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool, which addresses the following domains: selection bias (randomisation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting) and other bias. Judgements were assigned as recommended in the Cochrane Handbook of Systematic Reviews of Interventions Section 8.5 (Higgins 2011). We resolved disagreements through discussion. We have fully described all judgements and summarised our conclusions in the 'Risk of bias' table in the Characteristics of included studies table.
Measures of treatment effect
For dichotomous data, we used the numbers of events in the intervention and control groups of each study to calculate Mantel‐Haenszel risk ratios (RR). For continuous data, if all studies reported the outcomes on the same scale, we calculated mean difference (MDs) between treatment groups. If similar outcomes were reported on different scales, we calculated the standardised mean difference (SMD). We treated ordinal data (e.g. quality of life scores) as continuous data. We presented 95% confidence intervals (CI) for all outcomes.
Where data to calculate RRs, MDs or SMDs were not available, we utilised the most detailed numeric data available that could facilitate similar analyses of included studies.
Unit of analysis issues
The analysis was per woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis where possible (i.e. including all randomised participants in analysis, in the groups to which they were randomised). Attempts were made to obtain missing data from the original trialists (i.e. from Ali 2013). Where these were unobtainable, we undertook analysis only of the available data. Any imputation undertaken was be subjected to sensitivity analysis (see below).
If studies reported sufficient detail to calculate MDs or SMDs but no information on associated standard deviation (SD), we assumed the outcome to have a SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We were unable to assess heterogeneity in this update of the review. In future updates, if sufficient studies are available, we will consider whether the clinical and methodological characteristics of the included studies are sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We will assess statistical heterogeneity by the measure of the I2 statistic. An I2 statistic greater than 50% will indicate substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. We had planned that, if there were 10 or more trials in an analysis, we would produce a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies). We were unable to make this assessment in this update of the review. In future updates, we will seek to explore publication bias where sufficient trials are available.
Data synthesis
Where studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons:
COCP versus placebo/no treatment;
COCP versus other medical treatment.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analysis to determine the separate evidence within the following subgroups:
type of COCP (type A versus type B).
When we detected substantial heterogeneity, we planned to explore possible explanations in subgroup or sensitivity analyses, or both. There was insufficient evidence for us to explore subgroups in this update of the review.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcome of this review to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. We considered whether the review conclusions would have changed if:
eligibility was restricted to studies without high risk of bias;
a random‐effects model had been adopted.
Due to insufficient evidence, we were unable to explore sensitivity analyses in this update of the review.
Overall quality of the body of evidence: 'Summary of findings' tables
Two review authors (JB and TC) independently prepared 'Summary of findings' tables using GRADE software (GRADEpro GDT 2015). These tables evaluated the overall quality of the body of evidence for the primary review outcome (self‐reported pain (dysmenorrhoea) at the end of treatment) for the main review comparisons (COCP versus placebo/no treatment and COCP versus other medical therapies) using GRADE criteria (study limitations, consistency, imprecision, indirectness and publication bias). Judgements about quality (high, moderate, low or very low) were justified and incorporated into the results for each outcome.
Results
Description of studies
Results of the search
The search retrieved 50 articles. Twenty‐three articles were potentially eligible and were retrieved in full text. Upon closer examination, 13 articles (12 studies) did not meet the inclusion criteria due to use of hormonal treatment as an adjunct to surgery. Five studies (six publications) met our inclusion criteria (Ali 2013; Guzick 2011; Harada 2008; Harada 2017; Vercellini 1993). Vercellini 1993 was identified in the original search conducted in 1996, and was included in the previous version of this review (Davis 2007). We excluded 10 studies due to ineligible study design, ineligible population or ineligible treatment (Figure 1). See: Characteristics of included studies table and Characteristics of excluded studies table. There were no ongoing studies identified.
1.
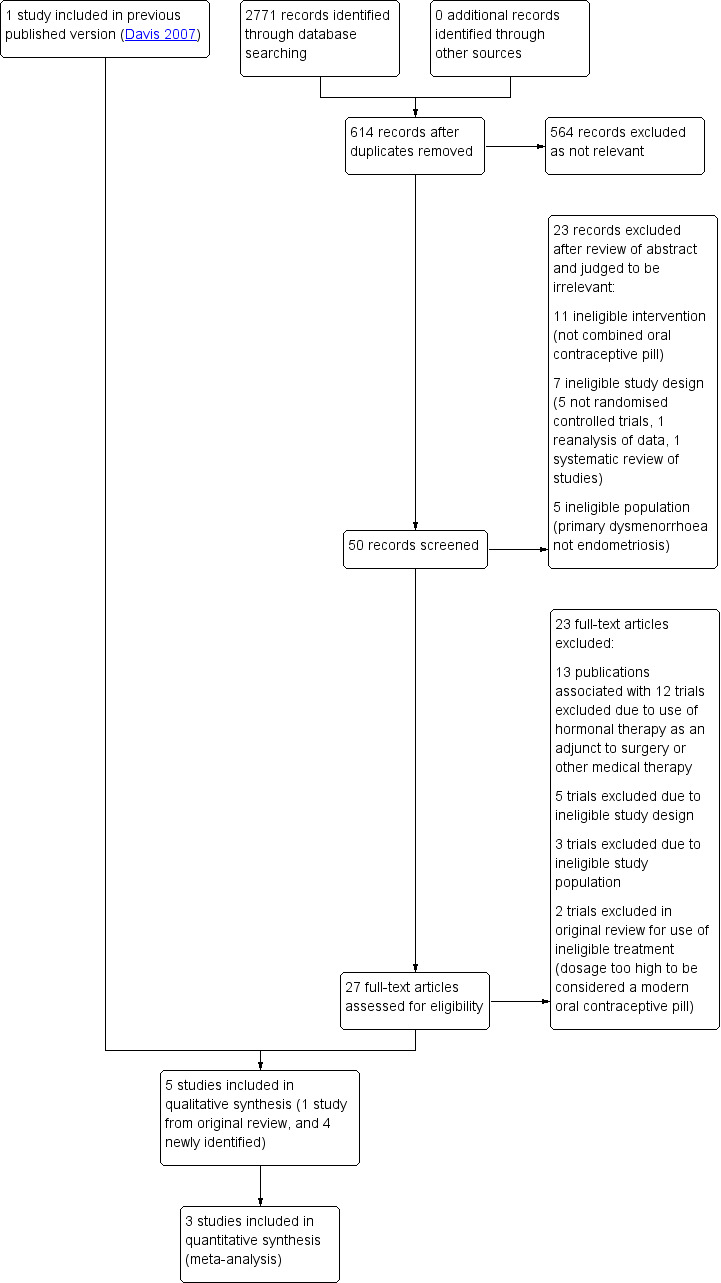
Study flow diagram.
Included studies
Study design and setting
The search identified five parallel‐design RCTs conducted in Egypt (Ali 2013), US (Guzick 2011), Japan (Harada 2008; Harada 2017), and Italy (Vercellini 1993). There was one single‐centre trial (Vercellini 1993) and three multi‐centre trials (Guzick 2011; Harada 2008; Harada 2017) conducted in hospitals. One trial was only available as an abstract and did not specify details of trial site (Ali 2013).
Participants
The studies included 612 women experiencing pelvic pain associated with endometriosis (Ali 2013: 150 women, Guzick 2011: 47 women, Harada 2008: 100 women, Harada 2017: 258 women; Vercellini 1993: 57 women).
Mean (± SD) ages ranged across studies from 27 ± 5 years (Vercellini 1993) to 35.7 ± 6.9 years (Harada 2017). The proportion of parous women included in each trial was comparable (Vercellini 1993: 26%; Guzick 2011: 28%; Harada 2008: 30%). Detailed information about participant demographics was not available in the Ali 2013 abstract. Harada 2017 did not report parity.
Guzick 2011 reported that endometriosis was diagnosed by laparoscopy or laparotomy within three years of trial entry. Either histology had to be consistent with endometriosis or operative records had to indicate visual evidence of lesions consistent with endometriosis. Harada 2008 and Harada 2017 reported that endometriosis was diagnosed by laparoscopy or laparotomy or endometrioma were diagnosed by ultrasound scan or magnetic resonance imaging. Vercellini 1993 reported that diagnosis of endometriosis was by laparoscopy or laparotomy. Ali 2013 provided no details for how endometriosis was diagnosed.
Interventions
The durations of intervention were three months (Ali 2013), four months (Harada 2008), six months (Harada 2017; Vercellini 1993), and 11 months (Guzick 2011).
Three trials used a monophasic COCP consisting of ethinylestradiol 0.035 mg and norethisterone 1 mg (Ali 2013; Guzick 2011; Harada 2008), one trial used a formulation of ethinylestradiol 0.020 mg and desogestrel 0.15 mg with the option to increase the dosage of ethinylestradiol to 0.030 mg if spotting or breakthrough bleeding occurred (Vercellini 1993), and the fifth trial used ethinylestradiol 0.02 mg and drospirenone 3 mg in a flexible extended regimen.
Two trials used continuous (daily) administration for the duration of the intervention (Ali 2013; Guzick 2011), and three trials used cyclical administration (21 days of active pills and seven days of placebo tablets) (Harada 2008; Harada 2017; Vercellini 1993).
Comparisons
Three trials used a gonadotropin‐releasing hormone analogue as the comparison (leuprolide 11.25 mg intramuscularly 12 weekly (Guzick 2011); leuprolide 3.75 mg intramuscularly monthly (Ali 2013); goserelin 3.6 mg subcutaneously (Vercellini 1993)).
Two trials used an inactive placebo of identical appearance as the comparison to facilitate blinding (Harada 2008; Harada 2017). Participants were also blinded in the Guzick 2011 trial and this was facilitated by administering a normal saline injection every 12 weeks to the women in the COCP group, and administering a daily oral "hormonal add‐back" of norethisterone acetate 5 mg identical in appearance to the COCP to the women in the leuprolide group.
Outcomes
Four trials used the Biberoglu and Behrman (Biberoglu 1981) verbal rating score (Ali 2013; Guzick 2011; Vercellini 1993) or a modified version of it (Harada 2008) to assess pain at the end of treatment, as well as other visual or numerical pain ratings methods. Harada 2017 reported data on reduction in pain from baseline to end of treatment. Harada 2008; Harada 2017; and Vercellini 1993 reported adverse effects of treatment. Guzick 2011 included data on adverse effects but this was not in a format that could be included in a meta‐analysis. There were no available data for meta‐analysis from the Ali 2013 trial, as this was only available in abstract form.
Excluded studies
We excluded 23 studies, for the following reasons.
Five studies had ineligible study design. Following review of the full‐text articles, we confirmed that four studies were not RCTs (Caruso 2016; Kitawaki 2012; Tanaka 2016; Taniguchi 2015), and one was a secondary report of a larger non‐randomised trial (Fedele 2008).
Three trials were conducted in healthy women without endometriosis, or with primary dysmenorrhoea but no identifiable pelvic disease (Caruso 2011; Portman 2011; Strowitzki 2012).
Twelve trials (13 publications) used hormonal therapy as an adjunct to surgery (Cheewadhanaraks 2012; Cucinella 2013; Granese 2015; Moawad 2012; Muzii 2011; Seracchioli 2010; Sesti 2007; Vercellini 2005; Vercellini 2002; Xu 2011; Zhu 2014), or other medical therapy (Long 2010).
Two trials used high doses of hormone that were not comparable to modern low‐dose contraceptive preparations (Fedele 1989; Shturkalev 1970), and so did not meet the inclusion criteria.
Risk of bias in included studies
2.
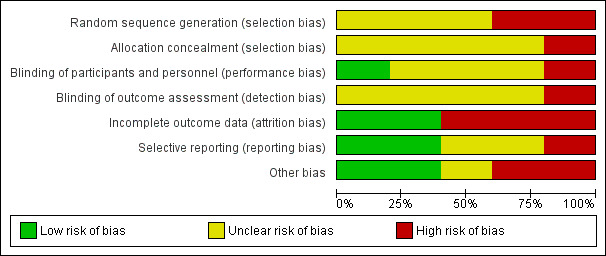
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
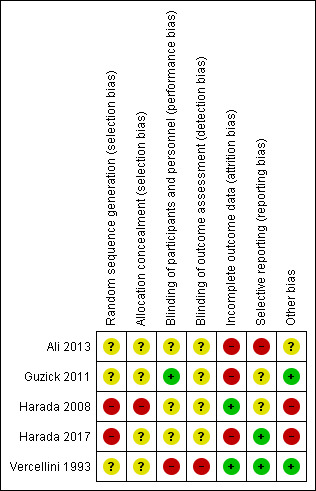
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Random sequence generation
Three trials provided insufficient details and were at unclear risk of bias related to random sequence generation (Ali 2013; Guzick 2011; Vercellini 1993). Harada 2008 and Harada 2017 were at high risk of bias for random sequence generation as randomisation was conducted by the pharmaceutical company funding the trial.
Allocation concealment
Four trials provided insufficient details and were at unclear risk of bias related to allocation concealment (Ali 2013; Guzick 2011; Harada 2017; Vercellini 1993). Harada 2008 stated that allocation concealment was accomplished by the pharmaceutical company, and was not broken until after all data were collected. This was at high risk of bias for this domain. The company funding the trial was also responsible for allocation concealment; no details were provided as to whether sequentially numbered opaque envelopes were used.
Blinding
Participants were blinded in Guzick 2011 and the trial was at low risk for performance bias. Harada 2008 and Harada 2017 stated that participants and clinicians were blinded by using a placebo. However, as the trial was sponsored by the pharmaceutical company who were also responsible for randomisation and allocation concealment in Harada 2008, it is possible that there was some communication between the clinicians and the sponsors. We have judged this trial at unclear risk of performance bias. Guzick 2011; Harada 2008; and Harada 2017 did not stipulate that outcome assessors were blinded, and were at unclear risk of detection bias. Vercellini 1993 was at high risk of performance bias and detection bias as participants and clinicians were aware of the treatment allocation and blinding was not undertaken, due to the nature of the treatment administration (injection versus oral). Ali 2013 was only available as a conference abstract and provided no details on numbers of participants allocated to each group or final number analysed.
Incomplete outcome data
Three trials were at high risk of attrition bias. Ali 2013 was at high risk of bias as no details were provided on allocation to groups for final number of women analysed. Guzick 2011 was at high risk of attrition bias. Of the 47 women randomised, seven dropped out immediately (three in the OCP group and four in the leuprolide group), and only 24 women completed the trial although it is unclear which groups they were allocated to. Harada 2017 reported that 20% in each group discontinued the study by 24 weeks of treatment.
Seven of 57 women randomised in the Vercellini 1993 trial did not complete follow‐up (four in the COCP group, three in the goserelin group). This was rated at low risk of attrition bias. Fourteen of the 100 women randomised discontinued the Harada 2008 trial (seven in each group) but had data included in the analysis. This was rated at low risk for attrition bias.
Selective reporting
None of the trials had published protocols. Vercellini 1993 and Harada 2017 were at low risk of selective reporting bias as all prespecified outcomes were reported. Harada 2008 was at unclear risk of selective reporting as there was no protocol and the trial did not appear to have been registered. Guzick 2011 was at unclear risk of selective reporting bias as adverse effects were reported that were not prespecified. The Ali 2013 trial was at high risk of bias as it was only available as a conference abstract and reported only one outcome.
Other potential sources of bias
Guzick 2011 and Vercellini 1993 were at low risk of other potential sources of bias. The Ali 2013 trial at unclear risk of bias as there was insufficient information to enable a judgement. Harada 2008 and Harada 2017 were at high risk of bias as authors received consulting fees/payments from the pharmaceutical company that also provided the randomisation or allocation concealment (or both) service.
Effects of interventions
1. Combined oral contraceptive pill versus placebo/no treatment
Two studies with 354 women compared COCP with placebo (Harada 2008; Harada 2017).
Primary outcome
1.1. Self‐reported pain (dysmenorrhoea) at the end of treatment
Treatment with COCP was associated with a lower score on the Dysmenorrhoea verbal rating scale compared with placebo (MD ‐1.30 points, 95% CI ‐1.84 to ‐0.76; 1 RCT, 96 women; very low quality evidence), a lower score on the Dysmenorrhoea Visual Analogue Scale compared with placebo (MD ‐23.68 points, 95% CI ‐28.75 to ‐18.62, 2 RCTs, 327 women; very low quality evidence) at the end of treatment and a greater reduction in menstrual pain from baseline to the end of treatment (MD 2.10 points, 95% CI 1.38 to 2.82; 1 RCT, 169 women; very low quality evidence) (Analysis 1.1; Figure 4).
1.1. Analysis.
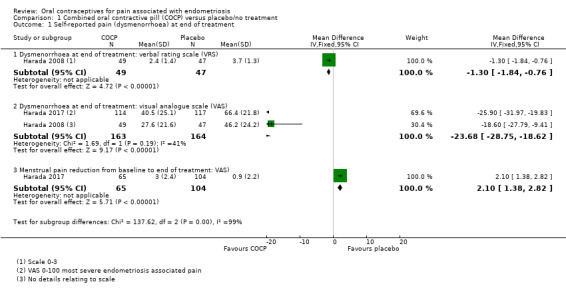
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 1 Self‐reported pain (dysmenorrhoea) at end of treatment.
4.
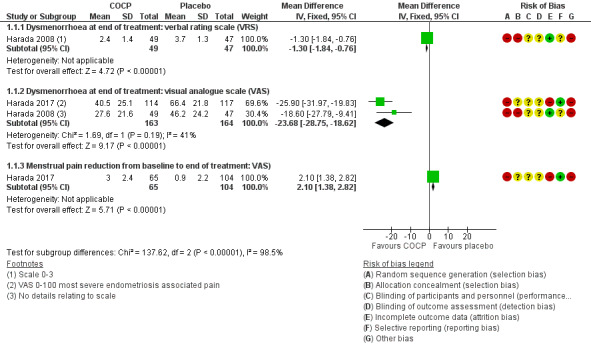
Forest plot of comparison: 1 Combined oral contractive pill (COCP) versus placebo/no treatment, outcome: 1.1 Self‐reported pain (dysmenorrhoea) at end of treatment.
Secondary outcomes
1.2. Cyclical pain (non‐menstrual)
At the end of treatment, there was no clear evidence of a difference between women treated with COCP and women treated with placebo for the non‐menstrual pain verbal rating scale (MD 0.10, 95% CI ‐0.48 to 0.68; 1 RCT, 96 women) or for the non‐menstrual pain visual analogue scale (MD ‐1.90, 95% CI ‐11.72 to 7.92; 1 RCT, 96 women). There was a greater mean reduction in menstrual pain from baseline to the end of treatment for women in the COCP group compared with placebo (MD 1.00 points, 95% CI 0.30 to 1.70; 1 RCT, 212 women) (Analysis 1.2).
1.2. Analysis.
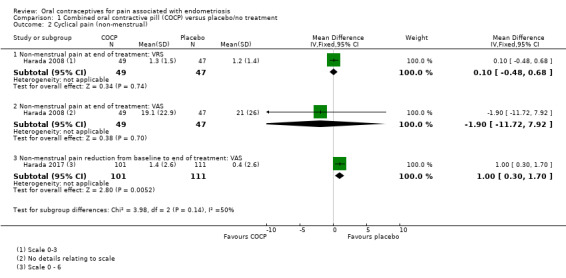
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 2 Cyclical pain (non‐menstrual).
1.3. Lower abdominal or pelvic pain of a non‐cyclical nature
We found no data on lower abdominal or pelvic pain of a non‐cyclical nature.
1.4. Dyspareunia (pain during sexual intercourse)
Women in the COCP group reported a greater reduction in 'severest dyspareunia' from baseline to the end of treatment compared with placebo (MD 1.40 points, 95% CI 0.46 to 2.34; 1 RCT, 89 women) (Analysis 1.3).
1.3. Analysis.
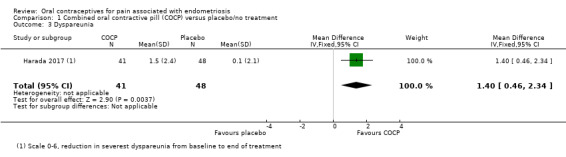
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 3 Dyspareunia.
1.5. Postcoital pain (pain following sexual intercourse)
We found no data on postcoital pain.
1.6. Dyschezia (pain on defecation)
Women in the COCP group reported a greater reduction in the 'severest defecation pain' from baseline to the end of treatment compared with placebo (MD 1.20 points, 95% CI 0.56 to 1.84; 1 RCT, 231 women) (Analysis 1.4).
1.4. Analysis.
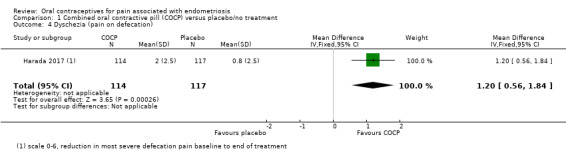
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 4 Dyschezia (pain on defecation).
1.7. Any other pain symptom ascribed to endometriosis
We found no data on any other pain symptom ascribed to endometriosis.
1.8. Participant satisfaction
Women in the COCP group reported a greater level of satisfaction (very highly/highly satisfied) compared with placebo (RR 4.24, 95% CI 2.44 to 7.37; 1 RCT, 258 women) (Analysis 1.5).
1.5. Analysis.
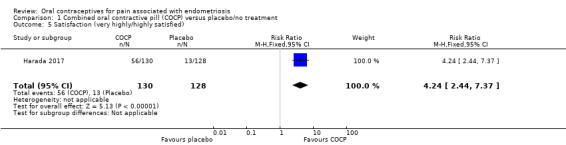
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 5 Satisfaction (very highly/highly satisfied).
1.9. Withdrawal from treatment group
There was no clear evidence of a difference in withdrawal from the study between the COCP and placebo groups (RR 1.34, 95% CI 0.83 to 2.18; 2 RCTs, 354 women) (Analysis 1.6).
1.6. Analysis.
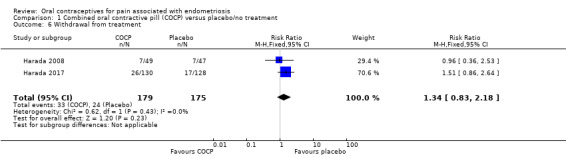
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 6 Withdrawal from treatment.
1.10. Adverse effects occurring during therapy
Pregnancy
There was no clear evidence of a difference between COCP and placebo groups for pregnancy (RR 2.88, 95% CI 0.12 to 68.98; 1 RCT, 96 women). There was only one event reported in the COCP group and no events in the placebo group.
Spotting/irregular bleeding/menorrhagia
Women treated with COCP were more likely to experience spotting, irregular bleeding or menorrhagia compared with women treated with placebo (RR 2.44, 95% CI 1.44 to 4.15; 2 RCTs, 354 women).
Nausea
Women treated with COCP were more likely to experience nausea compared with women treated with placebo (RR 4.14, 95% CI 1.79 to 9.54; 2 RCTs, 354 women).
Any treatment‐associated adverse effect
Women treated with COCP were more likely to experience any treatment‐associated adverse effect compared with women treated with placebo (RR 1.17, 95% CI 1.00 to 1.36; 1 RCT, 258 women). (Analysis 1.7).
1.7. Analysis.
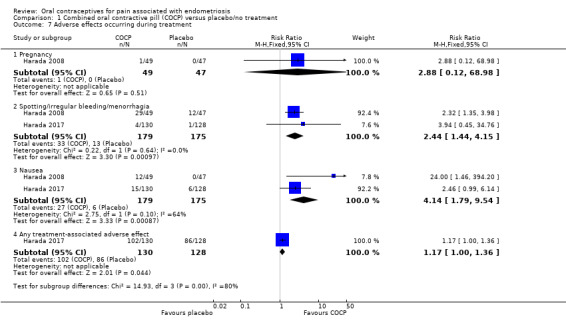
Comparison 1 Combined oral contractive pill (COCP) versus placebo/no treatment, Outcome 7 Adverse effects occurring during treatment.
1.11 Adverse effects persisting after treatment
We found no data on adverse effects persisting after treatment.
1.12. Economic evaluations
We found no data on economic evaluations.
2. Combined oral contraceptive pill versus other medical therapies
One study (57 women) compared cyclic low‐dose monophasic COCP, containing ethinylestradiol 0.02 mg plus desogestrel 0.15 mg with goserelin 3.6 mg subcutaneous depot formulation monthly for six months (Vercellini 1993). Two studies compared COCP (ethinylestradiol 0.035 mg plus norethisterone 1 mg) with leuprolide 11.25 mg intramuscularly 12 weekly (Ali 2013; Guzick 2011). There were no data from Ali 2013 and Guzick 2011 that could be included in a meta‐analysis although there were declines in Biberoglu and Behrman pain scores, Numerical Rating Scores and Beck Depression Inventory scores from baseline in both treatment groups, but no differences between groups, reported in Guzick 2011.
Primary outcome
2.1. Self‐reported pain (dysmenorrhoea) at the end of treatment
At the end of treatment, no women in the goserelin group reported dysmenorrhoea as they were amenorrhoeic, therefore, no direct comparison could be made between groups. In the COCP group, the visual analogue scale (mean ± SD) was 3.7 ± 2.1 (24 women) and the verbal rating scale (mean ± SD) was 2.4 ± 1.7 (24 women). At six months' follow‐up, there was no clear evidence of a difference between women treated with COCP and women treated with goserelin for measures of dysmenorrhoea on a visual analogue scale (MD ‐0.10, 95% CI ‐1.28 to 1.08; 1 RCT, 50 women; very low quality evidence) or a verbal rating scale (MD ‐0.10, 95% CI ‐0.99 to 0.79; 1 RCT, 50 women; very low quality evidence) (Analysis 2.1; Figure 5).
2.1. Analysis.
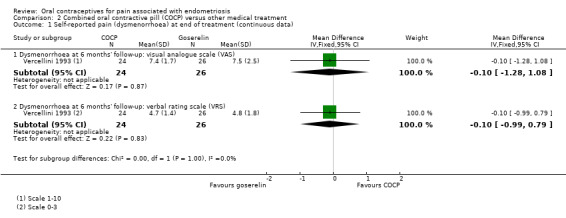
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data).
5.
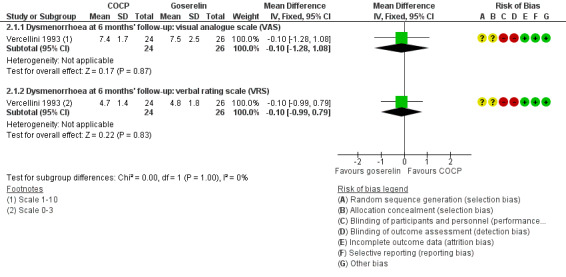
Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data).
At the end of treatment, all women in the goserelin group were amenorrhoeic and, therefore, no comparison could be made between the groups. In the COCP group, 17/24 women reported mild or complete absence of dysmenorrhoea at the end of treatment and 3/24 women reported complete absence of pain. At six months' follow‐up, there was no clear evidence of a difference between the COCP and goserelin groups for reporting mild or zero pain using a visual analogue scale (5/24 women with COCP versus 6/26 women with goserelin; RR 0.90, 95% CI 0.32 to 2.58; 1 RCT, 50 women) or complete absence of pain (0/24 women with COCP and 1/26 women with goserelin; RR 0.36, 95% CI 0.02 to 8.43; 1 RCT, 50 women; very low quality evidence) (Analysis 2.2; Figure 6).
2.2. Analysis.
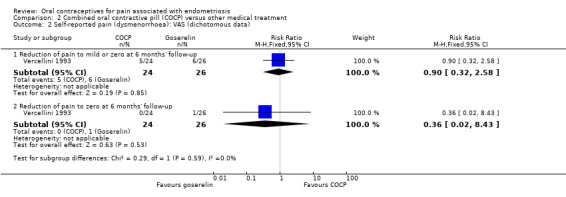
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data).
6.
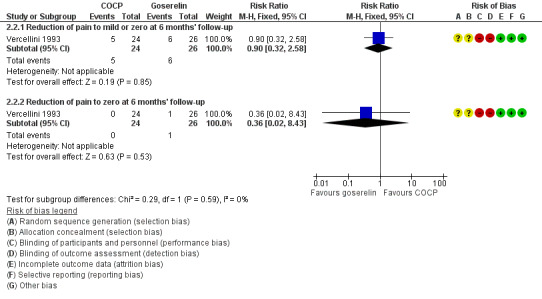
Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data).
At the end of treatment, all women in the goserelin group were amenorrhoeic and therefore no comparison could be made between the groups. In the COCP group, 18/24 women reported mild or complete absence of dysmenorrhoea at the end of treatment and 3/24 women reported complete absence of pain. At six months' follow‐up, there was no clear evidence of a difference between the COCP and goserelin groups for reporting mild or zero pain using a verbal rating scale (17/22 women with COCP versus 18/22 women with goserelin; RR 0.94, 95% CI 0.70 to 1.28; 1 RCT, 44 women) or complete absence of pain (24/24 women with OCP versus 25/25 women with goserelin; RR 1.00, 95% CI 0.93 to 1.08; 1 RCT, 49 women; low quality evidence) (Analysis 2.3; Figure 7).
2.3. Analysis.
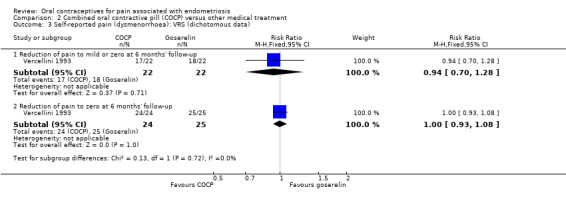
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data).
7.
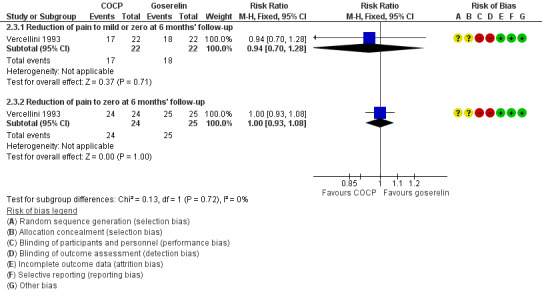
Forest plot of comparison: 2 Combined oral contractive pill (COCP) versus other medical treatment, outcome: 2.3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data).
Secondary outcomes
2.2. Cyclical pain (non‐menstrual)
Overall non‐menstrual pain
At the end of treatment, there was no clear difference between women treated with COCP and women treated with goserelin for non‐menstrual pain using a visual analogue scale (MD ‐0.20, 95% CI ‐1.51 to 1.11; 1 RCT, 50 women) or using a verbal rating scale (MD 0.40, 95% CI ‐0.51 to 1.31; 1 RCT, 50 women).
At six months' follow‐up, there was no clear difference between women treated with COCP and women treated with goserelin for non‐menstrual pain using a visual analogue scale (MD ‐0.30, 95% CI ‐1.85 to 1.25; 1 RCT, 50 women) or using a verbal rating scale (MD 0.00, 95% CI ‐1.08 to 1.08; 1 RCT, 50 women) (Analysis 2.4).
2.4. Analysis.
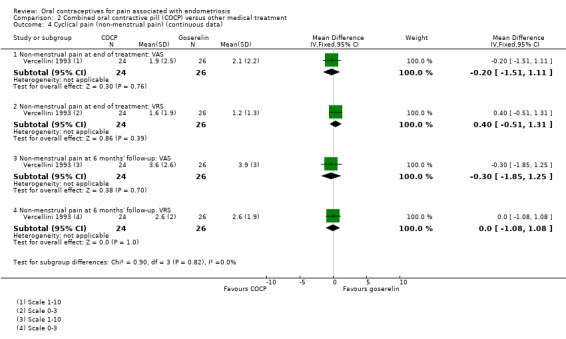
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 4 Cyclical pain (non‐menstrual pain) (continuous data).
Mild or zero non‐menstrual pain or complete absence of pain
At the end of treatment, there was no clear difference between women treated with COCP and women treated with goserelin for mild or zero non‐menstrual pain (RR 0.99, 95% CI 0.81 to 1.21; 1 RCT, 50 women) or complete absence of pain (RR 0.98, 95% CI 0.51 to 1.89; 1 RCT, 50 women) using a visual analogue scale or for mild or zero non‐menstrual pain (RR 0.99, 95% CI 0.84 to 1.17; 1 RCT, 50 women) or complete absence of pain (RR 0.98, 95% CI 0.51 to 1.89; 1 RCT, 50 women) using a verbal rating scale (Analysis 2.5).
2.5. Analysis.
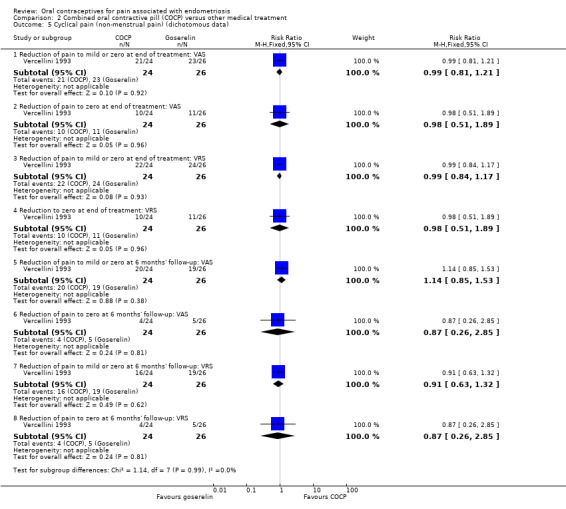
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 5 Cyclical pain (non‐menstrual pain) (dichotomous data).
At six months' follow‐up, there was no clear difference between women treated with COCP and women treated with goserelin for mild or zero non‐menstrual pain (RR 1.14, 95% CI 0.85 to 1.53; 1 RCT, 50 women) or complete absence of pain (RR 0.87, 95% CI 0.26 to 2.85; 1 RCT, 50 women) using a visual analogue scale or for mild or zero non‐menstrual pain (RR 0.91, 95% CI 0.63 to 1.32; 1 RCT, 50 women) or complete absence of pain (RR 0.87, 95% CI 0.26 to 2.85; 1 RCT, 50 women) using a verbal rating scale (Analysis 2.5).
2.3. Lower abdominal or pelvic pain of a non‐cyclical nature
We found no data on lower abdominal or pelvic pain of a non‐cyclical nature.
2.4. Dyspareunia (pain during sexual intercourse)
Overall dyspareunia
At the end of treatment, there was an increase in the experience of dyspareunia in women treated with COCP compared with women treated with goserelin using a visual analogue scale (MD 1.80, 95% CI 0.18 to 3.42; 1 RCT, 43 women) although there was no evidence of a difference between groups for dyspareunia using a verbal rating scale (MD 0.10, 95% CI ‐0.41 to 0.61; 1 RCT, 43 women) (Analysis 2.6).
2.6. Analysis.
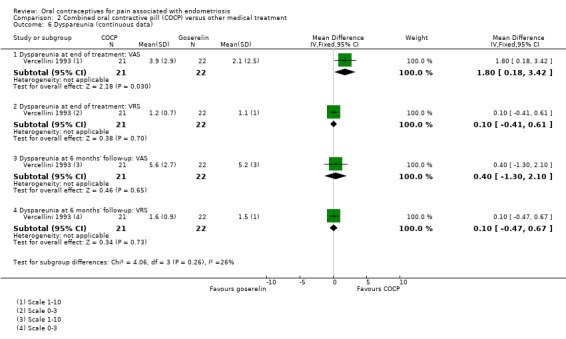
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 6 Dyspareunia (continuous data).
At six months' follow‐up, there was no clear difference between women treated with COCP and women treated with goserelin for dyspareunia using a visual analogue scale (MD 0.40, 95% CI ‐1.30 to 2.10; 1 RCT, 43 women) or a verbal rating scale (MD 0.10, 95% CI ‐0.47 to 0.67; 1 RCT, 43 women) (Analysis 2.6).
Mild or zero dyspareunia or complete absence of dyspareunia
At the end of treatment, there was no clear difference between women treated with COCP and women treated with goserelin for mild or zero dyspareunia (RR 0.73, 95% CI 0.53 to 1.02; 1 RCT, 43 women) or complete absence of dyspareunia (RR 0.52, 95% CI 0.19 to 1.48; 1 RCT, 43 women) using a visual analogue scale or mild or zero dyspareunia (RR 0.96, 95% CI 0.56 to 1.65; 1 RCT, 25 women) or complete absence of dyspareunia (RR 1.35, 95% CI 0.99 to 1.84; 1 RCT, 38 women) using a verbal rating scale (Analysis 2.7).
2.7. Analysis.
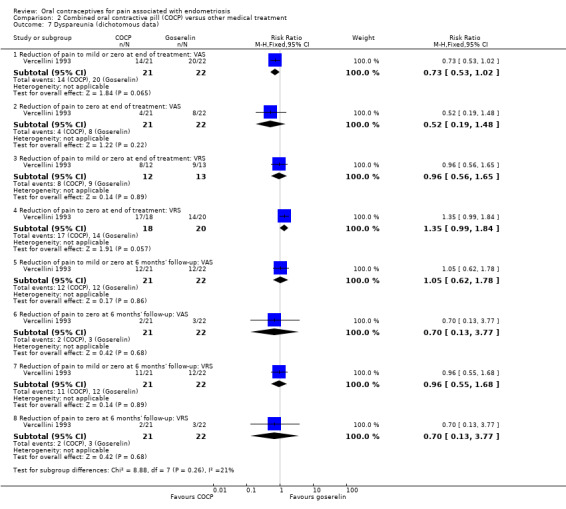
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 7 Dyspareunia (dichotomous data).
At six months' follow‐up, there was no clear difference between women treated with COCP and women treated with goserelin for mild or zero dyspareunia (RR 1.05, 95% CI 0.62 to 1.78; 1 RCT, 43 women) or complete absence of pain (RR 0.70, 95% CI 0.13 to 3.77; 1 RCT, 43 women) using a visual analogue scale or mild or zero dyspareunia (RR 0.96, 95% CI 0.55 to 1.68; 1 RCT, 43 women) or complete absence of pain (RR 0.70, 95% CI 0.13 to 3.77; 1 RCT, 43 women) using a verbal rating scale (Analysis 2.7).
2.5. Postcoital pain (pain following sexual intercourse)
We found no data on postcoital pain.
2.6. Dyschezia (pain on defecation)
We found no data on dyschezia.
2.7. Any other pain symptom ascribed to endometriosis
We found no data on any other pain symptom ascribed to endometriosis.
2.8. Participant satisfaction
We found no data on participant satisfaction.
2.9. Withdrawal from treatment group
There was no clear evidence of a difference between the COCP group and the goserelin group for withdrawal from treatment (RR 1.38, 95% CI 0.34 to 5.62; 1 RCT, 57 women). Seven women in total withdrew from the study: because of pregnancy (one woman), lost to follow‐up (one woman) and use of additional hormonal therapy after the end of treatment (one woman) in the goserelin group; and lost to follow‐up (one woman), additional hormonal therapy (two women) and adverse effects (one woman) in the COCP group (Analysis 2.8).
2.8. Analysis.
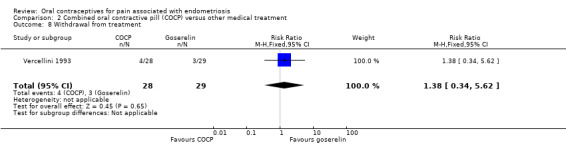
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 8 Withdrawal from treatment.
2.10. Adverse effects occurring during therapy
Women in the COCP group were less likely to experience hot flushes than women in the goserelin group (RR 0.04, 95% CI 0.01 to 0.30; 1 RCT, 57 women).
There was no clear evidence of a difference between the COCP group and the goserelin group for any of the other adverse effects reported including insomnia (RR 0.07, 95% CI 0.00 to 1.15; 1 RCT, 57 women); spotting/irregular bleeding (RR 1.21, 95% CI 0.46 to 3.15; 1 RCT, 57 women); decreased libido (RR 0.69, 95% CI 0.22 to 2.19; 1 RCT, 57 women); vaginal dryness (RR 0.09, 95% CI 0.01 to 1.63; 1 RCT, 57 women); mood changes (RR 1.04, 95% CI 0.34 to 3.19; 1 RCT, 57 women); headache (RR 1.55, 95% CI 0.49 to 4.92; 1 RCT, 57 women); paraesthesia (RR 0.35, 95% CI 0.04 to 3.12; 1 RCT, 57 women); breast tenderness (RR 1.73, 95% CI 0.45 to 6.55; 1 RCT, 57 women); weight gain (RR 2.07, 95% CI 0.41 to 10.43; 1 RCT, 57 women); peripheral oedema (RR 1.04, 95% CI 0.07 to 15.77; 1 RCT, 57 women); joint pain (RR 0.34, 95% CI 0.01 to 8.12; 1 RCT, 57 women) (Analysis 2.9).
2.9. Analysis.
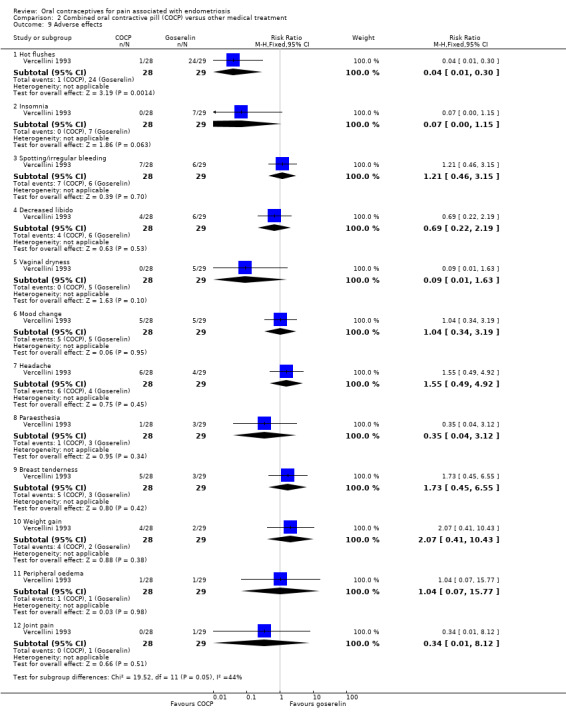
Comparison 2 Combined oral contractive pill (COCP) versus other medical treatment, Outcome 9 Adverse effects.
2.11. Adverse effects persisting after treatment
We found no data on adverse effects persisting after treatment.
2.12. Economic evaluations
We found no data on economic evaluations.
3. Combined oral contraceptive pill versus conservative surgical treatment
We found no studies comparing COCP versus conservative surgical treatment.
Discussion
Endometriosis is a recurring disease and medical therapy should be viewed as symptom control rather than a cure. At present the use of GnRH analogues is limited to six months because of associated bone loss. The duration of other medical treatments is also limited to six months in the first instance because of unwanted metabolic effects. The OCP has the great advantage that it can be taken for prolonged periods provided it is not contraindicated. Although serious adverse effects, such as thromboembolic episodes, may occur with treatment, the risk is estimated to be only 7/10,000 to 10/10,000 women per year (Bateson 2016), the highest risk being within the first year of use. The OCP is widely used by many women long term and risks are well known and accepted by doctors and women.
Summary of main results
Two trials including 354 women compared the COCP with placebo. The COCP was associated with an improvement in self‐reported pain (dysmenorrhoea) at the end of treatment. There were also improvements in cyclical non‐menstrual pain, dyspareunia and dyschezia. Women in the COCP group were more likely to report being very highly or highly satisfied compared with placebo. Spotting, irregular bleeding or menorrhagia, and nausea were more commonly associated with the COCP compared with placebo.
Three studies compared COCP with another medical treatment. Data suitable for meta‐analysis were only available from one trial that compared the COCP with goserelin (Vercellini 1993). There was no clear evidence of a difference between groups for self‐reported measures of dysmenorrhoea pain reduction or non‐menstrual pain reduction. At the end of treatment, COCP was associated with an increase in dyspareunia as reported using a visual analogue scale; however, at six‐month follow‐up this difference was no longer detected. COCP was associated with a reduced risk of hot flushes compared with goserelin and may, therefore, be an alternative option.
No studies compared COCP with conservative surgical treatment were identified.
Overall completeness and applicability of evidence
There continues to be surprisingly little literature about the treatment of endometriosis with OCPs despite their apparent widespread use in clinical practice. Our search strategy identified only three studies that could be included in a meta‐analysis from the five overall studies. Therefore, we were unable to comment on most of the comparisons identified for our objectives. There were no data for the comparison of COCP versus conservative surgical treatment.
Two trials of 354 women, both conducted in Japan, compared COCP with placebo. The findings are not likely to be generalisable as, due to the limited evidence, we do not know if different formulations of COCP would have different effects. The formulation used in the Japanese studies may not be readily available globally.
In the one study of COCP compared with goserelin, the power calculation became invalid as a result of a higher than expected recurrence rate of pain in the goserelin group (77% recurrence rather than the 35% that was used in the power calculation), rendering the study underpowered. Hence, the study may have failed to detect a difference in efficacy between COCP and goserelin. This study, which was conducted in Italy, is also unlikely to be generalisable to other settings.
This review focused on the effectiveness of OCPs for treating pain associated with endometriosis. There are other formulations of the combined hormonal contraception such as the transdermal patch, vaginal ring or combined injectable contraceptives that were not covered in this review but should be considered in future updates.
Quality of the evidence
We prepared 'Summary of findings' tables using GRADEpro and Cochrane methods (Table 1; Table 2). These tables evaluated the overall quality of the body of evidence for the main review outcome (pain associated with endometriosis) for the main review comparison that had data available (COCP versus placebo and COCP versus goserelin). We assessed the quality of the evidence using GRADE criteria (risk of bias, consistency of effect, imprecision, indirectness and publication bias). Two review authors independently judged quality of evidence (high, moderate, low or very low), with disagreements resolved by discussion. Judgements were justified, documented and incorporated into reporting of results for the outcome.
We judged the evidence for the comparison of COCP with placebo to be very low quality overall. We judged the evidence for the comparison of COCP with other medical treatment to be low or very low quality overall.
One of the main concerns of the two Japanese trials comparing COCP with placebo was that the trials were supported by a pharmaceutical company that participated in the trial design and managed operational aspects of the trial process, including data collection, analysis and writing of the report (Harada 2008; Harada 2017). Authors also received payment from the company.
Potential biases in the review process
Potential biases in the review process were minimised by searching published and unpublished literature from a variety of sources with no restrictions on date of publication or language. At least two review authors independently extracted data and conducted the risk of bias assessment.
We were unable to judge the potential effect of publication bias, we identified fewer than 10 trials.
Agreements and disagreements with other studies or reviews
Two reviews of available evidence concluded that oral contraceptives were effective for first‐line treatment of pain associated with endometriosis but noted that this was based on limited evidence (Al‐Jefout 2011; Zito 2014). Neither of these reviews identified additional studies that were not identified and included in this review. We had included the most recent study by Harada 2017.
Authors' conclusions
Implications for practice.
Based on the limited evidence from two trials and limited data for the prespecified outcomes for this review, there is insufficient evidence to make a judgement on the effectiveness of the combined oral contraceptive pill (COCP) compared with placebo.
Based on the limited evidence from one small trial that was at high risk of bias, there is insufficient evidence to make a judgement on the effectiveness of COCP compared with other medical treatments. Only one comparison was possible, with the medical intervention being goserelin.
Given the widespread and recommended use of the COCP in this setting, clinicians need to make decisions based on other available evidence.
Implications for research.
Endometriosis is a common disease and the treatments currently available have unpleasant adverse effects. For all hormonal treatments other than COCP, the duration of therapy is limited, at least theoretically. It is well known that symptoms frequently recur when medication is stopped. If COCP could be shown to be at least as effective and safe as the existing well‐recognised therapies, it would offer a wider choice of treatment options, including long‐term symptom control. Acceptability from the perspective of the consumer should be considered when designing these studies.
There continues to be a need for larger studies of oral contraceptive pill use for pain in endometriosis, accompanied by measures of participant satisfaction. However, the tendencies to treat endometriosis‐associated symptoms in the absence of a surgical diagnosis and surgically confirmed disease at the initial laparoscopy may make such studies difficult to perform and funding is likely to be problematical. Studies that compare COCP with other hormonal contraceptives, such as the levonorgestrel‐releasing intrauterine system, would be welcomed but it is more likely that these will be undertaken in the field of secondary prevention rather than primary treatment reflecting current clinical practice.
We found no studies comparing COCP and conservative surgical treatment.
Further research is needed to evaluate fully the role of oral contraceptive pills in managing pain‐related symptoms associated with endometriosis. There are other formulations of the combined hormonal contraception such as the transdermal patch, vaginal ring or combined injectable contraceptives that were not covered in this review but should be considered in future updates.
What's new
| Date | Event | Description |
|---|---|---|
| 20 March 2018 | New citation required but conclusions have not changed | The addition of 4 studies at this update did not lead to a change in the conclusions of this review. |
| 20 March 2018 | New search has been performed | Review updated in 2018 and four new studies added (Ali 2013; Guzick 2011; Harada 2008; Harada 2017). |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 20 June 2011 | New search has been performed | 'Summary of findings' table added to review |
| 10 November 2008 | Amended | Title changed from "Modern combined oral contraceptives for pain associated with endometriosis" |
| 7 November 2008 | Amended | Converted to new review format |
| 17 May 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Thank you to the editorial base in Auckland.
2018 update: We would like to acknowledge the contributions of L‐J Davis, SS Kennedy and J Moore to previous versions of this review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
Searched 19 October 2017
Procite platform
Keywords CONTAINS "endometriosis" or "Endometriosis‐Symptoms" or "pelvic pain" or "dyspareunia" or "dyschezia" or Title CONTAINS" endometriosis" or "Endometriosis‐Symptoms" or "pelvic pain" or "dyspareunia" or "dyschezia"
AND
Keywords CONTAINS "Oral Contraception" or "oral contraceptive" or "oral contraceptive pill" or "Oral Contraceptive Agent" or "oral contraceptives" or "oral estradiol" or "oral estrogen" or "OCP" or "oestrogen plus progestagen" or "ethinyl estradiol + drospirenone" or "ethinyl estradiol‐cyproterone acetate" or "ethinyl‐estradiol" or "desogestral" or "desogestrel" or "Levonorgestrel" or "Norgestimate" or "Norgestrel" or "Norethisterone" or "noresthisterone" or "combined oral contraceptives" or Title CONTAINS "Oral Contraception" or "oral contraceptive" or "oral contraceptive pill" or "Oral Contraceptive Agent" or "oral contraceptives" or "oral estradiol" or "oral estrogen" or "OCP" or "oestrogen plus progestagen" or "ethinyl estradiol + drospirenone" or "ethinyl estradiol‐cyproterone acetate" or "ethinyl‐estradiol" or "desogestral" or "desogestrel" or "Levonorgestrel" or "Norgestimate" or "Norgestrel" or "Norethisterone" or "noresthisterone" or "combined oral contraceptives" (91 hits)
Appendix 2. CENTRAL CRSO search strategy
Searched 19 October 2017
Web platform
#1MESH DESCRIPTOR Endometriosis EXPLODE ALL TREES (536) #2Endometrio*:TI,AB,KY (1507) #3Dyspareunia:TI,AB,KY (616) #4Dyschezia:TI,AB,KY (25) #5(pelvic pain):TI,AB,KY 960 #6#1 OR #2 OR #3 OR #4 OR #5 (2656) #7MESH DESCRIPTOR Contraceptives, Oral EXPLODE ALL TREES (3262) #8MESH DESCRIPTOR Contraceptives, Oral, Combined EXPLODE ALL TREES (698) #9MESH DESCRIPTOR Ethinyl Estradiol‐Norgestrel Combination EXPLODE ALL TREES (67) #10MESH DESCRIPTOR Contraceptives, Oral, Hormonal EXPLODE ALL TREES (242) #11MESH DESCRIPTOR Contraceptives, Oral, Sequential EXPLODE ALL TREES (32) #12MESH DESCRIPTOR Contraceptives, Oral, Synthetic EXPLODE ALL TREES (2643) #13MESH DESCRIPTOR Desogestrel EXPLODE ALL TREES (349) #14MESH DESCRIPTOR Levonorgestrel EXPLODE ALL TREES (628) #15MESH DESCRIPTOR Norgestrel EXPLODE ALL TREES (848) #16(oral contracept*):TI,AB,KY or (contraceptive pill*):TI,AB,KY (2416) #17OCP*:TI,AB,KY (160) #18(ethinyl estradiol):TI,AB,KY (1390) #19desogestrel:TI,AB,KY (534) #20dienogest:TI,AB,KY (138) #21levonorgestrel:TI,AB,KY (1218) #22norgestrel:TI,AB,KY (434) #23(estrogen* or oestrogen*):TI,AB,KY (10437) #24(Progestin* or Progest?gen*):TI,AB,KY (2074) #25MESH DESCRIPTOR Norethindrone EXPLODE ALL TREES (707) #26Norethindrone:TI,AB,KY (807) #27norethisterone:TI,AB,KY (731) #28gestodene:TI,AB,KY (240) #29MESH DESCRIPTOR Ethinyl Estradiol EXPLODE ALL TREES (1165) #30MESH DESCRIPTOR Norgestrienone EXPLODE ALL TREES (33) #31(ethinyl oestradiol or ethinylestradiol):TI,AB,KY (909) #32#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 (14739) #33 #6 AND #32 (527)
Appendix 3. MEDLINE search strategy
Searched from 1946 to 19 October 2017
Ovid platform
1 exp contraceptives, oral/ (47054) 2 exp contraceptives, oral, combined/ or exp ethinyl estradiol‐norgestrel combination/ or contraceptives, oral, hormonal/ or contraceptives, oral, sequential/ or exp contraceptives, oral, synthetic/ or exp desogestrel/ or exp levonorgestrel/ or exp norgestrel/ (31520) 3 (oral contracept$ or contraceptive pill*).tw. (27438) 4 OCP$.tw. (3803) 5 ethinyl estradiol.tw. (3985) 6 desogestrel.tw. (1152) 7 dienogest.tw. (397) 8 levonorgestrel.tw. (4445) 9 norgestrel.tw. (1143) 10 (estrogen$ or oestrogen$).tw. (154616) 11 (Progestin$ or Progest?gen$).tw. (18783) 12 exp Norethindrone/ (4456) 13 norethisterone.tw. (2066) 14 gestodene.tw. (801) 15 exp ethinyl estradiol/ or exp norgestrienone/ (11354) 16 (ethinyl oestradiol or ethinylestradiol).tw. (3066) 17 or/1‐16 (213655) 18 exp Endometriosis/ (21265) 19 Endometrio*.tw. (28025) 20 Dyspareunia.tw. (3573) 21 Dyschezia.tw. (250) 22 pelvic pain.tw. (8103) 23 or/18‐22 (40610) 24 17 and 23 (4578) 25 randomized controlled trial.pt. (497191) 26 controlled clinical trial.pt. (99259) 27 randomized.ab. (434049) 28 placebo.tw. (208224) 29 clinical trials as topic.sh. (195576) 30 randomly.ab. (299103) 31 trial.ti. (196021) 32 (crossover or cross‐over or cross over).tw. (80882) 33 or/25‐32 (1240442) 34 exp animals/ not humans.sh. (4679127) 35 33 not 34 (1143190) 36 24 and 35 (529)
Appendix 4. Embase search strategy
Searched from 1946 to 19 October 2017
Ovid platform
1 exp ENDOMETRIOSIS/ (32243) 2 Endometriosis.tw. (27961) 3 Dyspareunia.tw. (5918) 4 Dyschezia.tw. (466) 5 pelvic pain.tw. (12164) 6 or/1‐5 (47558) 7 exp oral contraceptive agent/ (57765) 8 exp ethinylestradiol plus norelgestromin/ or exp ethinylestradiol plus norethisterone/ or exp ethinylestradiol plus norethisterone acetate/ or exp ethinylestradiol plus norgestimate/ or exp ethinylestradiol plus norgestrel/ or exp estrogen/ or exp gestagen/ (312716) 9 exp dienogest/ (1013) 10 exp dienogest plus ethinylestradiol/ (124) 11 dienogest.tw. (674) 12 (oral contracept$ or contraceptive pill*).tw. (27835) 13 OCP$.tw. (4656) 14 ethinyl estradiol.tw. (3159) 15 desogestrel.tw. (1190) 16 levonorgestrel.tw. (5210) 17 norgestrel.tw. (732) 18 (estrogen$ or oestrogen$).tw. (170735) 19 (Progestin$ or Progest?gen$).tw. (19282) 20 exp norethisterone/ (6700) 21 norethisterone.tw. (1939) 22 exp ethinylestradiol/ (16412) 23 ethinylestradiol.tw. (3166) 24 or/7‐23 (411964) 25 6 and 24 (8706) 26 Clinical Trial/ (949969) 27 Randomized Controlled Trial/ (471914) 28 exp randomization/ (75860) 29 Single Blind Procedure/ (29732) 30 Double Blind Procedure/ (140776) 31 Crossover Procedure/ (53437) 32 Placebo/ (300796) 33 Randomi?ed controlled trial$.tw. (168408) 34 Rct.tw. (25850) 35 random allocation.tw. (1695) 36 randomly allocated.tw. (28434) 37 allocated randomly.tw. (2269) 38 (allocated adj2 random).tw. (785) 39 Single blind$.tw. (19880) 40 Double blind$.tw. (175965) 41 ((treble or triple) adj blind$).tw. (717) 42 placebo$.tw. (256628) 43 prospective study/ (405705) 44 or/26‐43 (1812459) 45 case study/ (50227) 46 case report.tw. (340144) 47 abstract report/ or letter/ (1013008) 48 or/45‐47 (1395186) 49 44 not 48 (1766305) 50 25 and 49 (1711)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 19 October 2017
Ovid platform
1 exp Oral Contraceptives/ (871) 2 oral contracept$.tw. (1460) 3 OCP$.tw. (333) 4 ethinyl estradiol.tw. (97) 5 desogestrel.tw. (14) 6 levonorgestrel.tw. (87) 7 norgestrel.tw. (12) 8 (estrogen$ or oestrogen$).tw. (7953) 9 (Progestin$ or Progest?gen$).tw. (789) 10 exp Estradiol/ (2934) 11 Estradiol.tw. (5624) 12 norethisterone.tw. (23) 13 gestodene.tw. (8) 14 ethinylestradiol.tw. (59) 15 or/1‐14 (12986) 16 exp Dyspareunia/ (247) 17 Endometriosis.tw. (222) 18 Dyspareunia.tw. (540) 19 Dyschezia.tw. (7) 20 pelvic pain.tw. (514) 21 or/16‐20 (1190) 22 15 and 21 (78)
Appendix 6. CINAHL search strategy
Searched from 1961 to 19 October 2017
Ebsco platform
| # | Query | Results |
| S36 | S23 AND S35 | 174 |
| S35 | S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 | 1,169,103 |
| S34 | TX allocat* random* | 7,289 |
| S33 | (MH "Quantitative Studies") | 16,561 |
| S32 | (MH "Placebos") | 10,403 |
| S31 | TX placebo* | 47,658 |
| S30 | TX random* allocat* | 7,289 |
| S29 | (MH "Random Assignment") | 44,312 |
| S28 | TX randomi* control* trial* | 132,934 |
| S27 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 912,856 |
| S26 | TX clinic* n1 trial* | 212,303 |
| S25 | PT Clinical trial | 80,036 |
| S24 | (MH "Clinical Trials+") | 222,972 |
| S23 | S16 AND S22 | 598 |
| S22 | S17 OR S18 OR S19 OR S20 OR S21 | 7,342 |
| S21 | TX pelvic pain | 3,154 |
| S20 | TX Dyschezia | 26 |
| S19 | TX Dyspareunia | 1,067 |
| S18 | TX Endometrio* | 3,828 |
| S17 | (MM "Endometriosis") | 1,879 |
| S16 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 | 24,946 |
| S15 | TX ethinylestradiol | 155 |
| S14 | TX gestodene | 39 |
| S13 | TX norethisterone | 128 |
| S12 | TX Norethindrone | 85 |
| S11 | TX Progestin* or TX Progest?gen* | 1,863 |
| S10 | TX estrogen* or oestrogen* | 16,303 |
| S9 | TX norgestrel | 21 |
| S8 | TX levonorgestrel | 1,485 |
| S7 | TX desogestrel or TX dienogest | 163 |
| S6 | TX ethinyl estradiol | 320 |
| S5 | TX OCP* | 356 |
| S4 | TX oral contracept* | 6,785 |
| S3 | (MM "Levonorgestrel") | 679 |
| S2 | (MM "Estrogens") | 3,098 |
| S1 | (MM "Contraceptives, Oral+") OR (MM "Contraceptives, Oral Combined") | 7,394 |
Appendix 7. Trial Registries keyword search
Searched 19 October 2017
Web platform
The keywords 'endometriosis AND oral contraceptive' were used to search:
ClinicalTrials.gov (a service of the US National Institutes of Health) (www.clinicaltrials.gov);
World Health Organization Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/Default.aspx).
Data and analyses
Comparison 1. Combined oral contractive pill (COCP) versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self‐reported pain (dysmenorrhoea) at end of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dysmenorrhoea at end of treatment: verbal rating scale (VRS) | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.84, ‐0.76] |
| 1.2 Dysmenorrhoea at end of treatment: visual analogue scale (VAS) | 2 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐23.68 [‐28.75, ‐18.62] |
| 1.3 Menstrual pain reduction from baseline to end of treatment: VAS) | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | 2.1 [1.38, 2.82] |
| 2 Cyclical pain (non‐menstrual) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Non‐menstrual pain at end of treatment: VRS | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.48, 0.68] |
| 2.2 Non‐menstrual pain at end of treatment: VAS | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐11.72, 7.92] |
| 2.3 Non‐menstrual pain reduction from baseline to end of treatment: VAS | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.30, 1.70] |
| 3 Dyspareunia | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.46, 2.34] |
| 4 Dyschezia (pain on defecation) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.56, 1.84] |
| 5 Satisfaction (very highly/highly satisfied) | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [2.44, 7.37] |
| 6 Withdrawal from treatment | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.83, 2.18] |
| 7 Adverse effects occurring during treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pregnancy | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 68.98] |
| 7.2 Spotting/irregular bleeding/menorrhagia | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.44, 4.15] |
| 7.3 Nausea | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [1.79, 9.54] |
| 7.4 Any treatment‐associated adverse effect | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.36] |
Comparison 2. Combined oral contractive pill (COCP) versus other medical treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self‐reported pain (dysmenorrhoea) at end of treatment (continuous data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dysmenorrhoea at 6 months' follow‐up: visual analogue scale (VAS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.28, 1.08] |
| 1.2 Dysmenorrhoea at 6 months' follow‐up: verbal rating scale (VRS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.99, 0.79] |
| 2 Self‐reported pain (dysmenorrhoea): VAS (dichotomous data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Reduction of pain to mild or zero at 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.32, 2.58] |
| 2.2 Reduction of pain to zero at 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.43] |
| 3 Self‐reported pain (dysmenorrhoea): VRS (dichotomous data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Reduction of pain to mild or zero at 6 months' follow‐up | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.28] |
| 3.2 Reduction of pain to zero at 6 months' follow‐up | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 4 Cyclical pain (non‐menstrual pain) (continuous data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Non‐menstrual pain at end of treatment: VAS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.51, 1.11] |
| 4.2 Non‐menstrual pain at end of treatment: VRS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.51, 1.31] |
| 4.3 Non‐menstrual pain at 6 months' follow‐up: VAS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.85, 1.25] |
| 4.4 Non‐menstrual pain at 6 months' follow‐up: VRS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.08, 1.08] |
| 5 Cyclical pain (non‐menstrual pain) (dichotomous data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Reduction of pain to mild or zero at end of treatment: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 5.2 Reduction of pain to zero at end of treatment: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.89] |
| 5.3 Reduction of pain to mild or zero at end of treatment: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.17] |
| 5.4 Reduction to zero at end of treatment: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.89] |
| 5.5 Reduction of pain to mild or zero at 6 months' follow‐up: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.53] |
| 5.6 Reduction of pain to zero at 6 months' follow‐up: VAS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.85] |
| 5.7 Reduction of pain to mild or zero at 6 months' follow‐up: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.32] |
| 5.8 Reduction of pain to zero at 6 months' follow‐up: VRS | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.85] |
| 6 Dyspareunia (continuous data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Dyspareunia at end of treatment: VAS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.18, 3.42] |
| 6.2 Dyspareunia at end of treatment: VRS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.41, 0.61] |
| 6.3 Dyspareunia at 6 months' follow‐up: VAS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.30, 2.10] |
| 6.4 Dyspareunia at 6 months' follow‐up: VRS | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.47, 0.67] |
| 7 Dyspareunia (dichotomous data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Reduction of pain to mild or zero at end of treatment: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.02] |
| 7.2 Reduction of pain to zero at end of treatment: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.48] |
| 7.3 Reduction of pain to mild or zero at end of treatment: VRS | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.56, 1.65] |
| 7.4 Reduction of pain to zero at end of treatment: VRS | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.84] |
| 7.5 Reduction of pain to mild or zero at 6 months' follow‐up: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.78] |
| 7.6 Reduction of pain to zero at 6 months' follow‐up: VAS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.77] |
| 7.7 Reduction of pain to mild or zero at 6 months' follow‐up: VRS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.68] |
| 7.8 Reduction of pain to zero at 6 months' follow‐up: VRS | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.77] |
| 8 Withdrawal from treatment | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.34, 5.62] |
| 9 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Hot flushes | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.30] |
| 9.2 Insomnia | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.15] |
| 9.3 Spotting/irregular bleeding | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.46, 3.15] |
| 9.4 Decreased libido | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.19] |
| 9.5 Vaginal dryness | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.63] |
| 9.6 Mood change | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.34, 3.19] |
| 9.7 Headache | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.49, 4.92] |
| 9.8 Paraesthesia | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.12] |
| 9.9 Breast tenderness | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.45, 6.55] |
| 9.10 Weight gain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.41, 10.43] |
| 9.11 Peripheral oedema | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.77] |
| 9.12 Joint pain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 2013.
| Methods | Parallel randomised controlled trial. | |
| Participants | 150 women randomised. Inclusion criteria: no details. Exclusion criteria: no details. Setting: Egypt. Timing: no details. |
|
| Interventions | Monophasic oral contraceptive norethisterone 1 mg + ethinylestradiol 35 μg (PO). Leuprolide 3.75 mg (IM). 3 months' treatment. |
|
| Outcomes | Biberoglu and Behram pain scores. | |
| Notes | Conference abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized;" no other details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated double blind but no details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated double blind but no details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details on allocation to groups for final number of women analysed. |
| Selective reporting (reporting bias) | High risk | Protocol not available. Conference abstract with only 1 outcome reported. |
| Other bias | Unclear risk | Unable to judge due to lack of information. |
Guzick 2011.
| Methods | Parallel randomised controlled trial. | |
| Participants | 47 women with endometriosis‐associated pain. Inclusion criteria: > 18 years of age, premenopausal, pelvic pain ≥ 3 months' duration, diagnosis of endometriosis by laparoscopy or laparotomy within 3 years of study entry, moderate‐to‐severe pelvic pain associated with endometriosis, willingness to comply with study protocol. Exclusion criteria: use of OCPs within 1 month of trial enrolment, use of leuprolide within previous 3 months if given monthly or 5 months if given 3 monthly, any contraindication to the use of OCPs or GnRH analogues, history of hysterectomy or bilateral salpingo‐oophrectomy, pregnant, breastfeeding, serious mental or chronic condition that would prevent the completion of the study. Setting: medical centre, USA. Timing: 2005‐2008. |
|
| Interventions | Monophasic oral contraceptive (norethisterone 1 mg + ethinylestradiol 35 μg) given daily + placebo Leuprolide, 11.25 mg depot IM every 12 weeks with hormonal add‐back using norethisterone acetate 5 mg PO, daily + placebo; 48 weeks' treatment. |
|
| Outcomes | Biberoglu and Behrman pain scores, NRS, BDI and ISS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized;" no other details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sample size calculation suggested 188 women required. Only 47 women randomised and 7 dropped out immediately after screening (3 from COCP group and 4 from leuprolide group). Only 24 women completed the trial but unclear which groups they were in. Reasons given included lack of improvement in pain symptoms, adverse effects and a decision to proceed with alternative therapies such as surgery. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Adverse effects reported that were not prespecified in the study methods section. |
| Other bias | Low risk | Groups were balanced at baseline. |
Harada 2008.
| Methods | Multicentre (18 sites), parallel randomised controlled trial. | |
| Participants | 100 women randomised. Mean age: 31.7 (SD 5.6) years in OCP group; 31.5 (SD 6.3) years in placebo group. Inclusion criteria: aged ≥ 18 years; regular menstrual cycles (28 ± 2 days); symptomatic endometriosis (diagnosed by laparoscopy or laparotomy) or ovarian endometrioma (diagnosed by ultrasound or magnetic resonance imaging); normal cervical and endometrial smear cytology; moderate or severe dysmenorrhoea (evaluated by a modified pain scale) and no medical or surgical treatment for endometriosis within 8 weeks before entry into study, including hormonal agents, such as OCP, GnRH analogue and danazol. Exclusion criteria: no details. Setting: Japan. Timing: no details. |
|
| Interventions | 4 cycles of treatment. Monophasic OCP (ethinylestradiol 0.035 mg + norethisterone 1 mg) for 21 days + placebo for 7 days. Placebo for 28 days. Women could continue to use analgesia of their choice during study. |
|
| Outcomes | VRS and VAS to measure the severity of disability because of dysmenorrhoea in daily life and non‐menstrual pain, and the women's use of analgesics. Clinical evaluation of pelvic induration and size of ovarian endometrioma. | |
| Notes | All authors received consulting fees from Nobelpharma Co., Ltd. Tokyo, Japan, the pharmaceutical company provided the randomisation service. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomly assigned." "Randomization was done by the pharmaceutical company...using the permuted block method." The pharmaceutical company appeared to have been responsible for randomisation of a trial of their own drug. |
| Allocation concealment (selection bias) | High risk | "Randomization was done by the pharmaceutical company." "Allocation concealment was accomplished centrally by the company, not broken until after all data were collected." The pharmaceutical company appeared to have been responsible for allocation concealment. There were no details as to whether sequentially number opaque envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Both the patients and the doctors were blinded regarding the medication." As the trial was funded by the pharmaceutical company that was responsible for randomisation and allocation concealment, it is likely that it may also have communicated with the doctors who were involved in conducting the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 51 women randomised to OCP; 1 became pregnant and 1 was lost to follow‐up. 49 randomised to placebo; 2 lost to follow‐up. 14 women (7 in each group) discontinued study but had data included in the analysis. 4 women in OCP group were discontinued because of adverse effects (1 rupture of ovarian cyst; 1 nausea and headache; 1 ovarian haemorrhagic cyst; 1 oedema), 2 women were lost to follow‐up, and 1 woman took a prohibited drug. 7 women in placebo group terminated: 3 had adverse effects (1 oedema and headache; 1 ovarian haemorrhagic cyst; 1 worsened dysmenorrhoea), 3 were lost to follow‐up, and 1 used a prohibited drug. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All prespecified outcomes appeared to be reported. |
| Other bias | High risk | Some women had radiological diagnosis of endometriosis rather than surgical diagnosis as most women had endometrioma. Groups were balanced at baseline. All authors received consulting fees from Nobelpharma Co., Ltd. Tokyo, Japan, the pharmaceutical company that provided the randomisation service. |
Harada 2017.
| Methods | Parallel, multicentre (32 sites), randomised controlled trial. 3 treatment arms (1 not used in this review). |
|
| Participants | Inclusion criteria: aged ≥ 20 years with a clinical diagnosis of endometriosis who had pelvic tenderness, induration in the cul de sac or uterine immobility; diagnosed as having endometriosis by laparotomy/laparoscopy or by identification of endometriomas. Pelvic pain (defined as a VAS score ≥ 40 mm) and regular menstrual cycles (25‐38 days) during the baseline observation phase and a normal or clinically insignificant cervical smear not requiring further follow‐up collected during screening or documented within the previous 6 months. Women willing to use a barrier method of contraception for duration of study. Exclusion criteria: surgical treatment for endometriosis by laparotomy or laparoscopy within 2 months of start of study; antiphospholipid antibody syndrome; requirement for analgesics for medical reasons other than relief of endometrial pain during study (occasional use was permitted; prophylaxis was not permitted); aged ≥ 40 years with endometriomas for which the largest diameter was > 10 cm; endometriomas containing solid components; any disease or condition that was likely to worsen under hormonal treatment; receipt of hormone preparations containing progestin, oestrogen, GnRH analogues, testosterone derivatives, oestrogen antagonists, aromatase inhibitors, medications or their derivatives that were presumed to affect the secretion of sex hormones, or St John's Wort within 2 months prior to the baseline observation phase; pregnant or breastfeeding; undiagnosed abnormal vaginal bleeding; known hypersensitivity to any ingredient in study drug; and aged > 35 years who smoked or those of any age who smoked ≥ 15 cigarettes/day. Setting: Japan. Timing: October 2012 to December 2014. |
|
| Interventions | Ethinylestradiol 20 μg + drospirenone 3 mg (FlexibleMIB) (130 women), 1 tablet per day. Treatment began between the first and fifth day of menstruation. Tablets administered continuously for 120 days, followed by a 4‐day tablet‐free interval. In the event of ≥3 consecutive days of spotting or bleeding (or both) on days 25‐120 of the cycle, women began and completed the 4‐day tablet‐free interval, then started next cycle of treatment. Placebo (129 women), 1 tablet daily following the same instructions as the FlexibleMIB group for 24 weeks, after which these women were changed to FlexibleMIB for the open‐label extension phase. There was also an unblinded reference arm that received dienogest (53 women). This arm was used as the reference for the extension phase and was, therefore, not included in this analysis. Randomisation was 2.5:2.5:1. |
|
| Outcomes | Primary outcome: change in most severe endometriosis associated pelvic pain (VAS scale 0‐100 mm). Secondary outcomes: pelvic pain, dyspareunia, defecation pain, rated 0‐10 or by VAS) induration in the cul de sac, limitation of uterine mobility, pelvic tenderness, size and number of endometriomas, endometrial thickness, serum oestrogen and progesterone levels. Clinical Global Improvement/Change subscale of the Clinical Global Impression rating scale. Treatment satisfaction. Adverse events. |
|
| Notes | At the end of the blinded 24‐week treatment phase, the arms were unblinded and the women who had been allocated to the placebo group were given the OCP for an extension phase of the trial. Trial registration: NCT01697111. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was via an Interactive Web Response system, with numbers generated by a Randomization Management Group from Bayer and was stratified by baseline visual analog scale (VAS) score <60mm vs 60mm or more." Randomisation is conducted by the pharmaceutical company sponsoring and running the trial. No details on how random numbers were generated. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States that placebo tablets were the same as FlexibleMIB to maintain masking but paper does not state who was blinded. Blinding was unmasked at the end of the treatment phase and women in the placebo group changed to FlexibleMIB in the extension phase. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% in each group discontinued the study by 24 weeks of treatment. OCP: 130 women. 26 withdrawals: adverse event (12), withdrawal of consent (11), protocol deviation (2), wish for pregnancy (1). Placebo: 1 women did not receive treatment. 128 women. 17 withdrawals: adverse event (2), withdrawal of consent (9), lost to follow‐up (2), protocol deviation (1), wish for pregnancy (0), other (3). |
| Selective reporting (reporting bias) | Low risk | Outcomes appeared matched those of manuscript and those registered in the clinical trials registry. |
| Other bias | High risk | Declaration in paper stated that the trial was supported by Bayer Yakuhin Ltd., which participate in trial design and managed all operational aspects of the study, including monitoring data collection, statistical analysis and writing the report. The first author reported that they received advisory fees from Bayer, 1 author received speakers bureau fees from multiple pharmaceutical companies including Bayer. The other authors reported that they had nothing to disclose. |
Vercellini 1993.
| Methods | Parallel randomised controlled trial. | |
| Participants | 57 women with laparoscopically diagnosed endometriosis and ≥ 1 moderate or severe pain symptom as judged by both a VRS and VAS. Exclusion criteria: any treatment for endometriosis other than non‐steroidal anti‐inflammatory drugs in the preceding 3 months; ≥ 1 moderate or severe symptom on 2 separate pain scales. Setting: University Hospital, Milan, Italy. Timing: not stated. |
|
| Interventions | Goserelin 3.6 mg subcutaneous depot formulation monthly for 6 months. Cyclic low‐dose monophasic OCP, containing ethinylestradiol 0.02 mg + desogestrel 0.15 mg (dose increased to ethinylestradiol 0.03 mg if spotting occurred). | |
| Outcomes | Change in pain scores (VAS and VRS) after 6 months' treatment and after 6 months' follow‐up. Occurrence of adverse effects. | |
| Notes | Power calculation completed. Funded by a grant from the Italian National Research Council Applied Project "Prevention and Control of Disease Factors" subproject 5 (Fertility Control), grant no 91.00131.PF41.115.05532, Milan Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that "a randomization list" was used to allocate participants to treatment group but no further details were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of method used to conceal sequence of allocation to treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the treatment administration (injection vs oral tablet) blinding of study participants or investigators was not undertaken. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details provided of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29 women were randomised to the goserelin group and data were presented for 26 women. There was 1 loss to follow‐up and 2 women did not complete the follow‐up ‐ 1 due to pregnancy and 1 requested additional hormonal therapy at end of treatment period. 28 women were randomised to OCP group and data were presented on 24 women. There was 1 loss to follow‐up. 1 woman withdrew because of headaches, and 2 women did not complete follow‐up due to requesting additional hormonal therapy at end of treatment period. |
| Selective reporting (reporting bias) | Low risk | Protocol did not appear to be available; however, all outcomes prespecified in the materials and methods section of the article were reported on. |
| Other bias | Low risk | Groups appeared balanced for age and parity. |
BDI: Beck Depression Inventory; GnRH: gonadotrophin‐releasing hormone; IM: intramuscular; ISS: Index of Sexual Satisfaction; NRS: numerical rating score; OCP: oral contraceptive pill; PO: orally; SD: standard deviation; VAS: visual analogue scale; VRS: verbal rating scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Caruso 2011 | Ineligible study population. Participants were healthy women without endometriosis. |
| Caruso 2016 | Ineligible study design. Not a randomised controlled trial. |
| Cheewadhanaraks 2012 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Cucinella 2013 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Fedele 1989 | Dose of cyproterone acetate used was 27 mg. Dose in modern contraceptive (Dianette) is 2 mg. Therefore, therapeutic effect of trial medication unlikely to be extendable to modern low‐dose contraceptives. |
| Fedele 2008 | Ineligible study design. Secondary report detailing cytoscopic changes in a small subgroup of women with bladder endometriosis participating in a larger non‐randomised trial. |
| Granese 2015 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Kitawaki 2012 | Ineligible study design. Following correspondence with authors, it was established that participants were allocated to treatment group based on chart number, not a randomised control trial. |
| Long 2010 | Hormonal therapy used as an adjunct to other medical treatment, a criterion for exclusion. |
| Moawad 2012 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Muzii 2011 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Portman 2011 | Ineligible study population. Participants were women with menstrual‐related pelvic pain without any identifiable pelvic disease. |
| Seracchioli 2010 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Sesti 2007 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Shturkalev 1970 | Trial published in Bulgarian. English abstract stated that the included oral contraceptive was Ovostat, which contained ethinylestradiol 0.05 mg; therefore, did not meet inclusion criteria of a 'modern' oral contraceptive. |
| Strowitzki 2012 | Ineligible study population. Participants were women with primary dysmenorrhoea without any identifiable pelvic disease. |
| Tanaka 2016 | Ineligible study design. Not a randomised controlled trial. |
| Taniguchi 2015 | Ineligible study design. Not a randomised controlled trial. |
| Vercellini 2002 | Medical treatment used as an adjunct to surgery, a criterion for exclusion. |
| Vercellini 2005 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Xu 2011 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
| Zhu 2014 | Hormonal therapy used as an adjunct to surgery, a criterion for exclusion. |
Differences between protocol and review
In the 2018 update of this review we:
removed the restriction on the duration of treatment that was previously an exclusion for participants;
changed the presentation of data from odds ratio to risk ratios for dichotomous data to comply with current Cochrane Gynaecology and Fertility Group guidance;
changed the primary outcome from "Pain symptoms of endometriosis and dysmenorrhoea: recurrence, frequency and severity (pain scores, days lost off work, use of pain killers)" to "self‐reported pain (dysmenorrhoea) at the end of treatment (as defined by trialists)."
In the 'Participants' section we clarified that trials reporting on women with endometrial deposits outside the uterus were excluded.
Contributions of authors
JB, AP, TC: identified the trials for inclusion, extracted and entered data, and assessed trials for risk of bias.
SD and AP were available for content expertise, to assist with any disagreements in assessment by JB and TC, and both made comments and contributions to draft and final versions of the review update.
Sources of support
Internal sources
AP: University of Cambridge, UK.
JM and SK: University of Oxford, UK.
External sources
LJD Peninsula Medical School Foundation Bursary, UK.
LJD National Birthday Trust Fund, Wellbeing of Women, UK.
Declarations of interest
JB: none known.
TC: none known.
SD: none known.
AP: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ali 2013 {published data only}
- Ali AF, Farid LA, Fouad M, Omar ED. Continuous oral contraception and leuprolide in the treatment of endometriosis associated pelvic pain. Journal of Endometriosis 2013;5 (Suppl 1). [Google Scholar]
- Ali AFM, Farid LA, Fouad M, Daly O. Continuous oral contraception and leuprolide in the treatment of endometriosis‐associated pelvic pain. European Journal of Contraception and Reproductive Health Care 2013;18 (Suppl 1):S257. [Google Scholar]
Guzick 2011 {published data only}
- Guzick DS, Huang LS, Broadman BA, Nealon M, Hornstein MD. Randomized trial of leuprolide versus continuous oral contraceptives in the treatment of endometriosis‐associated pelvic pain. Fertility and Sterility 2011;95(5):1568‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harada 2008 {published data only}
- Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low‐dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo‐controlled, double‐blind, randomized trial. Fertility and Sterility 2008;90(5):1583‐88. [DOI] [PubMed] [Google Scholar]
Harada 2017 {published data only}
- Harada T, Kosaka S, Ellisen J, Yasuda M, Ito M, Momoeda M. Ethinylestradiol 20 μg/drospireone 3 mg in a flexible extended regiment for the management of endometriosis‐associated pelvic pain: a randomized controlled trial. Fertility and Sterility 2017;108(5):798‐805. [DOI: 10.1016/j.fertstert.2017.07.1165] [DOI] [PubMed] [Google Scholar]
Vercellini 1993 {published data only}
- Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani PG. A gonadotrophin‐releasing hormone agonist versus a low‐dose oral contraceptive for pelvic pain associated with endometriosis. Fertility and Sterility 1993;60(1):75‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Caruso 2011 {published data only}
- Caruso S, Iraci Sareri M, Agnello C, Romano M, Lo Presti L, Malandrino C, et al. Conventional vs. extended‐cycle oral contraceptives on the quality of sexual life: comparison between two regimens containing 3 mg drospirenone and 20 micro g ethinyl estradiol. Journal of Sexual Medicine 2011;8(5):1478‐85. [DOI] [PubMed] [Google Scholar]
Caruso 2016 {published data only}
- Caruso S, Iraci M, Cianci S, Fava V, Casella E, Cianci A. Comparative, open‐label prospective study on the quality of life and sexual function of women affected by endometriosis‐associated pelvic pain on 2 mg dienogest/30 micro g ethinyl estradiol continuous or 21/7 regimen oral contraceptive. Journal of Endocrinological Investigation 2016;39(8):923‐31. [DOI] [PubMed] [Google Scholar]
Cheewadhanaraks 2012 {published data only}
- Cheewadhanaraks S, Choksuchat C, Dhanaworavibul K, Liabsuetrakul T. Postoperative depot medroxyprogesterone acetate versus continuous oral contraceptive pills in the treatment of endometriosis‐associated pain: a randomized comparative trial. Gynecologic and Obstetric Investigation 2012;74(2):151‐6. [DOI] [PubMed] [Google Scholar]
Cucinella 2013 {published data only}
- Cucinella G, Granese R, Calagna G, Svelato A, Saitta S, Tonni G, et al. Oral contraceptives in the prevention of endometrioma recurrence: does the different progestins used make a difference?. Archives of Gynecology & Obstetrics 2013;288(4):821‐7. [DOI] [PubMed] [Google Scholar]
Fedele 1989 {published data only}
- Fedele L, Arcaini L, Bianchi S, Baglioni A, Vercillini P. Comparison of cyproterone acetate and danazol in the treatment of pelvic pain associated with endometriosis. Obstetrics and Gynecology 1989;73(6):1000‐4. [DOI] [PubMed] [Google Scholar]
Fedele 2008 {published data only}
- Fedele L, Bianchi S, Montefusco S, Frontino G, Carmignani L. A gonadotropin‐releasing hormone agonist versus a continuous oral contraceptive pill in the treatment of bladder endometriosis. Fertility and Sterility 2008;90(1):183‐4. [DOI] [PubMed] [Google Scholar]
Granese 2015 {published data only}
- Granese R, Perino A, Calagna G, Saitta S, Franciscis P, Colacurci N, et al. Gonadotrophin‐releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi‐center randomized trial. Acta Obstetricia et Gynecologica Scandinavica 2015;94(6):637‐45. [DOI] [PubMed] [Google Scholar]
Kitawaki 2012 {published and unpublished data}
- Kitawaki J, Kusuki I, Suganuma I, Sasaki A, Itoh F, Matsuo S, et al. Long‐term suppression of endometriosis associated pelvic pain with sequential administration of a gonadotropin releasing hormone agonist followed by danazol, oral contraceptives or dienogest. Journal of Endometriosis 2012;4(3):150. [Google Scholar]
Long 2010 {published data only}
- Long QQ, Zhang SF, Han Y, Chen H, Li XL, Hua KQ, et al. Clinical efficacy and safety of gonadotropin releasing hormone agonist combined with estrogen‐dydrogesteronea in treatment of endometriosis. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2010;45(4):247‐51. [PubMed] [Google Scholar]
Moawad 2012 {published data only}
- Moawad A, Salah A, Abou‐Ria H, Abd‐Elzaher M, Madkour W. Is continuous use of long term OCP's more significant than cyclic use in prevention of endometrioma recurrence after laparoscopic excision? A randomized controlled trial. Human Reproduction 2012;27(Suppl 2):ii66‐7. [Google Scholar]
Muzii 2011 {published data only}
- Muzii L, Maneschi F, Marana R, Porpora MG, Zupi E, Bellati F, et al. Oral estroprogestins after laparoscopic surgery to excise endometriomas: continuous or cyclic administration? Results of a multicenter randomized study. Journal of Minimally Invasive Gynecology 2011;18(2):173‐8. [DOI] [PubMed] [Google Scholar]
Portman 2011 {published data only}
- Portman D, Reape K, Hait H, Howard B. Reduction in dysmenorrhea severity in women using a 91‐day extended regimen oral contraceptive compared to a 28‐day regimen oral contraceptive for the treatment of cyclic pelvic pain. Fertility and Sterility 2011;96(3 Suppl):S110. [Google Scholar]
Seracchioli 2010 {published data only}
- Seracchioli R, Mabrouk M, Frasca C, Manuzzi L, Montanari G, Keramyda A, et al. Long‐term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertility and Sterility 2010;93(1):52‐6. [DOI] [PubMed] [Google Scholar]
- Seracchioli R, Mabrouk M, Frasca C, Manuzzi L, Savelli L, Venturoli S. Long‐term oral contraceptive pills and postoperative pain management after laparoscopic excision of ovarian endometrioma: a randomized controlled trial. Fertility and Sterility 2010;94(2):464‐71. [DOI] [PubMed] [Google Scholar]
Sesti 2007 {published data only}
- Sesti F, Pietropolli A, Capozzolo T, Broccoli P, Pierangeli S, Bollea MR, et al. Hormonal suppression treatment or dietary therapy versus placebo in the control of painful symptoms after conservative surgery for endometriosis stage III‐IV. A randomized comparative trial. Fertility and Sterility 2007;88(6):1541‐7. [DOI] [PubMed] [Google Scholar]
Shturkalev 1970 {published data only}
- Shturkalev I, Katsulov A. Results of clinical trials of Ovostat as a contraceptive and therapeutic agent. Akusherstvo i Ginekologiia 1970;9(5):383‐7. [PubMed] [Google Scholar]
Strowitzki 2012 {published data only}
- Strowitzki T, Kirsch B, Elliesen J. Efficacy of ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen in women with moderate‐to‐severe primary dysmenorrhoea: An open‐label, multicentre, randomised, controlled study. Journal of Family Planning and Reproductive Health Care 2012;38(2):94‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2016 {published data only}
- Tanaka Y, Mori T, Ito F, Koshiba A, Kusuki I, Kitawaki J. Effects of low‐dose combined drospirenone‐ethinylestradiol on perimenstrual symptoms experienced by women with endometriosis. International Journal of Gynecology and Obstetrics 2016;135(2):135‐9. [DOI] [PubMed] [Google Scholar]
Taniguchi 2015 {published data only}
- Taniguchi F, Enatsu A, Ota I, Toda T, Arata K, Harada T. Effects of low dose oral contraceptive pill containing drospirenone/ethinylestradiol in patients with endometrioma. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2015;191:116‐20. [DOI] [PubMed] [Google Scholar]
Vercellini 2002 {published data only}
- Vercellini P, Giorgi O, Mosconi P, Stellato G, Vicentini S, Crosignani PG. Cyproterone acetate versus a continuous monophasic oral contraceptive in the treatment of recurrent pelvic pain after conservative surgery for symptomatic endometriosis. Fertility and Sterility 2002;77(1):52‐61. [DOI] [PubMed] [Google Scholar]
Vercellini 2005 {published data only}
- Vercellini P, Pietropaolo G, Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen‐progestogen combination versus low‐dose norethindrone acetate. Fertility and Sterility 2005;84(5):1375‐87. [DOI] [PubMed] [Google Scholar]
Xu 2011 {published data only}
- Xu XW, Wang LD, Zhu XQ, Yan LZ, Guan YT, Zhu SC, et al. Levonorgestrel‐releasing intrauterine system and combined oral contraceptives as conservative treatments for recurrent ovarian endometriosis: a comparative clinical study. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2011;91(15):1047‐50. [PubMed] [Google Scholar]
Zhu 2014 {published data only}
- Zhu S, Liu D, Huang W, Wang Q, Zhou L, Feng G. Post‐laparoscopic oral contraceptive combined with Chinese herbal mixture in treatment of infertility and pain associated with minimal or mild endometriosis: a randomized controlled trial. BMC Complementary and Alternative Medicine 2014;14(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahangari 2014
- Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician 2014;17(2):E141‐7. [PubMed] [Google Scholar]
Al‐Jefout 2011
- Al‐Jefout M. Brief update on endometriosis treatment. Middle East Fertility Society Journal 2011;16(3):167‐74. [Google Scholar]
ASRM 1997
- American Society of Reproductive Medicine. Revised American Society of Reproductive Medicine Classification of Endometriosis: 1996. Fertility and Sterility 1997;67(5):817‐21. [DOI] [PubMed] [Google Scholar]
Barlow 2005
- Barlow DH, Kennedy S. Endometriosis: new genetic approaches and therapy. Annual Review of Medicine 2005;56:345‐56. [DOI] [PubMed] [Google Scholar]
Bateson 2016
- Bateson D, Butcher BE, Donovan C, Farrell L, Kovacs G, Mezzini T, et al. Risk of venous thromboembolism in women taking the combined oral contraceptive: a systematic review and meta‐analysis. Australian Family Physician 2016;45(1‐2):59‐64. [PubMed] [Google Scholar]
Biberoglu 1981
- Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: short‐term and long‐term effectiveness. American Journal of Obstetrics and Gynecology 1981;139:645. [DOI] [PubMed] [Google Scholar]
Crosignani 2006
- Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Human Reproduction Update 2006;12(2):179‐89. [DOI] [PubMed] [Google Scholar]
Dunselman 2014
- Dunselman GA, Vermeulen N, Becker C, Calhaz‐Jorge C, D'Hooghe T, Bie B, et al. European Society of Human Reproduction Embryology. ESHRE guideline: management of women with endometriosis. Human Reproduction 2014;29(3):400‐12. [DOI] [PubMed] [Google Scholar]
Fauconnier 2005
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and its implications. Human Reproduction Update 2005;11(5):595‐606. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster. GRADEpro GDT. Version accessed 10 November 2017. Hamilton (ON): GRADE Working Group, McMaster, 2015. Available at gradepro.org.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jacobson 2002
- Jacobson TZ, Barlow DH, Koninkx PR, Olive D, Farquhar C. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001300] [DOI] [PubMed] [Google Scholar]
Johnson 2006
- Johnson N, Farquhar C. Endometriosis. Clinical Evidence 2006;15:2449‐64. [PubMed] [Google Scholar]
Jones 2004
- Jones G, Jenkinson C, Kennedy S. The impact of endometriosis upon quality of life: a qualitative analysis. Journal of Psychosomatic Obstetrics and Gynaecology 2004;25:123‐33. [DOI] [PubMed] [Google Scholar]
Kennedy 2006
- Kennedy 2000 on behalf of the Guidelines and Audit committee of the Royal College of Obstetricians and Gynaecologist. The investigation and management of endometriosis 24. Royal College of Obstetricians and Gynaecologists: Green Top Guidelines 2006.
Kistner 1958
- Kistner RW. The use of newer progestins in the treatment of endometriosis. American Journal of Obstetrics and Gynecology 1958;75:264‐78. [DOI] [PubMed] [Google Scholar]
Kistner 1959
- Kistner RW. Treatment of endometriosis by inducing pseudo‐pregnancy with ovarian hormones. Fertility and Sterility 1959;10:539‐54. [Google Scholar]
Lancaster 1995
- Lancaster JM, Prentice A, Smith SK. Successful medical treatment of sub‐diaphragmatic endometriosis. Journal of Obstetrics and Gynaecology 1995;15:206‐9. [Google Scholar]
Meresman 2002
- Meresman GF, Auge L, Baranoa RI, Lombardi E, Tesone M, Sueldo C. Oral contraceptives suppress cell proliferation and enhance apoptosis of ectopic endometrial tissue from patients with endometriosis. Fertility and Sterility 2002;77(6):1141‐7. [DOI] [PubMed] [Google Scholar]
Vessey 1993
- Vessey MP, Villard‐Mackintosh L, Painter R. Epidemiology of endometriosis in women attending family planning clinics. BMJ 1993;306:182‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yap 2004
- Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003678.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zito 2014
- Zito G, Luppi S, Giolo E, Martinelli M, Venturin I, Lorenzo G, et al. Medical treatments for endometriosis‐associated pelvic pain. BioMed Research International 2014;2014:191967. [DOI: 10.1155/2014/191967] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Davis 1997
- Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD001019] [DOI] [Google Scholar]
Davis 2007
- Davis L, Kennedy SS, Moore J, Prentice A. Oral contraceptives for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001019.pub2] [DOI] [PubMed] [Google Scholar]


