Abstract
Background
Recent progress in understanding the genetic basis of breast cancer and widely publicized reports of celebrities undergoing risk‐reducing mastectomy (RRM) have increased interest in RRM as a method of preventing breast cancer. This is an update of a Cochrane Review first published in 2004 and previously updated in 2006 and 2010.
Objectives
(i) To determine whether risk‐reducing mastectomy reduces death rates from any cause in women who have never had breast cancer and in women who have a history of breast cancer in one breast, and (ii) to examine the effect of risk‐reducing mastectomy on other endpoints, including breast cancer incidence, breast cancer mortality, disease‐free survival, physical morbidity, and psychosocial outcomes.
Search methods
For this Review update, we searched Cochrane Breast Cancer's Specialized Register, MEDLINE, Embase and the WHO International Clinical Trials Registry Platform (ICTRP) on 9 July 2016. We included studies in English.
Selection criteria
Participants included women at risk for breast cancer in at least one breast. Interventions included all types of mastectomy performed for the purpose of preventing breast cancer.
Data collection and analysis
At least two review authors independently abstracted data from each report. We summarized data descriptively; quantitative meta‐analysis was not feasible due to heterogeneity of study designs and insufficient reporting. We analyzed data separately for bilateral risk‐reducing mastectomy (BRRM) and contralateral risk‐reducing mastectomy (CRRM). Four review authors assessed the methodological quality to determine whether or not the methods used sufficiently minimized selection bias, performance bias, detection bias, and attrition bias.
Main results
All 61 included studies were observational studies with some methodological limitations; randomized trials were absent. The studies presented data on 15,077 women with a wide range of risk factors for breast cancer, who underwent RRM.
Twenty‐one BRRM studies looking at the incidence of breast cancer or disease‐specific mortality, or both, reported reductions after BRRM, particularly for those women with BRCA1/2 mutations. Twenty‐six CRRM studies consistently reported reductions in incidence of contralateral breast cancer but were inconsistent about improvements in disease‐specific survival. Seven studies attempted to control for multiple differences between intervention groups and showed no overall survival advantage for CRRM. Another study showed significantly improved survival following CRRM, but after adjusting for bilateral risk‐reducing salpingo‐oophorectomy (BRRSO), the CRRM effect on all‐cause mortality was no longer significant.
Twenty studies assessed psychosocial measures; most reported high levels of satisfaction with the decision to have RRM but greater variation in satisfaction with cosmetic results. Worry over breast cancer was significantly reduced after BRRM when compared both to baseline worry levels and to the groups who opted for surveillance rather than BRRM, but there was diminished satisfaction with body image and sexual feelings.
Seventeen case series reporting on adverse events from RRM with or without reconstruction reported rates of unanticipated reoperations from 4% in those without reconstruction to 64% in participants with reconstruction.
In women who have had cancer in one breast, removing the other breast may reduce the incidence of cancer in that other breast, but there is insufficient evidence that this improves survival because of the continuing risk of recurrence or metastases from the original cancer. Additionally, thought should be given to other options to reduce breast cancer risk, such as BRRSO and chemoprevention, when considering RRM.
Authors' conclusions
While published observational studies demonstrated that BRRM was effective in reducing both the incidence of, and death from, breast cancer, more rigorous prospective studies are suggested. BRRM should be considered only among those at high risk of disease, for example, BRCA1/2 carriers. CRRM was shown to reduce the incidence of contralateral breast cancer, but there is insufficient evidence that CRRM improves survival, and studies that control for multiple confounding variables are recommended. It is possible that selection bias in terms of healthier, younger women being recommended for or choosing CRRM produces better overall survival numbers for CRRM. Given the number of women who may be over‐treated with BRRM/CRRM, it is critical that women and clinicians understand the true risk for each individual woman before considering surgery. Additionally, thought should be given to other options to reduce breast cancer risk, such as BRRSO and chemoprevention when considering RRM.
Plain language summary
Women should be aware of their true risk of developing breast cancer and the limitations of current evidence when considering risk‐reducing mastectomy
Review question
We reviewed the evidence on whether risk‐reducing mastectomy (RRM) reduces death rates from any cause in women who have never had breast cancer and in women who have a history of breast cancer in one breast. Also, we reviewed the effect of RRM on other endpoints, including breast cancer incidence, breast cancer mortality, disease‐free survival, physical morbidity, and psychosocial outcomes.
Background
Recent progress in understanding the genetic basis of breast cancer and widely publicized reports of celebrities undergoing RRM have increased interest in it as a method of preventing breast cancer.
Study characteristics
Sixty‐one studies presented data on 15,077 women with a wide range of risk factors for developing breast cancer, who underwent RRM. Risk‐reducing mastectomy could include either surgically removing both breasts to prevent breast cancer (bilateral risk‐reducing mastectomy or BRRM), or removing the disease‐free breast in women who have had breast cancer in one breast to reduce the incidence of breast cancer in the other breast (contralateral risk‐reducing mastectomy or CRRM). The evidence is current to July 2016.
Key results
The BRRM studies reported that it reduced the incidence of breast cancer or the number of deaths or both, but many of the studies have methodological limitations. After BRRM, most women are satisfied with their decision, but reported less satisfaction with cosmetic results, body image, and sexual feelings. One of the complications of RRM was the need for additional unanticipated surgeries, particularly in women undergoing reconstruction after RRM. However, most women also experienced reduced worry of developing and dying from breast cancer along with diminished satisfaction with body image and sexual feelings
In women who have had cancer in one breast, removing the other breast (CRRM) may reduce the incidence of cancer in that other breast, but there is insufficient evidence that this improves survival because of the continuing risk of recurrence or metastases from the original cancer.
While published observational studies demonstrated that BRRM was effective in reducing both the incidence of, and death from, breast cancer, more rigorous prospective studies are suggested. BRRM should be considered only among those at high risk of disease, for example, carriers of mutations in the breast cancer genes, BRCA1 and BRCA2. CRRM was shown to reduce the incidence of contralateral breast cancer (CBC), but there is insufficient evidence that CRRM improves survival, and studies that control for multiple variables that can affect results are recommended. It is possible that selection bias in terms of healthier, younger women being recommended for or choosing CRRM produces better overall survival numbers for CRRM.
Quality of evidence
Just over half of the studies were found to have a low risk of selection bias, that is, studies adjusting for systematic differences in prognosis or treatment responsiveness between the groups, and similarly, 60% had a low risk of detection bias, that is, studies considered systematic differences in the ways the outcomes were measured and detected. The primary cause for both selection bias and detection bias was not controlling for all major confounding factors, e.g., risk factors or having bilateral risk‐reducing salpingo‐oophorectomy (BRRSO ‐ surgery to remove fallopian tubes and ovaries) in the subject and control groups. Performance bias (validation of the risk‐reducing mastectomy) was not problematic, as most studies were based on surgical reports; three relied on self‐reports and eight were unclear because of multiple sources of data and/or broad timeframe. Attrition bias was at high risk or unclear in approximately 13% of the studies. The mean or median follow‐up period reported was from 1 ‐ 22 years.
Conclusions
Given the number of women who may be over‐treated with BRRM/CRRM, it is critical that women and clinicians understand the true risk for each individual woman before considering surgery. Additionally, thought should be given to other options to reduce breast cancer risk, such as BRRSO and chemoprevention, when considering RRM.
Background
Description of the condition
This is an update of a Cochrane Review first published in 2004 and previously updated in 2006 and 2010.
Breast cancer is the most common cause of cancer death worldwide for women, and the fifth most common cancer overall, with around 522,000 deaths from breast cancer in 2012 (15% of female deaths and 6% of the total) (Ferlay 2013). Breast cancer is the most common malignancy worldwide for women, with an estimated number of incident cases in 2012 of around 1.7 million, and is the most common cancer in women in both high‐income, and middle‐ and lower‐income regions in the world (GLOBOCAN 2012). For those with BRCA1/2 mutations, the risks are higher than for the average woman; Kuchenbaecker 2017 reported that "the cumulative breast cancer risk to age 80 years was 72% (95% CI 65% to 79%) for BRCA1 and 69% (95% CI 61% to 77%) for BRCA2 carriers" in a large study with subjects from multiple western countries. The Global Cancer Observatory data as of 2012 show that the estimated age‐standardized rate of incident cases of breast cancer has been increasing across most countries that submit data to it; however, at the same time, the estimated age‐standardized rate of deaths from breast cancer has been decreasing for most countries that submit data to the Global Cancer Observatory.
More recent data for selected countries show that breast cancer is still a major issue. The American Cancer Society estimates for 2017 that new cases of breast cancer for both sexes combined in the USA will be about 255,000, which will be the highest for all cancer types. The estimated deaths for 2017 in the USA will be about 41,000, which will be the fourth highest among all cancer types (ACS 2017).
Description of the intervention
Recent progress in understanding the genetic basis of certain breast cancers has led to increased interest in predicting breast cancer development and identifying women at high risk through the use of molecular methods. Women at high risk are particularly interested in preventing or reducing the risk of the subsequent development of breast cancer. Risk‐reducing mastectomy (RRM) is among the alternatives usually offered for this purpose. The most relevant change since this review was originally published is the widespread availability and increase in use of genetic testing for women seeking information on their breast cancer risk.
High‐risk women, who have no previous personal history of breast cancer, may consider bilateral risk‐reducing mastectomy (BRRM) as a means of primary prevention of the disease. A woman's decision to have BRRM is found to be strongly correlated with her BRCA1 or 2 mutation test results and with a physician's recommendation to have genetic testing or BRRM (Schwartz 2004).
Likewise, women who were previously diagnosed with a breast cancer in one breast and thus are at higher risk of developing a primary cancer in the other (contralateral) breast, may consider risk‐reducing mastectomy of that breast (CRRM) as an option to prevent the occurrence of a second breast cancer. The risk of contralateral breast cancer in women with hereditary/familial non‐BRCA1/2 primary breast cancer is five times greater than the expected incidence based on SEER (Surveillance, Epidemiology and End Results collected in the USA) data (Shadehi 2005). In addition, a study of 6294 participants diagnosed under 50 years of age reported, “Age at first breast cancer is a strong risk factor for cumulative contralateral breast cancer risk in BRCA1/2 mutation carriers." and "Those diagnosed before age 41 years had a 10‐year cumulative contralateral breast cancer risk of 23.9% (BRCA1: 25.5%; BRCA2: 17.2%) compared with 12.6% (BRCA1: 15.6%; BRCA2: 7.2%) for those 41 to 49 years of age (P = .02)” (Van den Broek 2016). However, if there is no family history of breast cancer, the incidence of contralateral breast cancer is a rare event estimated to occur in 2.7% of women with breast cancer (Herrinton 2005) after 4.8 years of follow‐up.
In the past, RRM has been performed on women with any family history of breast cancer, painful breasts, cancer phobia, and history of breast biopsies (with or without proliferating disease). Recently, consideration for the procedure has tended to focus on women at high risk as determined by the identified presence of genetic mutations of the BRCA1 or 2 genes, both of which are associated with increased risk of breast cancer, or by statistical models of risk such as the Gail model (Gail 1994) or other methods of estimating susceptibility. Much of the data used in this review did not allow subset identification by genetic testing.
How the intervention might work
As a preventive measure, risk‐reducing mastectomy remains controversial. Potential benefits include a reduction of risk of breast cancer and increase in psychological peace of mind. Potential disadvantages include the invasiveness of the procedure and consequent morbidity, as well as diminished satisfaction with body image and reduced tactile sensations in the breast. A paradox now exists in which the surgical management of invasive breast cancer has become less radical, with many women opting for breast‐conserving surgery, while removal of the breast is used for breast cancer prevention. Furthermore, no mastectomy can remove all breast tissue, and therefore cannot eliminate all risk of breast cancer, even if this surgery is shown to be effective in reducing one's risk. In addition, RRM may cause significant physical morbidity or affect women's quality of life, or both. Because no test is available that can determine which women will actually develop breast cancer in the absence of RRM, it is likely that many individuals will undergo RRM needlessly. Also, RRM is not the only alternative for women at high risk of breast cancer. Other possible options of variable demonstrated efficacy include one, or a combination of chemoprevention with drugs such as tamoxifen and aromatase inhibitors, close surveillance with frequent clinical examinations and imaging studies, or oophorectomy (removal of ovaries) (Evans 2013; Heemskerk‐Gerritsen 2015; Ingham 2013; Kiely 2010; Metcalfe 2004a; Van Sprundel 2005).
Given the drastic and irreversible nature of RRM, it is essential that women contemplating this procedure be able to make informed decisions based upon the best available evidence, consider both the benefits and limitations of the procedure, and weigh the risks and benefits of other alternatives. RRM can have a negative impact on self‐esteem, sexual relations and satisfaction with body appearance (Brandberg 2008; Brandberg 2012; Bresser 2006; Frost 2000; Frost 2005; Gahm 2010; Gopie 2013; Unukovych 2012).
Why it is important to do this review
This review evaluates the existing research literature on the effectiveness of RRM in terms of overall mortality, breast cancer mortality, breast cancer incidence, disease‐free survival, physical morbidity, and quality of life among both disease‐free women and women with disease in one breast who had elective RRM in the other, non‐diseased breast. Other reviews of the scientific literature concerning RRM have been conducted (Anderson 2001; Barry 2011; Brewster 2011; Eisen 2000; Fayanju 2014; Hartmann 2004; Stefanek 2001; Yao 2010), however, these reviews have lacked a systematic search strategy, an assessment of methodological quality of the included studies, or a comprehensive scope including both physical and psychosocial outcomes.
Objectives
(i) To determine whether risk‐reducing mastectomy reduces death rates from any cause in women who have never had breast cancer and in women who have a history of breast cancer in one breast, and (ii) to examine the effect of risk‐reducing mastectomy on other endpoints, including breast cancer incidence, breast cancer mortality, disease‐free survival, physical morbidity, and psychosocial outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomized trials as they provide the highest level of evidence. Because we knew it was unlikely that any would be found, we expanded our criteria to include studies of any design type including cohort, case‐control studies, case series, and longitudinal observational studies that had at least 20 participants. We included studies conducted during any time period, in any country and reported in English.
Types of participants
Participants comprised women at risk from breast cancer. This included women with a positive family history of breast cancer, BRCA1/2 mutation carriers, previous cancer in one breast, previous multiple breast biopsies, and previous diagnosis of lobular carcinoma in situ, atypical hyperplasia, or proliferating breast disease. The authors of each reported study defined a positive family history, and the definitions are provided in the Characteristics of included studies tables.
Types of interventions
We included all types of risk‐reducing mastectomy (RRM), including subcutaneous mastectomy, total or simple mastectomy, modified radical mastectomy, and radical mastectomy.
Types of outcome measures
Primary outcomes
All‐cause mortality
Secondary outcomes
Beast cancer mortality
Disease‐free survival (e.g. disease‐specific (breast cancer), any disease‐free, all‐cause survival, overall survival)
Breast cancer incidence
Physical morbidity (e.g. postoperative complications, surgical complications, infections, necrosis, hematoma)
Quality of life (including satisfaction with the decision to have RRM, satisfaction with cosmetic outcome, satisfaction with the medical process, psychological well‐being, impact on body image, and impact on primary relationships and sexuality)
We did not pre‐specify exclusion criteria related to duration of follow‐up, but this information is available for each study in the summary table.
Search methods for identification of studies
Electronic searches
For the review update, we performed the following searches.
The Cochrane Breast Cancer (CBCG) Specialized Register (searched 4 May 2016) . Details of the search strategies used by the Group for the identification of studies and the procedure used to code references are outlined in the Group's module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). We extracted trials coded with the key words “breast cancer unspecified”, “high risk”, “history”, “surgery”, “mastectomy”, “risk‐reducing mastectomy”, “radical mastectomy”, “modified radical mastectomy”, “simple mastectomy” and “total mastectomy”.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5). See Appendix 1 for the full search strategy.
MEDLINE OvidSP (1946 to 14 July 2016). We used a revised search strategy for searching the 2012 to 2016 period. See Appendix 2 for search details.
Embase OvidSP (1974 to 14 July 2016). We used a revised search strategy for searching the 2012 to 2016 period. See Appendix 3 for search details.
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/AdvSearch.aspx) for all prospectively registered and ongoing trials (searched 4 May 2016). See Appendix 4 for the search strategy.
ClinicalTrials.gov (clinicaltrials.gov/ct2/home) clinical trials registry (searched 4 May 2016). See Appendix 5 for the search strategy.
Data collection and analysis
Selection of studies
After excluding all non‐English language studies from the citation lists produced by the searches, we divided the remaining English‐language studies into sections of a manageable size, and at least two group members independently examined each abstract to determine whether reports appeared to meet our inclusion criteria. Those two individuals resolved any differences by discussion. We obtained copies of the reports that appeared to meet the inclusion criteria for closer examination, and two members of the group examined each one. Two members of the group also examined information obtained about additional studies. The entire group reviewed all potentially eligible reports and made a final decision as to which should be included in the review.
Data extraction and management
The entire group agreed upon uniform criteria for data extraction before the process began. At least two group members independently examined and extracted data from each report included in the review. Two members of the group resolved any differences by discussion and consensus. The entire group made final decisions as to presentation of the data in the review and the Characteristics of included studies tables.
Because of the diversity of the included studies, statistical pooling of the data was not appropriate. We reported information on study design, study population, interventions used, outcomes reported, and methodological study quality or possible biases. Women who have had breast cancer in one breast arguably were different from women who were at high risk but had never had breast cancer. Therefore, we presented information separately on these groups.
Assessment of risk of bias in included studies
There were no randomized studies included in this review. Therefore, three domains of bias typically included in a Cochrane Review ‐ adequate sequence generation, allocation concealment and blinding ‐ are not applicable to this review (Higgins 2011). Rather, three review authors assessed the methodological quality of the included studies to determine whether or not the methods used sufficiently minimized selection bias, performance bias, detection bias, and attrition bias (Clarke 2002). We defined selection bias as systematic differences between comparison groups in prognosis or responsiveness to treatment. Typically, randomization is the method used to reduce selection bias. However, in observational studies, controlling for variables that may influence the results is the major way to reduce selection bias. We defined performance bias as systematic differences in care provided apart from the intervention being evaluated and detection bias as systematic differences between comparison groups in how outcomes were ascertained, diagnosed or verified. We defined attrition bias as systematic differences between comparison groups in withdrawals or exclusions of participants from the results of a study.
For studies with a comparison group (cohort studies or case series with a statistically modeled comparison group), we used the following questions to operationally apply the above definitions.
Selection bias: were key risk/protective factors (confounders/co‐interventions) adjusted for to ensure comparability between groups? We identified key risk/protective factors from review articles on the topic. For breast cancer incidence, Lise 1997 proposed the following as important factors: age, number of biopsies and histological status of previous biopsies, family history, use of other preventive options such as tamoxifen or oophorectomy, BRCA (breast cancer gene mutation) status, LCIS (lobular carcinoma in situ) status. For mortality, Chang 2003 proposed the following as important prognostic variables: age, stage at diagnosis, treatment, ER (estrogen receptor) status, HER2 (human epidermal growth factor receptor 2) status, and number of positive nodes. For incidence in contralateral studies, which is substantially affected by the features of the previous cancer, Eisen 2000 and Lopez 1996 considered the following to be important variables: stage of the previous carcinoma and the presence of multifocal (two or more individual cancers in one breast) breast cancer in the ipsilateral (same) breast, carcinoma in situ, atypical ductal or lobular hyperplasia in the remaining breast, strong family history of breast cancer, and BRCA mutation status, if known. For psychosocial studies, in which there are fewer known factors associated with RRM and outcome, we deemed the pre‐existence of psychological morbidity as the major variable.
Performance bias: was the intervention (RRM) confirmed in an objective way (i.e. medical or surgical records) and not determined exclusively by self‐report?
Detection bias: was the outcome assessed in a valid way (e.g. validated pre/post instruments for psychosocial measures, medical records for incidence, medical/death records for vital status) and in the same way for both groups? Were the outcome assessors masked to the treatment that each participant received?
Attrition bias: was there a low dropout rate or were dropouts/withdrawals sufficiently accounted for, or both, so that the reviewer was convinced that differential reasons for dropping out did not occur?
For studies without a comparison group (convenience samples or case series without statistically modeled comparison groups), assessment questions for performance bias, detection bias and attrition bias remained the same. However, selection bias is a term that specifically pertains to assessing comparability between groups. Because there were no comparison groups in these studies, we used the term 'preferential selection' for selection bias, so as not to confuse the terminology with 'selection bias' used in studies with a comparison group. Item 1, preferential selection, asked the following question: was there evidence of a consecutive sample, or a clearly defined patient population (e.g. patients at a particular clinic at a particular time period) or some other method to minimize the chance that clinicians preferentially selected patients with favorable outcomes or that patients with better outcomes volunteered (healthy volunteer bias)?
From these checklists representing the four possible sources of bias, at least two review authors rated all studies on all items. We compared results and resolved differences by discussion to arrive at consensus (see 'Risk of bias tables in Characteristics of included studies tables).
Classification of study designs
We included various study designs and define them as follows.
Case series: a report on a consecutive collection of patients treated in a similar manner without a concurrent control group (Haynes 1990)
Convenience sample: individuals or groups selected at the convenience of the investigator or primarily because they were available at a convenient time or place (Haynes 1990)
Prospective cohort study: a group of exposed and non‐exposed individuals that have been followed over time to compare incidence (or rate of death from disease) between the groups (Gordis 1996). In prospective cohort studies, the recruitment, exposure/intervention, and outcomes must all have occurred after setting up the study; in a longitudinal cohort study, participants are followed over time with continuous or repeated monitoring of risk factors or health outcomes, or both;
Retrospective cohort study: a group of exposed and non‐exposed individuals that have been followed over time to compare incidence (or rate of death from disease) between the groups (Gordis 1996). In retrospective cohort studies, outcomes can have occurred prior to setting up the study or be collected afterwards, or both.
Results
Description of studies
Results of the search
The latest searches identified 2492 citations. We also reviewed again the 39 included studies from the previous version of the review and removed six small studies with fewer than 20 participants, and reclassified two reports as part of other studies. Teams of two people reviewed titles and abstracts of each citation. There was no duplicate detection step, as the review authors reviewed the citations returned from each database separately (i.e. did not combine citations to a larger single file). The majority of citations were excluded because the citation did not appear relevant. We retrieved as possibly relevant and reviewed 158 full‐text reports. Of these, 30 studies met the inclusion criteria, giving a total of 61 included studies in this review. The PRISMA flowchart (Figure 1) outlines the process and shows the combined original and new numbers (Moher 2009).
1.

6Study flow diagram.
Included studies
Study design
None of the studies involved controlled clinical trials, either randomized or non‐randomized. The 61 studies included had the following study designs.
Six studies (Hatcher 2001; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015; Meijers‐Heijboer 2001; Rebbeck 2004 (also had retrospective results); Skytte 2011) were prospective cohort studies. Klijn 2004 reports on Meijers‐Heijboer 2001 BRRM participants at 4.8 years.
Twenty‐three studies (Barton 2005; Bedrosian 2010; Boughey 2010; Bresser 2006; de la Pena‐Salcedo 2012; Gahm 2007; Gahm 2010; Geiger 2005; Geiger 2007; Hartmann 1999a (also had case series data); Herrinton 2005; Ingham 2013; Kiely 2010; King 2011a; Koskenvuo 2014; Lee 1995; Metcalfe 2014; Mutter 2015; Peralta 2000; Pesce 2014; Van Sprundel 2005; Zeichner 2014; Zion 2003) were retrospective cohort designs. Boughey 2015 updates Frost 2005; Gahm 2013 expanded the results found in Gahm 2010.
Twenty‐eight of the studies (Altschuler 2008; Arver 2011; Brandberg 2008; Brewster 2012; Chung 2012; Contant 2002; Evans 1999; Frost 2000; Frost 2005; Gabriel 1997; Geiger 2006; Goldflam 2004; Gopie 2013; Hartmann 1999a; Hartmann 2001; Hopwood 2000; Horton 1978; Isern 2008 (except for age‐matched population for Short Form 36 Health Survey Questionnaire); Jatoi 2014; Kass 2010; Kruper 2014; Leis 1981; McDonnell 2001; Metcalfe 2004b; Metcalfe 2005; Miller 2013; Pennisi 1989; Unukovych 2012) were quantitative case series studies.
Hartmann 1999b included a retrospective cohort study and a case series. We determined that Frost 2011 was an update of Frost 2005, which reports on CRRM patients at 10.3 years' follow‐up. Frost 2011 reports on the participants they could find from Frost 2005 at 20.3 years. Brandberg 2012 reported additional information on the participants in Brandberg 2008. We determined that Heemskerk‐Gerritsen 2013 was a follow‐up of Heemskerk‐Gerritsen 2007. Metcalfe 2004a reported on CRRM incidence at a mean of 9.2 years and Metcalfe 2014 reports on patients at a median of 14.3 years.
Two studies were longitudinal prospective observational studies (Den Heijer 2012; Evans 2013).
Three studies (Borgen 1998; Hwang 2016; Montgomery 1999) were convenience samples. Additional features of each study (risk definitions, follow‐up times, and attrition rates) are found in Characteristics of included studies.
Characteristics of participants
Twenty‐one of the studies (Arver 2011; Barton 2005; Borgen 1998; Brandberg 2008; Frost 2000; Gahm 2007; Gahm 2010; Geiger 2005; Geiger 2007; Gopie 2013; Hartmann 1999a [two studies in the same report]; Hartmann 2001; Hatcher 2001; Heemskerk‐Gerritsen 2013;Hopwood 2000; Ingham 2013; Meijers‐Heijboer 2001; Metcalfe 2004b; Metcalfe 2005; Rebbeck 2004; Skytte 2011) involved women with no previous diagnosis of breast cancer who underwent bilateral risk‐reducing mastectomy to reduce their risk of getting breast cancer.
Twenty‐six of the studies (Bedrosian 2010; Boughey 2010; Brewster 2012; Chung 2012; Evans 2013; Frost 2005; Geiger 2006; Goldflam 2004; Heemskerk‐Gerritsen 2015; Herrinton 2005; Hwang 2016; Jatoi 2014; Kiely 2010; King 2011a; Kruper 2014; Lee 1995; Leis 1981; McDonnell 2001; Metcalfe 2014; Miller 2013; Montgomery 1999; Peralta 2000; Pesce 2014; Unukovych 2012; Van Sprundel 2005; Zeichner 2014) were of women with a previous diagnosis of breast cancer in one breast who underwent a risk‐reducing mastectomy of the contralateral breast to reduce the risk of getting a primary breast cancer in the other breast.
Twelve studies (Altschuler 2008; Bresser 2006; Contant 2002; de la Pena‐Salcedo 2012; Den Heijer 2012; Evans 1999; Horton 1978; Isern 2008; Kass 2010; Mutter 2015; Pennisi 1989; Zion 2003) included participants who had bilateral risk‐reducing mastectomies as well as some who had contralateral risk‐reducing mastectomies.
Two additional studies (Gabriel 1997; Koskenvuo 2014) did not specify whether the study participants had bilateral or contralateral risk‐reducing mastectomies.
Characteristics of interventions
Collectively, these studies presented data for 15,077 unique women who had risk‐reducing mastectomies. There are a number of studies in which the participants of one study were also included in another study and this is noted in the review in Table of Characteristics of included studies. Participants in the studies of Frost 2000; Gabriel 1997; Hartmann 2001; McDonnell 2001; and Zion 2003 had overlap with the participants in the Hartmann 1999a study. Geiger 2006 participants were a subset of Herrinton 2005; Barton 2005 participants were a subset of Geiger 2005, as were Geiger 2007 participants; and Metcalfe 2005 participants were a subset of Metcalfe 2004b. Altschuler 2008 included 519 participants from Geiger 2006; Arver 2011 included 24 participants from Gahm 2007; Boughey 2010 included duplicate participants from Mutter 2015, Frost 2005, and McDonnell 2001; Klijn 2004 is an update of participants in Meijers‐Heijboer 2001. Care was taken to try not to include a participant in the count more than once. Consequently, the patients in Bedrosian 2010 (8,902), Jatoi 2014 (25,962), and Kruper 2014 (26,526), all CRRM studies obtained from SEER records, and the Hwang (7,619) study from Army of Women were not counted in the totals because it cannot be determined which of these patients are unique to Bedrosian, Hwang, Jatoi or Kruper and which have been reported in other included studies.
Of the 15,077 women, data were presented for 5,367 participants who had BRRM. The number of women involved in studies involving bilateral mastectomy and assessing physical outcomes is 4,340 (Arver 2011; Barton 2005; Contant 2002; de la Pena‐Salcedo 2012: Den Heijer 2012; Evans 1999; Gahm 2007; Geiger 2005; Hartmann 1999a; Hartmann 2001; Heemskerk‐Gerritsen 2013; Horton 1978; Ingham 2013, Kass 2010; Meijers‐Heijboer 2001; Pennisi 1989; Rebbeck 2004; Skytte 2011; Zion 2003); 460 women participated in studies looking at quality of life or other psychological or social outcomes (Altschuler 2008; Brandberg 2008; Bresser 2006; de la Pena‐Salcedo 2012; Frost 2000; Geiger 2007; Gopie 2013; Hatcher 2001; Hopwood 2000; Metcalfe 2005; Montgomery 1999); and finally, 567 participants were involved in studies that presented information concerning both physical and psychological outcomes (Borgen 1998; de la Pena‐Salcedo 2012; Den Heijer 2012; Gahm 2010; Isern 2008; Metcalfe 2004b).
The number of women participating in studies of CRRM is 9,900. The number of women involved in studies having contralateral mastectomy and assessing physical outcomes is 8,891, not counting the large numbers from studies using SEER or Army of Women data (Bedrosian 2010; Boughey 2010; Bresser 2006; Brewster 2011; Chung 2012; Contant 2002; Evans 1999; Evans 2013; Frost 2005; Geiger 2006; Goldflam 2004; Heemskerk‐Gerritsen 2007; Heemskerk‐Gerritsen 2015; Herrinton 2005; Horton 1978; Jatoi 2014; Kass 2010; Kiely 2010; King 2011a; Kruper 2014; Lee 1995; Leis 1981; McDonnell 2001; Metcalfe 2014; Miller 2013; Mutter 2015; Pennisi 1989; Peralta 2000; Pesce 2014; Van Sprundel 2005; Zeichner 2014; Zion 2003); 900 women participated in studies looking at quality of life or other psychological or social outcomes (Altschuler 2008; Bresser 2006; Frost 2005; Geiger 2006; Hwang 2016; Montgomery 1999;Unukovych 2012); and 71 women in two studies (de la Pena‐Salcedo 2012; Isern 2008) presented information on both physical and psychological outcomes.
In two studies assessing physical outcomes, the type of RRM could not be determined (Gabriel 1997; Koskenvuo 2014).
Outcomes reported
Twenty studies reported on all‐cause mortality, the primary outcome for this review (Boughey 2010; Brewster 2012; Chung 2012; Evans 2013; Geiger 2005; Goldflam 2004;Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015; Herrinton 2005; Ingham 2013; Jatoi 2014; Kiely 2010; Klijn 2004 [Meijers‐Heijboer 2001]; Kruper 2014; Metcalfe 2014; Peralta 2000; Pesce 2014; Van Sprundel 2005; Zeichner 2014). However, most available data were for secondary outcomes.
Fourteen studies provided data for breast cancer mortality (Goldflam 2004; Hartmann 1999a [2 studies in the same report]; Heemskerk‐Gerritsen 2013; Herrinton 2005; Jatoi 2014; King 2011a; Kruper 2014; Lee 1995; Meijers‐Heijboer 2001; Metcalfe 2014; Mutter 2015; Pennisi 1989; Peralta 2000; Van Sprundel 2005). Two studies reported on breast cancer mortality that combined patients with RRM plus risk‐reducing salpingo‐oophorectomy (RRSO) (Evans 2013; Ingham 2013).
Twenty‐four of the studies reported data concerning incidence of breast cancer (Arver 2011; Borgen 1998; Brewster 2012; Contant 2002; Evans 1999; Geiger 2005; Hartmann 1999a; Hartmann 2001; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015; Herrinton 2005; Horton 1978; Kass 2010; Kiely 2010; King 2011a; Koskenvuo 2014; McDonnell 2001; Meijers‐Heijboer 2001; Mutter 2015; Pennisi 1989; Peralta 2000; Rebbeck 2004; Skytte 2011; Van Sprundel 2005).
Ten studies included data for disease‐free survival (Bedrosian 2010; Brewster 2012; Chung 2012; Evans 2013; Lee 1995; Leis 1981; Mutter 2015; Peralta 2000; Van Sprundel 2005; Zeichner 2014).
Sixteen studies reported data concerning physical morbidity (Arver 2011; Barton 2005; Contant 2002; de la Pena‐Salcedo 2012; Den Heijer 2012; Frost 2005; Gabriel 1997; Gahm 2007; Gahm 2010; Goldflam 2004; Heemskerk‐Gerritsen 2007; Isern 2008; Koskenvuo 2014;Metcalfe 2004b; Miller 2013; Zion 2003).
Twenty studies reported data concerning quality of life, psychological morbidity, or other assessments of emotional or social function (Altschuler 2008; Borgen 1998; Brandberg 2008; Bresser 2006; de la Pena‐Salcedo 2012; Den Heijer 2012; Frost 2000; Frost 2005; Gahm 2010; Geiger 2006; Geiger 2007; Gopie 2013; Hatcher 2001; Hopwood 2000; Hwang 2016; Isern 2008; Metcalfe 2004b; Metcalfe 2005; Montgomery 1999; Unukovych 2012). One caveat: some reports on psychosocial outcomes may have been missed because PsycINFO was not searched.
Excluded studies
We excluded six previously included studies from this update because each had fewer than 20 participants (Babiera 1997; Josephson 2000; Lodder 2002; Lloyd 2000; Mulvihill 1982; Stefanek 1995).
Risk of bias in included studies
The methodological quality varied among studies (Characteristics of included studies). The most common source of potential bias was selection bias because 30 of the 61 studies either did not adjust for potential confounding factors or failed to adjust for all of the major variables associated with a particular outcome (Figure 2 and Figure 3). The results of these studies, therefore, were potentially confounded by other risk or confounding factors. Performance bias (assessment of the RRM) was generally not problematic, as studies were based on surgical reports and did not rely on self‐reports except for three studies (Borgen 1998; Hwang 2016; Montgomery 1999). There were five studies in which the performance bias was unclear because of data sources or age of collected data: (Evans 2013;Hwang 2016;Mutter 2015;Pesce 2014;Zeichner 2014). The potential for detection bias varied among the 61 studies, with 19 of them (Altschuler 2008;Borgen 1998;Brandberg 2008;Bresser 2006;Contant 2002;Frost 2000;Frost 2005;Gahm 2010;Geiger 2007; Hopwood 2000;Kass 2010;Kiely 2010;Lee 1995;Leis 1981;Metcalfe 2004b;Montgomery 1999;Peralta 2000;Skytte 2011; Zeichner 2014) having potential bias, and with the risk unclear in four studies (Evans 1999;Kruper 2014;Miller 2013;Pennisi 1989). Common sources of potential detection bias were recall bias in quality of life assessment (in which participants were asked to rate their psychological status both before and after RRM) and assessment of disease‐free survival (in which regular intervals of follow‐up to detect recurrence of disease were not typically specified in CRRM studies). Furthermore, studies generally did not report blinding or masking the study outcomes assessor or medical records extractor when determining cause of death from the medical record, another potential source of detection bias. Attrition bias was of concern in only 13 studies (Altschuler 2008;Brandberg 2008;Gahm 2010; Geiger 2007; Gopie 2013;Hopwood 2000;Leis 1981;Metcalfe 2004b;Metcalfe 2005;Montgomery 1999; Pennisi 1989; Skytte 2011) (Figure 2) and unclear in two additional studies (Bedrosian 2010;Metcalfe 2014), as most studies accounted for all the participants in the initial sample they specified. However, in many cases there was no way to tell whether the number reported for the original cohort was correct.
2.
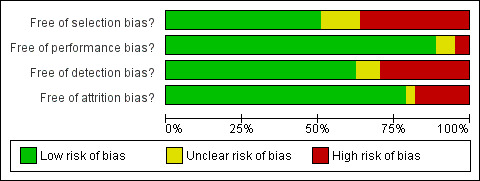
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Participants who choose to undergo BRRM to reduce the risk of having an initial breast cancer diagnosed are very likely different in characteristics from those who already had an initial diagnosis of cancer in one breast and then choose CRRM to reduce the risk of a primary breast cancer in the other breast. In light of this, we have reported the data for outcomes for BRRM and CRRM separately where possible.
A. Bilateral risk‐reducing mastectomy
Twenty‐one studies involved participants who had BRRM only (Arver 2011; Barton 2005; Borgen 1998; Brandberg 2008; Frost 2000; Gahm 2007; Gahm 2010; Geiger 2005; Geiger 2007; Gopie 2013; Hartmann 1999a (two studies in the same report); Hartmann 2001; Hatcher 2001; Heemskerk‐Gerritsen 2013;Hopwood 2000; Ingham 2013; Meijers‐Heijboer 2001; Metcalfe 2004b; Metcalfe 2005; Rebbeck 2004; Skytte 2011). Two studies (Ingham 2013; Kass 2010) included some participants who had BRRM and RRSO.
1. All‐cause mortality
Two of the 21 studies (Heemskerk‐Gerritsen 2013; Klijn 2004) reported all‐cause mortality data. Heemskerk‐Gerritsen 2013 was a quantitative case series and Klijn 2004, a follow‐up to Meijers‐Heijboer 2001, was a prospective cohort study.
BRCA1 and BRCA2 mutations
With a median follow‐up for the BRRM group of 8.5 years and 4.1 years for the control group, Heemskerk‐Gerritsen 2013, reporting on BRCA1/2 women, found all‐cause mortality hazard ratio (HR) for the BRRM group = 0.20 (95% confidence interval (CI) 0.02 to 1.68). All‐cause mortality rates per 1000 person‐years of observation were BRRM = 0.7, control 2.7, HR 0.20 (95% CI 0.02 to 1.68). Ten‐year overall survival for the BRRM participants was 99%, while that for the controls was 96%.
Klijn 2004 also studied BRCA1/2 women and reported that, after 4.8 years of follow‐up for the RRM group and 3.5 years for the surveillance group, there were no deaths among the 113 BRRM women, but two of the 173 women in the surveillance group had died.
2. Breast cancer (disease‐specific) mortality
Five studies (Geiger 2005; Hartmann 1999a (two studies in the same report); Heemskerk‐Gerritsen 2013; Ingham 2013; Meijers‐Heijboer 2001) reported data concerning the effect of BRRM on breast cancer mortality. See Table 1.
1. Mortality: bilateral risk‐reducing mastectomy (BRRM).
| Study | Outcome | Length of follow‐up | Attrition | Study details |
|
Geiger 2005 BRRM |
BRRM group: 0/276 deaths (0.0%)
Controls: 1600/666,800 deaths (0.2%) HR = 0.005 (95% CI 0.001 to 0.044) |
Mean
BRRM: 10.3 years Controls: 6.2 years |
None | 65% of women with BRRM (276) had multiple risk factors versus 12% of those without BRRM (196); see Characteristics of included studies for risk factors. An estimate based on the Gail Model, 15 BC cases were expected in the participant cohort. Absolute risk of BC death in the non‐BRRM women was relatively low. |
|
Hartmann 1999a BRRM USA |
Women at high risk
BRRM group: 2/214 deaths
Comparison group: 90/403 deaths Using 3 different methods to calculate incidence taking into account ascertainment bias, the risk of death was reduced by 81%‐94% Most conservative estimate for high risk: % reduction = 80.9% (95% CI 31.4% to 97.7%) Moderate risk: BRRM: 0 of 425 Predicted incidence of death: 10.4 of 214 % reduction = 100% (95% CI 70% to 100%) |
Median follow‐up was 14 years | None | See Table 2 for study population details and definitions of 'high risk' and 'moderate risk' |
|
Heemskerk‐Gerritsen 2013 BRRM |
10‐year BC‐free survival BRRM group = 100% Control group = 74% Deaths due to BC BRRM group = 1 . Control group = 6. All‐cause mortality BRRM group HR 0.20 (95% CI 0.02 to 1.68) BC‐specific mortality BRRM group HR 0.29 (95% CI 0.03 to 2.61) All‐cause mortality Rates per 1000 person‐years of observation: BRRM = 0.7 Controls = 2.7 HR 0.20 (95% CI 0.02 to 1.68) 10‐year overall survival BRRM = 99% Control = 96% Participants were BRCA1/2 mutation carrier women |
Median follow‐up BRRM = 8.5 years Controls = 4.1 years |
None | |
|
Ingham 2013 BRRM |
Survival BRRM (58) in BRCA1/2 carriers was not significantly associated with improved survival (HR 0.25, 95% CI 0.03 to 1.81, P = 0.14) 10‐year survival for BRCA1/2 carriers with BRRM only was 98.1% (95% CI 87.1 to 99.7%) and the 20‐year survival was the same. The combined survival result for BRCA carriers and untested 1st‐degree relatives with BRRM only (68) was HR 0.25 (0.03 to 1.80, P = 0.14) 10‐ and 20‐year survival was 98.4% (88.9 to 99.8%) Matched analysis where each individual with BRRM was matched by date of birth, gene and whether each had undergone BRRSO to an individual who did not undergo BRRM with a proportional hazard model fit to these data failed to yield a significant effect of BRRM (HR 0.28, 95% CI 0.06 to 1.35) For those undergoing just BRRM compared with no BRRSO, a borderline significant result was obtained: HR 0.12 (95% CI 0.02 to 1.01) In those who had BRRM plus BRRSO (68) there was a significant survival advantage (HR 0.14 (0.02 to 1.02) P = 0.02 Only BRRSO (108) was significantly associated with improved survival (HR 0.22 (0.08to0.61) P =0.002 |
Median duration of follow‐up (from ascertainment to death or loss to follow‐up) was 13.3 years | None reported | |
|
Meijers‐Heijboer 2001 BRRM Netherlands Follow up Klijn 2004 (Meijers‐Heijboer 2001) |
BRRM group: 0/76 deaths
Surveillance group: 1/63 deaths RR 0.28 (95% CI 0.01 to 6.68), P = 0.43 In a later follow up overall survival: BRRM group: 0 of 113 died No‐BRRM group: 2 of 173 died Participants and controls were BRCA1/2 carriers |
Mean follow‐up of 3.0 ± 1.5 years | None | |
|
Mutter 2015 BRRM CRRM |
5‐year disease‐free survival estimate = 69% overall (95% CI 52% to 94%) 5‐year disease‐free survival estimate for the 11 women with isolated loco‐regional BC after BRRM = 90% (95% CI 73% to 100%) 5‐year disease‐free survival estimate for the 11 women with isolated loco‐regional BC after CRRM = 52% (95% CI 29% to 94%). This is not statistically significantly different to the BRRM rate (P = 0.23) (Figure 1 shows the Kaplan‐Meier curve for disease‐free survival. Figure 2 shows the Kaplan Meier curve for disease‐free survival for CRRM vs BRRM in the paper) |
Median follow‐up = 22 years Range = 3‐34 years |
None | |
|
Pennisi 1989 BRRM and CRRM combined |
BRRM/CRRM: 3 of the 1500 participants died from BC No comparison group |
70% followed for 9 years | 30% were lost to follow‐up | 1500 patients from 165 plastic surgeons who had subcutaneous RRM and were registered with the Subcutaneous Mastectomy Data Evaluation Center 78 (5.2%) participants had obscure carcinoma and 51 (3.4%) had LCIS at the time of surgery and were included in the study. Among the 139 participants who had CRRM, 4 (3%) had BC and 5 (3.6%) had LCIS and were included in the study. 300 (20%) had a 1st‐degree relative with BC and 21% had a history of 2nd‐degree maternal or paternal relatives with a history of BC. Skin necrosis occurred in 5% of the participants |
BRRSO: bilateral risk‐reducing salpingo‐oophorectomy BRRM: bilateral risk‐reducing mastectomy CI: confidence interval CRRM: contralateral risk‐reducing mastectomy HR: hazard ratio LCIS: lobular carcinoma in situ RR: relative risk RRM: risk‐reducing mastectomy
BRCA1 and BRCA2 mutations
Two studies reported on women with BRCA1/2 mutations. Heemskerk‐Gerritsen 2013 reported on 212 women who had BRRM and 358 controls who had surveillance only. There was one death due to breast cancer in the BRRM group, and six in the control group. Meijers‐Heijboer 2001 reported no deaths due to breast cancer among the 76 women who underwent BRRM at three‐years' follow‐up, but one breast cancer death among 63 women who chose surveillance.
In a retrospective cohort study, Ingham 2013 reported on 58 BRCA1/2 carriers with BRRM, and found it was not significantly associated with improved survival (HR 0.25, 95% CI 0.03 to 1.81, P = 0.14). Ten‐year survival in the study was 98.1% (95% CI 87.1% to 99.7%) and the 20‐year survival was the same. The survival results when combining BRCA carriers and untested first‐degree relatives with BRRM (68 participants) were HR 0.25 (95% CI 0.03 to 1.80, P = 0.14); 10‐ and 20‐year survival was 98.4% (95% CI 88.9% to 99.8%).
Ingham 2013 also looked at BRCA1/2 mutation carriers who had BRRM and bilateral RRSO (BRRSO) (68 participants); for them, there was a significant survival advantage (HR 0.14, 95% CI 0.02 to 1.02, P = 0.02). However, in matched analysis, where each individual with BRRM was matched by date of birth, gene, and whether each had undergone BRRSO to an individual who did not undergo BRRM, with a proportional hazard model fit to these data failed to yield a significant effect of BRRM (HR 0.28, 95% CI 0.06 to 1.35). For those undergoing just BRRM compared with no risk‐reducing surgery, a borderline significant result was obtained: HR 0.12 (95% CI 0.02 to 1.01). Only BRRSO (108 participants) was significantly associated with improved survival (HR 0.22, 95% CI 0.08 to 0.61, P = 0.002.
High risk (strong family history, but not necessarily BRCA1/2 mutation carriers)
Hartmann 1999a followed 639 women at "high and moderate" risk of developing breast cancer. The median length of follow‐up was 14 years. Of the 214 participants at high risk (as defined in Table 2) of breast cancer, two subsequently developed and died of the disease, compared to 90 deaths in the control group (participants' sisters). Depending on the statistical model used, the study reported an 81% to 94% reduction in risk of dying from breast cancer following BRRM.
2. Incidence: bilateral risk‐reducing mastectomy (BRRM).
| Study | Incidence | Length of follow‐up | Attrition | Study details |
|
Arver 2011 BRRM |
BC incidence 0 of 223 high‐risk women developed BC 12 cases expected in 223 women without BRRM per BOADICEA model |
Mean 6.6 years; 2.1‐14.0 years (1468 women years) |
None | 129 of the women were BRCA1/2+ |
|
Borgen 1998 BRRM |
BRRM: 3/370 | Mean 14.8 years (range 0.2‐51.5 years) | Not applicable | Incidental carcinoma was identified in 14 of the 370 (4%) and they were included in the study. |
|
Contant 2002 BRRM |
BC incidence BRRM = 0 of 79 with no previous history of BC (2 had DCIS previously) | Median 2.5 years | None | Some of the participants also had BRRSO. |
|
Evans 1999 BRRM and CRRM combined |
Incidence of BC CRRM/BRRM: 0/400 woman years Comparison group statistically simulated using the Claus model: 4/400 woman years were expected |
Mean 2.2 years (400 women years) | None | Women were from 10 European cancer centers that offer risk assessment and counselling services to women with a lifetime risk of BC from 25%‐80% using the Claus data. Study authors stated that follow‐up for > 5 years would be necessary to address the issue of risk reduction. Note: this study contained a small group of CRRM patients; however, results are not presented separately. Due to the preponderance of BRRM, the study is reported with BRRM incidence results. |
|
Geiger 2005 BRRM |
BC incidence
BRRM = 1/276 (0.4%)
Controls (calculated) = 26,800/666,800 (4.0%) HR = 0.005 (95% CI 0.001 to 0.044) HR stratified by birth year |
Mean
10.3 years for BRRM 6.2 years for no BRRM |
None | BRRM reduced occurrence of BC in high‐risk women treated in community practices by 95%. Gail model suggests that 15 BCs should have occurred without BRRM in the 214 women who fell within the age range of the model. 12 cases diagnosed within 60 days of BRRM considered incidental and not included as failures. Multiple risk factors: BRRM = 65% Controls = 12% Mean age at BRRM was 45 years (range 23‐74 years). Of controls who developed BC, 22% had 1st‐degree relative with BC. |
|
Hartmann 1999a BRRM |
Moderate‐risk women
BRRM: 4/425
Comparison group statistically simulated using the Gail model: 37.4/425 RR = 89.5% High‐risk women BRRM: 3/214 3 comparison groups all simulated from probands' sisters' BC rates:
Using 3 different methods to calculate incidence taking into account ascertainment bias, the expected incidents among the 214 high‐risk probands ranged from 30.0 to 52.9/214 Most conservative estimate was % difference = 90.0 (95% CI 70.8 to 97.9) |
Median 14 years | None | To be classified as high risk, women had to meet 1 of the following criteria: ≥ 2 1st‐degree relatives with BC; 1 first‐degree relative and ≥ 2 second‐ or third‐degree relatives with BC; 1 first‐degree relative with BC before the age of 45 and one other relative with BC; 2 second‐degree or third‐degree relatives with BC and ≥ 1 with ovarian cancer; 1 second or third‐degree relative with BC and ≥ 2 with ovarian cancer; ≥ 3 second or third‐degree relatives with BC; 1 first‐degree relative with bilateral BC. 2 women in the high‐risk group developed ovarian cancer. All 7 who developed BC had subcutaneous mastectomies. But there was no significant difference in outcome between the group with subcutaneous mastectomies compared to those who had total mastectomies. Median time to development of BC was 6 years. At the time of the study, tissue was available for pathological review for 603 of the women. 2 invasive cancers were identified during the review. One of the two women had developed BC 3 years after the BRRM. |
|
Hartmann 2001 BRRM |
Participants with BRCA1/2 mutations
BRRM: 0/26
2 statistically simulated comparison groups: Simulated group 1 ‐ Easton penetrance model: 9.37/26 RR = 100% (95% CI 51.0 to 100.0) Simulated group 2 ‐ Struewing penetrance model: 6.52/26 RR = 100% (95% CI 54.1 to 100.0) |
13.4 years (range 5.8‐28.5 years) | None | Participants were a subset of the 214 high‐risk women who were participants in Hartmann 1999a. 26 had alterations in BRCA1 or BRCA2. 8 of the original 214 participants in the cohort had died at the time this study began: 2 from BC, 1 from ovarian cancer. The woman with ovarian cancer had a deleterious BRCA1 mutation. |
|
Heemskerk‐Gerritsen 2007 BRRM Preceded Heemskerk‐Gerritsen 2013 |
BCincidence: BRRM group: 1/177 |
Median 4.5 years |
None | 86 of 177 women in BRRM group also had BRRSO |
|
Heemskerk‐Gerritsen 2013 BRRM |
BRRM group = 0 incidence (incidence rate per 1000 women = 0) Control group = 57 women with BC (incidence rate per 1000 women = 28) Metastatic BC: 4 of 51 women diagnosed with invasive BC developed metastatic BC. All were BRCA1 mutation carriers Participants were BRCA1/2 mutation carrier women |
Median BRRM = 8.5 years Controls = 4.1 years |
None | |
|
Horton 1978 BRRM and CRRM |
BRRM: 0/93 CRRM: 0/11 | Mean 3.1 years (range 1 month‐10 years) | None | Note: this study contained a small group of CRRM patients; however, results were not presented separately. Due to the preponderance of BRRM, the study is reported with BRRM incidence results. |
|
Kass 2010 BRRM |
BC incidence: BRCA1/2 + BRRM group: 0 of 147 |
Mean 6.1 years (SE 3.4) was longer in BRCA1 carriers compared with the BRCA2 carriers with 3.7 years (SE 3.1) |
None | Confounding factor: 80 BRRM women (54%) opted for BRRSO. In 24 of them, this procedure was conducted a mean of 2 years before their BRRM. |
|
Koskenvuo 2014 RRM |
1/52 participants had metastatic axillary lymph nodes 45 months post‐BRRM Participants were 105 BRCA1 mutation carriers and 92 BRCA2 mutation carriers |
Median after RRM was 52 months (range: 1‐133 months) | None – only followed specific group | 33/52 of the women who had RRM also had BRRSO |
|
Meijers‐Heijboer 2001 BRRM Klijn 2004 (follow‐up to Meijers‐Heijboer 2001) |
BC incidence BRRM: 0/76 Surveillance arm: 8/63 BRRM significantly (P = 0.003) decreased incidence of BC at 3 years' follow‐up. HR 0 (95% C.I. 0.0 to 0.36) P = 0.003 Actuarial 5‐year BC incidence BRRM group: 1 /73 developed distance metastasis No‐BRRM group: 24 of 173 developed BC (17%) P = 0.01 HR = 0.07 Adjusting for BRRSO: P = 0.02 |
Mean 3.0 +/‐1.5 years Median BRRM group 4.8 years Comparison group: 3.5 years |
None Unknown |
Using the surveillance group, the authors estimate the 5‐year risk of BC was 24 +/‐ 9% The ratio of observed occurrences to expected occurrences in the surveillance group was 1.2 (8 vs 6.7). Significantly more women in the BRRM arm than in the surveillance arm also had BRRSO (44 vs 27 (58% vs 38%)). MRI detected 6 of the 6 cancers screened. Mammography detected 2 of the 8 cancers screened |
|
Mutter 2015 BRRM or CRRM |
Out of 1065 BRRM, 13 had an incidence of BC. Median time to develop BC = 6 years. Of the 13 cases, 10 were local disease only, 1 was auxiliary BC of unknown primary disease, and 2 were synchronous local and distant disease. See Table 2 (in paper) for full details. Out of 1643 CRRM, 12 had an incidence of BC. Median time to develop BC = 8 years. Of the 12 cases, 7 were local disease only, 1 was local and regional disease, 3 were auxiliary BC of unknown primary disease, and 1 was synchronous local and distant disease. See Table 2 for full details. |
Median 6.1 years |
None | Collected data on women at the Mayo Clinic between 1 January 1960‐31 December 1993. BC developed ipsilateral to the RRM in 25 participants (13 after BRRM; 12 after therapeutic mastectomy and CRRM). The study utilized a study‐specific questionnaire (sent from 1995‐1997), and follow‐up surveys at 10 and 20 years after RRM. |
|
Pennisi 1989 BRRM and CRRM combined |
BRRM/CRRM: 6/1500 developed BC |
70% of participants were followed for 9 years | 30% were lost to follow‐up | 1500 patients from 165 plastic surgeons who had subcutaneous RRM and were registered with the Subcutaneous Mastectomy Data Evaluation Center. 78 (5.2%) participants had obscure carcinoma and 51 (3.4%) had LCIS at the time of surgery and were included in the study. Among the 139 patients who had CRRM, 4 (3%) had BC and 5 (3.6%) had LCIS and were included in the study. 300 (20%) had a 1st‐degree relative with BC and 21% had a history of 2nd‐degree maternal or paternal relatives with a history of BC. Note: this study contained a small group of CRRM patients; however, results were not presented separately. Due to the preponderance of BRRM, the study is reported with BRRM incidence results. |
|
Rebbeck 2004 BRRM |
BC incidence in BRCA1/2 carriers Analysis 1 ‐ participants may have had BRRSO BRRM: 2/102 (0.02%) (2.3 and 9.2 years after BRRM) Controls: 184/378 (48.7%) HR 0.05 (95% CI 0.01 to 0.22); P < 0.0001 Analysis 2 ‐ no BRRSO BRRM: 2/59 Controls: 149/305 HR 0.09 (95% CI 0.02 to 0.38), P < 0.001 Analysis 3 ‐ participants may have had BRRSO BRRM: 0/24 Controls: 24/107 P < 0.0001 Analysis 4 ‐ no BRRSO BRRM: 0/19 Controls: 19/69 P < 0.0001 |
Mean
5.5 years post BRRM for all cases 6.7 years for all controls |
None | Mean age at time of BRRM was 38.1 years. Follow‐up of controls began at mean age of 36.3 years Participants in Analyses 1 and 2 may have had BRRM before ascertainment; in Analyses 3 and 4, participants had BRRM after ascertainment. BRRM reduced the risk of BC by approximately 95% in BRCA1/2 carriers with prior or concurrent BRRSO and by approximately 90% in women without BRRSO. |
|
Skytte 2011 BRRM |
BC incidence: The annual incidence of BC was: BRRM group ‐ 0.8% (3 of 96 women) No‐BRRM group ‐ 1.7% (16 of 211 women) HR 0.394 (95% CI 0.115 to 1.355; P = 0.14) Protective effect but not significant |
Median: BRRM group from BRRM to diagnosis or end of study ‐ 3.94 years (378.7 women‐years divided by 96 participants) No‐BRRM group from date of disclosure of genetic testing to BRRM, diagnosis, or end of study ‐ 4.43 years (934.6 women years divided by 211 controls) |
None |
BC: breast cancer BRRM: bilateral risk‐reducing mastectomy BRRSO: bilateral risk‐reducing salpingo‐oophorectomy CRRM: contralateral risk‐reducing mastectomy DCIS: ductal carcinoma in situ HR: hazard ratio LCIS: lobular carcinoma in situ RRM: risk‐reducing mastectomy
Moderate risk
There were no deaths reported for the 425 participants in the "moderate risk" group (Hartmann 1999a) compared to an expected 10.4 deaths using the Gail model. The reduction in risk for the moderate risk group, therefore, was 100%.
Geiger 2005 reported no deaths after 10 years of follow‐up among 276 women who had BRRM compared to a calculated death rate of 1600/666,800 (0.2%) in matched controls, despite the fact that 65% of the participants had multiple breast cancer risk factors versus 12% of the controls.
Heemskerk‐Gerritsen 2013 reported breast cancer‐specific mortality as one death in 212 women with BRRM and six out of 358 controls; HR for the BRRM group = 0.29 (95% CI 0.03 to 2.61).
3. Disease‐free survival
BRCA1 and BRCA2 mutations
Heemskerk‐Gerritsen 2013, looking at BRCA1/2 women, reported that 10‐year breast‐cancer‐free survival for the BRRM group (212 participants) was 100%; the 10‐year breast‐cancer‐free survival for the control group (358 participants) was 74%.
4. Breast cancer incidence
Sixteen studies included data concerning the effects of BRRM on the incidence of breast cancer (Arver 2011; Borgen 1998; Contant 2002; Evans 1999; Geiger 2005; Hartmann 1999a; Hartmann 2001: Heemskerk‐Gerritsen 2013; Horton 1978; Kass 2010; Koskenvuo 2014; Meijers‐Heijboer 2001; Mutter 2015; Pennisi 1989; Rebbeck 2004; Skytte 2011). Seven studies dealt with women who had BRCA1/2 mutations, six dealt with high‐risk women, and the risk was unknown in three. See Table 2.
BRCA1 and BRCA2 mutations
For a number of years, genetic testing for BRCA1/2 mutations has been able to identify women who are considered at high risk of developing breast cancer. The participants in seven studies (Arver 2011; Hartmann 2001; Heemskerk‐Gerritsen 2013; Kass 2010; Meijers‐Heijboer 2001; Rebbeck 2004; Skytte 2011) were all or included some women with BRCA1/2 mutations.
Arver 2011, in a retrospective case series, reported no incidence of breast cancer (0 of 223) in high‐risk women (129 of whom were BRCA1/2 mutation‐positive) following BRRM versus an expected 12 cases (per the BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm) model) after a mean follow‐up of 6.6 years (2.1 to 14.0 years, 1468 women‐years).
Hartmann 2001 reported no incidence of breast cancer (0 of 26) following BRRM versus an expected incidence of 6 to 9 cancers in 26 women with BRCA1/2 mutations. Various statistical models were used to estimate the expected number of breast cancers and relative risk reduction, which ranged from 85% (95% CI 15.6% to 99.6%) to 100% (95% CI 54.1% to 100.00%). The follow‐up time ranged from 5.8 to 28.5 years, with a median follow‐up of 13.4 years.
Heemskerk‐Gerritsen 2013, in a prospective case series, reported on women who tested positive for BRCA1/2 mutations and who had BRRM (212 participants), with a control group of 358 women who had surveillance only. With a median follow‐up of 8.5 years for the BRRM women and 4.1 years for the control group women, the incidence rate per 1000 BRRM women was zero; for the control group, there were 57 women with breast cancer, for an incidence rate per 1000 women of 28. There were 51 women diagnosed with invasive breast cancer; of those, four women (all BRCA1 mutation carriers) developed metastatic breast cancer.
Kass 2010, in a retrospective series, reported on 147 asymptomatic BRCA1/2 mutation carriers who had BRRM after a normal surveillance round including breast magnetic resonance imaging. The breast cancer incidence was 0 out of 147 after a mean follow‐up time of 6.1 years (standard error (SE) 3.4) for BRCA1 carriers and 3.7 years (SE 3.1) in BRCA2 carriers. A confounding factor in this study was that 80 (54%) of these women had BRRSO.
Meijers‐Heijboer 2001 conducted a prospective cohort study comparing BRCA1/2 mutation positive women choosing BRRM with those choosing surveillance. There was a significant difference (0 of 76 versus 8 of 63, P = 0.003) in incidence of breast cancer in the BRRM group. Thus, the study reported a 100% reduction in estimated risk of breast cancer incidence at three years of follow‐up. Klijn 2004 reported that one of 73 participants who had BRRM developed distant metastasis, but 24 of 173 women in the surveillance group developed breast cancer. In the surveillance group, the actuarial (insurance calculation) five‐year incidence of breast cancer was 17%, which was significantly (P = 0.01) different from the BRRM group incidence rate (HR = 0.07). After adjusting for risk‐reducing oophorectomy, the result was significant (P = 0.02).
Rebbeck 2004 did both a prospective and retrospective analysis of BRCA1/2 mutation carriers. In the retrospective analysis, among the 102 carriers who selected BRRM, two developed breast cancer in the five‐year follow‐up period versus 184 of 378 (48.7%) who did not select BRRM (P < 0.0001). Excluding women who had BRRSO, the incidence of breast cancer in the BRRM group compared to the controls remains significant (2/59 versus 149/305, P < 0.001). Analyzing those participants who selected BRRM after determining their BRCA1/2 status, the reduction of the incidence of breast cancer remained significant with or without BRRSO (0/24 versus 24/107 (P < 0.0001) and 0/19 versus 19/69 (P < 0.0001)).
Skytte 2011 conducted a prospective cohort study of 307 women with BRCA1/2 mutations. Ninety‐six women opted for BRRM, and their median time of follow‐up was 3.94 years from either the time of their BRRM until breast cancer diagnosis, the date of death, or the end of the study. The 211 women who opted not to have BRRM were followed for 4.43 years from their BRCA or genetic testing date to clinically indicated mastectomy diagnosis, or end of study. The annual incidence of breast cancer in the BRRM group was 0.8% (3 of 96 women, all of whom were BRCA1 mutation carriers); for the non‐BRRM group, it was 1.7% (16 of 211 women, 12 of whom were BRCA1 mutation carriers) (HR = 0.394), which shows a protective effect but is not statistically significant.
High risk (strong family history, but not necessarily BRCA1/2 mutation carriers)
Contant 2002 reported no incidence of breast cancer within 2.8 years of follow‐up after BRRM among 79 women who were BRCA1/2 mutation carriers or had a 50% risk for breast cancer.
Hartmann 1999a used a retrospective cohort design to determine risk among the "high risk" group, with sisters acting as controls. High risk was defined as having a strong family history of breast cancer and did not exclude women with BRCA1/2 mutations. (See Table 2 for high‐risk criteria.) This study reported that three participants developed breast cancer after surgery compared to an expected incidence of 30 to 52.9 cancers. Thus, there was a 90% to 94% reduction in incidence for this group.
Moderate risk
It was reported in Geiger 2005 that BRRM significantly reduced breast cancer in the participants who selected BRRM compared to the control group based on a record review of 666,800 women (1/276 versus calculated 26,800/666,800; HR 0.005, 95% CI 0.001 to 0.044).
Hartmann 1999a compared incidence from a case series to expected incidence using the Gail model for moderate‐risk women, and this approach indicated significantly reduced incidence of breast cancer following BRRM. Among the moderate‐risk group, four participants later developed breast cancer compared to an estimate of 37.4 based on the Gail model, a reduction of 89.5%. The median follow‐up for all participants was 14 years, with 99% followed for at least two years.
The two remaining studies did not provide detail on risk assessment. Borgen 1998, in a convenience sample, reported that three of 370 women having BRRM, or less than 1%, subsequently were diagnosed with breast cancer. Follow‐up ranged from 0.2 to 51.5 years with a mean of 14.8 years. Evans 1999 used a case series and compared actual incidence to expected incidence based on the Claus model, but the follow‐up time was short, only 2.2 years.
5. Physical morbidity
Seven studies (Arver 2011; Barton 2005; Gabriel 1997; Gahm 2007; Gahm 2010; Metcalfe 2004b; Zion 2003) focused on physical morbidity following BRRM (and/or CRRM in studies where the numbers were combined or it is unclear) with breast reconstruction. See Table 3.
3. Physical morbidity.
| Study | Outcome | Follow‐up time | Attrition | Study details |
|
Arver 2011 BRRM |
Reoperations 142 of 223 (64%) women had unanticipated secondary operations Complications 115/223 (52%) experienced one or more early complications (< 30 days): Partial skin necrosis/epidermolysis 63 (29.9) Wound infection 38 (17.0) Hematoma, evacuated 18 (8.1) Seroma, evacuated 17 (7.6) Wound rupture 8 (3.6) Blood loss requiring transfusion 20 (9.0) Non‐breast related complication 7 (3.1) 62/209 (29.8%) women had ≥ 1 implant complications Capsular contracture requiring surgery 29 (13.9) Implant loss due to infection/necrosis 21 (10.1) Implant rupture 14 (6.7) Expander port leakage 12 (7.3) 7/12 women had ≥ 1 flap‐related complications Reoperation due to anastomotic failure 4 (33.3) Partial flap failure 4 (33.3) Complete flap failure 1 (8.3) Donor site infection/necrosis 3 (25.0) 22 (9.9%) women had late (> 30 days) wound infection |
Mean 6.6 years; 2.1‐14.0 years (1468 women years) |
None | Women with a BMI of 25‐30 had a higher proportion of infections than women with BMI < 25 (36% vs 15%) and it increased further for women with BMI > 30 (73%), P < 0.001 The proportion of implant loss increased with increasing weight as well (5% if BMI < 25, 16% if BMI 25–30, and 27% if BMI > 30, P = 0.008) Wound necrosis/epidermolysis was more common in smokers than in nonsmokers (68% vs 16%, P = 0.007) |
|
Barton 2005 BRRM |
Complications following BRRM 172/269 (64%) had ≥ 1 169 (63.8%) local 32 (11.9%) systemic Number of participants having complications 21/28 (75%) autogenous tissue graft 122/186 (66%) implants 29/55 (53%) no reconstruction Mean number of reoperations per participant 0.27 no reconstruction 5.6 implants 6.7 autogenous tissue graft |
Mean 7.4 years | None | 9 (3.3%) developed lymphedema Reoperations included anticipated procedures i.e. inflation of expander, nipple reconstruction Timing of reconstruction was borderline significant when comparing immediate with delayed reconstruction (80.6% versus 64.0% (P = 0.055)) 10% of women had at least 1 complication noted more than once, thus possibly chronic with pain being the most common repeated complication When comparing participants by 5‐year time periods, there was a trend toward more complications in the more recent time periods |
|
Contant 2002 BRRM and CRRM combined |
Reoperations 2/9 (22%) who did not have reconstruction after BRRM or CRRM had unanticipated re operations 30/103 (29%) who had reconstruction after BRRM or CRRM had unanticipated complications: 21 complications within 6 weeks of surgery 23 complications > 6 weeks after surgery Some participants had more than 1 complication. 34/44 (77%) of the complications required additional surgery |
Median 2.8 years | None | 10 instances of bleeding required surgery 8/14 cases of prosthesis capsular contracture required surgery 10 prostheses were removed: 7 due to infection, 2 due to wound necrosis and 1 due to pain |
|
de la Pena‐Salcedo 2012 BRRM/CRRM |
Complications 7 of 64 (10.9%) reconstructed breasts had short‐term (undefined) complications: 4 capsular contracture 2 hematomas 1 infection Esthetic outcome assessed by plastic surgeon not associated with the intervention: on scale of 1 (unesthetic) to 10 (esthetic) the overall esthetic index = 8.8 with scores ranging from 6‐10 |
Mean 12 years |
None | All breasts were reconstructed |
|
Den Heijer 2012 BRRM/CRRM |
11 women (31%) underwent additional surgeries after the primary RRM | 7‐9 years | None | |
|
Frost 2005 CRRM |
Reoperations 157/583 (27%) women had 213 unanticipated reoperations following CPM, of these 113 (72%) were implant related including: 75 implant failures 47 esthetic implant concerns 9 silicone anxiety 43% of subcutaneous mastectomy women had reoperations 15% of women with simple mastectomy had reoperations P < 0.0001 |
Mean 10.3 years | Of original 792 who had the procedure, 621 were living at time of study and 583 (94%) competed study questionnaire | These participants are all part of the cohort in McDonnell 2001 98% of women with subcutaneous mastectomy had reconstruction 48% of women with simple mastectomy had reconstruction |
|
Frost 2011 (follow‐up to Frost 2005) CRRM |
Reoperations after reconstruction 115 (54%) had no reoperations 70 (33.3%) had 1 reoperation 25 (11.9%) had > 1 reoperations Among those with reconstruction, 45% underwent ≥ 1 reoperations, and satisfaction was lower in women with reoperation than those without (P = 0.04) |
Mean 20.2 (11.4‐44.5) years post CRRM |
Of the 487 women in Frost 2005 who were still alive, 269 (55%) responded to second survey | |
|
Gabriel 1997 BRRM or CRRM |
Complications, defined as events requiring surgical interventions, involved 274 (18.8%) of the 1454 breasts with implants and 321 (18.8%) of the 1703 implants By 5 years, the number of implants with complications was nearly 3 times as high in cancer and risk‐reducing groups as the cosmetic group: Cancer group: 34.0% of 125 (95% CI 27.2% to 41.3%) Risk‐reducing group: 30.4% of 92 (95% CI 231% to 38.4%) Cosmetic group: 12.0% of 532 (95% CI 9.1%, 15.2%) The 3 most frequent problems were:
|
Mean
7.8 years (range 0‐7.8 years; 5847 person years) For analysis, follow‐up period was 5 years |
None | 208 of the 749 (27.8%) underwent 450 additional surgical procedures within 5 years. 91 of 450 (20.2%) of the procedures were anticipated (staged procedures, participant's request for size change or esthetic improvement) and 359 had clinical indications and were performed in 178 (23.8%) of the women. Despite number of complications, study author cautions that study did not evaluate participants' overall satisfaction with their implants or the effects of these events on participants' overall health status |
|
Gahm 2007 BRRM |
BRRM group: 24 women who had BRRM with immediate reconstruction < 2 years before ascertainment Comparison group: 16 women with no BC Sensitivity in reconstructed breasts Touch: significantly reduced sensitivity to touch in BRRM group compared with comparison group (P < 0.001) Cold BRRM group had significantly lower thresholds to cold stimuli than comparison group (P < 0.001). The threshold level was a mean of 8° C lower in BRRM participants than controls (20.6° C and 28.8° C) Warmth Significantly higher thresholds to warmth were found in BRRM group than in comparison group (P < 0.001). The threshold level for warmth was a mean of 9.2° C higher in the BRRM participant group than in the control group (36.3° C and 45.5° C) Sexual feelings 4 of 18 in BRRM group reported that they could experience sexual feelings in the reconstructed breasts Discomfort 66% of BRRM group said that they experienced spontaneous or stimulus‐evoked discomfort, or both, in the reconstructed breasts |
Mean 5 years |
None | |
|
Gahm 2013 BRRM Follow‐up Gahm 2007 |
Touch The results of Optihair von Frey Filament testing demonstrated significantly reduced touch sensitivity postoperatively compared to that observed preoperatively in the breast skin (P < 0.0001) Cold and warmth The postoperative perception thresholds to cold stimuli were significantly lower than preoperatively (P < 0.001) There were significantly higher thresholds to warmth postoperatively (P < 0.001) Sexual feelings 33 of the 46 participants reported a lost or decreased ability to experience sexual feelings in the reconstructed breasts after surgery These findings were also reported in Gahm 2007 |
Mean 29 months (24‐49) |
No attrition | |
|
Gahm 2010 BRRM |
Corrective surgical procedures 35/55 participants (59%) had ≥ 1 postoperative corrective surgery Infection in 55 participants 11 participants had postoperative infections: 3 had implant extraction 4 had hematomas 2 had acute evacuation 2 had flap necrosis Pain 38/55 participants (69%) reported pain in their reconstructed breasts 20/55 participants (36%) reported that pain in their reconstructed breasts affected their sleep 12/55 (22%) reported that pain in their reconstructed breasts affected their daily activities |
Mean 29 months (24‐49) |
55 of 59 participants (93%) returned questionnaire on pain, discomfort, sexuality and feelings of regret 37 of 59 participants (64%) returned the Swedish Short Form‐36 survey on health‐related QoL. |
|
|
Goldflam 2004 CRRM |
Complications that occurred in participants with CRRM
39/239 (16.3%) had complications:
20 (8.4%) were in breast with primary cancer
15 (6.3%) on CRRM side
4 (1.7%) in both Types of complications in 239 participants: Infection: 7 (2.9%) Flap loss: 1 (0.4%) Mastectomy skin flap necrosis: 8 (3.4%) Reoperation bleeding: 9 (3.8%) Reoperation, other: 7 (2.9%) Combination (flap loss/necrosis): 7 (2.9%) |
Mean
7.8 years (1846 person‐years) |
None | |
|
Heemskerk‐Gerritsen 2007 BRRM & CRRM combined before Heemskerk‐Gerritsen 2013 |
Reconstructive breast surgery complications Of the 276 women opting for breast reconstruction, 137/276 (49.6%) registered ≥ 1 complications, totaling 215 complications Surgical re‐interventions were performed in 153 of the 215 complications; 124 for complications later than 6 weeks postoperatively |
Median 4.5 years |
None | |
|
Isern 2008 BRRM and CRRM combined |
Appearance of breast Asymmetry between the breasts was found among 17 (32%) of the women Reoperations 4/61 participants required reoperation within 6 weeks of surgery 7/61 participants developed late complications, 5 of which had re operations Another 7 women (11%) had cosmetic corrections |
Median 42 months (7‐99) |
7 of 61 (11%) eligible women did not participate in follow‐up | |
|
Koskenvuo 2014 BRRM or CRRM |
26 surgical complications in 21 participants that resulted in 20 reoperations. Frequency of complications was 33% (26/80) per operated breast and 40% (21/52) per participant In the group with reconstruction with autologous flaps, there were 11 (28%) complications in total; in the group of implant‐based reconstruction, complications were recorded in 13 (42%) breasts The most common complication was wound infection, others were seroma, hematoma, skin edge necrosis, blood supply problem, total flap loss, and implant loss 5 reconstructions failed and were corrected with re‐reconstruction In the 10 participants who had previously had BCS, there were 4 cases of minor complications |
Median after RRM was 52 months (range: 1‐133 months) | None – only followed specific group | 10 of the participants with BRRM had previously had BCS on a cancerous breast then decided to have RRM on that breast |
|
Metcalfe 2004b BRRM |
60 women who had BRRP provided medical history information through postoperative postal questionnaire 38 (64.4%) reported postsurgical symptoms: 27 (45%) reported numbness 7 (12%) reported pain 7 (12%) reported tingling 7 (12%) reported infection 2 (3%) reported swelling 2 (3%) reported breast hardness 1 each reported hematoma, failed reconstruction, breathing complications, thrombosis or pulmonary embolism |
Mean 52.2 months (range 6 to 117 months) | 60 of 75 returned completed questionnaire | Number of symptoms reported: 18 women reported 1 symptom 15 women reported 2 symptoms 5 women reported 3 symptoms |
|
Metcalfe 2014 CRRM |
Overall survival of BRCA1/2+ or high risk at 20 years CRRM 88% (95% CI 83 – 93%); No CRRM 66% (59 – 73%). The adjusted hazard ratio for women with CRRM associated w/48% reduction in death from breast cancer (HR 0.52, 95% CI 0.29 ‐ .93, P = 0.03). Propensity score adjusted analysis of 79 matched pairs (CRRM vs. No CRRM), the association was not significant (0.60, 0.34 – 1.06, P= 0.08). Adjusted hazard ration for CRRM compared with No CRRM was 0.58 (‐.34 0 0.97, P = 0.04 for entire study period and 0.36 (0.13 – 0.96, P = 0.04) for the second 10 yrs of follow‐up. The association between contralateral mastectomy and death from breast cancer in the first 10 years from diagnosis was not statistically significant in either the univariable or multivariable analysis. 20‐yr breast‐cancer specific mortality for No CRRM = 31%; CRRM women had a 48% reduction in risk of mortality vs. No CRRM women over 20‐year period. |
Median 14.3 years | Average time from diagnosis to CRRM was 2.3 years. Mean time to death from diagnosis 7.1 years (range 0.7 – 19.3 years). Some of the CBC cases were diagnosed within 1 ‐ 2 months (0.01 years) of original diagnosis of BC, less than the commonly used second new breast cancer diagnoses at ≤6 months, and more correctly should be classified as bilateral breast cancer. This classification then could have overstated the incidence of CBC in the No CRRM group. Metachronous contralateral breast cancer (CBC) is defined as a tumor in the opposite breast which was diagnosed more than 6 months following the detection of the first cancer. |
|
|
Miller 2013 CRRM |
Complications in CRRM group vs UM group (no CRRM) were 41.6% (112) vs 28.6% (87), P = 0.001 Major complications (including re operations, rehospitalizations, flap and/or implant loss): CRRM 13.9% (29) No CRRM 4.1% (16) P < 0.001 After adjusting for age, BMI, smoking history, diabetes, AJCC stage, previous radiation, type of reconstruction, and adjuvant therapy, CRRM participants were 2.7 times more likely to have major complications (OR 2.66, 95% CI 1.37 to 5.19, P = 0.004) The most frequent major complications were fixed tissue expander or implant removal in CRRM participants (17.3%) and seroma requiring reoperation in UM participants (5.9%) CRRM participants were 1.5 times more likely to have any complication than no‐CRRM participants: (OR 1.53, 95% CI 1.04 to 2.25, P = 0.029) The rates of any and major complication were significantly higher in participants with reconstructed versus non‐reconstructed breasts, 37.8% vs 23.7% (P = 0.001) and 10.2% vs 2.0% (P = 0.001), respectively Among those who did not undergo reconstruction, 42.9% of CRRM participants had any complications vs 21.5% of UM participants (P = 0.029) Of those who had reconstruction, 87/209 (41.6%) had any complication; breast site complications were on cancer side in 29 (39.7%) and on CRRM side in 27 (37%) participants Minor complications included minor infections, necrosis, and delayed wound healing Univariate analysis showed that CRRM (P = 0.001), type of reconstruction (P = 0.001), and smoking history (P = 0.007) were significantly associated with any complication |
1 year | N/A | Complications in CRRM participants not having reconstruction were about twice the amount of those in UM participants, which is logical, since twice as many breasts were removed. |
|
Zion 2000 BRRM Preceeded Zion 2003 |
290 of the 591 (49%) had unanticipated reoperation (UR). For all 591 women, the average UR per person was 0.96 (SD 1.32) Reasons for UR were: 22% ‐ immediate postoperative complications 46% ‐ implant‐related issues 32% ‐ esthetic concerns |
Mean 14.2 years | None | Physical morbidity assessed by review of medical records and patient interviews to assess complications leading to surgical procedures that were not part of the standard breast implantation protocol. Median time to UR was 1.3 years with 42% occurring within 1 year of breast reconstruction. Of 1182 implants originally placed, 432 (37%) were removed and 389 new implants were placed. Note: some of these participants are probably the same as some of the participants in the Gabriel 1997 study |
|
Zion 2003 BRRM and CRRM |
Reoperations performed after BRRM or CRRM with or without implant reconstruction 8/39 (21%) BRRM without reconstruction 36% within 1 year 65% with 5 years 14 total re operations in 21 years 10/279 (4%) CRRM without reconstruction 82% within 1 year 11 total re operations in 13 years 311/593 (52%) BRRM + reconstruction 28% within 1 year 41% within 5 years 152 women (26%) had 1 reoperation 159 women (27%) had ≥ 2 reoperations 605 total reoperations 189/506 (37%) CRRM + reconstruction 22.4% by the first year 32.4% within 5 years 142 women (28%) had 1 reoperation 47 women (9%) had ≥ 2 Rate of reoperation BRRM plus reconstruction versus no reconstruction was RR 13.0 (95% CI 8.6 to 19.7) Rate of reoperation in CRRM plus reconstruction versus no reconstruction was RR 7.7 (95% CI 5.1 to 11.7) Rate of reoperation in the no reconstruction group was greater among the BRRM (P < 0.01) and SCM (P < 0.01) women than in CRRM RR for reoperation in BRRM versus CRRM was 7.9 (95%CI 3.6 to 17.4) RR for reoperation in SCM versus TM was 19.5 (95%CI 8.8 to 43.4) Implants were removed from women with reconstruction: BRRM = 194/593 (33%) CRRM = 74/311 (24%) In BRRM group, nulliparous women had significantly fewer reoperations RR 0.68 (95% CI 0.50 to 0.92) |
Median
14.2 years for BRRM 8.8 years for CRRM 15.0 years for no reconstruction |
None | Reasons for reoperation included; immediate postoperative complications, implant‐related issues, esthetic concerns, and nodule removal. Postoperative complications resulted in 9% to 12% of all re operations in the reconstruction group and 28% of all re operations in the no‐reconstruction group. Approximately 50% to 60% of reoperation indications concerned implants, 33% were removed and replaced, 4% were removed with no replacement. 92% of BRRM group and 96% of CRRM group had reconstruction within 2 weeks of RRM Median ages at reconstruction for BRRM and CRRM were 42 and 46 years, respectively. Median time for BRRM from reconstruction to first reoperation was 10.4 months, with 26% within 6 months of reconstruction. Median time for CRRM to first reoperation was 7.8 months, with 22.4% occurring in the first year. There was a trend for women in BRRM group to have more reoperations in the more recent years of the study. Rate of reoperations in the CRRM reconstruction group was not statistically different when comparing SCM versus TM Study authors could not distinguish reliably between medically necessary and elective reoperations, so all reoperations were tallied. |
BCS: breast conserving surgery BMI: body mass index BRRM: bilateral risk‐reducing mastectomy BRRSO: bilateral risk‐reducing salpingo‐oophorectomy CBC: contralateral breast cancer CRRM: contralateral risk‐reducing mastectomy ILC: invasive lobular cancer OR: odds ratio RR: risk ratio RRM: risk‐reducing mastectomy SCM: subcutaneous mastectomy TM: total mastectomy QoL: quality of life UM: unilateral mastectomy UR: unanticipated reoperations
Arver 2011 conducted a retrospective series on 223 high‐risk women in Sweden with a mean follow‐up of 6.6 years; 142 women (64%) had unanticipated secondary operations. Sixty‐two of the women had one or more implant complications (capsular contraction, implant loss or rupture, expander port leakage), seven women had one or more flap‐related complications (anastigmatic failure, partial flap failure, complete flap failure, donor site infection/necrosis), and 22 women had late (> 30 days) wound infection.
Barton 2005 gathered data through chart review, which showed that 64% (172 of 269) of the women having BRRM reported having one or more complications, with slightly more than half reporting pain as a complication.
Gabriel 1997 defined physical morbidity as "complications leading to unanticipated surgical interventions following breast implant." At five years, 34% (43 of 125) (95% CI 27.2 to 41.3) of women with cancer had complications compared to 30.4% (28 of 92) (95% CI 23.1 to 38.4) of women having risk‐reducing surgery and 12.0% (64 of 532) (95% CI 9.1 to 15.2) of women having implants for cosmetic reasons.
Gahm 2010 reported results from a questionnaire from 55 of 59 Swedish women who underwent BRRM and immediate reconstruction from 2004 to 2006, with mean follow‐up of 29 months. Thirty‐five participants (59%) reported one or more postoperative corrective surgeries. Twenty‐two participants had postoperative infections (resulting in implant extraction, hematomas, acute evacuation, flap necrosis) and 38 of the 55 (69%) reported pain in their reconstructed breasts. Of those, 20 participants (36%) reported that pain in their reconstructed breasts affected their sleep and 12 (22%) reported that the pain affected their daily activities.
In a follow‐up of Gahm 2010, Gahm 2013 reported on Optihair von Frey Filament testing on 46 of the 59 women and demonstrated significantly reduced touch sensitivity postoperatively compared to that observed preoperatively in the breast skin (P < 0.0001). The postoperative perception thresholds to cold stimuli were significantly lower than preoperatively (P < 0.001). There were significantly higher thresholds to warmth postoperatively (P < 0.001).
Earlier, Gahm 2007 followed a smaller group of 24 women, also in Sweden, two years post‐BRRM between 1993 and 2005, with the same results. They experienced significantly reduced sensitivity to touch compared to controls (P < 0.001), significantly lower thresholds to cold stimuli (P < 0.001), and significantly higher thresholds to warmth (P < 0.001). Sixty‐six percent of participants experienced spontaneous or stimulus‐evoked discomfort in the reconstructed breasts.
Metcalfe 2004b used a questionnaire mailed to women 6 to 117 months after having BRRM. Post‐surgical symptoms were reported by 38 of 60 women (64.4%) including numbness (45%), pain (12%), tingling (12%), infection (12%), swelling (3%) and breast hardness (3%).
Zion 2003 updated data provided in the Zion 2000 abstract after a mean follow‐up of 10.3 years on physical morbidity, defined as unanticipated reoperations done for immediate postoperative complications following BRRM with reconstruction; 311 of the 593 participants, or 52%, had unanticipated operations following the initial surgery. The reasons for the subsequent surgeries included the following: immediate postoperative complications, implant‐related issues, and aesthetic concerns. Earlier, Zion 2000 reported that 432 of 1182 (37%) original implants were removed, with 90% of those being replaced. The percentage of reoperations following BRRM without reconstruction was 21% (8/39).
6. Quality of life/psychological morbidity
Eleven studies (Altschuler 2008; Borgen 1998; Brandberg 2008; Frost 2000; Gahm 2010; Geiger 2007; Gopie 2013; Hatcher 2001; Hopwood 2000; Metcalfe 2004b; Metcalfe 2005) presented data concerning psychosocial outcomes (satisfaction with decision, satisfaction with cosmetic result, satisfaction with the medical process, or other assessments of emotional or social function) (see Table 4). Data are derived from different sources, ranging from participant‐generated written responses to questionnaires to transcribed oral responses from in‐depth personal interviews. The results of these studies varied.
4. Quality of life.
| Study | Outcome | Follow‐up | Attrition | Study details |
|
Altschuler 2008 BRRM and CRRM |
General satisfaction among BRRM participants in response to closed‐end questions: 91/117 (77.7%) expressed general satisfaction 18/117 (15.4%) expressed general dissatisfaction 8 (6.9%) did not respond General satisfaction among CRRM participants in response to closed‐end questions: 401/567 (70.7%) expressed general satisfaction 60/567 (10.6%) expressed general dissatisfaction 102 (18%) did not respond |
Median 9 years (3‐22) postmastectomy |
Women responding to closed‐ended questionnaire: BRRM 117 of 195 CRRM 567 of 772 Women responding to 2 open‐ended questions: BRRM 78 of the 117 CRRM 249 of the 567 |
Among women who were generally satisfied with RRM, 30/91 BRRM participants had negative comments about such topics as implants, body image, sexuality or emotional concerns. 75/401 CRRM participants made similar negative comments. “These findings suggest that even among women who report general satisfaction with their decision to have risk‐reducing mastectomy via closed‐ended survey questions, lingering negative psychosocial outcomes can remain, particularly among women with bilateral risk‐reducing mastectomy. This dichotomy could be an important factor to discuss in counselling women considering the procedure.” |
|
Borgen 1998 BRRM |
Decision satisfaction
Most women were satisfied with BRRM: 21/370 (5%) regretted their decision to have RRM, with 19 of them among the 255 for whom the discussion about RRM was initiated by their physicians. Of the 21 with regrets: 10/21 (48%) had major regrets and would not undergo BRRM again 7/21 (33%) had minor regrets 4/21(19%) did not report level of regrets 19/21 (90%) of women who were unhappy with BRRM results did not have preoperative counselling Cosmetic satisfaction Of the 331 who responded about cosmetic results: 116/331 (35%) reported excellent results 163/331 (49%) reported acceptable results 52/331 (16%) reported unacceptable results |
Mean 14.8 years since surgery (range 0.2‐51.5 years) | Not applicable | QoL/satisfaction assessed by survey regarding satisfaction and regrets with BRRM. There was no mention of whether the survey was validated. 336 participants were selected from a group of 817 volunteers who responded to an invitation in the popular press to join the National risk‐reducing Mastectomy Registry. 34 participants were recruited from the study authors' practice or the NY Metropolitan Breast Cancer Group. Women with LCIS were excluded. 220 of the 370 (59%) reported having at least 1 first‐degree relative diagnosed with BC. 255 of 370 (69%) reported the discussion to have BRRM was initiated by their physician, while 108 (29%) initiated the discussion themselves. 5 did not recall who initiated the discussion. Mean age of participants with regrets was 45 and group overall was younger than those who were satisfied with BRRM. Incidental carcinoma was identified in 14 of the 370 (4%) and they were included in the study. |
|
Boughey 2015 CRRM Follow‐up to Frost 2005, Frost 2011 |
269 unilateral BC patients with a family history of BC who underwent CRRM between 1960 and 1993 were surveyed: 210 (78 %) reconstruction 59 (22 %) no reconstruction Satisfaction: P = 0.03 89% (187) reconstruction were satisfied with CRRM 95% (56) no reconstruction were satisfied with CRRM Choose CRRM again: P = 0.10 92% (193) reconstruction were satisfied with CRRM 93% (55) no reconstruction were satisfied with CRRM Positive feelings of body image remained significantly higher in reconstruction vs no reconstruction P = 0.01 |
Median 18.4 years; mean 20.2 years after CRRM | No attrition reported | |
|
Brandberg 2008 BRRM |
Body Image Score (BIS) No statistical significant difference in summated BIS mean scores between the 6‐month (mean, 4.57; SE, 0.56) and the 1‐year assessments (mean, 3.71; SE, 0.45) Sexual pleasure (SAQ) Among sexually active women pleasure decreased statistically significantly from the assessment before BRRM to the 1‐year assessment (df (2, 27); F, 5.839, P = .005) Anxiety (HAD) Anxiety decreased over time (df (2, 53); F, 8.53, P = 0 0004). Depression (HAD) No statistical significant difference was found for depression |
1 year | 81 of 90 women responded to questionnaires before BRRM 71 of the 81 responded to questionnaires 6 months post‐BRRM 65 of the 71 responded to questionnaires 1 year post‐BRRM |
“One drawback of this study is that cancer‐specific worries were not measured, an important issue when assessing distress among women with hereditary cancer syndromes. Thus, the conclusions concern general anxiety and depression.” “There are missing questionnaires at each of the assessment points, making the group that could be analyzed over time small and provides limited power to determine statistically significant differences.” 24/98 (25%) had BRRSO, known to affect sexuality prior to BRRM |
|
Brandberg 2012 BRRM Follow‐up of Brandberg 2008 |
80 of 91 women (88%) responded to the questionnaire before BRRM, 73/91 (80%) 6 months after BRRM, and 67/91 (74%) at the 1‐year assessment. Participants scored the cosmetic results of BRRM items with 7 responses categorized as follows: 1‐3 = negative, 4 = intermediate, 5‐7 = positive. Association between the “correspondence between the overall results and expectations before BRRM” with mutation status BRCA1/2 carriers: 16 (52%) positive response 15 (48%) negative response Non‐carriers: 26 (76%) positive response 8 (24%) negative response P = 0.039 Size of breast Most women (range 83%‐90%, n = 58‐70) were satisfied with the size of their breasts Softness of breasts 20 women (51% of those who responded to this item) responded at the 1‐year assessment that they were satisfied with the softness of both breasts. 19 women (49%) indicated that at least one breast was “too hard”, and of these women, 14 (36%) stated that both breasts were too hard. |
1 year | 80 women (88%) responded to the questionnaire before RRM, 73 (80%) 6 months after RRM, and 67 (74%) at the 1‐year assessment |
Same participants as Brandberg 2008 |
|
Bresser 2006 BRRM and CRRM |
Responses to questions about satisfaction with RRM and breast reconstruction, its impact on sexual relationships using a self‐reporting questionnaire
68/113 (60%) satisfied with RRM and reconstruction
106/112 (95%) would choose RRM again
89/112 (80%) would choose same type of reconstruction again
95/112 (85%) felt sufficiently informed before surgery
10/77 (13%) experienced positive changes in sexual relationship
40/90 (44%) experienced adverse changes in sexual relationship Satisfaction with the result of breast reconstruction 68 (60%) = satisfied participants 45 (40%) = non‐satisfied participants Comparing the 45 non‐satisfied with the 68 satisfied participants, there were statistically significant differences in these psychosocial factors:
7 women (18%) who were not satisfied with their breast reconstruction would not opt for reconstruction again (P = 0.01). 90 women answered questions about impact onsexual relationships; of those, 40 (44%) reported that RRM negatively affected their sexual relationship. That outcome is also associated with other adverse effects as compared to the 50 women who were not negatively affected including: 12 (30%) felt insufficiently informed about the procedure and possible results (P = 0.01) 18 (45%) said surgery did not meet their expectations (P= 0.01) 18 (45%) experienced more limitations in daily life (P = 0.01) 20/27 (74%) who answered the question perceived an adverse change in partner’s perception of sexual relationship (P = 0.001) |
Median 3 years | None | 65 women also had BRRSO either before, simultaneously, or after RRM. There was an absence of a relationship between satisfaction with RRM & reconstruction and changes in sexual relationship. It may be impossible to distinguish between RRM and reconstruction effects on women. |
|
de la Pena‐Salcedo 2012 BRRM/CRRM |
Satisfaction with RRM Of 52 participants undergoing RRM: 39 (75.00%) reported being highly satisfied 10 (19.23%) reported being partially satisfied 3 (5.76%) reported being unsatisfied |
Mean 0‐12 years |
None | |
|
Den Heijer 2012 BRRM/CRRM |
36 of 52 women at high‐risk for hereditary breast/ovarian cancer who had BRRM/CRRM with/without reconstruction Participants were assessed at 2‐4 weeks (T0) before RRM (T1), 6 months after RRM and 6‐9 years (T2) after RRM General distress levels scores went down: From T0‐T1 9.91‐7.45, P = 0.03 From T1‐T2 7.45‐6.58, P = 0.01 BC specific stress level scores went down: From T0‐T1 22.7‐12.9, P = 0.01 From T1 to T2 12.9‐6.1, P = 0.01 General body image scores fluctuated, declining and then improving but not to pre‐op levels: From T0‐T1 10.7‐12.4 P =0.02 From T1‐T2 12.4‐11.7, P = 0.18 Breast‐related body image scales fluctuated, improving and then declining: From T0 to T1 5.0‐6.7, P =0.01 From T1 to T2 6.7‐5.9, P = 0.03 |
6‐9 years | None | Study used validated assessments: Utrecht Coping List (UCL), Impact of Events Scale (IES), and HADS and Body Image Scale 75% of women were BRCA1/2+ |
|
Frost 2000 BRRM |
Decision satisfaction
393/562 (70%) were either satisfied or very satisfied with their BRRM
69/562 (11%) were neutral
107/562 (19%) were dissatisfied or very dissatisfied
383/572 (67%) indicated they would definitely or probably choose BRRM again There was correlation between lower level of satisfaction and physician's advice being given as the primary reason for choosing BRRM. Cancer worry 423/572 (74%) reported a diminished level of emotional concern about developing BC 520/572 (91%) of the women reported no change or favorable effect on emotional stability 52/572 (9%) reported adverse effect in level of emotional stability 492/572 (86%) of the women reported no change or favorable effect on stress levels 80/572 (14%) reported adverse effect in level of stress Body image 275/572 (48%) reported no change in their level of satisfaction with their physical appearance 92/572 ( 16%) reported favorable effects 206/572 (36%) reported diminished satisfaction with their physical appearance 429/572 (75%) of the women reported no change or favorable effect in feelings of femininity 132/572 (23%) reported adverse effect in feelings of femininity Variable most strongly associated with patient satisfaction after BRRM was satisfaction with body appearance: 469/572 (82%) of the women reported no change or favorable effect in self‐esteem 103/572 (18%) reported adverse effect in self‐esteem Sexuality 440/572 (77%) of the women reported no change or favorable effect 132/572 (23%) reported adverse effect in sexual relationships |
Mean 14.5 years after surgery | 572 of 609 (94%) completed the questionnaire | Patient satisfaction assessed by questionnaire to evaluate long‐term satisfaction, and psychological and social function. The 609 women were a subset of 639 participants in Hartmann 1999a study known to be alive and were recruited to complete a study questionnaire after their BRRM to evaluate their long‐term satisfaction, and psychological and social function. Family history was the most common number one reason given for having a BRRM, followed by physicians' advice and nodular breasts. Because reason for choosing BRRM was not collected pre‐operatively, authors are concerned that recall of reason for choosing BRRM may have been colored by subsequent experience. 100% of the 19 women who did not have reconstruction reported being very satisfied or satisfied, and using multiple regression analysis showed there was an association between satisfaction and no reconstruction. |
|
Frost 2005 CRRM |
Psychosocial outcomes among 583 women with CRRM after BC diagnosis at a single institution between 1960‐1993:
42% subcutaneous mastectomy + reconstruction
1% had subcutaneous mastectomy
27% had total mastectomy + reconstruction
30% had total mastectomy Most frequent reasons cited for having CRRM: 72% cancer in the other breast 59% physician’s advice 40% family history Satisfaction with CRRM: 83% were either satisfied or very satisfied 8% were neutral 9% were dissatisfied or very dissatisfied Percent of women dissatisfied or very dissatisfied with CRRM by type of surgery: 13% of women who had subcutaneous mastectomy 6% of those who had total mastectomy Percent of women indicating they would definitely or probably choose CRRM again: 75% of women who had subcutaneous mastectomy 89% of those who had total mastectomy 74% reported a diminished level of emotional concern about developing BC Level of satisfaction with their physical appearance: 48% reported no change 16% reported favorable effects 36% reported diminished satisfaction 33% of the women reported body image was negatively affected 26% reported adverse effects in feelings of femininity 23% reported adverse effects in sexual relationships 17% reported adverse effects in level of stress 12% reported adverse effects in level of emotional stability 17% reported adverse effects in self‐esteem |
Mean 10.3 years | Of original 792 who had the procedure, 621 were living at time of study and 583 (94%) competed study questionnaire | These participants are all part of the cohort in McDonnell 2001. There was correlation between dissatisfaction with CRRM and dissatisfaction with cosmetic results, adverse symptoms and complications, and diminished body image. There was moderate correlation between satisfaction with CRRM and satisfaction with body image, favorable feelings of femininity, self‐esteem, decreased levels of stress, and favorable sexual relationships. There was an absence of a relationship between satisfaction with RRM and reconstruction and changes in sexual relationships. Women less likely to choose CRRM again had strong association with diminished sexual relationships, having a subcutaneous mastectomy, diminished feelings of femininity and not being married. |
|
Frost 2011(follow‐up to Frost 2005) CRRM |
Satisfaction with CRRM: 90% of women were satisfied or very satisfied with the decision to undergo CRRM. Women with reconstruction had significantly lower satisfaction than women without reconstruction (P = 0.03) Choose CRRM: 92% of women reported that, knowing what they do now, they definitely or probably would choose CRRM again. Adverse effects: body appearance 31% feelings of femininity 24% sexual relationships 23% Informed decision: 93% reported they felt they made an informed choice about their CRRM |
Mean 20.2 (11.4‐44.5) years post CRRM |
Of the 487 women in Frost 2005 who were still alive, 269 (55%) responded to second survey. | Those who responded to the second survey also expressed more satisfaction (P = 0.004) on the first survey and were more likely to choose CRRM again (P = 0.001) |
|
Gahm 2010 BRRM |
Pain and discomfort in the breast: 38 of 55 participants (69%) reported pain in the breast most frequently triggered by pressure and physical activity 39 participants (71%) expressed discomfort in the breasts and the most frequent sensations were numbness, tingling, and squeezing, which were triggered by touch, physical activity, or pressure Sexuality: The ability to feel sexual sensations in the breasts was totally lost in 25 (45%) participants and substantially impaired in an additional 22 participants. There was a significant negative change in the breasts' sexual importance before BRRM (OR = 38.253; Wald 95% CI 8.315 to 1.807, P = 0.007) A significant negative change in sexual enjoyment relating to breasts' sexual importance after BRRM (OR = 24.355; Wald 95% CI 5.713 to 1.340, P = 0.019) Bodily pain: Participants reported significantly higher mean scores in the bodily pain domain than did the control group (P = 0.002) |
Mean 29 months (24‐49) |
55 of 59 participants (93%) returned questionnaire on pain, discomfort, sexuality and feelings of regret. 37 of 59 participants (64%) returned questionnaire the Swedish Short Form‐36 survey on health‐related QoL. |
|
|
Geiger 2006 CRRM |
BC concerns
CRRM = 257/511 (50.3%) very concerned or concerned
No CRRM = 45/61 (73.8%) very concerned or concerned
P < 0.001 CRRM = 371/429 (86.4%) very satisfied or satisfied with decision for CRRM Contentment with QoL CRRM = 396/519 (76.3%) No CRRM = 46/61 (75.4%) Self‐conscious about appearance: CRRM = 108/510 (21.1%) No CRRM = 9/60 (15.0%) P = 0.263 Satisfied with appearance when dressed: CRRM = 307/518 (59.3%) No CRRM = 34/61 (55.7%) P = 0.596 Satisfied with sex life: CRRM = 194/474 (40.9%) No CRRM = 23/57 (40.3%) P = 0.933 (49 women did not answer this question) Excellent, very good or good perception of health: CRRM = 418/516 (81.0%) No CRRM = 53/61 (86.9%) |
4‐20 years | Excluded women who were deceased, whose physicians declined their participation, and those with invalid address. Also excluded those who returned questionnaires with more than 25% questions not answered. | There was no statistically significant difference between CRRM and no‐CRRM participants for psychosocial factors: contentment with QoL, satisfaction with CRRM decision, self‐conscious about appearance, satisfied with appearance when dressed, satisfied with sex life and perception of general health. Cohorts from multiple community‐based healthcare delivery systems so results are more likely to apply to a broad range of women with BC than those studies in which participants were recruited from BC centers or through relatives with BC (per author). Could not determine whether the degree to which contentment, satisfaction with life and appearance changed from before and after CRRM, since there was no pre‐CRRM psychosocial assessment. |
|
Geiger 2007 BRRM |
48 women BRCA1/2+ or with a high‐risk family who opted for BRRM + IBR (immediate breast reconstruction) responded to questionnaires Satisfaction with decision: 85 of 106 BRRM women (85%) reported they were very satisfied or satisfied with their decision to have BRRM Contentment with QoL: BRRM: 65 of 106 women (61.4%) reported high contentment with QoL No BRRM: 38 of 62 women (61.4%) reported high contentment with QoL (P = 1.0) Concern about BC: BRRM: 59 of 106 (56.7%) reported they were very concerned or concerned about BC No BRRM: 39 of 62 women (62.9%) reported they were very concerned or concerned about BC Psychosocial outcomes: Psychosocial outcomes did not vary between women who underwent BRRM who did and did not have breast reconstruction (data not shown) |
Not stated but the BRRMs were performed between 1979‐1999 | 312 of the 482 women in the Geiger 2005 study were contacted by mail and 181 (58.0%) returned surveys 25 women (16 BRRM and 9 no‐BRRM) at 3 healthcare delivery systems were excluded because the Institutional Review Boards required that women be excluded if their physicians declined to give approval for their recruitment. |
Qualifying BC risk factors included a family history of BC, a personal history of atypical hyperplasia, one or more benign breast biopsies, lobular carcinoma in situ, micro‐calcifications, or ovarian cancer. 65% (69 of 106) of BRRM participants had a 1st‐degree family member with BC, while 14.5% (9 of 62) of the controls had a 1st degree relative with BC. P < 0.001 Respondents and non‐respondents did not differ in demographic characteristics or family history of BC, whether including deceased non‐respondents or limiting the comparison to living participants 13 surveys in which ≥ 25% of the questions were not answered were excluded from the analysis |
|
Gopie 2013 BRRM |
Body Image: Using a scale of 1‐5, body image declined from T0 to T1 from 3.8‐3.3 (P < 0.001) Continued to decrease T0 to T2 from 3.8‐3.5 (P = 0.06) Satisfaction with partnership relationship did not significantly change from T0 to T1 (P = 0.79) Sexual satisfaction tended to decrease from T0‐T1 (P = 0.07) Continued to decrease T1‐T2 (P = 0.06) Cancer distress: declined significantly from T0‐T1 (P = 0.001) General mental health: improved from T0‐T1 (P = 0.02) General physical health: significantly declined from T0‐T1 (P = 0.001) Appearance of breasts: at T2 there was a significant increase in the proportion of women who reported they were not happy with the appearance of their breasts compared to T1 (P = 0.001) |
Median 29 months (24‐49) | No attrition | |
|
Hatcher 2001 BRRM |
Psychological morbidity/anxiety treatment group
In the 79 women who chose BRRM, anxiety decreased significantly from 41/71 (58%) preoperatively to 29/71 (41%) 6 months postoperatively (P = 0.04) and remained low at 18 months postoperatively Comparison group Psychological morbidity showed a trend towards a decrease in the 64 women who declined BRRM from baseline 57% (31/54) versus 43% (23/54) at 6 months (% diff = 14; 95% CI 0 to 29, P= 0.08). Changes from baseline 57% (29/52) versus 18 months 41% (21/52) (% diff = 16%; 95% CI 2 to 33, P= 0.11) Cancer worry Significantly more women in the BRRM group 24/74 or 32% compared to the no BRRM group 6/58 or 10% were likely to believe that it was inevitable that they would develop BC (P = 0.03) Sexuality The degree of sexual pleasure did not change significantly in either group Body image Body image questionnaires given at the 6‐ and 18‐month postoperative interviews to acceptors showed no difference in median score of 4 for body image on a scale of 0‐30 with 0 being the most positive |
Those choosing BRRM were interviewed again at 6 and 18 months postoperatively. Those declining or deferring were re‐interviewed at 18 months after the first interview | 11/168 were lost to contact before completing assessment | Participants were assessed with 6 questionnaires measuring general health, anxiety, sexual activity, coping, risk perception and body image. A score of ≥ 4 on the General Health Questionnaire (GHQ) defined possible psychological morbidity. Participants were identified from cohort of 168 women having a family history of BC or having sufficiently high risk factors for BRRM to be offered. They were followed prospectively with baseline data being collected prior to having BRRM. The comparison group is women who considered BRRM, but declined. Of these, 154 were recruited for the study. Eleven deferred their decisions whose results were not reported. Baseline statistical analysis included all women who completed the assessment at the first interview. In subsequent analyses, only those women who completed assessments at each time point were included. Most women in both groups were employed and had children. The median age of acceptors was 38 and for decliners was 40. Psychological morbidity decreased significantly over time among acceptors, and the longer the time from surgery, the greater the decline. 29% of the acceptors had genetic testing versus 5% of the decliners. |
|
Hopwood 2000 BRRM |
Cancer worry
47 of 49 returned General Health Questionnaires (GHQ) able to be evaluated 1 year postoperatively 8/47 (17%) scored > 9 in a range of 0‐28 suggesting "case" level distress. Body image All 49 returned Body Image Scale (BIS) questionnaires one year post‐operatively. 6/49 (12%) reported moderately changed or very much changed overall in body image on 10 items More than half of the women reported a change from little to very much for 3 items: 27/49 (55.1%) felt less sexually attractive 26/49 (53.1%) felt self‐conscious about appearance 26/49 (53.1%) feel less physically attractive |
19/49 women had 1‐ and 2‐year assessments 9/49 women had 1‐, 2‐ and 3‐year assessments |
19/49 women had 1‐ and 2‐year assessments 9/49 women had 1‐, 2‐ and 3‐year assessments |
QoL measured by General Health Questionnaire (GHQ) and Body Image Scale (BIS) to assess mental health and body image 1 year postoperatively. Participants were recruited from a group of 76 women who had BRRM. 7 of 45 women required further psychiatric help. 3 of the 7 were given antidepressant medication. Complications from surgery accounted for 4 of the 7 women needing psychiatric help. Surgical complications e.g. skin necrosis, nipple loss, infection and pain, accounted for some of the highest GHQ and BIS scores. |
|
Hwang 2016 CRRM |
1598 volunteers from Army of Women aged ≥ 18 years who reported a history of BC surgery and reported having CRRM completed a survey Choosing CRRM Those who chose CRRM were: younger than no‐CRRM (53.7 vs 59.2 years, P < .001) married (76% vs 71%, P < .001) higher income (P < .001) more likely to have reconstruction than no‐CRRM (OR 1.72, 95% CI 1.43 to 2.08) CRRM was associated with a higher breast satisfaction score than no CRRM (62.0 v 59.9, P = 0.0043) CRRM women had lower physical well‐being scores than no‐CRRM women (74.5 v 76.8, P < 0.001) and lower psychosocial well‐being (71.7 v 73.9, P = 0.0051) |
Ranged from < 1 year to > 20 years | None | Used the BREAST‐Q, a well‐validated breast surgery outcomes patient‐reporting tool |
|
Isern 2008 BRRM & CRRM |
HADS Anxiety: 78% (n = 42) of the women were regarded as non‐cases concerning anxiety (score ≤ 8), 13% (n = 7) as doubtful cases (score 9‐10) and 9% (n = 5) as definite cases (score ≥ 11) Depression: 98% (n = 53) non‐cases and 2% (n = 1) definite cases Patient satisfaction "The women in our study reported higher levels of general satisfaction (92%) than aesthetic satisfaction (74%)" |
Median 42 months (7‐99) |
7 of 61 (11%) eligible women did not participate in follow‐up | |
|
Metcalfe 2004b BRRM |
Satisfaction with BRRM assessed through postoperative postal questionnaire. 60 women completed satisfaction questionnaire: 48 (80%) reported being extremely satisfied with their decision 10 (17%) reported being satisfied with their decision 2 (3%) reported neither satisfaction nor dissatisfaction with their decision 57 completed Impact of Event scale questionnaire measuring current distress related to having a family history of BC: 4 (7%) scored > the clinical cut‐off of 20 on the intrusion subscale 5 (8.8%) scored > 20 on the avoidance scale Women with a higher perceived risk of BC had more intrusive cancer‐related thoughts (P = 0.05) 59 completed the Sexual Activity Questionnaire: 40 (66.7%) reported being sexually active 40 (66.7%) reported that BRRM did not impact their sexual lives 19 (31.7%) reported it worsened their sexual lives 1 (1.7%) reported it improved their sexual lives 60 women completed the Body Image After Breast Cancer questionnaire: 29 (48.3%) reported no change in their self‐image 14 (23.3%) reported a worse self‐image 17 (28.3%) reported an improved self‐image 59 women completed the Brief Symptom Inventory, a measure of current psychological status: 19 (32.2%) of the women had levels of psychological distress symptoms consistent with the need for psychological counselling |
Mean 52.2 months (range 6‐117 months) | ||
|
Metcalfe 2005 BRRM |
Analysis of questionnaire data in Metcalfe 2004b identified two significant predictors for QoL for women had undergone BRRM:
Both of these factors are also associated with perceived risk of BC |
Mean 52.2 months after RRM (range 6‐117) | 15 of the 75 women who agreed to participate did not return questionnaires | Participants were the same women as those in Metcalfe 2004b. An increase in either the psychological distress or vulnerability score lowered the above average QoL score. Vulnerability includes feelings of susceptibility of the body to illness and cancer, as well as feelings of invasion of the body and a loss of trust in the body. |
|
Montgomery 1999 CRRM |
Decision satisfaction
Most women were satisfied with CRRM. 18 of 296 (6%) regretted their decision to have CRRM with 11/296 (5%) of them among the 212 who said the discussion about CRRM was initiated by the physician. Cosmetic satisfaction 12/111 who had reconstruction had regrets 6/185 who did not have reconstruction had regrets (RR 0.30, 95% CI 0.12 to 0.78, P = 0.01) 88/111 (79%) who underwent reconstruction reported their cosmetic results were excellent or acceptable 18/111 (16%) said cosmetic results were unacceptable, but only 12 of them also had regrets 5/111 (5%) did not report satisfaction 6/111 (5.4%) said they would not chose CRRM again if they had known the cosmetic outcome Sexuality The reasons given by the 18 women with regrets were: 7/18 (39%) cosmetic results 4/18 (22%) diminished sense of sexuality 4/18 (22%) lack of education about alternatives 3/18 (17%) other reasons |
Mean 4.9 years since surgery (range 0.25‐43.8 years) | 50 women of 346 did not respond to questionnaire | QoL/satisfaction assessed by survey regarding satisfaction and regrets with RRM. 346 participants were selected from a group of 817 volunteers who responded to an invitation in the popular press to join the National risk‐reducing Mastectomy Registry and who had CRRM. Insurance companies overwhelmingly provided coverage for CRRM in 276 women (93%) Regrets were less common, but not statistically significant, among women with whom the discussion to have CRRM was initiated by the physician (11/212 or 5%) than among women who initiated the discussion themselves (7/84 or 8%) |
|
Unukovych 2012 CRRM |
60 of 69 consecutive patients with a confirmed family history of BC who underwent CRRM were surveyed. Body Pain After increasing at 6 months, at 2 years after CRRM the comparison between participant and normative data revealed statistically significant difference in the bodily pain subscale favoring the participants (P = 0.007) Anxiety and depression No statistically significant differences between preoperative and postoperative mean levels were found for anxiety or depression. Problems with appearance Two years after CRRM > 50% of the women reported problems with appearance and with the scars, felt less attractive and feminine. |
2 years | 45 participants (75%) responded before CRRM, 49 (82%) at 6 months, and 45 (75%) at 2 years after CRRM | “According to the policy at the Karolinska University Hospital, CPM is not performed concurrently with BC surgery. Primary BC and its treatment should be prioritized. In our experience, patients opting for CPM at the time of BC surgery are seldom prepared; their risk of contralateral events has not been established, as they have not undergone oncogenetic investigation with mutation screening.” |
BC: breast cancer CBC: contralateral breast cancer BRRM: bilateral risk‐reducing mastectomy BRRSO: bilateral risk‐reducing salpingo‐oophorectomy BSO: bilateral salpingo oophorectomy CRRM: contralateral risk‐reducing mastectomy HAD(S): Hospital Anxiety Depression (Scale) IBR ‐ immediate breast reconstruction LCIS: lobular carcinoma in situ OR: odds ratio QoL: quality of life RR: risk ratio RRM: risk‐reducing mastectomy SE: standard error UM: unilateral mastectomy
a. Predictors of quality of life
Gopie 2013 reported that general mental health improved from preoperatively to six months postoperatively (P = 0.02) and general physical health significantly declined during the same period (P = 0.001). In analyzing scores from the Quality of Life Index (QLI) questionnaire by 59 women who had BRRM compared with their responses in four other psychosocial questionnaires, Metcalfe 2005 showed there were two significant predictors of quality of life: psychological distress (global severity index) and one subscale of body image (vulnerability). Psychological distress was defined as women who continue to perceive they have a high risk for breast cancer following BRRM. Vulnerability was defined as feelings of susceptibility of the body to illness and cancer, as well as feelings of invasion of the body and a loss of trust in the body as a healthy and functioning organ. Every one unit of increase in these two scores was correlated to a decrease in quality‐of‐life scores by 74% and 13%, respectively.
b. Satisfaction with decision/general satisfaction
None of these studies compared satisfaction with decision between women who chose surveillance and those who chose RRM. The studies found that the majority of women who had BRRM reported satisfaction with their decision. Most of the women, when asked, said they would recommend the surgery to other women with the same risk (Metcalfe 2004b), would chose BRRM again (Borgen 1998; Frost 2000), had no regrets about their decision (Borgen 1998) or were satisfied with their decision (Geiger 2007; Metcalfe 2004b). In Geiger 2007, 85 of 106 (84.2%) women reported they were very satisfied or satisfied with their decision to have BRRM. Only a small minority of women reported dissatisfaction. Borgen 1998 found that 5% (21 of 370) of women in the study regretted their decision to have BRRM. Nineteen of the 21 women with regrets reported that the physician had initiated the discussion of BRRM. Frost 2000 similarly found a correlation between dissatisfaction and listing physician's advice as the primary reason for BRRM.
General satisfaction was reported by 77.7% (91/117) of the women in Altschuler 2008, with 15.4% (18 of 117) expressing general dissatisfaction and 6.9% (8 women) not responding to the question.
c. Satisfaction with cosmetic outcome
Cosmetic satisfaction generally pertained to satisfaction with breast reconstruction, and these results were less consistently favorable than satisfaction with the decision to have BRRM. Frost 2000 reported 70% of women (393 of 562) were either "satisfied" or "very satisfied" with BRRM, 11% (69 of 562) neutral, and 19% (107 of 562) "dissatisfied" or "very dissatisfied." Although 'satisfaction' in this study was a general question that could be interpreted by the respondent in any domain of satisfaction, the highest correlates to satisfaction were cosmetic results. For example, increased satisfaction with physical appearance and fewer problems with implants were highly significantly associated with BRRM satisfaction.
Brandberg 2012 reported on 80 of 91 women (88%) who responded to a questionnaire before BRRM, 73 out of 91 (80%) six months post‐BRRM, and 67 out of 91 (74%) at the one‐year assessment. Most women were satisfied with the size of their breasts (range 83% to 90%, n = 58 to 70). Twenty women (51% of those who responded to this item) said at the one‐year assessment that they were satisfied with the softness of both breasts. Nineteen women (49%) indicated that at least one breast was “too hard,” and of these women, 14 (36%) stated that both breasts were too hard
Gopie 2013 reported on 48 women who had BRRM and, after reconstruction, showed a significant increase in the proportion of women who reported that they were not happy with the appearance of their breasts (P = 0.001).
Hopwood 2000 reported that 16% (7 of 45 women) required further psychiatric help following BRRM, and the psychiatric distress was associated with surgical morbidity. Borgen 1998 reported that 16% (52 of 331 women) found the cosmetic results of their BRRM unacceptable.
Another important aspect of cosmetic satisfaction is the level of satisfaction among those women who opted for BRRM without reconstruction. While the majority of women chose BRRM with reconstruction, the minority who did not choose reconstruction appeared to be highly satisfied with their cosmetic decision. Frost 2000 showed that choosing not to have reconstruction was positively correlated with satisfaction (P = 0.001). In Geiger 2007, general psychosocial outcomes did not vary between women with BRRM who did and did not have reconstruction, although the data were not shown.
d. Psychological well‐being/cancer‐related anxiety
Sixty‐five of 106 women (61.4%) who had BRRM in Geiger 2007 reported high contentment with quality of life, compared with 61% (38 of 62) among women who opted not to have BRRM (P = 0.1). Fifty‐nine of the BRRM women (56.7%) and 39 of no‐BRRM women (62.9%) reported that they were very concerned or concerned about breast cancer.
In Brandberg 2008, anxiety decreased over time (df (2, 53); F, 8.53, P = 0 0004). However, “…cancer‐specific worries were not measured, … Thus, the conclusions concern general anxiety and depression.”
Gopie 2013 reported that cancer distress declined significantly from preoperatively to six months postoperatively (P = 0.001).
Frost 2000 reported a diminished level of emotional concern about developing breast cancer in 74% (423 of 572) of those having BRRM and neutral or favorable effects on emotional stability in 91% (520 of 572). In this same study, 86% (492 of 527) indicated no change or favorable effects on stress.
Hatcher 2001 reported that psychological morbidity for acceptors (those who had BRRM) decreased significantly (from 41/71 to 29/71 (P = 0.04)) at six months postoperatively and decreased less for decliners (those who decided not have BRRM) in the same time period.
Measuring current psychological status, Metcalfe 2004b found that 32.2% (19/59) of women who had BRRM had psychological distress symptoms consistent with the need for psychological counselling after a mean follow‐up of 52.2 months. A weakness of this finding is that there is no presurgical baseline data for comparison.
e. BRCA1 and BRCA2 mutations
Brandberg 2012 recorded the association between the “correspondence between overall results and expectations before BRRM” with mutation status. BRCA1/2 carriers had 16 (52%) positive responses and 15 (48%) negative responses; non‐carriers had 26 (76%) positive responses and 8 (24%) with negative responses (P = 0.039).
f. Body image/sexuality
There was no statistically significant difference in summated mean Body Image Score results between the six‐month and one‐year assessment in Brandberg 2008. Gopie 2013 reported that, using a scale of 1 to 5, body image declined from 3.8 to 3.3 between just postoperatively to six months postoperatively (P = < 0.001) and continued to decrease from 12 months postoperatively 3.8 to 3.5 (P = 0.06). In another study (Metcalfe 2004b), the impact of surgery on body image varied; 17 out of 60 women (28.3%) reported improved self‐image while 14 out of 60 women (23.3%) reported diminished self‐image.
Issues about sexuality and body image/femininity were addressed in many studies. Responses about sexuality ranged from no one reporting change in sexual activity or pleasure following BRRM (Hatcher 2001), to 23% (132 of 572) reporting adverse effect on sexual relationships (Frost 2000), 31.7% (19/59) reporting worsened sexual lives (Metcalfe 2004b), pleasure among sexually active women decreasing statistically significantly from the assessment before BRRM to the one‐year assessment (df (2, 27); F, 5.839, P = .0005) in Brandberg 2008, to 55.1% (27 of 49) reporting feeling less sexually attractive (Hopwood 2000). Gopie 2013 reported that sexual satisfaction tended to decrease from preoperatively to six months postoperatively (P = 0.07) and continued to decrease through 12 months postoperatively (P = 0.06). Furthermore, 23% (132/572) of participants in the Frost 2000 study reported adverse effects in feelings of femininity, and 12% (6 of 49) of those in the Hopwood 2000 study reported moderate or much negative change in body image.
In Gahm 2010, the ability to feel sexual sensations in the breast was totally lost in 25 (45%) of participants and substantially impaired in an additional 22 participants. There was a significant negative change in the breasts’ sexual importance before BRRM (odds ratio (OR) 38.253, Wald 95% CI 8.315 to 1.807, P = 0.007) as well as a significant negative change in sexual enjoyment relating to the breasts’ sexual importance after BRRM (OR 24.355, Wald 95% CI 5.713 to 1.340, P = 0.019). In relation to this, 38 of 55 participants (69%) reported pain in the breast most frequently triggered by pressure and physical activity. Thirty‐nine participants (71%) expressed discomfort in the breasts, and the most frequent sensations were numbness, tingling, and squeezing, which were triggered by touch, physical activity, or pressure. Brandberg 2012 reported that a majority of women in the study (73%) responded that they did not have any, or had only minor, sensitivity in the breasts at both assessment points, 52 of 71 participants (73%) at six months post‐RRM and 47 of 64 participants (73%) at one year.
g. Impact on interpersonal relationships
Only one study (Gopie 2013) reported on impact on interpersonal relationships. That study reported that satisfaction with partnership relationship did not significantly change from preoperatively to six months postoperatively (P = 0.79).
B. Contralateral risk‐reducing mastectomy
Twenty‐six studies involved only participants with a previous diagnosis of breast cancer in one breast who chose to undergo a contralateral risk‐reducing mastectomy (CRRM) in the other breast (Bedrosian 2010; Boughey 2010; Brewster 2012; Chung 2012; Evans 2013; Frost 2005; Geiger 2006; Goldflam 2004; Heemskerk‐Gerritsen 2015; Herrinton 2005; Hwang 2016; Jatoi 2014; Kiely 2010; King 2011a; Kruper 2014; Lee 1995; Leis 1981; McDonnell 2001; Metcalfe 2014; Miller 2013; Montgomery 1999; Peralta 2000; Pesce 2014; Unukovych 2012; Van Sprundel 2005; Zeichner 2014). Four additional studies (Altschuler 2008; Heemskerk‐Gerritsen 2007; Kass 2010; Zion 2003) included both BRRM and CRRM participants, but their results were separated according to BRRM or CRRM, so only their CRRM results are reported here, for a total of 30 studies.
1. All‐cause mortality
Fifteen studies (Boughey 2010; Brewster 2012; Chung 2012; Evans 2013; Goldflam 2004; Heemskerk‐Gerritsen 2015; Herrinton 2005; Jatoi 2014; Kiely 2010; Kruper 2014; Metcalfe 2014; Peralta 2000; Pesce 2014; Van Sprundel 2005;Zeichner 2014) reported all‐cause mortality (see Table 5). Boughey 2010 reported on 385 women with median follow‐up of 17.3 years. The 10‐year overall survival for those who had CRRM (128 of 385 died) as opposed to the non‐CRRM group (162 of 385 died) with a HR of 0.68 (95% CI 0.54 to 0.86, P = 0.001) resulted in a 10‐year survival after a multivariate analysis of HR 0.77 (95% CI 0.60 to 0.98, P = 0.03). Goldflam 2004 found the all‐cause mortality following CRRM was 5.8% (14/239) after a mean follow‐up of 7.8 years. Herrinton 2005 showed improved survival following CRRM with a HR of 0.60 (95% CI 0.50 to 0.72) for CRRM participants versus no CRRM for women with breast cancer in one breast. Zeichner 2014 reported on 237 participants younger than 40 years with breast cancer, 42 having CRRM and 195 with No CRRM. Overall survival at 10 years for the CRRM versus no CRRM was HR 2.35 (95% CI 1.02 to 5.41, P = 0.046), with five deaths (11.9%) in the CRRM group versus 51 (26.2%) in the no‐CRRM group (P = 0.05).
5. Mortality: contralateral risk‐reducing mastectomy (CRRM).
| Study | Survival | Follow‐up | Attrition | Study details |
|
Bedrosian 2010 CRRM |
CRRM group = 8902 participants Comparison group = 107,106 patients from SEER diagnosed with BC 1996‐2003 data Disease‐specific survival CRRM was associated with improved disease‐specific survival for women with stages I–III BC (HR of death 0.63, 95% CI 0.57 to 0.69; P < 0.001) On adjusted analysis, the cancer‐related survival associated with CRRM declined with age < 50 years: modest risk reduction (HR of death 0.84, 95% CI 0.72 to 0.97; P = 0.02) > 60 years: no risk reduction from CRRM (HR of death 0.88, 95% CI 0.75 to 1.03; P = 0.13) Disease‐specific mortality Women diagnosed aged < 50 years: Stage I/II ER‐negative BC had a reduction in the risk of associated with CRRM (HR for death 0.68, 95% CI 0.53 to 0.88; P = 0.004) Stage I/II ER‐positive BC had no reduction in the risk of associated with CRRM (HR for death 0.88, 95% CI 0.66 to 1.17, P = 0.38) |
Median 47 months |
Unknown – if participants migrated out of SEER regions they would be missed. | Participants who elected CRRM were more likely to be younger and to have earlier‐stage disease P < .001 for each) The study author writes: “However, despite these efforts, a causal relationship between survival and CRRM cannot be proved, that is only possible in a randomized controlled trial, unlikely to be completed in the foreseeable future.” |
|
Boughey 2010 CRRM |
CRRM group: 128 of 385 died No‐CRRM group: 162 of 385 died 10‐year overall survival: HR 0.68 (95% CI 0.54 to 0.86, P = 0.001) 10‐year survival after multivariate analysis: HR 0.77 (95% CI 0.60 to 0.98, P = 0.03) Disease‐free survival: HR 0.66 (95% CI 0.53 to 0.82, P = 0.0002) Disease‐free survival after multivariate analysis: HR 0.67 (95% CI 0.54 to 0.84, P = 0.0005) BC‐free survival after multivariate analysis: HR 0.82 (95% CI 0.59 to 1.14, P = 0.24) |
Median 17.3 years (< 1‐38.8 years) |
None for overall survival and disease‐free survival. For BC‐free survival, the cause of death for 23 of 128 deaths in CRRM group and 49 of 162 deaths in no‐CRRM group was unknown and these participants were excluded |
“Other variables significant in this (10‐year survival) multivariate model included age and tumor stage, as well as having more than two positive nodes versus negative nodes, and undergoing oophorectomy for a malignancy.” |
|
Brewster 2012 CRRM |
Summary result: "In the adjusted multivariate models, patients who underwent CRRM had longer overall survival than did patients who did not undergo CRRM" Disease‐free survival: All patients adjusted model favored CRRM, with HR 0.75 (95% CI 0.59 to 0.97). All patients matched model did not showed a significant difference, with HR 0.77 (95% CI 0.53 to 1.13). Hormone receptor‐positive adjusted and matched models did not show a significant difference. Hormone receptor‐negative adjusted model favored CRRM, with HR 0.60 (95% CI 0.38 to 0.95), and matched model HR of 0.48 (95% CI 0.22 to 1.01). Overall survival: all patients adjusted model favored CRRM, with HR 0.74 (95% CI 0.56 to 0.99), but matched model was not statistically significant. Hormone receptor‐positive models were not statistically significant. Hormone receptor‐negative adjusted model favored CRRM, with HR 0.58 (0.36‐0.96), but the matched model was not statistically significant Women with clinical Stage I‐III primary unilateral invasive BC |
Median follow‐up overall = 4.5 years. Median follow‐up for CRRM = 4.4 years. Median follow‐up for controls = 4.6 years. | None | All patients adjusted model: adjusted for stage, nuclear grade, hormone receptor status, and chemotherapy history. All patients matched model: matched by propensity score. See Tables 2 and 3 (in paper) for all results. |
|
Chung 2012 CRRM |
Summary result: CRRM was not a significant predictor of overall survival, disease‐free survival, distant metastasis‐free survival, or local recurrence‐free survival See Figure 2 (in paper) for the 4 Kaplan‐Meier survival curves Overall survival curve difference P = 0.415 Disease‐free survival curve difference P = 0.081 Local recurrence‐free survival curve difference P = 0.225 Distant metastasis‐free survival curve difference P = 0.417 Participants had unilateral Stage 0‐III BC |
Median follow‐up = 61 months | None | All‐patients adjusted model: adjusted for stage, nuclear grade, hormone receptor status, and chemotherapy history All‐patients matched model: matched by propensity score. |
|
Evans 2013 CRRM |
All deaths: CRRM = 9/105; controls = 180/473 Deaths from BC: CRRM = 8/105; controls = 150/473 Other deaths: CRRM = 0; controls = 10 10 year survival (%): CRRM and BRRSO = 92% (HR 0.16, 95% CI 0.06‐0.44) CRRM no BRRSO = 83% (HR 0.48, 95% CI 0.19‐1.14) BRRSO no CRRM = 81% (HR 0.46, 95% CI 0.27‐0.78) No surgery = 65% (no HR) Figure 2 shows the survival curves for CRRM vs no‐CRRM groups. Adjusted analysis: "After adjusting for potential confounders only CRRM (HR 0.28, 95% CI 0.14 to 0.55) and BRRSO (HR 0.34, 95% CI 0.21 to 0.55) were independently predictive of improved survival." Table 2 shows the detailed results for CRRM vs no‐CRRM groupings CRRM group: all deaths = 9; deaths from BC = 8; deaths other = 1; 10‐year survival = 89% Control group: all deaths = 26; deaths from BC = 24; other deaths = 2 10‐year survival = 71%. Figure 3 (in paper) shows the survival curves for CRRM vs no CRRM for BRCA1/2 mutation carriers. HR 0.37 (95% CI 0.17 to 0.80), P = 0.008 Women tested positive for pathogenic mutation in BRCA1 or BRCA2 |
Median follow‐up was 9.7 years in the CRRM group and 8.6 in the non‐ CRRM group |
None | The authors noted: "Although women with CRRM had apparently reduced BC and non‐BC mortality this result is potentially confounded by several factors including:
|
|
Goldflam 2004 CRRM |
All‐cause mortality
CRRM group = 14/239 (5.8%) BC‐specific mortality CRRM group = 8/239 (2.5%) |
Mean = 7.8 years or 1846 person‐years | None | It is not clear whether one of the BC deaths was the 1 participant who developed CBC after CRRM or not. The other 7 had to be deaths from the primary cancer |
|
Heemskerk‐Gerritsen 2015 CRRM |
Mortality due to BC in BRCA1/2 participants: CRRM = 4/242; BRCA1/2 controls = 16/341 All‐cause mortality: mortality was lower in the CRRM group (19 in CRRM vs 65 in controls; 21.6 vs 9.6 per person‐years of observation). Cox analysis yielded an HR 0.49 (95% CI 0.29 to 0.82) adjusted for BRRSO 10‐year survival: fewer women died in the CRRM group (8% vs 19%, P < 0.001) 15‐year survival: better in the CRRM group (86%) than in the control group (74%) Survival curves (Kaplan‐Meier) are shown in Figure 1. Time to onset of BC statistically significantly favored CRRM (P logrank < 0.001). Death by all causes favored CRRM (P logrank < 0.119) but not statistically significantly. Figure 2 (in paper) shows the survival curves. Figure 3 (in paper) shows the stratified HR. All of the participants in this study were BRCA1/2 positive |
Median follow‐up for CRRM = 11.4 years Median follow‐up for control = 11.3 years |
None | There is a lack of data on BC‐specific mortality. |
|
Herrinton 2005 CRRM |
All‐cause mortality
CRRM group = 118/908 (13%)
No‐CRRM group = 9971/46,368 (20.5%) HR = 0.60 (95% CI 0.50 to 0.72) BC‐specific mortality CRRM group = 74/908 (8.1%) No‐CRRM group = 5437/46,368 (11.7%) HR 0.57 (95% CI 0.45 to 0.72) |
5.7 years for CRRM 4.8 years for no CRRM |
None | BC mortality was lowest among women diagnosed between ages 40‐49 (HR = 0.77). BC mortality decreased with later year of diagnosis: < 1985: HR = 0.17 1995‐1999: HR = 0.04 Cause of death was unknown for 2 CRRM women (0.2%) and 494 no‐CRRM women (1%) |
|
Jatoi 2014 CRRM |
All‐cause mortality For all participants, all‐cause mortality rate 14.3% 5‐year BC mortality rate 7.9% and 5‐year non‐BC mortality rate 5.7% CRRM was associated with: lower all‐cause mortality (HR 0.83, 95% CI 0.80 to 0.88) lower BC‐specific mortality (HR 0.84, 95% CI 0.79 to 0.89) lower non‐cancer mortality (HR 0.71, 95% CI 0.64 to 0.80) 5‐year hazard of death Association between CRRM and lower BC‐specific, overall, and non‐cancer mortality persists even after adjusting for stage Women diagnosed with ductal or lobular BC Stage I‐III |
5 years | N/A | Validity of observational studies addressing effect of CRRM on BC mortality remains an important consideration. The relationship between CRRM and non‐cancer mortality was stronger than either all‐cause or BC‐specific mortality, suggesting an underlying selection bias for treating potentially healthier women with CRRM |
|
Kiely 2010 CRRM |
All‐cause survival at last follow‐up: CRRM group: 144 of 154 women (93.5%) were alive No‐CRRM group: 800 of 864 women (92.6%) were alive |
Median 11.1 years; 8 years for CRRM group 11.7 for no‐CRRM group |
None | Confounding factor BRRSO: CRRM group: 86 of 154 women (59%) also had BRRSO No‐CRRM group: 240 of 864 women (24%) also had BRRSO |
|
King 2011a CRRM |
BC‐free survival: At last follow‐up: CRRM group: 91% alive without disease No‐CRRM group: 84% alive without disease Kaplan‐Meier analysis P = 0.02 Multivariate Cox regression, adjusting for age and treatment factors (chemotherapy, radiotherapy, and MRI) demonstrated no difference in subsequent BC event rates between groups (P = 0.23) |
Median 4.4 years (0.18‐11.70 years) for CRRM group and 6.8 years (0.33‐12.20 years) for no‐CRRM group |
None | |
|
Kruper 2014 CRRM |
CRRM when compared to no CRRM was associated with: improved disease‐specific survival (DSS) (HR 0.86, 95% CI 0.79 to 0.93) and greater overall survival (OS) (HR 0.76, 95% CI 0.71 to 0.81) Participants diagnosed from 2007‐2010 had improved DSS (HR 0.87, 95% CI 0.78 to 0.98) and OS (HR 0.89, 95% CI 0.81 to 0.98) compared with those diagnosed 1998‐2006 CRRM decreased risk of overall death by 24%. 3‐, 5‐, and 10‐year DSS and OS were greater for CRRM vs No CRRM Removing CBC cases from analysis had little impact on CRRM DSS (HR 0.86, 95 % CI 0.79 to 0.93) and OS (HR 0.77, 95% CI 0.72 to 0.82) suggesting that prevention of CBC by CRRM does not explain the observed survival benefit. |
NA | N/A | Diagnosis time period divided into 1998‐2006 and 2007‐2010 to control for 11/2006 adoption of use of trastuzumab in adjuvant setting. CRRM rates increased from 5% in 1998 to 28% in 2010; part of the increase may reflect changes in coding in SEER data (possible Reporting bias). Differences across groups in OS were greater than group differences in DSS, consistent with selection bias. Possible that observed survival benefits may be result of healthier people choosing or being recommended for CRRM rather than actual benefit of CRRM over SM. |
|
Lee 1995 CRRM |
15‐year disease‐specific survival
CRRM or biopsy = 105 participants
No CRRM (surveillance) = 299 There was a statistically significant 15‐year survival advantage in CRRM or biopsy (P = 0.01 after adjusting for age) |
Mean = 6 years Median = 5.3 years | None | Participants had unilateral ILC. Participants in the CRRM group were significantly younger and a significantly greater proportion had multifocal lesions than in the no‐CRRM group. Results were age adjusted. Those getting CRRM and those only getting biopsies were lumped together the 'treatment group.' There are no statistical analyses of just the CRRM group alone. |
|
Leis 1981 CRRM |
Disease‐Free Survival: Among the 58 patients followed for 10 or more years, the no‐evidence‐of‐disease survival was 93.1% (54 of 58) |
10 years | 68 of 127 patients lost to follow‐up before 10 years | |
|
Metcalfe 2014 CRRM |
Overall survival of BRCA1/2+ or high risk at 20 years CRRM 88% (95% CI 83% to 93%) No CRRM 66% (59% to 73%) The adjusted HR for women with CRRM associated with 48% reduction in death from BC (0.52, 95% CI 0.29 to 0.93, P = 0.03). Propensity score‐adjusted analysis of 79 matched pairs (CRRM vs no CRRM), the association was not significant (HR 0.60, 95% CI 0.34 to 1.06, P = 0.08). Adjusted HR for CRRM compared with No CRRM was 0.58 (0.34 to 0.97, P = 0.04 for entire study period and 0.36 (0.13 to 0.96, P = 0.04) for the second 10 years of follow‐up. The association between contralateral mastectomy and death from BC in the first 10 years from diagnosis was not statistically significant in either the univariant or multivariable analysis. 20‐year BC‐specific mortality for no CRRM = 31%; CRRM women had a 48% reduction in risk of mortality vs no‐CRRM women over 20‐year period. Women with a family history of Stage I or II BC at 65 or less and BRCA1/2 mutation carriers |
The median follow‐up time was 14.3 years (range 0.1‐20.0 years); Mean was 13.0 years | Average time from diagnosis to CRRM was 2.3 years. Mean time to death from diagnosis 7.1 years (range 0.7 to 19.3 years). Some of the CBC cases were diagnosed within 1‐2 months (0.01 years) of original diagnosis of BC; they should be classified as bilateral BC as they occurred within less time than the commonly used definition of CBC as being breast cancer in the contralateral breast ≤ 6 months after the primary BC This classification then could have overstated the incidence of CBC in the no‐CRRM group. |
|
|
Peralta 2000 CRRM |
Disease‐Free Survival: At 15 years disease‐free survival (contralateral or primary): CPM group = 55% (95% C.I. 38%‐69%) Controls = 28% (95% C.I. 19%‐36%) (P=0.01). 15‐year all‐cause survival CRRM group 64% (41/64) (95% CI 45% to 78%) No‐CRRM group 49% (87/182) (95% CI 39% to 58%) P = 0.26 15‐year disease‐specific survival in women with Stage 0, I or II BC CRRM group 71% (45/64) (95% CI 52% to 84%) No CRRM (surveillance) 53% (96/182) (95% CI 42% to 62%) P = 0.06 |
Median
6.2 years for CRRM group 6.8 years for no‐CRRM group |
None | Comparison group participants were matched for age, stage of disease at diagnosis, presence of LCIS, chemotherapy and tamoxifen therapy from among 2852 participants who underwent mastectomy between 1 January 1973 and 30 September 1998 at 1 institution. 71% having CRRM had immediate reconstruction |
|
Pesce 2014 CRRM |
CRRM had better survival than unilateral mastectomy, without adjustments (P < 0.001) Unadjusted Kaplan‐Meier survival curve showed CRRM was statistically significantly better than unilateral mastectomy (P = 0.0002). (Figure 1 in the paper) There was no statistically significant difference inoverall survival between CRRM and unilateral mastectomy after adjusting for various factors. HR 0.93 (95% CI 0.79 to 1.09, P = 0.38). The adjustment factors were: age, race, insurance status, co‐morbidities, year of diagnosis, facility type, facility location, ER status, tumor size, node status, grade, histology, and use of adjuvant radiation and chemo‐hormonal therapy. See Figure 3 in the paper. Unadjusted Kaplan‐Meier survival curve showed no difference between groups for ER‐negative patients (P = 0.432). (Figure 2 in the paper) |
Median follow‐up = 6.1 years | None | |
|
Van Sprundel 2005 CRRM |
All‐cause mortality in BRCA1/2 carriers
CRRM survival = 94%
Surveillance group survival = 77%
P = 0.03 BC mortality in BRCA1/2 carriers BC‐specific survival was not significantly better in the CRRM group without BRRSO P = 0.11) Participants who had CRRM and BRRSO had significantly better survival than those who did not have BRRSO: all‐cause survival: HR 0.12 (95% CI 0.03 to 0.46) BC survival: HR 0.16 (95% CI 0.04 to 0.61) |
Mean = 3.5 years | None | Significant overall survival advantage in CRRM group mostly due to higher mortality related to primary BC and ovarian cancer in surveillance group CRRM effect on overall survival not significant in participants who had BRRSO after adjustment for BRRSO; only BRRSO led to significant improvement of overall survival |
|
Zeichner 2014 CRRM |
237 participants < 40 years with BC, 42 CRRM, 195 no CRRM. CRRM participants had significantly smaller tumors (0‐2 cm. 41.7% vs 24.8%, P = 0.04) Overall 5‐ and 10‐year disease‐free survival for the 42 CRRM participants was 81.3% and 73.3%, respectively The 5‐ and 10‐year breast‐cancer specific overall survival for the 42 CRRM participants was 86.1% and 77.6%, respectively Overall survival at 10 years for CRRM vs no CRRM HR 2.35 (95% CI 1.02 to 5.41) P = 0.046 Participants in the CRRM group had 5 deaths (11.9%) vs 51 (26.2%) P = 0.05) Participants were women with BC age < 40 |
Median = 93 months (1‐383 months) | N/A | There are major differences in follow‐up time that could contribute to detection bias. 95.2% of CRRM participants were followed for 3‐13 years vs only 30% of the no‐CRRM. 60% of the no‐CRRM participants were followed for 13‐23 years vs only 4.8% of CRRM participants. Thus the no‐CRRM participants had longer to be reported dead |
BC: breast cancer BRRSO: bilateral risk‐reducing salpingo‐oophorectomy CBC: contralateral breast cancer CI: confidence interval CRRM: contralateral risk‐reducing mastectomy ER: estrogen receptor HR: hazard ratio ILC: invasive lobular cancer LCIS: lobular carcinoma in situ MRI: magnetic resonance imaging OS: overall survival RR: relative risk
Brewster 2012 studied 532 women who had CRRM versus 335 women with no CRRM, resulting in variable findings. The all‐patients adjusted model favored CRRM for overall survival, with HR 0.74 (95% CI 0.56 to 0.99), but the matched model (which used 497 CRRM versus 497 no CRRM) was not statistically significant, with HR 0.77 (95% CI 0.53 to 1.13). However, in the adjusted multivariate models, participants who underwent CRRM had longer overall survival than did participants who did not. Hormone receptor‐positive adjusted and matched models were not statistically significant; the hormone receptor‐negative adjusted model favored CRRM, with HR 0.58 (95% CI 0.36 to 0.96).
Chung 2012 reported on women with Stage 0 to III unilateral breast cancer, 177 and non‐breast cancer in the CRRM group versus 178 controls. The overall survival curve difference was P = 0.415. CRRM was not a significant predictor of overall survival.
Kiely 2010 reported on 1018 women with a family history of breast cancer with a median follow‐up of 11.1 years (eight years for 154 in the CRRM group and 11.7 years for the non‐CRRM group). At last follow‐up, there was no apparent difference in survival as 144 of 154 women in the CRRM group (93.5%) and 800 of 864 women (92.6%) in the non‐CRRM group were alive.
In a retrospective case control study of 25,961 women who had CRRM in the first course of treatment for breast cancer and 423,217 women treated for breast cancer but no CRRM, Jatoi 2014 found that for all participants, the all‐cause mortality rate was 14.3% with five years of follow‐up (breast cancer mortality rate was 7.9% versus the non‐breast cancer mortality rate of 5.7%). CRRM was associated with lower all‐cause mortality (HR 0.83, 95% CI 0.80, 0.88).
Kruper 2014 reported on 26,526 women from the SEER database with unilateral breast cancer who had CRRM and 138,826 who had no CRRM. When comparing CRRM to no CRRM, there was greater overall survival (HR 0.76, 95% CI 0.71 to 0.81) for CRRM women. Participants diagnosed with breast cancer from 2007 to 2010 had improved overall survival (HR 0.89, 95% CI 0.81 to 0.98) compared with those diagnosed 1998 to 2006. CRRM decreased the risk of overall death by 24%; 3‐, 5‐, and 10‐year overall survival was greater for CRRM women versus no CRRM. However, removing contralateral breast cancer cases from the analysis had little impact on CRRM overall survival (HR 0.77, 95% CI 0.72 to 0.82), suggesting that prevention of contralateral breast cancer by CRRM does not explain the observed survival benefit.
Peralta 2000 reported data on 246 participants with at least one first‐ or second‐degree relative with breast cancer for overall survival at 15 years. Overall survival for participants having CRRM was 64% (41 of 64) versus 48% (87 of 182) for those in the comparison group after controlling for multiple prognostic factors. This difference was not significant (P = 0.26).
Pesce 2014 also used a large database (the USA National Cancer Database) to identify 10,289 women who had unilateral treatment mastectomy (UM) and 4338 who had CRRM. Those with CRRM had better survival than UM without adjustments (P < 0.001); the unadjusted Kaplan‐Meier survival curve showed CRRM was statistically significantly better than UM (P = 0.0002). However, there was no statistically significant difference in overall survival between CRRM and UM after adjusting for various factors such as age, race, insurance status, co‐morbidities, year of diagnosis, facility type, facility location, ER status, tumor size, node status, grade, histology, and use of adjuvant radiation and chemo‐hormonal therapy (HR 0.93, 95% CI 0.79 to 1.09, P = 0.38).
BRCA1 and BRCA2 mutations
Evans 2013 reported on BRCA1/2 mutation carriers with breast cancer: 105 women had CRRM, 473 had No CRRM, and 120 BRRSO only. All deaths were: CRRM = 9/105, controls = 180/473 after a median follow‐up of 9.7 years in the CRRM group and 8.6 in the non‐CRRM group with a HR 0.37 (95% CI 0.17 to 0.80, P = 0.008.) The 10‐year survival figures were: CRRM and BRRSO = 92% (HR 0.16, 95% CI 0.06 to 0.44); CRRM no BRRSO = 83% (HR 0.48, 95% CI 0.19 to 1.14); BRRSO no CRRM = 81% (HR 0.46, 95% CI 0.27 to 0.78); no CRRM = 65% (no HR). In the adjusted analysis, after adjusting for potential confounders, only CRRM (HR 0.28, 95% CI 0.14 to 0.55) and BRRSO (HR 0.34, 95% CI 0.21 to 0.55) were independently predictive of improved survival.
In the Heemskerk‐Gerritsen 2015 study of BRCA1/2‐positive women, all‐cause mortality was lower in the CRRM group (242 women) than the control (surveillance; 341 women) group, with 19 in CRRM versus 65 in controls, 21.6 versus 9.6 per person‐years of observation. For 10‐year survival, fewer women died in the CRRM group (8% versus 19%, P < 0.001); the 15‐year survival was also better in the CRRM group (86%) than in the control group (74%). In the survival curves (Kaplan‐Meier), death by all causes favored CRRM (P logrank < 0.119) but was not statistically significant.
Metcalfe 2014 showed, in a study of 390 women with Stage I or II breast cancer, carriers of BRCA1/2 mutations or untested with a family history of breast cancer and/or BRCA1/2 mutations (181 with CRRM and 209 with unilateral mastectomy only), that the overall survival of BRCA1/2 or high‐risk women at 20 years' follow‐up was CRRM 88% (95% CI 83% to 93%) and 66% (95% CI 59% to 73%) for no CRRM. However, with the propensity score adjusted analysis of 79 matched pairs (CRRM versus no CRRM), the association was not significant (0.60, 0.34 to 1.06, P = 0.08). The adjusted HR for CRRM compared with no CRRM was 0.58 (95% CI 0.34 to 0.97, P = 0.04) for the entire study period and 0.36 (95% CI 0.13 to 0.96, P = 0.04) for the second period of 10 to 20 years of follow‐up.
A study of BRCA1/2 carriers (Van Sprundel 2005) showed improved survival for CRRM participants (94% versus 77%, P = 0.03) but this was mostly due to higher mortality related to primary breast cancer and ovarian cancer. After adjusting for BRRSO, the CRRM effect on all‐cause mortality was no longer significant.
2. Breast cancer (disease‐specific) mortality
Nine studies (Bedrosian 2010; Evans 2013; Goldflam 2004; Herrinton 2005; Jatoi 2014; King 2011a; Lee 1995; Metcalfe 2014; Peralta 2000) provided data on breast cancer mortality, and the results were inconsistent among studies (see Table 5). Five studies were retrospective cohort studies (Herrinton 2005; King 2011a; Lee 1995; Metcalfe 2014; Peralta 2000) comparing women who had chosen CRRM to a group of women who had elected not to undergo CRRM. Bedrosian 2010 compared his results to SEER data. Evans 2013; King 2011a; Peralta 2000; Herrinton 2005 and Metcalfe 2014 attempted to balance the two groups by adjustments in the analysis for multiple confounders.
Bedrosian 2010 found that CRRM was associated with improved disease‐specific survival in specific patient populations: for women with stages I‐III breast cancer (HR of death 0.63, 95% CI 0.57 to 0.69, P < 0.001); on adjusted analysis, the cancer‐related survival associated with CRRM declined with age; women under 50 years of age had a modest reduction (HR of death 0.84, 95% CI = 0.72 to 0.97, P = 0.02); whereas women aged over 60 years had no risk reduction (HR of death 0.88, 95% CI 0.75 to 1.03, P = 0.13); women diagnosed at under 50 years of age with Stage I or II estrogen receptor (ER)‐negative breast cancer had a reduction in risk with CRRM (HR for death 0.68, 95% CI 0.53 to 0.88, P = 0.004); in contrast, women with stage I or II ER‐positive breast cancer and CRRM had no reduction in risk (HR for death 0.88, 95% CI 0.66 to 1.17, P = 0.38).
Goldflam 2004 reported a breast cancer mortality rate of 2.5% (8/239) in women with Stage 0 to II breast cancer after a mean follow‐up of 7.8 years.
Herrinton 2005 reported a significant difference in breast cancer mortality (HR 0.57, 95% CI 0.45 to 0.72) in comparing CRRM women (74/908 (8.1%)) and no‐CRRM women (5437/46,368 (11.7%)) after approximately five years.
Jatoi 2014 found that CRRM (25,961/423,217 women with Stage I to III breast cancer) was associated with lower breast cancer‐specific mortality (HR 0.84, 95% CI 0.79 to 0.89) and lower non‐cancer mortality (HR 0.71, 95% CI 0.64 to 0.80), five‐year hazard of death. The association between CRRM and lower breast‐cancer specific mortality persisted even after adjusting for stage.
King 2011a showed that 91% (383/407) of CRRM women with Stage 0 to II breast cancer were alive without disease at median follow‐up of 4.4 years and 84% (2297/23,572) of the no‐CRRM group were alive after median follow‐up of 6.8 years (Kaplan‐Meier analysis P = 0.02). However, after multivariate Cox regression, adjusting for age and treatment factors (chemotherapy, radiotherapy, and magnetic resonance imaging (MRI)) demonstrated no difference in subsequent breast cancer event rates between groups (P = 0.23).
Lee 1995 reported a significant survival advantage for those who had CRRM or biopsy in the contralateral breast at 15 years' follow‐up.
At 15 years of follow‐up, Peralta 2000 found that there was a tendency toward improved disease‐specific survival (P = 0.06) only among the subgroup of participants with initial diagnoses of Stage 0, I or II breast cancer: 71% (95% CI 52% to 84%) versus 53% (95% CI 42% to 62%).
BRCA1 and BRCA2 mutations
Evans 2013 reported results as follows for the CRRM group (105 participants) of BRCA1/2 mutation carriers, all deaths = 9 of 105 participants, deaths from breast cancer = 8/105; deaths from other cause = 1. The 10‐year survival was 89%. In the control group (473 participants), all deaths = 26, deaths from breast cancer = 24, and other deaths = 2. The 10‐year survival was 71%.
Metcalfe 2014 showed that the adjusted hazard ratio for women with CRRM and BRCA1/2 mutations or high risk was associated with a 48% reduction in death from breast cancer (HR 0.52, 95% CI 0.29 ‐ 0.93, P = 0.03). However, the propensity score adjusted analysis of 79 matched pairs (CRRM versus no CRRM), the association was not significant (HR 0.60, 0.34 to 1.06, P = 0.08). The association between CRRM and death from breast cancer in the first 10 years from diagnosis was not statistically significant in either the univariable or multivariable analysis. However, the 20‐year breast cancer‐specific mortality for no CRRM was 31%; CRRM women had a 48% reduction in risk of mortality versus no‐CRRM women over a 20‐year period.
3. Disease‐free survival/recurrence
Eleven studies (Boughey 2010; Brewster 2012; Chung 2012; Heemskerk‐Gerritsen 2015; Kiely 2010; King 2011a; Kruper 2014; Leis 1981; Peralta 2000; Van Sprundel 2005;Zeichner 2014) reported varying results on data for disease‐free survival (see Table 5). Follow‐up intervals were not standardized in any of these studies for the groups except for Brewster 2012 and Heemskerk‐Gerritsen 2015. Heemskerk‐Gerritsen 2015 also adjusted the analysis for the confounder of BRRSO. Therefore, no disease‐free survival estimate has attempted to minimize the potential detection bias of "the more frequently one looks, the more chances of finding something". Conversely, if one does not look, it can appear that the person has not relapsed (Johnson 2003).
In terms of disease‐free survival in Brewster 2012, the all‐patients adjusted model favored CRRM, with HR 0.75 (0.59 to 0.97), while the all‐patients matched model did not show a significant difference, with HR 0.77 (0.53 to 1.13). Hormone receptor‐positive adjusted and matched models did not show a significant difference; the hormone receptor‐negative adjusted model favored CRRM, with HR 0.60 (95% CI 0.38 to 0.95) and the matched model HR of 0.48 (95% CI 0.22 to 1.01).
Boughey 2010 showed that breast cancer recurrence was 24% (104 of 385) in the CRRM group as opposed to 32% (123 of 385) in the no‐CRRM group after 17.3 years of follow‐up. There were 148 breast cancer events, including local and distant recurrences and death in the CRRM group plus 201 events among the no‐CRRM group, the difference being statistically significant (HR 0.66, 95% CI 0.53 to 0.82, P = 0.0002). It remained significant after multivariate analysis (HR 0.67, 95% CI 0.54 to 0.84, P = 0.0005).
Chung 2012 was a retrospective study that looked at 177 women with CRRM and 178 controls with a median follow‐up of 61 months. CRRM was not a significant predictor of overall survival, disease‐free survival, distant metastasis‐free survival or local recurrence‐free survival. The Kaplan‐Meier survival curves showed the disease‐free survival curve difference P = 0.081. Both the local recurrence‐free survival curve difference (P = 0.225) and the distant metastasis‐free survival curve difference (P = 0.417) were not statistically significant between the groups.
Kiely 2010 reported a systemic recurrence rate of 6.2 per 1000 women‐years for CRRM women and 10.4 per 1000 women‐years for non‐CRRM women (P = 0.04). However, there was a confounding factor that, in the CRRM group, 86 of 154 women (59%) had also had BRRSO and only 240 of 864 women in the non‐CRRM (24%) had also had BRRSO.
King 2011a found that, at last follow‐up for women with CRRM, 91% were alive without disease as opposed to those without CRRM (84% alive, P = 0.02).
CRRM when compared to no CRRM was associated with improved disease‐specific survival in Kruper 2014 (HR 0.86, 95% CI 0.79 to 0.93). Women diagnosed from 2007 to 2010 had improved disease‐specific survival (HR 0.87, 95% CI 0.78 to 0.98) compared with those diagnosed 1998 to 2006. Disease‐specific survival at three, five, and 10 years was greater for CRRM versus no CRRM. However, removing the contralateral breast cancer cases from the analysis had little impact on CRRM disease‐specific survival (HR 0.86, 95% CI 0.79 to 0.93), suggesting that the prevention of contralateral breast cancer by CRRM does not explain the observed survival benefit.
Leis 1981 reported in a case series that, among 58 women who were followed for 10 or more years, disease‐free survival was 93.1% (54 of 58). Data were not reported for the 68 women who received CRRM but were not followed for at least 10 years.
Peralta 2000 reported that at 15 years, disease‐free survival for the group receiving CRRM was 55% (95% CI 38% to 69%) compared to 28% for the control group (95% CI 19% to 36%). The difference was statistically significant (P = 0.01).
The overall five‐ and 10‐year disease‐free survival for the 42 CRRM participants in Zeichner 2014 was 81.3% and 73.3%, respectively. However, compared to the 195 no‐CRRM participants, the CRRM participants had significantly smaller tumors (0 cm to 2 cm; 41.7% versus 24.8%, P = 0.04).
BRCA1 and BRCA2 mutations
In a study of BRCA1/2 mutation carriers, Van Sprundel 2005 found there was no improved survival (P = 0.11) in the CRRM group without BRRSO. Participants who had CRRM and BRRSO had significantly better disease‐free survival (HR 0.16, 95% CI 0.04 to 0.61) than those who did not have BRRSO.
Heemskerk‐Gerritsen 2015 also followed women with BRCA1/2 mutations, 242 women with CRRM and 341 without (controls), with a median follow‐up for CRRM of 11.4 years and for controls of 11.3 years. Time to onset of breast cancer statistically significantly favored CRRM (P logrank < 0.001). Cox analysis adjusted for BRRSO for mortality yielded a HR 0.49 (95% CI 0.29 to 0.82) which favors CRRM.
4. Incidence of breast cancer
Seventeen studies (Bedrosian 2010; Boughey 2010; Brewster 2012; Chung 2012; Contant 2002; Evans 2013; Goldflam 2004; Heemskerk‐Gerritsen 2013; Herrinton 2005; Kass 2010; Kiely 2010; King 2011a; Kruper 2014; McDonnell 2001; Peralta 2000; Van Sprundel 2005;Zeichner 2014) reported data for contralateral breast cancer incidence after CRRM, with 11 having controls showing significantly lower breast cancer incidence in those who had CRRM (see Table 6).
6. Incidence in contralateral breast: contralateral risk‐reducing mastectomy (CRRM).
| Study | Incidence | Follow‐up time | Attrition | Study details | |
|
Bedrosian 2010 CRRM |
Incidence of CBC In women with early‐stage ER‐negative disease, the cumulative incidence of CBC was: CRRM group 0.16% No‐CRRM group 0.90% P = 0.05 In women with early‐stage ER‐positive cancer, the cumulative incidence of CBC was: CRRM 0.13% no CRRM 0.46% P = 0 .07 |
Median 47 months |
Unknown – if women migrated out of SEER regions they would be missed | Study author theorizes that lower baseline risk of CBC in early‐stage ER‐positive women may account for the lack of benefit associated with CRRM in young women with early‐stage ER‐positive disease. The study author anticipates that with longer durations of follow‐up, the benefit associated with CRRM in ER‐negative women will increase. |
|
|
Boughey 2010 CRRM |
Incidence of CBC: CRRM group: 2 of 385 No CRRM: 31 of 385 HR 0.05 (95% CI 0.01 to 0.22, P = 0.0001) Incidence of CBC after multivariate analysis: HR 0.05 (95% CI 0.01 to 0.19, P = 0.0001) BCrecurrence: CRRM group: 104 of 385 No‐CRRM group: 123 of 385 |
Median 17.3 years (< 1‐38.8 years) |
None | CBC multivariate analysis adjusted for age, stage, nodal status and 1st‐degree family history | |
|
Brewster 2012 CRRM |
Incidence of CBC 1/532 CBC in CRRM group. 67/335 CBC in control group Participants women with clinical Stage I‐III primary unilateral invasive BC |
Median Overall = 4.5 years CRRM = 4.4 years Controls = 4.6 years |
None | Participants were women with clinical Stage I‐III primary unilateral invasive BC | |
|
Chung 2012 CRRM |
Incidence of CBC 0/177 of CRRM participants developed CBC 3/178 control group women developed CBC |
Median = 61 months (range 2‐171 months) |
None | Overall, there were 68 of 355 participants (19.1%) with ductal carcinoma in situ, 148 of 355 (41.7%) with Stage I invasive BC, 138 of 355 (38.9%) with Stage II, and only 1 of 355 (0.003%) presented with Stage III disease | |
|
Contant 2002 CRRM |
Incidence of CBC CRRM: 5 of 29 (17.2%) with previous BC had visceral metastatic disease | Median 2.8 years | None | The study author did not report which of the 29 participants also had BRRSO It was not reported whether the 5 women with disease had CBC or recurrence from their primary disease |
|
|
Evans 2013 CRRM |
Incidence of CBC CRRM group: CBC = 6/105 (5.7%) Control group: CBC = 35/473 (7.4%) |
Median CRRM = 8.8 years. Medical follow‐up for non‐CRRM group = 7.3 years |
None | ||
|
Goldflam 2004 CRRM |
Incidence of CBC
CRRM: 1/239 developed CBC (0.4%) |
Mean 7.8 years (1846 person‐years) | None | Risk factors for CBC determined using Gail model; information on risk obtained for 157 of 239 participants. Median 5‐year risk was 1.3% (0.2‐12.2%) 48 had risk of ≥ 1.67% with 58.6% of these participants having a family history of BC. Used 2 methods to calculate the number of expected CBC without CRRM:
> 90% reduction in incidence of clinically detected CBC from that expected |
|
|
Heemskerk‐Gerritsen 2015 CRRM Follow‐up to Heemskerk‐Gerritsen 2007 |
Incidence of CBC CRRM group = 4/242 Controls = 64/341 |
Median CRRM = 11.4 years Control = 11.3 years |
None | All of the participants in this study were BRCA1/2 positive | |
|
Herrinton 2005 CRRM |
Incidence of CBC
CRRM: 5/1072
No CRRM: 69/317 HR = 0.03 (95% CI 0.006‐0.13) |
Median
5.7 years for CRRM 4.8 years for no CRRM |
None | Women without CRRM who developed CBC were over‐sampled by age and outcome for the no‐CRRM group to maintain the power of the study but avoid the cost of collecting detailed covariate information from 55,328 charts, resulting in 317 participants. The 69 no‐CRRM participants who developed CBC were over‐sampled by a factor of 10 | |
|
Kass 2010 CRRM |
BC incidence: BRCA1/2 + CRRM group: 1 of 107 |
Mean CRRM BRCA1 carriers was 5.8 years (SE 3.4) versus 4.2 years (SE 3.0) in BRCA2 carriers |
None | ||
|
Kiely 2010 CRRM |
Incidence of CBC: CRRM: 1 of 154 had a chest wall event No CRRM: 177 of 864 women had a contralateral BC event (invasive or in situ) P < 0.0001 Recurrence: Systemic recurrence rate: CRRM group: 6.2 per 1000 women‐years No‐CRRM group: 10.4 per 1000 women‐years P = 0.04 |
Median 11.1 years; 8 years for CRRM group and 11.7 for no‐CRRM group |
None | Confounding factor BRRSO: CRRM group: 86/154 women (59%) also had BRRSO No‐CRRM group: 240/864 women (24%) also had BRRSO |
|
|
King 2011a CRRM |
Incidence of CBC: CRRM group: 0 of 407 No‐CRRM group: 14 of 2572 P = 0.02 Multivariate Cox regression, adjusting for age and treatment factors (chemotherapy, radiotherapy, and MRI) demonstrated no difference in subsequent BC event rates between groups (P = 0.23) |
Median CRRM group 4.4 years (0.18‐11.70 years) No‐CRRM group 6.8 years (0.33‐12.20 years) |
At the last follow‐up, 91% of the participants in the CRRM group and 84% of the participants in the non‐CRRM group were alive without disease | “In our series, the incidence of CBC among women not having CRRM (0.5%) was 17‐fold less than the incidence of distant metastases (7%) and seven‐fold less than the incidence of loco‐regional recurrence (3%).” | |
|
Kruper 2014 CRRM |
Incidence of CBC Occurred in 1.6% (829) of cohort |
N/A | N/A | ||
|
Leis 1981 CRRM |
Incidence at 10 years CRRM: 4/58 No comparison group | 10 years | 69/127 (54%) participants not accounted for at 10 years | 25 of 127 (19.7%) had unsuspected cancer in the contralateral breast at the time of CRRM; 11 were invasive and 14 were non‐invasive | |
|
McDonnell 2001 CRRM |
Incidence of CBC Premenopausal women Adjusted for adjuvant therapy and tamoxifen CRRM: 6/388 Comparison group statistically simulated occurrences expected = 106.2/388 Risk reduction = 94.4% (95% CI 87.7% to 97.9%) Not adjusting for adjuvant therapy or tamoxifen CRRM: 6/388 Comparison group statistically simulated occurrences expected = 115/388 Risk reduction = 94.8% (95% CI 88.6% to 98.1%) Postmenopausal women Adjusted for adjuvant therapy and tamoxifen CRRM: 2/357 Comparison group statistically simulated occurrences expected = 50.3/357 Risk reduction = 96.0% (95% C.I. 85.6% to 99.5%) Not adjusting for adjuvant therapy or tamoxifen: CRRM 54/357 Comparison group statistically simulated occurrences expected = 54/357 Risk reduction = 96.3% |
Median
10 years 98% of participants were followed at least 2 years |
None | Total occurrences of contralateral cancers among all women was 8/745. 742 of the participants fit the Anderson model definition of positive family history, which requires one of the 3 types of pedigrees: parent affected, sibling affected or 2nd‐degree relative affected. 3 women who developed CBC after CRRM and whose family pedigree was unclear were included to make the calculated risk reductions conservative. The median time from mastectomy to development of BC was 2 years (range 1‐18 years) "Adjusted for treatment" means adjusted for adjuvant therapy and tamoxifen. Comparison group was statistically simulated using age‐adjusted life tables. 4 of the cancers were diagnosed within 2 years of CRRM, suggesting that the cancer may have been present but not detected at that time. |
|
|
Metcalfe 2014 CRRM |
Incidence of CBC Women from BRCA1/BRCA2 mutation carrier families diagnosed with Stage1/2 from 1975‐2000, age < 66, and were mutation carriers or untested CRRM: 1/146 No CRRM: 97/336 HR = 0.03, P = 0.0005 There was a moderate decrease in risk of CBC associated with use of Tamoxifen (HR = 0.59; 95% CI 0.035, 1.01; P = 0.05). BRRSO was associated with a 59% reduction in the risk of CBC (HR = 0.41; 95% CI 0.18 to 0.90) |
Mean 9.2 years | Mean interval from diagnosis of first BC to diagnosis of CBC was 5.5 years (0.1 to 16.2) for no‐CRRM women. The 5‐year actuarial risk for CBC without CRRM was 16.9% (95% CI 10.5%, 23.2%) and the 10 year actuarial risk was 29.5% (95% CI 20.6%, 38.3%). There was a moderate decrease in risk of CBC associated with use of Tamoxifen (HR = 0.59; 95% CI 0.035, 1.01; P = 0.05). Oophorectomy was associated with a 59% reduction in the risk of CBC (HR = 0.41; 95% CI 0.18, 0.90). |
||
|
Peralta 2000 CRRM |
Incidence of BC CRRM: 0/64 No CRRM (surveillance): 36/182 Risk reduction 0.04 (95% CI 0.00 to 0.62), P = 0.02 |
Median
6.2 years for CRRM group 6.8 years for no‐CRRM group |
None | Comparison group participants were matched for age, stage of disease at diagnosis, presence of LCIS, chemotherapy and tamoxifen therapy from among 2852 women who underwent mastectomy between 1 January 1973 and 30 September 1998 at 1 institution 71% had immediate reconstruction |
|
|
Van Sprundel 2005 CRRM |
Incidence of CBC BRCA1 or BRCA2 mutation CRRM: 1/75 (1.3%) Surveillance group: 6/43 (14.0%) P < 0.001 |
Mean 3.5 years | None | CRRM reduced risk of CBC by 91% independent of the impact of BRRSO. Period from diagnosis of first BC until end of follow‐up was 7.4 years for the CRRM group and 10.5 years for the surveillance group |
|
|
Zeichner 2014 CRRM |
Incidence of CBC CRRM: 6/42 (14.3%) No CRRM: 60/195 (30.8%) P = 0.03 Participants were women with BC aged < 40 years |
Median 93 months (1‐383 months) |
None | There were major differences in follow‐up time that could contribute to detection bias. 95.2% of CRRM participants were followed for 3‐13 years vs only 30% of the no‐CRRM. 60% of the no‐CRRM participants were followed for 13‐23 years vs only 4.8% of CRRM participants |
BC: breast cancer BRRSO: bilateral risk‐reducing oophorectomy CBC: contralateral breast cancer CRRM: contralateral risk‐reducing mastectomy ER: estrogen receptor HR: hazard ratio ILC: invasive lobular cancer LCIS: lobular carcinoma in situ MRI: magnetic resonance imaging RR: relative risk SE: standard error
Bedrosian 2010 used SEER data and found that, in women with early‐stage ER‐negative cancer and CRRM, the cumulative incidence of contralateral breast cancer was 0.16% as opposed to the no‐CRRM group that was 0.90% (P = 0.05). In women with early‐stage ER‐positive cancer, the cumulative incidence of contralateral breast cancer was 0.13% for the CRRM group and 0.46% in the no‐CRRM group (P = 0.07).
Boughey 2010 found that the incidence of contralateral breast cancer in the CRRM group was two out of 385 women, and in the no‐CRRM group, it was 31 of 385 women (HR 0.05, 95% CI 0.01 to 0.22, P = 0.0001). The incidence of contralateral breast cancer after multivariate analysis was HR 0.05 (95% CI 0.01 to 0.19, P = 0.0001).
In Brewster 2012, there was one incidence of contralateral breast cancer in the CRRM group of 532, and 67 contralateral breast cancer in the control group of 335.
Chung 2012 found that three out of 178 control‐group women developed contralateral breast cancer versus none of the 177 CRRM women.
Herrinton 2005 and Metcalfe 2014 both found a HR of 0.03 for their CRRM treatment groups.
Kiely 2010 found the incidence of contralateral breast cancer in the CRRM group was one chest wall event in 154 participants versus 177 of 864 women without CRRM who had an invasive or in situ event (P < 0.0001).
King 2011a found no incidence of contralateral breast cancer in the CRRM group of 407 women, and 14 of 2572 (P = 0.02) in the no‐CRRM group. However, multivariate Cox regression adjusting for age and treatment factors (chemotherapy, radiotherapy, and MRI) showed no difference in subsequent breast cancer event rates between the groups (P = 0.23).
Kruper 2014 found that contralateral breast cancer occurred in 1.6% (829) of the cohort.
McDonnell 2001 reported on a case series of 745 women (388 premenopausal, 357 postmenopausal) who underwent CRRM and were followed for a median of 10 years. Eight of these women later developed breast cancer in the contralateral breast; six of the eight were premenopausal. The expected contralateral incidence in premenopausal women, adjusted for treatment with tamoxifen and adjuvant therapy, was 106.2/388. Thus, the adjusted reduction in breast cancer incidence among premenopausal women was reported as 94.4%. Two of 357 postmenopausal women developed contralateral breast cancer following CRRM. The expected incidence, adjusted for treatment with tamoxifen and adjuvant therapy, was 50.3 of 357, an adjusted reduction in breast cancer incidence of 96%. Unadjusted estimates of reductions in breast cancer risk were virtually the same. These estimated differences were all statistically significant (P < 0.05).
In an earlier 2004 report, Metcalfe 2004a reported a 59% reduction in contralateral breast cancer associated with women who had BRRSO (HR 0.41, 95% CI 0.18 to 0.90).
Peralta 2000 reported that none of 64 participants who had CRRM subsequently developed contralateral breast cancer compared to 36 of 182 control participants (19.8%). This difference in incidence was significant (P = 0.02).
Zeichner 2014 reported that the participants in the CRRM group had fewer recurrences. CRRM group had six recurrences out of 42 women (14.3%) versus no‐CRRM group, which had 60 out of 195 women (30.8%).
BRCA1 and BRCA2 mutations
In Evans 2013, there were six incidences of contralateral breast cancer in the CRRM group of 105 women (5.7%), and in the control group there were 35 incidences in 473 women (7.4%).
In Heemskerk‐Gerritsen 2013, with all participants BRCA1/2 mutation positive, the CRRM group had 4 incidences of contralateral breast cancer in 242 women, whereas the control group had 64 in 341 women.
Kass 2010 found that there was one contralateral breast cancer incidence in a group of 107 BRCA1/2‐mutation participants, with a mean follow‐up for BRCA1 carriers of 5.8 years and 4.2 years' follow‐up in BRCA2 carriers.
Van Sprundel 2005 data on BRCA1/2 mutation carriers indicated a 1.3% incidence of breast cancer after CRRM versus 14% (6/43) incidence for the surveillance group (P < 0.001).
5. Physical morbidity
Four of the studies (Frost 2005; Goldflam 2004; Miller 2013; Zion 2003) reported data for physical morbidity (see Table 3). Frost 2005 reported that 27% of participants (157/583) had unanticipated re operations following CRRM with or without reconstruction, with 72% of these related to implants. Reoperations were reported by Zion 2003 in 37% (189/506) of women who had reconstruction. Goldflam 2004 found 16.3% of participants (39/239) had complications following CRRM including re operations, bleeding, necrosis and infection.
Miller 2013 reported on complications in CRRM women (209) versus those who had unilateral treatment mastectomy (UM n = 391). Complications in the CRRM group versus UM group were 41.6% (112) versus 28.6% (87), P = 0.001. Of those who had reconstruction, 87 of 209 (41.6%) had any complication; breast site complications were on the cancer side in 29 (39.7%) and on the CRRM side in 27 (37%) patients. Among those who did not have reconstruction, 42.9% of CRRM patients had any complications versus 21.5% of UM patients (P = 0.029). Major complications, including re operations, rehospitalizations, flap and/or implant loss in reconstruction, were: CRRM – 13.9% (29); UM – 4.1% (16), P < 0.001. The most frequent major complications were fixed tissue expander or implant removal in CRRM patients (17.3%) and seroma requiring reoperation in UM patients (5.9%). Minor complications included minor infections, necrosis, and delayed wound healing. Univariate analysis showed that CRRM (P = 0.001), type of reconstruction (P = 0.001), and smoking history (P = 0.007) were significantly associated with any complication. After adjusting for age, BMI, smoking history, diabetes, American Joint Committee on Cancer (AJCC) stage, previous radiation, type of reconstruction, and adjuvant therapy, CRRM patients were 2.7 times more likely to have major complications (OR 2.66, 95% CI 1.37 to 5.19, P = 0.004). CRRM patients were 1.5 times more likely to have any complications than UM patients (OR 1.53, 95% CI 1.04 to 2.25, P = 0.029).
6. Quality of life/psychological morbidity
Seven studies (Altschuler 2008; Boughey 2015; Frost 2005; Geiger 2006; Hwang 2016; Montgomery 1999;Unukovych 2012) presented data concerning quality of life, satisfaction with the mastectomy, or other assessments of emotional or social function following CRRM (see Table 4). One study (Hwang 2016) also looked at who chose CRRM and whether receipt of CRRM affects quality‐of‐life outcomes.
a. Satisfaction with decision
Altschuler 2008 reported general satisfaction among CRRM participants in response to a closed‐end question in a questionnaire; 401 of 567 women (70.7%) expressed general satisfaction, 60 women (10.6%) expressed general dissatisfaction, and 102 (18%) did not respond to that question.
Three studies (Frost 2005; Geiger 2006; Montgomery 1999) and two follow‐ups (Boughey 2015; Frost 2011) had data on satisfaction with decision. Frost 2005 found 83% of 583 women who had CRRM were satisfied with their decision after a mean follow‐up of 10.3 years; Geiger 2006 reported 86.4% of women (371/429) were satisfied. Montgomery 1999 reported that the majority of women in the study were satisfied with their decision; only 6% (18 of 296) regretted their decision, with cosmetic results being the number one reason cited.
Frost 2011 found that 90% of the 269 women who were Frost 2005's participants responding to a questionnaire were satisfied or very satisfied with their decision after mean follow‐up of 20.2 years post CRRM, with 92% of women reporting that, knowing what they do now, they definitely or probably would choose CRRM again. It was also found that women with reconstruction had significantly lower satisfaction than women without reconstruction (P = 0.03). Boughey 2015, in a later follow‐up to Frost 2011, reported on the 269 respondents at 20 years, 210 of whom (78%) had reconstruction and 59 (22%) with no reconstruction. Of those who had had reconstruction, 89% (187 women) were satisfied with CRRM, and 95% of those with no reconstruction (56 women) were satisfied (P = 0.03). Of those who had had reconstruction, 92% (193) would choose CRRM again, and 93% (55) of those with no reconstruction would choose CRRM again (P = 0.10).
Regrets were more common in women with whom the discussion to have CRRM was initiated by the physician than in women who initiated the discussion themselves, Montgomery 1999 found. The study did not compare satisfaction with decision between women who chose surveillance and those who chose RRM. Frost 2005 also found that there was a difference between the women who had a subcutaneous mastectomy and those who had a total mastectomy when asked if they would chose to have CRRM again (75% versus 89%).
b. Satisfaction with cosmetic outcome
Five studies (Frost 2005; Geiger 2006; Hwang 2016; Montgomery 1999;Unukovych 2012) and two follow‐ups (Boughey 2015; Frost 2011) reported on satisfaction with cosmetic results.
In the Frost 2005 study, 36% of 583 women reported a diminished satisfaction with their physical appearance. Six years later, Frost 2011 found that, of the 269 women who responded to new questionnaires, 31% still felt that body appearance was one of the 'adverse effects' of the procedure. Of those women who had had CRRM, 92% continued (after mean follow‐up of 20.2 years) to feel they had made an informed decision. Positive feelings of body image remained significantly higher in those who chose reconstruction versus no reconstruction (P = 0.01), Boughey 2015 found. Hwang 2016 found that, in those women who had reconstruction, CRRM was associated with a higher breast satisfaction score (62.0 versus 59.9, P = 0.0043) than those who did not have reconstruction.
Comparing participants who accepted CRRM versus those who did not concerning being self‐conscious about their appearance, Geiger 2006 found that there was not a statistically significant difference, with 21.1% (108/510) acceptors and 15% (9/60) of non‐acceptors being self‐conscious (P = 0.263). Unukovych 2012 found that two years after CRRM, more than 50% of the women reported problems with appearance and the scars, and felt less attractive and feminine.
Montgomery 1999 reported that 16% (18 of 111) of those who had reconstruction found the cosmetic results of their reconstruction following CRRM unsatisfactory. As with BRRM, there seemed to be correlation between satisfaction and reconstruction. Montgomery 1999 also found a correlation between having reconstruction and having regrets. The 185 women who opted not to have reconstruction after CRRM had significantly less regret than those who opted for reconstruction (P = 0.01).
c. Psychological well‐being/cancer‐related anxiety
Four studies reported psychological well‐being/cancer‐related anxiety (Frost 2005; Geiger 2006;Hwang 2016;Unukovych 2012). In one study with controls (Geiger 2006), four to 20 years after their decision to have CRRM or not, there was a significant difference between CRRM acceptors and CRRM decliners as to breast cancer concerns, with 50.3% (257/511) versus 73.8% (45/61) expressing concern, respectively (P < 0.001). When asked about contentment with their quality of life, the study found no difference between CRRM acceptors and CRRM decliners, 76.3% versus 75.4%, respectively. Frost 2005 found 74% of the women who had CRRM reported a diminished level of emotional concern about developing breast cancer. Unukovych 2012 found no statistically significant differences between preoperative and postoperative mean levels found for anxiety or depression in 60 women.
Hwang 2016 found that those who had reconstruction after CRRM versus those who did not scored lower in physical well‐being (74.5 versus 76.8, P < 0.001) and lower psychosocial well‐being (71.7 versus 73.9, P = .0051). However, psychosocial well‐being and breast satisfaction were higher overall in women with CRRM (BREAST‐Q scores + 1.80 and 1.49, respectively).
d. Body image/sexuality
Two studies (Frost 2005; Geiger 2006) reported on body image and sexuality issues. Frost 2005 found a number of adverse physical effects among 583 women who had CRRM: 33% reported their body image was negatively affected; 26% felt less feminine; 23% had an adverse effect on their sexual relations; and 12% reported adverse effects on their emotional stability. In Frost 2011, 269 women who chose CRRM continued to say that CRRM had an adverse effect on feelings of femininity (24%) and sexual relationships (23%).
In the one study that had controls, Geiger 2006 found no difference between CRRM acceptors and CRRM decliners regarding their satisfaction with their sexual lives (40.9% versus 40.3%, respectively).
e. Health‐related quality of life
Unukovych 2012 found that body pain for those undergoing CRRM increased at six months postsurgery; at two years after CRRM the comparison between participant and normative data revealed a statistically significant difference in the bodily pain subscale favoring the participants (P = 0.007).
C. Combined bilateral and contralateral risk‐reducing mastectomy
Twelve studies (Altschuler 2008; Bresser 2006; Contant 2002; de la Pena‐Salcedo 2012; Den Heijer 2012; Evans 1999; Horton 1978; Isern 2008; Kass 2010; Mutter 2015; Pennisi 1989; Zion 2003) included participants receiving BRRM as well as participants receiving CRRM. Four studies (Altschuler 2008; Heemskerk‐Gerritsen 2007; Kass 2010; Zion 2003) separated BRRM and CRRM participants when reporting data and those results are reported above. Collectively, the other 10 studies involved 2157 participants; 1809 of them (83.7%) received BRRM, 348 (16.3%) received CRRM, and 36 were unclear.
1. All‐cause mortality
One study (Pennisi 1989) found that, of the 70% of 1500 participants who were followed for nine years, 0.3% died of "other causes". These are the only data provided concerning mortality from causes other than breast cancer. There are no data for all‐cause mortality.
2. Breast cancer (disease‐specific) mortality
One study (Pennisi 1989) reported that three of the 1500 participants receiving risk‐reducing surgery subsequently died of breast cancer. Thirty percent of participants were lost to follow‐up, however.
3. Disease‐free survival
Mutter 2015 reported that 13 of 1065 women with BRRM and 12 of 1643 with CRRM developed breast cancer in the risk‐reducing mastectomy breast with a median follow‐up of 22 years. Using a Kaplan‐Meier curve for disease‐free survival, the five‐year disease‐free survival estimate was 69% overall (95% CI 52% to 94%). When separated by RRM type, the five‐year disease‐free survival estimate for the 11 women with isolated loco‐regional breast cancer after BRRM was 90% (95% CI 73% to 100%); the five‐year disease‐free survival estimate for the 11 women with isolated loco‐regional breast cancer after CRRM was 52% (95% CI 29% to 94%). This was not statistically different to the BRRM rate (P = 0.23).
4. Incidence of breast cancer
Five studies reported data on breast cancer incidence, and all five reported few cases following risk‐reducing surgery.
Evans 1999 reported data on 178 participants: 141 received BRRM and 37 received CRRM. No breast cancers developed after surgery in the participants who had risk‐reducing mastectomy, although the authors estimated that four cases would have been expected. Follow‐up was less than five years.
Horton 1978 followed 104 women: 93 received BRRM and 11 received CRRM. No breast cancer developed in any participant following risk‐reducing surgery.
Mutter 2015 reported that, out of 1065 women with BRRM, 13 had an incidence of breast cancer; median time to develop breast cancer was six years. Of the 13 cases, 10 were local disease only, one was auxiliary breast cancer of unknown primary disease, and two were synchronous local and distant disease. Twelve of 1643 women with CRRM had a breast cancer incidence; median time to develop breast cancer was eight years. Of the 12 cases, seven were local disease only, one was local and regional disease, three were auxiliary breast cancer of unknown primary disease, and one was synchronous local and distant disease.
Pennisi 1989 followed 1500 participants: 1361 received BRRM and 139 received CRRM. Six of the 1500 participants (0.4%) developed breast disease following surgery. However, 30% of the participants were lost to follow‐up.
BRCA1 and BRCA2 mutations
Koskenvuo 2014 reported on a retrospective cohort of 136 BRCA1/2 mutation carriers with a median follow‐up of 52 months, of whom 52 had RRM. Thirty‐three of the 52 women also had RRSO. Forty‐five months postsurgery, one of the 52 participants had metastatic axillary lymph nodes.
5. Physical morbidity
Six studies (Contant 2002; de la Pena‐Salcedo 2012; Den Heijer 2012; Heemskerk‐Gerritsen 2007; Isern 2008; Pennisi 1989) reported on physical morbidity (see Table 3); four studies (de la Pena‐Salcedo 2012; Den Heijer 2012; Heemskerk‐Gerritsen 2007 (earlier report of Heemskerk‐Gerritsen 2013andHeemskerk‐Gerritsen 2015);Koskenvuo 2014) reported on physical morbidity only in a combined fashion, whereas the rest of the data were separated out. Heemskerk‐Gerritsen 2007 reported that, of 276 women opting for breast reconstruction, 137 (49.6%) recorded one or more complications, for a total of 215 complications in all. Surgical re operations were performed in 153 of the 215 complications (71%), 124 of which were for complications later than six weeks postoperatively. de la Pena‐Salcedo 2012 reported on 40 participants with CRRM and 12 with BRRM for a total of 64 breasts. Seven of the 64 (10.9%) reconstructed breasts had short‐term (undefined) complications: four capsular contractures, two hematomas, and one infection. Of 36 women who had RRM with/without reconstruction or BRRSO in Den Heijer 2012, 11 women (31%) underwent additional surgeries after the primary RRM.
One study (Pennisi 1989) reported that 5% of 139 participants receiving risk‐reducing surgery developed skin necrosis. Contant 2002 found that 30 of 103 women (29%) who had RRM with reconstruction had postoperative complications, with 77% of the complications requiring surgery. Among those who did not have reconstruction, two of nine participants (22%) required reoperation. Isern 2008 found that four of 61 participants required reoperation within six weeks of surgery; seven of 61 participants developed late complications, for which five had reoperation. Another seven women (11%) had cosmetic corrections.
BRCA1 and BRCA2 mutations
Koskenvuo 2014 reported on a cohort of 52 women with BRCA1/2 mutations who had RRM with a median follow‐up of 52 months. Ten of the participants had previously had breast‐conserving surgery (BCS) on a cancerous breast, then decided to have RRM on that breast. There were 26 surgical complications in 21 participants that resulted in 20 reoperations. The frequency of complications was 33% (26/80) per operated breast and 40% (21/52) per participant. In the group with reconstruction with autologous flaps, there were 11 (28%) complications in total; in the group of implant‐based reconstruction, complications were recorded in 13 (42%) breasts, with the most common complication being wound infection (others were seroma, hematoma, skin edge necrosis, blood supply problem, total flap loss and implant loss). In the 10 participants who had previously had BCS, there were four cases of minor complications. Five reconstructions failed and were corrected with re‐reconstruction.
6. Quality of life/psychological morbidity
Four studies (Bresser 2006; de la Pena‐Salcedo 2012; Den Heijer 2012; Isern 2008) presented data concerning quality of life, satisfaction with the mastectomy, or other assessments of emotional or social function (see Table 4).
a. Satisfaction with cosmetic outcome
Bresser 2006 found that, among women who had reconstruction, 68/113 (60%) were satisfied and 45/113 (40%) were unsatisfied with the result. There were statistically significant differences for the unsatisfied women as compared to the satisfied for feeling less informed (P = 0.02), reporting more complications (P = 0.01), and seven women (15.5%) would not opt for reconstruction again (P = 0.01). de la Pena‐Salcedo 2012 found that, of 52 participants undergoing RRM, 39 (75.0%) reported being highly satisfied, 10 (19.23%) reported being partially satisfied, and three (5.76%) reported being unsatisfied. Isern 2008 reported that asymmetry between the breasts was found among 17 (32%) of the women. The women in that study reported higher levels of general satisfaction (92%) than aesthetic satisfaction (74%).
b. Satisfaction with the medical process
Bresser 2006 reported that, among the 112 women who reported being satisfied with their RRM, 17 (15%) said they did not feel sufficiently informed. The same study also reported that the percentage was greater among 40 women who reported that their RRM negatively impacted their sexual relationships, with 30% (12/40) reporting that they felt insufficiently informed about the procedure and possible results.
c. Body image/sexuality
Bresser 2006 found 44% (40/90) of the women reported RRM negatively affected their sexual relationship. This finding was significantly correlated to feeling insufficiently informed (P = 0.01) and reporting that the surgery did not meet their expectations (P = 0.01). General body image scores in Den Heijer 2012 fluctuated, declining and then improving, but not to preoperative levels. From two to four weeks preoperatively to six months after RRM, the general body image scores were 10.7 to 12.4 (P = 0.02), and from six to nine years after RRM, the general body image scores were 12.4 to 11.7 (P = 0.18). Breast‐related body image scales also fluctuated, improving and then declining, from preoperatively to six months postoperatively 5.0 to 6.7 ( P = 0.01), and from six months, to six to nine years postoperatively 6.7 to 5.9 (P = 0.03).
d. Psychological well‐being/cancer‐related anxiety
Using the Hospital Anxiety and Depression Scale, Isern 2008 found after median follow‐up time of 42 months that 42 of 61 women (78%) screened for anxiety were regarded as non‐cases concerning anxiety, seven women (13%) doubtful cases, and five (9%) as definite cases of anxiety. In terms of depression, there were 53 (98%) non‐cases and one (2%) definite case. Den Heijer 2012 found that general distress level scores went down from preoperatively to six months postoperatively (9.91 to 7.45, P = 0.03), and from six months to six to nine years postoperatively (7.45 to 6.58, P = 0.01). Breast cancer‐specific stress level scores went down from preoperatively to six months postoperatively (22.7 to 12.9, P = 0.01) and from six months, to six to nine years postoperatively (12.9 to 6.1, P = 0.01).
Discussion
Summary of main results
Bilateral risk‐reducing mastectomy incidence and mortality
The findings of the studies involving women with no previous history of breast cancer who underwent BRRM were consistent in showing a reduced incidence of breast cancer or reduced breast cancer mortality, or both, particularly in women at high risk for the disease. One study reported reductions in risk of death as high as 94% following BRRM (Hartmann 1999a) for high‐risk women when compared to a control group of participants’ sisters, and another showed 100% reduction for women with a moderate risk of the disease (Geiger 2005). Ten‐year overall survival for the BRRM participants was 99%, while that for the controls was 96% in Heemskerk‐Gerritsen 2013.
Two studies reported reduction in incidence of breast cancer following BRRM as high as 100% (Hartmann 2001; Meijers‐Heijboer 2001), and in high‐risk women, Arver 2011 found an incidence of 0 of 223 against 12 expected cases. Heemskerk‐Gerritsen 2013 also showed no incidence after BRRM versus 57 women in the control group. Other reports (Klijn 2004) showed lower but specific reductions, such as one of 73 BRRM participants developing breast cancer versus 23 of 173 non‐BRRM participants. However, Skytte 2011 found an annual incidence of breast cancer of 0.8% in the BRRM group and 1.7% in the non‐BRRM group, which was a protective effect but not significant.
Data from breast reduction surgery adds biological plausibility to the theory that reducing the amount of breast tissue reduces the risk of breast cancer. Studies by Baasch 1996, Brinton 2001, and Fryzek 2005 reported that women who underwent breast reduction surgery had a lower incidence of breast cancer compared to the expected number of cases.
BRCA1 and BRCA2 mutations
For women with BRCA1/2 mutations, Heemskerk‐Gerritsen 2013 reported that deaths due to breast cancer in the BRRM group were 0.5%, and in the control group were 1.7%. Meijers‐Heijboer 2001 reported no deaths due to breast cancer among the 76 women who underwent BRRM at three‐years' follow‐up, but one breast cancer death among 63 women who chose surveillance.
The Ingham 2013 study clearly separated out results for BRCA1/2 carriers who either chose risk‐reducing surgery or not: for those undergoing BRRM compared with no risk‐reducing surgery, a borderline significant result was obtained; BRRM plus BRRSO showed significant survival advantage; only BRRSO alone was significantly associated with improved survival. Thus, the survival advantage could be attributed to BRRSO, not BRRM.
It should be noted that, among the 214 high‐risk women (determined by family history but not necessarily BRCA1/ 2 mutation carriers) who underwent BRRM in the Hartmann 1999a study, it has been estimated that most of the women would not have died from breast cancer in any case (Ernster 1999). Even BRCA1/2 mutations have incomplete penetrance estimated at 70%, and thus 30% of BRRMs in carriers will be non‐therapeutic and unnecessary (Rookus 2002). However, there is disagreement on how to manage these high‐risk BRCA mutation carriers; Rookus also notes, "... the ineffectiveness of surveillance, and the high lethality by late diagnosis are the main argues [sic] for the recommendation of risk‐reducing surgery as a reasonable strategy." In contrast, Burness 2011 felt that “Screening with MRI and mammography beginning at 25 years of age results in a similar survival benefit to (RRM), and MRI screening is generally accepted to be cost effective in BRCA mutation carriers”.
Contralateral risk‐reducing mastectomy incidence and mortality
The most significant question about CRRM is whether it improves survival for women who already have a diagnosis of breast cancer, since CRRM does not alter the outcome of the original breast cancer. The validity of observational studies addressing the effect of CRRM on breast cancer mortality remains an important consideration.
One study (Kiely 2010) found no apparent difference in survival between the CRRM group (93.5%) and the non‐CRRM group (92.6%). Results for breast cancer mortality vary among 11 other CRRM studies, and this could be partially explained by various confounding factors such as selection bias, including age and/or other concurrent treatments undertaken, and when matched analysis was conducted the advantage disappeared.
Three studies (Herrinton 2005; Jatoi 2014; Kruper 2014) found evidence, when analyzing survival data, that the survival advantage may be due to selection bias, with healthier, younger women selecting CRRM. Herrinton 2005 found significantly improved all‐cause and breast cancer mortality for the CRRM group when compared to the group who did not select CRRM. It should be noted that the women selecting CRRM may have had less comorbidity, as they had a 27% lower risk of death from other causes than the women who did not select CRRM. CRRM compared to no CRRM was associated with improved disease‐specific survival and greater overall survival in Kruper 2014. Removing the contralateral breast cancer cases from the analysis had little impact on CRRM disease‐specific survival and overall survival, suggesting that prevention of contralateral breast cancer by CRRM does not explain the observed survival benefit. Also, differences across groups in overall survival were greater than group differences in disease‐free survival, consistent with selection bias. Therefore, it is possible that the observed survival benefits might be the result of healthier people choosing or being recommended for CRRM rather than the actual benefit of CRRM over single‐treatment mastectomy. Jatoi 2014 found that CRRM was associated with lower all‐cause, breast‐cancer specific, and non‐cancer mortality, which persisted after adjusting for stage. However, the relationship between CRRM and non‐cancer mortality was stronger than either all‐cause or breast cancer‐specific mortality, suggesting an underlying selection bias for treating potentially healthier women with CRRM
Two other studies (Brewster 2012; Pesce 2014) performed matched analysis between CRRM and no‐CRRM controls, and in each case, the CRRM survival advantage was no longer significant. Brewster 2012 found that participants who had CRRM had longer overall survival than participants who did not in the adjusted multivariate models, but the matched model was not statistically significant. Pesce 2014 found the participants with CRRM had better survival than those with unilateral mastectomy without adjustments, but there was no statistically significant difference in overall survival between CRRM and unilateral mastectomy after adjusting for various factors. Metcalfe 2014 reported a survival advantage for CRRM participants in the second decade after surgery. However, when propensity scores were calculated for 79 matched pairs, the survival advantage was no longer significant. Additionally, some of the contralateral breast cancer cases were diagnosed within one to two months (0.01 years) of original diagnosis of breast cancer, less than the commonly used second new breast cancer diagnoses at six months or less, and should have been excluded for having bilateral breast cancer.
There were three studies (Bedrosian 2010; Peralta 2000; Zeichner 2014) that looked at the impact of tumor size and breast cancer stage on survival results; a fourth study that did so (Van Sprundel 2005) is discussed under the BRCA1/2 heading. Bedrosian 2010 found CRRM was associated with improved disease‐specific survival only in participants with stages I to III breast cancer and declined with age, so those older than 60 had no risk reduction from the procedure, showing that the risk of mortality from contralateral disease needs to be weighed against risk of mortality from primary tumor metastases. Peralta 2000 controlled for prognostic factors (e.g. features of the primary tumor) when assessing whether CRRM improves survival. That study found no overall survival benefit at 15 years. When the same study assessed breast cancer (disease‐specific) survival, there was a significant benefit only for the subgroup of participants with early stages of disease (stages 0, 1, II). Van Sprundel 2005 attributed the significantly higher overall survival of the CRRM group in his study to the higher mortality in the surveillance group due to their primary breast cancers and ovarian cancer. The CRRM participants had significantly smaller tumors than the no‐CRRM participants in Zeichner 2014, and there were major differences in follow‐up time that could have contributed to detection bias: 95.2% of CRRM participants were followed for 3 to 13 years versus only 30% of the no‐CRRM participants. Sixty percent of the no‐CRRM participants were followed for 13 to 23 years versus only 4.8% of the CRRM participants. Thus, the no‐CRRM participants had a longer time period for mortality to occur.
BRCA1 and BRCA2 mutations
Metcalfe 2014 also performed matched analysis between CRRM and no‐CRRM controls, and reported a survival advantage for CRRM participants in the second decade after surgery. However, when propensity scores were calculated for 79 matched pairs, the survival advantage was no longer significant. Additionally, some of the contralateral breast cancer cases were diagnosed within one to two months (0.01 years) of original diagnosis of breast cancer, less than the commonly used second new breast cancer diagnoses at six months or less, and should have been excluded for having bilateral breast cancer. Van Sprundel 2005, when looking at the impact of tumor size and BC stage on survival results, attributed the significantly higher overall survival of the CRRM group in his study to the higher mortality in the surveillance group due to their primary breast cancers and ovarian cancer.
BRRSO factor
BRRSO has been found to be a significant confounding factor in survival by four studies (Evans 2013; Heemskerk‐Gerritsen 2015; Metcalfe 2004a; Van Sprundel 2005). When controlling for BRRSO, Van Sprundel 2005 found significantly better survival for those who had CRRM and BRRSO compared with those who had CRRM only. This is consistent with Metcalfe 2004a finding that BRRSO was significantly associated with the reduction of incidence of contralateral breast cancer. Evans 2013 found that, after adjusting for potential confounders, only CRRM and BRRSO were independently predictive of improved survival. Therefore, although women with CRRM had apparently reduced breast‐cancer and non‐breast‐cancer mortality, the result is potentially confounded by concomitant BRRSO and the differences in median follow‐up (8.8 years for the CRRM group and 7.3 years for the non‐RRM group). Heemskerk‐Gerritsen 2015 found mortality was lower in the CRRM group (19 in CRRM versus 65 in controls), and Cox analysis yielded a HR 0.49 (95% CI 0.29 to 0.82) adjusted for BRRSO.
Thus, most CRRM studies failed to control for most prognostic factors regardless of the differences in baseline prognostic factors that were noted between CRRM and non‐CRRM participants. In a meta‐analysis of 14 CRRM studies, Fayanju 2014 found that the rate of contralateral breast cancer was very low whether the breast cancer participant had CRRM or not, suggesting that reported improved survival is not the result of CRRM. Yao 2010 commented on CRRM death risk versus index cancer risk, “One in 25 breast cancer survivors will develop a second primary breast cancer, either in the index breast or the contralateral breast, but contralateral cancers account for only 2.5% of breast cancer deaths”. Further caution is offered by Lise 1997 who recommends, "For women with previous breast cancer, their prognosis should be evaluated and if the risk of death from distant metastases exceeds that of a contralateral cancer, risk‐reducing mastectomy should not be considered."
Brewster 2011 noted that “The lack of information about the clinical value of CRRM in women with sporadic breast cancer is an important public health problem.” Also, “…with the increased use of adjuvant therapies, we would now expect to see a lower incidence of contralateral breast cancer than previously reported” (Quan 2008).
Psychological and physical morbidity
It should be noted that morbidity is an under‐reported aspect of research studies. What is reported in our included studies is only a portion of all included studies; some studies did not report on morbidity at all, a lost opportunity for researchers. Nonetheless, some trends can be observed regarding psychological and physical morbidity following RRM. In terms of feeling “at risk,” Van Dijk 2008 noted a statistically significant decrease in perceived risk simply after genetic counselling, especially for women at relatively low risk as opposed to very high‐risk women. RRM can also help women feel more in control of their health risk, “Patients believe that a CRRM offers them the opportunity to significantly diminish their risk of a second breast cancer and reassure them they did everything possible to reduce the risk” (Barry 2011). Hwang 2016 also found that the belief that CRRM can reduce breast cancer mortality persists despite studies showing little survival benefit.
Generally, women reported satisfaction with their decisions to have BRRM, but were less consistent in satisfaction with cosmetic outcome; diminished satisfaction often was due to surgical complications. Dissatisfaction with the decision to have BRRM was correlated in two studies with either the discussion being initiated by the physician or the physician's advice to have BRRM being the primary deciding factor for the woman. Again, because decision satisfaction data were only collected postsurgically, we do not know the extent to which recall bias or cognitive dissonance influenced dissatisfied participants' recollections of the physician's role in decision‐making. This correlation between regret and physician's role was not found to be true in the one CRRM study (Montgomery 1999) that looked at regrets. Women who made the decision alone with or without their physicians’ opinions were twice as likely to be satisfied with their CRRM six months postsurgery (OR 2.2, 95% CI 1.1 to 4.2) than those who shared the decision‐making with their physician (Nekhlyudov 2005). “It is important that providers spend time with all women before and after CRRM to assess their knowledge and correct any misconceptions” (Nekhlyudov 2005). The decision to have RRM may be affected by anticipated regret, “…risk‐management preference was strongly correlated with anticipated feelings of regret; that is, the amount of regret women think they would have if they were diagnosed with breast cancer after rejecting the option of (RRM)” (Van Dijk 2008).
Hwang 2016 found a difference in the women who selected CRRM versus those who did not. Those who chose CRRM were younger (53.7 versus 59.2 years, P < .001), married (76% versus 71%, P < .001), higher income (P < .001), and more likely to have reconstruction than no CRRM (OR, 1.72, 95% CI 1.43 to 2.08). Pesce 2014 found a statistically significant different between groups, with about half the CRRM group having Stage I.
With regard to emotional well‐being, most women recover well postoperatively, reporting reduced cancer worry and showing reduced psychological morbidity from their baseline measures (Hatcher 2001), but exceptions also have been noted. Metcalfe 2004b reported that 32% of women who had BRRM showed levels of psychological symptoms consistent with the need for psychological counselling based on responses to the Brief Symptom Inventory. However, no preoperative data were gathered to determine if these symptoms were caused by the BRRM or other factors. Psychosocial outcomes may have long‐term effects, “…even among women who report general satisfaction with their decision to have (RRM)…lingering negative psychosocial outcomes can remain, particularly among women with (BRRM). This dichotomy could be an important factor to discuss in counselling women considering the procedure” (Altschuler 2008). In terms of physical well‐being, Gahm 2013 found differences in preoperative and postoperative perceptions of cold, warmth, and touch in those with BRRM, as well as loss or decrease in sexual feelings in the reconstructed breasts. Gopie 2013 found that women at high risk for breast cancer who had BRRM with reconstruction had significantly improved cancer distress and general physical health; they also had significantly diminished body image and satisfaction with the appearance of their breasts.
Hwang 2016 reported psychosocial well‐being increased in those women with and without CRRM when surveyed five and 10 years after treatment. CRRM was associated with a higher breast satisfaction score) at the cost of lower physical well‐being and lower psychosocial well‐being. The study showed that CRRM was independently associated with higher BREAST‐Q scores for psychosocial well‐being and breast satisfaction; however, “…the magnitude of benefit was small and may be clinically negligible compared with the much greater favorable impact of breast reconstruction.”
Unukovych 2012 reported that, two years after CRRM, more than 50% of the women from families with a history of breast cancer reported problems with appearance and with the scars, and felt less attractive and feminine. Den Heijer 2012, reporting on women at high risk from breast or ovarian cancer, or both, who had undergone RRM, found when comparing pre‐ and post‐operative responses, that the women’s general and breast cancer‐specific stress levels were statistically diminished as well as their breast body image.
Studies looking at physical morbidity following RRM reported that a high proportion of participants found they had unanticipated surgical interventions. Arver 2011 found that 64% of women had unanticipated secondary operations after RRM. Implants were a major source for the reoperations. Zion 2000 reported 37% of the original implants were subsequently removed, and Frost 2005 found that 72% of reoperations were implant related. Of 276 women opting for reconstruction after RRM in Heemskerk‐Gerritsen 2007, 137 of them (49.6%) registered one or more complications requiring surgical interventions for 153 of the complications. Frost 2011 reported that, among those women having reconstruction after CRRM, 45% underwent one or more re operations, and satisfaction was lower in women with re operations than in those without). Miller 2013 found that complications in CRRM participants having reconstruction were about twice the amount of those in participants with only a treatment mastectomy, but this is logical, since twice as many breasts were removed and reconstructed. The second‐most frequent problem in the CRRM group was tissue expander or implant requiring removal, which is a reconstruction problem, not a RRM problem.
In an update of Brandberg 2008, the study author reported that at six months postoperatively, 73% of the BRRM women with hereditary risk for breast cancer responded that they did not have any, or had only minor, sensitivity in the breasts at two assessment points, which continued at one year.
Physical condition at the time of RRM also can affect morbidity. For instance, Arver 2011 found that women with a BMI of 25 to 30 had a higher proportion of infections after RRM than women with a BMI less than 25 (36% versus 15%), and the proportion of implant loss increased with increasing weight (5% if BMI was less than 25, 16% if BMI was 25 to 30, and 27% if BMI was more than 30, P = 0.008). Crosby 2011 also found BMI was consistently predictive of postoperative complications in CRRM participants having reconstruction by both univariate (OR 1.28, 95% CI 1.11 to 1.47, P = 0.006) and multivariate analysis (OR 1.32, 95% CI 1.15 to 1.53) for every 5‐unit increase. Arver 2011 also found that wound necrosis/epidermolysis was more common in smokers than in nonsmokers (68% versus 16%, P = 0.007). Smoking history was also one of the factors significantly associated with any complications in Miller 2013.
Quality of the evidence
Bilateral risk‐reducing mastectomy incidence and mortality
The findings of the review on BRRM should be taken in the context of the methodological limitations of many of the older studies but which have been adjusted for in many of the more recent studies. Two older studies included women who would no longer be considered high risk (Horton 1978; Pennisi 1989). Two studies (Borgen 1998; Montgomery 1999) recruited participants from adverts in the public press, and therefore posed the risk of healthy volunteer bias. The selection criteria for controls in a study by Rebbeck 2004 had a risk for selection bias; 25 women were excluded from Geiger 2007 because their physicians declined to give approval for their recruitment. A study by Pennisi 1989 had a 30% attrition rate, posing the possibility of attrition bias; participant numbers responding to questionnaires in Brandberg 2008; Gahm 2010 and Geiger 2007 were variable for unknown reasons. Brandberg 2008; Klijn 2004 (a follow‐up to Meijers‐Heijboer 2001), and Skytte 2011 all included patients with BRRSO, which posed a risk for detection bias. However, this possible bias was adjusted for in Ingham 2013 by using matched analysis, so that the effects of BRRSO on survival could be accounted for. For other studies, follow‐up times were of durations of less than five years (Contant 2002; Evans 1999; Heemskerk‐Gerritsen 2007; Heemskerk‐Gerritsen 2015; Meijers‐Heijboer 2001; Van Sprundel 2005). Many of the newer studies had longer follow‐up times, such as medians of 8.5 years (Heemskerk‐Gerritsen 2013) and 13.3 years (Ingham 2013).
Klaren 2003 discusses the difficulty of designing a study on the efficacy of RRM because of a variety of potential biases associated with the selection of study participants and controls. Many studies of risk‐reducing surgery are family based or health center based and can include relatives (Bresser 2006;Metcalfe 2004b; Metcalfe 2005; Metcalfe 2014). If events related to cancer within a family influence the behavior of more than one family member in the study, and these events are assumed to be independent, familial event bias can occur. In Heemskerk‐Gerritsen 2013 there were some differences in the proportion of age groupings, those who received radiotherapy, chemotherapy, and BRRSO.
Furthermore, many studies lacked a comparison group. Three of the studies (Evans 1999; Hartmann 1999a; Hartmann 2001) employed statistical modeling to simulate a comparison group, and this approach allowed the researchers to estimate the risk reduction attributable to BRRM. These all found risk reductions in the BRRM group for both incidence and mortality.
The identification of gene mutations associated with breast cancer has resulted in renewed interest in BRRM as a preventive therapy. Much of the data used in this review did not allow subset identification by genetic testing, although a number of studies included participants who had been or considered being tested for BRCA1/2 mutations. As expected, our systematic review on BRRM did not identify any randomized controlled trials, nor is it likely that there will be any in the future, as probably few women would agree to be randomized to either BRRM or surveillance. Although not optimal in terms of the reliability and validity of the information collected, a number of non‐randomized studies were available to assist women in assessing the effectiveness of the procedure.
Contralateral risk‐reducing mastectomy incidence and mortality
Studies of CRRM were also subject to methodological limitations leading to selection, detection, or attrition bias. Some studies (Contant 2002; Goldflam 2004; Leis 1981; Montgomery 1999; Pennisi 1989) had high dropout rates or lacked a comparison group, or both. Bedrosian 2010 and Jatoi 2014 used SEER data; if participants migrated out of SEER regions, they would be missed in follow‐up, thus creating possible attrition bias. Kruper 2014 also used SEER data, and noted that changes in coding granularity might have affected reporting of rates of single mastectomy or CRRM. Frost 2011 reported on a survey mailed to women from Frost 2005 who were still alive; only 55% of those women responded, thus contributing to selection bias. Hwang 2016 surveyed volunteers from the Army of Women, which has a relatively affluent, well‐educated population (selection bias), and also self‐reported CRRM without confirmation with medical records (performance bias). Lee 1995 combined women who had CRRM with women who had a biopsy of the contralateral breast in the study group, and thus the risk exclusively for CRRM women could not be ascertained. The CRRM cohort in Boughey 2010 all had a family history of breast cancer, but only 34.8% of the non‐CRRM cohort did, and the proportion of those with first‐degree family history was also skewed (46.2% in the CRRM cohort versus 21.6% in the non‐CRRM cohort). Zeichner 2014 had significant differences in the length of follow‐up in the two groups, with 95.2% of CRRM participants followed for three to 13 years versus 30% of the no‐CRRM participants.
There is potential selection bias in Metcalfe 2014, as some of the contralateral breast cancer cases were diagnosed within one to two months (0.01 years) of original diagnosis of breast cancer, less than the commonly used second new breast cancer diagnoses at six months or less, and more correctly should be classified as bilateral breast cancer. This classification then could have overstated the incidence of contralateral breast cancer in the no‐CRRM group.
Efforts to control for important confounding factors varied among the studies. McDonnell 2001 and Peralta 2000 used multivariate analyses to adjust for chemotherapy and tamoxifen therapy, while only Peralta 2000 adjusted for stage of primary tumor. Both of the studies assessing incidence of cancer in the contralateral breast while controlling for chemotherapy and tamoxifen use (McDonnell 2001; Peralta 2000) reported markedly reduced incidences of breast cancer in the contralateral breast following CRRM. This is consistent with the BRRM and breast reduction surgery findings that reducing breast tissue can reduce risk of breast cancer incidence. Kass 2010 and Kiely 2010 included participants who also had BRRSO, with no information on how that confounded the results of CRRM. Evans 2013, however, used two types of controls – those with BRRSO but no CRRM, and those with no RRS. There were differences in the CRRM groups and the control groups in Chung 2012 as to the presence of BRCA mutations and the percentage of family history that could have biased the amount of effect. There was also the potential selection bias of healthier women having CRRM as in Jatoi 2014 and Kruper 2014. In Heemskerk‐Gerritsen 2015, there were differences in the proportion of age groups in the controls versus the RRM group. Zeichner 2014 had statistically significant differences between CRRM and no‐CRRM groups for tumor size, lymph node status, and radiotherapy treatment.
Psychological and physical morbidity
The decision to have RRM involves issues other than the surgical procedure. One of our objectives was to examine quality of life issues postoperatively. For this group of studies, the most common methodological limitation was failure to address recall bias. Ten studies (Altschuler 2008; Borgen 1998; Frost 2000; Frost 2005; Frost 2011; Gahm 2010; Geiger 2006; Hopwood 2000; Metcalfe 2004b; Metcalfe 2005) collected only retrospective data, often asking participants to remember what their psychological state or body image was prior to surgery and comparing it with after surgery. However, there were pre‐ and postoperative evaluations conducted in Brandberg 2012; Den Heijer 2012; Gopie 2013; and Unukovych 2012. Two studies (Geiger 2006; Geiger 2007) had a control group of women not opting for RRM to evaluate whether changes noted were due to the surgery or some other factor and found no difference in contentment with quality of life between CRRM participants and no‐CRRM controls.
Another common limitation was that some studies that assessed participants' satisfaction reported having used an invalidated patient satisfaction instrument that has been known to overestimate the level of satisfaction (Rubin 1991; Ware 1988). Brandberg 2008 used a sexual activity questionnaire that had no formal validation or reliability testing for the Swedish translation that was used; also “There are missing questionnaires at each of the assessment points, making the group that could be analyzed over time small and provides limited power to determine statistically significant differences” (Brandberg 2008). There were no pre‐CRRM assessments of psychosocial factors for comparison and it is unknown whether the questionnaire used had been tested for reliability or validity in Altschuler 2008. However, Boughey 2015; Brandberg 2012; de la Pena‐Salcedo 2012; Den Heijer 2012; Gopie 2013; and Unukovych 2012 all used validated instruments, and although Hwang 2016 participants may have had recall bias, the questionnaire they used was also validated.
It is surprising that decision satisfaction was so high, especially since the authors of the largest study of 425 women at "moderate risk" stated that many of the women in their moderate‐risk group would "not now be considered to have a markedly elevated risk of breast cancer" (Hartmann 1999b). Stefanek 2001 noted that it is not uncommon for a person to wonder if the surgery has been "wasted" (Newman 2001). While the high decision satisfaction may be real, it may also be due to positive response bias from cognitive dissonance, a phenomenon documented in invalidated patient satisfaction measurements (Carr‐Hill 1992) and an issue particularly relevant to surgical decision satisfaction (Homer 2000). von Oostrom 2003 writes, “Cognitive dissonance theory suggests that an autonomously made decision will be positively evaluated, especially when the decision is difficult to change.” Altschuler 2008 makes an important observation concerning decision satisfaction: “These findings suggest that even among women who report general satisfaction with their decision to have RRM via closed‐ended survey questions, lingering negative psychosocial outcomes can remain, particularly among women with BRRM. This dichotomy could be an important factor to discuss in counselling women considering the procedure.”
Women also need to understand that even when breast cancer is detected early through screening it still requires surgery and one or more of the following adjuvant (or neoadjuvant) therapies to increase the chances of a cure: chemotherapy, radiotherapy, and endocrine therapy, and possibly immunotherapy. These therapies also have their own side effects. So for some women, undergoing RRM in order to reduce the risk of developing breast cancer in the future may be preferable to living through a breast cancer diagnosis and the subsequent treatment required to reduce the risk of recurrence.
Authors' conclusions
Implications for practice.
Bilateral risk‐reducing mastectomy
Overall, while a number of case series and retrospective cohort studies indicate that bilateral risk‐reducing mastectomy (BRRM) is effective in reducing both incidence and death from breast cancer, various biases in the studies warrant caution in broadly applying these results. The state of the science is far from exact in predicting who will develop or die from breast cancer. By one estimate, most high‐risk women (determined by strong family history but not necessarily BRCA1/2 mutation carriers) who had BRRM would not have died from breast cancer even without the surgery.
BRRM is a radical surgical procedure to be considered only by those women at high risk, as it is not a procedure that should be routinely considered by women with an average risk of breast cancer. Even for BRCA mutation carriers, BRRM needs to be presented as an option along with other risk‐management strategies including risk‐reducing salpingo‐oophorectomy (RRSO), chemoprevention and breast screening. BRRM clearly reduces the incidence of breast cancer, but women also need to understand the risks, including psychological and physical harms.
Given the number of women who may be overtreated with BRRM or contralateral risk‐reducing mastectomy (CRRM), it is critical that women and clinicians understand the true risk for each individual woman before considering surgery, especially in consideration of comorbidities or lifestyle choices, or both. The paradox is that many women with breast cancer have breast‐conserving surgery, while BRRM removes the breasts of those who do not have breast cancer.
Contralateral risk‐reducing mastectomy
For women who have already been diagnosed with a primary tumor, the data show a reduction of incidence of contralateral breast cancer following CRRM. While it appears that CRRM reduces the incidence of cancer in the contralateral breast, there is limited evidence about whether, and for whom, CRRM may actually improve survival. There is increasing evidence that the survival data may be skewed by the evidence that women who have CRRM may be younger and healthier, with fewer comorbidities, than those who have unilateral treatment mastectomies only. The amount and quality of information given to women about CRRM should be improved in order to allow women consider properly the risk of mortality from contralateral disease versus from their primary breast cancer and mortality from tumor metastases.
Psychological and physical morbidity
The women who selected BRRM tended to be more anxious and more likely to believe it was inevitable that they would develop breast cancer. The surgery tended to reduce anxiety in these women. Understanding their true risk may reduce the anxiety and perception of inevitability of some of these women. Genetic counselling about risk can also change risk perception.
Regarding psychosocial outcomes, women generally reported satisfaction with their decision to have risk‐reducing mastectomy (RRM), but were less consistently favorable regarding the cosmetic outcome. Often, diminished cosmetic satisfaction was associated with surgical complications or reconstruction, or both. Therefore, physical morbidity, lifestyle choices and postoperative surgical complications are factors that should not be overlooked when making a decision about RRM.
With regards to emotional well‐being, most women recover well postoperatively, reporting reduced cancer worry and showing reduced psychological morbidity from their baseline measures, but exceptions were also noted. Of the psychosocial outcomes measured, body image and feelings of femininity were the most often adversely affected.
Beyond the informational needs, there is an emotional dimension to RRM, and Lloyd 2000 suggests psychological support should be part of the entire process from decision making to resuming life after surgery. Psychosocial outcomes may have long‐term effects, even in women who report satisfaction with their decision. Some of these women may have negative psychosocial outcomes, and this dichotomy should be considered by healthcare professionals when making decisions with individual women. These views are supported by findings that there are some differences between women who select BRRM (acceptors) and those that consider it but do not choose to have BRRM (decliners). Those selecting BRRM exhibited more anxiety‐relieving behavior, were more anxious and were more likely to feel it was inevitable that they would get breast cancer than decliners.
Decision making
Any woman with increased risk of breast cancer should consider having a discussion about the options and benefits of RRM, including her absolute risk of breast cancer, the benefits of RRM, and the potential harms (multiple surgeries, surgical complications, the possibility of chronic pain, impact on sexual function, and possible poor cosmetic outcome). The most important practice implications of these findings are that providers should offer understandable and complete information for women who are making their decision about whether to have RRM, and should ensure psychosocial support for the woman throughout the process. With genetic testing becoming more accessible, it will become even more important for clinicians to help women understand their lifetime risk of breast cancer and to counsel them on the benefits and harms of RRM. Women also need to understand that most women diagnosed with early breast cancer do not die from breast cancer, but the treatment required to achieve a cure can be quite extensive with many side effects.
Information on RRM and reconstruction is often given to women at the moment that the urge to survive predominates. It is possible that the "urge to reduce anxiety, remain healthy and survive" outweighs the possible negative outcomes of RRM and reconstruction (Bresser 2006). Also, “…the internet, combined with celebrity endorsements, has made the option of [RRM and] breast reconstruction more socially acceptable and an alternative to lifelong screening for many women” (Barry 2011).
Studies show that many women considering RRM can highly overestimate their risk of disease (Metcalfe 2002). Women considering BRRM should not only understand the risk of breast cancer, but also understand that many women having BRRM would not have died from breast cancer even without having the surgery. Women considering CRRM after a primary diagnosis of breast cancer should understand that there are few, good, long‐term data to indicate that CRRM, in and of itself, will improve survival. In a study of why women chose CRRM, Yao 2016 said: “The most common reason that women choose CPM [CRRM] is based on misperceptions about CPM’s effect on survival and overestimation of their contralateral breast cancer risk.” Given the available evidence, if RRM is considered at all, it should only be considered by women at high risk, e.g., BRCA mutation carriers with high‐penetrance mutations.".
There is often confusion about what 'risk' means for those women considering RRM, especially the difference between absolute risk and relative risk. It is important, therefore, that risk is translated into understandable terminology. With the field of breast cancer treatment changing rapidly, knowing her risk of developing the disease in the next 10 years might help a woman decide whether to have RRM now or postpone her decision for a few years to see what new preventions or treatments might become available. Consideration of other possible options of variable demonstrated efficacy, for example, tamoxifen, BRRSO, or simply surveillance, may also play a role in decision making.
In the end, this is a highly personal decision. Also, because both subcutaneous and total mastectomies result in incomplete removal of all breast tissue, women need to know that breast cancer can still occur after RRM (Eisen 2000). Ghosh 2002 suggests "risk‐reducing mastectomy" is a better term than "prophylactic mastectomy", since 'risk‐reducing' implies a reduction of risk rather than elimination of risk as prophylactic does (in the older studies in this review, the term 'prophylactic' is used, but risk‐reducing is now considered the proper term). Finally, women need to know that morbidity resulting in unanticipated reoperations is not uncommon with RRM.
For some women, avoiding the diagnosis and subsequent treatment of breast cancer is just as important as avoiding death from breast cancer. Many women overestimate their risk of dying from breast cancer, and many women underestimate the morbidity from RRM with or without reconstruction; clinicians need to help women understand the risks in order to make informed decisions. A decision aid to help women considering RRM weigh the benefits and harms of the options as they pertain to her, would be of tremendous help to these women.
Implications for research.
The benefits of BRRM relative to chemoprevention are unclear because there are no prospective, randomized trials comparing the two. This is also the case for CRRM; Bedrosian 2010 stated that “…despite these efforts, a causal relationship between survival and CRRM cannot be proved, that is only possible in a randomized controlled trial, unlikely to be completed in the foreseeable future.” While others call for randomized controlled trials (RCTs) (Palmieri 1999), it is very unlikely that a RCT will ever be conducted given the radical nature of the procedure.
In the absence of RCTs, research can be improved by the use of population‐based, prospective data that are collected on all women, such as in the Scandinavian prospective cohort study (Meijers‐Heijboer 2001). Such studies should adequately adjust for other variables that may influence the outcome, include morbidity data, confounding therapies, and have sufficient follow‐up time. As a short‐term goal, authors of the included studies are encouraged to update their findings and control for major confounders in the analyses, a major limitation of the published studies thus far. Studies of the effectiveness of RRM with and without RRSO need to be conducted, the data separated out and controlled for in future studies or analyses.
Physical morbidity was not uncommon following RRM, and many women underwent unanticipated reoperations, usually due to problems with reconstruction. These data should be updated to reflect changes in surgical procedures and reconstruction. Patient satisfaction was the least favorable regarding feeling of support provided by healthcare practitioners when providing risk assessment information. Further research needs to focus on how to make this information more understandable and how to minimize patients' stress when receiving it.
Establishing a RRM registry that includes all cases of RRM and certain details about those undergoing the procedure has been proposed by some as a way to glean important RRM information in the absence of a RCT. Without adequate legal protections, inclusion in such a registry could have adverse consequences for participants (and possibly their families) with respect to insurance and employment discrimination; however, with the establishment of Health Insurance Portability and Accountability Act (HIPPA) regulations, the passage of the Genetic Information Nondiscrimination Act (GINA) in 2008, and the passage of the Patient Protection and Affordable Care Act in 2010 in the USA, these concerns have been somewhat addressed and diminished. Similar legal issues could exist for the establishment of a tissue bank in conjunction with a registry that would shed light on whether certain mutations are most likely to manifest in breast cancer in spite of RRM. However, RRM and tissue bank registries could help relieve some of the inconsistencies in reporting of procedures and outcomes in published articles. We have found it difficult in some cases to determine if follow‐up reports are truly a continuation of the same participant group or not.
Prospective studies that collect baseline information prior to the intervention using validated instruments are needed to better understand the psychological impact of RRM. There also needs to be more understanding of the emotional impact on women of having the surgery in order to better support those women who choose it. As Brandberg 2008 noted, “One drawback of this study is that cancer‐specific worries were not measured, an important issue when assessing distress among women with hereditary cancer syndromes.” Future research could also focus on developing a screening tool that can predict those who are at risk for high emotional distress and, hence, may need additional supportive services.
Little is reported about the psychosocial impact of BRRM and CRRM on the people who have primary relationships with women undergoing the surgery. While a high‐risk woman may accept and adjust to the cosmetic and sexuality side effects of RRM because of the peace of mind it offers, how her partner adjusts is unknown. Future studies should include interviews with those in primary relationships with women undergoing RRM. Finally, the study finding by Josephson 2000 that most women were dissatisfied with the psychological support provided by healthcare personnel during risk counselling demonstrated that little is known about what creates an optimal counselling and decision‐making environment.
What's new
| Date | Event | Description |
|---|---|---|
| 10 January 2019 | Review declared as stable | Due to the complexity of this topic and studies included in this review, Cochrane has advised that additional revisions on this topic are required. Rather than updating the review, the topic will be split into two new review topics presenting the evidence separately for women diagnosed with breast cancer compared to those unaffected. The two new titles to address this topic will be: (1) Women with a previous or current diagnosis of breast cancer with or without a risk factor and (2) Women without breast cancer with a risk factor (e.g. BRCA1/2 mutation carriers) |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 9 July 2016 | New citation required but conclusions have not changed | Conclusions are unchanged |
| 9 July 2016 | New search has been performed | Performed searches for new studies on 9 July 2016. Thirty new studies were included in the review since the previous version of this review. We removed six small studies with fewer than 20 participants from the review. |
| 14 July 2010 | New citation required but conclusions have not changed | Sixteen new studies were incorporated into the updated review. Conclusions not changed. |
| 14 June 2006 | New search has been performed | Performed search for new studies on the 14th June 2006. |
| 23 June 2004 | New citation required and conclusions have changed | Publication of review |
| 30 August 2000 | Amended | Publication of protocol |
Notes
Due to the complexity of this topic and studies included in this review, Cochrane has advised that additional revisions on this topic are required. Rather than updating the review, the topic will be split into two new review topics presenting the evidence separately for women diagnosed with breast cancer compared to those unaffected. The two new titles to address this topic will be: (1) Women with a previous or current diagnosis of breast cancer with or without a risk factor and (2) Women without breast cancer with a risk factor (e.g. BRCA1/2 mutation carriers).
Acknowledgements
Assistance provided to the Review author team for the original review can be viewed in Lostumbo 2010. We would like to thank Dr Kay Dickersin, from the United States Cochrane Center, and Sharon Parker, Melina Willson, Slavica Berber, and all the Cochrane Breast Cancer editors for their comments and suggestions. We also are most grateful for the technical assistance extended by Kristina Lindsey, from the United States Cochrane Center, to update the previous review in Review Manager 5. Finally, we would like to dedicate this review to the memory of Mrs Annette Drummond, a long‐standing breast cancer advocate, friend, and colleague, who tirelessly worked with us on this review until her death from breast cancer in 2002.
Appendices
Appendix 1. CENTRAL Search
We searched Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) via the Cochrane Library using following search strategy:
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer* #3 breast near neoplasm* #4 breast near carcinoma* #5 breast near tumour* #6 breast near tumour* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Mastectomy] explode all trees #9 MeSH descriptor: [Mastectomy, Subcutaneous] explode all trees #10 MeSH descriptor: [Mastectomy, Extended Radical] explode all trees #11 MeSH descriptor: [Mastectomy, Segmental] explode all trees #12 MeSH descriptor: [Mastectomy, Radical] explode all trees #13 MeSH descriptor: [Mastectomy, Modified Radical] explode all trees #14 MeSH descriptor: [Mastectomy, Simple] explode all trees #15 #8 or #9 or #10 or #11 or #12 or #13 or #14 #16 mastectomy* #17 MeSH descriptor: [Mammaplasty] explode all trees #18 mammaplasty* or mammoplasty* #19 #15 or #16 or #17 or #18 #20 prophylac* or prophylaxis or prevent* or risk‐reducing #21 #19 and #20 #22 prophylactic* next (surger* or resect* or mastectom* or mammaplast* or mammoplast*) #23 prevent* next (surger* or resect* or mastectom* or mammaplast* or mammoplast*) #24 risk‐reducing* next (surger* or resect* or mastectom* or mammaplast* or mammoplast*) #25 #22 or #23 or #24 #26 #21 or #25 #27 #7 and #26 Publication Year from 2012 to 2016
Appendix 2. MEDLINE Search
For this review, the first updated search of MEDLINE was conducted in February 2012 using following search strategy:
| # | Searches |
| 1 | exp Breast Neoplasms/ |
| 2 | prophylac$.mp. |
| 3 | prophylaxis.mp. |
| 4 | subcutaneous.mp. |
| 5 | hypertrophy.mp. |
| 6 | 2 or 3 or 4 or 5 |
| 7 | exp Mastectomy, Subcutaneous/ or exp Mastectomy, Extended Radical/ or exp Mastectomy, Segmental/ or exp Mastectomy/ or exp Mastectomy, Radical/ or exp Mastectomy, Modified Radical/ or exp Mastectomy, Simple/ |
| 8 | mastectom$.mp. |
| 9 | exp Mammaplasty/ |
| 10 | mammoplast$.mp. |
| 11 | 7 or 8 or 9 or 10 |
| 12 | (risk‐reducing adj surgery).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 13 | (risk‐reducing adj surgeries).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 14 | (preventive adj resection).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 15 | (preventive adj mastectomy).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 16 | (risk‐reducing adj treatment#).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 17 | 12 or 13 or 14 or 15 or 16 |
| 18 | 1 and 6 and 11 |
| 19 | 1 and 17 |
| 20 | 18 or 19 |
| 21 | limit 20 to (humans and yr="2006 ‐Current") |
The second search of MEDLINE was conducted on 14 July 2016 using following search strategy:
Case‐Control Studies/
Control Groups/
Matched‐Pair Analysis/
Retrospective Studies/
((case* adj5 control*) or (case adj3 comparison*) or control group*).ti,ab.
or/1‐5
Cohort Studies/
Longitudinal Studies/
Follow‐Up Studies/
Prospective Studies/
Retrospective Studies/
cohort.ti,ab.
longitudinal.ti,ab.
prospective.ti,ab.
retrospective.ti,ab.
or/7‐15
exp Breast Neoplasms/
(breast adj6 cancer$).tw.
(breast adj6 neoplasm$).tw.
(breast adj6 carcinoma$).tw.
(breast adj6 tumo?r$).tw.
or/17‐21
exp Mastectomy, Subcutaneous/ or exp Mastectomy, Extended Radical/ or exp Mastectomy, Segmental/ or exp Mastectomy/ or exp Mastectomy, Radical/ or exp Mastectomy, Modified Radical/ or exp Mastectomy, Simple/
mastectom$.tw.
exp Mammaplasty/
mamm?plast$.tw.
or/23‐26
prophylac$.tw.
prophylaxis.tw.
prevent$.tw.
risk‐reducing.tw.
28 or 29 or 30 or 31
27 and 32
(prophylactic$ adj (surger$ or resect$ or mastectom$ or mamm?plast$)).tw.
(prevent$ adj (surger$ or resect$ or mastectom$ or mamm?plast$)).tw.
(risk‐reducing adj (surger$ or resect$ or mastectom$ or mamm?plast$)).tw.
34 or 35 or 36
exp Genes, BRCA1/
exp Genes, BRCA2/
(BRCA1 or BRCA2).tw.
exp Genetic Predisposition to Disease/
genetic risk.tw.
38 or 39 or 40 or 41 or 42
27 and 43
33 or 37 or 44
22 and 45
46 and (6 or 16)
Appendix 3. Embase Search
For this review, the first updated search of Embase was conducted in February 2012 via Embase.com using the following search strategy:
'breast neoplasm'
'breast cancer'/exp OR 'breast cancer'
'breast carcinoma'/exp OR 'breast carcinoma'
'breast neoplasm'
'breast neoplasia'
'breast tumour'
'breast tumor'/exp OR 'breast tumor'
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
prophylac*
'prophylaxis'/exp OR prophylaxis
'hypertrophy'/exp OR hypertrophy
'subcutaneous'/exp OR subcutaneous
#9 OR #10 OR #11 OR #12
mastectom*
mammoplast*
'lymph node dissection'/exp OR 'lymph node dissection'
#14 OR #15 OR #16
partial
'recurrence'/de OR recurrence
'counseling'/de OR counseling
bilateral
'cancer survival'/exp OR 'cancer survival'
'cancer mortality'/exp OR 'cancer mortality'
#18 OR #19 OR #20 OR #21 OR #22 OR #23
'prophylatic surgery'
'risk‐reducingsurgeries'
'preventive resection'
'preventive mastectomy'
'risk‐reducingtreatment?'
#25 OR #26 OR #27 OR #28 OR #29
#8 AND #13 AND #17 AND #24
#8 AND #24 AND #30
#31 OR #32
#33 AND [humans]/lim AND [embase]/lim AND [2006‐2012]/py
The second search of Embase was conducted on 14 July 2016 via OvidSP using following search strategy:
exp case control study/
case control study.ti,ab.
((case control or case base or case matched or retrospective) adj1 (analys* or design* or evaulation* or research or stud* or survey* or trial*)).ti,ab.
or/1‐3
exp retrospective study/
exp prospective study/
((cohort or concurrent or incidence or longitudinal or followup or 'follow up' or prospective or retrospective) adj1 (analys* or design* or evaluation* or research or stud* or survey* or trial*)).ti,ab.
or/5‐7
exp breast/
exp breast disease/
(9 or 10) and exp neoplasm/
exp breast tumor/
exp breast cancer/
exp breast carcinoma/
(breast$ adj5 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab.
or/11‐15
exp partial mastectomy/ or exp subcutaneous mastectomy/ or exp mastectomy/ or exp segmental mastectomy/
mastectom$.tw.
exp breast reconstruction/
mamm?plast$.tw.
17 or 18 or 19 or 20
prophylac$.tw.
prophylaxis.tw.
prevent$.tw.
risk‐reducing.tw.
22 or 23 or 24 or 25
21 and 26
(prophylactic$ adj (surger$ or resect$ or mastectom$ or mamm?plast$)).tw.
(prevent$ adj (surger$ or resect$ or mastectom$ or mamm?plast$)).tw.
(risk‐reducing adj (surger$ or resect$ or mastectom$ or mamm?plast$)).tw.
28 or 29 or 30
(BRCA1 or BRCA2).tw.
exp genetic risk/
exp disease predisposition/
genetic risk.tw.
32 or 33 or 34 or 35
21 and 36
27 or 31 or 37
16 and 38
39 and (4 or 8)
Appendix 4. WHO ICTRP Search
The first updated search for this review was performed in February 2012 using the following search strategy:
Advanced Search:
1. Title: risk‐reducing mastectomy for the prevention of breast cancer
Recruitment Status: ALL
2. Condition: breast cancer OR breast cancers OR breast carcinoma OR breast carcinomas OR breast neoplasm OR breast neoplasms
Intervention: mastectomy OR risk‐reducing mastectomy OR risk‐reducing surgery OR risk‐reducing mastectomies OR risk‐reducing surgeries OR preventative mastectomy OR preventative surgery OR preventative mastectomies OR preventative surgeries OR risk‐reducing resection OR risk‐reducing resections OR preventative resection OR preventative resections
Recruitment Status: ALL
Second search was performed in May 2016 using following search strategy:
Basic search:
1. risk‐reducing mastectomy
2. prophylactic mastectomy
3. breast cancer AND mastectomy AND risk
Advanced Search:
Condition: breast cancer OR breast carcinoma OR breast neoplasm
Intervention: prophylactic surgery OR prophylactic resection OR preventative mastectomy OR preventative surgery OR preventative resection OR risk‐reducing mastectomy OR risk‐reducing surgery OR risk‐reducing resection
Recruitment Status: ALL
Appendix 5. ClinicalTrials.gov Search
ClinicalTrials.gov was searched in May 2016 using following search strategy:
Basic search:
1. risk‐reducing mastectomy
2. prophylactic mastectomy
3. breast cancer AND mastectomy AND risk
Advanced Search:
1. Recruitment: All studies
Study results: All studies
Study type: All studies
Gender: All studies
Conditions: breast cancer OR breast neoplasm
Interventions: prophylactic surgery OR prophylactic resection OR preventative mastectomy OR preventative surgery OR preventative resection OR risk‐reducing mastectomy OR risk‐reducing surgery OR risk‐reducing resection
2. Search terms: prophylaxis OR prophylactic OR preventative OR risk
Recruitment: All studies
Study results: All studies
Study type: All studies
Gender: All studies
Conditions: breast cancer OR breast neoplasm
Interventions: mastectomy
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altschuler 2008.
| Methods | Retrospective series | |
| Participants | 684 women 3–22 years after they had had RRM between 1979 and 1999, who were aged 18–80 years at 6 community health centers were mailed surveys | |
| Interventions | BRRM = 177 CRRM = 567 |
|
| Outcomes | Quality of life ‐ general satisfaction | |
| Notes | 519 CRRM subjects duplicates of Geiger 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. These were women who had RRM at least 3 years previously in 6 health systems and were selected from a medical record review from the Cancer Research Network database. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way, via medical record review from the Cancer Research Network database. |
| Free of detection bias? | High risk | There were no pre‐CRRM assessments of psychosocial factors for comparison and it is unknown whether the questionnaire used had been tested for reliability or validity |
| Free of attrition bias? | High risk | 78 BRRM and 205 CRRM women did not respond to closed‐ended questionnaire A further 39 BRRM and 318 women did not respond to 2 open‐ended questions |
Arver 2011.
| Methods | Retrospective series | |
| Participants | 223 high‐risk women (> 20%) without a previous breast malignancy who had BRRM performed at 8 hospitals in Sweden between 1995 and 2005 | |
| Interventions | BRRM | |
| Outcomes | BC incidence Physical morbidity ‐ complications, reoperations |
|
| Notes | Includes 24 Gahm 2007 subjects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. These were “Women without a previous breast malignancy who had undergone BRRM in Sweden between January 1, 1995 and December 31, 2005”. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. “Oncologists and geneticists at the university hospitals throughout Sweden … were asked to identify women who had been referred for risk‐reducing surgery”. |
| Free of detection bias? | Low risk | The outcomes were assessed in a valid way. A questionnaire was developed in collaboration with collaborator input. “Data were derived from patient charts, the regional Oncology Centers, and the nationwide Cause of Death Register”. |
| Free of attrition bias? | Low risk | There was a low dropout rate and dropouts/withdrawals were sufficiently accounted for. Only 15% of cases did not have genetic screening, and 1 woman declined mapping. |
Barton 2005.
| Methods | Retrospective cohort | |
| Participants | Family history/risk ‐ not reported | |
| Interventions | BRRM = 269 in 6 community‐based health plans from 1979‐1998 | |
| Outcomes | Physical morbidity | |
| Notes | Same subjects as Geiger 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Used automated data source, hospital data, chart elements for eligibility. Excluded women with previous cancer using cancer registry. |
| Free of performance bias? | Low risk | Used charts to confirm complications |
| Free of detection bias? | Low risk | Used validated quality control programs for medical record abstractions |
| Free of attrition bias? | Low risk | No dropouts noted, recorded length of follow‐up |
Bedrosian 2010.
| Methods | Retrospective population cohort | |
| Participants | Unilateral breast cancer Stage 0‐III | |
| Interventions | CRRM ‐ 8902 SEER patients Controls ‐ controls 98,204 (91.7%) SEER patients who had unilateral mastectomy |
|
| Outcomes | Disease‐specific survival 5‐year survival BC incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. The study identified the relevant population in the SEER database using explicit inclusion and exclusion criteria. Baseline characteristics of patients between the groups were not statistically significantly different. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. The study used the SEER database to confirm RRM. |
| Free of detection bias? | Low risk | The outcomes were assessed in a valid way. The study used SEER database data. |
| Free of attrition bias? | Unclear risk | Unknown – if patients migrated out of SEER regions they would be missed. |
Borgen 1998.
| Methods | Convenience sample | |
| Participants | Family history/risk ‐ 220 (69%) reported having at least one 1st degree relative with breast cancer | |
| Interventions | BRRM = 370 | |
| Outcomes | Quality of life BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Because the participants responded to advertisements, those who responded could be different in some important way than those who did not respond. |
| Free of performance bias? | High risk | The participants self‐reported having had a BRRM |
| Free of detection bias? | High risk | The participants reported about their regrets and satisfaction of having BRRM. There is possible recall bias from collecting all psychological data postoperatively. |
| Free of attrition bias? | Low risk | The report only included women who responded to the questionnaire. |
Boughey 2010.
| Methods | Retrospective cohort | |
| Participants | Participants: 385 women with stage I or II BC and a family history of BC, who underwent therapeutic mastectomy and CRRM between 1971 and 1993 at one institution. Controls: 385 participants matched on age at diagnosis, tumor stage, nodal status, and year of diagnosis who underwent therapeutic mastectomy only at 1 institution |
|
| Interventions | CRRM | |
| Outcomes | BC Incidence Overall survival Disease‐specific survival Disease‐free survival |
|
| Notes | Same participants as McDonnell 2001, which reports on BC incidence Same participants as Frost 2005, which reports on physical morbidity & QoL |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | All of the CRRM cohort had a family history of BC, whereas only 34.8% of the no‐CRRM group did (P < 0.0001). Similarly, there was a statistically significant difference in the proportion with a first‐degree family history (46.2% CRRM vs 21.6% No CRRM, P < 0.0001) |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. Participants' medical records were reviewed |
| Free of detection bias? | Low risk | The outcomes were assessed in a valid way. Participants’ medical records were reviewed, and follow‐up information was obtained from the Mayo Cancer Registry. For participants not from the Mayo Clinic, “a study‐specific questionnaire was used to collect data”. |
| Free of attrition bias? | Low risk | There did not seem to be any attrition or dropouts. |
Brandberg 2008.
| Methods | Prospective series | |
| Participants | 90 of 98 consecutive women with a hereditary risk of BC who underwent BRRM with reconstruction during October 1997 to December 2005 following counseling about the impact of BRRM | |
| Interventions | BRRM plus reconstruction | |
| Outcomes | QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. “Consecutive women who had BRRM including breast reconstruction between October 1997 and December 2005 were eligible.” |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. All women underwent BRRM at 1 institution (the Karolinka University Hospital) and participated in the same patient consultation program. |
| Free of detection bias? | High risk | The Sexual Activity Questionnaire (SAQ) had no formal validation or reliability testing on the Swedish translation. 24 of 98 (25%) had BRRSO, known to affect sexuality prior to BRRM. |
| Free of attrition bias? | High risk | 65 of the 90 responded to questionnaires 1 year post‐BRRM, “…making the group that could be analyzed over time small and provides limited power to determine statistically significant differences.” |
Bresser 2006.
| Methods | Retrospective cohort study | |
| Participants | Family history/risk ‐ BRCA1/2 carriers or women with 50% risk of BC | |
| Interventions | BRRM or CRRM with reconstruction = 114 women at 1 institution between 1994‐2002 who completed a questionnaire 37 respondents with BC 77 respondents without BC |
|
| Outcomes | QoL | |
| Notes | 136 women were sent the questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Participants were recruited through BC relatives. |
| Free of performance bias? | Low risk | Used database of follow‐up study of RRM at institution |
| Free of detection bias? | High risk | The questionnaire used had not been tested for reliability or validity and there were no pre‐CRRM assessments of psychosocial factors for comparison. |
| Free of attrition bias? | Low risk | Accounted for 2 participants who had moved; some participants did not answer all questions, but respondents and non‐respondents did not differ demographically |
Brewster 2012.
| Methods | Retrospective series | |
| Participants | The prospective Breast Cancer Management System database of The University of Texas MD Anderson Cancer Center was used to identify women with clinical stage 1‐3 primary unilateral invasive BC who underwent a mastectomy between June 1997 and August 2009. Excluded women were: bilateral BC; contralateral invasive or DCIS incidentally discovered at the time of CRRM. 532 CRRM vs 335 no‐CRRM. Matched analysis used 497 CRRM vs 497 no‐CRRM. |
|
| Interventions | CRRM | |
| Outcomes | Disease‐free survival Overall survival BC incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | The study used explicit inclusion and exclusion criteria. The study used statistical methods to balance the demographic and clinical characteristics between CRRM and control groups (i.e. created “matched patients”) |
| Free of performance bias? | Low risk | The study used various database sources via direct review of medical records and linkage to registry data during the follow‐up period. The treatments were all performed at a single institution within a relatively moderate timeframe (i.e. 12‐year period). |
| Free of detection bias? | Low risk | The study used various database sources via direct review of medical records and linkage to registry data during the follow‐up period. The treatments were all performed at a single institution within a relatively moderate timeframe (i.e. 12‐year period). |
| Free of attrition bias? | Low risk | There was database and registry data on all included participants. |
Chung 2012.
| Methods | Retrospective case control | |
| Participants | Used the John Wayne Cancer Institute Prospective Breast Database to identify women diagnosed with unilateral stage 0‐3 BC who had CRRM at the John Wayne Cancer Institute between Jan 1995 and Nov 2008. Women were excluded if they had clinically detected concurrent bilateral malignancies or had previous mastectomy. Women who underwent a unilateral total mastectomy for unilateral BC were the control group. CRRM = 177. Controls = 178. | |
| Interventions | CRRM with or without immediate reconstruction | |
| Outcomes | Disease‐free survival Overall survival Distant metastases‐free survival |
|
| Notes | Overall, there were 68 of 355 participants (19.1%) with DCIS, 148 of 355 (41.7%) with stage I invasive BC, 138 of 355 (38.9%) with stage II, and only 1 of 355 (0.003%) presented with stage III disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Unclear risk | Table 2 shows that there is a statistically significant difference between CRRM and control groups for the presence of BRCA mutation (e.g. 80% are negative in the CRRM group vs 94% are negative in the control group) and women with a family history of BC (64% had a history in the CRRM group vs 41% had a history in the control group) |
| Free of performance bias? | Low risk | All participants were treated at a single institution within a relatively moderate timeframe (i.e. 13‐year period). |
| Free of detection bias? | Low risk | The study relied on objective clinical results as reported in the database. Survival is an objective measure. |
| Free of attrition bias? | Low risk | There was database data on all included participants. |
Contant 2002.
| Methods | Retrospective case series | |
| Participants | Family history/risk ‐ BRCA1/2 mutation carriers or 50% risk carriers of a germ‐line mutation based on BC in their mothers | |
| Interventions | BRRM or CRRM = 122 high‐risk participants who underwent RRM at one institution between December 1993 and December 1999 BRRM = 83 who had no previous BC (2 had DCIS) CRRM + full removal of diseased breast if previously treated with breast‐conserving surgery = 29 who had previous BC |
|
| Outcomes | BC incidence Physical morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Used medical records of all women who chose RRM at Family Cancer Clinic |
| Free of performance bias? | Low risk | Used medical records at clinic |
| Free of detection bias? | High risk | There was no information on how BRRSO performed on some participants, which confounded the data |
| Free of attrition bias? | Low risk | No dropouts, only deaths recorded that stopped follow‐up |
de la Pena‐Salcedo 2012.
| Methods | Retrpospective cohort | |
| Participants | 52 patients: 40 CRRM and 12 BRRM = 64 breasts removed prophylactically from 1/1/1985 to 12/31/2010 | |
| Interventions | BRRM & CRRM, all with reconstruction | |
| Outcomes | Physical morbidity QoL |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | All patients who had PM from 1/1/1985 to 12/31/2010 and did not have exclusion factors |
| Free of performance bias? | Low risk | The participants were included based on a review of medical records |
| Free of detection bias? | Low risk | The outcomes were based on a review of medical records |
| Free of attrition bias? | Low risk | No attrition was reported |
Den Heijer 2012.
| Methods | Longitudinal prospective observational series | |
| Participants | 36 of 52 women at high‐risk for hereditary breast/ovarian cancer at the Family Cancer Clinic of the Erasmus MC – Daniel den Hoed Cancer Centre who had RRM with/without reconstruction or BRRSO from 1999‐2003 and participated in a previous study, who were asked in 2007 to participate in a long‐term follow‐up if they had not developed a new cancer or recurrence since their RRM. Participants were assessed at 2‐4 weeks (T0) before RRM (T1), 6 months after RRM and 6‐9 years (T2) after RRM. | |
| Interventions | BRRM or CRRM with or without reconstruction and/or BPSO | |
| Outcomes | Psychological distress Body image Morbidity |
|
| Notes | 13 (36%) had history of BC or ovarian cancer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Unclear risk | 16 invited women did not participate. Reasons given include complications leading to removal of prosthesis, diagnosis of ovarian cancer. |
| Free of performance bias? | Low risk | The participants were invited based on a review of medical records. |
| Free of detection bias? | Low risk | The outcomes were assessed in a valid way. These surveys used several standardized, validated and reliable scales to measure various outcomes of interest |
| Free of attrition bias? | Low risk | All of the participants returned the surveys. |
Evans 1999.
| Methods | Case series | |
| Participants | Family history/risk ‐ women with a lifetime risk of BC ranging from 25%‐80% using the Claus data | |
| Interventions | BRRM = 141
CRRM = 37 Comparison group: statistically modeled group based on the Claus model presuming no RRM |
|
| Outcomes | BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Only women who had undergone BRRM at the participating centers were included. |
| Free of performance bias? | Low risk | Participants had had BRRM at participating centers |
| Free of detection bias? | Unclear risk | It is uncertain how the estimation of risk is affected by use of the Claus model. |
| Free of attrition bias? | Low risk | The 174 women were followed for 400 women years. |
Evans 2013.
| Methods | Longitudinal prospective observational | |
| Participants | Women diagnosed with first unilateral BC after 1 January 1985 and before 31 December 2010, and who tested positive for pathogenic mutation in BRCA1 or BRCA2. A total of 718 women were eligible. CRRM = 105. No surgery (control 1) = 473. BRRSO but no CRRM (control 2) = 120. | |
| Interventions | CRRM | |
| Outcomes | Overall survival Time to recurrence Recurrences Mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | The study used explicit inclusion and exclusion criteria. All cases of BC were confirmed by hospital/pathology records, or from Regional Cancer Registries |
| Free of performance bias? | Unclear risk | Participants were included via a pathology and hospital record review. All participants were treated within a relatively moderate timeframe (i.e. 31‐year period) |
| Free of detection bias? | Low risk | Women were followed up throughout the study, and dates of deaths were obtained from the cancer registry or from death certificates. Survival is an objective measure. |
| Free of attrition bias? | Low risk | No attrition was reported |
Frost 2000.
| Methods | Case series | |
| Participants | Family history/risk ‐ all had a family history of BC 35% high risk ‐ had a pedigree consistent with a single‐gene autosomal dominant predisposition to BC 65% moderate risk |
|
| Interventions | BRRM = 609 | |
| Outcomes | QoL | |
| Notes | Duplicate subjects of Hartmann 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | All 609 living women who had BRRM at the Mayo Clinic from 1960‐1993 were invited to participate |
| Free of performance bias? | Low risk | Chart review identified women who had BRRM at the institution, who were then invited to participate |
| Free of detection bias? | High risk | There is possible recall bias from collecting all psychological data postoperatively. |
| Free of attrition bias? | Low risk | 572 of the 609 (94%) women sent the questionnaire responded |
Frost 2005.
| Methods | Retrospective case series | |
| Participants | Family history/risk ‐ had a personal and a family history of BC | |
| Interventions | CRRM ‐ 583 women who had CRRM between 1960 to1993 at a single institution | |
| Outcomes | QoL Physical morbidity | |
| Notes | Follow‐up report Frost 2011 Same participants as McDonnell 2001, which reports on BC incidence Same participants as Boughey 2010, which reports on survival and incidence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Women identified through Mayo Clinic Surgical Index who had CRRM |
| Free of performance bias? | Low risk | Used medical records |
| Free of detection bias? | High risk | There were no pre‐CRRM assessments of psychosocial factors for comparison and there is possible recall bias from collecting all psychosocial data postoperatively. |
| Free of attrition bias? | Low risk | Sent questionnaire to those known to be alive at time of study; 94% of women answered |
Gabriel 1997.
| Methods | Case series | |
| Participants | Women having breast implant surgery Family history/risk ‐ not reported | |
| Interventions | Breast implant surgery from RRM = 92 from cancer = 125 for cosmesis = 532 | |
| Outcomes | Physical morbidity | |
| Notes | Duplicate subjects with Hartmann 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Only women who had breast implants at the Mayo Clinic and who lived in the Olmsted County, MN, USA, were invited to participate. |
| Free of performance bias? | Low risk | Nurse‐abstractors reviewed charts to verify surgical procedures and complications. |
| Free of detection bias? | Low risk | Nurse‐abstractors reviewed charts to verify surgical procedures and complications. |
| Free of attrition bias? | Low risk | Mean follow‐up 7.8 years (0‐25.8 years) |
Gahm 2007.
| Methods | Retrospective cohort | |
| Participants | Participants: 24 women with an increased risk from BRCA1 or BRCA2 mutations or a family pattern indicating genetic inherited BC who underwent BRRM and immediate reconstruction from 1993‐2005 and were 2 years' post‐BRRM. Controls: 16 women who had had no previous breast surgery |
|
| Interventions | BRRM and immediate reconstruction | |
| Outcomes | Physical morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. The study used explicit inclusion criteria and women who all attended the Karolinska University Hospital. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. All women had BRRM and immediate reconstruction with implants at a single institution (Karolinska University Hospital). One surgeon performed the majority of the BRRM, and one surgeon did all the reconstructions. |
| Free of detection bias? | Low risk | The outcomes were assessed in a valid way. All patients completed a baseline questionnaire, and all had subsequent sensory testing done under similar conditions. |
| Free of attrition bias? | Low risk | There was a low dropout rate and dropouts/withdrawals were sufficiently accounted for. |
Gahm 2010.
| Methods | Retrospective cohort | |
| Participants | A total of 59 consecutive patients with an increased risk of BC but without a personal history of BC underwent BRRM and immediate reconstruction between 2004 and 2006 at one institution. Reference sample: 1725 women from the general population used in previous study |
|
| Interventions | BRRM and immediate reconstruction | |
| Outcomes | Physical morbidity QoL |
|
| Notes | Some participants may also be participants in Gahm 2007 and Anvers 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. The study used explicit inclusion criteria and women who all attended the Karolinska University Hospital. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. All women had BRRM and immediate reconstruction with implants at a single institution (Karolinska University Hospital). One surgeon performed the majority of the BRRM, and one surgeon did all the reconstructions. |
| Free of detection bias? | High risk | There is no information on how BRRSO performed on 21 of the participants confounded the results |
| Free of attrition bias? | High risk | The response rate on the SF‐36 in study II was low, (64%) |
Geiger 2005.
| Methods | Retrospective cohort study | |
| Participants | Family history/risk ‐ women with family history of BC especially first‐degree relatives or high risk defined as atypical hyperplasia, > one breast biopsy, LCIS, micro‐calcifications, or ovarian cancer | |
| Interventions | BRRM = 276 women aged 18‐80 years who enrolled in 6 health plans of the NCI Cancer Research Network between 1979‐1998 Controls = 196 random sample women taken from 689 eligible women representing underlying cohort of 666,800 women with elevated risk for BC without RRM |
|
| Outcomes | BC mortality BC incidence | |
| Notes | Many of these participants also participants in Geiger 2007, which reported on QoL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Accepters had more risk factors for BC than decliners (controls) |
| Free of performance bias? | Low risk | Used 6 health plans of Cancer Research Network, their computerized data and medical records to select high‐risk women with BRRM and without |
| Free of detection bias? | Low risk | Used hospitalization, cancer registry & ambulatory care data; oversampled women without BRRM; used PHREG procedure for analysis and Gail model to determine BRRM efficacy |
| Free of attrition bias? | Low risk | No attrition noted; originally excluded women with history of BC. Censoring occurred at several measures. |
Geiger 2006.
| Methods | Retrospective case series | |
| Participants | 637 women diagnosed with BC between 1979‐1999 at 1 of 6 health care systems in NCI Cancer Research Network, who participated in Herrinton 2005 study and returned surveys mailed to them. Family history/risk ‐ not reported | |
| Interventions | CRRM acceptors = 519 CRRM decliners = 61 | |
| Outcomes | QoL | |
| Notes | Same participants as Herrinton 2005, which reported on BC mortality and incidence; 877 women were mailed surveys | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Deceased women could not be surveyed, which could have skewed QoL data. Also, IRBs at 4 of 6 centers excluded women if their physicians declined to approve their enrolment; reason for refusal unknown ‐ poor physical health, mental health, etc. CRRM selectors were more likely to be white than non‐selectors (86.1% vs 72.1%) and have higher BMI (BMI > 30 for 31.1% versus 18.0%) |
| Free of performance bias? | Low risk | Data confirmed by medical record review, including verification that CRRM done for prophylaxis |
| Free of detection bias? | Low risk | Data collection modeled on method of Dillman from mailed surveys; used items from Functional Assessment of Cancer Therapy – Breast Cancer and other various cited scales. Also pilot tested survey before use |
| Free of attrition bias? | Low risk | Respondents and non‐respondents (women from previous study with exclusions for deceased, physician denial of access, invalid addresses) did not differ in demographic characteristics or family history of BC. Respondents who omitted more than 25% of questions were eliminated from analysis. |
Geiger 2007.
| Methods | Retrospective cohort | |
| Participants | 195 of original 276 women reported in Geiger 2005 who had at least one qualifying BC risk factor noted in their medical record, and had no personal history of BC and who had bilateral subcutaneous or more extensive RRMs from 1979‐1999 Controls: 117 of original 206 controls in Geiger 2005 selected from a random sample of women at elevated BC risk but with no RRMs, frequency‐matched within each healthcare delivery system by year of birth were mailed the survey. |
|
| Interventions | BRRM | |
| Outcomes | QoL | |
| Notes | These participants also participants in Geiger 2007, which reported on mortality and incidence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | 16 BRRM ad 9 no‐BRRM women were excluded at 3 healthcare delivery systems. Institutional Review Boards required that women be excluded if their physicians declined to give approval for their recruitment |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. Relevant procedures were confirmed by medical record review and verification of BRRM. |
| Free of detection bias? | Unclear risk | “We found that concern about breast cancer exists in similar percentages among women with and without bilateral risk‐reducing mastectomy, but in the absence of a baseline measure, our study is unable to examine a reduction in concern as a result of the procedure.” |
| Free of attrition bias? | High risk | 312 of the 482 women in the Geiger 2005 study were contacted by mail and 181 (58.0%) returned surveys, 60% BRRM and 54.7% no‐BRRM |
Goldflam 2004.
| Methods | Retrospective case series | |
| Participants | 239 women who had CRRM at one center between 1987‐1997; all had unilateral primary BC, tumor staged as 0‐II, with no clinical or radiological findings in the contralateral breast prior to surgery | |
| Interventions | CRRM | |
| Outcomes | All‐cause mortality BC mortality Physical morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Used databases maintained by Dept. of Medical Informatics at cancer center to identify women who had bilateral mastectomies and exclude those with bilateral BC. However, likely that participants in CRRM group were selected for higher percent risk of CBC |
| Free of performance bias? | Low risk | Used medical records, operative reports, pathology reports to confirm BC |
| Free of detection bias? | Low risk | Used medical records, pathology reports to confirm contralateral BC or not; used several studies based on SEER data plus Gail model to calculate number of CBCs if no CRRM. Some self‐reporting of cancer status from participants not followed up at institution. |
| Free of attrition bias? | Low risk | No attrition noted; some participants on record had died at time of study |
Gopie 2013.
| Methods | Prospective series | |
| Participants | 48 women BRCA1/2+ or with a high‐risk family who opted for BRRM + immediate breast reconstruction approached from December 2007‐May 2010 completed questionnaires pre‐operatively T0, 6 months post‐op T1 and after completing breast reconstruction T2 | |
| Interventions | BRRM | |
| Outcomes | Body images Sexual and partner relationship satisfaction General physical and mental health QoL BC‐specific distress |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was invited to participate pre‐operatively. Two were later excluded when BC was discovered in the mastectomy specimen. |
| Free of performance bias? | Low risk | The participants who responded to questionnaires were included. |
| Free of detection bias? | Low risk | The outcomes were assessed in a valid way. The questionnaires used were standardized, validated and reliable scales to measure various outcomes of interest. |
| Free of attrition bias? | High risk | 23 of 73 (31%) women declined to participate and 2 were excluded. 16 of the 48 (33%) remaining participants dropped out; 7 stopped participating, 9 did not respond to ≥ 1 questionnaires at T1 and/or T2. More dropouts had unfinished breast reconstructions at the end of the study (69% vs 31%; P = 0.001) |
Hartmann 1999a.
| Methods | Case series and retrospective cohort study (1 paper, 2 studies) | |
| Participants | Family history/risk ‐ all with a family history of BC were included | |
| Interventions | BRRM (subcutaneous or total) = 639
High risk = 214
Comparison group: sisters without BRRM = 403 Moderate risk = 425 Comparison group: statistically modeled group based on Gail model presuming no BRRM |
|
| Outcomes | BC incidence BC mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Study did not control for other important preventive measures besides RRM that may have been used by this population including oophorectomy and chemoprevention. |
| Free of performance bias? | Low risk | Chart review identified patients who had BRRM at the Mayo Clinic |
| Free of detection bias? | Low risk | Chart review provided incidence and survival information. |
| Free of attrition bias? | Low risk | 14 (2%) eligible women could not be found, and 32 women (5%) refused to participate but their medical records were available. |
Hartmann 2001.
| Methods | Case series | |
| Participants | 26 women who tested positive for BRCA1 or BRCA2 mutations | |
| Interventions | BRRM Comparison group: statistically modeled group using Struewing and Easton models presuming no BRRM | |
| Outcomes | BC incidence | |
| Notes | Duplicates subjects of Hartmann 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | The proband were the high‐risk participants from Hartmann 1999a. Controls were sisters of 82 of the probands |
| Free of performance bias? | Low risk | Blood samples from 176 of the 214 participants were available and tested for BRCA1/2 |
| Free of detection bias? | Low risk | Chart review provided incidence and survival information |
| Free of attrition bias? | Low risk | No attrition was reported. |
Hatcher 2001.
| Methods | Prospective cohort study | |
| Participants | Family history/risk ‐ all had a family history of BC or other high‐risk factors (undefined) | |
| Interventions | BRRM = 79 Comparison group: surveillance = 64 | |
| Outcomes | QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | 5% of the decliners had genetic testing versus 29% of the acceptors. We do not know if there were different baseline risks. |
| Free of performance bias? | Low risk | All participants were women at high risk for BC, who were referred by clinicians working in 20 participating centres throughout the UK and were offered the option of having BRRM |
| Free of detection bias? | Low risk | Participants were screened pre‐ and postoperatively using standardized questionnaires |
| Free of attrition bias? | Low risk | At least 89% of participants and controls completed 3 questionnaires. Response data were provided for 3 other questionnaires. |
Heemskerk‐Gerritsen 2013.
| Methods | Prospective cohort | |
| Participants | Used an institutional Family Cancer Clinic registry database to identify eligible women, who were those with no history of cancer at the time of DNA testing and had both breasts and both ovaries in situ at the time of DNA testing. Women with symptomatic BC before the first screening round were excluded. BRRM = 212 Control (surveillance) = 358 |
|
| Interventions | BRRM | |
| Outcomes | Mortality BC incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Unclear risk | There were some minor differences in the proportion of age groups in the BRRM vs control groups. 30‐39‐year‐olds made up 49% of the BRRM group, but only 32% of the control group. The BRRM group also had more RRSO (54%) compared to the control group (38%). |
| Free of performance bias? | Low risk | Participants were included based on explicit selection criteria for a registry data analysis from a single institution. |
| Free of detection bias? | Low risk | The outcomes are objective measures and were all done by a single institution. |
| Free of attrition bias? | Low risk | The study’s inclusion criteria stated that women had to have follow‐up at the Family Cancer Clinic to be included in this analysis. |
Heemskerk‐Gerritsen 2015.
| Methods | Prospective cohort | |
| Participants | Used a combination of an ongoing nationwide Dutch study on risk assessment and gene‐environment interactions, Clinical Genetics/Family Cancer Clinics, Netherlands Cancer Institute, Foundation for the detection of Hereditary Tumors databases, and linkage to the Netherlands Cancer Registry and the Netherlands Pathology Database to identify eligible women were those with proven BRCA1 or BRCA2 female mutation carriers with BC diagnosed during the period 1980–2011 with no history of bilateral BC or ovarian cancer, no evidence of distant disease activity, and at least 1 unaffected breast in situ. CRRM = 242 Control (surveillance) = 341 |
|
| Interventions | CRRM | |
| Outcomes | Incidence of contralateral BC Mortality Overall survival |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Unclear risk | Table 1 shows there are some differences in the proportion of population age groupings, surgery for primary BC, radiotherapy, chemotherapy, and RRSO. |
| Free of performance bias? | Low risk | Yes participants were included via a pathology and hospital record review. All participants were treated within a relatively moderate timeframe (i.e. 21‐year period). Because of the multiple sources of data, it is unclear how data collection was standardised across different sources. However because of the objective and fairly standard data being collected it is assumed that these data items are valid across all oncological databases in the Netherlands. |
| Free of detection bias? | Low risk | Data were collected from medical records from participating clinics and hospitals, and through data linkage to the Netherlands Cancer Registry and Netherlands Pathology Database. Survival, mortality, and incidence are object measures. |
| Free of attrition bias? | Low risk | The study authors reported that they excluded 85 participants based on missing baseline or outcomes data |
Herrinton 2005.
| Methods | Retrospective cohort study | |
| Participants | Family history/risk ‐ not reported | |
| Interventions |
Part 1
56,400 women from 6 health maintainance organizations diagnosed with BC between 1979‐1999
CRRM = 1072
No CRRM = 317 selected by over‐sampling CBC patients from 55,328 eligible control cases Part 2 47,276 women from 4 HMOs diagnosed with BC between 1979‐1999 CRRM = 908 No CRRM = 46,368 |
|
| Outcomes |
Part 1
BC incidence Part 2 All‐cause mortality BC mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Incidence data ‐ women without CRRM who developed CBC were over‐sampled by age and outcome for the no‐CRRM group to maintain the power of the study but to avoid the cost of collecting detailed covariate information from 55,328 charts, resulting in 317 participants. Mortality data ‐ the women selecting CRRM may have had less comorbidity as they had a 27% lower risk of death from other causes than the women who did not select CRRM. Also, more women who had CRRM also had mastectomies (95%) as initial treatment rather than BCS compared to no‐CRRM women (53%) and fewer (7%) had radiation compared to the no‐CRRM women (26%.) |
| Free of performance bias? | Low risk | Used computerized data confirmed by chart review to confirm CRRM or no CRRM |
| Free of detection bias? | Low risk | Used computerized HMO databases, medical charts, cancer registry and state mortality files. Two abstractors reviewed all charts |
| Free of attrition bias? | Low risk | No “dropouts” noted. Follow‐up to date of death, disenrolment from HMO (15% of study population) or last contact |
Hopwood 2000.
| Methods | Case series | |
| Participants | 49 women with a family history of BC and who had a > 1:4 lifetime risk of BC | |
| Interventions | BRRM (subcutaneous with nipples preserved) | |
| Outcomes | QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | All women having RRM at participating institutions in Manchester, UK were sent 2 questionnaires at least at least 6 months postoperatively. |
| Free of performance bias? | Low risk | All participants were part of a standard RRM post‐operative protocol |
| Free of detection bias? | High risk | This is entirely retrospective data. Baseline measures were not collected. There was possible recall bias from collecting all psychological data postoperatively. |
| Free of attrition bias? | High risk | The study proposed to measure changes over time; however, of the original 49, only 19 had follow‐up data for years 1 and 2; only 9 had follow‐up data for years 1, 2, 3. Reasons for dropping out were not stated. |
Horton 1978.
| Methods | Case series | |
| Participants | Family history/risk ‐ 54 had family history of BC, others had benign diseases that were not considered risk factors for BC by today's standards | |
| Interventions | BRRM = 93 CRRM = 11 | |
| Outcomes | BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Women were included in this cohort who had benign diseases that were not considered risk factors for BC by today's standards. |
| Free of performance bias? | Low risk | Participants were all patients of the study authors |
| Free of detection bias? | Low risk | Participants were all patients of the study authors |
| Free of attrition bias? | Low risk | Mean follow‐up 3.1 years (1 month –10 years) |
Hwang 2016.
| Methods | Retrospective questionnaire | |
| Participants | 7619 volunteers from Army of Women aged ≥ 18 years with reported history of BC surgery completed survey; 1598 (21%) reported CRRM and 2379 (31%) reported no CRRM but treatment mastectomy only 3470 (46%) reported BCS and were excluded 87.3% had CRRM at time of initial treatment and 10.5% had CRRM at time of recurrence or new primary diagnosis |
|
| Interventions | CRRM | |
| Outcomes | QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Cohort recruited through a call‐to‐action email sent to all Army of Women members from AOW, (relatively affluent, well‐educated population who join AoW) |
| Free of performance bias? | High risk | CRRM only self‐reported, no medical record data |
| Free of detection bias? | Low risk | Used validated questionnaires (BREAST‐Q) administered electronically |
| Free of attrition bias? | Low risk | All women who volunteered to complete surveys did so |
Ingham 2013.
| Methods | Retrospective cohort | |
| Participants | 691 female BRCA1/2 mutation carriers without breast or ovarian cancer at time of family referral to one Genetic Medicine center between February 1980–December 2011 (346 BRCA1, 345 BRCA2) 457 did not have any risk‐reducing surgery; 58 had BRRM only, 68 had both BRRM & RRSO, 108 RRSO only |
|
| Interventions | BRRM & RRSO | |
| Outcomes | Survival | |
| Notes | Female first‐degree relatives (FDRs) without predictive genetic testing who otherwise met eligibility criteria were also included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Patients identified from Genetic Medicine database, genetic status confirmed, plus female first‐degree relatives who had not been tested but met “alive & unaffected at time of family referral” to control for testing bias |
| Free of performance bias? | Low risk | Used medical records from Manchester Genetic Medicine database. Also used “family files” or records at North West Cancer Intelligence Service for cancer breast confirmation or National Health Service records |
| Free of detection bias? | Low risk | Used medical records from Manchester Genetic Medicine database. Also used “family files” or records at North West Cancer Intelligence Service for cancer breast confirmation or National Health Service records |
| Free of attrition bias? | Low risk | Retrospective analysis so no dropouts; women censored at date of last follow‐up (last contact with genetics dept. or other NHS service) or date of death |
Isern 2008.
| Methods | Retrospective cohort for SF‐36 Questionnaire, series for other outcomes | |
| Participants | 54 of 61 otherwise healthy women with an increased risk of developing BC underwent RRM and immediate breast reconstruction at 1 institution between 1995 and April 2003 | |
| Interventions | 30 had BRRM 31 had CRRM ‐ 10 had earlier BC with breast‐conserving surgery |
|
| Outcomes | Physical morbidity QoL |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. Inclusion criteria were adequately described and relevant. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. Treatment histories were adequately described and relevant. All women underwent surgery at one institution. |
| Free of detection bias? | Low risk | The outcomes were assessed in a valid way. All assessments were conducted at one institution by a surgeon “who had not carried out the operations”. The questionnaire utilized an adapted questionnaire and 2 standardised QoL questionnaires. |
| Free of attrition bias? | Low risk | There was a low dropout rate and dropouts/withdrawals were sufficiently accounted for. Only 2 data points (answers to questions) were missing. |
Jatoi 2014.
| Methods | Retrospective case control | |
| Participants | 449,178 women diagnosed with BC stage I‐III ductal or lobular from 1 January 1998‐31 December 2010, aged 18–90 years from SEER data 25,961 (5.8%) had CRRM in first course of treatment 423,217 women were treated for BC but no CRRM |
|
| Interventions | CRRM | |
| Outcomes | All‐cause mortality BC‐specific mortality Non‐cancer‐related mortality |
|
| Notes | The study author wrote: “Thus, the reported associations between CPM and reductions in mortality might at least partly be attributable to selection bias.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Unclear risk | Used SEER data, excluded women with unknowns in survival length, confirmation of tumor, bilateral BC diagnosis. However, point of article that there may be selection bias for participants chosen for CRRM. Demonstrated a strong association between CRRM and reduced non‐cancer mortality suggesting selection bias of healthier women for CRRM. |
| Free of performance bias? | Low risk | Used SEER data for demographic confirmation, tumor characteristics, (no HER2 status info available) death certificates |
| Free of detection bias? | Low risk | Used SEER data |
| Free of attrition bias? | Low risk | See exclusions listed above |
Kass 2010.
| Methods | Retrospective series | |
| Participants | 254 consecutive BRCA1/2 gene mutation carriers that had RRM after a normal surveillance round including breast‐magnetic resonance imaging were identified. | |
| Interventions | 147 asymptomatic carriers underwent BRRM 107 symptomatic women had CRRM after a mean cancer‐free interval of 3.6 years |
|
| Outcomes | BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. Explicit inclusion and exclusion criteria were applied to the database of the Netherlands Cancer Institute – Antoni van Leeuwenhoek Hospital to find relevant women for the study. Included women had to have completed at least 1 surveillance round. 8 women were excluded for relevant reasons. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. Treatments were confirmed from the database of the Netherlands Cancer Institute – Antoni van Leeuwenhoek Hospital. Women were treated by the same team of surgeons. |
| Free of detection bias? | High risk | There is no information on how BRRSO performed on 54% of the participants confounded the results |
| Free of attrition bias? | Low risk | There were no dropouts. |
Kiely 2010.
| Methods | Retrospective cohort | |
| Participants | 1018 women from Australia and New Zealand with high familial risk of BC who had unilateral BC | |
| Interventions | Participants: 154 women who had CRRM Controls: 864 women who had no CRRM |
|
| Outcomes | Overall survival BC incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. Relevant inclusion and exclusion criteria were applied to participants in a national BC database to select women for this study. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. “Pathology and surgical reports were obtained where possible to verify cancer events and surgeries”. |
| Free of detection bias? | High risk | There is no information on how BRRSO performed on 59% of the CRRM participants and 24% of the no‐CRRM controls confounded the results. |
| Free of attrition bias? | Low risk | There was a low dropout rate and dropouts/withdrawals were sufficiently accounted for. |
King 2011a.
| Methods | Retrospective cohort | |
| Participants | 2979 women with unilateral stage 0‐III BC who underwent mastectomy for their index BC from 1997‐2005 at 1 institution | |
| Interventions | Participants: 407 participants underwent CRRM within 1 year of treatment Controls: 2572 had no CRRM |
|
| Outcomes | Disease‐free survival BC incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. Explicit inclusion and exclusion criteria were applied to institutional databases to identify relevant participants. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. Treatment data were collected from the institutional databases. All women were treated at a single institution – Memorial Sloan‐Kettering Cancer Center. Women were treated by 13 specialized breast surgeons in the specified timeframe. |
| Free of detection bias? | Low risk | The outcomes were assessed in a valid way. Outcome data were collected from the institutional databases. |
| Free of attrition bias? | Low risk | There were no dropouts. |
Koskenvuo 2014.
| Methods | Retrospective cohort | |
| Participants | 52 women in cohort of 136 BRCA1/2 mutation carriers who had RRM surgery and/or were followed up at the Helsinki University Central Hospital (HUCH) | |
| Interventions | RRM | |
| Outcomes | Complications BC incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Included all BRCA1/2 mutation carriers who had follow‐up or surgery at HUCH from January 1997–March 2010 |
| Free of performance bias? | Low risk | Used patient records from Breast Surgery Unit and Dept. of Plastic Surgery |
| Free of detection bias? | Low risk | Used patient records from Breast Surgery Unit and Dept. of Plastic Surgery |
| Free of attrition bias? | Low risk | Excluded men, those followed‐up or treated at other hospitals, mutation carriers who previously had bilateral treatment mastectomy, participants diagnosed during preoperative imaging |
Kruper 2014.
| Methods | Retrospective case‐control | |
| Participants | Women from SEER database 1998–2010 with unilateral BC aged 18–90 years who had treatment mastectomy 26,526 – CRRM 138,826 ‐ no CRRM |
|
| Interventions | CRRM | |
| Outcomes | Disease‐specific survival Overall survival |
|
| Notes | Excluded cases of CBC diagnosed < 3 months after initial diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Used SEER data, divided participants by diagnosis period (1998–2006; 2007–2010) to control for differences for adoption of trastuzumab therapy in 2006 |
| Free of performance bias? | Low risk | Used SEER data |
| Free of detection bias? | Unclear risk | Changes in coding granularity might have affected reporting of rates of single mastectomy or CRRM |
| Free of attrition bias? | Low risk | Used SEER data |
Lee 1995.
| Methods | Retrospective cohort study | |
| Participants | Family history/risk CRRM: 14/84 (13%) had a family history of BC (undefined) Comparison group: 28/299 (9%) had a family history of BC (undefined) | |
| Interventions | CRRM = 84
Undirected contralateral biopsies = 21 Comparison group: surveillance with no CRRM = 299 |
|
| Outcomes | BC survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | The study only adjusted for age, not the other major confounders. Also, the treatment group included those undergoing contralateral RRM as well as those having biopsies. It is unclear how including those with only biopsies may have biased the results. |
| Free of performance bias? | Low risk | Patient case histories at the Mayo Clinic were reviewed to identify all those who had invasive lobular carcinoma between 1978 and 1991. |
| Free of detection bias? | High risk | The RRM group was combined with those receiving biopsies; therefore, the risk in the RRM group is not ascertainable. |
| Free of attrition bias? | Low risk | No attrition with a mean follow‐up time 6 years (median, 5.3 years) for all participants |
Leis 1981.
| Methods | Case series | |
| Participants | Family history/risk ‐ all high risk (undefined) | |
| Interventions | CRRM = 127 | |
| Outcomes | Disease‐free survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Women were included in this cohort who had benign diseases that were not considered risk factors for BC by today's standards. |
| Free of performance bias? | Low risk | Participants were 127 women who had CRRM and were patients of the study authors |
| Free of detection bias? | High risk | Valid disease‐free survival estimates depend on all participants getting assessed for disease at regular, fixed intervals. It was not mentioned whether or not this occurred. |
| Free of attrition bias? | High risk | There were 69/127 participants who were not accounted for |
McDonnell 2001.
| Methods | Case series | |
| Participants | Family history/risk ‐ all with a first BC, who had a family history of breast or ovarian cancer Participants underwent CRRM between 1960 and 1993 388 = pre‐menopausal 357 = post‐menopausal |
|
| Interventions | CRRM (41% subcutaneous, 59% total) = 745 Comparison group: simulated from age‐adjusted life tables presuming no CRRM | |
| Outcomes | BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | The analysis did not sufficiently control for confounding factors (i.e. histology or stage of primary tumor). |
| Free of performance bias? | Low risk | Participants identified through search of Surgical Index Recording System for all participants having a CRRM from 1960‐1993. |
| Free of detection bias? | Low risk | Medical record information was available for all participants. |
| Free of attrition bias? | Low risk | Median length of follow‐up was 10 years. Questionnaires completed for 90.3% of the participants |
Meijers‐Heijboer 2001.
| Methods | Prospective cohort study | |
| Participants | 139 women who had BRCA 1 or 2 mutations | |
| Interventions | BRRM (simple total) = 76
Comparison group: close observation = 63 Close observation defined as monthly breast self‐examination, clinical breast examination every 6 months, and yearly mammography |
|
| Outcomes | BC incidence BC mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | While the study authors controlled for some factors such as age and oophorectomy status, adjustment of other important factors was not reported. |
| Free of performance bias? | Low risk | Only women who tested positive for BRCA1/2 were invited to participate in BC surveillance program. Participants were those who had BRRM. |
| Free of detection bias? | Low risk | Vital status and the occurrence of cancer was extracted from the women’s medical files. |
| Free of attrition bias? | Low risk | No women were lost to follow‐up after BRRM |
Metcalfe 2004b.
| Methods | Retrospective case series | |
| Participants | Family history/risk ‐ not reported | |
| Interventions | BRRM = 60 women with no history of BC who had BRRM between 1991‐2000 in Ontario, Canada and returned ≥ 1 of 5 questionnaires sent to them | |
| Outcomes | QoL Physical morbidity | |
| Notes | 75 women were sent questionnaires | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Physicians for 20 of 122 potential participants did not give permission to contact their patients. |
| Free of performance bias? | Low risk | Access to the Ontario Ministry of Health’s database for hospital procedures and diagnosis codes |
| Free of detection bias? | High risk | There were no pre‐CRRM assessments of psychosocial factors for comparison. |
| Free of attrition bias? | High risk | 15 of 75 participants did not return questionnaires. |
Metcalfe 2005.
| Methods | Retrospective case series | |
| Participants | Family history/risk ‐ 13 were BRCA1/2 mutation carriers, 33 had strong family history (1 first degree relative or 2 second degree relatives with BC plus diagnosis < 51, ovarian cancer or male BC) and 14 had limited family history | |
| Interventions | BRRM = 60 women with no history of BC who had BRRM between 1991‐2000 in Ontario, Canada and returned ≥ 1 of 5 questionnaires sent to them | |
| Outcomes | QoL | |
| Notes | Same participants as Metcalfe 2004b; 75 women were sent the questionnaires | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Physicians for 20 of 122 potential participants did not give permission to contact their patients. |
| Free of performance bias? | Low risk | Hospital procedure & diagnosis codes for RRM used, then medical charts to confirm |
| Free of detection bias? | Low risk | Used indices and questionnaires developed and used for other studies and referenced – QoL Index, Brief System Inventory, Body Image After Breast Cancer, Impact of Event Scale, Social Support Questionnaire |
| Free of attrition bias? | High risk | 15 of 75 participants did not return questionnaires |
Metcalfe 2014.
| Methods | Retrospective cohort study | |
| Participants | Family history/risk ‐ BRCA1/BRCA2 mutation carrier families | |
| Interventions | 482 women from BRCA1/BRCA2 mutation carrier families diagnosed with S I or II BC from 1975‐2000, age < 66, and were mutation carriers or untested CRRM = 146 No CRRM = 336 |
|
| Outcomes | BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Some of the CBC was diagnosed within 1‐2 months of original diagnosis of BC, less than the commonly used second new BC diagnoses at ≤ 6 months, and more correctly should be classified as bilateral BC. This classification then could have overstated the incidence of CBC in the no‐CRRM group. There are also some discrepancies in reporting the date of diagnosis. In the Methods section it says "...diagnosis of SI or SII BC at age 65 or less, between 1975 and 2008". But in the results section it says “The women were given a diagnosis between 1977 and 2009 …”. Also in the Methods section it is stated that “…54 (14%) were not tested.” But in the Strengths and Limitations section it is stated that “…53 women included in this study who did not undergo genetic testing …”. This small discrepancy in numbers could have a major impact on the long‐term results due to the small denominator on which these are based. |
| Free of performance bias? | Low risk | Medical records were obtained from the hospital where the CRRM was performed. |
| Free of detection bias? | Low risk | Medical treatment records and pathology documents were reviewed. |
| Free of attrition bias? | Unclear risk | This report is a follow‐up to Metcalfe 2004a that reported on 482 women (CRRM = 146; no CRRM = 336), 92 more than in the update. This report does not say what happened to the 92 participants in the 2004a report although in the update, women who had breast‐conserving surgery were excluded, which may explain the difference. |
Miller 2013.
| Methods | Retrospective case control | |
| Participants | 600 women with unilateral BC who had treatment mastectomy at 1 institution between January 2009 and March 2012. 391 (65%) had unilateral mastectomy (UM) 209 (35%) had RRM |
|
| Interventions | CRRM | |
| Outcomes | Complications | |
| Notes | Mean age in CRRM group was 50 years, in UM group, 62 years (P = 0.001) CRRM participants were diagnosed at an earlier AJCC stage than UM participants (P= 0.017) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | All participants treated for BC with unilateral mastectomy or CRRM in 1 health system between January 2009 – March 2012 were included. |
| Free of performance bias? | Low risk | Used medical records for treatment and complications. However, some complications in outpatient setting may have been underreported. |
| Free of detection bias? | Unclear risk | Medical records used; however, a standardized system for complication classification was not used. Also, not divided into early/late complications during 1‐year follow‐up |
| Free of attrition bias? | Low risk | None reported |
Montgomery 1999.
| Methods | Convenience sample | |
| Participants | Family history/risk ‐ 30% reported having at least one 1st degree relative with BC | |
| Interventions | CRRM = 296 | |
| Outcomes | QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Because the participants responded to advertisements, respondents may have been different in some important way than the non‐respondents. |
| Free of performance bias? | High risk | The participants self‐reported having had a BRRM |
| Free of detection bias? | High risk | There was a possibility of recall bias by asking all QoL questions after the surgery only. |
| Free of attrition bias? | High risk | 50 women of 346 did not respond to questionnaire |
Mutter 2015.
| Methods | Retrospective longitudinal cohort | |
| Participants | Collected data on women at the Mayo Clinic between 1 January 1960‐31 December 1993 BC developed ipsilateral to the RRM in 25 participants (13 after BRRM; 12 after therapeutic mastectomy and CRRM). The study utilized a study‐specific questionnaire (sent from 1995‐1997), and follow‐up surveys at 10 and 20 years after RRM. All participants who underwent RRM were followed up yearly through the Mayo Clinic Cancer Registry for subsequent BC events and outcomes. |
|
| Interventions | BRRM CRRM |
|
| Outcomes | Incidence Disease‐free survival |
|
| Notes | Subjects also included in Hartmann 1999 and McDonnell 2001 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Unclear risk | This paper doesn't report on the entire cohort. Table 1 reports on the characteristics of the 25 women who developed BC after RRM, and shows that there were baseline differences in family history status, type of mastectomy undertaken, and breast reconstruction choice, although it is not clear if these are statistically significant. |
| Free of performance bias? | Unclear risk | Because of the 34‐year timeframe (1960‐1993) it is unclear how RRM techniques, and adjuvant therapies, changed over that time period, and how this may have affected the clinical outcomes. |
| Free of detection bias? | Low risk | A survey was sent to all women or their next‐of‐kin, and data were also collected via the Mayo Clinic Cancer Registry. Nurses called participants to follow up on surveys. All medical records were also reviewed. |
| Free of attrition bias? | Low risk | No attrition was reported (there were only 25 participants in this study). |
Pennisi 1989.
| Methods | Case series | |
| Participants | Family history/risk ‐ not reported | |
| Interventions | BRRM (subcutaneous) = 1361 CRRM = 139 | |
| Outcomes | BC incidence BC mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Women were included in this cohort who had benign breast diseases that are not considered risk factors for BC by today's standards. |
| Free of performance bias? | Low risk | 165 plastic surgeons provided patients’ histories and follow‐up reports on 1500 patients who underwent subcutaneous mastectomy. |
| Free of detection bias? | Unclear risk | Not enough details provided |
| Free of attrition bias? | High risk | The 30% loss to follow‐up increased risk of attrition bias |
Peralta 2000.
| Methods | Retrospective cohort study | |
| Participants | Family history/risk ‐ CRRM
23 (36%) had at least one 1st degree relative with BC
19 (29%) had at least one 2nd degree relative with BC Comparison group 35 (19.5%) had at least one 1st degree relative with BC 47 (26.1%) had at least one 2nd degree relative with BC |
|
| Interventions | CRRM = 64 with primary BC Comparison group: primary BC and no CRRM = 82 | |
| Outcomes | BC incidence Disease‐free survival BC survival All‐cause survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | This study did not adjust for all major confounders. |
| Free of performance bias? | Low risk | Information on participants’ medial history was obtained from a computerized prospective database. |
| Free of detection bias? | High risk | Valid disease‐free survival estimates depend on participants in both groups getting assessed at the same fixed intervals. Therefore, we do not know the validity of these data as intervals were not mentioned. |
| Free of attrition bias? | Low risk | Mean follow‐up was 6.8 years (0.3‐23.6 years) |
Pesce 2014.
| Methods | Retrospective longitudinal cohort | |
| Participants | Used the USA National Cancer Database (NCDB), which is a nationwide dataset that reports from about 1450 hospitals with the American College of Surgeon's Commission on Cancer accredited cancer programs to identify participants 10,289 women: (70.3%) underwent unilateral mastectomy and 4338 (29.7 %) women underwent CRRM |
|
| Interventions | CRRM | |
| Outcomes | Overall survival | |
| Notes | The NCDB does not collect data on disease‐free survival or BC‐specific mortality, so the results can only be interpreted from an all‐cause mortality standpoint. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Unclear risk | The paper selectively looked at women ≤ 45 years. Table 1 shows that there is a statistically significant difference between CRRM and unilateral mastectomy in the proportion of women in certain age groupings, as well as a statistically significant difference in the proportion of races included between the groups. There was a statistically significant different between groups for stage 1 and 2 BC, with about half having stage 1 BC in the CRRM group but only 1/3 of women having stage 1 in the unilateral mastectomy group. There are also statistically significant differences between groups for the tumor sizes, lymph node status, and radiotherapy treatment. |
| Free of performance bias? | Unclear risk | The NCDB does not collect information about the type of chemotherapy, radiotherapy, or hormonal therapy used. Given that > 70% of both groups had chemotherapy and about 50% of both groups had hormone therapy, the type of therapy could be an important confounding treatment factor in influencing how effective RRM is. |
| Free of detection bias? | Low risk | Women were followed up throughout the study, and data were obtained from the NCDB. Survival is an objective measure. |
| Free of attrition bias? | Low risk | No attrition was reported. |
Rebbeck 2004.
| Methods | Prospective and retrospective cohort studies | |
| Participants | Family history/risk ‐ BRCA1/2 mutation carriers | |
| Interventions | 483 BRCA1/2 mutation carriers identified from 11 North American and European institutions (The PROSE Study Group) BRRM = 105 Matched controls = 378 women alive, cancer free, had both breasts at the time of the matched participants’ BRRM |
|
| Outcomes | BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | High risk | Matched controls were excluded if prior or concurrent BC at time of matched participant’s BRRM, but accepted as controls if they had BC when they were first seen at the center and enrolled. This could artificially increase the number of BCs in the controls and cause the overestimation of the benefit of BRRM. |
| Free of performance bias? | Low risk | BRCA1/2 mutation status confirmed by direct mutation testing; used medical center records for BRRM/no BRRM and/or BRRSO |
| Free of detection bias? | Low risk | Confirmed BC or none using pathology reports and/or cancer registries |
| Free of attrition bias? | Low risk | Historical cohort used (patients with RRM who could be matched with controls) censored at date of death, last contact, breast or ovarian cancer |
Skytte 2011.
| Methods | Prospective cohort | |
| Participants | 307 with BRCA1/2 mutation found between January 1996–February 2008 observed prospectively from time of positive mutation test and who had not had BSO prior to testing | |
| Interventions | Participants: 96 women who eventually had a BRRM contributed time at‐risk in the ‘no mastectomy’ group until the time point at which they underwent mastectomy. Thereafter, they belonged to the mastectomy group. Controls: 211 women who did not opt for mastectomy in this study period, all at‐risk time was assigned to the ‘no mastectomy’ group. |
|
| Outcomes | BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | A consecutive sample of a clearly defined population was chosen. Explicit inclusion and exclusion criteria were applied retrospectively to participants from a registry. |
| Free of performance bias? | Low risk | Exposure was confirmed in an objective way. Treatments were identified from a registry. Follow‐up data were also obtained from a registry as well as prospectively. |
| Free of detection bias? | High risk | While women who had BSO prior to genetic testing were excluded, some women had BSO after inclusion in the study and the effect was not accounted for. |
| Free of attrition bias? | Low risk | There were no dropouts. |
Unukovych 2012.
| Methods | Prospective series | |
| Participants | 60 of 69 consecutive patients with a confirmed family history of BC who underwent CRRM at Karolinska University Hospital, Department of Reconstructive Plastic Surgery, from January 1998‐June 2008 agreed to participate in the study. 60 of the 69 completed the questionnaires. | |
| Interventions | CRRM | |
| Outcomes | Health‐related QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Unclear risk | 69 of 91 consecutive patients with a confirmed family history who underwent CRRM at Karolinska University Hospital, Department of Reconstructive Plastic Surgery, from January 1998‐June 2008 agreed to participate in the study. 10 of the 91 were not invited due to administrative failure. |
| Free of performance bias? | Low risk | Before CRRM the women were referred a psychologist. At the end of the consultation, each participant was invited to participate in the questionnaire study. Those wishing to participate were handed a packet of questionnaires to complete. |
| Free of detection bias? | Low risk | 4 validated questionnaires were used: the SF‐36, the Hospial Anxiety and Depression Scale (HAD), the Body Image Scale (BIS), and the Sexual Activity Questionnaire (SAQ) |
| Free of attrition bias? | Low risk | 45 participants (75%) responded before CRRM, 49 (82%) at 6 months, and 45 (75%) at 2 years after CRRM |
Van Sprundel 2005.
| Methods | Retrospective cohort study | |
| Participants | Family history/risk ‐ BRCA1/2 mutation carriers | |
| Interventions | 148 BRCA1/2 mutation carriers (115 and 33, respectively) previously treated for unilateral BC at 2 medical centers CRRM = 79 Intense surveillance (monthly BSE, semi‐annual clinical breast exam, yearly mammography) = 69 |
|
| Outcomes | All‐cause mortality BC mortality Disease‐free survival BC incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | All women who had unilateral BC and identified as BRCA1/2 mutations were included. However, all Dutch women with BRCA mutations are enrolled in surveillance; not clear if difference between those who chose CRRM and those who continued surveillance |
| Free of performance bias? | Low risk | All data extracted from medical files, operation and pathology reports |
| Free of detection bias? | Low risk | Did evaluate many factors as confounders, adjusted for effect of BRRSO |
| Free of attrition bias? | Low risk | Fairly short follow‐up, no attrition except death |
Zeichner 2014.
| Methods | Retrospective cohort | |
| Participants | 237 of 481 patients with BC age < 40 at single US medical center between 1 January 1980‐31 December 2010 with last follow‐up April 2013 42 were identified as having undergone CRRM 195 were confirmed as no‐CRRM during the observation period Patients who were male, lost to follow‐up, and/or had a history of de‐novo metastases, secondary cancers, bilateral BCs, and one‐time consults, were excluded from the study |
|
| Interventions | CRRM | |
| Outcomes | Disease‐free survival Overall survival |
|
| Notes | The CRRM group had a significantly higher percentage of participants who were diagnosed between 2000 and 2010 (40/42 (95.2%) vs 78/195(40%) no CRRM, P = 0.0001) Abstract states 481 prospective patients, text states 480 prospective patients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | All patients diagnosed at one center in given time period. Excluded men, those lost to follow‐up (no numbers given), history of de novo metastasis, secondary cancers, bilateral BC and consults only |
| Free of performance bias? | Unclear risk | Does not specifically state, but assume medical records used to obtain information |
| Free of detection bias? | High risk | There were significant differences in the length of follow‐up in the two groups. 95.2% of CRRM participants were followed for 3‐13 years vs 30% of the no CRRM. 60% of the no‐CRRM participants were followed for 13‐23 years vs only 4.8% of CRRM participants followed that long so the no CRRM had longer to die. |
| Free of attrition bias? | Low risk | Retrospective study, no attrition reported |
Zion 2003.
| Methods | Retrospective cohort study | |
| Participants | Family history/risk ‐ not reported | |
| Interventions | 1417 women who had BRRM or CRRM at 1 institution between 1960‐1993 BRRM = 593 with reconstruction CRRM = 506 with reconstruction BRRM = 39 without reconstruction CRRM = 279 without reconstruction |
|
| Outcomes | Physical morbidity | |
| Notes |
Zion 2000 reported on same CRRM participants in an abstract Some BRRM participants are the same as Hartmann 1999a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Free of selection bias? | Low risk | Reoperations compared only among implant reconstruction group and no reconstruction group, not autologous tissue reconstruction with less reoperation rate |
| Free of performance bias? | Low risk | Used patient survey with responses validated by medical records |
| Free of detection bias? | Low risk | Used medical records, Cox regression & Anderson‐Gill models to assess risk of reoperations |
| Free of attrition bias? | Low risk | 92% of participants answered surveys, medical records available for all; no reason given for non‐responders |
Key to abbreviations: AJCC ‐ American Joint Committee on Cancer BC ‐ breast cancer BCS ‐ breast conserving surgery BMI ‐ body mass index BRRM ‐ bilateral risk‐reducing mastectomy (B)RRSO ‐ (bilateral) risk‐reducing salpingo‐oophorectomy BSE ‐ breast self examination CBC ‐ contralateral breast cancer CRRM ‐ contralateral risk‐reducing mastectomy DCIS ‐ ductal carcinoma in situ ILC ‐ invasive lobular cancer LCIS ‐ lobular carcinoma in situ QoL ‐ quality of life RRM ‐ risk‐reducing mastectomy SF‐36 ‐ Short‐Form 35 Health Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott 2011 | About patients' estimations of risk for BC |
| Ager 2016 | About choosing CRRM |
| Alamounti 2015 | About surgical technique |
| Antill 2006 | About decision making |
| Ariyan 1985 | No original patient data presented |
| Babiera 1997 | Fewer than 20 participants |
| Barry 2012 | About incidence of CBC at time of CRRM |
| Bebbington Hatcher 2003 | No original patient data presented |
| Blackburn 2016 | About surgical technique |
| Borreani 2014 | Unable to separate data for RRM participants from other surgery participants |
| Bostwick 1980 | Surgical technique article |
| Brekelmans 2006 | About BRCA1 BC |
| Brinton 2001 | Breast reduction surgery |
| Brown 2005 | About genetic counseling |
| Buehler 1983 | About assessing risk |
| Collins 2013 | About risk‐reduction methods with no data specific to RRM |
| Cortesi 2014 | About impact of rapid genetic testing on decision making for RRM |
| Dikmans 2016 | About surgical technique |
| Dinner 1981 | Surgical technique article |
| Domchek 2011 | No data on RRM alone, intermingled with BRRSO |
| Eisinger 2001 | Physician and patient attitudes about RRM |
| Evans 2005 | About decision making |
| Fowbie 2015 | About surgical technique |
| Fu 2015 | About choosing CRRM |
| Graham 2015 | About neo‐adjuvant therapy |
| Gschwantier 2016 | About surgical technique |
| Hagen 2014 | Data combine participants with BC and without BC whether they had RRM or not |
| Han 2011 | About who chooses RRM |
| Heiniger 2015 | < 20 RRM participants |
| Hoffman 1982 | About indications for risk |
| Horton 1988 | Surgical technique article |
| Houn 1995 | Survey of physicians about RRM |
| Jarrett 1982 | Surgical technique article |
| Jones 2009 | About who chooses RRM |
| Josephson 2000 | < 20 participants |
| Katapodi 2004 | About perceived risk of BC |
| Kheirelseid 2011 | About incidence of breast cancer |
| King 2011b | About presence of occult BC in CRRM patients |
| Klitzman 2010 | About decision making |
| Kurian 2005 | About breast screening |
| Leis 1980 | About incidence of CBC |
| Lerman 1996 | Predictors of genetic testing not RRM |
| Litton 2009 | Patients' perception of their risk of BC |
| Lloyd 2000 | < 20 participants |
| Lodder 2002 | < 20 participants |
| Lynch 1991 | Original patient data presented only on predictors of compliance with surveillance |
| Lynch 2006 | About genetic testing |
| Madlensky 2005 | About prevention of BC |
| McAvoy 1979 | No original patient data presented |
| McCready 2007 | Review of risk factors for CBC |
| Meijers‐Heijboer 2003 | No data on objectives of the review |
| Metcalfe 2004c | Satisfaction with breast reconstruction following RRM |
| Metcalfe 2008a | Predictors of having CRRM in women with a BRCA1/2 mutation |
| Metcalfe 2008b | International rates of uptake of RRM in BRCA1/2 carriers |
| Metcalfe 2011a | Risk of ipsilateral BC in BRCA1/2 carriers |
| Metcalfe 2011b | Predictors of CBC in BRCA1/2 carriers |
| Meyer 1986 | No original patient data presented |
| Mulvihill 1982 | < 20 participants |
| Narod 2011 | No original patient data, used mortality tables |
| Narod 2014 | Chapter of a book |
| Nekhlyudov 2005 | About decision making |
| Osman 2013 | About surgical technique |
| Patenaude 2008 | Need for psychological support for RRM patients |
| Payne 2000 | No original patient data presented |
| Pennisi 1984 | More recent data on same cohort presented in article included in review |
| Petit 2002 | No original patient data presented |
| Phillips 2006 | Use of risk‐management strategies |
| Rhiem 2012 | Data on risk of CBC |
| Ringberg 1982 | Incidence of occult CBC |
| Roberts 2014 | About cost‐effectiveness of RRM |
| Roberts 2015 | Complications and reoperations after any mastectomy |
| Roinick 2007 | What women wished they knew about RRM |
| Rubin 1979 | Surgical technique article |
| Rueth 2011 | About pre‐operative risk‐assessment for women undergoing BRRM |
| Sakorafas 2002 | No original patient data presented |
| Salhab 2010 | Review article |
| Schwartz 2004 | About BRCA1/2 testing |
| Scott 2003 | About decision making |
| See 2005 | About decision making |
| Snyderman 1984 | About decision making |
| Spear 2008 | About reconstruction |
| Specht 2004 | About personal health behavior |
| Stalmeier 2009 | Evaluation of decision aids for high‐risk women considering RRM and/or BRRSO |
| Stefanek 1995 | < 20 participants |
| Stolier 2005 | About decision making |
| Stuckey 2010 | About who chooses BRRM |
| Temple 1991 | Surgical technique article |
| Tercyak 2007 | About impact of genetic testing and choosing CRRM |
| Theogaraj 1973 | Surgical technique |
| Tuttle 2007 | Review of SEER data on CRRM |
| Unic 1998 | About assessing risk |
| Van Dijk 2003 | About perceived risk of BC |
| von Smitten 2001 | No original patient data presented |
| Wang 2015a | Who chooses CRRM |
| Wapnir 1990 | No original patient data presented |
| Wasteson 2011 | < 20 participants |
| Yarbro 1985 | On pathophysiology of BC |
| Yi 2010 | About who chose CRRM |
| Zendejas 2011 | About cost/benefit analysis of CRRM |
BC ‐ breast cancer CBC ‐ contralateral breast cancer RRM ‐ risk‐reducing mastectomy
Contributions of authors
N Carbine reviewed reports for inclusion, extracted data from reports, contributed conceptually to the formation of the paper, assessed the methodological quality of included studies, wrote and edited the paper, and participated in all key discussions regarding the paper.
L Lostumbo reviewed reports for inclusion, extracted data from reports, created the tables, contributed conceptually to the formation of the paper, assessed the methodological quality of included studies, edited sections of the manuscript, and participated in all key discussions regarding the paper.
J Wallace reviewed reports for inclusion, contributed conceptually to the formation of the paper, assessed the methodological quality of included studies, and participated in key discussions regarding the paper.
H Ko reviewed reports for inclusion, extracted data from reports, created the tables, contributed conceptually to the formation of the paper, revised the figures, assessed the methodological quality of included studies, and participated in all key discussions regarding the paper.
Declarations of interest
N Carbine: none L Lostumbo: none J Wallace: none H Ko: none
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Altschuler 2008 {published data only}
- Altschuler A, Nekhlyudov L, Rolnick SJ, Greene SM, Elmore JG, West CN, et al. Positive, negative, and disparate‐‐women's differing long‐term psychosocial experiences of bilateral or contralateral prophylactic mastectomy. Breast Journal 2008;14(1):25‐32. [DOI] [PubMed] [Google Scholar]
Arver 2011 {published data only}
- Arver B, Isaksson K, Atterhem H, Baan A, Bergkvist L, Brandberg Y, et al. Bilateral prophylactic mastectomy in Swedish women at high risk of breast cancer: a national survey. Annals of Surgery 2011;253(6):1147‐54. [DOI] [PubMed] [Google Scholar]
Barton 2005 {published data only}
- Barton MB, West CN, Liu IL, Harris EL, Rolnick SJ, Elmore JG, et al. Complications following bilateral prophylactic mastectomy. Journal of the National Cancer Institute. Monographs 2005;35:61‐6. [DOI] [PubMed] [Google Scholar]
Bedrosian 2010 {published data only}
- Bedrosian I, Hu CY, Chang GJ. Population‐based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. Journal of the National Cancer Institute 2010;102(6):401‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borgen 1998 {published data only}
- Borgen PI, Hill AD, Tran KN, Zee KJ, Massie MJ, Payne D, et al. Patient regrets after bilateral prophylactic mastectomy. Annals of Surgical Oncology 1998;5(7):603‐6. [DOI] [PubMed] [Google Scholar]
Boughey 2010 {published data only}
- Boughey JC, Hoskin TL, Degnim AC, Sellers TA, Johnson JL, Kasner MJ, et al. Contralateral prophylactic mastectomy is associated with a survival advantage in high‐risk women with a personal history of breast cancer. Annals of Surgical Oncology 2010;17(10):2702‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandberg 2008 {published data only}
- Brandberg Y, Arver B, Johansson H, Wickman M, Sandelin K, Liljegren A. Less correspondence between expectations before and cosmetic results after risk‐reducing mastectomy in women who are mutation carriers: a prospective study. European Journal of Surgical Oncology 2012;38(1):38‐43. [DOI] [PubMed] [Google Scholar]
- Brandberg Y, Sandelin K, Erikson S, Jurell G, Liljegren A, Lindblom A, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1‐year follow‐up study. Journal of Clinical Oncology 2008;26(24):3943‐9. [DOI] [PubMed] [Google Scholar]
Bresser 2006 {published data only}
- Bresser PJ, Seynaeve C, Gool AR, Brekelmans CT, Meijers‐Heijboer H, Geel AN, et al. Satisfaction with prophylactic mastectomy and breast reconstruction in genetically predisposed women. Plastic and Reconstructive Surgery 2006;117(6):1675‐82; discussion 1683‐4. [DOI] [PubMed] [Google Scholar]
Brewster 2012 {published data only}
- Brewster AM, Bedrosian I, Parker PA, Dong W, Peterson SK, Cantor SB, et al. Association between contralateral prophylactic mastectomy and breast cancer outcomes by hormone receptor status. Cancer 2012;118(22):5637‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2012 {published data only}
- Chung A, Huynh K, Lawrence C, Sim MS, Giuliano A. Comparison of patient characteristics and outcomes of contralateral prophylactic mastectomy and unilateral total mastectomy in breast cancer patients. Annals of Surgical Oncology 2012;19(8):2600‐6. [DOI] [PubMed] [Google Scholar]
Contant 2002 {published data only}
- Contant CM, Menke‐Pluijmers MB, Seynaeve C, Meijers‐Heijboer EJ, Klijn JG, Verhoog LC, et al. Clinical experience of prophylactic mastectomy followed by immediate breast reconstruction in women at hereditary risk of breast cancer (HB(O)C) or a proven BRCA1 and BRCA2 germ‐line mutation. European Journal of Surgical Oncology 2002;28(6):627‐32. [DOI] [PubMed] [Google Scholar]
de la Pena‐Salcedo 2012 {published data only}
- Pena‐Salcedo JA, Soto‐Miranda MA, Lopez‐Salguero JF. Prophylactic mastectomy: is it worth it?. Aesthetic Surgery Journal 2012;36(1):140‐8. [DOI] [PubMed] [Google Scholar]
Den Heijer 2012 {published data only}
- Heijer M, Seynaeve C, Timman R, Duivenvoorden HJ, Vanheusden K, Tilanus‐Linthorst M, et al. Body image and psychological distress after prophylactic mastectomy and breast reconstruction in genetically predisposed women: a prospective long‐term follow‐up study. European Journal of Cancer 2012;48(9):1263‐8. [DOI] [PubMed] [Google Scholar]
Evans 1999 {published data only}
- Evans DG, Anderson E, Lalloo F, Vasen H, Beckmann M, Eccles D, et al. Utilisation of prophylactic mastectomy in 10 European centres. Disease Markers 1999;15(1‐3):148‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2013 {published data only}
- Evans DG, Ingham SL, Baildam A, Ross GL, Lalloo F, Buchan I, et al. Contralateral mastectomy improves survival in women with BRCA1/2‐associated breast cancer. Breast Cancer Research and Treatment 2013;140(1):135‐42. [DOI] [PubMed] [Google Scholar]
Frost 2000 {published data only}
- Frost MH, Schaid DJ, Sellers TA, Slezak JM, Arnold PG, Woods JE, et al. Long‐term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA 2000;284(3):319‐24. [DOI] [PubMed] [Google Scholar]
Frost 2005 {published data only}
- Boughey JC, Hoskin TL, Hartmann LC, Johnson JL, Jacobson SR, Degnim AC, et al. Impact of reconstruction and reoperation on long‐term patient‐reported satisfaction after contralateral prophylactic mastectomy. Annals of Surgical Oncology 2015;22(2):401‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost MH, Hoskin TL, Hartmann LC, Degnim AC, Johnson JL, Boughey JC. Contralateral prophylactic mastectomy: long‐term consistency of satisfaction and adverse effects and the significance of informed decision‐making, quality of life, and personality traits. Annals of Surgical Oncology 2011;18(11):3110‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost MH, Slezak JM, Tran NV, Williams CI, Johnson JL, Woods JE, et al. Satisfaction after contralateral prophylactic mastectomy: the significance of mastectomy type, reconstructive complications, and body appearance. Journal of Clinical Oncology 2005;23(31):7849‐56. [DOI] [PubMed] [Google Scholar]
Gabriel 1997 {published data only}
- Gabriel SE, Woods JE, O'Fallon WM, Beard CM, Kurland LT, Melton LJ 3rd. Complications leading to surgery after breast implantation. New England Journal of Medicine 1997;336(10):677‐82. [DOI] [PubMed] [Google Scholar]
Gahm 2007 {published data only}
- Gahm J, Jurell G, Wickman M, Hansson P. Sensitivity after bilateral prophylactic mastectomy and immediate reconstruction. Scandinavian Journal of Plastic & Reconstructive Surgery & Hand Surgery 2007;41(4):178‐83. [DOI] [PubMed] [Google Scholar]
Gahm 2010 {published data only}
- Gahm J, Hansson P, Brandberg Y, Wickman M. Breast sensibility after bilateral risk‐reducing mastectomy and immediate breast reconstruction: a prospective study. Journal of Plastic, Reconstructive and Aesthetic Surgery 2013;66(11):1521‐7. [DOI] [PubMed] [Google Scholar]
- Gahm J, Wickman M, Brandberg Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer‐‐prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. Breast 2010;19(6):462‐9. [DOI] [PubMed] [Google Scholar]
Geiger 2005 {published data only}
- Geiger AM, Yu O, Herrinton LJ, Barlow WE, Harris EL, Rolnick S, et al. A population‐based study of bilateral prophylactic mastectomy efficacy in women at elevated risk for breast cancer in community practices. Archives of Internal Medicine 2005;165(5):516‐20. [DOI] [PubMed] [Google Scholar]
Geiger 2006 {published data only}
- Geiger AM, West CN, Nekhlyudov L, Herrinton LJ, Liu IL, Altschuler A, et al. Contentment with quality of life among breast cancer survivors with and without contralateral prophylactic mastectomy. Journal of Clinical Oncology 2006;24(9):1350‐6. [DOI] [PubMed] [Google Scholar]
Geiger 2007 {published data only}
- Geiger AM, Nekhlyudov L, Herrinton LJ, Rolnick SJ, Greene SM, West CN, et al. Quality of life after bilateral prophylactic mastectomy. Annals of Surgical Oncology 2007;14(2):686‐94. [DOI] [PubMed] [Google Scholar]
Goldflam 2004 {published data only}
- Goldflam K, Hunt KK, Gershenwald JE, Singletary SE, Mirza N, Kuerer HM, et al. Contralateral prophylactic mastectomy. Predictors of significant histologic findings. Cancer 2004;101(9):1977‐86. [DOI] [PubMed] [Google Scholar]
Gopie 2013 {published data only}
- Gopie JP, Mureau MA, Seynaeve C, Ter Kuile MM, Menke‐Pluymers MB, Timman R, et al. Body image issues after bilateral prophylactic mastectomy with breast reconstruction in healthy women at risk for hereditary breast cancer. Familial cancer 2013;12(3):479‐87. [DOI] [PubMed] [Google Scholar]
Hartmann 1999a {published data only}
- Hartmann LC, Schaid DJ, Woods JE, Crotty TP, Myers JL, Armold PG, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. New England Journal of Medicine 1999;340(2):77‐84. [DOI] [PubMed] [Google Scholar]
Hartmann 2001 {published data only}
- Hartmann LC, Sellers TA, Schaid DJ, Frank TS, Soderberg CL, Sitta D, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. Journal of the National Cancer Institute 2001;93(21):1633‐7. [DOI] [PubMed] [Google Scholar]
Hatcher 2001 {published data only}
- Hatcher M, Fallowfield L, A'Hern R. The psychological impact of bilateral prophylactic mastectomy: prospective study using questionnaires and semistructured interviews. BMJ 2001;322(7278):76‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heemskerk‐Gerritsen 2013 {published data only}
- Heemskerk‐Gerritsen BA, Brekelmans CT, Menke‐Pluymers MB, Geel AN, Tilanus‐Linthorst MM, Bartels CC, et al. Prophylactic mastectomy in BRCA1/2 mutation carriers and women at risk of hereditary breast cancer: long‐term experiences at the Rotterdam Family Cancer Clinic. Annals of Surgical Oncology 2007;14(12):3335‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk‐Gerritsen BA, Menke‐Pluijmers MB, Jager A, Tilanus‐Linthorst MM, Koppert LB, Obdeijn IM, et al. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Annals of Oncology 2013;24(8):2029‐35. [DOI] [PubMed] [Google Scholar]
Heemskerk‐Gerritsen 2015 {published data only}
- Heemskerk‐Gerritsen BA, Rookus MA, Aalfs CM, Auseums MG, Collée JM, Jansen L, et al. Improved overall survival after contralateral risk‐reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. International Journal of Cancer 2015;136(3):668‐77. [DOI] [PubMed] [Google Scholar]
Herrinton 2005 {published data only}
- Herrinton LJ, Barlow WE, Yu O, Geiger AM, Elmore JG, Barton MB, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a Cancer Research Network project. Journal of Clinical Oncology 2005;23(19):4275‐86. [DOI] [PubMed] [Google Scholar]
Hopwood 2000 {published data only}
- Hopwood P, Lee A, Shenton A, Baildam A, Brain A, Lalloo F, et al. Clinical followup after bilateral risk reducing (prophylactic) mastectomy: mental health and body image outcomes. Psycho‐oncology 2000;9(6):462‐72. [DOI] [PubMed] [Google Scholar]
Horton 1978 {published data only}
- Horton CE, Rosato FE, Schuler FA 3d, McGraw J. Postmastectomy reconstruction. Annals of Surgery 1978;188(6):773‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hwang 2016 {published data only}
- Hwang ES, Locklear TD, Rushing CN, Samsa G, Abernethy AP, Hyslop T, et al. Patient‐reported outcomes after choice for contralateral prophylactic mastectomy. Journal of Clinical Oncology 2016;34(13):1518‐27. [DOI] [PubMed] [Google Scholar]
Ingham 2013 {published data only}
- Ingham SL, Sperrin M, Baildam A, Ross GL, Clayton R, Lalloo F, et al. Risk‐reducing surgery increases survival in BRCA1/2 mutation carriers unaffected at time of family referral. Breast Cancer Research & Treatment 2013;142(3):611‐8. [DOI] [PubMed] [Google Scholar]
Isern 2008 {published data only}
- Isern AE, Tengrup I, Loman N, Olsson H, Ringberg A. Aesthetic outcome, patient satisfaction, and health‐related quality of life in women at high risk undergoing prophylactic mastectomy and immediate breast reconstruction. Journal of Plastic, Reconstructive & Aesthetic Surgery 2008;61(10):1177‐87. [DOI] [PubMed] [Google Scholar]
Jatoi 2014 {published data only}
- Jatoi I, Parsons HM. Contralateral prophylactic mastectomy and its association with reduced mortality: evidence for selection bias. Breast Cancer Research & Treatment 2014;148(2):389‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kass 2010 {published data only}
- Kaas R, Verhoef S, Wesseling J, Rookus MA, Oldenburg HS, Peeters MJ, et al. Prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers: very low risk for subsequent breast cancer. Annals of Surgery 2010;251(3):488‐92. [DOI] [PubMed] [Google Scholar]
Kiely 2010 {published data only}
- Kiely BE, Jenkins MA, McKinley JM, Friedlander ML, Milne RL, McLachland SA, et al. Contralateral risk‐reducing mastectomy in BRCA1 and BRCA2 mutation carriers and other high‐risk women in the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab). Breast Cancer Research and Treatment 2010;120(3):715‐23. [DOI] [PubMed] [Google Scholar]
King 2011a {published data only}
- King TA, Sakr P, Patil S, Gurevich I, Stempel M, Sampson M, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. Journal of Clinical Oncology 2011;29(16):2158‐64. [DOI] [PubMed] [Google Scholar]
Koskenvuo 2014 {published data only}
- Koskenvuo L, Svarvar C, Suominen S, Aittomaki K, Jahkola T. The frequency and outcome of breast cancer risk‐reducing surgery in Finnish BRCA1 and BRCA2 mutation carriers. Scandinavian Journal of Surgery 2014;103(1):34‐40. [DOI] [PubMed] [Google Scholar]
Kruper 2014 {published data only}
- Kruper L, Kauffmann RM, Smith DD, Nelson RA. Survival analysis of contralateral prophylactic mastectomy: a question of selection bias. Annals of Surgical Oncology 2014;21(11):3348‐56. [DOI] [PubMed] [Google Scholar]
Lee 1995 {published data only}
- Lee JS, Grant CS, Donohue JH, Crotty TB, Harmsen WS, Ilstrup DM. Arguments against routine contralateral mastectomy or undirected biopsy for invasive lobular breast cancer. Surgery 1995;118(4):640‐7. [DOI] [PubMed] [Google Scholar]
Leis 1981 {published data only}
- Leis HP, Cammarata A, LaRaja R, Reed L, Cleary J, Makoon‐Singh E. Bilateral breast cancer. Breast 1981;7(4):13‐7. [Google Scholar]
McDonnell 2001 {published data only}
- McDonnell SK, Schaid DJ, Myers JL, Grant CS, Donohue JH, Woods JE, et al. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. Journal of Clinical Oncology 2001;19(19):3938‐43. [DOI] [PubMed] [Google Scholar]
Meijers‐Heijboer 2001 {published data only}
- Klijn JGM, Geel AN, Meijers‐Heijboer H, Tilanus‐Linthorst M, Bartels CCM, Crepin CMG, et al. Results of extended series on prophylactic mastectomy versus surveillance in BRCA1/2 mutation carriers in Rotterdam. 27th Annual The Charles A. Coltman, Jr. San Antonio Breast Cancer Symposium (SABCS) 2004;1:S10. [Google Scholar]
- Meijers‐Heijboer H, Geel B, Putten WL, Henzen‐Logmans SC, Seynaeve C, Menke‐Pluymers MB, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. New England Journal of Medicine 2001;345(3):159‐64. [DOI] [PubMed] [Google Scholar]
Metcalfe 2004b {published data only}
- Metcalfe KA, Esplen MJ, Goel V, Narod SA. Psychosocial functioning in women who have undergone bilateral prophylactic mastectomy. Psycho‐oncology 2004;13(1):14‐25. [DOI] [PubMed] [Google Scholar]
Metcalfe 2005 {published data only}
- Metcalfe KA, Esplen MJ, Goel V, Narod SA. Predictors of quality of life in women with a bilateral prophylactic mastectomy. Breast Journal 2005;11(1):65‐9. [DOI] [PubMed] [Google Scholar]
Metcalfe 2014 {published data only}
- Metcalfe K, Gershman S, Ghadirian P, Lynch HT, Snyder C, Tung N, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 2014;348:g226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Journal of Clinical Oncology 2004;22(12):2328‐35. [DOI] [PubMed] [Google Scholar]
Miller 2013 {published data only}
- Miller ME, Czechura T, Martz B, Hall ME, Pesce C, Jaskowiak N, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Annals of Surgical Oncology 2013;20(13):4113‐20. [DOI] [PubMed] [Google Scholar]
Montgomery 1999 {published data only}
- Montgomery LL, Tran KN, Heelan MC, Zee KJ, Massie MJ, Payne DK, et al. Issues of regret in women with contralateral prophylactic mastectomies. Annals of Surgical Oncology 1999;6(6):546‐52. [DOI] [PubMed] [Google Scholar]
Mutter 2015 {published data only}
- Mutter RW, Frost MH, Hoskin TL, Johnson JL, Hartmann LC, Boughey JC. Breast cancer after prophylactic mastectomy (bilateral or contralateral prophylactic mastectomy), a clinical entity: presentation, management, and outcomes. Breast Cancer Research & Treatment 2015;153(1):183‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pennisi 1989 {published data only}
- Pennisi VR, Capozzi A. Subcutaneous mastectomy data: a final statistical analysis of 1500 patients. Aesthetic Plastic Surgery 1989;13(1):15‐21. [DOI] [PubMed] [Google Scholar]
Peralta 2000 {published data only}
- Peralta E, Ellenhorn J, Wagman L, Dagis A, Anderson J, Chu D. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. American Journal of Surgery 2000;180(6):439‐45. [DOI] [PubMed] [Google Scholar]
Pesce 2014 {published data only}
- Pesce C, Liederbach E, Wang C, Lapin B, Winchester DJ, Yao K. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor‐negative breast cancer. Annals of Surgical Oncology 2014;21(10):3231‐9. [DOI] [PubMed] [Google Scholar]
Rebbeck 2004 {published data only}
- Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, 't Veer L, Garber JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. Journal of Clinical Oncology 2004;22(6):1055‐62. [DOI] [PubMed] [Google Scholar]
Skytte 2011 {published data only}
- Skytte AB, Cruger D, Gerster M, Laenkholm AV, Lang C, Brondum‐Nielsen K, et al. Breast cancer after bilateral risk‐reducing mastectomy. Clinical Genetics 2011;79(5):431‐7. [DOI] [PubMed] [Google Scholar]
Unukovych 2012 {published data only}
- Unukovych D, Sandelin K, Liljegren A, Arver B, Wickman M, Johansson H, et al. Contralateral prophylactic mastectomy in breast cancer patients with a family history: a prospective 2‐years follow‐up study of health related quality of life, sexuality and body image. European Journal of Cancer 2012;48(17):3150‐6. [DOI] [PubMed] [Google Scholar]
- Unukovych D, Wickman M, Sandelin K, Arver B, Johansson H, Brandberg Y. Associations between reoperations and psychological factors after contralateral risk‐reducing mastectomy: a two‐year follow‐up study. International Journal of Breast Cancer 2016;2016(ID 4604852):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Sprundel 2005 {published data only}
- Sprundel TC, Schmidt MK, Rookus MA, Brohet R, Asperen CJ, Rutgers EJ, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. British Journal of Cancer 2005;93(3):287‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeichner 2014 {published data only}
- Zeichner SB, Zeichner SB, Ruiz AL, Markward NJ, Rodriguez E. Improved long‐term survival with contralateral prophylactic mastectomy among young women. Asian Pacific Journal of Cancer Prevention 2014;15(3):1155‐62. [DOI] [PubMed] [Google Scholar]
Zion 2003 {published data only}
- Zion S, Slezak J, Schaid D, Frost M, McDonnell S, Woods J, et al. Surgical morbidities following bilateral prophylactic mastectomy. ASCO Abstract No: 1730 Category: Health Services. 2000.
- Zion SM, Slezak JM, Sellers TA, Woods JE, Arnold PG, Petty PM, et al. Reoperations after prophylactic mastectomy with or without implant reconstruction. Cancer 2003;98(10):2152‐60. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbott 2011 {published data only}
- Abbott A, Rueth N, Pappas‐Varco S, Kuntz K, Kerr E, Tuttle T. Perceptions of contralateral breast cancer: an overestimation of risk. Annals of Surgical Oncology 2011;18(11):3129‐36. [DOI] [PubMed] [Google Scholar]
Ager 2016 {published data only}
- Ager B, Butow P, Jansen J, Phillips KA, Porter D. Contralateral prophylactic mastectomy (CPM): a systematic review of patient reported factors and psychological predictors influencing choice and satisfaction. Breast 2016;28:107‐20. [DOI] [PubMed] [Google Scholar]
Alamounti 2015 {published data only}
- Alamouti R, Hachach‐Haram N, Farhadi J. Multidisciplinary management of risk‐reducing mastectomy and immediate reconstruction: treatment algorithm and patient satisfaction. European Journal of Plastic Surgery 2015;38(5):385‐90. [Google Scholar]
Antill 2006 {published data only}
- Antill Y, Reynolds J, Young MA, Kirk J, Tucker K, Bogtstra T, et al. Risk‐reducing surgery in women with familial susceptibility for breast and/or ovarian cancer. European Journal of Cancer 2006;42(5):621‐8. [DOI] [PubMed] [Google Scholar]
Ariyan 1985 {published data only}
- Ariyan S. Prophylactic mastectomy for precancerous and high‐risk lesions of the breast. Canadian Journal of Surgery 1985;28(3):262‐4. [PubMed] [Google Scholar]
Babiera 1997 {published data only}
- Babiera GV, Lowy AM, Davidson BS, Singletary SE. The role of contralateral prophylactic mastectomy in invasive lobular carcinoma. Breast Journal 1997;3(1):2‐6. [Google Scholar]
Barry 2012 {published data only}
- Barry PN, Johnson RR, Harkenrider MM, Freeman AB, Kruse B, Wilson MR, et al. Contralateral prophylactic mastectomy: clinical and pathological features from a prospective database. American Journal of the Medical Sciences 2012;344(6):452‐6. [DOI] [PubMed] [Google Scholar]
Bebbington Hatcher 2003 {published data only}
- Bebbington Hatcher M, Fallowfield LJ. A qualitative study looking at the psychosocial implications of bilateral prophylactic mastectomy. Breast 2003;12(1):1‐9. [DOI] [PubMed] [Google Scholar]
Blackburn 2016 {published data only}
- Blackburn A, Taghizadeh R, Hughes D, O'Donoghue JM. Prevention of perioperative limb neuropathies in abdominal free flap breast reconstruction. Journal of Plastic, Reconstructive and Aesthetic Surgery 2016;69(1):48‐54. [DOI] [PubMed] [Google Scholar]
Borreani 2014 {published data only}
- Borreani C, Manoukian S, Bianchi E, Brunelli C, Peissel B, Caruso A, et al. The psychological impact of breast and ovarian cancer preventive options in BRCA1 and BRCA2 mutation carriers. Clinical Genetics 2014;85(1):7‐15. [DOI] [PubMed] [Google Scholar]
Bostwick 1980 {published data only}
- Bostwick J. Reconstruction of the breast. Acta Chirurgica Belgica 1980;79(2):125‐9. [PubMed] [Google Scholar]
Brekelmans 2006 {published data only}
- Brekelmans CT, Seynaeve C, Menke‐Pluymers M, Brüggenwirth HT, Tilanus‐Linthorst MM, Bartels CC, et al. Survival and prognostic factors in BRCA1‐associated breast cancer. Annals of Oncology 2006;17(3):391‐400. [DOI] [PubMed] [Google Scholar]
Brinton 2001 {published data only}
- Brinton LA, Persson I, Boice JD Jr, McLaughlin JK, Fraumeni JF Jr. Breast cancer risk in relation to amount of tissue removed during breast reduction operations in Sweden. Cancer 2001;91(3):478‐83. [PubMed] [Google Scholar]
Brown 2005 {published data only}
- Brown KL, Hutchison R, Zinberg RE, McGovern MM. Referral and experience with genetic testing among women with early onset breast cancer. Genetic Testing 2005;9(4):301‐5. [DOI] [PubMed] [Google Scholar]
Buehler 1983 {published data only}
- Buehler PK. Patient selection for prophylactic mastectomy: who is at high risk?. Plastic and Reconstructive Surgery 1983;72(3):324‐34. [DOI] [PubMed] [Google Scholar]
Collins 2013 {published data only}
- Collins IM, Milne RL, Weideman PC, McLachlan SA, Friedlander ML, Hopper JL, et al. Preventing breast and ovarian cancers in high‐risk BRCA1 and BRCA2 mutation carriers. Medical Journal of Australia 2013;199(10):680‐3. [DOI] [PubMed] [Google Scholar]
Cortesi 2014 {published data only}
- Cortesi L, Razzaboni E, Toss A, Matteis E, Marchi I, Medici V, et al. A rapid genetic counselling and testing in newly diagnosed breast cancer is associated with high rate of risk‐reducing mastectomy in BRCA1/2‐positive Italian women. Annals of Oncology 2014;25(1):57‐63. [DOI] [PubMed] [Google Scholar]
Dikmans 2016 {published data only}
- Dikmans RE, Morabit F, Ottenhof MJ, Tuinder SM, Twisk JW, Moues C, et al. Single‐stage breast reconstruction using Strattice: a retrospective study. Journal of Plastic, Reconstructive and Aesthetic Surgery 2016;69(2):227‐33. [DOI] [PubMed] [Google Scholar]
Dinner 1981 {published data only}
- Dinner MI, Labandter HP. Total mammary adenectomy with histologic evaluation and immediate reconstruction. Plastic and Reconstructive Surgery 1981;68(4):505‐11. [DOI] [PubMed] [Google Scholar]
Domchek 2011 {published data only}
- Domchek SM, Friebel TM, Singer CF, Gareth Evans D, Lynch HT, Isaacs C, et al. Association of risk‐reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. Journal of the American Medical Association 2010;304(9):967‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisinger 2001 {published data only}
- Eisinger F, Stoppa‐Lyonnet D, Lasset C, Vennin P, Chabal F, Noguès C, et al. Comparison of physicians' and cancer prone women's attitudes about breast/ovarian prophylactic surgery. Results from two national surveys. Familial Cancer 2001;1(3‐4):157‐62. [DOI] [PubMed] [Google Scholar]
Evans 2005 {published data only}
- Evans DG, Lalloo F, Hopwood P, Maurice A, Baildam A, Brain A, et al. Surgical decisions made by 158 women with hereditary breast cancer aged <50 years. European Journal of Surgical Oncology 2005;31(10):1112‐8. [DOI] [PubMed] [Google Scholar]
Fowbie 2015 {published data only}
- Fowbie B, Park C, Wang F, Peled A, Alvarado M, Ewing C, et al. Rates of reconstruction failure in patients undergoing immediate reconstruction with tissue expanders and/or implants and postmastectomy radiation therapy. International Journal of Radiation Oncology, Biology, Physics 2015;92(3):634‐41. [DOI] [PubMed] [Google Scholar]
Fu 2015 {published data only}
- Fu Y, Zhuang Z, Dewing M, Apple S, Chang H. Predictors for contralateral prophylactic mastectomy in breast cancer patients. International Journal of Clinical and Experimental Pathology 2015;8(4):3748‐64. [PMC free article] [PubMed] [Google Scholar]
Graham 2015 {published data only}
- Graham PJ, Brar MS, Foster T, McCall M, Bouchard‐Fortier A, Temple W, et al. Neoadjuvant chemotherapy for breast cancer, is practice changing? A population‐based review of current surgical trends. Annals of Surgical Oncology 2015;22(10):3376‐82. [DOI] [PubMed] [Google Scholar]
Gschwantier 2016 {published data only}
- Gschwantler‐Kaulich D, Schrenk P, Bjelic‐Radisic V, Unterrieder K, Leser C, Fink‐Retter A, et al. Mesh versus acellular dermal matrix in immediate implant‐based breast reconstruction ‐ a prospective randomized trial. European Journal of Surgical Oncology 2016;42(5):665‐71. [DOI] [PubMed] [Google Scholar]
Hagen 2014 {published data only}
- Hagen AI, Maehle L, Veda N, Vetti HH, Stormorken A, Ludvigsen T, et al. Risk reducing mastectomy, breast reconstruction and patient satisfaction in Norwegian BRCA1/2 mutation carriers. Breast 2014;23(1):38‐43. [DOI] [PubMed] [Google Scholar]
Han 2011 {published data only}
- Han E, Johnson N, Glissmeyer M, Wagie T, Carey B, DelaMelena T, et al. Increasing incidence of bilateral mastectomies: the patient perspective. American Journal of Surgery 2011;201(5):615‐8. [DOI] [PubMed] [Google Scholar]
Heiniger 2015 {published data only}
- Heiniger L, Butow PN, Coll J, Bullen T, Wilson J, Baylock B, et al. Long‐term outcomes of risk‐reducing surgery in unaffected women at increased familial risk of breast and/or ovarian cancer. Familial Cancer 2015;14(1):105‐15. [DOI] [PubMed] [Google Scholar]
Hoffman 1982 {published data only}
- Hoffman S, Pressman PI. Prophylactic mastectomy. The Mount Sinai Journal of Medicine (New York) 1982;49(2):102‐9. [PubMed] [Google Scholar]
Horton 1988 {published data only}
- Horton CE, Dascombe WH. Total mastectomy: indications and techniques. Clinics in Plastic Surgery 1988;15(4):677‐87. [PubMed] [Google Scholar]
Houn 1995 {published data only}
- Houn F, Helzlsouer KJ, Friedman NB, Stefanek ME. The practice of prophylactic mastectomy: a survey of Maryland surgeons. American Journal of Public Health 1995;85(6):801‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarrett 1982 {published data only}
- Jarrett JR, Cutler RG, Teal DF. Aesthetic refinements in prophylactic subcutaneous mastectomy with submuscular reconstruction. Plastic and Reconstructive Surgery 1982;69(4):624‐31. [DOI] [PubMed] [Google Scholar]
Jones 2009 {published data only}
- Jones NB, Wilson J, Koture L, Stephens J, Farrar WB, Agnese DM. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Annals of Surgical Oncology 2009;16(10):2691‐6. [DOI] [PubMed] [Google Scholar]
Josephson 2000 {published data only}
- Josephson U, Wickman M, Sandelin K. Initial experiences of women from hereditary breast cancer families after bilateral prophylactic mastectomy: a retrospective study. European Journal of Surgical Oncology 2000;26(4):351‐6. [DOI] [PubMed] [Google Scholar]
Katapodi 2004 {published data only}
- Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta‐analytic review. Preventive Medicine 2004;38(4):388‐402. [DOI] [PubMed] [Google Scholar]
Kheirelseid 2011 {published data only}
- Kheirelseid EA, Jumustafa H, Miller N, Curran C, Sweeney K, Malone C, et al. Bilateral breast cancer: analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Research & Treatment 2011;126(1):131‐40. [DOI] [PubMed] [Google Scholar]
King 2011b {published data only}
- King TA, Gurevich I, Sakr R, Patil S, Stempel M, Morrow M. Occult malignancy in patients undergoing contralateral prophylactic mastectomy. Annals of Surgery 2011;254(1):2‐7. [DOI] [PubMed] [Google Scholar]
Klitzman 2010 {published data only}
- Klitzman R, Chung W. The process of deciding about prophylactic surgery for breast and ovarian cancer: patient questions, uncertainties, and communication. American Journal of Medical Genetics 2010;152A(1):52‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurian 2005 {published data only}
- Kurian AW, Hartman AR, Mills MA, Ford JM, Daniel BL, Plevritis SK. Opinions of women with high inherited breast cancer risk about prophylactic mastectomy: an initial evaluation from a screening trial including magnetic resonance imaging and ductal lavage. Health Expectations 2005;8(3):221‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leis 1980 {published data only}
- Leis HP Jr. Managing the remaining breast. Cancer 1980;46(4 Suppl):1026‐30. [DOI] [PubMed] [Google Scholar]
Lerman 1996 {published data only}
- Lerman C, Narod S, Schulman K, Hughes C, Gomez‐Caminero A, Bonney G, et al. BRCA1 testing in families with hereditary breast‐ovarian cancer. A prospective study of patient decision making and outcomes. JAMA 1996;275(24):1885‐92. [PubMed] [Google Scholar]
Litton 2009 {published data only}
- Litton JK, Westin SN, Ready K, Sun CC, Peterson SK, Meric‐Bernstam F, et al. Perception of screening and risk reduction surgeries in patients tested for a BRCA deleterious mutation. Cancer 2009;115(8):1598‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd 2000 {published data only}
- Lloyd SM, Watson M, Oaker G, Sacks N, Querci della Rovere U, Gui G. Understanding the experience of prophylactic bilateral mastectomy: a qualitative study of ten women. Psycho‐oncology 2000;9(6):473‐85. [DOI] [PubMed] [Google Scholar]
Lodder 2002 {published data only}
- Lodder LN, Frets PG, Trijsburg RW, Meijers‐Heijboer EJ, Klijn JG, Seynaeve C, et al. One year follow‐up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Research & Treatment 2002;7(2):97‐112. [DOI] [PubMed] [Google Scholar]
Lynch 1991 {published data only}
- Lynch HT, Watson P, Conway TA, Lynch J. Monitoring high risk women: psychological aspects. Developments in Oncology 1991;62:191‐205. [Google Scholar]
Lynch 2006 {published data only}
- Lynch HT, Snyder C, Lynch JF, Karatoprakli P, Trowonou A, Metcalfe K, et al. Patient responses to the disclosure of BRCA mutation tests in hereditary breast‐ovarian cancer families. Cancer Genetics and Cytogenetics 2006;165(2):91‐7. [DOI] [PubMed] [Google Scholar]
Madlensky 2005 {published data only}
- Madlensky L, Flatt SW, Bardwell WA, Rock CL, Pierce JP, WHEL Study group. Is family history related to preventive health behaviors and medical management in breast cancer patients?. Breast Cancer Research and Treatment 2005;90(1):47‐54. [DOI] [PubMed] [Google Scholar]
McAvoy 1979 {published data only}
- McAvoy J. Subcutaneous mastectomy in the treatment of the high‐risk female‐‐is emotionalism obscuring the facts?. Annals of Plastic Surgery 1979;3(3):219‐26. [DOI] [PubMed] [Google Scholar]
McCready 2007 {published data only}
- McCready DR, Escallon J. Clinical considerations regarding contralateral prophylactic mastectomy. Women's Health 2007;3(1):39‐43. [DOI] [PubMed] [Google Scholar]
Meijers‐Heijboer 2003 {published data only}
- Meijers‐Heijboer H, Brekelmans CT, Menke‐Pluymers M, Seynaeve C, Baalbergen A, Burger C, et al. Use of genetic testing and prophylactic mastectomy and oophorectomy in women with breast or ovarian cancer from families with a BRCA1 or BRCA2 mutation. Journal of Clinical Oncology 2003;21(9):1675‐81. [DOI] [PubMed] [Google Scholar]
Metcalfe 2004c {published data only}
- Metcalfe KA, Semple JL, Narod SA. Satisfaction with breast reconstruction in women with bilateral prophylactic mastectomy: a descriptive study. Plastic and Reconstructive Surgery 2004;114(2):360‐6. [DOI] [PubMed] [Google Scholar]
Metcalfe 2008a {published data only}
- Metcalfe KA, Lubinski J, Ghadirian P, Lynch H, Kim‐Sing C, Friedman E, et al. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: The Hereditary Breast Cancer Clinical Study Group. Journal of Clinical Oncology 2008;26(7):1093‐7. [DOI] [PubMed] [Google Scholar]
Metcalfe 2008b {published data only}
- Metcalfe KA, Birenbaum‐Carmeli D, Lubinski J, Gronwald J, Lynch H, Moller P, et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. International Journal of Cancer 2008;122(9):2017‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metcalfe 2011a {published data only}
- Metcalfe K, Lynch HT, Ghadirian P, Tung N, Kim‐Sing C, Olopade OI, et al. Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Research & Treatment 2011;127(1):287‐96. [DOI] [PubMed] [Google Scholar]
Metcalfe 2011b {published data only}
- Metcalfe K, Gershman S, Lynch HT, Ghadirian P, Tung N, Kim‐Sing C, et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. British Journal of Cancer 2011;104(9):1384‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyer 1986 {published data only}
- Meyer L, Ringberg A. A prospective study of psychiatric and psychological sequelae of bilateral subcutaneous mastectomy. Scandinavian Journal of Plastic and Reconstructive Surgery 1986;20(1):101‐7. [DOI] [PubMed] [Google Scholar]
Mulvihill 1982 {published data only}
- Mulvihill JJ, Safyer AW, Bening JK. Prevention in familial breast cancer: counseling and prophylactic mastectomy. Preventive Medicine 1982;11(5):500‐11. [DOI] [PubMed] [Google Scholar]
Narod 2011 {published data only}
- Narod SA. The impact of contralateral mastectomy on mortality in BRCA1 and BRCA2 mutation carriers with breast cancer. Breast Cancer Research & Treatment 2011;128(2):581‐3. [DOI] [PubMed] [Google Scholar]
Narod 2014 {published data only}
- Narod SA. Bilateral breast cancers. Nature Reviews Clinical Oncology 2014;11:157‐66. [DOI] [PubMed] [Google Scholar]
Nekhlyudov 2005 {published data only}
- Nekhlyudov L, Bower M, Herrinton LJ, Altschuler A, Greene SM, Rolnick S, et al. Women's decision‐making roles regarding contralateral prophylactic mastectomy. Journal of the National Cancer Institute. Monographs 2005;35:55‐60. [DOI] [PubMed] [Google Scholar]
Osman 2013 {published data only}
- Osman F, Saleh F, Jackson TD, Corrigan MA, Cil T. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: an analysis of the NSQIP database. Annals of Surgical Oncology 2013;20(10):3212‐7. [DOI] [PubMed] [Google Scholar]
Patenaude 2008 {published data only}
- Patenaude AF, Orozco S, Li X, Kaelin CM, Gadd M, Matory Y, et al. Support needs and acceptability of psychological and peer consultation: attitudes of 108 women who had undergone or were considering prophylactic mastectomy. Psycho‐oncology 2008;17(8):831‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Payne 2000 {published data only}
- Payne DK, Biggs C, Tran KN, Borgen PI, Massie MJ. Women's regrets after bilateral prophylactic mastectomy. Annals of Surgical Oncology 2000;7(2):150‐4. [DOI] [PubMed] [Google Scholar]
Pennisi 1984 {published data only}
- Pennisi VR, Capozzi A. Subcutaneous mastectomy: an interim report on 1,244 patients. Annals of Plastic Surgery 1984;12(4):340‐7. [DOI] [PubMed] [Google Scholar]
Petit 2002 {published data only}
- Petit JY, Greco M, EUSOMA. Quality control in prophylactic mastectomy for women at high risk of breast cancer. European Journal of Cancer 2002;38(1):23‐6. [DOI] [PubMed] [Google Scholar]
Phillips 2006 {published data only}
- Phillips KA, Jenkins MA, Lindeman GJ, McLachlan SA, McKinely JM, Weideman PC, et al. Risk‐reducing surgery, screening and chemoprevention practices of BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Clinical Genetics 2006;70(3):198‐206. [DOI] [PubMed] [Google Scholar]
Rhiem 2012 {published data only}
- Rhiem K, Engel C, Graeser M, Zachariae S, Kast K, Kiechle M, et al. The risk of contralateral breast cancer in patients from BRCA1/2 negative high risk families as compared to patients from BRCA1 or BRCA2 positive families: a retrospective cohort study. Breast Cancer Research 2012;14(6):R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringberg 1982 {published data only}
- Ringberg A, Palmer B, Linell F. The contralateral breast at reconstructive surgery after breast cancer operation‐‐a histopathological study. Breast Cancer Research and Treatment 1982;2(2):151‐61. [DOI] [PubMed] [Google Scholar]
Roberts 2014 {published data only}
- Roberts A, Habibi M, Frick KD. Cost‐effectiveness of contralateral prophylactic mastectomy for prevention of contralateral breast cancer. Annals of Surgical Oncology 2014;21(7):2209‐17. [DOI] [PubMed] [Google Scholar]
Roberts 2015 {published data only}
- Roberts A, Baxter N, Camacho X, Lau C, Zhong T. Once is rarely enough: a population‐based study of reoperations after postmastectomy breast reconstruction. Annals of Surgical Oncology 2015;22(10):3302‐7. [DOI] [PubMed] [Google Scholar]
Roinick 2007 {published data only}
- Rolnick SJ, Altschuler A, Nekhlyudov L, Elmore JG, Green SM, Harris EL, et al. What women wish they knew before prophylactic mastectomy. Cancer Nursing 207;30(4):285‐91. [DOI] [PubMed] [Google Scholar]
Rubin 1979 {published data only}
- Rubin LR. A preventive mastectomy with immediate reconstruction for the high‐risk breast cancer patient. Clinics in Plastic Surgery 1979;6(1):107‐19. [PubMed] [Google Scholar]
Rueth 2011 {published data only}
- Rueth NM, McMahon M, Arrington AK, Swenson K, Leach J, Tuttle TM. Preoperative risk assessment among women undergoing bilateral prophylactic mastectomy for cancer risk reduction. Annals of Surgical Oncology 2011;18(9):2515‐20. [DOI] [PubMed] [Google Scholar]
Sakorafas 2002 {published data only}
- Sakorafas GH. Women at high risk for breast cancer: preventive strategies. The Mount Sinai Journal of Medicine (New York) 2002;69(4):264‐6. [PubMed] [Google Scholar]
Salhab 2010 {published data only}
- Salhab M, Bismohun S, Mokbel K. Risk‐reducing strategies for women carrying BRCA1/2 mutations with a focus on prophylactic surgery. BMC Women's Health 2010;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2004 {published data only}
- Schwartz MD, Lerman C, Brogan B, Peshkin BN, Halbert CH, DeMarco T, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. Journal of Clinical Oncology 2004;22(10):1823‐9. [DOI] [PubMed] [Google Scholar]
Scott 2003 {published data only}
- Scott CI, Iorgulescu DG, Thorne HJ, Henderson MA, Phillips KA, Kathleen Cuningham Foundation Consortium for Familial Breast Cancer (kConFab). Clinical, pathological and genetic features of women at high familial risk of breast cancer undergoing prophylactic mastectomy. Clinical Genetics 2003;64(2):111‐21. [DOI] [PubMed] [Google Scholar]
See 2005 {published data only}
- See HT, Cheung YB, Yong F, Khoo KS, Ang P. Acceptance of prophylactic surgery and chemoprevention of cancer in Singapore ‐ a survey. Annals of the Academy of Medicine (Singapore) 2005;34(3):238‐42. [PubMed] [Google Scholar]
Snyderman 1984 {published data only}
- Snyderman RK. Prophylactic mastectomy: pros and cons. Cancer 1984;53(3 Suppl):803‐8. [DOI] [PubMed] [Google Scholar]
Spear 2008 {published data only}
- Spear SL, Schwarz KA, Venturi ML, Barbosa T, Al‐Attar A. Prophylactic mastectomy and reconstruction: clinical outcomes and patient satisfaction. Plastic & Reconstructive Surgery 2008;122(1):1‐9. [DOI] [PubMed] [Google Scholar]
Specht 2004 {published data only}
- Specht MC, Borgen PI, Fey J, Zhang Z, Sclafani L. Personal health behaviors in women who have undergone risk‐reducing mastectomy. American Journal of Surgery 2004;188(4):448‐9. [DOI] [PubMed] [Google Scholar]
Stalmeier 2009 {published data only}
- Stalmeier PF, Roosmalen MS. Concise evaluation of decision aids. Patient Education & Counseling 2009;74(1):104‐9. [DOI] [PubMed] [Google Scholar]
Stefanek 1995 {published data only}
- Stefanek ME, Helzlsouer KJ, Wilcox PM, Houn F. Predictors of satisfaction with bilateral prophylactic mastectomy. Preventive Medicine 1995;24(4):412‐9. [DOI] [PubMed] [Google Scholar]
Stolier 2005 {published data only}
- Stolier AJ, Corsetti RL. Newly diagnosed breast cancer patients choose bilateral mastectomy over breast‐conserving surgery when testing positive for a BRCA1/2 mutation. The American Surgeon 2005;71(12):1031‐3. [PubMed] [Google Scholar]
Stuckey 2010 {published data only}
- Stuckey A, Dizon D, Scalia WJ, Kent J, Tejada‐Berges T, Gass J, et al. Clinical characteristics and choices regarding risk‐reducing surgery in BRCA mutation carriers. Gynecologic and Obstetric Investigations 2010;69(4):270‐3. [DOI] [PubMed] [Google Scholar]
Temple 1991 {published data only}
- Temple WJ, Lindsay RL, Magi E, Urbanski SJ. Technical considerations for prophylactic mastectomy in patients at high risk for breast cancer. American Journal of Surgery 1991;161(4):413‐5. [DOI] [PubMed] [Google Scholar]
Tercyak 2007 {published data only}
- Tercyak KP, Peshkin BN, Brogan BM, DeMarco T, Pennanen MF, Willey SC, et al. Quality of life after contralateral prophylactic mastectomy in newly diagnosed high‐risk breast cancer patients who underwent BRCA1/2 gene testing. Journal of Clinical Oncology 2007;25(3):285‐91. [DOI] [PubMed] [Google Scholar]
Theogaraj 1973 {published data only}
- Theogaraj SD. Cancer prophylaxis by subcutaneous mastectomy. Indications and recent trends in reconstruction. Virginia Medical Monthly 1973;100(6):545‐50. [PubMed] [Google Scholar]
Tuttle 2007 {published data only}
- Tuttle TM, Haberman EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. Journal of Clinical Oncology 2007;25(33):5203‐9. [DOI] [PubMed] [Google Scholar]
Unic 1998 {published data only}
- Unic I, Stalmeier PF, Verhoef LC, Daal WA. Assessment of the time‐tradeoff values for prophylactic mastectomy of women with a suspected genetic predisposition to breast cancer. Medical Decision Making 1998;18(3):268‐77. [DOI] [PubMed] [Google Scholar]
Van Dijk 2003 {published data only}
- Dijk S, Otten W, Zoeteweij MW, Timmermans DR, Asperen CJ, Breuning MH, et al. Genetic counselling and the intention to undergo prophylactic mastectomy: effects of a breast cancer risk assessment. British Journal of Cancer 2003;88(11):1675‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Smitten 2001 {published data only}
- Smitten K. Prophylactic breast surgery for women with BRCA1 and BRCA2 germline mutations. Tumori 2001;87(4):S13‐5. [PubMed] [Google Scholar]
Wang 2015a {published data only}
- Wang F, Amara D, Peled AW, Sbitany H, Foster RD, Ewing CA, et al. Negative genetic testing does not deter contralateral prophylactic mastectomy in younger patients with greater family histories of breast cancer. Annals of Surgical Oncology 2015;22(10):3338‐45. [DOI] [PubMed] [Google Scholar]
Wapnir 1990 {published data only}
- Wapnir IL, Rabinowitz B, Greco RS. A reappraisal of prophylactic mastectomy. Surgery, Gynecology & Obstetrics 1990;171(2):171‐84. [PubMed] [Google Scholar]
Wasteson 2011 {published data only}
- Wasteson E, Sandeline K, Brandberg Y, Wickman M, Arver B. High satisfaction rate ten years after bilateral prophylactic mastectomy ‐ a longitudinal study. 2011 European Journal of Cancer;20(4):508‐13. [DOI] [PubMed] [Google Scholar]
Yarbro 1985 {published data only}
- Yarbro JW. Breast cancer: the new biology in conflict with the old dogma. Seminars in Oncology Nursing 1985;1(3):157‐62. [DOI] [PubMed] [Google Scholar]
Yi 2010 {published data only}
- Yi M, Hunt KK, Arun BK, Bedrosian I, Barrera AG, Do KA, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prevention Research 2010;3(8):1026‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zendejas 2011 {published data only}
- Zendejas B, Moriarty JP, O'Byrne J, Degnim AC, Farley DR, Boughey JC. Cost‐effectiveness of contralateral prophylactic mastectomy versus routine surveillance in patients with unilateral breast cancer. Journal of Clinical Oncology 2011;29(22):2993‐3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACS 2017
- American Cancer Society. Cancer Statistics Center. cancerstatisticscenter.cancer.org/# 2017.
Anderson 2001
- Anderson BO. Prophylactic surgery to reduce breast cancer risk: A brief literature review. The Breast Journal 2001;7(5):321‐30. [DOI] [PubMed] [Google Scholar]
Baasch 1996
- Baasch M, Nielsen SF, Engholm G, Lund K. Breast cancer incidence subsequent to surgical reduction of the female breast. British Journal of Cancer 1996;73(7):961‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry 2011
- Barry M, Sacchini V. When is contralateral mastectomy warranted in unilateral breast cancer?. Expert Review of Anticancer Therapy 2011;11(8):1209‐14. [DOI] [PubMed] [Google Scholar]
Boughey 2015
- Boughey JC, Hoskin TL, Hartmann LC, Johnson JL, Jacobson SR, Degnim AC, et al. Impact of reconstruction and reoperation on long‐term patient‐reported satisfaction after contralateral prophylactic mastectomy. Annals of Surgical Oncology 2015;22(2):401‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandberg 2012
- Brandberg Y, Arver B, Johansson H, Wickman M, Sandelin K, Liljegren A. Less correspondence between expectations before and cosmetic results after risk‐reducing mastectomy in women who are mutation carriers: a prospective study. European Journal of Surgical Oncology 2012;38(1):38‐43. [DOI] [PubMed] [Google Scholar]
Brewster 2011
- Brewster AM, Parker PA. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncology 2011;16(7):935‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burness 2011
- Burness ML, Olopade OI. Is screening with magnetic resonance imaging in BRCA mutation carriers a safe and effective alternative to prophylactic mastectomy?. Journal of Clinical Oncology 2011;29(13):1652‐4. [DOI] [PubMed] [Google Scholar]
Carr‐Hill 1992
- Carr‐Hill RA. The measurement of patient satisfaction. Journal of Public Health Medicine 1992;14(3):236‐49. [PubMed] [Google Scholar]
Chang 2003
- Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer 2003;97(3):545‐53. [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD (eds.). Assessment of study quality. Cochrane reviewers Handbook 4.1.5 [updated April 2002]; Section 4. 2002, issue 2.
Crosby 2011
- Crosby MA, Garvey PB, Selber JC, Adelman DM, Sacks JM, Villa MT, et al. Reconstructive outcomes in patients undergoing contralateral prophylactic mastectomy. Plastic & Reconstructive Surgery 2011;12(5):1025‐33. [DOI] [PubMed] [Google Scholar]
Eisen 2000
- Eisen A, Rebbeck TR, Wood WC, Weber BL. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. Journal of Clinical Oncology 2000;18(9):1980‐95. [DOI] [PubMed] [Google Scholar]
Ernster 1999
- Ernster VL. Prophylactic mastectomy in women with a high risk of breast cancer. New England Journal of Medicine 1999;340(23):1838. [PubMed] [Google Scholar]
Fayanju 2014
- Fayanju OM, Stoll CRT, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta‐analysis. Annals of Surgery 2014;260:1000‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2013
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality worldwide. IARC CancerBase globocan.iarc.fr 2013; Vol. 11.
Frost 2011
- Frost MH, Hoskin TL, Hartmann LC, Degnim AC, Johnson JL, Boughey JC. Contralateral prophylactic mastectomy: long‐term consistency of satisfaction and adverse effects and the significance of informed decision‐making, quality of life, and personality traits. Annals of Surgical Oncology 2011;18(11):3110‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fryzek 2005
- Fryzek JP, Ye W, Nyren O, Tarone RE, Lipworth L, McLaughlin JK. A nationwide epidemiologic study of breast cancer incidence following breast reduction surgery in a large cohort of Swedish women. Breast Cancer Research and Treatment 2006;97(2):131‐4. [DOI] [PubMed] [Google Scholar]
Gahm 2013
- Gahm J, Hansson P, Brandberg Y, Wickman M. Breast sensibility after bilateral risk‐reducing mastectomy and immediate breast reconstruction: a prospective study. Journal of Plastic, Reconstructive & Aesthetic Surgery 2013;66(11):1521‐7. [DOI] [PubMed] [Google Scholar]
Gail 1994
- Gail MH, Benichou J. Validation studies on a model for breast cancer risk. Journal of the National Cancer Institute 1994;86(8):573‐5. [DOI] [PubMed] [Google Scholar]
Ghosh 2002
- Ghosh K, Hartmann LC. Current status of prophylactic mastectomy. Oncology 2002;16(10):1319‐25. [PubMed] [Google Scholar]
GLOBOCAN 2012
- Global Cancer Observatory, International Agency for Research on Cancer. gco.iarc.fr/ date accessed 28 May 2017.
Gordis 1996
- Gordis L. Epidemiology. Philadelphia: WB Saunders, 1996:116‐8. [Google Scholar]
Hartmann 1999b
- Hartmann LC, Schaid DJ, Sellers TA. Prophylactic mastectomy in women with a high risk of breast cancer (The authors reply). New England Journal of Medicine 1999;340(23):1839. [PubMed] [Google Scholar]
Hartmann 2004
- Hartmann LC, Degnim A, Schaid DJ. Prophylactic mastectomy for BRCA1/2 carriers: progress and more questions. Journal of Clinical Oncology 2004;22(6):981‐3. [DOI] [PubMed] [Google Scholar]
Haynes 1990
- Haynes RB, Mulrow CD, Huth EJ, Altman DG, Gardner MJ. More informative abstracts revisited. Annals of Internal Medicine 1990;113(1):69‐76. [DOI] [PubMed] [Google Scholar]
Heemskerk‐Gerritsen 2007
- Heemskerk‐Gerritsen BA, Brekelmans CT, Menke‐Pluymers MB, Geel AN, Tilanus‐Linthorst MM, Bartels CC, et al. Prophylactic mastectomy in BRCA1/2 mutation carriers and women at risk of hereditary breast cancer: long‐term experiences at the Rotterdam Family Cancer Clinic. Annals of Surgical Oncology 2007;14(12):3335‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Homer 2000
- Homer JJ, Sheard CE, Jones NS. Cognitive dissonance, the placebo effect and the evaluation of surgical results. Clinical Otolaryngology and Allied Sciences 2000;25(3):195‐9. [DOI] [PubMed] [Google Scholar]
Johnson 2003
- Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. Journal of Clinical Oncology 2003;21(7):1404‐11. [DOI] [PubMed] [Google Scholar]
Klaren 2003
- Klaren HM, Van't Veer LJ, Leeuwen FE, Rookus MA. Potential for bias in studies on efficacy of prophylactic surgery for BRCA1 and BRCA2 mutation. Journal of the National Cancer Institute 2003;95(13):941‐7. [DOI] [PubMed] [Google Scholar]
Klijn 2004
- Klijn JGM, Geel AN, Meijers‐Heijboer H, Tilanus‐Linthorst M, Bartels CCM, Crepin CMG, et al. Results of extended series on prophylactic mastectomy versus surveillance in BRCA1/2 mutation carriers in Rotterdam. 27th Annual The Charles A. Coltman, Jr. San Antonio Breast Cancer Symposium (SABCS). 2004; Vol. 1:S10.
Kuchenbaecker 2017
- Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos‐Blom MJ, et al. Risks of breast, ovarian and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017;317(23):2402‐16. [DOI] [PubMed] [Google Scholar]
Lise 1997
- Lise M, Zavagno G, Meggiolaro F. Prophylactic mastectomy in women at high risk of breast cancer. Forum 1997;7.1(2 Suppl):112‐6. [Google Scholar]
Lopez 1996
- Lopez MJ, Porter KA. The current role of prophylactic mastectomy. The Surgical Clinics of North America 1996;76(2):231‐42. [DOI] [PubMed] [Google Scholar]
Metcalfe 2002
- Metcalfe KA, Narod SA. Breast cancer risk perception among women who have undergone prophylactic bilateral mastectomy. Journal of National Cancer Institute 2002;94(20):1564‐9. [DOI] [PubMed] [Google Scholar]
Metcalfe 2004a
- Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Journal of Clinical Oncology 2004;22(12):2328‐35. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newman 2001
- Newman L. Despite efficacy of prophylactic mastectomy, procedure finds few enthusiasts. Journal of the National Cancer Institute 2001;93(5):338‐9. [DOI] [PubMed] [Google Scholar]
Palmieri 1999
- Palmieri C. Trial of prophylactic mastectomy is needed. BMJ 1999;318(7197):1556‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quan 2008
- Quan G, Pommier SJ, Pommier RF. Incidence and outcomes of contralateral breast cancers. American Journal of Surgery 2008;195(5):645‐50. [DOI] [PubMed] [Google Scholar]
Rookus 2002
- Rookus DH, Kappas AM, Tsianos E. Role of surgery in the prophylaxis of hereditary cancer syndromes. Annals of Surgical Oncology 2002;9(7):607‐9. [DOI] [PubMed] [Google Scholar]
Rubin 1991
- Rubin HR, Wu AW. Patient satisfaction: its importance and how to measure it. In: Gitnick G editor(s). The Business of Medicine: A Physician's Guide. New York: Elsevier Science Publishing Co, 1991:397‐409. [Google Scholar]
Shadehi 2005
- Shahedi K, Emanuelsson M, Wiklund F, Gronberg H. High risk of contralateral breast carcinoma in women with hereditary/familial non‐BRCA1/BRCA2 breast carcinoma. Cancer 2005;106(6):1237‐42. [DOI] [PubMed] [Google Scholar]
Stefanek 2001
- Stefanek M, Hartmann L, Nelson W. Risk‐reduction mastectomy: clinical issues and research needs. Journal of the National Cancer Institute 2001;93(17):1297‐306. [DOI] [PubMed] [Google Scholar]
Van den Broek 2016
- Broek AJ, 't Veer LJ, Hooning MJ, Cornelissen S, Broeks A, Rutgers EJ, et al. Impact of age at primary breast cancer on contralateral breast cancer risk in BRCA1/2 mutation carriers. Journal of Clinical Oncology 2016;34(5):409‐18. [DOI] [PubMed] [Google Scholar]
Van Dijk 2008
- Dijk S, Roosmalen MS, Otten W, Stalmeier PF. Decision making regarding prophylactic mastectomy: stability of preferences and the impact of anticipated feelings of regret. Journal of Clinical Oncology 2008;26(14):2358‐63. [DOI] [PubMed] [Google Scholar]
von Oostrom 2003
- Iris van Oostrom, Hanne Meijers‐Heijboer, Litanja N. Lodder, Hugo J. Duivenvoorden, Arthur R. van Gool, Caroline Seynaeve, Conny A. van der Meer, Jan G.M. Klijn, Bert N. van Geel, Curt W. Burger, Juriy W. Wladimiroff, Aad Tibben. Iris van Oostrom, Hanne Meijers‐Heijboer, Litanja N. Lodder, Hugo J. Duivenvoorden, Arthur R. van Gool, Caroline Seynaeve,Conny A. van der Meer, Jan G.M. Klijn, Bert N. van Geel, Curt W. Burger, Juriy W. Wladimiroff, and Aad Tibben. J Clin Oncol 2003;21:3867‐3874. [DOI] [PubMed] [Google Scholar]
Ware 1988
- Ware JE, Hays RD. Methods for measuring patient satisfaction with specific medical encounters. Medical Care 1988;26(4):393‐402. [DOI] [PubMed] [Google Scholar]
Yao 2010
- Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998‐2007. Annals of Surgical Oncology 2010;17(10):2564‐2. [DOI] [PubMed] [Google Scholar]
Yao 2016
- Yao K, Sisco M, Bedrosian I. Contralateral prophylactic mastectomy: current perspectives. International Journal of Women's Health 2016;8(3):213‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zion 2000
- Zion S, Slezak J, Schaid D, Frost M, McDonnell S, Woods J, et al. Surgical morbidities following bilateral prophylactic mastectomy. ASCO Abstract No: 1730 Category: Health Services. 2000.
References to other published versions of this review
Lostumbo 2010
- Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD002748.pub3] [DOI] [PubMed] [Google Scholar]


