Abstract
Background
This is an updated version of the Cochrane Review previously published in Issue 3, 2015.
The incidence of seizures following supratentorial craniotomy for non‐traumatic pathology has been estimated to be between 15% to 20%; however, the risk of experiencing a seizure appears to vary from 3% to 92% over a five‐year period. Postoperative seizures can precipitate the development of epilepsy; seizures are most likely to occur within the first month of cranial surgery. The use of antiepileptic drugs (AEDs) administered pre‐ or postoperatively to prevent seizures following cranial surgery has been investigated in a number of randomised controlled trials (RCTs).
Objectives
To determine the efficacy and safety of AEDs when used prophylactically in people undergoing craniotomy and to examine which AEDs are most effective.
Search methods
For the latest update we searched the following databases on 26 June 2017: Cochrane Epilepsy Group Specialized Register, the CENTRAL, MEDLINE, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP). We did not apply any language restrictions.
Selection criteria
We included RCTs of people with no history of epilepsy who were undergoing craniotomy for either therapeutic or diagnostic reasons. We included trials with adequate randomisation methods and concealment; these could either be blinded or unblinded parallel trials. We did not stipulate a minimum treatment period, and we included trials using active drugs or placebo as a control group.
Data collection and analysis
Three review authors (JW, JG, YD) independently selected trials for inclusion and performed data extraction and risk of bias assessments. We resolved any disagreements through discussion. Outcomes investigated included the number of participants experiencing seizures (early (occurring within first week following craniotomy), and late (occurring after first week following craniotomy)), the number of deaths and the number of people experiencing disability and adverse effects. Due to the heterogeneous nature of the trials, we did not combine data from the included trials in a meta‐analysis; we presented the findings of the review in narrative format. Visual comparisons of outcomes are presented in forest plots.
Main results
We included 10 RCTs (N = 1815), which were published between 1983 and 2015. Three trials compared a single AED (phenytoin) with placebo or no treatment. One three‐armed trial compared two AEDs (phenytoin, carbamazepine) with no treatment. A second three‐armed trial compared phenytoin, phenobarbital with no treatment. Of these five trials comparing AEDs with placebo or no treatment, two trials reported a statistically significant advantage for AED treatment compared to controls for early seizure occurrence; all other comparisons showed no clear or statistically significant differences between AEDs and control treatment. None of the trials that were head‐to‐head comparisons of AEDs (phenytoin versus sodium valproate, phenytoin versus phenobarbital, levetiracetam versus phenytoin, zonisamide versus phenobarbital) reported any statistically significant differences between treatments for either early or late seizure occurrence.
Incidences of death were reported in only five trials. One trial reported statistically significantly fewer deaths in the carbamazepine and no‐treatment groups compared with the phenytoin group after 24 months of treatment, but not after six months of treatment. Incidences of adverse effects of treatment were poorly reported; however, three trials did show that significantly more adverse events occurred on phenytoin compared to valproate, placebo, or no treatment. No trials reported any results relating to functional outcomes such as disability.
We considered the evidence to be of low quality for all reported outcomes due to methodological issues and variability of comparisons made in the trials.
Authors' conclusions
There is limited, low‐quality evidence to suggest that AED treatment administered prophylactically is either effective or not effective in the prevention of postcraniotomy (early or late) seizures. The current evidence base is limited due to the different methodologies employed in the trials and inconsistencies in the reporting of outcomes including deaths and adverse events. Further evidence from good‐quality, contemporary trials is required in order to assess the clinical effectiveness of prophylactic AED treatment compared to placebo or no treatment, or other AEDs in preventing postcraniotomy seizures in this select group of patients.
Plain language summary
The use of antiepileptic drugs to prevent seizures following brain surgery
Review question
This Cochrane Review examines the evidence for the effectiveness and safety of antiepileptic drugs (AEDs) when they are given to people who do not have epilepsy to prevent them experiencing seizures after craniotomy surgery (a type of brain surgery commonly used to remove brain tumours). We also planned to assess whether any particular AED is more effective in preventing seizures after craniotomy surgery.
Background
People who undergo a type of brain surgery known as craniotomy may be at increased risk of experiencing seizures after craniotomy surgery. AEDs have been used in trials to prevent seizures occurring after surgery in people with no previous history of epilepsy. A small number of trials have compared different AED treatments against each other, while others have compared AEDs to a placebo (a pill that contains no medicine) or no‐treatment group.
Study characteristics
The evidence is current to June 2017. Ten trials met our inclusion criteria, and included 1815 people. Three trials compared phenytoin (an AED) with a placebo or no treatment. One trial compared the AEDs phenytoin or carbamazepine with no treatment. One trial compared the AEDs phenytoin or phenobarbital with no treatment. Five other trials were head‐to‐head trials (where one drug is directly compared against another drug) of AEDs (phenytoin versus valproate; zonisamide versus phenobarbital and levetiracetam versus phenytoin).
Key findings
We did not find any consistent evidence to suggest that preventative AED treatments are effective in reducing the number of seizures that occurred postsurgery, deaths or adverse effects.
Quality of the evidence
Taking all the trials together, we considered that the quality of the evidence was low due to potential problems with the designs of the trials. Also the differences in the designs of the trials relating to the treatments examined and the results reported meant that it was difficult to compare results across trials. Further good‐quality studies are needed to validate the findings mentioned above.
Summary of findings
Summary of findings for the main comparison. Antiepileptic drugs as prophylaxis for postcraniotomy seizures.
| Antiepileptic drugs as prophylaxis for postcraniotomy seizures | ||||||
|
Patient or population: people with postcraniotomy seizures
Settings: hospital setting
Intervention: antiepileptic drugs Control: another antiepileptic drug, placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antiepileptic drugs | |||||
|
Early seizures
(number of people with seizures) Follow‐up: up to 1 week |
See comment | See comment | Not estimable | 1539 (9 trials) | ⊕⊕⊝⊝ lowa,b | 7 trials found no significant differences across comparisons examined: phenytoin vs no treatment, phenobarbital or phenytoin vs no treatment, phenytoin vs valproate, levetiracetam vs phenytoin and zonisamide vs phenobarbital. 2 trials found a significantly lower number of seizures following use of phenytoin vs no treatment.c |
|
Late seizures
(number of people with seizures) Follow‐up: 1 week up to 4 years (median) |
See comment | See comment | Not estimable | 798 (5 trials) | ⊕⊕⊝⊝ lowa,b | All trials found no significant differences across comparisons examined; phenytoin vs placebo or no treatment, phenobarbital or phenytoin vs no treatment, phenytoin vs valproate, zonisamide vs phenobarbital. |
|
Death
(number of deaths) Follow‐up: up to 4 years (median) |
See comment | See comment | Not estimable | 1016 (5 trials) | ⊕⊕⊝⊝ lowa,b | 4 trials found no significant differences over comparisons: phenytoin vs valproate, zonisamide vs phenobarbital; levetiracetam vs phenytoin and phenytoin vs placebo. 1 trial found significantly fewer deaths in the carbamazepine and the no‐treatment group at 24 months compared to phenytoin.d This trial showed no significant difference between the interventions at 6 months. |
|
Functional outcome (number of people with disabilities) Follow‐up: NA |
See comment | See comment | Not estimable | NA | NA | No included studies reported a functional outcome. |
|
Adverse effects
(number of people with adverse events) Follow‐up: up to 12 months |
See comment | See comment | Not estimable | 1165 (8 trials)Text in Effects of interventions gives some different numbers of participants. I have amended to what I think it should be and used tracked changes and highlighting to make the changes visible. Please check and amend as necessary. | ⊕⊕⊝⊝ lowa,b | Most trials found low numbers of adverse effects, and five trials found that no significant differences across comparisons were reported. Two trials found that significantly more adverse events were reported on phenytoin compared to placebo or no treatmente and one trial found that significantly more adverse events were reported on phenytoin compared valproate.f |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NA: not applicable | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded once due to risk of bias: methodological biases identified in trials (no allocation concealment, one study unblinded, unclear methods of dealing with missing data). bDowngraded once due to inconsistency: all trials differed in comparisons made. cLee 1989 and North 1983 found number of seizures was significantly lower in the phenytoin group compared to placebo group. dFoy 1992 found large differences in the number of deaths between treatment groups. Statistical significance level unreported. eNorth 1983 and Wu 2013 reported significantly higher overall adverse events in the phenytoin group compared to placebo or no treatment respectively. fZhang 2000 reported significantly higher overall adverse events in the phenytoin group compared to valproate group.
Background
This is an updated version of the Cochrane Review previously published in Issue 3, 2015.
Description of the condition
The incidence of epilepsy following supratentorial craniotomy for non‐traumatic pathology has been estimated to be 15% to 20% (Foy 1981); however, due to the nature of the underlying disease for which surgery is undertaken, the risk of postcraniotomy seizures appears to vary from 3% to 92% over a five‐year period. It is likely that such seizures may cause epilepsy in previously unaffected people. The probability of de novo seizures occurring in people who have no history of epilepsy decreases over time after surgery. The highest incidence of postoperative epilepsy (two‐thirds of the seizures) occurs within the first month after cranial surgery (North 1983), and 75% of those who develop epilepsy do so within one year of surgery. Few people (approximately 8%) have their first seizure more than two years after surgery. The risk of seizures for particular groups of people is higher for some groups than others; for example, people who suffer from an abscess continue to run a high risk of developing epilepsy (92%) after five years, whilst for those with an arteriovenous malformation who have had a spontaneous intracerebral haematoma, the overall risk does not fall below 10% between year two and year five after surgery (Shaw 1991).
Description of the intervention
Due to the risk of postoperative seizures, the prophylactic use of antiepileptic drugs (AEDs) has been advocated for people undergoing cranial surgery. However, it is also argued that AEDs should not be used prophylactically, but should only be administered following at least one seizure (Temkin 2002). Other investigators maintain that early postoperative seizures do not justify the diagnosis of epilepsy and only late seizures are considered to be true epilepsy (Manaka 2003).
How the intervention might work
Uncontrolled retrospective trials support the use of AED treatment in people with a predisposition towards developing postoperative seizures (Matthew 1980), and data from pathological trials suggest that certain AEDs could have a neuro‐protective action on damaged cerebral cortex (Calabresi 2003).
Why it is important to do this review
To inform decision making regarding the prophylactic use of AEDs for people undergoing craniotomy, reliable high‐quality evidence is required. Benefits and harms and any trade‐offs between these need to be examined carefully. Potential benefits include reduced short‐term seizure recurrence, reduced long‐term epilepsy rates, and better surgical outcome and quality of life. Harms include adverse effects and poorer surgical outcome. This Cochrane Review will provide a summary of the currently available evidence from randomised controlled trials (RCTs) regarding the prophylactic use of AEDs for people undergoing craniotomy by examining the following outcomes: occurrence of early and late seizures, occurrence of death, functional disability and occurrence of adverse events.
Objectives
To determine the efficacy and safety of AEDs when used prophylactically in people undergoing craniotomy and to examine which AEDs are most effective.
Methods
Criteria for considering studies for this review
Types of studies
RCTs
Double‐blinded, single‐blinded or unblinded trials
Placebo‐controlled, active drug‐control group or no‐treatment control group
Types of participants
People of any age and either gender undergoing a supratentorial or infratentorial craniotomy for either therapeutic or diagnostic reasons for all pathologies, who have had no history of seizures or prior exposure to AEDs. We excluded people with traumatic brain injuries from this review.
Types of interventions
The active treatment groups received treatment with any AED administered prior to or immediately postcraniotomy
The control groups received matched placebo, different AEDs or no treatment
Types of outcome measures
Primary outcomes
1. Early seizures
The proportion of people experiencing seizures occurring within the first week following craniotomy.
2. Late seizures
The proportion of people experiencing seizures occurring after the first week following craniotomy, including follow‐up period of one, two and five years postoperatively.
Secondary outcomes
1. Death
The proportion of deaths that occurred within the treatment period or during follow‐up.
2. Functional outcome
The proportion of people who experienced disability (partially or fully dependent on others in normal activities of daily living).
3. Adverse effects
The proportion of people who experienced any of the following adverse events.
Skin irritation
Dizziness
Fatigue
Nausea
Headache
In addition, we decided to look at the proportion of people who experienced the five most common adverse effects mentioned in the included trials if these differed from the list above.
Search methods for identification of studies
Electronic searches
We ran searches for the original review in January 2012 and subsequent searches in September 2012, August 2014, August 2016, and June 2017. For the latest update we searched:
The Cochrane Epilepsy Group Specialized Register (26 June 2017) using the search strategy outlined in Appendix 1.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017 issue 6) via the Cochrane Register of Studies Online (CRSO), using the search strategy outlined in Appendix 2.
MEDLINE (Ovid, 1946 to 26 June 2017) using the search strategy outlined in Appendix 3.
ClinicalTrials.gov (26 June 2017) using the search strategy outlined in Appendix 4.
WHO International Clinical Trials Registry Platform (ICTRP, 26 June 2017) using the search strategy outlined in Appendix 5.
We did not impose any language restrictions.
Searching other resources
We reviewed the reference lists of retrieved trials to check for additional reports of relevant studies.
Data collection and analysis
Selection of studies
Three review authors (JW, JG and YD) independently assessed articles for inclusion. We resolved any disagreements through discussion, and failing this, we sought the opinion of a fourth review author (AM). The same review authors independently carried out data extraction and assessed risk of bias. Again, we resolved any disagreements through discussion. Failing this, we sought the opinion of the fourth review author (AM).
Data extraction and management
We extracted the following information for each trial using a data extraction sheet.
Methodology/trial design
Method of randomisation and concealment
Method of blinding
Number of people excluded from analyses
Duration of baseline, treatment and follow‐up periods
Type of AED and dose tested
Time of treatment commencement
Participant demographics
Total number of people randomised to each group
Age/gender
Pathological group
Type of surgery
Site of lesion
Number of people with previous acute symptomatic seizures
Results
Sample size
Summary data for each intervention
For all trials we attempted to confirm the above information with trial authors or researchers, and sponsors.
Assessment of risk of bias in included studies
Three review authors (JW, JG and YD) independently assessed the risk of bias for each trial using the Cochrane 'Risk of bias' tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We rated the included trials as low, high or unclear on six domains applicable to RCTs: randomisation method, allocation concealment, blinding methods, incomplete outcome data, selective outcome reporting and other sources of bias (Assessment of risk of bias in included studies).
Measures of treatment effect
We have presented treatment effects as they were reported in the original reports. In this latest update, where data for each trial are entered into Data and analyses tables to allow for visual comparisons of results across trials, we have presented results for all dichotomous outcomes as risk ratios (RR) and 95% confidence intervals (CI).
Unit of analysis issues
The unit of allocation and analysis had to be the individual for all included trials, therefore cluster‐RCTs were not an eligible design. Due to the acute nature of postcraniotomy seizures, cross‐over designs were also not a suitable design.
For included trials with more than two treatment arms (e.g. AED1 versus AED2 versus placebo), we considered pairs of interventions in separate head‐to‐head comparisons (see Effects of interventions).
Dealing with missing data
We recorded attrition rates reported in each trial and if appropriate, attempted to contact original trial authors where the extent of missing data was unclear. In order to allow an intention‐to‐treat analysis within this review, we collected data by allocated treatment groups, irrespective of compliance, later exclusion (regardless of cause) or loss to follow‐up.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the differences in trial characteristics in order to inform decisions regarding the combination of trial data (Higgins 2002). Due to high levels of clinical heterogeneity, we did not synthesis any outcome data, If we had performed meta‐analysis, we would have estimated heterogeneity statistically using a Chi2 test for heterogeneity (with a conservative judgement of P value < 0.1 suggesting heterogeneity) and the I2 statistic, interpreted as follows (Deeks 2011):
might not be important (I2 values 0% to 40%);
may represent moderate heterogeneity (I2 values 30% to 60%);
may represent substantial heterogeneity (I2 values 50% to 90%); and
considerable heterogeneity (I2 values 75% to 100%).
Assessment of reporting biases
We examined reporting biases, such as publication bias, by identifying specific aspects of each trial (e.g. sponsors of the research, research teams involved).
Data synthesis
It was not possible to synthesise outcome data as we considered meta‐analysis to be inappropriate given the differences across trials in AED treatment, trial intervention characteristics and control groups (see Table 3).
1. Comparison of treatment protocols.
| Study | Intervention and daily dose (N) | Comparator(s) and daily dose (N) | Time of administration (reoperation/postoperation) | Treatment duration | Measurement period reported ‐ early | Measurement period reported ‐ late | Analysis |
| Beenen 1999 | PHT 300 mg (N = 50) |
VAL 1500 mg/day (N = 50) | Post‐op | 12 months | 1 week | 2 weeks to 12 months | ITT |
| Foy 1992 | PHT 300 mg
6‐months (N = 55) 24‐months (N = 56) |
CBZ 600 mg
6 months (N = 50) 24 months (N = 56) No treatment (N = 59) |
Pre‐op
and post‐op (pre‐ and post‐op doses differed) |
6 months 24 months | Not reported | 4 years (median) | ITT |
| Franceschetti 1990 | PHT 5 mg/kg (N = 16) |
PB 2 mg/kg (N = 25) No treatment (N = 22) | Pre‐op
and post‐op (pre‐ and post‐op doses differed) |
Unclear | 1 week | Unclear | No ITT 24 participants lost to follow‐up (for late seizure) |
| Fuller 2013 | LEV 250‐500 mg daily (N = 39) |
PHT 300 mg daily (N = 42) |
Pre‐op and post‐op |
90 days | 3 days | 90 days | Not ITT Only participants receiving 1 dose were analysed |
| Iuchi 2015 | LEV 500 mg daily (no prior seizure subgroup = 52) |
PHT 15‐18 mg/kg IV daily and 250 mg single oral dose (no prior seizure subgroup = 58) |
After anaesthesia induction and post‐op | 7 days | 7 days | Not measured | Not ITT. 1 participant was excluded from the analysis postrandomisation. Lesion was found to be not neoplastic. |
| Lee 1989 | PHT 5‐6 mg/kg (N = 189) | Placebo (N = 185) | Pre‐op
and post‐op (pre‐ and post‐op doses differed) |
3 days | 3 days | Not measured | ITT unclear Randomised = 400 but 26 deaths prior to treatment |
| Nakamura 1999 | ZNS 200 mg (N = 129) | PB 80 mg (N = 126) | Pre‐op
and post‐op (doses changed across course of trial) |
12 months | Not reported | 1‐12 months | ITT for 255 participants who received treatment 23 randomised participants were excluded prior to treatment |
| North 1983 | PHT 300 mg (N = 140) | Placebo (N = 141) | Post‐op | 12 months | 1 week | 12 months | ITT |
| Wu 2013 | PHT 300 mg (N = 62) |
No treatment (N = 61) |
Pre‐op and post‐op |
7 days | 7 days | > 30 days | ITT |
| Zhang 2000 | PHT 10 mg/kg 3 x daily (oral) or 5 mg/kg IV (N = 72) |
VAL 30 mg/kg 3 x daily (oral) or 20 mg/kg (IV) (N = 80) |
Pre‐op and post‐op |
1 month | 7 days | > 3 months | ITT unclear Numbers included in final analyses not reported |
CBZ: carbamazepine ITT: intention‐to‐treat LEV: levetiracetam PB: phenobarbital PHT: phenytoin VAL: valproate ZNS: zonisamide
We have presented study‐specific results for the following comparisons.
Treatment group versus control group on early seizures
Treatment group versus control group on late seizures
Treatment group versus control group on number of deaths
Treatment group versus control group on functional outcome
Treatment group versus control group on adverse effects (for each adverse effect see Types of outcome measures)
We stratified each comparison by type of drug and control group (i.e. placebo, other AED or no treatment) and presented the study‐specific results for comparison without synthesising in Data and analyses.
Subgroup analysis and investigation of heterogeneity
We did not plan any subgroup analyses a priori. The main sources of heterogeneity anticipated were the different trial interventions and control groups (considered within separate comparisons in this review) and different time points of measures (considered within different outcomes of this review).
Sensitivity analysis
We considered a sensitivity analysis of the primary outcomes of the review (where possible) based on the methodological quality of the studies, restricting meta‐analysis to only studies with a globally low risk of bias. However, given the minimal amount of data available for each comparison and the fact that we considered only two studies to have a low risk of bias due to lack of blinding, we did not deem this sensitivity analysis appropriate.
Summary of findings and quality of the evidence (GRADE)
Due to the variability of interventions and control groups within the included studies in this review, we have presented a single 'Summary of findings' table for all comparisons considered within this review (Schünemann 2011; Table 1).
We have included all primary and secondary outcomes in the 'Summary of findings' table. We determined the quality of the evidence using the GRADE approach (GRADE Working Group 2004), and downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results and high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
Our searches identified 157 records from the databases outlined in the Electronic searches section. We identified 10 additional records through the reference lists of the included trials. Eighty‐seven records remained after we removed duplicates, and we screened all for inclusion in the review. We excluded 60 records at this point, leaving 27 full‐text articles to be assessed for eligibility. Following this, we excluded 15 full‐text articles (see Figure 1 and Characteristics of excluded studies for reasons for exclusion). We included 10 trials from 12 reports in a narrative synthesis.
1.
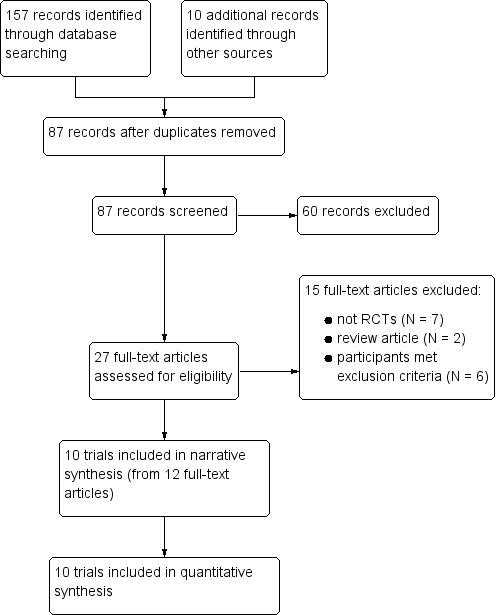
Study flow diagram
Included studies
We identified 10 parallel RCTs (Beenen 1999; Foy 1992; Franceschetti 1990; Fuller 2013; Iuchi 2015; Lee 1989; Nakamura 1999; North 1983; Wu 2013; Zhang 2000), examining the effectiveness of AEDs on postcraniotomy seizures in 1815 people. We have presented treatment protocols of the 10 included trials in Table 3.
The treatment periods varied across trials from three days to 24 months; in one trial the treatment period was unclear (Franceschetti 1990). People were excluded from six of the trials if they were taking AEDs already or if they had a history of epilepsy (Beenen 1999; Foy 1992; Lee 1989; Nakamura 1999; North 1983; Zhang 2000). One trial (Franceschetti 1990), included both people who had pre‐operative seizures (Group A) and those who did not (Group B). They analysed Group A and Group B separately compared to controls. We only extracted data pertaining to Group B to be included within this Cochrane Review, as Group A did not meet our inclusion criteria. One trial (Iuchi 2015), included people who had pre‐operative seizures, but the trial authors provided the results of a subgroup analysis for participants with no pre‐operative seizures.
Beenen 1999 was a single‐centre trial with a treatment period of 12 months. People aged between 18 and 80 years who were undergoing supratentorial craniotomy were eligible for inclusion in the trial. 100 patients were randomised: 50 to phenytoin 100 mg and 50 to valproate 500 mg treatment. They administered both treatments intravenously immediately postoperatively in a recovery room. Outcomes reported included early and late seizures, death and adverse effects. They did not report any data for functional outcome.
Foy 1992 was a single‐centre, head‐to‐head, three‐armed trial with a treatment period of either six or 24 months, and follow‐up of three years to a maximum of eight years. People aged over 16 years undergoing supratentorial craniotomy were eligible for inclusion in the trial. The trial authors randomised 276 patients: 55 to phenytoin for a six‐month treatment period, 56 to phenytoin for a 24‐month treatment period, 50 to carbamazepine for a six‐month treatment period, 56 to carbamazepine for a 24‐month treatment period and 59 to no treatment. Phenytoin (15 mg/kg) was administered 24 hours pre‐operation and increased to 100 mg eight‐hourly thereafter. Administration of carbamazepine (200 mg) was every six hours for the 24 hours immediately pre‐operatively and every eight hours thereafter. Outcomes reported included number of participants with seizures and death. The trial did not differentiate between early and late seizures, and no data were reported for functional outcome or adverse effects. All data were reported at six months into the treatment.
Franceschetti 1990 was a single‐centre, head‐to‐head, three‐armed trial that included a no‐treatment group. The duration of treatment is unclear. The trial randomised people undergoing surgery for supratentorial neoplasms; those with a history of seizures formed Group A and those with no history of seizures formed Group B. Group A participants were not eligible for inclusion in this review but there were 63 people randomised to Group B: 25 to phenobarbital, 16 to phenytoin and 22 to no treatment. The phenobarbital (4 mg/kg) was intravenously administered daily for five days and then decreased to 2 mg/kg daily via oral administration. Phenytoin (10 mg/kg) was intravenously administered daily for five days and then decreased to 5 mg/kg daily via oral administration. Outcomes reported included early and late seizures. Minimal data on adverse effects were presented.
Fuller 2013 was a single‐centre, head‐to‐head, two‐arm trial with a treatment period of 90 days. People aged 18 years and over undergoing craniotomy were eligible for inclusion in the trial. The trial randomised 81 people, 39 to levetiracetam and 42 to phenytoin. They administered levetiracetam (250 mg to 500 mg) twice daily, either intravenously or orally (one pre‐operative dose was required) and phenytoin (1000 mg loading dose followed by 300 mg) daily. Outcomes measured included discontinuation of treatment due to side effects, and clinically undesirable event and seizure occurrence. Outcomes were reported at three days and at 90 days.
Iuchi 2015 was a single‐centre, head‐to‐head, two‐arm trial with a treatment period and follow‐up of seven days. People aged 16 years and over with supratentorial tumours undergoing craniotomy were eligible for inclusion in the trial. The trial randomised a total of 147 people, including 110 people with no history of seizures. Of these, 52 people received levetiracetam and 58 people received phenytoin; levetiracetam (500 mg) was administered twice daily after anaesthesia induction either by suppository or orally, and phenytoin (15 to 18 mg/kg intravenously after induction of anaesthesia and continued at 5 mg/kg to 7.5 mg/kg daily intravenously or 250 mg orally). Outcomes measured included seizure occurrence and adverse events. Outcome data were reported at seven days. No data on functional outcomes were collected.
Lee 1989 was a placebo‐controlled trial with a treatment period of three days. The number of participating treatment centres is unclear. Adults receiving intracranial, supratentorial surgery were eligible to take part in the trial. The trial authors selected and randomised 400 patients for participation, however, 26 early deaths occurred, leaving 189 people randomised to phenytoin and 185 people to placebo. Phenytoin (15 mg/kg) was administered 15 to 20 minutes prior to wound closure followed by intravenous phenytoin (5 mg/kg to 6 mg/kg) three times daily for the first three postoperative days. Outcomes measured included number of seizures occurring within the three days of the trial. Data for late seizures, death, functional outcome and adverse effects were not recorded.
Nakamura 1999 was a multi‐centre, head‐to‐head trial with a treatment phase of one year and a follow‐up after two years postmedication. Adults undergoing craniotomy for cerebral tumours, cerebrovascular disease and head trauma were selected for eligibility. The trial randomised 278 people: 129 to zonisamide (100 mg twice daily) and 126 to phenobarbital (40 mg twice daily). However, 23 participants (12 randomised to zonisamide and 11 randomised to phenobarbital) were excluded from the final analysis due to protocol violations. Both drugs were administered orally, at least one week before surgery and then increased (zonisamide to 100 mg three or four times daily and phenobarbital to 40 mg three or four times daily) for one year followed by a tapering period of six months (three months at 100 mg (zonisamide) or 40 mg (phenobarbital) twice daily then three months at 100 mg (zonisamide) or 40 mg (phenobarbital) once daily). Outcomes reported were seizure frequency, death (during follow‐up period only) and adverse effects. No data were collected on functional outcome.
North 1983 was a single‐centre, placebo‐controlled trial with a treatment period of 12 months. People undergoing supratentorial operation (either burr hole, craniectomy or osteoplastic flap procedures) were eligible for inclusion in the trial. The trial authors randomised 281 people: 140 to phenytoin and 141 to placebo. Phenytoin (250 mg twice daily) was administered in a recovery room intravenously, and then continued with oral medication (100 mg three times daily) for one year. Outcomes reported were early and late seizures, death and adverse effects. No data were collected on functional outcomes.
Wu 2013 was a single‐centre, no‐treatment controlled trial with a treatment period of seven days. People with supratentorial tumours were eligible for inclusion in the trial. The trial authors randomised 123 people, 62 to phenytoin and 61 to a no‐treatment control group . Following a pre‐operative loading dose of 15 mg/kg, phenytoin (100 mg) was administered every eight hours to the treatment group. Outcomes reported were seizure occurrence and adverse reactions. No data relevant to functional outcomes were reported.
Zhang 2000 was a single‐centre, head‐to‐head trial with a treatment period of one month. The trial randomised 152 people undergoing craniotomy for differing pathologies, 72 to phenytoin and 80 to valproate. Treatment with phenytoin (10 mg/kg) and valproate (30 mg/kg) was given orally three times daily for seven days before surgery. Outcome measures included seizure occurrence and adverse effects of treatment. No data relevant to functional outcomes were reported.
Excluded studies
Overall we excluded 15 full‐text articles for the following reasons: seven were not RCTs (Baker 1995; Boarini 1985; De Santis 1996; Grobelny 2009; Hayashi 1999; Murri 1992; Notani 1984), two were review articles (Manaka 2003; Shaw 1991), and six studies had participants that did not meet our inclusion criteria (De Santis 2002; Levati 1996; Lim 2009; Temkin 1990; Temkin 1999; Tsolaki 1987).
Risk of bias in included studies
See Characteristics of included studies tables and Figure 2 for 'Risk of bias' judgements.
2.
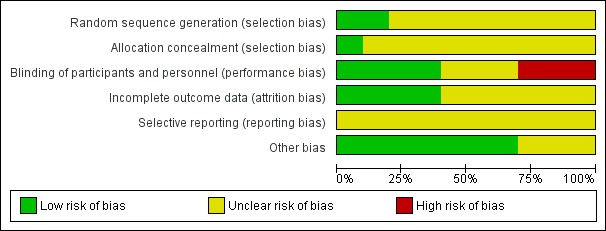
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials
Allocation
For sequence generation, we rated two trials at low risk of bias (Beenen 1999; Foy 1992), and eight trials at unclear risk of bias (Franceschetti 1990; Fuller 2013; Iuchi 2015Lee 1989; Nakamura 1999; North 1983; Wu 2013; Zhang 2000). We did not rate any trials at high risk of bias.
For allocation concealment, we rated one study at low risk of bias (Beenen 1999), and nine trials (Foy 1992; Franceschetti 1990; Fuller 2013; Iuchi 2015Lee 1989; Nakamura 1999; North 1983; Wu 2013; Zhang 2000), at unclear risk of bias due to the lack of detail of these methods.
Blinding
We rated four trials at low risk of bias due to the methods of blinding employed (Beenen 1999; Lee 1989; Nakamura 1999; North 1983). We rated three trials at unclear risk of bias (Franceschetti 1990; Fuller 2013; Zhang 2000), and three trials at high risk of bias, as only the outcome assessor appeared to be blinded in two trials (Foy 1992; Wu 2013), and the other trial was unblinded (Iuchi 2015).
Incomplete outcome data
We rated four trials at low risk of bias due to having no missing data (Beenen 1999; Iuchi 2015; North 1983; Wu 2013). We rated six trials at unclear risk of bias due to lack of detail regarding the analysis (Foy 1992; Franceschetti 1990; Fuller 2013; Lee 1989; Nakamura 1999; Zhang 2000). We did not rate any trials at high risk of bias.
Selective reporting
We rated all of the included trials at unclear risk of bias due to the lack of protocols available for comparison. We requested protocols from the trial authors if contact details were available, however we did not receive any responses.
Other potential sources of bias
We rated seven trials at low risk of bias as we did not identify any other bias (Beenen 1999; Franceschetti 1990; Fuller 2013; Iuchi 2015; Lee 1989; Nakamura 1999; North 1983). We rated three trials at unclear risk of bias (Foy 1992; Wu 2013; Zhang 2000).
Effects of interventions
See: Table 1
Due to the variety of head‐to‐head drug comparisons within the included trials (see Table 3 for a comparison of treatment protocols), we have presented the effects of the interventions by outcome measure as opposed to comparisons under trial.
Seizures
See Table 4 for individual trial results, and see Analysis 1.1 for comparative results for all seizures, Analysis 1.2 for early seizures and Analysis 1.3 for late seizures.
2. Study results for seizure data.
| Trial | All seizuresa | Early seizuresa | Late seizuresa | ||||||
| AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | |
| Beenen 1999b (PHT vs VAL) | PHT: 7/50 (14%) | VAL: 7/50 (14%) | ‐ | PHT: 4/50 (8%) |
VAL: 2/50 (4%) |
‐ | PHT: 3/50 (6%) | VAL: 5/50 (10%) | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 6 months) | CBZ: 21/50 (42%) |
PHT: 21/55 (38%) | NT: 25/59 (42%) | NR | NR | ‐ | NR | NR | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 24 months) | CBZ: 20/56 (36%) | PHT: 16/56 (29%) | NT: 25/59 (42%) | NR | NR | ‐ | NR | NR | ‐ |
| Franceschetti 1990c (PB vs PHT vs NT) | Total in the PB and PHT groups: 6/41 (15%) | NT: 7/22 (32%) | Total in the PB and PHT groups: 3/41 (17%) |
NT: 4/22 (18%) | PB 2/15 (13%) | PHT 1/10 (10%) | NT: 3/14 (21%) | ||
| Fuller 2013 (LEV vs PHT) | LEV: 0/39 (0%) | PHT: 6/42 (14%) | ‐ | LEV: 0/39 (0%) | PHT: 6/42 (14%) | ‐ | NR | NR | ‐ |
| Iuchi 2015 (LEV vs PHT) | LEV: 1/53 (2%) | PHT: 8/58 (14%) | ‐ | LEV: 1/53 (2%) | PHT: 8/58 (14%) | ‐ | NR | NR | ‐ |
| Lee 1989b (PHT vs placebo) | PHT: 2/189 (1%) | ‐ | Placebo: 9/185 (5%) |
PHT: 2/189 (1%) | Placebo: 9/185 (5%) | NR | NR | ‐ | |
| Nakamura 1999c (ZNS vs PB) | ZNS: 13/129 (10%) | PB: 11/126 (9%) | ‐ | ZNS: 6/129 (5%) | PB: 3/126 (2%) |
‐ | ZNS: 7/129 (5%) |
PB: 8/126 (6%) |
‐ |
| North 1983b (PHT vs placebo) | PHT: 18/140 (13%) |
‐ | Placebo: 26/141 (18%) |
PHT: 4/140 (3%) |
‐ | Placebo: 14/141 (10%) |
PHT: 14/140 (10%) | Placebo: 12/141 (9%) |
|
| Wu 2013 (PHT vs NT) | PHT: 15/62 (24%) | ‐ | NT: 11/61 (18%) | PHT: 2/62 (3%) |
‐ | NT: 5/61 (8%) | PHT: 13/62 (21%) |
‐ | NT: 6/61 (10%) |
| Zhang 2000 (PHT vs VAL) | PHT: 6/72 (8%) |
VAL: 9/80 (11%) | ‐ | PHT: 6/72 (8%) |
VAL: 9/80 (11%) | ‐ | NR | NR | |
AED: antiepileptic drug; CBZ: carbamazepine; LEV: levetiracetam; NR: not reported; NT: no treatment; PB: phenobarbital; PHT: phenytoin; VAL: valproate; ZNS: zonisamide
aSee Analysis 1.1 for comparative results for all seizures, Analysis 1.2 for early seizures and Analysis 1.3 for late seizures. bResults from these trials reported the number of participants who had seizures out of the number of participants randomised. However loss to follow‐up during the trial was unclear. cResults from the trials only reported the number of participants who had seizures out of the number of participants who were followed up. Foy 1992 followed up 39 participants for late seizures. Franceschetti 1990 reported combination of PB and PHT results, it is not possible to differentiate between groups on seizure outcome for all seizures and early seizures.
1.1. Analysis.
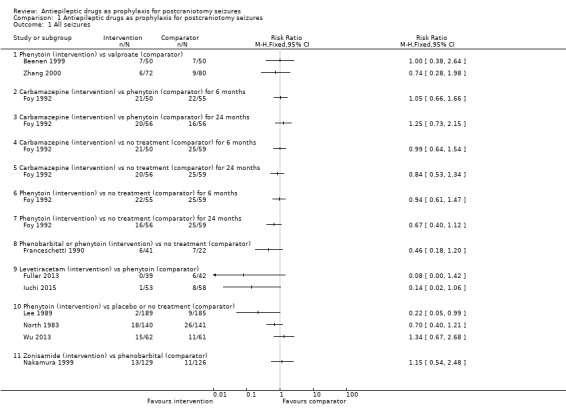
Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 1 All seizures.
1.2. Analysis.
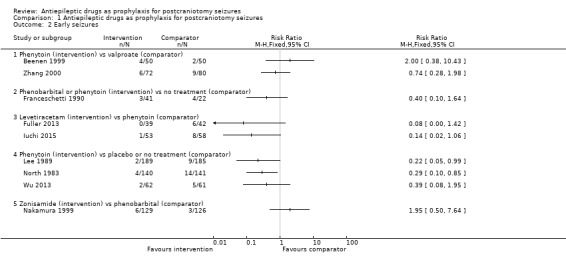
Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 2 Early seizures.
1.3. Analysis.
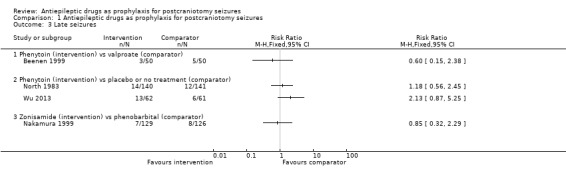
Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 3 Late seizures.
Any seizures
All 10 trials, with a total of 1815 participants, reported results for the proportion of people experiencing any seizures. Foy 1992 reported only occurrence of seizures (without the time frame); they found no statistically significant differences between any of the treatment groups. All other trials reported whether the seizures were early (i.e. within one week) or late (i.e. after one week).
Early seizures
Nine trials (N = 1539) reported the number of people experiencing early seizures.
Phenytoin versus placebo or no treatment
Lee 1989 reported two seizures in 189 participants (1%) in the phenytoin group compared to nine seizures in 185 participants (5%) in the placebo group. North 1983 reported four early seizures in 140 participants (3%) in the phenytoin group compared to 14 seizures in 141 participants (10%) in the placebo group. Wu 2013 reported two early seizures in 62 participants (3%) in the phenytoin group compared to five in 61 participants (8%) in the no‐treatment group. Lee 1989 and North 1983 reported a statistically significant difference in favour of treatment with phenytoin. Within the Lee 1989 published paper, the trial authors reported no statistically significant difference between phenytoin and placebo, however, they applied a Yates correction to their analysis methods, which may have led to the difference in the results between the published paper and this review.
Phenobarbital or phenytoin versus no treatment
Franceschetti 1990 reported three early seizures occurring in 41 participants (17%) in the phenobarbital and phenytoin groups and four in 22 participants (18%) in the no‐treatment group. The difference in early seizures between the two groups was not statistically significant.
Phenytoin versus valproate
Beenen 1999 reported four early seizures in 50 participants (8%) in the phenytoin group compared to two in 50 participants (14%) in the valproate group. Zhang 2000 reported six early seizures in 72 participants (8%) in the phenytoin group compared to nine in 80 participants (11%) in the valproate group. Neither trial found a statistically significant difference in early seizures between the two treatment groups.
Levetiracetam versus phenytoin
Fuller 2013 reported no early seizures in 39 participants in the levetiracetam group and six in 42 participants (14%) in the phenytoin group. Iuchi 2015 reported one early seizure in 53 participants (2%) in the levetiracetam group and eight in 58 participants (14%) in the phenytoin group. Within this Cochrane Review, neither result was statistically significant. However, within both of the published reports of the trials, the trial authors noted a statistically significant advantage of treatment with levetiracetam. We believe that the differences between the published reports and this review are due to different measures being used; this review uses risk ratios, whilst Fuller 2013 used log‐rank methods and Iuchi 2015 reported odds ratios.
Zonisamide versus phenobarbital
Nakamura 1999 reported six early seizures in 129 participants (5%) in the zonisamide group compared to three in 126 participants (2%) in the phenobarbital group. The difference in early seizures between the two groups was not statistically significant.
Summary
Overall, the quality of the evidence for this outcome was low due to unclear risk of bias and variability of treatment protocols in the included trials. Two trials reported a significant difference between AED treatment and no treatment or placebo for early seizure occurrence (Lee 1989; North 1983). No significant differences between the treatments were reported in the other trials (Analysis 1.2).
Late seizures
Five trials, with a total of 798 participants, reported the number of people experiencing late seizures.
Phenytoin versus placebo or no treatment
North 1983 reported 14 late seizures in 140 participants (10%) in the phenytoin group compared to 12 late seizures in 141 participants (9%) in the control group at 12 months. Wu 2013 reported 13 late seizures in 62 participants (21%) in the phenytoin group compared to six late seizures in 61 participants (10%) in the control group beyond 30 days. The difference in late seizures between the two groups was not statistically significant.
Phenobarbital or phenytoin versus no treatment
The Franceschetti 1990 trial only followed up 39 participants, and reported two late seizures in 25 participants (13%) in the phenobarbital group, one late seizure in 10 participants (10%) in the phenytoin group and three late seizures in 14 participants (21%) in the no‐treatment group. The timing of the follow‐up is unclear. The difference in late seizures between the treatment and no‐treatment groups was not statistically significant.
Phenytoin versus valproate
Beenen 1999 reported three late seizures in 50 participants (6%) in the phenytoin group compared with five late seizures in 50 participants (10%) in the valproate group at up to 12 months.
Zonisamide versus phenobarbital
Nakamura 1999 reported seven late seizures in 129 participants (5%) in the zonisamide group and eight late seizures in 126 participants (6%) in the phenobarbital group at 12 months. The difference in late seizures between the two groups was not statistically significant.
Summary
Overall, the quality of the evidence for this outcome was low due to risk of bias and variability of treatment protocols in the included trials. None of the trials that reported data for late seizures found any statistically significant differences between treatment and controls (Analysis 1.3).
Deaths
Five trials, with a total of 1016 participants, reported the number of deaths that occurred during the trials. See Table 5 for individual trial results and see Analysis 1.4 for comparative results for deaths.
3. Results for deaths and adverse events.
| Trial | Deathsa | Adverse eventsa | ||||
| AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | |
| Beenen 1999b (PHT vs VAL) | PHT: 13/50 (26%) | VAL: 10/50 (20%) | ‐ | PHT: 9/50 (18%) | VAL: 4/50 (8%) | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 6 months) | CBZ: 9/50 (18%) | PHT: 15/55 (27%) | NT: 13/59 (22%) | NR | NR | NR |
| Foy 1992b (CBZ vs PHT vs NT for 24 months) | CBZ: 10/56 (18%) | PHT: 27/56 (48%) | NT: 13/59 (22%) | NR | NR | NR |
| Franceschetti 1990c (PB vs PHT vs NT) | NR | NR | NR | PB: 1/15 (7%) | PHT: 3/10 (30%) | NR |
| Fuller 2013 (LEV vs PHT) | LEV: 3/39 (8%) | PHT: 5/42 (12%) | ‐ | LEV: 22/39 (56%) | PHT: 18/42 (43%) | ‐ |
| Iuchi 2015 (LEV vs PHT) | NR | NR | ‐ | LEV: 3/73 (4%) | PHT: 8/73 (11%) | ‐ |
| Lee 1989b (PHT vs placebo) | NR | NR | ‐ | NR | NR | ‐ |
| Nakamura 1999c (ZNS vs PB) | ZNS: 8/112 (7%) | PB: 13/107 (12%) | ‐ | ZNS: 28/129 (22%) | PB: 30/126 (24%) | ‐ |
| North 1983b (PHT vs placebo) | PHT: 20/140 (14%) | ‐ | Placebo: 24/141 (17%) | PHT: 12/140 (9%) | Placebo: 3/141 (2%) |
|
| Wu 2013 (PHT vs NT) | NR | ‐ | NR | PHT: 11/62 (18%) | ‐ | NT: 0/61 (0%) |
| Zhang 2000 (PHT vs VAL) | NR | NR | ‐ | PHT: 11/72 (15%) | VAL: 2/80 (3%) | ‐ |
AED: antiepileptic drug; CBZ: carbamazepine; LEV: levetiracetam; NR: not reported; NT: no treatment; PB: phenobarbital; PHT: phenytoin; VAL: valproate; ZNS: zonisamide.
aSee Analysis 1.4 for comparative results for deaths and Analysis 1.5 for adverse events. bResults from these trials reported the number of participants who died or experienced adverse events out of the number of participants randomised. However, loss to follow‐up during the trial was unclear. cResults from the trials only reported the number of participants who died or experienced adverse events out of the number of participants who were followed up.
1.4. Analysis.
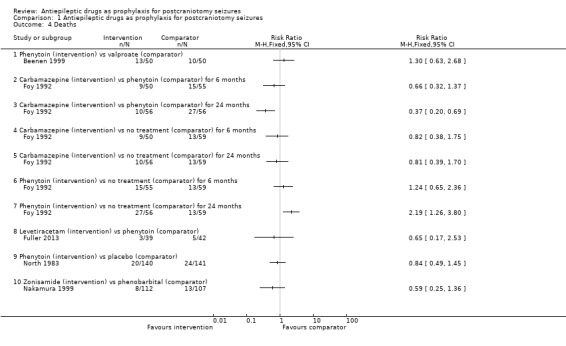
Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 4 Deaths.
Five trials did not present data for the outcome of death (Franceschetti 1990; Iuchi 2015; Lee 1989; Wu 2013; Zhang 2000).
Phenytoin versus placebo or no treatment
North 1983 reported 20 deaths in 140 participants (14%) in the phenytoin group and 24 deaths in 141 participants (17%) in the placebo group.
Carbamazepine versus phenytoin versus no treatment
Foy 1992 reported nine deaths in 50 participants (18%) in the carbamazepine group, 15 deaths in 55 participants (27%) in the phenytoin group and 13 deaths in 59 participants (22%) in the no‐treatment group at six months. At 24 months, Foy 1992 reported 10 deaths in 56 participants (18%) in the carbamazepine group, 27 deaths in 56 participants (48%) in the phenytoin group and 13 deaths in 59 participants (22%) in the no‐treatment group. The number of deaths in the phenytoin group was significantly higher than the other treatment groups.
Phenytoin versus valproate
Beenen 1999 reported 13 deaths in 50 participants (26%) in the phenytoin group and 10 deaths in 50 participants (20%) in the valproate group.
Levetiracetam versus phenytoin
Fuller 2013 reported three deaths in 39 participants (8%) in the levetiracetam group and five deaths in 42 participants (12%) in the phenytoin group.
Zonisamide versus phenobarbital
Nakamura 1999 reported eight deaths in 112 participants (7%) in the zonisamide group and 13 deaths in 107 participants (12%) in the phenobarbital group.
Summary
Overall, the quality of the evidence for this outcome was low due to risk of bias and variability of treatment protocols in the included trials. One trial (Foy 1992), found significantly fewer deaths in the carbamazepine and the no‐treatment group at 24 months compared to phenytoin. This trial showed no significant difference between the interventions at six months (Analysis 1.4).
Functional outcome
No included trials reported any data or results for a functional outcome.
Adverse effects
Eight trials, with a total of 1165 participants, reported the number of people experiencing adverse events during the trials. See Table 5 for individual trial results and see Analysis 1.5 for comparative results for adverse events. No adverse effects data from the two remaining trials were provided (Foy 1992; Lee 1989).
1.5. Analysis.
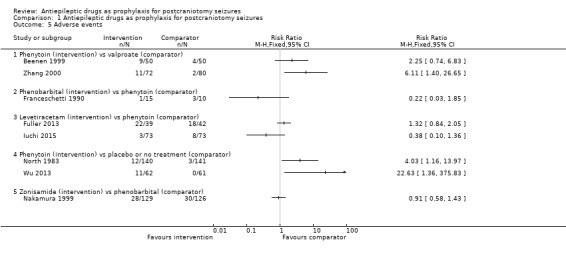
Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 5 Adverse events.
Phenytoin versus valproate
Beenen 1999 reported that four out of 50 participants experienced a skin reaction, three out of 50 participants experienced liver dysfunction, one out of 50 participants experienced thrombopenia, and there was one case of nausea within the phenytoin group (N = 50). In the valproate group there were three cases of liver dysfunction and one case of a rise in liver enzymes (N = 50). Zhang 2000 reported eight cases of rash, one case of poisoning and two cases of liver damage in the phenytoin group, whilst in the valproate group, there was one case of rash and one case of mild liver damage.
Phenytoin versus placebo or no treatment
North 1983 reported eight cases of rash, one case of involuntary movements, one hirsutism, one headache and one case of discomfort of the face in the phenytoin group (N = 140) compared to one case of rash, one dizziness and one nausea in the placebo group (N = 141). Wu 2013 reported 11 participants with adverse effects of treatment in the phenytoin group (N = 62) and no participants in the NT group. The reported events included four cases of rash, four cases of increased liver function test values, two cases each of thrombocytopenia, confusion and aphasia, and one case each of decreased level of consciousness, nausea, vomiting, dry itchy skin, ataxia and photophobia.
Phenobarbital or phenytoin versus no treatment
Franceschetti 1990 reported minimal data on adverse effects, only that three out of 10 participants in the phenytoin group and one out of 10 participants in the phenobarbital group experienced neurological side effects.
Levetiracetam versus phenytoin
Fuller 2013 reported that a total of 22 out of 39 people taking levetiracetam experienced adverse events, eight experienced lethargy/tiredness or asthenia, four people experienced rash, one person had delirium, one had headache, one had pruritus and seven experienced mood/irritability problems. In the phenytoin group a total of 18 out of 42 people experienced adverse events, with five cases of rash/itch, three cases each of thrombophlebitis and mood/irritability problems, two cases each of drug intoxication and anaphylaxis, and one case each of ataxia, nausea, and lethargy/tiredness/asthenia.
Iuchi 2015 reported adverse effects for the overall trial population (N = 146) rather than the subgroup of participants with no prior history of seizures (N = 110). In the levetiracetam group, three participants experienced haematological toxicity, two participants in the phenytoin group experienced haematological toxicity, two people experienced Grade 3 hyponatraemia, two people experienced Grade 3 skin eruption and two people experienced atrial fibrillation.
Zonisamide versus phenobarbital
Nakamura 1999 reported two cases of somnolence and six cases of nausea in the zonisamide group (N = 129), and seven cases of somnolence and two cases of nausea in the phenobarbital group (N = 126). Overall they reported 28 adverse effects in 129 participants in the zonisamide group and 30 adverse effects out of 126 participants in the phenobarbital group.
Summary
Overall, the quality of the evidence for this outcome was low due to risk of bias and variability of treatment protocols in the included trials. Two trials (North 1983; Wu 2013) found that significantly more adverse events were reported on phenytoin compared to placebo or no treatment and one trial (Zhang 2000) found that significantly more adverse events were reported in the phenytoin group compared with participants treated with valproate (Analysis 1.5).
Discussion
Summary of main results
The 10 trials included in this Cochrane Review were all RCTs investigating the effects of a range of AEDs given either immediately before or after a craniotomy procedure to people with no previous history of seizures or exposure to AEDs.
For the outcome of incidence of seizures, overall most trials reported no significant difference between treatment with AEDs and no treatment, or between treatment with different AEDs. Only two trials reported any statistically significant findings. In Lee 1989 and North 1983, the incidence of early seizures was reduced in the AED group (phenytoin) compared to placebo (P = 0.05 and P = 0.02 respectively). Overall, the majority of results from the individual trials showed few significant differences between AED treatment participants and control participants for outcomes relevant to the number of deaths and adverse effects. However, one trial (Foy 1992), showed that significantly more deaths occurred on phenytoin than carbamazepine or no treatment at 24 months (P = 0.001 and P = 0.005 respectively) and three trials did show significant differences for adverse event outcomes (Fuller 2013; Wu 2013; Zhang 2000). None of the included trials examined participants' functional outcomes.
Overall completeness and applicability of evidence
The underlying pathologies for craniotomy surgery were mixed within the trials (e.g. tumour, abscess, meningioma), with a small percentage of participants having surgery as a result of head injuries. One study included a substantial proportion (210/374) of people with head‐injury (Lee 1989). This is a major limitation of this review as the objective is to examine outcomes for people undergoing craniotomy presenting with non‐trauma pathology. We acknowledge the possibility of differences in the risk of seizure postsurgery depending on the underlying pathology of the participant.
We were unable to meta‐analyse any of the data and structuring a narrative summary was difficult for a number of reasons: few trials were available under each comparison examined (see Data synthesis for list of comparisons under investigation) and the interventions varied substantially with regards to duration of treatment period, dose and method of drug administration, country, methodological rigour and underlying pathologies. Trials differed regarding their reporting of outcomes, one trial did not differentiate between early and late seizures, and information about adverse effects of treatment was very limited. Most trials had similar inclusion and exclusion criteria. People undergoing supratentorial craniotomy were randomised in seven of the 10 included trials, but Fuller 2013, Iuchi 2015 and Nakamura 1999 did not specify the type of surgery.
Quality of the evidence
The outcomes of the risk of bias assessments conducted for each trial are noteworthy. We rated most trials as unclear on several of the criteria due to lack of published information regarding methodological trial design. We rated only two of the 10 trials at low risk of bias due to the method used to generate the randomisation sequence (Beenen 1999; Foy 1992), and only one trial used adequate methods for concealing the allocation of intervention (Beenen 1999). Most trials used adequate methods for blinding participants and outcome assessors; however, one trial was unblinded (Foy 1992), and therefore we rated it at high risk of bias for this criteria. There were no protocols available for any of the trials, therefore assessing selective reporting across trials was rated as unclear. We rated several trials as unclear on how they managed missing data within their analyses. In most cases trials reported attrition and described the reasons for withdrawal.
Furthermore, variability of treatment protocols, particularly AED interventions examined and control groups used prevented us from conducting data synthesis and comparison of interventions and controls was difficult. Therefore, we rated the overall quality of the evidence for all outcomes provided by this review to be low.
Potential biases in the review process
We did not identify any biases in the review process. We conducted this review in line with Cochrane MECIR standards (MECIR 2016), and presented results in the most appropriate way possible, given the heterogeneity of the evidence.
Agreements and disagreements with other studies or reviews
A systematic review published in 1996 (Kuijlen 1996), assessed the effectiveness of prophylactic AED use in people undergoing supratentorial craniotomies. The review included three trials (Foy 1992; Lee 1989; North 1983), that were considered to be of satisfactory methodological quality. Kuijlen 1996 calculated odds ratios as a means of assessing the degree of association between treatment and the incidences of convulsions. The results of pooling the data from these three trials demonstrated no statistically significant difference between prophylaxis with AEDs and no treatment. The authors of Kuijlen 1996 noted that there were only a small number of trials available in this area. A systematic review published in 2017 (Islim 2017), assessed the use of prophylactic AEDs for people undergoing surgery for meningioma. It included 11 cohort trials (1143 participants) and the authors reported that there was no statistically significant difference between prophylaxis with AEDs and no treatment. They advised that good‐quality RCTs are needed for robust conclusions to be drawn.
Authors' conclusions
Implications for practice.
Our results from this review show that there is not enough evidence of sufficient quality available to suggest that antiepileptic drug (AED) treatment can or cannot be recommended to reduce postcraniotomy seizures. There is no evidence on which to base clinical practice.
Implications for research.
More trials are needed to better evaluate the effectiveness of prophylactic treatment with AEDs in preventing seizures following cranial surgery. These trials must address the methodological weaknesses and protocol inconsistencies we identified within this review including:
timing of AED administration (pre‐ or postsurgery);
adequate length of treatment and follow‐up period
head‐to‐head or other control group;
methodological aspects (well‐controlled trials with adequate methods employed for generating randomisation sequences and concealing allocation); and
outcome reporting (differentiating between early and late seizures, adverse effects of treatment, handling of missing data) or other important outcomes (functional outcomes in terms of activities of daily living including working, driving etc.) not currently addressed.
What's new
| Date | Event | Description |
|---|---|---|
| 26 June 2017 | New search has been performed | Searches updated on 26 June 2017; two new studies have been included (Iuchi 2015; Zhang 2000) |
| 26 June 2017 | New citation required but conclusions have not changed | Conclusions remain unchanged |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 2, 2013
| Date | Event | Description |
|---|---|---|
| 18 June 2015 | Amended | Minor corrections made |
| 4 August 2014 | New search has been performed | The searches were updated on 04 August 2014 |
| 4 August 2014 | New citation required but conclusions have not changed | Two new trials have been included (Fuller 2013; Wu 2013); conclusions remain unchanged |
Acknowledgements
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Epilepsy. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The review authors would like to thank Kee‐Hsin Chen from Taipei Medical University and Anna Lee and Veronica Lai at the Chinese University of Hong Kong for their help in providing an English translation of the Zhang 2000 paper. We also thank Angela Boland at the University of Liverpool for her comments on the final draft.
Appendices
Appendix 1. Cochrane Epilepsy Specialized Register search strategy
#1 MeSH DESCRIPTOR Craniotomy Explode All
#2 craniotom* OR postcraniotom*
#3 supratentorial NEXT surgery
#4 infratentorial NEXT surgery
#5 postoperative NEXT epilep*
#6 post‐operative NEXT epilep*
#7 postoperative NEXT seizure*
#8 post‐operative NEXT seizure*
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 INREGISTER AND >04/08/2014:CRSCREATED
#11 #9 AND #10
Appendix 2. CENTRAL via CRSO search strategy
The following was used in the latest update to search CENTRAL via the Cochrane Register of Studies Online (CRSO).
#1 MESH DESCRIPTOR Craniotomy EXPLODE ALL TREES
#2 craniotom* OR postcraniotom*
#3 supratentorial NEXT surgery
#4 infratentorial NEXT surgery
#5 #1 OR #2 OR #3 OR #4
#6 MESH DESCRIPTOR Seizures EXPLODE ALL TREES
#7 seizure*
#8 #6 OR #7
#9 #5 AND #8
#10 postoperative NEXT epilep*
#11 post‐operative NEXT epilep*
#12 postoperative NEXT seizure*
#13 post‐operative NEXT seizure*
#14 #9 OR #10 OR #11 OR #12 OR #13
#15 * NOT INMEDLINE AND 04/08/2014 TO 26/06/2017:CD
#16 #14 AND #15
The following was used previously to search CENTRAL via the Cochrane Library.
#1 MeSH descriptor Craniotomy explode all trees
#2 (craniotom*)
#3 (postcraniotom*)
#4 (supratentorial NEXT surgery)
#5 (infratentorial NEXT surgery)
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 MeSH descriptor Seizures explode all trees
#8 (seizure*)
#9 (#7 OR #8)
#10 (#6 AND #9)
#11 (postoperative NEXT epilep*)
#12 (post‐operative NEXT epilep*)
#13 (postoperative NEXT seizure*)
#14 (post‐operative NEXT seizure*)
#15 (#10 OR #11 OR #12 OR #13 OR #14)
Appendix 3. MEDLINE (Ovid) search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011).
1. exp Craniotomy/
2. (craniotom$ or postcraniotom$ or supratentorial surgery or infratentorial surgery).tw.
3. 1 or 2
4. exp Seizures/
5. seizure*.tw.
6. 4 or 5
7. 3 and 6
8. (postoperative epilep$ or postoperative seizure$).tw.
9. 7 or 8
10. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
11. clinical trials as topic.sh.
12. trial.ti.
13. 10 or 11 or 12
14. exp animals/ not humans.sh.
15. 13 not 14
16. 9 and 15
17. remove duplicates from 16
18. limit 17 to ed=20140804‐20170626
19. 17 not (1$ or 2$).ed.
20. 19 and (2014$ or 2015$ or 2016$ or 2017$).dc.
21. 18 or 20
Appendix 4. ClinicalTrials.gov search strategy
(post‐craniotomy seizures OR supratentorial craniotomy OR cranial surgery) AND antiepileptic drugs
Appendix 5. ICTRP search strategy
post‐craniotomy seizures AND antiepileptic drugs OR supratentorial craniotomy AND antiepileptic drugs OR cranial surgery AND antiepileptic drugs
Data and analyses
Comparison 1. Antiepileptic drugs as prophylaxis for postcraniotomy seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All seizures | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Phenytoin (intervention) vs valproate (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Carbamazepine (intervention) vs phenytoin (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Carbamazepine (intervention) vs phenytoin (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Carbamazepine (intervention) vs no treatment (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Carbamazepine (intervention) vs no treatment (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Phenytoin (intervention) vs no treatment (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Phenytoin (intervention) vs no treatment (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Phenobarbital or phenytoin (intervention) vs no treatment (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Levetiracetam (intervention) vs phenytoin (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Phenytoin (intervention) vs placebo or no treatment (comparator) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Early seizures | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Phenytoin (intervention) vs valproate (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Phenobarbital or phenytoin (intervention) vs no treatment (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Levetiracetam (intervention) vs phenytoin (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Phenytoin (intervention) vs placebo or no treatment (comparator) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Late seizures | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Phenytoin (intervention) vs valproate (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Phenytoin (intervention) vs placebo or no treatment (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Deaths | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Phenytoin (intervention) vs valproate (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Carbamazepine (intervention) vs phenytoin (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Carbamazepine (intervention) vs phenytoin (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Carbamazepine (intervention) vs no treatment (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Carbamazepine (intervention) vs no treatment (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Phenytoin (intervention) vs no treatment (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Phenytoin (intervention) vs no treatment (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Levetiracetam (intervention) vs phenytoin (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Phenytoin (intervention) vs placebo (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Phenytoin (intervention) vs valproate (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Phenobarbital (intervention) vs phenytoin (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Levetiracetam (intervention) vs phenytoin (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Phenytoin (intervention) vs placebo or no treatment (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beenen 1999.
| Methods | Randomised double‐blind, placebo‐controlled, single‐centre (Netherlands), parallel trial 2 treatment arms: PHT and VAL Allocation concealed using sealed envelopes, trial medication identical in pre‐coded packaged materials Treatment period: 12 months Follow up: 12 months |
|
| Participants | Adults aged 21‐78 (mean age in PHT arm = 55 years, mean age in VAL arm = 51 years) Overall 47 men and 53 women, all patients undergoing craniotomy for different pathological conditions. Participants were not taking AEDs prior to randomisation and had no history of seizures. 100 randomised: 50 to PHT and 50 to VAL |
|
| Interventions | Group 1: PHT 100 mg (IV) 3 times daily administered immediately postoperation in recovery room Group 2: VAL 500 mg (IV) 3 times daily administered immediately postoperation in recovery room Participants took medication in oral form as soon as was possible for 12 months |
|
| Outcomes | Primary outcome: drug efficacy (time of and number of seizures) Secondary outcomes: tolerability (number of withdrawals, adverse effects), death, QoL and cognitive functioning |
|
| Notes | ITT analysis employed for primary outcome, not for other outcomes (QoL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study used computer‐generated randomisation method |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, pre‐coded and packaged medication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adequate blinding techniques used for personnel and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study attrition reported, employed ITT analysis for primary outcome |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | Low risk | No other bias detected |
Foy 1992.
| Methods | Randomised, controlled, parallel, single‐centre (UK) trial 5 treatment arms: CBZ 6 months and 24 months, PHT 6 months and 24 months, no treatment Participants randomised in blocks of 5 from prepared lists Treatment period: 6 or 24 months Follow up: 3 to 8 years |
|
| Participants | Patients aged 16‐77 years (median 45 years), 134 men and 142 women all undergoing supratentorial craniotomy. Participants had no previous history of seizures. 276 randomised: 50 to CBZ (6 months), 56 to CBZ (24 months), 55 to PHT (6 months), 56 to PHT (24 months), 59 to no treatment |
|
| Interventions | Group 1: CBZ 200 mg/6 h for 24 h pre‐surgery, 200 mg/8 h thereafter for 6 months Group 2: CBZ 200 mg/6 h for 24 h pre‐surgery, 200 mg/8 h thereafter for 24 months Group 3: PHT 15 mg/kg 24 h pre‐surgery, 100 mg/8 h thereafter for 6 months Group 4: PHT 15 mg/kg 24 h pre‐surgery, 100 mg/8 h thereafter for 24 months Group 5: no treatment |
|
| Outcomes | Primary outcome: drug efficacy (number of seizures) Secondary outcomes: seizure freedom, death |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used blocks of 5 from prepared lists |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study attrition reported, however, missing data and ITT analysis is unclear within the text |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | Unclear risk | The first 102 patients were randomised to treatment with CBZ or PHT for 6 or 24 months. Since analysis showed little difference in the incidence of postoperative seizures in this group relative to a retrospective study of postoperative seizures, the subsequent patients were randomised equally between policies of no treatment, treatment with CBZ and treatment with PHT. |
Franceschetti 1990.
| Methods | Randomised, controlled parallel trial, single centre trial. 2 treatment arms, one no treatment arm No details available in text of randomisation or blinding methods employed Treatment period: unclear Follow up: >6months to <12 months |
|
| Participants | Mean age 55 years, 34 men and 29 women undergoing supratentorial craniotomy for neoplasms 128 patients entered trial Group A: 65 participants had pre‐operative seizures and were treated with AEDs (excluded from this review) Group B: 63 participants had no seizures prior to operation and were not taking any AEDs |
|
| Interventions | 3 treatment arms for Group B randomised participants: PB, PHT and no treatment Group 1: PB (4 mg/kg daily for 5 days), followed by 2 mg/kg daily Group 2: PHT (10 mg/kg daily for 5 days), followed by 5 mg/kg daily Group 3: no treatment |
|
| Outcomes | Primary outcomes: efficacy (number of seizures (early and late seizures), adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in text |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details in text |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition unreported, 24 participants with missing data for late seizure outcome |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | Low risk | No other bias detected |
Fuller 2013.
| Methods | Pragmatic, prospective, randomised, single‐centre (Australia) trial 2 treatment arms: LEV or PHT Block randomisation Treatment period: 90 days Follow up: 90 days |
|
| Participants | Patients aged 25‐89 years with neurosurgical indications requiring craniotomy for which perioperative IV seizure prophylaxis was routine or otherwise warranted. Participants must have been on no AED or stable dose AED(s) excluding study AEDs for 3 weeks before enrolment, and must not have contraindication to either study medication. 81 randomised: 39 to LEV, 42 to PHT |
|
| Interventions | Group 1: LEV 250‐500 mg daily IV or orally Group 2: PHT 300 mg (≤ 3 doses in 24 h) or 1000 mg (single dose) IV loading then 300 mg daily IV or orally ≤ 2 oral doses of allocated AEDs were allowed between randomisation and IV administration. 1 pre‐operative IV dose was required. Following IV AED administration, participants received the same medication orally. Additional PHT titration to therapeutic serum levels was allowed but not mandated. Doses were within standard range. After randomisation, treating teams made all decisions regarding study AED treatment including IV and oral durations, serologic monitoring, dose adjustment and cessation. |
|
| Outcomes | Primary outcome: discontinuation of study AED because of side effects Secondary outcome: seizure occurrence Outcomes reported at 3 days and 90 days |
|
| Notes | Not ITT analysis. The pragmatic nature of the trial is highlighted. The reviewers also note the length of active treatment in the trial. 4 people in the trial had prior seizures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of sequence generation not described. Block randomisation was reported as used. However, the paper reports that early during data collection the contractor communicated that "allocation was as follows: each 10 sequentially recruited patients were not internally randomised but received the same drug, determined by hat‐draw at enrolment of the first patient in each block, with eight blocks of 10 patients then two blocks of four to be randomised with equal probability." At study completion, impact of allocation procedure on bias was assessed by statistical comparison of baseline patient characteristics, with similar age and gender distribution and proportion of serious pathologies and death from underlying pathology found between treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | The allocation procedure was communicated to quarantined keeper for randomisation data. However, it is unclear what the procedure was. Also, see above note as to failure of block randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The investigation team conducting the information and consenting process, data collection, outcome adjudication and analysis was blinded. The quarantined keeper and liaison for randomisation data did not participate in recruitment, patient treatment, data collection, outcome assessment or analysis. Recruiting neurosurgical teams, including anaesthetists, were blinded to the allocation procedure. Otherwise the study was open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis not used. 81 people randomised, 74 were included in the analyses at 3 days and 61 at 90 days. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | Low risk | UCB Pharma provided funding and LEV |
Iuchi 2015.
| Methods | Randomised, prospective, parallel, single‐centre (Japan) trial 2 treatment arms: LEV or PHT Patients were randomised using sequentially numbered envelopes. Treatment period: 7 days. No further follow‐up |
|
| Participants | Patients aged ≥ 16 years with supratentorial tumours that required craniotomy. The history of seizures prior to surgery was not considered, but patients were excluded if they had a history of seizures and their seizures remained after medication with LEV or PHT. No known allergy to study treatments. 147 randomised: 73 to LEV, 74 to PHT A subgroup of participants (N = 110) had no prior history of seizures. |
|
| Interventions | In participants who had a history of seizures prior to surgery, and who received AEDs to control seizures, AEDs were continued until the day before surgery. Group 1: LEV 500 mg (initially by suppository and then orally) every 12 h Group 2: PHT 15‐18 mg IV after induction of anaesthesia and continued at 5‐7.5 mg per day. After participants were able to take oral medication, PHT was administered at 250 mg daily. This dose was sufficient to achieve a therapeutic plasma concentration in Japanese participants. Plasma concentrations of all participants were measured 2 h after the first administration of study drug |
|
| Outcomes | Primary outcome: occurrence of seizures Secondary outcomes: occurrence of haematological and non‐haematological adverse events |
|
| Notes | The overall trial population (N = 147) included participants with and without a history of seizures. The results for participants with no history of seizures (N = 110) is reported as a subgroup analysis. The trial authors note that the plasma concentration of participants taking PHT were at the lower limit of the therapeutic range. They also note that plasma concentration did not differ between participants who experienced and did not experience postoperative seizures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Individual randomised allocation was performed using sequentially numbered envelopes. It is not stated if the envelopes were opaque or sealed. |
| Allocation concealment (selection bias) | Unclear risk | Individual randomised allocation was performed using sequentially numbered envelopes. It is not stated if the envelopes were opaque or sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was excluded from the analysis as the lesion was found to be non‐neoplastic. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol is available. |
| Other bias | Low risk | None identified |
Lee 1989.
| Methods | Randomised, double‐blind, placebo‐controlled trial 2 treatment arms: PHT and placebo Patients randomised using random digits, all participants received identical medication Treatment period: 3 days. No follow‐up of participants |
|
| Participants | Adults, mean age 39.9 years (PHT) and 37.5 years (placebo) all undergoing intracranial, supratentorial surgery. Participants had no history of seizures and not taking AEDs prior to surgery. 400 participants randomised: 189 to PHT and 185 to placebo. 26 died prior to treatment |
|
| Interventions | Group 1: PHT 15 mg/kg for 15‐20 min prior to wound closure followed by 5‐6 mg/kg/day, 3 times daily in first 3 postoperative days Group 2: saline solution administered as described above |
|
| Outcomes | Primary outcome: efficacy (number of seizures at 3 days) | |
| Notes | 26 participants randomised died prior to treatment, excluded from all data exploration and analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used random digits, unclear how generated, whether open list, etc |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical medication used for both groups. Adequate blinding methods for key personnel and participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear details on study attrition rate and how data analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias detected |
Nakamura 1999.
| Methods | Randomised, double‐blind, controlled, multi‐centre (Japan) trial 2 treatment arms: ZNS and PB. Identical medication administered (no details of methods of randomisation). Treatment period: 1 year. Follow‐up period: 3 years |
|
| Participants | 278 patients who were scheduled to receive craniotomy for cerebral tumours, cerebrovascular diseases and head trauma, were randomised. 129 in ZNS group analysed, 126 in PB group were analysed |
|
| Interventions | Group 1: ZNS (100 mg twice daily) until 1 month after craniotomy Group 2: PB (40 mg twice daily) until 1 month after craniotomy In both groups dose was adjusted to therapeutic serum concentration and continued up to 1 year |
|
| Outcomes | Primary outcome: frequency of epilepsy Secondary outcome: drug concentration, adverse effects. |
|
| Notes | 23 'unsuitable cases' not included in the analysis. 36 cases not followed up for full 3 years of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in text |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical medication administered to both groups. Drug name blinded from participating institutions, only blood concentration values provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 23 cases not included within analysis, all excluded prior to treatment. 36 lost to follow‐up were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Trial reports data for overall adverse effects, only reports data for 2 individual adverse effects |
| Other bias | Low risk | No other bias detected |
North 1983.
| Methods | Randomised, double‐blinded, placebo‐controlled, parallel, single‐centre (Australia) trial 2 treatment arms: PHT and placebo Participants received identical medication (no details available of randomisation methods) Treatment period: 12 months Follow up: 12 months |
|
| Participants | Adults, mean age 46.7 years (PHT) and 50.21 years (placebo), all undergoing supratentorial craniotomy. Participants had no previous exposure to AEDs and no previous history of epilepsy. 281 patients were randomised: 140 to PHT and 141 to placebo |
|
| Interventions | Group 1: PHT 250 mg twice daily administered (IV) first dose administered in the recovery room postcraniotomy followed by oral medication 100 mg 3 times daily for 12 months Group 2: placebo medication administered as described above. |
|
| Outcomes | Primary outcome: efficacy (number of seizures) Secondary outcomes: survival time (number of days since randomisation to incidence of seizure or to 365 days in seizure‐free participants), adverse effects |
|
| Notes | 63 participants in PHT arm and 59 participants in placebo arm received intended treatment. All cases randomised were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in text |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical medication used for both groups. Only pharmacologist aware of serum drug levels and both PHT and placebo group participants had blood samples taken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported and ITT analysis employed. 6 participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol outlined within paper. All outcomes reported. Full protocol not available |
| Other bias | Low risk | No other bias detected |
Wu 2013.
| Methods | Prospective RCT. Single‐centre (USA) trial 2 study arms: PHT and no treatment Treatment period: 7 days at full dose followed by tapering Follow up: up to 12 months |
|
| Participants | Patients aged 16‐84, mean age 56 years (PHT) and 61 years (no treatment) with intraparenchymal supratentorial brain tumours either proven by biopsy to be a brain metastasis or a glioma, or with compelling CT or MRI evidence of metastasis or glioma. All participants had to be previously untreated with AEDs. 123 randomised: 62 to PHT and 61 to no treatment |
|
| Interventions | Group 1: PHT 100 mg every 8 h administered IV or oral Group 2: no treatment |
|
| Outcomes | Primary outcomes: seizure occurrence Secondary outcomes: adverse reactions to PHT |
|
| Notes | Measurements taken every 2‐3 months up to 12 months. Time points reported in study were 7 days and 30 days. Trial was stopped early due to few events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Other bias | Unclear risk | Trial stopped early due to futility, unclear if this introduced bias |
Zhang 2000.
| Methods | Prospective RCT (China) 2 treatment arms: PHT and VAL Treatment period: 1 month Follow up: 3 months |
|
| Participants | 152 patients undergoing craniotomy surgery for differing pathologies and who had no prior history of seizures or AED use. Participants in the PHT group (N = 72) had a mean age of 47.98 ± 9.67 and participants in the VAL group (N = 80) had a mean age of 46.15 ± 9.54 | |
| Interventions | Group 1: before surgery, oral PHT 10 mg/kg given 3 times a day for 7 days (plasma PHT concentration was then checked); after surgery, IV PHT 5 mg/kg given 3 times daily for 2 consecutive days, and then from third postoperative day onwards, a maintenance dose of oral PHT 5 mg/kg given 3 times daily for a month. Group 2: before surgery, oral 30 mg/kg VAL given 3 times daily; after surgery, IV 20 mg/kg VAL given three times daily for 2 consecutive days, and then from third postoperative day onwards, a maintenance dose of oral 20 mg/kg VAL given twice daily for a month. | |
| Outcomes | The primary objective was to compare the effects of PHT and VAL on postoperative epilepsy and toxicity, as well as the relationship with blood concentration. | |
| Notes | No information available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given |
| Selective reporting (reporting bias) | Unclear risk | No information given |
| Other bias | Unclear risk | Insufficient information to make a judgement |
AED: antiepileptic drug CBZ: carbamazepine CT: computerised tomography h: hour IV: intravenously ITT: intention‐to‐treat LEV: levetiracetam MRI: magnetic resonance imaging PB: phenobarbital PHT: phenytoin QoL: quality of life RCT: randomised controlled trial VAL: valproate ZNS: zonisamide
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baker 1995 | Not a RCT, retrospective trial |
| Boarini 1985 | Not a RCT, retrospective trial |
| De Santis 1996 | Not a RCT |
| De Santis 2002 | Participants taking AEDs prior to randomisation |
| Grobelny 2009 | Not a RCT, retrospective design |
| Hayashi 1999 | Not a RCT |
| Levati 1996 | Participants taking AEDs prior to randomisation |
| Lim 2009 | Participants taking AEDs and experiencing seizures prior to randomisation. |
| Manaka 2003 | Review paper |
| Murri 1992 | Not a RCT |
| Notani 1984 | Not a RCT |
| Shaw 1991 | Review paper |
| Temkin 1990 | No craniotomy surgery performed. People with brain injury |
| Temkin 1999 | No craniotomy surgery performed. People with brain injury |
| Tsolaki 1987 | Participants taking AEDs prior to study |
AED: antiepileptic drug RCT: randomised controlled trial
Differences between protocol and review
We were unable to make all the intended comparisons specified in the protocol due to lack of data.
In the 2018 update, where data for each trial are entered into Data and analyses tables to allow for a visual comparisons of results across trials, we have presented results for all dichotomous outcomes as risk ratios and 95% confidence intervals.
Contributions of authors
Jennifer Weston and Janette Greenhalgh carried out and completed the original review and the 2014 review update. Janette Greenhalgh, Yenal Dundar and Sarah Nevitt carried out the 2018 update. Anthony Marson supervised the review. Nikola Vojvodic, Aleksandar Ristic and Dragoslav Sokic developed the protocol for this review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research, UK.
Declarations of interest
JG: none known JW: none known YD: none known SN: none known AGM: a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is part funded by National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North West Coast (NIHR CLAHRC NWC).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Beenen 1999 {published data only}
- Beenen LF, Lindeboom J, Kasteleijn‐Nolst Trenité DG, Heimans JJ, Snoek FJ, Touw DJ, et al. Comparative double blind clinical trial of phenytoin and sodium valproate as anticonvulsant prophylaxis after craniotomy: efficacy, tolerability, and cognitive effects. Journal of Neurology, Neurosurgery, and Psychiatry 1999;67(4):474‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foy 1992 {published data only}
- Foy PM, Chadwick DW, Rajgopalan N, Johnson AL, Shaw MD. Do prophylactic anticonvulsant drugs alter the pattern of seizures after craniotomy?. Journal of Neurology, Neurosurgery, and Psychiatry 1992;55(9):753‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MDM, Foy P, Chadwick D. The effectiveness of prophylactic anticonvulsants following neurosurgery. Acta Neurochirurgica 1983;69(3‐4):253‐8. [DOI] [PubMed] [Google Scholar]
Franceschetti 1990 {published data only}
- Franceschetti S, Binelli S, Casazza M, Lodrini S, Panzica F, Pluchino F, et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochirurgica 1990;103(1‐2):47‐51. [DOI] [PubMed] [Google Scholar]
Fuller 2013 {published data only}
- Fuller KL, Wang YY, Cook MJ, Murphy MA, D'Souza WJ. Tolerability, safety, and side effects of levetiracetam versus phenytoin in intravenous and total prophylactic regimen among craniotomy patients: a prospective randomized study. Epilepsia 2013;54(1):45‐57. [DOI] [PubMed] [Google Scholar]
Iuchi 2015 {published data only}
- Iuchi T, Kuwabara K, Matsumoto M, Kawasaki K, Hasegawa Y, Sakaida T. Levetiracetam versus phenytoin for seizure prophylaxis during and early after craniotomy for brain tumours: a phase II prospective, randomised study. Journal of Neurology, Neurosurgery & Psychiatry 2015;86(10):1158‐62. [DOI] [PubMed] [Google Scholar]
Lee 1989 {published data only}
- Lee ST, Lui TN, Chang CN, Cheng WC, Wang DJ, Heimburger RF, et al. Prophylactic anticonvulsants for prevention of immediate and early postcraniotomy seizures. Surgical Neurology 1989;31(5):361‐4. [DOI] [PubMed] [Google Scholar]
Nakamura 1999 {published data only}
- Nakamura N, Ishijima B, Mayanagi Y, Manaka S. A randomized controlled trial of zonisamide in postoperative epilepsy: a report of the Cooperative Group Study. Japanese Journal of Neurosurgery 1999;8(10):647‐56. [Google Scholar]
North 1983 {published data only}
- North JB, Penhall RK, Hanieh A, Frewin DB, Taylor WB. Phenytoin and postoperative epilepsy. A double‐blind study. Journal of Neurosurgery 1983;58(5):672‐7. [DOI] [PubMed] [Google Scholar]
- North JB, Penhall RK, Hanieh A, Hann CS, Challen RG, Frewin DB. Postoperative epilepsy: a double‐blind trial of phenytoin after craniotomy. Lancet 1980;1(8165):384‐6. [DOI] [PubMed] [Google Scholar]
Wu 2013 {published data only}
- Wu AS, Trinh VT, Suki D, Graham S, Forman A, Weinberg JS, et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumours. Journal of Neurosurgery 2013;118(4):873‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2000 {published data only}
- Zhang Y, Zhou LF, Du GL, Gao L, Xu B, Xu J, et al. Phenytoin or sodium valproate for prophylaxis of postoperative epilepsy: a randomised comparison. Chinese Journal of Nervous and Mental Diseases 2000;26(4):231‐3. [Google Scholar]
References to studies excluded from this review
Baker 1995 {published data only}
- Baker CJ, Prestigiacomo CJ, Solomon RA. Short‐term perioperative anticonvulsant prophylaxis for the surgical treatment of low‐risk patients with intracranial aneurysms. Neurosurgery 1995;37(5):863‐70. [DOI] [PubMed] [Google Scholar]
Boarini 1985 {published data only}
- Boarini DJ, Beck DW, VanGilder JC. Postoperative prophylactic anticonvulsant therapy in cerebral gliomas. Neurosurgery 1985;16(3):290‐2. [DOI] [PubMed] [Google Scholar]
De Santis 1996 {published data only}
- Santis A, Baratta P, Bello L, Spagnoli D, Ceccarelli G, Songa V, et al. Early postoperative seizures and endovenous phenytoin. Preliminary clinical data. Journal of Neurosurgical Sciences 1996;40(3‐4):207‐12. [PubMed] [Google Scholar]
De Santis 2002 {published data only}
- Santis A, Villani R, Sinisi M, Stocchetti N, Perucca E. Add‐on phenytoin fails to prevent early seizures after surgery for supratentorial brain tumors: a randomized controlled study. Epilepsia 2002;43(2):175‐82. [DOI] [PubMed] [Google Scholar]
Grobelny 2009 {published data only}
- Grobelny BT, Ducruet AF, Zacharia BE, Hickman ZL, Andersen KN, Sussman E, et al. Preoperative antiepileptic drug administration and the incidence of postoperative seizures following bur hole‐treated chronic subdural hematoma. Journal of Neurosurgery 2009;111(6):1257‐62. [DOI] [PubMed] [Google Scholar]
Hayashi 1999 {published data only}
- Hayashi T, Hadeishi H, Kawamura S, Nonoyama Y, Suzuki A, Yasui N. Postoperative anticonvulsant prophylaxis for patients treated for cerebral aneurysms. Neurologia Medico‐Chirurgica 1999;39(12):828‐33. [DOI] [PubMed] [Google Scholar]
Levati 1996 {published data only}
- Levati A, Savoia G, Zoppi F, Boselli L, Tommasino C. Peri‐operative prophylaxis with phenytoin: dosage and therapeutic plasma levels. Acta Neurochirurgica 1996;138(3):274‐9. [DOI] [PubMed] [Google Scholar]
Lim 2009 {published data only}
- Lim DA, Tarapore P, Chang E, Burt M, Chakalian L, Barbaro N, et al. Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma‐related seizure control following craniotomy: a randomized phase II pilot study. Journal of Neuro‐Oncology 2009;93(3):349‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manaka 2003 {published data only}
- Manaka S, Ishijima B, Mayanagi Y. Postoperative seizures: epidemiology, pathology, and prophylaxis. Neurologia Medico‐Chirurgica (Tokyo) 2003;43(12):589‐600. [DOI] [PubMed] [Google Scholar]
Murri 1992 {published data only}
- Murri L, Arrigo A, Bonuccelli U, Rossi G, Parenti G. Phenobarbital in the prophylaxis of late posttraumatic seizures. Italian Journal of Neurological Sciences 1992;13(9):755‐60. [DOI] [PubMed] [Google Scholar]
Notani 1984 {published data only}
- Notani M, Kawamura H, Amano K, Tanikawa T, Kawabatake H, Iseki H, et al. The incidence of postoperative epilepsy and prophylactic anticonvulsants in patients with intracranial aneurysm. No Shinkei Geka 1984;12(3 Suppl):269‐74. [PubMed] [Google Scholar]
Shaw 1991 {published data only}
- Shaw MD, Foy PM. Epilepsy after craniotomy and the place of prophylactic anticonvulsant drugs: discussion paper. Journal of the Royal Society of Medicine 1991;84(4):221‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Temkin 1990 {published data only}
- Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double‐blind study of phenytoin for the prevention of post‐traumatic seizures. New England Journal of Medicine 1990;323(8):497‐502. [DOI] [PubMed] [Google Scholar]
Temkin 1999 {published data only}
- Temkin NR, Dikmen SS, Anderson GD, Wilensky AJ, Holmes MD, Cohen W, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. Journal of Neurosurgery 1999;91(4):593‐600. [DOI] [PubMed] [Google Scholar]
Tsolaki 1987 {published data only}
- Tsolaki M, Yannacou‐Peftoulidou M, Grammaticos P, Sofianos E, Foroglou G. Phenytoin plasma levels after intraoperative administration, for the prevention of post‐craniotomy seizures. Acta Neurochirurgica 1987;84(1‐2):36‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Calabresi 2003
- Calabresi P, Cupini LM, Centonze D, Pisani F, Bernardi G. Antiepileptic drugs as a possible neuroprotective strategy in brain ischemia. Annals of Neurology 2003;53(6):693‐702. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (Editors). Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Foy 1981
- Foy PM, Copeland GP, Shaw MDM. The incidence of postoperative seizures. Acta Neurochirurgica 1981;55(3‐4):253‐64. [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Islim 2017
- Islim IA, McEver S, Kusu‐Orker T, Jenkinson MD. The role of prophylactic antiepileptic drugs for seizure prophylaxis in meningioma surgery: a systematic review. Journal of Clinical Neuroscience 2017;43:47‐53. [DOI] [PubMed] [Google Scholar]
Kuijlen 1996
- Kuijlen J, Teernstra O, Kessels A, Herpers M, Beuls E. Effectiveness of antiepileptic prophylaxis used with supratentorial craniotomies: a meta‐analysis. Seizure 1996;5:291‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Matthew 1980
- Matthew E, Sherwin AL, Welner SA, Odusote K, Stratford JG. Seizures following intracranial surgery: incidence in the first post‐operative week. Canadian Journal of Neurological Sciences 1980;7(4):285‐90. [DOI] [PubMed] [Google Scholar]
MECIR 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London, Version 1.02 2016.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Temkin 2002
- Temkin N. Prophylactic anticonvulsants after neurosurgery. Epilepsy Currents 2002;2(4):105‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Pulman 2013
- Pulman J, Greenhalgh J, Marson AG. Antiepileptic drugs as prophylaxis for post‐craniotomy seizures. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007286.pub2] [DOI] [PubMed] [Google Scholar]
Vojvodic 2008
- Vojvodic N, Ristic A, Sokic D. Antiepileptic drugs as prophylaxis for postcraniotomy seizures. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weston 2015
- Weston J, Greenhalgh J, Marson AG. Antiepileptic drugs as prophylaxis for post‐craniotomy seizures. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007286.pub3] [DOI] [PubMed] [Google Scholar]


