Abstract
Background
As mortality secondary to acute infectious diarrhoea has decreased worldwide, the focus shifts to adjuvant therapies to lessen the burden of disease. Smectite, a medicinal clay, could offer a complementary intervention to reduce the duration of diarrhoea.
Objectives
To assess the effects of smectite for treating acute infectious diarrhoea in children.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Pubmed), Embase (Ovid), LILACS, reference lists from studies and previous reviews, and conference abstracts, up to 27 June 2017.
Selection criteria
Randomized and quasi‐randomized trials comparing smectite to a control group in children aged one month to 18 years old with acute infectious diarrhoea.
Data collection and analysis
Two review authors independently screened abstracts and the full texts for inclusion, extracted data, and assessed risk of bias. Our primary outcomes were duration of diarrhoea and clinical resolution at day 3. We summarized continuous outcomes using mean differences (MD) and dichotomous outcomes using risk ratios (RR), with 95% confidence intervals (CI). Where appropriate, we pooled data in meta‐analyses and assessed heterogeneity. We explored publication bias using a funnel plot.
Main results
Eighteen trials with 2616 children met our inclusion criteria. Studies were conducted in both ambulatory and in‐hospital settings, and in both high‐income and low‐ or middle‐income countries. Most studies included children with rotavirus infections, and half included breastfed children.
Smectite may reduce the duration of diarrhoea by approximately a day (MD ‐24.38 hours, 95% CI ‐30.91 to ‐17.85; 14 studies; 2209 children; low‐certainty evidence); may increase clinical resolution at day 3 (risk ratio (RR) 2.10, 95% CI 1.30 to 3.39; 5 trials; 312 children; low‐certainty evidence); and may reduce stool output (MD ‐11.37, 95% CI ‐21.94 to ‐0.79; 3 studies; 634 children; low‐certainty evidence).
We are uncertain whether smectite reduces stool frequency, measured as depositions per day (MD ‐1.33, 95% CI ‐2.28 to ‐0.38; 3 studies; 954 children; very low‐certainty evidence). There was no evidence of an effect on need for hospitalization (RR 0.93, 95% CI 0.75 to 1.15; 2 studies; 885 children; low‐certainty evidence) and need for intravenous rehydration (RR 0.77, 95% CI 0.54 to 1.11; 1 study; 81 children; moderate‐certainty evidence). The most frequently reported side effect was constipation, which did not differ between groups (RR 4.71, 95% CI 0.56 to 39.19; 2 studies; 128 children; low‐certainty evidence). No deaths or serious adverse effects were reported.
Authors' conclusions
Based on low‐certainty evidence, smectite used as an adjuvant to rehydration therapy may reduce the duration of diarrhoea in children with acute infectious diarrhoea by a day; may increase cure rate by day 3; and may reduce stool output, but has no effect on hospitalization rates or need for intravenous therapy.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Acute Disease; Antidiarrheals; Antidiarrheals/therapeutic use; Diarrhea; Diarrhea/therapy; Diarrhea/virology; Randomized Controlled Trials as Topic; Rotavirus Infections; Rotavirus Infections/complications; Silicates; Silicates/therapeutic use
Smectite for treating children with acute diarrhoea
What is the aim of this review?
The aim of this Cochrane Review was to find out if smectite (or diosmectite), a medicinal clay commonly prescribed to people who have diarrhoea in order to reduce their stool output, helps children with acute diarrhoea. We collected and analysed all relevant studies to answer this question and found 18 relevant studies.
Key messages
Giving smectite to children with acute diarrhoea may reduce its duration. However, more high‐quality studies are still needed, including studies that assess different causes of diarrhoea and the economic effects of this treatment.
What was studied in the review?
Acute diarrhoea is one of the most common diseases in children. It is usually caused by a viral infection. The main aim of treatment is to maintain a good level of hydration. This is achieved with oral rehydration solutions, and few children need to be hospitalized or require intravenous rehydration. Still, even with proper hydration, having loose stools is a burden for both parents and patients.
Smectite may help by reducing inflammation in the gut; by acting as a barrier to reduce the penetration of toxins; or by increasing water absorption.
What are the main results?
We found 18 relevant studies with 2616 children that were conducted in both high‐income and low‐ or middle‐income countries. These studies compared children receiving smectite with children receiving routine care or a placebo (a pill or liquid that contains no medicine). Eight studies were funded by the manufacturer.
Smectite may reduce the duration of diarrhoea by one day (low‐certainty evidence); may increase the number of children cured by day 3 (low‐certainty evidence); and may slightly reduce the quantity of loose stools (low‐certainty evidence).
We are uncertain whether smectite has an effect on how many stools children have (very low‐certainty evidence). It may not have an effect on how many children need to be hospitalized (low‐certainty evidence), and probably does not have an effect on how many children need intravenous rehydration (moderate‐certainty evidence).
We found no reports of serious adverse effects. Minor adverse effects included constipation, vomiting, and bad taste, but these did not differ between groups.
How up‐to‐date is this review?
We searched for studies published up to 27 June 2017.
Summary of findings
Summary of findings for the main comparison.
Smectite compared to control for acute infectious diarrhoea in children
| Smectite compared to control for acute infectious diarrhoea in children | ||||||
| Patient or population: acute infectious diarrhoea in children Setting: hospital and outpatients Intervention: smectite Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments (compared with control) | |
| Risk with control | Risk with smectite | |||||
| Duration of diarrhoea assessed with: clinical and parental assessment, measured in total hours Follow‐up: mean 1 week | The mean duration of diarrhoea ranged from 32.6 to 118.92 hours | MD 24.38 hours fewer (30.91 fewer to 17.85 fewer) | ‐ | 2209 (14 RCTs) | ⊕⊕⊝⊝ LOW1,2 | Smectite may reduce the duration of diarrhoea |
| Clinical resolution at day 3 assessed with: clinical assessment by parents and clinicians Follow‐up: mean 3 days | Study population | RR 2.10 (1.30 to 3.39) | 312 (5 RCTs) | ⊕⊕⊝⊝ LOW3,4 | Smectite may increase the resolution of diarrhoea by the third day | |
| 342 per 1000 | 718 per 1000 (445 to 1000) | |||||
| Stool frequency assessed with: clinical assessment as number of depositions per day Follow‐up: mean 1 week | The mean stool frequency was 0 depositions per day | MD 1.33 depositions per day fewer (2.28 fewer to 0.38 fewer) | ‐ | 954 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW5,6,7 | We are uncertain whether or not smectite reduces stool frequency |
| Stool output assessed with: grams of stool output per kg of body weight in a 72‐hour period Follow‐up: mean 1 week | The mean stool output ranged from 90.7 to 118.8 g/kg | MD 11.37 g/kg fewer (21.94 fewer to 0.79 fewer) | ‐ | 634 (3 RCTs) | ⊕⊕⊝⊝ LOW7,8 | Smectite may decrease stool output |
| Need for hospitalization Follow‐up: mean 1 week | Study population | RR 0.93 (0.75 to 1.15) | 885 (2 RCTs) | ⊕⊕⊝⊝ LOW6,9 | Smectite may make little or no difference in the need for hospitalization | |
| 85 per 1000 | 79 per 1000 (64 to 98) | |||||
| Need for intravenous access for rehydration Follow‐up: mean 1 week | Study population | RR 0.77 (0.54 to 1.11) | 81 (1 RCT) | ⊕⊕⊕⊝ MODERATE9 | Smectite probably makes little or no difference in the need for intravenoous access | |
| 676 per 1000 | 520 per 1000 (365 to 750) | |||||
| Adverse events – constipation Follow‐up: mean 1 week | Study population | RR 4.71 (0.56 to 39.19) | 128 (2 RCTs) | ⊕⊕⊝⊝ LOW3,9 | Smectite may make little or no difference in the appeareance of adverse events | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Death | ‐ | ‐ | ‐ | ‐ | ‐ | There were no deaths in the included studies |
| Serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | There were no serious side effects in the included studies |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Four trials are quasi‐randomized and without adequate blinding of participants. 2High heterogeneity (I2 = 96%) among studies that may be explained by differences in age and definition of resolution, although the effect in all studies points in the same direction. 3Three studies have high risk of selection bias, including one that is quasi‐randomized, and three did not perform adequate blinding of participants. 4High heterogeneity (I2 = 81%), although the effect in all studies points in the same direction. 5High heterogeneity (I2 = 97%), although all effects point in the same direction. 6Two of the three studies are classified as quasi‐randomized with inadequate blinding of participants. 7A wide CI that does not exclude the threshold of appreciable clinical benefit. 8One quasi‐randomized study was not pooled because the authors reported stool output as stool weight in total grams per day with an effect estimate favouring smectite (mean of 255.67 g in the smectite group versus 741.33 g in the control group) at day 3 of treatment. 9Wide CI that does not exclude an appreciable benefit or harm.
Background
Description of the condition
Acute diarrhoea is defined as the passage of unusually loose or watery stools, usually at least three times in a 24‐hour period, for less than 14 days (King 2003; WHO 2005; WHO/UNICEF 2013). Incidence of acute diarrhoea in children under five years of age is approximately two to three episodes per child per year (Walker 2013). The aetiology is usually infectious, and is usually transmitted by faecal‐oral route, or by contaminated water or food. Although most cases of acute diarrhoea are self limited, the most common complication is dehydration where children are at higher risk compared to adults. The objective of treatment in many countries is to relieve symptoms and avoid complications. In low‐ and middle‐income countries there are additional concerns to prevent dehydration and prevent the illness contributing to malnutrition. Therapeutic options for the latter objective include probiotics (Allen 2010), zinc (Lazzerini 2016), lactose‐free formula (MacGillivray 2013), antibiotics, and antidiarrhoeal agents such as loperamide, racecadotril, and smectite.
Description of the intervention
Smectite is a medicinal clay commonly prescribed to reduce stool output in people with diarrhoea. A survey conducted in 29 European countries with a response rate of 34% found that 22% of physicians (9% in Western European countries and 41% in Eastern European countries) would give smectite as an adjuvant treatment to children with gastroenteritis (Szajewska 2000). In France, the use of smectite by private paediatricians may be as high as 84% (Uhlen 2004). Another survey, conducted in Prague, Czech Republic, found that 45.7% of children with acute diarrhoea received smectite (Kudlova 2010). A survey carried out in 20 hospitals in two Chinese provinces found that smectite was prescribed to 59.3% of adults with acute infectious diarrhoea (Hou 2013).
How the intervention might work
Dioctahedral smectite, or diosmectite, is a natural clay formed from sheets of aluminium and magnesium silicate. Its proposed mechanism of action differs from other antidiarrhoeal agents such as loperamide, which is an opioid‐receptor agonist, and racecadotril, which acts as an enkephalinase inhibitor. Three possible mechanisms of action of smectite against diarrhoea have been proposed: an anti‐inflammatory activity, alteration of the gut mucus barrier to reduce penetration of toxins, and adsorptive properties. These mechanisms have been replicated mainly in vitro and in animal models (Dupont 2009). In theory, these mechanisms would reduce stool output in children, thereby providing symptomatic relief and possibly preventing dehydration.
Why it is important to do this review
In many countries, symptomatic relief of diarrhoea is important to the public. Smectite is one such option for providing this relief. Two previous systematic reviews including 13 randomized controlled trials published between 1986 and 2013 provide evidence that smectite reduces the frequency and duration of diarrhoea in children (Das 2015; Szajewska 2006). The only reported adverse event was constipation. Since acute diarrhoea is usually a self limited illness, provided the person is properly hydrated, it is important to assess the efficacy and safety of adjuvant therapies such as smectite. With the publication of recent trials, there was a need to update the evidence on this topic.
Objectives
To assess the effects of smectite for treating acute infectious diarrhoea in children.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized trials comparing children with acute diarrhoea treated with smectite against a control group.
Types of participants
We included trials evaluating children, aged one month to 18 years old, with clinically defined diarrhoea of less than 14 days duration, presumed to be caused by an infectious agent. We excluded studies with other causes of diarrhoea, such as chronic or antibiotic‐associated diarrhoea.
Types of interventions
We included trials assessing oral smectite against a control group, either placebo or no smectite. We did not exclude trials that administered other interventions, such as probiotics or zinc, provided that the intervention and control arms were treated identically.
Types of outcome measures
Primary outcomes
Duration of diarrhoea, measured in hours.
Clinical resolution at day 3 after starting treatment.
Secondary outcomes
Stool frequency, measured as number of depositions per day, on day 3 after starting treatment.
Stool output, measured in g or mL/kg per day.
Need for hospitalization.
Need for intravenous access for rehydration.
Death (from any cause or diarrhoea‐related).
-
Adverse events:
serious adverse events (life‐threatening events).
other adverse events (for example, constipation, vomiting, among others).
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (27 June 2017); Cochrane Central Register of Controlled Trials (CENTRAL) (27 June 2017), published in the Cochrane Library (2017, Issue 5); MEDLINE (Pubmed; 1946 to 27 June 2017); Embase (Ovid; 1974 to 27 June 2017); and LILACS (Latin American and Caribbean Health Sciences Literature) (1982 to 27 June 2017). We also searched the metaRegister of Controlled Trials (mRCT) (27 June 2017) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (27 June 2017) using ‘smectite' and ‘diosmectite' as search terms (Appendix 1).
Searching other resources
Conference proceedings
We searched the following conference proceedings of the last two years (2014 to 2016) for relevant abstracts.
Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC).
Infectious Diseases Society of America (IDSA) conferences.
International Congress on Infectious Diseases (ICID) from the International Society for Infectious Diseases (ISID).
Researchers and organizations
We contacted researchers, authors of included trials, other experts in the field of infectious diseases, and pharmaceutical companies that manufacture smectite.
Reference lists
We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Two review authors (GP and CC) independently screened the search results to identify potentially relevant trials and retrieved the full‐text articles of these trials. GP and CC independently applied the inclusion criteria using an eligibility form, resolving any differences by discussing them with a third review author (VP or IF). We scrutinized the trial reports to ensure that multiple publications from the same trial were included only once. We listed the excluded studies and the reasons for their exclusion in the ‘Characteristics of excluded studies' section. Finally, when we were unsure whether a trial should be included because further information was needed, we attempted to contact the trial authors for clarification and allocated the trial to the ‘Studies awaiting classification' section. We have presented an adapted PRISMA flowchart showing study selection (Liberati 2009).
Data extraction and management
Two review authors (GP and CC) independently extracted prespecified characteristics of each trial using a standardized, piloted data extraction form. We attempted to contact trial authors in cases of unclear or missing data. We extracted the following data.
The numbers of randomized and analysed participants in each treatment group for each outcome.
The mean and standard deviation (SD) for each treatment group for continuous outcomes, and the number of participants with the event for each treatment group for dichotomous outcomes. If these values were not explicitly presented, we attempted to transform data where possible from available numbers such as 95% confidence intervals (CIs), standard errors, range or test statistics (that is, t, F, Z scores, P values, etc.). We obtained the SD from 95% CIs in one study according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We imputed SDs for studies that did not present any measure of data dispersion. We extracted information from figures in three trials that presented the results in this format and did not provide numerical values for measures of dispersion (Dupont 2009a; Dupont 2009b; Pociecha 1998a; Pociecha 1998b), using the Plot Digitizer open source software (Jelicic 2016). Two trials presented the information using median and 95% CI and provided a Kaplan‐Meier curve with the data for both intervention and control group (Dupont 2009a; Dupont 2009b). We applied the Hozo and colleagues approach to calculate the best estimation of mean and SD (Hozo 2005).
Assessment of risk of bias in included studies
Two review authors (GP and CC) independently assessed the risk of bias of the included studies, resolving any disagreements by discussion with a third review author (VP or IF). We attempted to contact trial authors regarding unclear or unspecified information. We used the Cochrane ‘Risk of bias' assessment tool, which includes the following domains (Higgins 2011).
Sequence generation: describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment: describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
Blinding (masking) of participants, personnel, and outcome assessors: describe all measures used, if any, to mask trial participants, personnel, and outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended masking was effective.
Incomplete outcome data: describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition or exclusions where reported, and any re‐inclusions in analyses performed by the review authors.
Selective outcome reporting: state how the possibility of selective outcome reporting was examined by the review authors and what was found.
Other sources of bias: state any important concerns about bias not addressed in the other domains in the tool.
We assessed the risk of bias for each component using ‘yes', ‘no', or ‘unclear' to indicate a low, high, or unclear risk of bias, respectively. We have presented the ‘Risk of bias' assessment in a ‘Risk of bias' graph and the ‘Risk of bias' tables.
Certainty of the evidence
We have presented the certainty of the evidence according to the GRADE approach. Two review authors (GP and CC) independently rated the certainty of the evidence for each outcome. Since we included randomized controlled trials, which are considered as high certainty, review authors could downgrade the body of evidence depending on five criteria: limitations, inconsistency, indirectness, imprecision, and publication bias. Evidence could be upgraded if a large effect size was found, if there was a dose‐response association, or if trial authors considered plausible confounding factors. We have presented a summary of the evidence in a ‘Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and studies addressing each important outcome, and the rating of the overall certainty in effect estimates for each outcome. We used GRADEpro GDT to create the ‘Summary of findings' table (GRADEpro GDT).
Measures of treatment effect
For continuous outcomes, we used mean differences (MD) as the measure of effect with 95% CIs. For outcomes with different measurements, for example stool output, which can be measured in grams or mL per kg, we used standardized mean differences (SMD). For dichotomous outcomes, we used risk ratios (RR) as the measure of effect with 95% CIs.
Unit of analysis issues
Given the condition under study and the trial participants, we did not expect to find cluster randomized controlled trials or cross‐over trials. When we found trials with repeated measurements, we decided on a single time point (for example, diarrhoea resolution at day 3).
Dealing with missing data
When there were no missing data, we carried out analyses according to the intention‐to‐treat principle, that is all children were analysed according to the group to which they were initially randomized. If there were missing data, we attempted to contact trial authors to request any missing data. If the trial authors did not respond within four to eight weeks, we conducted the analyses based on only the available information.
Assessment of heterogeneity
We used forest plots to detect overlapping CIs, and applied the Chi2 test with a P value < 0.10 to indicate statistical significance for heterogeneity. We investigated inconsistency with the I2 statistic, considering a value from 0% to 40% as not important.
Assessment of reporting biases
We assessed reporting biases by examining asymmetry of funnel plots.
Data synthesis
One review author (GP) analysed the data using Review Manager 5 (RevMan 2014). When appropriate, we combined data by meta‐analysis using a fixed‐effect model. When we found inconsistency (I2 statistic > 40%) or heterogeneity (Chi2 test at a significant P value < 0.10), we combined the results using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We expected to perform subgroup analysis based on age groups, given that severity of disease might be different among infants, children, and adolescents. Since the higher burden and mortality of acute diarrhoea is in infants (Walker 2013), we analysed subgroups under and over two years of age.
Sensitivity analysis
We performed sensitivity analyses regarding risk of bias to investigate the robustness of the results, that is restricting the analysis by taking into account trials at low versus high or unclear risk of bias, as specified in the Assessment of risk of bias in included studies section. We explored if the following markers affected the direction of results: randomization, allocation concealment, blinding, follow‐up, and missing data. We also performed a sensitivity analysis excluding the trials that required estimations and figure extractions (Dupont 2009a; Dupont 2009b; Pociecha 1998a; Pociecha 1998b).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
Our search strategy identified 34 potentially relevant studies, of which 22 studies were screened in full text. Eighteen studies met the inclusion criteria, and four were excluded (Dupont 1991; Dupont 1992; Karas 1996; Madkour 1994). The study flow diagram is shown in Figure 1. One reference included two studies (Dupont 2009a; Dupont 2009b). Another study is presented in the results as two separate studies because data were divided by age group (Pociecha 1998a; Pociecha 1998b).
Figure 1.
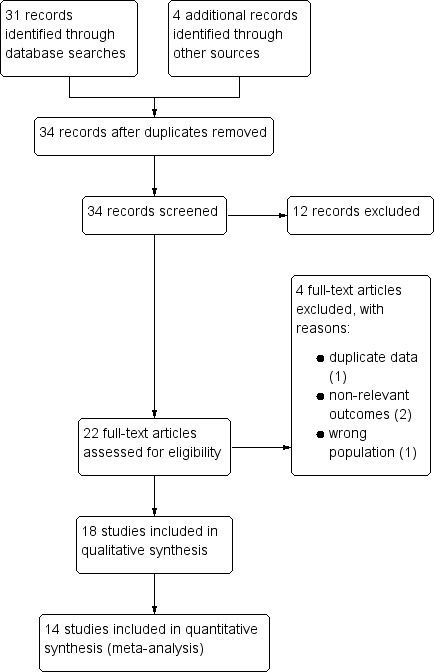
Study flow diagram.
Included studies
Study location
Eleven studies were conducted in low‐ or middle‐income countries: Peru, Malaysia, Egypt, Thailand, India, Pakistan, Indonesia, and China (Dupont 2009a; Dupont 2009b; Lachaux 1986; Lexomboon 1994; Madkour 1993; Mujawar 2012; Rehman 2013; Vivatvakin 1992; Wang 1995; Widiasa 2009; Zong 1997). Seven were conducted in high‐income countries: France, Italy, Lithuania, and Poland (Gilbert 1991; Guarino 2001; Lachaux 1986; Milocco 1999; Narkeviciute 2002; Pieścik‐Lech 2013; Pociecha 1998a; Pociecha 1998b). Most trials were conducted in hospitals, with two studies conducted in both hospital and an ambulatory setting (Madkour 1993; Wang 1995), three exclusively with outpatients (Guarino 2001; Lexomboon 1994; Mujawar 2012), and two that did not specify (Gilbert 1991; Zong 1997).
Participants
Most studies included infants aged one to 24 months. One study did not include infants (Mujawar 2012), and one did not report age (Wang 1995). Nine studies included children aged two to 12 years old. No trials included adolescents. Two trials included only males (Dupont 2009a; Dupont 2009b). One report divided its results into two age groups: less than 12 months and 13 to 36 months (Pociecha 1998a; Pociecha 1998b).
Two studies included exclusively breastfed infants (Dupont 2009a; Dupont 2009b), and seven studies included children who were breastfed (Lexomboon 1994; Osman 1992; Pieścik‐Lech 2013; Pociecha 1998a; Pociecha 1998b; Rehman 2013; Vivatvakin 1992; Widiasa 2009). One study excluded breastfed infants (Narkeviciute 2002). Thirteen trials reported rotavirus as the most frequent gastroenteritis aetiology. No studies included dysentery or bloody diarrhoea or children with cholera. One study included children with moderate malnutrition (Widiasa 2009), while the other studies excluded children with any degree of malnutrition.
Most trials defined diarrhoea as three or more loose stools, but the duration varied among studies: four defined it as less than two days (Guarino 2001; Lexomboon 1994; Mujawar 2012; Widiasa 2009); six as less than three days (Dupont 2009a; Dupont 2009b; Narkeviciute 2002; Pociecha 1998a; Pociecha 1998b; Rehman 2013; Vivatvakin 1992); one as less than four days (Lachaux 1986); five as less than five days (Madkour 1993; Milocco 1999; Pieścik‐Lech 2013; Wang 1995; Zong 1997); one as less than seven days (Osman 1992); and one referred to it as "recent" (Gilbert 1991).
Interventions
Doses of smectite varied between 1 g and 6 g per dose, and frequency of administration varied from once daily to every six hours. Most trials used 1.5 g per dose in infants less one year and 3 g in older infants or children. Two trials administered 3 g twice a day for three days, and then once a day in infants less than one year, and double the dose in older children (Dupont 2009a; Dupont 2009b). Five trials gave 1.5 g of smectite twice a day to infants less than one year, with double the dose for older children (Gilbert 1991; Guarino 2001; Milocco 1999; Pociecha 1998a; Pociecha 1998b; Wang 1995). Two studies gave a loading dose of 3 g (Lexomboon 1994; Narkeviciute 2002). Two trials administered smectite every eight hours (Mujawar 2012; Rehman 2013), and one study gave it every six hours (Madkour 1993). Two trials gave smectite every eight hours to children weighing less than 10 kg, and every six hours to children above 10 kg (Osman 1992; Vivatvakin 1992). Two studies gave Lactobacillus rhamnosus GG to both the intervention and the control group (Pieścik‐Lech 2013; Pociecha 1998a; Pociecha 1998b). Two studies did not report the dose (Widiasa 2009; Zong 1997).
The duration of treatment also differed among studies. Four studies gave smectite until recovery (Dupont 2009a; Dupont 2009b; Narkeviciute 2002; Pieścik‐Lech 2013); two administered the treatment for three days (Madkour 1993; Milocco 1999); five for five days (Mujawar 2012; Osman 1992; Rehman 2013; Vivatvakin 1992; Widiasa 2009); and one for six days (Pociecha 1998a; Pociecha 1998b). The remaining studies did not specify the duration of treatment.
Outcomes
Primary outcomes
Fifteen studies reported the duration of diarrhoea (Dupont 2009a; Dupont 2009b; Gilbert 1991; Guarino 2001; Lachaux 1986; Madkour 1993; Milocco 1999; Mujawar 2012; Narkeviciute 2002; Pieścik‐Lech 2013; Pociecha 1998a; Pociecha 1998b; Rehman 2013; Vivatvakin 1992; Widiasa 2009; Zong 1997), but the outcome was defined differently. Six trials defined it as time to the last loose stool (Guarino 2001; Madkour 1993; Narkeviciute 2002; Pieścik‐Lech 2013; Vivatvakin 1992; Widiasa 2009); three as time to first formed stool (Dupont 2009a; Lachaux 1986; Rehman 2013); one as time to first soft or formed stool (Dupont 2009b); three as time to normalization of stools (Gilbert 1991; Mujawar 2012; Pociecha 1998a; Pociecha 1998b); and two did not provide a clear definition (Milocco 1999; Zong 1997).
Five trials reported clinical resolution of diarrhoea at day 3 (Lachaux 1986; Lexomboon 1994; Madkour 1993; Osman 1992; Vivatvakin 1992).
Secondary outcomes
Four studies reported stool frequency: three as number of depositions per day (Guarino 2001; Madkour 1993; Osman 1992), and one as the total number of stools during follow‐up (Milocco 1999). Three trials reported stool output as grams per kilogram of child's weight at 72 hours (Dupont 2009a; Dupont 2009b), and one in grams per day (Osman 1992). Two studies reported need for hospitalization (Guarino 2001; Pieścik‐Lech 2013). One study reported need for intravenous access for rehydration (Pieścik‐Lech 2013). No studies reported deaths.
Risk of bias in included studies
See: Characteristics of included studies; Figure 2; Figure 3 for the risk of bias in included studies.
Figure 2.
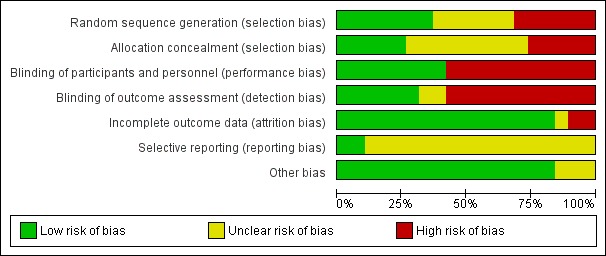
‘Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
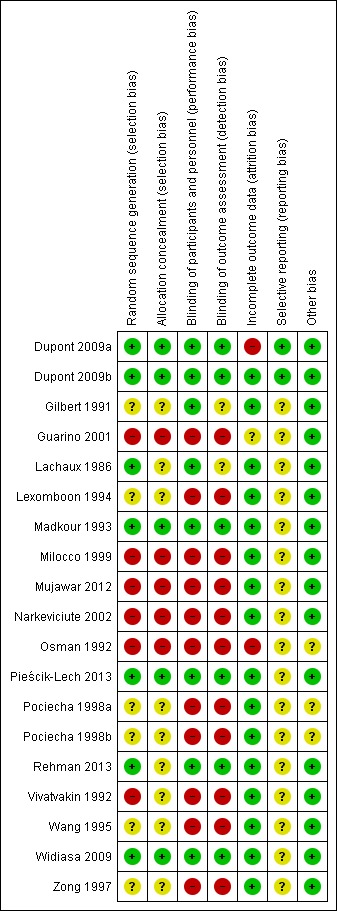
‘Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven studies had an adequate description of randomization method (Dupont 2009a; Dupont 2009b; Lachaux 1986; Madkour 1993; Pieścik‐Lech 2013; Rehman 2013; Widiasa 2009). In five trials the information about random allocation was unclear (Gilbert 1991; Lexomboon 1994; Pociecha 1998a; Pociecha 1998b; Wang 1995; Zong 1997). Five studies were quasi‐randomized trials in which children were allocated alternately, by birthday or serial number (Guarino 2001; Milocco 1999; Mujawar 2012; Narkeviciute 2002; Osman 1992). We suspected selection bias in one study as groups differed in the aetiology of diarrhoea, and the method of randomization was not described (Vivatvakin 1992).
Five studies adequately described allocation concealment (Dupont 2009a; Dupont 2009b; Madkour 1993; Pieścik‐Lech 2013; Widiasa 2009). We considered all quasi‐randomized trials as having high risk of bias regarding allocation concealment.
Blinding
Eight trials were reported as double‐blind and used a placebo as control (Dupont 2009a; Dupont 2009b; Gilbert 1991; Lachaux 1986; Madkour 1993; Pieścik‐Lech 2013; Rehman 2013; Widiasa 2009). The remaining trials were not blinded (Guarino 2001; Lexomboon 1994; Milocco 1999; Mujawar 2012; Narkeviciute 2002; Osman 1992; Pociecha 1998a; Pociecha 1998b; Vivatvakin 1992; Wang 1995; Zong 1997).
Incomplete outcome data
Fourteen trials had appropriate follow‐up and analysis of more than 90% of participants. Two included less than 90% in the analysis (Dupont 2009a; Osman 1992). In one trial information was insufficient to permit judgement (Guarino 2001).
Selective reporting
Two trials had a registered protocol (Dupont 2009a; Dupont 2009b).
Effects of interventions
See: Table 1
Primary outcomes
1.1 Duration of diarrhoea
Overall, duration of diarrhoea was reduced by approximately 24 hours (mean difference (MD) ‐24.38, 95% confidence interval (CI) ‐30.91 to ‐17.85; 14 trials; 2209 children, Analysis 1.1; low‐certainty evidence). There was significant heterogeneity (I2 = 96%). This high inconsistency was due to differences in effect size of the benefit, not because of opposing directions of effects (Figure 4).
Analysis 1.1.
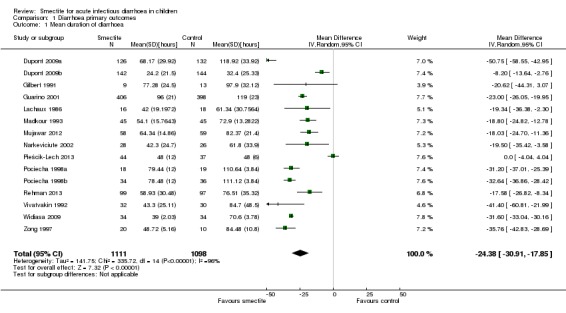
Comparison 1 Diarrhoea primary outcomes, Outcome 1 Mean duration of diarrhoea.
Figure 4.
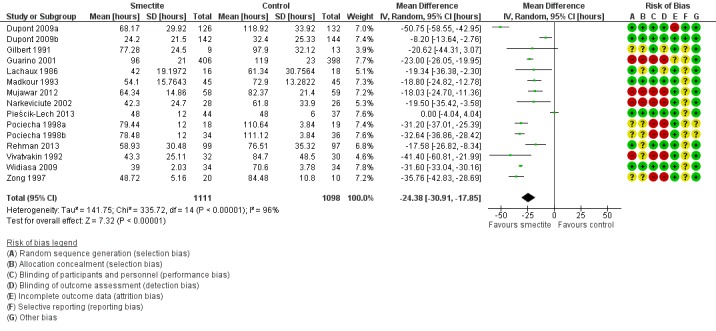
Forest plot of comparison: 1 Diarrhoea primary outcomes, outcome: 1.1 Mean duration of diarrhoea (hours).
A sensitivity analysis exploring the effect of randomization, allocation concealment, blinding, and follow‐up did not change the result of the meta‐analysis significantly. Sensitivity analysis excluding the trials that required estimations and figure extractions did not significantly change the result of the meta‐analysis (MD ‐22.07, 95% CI ‐30.38 to ‐13.76) (Dupont 2009a; Dupont 2009b; Pociecha 1998a; Pociecha 1998b).
On visual inspection, the funnel plot was roughly symmetric, with most studies centred together at the top, probably reflecting spuriously small standard deviations of the continuous outcome that is skewed (Figure 5).
Figure 5.
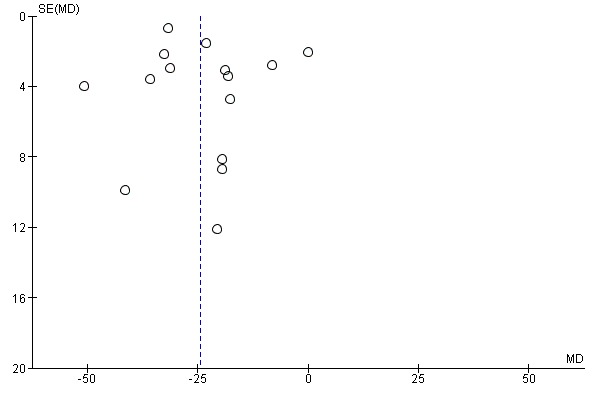
Funnel plot of comparison: 1 Diarrhoea primary outcomes, outcome: 1.1 Mean duration of diarrhoea (hours).
1.2 Duration of diarrhoea, infants less than two years
Five studies included only infants younger than two years (Gilbert 1991; Lachaux 1986; Madkour 1993; Rehman 2013; Vivatvakin 1992). One study reported results for infants less than 12 months (Pociecha 1998a). Smectite reduced the duration of diarrhoea by 24 hours (MD ‐24.11, 95% CI ‐31.35 to ‐16.87; 441 infants; Analysis 1.2). Other studies included both infants and children, but they did not provide enough information to be able to perform a subgroup analysis according to age (Figure 6).
Analysis 1.2.
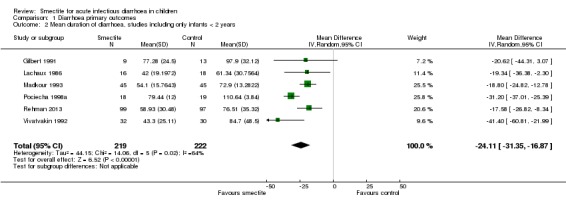
Comparison 1 Diarrhoea primary outcomes, Outcome 2 Mean duration of diarrhoea, studies including only infants < 2 years.
Figure 6.
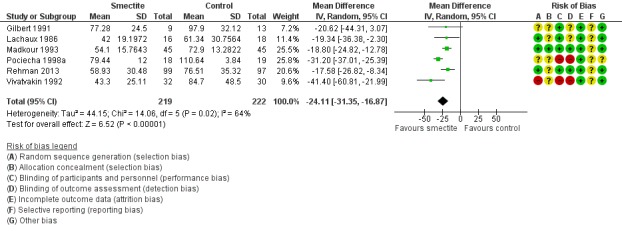
Forest plot of comparison: 1 Diarrhoea primary outcomes, outcome: 1.2 Mean duration of diarrhoea, studies including only infants < 2 years.
1.3 Clinical resolution at day 3 after starting treatment
Smectite increased the rate of clinical resolution at day 3 (risk ratio (RR) 2.10, 95% CI 1.30 to 3.39; 5 trials; 312 children; Analysis 1.3; low‐certainty evidence) (Figure 7). After performing a sensitivity analysis excluding trials with high risk of bias (Osman 1992; Vivatvakin 1992), the pooled effect was not significant (RR 1.90, 95% CI 0.96 to 3.77; 3 trials; 190 children).
Analysis 1.3.
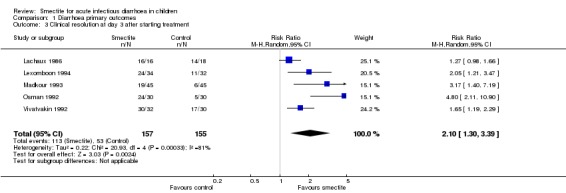
Comparison 1 Diarrhoea primary outcomes, Outcome 3 Clinical resolution at day 3 after starting treatment.
Figure 7.
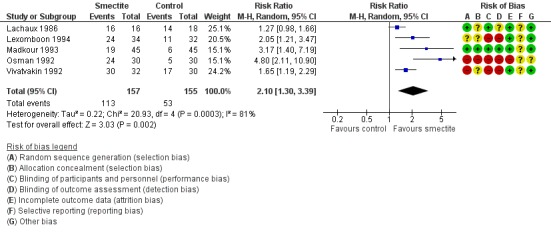
Forest plot of comparison: 1 Diarrhoea primary outcomes, outcome: 1.3 Clinical resolution at day 3 after starting treatment.
Secondary outcomes
2.1 Stool frequency
Three studies measured stool frequency as number of depositions per day, all of them reporting data on day 3 (Guarino 2001; Madkour 1993; Osman 1992). Smectite reduced stool frequency by one (MD ‐1.33, 95% CI ‐2.28 to ‐0.38; 3 trials; 954 children; Analysis 2.1; very low‐certainty evidence) (Figure 8). One study measured stool frequency as total number of depositions during follow‐up; the mean number of depositions was 10 in both groups (Milocco 1999).
Analysis 2.1.
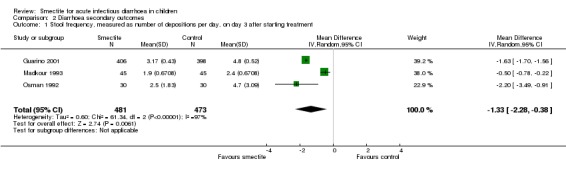
Comparison 2 Diarrhoea secondary outcomes, Outcome 1 Stool frequency, measured as number of depositions per day, on day 3 after starting treatment.
Figure 8.
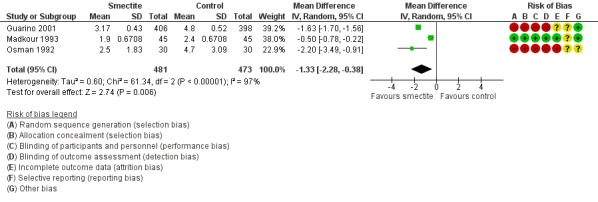
Forest plot of comparison: 2 Diarrhoea secondary outcomes, outcome: 2.1 Stool frequency, measured as number of depositions per day, on day 3 after starting treatment.
2.2 Stool output
Four studies evaluated stool output. Three studies reported cumulative stool output at 72 hours (Dupont 2009a; Dupont 2009b; Madkour 1993). Smectite reduced stool output by 11 g/kg (MD ‐11.37, 95% CI ‐21.94 to ‐0.79; 3 trials; 634 children; Analysis 2.2; low‐certainty evidence) (Figure 9). Another study was not pooled because the authors reported stool output as stool weight in total grams per day with an effect estimate favouring smectite (mean of 255.67 g in the smectite group versus 741.33 g in the control group) at day 3 of treatment (Osman 1992).
Analysis 2.2.
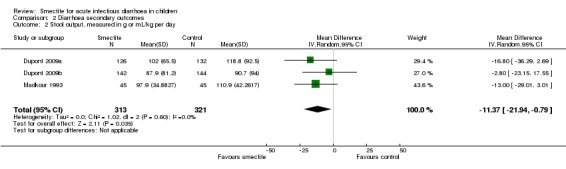
Comparison 2 Diarrhoea secondary outcomes, Outcome 2 Stool output, measured in g or mL/kg per day.
Figure 9.
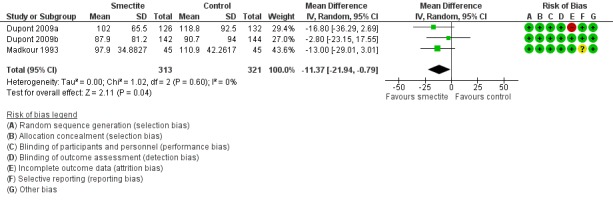
Forest plot of comparison: 2 Diarrhoea secondary outcomes, outcome: 2.2 Stool output, measured in g/kg at 72 hours.
2.3 Need for hospitalization
Two studies reported data on need for hospitalization. There was no evidence of benefit using smectite (RR 0.93, 95% CI 0.75 to 1.15; 2 trials; 885 children; Analysis 2.3; low‐certainty evidence) (Figure 10).
Analysis 2.3.
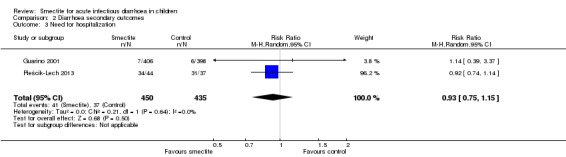
Comparison 2 Diarrhoea secondary outcomes, Outcome 3 Need for hospitalization.
Figure 10.
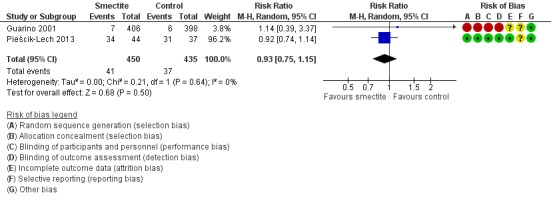
Forest plot of comparison: 2 Diarrhoea secondary outcomes, outcome: 2.3 Need for hospitalization.
2.4 Need for intravenous access for rehydration
There was no evidence of an effect on need for intravenous rehydration (RR 0.77, 95% CI 0.54 to 1.11; 1 trial; 81 children; Analysis 2.4; moderate‐certainty evidence).
Analysis 2.4.
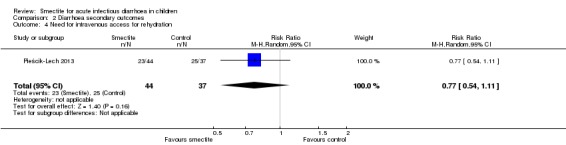
Comparison 2 Diarrhoea secondary outcomes, Outcome 4 Need for intravenous access for rehydration.
2.5 Death (from any cause or diarrhoea‐related)
No deaths were reported in any of the included trials.
2.6 Serious adverse events (life‐threatening events)
There were no reports of serious adverse events.
2.7 Other adverse events (constipation, vomiting)
The most commonly reported adverse effect was constipation. However, the risk of constipation using smectite was very uncertain due to imprecision, with very few events and wide confidence intervals (RR 4.71, 95% CI 0.56 to 39.19; 2 trials; 128 children; Analysis 2.5; low‐certainty evidence) (Figure 11). There were also no differences between groups regarding vomiting or fever. Another minor adverse event mentioned in trials was bad taste, but there were no specific numbers for the intervention and control groups.
Analysis 2.5.
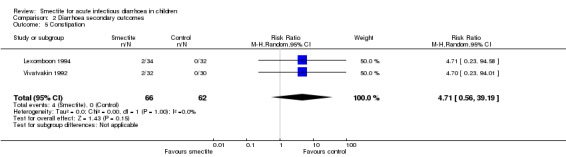
Comparison 2 Diarrhoea secondary outcomes, Outcome 5 Constipation.
Figure 11.
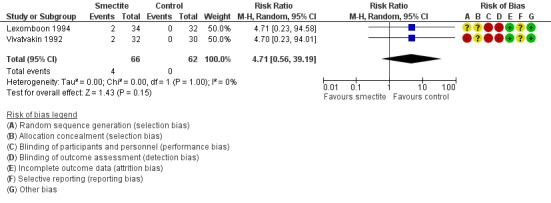
Forest plot of comparison: 2 Diarrhoea secondary outcomes, outcome: 2.5 Constipation.
Discussion
Summary of main results
We identified 18 studies that compared smectite to a control group. Overall, smectite reduced the duration of diarrhoea by approximately a day, increased clinical resolution by day 3, and had a modest benefit on stool frequency and output. This evidence of benefit persisted after a sensitivity analysis accounting for randomization method, even though five trials were quasi‐randomized. Eight trials reported the inclusion of breastfed infants.
There was no evidence of an effect on the need for hospitalization or intravenous rehydration, deaths, or serious side effects.
Overall completeness and applicability of evidence
Studies were conducted in diverse settings in both high‐income and low‐ or middle‐income countries, and including both ambulatory and hospital patients. Aetiology also varied, with most trials including a large proportion of children with rotavirus. Most studies excluded children with malnutrition. Most of the studies were funded by the industry. Although external funding and commercial interests are well recognized as a potential source of bias in clinical trials, most investigators provided reasonable information that shows that the manufacturers had no, or a very limited, active role in the design and conduct of the studies.
Quality of the evidence
We assessed the certainty of the evidence using the GRADE system, which is displayed in ‘Summary of findings' table 1 (Table 1). Overall, the certainty of the body of evidence ranged from very low to moderate. For our primary outcomes, the certainty of evidence was low mainly due to concerns of risk of bias and inconsistency of the results. Regarding risk of bias, we included four quasi‐randomized trials, and another four trials did not clearly describe the randomization process. Also, seven trials were not blinded.
The high heterogeneity observed may be due to differences in the definition of the condition, the age of participants, and the different aetiologies. In one study, Pieścik‐Lech 2013, another explanation for heterogeneity could be that both the intervention and the control group received a probiotic, but the other study that added a probiotic as a co‐intervention did not contribute to such inconsistency (Pociecha 1998a; Pociecha 1998b). The high inconsistency observed was mainly due to differences in effect size of the benefit and not because of opposing directions of effects.
Potential biases in the review process
We made every attempt to limit biases during the review process by ensuring a comprehensive search for potentially eligible studies. We believe that the authors’ independent assessments of eligibility of studies for inclusion and data extraction have minimized the potential for additional bias beyond that detailed in the ‘Risk of bias' tables in the Characteristics of included studies and in the funnel plot.
Agreements and disagreements with other studies or reviews
Our findings agree with those of previous systematic reviews (Das 2015; Szajewska 2006). Due to the differences in time of publication, our review includes more trials than the review by Szajewska 2006, and assesses the certainty of the evidence based on the GRADE approach. The review by Das 2015 included 13 out of the 18 studies that were included in this review. Szajewska 2006 reported a reduction of 22.7 hours in the duration of diarrhoea, while Das 2015 reported 22.39 hours. Szajewska 2006 and Das 2015 also report significant results for cure rate at day 3. While Das 2015 reported clinical resolution at day 5 and 7, we considered day 3 to be more clinically relevant.
Authors' conclusions
Smectite reduces the duration of symptoms of infectious diarrhoea by a day, and at least 17 hours, and increases clinical resolution at day 3. The effect on stool frequency and output is modest. Although smectite did not have an effect on other relevant outcomes such as the need for intravenous therapy or hospitalization, fewer hours of diarrhoea may be considered clinically significant in different settings and contexts, taking into account that most cases of infectious diarrhoea are self limited and resolve within three to five days with adequate hydration and medical care.
Further research with a focus on adequate randomization and blinding is needed. Future studies may explore the causes of heterogeneity in the effect of smectite, its possible benefit in vulnerable populations such as children under two years of age or with malnutrition, and its effect on certain specific aetiologies such as rotavirus or dysentery producing bacteria. Economic analyses will also provide information to guide practice in different countries or settings.
Acknowledgements
The editorial base for the Cochrane Infectious Diseases Group is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (Grant: 5242).
Appendices
Appendix 1. Search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | Embaseb | LILACSb |
| 1 | smectite | Smectite [Supplementary concept] | Smectite [Supplementary concept] | Smectite ti, ab | smectite |
| 2 | diosmectite | smectite* ti, ab | smectite* ti, ab | Diosmectite ti, ab | diosmectite |
| 3 | 1 or 2 | Diosmectite ti, ab | Diosmectite ti, ab | 1 or 2 | 1 or 2 |
| 4 | – | "smecta"[Supplementary Concept] | "smecta"[Supplementary Concept] | Limit 3 to human | ‐ |
| 5 | ‐ | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | ‐ | ‐ |
| 6 | ‐ | Limit 5 to humans | Limit 5 to humans | ‐ | ‐ |
| aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2011); upper case: MeSH or EMTREE heading; lower case: free text term. | |||||
Data and analyses
Comparison 1.
Diarrhoea primary outcomes
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 15 | 2209 | Mean Difference (IV, Random, 95% CI) | ‐24.38 [‐30.91, ‐17.85] |
| 2 Mean duration of diarrhoea, studies including only infants < 2 years | 6 | 441 | Mean Difference (IV, Random, 95% CI) | ‐24.11 [‐31.35, ‐16.87] |
| 3 Clinical resolution at day 3 after starting treatment | 5 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.30, 3.39] |
Comparison 2.
Diarrhoea secondary outcomes
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stool frequency, measured as number of depositions per day, on day 3 after starting treatment | 3 | 954 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.28, ‐0.38] |
| 2 Stool output, measured in g or mL/kg per day | 3 | 634 | Mean Difference (IV, Random, 95% CI) | ‐11.37 [‐21.94, ‐0.79] |
| 3 Need for hospitalization | 2 | 885 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 4 Need for intravenous access for rehydration | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.54, 1.11] |
| 5 Constipation | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 4.71 [0.56, 39.19] |
Differences between protocol and review
We added Ivan D Florez as an author.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 300 enrolled children Inclusion criteria: inpatients; well‐nourished male infants and children aged 1 to 36 months with watery diarrhoea < 3 days duration, with 3 watery stools per day and at least 1 watery stool in the past 24 hours; mild‐to‐moderate dehydration Exclusion criteria: severe dehydration or malnutrition, bloody diarrhoea, fever 39 ºC or higher, previous medications Breastfeeding: exclusively breastfed infants were excluded |
|
| Interventions | Intervention group: diosmectite. Dosage 3 g twice a day for 3 days, then 3 g daily for infants younger than 12 months. Double the dose for older children Control: placebo |
|
| Outcomes | Duration of diarrhoea (until first formed stool) Stool output in g/kg in the first 72 hrs |
|
| Notes | Location: Peru Setting: urban Cause of diarrhoea: rotavirus 22%. Other aetiologies not specified. Source of funding: industry Registration number: NCT00352716 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomized in sequential ascending order by a statistician |
| Allocation concealment (selection bias) | Low risk | Sponsor‐assigned biostatistician prepared a list of treatment allocation codes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was identical to diosmectite in size, weight, colour, smell, taste, and appearance, and was inert. Blinding seems appropriate. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind review of data by outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40 children (13%) were non‐adherent, and the rest analysed as per protocol. |
| Selective reporting (reporting bias) | Low risk | None detected. Registered trial |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 302 enrolled children Inclusion criteria: inpatients; well‐nourished male infants and children aged 1 to 36 months with watery diarrhoea < 3 days duration, with 3 watery stools per day and at least 1 watery stool in the past 24 hours; mild‐to‐moderate dehydration Exclusion criteria: severe dehydration or malnutrition, bloody diarrhoea, fever 39 ºC or higher, previous medications Breastfeeding: exclusively breastfed infants were excluded |
|
| Interventions | Intervention group: diosmectite. Dosage 3 g twice a day for 3 days, then 3 g daily for infants younger than 12 months. Double the dose for older children Control: placebo |
|
| Outcomes | Duration of diarrhoea (until first soft or formed stool) Stool output in g/kg in the first 72 hrs |
|
| Notes | Location: Malaysia Setting: urban Cause of diarrhoea: rotavirus 12%. Other aetiologies not specified. Source of funding: industry Registration number: NCT00352989 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomized in sequential ascending order by a statistician |
| Allocation concealment (selection bias) | Low risk | Sponsor‐assigned biostatistician prepared a list of treatment allocation codes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was identical to diosmectite in size, weight, colour, smell, taste, and appearance, and was inert. Blinding seems appropriate. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind review of data by outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 children (5%) were non‐adherent, and the rest analysed as per protocol. |
| Selective reporting (reporting bias) | Low risk | None detected. Registered trial |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 56 enrolled children Inclusion criteria: inpatients; children aged 2 to 24 months with moderate‐to‐severe acute diarrhoea Exclusion criteria: malnutrition Breastfeeding: not specified |
|
| Interventions | Intervention group: diosmectite. Dosage 1.5 g twice a day for infants younger than 12 months. Double the dose for older children Control: placebo Another control group received loperamide 0.11 mg/kg every 8 hours. |
|
| Outcomes | Duration of diarrhoea (time to normalization of stools) Stool frequency on day 5 |
|
| Notes | Location: France Setting: urban Cause of diarrhoea: rotavirus 18%, Staphylococcus aureus 3%, Escherichia coli 3%, Campylobacter spp. 3%, Candida spp. 1% Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomized but no method described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo; probably adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per‐protocol analysis. 4 children (7%) excluded and not analysed. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Quasi‐randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 804 enrolled children Inclusion criteria: outpatients; well‐nourished children aged 3 to 60 months with acute diarrhoea of mild‐to‐moderate severity < 2 days duration, with 3 watery stools per day Exclusion criteria: malnutrition, chronic diseases, previous medications Breastfeeding: not specified |
|
| Interventions | Intervention group: diosmectite. Dosage 1.5 g twice a day for infants younger than 12 months. Double the dose for older children Control: no medication |
|
| Outcomes | Duration of diarrhoea (from first to the last liquid–loose stool output preceding the return of normal stools) Diarrhoea at day 7 Vomiting Fever Hospitalization rate |
|
| Notes | Location: Italy Setting: urban Cause of diarrhoea: rotavirus 4%, not specified bacterial aetiology 1% Source of funding: industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized. Participants selected in sequential one‐to‐one basis. |
| Allocation concealment (selection bias) | High risk | Not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 36 enrolled infants Inclusion criteria: inpatients; infants aged 2 to 24 months with acute watery diarrhoea < 4 days duration, with mild‐to‐moderate dehydration Exclusion criteria: previous medications, concomitant illness Breastfeeding: not stated |
|
| Interventions | Intervention group: diosmectite. Dosage 3 g per day to infants < 1 year, 6 g per day to infants > 1 year. Duration not stated. Control: placebo |
|
| Outcomes | Duration of diarrhoea (from first drug administration to last liquid stool before a formed one) Clinical resolution at day 3 and 5 Adverse events |
|
| Notes | Location: France Setting: urban Cause of diarrhoea: rotavirus 77% Source of funding: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated as "drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo; probably adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Use of placebo; probably adequate blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 loss per group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: 5 days |
|
| Participants | Number: 66 enrolled children Inclusion criteria: outpatients; well‐nourished children aged 1 to 24 months with acute diarrhoea < 2 days duration, with 3 watery stools within 24 hours Exclusion criteria: severe dehydration, dysentery, fever higher than 38.5 ºC, previous medications Breastfeeding: included |
|
| Interventions | Intervention group: diosmectite. Dosage: loading dose of 3 g, then 1.5 g twice a day Control: no medication |
|
| Outcomes | Diarrhoea at day 3 and 5 Tolerability |
|
| Notes | Location: Thailand Setting: urban Cause of diarrhoea: rotavirus 27%, Campylobacter jejuni 8%, enteropathogenic Escherichia coli 5%, Salmonella spp. 6%, Shigella spp. 3%, Plesiomonas shigelloides 2% Source of funding: industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomized. No method described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: until recovery from diarrhoea |
|
| Participants | Number: 90 enrolled children Inclusion criteria: inpatients; well‐nourished male children aged 3 to 24 months with watery diarrhoea < 5 days duration, with dehydration of any severity Exclusion criteria: prolonged diarrhoea, malnutrition, major illnesses Breastfeeding: not specified |
|
| Interventions | Intervention group: diosmectite. Dosage 1.5 g, 4 times daily for 3 days Control group: placebo |
|
| Outcomes | Duration of diarrhoea (from enrolment to last liquid stool) Frequency of diarrhoea Duration of vomiting Feeding pattern |
|
| Notes | Location: Egypt Setting: urban Cause of diarrhoea: rotavirus 16%. Other aetiologies not specified. Source of funding: WHO and industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization by Diarrheal Disease Control Programme of the WHO |
| Allocation concealment (selection bias) | Low risk | Numerically coded envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Quasi‐randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 35 enrolled children Inclusion criteria: inpatients; children aged 0 to 60 months with watery diarrhoea < 5 days duration Exclusion criteria: not stated Breastfeeding: not specified |
|
| Interventions | Intervention group: diosmectite. Dosage 1.5 g twice a day for infants younger than 12 months. Double the dose for older children Control: no medication |
|
| Outcomes | Number of stools at 48 hrs Duration of diarrhoea (not clearly defined) Fever, vomiting, weight loss |
|
| Notes | Location: Italy Setting: urban Cause of diarrhoea: rotavirus 23%, Salmonella spp. 11%, Cryptosporidium 6% Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants selected "alternatively"; not truly random. |
| Allocation concealment (selection bias) | High risk | Not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 losses to follow‐up in intervention group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Quasi‐randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 117 enrolled children Inclusion criteria: outpatients; well‐nourished children aged 24 to 60 months with watery diarrhoea < 2 days duration; mild‐to‐moderate dehydration Exclusion criteria: bloody diarrhoea, chronic illness, previous medications Breastfeeding: not specified |
|
| Interventions | Intervention group: diosmectite. Dosage 1.5 g thrice a day for 5 days Control group: no medication |
|
| Outcomes | Duration of diarrhoea (until normal stool consistency) Complications: severe dehydration, severe dysentery, respiratory infection, and anaemia |
|
| Notes | Location: India Setting: urban Cause of diarrhoea: not specified Source of funding: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not truly random; participants selected by serial number. |
| Allocation concealment (selection bias) | High risk | Not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. 8 children (7%) were lost to follow‐up and were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Quasi‐randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 54 enrolled children Inclusion criteria: inpatients; well‐nourished infants and children aged 6 to 48 months with watery diarrhoea < 3 days duration, with 3 watery stools per day; mild‐to‐moderate dehydration Exclusion criteria: severe dehydration, malnutrition, chronic or concomitant illness Breastfeeding: excluded |
|
| Interventions | Intervention group: diosmectite. Dosage: loading dose of 3 g; 1.5 g, 3 times a day for children < 10 kg, 4 times a day for > 10 kg Control: no medication |
|
| Outcomes | Duration of diarrhoea (time to last watery/semiliquid stool) Length of stay |
|
| Notes | Location: Lithuania Setting: urban Cause of diarrhoea: rotavirus 70%, enteropathogenic Escherichia coli 4%, Campylobacter spp. 8% Source of funding: industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not truly random; participants selected by birthday. |
| Allocation concealment (selection bias) | High risk | Not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Quasi‐randomized controlled trial Length of follow‐up: 5 days |
|
| Participants | Number: 71 infants and children Inclusion criteria: inpatients; infants and children (no age limit specified, mean age of 13 months) with acute watery diarrhoea < 7 days duration, with mild‐to‐moderate dehydration Exclusion criteria: systemic illness; previous use of antibiotics or antidiarrhoeal agents; malnutrition Breastfeeding: included |
|
| Interventions | Intervention group: diosmectite. Dosage 1.5 g every 8 hours to infants < 10 kg, 1.6 g every 6 hours to infants > 10 kg for a maximum of 5 days Control: no medication |
|
| Outcomes | Clinical resolution (return of stools to previous formed consistency and average number of frequency) Stool output (g/kg) Stool frequency |
|
| Notes | Location: Egypt Setting: urban Cause of diarrhoea: rotavirus 43%, bacterial (not specified) 23% Source of funding: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not truly random; participants selected alternately. |
| Allocation concealment (selection bias) | High risk | Not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per‐protocol analysis with 4 exlusions in intervention group (12%) and 7 losses in control group (19%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Unclear risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: 7 days |
|
| Participants | Number: 88 enrolled children Inclusion criteria: inpatients/outpatients; well‐nourished infants and children aged 4 to 60 months with watery diarrhoea < 5 days duration, with 3 watery stools per day Exclusion criteria: recent history of diarrhoea, chronic diseases Breastfeeding: included |
|
| Interventions | Intervention group: diosmectite, dose 3 g once daily until diarrhoea stopped, plus Lactobacillus GG, dose of 6 x 109 colony forming units, once daily for 7 days Control group: placebo plus Lactobacillus GG |
|
| Outcomes | Duration of diarrhoea (time from randomization until the last watery stool, or at least 12 h with no stool) Stool frequency Consistency of stools Need for antibiotic therapy Diarrhoea recurrence Need for hospitalization Need for intravenous rehydration therapy |
|
| Notes | Location: Poland Setting: urban Cause of diarrhoea: rotavirus 60%, adenovirus 5%, Salmonella spp. 5%, Staphylococcus aureus 3%, enteropathogenic Escherichia coli 1% Source of funding: Medical University of Warsaw |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization |
| Allocation concealment (selection bias) | Low risk | Randomization prepared by independent investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per‐protocol analysis with 7 losses in control group (8%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: 28 days |
|
| Participants | Number: 56 enrolled infants Inclusion criteria: inpatients; well‐nourished infants ≤ 12 months with watery diarrhoea of rotaviral aetiology < 3 days duration, with moderate dehydration Exclusion criteria: chronic diseases, aetiologies other than rotavirus Breastfeeding: included |
|
| Interventions | Intervention group: diosmectite, dose 1.5 g twice daily for 6 days, plus Lactobacillus GG in "age dependent dose" Control group: Lactobacillus GG A third group received polyvinylpolypyrrolidone plus Lactobacillus GG. |
|
| Outcomes | Duration of intravenous rehydration Duration of fever and vomiting Duration of diarrhoea (time to normalization of consistency of the stool or a day without stool) Need of hospitalization after discharge |
|
| Notes | Location: Poland Setting: urban Cause of diarrhoea: rotavirus 100% Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomized. No method described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Unclear risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: 28 days |
|
| Participants | Number: 105 enrolled infants Inclusion criteria: inpatients; well‐nourished infants > 12 months with watery diarrhoea of rotaviral aetiology < 3 days duration, with moderate dehydration Exclusion criteria: chronic diseases, aetiologies other than rotavirus Breastfeeding: included |
|
| Interventions | Intervention group: diosmectite, dose 3 g twice daily for 6 days, plus Lactobacillus GG in "age dependent dose" Control group: Lactobacillus GG A third group received polyvinylpolypyrrolidone plus Lactobacillus GG. |
|
| Outcomes | Duration of intravenous rehydration Duration of fever and vomiting Duration of diarrhoea (time to normalization of consistency of the stool or a day without stool) Need of hospitalization after discharge |
|
| Notes | Location: Poland Setting: urban Cause of diarrhoea: rotavirus 100% Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomized. No method described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Unclear risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: 6 days |
|
| Participants | Number: 206 enrolled children Inclusion criteria: inpatients; well‐nourished infants and children aged 6 to 24 months with watery diarrhoea < 3 days duration, with 3 watery stools per day and at least 1 in the past 24 hours; mild‐to‐severe dehydration Exclusion criteria: bloody diarrhoea, medications, malnutrition, systemic infection Breastfeeding: included |
|
| Interventions | Intervention group: diosmectite, dose 1 g in infants < 12 months and 1.5 g in older children, every 8 hours, plus zinc (dose not specified) for 5 days Control group: placebo plus zinc |
|
| Outcomes | Duration of diarrhoea (until first stool of pre‐diarrhoeal consistency) | |
| Notes | Location: Pakistan Setting: urban Cause of diarrhoea: not specified Source of funding: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by lottery |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. Mentions only "lottery method". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per‐protocol analysis. 10 losses to follow‐up (5%), 4 in intervention group, 6 in control group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: 5 days |
|
| Participants | Number: 62 enrolled children Inclusion criteria: inpatients; infants/children aged 1 to 24 months with acute secretory diarrhoea < 3 days duration, with 3 watery stools per day Exclusion criteria: severe dehydration, third‐degree malnutrition, other medications, chronic illnesses Breastfeeding: included |
|
| Interventions | Intervention group: diosmectite. Dosage 1.5 g, every 12 hrs for infants < 3 kg; every 8 hrs for infants 4 to 10 kg; every 6 hrs for children 11 to 15 kg, for a maximum of 5 days Control: no medication |
|
| Outcomes | Duration of diarrhoea (from first intervention dose until last liquid stool) Number of stools Change in weight Oral liquid intake |
|
| Notes | Location: Thailand Setting: urban Cause of diarrhoea: rotavirus in 3% of children in intervention group, 19% in control group. Stool cultures were reported positive for Salmonella and Aeromonas spp. in 7% and 9% of children in the control and study group, respectively, but numbers of each bacterial aetiology per group were not stated. Source of funding: industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Stated as randomized, but no method of randomization described. Selection bias is suspected as groups were different in the aetiology of diarrhoea. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases were detected. |
| Methods | Randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 55 enrolled children Inclusion criteria: acute diarrhoea < 5 days duration. No age limit or other inclusion criteria stated. Exclusion criteria: not stated Breastfeeding: not stated |
|
| Interventions | Intervention group: diosmectite. Dosage 3 g per day in infants < 1 year old, 6 g per day in > 1 year Control: Bismuth complex. Dosage 5 mL, 3 times per day |
|
| Outcomes | Clinical resolution at day 5 | |
| Notes | Location: China Setting: not clear Cause of diarrhoea: not reported Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomization described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: until recovery |
|
| Participants | Number: 68 enrolled infants Inclusion criteria: inpatients; infants aged 6 to 12 months with watery diarrhoea < 2 days duration, with 3 watery stools per day and mild‐to‐moderate dehydration Exclusion criteria: severe malnutrition, bloody diarrhoea, severe disease Breastfeeding: included |
|
| Interventions | Intervention group: diosmectite. Dose not specified. Control group: placebo |
|
| Outcomes | Duration of diarrhoea (time until normal consistency and frequency) | |
| Notes | Location: Indonesia Setting: urban Cause of diarrhoea: not specified Source of funding: industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Low risk | Coded, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
| Methods | Randomized controlled trial Length of follow‐up: not stated |
|
| Participants | Number: 45 enrolled children Inclusion criteria: infants and children aged 2 to 30 months with watery diarrhoea of < 5 days duration Exclusion criteria: not specified Breastfeeding: not reported |
|
| Interventions | Intervention group: diosmectite. Dose not specified. Control: lactein tablet. Dose not specified. A third group received diosmectite and antibiotics. |
|
| Outcomes | Duration of diarrhoea (not clearly defined) | |
| Notes | Location: China Setting: unclear Cause of diarrhoea: rotavirus 100% Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomization described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol registered. |
| Other bias | Low risk | No other biases detected. |
WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dupont 1991 | Wrong outcome: permeability to mannitol and lactulose |
| Dupont 1992 | Wrong outcome: permeability to mannitol and lactulose |
| Karas 1996 | Wrong population: neonates |
| Madkour 1994 | Duplicate |
Contributions of authors
Giordano Pérez‐Gaxiola and Carlos Cuello‐García prepared the protocol and manuscript. Ivan D Florez checked the protocol and manuscript, and provided advice. Víctor Pérez‐Pico checked the protocol and provided advice as an infectious diseases specialist.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Grant: 5242
Declarations of interest
Giordano Pérez‐Gaxiola, Carlos A Cuello‐García, Ivan D Florez, Víctor M Pérez‐Pico: we certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of this Cochrane Review (for example, employment, consultancy, stock ownership, honoraria, or expert testimony).
Unchanged
References
References to studies included in this review
- Dupont C, Foo JL, Garnier P, Moore N, Mathiex‐Fortunet H, Salazar‐Lindo E, Peru and Malaysia Diosmectite Study Groups. Oral diosmectite reduces stool output and diarrhea duration in children with acute watery diarrhea. Clinical Gastroenterology and Hepatology 2009;7(4):456‐62. [DOI] [PubMed] [Google Scholar]
- Dupont C, Foo JL, Garnier P, Moore N, Mathiex‐Fortunet H, Salazar‐Lindo E, Peru and Malaysia Diosmectite Study Groups. Oral diosmectite reduces stool output and diarrhea duration in children with acute watery diarrhea. Clinical Gastroenterology and Hepatology 2009;7(4):456‐62. [DOI] [PubMed] [Google Scholar]
- Gilbert B, Liendhardt A, Palomera S, Barberis L, Borreda D. The efficacy of smectite in acute infantile diarrhea compared to a placebo and loperamide. Annales de Pediatrie 1991;38(9):633‐6. [PubMed] [Google Scholar]
- Guarino A, Bisceglia M, Castellucci G, Iacono G, Casali LG, Bruzzese E, et al. Smectite in the treatment of acute diarrhea: a nationwide randomized controlled study of the Italian Society of Pediatric Gastroenterology and Hepatology (SIGEP) in collaboration with primary care pediatricians. Journal of Pediatric Gastroenterology and Nutrition 2001;32(1):71‐5. [DOI] [PubMed] [Google Scholar]
- Lachaux A, Danzon A, Collet JP, Descos B, Hermier M. Acute infantile diarrhoea. Role of treatment with smectite as complement to rehydration. Randomised double‐blind study. International Review of Pediatrics 1986;163:29‐31. [Google Scholar]
- Lexomboon U, Harikul S, Lortholary O. Control randomized study of rehydration/rehydration with dioctahedral smectite in ambulatory Thai infants with acute diarrhea. Southeast Asian Journal of Tropical Medicine and Public Health 1994;25(1):157‐62. [PubMed] [Google Scholar]
- Madkour AA, Madina EMH, el‐Azzouni OEZ, Amer MA, El‐Walili TM, Abbass T. Smectite in acute diarrhea in children: a double‐blind placebo‐controlled clinical trial. Journal of Pediatric Gastroenterology and Nutrition 1993;17(2):176‐81. [DOI] [PubMed] [Google Scholar]
- Milocco C, Bolis A, Rizzo V, Suprani T, Cerasoli G, Marani M, et al. Evaluation of diosmectite in acute infantile diarrhoea. La Pediatria Medica e Chirurgica 1999;21(3):129‐33. [PubMed] [Google Scholar]
- Mujawar QM, Naganoor R, Ali MD, Malagi N, Thobbi AN. Efficacy of dioctahedral smectite in acute watery diarrhea in Indian children: a randomized clinical trial. Journal of Tropical Pediatrics 2012;58(1):63‐7. [DOI] [PubMed] [Google Scholar]
- Narkeviciute I, Rudzeviciene O, Leviniene G, Mociskiene K, Eidukevicius R. Management of Lithuanian children's acute diarrhoea with Gastrolit solution and dioctahedral smectite. European Journal of Gastroenterology & Hepatology 2002;14(4):419‐24. [DOI] [PubMed] [Google Scholar]
- Osman GAH, Mahomoud SAR, El‐Shakankiry HM, Abulhana SM. An adsorbent! Role in the management of acute diarrhea in infants and children. Ain Shams Medical Journal 2991;13(10‐1):12665‐76. [Google Scholar]
- Pieścik‐Lech M, Urbańska M, Szajewska H. Lactobacillus GG (LGG) and smectite versus LGG alone for acute gastroenteritis: a double‐blind, randomized controlled trial. European Journal of Pediatrics 2013;172(2):247‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pociecha W, Balcerska A. Influence of the mucoprotective drugs on the clinical course of children's rotaviral gastroenteritis. Gastroenterologia Polska 1998;5(6):533‐42. [Google Scholar]
- Pociecha W, Balcerska A. Influence of the mucoprotective drugs on the clinical course of children's rotaviral gastroenteritis. Gastroenterologia Polska 1998;5(6):533‐42. [Google Scholar]
- Rehman A, Ahmad M, Chaudhry TA, Tehseen SA. The efficacy of diosmectite in admitted children having acute watery diarrhea with dehydration. Pakistan Paediatric Journal 2013;37(4):195‐6. [Google Scholar]
- Vivatvakin B, Jongpipatvanich S, Harikul S, Eksaengri P, Lortholary O. Control study of oral rehydration solution (ORS)/ORS + dioctahedral smectite in hospitalized Thai infants with acute secretory diarrhea. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(3):414‐9. [PubMed] [Google Scholar]
- Wang JX, Fang HS. Intestinal microecological observation of smectite in the treatment of diarrhea in children. Chinese Journal of Clinical Pharmacology 1995;11(3):134‐7. [Google Scholar]
- Widiasa AAM, Soetjiningsih S, Karyana PG. Efficacy of dioctahedral smectite in infants with acute diarrhea: a double blind randomized controlled trial. Paediatrica Indonesiana 2009;49(1):97‐103. [Google Scholar]
- Zong S, Chao YM, Qu Y, Liu L. Study on smecta therapy for acute diarrhea in infants. Journal of Tianjin Medical University 1997;3:60‐3. [Google Scholar]
References to studies excluded from this review
- Dupont D, Moreno JL, Barau E, Thiane E, Plique O. Intestinal permeability to lactulose and mannitol during treatment by diosmectite in acute diarrhea in children a double blind vs. placebo study. Gastroenterology 1991;100(5 Pt 2):A684. [Google Scholar]
- Dupont C, Moreno JL, Barau E, Bargaoui K, Thiane E, Plique O. Effect of diosmectite on intestinal permeability changes in acute diarrhea: a double‐blind placebo‐controlled trial. Journal of Pediatric Gastroenterology and Nutrition 1992;14(4):413‐9. [DOI] [PubMed] [Google Scholar]
- Karas J. Smecta pulvis and its place in the treatment of acute rotavirus gastroenteritis of neonates. Československá Pediatrie 1996;51(2):85‐90. [Google Scholar]
- Madkour AA. Placebo‐controlled, double‐blind clinical trial of smectite in acute pediatric diarrhea. Fortschritte der Medizin 1994;112(24):6p. [PubMed] [Google Scholar]
Additional references
- Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD003048.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RR, Sankar J, Naik SS. Efficacy and safety of diosmectite in acute childhood diarrhoea: a meta‐analysis. Archives of Disease in Childhood 2015;100(7):704‐12. [DOI] [PubMed] [Google Scholar]
- Dupont C, Vernisse B. Anti‐diarrheal effects of diosmectite in the treatment of acute diarrhea in children: a review. Paediatric Drugs 2009;11(2):89‐99. [DOI: 10.2165/00148581-200911020-00001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime, Inc.). Available from gradepro.org. GRADEpro GDT: GRADEpro Guideline Development Tool. Hamilton, ON: McMaster University (developed by Evidence Prime, Inc.). Available from gradepro.org, 2015.
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Hou FQ, Wang Y, Li J, Wang GQ, Liu Y. Management of acute diarrhea in adults in China: a cross‐sectional survey. BMC Public Health 2013;13:41. [DOI: 10.1186/1471-2458-13-41] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelicic Kadic A, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. Journal of Clinical Epidemiology 2016;74:119‐23. [DOI] [PubMed] [Google Scholar]
- King CK, Glass R, Bresee JS, Duggan C, Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recommendations and Reports 2003;52(RR‐16):1‐16. [PubMed] [Google Scholar]
- Kudlova E. Home management of acute diarrhoea in Czech children. Journal of Pediatric Gastroenterology and Nutrition 2010;50(5):510‐5. [DOI: 10.1097/MPG.0b013e3181b7a691] [DOI] [PubMed] [Google Scholar]
- Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD005436.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray S, Fahey T, McGuire W. Lactose avoidance for young children with acute diarrhoea. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD005433.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Szajewska H, Hoekstra JH, Sandhu B. Management of acute gastroenteritis in Europe and the impact of the new recommendations: a multicenter study. The Working Group on Acute Diarrhoea of the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2000;30(5):522‐7. [PUBMED: 10817282] [DOI] [PubMed] [Google Scholar]
- Szajewska H, Dziechciarz P, Mrukowicz J. Meta‐analysis: smectite in the treatment of acute infectious diarrhoea in children. Alimentary Pharmacology & Therapeutics 2006;23(2):217‐27. [PUBMED: 16393300] [DOI] [PubMed] [Google Scholar]
- Uhlen S, Toursel F, Gottrand F, Association française de pédiatrie ambulatoire. Treatment of acute diarrhea: prescription patterns by private practice pediatricians [Traitement des diarrhées aiguës: les habitudes de prescription des pédiatres libéraux]. Archives de pédiatrie: organe officiel de la Sociéte française de pédiatrie 2004;11(8):903‐7. [PUBMED: 15288079] [DOI] [PubMed] [Google Scholar]
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381(9875):1405‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The Treatment of Diarrhoea: a Manual for Physicians and Other Senior Health Workers. Geneva: World Health Organization, 2005. [Google Scholar]
- World Health Organization/UNICEF. Ending Preventable Child Deaths From Pneumonia and Diarrhoea by 2025: the Integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). Geneva: World Health Organization, 2013. [Google Scholar]


