Abstract
Background
Epidural analgesia is a central nerve block technique achieved by injection of a local anaesthetic close to the nerves that transmit pain, and is widely used as a form of pain relief in labour. However, there are concerns about unintended adverse effects on the mother and infant. This is an update of an existing Cochrane Review (Epidural versus non‐epidural or no analgesia in labour), last published in 2011.
Objectives
To assess the effectiveness and safety of all types of epidural analgesia, including combined‐spinal‐epidural (CSE) on the mother and the baby, when compared with non‐epidural or no pain relief during labour.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register (ClinicalTrials.gov), the WHO International Clinical Trials Registry Platform (ICTRP) (30 April 2017), and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials comparing all types of epidural with any form of pain relief not involving regional blockade, or no pain relief in labour. We have not included cluster‐randomised or quasi‐randomised trials in this update.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risks of bias, extracted data and checked them for accuracy. We assessed selected outcomes using the GRADE approach.
Main results
Fifty‐two trials met the inclusion criteria and we have included data from 40 trials, involving over 11,000 women. Four trials included more than two arms. Thirty‐four trials compared epidural with opioids, seven compared epidural with no analgesia, one trial compared epidural with acu‐stimulation, one trial compared epidural with inhaled analgesia, and one trial compared epidural with continuous midwifery support and other analgesia. Risks of bias varied throughout the included studies; six out of 40 studies were at high or unclear risk of bias for every bias domain, while most studies were at high or unclear risk of detection bias. Quality of the evidence assessed using GRADE ranged from moderate to low quality.
Pain intensity as measured using pain scores was lower in women with epidural analgesia when compared to women who received opioids (standardised mean difference ‐2.64, 95% confidence interval (CI) ‐4.56 to ‐0.73; 1133 women; studies = 5; I2 = 98%; low‐quality evidence) and a higher proportion were satisfied with their pain relief, reporting it to be "excellent or very good" (average risk ratio (RR) 1.47, 95% CI 1.03 to 2.08; 1911 women; studies = 7; I2 = 97%; low‐quality evidence). There was substantial statistical heterogeneity in both these outcomes. There was a substantial decrease in the need for additional pain relief in women receiving epidural analgesia compared with opioid analgesia (average RR 0.10, 95% CI 0.04 to 0.25; 5099 women; studies = 16; I2 = 73%; Tau2 = 1.89; Chi2 = 52.07 (P < 0.00001)). More women in the epidural group experienced assisted vaginal birth (RR 1.44, 95% CI 1.29 to 1.60; 9948 women; studies = 30; low‐quality evidence). A post hoc subgroup analysis of trials conducted after 2005 showed that this effect is negated when trials before 2005 are excluded from this analysis (RR 1.19, 95% CI 0.97 to 1.46). There was no difference between caesarean section rates (RR 1.07, 95% CI 0.96 to 1.18; 10,350 women; studies = 33; moderate‐quality evidence), and maternal long‐term backache (RR 1.00, 95% CI 0.89 to 1.12; 814 women; studies = 2; moderate‐quality evidence). There were also no clear differences between groups for the neonatal outcomes, admission to neonatal intensive care unit (RR 1.03, 95% CI 0.95 to 1.12; 4488 babies; studies = 8; moderate‐quality evidence) and Apgar score less than seven at five minutes (RR 0.73, 95% CI 0.52 to 1.02; 8752 babies; studies = 22; low‐quality evidence). We downgraded the evidence for study design limitations, inconsistency, imprecision in effect estimates, and possible publication bias.
Side effects were reported in both epidural and opioid groups. Women with epidural experienced more hypotension, motor blockade, fever, and urinary retention. They also had longer first and second stages of labour, and were more likely to have oxytocin augmentation than the women in the opioid group. Women receiving epidurals had less risk of respiratory depression requiring oxygen, and were less likely to experience nausea and vomiting than women receiving opioids. Babies born to women in the epidural group were less likely to have received naloxone. There was no clear difference between groups for postnatal depression, headache, itching, shivering, or drowsiness. Maternal morbidity and long‐term neonatal outcomes were not reported.
Epidural analgesia resulted in less reported pain when compared with placebo or no treatment, and with acu‐stimulation. Pain intensity was not reported in the trials that compared epidural with inhaled analgesia, or continuous support. Few trials reported on serious maternal side effects.
Authors' conclusions
Low‐quality evidence shows that epidural analgesia may be more effective in reducing pain during labour and increasing maternal satisfaction with pain relief than non‐epidural methods. Although overall there appears to be an increase in assisted vaginal birth when women have epidural analgesia, a post hoc subgroup analysis showed this effect is not seen in recent studies (after 2005), suggesting that modern approaches to epidural analgesia in labour do not affect this outcome. Epidural analgesia had no impact on the risk of caesarean section or long‐term backache, and did not appear to have an immediate effect on neonatal status as determined by Apgar scores or in admissions to neonatal intensive care. Further research may be helpful to evaluate rare but potentially severe adverse effects of epidural analgesia and non‐epidural analgesia on women in labour and long‐term neonatal outcomes.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Analgesia, Epidural; Analgesia, Epidural/adverse effects; Delivery, Obstetric; Labor Pain; Labor, Obstetric; Analgesia, Obstetrical; Analgesia, Obstetrical/adverse effects; Analgesia, Obstetrical/methods; Cesarean Section; Cesarean Section/statistics & numerical data; Intensive Care Units, Neonatal; Intensive Care Units, Neonatal/statistics & numerical data; Patient Satisfaction; Patient Satisfaction/statistics & numerical data; Randomized Controlled Trials as Topic; Risk
Plain language summary
Epidurals for pain relief in labour
What is the issue?
We set out to assess the effectiveness of all kinds of epidural analgesia (including combined‐spinal‐epidural) on the mother and the baby, when compared with non‐epidural or no pain relief during labour.
Why is this important?
Pain relief is important for women in labour. Pharmacological methods of pain relief include breathing in of nitrous oxide, injection of opioids and local analgesia with an epidural for a central nerve block. Epidurals are widely used for pain relief in labour and involve an injection of a local anaesthetic into the lower region of the back close to the nerves that transmit pain. Epidural solutions are given by bolus injection (a large, rapid injection), continuous infusion or using a patient‐controlled pump. Lower concentrations of local anaesthetic when given together with an opiate allow women to maintain the ability to move around during labour and to actively participate in the birth. Combined‐spinal‐epidural involves a single injection of local anaesthetic or opiate into the cerebral spinal fluid for fast onset of pain relief, as well as insertion of the epidural catheter for continuing pain relief. Side effects such as itchiness, drowsiness, shivering and fever have been reported. Rare but potentially severe adverse effects of epidural analgesia can occur, such as severe long‐lasting headache after the injection, or nerve injury.
What evidence did we find?
We searched for evidence in April 2017 and identified 40 trials, involving over 11,000 women, that contributed information to this review. The trials varied in the quality of their methods.
All but six studies compared epidural analgesia with injected opioid drugs. Epidurals may relieve labour pain more effectively than opioids, and more women may be more satisfied with epidural as pain relief. Overall, women using epidural analgesia may be more likely to require forceps or ventouse to assist with the birth when compared with opioid drugs. However we did not see this effect in studies conducted since 2005, where the use of lower concentrations of local anaesthetic and more modern epidural techniques such as patient‐controlled epidural analgesia (PCEA) were more likely. Epidural in comparison to opioids probably makes little or no difference to caesarean section rates, women with long‐term backache, effects on the baby at birth or the number of babies who were admitted to neonatal intensive care.
Women who used epidurals can have problems passing urine and can suffer fever. There are highly variable findings such as a longer labour, experiencing very low blood pressure, and being unable to move for a period of time after the birth (motor blockade), probably due to higher concentrations of local anaesthetic being used in the epidural or the use of epidural infusions rather than epidural doses of pain relief administered at intervals. However, women who received opioid drugs also showed some side effects such as a slowing of their breathing so that they needed to wear an oxygen mask, and more nausea and vomiting. More babies whose mothers received opioids were given a drug to counteract the effects of the opioids. There was no difference between women in the epidural or opioid groups for postnatal depression, headaches, itching, shivering, or drowsiness.
Women with epidurals reported less pain compared to women with placebo or no treatment, or acu‐stimulation. Pain was not reported in the trials that compared epidural with inhaled analgesia, or continuous support.
What does this mean?
Epidurals may reduce pain during labour more effectively than any other form of pain relief, and may increase maternal satisfaction with pain relief. However, some women who have an epidural instead of opioid drugs may be more likely to have an assisted vaginal birth, but this finding probably reflects the higher concentrations of local anaesthetics used traditionally rather than the low concentrations of modern epidurals. Further research would be helpful, using more consistent measures of reducing the adverse outcomes with epidurals.
Summary of findings
Summary of findings for the main comparison. Epidural compared to opioids in labour (maternal outcomes).
| Epidural compared to opioids in labour (maternal outcomes) | ||||||
| Patient or population: women in labour Setting: hospital setting in Canada, China, Denmark, Egypt, Finland, France, India, Israel, Kuwait, Malaysia, Netherlands, Norway, Sweden, United Kingdom, and United States Intervention: epidural Comparison: opioids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with opioids | Risk with epidural | |||||
| Pain intensity measured using pain score in labour (lower scores = less pain) | SMD 2.64 lower (4.56 lower to 0.73 lower) | ‐ | 1133 (5 RCTs) | ⊕⊕⊝⊝ Low 1, 2 | ‐ | |
| Satisfaction with pain relief ‐ proportion rating excellent or very good | Study population | Average RR 1.47 (1.03 to 2.08) | 1911 (7 RCTs) | ⊕⊕⊝⊝ Low 1, 2 | ‐ | |
| 500 per 1000 | 735 per 1000 (515 to 1000) | |||||
| Assisted vaginal birth | Study population | RR 1.44 (1.29 to 1.60) | 9948 (30 RCTs) | ⊕⊕⊝⊝ Low 1, 3 | ‐ | |
| 99 per 1000 | 142 per 1000 (127 to 158) | |||||
| Caesarean section | Study population | RR 1.07 (0.96 to 1.18) | 10,350 (33 RCTs) | ⊕⊕⊕⊝ Moderate 1 | ‐ | |
| 114 per 1000 | 122 per 1000 (110 to 135) | |||||
| Side effects (maternal) ‐ long‐term backache | Study population | RR 1.00 (0.89 to 1.12) | 814 (2 RCTs) | ⊕⊕⊕⊝ Moderate 1 | ‐ | |
| 585 per 1000 | 585 per 1000 (520 to 655) | |||||
| Admission to special care baby unit/neonatal intensive care unit (as defined by trialists) | Study population | RR 1.03 (0.95 to 1.12) | 4488 (8 RCTs) | ⊕⊕⊕⊝ Moderate 1 | ‐ | |
| 204 per 1000 | 210 per 1000 (194 to 228) |
|||||
| Apgar score less than 7 at 5 minutes | Study population | RR 0.73 (0.52 to 1.02) | 8752 (22 RCTs) | ⊕⊕⊝⊝ Low 1, 4 | ‐ | |
| 17 per 1000 | 12 per 1000 (9 to 17) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded due to limitation of study design (‐1). 2Severe unexplained heterogeneity (‐1). 3Funnel plot suggests possible publication bias (‐1). 4Wide confidence interval crossing the line of no effect (‐1).
Background
This review was last updated (Anim‐Somuah 2011) as one of a series of Cochrane Reviews examining pain management in labour. These reviews contributed to an overview of systematic reviews of pain management for women in labour (Jones 2012), and shared a generic protocol (Jones 2011). This current review is an update from the previous version (Anim‐Somuah 2011).
Description of the condition
Pain relief is an important issue for women in labour. The level of pain experienced and the effectiveness of pain relief may influence a woman's satisfaction with labour and the birth and may have immediate and long‐term emotional and psychological effects (Christiansen 2002). The type of pain relief used in labour may impact on breastfeeding and mother‐infant interaction (Walker 1997).
Women experience varying degrees of pain in labour and exhibit an equally varying range of responses to it. An individual's reaction to the pain of labour may be influenced by the circumstances of her labour, the environment, her cultural background, preparation for labour and the support available to her (Brownridge 1991; McCrea 2000; Rowlands 1998). Need for pain relief in labour is also influenced by the type of onset of labour (spontaneous or induced) and medical interventions such as instrumental vaginal delivery and episiotomy. Several methods of relieving pain in labour and various coping strategies have been advocated, ranging from limited intervention such as breathing exercises to medical techniques like epidural analgesia. Regardless of the intensity of the pain experienced and response generated, it is important that whatever method is used to ameliorate maternal discomfort, it is both effective and safe for the mother and baby.
Relaxation therapies, distraction techniques, hypnosis Madden 2016) and continuous support (Bohren 2017) are believed to help women in labour to use their own resources to cope with pain. Other non‐pharmacological methods used for relieving pain include acupressure, acupuncture, reflexology, aromatherapy, transcutaneous electrical nerve stimulation and intradermal injection of sterile water (Martensson 1999). Reported effectiveness of these methods varies (Dowswell 2009; Ranta 1994; Smith 2011a; Smith 2011b). There are data to show that women who have continuous intrapartum support are less likely to have pain relief in labour (Bohren 2017; Lieberman 2002), and measures, such as labouring in water, massage, acupuncture and hypnosis, may be helpful therapies for pain management in labour (Chang 2002; Cluett 2009; Cyna 2004). Efficacy of other methods such as audioanalgesia and music therapy remains to be assessed (Cluett 2009). Pharmacological methods like inhalation of nitrous oxide, parenteral injection of opioids and regional analgesia in the form of epidural and combined spinal epidural are also commonly used to relieve pain in labour.
Description of the intervention
Epidural analgesia was first used in obstetric practice in 1946 and its use in labour has steadily increased over the past 20 years, with more than 20% of women in the UK, 60% in the USA and increasing numbers of women in China choosing this form of pain relief (DOH 2005; Grant 2015; Hu 2016; Sng 2015). However, there is considerable variation in the availability and use of epidural analgesia between hospitals in the same country and between different countries across the world.
Epidural analgesia is a central nerve blockade technique, which involves the injection of a local anaesthetic with or without an adjunct such as the opioid fentanyl, into the epidural space of the lower region of the spine close to the nerves that transmit painful stimuli from the contracting uterus and birth canal. Protocols for the care of women using epidural analgesia vary among hospitals. Epidural solutions are administered either by bolus, continuous infusion or patient‐controlled pump. An intermittent technique involves injections of local anaesthetic through a catheter positioned in the epidural space. Boluses of higher concentrations, as used in the earlier years, have been associated with a dense motor block resulting in reduced mobility, decreased pelvic tone and loss of the bearing‐down sensations usually experienced in the second stage of labour (Thornton 2001). More recently there has been a trend to use a lower concentration of local anaesthetic in combination with a variety of opiates; these combinations provide analgesic effect while allowing the woman to maintain some motor function, such as the ability to move during her labour and retain her ability to bear down (COMET 2001; Russell 2000; Sng 2015), and avoid an assisted vaginal birth such as the use of forceps. Combined‐spinal‐epidural (CSE) involves a single injection of local anaesthetic or opiate or both into the cerebral spinal fluid, as well as insertion of the epidural catheter. CSE combines the advantages of spinal analgesia (faster onset of pain relief,from the time of injection and more reliable analgesia) with the advantages of epidural analgesia, such as continuing pain relief, potentially maintained throughout the entire duration of labour (Simmons 2012; Sng 2015)
How the intervention might work
Epidural analgesia is considered to be the most effective method for reducing pain in labour (Brownridge 1991; Howell 2001). The anaesthetic inhibits nerve conduction by blocking sodium channels in nerve membranes, thereby preventing the propagation of nerve impulses along these fibres. Blocking of painful impulses from the nerves as they cross the epidural space results in analgesia which is usually apparent within 10 to 20 minutes of administration. The anaesthetic placed in the epidural space exerts a concentration‐specific effect, affecting all the types of sensation of the blocked nerves to varying degrees, such that administration of a lower‐dose anaesthetic (e.g. 0.125% bupivacaine, 0.1% or 0.2% ropivacaine) selectively blocks painful stimuli whilst largely preserving motor function. Traditionally, higher doses of local anaesthetic were used, leading to excessive motor blockade that limited mobility in labour (Sng 2015). Epidural analgesia allows the woman to remain alert during labour. The regional administration of epidural drugs may help avoid some systemic side effects of analgesic medication on the baby, such as opioid‐induced neonatal respiratory depression. A functioning epidural allows the option of regional anaesthesia for interventions such as caesarean section or manual removal of retained placenta, thereby avoiding the risks associated with general anaesthesia (Hibbard 1996). However, spinal anaesthesia can also be used for this purpose.
Why it is important to do this review
Although epidural analgesia usually provides effective pain relief in labour, it may be associated with unwanted effects for the mother and baby. Reported maternal complications may include hypotension (a reduction in maternal blood pressure (BP)). Severe sudden hypotension (sometimes defined as more than a 20% decrease in baseline BP) may result in a clinically significant decrease in utero‐placental blood flow, which could potentially affect delivery of oxygen to the baby. This may especially compromise a baby with inadequate reserves (Vincent 1998). For this reason, intravenous fluids may be given before administering the epidural drugs (fluid preload) to attenuate the decrease in maternal BP. Side effects such as itchiness, drowsiness, shivering and fever have also been reported (Buggy 1995; Eberle 1996). Women may develop urinary retention while using epidural analgesia. This may necessitate the insertion of a catheter to drain the bladder. Urinary retention in the postpartum period has been attributed to long labours in women using epidural analgesia (Liang 2002). Less common side effects reported are accidental puncture of the dura, which can cause severe headache (post‐dural puncture headache (1%) (Stride 1993)). This resolves spontaneously in some women; however, a blood patch may be needed when the headache is persistent. This involves a sterile injection of 15 mL to 20 mL of the woman's fresh blood into the epidural space (Bromage 1999; Vincent 1998). This resolves the headache for 60% of women.
Epidural analgesia may influence the course of labour. There have been suggested associations with malpositions of the fetal head, prolonged labour, increased use of oxytocin and of instrumental deliveries (Eberle 1996);. Effects of epidural analgesia on the neonate may be mixed. Higher cord pH values and less naloxone use at birth have been reported (Halpern 1998), as has a greater need for neonatal resuscitation (COMET 2001). It has been suggested that babies of women who use epidural analgesia may be more prone to low blood sugar in the first hours after birth (Swanström 1981b).
Objectives
To assess the effectiveness and safety of all types of epidural analgesia, including combined‐spinal‐epidural (CSE) on the mother and the baby, when compared with non‐epidural or no pain relief during labour.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing all types of epidural analgesia including CSE with alternative forms of pain relief not involving regional analgesia or no pain relief in labour. We included abstracts of unpublished manuscripts of RCTs, provided there was sufficient information to assess eligibility and risk of bias, and excluded quasi‐randomised trials. We excluded studies with a high level of attrition (more than 25%). We have not included cluster‐randomised trials in this update.
Types of participants
Pregnant women requesting pain relief in labour, regardless of parity and whether labour was spontaneous or induced.
Types of interventions
We considered all forms of epidural administration, compared with any form of pain relief not involving regional blockade, or no pain relief. Trials comparing different techniques of epidural are the subject of another review (Simmons 2012).
The previous version of this review was one in a series of Cochrane Reviews examining pain management in labour that contributed to an overview of systematic reviews. They shared a generic protocol. To avoid duplication, the different methods of pain management were listed in a specific order, from 1 to 15. Individual reviews focusing on particular interventions included comparisons with only the intervention above it on the list. The list is as follows:
Placebo/no treatment
Hypnosis (Madden 2016)
Biofeedback (Barragán 2011)
Intracutaneous or subcutaneous sterile water injection (Derry 2012)
Immersion in water (Cluett 2009)
Aromatherapy (Smith 2011a)
Relaxation techniques (yoga, music, audio) (Smith 2018a)
Acupuncture or acupressure (Smith 2011b)
Manual methods (massage, reflexology) (Smith 2018b)
Transcutaneous electrical nerve stimulation (TENS) (Dowswell 2009)
Inhaled analgesia (Klomp 2012)
Opioids (Ullman 2010)
Non‐opioid drugs (Othman 2011)
Local anaesthetic nerve blocks (Novikova 2011)
Epidural (including combined spinal epidural) (Simmons 2012)
Accordingly, where data are available, this review includes comparisons of any form of epidural administration, compared with: 1. placebo/no treatment; 2. hypnosis; 3. biofeedback; 4. intracutaneous or subcutaneous sterile water injection; 5. immersion in water; 6. aromatherapy; 7. relaxation techniques (yoga, music, audio); 8. acupuncture or acupressure; 9. manual methods (massage, reflexology); 10. TENS; 11. inhaled analgesia; 12. opioids; 13. non‐opioid drugs; and 14. local anaesthetic nerve blocks.
Types of outcome measures
Primary outcomes
Effectiveness of interventions
Pain intensity (as defined by trialists) Satisfaction with pain relief (as defined by trialists) Sense of control in labour (as defined by trialists) Satisfaction with childbirth experience (as defined by trialists) Need for other means of pain relief
Safety of interventions
Effect (negative) on mother/baby interaction Breastfeeding (at specified time points) Assisted vaginal birth Caesarean section Side effects (for mother)
Long‐term backache (as defined by trial authors)
Maternal hypotension (as defined by authors)
Postnatal depression (authors' definition, treatment for depression or self‐reported)
Motor blockade
Respiratory depression requiring oxygen administration
Uterine rupture
Headache
Headache requiring blood patch
Venous thromboembolic events
Perineal trauma requiring suturing
Nausea or vomiting or both
Itching
Fever
Shivers
Drowsiness
Urinary retention
Catheterisation during labour
Other morbidity (e.g. impaired consciousness, meningitis, intensive care unit admission, paralysis)
Malposition (as defined by trial authors)
Surgical amniotomy
Side effects (for baby)
Acidosis, as defined by cord blood arterial pH less than 7.2
Acidosis, as defined by cord blood arterial pH less than 7.15
Naloxone administration
Neonatal hypoglycaemia (less than or equal to 1.67 mmol/l)
Birth trauma
Long‐term neonatal complication
Meconium staining of liquor
Admission to special care baby unit/neonatal intensive care unit (as defined by trialists) Apgar score less than seven at five minutes Poor infant outcomes at long‐term follow‐up (as defined by trialists, e.g. seizures, disability in childhood)
Other outcomes
Cost (as defined by trialists)
Secondary outcomes
Length of first stage of labour Length of second stage of labour Oxytocin augmentation Caesarean section for fetal distress Caesarean section for dystocia
Search methods for identification of studies
The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
[For this update], We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (30 April 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification).
We also searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (30 April 2017) (See: Appendix 1 for search methods used).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeAnim‐Somuah 2011. For this update, we used the following methods for assessing the trial reports that we identified as a result of the updated search. The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager 5 software (RevMan 2014) and checked them for accuracy.
When information about any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risks of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis, done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Assessment of the quality of the evidence using the GRADE approach
For this update we assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison: epidural anaesthesia versus opioids.
Pain intensity (as defined by trialists)
Satisfaction with pain relief (as defined by trialists)
Assisted vaginal birth
Caesarean section
Side effects (for mother): long‐term backache (as defined by trialists)
Admission to special care baby unit/neonatal intensive care unit (as defined by trialists)
Apgar score less than seven at five minutes
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create a ’Summary of findings’ table. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes, using the GRADE approach. This uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as a summary risk ratio (RR) with a 95% confidence interval (CI).
Continuous data
We used the mean difference (MD) if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We include no cluster‐randomised trials in this update (2018). In future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial if possible, from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and if we consider the interaction between the effect of intervention and the choice of randomisation unit to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over designs are not a valid study design for this review.
Other unit of analysis issues
Multiple pregnancies
Most of the data in the review are from trials recruiting women with singleton pregnancies only, and in those trials which included women with multiple pregnancies or which did not specify whether such women were included, the number of such pregnancies was likely to be a small proportion of the sample. We therefore did not adjust findings for multiple pregnancies to take account of possible non‐independence of outcomes for babies from the same pregnancy.
Trials with more than two treatment groups
In this update, trials with more than two treatment groups contributed data into different comparisons and so unit‐of‐analysis errors were not an issue. In future updates, where necessary, we plan to follow the methods as described in the Cochrane Handbook (16.5.4) in order to avoid unit‐of‐analysis errors (combine groups to create a single pair‐wise comparison or select one pair of interventions and exclude others).
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if we include more eligible studies, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect, by using sensitivity analysis.
For all outcomes, we conducted analyses as far as possible on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if I2 was greater than 30% and either Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis for important outcomes we investigated reporting biases (such as publication bias) using funnel plots. We assessed the funnel plots' asymmetry visually. Where asymmetry was suggested by a visual assessment, we reported this in the Results. We may perform exploratory analyses to investigate the asymmetry in future updates. Funnel plots are displayed for GRADE outcomes.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we found substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We considered the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, we present the results as the average treatment effect with a 95% confidence interval, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
We planned to investigate substantial heterogeneity using subgroup analyses.
For the primary outcomes, where data were available, we planned the following subgroup analyses:
Spontaneous labour versus induced labour.
Primigravida versus multiparous.
Term versus preterm birth.
Continuous support in labour versus no continuous support.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and to report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 value. We did not carry out planned subgroup analyses because a complete breakdown of the separate subgroup categories was rarely provided.
We conducted a post hoc subgroup analysis of trials conducted after 2005 for the outcome of assisted vaginal birth for the main comparison of epidural versus opioids, in response to peer referee comments.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of risks of bias assessed by concealment of allocation, high attrition rates, or both, with studies of high or unclear risk of bias being excluded from the analyses in order to assess whether this made any difference to the overall result. We conducted this sensitivity analysis, where possible, for the outcomes maternal satisfaction with pain relief; and need for additional means of pain relief.
Results
Description of studies
Results of the search
See: Figure 1.
1.

Study flow diagram.
We retrieved 424 trial reports to assess in the April 2017 search, including two trials (three reports) that were awaiting further classification in the previous version of the review (Moreno 1997; Vavrinkova 2005). We screened out 379 reports and assessed 45 full‐text trial reports. Some trials had more than one report.
We included 16 new trials (in 24 reports) and 13 of these trials contributed data to this update (De Orange 2011; Douma 2011; Freeman 2014; Genc 2015; Ismail 2012; Jaitley 2011; Khadem 2013; Liu 2015; Logtenberg 2017; Sabry 2011; Stocki 2011; Tveit 2012; Xing 2015). Chen 2008b and Jain 2012 did not contribute data. One report was an additional study to a trial that was already included in the review and did not contribute any new data (Evron 2008). We excluded nine studies (in 13 reports) and added an additional report to an already excluded trial. Six trials (in eight reports) are awaiting further classification due to lack of information to enable assessment. We have attempted to contact and are awaiting response from three trial authors (Antipin 2014; Gupta 2016 ; Weissman 2006), but were unable to find contact details for the remaining three (Kamali 2016; Marshalov 2012; Vavrinkova 2005).
We have also excluded Chen 2000, which was not a randomised controlled trial (RCT), and Nafisi 2006 which was a quasi‐RCT, but were both included in the last update.
Overall, this review now includes 52 studies, 12 of which did not contribute data (Camann 1992; Chen 2008a; Chen 2008b; Evron 2007; Jain 2012; Lian 2008; Morris 1994; Rabie 2006; Scavone 2002; Shifman 2007; Sullivan 2002; Witoonpanich 1984). Of these 12, two (Chen 2008b; Jain 2012) did not report any outcomes of this review; four (Camann 1992; Evron 2007; Lian 2008; Shifman 2007) did not report them in a format that could be included in the analysis; there were limited data from only an abstract in five (Chen 2008a; Rabie 2006; Scavone 2002; Sullivan 2002; Witoonpanich 1984), and one study (Morris 1994) reported unclear cross‐over data.
Included studies
Of the 40 RCTs contributing data, 34 compared epidural with opioids, seven trials compared epidural with no analgesia, one trial compared epidural with acu‐stimulation, one trial compared epidural with inhaled analgesia, and one trial compared epidural with continuous midwifery support and other analgesia. Three trials (Jaitley 2011; Liu 2015; Long 2003) included multiple intervention arms and contributed data to epidural compared with opioids, and epidural compared with no analgesia. Liu 2015 also contributed data to epidural compared with acu‐stimulation.
The trials that did not contribute data compared epidural with: no analgesia (four trials) (Chen 2008a; Chen 2008b; Lian 2008; Shifman 2007); intravenous (IV) sufentanil (one trial) (Camann 1992); intramuscular (IM) tramadol (one trial) (Jain 2012); PCA pethidine (one trial) (Evron 2007); IV and IM hydromorphone (two trials) (Scavone 2002; Sullivan 2002); IV fentanyl (one trial) (Morris 1994); PCA remifentanil (one trial) (Rabie 2006); and IM pethidine or pentazocine (one trial) (Witoonpanich 1984).
Settings
All trials were conducted in a hospital setting.
Trials comparing epidural and opioid analgesia took place in the USA (5167 women) (Bofill 1997; Clark 1998; Gambling 1998; Head 2002; Hogg 2000; Lucas 2001; Ramin 1995; Sharma 1997; Sharma 2002; Thorp 1993), the Netherlands (1948 women) (Douma 2011; Freeman 2014; Logtenberg 2017), Egypt (90 women) (El‐Kerdawy 2010; Sabry 2011), Israel (313 women) (Evron 2008; Stocki 2011), France (90 women) (Grandjean 1979), Canada (477 women) (Halpern 2004; Muir 1996; Muir 2000), United Kingdom (985 women) (Howell 2001; Loughnan 2000), Kuwait (1140 women) (Ismail 2012), India (216 women) (Jain 2003; Jaitley 2011), Malaysia (192 women) (Jalil 2009), China (200 women) (Liu 2015; Long 2003), Denmark (112 women) (Philipsen 1989), Sweden (28 women) (Thalme 1974), Norway (39 women) (Tveit 2012), and Finland (72 women) (Nikkola 1997; Volmanen 2008).
Eleven trials took place between 1990 and 2000: Bofill 1997 1995 ‐ 1996; Clark 1998 1995 ‐ 1996; Gambling 1998 1994 ‐ 1995; Halpern 2004 1997 ‐ 1999; Howell 2001 1992 ‐ 1997; Loughnan 2000 1992 ‐ 1995; Lucas 2001 1996 ‐ 1998; Ramin 1995 1993 ‐ 1994; Sharma 1997 1995 ‐ 1996; Sharma 2002 1998 ‐ 2000; Thorp 1993 1990 ‐ 1992. Six trials took place between 2000 and 2010: Douma 2011 2008 ‐ 2010; Evron 2008 2003; Jalil 2009 2005 ‐ 2006; Sabry 2011 2008 ‐ 2009; Stocki 2011 2010. Ismail 2012 was conducted between 2009 and 2011. Three trials took place between 2010 ‐ 2013: Freeman 2014 2011 ‐ 2012; Liu 2015 2010 ‐ 2013; Logtenberg 2017 2012 ‐ 2013. Dates were not stated in 14 trials (El‐Kerdawy 2010; Grandjean 1979; Head 2002; Hogg 2000; Jain 2003; Jaitley 2011; Long 2003; Muir 1996; Muir 2000; Nikkola 1997; Philipsen 1989; Thalme 1974; Tveit 2012; Volmanen 2008).
Trials comparing epidural and placebo or no treatment took place in China (508 women) (Liu 2015; Long 2003; Xing 2015), and one was set in Brazil (70 women) (De Orange 2011), Turkey (100 women) (Genc 2015), India (90 women) (Jaitley 2011), and Mexico (129 women) (Morgan‐Ortiz 1999). One study took place from 1997 to 1998 (Morgan‐Ortiz 1999), three studies were all conducted between 2010 and 2014: De Orange 2011 2010; Genc 2015 2012 ‐ 2014; Liu 2015 2010 – 2013; Xing 2015 2013 ‐ 2014, and two studies did not specify the study dates (Jaitley 2011; Long 2003).
One trial Liu 2015 (2010 ‐ 2013) conducted in China also compared epidural and acu‐stimulation.
One trial (Khadem 2013), comparing epidural and inhaled analgesia, took place in Iran between 2010 and 2011.
One trial (Dickinson 2002), comparing epidural and continuous support with other analgesia, was conducted in Australia between 1997 and 1999.
Funding and declarations of interest
The majority of trials did not state funding sources. Four were funded by the hospitals in which the trials took place: Bofill 1997 was by the Vicksburg Hospital Medical Foundation; Douma 2011 the Department of Anesthesiology, Leiden University Medical Centre; Sharma 2002 was solely from institutional or departmental sources of their host hospital; Tveit 2012 was by the Sorlandet Hospital HF, Sorlandets Kompetansefond and Helse Sor‐Ost, Norway. Most were funded by medical research grants, foundations, or combinations of the two: Dickinson 2002 was funded by National Health and Medical Research Council Grant 970076, Australia; Evron 2008 was supported by National Institute for Health Grant GM 061655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY); Freeman 2014 grant from ZonMW (Dutch Organization for Health Care Research and Development); Halpern 2004 was supported by Physicians Services Incorporated Foundation, Toronto; Alberta Heritage Fund; Clinical Teaching and Research Grant, College of Medicine, University of Saskatchewan; Medical Services Incorporated of Alberta; Grace Maternity Research Foundation Grant; and Dalhousie University Department of Anaesthesia; Howell 2001 was funded by WellBeing, a grant from the North Staffordshire Medical Institute, and a grant from the NHS(E) West Midlands Research and Development Programme; Jalil 2009 was funded by a short‐term grant from the Universiti Sains Malaysia; Khadem 2013 by the Women’s Health Research Center of Mashhad University of Medical Sciences; Loughnan 2000 by the National Health Service Executive, North Thames; Nikkola 1997 was supported by funds from Instrumentarium Research Foundation, Finland and funds from Turku University Hospital, Finland; Stocki 2011 this study was supported by a research grant for Anesthesiologists from the Hadassah Hebrew University Medical Center, Jerusalem; Thalme 1974 supported by a grant from the Swedish Medical Research Council; and Xing 2015 was supported by the Scientific and Technological Key Project of Nanning City (no. 20133189). Sabry 2011 was self‐funded.
De Orange 2011, Douma 2011, Evron 2008, Freeman 2014, Genc 2015, Ismail 2012, Sabry 2011, Xing 2015 all stated they had no conflicts of interest to declare. Stocki 2011 reported that two authors received money for travel to conference to present the paper from Oridion®, a company who had provided equipment for their trial. No other trials stated whether or not they had conflicts to declare.
Participants
Twenty of the 40 trials recruited primiparous women (Bofill 1997; Clark 1998; Dickinson 2002; El‐Kerdawy 2010; Genc 2015; Halpern 2004; Howell 2001; Ismail 2012; Jain 2003; Khadem 2013; Loughnan 2000; Morgan‐Ortiz 1999; Muir 1996; Muir 2000; Nikkola 1997; Sabry 2011; Sharma 2002; Thalme 1974; Thorp 1993; Xing 2015); three stated that they recruited multiparous women (Grandjean 1979; Jalil 2009; Stocki 2011); five recruited both primiparous and multiparous women (Gambling 1998; Jaitley 2011; Lucas 2001; Philipsen 1989; Sharma 1997); and parity was not reported in the remaining 12 trials. Most of the trials included women at more than 36 weeks' gestation in spontaneous labour with no obstetric or medical complications. Exceptions were Dickinson 2002 and Loughnan 2000, who included women in both spontaneous and induced labours; Lucas 2001, who recruited only women with pregnancy‐induced hypertension in both spontaneous and induced labours; Freeman 2014 and Logtenberg 2017, who recruited from 32 weeks' gestation; and Head 2002, Hogg 2000 and El‐Kerdawy 2010, who included only women with pre‐eclampsia at more than 24 weeks' gestation in labour.
Interventions and comparisons
Epidural analgesia compared with opioid analgesia (34 trials involving 10,440 women)
Epidural techniques and drugs varied between the trials. Ten trials administered a fluid preload (Bofill 1997; Clark 1998; Gambling 1998; Head 2002; Jalil 2009; Lucas 2001; Philipsen 1989; Sharma 1997; Sharma 2002; Thalme 1974). Bupivacaine or levobupivacaine was used for the epidural analgesia in most of the trials when reported. Exceptions were Grandjean 1979, which used lignocaine, and Long 2003 using ropivacaine. In Evron 2008 epidural analgesia was given with ropivacaine, with or without a combination of IV remifentanil or acetaminophen. The agents used in the epidural were not mentioned in two trials (Freeman 2014; Hogg 2000). Bupivacaine was supplemented with fentanyl in nine of the trials (El‐Kerdawy 2010; Gambling 1998; Halpern 2004; Head 2002; Jain 2003; Lucas 2001; Sharma 1997; Sharma 2002; Volmanen 2008), with pethidine in one (Muir 1996), and with tramadol in another (Jaitley 2011). Levobupivacaine was supplemented with fentanyl in one trial (Ismail 2012), in a continuous infusion. Continuous infusion was reported in another 12 studies (Bofill 1997; El‐Kerdawy 2010; Gambling 1998; Head 2002; Jain 2003; Jalil 2009; Logtenberg 2017; Lucas 2001; Ramin 1995, Sharma 1997; Sharma 2002; Tveit 2012). In all these trials, except for Jalil 2009, Logtenberg 2017, and Tveit 2012, a bolus of 0.25% of bupivacaine was used followed by infusion of 0.0125 % to maintain epidural analgesia. Jalil 2009, Logtenberg 2017, and Tveit 2012 used a bolus dose of 0.2% ropivacaine, followed by continuous epidural infusion of 0.2% ropivacaine either with fentanyl (Jalil 2009; Tveit 2012), or sufentanil (Logtenberg 2017). Two trials used a much higher concentration of bupivacaine: Philipsen 1989 used 0.375% bupivacaine and Nikkola 1997 used 0.5%. Patient‐controlled epidural analgesia (PCEA) was used in seven trials (Evron 2008; Halpern 2004; Liu 2015; Long 2003; Muir 1996; Sharma 2002; Stocki 2011). Only four of the trials (Gambling 1998; Ismail 2012; Long 2003; Sabry 2011) used combined‐spinal epidural. In Gambling 1998 spinal block was achieved with sufentanil alone and epidural infusion was started immediately following the intrathecal administration of the opoid, whereas the spinal block in Long 2003 was achieved with ropivacaine supplemented with fentanyl and epidural analgesia was given only after dissipation of the spinal analgesia. Levobupivacaine and fentanyl (total volume of 2 mL) were injected intrathecally and the spinal needle was removed in Ismail 2012. Sabry 2011 was a multi‐armed trial with four epidural arms: two arms with combined spinal epidural, and two epidural arms; and the analgesia in each arm was either bupivacaine and fentanyl, or lidocaine and fentanyl. For this review, we combined the four arms. Epidural use was discontinued in the second stage of labour in three studies (Loughnan 2000; Nikkola 1997; Philipsen 1989).
Opioids compared included: pethidine (16 trials, 6494 women) (Clark 1998; Gambling 1998; Head 2002; Hogg 2000; Howell 2001; Jalil 2009; Loughnan 2000; Lucas 2001; Muir 1996; Philipsen 1989; Ramin 1995; Sabry 2011; Sharma 1997; Sharma 2002; Thalme 1974; Thorp 1993); butorphanol (one trial, 100 women) (Bofill 1997); fentanyl (three trials, 447 women) (Halpern 2004; Muir 2000; Nikkola 1997); remifentanil (nine trials, 3462 women) (Douma 2011; El‐Kerdawy 2010; Evron 2008; Freeman 2014; Ismail 2012; Logtenberg 2017; Stocki 2011; Tveit 2012; Volmanen 2008); phenoperidine (one trial, 90 women) (Grandjean 1979); tramadol (one trial, 90 women) (Jaitley 2011); pethidine and tramadol (one trial, 126 women) (Jain 2003); pethidine or no analgesia (one trial, 80 women) (Long 2003); and ondansetron, or acu‐stimulation, or no analgesia (one trial, 120 women) (Liu 2015). Opioids were administered as patient‐controlled intravenous analgesia (PCIA) (19 trials) (Douma 2011; El‐Kerdawy 2010; Freeman 2014; Halpern 2004; Head 2002; Hogg 2000; Ismail 2012; Liu 2015; Logtenberg 2017; Long 2003; Lucas 2001; Muir 1996; Muir 2000; Nikkola 1997; Sharma 1997; Sharma 2002; Stocki 2011; Tveit 2012; Volmanen 2008), IV injection (9 trials) (Bofill 1997; Clark 1998; Evron 2008; Gambling 1998; Grandjean 1979; Jaitley 2011; Ramin 1995; Sabry 2011; Thorp 1993), and IM injection (five trials) (Howell 2001; Jain 2003; Jalil 2009; Loughnan 2000; Philipsen 1989). The route of administration was unclear in one trial (Thalme 1974).
Epidural analgesia compared with no analgesia or placebo (seven trials involving 897 women)
All seven of these trials used bupivacaine or ropivacaine for the epidural analgesia. Ropivacaine was supplemented with sufentanil in one trial (Liu 2015). Bupivacaine was supplemented with fentanyl bolus injections in Genc 2015, and with tramadol in Jaitley 2011. Morgan‐Ortiz 1999 used bupivacaine but gave no further information about the epidural. PCEA was used in two trials (Liu 2015; Long 2003). Long 2003 also used combined‐spinal‐epidural, along with De Orange 2011 and Xing 2015. In De Orange 2011 spinal block was achieved with bupivacaine and sufentanil and epidural infusion was started immediately following the intrathecal administration, whereas the spinal block in Long 2003 was achieved with ropivacaine supplemented with fentanyl and epidural analgesia was given only after dissipation of the spinal analgesia. Xing 2015 injected sufentanil intrathecally until the visual analogue scale (VAS) was three or higher, when a continuous infusion of ropivacaine and sufentanil began.
Comparison groups were not well described in two trials (190 women: Genc 2015; Jaitley 2011), although none of the women in the comparison groups had epidural analgesia. It appears that women in the control group of Jaitley 2011 did not receive pain relief; It is unclear if these women were able to request analgesia or if they were restricted to no analgesia. Women in the control groups of Liu 2015, Long 2003, Morgan‐Ortiz 1999 and Xing 2015 had no analgesia (four trials, 637 women). Both epidural and control group in De Orange 2011 were given continuous support during delivery by a doula or trained lay person, and had access to Swiss exercise balls, massage, and music therapy (one trial, 70 women).
Epidural analgesia compared with acu‐stimulation (one trial involving 60 women)
Liu 2015 used ropivacaine supplemented with sufentanil in PCEA compared with acu‐stimulation. The women in the acu‐stimulation group received pulse stimulus at acupoints – Jiaji points (T 10‐L 3) and Ciliao (BL 32). Stimulation was delivered at 100 Hz with burst frequency 2 Hz, intensity 15‐30 mA, for a duration of 30 minutes.
Epidural analgesia compared with inhaled analgesia (one trial involving 86 women)
One trial (Khadem 2013), compared epidural with inhaled nitrous oxide. Following a fluid preload, bupivacaine with fentanyl was given to women at 5 cm dilatation followed by an increase in bupivacaine concentration if required. Women in the nitrous oxide group, inhaled the gas with a mask throughout each contraction and breathed room air between the contractions. Two women were excluded because of "giddiness" due to the nitrous oxide.
Epidural analgesia compared with continuous care (one trial involving 992 women)
One trial (Dickinson 2002), compared combined‐spinal‐epidural with fentanyl and bupivacaine in nulliparous women. A fluid preload was given to the women in the epidural group. Following onset of analgesia, the women controlled the epidural until the birth with bupivacaine and pethidine. The comparison group received one‐to‐one continuous midwifery support along with usual analgesia choices such as IM pethidine, nitrous oxide inhalation, TENS, and/or non‐pharmacological forms of pain relief as requested.
Outcomes
The following primary outcomes were reported in the included trials: pain intensity (12 trials); maternal satisfaction with pain relief (17 trials); sense of control in labour (two trials); satisfaction with the childbirth experience (one trial); need for additional means of pain relief (19 trials); breastfeeding (one trial); assisted vaginal birth (34 trials); caesarean section (38 trials); Side effects for mother: long‐term backache (three trials); maternal hypotension (10 trials); postnatal depression (one trial); motor blockade (three trials); respiratory depression requiring oxygen (five trials); headache (five trials); perineal trauma requiring suturing (two trials); nausea and vomiting (17 trials); itching (eight trials); fever (10 trials); shivering (two trials); drowsiness (seven trials); urinary retention (five trials); catheterisation (two trials); malposition (four trials); surgical amniotomy (two trials); Side effects for baby: acidosis arterial pH less than 7.2 (eight trials); acidosis arterial pH less than 7.15 (three trials); naloxone administration (10 studies); meconium staining (five trials); admission to special care baby unit (eight trials); Apgar score of less than seven at five minutes (23 trials).
No trial reported on the following primary outcomes: uterine rupture, headache requiring blood patch, venous thromboembolic events,other maternal morbidity (e.g. impaired consciousness, meningitis, intensive care unit admission, paralysis; effect (negative) on mother/baby interaction; neonatal hypoglycaemia, birth trauma, long‐term neonatal complication, and cost.
The following secondary outcomes were reported in the meta‐analysis: length of first stage of labour (10 studies); length of second stage of labour (18 studies); oxytocin augmentation (22 trials); caesarean section for fetal distress (13 trials); and caesarean section for dystocia (14 trials).
SeeCharacteristics of included studies for details of the individual trials.
Excluded studies
We excluded 38 studies (52 publications) for the following reasons.
Not RCT or inadequate randomisation (Anwar 2015; Buchan 1973; Chen 2000; Cutura 2011; Jouppila 1976; Jouppila 1980; Leong 2000; Moreno 1997; Noble 1971; Ryhanen 1984; Solek‐Pastuszka 2009; Stourac 2014; Tugrul 2006; Wassen 2015)
All women received epidural and interventions did not satisfy review's inclusion criteria (Abboud 1982; Ginosar 2002; Ginosar 2003; Gupta 2013; Hood 1993; John 2013; Justins 1983; Kujansuu 1987; Lassner 1981; MacKenzie 1996; Martin 2003; McGrath 1992; Polley 2000; Wong 2005; Wong 2009)
Interventions did not satisfy review's inclusion criteria (Manninen 2000)
Quasi‐randomised trials (Kurjak 1974; Nafisi 2006; Neri 1986; Swanström 1981)
Intervention was post‐caesarean not in labour (Zakowski 1994)
High exclusion rate from analysis (Revill 1979 (28%); Robinson 1980 (30%); Intention‐to‐treat analysis not used (Robinson 1997)
SeeCharacteristics of excluded studies for details of the individual studies.
Risk of bias in included studies
See Figure 2; Figure 3 for 'Risk of bias' graph and 'Risk of bias' summary figures.
2.
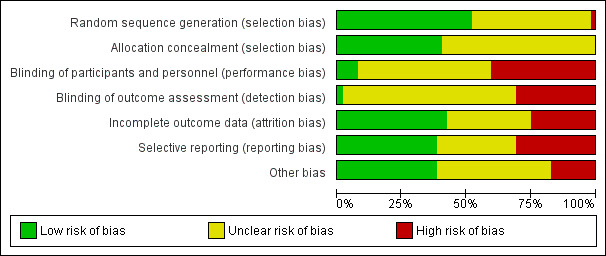
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
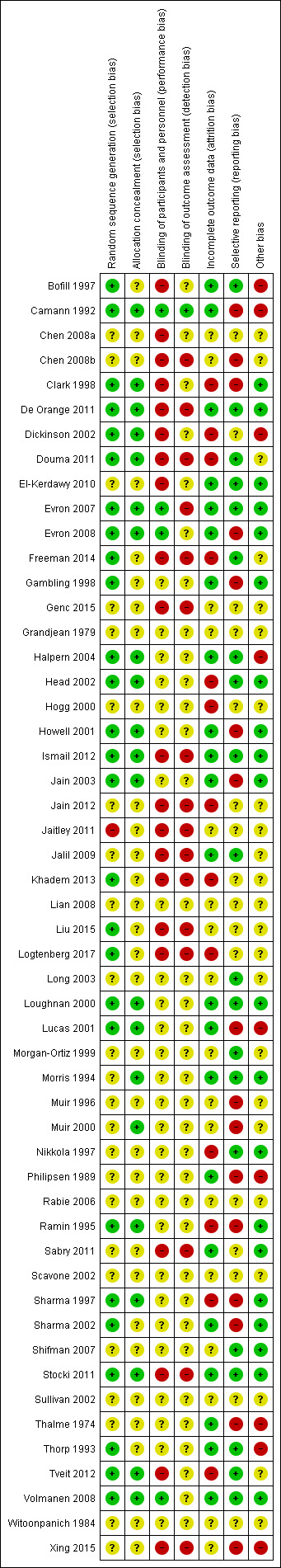
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All included studies stated that women were randomly allocated to epidural analgesia and control groups. Out of the 40 trials contributing data, information about generation of the randomisation sequence was clearly described in 25 studies (low risk). Of these, 20 trials used computerised randomisation (Bofill 1997; Clark 1998; De Orange 2011; Douma 2011; Evron 2008; Freeman 2014; Gambling 1998; Halpern 2004; Head 2002; Howell 2001; Ismail 2012; Logtenberg 2017; Loughnan 2000; Lucas 2001; Ramin 1995; Sharma 1997; Sharma 2002; Thorp 1993; Tveit 2012; Volmanen 2008). Randomisation was achieved with random number tables in two studies (Jain 2003; Liu 2015); using a blocked group in one study (Dickinson 2002); using random numbers generated by a calculator in one study (Khadem 2013); and shuffling cards in groups of eight in another study (Stocki 2011). We assessed the randomisation sequence as being at high risk of bias in one study; Jaitley 2011 reported using randomisation but also stated that the intervention group was subdivided with no reference to randomisation method. Sequence generation was not described clearly in the remaining 14 studies which we assessed to be at unclear risk of bias (El‐Kerdawy 2010; Genc 2015; Grandjean 1979; Hogg 2000; Jalil 2009; Long 2003; Morgan‐Ortiz 1999; Muir 1996; Muir 2000; Nikkola 1997; Philipsen 1989; Sabry 2011; Thalme 1974; Xing 2015).
Allocation concealment
We assessed allocation concealment as being at low risk of bias in 19 of the 40 trials and described as using "sequentially numbered sealed opaque envelopes" or "sealed opaque envelopes" (Clark 1998; De Orange 2011; Dickinson 2002; Douma 2011; Evron 2008; Halpern 2004; Head 2002, Howell 2001; Ismail 2012; Jain 2003; Logtenberg 2017; Loughnan 2000; Lucas 2001; Muir 2000; Ramin 1995; Sharma 1997; Stocki 2011; Tveit 2012; Volmanen 2008). In the remaining 21 trials the methods used to conceal allocation were not described or the methods were not clear (Bofill 1997; El‐Kerdawy 2010; Freeman 2014; Gambling 1998; Genc 2015; Grandjean 1979; Hogg 2000; Jaitley 2011; Jalil 2009; Khadem 2013; Liu 2015; Long 2003; Morgan‐Ortiz 1999; Muir 1996; Nikkola 1997; Philipsen 1989; Sabry 2011; Sharma 2002; Thalme 1974; Thorp 1993; Xing 2015).
Blinding
Participants and personnel
We rated two studies at low risk of performance bias: Evron 2008 used a PCIA syringe filled with a saline infusion; and Volmanen 2008 reported that both women and staff were blinded as to which medication was administered. We have noted where there had been any attempt to blind study participants, caregivers or outcome assessors to group allocation. With a complex intervention such as an epidural analgesia, it is often not feasible to blind women or staff to group assignment; 18 trials did not blind women or staff (Bofill 1997; Clark 1998; De Orange 2011; Dickinson 2002; Douma 2011; El‐Kerdawy 2010; Freeman 2014; Genc 2015; Ismail 2012; Jaitley 2011; Jalil 2009; Khadem 2013; Liu 2015; Logtenberg 2017; Sabry 2011; Stocki 2011; Tveit 2012; Xing 2015), and blinding was not clear in the remaining 20 trials.
Outcome assessment
Outcome assessors were not blinded in 13 trials (De Orange 2011; Douma 2011; Freeman 2014; Genc 2015; Ismail 2012; Jaitley 2011; Jalil 2009; Khadem 2013; Liu 2015; Logtenberg 2017; Sabry 2011; Stocki 2011; Xing 2015), and it was unclear whether the remaining 27 trials attempted to blind outcome assessors.
Incomplete outcome data
Intention‐to‐treat analysis was used in all included trials for outcome data extracted. Nineteen trials had low or no loss to follow‐up and we assessed them as being at low risk of attrition bias (Bofill 1997; De Orange 2011; El‐Kerdawy 2010; Evron 2008; Gambling 1998; Halpern 2004; Howell 2001; Ismail 2012; Jain 2003; Jalil 2009; Loughnan 2000; Lucas 2001; Philipsen 1989; Sabry 2011; Sharma 2002; Stocki 2011; Thalme 1974; Thorp 1993; Volmanen 2008). Loss to follow‐up was present in 12 high‐risk studies due to high numbers of women not receiving the allocated intervention (Clark 1998; Dickinson 2002; Head 2002; Hogg 2000; Nikkola 1997; Ramin 1995; Sharma 1997), or for reasons which are not adequately explained (Douma 2011; Freeman 2014; Khadem 2013; Logtenberg 2017; Tveit 2012). Small numbers of exclusions and inadequate reporting of loss to follow‐up was observed in the remaining trials (Genc 2015; Grandjean 1979; Jaitley 2011; Liu 2015; Long 2003; Morgan‐Ortiz 1999; Muir 1996; Muir 2000; Xing 2015).
Selective reporting
For 17 of the trials, all prespecified outcomes from the methods section were reported within the results (low risk) (Bofill 1997; De Orange 2011; Douma 2011; El‐Kerdawy 2010; Freeman 2014; Halpern 2004; Head 2002; Ismail 2012; Jalil 2009; Long 2003; Loughnan 2000; Morgan‐Ortiz 1999; Nikkola 1997; Stocki 2011; Thorp 1993; Tveit 2012; Volmanen 2008). Fourteen of the studies either failed to report on outcomes which were prespecified within the Methods section or the reported outcomes were incomplete such that data could not be analysed (high risk) (Clark 1998; Evron 2008; Gambling 1998; Howell 2001; Jain 2003; Lucas 2001; Muir 1996; Muir 2000; Philipsen 1989; Ramin 1995; Sharma 1997; Sharma 2002; Thalme 1974; Xing 2015). The remaining nine studies provided insufficient information to make a judgement on selective reporting bias and we judged them to be at unclear risk of bias (Dickinson 2002; Genc 2015; Grandjean 1979; Hogg 2000; Jaitley 2011; Khadem 2013; Liu 2015; Logtenberg 2017; Sabry 2011).
Other potential sources of bias
Other potential sources of bias included imbalanced groups (Bofill 1997; Lucas 2001), trials stopping early before required sample size were recruited (Halpern 2004; Thorp 1993), high cross‐over rates (Dickinson 2002; Philipsen 1989), failure to report on assisted vaginal births for longer second stage of labour (Thalme 1974), and general poor reporting (Xing 2015). No other potential sources of bias were evident in 17 of the trials (Clark 1998; De Orange 2011; El‐Kerdawy 2010; Evron 2008; Gambling 1998; Head 2002; Howell 2001; Ismail 2012; Jain 2003; Loughnan 2000; Nikkola 1997; Ramin 1995; Sabry 2011; Sharma 1997; Sharma 2002; Stocki 2011; Volmanen 2008), and there was insufficient information in the remaining 15 trials (Douma 2011; Freeman 2014; Genc 2015; Grandjean 1979; Hogg 2000; Jaitley 2011; Jalil 2009; Khadem 2013; Liu 2015; Logtenberg 2017; Long 2003; Morgan‐Ortiz 1999; Muir 1996; Muir 2000; Tveit 2012).
Effects of interventions
See: Table 1
1. Epidural versus opioids (34 trials involving 10,440 women)
Primary outcomes
Effects of interventions
Pain intensity (as defined by trialists)
Different tools including visual analogue scores (VAS) were used to measure pain intensity, ranging from 0 to 10 and 0 to 100. For all the comparisons in general a lower pain score represented less pain intensity.
Lower pain scores were reported in the epidural group than in the opioids group (standardised mean difference (SMD) ‐2.64, 95% confidence interval (CI) ‐4.56 to ‐0.73; random‐effects; 1133 women; studies = 5; I2 = 98%; Analysis 1.1; low‐quality evidence) but heterogeneity was very high for this outcome and different VAS used may have contributed to heterogeneity.
1.1. Analysis.
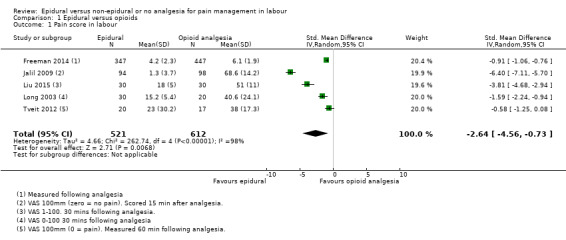
Comparison 1 Epidural versus opioids, Outcome 1 Pain score in labour.
Satisfaction with pain relief (as defined by trialists)
There was high heterogeneity in all outcomes relating to maternal satisfaction which included more than one study in the meta‐analysis, so we used random‐effects analysis throughout, and the results should be interpreted with caution. Women's satisfaction with pain relief in labour favoured epidural (lower score = more satisfied) (mean difference (MD) ‐3.36 VAS score, 95% CI ‐5.41 to ‐1.31; random‐effects; 1166 women; studies = 3; I2 = 98%; Tau2 = 3.14; Chi2 = 117.61; P < 0.00001; Analysis 1.3). Epidural was favoured in perception of pain relief in both first (MD ‐12.05 VAS score, 95% CI ‐19.35 to ‐4.75; random‐effects; 194 women; studies = 3; I2 = 68%; Tau2 = 27.96; Chi2 = 6.23; P = 0.04; Analysis 1.4), and second stages of labour (MD ‐20.75 VAS score, 95% CI ‐22.50 to ‐19.01; 164 women; studies = 2; I2 = 26%; Analysis 1.5) (lower VAS score = lower perception of pain). More women (707/931 compared to 490/980) in the epidural group rated their pain relief as excellent or very good (average risk ratio (RR) 1.47, 95% CI 1.03 to 2.08; 1911 women; studies = 7; I2 = 97%; Tau2 = 0.19; Chi2 = 201.68; P < 0.00001; Analysis 1.6; low‐quality evidence), and reported higher satisfaction scores with pain relief than those receiving opioids (SMD 0.51, 95% CI 0.10 to 0.91; random‐effects; 3171 women; studies = 7; I2 = 95%; Tau2 = 0.26; Chi2 = 132.17; P < 0.00001; Analysis 1.7). Heterogeneity was high for this outcome, with large differences between size and direction of effect. One study measured the time (minutes) from administration to when the women reported satisfaction with the pain relief, which was less in the epidural group (MD ‐6.70 minutes, 95% CI ‐8.02 to ‐5.38; 82 women; Analysis 1.8).
1.3. Analysis.
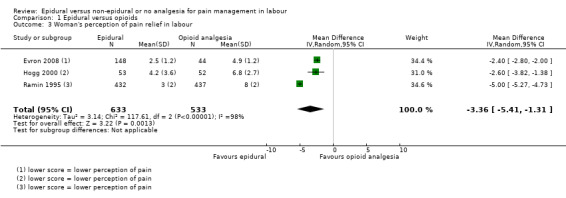
Comparison 1 Epidural versus opioids, Outcome 3 Woman's perception of pain relief in labour.
1.4. Analysis.
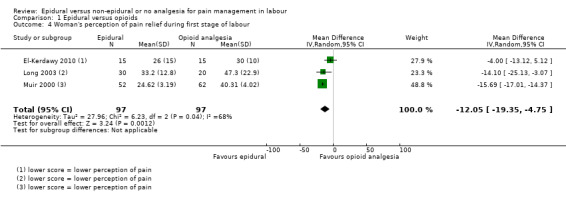
Comparison 1 Epidural versus opioids, Outcome 4 Woman's perception of pain relief during first stage of labour.
1.5. Analysis.

Comparison 1 Epidural versus opioids, Outcome 5 Woman's perception of pain relief during the second stage of labour.
1.6. Analysis.
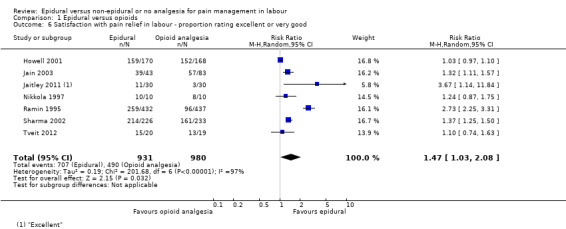
Comparison 1 Epidural versus opioids, Outcome 6 Satisfaction with pain relief in labour ‐ proportion rating excellent or very good.
1.7. Analysis.
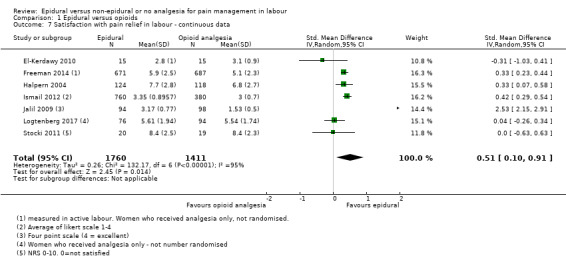
Comparison 1 Epidural versus opioids, Outcome 7 Satisfaction with pain relief in labour ‐ continuous data.
1.8. Analysis.
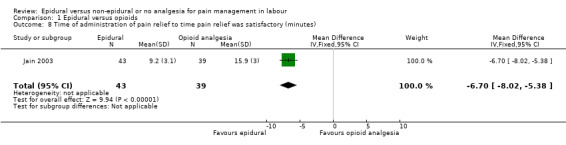
Comparison 1 Epidural versus opioids, Outcome 8 Time of administration of pain relief to time pain relief was satisfactory (minutes).
Sense of control in labour (as defined by trialists)
There was no clear difference between the groups for women reporting poor control in labour (RR 1.17, 95% CI 0.62 to 2.21; 344 women; studies = 1; Analysis 1.9).
1.9. Analysis.
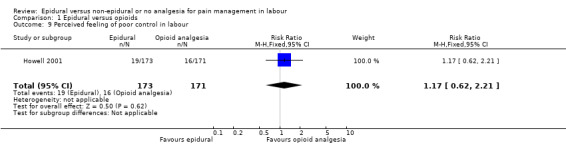
Comparison 1 Epidural versus opioids, Outcome 9 Perceived feeling of poor control in labour.
Satisfaction with childbirth experience (as defined by trialists)
There was no clear difference between the groups for women reporting satisfaction with the childbirth experience (proportions rating satisfied or very satisfied) (RR 0.95, 95% CI 0.87 to 1.03; 332 women; studies = 1; Analysis 1.10).
1.10. Analysis.
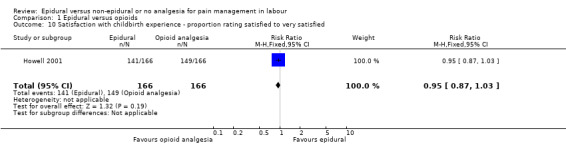
Comparison 1 Epidural versus opioids, Outcome 10 Satisfaction with childbirth experience ‐ proportion rating satisfied to very satisfied.
Need for other means of pain relief
Fewer women in the epidural group required additional analgesia (average RR 0.10, 95% CI 0.04 to 0.25; 5099 women; studies = 16; I2 = 73%; Tau2 = 1.89; Chi2 = 52.07; P < 0.00001; Analysis 1.11). Heterogeneity was high in this outcome; no trial favoured opioids but six reported no clear difference between groups. The funnel plot for this outcome was asymmetrical, suggesting that the effect size was more pronounced in small studies. The three trials that carried slightly more weight in the analysis showed a smaller effect (funnel plot not shown).
1.11. Analysis.
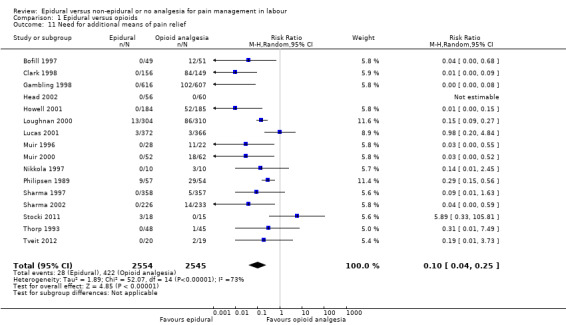
Comparison 1 Epidural versus opioids, Outcome 11 Need for additional means of pain relief.
Safety of interventions
Assisted vaginal birth
The assisted vaginal birth rate was higher in the epidural group (RR 1.44, 95% CI 1.29 to 1.60; 9948 women; studies = 30; Analysis 1.12; low‐quality evidence). The funnel plot for this outcome (Figure 4) suggests some publication bias.
1.12. Analysis.

Comparison 1 Epidural versus opioids, Outcome 12 Assisted vaginal birth.
4.
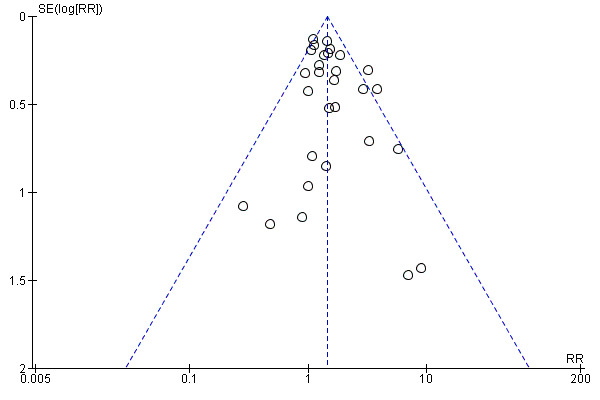
Funnel plot of comparison: 1 Epidural versus opioids, outcome: 1.12 Assisted vaginal birth.
Post hoc subgroup analysis of trials conducted after 2005 showed no effect on assisted vaginal birth between epidural and non‐epidural groups
Caesarean section
The caesarean section rate was no different between the groups (RR 1.07, 95% CI 0.96 to 1.18; 10,350 women; studies = 33; Analysis 1.13; moderate‐quality evidence). The funnel plot for this outcome (Figure 5) appears to be symmetrical.
1.13. Analysis.
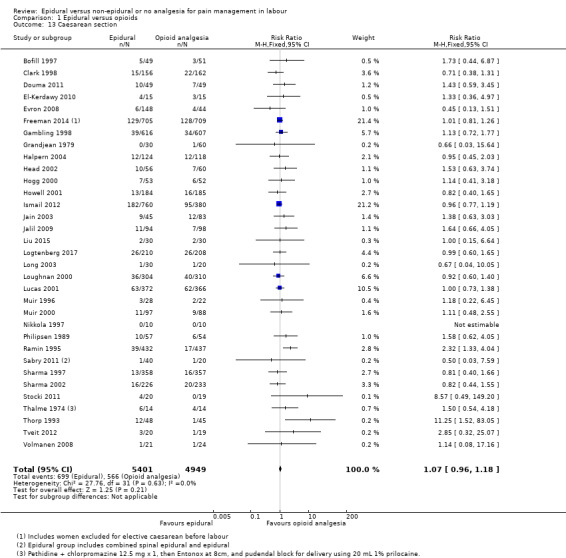
Comparison 1 Epidural versus opioids, Outcome 13 Caesarean section.
5.

Funnel plot of comparison: 1 Epidural versus opioids, outcome: 1.13 Caesarean section.
Effect (negative) on mother/baby interaction and Breastfeeding (at specified time points) were not reported by any study under this comparison.
Side effects (for mother)
Long‐term backache (as defined by trial authors)
There was no clear difference between the groups for women reporting long‐term backache (RR 1.00, 95% CI 0.89 to 1.12; 814 women; studies = 2; Analysis 1.14; moderate‐quality evidence).
1.14. Analysis.
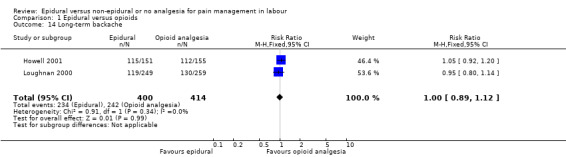
Comparison 1 Epidural versus opioids, Outcome 14 Long‐term backache.
Maternal hypotension (as defined by authors)
More women experienced hypotension in the epidural group than in the opioid group (average RR 11.34, 95% CI 1.89 to 67.95; 4212 women; studies = 10; I2 = 87%; Tau2 = 6.64; Chi² = 66.89; P < 0.00001; Analysis 1.15), but heterogeneity was high for this outcome. The funnel plot was difficult to interpret because there were only 10 trials contributing data, but there does appear to be some asymmetry suggesting possible publication bias (funnel plot not shown).
1.15. Analysis.
Comparison 1 Epidural versus opioids, Outcome 15 Hypotension as defined by trial authors.
Postnatal depression (authors' definition, treatment for depression or self‐reported)
There was no clear difference between the groups for women who developed postnatal depression (RR 0.63, 95% CI 0.38 to 1.05; 313 women; studies = 1; Analysis 1.16).
1.16. Analysis.

Comparison 1 Epidural versus opioids, Outcome 16 Postnatal depression (authors definition, on medication, or self‐reported).
Motor blockade
Twenty‐three out of 125 women in the epidural group experienced a motor blockade (RR 31.71, 95% CI 4.16 to 241.99; 322 women; studies = 3; Analysis 1.17).
1.17. Analysis.
Comparison 1 Epidural versus opioids, Outcome 17 Motor blockade.
Respiratory depression requiring oxygen administration
Fewer women in the epidural group experienced respiratory depression requiring oxygen administration (average RR 0.23, 95% CI 0.05 to 0.97; 2031 women; studies = 5; I2 = 42%; Tau2 = 0.81; Chi2 = 3.48; P = 0.18; Analysis 1.18). Statistical heterogeneity was present for this outcome.
1.18. Analysis.
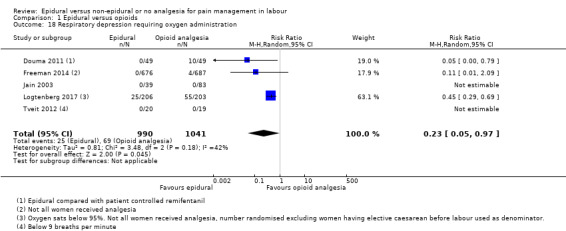
Comparison 1 Epidural versus opioids, Outcome 18 Respiratory depression requiring oxygen administration.
Headache
There was no clear difference between the groups on incidence of headaches (RR 1.06, 95% CI 0.74 to 1.54; 1938 women; studies = 4; Analysis 1.19).
1.19. Analysis.

Comparison 1 Epidural versus opioids, Outcome 19 Headache.
Perineal trauma requiring suturing
There was no clear difference between the groups for women who had perineal tears that required suturing (RR 1.05, 95% CI 0.93 to 1.18; 369 women; studies = 1; Analysis 1.20).
1.20. Analysis.
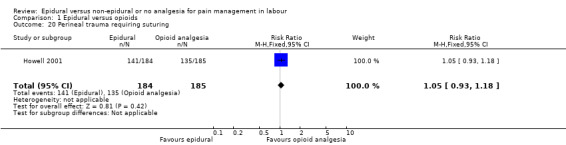
Comparison 1 Epidural versus opioids, Outcome 20 Perineal trauma requiring suturing.
Nausea or vomiting or both
Fewer women in the epidural group experienced nausea and vomiting (average RR 0.62, 95% CI 0.45 to 0.87; 4440 women; studies = 15; I2 = 70%; Tau2 = 0.24; Chi2 = 46.51, df = 14; P < 0.0001; Analysis 1.21), although heterogeneity was high for this outcome and the results should be interpreted with caution. The funnel plot does not appear to be symmetrical, which suggests that publication bias could be present (funnel plot not shown).
1.21. Analysis.
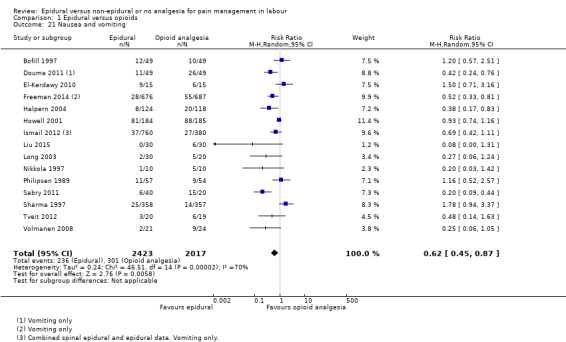
Comparison 1 Epidural versus opioids, Outcome 21 Nausea and vomiting.
Itching
There was no clear difference between the groups for women who reported itching (average RR 1.19, 95% CI 0.81 to 1.77; 2900 women; studies = 8; Analysis 1.22).
1.22. Analysis.
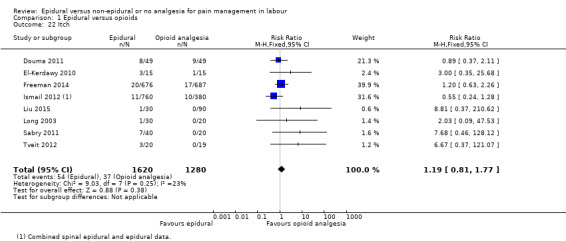
Comparison 1 Epidural versus opioids, Outcome 22 Itch.
Fever
More women experienced fever above 38 ºC in the epidural group (average RR 2.51, 95% CI 1.67 to 3.77; 4276 women; studies = 9; I2 = 66%; Tau2 = 0.21; Chi2 = 23.24; P = 0.003; Analysis 1.23), although heterogeneity was high for this outcome and the results should be interpreted with caution. The funnel plot was difficult to interpret because only 10 trials contributed data, but there does appear to be some asymmetry, suggesting possible publication bias (funnel plot not shown).
1.23. Analysis.
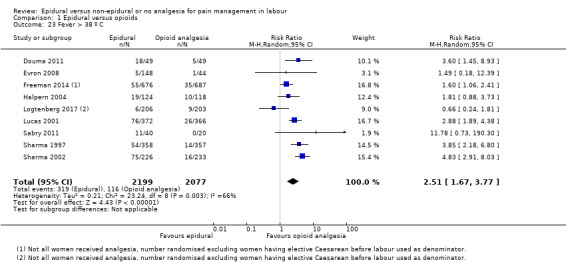
Comparison 1 Epidural versus opioids, Outcome 23 Fever > 38 º C.
Shivers
One small trial found no clear difference between groups for women shivering (RR 5.00, 95% CI 0.27 to 92.62; 20 women; studies = 1; Analysis 1.24).
1.24. Analysis.
Comparison 1 Epidural versus opioids, Outcome 24 Shivering.
Drowsiness
There was no clear difference between groups for drowsiness (average RR 0.48, 95% CI 0.17 to 1.33; 740 women; studies = 6; I2 = 92%; Tau2 = 1.07; Chi2 = 59.48; P < 0.00001; Analysis 1.25), although heterogeneity was high for this outcome and results should be interpreted with caution.
1.25. Analysis.
Comparison 1 Epidural versus opioids, Outcome 25 Drowsiness.
Urinary retention and catheterisation during labour
More women in the epidural group had urinary retention compared with the opioid group (RR 14.18, 95% CI 4.52 to 44.45; 343 women; studies = 4; Analysis 1.26). One trial reported catheterisation in labour but detected no clear difference between groups (RR 5.68, 95% CI 0.71 to 45.68; 111 women; studies = 1; Analysis 1.27).
1.26. Analysis.
Comparison 1 Epidural versus opioids, Outcome 26 Urinary retention.
1.27. Analysis.
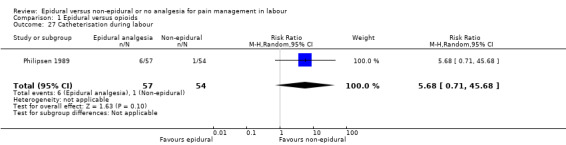
Comparison 1 Epidural versus opioids, Outcome 27 Catheterisation during labour.
Malposition (as defined by trial authors)
Malposition appears to be more common in the epidural group, but the lower limit of the CI just crosses the line of no effect, so this result is unclear (RR 1.40, 95% CI 0.98 to 1.99; 673 women; studies = 4; Analysis 1.28).
1.28. Analysis.
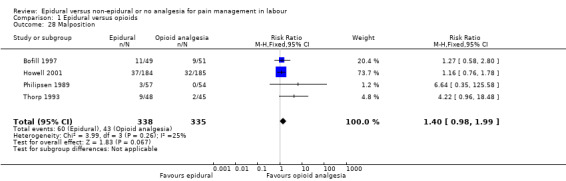
Comparison 1 Epidural versus opioids, Outcome 28 Malposition.
Surgical amniotomy
Two studies reported this outcome and found no clear difference between the groups (average RR 1.03, 95% CI 0.74 to 1.43; 211 women; studies = 2; I2 = 81%; Tau2 = 0.05; Chi2 = 5.18; P = 0.02; Analysis 1.29). There was substantial heterogeneity in this analysis.
1.29. Analysis.
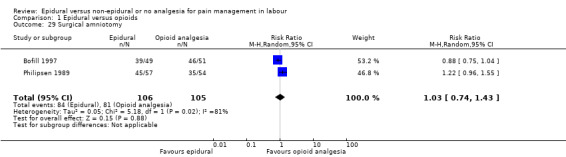
Comparison 1 Epidural versus opioids, Outcome 29 Surgical amniotomy.
Uterine rupture, Headache requiring blood patch, Venous thromboembolic events and Other morbidity were not reported in these trials.
Side effects (for baby)
Acidosis as defined by cord blood arterial pH less than 7.2
Fewer babies born to the women with epidural analgesia had low arterial cord pH of below 7.2 compared with those who received opioids (RR 0.81, 95% CI 0.69 to 0.94; 4783 babies; studies = 8; Analysis 1.30).
1.30. Analysis.
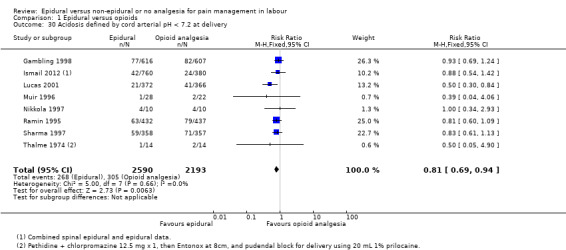
Comparison 1 Epidural versus opioids, Outcome 30 Acidosis defined by cord arterial pH < 7.2 at delivery.
Acidosis as defined by cord blood arterial pH less than 7.15
There was no clear difference between groups in babies who were born with very low arterial blood pH of below 7.15 (RR 1.17, 95% CI 0.64 to 2.14; 480 babies; studies = 3; Analysis 1.31).
1.31. Analysis.
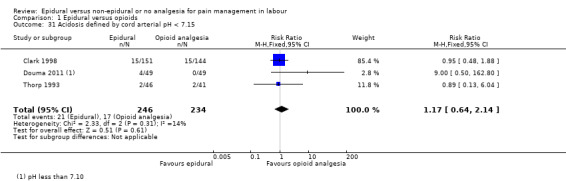
Comparison 1 Epidural versus opioids, Outcome 31 Acidosis defined by cord arterial pH < 7.15.
Naloxone administration
Fewer babies of women in the epidural group required naloxone administration (RR 0.15, 95% CI 0.10 to 0.23; 2645 babies; studies = 10; Analysis 1.32). The funnel plot was difficult to interpret because only 10 trials contributed data, but there does appear to be some asymmetry, suggesting publication bias (funnel plot not shown).
1.32. Analysis.
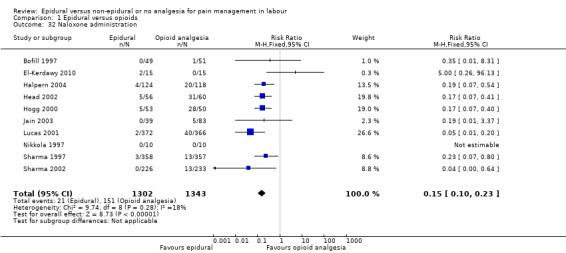
Comparison 1 Epidural versus opioids, Outcome 32 Naloxone administration.
Meconium staining of liquor
Meconium staining of liquor was observed in similar numbers across the two groups (RR 1.01, 95% CI 0.84 to 1.21; 2295 women; studies = 5; Analysis 1.33).
1.33. Analysis.
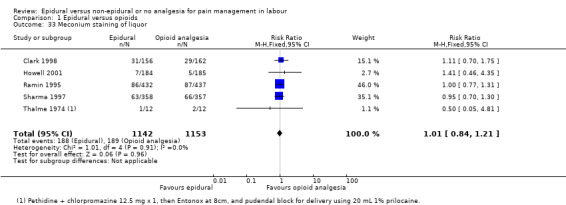
Comparison 1 Epidural versus opioids, Outcome 33 Meconium staining of liquor.
Side effects of Neonatal hypoglycaemia (less than or equal to 1.67 mmol/l), Birth trauma, and Long‐term neonatal complication were not reported in these trials.
Admission to special care baby unit/neonatal intensive care unit (as defined by trialists)
There was no clear difference between groups in babies who were admitted to special care baby unit (RR 1.03, 95% CI 0.95 to 1.12; 4488 babies; studies = 8; Analysis 1.34; moderate‐quality evidence).
1.34. Analysis.
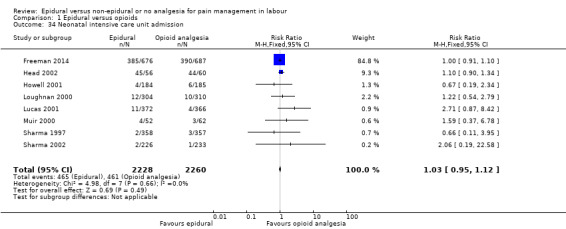
Comparison 1 Epidural versus opioids, Outcome 34 Neonatal intensive care unit admission.
Apgar score less than seven at five minutes
There was no clear difference between groups in babies who had low Apgar scores at five minutes (RR 0.73, 95% CI 0.52 to 1.02; 8752 babies; studies = 22; Analysis 1.35; low‐quality evidence). The funnel plot does not show signs of publication bias (Figure 6).
1.35. Analysis.
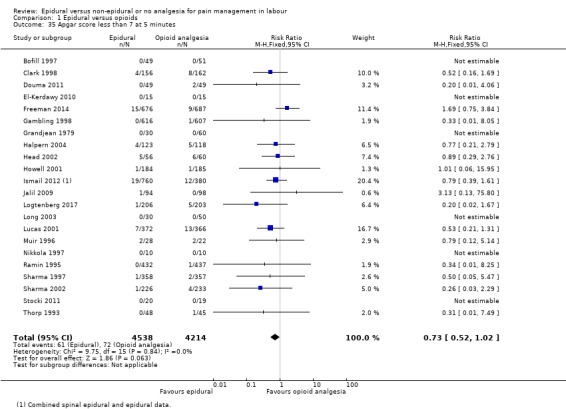
Comparison 1 Epidural versus opioids, Outcome 35 Apgar score less than 7 at 5 minutes.
6.
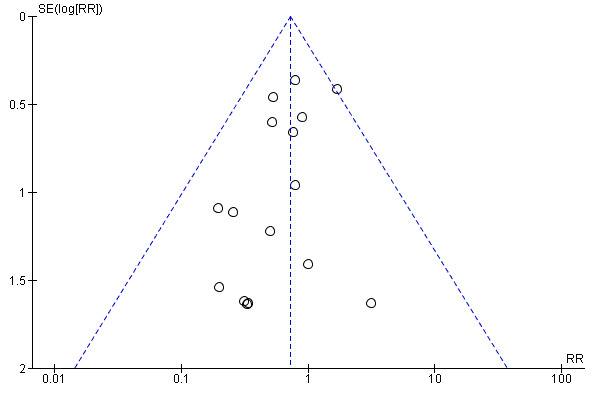
Funnel plot of comparison: 1 Epidural versus opioids, outcome: 1.35 Apgar score less than 7 at 5 minutes.
Poor infant outcomes at long‐term follow‐up (as defined by trialists, e.g. seizures, disability in childhood) were not reported in these trials.
Other outcomes
Cost (as defined by trialists) was not reported in these trials.
Secondary outcomes
Length of first and second stages of labour
Both first and second stages of labour were shorter for the women who received opioids (MD 32.28 minutes, 95% CI 18.34 to 46.22; 2259 women; studies = 9; Analysis 1.36; and MD 15.38 minutes, 95% CI 8.97 to 21.79; random‐effects; 4979 women; studies = 16; I2 = 88%; Tau2 = 112.92; Chi2 = 130.33; P < 0.00001; Analysis 1.37, respectively). Heterogeneity was particularly high for second stage where three trials appeared to favour epidural. Both funnel plots appear to show asymmetry and publication bias is possible (funnel plots not shown).
1.36. Analysis.
Comparison 1 Epidural versus opioids, Outcome 36 Length of first stage of labour (minutes).
1.37. Analysis.
Comparison 1 Epidural versus opioids, Outcome 37 Length of second stage of labour (minutes).
Oxytocin augmentation
Oxytocin augmentation occurred more in the epidural group, but the lower limit of the confidence interval touches the line of no effect (average RR 1.12, 95% CI 1.00 to 1.26; 8351 women; studies = 19; I2 = 80%; Tau2 = 0.04; Chi2 = 89.51; P < 0.00001; Analysis 1.38), although again, heterogeneity was high and results should be interpreted with caution. The funnel plot showed some asymmetry, which suggests possible publication bias (funnel plot not shown).
1.38. Analysis.
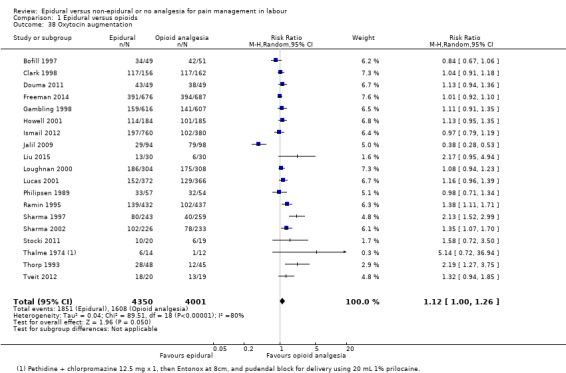
Comparison 1 Epidural versus opioids, Outcome 38 Oxytocin augmentation.
Caesarean section for fetal distress and caesarean section for dystocia
There was no clear difference between the groups for caesarean section for fetal distress (RR 1.32, 95% CI 0.97 to 1.79; 5753 women; studies = 12; Analysis 1.39) or for dystocia (RR 0.93, 95% CI 0.79 to 1.11; 5938 women; studies = 13; Analysis 1.40). Funnel plots for these outcomes did not appear to show signs of asymmetry (funnel plots not shown).
1.39. Analysis.
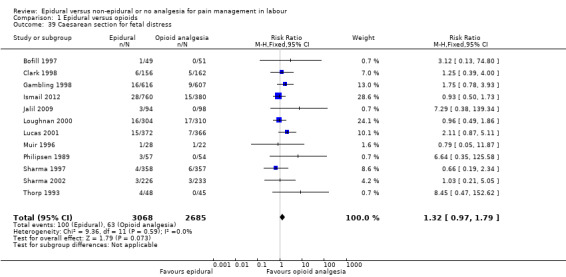
Comparison 1 Epidural versus opioids, Outcome 39 Caesarean section for fetal distress.
1.40. Analysis.
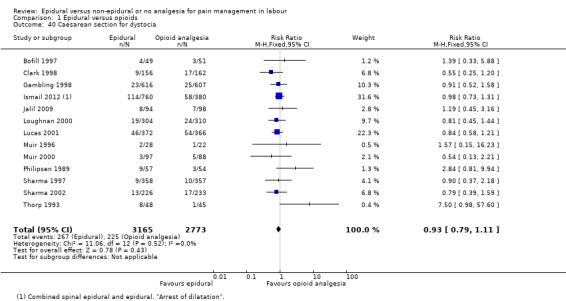
Comparison 1 Epidural versus opioids, Outcome 40 Caesarean section for dystocia.
2. Epidural versus placebo/no‐treatment (seven trials involving 897 women)
Primary outcomes
Pain intensity (as defined by trialists)
Women in the epidural group experienced reduced pain compared to those in the placebo or no‐treatment group (SMD ‐9.55, 95% CI ‐12.91 to ‐6.19; 120 women; studies = 2; I2 = 84%; Tau2 = 4.97; Chi2 = 6.40; P = 0.01; Analysis 2.1). There was, however, substantial heterogeneity between the studies. Another single study suggests that the perception of pain intensity was low among women who received epidural both during the first stage of labour (lower VAS score = less pain) (MD ‐55.90 VAS score, 95% CI ‐61.09 to ‐50.71; 60 women; Analysis 2.2) as well as during the second stage of labour (MD ‐55.70 VAS score, 95% CI ‐63.54 to ‐47.86; 60 women; Analysis 2.3). Nonetheless, there was no clear difference in pain intensity between groups in one study (RR 0.03, 95% CI 0.00 to 0.41; 60 women; Analysis 2.4).
2.1. Analysis.
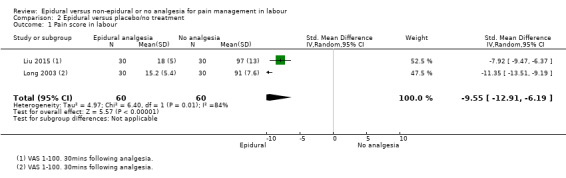
Comparison 2 Epidural versus placebo/no treatment, Outcome 1 Pain score in labour.
2.2. Analysis.
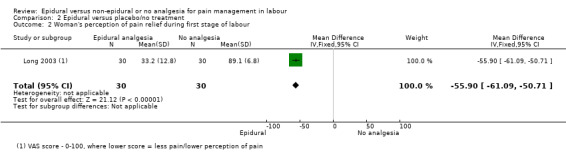
Comparison 2 Epidural versus placebo/no treatment, Outcome 2 Woman's perception of pain relief during first stage of labour.
2.3. Analysis.
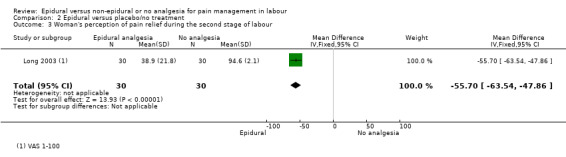
Comparison 2 Epidural versus placebo/no treatment, Outcome 3 Woman's perception of pain relief during the second stage of labour.
2.4. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 4 Pain intensity.
Satisfaction with pain relief (as defined by trialists)
A higher proportion of women in the epidural group rated their satisfaction with pain relief as excellent or very good (RR 1.32, 95% CI 1.05 to 1.65; 70 women; studies = 1; Analysis 2.5).
2.5. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 5 Satisfaction with pain relief in labour ‐ proportion rating excellent or very good.
Sense of control in labour (as defined by trialists)
There was no clear difference in this outcome between the groups (RR 0.89, 95% CI 0.52 to 1.50; 130 women; studies = 2; Analysis 2.6).
2.6. Analysis.
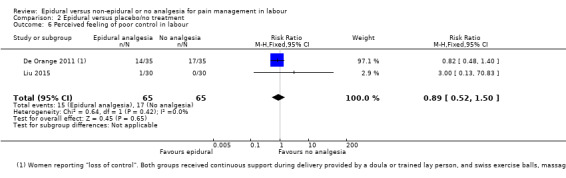
Comparison 2 Epidural versus placebo/no treatment, Outcome 6 Perceived feeling of poor control in labour.
Need for other means of pain relief
There was no clear difference in this outcome between the groups (RR 0.14, 95% CI 0.02 to 1.14; 355 women; studies = 2; Analysis 2.7).
2.7. Analysis.
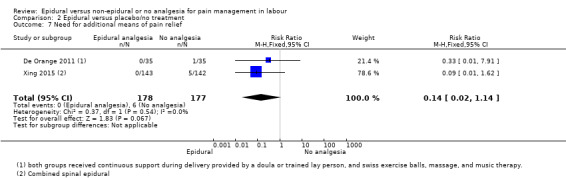
Comparison 2 Epidural versus placebo/no treatment, Outcome 7 Need for additional means of pain relief.
Satisfaction with childbirth experience (as defined by trialists) was not reported in any trial.
Safety of interventions
Assisted vaginal birth
There was no clear difference in this outcome between the groups (average RR 3.41, 95% CI 0.62 to 18.80; 515 women; studies = 4; I2 = 30%; Tau2 = 0.69; Chi2 = 2.84; P = 0.24; Analysis 2.8).
2.8. Analysis.
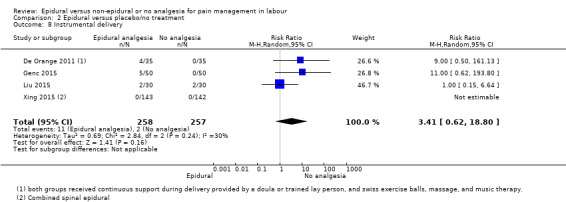
Comparison 2 Epidural versus placebo/no treatment, Outcome 8 Instrumental delivery.
Caesarean section
Fewer women in the epidural group underwent caesarean section compared to women in the placebo or no‐treatment group (average RR 0.46, 95% CI 0.23 to 0.90; 578 women; studies = 5; Analysis 2.9).
2.9. Analysis.
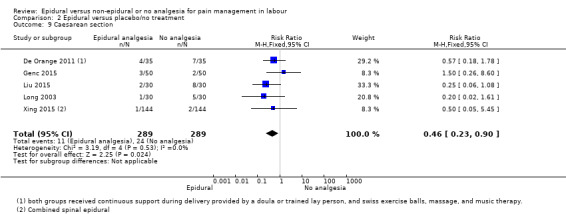
Comparison 2 Epidural versus placebo/no treatment, Outcome 9 Caesarean section.
Effect (negative) on mother/baby interaction, Breastfeeding (at specified time points) were not reported in any trial.
Side effects (for mother)
Motor blockade, Headache
The included study did not report any events for the above outcomes in either group (Analysis 2.10; Analysis 2.11).
2.10. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 10 Motor blockade.
2.11. Analysis.

Comparison 2 Epidural versus placebo/no treatment, Outcome 11 Headache.
Perineal trauma requiring suturing
There was no clear difference in this outcome between the groups (RR 0.86, 95% CI 0.50 to 1.50; 285 women; studies = 1; Analysis 2.12).
2.12. Analysis.
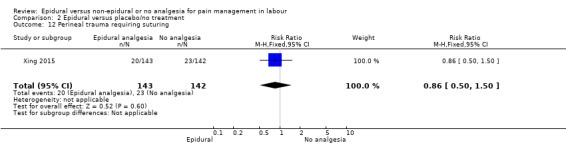
Comparison 2 Epidural versus placebo/no treatment, Outcome 12 Perineal trauma requiring suturing.
Nausea and/or vomiting
There was no clear difference in this outcome between the groups (average RR 11.00, 95% CI 0.62 to 193.80; 160 women; studies = 2; Analysis 2.13).
2.13. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 13 Nausea and vomiting.
Itching
There was no clear difference in this outcome between the groups (RR 3.00, 95% CI 0.13 to 70.83; 60 women; studies = 1; Analysis 2.14).
2.14. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 14 Itch.
Fever
There was no clear difference in this outcome between the groups (RR 11.00, 95% CI 0.63 to 191.69; 70 women; studies = 1; Analysis 2.15).
2.15. Analysis.
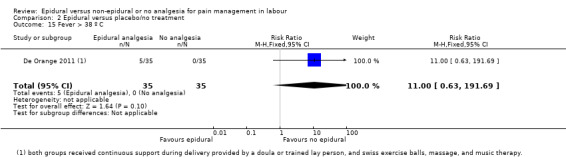
Comparison 2 Epidural versus placebo/no treatment, Outcome 15 Fever > 38 º C.
Shivers
Women in the epidural group experienced higher incidence of shivering compared to women in the placebo or no‐treatment group (RR 8.00, 95% CI 1.04 to 61.62; 100 women; studies = 1; Analysis 2.16).
2.16. Analysis.
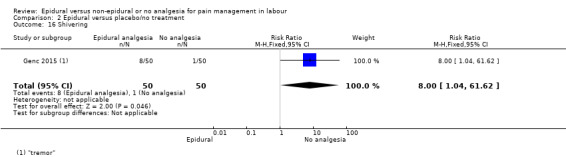
Comparison 2 Epidural versus placebo/no treatment, Outcome 16 Shivering.
Drowsiness
There was no clear difference in this outcome between the groups (RR 7.00, 95% CI 0.37 to 132.10; 100 women; studies = 1; Analysis 2.17).
2.17. Analysis.
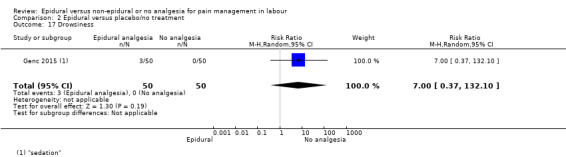
Comparison 2 Epidural versus placebo/no treatment, Outcome 17 Drowsiness.
Urinary retention
There was no clear difference in this outcome between the groups (RR 3.00, 95% CI 0.32 to 28.21; 160 women; studies = 2; Analysis 2.18).
2.18. Analysis.
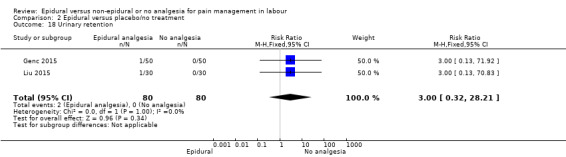
Comparison 2 Epidural versus placebo/no treatment, Outcome 18 Urinary retention.
Maternal hypotension (as defined by authors), Postnatal depression (authors' definition, treatment for depression or self‐reported), Long‐term backache (as defined by trial authors), Respiratory depression requiring oxygen administration, Uterine rupture, Headache requiring blood patch, Venous thromboembolic events were not reported in any trial. Catheterisation during labour, Other morbidity (e.g. impaired consciousness, meningitis, intensive care unit admission, paralysis), Malposition (as defined by trial authors), Surgical amniotomy were not reported in these trials.
Side effects (for baby)
Apgar score less than seven at five minutes
The included study did not report any events for the above outcome in either group (Analysis 2.19).
2.19. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 19 Apgar score less than 7 at 5 minutes.
Acidosis as defined by cord blood arterial pH less than 7.2, Acidosis as defined by cord blood arterial pH less than 7.15, Naloxone administration, Neonatal hypoglycaemia (less than or equal to 1.67 mmol/l), Birth trauma, Long‐term neonatal complication, Meconium staining of liquor, Admission to special care baby unit/neonatal intensive care unit (as defined by trialists), Poor infant outcomes at long‐term follow‐up (as defined by trialists, e.g. seizures, disability in childhood) were not reported in any trial under this comparison.
Other outcomes
Cost (as defined by trialists) was not reported in the included studies.
Secondary outcomes
Length of first stage of labour, length of second stage of labour, oxytocin augmentation, caesarean section for fetal distress, caesarean section for dystocia
There was no clear difference between the groups for length of first stage of labour (minutes) (MD ‐55.09 minutes, 95% CI ‐186.26 to 76.09; random‐effects; 189 women; studies = 2; I2 = 92%; Tau2 = 8236.28; Chi2 = 12.10; P = 0.0005; Analysis 2.20); length of second stage of labour (MD 7.66 minutes, 95% CI ‐6.12 to 21.45; random‐effects; 344 women; studies = 4; I2 = 78%; Tau2 = 148.06; Chi2 = 13.87; P = 0.003; Analysis 2.21); oxytocin augmentation (RR 0.89, 95% CI 0.63 to 1.24; 415 women; studies = 3; Analysis 2.22); caesarean section for fetal distress (RR 1.00, 95% CI 0.06 to 15.55; 100 women; studies = 1; Analysis 2.23); caesarean section for dystocia (RR 2.00, 95% CI 0.19 to 21.36; 100 women; studies = 1; Analysis 2.24). There was substantial heterogeneity present for the length of first and second stages of labour.
2.20. Analysis.

Comparison 2 Epidural versus placebo/no treatment, Outcome 20 Length of first stage of labour (minutes).
2.21. Analysis.

Comparison 2 Epidural versus placebo/no treatment, Outcome 21 Length of second stage of labour (minutes).
2.22. Analysis.
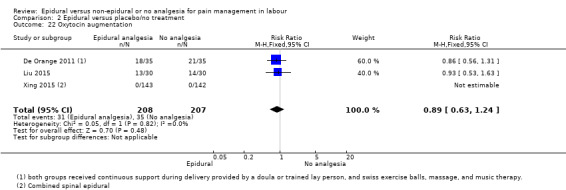
Comparison 2 Epidural versus placebo/no treatment, Outcome 22 Oxytocin augmentation.
2.23. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 23 Caesarean section for fetal distress.
2.24. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 24 Caesarean section for dystocia.
3. Epidural versus Acu‐stimulation (one trial involving 60 women)
Primary outcomes
Effects of interventions
Pain intensity (as defined by trialists)
Women in the epidural group reported lower pain scores in labour than those in the acu‐stimulation group (SMD ‐53.00, 95% CI ‐57.98 to ‐48.02; Analysis 3.1).
3.1. Analysis.
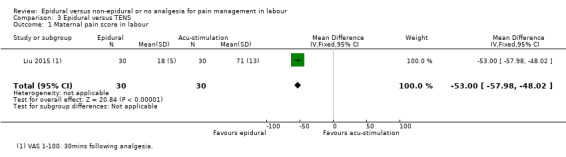
Comparison 3 Epidural versus TENS, Outcome 1 Maternal pain score in labour.
This trial did not report Satisfaction with pain relief (as defined by trialists), Sense of control in labour (as defined by trialists), Satisfaction with childbirth experience (as defined by trialists), or Need for other means of pain relief.
Safety of interventions
Assisted vaginal birth and caesarean section
There was no clear difference between groups for assisted vaginal birth (RR 1.00, 95% CI 0.15 to 6.64; Analysis 3.2) or caesarean section (RR 2.00, 95% CI 0.19 to 20.90; Analysis 3.3).
3.2. Analysis.
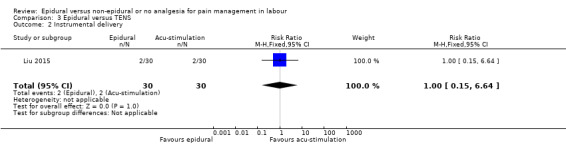
Comparison 3 Epidural versus TENS, Outcome 2 Instrumental delivery.
3.3. Analysis.
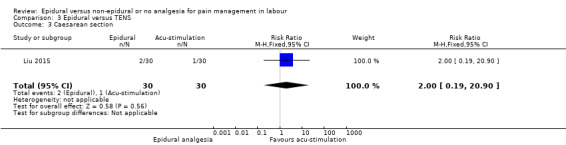
Comparison 3 Epidural versus TENS, Outcome 3 Caesarean section.
Effect (negative) on mother/baby interaction and Breastfeeding (at specified time points) were not reported in this trial.
Side effects (for mother)
There was no clear difference between group for maternal hypotension (RR 3.00, 95% CI 0.13 to 70.83; Analysis 3.4) or urinary retention (RR 3.00, 95% CI 0.13 to 70.83; Analysis 3.5). There were no reports of nausea or vomiting in either group (Analysis 3.6). These were the only side effects reported in this trial.
3.4. Analysis.

Comparison 3 Epidural versus TENS, Outcome 4 Hypotension as defined by trial authors.
3.5. Analysis.
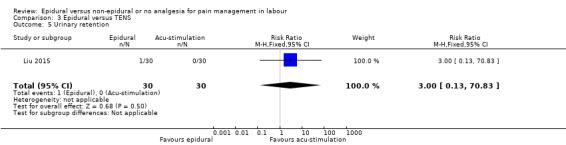
Comparison 3 Epidural versus TENS, Outcome 5 Urinary retention.
3.6. Analysis.

Comparison 3 Epidural versus TENS, Outcome 6 Nausea and vomiting.
No Side effects (for baby), Neonatal outcomes or Other outcomes were reported.
Secondary outcomes
Length of second stage of labour
Shorter second stages were reported for women in the acu‐stimulation group (minutes) (MD 17.90 minutes, 95% CI 5.66 to 30.14; Analysis 3.7).
3.7. Analysis.
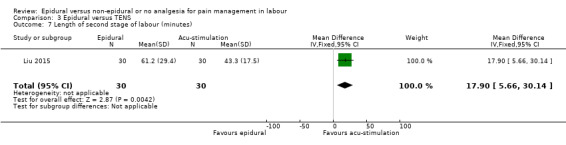
Comparison 3 Epidural versus TENS, Outcome 7 Length of second stage of labour (minutes).
Oxytocin augmentation
There was no clear difference between groups in the proportion of women receiving oxytocin augmentation (RR 1.08, 95% CI 0.59 to 1.97; Analysis 3.8).
3.8. Analysis.
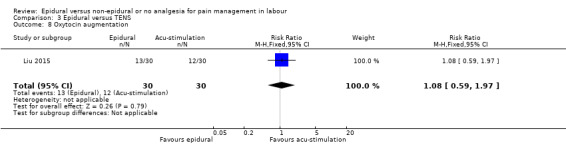
Comparison 3 Epidural versus TENS, Outcome 8 Oxytocin augmentation.
Length of first stage of labour, Caesarean section for fetal distress, and Caesarean section for dystocia were not reported in this trial.
4. Epidural versus inhaled analgesia (one trial involving 86 women)
Only one trial contributed to this comparison and reported only two outcomes relevant to this review.
Satisfaction with pain relief
More women rated epidural very good or excellent compared with inhaled analgesia (RR 2.18, 95% CI 1.31 to 3.62; Analysis 4.1).
4.1. Analysis.
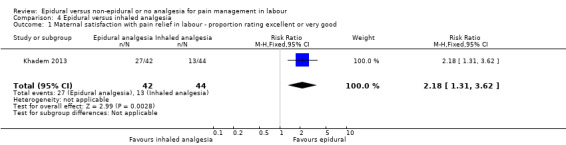
Comparison 4 Epidural versus inhaled analgesia, Outcome 1 Maternal satisfaction with pain relief in labour ‐ proportion rating excellent or very good.
Caesarean section
There was no clear difference between the groups in caesarean section rate (RR 0.63, 95% CI 0.16 to 2.47; Analysis 4.2).
4.2. Analysis.
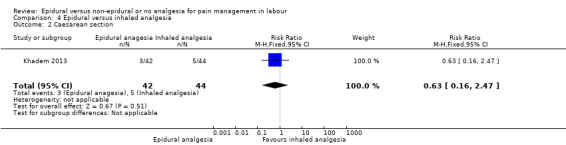
Comparison 4 Epidural versus inhaled analgesia, Outcome 2 Caesarean section.
5. Epidural versus continuous support (one trial involving 992 women)
The comparison group in this study (Dickinson 2002) received one‐to‐one continuous midwifery support along with usual analgesia choices such as IM pethidine, nitrous oxide inhalation, TENS, and/or non‐pharmacological forms of pain relief.
Primary outcomes
Effects of interventions
Satisfaction with pain relief
All women in the epidural group and 494 out of 499 women in the non‐epidural group rated their pain relief as 'excellent or very good' (RR 1.01, 95% CI 1.00 to 1.02; Analysis 5.1). No women in the epidural group requested other means of pain relief compared to 262 out of 499 in the non‐epidural group (RR 0.00, 95% CI 0.00 to 0.03; Analysis 5.2).
5.1. Analysis.
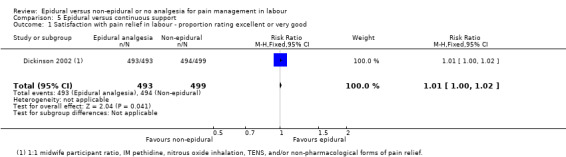
Comparison 5 Epidural versus continuous support, Outcome 1 Satisfaction with pain relief in labour ‐ proportion rating excellent or very good.
5.2. Analysis.
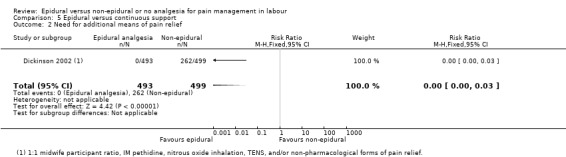
Comparison 5 Epidural versus continuous support, Outcome 2 Need for additional means of pain relief.
Pain intensity (as defined by trialists), Sense of control in labour (as defined by trialists), and Satisfaction with childbirth experience (as defined by trialists) were not reported in this trial.
Safety of interventions
Assisted vaginal birth and caesarean section
There was no clear difference between groups for assisted vaginal birth (RR 1.16, 95% CI 0.96 to 1.39; Analysis 5.3) and caesarean section (RR 1.21, 95% CI 0.91 to 1.62; Analysis 5.4).
5.3. Analysis.
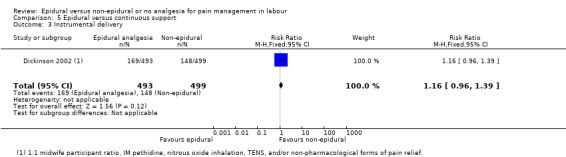
Comparison 5 Epidural versus continuous support, Outcome 3 Instrumental delivery.
5.4. Analysis.
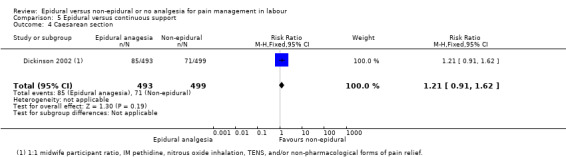
Comparison 5 Epidural versus continuous support, Outcome 4 Caesarean section.
Effect (negative) on mother/baby interaction and Breastfeeding (at specified time points) were not reported in this trial.
Side effects (for mother)
There was no clear difference between groups for women with long‐term backache (RR 0.88, 95% CI 0.69 to 1.11; Analysis 5.5), headaches (RR 0.96, 95% CI 0.79 to 1.17; Analysis 5.6), and nausea and vomiting (RR 1.12, 95% CI 0.80 to 1.57; Analysis 5.7). More women in the epidural group were catheterised during labour (RR 1.16, 95% CI 1.04 to 1.29; Analysis 5.8). No other maternal side effects were reported in the trial.
5.5. Analysis.
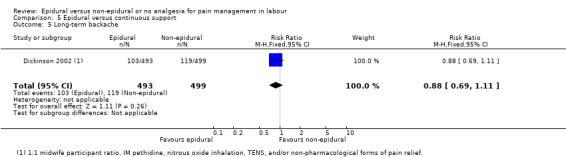
Comparison 5 Epidural versus continuous support, Outcome 5 Long‐term backache.
5.6. Analysis.
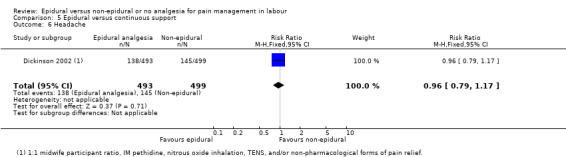
Comparison 5 Epidural versus continuous support, Outcome 6 Headache.
5.7. Analysis.
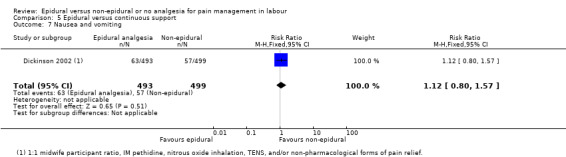
Comparison 5 Epidural versus continuous support, Outcome 7 Nausea and vomiting.
5.8. Analysis.
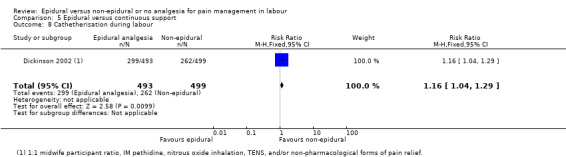
Comparison 5 Epidural versus continuous support, Outcome 8 Cathetherisation during labour.
Side effects (for baby)
Side effects, Admission to special care baby unit/neonatal intensive care unit, and Poor infant outcomes at long‐term follow‐up were not reported in this trial. There was no clear difference between groups in Apgar scores of less than seven at five minutes (RR 2.02, 95% CI 0.61 to 6.68; Analysis 5.9).
5.9. Analysis.
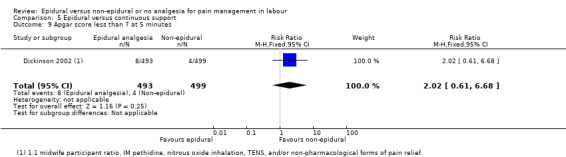
Comparison 5 Epidural versus continuous support, Outcome 9 Apgar score less than 7 at 5 minutes.
Other outcomes
Cost was not reported in this trial.
Secondary outcomes
No secondary outcomes were reported in this trial.
Subgroup analysis
We did not carry out planned subgroup analyses because a complete breakdown of the separate subgroup categories was not provided.
We conducted one post hoc subgroup analysis of trials conducted after 2005, to assess whether more recent modern epidural techniques still showed an increased incidence of assisted vaginal birth
Sensitivity analysis
We conducted sensitivity analyses for two primary outcomes: Satisfaction with pain relief; and Need for other means of pain relief. We excluded from the analysis studies with a high or unclear risk of bias for allocation concealment or incomplete outcome data for these two outcomes. There were sufficient studies in comparisons 1, 2, and 5 to conduct this sensitivity analysis.
Comparison 1. Epidural versus opioids
Removing studies with unsatisfactory allocation concealment in Satisfaction with pain relief had an impact on the pooled effect size, resulting in no clear difference between the groups (average RR 1.42, 95% CI 0.70 to 2.92; 1372 women; studies = 4; I2 = 99%; Analysis 1.41), albeit with substantial heterogeneity. We noted a similar finding when studies with incomplete outcome data were removed (average RR 1.23, 95% CI 0.97 to 1.55; 923 women; studies = 3; I2 = 94%; Analysis 1.42).
1.41. Analysis.

Comparison 1 Epidural versus opioids, Outcome 41 Sensitivity analysis ‐ allocation concealment: Maternal satisfaction with pain relief in labour ‐ proportion rating excellent or very good.
1.42. Analysis.

Comparison 1 Epidural versus opioids, Outcome 42 Sensitivity analysis ‐ incomplete outcome data: Maternal satisfaction with pain relief in labour ‐ proportion rating excellent or very good.
Removing the studies with unsatisfactory allocation concealment (average RR 0.12, 95% CI 0.03 to 0.53; 3043 women; studies = 9; I2 = 75%; Analysis 1.43) and incomplete outcome data (average RR 0.15, 95% CI 0.05 to 0.45; 3740 women; studies = 9; I2 = 78%; Analysis 1.44) for the outcome Need for other pain relief made little difference to the meta‐analyses.
1.43. Analysis.

Comparison 1 Epidural versus opioids, Outcome 43 Sensitivity analysis ‐ allocation concealment: Need for additional means of pain relief.
1.44. Analysis.
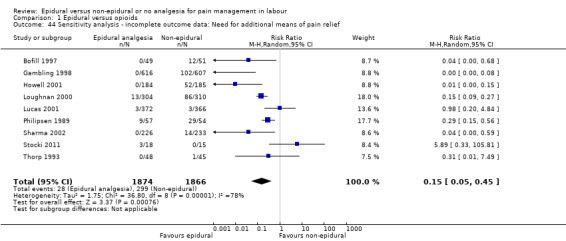
Comparison 1 Epidural versus opioids, Outcome 44 Sensitivity analysis ‐ incomplete outcome data: Need for additional means of pain relief.
Comparison 2. Epidural versus placebo/no treatment
The sensitivity analysis for Need for additional pain relief widened CIs and the effect of epidural remained unclear, when studies with unsatisfactory allocation concealment (RR 0.33, 95% CI 0.01 to 7.91; 70 women; studies = 1; Analysis 2.25), and incomplete outcome data (RR 0.33, 95% CI 0.01 to 7.91; 70 women; studies = 1; Analysis 2.26) were removed. Only one study contributed to the analysis for Satisfaction with pain relief, so a sensitivity analysis was not appropriate.
2.25. Analysis.
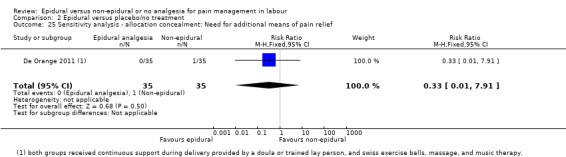
Comparison 2 Epidural versus placebo/no treatment, Outcome 25 Sensitivity analysis ‐ allocation concealment: Need for additional means of pain relief.
2.26. Analysis.
Comparison 2 Epidural versus placebo/no treatment, Outcome 26 Sensitivity analysis ‐ incomplete outcome data: Need for additional means of pain relief.
Discussion
Summary of main results
We include data from 40 trials, involving over 11,000 women. Thirty‐four trials compared epidural with opioids, seven trials compared epidural with no analgesia, one trial compared epidural with acu‐stimulation, one trial compared epidural with inhaled analgesia, and one trial compared epidural with continuous midwifery support and other analgesia.
Comparing epidural with opioids, women with epidural analgesia reported lower pain intensity as expressed by lower pain scores and a higher proportion were satisfied with their pain relief, reporting it to be "excellent or very good". There was a substantial amount of statistical heterogeneity in both these outcomes. More women in the epidural group experienced assisted vaginal birth, although there appears to be a larger effect reported in the small studies contributing data to this outcome, so the results should be interpreted with caution. In addition, post hoc subgroup analysis showed that this effect was no longer present in studies after 2005, suggesting that more modern techniques of epidural analgesia such as using lower doses of local anaesthetic and avoiding epidural infusions may not affect this outcome. There were no clear differences between caesarean section rates, and maternal long‐term backache. There were also no clear differences between groups for the neonatal outcomes, admission to neonatal intensive care unit and Apgar score less than seven at five minutes. We downgraded evidence for study design, inconsistency, imprecision in effect estimates, and possible publication bias.
Side effects were reported in both epidural and opioid groups. Women with epidural experienced more hypotension, motor blockade, fever, and urinary retention. They also had longer first and second stages of labour, and were more likely to have oxytocin augmentation than the women in the opioid group. The women in the opioid group had more respiratory depression requiring oxygen, and nausea and vomiting. Babies born to women in the opioid group were more likely to have had naloxone administration. There was no clear difference between groups for postnatal depression, headache, itching, shivering, or drowsiness. Maternal morbidity and long‐term neonatal outcomes were not reported.
We detected substantial heterogeneity for many outcomes in the epidural versus opioid comparison. Exploration of heterogeneity was not possible using subgroup analysis, but we investigated the effect of trial quality using prespecified sensitivity analysis. Heterogeneity could not be explained by sensitivity analyses. Varying epidural protocols and different types of opioids used may have contributed to heterogeneity. There was considerable variation in outcome measures in trials reporting women's satisfaction with pain relief, as previously discussed. None of the trials reporting maternal hypotension gave their definitions for this outcome, so there may be substantial differences here. Heterogeneity for the outcomes of length of labour and use of oxytocin augmentation may be explained by variations in clinical practice as to when labour begins and when oxytocin is required.
Epidural analgesia resulted in less reported pain when compared with placebo or no treatment, and acu‐stimulation. Pain intensity was not reported in the trials that compared epidural with inhaled analgesia, or continuous support. Maternal satisfaction was greater in epidural groups when compared with no treatment/placebo, inhaled analgesia or continuous support, although most of both epidural and continuous support trials reported their pain relief to be excellent. Few trials reported on serious maternal side effects.
Overall completeness and applicability of evidence
Some limitations of our analysis should be noted. Eleven studies reported women's perception of pain as an outcome but we could not extract the data from these studies for meta‐analysis, because trials measured this outcome differently and reported the data in a format not compatible with the software used. These studies used various forms of VAS scores as a way of measuring women's perception of pain, but it was not possible to extract the data presented. In three of the studies (Bofill 1997; Sharma 1997; Sharma 2002) data were in graphical representation only. For two of the studies (Dickinson 2002; Muir 1996), it was unclear as to whether the data presented were means or medians. Philipsen 1989 used medians; Gambling 1998, Nikkola 1997 and Thorp 1993 measured this outcome at different time intervals and we therefore could not combine the data. Two studies (Jain 2003; Loughnan 2000), presented their data as the number of women experiencing different levels of pain. Trials varied in the characteristics of participants, labour management protocols and the epidural regimen and opioids used. These factors may influence the course of labour, pain relief requirements and outcomes such as duration of labour, oxytocin augmentation and instrumental delivery. Combining studies using a high concentration of a local anaesthetic agent for epidural analgesia with low‐concentration techniques, and combining studies maintaining a block in the second stage of labour to those discontinuing may influence some outcomes, in particular the duration of labour and assisted vaginal births. We had planned subgroup analyses based on parity, spontaneous labour versus induced labour, term versus preterm, continuous support in labour versus no continuous support, in an attempt to explore whether these variations had any effect on the results. However, data on the separate subgroups were rarely provided and so it was not possible to conduct any subgroup analysis.
Epidural dose and technique impact have been shown to affect the incidence of assisted vaginal birth when more concentrated epidural solutions are used (COMET 2001; Sultan 2013). Most women in the control group were randomised to opioids and the effect on some outcomes may therefore be applicable to the use of opioids in labour rather than to all other non‐epidural forms of analgesia or no pain relief. Some women randomised to non‐epidural analgesia received epidural as well. To a lesser extent, some women in the epidural arm did not receive the intervention, due to rapid labour. We included only data based on an intention‐to‐treat analysis. However, this approach may make the results difficult to interpret.
The evidence presented in this review needs to be interpreted taking these limitations into account.
Quality of the evidence
Risk of bias varied throughout the included trials (see Figure 2; Figure 3). Most of the trials were not well reported and were assessed to be at unclear risk of bias in many domains. Only two trials contributing data were assessed as using inadequate random sequence generation, and all trials either concealed allocation or did not report this domain clearly. No trial reported blinding outcome assessors and only two blinded participants and staff, although the nature of many of the interventions made this difficult. We rated most trials at low risk of attrition and reporting biases, although some were at high or unclear risk of both.
We assessed the quality of the evidence of seven outcomes comparing epidural and opioid analgesia using the GRADE approach (see Table 1). We graded evidence for pain intensity, satisfaction with pain relief, assisted vaginal birth, and Apgar score less than seven at five minutes as low quality. We rated caesarean section, side effects ‐ long‐term backache, and admission to neonatal intensive care unit as moderate‐quality evidence. We downgraded evidence for study design limitations, high statistical heterogeneity, imprecision of effect estimates, and possible publication bias.
Potential biases in the review process
We took steps to minimise bias, although we are aware that bias may be present in our review. Two review authors independently assessed studies for eligibility and extracted the data as necessary. We resolved discrepancies through discussion or if required we consulted a third review author. Two review authors also performed GRADE assessments independently and resolved discrepancies though discussion.
Agreements and disagreements with other studies or reviews
An extensive body of evidence exists assessing pharmacological methods of pain relief, include inhalation of nitrous oxide (Klomp 2012), opioids (Ullman 2010), and local anaesthetic nerve block (Novikova 2011). This review is an update of the previous version of the Cochrane Review of epidural versus non‐epidural or no analgesia in labour. Thirteen new studies have provided additional data to the review. The addition of these new data has not greatly altered the conclusions of this review, other than low‐quality evidence showing that epidural analgesia is effective in increasing maternal satisfaction with pain relief. Six additional trials, awaiting clarification (Antipin 2014; Gupta 2016; Kamali 2016; Marshalov 2012; Vavrinkova 2005; Weissman 2006), may be included in future updates, and have the potential to alter the current conclusions of the review.
Authors' conclusions
Implications for practice.
Low‐quality evidence shows that epidural analgesia may be more effective in reducing pain during labour and increasing maternal satisfaction with pain relief than non‐epidural methods. Although overall there appears to be an increase in assisted vaginal birth when women have epidural analgesia, a post hoc subgroup analysis shows that this effect is not seen in recent studies (after 2005), suggesting that modern approaches to epidural analgesia in labour do not affect this outcome. Epidural analgesia had no impact on the risk of caesarean section or long‐term backache, and did not appear to have an immediate effect on neonatal status as determined by Apgar scores or in admissions to neonatal intensive care. Further research may be helpful to evaluate rare but potentially severe adverse effects of epidural analgesia and non‐epidural analgesia on women in labour and long‐term neonatal outcomes.
Implications for research.
To facilitate future meta‐analyses, we advise standardisation of outcomes and outcome measures in trials. Despite a large number of randomised trials including many women, none of the included studies reported on rare but serious adverse effects. Some of these data may be better obtained from large case series. There was little evidence of immediate adverse effects on the baby, but long‐term consequences are still not known.
Further research is needed to minimise the adverse effects of epidural analgesia in women who choose epidural as their method of pain relief and to evaluate rare but potentially severe adverse effects of epidural analgesia. Research to elucidate optimal concentration of epidural infusions is also needed.
Feedback
Olsen, April 1998
Summary
Abstract: The section main results should use consistent terminology. The effect on pain relief was only reported in one small trial, and this should be referred to in the same way as for the adverse effects.
The conclusion is also inconsistent. A suggestion for the first sentence: 'Epidural analgesia is an effective method of pain relief during labour, but is associated with longer first and second stages of labour, increased oxytocin use, malrotation, instrumental delivery and Caesarean section; women should be counselled about these risks before labor.' The more rare maternal side effects could also be mentioned.
Background: The statements about epidural as effective form of pain relief are not justified or referenced. It is unclear whether more evidence to support this effect is necessary.
Reply
Pain relief has now been reported in four studies, all of which showed epidural to be better than non‐epidural analgesia. The review has been amended to take account of this, and the other comments.
[Summary of response from Charlotte Howell, May 1999]
Contributors
Summary of comments received from Ole Olsen, April 1998.
Vickers, August 1999
Summary
Results and discussion: The interpretation of the summary statistic for Caesarean section is misleading. The lower limit of the 95% confidence interval is just below one (relative risk 1.27, 95% confidence interval 0.93‐1.74), and so does not achieve statistical significance. The authors conclude 'there is no significant increase in the Caesarean section rate', but this under rates the clinical importance of these data. It is not usual to demand statistically significant differences between groups before considering it worth mentioning a possible adverse event to a patient. The most likely effect is an increase of 25%, but this may be as much as 75% and a small, 10%, decrease in the risk of Caesarean section is also possible.
Women considering their choice of pain relief should be warned that epidural analgesia probably increases their risk of having a Caesarean section.
Reply
This broader interpretation of the confidence intervals has been incorporated.
(Summary of response from M Anim‐Somuah, April 2005.)
Contributors
Summary of comments received from Andrew Vickers, August 1999.
Vickers, April 2001
Summary
Update on previous comment
The reviewer stated in February 2000 that "This broader interpretation of the confidence intervals will be incorporated into the next update of the review." In April 2001 this has yet to be done. The review continues to be misleading in stating that epidurals do not increase rates of caesarean section.
Reply
The review has now been updated. With addition of new trials, the overall relative risk of caesarean section for women allocated epidural rather than other forms of analgesia was 1.07, 95% CI 0.93 to 1.23. The implications are discussed in the review.
(Summary of response from M. Anim‐Somuah, April 2005.)
Contributors
Summary of comments from Andrew Vickers, April 2001.
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2017 | New search has been performed | Search updated and 45 trial reports assessed; 16 new trials were included and 13 of these contributed data to this update. Altogether, this version includes data from 40 trials. We excluded two trials included in last update. |
| 30 April 2017 | New citation required but conclusions have not changed | 13 new trials contribute data; conclusions are similar to previous versions of the review. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 19 July 2011 | New search has been performed | Search updated 31 March 2011. We have included data from 17 new studies. These changes have not altered the conclusions of the review. Outcomes included and methods used for subgroup and sensitivity analyses have changed slightly since the last update ‐ seeDifferences between protocol and review. |
| 19 July 2011 | New citation required but conclusions have not changed | A new author helped update the review. |
| 22 June 2010 | Amended | Search updated. Twenty‐six reports added to Studies awaiting classification. |
| 21 August 2008 | Amended | Converted to new review format. |
| 16 August 2005 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
C Howell (CH) prepared the first version of the review (Howell 1999).
We thank Angela Gonzales and Alison Ledward for assistance with translation. We thank Leanne Jones for her contribution as an author on the previous version of this review (Anim‐Somuah 2011).
We also thank Therese Dowswell and Lambert Felix from Cochrane Pregnancy and Childbirth for their input in preparing the 2018 update.
As part of the prepublication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, through Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
epidural AND labo(u)r
Data and analyses
Comparison 1. Epidural versus opioids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score in labour | 5 | 1133 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐4.56, ‐0.73] |
| 2 Pain intensity severe or intolerable | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Woman's perception of pain relief in labour | 3 | 1166 | Mean Difference (IV, Random, 95% CI) | ‐3.36 [‐5.41, ‐1.31] |
| 4 Woman's perception of pain relief during first stage of labour | 3 | 194 | Mean Difference (IV, Random, 95% CI) | ‐12.05 [‐19.35, ‐4.75] |
| 5 Woman's perception of pain relief during the second stage of labour | 2 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐20.75 [‐22.50, ‐19.01] |
| 6 Satisfaction with pain relief in labour ‐ proportion rating excellent or very good | 7 | 1911 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.03, 2.08] |
| 7 Satisfaction with pain relief in labour ‐ continuous data | 7 | 3171 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.10, 0.91] |
| 8 Time of administration of pain relief to time pain relief was satisfactory (minutes) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐8.02, ‐5.38] |
| 9 Perceived feeling of poor control in labour | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.62, 2.21] |
| 10 Satisfaction with childbirth experience ‐ proportion rating satisfied to very satisfied | 1 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.03] |
| 11 Need for additional means of pain relief | 16 | 5099 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.04, 0.25] |
| 12 Assisted vaginal birth | 30 | 9948 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.29, 1.60] |
| 13 Caesarean section | 33 | 10350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.96, 1.18] |
| 14 Long‐term backache | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 15 Hypotension as defined by trial authors | 10 | 4212 | Risk Ratio (M‐H, Random, 95% CI) | 11.34 [1.89, 67.95] |
| 16 Postnatal depression (authors definition, on medication, or self‐reported) | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.38, 1.05] |
| 17 Motor blockade | 3 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 31.71 [4.16, 241.99] |
| 18 Respiratory depression requiring oxygen administration | 5 | 2031 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.05, 0.97] |
| 19 Headache | 4 | 1938 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.54] |
| 20 Perineal trauma requiring suturing | 1 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.18] |
| 21 Nausea and vomiting | 15 | 4440 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.87] |
| 22 Itch | 8 | 2900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.81, 1.77] |
| 23 Fever > 38 º C | 9 | 4276 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [1.67, 3.77] |
| 24 Shivering | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 92.62] |
| 25 Drowsiness | 6 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.17, 1.33] |
| 26 Urinary retention | 4 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.18 [4.52, 44.45] |
| 27 Catheterisation during labour | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 5.68 [0.71, 45.68] |
| 28 Malposition | 4 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.98, 1.99] |
| 29 Surgical amniotomy | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.74, 1.43] |
| 30 Acidosis defined by cord arterial pH < 7.2 at delivery | 8 | 4783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.69, 0.94] |
| 31 Acidosis defined by cord arterial pH < 7.15 | 3 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.64, 2.14] |
| 32 Naloxone administration | 10 | 2645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.10, 0.23] |
| 33 Meconium staining of liquor | 5 | 2295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.21] |
| 34 Neonatal intensive care unit admission | 8 | 4488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.95, 1.12] |
| 35 Apgar score less than 7 at 5 minutes | 22 | 8752 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.52, 1.02] |
| 36 Length of first stage of labour (minutes) | 9 | 2259 | Mean Difference (IV, Fixed, 95% CI) | 32.28 [18.34, 46.22] |
| 37 Length of second stage of labour (minutes) | 16 | 4979 | Mean Difference (IV, Random, 95% CI) | 15.38 [8.97, 21.79] |
| 38 Oxytocin augmentation | 19 | 8351 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.00, 1.26] |
| 39 Caesarean section for fetal distress | 12 | 5753 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.97, 1.79] |
| 40 Caesarean section for dystocia | 13 | 5938 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.11] |
| 41 Sensitivity analysis ‐ allocation concealment: Maternal satisfaction with pain relief in labour ‐ proportion rating excellent or very good | 4 | 1372 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.70, 2.92] |
| 42 Sensitivity analysis ‐ incomplete outcome data: Maternal satisfaction with pain relief in labour ‐ proportion rating excellent or very good | 3 | 923 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.97, 1.55] |
| 43 Sensitivity analysis ‐ allocation concealment: Need for additional means of pain relief | 9 | 3043 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.53] |
| 44 Sensitivity analysis ‐ incomplete outcome data: Need for additional means of pain relief | 9 | 3740 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.05, 0.45] |
1.2. Analysis.
Comparison 1 Epidural versus opioids, Outcome 2 Pain intensity severe or intolerable.
Comparison 2. Epidural versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score in labour | 2 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐9.55 [‐12.91, ‐6.19] |
| 2 Woman's perception of pain relief during first stage of labour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐55.90 [‐61.09, ‐50.71] |
| 3 Woman's perception of pain relief during the second stage of labour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐55.70 [‐63.54, ‐47.86] |
| 4 Pain intensity | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.41] |
| 5 Satisfaction with pain relief in labour ‐ proportion rating excellent or very good | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.05, 1.65] |
| 6 Perceived feeling of poor control in labour | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.52, 1.50] |
| 7 Need for additional means of pain relief | 2 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.14] |
| 8 Instrumental delivery | 4 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 3.41 [0.62, 18.80] |
| 9 Caesarean section | 5 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.23, 0.90] |
| 10 Motor blockade | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Headache | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Perineal trauma requiring suturing | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.50, 1.50] |
| 13 Nausea and vomiting | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [0.62, 193.80] |
| 14 Itch | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 15 Fever > 38 º C | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.63, 191.69] |
| 16 Shivering | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [1.04, 61.62] |
| 17 Drowsiness | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.37, 132.10] |
| 18 Urinary retention | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 28.21] |
| 19 Apgar score less than 7 at 5 minutes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Length of first stage of labour (minutes) | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐55.09 [‐186.26, 76.09] |
| 21 Length of second stage of labour (minutes) | 4 | 344 | Mean Difference (IV, Random, 95% CI) | 7.66 [‐6.12, 21.45] |
| 22 Oxytocin augmentation | 3 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.24] |
| 23 Caesarean section for fetal distress | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| 24 Caesarean section for dystocia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| 25 Sensitivity analysis ‐ allocation concealment: Need for additional means of pain relief | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 26 Sensitivity analysis ‐ incomplete outcome data: Need for additional means of pain relief | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
Comparison 3. Epidural versus TENS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal pain score in labour | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐53.00 [‐57.98, ‐48.02] |
| 2 Instrumental delivery | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.64] |
| 3 Caesarean section | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 4 Hypotension as defined by trial authors | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 5 Urinary retention | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 6 Nausea and vomiting | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Length of second stage of labour (minutes) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 17.90 [5.66, 30.14] |
| 8 Oxytocin augmentation | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.59, 1.97] |
Comparison 4. Epidural versus inhaled analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal satisfaction with pain relief in labour ‐ proportion rating excellent or very good | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.31, 3.62] |
| 2 Caesarean section | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.16, 2.47] |
Comparison 5. Epidural versus continuous support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Satisfaction with pain relief in labour ‐ proportion rating excellent or very good | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [1.00, 1.02] |
| 2 Need for additional means of pain relief | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.00 [0.00, 0.03] |
| 3 Instrumental delivery | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.96, 1.39] |
| 4 Caesarean section | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.91, 1.62] |
| 5 Long‐term backache | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.11] |
| 6 Headache | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.17] |
| 7 Nausea and vomiting | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.80, 1.57] |
| 8 Cathetherisation during labour | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.04, 1.29] |
| 9 Apgar score less than 7 at 5 minutes | 1 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.61, 6.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bofill 1997.
| Methods | Computer‐generated list of random numbers were prepared by an uninvolved 3rd party. Randomisation was accomplished by selection of the next in a series of opaque, sealed envelopes. All women were accounted for. Intention‐to‐treat analysis was used. | |
| Participants | 100 women recruited (epidural N = 49, narcotics N = 51) Eligibility: nulliparous women at 36 ‐ 42 weeks' gestation, in spontaneous labour (at least 4 cm dilated) Exclusion: women with insulin‐dependant diabetes, chronic hypertension, PIH or twin pregnancy | |
| Interventions | Epidural: preload given 500 ‐ 1000 mL sodium lactate 0.25% bupivacaine ± 50 ‐ 100 mg fentanyl until T10 sensory analgesia achieved, then continuous infusion 0.125% bupivacaine with 1.5 mg/mL fentanyl. Continued in 2nd stage Narcotic: 1 ‐ 2 mg butophanol (1 ‐ 2 hourly) IV | |
| Outcomes | Maternal: pain scores measured hourly, length of 1st and 2nd stage of labour, oxytocin in labour, malposition, amniotomy, nausea and vomiting, operative vaginal delivery, caesarean section, caesarean section for dystocia and fetal distress Neonatal: Apgar scores (mean), arterial cord pH, naloxone administration | |
| Notes | University of Mississippi, USA
Active management of labour protocol. 33 of 39 operative vaginal deliveries in epidural group and 17 of 28 operative vaginal deliveries in opoid group were performed for purposes of resident training.
12 (24%) women randomised to narcotic received epidural as well, due to inadequate pain relief. 2 women randomised to epidural delivered before receiving it. Dates: Trial carried out 1995 ‐ 1996 Funding: Supported by the Vicksburg Hospital Medical Foundation Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Selection of the next in a series of opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women in the epidural group were delivered before obtaining regional analgesia and 12 women in the parenteral analgesia received "epidural rescue", but these participants remain in their group for all statistical considerations. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the Methods section have been reported on in the results. |
| Other bias | High risk | More white women in the narcotic group (P = 0.008) |
Camann 1992.
| Methods | All participants randomised according to a random number scheme with instructions contained in sequentially numbered, opaque envelopes | |
| Participants | 24 women were recruited (sufentanil intrathecal N = 9, epidural N = 8, IV N = 7). Eligibility: ASA physical status 1 or 2 parturients requesting epidural analgesia during active labour. All participants were at term and had uncomplicated pregnancies and normal fetal heart tracings. Exclusion: not reported | |
| Interventions | Sufentanil 10 µg either intrathecally (N = 9), epidurally (N = 8) or intravenously (N = 7), using a CSE technique. The sufentanil was administered alone without concomitant local anaesthetics. Participants could request additional analgesia (bupivacaine 0.25% via the epidural catheter) if pain relief was unsatisfactory by 15 mins after injection of study drug. | |
| Outcomes |
|
|
| Notes | Brigham and Women's Hospital, Harvard Medical School, USA
The study was terminated early "We had originally planned to enrol more patients in this protocol but terminated the study when it became clear that a large number of the subjects had clearly unsatisfactory analgesia" page 885, 1st paragraph within Discussion Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to a random number scheme. |
| Allocation concealment (selection bias) | Low risk | All participants randomised in a double‐blind fashion according to a random number scheme with instructions contained in sequentially numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Presume so ‐ states "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Probably ‐ "All injectates were prepared by an anaesthesiologist not involved in subsequent data collection" ‐ implies people collecting data would not have been aware of drug allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised appear to have been accounted for within the results, although only a small number of women were recruited because the study was stopped early. |
| Selective reporting (reporting bias) | High risk | They did not report the results for the following outcomes:
|
| Other bias | High risk | The study was stopped early because "it became clear that a large number of the subjects had clearly unsatisfactory analgesia" so the number of participants in the study was small. |
Chen 2008a.
| Methods | RCT Parallel design Single centre Tongji Hospital, Wuhan, Hubei, China |
|
| Participants | 200 women were randomly divided into 2 groups. Group 1 (N = 100) ‐ labour analgesia group ‐ ropivacaine 3.75 mg and fentanyl 20 µg injected into subarachnoid space while utero‐cervical was opened 2 ‐ 3 cm and then ropivacaine 0.1% plus fentanyl 2 µg/mL was used in epidural space. Group II (N = 100) ‐ natural delivery without analgesia Eligibility: ASA physical status I ‐ II parturients Exclusion: not reported |
|
| Interventions | Group 1 (N = 100) ‐ labour analgesia group ‐ ropivacaine 3.75 mg and fentanyl 20 µg injected into subarachnoid space while utero‐cervical was opened 2 ‐ 3 cm and then ropivacaine 0.1% plus fentanyl 2 µg/mL was used in epidural space. Group II ‐ natural delivery without analgesia |
|
| Outcomes |
|
|
| Notes | Abstract only ‐ so results limited Dates: Not stated Funding: Not stated Declarations of Interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Chen 2008b.
| Methods | Reported to be single‐centre RCT with individual allocation but no information on methods | |
| Participants | 124 women anticipating vaginal delivery were recruited and divided into 2 groups: PCA epidural ropivacaine (N = 75), versus 'no pain relieving methods' (N = 49). Eligibility: women anticipating vaginal delivery (it was not clear whether any women subsequently had CS) Exclusion: pregnancy complications |
|
| Interventions | Group 1 ‐ PCA epidural Ropivacaine ‐ 3 mL ropivacaine (0.125%) injected through an epidural catheter and another 12 mL 5 minutes later if there was no total spinal anaesthesia. The block level was controlled to be below the T10 level. Then 5 mL (0.104 mg/min) per hour until full dilatation. (N = 75). Group 2 ‐ control ‐ "no pain relieving measures” (N = 49) |
|
| Outcomes | Prolactin levels at delivery and 2 hours later and time of the start of lactation Mean newborn weight reduction in the 1st day following delivery. No relevant outcomes reported |
|
| Notes | Trial conducted in China, women attending a hospital in Bejing Dates of trial: January 2006 – June 2007 Funding: not stated Conflicts of Interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Translated notes state "controlled clinical trial" and that women were “randomly divided” into groups but no further information. |
| Allocation concealment (selection bias) | Unclear risk | Translated notes state "controlled clinical trial" and that women were “randomly divided” into groups but no further information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding: women in the control group had no analgesia whereas the intervention group had epidural; staff would be aware of study group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated, although outcomes were measured immediately after delivery, so it is likely outcome assessors were aware of analgesia. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not stated whether or not there was any loss to follow‐up, translated notes report that numbers in tables report the same numbers as those randomised. Intention‐to‐treat analysis: not reported. It was not stated whether any women had CS or whether these women were excluded post‐randomisation. |
| Selective reporting (reporting bias) | High risk | We have no protocol and assessment is from stated notes. Although this was reported as an RCT there was considerable imbalance between groups (75 vs 49). There was no explanation for this. |
| Other bias | Unclear risk | Insufficient information to make a judgement. The control group were reported to receive no analgesia; it is not clear whether this was at the point of randomisation or whether women requesting pain relief were denied it. |
Clark 1998.
| Methods | Computer‐generated, random‐number tables, group assignments were placed in sealed, opaque, sequentially‐numbered envelopes. All women accounted for. Intention‐to‐treat analysis used. | |
| Participants | 318 women recruited (epidural N = 156, meperidine N = 162) Eligibility: nulliparous women in spontaneous labour (at least 50% cervical effacement or ruptured membranes, at least 2 contractions every 15 mins) at 36 weeks' gestation or more, vertex presentation Exclusion: maternal or fetal conditions precluding trial of labour, thrombocytopenia or coagulation disorder, or multiple pregnancy | |
| Interventions | Epidural: IV fluid bolus of 1 litre normal saline solution following by placement of the epidural catheter through the L2 ‐ 3 or L3 ‐ 4 interspace. A test dose of 3 mL 1% lignocaine with epinephrine was administered, followed by 9 mL 0.25% bupivacaine with 50 µg fentanyl in 3 divided doses at 10‐min intervals; if vital signs remained stable during the subsequent 15 mins, a continuous infusion of 0.125% bupivacaine with 1 µg/mL fentanyl was initiated at 12 mL/hr and titrated to maintain anaesthesia to the T10 dermatome level. IV meperidine: 50 to 75 mg meperidine every 90 mins as needed. These participants did not receive pre‐analgesic hydration. | |
| Outcomes | Maternal: oxytocin use, length of 1st and 2nd stages of labour, 2nd stage labour, mode of delivery, caesarean for dystocia, caesarean for fetal distress Neonatal: Apgar score at 5 minutes, meconium, umbilical cord pH/BE (arterial and venous), umbilical artery pH < 7.15 | |
| Notes | University of Louisville Hospital, Kentucky, USA
84 (52%) women in opioid group did not receive intervention (no reason given in paper), but received an epidural. 9 women in epidural group did not receive intervention (5 inability to site catheter, 4 delivered before epidural inserted). Dates: Trial carried out 1995 ‐ 1996 Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially‐numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Because of the large number of cross‐over participants (52%), the data were subsequently analysed with respect to those who were compliant with the assigned analgesic method. 78 of 162 (48.1%) received IV meperidine and 147 of 156 (94.2%) of the epidural group. |
| Selective reporting (reporting bias) | High risk | Additional outcomes reported in tables (Apgar scores, meconium) not specified in the Methods section. |
| Other bias | Low risk | 10 participants were excluded from the data analysis because of protocol violations. |
De Orange 2011.
| Methods | Randomised controlled trial using individual randomisation | |
| Participants | 70 women randomised (combined spinal anaesthesia, n = 35; continuous support, n = 35). Women admitted to the antepartum unit of the Instituto de Medicina Integral Prof Fernando Figueira, Brazil Eligibility: pregnant women, singleton, full‐term fetus with cephalic presentation and cervical dilatation of 3 ‐ 6 cm Excluded: women with fever before or at the time of randomisation, those using antibiotics, those with high‐risk pregnancies (placenta previa, placental abruption, severe pre‐eclampsia/eclampsia, premature delivery, HIV‐positive), and those with an indication of immediate caesarean section |
|
| Interventions | CSE anaesthesia: 2.5 mg of 0.5% heavy bupivacaine associated with 5 mg of sufentanil was injected into the subarachnoid space. Immediately afterwards, the epidural space was punctured using an 18 G Tuohy needle and a catheter was inserted into the same interspinous space used for subarachnoid puncture. Only 30 mins after subarachnoid puncture, administration of 5 mL of a solution containing 0.05% bupivacaine and sufentanil 0.2 mg mL–1 was initiated through the epidural catheter. This solution was administered intermittently every 30 mins until delivery of the infant. CSE was initiated only when requested by participants. 1 woman did not request epidural. Women in both groups received continuous support during delivery provided by a doula or trained lay person, and Swiss exercise balls, massage, and music therapy. |
|
| Outcomes | Satisfaction Loss of control Mode of birth Oxytocin augmentation Fever |
|
| Notes | Setting: hospital in Brazil Dates of trial: February – May 2010 Funding: unclear CoI: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers generated using the Random Allocation Software program |
| Allocation concealment (selection bias) | Low risk | Sealed open envelopes contained the allocation group to which each participant was to be assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible ‐ women and staff would have been aware of intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor: insufficient information, labour outcome probably assessed by caregivers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up occurred after randomisation. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported. |
| Other bias | Low risk | Women in both the groups were balanced for all the baseline characteristics. |
Dickinson 2002.
| Methods | Randomly selected from block group of sealed, opaque envelopes. Primary analysis: intention‐to‐treat analysis. Secondary analysis of compliant participants only, randomisation stratification into spontaneous and induced labour. All women accounted for | |
| Participants | 992 women recruited (epidural N = 493, continuous midwifery support group N = 499) Eligibility: nulliparous women at term with singleton cephalic presentation in spontaneous labour (cervix < 5 cm dilated) and induced labour |
|
| Interventions | CSE: needle‐through‐needle approach. Preload 500 ‐ 1000 mL crystalloids. Spinal block achieved with fentanyl 25 micrograms and bupivacaine 2 mg. Following onset of analgesia epidural catheter dosed with 0.125% bupivacaine ‐6 mL then participant‐controlled epidural analgesia until delivery with 0.1% bupivacaine and 2 micrograms of pethidine. 136 women did not receive epidural. Continuous midwifery support group was 1:1 midwife:participant ratio, IM pethidine, nitrous oxide inhalation, TENS, and/or non‐pharmacological forms of pain relief. | |
| Outcomes | Maternal: pain scores, caesarean section, duration of 1st and 2nd stages of labour. operative vaginal delivery, vomiting, catheterisation during labour, fever (> 37.5 ºC) and satisfaction with childbirth (median VAS); breastfeeding reported on compliant participants only
Neonatal: Apgar scores, cord pH Long‐term outcomes (Orlikowski 2006) ‐ back pain, headache, migraine, mod‐severe back pain, severe headache, severe migraine before pregnancy, during pregnancy, and at 2 (N = 576) and at 6 months (N = 521) postpartum |
|
| Notes | King Edward Memorial Hospital for Women, Perth, Western Australia, between May 1997 and October 1999 Funding: supported by NH&MRC Grant 970076 Conflicts of interest: not mentioned 137 (27%) women randomised to epidural received continuous midwifery support. 306 (62%) women randomised continuous midwifery support received epidural. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Selection from a blocked group of 8 sealed opaque envelopes replenished from blocks of 12 |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women were encouraged to manage their labour with the assistance of a midwife and with the intention of avoiding the use of epidural analgesia. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The cross‐over rate from the EPI to the CMS group was 27.8% (N = 137) and cross‐over rate from CMS to EPI analgesia was 61.3% (N = 306). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | As the compliance rate was approximately 40% in the CMS group and 75% in the EPI group, it would not be possible to distinguish between the caesarean section rates, as hypothesised, without 12,000 participants. As it was not feasible to recruit the number of women required to demonstrate such a difference, enrolment into the trial was stopped. |
Douma 2011.
| Methods | RCT with individual randomisation Participants randomly allocated to 2 intervention groups, control group recruited by observational cohort. Control group data not included in this review. |
|
| Participants | 116 women recruited to 2 treatment groups but data reported only for 98 (epidural analgesia n = 49; intravenous remifentanil patient‐controlled analgesia n = 49). Eligibility: women who are classed as ASA class I or II parturients with a singleton pregnancy, between 37 and 42 weeks of gestation Excluded: BMI ≥ 40 kg/m2, insulin‐dependent diabetes, severe pre‐eclampsia (proteinuria ≥ 5 g/24 hr), use of antibiotics during delivery, initial maternal SpO2 < 98%, initial maternal temperature ≥ 38 oC, cervical dilation of > 7 cm and ruptured membranes for > 24 hrs at the time of inclusion. If delivery occurred within 1 hr of starting the study, women were excluded from analysis. |
|
| Interventions | Epidural analgesia: EA (n = 49) A catheter was inserted at the L2 – 3 or L3 – 4 interspace using a 17‐gauge Tuohy needle. Parturients received a loading dose of ropivacaine 25 mg (0.2% ropivacaine 12.5 mL), followed by a continuous infusion of 0.1% ropivacaine and sufentanil 0.5 µg/mL at 10 mL/h. In case of inadequate analgesia, additional 10 mL boluses were given. In case of epidural catheter dislodgement, the catheter was replaced. rPCA (n = 49) Received a 40 µg bolus (lockout 2 mins, bolus duration 36 s) using a Graseby 3300 syringe pump. The maximum dose permitted was 1200 µg/h. No background infusion was added. Because of concerns about the potential for neonatal respiratory depression, the pump was stopped when the woman reached full cervical dilatation. When parturients were dissatisfied with analgesia, EA was offered as alternative. |
|
| Outcomes | Mode of birth Side effects Apgar scores Umbilical cord gases Duration of labour Satisfaction scores |
|
| Notes | Country and setting: Netherlands, Leiden University Medical Center Dates of trial: November 2008 – October 2010 Funding: Department of Anesthesiology, Leiden University Medical Centre Conflicts of interest: none Neonatal fever 2/49 EA; 2/49 rPCA Sepsis follow‐up 4/49 EA; 3/49 rPCA Positive blood culture 0/49 EA; 0/49 rPCA Overall satisfaction measured post‐delivery 8.4 (SD 1.2)/49 EA; 8.1 (SD 1.2)/49 rPCA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Randomisation list was kept in a numbered opaque sealed envelope that was opened upon the request for analgesia. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible for these interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information on who collected or analysed the outcome given, but probably collected by care provider in labour Satisfaction score was by self‐administered questionnaire |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 women were excluded from the analysis in EA group: 7 delivered < 1 hour of analgesia; 3 met exclusion criteria post‐randomisation but reasons not explicit. 8 women were excluded from the analysis in rPCA group: 6 delivered < 1 hr; 2 "met exclusion criteria". "Continuous saturation data were not always available and this information is reported for only 114 women." 1 women lost to follow‐up in labour in each group. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported as per protocol. |
| Other bias | Unclear risk | Similar baseline characteristics. Some reporting of results is not clear. |
El‐Kerdawy 2010.
| Methods | RCT Parallel design Single centre Cairo, Egypt |
|
| Participants | 30 nulliparous pre‐eclamptic parturient women were randomly divided into 2 equal groups. Epidural group: N = 15 Remifentanil group: N = 15 Eligibility: ≧ 32 weeks' gestation, normal cephalic presentation, < 5 cm cervical dilatation, clinical diagnosis of pre‐eclampsia Exclusion: remifentanil allergy, progression to eclampsia, evidence of increased intracranial pressure or focal neurologic deficit, women with a platelet count of less than 80 x 109/L, or evidence of pulmonary oedema, non‐reassuring fetal heart rate tracing requiring imminent delivery |
|
| Interventions | Epidural group (N = 15): received epidural analgesia according to a standardised protocol using bupivacaine plus fentanyl. Remifentanil group (N = 15): PCA was set up to deliver remifentanil 0.5 µg/kg as a loading bolus infused over 20 s, lockout time of 5 mins, PCA bolus of 0.25 µg/kg, continuous background infusion of 0.05 µg/kg/min, and maximum dose is 3 mg in 4 hrs. Women were advised to start the PCA bolus when they felt signs of a coming uterine contraction. |
|
| Outcomes |
7. Maternal side effects:
8. Assisted vaginal delivery 9. Caesarean section 10. Normal delivery |
|
| Notes | Cairo University, Egypt Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported. |
| Other bias | Low risk | Baseline characteristics of groups were similar. |
Evron 2007.
| Methods | RCT Parallel design Single centre Department of Obstetrics and Gynecology, The Edith Wolfson Medical Center, Israel |
|
| Participants | 60 women recruited ‐ 4 excluded (epidural N = 29, iv meperidine N = 27) Eligibility: healthy, ASA physical status I and II primiparous women in spontaneous labour with singleton cephalic presentation at term Exclusion: not stated |
|
| Interventions | PCEA with 0.2 % ropivacaine (N = 29) Patient‐controlled IV analgesia (PCA) with meperidine (N = 27) |
|
| Outcomes |
|
|
| Notes | Department of Obstetrics and Gynecology, The Edith Wolfson Medical Center, Israel 4 exclusions (3 caesarean deliveries performed for non‐reassuring FHRs and 1 parturient in the meperidine group demanded epidural analgesia) Dates: Trial carried out February to September 2003 Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on computer‐generated codes. |
| Allocation concealment (selection bias) | Low risk | Randomisation was based on computer‐generated codes, maintained in sequentially numbered opaque envelopes until just before use. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dummy IV saline and dummy epidural catheter were used. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Pathologist who examined placenta and umbilical cord was blinded to parturient's temperature. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 exclusions (3 caesarean deliveries performed for non‐reassuring FHRs and 1 parturient in the meperidine group demanded epidural analgesia) ‐ outcome data available for all remaining participants (N = 56). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported within the Methods section are available within the Results. |
| Other bias | Low risk | Baseline characteristics similar between groups. |
Evron 2008.
| Methods | RCT Parallel design Single centre The Wolfson Medical Center, affiliated to Tel‐Aviv University, Israel |
|
| Participants | 213 women recruited to the study, 201 completed it. The remaining 12 completed the delivery quickly and did not require any analgesia. All participants (N = 192) with at least 2 hrs of labour were included in the data analysis. Analgesia was randomly provided for 1 of 4 treatment groups:
Eligibility: healthy women with singleton cephalic presentation at term and presenting in spontaneous active labour. Exclusion: Women were excluded if they initially had a fever (oral temperature ≥ 38 °C), signs of infection, or ruptured membranes for more than 24 hrs. Also excluded if caesarean delivery was anticipated. |
|
| Interventions | Analgesia was randomly provided for 1 of 4 treatment groups:
|
|
| Outcomes |
|
|
| Notes | The Wolfson Medical Center, affiliated to Tel‐Aviv University, Israel The remaining 12 completed the delivery quickly and did not require any analgesia. All participants (N = 192) with at least 2 hrs of labour were included in the data analysis. Dates: Not stated Funding: "Supported by NIH Grant GM 061655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). Mallinckrodt Anesthesiology Products, Inc. (St. Louis, MO) donated the thermocouples we used. Exergen, Inc. (Boston, MA) donated the infrared skin‐temperature thermometer." Declarations of Interest: "None of the authors has any personal financial interest in this research." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on computer‐generated codes. |
| Allocation concealment (selection bias) | Low risk | Randomisation was based on computer‐generated codes that were maintained in sequentially numbered opaque envelopes until just prior to use. The randomisation envelopes were opened and the designated treatment started when the visual analogue pain score (VAPS) reached 30 mm. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The treatment regimen was blinded for the evaluator anaesthesiologists by using 2 patient‐controlled analgesia machine devices (PCIA and PCEA) for every participant. A "dummy" IV saline infusion (PCIA) was attached to parturients with PCEA and the other was a "dummy" epidural catheter attached superficially to the skin and connected to a PCEA syringe in the group with PCIA with remifentanil. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Pathologist was blinded to participant group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 213 women recruited to the study, 201 completed it. The remaining 12 completed the delivery quickly and did not require any analgesia. All patients (N = 192) with at least 2 hrs of labour were included in the data analysis. |
| Selective reporting (reporting bias) | High risk | Actual figures for Apgar scores, heart rate, blood pressure and oxygen saturation not given ‐ just mentioned in narrative, last paragraph page 108 before Discussion. |
| Other bias | Low risk | All groups appear to be similar according to baseline characteristics. |
Freeman 2014.
| Methods | Multicentre randomised controlled trial with individual randomisation | |
| Participants | 1414 women randomised (remifentanil PCA n = 709; epidural n = 705) (data analysed for 3158 women). Dutch consortium for women’s health and reproductivity. Academic hospitals, and general hospital Eligibility: women in secondary and tertiary care (intermediate or high risk), i.e. they have illnesses in their medical history that can affect pregnancy or that are affected by pregnancy or if they have complications in this or previous pregnancies or deliveries. Women were eligible to participate if they were healthy or had a mild systemic disease, aged 18 or older, and were scheduled to deliver vaginally after 32 weeks. Excluded: contradictions for epidural analgesia or hypersensitivity to 1 of the drugs used |
|
| Interventions | Epidural analgesia: women could request this when they requested pain relief, according to local protocol. If pain relief was judged inadequate, women could receive patient‐controlled remifentanil instead of epidural analgesia. Remifentanil: patient‐controlled device was programmed to deliver 30 μg remifentanil (solution 20 µg/mL) on request with a lockout time of 3 mins. The dose could be increased to 40 μg in case of insufficient pain relief or decreased to 20 μg in case of excessive side effects. If pain relief was inadequate, women could request epidural analgesia. They were advised to discontinue using the device during the 2nd stage of labour to minimise the risk of neonatal side effects. Women did not receive any advice about continuing epidural analgesia during 2nd stage of labour. "Of the 709 women randomised to patient controlled remifentanil, 447 (65%) actually received analgesia during labour, compared with 52% (347) in the epidural analgesia group (relative risk 1.32, 95% confidence interval 1.18 to 1.48)." For data analysis in this review we used the number randomised. We did not count the women removed for elective caesarean section. Denominators used: Epidural ‐ 676 women; remifentanil ‐ 687 Difficult to interpret as only 347/676 received epidural, and 447/687 received rPCA. |
|
| Outcomes | Mode of birth Satisfaction scores Oxytocin augmentation Maternal hypotension Maternal respiratory depression Side effects Apgar scores Admission to neonatal special care |
|
| Notes | Country and setting: Netherlands, secondary care Dates of trial: May 2011 – October 2012 Funding: grant from ZonMW (Dutch Organization for Health Care Research and Development) Conflict of interest declared. All authors completed the ICMJE uniform disclosure form: "no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomised programme, randomised in fixed blocks of 3, stratified for centre and parity |
| Allocation concealment (selection bias) | Unclear risk | Allocation code appears after a participant’s initials were entered in to the randomisation programme. Research nurses/midwives as well as attending medical staff performed randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible because of the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There is no information on who assessed or analysed the outcomes. Labour outcomes likely to have been recorded by caregiver. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 709 allocated to remifentanil, 22 were excluded from final analysis due to elective planned caesarean, while in the epidural group 29 were excluded due to elective planned caesarean. In the epidural group, 3 women were lost to follow‐up, while 2 withdrew informed consent after randomisation. Used multiple imputation to correct for missing primary outcome data, imputed missing AUC values for satisfaction with pain relief and pain intensity using 20 imputed datasets. Other missing values were not imputed. Some outcomes were only reported for the women who received the analgesia. Number randomised was used for this review. |
| Selective reporting (reporting bias) | Low risk | Protocol is available and all prespecified outcomes are reported in the main trial. |
| Other bias | Unclear risk | No baseline imbalance but denominators unclear following exclusions for CS. Not all women received analgesia allocated. |
Gambling 1998.
| Methods | Computer‐generated, in groups of 100, allocation was secured in a numbered and sealed envelope. Intention‐to‐treat analysis used. All women accounted for. | |
| Participants | 1223 women recruited (epidural N = 616, meperidine = 607). Eligibility: nulliparous and parous women in spontaneous labour (regular contractions, at least 3 cm dilated), singleton, cephalic presentation, cervix < 5 cm dilated Exclusion: pregnancy complication (not specified), more than 5 cm dilated, multiple pregnancy, non‐cephalic presentation | |
| Interventions | CSE: preload with 500 mL sodium lactate. Catheter L2 ‐ 3 or L3 ‐ 4 interspace. Spinal block with 10 µg sufentanil in 2 mL normal saline. Needle‐through‐needle approach. Following dissipation of spinal analgesia, epidural analgesia achieved with 0.25% bupivacaine in 3 ‐ 5 mL increments to achieve T10 ‐ T8 sensory level. This was followed by epidural infusion 0.125% bupivacaine and 2 microgram per mL fentanyl at 8 mL/h. Rate of infusion halved during 2nd stage of labour. Meperidine group: 50 mg meperidine + 25 mg promethazine hydrochloride intravenously. Further 50 mg IV meperidine on request hourly to a maximum of 200 mg in 4 hrs. All women had IV fluid administration. | |
| Outcomes | Maternal: intrapartum visual analogue pain score and postpartum overall satisfaction with labour analgesia, oxytocin, mode of delivery, hypotension, meconium, surgical amniotomy, motor block, fever, itch, operative vaginal delivery Neonatal: Apgar score, birthweight, cord arterial pH | |
| Notes | University of Texas, USA. Amniotomy routinely performed in active labour when fetal head is well applied to cervix. Intrauterine pressure catheter used to assess adequacy of contraction if progress < 1 cm/hr and oxytocin augmentation employed if uterine pressure < 200 montevideo units.
216 (35%) women randomised to epidural did not receive it (82 received meperidine, 52 declined any analgesia, 43 rapid delivery, 39 non‐study drug used). For 255 (42%) women randomised to meperidine: 102 received epidural as well, 57 received epidural only, 42 declined any analgesia, 30 rapid delivery, 24 non‐study drug used. Dates: Trial carried out 1994 ‐ 1995 Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated in groups of 100 |
| Allocation concealment (selection bias) | Unclear risk | Numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Cross‐over participants were analysed in their original groups. |
| Selective reporting (reporting bias) | High risk | Additional outcome (Apgar score) reported in tables not specified in the Methods section. |
| Other bias | Low risk | None evident |
Genc 2015.
| Methods | Reported to be a prospective randomised controlled study but methods not described. Women randomised into equal‐sized groups. | |
| Participants | 100 women randomised (epidural, N = 50; no epidural, N = 50) Eligibility: healthy, nulliparous women in active labour with 3 ‐ 5 cm cervical dilatation, 3 ‐ 5 contractions in 10 mins, healthy with singleton fetus at term (37 ‐ 41 weeks’ gestation), no evidence of cephalopelvic disproportion Exclusion: amniotic fluid deficiency or fetal heart rate non‐reactivity |
|
| Interventions | Group 1: epidural. N = 50 2 cc test with 40 mg lidocaine; after 5 mins provided woman had no motor block and experienced pain relief, 4 cc of 0.5 bupivacaine and 50 mg of fentanyl were diluted in 0.9% saline and administered as a bolus injection. 5 ‐ 10 cc further administered as needed. Women were in bed in left lateral position. If they had fewer than 3 contractions in 10 mins labour was augmented with oxytocin. Group 2: not described. No epidural analgesia. N = 50 It was not clear whether women received other pharmacological analgesia or whether the same protocol was followed in case of any delay in labour. |
|
| Outcomes | Mode of birth Side effects Duration of labour Hypotension |
|
| Notes | Trial conducted at hospital in Izmir, Turkey. Dates of trial: July 2012 ‐ August 2014 Funding: not reported Conflicts of Interest: the authors reported no conflicts of interest. It was stated for women in the epidural group that if contractions were less than 3 in 10 mins oxytocin was administered. Not clear if the same protocol was used for the control group, so length of 1st stage may be meaningless (more in the ED group may have had oxytocin – this was not clear). Epidural mean 217.9 min (166.33); no epidural 258.87 (158.48) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described although there were equal‐sized groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and staff would be aware of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Most outcomes were recorded during labour by staff providing care, so susceptible to bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Women who had CS were excluded from the analysis. No other loss to follow‐up was reported. Not clear |
| Selective reporting (reporting bias) | Unclear risk | We have no protocol for this study. The intervention was not well described for the comparison group. There did not seem to be a power calculation. |
| Other bias | Unclear risk | The main outcome was duration of labour. There was a clear description of what happened for any delay for women in the intervention group (oxytocin augmentation). It was not clear that women in the non‐epidural group had the same treatment in case of delay. |
Grandjean 1979.
| Methods | Random allocation by drawing lots. All women accounted for | |
| Participants | 90 women recruited (epidural N = 30, phenoperidine N = 30, no analgesia N = 30) Eligibility: women at 38 ‐ 42 weeks' gestation, para 1 or para 2 in spontaneous labour, at 4 cm dilatation with no obstetric complications | |
| Interventions | Epidural: preload not mentioned. Epidural delivery of 12 mL of 1.5% lidocaine in 1:20,000 adrenaline. Followed by top‐ups of 6 mL lignocaine as needed Phenoperidine: IV injection of 1 mg followed by infusion of 34 micrograms per min, with 3l/min humidified oxygen intranasally | |
| Outcomes | Maternal: mode of delivery, blood gases and pH Fetal/neonatal: fetal heart rate, Apgar scores, fetal blood pH and gases, umbilical artery pH | |
| Notes | Toulouse, France
Paper does not state if any women did not receive their allocated treatment. Dates: Year trial carried out not stated Funding: Not stated in translation Declarations of Interest: Not stated in translation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Participants were drawn by lots, no further information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Halpern 2004.
| Methods | RCT Parallel design Multicentre Canada |
|
| Participants | 242 parturients enrolled and assigned to the PCIA group (N = 118) and the PCEA group (N = 124) Eligibility: nulliparous women with healthy term (37 ‐ 42 weeks' gestation) pregnancies from 4 tertiary‐care Canadian centres. ASA I or II in spontaneous labour with singleton pregnancy in vertex presentation Exclusion: pre‐eclampsia, antenatal haemorrhage, a BMI > 35 kg/m2, multiple gestation, abnormal presentation, known fetal anomalies, or fetal distress |
|
| Interventions | Patient‐controlled epidural analgesia (PCEA) with 0.08% bupivacaine and fentanyl 1.6 µg/mL; N = 124 Patient‐controlled IV opioid analgesia (PCIA) with fentanyl; N = 118 |
|
| Outcomes |
|
|
| Notes | Multicentre ‐ 4 tertiary‐care centres, Canada 51 participants (43%) in the PCIA group received epidural analgesia: 39 (33%) because of inadequate pain relief and 12 (10%) to facilitate operative delivery. Dates: Trial carried out September 1997 ‐ December 1999 Funding: Supported by Physicians Services Incorporated Foundation, Toronto; Alberta Heritage Fund; Clinical Teaching and Research Grant, College of Medicine, University of Saskatchewan; Medical Services Incorporated of Alberta; Grace Maternity Research Foundation Grant; and Dalhousie University Department of Anaesthesia Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned to one of two treatment allocations by using a computer‐generated random number system." |
| Allocation concealment (selection bias) | Low risk | "Each centre was randomised separately at a central location. Each centre received sealed, consecutively numbered opaque envelopes that were randomised in blocks of 20." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data were analysed according to group assignment (intention‐to‐treat). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported within the Methods section are available within the Results. |
| Other bias | High risk | According to sample size calculation ‐ 485 participants per group needed ‐ actually recruited 242 patients. "A priori we decided to inspect neonatal data after enrolling 200 patients to ensure neonatal safety. We also decided to stop the study after 2 yr of enrolment, regardless of the number of patients." All groups appear to be similar according to baseline characteristics. |
Head 2002.
| Methods | Computer‐generated block randomisation, stratified according to gestational age (< 35 weeks versus ≥ 35 weeks). Numbered, sealed, opaque envelopes. Intention‐to‐treat analysis used. All women accounted for | |
| Participants | 116 women recruited (meperidine N = 60, epidural N = 56). Eligibility: women > 24 weeks' gestation with severe pre‐eclampsia having singleton vertex presentation and at least 2 cm dilated to 6 cm cervical dilatation | |
| Interventions | Epidural: preload 250 ‐ 500 mL sodium lactate over 20 mins. Epidural catheter placed in L3 ‐ L4 interspace. Test dose of 0.25% bupivacaine 3 mL, then incremental bolus doses of 3 ‐5 mL 0.25% bupivacaine to obtain T‐10 sensory level, maintained by continuous infusion of 0.125% bupivacaine with 2 microgram fentanyl at rate of 10 mL/hr. Meperidine: PCA IV meperidine dose of 10 mg and lockout interval of 10 mins. Maximum dose of 240 mg in 6 hrs also had IV promethazine 25 mg 4‐hourly. All women received IV crystalloid 100 mL/h and magnesium sulphate 4 g bolus followed by infusion of 2 g/hr til 24 hrs postpartum. | |
| Outcomes | Maternal: intrapartum visual analogue pain score, mode of delivery, woman's satisfaction with pain relief, hypotension, headache, eclampsia, acute renal dysfunction Neonatal: Apgar scores, seizure, naloxone administration, neonatal intensive care admission, fetal heart rate abnormalities, umbilical cord pH, birthweight | |
| Notes | Alabama, USA
42 women in the epidural group and 41 women, in control group received opioid prior to randomisation. 25 women in epidural group and 19 women in control group received hydralazine.
7 women did not receive their allocated treatment (5 from opioid group). 1 woman randomised to opioid had epidural as well.
1 woman randomised to opioid had epidural instead.
Year trial carried out not stated. Dates: "42 month study period" Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated block randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Consecutively‐numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 women did not receive the assigned treatment, 3 in the epidural group and 7 in the opioid group. Rapid labour was the most common event that precluded the assigned treatment (epidural, n = 3 versus opioid, n = 5). 1 woman assigned to the opioid group received epidural analgesia at the discretion of the attending anaesthesiologist. Another woman who was assigned to opioids received epidural analgesia after experiencing severe nausea. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the Methods section have been reported on in the Results section. |
| Other bias | Low risk | None evident |
Hogg 2000.
| Methods | "Randomized clinical trial." No further detail in abstract. Intention‐to‐treat analysis used. All women accounted for | |
| Participants | 105 women recruited (epidural N = 53, meperidine N = 52) Eligibility: labouring women with severe pre‐eclampsia at > 24 weeks' gestation | |
| Interventions | Epidural analgesia versus IV PCA with meperidine. No further information in abstract | |
| Outcomes | Maternal: caesarean section, pain score, satisfaction score, maternal ephedrine administration Neonatal: naloxone administration, birthweight, cord pH, NICU admission, deaths | |
| Notes | Birmingham, Alabama, USA
8 of the 105 women did not receive assigned treatment due to rapid labour. 2 in the meperidine group received epidural as well.
Year trial carried out not stated Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 participants did not received the assigned intervention. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Howell 2001.
| Methods | Computer‐generated randomisation at the time of request for pain relief. Intention‐to‐treat analysis used. Outcome assessor for backache blinded. All women accounted for, with the exception of backache (17% loss to follow‐up at 26 months). | |
| Participants | 369 women recruited (epidural N = 184, non‐epidural N = 185). Eligibility: labouring nulliparous women at term with singleton pregnancy and cephalic presentation, with no contraindication to either form of analgesia. | |
| Interventions | Preload not stated. 10 mL of 0.25% bupivacaine. Followed by top‐ups of 0.25% 5 ‐ 10 mL as required. Pethidine: 50 ‐ 100 mg IM pethidine, repeated according to standard midwifery practice. Women in both groups allowed to use Entonox. | |
| Outcomes | Maternal: mode of delivery, length of labour, use of oxytocin, maternal satisfaction with pain relief, backache, postnatal depression, not feeling in control, drowsiness, concerns regarding pain relief, catheterisation postdelivery, postnatal haemoglobin, maternal blood loss at delivery Fetal/neonatal: Apgar scores, umbilical cord pH | |
| Notes | North Staffordshire, UK
52 (28%) women randomised to non‐epidural received epidural. 61 (33%) women randomised to epidural did not receive it. Dates: Trial carried out 1992 ‐ 1997 Funding: "The study was funded by WellBeing, and Ms P. Upton was supported by a grant from the North Staffordshire Medical Institute. The clinical trials work of Mr Richard Johanson and Ms Linda Lucking is supported by a grant from the NHS(E) West Midlands Research and Development Programme." Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocation was displayed on the computer screen. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Women in the epidural and non‐epidural groups remain in the group to which they were initially allocated, regardless of the eventual method of pain relief given during labour. |
| Selective reporting (reporting bias) | High risk | Outcomes not all prespecified in Methods section. |
| Other bias | Low risk | None evident |
Ismail 2012.
| Methods | 3‐armed RCT with individual randomisation 3 blocks of 380 participants. |
|
| Participants | 1140 women recruited (epidural anaesthesia, N = 380; remifentanil group by patient‐controlled IV analgesia, N = 380; combined spinal‐epidural, N = 380). Eligibility: healthy nulliparous pregnant women (with term, singleton pregnancies), who spontaneously went into established labour (with at least 2 painful uterine contractions in 10 mins and the cervix is at least 80% effaced and up to 3 cm dilated) and requesting labour analgesia. Exclusion: (1) Allergy to opioids, a history of the use of centrally‐acting drugs of any sort, chronic pain, and psychiatric diseases records (2) Participants < 18 years or > 40 years (3) Those who were not willing to or could not finish the whole study (4) Alcohol‐ or opioid‐dependent women were excluded for their influence on the analgesic efficacy of the epidural analgesics (5) Women with a non‐vertex presentation or scheduled induction of labour (6) Women with diabetes mellitus and pregnancy‐induced hypertension (7) Twin gestation and breech presentation (8) Any contraindication to neuraxial or systemic opioid analgesia (9) Cervical dilation of 4 cm or more (10) Estimated fetal weight above 4000 g and abnormal fetal heart rate tracing on admission |
|
| Interventions | Group 1: epidural anaesthesia (N = 380) All blocks were performed in the sitting position. The epidural space was located at the L3 – L4 interspace using loss of resistance to air (an 18‐gauge Tuohy needle was used). In both groups, a 3‐mL epidural test dose of 2% lidocaine was given through the epidural catheter. In the EA (Group I), after the test dose, an 8‐mL dose of 0.125 % levobupivacaine with 2 lg/mL fentanyl was administered through the epidural catheter. Then the catheter was connected to an electronic pump set to deliver a continuous infusion of 8 mL/hr of 0.125 % levobupivacaine and 2 lg/mL fentanyl. Further boluses of 5 – 10 mL of 0.125 % levobupivacaine were given by the attending anaesthesiologist upon request. Group 2: remifentanil group by patient‐controlled IV analgesia (N = 380) The PCIA device was set to deliver 0.1 ug/kg of Ultiva (remifentanil hydrochloride, Glaxo Operations UK Ltd, Barnard Castle, Durham, UK), diluted with saline and given as a solution of 25 ug/mL as a bolus infused during a period of 1 min, with a lockout time of 1 min, into an IV catheter attached to a 1‐way line providing continuous infusion of saline at approximately 100 mL/hr. During the study, the IV PCIA bolus was increased following a dose escalation scheme (0.1 – 0.2 – 0.3 – 0.5 – 0.7 – 0.9 ug/kg) after every 2nd contraction until the parturient answered ‘no’ to the question whether she would like to get more efficient pain relief or until a maximum dose of 0.9 ug/kg was achieved. Group 3: combined spinal–epidural (N = 380) A needle‐through‐needle technique was performed with 2 mg levobupivacaine and 15 lg fentanyl (total volume of 2 mL) injected intrathecally and the spinal needle removed. Then the epidural catheter was inserted and connected to an electronic pump set to deliver the same previously‐mentioned mixture. |
|
| Outcomes | Pain score Mode of birth Oxytocin augmentation Side effects Duration of labour Satisfaction with pain relief Apgar scores Cord blood gases |
|
| Notes | Motor block levels according to the Bromage scale (Groups I and III) and sedation levels according to the Ramsay scale (Group II) were observed. Decisions regarding obstetric management were made by the obstetricians. Artificial rupture of membranes was performed (if there was no ROM), and oxytocin infusions were titrated according to our hospital protocol. All participants had continuous external electronic fetal heart rate monitoring and tocodynamometry. Trial conducted at TAIBA Hospital in Kuwait. Dates of trial: September 2009 ‐ August 2011 Funding: not stated Conflicts of Interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomised (in 3 blocks of 380 participants per block) through a computer‐generated, random‐number list to receive either EA (Group I), or patient‐controlled IV analgesia (PCIA) with remifentanil (Group II) or combined spinal–epidural (CSE) analgesia (Group III). The random‐number list was generated by means of the QuickCalcs (GraphPad Software Inc., La Jolla, CA, USA). |
| Allocation concealment (selection bias) | Low risk | The group assignment numbers were sealed in an envelope and kept by the study supervisor. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind participants or caregivers as mode of administration varied. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome data reported to be collected at time of delivery or the day after by assessors not involved in the woman’s care. However, labour outcomes would be recorded by staff providing care. ? e.g. VAS completed hourly during labour. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No post‐randomisation exclusions, no loss to follow‐up reported. 320 excluded prior to randomisation because they did not fit the inclusion criteria. Intention‐to‐treat analysis: no loss to follow‐up or protocol deviations reported (it was not clear how many women actually received the allocated analgesia). |
| Selective reporting (reporting bias) | Low risk | No, no protocol available but all outcomes reported from Methods text and all expected outcomes reported. |
| Other bias | Low risk | No baseline imbalance. Funding source not disclosed |
Jain 2003.
| Methods | Randomisation with Tippets random number table into 3 groups. Allocation was concealed using sealed, opaque envelopes (information obtained directly from trial authors). All women accounted for | |
| Participants | 126 women recruited (epidural N = 43, meperidine N = 39, tramadol N = 44) Eligibility: nulliparous women in spontaneous labour at > 36 weeks' gestation with singleton pregnancy and cephalic presentation Exclusion: cervical dilatation more than 5 cm, evidence of cephalic disproportion, utero placental insufficiency, any medical/surgical complications | |
| Interventions | Preload not mentioned. Test dose 0.25% bupivacaine with adrenaline 1:200,000. Followed by 10 mL bolus of 0.15% bupivacaine and 30 micrograms fentanyl. If further analgesia required after 2 hrs same bolus given. If within 2 hrs the fentanyl reduced to 15 µg, if > 2 top‐ups requested in 1 hr, a continuous infusion of 0.1% bupivacaine and 1 µg fentanyl per mLbegun at rate of 10 mL/hr. Meperidine: 50 ‐ 100 mg IM depending on maternal weight, repeated 4‐hourly. If analgesia requested in < 4 hrs, 1 of above dose is given. Each injection of meperidine is given with 25 mg promethazine. No meperidine is given after cervical dilatation of 8 cm. Tramadol: IM injection of 1 mg/kg weight and not exceeding 200 mg in 24 hrs | |
| Outcomes | Maternal: mode of delivery, pain score, maternal satisfaction with pain relief, duration of 1st and 2nd stages of labour, hypotension, urinary retention, respiratory depression, desire to use same pain relief in future Neonatal: Apgar score, cord pH, naloxone administration | |
| Notes | Chandigarh, India
All women received assigned allocation.
Year trial carried out not stated Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Tippets random table |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using sealed, opaque envelopes (information obtained directly from trial authors). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 from group I delivered by caesarean section before analgesia could be given. |
| Selective reporting (reporting bias) | High risk | Outcomes documented in Methods section not reported ‐ PPH and neonatal sepsis |
| Other bias | Low risk | None evident |
Jain 2012.
| Methods | RCT. Unit of randomisation not clear, probably individual | |
| Participants | 36 women (Samanta), and 20 women (Jain) randomised (epidural N = ?, tramadol N = ?). Eligibility: pregnant women at term gestation with sonographic evidence of umbilical artery systolic‐diastolic ratio ≥ 3 (FGR) |
|
| Interventions | Epidural parturients received an incremental bolus of 10 mL ropivacaine 0.1% with 2ì/mL fentanyl followed by 5 ‐ 15 mL/hr continuous infusion of the same drug. Tramadol parturients received intramuscular tramadol 1 mg/kg repeated every 4 hrs. | |
| Outcomes | Changes in doppler pulsality index Apgar scores Cord blood gases |
|
| Notes | Authors contacted for more information Trial conducted in India but no further detail given. Dates of trial: not stated Funding: not stated Conflicts of Interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Infeasible to blind this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome collection during labour so likely recorded by staff providing care that would be aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Jain reports 20 women randomised – data only analysed for 14. Samanta reports 36 women randomised – data only analysed for 30. Not clear how many women were in this study or why 6 were excluded post‐randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess from abstract |
| Other bias | Unclear risk | Unable to assess from abstract |
Jaitley 2011.
| Methods | 3‐arm RCT with individual randomisation | |
| Participants | 90 women randomised (tramadol via epidural N = 30, tramadol IV N = 30, control N = 30) Eligibility: 37 ‐ 41 weeks of pregnancy, primipararous and multipararous women in established active stage of labour (uterine contraction 2 per 10 mins, lasting for 30 to 40 s and cervical dilation > 3 cm) with vertex presentation and willing for analgesia Exclusion: malpresentation, cephalopelvic disproportion, previous caesarean section, antepartum haemorrhage, any medical complications (diabetes, asthma, primary pulmonary hypertension, hypertensive disorders of pregnancy, etc.) |
|
| Interventions | Tramadol via epidural: tramadol in doses of 1 mg/kg body weight along with 8 ‐ 10 mL of 0.25% bupivacaine was given by epidural route, N = 30 Tramadol IV: tramadol in doses of 1 mg/kg body weight IV bolus and 100 mg in 500 ml Ringer’s lactate drip at the rate of 8 ‐ 24 drops/min was given, N = 30 Control Group: control not described. No information whether women in the control group received any analgesia or whether they were denied analgesia (all of this group reported moderate to intolerable pain), N = 30 |
|
| Outcomes | Pain intensity Satisfaction with pain relief Spontaneous birth |
|
| Notes | No information whether women in the control group received any analgesia or whether they were denied analgesia (all of this group reported moderate to intolerable pain). Conducted in the Department of Obstetrics & Gynaecology, S.N.Medical College, Agra, India Dates of trial: not stated. Study accepted by journal 2010 Funding: not stated Conflicts of Interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | States “randomly divided”, although also says that the study group was subdivided into 2 groups. Not clear whether this was done randomly or not, or how many went into each group (although in tables results are reported for 30 women in each group). |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Infeasible to blind women or staff |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned – probably high, as outcomes relate to labour and staff providing care would also have recorded outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears to report all, but numbers in each group not clearly stated. Not clear in tables whether all data are reported. Unclear if ITT |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not prespecified in Method text |
| Other bias | Unclear risk | Similar baseline characteristics. The methods were generally not clear |
Jalil 2009.
| Methods | RCT with individual randomisation | |
| Participants | 192 women randomised (epidural n = 94; pethidine n = 98) Eligible: women in labour with ASA 1 ‐ 11, gravida 2 ‐ 5 with tested pelvis, spontaneous onset of labour, age between 18 ‐ 40 years old, singleton fetus with cephalic presentation, presenting OS 3 ‐ 5 cm, height more than 150 cm, and weight less than 100 kg Excluded: bad obstetric history, post‐date, history of allergy to local anaesthetic, patient refusal, failed epidural and those who had contraindications for epidural analgesia |
|
| Interventions | Epidural (n = 94) Received IV fluid bolus of at least 500 mL of Ringer’s Lactate solution. Lumber epidural analgesia was achieved using an indwelling catheter inserted by 18‐gauge Tuohy needle at L2 ‐ L3 or L3 ‐ L4 interspaces. A 3‐mL test dose of 0.2% ropivacaine was given followed by a bolus dose making the total dose of 12 mL. This was followed by continuous epidural infusion of 0.2% ropivacaine with 2 ug/mL fentanyl at 7 ‐ 10 mL/hr. Pethidine IM (n = 98) 75 ‐ 100 mg IM pethidine with 25 mg promethazine hydrochloride at first request of pain relief. Additional 75 mg of pethidine were given by request to a maximum of 300 mg in 4 hrs. Both groups were able to self‐administer nitrous oxide. |
|
| Outcomes | Mode of birth Oxytocin administration Apgar scores Pain score Satisfaction score Duration of labour |
|
| Notes | Setting: hospital setting in Malaysia Dates of trial: 2005 ‐ 2006 Funding: Universiti Sains Malaysia short‐term grant no. 304/ppsp/613131 Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A trained staff nurse would choose an envelope to allocate the patient randomly (closed envelope technique).” |
| Allocation concealment (selection bias) | Unclear risk | “A trained staff nurse would choose an envelope to allocate the patient randomly (closed envelope technique).” It was not stated how the sequence was generated, whether the envelopes were sealed, in sequential order and all accounted for. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors say women were blinded to the expected effects of epidural but they cannot have been blind to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not clear who recorded outcomes, assuming it was care provider in labour |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported, data reported for each participant. It was not stated if there were any missing data for any outcomes. |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the Methods are all reported clearly. We did not have a study protocol. All expected outcomes reported. |
| Other bias | Unclear risk | Baseline characteristics similar in both groups. The clinical management of women in the 2 groups varied and this made it difficult to interpret some results. |
Khadem 2013.
| Methods | RCT with individual randomisation | |
| Participants | 86 women randomised (epidural n = 42; inhaled nitrous oxide n = 44) Eligibility: nulliparous women, consent given for analgesia, no contraindication for vaginal delivery, single pregnancy, gestational age ≥ 37 weeks, cephalic presentation, active phase of labour (cervical dilatation 3 ‐ 5 cm with contractions occurring at least once every 3 mins), no contraindication for regional analgesia (coagulopathy disorder, infections in the site of catheter insertion, and haemodynamic instability) Excluded: labour arrest, maternal or fetal problems which need caesarean, previous caesarean |
|
| Interventions | Epidural (n = 42) Epidural group were placed in sterile conditions, and after hydration by 500 mL ringer lactate, epidural was entered to epidural space from lumbar site L3 ‐ L4 or L4 ‐ L5 with Tuohy needle size 18, then it was entered 4 ‐ 6 cm into the space and then epidural needle was removed and catheter was fixed in the site using suture. The participant was controlled in the view of labour development and fetal heart monitoring. When dilatation was 5 cm, 1st dose including bupivacaine 0.125%, fentanyl 1 μg/mL in volume of 8 ‐ 10 mL was injected at the beginning. Then dilution solution was infused with speed of 8 ‐ 15 mL/h related to the participant’s need. If it was required, the concentration of bupivacaine was increased to 0.25%. Inhaled nitrous oxide (n = 44, data for 42) Inhaled nitrous oxide by a mask simultaneously with beginning of feeling contraction by mother. In pain intervals, mask was removed and room air was inhaled by mother. "2 mothers didn't continue the study due to giddiness and they were excluded from the study." |
|
| Outcomes | Satifaction with pain relief Mode of birth |
|
| Notes | Describe setting: hospital setting in Iran Dates of trial: 10 May 2010 – 10 May 2011 Funding: Women’s Health Research Center of Mashhad University of Medical Sciences Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly divided into 2 groups by means of random numbers of calculator |
| Allocation concealment (selection bias) | Unclear risk | Concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Infeasible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned, assuming not blinded and caregiver collected information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 women withdrew consent following randomisation – no reason given. Women who had a caesarean section for fetal distress were excluded, although unclear how many women this applied to. 2 women were excluded due to ‘giddiness’ in the Entonox group. Difficult to assess this domain due to poor reporting. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and although outcomes are reported as prespecified in Methods section, outcome data are not reported clearly |
| Other bias | Unclear risk | Similar baseline characteristics but poor reporting |
Lian 2008.
| Methods | RCT Parallel design China |
|
| Participants | 75 voluntary pregnancies were randomised: group A (N = 25), Group B (N = 25), Group C (N = 25). Eligibility: ASA I ‐ II, primiparous with completely normal pregnancy and labour stage of cervical os opening 2 ‐ 3 cm Exclusion: not reported (abstract only) |
|
| Interventions | Group A (N = 25) ‐ control ‐ no medicine to ease pain Group B (N = 25) ‐ epidural analgesia ‐ combination of ropivacaine and fentanyl firstly with a dose of 10 mL by way of cavitas epiduralis, then additional 5 mL was carried over with the assurance of uncavitas subarachnoidealis Group C (N = 25) ‐ CSE analgesia |
|
| Outcomes |
|
|
| Notes | Data limited as only abstract available. Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only available |
| Allocation concealment (selection bias) | Unclear risk | Abstract only available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Abstract only available. Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only available. Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only available |
| Selective reporting (reporting bias) | Unclear risk | Abstract only available |
| Other bias | Unclear risk | Abstract only available |
Liu 2015.
| Methods | Reported to be randomised trial with individual women randomised. | |
| Participants | 120 women randomised (epidural PCEA, N = 30; PCIA ondansetron, N = 30; Acu‐stimulation, N = 30; no analgesia, N = 30) Eligibility: no previous poor obstetric outcome, no experience of Hans acupoint nerve stimulator and TENS, term pregnancy (> 37 weeks’ gestation), active stage of 1st stage with cervical dilatation 3 cm Exclusion: allergy to study drugs, maternal morbidity such as mental or neurological disease affecting evaluation of pain, pregnancy complications such as gestational hypertension, gestational diabetes, gestational thyroid disease, had already taken analgesia or had long‐term use of analgesic drugs, had already used sedative drugs in labour, had low or high BMI (< 18.5 or > 25 kg/m2) |
|
| Interventions | 3 study groups: all treatments stopped at full dilatation. 30 women in each Group 1 ‐ epidural PCEA. Combined spinal 3 mg ropivacaine, epidural 100 mL 0.1% ropivacaine and 50 mcg of sufentanil; background infusion 5 mL, PCA dose 5 mL with 10 minute lockout Group 2 ‐ PCIA ondansetron 8 mg, 5 mins later 1.5 mg/kg tramadol, with 50 mL 0.7 tramadol and 8 mg ondansetron background and 2 mL PCA dose, with 10‐min lockout Group 3 ‐ Acu‐stimulation. Pulse stimulus at acupoints – Jiaji points (T 10 ‐ L3) and Ciliao (BL 32). 100 Hz with burst frequency 2 Hz, intensity 15 ‐ 30 mA, pulse duration 30 minutes Group 4 ‐ control. No analgesia |
|
| Outcomes | Pain Duration of labour Mode of birth Oxytocin augmentation Maternal hypotension Side effects Neonatal asphyxia |
|
| Notes | Trial conducted at hospital in Bejing, China. Dates of trial: August 2010 – November 2013 Funding: not stated Conflicts of Interest: not reported Data from groups 2, 3, and 4 combined to form overall comparison group. Maternal hypotension EA 1/30, Control 0/90 Neonatal asphyxia EA 1/30, Control 6/90 Pain after 1 hour EA 20 (6), Acu 65 (12), Opiate 45 (8), Control 97 (14) Duration of 1st stage EA 423.3 (181.2), Acu 430.1 (119.8), Opiate 425.2 (198.7), Control 439.6 (200.3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reports using random‐number tables |
| Allocation concealment (selection bias) | Unclear risk | Not described, reports using random‐number tables but there were 4 equal‐sized study groups (30 women in each) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and caregivers would be aware of interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported although most outcomes were recorded in labour by staff providing care. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were some discrepancies between tables, While the study flow diagram suggests there were 40 women in each group, the results tables report results for 120 women (30 in each group). There was no report of any missing data. The denominators for mean duration of labour appear to include all women (i.e. women having CS were not excluded). |
| Selective reporting (reporting bias) | Unclear risk | No protocol. There was no power calculation. |
| Other bias | Unclear risk | Groups appeared similar at baseline. The equal‐sized study groups, discrepancies between tables and lack of clarity regarding denominators make the results difficult to interpret. |
Logtenberg 2017.
| Methods | Multicentre open‐label randomised trial with individual randomisation (described as randomised equivalence trial) in 18 midwifery practices in the Netherlands, positioned within the Dutch Obstetric Consortium for women’s health research | |
| Participants | 418 randomised before labour (IV remifentanil, n = 208; epidural, n = 210). Eligibility: low‐risk women beyond 32 weeks of gestation under the care of primary‐care midwives were eligible. Excluded: women < 18 years, women with a contraindication for epidural analgesia or a hypersensitivity to opioid and women in whom labour had already started were not eligible. |
|
| Interventions | Intravenous remifentanil patient‐controlled analgesia (RPCA) (n = 208 – 203 analysed): Intravenous remifentanil 30‐lg boluses (solution 20 lg/mL) with a lockout time of 3 mins and without background infusion. A doctor or a midwife and a nurse were responsible for providing and monitoring the RPCA. The RPCA was administered by the parturient herself after instruction on how to use RPCA in the most beneficial way, which is to use the bolus dose just before the anticipated contraction. It was possible to increase the bolus dosage to 40 lg in case of insufficient pain relief, or to decrease the dose to 20 lg in case of excessive side effects. Epidural anaesthesia (n = 210 – 206 analysed): EA with a loading dose of 25 mg (12.5 mL ropivacaine 0.2%) and continuous infusion of ropivacaine 0.1% plus sufentanil 0.5 lg/mL was administered. Continuous infusion was used at a variable rate defined by the anaesthetist and the local protocol. Additional boluses were used for inadequate levels of analgesia. |
|
| Outcomes | Pain intensity Satisfaction Mode of birth Maternal respiratory depression Headache Fever PPH Apgar scores Duration of 2nd stage |
|
| Notes | Country and setting: Netherlands Dates of trial: November 2012 ‐ June 2013 Funding: no funding sources stated. “For this study we did not receive funding or supplies (such as financial supply or supply of drugs).” ZonMW (www.zonmw.nl) Dossier number 80‐82310‐97‐11039 Conflicts of interest: stated on website – cannot find. Only 94/203 received RPCA, and 76/206 received EA ‐ authors contacted for more information. Data for pain intensity reported as "area under the curve" ‐ unusable data. Reports that "among women who actually received analgesia scores for satisfaction with pain relief were significantly lower in the rPCA group compared with the EA group." 23/94 women in Epidural group, and 35/76 women in rPCA reported satisfaction with analgesia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed using a web‐based randomisation programme stratified for midwifery practice and parity Randomisation was done before labour. |
| Allocation concealment (selection bias) | Unclear risk | "Both the woman and the midwife knew the randomisation allocation in case a request for pain relief should occur during labour." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and midwife knew the randomisation allocation. Not feasible to blind these interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned, assumed not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 removed after randomisation for elective section (5 in RPCA group, 4 in EA group). “If analgesia with the randomly allocated pain method was insufficient according to the woman, a switch to the other trial arm was allowed.” Reported to be intention‐to‐treat with only ElCS women excluded from analysis, although side effects were only reported for those women receiving allocated intervention? There were missing data for some outcomes. Some outcomes were only reported for the women who received the analgesia. We used number randomised for this review. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. The primary outcome was changed before analysis from satisfaction with pain relief at given time points to area under the curve. |
| Other bias | Unclear risk | Similar baseline characteristics 94/203 women in RPCA group received analgesia (105 requested pain relief). 76/206 women in epidural group received analgesia (101 requested pain relief). Results from this study were very difficult to interpret as fewer than half of the women received the allocated intervention. (Authors contacted for more information) |
Long 2003.
| Methods | "Randomly divided into 3 groups." No further information. Intention‐to‐treat analysis used. All women accounted for | |
| Participants | 80 women recruited (CSE N = 30, tramadol N = 20, no analgesia N = 30). Eligibility: women at 37 ‐ 41 weeks' gestation in spontaneous, uncomplicated labour, aged between 23 and 32 years, ASA I ‐ II and expected to have vaginal delivery Exclusion: ASA physical status at least III, clinical contraindications to epidural |
|
| Interventions | Group 1 CSE: preload not mentioned, spinal administration of 2.5 mg ropivacaine with 5 micrograms of fentanyl. Epidural mixture of 0.1% ropivacaine and 1.5 micrograms of fentanyl PCEA infusing at 4 mL/h with PCEA dose of 4 mL and lockout time of 15 mins Group 2 Tramadol: 1 mg/kg loading dose IV followed by PCIA with 0.75% tramadol. PCA dose of 2 mL infusing at 2 mL/hr with 10 mins lockout, maximum dose of 400 mg. 5 mg navoban given IV to prevent nausea and vomiting Group 3 received no analgesia. | |
| Outcomes | Maternal: pain scores, motor block assessed with modified Bromage score, duration of 1st and 2nd stages of labour, caesarean section, sedation, nausea and vomiting, urinary retention, post‐dural puncture headache Neonatal: Apgar score | |
| Notes | Beijing, China
Paper does not state if any women did not receive their allocated treatment. Trial did not record side effect data for no‐analgesia group. Dates: Year trial carried out not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly divided in to 3 groups. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | All outcomes in the Methods section have been reported on in the Results section. |
| Other bias | Unclear risk | Insufficient information |
Loughnan 2000.
| Methods | Computerised random‐number allocation, sealed, opaque envelopes. Intention‐to‐ treat analysis used; however, backache (at 6 months) analysed on data of women who responded to questionnaire only. Secondary analysis based on actual analgesia received. All women accounted for, with the exception of backache (17% loss to follow‐up at 6 months). | |
| Participants | 614 women recruited (epidural N = 304, pethidine N = 310). Eligibility: nulliparous women with term singleton pregnancy, cephalic presentation, in spontaneous or induced labour, with no evidence of cephalic pelvic disproportion Exclusion: any medical/obstetric complications. | |
| Interventions | Epidural: 0.25% bupivacaine 10 mL followed by infusion of 0.125% bupivacaine at 10 mL/hr until 2nd stage Lignocaine 2% was administered for instrumental or caesarean delivery Pethidine: 100 mg IM injection | |
| Outcomes | Maternal: mode of delivery, long‐term backache, duration of 1st and 2nd stages of labour, oxytocin augmentation, pain scores Neonatal: admission to NICU | |
| Notes | Northwick Park, England
86 (28%) women randomised to pethidine received epidural as well. 89 (29%) of women on pethidine received epidural instead and 3 used Entonox.
13 (4%) women randomised to epidural received pethidine as well, 44 (14%) received pethidine alone and 3 used Entonox alone. Dates: Trial carried out 1992 ‐ 1995 Funding: National Health Service Executive, North Thames Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised number generation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed in their original groups with no loss |
| Selective reporting (reporting bias) | Low risk | All outcomes in the Methods section have been reported on in the Results section. |
| Other bias | Low risk | No obvious signs of other bias |
Lucas 2001.
| Methods | Computer‐generated numbers, in opaque, sealed envelopes Intention‐to‐treat analysis used. All women accounted for | |
| Participants | 738 women randomised (epidural N = 372, meperidine PCIA N = 366) Eligibility: parous and nulliparous women with PIH (diastolic at least 90 mmHg) in spontaneous or induced labour 20 women in the epidural group and 18 in the control group had gestation < 36 weeks. Exclusion: chronic hypertension, or received any analgesia/sedation prior | |
| Interventions | Epidural: preload with 500 mL sodium lactate. Epidural analgesia achieved with boluses of 0.25% bupivacaine to T10 level of sensory analgesia, followed by continuous infusion of 0.125% bupivacaine with 2 mg/mL of fentanyl titrated to maintain analgesia Meperidine: IV bolus of 50 mg meperidine with 25 mg promethazine followed by PCA infusion up to 15 mg every 10 mins All women received a loading dose of IM magnesium sulphate 10 g and maintenance dose of 5 g every 4 hrs to prevent eclampsia | |
| Outcomes | Maternal: duration of 1st and 2nd stages of labour, hypotension, fever, oxytocin augmentation, mode of delivery, ephedrine use, pulmonary oedema, postpartum oliguria, postpartum weight loss Neonatal: Apgar scores, umbilical artery pH, naloxone administration, birthweight, NICU, ventilation/24 hrs | |
| Notes | Texas, USA
3 women in each group required additional analgesia. Dates: Trial carried out 1996 ‐ 1998 Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77 women did not receive treatment as specified by the protocol, but analyses were reported as to intention‐to‐treat. |
| Selective reporting (reporting bias) | High risk | Outcomes documented in Methods section not reported ‐ serial laboratory values that included haematocrit level, platelet count, creatinine level, and liver enzymes. |
| Other bias | High risk | Nulliparous women, more of whom were assigned to the PCIA group (P = 0.005). |
Morgan‐Ortiz 1999.
| Methods | "Randomised into 2 groups", no further information given. Intention‐to‐treat analysis used | |
| Participants | 129 women recruited (epidural N = 69, no analgesia N = 63) Eligibility: primiparous women in "beginning of active phase of labour". | |
| Interventions | Epidural bupivacaine versus no analgesia. No further information in abstract | |
| Outcomes | Maternal: duration of 1st and 2nd stages of labour, pain scores Neonatal: Apgar scores, Silverman score | |
| Notes | Sinaloa, Mexico
Paper does not state if any women did not receive their allocated treatment. Dates: Trial carried out 1997 ‐ 1998 Funding: Not stated in translation Declarations of Interest: Not stated in translation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised into 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Randomly divided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | All outcomes in the Methods section have been reported on in the Results section. |
| Other bias | Unclear risk | Insufficient information |
Morris 1994.
| Methods | RCT Double‐blind, randomised, cross‐over fashion Single centre University of Saskatchewan, Canada |
|
| Participants | 100 labouring parturients assigned to IV fentanyl group (N = 50) or the epidural fentanyl group (N = 50) Eligibility: ASA I and II labouring parturients requesting epidural Exclusion: "There were no specific exclusion criteria apart from drug allergy". |
|
| Interventions | IV fentanyl group (N = 50) ‐ 100 µg fentanyl IV and saline by an epidural catheter Epidural fentanyl group (N = 50) ‐ saline IV and 100 µg fentanyl by an epidural catheter |
|
| Outcomes |
|
|
| Notes | Single centre ‐ Canada Cross‐over ‐ at 2 hrs those participants who had not yet delivered were crossed over to the other study medication by the alternate route. Out of 100 labouring parturients, 41 crossed over to receive fentanyl by the alternate route ‐ does not specify how many from each group crossed over. Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | An anaesthetist initially prepared the syringes with either fentanyl or saline. These were then allocated by a separate study nurse: "These syringes together with labels enclosed in a randomisation envelope were given to an attending nurse who then re‐labelled the syringes, "epidural" or "intravenous", according to instructions within the envelope". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "An anaesthetist blinded to the route of administration questioned each patient with regard to changes in analgesia, level of sedation, dizziness, or euphoria. He or she then guessed as to whether this patient had received intravenous fentanyl" within abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised appear to have been accounted for within the results ‐ although 41 crossed over and it does not specify from and to which group. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported within the Methods section are available within the Results. |
| Other bias | Low risk | Baseline characteristics of groups were similar ‐ "There were no differences between the groups at initial randomisation with regard to age, height, weight, parity, or racial origin (Table I)". |
Muir 1996.
| Methods | Women "prospectively randomised", no further information given | |
| Participants | 50 women recruited (epidural N = 28, meperidine N = 22) Eligibility: uncomplicated primiparous women in spontaneous labour | |
| Interventions | Epidural method: preload not stated Bupivacaine 0.125% with adrenaline, 10 ‐ 15 mL, plus pethidine 25 mg, followed by PCA (bupivacaine 0.125% with adrenaline plus pethidine 0.5 mg/mL, 4 mL boluses, lockout 15 mins) 2nd stage: epidural use not stated Control method: IV pethidine by PCA pump (up to 1 mg/kg loading dose, followed by 10 mg boluses, lockout 10 mins | |
| Outcomes | Maternal: pain scores, motor and sensory block, duration of labour, cervical dilation, use of oxytocin, mode of delivery, maternal satisfaction, temperature Neonatal: Apgar score, cord pH < 7.15 (epidural 1/28, control 2/22) and NACS score at 2 and 24 hrs | |
| Notes | Canada
11 (50%) women randomised to meperidine received epidural.
An additional 3 women were enrolled into the trial, all were excluded for technical or equipment failures (group not stated). Dates: Year trial carried out not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | High risk | Outcome documented in Methods section not reported ‐ oxytocin use |
| Other bias | Unclear risk | Insufficient information |
Muir 2000.
| Methods | Participants randomly assigned to receive PCEA or PCIA. Computer‐generated random number system concealed in consecutively‐numbered sealed, opaque envelopes (further information was obtained directly from trial authors). Intention‐to‐treat analysis was used. All women accounted for | |
| Participants | 185 women recruited (epidural = 97, IV fentanyl = 88) Eligibility: healthy, nulliparous, spontaneous labour, requesting analgesia Exclusions: any condition known to increase incidence of operative delivery | |
| Interventions | Epidural: 0.08% bupivacaine + 1.67 mcg/mL fentanyl ‐ loading dose of 10 ‐ 15 mL followed by 5 mL every 10 minutes as needed IV fentanyl ‐ loading dose of 1 ‐ 2 µg followed by 50 µg every 10 mins as needed | |
| Outcomes | Maternal: pain scores, satisfaction with analgesia, need for further analgesia, duration of analgesia, caesarean section rate Infant: Apgar scores, NICU admission, cord pH, neuro‐adaptive scores, cord fentanyl levels | |
| Notes | Canada. Multicentre trial. 18 (20%) women in the IV fentanyl group received an epidural also. Dates: Year trial carried out not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random‐number system concealed in consecutively‐numbered sealed, opaque envelopes (further information was obtained directly from trial authors). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | High risk | Safety outcomes documented in Methods section not reported. |
| Other bias | Unclear risk | Insufficient information |
Nikkola 1997.
| Methods | "Randomised" method not specified Intention‐to‐treat analysis used. All women were accounted for. | |
| Participants | 20 women recruited (epidural N = 10, fentanyl N = 10) Healthy primigravidas, aged 20 ‐ 35 years Exclusion: complications of pregnancy, regular use of drugs and chronic disease | |
| Interventions | Epidural: preload unknown 6 mL 0.5% bupivacaine initially. Intermittent top‐ups with 4 mL (only 1st stage) IV narcotic: fentanyl 50 mg initially. PCA delivered. 20 mg boluses (only 1st stage) | |
| Outcomes | Maternal: VAS pain score, side effects, length of labour after analgesia, mode of delivery, heart rate, oxygen saturation Fetal/neonatal: CTG variability, Apgar score, cord pH arterial and venous, Amiel‐Tison's neurological score, birthweight | |
| Notes | Finland
4 (40%) women randomised to fentanyl received epidural as well Dates: Not stated Funding: "supported by funds from Instrumentarium Research Foundation, Finland and funds from Turku University Hospital, Finland" Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 of 10 participants in fentanyl group received epidural because of unsatisfactory pain relief and 1 because fentanyl prolonged delivery. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the Methods section have been reported on in the Results. |
| Other bias | Low risk | |
Philipsen 1989.
| Methods | "Randomly assigned by random numbers, contained in sealed, consecutively opened envelopes." 1 woman in non‐epidural group lost to follow‐up. Intention‐to‐treat analysis used | |
| Participants | 112 women recruited (epidural N = 57, pethidine N = 55) Eligibility: 37 ‐ 42 weeks' gestation, no medical/obstetric abnormality, in early spontaneous labour, no scars on uterus, 104/112 primiparous | |
| Interventions | Epidural method: preload given. Bupivacaine 0.375% (1 mL per 10 kg) by intermittent top‐up. T10 ‐ L1 block.
2nd stage: epidural use discontinued
Control method: pethidine 75 mg IM (x 1 ‐ 2) All women offered nitrous oxide/oxygen inhalation on demand, and pudendal block (20 mL mepivacaine) in 2nd stage |
|
| Outcomes | Maternal: pain, hypotension, nausea and vomiting, urinary retention, sleepiness, motor blockade, length of 1st stage of labour, duration of 2nd stage of labour, position of fetal head at delivery, mode of delivery, maternal memory of labour Fetal/neonatal: fetal heart rate abnormality, Apgar score at 5 minutes, cord venous pH, neurobehavioural abnormalities | |
| Notes | Denmark
9 (16%) women randomised epidural and 29 (53%) women randomised pethidine had entonox also. Dates: Year trial carried out not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Sealed, consecutively opened envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant loss |
| Selective reporting (reporting bias) | High risk | Additional outcomes reported in tables not specified in the Methods section. |
| Other bias | High risk | 9 participants in the epidural group and 29 in the pethidine group used nitrous oxide. The participants' pain scores in stage 2 were found equal in the 2 groups, 86% in the pethidine versus 85% in the epidural group had a pudendal block. |
Rabie 2006.
| Methods | RCT Parallel design Single centre Riyadh, Saudi Arabia |
|
| Participants | 30 pregnant women were randomised to Group EP (N = 15) ‐ epidural or Group R ‐ (N = 15). Eligibility: ASA I or II with no obstetric complications or contraindication to remifentanil or epidural analgesia Exclusion: not reported |
|
| Interventions | Group EP (N = 15) ‐ epidural analgesia ‐ epidural infusion of bupivacaine 1% plus 2 µg/mL of fentanyl Group R (N = 15) ‐ PCA remifentanil ‐ with a bolus of 0.4 µg kg‐1 over 20 s and a lockout period of 1 min as an analgesia for labour |
|
| Outcomes |
|
|
| Notes | Abstract only, so data limited Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Ramin 1995.
| Methods | RCT Parallel design Single centre University of Texas Southwestern Medical Center, Dallas |
|
| Participants | 1330 women with uncomplicated term pregnancies were randomised to be offered epidural (N = 664) or IV analgesia (N = 666) ‐ 65% of each randomisation group accepted the allocated treatment ‐ epidural group (N = 432), IV group (N = 437). Eligibility: women with normal pregnancies presenting in spontaneous labour Exclusion: women with an identified pregnancy complication, cervical dilatation > 5 cm, or other than singleton cephalic gestations were excluded. |
|
| Interventions | Epidural analgesia ‐ epidural bupivacaine‐fentanyl ‐ at participant's 1st request for pain relief, a 3 mL test dose of 0.25% bupivacaine was given, followed by further 3‐mL increments to achieve a bilateral T‐10‐ sensory level. This was followed by a continuous epidural infusion of 0.125% bupivacaine with 2 µg/mL fentanyl at 8 ‐ 10 mL/hr. The infusion was titrated to achieve a maximum T‐8 sensory level. Additional boluses of fentanyl or bupivacaine or both were injected to overcome inadequate analgesia. IV analgesia ‐ IV meperidine ‐ 50 mg with 25 mg of promethazine hydrochloride IV at 1st request for pain relief. Additional 50 mg doses of meperidine were given on request, to a maximum of 200 mg in 4 hrs. When pain relief was inadequate, epidural analgesia was administered on patient request. |
|
| Outcomes |
|
|
| Notes | Single centre, Dallas, USA 2680 offered participation, 1330 (51%) accepted. 1279 who did not consent to participate were demographically similar to those accepting of 1330 ‐ 664 randomised to epidural ‐ but 232 (35%) never received allocated treatment ‐ half had refused offer of epidural and the remainder progressed to delivery before epidural analgesia could be initiated. 666 women randomised to meperidine IV, but 229 (34%) were not treated ‐ 103 of this group requested epidural after finding meperidine to be inadequate. Dates: Trial carried out November 1 1993 ‐ April 30 1994 Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was computer‐derived in blocks of 20." |
| Allocation concealment (selection bias) | Low risk | Women were randomly assigned using numbered, sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a substantial loss of participants from both groups due to them not following the allocated protocol (35% loss in epidural group, 34% loss in the IV group). All but 1 set of results are therefore based on the available data ‐ operative delivery for dystocia was only outcome analysed on an intention‐to‐treat basis "when comparing the two groups on an intention‐to‐treat basis". However, they do say that a "multivariate analysis of the entire cohort was performed to control for confounding effects of other variables, particularly parity." The results of this cohort analysis are consistent with the labour outcome difference observed between the 2 allocation‐compliant treatment groups for the outcomes of caesarean section and operative delivery for dystocia. |
| Selective reporting (reporting bias) | High risk | Hypotension ‐ described as an outcome in Methods ‐ but not within the Results. All other prespecified outcomes reported within the Methods section are available within the Results. |
| Other bias | Low risk | None evident |
Sabry 2011.
| Methods | Randomised controlled trial (prospective parallel single‐blind) with individual randomisation Set in hospital in Egypt. |
|
| Participants | 60 women randomised to receive combined spinal epidural or epidural with bupivacaine or lidocaine (10 women received each method), or IV pethidine (20 women). Full‐term nulliparous women in active labour with cervical dilatation of 5 cm and cephalic‐presenting fetus. Exclusion criteria: women who had diabetes, neurological disease, pre‐eclampsia, or those who received parenteral analgesics or those with contraindication to epidural or spinal analgesia, or sensitivity to local anaesthetics or opioids were excluded |
|
| Interventions | Group 1 (CSE1) – CSE, bupivacaine, (n = 10): CSE analgesia, 25 µg fentanyl were injected intrathecally and a bolus dose of 10 mL of 0.5% lidocaine injected epidurally. Top‐ups of 5 ‐ 10 mL of 0.5 ‐ 0.8% of lidocaine injected epidurally upon request Group 2 (CSE2) – CSE, lidocaine (n = 10): received CSE analgesia, 25 µg fentanyl were injected intrathecally and a bolus dose of 10 mL of 0.0625% bupivacaine injected epidurally. Top‐ups of 5 ‐ 10 mL of 0.0625 ‐ 0.8% of lidocaine injected epidurally upon request Group 3 (E1) – Epidural, bupivacaine (n = 10): received epidural analgesia, 50 µg fentanyl were injected epidurally together with a bolus dose of 10 mL of 0.5% lidocaine. Top‐ups of 5 ‐ 10 mL of 0.5 ‐ 0.8% of lidocaine injected epidurally upon request Group 4 (E2) – Epidural, lidocaine (n = 10): received epidural analgesia, 50 µg fentanyl were injected epidurally together with a bolus dose of 10 mL of 0.125 – 0.25% bupivacaine injected epidurally upon request Group 5 (IV) (n = 20): 50 mg of IV pethidine administered as a loading dose, followed by 0.5 mg/kg, with a maximum limit of 130 mg 4 epidural groups were combined in data and analysis, and compared with IV pethidine. |
|
| Outcomes | SD for VAS pain score, number of top‐ups, degree of motor block | |
| Notes | Dates of trial: January 2008 – January 2009 Funding: self‐funded Conflicts of interest: none Maternal hypotension EA: 9/40; IV 0/20 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There is no information provided; probably labour outcomes would be collected by caregiver. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that the data for all the women who were randomised are available. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes prespecified in protocol are reported except for modified Bromage scale and time from analgesia to birth. |
| Other bias | Low risk | Demographic variables were comparable between the groups, haemodynamic changes did not differ among the groups. |
Scavone 2002.
| Methods | RCT Parallel design Single centre Northwestern University Medical School, Chicago, USA |
|
| Participants | 100 healthy nulliparous women at term in spontaneous labour or with spontaneous rupture of membranes randomised to intrathecal opioid CSE (N = 49) or systemic opioid (N = 51) Eligibility: nulliparous, healthy at term in spontaneous labour or with spontaneous rupture of membranes requesting labour analgesia < 4 cm cervical dilatation Exclusion: not reported |
|
| Interventions | Intrathecal opioid ‐ as part of a combined spinal/epidural technique (fentanyl 25 µg followed by epidural test dose of 3 mL ‐ 1.5% lidocaine with epinephrine 15 µg) N = 49. Systemic opioid ‐ (hydromorphone 1 mg IV and 1 mg IM) N = 51 |
|
| Outcomes |
|
|
| Notes | Abstract only ‐ so limited data. Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "A perinatologist blinded to patient group examined the heart rate and contraction pattern abnormalities." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Sharma 1997.
| Methods | Randomised sequence was computer‐derived in blocks of 20, with numbered, opaque sealed envelopes. Intention‐to‐treat analysis used. All women accounted for | |
| Participants | 715 women recruited (epidural N = 358, IV meperidine analgesia N = 357) Eligibility: mixed‐parity women in spontaneous labour at term | |
| Interventions | Epidural: preload given Continuous infusion with 0.125% bupivacaine with 2 µg/mL fentanyl. 68% complied with protocol IV narcotic: PCA with meperidine. Additional doses given on request. | |
| Outcomes | Maternal: visual analogue pain scores, length of labour, oxytocin augmentation, fever > 38º centigrade, mode of delivery Fetal/neonatal: meconium in labour, non‐reassuring CTG, Apgar scores, cord pH, naloxone, NICU | |
| Notes | Texas, USA
8 (2%) women randomised to epidural received meperidine instead.
5 (1%) women randomised to meperidine received epidural as well. Dates: Trial carried out 1995 ‐ 1996 Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was computer‐derived in blocks of 20. |
| Allocation concealment (selection bias) | Low risk | Numbered and sealed opaque envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 357 women who were allocated to receive epidural analgesia, 259 completed the study as allocated. Of the 98 women (28%) who did not comply with the patient controlled IV analgesia protocol, 73 progressed rapidly to delivery before receiving analgesia, 20 refused analgesia, and 5, who received meperidine as randomised, later crossed over to epidural analgesia. Of the 358 women who were allocated to receive patient‐ controlled IV analgesia, 243 completed the study as allocated. Of the 115 women (32%) who did not comply with the patient protocol, 87 progressed rapidly to delivery before receiving analgesia, 37 refused analgesia. |
| Selective reporting (reporting bias) | High risk | Some outcomes reported in tables not specified in the Methods section |
| Other bias | Low risk | |
Sharma 2002.
| Methods | Computer‐generated randomisation numbers in sealed envelopes Intention‐to‐treat analysis used. All women accounted for | |
| Participants | 459 women recruited (epidural N = 226, meperidine N = 233) Eligibility: nulliparous, singleton, at term, spontaneous labour, cephalic presentation | |
| Interventions | Epidural: preload given 500 mL sodium lactate. Test dose of 3 mL of 1% lidocaine with epinephrine, then 0.25% bupivacaine in 3 mL increments till T‐10 sensory level analgesia. Then infusion of 0.0625% bupivacaine with 2 µg/mL fentanyl at 6 mL/h with 5 mL boluses every 15 min prn using PCA pump Meperidine: 50 mg IV with 25 mg promethazine followed by PCA pump delivering 15 mg meperidine every 15 mins until delivery. Additional 25 mg are given on request, maximum of 100 mg in 2 hrs. | |
| Outcomes | Maternal: fever, hypotension, oxytocin augmentation, instrumental delivery Infant: Apgar scores, umbilical artery pH, fetal heart abnormalities, birthweight | |
| Notes | Texas, USA
24 women (12 in each group) received another form of analgesia. An additional 14 women in the meperidine group received epidural as well. Dates: Trial carried out 1998 ‐ 2000 Funding: "Support was provided solely from institutional and/or departmental sources." Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐derived in blocks of 20 |
| Allocation concealment (selection bias) | Unclear risk | Numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 who received IV meperidine as randomised crossed over to epidural analgesia because of inadequate pain relief, and 24 women refused their allocated analgesia and received other analgesia. All included in the intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Outcomes not prespecified in Methods section, other than caesarean section |
| Other bias | Low risk | |
Shifman 2007.
| Methods | RCT Parallel design Single centre Russia |
|
| Participants | 90 healthy pregnant women ‐ randomised into 3 groups, 30 in each group Eligibility: healthy pregnant women Exclusion: women with a history of chronic back pain or neurological illnesses or symptoms and women who had already given birth before or with pregnancy and birth complications |
|
| Interventions | Group 1 ‐ epidural analgesia (N = 30) ‐ 1% lidocaine Group 2 ‐ epidural analgesia (N = 30) ‐ 0.2% ropivacaine Group 3 ‐ control ‐ (N = 30) ‐ no epidural |
|
| Outcomes | Caesarean section Transient neurological symptoms (2 days after labour) ‐ included symmetric pain and/or dysthaesia in the buttocks, lower lumbar region, and/or legs |
|
| Notes | Faculty of Anaesthesiology, Russian University of Friendship Between Nations in Moscow. Paper in Russian ‐ sections translated Dates: Not stated in translation Funding: Not stated in translation Declarations of Interest: Not stated in translation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Two days after the EA, a blind observer asked patients questions using the BG Cramer table. The observer was not informed about the treatment received by the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total for control group for caesarean section rate is given as 50 in totals ? only 30 in original group (from translation ? so may be a typo). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported. |
| Other bias | Low risk | Not evident |
Stocki 2011.
| Methods | Single‐centre, individually‐randomised non‐blinded controlled non‐inferiority design trial | |
| Participants | 40 women randomised (39 analysed) (remifentanil PCIA, N = 20; patient controlled epidural, N = 20) Eligibility: healthy, ASA physical status class I or II, age 18 ‐ 40 years, body weight < 110 kg, gestational age > 36 completed weeks, with singleton pregnancy and vertex presentation Excluded: contraindication to epidural analgesia, opioid administration in the previous 2 hours, previous uterine surgery, pre‐eclampsia, inability to understand the consent form, nasal obstruction for any reason and medical indication for epidural analgesia (e.g. cardiac disease, suspected difficult airway), or non‐reassuring fetal heart rate tracing |
|
| Interventions | Group 1: Patient‐controlled remifentanil (N = 20 with 19 analysed) The bolus dose was titrated to effect from 20 mcg up to a maximum of 60 mcg as required; the lockout interval was initially set at 2 min, without a background infusion. The PCIA bolus/lockout interval was titrated to an end point of either participant comfort, or a maximal bolus dose of 60 mcg/minimal lockout interval of 1 min by the recruiting anaesthetist at any time during labour. The PCIA pump tubing was “piggybacked” into the distal‐most port of the mainline IV fluid tubing. The mainline tubing contained an antireflux valve designed to prevent remifentanil inadvertently backing up in the IV line during administration. The recruiting anaesthetist (a resident performing a mandatory research project) remained by the woman’s bedside until the end of treatment with remifentanil. Group 2: Patient‐controlled epidural (N = 20) For women randomised to receive epidural analgesia, a 17‐gauge Tuohy needle was inserted by the midline approach using loss of resistance to air at intervertebral space L3 ‐ 4 or L2 ‐ 3. An incremental initial loading dose of 15 mL of 0.1% bupivacaine with 50 mcg fentanyl was administered followed by patient‐controlled epidural analgesia infusion of 0.1% bupivacaine with 2 mcg/mL fentanyl: basal infusion of 5 mL/hr, patient‐controlled bolus 10 mL, and lockout interval 20 minutes. Additional epidural bolus doses (either 0.1% bupivacaine 10 mL during the 1st stage of labour or 1% lidocaine 8 mL during the 2nd stage of labour) were administered by the anaesthetist to treat breakthrough pain. If epidural analgesia failed, the epidural catheter was reinserted. After epidural analgesia administration, the recruiting anaesthetist remained by the woman’s bedside for the 1st hour and then remained in the labour ward, dedicated to her care, until delivery. |
|
| Outcomes | Need for further analgesia Mode of birth Oxytocin augmentation Respiratory depression Side effects Apgar scores Neonatal resuscitation Pain change scores Duration of labour Satisfaction scores Cord blood gases |
|
| Notes | Conducted in tertiary hospital in Jerusalem, Israel Dates of trial: February 2010 – August 2010 Funding: this study was supported by a research grant for Anesthesiologists from the Hadassah Hebrew University Medical Center, Jerusalem, Israel. Oridion® provided the capnography equipment, developed the dedicated software, and provided the mathematician who performed data extraction. Neither the funding body nor Oridion® had a role in study design, data interpretation, writing of the manuscript, or manuscript submission for publication. Conflicts of Interest: 2 authors received money from Oridion® for travel to conference to present paper. High level of women receiving oxytocin, so 1st stage of labour data not reported in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling cards. Group of 8 – 4 each of intervention and control |
| Allocation concealment (selection bias) | Low risk | Randomisation and group allocation were determined: cards were divided into groups of 8 cards. Each group contained 4 allocation cards for remifentanil and 4 allocation cards for epidural analgesia (ratio 1:1), and 8 opaque envelopes numbered in groups from 1 – 8, 9 – 16, etc. were assigned to each group of cards. The cards were placed face down, manually shuffled, randomly selected, and then inserted into the numbered, opaque envelopes by a person not involved in the study. These envelopes were then sealed. Treatment assignment was revealed by breaking the seal of an envelope in consecutive order from number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants would be aware of allocation and were informed that they could cross over if analgesia not effective. Caregiver would be aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. Staff providing care would record labour outcomes. Outcome for infant respiratory rate observed by assessor blind to study hypothesis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 58 declined to participate. 40 randomised. 1 in RPCA group was excluded by obstetrician request. 3 in Remifentanil group crossed over to epidural, 1 epidural crossed to remifentanil due to failure of allocated analgesia. These women are excluded for some analyses. Intention‐to‐treat analysis: yes |
| Selective reporting (reporting bias) | Low risk | Appear to all be reported. No protocol available but all expected outcomes reported. Power calculation for non‐inferiority design |
| Other bias | Low risk | Similar baseline characteristics and other bias not apparent |
Sullivan 2002.
| Methods | RCT Parallel design Single centre Chicago, Illinois, USA |
|
| Participants | 180 healthy nulliparous women were randomised, to either systemic opioids (N = 70) or intrathecal opioids as part of a CSE technique (N = 80). Eligibility: term in spontaneous labour or with spontaneous rupture of membranes and requested labour analgesia prior to 4 cm of cervical dilatation. All received oxytocin to augment labour. |
|
| Interventions | Group SYS ‐ systemic opioids ‐ hydromorphone 1 mg IV/1 mg IM (N = 70) Group IT ‐ intrathecal opioids as part of a combined spinal epidural technique ‐ intrathecal fentanyl 25 µg plus epidural test dose of lidocaine 45 mg with epinephrine 15 µg (N = 80) |
|
| Outcomes | Oxytocin infusion rates ‐ recorded for 2‐hr period (1 hr prior to and 1 hr after the initiation of labour analgesia) | |
| Notes | Dept of Anesthesiology, Northwestern University Medical School, Chicago, Illinois, USA Abstracts only, so data limited. Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Thalme 1974.
| Methods | "Randomly allotted", using sealed envelopes drawn by a midwife Intention‐to‐treat analysis used. All women accounted for | |
| Participants | 28 women recruited (epidural N = 14, meperidine N = 14) Eligibility: nulliparous women aged 18 ‐ 35 years at 37 ‐ 41 weeks' gestation in spontaneous labour with no medical or obstetric complications | |
| Interventions | Epidural method: preload given. Bupivacaine 0.25% with adrenaline 6 ‐ 8 mL by intermittent top‐up. Level of block not known 2nd stage: epidural use continued Control method: pethidine 100 mg x 1 (route not stated), chlorpromazine 12.5 mg x 1, then entonox at 8 cm, and pudendal block for delivery using 20 mL 1% prilocaine | |
| Outcomes | Maternal: duration of 1st and 2nd stages of labour, oxytocin augmentation, acid/base values, mode of delivery Fetal/neonatal: fetal heart rate abnormality, meconium, acid/base values, Apgar scores, blood chemistry, Silverman‐Anderson score to assess breathing performance, rectal temperature | |
| Notes | Sweden
Paper did not state if any women did not receive their allocated treatment. Dates: Year trial carried out not stated Funding: "supported by a grant from the Swedish Medical Research Council" Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allotted to 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants were removed from the study: 2 from the control group and 2 from the epidural group, because of moderate to pronounced dysmaturity of the baby or clinical appearance of the baby indicated a gestational age of 36 weeks. |
| Selective reporting (reporting bias) | High risk | Outcomes not all prespecified in Methods section |
| Other bias | High risk | The tendency to increased duration of the 2nd stage after epidural block made the prophylactic use of vacuum extraction necessary to exclude the deleterious effect on the fetus of a 2nd stage exceeding 1 hr, but author continued to report on duration of the 2nd stage but not the instrumental delivery. |
Thorp 1993.
| Methods | Randomisation to treatment by sealed envelopes. Randomisation sequence derived from a computer‐generated random number table Intention‐to‐treat analysis used. All women accounted for | |
| Participants | 93 women recruited (epidural N = 48, control N = 45) Eligibility: uncomplicated pregnancies at 37 ‐ 42 weeks' gestation, spontaneous labour, nulliparous women | |
| Interventions | Epidural method. Preload not mentioned. Bupivacaine 0.25% bolus dose followed by 0.25% bupivacaine infusion. Block to T10 ‐ T12 2nd stage: epidural use continued Control: 75 mg pethidine and 25 mg promethazine IV every 90 mins as required | |
| Outcomes | Maternal: length of 1st and 2nd stages of labour, oxytocin augmentation, method of delivery, pain scores Fetal/neonatal: presence of meconium, Apgar scores, umbilical cord blood gases, neurologic adaptive capacity score | |
| Notes | USA
1 woman randomised to narcotic received epidural as well.
1 woman randomised to epidural never received it.
Trial terminated early following preliminary analysis, showing increase in caesarean delivery in epidural group Dates: Trial took place 1990 ‐ 1992 Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of the participants and crossed‐over participants analysed in their original groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported within the Methods section are available within the Results. |
| Other bias | High risk | Study was terminated early because after 93 participants entered in the trial statistically significant increase in the rate of caesarean sections seen in the epidural group. Initially 100 participants in each arm intended |
Tveit 2012.
| Methods | Randomised controlled trial with individual randomisation | |
| Participants | 39 women randomised (epidural analgesia n = 20; IV remifentanil n = 19) Eligibility: ASA I or II with normal singleton pregnancies, regular uterine contractions, cervical dilatation > 2 cm, anticipated vaginal delivery, fetus without suspected abnormality, normal fetal cardiotocographic pattern, no complications during pregnancy and gestation age 37 ‐ 40 weeks Excluded: women were excluded if they requested EDA, had received pethidine < 8 hrs before the study period or if there were contraindications to remifentanil. |
|
| Interventions | Remifentanil group: n = 19 Women received remifentanil hydrochloride diluted in "physiological saline to a concentration of 50 µg/mL, given as stepwise bolus doses with no background infusions. The starting bolus dose was 0.15 µg/kg, with increasing dose steps of 0.15 µg/kg and no maximum limit. The dose was allowed to be increased or decreased every 15th minute according to women’s request for dose adjustment. VAS pain score, and side effects. The lock‐out period was 2 mins." Remifentanil was administered using a PCA pump with a bolus infusion speed of 2 mL/min (100 µg/min). Epidural analgesia group: n = 20 Women had an epidural catheter inserted in the midline at L2 ‐ L3/L3 ‐ 4 by the investigator, received a continuous epidural infusion of ropivacaine 1 mg/mL and fentanyl 2 µg/ml (‘walking epidural’). An initial bolus dose of 10 mL, followed by a 5 mL top‐up after 5 min (total 15 mL) was given before the start of infusion (10 mL/hr). Midwife could adjust the infusion dose (5 ‐ 15 mL/hr) and give rescue doses (5 mL) if needed. EA group was managed in accordance with the local protocol. |
|
| Outcomes | Satisfaction scores Oxytocin augmentation Side effects Duration of labour |
|
| Notes | Country and setting: Norway Dates of trial: Not reported Funding: Sorlandet Hospital HF, Sorlandets Kompetansefond and Helse Sor‐Ost, Norway Conflicts of interest: none declared Duration of 1st stage not reported in review due to large number of women having oxytocin Sense of control in labour |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were kept in a sealed envelopes until recruitment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "after the study, two additional obstetricians, blinded to the analgesia method and neonatal outcome, independently evaluated fetal heart rate recordings." It was unclear for other outcomes; anaesthesiologist collected the pain scores. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No loss to follow‐up 2 women in the remifentanil group discontinued intervention and received epidural. They were excluded from the analysis, although fig 1 reports that none were excluded. |
| Selective reporting (reporting bias) | Low risk | Protocol not seen but outcomes prespecified in Methods and appear to all be reported. |
| Other bias | Unclear risk | Underpowered because of finishing trial early due to technical faults. Baseline characteristics appear similar. |
Volmanen 2008.
| Methods | RCT Parallel design Single centre University of Oulo, Finland |
|
| Participants | 52 women randomly allocated to remifentanil (N = 27) and to epidural analgesia (N = 25) Eligibility: healthy term parturients with uncomplicated singleton pregnancies, 1st stage of labour with normal cephalic presentation and no prior administration of opioid analgesia for at least 4 hrs or regional analgesia Exclusion: not reported |
|
| Interventions | IV patient‐controlled analgesia (IV PCA) with remifentanil ‐ PCA dose given over 1 min with a lockout time of 1 min. Dose was increased starting from the bolus of 0.1 µg/kg and following a dose escalation scheme up until the individual‐effective dose was reached. Epidural analgesia with 20 mL Levobupivacaine 0.625 mg/mL and fentanyl 2 µg/mL in saline |
|
| Outcomes |
|
|
| Notes | 7 participants not included in the analysis: remifentanil group (N = 3) ‐ discontinued due to entering 2nd stage of labour; epidural group (N = 4) ‐ 3 discontinued due to entering 2nd stage of labour, 1 did not receive allocated intervention due to dural tap. Department of Anaesthesia & Intensive Care, University of Oulo, Finland Dates: Dates not stated. Accepted for publication 2007 Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list that was stratified according to parity |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes numbered according to computer‐generated list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both the parturient and all the personnel present during the study were blinded as to which medication was used during the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Fetal heart rate tracings were analysed by an obstetrician blinded to analgesia group and outcome of the newborn. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT flowchart outlining numbers allocated, followed‐up and analysed. Reasons for not including in the analysis clearly documented: remifentanil group (N = 3) ‐ discontinued due to entering 2nd stage of labour; epidural group (N = 4)‐ 3 discontinued due to entering 2nd stage of labour, 1 did not receive allocated intervention due to dural tap. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported within the Methods section are available within the Results. |
| Other bias | Low risk | Baseline characteristics similar, apart from more nausea before the study in the remifentanil group (N = 9) versus none in epidural group. |
Witoonpanich 1984.
| Methods | RCT Parallel design Single centre Chula‐longkorn University, Thailand |
|
| Participants | 62 pre‐eclamptic women 16 ‐ 29 years (21 ± 4.23), primigravida, in labour at term randomised to study group (N = 31) continuous lumbar epidural analgesia, or control group (N = 31) pethidine or pentazocine intramuscularly | |
| Interventions | Continuous lumbar epidural analgesia ‐ standard precautions for epidural analgesia were taken throughout, bupivacaine (marcain) was intermittently given to provide painless labour (N = 31). Pethidine or pentazocine given intramuscularly (N = 31) |
|
| Outcomes | Blood pressure | |
| Notes | Abstract only ‐ data limited. Dates: Not stated Funding: Not stated Declarations of Interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Xing 2015.
| Methods | Reported to be RCT with individual randomisation | |
| Participants | 308 women randomised but data only for 285 (CSEA n = 143; control n = 142) Eligible: primiparous women who (1) were 22 – 30 years old; (2) were 155 – 165 cm tall; (3) were assigned a score of I or II on the ASA scale; and (4) gave birth by vaginal delivery to a live, single, mature fetus (38 ‐ 40 wks) in the vertex position with (5) a neonatal weight of 2900 – 3500 g Excluded: (1) history of chronic cough; (2) chronic constipation or pelvic organ resection; (3) family history of urinary incontinence; (4) pelvic organ prolapse; (5) any systemic disease before delivery; or (6) a history of surgery, trauma, tumour or deformity of lumbar vertebrae |
|
| Interventions | Combined spinal‐epidural analgesia group (n = 143) Women in the CSEA group received CSEA during labour. An intravenous line was established when the cervical opening measured 1 – 2 cm. Then sufentanil (5 – 7 μg) was injected intrathecally. When the visual analogue pain score was 3 or higher, a mixture of ropivocaine (0.143%) and sufentanil (0.3 μg/mL) was continuously infused into the epidural space using an analgesia pump until the cervix was fully dilated. Load capacity was 5 mL. The analgesic plane was controlled under T10. Control group (n = 142) Women in the control group were not provided any analgesia during labour. |
|
| Outcomes | Need for other analgesia Mode of birth Oxytocin administration Duration of labour Perineal injury |
|
| Notes | Setting: Maternal and Child Health Hospital of Nanning, China Dates of trial: June 2013 and June 2014 Funding: this work was supported by the Scientific and Technological Key Project of Nanning City (no. 20133189). Conflicts of interest: the authors have declared that no competing interests exist. Duration of 1st and 2nd stage reported but not clear if it is median and IQR or range |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described, although consecutive sampling is mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Staff and women knew allocation due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Labour outcomes probably reported by caregiver in labour. Outcome assessor testing pelvic floor strength 6 ‐ 8 weeks post‐delivery was blinded but this outcome is not relevant to this review. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Following randomisation 2 women in each group were lost to follow up. “Another 12 women were excluded because they reported feeling vaginal fullness at an inflation volume <15 ml or >30 ml…. Three were delivered by caesarean and 4 fetal weight were outside the range of 2900–3500 g. These 7 women were also excluded.” 12/155 women in the CSEA group were excluded in total. 11/153 women in the control group were excluded. 5 women in the control group were given epidural on request but were analysed in the control group (ITT). Reasons given for exclusions, may have introduced attrition bias. |
| Selective reporting (reporting bias) | High risk | Duration of labour was added post hoc as the protocol was registered after the trial was completed. |
| Other bias | High risk | No baseline characteristic imbalances apparent. Methods not reported well (in the CONSORT checklist, authors have reported that they have provided information 8 – 10 on pages 4 and 5 in the report, but the information is missing). |
ASA: American Society of Anesthesiologist AUC: area under the curve BE: base excess BMI: body mass index CMS: continuous midwifery support CS: Caesarean section CSE: combined spinal‐epidural CTG: cardiotocography EPI: epidural FGR: fetal growth restriction FHR: fetal heart rate hr: hour IM: intramuscular IV: intravenous
min: minutes NACS: Neurological Adaptive Capacity Score NICU: neonatal intensive care unit NO: Nitric oxide PCA: participant‐controlled analgesia PCEA: participant‐controlled epidural analgesia PCIA: participant‐controlled intravenous analgesia PIH: pregnancy‐induced hypertension PPH: postpartum haemorrhage PRL: prolactin RCT: randomised controlled trial ROM: rupture of membrane s: seconds SD: standard deviation TENS: transcutaneous electrical nerve stimulation VAS: visual analogue scores
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abboud 1982 | This study was designed to assess the effect on beta‐endorphin levels, of momentarily withholding local anaesthetic after insertion of the catheter into the epidural space. |
| Anwar 2015 | This is not an RCT. Women were divided into 2 groups, 50 each "as per convenience". |
| Buchan 1973 | Excluded because the method of randomisation was not adequate (alternate allocation). Epidural bupivacaine (N = 10) compared with intramuscular pethidine (N = 10). Outcomes include corticosteroid levels and mode of delivery. |
| Chen 2000 | Control group were not randomised, 2 epidural groups were randomised. Comparisons of interest therefore not randomised and study does not satisfy eligibility criteria. |
| Cutura 2011 | This is not an RCT. This was not a trial; it looked at 200 women, 100 chose EA and 100 other analgesia. It was looking at factors associated with choosing EA. |
| Ginosar 2002 | Excluded because all women received epidural bupivacaine until pain free (N = 48), then randomised to IV fentanyl or epidural fentanyl (abstract of study published in full in 2003). |
| Ginosar 2003 | Excluded because both groups received lumbar epidural analgesia with 20 ‐ 30 mL bupivacaine until pain‐free then randomised to IV fentanyl infusion and epidural fentanyl infusion. |
| Gupta 2013 | Excluded because both groups received epidural. This study was looking at paracetamol as an adjuvant therapy. |
| Hood 1993 | Excluded because both experiment and control groups had regional procedure although saline was control. This study compared epidural bupivacaine (N = 14) with epidural saline (N = 14) for 60 minutes after insertion of the epidural catheter. The outcome of interest was fetal heart rate changes. |
| John 2013 | Excluded because interventions do not meet review criteria. Denominators not clear, brief abstract. No relevant outcome data |
| Jouppila 1976 | Excluded because the method of randomisation was not adequate (alternate allocation). Epidural bupivacaine (N = 14) compared with intramuscular pethidine (N = 14). Outcomes include duration of labour, growth hormone, insulin, fetal/infant outcomes and mode of delivery. |
| Jouppila 1980 | Excluded because the method of randomisation was not adequate (alternate allocation). Epidural bupivacaine (N = 8) compared with intramuscular pethidine (N = 10). Outcomes include duration of labour, prolactin, fetal/infant outcomes and mode of delivery. |
| Justins 1983 | Excluded because all participants were given epidural test dose followed by either intramuscular fentanyl or epidural fentanyl. Outcomes included duration of analgesia, hypotension, itching, bladder dysfunction and neonatal Apgar scores in correlation with plasma fentanyl concentration. |
| Kujansuu 1987 | Excluded because trial compared epidural with paracervical epidural. |
| Kurjak 1974 | Quasi‐randomised Epidural bupivacaine (N = 224), control group (N = 224) conventional analgesia. Most participants in the control group had pethidine 150 mg/4 hours. The rest had nitrous oxide or no analgesia. Outcomes include maternal and umbilical arterial blood acid‐base status, fetal heart rate changes, fetal blood pH, Apgar scores. |
| Lassner 1981 | Excluded because study compared epidural morphine (N = 13) with epidural saline (N = 12), with both groups receiving epidural bupivacaine at some stage in labour. |
| Leong 2000 | Not RCT. All participants were offered epidural analgesia in labour and those who accepted formed the epidural group (N = 55), those who declined epidural analgesia were controls (N = 68). Outcomes included duration of labour, oxytocin augmentation and mode of delivery. |
| MacKenzie 1996 | All participants had epidural bupivacaine in labour prior to randomisation to continuous infusion of epidural bupivacaine and fentanyl (N = 7) or IV fentanyl (N = 6). Outcomes included fentanyl concentration in maternal and cord blood. |
| Manninen 2000 | Excluded because intervention is not relevant. |
| Martin 2003 | Both groups received epidural analgesia. |
| McGrath 1992 | The study randomised participants to epidural analgesia or nalbuphine intravenously with the intention of providing all women with epidural analgesia later in labour. The outcome of interest was fetal heart rate changes in the 1st hour after randomisation. |
| Moreno 1997 | Excluded because this was not an RCT. |
| Nafisi 2006 | Quasi‐randomised. Odd and even numbers used for allocation This study compared epidural (N = 197) with IV meperidine (N = 198). |
| Neri 1986 | Quasi‐randomised (information from authors) N = 104 This study compared epidural analgesia (N = 52) with apresoline and magnesium sulphate (N = 52) in the management of women with pre‐eclampsia. Outcomes include change in blood pressure, mode of delivery, Apgar scores, neonatal jaundice and respiratory depression at birth. |
| Noble 1971 | Excluded because the method of randomisation was not adequate (allocation by case record number). Epidural bupivacaine (N = 125) compared with intramuscular pethidine (N = 120). Outcomes include duration of labour, maternal hypotension, fetal/infant outcomes and mode of delivery. |
| Polley 2000 | Excluded because both groups received epidural analgesia. |
| Revill 1979 | Excluded because more than 28% of women excluded from analysis. Out of 386 randomised only 132 completed interviews in their allocated groups. Outcomes include pain scores, satisfaction with analgesia, and concerns of analgesic effects on the baby. |
| Robinson 1980 | Excluded because more than 30% of women excluded from analysis. Out of approximately 300 women initially randomised at antenatal visit into the 2 groups, only 93 completed the interviews having used only the analgesic allocated to them. The large proportion excluded compromises the reliability of the results. Epidural bupivacaine (N = 45) was compared with intramuscular pethidine (N = 48). Outcomes include duration of labour, mode of delivery and maternal pain/discomfort, nausea, sleepiness, backache, satisfaction and worry over baby. |
| Robinson 1997 | Intention‐to‐treat analysis not used. 153 participants randomly allocated to low extra‐dural analgesia with 0.125% bupivacaine with 50 µg fentanyl followed by 0.1% bupivacaine with 2 µg/mL fentanyl top‐ups (N = 89), and IM pethidine 100 mg (N = 64). Outcomes were pain relief scores, mode of delivery, duration of 1st and 2nd stages of labour. |
| Ryhanen 1984 | Excluded because the method of randomisation was not adequate (alternate allocation). Epidural bupivacaine (N = 5) compared with intramuscular pethidine (N = 5). Outcomes include duration of labour, plasma leukocyte counts, fetal/infant outcomes. |
| Solek‐Pastuszka 2009 | Not a randomised controlled trial |
| Stourac 2014 | Excluded because women chose intervention, therefore this was not a randomised trial. |
| Swanström 1981 | Quasi‐randomised (running order). 80 women. Epidural (N = 37), paracervical N = 16; control group (N = 27) (further data from authors). Outcomes include duration of 1st and 2nd stages of labour, oxytocin augmentation, Apgar scores, neonatal jaundice, neurological outcomes at 6/18 months. |
| Tugrul 2006 | Not a randomised controlled trial. |
| Wassen 2015 | Excluded because this was not an RCT. |
| Wong 2005 | Excluded because both groups received epidural analgesia: women randomly assigned to receive intrathecal fentanyl or systemic hydromorphone at the 1st request of analgesia ‐ but epidural analgesia was initiated in the intrathecal group at the 2nd request for analgesia and in the systemic group at a cervical dilatation of 4.0 cm or greater or at the 3rd request for analgesia. |
| Wong 2009 | Excluded because both groups received epidural analgesia: participants were randomised to neuraxial (early) or systemic opioid (late) analgesia at the 1st analgesia request. Patient‐controlled epidural analgesia was initiated in the early group at the 2nd analgesia request and in the late group at cervical dilation of 4 cm or greater or at the 3rd analgesia request. |
| Zakowski 1994 | Excluded because compared epidural morphine to IV morphine postoperative analgesia in women who had elective Caesarean delivery. All participants had received epidural lidocaine preoperatively, epidural morphine (N = 8) IV morphine (N = 8). Outcomes were plasma and urinary morphine concentration. |
h: hours IM: intramuscular IV: intravenous RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Antipin 2014.
| Methods | It was not clear that this was a trial; women were "randomized to 3 groups ‐ one was "refused pain relief". |
| Participants | Women in the 1st stage of labour |
| Interventions | Group 1. Epidural Group 2. paravertebral block Group 3. "refused analgesia" |
| Outcomes | Duration of labour |
| Notes | Authors contacted ‐ awaiting response Unclear if this is an RCT. |
Gupta 2016.
| Methods | Reports to be RCT |
| Participants | Women in labour at term |
| Interventions | Group 1. Epidural Group 2. No epidural |
| Outcomes | Duration of labour Mode of birth |
| Notes | Author contacted for more information on methods It was not clear that this was an RCT. |
Kamali 2016.
| Methods | Reported to be trial |
| Participants | Women at term |
| Interventions | Group 1. Epidural Group 2. Entonox Group 3. no analgesia |
| Outcomes | No results ‐ trial registration |
| Notes |
Marshalov 2012.
| Methods | Reported to be RCT. Abstract only |
| Participants | Women in labour |
| Interventions | Epidural versus opiate analgesic (not specified) |
| Outcomes | Intra‐abdominal pressure, pain intensity |
| Notes | Unable to find author contact details |
Vavrinkova 2005.
| Methods | Methods not described, possibly RCT |
| Participants | Women in labour |
| Interventions | Epidural versus IV nalbuphine, or pethidine |
| Outcomes | Pain and Apgar score |
| Notes | Unable to find email address to contact authors |
Weissman 2006.
| Methods | Randomised, parallel‐assignment, open‐label controlled trial |
| Participants | Target number: 60 randomised Inclusion criteria: not clear Exclusion criteria: all parturients with cardiac disease, neurological disease, endocrine disease, diabetes, hypertension or any parturients being treated with medications that might effect the cardiovascular autonomic system |
| Interventions | Epidural versus IV Meperidine |
| Outcomes | Not stated |
| Notes | Predicted start and finish: March ‐ December 2006 Contacted study hospital for further information 15 June 2017 ‐ awaiting response |
Differences between protocol and review
The 2011 version of this review was one of a series of Cochrane Reviews examining pain management in labour. These reviews contributed to an overview of systematic reviews of pain management for women in labour (Jones 2012), and shared a generic protocol (Jones 2011). In order to adhere to the generic protocol the outcomes included and methods used for subgroup and sensitivity analyses were revised in the 2011 version of this review to comply with the generic protocol. In this updated review (2018) separate comparisons examine epidural versus opioids, versus placebo or no treatment, versus acu‐stimulation, and versus continuous support. This version of the review includes GRADE assessments for important outcomes, and a 'Summary of findings' table for the main comparison. We conducted a post hoc subgroup analysis of trials conducted after 2005 for the outcome of assisted vaginal birth for the main comparison of epidural versus opioids, in response to peer referee comments.
For the 2018 update, we include an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Contributions of authors
M Anim‐Somuah (MA) is responsible for this current update. A Cuthbert (AC) and R Smyth (RS) updated the Background and Methods sections, and MA, RS and AC assessed new studies for inclusion and extracted all the data independently. AC entered the data into RevMan and MA and RS double‐checked them. AC, MA, RS and Allan M Cyna (AMC) contributed to the Results, Discussion and Authors' conclusions.
Sources of support
Internal sources
No sources of support supplied
External sources
2017 Update ‐ WHO UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Millicent Anim‐Somuah: None known
Rebecca MD Smyth: None known
Allan M Cyna: None known
Anna Cuthbert: I am a research associate working in the editorial base of Cochrane Pregnancy and Childbirth. I am employed by the University of Liverpool to work as a research associate in Cochrane Pregnancy and Childbirth (who receives infrastructure funding from the NIHR, UK).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bofill 1997 {published data only}
- Bofill JA, Vincent RD, Ross EL, Martin RW, Normal PF, Werhan CF, et al. Nulliparous active labor, epidural analgesia, and cesarean delivery for dystocia. American Journal of Obstetrics and Gynecology 1997;177(6):1465‐70. [DOI] [PubMed] [Google Scholar]
Camann 1992 {published data only}
- Camann WR, Denney RA, Holby ED, Datta S. A comparison of intrathecal, epidural and intravenous sufentanil for labor analgesia. Anesthesiology 1992;77(5):884‐7. [DOI] [PubMed] [Google Scholar]
Chen 2008a {published data only}
- Chen Z, Tian Y, Li X, Luo F. Effect of analgesia with combined spinal‐epidural block on maternal serum prolactin. Anesthesiology 2008;109:A580. [Google Scholar]
Chen 2008b {published data only}
- Chen YM, Li Z, Wang AJ, Wang JM. Effect of labor analgesia with ropivacaine on the lactation of parturients. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2008;43(7):502‐5. [PubMed] [Google Scholar]
Clark 1998 {published data only}
- Clark A, Carr D, Loyd G, Cook V, Spinnato J. The influence of epidural analgesia on cesarean delivery rates: a randomised, prospective clinical trial. American Journal of Obstetrics and Gynecology 1998;179:1527‐33. [DOI] [PubMed] [Google Scholar]
De Orange 2011 {published data only}
- Orange FA, Passini RJ, Amorim MM, Almeida T, Barros A. Combined spinal and epidural anaesthesia and maternal intrapartum temperature during vaginal delivery: a randomized clinical trial. British Journal of Anaesthesia 2011;107(5):762‐8. [DOI] [PubMed] [Google Scholar]
- Orange FA, Passini R Jr, Melo AS, Katz L, Coutinho IC, Amorim MM. Combined spinal‐epidural anesthesia and non‐pharmacological methods of pain relief during normal childbirth and maternal satisfaction: a randomized clinical trial. Revista Da Associacao Medica Brasileira 2012;58(1):112‐7. [PubMed] [Google Scholar]
Dickinson 2002 {published data only}
- Dickinson JE, Paech MJ, McDonald SJ, Evans SF. Maternal satisfaction with childbirth and intrapartum analgesia in nulliparous labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43(6):463‐8. [DOI] [PubMed] [Google Scholar]
- Dickinson JE, Paech MJ, McDonald SJ, Evans SF. The impact of intrapartum analgesia on labour and delivery outcomes in nulliparous women. Australian and New Zealand Journal of Obstetrics & Gynaecology 2002;42(1):59‐66. [DOI] [PubMed] [Google Scholar]
- Henderson JJ, Dickinson JE, Evans SF, McDonald SJ, Paech MJ. Impact of intrapartum epidural analgesia on breast‐feeding duration. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43(5):372‐7. [DOI] [PubMed] [Google Scholar]
- Orlikowski CE, Dickinson JE, Paech MJ, McDonald SJ, Nathan E. Intrapartum analgesia and its association with post‐partum back pain and headache in nulliparous women. Australian and New Zealand Journal of Obstetrics and Gynaecology 2006;46(5):395‐401. [DOI] [PubMed] [Google Scholar]
Douma 2011 {published data only}
- Douma MR, Middeldorp JM, Verwey RA, Dahan A, Stienstra R. A randomised comparison of intravenous remifentanil patient‐controlled analgesia with epidural ropivacaine/sufentanil during labour. International Journal of Obstetric Anesthesia 2011;20(2):118‐23. [DOI] [PubMed] [Google Scholar]
- Douma MR, Stienstra R, Middeldorp JM, Arbous MS, Dahan A. Differences in maternal temperature during labour with remifentanil patient‐controlled analgesia or epidural analgesia: a randomised controlled trial. International Journal of Obstetric Anesthesia 2015;24(4):313‐22. [DOI] [PubMed] [Google Scholar]
El‐Kerdawy 2010 {published data only}
- El‐Kerdawy H, Farouk A. Labor analgesia in preeclampsia: remifentanil patient controlled intravenous analgesia versus epidural analgesia. Middle East Journal of Anesthesiology 2010;20(4):539‐45. [PubMed] [Google Scholar]
Evron 2007 {published data only}
- Evron S, Koren R, Parameswaran R, Avinoah I, Ezri T, Sadan O, et al. Immunohistochemical localization of activin subunit in human placenta: correlation with elevated temperature and placental infection in parturients laboring under epidural analgesia [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S188. [Google Scholar]
- Evron S, Parameswaran R, Zipori D, Ezri T, Sadan O, Koren R. Activin beta A in term placenta and its correlation with placental inflammation in parturients having epidural or systemic meperidine analgesia: a randomized study. Journal of Clinical Anesthesia 2007;19(3):168‐74. [DOI] [PubMed] [Google Scholar]
Evron 2008 {published data only}
- Evron S, Ezri T, Protianov M, Muzikant G, Sadan O, Herman A, et al. The effects of remifentanil or acetaminophen with epidural ropivacaine on body temperature during labor. Journal of Anesthesia 2008;22(2):105‐11. [DOI] [PubMed] [Google Scholar]
- Evron S, Koren R, Parameswaran R, Avinoah I, Ezri T, Sadan O, et al. Immunohistochemical localization of activin subunit in human placenta: correlation with elevated temperature and placental infection in parturients laboring under epidural analgesia [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S188. [Google Scholar]
Freeman 2014 {published data only}
- Freeman L, Bloemenkamp K, Franssen M, Papatsonis D, Hollmann M, Woiski M, et al. Remifentanil patient controlled analgesia versus epidural analgesia in labor; a randomized controlled trial. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S37. [Google Scholar]
- Freeman LM, Bloemenkamp KW, Franssen MT, Papatsonis DN, Hajenius PJ, Hollmann MW, et al. Patient controlled analgesia with remifentanil versus epidural analgesia in labour: randomised multicentre equivalence trial. BMJ (Clinical Research Ed.) 2015;350:h846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LM, Bloemenkamp KW, Franssen MT, Papatsonis DN, Hajenius PJ, Huizen ME, et al. Remifentanil patient controlled analgesia versus epidural analgesia in labour. A multicentre randomized controlled trial. BMC Pregnancy and Childbirth 2012;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gambling 1998 {published data only}
- Gambling DR, Sharma SK, Ramin SM, Lucas MJ, Leveno KJ, Wiley J, et al. A randomized study of combined spinal‐epidural analgesia versus intravenous meperidine during labor: impact on cesarean delivery rate. Anesthesiology 1998;89(6):1336‐44. [DOI] [PubMed] [Google Scholar]
Genc 2015 {published data only}
- Genc M, Sahin N, Maral J, Celik E, Kar AA, Usar P, et al. Does bupivacaine and fentanyl combination for epidural analgesia shorten the duration of labour?. Journal of Obstetrics and Gynaecology 2015;35(7):672‐5. [DOI] [PubMed] [Google Scholar]
Grandjean 1979 {published data only}
- Grandjean H, Mouzon J, Cabot JA, Desprats R, Pontonnier G. Peridural analgesia and by phenoperidine in normal labor. Therapeutic trial with a control series. Archives Francaises de Pediatrie 1979;36(9 Suppl):LXXV‐LXXXI. [PubMed] [Google Scholar]
Halpern 2004 {published data only}
- Halpern SH, Muir H, Breen TW, Campbell DC, Barrett J, Liston R, et al. A multicentre randomized controlled trial comparing patient‐controlled epidural with intravenous analgesia for pain relief in labour. Anaesthesia & Analgesia 2004;99:1532‐8. [DOI] [PubMed] [Google Scholar]
Head 2002 {published data only}
- Head B, Owen J, Vincent R, Shih G, Chestnut D, Hauth J. A randomized trial of intrapartum analgesia in women with severe preeclampsia. Obstetrics & Gynecology 2002;99(3):452‐7. [DOI] [PubMed] [Google Scholar]
Hogg 2000 {published data only}
- Hogg B, Owen J, Shih G, Vince R, Chestnut D, Hauth JC. A randomised control trial of intrapartum analgesia in women with severe preeclampsia (abstract). American Journal of Obstetrics and Gynecology 2000;182:S148. [DOI] [PubMed] [Google Scholar]
- Kenyon AP, Shennan A. Cesarean delivery rate among women with severe hypertensive disease: possible reduction with epidural anaesthesia? [letter]. American Journal of Obstetrics and Gynecology 2001;184(3):514. [DOI] [PubMed] [Google Scholar]
- Shih GH, Vincent RD, Chestnut DH, Hogg MD. Intrapartum analgesia for severe preeclampsia. Anesthesiology 2000;92 Suppl:A50. [Google Scholar]
Howell 2001 {published data only}
- Howell C, Kidd C, Roberts W, Johanson R, Upton P, Jones P, et al. Pain relief study: a randomised controlled trial of epidural versus pethidine analgesia in labour. British Journal of Obstetrics and Gynaecology 1998;105:Suppl 17:88. [DOI] [PubMed] [Google Scholar]
- Howell CJ, Dean T, Lucking L, Dziedzic K, Jones PW, Johanson RB. Randomised study of long term outcome after epidural versus non‐epidural analgesia during labour [abstract]. Obstetrics & Gynecology 2003;101(1):195‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell CJ, Dean T, Lucking L, Dziedzic K, Jones PW, Johanson RB. Randomised study of long term outcome after epidural versus non‐epidural analgesia during labour. [Erratum appears in BMJ 2002 sep 14;325(7364):580.]. BMJ 2002;325(7360):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell CJ, Kidd C, Roberts W, Upton P, Lucking L, Jones PW, et al. A randomised control trial of epidural compared with non‐epidural analgesia in labour. BJOG: an international journal of obstetrics and gynaecology 2001;108(1):27‐33. [DOI] [PubMed] [Google Scholar]
Ismail 2012 {published data only}
- Ismail MT, Hassanin MZ. Neuraxial analgesia versus intravenous remifentanil for pain relief in early labor in nulliparous women. Archives of Gynecology and Obstetrics 2012;286(6):1375‐81. [DOI] [PubMed] [Google Scholar]
Jain 2003 {published data only}
- Jain S, Arya S, Gopalan S, Jain V. Analgesic efficacy of intramuscular opioids versus epidural analgesia in labor. International Journal of Gynecology & Obstetrics 2003;83(1):19‐27. [DOI] [PubMed] [Google Scholar]
Jain 2012 {published data only}
- Jain K, Samnta S, Bhardwaj N. Continous labour epidural analgesia with ropivacaine improves uteroplacental blood flow in growth restricted fetuses with impaired doppler umbilical blood flow: a randomised control trial. International Association for the Study of Pain (IASP) 14th Congress on Pain; 2012 Aug 27‐31; Milan, Italy. 2012.
- Samanta S, Jain K, Bhardwaj N, Jain V, Singla V. Doppler velocimetric changes following labour epidural analgesia in growth restricted fetuses with impaired umbilical blood flow: A randomised controlled trial. Journal of Obstetric Anaesthesia and Critical care 2013;31(1):57. [Google Scholar]
Jaitley 2011 {published data only}
- Jaitley A, Singh S, Srivastava U, Nagrath A, Prajapati NC, Singh R. A comparison between epidural and IV tramadol for painless labor and effect on perinatal outcome. Journal of Obstetrics and Gynecology of India 2011;61(1):42‐7. [Google Scholar]
Jalil 2009 {published data only}
- Jalil NA, Omar M. Does ropivacaine 0.2% with fentanyl change the labour epidural profile?. International Medical Journal 2009;16(2):149‐55. [Google Scholar]
Khadem 2013 {published data only}
- Khadem N, Zirak N, Soltani G, Sahebdelfar N, Shamloo AS, Ebrahimzadeh S. Comparison of epidural analgesia versus Entonox for labor analgesia in nulliparous women. Journal of Surgery and Trauma 2013;1(1):1‐5. [Google Scholar]
Lian 2008 {published data only}
- Lian Q, Ye X. The effects of neuraxial analgesia of combination of ropivacaine and fentanyl on uterine contraction. Anesthesiology 2008;109:A1332. [Google Scholar]
Liu 2015 {published data only}
- Liu Y, Xu M, Che X, He J, Guo D, Zhao G, et al. Effect of direct current pulse stimulating acupoints of JiaJi (T10‐13) and Ciliao (BL 32) with Han's Acupoint Nerve Stimulator on labour pain in women: a randomized controlled clinical study. Journal of Traditional Chinese Medicine 2015;35(6):620‐5. [DOI] [PubMed] [Google Scholar]
Logtenberg 2017 {published data only}
- Logtenberg A, Oude Rengerink K, Verhoeven C, Mol BW. Remifentanil patient controlled analgesia versus epidural analgesia during labour: a randomised trial (NTR3687). Regional Anesthesia and Pain Medicine 2014;39(5 Suppl 1):E241‐E242. [Google Scholar]
- Logtenberg SL, Oude Rengerink K, Post JA, Verhoeven CJ, Freeman LM, Middeldorp JM, et al. Labour pain with remifentanil patient‐controlled analgesia versus epidural analgesia: a randomised equivalence trial. BJOG: an international journal of obstetrics and gynaecology 2017;124(4):652‐60. [DOI] [PubMed] [Google Scholar]
Long 2003 {published data only}
- Long J, Yue Y. Patient controlled intravenous analgesia with tramadol for pain relief. Chinese Medical Journal 2003;116(11):1752‐5. [PubMed] [Google Scholar]
Loughnan 2000 {published data only}
- Loughnan B, Carli F, Romney M, Dore C, Gordon H. A large randomised controlled trial comparing epidural bupivacaine with intramuscular pethidine for pain relief in labour in primiparous women. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):44. [Google Scholar]
- Loughnan B, Carli F, Romney M, Dore C, Gordon H. A large randomised controlled trial comparing epidural bupivacaine with intramuscular pethidine for pain relief in labour in primiparous women. British Journal of Anaesthesia 1998;80(5 Suppl):151‐2. [Google Scholar]
- Loughnan B, Carli F, Romney M, Dore C, Gordon H. Epidural analgesia and backache: a randomized comparison with intramuscular meperidine for analgesia during labour. British Journal of Anaesthesia 2002;89(3):466‐72. [PubMed] [Google Scholar]
- Loughnan BA, Carli F, Romney M, Dore C, Gordon H. The influence of epidural analgesia on the development of new backache in primiparous women: report of a randomized controlled trial. International Journal of Obstetric Anesthesia 1997;6:203‐4. [Google Scholar]
- Loughnan BA, Carli F, Romney M, Dore CJ, Gordon H. Randomized controlled comparison of epidural bupivacaine versus pethidine for analgesia in labour. British Journal of Anaesthesia 2000;84(6):715‐9. [DOI] [PubMed] [Google Scholar]
Lucas 2001 {published data only}
- Lucas M, Sharma S, Leveno K, Ramin S, Wiley J, Sidawi J. A randomized trial of labor epidural analgesia in women with preeclampsia. Anesthesiology 1998;88(4 Suppl):A25. [Google Scholar]
- Lucas M, Sharma S, McIntire D, Sidawi E, Ramin S, Leveno K, et al. A randomized trial of epidural analgesia on pregnancy‐induced hypertension. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S18. [DOI] [PubMed] [Google Scholar]
- Lucas M, Sharma S, McIntire D, Wiley J, Sidawi J, Ramin S, et al. A randomized trial of labor analgesia in women with pregnancy‐induced hypertension. American Journal of Obstetrics and Gynecology 2001;185(4):970‐5. [DOI] [PubMed] [Google Scholar]
Morgan‐Ortiz 1999 {published data only}
- Morgan‐Ortiz F, Quintero‐Ledezma J, Pérez‐Sotelo JA, Trapero‐Morales M. Evolution and quality care of labour and delivery in primiparous patients who underwent early obstetric analgesia. Ginecologia y Obstetricia de Mexico 1999;67:522‐6. [PubMed] [Google Scholar]
Morris 1994 {published data only}
- Morris GF, Gore‐Hickman W, Lang SA, Yip RW. Can parturients distinguish between intravenous and epidural fentanyl?. Canadian Journal of Anaesthesia 1994;41(8):667‐72. [DOI] [PubMed] [Google Scholar]
Muir 1996 {published data only}
- Muir HA, Shukla R, Liston R, Writer D. Randomised trial of labor analgesia: a pilot study to compare patient‐controlled epidural analgesia to determine if analgesic method affects delivery outcome. Canadian Journal of Anaesthesia 1996;43(5):A60. [Google Scholar]
Muir 2000 {published data only}
- Halpern S, Breen T, Campbell DC, Blanchard W. Epidural PCA fentanyl/bupivacaine vs IV PCA fentanyl: neonatal effects. Anesthesiology 1999;90(4 Suppl):A19. [Google Scholar]
- Halpern S, Muir H, Breen T, Campbell DC. Randomised controlled trials in obstetrical anesthesia‐what did the patients think?. Anesthesiology 1999;91(3A):A1068. [Google Scholar]
- Muir HA, Breen T, Campbell DC, Halpern S, Blanchard W. Is intravenous PCA fentanyl an effective method for providing labor analgesia?. Anesthesiology 1999;90(4 Suppl):A28. [Google Scholar]
- Muir HD, Breen T, Campbell D, Halpern S, Liston R, Blanchard W. A multi centre study of the effects of analgesia on the progress of labour (abstract). Anesthesiology 2000;92 Suppl:A23. [Google Scholar]
Nikkola 1997 {published data only}
- Nikkola EM, Ekblad UU, Kero PO, Alihanka JJ, Salonen MA. Intravenous fentanyl PCA during labour. Canadian Journal of Anaesthesia 1997;44(12):1248‐55. [DOI] [PubMed] [Google Scholar]
Philipsen 1989 {published data only}
- Philipsen T, Jensen NH. A randomised study comparing epidural block and pethidine as analgesic in labour. World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Brazil. 1988:378‐9.
- Philipsen T, Jensen NH. Epidural block or parenteral pethidine as analgesic in labour; a randomized study concerning progress in labour and instrumental deliveries. European Journal of Obstetrics & Gynecology and Reproductive Biology 1989;30(1):27‐33. [DOI] [PubMed] [Google Scholar]
- Philipsen T, Jensen NH. Maternal opinion about analgesia in labour and delivery. A comparison of epidural blockade and intramuscular pethidine. European Journal of Obstetrics & Gynecology and Reproductive Biology 1990;34(3):205‐10. [DOI] [PubMed] [Google Scholar]
Rabie 2006 {published data only}
- Rabie ME, Negmi HH, Moustafa AM, Al Oufi H. Remifentanil by patient controlled analgesia compared with epidural analgesia for pain relief in labour [abstract]. Regional Anesthesia and Pain Management 2006;31(5 Suppl 1):52. [Google Scholar]
Ramin 1995 {published data only}
- Ramin S, Gambling DR, Lucas MJ, Sharma SK, Sidawi JE, Leveno KJ. Randomised trial of epidural versus intravenous analgesia during labour. Obstetrics & Gynecology 1995;86(5):783‐9. [DOI] [PubMed] [Google Scholar]
Sabry 2011 {published data only}
- NCT01290289. Analgesia in labor, a prospective parallel single blind study to compare regional analgesia (combined spinal epidural analgesia (cse), epidural analgesia (e)) and intravenous (iv) pethidine analgesia. clinicaltrials.gov/show/NCT01290289 (first received 4 Feb 2011).
- Sweed N, Sabry N, Azab T, Nour S. Regional versus IV analgesics in labor. Minerva Medica 2011;102(5):353‐61. [PubMed] [Google Scholar]
Scavone 2002 {published data only}
- Scavone BM, Sullivan JT, Peaceman AM, Strauss‐Hoder TP, Wong CA. Fetal heart rate and uterine contraction pattern abnormalities after combined spinal/epidural vs systemic labor analgesia [abstract]. Anesthesiology 2002;96 Suppl:Abstract no: A1049. [Google Scholar]
Sharma 1997 {published data only}
- Alexander JM, Sharma SK, McIntire DD, Wiley J, Leveno KJ. Intensity of labor pain and cesarean delivery. Anesthesia & Analgesia 2001;92(1):1524‐8. [DOI] [PubMed] [Google Scholar]
- Alexander JM, Sharma SK, McIntire DD, Wiley J, Leveno KJ. The effect of parity on labour pain. American Journal of Obstetrics and Gynecology 2000;182:S134. [Google Scholar]
- Philip J, Alexander J, Ramin S, Sharma S, McIntire D, Leveno K. Epidural analgesia during labor may be an independent source of maternal fever. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S175. [Google Scholar]
- Philip J, Alexander JM, Sharma SK, Leveno KJ, McIntire DD, Wiley J. Epidural analgesia during labour and maternal fever. Anesthesiology 1999;90(5):1271‐5. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Sidawi JE, Ramin SM, Lucas MJ, Leveno KJ, Cunningham FG. A randomised trial of epidural versus patient‐controlled meperidine analgesia during labour. Anesthesiology 1997;87(3):487‐94. [DOI] [PubMed] [Google Scholar]
Sharma 2002 {published data only}
- Alexander JM, Sharma SK, McIntire DD, Leveno KJ. Epidural analgesia lengthens the friedman active phase of labor [abstract]. Anesthesiology 2002;96(Suppl 1):P40. [DOI] [PubMed] [Google Scholar]
- Alexander JM, Sharma SK, McIntire DD, Leveno KJ. Epidural analgesia lengthens the friedman active phase of labour. Obstetrics & Gynecology 2002;100(1):46‐50. [DOI] [PubMed] [Google Scholar]
- Hill J, Alexander J, Sharma S, McIntire D, Leveno K. The effects of low‐dose epidural technique for labor analgesia on fetal heart rate (fhr) [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S206. [Google Scholar]
- Hill JB, Alexander JM, Sharma SK, McIntire DD. A comparison of the effects of epidural and meperidine analgesia during labor on fetal heart rate. Obstetrics & Gynecology 2003;102(2):333‐7. [DOI] [PubMed] [Google Scholar]
- Sharma S, Leveno K, Messick G, Alexander J, Sidawi J, Wiley J. A randomized trial of patient‐controlled epidural versus patient‐controlled intravenous analgesia during labor [abstract]. Anesthesiology 2000;92 Suppl:A22. [Google Scholar]
- Sharma SK, Alexander JM, Messick G, Bloom SL, McIntire DD, Wiley J, et al. Cesarean delivery: a randomized trial of epidural analgesia versus intravenous meperidine analgesia during labor in nulliparous women. Anesthesiology 2002;96(3):546‐51. [DOI] [PubMed] [Google Scholar]
Shifman 2007 {published data only}
- Shifman EM, Butrov AV, Floka SE, Got IB. Transient neurological symptoms in puerperas after epidural analgesia during labor. Anesteziologiia i Reanimatologiia 2007, issue 6:17‐20. [PubMed]
Stocki 2011 {published data only}
- Stocki D, Matot I, Einav S, Eventov‐Friedman S, Ginosar Y, Weiniger CF. A randomized controlled trial of the efficacy and respiratory effects of patient‐controlled intravenous remifentanil analgesia and patient‐controlled epidural analgesia in laboring women. Anesthesia & Analgesia 2014;118(3):589‐97. [DOI] [PubMed] [Google Scholar]
Sullivan 2002 {published data only}
- Stocki D, Matot I, Weiniger CF. A prospective randomized controlled trial to compare the efficacy and safety of remifentanil IV PCA to epidural PECA in labor analgesia. European Journal of Anaesthesiology 2011;28 Suppl:164‐5. [Google Scholar]
- Sullivan JT, Scavone BM, McCarthy RJ, Wong CA. Does type of labor analgesia alter the pattern of oxytocin use? [abstract]. Anesthesiology 2002;96(Suppl 1):Abstract no: P48. [Google Scholar]
- Sullivan JT, Scavone BM, McCarthy RJ, Wong CA. Neuraxial labor analgesia is associated with an altered pattern of oxytocin use [abstract]. Anesthesiology 2002;96 Suppl:Abstract no: A1039. [Google Scholar]
Thalme 1974 {published data only}
- Thalme B, Belfrage P, Raabe N. Lumbar epidural analgesia in labour: I. Acid‐base balance and clinical condition of mother, fetus and newborn child. Acta Obstetricia et Gynecologica Scandinavica 1974;53(1):27‐35. [DOI] [PubMed] [Google Scholar]
Thorp 1993 {published data only}
- Thorp JA, Hu D, Albin R, McNitt J, Meyer BA, Cohen GR, et al. The effect of intrapartum epidural analgesia on nulliparous labor: a randomized controlled prospective trial. American Journal of Obstetrics and Gynecology 1993;168:319. [DOI] [PubMed] [Google Scholar]
- Thorp JA, Hu DH, Albin RM, McNitt J, Meyer BA, Cohen GR, et al. The effect of intrapartum epidural analgesia on nulliparous labor: a randomized, controlled, prospective trial. American Journal of Obstetrics and Gynecology 1993;169(4):851‐8. [DOI] [PubMed] [Google Scholar]
Tveit 2012 {published data only}
- Tveit TO, Seiler S, Halvorsen A, Rosland JH. Labour analgesia: A randomised, controlled trial comparing intravenous remifentanil and epidural analgesia with ropivacaine and fentanyl. European Journal of Anaesthesiology 2012;29(3):129‐36. [DOI] [PubMed] [Google Scholar]
Volmanen 2008 {published data only}
- Volmanen P, Sarvela J, Akural EI, Raudaskoski T, Korttila K, Alahuhta S. Intravenous remifentanil vs. epidural levobupivacaine with fentanyl for pain relief in early labour: a randomised, controlled, double‐blinded study. Acta Anaesthesiologica Scandinavica 2008;52(2):249‐55. [DOI] [PubMed] [Google Scholar]
Witoonpanich 1984 {published data only}
- Witoonpanich P, Surapong K. Control of hypertension in labouring preeclamptics. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:230.
Xing 2015 {published data only}
- Xing JJ, Liu XF, Xiong XM, Huang L, Lao CY, Yang M, et al. Effects of combined spinal‐epidural analgesia during labor on postpartum electrophysiological function of maternal pelvic floor muscle: a randomized controlled trial. Plos One 2015;10(9):e0137267. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abboud 1982 {published data only}
- Abboud TK, Sarkis F, Goebelsmann U, Hung TT, Henriksen EH. Effects of epidural anesthesia during labor on maternal plasma B‐endorphin levels. Anesthesiology 1982;57:A382. [DOI] [PubMed] [Google Scholar]
Anwar 2015 {published data only}
- Anwar S, Anwar MW, Ayaz A, Danish N, Ahmad S. Effect of epidural analgesia on labor and its outcomes. Journal of Ayub Medical College, Abbottabad: JAMC 2015;27(1):146‐50. [PubMed] [Google Scholar]
Buchan 1973 {published data only}
- Buchan PC, Milne MK, Browning MC. The effect of continuous epidural blockade on plasma 11‐hydroxy‐corticosteroid concentrations in labour. Journal of Obstetrics and Gynaecology of the British Commonwealth 1973;80:974‐7. [DOI] [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen LK, Hsu HW, Lin CJ, Huang CH, Tsai SK, Lee CN, et al. Effects of epidural fentanyl on labor pain during the early period of the first stage of induced labor in nulliparous women. Journal of the Formosan Medical Association 2000;99(7):549‐53. [PubMed] [Google Scholar]
Cutura 2011 {published data only}
- Cutura N, Soldo V, Milovanovic SR, Orescanin‐Dusic Z, Curkovic A, Tomovic B, et al. Optimal dose of an anesthetic in epidural anesthesia and its effect on labor duration and administration of vacuum extractor and forceps. Clinical & Experimental Obstetrics & Gynecology 2011;38(3):247‐50. [PubMed] [Google Scholar]
Ginosar 2002 {published data only}
- Ginosar Y, Columb M, Cohen SE, Mirikatani E, Tingle MS, Ratner EF, et al. Epidural fentanyl infusions in the presence of local anesthetics exert segmental analgesia: an MLAC infusion study in nulliparous labour [abstract]. Anesthesiology 2002;96(Suppl 1):P‐62. [DOI] [PubMed] [Google Scholar]
Ginosar 2003 {published data only}
- Ginosar Y, Columb MO, Cohen SE, Mirikatani E, Tingle MS, Ratner EF, et al. The site of action of epidural fentanyl infusions in the presence of local anesthetics: a minimum local analgesic concentration infusion study in nulliparous labor. Anesthesia & Analgesia 2003;97:1439‐45. [DOI] [PubMed] [Google Scholar]
Gupta 2013 {published data only}
- CTRI/2013/09/003968. Intravenous paracetamol as an adjunct to patient‐controlled epidural analgesia with levobupivacaine and fentanyl in labour: a randomised controlled trial. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=6573&EncHid=&modid=&compid=%27,%276573det%27 (first received 11 September 2011).
- Gupta N, Gupta S, Agarwal A, Agarwal S, Dwivedi S, Singh A. To study the painless labour by epidural analgesia and its effects on cardiotocographic parameters and labour. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(4):666‐70. [Google Scholar]
Hood 1993 {published data only}
- Hood DD, Parker RL, Meis PJ. Epidural bupivacaine does not effect fetal heart rate tracing. Anesthesiology 1993;79:3A. [Google Scholar]
John 2013 {published data only}
- John C, Fyneface‐Ogan S, Enyindah C. A comparison of the effect of spinal analgesia and sedo‐analgesia on maternal cortisol levels during labour. 1st FIGO African Regional Conference of Gynecology and Obstetrics; 2013 Oct 2‐5; Addis Ababa, Ethiopia. 2013.
Jouppila 1976 {published data only}
- Joupila R, Hollmen A. The effect of segmental analgesia on maternal and foetal acid‐base balance, lactate, serum potassium and creatinine phosphokinase. Acta Anaesthesiologica Scandinavica 1976;20:259‐68. [DOI] [PubMed] [Google Scholar]
- Jouppila R. The effect of segmental epidural analgesia on maternal growth hormone, insulin, glucose and free fatty acids during labour. Annales Chirurgiae et Gynaecologiae 1976;65(6):398‐404. [PubMed] [Google Scholar]
Jouppila 1980 {published data only}
- Jouppila R, Jouppila P, Moilanen K, Pakarinen A. The effect of segmental epidural analgesia on maternal prolactin during labour. British Journal of Obstetrics and Gynaecology 1980;87:234‐8. [DOI] [PubMed] [Google Scholar]
Justins 1983 {published data only}
- Justins DM, Knott C, Luthman J, Reynolds F. Epidural vs intramuscular fentanyl; anaesthesia and pharmacokinetics in labour. Anaesthesia 1983;38(10):937‐42. [DOI] [PubMed] [Google Scholar]
Kujansuu 1987 {published data only}
- Kujansuu E. Paracervical and epidural anaesthesia in labour pain [Paraservikaali‐ ja epiduraalipuudutus synnytyskivun lievityksessä]. Suomen Lääkärilehti 1987;42:2038‐9. [Google Scholar]
Kurjak 1974 {published data only}
- Kurjak A, Beazley JM. The effect of continuous lumbar epidural analgesia on the fetus, newborn child and the acid‐base status of maternal blood. Acta Medica Iugoslavica 1974;28:15‐26. [PubMed] [Google Scholar]
Lassner 1981 {published data only}
- Lassner J, Barrier G, Talafre ML, Durupty D. Failure of extradural morphine to provide adequate pain relief in labour. British Journal of Anaesthesia 1981;53:112P. [Google Scholar]
Leong 2000 {published data only}
- Leong EW, Sivanesaratnam V, Oh LL, Chan YK. Epidural analgesia in primigravidae in spontaneous labour at term: a prospective study. Journal of Obstetrics & Gynaecology Research 2000;26(4):271‐5. [DOI] [PubMed] [Google Scholar]
MacKenzie 1996 {published data only}
- MacKenzie P, James K, Bower S, McGrady E, Patrick A. Plasma fentanyl levels during epidural and intravenous fentanyl infusion for labour analgesia [abstract]. International Journal of Obstetric Anesthesia 1996;5:218‐9. [Google Scholar]
Manninen 2000 {published data only}
- Manninen T, Aantaa R, Salonen M, Pirhonen J, Palo P. A comparison of the hemodynamic effects of paracervical block and epidural anesthesia for labor analgesia. Acta Anaesthesiologica Scandinavica 2000;44(4):441‐5. [DOI] [PubMed] [Google Scholar]
Martin 2003 {published data only}
- Martin GC, Beilin Y, Holzman IR, Ekwa‐Ekoko C. Is there an effect of giving epidural fentanyl during labor on breastfeeding? [abstract]. Pediatric Research 2003;53 Suppl:93. [Google Scholar]
McGrath 1992 {published data only}
- McGrath J, Chestnut D, Debruyn C. The effect of epidural bupivacaine versus intravenous nalbuphine on fetal heart rate during labor. Anesthesiology 1992;77(3A):A984. [Google Scholar]
Moreno 1997 {published data only}
- Moreno I, Puertas A, Mino M, Lopez JC, Manzanares S, Carrillo MP, et al. Influence of epidural analgesia on the labour induced by premature rupture of membranes. Acta Ginecologica 1997;54:211‐4. [Google Scholar]
- Puertas A, Mino M, Moreno M, Rodriguez C, Miranda J, Herruzo A. Influence of the epidural anaesthesia in the oxytocin labour induction of premature rupture of membranes. Prenatal and Neonatal Medicine 1996;1(Suppl 1):88. [Google Scholar]
Nafisi 2006 {published data only}
- Nafisi S. Effects of epidural lidocaine analgesia on labor and delivery: a randomized, prospective, controlled trial. BMC Anesthesiology 2006;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neri 1986 {published data only}
- Neri A, Nitke S, Lachman E, Ovadia J. Lumbar epidural analgesia in hypertensive patients during labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1986;22(1‐2):1‐6. [DOI] [PubMed] [Google Scholar]
Noble 1971 {published data only}
- Noble AD, Craft IL, Bootes JA, Edwards PA, Thomas DJ, Mills KL. Continuous lumbar epidural analgesia using bupivacaine: a study of the fetus and newborn child. Journal of Obstetrics and Gynaecology of the British Commonwealth 1971;78(6):559‐63. [DOI] [PubMed] [Google Scholar]
Polley 2000 {published data only}
- Polley LS, Columb MO, Naughton NN, Wagner DS, Dorantes DM, Ven CJ. Effect of intravenous versus epidural fentanyl on the minimum local analgesic concentration of epidural bupivacaine in labor. Anesthesiology 2000;93(1):122‐8. [PUBMED: 10861155] [DOI] [PubMed] [Google Scholar]
Revill 1979 {published data only}
- Revill S. Pain relief in labour: what the patient requires. First European Congress of Obstetrical Anaesthesia and Analgesia; 1979; Birmingham, UK. 1979:1‐16.
Robinson 1980 {published data only}
- Robinson JO, Rosen M, Evans JM, Revill SI, David H, Rees GA. Maternal opinion about analgesia for labour. A controlled trial between epidural block and intramuscular pethidine combined with inhalation. Anaesthesia 1980;35(12):1173‐81. [DOI] [PubMed] [Google Scholar]
Robinson 1997 {published data only}
- Robinson PN, Romney M, Gordon H, Loughnan BA. Outcome of labour using low dose extradural bupivacaine compared with intramuscular pethidine. British Journal of Anaesthesia 1997;79(5):675P. [Google Scholar]
Ryhanen 1984 {published data only}
- Ryhanen P, Jouppila R, Lanning M, Jouppila P, Hollmen A, Kouvalainen K. Effect of segmental epidural analgesia on changes in peripheral blood leucocyte counts, lymphocyte subpopulations, and in vitro transformation in healthy parturients and their newborns. Gynecologic and Obstetric Investigation 1984;17(4):202‐7. [DOI] [PubMed] [Google Scholar]
Solek‐Pastuszka 2009 {published data only}
- Solek‐Pastuszka J, Kepinski S, Makowski A, Celewicz Z, Zukowski M, Safranow K, et al. Patient‐controlled continuous epidural analgesia vs intravenous remifentanil infusion for labour anaesthesia. Anestezjologia Intensywna Terapia 2009;41(2):84‐8. [PubMed] [Google Scholar]
Stourac 2014 {published data only}
- Stourac P, Suchomelova H, Stodulkova M, Huser M, Krikava I, Janku P, et al. Comparison of parturient‐controlled remifentanil with epidural bupivacain and sufentanil for labour analgesia: randomised controlled trial. Biomedical Papers: Journal of the Palacky University 2014;158(2):227‐32. [DOI] [PubMed] [Google Scholar]
Swanström 1981 {published data only}
- Bratteby LE. Short‐ and long‐term effects on the infant of obstetric regional anesthesia. Acta Anaesthesiologica Scandinavica 1983;27:36. [Google Scholar]
- Bratteby LE, Andersson L, Swanström S. Effect of obstetrical regional analgesia on the change in respiratory frequency in the newborn. British Journal of Anaesthesia 1979;51:41‐5. [Google Scholar]
- Swanström S, Bratteby LE. Metabolic effects of obstetric regional analgesia and of asphyxia in the newborn infant during the first two hours after birth. I. Arterial blood glucose concentrations. Acta Paediatrica Scandinavica 1981;70(6):791‐800. [DOI] [PubMed] [Google Scholar]
- Swanström S, Bratteby LE. Metabolic effects of obstetric regional analgesia and of asphyxia in the newborn infant during the first two hours after birth. II. Arterial plasma concentrations of glycerol, free fatty acids and beta‐hydroxybutyrate. Acta Paediatrica Scandinavica 1981;70(6):801‐9. [DOI] [PubMed] [Google Scholar]
- Swanström S, Bratteby LE. Metabolic effects of obstetric regional analgesia and of asphyxia in the newborn infant during the first two hours after birth. III. Adjustment of arterial blood gases and acid‐base balance. Acta Paediatrica Scandinavica 1981;70(6):811‐8. [DOI] [PubMed] [Google Scholar]
Tugrul 2006 {published data only}
- Tugrul S, Oral O, Bakacak M, Uslu H, Pekin O. Effects of epidural analgesia using ropivacaine on the mother and the newborn during labor. Saudi Medical Journal 2006;27(12):1853‐8. [PubMed] [Google Scholar]
Wassen 2015 {published data only}
- Bonouvrie K, Bosch A, Roumen FJ, Kuijk SM, Nijhuis JG, Evers SM, et al. Epidural analgesia during labour, routinely or on request: a cost‐effectiveness analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;207:23‐31. [DOI] [PubMed] [Google Scholar]
- Bosch AA, Goossens M, Bonouvrie K, Grimm B, Nijhuis JG, Roumen FJ, et al. Maternal quality of life in routine labor epidural analgesia versus labor analgesia on request, results of a randomized trial. Journal of Maternal‐Fetal and Neonatal Medicine 2016;29(Suppl 1):186‐7. [DOI] [PubMed] [Google Scholar]
- Wassen M, Smits L, Scheepers H, Marcus M, Neer J, Nijhuis J, et al. Routine labour epidural analgesia versus labour analgesia on request: a randomised non‐inferiority trial. BJOG: an International Journal of Obstetrics and Gynaecology 2015;122(3):344‐50. [DOI] [PubMed] [Google Scholar]
Wong 2005 {published data only}
- Scavone BM, McCarthy RJ, Wong CA, Sullivan JT. The influence of time of day of administration on duration of opioid labor analgesia. Anesthesia & Analgesia 2010;111(4):986‐91. [DOI] [PubMed] [Google Scholar]
- Wong CA, Scavone BM, Peaceman AM, McCarthy RJ, Sullivan JT, Diaz NT, et al. The risk of caesarean delivery with neuroaxial analgesia given early versus late in labour. New England Journal of Medicine 2005;352(7):655‐65. [DOI] [PubMed] [Google Scholar]
- Wong CA, Scavone BM, Sullivan JT, Strauss‐Hoder TP, McCarthy RJ. Randomized trial of neuraxial vs systemic analgesia for latent phase labor: effect on incidence of cesarean delivery [abstract]. Anesthesiology 2002;96 Suppl:Abstract no: A1047. [Google Scholar]
Wong 2009 {published data only}
- Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstetrics & Gynecology 2009;113(5):1066‐74. [DOI] [PubMed] [Google Scholar]
- Wong CA, Scavone BM, Sullivan JT, Ebarvia MJ, McCarthy RJ. The risk of cesarean delivery with early neuraxial analgesia in nulliparous induction of labor. Anesthesiology 2007;107:Abstract no: A1204. [Google Scholar]
- Wong CA, Sullivan JT, McCarthy RJ, Scavone BM, Patel R, Ebarvia MJ. Randomized trial of neuraxial vs. systemic analgesia for labor induction: effect on incidence of cesarean delivery [abstract]. Anesthesiology 2007;106(Suppl 1):21. [Google Scholar]
Zakowski 1994 {published data only}
- Zakowski MI, Ramanathan S, Sutin KM, Grant GJ, Turndorf H. Pharmacokinetic profile of morphine in parturients following intravenous or epidural administration. Regional Anesthesia 1994;19:119‐25. [Google Scholar]
References to studies awaiting assessment
Antipin 2014 {published data only}
- Antipin EE, Uvarov DN, Nedashkovskii EV, Kushev IP. Epidural analgesia in the first stage of labor‐‐is there an alternative?. Anesteziologiia I Reanimatologiia 2014;2014(1):18‐22. [PubMed] [Google Scholar]
Gupta 2016 {published data only}
- Gupta K, Mitra S, Kazal S, Saroa R, Ahuja V, Goel P. I.V. paracetamol as an adjunct to patient‐controlled epidural analgesia with levobupivacaine and fentanyl in labour: a randomized controlled study. British Journal of Anaesthesia 2016;117(5):617‐22. [DOI] [PubMed] [Google Scholar]
Kamali 2016 {published data only}
- IRCT2016051820258N9. Clinical trial comparison of labor phases in painless delivery with epidural analgesia and Entonox. en.search.irct.ir/view/30306 (first received 17 Nov 2016).
Marshalov 2012 {published data only}
- Marshalov D, Salov I, Shifman E, Petrenko A. Influence of epidural analgesia on abdominal wall pain tension and level of abdominal pressure in labor. Regional Anesthesia and Pain Medicine 2012;37(7 Suppl):E278. [Google Scholar]
Vavrinkova 2005 {published data only}
- Vavrinkova B, Oborna L, Binder T, Horak J. Nalbuphine in obstetrical analgesia [Nalbuphine v porodnicke analgezii]. Ceska Gynekologie 2005;70(3):180‐3. [PubMed] [Google Scholar]
Weissman 2006 {published data only}
- NCT00296751. Epidural analgesia versus iv meperidine for labor pain control. objective evaluation of the pain intensity influence on the autonomic nervous system. clinicaltrials.gov/ct2/show/record/NCT00296751 (first received 24 Feb 2006).
Additional references
Barragán 2011
- Barragán LIM, Solà I, Juandó PC. Biofeedback for pain management during labour. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD006168.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohren 2017
- Bohren MA, Hofmeyr GJ, Sakala C, Fukuzawa RK, Cuthbert A. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD003766.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bromage 1999
- Bromage PR. Neurologic complications of labour, delivery, and regional anesthesia. In: Chestnut DH editor(s). Obstetric Anesthesia, Principles and Practice. 2nd Edition. Mosby, 1999:639‐59. [Google Scholar]
Brownridge 1991
- Brownridge P. Treatment options for the relief of pain during childbirth. Drugs 1999;41(1):69‐80. [DOI] [PubMed] [Google Scholar]
Buggy 1995
- Buggy D, Gardiner J. The space blanket and shivering during extradural analgesia in labour. Acta Anaesthesiologica Scandinavica 1995;39(4):551‐3. [DOI] [PubMed] [Google Scholar]
Chang 2002
- Chang MY, Wang SY, Chen CH. Effects of massage on pain and anxiety during labour: a randomized controlled trial in Taiwan. Journal of Advanced Nursing 2002;38(1):68‐73. [DOI] [PubMed] [Google Scholar]
Christiansen 2002
- Christiansen P, Klostergaard KM, Terp MR, Poulsen C, Agger AO, Rasmussen KL. Long‐memory of labour pain. Ugeskrift for Laeger 2002;164(42):4927‐9. [PubMed] [Google Scholar]
Cluett 2009
- Cluett ER, Burns E. Immersion in water in labour and birth. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000111.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
COMET 2001
- Comparative Obstetric Mobile Epidural Trial (COMET) Study Group UK. Effect of low dose mobile versus traditional epidural techniques on mode of delivery: a randomised control trial. Lancet 2001;358(9275):19‐23. [DOI] [PubMed] [Google Scholar]
Cyna 2004
- Cyna AM, McAuliffe GL, Andrew MI. Hypnosis for pain relief in labour and childbirth: a systematic review. British Journal of Anaesthesia 2004;93(4):505‐11. [DOI] [PubMed] [Google Scholar]
Derry 2012
- Derry S, Straube S, Moore RA, Hancock H, Collins SL. Intracutaneous or subcutaneous sterile water injection compared with blinded controls for pain management in labour. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009107.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
DOH 2005
- Department of Health. Statistical Bulletin‐NHS Maternity Statistics, England:2003‐2004. London, UK: Department of Health, 2004. [Google Scholar]
Dowswell 2009
- Dowswell T, Bedwell C, Lavender T, Neilson JP. Transcutaneous electrical nerve stimulation (TENS) for pain relief in labour. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eberle 1996
- Eberle RL, Norris MC. Labour analgesia. A risk‐benefit analysis. Drug Safety 1996;14(4):239‐51. [DOI] [PubMed] [Google Scholar]
Grant 2015
- Grant EN, Tao W, Craig M, McIntire D, Leveno K. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG 2015;122(3):288‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halpern 1998
- Halpern SH, Leighton BL, Ohlsson A, Barrett JF, Rice A. Effect of epidural analgesia on the progress of labour: a meta‐analysis. JAMA 1998;280(24):2105‐10. [DOI] [PubMed] [Google Scholar]
Hibbard 1996
- Hibbard BM, Anderson MM, Drife JO, Tighe JR, Gordon G, Willatts S, et al. Deaths associated with anaesthesia. In: Rubery E, Bourdillon P editor(s). Report on Confidential Enquiries into Maternal Deaths in the United Kingdom 1991‐1993. Norwich: HMSO, 1996:87‐102. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hu 2016
- Hu LQ, Flood P, Li Y, Tao W, Zhao P, Xia Y, et al. No pain labor & delivery: a global health initiative's impact on clinical outcomes in China. Anesthesia & Analgesia 2016;122(6):1931‐8. [DOI] [PubMed] [Google Scholar]
Jones 2011
- Jones L, Dou L, Dowswell T, Alfirevic Z, Neilson James P. Pain management for women in labour: generic protocol. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD009167] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2012
- Jones L, Othman M, Dowswell T, Alfirevic Z, Gates S, Newburn M, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klomp 2012
- Klomp T, Poppel M, Jones L, Lazet J, Nisio M, Lagro‐Janssen AL. Inhaled analgesia for pain management in labour. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009351.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2002
- Liang CC, Wong SY, Tsay PT, Chang SD, Tseung LH, Wang MF, et al. The effect of epidural analgesia on postpartum urinary retention in women who deliver vaginally. International Journal of Obstetric Anesthesia 2002;11:164‐9. [DOI] [PubMed] [Google Scholar]
Lieberman 2002
- Lieberman E, O'Donoghue C. Unintended effects of epidural analgesia during labour. American Journal of Obstetrics and Gynecology 2002;186(5):S31‐S64. [DOI] [PubMed] [Google Scholar]
Madden 2016
- Madden K, Middleton P, Cyna AM, Matthewson M, Jones L. Hypnosis for pain management during labour and childbirth. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD009356.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martensson 1999
- Martensson L, Wallin G. Labour pain treated with cutaneous injection of sterile water: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1999;106:633‐67. [DOI] [PubMed] [Google Scholar]
McCrea 2000
- McCrea H, Wright ME, Stringer M. Psychosocial factors influencing personal control in pain relief. International Journal of Nursing Studies 2000;37:493‐503. [DOI] [PubMed] [Google Scholar]
Novikova 2011
- Novikova N, Cluver C. Local anaesthetic nerve block for pain management in labour. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Othman 2011
- Othman M, Jones L, Neilson JP. Non‐opioid drugs for pain management in labour. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009223] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranta 1994
- Ranta P, Jouppila P, Spalding M, Kangas‐Saarela T, Hollmén A, Jouppila R. Parturients' assessment of water blocks, pethidine, nitrous oxide, paracervical and epidural blocks in labour. International Journal of Obstetric Anesthesia 1994;3(4):193‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowlands 1998
- Rowlands S, Permezel M. Physiology of pain in labour. Bailliere's Clinical Obstetrics and Gynaecology 1998;12(3):347‐62. [DOI] [PubMed] [Google Scholar]
Russell 2000
- Russell R. The effects of regional analgesia on the progress of labour and delivery. British Journal of Anaesthesia 2000;84(6):799‐12. [DOI] [PubMed] [Google Scholar]
Simmons 2012
- Simmons SW, Taghizadeh N, Dennis AT, Hughes D, Cyna AM. Combined spinal‐epidural versus epidural analgesia in labour. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD003401.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2011a
- Smith CA, Collins CT, Crowther CA. Aromatherapy for pain management in labour. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2011b
- Smith CA, Collins CT, Crowther CA, Levett KM. Acupuncture or acupressure for pain management in labour. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009232] [DOI] [PubMed] [Google Scholar]
Smith 2018a
- Smith CA, Levett KM, Collins CT, Armour M, Dahlen HG, Suganuma M. Relaxation techniques for pain management in labour. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD009514.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2018b
- Smith CA, Levett KM, Collins CT, Dahlen HG, Ee CC, Suganuma M. Massage, reflexology and other manual methods for pain management in labour. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD009290.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sng 2015
- Sng Ban L, Kwok Sarah C, Sia Alex T H. Modern neuraxial labour analgesia. Current Opinion in Anaesthesiology 2015;28(3):285‐9. [DOI] [PubMed] [Google Scholar]
Stride 1993
- Stride PC, Cooper GM. Dural tap revisited: a 20 year survey from Birmingham Maternity Hospital. Anaesthesia 1993;48(3):247‐55. [DOI] [PubMed] [Google Scholar]
Sultan 2013
- Sultan P, Murphy C, Halpern S, Carvalho B. The effect of low concentrations versus high concentrations of local anesthetics for labour analgesia on obstetric and anesthetic outcomes: a meta‐analysis. Canadian Journal of Anaesthesia 2013;60(9):840‐54. [DOI] [PubMed] [Google Scholar]
Swanström 1981b
- Swanström S, Bratteby LE. Metabolic effects of obstetric regional analgesia and of asphyxia in the newborn infant during the first two hours after birth. I. Arterial blood glucose concentrations. Acta Paediatrica Scandinavica 1981;70(6):791‐800. [DOI] [PubMed] [Google Scholar]
Thornton 2001
- Thornton JG. Reducing likelihood of instrumental delivery with epidural anaesthesia. Lancet 2001;358(9275):2. [DOI] [PubMed] [Google Scholar]
Ullman 2010
- Ullman R, Smith LA, Burns E, Mori R, Dowswell T. Parenteral opioids for maternal pain management in labour. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007396.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vincent 1998
- Vincent RD, Chestnut DH. Epidural analgesia during labour. American Family Physician 1998; Vol. 58, issue 8:1785‐92. [PubMed]
Walker 1997
- Walker M. Do labour medications affect breastfeeding. Journal of Human Lactation 1997;13(2):131‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Anim‐Somuah 2005
- Anim‐Somuah M, Smyth RMD, Howell CJ. Epidural versus non‐epidural or no analgesia in labour. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD000331.pub2] [DOI] [PubMed] [Google Scholar]
Anim‐Somuah 2011
- Anim‐Somuah M, Smyth RMD, Jones L. Epidural versus non‐epidural or no analgesia in labour. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000331.pub3] [DOI] [PubMed] [Google Scholar]
Howell 1999
- Howell CJ. Epidural vs non‐epidural analgesia in labour. [revised 06 May 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.


