Abstract
When recruiting research participants through central cancer registries, high response fractions help ensure population-based representation. We conducted multivariable mixed-effects logistic regression to identify case and study characteristics associated with making contact with and obtaining cooperation of Utah cancer cases using data from 17 unique recruitment efforts undertaken by the Utah Cancer Registry (2007–2016) on behalf of the following studies: A Population-Based Childhood Cancer Survivors Cohort Study in Utah, Comparative Effectiveness Analysis of Surgery and Radiation for Prostate Cancer (CEASAR Study), Costs and Benefits of Follow-up Care for Adolescent and Young Adult Cancers, Study of Exome Sequencing for Head and Neck Cancer Susceptibility Genes, Genetic Epidemiology of Chronic Lymphocytic Leukemia, Impact of Remote Familial Colorectal Cancer Risk Assessment and Counseling (Family CARE Project), Massively Parallel Sequencing for Familial Colon Cancer Genes, Medullary Thyroid Carcinoma (MTC) Surveillance Study, Osteosarcoma Surveillance Study, Prostate Cancer Outcomes Study, Risk Education and Assessment for Cancer Heredity Project (REACH Project), Study of Shared Genomic Segment Analysis and Tumor Subtyping in High-Risk Breast-Cancer Gene Pedigrees, Study of Shared Genomic Segment Analysis for Localizing Multiple Myeloma Genes. Characteristics associated with lower odds of contact included Hispanic ethnicity (odds ratio (OR) = 0.34, 95% confidence interval (CI): 0.27, 0.41), nonwhite race (OR = 0.46, 95% CI: 0.35, 0.60), and younger age at contact. Years since diagnosis was inversely associated with making contact. Nonwhite race and age ≥60 years had lower odds of cooperation. Study features with lower odds of cooperation included longitudinal design (OR = 0.50, 95% CI: 0.41, 0.61) and study brochures (OR = 0.70, 95% CI: 0.54, 0.90). Increased odds of cooperation were associated with including a questionnaire (OR = 3.19, 95% CI: 1.54, 6.59), postage stamps (OR = 1.60, 95% CI: 1.21, 2.12), and incentives (OR = 1.62, 95% CI: 1.02, 2.57). Among cases not responding after the first contact, odds of eventual response were lower when >10 days elapsed before subsequent contact (OR = 0.71, 95% CI: 0.59, 0.85). Obtaining high response is challenging, but study features identified in this analysis support better results when recruiting through central cancer registries.
Keywords: epidemiologic research design, methods, neoplasms, patient participation rates, registries, research subject recruitment, surveys and questionnaires
Central cancer registries are important for monitoring cancer incidence and mortality trends. Because central registries, unlike hospital registries, are population-based, they provide unbiased sampling frames (1, 2) for ascertaining and recruiting individuals diagnosed with cancer for research (3–9). Registry-based research has been instrumental in promoting preventive behaviors (10), evaluating treatment outcomes (11–13), and assessing quality-of-life and other outcomes among cancer survivors (14–17).
However, some researchers have expressed concern about low participation when recruiting via cancer registries (18, 19). Nonresponse bias might also be problematic in cancer-related patient-reported outcomes research (20). Survey methodology and general biomedical research have demonstrated that response fractions have declined over time (21–26) and increased effort is required to recruit participants (27). There is widespread concern that low response could produce nonrepresentative results (19, 20, 24–26, 28, 29).
We examined data from studies seeking to recruit cancer cases, initiated by the Utah Cancer Registry from 2007 to 2016, to ascertain factors associated with recruitment outcomes. We aimed to identify both case and study characteristics that predicted the ability to contact and recruit cancer cases. By analyzing multiple recruitment efforts with varying features and populations, we sought to provide a comprehensive analysis to inform future registry-based recruiting.
Past research indicates that demographic characteristics influence cancer registry–based recruitment. Non-Hispanic whites had higher participation than other racial or ethnic groups (3, 4, 19, 30–32) and were more likely to be located and successfully contacted than Hispanic (33) or black persons (32). In most studies, women consented in greater proportions (30, 31) and were easier to locate (33) than men, while others found men more likely to participate (34). Several registry-based studies showed that younger individuals were more likely to respond than older individuals, although the ages classified as “younger” varied (2–4, 19, 30, 32). Conversely, another study found those diagnosed before age 40 years were less likely to respond than those 50 years or older (18), and in another the youngest and the oldest were underrepresented among participants (35). Younger individuals (diagnosed between ages 20 and 44 years) were also more likely to be lost to follow-up than those diagnosed at 65 years or older (33) and were less likely to be contacted (32).
Cancer characteristics have also been relevant to recruitment outcomes. Individuals diagnosed at an advanced stage were less likely to respond than those with less advanced disease (3, 4, 30), and response differed by cancer site (4, 30, 36). Greater time between diagnosis and time of study made it more difficult to locate and recruit cases (2, 3, 20, 30, 34, 37).
Study implementation processes are also relevant for registry-based recruitment outcomes. Including a questionnaire in the recruitment packet, rather than providing it after obtaining consent, increased response among controls in a case-control study of risk factors for breast cancer (38). Another study comparing questionnaires of varying lengths (10 vs. 16 pages) found no difference in response fractions (4). Additionally, a $20.00 gift card resulted in more responses than $10.00 gift cards or a $100.00 lottery (3), whereas another study showed no difference in response when using $5.00 compared with $3.00 (4). Others have noted the importance of interpersonal telephone contact (7); inclusion of telephone contacts, compared with only sending letters, was shown to improve response (6).
These prior examinations elucidate factors influencing registry-based recruitment outcomes, but most report on single studies, and some describe recruitment that took place over 20 years ago. We aimed to expand on prior work by evaluating multiple factors in recent registry-initiated recruitment across multiple studies. We utilized data from 17 studies that recruited via the Utah Cancer Registry during 2007–2016 to determine what case and study characteristics are associated with the registry’s ability to contact and recruit subjects diagnosed with cancer.
METHODS
Studies and recruitment process
We used data from all studies that initiated contact with potential participants through the Utah Cancer Registry during 2007–2016 and maintained accurate records of response outcomes (n = 17 distinct recruitment efforts). Study designs and results for most of these have been reported (10–12, 31, 39–49). Each study was approved by the relevant institution’s institutional review board.
Utah state policy governing the use of surveillance data for research recruitment requires that initial contact be made by the data steward, in this case the cancer registry. For all studies, the first attempted contact with potential participants was a packet mailed to the last known residential address in registry records. All packets contained at minimum a letter introducing the study, either a questionnaire or a form to indicate permission or refusal for the researcher to contact the individual about the study, and a return envelope. The specific contents of the packet and the next steps of the contact process differed across studies, depending on research protocol and study resources. If no response was received following the first mailing, all protocols involved additional contact attempts, using telephone and/or mail. All recruitment materials and telephone calls were in English. If the registry staff found that the address in registry records was no longer valid, they attempted to find updated contact information using a variety of sources, including contacting physicians’ offices, the voter registration database, and the White Pages or online directory searches. Registry staff documented contact attempts and outcomes in a study tracking database.
Variables
We obtained individuals’ stage at diagnosis and demographic information from the cancer registry variables. Demographic variables included age at recruitment, years between diagnosis and recruitment, sex, Hispanic ethnicity, and race (due to small numbers, all races other than white were grouped for analysis). We classified geography as nonmetropolitan or metropolitan using the 2010 Rural-Urban Commuting Area codes associated with cases’ diagnosis Zone Improvement Plan (ZIP) codes (codes 1–3 = metropolitan; codes 4–10 = nonmetropolitan).
Using the registry’s study documentation, we constructed study-level variables representing protocol features (Table 1). Certain variables described the contents of the recruitment packet, accounting whether each of the following was included: a questionnaire, an informed consent form for human subjects research, and a study brochure. The brochure, if included, described study objectives, eligibility criteria, what participation entailed, and protection of confidentiality. Postage type was another variable, with one strategy being use of first-class stamps on outgoing and return envelopes and the alternative being outgoing bulk-rate mail and business-reply return envelopes. Several variables represented characteristics of studies as described in the recruitment materials, including whether the study involved collection of biospecimens, whether the study involved future follow-up after initial participation (longitudinal observational and intervention studies), whether the researchers wished to also recruit relatives of the cancer case, and whether a gift card or monetary incentive (ranging from $15 to $75) was promised for study participation.
Table 1.
Characteristics of 17 Recruitment Efforts Undertaken by the Utah Cancer Registry, 2007–2016
| Study Characteristic | Recruitment Efforts | Cases | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Cancer site included | ||||
| Breast | 4 | 23.5 | 5,418 | 54.5 |
| Colorectal | 2 | 11.8 | 2,115 | 21.3 |
| Prostate | 2 | 11.8 | 763 | 7.7 |
| Hematologic or lymphoma | 3 | 17.6 | 833 | 8.4 |
| Other sites and/or multiple sites | 6 | 35.3 | 818 | 8.2 |
| Recruitment packet | ||||
| Type of recruitment packet | ||||
| Permission-to-contact form | 14 | 82.4 | 8,724 | 87.7 |
| Questionnaire | 3 | 17.6 | 1,223 | 12.3 |
| Consent form included in packet | ||||
| No | 15 | 88.2 | 8,989 | 90.4 |
| Yes | 2 | 11.8 | 958 | 9.6 |
| Study brochure included in packeta | ||||
| No | 2,425 | 24.4 | ||
| Yes | 7,522 | 75.6 | ||
| Envelope postage | ||||
| Stamps, outgoing and return | 3 | 17.6 | 6,582 | 66.2 |
| Bulk rate/business reply | 14 | 82.4 | 3,365 | 33.8 |
| Follow-up methods for nonrespondersb,c | ||||
| Days between first and second contactd | ||||
| ≤10 | 5,677 | 74.1 | ||
| ≥11 | 1,982 | 25.9 | ||
| Second contact mode | ||||
| Telephone | 6,594 | 84.9 | ||
| 1,173 | 15.1 | |||
| Study description in recruitment packet | ||||
| Biospecimen collection | ||||
| No | 10 | 58.8 | 8,109 | 81.5 |
| Yes | 7 | 41.2 | 1,838 | 18.5 |
| Future follow-up participation | ||||
| No | 15 | 88.2 | 7,846 | 78.9 |
| Yes | 2 | 11.8 | 2,101 | 21.1 |
| Study also recruiting relatives | ||||
| No | 11 | 64.7 | 2,468 | 24.8 |
| Yes | 6 | 35.3 | 7,479 | 75.2 |
| Offers incentive for participation | ||||
| No | 13 | 76.5 | 9,276 | 93.3 |
| Yes | 4 | 23.5 | 671 | 6.7 |
a Several recruitment protocols were modified while recruitment was active, resulting in variation in brochure use (some individuals received a brochure and others did not). Therefore, individual cases can be classified as having received a brochure or not, but recruitment efforts cannot be clearly classified as yes or no for use of a brochure.
b For both follow-up methods, variables (days between contact and mode of second contact) and values varied across cases within each study, so we cannot classify recruitment efforts in a single category.
c Counts and percentages of cases for the 2 follow-up methods’ variables are based only on the subsample of cases who did not respond to first contact but were subsequently recontacted (n = 7,767).
d The number of cases for days between first and second contact is less than 7,767 due to missing second contact date data for 108 cases.
We obtained contact attempt and outcome data for each case from the registry’s tracking databases. A small number of cases with inadequate record keeping were excluded (n = 37). If an individual was sampled for more than 1 study (n = 1,097), we included data only from the first study recruitment attempt.
Upon completion of the registry’s recruitment process, staff assigned each case 1 of 5 final outcome codes: responded, refused, no response, unable to locate, or ineligible. “Responded” indicated positive response; depending on the study, this entailed providing permission for the investigator to contact the case about the study, returning a completed questionnaire, or returning a completed questionnaire and a signed consent form. For studies for which the registry sought return of a permission-to-contact form, the investigator’s staff subsequently approached the individual to obtain consent.
The “refused” code was assigned if a permission form was returned marked “no” or if the individual verbally refused by telephone. “Unable to locate” was used if mail sent to the residential address was returned as undeliverable, and staff were not able to identify a new address. “No response” was used if mail was not returned as undeliverable, but no written response was received, and the individual did not state refusal by telephone. “Ineligible” was assigned to any case found to not meet eligibility criteria. The most common reason for ineligibility was death before study recruitment began. Ineligible cases were not considered further for this analysis.
We were interested in identifying factors predicting 2 steps of the recruitment process, defined as 2 separate outcomes for analysis. The first was ability to make confirmed, active contact with the case (“contact”). A case was classified as contacted if the registry either received a positive response or refusal by mail or spoke to the case via telephone. Cases were classified as not contacted if the registry was unable to locate and provide recruitment materials to them, or if materials were mailed to the case but the registry received no mailed response and was unable to reach the case by telephone.
The second step, “cooperation,” was defined as, among those cases who were successfully contacted, obtaining a positive response to the recruitment attempt. For studies that included a questionnaire in the recruitment packet, returning the completed questionnaire (and consent form, if applicable) was classified as cooperation. For studies that sent a permission-to-contact form, agreeing to be contacted by the researcher was classified as cooperation. We use the term “cooperation” as opposed to “participation” because, as noted above, in some studies the registry did not consent individuals for study participation. Cases who were successfully contacted by either recruitment approach but who refused were classified as “did not cooperate.” A small number of cases (n = 156) can be described as “passive refusals”—individuals who were reached by telephone but provided neither positive response nor specific refusal and who provided no subsequent response. We classified them as refusals; alternative models in which we classified them as nonresponses produced similar results. Whereas both contact and cooperation steps must be successful in order to recruit participants, different individual characteristics and study features contribute to each of these outcomes (50, 51).
Statistical analysis
We first performed simple cross-tabulations of recruitment outcome codes (responded, refused, no response, or unable to locate) according to individual-level variables. We calculated odds ratios and 2-sided 95% confidence intervals using mixed effects logistic regression to identify relevant predictors of our primary outcomes of contact and cooperation in multivariable models. Based on prior literature, we anticipated that demographic and diagnosis characteristics would predict both contact and cooperation. Study characteristics were expected to be factors individuals consider when deciding whether to cooperate, but not relevant for predicting contact. Therefore, the cooperation model contained both individual- and study-level variables while the contact model only included individual-level variables. We accounted for grouping of individuals according to recruitment effort by treating the recruitment effort as a random effect. Individuals’ characteristics were treated as fixed effects. In both models, we controlled for year of study recruitment. Because study and cancer site were collinear, we excluded cancer site from the models. We conducted all analyses using Stata, version 13 (StataCorp LLC, College Station, Texas) (52).
RESULTS
Analyses included 9,947 individuals with a cancer diagnosis reported to the registry. Women comprised 73.7% of the sample because several large breast cancer studies were included (Table 1). Excluding breast and prostate studies, the remaining sample was 55.8% female. Hispanics comprised 4.7% of cases, and 2.5% were of a race other than white. Most cases resided in metropolitan areas (84.9%). A majority (57.5%) were aged 50–69 years at recruitment, 16.9% were younger than 50, and 25.6% were 70 or older. Only 7.4% of cases were selected for recruitment within 2 years of diagnosis. Several of the studies focused on long-term survivors, and therefore 22.5% of cases were 15 or more years from diagnosis. A majority of cases were diagnosed at local stage (51.5%), followed by regional stage (27.9%). Distributions of cases and recruitment efforts according to study characteristics are displayed in Table 1 and Table 2.
Table 2.
Distribution of Cancer Cases According to Recruitment Outcomes and Odds Ratios for Patient Characteristics in Multivariable Models Predicting Contact and Cooperation, 17 Recruitment Efforts Undertaken by the Utah Cancer Registry, 2007–2016
| Variable | Case Distribution | Multivariable Modela | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Cases | Not Contactedb | Contactedc | Contactd | Cooperatione,f | ||||||||
| Refusal | Cooperation | |||||||||||
| No. | % | No. | % | No. | % | No. | % | OR | 95% CI | OR | 95% CI | |
| Sex | ||||||||||||
| Female | 7,333 | 73.7 | 1,992 | 27.2 | 1,574 | 21.5 | 3,767 | 51.4 | 0.97 | 0.83, 1.14 | 0.89 | 0.75, 1.06 |
| Male | 2,614 | 26.3 | 592 | 22.6 | 673 | 25.8 | 1,349 | 51.6 | 1.00 | Referent | 1.00 | Referent |
| Ethnicityg | ||||||||||||
| Hispanic | 468 | 4.7 | 235 | 50.2 | 62 | 13.3 | 171 | 36.5 | 0.34 | 0.27, 0.41 | 1.12 | 0.80, 1.56 |
| Non-Hispanic | 9,447 | 95.3 | 2,341 | 24.8 | 2,178 | 23.1 | 4,928 | 52.2 | 1.00 | Referent | 1.00 | Referent |
| Race | ||||||||||||
| Other race | 250 | 2.5 | 114 | 45.6 | 63 | 25.2 | 73 | 29.2 | 0.46 | 0.35, 0.60 | 0.44 | 0.30, 0.63 |
| White | 9,697 | 97.5 | 2,470 | 25.5 | 2,184 | 22.5 | 5,043 | 52.0 | 1.00 | Referent | 1.00 | Referent |
| Recruitment age, years | ||||||||||||
| <40 | 482 | 4.8 | 213 | 44.2 | 43 | 8.9 | 226 | 46.9 | 0.58 | 0.44, 0.75 | 1.74 | 1.16, 2.62 |
| 40–49 | 1,196 | 12.0 | 371 | 30.0 | 164 | 13.7 | 661 | 55.3 | 0.75 | 0.63, 0.89 | 1.38 | 1.10, 1.72 |
| 50–59 | 2,995 | 30.1 | 772 | 25.8 | 590 | 19.7 | 1,633 | 54.5 | 1.00 | Referent | 1.00 | Referent |
| 60–69 | 2,726 | 27.4 | 666 | 24.4 | 651 | 23.9 | 1,409 | 51.7 | 1.20 | 1.05, 1.37 | 0.81 | 0.70, 0.94 |
| 70–79 | 1,587 | 16.0 | 335 | 21.1 | 443 | 27.9 | 809 | 51.0 | 1.50 | 1.26, 1.79 | 0.60 | 0.50, 0.72 |
| ≥80 | 961 | 9.7 | 227 | 23.6 | 356 | 37.0 | 378 | 39.3 | 1.36 | 1.09, 1.69 | 0.31 | 0.25, 0.39 |
| Years since diagnosis | ||||||||||||
| ≤2 | 738 | 7.4 | 89 | 12.1 | 202 | 27.4 | 447 | 60.6 | 0.95 | 0.61, 1.48 | 0.96 | 0.62, 1.50 |
| 3–4 | 1,886 | 19.0 | 428 | 22.7 | 428 | 22.7 | 1,030 | 54.6 | 1.00 | Referent | 1.00 | Referent |
| 5–9 | 2,965 | 29.8 | 849 | 28.6 | 617 | 20.8 | 1,499 | 50.6 | 0.74 | 0.63, 0.88 | 0.99 | 0.83, 1.18 |
| 10–14 | 2,123 | 21.3 | 554 | 26.1 | 486 | 22.9 | 1,083 | 51.0 | 0.69 | 0.58, 0.81 | 0.88 | 0.73, 1.06 |
| ≥15 | 2,235 | 22.5 | 664 | 29.7 | 514 | 23.0 | 1,057 | 47.3 | 0.54 | 0.45, 0.65 | 0.87 | 0.71, 1.06 |
| Geographyh | ||||||||||||
| Metropolitan | 8,424 | 84.9 | 2,202 | 26.1 | 1,901 | 22.6 | 4,321 | 51.3 | 1.00 | Referent | 1.00 | Referent |
| Nonmetropolitan | 1,497 | 15.1 | 373 | 24.9 | 341 | 22.8 | 783 | 52.3 | 1.01 | 0.88, 1.16 | 1.05 | 0.90, 1.22 |
| Cancer stagei | ||||||||||||
| In situ | 748 | 8.3 | 199 | 26.6 | 155 | 20.7 | 394 | 52.7 | 1.17 | 0.97, 1.41 | 0.99 | 0.80, 1.23 |
| Local | 4,656 | 51.5 | 1,231 | 26.4 | 1,073 | 23.1 | 2,352 | 50.5 | 1.00 | Referent | 1.00 | Referent |
| Regional | 2,518 | 27.9 | 627 | 24.9 | 547 | 21.7 | 1,344 | 53.4 | 1.18 | 1.05, 1.33 | 1.11 | 0.97, 1.26 |
| Distant | 996 | 11.0 | 303 | 30.4 | 223 | 22.4 | 470 | 47.2 | 1.25 | 0.98, 1.59 | 0.92 | 0.74, 1.16 |
| Unstaged | 117 | 1.3 | 44 | 37.6 | 22 | 18.8 | 51 | 43.6 | 0.80 | 0.53, 1.21 | 1.25 | 0.72, 2.16 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Odds ratios and 95% confidence intervals from multivariable mixed-effects models with recruitment effort as random effect and all other variables as fixed effects. Multivariable models also included individuals’ year of selection for study recruitment.
b “Not contacted” includes both cases the registry was unable to locate to send mailings to and cases for whom mail was sent but no response was received, and we were unable to reach via telephone.
c “Contacted” includes cases for whom the registry received either a positive/cooperative response or refusal via mail or spoke to the case over the telephone.
d The multivariable model for “contact” predicted making contact, compared with not contacted.
e The multivariable model for “cooperation” included only cases who were successfully contacted (interacted with) and compares cases who cooperated with cases who refused (actively or passively).
f The model predicting cooperation also includes all of the study characteristic variables (presented in Table 3).
g Ethnicity was unknown for 32 cases.
h Geography could not be ascertained for 26 cases due to missing diagnosis address data.
i Cancer stage was not recorded for 912 cases.
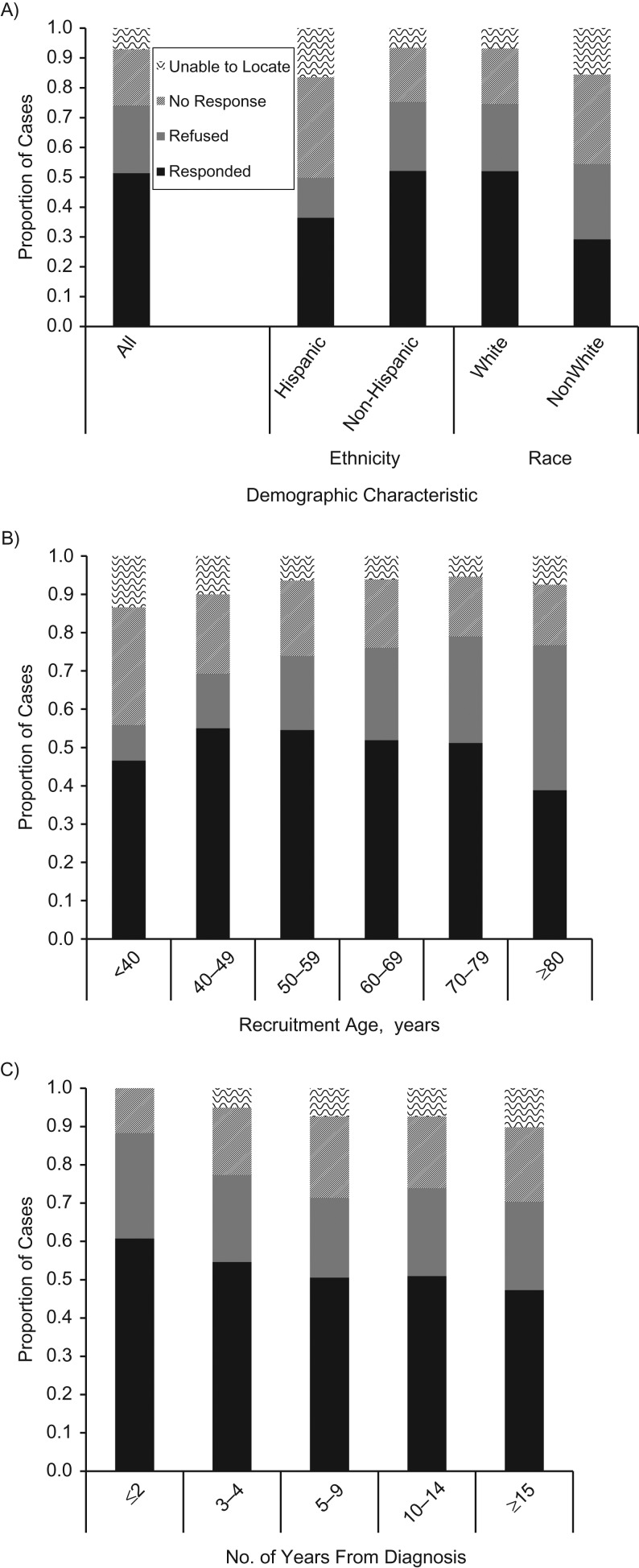
Overall, 5,116 (51.4%) cases had a final outcome of positive response to recruitment efforts (Figure 1), making the combined response fraction for all studies 51.4% (“Response Rate 2” of the American Association of Public Opinion Research) (53). Slightly more cases refused (22.6%) than did not respond (18.9%). Only 44 of the 2,247 refusals (2.0%) were noted as “irate” or “angry” refusals. Another 7.1% were not located and thus not contacted. Response outcomes were particularly variable based on ethnicity, race, age, and years since diagnosis (Figure 1). Response outcomes also varied by cancer site, but because site was highly collinear with study we did not include it in multivariable models.
Figure 1.
Recruitment outcomes according to demographic characteristics in 17 recruitment efforts undertaken by the Utah Cancer Registry, 2007–2016. Outcomes are shown according to ethnicity and race (A); age at recruitment, in years (B); and number of years since cancer diagnosis (C). Cases were coded as a refusal if they actively declined to participate, either in writing by mail or verbally by telephone. Cases labeled as no response were sent mailings (and no undeliverable mail was returned to the registry), but they did not provide any active refusal either through mail or telephone. A small number of passive refusals (cases who were successfully reached via telephone but did not provide active refusal or response, n = 156) are classified as refusals. The proportion of cases for the category of “unable to locate” among those ≤2 years from diagnosis have been suppressed due to small cell count (<5). The unable-to-locate and no-response categories equate to “not contacted,” and the categories of refused and responded equate to “contacted,” in the multivariable model predicting contact. The outcome of responded equates to “cooperation,” which is compared with the outcome of refused (“did not cooperate”) in the multivariable model predicting cooperation.
In a multivariable model, sex was not a significant predictor of making contact (Table 2). The registry was significantly less likely to contact Hispanics than non-Hispanics (odds ratio (OR) = 0.34, 95% confidence interval (CI): 0.27, 0.41) and individuals of a racial group other than white compared with whites (OR = 0.46, 95% CI: 0.35, 0.60). Nonmetropolitan residence did not significantly predict contact. Regional stage at diagnosis had higher odds of contact than local (OR = 1.18, 95% CI: 1.05, 1.33).
Younger individuals (<40 or 40–49 years) showed decreased odds of contact compared with those aged 50–59 years (for those <40 years of age, OR = 0.58, 95% CI: 0.44, 0.75; 40–49 years, OR = 0.75, 95% CI: 0.63, 0.89). Odds of contact increased with age and remained high for those aged 80 years or older compared with those aged 50–59 years. With more years between diagnosis and recruitment, the odds of successful contact became lower. After 15 or more years, odds of contact were significantly lower compared with contacting cases within 3–4 years of diagnosis (OR = 0.54, 95% CI: 0.45, 0.65). Odds of contact decreased over the 10-year period evaluated.
In the multivariable cooperation model, women exhibited a lower, although nonsignificant, likelihood of cooperation compared with men. Ethnicity was not significantly associated with cooperation among those contacted (OR = 1.12, 95% CI: 0.80, 1.56). Individuals of a race other than white were significantly less likely to cooperate (OR = 0.44, 95% CI: 0.30, 0.63). Nonmetropolitan residence and stage at diagnosis did not significantly predict cooperation. Among those contacted, younger age was significantly associated with increased odds of cooperation whereas older age decreased odds of cooperation. Years since diagnosis did not significantly influence odds of cooperation. Odds of cooperation decreased over the 10-year period.
Among study-level variables examined in the multivariable cooperation model (Table 3), sending a questionnaire in the recruitment packet obtained higher odds of cooperation than sending only a permission-to-contact form (OR = 3.19, 95% CI: 1.54, 6.59). The addition of a consent form decreased odds of cooperation (OR = 0.29, 95% CI: 0.16, 0.52), as did including a study brochure (OR = 0.70, 95% CI: 0.54, 0.90). Using first-class postage stamps increased odds of cooperation compared with bulk-rate and business-reply postage (OR = 1.60, 95% CI: 1.21, 2.12). Study descriptions mentioning collection of biological samples or recruitment of cancer cases’ relatives did not significantly influence odds of cooperation. Studies described as involving follow-up participation had lower odds of cooperation than nonlongitudinal studies (OR = 0.50, 95% CI: 0.41, 0.61). Recruitment materials promising an incentive for study participation increased odds of cooperation (OR = 1.62, 95% CI: 1.02, 2.57).
Table 3.
Distribution of Contacted Cancer Cases According to Recruitment Outcomes and Odds Ratios for Study Characteristics From a Multivariable Model Predicting Cooperation, 17 Recruitment Efforts Undertaken by the Utah Cancer Registry, 2007–2016
| Variable | Case Distribution | Multivariable Modela | ||||
|---|---|---|---|---|---|---|
| Refusalb | Cooperationc | Cooperationd | ||||
| No. | % | No. | % | OR | 95% CI | |
| Recruitment packet | ||||||
| Permission-to-contact form | 1,948 | 30.4 | 4,464 | 69.6 | 1.00 | Referent |
| Questionnaire | 299 | 31.4 | 652 | 68.6 | 3.19 | 1.54, 6.59 |
| Consent form | ||||||
| No | 1,989 | 29.9 | 4,656 | 70.1 | 1.00 | Referent |
| Yes | 258 | 35.9 | 460 | 64.1 | 0.29 | 0.16, 0.52 |
| Brochure | ||||||
| No | 531 | 30.5 | 1,210 | 69.5 | 1.00 | Referent |
| Yes | 1,716 | 30.5 | 3,906 | 69.5 | 0.70 | 0.54, 0.90 |
| Postage | ||||||
| Bulk rate/business reply | 790 | 32.4 | 1,647 | 67.6 | 1.00 | Referent |
| Stamps, outgoing and return | 1,457 | 29.6 | 3,469 | 70.4 | 1.60 | 1.21, 2.12 |
| Biospecimen collection | ||||||
| No | 1,774 | 29.5 | 4,232 | 70.5 | 1.00 | Referent |
| Yes | 473 | 34.9 | 884 | 65.1 | 1.31 | 0.90, 1.90 |
| Future follow-up participation | ||||||
| No | 1,580 | 27.9 | 4,076 | 72.1 | 1.00 | Referent |
| Yes | 667 | 39.1 | 1,040 | 60.9 | 0.50 | 0.41, 0.61 |
| Recruitment of relatives | ||||||
| No | 591 | 32.4 | 1,235 | 67.6 | 1.00 | Referent |
| Yes | 1,656 | 29.9 | 3,881 | 70.1 | 0.88 | 0.64, 1.21 |
| Incentive | ||||||
| No | 2,079 | 30.6 | 4,723 | 69.4 | 1.00 | Referent |
| Yes | 168 | 29.9 | 393 | 70.1 | 1.62 | 1.02, 2.57 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Odds ratios and 95% confidence intervals from multivariable mixed-effects models with recruitment effort as random effect and all other variables as fixed effects. The multivariable model also included patient characteristics (presented in Table 2) and individuals’ year of selection for study recruitment.
b “Refusal” refers to the recruitment outcome of refusing participation among cases who were successfully contacted.
c “Cooperation” refers to the recruitment outcome of providing a positive response among cases who were successfully contacted.
d The model for “cooperation” included only cases who were successfully contacted, and it compared cases who cooperated with cases who refused (actively or passively).
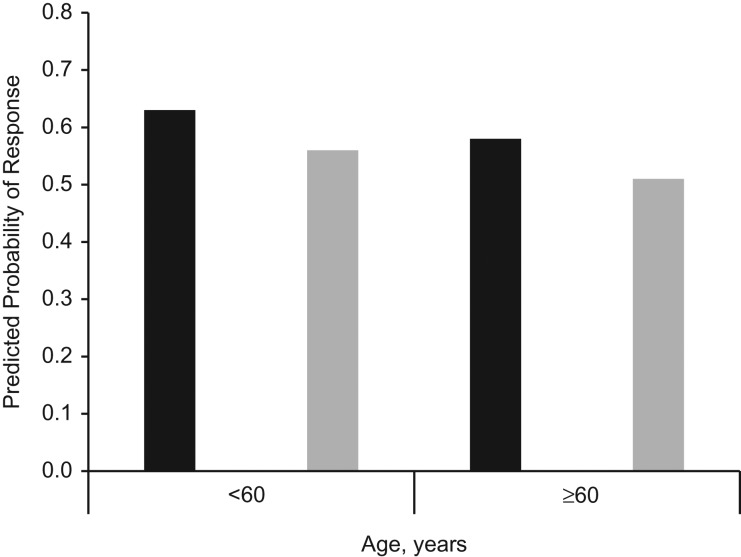
We also assessed “overall response,” comparing obtaining a positive response to recruitment efforts with refusals and nonresponses combined. Figure 2 displays predicted probabilities of a positive response from a multivariable model, classified by several relevant predictors. The probability of response was 0.63 for contacting individuals under age 60 within 5 years of diagnosis, including a questionnaire, and promising an incentive. In contrast, the probability of response was 0.51 for individuals over age 60 contacted after 5 or more years, and not providing a questionnaire or incentive.
Figure 2.
Predicted probability of positive response (compared with refusal or no response) according to selected cancer-case characteristics and study-implementation characteristics for 17 recruitment efforts undertaken by the Utah Cancer Registry, 2007–2016. Predicted probabilities are based on a multivariable model that adjusted for all patient and study-level variables included in the cooperation model. Black bars represent the probability of response when the following 3 conditions were met: Cases were recruited within 5 years of diagnosis, the recruitment packet for the study included a questionnaire, and an incentive was offered for study participation. Gray bars represent the probability of response when the alternate conditions were present: Cases were recruited 5 or more years after diagnosis, the recruitment packet did not contain a questionnaire, and no incentive was offered for participation.
To inform future recruitment efforts, we also examined follow-up methods for cases not heard from after the initial packet was mailed. Excluding unlocated cases, 15.5% of individuals responded positively or refused after receiving the first recruitment packet, before any follow-up calls or letters were delivered. Of these, 83.9% provided a positive response and 16.1% refused. Because a majority of individuals who eventually cooperated did so only after multiple contact attempts, we assessed whether methods of the second contact influenced the likelihood of obtaining a positive response (compared with refusing or no response) among those who did not reply after the first mailing. While mode of second contact (telephone or mail) was not a significant predictor of positive response, the timing of the second contact was relevant. Waiting more than 10 days to recontact after sending the initial packet resulted in decreased odds of positive response (OR = 0.71, 95% CI: 0.59, 0.85) compared with recontacting within 10 days of the first mailing.
To assess final consent outcomes for studies in which our outcome of cooperation entailed obtaining permission for investigators to contact cancer cases, we obtained from each study that maintained these records the number of cases that eventually enrolled in response to the study’s recruitment efforts. Among the studies that attempted to recruit all cases passed along from the registry (n = 8), 83.0% of the cancer cases consented to participate. The median study response fraction was 76.4%, with a range of 43.8%–86.4%.
DISCUSSION
Given trends of decreasing research participation (21–26), we sought to identify factors associated with cancer registry–based recruitment efforts. The average response fraction for 17 recruitment efforts sampling nearly 10,000 individuals diagnosed with various cancers was 50.4%. Both individual and study characteristics influenced the likelihood of successfully contacting cases and obtaining their cooperation. The results provide an updated assessment of the feasibility of recruitment through a cancer registry and useful information for investigators for understanding expected response patterns and assessing the potential implications they have for response bias.
Consistent with earlier studies (3, 4, 19, 30–32), we found recruitment efforts to be less successful for Hispanics and individuals of racial groups other than white; in multivariable models, both Hispanics and nonwhites were more difficult to contact than non-Hispanic whites. Among individuals contacted, nonwhites, but not Hispanics, had lower odds of cooperation than whites. Prior studies have suggested using culturally tailored materials and interpersonal contact (6), or even in-person visits (27), to more effectively recruit Hispanic cases. There is a need for more research to identify how to overcome barriers to effective recruitment of Hispanic and nonwhite individuals.
Some earlier registry-based studies found that younger individuals are more likely to participate than older people (2–4, 19, 30, 32). We also found that younger ages are associated with increased odds of cooperation, whereas older ages have decreased odds of cooperation. Like some other investigators (32, 33), we observed the opposite trend when predicting ability to contact cases; our results showed increased odds of successful contact for those aged 60 years or older and decreased odds among those under 50 years of age. Thus, the relationship between age and recruitment is complex.
Odds of successfully contacting cancer cases also decreased when 5 or more years had passed since diagnosis. Others have also found it more difficult to locate and recruit cancer cases as more time passes since their diagnosis (3, 20, 30, 34). Given these findings and the growing interest in research on cancer survivors, it is important that investigators recognize that long periods of time since diagnosis might make recruitment more difficult. However, we found that individuals who are further from diagnosis are no less likely than those closer to diagnosis to cooperate once successfully contacted.
The challenges in contacting younger adults and nonwhite individuals could in part be explained by greater residential mobility within 5 years among these demographic groups (54). Additionally, telephone surveyors have found it more difficult to contact younger populations and easier to reach older individuals (55, 56). Our data are consistent with this trend. Longer time since diagnosis would also increase the likelihood that the individual had moved and that the residential address reported to the registry at time of diagnosis is outdated. A limitation of our study is that when potential participants could not be contacted, efforts to trace them to new addresses varied by study and were not well documented. Several registries have recently started using Accurint (LexisNexis, New York, New York) for this purpose; it would be worthwhile to evaluate its effectiveness relative to other methods.
Studies described as longitudinal had lower odds of cooperation. Perhaps individuals perceived a longitudinal study as being more burdensome. There was no negative association between cooperation and mentioning collection of biospecimens; this null result is consistent with other findings (57). Although some literature suggested that mentioning recruitment of relatives would negatively influence response outcomes (58, 59), it did not influence odds of cooperation in this analysis. This difference could be explained by the high interest in genealogy in Utah.
Our results also demonstrated that methods commonly used for increasing response in survey research are also applicable for registry-based study recruitment. These methods include the use of first-class postage stamps (58, 60). Incentives are another such method. All incentives used by studies we examined were postincentives—those that are promised upon study completion—a method found to be less effective than unconditional preincentives (58, 61–64). Nevertheless, the promised incentives significantly increased odds of cooperation in our analysis. A prior study found pre- and postincentives to be equally effective among cancer survivors (65), suggesting that further investigation of optimal incentive methods and motivating factors for cooperation with registry-based recruitment studies is warranted.
Consistent with earlier findings on the benefits of including questionnaires at the outset, rather than first obtaining consent then providing the questionnaire (38), we found that including a questionnaire in the recruitment packet positively influenced cooperation compared with asking only for individuals’ permission for the study staff to contact them about study participation. Questionnaires might help recipients better understand what the study will entail and give the sense that study participation will be easy and immediate.
Including either a study brochure or a consent form that must be signed both resulted in reduced odds of cooperation. Prior research showed no association between brochures and participation (66–68). We postulate that there could be a fine line between providing just enough information about what a study will entail to encourage cooperation and providing too much information, which might be overwhelming. There is mixed evidence regarding whether recruitment material language, style, and content influence individuals’ likelihood of participating in health-based studies (69–73). Future studies should determine the optimal type and amount of study information to include in recruitment materials, and whether increasing awareness of registries influences cooperation.
Although others have suggested that telephone contact (6, 20) and in-person contact (36) are helpful for registry-based recruitment, we found no significant difference between telephone contact and another mailed letter for those who did not respond to the initial recruitment packet. However, we did observe that the length of time between the first and second contact was relevant for predicting positive response among those not responding to the first recruitment contact. Waiting more than 10 days before making follow-up contact decreased odds of response compared with follow-up within 10 days of the first mailing. Therefore, we believe it is advantageous to not allow too much time to pass between contact attempts with cancer cases.
By considering outcomes from multiple studies over a recent 10-year period, we have provided a comprehensive assessment of determinants of success of recruitment conducted via a cancer registry. This set of studies included diverse study objectives, cancer sites, and implementation features. By utilizing data from multiple studies, we were able to examine both individual demographic features and study-level variables simultaneously. However, there are limitations of this analysis that are important to recognize. First, our data are from a single registry, and the demographic characteristics of Utah and its cancer population are somewhat different from those in other states (74, 75), so results might not generalize to other registries. Further, there is variation in state and institutional review board policies governing registry-based research (1, 76); recruitment procedures might operate differently elsewhere. Additionally, for many of the analyzed studies, the outcome of cooperation entailed obtaining permission for investigators to contact the cancer case. This outcome is not the same as study enrollment. However, the available data suggest that over 80% of the cases who cooperated at this step eventually enrolled in the study.
Experimentation with random assignment is warranted to further explore the ways various study features relate to recruitment outcomes. Nevertheless, by evaluating multiple relevant individual- and study-level factors, this analysis provides useful information for cancer investigators seeking access to population-based samples, and it provides an updated assessment of the feasibility of study recruitment through a cancer registry. In order to design studies to maximize recruitment outcomes, investigators should consider tailoring recruitment efforts to underrepresented demographic groups, using proven survey-recruitment techniques such as incentives, stamps, and carefully timed follow-up contacts, as well as delivering questionnaires with requests to participate.
ACKNOWLEDGMENTS
Author affiliations: Division of Epidemiology, Department of Internal Medicine, School of Medicine, University of Utah, Salt Lake City, Utah (Morgan M. Millar, Lisa A. Cannon-Albright, Deborah W. Neklason, Carol Sweeney); Utah Cancer Registry, University of Utah Health, University of Utah, Salt Lake City, Utah (Morgan M. Millar, Mia Hashibe, Sandra L. Edwards, Carrie Bateman, Marjorie E. Carter, Carol Sweeney); Department of Biostatistics and Epidemiology, School of Public Health, Rutgers University, New Brunswick, New Jersey (Anita Y. Kinney); Rutgers Cancer Institute of New Jersey, Rutgers Health, Rutgers University, New Brunswick, New Jersey (Anita Y. Kinney, Antoinette M. Stroup); Division of Hematology and Hematological Malignancies, Department of Internal Medicine, School of Medicine, University of Utah, Salt Lake City, Utah (Nicola J. Camp); Cancer Control and Population Sciences Program, Huntsman Cancer Institute, University of Utah Health, University of Utah, Salt Lake City, Utah (Nicola J. Camp, Lisa A. Cannon-Albright, Mia Hashibe, Anne C. Kirchhoff, Deborah W. Neklason, Carol Sweeney); Division of Public Health, Department of Family and Preventive Medicine, School of Medicine, University of Utah, Salt Lake City, Utah (Mia Hashibe); Urologic Surgery, Department of Urology, Vanderbilt University Medical Center, Vanderbilt University, Nashville, Tennessee (David F. Penson); Center for Surgical Quality and Outcomes Research, Vanderbilt Institute for Medicine and Public Health, Vanderbilt University Medical Center, Vanderbilt University, Nashville, Tennessee (David F. Penson); Division of Pediatric Hematology and Oncology, Department of Pediatrics, School of Medicine, University of Utah, Salt Lake City, Utah (Anne C. Kirchhoff); Department of Epidemiology, RTI Health Solutions, RTI International, Research Triangle Park, North Carolina (Alicia W. Gilsenan); Safety, Epidemiology, and Risk Management, United BioSource Corporation, Blue Bell, Pennsylvania (Gretchen S. Dieck); Division of Cancer Epidemiology, Rutgers School of Public Health, Rutgers University, New Brunswick, New Jersey (Antoinette M. Stroup); and New Jersey State Cancer Registry, New Jersey Department of Health, Trenton, New Jersey (Antoinette M. Stroup).
This work was supported by the National Cancer Institute at the National Institutes of Health (grants R01CA134674, R01CA163353, and R21CA152336 to N.J.C.; R01CA164138 to L.A.C-A. and S.V. Tavtigian; R21CA185811 to M.H.; R01CA125194-0305 and 1R01CA129142 to A.Y.K.; R21CA205796 to D.W.N.; and P30CA042014 to the Shared Resources at the Huntsman Cancer Institute); the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (contract HHSN261201300017I to the Utah Cancer Registry); the National Institute of Dental and Craniofacial Research at the National Institutes of Health (grant R01DE023414 to M.H.); the Agency for Healthcare Research and Quality at the National Institutes of Health (grants 1R01HS019356 and 1R01HS022640 to D.F.P.); the Patient-Centered Outcomes Research Institute (contract CE12-11-4667 to D.F.P.); Eli Lilly & Company (to RTI Health Solutions); the Centers for Disease Control and Prevention’s National Program of Cancer Registries (cooperative agreement NU58DP0063200-01 to the Utah Cancer Registry); the University of Utah Department of Pediatrics (to A.C.K.); the Huntsman Cancer Institute’s Cancer Control and Population Sciences Pilot Grant Program (to A.C.K.); Primary Children’s Medical Foundation Career Development Award (to A.C.K.); and the Huntsman Cancer Foundation (to the Utah Cancer Registry).
We thank the Utah Cancer Registry study coordinators, who contacted cancer cases for these studies, and C. Janna Harrell, who created the initial tracking database utilized by the registry, all of whose efforts made this study possible. A.W.G. also thanks Kirk Midkiff and David Harris for their leadership and conduct of the RTI study.
An earlier version of this manuscript was presented at the 2017 Annual Conference for the American Association of Public Opinion Research, May 18–21, 2017, New Orleans, Louisiana.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- OR
odds ratio
REFERENCES
- 1. Beskow LM, Sandler RS, Weinberger M. Research recruitment through US central cancer registries: balancing privacy and scientific issues. Am J Public Health. 2006;96(11):1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinton-McHarg T, Carey M, Sanson-Fisher R, et al. Recruitment of representative samples for low incidence cancer populations: do registries deliver? BMC Med Res Methodol. 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carpentier MY, Tiro JA, Savas LS, et al. Are cancer registries a viable tool for cancer survivor outreach? A feasibility study. J Cancer Surviv. 2013;7(1):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kelly BJ, Fraze TK, Hornik RC. Response rates to a mailed survey of a representative sample of cancer patients randomly drawn from the Pennsylvania Cancer Registry: a randomized trial of incentive and length effects. BMC Med Res Methodol. 2010;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sweeney C, Edwards S, Baumgartner KB, et al. Recruiting Hispanic women for a population-based study: validity of surname search, and characteristics of non-participants. Am J Epidemiol. 2007;166(10):1210–1219. [DOI] [PubMed] [Google Scholar]
- 6. Ramirez AG, Miller AR, Gallion K, et al. Testing three different cancer genetics registry recruitment methods with Hispanic cancer patients and their family members previously registered in local cancer registries in Texas. Public Health Genomics. 2008;11(4):215–223. [DOI] [PubMed] [Google Scholar]
- 7. Pal T, Rocchio E, Garcia A, et al. Recruitment of black women for a study of inherited breast cancer using a cancer registry-based approach. Genet Test Mol Biomarkers. 2011;15(1–2):69–77. [DOI] [PubMed] [Google Scholar]
- 8. Newcomb PA, Love RR, Phillips JL, et al. Using a population-based cancer registry for recruitment in a pilot cancer control study. Prev Med. 1990;19(1):61–65. [DOI] [PubMed] [Google Scholar]
- 9. Pakilit AT, Kahn BA, Petersen L, et al. Making effective use of tumor registries for cancer survivorship research. Cancer. 2001;92(5):1305–1314. [DOI] [PubMed] [Google Scholar]
- 10. Kinney AY, Boonyasiriwat W, Walters ST, et al. Telehealth personalized cancer risk communication to motivate colonoscopy in relatives of patients with colorectal cancer: the Family CARE randomized controlled trial. J Clin Oncol. 2014;32(7):654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barocas DA, Chen V, Cooperberg M, et al. Using a population-based observational cohort study to address difficult comparative effectiveness research questions: the CEASAR study. J Comp Eff Res. 2013;2(4):445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffman RM, Koyama T, Fan KH, et al. Mortality after radical prostatectomy or external beam radiotherapy for localized prostate cancer. J Natl Cancer Inst. 2013;105(10):711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caan B, Sternfeld B, Gunderson E, et al. Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States). Cancer Causes Control. 2005;16(5):545–556. [DOI] [PubMed] [Google Scholar]
- 15. Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. [DOI] [PubMed] [Google Scholar]
- 16. Bellizzi KM, Smith A, Schmidt S, et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer. 2012;118(20):5155–5162. [DOI] [PubMed] [Google Scholar]
- 17. Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer. 2011;117(9):1994–2003. [DOI] [PubMed] [Google Scholar]
- 18. Hall AE, Sanson-Fisher RW, Lynagh MC, et al. Format and readability of an enhanced invitation letter did not affect participation rates in a cancer registry-based study: a randomized controlled trial. J Clin Epidemiol. 2013;66(1):85–94. [DOI] [PubMed] [Google Scholar]
- 19. Oral E, Simonsen N, Brennan C, et al. Unit nonresponse in a population-based study of prostate cancer. PLoS One. 2016;11(12):e0168364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wakefield CE, Fardell JE, Doolan EL, et al. Participation in psychosocial oncology and quality-of-life research: a systematic review. Lancet Oncol. 2017;18(3):e153–e165. [DOI] [PubMed] [Google Scholar]
- 21. Curtin R, Presser S, Singer E. Changes in telephone survey non-response over the past quarter century. Public Opin Q. 2005;69(1):87–98. [Google Scholar]
- 22. National Research Council Nonresponse in Social Science Surveys: A Research Agenda. Washington, DC: The National Academies Press; 2013. [Google Scholar]
- 23. Brick JM, Williams D. Explaining rising nonresponse rates in cross-sectional surveys. Ann Am Acad Pol Soc Sci. 2012;645(1):36–59. [Google Scholar]
- 24. Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–653. [DOI] [PubMed] [Google Scholar]
- 25. Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol. 2006;163(3):197–203. [DOI] [PubMed] [Google Scholar]
- 26. Tolonen H, Helakorpi S, Talala K, et al. 25-year trends and socio-demographic differences in response rates: Finnish adult health behaviour survey. Eur J Epidemiol. 2006;21(6):409–415. [DOI] [PubMed] [Google Scholar]
- 27. Rogers A, Murtaugh MA, Edwards S, et al. Contacting controls: are we working harder for similar response rates, and does it make a difference? Am J Epidemiol. 2004;160(1):85–90. [DOI] [PubMed] [Google Scholar]
- 28. Olson SH. Reported participation in case-control studies: changes over time. Am J Epidemiol. 2001;154(6):574–581. [DOI] [PubMed] [Google Scholar]
- 29. Drivsholm T, Eplov LF, Davidsen M, et al. Representativeness in population-based studies: a detailed description of non-response in a Danish cohort study. Scand J Public Health. 2006;34(6):623–631. [DOI] [PubMed] [Google Scholar]
- 30. Smith T, Stein KD, Mehta CC, et al. The rationale, design and implementation of the American Cancer Society’s studies of cancer survivors. Cancer. 2007;190(1):1–12. [DOI] [PubMed] [Google Scholar]
- 31. Simmons RG, Lee YC, Stroup AM, et al. Examining the challenges of family recruitment to behavioral intervention trials: factors associated with participation and enrollment in a multi-state colonoscopy intervention trial. Trials. 2013;14:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moorman PG, Newman B, Millikan RC, et al. Participation rates in a case-control study: the impact of age, race, and race of interviewer. Ann Epidemiol. 1999;9(3):188–195. [DOI] [PubMed] [Google Scholar]
- 33. Arora NK, Hamilton AS, Potosky AL, et al. Population-based survivorship research using cancer registries: a study of non-Hodgkin’s lymphoma survivors. J Cancer Surviv. 2007;1(1):49–63. [DOI] [PubMed] [Google Scholar]
- 34. Mols F, Oerlemans S, Denollet J, et al. Type D personality is associated with increased comorbidity burden and health care utilization among 3080 cancer survivors. Gen Hosp Psychiatry. 2012;34(4):352–359. [DOI] [PubMed] [Google Scholar]
- 35. Girgis A, Boyes A, Sanson-Fisher RW, et al. Perceived needs of women diagnosed with breast cancer: rural versus urban location. Aust N Z J Public Health. 2000;24(2):166–173. [DOI] [PubMed] [Google Scholar]
- 36. Heiden TL, Bailey HD, Armstrong BK, et al. Participation in paediatric cancer studies: timing and approach to recruitment. BMC Res Notes. 2013;6:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Midkiff KD, Andrews EB, Gilsenan AW, et al. The experience of accommodating privacy restrictions during implementation of a large-scale surveillance study of an osteoporosis medication. Pharmacoepidemiol Drug Saf. 2016;25(8):960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rogers PA, Haddow L, Thomson AK, et al. Including questionnaires with the invitation package appeared to increase the response fraction among women. J Clin Epidemiol. 2012;65(6):696–699. [DOI] [PubMed] [Google Scholar]
- 39. Slager SL, Rabe KG, Achenbach SJ, et al. Genome-wide association study identifies a novel susceptibility locus at 6p21.3 among familial CLL. Blood. 2011;117(6):1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Camp NJ, Parry M, Knight S, et al. Fine-mapping CASP8 risk variants in breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(1):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andreotti G, Birmann B, De Roos AJ, et al. A pooled analysis of alcohol consumption and risk of multiple myeloma in the international multiple myeloma consortium. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1620–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Curtin K, Lin WY, George R, et al. Meta association of colorectal cancer confirms risk alleles at 8q24 and 18q21. Cancer Epidemiol Biomarkers Prev. 2009;18(2):616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kinney AY, Butler KM, Schwartz MD, et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst. 2014;106(12):dju328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boonyasiriwat W, Hung M, Hon SD, et al. Intention to undergo colonoscopy screening among relatives of colorectal cancer cases: a theory-based model. Ann Behav Med. 2014;47(3):280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kirchhoff AC, Montenegro RE, Warner EL, et al. Childhood cancer survivors’ primary care and follow-up experiences. Support Care Cancer. 2014;22(6):1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smits-Seemann RR, Kaul S, Zamora ER, et al. Barriers to follow-up care among survivors of adolescent and young adult cancer. J Cancer Surviv. 2017;11(1):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andrews EB, Gilsenan AW, Midkiff K, et al. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res. 2012;27(12):2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. E Koro C, M Hale P, Ali A, et al. The rationale, objectives, design and status of the Medullary Thyroid Carcinoma (MTC) surveillance study: a case-series registry [poster 357]. Presented at the 86th Annual Meeting of the American Thyroid Association, Denver, CO, September 2016.
- 49. Soisson S, Ganz PA, Gaffney D, et al. Long-term cardiovascular outcomes among endometrial cancer survivors in a large, population-based cohort study. J Natl Cancer Inst. 2018;110(12):1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Watson N, Wooden M. Identifying factors affecting longitudinal survey response In: Lynn P, ed. Methodology of Longitudinal Surveys. West Sussex, UK: Wiley; 2009:157–182. [Google Scholar]
- 51. Gray R, Campanelli P, Deepchand K, et al. Exploring survey non-response: the effect of attrition on a follow-up of the 1984–85 health and life style survey. Statistician. 1996;45(2):163–183. [Google Scholar]
- 52. Stata Corporation Stata 13 base reference manual. College Station, TX: Stata Press; 2013. [Google Scholar]
- 53. American Association for Public Opinion Research Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 9th ed Oakbrook Terrace, IL: American Association for Public Opinion Research; 2016. [Google Scholar]
- 54. Ihrke DK, Faber CS. Geographical Mobility: 2005 to 2010 Washington, D.C.: US Census Bureau; 2012. (Current population reports, P20-567). https://www.census.gov/library/publications/2012/demo/p20-567.html. Published December 2012. Accessed June 20, 2018.
- 55. Keeter S, Hatley N, Kennedy C, et al. What low response rates mean for telephone surveys Washington, DC: Pew Research Center. http://www.pewresearch.org/methods/2017/05/15/what-low-response-rates-mean-for-telephone-surveys/. Published May 15, 2017. Accessed October 10, 2018.
- 56. Matias-Guiu J, Serrano-Castro PJ, Mauri-Llerda JA, et al. Analysis of factors influencing telephone call response rate in an epidemiological study. ScientificWorldJournal. 2014;2014:179375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Colt JS, Wacholder S, Schwartz K, et al. Response rates in a case-control study: effect of disclosure of biologic sample collection in the initial contact letter. Ann Epidemiol. 2005;15(9):700–704. [DOI] [PubMed] [Google Scholar]
- 58. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harris SA, Boucher BA, Cotterchio M. Will women diagnosed with breast cancer provide biological samples for research purposes? PLoS One. 2015;10(6):e0127994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fox RJ, Crask MR, Kim J. Mail survey response rate: a meta-analysis of selected techniques for inducing response. Public Opin Q. 1988;52(4):467–491. [Google Scholar]
- 61. Church AH. Estimating the effect of incentives on mail survey response rates: a meta-analysis. Public Opin Q. 1993;57(1):62–79. [Google Scholar]
- 62. James JM, Bolstein R. Large monetary incentives and their effect on mail survey response rates. Public Opin Q. 1992;56(4):442–453. [Google Scholar]
- 63. Leung GM, Johnston JM, Saing H, et al. Prepayment was superior to postpayment cash incentives in a randomized postal survey among physicians. J Clin Epidemiol. 2004;57(8):777–784. [DOI] [PubMed] [Google Scholar]
- 64. Alexander GL, Divine GW, Couper MP, et al. Effect of incentives and mailing features on online health program enrollment. Am J Prev Med. 2008;34(5):382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Evans BR, Peterson BL, Demark-Wahnefried W. No difference in response rate to a mailed survey among prostate cancer survivors using conditional versus unconditional incentives. Cancer Epidemiol Biomarkers Prev. 2004;13(2):277–278. [DOI] [PubMed] [Google Scholar]
- 66. Nakash RA, Hutton JL, Jørstad-Stein EC, et al. Maximising response to postal questionnaires—a systematic review of randomised trials in health research. BMC Med Res Methodol. 2006;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Parkes R, Kreiger N, James B, et al. Effects on subject response of information brochures and small cash incentives in a mail-based case-control study. Ann Epidemiol. 2000;10(2):117–124. [DOI] [PubMed] [Google Scholar]
- 68. Youl PH, Janda M, Lowe JB, et al. Does the type of promotional material influence men’s attendance at skin screening clinics? Health Promot J Austr. 2005;16(3):229–232. [DOI] [PubMed] [Google Scholar]
- 69. Steckelberg A, Hülfenhaus C, Haastert B, et al. Effect of evidence based risk information on “informed choice” in colorectal cancer screening: randomised controlled trial. BMJ. 2011;342:d3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parker A, Knapp P, Treweek S, et al. The effect of optimised patient information materials on recruitment in a lung cancer screening trial: an embedded randomised recruitment trial. Trials. 2018;19:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Masser BM, France CR, Himawan LK, et al. The impact of the context and recruitment materials on nondonors’ willingness to donate blood. Transfusion. 2016;56(12):2995–3003. [DOI] [PubMed] [Google Scholar]
- 72. Hirschey R, Lipkus I, Jones L, et al. Message framing and physical activity promotion in colorectal cancer survivors. Oncol Nurs Forum. 2016;43(6):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. United States Census Bureau American FactFinder: comparative demographic estimates 2013–2017 American Community Survey 5-year estimates. https://factfinder.census.gov/bkmk/table/1.0/en/ACS/17_5YR/CP05/0100000US|0400000US49. Published 2017. Accessed September 6, 2018.
- 75. Sweeney C, Herget KA, Otto VY et al. Cancer in Utah: An overview of incidence and mortality 2004–2013 Salt Lake City, UT: Utah Cancer Registry. https://uofuhealth.utah.edu/utah-cancer-registry/docs/cancerinutah-2004-2013.pdf. Published 2016. Accessed October 10, 2018.
- 76. Beskow LM, Sandler RS, Millikan RC, et al. Patient perspectives on research recruitment through cancer registries. Cancer Causes Control. 2005;16(10):1171–1175. [DOI] [PubMed] [Google Scholar]