Abstract
Objective:
The NMDA receptor antagonist ketamine produces rapid and sustained antidepressant actions even in treatment-resistant depressed patients. Vascular endothelial growth factor (VEGF) has been implicated in the effects of conventional monoamine-based antidepressants, but the role of VEGF in the rapid antidepressant actions of ketamine remains unclear. Here, we examined whether neuronal VEGF signaling in the medial prefrontal cortex (mPFC) mediates the rapid antidepressant actions of ketamine.
Method:
For these studies we used a combination of approaches, including conditional, neuron-specific knockout of VEGF or its receptor Flk-1, antibody neutralization, viral-mediated knockdown of Flk-1, and pharmacological inhibitors. Further in vitro and in vivo experiments were performed to examine whether neuronal VEGF signaling was required for the neurotrophic and synaptogenic actions of ketamine that underlie its behavioral actions.
Results:
The results demonstrate that the behavioral actions of systemic ketamine are blocked by forebrain excitatory neuron-specific deletion of either VEGF or Flk-1, or by intra-mPFC infusion of a VEGF neutralizing antibody. Moreover, intra-mPFC infusions of VEGF are sufficient to produce rapid ketamine-like behavioral actions, and these effects are blocked by neuron-specific Flk-1 deletion. The results also show that local knockdown of Flk-1 in mPFC excitatory neurons in adulthood blocks the behavioral effects of systemic ketamine. Moreover, inhibition of neuronal VEGF signaling blocks the neurotrophic and synaptogenic effects of ketamine.
Conclusions:
Together, these findings indicate that neuronal VEGF-Flk-1 signaling in the mPFC plays an essential role in the antidepressant actions of ketamine.
Introduction
Major depressive disorder is one of the most widespread psychiatric illnesses, affecting an estimated 300 million people worldwide (1), with enormous personal and socioeconomic consequences (2). Neuroimaging studies demonstrate reduced volume of the prefrontal cortex (PFC) and hippocampus (3, 4), where neuronal atrophy and glial loss have been reported in postmortem brains from depressed subjects and rodent chronic stress models (5, 6). Although the mechanisms underlying the pathophysiology and treatment of depression are still unknown, there is growing evidence for a role of growth factors, notably brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF). Chronic stress and depression decrease the expression and function of BDNF and/or VEGF in the PFC and hippocampus (7–11); VEGF is also decreased in cerebrospinal fluid of patients who have attempted suicide (12). In contrast, chronic, but not acute, treatment with typical antidepressants, notably monoamine reuptake inhibitors, increases BDNF and VEGF expression (7, 13–17), and blockade of BDNF or VEGF signaling attenuates the antidepressant effects of these treatments (13, 17, 18). These findings support a neurotrophic hypothesis of depression that reduced neurotrophic factor levels are strongly linked with the structural alterations caused by chronic stress and depression, and conversely that antidepressants act at least in part by producing the opposite effects via induction of BDNF and VEGF expression.
In contrast to the delayed response of weeks to months for the action of typical antidepressants, a single subanesthetic dose of ketamine, an NMDA receptor antagonist, produces rapid (within hours) and sustained (up to a week) antidepressant actions even in treatment-resistant depressed patients (5, 19). Preclinical studies have reported that low-dose ketamine produces rapid and sustained antidepressant behavioral responses and increases the number and function of synapses in medial PFC (mPFC) pyramidal neurons, reversing the synaptic loss caused by chronic stress (20, 21); these effects are dependent on the processing and activity-dependent release of BDNF (22, 23). Together, these findings indicate that the neurotrophic responses are associated with the behavioral actions of ketamine, but it remains unclear whether VEGF signaling is also involved. VEGF is a pleiotrophic growth factor expressed by neurons and glia, as well as vascular endothelial cells (18), and has potent neurotrophic activity (24). Here, we examined the role of neuronal VEGF signaling in the behavioral and neurotrophic effects of ketamine.
Method
Animals
Male wildtype, CaMKIIα-Cre (25), Flk-1flox/flox (26), VEGFflox/flox (27), CaMKIIα-Cre:Flk-1flox/flox (hereafter, Flk-1NEURON−/−) and CaMKIIα-Cre:VEGFflox/flox (hereafter, VEGFNEURON−/−) mice and Sprague-Dawley rats were used. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committee.
Surgery and drug infusion
Guide cannulas were implanted bilaterally into the mPFC (rats, 3.0 mm rostral, ±1.0 mm lateral, 4.0 mm ventral to bregma; mice, 1.8 mm rostral, ±0.4 mm lateral, 2.5 mm ventral to bregma) or dorsal striatum (rats, 1.6 mm rostral, ±1.9 mm lateral, 5.0 mm ventral to bregma). Rats were bilaterally infused with a VEGF neutralizing antibody or rat recombinant VEGF164 using an injector that protruded 0.5 mm beyond the tip of the guide cannula (0.5 μL/side; 0.25 μL/min). Mice were bilaterally infused with ketamine or mouse recombinant VEGF164 using an injector that protruded 0.3 mm beyond the tip of the guide cannula (0.2 μL/side; 0.2 μL/min).
AAV2shFlk−1 preparation and intra-mPFC viral infusion
Sense and antisense oligonucleotides encoding the Flk-1 short hairpin RNA (shFlk-1) were ligated into a plasmid (pshFlk-1) designed to restrict shRNA expression to cells that express Cre recombinase as previously reported (28). This plasmid allows U6 promoter-dependent shFlk-1 expression only when cytomegalovirus promoter-enhanced green fluorescent protein protein (CMV-EGFP) reporter/stop cassette is eliminated by Cre-dependent recombination (Figure 4B).
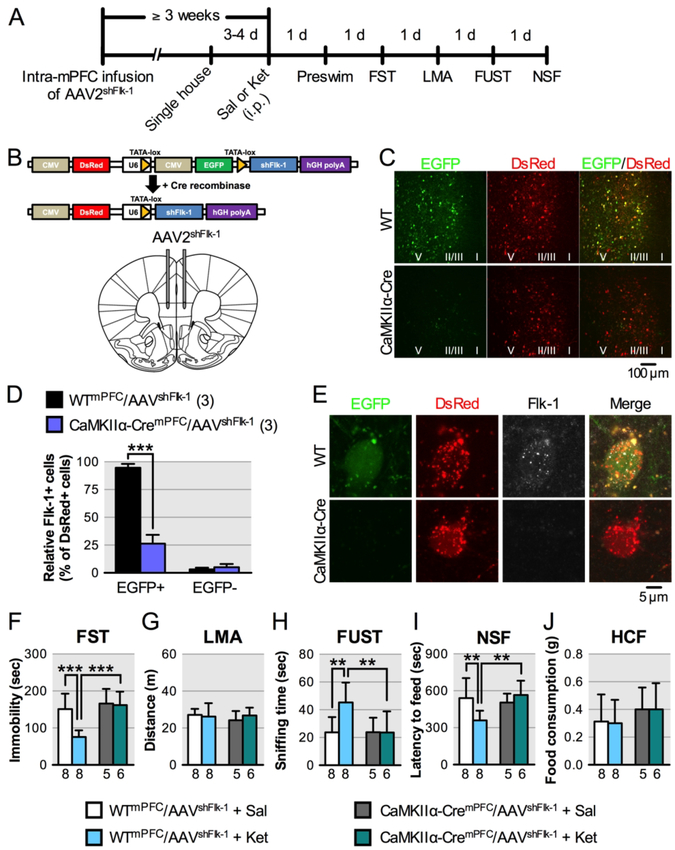
FIGURE 4. Effect of local knockdown of Flk-1 in mPFC pyramidal neurons on the behavioral effects of ketaminea.
a(A) Experimental timeline for behavioral testing starting ≥ 3 weeks after intra-mPFC infusion of AAV2shFlk−1 and 1 day after i.p. injection of either saline or ketamine (10 mg/kg). (B) Diagram of pshFlk-1 construct and Cre-induced recombination enabling U6 driven expression of shFlk-1. AAV2shFlk−1 was bilaterally infused into the mPFC of WT or CaMKIIα-Cre mice. (C) Representative images of the mPFC of WTmPFC/AAV2shFlk−1 (n = 16) and CaMKIIα-CremPFC/AAV2shFlk−1 mice (n = 11). Approximate laminar regions (I-V) are noted. (D) The relative number of Flk-1+/EGFP+/DsRed+ (t4 = 13.7, p = 0.0002) and Flk-1+/EGFP-/DsRed+ cells (t4 = 1.06, p = 0.348) in the mPFC of WTmPFC/AAV2shFlk−1 (n = 3; 54.7 ± 22.3 DsRed+ cells/mouse (total 164 cells) were analyzed) and CaMKIIα-CremPFC/AAV2shFlk−1 mice (n = 3; 62.3 ± 12.1 DsRed+ cells/mouse (total 187 cells) were analyzed). (E) Representative images of EGFP, DsRed expression and Flk-1 immunolabeling in mPFC neurons from WTmPFC/AAV2shFlk−1 and CaMKIIα-CremPFC/AAV2shFlk−1 mice. (F) Immobility time in the forced swim test (FST) 2 days after i.p. injection (interaction, F1,23 = 6.88, p = 0.015, n = 5–8). (G) Locomotor activity (LMA) 3 days after i.p. injection (interaction, F1,23 = 0.776, p = 0.39, n = 5–8). (H) Time spent sniffing female urine in the female urine sniffing test (FUST) 4 days after i.p. injection (interaction, F1,23 = 4.51, p = 0.045, n = 5–8). (I) Latency to feed in the novelty-suppressed feeding test (NSF) 5 days after i.p. injection (interaction, F1,23 = 6.84, p = 0.016, n = 5–8). (J) Home cage feeding (HCF) just after the NSF (interaction, F1,23 = 0.00778, p = 0.93, n = 5–8). Data are expressed as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
shFlk-1:
Sense: 5’-TTAAACCGGGATGTGAAACCCTTTCTTCAAGAGAGAAAGGGTTTCACATCCCGGTTTATTTTTTC-3’
Antisense: 5’-TCGAGAAAAAATAAACCGGGATGTGAAACCCTTTCTCTCTTGAAGAAAGGGTTTCACATCCCGGTTTAA-3’
To package into AAV2shFlk−1, HEK293 cells were transfected with pHelper, pAAV2-RC and pshFlk-1 constructs using Lipofectamine 2000 (Life Technologies). Forty-eight hours after transfection, AAV2shFlk−1 particles were purified, resuspended, and stored at −80ºC. Mice were infused with AAV2shFlk−1 bilaterally into the mPFC (2.0 mm rostral, ±0.3 mm lateral, 2.8 mm ventral to bregma; 1 μL; 0.15 μL/min).
Behavioral testing
The forced swim test, novelty-suppressed feeding test, and female urine sniffing test were performed as previously described (17, 22, 28–31). Forced swim test: 24 h after preswim, each animal was placed in a swim cylinder for 10 min and videotaped. The immobility time was scored during 2–6 min. Novelty-suppressed feeding test: animals were food-deprived overnight and placed in an open field with a small amount of food in the center. The latency to feed was measured. Female urine sniffing test: animals were exposed to a cotton-tipped applicator infused with fresh urine from females of the same strain for 5 min. The time spent sniffing the cotton-tipped applicator was measured. Tail suspension test: each mouse was suspended by its tail for 6 min and videotaped. The immobility time was measured during the last 4 min. Locomotor activity: each animal was placed in a testing chamber for 20 min. The total distance traveled (mice) or the number of beam breaks (rats) was measured.
Immunohistochemistry
The number of c-Fos-positive cells was counted in the regions near the infusion sites in the mPFC from two coronal sections (40 μm) per rat after 3–3’-diaminobenzidine staining.
Immunofluorescence
Free-floating sections (30 μm) were incubated with the following primary antibodies: rabbit anti-CaMKII, mouse anti-CaMKII, goat anti-mouse Flk-1, mouse anti-glucose transporter 1 (GLUT1), and rabbit anti-VEGF, and then incubated with secondary antibodies. To quantify knockdown the number of Flk-1-positive (Flk-1+) cells were counted and calculated as the percent of total DsRed+ cells.
Golgi staining and spine density analysis
Golgi staining was performed using FD Rapid GolgiStain kit (FD NeuroTechnologies) according to the manufacturer’s instruction. Spine density on the primary and secondary dendritic branches of the apical tuft of layer V pyramidal neurons in the infralimbic and prelimbic regions of the mPFC was analyzed using Neurolucida 10 (MBF Bioscience).
Sholl analysis
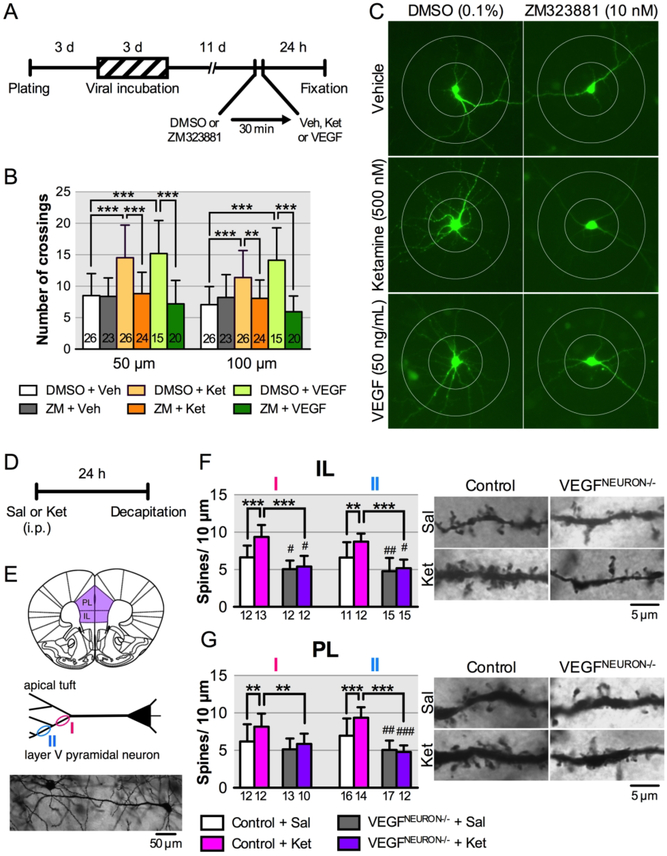
Dendritic complexity in primary cortical neurons was analyzed as previously described (32). Cortical neurons were dissected from E18 rat embryos, incubated with AAV2-EGFP for 72 h, and treated 500 nM ketamine or 50 ng/mL VEGF with or without an Flk-1 inhibitor ZM323881. After 24-h incubation, the number of dendritic crossings at 50 and 100 μm distances from the soma was measured.
Statistics
Data are expressed as mean ± SD. Data were analyzed by unpaired t-test, one-way ANOVA, or two-way ANOVA followed by Fisher’s LSD test using GraphPad Prism 6 (GraphPad Software). Differences with p < 0.05 were considered statistically significant.
Detailed methods are available as online supplemental data.
RESULTS
Neuronal VEGF signaling mediates the antidepressant-like behavioral effects of ketamine
To investigate the role of neuronal VEGF signaling in the antidepressant-like behavioral effects of ketamine, we engineered mice with neuron-specific deletion of VEGF and Flk-1 in forebrain, referred to as Flk-1NEURON−/− and VEGFNEURON−/− (Figure 1A, B). Both Flk-1 and VEGF are expressed in pyramidal neurons in the mPFC and hippocampus, and neuronal deletion of Flk-1 and VEGF in Flk-1NEURON−/− and VEGFNEURON−/− mice, respectively was confirmed by immunohistochemistry (Figure S1–4). Flk-1NEURON−/− and VEGFNEURON−/− mice exhibited normal ambulation and mild anxiety-like behaviors (Figure S5). Flk-1NEURON−/−, VEGFNEURON−/− and littermate controls (Flk-1flox/flox and VEGFflox/flox, but CaMKIIα-Cre negative) were injected with ketamine (10 mg/kg, i.p.), and tested in the forced swim test and novelty-suppressed feeding test 2 and 5 days later, respectively (Figure 1C). In the forced swim test, a model of behavioral despair, a single dose of ketamine significantly decreased immobility in control mice, and this effect was fully blocked in Flk-1NEURON−/− or VEGFNEURON−/− mice (Figure 1D, G); similar effects were observed in preswim conducted 1 day after ketamine (Figure S6A, B). There were no significant differences in immobility between saline-treated Flk-1flox/flox and Flk-1NEURON−/− mice or between saline-treated VEGFflox/flox and VEGFNEURON−/− mice. In the novelty-suppressed feeding test, a model in which increased latency to feed is associated with anxiety, a single dose of ketamine decreased latency to feed in Flk-1flox/flox control mice; Flk-1NEURON−/− mice, both saline- and ketamine-injected, displayed significantly decreased latency compared with saline-injected Flk-1flox/flox control mice (Figure 1E), making the results difficult to interpret. There was no effect observed in saline-injected VEGFNEURON−/− mice in latency to feed, and the ketamine-mediated reduction in latency to feed was completely blocked (Figure 1H). There were no significant effects of ketamine or Flk-1NEURON−/− and VEGFNEURON−/− deletion on home cage feeding demonstrating that these treatments do not produce a general effect on feeding behavior (Figure 1F, I).
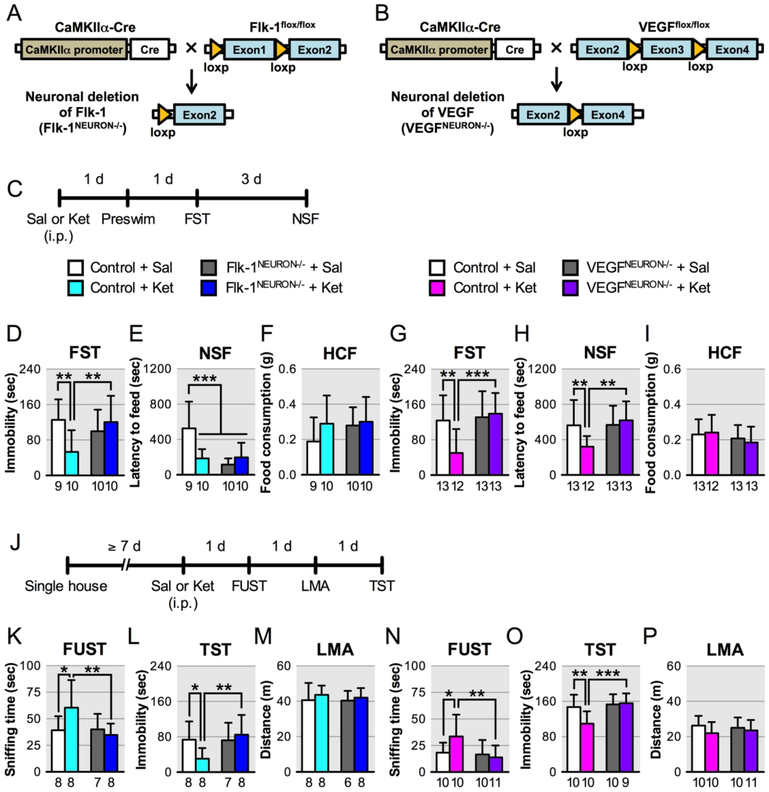
FIGURE 1. Effects of neuron-specific knockout of VEGF or Flk-1 on the behavioral effects of ketaminea.
aSchematic representation of neuronal deletion of Flk-1 (A) and VEGF (B). (C) Experimental timeline for behavioral testing starting 1 day after i.p. injection of either saline or ketamine (10 mg/kg) in Flk-1NEURON−/−, VEGFNEURON−/− mice and littermate controls. (D, G) Immobility time in the forced swim test (FST) 2 days after i.p. injection (D, interaction, F1,35 = 8.01, p = 0.0077, n = 9–10; G, interaction, F1,47 = 7.15, p = 0.010, n = 12–13). (E, H) Latency to feed in the novelty-suppressed feeding test (NSF) 5 days after i.p. injection (E, interaction, F1,35 = 13.4, p = 0.0008, n = 9–10; H, interaction, F1,47 = 5.69, p = 0.021, n = 12–13). (F, I) Home cage feeding (HCF) just after the NSF (F, interaction, F1,35 = 0.857, p = 0.36, n = 9–10; I, interaction, F1,47 = 0.476, p = 0.49, n = 12–13). (J) Experimental timeline for behavioral testing starting 1 day after i.p. injection of either saline or ketamine (10 mg/kg) in single-housed Flk-1NEURON−/−, VEGFNEURON−/− mice and littermate controls. (K, N) Time spent sniffing female urine in the female urine sniffing test (FUST) 1 day after i.p. injection of either saline or ketamine (K, interaction, F1,27 = 4.55, p = 0.042, n = 7–8; N, interaction, F1,37 = 4.15, p = 0.049, n = 10–11). (L, O) Immobility time in the tail suspension test (TST) 3 days after i.p. injection (L, interaction, F1,27 = 4.12, p = 0.052, n = 7–8; O, interaction, F1,35 = 5.96, p = 0.020, n = 9–10). (M, P) Locomotor activity (LMA) 2 days after i.p. injection (M, F1,26 = 0.0696, p = 0.79, n = 6–8; P, F1,37 = 0.57, p = 0.45, n = 10–11). Data are expressed as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
We also conducted additional tests for reward/anhedonia (female urine sniffing test), and despair-like behavior (tail suspension test), as well as locomotor activity in separate Flk-1NEURON−/− and VEGFNEURON−/− cohorts (Figure 1J). In the female urine sniffing test, ketamine significantly increased female urine sniffing time in Flk-1flox/flox and VEGFflox/flox control mice, but had no effect in Flk-1NEURON−/− or VEGFNEURON−/− mice (Figure 1K, N). In the tail suspension test, ketamine decreased immobility in control mice, and this effect was blocked in Flk-1NEURON−/− or VEGFNEURON−/− mice (Figure 1L, O). There were no effects of genotype or ketamine on locomotor activity (Figure 1M, P). These results indicate that neuronal VEGF-Flk-1 signaling in the forebrain contributes to the behavioral actions of ketamine.
Because BDNF signaling is required for the behavioral and synaptic actions of ketamine (22, 23), we also examined the influence of Flk-1 or VEGF deletion on BDNF, total TrkB, and phosphorylated/activated TrkB (pTrkB). Immunoblot analysis shows that there were no significant differences in basal levels of BDNF, TrkB and pTrkB in PFC of Flk-1NEURON−/− or VEGFNEURON−/− mice compared to control mice (Figure S7B-K). In addition, there were no significant effects of ketamine (1 h) on levels of BDNF, TrkB and pTrkB in either Flk-1NEURON−/− or VEGFNEURON−/− deletion mutant mice, or in controls (Figure S7B-K).
Prefrontal VEGF release is required for the antidepressant-like behavioral effects of ketamine
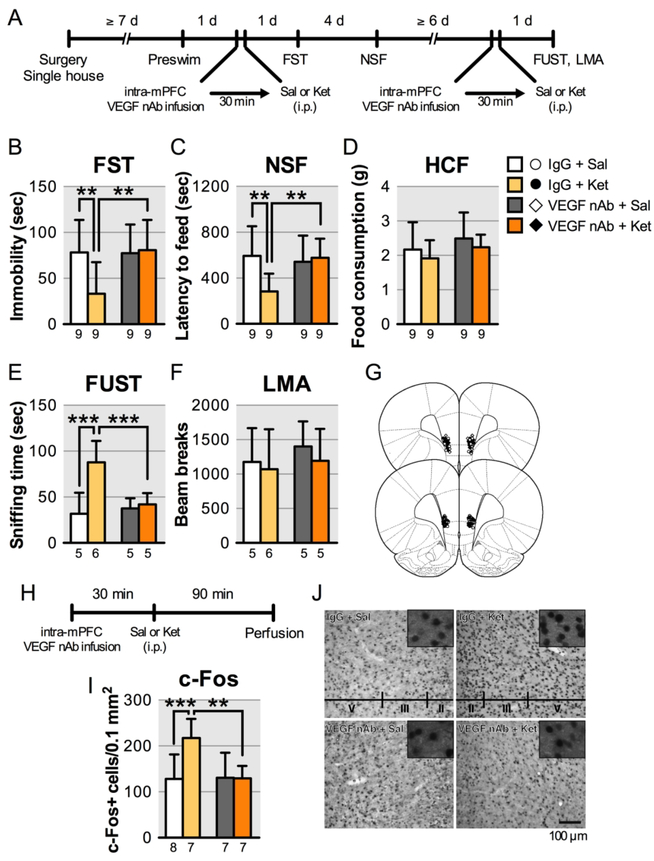
Our previous studies reveal that the mPFC is necessary and sufficient for the actions of ketamine (20, 22, 30). To examine VEGF in mPFC, rats were infused with a VEGF neutralizing antibody (0.2 μg/side) into the mPFC 30 min before ketamine (10 mg/kg, i.p.) and tested 1 and 5 days later (Figure 2A). In control IgG-infused rats, ketamine produced significant behavioral effects in the forced swim test and novelty-suppressed feeding test (Figure 2B, C), and these effects were blocked in VEGF neutralizing antibody-infused rats. There were no effects of ketamine and VEGF neutralizing antibody infusion on home cage feeding (Figure 2D). A subset of rats received a second intra-mPFC infusion of the VEGF neutralizing antibody and i.p. injection after a ≥ 6-day interval and were tested in the female urine sniffing test 1 day later. Ketamine significantly increased time spent sniffing female urine in IgG-infused rats, and VEGF neutralizing antibody completely blocked this effect (Figure 2E). These treatments did not influence locomotor activity conducted 1.5 h after the female urine sniffing test (Figure 2F). We also show that ketamine significantly induced c-Fos expression, an immediate early gene marker of neuronal activation, in the mPFC of IgG-infused rats, replicating our previous report (30), and VEGF neutralizing antibody-infusion blocked this effect (Figure 2I, J).
FIGURE 2. Effect of intra-mPFC infusion of VEGF nAb on the behavioral effects of ketamine in ratsa.
a(A) Experimental timeline for behavioral testing starting 1 day after intra-mPFC infusion of either control IgG (0.2 μg/side) or VEGF nAb (0.2 μg/side) and i.p. injection of either saline or ketamine (10 mg/kg). (B) Immobility time in the forced swim test (FST) 1 day after intra-mPFC infusion and i.p. injection (interaction, F1,32 = 4.64, p = 0.039, n = 9). (C) Latency to feed in the novelty-suppressed feeding test (NSF) 5 days after intra-mPFC infusion and i.p. injection (interaction, F1,32 = 6.28, p = 0.018, n = 9). (D) Home cage feeding (HCF) just after the NSF (interaction, F1,32 = 0.000248, p = 0.99, n = 9). (E) Time spent sniffing female urine in the female urine sniffing test (FUST) 1 day after the second intra-mPFC infusion and i.p. injection (interaction, F1,17 = 10.1, p = 0.0056, n = 5–6). (F) Locomotor activity (LMA) 1 day after the second intra-mPFC infusion and i.p. injection (interaction, F1,17 = 0.0583, p = 0.81, n = 5–6). (G) Schematic representation of mPFC infusion sites. (H) Experimental timeline for c-Fos quantification. (I) The number of c-Fos-positive cells in the mPFC (interaction, F1,25 = 7.08, p = 0.013, n = 7–8). (J) Representative images of c-Fos expression in the mPFC of each group. Data are expressed as means ± SD. **p < 0.01, ***p < 0.001.
Previous studies demonstrate that signaling required for the synaptic and behavioral actions of ketamine occurs within the first 2 h (20). Intra-mPFC infusion of VEGF neutralizing antibody 2-h after ketamine failed to block the behavioral effects of ketamine in the forced swim test, novelty-suppressed feeding test, and female urine sniffing test, indicating that VEGF is required for this initial critical signaling period (Figure S8).
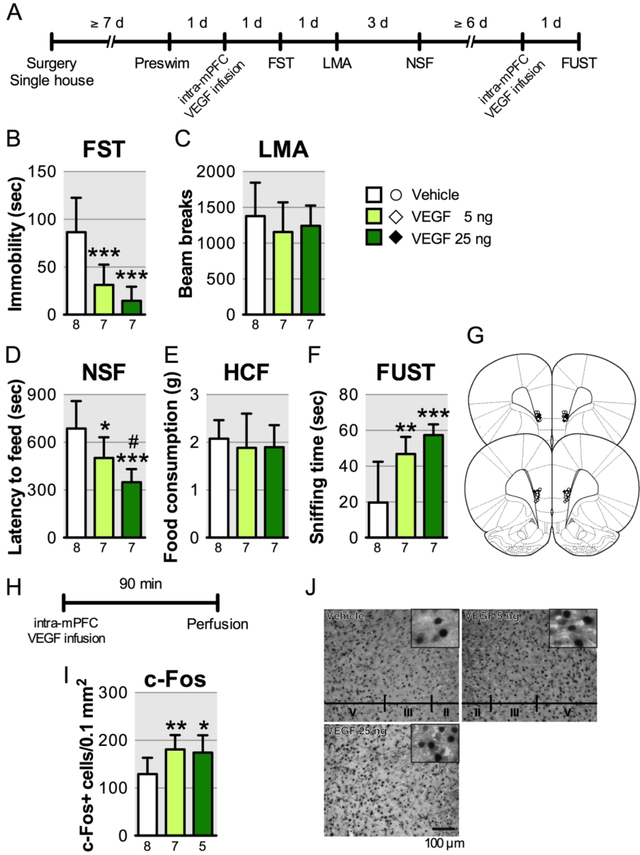
To determine whether increased VEGF signaling in the mPFC is sufficient to produce rapid behavioral effects similar to ketamine in the forced swim test, novelty-suppressed feeding test, and female urine sniffing test, rats were infused with recombinant VEGF164, the predominant VEGF isoform, and subjected to behavioral testing (Figure 3A). Intra-mPFC infusion of VEGF164 (5 or 25 ng/side) significantly decreased immobility in the forced swim test (Figure 3B) and latency to feed in the novelty-suppressed feeding test (Figure 3D); there were no effects on locomotor activity or home cage feeding (Figure 3C, E). In the female urine sniffing test, a second intra-mPFC VEGF164 infusion (both doses) significantly increased female urine sniffing time (Figure 3F). Intra-mPFC VEGF164 infusion also significantly increased c-Fos-positive cells in the mPFC (Figure 3H-J). Moreover, further analysis shows that there were no significant effects of ketamine on VEGF levels in PFC of C57BL/6J mice 0.5 and 1 h after injection (Figure S9). Together these findings indicate that elevated VEGF release, but not expression, in the mPFC is necessary and sufficient to produce ketamine-like behavioral actions via increased neuronal activation.
FIGURE 3. The behavioral actions of intra-mPFC infusion of VEGF164 in ratsa.
a(A) Timeline for behavioral testing starting 1 day after intra-mPFC infusion of either vehicle or VEGF164 (5 or 25 ng/side). (B) Immobility time in the forced swim test (FST) 1 day after intra-mPFC infusion (F2,19 = 15.6, p < 0.0001, n = 7–8). (C) Locomotor activity (LMA) 2 days after intra-mPFC infusion (F2,19 = 0.598, p = 0.56, n = 7–8). (D) Latency to feed in the novelty-suppressed feeding test (NSF) 5 days after intra-mPFC infusion (F2,19 = 11.7, p = 0.0005, n = 7–8). (E) Home cage feeding (HCF) just after the NSF (F2,19 = 0.298, p = 0.75, n = 7–8). (F) Time spent sniffing female urine in the female urine sniffing test (FUST) 1 day after the second intra-mPFC infusion (F2,19 = 12.5, p = 0.0003, n = 7–8). (G) Schematic representation of mPFC infusion sites. (H) Timeline for c-Fos quantification. (I) The number of c-Fos-positive cells in the mPFC (F2,17 = 5.18, p = 0.018, n = 5–8). (J) Representative images of c-Fos expression in the mPFC of each group. Data are expressed as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. #p < 0.05 relative to VEGF164 5 ng.
To investigate regional selectivity, we tested infusions of VEGF164 (25 ng/side) into the dorsal striatum on the same behaviors (Figure S10). Intra-striatal infusion of VEGF164 did not produce any significant behavioral changes.
Neuronal Flk-1 signaling in the mPFC is required for the antidepressant-like behavioral effects of ketamine
First, we examined the effects of intra-mPFC ketamine infusion (10 ng/side) in Flk-1NEURON−/− mice (Figure S11A). In the forced swim test, intra-mPFC ketamine infusion significantly decreased immobility in control mice, but had no effects in Flk-1NEURON−/− mice (Figure S11B); similar effects were observed in the preswim (Figure S6C). Intra-mPFC ketamine infusion did not influence locomotor activity (Figure S11C). In the female urine sniffing test, intra-mPFC ketamine infusion significantly increased time spent sniffing female urine in control mice, and this effect was blocked in Flk-1NEURON−/− mice (Figure S11D). We also found that the behavioral effects of intra-mPFC VEGF164 infusion (5 ng/side) in the forced swim test and female urine sniffing test were blocked in Flk-1NEURON−/− mice (Figure S6D, S11F-I).
To confirm that prefrontal VEGF-Flk-1 signaling specifically in mPFC excitatory neurons mediates the behavioral effects of ketamine in the forced swim test, female urine sniffing test, and novelty-suppressed feeding test, the influence of selective Flk-1 knockdown in mPFC CaMKIIα-positive neurons was evaluated (Figure 4A). The AAV2shFlk−1 construct constitutively expresses DsRed and contains a CMV-EGFP cassette disrupting the expression of shFlk-1, which silences Flk-1 mRNA. Upon Cre-dependent recombination, the CMV-EGFP cassette is excised, allowing expression of DsRed only, along with shFlk-1 (Figure 4B). Infusion of AAV2shFlk−1 into the mPFC of CaMKIIα-Cre (CaMKIIα-CremPFC/AAV2shFlk−1) mice resulted in recombination in the majority of CaMKIIα-positive neurons (EGFP-/DsRed+) in the infralimbic and prelimbic regions of the mPFC, with no recombination observed in the mPFC of AAV2shFlk−1-infused wildtype (WTmPFC/AAV2shFlk−1) mice (EGFP+/DsRed+; Figure 4C). Flk-1 immunostaining demonstrated that the number of Flk-1+/EGFP+/DsRed+ cells in CaMKIIα-CremPFC/AAV2shFlk−1 mice, compared to WTmPFC/AAV2shFlk−1 mice was decreased by approximately 75% (Figure 4D, E). Moreover, the numbers of Flk-1+/EGFP-/DsRed+ cells in either CaMKIIα-CremPFC/AAV2shFlk−1 or WTmPFC/AAV2shFlk−1 mice were ess than 10% (Figure 4D). These data confirm that Flk-1 knockdown successfully occurred in most of recombined (EGFP-/DsRed+) cells in CaMKIIα-CremPFC/AAV2shFlk−1 mice.
In the forced swim test, ketamine significantly decreased immobility in WTmPFC/AAV2shFlk−1 mice, but this effect was blocked in CaMKIIα-CremPFC/AAV2shFlk−1 mice (Figure 4F); similar effects were observed in the preswim (Figure S6E); there were no effects on locomotor activity (Figure 4G). In the female urine sniffing test, ketamine induction of time spent sniffing female urine in WTmPFC/AAV2shFlk−1 mice was completely blocked in CaMKIIα-CremPFC/AAV2shFlk−1 mice (Figure 4H). In the novelty-suppressed feeding test, ketamine significantly decreased latency to feed in WTmPFC/AAV2shFlk−1 mice, and this effect was fully blocked in CaMKIIα-CremPFC/AAV2shFlk−1 mice (Figure 4I) with no change in home cage feeding (Figure 4J). Unlike the novelty-suppressed feeding results in Flk-1NEURON−/− mice (Figure 1C), there was no effect on latency after Flk-1 knockdown in vehicle treated CaMKIIα-CremPFC/AAV2shFlk−1 mice. These results demonstrate that the behavioral effects of ketamine in these models are mediated by mPFC neuronal Flk-1 signaling.
Ketamine exerts neurotrophic and synaptogenic effects through neuronal VEGF signaling
Ketamine increases spine density and synaptic function in the mPFC layer V pyramidal neurons, which underlies it’s antidepressant effects (20). Here, we tested the role of neuronal VEGF signaling in the structural and synaptic effects of ketamine in primary neuronal cultures and in the mPFC. We measured green fluorescent protein-labeled neuronal branching in rat primary cortical neurons 24-h after ketamine (500 nM) or VEGF164 (50 ng/mL) incubation, with or without the selective Flk-1 inhibitor ZM323881 (10 nM, in 0.1% DMSO, 30-min pre-incubation) (Figure 5A). Ketamine or VEGF164 significantly increased the number of dendrite branch crossings compared with vehicle control at both 50- and 100-μm distances from the soma, and these effects were blocked by ZM323881 (Figure 5B, C). For in vivo studies, control or VEGFNEURON−/− mice were injected with ketamine (10 mg/kg), and then 24 h later, brains were collected and processed for Golgi staining to visualize and analyze spine density in the apical tuft of mPFC layer V pyramidal neurons (Figure 5D, E). There was a small but significant decrease in spine density in the apical tuft (measured at levels I and II as indicated) in both the infralimbic and prelimbic regions of the mPFC in saline-injected VEGFNEURON−/− mice compared with control mice, with the exception of the more primary tuft of the prelimbic region (level I), indicating impairment of basal synaptogenesis in the mPFC of VEGFNEURON−/− mice (Figure 5F, G). Ketamine significantly increased spine density of layer V pyramidal neurons in both the infralimbic and prelimbic regions in control mice, but these effects were completely blocked in VEGFNEURON−/− mice (Figure 5F, G). These results indicate that ketamine requires neuronal VEGF signaling to produce morphometric/neurotrophic and synaptogenic effects.
FIGURE 5. Effect of blockade of neuronal VEGF signaling on the neurotrophic and synaptogenic effects of ketaminea.
a(A) Experimental timeline for dendritic morphology in rat primary cultured cortical neurons. (B) The number of dendritic crossings at 50 and 100 μm distances from the soma after 24-h vehicle, ketamine (500 nM) or VEGF164 (50 ng/mL) treatment with 30-min pre-incubation of 0.1% DMSO with or without ZM323881 (10 nM; 50 μm, interaction, F2,128 = 11.0, p < 0.0001; 100 μm, interaction, F2,128 = 17.2, p < 0.0001, n = 15–26). (C) Representative images of EGFP-expressing rat primary cortical neurons from each group with concentric circles (100- and 200-μm diameter). (D) Experimental timeline for dendritic spine analysis. (E) Spine density was analyzed on primary (I) and secondary (II) branches of the apical tuft of layer V pyramidal neurons in the infralimbic (IL; n = 11–15 branches from 6 cells from 3 mice/group) and prelimbic (PL; n =10–17 branches from 6 cells from 3 mice/group) mPFC. Bottom, representative image of a Golgi-stained layer V pyramidal neuron. (F) Left, spine density of infralimbic pyramidal neurons (I, interaction, F1,45 = 8.45, p = 0.0056, n = 12–13; II, interaction, F1,49 = 4.06, p = 0.049, n = 11–15). Right, representative images of the apical tuft segments. (G) Left, spine density of prelimbic pyramidal neurons (I, interaction, F1,43 = 1.51, p = 0.23, main effect of ketamine, F1,43 = 6.72, p = 0.013, main effect of genotype, F1,43 = 10.6, p = 0.0022, n = 10–13; II, interaction, F1,55 = 10.2, p = 0.0023, n = 12–17). Right, representative images of the apical tuft segments. Data are expressed as means ± SD. **p < 0.01, ***p < 0.001; #p < 0.05, ##P < 0.01, ###P < 0.001 relative to Control+Sal mice.
DISCUSSION
The results demonstrate that neuronal VEGF-Flk-1 signaling in the mPFC plays a pivotal role in the antidepressant-like behavioral and neurotrophic actions of ketamine. We also found that a single intra-mPFC infusion of ketamine or VEGF164 mimics the behavioral effects of systemic ketamine in a neuronal Flk-1-dependent manner. These findings indicate that neuronal VEGF-Flk-1 signaling in the mPFC is necessary and sufficient for the rapid and sustained antidepressant-like behavioral actions of ketamine. Alternative splicing of the VEGF gene results in various isoforms of different amino acid lengths, with VEGF120 and VEGF164 the most abundant (~20% and 75%, respectively) in adult mouse brain (33). Both VEGF120 and VEGF164 bind two high affinity tyrosine kinase receptors, Flt-1 (VEGFR1) and Flk-1 (VEGFR2), but only VEGF164 binds to the co-receptors neuropilin-1 and −2 (34). Our results demonstrate that Flk-1 is required for the behavioral actions of ketamine and VEGF164, although we cannot rule out the contribution of Flt-1 and neuropilin-1 and −2.
While VEGF-Flk-1 signaling is required for the actions of ketamine, the results demonstrate that blockade of neuronal VEGF-Flk-1 signaling did not result in increased immobility in the forced swim and tail suspension tests, increased latency to feed in the novelty-suppressed feeding test, or decreased time in the female urine sniffing test. These findings indicate that under controlled environmental, non-stress conditions, loss of neuronal VEGF-Flk-1 signaling is not sufficient to produce these behavioral changes. Similar findings have been observed in BDNF mutant mice; only when these mice are exposed to stress is depressive vulnerability observed (35–37). This could be due to the simultaneous actions of multiple neurotrophic factors, such that loss of one (e.g., BDNF or VEGF) is insufficient to produce behavior deficits. However, a recent study reported that viral-mediated VEGF knockdown in the hippocampus increased immobility and latency to feed in the forced swim test and novelty-suppressed feeding test, respectively (38); this could possibly be due to nonselective VEGF knockdown, in glia and endothelial cells as well as neurons, whereas deletion in the current study was specific to neurons. The latter study also reported that nonselective VEGF knockdown in hippocampus reduced but did not block the behavioral actions of ketamine. Further studies are needed to determine if blockade of both VEGF and BDNF signaling in neurons and/or glial and endothelial cells in mPFC is sufficient to produce behavior deficits. In addition, future studies are needed to determine if knockdown of neuronal VEGF-Flk-1 signaling increases susceptibility to stress in rodent models such as chronic unpredictable stress or social defeat (21), and if the behavioral actions of ketamine in these models require VEGF-Flk-1 signaling.
Neuron-specific deletion of Flk-1 or VEGF did not result in overt developmental phenotypes although there were some subtle effects. Basal spine density in layer V pyramidal neurons was decreased and open arm time in the elevated plus maze was reduced in the VEGFNEURON−/− mice; in Flk-1NEURON−/− mice preswim immobility was increased, latency to feed in the novelty-suppressed feeding test was decreased (saline- and/or ketamine-injected), and center time in the open field test decreased. The mechanisms underlying these effects are unknown, but may be related to neuronal Flk-1 deletion throughout the forebrain, as region specific, viral-mediated Flk-1 knockdown in mPFC had no effect on forced swim test or novelty-suppressed feeding behaviors. Moreover, these effects were not observed in VEGFNEURON−/− mice. Future studies using viral-mediated Flk-1 knockdown are needed to determine if other forebrain regions could explain the complex phenotypes shown in the Flk-1NEURON−/− mice. Alternatively, these behavioral differences could be related to the housing conditions: single-housed Flk-1flox/flox/Flk-1NEURON−/− mice were more active than VEGFflox/flox/VEGFNEURON−/− mice in the locomotor activity and tail suspension test, but there was no difference in locomotor activity when these lines were group housed. Also, although Flk-1flox/flox and VEGFflox/flox mice were backcrossed onto C57BL/6J mice, there could still be genetic variation that could account for the locomotor differences. Notably, the Flk-1flox/flox line includes background of the outbred ICR strain (26) that shows higher locomotor activity in a novel environment than C57BL/6J mice (39). Despite these baseline differences, the controls for both lines show responses to ketamine that are blocked in the littermate knockout mice, demonstrating the utility of these lines for our studies of neuronal VEGF and Flk-1. Taken together, the conditional, cell-specific knockout approach provides strong evidence that forebrain excitatory neurons are both the source and target of VEGF that is released in response to ketamine.
Analysis of c-Fos shows that VEGF-dependent neuronal activation in the mPFC is associated with the behavioral actions of ketamine. This is consistent with our previous results demonstrating that neuronal silencing of the infralimbic mPFC blocks the induction of c-Fos and the behavioral effects of ketamine, and that in vivo optogenetic stimulation of the mPFC produces ketamine-like behavioral responses in the forced swim test, novelty-suppressed feeding test, and sucrose preference test (a test for reward/anhedonia) in rats (30). Consistent with these data, the current study shows that infusion of a VEGF neutralizing antibody into the mPFC prior to ketamine administration completely suppresses both the behavioral responses and c-Fos. In contrast, infusion of the VEGF neutralizing antibody into the mPFC 2-h post-ketamine administration did not block the behavioral effects of ketamine. Moreover, ketamine did not alter VEGF levels in the PFC within 1 h. Taken together, the results indicate that ketamine rapidly and transiently increases VEGF release, but not protein levels, in the mPFC, and that VEGF-Flk-1 signaling during the initial 2-h period is required for the rapid and sustained behavioral actions of ketamine (Figure 6). Previous studies report that ketamine increases levels of VEGF as well as BDNF in the hippocampus, but these studies did not test if hippocampal VEGF or BDNF is required for the actions of ketamine (38, 40), as was done in the current study and in our previous work for BDNF (22, 23). These findings indicate that rapid increases in VEGF and BNDF release, but not expression, in the mPFC at early time points less than 2 h post-injection are critical for the behavioral effects of ketamine.
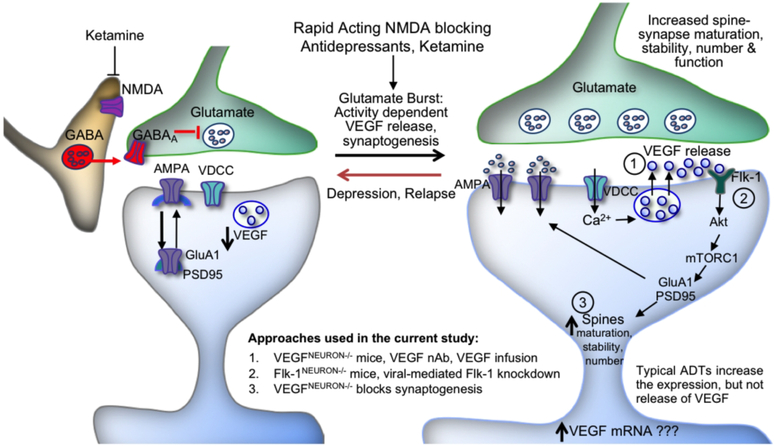
FIGURE 6. Model for the cellular actions of ketamine: regulation of VEGF release and Flk-1 signalinga.
aKetamine blockade of NMDA receptors located on GABAergic interneurons results in disinhibition and a rapid but transient glutamate burst that activates AMPA receptors. This leads to stimulation of L-type voltage-dependent calcium channels (VDCCs) and influx of Ca2+ that stimulates VEGF release, which stimulates Flk-1 signaling, including activation of mTORC1 pathway. The mTORC1 pathway controls the translation and synthesis of synaptic proteins, including GluA1 and PSD95, which are required for increases in synaptogenesis and spine maturation. These cellular events are associated with the rapid and sustained antidepressant actions of ketamine. Previous studies demonstrate that BDNF release also plays an essential role in the rapid and sustained actions of ketamine (see discussion). Abbreviations: VDCC, voltage dependent calcium channel; NMDA, N-methyl-D-aspartate receptor; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GluA1, subtype of AMPA receptor; PSD95, postsynaptic density protein 95; Akt, a serine/threonine protein kinase; mTORC1, mechanistic target of rapamycin complex 1; VEGF nAb, VEGF neutralizing antibody; ADT, antidepressant.
Previous studies demonstrate that ketamine rapidly increases the number and function of spine synapses on layer V pyramidal neurons in the mPFC through activity-dependent BDNF release and activation of the mechanistic target of rapamycin complex 1 (mTORC1) (5, 20, 23). The current results demonstrate that VEGF-Flk-1 signaling is sufficient to increase dendrite complexity in primary neurons, consistent with a previous report (24). VEGF-Flk-1 is also necessary for ketamine induction of dendrite complexity and spine synapse number in layer V mPFC pyramidal neurons as these effects were blocked by pharmacological inhibition of Flk-1 and in VEGFNEURON−/− mice, respectively. These results indicate that neuronal VEGF, as well as BDNF (22, 23) signaling play key roles in the synaptogenic effects of ketamine. In addition, spine density is decreased in mPFC of VEGFNEURON−/− mice, similar to what is seen with chronic stress (21, 41, 42), although these mice do not display behavior deficits in models of despair, anxiety, and motivation/reward. Similar morphological, but not behavioral deficits (i.e., despair in the forced swim test) have been reported in BDNF Val66Met knock-in mice in which the secretion of BDNF is impaired (23). These findings suggest that reductions of both VEGF and BDNF signaling are required to produce behavior deficits, as deletion or inhibition of only one pathway is insufficient. Whether these trophic factors act in parallel or sequentially remains to be determined. BDNF is reported to stimulate VEGF expression via mTORC1 in neuroblastoma cells, supporting the possibility that VEGF-Flk-1 signaling is downstream of BDNF (43). However, it is also possible that ketamine-induction of neuronal activity causes release of both BDNF and VEGF that then act simultaneously.
In summary, the current findings demonstrate that VEGF signaling in the mPFC plays an essential role in the behavioral and synaptic effects of ketamine and may have important implications for understanding the pathophysiology and treatment of depression. Although the role of VEGF in depressed patients remains unknown, a recent genome-wide association study reveals that a VEGF-related single-nucleotide polymorphism (SNP) rs4416670 is associated with increased risk for depression (44), and the minor C allele of this SNP is associated with decreased levels of VEGF (45). Together, these findings raise the possibility that patients with the C allele and reduced levels of VEGF would not respond to ketamine to the same extent as patients with the major T allele SNP. Further studies of the impact of this SNP in humans as well as in rodent models could test this interesting hypothesis. In addition, the current findings could help identify novel VEGF-Flk-1 signaling sites to target for the development of new rapid acting antidepressants.
Supplementary Material
Previous presentation.
Neuroscience 2015 (the 45th Annual Meeting of the Society for Neuroscience), Chicago, IL, October 17–21, 2015
The 90th Annual Meeting of the Japanese Pharmacological Society, Nagasaki, Japan, March 15–17, 2017
The 40th Annual Meeting of the Japan Neuroscience Society, Chiba, Japan, July 20–23, 2017
The 47th Annual Meeting of the Japanese Society for Neuropsychopharmacology, Sapporo, Japan, September 28–30, 2017
Disclosures and acknowledgments
This study was supported by NIMH grants MH045481 (R.S.D.), MH093897 (R.S.D.), MH077681 (M.R.P.), the Uehara Memorial Foundation fellowship (S. Deyama), and the state of Connecticut. The authors declare no competing financial interests.
REFERENCES
- 1.World Health Organization (WHO): Depression. Fact sheet/March 2018. http://www.who.int/news-room/fact-sheets/detail/depression, Accessed July 7, 2018
- 2.Greenberg PE, Fournier AA, Sisitsky T, et al. : The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015; 76:155–162 [DOI] [PubMed] [Google Scholar]
- 3.Drevets WC, Price JL, Simpson JR Jr., et al. : Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824–827 [DOI] [PubMed] [Google Scholar]
- 4.Wise T, Radua J, Via E, et al. : Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry 2017; 22:1455–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duman RS, Aghajanian GK, Sanacora G, et al. : Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22:238–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. : Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 1999; 45:1085–1098 [DOI] [PubMed] [Google Scholar]
- 7.Duman RS, Monteggia LM: A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006; 59:1116–1127 [DOI] [PubMed] [Google Scholar]
- 8.Karege F, Vaudan G, Schwald M, et al. : Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 2005; 136:29–37 [DOI] [PubMed] [Google Scholar]
- 9.Qi XR, Zhao J, Liu J, et al. : Abnormal retinoid and TrkB signaling in the prefrontal cortex in mood disorders. Cereb Cortex 2015; 25:75–83 [DOI] [PubMed] [Google Scholar]
- 10.Elfving B, Plougmann PH, Wegener G: Differential brain, but not serum VEGF levels in a genetic rat model of depression. Neurosci Lett 2010; 474:13–16 [DOI] [PubMed] [Google Scholar]
- 11.Heine VM, Zareno J, Maslam S, et al. : Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci 2005; 21:1304–1314 [DOI] [PubMed] [Google Scholar]
- 12.Isung J, Aeinehband S, Mobarrez F, et al. : Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry 2012; 2:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteggia LM, Barrot M, Powell CM, et al. : Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A 2004; 101:10827–10832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nibuya M, Morinobu S, Duman RS: Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995; 15:7539–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nibuya M, Nestler EJ, Duman RS: Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 1996; 16:2365–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saarelainen T, Hendolin P, Lucas G et al. : Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 2003; 23:349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warner-Schmidt JL, Duman RS: VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A 2007; 104:4647–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene J, Banasr M, Lee B, et al. : Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology 2009; 34:2459–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarate CA Jr., Singh JB, Carlson PJ, et al. : A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63:856–864 [DOI] [PubMed] [Google Scholar]
- 20.Li N, Lee B, Liu RJ, et al. : mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329:959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N, Liu RJ, Dwyer JM, et al. : Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69:754–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepack AE, Fuchikami M, Dwyer JM, et al. : BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2015; 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu RJ, Lee FS, Li XY, et al. : Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 2012; 71:996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenstein JM, Mani N, Khaibullina A, et al. : Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci 2003; 23:11036–11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casanova E, Fehsenfeld S, Mantamadiotis T, et al. : A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis 2001; 31:37–42 [DOI] [PubMed] [Google Scholar]
- 26.Albuquerque RJ, Hayashi T, Cho WG, et al. : Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med 2009; 15:1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerber HP, Hillan KJ, Ryan AM, et al. : VEGF is required for growth and survival in neonatal mice. Development 1999; 126:1149–1159 [DOI] [PubMed] [Google Scholar]
- 28.Wohleb ES, Wu M, Gerhard DM, et al. : GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest 2016; 126:2482–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutheil S, Ota KT, Wohleb ES, et al. : High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology 2016; 41:1874–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchikami M, Thomas A, Liu R, et al. : Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci U S A 2015; 112:8106–8111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ota KT, Liu RJ, Voleti B, et al. : REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 2014; 20:531–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dwyer JM, Maldonado-Aviles JG, Lepack AE, et al. : Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior. Proc Natl Acad Sci U S A 2015; 112:6188–6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng YS, Rohan R, Sunday ME, et al. : Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn 2001; 220:112–121 [DOI] [PubMed] [Google Scholar]
- 34.Neufeld G, Cohen T, Gengrinovitch S, et al. : Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999; 13:9–22 [PubMed] [Google Scholar]
- 35.Advani T, Koek W, Hensler JG: Gender differences in the enhanced vulnerability of BDNF+/− mice to mild stress. Int J Neuropsychopharmacol 2009; 12:583–588 [DOI] [PubMed] [Google Scholar]
- 36.Duman CH, Schlesinger L, Kodama M, et al. : A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry 2007; 61:661–670 [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Wang DD, Wang Y, et al. : Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 2012; 32:4092–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi M, Lee SH, Chang HL, et al. : Hippocampal VEGF is necessary for antidepressant-like behaviors but not sufficient for antidepressant-like effects of ketamine in rats. Biochim Biophys Acta 2016; 1862:1247–1254 [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi T, Nagasawa M, Ikeda H, et al. : Manipuration of dopamine metabolism contributes to attenuating innate high locomotor activity in ICR mice. Behav Brain Res 2017; 328:227–234 [DOI] [PubMed] [Google Scholar]
- 40.Autry AE, Adachi M, Nosyreva E, et al. : NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011: 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu CT, Scheuing L, Liu G, et al. : The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int J Neuropsychopharmacol 2015; 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu RJ, Aghajanian GK: Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 2008; 105:359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura K, Martin KC, Jackson JK, et al. : Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res 2006; 66:4249–4255 [DOI] [PubMed] [Google Scholar]
- 44.Xie T, Stathopoulou MG, de Andrés F, et al. ; VEGF-related polymorphisms identified by GWAS and risk for major depression. Transl Psychiatry 2017; 7:e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debette S, Visvikis-Siest S, Chen MH, et al. : Identification of cis- and trans-acting genetic variants explaining up to half the variation in circulating vascular endothelial growth factor levels. Circul Res 2011; 109:554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.