Abstract
Purpose:
Many transformed cells and embryonic stem cells are dependent on the biosynthesis of the universal methyl-donor S-adenosylmethionine (SAM) from methionine by the enzyme MAT2A to maintain their epigenome. We hypothesized that cancer stem cells (CSCs) rely on SAM biosynthesis and that the combination of methionine depletion and MAT2A inhibition would eradicate CSCs.
Methods:
Human triple (ER/PR/HER2)-negative breast carcinoma (TNBC) cell lines were cultured as CSC-enriched mammospheres in control or methionine-free media. MAT2A was inhibited with siRNAs or cycloleucine. The effects of methionine restriction and/or MAT2A inhibition on the formation of mammospheres, the expression of CSC markers (CD44hi/C24low), MAT2A and CSC transcriptional regulators, apoptosis induction, and histone modifications were determined. A murine model of metastatic TNBC was utilized to evaluate the effects of dietary methionine restriction, MAT2A inhibition and the combination.
Results:
Methionine restriction inhibited mammosphere formation and reduced the CD44hi/C24low CSC population; these effects were partly rescued by SAM. Methionine depletion induced MAT2A expression (mRNA and protein) and sensitized CSCs to inhibition of MAT2A (siRNAs or cycloleucine). Cycloleucine enhanced the effects of methionine depletion on H3K4me3 demethylation and suppression of Sox9 expression. Dietary methionine restriction induced MAT2A expression in mammary tumors, and the combination of methionine restriction and cycloleucine was more effective than either alone at suppressing primary and lung metastatic tumor burden in a murine TNBC model.
Conclusions:
Our findings point to SAM biosynthesis as a unique metabolic vulnerability of CSCs that can be targeted by combining methionine depletion with MAT2A inhibition to eradicate drug resistant-CSCs.
Keywords: methionine, breast cancer, S-adenosylmethionine, nutrition, cancer stem cell, therapeutics
Introduction
The essential sulfur-containing amino acid methionine plays a critical role in maintaining the pluripotency of embryonic stem (ES) cells and induced pluripotent stem (iPS) cells [1, 2]. Methionine is converted to S-adenosylmethionine (SAM) by a family of conserved methionine adenosyltransferase (MAT) enzymes whose catalytic subunits are derived from two genes, the liver-specific Mat1a and the ubiquitously expressed Mat2a genes [3]. SAM is the universal methyl-donor for a broad range of methyltransferases involved in DNA and histone methylation of the epigenome [4]. Depletion of SAM by a variety of strategies, including methionine deprivation, leads to demethylation of a specific histone modification (H3K4me3), a conserved epigenetic mark that transcriptionally activates gene networks that regulate pluripotency [1, 5, 6]. Transient methionine restriction induces differentiation of ES and iPS cells, while prolonged methionine restriction activates p53-dependent apoptosis in these cells [1]. In this way, methionine metabolism directly links the nutrient status of stem cells to the epigenetic regulation of pluripotency.
Intriguingly, many tumor cells are also dependent on methionine for cell proliferation and survival [7]. Methionine restriction activates cell cycle arrest and/or apoptosis in a broad range of transformed cells and inhibits tumor growth in diverse murine models [8–11]. These effects of methionine depletion are rescued by homocysteine supplementation in normal cells but not transformed cells [12]. However, the molecular mechanisms underlying the “methionine dependence” of cancer are poorly understood. Although clinical trials in advanced solid tumors have demonstrated the safety of dietary methionine restriction alone or in combination with cytotoxic agents, these studies have failed to demonstrate therapeutic efficacy [13–15]. We have recently demonstrated that methionine restriction “primes” triple (estrogen receptor, progesterone receptor and HER2)-negative breast tumors to respond to pro-apoptotic TRAIL receptor agonists by increasing cell surface expression of TRAIL receptor-2 (TRAIL-R2 or DR5) [16]. Dietary methionine restriction enhances the activity of TRAIL receptor agonists in a murine model of metastatic triple-negative breast cancer. In principle, this “metabolic priming” approach could be used to target other stress response pathways activated by methionine restriction to selectively enhance the therapeutic efficacy of this dietary intervention.
Given the dependence of both stem cells and cancer cells on methionine, we postulated that cancer stem cells (CSCs), rare self-renewing cells within tumors that are likely responsible for treatment resistance and tumor progression [17], might be especially vulnerable to methionine depletion. Moreover, because methionine restriction induces expression of MAT2A as a homeostatic response to preserve SAM levels [1, 18], we hypothesized that methionine restriction primes CSCs to MAT2A inhibition. Here we report that methionine restriction inhibits the formation of CSC-enriched mammospheres and reduces the population of CD44hi/CD24low CSCs. These effects are partly rescued by SAM supplementation. Methionine depletion induces MAT2A expression and sensitizes CSCs to inhibition of MAT2A expression or activity. The MAT2A inhibitor cycloleucine augments the effects of methionine depletion on H3K4me3 demethylation. Moreover, the combination of dietary methionine restriction and cycloleucine is more effective than either individual intervention at suppressing primary and lung metastatic tumor burden in a murine model. Taken together, our findings point to SAM biosynthesis as a novel metabolic vulnerability of CSCs and indicate that MAT2A inhibition selectively enhances the antitumor activity of methionine depletion.
Methods and Materials
Cell culture and reagents
Human MDA-MB-231 and GILM2 TNBC cells stably expressing mCherry were cultured as described [19, 20]. BT20 TNBC cells were grown in MEM medium supplemented with 10% FBS, 1% sodium pyruvate, 1% NEAA, 2% sodium bicarbonate and 100 IU/mL penicillin/streptomycin (Thermo Fisher Scientific). Cell lines were authenticated by STR analyses. Cycloleucine and SAM were purchased from Sigma-Aldrich.
Mammosphere assay
TNBC cells (1 × 104 cells per well) were seeded in 6-well ultra-low attachment plates (Corning) in mammosphere medium composed of serum-free RPMI containing 1% methylcellulose, 10 ng/mL basic fibroblast growth factor (bFGF/FGF2), 20 ng/mL epidermal growth factor (EGF), 2% B-27 supplements, 10 μg/mL human insulin and 100 IU/mL penicillin/streptomycin (Thermo Fisher Scientific). Experiments were performed in control or methionine-free (0% Met) mammosphere media with or without SAM (100 μM) and cycloleucine (50 mM). Three hundred μl of fresh media was added to each well every day (without removing the old media). Mammospheres ≥ 50 μm in diameter were manually counted in ten randomly selected 10× fields per well (Nikon Eclipse TS100 Inverted Microscope) after the indicated number of days.
CD24 and CD44 cell surface expression
To measure cell surface expression of CD24 and CD44, TNBC mammospheres cultured for 6 days were collected, washed with PBS and then enzymatically dissociated with 0.05% trypsin/0.25% EDTA. Cells were pelleted by centrifugation at 500 × g for 5 minutes and resuspended in 10 μL fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD24 monoclonal antibody and allophycocyanin (APC)-conjugated mouse anti-human CD44 monoclonal antibody (BD Pharmingen). After incubating cells for 30 minutes at 4°C in the dark, cells were washed and then analyzed by flow cytometry.
MAT2A silencing
siRNAs targeting the sequences CACACAAGCUAAAUGCCAA (si-MAT2A-1) or CAGUUGUGCCUGCGAAAUA (si-MAT2A-2) in the human Mat2a gene and non-silencing control siRNA were purchased from Sigma-Aldrich. Cells were transfected with siRNAs using Lipofectamine RNAiMAX Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Immunoblotting
Whole-cell lysates were immunoblotted as described [21] using primary Abs against MAT2A, MAT1A, β-actin (Sigma-Aldrich), Sox9, and KLF4 (Cell Signaling).
Real-time PCR
Real-time PCR for MAT2A and GAPDH was performed as described previously [16]. Primers for MAT2A (forward 5-GACATTGGTGCTGGAGACCA, reverse 5-ACTCTGATGGGAAGCACAGC) were purchased from Integrated DNA Technologies and real-time PCR was performed using a comparative Ct method to normalize RNA expression in samples to the controls in each experiment.
Annexin V labeling
For the Annexin V apoptosis assay, TNBC mammospheres cultured for 6 days were collected, washed with PBS and then enzymatically dissociated with 0.05% trypsin/0.25% EDTA. Cells were pelleted by centrifugation at 500 × g for 5 minutes and then analyzed by flow cytometry using the Annexin-PE Apoptosis Detection Kit (BD Bioscience).
Immunoblotting for histones and histone modifications
Mammospheres were incubated on ice in RIPA buffer (50 mM Tris, 150 mM NaCl, 1% NP40, 0.5% Na-DOC, 0.4 mM phenylmethanesulfonyl fluoride, 2X protease inhibitor cocktail) and the lysates spun at 21,000 × g. The supernatant protein concentration was quantified (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific). Pellets (containing histones) were resuspended and boiled in 2X SDS loading dye at amounts proportional to the supernatant protein concentration. Equal amounts of pellet lysate were separated on 15% SDS-PAGE gels and transferred onto nitrocellulose membrane in 1X Towbin/20% methanol. Membranes were blocked in 5% milk/TBS-T and incubated overnight in primary Abs against K4me3 (Active Motif), K9me3 (Abcam), K27me3 (Millipore), H3 [22] or H4, [23] in 5% milk/TBS-T. Membranes were washed with TBS-T, incubated with goat anti-rabbit IgG-HRP (BioRad 1706515) in 5% milk/TBS-T, washed with TBS-T, and HRP was visualized by chemiluminescence (SuperSignal™ West Pico, Thermo Fisher Scientific).
Murine model of metastatic TNBC
GILM2-mCherry TNBC cells (2 × 106) were resuspended in Matrigel (BD Biosciences) and injected into the lactiferous ducts of both 4th mammary glands in 4- to 5-week-old female NOD scid IL2 receptor γ chain knockout (NSG) mice (Jackson Laboratory). Mice were then randomly divided into four treatment groups (10 mice per group): control diet plus vehicle, control diet plus cycloleucine (25 mg/kg twice weekly for 4 doses), methionine-free diet (0% methionine, Teklad TD.140119) plus vehicle, or methionine-free diet plus cycloleucine (25 mg/kg twice weekly for 4 doses). Mice were started on their respective diets 2.5 weeks after tumor cell inoculation, and the diets were continued throughout the treatment period. Mammary tumor volume was calculated using calipers as described [24]. Lung metastatic burden was visualized by fluorescence microscopy in isolated whole lungs and scored using NIH ImageJ analysis as described [24]. For immunoblots and mass spectrometry analysis, a separate cohort of female NSG mice with orthotopic mammary xenograft tumors (n= 3 mice per group) were placed on a control diet or methionine-free diet for 2 weeks, after which time the mice were euthanized and tumors were harvested and frozen for immunoblot analysis. Blood was collected into EDTA tubes at the end of the experiment; samples were centrifuged for 10 minutes at 2,000 × g at 4°C. All animal experiments were carried out as part of an IACUC-approved protocol at the University of Wisconsin-Madison.
Metabolite sample preparation and mass spectrometry analysis
Frozen plasma samples (50 μl) were combined with 200 μL cold acetone and 5 μL tris(2-carboxyethyl)phosphine (TCEP, 2 mM), vortexed for 5 minutes, and centrifuged for 15 minutes at 13,400 × g at 4°C. Metabolite-containing extract (80 μL) was combined with 20 μL dilution buffer (60% Acetonitrile, 1 mM TCEP), and 2 μL internal standard (5 μM heavy methionine, 1 μM heavy SAM). Standard curves for each metabolite were prepared with equivalent addition of internal standard. Targeted LC-MS/MS experiments were performed using a TSQ Quantiva Triple Quadrupole mass spectrometer and Ultimate 3000 RSLC Binary Pump (Thermo Fisher Scientific). Analytes were injected and separated on an ACQUITY BEH Amide column (Waters) heated to 35°C. Mobile phase A consisted of 10 mM ammonium formate with 0.1% formic acid and mobile phase B was 95% acetonitrile with 10 mM ammonium formate with 0.1% formic acid. An 11-min gradient starting with 95% B and ending with 40% B was employed with an 18 minute total method runtime. Selected reaction monitoring (SRM) scans were taken of selected metabolites using 2–3 transitions per metabolite using previously optimized transitions for each target. The total cycle time was 1 sec. The mass spectrometer was equipped with a heated electrospray ionization source operated in positive mode using a spray voltage of 3500 V. Sheath, auxiliary, and sweep gases were held at 25, 13, and 1 arbitrary units, respectively. The ion transfer tube was heated to 342 °C and the vaporizer temperature was set to 358 °C. Q1 and Q3 resolution were set to 0.7 and 2 full width at half maximum. CID gas was set to 1.5 mTorr and 16 V was used for the source fragmentation parameter. LC-MS/MS data were processed using TraceFinder software version 4.0 (Thermo Fisher Scientific). Metabolite peak areas were normalized using internal standards, and metabolites were quantified with standard curves.
Tumor apoptosis assay
The percentage of tumor cells that were positive for active caspase-3 was determined by immunohistochemistry of formalin-fixed, paraffin-embedded tumor tissue sections using an Ab against cleaved caspase-3 (Cell Signaling) as described [24].
Statistics
The statistical significance of differences between groups was determined by ANOVA with Bonferroni posttests using GraphPad Prism 4 software.
Results
Methionine restriction inhibits CSCs by a SAM-dependent mechanism
To explore the role of methionine metabolism in CSCs, we grew TNBC cells in ultra-low attachment plates in normal mammosphere growth media or methionine-free mammosphere growth media with or without supplemental SAM. Methionine depletion resulted in a robust decrease in the number of mammospheres in three different human TNBC cell lines (Fig 1a). Moreover, SAM supplementation largely rescued the effects of methionine restriction in these mammosphere assays. Consistent with these findings, mammospheres grown in methionine-free media exhibited a reduction in the population of CD44hi/CD24low cells that are enriched for CSCs (Fig. 1b). SAM supplementation at least partly rescued the effects of methionine restriction on the CD44hi/CD24low population. Collectively, these results indicate that methionine depletion inhibits CSCs by a SAM-dependent mechanism.
Figure 1. Methionine restriction inhibits CSCs by a SAM-dependent mechanism.
a, Mammosphere assay in TNBC cells. MDA-MB-231, GILM2 and BT20 TNBC cells were cultured in ultra-low attachment plates in normal (control) or methionine-free mammosphere growth media (0% Met) with or without SAM (100 μM). Top panel, representative images showing mammospheres at day 7. Bottom panel, mammospheres were scored in ten fields from each well (mean ± SEM, n =3) *, P < 0.05, ***, P < 0.001 versus mammospheres cultured in control media without SAM. b, Flow cytometry analysis of CD44 and CD24 expression in primary mammospheres from TNBC cells grown for 6 days in control or methionine-free media with or without SAM (100 μM).
Methionine restriction induces MAT2A and potentiates the effect of MAT2A silencing on CSCs
Consistent with prior reports [1, 18], methionine restriction increased MAT2A protein and mRNA levels (Fig. 2a, left and right panels, respectively) in TNBC mammospheres, but did not alter MAT1A protein levels. To determine the functional relevance of the observed induction in MAT2A in mammospheres, we silenced MAT2A expression in mammospheres by transfecting them with one of two different siRNAs targeting MAT2A (si-MAT2A-1 or si-MAT2A-2). Both siRNAs robustly reduced the expression of MAT2A protein in all three TNBC cell lines compared to a non-silencing control (C) siRNA (Fig. 2b). Silencing MAT2A modestly inhibited mammosphere formation and potentiated the effects of methionine restriction on mammospheres (Fig. 2c). Taken together, these results indicate that SAM biosynthesis is required for CSC survival and point to this pathway as a targetable metabolic vulnerability.
Figure 2. Methionine restriction induces MAT2A expression and potentiates the effect of MAT2A silencing on CSCs.
a, Methionine deprivation increases MAT2A protein and mRNA levels. Left panel, immunoblot analysis of MAT2A and MAT1A protein expression in TNBC mammospheres grown in control or methionine-free mammosphere media for 4 days. Right panel, real-time PCR analysis of MAT2A mRNA levels in TNBC mammospheres grown in control or methionine-free mammosphere media for 72 hours. MAT2A mRNA levels were normalized to expression in TNBC cells cultured in control media. b, Immunoblot analysis of MAT2A expression in TNBC cells transfected with a scrambled control siRNA or two different siRNAs targeting MAT2A (MAT2A-1 and MAT2A-2) 48 hours after transfection. c, Mammosphere assay in TNBC cells transfected with a scrambled control siRNA or two different siRNAs targeting MAT2A (MAT2A-1 and MAT2A-2). The next day, cells were collected, seeded in ultra-low attachment plates in control or methionine-free mammosphere media (0% Met) and grown for 4 days. Left panel, representative images showing mammospheres at day 4. Right panel, mammospheres were scored in 10 fields from each well (mean ± SEM, n = 3). *, P < 0.05, **, P < 0.01, ***, P < 0.001 versus the indicated comparisons.
Methionine restriction potentiates the effect of the MAT2A inhibitor cycloleucine on CSCs
Based on our siRNA results, we postulated that cycloleucine, an amino acid analog that competitively inhibits MAT activity [25], would enhance the inhibitory effects of methionine restriction on CSCs. Consistent with our hypothesis, methionine restriction or cycloleucine individually inhibited mammospheres, but the combination of methionine restriction and cycloleucine virtually eradicated mammospheres (Fig. 3a). Cycloleucine augmented apoptosis induction by methionine restriction in TNBC mammospheres with effects ranging from additive (MDA-MB-231 cells) to synergistic (GILM2 and BT20) (Fig. 3b). Moreover, cycloleucine potentiated the cytotoxicity of methionine depletion against adherent TNBC cells (Fig. S1). To determine the specific role of SAM depletion in the observed effects, we added SAM to TNBC mammospheres treated with methionine restriction, cycloleucine or the combination. Notably, SAM supplementation at least partly rescued the inhibitory effects of methionine depletion and cycloleucine on TNBC mammospheres (Fig. 3c). Collectively, these results indicate that cycloleucine potentiates the effects of methionine restriction on CSCs by a SAM-dependent mechanism.
Figure 3. Methionine restriction enhances the effects of the MAT2A inhibitor cycloleucine on CSCs.
a, Mammosphere assay of TNBC cells grown in ultra-low attachment plates in control or methionine-free mammosphere media (0% Met) with or without cycloleucine (CL, 50 mM). Top panel, representative images showing mammospheres at day 7. Bottom panel, mammospheres were scored in ten fields from each well (mean ± SEM, n =3) ***, P < 0.001 versus mammospheres grown in control media without cycloleucine. b, TNBC cells were cultured in ultra-low attachment plates in control or methionine-free mammosphere media (0% Met) with or without CL (50 mM) for 5 days. Apoptosis was measured by Annexin V labeling using flow cytometry. c, TNBC cells were cultured in ultra-low attachment plates in control or methionine-free mammosphere media (0% Met) with or without SAM (100 μM), CL (50 mM), or both for 7 days. Mammospheres were scored in ten fields from each well (mean ± SEM, n =3) *, P < 0.05, ***, P < 0.001 versus mammospheres grown in control media or the indicated comparisons.
Methionine restriction augments the effects of cycloleucine in suppressing histone methylation
Nutrient availability of Met and SAM have been directly linked to changes in histone H3 K4, K9 and K27 trimethylation [1, 5, 26]. Although some TNBC mammospheres cultured in the presence of cycloleucine or in methionine-free media exhibited modest reductions in H3K4me3, H3K9me3 and H3K27me3 levels (Fig. 4a), the combination of methionine restriction and cycloleucine led to marked demethylation of H3K4me3 in all of the TNBC mammospheres. We next examined the effects of these interventions on the expression of the breast CSC transcriptional regulators Sox9 and KLF4 [27, 28]. Notably, methionine restriction and the combination of methionine restriction and cycloleucine consistently reduced Sox9 protein levels but had more variable effects on KLF4 levels (Fig. 4b). Taken together, these findings suggest that the combination of methionine restriction and cycloleucine robustly induces demethylation of H3K4me3 and suppresses expression of a subset of breast CSC transcriptional regulators.
Figure 4. Methionine restriction augments the effects of cycloleucine in suppressing specific histone methylation.
TNBC cells were grown in ultra-low attachment plates in control or methionine-free mammosphere media (0% Met) with or without SAM (100 μM) or CL (50 mM) for 4 days. a, Immunoblot analysis of histone H3 methylation. b, Immunoblot analysis of the breast CSC regulators Sox9 and KLF4.
Dietary methionine restriction enhances the antitumor effects of cycloleucine in vivo
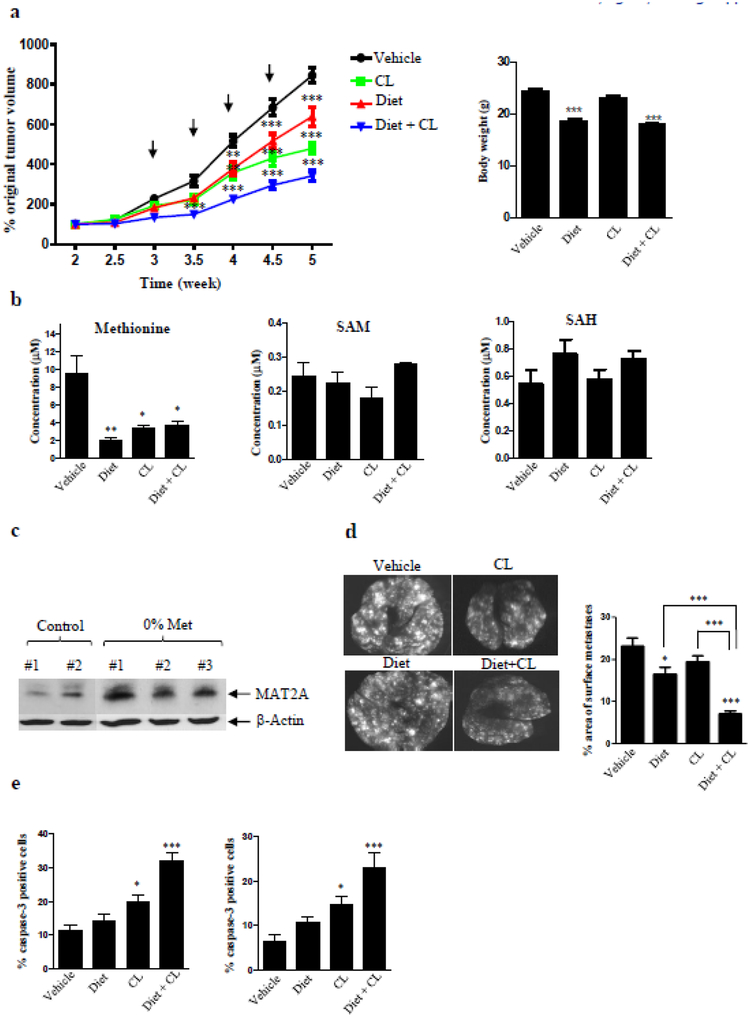
To examine the antitumor activity of dietary methionine restriction, cycloleucine or the combination in vivo, female NSG mice with orthotopic GILM2-mCherry mammary tumors were fed a control or methionine-free diet and treated with vehicle or cycloleucine (25 mg/kg twice weekly for 4 doses). Both the methionine-free diet and cycloleucine alone inhibited mammary tumor growth compared to vehicle-treated mice on a control diet, but the combination of methionine-free diet plus cycloleucine was more effective than either individual intervention (Fig. 5a, left panel). Mice on the methionine-free diet receiving vehicle or cycloleucine exhibited modest weight loss at the end of the study, while cycloleucine treatment itself did not result in weight loss (Fig. 5a, right panel). Plasma levels of methionine, but not SAM or SAH, were significantly reduced in all three intervention groups (Fig. 5b), validating the efficacy of each intervention. Consistent with our in vitro findings, dietary methionine restriction increased protein levels of MAT2A in mammary tumors (Fig. 5c). The combination of dietary methionine restriction and cycloleucine was more effective than either individual treatment at suppressing lung metastases (Fig. 5d) and inducing apoptosis in primary tumors and lung metastases as determined by active caspase-3 immunostaining (Fig. 5e). These findings indicate that dietary methionine restriction enhances the antitumor effects of cycloleucine by inducing its molecular target MAT2A.
Figure 5. Dietary methionine restriction enhances the antitumor effects of cycloleucine in vivo.
Female NSG mice with orthotopic GILM2-mCherry mammary tumors were randomized into four groups (10 mice per group): control diet plus vehicle, control diet plus cycloleucine (25 mg/kg twice weekly for 4 doses, indicated by arrows), methionine-free diet plus vehicle, or methionine-free diet plus cycloleucine (25 mg/kg twice weekly for 4 doses). Mice were started on their respective diets 2 weeks after tumor cell inoculation, and the diets were continued throughout the treatment period. a, Left panel, percentage original mammary tumor volume (at 2 weeks) in each treatment group (mean ± SEM, n = 10 mice per group). Right panel, body weight of mice at the end of the study. b, Plasma levels of Met, SAM and SAH in the mice in each treatment group after two weeks (mean ± SEM, n = 3 mice per group). c, Immunoblot analysis of MAT2A expression in mammary tumors from mice receiving control diet or methionine-free diet for 2 weeks. d, Left panel, representative whole lung images by fluorescence microscopy. Right panel, the percentage of the surface area occupied by lung metastases (mean ± SEM, n = 10 mice per group). e, the percentage active caspase-3-positive tumor cells in mammary tumors (left panel) or metastatic lung tumors (right panel) at the end of the study (mean ± SEM, n = 3 tumors per group). In all panels, *, P < 0.05, **, P < 0.01, ***, P < 0.001 versus vehicle-treated mice or the indicated comparison.
Discussion
We have demonstrated that CSCs are dependent on SAM biosynthesis for self-renewal and survival. Several lines of evidence support this conclusion. First, methionine depletion inhibits mammosphere formation and reduces the population of CD44hi/CD24low CSCs. Strikingly, SAM supplementation at least partly rescues the effects of methionine restriction on CSCs, indicating that CSCs rely on SAM rather than methionine per se for self-renewal and survival. Second, methionine depletion leads to induction of the Mat2a gene and increased MAT2A protein levels as a homeostatic response to attempt to preserve SAM levels in the context of reduced methionine availability. Our findings are consistent with prior reports describing MAT2A induction in response to methionine restriction [1, 18]. Third, dual targeting of SAM biosynthesis by combining methionine restriction with MAT2A inhibition using siRNAs or cycloleucine results in more robust elimination of CSCs, apoptosis induction and demethylation of H3K4me3 than either intervention alone. Fourth, SAM supplementation partly rescues the effects of combined methionine depletion and MAT2A inhibition on CSCs, underscoring the essential role of SAM in CSC survival and methylation of H3K4me3.
Mechanistically, inhibition of SAM biosynthesis activates apoptosis in a subset of CSCs and inhibits CSC self-renewal, which may reflect at least partly the observed reduction in H3K4me3 levels, an epigenetic mark linked to activation of pluripotency gene networks that is highly sensitive to nutrient availability of methionine [1, 5, 26]. The observed robust reduction in levels of Sox9, a transcriptional regulator of CSC self-renewal, in response to inhibition of SAM biosynthesis may contribute to the depletion of CSCs, although we have not specifically assessed the functional relevance of this alteration in these studies. By targeting a metabolic liability of CSCs, inhibition of SAM biosynthesis has a major therapeutic advantage over cytotoxic drugs, which are inactive against CSCs [17].
We have also demonstrated that dual inhibition of SAM biosynthesis inhibits mammary tumor growth and lung metastases more robustly than methionine restriction or cycloleucine individually in a murine TNBC model that recapitulates many clinical features of this disease. Dual targeting also augmented apoptosis induction in primary and metastatic tumors. Consistent with our findings in cell-based models, dietary methionine restriction induced the expression of MAT2A in mammary tumors, providing additional mechanistic insights into the enhanced efficacy of dual targeting of SAM biosynthesis. Notably, dietary methionine restriction, cycloleucine treatment and the combined treatment reduced plasma methionine levels but did not significantly affect plasma SAM or SAH levels. Although cycloleucine was largely abandoned as a chemotherapy drug due to CNS toxicity, we used much lower doses (50 mg/kg twice weekly × 4) than the reported LD50 in mice (120–140 mg/kg/d × one week) [29–31]. At the doses used in these studies, cycloleucine was well tolerated and did not cause weight loss, although methionine restriction did result in modest weight loss. Additional higher affinity MAT2A inhibitors have recently been described [32], and one such MAT2A inhibitor (AG-270) is currently being evaluated in a phase I clinical trial in patients with lymphoma or solid tumors (NCT03435250, Clinical trials.gov). Hence, additional MAT2A inhibitors that may have an improved therapeutic index will be available for future clinical translation. Moreover, dietary methionine restriction reduces visceral fat and improves glucose homeostasis [33, 34], suggesting that these systemic effects may also contribute to the antitumor activity of this intervention. Taken together, our results provide proof-of-principle preclinical evidence in support of dual targeting of SAM biosynthesis by combining methionine restriction and MAT2A inhibition.
The proposed dual targeting of SAM biosynthesis aligns nicely with our recently described “metabolic priming” strategy. We reported previously that methionine restriction enhances cell surface expression of the cell death receptor TRAIL-R2, rendering triple-negative breast tumors more vulnerable to TRAIL-R2 agonists [16]. In an analogous manner, methionine stress activates MAT2A gene expression in TNBC cells, thereby “priming” them to respond to MAT2A inhibition by undergoing apoptosis or differentiation. MAT2A is often aberrantly expressed in human tumors in response to HIF-1α, IGF-1, Nrf2 and EGF and promotes cell growth and drug resistance [3, 35, 36]. Moreover, deregulated MAT2A expression has been linked to tamoxifen-resistance in breast cancer [37]. Silencing MAT2A inhibits proliferation and induces apoptosis in carcinoma cells [38]. Collectively, these findings point to MAT2A as a promising therapeutic target in cancer.
In summary, we have identified SAM biosynthesis as a previously unrecognized metabolic vulnerability of CSCs. Methionine restriction induces MAT2A expression, thereby providing an explanation for the robust synergy between methionine depletion and MAT2A inhibition/silencing in suppressing CSC survival and SAM-dependent epigenetic alterations such as H3K4me3, which have been linked to pluripotency [6]. Dual targeting of SAM biosynthesis inhibits mammary tumor growth and lung metastasis in vivo more robustly than methionine restriction or cycloleucine alone. Our preclinical results lay the foundation for translating these insights into novel strategies to eradicate drug resistant-CSCs by targeting their unique metabolic requirements.
Supplementary Material
Acknowledgements
We are indebted to members of the Cryns lab for their critical reading of the manuscript.
Funding
This work was supported by grants from the Breast Cancer Research Foundation (VLC), V Foundation for Cancer Research (VLC), the Sidney Kimmel Foundation for Cancer Research (PWL), Wisconsin Partnership Program (VLC), National Institutes of Health grant P41GM108538 (JJC), University of Wisconsin Comprehensive Cancer Center Pilot Award (VLC and PWL) and P30CA14520 core facility support. We also acknowledge financial support from the Morgridge Institute for Research Metabolism Theme.
Footnotes
Conflict of interest
JJC is a consultant for Thermo Fisher Scientific. The other authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Shiraki N, Shiraki Y, Tsuyama T, Obata F, Miura M, Nagae G, et al. (2014) Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab 19:780–794. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekaran S, Zhang J, Sun Z, Zhang L, Ross CA, Huang YC, et al. (2017) Comprehensive mapping of pluripotent stem cell metabolism using dynamic genome-scale network modeling. Cell Rep 21:2965–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldonado LY, Arsene D, Mato JM, Lu SC (2018) Methionine adenosyltransferases in cancers: Mechanisms of dysregulation and implications for therapy. Exp Biol Med (Maywood) 243:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locasale JW (2013) Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13:572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, et al. (2013) Influence of threonine metabolism on S-adenosylmethionine and histone methylation. 339:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, et al. (2011) Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell 145:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreis W, Baker A, Ryan V, Bertasso A (1980) Effect of nutritional and enzymatic methionine deprivation upon human normal and malignant cells in tissue culture. Cancer Res 40:634–641. [PubMed] [Google Scholar]
- 8.Guo H, Lishko VK, Herrera H, Groce A, Kubota T, Hoffman RM (1993) Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res 53:5676–5679. [PubMed] [Google Scholar]
- 9.Hoshiya Y, Guo H, Kubota T, Inada T, Asanuma F, Yamada Y, et al. (1995) Human tumors are methionine dependent in vivo. Anticancer Res 15:717–718. [PubMed] [Google Scholar]
- 10.Lu S, Hoestje SM, Choo E, Epner DE (2003) Induction of caspase-dependent and -independent apoptosis in response to methionine restriction. Int J Oncol 22:415–420. [PubMed] [Google Scholar]
- 11.Mecham JO, Rowitch D, Wallace CD, Stern PH, Hoffman RM (1983) The metabolic defect of methionine dependence occurs frequently in human tumor cell lines. Biochem Biophys Res Commun 117:429–434. [DOI] [PubMed] [Google Scholar]
- 12.Halpern BC, Clark BR, Hardy DN, Halpern RM, Smith RA (1974) The effect of replacement of methionine by homocystine on survival of malignant and normal adult mammalian cells in culture. Proc Natl Acad Sci USA 71:1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durando X, Farges MC, Buc E, Abrial C, Petorin-Lesens C, Gillet B, et al. (2010) Dietary methionine restriction with FOLFOX regimen as first line therapy of metastatic colorectal cancer: a feasibility study. Oncol 78:205–209. [DOI] [PubMed] [Google Scholar]
- 14.Epner DE, Morrow S, Wilcox M, Houghton JL (2002) Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr Cancer 42:158–166. [DOI] [PubMed] [Google Scholar]
- 15.Thivat E, Durando X, Demidem A, Farges MC, Rapp M, Cellarier E, et al. (2007) A methionine-free diet associated with nitrosourea treatment down-regulates methylguanine-DNA methyl transferase activity in patients with metastatic cancer. Anticancer Res 27:2779–2783. [PubMed] [Google Scholar]
- 16.Strekalova E, Malin D, Good DM, Cryns VL (2015) Methionine deprivation induces a targetable vulnerability in triple-negative breast cancer cells by enhancing TRAIL receptor-expression. Clin Cancer Res 21:2780–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibue T, Weinberg RA (2017) EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 14:611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Chantar ML, Latasa MU, Varela-Rey M, Lu SC, Garcia-Trevijano ER, Mato JM, et al. (2003) L-methionine availability regulates expression of the methionine adenosyltransferase 2A gene in human hepatocarcinoma cells: role of S-adenosylmethionine. J Biol Chem 278:19885–19890. [DOI] [PubMed] [Google Scholar]
- 19.Malin D, Strekalova E, Petrovic V, Deal AM, Al Ahmad A, Adamo B, et al. (2014) αB-crystallin: a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res 20:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malin D, Strekalova E, Petrovic V, Rajanala H, Sharma B, Ugolkov A, et al. (2015) ERK-regulated αB-crystallin induction by matrix detachment inhibits anoikis and promotes lung metastasis in vivo. Oncogene 34:5626–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, et al. (2006) αB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest 116:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu C, Jain SU, Hoelper D, Bechet D, Molden RC, Ran L, et al. (2016) Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science 352:844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoelper D, Huang H, Jain AY, Patel DJ, Lewis PW (2017) Structural and mechanistic insights into ATRX-dependent and -independent functions of the histone chaperone DAXX. Nat Commun 8:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malin D, Chen F, Schiller C, Koblinski J, Cryns VL (2011) Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer. Clin Cancer Res 17:5005–5015. [DOI] [PubMed] [Google Scholar]
- 25.Sufrin JR, Coulter AW, Talalay P (1979) Structural and conformational analogues of L-methionine as inhibitors of the enzymatic synthesis of S-adenosyl-L-methionine. IV. Further mono-, bi- and tricyclic amino acids. Mol Pharmacol 15:661–677. [PubMed] [Google Scholar]
- 26.Mentch SJ, Mehrmohamadi M, Huang L, Liu X, Gupta D, Mattocks D, et al. (2015) Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metab 22:861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeselsohn R, Cornwell M, Pun M, Buchwalter G, Nguyen M, Bango C, et al. (2017) Embryonic transcription factor SOX9 drives breast cancer endocrine resistance. Proc Natl Acad Sic USA 114:E4482–E4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu F, Li J, Chen H, Fu J, Ray S, Huang S, et al. (2011) Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene 30:2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greco CM, Powell HC, Garrett RS, Lampert PW (1980) Cycloleucine encephalopathy. Neuropathol Appl Neurobiol 6:349–360. [DOI] [PubMed] [Google Scholar]
- 30.Guarino AM, Rozencweig M, Kline I, Penta JS, Venditti JM, Lloyd HH, et al. (1979) Adequacies and inadequacies in assessing murine toxicity data with antineoplastic agents. Cancer Res 39:2204–2210. [PubMed] [Google Scholar]
- 31.Savlov ED, MacIntyre JM, Knight E, Wolter J (1981) Comparison of doxorubicin with cycloleucine in the treatment of sarcomas. Cancer Treat Rep 65:21–27. [PubMed] [Google Scholar]
- 32.Quinlan CL, Kaiser SE, Bolanos B, Nowlin D, Grantner R, Karlicek-Bryant S, et al. (2017) Targeting S-adenosylmethionine biosynthesis with a novel allosteric inhibitor of Mat2A. Nat Chem Biol 13:785–792. [DOI] [PubMed] [Google Scholar]
- 33.Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW (2014) Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 63:3721–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D, Yang SE, Miller BR, Wisinski JA, Sherman DS, Brinkman JA, et al. (2018) Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Liu L, Zhao Y, Zhang J, Wang D, Chen J, et al. (2011) Hypoxia induces genomic DNA demethylation through the activation of HIF-1alpha and transcriptional upregulation of MAT2A in hepatoma cells. Mol Cancer Ther 10:1113–1123. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Li TW, Peng J, Mato JM, Lu SC (2011) Insulin-like growth factor 1 activates methionine adenosyltransferase 2A transcription by multiple pathways in human colon cancer cells. Biochem J 436:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phuong NT, Kim SK, Im JH, Yang JW, Choi MC, Lim SC, et al. (2016) Induction of methionine adenosyltransferase 2A in tamoxifen-resistant breast cancer cells. Oncotarget 7:13902–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Wu K, Zhu Y, He Y, Wu J, Liu Z (2007) Silencing MAT2A gene by RNA interference inhibited cell growth and induced apoptosis in human hepatoma cells. Hepatol Res 37:376–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.