Abstract
Purpose
Ductal Carcinoma in situ (DCIS) is a non-invasive form of breast cancer which could progress to or recur as invasive breast cancer. The underlying molecular mechanism of DCIS progression is yet poorly understood, and appropriate biomarkers to distinguish benign form of DCIS from potentially invasive tumor are urgently needed.
Methods
To identify the key regulators of DCIS progression, we performed gene expression analysis of syngeneic breast cancer cell lines MCF10A, DCIS.com and MCF10CA and cross-referenced the targets with patient cohort data.
Results
We identified ID2 as a critical gene for DCIS initiation and found that ID2 promoted DCIS formation by enhancing cancer stemness of premalignant cells. ID2 also plays a pivotal role in survival of the aggressive cancer cells. In addition, we identified INHBA and GJB2 as key regulators for the transition of benign DCIS to aggressive phenotype. These two genes regulate migration, colonization and stemness of invasive cancer cells. Upregulation of ID2 and GJB2 predicts poor prognosis after breast conserved surgery. Finally, we found a natural compound Helichrysetin as ID2 inhibitor which suppresses DCIS formation in vitro and in vivo.
Conclusion
Our results indicate that ID2 is a key driver of DCIS formation and therefore is considered to be a potential target for prevention of DCIS, while INHBA and GJB2 play vital roles in progression of DCIS to IDC and they may serve as potential prognosis markers.
Keywords: Breast cancer, DCIS, IDC, cancer prevention
Introduction
Ductal carcinoma in situ (DCIS) is a noninvasive form of breast cancer that accounts for 20% to 25% of all newly diagnosed breast cancers in the United States and 17% to 34% of mammography-detected cases [1]. The incidence of DCIS has risen from 5.8 to 32.5 per 100,000 women from 1975 to 2003 primarily due to increased mammography screening [2]. Subsequently, the incidence from 2004 to 2014 has been relatively stable, varying from 32.6 to 37.0 per 100,000 women[2]. DCIS is generally treated by surgical resection of the primary tumor followed by radiotherapy and tamoxifen treatment. Radiation after lumpectomy significantly decreases ipsilateral recurrence rate, whereas tamoxifen treatment significantly decreases both ipsilateral and contralateral recurrence [1, 3]. Unfortunately, disease recurrence develops in 30% of DCIS patients who undergo breast conserving surgery without radiotherapy or tamoxifen treatment, with 20% ipsilateral recurrence and 10% contralateral recurrence[3]. Importantly, 50% of the recurrent DCIS are found to be invasive breast cancer[3].
One important goal of DCIS research is to develop effective measures to prevent DCIS progression and recurrence in high risk populations and thus reduce overall breast cancer incidence. Only a few clinical trials have focused on DCIS prevention in the past decades with limited outcomes [4, 5]. Tamoxifen can reduce ER positive breast cancer but not ER negative breast cancer while raloxifene only works with postmenopausal patients. Aromatase inhibitors and NASID such as aspirin need to be evaluated further [6]. Moreover, recurrence of DCIS could progress from undetected lesions instead of residues or disseminated cells from the original tumor. To address these issues, it is critical to decipher the exact molecular mechanism by which normal epithelial cells progress to DCIS and malignant tumors. Compounds targeting such pathway will potentially prevent DCIS initiation and progression.
In this study, alteration of gene expression during DCIS formation and progression were characterized in two different systems: syngeneic cell lines and patient cohort data consisting of normal, DCIS, and IDC tissue samples. The spontaneously immortalized human breast epithelial cell line MCF10A was established by S.C. Brooks and colleagues in 1990[7]. The F.R. Miller group transfected constitutively activated HRAS into MCF10A cells and established the premalignant MCF10AT cell line[8]. MCF10AT cells are able to form small nodules in nude mice that progress to DCIS or IDC at low rates and after long lag periods in vivo. The DCIS.com cell line, which forms DCIS in vivo, was established after two passages of lesions formed by MCF10AT cells in nude mice[9]. Similarly, the MCF10CA cell line, which forms IDC and metastasizes to distant sites, was established after repeatedly passaging MCF10AT cells in nude mice[10]. Therefore, these syngeneic cell lines, MCF10A, MCF10AT, DCIS.com, and MCF10CA, provide a unique set of tools for investigating the molecular signature of DCIS initiation and progression (Fig.1a).
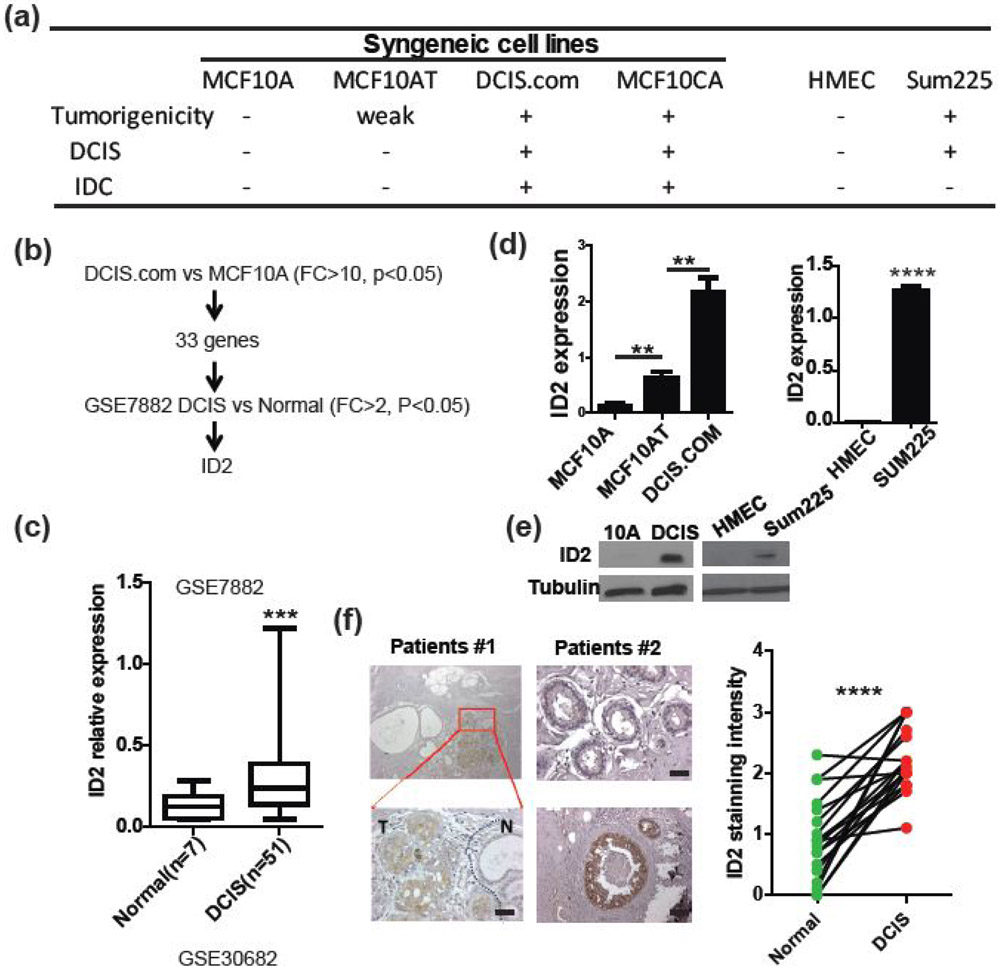
Figure 1. ID2 is upregulated in DCIS.
(a) Summary of cell line used in this study and their tumorigenicity. (b) The expression profiles of syngeneic cell lines, MCF10A and DCIS.com were examined by the Affymetrix expression array. Differentially upregulated genes in human DCIS compared to normal cells were identified in the GSE7882 database, (c) ID2 expression was significantly upregulated in patients with DCIS examined by unpaired t test with Welch’s correction. (d-e) ID2 expression was examined in MCF10A, MCF10AT, DCIS.com, HMEC, and SUM225 cells by (d) qRT-PCR and (e) western blot. (f) ID2 expression was examined in normal tissues and in DCIS from patients with breast cancer by immunohistochemistry using an antibody against ID2. Two representative cases are shown (n = 19). Quantification of immunohistochemistry is shown in right panel. Scale bar, 50μm. **p<0.001.
Methods
Cell culture and reagents
Human breast epithelial or carcinoma cell lines, MCF10A, MCF10AT and MCF10CA1a were purchased from American Type Culture Collection. DCIS.com was purchased from Asterand, Inc . Human mammary epithelial cells (HMEC) cell line was purchased from Lonza. Sum225 was obtained from Dr. Fariba Behbod in university of Kansas medical center. MCF10AT, DCIS.com and MCF10CA1 were cultured in RPMI medium supplemented with 10% FBS, streptomycin (100 mg/ml) and penicillin (100 units/ml). MCF10A and HMEC were cultured with human mammary epithelial growth medium (Lonza). Sum225 was cultured in DMEM/F12 medium supplemented with 5%FBS, streptomycin (100 mg/ml), penicillin (100 units/ml), insulin (5ug/ml), Hydrocortisone ( 1ug/ml) and HEPES( 10mM). All cells were grown at 37 °C in a 5% CO2 atmosphere.
Plasmids and reagents
Lentiviral vectors expressing shRNA for ID2, GJB2, and INHBA were obtained from Dharmacon (shID2-# RHS4533-EG3398, shGJB2-# RHS4533-EG2706 and shINHBA-# RHS4533-EG3624). The plasmid expressing ID2 was purchased from Origene. A mixture of 3 or 4 individual shRNA was used for each gene.
Western blot
Western blot analysis was performed as described previously (34), using antibodies against ID2 (1/200; Abcam), SOX2 (1/1000, Cell Signaling Technology), Tubulin (1/1000, Cell Signaling Technology) and GAPDH (1/5,000; Cell Signaling Technology).
Quantitative real-time PCR
Total RNAs were isolated from cells and reverse transcribed as described previously [11]. The cDNA was then amplified with a pair of forward and reverse primers for the following genes:
ID2-F 5’ TCAGCACTTAAAAGATTCCGTG3’
ID2-R 5’ GACAGCAAAGCACTGTGTGG3’
INHBA-F 5’ GGAGTGTGATGGCAAGGTCA3’
INHBA-R 5’ ACATGGGTCTCAGCTTGGTG3’
GJB2-F 5’ CGGTTAAAAGGCGCCACGG3’
GJB2-R 5’ ACGGTGAGCCAGATCTTTCC3’
SOX2-F 5’ GGGAAATGGGAGGGGTGCAAAAGAGG3’
SOX2-R 5’ TTGCGTGAGTGTGGATGGGATTGGTG3’
PCR array
The PCR array analysis was performed by following manufacture’s manual (Qiagen# PAHS-176Z). The PCR array contains 84 cancer stem cell related genes and 5 housekeeping genes. Briefly, cDNA was prepared by RT2 first strand kit( Qiagen# 330401) and subjected to PCR array analysis. Fold changes were calculated by following manufacture’s manual (www.SABiosciences.com/pcrarraydataanalysis.php).
Clinical samples
Human serum samples were obtained from surgical pathology archives of the, Wake Forest Baptist Comprehensive Cancer Center (WFBCCC). Human DCIS and breast cancer samples were obtained from Cooperative Human Tissue Network (CHTN) and Tumor Tissue and Pathology Shared Resource at WFBCCC. All tissue sections were obtained by surgical resection.
Immunohistochemistry
Paraffin fixed tissues were cleared with xylene and ethanol, washed in water and incubated in 2.5uM sodium citrate in 95 °C for 30min. After cooling down, the sections were then washed with PBS and blocked with 2% BSA for 1 hour and incubated with anti-ID2 (1/500: Abcam) antibody for 12 hrs at 4 °C. Samples were then incubated with anti-rabbit IgG (Dako) for 1hr at room temperature and stained with chromogen (Dako).
Mammosphere assay
1000 cells were plated in ultra-low attachment plate (Corning, 3473) in mammosphere forming medium containing DMEM/F12 supplemented with 2% B27 (Invitrogen), 20ng/ml EGF and 4μg/ml Insulin (Sigma). The numbers of mammospheres were counted and images were taken at day 14.
3D colony formation assay
1000 cells were seeded on top of 5% soft agar and cultured in medium containing 0.2% soft agar. Colonies were counted after 1 week.
Induced-Fit docking
All the compounds were studied by Induced-Fit Docking (IFD) studies in Maestro (Schrodinger LLE) and their binding modes were analyzed. Compounds which block the hydrogen bonding interactions between Leu49β-Gln76α and Gln76α-Tyr 71β act could prevent dimer formation.
Gene-expression microarray profiling
RNAs were extracted, labeled and hybridized to Human Gene 1.0 ST array (Affymetrix) using the manufacturer’s protocol. Normalization of the data was performed using the RMA algorithm.
Animal experiments
All animal experiments were done in accordance with a protocol approved by the Wake Forest Institutional Animal Care and Use Committee. For mammary fat pad injection, 106 cells were injected to the fourth mammary fat pad of nude mouse. Mice were imaged by IVIS twice a week after injection.
Statistical analysis
All analysis was calculated by GraphPad Prism. Data are presented as mean+ s.d. The p-value is calculated by an unpaired Student’s t-test without special address. Significance between each groups were represented as *p<0.05, **p<0.01.
Results
ID2 is upregulated in DCIS
To study the molecular mechanisms of DCIS initiation and progression, we performed comprehensive mRNA expression profiling of MCF10A, DCIS.com, and MCF10CA1a (MCF10CA) cell lines. First, we focused on genes that mediate DCIS initiation. We found 33 genes were upregulated in DCIS.com compared to MCF10A cells (fold change (FC)>10, p<0.05, FDR<0.25). Of those genes, nine also showed increased expression levels in MCF10CA cells (FC>1, p<0.05,) and 24 showed decreased expression levels in MCF10CA cells compared to DCIS.com cells (FC<1, p<0.05). To further examine the expression of these genes in patients, a clinical cohort (GSE7882) that included expression profiles of 51 DCIS patients and 7 healthy donors contributed by Davis SR et al. was used because they conducted their experiments using microdissected samples to minimize stromal component[12]. Only inhibitor of DNA binding 2 (ID2) gene was found to be significantly upregulated (FC>2, P<0.05, FDR<0.01) in DCIS patients compared to normal donors (Fig. 1b, c). We found a very consistent low expression of ID2 in seven healthy breast tissue regardless of low sample number. ID2 is a well-known stem cell factor, which binds to DNA and regulates transcription of target genes[13]. However, the role of ID2 in breast cancer has not been well understood and apparent conflicting reports exist [14-16]. Moreover, the role of ID2 in DCIS progression is virtually unknown. As our screening results showed that ID2 was upregulated in DCIS compared to normal, we first confirmed the expression of ID2 in MCF10A, MCF10AT and DCIS.com cell lines by qRT-PCR and western blot (Fig. 1d, e). We also found that ID2 expression was upregulated in another DCIS cell line, Sum225, compared to HMEC (Fig. 1d, e). In addition, ID2 expression was significantly elevated in human DCIS compared to paired normal gland when examined by immunohistochemistry (Fig. 1f). These results suggest that ID2 could play a role in the initiation of DCIS.
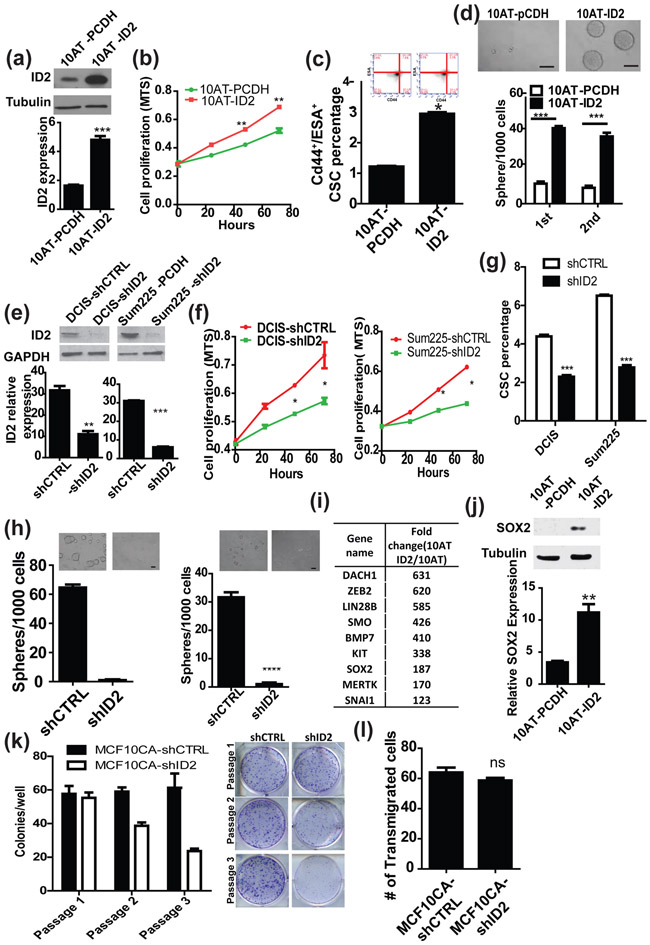
Figure 2. ID2 promotes stemness and proliferation of pre-malignant cells in vitro.
(a) Ectopic expression of ID2 in MCF10AT cells was confirmed by western blot (upper panel) and qRT-PCR (lower panel). (b) Cell proliferation was examined by MTS assay in MCF10AT and MCF10AT-ID2 cells. (c) The population of tumor initiating cells in both MCF10AT and MCF10AT-ID2 cell lines were examined by FACS using the stem cell markers, CD24low/CD44high/ESAhigh. (d) Mammosphere formation assay of MCF10AT and MCF10AT-ID2 cells. The number of mammospheres was counted under a microscope. The test was performed for two cycles of the culture. Scale bar, 100μm (e) ID2 expression was knocked down by shRNA in DCIS.com and Sum225 cells and was confirmed by qRT-PCR and western blot. (f) Cell proliferation was examined by MTS assay in DCIS-shCTRL, DCIS-shID2, Sum225-shCTRL and Sum225-shID2 cells. (g) The population of tumor initiating cells in DCIS-shCTRL, DCIS-shID2, Sum225-shCTRL and Sum225-shID2 cells cell lines were examined by FACS using the stem cell markers, CD24low/CD44high/ESAhigh. (h) Mammosphere formation assay of DCIS-shCTRL, DCIS-shID2, Sum225-shCTRL and Sum225-shID2. The number of mammospheres was counted under a microscope. Scale bar, 100μm (i) PCR array results MCF10AT-pCDH and MCF10AT-ID2 cells. (j) SOX2 expression in MCF10AT-pCDH and MCF10AT-ID2 cells was examined by qRT-PCR and western blot. (k) MCF10CA-shCTRL and MCF10CA-shID2 cells were seeded at 500 cells per well in a 6-well plate. Colonies were counted after 72 hours. (l) MCF10CA-shCTRL and MCF10CA-shID2 cells were seeded at 1,000 cells per well in invasion chamber. Cells that invaded into matrix-gel were counted under microscope after 24 hours.
ID2 promotes proliferation and stemness of pre-malignant cells in vitro
To investigate the roles of ID2 in DCIS formation, we overexpressed ID2 in MCF10A cells followed by injection of the established cell line into nude mice. However, MCF10A-ID2 cells failed to generate tumors (data not shown), suggesting that ID2 expression alone may not be enough to drive tumor initiation and that ID2 may need co-operation with other oncogenes to accelerate tumor progression. Therefore, we ectopically expressed ID2 in MCF10AT cells which derived from MCF10A by introducing constitutively activated RAS, and validated ID2 expression by qRT-PCR and western blot (Fig. 2a). The ectopic expression of ID2 significantly promoted cell proliferation of the pre-malignant MCF10AT cells (Fig. 2b). As ID2 is considered a stem cell factor, we also examined the effect of ID2 on the cancer stem cell population and self-renewal ability of MCF10AT cells by FACS analysis and mammosphere forming assay, respectively. We found that the ectopic expression of ID2 significantly increased the CD24low/CD44high/ESAhigh cancer stem cell population and the number of spheres compared to control groups (Fig. 2c, d). In addition, we knocked-down the ID2 genes in DCIS.com and Sum225 (Fig. 2e), and examined their effects on cancer stem cells. As shown in Figure 2f–h knockdown of ID2 significantly decreased cell proliferation, cancer stem cell population, and mammosphere formation of DCIS.com cells. Because ID2 is considered to be one of the major regulators of dedifferentiation [17, 18], we performed a PCR array analysis to identify genes related to cancer stemness that are regulated by ID2. We found that several stem cell factors, including SOX2, SMO, and ZEB2, were upregulated in ID2 overexpressing cells (Fig.2i)[19]. We further confirmed SOX2 upregulation by qRT-PCR and western blot (Fig. 2j).
Because DCIS is considered as the precursors of IDC, ID2 may also mediate progression of DCIS to IDC. Therefore, we knocked down ID2 in MCF10CA cells and found that silencing ID2 decreased plating efficiency of MCF10CA cells but not their invasive ability (Fig2, k, l). These results strongly suggest that upregulation of ID2 promotes the initiation of DCIS through expansion of the cancer stem cell population and that ID2 also required for cell proliferation of IDC.
ID2 promotes DCIS formation in vivo
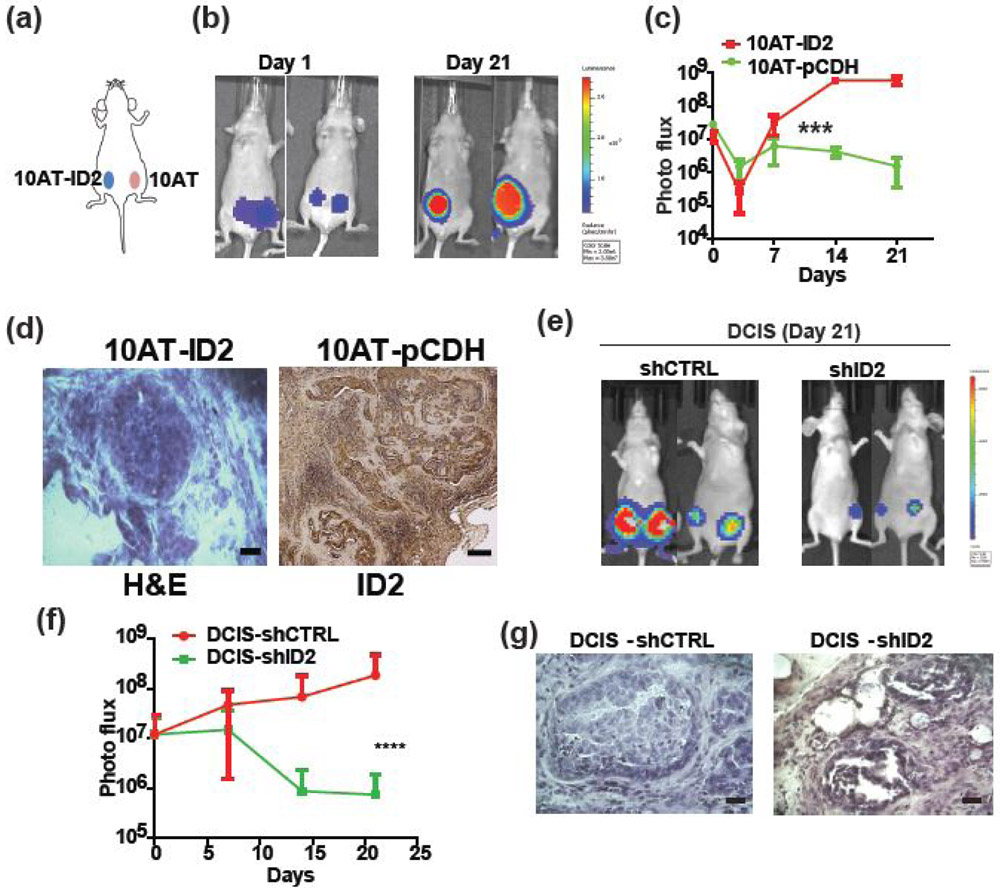
To examine whether ID2 drives DCIS formation in vivo, we implanted MCF10AT and MCF10AT-ID2 cells into the mammary fat pad of nude mice (Fig. 3a). The MCF10AT cells failed to establish tumors in 3 weeks, whereas the MCF10AT-ID2 cells formed tumors efficiently with 100% incidence (Fig. 3b, c). H&E staining of tumors derived from MCF10A-ID2 revealed that tumors cells were confined within the basement membrane and showed typical pathological features of DCIS (Fig. 3d left). In addition, we found that ID2 was indeed highly expressed in rare tumor tissue formed by 10AT cells, indicating ID2 is required for the tumor initiation. (Fig. 3d right). In contrast, knockdown of ID2 in DCIS.com cells significantly suppressed overall tumor growth (Fig. 3e-g). Moreover, 4 out of 10 injected fat pads failed to form tumors even after 8 weeks. These results indicate that ID2 is a crucial factor in DCIS formation in vivo.
Figure 3. ID2 promotes DCIS formation in vivo.
(a) MCF10AT-ID2 and MCF10AT-pCDH (control) cells were implanted in the same mouse into the left and right side of the mammary fat pad, respectively (n = 10, left panel). (b) Tumor growth was monitored with the IVIS imaging system for up to 3 weeks. Representative IVIS images on day 1 and day 21 are shown in the right panels. The growth kinetics of tumor are shown in (c). (d) The tumors generated by MCF10AT-ID2 cells in nude mice were sectioned and H&E stained (left panel). Tumor formed by MCF10AT showed strong ID2 expression. Scale bar, 100μm. (e) DCIS-shCTRL and DCIS-shID2 cells were implanted into the mammary fat pad and monitored with the IVIS imaging system for 3 weeks. Representative images of tumor signal at day 21. (f) Growth kinetic of tumors. (g) The tumors generated by DCIS-shCTRL and DCIS-shID2 cells in nude mice were sectioned and H&E stained. Scale bar, 100μm.
Aggressive genes are upregulated in invasive breast cancer
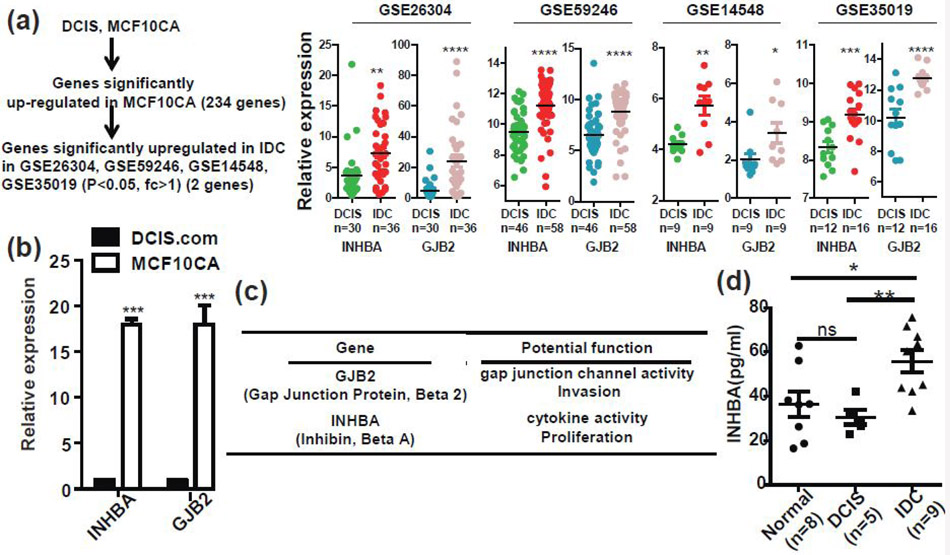
Although we have shown that ID2 plays a critical role in DCIS, our results suggest that other genes are involved in the progression of ID2-mediated DCIS to IDC. To elucidate the molecular mechanisms that promote the progression of DCIS to IDC, we compared the DCIS.com and MCF10CA for mRNA expression profiles and found that 234 genes were upregulated in MCF10CA cells (Fig. 4a). We cross-verified expression of these genes in four clinical cohorts, GSE26304, GSE59246, GSE14548, and GSE35019 individually and found that only gap junction protein beta 2 (GJB2) and inhibin beta A subunit (INHBA) were significantly upregulated in invasive breast cancer patients compared to DCIS patients in all four cohorts (Fig. 4a). We confirmed the expression of GJB2 and INHBA in DCIS.com and MCF10CA cells by qRT-PCR (Fig. 4b). GJB2 and INHBA are known key regulators of cancer progression (Fig. 4c)[20-22]. INHBA encodes the α chain of inhibin B, a hormone that is detectable in serum[23]. Therefore, we examined the level of INHBA in serum from healthy donors, DCIS patients, and invasive breast cancer patients. As shown in Figure 4d, INHBA levels in invasive breast cancer patients were found to be significantly higher than healthy donors and DCIS patients, suggesting that INHBA may serve as a noninvasive biomarker for detection of IDC.
Figure 4. GJB2 and INHBA are upregulated in invasive breast cancer.
(a) The expression profiles of syngeneic cell lines, DCIS.com and MCF10CA, were examined by the Affymetrix microarray. Two hundred and thirty-four genes were significantly upregulated in MCF10CA cells (p<0.05). The expression status of these genes in human breast DCIS and invasive carcinoma were examined using the GSE26304, GSE59246, GSE14548, and GSE35019 cohorts. Among the 234 genes, two genes (GJB2 and INHBA) were significantly upregulated in patients with invasive ductal carcinoma. (b) The expression of INHBA and GJB2 were examined in DCIS.com and MCF10CA cells by qRT-PCR. (c) Summary of the functions of INHBA and GJB2. (d) Serum from normal subjects (n = 8), patients with DCIS (n = 5), and patients with invasive cancer (n = 9) was examined for the level of INHBA protein using ELISA assay kits.
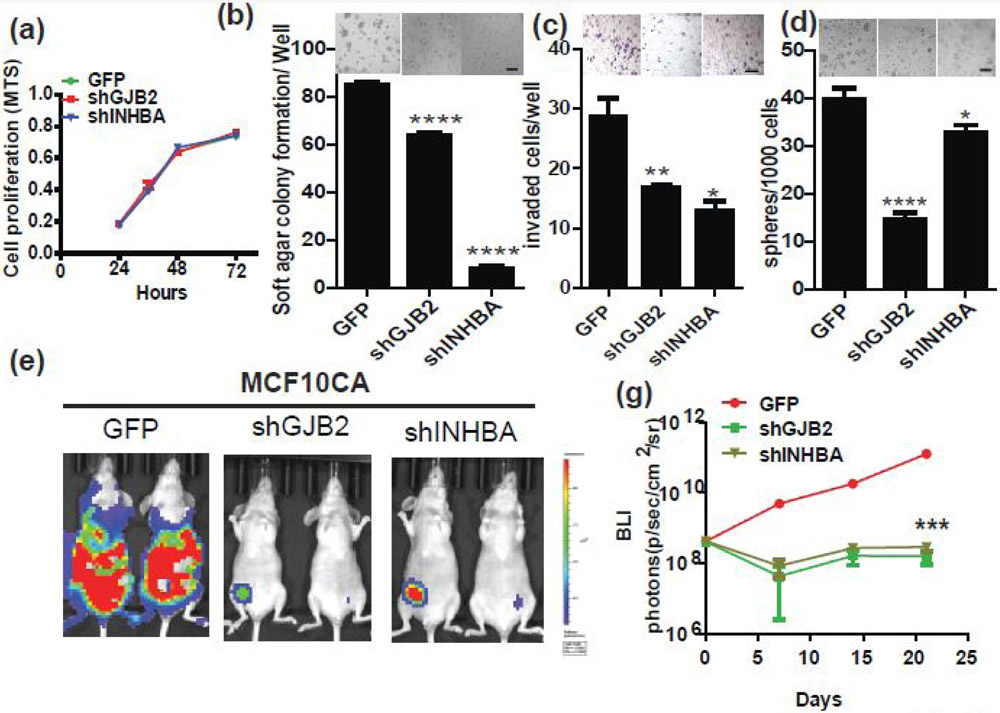
To understand the function of INHBA and GJB2 genes in early stage breast cancer progression, we knocked each gene down separately in MCF10CA cells by shRNA and examined the cancer-associated phenotypes, including proliferation, 3D growth, invasion, anoikis resistance, and self-renewal. As shown in Figure 5a-d, we found that knockdown of INHBA and GJB2 significantly suppressed 3D growth, invasion, and mammosphere formation abilities. Consistent with previously finding that GJB2 regulates self-renew ability, knockdown of GJB2 in MCF10CA cells dramatically decreased number of spheres. Of note, neither of these genes affected 2D growth (Fig. 5a). We also examined in vivo growth of these cell lines by transplanting them into mammary fat pads of nude mice (Fig. 5e,f) and found that knockdown of either gene significantly suppressed in vivo growth of MCF10CA cells. Collectively, these results suggest that INHBA and GJB2 are important aggressive driver genes of ID2-mediated DCIS into IDC.
Figure 5. GJB2 and INHBA are crucial for aggressiveness of IDC.
(a-d) shRNAs to GJB2 or INHBA were introduced by lentivirus into MCF10CA cells and the effects of knockdown on (a) cell proliferation, (b) 3D soft agar colony formation, (c) matrigel invasion, and (d) mammosphere formation were examined. (e) MCF10CA-GFP (control), MCF10CA-shGJB2 and MCF10CA-shINHBA cells were implanted into the mouse mammary fat pad and the tumor growth was monitored by the IVIS imaging system for 21 days. Representative images are shown. (f) Growth kinetics of the tumors.
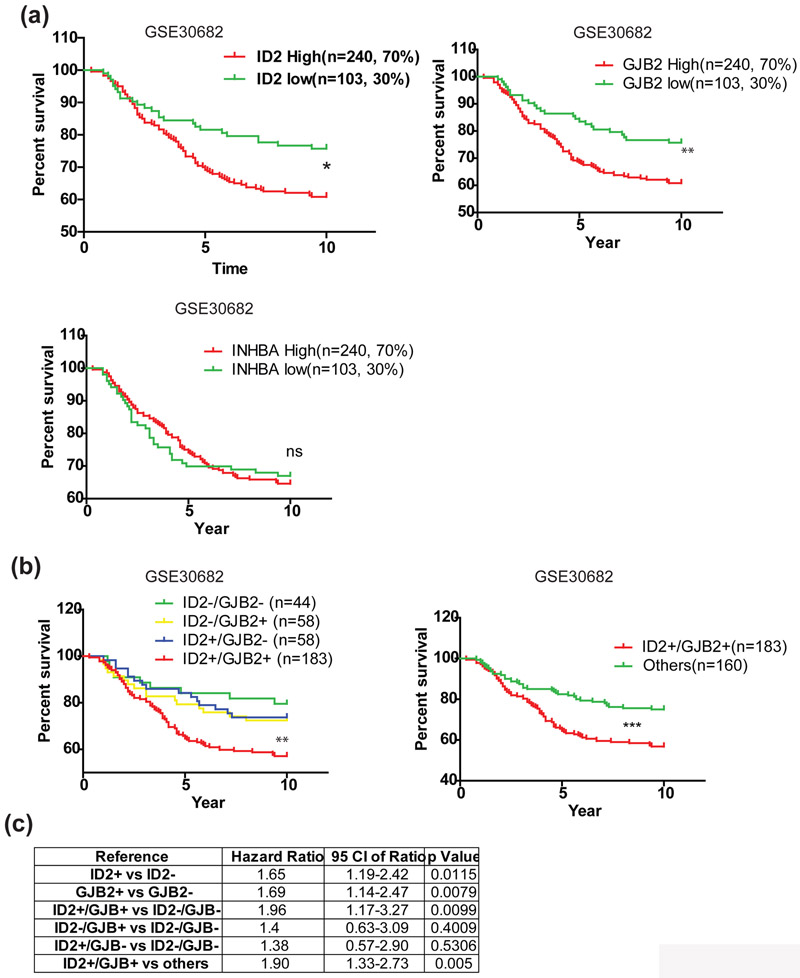
ID2 and GJB2 expression predicts poor prognosis
Pre-malignant regulators are potential prognostic markers. Therefore, we examined prognostic value of ID2, INHBA and GJB2 in a clinical cohort of 343 early stage breast cancer patients of which 64% are DCIS (GSE30682). We found that patients with high expression of ID2 and GJB2 but not INHBA were more likely to have had recurrence after breast conserved surgery (Fig. 6a). A recent study shows that GJB2, also known as CX26, drives self-renewal in triple negative breast cancer via NANOG and focal adhesion kinase (FAK) [24]. As ID2 and GJB2 both regulate stemness, patients with high expression of ID2 and GJB2 are more likely to harbor a high population of cancer stem cell and may have a higher chance of recurrence and poor prognosis. Indeed, combination of ID2 and GJB2 expression showed a higher predictive value of patient outcome than either ID2 or GJB2 expression alone (Fig. 6b, c). These results suggest that a patient with high expression ID2 and GJB2 has high risk of recurrence and those patients are candidates for preventive therapy after conserved surgery.
Figure 6. High expression of ID2 and GJB2 indicates poor prognosis.
(a) Local recurrence-free survival of early stage breast cancer patients after surgery. Patients were divided into groups based on their ID2, GJB2 or INHBA expression respectively. Patients with high and medium expression were considered as positive expression group and patients with low expression were considered as negative group. * p<0.05, log-rank (Mantel-Cox) test. (b) Local recurrence-free survival of early stage breast cancer patients after surgery. Patients were divided into four (left panel) or two groups (right panel) based on their ID2 and GJB2 expression. ** p<0.01, log-rank (Mantel-Cox) test. (c) Summary of hazard ratio and p value.
Helichrysetin inhibits DCIS formation
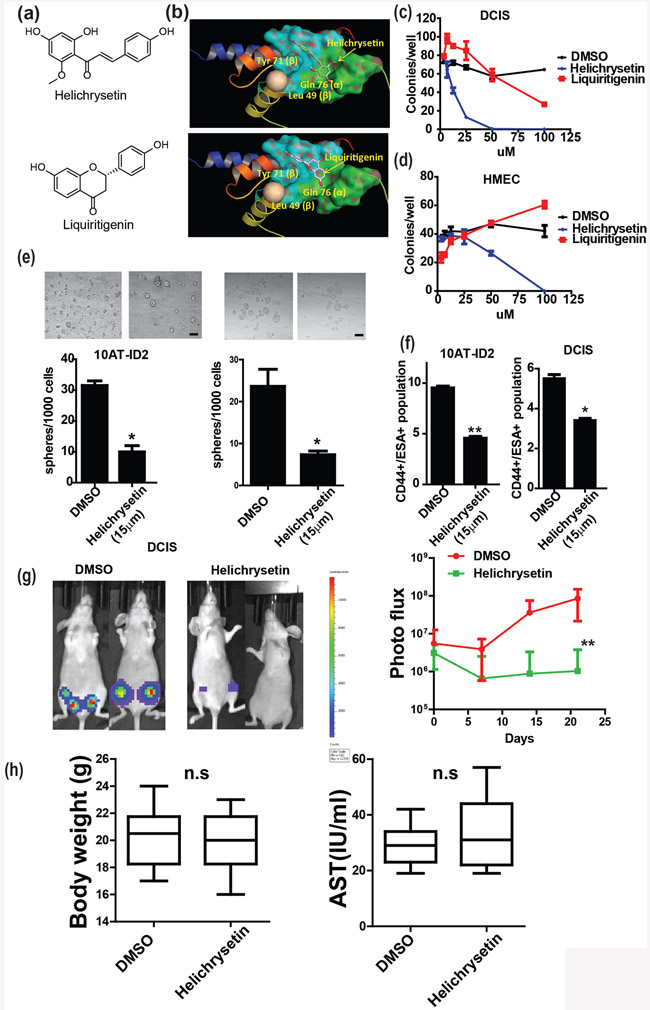
We have demonstrated that ID2 is a key initiating factor of DCIS and promotes the self-renewal of cancer stem cells, which is linked to poor prognosis. Interestingly, ID2 depletion in an inducible whole-body ID2 knockout mouse is very well tolerated[25]. Therefore, ID2 is an excellent target for prevention of early stage breast cancer among high risk populations. To identify a selective drug for ID2, we conducted an in silico screening of 650 natural products that were available at the National Center for Natural Product Research at the University of Mississippi. The crystal structure of ID2 was previously determined [26]; therefore, we performed a virtual docking screening and identified two potential compounds that target ID2, Helichrysetin and Liquiritigenin (Fig. 7a). Our modeling indicated that these compounds dock in the pocket of ID2 at key residues (Leu 49, Tyr 71 of the β-unit, and Gln 76 of the α-unit) that are involved in the dimerization of ID2 (Fig. 7b). We examined the effect of these compounds in DCIS.com cells and HMEC cells and found that only Helichrysetin significantly decreased colony forming ability in DCIS.com cells but not HMEC cells (Fig. 7c, d). Because ID2 promotes cancer stemness, we also evaluated the efficacy of Helichrysetin on the self-renewal ability of cancer stem cells and found that Helichrysetin significantly suppressed mammosphere formation and decreased the population of cancer stem cells in both DCIS.com and MCF10AT-ID2 cells (Fig. 7e, f). Moreover, we found that Helichrysetin significantly suppressed DCIS growth in vivo with no significant side effects (Fig. 7g,h).
Figure 7. Helichrysetin suppresses DCIS by targeting ID2.
(a)To identify selective inhibitors of ID2, we performed an in silico screen of 650 natural compounds using a computational virtual docking program and identified two compounds, Helichrysetin and Liquiritigenin. The chemical structures of these compounds are shown. (b) 3D docking structures of the ID2 protein with Helichrysetin or Liquiritigenin are shown. (c) DCIS.com cells were treated with DMSO, Helichrysetin, or Liquiritigenin at 0, 5, 15, 25, 50 and 100 μM for 48 hours and plated for colony formation assays. Colonies were counted after 7 days of culture. (d) HMEC cells were treated with DMSO, Helichrysetin, or Liquiritigenin at 0, 5, 15, 25, 50 and 100 μM for 48 hours and plated for colony formation assays. Colonies were counted after 7 days of culture. (e) Number of spheres was counted after 7 days. Scale bar, 100μm. (f) The cancer stem cell population was examined by FACS using the stem cell markers, CD24low/CD44high/ESAhigh. (g) DCIS.com cells were injected into the mouse mammary fat pad. DMSO or Helichrysetin (10 mg/kg) were administered to tumor bearing mice by intraperitoneal injection every 3 days. Representative images and growth kinetics are shown. (h) body weight of control and treatment groups were examined (left panel). AST levels were examined in the serum of control and treatment groups at the end point (right panel).
Discussion
We have shown that ID2 regulates initiation and progression of DCIS and that high expression of ID2 indicates poor survive in early stage breast cancer. There are a few of research focus on role of ID2 in invasive breast cancer. Several groups reported that high ID2 level in breast cancer was correlated with non-invasiveness and a favorable prognosis [14, 15] [27]. On the other hand, Kai L. et al. reported that ID2 predicted poor prognosis in breast cancer and inhibited E-cadherin expression [16]. However, these conclusions were not supported by paired clinical samples, statistical analysis or in vivo experiments. In our study, we focused on ID2 expression in DCIS compared to normal and we found that ID2 expression was significantly upregulated in DCIS compared to adjacent normal. We also found that ID2 regulates tumor growth in vivo. These results strongly support that ID2 indeed drives early stage breast cancer progression.
ID2 is known to be upregulated in cancer through multiple mechanisms [28-30]. In this study, we found that the RNA level of ID2 was highly upregulated, suggesting transcriptional or mRNA level regulation of ID2 expression. It is known that ID2 can be upregulated by transcription factors, such as HIF1 and TWIST, and proteins, such as BMP7 and TGF-β [31-33]. Interestingly, these factors are often upregulated in DCIS and IDC and may lead to upregulation of ID2 in breast cancer.
We found that two genes, GJB2 and INHBA, are upregulated in breast cancer, and overexpression of these genes contributes to an aggressive phenotype. However, with the exception of the functional assays reported here, the roles of INHBA and GJB2 in breast cancer progression are not well studied. GJB2 and INHBA previously have been shown as one of the prognosis markers of the survival in pancreatic and colorectal cancer respectively [22] [34]. Several studies have identified the association of INHBA expression with invasion or poor survival in cancer [22, 35, 36]. In this study, we not only found that INHBA level was associated with cancer aggressiveness, but we also found that serum INHBA level may be a potential diagnostic marker of invasive breast cancer. In 88% of invasive breast cancer patients, the INHBA serum level was higher than 40 ng/ml. However, only 23% of healthy donors or DCIS patients had serum INHBA levels higher than 40 ng/ml. Inhibin A and inhibin B levels have been used as clinical tumor diagnostic markers for ovarian cancer[37, 38]. Our results suggest that INHBA may be a promising diagnostic marker for breast cancer progression. However, inhibin level is strongly affected by menstrual cycle [38, 39], and, therefore, large scale studies with known menstrual cycle status are needed to clarify the predictive value of serum INHBA level in breast cancer.
We also found that the cell adhesion protein GJB2 is upregulated in IDC compared to DCIS. Even though recent study has addressed the role of GJB2 on cancer stemness in breast cancer, our result suggests that GJB2 regulates a broad spectrum of biological process besides cancer stemness. It is noteworthy that a recent study has shown that metastatic cancer cells communicate with stroma cells through gap junctions [40] in which GJB2 plays an important role. This finding suggests that upregulation of GJB2 may enable cancer cells to communicate with stromal cells and aid cancer cell survival in the new microenvironment. Additionally, enhanced gap junction communication between cancer cells could also be mediated by GJB2 to augment cancer invasion. Cancer cells are known to invade as a group, termed collective migration, rather than as a single cell[41]. Gap junction communication established by GJB2 may enable multicellular organization and collective migration.
Our results also suggest that ID2 is not a driver of tumor cell invasion, which is consistent with previously study [14, 15]. However, the knockdown of ID2 by shRNA led to growth retardation in aggressive breast cancer cells. Furthermore, treatment with Helichrysetin to target ID2 in a xenograft model significantly suppressed or delayed breast cancer development. These findings suggest that targeting ID2-positive cells could prevent DCIS progression. Collectively, our results data suggest that suppression of ID2 by Helichrysetin is a feasible option for prevention of DCIS considering the minimum toxicity of the compound. Delivering drugs specifically to the tumor could further increase the specificity and efficacy of treatment.
Conclusion
We have identified ID2 as a key factor of DCIS initiation and GJB2 and INHBA as aggressive factors during progression of ID2-mediated DCIS to IDC. ID2 is capable of promoting the cancer stem cell population in pre-malignant cells and leads to DCIS formation. Concomitant upregulation of GJB2 further enhanced the stem cell population. High expression of ID2 and GJB2 indicates poor prognosis after conserved surgery in early stage breast cancer.
Acknowledgements
We thank Dr. Fariba Behbod for the Sum225 cell line.
Funding: This study was funded by This work was supported by NIH grant R01CA173499, R01CA185650 and R01CA205067 to K.W, a pilot grant from Comprehensive Cancer Center of Wake Forest University to F.X. Y.L is supported by NIH fellowship F31CA200286. The Tumor Tissue and Pathology Shared Resources, and Biostatistics/Bioinformatics Shared Resource are supported by the Comprehensive Cancer Center of Wake Forest University NCI, National Institutes of Health Grant (P30CA012197).
List of abbreviations
- DCIS
Ductal carcinoma in situ
- IDC
Invasive ductal carcinoma
- CI
Confidence interval
- ID2
Inhibitor of DNA binding 2
- INHBA
inhibin beta A subunit
- GJB2
Gap junction protein beta 2
- SOX2
SRY-Box 2
- ZEB
Zinc Finger E-Box Binding Homeobox
- BMP7
Bone Morphogenetic Protein 7
- TGF-β
Transforming Growth Factor Beta
- TWIST
Twist Family BHLH Transcription Factor
- TCGA
The cancer genome atlas
Footnotes
Conflict of Interest: all of the authors declare no conflict of interest.
Ethical approval : All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants by any of the authors.
Reference
- 1.Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, Land SR, Margolese RG, Swain SM, Costantino JP et al. : Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 2011, 1036:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N NA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA SEER Cancer Statistics Review, 1975-2014, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 3.Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, Forbes JF, Bishop H, Fentiman IS, George WD: Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 2011, 121:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J et al. : Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998, 9018:1371–1388. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, Boyle P: Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003, 3619354:296–300. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ et al. : Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 2009, 105:501–507. [DOI] [PubMed] [Google Scholar]
- 7.Soule HD, Maloney TM, Wolman SR, Peterson WD Jr., Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC: Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res 1990, 5018:6075–6086. [PubMed] [Google Scholar]
- 8.Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR: MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol 1996, 1481:313–319. [PMC free article] [PubMed] [Google Scholar]
- 9.Miller FR, Santner SJ, Tait L, Dawson PJ: MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 2000, 9214:1185–1186. [DOI] [PubMed] [Google Scholar]
- 10.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, Wolman SR, Heppner GH, Miller FR: Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat 2001, 652:101–110. [DOI] [PubMed] [Google Scholar]
- 11.Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, Saito K, Commes T, Hayashi S, Watabe M et al. : The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res 2003, 638:1731–1736. [PubMed] [Google Scholar]
- 12.Balleine RL, Webster LR, Davis S, Salisbury EL, Palazzo JP, Schwartz GF, Cornfield DB, Walker RL, Byth K, Clarke CL et al. : Molecular grading of ductal carcinoma in situ of the breast. Clin Cancer Res 2008, 1424:8244–8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perk J, Iavarone A, Benezra R: Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer 2005, 58:603–614. [DOI] [PubMed] [Google Scholar]
- 14.Stighall M, Manetopoulos C, Axelson H, Landberg G: High ID2 protein expression correlates with a favourable prognosis in patients with primary breast cancer and reduces cellular invasiveness of breast cancer cells. Int J Cancer 2005, 1153:403–411. [DOI] [PubMed] [Google Scholar]
- 15.Itahana Y, Singh J, Sumida T, Coppe JP, Parrinello S, Bennington JL, Desprez PY: Role of Id-2 in the maintenance of a differentiated and noninvasive phenotype in breast cancer cells. Cancer research 2003, 6321:7098–7105. [PubMed] [Google Scholar]
- 16.Li K, Yao L, Chen L, Cao ZG, Yu SJ, Kuang XY, Hu X, Shao ZM: ID2 predicts poor prognosis in breast cancer, especially in triple-negative breast cancer, and inhibits E-cadherin expression. Onco Targets Ther 2014, 7:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker-Herman S, Lantner F, Shachar I: Id2 negatively regulates B cell differentiation in the spleen. J Immunol 2002, 16811:5507–5513. [DOI] [PubMed] [Google Scholar]
- 18.Damdinsuren B, Nagano H, Kondo M, Yamamoto H, Hiraoka N, Yamamoto T, Marubashi S, Miyamoto A, Umeshita K, Dono K et al. : Expression of Id proteins in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Int J Oncol 2005, 262:319–327. [PubMed] [Google Scholar]
- 19.Reya T, Morrison SJ, Clarke MF, Weissman IL: Stem cells, cancer, and cancer stem cells. Nature 2001, 4146859:105–111. [DOI] [PubMed] [Google Scholar]
- 20.Castellana B, Escuin D, Peiro G, Garcia-Valdecasas B, Vazquez T, Pons C, Perez-Olabarria M, Barnadas A, Lerma E: ASPN and GJB2 Are Implicated in the Mechanisms of Invasion of Ductal Breast Carcinomas. J Cancer 2012, 3:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezumi K, Yamamoto H, Murata K, Higashiyama M, Damdinsuren B, Nakamura Y, Kyo N, Okami J, Ngan CY, Takemasa I et al. : Aberrant expression of connexin 26 is associated with lung metastasis of colorectal cancer. Clin Cancer Res 2008, 143:677–684. [DOI] [PubMed] [Google Scholar]
- 22.Okano M, Yamamoto H, Ohkuma H, Kano Y, Kim H, Nishikawa S, Konno M, Kawamoto K, Haraguchi N, Takemasa I et al. : Significance of INHBA expression in human colorectal cancer. Oncol Rep 2013, 306:2903–2908. [DOI] [PubMed] [Google Scholar]
- 23.Burger HG, Igarashi M: Inhibin: definition and nomenclature, including related substances. Endocrinology 1988, 1224:1701–1702. [DOI] [PubMed] [Google Scholar]
- 24.Thiagarajan PS, Sinyuk M, Turaga SM, Mulkearns-Hubert EE, Hale JS, Rao V, Demelash A, Saygin C, China A, Alban TJ et al. : Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nat Commun 2018, 91:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawlins EL, Clark CP, Xue Y, Hogan BL: The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 2009, 13622:3741–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong MV, Jiang S, Palasingam P, Kolatkar PR: A divalent ion is crucial in the structure and dominant-negative function of ID proteins, a class of helix-loop-helix transcription regulators. PLoS ONE 2012, 710:e48591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen XF, Chen M, Wu Y, Chen MN, Glogowska A, Klonisch T, Zhang GJ: Inhibitor of DNA Binding 2 Inhibits Epithelial-Mesenchymal Transition via Up-Regulation of Notch3 in Breast Cancer. Transl Oncol 2018, 115:1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleeff J, Ishiwata T, Friess H, Buchler MW, Israel MA, Korc M: The helix-loop-helix protein Id2 is overexpressed in human pancreatic cancer. Cancer Res 1998, 5817:3769–3772. [PubMed] [Google Scholar]
- 29.Lasorella A, Boldrini R, Dominici C, Donfrancesco A, Yokota Y, Inserra A, Iavarone A: Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res 2002, 621:301–306. [PubMed] [Google Scholar]
- 30.Coma S, Amin DN, Shimizu A, Lasorella A, Iavarone A, Klagsbrun M: Id2 promotes tumor cell migration and invasion through transcriptional repression of semaphorin 3F. Cancer Res 2010, 709:3823–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lofstedt T, Jogi A, Sigvardsson M, Gradin K, Poellinger L, Pahlman S, Axelson H: Induction of ID2 expression by hypoxia-inducible factor-1: a role in dedifferentiation of hypoxic neuroblastoma cells. J Biol Chem 2004, 27938:39223–39231. [DOI] [PubMed] [Google Scholar]
- 32.Isenmann S, Arthur A, Zannettino AC, Turner JL, Shi S, Glackin CA, Gronthos S: TWIST family of basic helix-loop-helix transcription factors mediate human mesenchymal stem cell growth and commitment. Stem Cells 2009, 2710:2457–2468. [DOI] [PubMed] [Google Scholar]
- 33.Izumi N, Mizuguchi S, Inagaki Y, Saika S, Kawada N, Nakajima Y, Inoue K, Suehiro S, Friedman SL, Ikeda K: BMP-7 opposes TGF-beta1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol Physiol 2006, 2901:L120–126. [DOI] [PubMed] [Google Scholar]
- 34.Zhu T, Gao YF, Chen YX, Wang ZB, Yin JY, Mao XY, Li X, Zhang W, Zhou HH, Liu ZQ: Genome-scale analysis identifies GJB2 and ERO1LB as prognosis markers in patients with pancreatic cancer. Oncotarget 2017, 813:21281–21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Watkinson J, Varadan V, Anastassiou D: Multi-cancer computational analysis reveals invasion-associated variant of desmoplastic reaction involving INHBA, THBS2 and COL11A1. BMC Med Genomics 2010, 3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan DW, Tang HM, Peng ZH: Upregulated INHBA expression is associated with poor survival in gastric cancer. Med Oncol 2012, 291:77–83. [DOI] [PubMed] [Google Scholar]
- 37.Mom CH, Engelen MJ, Willemse PH, Gietema JA, ten Hoor KA, de Vries EG, van der Zee AG: Granulosa cell tumors of the ovary: the clinical value of serum inhibin A and B levels in a large single center cohort. Gynecol Oncol 2007, 1052:365–372. [DOI] [PubMed] [Google Scholar]
- 38.Robertson DM, Pruysers E, Jobling T: Inhibin as a diagnostic marker for ovarian cancer. Cancer Lett 2007, 2491:14–17. [DOI] [PubMed] [Google Scholar]
- 39.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L: Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 1999, 8411:4025–4030. [DOI] [PubMed] [Google Scholar]
- 40.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob L, Patwa R, Shah H, Xu K et al. : Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016, 5337604:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedl P, Gilmour D: Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009, 107:445–457. [DOI] [PubMed] [Google Scholar]