Abstract
Research on changes in personality and behavior following brain damage has focused largely on negative outcomes, such as increased irritability, moodiness, and social inappropriateness. However, clinical observations suggest that some patients may actually show positive personality and behavioral changes following a neurological event. In the current work, we investigated neuroanatomical correlates of positive personality and behavioral changes following a discrete neurological event (e.g., stroke, benign tumor resection). Patients (N=97) were rated by a well-known family member or friend on five domains of personality and behavior: social behavior, irascibility, hypo-emotionality, distress, and executive functioning. Ratings were acquired during the chronic epoch of recovery, when psychological status was stabilized. We identified patients who showed positive changes in personality and behavior in one or more domains of functioning. Lesion analyses indicated that positive changes in personality and behavior were most consistently related to damage to the bilateral frontal polar regions and the right anterior dorsolateral prefrontal region. These findings support the conclusion that improvements in personality and behavior can occur after a neurological event, and that such changes have systematic neuroanatomical correlates. Patients who showed positive changes in personality and behavior following a neurological event were rated as having more disturbed functioning prior to the event. Our study may be taken as preliminary evidence that improvements in personality and behavior following a neurological event may involve dampening of (premorbidly) more extreme expressions of emotion.
Keywords: personality, behavior, lesion, neurological event
Introduction
Previous investigations of changes in personality and behavior in patients who have sustained a neurological event (e.g. stroke, surgery, traumatic brain injury) have focused largely on negative outcomes in various domains of functioning. Links between damage to certain regions of the brain and particular changes in personality and behavior have been extensively documented. For example, increases in irritability, moodiness, and social inappropriateness have long been reported in patients with prefrontal damage (e.g., Stuss & Benson, 1986; Stuss & Knight, 2002). Such work has reported behavioral dysfunction characterized in part by impairments in the ability to monitor behavior and attitudes of apathy in patients with acquired prefrontal dysfunction (Stuss & Benson, 1984; Stuss & Knight, 2002). Patients with damage to the ventromedial prefrontal cortex, in particular, often exhibit clusters of symptoms thought to resemble psychopathy, such as generally dampened emotional experience, poor emotional modulation, impaired decision-making, impaired goal-directed behavior, and a striking lack of insight (e.g., Barrash, Tranel, & Anderson, 2000; Koenigs & Tranel, 2006). This acquired dysfunction, commonly referred to as “pseudopsychopathy” or “acquired sociopathy,” is typically enduring and can be highly problematic for rehabilitation, return to gainful employment, and interpersonal functioning (e.g., Tranel, Bechara, & Damasio, 2010).
It is not surprising that most of the existing literature has examined negative changes in personality and behavior following lesion onset – such changes have major life implications for the patients who suffer them and for the patients’ caregivers. However, investigating negative changes in personality and behavior following brain damage may only capture part of the story. An important paradox has been noted in the literature, specifically, that on occasion there are improvements in patients’ functions, in the opposite direction of the impairments that are more typically associated with damage to particular brain regions (e.g., Kapur, 1996). In line with this, clinical observations suggest that patients sometimes show improvements in personality and behavioral characteristics following a neurological event (e.g., Koenigs & Tranel, 2006). Taking advantage of a large database of patients with focal lesions who have available personality and behavior ratings for before and after lesion onset, in the current study we aimed to systematically examine improvements in personality and behavior following a neurological event. The primary objectives were to: (1) Investigate the occurrence and nature of improvements in personality and behavior following a neurological event; and (2) Determine if there are systematic neuroanatomical correlates associated with improvements in personality and behavior following a neurological event.
Materials and Methods
2.1. Participants
Participants were 97 patients (53 men and 44 women) enrolled in the Iowa Neurological Patient Registry of the Division of Cognitive Neuroscience at the University of Iowa. All patients experienced a discrete neurological event resulting in permanent focal, stable brain damage. Detailed neuropsychological and neuroanatomical data were collected for these patients, using the Benton Neuropsychology Laboratory and Laboratory of Brain Imaging and Cognitive Neuroscience standard protocols (Barrash et al., 2011; Tranel, 2009). Demographic and cognitive information is provided in Table 1. All participants gave written informed consent at the time of their enrollment in the Iowa Neurological Patient Registry and the study was approved by the University of Iowa Institutional Review Board.
Table 1.
Demographic and cognitive characteristics N= 97 (female= 44)
| M(SD) | min | max | |
|---|---|---|---|
| Age at ISPC (years) | 56.68(13.00) | 26.79 | 85.09 |
| Age at lesion onset (years) | 50.59(14.08) | 16.14 | 76.06 |
| Chronicity of lesion (months)* | 72.73(85.40) | 3.23 | 318.47 |
| Education (years) | 13.79(2.48) | 8.00 | 20.00 |
| Full Scale IQ** | 101.18(13.81) | 74.00 | 135.00 |
| Premorbid IQ*** | 98.71(11.45) | 74.00 | 131.00 |
participant age at the time the personality and behavior measure used in the current study (ISPC) was collected
at time of ISPC; IQ measured using WAIS-III, WAIS-R, WAIS-IV
88 scores for Premorbid IQ were derived from the reading subtest of the WRAT-3 (n= 67) or the WRAT-4 (n= 21), 8 scores were derived from the Barona equation (Barona, Reynolds, & Chastain, 1984), and 1 score was derived from the National Adult Reading Test (Nelson & Wilson, 1991)
2.2. Procedures
All data used in this study were obtained in the chronic epoch (at least 3 months post onset of lesion), when psychological and behavioral status as related to an acute neurological event has mostly stabilized.
Iowa Scales of Personality Change:
The Iowa Scales of Personality Change (ISPC) was used to assess changes in personality and behavior following a neurological event from an informant perspective (Barrash, Anderson, Jones, & Tranel, 1997; Barrash et al., 2011). Previous work has shown that this is a valid and reliable measure of changes in personality and behavior following a neurological event, with interrater agreement ranging from 0.80- 0.96 (Barrash et al., 1997; Barrash et al., 2011). The ISPC is a standardized measure of 26 personality and behavioral characteristics that may change following a neurological event. Using principle components analysis, these 26 characteristics have been collapsed into five domains of psychological functioning: social behavior, irascibility, hypo-emotionality, distress, and executive functioning (Barrash et al., 2011). The ISPC items included in the current study are listed in Table 2. Three items on the ISPC (“lack of stamina,” “suspiciousness,” “obsessiveness”) were not included in the current study because they do not load onto any of the five domains of psychological functioning on the ISPC (Barrash et al., 2011). One item, “lack of insight,” was not included because it only pertains to current functioning, rather than changes in functioning from before to after a neurological event.
Table 2.
Iowa Scales of Personality Change Items
| Social Behavior | Irascibility | Hypo-emotionality | Distress | Executive Functioning |
|---|---|---|---|---|
| Insensitivity | Irritability | Blunted affect | Depression | Lack of initiative |
| Social inappropriateness | Lability | Social withdrawal | Anxiety | Perseveration |
| Aggression | Inflexibility | Apathy | Dependency | Impulsivity |
| Inappropriate affect | Impatience | Vulnerability to pressure | Lack of persistence Lack of planning Poor judgment Indecisiveness |
To complete the ISPC, informants rated patients on each item, assessing the person’s personality characteristics and behavior prior to their neurological event (“Before” rating) and the person’s personality characteristics and behavior after their neurological event (“Now” rating). All informants in the current study were close family members or friends of the patients and knew the patients well before and after the neurological event (see Table 3 for informant characteristics). Informant-ratings, especially ratings from a well-known family member or friend, can provide information that yields predictive validities incremental to and often substantially greater than self-reports and may be particularly useful in evaluating changes in personality and behavior in neurological patients with brain lesions (Achenbach, Krukowski, Dumenci, & Ivanova, 2005). Informant measures are not only strong predictors of patient behavior (Connelly & Ones, 2010), but also help circumvent a possible lack of insight that could limit the ecological validity of self-report measures from patients with brain lesions (Barrash et al., 2011).
Table 3.
Informant characteristics, N=97
| Rater relationship | N | ||
|---|---|---|---|
| Spouse | 73 | ||
| Child | 9 | ||
| Sibling | 3 | ||
| Parent | 5 | ||
| Friend | 7 | ||
| M(SD) | Min | Max | |
| Rater years known | 34.62(15.50) | 4 | 66 |
On the ISPC, the “Before” rating requires informants to think back to how the patient was before the neurological event, whereas the “Now” rating requires informants to assess the patient’s current personality and behavior. Thus, current personality and behavior ratings reflect stabilized traits in the chronic epoch of recovery following a neurological event. Ratings were made on a 7-point scale, with 3 reflecting the average or usual amount of the characteristic for a typical healthy adult. Higher ratings indicate an increasing degree of disturbance or dysfunction in that characteristic (Barrash et al., 2000).
Grouping participants:
Participants were categorized as “improved” or “impaired,” using data from the ISPC. Changes in functioning were calculated based on informant ratings of personality and behavior “Now” and “Before” (“Now” score − “Before” score = “Change” score). (As noted, higher scores on the “Now” and “Before” scales indicate higher levels of dysfunction.) As such, a negative change score indicates improvement on a given personality or behavior characteristic. In the current study, patients with an average change score of −1 or less (higher negative numbers) on the overall ISPC (N=5) or on the dimensions of social behavior (N=7), irascibility (N=16), hypo-emotionality (N=10), distress (N=9), or executive functioning (N=6) were categorized as “improved.” It is important to note that the classification approach used in this study is descriptive and exploratory, attempting to capture improvement in personality and behavior as broadly as possible. This classification approach results in several patients being included in multiple subgroups. This is explained carefully throughout the results below. In addition, we want to underscore that the N’s for the groups listed here are not entirely independent of one another. The sum total of patients who improved in personality and behavior was 22, with 10 patients improving in only one domain, 5 patients improving in 2 domains, 3 patients improving in 3 domains, 1 patient improving in 4 domains, and 3 patients improving in all 5 domains.
Patients with an average change score of +1 or greater (higher positive numbers) on the overall ISPC (N=37) or on the dimensions of social behavior (N=32), irascibility (N=39), hypo-emotionality (N=25), distress (N=44), or executive functioning (N=48) were categorized as “impaired.” As with the improved groupings, the groups for impairment in personality and behavior are not entirely independent of one another. In all, 54 patients were impaired on at least one dimension of personality and behavior and did not improve on any dimension.
Patients with an average change score between −0.99 and +0.99 were considered not to have changed in terms of personality or behavior following a neurological event and were grouped into a “no change” category (N= 21). Patients with no change in personality and behavior were not examined in lesion mapping analyses.
Lesion characteristics and mapping:
Basic lesion etiology and laterality information for the entire sample of 97 is presented in Table 4.
Table 4.
Lesion characteristics, N=97
| Lesion etiology | Improved | Impaired | No change | Total |
|---|---|---|---|---|
| N | N | N | N | |
| CVA, non-hemorrhage | 7 | 26 | 9 | 42 |
| CVA, hemorrhage | 6 | 12 | 6 | 24 |
| AVM resection | 0 | 2 | 0 | 2 |
| Neurosurgery, benign tumor (meningioma) | 6 | 9 | 3 | 18 |
| Herpes simplex encephalitis | 1 | 0 | 2 | 3 |
| TBI with focal contusion | 2 | 5 | 1 | 8 |
| Lesion laterality | N | N | N | N |
| Left | 10 | 28 | 11 | 49 |
| Right | 6 | 17 | 8 | 31 |
| Bilateral | 6 | 9 | 2 | 17 |
Lesion mapping was based on structural MRI data or CT data (when MRI was contraindicated) collected in the chronic epoch of recovery, and was conducted using the MAP-3 procedures (Damasio & Frank, 1992). Each lesion was reconstructed using Brainvox (Frank, Damasio, & Grabowski, 1997) and manually mapped onto a common template brain (Fiez, Damasio, & Grabowski, 2000). Figure 1 depicts the lesion distribution for the entire sample.
Figure 1.
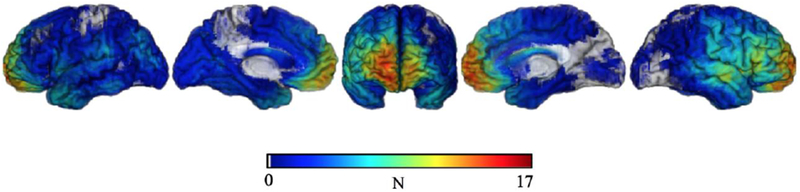
Overlap map depicting lesion distribution for the entire sample.
Proportional subtraction analytical methods were used to investigate areas of damage associated with improvement. As described by Rudrauf et al. (2008), voxelwise proportional differences in lesion locations were calculated by subtracting: (patients who improved and had overlapping lesions/all patients who improved) – (patients who were impaired and had overlapping lesions/all patients who were impaired). This approach takes into account all possible combinations of the lesion-improvement relationship. Specifically, proportional subtraction analyses account for: 1. Patients with lesions in a particular location and an improvement, 2. Patients with lesions in a particular area and no improvement, 3. Patients with no lesions in a particular area and an improvement, and 4. Patients with no lesions at a particular area and no improvement. In addition, proportional subtraction analyses account for uneven numbers of patients who improved compared to those who became impaired.
The resulting proportional lesion overlap maps convey descriptive information regarding the degree of difference in lesion location between patients who improved in personality and behavior compared to those who were impaired. Given common issues related to lesion studies (e.g., small N’s, lack of satisfactory effective coverage of all brain areas), it is particularly important to have a useful descriptive measure to illustrate the lesion-improvement relationship. The presented lesion overlap maps are descriptive rather than statistical tools and do not provide a formal statistic per se. Rather, the proportional lesion overlap maps (presented in the Results) provide a descriptive index of the magnitude of the effects. This measure goes beyond a simple subtraction, accounting for the false positive and the false negative cases in addition to the true positive and the true negative cases.
Warm colors on the lesion subtraction maps indicate areas in which proportionally more patients with improvements had damage in a given voxel compared to patients with impairments. Given our particular interest in the neuroanatomical correlates associated with improvements in personality and behavior following a neurological event, the presented results primarily focus on lesion overlap and improvement in personality and behavior. In particular, the presented overlap maps reflect neuroanatomical areas in which patients who showed improvements in personality and behavior were more likely to have a lesion as compared to patients who showed impairments in personality and behavior.
Results:
3.1. Demographic differences by group and by dimension of personality and behavior
There were no significant differences on basic demographic and cognitive characteristics, lesion etiology or laterality, or informant characteristics between patients who improved, were impaired, or experienced no change in personality and behavior.
Examination of changes in personality and behavior in each domain of the ISPC revealed no significant correlations between changes in any domain of personality and behavior and patient age at the time of ISPC administration, age at lesion onset, chronicity of lesion, education, full scale IQ, or premorbid IQ (after Bonferroni corrections for multiple comparisons). In addition, no significant gender differences emerged in any domain of personality and behavior. Informant characteristics were not significantly related to changes in social behavior, irascibility, hypo-emotionality, or distress. Ratings of changes in executive functioning were significantly affected by the type of informant relationship, H(4) = 9.85, p = 0.03. However, post hoc analyses corrected for multiple comparisons revealed no significant differences in rating of executive functioning between spouses, children, siblings, parents, and friends. No significant differences in irascibility, hypo-emotionality, distress, or executive functioning emerged based on lesion laterality. A significant relationship between social behavior and lesion laterality emerged, H(2) = 6.00, p = 0.05; however, post hoc analyses corrected for multiple comparisons did not survive, showing no significant differences between left, right, and bilateral lesions in changes in social behavior. Similarly, no significant differences based on lesion etiology emerged in social behavior, irascibility, hypo-emotionality, or distress. A significant relationship emerged between lesion etiology and changes in executive functioning, H(5) = 13.44, p = 0.02. Post hoc analyses revealed a significant difference in executive functioning between patients with who had experienced a TBI compared to those who had undergone a tumor resection (p = 0.03). These analyses investigating differences between domains of personality and behavior and demographic and cognitive variables should be interpreted cautiously, as group sizes were variable and analyses were generally underpowered.
3.2. Improved patient characteristics, by ISPC domain
The following results examine characteristics of patients who improved in personality and behavior following a neurological event. Specific patient characteristic for each ISPC domain are presented in Tables 5–10. As noted above, the various subgroups of patients are not entirely independent (some patients are in more than one group).
Table 5.
Improved overall , N = 5 (female = 4)
| Demographic and cognitive characteristics | |||
|---|---|---|---|
| M(SD) | Min | Max | |
| Age at ISPC (years) | 55.78(2.26) | 33.1 | 70.95 |
| Age at lesion onset (years) | 48.46(9.00) | 26.14 | 70.54 |
| Chronicity of lesion (months)* | 84.35(53.78) | 4.97 | 297.47 |
| Education (years) | 12.80(0.58) | 12.00 | 15.00 |
| Full Scale IQ | 102.60(4.52) | 87.00 | 113.00 |
| Informant characteristics | |||
| Rater relationship | N | ||
| Spouse | 3 | ||
| Child | 1 | ||
| Sibling | 1 | ||
| Parent | 0 | ||
| Friend | 0 | ||
| Rater years known | M(SD) 39.20(8.38) |
Min 15 |
Max 58 |
| Lesion characteristics | |||
| Lesion Etiology | N | ||
| CVA, non-hemorrhage | 1 | ||
| CVA, hemorrhage | 2 | ||
| AVM resection | 0 | ||
| Neurosurgery, benign tumor (meningioma) | 2 | ||
| Herpes simplex encephalitis | 0 | ||
| TBI with focal contusion | 0 | ||
| Lesion Laterality | N | ||
| Left | 1 | ||
| Right | 1 | ||
| Bilateral | 3 | ||
at time of ISPC
Table 10.
Improved executive functioning, N = 6 (female = 4)
| Demographic and cognitive characteristics | |||
|---|---|---|---|
| M(SD) | Min | Max | |
| Age at ISPC (years) | 55.65(5.72) | 33.10 | 70.95 |
| Age at lesion onset (years) | 47.44(7.42) | 26.14 | 70.54 |
| Chronicity of lesion (months)* | 98.55(46.15) | 4.97 | 297.47 |
| Education (years) | 12.33(0.67) | 10.00 | 15.00 |
| Full Scale IQ | 99.17(5.04) | 82.00 | 113.00 |
| Informant characteristics | |||
| Rater relationship | N | ||
| Spouse | 4 | ||
| Child | 1 | ||
| Sibling | 1 | ||
| Parent | 0 | ||
| Friend | 0 | ||
| Rater years known | M(SD) 39.83(6.87) |
Min 15 |
Max 58 |
| Lesion characteristics | |||
| Lesion Etiology | N | ||
| CVA, non-hemorrhage | 2 | ||
| CVA, hemorrhage | 2 | ||
| AVM resection | 0 | ||
| Neurosurgery, benign tumor (meningioma) | 2 | ||
| Herpes simplex encephalitis | 0 | ||
| TBI with focal contusion | 0 | ||
| Lesion Laterality | N | ||
| Left | 1 | ||
| Right | 2 | ||
| Bilateral | 3 | ||
at time of ISPC
3.3. Neuroanatomical correlates of improvements in personality and behavior following a neurological event
As noted in the Methods, warm colors on the lesion subtraction maps indicate areas in which proportionally more patients with improvements had damage in a given voxel compared to patients with impairments.
Improved, overall:
As shown in Figure 2, compared to patients with acquired impairment, patients who improved on the ISPC overall had the greatest lesion overlap, corresponding to the red portion of the color spectrum, in the bilateral frontal polar region (with more overlap observed in the right hemisphere), extending medially and laterally (areas 10, 11, 25), and in right anterior dorsolateral prefrontal cortex, extending dorsomedially (area 9). Some overlap, corresponding to the orange and yellow hues, also emerged in the right anterior cingulate cortex (areas 24, 32, 33) and in the left superior temporal gyrus (area 22).
Figure 2.

Proportional subtraction overlap map depicting brain damage in patients who improved overall on the ISPC.
Improved, social behavior:
As shown in Figure 3, compared to patients with acquired impairment, patients who improved in the domain of social behavior (i.e. were rated as having fewer social problems postmorbidly) had greatest lesion overlap, corresponding to the red hue, in the bilateral frontal polar region, extending medially and laterally (area 10, 11, 25) and right anterior dorsolateral prefrontal cortex, extending dorsomedially (area 9). Moderate overlap, corresponding to the orange and yellow hues, also emerged in the left inferior portions of supplementary motor area, primary motor cortex, and primary somatosensory cortex (areas 6, 4, 3, 1, 2); Broca’s area (areas 44, 45); left superior temporal gyrus (area 22); and right medial temporal region (areas 20, 36, 35, 28).
Figure 3.

Proportional subtraction overlap map depicting brain damage in patients who improved in the domain of social behavior on the ISPC.
Improved, irascibility:
As shown in Figure 4, strong differences in lesion overlap did not emerge in patients who improved in the domain of irascibility (i.e. were rated as becoming less irascible postmorbidly) compared to patients with acquired impairment. However, some moderate overlap, corresponding to the orange and yellow hues, emerged in the right frontal polar region, extending medially and laterally (areas 10, 11); right anterior dorsolateral prefrontal cortex, extending dorsomedially (area 9); Broca’s area (areas 44, 45); right medial temporal region (areas 20, 36, 35, 28); and left superior temporal gyrus (area 22).
Figure 4.

Proportional subtraction overlap map depicting brain damage in patients who improved in the domain of irascibility on the ISPC.
Improved, hypo-emotionality:
As shown in Figure 5, compared to patients with acquired impairment, patients who improved in the domain of hypo-emotionality (i.e. were rated as becoming less withdrawn, emotionally blunted, etc., postmorbidly) had greatest lesion overlap, corresponding to the red hue, in the bilateral frontal polar region, extending medially and laterally (area 10, 11, 25); right anterior dorsolateral prefrontal cortex, extending dorsomedially (area 9); and right anterior cingulate cortex (areas 24, 32, 33). Some overlap, corresponding to the orange and yellow hues, also emerged in the right temporoparietal junction (area 39); right portions of primary motor cortex (area 4); and left posterior superior temporal gyrus (area 22).
Figure 5.

Proportional subtraction overlap map depicting brain damage in patients who improved in the domain of hypo-emotionality on the ISPC.
Improved, distress:
As shown in Figure 6, compared to patients with acquired impairment, patients who improved in the domain of distress (i.e. were rated as becoming less distressed postmorbidly) had greatest lesion overlap, corresponding to red hues, in the bilateral frontal polar region, extending medially and laterally (area 10, 11, 25); right anterior dorsolateral prefrontal cortex, extending dorsomedially (area 9); and right anterior cingulate cortex (areas 24, 32, 33). More moderate overlap, corresponding to the orange and yellow hues, emerged in the right temporoparietal junction (area 39) and right portions of primary motor cortex (area 4).
Figure 6.

Proportional subtraction overlap map depicting brain damage in patients who improved in the domain of distress on the ISPC.
Improved, executive functioning:
As shown in Figure 7, compared to patients with acquired impairment, patients who improved in the domain of executive functioning (i.e. were rated as having less executive functioning problems postmorbidly) had greatest lesion overlap, corresponding to the red hues, in the bilateral frontal polar region, extending medially and laterally (area 10, 11, 25) and right dorsolateral prefrontal cortex, extending dorsomedially (area 9). Some overlap, corresponding to the orange and yellow hues, emerged in the right anterior cingulate cortex (areas 24, 32, 33); right temporoparietal junction (area 39); right posterior superior temporal gyrus (areas 22); and right inferior portions of primary motor cortex and primary somatosensory cortex (areas 4, 3, 1, 2).
Figure 7.

Proportional subtraction overlap map depicting brain damage in patients who improved in the domain of executive functioning on the ISPC.
3.3. Neuroanatomical similarities and differences, by dimension of personality and behavior
Neuroanatomically, patients with improvements in personality and behavior following a neurological event showed consistently greater lesion overlap in the bilateral frontal polar regions (areas 10, 11, 25), with highest degrees of overlap on the right side, and in the right anterior dorsolateral prefrontal cortex, extending dorsomedially (area 9), compared to patients with impairments in personality and behavior.
Notable nuances emerged in lesion location across the different domains of personality and behavior, as follows:
Patients who improved in hypo-emotionality, distress, and executive functioning showed higher lesion overlap in the right anterior cingulate cortex (areas 24, 32, 33) compared to patients who were impaired in those domains. In addition, patients who improved in hypo-emotionality, distress, and executive-functioning showed higher lesion overlap in the right temporoparietal junction (area 39) and in the right portions of primary motor cortex (area 4). Patients who improved in social behavior and irascibility did not show these same patterns of lesion location.
Patients who improved in social behavior and irascibility showed higher lesion overlap in the left inferior portions of supplementary motor area, primary motor cortex, and primary somatosensory cortex (areas 6, 4, 3, 1, 2); Broca’s area (areas 44, 45); and right medial temporal region (areas 20, 36, 35, 28) compared to patients who were impaired in those domains. Patients who improved in hypo-emotionality, distress, and executive functioning did not show this same pattern of lesion location.
Patients who improved in social behavior, irascibility, and hypo-emotionality showed higher lesion overlap in the left posterior superior temporal gyrus (area 22) compared to patients who were impaired in those domains. Patients who improved in distress and executive functioning did not show this same pattern of lesion location.
Patients who improved in executive functioning showed higher lesion overlap in right posterior superior temporal gyrus (areas 22) compared to patients who were impaired in executive functioning. Patients who improved in social behavior, irascibility, hypo-emotionality, and distress did not show this same pattern of lesion location.
While a thorough investigation of differences between patients who improved, were impaired, or showed no changes in personality and behavior following a neurological event is beyond the scope of the current work, examination of lesion overlap maps by group (Supplementary Figures 1–6) suggests that in the current study, across all domains of personality and behavior, higher degrees of overlap were generally apparent in the right frontal cortex in patients who showed improvements, compared to those who showed impairments or no change.
3.4. Case studies
Patients 3534, 2021, and 2410 are illustrative cases of patients who improved in personality and behavior following a neurological event. In all three cases, informants reported notable improvements in all 5 domains of personality and behavior (i.e. social behavior, irascibility, hypo-emotionality, distress, and executive functioning). All three patients have lesions in anterior frontal regions (see Figure 8).
Figure 8.

a. Lesion map for patient 3534 in lateral, medial, and coronal views. b. Lesion map for patient 2021 in lateral, medial, and coronal views. c. Lesion map for patient 2410 in lateral, medial, and coronal views.
Patient 3534 is a right handed woman with a high school level education who underwent a bifrontal meningioma resection at age 70. This case is notable for numerous personality changes following tumor resection, in a strikingly positive direction. According to the patient’s spouse who knew the patient for 58 years, in the years prior to the discovery of the tumor, 3534 showed high levels of irritability and outspokenness, low emotion, and excessive sleeping. The patient’s husband characterized her personality before her tumor resection as “stern.” In particular, the patient’s husband rated her as having suboptimal functioning in social behavior (M= 3.50), irascibility (M= 5.25), hypo-emotionality (M=3.67), distress (M=4.25), and executive functioning (M=5.43), in the years prior to discovery of the tumor. In the postmorbid epoch, the patient’s husband indicated that she is “happier, more outgoing and more talkative than ever before.” Patient records indicate that following surgery both the patient and her husband reported that she smiles more, laughs more, has good energy, and is more socially outgoing. All informant ratings on domains of personality and behavior following surgery were within a normal range of functioning (i.e. mean ISPC domain score ≤ 3). Clinician notes indicate that during an interview in the chronic epoch of recovery, the patient had broad, mostly bright affect. The patient had generally normal cognitive ability following surgery (e.g., Full Scale IQ = 110).
Similar to patient 3534, patient 2021 is a right handed woman with a high school education who underwent a bifrontal meningioma resection at age 66. Patient 2021 also showed various improvements in personality and behavior following surgery, as indicated by her spouse who knew her for 55 years. According to her spouse, prior to surgery the patient had suboptimal functioning in social behavior (M= 5.00), irascibility (M=5.25), hypo-emotionality (M=6.33), distress (M=5.00), and executive functioning (M=6.00). Clinical records show notable stable impairments in objective measures of executive function in the chronic epoch of recovery following surgery, but informant report suggests general improvement in all domains of personality and behavior. All ratings of personality and behavior were within a normal range of functioning following the neurological event (i.e. mean ISPC domain score ≤ 3). Clinical observations and informant reports indicate that following surgery, the patient had a pleasant and bright affect. While some limitations in awareness/concern were noted, the patient maintained generally normal cognitive functioning following surgery (e.g., Full Scale IQ = 101).
Unlike patients 3524 and 2021, patient 2410 is a right handed male, age 30, who experienced a subarachnoid hemorrhage and right frontal craniotomy for clipping of an anterior communicating artery (ACoA) aneurysm. His spouse, who knew him for 15 years, reported notable improvement in all domains of personality and behavior following his neurological event. According to his spouse, prior to his neurological event the patient had suboptimal functioning in social behavior (M= 4.25), irascibility (M=5.75), hypo-emotionality (M=5.00), distress (M=3.25), and executive functioning (M=4.14). Following his neurological event, the patient’s spouse reported normal functioning in all domains of personality and behavior (mean ISPC domain score ≤ 3). Both 2410 and his spouse reported that premorbidly, 2410 would express anger and frustration on a frequent basis (e.g. complaining about job; short temper with daughter). Following his aneurysm and ACoA clipping, however, he is “more passive and easy going.” In addition, prior to his aneurysm and ACoA clipping, 2410 and his spouse reported that he was generally “mopey.” Now, he laughs and jokes and seems generally more content. Overall, 2410 and his spouse reported that the observed changes in personality and behavior have been quite positive. Although clinical notes indicate some mild impairments in free recall, his overall cognitive ability fell within a low average range (Full Scale IQ = 87).
In all of the cases described above, dramatic improvements in personality and behavior followed a major neurological event. As illustrated, no single demographic or etiological characteristic would appear to account for the observed improvements. Rather, these cases indicate that a complex interaction of factors, including lesion location, most likely contribute to improvements in personality and behavior following a neurological event.
Discussion
The current work systematically investigated improvements in personality and behavior following a neurological event. Using behavioral and neuroanatomical data, we aimed to examine the phenomenon of improvement in personality and behavior following a neurological event and to determine if there are systematic neuroanatomical correlates associated with improvement. Our data suggest that while improvements in personality and behavior are not common in patients following a neurological event, they do occur. In some cases, these changes can be notable: several of our patients, exemplified in the three case studies we elaborated, showed major positive changes in multiple aspects of personality and behavioral functioning. While previous studies have primarily focused on impairments in functioning following neurological events, the current study indicates that improvements in personality and behavior may also occur following a brain lesion.
Given the finding that improvements in personality and behavior do occur on occasion in patients who have experienced a neurological event, we aimed to determine if there are systematic neuroanatomical correlates associated with this phenomenon. While improvements in personality and behavior following a neurological event are undoubtedly the product of a combination of factors, our findings illustrate that improvements in personality and behavior following a lesion, as compared to impairments, are more likely to be associated with damage to specific areas of the prefrontal cortex. Specifically, proportional subtraction lesion maps showed that compared to patients with impairments in personality and behavior, those with improvement in personality and behavior had consistently greater lesion overlap in the bilateral frontal polar regions, with highest degrees of overlap on the right side, and in the right anterior dorsolateral prefrontal cortex, extending dorsomedially. This pattern was evident across all five domains of personality and behavior measured using the ISPC. In addition, a consistent pattern emerged such that patients with improvement in personality and behavior showed higher levels of overlap in the right anterior cingulate cortex, right temporoparietal junction, right inferior portions of primary motor cortex, right posterior superior temporal gyrus, right medial temporal regions, left superior temporal gyrus, left inferior motor areas and somatosensory cortex, and Broca’s area. These areas of overlap varied in consistency and intensity across domains of personality and behavior.
This work expands on previous findings from our lab and others, which have primarily focused on impairments in personality and behavior following a neurological event. The current work suggests that, on some occasions, lesions that are at first glance somewhat similar in location to those previously implicated in impaired functioning may be related to improved functioning. Patient EVR (Eslinger & Damasio, 1985) provides a famous example of a patient who experienced dramatic impairments in personality and behavior (particularly social behavior) following a bilateral ventromedial prefrontal cortex (vmPFC) lesion. Similarly, Barrash et al. (2000) showed that patients with bilateral vmPFC lesions tend to show impairments in personality and behavior compared to patients with unilateral vmPFC lesions or lesions outside the vmPFC. Although there is an ostensible discrepancy between previous work and the current findings, a nuanced examination of these results suggests some important differences. Previous cases (of impairments following lesion onset) tend to have 1) bilateral vmPFC damage that is concentrated in the ventral and medial-to-posterior part of vmPFC, and 2) well-adjusted, entirely normal premorbid personality and social functioning. By contrast, our current cases of improvements following lesion onset tend to have 1) right-sided anterior frontal damage, and 2) less well-adjusted premorbid personalities. Further work will be needed to establish more definitively whether there are systematic lesion differences between patients who get worse and patients who get better, and to establish how premorbid personality and emotion are related to improvement in personality and behavior following a lesion.
While perhaps somewhat counterintuitive, the notion that improvements in personality and behavior can occur following a neurological event is actually not new. In fact, the idea that altering neural circuits can lead to improved psychological outcomes is consistent with the underlying logic of neurosurgical interventions for psychiatric disorders. Although rarely performed today, procedures such as frontal lobotomies and amygdalectomies were often used in the past to treat serious psychiatric disorders. Such procedures were shown to reduce pathological personality and behavior characteristics, such as violence (Freeman, Watts, & Hunt, 1942). Pioneers in psychosurgery, such as Egas Moniz and Walter Freeman, championed the use of frontal lobotomies as an effective treatment for severely disturbed patients suffering from a range of disorders, including schizophrenia and severe anxiety (Freeman, 1960; Freeman & Watts, 1937, 1942; Freeman et al., 1942; Moniz, 1937). The use of such methods illustrates the longstanding idea that disruptions in neural circuitry can lead, in selected instances, to improvement in psychological functioning.
Today, more refined surgical procedures, such as stereotactic subcaudate tractotomy, anterior cingulotomy, stereotactic limbic leucotomoy, and anterior capsulotomy, deep brain stimulation (DBS), or electroculvulsive therapy (ECT), are sometimes used in patients with severe, intractable mental illness (e.g., depression, OCD), who have not responded to other treatment options (Coenen & Honey, 2009; Schoene-Bake et al., 2010; Lippitz, Mindus, Meyerson, Kihlström, & Lindquist, 1999; Christmas et al., 2010; Greenberg et al., 2010; Mayberg et al., 2005; Rück et al., 2008). Resting on the principle that interruption in aberrant neural functioning might lead to behavioral improvements, these treatments target brain areas associated with the particular disorder and alter the functioning either with lesion or electrical stimulation in those regions. Research shows that such interventions can result in improvements in symptoms and mood (e.g. Abelson et al., 2005). Although there has been a resurgence of interest in neurosurgery for psychiatric disorders and there has been more widespread use of DBS and ECT, these procedures are still generally reserved for patients with severe, incapacitating mental illness who have failed to improve adequately following aggressive use of behavioral and medication treatments (Greenberg et al., 2010).
While typically used as last resorts, these treatments have been shown to be effective in patients with symptoms refractory to more standard treatments (Dougherty et al., 2002; Nuttin, Cosyns, Demeulemeester, Gybels, & Meyerson, 1999). The most common surgical targets for depression and OCD include the anterior cingulate cortex and frontobasal subcortical white matter (Coenen & Honey, 2009; Schoene-Bake et al., 2010). Our findings are in line with this literature, implicating similar regions (when damaged) in improvement in personality and behavior.
In the current work, patients with improvement in personality and behavior had consistently greater lesion overlap in the bilateral frontal polar regions and in the right anterior dorsolateral prefrontal cortex. Work examining psychiatric illness suggests that aberrant connectivity between neural circuits involving the frontal cortex and other brain areas may play an important role in psychological dysfunction (e.g. Sylvester et al., 2012). Although findings have been mixed, with some studies showing hypoactivation in frontal regions in illnesses such as depression and eating disorders (Donofry, Roecklein, Wildes, Miller, & Erickson, 2016), and other studies showing hyperconnectivity between portions of the frontal cortex and various other brain regions in illnesses such as depression, anxiety, and bipolar disorder (Liao et al., 2010; Sheline, Price, Yan, & Mintun, 2010; Wessa et al., 2007), the literature as a whole suggests that altered activation patterns in networks involving the anterior frontal region (e.g. frontal-striatal network, frontal-parietal network, default mode network) are related to psychological dysfunction.
Given that aberrant functional connectivity in frontal regions is associated with psychological dysfunction, it follows that disturbance to a dysfunctional network following a lesion may, in some cases, prove beneficial. For example, hyperconnectivity between the dorsal medial prefrontal cortex and three important neural networks (the cognitive control network, default mode network, and affective network) is associated with major depression, and may provide a potential mechanistic explanation for symptoms such as rumination, excessive self-focus, increased vigilance, and emotional dysregulation seen in patients with depression (Sheline et al., 2010). Reasonably, an interruption to such dysfunctional neural networks might help improve depressive symptoms.
The current understanding of the relationship between functional connectivity and psychiatric illness is nascent. Mixed findings presumably indicate that our levels of measurement are not yet precise enough to draw a definitive picture of the exact regions and the direction of dysfunction (hypoactivation or hyperactivation) implicated in psychological illness. However, work showing hyperconnectivity in frontal regions in patients with depression, anxiety, and bipolar disorder, dovetails with the current findings that lesions in the frontal pole and right anterior dorsolateral prefrontal cortex can, on occasion, lead to improvements in personality and behavior. In addition, older work examining patients with unilateral prefrontal lesions has shown that while right-sided lesions are generally associated with symptoms of dampened emotional reactivity or indifference, left-sided lesions are generally associated with exaggerated emotional reactivity (Gainotti, 1969). We hypothesize that damage to the anterior frontal lobe, in some patients who may have had overactive neural networks resulting in symptoms such as rumination or excessive worry, actually reduces depression or anxiety by dampening maladaptive emotional reactivity. Similarly, a slight loss of inhibition resulting from damage to the anterior frontal region may help certain patients experience more positive emotions.
While no patients in the current study had premorbid psychiatric conditions or mental illness, patients did range in terms of premorbid levels of the various personality and behavior characteristics we studied. Interestingly, as illustrated in the case studies presented in the current work, patients who improved in personality and behavior appeared to have suboptimal premorbid personality and behavior characteristics (i.e. ISPC “Before” ratings > 3) (see Supplementary Table 1). Future studies could investigate the observation that people with somewhat negative premorbid proclivities might become less negative following a neurological event. One potential explanation for this observation is that in patients with somewhat negative personality and behavior characteristics, a disruption in neural circuitry may allow for a dampening of more extreme expressions of personality and behavior.
Although limited, some previous work has identified improvements in personality and behavior following brain injury or lesion. For example, research shows that damage to particular regions of the prefrontal cortex or the anterior temporal region may confer resistance to depression or PTSD, respectively (Koenigs, Huey, Calamia, et al., 2008; Koenigs, Huey, Raymont, et al., 2008). In addition, damage to the insular cortex is associated with a disruption of smoking addiction (Naqvi, Rudrauf, Damasio, & Bechara, 2007). Specifically, patients addicted to smoking with brain damage involving the insula were more likely than patients addicted to smoking with brain damage that did not involve the insula to quit smoking easily, immediately, without relapse, and without the persistent urge to smoke (Naqvi et al., 2007). Higher levels of religiosity have been reported following focal damage to the ventromedial prefrontal cortex (Asp, Ramchandran, & Tranel, 2012). Finally, in a study examining personality changes following stroke, it was noted that occasionally caregivers viewed certain aspects of personality change as positive (e.g. patients became less bored, unhappy, worried, irritable, quick tempered; Stone et al., 2004). The current findings are in line with this previous work and contribute to the small literature regarding improvements in personality and behavior following a neurological event.
Limitations of the current work should be noted. First, the current work was exploratory, and results should be interpreted cautiously. Although our data provide evidence that, on occasion, improvements in personality and behavior are apparent in patients following a neurological event, the number of patients who showed improvements was small compared to those who showed impairments. In addition, the current sample was fairly homogenous in terms of race and ethnicity. Future work could investigate a larger, more diverse, sample of patients who show improvements in personality and behavior following a neurological event. Moreover, while consistent patterns of neuroanatomical correlates to improvements in personality and behavior following a neurological event emerged, these findings were largely descriptive. Future work could aim to quantify the observed differences in the neuroanatomical correlates of improvement in personality and behavior, as compared to impairments in personality and behavior in patients with brain damage.
Whereas most previous work has focused on the relation between brain damage and impairments in various domains of functioning, the current study systematically investigated the phenomenon of improvement in personality and behavior following a neurological event. The fact that, on occasion, patients do improve in various domains of personality and behavior, as reported by an informant, was established in the current work. Our investigation revealed that such improvements may be related, in part, to lesion location. This work provides groundwork for future studies to investigate specific hypotheses regarding improvements in personality and behavior following a neurological event.
Supplementary Material
Table 6.
Improved social behavior , N = 7 (female = 5)
| Demographic and cognitive characteristics | |||
|---|---|---|---|
| M(SD) | Min | Max | |
| Age at ISPC (years) | 57.85(6.20) | 33.10 | 72.17 |
| Age at lesion onset (years) | 51.18(7.80) | 26.14 | 71.14 |
| Chronicity of lesion (months)* | 68.06(39.03) | 4.97 | 297.47 |
| Education (years) | 13.71(0.84) | 12.00 | 18.00 |
| Full Scale IQ | 100.71(2.71) | 87.00 | 110.00 |
| Informant characteristics | |||
| Rater relationship | N | ||
| Spouse | 5 | ||
| Child | 0 | ||
| Sibling | 1 | ||
| Parent | 1 | ||
| Friend | 0 | ||
| Rater years known | M(SD) 41.71(5.98) |
Min 15 |
Max 58 |
| Lesion characteristics | |||
| Lesion Etiology | N | ||
| CVA, non-hemorrhage | 0 | ||
| CVA, hemorrhage | 3 | ||
| AVM resection | 0 | ||
| Neurosurgery, benign tumor (meningioma) | 3 | ||
| Herpes simplex encephalitis | 0 | ||
| TBI with focal contusion | 1 | ||
| Lesion Laterality | N | ||
| Left | 2 | ||
| Right | 2 | ||
| Bilateral | 3 | ||
at time of ISPC
Table 7.
Improved irascibility, N = 16 (female = 8)
| Demographic and cognitive characteristics | |||
|---|---|---|---|
| M(SD) | Min | Max | |
| Age at ISPC (years) | 56.29(3.26) | 33.10 | 72.17 |
| Age at lesion onset (years) | 50.58(4.10) | 26.14 | 71.14 |
| Chronicity of lesion (months)* | 68.79(21.94) | 3.83 | 297.47 |
| Education (years) | 14.56(0.71) | 11.00 | 20.00 |
| Full Scale IQ | 103.94(3.45) | 74.00 | 124.00 |
| Informant characteristics | |||
| Rater relationship | N | ||
| Spouse | 11 | ||
| Child | 1 | ||
| Sibling | 1 | ||
| Parent | 1 | ||
| Friend | 2 | ||
| Rater years known | M(SD) 32.00(4.675) |
Min 4 |
Max 66 |
| Lesion characteristics | |||
| Lesion Etiology | N | ||
| CVA, non-hemorrhage | 5 | ||
| CVA, hemorrhage | 4 | ||
| AVM resection | 0 | ||
| Neurosurgery, benign tumor (meningioma) | 4 | ||
| Herpes simplex encephalitis | 1 | ||
| TBI with focal contusion | 2 | ||
| Lesion Laterality | N | ||
| Left | 7 | ||
| Right | 5 | ||
| Bilateral | 4 | ||
at time of ISPC
Table 8.
Improved hypo-emotionality, N = 10 (female = 4)
| Demographic and cognitive characteristics | |||
|---|---|---|---|
| M(SD) | Min | Max | |
| Age at ISPC (years) | 55.22(3.88) | 33.10 | 70.95 |
| Age at lesion onset (years) | 49.51(4.51) | 28.72 | 70.54 |
| Chronicity of lesion (months)* | 68.51(26.89) | 4.97 | 259.37 |
| Education (years) | 12.50(0.67) | 10.00 | 18.00 |
| Full Scale IQ | 98.40(4.80) | 82.00 | 120.00 |
| Informant characteristics | |||
| Rater relationship | N | ||
| Spouse | 9 | ||
| Child | 1 | ||
| Sibling | 0 | ||
| Parent | 0 | ||
| Friend | 0 | ||
| Rater years known | M(SD) 35.10(4.59) |
Min 15 |
Max 58 |
| Lesion characteristics | |||
| Lesion Etiology | N | ||
| CVA, non-hemorrhage | 3 | ||
| CVA, hemorrhage | 3 | ||
| AVM resection | 0 | ||
| Neurosurgery, benign tumor (meningioma) | 4 | ||
| Herpes simplex encephalitis | 0 | ||
| TBI with focal contusion | 0 | ||
| Lesion Laterality | N | ||
| Left | 4 | ||
| Right | 1 | ||
| Bilateral | 5 | ||
at time of ISPC
Table 9.
Improved distress, N = 9 (female = 6)
| Demographic and cognitive characteristics | |||
|---|---|---|---|
| M(SD) | Min | Max | |
| Age at ISPC (years) | 56.43(3.98) | 33.10 | 70.95 |
| Age at lesion onset (years) | 49.62(5.27) | 26.14 | 70.54 |
| Chronicity of lesion (months)* | 82.28(32.74) | 3.83 | 297.47 |
| Education (years) | 13.56(0.87) | 10.00 | 19.00 |
| Full Scale IQ | 98.33(4.87) | 74.00 | 117.00 |
| Informant characteristics | |||
| Rater relationship | N | ||
| Spouse | 6 | ||
| Child | 1 | ||
| Sibling | 1 | ||
| Parent | 0 | ||
| Friend | 1 | ||
| Rater years known | M(SD) 35.33(6.61) |
Min 4 |
Max 58 |
| Lesion characteristics | |||
| Lesion Etiology | N | ||
| CVA, non-hemorrhage | 2 | ||
| CVA, hemorrhage | 2 | ||
| AVM resection | 0 | ||
| Neurosurgery, benign tumor (meningioma) | 4 | ||
| Herpes simplex encephalitis | 0 | ||
| TBI with focal contusion | 1 | ||
| Lesion Laterality | N | ||
| Left | 2 | ||
| Right | 3 | ||
| Bilateral | 4 | ||
at time of ISPC
Acknowledgments
This work was supported by Kiwanis International, the James S. McDonnell Foundation [grant number McDonnell UHC-Collab 220020387], NIMH (1 P50 MH094258), and the National Institutes of Health Predoctoral Training Grant (T32-GM108540).
Footnotes
Conflicts of interest: none
Contributor Information
Marcie L. King, Email: Marcie-king@uiowa.edu.
Daniel Tranel, Email: Daniel-tranel@uiowa.edu.
References
- Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, … & Giordani B (2005). Deep brain stimulation for refractory obsessive-compulsive disorder. Biological Psychiatry, 57(5), 510–516. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Krukowski RA, Dumenci L, & Ivanova MY (2005). Assessment of adult psychopathology: meta-analyses and implications of cross-informant correlations. Psychological Bulletin, 131(3), 361–382. [DOI] [PubMed] [Google Scholar]
- Asp E, Ramchandran K, & Tranel D (2012). Authoritarianism, religious fundamentalism, and the human prefrontal cortex. Neuropsychology, 26(4), 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barona A, Reynolds CR, & Chastain R (1984). A demographically based index of premorbid intelligence for the WAIS—R. Journal of Consulting and Clinical Psychology, 52(5), 885. [Google Scholar]
- Barrash J, Anderson SW, Jones RD, & Tranel D (1997). The Iowa rating scales of personality change: reliability and validity. Journal of the International Neuropsychological Society, 3(1), 27–28. [Google Scholar]
- Barrash J, Asp E, Markon K, Manzel K, Anderson SW, & Tranel D (2011). Dimensions of personality disturbance after focal brain damage: Investigation with the Iowa Scales of Personality Change. Journal of Clinical and Experimental Neuropsychology, 33(8), 833–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Tranel D, & Anderson SW (2000). Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology, 18(3), 355–381. [DOI] [PubMed] [Google Scholar]
- Christmas D, Eljamel MS, Butler S, Hazari H, MacVicar R, Steele JD, … & Matthews K (2010). Long term outcome of thermal anterior capsulotomy for chronic, treatment refractory depression. Journal of Neurology, Neurosurgery & Psychiatry, 82(6), 594–600. doi: 10.1136/jnnp.2010.217901 [DOI] [PubMed] [Google Scholar]
- Coenen VA, & Honey CR (2009). Ablative procedures for depression In Textbook of Stereotactic and Functional Neurosurgery (pp. 2943–2951). Springer; Berlin Heidelberg. [Google Scholar]
- Connelly BS, & Ones DS (2010). An other perspective on personality: meta-analytic integration of observers’ accuracy and predictive validity. Psychological Bulletin, 136(6), 1092–1122. [DOI] [PubMed] [Google Scholar]
- Damasio H, & Frank R (1992). Three-dimensional in vivo mapping of brain lesions in humans. Archives of Neurology, 49(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Donofry SD, Roecklein KA, Wildes JE, Miller MA, & Erickson KI (2016). Alterations in emotion generation and regulation neurocircuitry in depression and eating disorders: A comparative review of structural and functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 68, 911–927. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Baer L, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, … & Rauch SL (2002). Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. American Journal of Psychiatry, 159(2), 269–275. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, & Damasio AR (1985). Severe disturbance of higher cognition after bilateral frontal lobe ablation patient EVR. Neurology, 35(12), 1731–1731. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Damasio H, & Grabowski TJ (2000). Lesion segmentation and manual warping to a reference brain: Intra‐and interobserver reliability. Human brain mapping, 9(4), 192–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, & Grabowski TJ (1997). Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage, 5(1), 13–30. [DOI] [PubMed] [Google Scholar]
- Freeman W (1960). Psychosurgery. American Journal of Psychiatry, 116(7), 601–604. [DOI] [PubMed] [Google Scholar]
- Freeman W, & Watts JW (1937). Prefrontal lobotomy in the treatment of mental disorders. Southern Medical Journal, 30(1), 23–31. [Google Scholar]
- Freeman W, & Watts JW (1942). Psychosurgery. Oxford, England: Charles C. Thomas. [Google Scholar]
- Freeman W, Watts JW, & Hunt TC (1942). Psychosurgery: Intelligence, emotion, and social behavior following prefrontal lobotomy for mental disorders. London, England: Baillière, Tindall & Cox. [Google Scholar]
- Gainotti G (1969). Reactions “catastrophiques” et manifestations d’indifférence au cours des atteintes cérébrales. Neuropsychologia, 7, 195–204. [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DA, Rezai AR, Friehs GM, Okun MS, … & Malloy PF (2010). Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Molecular Psychiatry, 15(1), 64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N (1996). Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain, 119, 1775–1790. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, & Grafman J (2008). Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. Journal of Neuroscience, 28(47), 12341–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, & Grafman J (2008). Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nature Neuroscience, 11(2), 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, & Tranel D (2006). Pseudopsychopathy: A perspective from cognitive neuroscience In Zald DH & Rauch SL (Eds.), The orbitofrontal cortex (597–619). New York, NY: Oxford University Press. [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, … & Gong Q (2010). Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage, 52(4), 1549–1558. [DOI] [PubMed] [Google Scholar]
- Lippitz BE, Mindus P, Meyerson BA, Kihlström L, & Lindquist C (1999). Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery, 44(3), 452–458. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, … & Kennedy SH (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45(5), 651–660. [DOI] [PubMed] [Google Scholar]
- Moniz E (1937). Prefrontal leucotomy in the treatment of mental disorders. American Journal of Psychiatry, 93(6), 1379–1385. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, & Bechara A (2007). Damage to the insula disrupts addiction to cigarette smoking. Science, 315(5811), 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, Wilson J (1991) National Adult Reading Test (NART), NFER-Nelson, Windsor, UK. [Google Scholar]
- Nuttin B, Cosyns P, Demeulemeester H, Gybels J, & Meyerson B (1999). Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. The Lancet, 354(9189), 1526. [DOI] [PubMed] [Google Scholar]
- Rück C, Karlsson A, Steele JD, Edman G, Meyerson BA, Ericson K, . . . Svanborg P (2008). Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients. Archives of General Psychiatry, 65(8), 914–921. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Bruss J, Tranel D, Damasio H, & Grabowski TJ (2008). Thresholding lesion overlap difference maps: application to category-related naming and recognition deficits. Neuroimage, 41(3), 970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene-Bake JC, Parpaley Y, Weber B, Panksepp J, Hurwitz TA, & Coenen VA (2010). Tractographic analysis of historical lesion surgery for depression. Neuropsychopharmacology, 35(13), 2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, & Mintun MA (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences, 107(24), 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Townend E, Kwan J, Haga K, Dennis M, & Sharpe M (2004). Personality change after stroke: some preliminary observations. Journal of Neurology, Neurosurgery & Psychiatry, 75(12), 1708–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, & Benson DF (1984). Neuropsychological studies of the frontal lobes. Psychological Bulletin, 95(1), 3–28. doi: 10.1037/0033-2909.95.1.3 [DOI] [PubMed] [Google Scholar]
- Stuss DT, & Benson DF (1986). The frontal lobes. New York, NY: Raven Press. [Google Scholar]
- Stuss DT, & Knight RT (2002). Principles of frontal lobe function. New York, NY: Oxford University Press. [Google Scholar]
- Sylvester C, Corbetta M, Raichle M, Rodebaugh T, Schlaggar B, Sheline Y, . . . Lenze E (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences, 35(9), 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D (2009). The Iowa-Benton school of neuropsychological assessment In Grant I & Adams K (Eds.), Neuropsychological assessment of neuropsychiatric and neuromedical disorders (66–83). USA: Oxford University Press. [Google Scholar]
- Tranel D Bechara A, & Damasio AR (2010). Acquired sociopathy In Barch D (Ed.), Cognitive and affective neurscience of psychopathology. New York: Oxford University Press. [Google Scholar]
- Wessa M, Houenou J, Paillère-Martinot M-L, Berthoz S, Artiges E, Leboyer M, & Martinot J-L (2007). Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. American Journal of Psychiatry, 164(4), 638–646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


