Abstract
Chymases, a family of serine proteases with chymotryptic activity, play a significant role in cardiac angiotensin II (Ang II) formation from its substrate Ang-(1-12) in both human and rodent models. No studies, to date, have assessed the differences in enzymatic activity among these isoforms in Ang II formation, particularly in the cardiomyocyte (CM). Using PCR and DNA sequencing, we demonstrated that MCP-1, MCP-2, MCP-4, and MCP-5 mRNAs are expressed in the CM of both spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto rats (WKY). While rMCP-1 and rMCP-5 gene transcripts were higher than that of other isoforms in both rat strains, WKY CM exhibits higher levels of rMCP-1 and rMCP-5 mRNAs compared to the SHR CM. Ovariectomy (OVX) increased the expression of rMCP-1 and rMCP-5 mRNAs in WKY. In SHR, OVX was associated with a blunted increase in rMCP-1 mRNA compared to OVX normotensive WKY. Chymase activity, measured as Ang II formation from Ang-(1-12), significantly correlated with rMCP-1 and rMCP-5 mRNA expression in both rat strains. Both rMCP-1 and rMCP-5 mRNA expressions were positively correlated with progressive diastolic dysfunction (increasing the ratio of early mitral inflow velocity-to-early mitral annular velocity, E/e’) and expanding chamber dimensions or increasing left ventricular internal diameter end diastole (LVIDd). These data show rMCP-1 and rMCP-5 as the Ang II forming chymase isoforms participating in the loss of normal cardiac function due to OVX in rodents.
Keywords: mast cell protease, chymase, cardiomyocyte, hypertension, ovariectomy, Angiotensin-(1-12)
Introduction
Decades of progress have been made since the introduction of angiotensin converting enzyme (ACE) inhibitors, almost a half century ago [1, 2], in characterizing the renin angiotensin system (RAS) as a major contributor to cardiovascular disease (CVD) pathogenesis, including hypertension [3], myocardial infarction [4], and heart failure [5]. Yet, the clinical benefits gained from RAS blockers in halting or reversing CVD progression has been less than anticipated [6–8]. We [9, 10] have called attention to the most likely factors accounting for the disconnection between scientific knowledge of RAS mechanisms and the clinical efficacy of drugs that suppress the enzymatic activity of renin, ACE, and angiotensin II (Ang II) receptors. As reviewed elsewhere [9, 10], the disconnect may be related to a limited ability of these drugs to enter the intracellular spaces at which Ang II is formed, as well as critical differences in the identity of the major tissue Ang II forming enzymes in humans and rodents.
Since Urata et al. [11, 12] original studies, a robust literature shows that chymase, not ACE, is the major Ang II-forming enzyme in human tissues. As thoroughly reviewed most recently by Dell’Italia et al. [13], ubiquitous chymase expression in mast cells, cardiac myocytes, cardiac fibroblasts, and coronary artery endothelial cells suggests that this enzyme is a key biotransformation component accounting for Ang II production from either angiotensin I (Ang I) or the tissue angiotensin-(1-12) [Ang-(1-12)] substrate [14, 15]. Clarifying the contributions of chymase to human disease is complicated by the differential expression and activity of chymase isoforms among humans, rats, and mice. The name chymase refers to a family of serine proteases with chymotryptic activity [16]. Although chymases evolved from a common tree-ancestor with chymotryptic substrate specificity, functional plasticity among chymases is associated with lost catalytic activity or changed specificity to elastolytic or Leucine-selective activity [17]. Issues associated with arbitrary nomenclature approaches and a bewildering number and variety of chymase isoforms with partial or no chymotryptic activity represent obstacles to a definitive understanding of chymase participation in CVD [13].
Studies underscore a critical role of chymase as a mediator of oxidative stress and a causative factor of arrhythmias [14,18]. The recent publication of an orally active chymase inhibitor (BAY 1142524) as a first-class treatment for left ventricular dysfunction after a myocardial infarction [19] now highlights the need for a more precise identification of cardiac chymase isoforms expressions in rat genetic models of hypertension, as the SHR is universally accepted to express many of the features found in the diseased human left ventricle [20]. A resurging interest in understanding the contribution of non-canonical cardiac chymase-dependent Ang II production has been reviewed recently [13]. A PubMed review of publications with chymase as the search term produced 201 papers between 2016 and 2018. Progress in the design of chymase inhibitors and their mechanism of action will require knowledge of the various isoforms possessing hydrolytic activity on Ang-(1-12) and Ang I as some of the β-chymase isoforms both form and degrade Ang II [21].
While human tissue expresses only the α-form of chymase, multiple chymase isoforms, including α- and -forms, are found in rodents. Five of the ten rat mast cell proteases (rMCP-1, rMCP-2, rMCP-3, rMCP-4, and rMCP-5) display chymase activity, and rats express predominantly the -chymase isoforms [22], No studies to date have assessed the differences in enzymatic activity among these various isoforms in Ang-(1-12) metabolism, while data addressing the cleavage activity of -chymase isoforms in rodents remains fragmented. Data suggest that rMCP-5 is the equivalent to rat α-chymase and the homologue to the single human chymase, whereas mouse MCP-4 converts Ang I into Ang II [21].
Previous studies from our laboratory found that ovarian hormones regulate the expression and activity of cardiac rMCP-1 in the SHR [23], and that ovariectomy (OVX) augments cardiac chymase activity in both Wistar Kyoto rats (WKY) and SHR [23]. The present study was designed to identify: 1) other isoforms of rat mast cell serine proteases expressed in cardiomyocytes (CM); 2) which chymase isoforms correlate with indices of cardiac function and Ang II forming activity in the CM; and 3) the impact of ovarian hormone depletion on the expression of chymase isoforms in the CM. Emphasis in using the dodecapeptide Ang-(1-12) as the substrate for assessing chymase hydrolytic activity is borne from our studies showing a preferential affinity of chymase for Ang-(1-12) hydrolysis in human and rat hearts and evidence that this extended form of Ang I may be the principal source for paracrine/intracrine cardiac Ang II actions [10, 14, 15, 24].
Methods
Animals
Ten-week-old female SHR and WKY rats were purchased from Charles River, Inc. (Needham, MA) and housed in pairs in a facility on a 12-hr light/dark cycle with ad libitum access to soy- free rat chow (Teklad Global 16% Protein Rodent Diet, Envigo, Madison, WI) and tap water. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences (protocol # A14-064). All possible steps were taken to avoid animal suffering at each stage of the experiment.
Experimental protocol
At 12 weeks of age, SHR and WKY rats were randomly assigned to undergo either bilateral ovariectomy (OVX: WKY, n = 6; SHR, n = 6) or sham surgery (Intact: WKY, n = 5; SHR, n = 6). Bilateral OVX was performed under 2% isoflurane anesthesia, as previously described [25]. The absence of ovaries and decrease in uterine weight upon postmortem examination were used to confirm the efficacy of OVX [25]. Eight weeks after OVX or sham surgery, rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p., Akorn Inc., Lake Forest, IL), and the heart was removed for CM isolation.
Echocardiographic evaluation
Left ventricular (LV) function and dimensions were assessed prior to the protocol termination in animals anesthetized with an isoflurane (1.5%) oxygen mixture by nose cone using a commercially available echocardiograph equipped with both PureWave 12-4 MHz sector and 15-7 MHz linear transducers (CX50 Compact Xtreme System; Philips Medical Systems, Amsterdam, The Netherlands) by the investigator (LG), who was blinded to the experimental groups.
Cell isolation procedure
Cardiac myocytes were isolated as reported previously [26, 27]. Briefly, each rat heart was cannulated through the aorta on an Easycell System for Cardiomyocyte Isolation (Harvard Apparatus, Holliston, MA) and perfused at 37°C with calcium-free buffer for 5 min, and then switched to digestion buffer for 20-25min. The heart was pulled from the cannula and the ventricles were transferred to a 60-mm sterile dish containing 11 mL of transfer buffer and cut into small pieces. The minced tissue was incubated in a 37°C water bath for 10 min. The cell suspension was filtered through a 100-μm mesh cell strainer (BD Biosciences, San Jose, CA) to remove tissue debris and spun at 420 rpm at room temperature for 2 min. The resultant pellet portion underwent further processing for CM chymase activity and for CM mRNA expression of rMCPs.
Chymase enzymatic activity in CM
Chymase activity in CMs was determined by HPLC, as previously described [15]. Because cardiac chymase adheres to proteoglycans within the cell membrane, a 1M NaCl buffer solution was substituted for the typical normal salt (125 mM NaCl) buffer for the determination of chymase activity. Radiolabeled (125I) (Perkin Elmer Life and Analytical Sciences, Inc., Waltham, MA) rat Ang-(1-12) (GenScript USA, Inc., Piscataway, NJ) was used as a substrate for the determination of chymase activity. Selection of Ang-(1-12) as the substrate for the chymase activity assay is based on our previous demonstration of higher enzymatic activity of rat chymase with Ang-(1-12) compared to Ang I [15].
RT-PCR
Total RNA was isolated from CM using the RNeasy Lipit Tissue Mini Kit (Qiagen, Inc., Germantown, MD) and further purified using RNeasy MinElute Cleanup Kit (Qiagen, Inc.) followed by quality assessment on an Agilent 2100 bioanalyzer, as described previously [28, 29]. Complimentary first-strand DNA was synthesized from oligo (dT) primed total RNA using the Omniscript RT kit (Qiagen, Inc.). PCR amplification of rMCPs and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were performed under the following PCR conditions: initial denaturation at 95°C for 10 min, followed by 30 cycles of 94°C for 30 sec, 60- 64°C for 30 sec, and 72°C for 30 sec. PCR products were visualized on 1.5% agarose gel (Sigma, St. Louis, MO) stained with ethidium bromide and observed under ultraviolet light. PCR products were isolated and purified using Qiaguick Gel Extraction Kit (Qiagen, Inc.) for DNA sequencing by Eton Bioscience, Inc. (Research Triangle Park, NC).
Real-time quantitative PCR
Relative quantification of rMCP mRNA levels by real-time qPCR was performed using a SYBR Green PCR kit (Qiagen, Inc.) [28, 29]. Amplification and detection were performed with the QuantStudio 3 Real-Time PCR System (Applied Biosystems, Foster City, CA). Sequence- specific oligonucleotide primers were designed according to published GenBank sequences and confirmed with OligoAnalyzer 3.0 (Table 1). Relative target mRNA levels in each sample were normalized to GAPDH. Expression levels are reported relative to the geometric mean of the control group.
Table 1.
Sequence of PCR Primers (5′−3′)
| Gene | Oligonucleotide sequence | GenBank Locus | Position | Product size (bp) |
|---|---|---|---|---|
| GAPDH | accatcttccaggagcgaga | NM_017008.4 | 292–311 | 114 |
| caggtgagccccagccttct | 405–386 | |||
| rMCP-1 | tggtgtggtccatggtattg | NM_017145.2 | 659–678 | 93 |
| ttaatccagggcacataggg | 751–732 | |||
| rMCP-2 | ggtcatctgtggtgggtttc | NM_172044.1 | 141–160 | 126 |
| ttctgctgtgtggattctcg | 266–247 | |||
| rMCP-4 | aatgaaggccctgctattcc | NM_019321.2 | 13–32 | 117 |
| ttcagaagggccatgtaagg | 129–110 | |||
| rMCP-5 | tctggaggacctctcctgtg | NM_013092.1 | 641–660 | 66 |
| tgcattcggatgtacgtagg | 706–687 | |||
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; rMCP, rat mast cell protease
Since the capacity of sample number in the QuantStudio 3 Real-Time PCR System is limited (maximum of 48 duplicated samples including negative control in a 96-well plate), we performed two sets of real-time PCR runs in this study. The first set including one PCR run was to compare four rMCP isoforms in cardiomyocytes from ovary-intact WKY rats and SHR. The second set including 4 PCR runs (for rMCP-1, rMCP-2, rMCP-4, and rMCP-5, respectively) compared mRNA levels, for each rMCP isoform, in CMs from ovariectomized with that from sham-operated SHR and WKY rats.
Statistics
All values are expressed as mean ± SEM. One-way ANOVA was used to determine the significance of differences among groups. Significant interactions between groups were determined using Tukey post-hoc tests. Linear regression analysis was used to determine the relationships between rMCP mRNAs and chymase activity in CMs and echocardiographic- derived indices of filling pressure and left ventricular internal diameter end diastole (LVIDd). Data were analyzed using GraphPad Prism Version 6 software (GraphPad Software, Inc., La Jolla, CA). Differences for all tests were considered significant at P < 0.05.
Results
rMCP Expression in rat cardiomyocytes
Three methods (standard PCR, real-time PCR melting curve, and PCR product sequencing) were used to identify the chymase isoforms in CMs from WKY and SHR. RT-PCR detected 93-, 126-, 117-, and 66-bp fragments of rat MCP-1, MCP-2, MCP-4, and MCP-5 mRNA, respectively (Fig. 1A). A single peak in the melting curves of real-time PCR confirmed the specificity of the PCR products for each rMCP isoform (Fig. 1B). To further confirm specificity, we isolated PCR products from the agarose gel for DNA sequencing. The sequence of PCR products showed 100% matching with published sequences for each rMCP isoform (Fig. 1C). Specific PCR products for rMCP-3 were not detected in CMs, despite confirmation attempts using three different pairs of primers (data not shown).
Fig. 1.
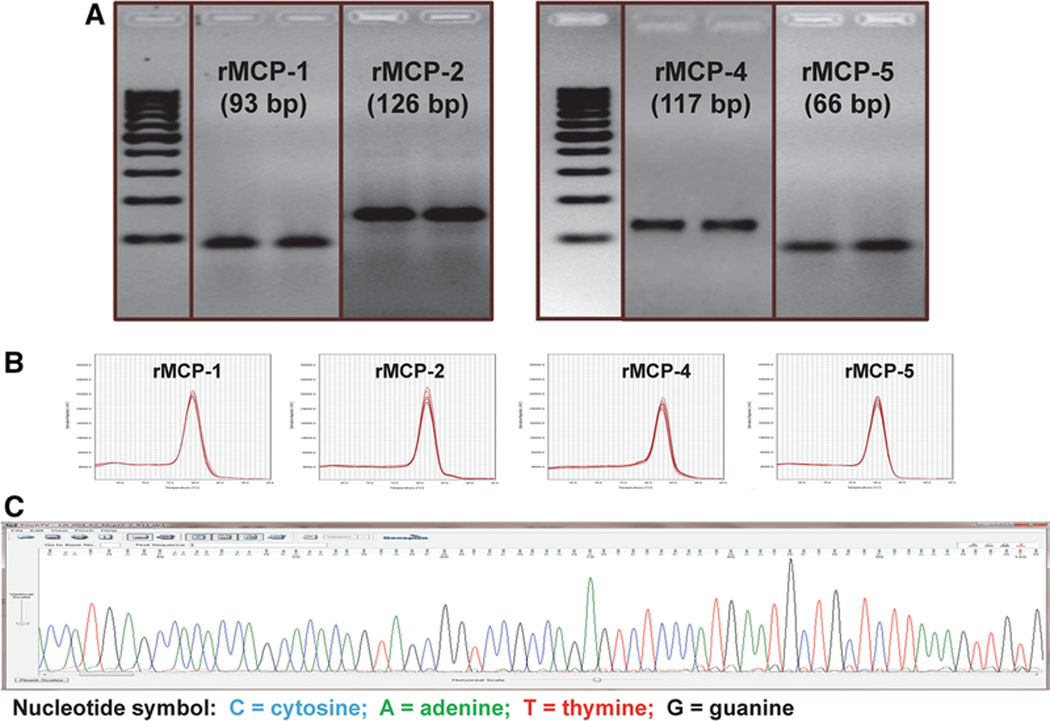
Mast cell protease isoforms expressed in cardiomyocytes (CM) from adult female WKY rats. (A) Agarose gel electrophoresis of PCR products of rMCP-1, rMCP-2, rMCP-4, and rMCP- 5.Two different rat samples were used for each isoform assay. (B) A single peak in the melting curves of real-time PCR for rMCPs from CM. (C) A representative image of the original data from PCR product sequencing
Comparison of rMCP isoforms in cardiomyocytes from WKY and SHR by real-time PCR
In WKY rats, the mRNA expression levels of each rMCP isoform were compared in CMs from female WKY and SHR. WKY CMs showed higher expression of rMCP-1, and rMCP-5 mRNAs compared to SHR (Fig. 2). rMCP-2 and rMCP-4 gene transcripts showed substantially lower expression compared to rMCP-1 and rMCP-5 mRNAs in either WKY or SHR (Fig. 2).
Fig. 2.

Differential expression of mast cell protease (MCP) isoforms mRNAs in adult female WKY and SHR. Values are means ± SE. n=5 for WKY rats and n=6 for SHR. *, P < 0.02 compared to corresponding MCP isoform in WKY
rMCP mRNA Levels in CM of ovariectomized or sham-operated SHR and WKY rats
Results from real-time PCR showed that OVX was associated with increased mRNA expression of rMCP-1 and rMCP-5 chymase isoforms in WKY rats (Fig. 3). In SHR, loss of ovarian hormones by OVX was associated with a blunted increase in rMCP-1 mRNA expression (Fig. 3). Expression levels of rMCP-2 and rMCP-4 mRNAs were not affected by OVX in either SHR or WKY rats.
Fig. 3.

Scattergram of individual values and mean ± SEM of mRNA levels for four mast cell protease (MCP) isoforms in cardiac myocytes of WKY and SHR exposed to sham operation or ovariectomy (OVX). Values are means ± SE. n=5-6/group.
Correlations between rMCP mRNA expression and chymase activity in cardiomyocytes
Chymase activity was measured as the amount of Ang II formation generated with 125I-labeled Ang-(1-12) as substrate [15]. Pooled data from both sham and OVX WKY and SHR revealed significant positive correlations between chymase activity and rMCP-1 and rMCP-5 mRNA expression levels (Fig. 4). Chymase activity and rMCP-2 and rMCP-4 expression levels were also positively correlated but the changes did not reach statistical significance (rMCP-2 vs. chymase activity: R= 0.467, P=0.059; rMCP-4 vs. chymase activity R=0.430, P=0.051).
Fig. 4.

Chymase activity assessed as the rate of 125I-angiotensin-(1-12) metabolism into 125I-Ang II correlates with mRNA levels of rMCP-1 and rMCP-5. Data include all matched observations from the entire cohort of WKY and SHR subjected to either sham-operation or ovariectomy. Solid circle: WKY-sham; open circle: WKY-OVX; solid triangle: SHR-sham; open-triangle: SHR-OVX.
Correlations between rMCPs mRNA expression in cardiomyocytes, diastolic function, and LV chamber dimensions
Figure 5 shows that mRNA levels of rMCP-1 and rMCP-5 in CMs were positively correlated with progressive diastolic dysfunction (increasing E/e’) and expanding LVIDd, whereas rMCP-2 mRNA expression in CMs lacks an association with E/e’ and LVIDd. Increasing levels of rMCP- 4 expression correlated with increasing LVIDd (R= 0.507, P = 0.016) but not E/e’ (data not shown). None of the rMCP isoform mRNA levels were significantly correlated with other echocardiograph indices including LV fractional shortening, posterior wall thickness, relative wall thickness, or left ventricular internal diameter at end-systole (data not shown).
Fig. 5.
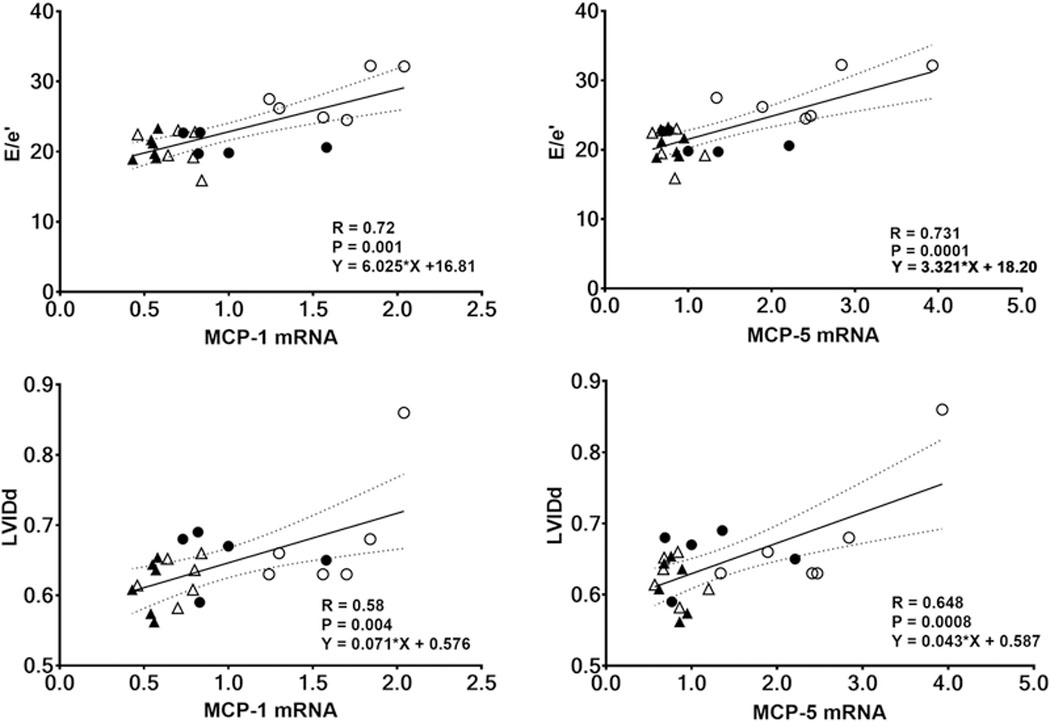
Echocardiographic indices of cardiac diastolic function (E/e’) and chamber dimensions (LVIDd) correlate with two chymase gene transcript isoforms (rMCP-1 and rMCP-5). Data include all matched observations from the entire cohort of WKY and SHR subjected to either sham-operation or ovariectomy. Solid circle: WKY-sham; open circle: WKY-OVX; solid triangle: SHR-sham; open-triangle: SHR-OVX.
Discussion
This study is the first systematic analysis of the principal chymase isoforms found in rodent CMs and their changed expression in response to ovarian hormone status in an established hypertensive rat model (SHR) and a normotensive control (WKY rat). The identification of gene transcripts for both the α- (rMCP-5) and β- (rMCP-1, rMCP-2, and rMCP-4) isoforms of chymase in CMs isolated from these strains reveals that the contractile and relaxant units of the rodent heart are a source for the chymase gene in addition to mast cells, cardiac fibroblasts, or even vascular endothelial cells [13, 30]. Quantitative differences in chymase isoform expression were detected in gonad-intact WKY and SHR. rMCP-1 and rMCP-5 were the predominant chymase isoforms in WKY and SHR while expression levels of all rMCPs were significantly lower in CMs from SHR. The current findings expand on our previous observation that OVX in WKY is associated with increased chymase enzymatic activity [23]through demonstrating that this effect is due to a robust increase in rMCP-1 and rMCP-5 mRNA levels in CMs of WKY. A comparatively modest increase in rMCP-1 in CMs was seen in OVX SHR, with no change in rMCP-5 with OVX.
The differential catalytic correlation between Ang II-forming activity and mRNAs of the different isoforms found in WKY and SHR CM further suggest that rMCP-1 and rMCP-5 are the isoforms most likely involved in Ang II cardiac mechanisms. Our previous work showed cardiac chymase activity for conversion of Ang-(1-12) to Ang II was 8- to 15-fold higher than the hydrolytic activity for conversion of the Ang I substrate in the heart and lung tissue of SHR [15]. Plotting Ang II production during incubation with Ang-(1-12) against rMCP-1 and rMCP-5 mRNA expression suggests that these isoforms have a similar capacity to metabolize Ang-(1-12). The current findings are of considerable importance as inconsistencies in nomenclature and the extensive number and variety of chymases [16], as well as variations in chymotryptic activity, have stymied efforts to understand their functional contribution to heart and vascular pathological remodeling. This first quantitative demonstration of differential expression and activity of α- and β- chymase isoforms in CMs of an established rat model of primary hypertension and its normotensive counterpart suggest a role for specific chymases in the development of heart disease. Future studies will need to consider these findings in the design of drugs able to specifically inhibit either the expression of the protein or the Ang II-forming activity of chymases.
Rat MCP-5 is the homolog to human α-chymase gene [16, 21], while MCP-4 is considered to be most similar to the human chymase form in terms of hydrolytic activity and tissue distribution [16]. Enzymatic characterization of chymase hydrolytic activity shows that all isoforms cleave amino acids at active site position His65, Asp109, and Ser202 [31]. Nevertheless, preferred cleavage sites and target proteins may differ in terms of tissues, charge, heparin binding, substrate specificity, inhibitor susceptibility, and structure [31]. In agreement with Caughey et al. [21], we showed that both α (rMCP-5) and β (rMCP-1) forms of rat chymase possess Ang II forming activity.
While we did not investigate the effects of estrogen status on the contractile and relaxant functions of cardiomyocytes in this study, others show that estrogen is involved in the regulation of cardiomyocyte contraction. Parks et al. [32] reported gender differences in the parameters of cardiac excitation-contraction coupling between male and female hearts and cultured myocytes [32]. Ribeiro et al. [33] demonstrated that myocardial contractile dysfunction induced by ovariectomy in rats was prevented by the Ang II type 1 receptor blocker, losartan, suggesting a role for Ang II in OVX-induced cardiomyocyte contractile dysfunction. Based on our findings, the effects of estrogen on cardiomyocyte contraction might be through its regulation on Ang-1– 12/chymase/Ang II forming pathway in cardiomyocytes. However, confirmation of this concept will require further investigation, along with approaches aimed at blocking or knocking down each MCP isoform in cardiomyocytes.
Besides the functions of converting cardiac Ang-(1-12) to Ang II, rMCPs also play important roles in tissue remodeling through Ang II-independent pathways [13]. The absence of a significant association of rMCP-2 and rMCP-4 mRNA with chymase activity suggests that these isoforms do not have a critical role in cardiac Ang II generation from Ang-(1-12). Very little is known about the biological function of rMCP-2 and rMCP-4 in the heart. rMCP-2 has been reported to cleave cell adhesion and junction proteins and have an important role in regulating intestinal permeability [34]. It is not clear if rMCP-2 also affects cardiac remodeling and heart function through similar mechanisms. In a detailed substrate specificity study of rMCP-4 using peptide phage display analysis, Karlson et al. [31] identified the procollagen C- proteinase enhancer protein as a potential substrate. Its cleavage by rMCP-4 may result in decreased amounts of collagen. Another potential substrate of rMCP-4 is plasminogen activator inhibitor-1 (PAI-1). Destruction of PAI-1 by rMCP-4 could lead to an increase in plasmin formation, which in turn may increase the proteolytic degradation of the extracellular matrix. The positive correlation between rMCP-4 mRNA expression and LVIDd might be due to these degrading effects of rMCP-4 on the cardiac extracellular matrix and collagen deposition.
The involvement of mast cell protease in the development of heart disease, including cardiac hypertrophy and heart failure, is considered by some scientists to be at the centerpiece of its pathogenesis [35]. The ubiquitous distribution of Ang-(1-12), the preferred rat cardiac chymase substrate, has been found in various tissues and plasma [36–38]. Demonstration of increased Ang-(1-12) expression and concentrations in the hypertrophic heart of the SHR suggest a critical contribution of this novel substrate to the pathogenesis of hypertension in this model [39]. The clinical relevance of these findings has been demonstrated by the presence of Ang-(1- 12) and chymase in human diseased left atrial appendages [14] and the left ventricle from normal subjects [24]. Taken together, these findings provide basic research evidence supporting the development of pharmaceuticals that target intracellular chymase-mediated Ang II formation to treat cardiovascular diseases. The recent publication of the pharmacokinetics, safety, and tolerability of an orally active chymase inhibitor for prevention of left ventricular failure post- myocardial infarction underscores the importance of accelerating research efforts in characterizing the role of chymase in cardiovascular disease [19].
Limitations and future studies
Although this study was done in female rats, estrogens have been shown to protect both female and male hearts from various stresses [40–42], even though the defense mechanisms involved might be different [43]. Indeed, it would be interesting to investigate rMCPs expression levels in cardiomyocytes from male rats and their corresponding roles in sex-specific cardiac function and morphology. Correspondingly, future studies examining the effect of estrogen treatment in OVX rats will be important to confirm that estrogen, as opposed to other ovarian hormones, influences rMCP expression levels in female cardiomyocytes. Another limitation is that protein expression levels of rMCPs were not assessed in this study. While anti-rMCP5 (rat chymase 1) antibody exists, no specific anti-rMCP1 antibody is commercially available. Since the quantity of CMs isolated from each rat was sufficient only for mRNA and chymase activity assays, immunoblots and immunohistochemical measures were not performed.
In summary, this study demonstrated that rMCP-1, rMCP-2, rMCP-4, and rMCP-5 are expressed in CMs of both WKY and SHR, their expression levels are differentially affected by blood pressure and ovarian hormone status, and differentially correlate with diastolic function and end diastolic LV dimension. The exact contribution of these rMCPs to heart disease causation or compensation needs further study with CM-specific knockdown or overexpression models.
Acknowledgments
We thank Marina Lin for her technical assistance.
Funding information
This work was funded by grants from the National Heart Lung and Blood Institute (CMF and LG) P01-HL051952 and the National Institute on Aging (LG) AG033727 of the National Institute of Health.
Footnotes
Declarations of interest
Authors declare that there are no competing interests associated with the manuscript.
Compliance with Ethical Standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Ondetti MA (1991) Angiotensin converting enzyme inhibitors. An overview. Hypertension 18:III134–nI135. [DOI] [PubMed] [Google Scholar]
- 2.Ondetti MA, Williams NJ, Sabo EF, Pluscec J, Weaver ER, Kocy O (1971) Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry 10:4033–4039. [DOI] [PubMed] [Google Scholar]
- 3.Carretero OA, Oparil S (2000) Essential hypertension. Part I: definition and etiology. Circulation 101:329–335. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA (1995) ACE inhibition in acute myocardial infarction. N Engl J Med 332:118–120. [DOI] [PubMed] [Google Scholar]
- 5.Hood WB Jr (1993) Role of converting enzyme inhibitors in the treatment of heart failure. J Am Coll Cardiol 154A–157A. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario CM, Mullick AE (2017) Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol Res 125:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyes S, Varagic J, Ahmad S, VonCannon J, Kon ND, Wang H, Groban L, Cheng CP, Dell’Italia LJ, Ferrario CM (2017) Novel Cardiac Intracrine Mechanisms Based on Ang- (1–12)/Chymase Axis Require a Revision of Therapeutic Approaches in Human Heart Disease. Curr Hypertens Rep 19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusing R (2016) Mega clinical trials which have shaped the RAS intervention clinical practice. Ther Adv Cardiovasc Dis 10:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrario CM (2010) Addressing the theoretical and clinical advantages of combination therapy with inhibitors of the renin-angiotensin-aldosterone system: antihypertensive effects and benefits beyond BP control. Life Sci 86:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, Collawn JF, Dell Italia LJ (2016) Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol 311 :H404–H414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urata H, Healy B, Stewart RW, Bumpus FM, Husain A (1990) Angiotensin II-forming pathways in normal and failing human hearts. Circ Res 66:883–890. [DOI] [PubMed] [Google Scholar]
- 12.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A (1990) Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem 265: 22348–22357. [PubMed] [Google Scholar]
- 13.Dell’Italia LJ, Collawn JF, Ferrario CM (2018) Multifunctional role of chymase in acute and chronic tissue injury and remodeling. Circ Res 122:319–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM (2011) Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue. PLoS One 6:e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad S, Varagic J, VonCannon JL, Groban L, Collawn JF, Dell’Italia LJ, Ferrario CM (2016) Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1–12) metabolizing enzyme. Biochem Biophys Res Commun 478:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caughey GH (2013) Chymases In: Rawlings NDSG, editor. Handbook of Proteolytic Enzymes, 3rd edn Academic Press, London, pp 2675–2682. [Google Scholar]
- 17.Caughey GH, Beauchamp J, Schlatter D, Raymond WW, Trivedi NN, Banner D, Mauser H, Fingerle J (2008) Guinea pig chymase is leucine-specific: a novel example of functional plasticity in the chymase/granzyme family of serine peptidases. J Biol Chem 283:13943–13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai S, Jin D (2016) Improvement of cardiovascular remodelling by chymase inhibitor. Clin Exp Pharmacol Physiol 43:387–393. [DOI] [PubMed] [Google Scholar]
- 19.Kanefendt F, Thuss U, Becka M, Boxnick S, Berse M, Schultz A, Otto C (2018) Pharmacokinetics, safety, and tolerability of the novel chymase inhibitor BAY 1142524 in healthy male volunteers. Clin Pharmacol Drug Dev. 2018. E-pub Ahead of Print doi: 10.1002/cpdd.579. [DOI] [PubMed] [Google Scholar]
- 20.Susic D, Frohlich ED (2011) Hypertensive cardiovascular and renal disease and target organ damage: Lessons from animal models. Cardiorenal Med 1:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caughey GH, Raymond WW, Wolters PJ (2000) Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta 1480:245–257. [DOI] [PubMed] [Google Scholar]
- 22.Lützelschwab C, Pejler G, Aveskogh M, Hellman L (1997) Secretory granule proteases in rat mast cells. Cloning of 10 different serine proteases and a carboxypeptidase A from various rat mast cell populations. J Exp Med 185:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad S, Sun X, Lin M, Varagic J, Zapata-Sudo G, Ferrario CM, Groban L, Wang H (2018) Blunting of estrogen modulation of cardiac cellular chymase/RAS activity and function in SHR. J Cell Physiol 233:3330–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad S, Wei CC, Tallaj J, Dell’Italia LJ, Moniwa N, Varagic J, Ferrario CM (2013) Chymase mediates angiotensin-(1–12) metabolism in normal human hearts. J Am Soc Hypertens 7:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jessup JA, Wang H, MacNamara LM, Presley TD, Kim-Shapiro DB, Zhang L, Chen AF, Groban L (2013) Estrogen therapy, independent of timing, improves cardiac structure and function in oophorectomized mRen2.Lewis rats. Menopause 20:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Cheng HJ, Zhou P, Kitzman DW, Ferrario CM, Li WM, Cheng CP (2017) Cellular basis of angiotensin-(1–7)-induced augmentation of left ventricular functional performance in heart failure. Int J Cardiol 236:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P, Cheng CP, Li T, Ferrario CM, Cheng HJ (2015) Modulation of cardiac L-type Ca2+ current by angiotensin-(1–7): normal versus heart failure. Ther Adv Cardiovasc Dis 9:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Sun X, Chou J, Lin M, Ferrario CM, Zapata-Sudo G, Groban L. Inflammatory and mitochondrial gene expression data in GPER-deficient cardiomyocytes from male and female mice. Data Brief 10:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Jessup JA, Zhao Z, Da Silva J, Lin M, MacNamara LM, Ahmad S, Chappell MC, Ferrario CM, Groban L (2013) Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2.Lewis rats. PLoS One 8:e76992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu L, Wei CC, Powell PC, Bradley WE, Ahmad S, Ferrario CM, Collawn JF, Dell’Italia LJ (2016) Increased fibroblast chymase production mediates procollagen autophagic digestion in volume overload. J Mol Cell Cardiol 92:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlson U, Pejler G, Froman G, Hellman L (2002) Rat mast cell protease 4 is a beta- chymase with unusually stringent substrate recognition profile. J Biol Chem 277:18579–18585. [DOI] [PubMed] [Google Scholar]
- 32.Parks RJ, Howlett SE (2013) Sex differences in mechanisms of cardiac excitation- contraction coupling. Pflugers Arch 465:747–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro RF Jr, Pavan BM, Potratz FF, Fiorim J, Simoes MR, Dias FM, Lima FL, Fernandes AA, Vassallo DV, Stefanon I (2012) Myocardial contractile dysfunction induced by ovariectomy requires at1 receptor activation in female rats. Cell Physiol Biochem 30:1–12. [DOI] [PubMed] [Google Scholar]
- 34.Fu Z, Thorpe M, Hellman L (2015) rMCP-2, the Major Rat Mucosal Mast Cell Protease, an Analysis of Its Extended Cleavage Specificity and Its Potential Role in Regulating Intestinal Permeability by the Cleavage of Cell Adhesion and Junction Proteins. PLoS One 10:e0131720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS (2011) Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res 89:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR (2009) Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol 296:H1184–H1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagata S, Kato J, Kuwasako K, Asami M, Kitamura K (2012) Plasma and tissue concentrations of proangiotensin-12 in rats treated with inhibitors of the renin- angiotensin system. Hypertens Res 35:234–238. [DOI] [PubMed] [Google Scholar]
- 38.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K (2006) Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 350:1026–1031. [DOI] [PubMed] [Google Scholar]
- 39.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K Ferrario CM (2008) Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 294:H2614–H2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner JD, Murray DB, Voloshenyuk TG, Brower GL, Bradley JM, Janicki JS (2010) Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol 298:H497–H504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baka T, Hodosy J, Krajcirovicova K, Repova K, Aziriova S, Domonkos E, Borbelyova V, Slavkovsky P, Zorad S, Celec P, Paulis L, Simko F (2018) 17β-Estradiol treatment reversed left ventricular dysfunction in castrated male rats: an echocardiographic study. Can J Physiol Pharmacol 96:850–854. [DOI] [PubMed] [Google Scholar]
- 42.Soliman M (2015) Protective Effects of Estradiol on Myocardial Contractile Function Following Hemorrhagic Shock and Resuscitation in Rats. Chin Med J (Engl) 128:2360–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devanathan S, Whitehead T, Schweitzer GG, Fettig N, Kovacs A, Korach KS, Finck BN, Shoghi KI (2014) An animal model with a cardiomyocyte-specific deletion of estrogen receptor alpha: functional, metabolic, and differential network analysis. PLoS One 9:e101900. [DOI] [PMC free article] [PubMed] [Google Scholar]


