Abstract
Background
This study measured longitudinal DNA methylation dynamics at growth-related genes during childhood, and then tested whether DNA methylation at various stages of childhood was associated with obesity status.
Methods
Using neonatal bloodspot (n=132) and matched childhood blood samples (n=65), DNA methylation was quantified at a repetitive element (LINE-1), two imprinted genes (IGF2, H19), and four non-imprinted genes (LEP, PPARA, ESR1, SREBF1) related to growth and adiposity. Logistic regression was used to test whether neonatal bloodspot DNA methylation at target genes was associated with log odds of obesity (Y/N) in children recruited from three age groups – 12–24 months old (n=40), 3–5 years of age (n=40), and 10–12 years of age (n=52).
Results
In 3–5 year olds, neonatal bloodspot LINE-1 methylation was negatively associated with obesity (log odds = −0.40, p=0.04). Across childhood age group in matched blood samples, DNA methylation levels in blood decreased (p<0.05) at LINE-1, PPARA, ESR1, SREBF1, IGF2, and H19, and increased (p<0.05) at LEP.
Conclusions
Our results suggest that age-related epigenetic changes occur at growth-related genes in the first decade of life, and that gene-specific neonatal bloodspot DNA methylation may be a useful biomarker of obesity likelihood during childhood.
Introduction
Despite multiple public health initiatives aimed at improving metabolic health in the U.S., obesity rates have continued to climb over the past few decades, reaching 39.8% in 2015–2016 (1). Obesity is associated with a number of chronic diseases, including heart disease, hypertension, some cancers, and type 2 diabetes (2). Previous research has shown that the best predictors of adult obesity are childhood obesity or a family history of obesity (3), suggesting that genetics plays a critical role in determining obesity status. However, reviews on this topic indicate that BMI-associated genetic variants only explain 0.66–2.70% of BMI variation, and that the available genetic information does not accurately reflect risk of obesity (3). This inconsistency suggests that there is critical role of the environment – including gene-environment interactions – in shaping obesity risk.
Epigenetics refers to heritable and potentially reversible changes in an organism’s gene regulation that occur independent of the DNA sequence. Epigenetic marks, including DNA methylation, have been shown to change in response to environmental factors during early development (4), adolescence (5), and even adulthood (6). Recent research has also shown that the epigenome is dynamic with age in human twin (7) and non-twin (8, 9) cohorts, a process that may play an important role in the development of chronic diseases. Reports suggest that both cross-sectional, gene-specific DNA methylation and rates of epigenetic aging are associated with obesity status in humans (10, 11), but it remains to be seen whether developmental programming of the epigenome at obesity-related genes could be associated with childhood obesity development. Here, we aim to shed light on this question by measuring longitudinal patterns of age-related DNA methylation at a number of obesity-related genes throughout childhood.
For this study, we examined DNA methylation levels at seven target gene regions – the LINE-1 repetitive element, IGF2, H19, PPARA, LEP, ESR1, and SREBF1. LINE-1, or long interspersed nuclear element 1, is a retrotransposon that comprises ~18% of the human genome (12). Given its prevalence across the genome, LINE-1 can be used as a surrogate for global DNA methylation levels (13). Apart from LINE-1, all of the other interrogated genes play a role in metabolism, growth, or development. Insulin-like growth factor II (IGF2) and H19 are well-characterized imprinted genes, in which parent-of-origin monoallelic expression is involved in the regulation of body composition and growth (14). PPARA is a non-imprinted gene that encodes the peroxisome proliferator-activated receptor alpha (PPAR-α) protein, a nuclear receptor that regulates fatty acid metabolism (15). LEP is a non-imprinted gene that encodes leptin, an adipokine involved in satiety signaling (16). ESR1 is a non-imprinted gene that encodes estrogen receptor alpha (ER-α), a transcription factor involved in regulation of energy homeostasis (17). Lastly, SREBF1 is a non-imprinted gene that encodes the Sterol regulatory element-binding transcription factor 1, a transcription factor involved in glucose metabolism and lipid homeostasis (18).
The Michigan Momentum Center Healthy Families (HF) Project is an interdisciplinary study that explores the obese phenotype among children in three age groups (12–24 months old (n=40), 3–5 years of age (n=40), or 10–12 years of age (n=52)) through measurements of weight status, biology, food environment, and parenting (19). Longitudinal paired DNA methylation levels were quantified from neonatal bloodspots and blood draws in childhood. This study design allows for a comprehensive examination of associations between age, the epigenome, and obesity during childhood. Here, we tested three hypotheses: 1. DNA methylation at growth-related genes at birth and during childhood will be associated with obesity status; 2. growth-related genes will exhibit age-related DNA methylation in childhood; 3. rates of age-related methylation will differ by childhood age group. In testing these hypotheses, we aim to identify early-life epigenetic biomarkers of childhood obesity onset, as well as determine whether age-related DNA methylation is occurring at obesity-related genes during the early stages of human life.
Methods
Healthy families study
The HF project recruited 40 families with children 12–24 months old (toddlers), 40 families with children 3–5.99 years old (preschool), and 52 families with children 10–12.99 years old (school-aged) within 1-hour driving distance to Ann Arbor, MI. During a home visit by a research assistant, families provided survey data, child and parent anthropometry measurements, and written, informed consent. Questionnaires were used to assess sociodemographic characteristics, which included measures of maternal education, child race/ethnicity, and child sex. Each child was weighed to the nearest 0.1 kg using a Detecto Portable Scale Model (Detecto, Cat. #DR550C) and measured to the nearest 0.1 cm using a Seca 214 portable stadiometer (Seca, Prod. #213 1821 009). For toddlers, length was measured using a Seca 417 infantometer (Seca, Prod. # 417 1821 009). Each individual was weighed twice; if the two readings were inconsistent by more than 0.1 kg, the individual was weighed two more times. For the height and length measurement, the individual’s position and posture were checked and the height/length measured twice; if the measurements differed by more than 0.5 cm, two more measurements were performed. Weight-for-length (WFL) or Body mass index (BMI) was then calculated. Separately, preschool and school-aged BMI z-score and toddler WFL z-score were derived using age- and sex-specific references from the US Centers for Disease Control (CDC) growth charts (20). Per CDC recommendations, for preschool and school-age children, obese was categorized as a BMI ≥ 95th percentile, and overweight as a BMI ≥ 85th percentile and <95th percentile for age and sex (21). For toddlers, obese was categorized as a WFL ≥ 95th percentile and overweight ≥ 85th percentile and <95th percentile for age and sex. Institutional Review Board (IRB) approval was obtained for all research practices (HUM00079730).
Neonatal bloodspots and blood collection
Neonatal bloodspots (n=132; collected within 24 hours of birth) for each recruited child were sourced from the Michigan Neonatal Biobank (MNB). Consent was obtained from all recruited families prior to bloodspot retrieval. Michigan Department of Health and Human Services (MDHHS) and University of Michigan (UM) IRB approvals were obtained for the Healthy Families project (UM: HUM00079730), DNA isolations from neonatal bloodspots (MDHHS: 201311–04-EA), and DNA isolations from blood draw samples (UM: HUM00086182). Before retrieval, MNB neonatal bloodspots were stored at different temperatures depending upon their collection date. As such, the current age of each recruited child corresponded to the storage method for their bloodspot – toddler and preschooler bloodspots were stored at −20°C, school-aged children’s bloodspots were stored at 4°C.Upon receiving the bloodspots, they were stored in their shipping bags at 4°C.
In addition to neonatal bloodspots, matched childhood blood samples were collected for recruited children by the Michigan Clinical Research Unit based on child assent (school-aged group)/parent consent (all ages). During this blood draw, 0.25–0.50 mL of blood (finger or heel poke) or 7 mL of blood (venous draw) were collected from the toddlers/preschoolers or school-aged children, respectively. Due to lack of consent, blood draw samples were only collected for approximately half of the recruited children (n=65). Across the three age groups – toddler, preschool, and school-aged – the childhood blood draw sample sizes were n=19, n=26, and n=20, respectively (Supplemental Table S1 (online)). After collection, all blood draw samples were stored at −80°C.
DNA isolation and bisulfite conversion
DNA was isolated from neonatal bloodspots using a modified version of the Oragene QIAamp DNA Micro Kit (Qiagen, Cat. #56304). To maximize DNA yields from bloodspots, a number of changes were made to the standard QIAamp DNA Micro Kit protocol. First, isolations were performed on four 3mm bloodspot punches rather than the recommended three punches. Second, to account for the increase in sample punches, all protocol buffer volumes were scaled up by 33%. Third, to ensure complete digestion, the heated incubation step was increased from 1 hour to overnight. Fourth, the two vortex mixing steps were increased to 2 minutes and 5 minutes, respectively. Fifth, the elution buffer was heated to 56°C prior to use. Finally, the final elution step was repeated to maximize yield. Across all bloodspot samples, average DNA yield was 19.3 ng/μL (S.D. = 9.3 ng/μL) for 25 μL samples.
For the 12–24 mo. and 3–5 yr. age groups, DNA was isolated from blood samples using the Qiagen DNA Blood Mini Kit (Qiagen, Cat. #51104). For the 10–12 yr. group, DNA was isolated from blood samples using the Qiagen Flexigene Kit (Qiagen, Cat. #51206). DNA purity and yield was measured using a NanoDrop spectrophotometer. All isolated DNA was stored at −80°C prior to use.
Genomic DNA from all samples was bisulfite converted using the Zymo Research 96-well EZ-methylation kit (Zymo Research, Cat. #D5004). Depending on the available yield of DNA, which varied across samples, bisulfite conversion was carried out on 0.2–1 μg of genomic DNA. To amplify bisulfite converted DNA, polymerase chain reaction (PCR) was performed using HotStarTaq master mix (Qiagen, Cat. #203443), RNAse-free water, forward primer (9 pmol), and biotinylated reverse primer (9 pmol). Total PCR volume was 35 μL per sample, and gel electrophoresis was used to verify PCR product identity.
Target gene pyrosequencing
Upon successful DNA purification, bisulfite conversion, and PCR amplification, % DNA methylation was quantified for a panel of target genes using quantitative DNA pyrosequencing assays specific to each gene (Supplemental Table S2 (online)). Methylation levels for all genes of interest were defined as the mean % methylation of all CpG sites in each gene’s PCR amplicon. Mean % methylation was used for all investigated loci in an effort to limit the effects of multiple testing. For LINE-1 (22), PPARA, ESR1, and LEP (23), pyrosequencing assays interrogated DNA methylation within the gene promoters. For the IGF2 and H19 imprinted genes, pyrosequencing assays interrogated differentially methylated regions within the imprinted IGF2 promoter (upstream of exon 3) and within the imprinting control region upstream of H19 (24). Finally, for the SREBF1 gene, the pyrosequencing assay interrogated DNA methylation at CpG sites within the gene body; one of these CpG sites is an RNA Pol2 binding site that has previously shown associations with vitamin B12 insufficiency (25).
Quantification of DNA methylation levels was performed using the Q96 PyroMark ID instrument (Qiagen). Percentage methylation at target CpG sites is calculated as the fraction of 5methylcytosine (5-mC) among all cytosines (methylated and unmethylated). All samples were run in duplicate, and mean methylation percentages were calculated as the mean of the technical replicates. Only replicates with coefficient of variation (CV) < 10% were included in final analyses. All pyrosequencing plates included 0%, 25%, 50%, 75%, and 100% methylation controls to ensure proper functioning of the instrument, and to provide baseline measures of methylation.
Statistical analysis
Simple summary statistics, including number of recruited individuals, sex ratios, and mean WFL or BMI z-score were calculated for each age group. For sex, child race/ethnicity, maternal education, and weight status category, a chi-squared test of equal proportions was computed to assess differences in distribution of sociodemographic characteristics across the three age groups. Analysis of variance (ANOVA) was used to test for significant differences in mean WFL or BMI z-score across the three age groups.
To test our first hypothesis, logistic regression models were used to examine the association between target gene bloodspot DNA methylation (% methylation) and obesity status (categorical outcome; obese vs. not obese). One model was run for all age groups combined, followed by separate, stratified models for each individual age group (toddler, pre-school, school-aged). As a secondary test of the association between DNA methylation and weight status, linear regression models were used to examine the association between target gene bloodspot DNA methylation (% methylation) and continuous WFL or BMI z-score. This modeling approach was repeated to examine associations between childhood blood draw DNA methylation and weight status.
For our second hypothesis, age-related methylation was measured as the absolute change in average DNA methylation for each gene from birth to follow-up in each of three age groups (toddler, preschool, school-aged). Linear mixed effect (LME) models were used to test for an association between percent DNA methylation and age at each gene region. Percent DNA methylation for all investigated loci was defined as the mean of all included CpG sites. Age, WFL or BMI z-score, and sex were included as explanatory variables in LME models. Linear mixed models for each target region also included a paired individual factor to account for matched, within-individual data. Given that each age group contained different individuals, separate age-related methylation models were run for each group. All models were run using the lme4 package in R 3.4.0 (http://www.r-project.org).
For the third hypothesis, childhood age group was delineated as a categorical variable. Using this new grouping variable, an age*group interaction term was included in the linear mixed effect models to test for differences in age-related methylation slope by recruited age group.
Significance levels were set at p ≤ 0.05 for all analyses. Results with p≤ 0.10 were considered suggestive of significance.
Results
Summary statistics
Mean weight-for-length (WFL) or BMI z-score and weight category distribution were not significantly different across the three age groups (Table 1). Child race/ethnicity was significantly different (p=0.013) in the toddler (12–24 months old) group compared to the total population, but maternal education and child sex ratio were not significantly different by age group (Supplemental Table S1 (online)).
Table 1. Healthy Families Summary Statistics by Age Group.
A total of n=132 bloodspots from children in three age groups were sourced from the Michigan Neonatal Biobank. WFL z-score, BMI z-score, and weight distributions were not significantly different by age.
| Variables | 12–24 months (n=40) |
3–5 years (n=40) |
10–12 years (n=52) |
|||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean | (SD) | Mean | (SD) | ||
| WFL or BMI z-score | 0.32 (0.94) | 0.38 | (1.06) | 0.24 | (1.28) | |
| 12–24 months (n=40) |
3–5 years (n=40) |
10–12 years (n=52) |
||||
|---|---|---|---|---|---|---|
| Count (n) | (%) | Count (n) | (%) | Count (n) | (%) | |
| Child Weight Class | ||||||
| Underweight | 1 | (2.5) | 2 | (5) | 5 | (9.6) |
| Normal | 30 | (75) | 28 | (70) | 30 | (57.7) |
| Overweight | 6 | (15) | 6 | (15) | 10 | (19.2) |
| Obese | 3 | (7.5) | 4 | (10) | 7 | (13.5) |
Associations between neonatal DNA methylation and childhood weight status
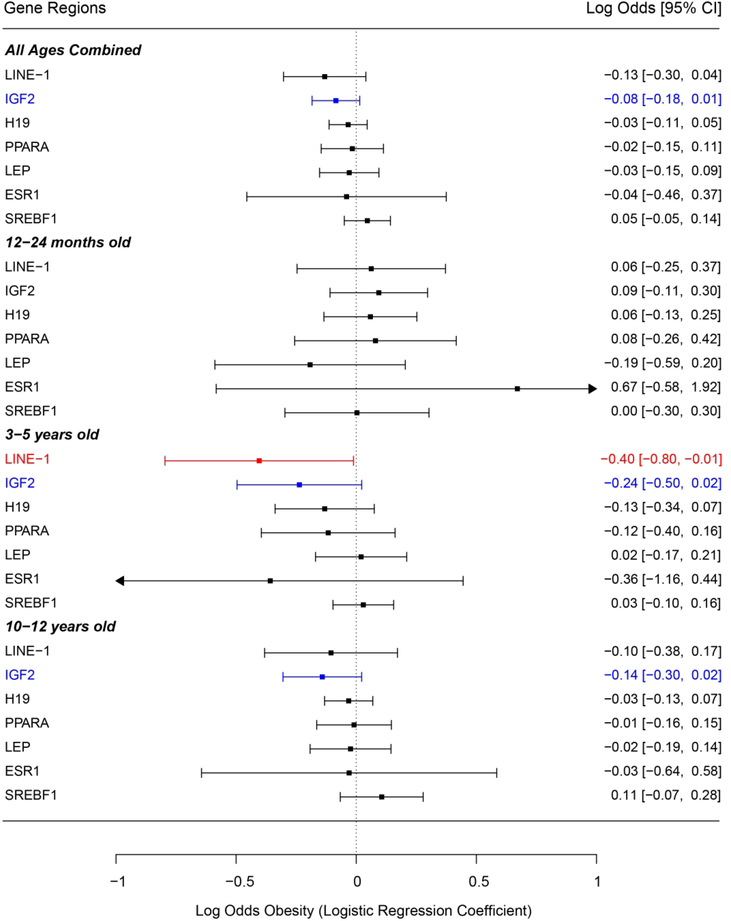
First, logistic regression models were used to examine the relationship between neonatal bloodspot target gene DNA methylation and dichotomous obesity (Y/N). Pyrosequencing replicates with CV < 10% were included in methylation analyses. No significant differences in CV% were observed across the bloodspot age groups. For the toddler group, no target genes demonstrated significant associations between bloodspot DNA methylation and obesity likelihood. However, in the preschool group, bloodspot LINE-1 methylation showed a significant negative association with obesity likelihood (log odds = −0.40, p=0.04) (Figure 1). In addition, preschool bloodspot IGF2 methylation had a marginally significant negative association with obesity likelihood (log odds = −0.24, p=0.07). Matching this pattern, the school-aged group showed a marginally significant negative association between bloodspot IGF2 methylation and obesity likelihood (log odds = −0.14, p=0.09). This negative marginal association between bloodspot IGF2 methylation and log odds of obesity also held when all age groups were combined in a single model (log odds = −0.08, p=0.09) (Figure 1). No other investigated loci showed significant associations between bloodspot DNA methylation and log odds of obesity in the preschool or school-aged groups.
Figure 1 – Associations between Bloodspot DNA Methylation and Log Odds of Obesity (Y/N).
Forest plot of logistic regression models examining associations between neonatal bloodspot DNA methylation at investigated target genes and log odds of obesity. Logistic regression coefficients are represented as log odds of obesity in the right column. The left column shows how the obesity outcome was grouped by age in the regression models. It also lists all target genes included in analyses. Associations that approach significance (p<0.10) are indicated in blue, and significant (p<0.05) associations are indicated in red. Arrows indicate that confidence interval extends beyond the scale of the plot.
Separate from the logistic regression analysis, linear regression models were used to examine the relationship between bloodspot DNA methylation and continuous WFL or BMI z-score (data not shown). For the toddler group, bloodspot LEP methylation showed a marginal negative association (β= −0.08, p=0.09) with WFL z-score, but no other investigated loci showed significant associations between bloodspot DNA methylation and WFL or BMI z-score in the toddler, preschool, or school-aged groups. This lack of significance also held true in models where all age groups were combined.
Associations between childhood DNA methylation and childhood weight status
Following up on the bloodspot DNA methylation results, simple logistic models were also used to determine whether childhood blood DNA methylation levels were associated with obesity likelihood. Just as in the bloodspot results, separate models were run for each age group. When combining all the age groups together into a single model, there was a marginally significant negative relationship between LEP methylation and obesity likelihood (log odds = −0.455, p=0.08) (Supplemental Figure S1 (online)). In the preschool group alone, we also found a marginal negative association between blood ESR1 methylation and obesity likelihood (log odds = −1.668, p=0.08) (Supplemental Figure S1 (online)). However, both of these results are difficult to interpret due to the very low sample size of the childhood blood draws.
Given the difficulty in interpreting the logistic regression results, follow-up linear regression models were used to examine the relationship between childhood blood DNA methylation and continuous WFL or BMI z-score. In the preschool group, blood PPARA methylation showed a significant positive association with BMI z-score (β= 0.210, p=0.01) (Supplemental Figure S2 (online)). While this result did not carry over to any of the other age groups, it is noteworthy that the preschool group had the largest childhood blood draw sample size. The remaining genetic loci did not demonstrate significant associations between DNA methylation and WFL or BMI z-score in any analysis.
Age-related DNA methylation in matched samples
Linear mixed effect (LME) models were used to model longitudinal patterns of age-related methylation for each target gene. Given that each age group contained different individuals, separate LME models were constructed for matched data in each age group (Table 2). Longitudinal changes in methylation reflect differences between neonatal bloodspot DNA (birth) and blood draw (childhood). All genes were modeled using the mean of all included CpG sites. For all age groups, LINE-1, H19, PPARA, LEP, and SREBF1 demonstrated significant age-related methylation (Table 2). In contrast, significance of age-related methylation at IGF2 and ESR1 differed by age, suggesting that patterns of age-related methylation are not static throughout childhood.
Table 2 – Age-related methylation by gene region and age group.
Linear mixed models were used to model age-related methylation across the three age groups. Separate models were run for each gene; beta coefficients and associated p-values for age are reported.
| Toddler Age-related Methylation | |||||
|---|---|---|---|---|---|
| Gene | N | Mean Bloodspot % Methylation (SE) | Mean 12–24 month old % Methylation (SE) | Methylation by Age - Beta coefficienta | p-value |
| LINE-1 | 18 | 80.44 (0.89) | 77.99 (0.23) | −2.431 | 0.011* |
| IGF2 | 18 | 49.10 (1.43) | 47.93 (0.59) | −1.147 | 0.310 |
| H19 | 18 | 58.14 (1.36) | 52.37 (0.56) | −5.774 | <0.001* |
| PPARA | 18 | 18.29 (0.88) | 14.45 (0.67) | −3.852 | 0.001* |
| LEP | 18 | 21.9 (0.69) | 26.93 (0.63) | 5.020 | <0.001* |
| ESR1 | 18 | 4.96 (0.23) | 4.40 (0.18) | −0.582 | 0.052 |
| SREBF1 | 13 | 50.52 (2.47) | 41.42 (2.91) | −11.750 | 0.005* |
| Preschool Age-related Methylation | |||||
|---|---|---|---|---|---|
| Gene | N | Mean Bloodspot % Methylation (SE) | Mean 3–5 year old % Methylation (SE) | Methylation by Age - Beta coefficienta | p-value |
| LINE-1 | 25 | 82.15 (0.66) | 77.68 (0.23) | −2.058 | <0.001* |
| IGF2 | 25 | 50.98 (0.91) | 47.06 (0.50) | −1.865 | <0.001* |
| H19 | 25 | 61.92 (1.39) | 52.36 (0.45) | −4.652 | <0.001* |
| PPARA | 23 | 21.62 (0.74) | 12.58 (0.49) | −4.890 | <0.001* |
| LEP | 23 | 22.05 (1/19) | 28.59 (0.65) | 3.297 | <0.001* |
| ESR1 | 23 | 5.42 (0.28) | 4.54 (0.18) | −0.430 | 0.008* |
| SREBF1 | 22 | 47.85 (1.49) | 37.89 (1.65) | −5.306 | <0.001* |
| School-Aged Age-related Methylation | |||||
|---|---|---|---|---|---|
| Gene | N | Mean Bloodspot % Methylation (SE) | Mean 10–12 year old % Methylation (SE) | Methylation by Age - Beta coefficienta | p-value |
| LINE-1 | 17 | 81.95 (0.73) | 79.69 (0.49) | −0.843 | 0.018* |
| IGF2 | 16 | 49.34 (1.71) | 45.30 (0.48) | −1.779 | 0.013* |
| H19 | 16 | 63.23 (3.51) | 52.81 (0.59) | −4.683 | 0.002* |
| PPARA | 16 | 19.98 (2.04) | 10.06 (0.47) | −3.607 | 0.002* |
| LEP | 13 | 19.52 (2.27) | 27.68 (0.74) | 2.184 | 0.047* |
| ESR1 | 15 | 4.71 (0.29) | 5.08 (0.17) | 0.120 | 0.337 |
| SREBF1 | 12 | 51.11 (2.08) | 36.38 (1.55) | −5.305 | <0.001* |
Beta coefficient for age predictor in linear mixed model
p<0.05.
Age-related DNA methylation by childhood age group
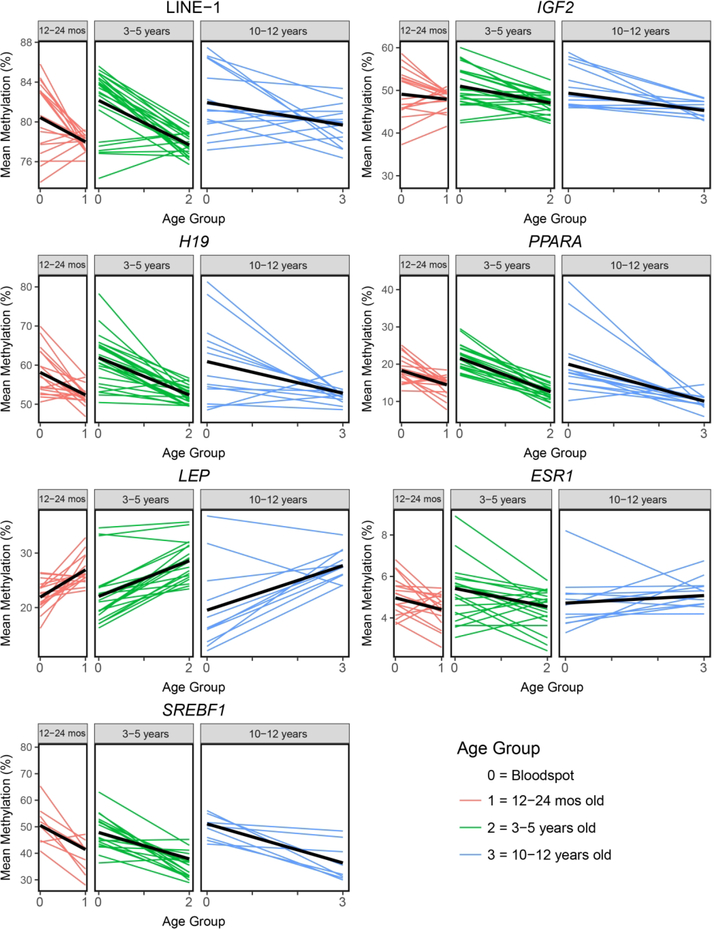
To further test whether age-related methylation varied by recruited age group, an age*group interaction term was included in linear mixed effect models for each target gene. For most of the genes, the age*group interaction term was not significant. However, both ESR1 (β=0.364; p=0.007) and LINE-1 (β=1.021; p=0.003) showed significant age*group interaction terms in the mixed models. At these genes, the slope of age-related methylation varied across the three age groups, showing a lower intensity of effect with age (Figure 2). This may reflect region-specific, controlled attenuation of age-related methylation that occurs throughout childhood.
Figure 2 – Age-related methylation at seven target loci by age group.
Spaghetti plots are used to visualize age-related methylation for all individuals at each gene region. The three separate age groups are represented in different boxes, as well as by different colors. Thick black lines correspond to linear regression lines for association between age and mean methylation at each gene region. Age groups are indicated as 0, 1, 2, and 3, which correspond to bloodspot (time=0) and follow-up at one of three age groups -- 12–24 months old (time=1), 3–5 years old (time=2), and 10–12 years old (time=3). All seven target genes demonstrated significant (p<0.05) age-related methylation between birth and at least one follow-up age.
Discussion
Associations between DNA methylation and childhood weight status
Neonatal bloodspot DNA methylation at the LINE-1 repetitive element and IGF2 imprinted locus demonstrated significant and marginally significant negative associations with obesity likelihood in preschool children, respectively. These results, while not apparent in the other investigated loci, suggest that neonatal epigenetic biomarkers may be associated with childhood obesity. While these are only preliminary results from a small number of individuals, to our knowledge, this is the first time that neonatal bloodspot DNA methylation status has been linked to obesity likelihood in matched individuals during childhood.
The significant bloodspot locus, LINE-1, is an active retrotransposon that comprises ~18% of the human genome (12). DNA methylation acts as a repressive mark at retrotransposons, blocking these repetitive elements from either duplicating in the genome or generating chimeric fusion transcripts (26). Research indicates that decreases in LINE-1 methylation can increase retrotransposon activity, leading to a reduction in overall genomic stability (12). Separate from its intrinsic retrotransposon activity, LINE-1 methylation is prevalent enough in the genome that it also serves as a useful surrogate for global methylation levels (13). As such, the negative association between LINE-1 methylation and log odds of obesity in the preschool group may reflect a global link between lower DNA methylation and obesity likelihood during a specific phase of childhood.
Distinct from LINE-1, IGF2 is an imprinted gene that encodes insulin-like growth factor II, a developmental growth factor that is active throughout life in humans (27). As an imprinted gene, IGF2 demonstrates parent-of-origin-dependent mono-allelic expression, a pattern at least partially controlled via differentially methylated regions (DMRs) (28). Recent studies demonstrate associations between developmental IGF2 DMR methylation and newborn growth indices; however, directionality of these effects varies by study, making interpretation difficult. For example, one study found a significant association between lower umbilical cord blood IGF2 DMR methylation and higher plasma IGF2 protein level, as well as a positive association between plasma IGF2 protein levels and birth weight (29). Meanwhile, another group showed that greater placental IGF2 DMR methylation was positively correlated with newborn length, head circumference, and weight (30). Our results support the first study, showing a marginal link between lower bloodspot IGF2 DNA methylation and higher obesity likelihood during specific stages of childhood.
In the cross-sectional blood draw DNA methylation models, PPARA promoter methylation showed a significant positive association with BMI z-score in the preschool children (Supplemental Figure S2 (online)). PPARA encodes the peroxisome proliferator-activated receptor alpha (PPAR-α) protein, a nuclear receptor that regulates fatty acid metabolism (15). Greater expression of this gene leads to breakdown of fatty acids, potentially leading to reductions in dyslipidemia and obesity (15, 31). As such, it is expected that higher methylation at the PPARA promoter would be associated with lower PPARA gene expression and an increased risk of obesity. The significant positive association between preschool PPARA promoter methylation and BMI z-score matches this expectation, suggesting that PPARA promoter methylation may play a role in childhood obesity development. However, this result was not consistent across age groups, making interpretation of these data difficult.
Age-related methylation in matched samples
A number of human cohort studies have examined the effect of age on the epigenome, with most of the available literature utilizing cross-sectional samples from pediatric or elderly populations (32–34). Here, we utilized matched bloodspot and blood samples from children in multiple age groups to investigate the effects of aging on the epigenome throughout different phases of childhood. We found significant age-related methylation in all investigated genetic loci, with the directionality and magnitude of age effects varying by region. This matches previous studies, which have shown that the childhood methylome varies with age in a gene-specific fashion (9, 34). The directionality of age-related methylation was consistent across all three age groups for six out of the seven investigated loci, suggesting that aging effects during childhood are generally consistent at a given gene region.
Of the seven investigated loci, six showed age-related hypomethylation (LINE-1, IGF2, H19, PPARA, ESR1, SREBF1) and one showed age-related hypermethylation (LEP), suggesting a general pattern of age-related hypomethylation during childhood. This matches previous work in pediatric populations, which has shown a skew toward age-related hypomethylation during childhood aging (35), although the exact directionality of age-related methylation during childhood is gene-specific (36). Future work should further investigate whether the age-related hypomethylation during childhood is related to specific phenotypic outcomes.
Age-related methylation by childhood age group
Beyond demonstrating age-related methylation during childhood, we also showed that the magnitude of age-related methylation can vary by developmental stage. This is most apparent at LINE-1, LEP, and ESR1, where rates of age-related methylation significantly diminished with increasing age (Figure 2). These results indicate that rates of age-related methylation are not necessarily static during childhood, but may instead follow regulated trajectories. For example, the ESR1 gene shifted from significant age-related hypomethylation in preschool children to non-significant age-related hypermethylation in the school-aged children (Figure 2). This later-life pattern of hypermethylation was not unexpected, since previous studies in adult human colon tissue have demonstrated significant age-related hypermethylation at the ESR1 promoter (37). However, the shift from hypo- to hypermethylation with during childhood indicates that early-life may be a period of particular epigenetic volatility at the ESR1 promoter region. This idea matches evidence from previous studies, which show that patterns of age-related methylation are different during childhood and adulthood (34, 38), particularly at genes related to development (9, 39). Building on our results, future studies must not only capture cross-sectional measures of the epigenome early and late in life, but also characterize how longitudinal epigenetic marks vary during the child-to-adult transition.
Limitations and future directions
Our study was unique in many ways, but the findings are limited by the small sample sizes in analyses with the neonatal bloodspots (n=40, n=40, n=52) and especially in analyses with matching blood samples at childhood (n=16, n=26, n=20). While we observed several significant (p<0.05) and marginally significant (p<0.1) associations between target gene DNA methylation and childhood obesity outcomes, none would withstand correction for multiple testing. As such, it is possible that our significant results are false positives. To validate the biological plausibility of the observed associations, we recommend future longitudinal studies with increased cohort size and longer follow-up across successive life stages. Follow-up studies should also consider expanding beyond target gene pyrosequencing, instead measuring epigenome-wide DNA methylation from neonatal bloodspots. This may lead to the identification of additional gene regions where bloodspot DNA methylation predicts either childhood or adulthood obesity likelihood, strengthening the utility of bloodspot epigenetics as a clinically relevant tool for chronic disease risk estimation.
In addition to a small sample size, interpretation of the bloodspot DNA methylation results may be confounded by differences in storage conditions and handling of neonatal bloodspots across the age groups. As mentioned in the methods, the current age of each recruited child corresponded to the storage method for their bloodspot – toddler and preschooler bloodspots were stored at −20°C, school-aged children’s bloodspots were stored at 4°C. Previous research has compared the stability and quality of DNA isolated from fresh bloodspots, archival bloodspots stored at −20°C, and archival bloodspots stored at room temperature, showing that DNA yield is similar for all three types of bloodspot sample, but that DNA fragmentation is increased in archival bloodspots stored at room temperature (40). Based on this data, it’s likely that the school-aged group, where bloodspots were stored at 4°C, had increased DNA fragmentation compared to the younger age groups. However, given that the DNA pyrosequencing assays used in this study have small amplicons ranging from 93–383 bp, it is unlikely that increased rates of DNA fragmentation had a large effect on PCR amplification.
Given that contemporary blood draws were collected from different individuals across multiple age groups, comparisons of age-related methylation rates across the different stages of childhood are limited by inter-individual variability. However, within each age group, we used matched bloodspots and blood draw samples from the same individuals to directly measure the effects of aging on DNA methylation during childhood. As such, while comparisons across separate age groups are biased, the age-related methylation data still demonstrated intra-individual changes in DNA methylation during childhood. In addition to inter-individual variability, DNA methylation levels also vary by tissue, so it remains to be seen whether the documented target gene age-related methylation in blood is consistent in other human tissues during childhood. Additionally, we did not correct for cellular heterogeneity over time, and the documented age-related methylation could be a reflection of shifting blood cell types with age. Future work should determine white blood cell percentage estimates from both neonatal bloodspots and childhood blood samples, then adjust for bias using a normalization method. Despite this uncertainty, there were some distinct advantages to using blood samples. First, they provided a matched tissue to retroactively collected neonatal bloodspots, allowing for direct measurement of intra-individual age-related methylation during childhood. In addition, blood is minimally invasive to collect from a cohort of children and was the best available biological sample for children in the Healthy Families project.
Overall, we demonstrated negative associations between bloodspot target gene DNA methylation and obesity likelihood in preschool children, suggesting that the neonatal methylome may be useful tool for estimating obesity risk in childhood. In addition, all investigated genetic loci demonstrated significant age-related methylation during childhood, suggesting that patterns of epigenetic aging are established during the first decade of life. To improve the utility of these results, the dynamics of epigenome-wide bloodspot DNA methylation should be further evaluated in larger cohorts that examine specific exposure to environmental chemicals or modified behaviors during childhood.
Supplementary Material
Acknowledgements
We acknowledge the Michigan Neonatal Biobank for providing neonatal bloodspots.
Statement of Financial Support
Data for this study were collected as a part of the Healthy Families Project, funded by the Michigan Momentum Center. The work was supported by the MCubed program at the University of Michigan; the Michigan NIEHS Core Center P30 ES017885; and the T32 ES007062.
Footnotes
Disclosure statement
The authors declare no conflicts of interest.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016 Key findings Data from the National Health and Nutrition Examination Survey. 2017.
- 2.Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol 2010;316:104–108. [DOI] [PubMed] [Google Scholar]
- 3.Loos RJF, Janssens ACJW. Predicting Polygenic Obesity Using Genetic Information. Cell Metab 2017;25:535–543. [DOI] [PubMed] [Google Scholar]
- 4.Bernal AJ, Jirtle RL. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol 2010;88:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Essex MJ, Boyce WT, Hertzman C, et al. Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Dev 2013;84:58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tellez-Plaza M, Tang WY, Shang Y, et al. Association of global DNA methylation and global DNA hydroxymethylation with metals and other exposures in human blood DNA samples. Env Heal Perspect 2014;122:946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martino D, Loke YJ, Gordon L, et al. Longitudinal, genome-scale analysis of DNA methylation in twins from birth to 18 months of age reveals rapid epigenetic change in early life and pair-specific effects of discordance. Genome Biol 2013;14:R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madrigano J, Baccarelli A, Mittleman MA, et al. Aging and epigenetics: longitudinal changes in gene-specific DNA methylation. Epigenetics 2012;7:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urdinguio RG, Torró MI, Bayón GF, et al. Longitudinal study of DNA methylation during the first 5 years of life. J Transl Med 2016;14:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath S, Erhart W, Brosch M, et al. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci U S A 2014;111:15538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahl S, Drong A, Lehne B, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 2016;541:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodić N, Burns KH, Vallot C, Castoro R, Chung W. Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms? PLoS Genet 2013;9:e1003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang AS, Estécio MRH, Doshi K, Kondo Y, Tajara EH, Issa J-PJ. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res 2004;32:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang R-C, Galati JC, Burrows S, et al. DNA methylation of the IGF2/H19 imprinting control region and adiposity distribution in young adults. Clin Epigenetics 2012;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon M The role of PPARα in lipid metabolism and obesity: Focusing on the effects of estrogen on PPARα actions. Pharmacol Res 2009;60:151–159. [DOI] [PubMed] [Google Scholar]
- 16.Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: An epigenetic landscape. Life Sci 2015;140:57–63. [DOI] [PubMed] [Google Scholar]
- 17.Mauvais-Jarvis F Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 2011;22:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferré P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 2007;68:72–82. [DOI] [PubMed] [Google Scholar]
- 19.Acharya Y, Norton EC, Lumeng JC. The Effect of Financial Compensation on Willingness to Supply a Child?s Blood Sample: A Randomized Controlled Trial. Eval Health Prof 2017;40:359–371. [DOI] [PubMed] [Google Scholar]
- 20.Center for Health Statistics N. Vital and Health Statistics, Series 11, No. 246 (5/2002)--updated 6/30/2010. 2000 CDC Growth Charts for the United States: Methods and Development, 2000. (https://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf). [PubMed]
- 21.Centers for Disease Control and Prevention. Defining Childhood Obesity, 2017. (https://www.cdc.gov/obesity/childhood/defining.html).
- 22.Virani S, Dolinoy DC, Halubai S, et al. Delivery type not associated with global methylation at birth. Clin Epigenetics 2012;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesseur C, Armstrong DA, Paquette AG, Koestler DC, Padbury JF, Marsit CJ. Tissue-specific Leptin promoter DNA methylation is associated with maternal and infant perinatal factors. Mol Cell Endocrinol 2013;381:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoyo C, Murtha AP, Schildkraut JM, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 2011;6:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adaikalakoteswari A, Finer S, Voyias PD, et al. Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin Epigenetics 2015;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, Santos JH, Feng J, et al. A Novel Analytical Strategy to Identify Fusion Transcripts between Repetitive Elements and Protein Coding-Exons Using RNA-Seq. PLoS One 2016;11:e0159028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao W, D’Amore PA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev 2008;19:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobi EW, Slagboom PE, van Dongen J, et al. Prenatal Famine and Genetic Variation Are Independently and Additively Associated with DNA Methylation at Regulatory Loci within IGF2/H19. PLoS One 2012;7:e37933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyo C, Fortner K, Murtha AP, et al. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control 2012;23:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St-Pierre J, Hivert MF, Perron P, et al. IGF2 DNA methylation is a modulator of newborn’s fetal growth and development. Epigenetics 2012;7:1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung S, Kim YJ, Yang SJ, Lee Y, Lee M. Nutrigenomic Functions of PPARs in Obesogenic Environments. PPAR Res 2016;2016:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heyn H, Li N, Ferreira HJ, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci 2012;109:10522–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madrigano J, Baccarelli AA, Mittleman MA, et al. Aging and epigenetics: Longitudinal changes in gene-specific DNA methylation. Epigenetics 2012;7:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alisch RS, Barwick BG, Chopra P, et al. Age-associated DNA methylation in pediatric populations. Genome Res 2012;22:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C-J, Bonder MJ, Söderhäll C, et al. The emerging landscape of dynamic DNA methylation in early childhood. BMC Genomics 2017;18:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones MJ, Goodman SJ, Kobor MS. DNA methylation and healthy human aging. Aging Cell 2015;14:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaz AM, Wong C-J, Dzieciatkowski S, Luo Y, Schoen RE, Grady WM. Patterns of DNA methylation in the normal colon vary by anatomical location, gender, and age. Epigenetics 2014;9:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpkin AJ, Suderman M, Gaunt TR, et al. Longitudinal analysis of DNA methylation associated with birth weight and gestational age. Hum Mol Genet 2015;24:3752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acevedo N, Reinius LE, Vitezic M, et al. Age-associated DNA methylation changes in immune genes, histone modifiers and chromatin remodeling factors within 5 years after birth in human blood leukocytes. Clin Epigenetics 2015;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjoholm MIL, Dillner J, Carlson J. Assessing Quality and Functionality of DNA from Fresh and Archival Dried Blood Spots and Recommendations for Quality Control Guidelines. Clin Chem 2007;53:1401–1407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.