Abstract
Although the resting ankle–brachial index (ABI) is commonly used as a httptool to diagnose peripheral artery disease (PAD), several additional indices measured after exercise may have increased sensitivity for identifying PAD. The aim of this study was to determine the utility of resting ABI and three post-exercise physiological parameters for diagnosing PAD confirmed by arterial imaging studies. For each qualifying study, we assessed the performance measures for identifying PAD for resting ABI < 0.90, exercise ABI < 0.90, a decrease in ABI > 20% with exercise, and a decrease in ankle pressure > 30 mmHg with exercise. Of the 199 exams that met our inclusion criteria, imaging showed a > 75% stenotic lesion in at least one limb in 138 (69%) of patients. For stenoses > 75%, resting ABI < 0.90 had a sensitivity of 64% (95% CI: 56–72%) and exercise ABI < 0.90 had a sensitivity of 88% (95% CI: 82–93%). The sensitivity for a post-exercise ABI decrease > 20% was 67% (95% CI: 59–75%) and the sensitivity for a decrease in ankle pressure > 30 mmHg was 4% (95% CI: 2–9%). For individuals with a normal resting ABI but stenotic lesions > 75% confirmed by imaging (n=49), the addition of exercise ABI testing correctly identified an additional 25% of this population. Overall, exercise ABI < 0.90 exhibits a greater sensitivity for detecting PAD compared to resting ABI. Furthermore, exercise ABI < 0.90 had added clinical utility in patients with normal resting ABIs and was superior to other commonly used exercise indices.
Keywords: ankle–brachial index (ABI), peripheral artery disease (PAD), vascular imaging/diagnostics, vascular physiological testing
Introduction
The ankle–brachial index (ABI) is the ratio of the higher of the dorsalis pedis or posterior tibial pressures to the highest of the systolic blood pressures measured in both arms. Under normal conditions, the systolic blood pressure increases as the waveform propagates distally from the heart. This is due both to the incorporation of reflective waves from peripheral arterial beds1 as well as remodeling of the arterial wall in lower extremity arteries.2 Stenotic lesions in the lower extremity arteries disrupt distal waveform propagation and can lower the ABI depending on their hemodynamic significance. Since its initial description in 1950,3 the ABI has become the most common non-invasive means of detecting peripheral artery disease (PAD),4,5 and current professional guidelines recommend performing the test in all individuals with signs or symptoms of PAD to confirm the diagnosis using a cut-off of < 0.90.6
Some patients present with lower extremity symptoms concerning for PAD but with normal ABIs. In these instances, clinicians may use exercise testing followed by ABI measurements as a non-invasive means of diagnosing PAD. During exercise, central aortic pressure increases, and peripheral blood pressure at the ankles, decreases as arterial beds dilate and deliver more oxygenated blood to meet the metabolic demands of the leg muscles.7 In healthy patients, this leads to a small but detectable decrease in ABI following exercise.8,9 However, atherosclerotic plaques within the leg vessels cause an exaggerated drop in ankle pressure following exercise.10,11
Data on post-exercise thresholds for diagnosing PAD vary, in part due to differences in exercise protocols. Some studies suggest an ABI < 0.90 following exercise is diagnostic of PAD,7,12 while others demonstrate a > 20% decrease in ABI10, or an absolute decrease in ankle pressure > 30 mmHg13 in diseased limbs after exercise. Based on these findings, professional guidelines recommend all three thresholds as valid measures for diagnosing PAD.7,12 However, the data supporting each exercise parameter are limited, and many of these studies lack rigorous imaging confirmation of hemodynamically significant PAD. Therefore, we hypothesized that testing the performance of each measure along with angiographic confirmation of PAD would identify the most sensitive exercise ABI parameter for detecting PAD.
Methods
Study population and inclusion criteria
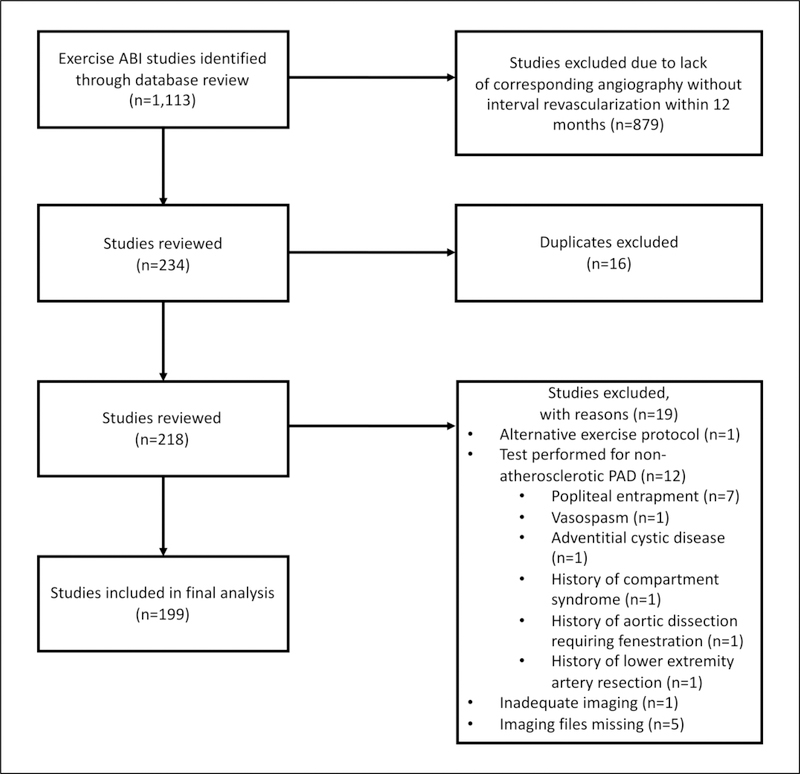
In this retrospective analysis, we reviewed all consecutive ABI studies (n=1113) performed in the vascular laboratory at Brigham and Women’s Hospital (Boston, MA, USA) from January 2000 to March 2015 (Figure 1). Studies were excluded if there was not a corresponding angiographic imaging study (computerized tomography (CTA), magnetic resonance (MRA), or invasive angiography) within 12 months of the ABI study or if patients had an intervening surgical or percutaneous revascularization (n=879). Of the remaining 234 studies, 16 duplicate tests were removed from the analysis, and 12 studies assessing non-atherosclerotic PAD were also excluded. Additionally, seven studies were excluded due to inadequate or missing imaging files or alternative exercise protocols. The final analysis consisted of 199 exercise ABI studies (398 limbs). The study was approved by the institutional review board of Brigham and Women’s Hospital.
Figure 1.
Flow chart for ABI study selection. ABI, ankle–brachial index; PAD, peripheral artery disease.
Exercise ABI protocol
At baseline, all patients underwent systolic blood pressure measurements of bilateral brachial, posterior tibial, and dorsalis pedis arteries using an 8 MHz Doppler probe and 10 cm cuff. Measurements were recorded using a MultiLab digital peripheral vascular diagnostic system (Unetixs Vascular, Inc., North Kingstown, RI, USA). The arm with the higher of the two brachial pressures and the location of the higher ankle pressure in each limb were used for subsequent measurements. Subjects then walked on a treadmill with a 10% gradient at 1.5 mph (24 km/h) for 5 minutes or until symptoms prevented them from continuing. The brachial and ankle pressures were again measured immediately following exercise and at 2 minute intervals for a total of 6 minutes or until they returned to their pre-exercise values. The ABI for each limb at rest and following exercise was calculated by dividing the higher ankle pressure by the higher of the two brachial pressures. For rest and exercise ABIs, we used a cut-off of 0.90.7,12 Additional exercise measures included a decrease in ABI > 20% after exercise or a decrease in ankle pressure > 30 mmHg after exercise.7
Angiographic confirmation of PAD
All CTA and MRA studies were interpreted by vascular radiology physicians at Brigham and Women’s Hospital at the time the study was performed. Stenotic lesions within each segment were measured using digital calipers. Invasive angiograms were reviewed in a blinded manner by the authors, and measurements were made using either digital or manual calipers (Prestige Medical, Northridge, CA, USA). Lesions were classified into the following categories: absent disease, < 50% stenosis, 50–75% stenosis, and > 75% stenosis/occluded. Anatomic lesions were categorized as aortoiliac (aortic bifurcation, common iliac, external iliac, and internal iliac arteries), femoropopliteal (common femoral, superficial femoral, profunda femoris, and popliteal arteries), and infrageniculate (anterior tibial, tibioperoneal trunk, posterior tibial, and peroneal arteries). In the event of a stenosis within a surgical bypass graft, the lesion was classified according to the most proximal corresponding native vessel affected.
Laboratory and demographic measures
Basic demographic data were collected from outpatient and inpatient documentation in the electronic medical record for all qualifying patients. Details on smoking status as well as diagnoses of hypertension, diabetes mellitus, coronary artery disease (CAD), and stroke were not considered beyond 12 months of the ABI study to ensure these data were representative of disease states at the time of testing. Diagnoses of hypertension, CAD, and stroke were defined by clinical documentation of these diseases in the medical history. Diabetes mellitus was confirmed by recording of this diagnosis in the medical record, documentation of oral or insulin therapy for diabetes, or a hemoglobin A1c ≥ 6.5%. There were missing data in 12 patients for hypertension, 11 patients for diabetes mellitus, CAD, and history of stroke, 17 patients for smoking status, 74 patients for total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides, and 75 patients for low-density lipoprotein (LDL) cholesterol. History of lower extremity revascularization was confirmed by review of the medical record and available imaging studies. All laboratory testing was performed at Brigham and Women’s Hospital. Similarly, laboratory measures were only included in the analysis if they were collected within 12 months of ABI testing.
Statistical analysis
Continuous data were summarized as mean and standard deviation. Categorical data were summarized as total number and percentages. Between-group differences were assessed by the two-sample t-test for continuous data and Fisher’s exact test for categorical data. The two-sided p-value cut-off for all analyses was 0.05. Patients were classified as having an abnormal ABI or exercise test if they were abnormal in one or both legs. Similarly, PAD was defined as an abnormal imaging study in one or both legs. Contingency tables were constructed for comparisons of patients with an abnormal ankle pressure index against PAD defined as a stenosis > 75%. Analyses were performed per patient, rather than per limb, and patients were classified based on the most severe stenosis at any given level. We used similar methods for comparisons of patients with PAD defined as any stenosis > 50%, and subgroup analyses were performed among individuals with a history of diabetes mellitus. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy along with exact binomial 95% confidence intervals (95% CI) were calculated for all test parameters. Additionally, we created receiver operating characteristic (ROC) curves for each exercise test parameter. All analyses were performed using Stata 14 software (StataCorp, College Station, NC, USA).
Results
Baseline characteristics of the study population are listed in Table 1. The majority of individuals were men. Comparing those with and without severe PAD (stenoses > 75%), there was no statistically significant difference in the prevalence of hypertension, diabetes, CAD, and stroke. Approximately 30% of the study cohort had prior surgical or percutaneous lower extremity intervention. MRA was the most common imaging modality used to confirm PAD followed by invasive angiography and CTA. In the total study population, the mean resting ABI in each limb was 0.91.
Table 1.
Baseline characteristics in individuals with severe PAD.
| Any stenosis > 75% n=138 | No stenosis > 75% n=61 | p | |
|---|---|---|---|
| Age, years, mean (SD) | 65 (11) | 57 (13) | 0.0001 |
| Men, n (%) | 82 (59) | 28 (46) | 0.09 |
| Hypertension, n (%) | 100 (72) | 34 (56) | 0.16 |
| Diabetes mellitus, n (%) | 42 (30) | 12 (20) | 0.29 |
| Coronary artery disease, n (%) | 5 (4) | 17 (28) | 0.39 |
| Prior stroke, n (%) | 6 (4) | 3 (6) | 0.78 |
| Prior revascularization, n (%) | 36 (26) | 24 (39) | 0.07 |
| Modality used to assess PAD | 0.75 | ||
| Invasive, n (%) | 34 (25) | 18 (30) | |
| Computerized tomography, n (%) | 13 (9) | 6 (10) | |
| Magnetic resonance imaging, n (%) | 91 (66) | 37 (61) | |
| Ever smoker, n (%) | 106 (77) | 48 (79) | 0.37 |
| Systolic blood pressure, mmHg, mean (SD) | 141 (19) | 137 (17) | 0.11 |
| Creatinine, mg/dL, mean (SD) | 1.02 (0.30) | 1.04 (0.36) | 0.82 |
| Total cholesterol, mg/dL, mean (SD) | 155 (40) | 183 (42) | 0.0005 |
| HDL-C, mg/dL, mean (SD) | 43 (14) | 50 (17) | 0.024 |
| LDL-C, mg/dL, mean (SD) | 82 (32) | 104 (34) | 0.0009 |
| Triglycerides, mg/dL, mean (SD) | 150 (88) | 167 (115) | 0.38 |
HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; PAD, peripheral artery disease.
Table 2 describes the most severe stenosis in either limb by anatomic distribution. Based on lower extremity angiographic imaging, 20% of study participants had either no disease or a maximum narrowing < 50%, and 69% of participants had a > 75% stenosis in at least one anatomic distribution (Table 2). Among detected stenoses, those classified as > 75% were most prevalent; these were most common in infrageniculate vessels followed by femoropopliteal and aortoiliac vessels. Overall, the most common abnormal test result was an exercise ABI < 0.90 (84%) followed by a 20% decrease in ABI with exercise (61%), a resting ABI < 0.90 (52%), and a 30 mmHg decrease in ankle pressure (4%) (data not shown).
Table 2.
Highest degree of stenosis in either leg in each arterial bed.
| No disease n (%) | < 50% stenosis n (%) | 50–75% stenosis n (%) | > 75% stenosis n (%) | |
|---|---|---|---|---|
| Aortoiliac | 75 (38) | 40 (20) | 33 (17) | 50 (25) |
| Femoropopliteal | 62 (33) | 28 (15) | 27 (14) | 72 (38) |
| Infrageniculate | 80 (43) | 14 (7) | 12 (6) | 82 (44) |
| Maximum severity of disease at any location | 18 (9) | 21 (11) | 22 (11) | 138 (69) |
Data are missing for one patient in the aortoiliac distribution, 10 patients in the femoropopliteal distribution, and 11 patients in the infrageniculate distribution due to incomplete imaging.
Among the parameters tested, exercise ABI < 0.90 was the most sensitive for diagnosing > 75% stenoses (88%, 95% CI: 82–93%), followed by a 20% decrease in ABI with exercise (67%, 95% CI: 59–75%), and resting ABI < 0.90 (64%, 95% CI: 56–72%) (Table 3). Change in ankle pressure exhibited poor sensitivity for > 75% stenoses (4%). In contrast, change in ankle pressure had the greatest specificity (97%, 95% CI: 89–100%), followed by resting ABI (75%, 95% CI: 63–86%), a 20% decrease in ABI (53%, 95% CI: 39–65%), and exercise ABI (26%, 95% CI: 16–39%). Results for PPV, NPV, and accuracy are also listed in Table 3. We found similar results for the diagnosis of a > 50% stenosis (Table 3). Additionally, all test parameters performed similarly for both > 75% and > 50% stenoses among individuals with diabetes mellitus (Supplemental Table 1).
Table 3.
Test characteristics of ankle pressure indices for identifying stenoses > 75% and > 50% in any limb and arterial bed in 199 subjects.
| Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | Accuracy (95% CI) | |
|---|---|---|---|---|---|
| Identifying stenosis > 75% | |||||
| Resting ABI < 0.90 | 64% (56–72%) | 75% (63–86%) | 86% (77–92%) | 48% (38–59%) | 68% (61–74%) |
| Exercise ABI < 0.90 | 88% (82–93%) | 26% (16–39%) | 73% (66–80%) | 50% (32–68%) | 69% (62–76%) |
| Exercise ankle pressure decrease > 30 mmHg | 4% (2–9%) | 97% (89–100%) | 75% (35–97%) | 31% (24–38%) | 32% (26–40%) |
| Exercise ABI > 20% decrease | 67% (59–75%) | 53% (39–65%) | 76% (68–84%) | 42% (30–53%) | 63% (56–70%) |
| Identifying stenosis > 50% | |||||
| 50% Resting ABI < 0.90 | 61% (53–68%) | 82% (67–93%) | 93% (87–97%) | 34% (24–44%) | 65% (58–74%) |
| Exercise ABI < 0.90 | 88% (81–92%) | 31% (17–48%) | 84% (77–89%) | 38% (21–56%) | 76% (70–82%) |
| Exercise ankle pressure decrease > 30 mmHg | 4% (1–8%) | 95% (82–99%) | 75% (35–97%) | 19% (14–26%) | 21% (16–28%) |
| Exercise ABI > 20% decrease | 65% (51–72%) | 54% (37–70%) | 85% (78–91%) | 27% (18–39%) | 63% (56–70%) |
ABI, ankle–brachial index.
Supplemental Figure 1 displays ROC curves for > 75% stenoses. Based on the area under the curve (AUC), resting ABI performed best (AUC=0.6995), followed by a decrease in exercise ABI > 20% (AUC=0.5993), exercise ABI (AUC=0.5732), and a decrease in ankle pressure > 30 mmHg (AUC=0.5054). We found similar results for > 50% stenoses (Supplemental Figure 2). In analyses restricted to individuals with diabetes, exercise ABI performed best for lesions > 75% (AUC=0.8040) (Supplemental Figure 3), whereas resting ABI performed best for lesions > 50% (0.7134) (Supplemental Figure 4).
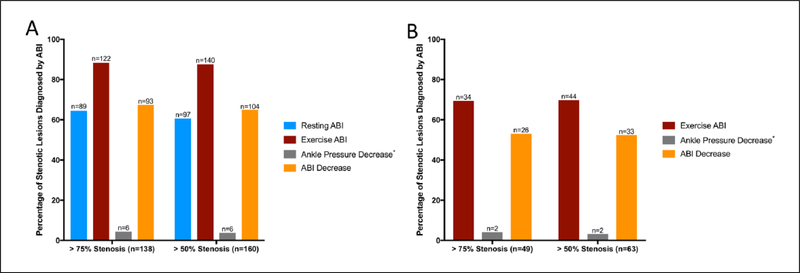
Figure 2 displays the results of both resting and exercise ABI parameters in all 199 exercise tests. In subjects with > 75% stenotic lesions, exercise ABI correctly diagnosed PAD in 122 individuals (Figure 2a). Compared to resting ABI, exercise ABI yielded a correct diagnosis of PAD in an additional 33 subjects. Resting ABI and percent change in ABI yielded similar numbers of correctly diagnosed individuals, while drop in ankle pressure only diagnosed six individuals. Overall, similar results were seen in individuals with > 50% stenoses (Figure 2a).
Figure 2.
Proportion of individuals with stenotic lesions diagnosed by ABI testing. (a) Performance of resting and post-exercise ABI parameters in all 199 individuals; (b) performance of post-exercise ABI parameters among 95 individuals with normal resting ABIs. *Measures missing from two cases. ABI, ankle–brachial index.
Incremental value of exercise ankle indices beyond resting ABI
Among individuals with normal resting ABI studies, an exercise ABI < 0.90 had the highest sensitivity (69%, 95% CI: 55–82%) of the exercise indices to identify a subject with a > 75% stenosis (Table 4). Both a 20% decrease in ABI and a change in ankle pressure with exercise exhibited poor sensitivity for these subjects (53% and 4%, respectively). We found similar results in limbs with > 50% stenoses and normal resting ABIs (Table 4). In terms of specificity, change in ankle pressure performed best (96%, 95% CI: 85–100%) followed by a 20% decrease in ABI (54%, 95% CI: 39–69%) and exercise ABI < 0.90 (35%, 95% CI: 21–50%). Additional performance measures are displayed in Table 4. Similar results were also seen with > 50% stenoses (Table 4).
Table 4.
Incremental value of exercise indices among the 95 subjects with normal resting ABIs (≥ 0.90).
| Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | Accuracy (95% CI) | |
|---|---|---|---|---|---|
| Identifying stenosis > 75% | |||||
| Exercise ABI < 0.90 | 69% (55–82%) | 35% (21–50%) | 53% (40–66%) | 52% (33–70%) | 53% (42–63%) |
| Exercise ankle pressure decrease > 30 mmHg | 4% (1–14%) | 96% (85–100%) | 50% (7–93%) | 48% (38–59%) | 48% (38–59%) |
| Exercise ABI > 20% decrease | 53% (38–68%) | 54% (39–69%) | 55% (40–70%) | 52% (37–67%) | 54% (43–64%) |
| Identifying stenosis > 50% | |||||
| Exercise ABI < 0.90 | 70% (57–81%) | 38% (21–56%) | 69% (56–80%) | 39% (22–58%) | 59% (48–69%) |
| Exercise ankle pressure decrease > 30 mmHg | 3% (0–11%) | 94% (79–99%) | 50% (7–93%) | 33% (23–43%) | 33% (24–44%) |
| Exercise ABI > 20% decrease | 52% (39–65%) | 56% (38–74%) | 70% (55–83%) | 38% (24–53%) | 54% (43–64%) |
ABI, ankle–brachial index.
Supplemental Figure 5 displays ROC curves for > 75% stenoses among individuals with normal resting ABIs. Overall, a 20% decrease in ABI exhibited the greatest accuracy (AUC=0.5370) followed by exercise ABI (AUC=0.5209) and drop in ankle pressure (AUC=0.4986). We found similar results for > 50% stenoses in individuals with normal resting ABIs (Supplemental Figure 6).
In 49 subjects with > 75% stenotic lesions but normal resting ABIs, exercise ABI correctly diagnosed PAD in an additional 34 cases (Figure 2b). A 20% decrease in ABI following exercise yielded an additional 26 correct diagnoses, while a decrease in ankle pressure only diagnosed two individuals. Overall, similar results were seen in individuals with > 50% stenoses.
Discussion
Our study demonstrated that an exercise ABI < 0.90 was more sensitive than resting ABI < 0.90 with a sensitivity of 88% for both > 50% and > 75% stenoses. Neither a decrease in ABI > 20% nor a decrease in ankle pressure > 30 mmHg was as sensitive as an exercise ABI < 0.90 for detecting PAD. Resting ABI and a post-exercise ABI decrease > 20% had similar sensitivities in our analysis, while a decrease in ankle pressure > 30 mmHg had a sensitivity of less than 5% for all degrees of stenotic lesions. Additionally, among individuals with severe PAD but normal resting ABIs, exercise ABI < 0.90 was the most sensitive post-exercise parameter for correctly reclassifying these individuals. The addition of exercise testing with a post-exercise ABI threshold of < 0.90 led to correct diagnoses of PAD in an additional 25% of individuals with > 50% stenoses and 28% of individuals with > 75% stenoses. Figure 2 summarizes the performance of each ABI parameter.
Not surprisingly, there was a trade-off between sensitivity and specificity in our analysis. Decrease in ankle pressure exhibited the greatest specificity for > 50% and > 75% stenoses in the total population as well as among individuals with normal resting ABIs. Additionally, based on ROC curve analysis, resting ABI had the best trade-off for sensitivity and specificity for > 50% and > 75% stenoses in the total study population. However, exercise ABI had the highest sensitivity. Among individuals with normal resting ABI, exercise ABI < 0.90 and an ABI decrease > 20% performed similarly in terms of accuracy. Nonetheless, given that exercise testing is typically used in individuals with suspected PAD, we feel sensitivity is the most clinically relevant performance parameter for this diagnostic test.
Studies testing ABI indices have evaluated distinct patient groups and have had deficiencies in their standards used to assess PAD. Early studies reported a sensitivity of resting ABI of up to 94–97% depending on the cut-off used.8,10 However, a contemporary review of eight published reports found the sensitivity of ABI ≤ 0.90 to be as variable as 15–79% for detecting PAD.14 One potential explanation for the discrepancy is the lack of consistent confirmation of PAD as well as differences in the study populations. In one analysis of 218 patients, 58 limbs met criteria for critical limb ischemia and therefore had a high pre-test probability for an abnormal ABI.10 Although angiography was used to confirm PAD in this study, only 33% of patients ultimately underwent angiography. Using an ABI cut-off of 0.97 with PAD confirmation by pulse wave Doppler waveform analysis, another study found a sensitivity of 79%.15 A large analysis of 585 patients who underwent color duplex ultrasound imaging for detection of atherosclerotic plaque found a sensitivity for ABI of 17%.16 Similarly, in a cohort of individuals who underwent whole-body MRA imaging, ABI had only a 15–20% sensitivity for detection of ≥ 50% peripheral stenoses.17
Prior studies assessing exercise ABI are similarly limited and often focus on asymptomatic individuals. One early study found no difference in sensitivity between rest and exercise ABI.10 In an analysis of 290 limbs in diabetic patients, the sensitivity of exercise ABI was only modestly increased compared to standard ABI (85% vs 83%) in limbs with PAD confirmed by duplex imaging.18 Another analysis of 631 randomly selected asymptomatic individuals found that there were fewer cases of abnormal exercise ABIs (n=27) compared to rest ABIs (n=66).13 None of the studies assessing the performance of exercise ABI testing has rigorously confirmed the presence of PAD with angiographic imaging. Furthermore, a variety of different post-exercise diagnostic thresholds have been proposed,10,11,13 and the most recent guidelines on the ABI test suggest that a post-exercise ABI < 0.90, a decrease in ABI > 20%, and a decrease in ankle pressure > 30 mmHg are all acceptable cut-offs.7,12 Other studies have similarly questioned the utility of these post-exercise thresholds. In a series of 7995 patients with normal resting ABI who also underwent exercise ABI testing, there was only a 58.4% chance of agreement among clinicians in diagnosing PAD when using the decrease in ABI > 20% or decrease in ankle pressure > 30 mmHg cut-offs.19
The 30 mmHg ankle pressure cut-off is largely based on an older study of 100 controls and 30 individuals with PAD.11 However, several methodologic limitations of this study are worth noting. The control individuals did not undergo imaging, and they were deemed free of disease based on history and physical examination alone. The PAD cohort was young (age range 38–72 years) compared to that of our analysis, and ABI has been shown to change with age.20,21 Additionally, the majority of individuals with PAD required aortic surgery, which suggests the disease distribution may be different from our own population in which infrageniculate disease was most prevalent.
In summary, our data support guideline recommendations that resting ABI should be the initial test for individuals with suspected PAD.6 However, if this testing is normal, our data support follow-up testing with an exercise ABI using a post-exercise threshold of < 0.90 given its high sensitivity. These findings have several important implications for clinical practice. First, in symptomatic patients with a high clinical suspicion for PAD, reflexively performing exercise ABI testing following a normal resting ABI may improve the sensitivity of screening. Given the high sensitivity of exercise ABI testing, our findings could potentially lead to a decrease in diagnostic imaging as a means of diagnosing PAD if validated in other cohorts.
Study limitations
Several limitations of our analysis warrant discussion. Despite the large number of exercise ABI studies initially screened, the final number used in the analysis with recent imaging available was relatively small, and there was inadequate clinical information available to further examine this potential source of selection bias. Presumably, many of the individuals who were not referred for angiographic imaging had both normal resting and exercise ABIs. Our study was retrospective in nature and limited to individuals with a high prevalence of PAD, and these findings may not apply to a healthier population. Although all individuals were referred for exercise testing due to a concern for PAD, the specific signs and symptoms that prompted testing were not consistently available. Additionally, repeat ABI measures were not performed, and some borderline measures may have been reclassified as abnormal if repeated.7 Data on severity of disease in some arterial beds were missing for some study participants, which could have introduced bias into our analyses. Nonetheless, the degree of missing data was small and ranged from 0.5% to 5.5% depending on the vascular bed. Finally, it is possible the severity of PAD changed in the interval between exercise ABI and angiography. However, we selected a maximum 12-month interval between exercise ABI and imaging because several previous studies suggest that clinically significant progression in PAD only occurs over a much longer time period.20,22 In addition, since exercise testing could occur before or after angiographic imaging, the impact of progressive disease would have been relatively balanced between the groups with ABI testing first compared to angiography first.
Conclusion
Our study provides quantitative evidence to guide current recommendations on exercise testing among individuals with suspected PAD but normal resting ABIs.6 The current guidelines suggest that post-exercise ABI < 0.90, an ABI decrease > 20%, and an ankle pressure decrease > 30 mmHg are all considered acceptable diagnostic thresholds,7,12 but in our study exercise ABI < 0.90 exhibited the best sensitivity overall and in patients with a normal resting ABI. Exercise ABI correctly diagnosed 25% more individuals with severe PAD compared to resting ABI.
Supplementary Material
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by Brigham and Women’s Hospital, National Institutes of Health (T32 HL007575) (Dr Aday), and the Clinical Science Research and Development Service of the VA Office of Research and Development. (grant nos. I01CX000440, I01CX001549) (Dr Kinlay).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
The supplementary material is available online with the article.
References
- 1.Safar ME, Protogerou AD, Blacher J. Statins, central blood pressure, and blood pressure amplification. Circulation 2009; 119: 9–12. [DOI] [PubMed] [Google Scholar]
- 2.Tsamis A, Stergiopulos N. Arterial remodeling in response to hypertension using a constituent-based model. Am J Physiol Heart Circ Physiol 2007; 293: H3130–H3139. [DOI] [PubMed] [Google Scholar]
- 3.Winsor T Influence of arterial disease on the systolic blood pressure gradients of the extremity. Am J Med Sci 1950; 220: 117–126. [DOI] [PubMed] [Google Scholar]
- 4.Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA 1969; 207: 1869–1874. [PubMed] [Google Scholar]
- 5.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45: S5–S67. [DOI] [PubMed] [Google Scholar]
- 6.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2017; 135: e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012; 126: 2890–2909. [DOI] [PubMed] [Google Scholar]
- 8.Ouriel K, Zarins CK. Doppler ankle pressure: An evaluation of three methods of expression. Arch Surg 1982; 117: 1297–1300. [DOI] [PubMed] [Google Scholar]
- 9.Carter SA. Response of ankle systolic pressure to leg exercise in mild or questionable arterial disease. N Engl J Med 1972; 287: 578–582. [DOI] [PubMed] [Google Scholar]
- 10.Ouriel K, McDonnell AE, Metz CE, et al. Critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery 1982; 91: 686–693. [PubMed] [Google Scholar]
- 11.Laing S, Greenhalgh RM. The detection and progression of asymptomatic peripheral arterial disease. Br J Surg 1983; 70: 628–630. [DOI] [PubMed] [Google Scholar]
- 12.Gerhard-Herman M, Gardin JM, Jaff M, et al. Guidelines for noninvasive vascular laboratory testing: A report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vasc Med 2006; 11: 183–200. [DOI] [PubMed] [Google Scholar]
- 13.Hoogeveen EK, Mackaay AJC, Beks PJ, et al. Evaluation of the one-minute exercise test to detect peripheral arterial disease. Eur J Clin Invest 2008; 38: 290–295. [DOI] [PubMed] [Google Scholar]
- 14.Xu D, Li J, Zou L, et al. Sensitivity and specificity of the ankle–brachial index to diagnose peripheral artery disease: A structured review. Vasc Med 2010; 15: 361–369. [DOI] [PubMed] [Google Scholar]
- 15.Stoffers HE, Kester AD, Kaiser V, et al. The diagnostic value of the measurement of the ankle-brachial systolic pressure index in primary health care. J Clin Epidemiol 1996; 49: 1401–1405. [DOI] [PubMed] [Google Scholar]
- 16.Flanigan DP, Ballard JL, Robinson D, et al. Duplex ultrasound of the superficial femoral artery is a better screening tool than ankle-brachial index to identify at risk patients with lower extremity atherosclerosis. J Vasc Surg 2008; 47: 789–792. [DOI] [PubMed] [Google Scholar]
- 17.Wikstrom J, Hansen T, Johansson L, et al. Ankle brachial index <0.9 underestimates the prevalence of peripheral artery occlusive disease assessed with whole-body magnetic resonance angiography in the elderly. Acta Radiol 2008; 49: 143–149. [DOI] [PubMed] [Google Scholar]
- 18.Allen J, Oates CP, Henderson J, et al. Comparison of lower limb arterial assessments using color-duplex ultrasound and ankle/brachial pressure index measurements. Angiology 1996; 47: 225–232. [DOI] [PubMed] [Google Scholar]
- 19.Mahe G, Pollak AW, Liedl DA, et al. Discordant diagnosis of lower extremity peripheral artery disease using American Heart Association postexercise guidelines. Medicine (Baltimore) 2015; 94: e1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bird CE, Criqui MH, Fronek A, et al. Quantitative and qualitative progression of peripheral arterial disease by non-invasive testing. Vasc Med 1999; 4: 15–21. [DOI] [PubMed] [Google Scholar]
- 21.Smith FB, Lee AJ, Price JF, et al. Changes in ankle brachial index in symptomatic and asymptomatic subjects in the general population. J Vasc Surg 2003; 38: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 22.Mohler ER, Bundens W, Denenberg J, et al. Progression of asymptomatic peripheral artery disease over 1 year. Vasc Med 2012; 17: 10–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.