Abstract
Background:
Of the many negative outcomes associated with gestational alcohol use, one that has received relatively little attention is preterm birth and its possible contribution to effects of prenatal alcohol exposure (PAE) on development. To examine the increased risk for premature delivery associated with PAE and the joint influence of preterm birth and alcohol on child outcomes, analysis was carried out in a longitudinal cohort recruited in Western Ukraine.
Methods
Alcohol-using women and low or non-drinking controls were identified prenatally for a clinical trial of multivitamins and minerals (MVM) in ameliorating effects of PAE. Women were interviewed to provide information about medical and social status and other drug use. At delivery, information was collected about infant (N=686) status including gestational age (GA) in weeks. Finally, 441 infants were followed to 6 months of age and cognitive (Mental Developmental Index (MDI)) and motor development (Psychomotor Developmental Index (PDI)) (measured using the Bayley Scales of Infant Development, 2nd Ed (BSID-II).
Results:
Seven percent were born at <37 weeks GA. The odds ratio for preterm delivery for Alcohol Exposed vs. Low/No Alcohol was 2.6 (95% Confidence Interval 1.37, 4.94) (p<.003); MVM supplements were associated with a lower rate of preterm delivery overall, but the relative proportion of preterm births did vary not by MVM supplement status between alcohol exposure groups. In mediation models of 6 month cognitive and motor development (Barron & Kenney, 1986), GA significantly mediated alcohol effects (MDI: Z=−2.64, p<.008; PDI: Z=−2.35, p<.02) although PAE independently affected both outcomes (MDI: t=−5.6, p<.000; PDI: t=−3.19, p<.002).
Conclusion:
Results suggest that PAE is associated with higher rates of preterm birth and that alcohol’s effect on development in infancy may be both direct and mediated by shortened length of gestation.
Keywords: Preterm birth, Prenatal alcohol exposure, smoking in pregnancy, child development
There has been limited examination of the relationship between maternal alcohol use in pregnancy and preterm birth, and the potential additional risks associated with an early delivery are usually not considered when evaluating the developmental problems associated with prenatal alcohol exposure (PAE). Full term birth is defined as a gestational age (GA) of 40 weeks (range: 37 to 42 weeks). Preterm birth is defined as birth at <37 weeks with those born between 34 and <37 weeks considered to be “late preterm” and those less than 34, “early preterm”. The great majority (~72% of all preterms) fall in the “late” range. While those born earlier are at highest risk for a range of complications (Rogers & Hintz, 2016), recent research has re-emphasized the developmental problems that can be associated with later preterm birth, as well (Ramachandrappa & Jain, 2009) leading to a reduction of elective deliveries in the weeks before term. Preterm birth is a major concern worldwide with the rate in the United States being around 10% (and among the highest in the world) (March of Dimes, 2016). Rates are generally lower in Europe and in 2010 were less than 7% in Ukraine (March of Dimes, 2010).
Research on the relationship between alcohol use and preterm birth has yielded mixed results. Janise et al (Janisse, Bailey, Ager, & Sokol, 2014) report that alcohol has not been definitively linked to preterm birth and other studies find no effects of low to moderate alcohol use on this outcome (Dale, Bakketeig, & Magnus, 2016; Lundsberg, Illuzzi, Belanger, Triche, & Bracken, 2015). In contrast, Sokol et al (Sokol et al., 2007) reported a relationship between high levels of alcohol use and extreme prematurity (defined in that paper as a gestational age of less than 32 weeks). They did not find a relationship with late preterm birth (32 to <37 weeks). Amount of alcohol use has also been considered in a recent meta-analysis of the limited body of research that addresses this issue (Patra et al., 2011), finding that the relative risk of preterm birth rose with the number of daily drinks. It is also possible that the combination of preterm birth and PAE may be associated with other risks that are detrimental to development.
Sokol and his colleagues (Sokol et al., 2007) noted some of the reasons why it has been difficult to investigate this issue including problems in accurately measuring maternal alcohol use retrospectively and unreliable measurement of gestational age as well as lack of control of other social and maternal factors possibly related to preterm birth. These include the family’s social status, maternal age and parity, other substance use, particularly cigarette use and exposure to cigarette smoking by other family members.
In the current study, we employed data from the alcohol and nutrition cohort (Chambers et al., 2014) recruited in Western Ukraine between 2007 and 2012. This data set provides an opportunity to examine the contribution of PAE to preterm birth and of alcohol-related preterm birth to later developmental outcomes. In this cohort, maternal alcohol use during pregnancy was documented at recruitment so that potential bias associated with retrospective recall following the birth of a preterm infant was eliminated. In addition to extensive information about alcohol, smoking and other drug use, the study collected information about social factors and maternal history and health that have been found to be associated with preterm birth in other contexts, e.g., (Huang, Zhu, Qu, & Mu, 2016). This cohort also allowed for the examination of a standard nutritional intervention in relation to PAE on preterm birth. Finally, because infants were followed longitudinally, it is possible to evaluate the joint contribution of preterm birth and PAE on infant development.
Hypotheses
The major goals of the analysis presented in this paper were to 1) determine whether prenatal alcohol exposure (PAE) is associated with preterm birth in this well characterized, prospectively followed cohort and to what extent other factors, in addition to alcohol, contributed to early delivery; and 2) to evaluate the impact of gestational age and prenatal alcohol exposure on child development at six months while controlling for other factors that might affect development at this age. It was hypothesized that some of the developmental effects attributable to PAE may be mediated through preterm birth.
Methods
Study Design:
A cohort study recruited 686 women of whom 342 drank at moderate to heavy levels, and 344 were controls with low drinking levels/abstaining. Another 50 women were recruited but excluded from this analysis due to inconsistent reports of alcohol use. Recruitment was at two sites in Western Ukraine located within clinical services, both affiliated with OMNI-Net, a network of educational and research sites that focus on the prevention of birth defects. Half of women within each of the exposure groups (high/moderately exposed and low/unexposed) were assigned randomly to receive a daily MVM supplement (an over-the-counter prenatal vitamin/mineral supplement), and half to standard of care (prenatal vitamins recommended but not provided). Half of the MVM-supplemented group also received a daily dose of 750 mg. of supplemental choline. When infants were born, information was collected from medical records and direct examination on growth, physical features associated with prenatal alcohol exposure, and other factors affecting development. A follow-up was done at approximately 6 months post-partum to evaluate infants’ cognitive and motor development.
Study Participants.
Women were recruited at their first prenatal visit to the Rivne Regional Medical Diagnostic Center or the Khmelnitsky Perinatal Center in Western Ukraine and provided informed consent at that time (The overall study was approved through the institutional review boards at the University of California San Diego and Lviv Medical University in Ukraine.) From 2007 to 2012, more than 13,000 women were screened and information on outcomes has been reported previously (Chambers et al., 2014). At the initial visit, nurse interviewers screened all women and provided information on the risks of alcohol consumption during pregnancy. Those in the alcohol group reported at least weekly binge-drinking (5+ drinks), at least five episodes of 3-4 standard drinks or at least 10 episodes of 1-2 standard drinks either in the month around conception or in the most recent month of pregnancy. Controls were recruited who met the following screening criteria (that is, no binge episodes, minimal or no alcohol in the month around conception, and no drinking in the most recent month of pregnancy). Once consented, women were assigned to a MVM intervention group (described above).
Measures.
Demographic information was obtained through a questionnaire at the nursing interview, and included family (e.g., maternal and paternal age) and pregnancy (e.g., parity) characteristics. Hollingshead (Hollingshead, 2011) social class ratings were calculated from education and occupational information. Questions on health-related activities included tobacco use, environmental tobacco exposure, use of vitamin and mineral supplements and folic acid, prenatally. Also recorded were data collection site (which of the two Ukrainian cities), and when prenatal care was initiated (gestational week) as a proxy for quality of prenatal care.
At delivery, information was obtained about infant’s GA, in weeks, as well as infant growth (Birthweight, Length, and Head Circumference). GA was assessed by taking the first day of last menstrual period (LMP) to calculate estimated due date based on menstrual cycle length. If no LMP date was available, GA was estimated from the earliest ultrasound generated date. If dates were discrepant, an algorithm was used to correct gestational dating by LMP for ultrasound dates.
Assessment of Maternal Alcohol Use.
Women who reported any lifetime drinking at the screening interview were asked by the study nurse during the enrollment interview about the number, volume, and type of alcoholic drinks consumed day by day in a typical week around conception (periconceptual) and in the most recent two weeks using a time-line follow back method (Sobell & Sobell, 2000). Quantity and frequency of alcohol use in responses to these questions was summarized as the average number of standard drinks per day over the period for which the mother was reporting as a reflection of the overall quantity of alcohol consumed (drinks/day). Absolute ounces of alcohol (oz/AA) consumed per time period was calculated as two standard drinks being equivalent to one ounce of absolute alcohol. In addition, to determine whether women’s drinking patterns could be classified as “high risk”, items were collected during the maternal interview that allowed the calculation of scores on two measures of risky drinking, the Alcohol Use Disorders Identification Test: AUDIT (Barbor, Higgins-Biddle, Saunders, & Monteiro, 2001) and the TWEAK (Russell et al., 1994) .
Follow up.
For the 6-month assessment, the Bayley Scales of Infant Development, 2nd Edition (BSID-II) (Bayley, 1993) was selected as the measure of development. This well standardized measure has been used worldwide in evaluating infants and was available in a Russian translation that would be accessible to Ukrainian users (most of whom are bilingual in Russian). The BSID-II measures Mental Development (problem solving and prelinguistic development) and Psychomotor Development (fine and gross motor skills) leading to standardized scores (Mental Development Index: MDI; and Psychomotor Development Index: PDI). The BSID-II was administered by Ukrainian child psychologists who were blind to the mother’s group status. Children were seen and tested individually in a private office while seated in their caregiver’s laps. Testing required 30 to 45 minutes time.
Data Analysis.
Data were collected on-site in Ukraine, entered into databases by local study staff and transmitted electronically to the investigators at the University of California at San Diego and Emory University, Atlanta, Georgia. Descriptive statistics were used to characterize the sample and calculate odds ratios. Analysis of variance and covariance were used to compare means of different alcohol use groups as well as those born pre and full term while controlling for potentially confounding variables as necessary. The following factors were evaluated as potential confounders: Data collection site, maternal and paternal age, parity, child sex, weeks of gestation when prenatal care was first sought (as a proxy for prenatal care), smoking in pregnancy (number of cigarettes a day), reported environmental tobacco exposure (ETS), Hollingshead social economic status (SES) and multivitamin use. Using regression models, alcohol exposure, measured as reported ounces of absolute alcohol a day (AA/day) both periconceptually and in the most recent two weeks was examined to evaluate impact of amount of alcohol exposure versus alcohol group status. Finally, regression models were tested that included both indicators of excessive alcohol use (AUDIT; TWEAK) and potential covariates to evaluate their contribution to outcomes.
At six months, to evaluate the hypothesis that observed effects of PAE on child behavioral outcomes are moderated by preterm birth, we carried out a series of regression equations following the guidelines of Baron and Kenny (Baron & Kenny, 1986). According to these guidelines, the following criteria must be met in order to show mediation: 1) the independent variable (PAE in oz AA/day) must show association with potential mediators (GA in weeks, SES, tobacco exposure), 2) the independent variable (PAE) must show association with the dependent variable (Infant MDI; PDI), 3) mediators (gestational age in weeks, SES, tobacco exposure) must show association with the dependent variable (infant development), and 4) the effect of the independent variable on the dependent variable must be significantly reduced when the mediator is added into the model. The current study used an algorithm recommend by Preacher and Hayes (Preacher & Hayes, 2008).
Results
Alcohol Use and Preterm Birth.
In the Ukrainian birth sample of 686, 48 infants or 6.99% were born at <37 weeks gestational age. In the total sample, among those in the alcohol use group 9.9% were preterm compared to 4.1% in the no or low-using group, for an odds ratio (OR) of preterm birth in the Alcohol group of 2.6 (95% Confidence Interval (CI) 1.37, 4.94) (p<.003). To determine the effect of nutritional supplements, the analysis was repeated in the group of 344 who had not received a supplement, finding that the rate in the Alcohol group was 11.7% and in the contrast group it was 4.9%, yielding an OR of preterm birth for the Alcohol group of 2.6 (95% CI: 1.16, 5.71) (p<.02). Thus, the nutritional intervention was associated with a lower rate of preterm birth overall (Controls: 3.1%; Alcohol users: 7.6%, OR: 2.55; 95% CI: 0.86, 7.51 ) but did not modify the relative proportions of preterm deliveries in the Alcohol versus no/low-alcohol groups.
Generalized linear regression was then used to model predictors of preterm birth in the birth cohort. Several different models were tested with weeks of gestational age as the outcome and data collection site, child sex, vitamin group status, parental age, SES, exposure to cigarette smoke either by maternal smoking or environmental exposure via paternal use, and several measure of alcohol use severity, AA/per day at periconceptually or in the two weeks before recruitment, and scores on the TWEAK and the AUDIT as predictors. Choline intervention was not found to be related to outcome; therefore, vitamin and mineral supplementation was collapsed across choline. Several variables, paternal smoking (environmental exposure), parental age, and child sex did not contribute significantly and were eliminated from subsequent models. Number of cigarettes smoked daily during pregnancy also did not contribute significantly.
In the best fitting model (X2 (5)=54.18, p<.000), gestational age at birth was predicted by Vitamin status (Wald X2 (1)=4.39, p<.04), socioeconomic status ((Wald X2 (1)=7.25, p<.007), timing of initiation of prenatal care (Wald X2 (1)=14.61, p<.000), and the AUDIT score ((Wald X2 (1)=5.07 p<.03). Substituting the TWEAK score for the Audit yielded a similar result (Wald X2 (1) =4.28, p<.04). In models that used only the amount of alcohol either at conception or at the time of enrollment, alcohol did not account for significant variance. (See Supplemental Tables).
Six Month Developmental Outcomes
Six Month Sample.
At approximately six months 441 families returned for evaluation of infant status. In comparison to those recruited, those returning for assessment were not more likely to be born preterm and there were no differences in mean gestational age (Followed: M GA=39.32 (SD=1.92); Not Followed: =39.11 (SD=2.29), F (1,682) =1.63, NS). Those followed were not more likely to have been assigned to MVM supplements than those lost to follow up (X2(1) <1, NS). There was no difference in amount of alcohol used either periconceptually or at the time of enrollment between these groups or in the number of cigarettes smoked. The groups did not differ in the sex of the index child or in the number of living children (parity). Differences were observed in that mothers returning for follow-up were slightly older (M age=26.39 (5.21) vs 25. 49 (5.25), F(1,684) =4.73, p<.03), were slightly higher in Hollingshead ratings of SES (M=37.47 (12.36) vs 33.06 (13.05), F(1,677) =18.97, p<.000), and had more years of education (M years= 13.99 (2.54) vs 12.71 (2.41), F(1,684) =41.60,p<.000). Characteristics of participants at six months are shown in Table 1.
Table 1:
Characteristics of Participants in Six Month Follow-Up (N=441)
| Measure | Control (n=239) | Alcohol-Exposed (n=202) | Statistic | p-value | ||
|---|---|---|---|---|---|---|
| FT (n=231) | PT (n=8) | FT (n=183) | PT (n=19) | |||
| Child’s Age (months)1 M (SD) | 6.43 (0.89) | 6.50 (0.54) | 6.37 (0.87) | 6.16 (1.17) | EToH:F(1,437)=1.09 Term:F(1,437)<1 ExT: F(1,437)<1 |
NS NS NS |
| % Male | 54.3% | 75% | 47% | 47.4% | EToH:X2
(1)<1 Term:X2(1)=2.81 |
NS .10 |
| GA at Birth M (SD) | 39.77 (1.18) | 34.41 (2.80) | 39.57 (1.19) | 33.38 (1.98) | EToH:F(1,437)=5.04 Term:F(1,437)=441.5 ExT: F(1,437)=2.29 |
.025 .000 .13 |
| Bayley MDI M (SD) | 90.99 (6.81) | 80.88 (16.33) | 88.85 (9.32) | 84.05 (15.03) | EToH:F(1,437)<1 Term:F(1,437)=15.99 ExT: F(1,437)=2.03 |
NS .000 .15 |
| Bayley PDI M (SD) | 90.36 (10.16) | 77.25 (12.78) | 88.07 (12.29) | 84.42 (17.24) | EToH:F(1,436)<1 Term:F(1,436)=11.32 ExT: F(1,436)=3.61 |
NS .001 .06 |
| % MVM Group | 45.9% | 25% | 42.6% | 42.1% | EToH:X2(1)<1 Term:X2(1)<1 |
NS NS |
| Measures Taken at Recruitment | ||||||
| Maternal age (Ys) M (SD) | 26.51 (4.63) | 27.75 (4.17) | 26.15 (5.92) | 26.79 (5.21) | EToH:F(1,437)<1 Term:F(1,437)<1 ExT: F(1,437)<1 |
NS NS NS |
| Parity M (SD) | 0.71 (1.06) | 0.75 (1.04) | 0.69 (0.99) | 1.32 (1.49) | EToH:F(1,437)=1.41 Term:F(1,437) =2.11 ExT: F(1,437)=1.68 |
NS NS NS |
| Prenatal care2 M (SD) | 8.96 (2.98) | 10.88 (1.73) | 10.82 (4.92) | 12.63 (6.39) | EToH:F(1,435)=4.23 Term:F(1,435)=4.50 ExT: F(1,435)<1 |
.04 .03 NS |
| ozAA/day Periconceptally M (SD) | 0.002 (0.01) | 0.0 (0.0) | 0.815 (0.97) | 0.814 (0.83) | EToH:F(1,436)=32.6 Term:F(1,436)<1 ExT: F(1,436)<1 |
.000 NS NS |
| ozAA/day at Enrollment M (SD) | 0.000 (0.003) | 0.0 (0.0) | 0.226 (0.51) | 0.255 (0.36) | EToH:F(1,437)=11.04 Term:F(1,437)<1 ExT: F(1,437)<1 |
.001 NS NS |
| Cigarettes per day/pregnancy | 0.14 (1.39) | 0.0 (0.0) | 1.79 (4.14) | 2.95 (4.22) | EToH:F(1,436)=12.68 Term:F(1,436)<1 ExT: F(1,436)=1.01 |
.000 NS NS |
| Paternal Age (Ys) M (SD) | 28.75 (5.28) | 29.50 (6.99) | 30.38 (6.88) | 32.83 (8.85) | EToH:F(1,435)=3.38 Term:F(1,435)=1.40 ExT: F(1,435)<1 |
.07 NS NS |
| SES: Hollingshead Score3 M (SD) | 41.6(11.63) | 38.25 (11.86) | 33.45 (10.77) | 24.95 (14.68) | EToH:F(1,433)=18.94 Term:F(1,433) =5.82 ExT: F(1,433)= 1.05 |
.000 .016 NS |
with Adjustment for gestational age at time of testing.
Weeks in pregnancy when prenatal care initiated
Measure of Socioeconomic status; higher scores reflect more status (Hollingshead, 2011)
Developmental Outcome at 6 Months.
Infants born preterm had lower Mental Development (F (1,436) =15.76, p<.000) and Psychomotor Development Indices, (F (1,434) =11.08, p<.001). Alcohol group, per se, was not significant although there was a trend toward an interaction between preterm birth and alcohol group (Table 1), for PDI (F (1,434) =3.79 p<.05). In generalized linear regression models that included data collection site, MVM status, Study Site, Gestational age, SES, child’s sex, number of cigarettes smoked, and parity, oz AA/day of alcohol reported in the periconceptual period was related significantly to both mental development (Beta=−2.55, Wald X2(1)=27.49, p<.000) and motor development (Beta=−1.71 Wald X2(1)=6.25, p<.01). Alcohol use at the time of enrollment also was related to mental (Beta=−3.48 Wald X2(1)=10.27, p<.001) and motor development (Beta=−3.48 Wald X2(1)=10.27, p<.001). (See Supplemental Tables for details).
Regression based mediation models (Baron & Kenny, 1986) were created using ozAA/week (both periconceptual and at enrollment) as the Independent variables (IV) and either Bayley Mental Development Index (MDI) or Psychomotor Development Index (PDI) as the Dependent variables (DV). Gestational age at birth, Hollingshead Rankings of SES and number of cigarettes smoked were tested as mediators in an analysis that permitted testing of multiple mediators (Preacher & Hayes, 2008). Results indicated that, as required, all potential mediators were related significantly to oz AA/day and Gestational age and SES to MDI and PDI. However, cigarette use was not related to outcome and was not included the final model. In both models (cognition, MDI, and motor, PDI), GA significantly mediated alcohol effects (MDI: Z=−2.64, p<.008; PDI: Z=−2.35, p<.02) while SES (p=.07) was not a mediator in 6-month developmental performance (Figures 1a and 1b). Alcohol exposure independently affected both outcomes (MDI: t=−5.6, p<.000; PDI: t=−3.19, p<.002). Both alcohol exposure around conception and reported at enrollment were evaluated in these models and the results of the model using periconceptual exposure are shown in Figures 1a and 1b. However, the model examining the mediation of alcohol at enrollment effects by gestational age and SES yielded similar results as shown in the following model summaries for MDI, R2=.148, F(3, 433)=25.11, p<.000, and for PDI, R2=.097, F(3, 432)=15.41, p<.000, with both GA and SES mediating the effects of PAE on outcomes.
Figure 1a:
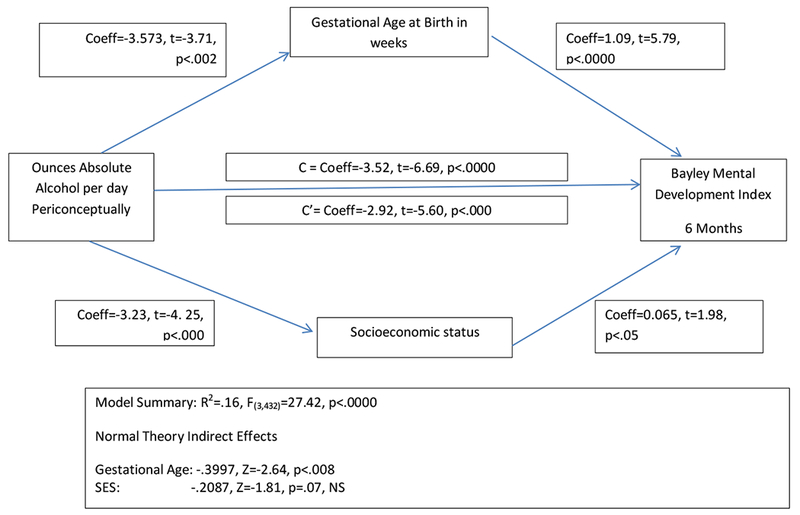
Model of the Impact of the Indirect Effects of Gestational Age and Socioeconomic Status on the Relationship Between Prenatal Alcohol Exposure and Cognitive Development at 6 Months of Age. Note that both factors are related to alcohol use and have a direct effect on child outcome but only Gestational Age is a mediator of the alcohol effect.
Figure 1b:

Model of the Impact of the Indirect Effects of Gestational Age and Socioeconomic Status on the Relationship Between Prenatal Alcohol Exposure and Motor Development at 6 Months of Age. Note that both factors are related to alcohol use and have a direct effect on child outcome but only Gestational Age is a mediator of the alcohol effect.
Conclusions
Due to its many negative consequences for infant health and development, reducing preterm birth is a major public health objective and one that has been challenging to achieve (Newnham et al., 2017). In 2010, during the time these data were collected, the March of Dimes (http://www.marchofdimes.org/mission/global-preterm.aspx#) reported that that the global average for preterm birth was 11%, the United States average was 12% and the Ukrainian average overall was 6.5%. There are many social and health-related factors associated with early delivery and although tobacco use is often mentioned, alcohol use is rarely discussed. However, in this sample, prenatal alcohol use, particularly “risky alcohol use” as indicated by the TWEAK and AUDIT screeners increased the risk of prematurity. Other factors, often associated with maternal alcohol abuse also contributed to these non-optimal results. In this sample, these factors include lower socioeconomic status (SES) as well as timing of prenatal care in that women who had higher SES and those who received earlier prenatal care were more likely to deliver term infants. It is also of interest that MVM supplementation reduced the preterm rate overall and are consistent with the findings of Avalos, et al (2011) from a study done in the Western United States. These results re-emphasize the importance of access to prenatal care as well as the value of screening women who are at high risk for problematic outcomes so that appropriate care can be provided.
The longitudinal nature of this sample allowed evaluation at 6 months of the joint impact of both alcohol exposure and early gestational age. Previous studies of the impact of PAE on development, including our own (Coles et al., 2015), have not emphasized the contribution of gestational age to problematic outcomes in alcohol-affected children and, therefore, may have failed to consider a factor that can heavily contribute to developmental problems. Given that these factors are correlated, mediation analysis provided the best way to examine their relative contributions. Results suggest that, while alcohol does have a direct effect on six-month developmental outcome, some of the impact of PAE is mediated through preterm birth in those children who are born preterm.
It is important to acknowledge limitations associated with this study. The developmental follow-up discussed here was limited to 6 months which is not sufficient to evaluate all of the potential deficits associated with the risk factors that were considered. Infant assessments at this age are relatively insensitive and some of the sequelae of alcohol exposure and preterm birth (e.g., academic problems, language delays) cannot be measured at six months. Factors such as maternal smoking and lower SES were also more common in families where the mother reported periconceptual and mid-pregnancy alcohol use. SES was observed to have its usual significant and independent effect on development, while, in this analysis, tobacco use was not significantly related to children’s developmental scores. It is also difficult to generalize from this Ukrainian sample, which has a relatively low rate of preterm birth, a population that is non-diverse, and universal health care, to the much more complex situation in other countries, including the United States.
These results do suggest that alcohol use in pregnancy must be considered as one of a constellation of risk factors for a woman or her child. It is likely that maternal alcohol use in pregnancy has both a direct teratogen effect and also serves as a potential marker for other contributing factors. It is important for clinician to be aware of these relationships, given the possibility that that the combination of preterm birth and PAE may be associated with other risks that are detrimental to development. In this paper, we demonstrate that cognitive and motor development may be negatively impacted in infants with both conditions but there are other consideration, as well. For instance, in the neonatal period, preterm infants have a higher risk for brain hemorrhage when exposed to alcohol (Holzman, Paneth, Little, & Pinto-Martin, 1995).
There may be a subset of alcohol-exposed infants whose development is impaired due to the accumulation of risk factors, including early delivery. By examining this accumulation of risk both during infancy and in the preschool period, it may be possible to screen for those children who are most vulnerable and who will require early intervention services most urgently. Attention to attendant risk factors, like access to prenatal care, also may help to ameliorate some of the negative consequences of alcohol exposure in this group.
Supplementary Material
Acknowledgements
All or part of this work was done in conjunction with the CIFASD. Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org.
This project was supported by NIH Research Grant #U01AA014835 funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the NIH Office of Dietary Supplements (ODS)
Christina Chambers, PI
We wish to acknowledge the contribution of:
OMNI-Net, Ukraine
Participating families and staff in Rivne and Khmelnytsky, Ukraine
References
- Avalos LA, Kaskutas L, Block G, Abrams B, & Li DK (2011) Does lack of multinutrient supplementation during early pregnancy increase vulnerability to alcohol-related preterm or small-for-gestational-age births? Matern Child Health J, 15 (8), 1324–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB and Monteiro M. AUDIT The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. Geneva: World Health Organization, 2001. (Second Edition). [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol, 51(6), 1173–1182. [DOI] [PubMed] [Google Scholar]
- Bayley N, Bayley Scales of Infant Development(BSID-2). 2nd ed. 1993, San Antonio, TX: Psychological Corporation. [Google Scholar]
- Chambers CD, Yevtushok L, Zymak-Zakutnya N, Korzhynskyy Y, Ostapchuk L, Akhmedzhanova D, … Wertelecki W (2014). Prevalence and predictors of maternal alcohol consumption in 2 regions of Ukraine. Alcohol Clin Exp Res, 38(4), 1012–1019. doi: 10.1111/acer.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, … Cifasd. (2015). Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Matern Child Health J, 19(12), 2605–2614. doi: 10.1007/s10995-015-1779-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale MT, Bakketeig LS, & Magnus P (2016). Alcohol consumption among first-time mothers and the risk of preterm birth: a cohort study. Ann Epidemiol, 26(4), 275–282. doi: 10.1016/j.annepidem.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (2011). Four Factor Index of Social Status. Yale Journal of Sociology, 8, 21–51. [Google Scholar]
- Holzman C, Paneth N, Little R, & Pinto-Martin J (1995). Perinatal brain injury in premature infants born to mothers using alcohol in pregnancy. Neonatal Brain Hemorrhage Study Team. Pediatrics, 95(1), 66–73. [PubMed] [Google Scholar]
- Huang J, Zhu T, Qu Y, & Mu D (2016). Prenatal, Perinatal and Neonatal Risk Factors for Intellectual Disability: A Systemic Review and Meta-Analysis. PLoS One, 11(4), e0153655. doi: 10.1371/journal.pone.0153655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janisse JJ, Bailey BA, Ager J, & Sokol RJ (2014). Alcohol, tobacco, cocaine, and marijuana use: relative contributions to preterm delivery and fetal growth restriction. Subst Abus, 35(1), 60–67. doi: 10.1080/08897077.2013.804483 [DOI] [PubMed] [Google Scholar]
- Lundsberg LS, Illuzzi JL, Belanger K, Triche EW, & Bracken MB (2015). Low-to-moderate prenatal alcohol consumption and the risk of selected birth outcomes: a prospective cohort study. Ann Epidemiol, 25(1), 46–54 e43. doi: 10.1016/j.annepidem.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March of Dimes (2010). from https://www.marchofdimes.org/mission/global-preterm.aspx#tabs-3
- March of Dimes (2016). from https://www.marchofdimes.org/materials/premature-birth-report-card-united-states.pdf
- Newnham JP, Kemp MW, White SW, Arrese CA, Hart RJ, & Keelan JA (2017). Applying Precision Public Health to Prevent Preterm Birth. Front Public Health, 5, 66. doi: 10.3389/fpubh.2017.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J, Bakker R, Irving H, Jaddoe VW, Malini S, & Rehm J (2011). Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG, 118(12), 1411–1421. doi: 10.1111/j.1471-0528.2011.03050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Ramachandrappa A, & Jain L (2009). Health issues of the late preterm infant. Pediatr Clin North Am, 56(3), 565–577, Table of Contents. doi: 10.1016/j.pcl.2009.03.009 [DOI] [PubMed] [Google Scholar]
- Rogers EE, & Hintz SR (2016). Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol, 40(8), 497–509. doi: 10.1053/j.semperi.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Russell M, Martier SS, Sokol RJ, Mudar P, Bottoms S, Jacobson S, & Jacobson J (1994). Screening for pregnancy risk-drinking. Alcohol Clin Exp Res, 18(5), 1156–1161. [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (2000). Alcohol Timeline followback (TLFB) In A. P. Association (Ed.), Handbook of Psychiatric Measures (pp. 477–479). Washington, DC: American Psychiatric Association. [Google Scholar]
- Sokol RJ, Janisse JJ, Louis JM, Bailey BN, Ager J, Jacobson SW, & Jacobson JL (2007). Extreme prematurity: an alcohol-related birth effect. Alcohol Clin Exp Res, 31(6), 1031–1037. doi: 10.1111/j.1530-0277.2007.00384.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


