Abstract
Study objective:
In emergency departments (EDs), diagnosis and treatment of Chlamydia trachomatis and Neisseria gonorrhoeae are challenging. We conducted a randomized clinical trial to assess rapid C trachomatis and N gonorrhoeae testing on overtreatment and undertreatment of women evaluated for C trachomatis and N gonorrhoeae.
Methods:
Women undergoing pelvic examinations and C trachomatis and N gonorrhoeae testing (n=254) were randomized to control or rapid test groups. The control group received standard-of-care C trachomatis and N gonorrhoeae nucleic acid amplification tests of endocervical specimens, with 2- to 3-day turnaround times. For the rapid test group, clinicians collected an extra endocervical swab for GeneXpert C trachomatis and N gonorrhoeae rapid testing, in addition to the standard-of-care nucleic acid amplification test swab. Rapid results were immediately provided, and all patients were treated according to providers’ clinical judgment.
Results:
In the rapid test group, 7.9% of patients had positive test results for C trachomatis; 3.9% had positive test results for N gonorrhoeae. In the control standard-of-care group, 10.2% of patients had positive nucleic acid amplification test results for C trachomatis; 5.5% had positive results for N gonorrhoeae. Undertreatment for both C trachomatis and N gonorrhoeae in the ED was 0% for the rapid test group and 43.8% for the control standard-of-care group. Clinicians overtreated 46.5% of uninfected standard-of-care control patients for C trachomatis compared with 23.1% of uninfected rapid test patients. For patients uninfected with N gonorrhoeae, clinicians overtreated 46.7% of standard-of-care control patients compared with 25.4% of rapid test patients. The length of stay did not differ significantly between groups.
Conclusion:
Rapid C trachomatis and N gonorrhoeae testing in the ED led to a significant reduction in overtreatment for women without infections compared with the standard-of-care control group. Additionally, in the rapid test group there was significant improvement in appropriate treatment for patients with infections.
INTRODUCTION
Background
Chlamydia trachomatis is the most commonly reported bacterial sexually transmitted infection, with approximately 1.6 million reported cases in the United States in 2016, representing an increase of 4.7% compared with the rate in 2015.1 In 2016, greater than 450,000 Neisseria gonorrhoeae cases were reported, representing an increase of 18.5% across the United States. Despite the Centers for Disease Control and Prevention’s (CDC’s) and many professional organizations’ recommendations that women with urogenital symptoms be tested diagnostically for C trachomatis and N gonorrhoeae and sexually active women younger than 25 years be screened annually for C trachomatis and N gonorrhoeae, diagnostic testing coverage remains suboptimal, especially in emergency departments (EDs), where the burden of infections is high.2–4 Although EDs are a common site for diagnosis and treatment of sexually transmitted infections, such as C trachomatis and N gonorrhoeae, reliable diagnosis for patients presenting to an ED with genitourinary complaints that warrant sexually transmitted infection testing has been challenged by the fact that recommended routine standard-of-care tests such as traditional nucleic acid amplification tests do not provide results within the timeframe of a typical ED visit. Nucleic acid amplification tests are sent and processed at a central laboratory and are not typically run immediately, meaning test results are not made available for 2 to 4 days.
Importance
ED providers make empiric treatment decisions, resulting in overtreatment of up to 30% of the population who do not have a sexually transmitted infection and undertreatment of up to 40% of patients with confirmed C trachomatis and N gonorrhoeae, with potential resultant morbidity and long-term adverse sequalae.3–8 Underuse of antibiotics for patients with sexually transmitted infections is known to lead to multiple medical complications, whereas inappropriate antibiotic use can cause unnecessary adverse effects and lead to increased antibiotic resistance in the community. Integrating a reliable and more rapid assay for the accurate diagnosis of C trachomatis and N gonorrhoeae in the ED could facilitate appropriate use of antibiotics and prevent the overuse of inappropriate antibiotic therapy for patients who are not infected, thus affecting both the patient and antibiotic stewardship.9–11
Goals of This Investigation
Newer, highly accurate rapid and point-of-care nucleic acid amplification tests, which can be run at or near the patient’s bedside in the ED, are now available and can provide timely results during the patient’s visit. Our goal was to evaluate the clinical effect of rapid sexually transmitted infection testing for C trachomatis and N gonorrhoeae in an ED, relative to standard of care. Our primary objective was powered to estimate overtreatment and our secondary objective was to estimate undertreatment for sexually transmitted infections in the ED.
MATERIALS AND METHODS
We conducted a prospective randomized clinical trial from April 2015 to May 2016 in an urban academic ED, which has approximately 70,000 visits per year and provides care to a primarily socioeconomically disadvantaged population. This clinical trial compared diagnosis and treatment of C trachomatis and N gonorrhoeae, using current standard-of-care nucleic acid amplification tests and the Food and Drug Administration-cleared GeneXpert rapid point-of-care C trachomatis and N gonorrhoeae nucleic acid amplification test (Xpert CT/NG; Cepheid Inc., Sunnyvale, CA). Previous clinical validation of the Xpert CT/NG for endocervical swabs showed a sensitivity of 98.7% and specificity of 99.4% for C trachomatis, and sensitivity of 100% and specificity of 100% for N gonorrhoeae.12 This study was approved by the Johns Hopkins University institutional review board.
Women aged 18 to 50 years and undergoing pelvic examination including C trachomatis and N gonorrhoeae testing as part of their ED standard of care were considered eligible for enrollment. Dedicated research staff screened potential patients for eligibility based on specific reported symptoms such as lower abdominal pain, vaginal pain, and vaginal discharge. An electronic medical record page alerted research staff when clinical sexually transmitted infection tests and procedures were ordered by a clinician as part of their clinical Standard-of-Care evaluation. Research staff approached clinicians and informed them of their patient’s eligibility for the study. The study took place according to the availability of research assistants, which covered approximately 10 hours per day, Monday through Friday. All eligible patients provided voluntary written informed consent before any study-related activities. Patients were excluded if they did not speak English, if they were unable to provide informed consent, and if they had a known pregnancy. Patients who provided consent were excluded as screen failures if the sexually transmitted infection test or pelvic examination was canceled, if standard-of-care cervical specimens for C trachomatis and N gonorrhoeae were not collected, or if they were determined to have been previously enrolled.
After written informed consent, participants were randomized with block randomization preassigned in concealed envelopes to either the control group or the rapid test group. Both the participant and her treating clinician were informed of the group assignment. After research staff collected baseline and demographic data, all participants underwent pelvic examination and specimen collectionby the clinician according to standard of care at our institution. For participants randomized to the control standard-of-care group, one standard-of-care C trachomatis and N gonorrhoeae endocervical swab was collected and sent for batch testing by nucleic acid amplification test (Aptima Combo2; Hologic, San Diego, CA), with a 2- to 3-day turnaround time. In accordance with clinical symptoms, patient report, or both, clinicians offered treatment for chlamydia and gonorrhea according to clinical judgement and CDC treatment guidelines, which are used to guide standard of care in this ED.13
For participants randomized to the rapid test group, one standard-of-care C trachomatis and N gonorrhoeae endocervical swab was collected for batch testing by nucleic acid amplification test, and an additional one was collected for rapid testing with the polymerase chain reaction-based GeneXpert C trachomatis and N gonorrhoeae instrument (Cepheid), with an approximate 90- to 100-minute turnaround time.14 Research staff labeled, transported, and processed the additional endocervical specimens for the rapid test group in a research area located in the central laboratory immediately, according to manufacturer’s instructions. Results from the rapid test were given to treating clinicians and entered into the medical record, and patients were treated according to the provider’s clinical judgment. Any rapid test samples that were invalid or indeterminate (reading invalid, error, or no result) were retested with the GeneXpert C trachomatis and N gonorrhoeae assay and the results were given to the treating clinicians when they became available. We did not ask patients in the rapid test group to wait for their results after they consented to the study. Patient dispositions were handled according to the provider’s clinical judgment, so some patients could have been discharged before rapid results were available.
Additionally, approximately 2 weeks after the initial ED visit, 2 trained research staff attempted to contact participants in both rapid test and control standard-of-care groups by telephone. During telephone follow-up, participants were asked whether they sought additional related care or treatment based on their C trachomatis and N gonorrhoeae diagnosis. The 2 research staff also reviewed the medical chart from the participants’ ED visit, using a structured data instrument to determine diagnosis of a sexually transmitted infection; antibiotic administration, prescription, and indication; results of standard-of-care testing; and length of stay in the ED. The results from the rapid test were recorded immediately on the structured data forms. The retrospective chart review elements of the study were confirmed by the second research staff. Classification of “appropriate” or “inappropriate” antibiotic treatment results was based on results of provision of treatment while the patient was in the ED, in accordance with recommended or alternative 2015 CDC sexually transmitted disease treatment guidelines.13 When post-ED visit attempts were made to contact untreated, infected, control standard-of-care patients, the women reported either no treatment (most) or were lost to follow-up, resulting in uneven follow-up completion. Thus, the decision was made to define treatment for infected patients as the time of the ED visit.
Primary Data Analysis
With an estimated C trachomatis and N gonorrhoeae combined prevalence of 10% and an overtreatment rate of 30%, a sample size of 120 controls and 120 rapid test group participants was needed to demonstrate a 50% decrease in overtreatment. Statistical analyses with SAS (version 9.4; SAS Institute, Inc., Cary, NC) were used to compare participants’ characteristics, as well as under- and overtreatment between groups. Overtreatment was defined as a C trachomatis— and N gonorrhoeae— negative patient who received antibiotic treatment in the ED. Rates of over- and undertreatment for C trachomatis and N gonorrhoeae were performed separately, as well as overall (for C trachomatis and N gonorrhoeae combined). Comparisons were made between the rapid test group and control standard-of-care group. Undertreatment was defined as a C trachomatis— and N gonorrhoeae—positive patient who did not receive appropriate antibiotic treatment as part of the ED visit between groups.
RESULTS
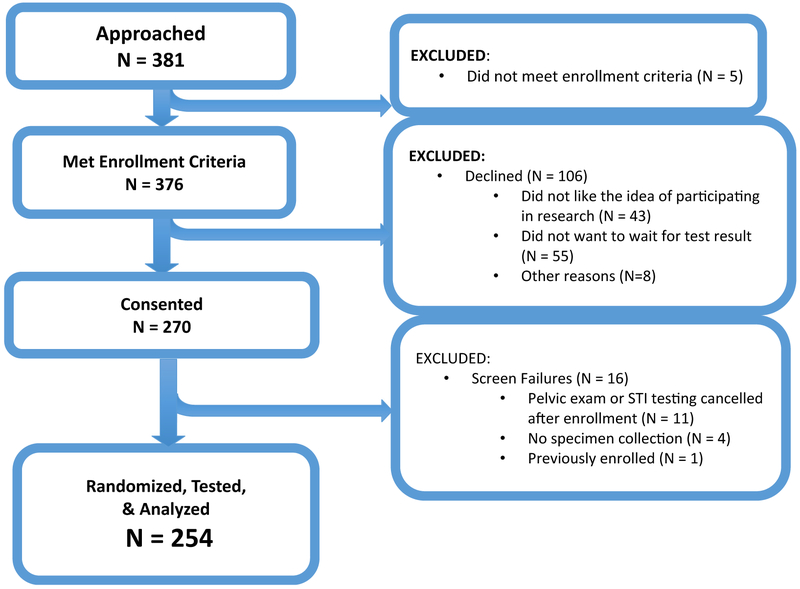
A total of 381 women were approached, and of these, 376 met enrollment criteria and 270 provided consent; after screen failures, 254 were randomized, 127 in the rapid test group and 127 in the control standard-of-care group (Figure 1). A summary of patient characteristics, medical history, and symptoms of patients in the rapid test group and control standard-of-care group indicated there were no significant differences in the 2 randomized groups (Table 1).
Figure 1.
Exclusion characteristics of potentially eligible patients who were approached for enrollment. STI, Sexually transmitted infection.
Table 1.
Characteristics, medical history, and symptoms of patients in the rapid test group and control groups.
| Characteristics | No. (%), n = 254 |
Group,No. (%) | Difference in Proportion (95% Cl) | |
|---|---|---|---|---|
| Rapid Test, n = 127 |
Control, n = 127 |
|||
| Age, y | ||||
| 18–24 | 113 (44.5) | 51 (40.2) | 62 (48.8) | −8.7 (−20.8 to 3.5) |
| 25–29 | 58 (22.8) | 30 (23.6) | 28 (22.0) | 1.6 (−8.8 to 11.9) |
| 30–34 | 44 (17.3) | 22 (17.3) | 22 (17.3) | 0.0 (−9.3 to 9.3) |
| 35–50 | 39 (15.4) | 24 (18.9) | 15 (11.8) | 7.1 (−1.7 to 15.9) |
| Race | ||||
| Black | 210 (82.7) | 104 (81.9) | 106 (83.5) | −1.6 (−10.9 to 7.7) |
| White | 36 (14.2) | 21 (16.5) | 15 (11.8) | 4.7 (−3.8 to 13.3) |
| Asian | 5 (2.0) | 1 (0.8) | 4 (3.1) | −2.4 (−5.8 to 1.0) |
| Other | 3 (1.2) | 1 (0.8) | 2 (1.6) | −0.8 (−3.4 to 1.9) |
| Ethnicity | ||||
| Hispanic | 6 (2.4) | 2 (1.6) | 4 (3.1) | −1.6 (−5.3 to 2.2) |
| Sexual behavior | ||||
| Condom use, always | 27 (10.6) | 14 (11.0) | 13 (10.2) | 0.8 (−6.8 to 8.4) |
| New sexual partner in the past 3 mo | 44 (17.3) | 26 (20.5) | 18 (14.2) | 6.3 (−3.0 to 15.6) |
| >1 sexual partner in the past 3 mo | 32 (12.6) | 21 (16.5) | 11 (8.7) | 7.9 (−0.2 to 16.0) |
| Having a sexual partner with an STI | 129 (50.8) | 65 (51.2) | 64 (50.4) | 0.8 (−11.5 to 13.1) |
| History of STIs | ||||
| Tested for an STI | 246 (96.9) | 122 (96.1) | 124 (97.6) | −1.6 (−5.9 to 2.7) |
| Chlamydia | 99 (39.0) | 45 (35.4) | 54 (42.5) | −7.1 (−19.1 to 4.9) |
| Gonorrhea | 59 (23.2) | 31 (24.4) | 28 (22.0) | 2.4 (−8.0 to 12.7) |
| Trichomonas | 73 (28.7) | 38 (29.9) | 35 (27.6) | 2.4 (−8.8 to 13.5) |
| Genital herpes | 16 (6.3) | 5 (3.9) | 11 (8.7) | −4.7 (−10.7 to 1.2) |
| Genital warts | 7 (2.8) | 6 (4.7) | 1 (0.8) | 3.9 (−0.1 to 7.9) |
| Syphilis | 3 (1.2) | 1 (0.8) | 2 (1.6) | −0.8 (−3.4 to 1.9) |
| PID | 46 (18.1) | 26 (20.5) | 20 (15.7) | 4.7 (−4.7 to 14.2) |
| HIV | 2 (0.8) | 2 (1.6) | 0 | 1.6 (−0.6 to 3.7) |
| Pubic lice | 8 (3.1) | 5 (3.9) | 3 (2.4) | 1.6 (−2.7 to 5.9) |
| Any STD | 161 (63.4) | 83 (65.4) | 78 (61.4) | 3.9 (−7.9 to 15.8) |
| Symptoms | ||||
| Lower abdominal pain | 180 (70.9) | 94 (74.0) | 86 (67.7) | 6.3 (−4.9 to 17.5) |
| Vaginal discharge | 128 (50.4) | 62 (48.8) | 66 (52.0) | −3.2 (−15.4 to 9.1) |
| Vaginal pain | 57 (22.4) | 26 (20.5) | 31 (24.4) | −3.9 (−14.2 to 6.3) |
| Vaginal itching | 59 (23.2) | 31 (24.4) | 28 (22.0) | −2.4 (−12.7 to 8.0) |
| Vaginal bleeding | 82 (32.3) | 43 (33.9) | 39 (30.7) | 3.2 (−8.3 to 14.6) |
| Fever | 32 (12.6) | 12 (9.4) | 20 (15.7) | −6.3 (−14.4 to 1.8) |
| Pain when urinating | 57 (22.4) | 26 (20.5) | 31 (24.4) | −3.9 (−14.2 to 6.3) |
| Frequent urination | 123 (48.4) | 58 (45.7) | 65 (51.2) | −5.5 (−17.8 to 6.8) |
| Lower back pain | 133 (52.4) | 64 (50.4) | 69 (54.3) | −3.9 (−16.2 to 8.3) |
| Pain during intercourse | 47 (18.5) | 24 (18.9) | 23 (18.1) | 0.8 (−8.8 to 10.3) |
PID, Pelvic inflammatory disease.
In the rapid test group, 7.9% of patients (10) had positive test results for C trachomatis and 3.9% (5) had positive test results for N gonorrhoeae. In the control standard-of-care group, 10.2% of patients (13) had positive results by nucleic acid amplification test for C trachomatis and 5.5% (7) had positive results for N gonorrhoeae (Table 2). Each individual in the rapid test group also had a standard-of-care nucleic acid amplification test, for which there was 100% agreement between the pairs of the rapid test result and the standard-of-care nucleic acid amplification test. There were 6 patients (2.4%) coinfected with both C trachomatis and N gonorrhoeae, 2 (1.6%) in rapid test group and 4 (3.1%) in control standard-of-care group.
Table 2.
Results of nucleic acid amplification tests for C trachomatis and N gonorrhoeae for the rapid test group and the control standard-of-care group.
| Rapid Test Group, No. (%) | Control Group, No. (%) | Total, No. (%) | |
|---|---|---|---|
| Enrolled | 127 | 127 | 254 |
| CT positive | 10 (7.9) | 13 (10.2) | 23 (9.1) |
| NG positive | 5 (3.9) | 7 (5.5) | 12 (4.7) |
CT, C trachomatis; NG, N gonorrhoeae.
There were 6 patients coinfected with both CT and NG.
All patients in the rapid test group who tested positive for either C trachomatis or N gonorrhoeae were treated appropriately for C trachomatis and N gonorrhoeae infections during the clinical encounter (Table 3). In the control standard-of-care group, 6 of 13 patients (46.2%) with C trachomatis—positive results did not receive appropriate antibiotic treatment in the ED (ie, undertreatment rate in the ED for the rapid test=0% [0/10] versus 46.2% [6/13]; undertreatment rate difference -46.2%; 95% confidence interval [CI] -73.3% to -19.2%). Also, in the control standard-of-care group, 4 of 7 patients (57.1%) with N gonorrhoeae were not treated with appropriate antibiotics in the ED (undertreatment rate for the rapid test=0% [0/5] versus 57.1% [4/7]; undertreatment rate difference -57.1%; 95% CI -93.8% to -20.5%). The overall undertreatment rate for both C trachomatis and N gonorrhoeae in the ED was 0% (0/13) for the rapid test group and 43.8% (7/16) for the control standard-of-care group (undertreatment rate difference -43.8%; 95% CI -68.1% to -19.4%).
Table 3.
Undertreatment of C trachomatis- and N gonorrhoeae-infected women and overtreatment of uninfected women in the rapid test group and standard-of-care control groups.
| Rapid Test Group | Standard-of-Care Control Group | |||
|---|---|---|---|---|
| Undertreatment, n/N (%) |
Overtreat merit, n/N (%) |
Undertreatment, n/N (%) |
Overtreatment, n/N (%) |
|
| CT | 0/10 | 27/117 (23.1) | 6/13 (46.2) | 53/114 (46.5) |
| NG | 0/5 | 31/122 (25.4) | 4/7 (57.1) | 56/120 (46.7) |
Undertreatment data are presented for CT- or NG-positive patients who did not receive antibiotic treatment according to the 2015 CDC treatment guidelines. Overtreatment data are presented for CT- or NG-negative patients who received antibiotic treatment. CT undertreatment rate difference was -46.2% (95% CI -73.3% to -19.2%). NG undertreatment rate difference was -57.1% (95% CI -93.8% to -20.5%). CT overtreatment rate difference was -23.4% (95% CI -35.3% to -11.5%). NG overtreatment rate difference was -21.3% (95% CI -33.1% to -9.5%).
Of patients who were not infected with C trachomatis, clinicians overtreated 23.1% (27/117) with antibiotics in the ED in the rapid test group compared with 46.5% (53/ 114) in the control standard-of-care group (overtreatment rate difference -23.4%; 95% CI -35.3% to -11.5%) (Table 3). Of patients who were not infected with N gonorrhoeae, clinicians overtreated 25.4% (31/122) in the rapid test group in the ED and 46.7% (56/120) in the control standard-of-care group (overtreatment rate difference -21.3%; 95% CI -33.1% to -9.5%).
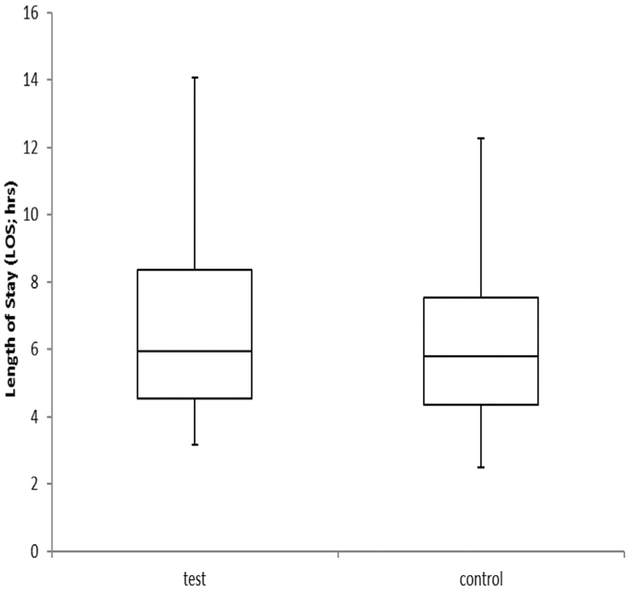
The median turnaround time for the rapid test results in the ED was 104 minutes, with an interquartile range of 99 to 108 minutes. The turnaround time was recorded by the research staff as “order to receipt of results.” In the rapid test group, 3 samples were identified as invalid, 1 sample was indeterminate, and 1 sample indicated no result on the first run. According to the manufacturer’s instructions, testing was repeated for all 5 samples, yielding valid results. In the control standard-of-care group, the ED length of stay ranged from 4.4 to 7.5 hours, with a median of 5.8 hours (Figure 2). In the rapid test group, the ED length of stay ranged from 4.6 to 8.4 hours, with a median of 5.9 hours. There were 14 patients in the rapid test group who were discharged before test results were available; however, none had C trachomatis or N gonorrhoeae. The box-and-whiskers plot indicates length of ED stay (Figure 2). The ED length of stay was not substantially different between the rapid test group and the control standard-of-care group (rapid test group 5.9 hours, interquartile range 4.6 to 8.6 hours; control standard-of-care group 5.8 hours, interquartile range 4.4 to 7.5 hours). In the control standard-of-care group, follow-up telephone calls to patients after discharge resulted in loss to follow-up (5 total), 1 patient receiving treatment for C trachomatis, 1 receiving no C trachomatis treatment, and 2 N gonorrhoeae-infected patients not receiving further medical treatment.
Figure 2.
Length of stay in the ED in the rapid test group compared with the control standard-of-care group. In the control standard-of-care group, the ED length of stay ranged from 4.4 to 7.5 hours, with a median of 5.8 hours. In the rapid test group, the ED length of stay ranged from 4.6 to 8.4 hours, with a median of 5.9 hours.
LIMITATIONS
Our study had limitations, which may prevent generalization to other populations and other EDs because this study was performed at only one urban inner-city ED. Because of constraints on study costs and timeline, the study was powered to demonstrate a difference in antibiotic overtreatment in infection-negative patients between the rapid testing and standard-of-care groups. Although we demonstrated significant findings in this primary objective, for overtreatment of sexually transmitted infections we were able to demonstrate a statistically significant difference in undertreatment only for overall disease-positive patients and for C trachomatis-positive patients. We were able to show only marginal significance in undertreatment in N gonorrhoeae—positive patients. A larger study may have demonstrated a stronger significant finding for the secondary objective of undertreatment for C trachomatis and N gonorrhoeae. Another limitation is that the success of the rapid test may be a reflection of the failure of the standard method because of our loss to follow-up. This failure to treat infected patients in the control standard-of-care group may have contributed to the success of the rapid test method.
DISCUSSION
This study demonstrated that the use of rapid C trachomatis and N gonorrhoeae testing in the ED led to a significant reduction in inappropriate overtreatment for sexually transmitted infections compared with that for the control standard-of-care group, as well as significant improvement in appropriate treatment for infected patients in the rapid testing group. EDs are important sites for the diagnosis and treatment of sexually transmitted infections, particularly among individuals with higher behavioral and demographic risks. Many challenges exist for treating sexually transmitted infections in EDs because results of diagnostic tests are usually not returned until after the patient leaves, making treatment decisions difficult for clinicians. Additionally, for infected patients who are cared for in ED settings, returning positive results to patients who were not treated at the clinical encounter and checking on treatment initiation is known to be very difficult because of unsuccessful telephone notifications of positive results, lack of callbacks, and loss to follow-up.15,16 This challenge was also noted in our study.
We believe our study adds to the existing literature because it demonstrated that on the basis of routine ED operations, the availability of a rapid test result could potentially make a difference in accurate treatment of infected women and prevention of overtreatment of uninfected women.
The lack of reliable and rapid test results often leads to overtreatment of patients who are not infected. The fact that the rapid test and the criterion standard comparator nucleic acid amplification test assays agreed completely for the rapid test group confirms the readability and accuracy of the rapid test as it was used in this study.12 Empiric or syndromic treatment often results in antibiotic overtreatment for suspected sexually transmitted infections for as many as 30% to 46% of patients and 15% to 40% of patients with confirmed C trachomatis and N gonorrhoeae not being treated.5–7,17 Medical record review from 2 inner-city EDs estimated an overtreatment rate of 86%.17 In another study, chart review of factors associated with over- and undertreatment of gonorrhea and chlamydia in 797 patients presenting to a public hospital ED with sexually transmitted infection exposure or urogenital symptoms found an overtreatment rate of 21.6% and an undertreatment rate of 43.4%, indicating that medical history and symptoms may not be a reliable approach for treatment decisions.18
These statistics have important implications both for patient care and public health. First, missed diagnosis in an individual can result in increased transmission of sexually transmitted infections (including HIV), with long-term adverse sequelae, including serious reproductive health complications. The CDC estimates that 20,000 women become infertile each year because of undiagnosed sexually transmitted infections.1 Second, inappropriate use of antibiotics not only leads to medical complications but also can lead to unnecessary adverse effects and increased antibiotic resistance in the community. Integrating a reliable and rapid test for C trachomatis and N gonorrhoeae in EDs where patients could be treated at the clinical visit could improve individual patient care and avoid overtreatment,15–17 thus enhancing antimicrobial stewardship.
With the increasing resistance to the fluoroquinolones and declining susceptibility to cefixime and azithromycin, dual therapy with ceftriaxone and azithromycin is now the only CDC-recommended treatment for gonorrhea.13 Continued monitoring of over- and undertreatment of patients attending EDs, as well as susceptibility patterns to these antibiotics, is critical and plays an important role in antibiotic stewardship. In our study, we found that knowing results from a rapid diagnostic test before a patient was discharged appeared to give clinicians useful treatment information. Patients in the control standard-of-care group whose test results were available only within the routine turnaround time of several days for nucleic acid amplification testing for C trachomatis and N gonorrhoeae were statistically more likely to receive presumptive overtreatment or complete lack of treatment before they left the ED. Because antimicrobial resistance remains an important consideration in the treatment of sexually transmitted infections, especially gonorrhea, our findings suggest that effort to advance the availability of rapid diagnostic instruments that can yield results within the timeframe of the patient’s visit could be a critical component of antibiotic stewardship by reducing antibiotic overusage.9–11
The prevalence of chlamydia and gonorrhea infections in our study was similar to that of other studies. A study with broad screening for asymptomatic chlamydia or gonorrhea in a pediatric ED found a positivity of 9.9%.6 That study excluded symptomatic patients, highlighting the importance of potential missed diagnoses among adolescents, who are at high risk of sexually transmitted infections. Previous reports of C trachomatis and N gonorrhoeae screening in EDs found that unrecognized rates of infections ranged from 38% to 82%.7,8 A recent study using the same rapid assay we used in our rapid test group reported a similar prevalence of 11% for chlamydia and 4% for gonorrhea, as well as good performance compared with the standard-of-care nucleic acid amplification test used, which was also the same assay used for our control standard-of-care group.14 Several studies have demonstrated that rapid testing for chlamydia and gonorrhea in EDs returns results faster, leading to significant increases of appropriate treatment.15,16 Rivard et al15 noted an odds ratio for appropriate treatment of 22.65. As did May et al,16 we corroborated what has been demonstrated at another center: that overtreatment can be prevented by use of rapid tests in the ED. Additionally, we showed that using rapid tests can improve appropriate treatment for more infected patients before they leave the ED.
Use of a rapid test can make treatment more precise. Financial costs are certainly an issue. The GeneXpert nucleic acid amplification test assay is certainly as accurate as any other nucleic acid amplification test assay that is commercially available and is more accurate than some. The assay platform is not as expensive as some large robotic platforms in use by many clinical laboratories. The cost per test cartridge is probably slightly more than that of other nucleic acid amplification test assays, but the volume ordered dictates the cost per test. The Current Procedural Terminology (CPT) code for insurance reimbursement isthe same for all nucleic acid amplification test assays. Although some laboratories and EDs are resistant to making changes, the rapid turn-around-time can offer advantages to both the laboratory and the clinic. Ideally, someone could run the Cepheid point-of-care test in the ED.
Many clinicians have concerns about integrating rapid testing and how that would affect the length of stay, a critical factor for our nation’s already crowded EDs. Integrating a rapid and accurate test in the ED clinic work flow early in the patient encounter should not affect clinic flow, especially if the diagnostic test were implemented into the clinical pathway in a way that was attentive to patient turnaround time. As observed in our study, using the rapid test did not substantially increase patients’ length of stay in the rapid test group compared with the control standard-of-care group. Often patients receiving sexually transmitted infection testing are undergoing other diagnostic testing for their symptoms, such as pelvic ultrasonography, or additional laboratory testing, which can be conducted simultaneously with the rapid sexually transmitted infection testing, thus minimizing the effect on length of stay. Further operational research in engineering clinical pathways for sexually transmitted infections could move testing to even earlier points in the patient’s evaluation (as has been successfully done for HIV testing).
Although changing testing procedures by laboratories is often difficult to achieve, demonstrating better patient care and precise treatment can help institute changes. As long as accuracy and financial costs can be maintained, which should certainly be possible with the GeneXpert assay, it would be advantageous for the patient and may prevent overtreatment of patients who do not have a sexually transmitted infection.
In summary, we demonstrated that using new rapid test diagnostic technology in the ED setting could promote correct antibiotic treatment for patients with sexually transmitted infection, as well as prevent empiric overtreatment for a substantial portion of women who are not infected, thus improving antibiotic stewardship.
Editor’s Capsule Summary.
What is already known on this topic
Treatment for sexually transmitted infections in the emergency department (ED) is usually presumptive because of delayed test results, leading to over- and undertreatment.
What question this study addressed
Women receiving pelvic examination and testing for chlamydia and gonorrhea (n=254) were randomized to routine testing (results in 2 to 3 days) versus rapid test results available in the ED. Treatment was at clinician discretion.
What this study adds to our knowledge
In the rapid testing group, 100% of patients with positive results for chlamydia or gonorrhea were treated versus 56% in the routine testing group. For patients uninfected, approximately 25% were unnecessarily treated in the rapid testing group versus 47% in the routine testing group.
How this is relevant to clinical practice
Rapid testing for chlamydia and gonorrhea could improve accuracy of ED treatment. The magnitude of improvement could depend on the effectiveness of follow-up procedures currently in place.
Funding and support:
By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. This study was funded by U54EB007958, National Institute of Biomedical Imagining and Bioengineering, National Institutes of Health (NIH); and U-01068613, NIH, National Institute of Allergy and Infectious Diseases. Cepheid Diagnostics provided diagnostic test cartridges.
Trial registration number: NCT02200224
Footnotes
All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016. Atlanta, GA: US Department of Health & Human Services; 2017. [Google Scholar]
- 2.National Committee for Quality Assurance. The state of the health care quality 2016. Available at: http://www.nchq.org/report-cards/health-plans/state-of-ealth-care-quality. Accessed July 27, 2017.
- 3.Jenkins WD, Zahnd W, Kovach R, et al. Chlamydia and gonorrhea screening in United States emergency departments. J Emerg Med. 2013;28:58–67. [DOI] [PubMed] [Google Scholar]
- 4.Mehta SD. Gonorrhea and chlamydia in emergency departments: screening, diagnosis, and treatment. Curr Infect Dis Rep. 2007;9:134–142. [DOI] [PubMed] [Google Scholar]
- 5.Borhart J, Birnbaumer DM. Emergency department management of sexually transmitted infections. Emerg Med Clin North Am. 2011;29:587–603. [DOI] [PubMed] [Google Scholar]
- 6.Schneider K, FitzGerald M, Byczkowski T, et al. Screening for asymptomatic gonorrhea and chlamydia in the pediatric emergency department. Sex Transm Dis. 2016;43:209–215. [DOI] [PubMed] [Google Scholar]
- 7.Mehta S, Rothman R, Kelen G, et al. Clinical aspects of diagnosis of gonorrhea and chlamydia infection in an acute care setting. Clin Infect Dis. 2001;32:655–659. [DOI] [PubMed] [Google Scholar]
- 8.Schechter-Perkins EM, Jenkins D, White LF, et al. Treatment of cases of Neisseria gonorrhoeae and Chlamydia trachomatis in emergency patients. Sex Transm Dis. 2015;42:353–357. [DOI] [PubMed] [Google Scholar]
- 9.Wi T, Lahra MM, Ndowa F, et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 2017;14:e1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz AR, Komeya AY, Kirkcaldy RD, et al. Cluster of Neisseria gonorrhoeae isolates with high-level azithromycin resistance and decreased ceftriaxone susceptibility, Hawaii, 2016. Clin Infect Dis. 2017;65:918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hook EW 3rd. Continued evolution of gonococcal antimicrobial resistance. Clin Infect Dis. 2017;65:924–926. [DOI] [PubMed] [Google Scholar]
- 12.Gaydos CA, Van Der Pol B, Jett-Goheen M, et al. Performance of the Cepheid CT/NG Xpert Rapid PCR test for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013;51:1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1–137.25590678 [Google Scholar]
- 14.Wilson SP, Vohra T, Goldberg J, et al. Reliable rapid assay for gonorrhea and chlamydia in the emergency department. J Emerg Med. 2017;53:890–895. [DOI] [PubMed] [Google Scholar]
- 15.Rivard K, Dumkow L, Draper H, et al. Impact of rapid diagnostic testing for chlamydia and gonorrhea on appropriate antimicrobial utilization in the emergency department. Diagn Microbiol Infect Dis. 2017;87: 175–179. [DOI] [PubMed] [Google Scholar]
- 16.May L, Ware CE, Jordan JA, et al. A randomized controlled trial comparing the treatment of patients tested for chlamydia and gonorrhea after a rapid polymerase chain reaction test versus standard of care testing. Sex Transm Dis. 2016;43: 290–295. [DOI] [PubMed] [Google Scholar]
- 17.Holley C, Van Pham T, Mezzadra H, et al. Overtreatment of gonorrhea and chlamydial infections in 2 inner-city emergency departments. Am J Emerg Med. 2015;33:1265–1268. [DOI] [PubMed] [Google Scholar]
- 18.Anaene M, Soyemi K, Caskey R. Factors associated with the overtreatment and under-treatment of gonorrhea and chlamydia in adolescents presenting to a public hospital emergency department. Int J Infect Dis. 2016;53:34–38. [DOI] [PubMed] [Google Scholar]