Abstract
The serotonin transporter (SERT) is one of the primary targets for medications to treat neuropsychiatric disorders and functions by exploiting pre-existing ion gradients of Na+, Cl−, and K+ to translocate serotonin from the synaptic cleft into the presynaptic neuron. Although recent hSERT crystal structures represent a milestone for structure-function analyses of mammalian neurotransmitter:sodium symporters, they are all derived from thermostabilized but transport-deficient constructs. Two of these structures are in complex with paroxetine, the most potent selective serotonin reuptake inhibitor known. In this study, by carrying out and analyzing the results of extensive and comparative molecular dynamics simulations while also re-evaluating the transport and binding properties of the thermostabilized constructs, we identified functionally important structural elements that are perturbed by these mutations, revealed unexpected dynamics in the central primary binding site of SERT, and uncovered a conceivable ambiguity in paroxetine’s binding orientation. We propose that the favored entropy contribution plays a significant role in paroxetine’s extraordinarily high affinity for SERT. Our findings lay the foundation for future mechanistic studies and rational design of high-affinity SERT inhibitors.
Keywords: Paroxetine, serotonin transporter, selective serotonin reuptake inhibitors, conformational thermostabilization, X-ray crystallography, molecular dynamics simulations
1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a crucial neurotransmitter that exerts a profound influence on a diverse range of fundamental physiological processes, including mood, anxiety, depression, learning, memory, neurodevelopment, appetite, circadian rhythm, digestion, thermoregulation, and vasoconstriction (Berger et al., 2009). Although more than 90% of 5-HT in the human body is found outside the central nervous system, mostly in the gastrointestinal tract and cardiovascular system, its role in the human brain has garnered more clinical attention primarily because of its involvement in the development of multiple neuropsychiatric disorders (Brummelte et al., 2017; Jacobs and Azmitia, 1992). Subsequent to its pre-synaptic release, 5-HT binds to and activates one or more of the thirteen different subtypes of human 5-HT receptors, leading to either excitatory or inhibitory neurotransmission depending on the specific receptor subtype (Nichols and Nichols, 2008). Despite the remarkable diversity of receptors responsible for mediating its downstream effects, there is only a single protein responsible for the reuptake of 5-HT from the synaptic cleft: the serotonin transporter (SERT) (Charnay and Leger, 2010). As the principal component for terminating 5-HT action, SERT is indispensable for shaping the duration and magnitude of serotonergic signaling. Given such a pivotal role, it is not surprising that mutations in SERT that adversely affect activity are strongly associated with numerous psychiatric ailments such as depression, panic attacks, obsessive-compulsive disorder (OCD), and autism; as such, SERT inhibitors are often used as first-line treatments for these patients (Daws and Gould, 2011; Hahn and Blakely, 2007; Pramod et al., 2013). Paroxetine ((3S,4R)-3-[(2H-1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine) is one of the most frequently prescribed and therapeutically effective selective serotonin reuptake inhibitors (SSRIs) (Bourin et al., 2001). Its high potency (<1 nM), high selectivity, and widespread clinical use have inspired detailed characterization of its binding mode in SERT (Davis et al., 2016; Nencetti et al., 2007; Nencetti et al., 2011; Sorensen et al., 2012; Tavoulari et al., 2009).
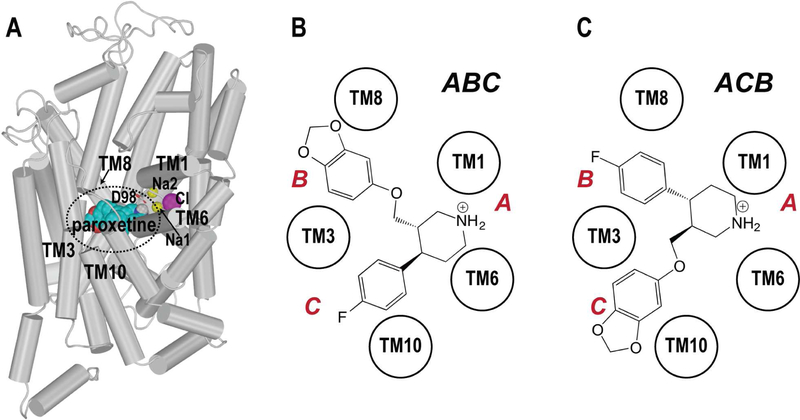
SERT is a member of the neurotransmitter:sodium symporter (NSS) family of secondary active transporters. Recent crystal structures revealed that SERT’s 12 transmembrane segments (TMs) are arranged in a so-called LeuT-like structural fold, characterized by two 5-TM inverted repeats (Coleman et al., 2016; Forrest et al., 2008; Yamashita et al., 2005). In these structures, SSRIs such as paroxetine and S-citalopram, are bound in the central primary binding (S1) site, in close association with two Na+ binding sites (Na1 and Na2) and a Cl− binding site (Coleman et al., 2016) (Figure 1A), although none of the individual structures possesses electron density for all three “coordinated” ions. The S1 site in NSS members can be divided into subsites A, B, and C, as first defined by Sørensen et al. (Sorensen et al., 2012). Subsite A is a polar region surrounding Asp98 (side chains from TMs 1, 6, and 8), whereas subsites B and C are the regions opposite to subsite A and are formed by residues from TMs 3 and 8 for subsite B and TMs 3, 6 and 10 for subsite C. The crystal structures of hSERT in complex with paroxetine are derived from thermostabilized but transport-deficient variants termed “ts3” (PDB ID 5I6X) (Coleman et al., 2016) and “Thr439-ts2” (PDB ID 6AWN) (Coleman and Gouaux, 2018) – see Table 1 for the list of the thermostabilizing mutations in each variant. In these two structures, the piperidine, benzodioxol, and fluorophenyl substituents of paroxetine reside in subsites A, B, and C of the S1 site, respectively – a pose we denote as ABC (Figure 1B).
Figure 1.
The two binding poses of paroxetine in the S1 site of hSERT. (A) The equilibrated model from “WT/paroxetine-ABC” simulations (see text) is viewed parallel to the membrane normal in a cylindrical representation, with the S1 site enclosed by a black dotted circle. The schematic orientations of the ABC and ACB poses of paroxetine in the S1 site are illustrated in panels (B) and (C), respectively. The locations of subsites A, B, and C are labeled with red letters.
Table 1. Functional characterization of hSERT constructs.
All experiments were performed six times (in triplicate) except for the ts2 paroxetine Kd, which was repeated three times (in triplicate). aValues represent the average ± SEM. bRelative surface expression compared to WT as assessed by surface biotinylation (see Methods). cCompared to WT Kd for paroxetine. dp = 0.0005, ep = 0.028, fp = 0.00004, gp = 0.05, and hp = 0.68. All p values determined by the Students unpaired t-test. Values less than or equal to 0.05 indicate a statistically significant difference.
| Construct | Mutations | Km (nM) | Vmax (pmol/min/105 cells) | Surface Expressionb | Kd for Paroxetine (nM) | Fold differencec |
|---|---|---|---|---|---|---|
| WT | 425 ± 30 | 2.29 ± 0.28 | 1.00 | 0.17 ± 0.03 | - | |
| ts2 | I291A/T439S | 2,414 ± 227d | 1.12 ± 0.12e | 1.13 ± 0.19 | 1.20 ± 0.14f | 7.1 |
| ts3 | Y110A/I291A/T439S | transport-deficient | 0.69 ± 0.14g | 4.1 | ||
| Thr439-ts2 | Y110A/I291A | transport-deficient | 0.19 ± 0.02h | 1.1 | ||
The binding mode of paroxetine in the S1 site of SERT was investigated concurrently in another study (Davis et al., 2016). Based on a synergistic combination of single-residue cross-species mutagenesis, flux assays, radioligand binding, and computational modeling, the benzodioxol and fluorophenyl substituents of paroxetine were proposed to reside in subsites C and B, respectively, an orientation “flipped” from that in the ABC pose (Davis et al., 2016) – we denote this pose as ACB (Figure 1C). In this pose, the fluorine of paroxetine is close to Ala169 in TM3. Mutating this residue to a negatively charged aspartate, the corresponding amino-acid residue in the SERTs from Drosophila and chicken, dramatically decreased the apparent affinity of paroxetine for hSERT-A169D. Thus, given the likely electrostatic repulsion between the negatively-charged Asp169 and electronegative fluorine, charge distribution was invoked as the primary determinant of the ACB pose (Davis et al., 2016).
Herein we propose that part of the discrepancy between the two studies may stem from the symmetrical physico-chemical properties of the paroxetine molecule. Along with remeasuring the 5-HT transport and paroxetine binding properties of the thermostabilized constructs, we carried out extensive molecular dynamics (MD) simulations comparing the ts3, Thr439-ts2, and wild-type (WT) hSERT variants in complex with paroxetine to investigate structural perturbations induced by the thermostabilizing mutations that adversely affect the activity of hSERT, and uncover clues within the S1 site that illuminate the mechanistic basis underlying paroxetine’s extraordinarily high affinity for hSERT as well as this SSRI’s putatively ambiguous binding modes.
2. Materials and Methods
2.1. Molecular dynamics simulations
We used the paroxetine-bound hSERT-ts3 crystal structure (PDB ID 5I6X) as the starting point for our modeling and MD simulations. The three thermostabilizing mutations were reverted back to their WT identities, and the missing sodium ion (Na2) was added according to its position in the S-citalopram-bound hSERT-ts3 complex (PDB ID 5I71). Pose ACB of paroxetine was selected from the results of docking the molecule into the hSERT S1 site using the induced-fit docking (IFD) protocol (Sherman et al., 2006) implemented in the Schrodinger suite (release 2016–4). hSERT models were placed into explicit 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine lipid bilayer (POPC) using the orientation of the structure 5I6X from the Orientation of Proteins in Membranes database (Lomize et al., 2006). Simple point charge (SPC) water model (Berendsen et al., 1981) was used to solvate the system, charges were neutralized, and 0.15 M NaCl was added. The total system size was ∼135000 atoms.
The OPLS3 force field (Harder et al., 2016) was used throughout this study. The initial parameters for paroxetine and its derivative, Br-paroxetine ((3S,4R)-3-((Benzo[d][1,3]dioxol-5-yloxy)methyl)-4-(4-bromophenyl)piperidine, Slack RD et al., manuscript in preparation), based on the default atom typing of OPLS3 were further optimized by the force field builder (Schrodinger release 2016–4).
Desmond MD system (D. E. Shaw Research, New York, NY) was used for the MD simulations. The system was initially minimized and equilibrated with restraints on the ligand heavy atoms and protein backbone atoms, followed by production runs at 310 K with all atoms unrestrained. The NPγT ensemble was used with constant temperature (310 K) maintained with Langevin dynamics. Specifically, 1 atm constant pressure was achieved with the hybrid Nose-Hoover Langevin piston method (Feller et al., 1995) on an anisotropic flexible periodic cell with a constant surface tension (x-y plane). After initial round of “WT/paroxetine-ABC” or “WT/paroxetine-ACB” simulations, the equilibrated systems were substituted/mutated in Maestro to introduce the Br-paroxetine analogue in either the ABC or ACB pose (“WT/Br-paroxetine-ABC” and “WT/Br-paroxetine-ACB”, respectively).
2.2. Conformational analysis
Distances and dihedral angles were calculated with MDTraj (version 1.7.2) (McGibbon et al., 2015) in combination with in-house Python scripts.
We developed the pairwise interaction analyzer for SERT (PIA-SERT), based on our previously-developed PIA-GPCR (Michino et al., 2017) and PIA-DAT (Abramyan et al., 2017). For analysis of coarse-grained interaction network of SERT, we defined the following structural elements: TM1i (the intracellular section (i) of TM1, residues 84–93), TM1m (the middle section (m) of TM1, residues 94–101), TM1e (the extracellular section (e) of TM1, residues 102–111), EL1 (the extracellular loop 1, residues 112–114), TM2e (residues 115–120), TM2m (residues 121–130), TM2i (residues 131–143), IL1 (residues 144–155), TM3i (residues 156–171), TM3m (residues 172–176), TM3e (residues 177–192), EL2 (residues 193–252), TM4e (residues 253– 261), TM4i (residues 262–270), IL2 (residues 271–276), TM5i (residues 277–280), TM5m (residues 281–289), TM5e (residues 290–299), EL3 (residues 300–322), TM6e (residues 323– 331), TM6m (residues 332–343), TM6i (residues 344–350), IL3 (residues 351–357). TM7i (residues 358–367), TM7m (residues 368–374), TM7e (residues 375–389), EL4a (residues 390– 401), EL4b (residues 402–420), TM8e (residues 421–433), TM8m (residues 434–442), TM8i (residues 443–452), IL4 (residues 453–461), TM9i (residues 462–471), TM9e (residues 472–482), EL5 (residues 483–486), TM10e (residues 487–495), TM10m (residues 496–500), TM10i (501– 513), IL5 (residues 514–535), TM11i (residues 536–546)., TM11e (residues 547–557), EL6 (residues 558–574), TM12e (residues 575–589), and TM12i (residues 590–601).
For the residue-level analysis, we focused on the amino acids located in the S1 binding site.
Data sets for conformational analyses were assembled as follows. We first combined data from individual trajectories into a common pool for each simulated condition. Then, for the distance and dihedral angle distribution analysis we extracted 500 bootstrapped samples of 5,000 random frames each for a given simulated condition and plotted averages and standard deviations of frequency distributions for those 500 samples. For the PIA-SERT and distance scatter plot analysis we used data from 5,000 random frames each for a given simulated condition. For the ligand pairwise RMSD calculations, the frames in each simulated condition were first separated into g- (between −120º and 0º), g+ (between 0º and 120º), and t (between - 180º and −120º and between 120º and 180º) rotamer states. For each state, we carried out 10 bootstrap samplings, and extract 500 frames for each sampling. For each of the 500-frame bootstraps, all the frames are aligned pairwise for the RMSD calculations, yielding a 500 × 500 matrix. The averages and standard deviations of the 10 bootstrapped samples were calculated and plotted.
2.3. Metadynamics simulations
Desmond (Bowers et al., 2006) (version 2.3, Schrodinger LLC, New York, NY, USA) was used for metadynamics runs. We used the Phe341 χ1 dihedral angle as our collective variable (CV). The height of the biasing Gaussian potential (“hill”) was 0.01 kcal/mol and the width was 5 degrees for the Phe341 χ1 dihedral angle. The data shown are the averages of one 16 and one 24ns runs, starting from various points within the trajectories of the indicated conditions.
2.4. Free energy perturbation calculations.
We assessed relative binding affinities of paroxetine and Br-paroxetine using the Schrodinger FEP+ method (Wang et al., 2015b). Calculations used either paroxetine or Br-paroxetine as the reference state, which were mutated to the corresponding perturbed states (i.e., either −F to −Br or −Br to −F). The calculations were performed for 5 or 10 ns using various frames from the trajectories as initial poses.
2.5. Quantum mechanical calculations
The electrostatic potential was calculated using Jaguar (version 9.4, Schrödinger, LLC, New York, NY, 2016). In this process, the geometries of paroxetine and Br-paroxetine were first optimized at the quantum mechanical level using B3LYP-density functional theory and the TZV** basis set implemented in Jaguar. Then the electrostatic potentials were calculated using the same functional theory and basis set and mapped onto the surfaces of constant electron density.
2.6. SERT steady-state kinetics
5-HT flux assays were performed as described previously (Davis et al., 2016) using transiently-transfected (20–24 hrs) T-REx-293 cells expressing hSERT-WT or -ts2 in 24-well poly-D-lysine coated plates at a density of 1×105 cells per well. Immediately prior to the assay, culture media was removed, cells were washed twice with 0.5 ml of a modified Ringer’s solution (pH 7.4) containing 5 mM Tris, 7.5 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, and then pre-incubated at room temperature in Ringer’s supplemented with 10 mM glucose, 100 µM pargyline, and 100 µM ascorbic acid. Uptake was initiated by replacing the supplemented Ringer’s in each well with one containing 0.025 – 20 µM [3H]5-HT (5-hydroxy[3H]tryptamine creatine sulfate [specific activity 45 Ci/mmol, Perkin Elmer, NET498]) and incubating for 5 min. Reactions were terminated by aspirating the assay solution from each well and washing three times with ice-cold Ringer’s (1 ml each time). Cells were lysed with 0.5 M NaOH (0.5 ml), after which the lysate was mixed with Opti-Fluor (PerkinElmer) liquid scintillation fluid and counted in a Beckman LS6500 counter. Specific [3H]5-HT transport was determined by subtracting the counts from control cells transiently transfected with an empty vector. Total uptake counts were always kept at less than 10% counts added to avoid ligand depletion. Experiments were performed six times, each time in triplicate, and the data fit to the Michaelis-Menten equation as implemented in GraphPad Prism 7.
2.7. SERT surface expression analysis
Relative expression of hSERT-WT versus -ts2 at the plasma membrane was quantitated by biotinylating surface proteins of intact cells with lysine-directed, membrane-impermeant sulfo-NHS-SS-Biotin (Pierce), as previously outlined (Henry et al., 2003). Note that the cells subjected to biotinylation originated from an aliquot of the same pool employed for the [3H]5-HT transport assays. Biotinylated proteins were captured with ImmunoPure immobilized streptavidin beads (Pierce), after which total and surface proteins were separated, run on SDS-PAGE, and immunoblotted. Westerns were probed with a hSERT monoclonal antibody (ST51–2, MabTechnologies) as well as an actin monoclonal antibody (PanActin Ab-5, ThermoFisher) followed by a LICOR donkey anti-mouse secondary antibody (LICOR-Biosciences). Blots were visualized on an Odyssey fluoroimager, and the images were analyzed with Image Studio Lite software (LICOR-Biosciences). The bands (pixel intensities of equal areas) for hSERT in the surface samples were compared for WT and ts2 to yield relative surface expression levels. The relative actin expression level was also compared to correct for any differences in the quantity of starting material.
2.8. [3H]Paroxetine radioligand binding
Crude membranes were isolated from transiently-transfected T-REx-293 cells expressing hSERT-WT, -ts2, -ts3, or −Thr439-ts2 (48 hrs) and resuspended in Ringer’s solution containing 20% glycerol. Aliquots were frozen in liquid nitrogen and stored at −80°C until needed. Binding was initiated by incubating the corresponding crude membranes in Ringer’s containing varying concentrations (0.02 to 25 nM) of [3H]-paroxetine (PerkinElmer NET869; 23.1 Ci/mmole) in borosilicate glass tubes for 1.5 hr at RT in the dark with continuous gyration. Nonspecific binding was measured from identical reactions supplemented with 200 µM cold paroxetine. To separate bound from free [3H]paroxetine, reactions were vacuum-filtered through a 96-well Multiscreen FB filter plate (MSFBN6B, Millipore) that had been pre-treated with 0.3% polyethyleneimine (freshly-made) for 6 hrs at RT and then washed. To prevent ligand depletion, the total amount of protein used in the binding assays was varied such that total counts bound were always less than 10% counts added. Experiments were performed at least 4 times, each time in triplicate, and data fit to a rectangular hyperbola, as implemented in GraphPad Prism 7.
3. Results
The three thermostabilizing mutations in the transport-deficient ts3 variant are Y110A in TM1e (see section 2.2 for our division of the subsegments), I291A in TM5e, and T439S in TM8m. Based on the ts3 crystal structure in complex with paroxetine oriented in the ABC pose (“x-ray ts3/paroxetine-ABC”; PDB ID 5I6X), we built the WT hSERT model in complex with paroxetine (“WT/paroxetine-ABC”). We then immersed both “ts3/paroxetine-ABC” and “WT/paroxetine-ABC” complexes in explicit lipid bilayers and collected multiple MD trajectories for each complex (see Methods and Table 2), and quantified the differences between these two complexes at both the subsegment and residue levels using the pairwise interaction analyzer for SERT (PIA-SERT, see section 2.2).
Table 2.
Simulated conditions and simulation lengths.
| Construct | Na2 | Ligand | Ligand pose | Number of trajectories | Lengths (µs) |
|---|---|---|---|---|---|
| ts3 | + | paroxetine | ABC | 2 | 7.17 |
| Thr439-ts2 | + | paroxetine | ABC | 3 | 2.01 |
| − | 3 | 1.98 | |||
| WT | + | paroxetine | ABC | 5 | 12.84 |
| ACB | 5 | 12.36 | |||
| Br-paroxetine | ABC | 5 | 11.61 | ||
| ACB | 5 | 8.37 | |||
| Total | 28 | 56.34 |
3.1. Characterization of the perturbations by thermostabilizing mutations near the S1 and Na2 sites reveal important structural elements for hSERT function
Our PIA-SERT analysis identified that the most prominent change between the simulations of ts3 and WT when bound to paroxetine in the ABC pose, is rearrangement of EL4a on the extracellular side (Figure 2A). Specifically, compared to ts3, residues Val394 to Ala398 in EL4a, which adopt a helical conformation, move away from the extracellular vestibule in WT. This upward movement of EL4a is associated with an outward tilting of TM1e (Figures 2B and C). A close examination shows that Tyr110 and Tyr107 of TM1e stack to form aromatic-hydrophobic interactions with Ala398 of EL4a in WT, whereas the Y110A mutation removes the stacking and results in dissociation of these two residues from Ala398 in both “x-ray ts3/paroxetine-ABC” structure and “ts3/paroxetine-ABC” simulations. The importance of this π-π stacking is illustrated by the fact that mutating Tyr107 to either Leu (Rannversson et al., 2015) or Cys (Henry et al., 2003) abolishes transport activity. Thus, the Y110A mutation appears to significantly disrupt the packing between EL4a and TM1e. Moreover, the sole addition of Y110A to the partially active ts2 construct (Table 1, see section 3.3 where we present the results of our own experiments to assess the functional properties of the hSERT variants), which harbors the I291A and T439S thermostabilizing mutations, to generate ts3, completely extinguishes activity (Coleman et al., 2016). The notion that distorted packing between EL4a and TM1e is responsible for the transport-deficient phenotype of ts3 is further supported by comparing the inward-open (PDB ID 3TT3) and outward-occluded/open (PDB IDs 2A65/3TT1) LeuT structures (Krishnamurthy and Gouaux, 2012; Yamashita et al., 2005). Specifically, coordinated rearrangements are observed between EL4a and TM1e, which are likely essential for the functionally-relevant conformational transitions.
Figure 2.
The significant conformational rearrangements in the “ts3/paroxetine-ABC” compared to the “WT/paroxetine-ABC” simulated condition. The analysis of the pairwise-distance differences among the subsegments (A) indicates EL4a moves away from, while TM6i gets closer to, other subsegments in WT compared to ts3. The equilibrated models from WT (B) and ts3 (C) simulations show that the packing between EL4a and TM1e is disrupted in ts3 compared to WT. Note the extracellular, middle, and intracellular subsegments are grouped together to better illustrate the conformational changes on either the extracellular or intracellular regions. The analysis of pairwise-distance differences among the Cα atoms of the S1 site residues (D) indicates significant changes near residues 341 and 343 in TM6m (darker orange and blue pixels). In particular, the shorter Phe341-Leu443 and the longer Val343-Ile172 distances in ts3, which are indicated by boxes in panel D, are illustrated in panels E and F. In panel F, these two pair-wise distances are indicated by the correspondingly colored arrows connecting the Cα atoms (in spheres) of these residues. In panels A and D, the distance difference matrices are calculated by subtracting the distances in “WT/paroxetine-ABC” from their corresponding ones in “ts3/paroxetine-ABC”, and the color is scaled from blue to orange corresponding to distance difference values (see the color ramp for each panel). Thus, the orange pixels indicate shorter distances and the blue pixels indicate longer distances in “ts3/paroxetine-ABC”.
In contrast to ts3, we found that in WT several hydrogen bonds (H-bonds) present in the WT LeuT structure (PDB ID 2A65) also exist on both the extracellular and intracellular sides of the S1 site. These include Tyr175-Glu493, Tyr176-Asp98, and Tyr350-Glu444 (Figure 3). The disruption of these interactions in the ts3 construct may originate from the T439S mutation in the S1 site.
Figure 3.
Conserved H-bond interactions form in the WT simulations. (A) A zoomed-in view of the S1 site shows the status of three conserved H-bond interactions (Tyr175-Glu493, Tyr176-Asp98, and Tyr350-Glu444) in each of the indicated conditions. The interactions that are persistently formed are indicated in dense dotted lines, whereas those intermittently formed are in loose dotted lines. (B) The distance distributions of the three indicated interactions. The curves are color coded as the colors of paroxetine molecules in panel A, the distances of these three pairs of residues in the crystal structures are indicated by dotted lines (black for ts3 and green for Thr439-ts2).
Thr439 is conserved among all SERTs. It is situated one helical turn above Glu444, and forms a H-bond with the backbone carbonyl of Gly435 in WT (Figure S1A). Such an intrahelical H-bond is capable of modulating the backbone conformation of an α-helix (Gray and Matthews, 1984; Shi et al., 2002). By contrast, this critical H-bond is absent in both the “x-ray ts3/paroxetine-ABC” structure and “ts3/paroxetine-ABC” simulations (Figure S1), where the sidechain of Ser439 adopts the trans χ1 rotamer. Consequently, the side-chain −OH of Ser439 instead forms a H-bond with the backbone carbonyl of Asn177 (Figure S1). Note Asn177 is immediately adjacent to Tyr175 and Tyr176. Thus, the altered H-bond configuration near residue position 439 by the Thr-to-Ser mutation may disrupt those of Tyr175-Glu493 and Tyr176-Asp98 on the extracellular side and Tyr350-Glu444 on the intracellular side of S1, thereby impairing the catalytic activity of ts2 (Table 1, see section 3.3). Such changes propagate to affect the overall shape of the S1 site, and we observe significant differences in the pair-wise residue distances in this site, specifically of residues in TM3 (Ala169, Ile172, and Tyr175) as well as those in the unwound region of TM6 at the bottom of the binding site (Phe341 and Val343) (Figure 2D). On the intracellular side, the absence of the Tyr350-Glu444 interaction is associated with a downward movement of TM6i in ts3 (see Figures 2A and 3A).
Na2 is coordinated by residues in TMs 1 and 8 in NSS members (Coleman et al., 2016; Malinauskaite et al., 2014; Penmatsa et al., 2013; Yamashita et al., 2005). In hSERT, these residues include the backbone carbonyl oxygens of Gly94 (TM1m), Val97 (TM1m), and Leu434 (TM8m); the side chain carboxylate of Asp437 (TM8m); and the side chain −OH from Ser438 (TM8m). Mutating either Asp437 or Ser438 either decreases or ablates transport depending on the substituting residue (Felts et al., 2014). In a recent study of MhsT, a bacterial homolog of SERT, we found that the interaction of Asn176 (TM5m) to Ser323, a Na2 coordinating residue that corresponds to Ser438 of SERT, is associated with the dissociation of bound Na+ from the Na2 site (Stolzenberg et al., 2017). We proposed that Thr284 in TM5m of SERT may play a similar role as Asn176 of MhsT (Shi and Weinstein, 2010; Stolzenberg et al., 2017).
By comparing the equilibrated models from the ts3 and WT simulations, we found that the side chains of Thr284 in TM5m and Asp437 of TM8m, both of which are associated with the Na2 binding site, adopt different conformations. In particular, in the ts3 simulations in which we added the missing Na+ in the Na2 site of “x-ray ts3/paroxetine-ABC”, the χ1 rotamer of Thr284 is in gauche+ and its −OH faces the Na2 site as in “x-ray ts3/paroxetine-ABC” and forms a H-bond with Asp437 (Figure 4A, B, and G). By contrast, in the WT simulations, this rotamer is in gauche- and the −OH rotates away from the Na2 site (Figure 4F and G). Interestingly, in the ts3 simulations, the distance between Na1 and Na2 increases – while Na1 remains stably bound in this condition, such an increased distance indicates that Na2 is slightly displaced from the original site and is not as tightly bound as in WT (Figure 4H).
Figure 4.
The Thr284-Asp437 interaction is affected by both thermostabilizing mutations and the presence of Na2. Comparing the structures and simulations with paroxetine in the ABC pose, the side chain −OH of Thr284 in TM5m faces the Na2 site in the ts3 and Thr439-ts2 structures (A, C), and forms an H-bond with Asp437 in TM8m in the ts3 simulations even in the presence of the bound Na2 (B). This −OH rotates away in the Thr439-ts2 (D) and WT (F) simulations in the presence of Na2, but not in the no-Na2 condition of Thr439-ts2 (E). Note that the T439S mutation (not shown) is two residues away from Asp437, while the impact of I291A may propagate from the conserved proline-kink in the TM5 (Pro288) to Thr284. (G) The Thr284 χ1 rotamer frequency distributions in the simulations of the ts3 (black curve), Thr439-ts2 (green solid and dashed curves, for the Na2-bound and -unbound conditions, respectively), and WT (cyan curves) conditions. Its values for the ts3 and Thr439-ts2 structures are indicated by the black and green dotted lines, perpendicular to the x-axis, respectively. (H) The distances between the side chain oxygens of Thr284 and Asp437 are plotted against the distances between Na1 and Na2 for the ts3 (black), the Na2-bound Thr439-ts2 (green), and WT (cyan) conditions.
3.2. The presence of Thr439 restores some conserved interactions and Na2 binding
Such a disruption of Na2 binding in “ts3/paroxetine-ABC” may result from either or both the I291A and T439S mutations. Residue 439 is just two residues away from Asp437, and the divergent χ1 rotamer preferences of Thr and Ser, i.e., gauche- of Thr439 in WT versus trans of Ser439 in ts3, which result in different H-bonding networks in this local region (Figure S1), are expected to alter the conformation of Asp437. Indeed, our analysis of the backbone dihedral angles showed that the presence of the H-bond between Thr439 and Gly435 in WT but not in ts3 (Figure S1) significantly affected the local backbone conformation including that of Asp437 (Table S1). Although residue 291 on the periphery of the transmembrane domain interacts directly with lipid molecules in our WT simulations, as residues 291 and 284 are located on either side of Pro288, the impact of I291A might propagate to residue 284 by reconfiguring around the highly conserved proline-kink in TM5 (Shi and Weinstein, 2010) and affect the interaction network associated with the Na2 binding.
A second crystal structure of hSERT in complex with paroxetine was recently published (PDB ID 6AWN) (Coleman and Gouaux, 2018) but at much lower resolution (3.62 Å). It harbors two of the three thermostabilizing mutations in ts3 - Y110A in TM1e and I291A in TM5e, whereas the S1 residue 439 is reverted to the original WT threonine (we refer to this construct as “Thr439-ts2”, Table 1). Note that this variant is also incapable of transport since it still possesses the Y110A mutation. To specifically evaluate the impact of the T439S mutation, we conducted MD simulations of “Thr439-ts2/paroxetine-ABC” (Table 2).
Curiously, the χ1 rotamer of Thr439 in the Thr439-ts2 crystal structure (PDB ID 6AWN) is in the trans orientation, identical to that of Ser439 in the ts3 structure (PDB ID 5I6X), even though the electron density for the Thr439 side chain is ambiguous (Figure S1C and D). However, in our WT and Thr439-ts2 simulations, this rotamer adopts the gauche- orientation, establishing an H-bond with the backbone carbonyl of Gly435 (Figure S1A and B). Note that in a helical context Thr would be in a high-energy state to have a trans χ1 rotamer (Shi et al., 2002). Consequently, in the Thr439-ts2 simulations, we found that the Tyr175-Glu493 and Tyr176-Asp98 interactions (see section 3.1), which are distorted in ts3, are restored as in WT (Figure 3).
To investigate the impact of T439S on Na2 binding, we comparatively simulated the “Thr439-ts2/paroxetine-ABC” complex in both the presence and absence of Na2 conditions. Interestingly, the Thr284-Asp437 interaction does not form in the presence of Na2 (akin to WT, Figure 4D), whereas it persistently forms in the absence of Na2 (akin to ts3, Figure 4E). In contrast to the ts3 condition simulated with Na2, we do not observe any weakening of Na2 binding in the Thr439-ts2 condition simulated with Na2, while the Thr284 χ1 rotamer in the latter condition is partially restored to the WT gauche- orientation (Figure 4G and H). Taken together, we conclude that Na2 binding in the ts3 construct is likely weakened, and that the T439S mutation may play a significant role in this effect, with a potentially minor contribution from the I291A mutation.
3.3. Functional re-analysis of the thermostabilized hSERT constructs
Our computational findings strongly suggest that the T439S mutation in the S1 site, which is present in both the ts2 and ts3 constructs, may alter paroxetine binding affinity. However, because ts2 and ts3 were reported to have paroxetine binding affinities virtually identical to that of WT (Coleman et al., 2016), we sought to re-analyze the functional parameters of the thermostabilized constructs for paroxetine binding as well as 5-HT transport. The results of these experiments are shown in Figure 5. Note that since ts3 and Thr439-ts2, both of which still harbor the Y110A mutation, are incapable of transport (Coleman and Gouaux, 2018; Coleman et al., 2016), we did not include them in our flux assays.
Figure 5.

Functional characterization of WT and thermostabilized hSERT constructs. (A) Steady-state kinetics for [3H]5-HT transport for WT (cyan) and ts2 (gray). (B) Saturation radioligand binding of [3H]paroxetine to WT (cyan), ts2 (gray), ts3 (black), and T439-ts2 (green). For the data in panel B, specific binding is expressed as the percent maximal binding for the indicated hSERT variant. The graphs are representative of the indicated experiments, each of which was performed in triplicate at least four separate times. Each data point (•) is the average of three replicates with the error bars denoting the standard error of the mean (SEM). Data were fit to either the Michaelis-Menten equation (panel A) or to a single-site rectangular hyperbola (panel B), as implemented in GraphPad Prism 7.
In contrast to previously-published data (Coleman and Gouaux, 2018; Coleman et al., 2016) we found that the ts2 and ts3 constructs possess lower affinities for paroxetine than does WT by approximately 7- and 4-fold, respectively (Figure 5B and Table 1). These lower affinities combined with our finding that the Thr439-ts2 variant binds to paroxetine almost as well as WT (Figure 5B, Table 1) suggest that the S1 site in ts2 and ts3 is indeed disrupted by the T439S mutation.
Further evidence for this disturbance is provided by the impaired transport activity exhibited by ts2. Specifically, its maximum velocity (Vmax) is approximately half that of WT (1.12 ± 0.12 versus 2.29 ± 0.28 pmol/min/105 cells; Figure 5A and Table 1), even though its surface expression level is similar (Figure S2). The fact that its corresponding Michaelis constant (Km) is about 6-fold higher than that of WT (2.41 ± 0.23 vs. 0.42 ± 0.03 µM) (Figure 5A, Table 1) reflects an approximately 12-fold lower catalytic efficiency.
3.4. The binding of paroxetine in hSERT is likely to have ambiguous poses
Determining the structure of a protein-ligand complex is extremely valuable, not only for understanding its relationship to biological activity but also for designing better therapeutics. Nevertheless, these inferences are exceedingly sensitive to X-ray data quality. At moderate-to-low resolution (worse than 3 Å), “fitting” a small molecule into the electron density can be subjective. Although the density may be adequate to identify the ligand binding site, it is not always sufficient to unambiguously orient the ligand in that site (Nicholls, 2017). The problem can be exacerbated when the crystallized construct does not recapitulate the function of the protein target, as is the case for ts3 and Thr439-ts2 (Figure 5B and Table 1).
Because the crystal structures of the hSERT-paroxetine complexes (PDB IDs 5I6X and 6AWN) were solved at resolutions of 3.14 Å and 3.62 Å, respectively, we evaluated the feasibility and stability of the ACB pose proposed by Davis et al. in the equilibrated “WT/paroxetine-ABC” model. Specifically, we replaced paroxetine in an equilibrated “WT/paroxetine-ABC” model with one in the ACB pose and carried out similar MD simulations (Table 2).
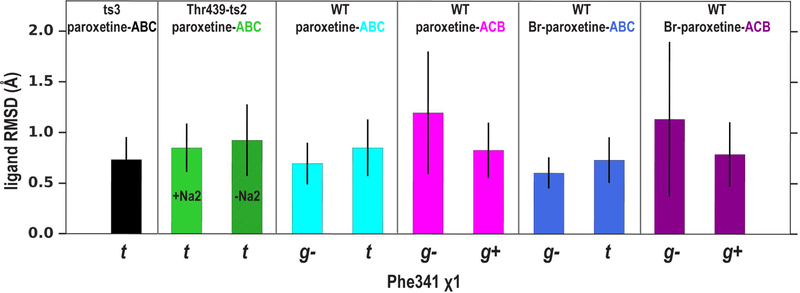
To the extent of our simulations, we found that paroxetine in the “WT/paroxetine-ACB” simulations reached a similar degree of stability compared to that in the “WT/paroxetine-ABC” simulations, as assessed from the ligand pair-wise root-mean-square deviation (RMSD) calculations (see Methods, Figure 6). Furthermore, in the “WT/paroxetine-ACB” simulations, the interactions near the S1 site, Tyr175-Glu493, Y176-Asp98, and Tyr350-Glu444, which were absent in “ts3/paroxetine-ABC”, persistently formed (Figure 3). Thus, the ACB pose can be easily adapted in the “WT/paroxetine-ABC” model without significantly disrupting the binding site and transporter conformation.
Figure 6.
The ABC and ACB poses reach comparable degrees of stability. Depending on the Phe341 χ1 rotamer, the paroxetine or Br-paroxetine poses in all the simulated conditions can reach similar degrees of stability as that of “WT/paroxetine-ABC”, measured by ligand pair-wise RMSDs (see Methods). The averages and standard deviations of the 10 bootstrapped samples (see section 2.2) were calculated and plotted.
Recently, a series of experiments used a genetically encoded photo crosslinking amino acid, specifically p-azido-L-phenylalanine (azF), to pinpoint the precise binding site of paroxetine (along with escitalopram) (Rannversson et al., 2017). While escitalopram crosslinked to two residues, Y95azF and F341azF, paroxetine crosslinked to only one, V501azF. Although the authors cautiously interpreted this result as support for the ABC pose, that conclusion would have to assume that V501azF had crosslinked to paroxetine’s fluorophenyl moiety given that the distance between these two functional groups is only 3.1 Å. However, it is equally possible that the V501azF had crosslinked to paroxetine’s benzodioxol, which would support the ACB pose. Thus, these data do not unequivocally distinguish between the two poses.
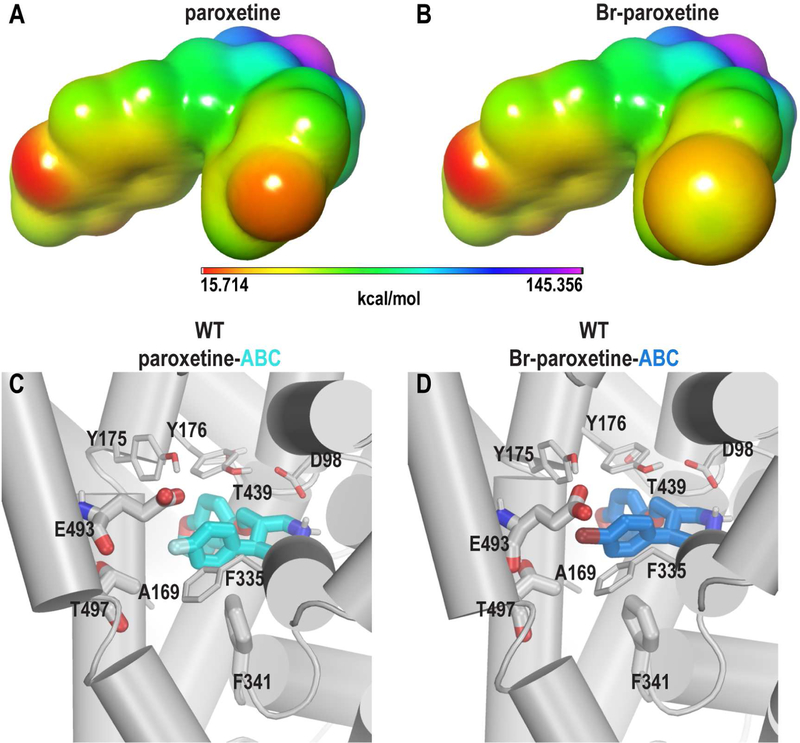
We reasoned that the ambiguity of paroxetine’s binding poses may be a consequence of this molecule’s symmetrical “V” shape as well as similar electronegative charge distributions at both ends of the “V” (Figure 7A). To subtly disturb this symmetry, we replaced the para-fluoro substituent of paroxetine with the sterically bulky and electron-rich but more polarizable bromo group, which affects both the shape and charge distribution of the molecule. In particular, the σ-hole of Br-substituent (Wilcken et al., 2013) results in a region of positive electrostatic potential (Figure 7B). Thus, Br-paroxetine was synthesized and experimentally characterized as a high affinity SERT inhibitor (Ki = 3.13 nM for inhibition of [3H]5-HT transport (Slack et al., manuscript in preparation)).
Figure 7.
Favorable interactions between the σ-hole of Br-paroxetine and residues in TM10. (A) and (B) display the results of quantum mechanical calculations for the electrostatic potential surfaces of paroxetine (A) and Br-paroxetine (B). Electrostatic potentials (kcal/mol) are superimposed onto a surface of constant electron density (0.001 e/bohr3) with the indicated colors. The most positive and negative potential regions are in deep-purple and red, respectively. Comparison of paroxetine (C) and Br-paroxetine (D) in their ABC poses shows that Br-paroxetine makes favorable interactions with the oxygen atoms of Glu493 and Thr497 in TM10 due to the presence of a σ-hole.
We investigated the impact of F- to Br- substitution on the binding poses in hSERT and built both “WT/Br-paroxetine-ABC” and “WT/Br-paroxetine-ACB” simulation systems (Table 2) based on the equilibrated “WT/paroxetine-ABC” and “WT/paroxetine-ACB” systems, respectively, and carried out extensive MD simulations using the OPLS3 force field that accounts for the σ-hole of halogens (see Figure S3 for the differences of atomic partial charges between paroxetine and Br-paroxetine) (Harder et al., 2016). When we calculated the ligand pair-wise RMSDs of both poses for Br-paroxetine, we found a very similar trend to paroxetine (Figure 6). Whereas our simulations reveal a similar binding pose ambiguity for Br-paroxetine as that for paroxetine, the σ-hole of the Br-substitution results in subtle but significant changes in its interactions with hSERT in the S1 site (see below).
3.5. Paroxetine’s extraordinarily high affinity may be associated with the dynamics of Phe341 in S1
Phe341 is located at the bottom of the S1 site, and the corresponding residues in LeuT and DAT have been found to play a critical role in the conformational transition of NSS members (Abramyan et al., 2017; LeVine and Weinstein, 2014; Singh et al., 2008; Wang et al., 2015a). In the current study, we observed that its χ1 rotamer spontaneously oscillates between trans and gauche- orientations in the “WT/paroxetine-ABC” but not in the “ts3/paroxetine-ABC” simulations (Figure 8A and B, Movie S1A, S1B, and S1C). Although such an oscillation appears to induce a slight movement of the bound paroxetine (Figure 8D and E), it does not significantly impact the overall conformation of the transmembrane domain (Figure 8G and H). Importantly, such dynamics in the “WT/paroxetine-ABC” is absent in the “ts3/paroxetine-ABC” simulations. When we calculated the ligand pair-wise RMSD of the ABC pose specifically in different χ1 rotamers of Phe341, we found that the ABC pose is similarly stable whether the rotamer assumes the trans or gauche- orientation in “WT/paroxetine-ABC” (Figure 6). In the “WT/paroxetine-ACB” simulations, we found that the Phe341 χ1 rotamer oscillates between the gauche- and gauche+ orientations and that this pose is also stable in either of these orientations (Figure 6). Notably, the conformational differences observed between the “WT/paroxetine-ABC” and “ts3/paroxetine-ABC” simulations are independent of whether the Phe341 χ1 in WT adopts the trans or gauche- rotamer (Figure S4). Thus, in addition to the altered S1 site (Figure 2D-F), the more dynamic conformation of Phe341 in WT results in a more favored entropy component in the binding free energy and may contribute to the more favored binding of paroxetine at WT than at ts3 (Figure 5B and Table 1).
Figure 8.
The side chain of Phe341 is more dynamic in “WT/paroxetine-ABC”. In “WT/paroxetine-ABC”, the Phe341 χ1 rotamer oscillates between trans and gauche- (B), whereas in “ts3/paroxetine-ABC” (A) and “WT/Br-paroxetine-ABC” (C), it does not. Such oscillations affect the RMSD of the ligand (D–F), but not that of the transmembrane domain (G–I). RMSDs were calculated against the last frame in each trajectory, based on the ligand heavy atoms (D–F) or the Cα atoms of TM 1–10 segments (G–I).
To further characterize the dynamics of Phe341 in different conditions, we used metadynamics free energy calculations (Laio and Parrinello, 2002) to compare the free energy landscape of the χ1 rotamer of Phe341 in the “ts3/paroxetine-ABC” and “WT/paroxetine-ABC” complexes. In metadynamics calculations, high energy barriers are overcome by adding small repulsive potentials to the underlying free energy minima to facilitate efficient exploration of the entire conformational space. The results of our metadynamics calculations revealed that the χ1 rotamer of Phe341 strongly prefers trans over gauche- in ts3 (the difference is 4.7 kcal/mol) as opposed to WT, where the energetic difference between the two rotamers is much smaller (2.1 kcal/mol) (Figure S5). Such energetic differences are consistent with the aforementioned observations in the conventional MD simulations in which multiple transitions occurred between these two rotamers in “WT/paroxetine-ABC” but none did in “ts3/paroxetine-ABC” (Figure 8A and B). Furthermore, in our ligand pair-wise RMSD calculations, the ABC pose in WT SERT is slightly more stable when the χ1 rotamer of Phe341 is in gauche- rather than trans, consistent with the moderate preference of gauche- in the metadynamics results (Figures 6 and S5).
Interestingly, by analyzing the resulting MD trajectories of “WT/Br-paroxetine-ABC” and “WT/Br-paroxetine-ACB” simulations, we found that, in both the ABC and ACB poses, Br-paroxetine significantly reduced the dynamics of Phe341 as well, such that Phe341 essentially remained in its starting χ1 rotamer throughout the entire simulations in all trajectories (Figure 8C, F, I, Movie S1A, S1B, and S1C). To quantify the energy barriers of the Phe341 χ1 rotamer changes in the presence of Br-paroxetine versus paroxetine, we carried out metadynamics calculations as well. We found that the height of the energy barrier for the transition from the trans to gauche- rotamer is 6.3 kcal/mol in the presence of Br-paroxetine, but is 4.5 kcal/mol in the presence of paroxetine, thereby making the conversion between gauche- and trans rotamers more difficult in the presence of Br-paroxetine (Figure S5).
To investigate how the F- to Br-substitution affects the ligand binding energy, we used the free energy perturbation method implemented in the Schrodinger suite (FEP+) (Wang et al., 2015b), which predicts the difference in binding energy of two ligands (∆∆G) rather than the difference in the absolute binding energy (∆G). In FEP+ calculations, we found that for both the ABC and ACB poses, the alchemical transformation from F- to Br-is energetically favorable, with ∆∆G values of −1.63±0.39 and −1.08±0.57 kcal/mol, respectively. These favorable ∆∆G changes are likely due to favorable enthalpic interactions between the Br-’s σ-hole (Figure 7B) and negatively charged oxygens of the nearby residues. In particular, we found that Br- makes slightly stronger interactions than does F- with the oxygen atoms of Glu493 and Thr497 in the ABC pose (Figures 7 and S6A, Movie S1A, S1B, and S1C), and with the oxygen atoms of Ala169, Thr439, and Gly442 in the ACB pose (Figure S6B).
These FEP+ calculations at the nanosecond timescale, however, cannot account for the disparate dynamics of paroxetine versus Br-paroxetine binding observed in the regular long MD simulations described above – note that the Phe341 side chain rotamer changes occurred on a microsecond timescale (Figure 8). Thus, comparing the Br-paroxetine binding to that of paroxetine, the combined effect of favored enthalpy changes with unfavored entropy changes, causes Br-paroxetine to bind to hSERT with slightly reduced binding affinity relative to paroxetine (Ki = 0.32 ± 0.03 nM vs. 0.07 ± 0.01 nM respectively, as assessed by [3H]paroxetine competition binding assays; Slack et al., manuscript in preparation).
4. Discussion
The hSERT crystal structures are the first of any mammalian NSS member to be solved. Compared to those of its prokaryotic counterparts, these structures should provide improved starting points to answer more sophisticated mechanistic queries. These questions may involve divergent regions of the protein for which the bacterial NSS structures prove to be less reliable templates, or the regions that are completely absent from the bacterial homologs. Furthermore, the molecular details of the ligand binding site revealed in the hSERT structures should be superior relative to those derived from homology models in drug discovery efforts. Nevertheless, this superiority also rests on any seemingly subtle yet still consequential disparities in the ligand binding pocket as well as other functionally relevant structural elements between the WT and thermostabilized variants. As all available SERT crystal structures are derived from thermostabilized, transport-deficient constructs, these subtle but important differences must be characterized, especially for a clinically significant symporter like SERT. In this study, our extensive and comparative MD simulations unmask the precise perturbations in the ligand binding pocket rendered by these mutations. They also reveal vital features in the interaction network of the transporter from the extracellular to intracellular side, the disruption of which would preclude proper protein function, as illustrated by the diminished catalytic activity for ts2 and the complete absence thereof for ts3. We have previously demonstrated that for the bacterial homologs of SERT, such transmembrane interaction networks are crucial for propagating the allosteric impact of substrate and ions in modulating the requisite conformational transitions in the transport cycle (Stolzenberg et al., 2017; Stolzenberg et al., 2016; Stolzenberg et al., 2015).
The “ts3/paroxetine-ABC” simulations were used as a control to evaluate the impact of immersing the complex in an explicit lipid bilayer on the hSERT structure. The consistency between the “x-ray ts3/paroxetine-ABC” structure and the equilibrated model from the “ts3/paroxetine-ABC” simulations (Figures 3, 4, 8 and S1), indicate that the observed conformational differences between the “ts3/paroxetine-ABC” and “WT/paroxetine-ABC” complexes are unlikely due to the environment or technical details, e.g. force field, in our simulations. Instead, the comparison of the equilibrated models of these two complexes more likely reveals that the mutations in the ts3 construct induce significant structural disparities in WT hSERT.
The side chain oscillation of Phe341 in the S1 site in the presence of paroxetine suggests that this SSRI has a nontrivial entropy component in its binding free energy at hSERT, which may also be reflected by its potential to bind in either the ABC or ACB pose. Thus, by integrating the results from long MD simulations, metadynamics simulations, FEP+, and ligand pair-wise RMSD calculations, we propose that this favored entropic component is related to paroxetine’s extraordinarily high affinity binding in hSERT. Indeed, substitution of paroxetine’s F- with Br-, despite improving enthalpy, reduces Phe341 dynamics and does not enhance its binding affinity at hSERT. This result is consistent with the finding from a previous thermodynamic analysis of ~100 protein-ligand complexes (Reynolds and Holloway, 2011). Specifically, this large-scale investigation revealed that enthalpy changes are negatively correlated with -T∆S in the binding free energy, i.e. ligands with favorable binding enthalpies are likely to possess unfavorable binding entropies (Reynolds and Holloway, 2011). Nevertheless, the stabilizing effect of Br-paroxetine on the S1 site suggests that it may be a suitable tool with which to crystallize a fully transport-competent hSERT.
5. Conclusions
Taken together, our resulting WT hSERT models and the unveiled dynamics in the S1 site establish the framework for future mechanistic studies and provide key insights for the rational design of high-affinity SERT inhibitors.
Supplementary Material
Highlights.
Important structural elements are perturbed by thermostabilizing mutations of SERT.
The symmetry of paroxetine molecule may result in ambiguous binding poses in SERT.
Paroxetine’s high affinity may be associated with the dynamics in the binding site.
Acknowledgements
Support for this research was provided by the National Institute on Drug Abuse–Intramural Research Program, Z1A DA000610-02 (A.H.N), and Z1A DA000606-03 (L.S.); the Goodman-Gilman Yale Scholar Award (S.K.S.); the Alfred P. Sloan Foundation (S.K.S.); The Brain and Behavior Research Foundation (S.K.S.); and the NIH/National Institute of Mental Health Grants R00MH083050 (S.K.S.) and R01MH100688 (S.K.S.) We thank Lingle Wang and Jeremie Vendome for validating our FEP+ results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramyan AM, Stolzenberg S, Li Z, Loland CJ, Noe F, Shi L, 2017. The Isomeric Preference of an Atypical Dopamine Transporter Inhibitor Contributes to Its Selection of the Transporter Conformation. ACS Chem Neurosci 8, 1735–1746. [DOI] [PubMed] [Google Scholar]
- Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J, 1981. Interaction Models for Water in Relation to Protein Hydration. In: Pullman B, (Ed), Intermolecular Forces: Proceedings of the Fourteenth Jerusalem Symposium on Quantum Chemistry and Biochemistry Held in Jerusalem, Israel, April 13–16, 1981 Springer Netherlands, Dordrecht, pp. 331–342. [Google Scholar]
- Berger M, Gray JA, Roth BL, 2009. The expanded biology of serotonin. Annu Rev Med 60, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Chue P, Guillon Y, 2001. Paroxetine: a review. CNS Drug Rev 7, 25–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers KJ, Chow E, Xu H, Dror RO, Eastwood MP, Gregersen BA, Klepeis JL, Kolossvary I, Moraes MA, Sacerdoti FD, Salmon JK, Shan Y, Shaw DE, 2006. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. ACM/IEEE SC 2006 Conference (SC’06), p. 43.
- Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF, 2017. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 342, 212–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay Y, Leger L, 2010. Brain serotonergic circuitries. Dialogues Clin Neurosci 12, 471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JA, Gouaux E, 2018. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nat Struct Mol Biol 25, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JA, Green EM, Gouaux E, 2016. X-ray structures and mechanism of the human serotonin transporter. Nature 532, 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Nagarajan A, Forrest LR, Singh SK, 2016. Mechanism of Paroxetine (Paxil) Inhibition of the Serotonin Transporter. Sci Rep 6, 23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Gould GG, 2011. Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacol Ther 131, 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller SE, Zhang Y, Pastor RW, Brooks BR, 1995. Constant pressure molecular dynamics simulation: The Langevin piston method. J Chem Phys 103, 4613–4621. [Google Scholar]
- Felts B, Pramod AB, Sandtner W, Burbach N, Bulling S, Sitte HH, Henry LK, 2014. The two Na+ sites in the human serotonin transporter play distinct roles in the ion coupling and electrogenicity of transport. J Biol Chem 289, 1825–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH, Rudnick G, 2008. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci U S A 105, 10338–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TM, Matthews BW, 1984. Intrahelical hydrogen bonding of serine, threonine and cysteine residues within alpha-helices and its relevance to membrane-bound proteins. J Mol Biol 175, 75–81. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD, 2007. The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol 47, 401–441. [DOI] [PubMed] [Google Scholar]
- Harder E, Damm W, Maple J, Wu C, Reboul M, Xiang JY, Wang L, Lupyan D, Dahlgren MK, Knight JL, Kaus JW, Cerutti DS, Krilov G, Jorgensen WL, Abel R, Friesner RA, 2016. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J Chem Theory Comput 12, 281–296. [DOI] [PubMed] [Google Scholar]
- Henry LK, Adkins EM, Han Q, Blakely RD, 2003. Serotonin and cocaine-sensitive inactivation of human serotonin transporters by methanethiosulfonates targeted to transmembrane domain I. J Biol Chem 278, 37052–37063. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC, 1992. Structure and function of the brain serotonin system. Physiol Rev 72, 165–229. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U, 2008. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J Biol Chem 283, 17691–17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Gouaux E, 2012. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laio A, Parrinello M, 2002. Escaping free-energy minima. Proc Natl Acad Sci U S A 99, 12562–12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine MV, Weinstein H, 2014. NbIT--a new information theory-based analysis of allosteric mechanisms reveals residues that underlie function in the leucine transporter LeuT. PLoS Comput Biol 10, e1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomize MA, Lomize AL, Pogozheva ID, Mosberg HI, 2006. OPM: orientations of proteins in membranes database. Bioinformatics 22, 623–625. [DOI] [PubMed] [Google Scholar]
- Malinauskaite L, Quick M, Reinhard L, Lyons JA, Yano H, Javitch JA, Nissen P, 2014. A mechanism for intracellular release of Na+ by neurotransmitter/sodium symporters. Nat Struct Mol Biol 21, 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGibbon RT, Beauchamp KA, Harrigan MP, Klein C, Swails JM, Hernandez CX, Schwantes CR, Wang LP, Lane TJ, Pande VS, 2015. MDTraj: A Modern Open Library for the Analysis of Molecular Dynamics Trajectories. Biophys J 109, 1528–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michino M, Boateng CA, Donthamsetti P, Yano H, Bakare OM, Bonifazi A, Ellenberger MP, Keck TM, Kumar V, Zhu C, Verma R, Deschamps JR, Javitch JA, Newman AH, Shi L, 2017. Toward Understanding the Structural Basis of Partial Agonism at the Dopamine D3 Receptor. J Med Chem 60, 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencetti S, Demontis GC, Mazzoni MR, Betti L, Banti I, Rossello A, Lapucci A, 2007. 3-[(Aryl)(4-fluorobenzyloxy)methyl]piperidine derivatives: high-affinity ligands for the serotonin transporter. J Pharm Pharmacol 59, 1439–1445. [DOI] [PubMed] [Google Scholar]
- Nencetti S, Mazzoni MR, Ortore G, Lapucci A, Giuntini J, Orlandini E, Banti I, Nuti E, Lucacchini A, Giannaccini G, Rossello A, 2011. Synthesis, molecular docking and binding studies of selective serotonin transporter inhibitors. Eur J Med Chem 46, 825–834. [DOI] [PubMed] [Google Scholar]
- Nicholls RA, 2017. Ligand fitting with CCP4. Acta Crystallogr D Struct Biol 73, 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD, 2008. Serotonin receptors. Chem Rev 108, 1614–1641. [DOI] [PubMed] [Google Scholar]
- Penmatsa A, Wang KH, Gouaux E, 2013. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramod AB, Foster J, Carvelli L, Henry LK, 2013. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med 34, 197–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannversson H, Andersen J, Bang-Andersen B, Stromgaard K, 2017. Mapping the Binding Site for Escitalopram and Paroxetine in the Human Serotonin Transporter Using Genetically Encoded Photo-Cross-Linkers. ACS Chem Biol 12, 2558–2562. [DOI] [PubMed] [Google Scholar]
- Rannversson H, Wilson P, Kristensen KB, Sinning S, Kristensen AS, Stromgaard K, Andersen J, 2015. Importance of the Extracellular Loop 4 in the Human Serotonin Transporter for Inhibitor Binding and Substrate Translocation. J Biol Chem 290, 14582–14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CH, Holloway MK, 2011. Thermodynamics of ligand binding and efficiency. ACS Med Chem Lett 2, 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman W, Day T, Jacobson MP, Friesner RA, Farid R, 2006. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem 49, 534–553. [DOI] [PubMed] [Google Scholar]
- Shi L, Liapakis G, Xu R, Guarnieri F, Ballesteros JA, Javitch JA, 2002. Beta2 adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J Biol Chem 277, 40989–40996. [DOI] [PubMed] [Google Scholar]
- Shi L, Weinstein H, 2010. Conformational rearrangements to the intracellular open states of the LeuT and ApcT transporters are modulated by common mechanisms. Biophys J 99, L103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Piscitelli CL, Yamashita A, Gouaux E, 2008. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen L, Andersen J, Thomsen M, Hansen SM, Zhao X, Sandelin A, Stromgaard K, Kristensen AS, 2012. Interaction of antidepressants with the serotonin and norepinephrine transporters: mutational studies of the S1 substrate binding pocket. J Biol Chem 287, 43694–43707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg S, Li Z, Quick M, Malinauskaite L, Nissen P, Weinstein H, Javitch JA, Shi L, 2017. The role of transmembrane segment 5 (TM5) in Na2 release and the conformational transition of neurotransmitter:sodium symporters toward the inward-open state. J Biol Chem 292, 7372–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg S, Michino M, LeVine MV, Weinstein H, Shi L, 2016. Computational approaches to detect allosteric pathways in transmembrane molecular machines. Biochim Biophys Acta 1858, 1652–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg S, Quick M, Zhao C, Gotfryd K, Khelashvili G, Gether U, Loland CJ, Javitch JA, Noskov S, Weinstein H, Shi L, 2015. Mechanism of the Association between Na+ Binding and Conformations at the Intracellular Gate in Neurotransmitter:Sodium Symporters. J Biol Chem 290, 13992–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavoulari S, Forrest LR, Rudnick G, 2009. Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J Neurosci 29, 9635–9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Penmatsa A, Gouaux E, 2015a. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wu Y, Deng Y, Kim B, Pierce L, Krilov G, Lupyan D, Robinson S, Dahlgren MK, Greenwood J, Romero DL, Masse C, Knight JL, Steinbrecher T, Beuming T, Damm W, Harder E, Sherman W, Brewer M, Wester R, Murcko M, Frye L, Farid R, Lin T, Mobley DL, Jorgensen WL, Berne BJ, Friesner RA, Abel R, 2015b. Accurate and reliable prediction of relative ligand binding potency in prospective drug discovery by way of a modern free-energy calculation protocol and force field. J Am Chem Soc 137, 2695–2703. [DOI] [PubMed] [Google Scholar]
- Wilcken R, Zimmermann MO, Lange A, Joerger AC, Boeckler FM, 2013. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J Med Chem 56, 1363–1388. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E, 2005. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature 437, 215–223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.