Abstract
Research on oxytocin (OT) has revealed a substantial involvement of this neuropeptide in social cognition processes and attachment behavior.
The rationale of the present project was to decipher the differential role of OT in basic social cognition processes towards non-erotic attachment stimuli vs. reproduction-related stimuli in human subjects.
In a randomized double-blind repeated-measures cross-over design, N = 82 participants were investigated twice and received either intranasal OT or placebo at the first assessment followed by placebo or OT at second assessment. Participants were presented with standardized pictures of parent-child dyads, romantic couples engaging in non-erotic or explicit sexual activities, and non-social pictures while we assessed pupil dilation and eye focus on specific pre-defined areas of interest. Multilevel analyses suggest that during the initial presentation, OT increased pupil dilation towards all categories of stimuli and led the eye focus towards the eyes and body regions, followed by a strong decrease in pupil dilation and fixations at the second session. These carry-over effects indicate that hormonal treatment at an initial contact to social stimuli can determine how these stimuli are processed later. These results might have implications for OT as a treatment in interventions with repeated exposure to social material.
Keywords: Eye-Tracking, Social Bonding, Social Perception, Sexual Stimuli, Parent-Child Attachment, Intranasal Oxytocin
Introduction
Our social interactions are natural consequences of what we perceive in our everyday social life (Dijksterhuis and Bargh, 2001) and are determined by our previous experiences, attitudes and interests. Therefore, appropriate social perception is the predisposition for adequate social behavior and is often impaired in clinical conditions such as autism (Auyeung et al., 2015). While in rodents and other non-primate mammals, social perception is mostly dependent on their high sensitive olfactory sensory system, primates’ brains specialized during evolution on the visual sensory system. Comparing primate species, the number of neurons in the visual system is positively correlated with the social group in which the species typically lives. With humans living in the largest group sizes, this is a further indicator for the substantial role of the visual basis of social information processing in the human brain (Barton, 1998). When analyzing neural response patterns to visual stimuli, research distinguishes between bottom-up processes (driven by means of physical salience of stimuli) and top-down processes (driven by cognitive and emotional processes) (Itti and Koch, 2001). The latter have been shown to be modulated through the medial prefrontal cortex (mPFC), cingulate and amygdala (Gamer, Schmitz, Tittgemeyer, & Schilbach, 2013; Nummenmaa and Calder, 2009). More precisely, frontal top-down control is assumed to regulate more indirect sympathetic arousal states that translate into coordinated response initiation (Moresi et al., 2008; Zekveld, Heslenfeld, Johnsrude, Versfeld, & Kramer, 2014).
Neurobiological research on the neuropeptide oxytocin (OT) has already proven its substantial involvement in socio-emotional cognition processes and suggests that OT modulates the underlying amygdala-driven networks in particular (Eckstein et al., 2017; Preckel, Scheele, Eckstein, Maier, & Hurlemann, 2015). On the one hand, basic social cognition, such as memory for social contents and socio-emotional learning (Cardoso, Orlando, Brown, & Ellenbogen, 2014; Eckstein et al., 2015; Hurlemann et al., 2010; Unkelbach, Guastella, & Forgas, 2008) are modulated by OT. On the other hand, OT seems to be involved in more complex social bonding processes, such as empathy (Abu-Akel, Palgi, Klein, Decety, & Shamay-Tsoory, 2015) and couple interaction (B. Ditzen et al., 2009; Hurlemann and Scheele, 2016). The establishment of social bonds requires healthy social cognition processes. To then maintain a stable bond seems to depend on the interaction of OT and reward-related (i.e. dopamine-driven) processes in the brain (Bosch et al., 2016; Skuse and Gallagher, 2009). Substantial research on the time-dynamics and the neuroendocrine systems underlying the social bonding process has been done in rodents and non-human primates (Amadei et al., 2017; Cavanaugh, Mustoe, Taylor, & French, 2014; Johnson and Young, 2015). This research suggests an effect of OT on sexual approach behavior and pair bonding but also parental bonding, which is mediated through the interaction of OT, dopamine and serotonin in specific brain regions (e.g. the nucleus accumbens) (Johnson and Young, 2017; Numan and Young, 2016; Rilling and Young, 2014). In monogamous prairie voles, for example, OT promotes reproductive behavior which is followed by subsequent pair bonding (Borrow and Cameron, 2012). Oxytocin not only facilitates approach to a potential partner and pair bonding behaviors, but also plays an essential role in non-sexual social recognition (Ferguson et al., 2000; Oettl et al., 2016; Scheele et al., 2012) and infant bonding in sheep (Keverne and Kendrick, 1992).
In humans, research using OT nasal spray suggests attachment- and bonding-relevant influences of central nervous system OT in parent-child bonding and adult couple relationships. More specifically, OT enhanced social behavior to support parent-infant bonding (Weisman, Zagoory-Sharon, & Feldman, 2012) and improved positive behavior during an instructed couple interaction task (Ditzen et al., 2009, 2012). Intranasal OT stimulated the reward system when viewing the partner’s face (Scheele et al., 2013) and increased the self-reported intensity of sexual experiences during instructed sexual activities (Behnia et al., 2014; Burri, Heinrichs, Schedlowski, & Kruger, 2008; Veening, De Jong, Waldinger, Korte, & Olivier, 2015; Zhang et al., 2015). Genetic analyses have revealed associations of OT receptor gene polymorphisms with adult pair bonding behavior (Walum et al., 2012) and most recently added evidence to the environmental influences on epigenetic modifications of the OT system (Feldman, Monakhov, Pratt, & Ebstein, 2016).
Notably, while OT seems specifically involved in social as compared to non-social processes (cognition, emotional responses and behavior), possible differences depending on the type of social bond have not been investigated extensively. Also, species-specific characteristics in social functioning limit the translation of non-human data to humans with pair bonding in animals being almost always combined with sexual reproduction, while this is not necessarily true for humans. This precise analysis of different relationship types is particularly relevant when focusing on the neurobiological mechanisms of mental disorders with involvement of the RDoC domain “social processes” (NIMH, RDoC, https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml). Here, with the question of how impairment in early-life social relationships will affect adult relationships central, it would be important to learn whether the same neurobiological processes are involved in different types of bonding relationships. Neuroendocrine theories on attachment and bonding propose a refined interplay of central nervous neuropeptides (e.g. OT, vasopressin), neurotransmitters (serotonin, dopamine) and steroids (e.g. estradiol, cortisol, testosterone) in the regulation of attachment bonds throughout the life span (Carter, 1998; de Boer, van Buel, & Ter Horst, 2012; Feldman, et al., 2016), both for parental attachment and pair bonding. However, while parent-child attachment is motivated by caregiving and characterized by asymmetric communication, the adult pair bond is motivated by the need for symmetric communication, and emotional and physical intimacy. In the current state of human research, it remains unclear whether OT directly and equally modulates cognition and behavior with different kinds of social bonding or whether OT acts on specific types or social categories of social bonding in specific ways.
Another central aspect which is currently unknown is the relative involvement of OT in the initiation vs. the maintenance of an attachment bond. Animal research has extensively investigated the role of OT in the formation of bonding behavior (see Insel and Young, 2001; Keverne and Kendrick, 1992) with some research exploring its role in maintaining long-term bonds (Bosch, et al., 2016; Bosch and Young, 2017). In human research (including our own research), however, usually a first-time and single presentation of social vs. non-social material is investigated under either OT or placebo (as reviewed in Shahrestani, Kemp, & Guastella, 2013). This design suffers from limited validity with respect to everyday life social interactions and long-term attachment bonds or the daily contact with one and the same person. Data from clinical trials (Guastella and Hickie, 2016) suggest, that - as in animal studies - OT might have an immediate and initial effect when the stimulus material is new, however, this effect might fade with repeated stimulus presentation.
Furthermore, recent research on the modulatory role of hormones suggests long-term and carryover effects of these hormones on subsequent behavior (e.g. the influence of sex-hormone levels early in the menstrual cycle on both affect and cognition at a later cycle phase, see (Beate Ditzen, Palm-Fischbacher, Gossweiler, Stucky, & Ehlert, 2017; Rupp and Wallen, 2007; Wallen and Rupp, 2010). Such effects can be identified using a repeated-measures design.
For all kinds of social interaction, the beginning and key element is social cognition. One valid and powerful measure of cognition processes is eye tracking. It is an objective method capable of assessing unconscious information processing; neurologically also controlled by top-down processes. While fixation patterns provide a valid measure of attention to specific cues (Nummenmaa and Calder, 2009), the pupil diameter is a neurophysiological assessment of autonomous arousal, which can be interpreted as vigilance and interest (Bradley, Miccoli, Escrig, & Lang, 2008). In developmental studies and research on autism spectrum disorders, eye tracking towards social stimuli has been used as an evaluation of social cognition capacities (Auyeung, et al., 2015) with eye and face regions of social stimuli assumed to hold most of the informative content.
Regarding the role of OT, there appear to be some inconsistencies across studies. Some studies report increased eye-gaze towards socially relevant stimuli, for example, in healthy volunteers as well as autism spectrum patients, OT facilitates the focus towards the eyes and mouth in pictures of faces (Andari et al., 2010; Auyeung, et al., 2015; Guastella, Mitchell, & Dadds, 2008). Other studies report that this focus depends on the emotional valence (positive vs. negative emotional content) of the pictures (Domes, Steiner, Porges, & Heinrichs, 2013; Guastella, Carson, Dadds, Mitchell, & Cox, 2009) and again others failed to show a modulatory influence of OT on eye movements at all (Alvares, Chen, Balleine, Hickie, & Guastella, 2012; Domes et al., 2013; Hubble et al., 2017; Lischke et al., 2012). An OT-induced increase in arousal towards socio-emotional stimuli of both positive and negative valence has been shown with eye tracking experiments and pupil dilation as an outcome measure (Leknes et al., 2012; Prehn et al., 2013). Pupil dilation has been interpreted as intensity marker of top-down attentional control (Alnaes et al., 2014) and locus coeruleus-driven noradrenergic neuromodulation (Murphy, O’Connell, O’Sullivan, Robertson, & Balsters, 2014). It can, thereby, provide information on central nervous system (CNS) arousal regulation.
From a methodological point of view it is important to note that all eye tracking studies on OT mainly used facial stimuli of portrayed persons displaying emotions rather than naturalistic social interaction scenes (in contrast, c.f. Rosler, End, & Gamer, 2017).
The rationale of the present project was to decipher the role of OT in basic social cognition processes, specifically towards bonding and sexual stimuli.
We hypothesized that intranasal OT would increase arousal towards social pictures in comparison to non-social pictures, as evident in pupil diameter. Moreover, OT should lead to increased gaze towards pre-defined areas of interest (eye region, faces) in the social pictures, specifically. We aimed to analyze differential effects towards specific categories of social bonding (namely non-erotic attachment vs. erotic stimuli).
Finally, we hypothesized more pronounced effects during the first stimulus presentation, as compared to repeated presentation, indicative of possible carry-over effects as previous studies point to the influence of hormonal state during the initial contact with social material on subsequent processing (Wallen and Rupp, 2010)..
Therefore, the eye movements and pupil dilation markers of healthy participants were investigated in response to the repeated presentation of social as compared to non-social stimuli after administration of intranasal OT or placebo (PL). The goal of this randomized placebo-controlled, double-blind, within-subject crossover study was to examine the impact of OT on stimulus-specific fixations and pupil diameter in men and women. The repeated stimulus presentation allowed for the analysis of carry-over effects and habituation processes.
Methods
Participants
In a repeated-measures design, eighty-four healthy heterosexual men and women participated in the study at two time points. N = 2 participants had to be excluded because of extremely short viewing durations (2 SD below the mean, N = 1), or eye tracker artifacts (N = 1). Data of 82 female (N = 41) and male (N = 41) adults aged 21 to 42 years (M ± SD: 25.93 ± 5.06) entered the final analysis (c.f. figure 1b). N = 3 participants missed the 2nd assessment, but were included into the analyses with missing values. N = 29 reported being in a romantic relationship currently, none had children of their own.
Figure 1. a) Experimental Procedure and (b) Randomized Cross-Over Design.
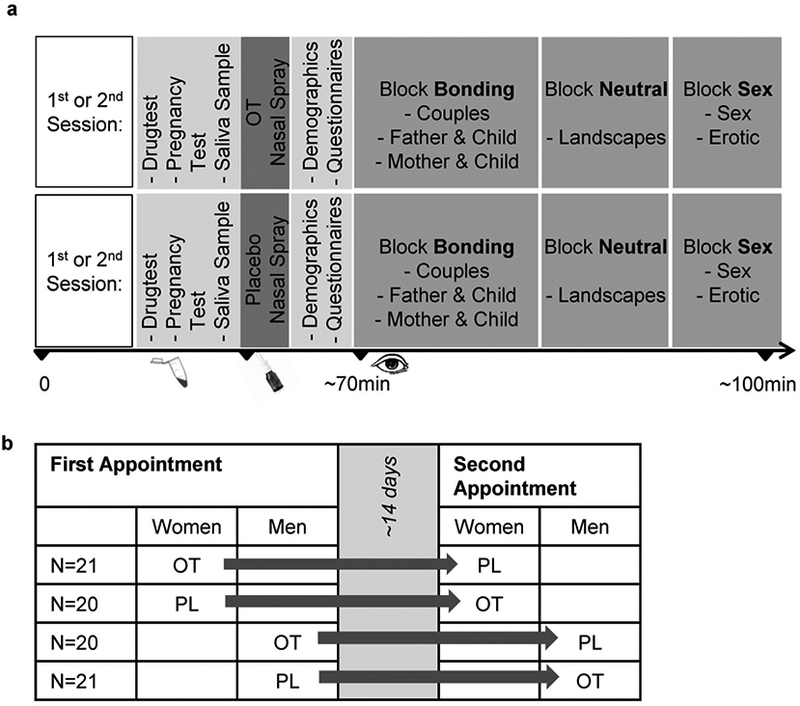
Participants performed two identically structured sessions approximately 14 days apart. They received either OT in the 1st session and PL in the 2nd session or vice versa in a double-blind manner before being presented blocks of bonding, neutral and sexual pictures.
Abbreviations: OT, Oxytocin, PL, Placebo
Prior to the experimental sessions a telephone screening was conducted to ensure none of the following exclusion criteria were met: Chronic physical or mental illness, regular smoking and alcohol consumption, medication intake (which included the intake of hormonal contraceptives), BMI above 25 or below 18, or drug abuse. For women, irregular menstrual cycle, hormonal contraception, pregnancy or breastfeeding served as exclusion criteria. All participants had normal or corrected-to-normal vision. All women were naturally cycling and scheduled balanced for cycle phase (50% were invited for the first session during the midluteal cycle phase and 50% during follicular phase according to self-report).
Participants were recruited via university advertisements and received either a financial incentive or study credits. All participants provided written informed consent before completing two experimental sessions separated by 10 to 18 days. Participants were randomly and double-blindly assigned to receiving OT in the first session and PL in the second session, or vice versa. In the first session OT was applied to 50% (N = 21 women, N = 20 men) of the participants and PL to the other 50% (N = 20 women, N = 21 men). The study was approved by the local and cantonal ethics committee as well as Swissmedic, was performed in compliance with the Declaration of Helsinki and monitored from the Clinical Trials Unit (CTU), Zurich University.
Procedure, Task, and Apparatus
The study was conducted in two identically structured experimental sessions on separate days, approximately 14 days apart, and took place between 3 and 9 pm (figure 1a). Prior to the investigation, participants received information about the experimental sessions along with instructions to abstain from smoking, caffeine, medication and alcohol or excessive sports on the days of the investigation. At the beginning of each appointment detailed information about the procedures and confidentiality was given and written consent was obtained. A multilevel drug screening (M-3–1-DT, Diagnostik Nord, Schwerin, Germany), and for women additionally, a pregnancy test was done (Evial, Inopharm, Muri/Bern, Switzerland). Participants gave a saliva sample for the assessment of gonadal hormones. Subsequently the participants self-administered either 24 international units (IU) of intranasal OT (Syntocinon Spray, Novartis, Basel, Switzerland) or PL (containing identical ingredients except for the peptide; Cantonal Pharmacy of Zurich) in three puffs per nostril. The trials then started 45 minutes after substance administration, with the actual eye tracking part of the investigation taking about 30 minutes. Procedures at first and second trial were identical, with exception of the order of the stimuli-presentation within the blocks.
Eye Tracking Session and Stimulus Material
The stimulus material consisted of 100 color photographs, made up of 69 social stimuli and 31 socially neutral stimuli (landscapes) that were selected after piloting. The social stimuli depicted interpersonal interactions: 16 parent-child interactions (8 mother-child/ 8 father-child), non-erotic adult attachment (17 images), adult erotic interaction (18 images), and adult sexual interaction (18 images). The following areas of interest (AOIs) were determined in all stimuli categories: face, eye and body of woman, man or child. For the erotic and sex stimuli breast and genital area were added as AOIs. Background served as non-social AOI (see example pictures in figure 2).
Figure 2. Example Stimuli and AOIs $$PARABREAKHERE$$a) + b) Category Attachment, c) Category Erotic, d) Category Mother-Child, d) Category Father-Child, f) Category Sex.

(GF = Woman’s Face, AF = Woman’s Eyes, KF = Woman’s Body, GM = Man’s Face, AM = Man’s Eyes, KM = Man’s Body, GK = Child’s Face, AK = Child’s Eyes)
The assessment took place in a quiet room with standardized light exposure. (See supplemental material for details.)
Statistical Analysis
As parameters of interest, we chose pupil diameter during stimulus presentation and fixations count towards specific areas of interest (AOIs), such as eyes, faces and body.
To reduce complexity of the data, we formed categories to integrate multiple pictures: Sexual and erotic pictures were statistically modeled as one sexual category. Adult and parent-child dyads were modeled as one bonding category.
In order to analyze the correspondence of repeated measures of pupil dilation and fixation count over the time course, we used multilevel linear modeling (also termed hierarchical or mixed modeling) with the statistical software R (Version 3.3.1 https://CRAN.R-project.org/) with the packages nlme (Pinheiro, Bates, DebRoy, & Sarkar, 2014) for multilevel modeling and MuMin for assessing pseudo-R-squared (Bartoń, 2013). We tested for models in which the slopes and intercepts for individual participants were set at random. AOI/category, treatment and time of assessment (first or second session) were chosen as predictors while including age and gender as covariates in the analyses. Background AOI and neutral category were used as reference categories (also called baseline category), i.e. all parameters were calculated in reference to the neutral category (landscapes, pupil dilation) or background AOI (fixation count).
Results
Pupil Dilation
Descriptive Statistics
Mean pupil dilations differed between categories, treatments and time of assessment (1st or 2nd session), with largest pupils to sex > erotic > attachment > parent-child > neutral, overall 1st session > 2nd session, and OT > PL (supplementary table S1 and figure 3). There was no difference between men and women in pupil dilation to the different categories.
Figure 3. Descriptive Values Pupil Dilation.
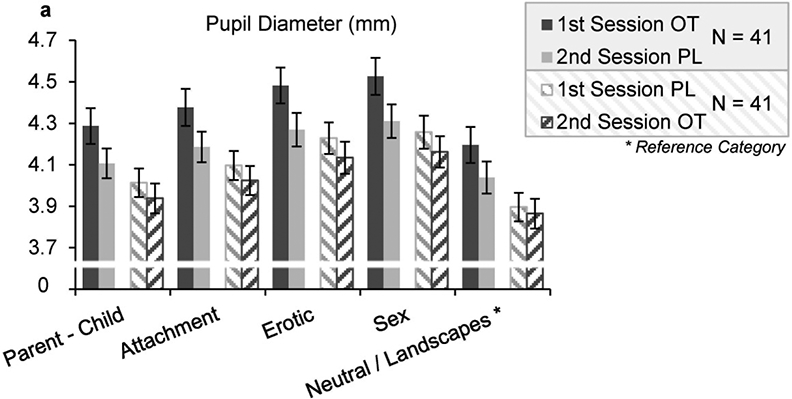
OT application at the 1st experimental session lead to enhanced pupil dilation in the first session; OT, Oxytocin, PL, Placebo; Error Bars indicate Standard Error of the Mean (SEM)
Statistical Modeling
Addressing the nested structure of the data, we performed multilevel modeling with repeated data entries nested within participants. In the intercept only model, pupil dilation was predicted by subject with an intra-class correlation (ICC) of r = .56.
We first examined whether category and time of assessment (session 1 or 2) were associated with pupil dilation (Model 1). This and all following models used the neutral condition as the reference category, allowed random intercepts and slopes per subject, and used stimulus duration, age and gender as covariates.
We then specified the following conditional model (Model 2): Pupil dilation was modeled as a function of treatment (OT or PL), time of assessment (session 1 or 2) and category (fixed effects). This model showed superior model fit (pseudo R2 = .74) and displayed significant fixed main effects for categories, treatment, stimulus duration and significant interactions of the categories * time of assessment, a significant interaction treatment * time of assessment (β = - 0.28, p = .03), yet no significant 3-way interactions or sex-differences (c.f. table 1 for all coefficients and figure 4 for the predicted values). The significant interaction indicates an increased pupil diameter at the first session under OT, followed by a stronger decline towards the second session for all categories. For PL in the first session, no such decline became evident.
Table 1.
Multilevel Models Predicting Pupil Dilation from Category, Time of Assessment and Treatment
| Model 1 Category * Time |
Model 2 Category * Time * Treatment |
|||
|---|---|---|---|---|
| Coefficients (± SD) | Coefficients (± SD) | |||
| Random effects | ||||
| Intercept | .43 | .41 | ||
| Assessment | .18 (−.17) | .17 (−.18) | ||
| Treatment | .08 (−.32) | |||
| Coefficients (± SEM) | t-value (df) | Coefficients (± SEM) | t-value (df) | |
| Fixed effects | ||||
| Intercept | 4.53 (.24) | 18.53 (15635) ** | 4.43 (.24) | 18.63 (15629)** |
| Category Bonding | .27 (.01) | 41.76 (15635)** | .29 (.01) | 32.27 (15629)** |
| Category Sexual | .45 (.01) | 68.28 (15635)** | .46 (.01) | 51.25 (15629)** |
| Treatment | .06 (.03) | 2.12 (15629)* | ||
| Assessment | −.04 (.02) | −2.36 (15635)* | .10 (.07) | 1.47 (15629) |
| Stimulus Duration | .00 (.00) | 23.66 (15635)** | .00 (00) | 23.62 (15629)** |
| Gender | −.03 (.09) | −.28 (82) | −.01 (.09) | −.06 (82) |
| Age | −.02 (.01) | −2.36 (82)* | −.02 (.01) | −2.18 (82)* |
| Category Bonding * Assessment | −.06 (.01) | −6.33 (15635)** | −.05 (.01) | −4.02 (15629)** |
| Category Sexual * Assessment | −.68(.01) | −7.74 (15635)** | −.07 (.01) | −5.85 (15629)** |
| Category Bonding * Treatment | −.04 (.01) | −3.22 (15629)** | ||
| Category Sexual * Treatment | −.03 (.01) | −2.57 (15629)** | ||
| Treatment * Assessment | −.28 (.13) | −2.17 (15629)* | ||
| Category Bonding * Treatment * Assessment | −.01 (.02) | −.70 (15629) | ||
| Category Sexual * Treatment * Assessment | .01 (.02) | 0.53 (15629) | ||
| Model fit AIC | 9,035 | 9,031 | ||
| Pseudo R-squared | .733 | .734 | ||
p < .05;
p < .01.
All estimates calculated by full maximum likelihood. Parameter estimates were calculated with category neutral serving as reference for the other categories; for assessment, 1st session serves as reference; for treatment, placebo serves as reference and for gender, female serves as reference.
Figure 4. Predicted Values Pupil Dilation.
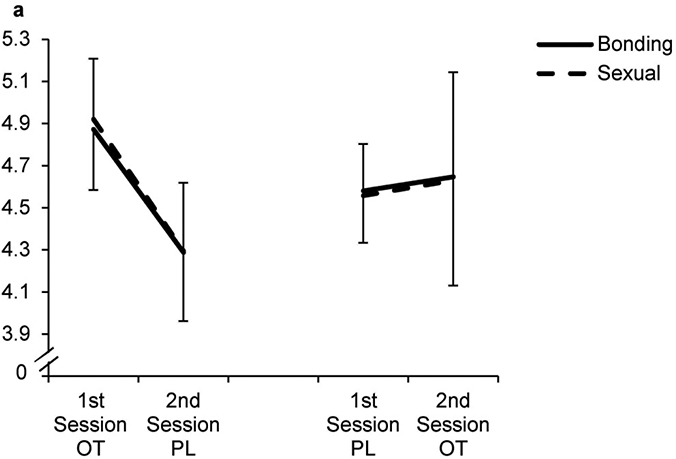
Multilevel analysis resulted in significant 2-way-interaction of treatment and time of assessment; neutral served as reference category; OT, Oxytocin, PL, Placebo; Error Bars indicate Standard Error of the Mean (SEM)
Fixation Count
Descriptive Statistics
Mean fixation time and fixation count were highly correlated (r = .86), so fixation count as a validated measure from eye tracking research was chosen as the outcome to interpret focus on the pre-defined AOIs.
Mean fixation count differed between AOIs and time of assessment (1st or 2nd session), with more fixations to child’s face > adult’s face > background > child’s eyes > genitals > adult’s body > adult’s eyes > child’s body, and overall 1st session > 2nd session (c.f. supplemental table S2 and figure 5). Men and women differed in their focus on the AOIs which suggests stronger interest in the AOIs of the displayed opposite sex person rather than the same-sex person.
Figure 5. Descriptive Values Fixation Count.
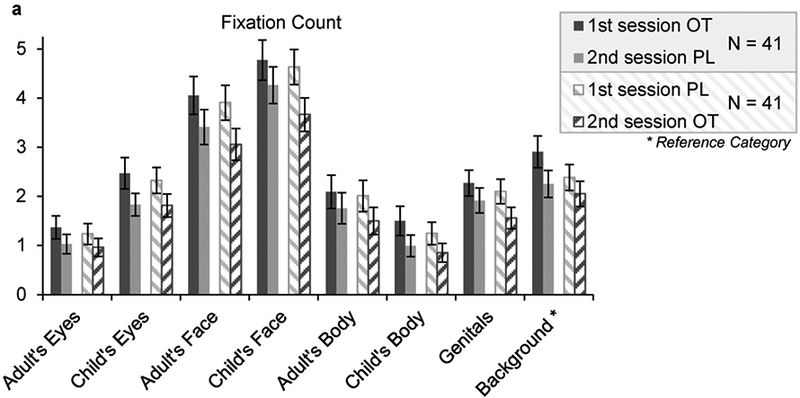
OT application at the 1st experimental session lead to enhanced fixation of (a) eye regions and (b) body regions OT, Oxytocin, PL, Placebo; Error Bars indicate Standard Error of the Mean (SEM)
Statistical Modeling
Addressing the nested structure of the data, we performed multilevel modeling with repeated data entries nested within participants. In the intercept-only model, fixation count was predicted by subject with an intra-class correlation (ICC) of r = .10.
We first examined whether AOI and time of assessment (session 1 or 2) were associated with fixation time (Model 1). This and all following models used the background AOI as reference category, i.e. all AOIs were contrasted to the background, allowed random intercepts and slopes per subject, and were controlled for age and gender.
We then specified the following conditional model (Model 2): Fixation time was modeled as a function of treatment (OT or PL), time of assessment (session 1 or 2), and AOI as fixed effects. This model showed superior model fit (pseudo R2 = .246) and displayed significant fixed main effects for AOIs and significant interactions of the AOI* time of assessment, AOI * treatment and significant 3-way interactions for AOI (adult’s eyes, child’s eyes, adult’s body) * treatment * time of assessment (cf. table 2 for all coefficients and figure 6 for the predicted values). The significant 3way- interactions indicate more fixations at the first session with OT-modulated slopes towards the 2nd session, different for some of the AOIs. There was no significant effect of sex, indicative of no difference between men and women.
Table 2.
Multilevel Models Predicting Fixation Count from AOI, Time of Assessment and Treatment
| Model 1 AOI * Time |
Model 2 AOI * Time * Treatment |
|||
|---|---|---|---|---|
| Coefficients (± SD) | Coefficients (± SD) | |||
| Random effects | ||||
| Intercept | .95 | .77 | ||
| Assessment | .73 (−.34) | .50 (.19) | ||
| Treatment | .58 (−.56) | |||
| Coefficients (± SEM) | t-value (df) | Coefficients (± SEM) | t-value (df) | |
| Fixed effects | ||||
| Intercept | 3.49 (.50) | 6.68 (86083)** | 3.03 (.48) | 6.31 (86067)** |
| AOI Adult’s Eyes | −1.35 (.04) | −33.13 (86083)** | −1.15 (.06) | −20.37 (86067)** |
| AOI Child’s Eyes | −.24 (.08) | −3.11 (86083)** | −.06 (.10) | −.54 (86067) |
| AOI Adult’s Face | 1.38 (.04) | 33.52 (86083)** | 1.55 (.06) | 27.37 (86067)** |
| AOI Child’s Face | 2.13 (.08) | 27.49 (86083) ** | 2.283 (.11) | 21.55 (86067)** |
| AOI Adult’s Body | −.57 (.04) | −14.44 (86083) ** | −.35 (.05) | 6.40 (86067)** |
| AOI Child’s Body | −1.28 (.08) | −16.57 (86083) | −1.13 (.11) | −10.68(86067)** |
| AOI Adult’s Genitals | −.47 (.06) | −8.21 (86083) ** | −.27 (.08) | −3.49 (86067)** |
| Treatment | .57 (.22) | 2.60 (86067) | ||
| Assessment | −.50 (.09) | −5.24 (86083) ** | −.13 (.21) | −.61 (86067) |
| Gender | .28 (.20) | 1.42 (83) | −.03 (.02) | −.61 (83) |
| Age | −.03 (.02) | −1.22 (83) | −5 (4) | −1.19 (83) |
| AOI Adult’s Eyes * Assessment | .19 (.06) | 3.21 (86083)** | −.08 (.08) | −1. (86067) |
| AOI Child’s Eyes * Assessment | −.08 (.10) | −.72 (86083) | −.34 (.15) | −2.24 (86067)* |
| AOI Adult’s Face* Assessment | −.26 (.06) | −4.04 (86083)** | −.35 (.08) | −4.423 (86067)** |
| AOI Child’s Face * Assessment | −.28 (.11) | −2.56 (86083)** | −.21 (.15) | −1.37 (86067) |
| AOI Adult’s Body * Assessment | .07 (.06) | 1.20 (86083) | −.12 (.08) | −1.55 (86067)** |
| AOI Child’s Body * Assessment | −.06 (.11) | .53 (86083) | −.11 (.16) | −.69 (86067) |
| AOI Adult’s Genitals * Assessment | −.07 (.08) | .87(86083) | −.02 (.11) | −.17 (86067) |
| AOI Adult’s Eyes * Treatment | −.42 (.08) | −5.16 (86067) | ||
| AOI Child’s Eyes * Treatment | −.38 (.15) | −2.47 (86067)* | ||
| AOI Adult’s Face* Treatment | −.37 (.08) | −4.45 (86067)** | ||
| AOI Child’s Face * Treatment | −.31 (.16) | −2.00 (86067)* | ||
| AOI Adult’s Body * Treatment | −.46 (.08) | −5.85 (86067)** | ||
| AOI Child’s Body * Treatment | −.30 (.16) | −1.94 (86067) | ||
| AOI Adult’s Genitals * Treatment | −.41 (.11) | −3.59 (86067)** | ||
| Treatment * Assessment | −.75 (.40) | −1.87 (86067) | ||
| AOI Adult’s Eyes * Treatment * Assessment | .55 (.11) | 4.76 (86067)** | ||
| AOI Child’s Eyes * Treatment * Assessment | .53 (.21) | 2.47 (86067)* | ||
| AOI Adult’s Face* Treatment * Assessment | .20 (.12) | 1.69 (86067) | ||
| AOI Child’s Face * Treatment * Assessment | −.11 (.22) | −.48 (86067) | ||
| AOI Adult’s Body * Treatment * Assessment | .41 (.11) | 3.59 (86067)** | ||
| AOI Child’s Body * Treatment * Assessment | .34 (.22) | 1.55 (86067) | ||
| AOI Adult’s Genitals * Treatment * Assessment | .21 (.16) | 1.29 (86067) | ||
| Model fit AIC | 399915 | 399878 | ||
| Pseudo R-squared | .245 | .246 | ||
p < .05;
p < .01.
All estimates calculated by full maximum likelihood. Parameter estimates were calculated with background serving as reference for AOIs; for assessment, 1st session serves as reference; for treatment, placebo serves as reference and for gender, female serves as reference.
Figure 6. Predicted Values Fixation Count.
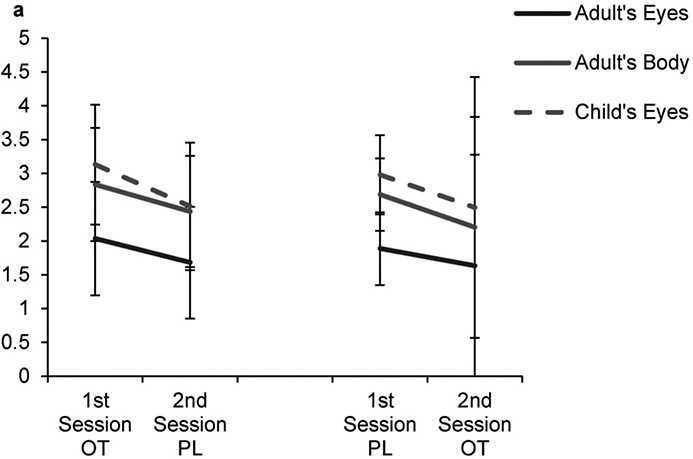
Multilevel analysis resulted in significant 3-way-interaction of treatment, time of assessment and AOI for the AOIs adult’s eyes, child’s eyes, adult’s body; background served as reference category; OT, Oxytocin, PL, Placebo; Error Bars indicate Standard Error of the Mean (SEM)
Discussion
The goal of this study was to systematically model the differential effect of intranasal OT administration on basic social cognition processes in different contexts. Using a rigorous randomized placebo-controlled, double-blind, within-subject crossover design in men and women, we were able to analyze drug effects in combination with intra-individual carry-over effects.
To summarize, our results indicate that OT increased arousal towards and focus on stimuli of all categories. This effect was particularly strong during the first presentation of the stimulus material and was not apparent when the stimuli were presented for the second time (see explanation of the carry-over effects below).
To the best of our knowledge, this is the first study using OT to influence the interest in parent-child bonding, pair bonding as well as sexual material. The results on the bonding material are in line with the beneficial effects of OT on social cognition processes and affiliation behavior in animals and humans (Hurlemann and Scheele, 2016; Lim and Young, 2006) though. With eye tracking, as a well-established non-invasive method to investigate cognition processes, salience and arousal (Itier and Batty, 2009; Nummenmaa and Calder, 2009), we found an increased arousal to and processing of novel social and non-social stimuli under OT in both men and women.
Pupil Dilation
Specifically, OT increased pupil dilation to all stimuli during their initial exposition. The overall enlargement in pupil dilation with increased sexual content of the stimulus material suggests augmented attentional focus (Alnaes, et al., 2014) and, assumingly, noradrenergic activation from the locus coeruleus (Murphy, et al., 2014) towards these stimuli. These overall results, thus, validate pupil dilation as a marker of central nervous arousal (Zekveld, et al., 2014).
Furthermore, the OT-induced general increase in pupil dilation is in line with previous studies on OT and face-processing (Leknes, et al., 2012; Prehn, et al., 2013), which showed stronger pupil dilation towards different categories of social stimuli after OT intake as well.
The interaction of stimulus type with OT and 1st vs. 2nd presentation can indicate an intensified processing or a salience effect with the social stimuli, while this interest seemed decreased during the subsequent PL session. OT has been discussed before as facilitator of salience but also social approach (Kemp and Guastella, 2010). In this context, the carry-over effect from the first to the second session might also be interpreted as a reduced effort necessary for recognition of the stimuli at the second presentation or marker of social adaptation, as proposed by Ma, Shamay-Tsoory, Han, & Zink (2016).
Areas of Interest (AOIs)
Previous research has shown that OT increased the focus on the eyes and the mouth in pictures and videos of faces. Some studies report increased eye-gaze to socially relevant stimuli in comparison to non-social stimuli (e.g. pictures of houses): In healthy volunteers and autism spectrum patients, OT facilitated the focus to eye and face regions (Andari, et al., 2010; Auyeung, et al., 2015; Guastella, et al., 2008). Specific effects of OT on the focus towards negative affective pictures have been reported (Guastella, et al., 2009), as well as an increased focus on both positive and neutral affective video sequences (Domes, Steiner, et al., 2013; Guastella, et al., 2009). In this study, we assumed that OT would affect the focus on the eyes, the mouth and areas of physical contact. This is supported by the clearly overall more fixations on these areas (and also the other areas) under OT in the first session. Moreover, the within-subject analyses suggest that in a time * OT interaction, slopes differed for some of the AOIs between the first and the second session. For instance for eyes and adult bodies, while OT increased the eye focus at the first session, it decreased the fixations in the subsequent PL session.
Stimulus Categories
Overall, the results support an effect of OT on arousal towards and the focus on complex social stimuli and interaction sequences. Results thereby extend our knowledge on the involvement of OT in social cognition processes to naturalistic sequences and beyond the eyes and faces as previously shown (Domes, Steiner, et al., 2013).
Notably, we did not observe differential effects of OT application on focus to bonding vs. sexual stimuli or differences between reactions to parent-child dyads in comparison to (erotic) adult dyads. Instead, OT increased pupil dilation in the present study in response to all kinds of new stimuli and focus towards all AOIs, and on a descriptive level, also on the background. These results might be interpreted in the light of research on endogenous OT activation and the context when endogenous OT is released:
Animal research has shown that endogenous OT within specific brain areas and into the periphery is released both during sexual arousal and social contact (Blaicher et al., 1999; de Jong et al., 2015; Veenema and Neumann, 2008). In humans, no in vivo data are available on central nervous system OT mechanisms, however, during sex, birth, and lactation increased OT has been found in the periphery (de Jong, et al., 2015; White-Traut et al., 2009). Previous research suggests that intranasal administration of the neuropeptide might to some extend mimic endogenous OT mechanisms. Based on this, we could assume that in a specific social context, OT might indeed increase overall alertness towards the situation however it might be the context that defines OT’s specific pro-social effects which have been reported in the past. This is in line with the heterogeneous findings in OT research reporting either global OT-effects or specific effects depending on the task (e.g. emotion recognition in faces, (Domes, Sibold, et al., 2013; Lischke, et al., 2012).
The present data do not support specific differences between types of human social relationships with regard to OT effects. While in monogamous animals, mating, reproduction and bonding are intertwined, humans engage in sexual activities without explicitly wishing to reproduce. In fact, a large part of pharmaceutical industry is focused on helping humans to prevent reproduction.
Although certainly influenced by (subtle) signals on reproductive health, such as age, physiognomy, or fitness (Symons, 1995; Wallen, 1995) heterosexual sexual interaction is frequently driven by the interest in sex without founding a family. In comparison, animals most likely do not actively decide to prevent reproduction; therefore humans and voles are hardly comparable in this context (Freeman and Young, 2016; Young, 2015). While a large body of human research indicates that OT plays a substantial role in parent-child bonding (Gordon, Zagoory-Sharon, Leckman, & Feldman, 2010; Weisman, et al., 2012), there is no data available about OT’s role in the wish for an own child or for interest in reproduction. Therefore, our data cannot answer the question whether OT facilitates both the interest in sexual activities and the willingness to reproduce. However, the present data clearly support theories on the shared underlying neuroendocrine mechanisms of parent-infant and adult attachment bonds (Carter, 1998; de Boer, et al., 2012; Feldman, et al., 2016). We could follow that learning processes throughout individual development establish a network of reward-relevant activation patterns in the CNS, which are driven by OT and other neuromodulators and that these pattern could be adapted and stabilized through adult attachment interactions (Feldman, 2017).
Effect of Substance Order and Carry-over Effects
It is worth discussing that in previous studies, effects of OT were exclusively tested when administered during the initial test session, when the stimuli were novel. Our design, however, allowed for possible within-subject and carry-over effects. Results show that pupil dilation was larger when OT was given first than when it was given subsequently. Furthermore, eye fixation on the pre-defined AOIs was increased when OT was given at the first stimulus presentation in comparison to PL and to subsequent stimulus presentation about two weeks later.
One possible explanation could be habituation. However, the PL data show that pupil diameter is even smaller when PL is given firstly than when PL is given second (see supplementary table S1), which would argue against overall habituation. Instead, as the second PL session was preceded by OT, a carry-over from the first OT session might explain the larger pupil dilation during the second test session. In contrast, a clear within-subject difference in pupil dilation occurs when OT is given first. When OT is given second, there is no difference between OT and PL.
In previous studies, an association between endogenous OT and prosocial cognition has been shown to be particularly pronounced in novel stimuli (Tops et al., 2013) and especially during the beginning of social relationships (Feldman, 2012). Furthermore, using repeated-measures paradigms, it has been shown before, that the hormonal state during first exposure influences subsequent interest in sexual stimuli (Wallen and Rupp, 2010) and the focus on male faces (Ditzen, et al., 2017). We here find that OT increases arousal towards and focus on attachment and sex related pictures when administered at the first contact with these pictures - but not during the second presentation of the stimulus material. These results support published research on the effects of one-time single dose OT administration. However, it remains to be investigated whether OT effects with repeated stimulus presentation a) make the processing of social stimuli in general more effective (Ma, et al., 2016), b) decrease interest in social stimuli, or c) just fade over time. Each adaptive process would then be associated with dynamic changes in the CNS OT receptor system as, indeed, is suggested by region-specific brain research in rodent populations (Ross et al., 2009). Such dynamics are of particular relevance when OT is considered a possible long-term treatment in socially impaired individuals.
Limitations
Intranasal OT has been shown to reach the central nervous system (Striepens et al., 2013), however, from nasal spray administration we cannot tell where exactly the supposed brain region specific effects take place. So far, no PET or SPECT tracer suitable for the human OT system has been identified (Freeman, Inoue, Smith, Goodman, & Young, 2014), which limits the region - or allows even cell type-specific analysis of OT - in the living human brain. Future fMRI research with combined eye tracking might allow for interpretation of specific network-activation to OT in the brain.
In the present study, OT explains less variance than other factors, such as stimulus category. This is in line with other studies reporting only small to medium effects of intranasal OT (Bakermans-Kranenburg and Van Ijzendoorn, 2013; Walum, Waldman, & Young, 2016) and underlines the context-specific mechanisms of OT. Therefore, our results should be interpreted with caution given the small effect sizes and small to moderate effects (Walum, et al., 2016). Considering this, it is particularly interesting that responses did not systematically differ between the different social categories. In this study, no systematic and modulating effect of menstrual cycle phase or sex-hormones was found. Although menstrual cycle phase was controlled for and only naturally cycling women were included in this study, the number of women in each cell was small, so that possible small effects might not have been detected. This is due to inconsistencies in self-report and endocrine markers in some of the female participants, which made it difficult to correctly assign their menstrual cycle phase at each measure.
Another relevant moderator, the relationship status (Scheele, et al., 2012), did not show any significant results in additional explorative analyses, which might as well be due to a lack of power in the small subgroup of pair-bonded individuals (N = 29).
Further characteristics of our sample limit the generalizability of our data: Our participants were heterosexual, had no children of their own and the women were all natural cycling. Given reports on the influential role of sexual orientation, parenthood and hormonal contraception (Scheele, Plota, Stoffel-Wagner, Maier, & Hurlemann, 2015; Thienel et al., 2014; Weisman, et al., 2012), we cannot extrapolate our findings to homosexuals, parents or women using hormonal contraception.
Taken together, our study provides substantial evidence to the modulating role of OT in social cognition processes related to both bonding and sexual reproduction. However these effects appear not specific to social in comparison to non-social cues but rather indicate an overall arousal following OT administration. Notably, the effects of OT seem limited to novel stimuli, which might indicate that OT facilitates effective processing and adaptation towards social stimuli in the long run. These results might help explain why initial clinical trials using OT as a therapeutic agent to increase sexual behavior and satisfaction have revealed mixed results only (Behnia, et al., 2014; Muin et al., 2015).
On a basic level, our results underscore the importance of OT in interaction with the given context, when specific effects on social behavior and social bonding are assessed. Above this, our data suggest long-lasting effects which are of particular importance for repeated exposures and need to be taken in account for treatment concepts to improve social functioning.
Supplementary Material
Acknowledgements
This work was supported by a Swiss National Science Foundation (SNF) Postdoctoral Research Fellowship to BD, SNF individual project funding (grant number SNF 105314 124627) to BD, and a Center for Behavioral Neuroscience (CBN) Venture Grant to BD, KW and LY. Additional support was provided by NIH grants P50MH100023 to LJY and ORIP/OD P51OD011132 to YNPRC. We gratefully acknowledge A. Isler, S. Joehl, D. Schneider, M. Prevost and I. Gold for their skilled support in running the study. The authors wish to thank S. Dubber for proofreading the manuscript.
Footnotes
Disclosures of Conflict of Interest
The authors report no competing biomedical financial interests or personal affiliations in connection with the content of this manuscript.
References
- Abu-Akel A, Palgi S, Klein E, Decety J, & Shamay-Tsoory S (2015). Oxytocin increases empathy to pain when adopting the other-but not the self-perspective. Social neuroscience, 10(1), pp. 7–15. [DOI] [PubMed] [Google Scholar]
- Alnaes D, Sneve MH, Espeseth T, Endestad T, van de Pavert SH, & Laeng B (2014). Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J Vis, 14(4) [DOI] [PubMed] [Google Scholar]
- Alvares GA, Chen NT, Balleine BW, Hickie IB, & Guastella AJ (2012). Oxytocin selectively moderates negative cognitive appraisals in high trait anxious males. Psychoneuroendocrinology, 37(12), pp. 2022–2031. [DOI] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Kwon YJ, Shpiner AC, Saravanan V, Mays WD, … Young LJ (2017). Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature, 546(7657), pp. 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, & Sirigu A (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences, 107(9), pp. 4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Lombardo M, Heinrichs M, Chakrabarti B, Sule A, Deakin J, … Sipple J (2015). Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Translational psychiatry, 5(2), p e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M, & Van Ijzendoorn M (2013). Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational psychiatry, 3(5), p e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoń K (2013). MuMIn: Multi-model inference R package version 1.9. 13 The Comprehensive R Archive Network (CRAN), Vienna, Austria [Google Scholar]
- Barton RA (1998). Visual specialization and brain evolution in primates. [10.1098/rspb.1998.0523]. Proceedings of the Royal Society o fLondon. Series B: Biological Sciences, 265(1409), p 1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia B, Heinrichs M, Bergmann W, Jung S, Germann J, Schedlowski M, … Kruger TH. (2014). Differential effects of intranasal oxytocin on sexual experiences and partner interactions in couples. Hormones and behavior, 65(3), pp. 308–318. [DOI] [PubMed] [Google Scholar]
- Blaicher W, Gruber D, Bieglmayer C, Blaicher AM, Knogler W, & Huber JC (1999). The role of oxytocin in relation to female sexual arousal. Gynecologic and obstetric investigation, 47(2), pp. 125–126. [DOI] [PubMed] [Google Scholar]
- Borrow AP, & Cameron NM (2012). The role of oxytocin in mating and pregnancy. Hormones and behavior, 61(3), pp. 266–276. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, … Rainnie DG (2016). Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology, 64, pp. 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, & Young LJ (2017). Oxytocin and social relationships: from attachment to bond disruption. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, & Lang PJ (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45(4), pp. 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri A, Heinrichs M, Schedlowski M, & Kruger TH (2008). The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology, 33(5), pp. 591–600. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Orlando MA, Brown CA, & Ellenbogen MA (2014). Oxytocin and enhancement of the positive valence of social affiliation memories: an autobiographical memory study. Social neuroscience, 9(2), pp. 186–195. [DOI] [PubMed] [Google Scholar]
- Carter CS (1998). Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology, 23(8), pp. 779–818. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, & French JA (2014). Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology, 49, pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer A, van Buel EM, & Ter Horst GJ (2012). Love is more than just a kiss: a neurobiological perspective on love and affection. Neuroscience, 201, pp. 114–124. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Menon R, Bludau A, Grund T, Biermeier V, Klampfl SM, … Neumann ID (2015). Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology, 62, pp. 381–388. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, & Bargh JA (2001). The perception-behavior expressway: Automatic effects of social perception on social behavior. Advances in experimental social psychology, 33, pp. 1–40. [Google Scholar]
- Ditzen B, Palm-Fischbacher S, Gossweiler L, Stucky L, & Ehlert U (2017). Effects of stress on women’s preference for male facial masculinity and their endocrine correlates. Psychoneuroendocrinology, 82, pp. 67–74. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Bodenmann G, Gabriel B, Ehlert U, & Heinrichs M (2009). Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry, 65(9), pp. 728–731. [DOI] [PubMed] [Google Scholar]
- Domes G, Sibold M, Schulze L, Lischke A, Herpertz S, & Heinrichs M (2013). Intranasal oxytocin increases covert attention to positive social cues. Psychological medicine, 43(08), pp. 1747–1753. [DOI] [PubMed] [Google Scholar]
- Domes G, Steiner A, Porges SW, & Heinrichs M (2013). Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology, 38(7), pp. 1198–1202. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, … Hurlemann R (2015). Oxytocin facilitates the extinction of conditioned fear in humans. Biological psychiatry, 78(3), pp. 194–202. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Markett S, Kendrick KM, Ditzen B, Liu F, Hurlemann R, & Becker B (2017). Oxytocin differentially alters resting state functional connectivity between amygdala subregions and emotional control networks: inverse correlation with depressive traits. NeuroImage [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Oxytocin and social affiliation in humans. Hormones and behavior, 61(3), pp. 380–391. [DOI] [PubMed] [Google Scholar]
- Feldman R (2017). The neurobiology of human attachments. Trends in Cognitive Sciences, 21(2), pp. 80–99. [DOI] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, & Ebstein RP (2016). Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biological Psychiatry, 79(3), pp. 174–184. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, & Winslow JT (2000). Social amnesia in mice lacking the oxytocin gene. Nature genetics, 25(3), pp. 284–288. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, & Young LJ (2014). The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology, 45, pp. 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, & Young LJ (2016). Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. Journal of neuroendocrinology, 28(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Schmitz AK, Tittgemeyer M, & Schilbach L (2013). The human amygdala drives reflexive orienting towards facial features. Curr Biol, 23(20), pp. R917–918. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, & Feldman R (2010). Oxytocin and the development of parenting in humans. Biological psychiatry, 68(4), pp. 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Carson DS, Dadds MR, Mitchell PB, & Cox RE (2009). Does oxytocin influence the early detection of angry and happy faces? Psychoneuroendocrinology, 34(2), pp. 220–225. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, & Hickie IB (2016). Oxytocin treatment, circuitry, and autism: a critical review of the literature placing oxytocin into the autism context. Biological Psychiatry, 79(3), pp. 234–242. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Dadds MR (2008). Oxytocin increases gaze to the eye region of human faces. Biological psychiatry, 63(1), pp. 3–5. [DOI] [PubMed] [Google Scholar]
- Hubble K, Daughters K, Manstead AS, Rees A, Thapar A, & van Goozen SH (2017). Oxytocin Reduces Face Processing Time but Leaves Recognition Accuracy and Eye-Gaze Unaffected. J Int Neuropsychol Soc, 23(1), pp. 23–33. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, … Maier W (2010). Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience, 30(14), pp. 4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, & Scheele D (2016). Dissecting the role of oxytocin in the formation and loss of social relationships. Biological Psychiatry, 79(3), pp. 185–193. [DOI] [PubMed] [Google Scholar]
- Insel TR, & Young LJ (2001). Opinion: The neurobiology of attachment. Nature reviews. Neuroscience, 2(2), p 129. [DOI] [PubMed] [Google Scholar]
- Itier RJ, & Batty M (2009). Neural bases of eye and gaze processing: the core of social cognition. Neuroscience & Biobehavioral Reviews, 33(6), pp. 843–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, & Koch C (2001). Computational modelling of visual attention. Nature reviews. Neuroscience, 2(3), p 194. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, & Young LJ (2015). Neurobiological mechanisms of social attachment and pair bonding. Current opinion in behavioral sciences, 3, pp. 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, & Young LJ (2017). Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neuroscience & Biobehavioral Reviews, 76, pp. 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, & Guastella AJ (2010). Oxytocin: prosocial behavior, social salience, or approach-related behavior? Biological psychiatry, 67(6), pp. e33–e34. [DOI] [PubMed] [Google Scholar]
- Keverne EB, & Kendrick KM (1992). Oxytocin Facilitation of Maternal Behavior in Sheepa. Annals of the New York Academy of Sciences, 652(1), pp. 83–101. [DOI] [PubMed] [Google Scholar]
- Leknes S, Wessberg J, Ellingsen D-M, Chelnokova O, Olausson H, & Laeng B (2012). Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions. Social cognitive and affective neuroscience, p nss062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, & Young LJ (2006). Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and behavior, 50(4), pp. 506–517. [DOI] [PubMed] [Google Scholar]
- Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, & Domes G (2012). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology, 37(4), pp. 475–481. [DOI] [PubMed] [Google Scholar]
- Ma Y, Shamay-Tsoory S, Han S, & Zink CF (2016). Oxytocin and social adaptation: Insights from neuroimaging studies of healthy and clinical populations. Trends in cognitive sciences, 20(2), pp. 133–145. [DOI] [PubMed] [Google Scholar]
- Moresi S, Adam JJ, Rijcken J, Van Gerven PWM, Kuipers H, & Jolles J (2008). Pupil dilation in response preparation. International Journal of Psychophysiology, 67(2), pp. 124–130. [DOI] [PubMed] [Google Scholar]
- Muin DA, Wolzt M, Marculescu R, Rezaei SS, Salama M, Fuchs C, … Bayerle-Eder M (2015). Effect of long-term intranasal oxytocin on sexual dysfunction in premenopausal and postmenopausal women: a randomized trial. Fertility and sterility, 104(3), pp. 715–723. e714. [DOI] [PubMed] [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, & Balsters JH (2014). Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum Brain Mapp, 35(8), pp. 4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, & Young LJ (2016). Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Hormones and behavior, 77, pp. 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, & Calder AJ (2009). Neural mechanisms of social attention. Trends in cognitive sciences, 13(3), pp. 135–143. [DOI] [PubMed] [Google Scholar]
- Oettl L-L, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, … Young WS (2016). Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron, 90(3), pp. 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, & Sarkar D (2014). R Core Team (2014) nlme: linear and nonlinear mixed effects models. R package version 3.1–117. See http://CRAN.R-project.org/package=nlme
- Preckel K, Scheele D, Eckstein M, Maier W, & Hurlemann R (2015). The influence of oxytocin on volitional and emotional ambivalence. Soc Cogn Affect Neurosci, 10(7), pp. 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn K, Kazzer P, Lischke A, Heinrichs M, Herpertz SC, & Domes G (2013). Effects of intranasal oxytocin on pupil dilation indicate increased salience of socioaffective stimuli. Psychophysiology, 50(6), pp. 528–537. [DOI] [PubMed] [Google Scholar]
- Rilling JK, & Young LJ (2014). The biology of mammalian parenting and its effect on offspring social development. Science, 345(6198), pp. 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler L, End A, & Gamer M (2017). Orienting towards social features in naturalistic scenes is reflexive. PLoS One, 12(7), p e0182037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, & Young LJ (2009). Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. Journal of Neuroscience, 29(5), pp. 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, & Wallen K (2007). Relationship between testosterone and interest in sexual stimuli: the effect of experience. Hormones and behavior, 52(5), pp. 581–589. [DOI] [PubMed] [Google Scholar]
- Scheele D, Plota J, Stoffel-Wagner B, Maier W, & Hurlemann R (2015). Hormonal contraceptives suppress oxytocin-induced brain reward responses to the partner’s face. Soc Cogn Affect Neurosci, 11(5), pp. 767774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Güntürkün O, Deutschländer S, Maier W, Kendrick KM, & Hurlemann R (2012). Oxytocin modulates social distance between males and females. Journal of Neuroscience, 32(46), pp. 16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O, … Hurlemann R (2013). Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proceedings of the National Academy of Sciences, 110(50), pp. 20308–20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, & Guastella AJ (2013). The Impact of a Single Administration of Intranasal Oxytocin on the Recognition of Basic Emotions in Humans: A Meta-Analysis. Neuropsychopharmacology, 38(10), pp. 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, & Gallagher L (2009). Dopaminergic-neuropeptide interactions in the social brain. Trends in Cognitive Sciences, 13(1), pp. 27–35. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, & Hurlemann R (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific reports, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons D (1995). Beauty is in the adaptations of the beholder: The evolutionary psychology of human female sexual attractiveness. Sexual nature, sexual culture, pp. 80–118. [Google Scholar]
- Thienel M, Heinrichs M, Fischer S, Ott V, Born J, & Hallschmid M (2014). Oxytocin’s impact on social face processing is stronger in homosexual than heterosexual men. Psychoneuroendocrinology, 39, pp. 194–203. [DOI] [PubMed] [Google Scholar]
- Tops M, Huffmeijer R, Linting M, Grewen KM, Light KC, Koole SL, … van IJzendoorn MH (2013). The role of oxytocin in familiarization-habituation responses to social novelty. Frontiers in psychology, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkelbach C, Guastella AJ, & Forgas JP (2008). Oxytocin selectively facilitates recognition of positive sex and relationship words. Psychological Science, 19(11), pp. 1092–1094. [DOI] [PubMed] [Google Scholar]
- Veenema AH, & Neumann ID (2008). Central vasopressin and oxytocin release: regulation of complex social behaviours. Progress in brain research, 170, pp. 261–276. [DOI] [PubMed] [Google Scholar]
- Veening J, De Jong T, Waldinger M, Korte S, & Olivier B (2015). The role of oxytocin in male and female reproductive behavior. European journal of pharmacology, 753, pp. 209–228. [DOI] [PubMed] [Google Scholar]
- Wallen K (1995). The evolution of female sexual desire. Sexual nature/sexual culture, pp. 57–79. [Google Scholar]
- Wallen K, & Rupp HA (2010). Women’s interest in visual sexual stimuli varies with menstrual cycle phase at first exposure and predicts later interest. Hormones and behavior, 57(2), pp. 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, … Westberg L (2012). Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biological psychiatry, 71(5), pp. 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Waldman ID, & Young LJ (2016). Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biological psychiatry, 79(3), pp. 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, & Feldman R (2012). Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biological psychiatry, 72(12), pp. 982–989. [DOI] [PubMed] [Google Scholar]
- White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, & Carter CS (2009). Detection of salivary oxytocin levels in lactating women. Developmental psychobiology, 51(4), pp. 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ (2015). Oxytocin, social cognition and psychiatry. Neuropsychopharmacology, 40(1), pp. 243–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekveld AA, Heslenfeld DJ, Johnsrude IS, Versfeld NJ, & Kramer SE (2014). The eye as a window to the listening brain: Neural correlates of pupil size as a measure of cognitive listening load. Neuroimage, 101, pp. 76–86. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Deiter F, Jung S, Heinrichs M, Schedlowski M, & Krüger H (2015). Differential Effects of Intranasal Oxytocin Administration On Sexual Functions in Healthy Females: a Laboratory Setting. European Psychiatry, 30, p 264. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


