Abstract
Nanoparticulate platforms have contributed significantly to the field of biomedical research, demonstrating advantages over traditional modalities in areas such as drug delivery, detoxification, and vaccination. When it comes to the design of nanoparticles, biomimetic strategies have become increasingly popular as a means of promoting effective interactions with biological systems. A recently developed cell membrane-coated nanoparticle platform can leverage the natural interactions that cells engage in with other cells, the extracellular matrix, and biomolecules in order to reduce undesirable nonspecific interactions, while increasing targetspecific interactions. Here, we discuss the current state of these biomimetic nanoparticles and highlight how they can be used for various biomedical applications.
Graphical abstract
Introduction
The use of nanotechnology in biomedical research has become increasingly popular over time [1], and the continual progression of nanoparticulate systems has helped to advance different fields, including cancer therapy [2], infectious disease treatment [3], and immune modulation [4]. Compared to traditional therapies, one advantage of nanoparticles is that they can be engineered and modified to enhance cargo release at a site of interest, which contributes to improved safety and reduced side effects [5]. Another benefit is their ability to vastly enhance the solubility and bioavailability of drug molecules. It is now known that the in vivo performance of a nanoparticulate therapy relies heavily on its ability to properly interact with biological systems [6]. Upon systemic administration, plasma proteins will rapidly adsorb onto the nanoparticle surface, which can not only deteriorate targeting capability, but can also dramatically increase immune clearance [7]. In fact, it is known that the vast majority of nanoparticles end up in the spleen and liver instead of in the tissue of interest [8], diminishing the effectiveness of drug payloads and introducing toxicity to healthy tissues. As such, the engineering of nanoscale platforms that can overcome such biological hurdles, while simultaneously exhibiting specific targeting capabilities, has remained of great interest to researchers working in the nanomedicine field.
Conventional biointerfacing strategies
Solving the challenge of nonspecific interactions encountered by nanoparticles remains a central challenge, and factors such as surface charge, size, and hydrophobicity all play a strong role in determining the fate of a nanoparticle after administration [9]. While a multitude of materials and synthesis methods have been explored, the gold standard for reducing unwanted biological interactions remains the use of polyethylene glycol (PEG) [10], a polymer that is capable of reducing plasma protein adsorption, thus shielding nanoparticles from immune cell detection and clearance. On the other end, increasing the specific interactions between a nanoparticle and its desired target is often accomplished by methods such as the chemical conjugation of moieties with affinity towards certain cells or tissues of interest. Commonly used targeting ligands include antibodies, peptides, aptamers, and small molecules [11,12]. Folate, a small molecule important for cell biosynthesis whose receptor is significantly overexpressed on many cancer types, has been widely used by nanoplatforms for tumor targeting [13]. Another commonly used molecule is the RGD peptide, which is well-known for its ability to target integrin proteins expressed on tumor vasculature, and this ligand has been used to promote the delivery of cargoes such as anti-angiogenic drugs or imaging contrast agents [14].
Cell membrane-coated nanoparticles
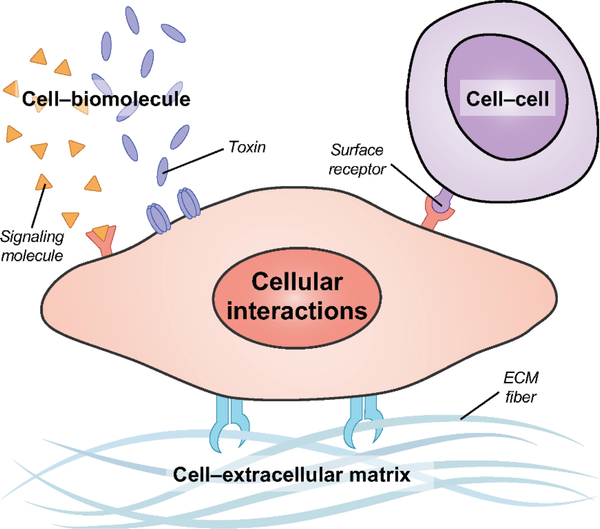
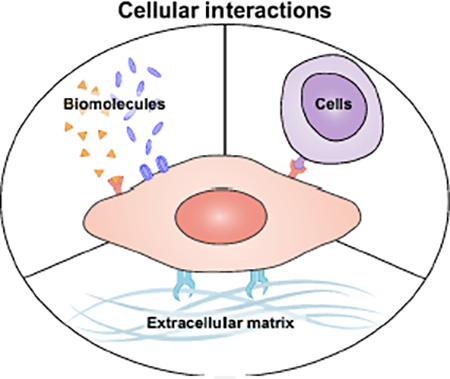
Although nanoplatforms employing conventional biointerfacing strategies have proven to be highly successful and are currently under clinical investigation [15], there are still many opportunities for improvement [16]. The bottom-up assembly of nanoparticles by synthetic techniques generally oversimplifies biological interactions, which are inherently multifaceted and complex, and these workflows usually require explicit identification of targeting specificities. It is for these reasons that biomimetic nanotechnologies inspired by nature have recently emerged as an attractive means of overcoming some of the drawbacks inherent to synthetic materials [17–19]. Such platforms focus on directly leveraging naturally occurring interactions that have already been highly optimized by the process of evolution, combining them with increasingly advanced bioengineering tools. In general, an effective strategy for biomimicry at the nanoscale has been to take design cues from the cell. As one of the most fundamental units of biology, cells participate in a number of different biological interactions, including with other cells, the extracellular matrix (ECM), and a wide range of biomolecules (Figure 1).
Figure 1.
Cellular interactions leveraged for biomimetic nanoparticle design. Cells physically participate in a number of interactions, including with other cells through the engagement of surface receptors by membrane-bound ligands, with components of the extracellular matrix (ECM), and with biomolecules such as those involved in endogenous signaling or toxic compounds secreted by other organisms.
Recently, a new biomimetic platform has been developed in which synthetic nanoparticulate cores are functionalized directly with a naturally derived cell membrane layer [20,21]. This top-down strategy, which involves the transfer of various components and structures found on the cell surface [22], enables the resulting cell membrane-coated nanoparticles to naturally interface with cells, ECM, and biomolecules in a multivalent manner. The membrane coating concept was first demonstrated using red blood cell (RBC) membrane cloaking to decrease nonspecific interactions. This helped to significantly improve nanoparticle blood residence by harnessing the inherent properties of RBCs [23]. The long circulation exhibited by RBC membrane-coated nanoparticles could in part be attributed to the CD47 self-marker protein present on the membrane surface, which inhibits macrophage uptake via interaction with signal-regulatory protein α on phagocytic cells [24]. It has since been shown that this membrane coating can be used to alter drug release kinetics [25], and additional functionality can be introduced through facile, non-disruptive means [26]. Since their initial conception, cell membrane-coated nanoparticles have been fabricated for different applications using a wide range of nanoparticulate cores [27–30]. The membranes from different source cells have also been employed in order to harness their unique interaction mechanisms [31–33]. In the following sections we will discuss the application of these biomimetic nanoparticles in several key areas of nanomedicine.
Targeted nanodelivery
Cell membrane-coated nanoparticles can be easily tailored for various delivery applications by altering the origin of the membrane coating (Figure 2). In one notable case, cancer cell membrane has been shown to enable homotypic targeting, where nanoparticles preferentially bind to their source cells [31]. Remarkably, precise homotypic targeting of membrane-coated nanoparticles can be achieved in vivo, even in the presence of a competing heterologous tumor [34], and this mechanism has been used across different tumor types [31,35,36]. Beyond the standard delivery of chemotherapeutics, this approach has also been adapted for use with more sophisticated tumor treatment strategies. For example, joint phototherapeutic and chemotherapeutic treatment was demonstrated using doxorubicin-loaded breast cancer cell membrane-coated gold nanocages, and this combination therapy resulted in a 5-fold decrease in tumor volume compared to free drug administration [37]. Similarly, a cervical cancer cell membrane-coated nanoparticle loaded with both a chemotherapeutic and a photothermal agent was used to successfully control tumor growth upon near-infrared irradiation [36]. Cancer cell membrane-coated nanoparticles have also been used to relieve tumor hypoxia, which promotes tumor progression and drug resistance. In one example, nanostructures loaded with doxorubicin and hemoglobin were cloaked with cancer cell membrane, enabling oxygenation of the tumor microenvironment [38]. These nanocarriers demonstrated an approximately 3-fold improvement in oxygen release compared to bare nanoparticles.
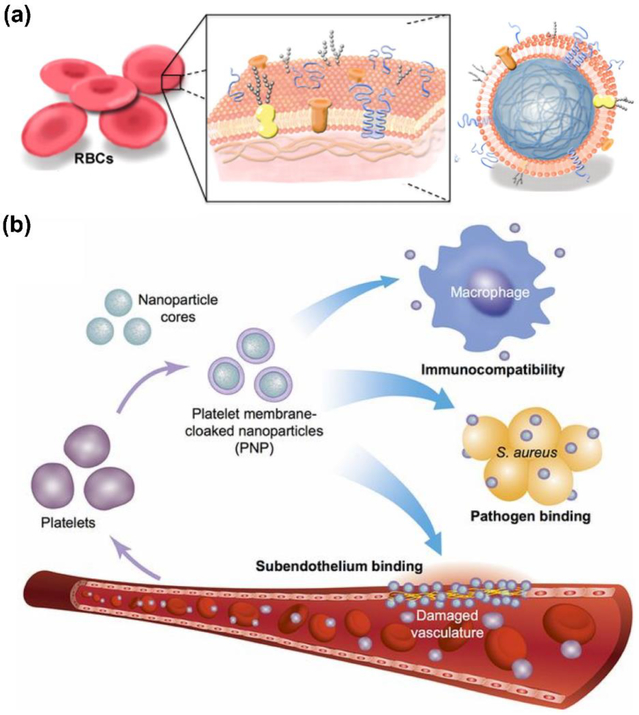
Figure 2.
Cell membrane-coated nanoparticles for nanodelivery. (a) RBC membrane-coated nanoparticles are functionalized with the surface markers from the original cell, which makes them well-suited for drug delivery due to their ability to circulate for extended periods of time in the bloodstream. Adapted with permission from [23]. (b) Platelet membrane-coated nanoparticles are immunocompatible and can target different disease substrates, including antibiotic-resistant bacteria and damaged vasculature. Adapted with permission from [32].
In addition to cancer cell membrane coatings, those derived from other cell types that play a role in tumorigenesis have also been used for tumor targeting. For example, by capitalizing on the affinity between white blood cells and some types of cancers, membrane-cloaked nanoparticles loaded with chemotherapeutics have been successfully fabricated for tumor-targeted drug delivery [39,40]. On mesenchymal stem cells, various cell surface moieties also participate in interactions with tumor cells, and this has likewise been exploited for biomimetic nanoparticle-based cancer therapy [41]. While tumors have been the most common focus for nanodelivery platforms, other types of target cells can also be of great therapeutic interest. Within populations of T cells and B cells, whose dysregulation can lead to autoimmune disease, the only distinctive feature between different clones are their antigen-specific receptors. Disease-causing T cell and B cell populations are thus extremely difficult to target, which has necessitated the use of broad immunosuppression in treating autoimmunity despite potential side effects [42]. As the targets of these immune cells are often other cells within the body, this has naturally provided an opportunity for biomimetic nanoformulations to address this challenge. In a proof-of-concept work, RBC membrane-functionalized nanoparticles were used to target RBC-specific B cells in a model of autoimmune hemolytic anemia [43]. The work demonstrated that the presentation of cognate antigens on nanoparticle surfaces is a viable means of engaging the corresponding receptor on a clonal population of malignant immune cells.
Biomimetic targeting using cell membrane coatings has also been effectively leveraged to treat bacterial infections. It is known that an important mechanism of action in many types of bacterial infections centers on adhesion to host cells [44]. To take advantage of this fact for Helicobacter pylori treatment, antibiotic-loaded polymeric cores were cloaked in membrane from gastric epithelial cells, which naturally adhere to the pathogen [45]. When delivered orally, the membrane-coated nanoformulation was able to outperform free drug, as well as a non-targeted nanoformulation. Along these lines, platelets, which bind to certain types of bacteria via their surface receptors [46], have been employed in a similar fashion. Drug-loaded polymeric cores were functionalized with platelet membrane and used to treat methicillin-resistant Staphylococcus aureus [32]. The platelet membrane-coated nanoparticles had significantly higher binding to the bacteria compared with their RBC membrane-coated counterparts. When used to treat systemic infection, the nanoformulation significantly reduced bacterial counts in most major organs, even compared with free drug administered at a 6-fold higher dosage.
Platelets are also unique in their ability to target various components of the ECM, which enables them to carry out their role in maintaining hemostasis [47]. By leveraging this biological affinity, platelet membranes have been used to design nanoformulations capable of addressing certain vascular disorders. In one instance, platelet membrane-coated nanoparticles were loaded with docetaxel, which can be used to prevent restenosis after arterial injury [48]. The nanoparticles were shown to effectively bind exposed collagen, helping to specifically deliver the therapeutic payload to the site of injury [32]. Ultimately, the nanoparticles helped to almost completely prevent restenosis in an animal model of vascular injury, with no significant occlusion observed compared with baseline controls. A similar platform was also used to achieve targeted delivery of a magnetic resonance imaging contrast agent, enabling the noninvasive detection of atherosclerotic lesions [49]. In a final example, engineered nanoplatelets were used to target fibrin-containing clots [50]. When further functionalized with tissue plasminogen activator, an enzyme capable of lysing clots, the nanoformulation exhibited high thrombus dissolution activity.
Detoxification
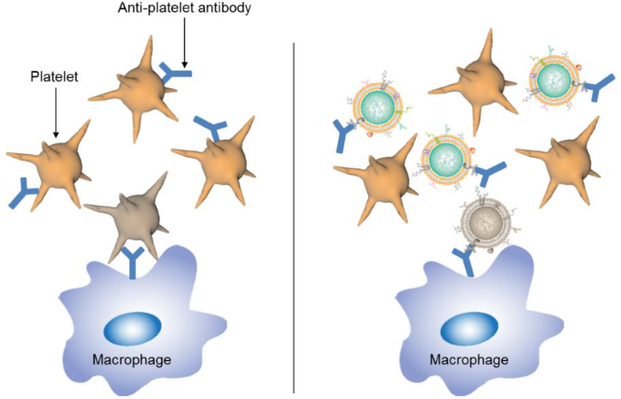
Functionalization with cell membrane coatings enables nanoparticles to interact with different types of biomolecules, oftentimes in a receptor-specific fashion. This has been leveraged by researchers in the design of nanosponges against various biological toxins (Figure 3), most of which must interact with cell membranes in order to exert their mechanism of action [51]. These nanosponges can act as biomimetic decoys that divert pathological proteins away from their intended targets, helping to alleviate disease symptoms for many difficult-to-treat conditions. This has been demonstrated in the case of type II hypersensitivities, such as autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia purpura (ITP), where autoantibodies attack healthy cells [52,53]. In AIHA, autoantibodies attack RBC surface antigens, resulting in opsonization and eventual phagocytosis [54]. Current clinical treatments are nonspecific and can be highly invasive, which can pose serious health risks for patients. It was demonstrated that nanosponges fabricated using RBC membrane could effectively bind antiRBC antibodies, helping to normalize blood parameters in an animal model of antibody-induced anemia [52]. Similarly, ITP is an immune disorder marked by low platelet counts due to the prevalence of anti-platelet autoantibodies [55], and it was shown that platelet-derived nanosponges could preserve platelet counts in an antibody-induced thrombocytopenia animal model [53]. Notably, treatment with the nanosponges helped to preserve hemostatic function, and treated mice exhibited normal clotting upon injury. A major advantage of this approach is that the membrane present on the nanoparticle surfaces contains all of the source cell’s naturally occurring surface antigens, enabling binding and neutralization regardless of the exact specificity of the autoimmune antibodies. More recently, this approach has been expanded from autoantibodies to endogenous cytokines using neutrophil-derived nanosponges, and this has major implications for the treatment of inflammatory disorders such as rheumatoid arthritis [56].
Figure 3.
Cell membrane-coated nanoparticles for detoxification. Platelet membrane-coated nanoparticles can bind to anti-platelet antibodies, diverting them away from healthy cells that would otherwise be opsonized and eliminated by the immune system. Adapted with permission from [53].
Virulence factors such as pore-forming toxins are a major component of bacterial secretions and animal venoms. Conventional methods for neutralization focus on individual molecular structures, which requires new treatments to be developed for each toxin. However, toxin nanosponges based on cell membrane coatings have emerged as a versatile strategy for the broad neutralization of toxins based on their function [51]. An RBC-based nanosponge has been shown to absorb multiple types of toxins, including α-hemolysin secreted by S. aureus, streptolysin-O from group A streptococcus, and melittin from honeybee venom [57,58]. These nanosponges were able to rescue mice that had been administered with a lethal bolus dose of toxin, highlighting the promise of this approach for treating bacterial infections and animal envenoming. In addition to exotoxins, it has also been shown that nanosponges are capable of neutralizing the biological activity of endotoxins such as lipopolysaccharide from gram-negative bacteria [33]. Nanosponges fabricated using macrophage membrane displayed endotoxin receptors, enabling them to prevent the immune activation that is characteristic of sepsis. Further, it was demonstrated that these nanoparticles could also bind to proinflammatory cytokines. When combined together, the ability to neutralize bacterial toxins and endogenous cytokines served to prevent unwanted immune activation in an animal model of sepsis, enhancing survival compared to nonspecific nanoparticle controls.
Vaccination
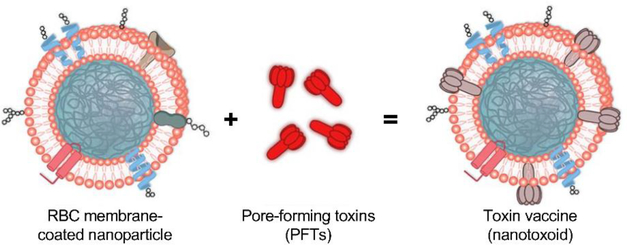
The successful detoxification of bacterial toxins using membrane-coated nanoparticles, which has been enabled by leveraging naturally occurring cell–biomolecule interactions, has also opened the door for the development of nanotoxoid formulations for antibacterial vaccination [59]. While conventional approaches for generating safe toxoids require denaturation methods that can alter the antigenicity of the original toxin, nanotoxoids synthesized using membrane-coated nanoparticle neutralization deliver toxins in their native form (Figure 4). In the first proof-of-concept work, RBC membrane-coated nanoparticles were used to neutralize staphylococcal α-hemolysin, and the resulting nanotoxoids were able to elicit potent and long-lasting antibody titers capable of neutralizing lethal doses of toxin in vivo [60]. It was later demonstrated that this approach could also be used to significantly attenuate live bacterial infections in a mouse model [61]. Because previous identification of the toxins is not required using this strategy, nanotoxoids can be easily created using crude mixtures of bacterium-secreted toxins as the antigenic material. When incubated with a hemolytic protein fraction from antibiotic-resistant bacteria, RBC-based nanosponges were able to completely inhibit the toxicity of the proteins [62]. When used to vaccinate mice, titers against multiple toxins were achieved, which helped to protect against live bacterial infection. This approach could easily be generalized to various cell membrane and bacterium combinations in the future. Beyond antibacterial applications, cell membrane-coated nanoparticles have also been used as anticancer vaccines. For example, cancer cell membrane-coated nanoparticles were loaded with an immunological adjuvant, and the resulting nanovaccine was able to interact with antigen-presenting cells to elicit multi-antigenic antitumor immunity [63]. When combined with checkpoint blockade therapy, the combination treatment was able to significantly control tumor growth in a therapeutic setting.
Figure 4.
Cell membrane-coated nanoparticles for toxoid vaccination. RBC membrane-coated nanoparticles can bind and neutralize pore-forming toxins secreted by bacteria. The resulting nanotoxoids can then be safely delivered as a vaccine, eliciting anti-toxin antibodies that can protect against infections. Adapted with permission from [60].
Conclusions
Cells, as one of the most fundamental units of biology, participate in a wide range of interactions within the body. Cell membrane-coated nanoparticles leverage these naturally occurring biological interactions in order to more effectively interface with other cells, the ECM, and various biomolecules. The cell–cell and cell–ECM interfaces are particularly important for delivery applications, where targeting specificity can help to more effectively localize payloads to specific organs or cell populations. Cell–biomolecule interactions are important for detoxification and vaccine design, and these are two areas in which cell membrane-coated nanoparticles hold significant potential. With the wide range of cell types that exist, membranes with unique surface marker profiles can be combined with almost any type of synthetic nanoparticulate core, depending on the desired application. In the future, it will also be possible to use fusion membranes to further fine-tune nanoparticle functionality [64]. All of this can be accomplished without having to specifically identify the exact proteins responsible, resulting in a function-driven approach to nanoengineering. It can be envisioned that continued research in biomimetic cell membrane coatings will lead to generalizable platforms with enhanced functionality that can be used to help tackle some of the most challenging problems in medicine.
Highlights.
Biological interactions on the nanoscale are critical for the in vivo performance of nanoparticles.
A central challenge is the reduction of nonspecific interactions while increasing specific interactions.
Biomimetic nanoengineering strategies are currently being developed to enhance nanoparticle functionality.
Cell membrane-coated nanoparticles have applications in drug delivery, detoxification, and vaccine design.
Acknowledgements
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Numbers HDTRA1–14-1–0064 and HDTRA1–18-1–0014, and the National Institutes of Health under Award Number R01CA200574.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Jong WH, Borm PJ: Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 2008, 3:133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain RK, Stylianopoulos T: Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010, 7:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh AJ, Kwon YJ: “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release 2011, 156:128–145. [DOI] [PubMed] [Google Scholar]
- 4.Fang RH, Zhang L: Nanoparticle-based modulation of the immune system. Annu Rev Chem Biomol Eng 2016, 7:305–326. [DOI] [PubMed] [Google Scholar]
- 5.Byrne JD, Betancourt T, Brannon-Peppas L: Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 2008, 60:1615–1626. [DOI] [PubMed] [Google Scholar]
- 6.Pelaz B, Charron G, Pfeiffer C, Zhao Y, de la Fuente JM, Liang XJ, Parak WJ, Del Pino P: Interfacing engineered nanoparticles with biological systems: anticipating adverse nano-bio interactions. Small 2013, 9:1573–1584. [DOI] [PubMed] [Google Scholar]
- 7.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Åberg C, Mahon E, Dawson KA: Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol 2013, 8:137–143. [DOI] [PubMed] [Google Scholar]
- 8.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE: Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29:1912–1919. [DOI] [PubMed] [Google Scholar]
- 9.Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M: Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater 2009, 8:543–557. [DOI] [PubMed] [Google Scholar]
- 10.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS: Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 2011, 6:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brannon-Peppas L, Blanchette JO: Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 2004, 56:1649–1659. [DOI] [PubMed] [Google Scholar]
- 12.Steichen SD, Caldorera-Moore M, Peppas NA: A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci 2013, 48:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Bennett A, Nees M, Fadeel B: In search of the holy grail: folate-targeted nanoparticles for cancer therapy. Biochem Pharmacol 2011, 81:976–984. [DOI] [PubMed] [Google Scholar]
- 14.Danhier F, Le Breton A, Préat V: RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm 2012, 9:2961–2973. [DOI] [PubMed] [Google Scholar]
- 15.Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D,Figa M, Figueiredo M, Horhota A, et al. : Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med 2012, 4:128ra–139.. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Lai SK: Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2015, 7:655–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehaini D, Fang RH, Zhang L: Biomimetic strategies for targeted nanoparticle delivery. Bioeng Transl Med 2016, 1:30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo JW, Irvine DJ, Discher DE, Mitragotri S: Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov 2011, 10:521–535. [DOI] [PubMed] [Google Scholar]
- 19.Kroll AV, Fang RH, Zhang L: Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjug Chem 2017, 28:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.**. Fang RH, Kroll AV, Gao W, Zhang L: Cell membrane coating nanotechnology. Adv Mater 2018, 30:1706759 In this review, the authors provide a comprehensive overview of cell membrane-coated nanoparticles, organized by their various applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao W, Zhang L: Coating nanoparticles with cell membranes for targeted drug delivery. J Drug Target 2015, 23:619–626. [DOI] [PubMed] [Google Scholar]
- 22.Fang RH, Jiang Y, Fang JC, Zhang L: Cell membrane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L: Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A 2011, 108:10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP: Role of CD47 as a marker of self on red blood cells. Science 2000, 288:2051–2054. [DOI] [PubMed] [Google Scholar]
- 25.Aryal S, Hu CM, Fang RH, Dehaini D, Carpenter C, Zhang DE, Zhang L: Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine (Lond) 2013, 8:1271–1280. [DOI] [PubMed] [Google Scholar]
- 26.Fang RH, Hu CM, Chen KN, Luk BT, Carpenter CW, Gao W, Li S, Zhang DE, Lu W, Zhang L: Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale 2013, 5:8884–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, et al. : Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol 2012, 8:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren X, Zheng R, Fang X, Wang X, Zhang X, Yang W, Sha X: Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92:13–24. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhang J, Chen W, Angsantikul P, Spiekermann KA, Fang RH, Gao W, Zhang L: Erythrocyte membrane-coated nanogel for combinatorial antivirulence and responsive antimicrobial delivery against Staphylococcus aureus infection. J Control Release 2017, 263:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao W, Hu CM, Fang RH, Luk BT, Su J, Zhang L: Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater 2013, 25:3549–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L: Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 2014, 14:2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, et al. : Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.*. Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang Q, Olson J, Luk BT, Zhang S, Fang RH, Gao W, Nizet V, et al. : Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci U S A 2017, 114:11488–11493. Macrophage membrane-coated nanoparticles are used to mitigate the immune activation that is characteristic of bacterial sepsis. This work demonstrates the utility of white blood cell membrane for the concurrent neutralization of both endotoxins and cytokines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.**. Zhu J-Y, Zheng D-W, Zhang M-K, Yu W-Y, Qiu W-X, Hu J-J, Feng J, Zhang X-Z: Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett 2016, 16:5895–5901. Using cancer cell membrane-coated nanoparticles, the preferential targeting of tumors for both drug delivery and imaging applications is demonstrated. This work leverages the homotypic targeting effect to localize membrane-coated nanoparticles to cells of the same origin. [DOI] [PubMed] [Google Scholar]
- 35.Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang P, Zhang Z, Yu H, Wang S, et al. : Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv Mater 2016, 28:9581–9588. [DOI] [PubMed] [Google Scholar]
- 36.Zhang N, Li M, Sun X, Jia H, Liu W: NIR-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery. Biomaterials 2018, 159:25–36. [DOI] [PubMed] [Google Scholar]
- 37.Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang Z, Yu H, Zhang P, Wang S, et al. : Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv Funct Mater 2016, 27:1604300. [Google Scholar]
- 38.Tian H, Luo Z, Liu L, Zheng M, Chen Z, Ma A, Liang R, Han Z, Lu C, Cai L: Cancer cell membrane-biomimetic oxygen nanocarrier for breaking hypoxia-induced chemoresistance. Adv Funct Mater 2017, 27:1703197. [Google Scholar]
- 39.Krishnamurthy S, Gnanasammandhan MK, Xie C, Huang K, Cui MY, Chan JM: Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale 2016, 8:6981–6985. [DOI] [PubMed] [Google Scholar]
- 40.Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, Li Y: Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016, 10:7738–7748. [DOI] [PubMed] [Google Scholar]
- 41.Gao C, Lin Z, Jurado-Sánchez B, Lin X, Wu Z, He Q: Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small 2016, 12:4056–4062. [DOI] [PubMed] [Google Scholar]
- 42.Bach JF: Immunosuppressive therapy of autoimmune diseases. Trends Pharmacol Sci 1993, 14:213–216. [DOI] [PubMed] [Google Scholar]
- 43.Luk BT, Jiang Y, Copp JA, Hu C-MJ, Krishnan N, Gao W, Li S, Fang RH, Zhang L: Biomimetic targeting of nanoparticles to immune cell subsets via cognate antigen interactions. Mol Pharm 2018, 15:3723–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pizarro-Cerda J, Cossart P: Bacterial adhesion and entry into host cells. Cell 2006, 124:715–727. [DOI] [PubMed] [Google Scholar]
- 45.Angsantikul P, Thamphiwatana S, Zhang Q, Spiekermann K, Zhuang J, Fang RH, Gao W,Obonyo M, Zhang L: Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection. Adv Ther 2018, 1:1800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siboo IR, Chambers HF, Sullam PM: Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect Immun 2005, 73:2273–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodvien R, Mielke CH: Role of platelets in hemostasis and thrombosis. Western J Med 1976, 125:181–186. [PMC free article] [PubMed] [Google Scholar]
- 48.Yasuda S, Noguchia T, Gohda M, Arai T, Tsutsui N, Nakayama Y, Matsuda T, Nonogi H: Local delivery of low-dose docetaxel, a novel microtubule polymerizing agent, reduces neointimal hyperplasia in a balloon-injured rabbit iliac artery model. Cardiovasc Res 2002, 53:481–486. [DOI] [PubMed] [Google Scholar]
- 49.*. Wei X, Ying M, Dehaini D, Su Y, Kroll AV, Zhou J, Gao W, Fang RH, Chien S, Zhang L: Nanoparticle functionalization with platelet membrane enables multifactored biological targeting and detection of atherosclerosis. ACS Nano 2018, 12:109–116. Using a platelet membrane coating, an imaging platform capable of localizing imaging payloads to atherosclerotic plaque is fabricated. This work highlights the utlity of platelet membrane for targeting disease-relevant substrates in cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Q, Qian C, Sun W, Wang J, Chen Z, Bomba HN, Xin H, Shen Q, Gu Z: Engineered nanoplatelets for enhanced treatment of multiple myeloma and thrombus. Adv Mater 2016, 28:9573–9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang RH, Luk BT, Hu CM, Zhang L: Engineered nanoparticles mimicking cell membranes for toxin neutralization. Adv Drug Deliv Rev 2015, 90:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Copp JA, Fang RH, Luk BT, Hu C-MJ, Gao W, Zhang K, Zhang L: Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci U S A 2014, 111:13481–13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei X, Gao J, Fang RH, Luk BT, Kroll AV, Dehaini D, Zhou J, Kim HW, Gao W, Lu W, et al. : Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials 2016, 111:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park SH: Diagnosis and treatment of autoimmune hemolytic anemia: classic approach and recent advances. Blood Res 2016, 51:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cines DB, Bussel JB, Liebman HA, Luning Prak ET: The ITP syndrome: pathogenic and clinical diversity. Blood 2009, 113:6511–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.**. Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, Fang RH, Gao W, Zhang L: Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol 2018, in press. Neutrophil membrane-coated nanoparticles are employed for the alleviation of disease symptoms in an animal model of rheumatoid arthritis. This work demonstrates the ability of membrane-coated nanosponges to interrupt endogenous immune signaling. [DOI] [PubMed] [Google Scholar]
- 57.*. Chen Y, Chen M, Zhang Y, Lee JH, Escajadillo T, Gong H, Fang RH, Gao W, Nizet V, Zhang L: Broad-spectrum neutralization of pore-forming toxins with human erythrocyte membrane-coated nanosponges. Adv Healthc Mater 2018, 7:e1701366 Using red blood cell membrane-coated nanosponges, a wide range of pore-forming toxins are successfully neutralized when administered to mice in lethal doses. This work highlights the broad, mechanism-based neutralization enabled by cell membrane coatings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu C-MJ, Fang RH, Copp J, Luk BT, Zhang L: A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol 2013, 8:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angsantikul P, Fang RH, Zhang L: Toxoid vaccination against bacterial infection using cell membrane-coated nanoparticles. Bioconjug Chem 2018, 29:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu C-MJ, Fang RH, Luk BT, Zhang L: Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol 2013, 8:933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang F, Fang RH, Luk BT, Hu CMJ, Thamphiwatana S, Dehaini D, Angsantikul P, Kroll AV, Pang ZQ, Gao WW, et al. : Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv Funct Mater 2016, 26:1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.**. Wei X, Gao J, Wang F, Ying M, Angsantikul P, Kroll AV, Zhou J, Gao W, Lu W, Fang RH, et al. : In situ capture of bacterial toxins for antivirulence vaccination. Adv Mater 2017, 29:1701644 Red blood cell membrane-coated nanoparticle-based nanotoxoids are fabricated using a crude mixture of toxins secreted by antibiotic-resistant bacteria. This work shows that the nanotoxoid strategy can safely be applied for anti-toxin vaccination, even when the exact profile and identify of bacteria-secreted toxins are unknown. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.*. Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, et al. : Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater 2017, 29:1703969 Cancer cell membrane-coated nanoparticles are loaded with a potent immunostimulatory adjuvant and used to treat tumors in conjunction with checkpoint blockade therapy. This is the first demonstration of employing membrane-coated nanoparticles for eliciting multi-antigenic antitumor immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.**. Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, et al. : Erythrocyte–platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater 2017, 29:1606209 Red blood cell and platelet membrane are fused together to fabricate nanoparticles with a hybrid coating. Combining the features from two different types of membrane has the potential to further enhance the functionality of future biomimetic nanoformulations. [DOI] [PMC free article] [PubMed] [Google Scholar]