Abstract
IL-32 exists as seven mRNA transcripts that can translate into distinct individual IL-32 variants with specific protein domains. These translated protein domains of IL-32 variants code for specific functions that allow for interaction with different molecules intracellularly or extracellularly. The longest variant is IL-32γ possessing 234 amino acid residues with all 11 protein domains, while the shortest variant is IL-32α possessing 131 amino acid residues with three of the protein domains. The first domain exists in 6 variants except IL-32δ variant, which has a distinct translation initiation codon due to mRNA splicing. The last eleventh domain is common domain for all seven IL-32 variants. Numerous studies in different fields, such as inflammation, autoimmunity, pathogen infection, and cancer biology, have claimed the specific biological activity of individual IL-32 variant despite the absence of sufficient data. There are 4 additional IL-32 variants without proper transcripts. In this review, the structural characteristics of seven IL-32 transcripts are described based on the specific protein domains.
Keywords: IL-32, Variants, mRNA transcript, Protein domains, mRNA splicing
SEVEN IL-32 VARIANTS WITH ELEVEN PROTEIN DOMAINS
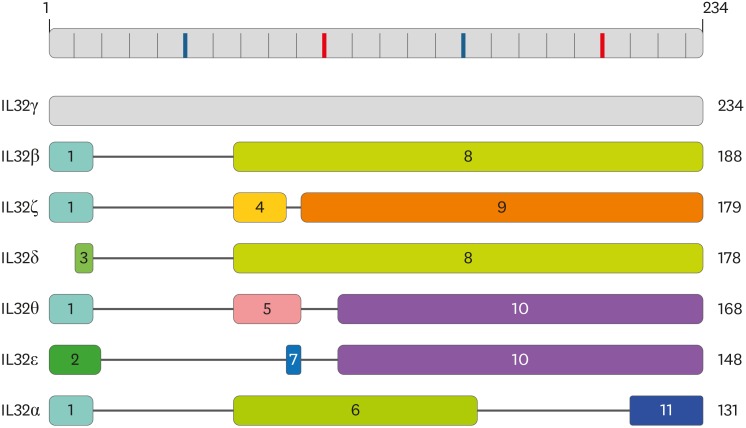
Seven IL-32 variant proteins are described in this review and each IL-32 variant is translated from its distinct messenger RNA (mRNA) transcript. An individual protein domain is numbered by the order of N-terminal initiation and early termination in conjunction with the gradated protein map on the top of Fig. 1. The seven IL-32 variant proteins are composed of any of 11 protein domains, which may be associated with a particular activity of individual IL-32 variant. The amino acid sequence of domain 3 and 11 exists in all seven IL-32 variants. Seven IL-32 variants were exhibited by the length of amino acid sequence and the number of amino acid residues is indicated on the right (Fig. 1). IL-32γ is the longest variant that consists of 234 amino acid residues while IL-32α is the shortest variant that consists of 131 amino acid residues. IL-32γ contains all of eleven protein domains and possesses the strongest biological activity in inducing inflammatory cytokines, whereas the shortest IL-32α variant contains three protein domains, domain 1, 6, and 11 (1). The biological activity of shortest IL-32α variant was originally examined because the yield of recombinant IL-32α protein was higher than other recombinant IL-32 variants protein (unpublished data). However, its activity in inducing inflammatory cytokines is weaker than IL-32γ variant (1,2,3).
Figure 1. Schematic drawing of seven IL-32 variants with gradated protein map. Seven IL-32 variants are composed of eleven protein domains. The longest variant is IL-32γ with 234 amino acid residues on the top while the shortest variant is IL-32α with 138 amino acid residues on the bottom. Each domain is numbered in line with the order of N-terminal initiation by earlier termination in conjunction with the gradated protein map at the top.
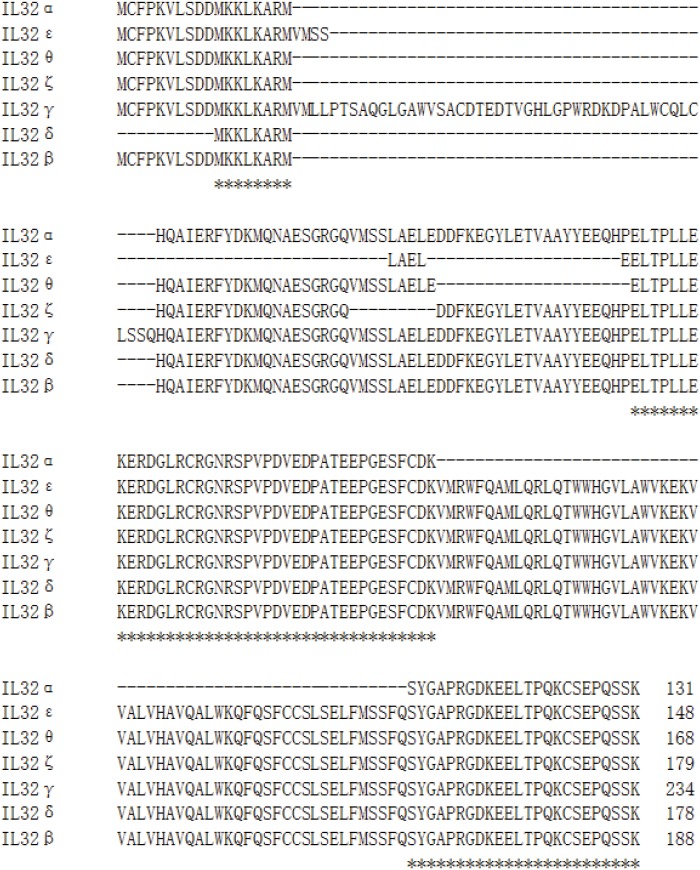
The original report characterized IL-32 as an inflammatory cytokine with four IL-32 variants such as IL-32α, IL-32β, IL-32γ, and IL-32δ (4). IL-32ε is the second shortest variant composed of 148 amino acid sequence containing protein domain 2, 7, and 10. The first domain of IL-32ε has four additional amino acid residues “VMSS” compared to the protein domain 1 of other variants. The protein domain 7 of IL-32ε is the shortest domain that consists of 4 amino acid residues “LAEL” (Fig. 2). IL-32δ has a distinct translation initiation codon due to mRNA splicing while it has domain 8 like IL-32β. Therefore, the biological activity of IL-32δ could be similar to IL-32β variant. It is necessary to investigate whether IL-32δ has a distinct transcriptional regulation since it uses a different translational initiation codon.
Figure 2. Multiple alignments of seven IL-32 variants using amino acid sequence. The amino acid sequences of seven IL-32 variants were aligned using program CLUSTALW at website (https://expasy.org/tools). The amino acid residues exist all of seven variants marked by symbol at the bottom.
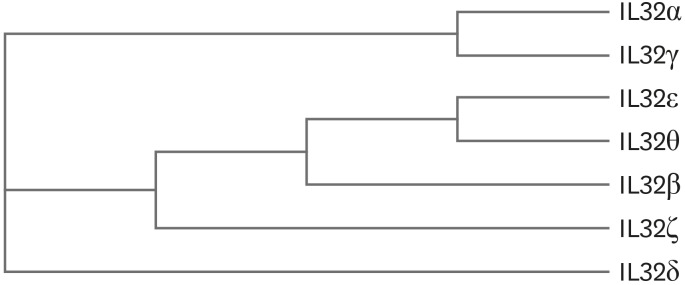
The third shortest variant is IL-32θ that is constituted by 168 amino acid residues. IL-32θ consists of protein domain 1, 5, and 10. The domain 10 exists in IL-32ε therefore IL-32θ probably has similar biological activity to IL-32ε. The phylogenetic tree of IL-32 variants in Fig. 3 suggested that IL-32θ is the closest variant to IL-32ε. These two variants are close to IL-32β. Interestingly, the result of phylogenetic tree showed that IL-32γ variant is the most close to IL-32α that is the shortest variant (Fig. 3). IL-32ζ consists of domain 1, 4, and 9 with 179 amino acid residues that is a single amino acid residue longer than IL-32δ though the first protein domain is different. IL-32ζ has unique domain 4 and 9, which are spliced from the domain 8 (Figs. 1 and 2).
Figure 3. The phylogenetic tree of seven IL-32 variants. The amino acid sequences of all seven IL-32 variants were used for drawing phylogenetic tree protein family. The program CLUSTALW was used at website (https://expasy.org/tools).
The second longest variant is IL-32β that consists of 188 amino acid residues. IL-32β variant has the common domain 1 that exists in four IL-32 variants such as IL-32α, IL-32β, IL-32θ, and IL-32ζ variant. IL-32β variant is constituted by two protein domain 1 and 8, which are spliced from IL-32γ variant since IL-32β transcript has been found in human IL-32γ variant overexpressed transgenic mouse (unpublished data). This data suggested that the splicing of full length IL-32γ transcript could generates IL-32β variant, which possesses 46 amino acid residues less than IL-32γ variant (Fig. 2). The biological activity of IL-32β is between IL-32α and IL-32γ (1).
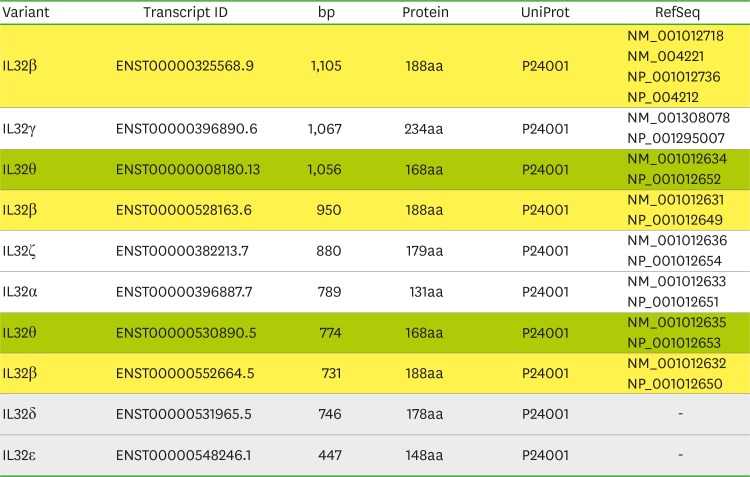
The mRNA transcript of seven IL-32 variants was pictured with essential information such as transcript and protein size in Fig. 4. The top row identified variant name, transcript identity (ID), mRNA transcript size by base pair (bp), protein length by the number of amino acid residue, accession number in UniPort, and accession number in database. IL-32β variant is the most abundant IL-32 transcript among seven IL-32 variant highlighted by yellow color that has four reference sequences in database. IL-32θ variant highlighted by green color has 2 reference transcripts with amino acid sequences in database. IL-32α, IL-32γ, and IL-32ζ variant have a single transcript with reference sequence, while IL-32δ and IL-32ε variant do not have the reference sequence of transcript in database (Fig. 4, highlighted by gray color).
Figure 4. The information of seven IL-32 transcripts. Four IL-32β variant transcripts with reference sequence in database are highlighted in yellow. IL-32β is the most abundant transcript among seven IL-32 variants. Next IL-32θ variant has 2 transcripts reference sequences in database highlighted in green. IL-32α, IL-32γ, and IL-32ζ have a single transcript with reference sequence in database whereas IL-32δ and IL-32ε do not have reference sequences in database highlighted with gray. The website (http://uswest.ensembl.org) information was modified and used for the Figure 4.
IL-32 VARIANTS IN DIFFERENT DISEASES
IL-32 is generally considered to be an inflammatory cytokine since early IL-32 research suggested that it is an inducer of TNFα. Therefore many investigators approached the effect of IL-32 in Th1 autoimmune-associated diseases such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis (5,6,7,8). The majority of clinical studies depend on the measurement of circulating IL-32 levels in the plasma or serum of patients comparing to normal individuals. The limitation of this type of study cannot distinguish IL-32 variants, which may possess a distinct potency in inflammatory activity as well as different functions in diseases.
Relatively small numbers of IL-32-associated Th2 autoimmune diseases have been reported (9,10,11). These studies were initiated later comparing to Th1 autoimmune diseases related with IL-32. Unlike IL-32-mediated Th1 autoimmune diseases, the mechanism of IL-32-associated Th2 autoimmune diseases is unclear and not well understood. The association of IL-32 activity in Th2 autoimmune disease may be an outcome of Th2 inflammation rather than a cause of the disease. Further investigation with specific IL-32 variants may clarify the role of IL-32 in Th2 autoimmune diseases, such as asthma and atopic dermatitis. Few reports with other autoimmune diseases have been studied with limited information (12).
Various reports with IL-32 in cancer have been investigated with the mouse model of human IL-32α, IL-32β, and IL-32γ variant overexpressed transgenic mice (5,13,14). These studies explained the importance of IL-32 role in cell growth by different mechanisms including cell apoptosis. IL-32 genes have been found in most mammals except rodent (15). Mouse IL-32 gene exists in chromosome, but its transcript has not found yet (unpublished data). Human IL-32 variants overexpressed transgenic mouse data have some limitation although human recombinant IL-32 variant proteins have activities with different mouse cell lines as well as primary immune cells such as bone marrow and spleen cells. It is necessary to identify a rodent transcript of IL-32 in order to generate genetically modified animal for mouse model of different diseases in the future.
Since IL-32 transcripts were originally found in activated T-cells or NK cells it has important functions in pathogen infections-associated with Th1-mediated immune response (16). The roles of IL-32 against viral and intracellular bacterial infections have been studied by different research groups (12,17,18,19,20,21,22). IL-32 is involved in immune cell differentiation and proliferation during pathogen infections. Th1-mediated immune responses after viral and intracellular bacterial pathogen infections activate T or NK cells producing IL-32 variants. The immune cells-produced IL-32 variants mainly act on myeloid type immune cells (1,2,10,23) due to expression of proteinase 3 (PR3), whereas lymphoid cells do not respond to IL-32 (unpublished data). PR3 is known as one of neutrophil serine proteinase expressed in monocyte, but not expressed in lymphoid cells. PR3 binds to IL-32 and modulate IL-32 activity. PR3 is the only molecule that was confirmed as an IL-32 interacting cell surface molecule with biochemical data (8,24). This data support that IL-32 exhibits biological activity with only myeloid cells in inducing inflammatory cytokines.
CONCLUSION
Over three hundred publications have been reported with IL-32 since the first publication in 2005. The most studied variant is IL-32γ followed by IL-32α and IL-32β variant. IL-32β variant is the most abundant variant in Fig. 4, though less numbers of studies. There is some limitation to identify IL-32 variant at protein level because the lack of specific antibody to detect IL-32 variant. IL-32 variant expression pattern and its regulation could be identified with variant specific primers rather than protein level. There are four additional IL-32 variants in the absence of report (data not shown). Further investigation of IL-32 variant expression pattern and characterization of IL-32 variant domain specific activity in human diseases will help understand to clarify IL-32 variant-associated autoimmune and infectious diseases.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF-2015R1A2A2A01003472, NRF-2014M3A6A4075058, and NRF-2015R1A2A1A15051472); BK21 Plus Project fund.
Abbreviations
- bp
base pair
- mRNA
messenger RNA
- PR3
expression of proteinase 3
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Methodology: Kim S, Lee S.
- Supervision: Kim S.
- Validation: Nguyen TT, Kim S, Shim S, Lee S, Lee Y, Jhun H.
- Writing - original draft: Sohn DH, Nguyen TT, Kim J, Kim S.
- Writing - review & editing: Azam T, Kim S.
References
- 1.Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, Choi WS, Kim BK, Lee CK, Yoon DY, et al. Identification of the most active interleukin-32 isoform. Immunology. 2009;126:535–542. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho KA, Jun YH, Suh JW, Kang JS, Choi HJ, Woo SY. Orientia tsutsugamushi induced endothelial cell activation via the NOD1-IL-32 pathway. Microb Pathog. 2010;49:95–104. doi: 10.1016/j.micpath.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, Turato G, Lokar-Oliani K, Papi A, Zuin R, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFα. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Bae S, Hong J, Ryoo S, Jhun H, Hong K, Yoon D, Lee S, Her E, Choi W, et al. Paradoxical effects of constitutive human IL-32γ in transgenic mice during experimental colitis. Proc Natl Acad Sci U S A. 2010;107:21082–21086. doi: 10.1073/pnas.1015418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S. Interleukin-32 in inflammatory autoimmune diseases. Immune Netw. 2014;14:123–127. doi: 10.4110/in.2014.14.3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon OC, Kim S, Hong S, Lee CK, Yoo B, Chang EJ, Kim YG. Role of IL-32 gamma on bone metabolism in autoimmune arthritis. Immune Netw. 2018;18:e20. doi: 10.4110/in.2018.18.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, Choi DK, Kwak A, Kim S, Nguyen TT, Gil G, Kim E, Yoo KH, Kim IA, Lee Y, et al. IL-32-induced inflammatory cytokines are selectively suppressed by α1-antitrypsin in mouse bone marrow cells. Immune Netw. 2017;17:116–120. doi: 10.4110/in.2017.17.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheon S, Lee JH, Park S, Bang SI, Lee WJ, Yoon DY, Yoon SS, Kim T, Min H, Cho BJ, et al. Overexpression of IL-32alpha increases natural killer cell-mediated killing through up-regulation of Fas and UL16-binding protein 2 (ULBP2) expression in human chronic myeloid leukemia cells. J Biol Chem. 2011;286:12049–12055. doi: 10.1074/jbc.M110.159756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong HJ, Oh HA, Lee BJ, Kim HM. Inhibition of IL-32 and TSLP production through the attenuation of caspase-1 activation in an animal model of allergic rhinitis by Naju Jjok (Polygonum tinctorium) Int J Mol Med. 2014;33:142–150. doi: 10.3892/ijmm.2013.1548. [DOI] [PubMed] [Google Scholar]
- 11.Bang BR, Kwon HS, Kim SH, Yoon SY, Choi JD, Hong GH, Park S, Kim TB, Moon HB, Cho YS. Interleukin-32γ suppresses allergic airway inflammation in mouse models of asthma. Am J Respir Cell Mol Biol. 2014;50:1021–1030. doi: 10.1165/rcmb.2013-0234OC. [DOI] [PubMed] [Google Scholar]
- 12.Bae S, Kang D, Hong J, Chung B, Choi J, Jhun H, Hong K, Kim E, Jo S, Lee S, et al. Characterizing antiviral mechanism of interleukin-32 and a circulating soluble isoform in viral infection. Cytokine. 2012;58:79–86. doi: 10.1016/j.cyto.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Jhun H, Choi J, Hong J, Lee S, Kwak A, Kim E, Jo S, Ryoo S, Lim Y, Yoon DY, et al. IL-32γ overexpression accelerates streptozotocin (STZ)-induced type 1 diabetes. Cytokine. 2014;69:1–5. doi: 10.1016/j.cyto.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Lee S, Kwak A, Kim E, Jo S, Bae S, Lee Y, Ryoo S, Choi J, Kim S. Interleukin-32γ transgenic mice resist LPS-mediated septic shock. J Microbiol Biotechnol. 2014;24:1133–1142. doi: 10.4014/jmb.1404.04012. [DOI] [PubMed] [Google Scholar]
- 15.Jaekal J, Jhun H, Hong J, Park S, Lee J, Yoon D, Lee S, Her E, Yang Y, Rho G, et al. Cloning and characterization of bovine interleukin-32 beta isoform. Vet Immunol Immunopathol. 2010;137:166–171. doi: 10.1016/j.vetimm.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992;148:597–603. [PubMed] [Google Scholar]
- 17.Bai X, Kim SH, Azam T, McGibney MT, Huang H, Dinarello CA, Chan ED. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J Immunol. 2010;184:3830–3840. doi: 10.4049/jimmunol.0901913. [DOI] [PubMed] [Google Scholar]
- 18.Bai X, Ovrutsky AR, Kartalija M, Chmura K, Kamali A, Honda JR, Oberley-Deegan RE, Dinarello CA, Crapo JD, Chang LY, et al. IL-32 expression in the airway epithelial cells of patients with Mycobacterium avium complex lung disease. Int Immunol. 2011;23:679–691. doi: 10.1093/intimm/dxr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenk M, Krutzik SR, Sieling PA, Lee DJ, Teles RM, Ochoa MT, Komisopoulou E, Sarno EN, Rea TH, Graeber TG, et al. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med. 2012;18:555–563. doi: 10.1038/nm.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semango G, Heinhuis B, Plantinga TS, Blokx WA, Kibiki G, Sonda T, Mavura D, Masenga EJ, Nyindo M, van der Ven AJ, et al. Exploring the role of IL-32 in HIV-related Kaposi sarcoma. Am J Pathol. 2018;188:196–203. doi: 10.1016/j.ajpath.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 21.Akuzum B, Kim S, Nguyen TT, Hong J, Lee S, Kim E, Kim J, Choi Y, Jhun H, Lee Y, et al. L1 recombinant proteins of HPV tested for antibody forming using sera of HPV quadrivalent vaccine. Immune Netw. 2018;18:e19. doi: 10.4110/in.2018.18.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai X, Aerts SL, Verma D, Ordway DJ, Chan ED. Epidemiologic evidence of and potential mechanisms by which second-hand smoke causes predisposition to latent and active tuberculosis. Immune Netw. 2018;18:e22. doi: 10.4110/in.2018.18.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci U S A. 2008;105:3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci U S A. 2006;103:3316–3321. doi: 10.1073/pnas.0511206103. [DOI] [PMC free article] [PubMed] [Google Scholar]