Abstract
Purpose
An unresolved inflammatory state contributes to the pathogenesis of periodontal disease and metabolic syndrome (MetS). Therefore, the purpose of this study was to evaluate the role of lipoxin A4 (LXA4), a proresolving lipid mediator, in the association between periodontal disease and MetS.
Methods
Sixty-seven patients with MetS and 65 patients without MetS were included in the study. Sociodemographic information was obtained via a questionnaire, and detailed medical diagnoses were made. Periodontal parameters (plaque index [PI], gingival index [GI], probing pocket depth [PD], and clinical attachment level [CAL]) and metabolic parameters were measured, and serum LXA4 levels were determined. The associations among MetS, periodontal parameters, and serum LX levels were evaluated by adjusted multivariate linear regression analyses.
Results
Patients with MetS were older and had a higher body mass index than patients without MetS. Periodontal parameters (PI, GI, PD, and CAL) were higher in patients with MetS than in those without MetS. Serum LXA4 levels were higher in patients without MetS. Multivariate linear regression analysis indicated a positive association between MetS and periodontal parameters (PD and CAL). Negative associations were established between MetS and LXA4 levels, and between LXA4 and periodontal parameters (PI, PD, and CAL).
Conclusions
The presence of higher values of periodontal parameters in patients with MetS and the negative relationship of LXA4 with MetS and periodontal disease may support the protective role of proresolving lipid mediators in the association between periodontal disease and MetS.
Keywords: Inflammation, Lipoxins, Metabolic syndrome X, Periodontal diseases
Graphical Abstract
INTRODUCTION
Metabolic syndrome (MetS), characterized by a constellation of metabolic risk factors including abdominal obesity, dyslipidemia, hypertension, and impaired fasting glucose, constitutes a major risk for the development of atherosclerotic cardiovascular diseases [1]. Periodontitis is a dysbiotic inflammatory disease that causes an exaggerated inflammatory response to pathogenic microbiota, resulting in loss of periodontal tissues [2]. Periodontitis may elevate the systemic inflammatory burden via ulcerations in the pocket wall [3], and it has been confirmed that the proinflammatory state caused by periodontitis can contribute to an increased risk of insulin resistance and cardiovascular disease [4].
A number of studies have indicated that patients with MetS have higher values of periodontal parameters [5,6,7,8,9,10], and many have shown that MetS affects periodontal disease [11,12]. Although the underlying pathologic mechanisms are controversial, the systemic release of periodontal bacteria [13], oxidative stress [14], and proatherogenic lipoproteins [15] may play a role in this association.
Low-grade systemic inflammation occurs in several diseases, such as coronary heart disease, diabetes mellitus, and hypertension, and an imbalance in oxidative and inflammatory response has been reported to play a role in the pathogenesis of those conditions [16]. The persistence of inflammation causes tissue destruction [17], and the complete resolution of an inflammatory response has been suggested as a necessity for ongoing health [18]. The functional return to homeostasis after an inflammatory injury is termed the resolution of inflammation, and it is mediated by proresolving lipid mediators. Lipoxins (LXs), which are a class of anti-inflammatory and proresolving lipid mediators derived from arachidonic acid, can inhibit leukocyte chemotaxis, adherence, and activation, and antagonize proinflammatory leukotrienes [16]. Furthermore, LXs have antioxidant activities [19] and are also known to induce neutrophil apoptosis [17]. Studies have revealed that LXs play a protective role in MetS [16,20] and periodontitis [21,22,23,24,25,26].
We hypothesized that lipoxin A4 (LXA4) may have a protective function in the association between MetS and periodontal disease. The aim of this study was to compare the periodontal status of patients with MetS and without MetS and to evaluate the potential role of LXA4 in this relationship.
MATERIALS AND METHODS
The study was approved by the Süleyman Demirel University Faculty of Medicine Clinical Research Ethics Committee, Isparta, Turkey (date: February 11, 2015, number: 33) in accordance with the Declaration of Helsinki, which was revised in 2013.
Sixty-seven patients who had MetS (32 men, 35 women) and 65 sex-matched persons who did not have MetS (32 men, 33 women) referred to Süleyman Demirel University Hospital, Department of Internal Medicine for a detailed medical examination received a consultation at Süleyman Demirel University Department of Periodontology. Written consent forms were obtained, and sociodemographic information including age (years), education level (primary school, high school, or university), monthly family income (<1,500, 1,500–2,500, or >2,500 Turkish lira [TRY]), and brushing frequency (2–3 times/day, 1 time/day, or <1 time/day) was collected via questionnaire.
Women who were pregnant or lactating at the time of the study; patients with a history of chemotherapy or radiotherapy, surgical menopause, and/or hormone replacement therapy; patients with any systemic disorders other than the MetS criteria that may have affected MetS or periodontal disease, such as respiratory, cardiovascular, liver, or renal diseases, osteopenia, or osteoporosis; patients using bisphosphonates; current or former smokers; patients who had undergone systemic antibiotic administration within the previous 3 months, periodontal treatment within the last 6 months, or drug-induced gingival enlargement; patients with aggressive periodontitis; and patients with <8 teeth (<2 teeth in each quadrant) were excluded.
Periodontal parameters
A periodontal examination of all teeth was conducted by 2 calibrated dentists (E.D. and B.D.), including measurements of the probing pocket depth (PD), clinical attachment level (CAL), plaque index (PI) [27], and gingival index (GI) [28]. PD and CAL were measured at 6 sites (buccal and lingual aspects, each with mesial, median, and distal points), and PI and GI were evaluated at 4 sites (mesio-buccal, mid-buccal, disto-buccal, and mid-lingual) around each tooth using a periodontal probe (Williams periodontal probe; Hu-Friedy, Chicago, IL, USA). Periodontal disease was diagnosed based on the classification of the American Academy of Periodontology [29]. The inter-examiner and intra-examiner correlation coefficients were found to be 0.85 and 0.90 for PD and 0.83 and 0.89 for CAL, respectively. Weighted kappa values (±1 mm) ranged from 0.84 to 0.92 for PD and 0.82 to 0.90 for CAL.
Metabolic parameters
Participants were instructed not to eat or drink for at least 8 hours prior to sampling. Anthropometric and metabolic parameters, including fasting blood glucose (FBG), glycated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol, were determined at Süleyman Demirel University Faculty of Medicine, Department of Internal Medicine, Division of Endocrinology. Body mass index (BMI) and waist circumference (WC) were measured. BMI was calculated as the body weight (kg) divided by the height squared (m2) [30]. Systolic and diastolic blood pressure (BP) measurements were taken in a sitting position from the participant's right arm [31]. MetS was determined using National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria. The NCEP ATP III criteria for MetS were met if a patient had 3 or more of the following risk factors: WC>88 cm in women and >102 cm in men; hypertension (systolic BP>130 and/or diastolic BP>85 mmHg) or a history of antihypertensive medication usage; hypertriglyceridemia (TG≥150 mg/dL) or a history of antilipemic medication usage; HDL<50 mg/dL in women and <40 mg/dL in men; and FBG≥110 mg/dL or on treatment for diabetes [1].
Serum LXA4 levels
After 8 hours of fasting, blood samples were collected from the antecubital vein and centrifuged at 3000 rpm for 15 minutes. Serum samples were collected in Eppendorf tubes and stored at −80°C until analysis. Serum LXA4 levels were determined using a commercial enzyme-linked immunosorbent assay kit (Cusabio Biotech, Catalog number: CSB-E09689h, Wuhan, China) according to the manufacturer's instructions based on the principle of the quantitative sandwich enzyme immunoassay technique. Color changes were measured spectrophotometrically at 450 nm with the correction wavelength set at 540 nm. The level of LXA4 was expressed as pg/mL.
Statistical analyses
A statistical package program (SPSS version 21.0 for Windows; IBM Corp., Armonk, NY, USA) was used for the statistical analyses. Sample size software (G*power version 3.1.9.2 for Windows; University of Kiel, Kiel, Germany) was used to determine the achieved power. A power of >95% at the α=0.05 level for periodontal parameters (GI, PD, CAL) and serum LXA4 levels was observed.
The normality of the distribution was analyzed using the Kolmogorov-Smirnov test, and the Levene test of homogeneity of variance was carried out. None of the evaluated parameters was normally distributed. The Mann-Whitney U test for BMI, FBG, HbA1c, serum lipid parameters, periodontal parameters, and serum LXA4 levels and the χ2 test for categorical variables were used for comparisons between groups.
Multivariate linear regression analyses were applied to characterize the associations among periodontal parameters, MetS, and serum LXA4 levels. The effect of covariates including age, BMI, and periodontal parameters, which were different between study groups, was adjusted. The following 3 models were created: model 1, adjusted for age and BMI; model 2, model 1 + MetS; model 3, model 1 + periodontal parameters. P values <0.05 were considered to indicate statistical significance.
RESULTS
While there were no significant differences regarding sex, family income, tooth brushing frequency, and education level (P>0.05), age and BMI were higher in the participants with MetS than in those without MetS (P=0.000). The frequency of the NCEP ATP III criteria was significantly higher in the MetS group (P<0.05). Metabolic parameters (FBG, HbA1c, TC, and TG) were higher in the participants with MetS than in those without MetS (P<0.05), and no significant difference regarding HDL and LDL was observed between groups (P>0.05) (Table 1).
Table 1. Sociodemographic and metabolic parameters of the groups.
| Variables | No MetS (n=65) | MetS (n=67) | P valuea) | |
|---|---|---|---|---|
| Age (yr) | 37.46±10.01 | 48.43±8.45 | 0.000 | |
| Gender | 0.866 | |||
| Women | 33 (50.8) | 35 (52.2) | ||
| Men | 32 (49.2) | 32 (47.8) | ||
| Education level | 0.063 | |||
| Primary school | 21 (32.3) | 30 (44.8) | ||
| High school | 25 (38.5) | 26 (38.8) | ||
| University | 19 (29.2) | 11 (16.4) | ||
| Family income (TRY) | 0.711 | |||
| <1,500 | 46 (70.8) | 44 (65.7) | ||
| 1,500–2,500 | 10 (15.4) | 14 (20.9) | ||
| >2,500 | 9 (13.8) | 9 (13.4) | ||
| BMI (kg/m2) | 24.77±4.08 | 31.77±6.74 | 0.000 | |
| Tooth brushing (times/day) | 0.887 | |||
| 2–3 | 9 (13.8) | 9 (13.4) | ||
| 1 | 16 (24.6) | 19 (28.4) | ||
| <1 | 40 (61.5) | 39 (58.2) | ||
| NCEP ATP III criteria | ||||
| High WC | 22 (25.9) | 63 (74.1) | 0.000 | |
| High BP or medication usage | 1 (3.4) | 28 (96.6) | 0.000 | |
| High TG or medication usage | 10 (20) | 40 (80) | 0.000 | |
| Low HDL | 21 (38.9) | 33 (61.1) | 0.048 | |
| High FBG or medication usage | 6 (11.1) | 48 (88.9) | 0.000 | |
| FBG | 103.86±64.15 | 173.01±90.68 | 0.000 | |
| HbA1c | 6.47±1.70 | 8.99±3.26 | 0.002 | |
| TC | 170.98±36.91 | 193.66±47.07 | 0.005 | |
| TG | 130.32±84.64 | 217.67±129.02 | 0.000 | |
| HDL | 44.35±9.38 | 44.34±11.83 | 0.960 | |
| LDL | 100.65±27.75 | 107.87±41.94 | 0.262 | |
Continuous variables are shown as unadjusted mean±standard deviation; categorical variables are shown as number (%). Bold denotes statistical significance at P<0.05.
MetS: metabolic syndrome, TRY: Turkish lira, BMI: body mass index, NCEP ATP III: National Cholesterol Education Program Adult Treatment Panel III, WC: waist circumference, BP: blood pressure, TG: triglyceride, HDL: high-density lipoprotein, FBG: fasting blood glucose, HbA1c: hemoglobin A1c, TC: total cholesterol, LDL: low-density lipoprotein.
a)P values were computed with the χ2 test for categorical variables or the Mann-Whitney U test for continuous variables.
The comparisons of periodontal parameters and serum LXA4 levels between the groups are presented in Table 2. In the MetS group, serum LXA4 levels were lower than in the group without MetS, while all the periodontal parameters and the prevalence of patients with periodontitis were higher (P<0.05).
Table 2. Comparisons of periodontal parameters and serum LXA4 levels between the groups.
| Variables | No MetS (n=65) | MetS (n=67) | P valuea) | |
|---|---|---|---|---|
| PI | 1.43±0.61 | 1.75±0.72 | 0.013 | |
| GI | 1.18±0.29 | 1.43±0.41 | 0.000 | |
| PD | 2.63±0.39 | 3.05±0.62 | 0.000 | |
| CAL | 2.73±0.62 | 3.43±0.94 | 0.000 | |
| LXA4 | 0.19±0.06 | 0.15±0.05 | 0.002 | |
| Periodontal diagnosis | 0.000 | |||
| Periodontitis | 24 (27.9) | 62 (72.1) | ||
| Gingivitis or healthy | 41 (89.1) | 5 (10.9) | ||
Continuous variables are shown as unadjusted mean±standard deviation; categorical variables are shown as number (%). Bold denotes statistical significance at P<0.05.
MetS: metabolic syndrome, PI: plaque index, GI: gingival index, PD: probing depth, CAL: clinical attachment level, LXA4: lipoxin A4.
a)P values were computed with the χ2 test for categorical variables or the Mann-Whitney U test for continuous variables.
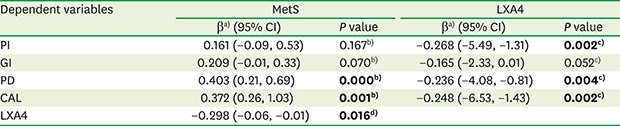
A positive relationship between periodontal parameters (PD and CAL) and MetS was demonstrated (P<0.05). In contrast, negative associations were found between periodontal parameters (PI, PD, and CAL) and serum LXA4 levels (P<0.05). Additionally, a negative relationship between MetS and serum LXA4 levels was identified (P<0.05) (Table 3).
Table 3. Multivariate linear regression analysis among periodontal parameters, MetS, and serum LXA4 levels.
| Dependent variables | MetS | LXA4 | ||
|---|---|---|---|---|
| βa) (95% CI) | P value | βa) (95% CI) | P value | |
| PI | 0.161 (−0.09, 0.53) | 0.167b) | −0.268 (−5.49, −1.31) | 0.002c) |
| GI | 0.209 (−0.01, 0.33) | 0.070b) | −0.165 (−2.33, 0.01) | 0.052c) |
| PD | 0.403 (0.21, 0.69) | 0.000b) | −0.236 (−4.08, −0.81) | 0.004c) |
| CAL | 0.372 (0.26, 1.03) | 0.001b) | −0.248 (−6.53, −1.43) | 0.002c) |
| LXA4 | −0.298 (−0.06, −0.01) | 0.016d) | ||
Bold denotes statistical significance at P<0.05.
MetS: metabolic syndrome, LXA4: lipoxin A4, PI: plaque index, GI: gingival index, PD: probing depth, CAL: clinical attachment level.
a)The standardized β coefficient represents the change in periodontal parameters and LXA4 levels (dependent variables) for each unit increase in the predictor variable (MetS or LXA4); b)Model 1 is adjusted for age and body mass index; c)Model 2 is adjusted for Model 1 + MetS; d)Model 3 is adjusted for Model 1 + periodontal parameters (PI, GI, PD, and CAL).
DISCUSSION
To our knowledge, this is the first study to evaluate the role of serum LXA4 levels in the relationship between MetS and periodontal disease. In this observational study, the higher values of periodontal parameters in the MetS group and positive associations between MetS and periodontal parameters (PD and CAL) support the proposal that MetS may contribute to periodontal status. Furthermore, the negative relationship between periodontal parameters (PI, PD, and CAL) and serum LXA4 levels, along with the negative association between serum LXA4 levels and MetS, may indicate that LXA4 plays a protective role, in accordance with our hypothesis.
Periodontal disease and MetS have some common shared risk factors. The potential underlying biological mechanisms in the relationship between periodontal disease and MetS need to be investigated, although systemic oxidative stress and an excessive inflammatory response are believed to be responsible for the relationship between periodontal disease and MetS [32]. Uncontrolled and unresolved inflammation has been shown to be involved in various chronic diseases such as diabetes mellitus, hypertension, and periodontitis [16]. In light of these studies, we hypothesized that LXA4, a proresolving lipid mediator, may play a role in the relationship between MetS and periodontal disease.
It has been suggested that deficient LXA4 levels may contribute to a chronic inflammatory state in adipose tissue and induce insulin resistance [20]. Enhancing the production of anti-inflammatory lipid mediators such as LXs may suppress the low-grade chronic inflammation seen in MetS [16]. Additionally, it has been reported that LXA4 may be useful for improving insulin sensitivity by decreasing adipose inflammation [33,34]. Decreased LXA4 levels in patients with MetS have been reported [20]. In a cohort study, Yu et al. [20] reported that low serum LXA4 levels were associated with an increased incidence of MetS. The results of our study support the findings of previous studies indicating a negative association between MetS and LXA4.
Periodontal diseases occur as a result of an immune response induced by microbial etiologic factors in order to protect the periodontal tissues. However, uncontrolled and unresolved inflammation seems to be the main factor contributing to the progression of periodontal disease and its commonality with other systemic diseases [35,36]. It has been reported that LXs play a protective role in periodontal inflammation and alveolar bone loss, and that sufficient quantities of LXs were capable of preventing periodontal tissue destruction [26]. Börgeson et al. [25] indicated that LXA4 may inhibit Porphyromonas gingivalis and can slow down the progression of chronic inflammatory diseases such as periodontitis and atherosclerosis. In an experimental periodontitis model, LXA4 administration improved periodontitis-related bone loss and clinical outcomes [26]. Similarly, a novel LX analog (benzo-LXA4) was reported to promote regeneration of hard and soft tissues lost to periodontitis [24]. It was found that patients with chronic periodontitis had lower gingival crevicular fluid LXA4 levels than patients with gingivitis [23]. Similarly to our results, Tarannum and Faizuddin [22] reported that patients with periodontitis had lower LXA4 ratios than controls, and negative correlations were found between LXA4 and PD and CAL. In contrast, our previous study reported increased LXA4 levels in systemically healthy patients with periodontitis than in controls [21]. In that study, we suggested that higher LXA4 levels may limit the severity of periodontal disease. It can be concluded from these studies that a failure to resolve inflammation in the periodontium may lead to periodontal disease destruction. Our current results, in which all possible confounders were adjusted, confirm those of previous studies that reported a protective role of LXs in periodontal disease pathogenesis.
A relationship between MetS and periodontal disease has been reported in many previous studies. Poor periodontal conditions were observed in patients with MetS [9]. Furthermore, when more metabolic risk factors were present, poorer periodontal parameters were reported [8,37,38]. Patients with MetS were reported to be nearly twice as likely to develop periodontitis than those without MetS [7], and it has been argued that periodontal disease should be considered part of MetS [39]. A positive association between MetS and periodontal parameters, including PD and CAL, was reported [5,6]. Additionally, an increase in PD was implied to affect MetS [10]. In a recent study, Kim et al. [40] reported a positive association between the severity of periodontitis and MetS in men. Additionally, it seemed that the prevalence of MetS (Table 1) and periodontitis (data not shown) increased with age in this study. Although some of these studies indicated that sex [40], periodontal disease severity [5,6,37,40], and the number of metabolic components [8,37,38] may play a role in this relationship, we found a positive association between MetS and periodontal disease independent of confounders such as age and BMI.
Our study was conducted on patients residing in the same local region of Turkey with a similar socioeconomic status and dietary habits; nonetheless, it has some limitations. In order to evaluate a causal relationship, it would be necessary to conduct a prospective cohort study among periodontally healthy patients and those with gingivitis or periodontitis; however, the goal of this observational study was to determine whether a causal relationship is plausible. We found that as age increased, the prevalence of MetS (Table 1) and periodontal disease severity (data not shown) increased. LXA4 levels may be affected by the synergistic effect of MetS and periodontal disease. In order to characterize the relationships among periodontal parameters, MetS, and LXA4 as precisely as possible, the effects of possible confounders such as age and BMI were adjusted via various regression models. However, the effects of drugs such as statins, duration of the disease, the number of MetS risk factors, and menopausal status, all of which may have affected the study results, were not evaluated. The mild to moderate periodontal status of the patients limited our ability to comment on the effects of periodontal disease severity on MetS and LXA4 levels.
In conclusion, the presence of higher values of periodontal parameters in patients with MetS, independent of confounders, and the finding of a negative relationship of LXA4 with MetS and periodontal disease may support the hypothesis that proresolving lipid mediators play a protective role in the association between periodontal disease and MetS. Larger population-based studies additionally evaluating periodontal disease severity will provide a better understanding of the pathogenetic mechanisms underlying this relationship.
ACKNOWLEDGMENTS
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article. We also thank Mr. Hakan Doğangönül for performing the laboratory analyses and Dr. Banu Kale for helping with the medical diagnoses.
Footnotes
- Conceptualization: Esra Sinem Kemer Doğan.
- Data curation: Esra Sinem Kemer Doğan, Burak Doğan.
- Formal analysis: Burak Doğan.
- Investigation: Esra Sinem Kemer Doğan, Burak Doğan.
- Methodology: Esra Sinem Kemer Doğan, Burak Doğan, Özlem Fentoğlu, Fatma Yeşim Kırzıoğlu.
- Writing - original draft: Esra Sinem Kemer Doğan, Burak Doğan.
- Writing - review & editing: Özlem Fentoğlu, Fatma Yeşim Kırzıoğlu.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40(Suppl 14):S113–S134. doi: 10.1111/jcpe.12059. [DOI] [PubMed] [Google Scholar]
- 5.Khader Y, Khassawneh B, Obeidat B, Hammad M, El-Salem K, Bawadi H, et al. Periodontal status of patients with metabolic syndrome compared to those without metabolic syndrome. J Periodontol. 2008;79:2048–2053. doi: 10.1902/jop.2008.080022. [DOI] [PubMed] [Google Scholar]
- 6.Fukui N, Shimazaki Y, Shinagawa T, Yamashita Y. Periodontal status and metabolic syndrome in middle-aged Japanese. J Periodontol. 2012;83:1363–1371. doi: 10.1902/jop.2012.110605. [DOI] [PubMed] [Google Scholar]
- 7.Nibali L, Tatarakis N, Needleman I, Tu YK, D'Aiuto F, Rizzo M, et al. Clinical review: association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:913–920. doi: 10.1210/jc.2012-3552. [DOI] [PubMed] [Google Scholar]
- 8.Shimazaki Y, Saito T, Yonemoto K, Kiyohara Y, Iida M, Yamashita Y. Relationship of metabolic syndrome to periodontal disease in Japanese women: the Hisayama Study. J Dent Res. 2007;86:271–275. doi: 10.1177/154405910708600314. [DOI] [PubMed] [Google Scholar]
- 9.Li P, He L, Sha YQ, Luan QX. Relationship of metabolic syndrome to chronic periodontitis. J Periodontol. 2009;80:541–549. doi: 10.1902/jop.2009.080387. [DOI] [PubMed] [Google Scholar]
- 10.Morita T, Yamazaki Y, Mita A, Takada K, Seto M, Nishinoue N, et al. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol. 2010;81:512–519. doi: 10.1902/jop.2010.090594. [DOI] [PubMed] [Google Scholar]
- 11.Thanakun S, Watanabe H, Thaweboon S, Izumi Y. Association of untreated metabolic syndrome with moderate to severe periodontitis in Thai population. J Periodontol. 2014;85:1502–1514. doi: 10.1902/jop.2014.140105. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe K, Cho YD. Periodontal disease and metabolic syndrome: a qualitative critical review of their association. Arch Oral Biol. 2014;59:855–870. doi: 10.1016/j.archoralbio.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi T, Bandow K, Kakimoto K, Machigashira M, Matsuyama T, Matsuguchi T. Oxidative stress causes alveolar bone loss in metabolic syndrome model mice with type 2 diabetes. J Periodontal Res. 2009;44:43–51. doi: 10.1111/j.1600-0765.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo M, Cappello F, Marfil R, Nibali L, Marino Gammazza A, Rappa F, et al. Heat-shock protein 60 kDa and atherogenic dyslipidemia in patients with untreated mild periodontitis: a pilot study. Cell Stress Chaperones. 2012;17:399–407. doi: 10.1007/s12192-011-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das UN. Lipoxins, resolvins, protectins, maresins and nitrolipids, and their clinical implications with specific reference to diabetes mellitus and other diseases: part II. Clin Lipidol. 2013;8:465–480. [Google Scholar]
- 17.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 18.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Kebir D, József L, Pan W, Wang L, Petasis NA, Serhan CN, et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu D, Xu Z, Yin X, Zheng F, Lin X, Pan Q, et al. Inverse Relationship between Serum Lipoxin A4 Level and the Risk of Metabolic Syndrome in a Middle-Aged Chinese Population. PLoS One. 2015;10:e0142848. doi: 10.1371/journal.pone.0142848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doğan B, Fentoğlu Ö, Kırzıoğlu FY, Kemer ES, Köroğlu BK, Aksu O, et al. Lipoxin A4 and neutrophil/lymphocyte ratio: a possible indicator in achieved systemic risk factors for periodontitis. Med Sci Monit. 2015;21:2485–2493. doi: 10.12659/MSM.895115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarannum F, Faizuddin M. Association between lipoxin A4 and interleukin-12 in gingival crevicular fluid: a preliminary investigation. J Periodontal Res. 2017;52:210–217. doi: 10.1111/jre.12383. [DOI] [PubMed] [Google Scholar]
- 23.Lütfioğlu M, Aydoğdu A, Sakallioğlu EE, Alaçam H, Pamuk F. Gingival crevicular fluid interleukin-8 and lipoxin A4 levels of smokers and nonsmokers with different periodontal status: a cross-sectional study. J Periodontal Res. 2016;51:471–480. doi: 10.1111/jre.12324. [DOI] [PubMed] [Google Scholar]
- 24.Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, et al. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res. 2015;94:148–156. doi: 10.1177/0022034514557331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Börgeson E, Lönn J, Bergström I, Brodin VP, Ramström S, Nayeri F, et al. Lipoxin A4 inhibits porphyromonas gingivalis-induced aggregation and reactive oxygen species production by modulating neutrophil-platelet interaction and CD11b expression. Infect Immun. 2011;79:1489–1497. doi: 10.1128/IAI.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 27.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 28.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 29.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO technical report series 894. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 31.Ahn YB, Shin MS, Byun JS, Kim HD. The association of hypertension with periodontitis is highlighted in female adults: results from the Fourth Korea National Health and Nutrition Examination Survey. J Clin Periodontol. 2015;42:998–1005. doi: 10.1111/jcpe.12471. [DOI] [PubMed] [Google Scholar]
- 32.Lamster IB, Pagan M. Periodontal disease and the metabolic syndrome. Int Dent J. 2017;67:67–77. doi: 10.1111/idj.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Börgeson E, McGillicuddy FC, Harford KA, Corrigan N, Higgins DF, Maderna P, et al. Lipoxin A4 attenuates adipose inflammation. FASEB J. 2012;26:4287–4294. doi: 10.1096/fj.12-208249. [DOI] [PubMed] [Google Scholar]
- 35.Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol. 2008;79(Suppl):1601–1608. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasturk H, Kantarci A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol 2000. 2015;69:255–273. doi: 10.1111/prd.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita T, Ogawa Y, Takada K, Nishinoue N, Sasaki Y, Motohashi M, et al. Association between periodontal disease and metabolic syndrome. J Public Health Dent. 2009;69:248–253. doi: 10.1111/j.1752-7325.2009.00130.x. [DOI] [PubMed] [Google Scholar]
- 38.Kushiyama M, Shimazaki Y, Yamashita Y. Relationship between metabolic syndrome and periodontal disease in Japanese adults. J Periodontol. 2009;80:1610–1615. doi: 10.1902/jop.2009.090218. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura F, Soga Y, Iwamoto Y, Kudo C, Murayama Y. Periodontal disease as part of the insulin resistance syndrome in diabetic patients. J Int Acad Periodontol. 2005;7:16–20. [PubMed] [Google Scholar]
- 40.Kim OS, Shin MH, Kweon SS, Lee YH, Kim OJ, Kim YJ, et al. The severity of periodontitis and metabolic syndrome in Korean population: the Dong-gu study. J Periodontal Res. 2018;53:362–368. doi: 10.1111/jre.12521. [DOI] [PubMed] [Google Scholar]


