Abstract
Photochemical conversion of CO2 into fuels has promise as a strategy for storage of intermittent solar energy in the form of chemical bonds. However, higher-energy-value hydrocarbons are rarely produced by this strategy, because of kinetic challenges. Here we demonstrate a strategy for green-light-driven synthesis of C1–C3 hydrocarbons from CO2 and H2O. In this approach, plasmonic excitation of Au nanoparticles produces a charge-rich environment at the nanoparticle/solution interface conducive for CO2 activation, while an ionic liquid stabilizes charged intermediates formed at this interface, facilitating multi-step reduction and C–C coupling. Methane, ethylene, acetylene, propane, and propene are photosynthesized with a C2+ selectivity of ~50% under the most optimal conditions. Hydrocarbon turnover exhibits a volcano relationship as a function of the ionic liquid concentration, the kinetic analysis of which coupled with density functional theory simulations provides mechanistic insights into the synergy between plasmonic excitation and the ionic liquid.
Subject terms: Photocatalysis, Energy, Nanoparticles
While light-driven conversion of CO2 and H2O directly into fuels affords an attractive means to store sunlight in chemical bonds, few systems produce high-value hydrocarbons. Here, authors show gold nanoparticles to reduce CO2 to multi-carbon products using visible light, ionic liquids, and H2O.
Introduction
Carbon dioxide (CO2) fixation is recognized to be a much-needed component of a carbon-neutral energy strategy1–4. Although CO2 is relatively unreactive, various catalytic processes triggered by heat (thermochemical)5–8, electricity (electrochemical)9–17, and light (photochemical)18–28 are being explored for activating CO2 and recycling it back to valuable petrochemicals. Sunlight-driven conversion of CO2 to fuels is particularly attractive as a means to store intermittent solar energy in the form of C–C and C–H bonds. Semiconductor and metal-catalyzed photoelectrolytic reduction of CO2 has shown promise; however, these processes have often required ultraviolet (UV) light and/or considerable electrical energy input, or they do not favor energy-rich hydrocarbon products. Longer-chain hydrocarbons possess higher energy densities. Moreover, hydrocarbons in the liquid state are easier to transport29,30. However, the formation of longer-chain hydrocarbons from CO2 requires multiple electron (e–) and proton (H+) transfer steps, as well as C–C bond formation9,31,32, which pose major kinetic bottlenecks.
Here we demonstrate a visible-light-driven route for the conversion of CO2 and H2O into C1–C3 hydrocarbons. The scheme does not involve the application of an electrochemical potential, UV light, high temperatures, hydrogen gas, or a sacrificial agent. It uses green light as the sole energy input and driving agent. The strategy employs plasmonic Au nanoparticles (NPs) of a pseudospherical shape and an average diameter of ~12 nm, as characterized previously28. Au NPs are known from electrochemical studies33 to activate CO2. The choice of Au NPs was further driven by the relative chemical stability of Au against bulk oxidation and photocorrosion; the other two common plasmonic metals, Ag and Cu, while electrocatalytically active for CO2 reduction, are prone to oxidation in air, water, and/or light excitation. The Au NPs possess a strong localized surface plasmon resonance (LSPR) band centered around 520 nm (Fig. 1a), which enables strong, resonant absorption of green light. The LSPR excitation of the NPs yields energetic electron–hole (e––h+) carriers via Landau damping. These e––h+ carriers were shown in recent studies to drive redox conversions28,34–36, especially the conversion of CO2 to methane and ethane under blue–green light28. However, in this past demonstration, isopropanol was used as a sacrificial h+ scavenger to facilitate e––h+ pair separation; otherwise, unproductive e––h+ recombination dominated. Thus, isopropanol served as the H+ source in this CO2 reduction scheme, which posed a major limitation for net energy storage.
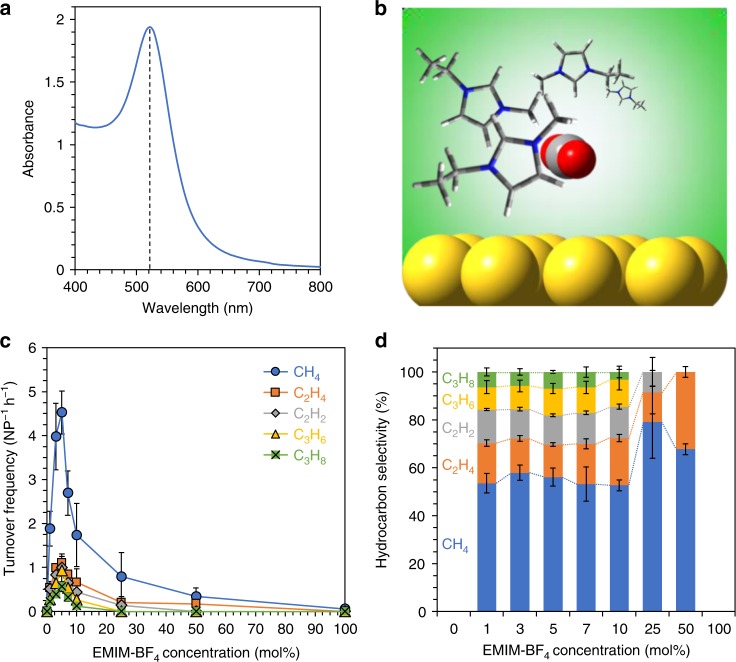
Fig. 1.
Ionic-liquid-promoted CO2 reduction to C1–C3 hydrocarbons using a plasmonic Au nanoparticle (NP) photocatalyst. a UV−vis extinction spectrum of a colloid of the Au NPs used for preparation of the photocatalyst film. The spectrum exhibits a localized surface plasmon resonance (LSPR) band centered around 520 nm, as indicated by the dotted line. b Scheme for CO2 conversion on plasmon-excited Au NPs promoted by an ionic liquid, EMIM-BF4. A continuous-wave (CW) laser of a wavelength of 532 nm and intensity of 1 W cm–2 was used as the light source for photoexcitation of Au NPs. EMIM-BF4 stabilizes CO2 and resulting adsorbates/intermediates on the photoexcited Au surface. c Turnover frequencies of hydrocarbon products formed in the CO2RR plotted as a function of the EMIM-BF4 concentration (mol%). The CO2 conversion activity peaks at 5 mol% of EMIM-BF4. d Hydrocarbon product selectivity as a function of EMIM-BF4 concentration (mol%). Each data point in c and d is the average of results from three identical trials and the error bar represents the SD of these measurements
The present strategy overcomes this drawback and uses water as the H+ source and does not require a sacrificial h+ scavenger, thus constituting a truly fuel-forming reaction. The enhanced reactivity was enabled by the use of an ionic liquid (IL) medium, specifically comprised 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIM-BF4). Our choice was motivated by examples from electrocatalytic CO2 reduction reaction (CO2RR) where the EMIM-BF4 electrolyte, owing to its highly ionic character, stabilizes the high-energy CO2•− radical anion intermediate formed in the reaction and decreases the overpotential needed for CO2RR37–42. In addition, EMIM-BF4 has a wide electrochemical window and high thermal stability43,44. In our photocatalytic scheme, the EMIM-BF4, as we find from kinetic analysis and density functional theory (DFT) simulations, promotes e– transfer at the interface of the photoexcited Au NP and adsorbed CO2 (Fig. 1b), obviating the need for a h+ scavenger or applied potential for e––h+ separation.
Results
IL-mediated plasmonic CO2 reduction
The photocatalyst had the form of a substrate-supported film of Au NPs immersed in an aqueous solution of EMIM-BF4 saturated with CO2 and contained inside a glass reactor (Supplementary Methods). The light excitation source comprised a continuous-wave (CW) laser of a wavelength of 532 nm light and an intensity of 1 W cm−2. Under CW excitation, the steady-state temperature of the reaction medium got moderately elevated to ~48 °C. Hydrocarbon products collected in the reactor headspace were measured (Supplementary Figs. 1−11) using a gas chromatograph (GC) equipped with a flame ionization detector. The EMIM-BF4 concentration was varied from 0 to 100 mol%, to find optimal conditions for CO2RR. In 1–10 mol% EMIM-BF4, the products of plasmon-excitation-driven CO2RR were found to be C1 (CH4), C2 (C2H4 and C2H2), and highly reduced C3 (C3H6 and C3H8) hydrocarbons (Fig. 1c, d and Supplementary Note 1). This product profile is quite striking when one considers that the major product in electrochemical CO2RR is carbon monoxide (CO) formed by 2e––2H+ reduction of CO2 (refs. 13–17). On the other hand, propane (C3H8), formed in our scheme, requires an overall 20e––20 H+ reduction and coupling of three CO2 molecules. Such generation of C3 hydrocarbons by artificial photosynthesis is challenging and therefore rare.
The CO2RR activity depends on the IL concentration (Fig. 1c). In pure water the activity was nil, whereas in 1 mol% EMIM-BF4 solution the generation of C1, C2, and C3 hydrocarbons was observed. The CO2RR activity, as quantified by turnover frequencies (TOFs) of the hydrocarbon products, increased dramatically with an increase in the EMIM-BF4 concentration. The highest activity was found at 5 mol% EMIM-BF4. Increasing the EMIM-BF4 concentration further resulted in a sharp drop in the CO2RR activity. In 100 mol% EMIM-BF4 solution, the activity was nil, similar to that in pure water. Thus, the CO2RR activity exhibits a volcano relationship as a function of the EMIM-BF4 concentration (Fig. 1c). At all EMIM-BF4 concentrations, where C1, C2, and C3 hydrocarbons were produced, the product selectivity was found to follow the order: C1 > C2 > C3. The selectivity for C2+ production is ~50% in 1−10 mol% EMIM-BF4 solution (Fig. 1d).
Non-hydrocarbon products were also characterized by a GC equipped with a thermal conductivity detector (TCD) (Supplementary Figs. 12–15). Considerable hydrogen (H2) production was measured (Supplementary Fig. 12), the TOF of which was 138.2 NP−1 h−1 in 5 mol% EMIM-BF4 solution, the IL concentration where CO2RR activity is the highest. The H2 likely originates from the competing reduction of H+ in the reaction medium (Supplementary Eq. (6)). In the GC-TCD measurements, there were no detection of CO (Supplementary Fig. 15), otherwise known to be a major product in electrocatalytic CO2RR on Au (refs. 13–17). Of the possible oxidation products, there was no measurable production of O2 (see Supplementary Information). H2O2 was detected (Supplementary Figs. 16–18) by the fluorogenic test employing a amplex red and horseradish peroxidase reagent45. Thus, the oxidation of H2O to H2O2 and H+ (2H2O → H2O2 + 2H+ + 2e−) is the likely oxidation half-reaction that consumes the photogenerated h+.
Control studies were performed, one without Au NPs, another without light, and a third without CO2. The conditions were otherwise maintained the same as those in the photoreaction tests and a 5 mol% EMIM-BF4 solution, found to be most optimal in the photoreaction tests, was employed. The control studies showed that the absence of any one of the components Au NPs, green light illumination, or CO2 resulted in nil hydrocarbon production, despite the use of 5 mol% EMIM-BF4 solution (Supplementary Fig. 19a–c). Thus, it is confirmed that the hydrocarbon production originates from green-light-driven CO2 reduction on Au NPs. The control study without light excitation was performed at an elevated temperature of 50 °C so as to mimic the steady-state bulk solution temperature of the reaction mixture in the photoreaction tests. The lack of CO2RR activity in this dark control study demonstrates that the CO2RR activity in the photoreaction tests does not originate from simply a photothermal effect of the light excitation. Rather a photoredox process facilitated by the Au NPs and the IL is responsible for the conversion of CO2 to hydrocarbons.
The plasmonic catalyst also exhibited stability and recyclability under the photoreaction conditions and IL media subjected on the catalyst. We tested the same substrate-supported Au NP film immersed in 5 mol% EMIM-BF4 over multiple cycles, each consisting of a 10 h photoreaction. The CO2RR activity and product selectivity, as determined from the TOFs of the hydrocarbon products, was maintained over the course of this multi-cycle test (Supplementary Fig. 20). As the NP film or EMIM-BF4 solution were not replenished between cycles, the maintenance of CO2RR activity over multiple cycles suggests that Au and EMIM-BF4 were not consumed, at any discernible levels, in the photoredox reaction.
The origin of products
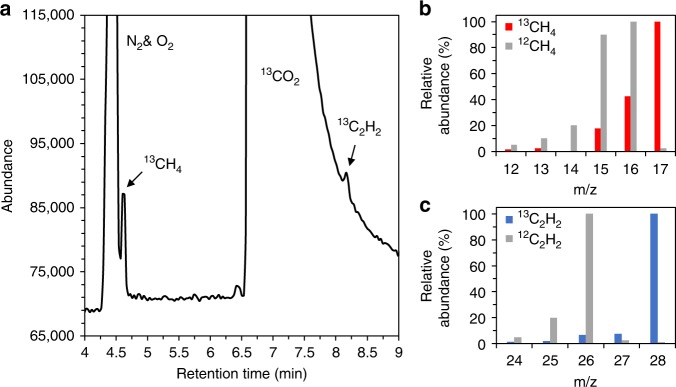
Given the hydrocarbon profile of the product mixture, it was necessary to go beyond the control studies described above and confirm more directly that CO2, rather than carbon contamination or photolysis of the EMIM-BF4, was the source of the hydrocarbon products. For this confirmation, 13C isotope labeling was employed (Fig. 2 and Supplementary Figs. 21 and 22). In this labeling study, 13CO2 was employed as the reactant instead of 12CO2, whereas all other conditions were kept the same as those in other photoreaction tests. GC-mass spectrometry (GC-MS) was used for identification of the hydrocarbon products generated in the photoreaction (Fig. 2a). The GC-MS analysis confirmed the presence of 13CH4 (Fig. 2b) and 13C2H2 (Fig. 2c), manifested by their characteristic mass fragmentation patterns, shifted to higher m/z compared with reference fragmentation patterns of 12CH4 and 12C2H2, respectively. Thus, isotope labeling confirms CO2 to be the origin of hydrocarbon products.
Fig. 2.
13CO2 isotopolog confirmation of CO2RR. a Total ion chromatogram (TIC) of the gaseous products from a 240 h long Au NP-photocatalyzed 13CO2RR in 5 mol% EMIM-BF4 solution under CW irradiation of 532 nm light (1 W cm–2). Peaks in the TIC appearing at retention times of 4.6 min and 8.2 min correspond to 13CH4 and 13C2H2. The basis for this assignment is provided in Supplementary Fig. 22. b Mass fragmentation pattern (red bars) acquired at a retention time of 4.6 min of the TIC shown in a. For comparison, a reference fragmentation pattern (gray bars) of 12CH4 from the National Institute of Standards and Technology (NIST) Chemistry WebBook is shown. Fragments at m/z = 14 and 18 in the experimental pattern were suppressed to remove the mass peaks contributed by N2 and moisture, respectively. c Mass fragmentation pattern (blue bars) acquired at a retention time of 8.2 min of the TIC shown in a. For comparison, a reference fragmentation pattern (gray bars) of 12C2H2 from the NIST Chemistry WebBook is shown. The fragment at m/z = 28 in the experimental pattern has relatively high abundance as compared with that of the reference fragmentation pattern due to the contribution of N2 from the atmosphere. Relative abundances in b and c were obtained from the measured abundances shown in Supplementary Fig. 21a, b, respectively. It is noteworthy that 13C2H4 was not resolved by GC-MS due to the likely overlap of the 13C2H4 peak with the broad, intense 13CO2 peak in the TIC (Supplementary Fig. 22b)
The role of the IL
We attempted to gain a mechanistic understanding of this catalytic scheme focusing on the question of how the IL promotes CO2RR activity. It was observed that the presence of EMIM-BF4 in the aqueous medium results in a considerably acidic pH (Supplementary Fig. 23): the 5 mol% EMIM-BF4 solution has a pH of 2.95. To determine whether this acidity is responsible for the enhanced CO2RR activity in a EMIM-BF4 solution, we performed a photoreaction in deionized water containing no EMIM-BF4 but with a pH of 2.93 achieved using acid (Supplementary Fig. 19d). All other conditions were kept the same as in the photoreactions in EMIM-BF4 solutions. In this EMIM-BF4-free photoreaction, no products were observed, which demonstrated that the high acidity or H+ concentration, [H+], of the EMIM-BF4-containing medium is not the sole cause of the enhanced CO2RR activity. EMIM-BF4 plays other role(s). It is possible, in principle, for EMIM-BF4, instead of H2O, to serve as the h+ acceptor; however, if this were the case, then the CO2RR activity would have been enhanced at higher EMIM-BF4 concentrations, in line with a study of a different plasmon excitation-catalyzed redox reaction36. Instead, we observed peak activity at a EMIM-BF4 concentration of 5 mol%, above which the activity drops steeply reaching nil in pure EMIM-BF4 wherein H2O is not available.
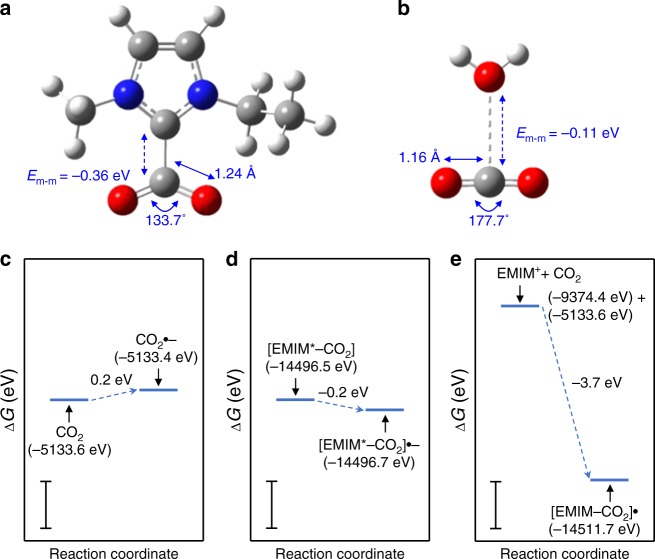
We hypothesized that the strongly ionic character of EMIM-BF4 plays a role in the activation of CO2, which is otherwise fairly redox inactive. CO2, however, is highly polarizable, as indicated by its quadrupole moment of −4.3 D Å (ref. 46). The interaction of EMIM-BF4 and CO2 was simulated by DFT. A past study suggests that CO2 can undergo complexation with the N-heterocyclic carbene, EMIM*, formed from EMIM+ by H+ loss42. We investigated using DFT the structure of such a [EMIM*-CO2] complex (Fig. 3a). The complex exhibits binding between the C atom of the CO2 and the C2 atom of the imidazole ring with an energy of intermolecular interaction, Em-m, of −0.36 eV. This interaction is stronger than, for instance, the interaction of an H2O molecule and CO2 (Fig. 3b). Unlike the latter case, complexation with EMIM* leads to considerable restructuring of the CO2 moiety. The CO2 moiety adopts a bent configuration with an O=C=O angle of 133.7° and C=O bonds lengthened to 1.24 Å. In fact, the geometry of the CO2 moiety in the complex closely mirrors that of the CO2•− anion radical, which has a bond angle of 137.8° and bond length of 1.23 Å (Supplementary Fig. 24). Moreover, from Mulliken charge partitioning analysis (Supplementary Fig. 25), the CO2 moiety in the [EMIM*-CO2] complex is found to have a net charge of −0.73, which indicates its partial anionic character.
Fig. 3.
The role of the ionic liquid in Au NP-photocatalyzed CO2RR. a,b DFT-optimized geometries of [EMIM*-CO2] (a) and [H2O-CO2] (b) complexes. C, H, O, and N atoms are depicted by gray, white, red, and blue spheres, respectively. Key bond lengths, bond angles, and the energy of intermolecular interaction, Em-m, are indicated for each of the complexes. c–e DFT-computed free energy cost, ∆G, of formation of the 1e− adduct of CO2 (c), 1e− adduct of [EMIM*-CO2] (d), and 1e− adduct of CO2 in the presence of EMIM+ (e). In the latter case, the 1e− adduct of CO2, CO2•−, is stabilized by complexation with EMIM+ as described by the net process: EMIM+ + CO2 + e−→ [EMIM-CO2]•. In c–e, the free energy of each species is indicated in parentheses. Scale bars are 1 eV in length
It is known that the energetic cost of the drastic structural reorganization from linear CO2 to the bent CO2•− anion radical poses a major barrier for e− acceptance by CO2 (refs. 37–42). However, our DFT calculations show that in its complex with EMIM*, the CO2 moiety is structurally pre-configured for e− acceptance. Consistent with this finding, 1e– addition to [EMIM*-CO2] is much more favorable as compared with 1e− addition to CO2 (Fig. 3c, d). Thus, it appears that EMIM-BF4 can promote the transfer of photogenerated e– from the Au NP to adsorbed CO2, which is otherwise a major kinetic bottleneck in the photocatalytic reduction process. Furthermore, it is plausible that the CO2•− anion radical formed on the Au surface by photo-initiated e– transfer process has an enhanced lifetime due to solvation or complexation by EMIM+ (Fig. 3e). A longer lifetime of this reactive intermediate would increase the probability of C–C coupling between the intermediates.
Empirical kinetic model
Although the DFT computations provide insight into the central role of EMIM-BF4 in CO2 activation, the volcano-type dependence of the CO2RR activity on the IL concentration deserves an explanation. From the hydrolysis of EMIM-BF4 known from past studies47–50:
| 1 |
where x = 1–4 and the complexation of CO2 with EMIM+ predicted in DFT simulations:
| 2 |
we postulate a rate determining step in the reaction of CO2 and H2O:
| 3 |
From this reaction equation, the concentration of the activated CO2 complex, [EMIM*-CO2], is expected to be directly proportional to [H+]x+1. Therefore, the [H+] determined from the measured pH of the EMIM-BF4 solution (Supplementary Fig. 23) serves as a proxy for the concentration of [EMIM*-CO2], based on which the [EMIM*-CO2] concentration is expected to be the highest in the EMIM-BF4 concentration range around 5 mol%. The higher the concentration of the activated [EMIM*-CO2] complex, the greater is the rate of CO2 conversion and also the higher the likelihood of C–C coupling required for C2+ production. Therefore, both the overall activity and the selectivity in favor of C2+ products are favorable in the 3–7 mol% EMIM-BF4 range, with the most optimal performance achieved at 5 mol% EMIM-BF4. On the other hand, the activated complex has zero concentration in pure water on one extreme and in pure EMIM-BF4 on the other extreme, which explains the nil turnover at these conditions. An additional reason for the drop in activity at higher EMIM-BF4 concentrations may be that the adsorption of BF4– to the Au NP surface (Supplementary Fig. 26) dominates at these concentrations to such an extent that the adsorption of CO2 and/or [EMIM*-CO2] to the Au surface is largely inhibited and so is the e− transfer to CO2.
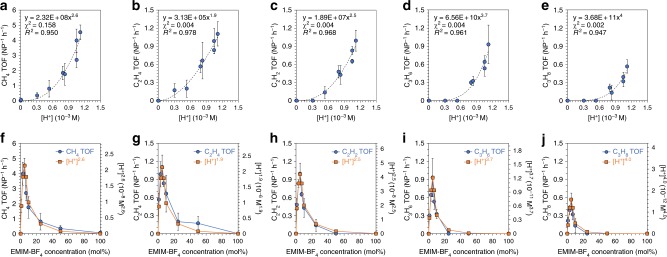
The CO2RR activity depends on the concentration of this activated complex to a high reaction order. This is best exemplified by the plots of TOF for each hydrocarbon as a function of the [H+] (Fig. 4a–e), which as explained above, serves as a proxy for the concentration of [EMIM*-CO2]. The pseudo-reaction order, n, is found to be 1.9 for C2H4, 2.5 for C2H2, 3.7 for C3H6, and 4.0 for C3H8. The fit for the CH4 TOF has a relatively high χ2-value, so the n of 2.7 estimated for CH4 has a lower confidence. In general, the pseudo-reaction order is higher for the longer hydrocarbons, which perhaps captures the need for multiple activated complexes to be available for undergoing coupling to C2 and C3 fragments. The high pseudo-reaction order for the C3 products goes hand-in-hand with an apparent threshold in [H+] below which the TOF is zero or below the detection limit (Fig. 4d, e). For each of the hydrocarbon products, the [H+] raised to the power of the corresponding n follows a volcano trend with respect to the EMIM-BF4 concentration, mirroring closely the trend in the TOF for that hydrocarbon (Fig. 4f–j).
Fig. 4.
Empirical kinetic model for the CO2RR. a–e For each of the hydrocarbon products, the TOF is plotted as a function of the H+ concentration, [H+], which serves as a proxy for the concentration of the [EMIM*-CO2] complex. A fit to a power-law function y = axn (dashed line) yields the fit parameter n, which represents the apparent reaction order in [H+]. The best-fit equation is indicated for each plot along with the values of χ2 and R2, which serve as metrics of the goodness-of-fit. f–j For each hydrocarbon product, the TOF plotted as a function of the EMIM-BF4 concentration follows a similar trend as the [H+]n, where n is the corresponding reaction order obtained from the plots in a–e. Each data point in a–j is the average of results from three identical trials and the error bar represents the SD of these measurements
Thus, we reported the green-light-driven synthesis of C1–C3 hydrocarbons from CO2 and water on plasmonic Au NPs in an IL medium. The resonant green light absorption of the plasmonic NPs and their ability to sustain electrostatically charged surfaces under resonant CW excitation are at the heart of the observed photoreactivity. The IL plays a synergistic role due to its complexation with the CO2, which preconfigures the CO2 for accepting e– from photoexcited Au NPs. The enhanced reactivity of CO2 in the presence of the IL obviates the need for an applied potential or a sacrificial scavenger. Although hydrocarbon production yields in the reaction need further optimization, the generation of propane by overall 20e−–20H+ reduction and coupling of three CO2 molecules is both striking and mechanistically rich. The precise intermediates and reaction pathways, including C–C coupling and dehydrogenation steps, which yield each of the hydrocarbons, deserve further elucidation. Beyond CO2 conversion studied here, ILs may have promise in other photocatalytic schemes where activation of relatively inert substrates and stabilization of high-energy charged intermediates is desirable.
Supplementary information
Acknowledgements
Funding for this work was provided by the Energy & Biosciences Institute (EBI) through the EBI-Shell program. GC-TCD calibration data were obtained from Andrew J. Wilson.
Author contributions
S.Y. performed all experimental studies, DFT computations, and data-analysis, and co-wrote the manuscript. P.K.J. conceived project and designed studies, analyzed results, and co-wrote the manuscript.
Data availability
All raw images and source data are available from the authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Jie Liu, Jianfang Wang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10084-5.
References
- 1.Lewis NS, Nocera DG. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA. 2006;103:15729–15735. doi: 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olah GA, Prakash GKS, Goeppert A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 2011;133:12881–12898. doi: 10.1021/ja202642y. [DOI] [PubMed] [Google Scholar]
- 3.Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature. 2012;488:294–303. doi: 10.1038/nature11475. [DOI] [PubMed] [Google Scholar]
- 4.Lewis NS. Research opportunities to advance solar energy utilization. Science. 2016;351:353. doi: 10.1126/science.aad1920. [DOI] [PubMed] [Google Scholar]
- 5.Bai S, et al. Highly active and selective hydrogenation of CO2 to ethanol by ordered Pd–Cu Nanoparticles. J. Am. Chem. Soc. 2017;139:6827–6830. doi: 10.1021/jacs.7b03101. [DOI] [PubMed] [Google Scholar]
- 6.Xie C, et al. Tandem catalysis for CO2 hydrogenation to C2–C4 hydrocarbons. Nano Lett. 2017;17:3798–3802. doi: 10.1021/acs.nanolett.7b01139. [DOI] [PubMed] [Google Scholar]
- 7.Kattel S, Ramírez PJ, Chen JG, Rodriguez JA, Liu P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science. 2017;355:1296–1299. doi: 10.1126/science.aal3573. [DOI] [PubMed] [Google Scholar]
- 8.Vogt C, et al. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018;1:127–134. doi: 10.1038/s41929-017-0016-y. [DOI] [Google Scholar]
- 9.Jiang K, et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 2018;1:111–119. doi: 10.1038/s41929-017-0009-x. [DOI] [Google Scholar]
- 10.He J, Dettelbach KE, Salvatore DA, Li T, Berlinguette CP. High-throughput synthesis of mixed-metal electrocatalysts for CO2 reduction. Angew. Chem. Int. Ed. 2017;56:6068–6072. doi: 10.1002/anie.201612038. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl KP, et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014;136:14107–14113. doi: 10.1021/ja505791r. [DOI] [PubMed] [Google Scholar]
- 12.Resasco J, et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 2017;139:11277–11287. doi: 10.1021/jacs.7b06765. [DOI] [PubMed] [Google Scholar]
- 13.Manthiram K, Surendranath Y, Alivisatos AP. Dendritic assembly of gold nanoparticles during fuel-forming electrocatalysis. J. Am. Chem. Soc. 2014;136:7237–7240. doi: 10.1021/ja502628r. [DOI] [PubMed] [Google Scholar]
- 14.Wuttig A, Yoon Y, Ryu J, Surendranath Y. Bicarbonate is not a general acid in Au-catalyzed CO2 electroreduction. J. Am. Chem. Soc. 2017;139:17109–17113. doi: 10.1021/jacs.7b08345. [DOI] [PubMed] [Google Scholar]
- 15.Wuttig A, Yaguchi M, Motobayashi K, Osawa M, Surendranath Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl Acad. Sci. USA. 2016;113:E4585–E4593. doi: 10.1073/pnas.1602984113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature. 2016;537:382–386. doi: 10.1038/nature19060. [DOI] [PubMed] [Google Scholar]
- 17.Mariano RG, McKelvey K, White HS, Kanan MW. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science. 2017;358:1187–1192. doi: 10.1126/science.aao3691. [DOI] [PubMed] [Google Scholar]
- 18.Zeng G, et al. Enhanced photocatalytic reduction of CO2 to CO through TiO2 passivation of InP in ionic liquids. Chem. Eur. J. 2015;21:13502–13507. doi: 10.1002/chem.201501671. [DOI] [PubMed] [Google Scholar]
- 19.Xing M, et al. Modulation of the reduction potential of TiO2-x by fluorination for efficient and selective CH4 generation from CO2 photoreduction. Nano Lett. 2018;18:4–10. doi: 10.1021/acs.nanolett.8b00197. [DOI] [PubMed] [Google Scholar]
- 20.Park H, Ou HH, Colussi AJ, Hoffmann MR. Artificial photosynthesis of C1-C3 hydrocarbons from water and CO2 on titanate nanotubes decorated with nanoparticle elemental copper and CdS quantum dots. J. Phys. Chem. A. 2015;119:4658–4666. doi: 10.1021/jp511329d. [DOI] [PubMed] [Google Scholar]
- 21.Rao H, Schmidt LC, Bonin J, Robert M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature. 2017;548:74–77. doi: 10.1038/nature23016. [DOI] [PubMed] [Google Scholar]
- 22.Niu K, et al. A spongy nickel-organic CO2 reduction photocatalyst for nearly 100% selective CO production. Sci. Adv. 2017;3:e1700921. doi: 10.1126/sciadv.1700921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neaţu Ş, Maciá-Agulló JA, Concepción P, Garcia H. Gold–copper nanoalloys supported on TiO2 as photocatalysts for CO2 reduction by water. J. Am. Chem. Soc. 2014;136:15969–15976. doi: 10.1021/ja506433k. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, et al. Plasmon-enhanced catalysis: distinguishing thermal and nonthermal effects. Nano Lett. 2018;18:1714–1723. doi: 10.1021/acs.nanolett.7b04776. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, et al. Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation. Nat. Commun. 2017;8:14542. doi: 10.1038/ncomms14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuChene JS, Tagliabue G, Welch AJ, Cheng WH, Atwater HA. Hot hole collection and photoelectrochemical CO2 reduction with plasmonic Au/p-GaN photocathodes. Nano Lett. 2018;18:2545–2550. doi: 10.1021/acs.nanolett.8b00241. [DOI] [PubMed] [Google Scholar]
- 27.Hou W, Pavaskar P, Goeppert A, Aykol M, Cronin SB. Photocatalytic conversion of CO2 to hydrocarbon fuels via plasmon-enhanced absorption and metallic interband transitions. ACS Catal. 2011;1:929–936. doi: 10.1021/cs2001434. [DOI] [Google Scholar]
- 28.Yu S, Wilson AJ, Heo J, Jain PK. Plasmonic control of multi-electron transfer and C-C coupling in visible-light-driven CO2 reduction on Au nanoparticles. Nano Lett. 2018;18:2189–2194. doi: 10.1021/acs.nanolett.7b05410. [DOI] [PubMed] [Google Scholar]
- 29.Yu S, Wilson AJ, Kumari G, Zhang X, Jain PK. Opportunities and challenges of solar-energy-driven carbon dioxide to fuel conversion with plasmonic catalysts. ACS Energy Lett. 2017;2:2058–2070. doi: 10.1021/acsenergylett.7b00640. [DOI] [Google Scholar]
- 30.Yang J, Guo Y, Lu W, Jiang R, Wang J. Emerging applications of plasmons in driving CO2 reduction and N2 fixation. Adv. Mater. 2018;30:1802227. doi: 10.1002/adma.201802227. [DOI] [PubMed] [Google Scholar]
- 31.Calle-Vallejo F, Koper MTM. Theoretical considerations on the electroreduction of CO to C2 species on Cu(100) electrodes. Angew. Chem. Int. Ed. 2013;52:7282–7285. doi: 10.1002/anie.201301470. [DOI] [PubMed] [Google Scholar]
- 32.Nie X, Esopi MR, Janik MJ, Asthagiri A. Selectivity of CO2 reduction on copper electrodes: the role of the kinetics of elementary steps. Angew. Chem. Int. Ed. 2013;52:2459–2462. doi: 10.1002/anie.201208320. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Li CW, Kanan MW. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 2012;134:19969–19972. doi: 10.1021/ja309317u. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y, Dumett Torres D, Jain PK. Activation energies of plasmonic catalysts. Nano Lett. 2016;16:3399–3407. doi: 10.1021/acs.nanolett.6b01373. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y, Wilson AJ, Jain PK. The nature of plasmonically assisted hot-electron transfer in a donor-bridge-acceptor complex. ACS Catal. 2017;7:4360–4365. doi: 10.1021/acscatal.7b01318. [DOI] [Google Scholar]
- 36.Kim Y, Smith JG, Jain PK. Harvesting multiple electron-hole pairs generated through plasmonic excitation of Au nanoparticles. Nat. Chem. 2018;10:763–769. doi: 10.1038/s41557-018-0054-3. [DOI] [PubMed] [Google Scholar]
- 37.Rosen Ba, et al. Ionic liquid–mediated selective conversion of CO2 to CO at low overpotentials. Science. 2011;334:643–644. doi: 10.1126/science.1209786. [DOI] [PubMed] [Google Scholar]
- 38.García Rey N, Dlott DD. Structural transition in an ionic liquid controls CO2 electrochemical reduction. J. Phys. Chem. C. 2015;119:20892–20899. doi: 10.1021/acs.jpcc.5b03397. [DOI] [Google Scholar]
- 39.Sun L, Ramesha GK, Kamat PV, Brennecke JF. Switching the reaction course of electrochemical CO2 reduction with ionic liquids. Langmuir. 2014;30:6302–6308. doi: 10.1021/la5009076. [DOI] [PubMed] [Google Scholar]
- 40.Asadi M, et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science. 2016;353:467–470. doi: 10.1126/science.aaf4767. [DOI] [PubMed] [Google Scholar]
- 41.Rosen BA, et al. In situ spectroscopic examination of a low overpotential pathway for carbon dioxide conversion to carbon monoxide. J. Phys. Chem. C. 2012;116:15307–15312. doi: 10.1021/jp210542v. [DOI] [Google Scholar]
- 42.Wang Y, et al. Activation of CO2 by ionic liquid EMIM–BF4 in the electrochemical system: a theoretical study. Phys. Chem. Chem. Phys. 2015;17:23521–23531. doi: 10.1039/C5CP02008E. [DOI] [PubMed] [Google Scholar]
- 43.Fredlake CP, Crosthwaite JM, Hert DG, Aki SNVK, Brennecke JF. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data. 2004;49:954–964. doi: 10.1021/je034261a. [DOI] [Google Scholar]
- 44.Zhang Y, Shi C, Brennecke JF, Maginn EJ. Refined method for predicting electrochemical windows of ionic liquids and experimental validation studies. J. Phys. Chem. B. 2014;118:6250–6255. doi: 10.1021/jp5034257. [DOI] [PubMed] [Google Scholar]
- 45.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 46.Pera-Titus M. Porous inorganic membranes for CO2 capture: present and prospects. Chem. Rev. 2014;114:1413–1492. doi: 10.1021/cr400237k. [DOI] [PubMed] [Google Scholar]
- 47.Rosen BA, Zhu W, Kaul G, Salehi-Khojin A, Masel RI. Water enhancement of CO2 conversion on silver in 1-ethyl-3-methylimidazolium tetrafluoroborate. J. Electrochem. Soc. 2012;160:H138–H141. doi: 10.1149/2.004303jes. [DOI] [Google Scholar]
- 48.Freire MG, Neves CMSS, Marrucho IM, Coutinho JAP, Fernandes AM. Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J. Phys. Chem. A. 2010;114:3744–3749. doi: 10.1021/jp903292n. [DOI] [PubMed] [Google Scholar]
- 49.Kumar B, et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 2013;4:1–8. [Google Scholar]
- 50.Deetlefs M, Pitner WR, Hardacre C. Quantification of halide in ionic liquids using ion chromatography. Anal. Chem. 2004;76:2118–2123. doi: 10.1021/ac035157z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw images and source data are available from the authors upon reasonable request.