Abstract
White-nose syndrome (WNS) caused by the fungus, Pseudogymnoascus destructans (Pd) has killed millions of North American hibernating bats. Currently, methods to prevent the disease are limited. We conducted two trials to assess potential WNS vaccine candidates in wild-caught Myotis lucifugus. In a pilot study, we immunized bats with one of four vaccine treatments or phosphate-buffered saline (PBS) as a control and challenged them with Pd upon transfer into hibernation chambers. Bats in one vaccine-treated group, that received raccoon poxviruses (RCN) expressing Pd calnexin (CAL) and serine protease (SP), developed WNS at a lower rate (1/10) than other treatments combined (14/23), although samples sizes were small. The results of a second similar trial provided additional support for this observation. Bats vaccinated orally or by injection with RCN-CAL and RCN-SP survived Pd challenge at a significantly higher rate (P = 0.01) than controls. Using RT-PCR and flow cytometry, combined with fluorescent in situ hybridization, we determined that expression of IFN-γ transcripts and the number of CD4 + T-helper cells transcribing this gene were elevated (P < 0.10) in stimulated lymphocytes from surviving vaccinees (n = 15) compared to controls (n = 3). We conclude that vaccination with virally-vectored Pd antigens induced antifungal immunity that could potentially protect bats against WNS.
Subject terms: Immunology, Fungal infection
Introduction
Since its discovery in New York in 20061, white-nose syndrome (WNS), caused by the fungus Pseudogymnoascus (Geomyces) destructans (Pd), has killed at least 7 million bats in the US2, causing significant population declines in numerous bat species. The once ubiquitous little brown bat (Myotis lucifugus) is particularly sensitive and has experienced severe population declines in many states3. This emerging disease may also have important economic implications as bats consume many crop and forests pests, providing key ecological services to US agriculture4. The fungus, which only infects bats during hibernation, is spreading across the US at an alarming rate, having reached Midwestern states by the winter of 2010 and Washington state in the winter of 20165.
Current proposed methods for control of WNS in bats include application of plant – derived compounds or bacteria to hibernating bats to directly inhibit the growth of the fungus6–10 or inhibition by bacterially produced volatile organic compounds11. While results from these studies show promise in the laboratory for reducing the growth of Pd, their practical application may be hindered by logistical factors such as the amount of agent needed or unintended consequences such as the disruption of skin flora or cave ecosystems. Furthermore, most proposed methods would require treatment of bats in the winter, and treatment application when bats are hibernating may be problematic or impractical for some species.
Vaccination may offer an alternative method of disease control. Historically, few fungal vaccines have been developed and used, but the increasing prevalence and severity of invasive fungal pathogens in both humans and animals has led to expanded research and many promising developments in this area12. For managing WNS in bats, practical delivery of an oral vaccine could be achieved through topical application via liquid gels or pastes that are ingested by bats during grooming; it could be applied months prior to hibernation, without requiring access to, and disruption of, cave ecosystems. Additionally, vaccination may generate long-lasting immunity without requiring repeated annual treatments. Delivering oral vaccines to wildlife has been successfully achieved through the use of poxviral vectors13,14 such as vaccinia (e.g. oral rabies vaccine for carnivores) and raccoon poxvirus (RCN; e.g. sylvatic plague vaccine for prairie dogs). Our previous studies have shown that attenuated RCN is a suitable vaccine vector for bats as it was able to infect Tadarida brasiliensis for a limited time period when given via the oronasal route and produced exogenous antigens at high levels without causing overt disease15. In addition, humoral immune responses were observed following mucosal exposure of RCN-vectored rabies vaccine in another bat species, Eptesicus fuscus16.
As a starting point in developing vaccines for WNS, we targeted two potentially immunogenic antigens from Pd. The first is calnexin (CAL), a highly conserved ascomycete fungal antigen that has been shown to protect mice against a variety of fungal pathogens17. Indeed, mouse T-cells specific for CAL18 proliferate in the presence of Pd17. The other, a subtilisin-like or serine protease (SP), also known as “destructin-1”, was identified in two separate studies as a major proteolytic component of Pd19,20, likely facilitating tissue invasion of the host. As a major component of the secretome of Pd, SP could be a desired target of the host’s specific immune response, as disrupting the activity of the protease may limit the ability of Pd to cause disease. Studies have shown that WNS induces anti-fungal immune responses in the skin and lymph nodes of hibernating bats, including an IL-17A response21,22. Because IL-17A is known to recruit and activate phagocytes targeting fungi18, results suggest that bats are mounting an adaptive immune response to Pd during hibernation21,22, although there is no evidence that this immunity is protective.
In this study, we assessed whether induction of specific immunity through vaccination can reduce the disease burden of WNS in a highly susceptible host, M. lucifugus. We predicted that the development of cell-mediated immune responses to specific antigens prior to infection with Pd would limit cutaneous invasion of the fungus, leading to milder disease presentation and greater survival rates among infected individuals. To test this, we conducted two vaccine trials in which we immunized captive M. lucifugus bats with different vaccine formulations via different routes of administration and challenged them with Pd prior to hibernation.
Results
Characterization of RCN expressing Pd antigens by PCR and western blot
We first identified a potential CAL-like protein expressed by Pd by using the basic local alignment search tool (BLAST, blast.ncbi.nlm.nih.gov) to search the Pd proteome for proteins with significant homology to the CAL from Paracoccidiodes brasiliensis (Pb), previously shown to be immunogenic and protective17. We found a 571 amino acid protein that shared 79% sequence similarity and was categorized in the calreticulin superfamily (Genbank: GMDG_03017). We generated RCN constructs expressing Pd CAL (RCN-CAL) and confirmed insertion by PCR (Supplementary Fig. S1). Expression was tested in vitro by western blot analysis using serum from CAL vaccinated mice (Supplementary Fig. S2). All RCN-CAL constructs produced an antigen specific band that was not present in cells infected with RCN expressing green fluorescent protein (GFP) and corresponded to the major immunoreactive band of 63 kDa from positive control CAL produced in E. coli previously17. Protein was found in the pellet fraction, as expected of a membrane bound protein. We also generated RCN expressing the “destructin-1” serine protease (RCN-SP), as this protein was hypothesized to be involved in development of WNS19,20. PCR confirmed insertion of the gene (Supplementary Fig. S1) and qPCR for SP specific RCN transcribed mRNA confirmed expression.
Vaccination and challenge of bats
Pilot vaccine trial
Bats were randomly assigned to groups of 10 or 11 for the following vaccine treatments in mid-November, 2015 (Table 1): CAL protein via intramuscular (IM) injection, RCN-CAL via intranasal (IN) injection, RCN-CAL and RCN-SP simultaneously injected IM, inactivated Pd culture injected intraperitoneally (IP), and a control group that received phosphate buffered saline (PBS) via IM injection. Bats were boosted (same route and dose) 22 days post-initial vaccination. Unfortunately, by this time, weight loss, non-specific morbidity, and mortality associated with husbandry issues began to occur (see Supplementary material for more details), complicating the trial. None of the bats developed pox lesions or had any signs of morbidity related to vaccination with RCN or other treatments, but challenge with Pd and hibernation were delayed until early February (60 days post-boost) when body condition and weights stabilized. By the time bats were challenged, average body weights of each group ranged from 7.95–9.62 g (but were not statistically different) and numbers of bats in each treatment group were diminished, particularly the control group (Table 1).
Table 1.
Mean weight (in grams) of Myotis lucifugus that received various treatments prior to challenge with Pseudogymnoascus destructans (Pd) and post-challenge survival.
| Treatment | Route | n | % survival after challenge | Mean weight at challenge (g) ± s.d. |
|---|---|---|---|---|
| PBS | IM | 3 | 0 | 8.68 ± 0.21 |
| CAL | IP | 7 | 0 | 7.95 ± 1.22 |
| RCN-CAL | IN | 8 | 25 | 9.09 ± 1.12 |
| RCN-CAL/RCN-SP | IM | 10 | 10 | 8.23 ± 1.28 |
| Inactivated Pd | IM | 9 | 44 | 9.62 ± 1.38 |
Treatments included phosphate buffered saline (PBS) as a negative control, calnexin (CAL) protein, raccoon poxvirus (RCN) expressing CAL, RCN expressing CAL and RCN expressing serine protease (SP) in combination, and inactvated Pd, administered via intramuscular (IM), intraperitoneal (IP), or intranasal (IN) routes.
Of 37 bats (31 adults and 6 juveniles) that were challenged with Pd and placed into the hibernation chamber, 3 died before day 40 post-inoculation (days 9, 22, and 28) and were excluded from further analyses (see Supplementary Table S1 for details). Only seven bats survived the entire 100-day hibernation period (ending in early May): four that received inactivated Pd (one individual was WNS positive), two that received RCN-CAL IN (both WNS positive) and one that received RCN-CAL/RCN-SP (WNS negative). No significant differences in survival were evident between treatment groups, but the numbers of animals in each treatment group were very low. Instead, survival strongly correlated with their weight when placed into the environmental chamber, with bats of lower weight being more likely to die before 100 days regardless of their treatment group (Fig. 1).
Figure 1.
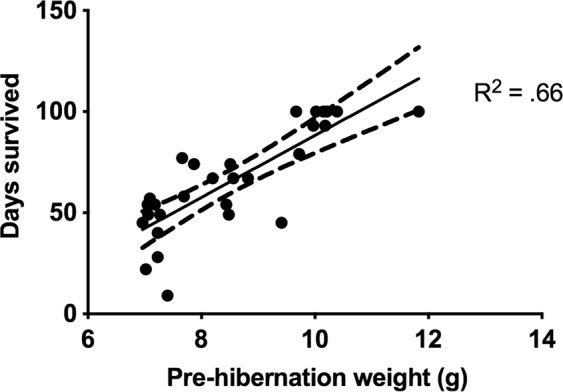
Myotis lucifugus survival was correlated to pre-hibernation weight. The number of days bats survived after challenge with Pseudogymnoascus destructans is plotted against pre-hibernation weight, with Pearson correlation test line and confidence intervals.
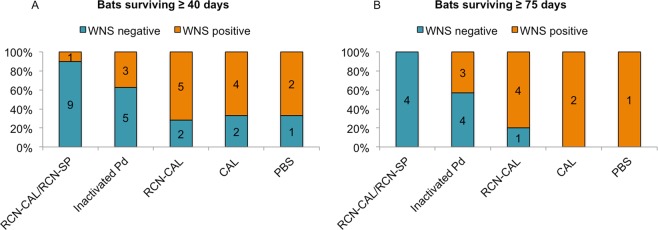
Fifteen of 34 bats examined developed histologic lesions characteristic of WNS23 (Fig. 2) as described in Supplementary materials and depicted in Supplementary Fig. S3). In addition to or in place of characteristic WNS lesions, a few bats had microscopic evidence of a resolving fungal infection, including the formation of neutrophilic pustules containing rare fungal hyphae (Supplementary Fig. S3). Lesions of WNS were evident in one bat found dead as early as day 45 post-challenge. Of those bats that survived past day 40 post-challenge (Fig. 2A), the proportion with WNS lesions was significantly lower (P < 0.05) in the RCN-CAL/RCN-SP group (1/10) compared to the groups that received RCN-CAL (5/7) or CAL protein (4/6), but not the inactivated Pd group (3/8). We did not have the power to detect differences between the PBS control group (2/3) and other treatment groups due to the small sample size of this group. For those surviving past 75 days (Fig. 2B), a similar result was obtained using Fisher’s exact test for small samples sizes. The proportion of bats positive for WNS was significantly lower (P = 0.007) in those that received RCN-CAL/RCN-SP (0/4) compared to the proportion of bats (7/8) that received RCN-CAL, CAL, or PBS, but not different than bats vaccinated with inactivated Pd (3/7). Pd DNA was detected by real-time PCR on all but one of 29 bats swabbed at death or euthanasia (Supplementary Table S1).
Figure 2.
Proportion of Myotis lucifugus positive and negative for white-nose syndrome (WNS) by histological examination after challenge with Pseudogymnoascus destructans (Pd) in hibernating bats surviving 40 days or more (A) or 75 days or more (B). Bats received either a combination of raccoon poxvirus (RCN) expressing calnexin (CAL) and RCN expressing serine protease (RCN-CAL/RCN-SP), inactivated Pd, RCN-CAL only, purified CAL protein, or phosphate buffered saline (PBS). Numbers indicate the total number of bats in each group.
Second vaccine trial
A second trial was conducted to further evaluate the most promising treatment in the pilot study, RCN-CAL/RCN-SP, for both its protective efficacy after Pd challenge and stimulation of anti-fungal immunity. In the second trial, all bats were juveniles, vaccinated the day after intake in mid-October, only once either IM or orally, and maintained in an active state for only 22 days before they were challenged with Pd and placed in an environmental chamber for hibernation in November. Controls received RCN expressing luciferase (luc), a neutral antigen, to control for the effects of the viral vector (Table 2).
Table 2.
Percent survival and mean weight (in grams) at challenge and at death or euthanasia in Myotis lucifugus vaccinated with raccoon poxvirus (RCN) expressing calnexin (RCN-CAL) and RCN expressing serine protease (RCN-SP) in combination, either via the oral route or intramuscular (IM) route, or RCN expressing luciferase (RCN-luc, control group).
| Treatment | Route | n | % survival after challenge | Mean weight at challenge (g) ± s.d. | Mean weight at death (g) ± s.d. |
|---|---|---|---|---|---|
| RCN-luc-control | IM/oral | 9 | 30 | 10.1 ± 0.9A | 5.3 ± 1.8A |
| RCN-CAL/RCN-SP | IM | 10 | 80 | 10.4 ± 1.1A | 6.1 ± 0.9AB |
| RCN-CAL/RCN-SP | oral | 8 | 88 | 10.5 ± 1.3A | 7.0 ± 0.8BC |
Bats were challenged with Pseudogymnoascus destructans three weeks after immunization and just prior to a 120 day hibernation period. Mean weights with different letters within a column are significantly different at P < 0.05.
Pre-challenge survival of bats in this trial was much better than the previous trial. Only two bats were excluded due to a failure to adapt to captivity (weight < 7 g). Mean weight at the beginning of hibernation was >10 g and not significantly different between groups (Table 2). Overall, post-challenge survival was much higher (18/27) than the previous trial, with vaccinees (regardless of route of immunization) surviving at a higher rate than controls (Fig. 3; P < 0.05). Mean end weight of bats (both live and dead, excluding one control too mummified for evaluation) was significantly higher in the oral vaccinees compared to controls, but IM vaccinees did not differ significantly from either of the other groups (Table 2).
Figure 3.
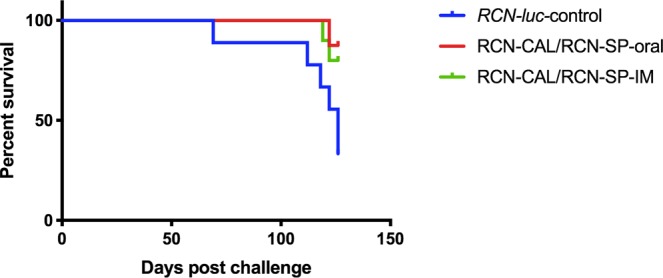
Comparison of survival of vaccinated and unvaccinated Myotis lucifugus. KaplanMeier survival analysis shows that bats vaccinated with a combination of raccoon poxvirus (RCN) expressing calnexin (RCN-CAL) and RCN expressing serine protease (RCN-SP), either via the oral route or intramuscular (IM) route, survived at a significantly (P = 0.01) higher rate than control bats receiving RCN expressing luciferase (RCN-luc).
All dead bats had evidence of Pd infection - lesions of WNS on histologic examination or obvious orange fluorescence under UV light indicative of Pd invasion – with the exception of one control bat that was found mummified on day 126 and was too decomposed for evaluation (Supplementary Table S2). All dead bats had lost >47% of their initial body weight (54.7% ± 7.6) and died between day 69 and 122. Most bats surviving to day 126 also had evidence of Pd infection (15/18), but they lost significantly (P < 0.0001) less weight (33.9% ± 8.5) compared to those that died. The average numbers of WNS lesions (after removing 3 statistical outliers, one in each treatment group, with >45 invasion sites) in all bats vaccinated with RCN-CAL/RCN-SP via oral (2.4 ± 2.3) or IM (3.1 ± 4.2) routes were lower (Fig. 4) but not statistically different than control bats sham-vaccinated with RCN-luc (8.4 ± 13.4).
Figure 4.
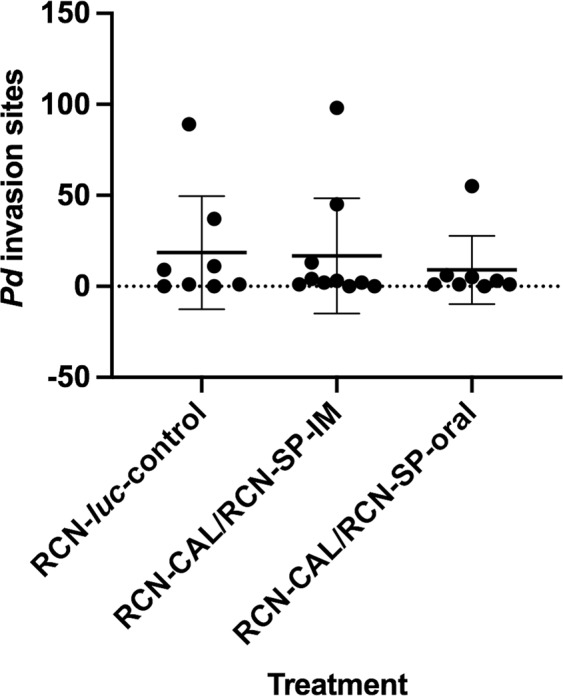
Number of Pseudogymnoascus destructans (Pd) invastion sites evident by histology on the wings of vaccinated and unvaccinated Myotis lucifugus after Pd challenge and hibernation.
Ex vivo cytokine expression by bat T cells
To look for evidence of T cell priming and function in surviving bats, we stimulated lymphoid tissue derived cells (pooled spleen and axillary lymph nodes) ex vivo and analyzed the cells for intracellular cytokine expression by flow cytometry. Since there are no commercially available anti-bat (i.e. M. lucifugus) antibodies for CD4, CD8, IFN-γ and IL-17A, we employed Prime Flow technology, which uses fluorescent in situ hybridization (Flow-FISH) to quantify gene expression at the mRNA level by flow cytometry. We also used anti-human anti-CD3 antibody that cross-reacts with bat T cells and anti-bat IgG for the detection of B cells24. Combined detection of protein (using cross-reactive antibodies) and mRNA (using Flow-FISH) at a single cell level has been described24.
Thirty percent or more of the stimulated CD4+ and CD8+ T cells from bats orally vaccinated with RCN-CAL/RCN-SP produced IFN-γ, whereas less than 1% produced IL-17A (Fig. 5 and Supplementary Fig. S4). The number of stimulated CD4+ T cells from orally vaccinated bats that produced IFN-γ was increased (P = 0.07) compared to RCN-luc controls (Fig. 5). Similar data were found for bat CD8+ cells (Supplementary Fig. 4), but the ability to detect differences between vaccinees and controls was limited by the low number of control bats. These data indicate that vaccination of bats with RCN-CAL/RCN-SP primarily drove a Th1 immune response that was augmented in oral vaccinees compared to controls, but not IM vacinees.
Figure 5.
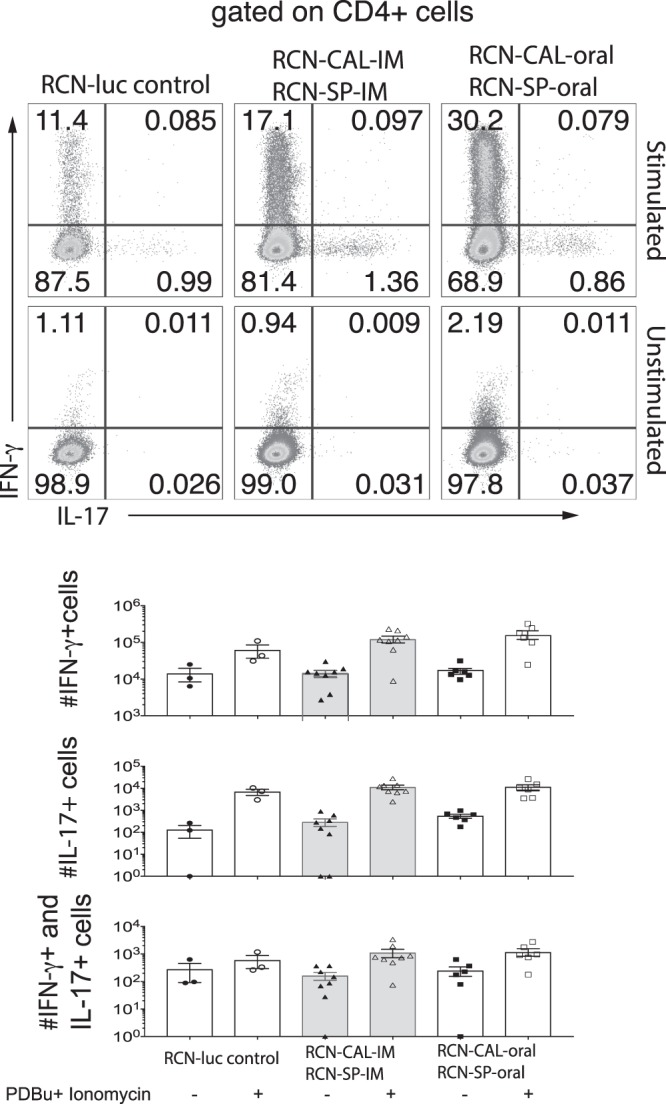
Cytokine expression by bat CD4+ T cells measured by Flow-FISH in vaccinated Myotis lucifugus that survived challenge with Pseudogymnoascus destructans (Pd). Bats had been vaccinated with raccoon poxviruses (RCN) expressing calnexin and serine protease (RCN-CAL/RCN-SP) via intramuscular (IM; n = 8) or oral (n = 7) administration, or sham vaccinated with RCN expressing luciferase (RCN-luc; n = 3). Bat splenocytes were stimulated with PDBu and ionomycin for 2 hours for RNA detection by Flow-FISH. Dot plots and histograms represent concatenates and averages of three to eight bats per group.
Since we used PDBu and ionomycin to stimulate bat T cells ex vivo, cytokine expression measured by Flow-FISH is due to all T cells that have been primed in the bats. To measure vaccine antigen-specific T cell responses, we restimulated primed lymphoid tissue derived cells ex vivo with CAL or inactived Pd for 48 h and measured cytokine transcript by RT-PCR. We analyzed and illustrated cytokine transcript expression relative to two denominators: 1) the RCN-luc control group and 2) medium control stimulation. Figure 6 shows the n-fold changes in cytokine expression vs. the RCN-luc control group. IFN-γ transcripts were elevated (P < 0.05) in bats vaccinated orally and IM with RCN-CAL/RCN-SP compared to those that received RCN-luc, independent of the ex vivo stimuli (medium, CAL and inactivated Pd), indicating that T cells had been induced in vivo to express the cytokine in a vaccine antigen-specific manner. The expression of IL-17A was not significantly altered in vaccinated vs. control bats. Figure 7 shows the n-fold changes in cytokine transcripts after ex vivo stimulation with CAL and inactivated Pd vs. medium control. The robust expression of IFN-γ in the RCN-CAL/RCN-SP vaccine recipients (Figs 6A and 7A) could not be further increased by ex vivo stimulation with CAL and inactivated Pd. In summary, the transcript analysis by Flow-FISH and real-time RT-PCR indicate a strong induction of IFN-γ expression by administration of RCN-CAL/RCN-SP.
Figure 6.
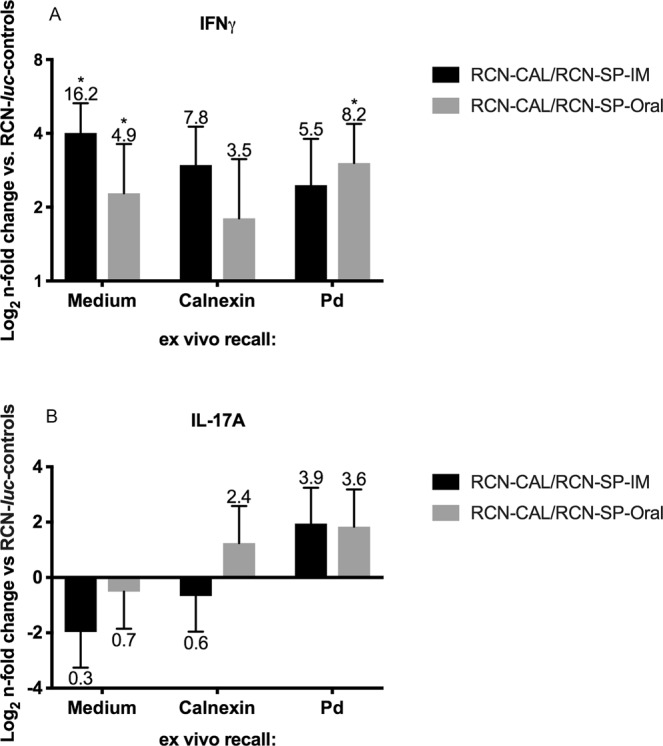
Expression of IFN-γ (A) and IL-17A (B), measured by RT-PCR, in pooled spleen and axillary lymph nodes of vaccinated Myotis lucifugus after challenge with Pseudogymnoascus destructans (Pd), and ex vivo recall with calnexin (CAL), Pd or medium (non-stimulated). Bats had been vaccinated with raccoon poxviruses (RCN) expressing CAL and serine protease (RCN-CAL/RCN-SP) via intramuscular (IM; n = 8) or oral (n = 7) administration, or sham vaccinated with RCN expressing luciferase (RCN-luc; n = 3). Bars and error bars represent average n-fold changes (indicated by the number above the error bar) and standard errors normalized to the RCN-luc group. Values marked with an asterisk are significantly different from the RCN-luc control group at a P < 0.05.
Figure 7.
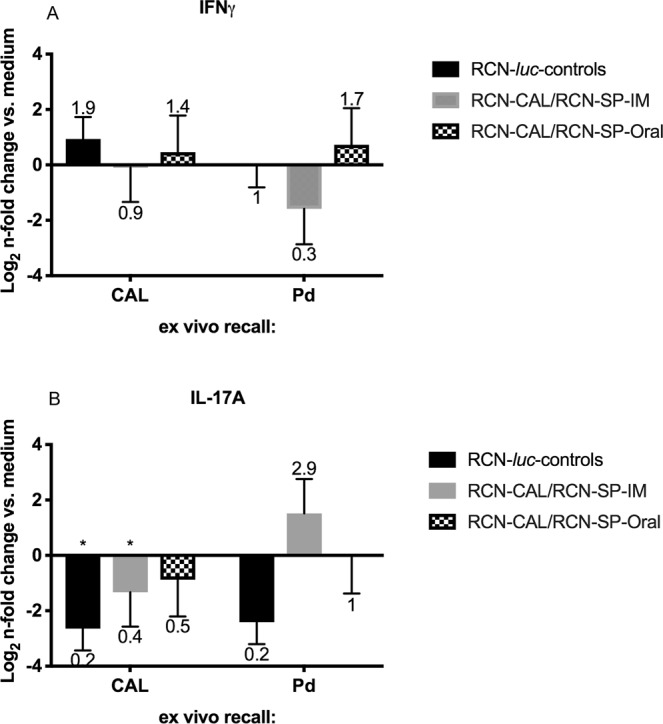
Expression of IFN-γ (A) and IL-17A (B), measured by RT-PCR, in pooled spleen and axillary lymph nodes of vaccinated Myotis lucifugus after challenge with Pseudogymnoascus destructans (Pd) and ex vivo recall with calnexin or Pd. Bats had been vaccinated with raccoon poxviruses (RCN) expressing calnexin and serine protease (RCN-CAL/RCN-SP), via intramuscular (IM; n = 8) or oral (n = 7) administration, or sham vaccinated with RCN expressing luciferase (RCN-luc; n = 3). Bars and error bars represent average n-fold changes (indicated by the number above the error bar) and standard errors, normalized to the non-stimulated (medium) control. Values marked with an asterisk are significantly different from the medium control at P < 0.05.
Discussion
Currently, few mitigation strategies are in use for limiting the spread and lethality of WNS in bats in North America. The trials described here are the first steps toward the development and testing of vaccination as a potential control method for WNS. Despite challenges in maintaining M. lucifugus in captivity, several findings are of note. Most importantly, rates of survival were higher in bats vaccinated with RCN-CAL/RCN-SP compared to controls that were challenged with Pd and placed in a hibernation chamber for 126 days. Orally vaccinated bats, in particular, had higher mean weights at death or euthanasia compared to controls, though all had evidence of Pd infection, and they had higher numbers of CD4 + T-cells producing IFN-γ. Similarly, in our pilot trial, a higher proportion of bats vaccinated with RCN-CAL/RCN-SP remained WNS negative 75 or more days post-challenge compared to other treatment groups, although the number of controls was small in that study. Further work is needed, but the combined results from the two trials provide evidence that vaccination can potentially protect bats from the effects of Pd infection. Vaccination may have slowed the growth of the fungus or reduced the severity, persistence, or discomfort of WNS lesions, thus decreasing arousal of bats during hibernation and enhancing their survival, even if the prevalence of the fungus on bats was unaffected.
Recent studies22,25 suggest that antibody-mediated immunity alone may not be protective against WNS, but until now, other mechanisms of immunity had not been studied. For several pathogenic mycoses, cell mediated immune responses have been shown to be crucial for protective immunity18,26,27. We employed Flow-FISH for the first time on M. lucifigus tissue to quantify immunologic responses on a per cell basis, because no commercial antibodies are currently available to demark little brown bat-specific CD4+ or CD8+ T cells and IFN-γ or IL-17A. Although we observed a trend (P = 0.07) towards increased expression of IFN-γ in surviving bats orally vaccinated with RCN-CAL/RCN-SP, the power to detect statistical differences between the vaccinees and controls was limited by the low numbers of surviving controls. Antigenic ex vivo stimulation with CAL and inactivated Pd indicated elevated IFN-γ transcripts in bats vaccinated orally and IM with RCN-CAL/RCN-SP compared to controls that received RCN-luc, independent of the ex vivo stimulus (medium, CAL and inactivated Pd), indicating that the T cells had been induced in vivo to express the cytokine in a vaccine antigen-specific manner. These cellular immune responses alone, or combined with antibody responses, may offer a basis by which recombinant RCN-CAL/RCN-SP may protect against WNS.
It is possible that some of the bats included in our initial trial may not have been naïve to Pd as the majority were adults (31/37 challenged) and evidence of Pd infection in the trapping location of our study bats was found the previous spring. If adult bats were previously primed to Pd, vaccination could have boosted their immune response. Alternatively, pre-existing exposure could potentially result in a maladaptive response to Pd with subsequent tissue damage and increased numbers of WNS cases. This latter outcome seems unlikely, as similar responses would have been expected in all three groups that received CAL, and that was not the case. Of the vaccinated adults, 80% of bats that received CAL (n = 5) and 67% that received RCN-CAL (n = 6) developed WNS, compared to 11% of bats that received RCN-CAL/RCN-SP (n = 9). In our second vaccine trial, only juveniles were included, as young animals typically respond more vigorously than adults to vaccination, including with other RCN vaccines28. Also, juveniles would not have been exposed to Pd the prior season, eliminating that variable.
Recombinant viral-vectored vaccines, such as RCN, have been shown to stimulate broad humoral and cellular immunity that provide long lasting protection15,29–33, particularly via mucosal delivery. Thus it is not surprising that oral vaccination appeared to be somewhat more effective than IM vaccination in our study. RCN has been shown to be safe and effective in a variety of species via the oronasal route, including domestic cats, piglets, dogs, raccoons, skunks, foxes, bobcats, rabbits, sheep, prairie dogs, non-human primates, chickens and bats, with none of the immunized animals showing clinical side effects15,30,34–42. Recently, a bait-delivered RCN-based sylvatic plague vaccine was evaluated in field trials in prairie dog colonies, demonstrating higher relative abundance and survival of prairie dogs on vaccine treated plots compared to paired placebo plots14. More importantly, an RCN-rabies vaccine construct was shown to protect E. fuscus (big brown bats) from rabies challenge after vaccination via both oronasal and topical application16, providing further support that an orally delivered RCN-based WNS vaccine may be feasible, once the most protective antigens are identified. In addition to CAL and SP, we are testing additional Pd antigens that could help slow the growth of Pd or reduce symptoms of infection that increase arousal of bats during hibernation, reducing their critical energy stores. Bats are naturally fastidious and spend a large amount of time grooming themselves43. Because of this, efficient vaccination of large groups of bats may be accomplished by applying an oral WNS vaccine topically in a liquid or paste vehicle, which would be consumed by bats during grooming.
In addition to identifying protective Pd antigens, other factors must be considered for further development of an RCN-based vaccine against WNS. Topical application will require an effective delivery vehicle that maintains vaccine titers, attaches well to the fur of target bat species, is sufficiently palatable to induce ingestion of the vehicle-vaccine mixture, and contains a tractable biomarker that allows easy distinction between vaccinated/unvaccinated animals. In addition, the optimal dose, number of applications, and timing of vaccination would need to be determined. These factors would be best determined in controlled field trials as maintaining insectivorous bats in captivity for long periods of time has proven difficult.
During our initial trial, problems were encountered in keeping M. lucifugus alive and healthy in a bio-secure environment, leading to diminished group sizes (see Supplement). The nature of the clinical signs and the fact that the greatest losses were encountered in the negative control group that received an IM dose of PBS, suggested that husbandry issues, particularly improper environmental humidity, were primarily responsible for the observed health problems, decreased feeding behavior, and weight loss, rather than the vaccines administrated. No evidence of poxvirus disease was seen clinically or on histologic evaluation in either study, and RCN has been shown to be safe in other bat species15. While most problems resolved prior to challenge in the pilot study, many of the bats apparently did not regain the levels of fat reserves necessary for hibernation, which likely explains the correlation between starting body weight and survival time during the challenge (Fig. 1). Reducing the time bats were kept in an active state in captivity to 3 weeks after vaccination in our second trial, significantly improved survival rates both pre- and post-challenge. This provides further confirmation of the safety of RCN for bats, and also suggests vaccination in the fall just prior to hibernation is a viable option for vaccinating bats in the field.
Measures for controlling WNS are crucially needed to help mitigate the loss of bat species threatened by this disease. Here we present several potential WNS vaccine candidates and preliminary evaluation of their safety in bats and efficacy in preventing WNS. Most importantly, these studies provide some evidence that immunity to WNS is possible, and therefore further development and testing of potential vaccines against Pd is warranted.
Materials and Methods
Ethical statement
Bats were captured and brought into captivity under a permit obtained from the Wisconsin Department of Natural Resources (WDNR), Endangered and Threatened Species (E/T) permit #922. All experimental procedures on bats were reviewed, approved, and performed in accordance with all relevant guidelines and regulations under University of Wisconsin (UW) animal care and use committee (protocol #V005277) or the US Geological Survey, National Wildlife Health Center (NWHC) animal care and use committee (protocol # EP170719). Construction of recombinant vaccine viruses was reviewed and approved by UW biosafety committee (protocol #B00000236).
Construction of RCN based vaccines
RCN was cultured in African Green Monkey kidney cells (ATCC #CCL-81) grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 1.5 g/l sodium bicarbonate, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and incubated at 37 °C in 5% CO2. Wild-type RCN was provided by the Centers for Disease Control (Atlanta, GA); the RCN-luciferase (luc) strain was previously described40.
Recombinant RCN viruses expressing Pd CAL (Genbank: GMDG_03017, RCN-CAL) or serine protease (Genbank: GMDG_06417, RCN-SP) were constructed as described previously31. Protein sequences were back-translated and codon-optimized for expression in vaccinia virus, then commercially synthesized. Genes were cloned into RCN specific transfer vectors to express in frame with a tPA secretory signal coding region and under the control of a poxvirus synthetic early-late promoter, with an internal ribosomal entry site expression enhancing element as previously described31. Expression was evaluated by western blot assay or RT-PCR.
Preparation of inactivated Pd and subunit protein vaccine
Generation and purification of CAL from Paracoccidiodes brasiliensis (Pb) was described previously17. Calnexin from Pd was cloned into the pET-28 expression by replacing the coding region of the Pb CAL with that of the Pd CAL by overlap extension PCR44. To generate inactivated Pd, cultures were grown as described below and hyphae and conidia harvested by scraping fungal material with a metal pick. The fungus was pulverized into a very fine powder in liquid nitrogen using a mortar and pestle and weighed. Petri dishes containing Pd were placed on a 212 nm ultraviolet light imager for 30 minutes to kill the fungus. The pulverized material was then suspended in cold PBS to a concentration of 400 mg/ml, and drawn through successively finer syringes until liquid was able to be easily drawn through a 27 gauge needle.
Preparation of Pd inoculum
Sabouraud dextrose plates containing chloramphenicol and gentamycin were inoculated with a mycelial plug from a starter culture of Pd and incubated for approximately 60 days at 7–10 °C. Sterile PBS containing 0.5% Tween-20 (PBST) was used to wash colony surfaces, followed by gentle rubbing with a sterile inoculating loop to dislodge conidia. Liquid containing conidia was transferred to a sterile tube on ice and then centrifuged at 3,500 RCF for 8 min; the supernatant was removed and the pellet re-suspended in cold sterile PBST. The suspension was passed through a 20 gauge needle several times to break apart aggregations. Conidia were enumerated using a hemocytometer, and the concentration adjusted to 2.5 × 104 conidia per μl using sterile PBST.
Vaccination and challenge of bats
Experimental animals
The first vaccine trial was conducted at the UW School of Veterinary Medicine, Charmany Instructional Facility (CIF). The second trial was conducted at NWHC.
For the first and second vaccine trials, male M. lucifugus were wild-caught at hibernacula in Dodge or Pierce County, Wisconsin, respectively by representatives of WDNR in late fall. Both adults (n = 44) and juveniles (n = 8) were captured for the first trial, but only juveniles (n = 29) were captured for the second trial. Wing bands were placed on each bat for identification. The bats were maintained under ABSL-2 conditions in 40 × 76 × 122 cm (378 liter) screen flight cages, kept within a 1.7 × 2.0 m greenhouse tent to allow increased humidity (85–95%) using warm mist humidifiers. Temperatures were maintained at 21–24 °C. On intake, bats were treated topically for parasites with selamectin. Bats were fed mealworms (Tenebrio molitor) supplemented with a vitamin and mineral mixture (Vionate®); water was available ad libitum. They were weighed frequently and any bats that had lost >0.3 g of body weight were hand fed with mealworms or liquid Carnivore Care™ diet if mealworms were refused.
Pilot vaccine trial
Bats were randomly assigned to groups of 10 or 11 for the following vaccine treatments in mid-November, 2015 (Table 1). One group (n = 10) received RCN-CAL at a dose of 108 plaque-forming units (PFU) administered IN under light anesthesia with isoflurane by drop-wise pipetting into both nostrils. A second group (n = 11) received both RCN-CAL and RCN-SP (108 PFU of each) injected IM into each separate rear deltoid muscle (RCN-CAL/RCN-SP). A third group (n = 10) was injected IM with an inactivated Pd preparation (20 mg in a volume of 50 µl). A fourth group (n = 11) received recombinant CAL protein (40 µg of both Pb and Pd CAL), mixed with alum in a total volume of 0.2 ml and injected IP. A negative control group (n = 10) was injected IM with PBS. Bats were boosted (same route and dose) 22 days post-initial vaccination. During vaccination, treatment groups were housed in separate cages to avoid cross infection with RCN constructs.
Due to health issues with the bats and difficulties in maintaining weight (see Supplement for details), the challenge was delayed until early February 2016. Bats (n = 37) were challenged with Pd 60 days after boosting. Pd inoculum was freshly prepared as above and kept on ice and in the dark. Before application to each bat, suspensions were thoroughly mixed by pipetting. When the mixture was uniform, 20 µL were pipetted onto the dorsal surface of the right wing between the 5th digit and the forearm while gently using the pipet tip to spread the liquid evenly across this area, so as not to damage the bat’s wing with the tip of the pipet. Once the liquid had been evenly spread, the wing was allowed to fold back into its resting position. Bats were then placed in an environmental chamber designed to mimic the conditions of stable low temperatures with high humidity found in caves during hibernation (Percival Model #I36NL). The chamber was maintained at 8.5 °C (±0.5 °C) and 90% (±1.0%) relative humidity. Bats were monitored daily by viewing through a window in the chamber door using red light to illuminate the interior. Any bats noted as down on the cage floor for >24 hours were retrieved and a full necropsy was performed. At 100 days, any surviving bats were euthanized and necropsied.
Seccond vaccine trial
Twenty-nine bats were randomly assigned to 3 treatment groups and immunized the day after capture. One group received both the RCN-CAL and RCN-SP vaccine constructs (107 PFU of each) via IM injection (n = 10) into each separate rear deltoid muscle (RCN-CAL/RCN-SP-IM). A second group received both constructs (same dosage) via oral administration (n = 9) in a 100 ul volume (RCN-CAL/RCN-SP-ORAL). The third group received RCN-luciferase (luc) via IM injection or oral administration, as a negative control, and results were combined for analysis. The bats were housed in separate cages to avoid cross contamination and hand fed every other day as described above. Three weeks after vaccination, bats were challenged with Pd as described above and randomly assigned into one of two cages, stratified by treatment, within an environmental chamber. The chamber was maintained at 7.0 °C (±0.5 °C) and 95% (±1.0%) relative humidity. Bats were monitored daily as described above, and at 126 days, surviving bats were euthanized and necropsied.
Verification of WNS
All bats found dead or euthanized at the end of each trial were examined for evidence of WNS via several methods.
Histopathology
We processed the portion of skin connecting the forelimbs with the hindlimbs (plagiopatagia) for histological assessment of WNS status as previously described23. Briefly, we dissected the plagiopatagia from both wings, rolled them onto 2 paraffin dowels 2.5 cm in length, fixed them in 10% neutral buffered formalin, trimmed into six sections equal in thickness, dehydrated, embedded in paraffin, sectioned at 5 μm, and stained for light microscopic examination with periodic acid-Schiff (PAS) staining method. This preparation technique yields slides with six whorls of wing membrane from each plagiopatagium. In the pilot trial, a board-certified veterinary pathologist (JL) screened all wing rolls by light microscopy and designated bats as positive or negative for WNS based on published criteria23,45,46. In the second study, a board-certified pathologist (MIA) screened by light microscopy a total of six whorls from the right wing of each bat included in the study at 400X for WNS lesions, using the same published criteria. When there were less than 6 rolled sections from the right wing available due to tissue processing artifacts, a complementary number of randomly selected sections were examined from the left wing. The number of WNS lesions in 6 rolls was counted for each bat. Briefly, WNS lesions in both vaccine trials included epidermal invasion by PAS-positive hyphae morphologically compatible with Pd forming, distinct cupping erosions (intact epidermal basement membrane) or ulcers (disrupted basement membrane and dermal invasion).
Ultraviolet light (UV) transillumination
In the second trial, we examined the wings of each bat using a 366 nm UV lamp and recorded the presence or absence of distinct orange–yellow fluorescence47, as well as the visual estimated percentage of wing surface affected (Supplementary Table S2).
Quantitative PCR
During necropsy, we swabbed both wings, placed the swabs in sterile water and stored them at −20 °C. Upon thawing, DNA was extracted using 250 μl of PrepMan® Ultra Sample Preparation Reagent and 100 mg of zirconium/silica beads; bead-beating steps were conducted using a FastPrep®-24 homogenizer. The presence of Pd DNA was determined using a quantitative real-time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Pd as described elsewhere48. Samples were considered positive with a threshold cycle (Ct) less than 40.
Characterization of the adaptive cellular immune response
Following euthanasia of the 18 bats that survived until the end of the second trial, we collected spleen and axillary lymph nodes from each. Splenocytes were collected and pooled with lymph node derived cells (lymphoid tissue derived cells). These pooled cells were used for the following assays:
Cytokine expression by bat T cells in peripheral lymphoid tissue using Flow-FISH
Single cell suspensions prepared in complete medium (RPMI + 10% FBS) were incubated with 50 nM PDBu and 0.5 µg/ml ionomycin for 2 hours before cytokine expression was measured by Flow-FISH. Stimulated cells were stained with fixable viability dye and surface antibody including anti-bat IgG (stains B cells). The intracellular detection of T cells with anti-CD3 antibody and staining with the PrimeFlow Target probes for CD4, CD8, IL-17 and IFN-γ was performed using PrimeFlow RNA assay kits and probe sets (Thermo Fisher Scientific) following the manufacturer’s protocol. The samples were recorded by flow cytometry and analyzed by FlowJo software. The gating strategy is shown in Fig. S4A. Briefly, single, live cells were gated for CD3+ cells (excluding B cells), then separated into CD4+ and CD8+ T cells and analyzed for IL-17A and IFN-γ production.
Cytokine transcript analysis in peripheral lymphoid tissue using quantitative RT-PCR
We incubated aliquots of 1 × 106 lymphoid tissue derived cells from each surviving bat (n = 18) with 10 µg/ml recombinant Pd CAL or with 1 mg/ml heat-inactivated Pd extract or 10% FBS/RPMI medium alone (unstimulated control) for 48 h at 37 °C, 5% CO2. After incubation we extracted the RNA using Qiagen RNeasy kits. Thereafter, genomic DNA was digested with Turbo DNase. Subsequently we carried out a second round of RNA purification over RNeasy columns before generating cDNA using iScript cDNA kit. Quantitative reverse RT-PCR was performed using SsoFast EvaGreen Supermix with the following primers optimized for little brown bat transcripts: IL-17A forward primer 5′-GCTTCTGTGAGAACTTCCTC-3′; IL-17A reverse primer 5′-CTTGTCCTCAGTATTTGGGC-3′; IFN-γ forward primer 5′-ACAGCAGCAACAGCAAAATG-3′; IFN-γ reverse primer 5′- TTTCCGCATCTTTGGGTTAG-3′. We used the following thermal cycle profile: 30 sec at 95 °C, 45 cycles for polymerase activation, 45 PCR cycles at 95 °C/10 sec (denaturation) and 55 °C/10 sec (annealing/extension), and melt curve (55 °C to 95 °C) on a Qiagen Rotor-Gene Q. Finally, we calculated the n-fold change of gene expression for stimulated (CAL or Pd extract) vs. non-stimulated (medium) lymphoid tissue derived cells using the 2−ddCt method49 normalizing the Ct values using the highly conserved mouse ortholog 18 S gene with forward primer 5′-CGCCGCTAGAGGTGAAATTCT-3′; reverse primer 5′-CGAACCTCCGACTTTCGTTCT-3′.
Statistical analyses
For challenge studies, survival among groups was compared using Kaplan-Meier survival curves analyzed by the log-rank test (Graph Pad Prism). Differences in the proportion of bats with WNS lesions evident upon histological examination were analyzed between treatment groups by regression using the package “rms” in R and contrast.rms to run specific contrasts of interest50 available from https://CRAN.R-project.org/package=rms or Fisher’s exact test for small sample sizes51. Mean number of Pd invasion sites identified by histology was analyzed between groups using ANOVA; values identified as outliers were removed. Flow-FISH and real-time RT-PCR data were statistically analyzed in Prism using Welch’s unpaired t-test, which does not assume equal standard deviation; values identified as outliers were removed. Preliminary analyses of survival and proportions of bats with WNS lesions in the second experiment did not detect a significant treatment effect by cage within the chamber, so the data were combined for further analyses. The datasets generated and/or analyzed during the current study are available in the USGS Science Base repository, 10.5066/P923NSSG.
Supplementary information
Supplementary Methods, Figures, and Tables
Acknowledgements
The authors are grateful to animal care staff from the UW Charmany Instructional Facility and the USGS National Wildlife Health Center for hand-feeding and caring for bats, C. Beekman for purified serine protease, R. Russell for statistical advice and editorial comments, and M. Verant for reviewing an earlier draft of the manuscript. Field assistance was provided by Heather Kaarakka and Jennifer Redell. The study was funded by the U.S. Geological Survey and the U.S Fish and Wildlife Service. Any use of trade, product, or firm names does not imply endorsement by the U.S. Government.
Author Contributions
T.E.R., B.K.B., M.W., R.C.A., B.S., B.K. and J.E.O. designed the studies. B.K.B., M.W., R.C.A., B.S., M.I.A., J.S.L., J.M.L., E.A.F., J.S.F., H.E.D., L.D.S.D., J.P.W., K.G., J.L.M. and J.P.W. conducted laboratory analyses or provided critical data and resources. T.E.R., B.K.B., M.W., M.I.A. and H.E.D. analyzed the data and wrote the manuscript, with contributions from all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43210-w.
References
- 1.Blehert DS, et al. Bat white-nose syndrome: an emerging fungal pathogen? Science. 2009;323:227. doi: 10.1126/science.1163874. [DOI] [PubMed] [Google Scholar]
- 2.Turner G, Reeder D, Coleman J. A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats, with a look at the future. Bat Research News. 2011;52:13–27. [Google Scholar]
- 3.Frick WF, et al. An emerging disease causes regional population collapse of a common North American bat species. Science. 2010;329:679–82. doi: 10.1126/science.1188594. [DOI] [PubMed] [Google Scholar]
- 4.Boyles JG, Cryan PM, McCracken GF, Kunz TH. Conservation. Economic importance of bats in agriculture. Science. 2011;332:41–42. doi: 10.1126/science.1201366. [DOI] [PubMed] [Google Scholar]
- 5.Lorch JM, et al. First detection of bat white-nose syndrome in western North America. mSphere. 2016;3:1. doi: 10.1128/mSphere.00148-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelison CT, et al. A preliminary report on the contact-independent antagonism of Pseudogymnoascus destructans by Rhodococcus rhodochrous strain DAP96253. BMC Microbiol. 2014;14:246. doi: 10.1186/s12866-014-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoyt JR, et al. Bacteria isolated from bats inhibit the growth of Pseudogymnoascus destructans, the causative agent of white-nose syndrome. PLoS One. 2015;10:e0121329. doi: 10.1371/journal.pone.0121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T, Chaturvedi V, Chaturvedi. S. Novel Trichoderma polysporum strain for the biocontrol of Pseudogymnoascus destructans, the fungal etiologic agent of bat white nose syndrome. PLoS One. 2015;10:e0141316. doi: 10.1371/journal.pone.0141316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boire N, et al. Potent inhibition of Pseudogymnoascus destructans, the causative agent of white-nose syndrome in bats, by cold-pressed, terpeneless, valencia orange oil. PLoS One. 2016;11:e0148473. doi: 10.1371/journal.pone.0148473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelison CT, Gabriel KT, Barlament C, Crow SA., Jr. Inhibition of Pseudogymnoascus destructans growth from conidia and mycelial extension by bacterially produced volatile organic compounds. Mycopathologia. 2014;177:1–10. doi: 10.1007/s11046-013-9716-2. [DOI] [PubMed] [Google Scholar]
- 11.Cheng TL, et al. Efficacy of a probiotic bacterium to treat bats affected by the disease white-nose syndrome. J. Appl. Ecology. 2017;54:701–708. doi: 10.1111/1365-2664.12757. [DOI] [Google Scholar]
- 12.Scorzoni L, et al. Antifungal therapy: new advances in the understanding and treatment of mycosis. Front. Microbiol. 2017;8:36. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cliquet F, Aubert M. Dev. Biol. (Basel) 2004. Elimination of terrestrial rabies in western European countries; pp. 185–204. [PubMed] [Google Scholar]
- 14.Rocke TE, et al. Sylvatic plague vaccine partially protects prairie dogs (Cynomys spp.) in field trials. EcoHealth. 2017;14:438–450. doi: 10.1007/s10393-017-1253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stading BR, et al. Infectivity of attenuated poxvirus vaccine vectors and immunogenicity of a raccoonpox vectored rabies vaccine in the brazilian free-tailed bat (Tadarida brasiliensis) Vaccine. 2016;34:5352–5358. doi: 10.1016/j.vaccine.2016.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stading B, et al. Protection of bats (Eptesicus fuscus) against rabies following topical or oronasal exposure to a recombinant raccoon poxvirus vaccine. PLoS Neglect. Trop. Dis. 2017;11:e0005958. doi: 10.1371/journal.pntd.0005958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wüthrich M, et al. Calnexin induces expansion of antigen-specific CD4(+) T cells that confer immunity to fungal ascomycetes via conserved epitopes. Cell Host Microbe. 2015;17:452–465. doi: 10.1016/j.chom.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wüthrich M, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pannkuk EL, Risch TS, Savary BJ. Isolation and identification of an extracellular subtilisin-like serine protease secreted by the bat pathogen Pseudogymnoascus destructans. PLoS One. 2015;10:e0120508. doi: 10.1371/journal.pone.0120508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donoghue AJ, et al. Destructin-1 is a collagen-degrading endopeptidase secreted by Pseudogymnoascus destructans, the causative agent of white-nose syndrome. Proc. Natl. Acad. Sci. USA. 2015;112:7478–7483. doi: 10.1073/pnas.1507082112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field KA, et al. The white-nose syndrome transcriptome: activation of anti-fungal host responses in wing tissue of hibernating little brown Myotis. PLoS Pathog. 2015;11:e1005168. doi: 10.1371/journal.ppat.1005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilley TM, et al. Immune responses in hibernating little brown myotis (Myotis lucifugus) with white-nose syndrome. Proc Biol Sci. B. 2017;284:20162232. doi: 10.1098/rspb.2016.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meteyer CU, et al. Histopathologic criteria to confirm white-nose syndrome in bats. J Vet. Diagn. Invest. 2009;21:411–414. doi: 10.1177/104063870902100401. [DOI] [PubMed] [Google Scholar]
- 24.Gomez JMM, et al. Phenotypic and functional characterization of the major lymphocyte populations in the fruit-eating bat Pteropus alecto. Sci Rep. 2016;6:37796. doi: 10.1038/srep37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson JS, et al. Antibodies to Pseudogymnoascus destructans are not sufficient for protection against white-nose syndrome. Ecol. Evol. 2015;5:2203–2214. doi: 10.1002/ece3.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 27.Cutler JE, Deepe GS, Jr., Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 2007;5:13–28. doi: 10.1038/nrmicro1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocke TE, et al. Age at vaccination may influence response to sylvatic plague vaccine (SPV) in Gunnison’s prairie dogs (Cynomys gunnisoni) EcoHealth. 2015;12:278–287. doi: 10.1007/s10393-014-1002-3. [DOI] [PubMed] [Google Scholar]
- 29.Rocke TE, et al. Further development of raccoon poxvirus-vectored vaccines against plague (Yersinia pestis) Vaccine. 2009;28:338–344. doi: 10.1016/j.vaccine.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 30.Osorio JE, et al. Raccoon poxvirus as a mucosal vaccine vector for domestic cats. J. Drug Target. 2003;11:463–470. doi: 10.1080/10611860410001670062. [DOI] [PubMed] [Google Scholar]
- 31.Kingstad-Bakke B, Brewoo JN, Mai le Q, Kawaoka Y, Osorio JE. Effects of route and coadministration of recombinant raccoon poxviruses on immune responses and protection against highly pathogenic avian influenza in mice. Vaccine. 2012;30:6402–6408. doi: 10.1016/j.vaccine.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Kingstad-Bakke B, Kamlangdee A, Osorio JE. Mucosal administration of raccoonpox virus expressing highly pathogenic avian H5N1 influenza neuraminidase is highly protective against H5N1 and seasonal influenza virus challenge. Vaccine. 2015;33:5155–5162. doi: 10.1016/j.vaccine.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Esposito JJ, Knight JC, Shaddock JH, Novembre FJ, Baer GM. Successful oral rabies vaccination of raccoons with raccoon poxvirus recombinants expressing rabies virus glycoprotein. Virology. 1988;165:313–316. doi: 10.1016/0042-6822(88)90692-7. [DOI] [PubMed] [Google Scholar]
- 34.Esposito, J. J., Chandler, F. W. & Baer, G. M. Oral immunization of animals with raccoon poxvirus expressing rabies virus glycoprotein in Vaccines-89: Modern approaches to new vaccines including prevention of AIDS (eds Lerner, R. A., Ginsberg, H., Chanock, R. M. & Brown, F.) 403–408 (Cold Spring Harbor Laboratory, 1989).
- 35.Fekadu M, et al. Oral vaccination of skunks with raccoon poxvirus recombinants expressing the rabies glycoprotein or the nucleoprotein. J. Wildl. Dis. 1991;27:681–684. doi: 10.7589/0090-3558-27.4.681. [DOI] [PubMed] [Google Scholar]
- 36.Knight JC, et al. Further analyses of the orthopoxviruses volepox virus and raccoon poxvirus. Virology. 1992;190:423–433. doi: 10.1016/0042-6822(92)91228-M. [DOI] [PubMed] [Google Scholar]
- 37.DeMartini JC, Bickle HM, Brodie SJ, He BX, Esposito JJ. Raccoon poxvirus rabies virus glycoprotein recombinant vaccine in sheep. Arch. Virol. 1993;133:211–222. doi: 10.1007/BF01309757. [DOI] [PubMed] [Google Scholar]
- 38.Hu L, Esposito JJ, Scott FW. Raccoon poxvirus feline panleukopenia virus FP2 recombinant protects cats against FPV challenge. Virology. 1996;218:248–252. doi: 10.1006/viro.1996.0186. [DOI] [PubMed] [Google Scholar]
- 39.Tripp DW, et al. Apparent field safety of a raccoon poxvirus-vectored plague vaccine in free-ranging prairie dogs (Cynomys spp.), Colorado, USA. J. Wildl. Dis. 2015;51:401–410. doi: 10.7589/2014-02-051. [DOI] [PubMed] [Google Scholar]
- 40.Hwa SH, Iams KP, Hall JS, Kingstad BA, Osorio JE. Characterization of recombinant raccoonpox vaccine vectors in chickens. Avian Dis. 2010;54:1157–1165. doi: 10.1637/9315-032410-Reg.1. [DOI] [PubMed] [Google Scholar]
- 41.Rocke TE, et al. Consumption of baits containing raccoon pox-based plague vaccines protects black-tailed prairie dogs (Cynomys ludovicianus) Vector Borne Zoonotic Dis. 2010;10:53–58. doi: 10.1089/vbz.2009.0050. [DOI] [PubMed] [Google Scholar]
- 42.Roper RL. Poxvirus safety analysis in the pregnant mouse model, vaccinia, and raccoonpox viruses. Methods Mol. Biol. 2017;1581:121–129. doi: 10.1007/978-1-4939-6869-5_7. [DOI] [PubMed] [Google Scholar]
- 43.Carter G, Leffer L. Social Grooming in Bats: Are Vampire Bats Exceptional? PLoS One. 2015;10:e0138430. doi: 10.1371/journal.pone.0138430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryksin AV, Matsumura I. Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. Biotechniques. 2010;48:463–465. doi: 10.2144/000113418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reeder DM, et al. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS ONE. 2012;7:e38920. doi: 10.1371/journal.pone.0038920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pikula J, et al. White-nose syndrome pathology grading in Nearctic and Palearctic bats. PLoS One. 2017;12:e0180435. doi: 10.1371/journal.pone.0180435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner GG, et al. Nonlethal screening of bat-wing skin with the use of ultraviolet fluorescence to detect lesions indicative of white-nose syndrome. J. Wildl. Dis. 2014;50:566–73. doi: 10.7589/2014-03-058. [DOI] [PubMed] [Google Scholar]
- 48.Muller LK, et al. Bat white-nose syndrome: a real-time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Geomyces destructans. Mycologia. 2013;105:253–259. doi: 10.3852/12-242. [DOI] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Harrell F. rms: Regression Modeling Strategies. R package version. 2017;5:1–1. [Google Scholar]
- 51.Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38:141–149. doi: 10.1093/biomet/38.1-2.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods, Figures, and Tables