Abstract
As robotic surgery has increased in popularity, the lack of haptic feedback has become a growing issue due to the application of excessive forces that may lead to clinical problems such as intraoperative and postoperative suture breakage. Previous suture breakage warning systems have largely depended on visual and/or auditory feedback modalities, which have been shown to increase cognitive load and reduce operator performance. This work catalogues a new sensing technology and haptic feedback system (HFS) that can reduce instances of suture failure without negatively impacting performance outcomes including knot quality. Suture breakage is common in knot- tying as the pulling motion introduces prominent shear forces. A shear sensor mountable on the da Vinci robotic surgical system’s Cadiere grasper detects forces that correlate to the suture’s internal tension. HFS then provides vibration feedback to the operator as forces near a particular material’s failure load. To validate the system, subjects tightened a total of four knots, two with the Haptic Feedback System (HFS) and two without feedback. The number of suture breakages were recorded and knot fidelity was evaluated by measuring knot slippage. Results showed that instances of suture failure were significantly reduced when HFS was enabled (p = 0.0078). Notably, knots tied with HFS also showed improved quality compared to those tied without feedback (p = 0.010). The results highlight the value of HFS in improving robotic procedure outcomes by reducing instances of suture failures, producing better knots, and reducing the need for corrective measures.
Index Terms-: haptic feedback, robotic surgery, force sensors, vibrotactile feedback, haptics, shear sensors
I. Introduction
TELEOPERATED robotic surgical systems have been steadily gaining popularity [1] since their FDA approval in 2000 [2]. Additional freedom of motion and the stable camera platform along with the recent introduction of automation into surgical subtasks [3] have greatly simplified many technically challenging laparoscopic operations [4]-[8]. Despite the many advantages of robotic systems, the current lack of comprehensive haptic feedback poses many significant problems that are not generally present in laparoscopic or open surgery [9]-[11]. Large force concentration at the tips of instrument graspers and limited depth perception have resulted in excessive frequent suture breakage intraoperatively and postoperatively, attributable both to excessive pulling [1], [12]-[14] and high grasping forces [15]. Suture failures have the potential to be both costly and dangerous, as they can complicate surgical procedures and necessitate additional corrective measures if detected intraoperatively [16]. When weakened sutures break inside the patient postoperatively, significant harm to the patient can result from peritonitis due to bowel anastomosis disruption [17] or hemorrhage from vascular anastomosis [18]. As the popularity of robotically aided surgery increases, it becomes increasingly important to develop technologies that can warn surgeons when sutures are close to breaking [19], [20]. It should be noted that the ultimate tensile strength of a suture is a function of both material type (absorbable or non-absorbable, monofilament or multifilament, etc.) and gauge (i.e. diameter size), and can be influenced by a multitude of different factors such as its orientation, the rate at which tension is applied, and potential material defects [21]–[23]. Tension is particularly important in suture tying procedures, as sutures are prone to failure due to the often-excessive forces produced by robotic graspers [24] and potential damage due to grasper teeth at points of manipulation [25]. Conversely, the possibility of post-operative knot slippage is present when insufficient force is applied to tighten the knot, resulting in further risk to the patient. For this reason, knot tying is one of the more challenging tasks in robotic surgery due to not only the complexity of forming a knot, but also because the surgeon needs to determine how hard to pull a suture such that the knot is sufficiently secure without weakening the suture material.
Several studies have demonstrated the repeatability of applied force and mechanical superiority of knots tied by hand in comparison with knots tied using either needle drivers, the da Vinci robot or in conventional laparoscopic surgery[26]-[29]. The clinical relevance of reducing incidence of suture breakage and improving knot quality has therefore been the motivation for several investigations into the development of sensing and feedback solutions for robotic knot tying tasks. Sensing technologies used up to date have had to either rely on modifications to the instruments or video processing to detect suture tension [30], [31]. Modifications to the instruments are valuable for early-stage research applications but do not translate well to clinical settings [30]. Sensors for clinical applications are limited by technical features including biocompatibility and sufficient robustness for autoclave or chemical sterilization methods as multiple-use devices [32]–[34]. Previous attempts at predicting suture breakage have measured the strain in sutures with visual analysis, but these approaches are limited due to their dependence on image quality, lighting conditions, and specific visual cues [31]. Other approaches have used instrument modifications including installation of strain gauges and sensors along the instrument shafts [30], but this approach requires modifications to the instruments and may be affected by movements along the trocar [35]. Finally, implementation of sensors using capacitive and piezoelectric variants have appeared in literature [36]-[40] but these sensors have not yet been designed for robotic surgical applications and, in most cases, do not function effectively in aqueous environments. Piezoresistive implementations such as the one proposed in this work provide a number of major benefits which make them ideal for robotic surgical applications: (1) they function well even if exposed to water and/or blood, (2) they are highly flexible, and (3) they require only a small number of simple interconnects. Therefore, this work focuses on sensing technologies aimed at addressing these limitations by utilizing an attachable, instrument tip-effector mounted, single-use (i.e. disposable) and cost-effective piezoresistive shear sensing mechanism. Additionally, by mounting the sensing component to the instrument tips, close to the interacting region, detected forces avoid compromising forces and measure with higher accuracy [41].
Beyond sensing, feedback mechanisms for applications in knot tying tasks have so far been largely limited to visual cues, which can be distracting and unintuitive. In fact, studies in this area have shown that haptic feedback is far superior for conveying tactile and kinesthetic information compared to sensory substitution by visual and auditory feedback methods [42], [43], noting that visual feedback is preferred over auditory feedback[29]. However, reliance on visual feedback has been shown to increase cognitive load[44], resulting in a tradeoff between task performance and time to completion[45]. Furthermore, training with haptics resulted in a shorter learning curve and higher consistency of performance for novices in complex laparoscopic knot-tying tasks[46]. Vibrotactile feedback has been shown to improve performance in a robotic needle-insertion task, yet vibrations were delivered the subjects’ wrists[47]. Utilizing a vibrotactile feedback solution installed on the master controls of the surgical robotic system is therefore proposed as an alternative and potentially more intuitive and effective haptic feedback solution.
The sensing and feedback technologies introduced in this work help complete a suture breakage warning system that is designed to reduce occurrences of suture failure by providing vibrotactile feedback to the operator’s fingertips when the tension approaches the suture’s failure load. This work relies on previously published data for suture failure of silk 3–0 sutures [21]. We hypothesize that such a system can reduce the incidence of suture failure without negatively affecting knot quality during robot-assisted knot tying tasks.
II. METHODS
A. Overview
In order to investigate the effectiveness of a suture breakage warning system, grasper mounted shear sensors were developed to detect shear forces on the end effectors of the da Vinci surgical system. These shear sensors were designed primarily for installation on the Cadiere forceps; however, they could be easily modified to fit other instruments as well. The goal of these sensors was to investigate the potential benefits of haptic feedback for knot tying tasks. To this end, the sensors were designed with the following goals in mind: (a) the sensor design must allow for potential miniaturization so that a final product can be made sufficiently small to fit on Cadiere graspers and not interfere with the ability to manipulate sutures (b) sensors must have sufficient friction at the surface to allow a firm grasp and be capable of detecting shear forces in at least one direction/axis, (c) the device must provide high dynamic sensing range and resolution to be effective in various applications for robot-assisted surgery. To meet these engineering requirements, a shear sensing mechanism was developed by utilizing two commercially available Tekscan A101 piezoresistive sensors.
B. Shear Sensor Design
The A101 sensor is one of the smallest commercially available piezoresistive sensors with a dynamic range of 0–44 N. The sensing area is 3.8 mm in diameter with the total sensor width of 7.6 mm which, for this application, was trimmed down to 6 mm (while maintaining water-tightness).
The sensing concept was comprised of two components, a static Outer Shell and an inner Movable Plate (see Fig. 1). The outer shell was kept from moving by tightly fitting the Cadiere grasper. The inner plate also had an opening in the center to allow the Cadiere grasper to freely pass through. This approach allowed the outer shell to remain static while the inner shell could move slightly from side to side. On each side of the inner component, an A101 sensor was sandwiched between the outer shell and an extruded area of the inner plate. A 20 shore A silicone rubber membrane with 0.5 mm thickness was placed between the sensor and the inner movable plate. The elasticity of this material allowed the inner plate to move back to the original center position after shear forces have been removed. The inner movable plate was also designed to be taller than the outer shell, ensuring that contact would be made only with this inner component. Applying shear to the top surface of the inner plate would push the inner component against the A101 sensor, leading to an increase in reported force values.
Fig. 1.
Uni-axial shear sensor design and how it responses to forces applied in one direction. Graph shows an example of how the sensor shows increasing Analog-to-Digital Converter (ADC) value in response to increasing shear and how it relaxes when the force is removed.
It is worth noting that while the inner component is referred to as a Movable Plate, in reality, the size of the inner movable plate and the outer shell are adjusted so that no movement/sliding of the inner plate can take place. This is necessary to avoid any shifts from static to dynamic friction which result in sudden changes in the forces detected by the A101 force sensors.
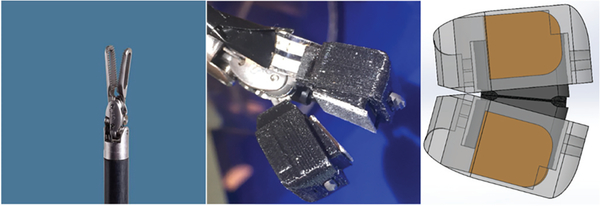
The surface of the inner component was developed with an angle to account for the tilt that exists on the surface of a Cadiere grasper. This was necessary to ensure that the two surfaces are aligned when the grasper is closed so as to provide an even, firm grasp across the whole surface of the grasper jaws (see Fig. 2).
Fig. 2.
Shear Sensors Installed on Cadiere Graspers.
The original da Vinci Cadiere graspers and, more importantly, the Large Needle Driver instrument that is primarily used during suturing, both have teeth-like structures that hold onto the sutures. In contrast, the 3D printed inner component, which is made of Polylactide (PLA), has a relatively smooth surface. Since the ultimate application of these shear sensors involved suture manipulation, a problem in benchtop tests was that the finer sutures (gauges 5–0, 4–0, and 3–0) would slip when placed in between the claws of the grasper. Although applying a sufficiently high normal force creates enough friction to hold onto the suture between this smooth surface, recordings made from benchtop tests using FlexiForce normal force sensors showed that the Cadiere graspers are only capable of applying up to 5 N of normal force. This is not sufficient to securely hold many commonly used sutures (such as Silk 3–0). To resolve this problem, a thin sheet of metal with a Corundum coated surface was cut and glued onto the surface of the inner component. This material selection was made such that the failure load of a suture using this surface would be similar to that of a da Vinci Large Needle Driver (~ 9 N for Silk 3–0 suture)[25], [48].
The total size of this sensing component was 14.3 mm × 10 mm, which was large enough to prevent movement through the 12mm diameter trocar. This is primarily due to limitations of the 3D printing technologies used for producing the sensor. Ultimately, a bi-axial and tri-axial optimized variations of this sensor can be developed using MEMs technologies, making this sensor small enough to be used in surgical environments.
C. Calibration
Calibration of the shear sensors was necessary to allow mapping between the digital values by the ADC and actual force values in Newtons. A readout circuit for the FlexiForce A101 sensors was used based on a simple voltage divider circuit and 10K resistors.
While the theoretical dynamic range of the sensors was 0 −44 N, the calibration was performed for forces below 10 N, as the sensors behave linearly at forces below 15 N. While the nonlinear (exponential) behavior of the sensors can easily be modeled, it was unnecessary for the purposes of this investigation. Using the 16-bit ADC this sensing solution achieved a resolution of 53mN. Since these sensors are designed primarily for use with finer sutures with failure loads below 15 N, this design choice was justifiable within the scope of this investigation.
Force measurements were performed using a Mark-10 Series 3 force gauge. A Silk 3–0 suture was tied to a hook on the force gauge. The other end of the suture was placed between the claws of the grasper. To prevent any minor suture slippage, a small knot was added to the distal end of the suture and positioned such that it would prevent any slipping after the claws were closed. The force gauge was then used to pull the suture; the corresponding force and ADC values were then recorded (see Fig. 3).
Fig. 3.
Shear Sensor Calibration. The ADC* value is on the x-axis and Force value is on the y-axis. ADC* is calculated by subtracting the baseline ADC value (0 N force) from the recorded ADC values. Sensors behave linearly for forces between 2 N - 10 N. Equation is used to program the haptics program to convert ADC values to the corresponding force value. Note: ADC values and the corresponding equation are used inside the HFS software in order to avoid reconfiguration of the software if the control unit (and therefore the reference voltage) were to change.
D. Sensor Implementation and Testing in Haptic Feedback System
Utilizing the developed shear sensors, a haptic feedback system was configured to deploy previously acquired suture failure load data, and to provide feedback when shear forces approached the breaking force of the suture[21]. Fig. 4 shows the configuration of the vibrotactile haptic feedback system for this study.
Fig. 4.
Control System of the Suture Breakage Warning System. Sensor to HFS controller communication delay (post decode) on local network was less 15ms on average (recorded using LEDs on sensor board & 120fps camera).
The shear sensor was installed on one side of the da Vinci Cadiere grasper. A similar component to the shear sensor but without the piezoresistive sensing component was installed on the other side of the grasper (see Fig. 5). For the purpose of these studies, this structure was extremely important for ensuring that the shear sensors were able to detect the forces applied to the sutures. If the other side of the grasper was not mounted with a sensor-like component with a movable inner plate, no shear forces could be detected. This behavior is due to the fact that a solid, non-movable component applying normal force to one side of a suture would prevent the suture from moving in either direction (assuming there was sufficient friction to prevent the suture from slipping). If the suture does not move, then shear forces cannot be detected by the shear sensor on the other side of the grasper. By placing two movable plates on the grasper, as the suture is pulled, both sides of the grasper shift, allowing the sensors to detect the applied force.
Fig. 5.
Uniaxial shear sensor installed on the right side of the Cadiere Grasper. A similar mechanism without the sensing component installed on the left side of the grasper.
The vibrotactile HFS was configured to provide vibration feedback at two levels of intensity. Vibration feedback was provided using an Eccentric Rotating Mass (ERM) vibration motor with intensity controlled using input voltage to the motor [49].
The ERM motor was installed on the da Vinci master controls such that the vibration is felt by the operator’s fingertips (Fig. 6). The first level was activated at 40% below the failure load of the suture while the second level, with a higher vibration intensity, activated at 10% below the suture’s failure load. For this study, Silk 3–0 sutures were used with failure loads of 9.77 N [21].
Fig. 6.
Vibration motor is installed on da Vinci master controls and provides feedback to the surgeon’s fingertips.
E. Experimental Protocol
A total of 15 novice subjects were instructed to tighten a knot until perceived as secure by pulling on the free ends of the suture (see Fig. 7). A repeated measures study design was used to increase statistical power and eliminate bias from intersubject variations. Subjects used the da Vinci IS1200 surgical system’s Cadiere graspers with a uniaxial shear sensor integrated onto the instrument of each arm. Previous research has shown that novice subject performance can benefit most from the presence of haptic feedback, whereas little improvement was observed in experts [15]. This is expected as surgeons with significant prior exposure to robotic surgical systems have learned to use visual cues to at least partially compensate for the handicap created by the lack of the sense of touch. However, for surgical residents and less experienced robotic surgeons, this handicap can reduce performance and impact clinical outcomes. Thus this study focuses on novice subjects who were familiar with operating the da Vinci system but had not received formal training and had less than 20 hours of experience working with a surgical robotic system.
Fig. 7.
(Right) Experimental set-up for the Suture Breakage Warning System study including four loose knots that were tightened by the subject. (Left) Increase in force on one of the sensors as the suture is pulled.
Each subject was provided with a setup containing four loose knots (i.e. 4 trials), two of which would be tightened while HFS was enabled and two of which would be tightened without any feedback. HFS was enabled in an alternating fashion with the order of HFS and no-feedback trials randomized to eliminate any bias towards trial conditions (i.e. Subjects completed trials 1 and 3 with HFS enabled and trials 2 and 4 without HFS, or trials 1 and 3 without HFS and trials 2 and 4 with HFS enabled).
The number of suture breakages was counted per trial, each trial having a maximum of two possible breakages (i.e. suture failures), one occurring from each arm. In addition, the knots were specifically made around a smooth, solid (polystyrene) cylindrical object to allow the knots to easily slide out after completion of the trial (see Fig. 6). For a quantitative assessment of knot quality, the knot was removed from the object and the total knot slippage (in mm) was measured while applying tension force to both ends. Applied shear forces were also recorded throughout the trial.
Furthermore, in real surgical conditions, multiple knots are usually made at the same position to ensure the stability of the anastomoses. To simulate this condition, subjects were instructed to tighten what was in fact the third knot, as the previous two knots were already made by the proctor to maintain consistency of tightness and secure the suture set up around the solid cylinder.
Work with human subjects was approved by the Institutional Review Board (IRB) under protocol #11–000077. The data for each of the two trials in the same condition (i.e. HFS vs. No Feedback) were averaged and statistical analysis was performed using Wilcoxon Signed Rank Test.
III. RESULTS
The study included a total of 60 trials, 30 of which utilized vibrotactile feedback and 30 of which received no feedback. A total of 9 instances of suture breakage occurred during the HFS trials, as opposed to 22 instances of failure without HFS during no feedback trials. Comparing the peak force for trials with and without suture breakage (see Fig. 8), significantly higher peak forces were observed for trials with suture failure (p = 0.017). Therefore, as expected, the data confirms that the primary cause of suture breakage was excessive force.
Fig. 8.
Peak shear force of trials with and without suture breakage compared.
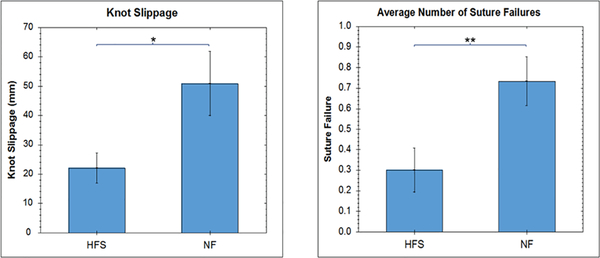
The average number of suture failures and the amount of knot slippage per subject is shown in Fig. 9. The average number of suture failure per subject is significantly higher when haptic feedback is not present (p = 0.0078). Interestingly, the amount of knot slippage is also significantly higher when haptic feedback is not present (p = 0.010).
Fig. 9.
Average number of suture failures/faults and knot slippage (in millimeters) per subject.
It is worth mentioning that all sutures failed at the point of contact with the grasper. This is consistent with observations made in previous experiments conducted with the da Vinci micro-needle driver [21].
IV. DISCUSSION
The results of the suture breakage experiments provide valuable insight into the benefits of haptic feedback for RMIS applications involving suturing. Considering that knot tying is one of the more challenging tasks laparoscopic and robotic procedures, providing feedback can be highly beneficial in improving performance and reducing suture breakage, especially in less experienced surgeons. The results confirm that the suture breakage warning system was highly effective in reducing the occurrence of suture breakage in the knot tying tasks. Having said that, while a significant reduction in the number of suture breakages can be seen when haptic feedback is present, the question does arise as to why any breakage occurred at all. The reason for this behavior may lie in the way novice subjects pulled the sutures. Many subjects pulled the sutures so rapidly that even though feedback was provided, as soon as the subject approached the suture’s failure load, he/she did not have sufficient time to respond to the feedback, thereby resulting in suture failure. There are two possible solutions to this problem. This first is to train subjects to perform a slow, gradual pull when tightening knots. The second is to build a more intelligent and adaptive feedback system which takes into consideration the rate at which shear force is increasing and provide feedback earlier (i.e., at a lower threshold).
In addition to reducing suture breakage, this study confirms that haptic feedback is also associated with improved knot quality. The reduction in knot slippage indicates that more secure knots were made when feedback was provided. This outcome can potentially have significant clinical benefits as it may reduce the occurrence of post-operative complications. There are however, two caveats that must be considered.
The first is that the solid rod used during this study as the base around which the knot were tied, was not a perfect representation of real tissue. While the utilization of the solid rods was the only reliable way to retract the knots after they’ve been tightened, it is worth noting that the elastic properties of tissue can have an impact on the knot tying process. In fact, the elastic properties of tissue can provide some visual feedback to assist the surgeon in assessing the knot stability [50]. Having said that, the repeated measures design of the study prevents this experimental design choice from having any impact on the statistical power of our experiments.
The second caveat is that considering that the lack of feedback resulted in the subject pulling harder and hence breaking the suture, one may expect that a knot produced without any HFS should be more secure than one created with HFS. Yet, the results indicate otherwise. One possible explanation could be random error due to, for instance, the quality of the underlying initial two knots, though the effect of such errors should have been eliminated considering the 60 trials performed in the study (p = 0.01). On the other hand, similar findings have also been reported in literature, where the knot quality does not appear to be the function of a sole variable consisting of magnitude of the pulling force [51], [52]. Hence, the underlying reason for this observation may lie in the way the suture’s structure fails under excessive load. Braided polybend sutures have been found to elongate significantly when sustaining excessive pulling forces [53]. For 3–0 silk sutures used in our experiments, the excessive forces applied to the suture may result in crack formation and mechanical failures within the suture material, hence weakening the entire structure of the knot. The results of these studies indicate that there may be an ideal tension for producing the best possible knot, one that does not result in structural changes to the suture, while also creating a tight enough knot that does not slip. In such a case, a vibrotactile feedback system can inform the surgeon as soon as he/she achieves ideal tension, thereby consistently producing the perfect knot.
It is worth mentioning that the development of the uniaxial shear sensor as part of this effort was aimed at providing the means for evaluating the potential benefits of a haptic feedback system in robotic suturing tasks. While this sensor design was shown to provide the necessary dynamic range and resolution for such an application, it is limited by both its uniaxial nature and its size. In particular, the sensor size was limiting for movement through the trocar and long-term manipulation of sutures and tissue. However, part of the initial goals of this investigation was to determine the viability of utilizing piezoresistive technologies for shear sensing in end-effector-mounted robotic surgical applications. The miniaturization of the sensors can be achieved in future studies using higher quality 3D printing and MEMS technologies, as these technologies have already proven to be advantageous in the context of medical robotics [54]. Additionally, it is worth mentioning that the uniaxial design can be particularly problematic in real surgical conditions because the surgeon rarely pulls the suture in only one direction. Rather, the actual tension in the suture is a combination of biaxial shear and normal forces applied to the sensors. Nevertheless, this uniaxial shear sensor as a proof of concept evidences the possibility for a biaxial and/or triaxial implementation based on the uniaxial design described in this work.
V. Conclusion
This investigation was aimed at identifying the potential benefits of haptic feedback systems in suturing applications for robotic surgery. Lack of haptic feedback has long been one the primary shortcomings of robotic surgical solutions. When compared to traditional open surgery, this limitation has resulted in reduced surgeon performance and undesirable outcomes for certain surgical tasks such as knot tying [27]. The goals of this work were to identify and evaluate a suture breakage warning system consisting of a force sensing and feedback components, designed for application in surgical robotic procedures. The results show that the sensor design and haptic feedback solutions are effective in reducing occurrence of suture breakage in knot tying tasks. More importantly, this work highlights the benefits of HFS for robotic suturing applications. Ultimately, proper implementation of a haptic feedback system for surgical robotics can lead to improved outcomes and may further expand the application of robotics surgical medicine.
Acknowledgments
This work was supported in part by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB19473–01.
Contributor Information
Ahmad Abiri, Center for Advanced Surgical and Interventional Technology, University of California, Los Angeles, CA 90095 USA and also the Henry Samueli School of Engineering, and the Department of Surgery, University of California, Los Angeles, CA 90095 USA..
Syed J. Askari, Center for Advanced Surgical and Interventional Technology, University of California, Los Angeles.
Anna Tao, Center for Advanced Surgical and Interventional Technology, University of California, Los Angeles..
Yen-Yi Juo, Department of Surgery, University of California, Los Angeles..
Yuan Dai, University of California, Los Angeles.
Jake Pensa, Center for Advanced Surgical and Interventional Technology, University of California, Los Angeles..
Robert Candler, Center for Advanced Surgical and Interventional Technology, University of California, Los Angeles, and also with the Henry Samueli School of Engineering and Applied Sciences, University of California, Los Angeles..
Erik P. Dutson, Center for Advanced Surgical and Interventional Technology, University of California, Los Angeles, and also with the Department of Surgery, University of California, Los Angeles.
Warren S. Grundfest, Center for Advanced Surgical and Interventional Technology, University of California, Los Angeles, and also with the Henry Samueli School of Engineering and Applied Sciences, University of California, Los Angeles.
References
- [1].Bhatia V and Tandon RK, “Stress and the gastrointestinal tract,” J.Gastroenterol. Hepatol, 20, no. 3, pp. 332–339, 2005. [DOI] [PubMed] [Google Scholar]
- [2].Barbash GI, Friedman B, Glied S. a, and Steiner C. a, “Factors associated with adoption of robotic surgical technology in US hospitals and relationship to radical prostatectomy procedure volume.,” Ann. Surg, 259, no. 1, pp. 1–6, 2014. [DOI] [PubMed] [Google Scholar]
- [3].Pedram SA, Ferguson P, Ma J, Dutson E, and Rosen J, “Autonomous suturing via surgical robot: An algorithm for optimal selection of needle diameter, shape, and path,” in Proceedings - IEEE International Conference on Robotics and Automation, 2017, pp. 2391–2398. [Google Scholar]
- [4].Ballantyne GH, “Robotic surgery, telerobotic surgery, telepresence, and telementoring: Review of early clinical results,” Surg. Endosc. Other Interv. Tech, 16, no. 10, pp. 1389–1402, 2002. [DOI] [PubMed] [Google Scholar]
- [5].Marescaux J et al. , “Transatlantic robot-assisted telesurgery.,” Nature, 413, no. 6854, pp. 379–80, 2001. [DOI] [PubMed] [Google Scholar]
- [6].Moorthy K et al. , “Dexterity enhancement with robotic surgery,” Surg. Endosc, 18, no. 5, pp. 790–795, May 2004. [DOI] [PubMed] [Google Scholar]
- [7].Munz Y et al. , “The benefits of stereoscopic vision in robotic-assisted performance on bench models.,” Surg. Endosc, 18, no. 4, pp. 611–6, 2004. [DOI] [PubMed] [Google Scholar]
- [8].Satava RM, “Surgical robotics: the early chronicles: a personal historical perspective.,” Surg. Laparosc. Endosc. Percutan. Tech, 12, no. 1, pp. 6–16, 2002. [DOI] [PubMed] [Google Scholar]
- [9].Aghajani Pedram S, Klatzky RL, and Berkelman P, “Torque Contribution to Haptic Rendering of Virtual Textures,” IEEE Trans. Haptics, pp. 1–1, 2017. [DOI] [PubMed] [Google Scholar]
- [10].Van Der Meijden OAJ and Schijven MP, “The value of haptic feedback in conventional and robot-assisted minimal invasive surgery and virtual reality training: A current review,” Surg. Endosc. Other Interv. Tech, 23, no. 6, pp. 1180–1190, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Abiri A et al. , “Visual-Perceptual Mismatch in Robotic Surgery,” Surg. Endosc, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cartmill JA, Shakeshaft AJ, Walsh WR, and Martin CJ, “HIGH PRESSURES ARE GENERATED AT THE TIP OF LAPAROSCOPIC GRASPERS,” ANZJ. Surg, 69, no. 2, pp. 127–130, 1999. [DOI] [PubMed] [Google Scholar]
- [13].M. CJ MARUCCI DAMIAND, SHAKESHAFT ANTHONYJ, CARTMILL JOHNA, COX MICHAELR, ADAMS STUARTG, “Grasper Trauma During Laparoscopic Cholecystectomy,” Aust NZJ Surg, 70, no. 8, pp. 578–581, 2000. [DOI] [PubMed] [Google Scholar]
- [14].De S, Rosen J, Dagan a., Hannaford B, Swanson P, and Sinanan M, “Assessment of Tissue Damage due to Mechanical Stresses,” Int. J. Rob. Res, 26, no. 11–12, pp. 1159–1171,2007. [Google Scholar]
- [15].CR W., “An investigation into the benefits of tactile feedback for laparoscopic, robotic, and remote surgery,” University of California, Los Angeles, 2013. [Google Scholar]
- [16].LeBlanc KA, “Laparoscopic incisional and ventral hernia repair: complications-how to avoid and handle.,” Hernia, 8, no. 4, pp. 323–331, 2004. [DOI] [PubMed] [Google Scholar]
- [17].Anup R and Balasubramanian K. a, “Surgical stress and the gastrointestinal tract.,” J.Surg. Res, 92, no. 2, pp. 291–300, 2000. [DOI] [PubMed] [Google Scholar]
- [18].Melinek J, Lento P, and Moalli J, “Postmortem analysis of anastomotic suture line disruption following carotid endarterectomy.,” J.Forensic Sci, 49, no. 5, pp. 1077–81, 2004. [PubMed] [Google Scholar]
- [19].Hirano Y, Ishikawa N, and Watanabe G, “Suture damage after grasping with EndoWrist of the da Vinci Surgical System.,” Minim. Invasive Ther. Allied Technol, 19, no. 4, pp. 203–6, 2010. [DOI] [PubMed] [Google Scholar]
- [20].Diks J, Nio D, Linsen MA, Rauwerda JA, and Wisselink W, “Suture damage during robot-assisted vascular surgery: is it an issue?,” Surg. Laparosc. Endosc. Percutan. Tech, 17, no. 6, pp. 524–527, 2007. [DOI] [PubMed] [Google Scholar]
- [21].Karabulut R, Sonmez K, Turkyilmaz Z, Bagbanci B, Basaklar a C., and Kale N, “An In Vitro and In Vivo Evaluation of Tensile Strength and Durability of Seven Suture Materials in Various pH and Different Conditions: An Experimental Study in Rats.,” IndianJ. Surg, 72, no. 5, pp. 386–90, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].von Fraunhofer J. a, Storey RS, Stone IK, and Masterson BJ, “Tensile strength of suture materials.,” J. Biomed. Mater. Res, 19, no. 5, pp. 595–600, 1985. [DOI] [PubMed] [Google Scholar]
- [23].Kim JC, Lee YK, Lim BS, Rhee SH, and Yang HC, “Comparison of tensile and knot security properties of surgical sutures,” J. Mater. Sci. Mater. Med, 18, no. 12, pp. 2363–2369, 2007. [DOI] [PubMed] [Google Scholar]
- [24].Abiri A et al. , “Tensile strength and failure load of sutures for robotic surgery,” Surg. Endosc. Other Interv. Tech, pp. 1–13,2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Diks J, Nio D, Linsen MA, Rauwerda JA, and Wisselink W, “Suture damage during robot-assisted vascular surgery: is it an issue?,” Surg. Laparosc. Endosc. Percutan. Tech, 17, no. 6, pp. 524–527, 2007. [DOI] [PubMed] [Google Scholar]
- [26].Livermore RW, Chong ACM, Prohaska DJ, Cooke FW, and Jones TL, “Knot security, loop security, and elongation of braided polyblend sutures used for arthroscopic knots.,” Am. J. Orthop. (Belle Mead. NJ)., 39, no. 12, pp. 569–576, 2010. [PubMed] [Google Scholar]
- [27].Kadirkamanathan SS, Shelton JC, Hepworth CC, Laufer JG, and Swain CP, “A comparison of the strength of knots tied by hand and at laparoscopy,” J. Am. Coll. Surg, 182, no. 1, pp. 46–54, 1996. [PubMed] [Google Scholar]
- [28].Kitagawa M, Okamura AM, Bethea BT, Gott VL, and Baumgartner W. a, “Analysis of Suture Manipulation Forces for Teleoperation with Force Feedback,” Fifth Int. Conf. Med. Image Comput. Comput. Assist. Interv, 2488, pp. 155–162, 2002. [Google Scholar]
- [29].Kitagawa M, Dokko D, Okamura AM, and Yuh DD, “Effect of sensory substitution on suture-manipulation forces for robotic surgical systems,” J. Thorac. Cardiovasc. Surg, 129, no. 1, pp. 151–158, 2005. [DOI] [PubMed] [Google Scholar]
- [30].Reiley CE, Akinbiyi T, Burschka D, Chang DC, Okamura AM, and Yuh DD, “Effects of visual force feedback on robot-assisted surgical task performance,” J. Thorac. Cardiovasc. Surg, 135, no. 1, pp. 196–202,2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Martell J, Elmer T, Gopalsami N, and Park YS, “Visual Measurement of Suture Strain for Robotic Surgery,” Comput. Math. Methods Med, 2011, pp. 1–9,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Okamura AM, “Haptic Feedback in Robot-Assisted Minimally Invasive Surgery,” Curr Opin Urol, 19, no. 1, pp. 102–107,2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Okamura AM, Vemer LN, Reiley CE, and Mahvash M, “Haptics for robot-assisted minimally invasive surgery,” in Springer Tracts in Advanced Robotics, 2010, 66, no. STAR, pp. 361–372. [Google Scholar]
- [34].Tiwana MI, Redmond SJ, and Lovell NH, “A review of tactile sensing technologies with applications in biomedical engineering,” Sensors Actuators APhys, 179, pp. 17–31,2012. [Google Scholar]
- [35].Westebring-van der Putten EP et al. , “Haptics in minimally invasive surgery - a review.,” Minim. Invasive Ther. Allied Technol, 17, no. 1, pp. 3–16, January 2008. [DOI] [PubMed] [Google Scholar]
- [36].Muhammad HB et al. , “Development of a bioinspired MEMS based capacitive tactile sensor for a robotic finger,” Sensors Actuators, A Phys, 165, no. 2, pp. 221–229, 2011. [Google Scholar]
- [37].Lee H, Chang S-I, Kim K-H, Kim S-J, Yun K-S, and Yoon E, “A modular expandable tactile sensor using flexible polymer,” 18th IEEE Int. Conf. Micro Electro Mech. Syst. 2005 MEMS 2005, pp. 642–645, 2005. [Google Scholar]
- [38].Leineweber M, Pelz G, Schmidt M, Kappert H, and Zimmer G, “New tactile sensor chip with silicone rubber cover,” Sensors Actuators, A Phys, 84, no. 3, pp. 236–245, 2000. [Google Scholar]
- [39].Dargahi J, “Piezoelectric tactile sensor with three sensing elements for robotic, endoscopic and prosthetic applications,” Sensors Actuators, A Phys, 80, no. 1, pp. 23–30, 2000. [Google Scholar]
- [40].Omata S and Terunuma Y, “New tactile sensor like the human hand and its applications,” Sensors Actuators A. Phys, 35, no. 1, pp. 9–15, 1992. [Google Scholar]
- [41].Enayati N, De Momi E, and Ferrigno G, “Haptics in Robot-Assisted Surgery: Challenges and Benefits.,” IEEE Rev. Biomed. Eng, PP, no. 99, p. 1, 2016. [DOI] [PubMed] [Google Scholar]
- [42].VITENSE HS, JACKO JA, and EMERY VK, “Multimodal feedback: an assessment of performance and mental workload,” Ergonomics, 46, no. 1–3, pp. 68–87, 2003. [DOI] [PubMed] [Google Scholar]
- [43].Talasaz A, Trejos AL, and Patel R, “The Role of Direct and Visual Force Feedback in Suturing using a 7-DOF Dual-Arm Teleoperated System,” IEEE Trans. Haptics, 1412, no. c, pp. 1–1, 2016. [DOI] [PubMed] [Google Scholar]
- [44].VITENSE HS, JACKO JA, and EMERY VK, “Multimodal feedback: an assessment of performance and mental workload,” Ergonomics, 46, no. 1–3, pp. 68–87, 2003. [DOI] [PubMed] [Google Scholar]
- [45].Tavakoli RVPM, “Robotic suturing forces in the presence of haptic feedback and sensory substitution,” Proc. 2005 IEEE Conf. Control Appl. 2005. CCA 2005., no. June 2014, pp. 1–6, 2005. [Google Scholar]
- [46].Zhou M, Tse S, Derevianko A, Jones DB, Schwaitzberg SD, and Cao CGL, “The Effect of Haptic Feedback on Laparoscopic Suturing and Knot-tying: A Learning Curve Study,” Proc. Hum. Factors Ergon. Soc. Annu. Meet, 52, no. 12, pp. 880–884, 2008. [Google Scholar]
- [47].Peddamatham S, Peine W, and Tan HZ, “Assessment of vibrotactile feedback in a needle-insertion task using a surgical robot,” Symp. Haptics Interfaces ‘Virtual Environ. Teleoperator Syst. 2008- Proceedings, Haptics, no. April 2008, pp. 93–99, 2008. [Google Scholar]
- [48].Abiri A et al. , “Tensile strength and failure load of sutures for robotic surgery,” Surg. Endosc, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].PrecisionMicroDrives, “Understanding ERM Vibration Motor Characteristics.” [Online]. Available: https://www.precisionmicrodrives.com/application-notes/ab-004-understanding-erm-vibration-motor-characteristics.
- [50].Konstantinova J, Jiang A, Althoefer K, Dasgupta P, and Nanay akkara T, “Implementation of tactile sensing for palpation in robot-assisted minimally invasive surgery: A review,” IEEE Sens. J, 14, no. 8, pp. 2490–2501,2014. [Google Scholar]
- [51].Türker M, Yalçinozan M, Çirpar M, Çetik Ö, and Kalaycioglu B, “Clamp fixation to prevent unfolding of a suture knot decreases tensile strength of polypropylene sutures,” Knee Surgery, Sport. Traumatol. Arthrosc, 20, no. 12, pp. 2602–2605, 2012. [DOI] [PubMed] [Google Scholar]
- [52].Neuhofer S, Wieser K, Lajtai G, M??ller D, Gerber C, and Meyer DC, “Surgical knot tightening: how much pull is necessary?,” Knee Surgery, Sport. Traumatol. Arthrosc, 22, no. 11, pp. 2849–2855, 2014. [DOI] [PubMed] [Google Scholar]
- [53].Wüst DM, Meyer DC, Favre P, and Gerber C, “Mechanical and Handling Properties of Braided Polyblend Polyethylene Sutures in Comparison to Braided Polyester and Monofilament Polydioxanone Sutures,” Arthrosc. J. Arthrosc. Relat. Surg, 22, no. 11, pp. 1146–1153, 2006. [DOI] [PubMed] [Google Scholar]
- [54].Isaac-Lowry OJ, Okamoto S, Pedram SA, Woo R, and Berkelman P, “Compact teleoperated laparoendoscopic single-site robotic surgical system: Kinematics, control, and operation,” International Journal of Medical Robotics and Computer Assisted Surgery, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]