Abstract
The improved safety profile and antifungal efficacy of liposomal amphotericin B (LAmB) compared to conventional amphotericin B deoxycholate (DAmB) is due to several factors including, its chemical composition, rigorous manufacturing standards, and ability to target and transit through the fungal cell wall. Numerous preclinical studies have shown that LAmB administered intravenously distributes to tissues frequently infected by fungi at levels above the minimum inhibitory concentration (MIC) for many fungi. These concentrations can be maintained from one day to a few weeks, depending upon the tissue. Tissue accumulation is dose-dependent with drug clearance occurring most rapidly from the brain and slowest from the liver and spleen. LAmB localizes in lung epithelial lining fluid, within liver and splenic macrophages and in kidney distal tubules. LAmB has been used successfully in therapeutic and prophylactic animal models to treat many different fungal pathogens, significantly increasing survival and reducing tissue fungal burden.
Keywords: liposomal amphotericin B, LAmB, preclinical, pharmacokinetics, pharmacodynamics
Amphotericin B (AmB) was introduced into clinical practice in 1959, and for more than 6 decades has remained an important life-saving drug for a wide range of endemic and opportunistic fungal diseases. Yet, the formidable nephrotoxicity of AmB, which became a larger clinical problem in the 1980s and 1990s with the global increase in the immunocompromised patient population and the rise in invasive fungal diseases, created a dire medical need for safer but equally effective treatment alternatives. This medical need eventually fueled the development of a new class of azole antifungals, the triazoles, and echinocandin antifungal agents, which have now largely replaced conventional amphotericin B deoxycholate (DAmB) as the preferred frontline therapy for common invasive fungal infections (IFIs) [1, 2].
However, these treatment alternatives to AmB have their own limitations. Triazole antifungals are predisposed to potentially serious pharmacokinetic (PK) drug interactions and hepatotoxicity. Voriconazole also carries the risk of visual hallucinations, solar hypersensitivity, and, in some instances, cutaneous malignancies. Fluconazole has an excellent safety profile but a limited antifungal spectrum. Similarly, echinocandins are primarily useful for the treatment of invasive candidiasis and must be administered intravenously. Perhaps most ominous is the emergence of resistance to triazoles and echinocandins in Candida spp. [3–7] and Aspergillus spp. [8], raising concerns about the future viability of these antifungal classes.
In hindsight, it was fortunate that an alternative strategy was pursued by several investigators to reduce the toxicity of AmB [9, 10], by considering alternate formulations of AmB to improve its therapeutic index. These research efforts ultimately led to the development and clinical introduction of 3 lipid formulations of AmB during the 1990s—liposomal amphotericin B (LAmB; AmBisome®), amphotericin B lipid complex (ABLC; Abelcet®), and amphotericin B colloidal dispersion (ABCD; Amphotec®). For the purpose of this article, the term “LAmB” refers exclusively to “AmBisome®.” All 3 formulations were approved based on their improved safety profile and demonstrated efficacy for IFIs, specifically in the salvage setting in patients who failed or were intolerant to conventional DAmB [9, 11–15]. In randomized, controlled clinical trials, LAmB was proven to be an effective agent for empirical antifungal therapy in persistently febrile neutropenic patients [16], cryptococcal meningitis [17], invasive aspergillosis [18], invasive candidiasis [19], and visceral leishmaniasis [20] and emerged as the most widely studied and prescribed lipid formulation of AmB.
Reformulation of AmB into a liposome carrier alters the PK and pharmacodynamic (PD) characteristics of AmB in ways that were probably not fully appreciated when LAmB was introduced more than 25 years ago [21]. This has led to persistent questions on how LAmB should be optimally dosed and whether its persistent antifungal effects in tissues may allow for less frequent dosing. Similarly, the field of antifungal PK/PD evaluation has matured over the past 2 decades with the development of relevant animal infection models and PK/PD modeling that have provided a clearer picture of how tissue PK characteristics of LAmB are important for understanding its antifungal efficacy and optimal dosing. In this article, we review the pharmacology and preclinical PK/PD of LAmB over the past 25 years and discuss how these preclinical data can improve dosing in the future.
HOW DOES THE LIPOSOME ALTER THE MECHANISM OF AMB ANTIFUNGAL ACTIVITY?
Several mechanisms have been proposed to explain the antifungal activity of AmB. The most widely cited mechanism is that through interaction with ergosterol in the fungal cell membrane, AmB molecules self-assemble as 4–12 subunit oligomers to form small (approximately 1 nm) membrane-permeabilizing ion channels that allow leakage of K+, Mg++, and organic substrates [22–24]. This mechanism would account for the rapid, concentration-dependent fungicidal activity of AmB and the still relatively low rates of resistance over the past 6 decades. Sokol-Anderson and colleagues demonstrated that AmB also has auto-oxidative properties that result in the generation of superoxide, hydrogen peroxide, and hydroxyl radicals that oxidize lipid membranes and lipoprotein receptors, impairing cell membrane function [25].
More recent studies suggest that the interaction of AmB with ergosterol, irrespective of pore formation, may be sufficient to produce fungicidal activity [26, 27]. AmB can adsorb to and sequester cell membrane ergosterol, causing destabilization of the cell membrane, or can aggregate around ergosterol at the cell membrane surface to act as a sponge that extracts ergosterol from the cell membrane [28].
The mechanism of action of LAmB depends on the presence of AmB in the liposome bilayer, the chemical composition of the liposome, its binding affinity for fungal cell walls [29], and its ability to transit intact through the cell wall and bind with ergosterol in the fungal cell membrane [30]. The lipids of LAmB include hydrogenated soy phosphatidylcholine with a gel-to- liquid crystalline phase transition temperature above 37°C [31], thus ensuring the stability of the liposomes when injected intravenously with minimal release of AmB into the circulation [32]. Distearoyl phosphatidylglycerol, another important liposome component, is similar in length to that of the hydrophobic region of AmB, with a net negative charge that allows the formation of an ion pair with the positively charged amino group of AmB [29]. The cholesterol in the liposome bilayer binds with AmB [33], enabling AmB to remain associated with the liposome rather than causing toxicity, which would follow if AmB were to be released from the liposome and instead bound to the cholesterol in mammalian cell membranes.
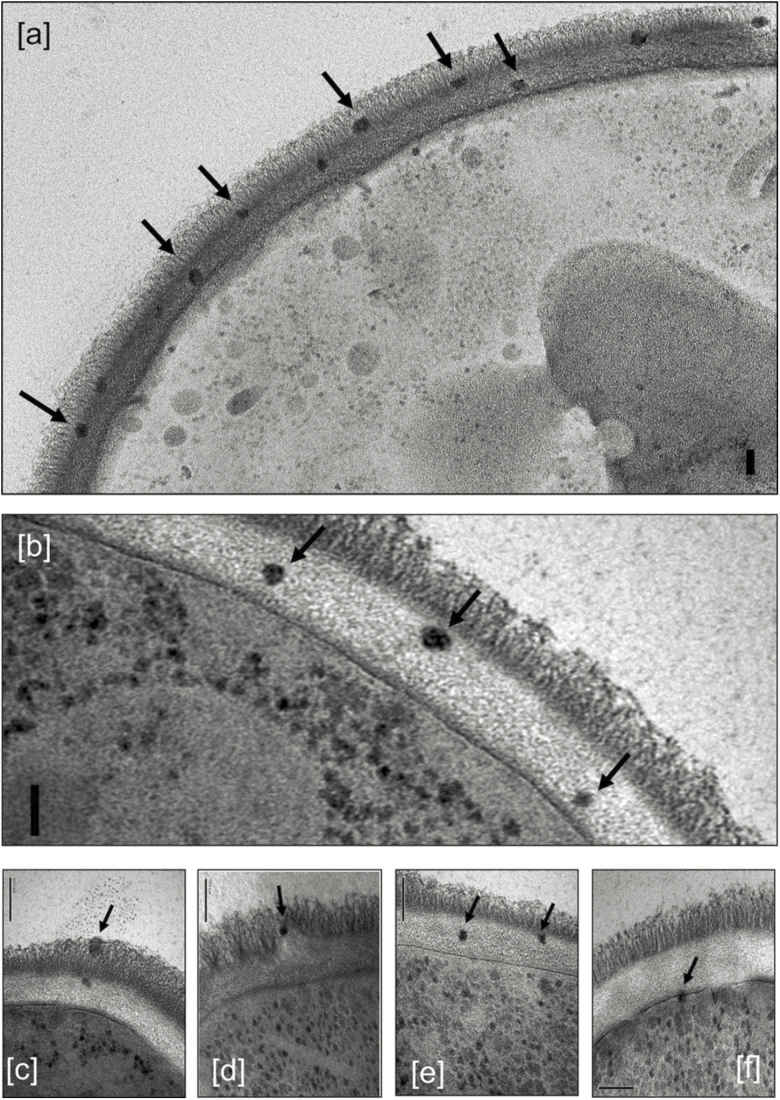
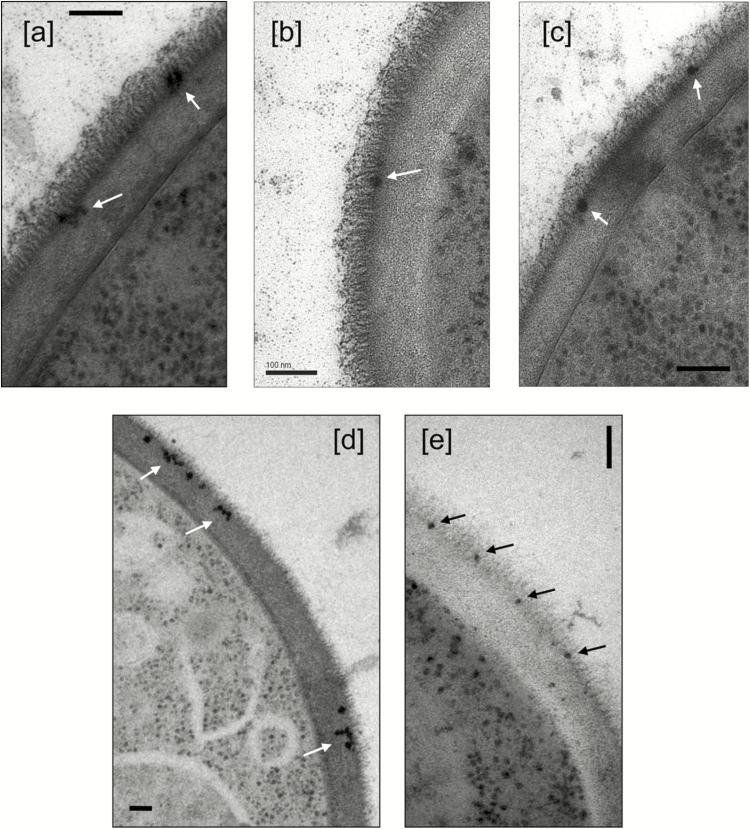
The chemical composition of LAmB, which is consistent as a result of its stringently regulated manufacturing conditions, results in AmB binding to the cell wall of yeasts and molds, both in vitro [34, 35] and in vivo [35–37], as demonstrated in studies that used fluorescent- and gold-labeled liposomes. Initially, it was hypothesized that following cell wall binding, AmB would be released from the liposome bilayer because of the 10-fold higher affinity of AmB for ergosterol in the fungal cell membrane compared with cholesterol in the liposomes. This would lead to the breakdown of liposomes at the outer portion of the cell wall and transit of free AmB through the cell wall to interact with ergosterol in the cell membrane. However, recent studies with Candida albicans and Cryptococcus neoformans, using cryofixation techniques with electron microscopy, have shown that the liposomes do not break down following binding to the fungal cell wall, but instead transit intact through the fungal cell wall (Figure 1), provided the fungus is not ergosterol deficient (Figure 2) [30]. These observations are important because they provide valuable insight into the viscoelastic properties of fungal cell walls. Since the diameter of the liposomes is 60–80 nm and the porosity of the cell wall has been estimated to be only about 5.8 nm, these results suggest that there is rapid cell wall remodeling that allows liposomes to move intact through the cell wall to ergosterol in the cell membrane, where it then releases AmB.
Figure 1.
Transmission electron microscopy images of Candida albicans SC5314 incubated with 12 µg/mL liposomal amphotericin B showing intact liposomes in the outer (A, C, and D) and inner (A, B, C, and E) cell wall and at the cell membrane (F), indicated by arrows. The granular particles in the cytoplasm are ribosomes, not liposomes. The bars represent 100 nm. Reproduced with permission from Walker L et al. MBio 2018; 9:e02383–17 [30].
Figure 2.
Liposomes with no incorporated amphotericin B (A–C) and an erg3-1 mutant (D) and erg11 mutant (E) of Candida albicans with liposomal amphotericin B both showing a deficiency in entering the inner cell wall layer. The bars represent 100 nm. Reproduced with permission from Walker L et al. MBio 2018; 9:e02383–17 [30].
Other lipid formulations of AmB have similar or different chemical compositions compared with LAmB, but these formulations exhibit different PK/PD characteristics and toxicity profiles [38, 39]. Consequently, the data presented with respect to LAmB cannot be extrapolated to other lipid formulations; this is true even if the formulation has the same lipid components as LAmB. The reason why the data cannot be extrapolated is the importance of controlling how the liposomes are assembled during manufacturing, since this is critical for ensuring the reduced toxicity and efficacy of the formulation. For example, when LAmB was compared with Anfogen (Genpharma, S.A., Argentina; a lipid formulation that has a chemical composition that is similar to that of LAmB but manufactured under different conditions), physical and biological testing demonstrated that LAmB batches had more consistent sizes than those of Anfogen. In addition, based on in vivo 50% lethal dose testing, Anfogen was at least 5-fold more toxic than LAmB and approximately 10-fold more toxic based on a red blood cell K+ release toxicity assay [38]. In a murine pulmonary aspergillosis model, LAmB treatment resulted in markedly better reduction of lung fungal burden compared with Anfogen, when administered at doses of 7.5 mg/kg and 15 mg/kg. Anfogen was also significantly more nephrotoxic than LAmB, with elevated levels of blood urea nitrogen and serum creatinine (Table 1) and extensive renal tubular necrosis seen on histological examination of tissue samples.
Table 1.
Blood Urea Nitrogen and Blood Creatinine Levels for Groups of Mice Infected With Aspergillus fumigatus and Treated 3 Times With the Indicated Doses of Liposomal Amphotericin B, Anfogen, and Control
| Measurement | Treatment Group | Concentration (mg/dL) in Samples From Mice Receiving 3 Doses of: | ||||
|---|---|---|---|---|---|---|
| Control (D5W) | 3 mg/kg | 5 mg/kg | 7.5 mg/kg | 15 mg/kg | ||
| Blood urea nitrogen | Control (D5W) | 12.80 ± 1.50 | … | … | … | … |
| Anfogen | … | 14.00 ± 1.30 | 22.20 ± 6.73 | 78.20a ± 8.09 | … | |
| LAmB | … | 12.60 ± 0.81 | 13.60 ± 1.03 | 12.00 ± 0.45 | 17.20 ± 0.92 | |
| Creatinine | Control (D5W) | 0.34 ± 0.02 | … | … | … | … |
| Anfogen | … | 0.36 ± 0.02 | 0.42 ± 0.02 | 0.64a ± 0.05 | … | |
| LAmB | … | 0.34 ± 0.02 | 0.38 ± 0.02 | 0.36 ± 0.02 | 0.38 ± 0.02 | |
Reproduced with Permission from Olson JA et al. Antimicrob Agents Chemother 2008; 52:259–68 [38]. Data are presented as means ± standard errors.
Abbreviations: D5W, dextrose 5% in water; LAmB, liposomal amphotericin B.
a P = .008 vs D5W; Mann–Whitney test.
HOW DOES INCORPORATION OF AMB IN THE LIPOSOME ALTER ITS TOXICITY?
Infusion-related Toxicities
Clinical use of AmB is often associated with severe infusion-related toxicities that can result in termination of treatment. These toxicities include fever, rigors, headache, arthralgia, nausea, vomiting, and hypotension and may be experienced by more than two-thirds of patients during the 2–6 hours of DAmB infusion [16, 40]. Because of the high frequency of infusion-related reactions, a standard practice in many institutions is to provide premedication drugs (ibuprofen, acetaminophen, antihistamines, hydrocortisone, or meperidine), as needed to ameliorate reactions. Although it is common protocol in hospitals to use acetaminophen and diphenhydramine, the rationale for the latter medication is not based on the known tumor necrosis factor-alpha (TNF-α) release that is associated with DAmB administration. While there are studies that clearly demonstrate the benefits of hydrocortisone (intravenous [IV] administration 1 mg/kg up to 50 mg IV) in preventing the infusion-related toxicity of DAmB, continued use of this strategy may result in adrenal insufficiency and chronic immunosuppression. A pooled analysis of premedication strategies has not identified a clinical benefit for routine antipyretic, anti-inflammatory, or antihistamine premedication [41].
Studies have been conducted to help elucidate the mechanism that underlies the infusion-related toxicities. AmB activates Toll-like receptor 2 (TLR2) microbial pattern recognition receptors through CD14-associated lipid rafts in mononuclear cells, which results in release of proinflammatory cytokines including TNF-α, interleukin (IL)-1β, IL-6, IL-8, and prostaglandin E2 [42, 43]. The onset of symptoms after infusion correlates with a rise in serum TNF-α, IL-1RA, and IL-6 [44]. It is unclear whether the infusion time of DAmB affects the frequency or severity of reactions, with some studies reporting higher rates of reactions with shorter infusions of 45 minutes vs 2-hours [45], while other studies have found no difference between 1- and 4-hour infusions [46, 47].
Encapsulation of AmB inside a liposome markedly reduces acute infusion-related toxicities [16, 48–50], confirmed by significantly lower rates of TNF-α, IL-1RA, and IL-6 liberation into the serum of patients who were administered LAmB vs DAmB or other lipid formulations [44]. Reduced immune activation has been linked to a reduction in TLR2 activation and proinflammatory cytokine elaboration by mononuclear cells by LAmB [51]. Notably, a reduced proinflammatory cytokine response may also reduce the risk of developing renal impairment during LAmB treatment [52]. This mechanism of increased nephrotoxicity may be related to DAmB-induced release of locally produced TNF-α in the renal parenchyma that would lead to increased afferent arteriolar vasoconstriction, decreased renal blood flow, and increased serum creatinine [53].
Similar to other particulate drug delivery systems, LAmB can be associated with a unique type 1 hypersensitivity reaction termed “complement activation-related pseudoallergy” (CARPA) [54]. This reaction results from activation of complement through both classic and alternative pathways, giving rise to C3a and C5a anaphylatoxins that trigger mast cell and basophil secretory responses. LAmB-triggered CARPA typically presents with a triad of symptoms known as severe acute infusion-related reactions (IRRs) including: (1) chest pain, dyspnea, hypoxia; (2) abdominal, flank, or leg pain; and (3) flushing and urticaria [49, 55]. Unlike classic AmB IRRs that develop over 2–6 hours, CARPA develops within the first 5 minutes of the first infusion and spontaneously resolves when the drug is stopped. In addition to discontinuing infusion, patients also appear to respond to IV administration of diphenhydramine, consistent with the mechanism of this reaction. The reaction may be milder or absent with repeated exposure; however, in some patients, the reaction may be sufficiently severe that further treatment should be avoided. The IRRs to ABLC are typically TNF-α driven and not the CARPA pattern. Consistent with this observation, current evidence suggests that patients who develop a CARPA reaction during LAmB treatment can be safely switched to ABLC [56].
Nephrotoxicity
Nephrotoxicity during DAmB therapy occurs through 2 mechanisms [57]. The first involves direct constriction of the renal arterioles, resulting in reduced renal perfusion and a drop in glomerular filtration rate (GFR) [58]. Patients with pre-existing decreased intravascular volume, hyponatremia, hypokalemia, and congestive heart failure are more likely to experience marked declines in GFR during AmB infusions. Tubuloglomerular feedback (TGF), a normal physiological response that causes afferent arteriolar vasoconstriction as a result of increased solute concentrations (especially a decreased Na+/K+ ratio) in the distal tubule, is also activated during AmB therapy, contributing to reduced GFR [59]. The signaling mediators of TGF at the afferent arteriole are thought to include calcium channels, TNF-α, and cyclic AMP. The practice of administering 500–1000 mL of normal saline in adults or 3 mEq/kg in children, referred to as “sodium loading,” immediately before and after AmB administration can reduce renal arteriolar vasoconstriction by increasing the solute concentration, especially the Na+/K+ ratio, and blunt TGF to maintain the GFR and restore electrolyte homeostasis [60]. In cases of myocardial dysfunction, the saline load can be infused over the course of 24 hours.
AmB can also cause direct damage to the distal tubular membranes of the kidney, presumably through its binding to cholesterol and formation of pores [61]. Pore formation reduces the ability of the tubular membrane to resorb electrolytes, resulting in loss of potassium and bicarbonate. As a result, hypokalemia and hypomagnesemia are frequently observed during DAmB treatment even before a decrease in GFR and an increase in serum creatinine are evident. The tubular toxicity of DAmB is most commonly evident as hypokalemia, and occurs in most patients who receive DAmB [61]. In approximately 5% of patients treated with DAmB for cryptococcal meningitis (at doses of 0.7–1.0 mg/kg/day), potassium supplementation, often as high as 80–120 mEq/day, is frequently required to reduce the risk of severe hypokalemia (<2.5 mmol/L) [62]. Distal tubular dysfunction also results in impaired resorption of magnesium [63], which complicates the ability to maintain potassium homeostasis. Magnesium deficiency allows excessive secretion of potassium through maxi-K channels in the distal tubules and collecting duct cells, thereby exacerbating hypokalemia until magnesium stores are replenished [64].
Compared with conventional DAmB, LAmB treatment has been associated with significantly lower rates of nephrotoxicity in preclinical animal models [65–67]. The reasons for the reduced nephrotoxicity of the liposomal formulation may include the preferential distribution of liposomes in organs rich in reticuloendothelial cells [53] and because AmB remains locked inside liposomes that do not undergo glomerular filtration due to the size of the particles [44]. However, free or readily diffusible AmB released from liposomes can still cause distal tubular damage, resulting in hypokalemia and decreased GFR, especially when LAmB is administered at higher than approved doses (>5 mg/kg/day) for prolonged periods (ie, >2 weeks) [18, 68, 69].
In several animal models, LAmB was less nephrotoxic than DAmB, although there was a slight rise in serum transaminases with prolonged administration [10, 65–67, 70–72]. Multiple-dose exposure studies in uninfected rats and beagle dogs in doses up to 20 mg/kg/day and 16 mg/kg/day, respectively, for 30 consecutive days revealed that LAmB had the same toxic effects as DAmB. Toxicity was linearly related to dosage, but appeared at much higher plasma exposures compared with those of DAmB [67, 71, 72].
In long-term exposure studies in rats given up to 12 mg/kg/day LAmB for 91 days, with a 30-day recovery period, chemical and histopathologic changes demonstrated that the kidneys and liver were the target organs for chronic toxicity. Nephrotoxicity was moderate (urea nitrogen ≤51 mg/dL; creatinine unchanged), and most toxic changes occurred early, stabilized by the end of dosing, and reversed during recovery with no delayed toxicities [66]. Much higher concentrations of LAmB were required to produce the deleterious effects on neutrophil function seen with DAmB [73]. Moreover, there is no experimental evidence to support impaired bacterial blood clearance by the mononuclear phagocytic cells after prolonged treatment with LAmB at clinically relevant doses [74].
HOW DOES THE LIPOSOME FORMULATION MODULATE THE IMMUNOLOGICAL ACTIVITY OF AMB?
It is reasonable to assume that some degree of AmB efficacy in vivo may be attributed to the ability of AmB to elicit a proinflammatory state in mononuclear and polymorphonuclear (PMN) leukocytes via CD14 and TLR2 signaling [42]. In neutrophils and macrophages, AmB enhances phagocytosis and the oxidative mechanisms of killing Aspergillus conidia [51].
Lipid formulations of AmB also display immunomodulatory activities for neutrophils, mononuclear cells, and pulmonary alveolar macrophages when incubated in vitro with medically important fungi. DAmB and ABLC additively augment the fungicidal activity of pulmonary alveolar macrophages against the conidia of Aspergillus fumigatus. DAmB, ABLC, and LAmB display similarly additive effects with polymorphonuclear leukocytes in damaging the hyphae of A. fumigatus [75]. When DAmB, ABLC, LAmB, and ABCD were studied in parallel against A. fumigatus and Fusarium solani with human neutrophils or mononuclear cells, the higher concentrations of the AmB lipid formulations elicited greater phagocyte-induced hyphal damage of both fungi than the lower concentrations [76]. At the same time, superoxide production was not affected by the lipid formulations, suggesting that enhanced nonoxidative mechanisms may contribute to the augmented hyphal damage.
Enhanced PMN leukocyte oxidative reactions may result in greater damage to host tissues in the absence of complementary nonoxidative mechanisms because products of oxidative stress impede phagocytic-dependent clearance of inflammatory products [77] and because excess production of reactive oxygen intermediates can adversely affect the ability of the host to oppose inflammatory pathology [78]. Consequently, the proinflammatory properties of AmB may be detrimental in fungal diseases with a component of inflammatory pathology. Balloy and colleagues demonstrated that although AmB reduced mortality in a chemotherapy (neutropenic) mouse model of invasive pulmonary aspergillosis, the antifungal drug had no discernable effect on mortality vs vehicle alone (control) in corticosteroid-immunosuppressed mice where disease pathology was driven by inflammation [79]. Using a model of Aspergillus pneumonia in T cell–depleted allogeneic transplanted, non-neutropenic mice, Bellocchio and colleagues observed 100% mortality and only a modest reduction in lung fungal burden following treatment with DAmB [51]. By contrast, treatment with LAmB at higher doses resulted in 100% mouse survival, with a significant accompanying reduction in lung fungal burden. The difference in antifungal activity and animal survival was attributed to the specific effects of the liposome, which attenuated the proinflammatory effects of AmB by diverting TLR2 signaling in neutrophils to TLR4 and by enhancing the nonoxidative mechanisms of neutrophil antifungal killing [51].
The immunomodulatory effects of liposomes in neutrophils were subsequently confirmed using LAmB as well as the empty (non-drug–containing) liposome [80]. In a corticosteroid-immunosuppressed mouse model, pretreatment with empty liposomes improved lung fungal clearance and animal survival following intranasal inoculation with A. fumigatus. The protective effect of the empty liposomes approached that of the 10 mg/kg/day LAmB dose and was significantly greater than the 1 mg/kg/day dose of DAmB. When neutrophils were collected and tested ex vivo for their ability to kill A. fumigatus hyphae, cells from animals treated with LAmB or empty liposomes exhibited a significantly greater ability to damage fungal hyphae compared with animals administered saline or DAmB [81].
These observations, in conjunction with results from other studies that have demonstrated that LAmB exerts additive activity with host immune cells against a variety of medically important fungi [75, 76, 81] and has potent anti-inflammatory and immunomodulatory activity [82–85], suggest that liposomes are not an inert carrier of AmB. Liposomes change how AmB interacts with the host immune system and, in preclinical models, engender more favorable antifungal effector mechanisms in the setting of excessive PMN-mediated damage to the lung.
HOW DOES THE LIPOSOME FORMULATION ALTER THE IN VITRO PHARMACODYNAMICS OF AMB?
In Vitro Susceptibility Testing
In vitro broth microdilution reference methods have been standardized for susceptibility testing of AmB against yeast and molds by the Clinical Laboratory Standards Institute (CLSI) [86, 87] and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [88, 89]. In general, AmB minimum inhibitory concentrations (MICs) determined using reference methods produce comparable results, although essential agreement is lower for Mucorales [90]. The CLSI has also developed standardized protocols for susceptibility testing of yeast by disk diffusion [87]. There are also several commercially available testing methods (eg, Sensititre YeastOne, VITEK 2, and Etest) that produce MICs in good agreement with reference methods.
Susceptibility testing with reference or commercial methods is always performed with analytical-grade AmB typically dissolved in dimethyl sulfoxide and not the commercial LAmB formulation. Direct testing of LAmB often results in MICs that are higher than those observed when MIC trays are prepared with analytical-grade powder [91–95].
Interpretation of AmB MIC data remains problematic because it is still unclear if current testing methods can reliably distinguish between susceptible and resistant isolates. Broth microdilution testing methods, in particular, often generate MICs that fall within a narrow range of dilutions (0.25–1.0 mg/L) that may be within the accepted error range of MICs tested for quality control strains [96]. Moreover, AmB-resistant strains are often not included in routine susceptibility testing for quality control. Finally, evidence concerning the correlation between AmB MICs and clinical outcome is inconsistent. A number of studies have found no correlation between AmB susceptibility and the clinical outcome of IFIs [97–102], while some studies have noted some correlation [103, 104].
The strongest evidence of in vitro, in vivo, and clinical correlation between AmB MICs and increased risk of treatment failure is with the intrinsically-resistant Aspergillus terreus species [105–107]. However, due to problems encountered with the broth media, resulting in narrowing of the MIC range, there are no validated CLSI interpretive breakpoints. Currently, the CLSI has not endorsed AmB susceptibility breakpoints, whereas EUCAST has proposed breakpoints of MIC ≤1 mg/L susceptible, >1 mg/L resistant for Candida spp. and a MIC ≤1 mg/L susceptible, >1 mg/L resistant for A. fumigatus and Aspergillus niger. Other molds known to have high in vitro AmB MICs that correlate with in vivo and clinical resistance include Fusarium spp., Pseudallescheria spp., and Lomentospora prolificans [108–110].
The in vitro, in vivo, and clinical resistance to AmB therapy has correlated more strongly with the minimum lethal concentration (MLC) or minimum fungicidal concentration (MFC) for several organisms, including Candida parapsilosis [111], Candida glabrata [112], and Trichosporon beigelii. The strongest predictors for microbiologic failure for candidemia in the study by Nguyen et al were 48-hour MLC (P < .001) and 24-hour MLC (P = .03) [112]. Trichosporon beigelii, which is resistant to the fungicidal effect of AmB in vitro and in vivo, emerged as a frequent cause of breakthrough fungemia in persistently neutropenic patients prior to the common use of fluconazole for antifungal prophylaxis [113]. Despite the low AmB MICs of <0.5 mg/mL, T. beigelii infections in persistently neutropenic rabbits were resistant to AmB and a multilamellar liposomal formulation of AmB [114], while triazoles were highly active in brain tissue and are now the preferred treatment for infections caused by this AmB-resistant pathogen.
Antifungal Activity in Biofilms
Fungal biofilms are resistant to varying degrees to both AmB and triazole antifungals. Extracellular (1→3)-β-D-glucans that make up a large part of the biofilm matrix directly bind and sequester AmB [115]. AmB MICs are 4- to 8-fold higher when the drug is tested in vitro against Candida spp. or Aspergillus spp. grown in biofilm vs planktonic conditions [116–118]. However, LAmB and ABLC largely retain antifungal activity against biofilm-embedded organisms, suggesting that the lipids may shield AmB from sequestration by the glucans of the biofilm matrix, and thus have better in vitro activity than AmB against biofilm-embedded fungi. In an in vitro model that simulated in vivo catheter lock therapy [119], 4 hours of LAmB exposure at a concentration of only 0.2 mg/mL reduced the metabolic activity of C. albicans, C. glabrata, and C. parapsilosis by at least 75% in 12-hour-old biofilms. In comparison, the same yeasts in 5-day-old biofilms were similarly susceptible to LAmB but only at 1.0 mg/mL.
In an in vivo study in rabbits with indwelling catheters containing 3-day-old C. albicans biofilms, LAmB at 10 mg/mL was locked in the catheter for 8 hours each day for 7 days [120]. At the end of the study, the liposome-treated catheters were free of biofilms and all catheter cultures were negative, while control catheters had many biofilm patches and all catheter segments yielded positive cultures for yeast.
Given the frequency of Candida infections in patients with urinary catheters, topical application of LAmB has also been examined in a preclinical model of ascending C. albicans urinary tract infection [121]. Administration of 200 μg LAmB transurethrally (drug lavage) every day for 5 days starting 24 hours post-yeast challenge reduced the yeast to undetectable levels in the bladder compared with the untreated mice that had about 1000 colony-forming units per gram in the bladder.
HOW DOES THE LIPOSOME FORMULATION ALTER THE IN VIVO PK/PD OF AMB?
Pharmacokinetics
AmB PK vary depending on the animal species. In general, after IV administration, AmB is primarily bound to lipoproteins, albumin, and erythrocytes [122]. Because of its limited solubility, free drug concentrations of AmB are limited to less than 1 mg/L [122]. Peak serum concentrations of AmB are achieved during the first hour, then rapidly fall to a plateau phase with levels of 0.2–0.5 mg/L in serum for approximately 24 hours, followed by a more prolonged terminal elimination phase that lasts several days [67]. This terminal elimination phase most likely represents the slow release of AmB from tissues.
In animal models, the highest concentrations of AmB are found in the liver, spleen, lung, and kidneys [123]. Concentrations of AmB in uninflamed meninges are 30- to 50-fold lower than concurrent serum levels [123], with minimal concentrations in the cerebrospinal fluid (0.002–0.010 mg/L) [124]. However, higher concentrations are detected in the brain parenchyma with persistent antifungal effects [125, 126]. The concentrations of AmB in brain tissue after administration of LAmB exceed those after administration of DAmB in experimental Candida meningoencephalitis [126]. With DAmB, concentrations in peritoneal, pleural, and joint fluids are less than 50% of concurrent serum levels [123]. Lung tissue concentrations are approximately 8-fold higher for DAmB and 4-fold lower for LAmB than concomitant serum concentrations, with 5-fold higher penetration of LAmB into the epithelial lining fluid (ELF) compared with DAmB and similar levels in the pulmonary alveolar macrophages [127]. AmB given intravenously does not penetrate the uninflamed eye but may be detected in the aqueous and vitreous humor when inflammation is present, with significantly higher levels of LAmB vs DAmB in both compartments [128].
The stability of LAmB after IV injection and the small size of the particles, along with targeting of LAmB to fungal cell walls, combine to facilitate penetration of the liposomes into many different tissues as mentioned above. This penetration has been reported in both uninfected and infected animal models and results in localization of the liposomes at sites of fungal infection in the lungs, liver, spleen, kidneys, and brain [129] (Table 2). Since the liposomes are less than 100 nm in size, they will initially bypass uptake by the macrophages in the reticuloendothelial system (RES) tissues. Over the next 24 hours, the circulating liposomes will be slowly taken up by the macrophages and can be found in highest concentrations in the liver and spleen. The delay in their removal by the RES leads to their distribution into the non-RES tissues of the lungs and kidneys, where they localize in the ELF and alveolar macrophages of the lungs [127], the distal tubules of the kidneys, and macrophages of the liver and spleen [139], with some minimal distribution into the brain. There is a nonlinear increase in drug concentration as the dose of LAmB is increased, and this is particularly important when the drug is given on a daily basis for a few days to several weeks to treat fungal infections. Overall, the relative concentration of LAmB in animal tissues, from highest to lowest, is liver = spleen >> kidneys > lungs > brain, and the levels achieved in the tissues are above the MIC for most fungi (Table 2). Based on preclinical studies, clearance of LAmB from these different organs varies from about 1 day for the brain, a few days for the lungs, to several weeks for the kidneys, spleen, and liver [129].
Table 2.
Tissue Concentrations of Amphotericin B Following Single or Multiple Doses of Intravenous Liposomal Amphotericin B in Infected Animals
| Animal Species | Infection Model | Time of Analysis Post-treatment (Hours) | Liposomal Amphotericin Dose, mg/kg (# Doses = ×) | Tissue | Amphotericin B Tissue Concentration (µg/g Tissue) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single-dose treatment | ||||||||||||
| Mouse | Systemic candidiasis | 4, 12, 24, 48 | 4 hours | 12 hours | 24 hours | 48 hours | van Etten et al (1995) [130] | |||||
| 7 (1 ×) | Liver | 64.6 | 85.2 | 97.8 | 82.3 | |||||||
| Spleen | 122 | 118 | 140 | 134 | ||||||||
| Kidney | 2.7 | 3.0 | 4.0 | 4 | ||||||||
| Lung | BLQ | 1.4 | 7.1 | 6.2 | ||||||||
| Mouse | Pulmonary aspergillosis | 4, 48 | 4 hours 0.96 17.6 | 48 hours 0.23 12.7 | Takemoto et al (2006) [37] | |||||||
| 1 (1 ×) | Lung | |||||||||||
| 10 (1 ×) | ||||||||||||
| Mouse | Pulmonary aspergillosis | 24 | 24 hours | Lewis et al (2007) [131] | ||||||||
| 5 (1 ×) | Lung | 3.3 | ||||||||||
| 10 (1 ×) | 15.6 | |||||||||||
| Mouse | Visceral leishmaniasis | 168 | 168 hours 0.26 6.79 |
Gershovich et al (2010) [132] Wasan et al (2009) [133] |
||||||||
| 2 (1 ×) | Liver | |||||||||||
| Spleen | ||||||||||||
| Multiple-dose treatment | ||||||||||||
| Mouse | Systemic candidiasis | 24, 336 | 24 hours 356 700 14.5 5.9 | 336 hours 170 243 7.8 BLQ | van Etten et al (1995) [130] | |||||||
| 7 (5 ×) | Liver | |||||||||||
| Spleen | ||||||||||||
| Kidney | ||||||||||||
| Lung | ||||||||||||
| Mouse | Pulmonary aspergillosis | 72 | 72 hours | Lewis et al (2007) [131] | ||||||||
| 5 (3 ×) | Lung | 8.2 | ||||||||||
| 10 (3 ×) | 13.0 | |||||||||||
| Mouse | Pulmonary aspergillosis | 24 | 24 hours 96.6 268 14.5 14.7 | Olson et al (2006) [134] | ||||||||
| 15 (3 ×) | Liver | |||||||||||
| Spleen | ||||||||||||
| Kidney | ||||||||||||
| Lung | ||||||||||||
| Mouse | Pulmonary mucormycosis | 24, 72, 120 | 24 hours | 72 hours | 120 hours | Lewis et al (2010) [135] | ||||||
| 5 (5 ×) | Lung | 1.4 | 1.0 | 3.7 | ||||||||
| 10 (5 ×) | 5.5 | 9.6 | 7.0 | |||||||||
| Mouse | Disseminated mucormycosis | 24 | 24 hours 5.8 10.4 BLQ BLQ | Ibrahim et al (2008) [136] | ||||||||
| 7.5 (2 ×) | Kidney | |||||||||||
| 15 (2 ×) | ||||||||||||
| 7.5 (2 ×) | Brain | |||||||||||
| 15 (2 ×) | ||||||||||||
| Mouse | Visceral leishmaniasis | 72, 1032, 2472 | 72 hours 1032 hours 2472 hours | Gangneux et al (1996) [137] | ||||||||
| 0.8 (6 ×) | Liver | 33.9 3.0 ND | ||||||||||
| 5 (6 ×) | 210 55.9 2.9 | |||||||||||
| 50 (6 ×) | 2575 808 215 | |||||||||||
| 0.8 (6 ×) | Spleen | 23.8 5.5 0.53 | ||||||||||
| 5 (6 ×) | 98.8 28.7 4.3 | |||||||||||
| 50 (6 ×) | 929 124 101 | |||||||||||
| 0.8 (6 ×) | Lung | ND ND ND | ||||||||||
| 5 (6 ×) | 1.6 ND ND | |||||||||||
| 50 (6 ×) | 35.9 5.0 1.6 | |||||||||||
| Rat | Systemic aspergillosis | 24 | 24 hours | Wasan et al(2007) [138] | ||||||||
| 5 (4 ×) | Liver | 110 | ||||||||||
| Spleen | 17.5 | |||||||||||
| Kidney | 1.1 | |||||||||||
| Lung | 2.6 | |||||||||||
| Heart | 0.6 | |||||||||||
| Brain | 0.7 | |||||||||||
| Rabbit |
Central nervous system candidiasis |
0.5 | 0.5 hours | Groll et al (2000) [126] | ||||||||
| 5 (7 ×) | Brain | 1.84 | ||||||||||
| Cerebrospinal fluid | BLQ | |||||||||||
Adapted from Adler-Moore JP et al. J Liposome Res 2017; 27:195–209, a Publication of Taylor & Francis Ltd (www.tandfonline.com) [129]
Abbreviations: BLQ, below the limits of quantification; ND, not determined.
To characterize single-dose plasma PK with tissue disposition of LAmB, investigators used healthy rabbits and administered LAmB IV at 0.5, 1.0, 2.5, 5, or 10 mg/kg or DAmB IV at 0.5, 1.0, or 1.5 mg/kg [65]. After a single 1 mg/kg dose of LAmB, the mean maximum concentration in serum (Cmax) was 26 ± 2.4 µg/mL and the mean area under the curve to infinity (AUC) was 60 ± 16 µg.h/mL, while a similar dose of DAmB achieved a significantly lower Cmax (4.7 ± 0.2 µg/mL) and a lower AUC (30.6 ± 2.2 µg.h/mL). Dose escalation of LAmB to 10 mg/kg resulted in a disproportionately higher Cmax (287 ± 14 µg/mL) and AUC (2223 ± 246 µg.h/mL), suggesting saturable elimination after a single dose. Whereas 2 of the 4 rabbits that received 1.5 mg/kg of DAmB died of acute cardiac toxicity, LAmB was administered without such toxicity at up to 10 mg/kg. After chronic dosing with LAmB at 5.0 mg/kg/day or DAmB at 1.0 mg/kg/day for 28 days, LAmB achieved peak levels of 122.8 ± 5.8 µg/mL and trough levels of 34.9 ± 1.8 µg/mL, while DAmB reached a peak of only 1.76 ± 0.11 µg/mL and a trough of 0.46 ± 0.04 µg/mL. Significant accumulations of AmB in the reticuloendothelial organs were observed, with 239 ± 39 µg/g in the liver after chronic dosing with LAmB, which was 7 times higher than the level in the liver of rabbits given chronic dosing with DAmB (33 ± 6 µg/g). However, accumulation in the kidneys remained 14-fold lower for LAmB vs DAmB (0.87 ± 0.61 µg/g vs 12.7 + 4.6 µg/g, respectively). During chronic dosing, nephrotoxicity occurred in only 1 in 4 animals treated with LAmB, while it occurred in all 4 animals that received DAmB.
Pharmacodynamics
In vivo, DAmB displays concentration-dependent PD that correlate with the ratio of total peak serum drug concentrations/MIC ratio for Candida [140] and Aspergillus [141] or AUC/MIC [93, 94]. In general, activity is maximized when the Cmax/MIC ratio surpasses 2 or when an AUC/MIC ratio, measured by bioassay, is greater than 10–50 depending on the organ investigated [94]. Al-Nakeeb and colleagues reported that in a murine model of invasive aspergillosis, near-maximal antifungal activity with DAmB was reached at an AUC/MIC of 13.6, which is well within clinically achievable exposures and typical MICs reported in human aspergillosis (AUC/MIC, 50) [142]. In the same model, near-maximal effects with LAmB dosing were observed with an AUC/MIC of 167, which was similar to mean AUC/MIC exposures (186 ± 96.2) predicted in 80 kg patients who received a 3 mg/kg/day dose of LAmB.
Given the significantly reduced toxicity of LAmB and the fact that the drug remains bioactive at antifungal inhibitory concentrations for more than 1 day in most tissues, investigators have used different animal models to study its prophylactic use. A single IV prophylactic dose of LAmB at 5, 10, or 20 mg/kg resulted in significantly prolonged survival when mice were subsequently challenged with C. albicans [143], Histoplasma capsulatum [143], or A. fumigatus [144] with reduced fungal burden in the kidneys, spleens, or lungs, respectively.
The efficacy of LAmB administered as a therapeutic drug has also been demonstrated in several models of IFIs in both normal and immunocompromised animals [9, 29, 145–149]. In the studies that examined different doses of LAmB from 1 mg/kg to as high as 30 mg/kg, given daily or every other day, doses that ranged from 5 to 15 mg/kg were found to be significantly more effective compared with controls when used to treat pulmonary aspergillosis [38, 135, 150, 151], systemic cryptococcosis [152, 153], systemic candidiasis [154, 155], pulmonary blastomycosis [156], pulmonary paracoccidioidomycosis [157], and the parasite infection visceral leishmaniasis [137] (Table 3).
Table 3.
Efficacy of Liposomal Amphotericin B and Deoxycholate Amphotericin B at Different Doses in Animal Models of Fungal Infection
| Tissue | Disease Model (Species) | Treatment | Dose (mg/kg) | % Survival | Log 10 Colony-forming Units | Reference |
|---|---|---|---|---|---|---|
| Lung | Aspergillosis (Rabbit) | LAmB | 0,a 1.0, 5.0, 10.0 | 7, 80, 100, 80 | 1.6-, 8-, 15-, 15-fold reduction compared with untreated animals | Francis et al (1994) [150] |
| DAmB | 1.0 | 30 | 15-fold reduction compared with untreated animals | |||
| Aspergillosis (Rat) | LAmB | 0, 1.0, 10.0 | 0, 0, 27 | 2.5, 0.9, 1.1 | Leenders et al (1996) [159] | |
| DAmB | 1.0 | 13 | 2.0 | |||
| Aspergillosis (Mouse) | LAmB | 0, 15 | 0, 86 | 4.5, 3.2 | Olson et al (2001) [160] | |
| ABLC | 15 | 29 | 4.5 | |||
| Aspergillosis (Mouse) | LAmB | 0, 6.05b | 40, 100 | 5.3, 0.54 | Allen et al (1994) [161] | |
| DAmB | 6.73b | 100 | 3.31 | |||
| Blastomycosis (Mouse) | LAmB | 0, 1.0, 3.0, 7.5, 15 | 0, 90, 100, 100, 100 | ---,c 6.53, 3.42, 0.22, 0.42 | Clemons et al (1993) [156] | |
| DAmB | 1.0 | 10 | 3.46 | |||
| Paracoccidioidomycosis (Mouse) | LAmB | 0, 0.6, 5.0, 15, 30 | 0, 7.1, 80, 79, 67 | ---,c 4.02, 5.09, 1.25, 0.56 | Clemons et al (1993) [157] | |
| DAmB | 0.6 | 47 | 7.11 | |||
| Brain | Coccidioidomycosis (Rabbit) | LAmB | 0, 7.5, 15, 22.5 | 37.5, 100, 100, 100 | 3.11, 1.18, 0.46, 1.11 | Clemons et al (2002) [162] |
| DAmB | 1.0 | 100 | 2.06 | |||
| Cryptococcosis (Mouse) | LAmB | 0, 3, 20, 30 | 0, 100, 100, 100 | 8.89, 5.96, 1.11, 0.61 | Albert et al (1995) [163] | |
| DAmB | 3.0 (intraperitoneal dosing) | 89 | 8.79 | |||
| Kidney | Candidiasis (Mouse) | LAmB | 0, 0.3, 7.0 | 10, 50, 100 | ---,c 3.96, 0.39 | Van Etten et al (1993) [155] |
| DAmB | 0.3 | 100 | 5.28 | |||
| Candidiasis (Rabbit) | LAmB | 0, 5.0 | 100, 100 | Significantly reduced (P < .01)d | Groll et al (2001) [164] | |
| DAmB | 1.0 | 100 | Significantly reduced (P < .001)d | |||
| ABLC | 5.0 | 100 | Not significantd | |||
| ABCD | 5.0 | 100 | Not significantd | |||
| Candidiasis (Mouse) | LAmB | 0, 1.0,e 5.0, 20.0 | 100, 100, 100, 100 | 6.22, 3.22, 3.46, 2.67 | Garcia et al (2000) [143] | |
| DAmB | 1.0 | 100 | 4.18 | |||
| Liver | Leishmaniasis | LAmB | 0, 0.04, 0.2, 1.0, 5.0 | 100, 100, 100, 100, 100 | 0, 15.8,f 41.2, 84.5, 99.8 | Croft et al (1991) [165] |
| DAmB | 0.04, 0.2, 1.0 | 100, 100, 100 | 3.4,f 22.0, 52.7 | |||
| Leishmaniasis | LAmB | 0, 0.8, 5.0, 50.0 | 100, 96, 96, 100 | 6.0, 1.0, 0, 0 | Gangneux et al (1996) [137] | |
| DAmB | 0.8 | 92 | 4.0 | |||
| Fusariosis | LAmB | 0, 3.0, 5.0, 10.0, 20.0 | 50, 100, 100, 100 | 4.8, 2.5, 2.7, 2.3, 2.4 | Ortoneda et al (2002) [166] | |
| DAmB | 1.5, 2.5 | 60, 100 | 4.2, 3.9 | |||
| Spleen | Histoplasmosis | LAmB | 0, 0.3, 0.6, 6.0 | 100, 100, 100, 100 | 8.68, 7.20, 6.66, 3.89 | Adler-Moore (1994) [35] |
| DAmB | 0.3, 0.6 | 100, 100 | 7.34, 6.30 | |||
| Leishmaniasis | LAmB | 0, 0.8, 5.0, 50.0 | 100, 96, 96, 100 | 7.0, 3.0, 0, 0 | Gangneux et al (1996) [137] | |
| DAmB | 0.8 | 92 | 6.5 | |||
| Fusariosis | LAmB | 0, 3.0, 5.0, 10, 20 | 50, 100, 100, 100 | 5.7, 4.5, 4.5, 3.2, 3.3 | Ortoneda et al (2002) [166] | |
| DAmB | 1.5, 2.5 | 60, 100 | 5.4, 5.3 | |||
| Mucosa | Vaginal candidiasis (Mouse) | LAmB | 0, 15, 20 | 100, 100, 100 |
3.26, 0, 0 (vaginal tissue) 3.0, 0, 0 (lavage) |
Gibbs et al (2002) [167] |
| Skin | Leishmaniasis | LAmB | 0, 6.25, 12.5, 25, 50 | 100, 100, 100, 100, 100 | +90,g +92, +45, –8, –68 | Yardley et al (1997) [168] |
| DAmB | 0.5 | 100 | +72g |
Adapted from Adler-Moore J and Proffit RT. Curr Opin Investig Drugs 2003; 4:179–85 [158].
a0 represents untreated control group throughout entire table.
bTotal dose as aerosol prophylaxis.
cColony-formimng units (CFUs) could not be determined, as all mice died by CFU assessment time.
dReductions in CFU compared with controls for significance.
eSingle-dose prophylaxis.
fPercentage clearance or inhibition of amastigotes in the liver.
gPercentage change in lesion size on day 24 post-treatment.
Abbreviations: ABCD, amphotericin B colloidal dispersion; ABLC, amphotericin B lipid complex; DAmB, deoxycholate amphotericin B; LAmB, liposomal amphotericin B.
The lung is an important site of IFIs because many fungi enter the host via the respiratory tract and spread locally and/or enter the bloodstream and disseminate to other organs. In preclinical single- and multidose distribution studies using equimolar doses of 1 mg/kg of AmB in uninfected mice and rats, lung levels achieved by LAmB were lower than those obtained by DAmB. However, after multiple dosing of LAmB at safely tolerated 5-fold higher doses, drug accumulation in the lung clearly exceeded that achieved by 1 mg/kg of DAmB [10].
Differences in lung distribution between DAmB and LAmB were examined in a lethal rabbit model of primary pulmonary aspergillosis that reproduced the persistent levels of profound granulocytopenia and the histopathologic features of bronchopneumonia, vascular invasion, and hemorrhagic infarction encountered in patients. Twenty-four hours after intratracheal A. fumigatus inoculation, groups of 5–18 profoundly granulocytopenic rabbits received LAmB at 1, 5, or 10 mg/kg/day, DAmB at 1 mg/kg/day, or normal saline for up to 10 days [150]. Surviving animals were euthanized 24 hours after the last dose was administered. Treatment with any dose of LAmB conferred significantly increased survival compared with treatment with the maximum tolerated dose of DAmB (1 mg/kg/day) and untreated controls. At 5 and 10 mg/kg/day, LAmB was more effective in reducing the number of viable organisms in the lung and in decreasing tissue injury. While animals treated with DAmB developed marked azotemia, as assessed by mean serum creatinine values at baseline and at end of treatment or death, the mean creatinine level remained normal in animals treated with 1 or 5 mg/kg of LAmB. However, at 10 mg/kg/day, significant increases in the mean serum creatinine occurred but without significant impact on survival. Thus, LAmB was significantly more effective and safer than DAmB for treatment of pulmonary aspergillosis in a rabbit model that mimicked the PK and PD in patients very closely.
A study was undertaken in healthy rabbits to compare the compartmentalized intrapulmonary PK of different AmB formulations including LAmB, DAmB, ABCD, and ABLC. This study showed strikingly different patterns among the different formulations at therapeutic dosages [127]. Cohorts of 3 to 7 catheterized rabbits received 1 mg/kg/day DAmB or 5 mg/kg/day of an AmB lipid formulation once daily for 8 days. Following serial plasma sampling, rabbits were euthanized 24 hours after the last dose, and ELF, pulmonary alveolar macrophages (PAM), and lung tissue were obtained. Mean (± standard deviation) AmB concentrations in lung tissue and PAM were highest in ABLC-treated animals, exceeding concurrent plasma levels by 70 fold and 375 fold, respectively. By comparison, drug concentrations in ELF were much lower than those achieved in lung tissue and PAM. Among the different cohorts, the highest ELF concentrations were found in LAmB-treated animals (2.28 ± 1.43 µg/mL) vs 0.44 ± 0.13, 0.68 ± 0.27, and 0.90 ± 0.28 μg/mL for DAmB, ABCD, and ABLC, respectively. In these experiments, only LAmB achieved an exposure that exceeded the proposed in vitro susceptibility breakpoints for AmB in all 3 compartments of the lung (Table 4).
Table 4.
Concentrations of Amphotericin B in Lung Tissue, Epithelial Lining Fluid, Pulmonary Alveolar Macrophages, and Peripheral Blood Monocytes After Once-daily Dosing for 8 Days
| Drug | Dose (mg/kg) | Mean Concentration ± Standard Deviation in: | ||||
|---|---|---|---|---|---|---|
| Lung Tissue (µg/g) | Epithelial Lining Fluid (µg/mL) | Pulmonary Alveolar Macrophages (µg/mL) | Peripheral Blood Monocytes (µg/mL) | Plasma (µg/mL) | ||
| Deoxycholate amphotericin B | 1 | 2.71 ± 1.22 | 0.44 ± 0.13 | 8.92 ± 2.84 | 1.20 ± 0.83 | 0.34 ± 0.07 |
| Amphotericin B colloidal dispersion | 5 | 6.29 ± 1.17 | 0.68 ± 0.27 | 5.43 ± 1.75 | 2.44 ± 1.90 | 0.37 ± 0.12 |
| Amphotericin B lipid complex | 5 | 16.26 ± 1.62 | 0.90 ± 0.28 | 89.1 ± 37 | 0.74 ± 0.42 | 0.24 ± 0.08 |
| Liposomal amphotericin B | 5 | 6.32 ± 0.57 | 2.28 ± 1.43 | 7.52 ± 2.50 | 1.51 ± 0.78 | 26.4 ± 4.99 |
Twenty-four hours after dosing. All values represent the means ± standard deviation from 3 to 7 rabbits in each dosing group. Plasma, concurrent plasma concentrations. Between-group comparisons using Dunn’s correction for multiple comparisons revealed significant differences in lung tissue concentrations between deoxycholate amphotericin B (DAmB)– and amphotericin B lipid complex (ABLC)–treated animals (P < .01), in epithelial lining fluid concentrations between DAmB– and Liposomal amphotericin B–treated animals (P < .01), and in pulmonary alveolar macrophages concentrations between amphotericin B colloidal dispersion– and ABLC–treated animals (P < .05). Reproduced with permission from Groll AH et al. Antimicrob Agents Chemother 2006; 50:3418–23 [127].
The central nervous system (CNS) is also an important target of IFIs [169, 170]. LAmB efficacy for several CNS animal infections has been demonstrated, including mucormycosis [136, 171], coccidioidal meningitis [162], cryptococcal meningitis [163], CNS aspergillosis [172], and Candida meningoencephalitis [126]. The CNS distribution and antifungal efficacy of LAmB were compared with other commercially available AmB formulations in a rabbit model of hematogenous C. albicans meningoencephalitis. Treatment with DAmB (1 mg/kg/day) or LAmB (5 mg/kg/day) yielded the highest Cmax, AUC0–24, and time above the MIC (Ttau > MIC) and led to complete eradication of C. albicans from brain tissue. By comparison, ABCD and ABLC (5 mg/kg/day each) were only partially effective. There was a strong correlation of Cmax, AUC0–24, Cmax/MIC, AUC0–24/MIC, and Ttau/MIC with clearance of C. albicans from brain tissue (P < .0002). Thus, there were strong concentration- and time-dependent correlations between plasma exposure and antifungal efficacy, indicating a potential advantage of LAmB for the treatment of CNS infections [126].
SUMMARY
The combination of LAmB’s unique chemical composition, rigorous manufacturing standards, and ability to target to and transit through fungal cell walls contribute to the improved safety profile and antifungal efficacy of this formulation compared with conventional DAmB. Based on results from numerous preclinical studies, LAmB given intravenously distributes to tissues most frequently infected by fungi, including the lungs, kidneys, liver, spleen, and brain, at drug levels that can be sustained above the MIC for 1 day to up to a few weeks depending on the tissue. Tissue accumulation and clearance with single or multiple IV administration is similar in uninfected and infected animals, with tissue accumulation being dose dependent and clearance fastest from the brain and slowest from the liver and spleen. In the lungs, the drug is primarily localized in the ELF; in the liver and spleen, it is mainly present in macrophages; and in the kidneys, it localizes to the distal tubules. It has been used successfully in both therapeutic and prophylactic animal models to treat yeast, mold, and endemic fungal pathogens, significantly increasing survival and reducing the residual fungal burden in target organs.
Notes
Acknowledgments.This supplement was made possible by funding from Gilead Sciences; however, Gilead had no input into the content. Editorial assistance in the preparation of this manuscript was provided by Christine Drewienkiewicz of OPEN Health Medical Communications (London, United Kingdom) and was funded by Gilead. T. J. W. was supported as a Scholar of the Henry Schueler Foundation for his work on this manuscript.
Disclaimer.The views, opinions, assumptions, or any other information set out in this article are solely those of the authors and should not be attributed to the funders or any person connected with the funders. Liposomal amphotericin B is not approved for prophylaxis.
Financial support.This work was supported by Gilead Sciences.
Supplement sponsorship.
Potential conflicts of interest.J. A.-M. has received grants, honoraria, and nonfinancial support from Gilead Sciences outside the submitted work and was involved in the discovery and development of liposomal amphotericin B by Vestar Inc. R. E. L. has received grants and personal fees from Gilead Sciences and Merck. R. J. M. B. has received grants and consulting fees from Gilead Sciences; has received unrestricted research grants and consulting fees from Astellas Pharma, Gilead Sciences, Merck Sharp & Dohme, and Pfizer; and has received consulting fees from F2G (all contracts were through Radboud UMC, and all payments were invoiced by Radboud UMC). B. J. A. R. has received grants and honoraria from Gilead Sciences outside the submitted work. A. H. G. has received grants for his institution from Gilead Sciences, Merck Sharp & Dohme, and Pfizer; has received personal fees from Amplyx, Astellas Pharma, Basilea Pharmaceutica, F2G, Gilead Sciences, Merck Sharp & Dohme, Schering-Plough, and SCYNEXIS outside the submitted work; and is on the speaker bureaus for Astellas Pharma, Basilea Pharmaceutica, Gilead Sciences, Merck Sharp & Dohme, Pfizer, Schering-Plough, and Zeneus/Cephalon. T. J. W. has received grants for his institution from Amplyx, Astellas Pharma, Merck, SCYNEXIS, Allergan, Medicines Company, Leadiant Biosciences, and Tetraphasel and has received honoraria from Astellas Pharma, Merck, SCYNEXIS, Allergan, Medicines Company, Gilead Sciences, and Leadiant Biosciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63:e1–60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrosky-Zeichner L. Candida glabrata and FKS mutations: witnessing the emergence of the true multidrug-resistant Candida. Clin Infect Dis 2013; 56:1733–4 . [DOI] [PubMed] [Google Scholar]
- 4. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A.. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 2017; 17:e383–92 . [DOI] [PubMed] [Google Scholar]
- 5. Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 2017; 64:134–40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamoth F, Kontoyiannis DP.. The Candida auris alert: facts and perspectives. J Infect Dis 2018; 217:516–20 . [DOI] [PubMed] [Google Scholar]
- 7. McCarthy MW, Walsh TJ.. Containment strategies to address the expanding threat of multidrug-resistant Candida auris. Expert Rev Anti Infect Ther 2017; 15:1095–9 . [DOI] [PubMed] [Google Scholar]
- 8. Verweij PE, Chowdhary A, Melchers WJ, Meis JF.. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 2016; 62:362–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Groll AH, Piscitelli SC, Walsh TJ.. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol 1998; 44:343–500 . [DOI] [PubMed] [Google Scholar]
- 10. Proffitt RT, Satorius A, Chiang SM, Sullivan L, Adler-Moore JP.. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother 1991; 28(Suppl B):49–61 . [DOI] [PubMed] [Google Scholar]
- 11. Ng TT, Denning DW.. Liposomal amphotericin B (AmBisome) therapy in invasive fungal infections. Evaluation of United Kingdom compassionate use data. Arch Intern Med 1995; 155:1093–8 . [PubMed] [Google Scholar]
- 12. Walsh TJ, Whitcomb P, Piscitelli S, et al. Safety, tolerance, and pharmacokinetics of amphotericin B lipid complex in children with hepatosplenic candidiasis. Antimicrob Agents Chemother 1997; 41:1944–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh TJ, Hiemenz JW, Seibel NL, et al. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis 1998; 26:1383–96 . [DOI] [PubMed] [Google Scholar]
- 14. Martino R, Cortés M, Subirá M, Parody R, Moreno E, Sierra J.. Efficacy and toxicity of intermediate-dose amphotericin B lipid complex as a primary or salvage treatment of fungal infections in patients with hematological malignancies. Leuk Lymphoma 2005; 46:1429–35 . [DOI] [PubMed] [Google Scholar]
- 15. Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH.. Amphotericin B: time for a new “gold standard.” Clin Infect Dis 2003; 37:415–25 . [DOI] [PubMed] [Google Scholar]
- 16. Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med 1999; 340:764–71 . [DOI] [PubMed] [Google Scholar]
- 17. Hamill RJ, Sobel JD, El-Sadr W, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis 2010; 51:225–32 . [DOI] [PubMed] [Google Scholar]
- 18. Cornely OA, Maertens J, Bresnik M, et al. ; AmBiLoad Trial Study Group Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis 2007; 44:1289–97 . [DOI] [PubMed] [Google Scholar]
- 19. Kuse ER, Chetchotisakd P, da Cunha CA, et al. ; Micafungin Invasive Candidiasis Working Group Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–27 . [DOI] [PubMed] [Google Scholar]
- 20. Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW.. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med 2010; 362:504–12 . [DOI] [PubMed] [Google Scholar]
- 21. Stone NR, Bicanic T, Salim R, Hope W.. Liposomal amphotericin B (AmBisome(®)): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016; 76:485–500 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cass A, Finkelstein A, Krespi V.. The ion permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol 1970; 56:100–24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grudzinski W, Sagan J, Welc R, Luchowski R, Gruszecki WI.. Molecular organization, localization and orientation of antifungal antibiotic amphotericin B in a single lipid bilayer. Sci Rep 2016; 6:32780 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Starzyk J, Gruszecki M, Tutaj K, et al. Self-association of amphotericin B: spontaneous formation of molecular structures responsible for the toxic side effects of the antibiotic. J Phys Chem B 2014; 118:13821–32 . [DOI] [PubMed] [Google Scholar]
- 25. Sokol-Anderson ML, Brajtburg J, Medoff G.. Amphotericin B-induced oxidative damage and killing of Candida albicans. J Infect Dis 1986; 154:76–83 . [DOI] [PubMed] [Google Scholar]
- 26. Boukari K, Balme S, Janot JM, Picaud F.. Towards new insights in the sterol/amphotericin nanochannels formation: a molecular dynamic simulation study. J Membr Biol 2016; 249:261–70 . [DOI] [PubMed] [Google Scholar]
- 27. Gray KC, Palacios DS, Dailey I, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 2012; 109:2234–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson TM, Clay MC, Cioffi AG, et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 2014; 10:400–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adler-Moore J, Proffitt RT.. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother 2002; 49(Suppl 1):21–30 . [DOI] [PubMed] [Google Scholar]
- 30. Walker L, Sood P, Lenardon MD, et al. The viscoelastic properties of the fungal cell wall allow traffic of AmBisome as intact liposome vesicles. MBio 2018; 9:e02383–17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papahadjopoulos D, Jacobson K, Nir S, Isac T.. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta 1973; 311:330–48 . [DOI] [PubMed] [Google Scholar]
- 32. Jensen GM, Bunch TH, Hu N, Eley CGS.. Process development and quality control of injectable liposome therapeutics. In: Jensen GM, Bunch TH, Hu N, Eley CGS, eds. Liposome technology. 3rd ed New York, NY: Informa Healthcare, 2006:297–310 . [Google Scholar]
- 33. Readio JD, Bittman R.. Equilibrium binding of amphotericin B and its methyl ester and borate complex to sterols. Biochim Biophys Acta 1982; 685:219–24 . [DOI] [PubMed] [Google Scholar]
- 34. Adler-Moore J, Proffitt RT.. AmBisome: a developmental case study of a liposomal formulation of the antifungal agent amphotericin B. In: Burgess DJ, ed. Parenteral dispersed systems: formulation, processing and performance. Chapter 14. Marcel Dekker. Boca Raton, FL: CRC Press, 2005:481–525 . [Google Scholar]
- 35. Adler-Moore J. AmBisome targeting to fungal infections. Bone Marrow Transplant 1994; 14(Suppl 5):S3–7 . [PubMed] [Google Scholar]
- 36. Adler-Moore JP, Proffitt RT.. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of amphotericin B. J Liposome Research 1993; 3:21 . [Google Scholar]
- 37. Takemoto K, Yamamoto Y, Ueda Y, Sumita Y, Yoshida K, Niki Y.. Comparative study on the efficacy of AmBisome and Fungizone in a mouse model of pulmonary aspergillosis. J Antimicrob Chemother 2006; 57:724–31 . [DOI] [PubMed] [Google Scholar]
- 38. Olson JA, Adler-Moore JP, Jensen GM, Schwartz J, Dignani MC, Proffitt RT.. Comparison of the physicochemical, antifungal, and toxic properties of two liposomal amphotericin B products. Antimicrob Agents Chemother 2008; 52:259–68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olson JA, Schwartz JA, Hahka D, et al. Toxicity and efficacy differences between liposomal amphotericin B formulations in uninfected and Aspergillus fumigatus infected mice. Med Mycol 2015; 53:107–18 . [DOI] [PubMed] [Google Scholar]
- 40. Gallis HA, Drew RH, Pickard WW.. Amphotericin B: 30 years of clinical experience. Rev Infect Dis 1990; 12:308–29 . [DOI] [PubMed] [Google Scholar]
- 41. Goodwin SD, Cleary JD, Walawander CA, Taylor JW, Grasela TH Jr. Pretreatment regimens for adverse events related to infusion of amphotericin B. Clin Infect Dis 1995; 20:755–61 . [DOI] [PubMed] [Google Scholar]
- 42. Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM.. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J Biol Chem 2003; 278:37561–8 . [DOI] [PubMed] [Google Scholar]
- 43. Gigliotti F, Shenep JL, Lott L, Thornton D.. Induction of prostaglandin synthesis as the mechanism responsible for the chills and fever produced by infusing amphotericin B. J Infect Dis 1987; 156:784–9 . [DOI] [PubMed] [Google Scholar]
- 44. Arning M, Kliche KO, Heer-Sonderhoff AH, Wehmeier A.. Infusion-related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses 1995; 38:459–65 . [DOI] [PubMed] [Google Scholar]
- 45. Ellis ME, al-Hokail AA, Clink HM, et al. Double-blind randomized study of the effect of infusion rates on toxicity of amphotericin B. Antimicrob Agents Chemother 1992; 36:172–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cleary JD, Weisdorf D, Fletcher CV.. Effect of infusion rate on amphotericin B-associated febrile reactions. Drug Intell Clin Pharm 1988; 22:769–72 . [DOI] [PubMed] [Google Scholar]
- 47. Cruz JM, Peacock JE Jr, Loomer L, et al. Rapid intravenous infusion of amphotericin B: a pilot study. Am J Med 1992; 93:123–30 . [DOI] [PubMed] [Google Scholar]
- 48. Wingard JR, White MH, Anaissie E, Raffalli J, Goodman J, Arrieta A; L Amph/ABLC Collaborative Study Group A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clin Infect Dis 2000; 31:1155–63 . [DOI] [PubMed] [Google Scholar]
- 49. Roden MM, Nelson LD, Knudsen TA, et al. Triad of acute infusion-related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis 2003; 36:1213–20 . [DOI] [PubMed] [Google Scholar]
- 50. Mistro S, Maciel Ide M, de Menezes RG, Maia ZP, Schooley RT, Badaró R.. Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis. Clin Infect Dis 2012; 54:1774–7 . [DOI] [PubMed] [Google Scholar]
- 51. Bellocchio S, Gaziano R, Bozza S, et al. Liposomal amphotericin B activates antifungal resistance with reduced toxicity by diverting Toll-like receptor signalling from TLR-2 to TLR-4. J Antimicrob Chemother 2005; 55:214–22 . [DOI] [PubMed] [Google Scholar]
- 52. Chai LY, Netea MG, Tai BC, et al. An elevated pro-inflammatory cytokine response is linked to development of amphotericin B-induced nephrotoxicity. J Antimicrob Chemother 2013; 68:1655–9 . [DOI] [PubMed] [Google Scholar]
- 53. Loo AS, Muhsin SA, Walsh TJ.. Toxicokinetic and mechanistic basis for the safety and tolerability of liposomal amphotericin B. Expert Opin Drug Saf 2013; 12:881–95 . [DOI] [PubMed] [Google Scholar]
- 54. Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology 2005; 216:106–21 . [DOI] [PubMed] [Google Scholar]
- 55. Seibel NL, Shad AT, Bekersky I, et al. Safety, tolerability, and pharmacokinetics of liposomal amphotericin B in immunocompromised pediatric patients. Antimicrob Agents Chemother 2017; 61:pii: e01477-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Farmakiotis D, Kyvernitakis A, Tarrand JJ, Kontoyiannis DP.. Early initiation of appropriate treatment is associated with increased survival in cancer patients with Candida glabrata fungaemia: a potential benefit from infectious disease consultation. Clin Microbiol Infect 2015; 21:79–86 . [DOI] [PubMed] [Google Scholar]
- 57. Sawaya BP, Briggs JP, Schnermann J.. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J Am Soc Nephrol 1995; 6:154–64 . [DOI] [PubMed] [Google Scholar]
- 58. Sawaya BP, Weihprecht H, Campbell WR, et al. Direct vasoconstriction as a possible cause for amphotericin B-induced nephrotoxicity in rats. J Clin Invest 1991; 87:2097–107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sabra R, Branch RA.. Amphotericin B nephrotoxicity. Drug Saf 1990; 5:94–108 . [DOI] [PubMed] [Google Scholar]
- 60. Branch RA. Prevention of amphotericin B-induced renal impairment. A review on the use of sodium supplementation. Arch Intern Med 1988; 148:2389–94 . [PubMed] [Google Scholar]
- 61. Hsuchen CC, Feingold DS.. Selective membrane toxicity of the polyene antibiotics: studies on natural membranes. Antimicrob Agents Chemother 1973; 4:316–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bicanic T, Bottomley C, Loyse A, et al. Toxicity of amphotericin B deoxycholate-based induction therapy in patients with HIV-associated cryptococcal meningitis. Antimicrob Agents Chemother 2015; 59:7224–31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barton CH, Pahl M, Vaziri ND, Cesario T.. Renal magnesium wasting associated with amphotericin B therapy. Am J Med 1984; 77:471–4 . [DOI] [PubMed] [Google Scholar]
- 64. Huang CL, Kuo E.. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–52 . [DOI] [PubMed] [Google Scholar]
- 65. Lee JW, Amantea MA, Francis PA, et al. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob Agents Chemother 1994; 38:713–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bekersky I, Boswell GW, Hiles R, Fielding RM, Buell D, Walsh TJ.. Safety, toxicokinetics and tissue distribution of long-term intravenous liposomal amphotericin B (AmBisome): a 91-day study in rats. Pharm Res 2000; 17:1494–502 . [DOI] [PubMed] [Google Scholar]
- 67. Bekersky I, Boswell GW, Hiles R, Fielding RM, Buell D, Walsh TJ.. Safety and toxicokinetics of intravenous liposomal amphotericin B (AmBisome) in beagle dogs. Pharm Res 1999; 16:1694–701 . [DOI] [PubMed] [Google Scholar]
- 68. Lanternier F, Poiree S, Elie C, et al. ; French Mycosis Study Group Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother 2015; 70:3116–23 . [DOI] [PubMed] [Google Scholar]
- 69. Walsh TJ, Goodman JL, Pappas P, et al. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob Agents Chemother 2001; 45:3487–96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gondal JA, Swartz RP, Rahman A.. Therapeutic evaluation of free and liposome-encapsulated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother 1989; 33:1544–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Boswell GW, Bekersky I, Buell D, Hiles R, Walsh TJ.. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob Agents Chemother 1998; 42:263–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boswell GW, Buell D, Bekersky I.. AmBisome (liposomal amphotericin B): a comparative review. J Clin Pharmacol 1998; 38:583–92 . [DOI] [PubMed] [Google Scholar]
- 73. Pallister CJ, Johnson EM, Warnock DW, Elliot PJ, Reeves DF.. In-vitro effects of liposome-encapsulated amphotericin B (AmBisome) and amphotericin B-deoxycholate (Fungizone) on the phagocytic and candidacidal function of human polymorphonuclear leucocytes. J Antimicrob Chemother 1992; 30:313–20 . [DOI] [PubMed] [Google Scholar]
- 74. van Etten EW, ten Kate MT, Snijders SV, Bakker-Woudenberg IA.. Administration of liposomal agents and blood clearance capacity of the mononuclear phagocyte system. Antimicrob Agents Chemother 1998; 42:1677–81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roilides E, Lyman CA, Filioti J, et al. Amphotericin B formulations exert additive antifungal activity in combination with pulmonary alveolar macrophages and polymorphonuclear leukocytes against Aspergillus fumigatus. Antimicrob Agents Chemother 2002; 46:1974–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dotis J, Simitsopoulou M, Dalakiouridou M, et al. Amphotericin B formulations variably enhance antifungal activity of human neutrophils and monocytes against Fusarium solani: comparison with Aspergillus fumigatus. J Antimicrob Chemother 2008; 61:810–7 . [DOI] [PubMed] [Google Scholar]
- 77. Reeves EP, Nagl M, Godovac-Zimmermann J, Segal AW.. Reassessment of the microbicidal activity of reactive oxygen species and hypochlorous acid with reference to the phagocytic vacuole of the neutrophil granulocyte. J Med Microbiol 2003; 52:643–51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11:275–88 . [DOI] [PubMed] [Google Scholar]
- 79. Balloy V, Chignard M.. The innate immune response to Aspergillus fumigatus. Microbes Infect 2009; 11:919–27 . [DOI] [PubMed] [Google Scholar]
- 80. Ben-Ami R, Lewis RE, Kontoyiannis DP.. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis 2008; 47:226–35 . [DOI] [PubMed] [Google Scholar]
- 81. Lewis RE, Chamilos G, Prince RA, Kontoyiannis DP.. Pretreatment with empty liposomes attenuates the immunopathology of invasive pulmonary aspergillosis in corticosteroid-immunosuppressed mice. Antimicrob Agents Chemother 2007; 51:1078–81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Eierman DF, Yagami M, Erme SM, et al. Endogenously opsonized particles divert prostanoid action from lethal to protective in models of experimental endotoxemia. Proc Natl Acad Sci U S A 1995; 92:2815–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Devine DV, Wong K, Serrano K, Chonn A, Cullis PR.. Liposome-complement interactions in rat serum: implications for liposome survival studies. Biochim Biophys Acta 1994; 1191:43–51 . [DOI] [PubMed] [Google Scholar]
- 84. Devine DV, Marjan JM.. The role of immunoproteins in the survival of liposomes in the circulation. Crit Rev Ther Drug Carrier Syst 1997; 14:105–31 . [PubMed] [Google Scholar]
- 85. Marjan J, Xie Z, Devine DV.. Liposome-induced activation of the classical complement pathway does not require immunoglobulin. Biochim Biophys Acta 1994; 1192:35–44 . [DOI] [PubMed] [Google Scholar]
- 86. Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. 4th ed (M27) Wayne, PA: CLSI, 2017:46 . [Google Scholar]
- 87. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antifungal susceptibility testing of yeasts. 1st ed (M60) Wayne, PA: CLSI, 2017:28 . [Google Scholar]
- 88. Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope W; EUCAST-AFST EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 2012; 18:E246–7 . [DOI] [PubMed] [Google Scholar]
- 89. Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW; European Committee on Antimicrobial Susceptibility Testing Subcommittee on Antifungal Susceptibility Testing (EUCAST-AFST) EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin Microbiol Infect 2012; 18:E248–50 . [DOI] [PubMed] [Google Scholar]
- 90. Chowdhary A, Singh PK, Kathuria S, Hagen F, Meis JF.. Comparison of the EUCAST and CLSI broth microdilution methods for testing isavuconazole, posaconazole, and amphotericin B against molecularly identified mucorales species. Antimicrob Agents Chemother 2015; 59:7882–7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Johnson EM, Ojwang JO, Szekely A, Wallace TL, Warnock DW.. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob Agents Chemother 1998; 42:1412–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pahls S, Schaffner A.. Comparison of the activity of free and liposomal amphotericin B in vitro and in a model of systemic and localized murine candidiasis. J Infect Dis 1994; 169:1057–61 . [DOI] [PubMed] [Google Scholar]
- 93. van Etten EW, ten Kate MT, Stearne LE, Bakker-Woudenberg IA.. Amphotericin B liposomes with prolonged circulation in blood: in vitro antifungal activity, toxicity, and efficacy in systemic candidiasis in leukopenic mice. Antimicrob Agents Chemother 1995; 39:1954–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anaissie E, Paetznick V, Proffitt R, Adler-Moore J, Bodey GP.. Comparison of the in vitro antifungal activity of free and liposome-encapsulated amphotericin B. Eur J Clin Microbiol Infect Dis 1991; 10:665–8 . [DOI] [PubMed] [Google Scholar]
- 95. Jessup C, Reyes G, Fothergill A, et al. A head-on comparison of the in vitro antifungal activity of conventional and lipid-based amphotericin B: a multicenter study. J Chemother 2000; 12:22–9 . [DOI] [PubMed] [Google Scholar]
- 96. Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 2008; 14:982–4 . [DOI] [PubMed] [Google Scholar]
- 97. Arechavala AI, Ochiuzzi ME, Borgnia MD, Santiso GM.. Fluconazole and amphotericin B susceptibility testing of Cryptococcus neoformans: results of minimal inhibitory concentrations against 265 isolates from HIV-positive patients before and after two or more months of antifungal therapy. Rev Iberoam Micol 2009; 26:194–7 . [DOI] [PubMed] [Google Scholar]
- 98. Rex JH, Pfaller MA, Barry AL, Nelson PW, Webb CD.. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Antimicrob Agents Chemother 1995; 39:40–4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Baddley JW, Marr KA, Andes DR, et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the transplant-associated infection surveillance network. J Clin Microbiol 2009; 47:3271–5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dannaoui E, Abdul M, Arpin M, et al. ; French Cryptococcosis Study Group Results obtained with various antifungal susceptibility testing methods do not predict early clinical outcome in patients with cryptococcosis. Antimicrob Agents Chemother 2006; 50:2464–70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Larsen RA, Bauer M, Pitisuttithum P, et al. Correlation of susceptibility of Cryptococcus neoformans to amphotericin B with clinical outcome. Antimicrob Agents Chemother 2011; 55:5624–30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Park BJ, Arthington-Skaggs BA, Hajjeh RA, et al. Evaluation of amphotericin B interpretive breakpoints for Candida bloodstream isolates by correlation with therapeutic outcome. Antimicrob Agents Chemother 2006; 50:1287–92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Antoniadou A, Kontoyiannis DP.. Status of combination therapy for refractory mycoses. Curr Opin Infect Dis 2003; 16:539–45 . [DOI] [PubMed] [Google Scholar]
- 104. Clancy CJ, Nguyen MH.. Correlation between in vitro susceptibility determined by E test and response to therapy with amphotericin B: results from a multicenter prospective study of candidemia. Antimicrob Agents Chemother 1999; 43:1289–90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lass-Flörl C, Kofler G, Kropshofer G, et al. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J Antimicrob Chemother 1998; 42:497–502 . [DOI] [PubMed] [Google Scholar]
- 106. Walsh TJ, Petraitis V, Petraitiene R, et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis 2003; 188:305–19 . [DOI] [PubMed] [Google Scholar]
- 107. Steinbach WJ, Benjamin DK Jr, Kontoyiannis DP, et al. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin Infect Dis 2004; 39:192–8 . [DOI] [PubMed] [Google Scholar]
- 108. Taj-Aldeen SJ. Reduced multidrug susceptibility profile is a common feature of opportunistic fusarium species: fusarium multi-drug resistant pattern. J Fungi (Basel) 2017; 3:1–15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lackner M, de Hoog GS, Verweij PE, et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother 2012; 56:2635–42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McCarthy MW, Katragkou A, Iosifidis E, Roilides E, Walsh TJ.. Recent advances in the treatment of scedosporiosis and fusariosis. J Fungi (Basel) 2018; 4:1–15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Seidenfeld SM, Cooper BH, Smith JW, Luby JP, Mackowiak PA.. Amphotericin B tolerance: a characteristic of Candida parapsilosis not shared by other Candida species. J Infect Dis 1983; 147:116–9 . [DOI] [PubMed] [Google Scholar]
- 112. Nguyen MH, Clancy CJ, Yu VL, et al. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J Infect Dis 1998; 177:425–30 . [DOI] [PubMed] [Google Scholar]
- 113. Walsh TJ, Melcher GP, Rinaldi MG, et al. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J Clin Microbiol 1990; 28:1616–22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Walsh TJ, Lee JW, Melcher GP, et al. Experimental Trichosporon infection in persistently granulocytopenic rabbits: implications for pathogenesis, diagnosis, and treatment of an emerging opportunistic mycosis. J Infect Dis 1992; 166:121–33 . [DOI] [PubMed] [Google Scholar]
- 115. Vediyappan G, Rossignol T, d’Enfert C.. Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to beta-glucans. Antimicrob Agents Chemother 2010; 54:2096–111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA.. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 2002; 46:1773–80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu W, Li L, Sun Y, et al. Interaction of the echinocandin caspofungin with amphotericin B or voriconazole against Aspergillus biofilms in vitro. Antimicrob Agents Chemother 2012; 56:6414–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ramage G, Jose A, Sherry L, Lappin DF, Jones B, Williams C.. Liposomal amphotericin B displays rapid dose-dependent activity against Candida albicans biofilms. Antimicrob Agents Chemother 2013; 57:2369–71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Toulet D, Debarre C, Imbert C.. Could liposomal amphotericin B (L-AMB) lock solutions be useful to inhibit Candida spp. biofilms on silicone biomaterials? J Antimicrob Chemother 2012; 67:430–2 . [DOI] [PubMed] [Google Scholar]
- 120. Schinabeck MK, Long LA, Hossain MA, et al. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob Agents Chemother 2004; 48:1727–32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Frazier C, Olson J, Adler-Moore J.. Effective treatment of azole resistant Candida albicans in a murine ascending urinary tract infection with liposomal amphotericin B delivered by bladder lavage. ASM Microbe, 2016. . [Google Scholar]
- 122. Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ.. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 2002; 46:834–40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Polak A. Pharmacokinetics of amphotericin B and flucytosine. Postgrad Med J 1979; 55:667–70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Perfect JR, Durack DT.. Treatment of experimental cryptococcal meningitis with amphotericin B, 5-fluorocytosine, and ketoconazole. J Infect Dis 1982; 146:429–35 . [DOI] [PubMed] [Google Scholar]
- 125. Livermore J, Howard SJ, Sharp AD, et al. Efficacy of an abbreviated induction regimen of amphotericin B deoxycholate for cryptococcal meningoencephalitis: 3 days of therapy is equivalent to 14 days. MBio 2014; 5:e00725–13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Groll AH, Giri N, Petraitis V, et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis 2000; 182:274–82 . [DOI] [PubMed] [Google Scholar]
- 127. Groll AH, Lyman CA, Petraitis V, et al. Compartmentalized intrapulmonary pharmacokinetics of amphotericin B and its lipid formulations. Antimicrob Agents Chemother 2006; 50:3418–23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Goldblum D, Rohrer K, Frueh BE, Theurillat R, Thormann W, Zimmerli S.. Corneal concentrations following systemic administration of amphotericin B and its lipid preparations in a rabbit model. Ophthalmic Res 2004; 36:172–6 . [DOI] [PubMed] [Google Scholar]
- 129. Adler-Moore JP, Proffitt RT, Olson JA, Jensen GM.. Tissue pharmacokinetics and pharmacodynamics of AmBisome® (L-AmBis) in uninfected and infected animals and their effects on dosing regimens. J Liposome Res 2017; 27:195–209 . [DOI] [PubMed] [Google Scholar]
- 130. van Etten EW, Otte-Lambillion M, van Vianen W, ten Kate MT, Bakker-Woudenberg AJ.. Biodistribution of liposomal amphotericin B (AmBisome) and amphotericin B-desoxycholate (Fungizone) in uninfected immunocompetent mice and leucopenic mice infected with Candida albicans. J Antimicrob Chemother 1995; 35:509–19 . [DOI] [PubMed] [Google Scholar]
- 131. Lewis RE, Liao G, Hou J, Chamilos G, Prince RA, Kontoyiannis DP.. Comparative analysis of amphotericin B lipid complex and liposomal amphotericin B kinetics of lung accumulation and fungal clearance in a murine model of acute invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2007; 51:1253–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gershkovich P, Wasan EK, Sivak O, et al. Visceral leishmaniasis affects liver and spleen concentrations of amphotericin B following administration to mice. J Antimicrob Chemother 2010; 65:535–7 . [DOI] [PubMed] [Google Scholar]
- 133. Wasan KM, Wasan EK, Gershkovich P, et al. Highly effective oral amphotericin B formulation against murine visceral leishmaniasis. J Infect Dis 2009; 200:357–60 . [DOI] [PubMed] [Google Scholar]
- 134. Olson JA, Adler-Moore JP, Schwartz J, Jensen GM, Proffitt RT.. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob Agents Chemother 2006; 50:2122–31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lewis RE, Albert ND, Liao G, Hou J, Prince RA, Kontoyiannis DP.. Comparative pharmacodynamics of amphotericin B lipid complex and liposomal amphotericin B in a murine model of pulmonary mucormycosis. Antimicrob Agents Chemother 2010; 54:1298–304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ibrahim AS, Gebremariam T, Husseiny MI, et al. Comparison of lipid amphotericin B preparations in treating murine zygomycosis. Antimicrob Agents Chemother 2008; 52:1573–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gangneux JP, Sulahian A, Garin YJ, Farinotti R, Derouin F.. Therapy of visceral leishmaniasis due to Leishmania infantum: experimental assessment of efficacy of AmBisome. Antimicrob Agents Chemother 1996; 40:1214–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wasan KM, Sivak O, Rosland M, Risovic V, Bartlett K.. Assessing the antifungal activity, pharmacokinetics, and tissue distribution of amphotericin B following the administration of Abelcet and AmBisome in combination with caspofungin to rats infected with Aspergillus fumigatus. J Pharm Sci 2007; 96:1737–47 . [DOI] [PubMed] [Google Scholar]
- 139. Smith PJ, Olson JA, Constable D, Schwartz J, Proffitt RT, Adler-Moore JP.. Effects of dosing regimen on accumulation, retention and prophylactic efficacy of liposomal amphotericin B. J Antimicrob Chemother 2007; 59:941–51 . [DOI] [PubMed] [Google Scholar]
- 140. Andes D, Stamsted T, Conklin R.. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob Agents Chemother 2001; 45:922–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wiederhold NP, Tam VH, Chi J, Prince RA, Kontoyiannis DP, Lewis RE.. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2006; 50:469–73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Al-Nakeeb Z, Petraitis V, Goodwin J, Petraitiene R, Walsh TJ, Hope WW.. Pharmacodynamics of amphotericin B deoxycholate, amphotericin B lipid complex, and liposomal amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother 2015; 59:2735–45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Garcia A, Adler-Moore JP, Proffitt RT.. Single-dose AmBisome (liposomal amphotericin B) as prophylaxis for murine systemic candidiasis and histoplasmosis. Antimicrob Agents Chemother 2000; 44:2327–32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lewis RE, Albert ND, Kontoyiannis DP.. Efficacy of single-dose liposomal amphotericin B or micafungin prophylaxis in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2008; 52:4178–80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Brajtburg J, Bolard J.. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev 1996; 9:512–31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Clemons KV, Stevens DA.. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy and virulence. Med Mycol 2005; 43(Suppl 1):S101–10 . [DOI] [PubMed] [Google Scholar]
- 147. Hiemenz JW, Walsh TJ.. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis 1996; 22(Suppl 2):S133–44 . [DOI] [PubMed] [Google Scholar]
- 148. Janknegt R, de Marie S, Bakker-Woudenberg IA, Crommelin DJ.. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. Clin Pharmacokinet 1992; 23:279–91 . [DOI] [PubMed] [Google Scholar]
- 149. Patterson TF. The future of animal models of invasive aspergillosis. Med Mycol 2005; 43(Suppl 1):S115–9 . [DOI] [PubMed] [Google Scholar]
- 150. Francis P, Lee JW, Hoffman A, et al. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J Infect Dis 1994; 169:356–68 . [DOI] [PubMed] [Google Scholar]
- 151. Olson JA, George A, Constable D, Smith P, Proffitt RT, Adler-Moore JP.. Liposomal amphotericin B and echinocandins as monotherapy or sequential or concomitant therapy in murine disseminated and pulmonary Aspergillus fumigatus infections. Antimicrob Agents Chemother 2010; 54:3884–94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Adler-Moore JP, Chiang SM, Satorius A, et al. Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome). J Antimicrob Chemother 1991; 28(Suppl B):63–71 . [DOI] [PubMed] [Google Scholar]
- 153. Clemons KV, Stevens DA.. Comparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob Agents Chemother 1998; 42:899–902 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Olson JA, Adler-Moore JP, Smith PJ, Proffitt RT.. Treatment of Candida glabrata infection in immunosuppressed mice by using a combination of liposomal amphotericin B with caspofungin or micafungin. Antimicrob Agents Chemother 2005; 49:4895–902 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. van Etten EW, van den Heuvel-de Groot C, Bakker-Woudenberg IA.. Efficacies of amphotericin B-desoxycholate (Fungizone), liposomal amphotericin B (AmBisome) and fluconazole in the treatment of systemic candidosis in immunocompetent and leucopenic mice. J Antimicrob Chemother 1993; 32:723–39 . [DOI] [PubMed] [Google Scholar]
- 156. Clemons KV, Stevens DA.. Therapeutic efficacy of a liposomal formulation of amphotericin B (AmBisome) against murine blastomycosis. J Antimicrob Chemother 1993; 32:465–72 . [DOI] [PubMed] [Google Scholar]
- 157. Clemons KV, Stevens DA.. Comparison of a liposomal amphotericin B formulation (AmBisome) and deoxycholate amphotericin B (Fungizone) for the treatment of murine paracoccidioidomycosis. J Med Vet Mycol 1993; 31:387–94 . [Google Scholar]
- 158. Adler-Moore J, Proffitt RT.. Effect of tissue penetration on AmBisome efficacy. Curr Opin Investig Drugs 2003; 4:179–85 . [PubMed] [Google Scholar]
- 159. Leenders AC, de Marie S, ten Kate MT, Bakker-Woudenberg IA, Verbrugh HA.. Liposomal amphotericin B (AmBisome) reduces dissemination of infection as compared with amphotericin B deoxycholate (Fungizone) in a rat model of pulmonary aspergillosis. J Antimicrob Chemother 1996; 38:215–25 . [DOI] [PubMed] [Google Scholar]
- 160. Olson JA, Adler-Moore JP.. Correlation between drug lung concentrations, colony forming units (CFU) and survival following AmBisome (AmBi) or Abelcet (Ablt) treatment of pulmonaryaspergillosis in immunosuppressed mice. ICAAC 2001; 41:J-1835 . [Google Scholar]
- 161. Allen SD, Sorensen KN, Nejdl MJ, Durrant C, Proffit RT.. Prophylactic efficacy of aerosolized liposomal (AmBisome) and non-liposomal (Fungizone) amphotericin B in murine pulmonary aspergillosis. J Antimicrob Chemother 1994; 34:1001–13 . [DOI] [PubMed] [Google Scholar]
- 162. Clemons KV, Sobel RA, Williams PL, Pappagianis D, Stevens DA.. Efficacy of intravenous liposomal amphotericin B (AmBisome) against coccidioidal meningitis in rabbits. Antimicrob Agents Chemother 2002; 46:2420–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Albert MM, Stahl-Carroll TL, Luther MF, Graybill JR.. Comparison of liposomal amphotericin B to amphotericin B for treatment of murine cryptococcal meningitis. J Mycol Médicale 1995; 5:1–6 . [Google Scholar]
- 164. Groll AH, Piscitelli SC, Walsh TJ.. Antifungal pharmacodynamics: concentration-effect relationships in vitro and in vivo. Pharmacotherapy 2001; 21:133–48S . [DOI] [PubMed] [Google Scholar]
- 165. Croft SL, Davidson RN, Thornton EA.. Liposomal amphotericin B in the treatment of visceral leishmaniasis. J Antimicrob Chemother 1991; 28(Suppl B):111–8 . [DOI] [PubMed] [Google Scholar]
- 166. Ortoneda M, Capilla J, Pastor FJ, Pujol I, Guarro J.. Efficacy of liposomal amphotericin B in treatment of systemic murine fusariosis. Antimicrob Agents Chemother 2002; 46:2273–5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gibbs DM, Olson JA, Aldler-Moore JP.. Clearance of Candida albicans from infected vaginal tissues of immunosuppressed mice following treatment with AmBisome. Am Soc Microbiol 2002; Abstract F48 . [Google Scholar]
- 168. Yardley V, Croft SL.. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother 1997; 41:752–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ.. Mold infections of the central nervous system. N Engl J Med 2014; 371:150–60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M.. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurol 2018; 17:362–72 . [DOI] [PubMed] [Google Scholar]
- 171. Luo G, Gebremariam T, Lee H, et al. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob Agents Chemother 2013; 57:3340–7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Clemons KV, Espiritu M, Parmar R, Stevens DA.. Comparative efficacies of conventional amphotericin b, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob Agents Chemother 2005; 49:4867–75 . [DOI] [PMC free article] [PubMed] [Google Scholar]