Abstract
Background
Resistance to latent Mycobacterium tuberculosis (M.tb) infection, identified by persistently negative tuberculin skin tests (TST) and interferon-gamma release assays (IGRA), after close contact with pulmonary tuberculosis (TB) patients has not been extensively characterized. Stability of this “resistance” beyond 2 years from exposure is unknown.
Methods
407 of 657 eligible human immunodeficiency virus (HIV)-negative adults from a TB household contact study with persistently negative TST (PTST−) or with stable latent M.tb infection (LTBI) were retraced 9.5 years (standard deviation = 3.2) later. Asymptomatic retraced contacts underwent 3 IGRAs and follow-up TST, and their M.tb infection status classified as definite/possible/probable.
Results
Among PTST− with a definite classification, 82.7% were concordantly TST−/ quantiferon-TB Gold− (QFT−), and 16.3% converted to TST+/QFT+ LTBI. Among original LTBI contacts, 83.6% remained LTBI, and 3.9% reverted their TST and were QFT−. Although TST and QFT concordance was high (κ = 0.78), 1.0% of PTST and 12.5% of original LTBI contacts could not be classified due to discordant TST and QFT results. Epidemiological variables did not differ between retraced PTST− and LTBI contacts.
Conclusion
Resistance to LTBI, defined by repeatedly negative TST and IGRA, in adults who have had close contact with pulmonary TB patients living in TB-endemic areas, is a stable outcome of M.tb exposure. Repeated longitudinal measurements with 2 different immune assays and extended follow-up provide enhanced discriminatory power to identify this resister phenotype and avoid misclassification. Resisters may use immune mechanisms to control aerosolized M.tb that differ from those used by persons who develop “classic” LTBI.
Keywords: TB exposure, case-contact study, resistance to infection, TB outcomes
Long-term resistance to latent M. tuberculosis infection, defined by persistent negative TST and interferon-gamma release assay, occurs in a subset of highly exposed adults in TB endemic settings. Identification of LTBI resisters requires follow-up and repeat TST and IGRA testing.
The natural history of tuberculosis (TB) is traditionally thought to follow a path from exposure to primary infection that often results in latent Mycobacterium tuberculosis (M.tb) infection (LTBI) from which a small fraction progresses to disease. A common view is that ultimately all heavily M.tb exposed adults become infected and develop LTBI, characterized by a positive tuberculin skin test (TST) and/or interferon-gamma release assay (IGRA) [1, 2].
Older literature, anecdotal reports, and recent studies suggest that a small but not rare number of persons who are heavily exposed to M.tb resist developing LTBI, that is, remain TST and IGRA negative over long periods of follow-up [3]. In a large TB household contact study in Kampala, Uganda, we found that ~9%–10% of contacts remained tuberculin skin test (TST) negative over a 2-year follow-up period [4]. Similarly, some miners in South Africa remain TST negative despite working for many years in one of the world’s highest M.tb transmission environments [5]. In addition, preliminary studies suggest that persons who do or do not develop LTBI in TB-endemic settings differ immunologically [6–10]. A persistently TST− person (PTST−) in a TB household may reflect a response to M.tb that differs immunologically from the person who develops LTBI. To determine the stability of the PTST− phenotype in contacts from an earlier TB contact study in Uganda, we retraced these contacts and characterized their M.tb infection status with 3 IGRAs and TST over a 1–2 year period. We sought to follow-up this cohort to better understand epidemiologic and immunologic factors that may differ between individuals susceptible and resistant to latent M.tb infection. These data also provide a framework for defining the LTBI resister phenotype (RSTR).
METHODS AND MATERIALS
Selection of TB Contacts for Retracing
TB contacts for retracing were participants in the Kawempe Community Health Study in Kampala, Uganda, who were enrolled between 2002 and 2012 (phase 1 in Figure 1A) [11, 12]. In brief, household contacts of people with culture-positive pulmonary TB were evaluated at baseline and every 3–6 months thereafter for up to 24 months for evidence of M.tb infection by TST and disease by symptoms, chest x-ray, and sputum cultures. Contacts with a negative TST at baseline were retested periodically thereafter and when they remained TST− for a minimum of 12 months or optimally 24 months termed persistently TST-negative (PTST−) [4, 12]. Contacts who had repeatedly negative TST but lost to follow-up before 12 months were considered TST− incomplete. Contacts who were TST+ upon enrollment were termed LTBI once active TB was excluded and were offered isoniazid preventive treatment. During this TB contact study, baseline clinical and epidemiological data were used to construct an epidemiologic risk score [4, 13], consisting of variables indicative of risk for M.tb exposure and infection, which has been used in other studies examining risk of LTBI and TB [14, 15]. This risk score was calculated differently for individuals who were children (age <15 years of age) vs adults (age ≥ 15), based on their respective proximity to the index case [4, 12]; details of these risk scores are found in Supplementary Methods.
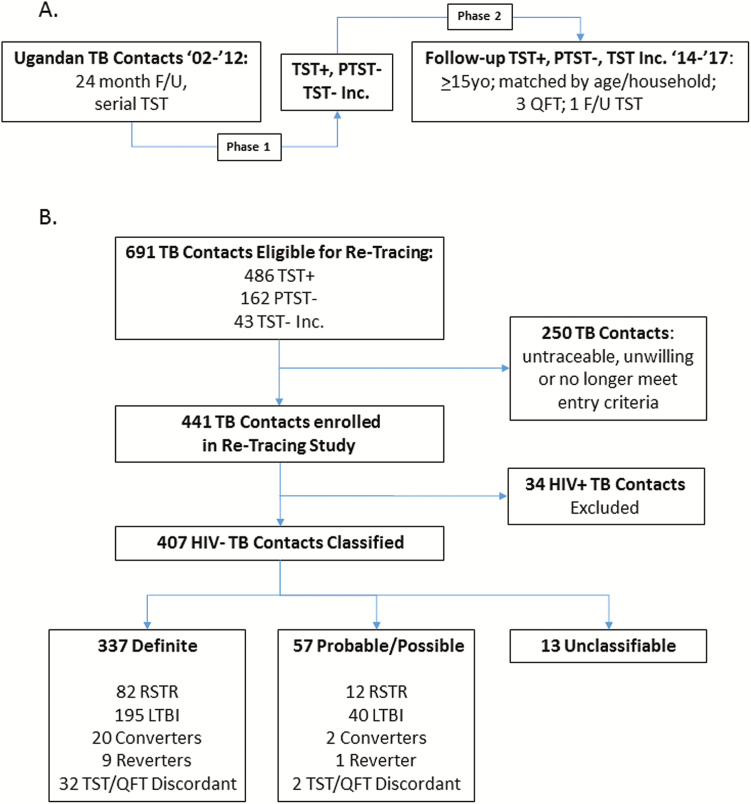
Figure 1.
Retracing study design and classification of retraced TB household contacts. A, Study design: TB household contacts with either persistently negative tuberculin skin tests (PTST−) or latent M.tb infection (LTBI) were identified in Kampala, Uganda between 2002 and 2012 (phase 1* [12]). PTST− and LTBI contacts from phase 1 were identified for retracing between 2014 and 2017, if they were > 15 years old in 2014, and matched by age bracket and household. Retraced contacts underwent 3 QFT tests and 1 TST (phase 2) over 1–2 years of additional follow-up. B, Classification of retraced contacts: PTST−, LTBI, or TST− contacts with less than 12 months of follow-up (TST incomplete [Inc]) in phase 1 were retraced and classified, based on 3 QFT from phase 2 and 2 TST from phases 1 and 2, into resister (RSTR; all tests negative), LTBI (all tests positive), converter (TST− in phase 1, QFT+/TST+ in phase 2), reverter (TST+ Inc phase 1, QFT−/TST−Inc phase 2) or TST/QFT discordant (TSTs consistent, QFTs consistent but in opposing directions). Among 250 subjects not consented, 6 had developed active TB between phases 1 and 2, 32 declined consent, 39 were unavailable, and 173 were untraceable. Contacts who developed TB were not eligible for retracing. The 34 HIV+ subjects excluded from further analysis included 27 who were HIV+ in phase 1 and 7 HIV conversions between phases 1 and 2. Classification as definite was based on availability and consistency of all 5 measures (2 TST and 3 QFT). Probable or possible classification was used when 1 assay was missing or equivocal. Unclassifiable subjects either didn’t complete phase 2 or had very inconsistent TST and QFT results (see Suppl. Methods). Abbreviations: F/U, follow up; HIV, human immunodeficiency virus; QFT, quantiferon-TB Gold; RSTR, resister phenotype; TB, tuberculosis; TST, tuberculin skin test.
Retracing Study
For retracing (phase 2 in Figure 1A), all PTST− and TST− incomplete subjects who were ≥15 years old in 2014 when the retracing study was initiated were eligible. TST+ subjects ≥15 years old were matched by age ±5 years and were either living in the house with a PTST− contact or had equal epidemiologic risk scores if not from the same TB household. Phase 2 sought a 2:1 ratio of TST+ to PTST− contacts. Upon retracing and consenting, subjects underwent a clinical evaluation including human immunodeficiency virus (HIV) testing and completed a questionnaire concerning M.tb exposure. Retraced contacts with a clinical history of TB between 2002 and 2014 were excluded. The retracting study’s evaluation of M.tb infection status consisted of 3 quantiferon-TB Gold (QFT) assays with the first at enrollment in the retracing study and the next 2 over 1–2 years followed by a TST after the last QFT. TST was performed by the Mantoux method (.1 mL of 5 tuberculin units of purified protein derivative, Tubersol; Connaught Laboratories Limited, Willowdale, Ontario, Canada). A positive TST was defined as an induration of 10 mm or greater for HIV− contacts, and 5 mm or greater in HIV+ individuals [16]. QFT was done according to manufacturer’s instructions [17]. QFT data were analyzed with the manufacturer’s software-generated standard curves, pass-fail criteria, and definitions. Test results were presented and analyzed as interferon-γ concentrations in international units per mL.
The retracing study protocol was reviewed and approved by the National AIDS Research Committee, The Uganda National Council on Science and Technology, and the institutional review board at University Hospitals Cleveland Medical Center. Written informed consent was obtained from all retraced household contacts.
Classification of Retraced Contacts
Classification of the M.tb infection status of retraced contacts was based on QFT, initial and follow-up TST, and determined to be definite, probable, possible, and unclassifiable. A “definite” classification required all 5 measurements (2 TST + 3 QFT) to be in agreement (classification schema in Supplementary Table S1). “Definite LTBI” required all TST and QFT to be positive. “Definite resisters” (RSTRs) were defined as PTST− or TST− incomplete contacts who remained TST− and had 3 negative QFT. “Definite converters” were PTST− but on retracing had 3 positive QFT and a positive TST. “Definite reverters” were TST+ but on retracing had 3 negative QFT, and negative TST. “TST/QFT discordants” had consistent TST but opposite QFT results. Contacts that did not meet the criteria for a definite classification were classified as probable, possible, or unclassifiable depending on available TST and QFT results (Supplementary Methods). TST conversion for HIV− contacts was defined as an original TST of <10 mm and a TST reaction of ≥10 mm upon subsequent testing with an increase of ≥6 mm [18]. A converter for this study required these TST criteria and positive QFT. TST reversion was defined as an original positive TST, which upon retesting had become <10 mm with a decrement of ≤3 mm. A reverter for this study required these TST criteria and negative QFT.
Plasma Cytokine Measurements
Plasma was collected at entry into the retracing study and examined with a multiplex microbead human cytokine and chemokine Luminex panel according to manufacturer’s instructions, from the first 31 definite RSTRs and 31 LTBIs. The Luminex panel included MIG, interleukin (IL)-18, tumor necrosis factor (TNF)-Rp75, BAFF, IL-15, interferon (IFN) alpha, GCSF, TNF-Rp55, IL-8, MCP1, IL-1 alpha, MIP1 alpha, IL-1ra, MIP1 beta, MCSF, IL-10, IL-1 beta, GM-CSF, IL-33, IL-12p70, IL-32, IL-3, IL-6, and TNF alpha. Cytokines were selected to minimize redundancy and represent immune pathways relevant to human responses to M.tb. Plates were read on a Luminex 200 (Millipore) instrument and analyzed with Luminex xPONENT Version 3.0 software.
Statistical Analysis
Subjects with “definite” RSTR and LTBI outcomes were compared with respect to demographic and epidemiologic characteristics using generalized estimating equations to account for clustering within household; the same approach was used to compare definite RSTRs and converters. Analyses of epidemiologic risk score were stratified by age in phase 1, because age determined the way risk was calculated (Supplementary Methods). Concordance between the TST and QFT at the last visit was examined by estimating the concordance rate and kappa statistic. The number of HIV+ subjects was too small after stratification by clinical group for statistical analyses and were excluded. A sensitivity analysis was conducted incorporating probable and possible outcomes, and results did not differ notably, so only analyses of definite outcomes are presented. Analyses were conducted using SPSS version 24.
RESULTS
Retracing Study and Classification of Retraced TB Contacts
We identified 691 TB contacts from phase 1 (Figure 1A) who were ≥15 years old at time of retracing and eligible for retracing according to the epidemiologic risk score criteria outlined in Methods. In sum, 486 had LTBI, 162 were PTST−, and 43 were TST negative incomplete (Figure 1B). Retracing (phase 2) started in July 2014 and ended in September 2017; 441 phase 1 contacts (63.8%) were enrolled with 250 contacts untraceable, unwilling, or otherwise unable to participate (Figure 1B). The average time between phase 1 enrollment and phase 2 commencement was 9.5 years (standard deviation [SD] = 3.2) with range shown in Supplementary Figure S1. Retraced contacts underwent clinical evaluation, QFT and HIV testing at baseline, 2 more QFT, and a final TST over a median of 17.7 months (SD = 8.5). In total, 34 phase 1 contacts were HIV+ and excluded from further analysis; 407 retraced HIV− contacts were classified for their M.tb infection status with levels of certainty (ie, definite, probable, possible, and unclassifiable; see Supplementary Methods for details) depending on agreement and completeness of TSTs and QFTs as converter, reverter, stable LTBI (LTBI), or resistance to LTBI (“resister” [RSTR], ie, PTST− who remained TST− and also were QFT−). A total of 337 subjects were classified as definite and composed of 82 RSTRs, 194 LTBIs, 20 converters, 9 reverters, and 32 TST/QFT discordant subjects (Figure 2B). Enumeration of HIV− retraced contacts with probable and possible levels of certainty are in Supplementary Table S2.
Figure 2.
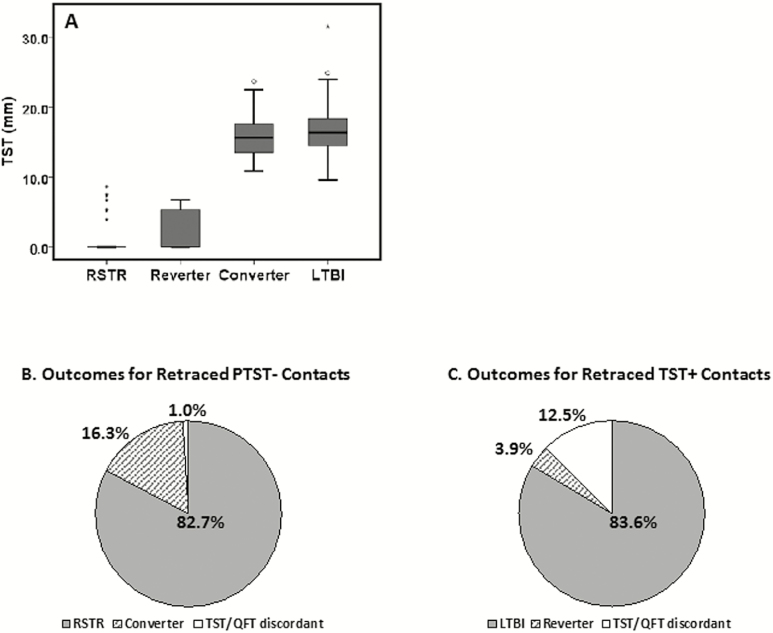
TST (mm) and outcomes after retracing PTST− and TST+ TB contacts. A, Boxplots illustrating 1st quartile, median, and 3rd quartile of TST in mm for definite RSTR, reverters, converters, and LTBI. B, Outcomes after retracing PTST− TB contacts. Definite RSTR: 5 of 5 tests (3 QFT, initial and retracing TST) negative. Definite converter: 3 of 3 QFT and follow-up TST were positive. TST/QFT discordant: 3 of 3 QFT positive but initial and retracing TST were negative. C, Outcomes after retracing of TST+ TB contacts. Definite LTBI: 5 of 5 tests (3 QFT, initial and retracing TST) positive. Definite reverter: 3 of 3 QFT and follow-up TST were negative. TST/QFT discordant: 3 of 3 QFT negative but initial and retracing TST were positive. Abbreviations: LTBI, latent M.tb infection; QFT, quantiferon-TB Gold; RSTR, resister phenotype; TB, tuberculosis; TST, tuberculin skin test.
Analysis of Retraced Contacts With Definite Classification
We next analyzed the retraced contacts with a definite classification (Figure 2A); 91.7% of RSTRs had a TST of 0 mm. Reverters had an average TST of 2.2 mm, with an average decrement of 12.8 mm (SD = 3.8, data not shown). Also, 62.5% of reverters had a TST of 0 mm induration. Converters and LTBI had similar TST indurations of 16.1 (SD = 3.3) and 16.7 (SD = 3.1) mm, respectively. For phase 1 PTST− contacts, 82.7% were QFT and repeat TST negative (Figure 2B and C). For phase 1 LTBI contacts, 83.6% were QFT and repeat TST positive. In sum, 16.3% of PTST− contacts were QFT positive and converted their TST; 3.9% of phase 1 LTBI contacts were QFT negative and reverted their TST. Among PTST− and phase 1 LTBI a number of contacts were identified for whom TST and QFT were discordant (eg, both TST were positive, yet 3 QFT were negative), with the proportion of TST+/QFT−greater than TST−/QFT+ (Table 1). When retraced contacts with probable and possible classifications are added to definites, proportions of RSTR, LTBI, reverter, and converter categories remain similar (Supplementary Table S3). Thus, RSTR and LTBI phenotypes are robust when a high concordance among multiple immune assays over a lengthy time period is used to characterize them.
Table 1.
Concordance Between Tuberculin Skin Test and Quantiferon-TB Gold at 3rd Retracing Visita
| QFT at 3rd Retracing Visit | TST at 3rd Retracting Visit | |
|---|---|---|
| TST+ | TST− | |
| QFT+ | 212 | 3 |
| QFT− | 29 | 88 |
Abbreviations: QFT, quantiferon-TB Gold; TST, tuberculin skin test.
aSubjects with definite outcomes.
Kappa = 0.778.
Concordance = 90.1%.
We next compared phase 2 RSTRs and LTBIs on a number of clinical and epidemiological parameters and found no significant differences between these groups on these key characteristics, consistent with our previous findings in PTST− (Table 2) [4]. Importantly, there was no significant difference in time elapsed since phase 1 enrollment or epidemiologic risk score. There is a slight trend toward significance in age distribution, which we examined further by 10-year age groupings (Supplementary Table S4). LTBIs were overall slightly older, but the oldest age group had equal proportions of RSTR and LTBI contacts, that is, the RSTR phenotype extended into late adulthood. We did not observe significant differences between RSTRs and converters in phase 1 (Table 3).
Table 2.
Comparison of Retraced Definite Resister Phenotype and Latent M.tb Infection Tuberculosis Contacts
| RSTRs (N = 82) |
LTBI (N = 194) |
OR | P-Value | |
|---|---|---|---|---|
| % Female | 47.6% | 48.5% | 0.977 | .937 |
| Current age (mean, SD) | 22.9 (10.1) | 24.12 (9.1) | 0.975 | .237 |
| Time since phase 1 enrolment (median, SD) | 9.2 (3.5) | 9.4 (3.2) | 0.982 | .693 |
| % BCG vaccinated | 62.2% | 69.1% | 0.783 | .471 |
| Epi risk score - children (median, SD)a | 6.00 (.9) | 6.00 (.8) | 0.867 | .531 |
| Epi risk score - adults (median, SD)a | 6.00 (1.1) | 6.00 (1.3) | 0.839 | .316 |
| Current BMI (mean, SD) | 22.3 (4.0) | 23.3 (4.8) | 0.965 | .305 |
| Contact with TB since original study | 0% | 2.6% | NA | .326 |
Abbreviations: BCG, bacille Calmette-Guérin; BMI, body mass index; Epi, epidemiologic; LTBI, latent M.tb infection; NA, not applicable; OR, odds ratio; RSTR, resister phenotype; SD, standard deviation; TB, tuberculosis.
aRisk score was generated based on the age of the contact during the original TB household contact study (phase 1); age determined the values of the score’s variables (eg, proximity to and characteristics of the index case). Analysis was stratified by age in phase 1, with children defined as <15 years old.
Table 3.
Comparison of Retraced Definite Resister Phenotype and Converter Contacts
| RSTRs (N = 82) |
Converters (N = 20) | OR | P-Value | |
|---|---|---|---|---|
| % Female | 47.6% | 60% | 0.559 | .260 |
| Current age (mean, SD) | 22.9 (10.1) | 23.9 (8.2) | 0.984 | .498 |
| Time since Phase 1 enrollment (median, SD) | 9.2 (3.5) | 10.9 (3.0) | 0.849 | .065 |
| % BCG vaccinated | 62.2% | 70.0% | 0.778 | .655 |
| Epi risk score-children (median, SD)a | 6.00 (.9) | 5.00 (1.0) | 1.758 | .230 |
| Epi risk score-adults (median, SD)a | 6.00 (1.1) | 7.00 (.5) | 0.504 | .204 |
| Current BMI (mean, SD) | 22.3 (4.0) | 23.8 (4.4) | 0.925 | .162 |
| Contact with TB case since original study | 0% | 0% | NA | NA |
Abbreviations: BCG, bacille Calmette-Guérin; BMI, body mass index; Epi, epidemiologic; NA, not applicable; OR, odds ratio; RSTR, resister phenotype; SD, standard deviation; TB, tuberculosis.
aRisk score was generated based on the age of the subject during the original study TB household contact study (phase 1); age determined the values of the variables of this score (eg, proximity to and characteristics of the index case). Analysis was stratified by age in phase 1, with children defined as <15 years old.
Requirement for Both TST and QFT to Identify RSTR
The importance of both TST and repeated QFT in evaluating outcomes should be noted. First, consistent with previous studies, there is a nonnegligible proportion of discordant readings between the 2 assays (~10% in individuals with definite outcomes; Table 2). Second, although the correlation among 3 QFT readings was high (>80%, Supplementary Table S5), there were occasionally inconsistent results across 3 QFTs. In 5% of retraced contacts, there was at least 1 indeterminate reading, and in 7% of subjects at least 1 QFT reading was inconsistent with the other 2 (data not shown). The advantage of multiple QFT assessments was to improve the precision of the certainty with which subjects could be classified. Quantitative QFT values are shown in Supplementary Table S6. Together, these data demonstrate that the ability to identify RSTRs in TB endemic settings requires repeated TST and QFT testing in addition to longitudinal follow-up.
Plasma Cytokine Responses
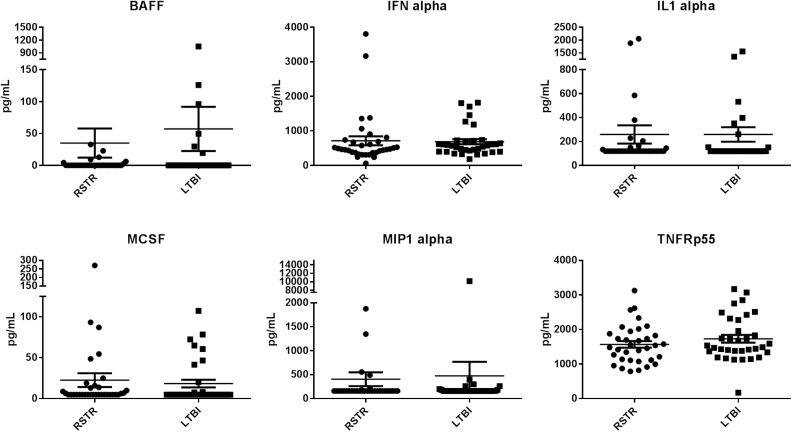
We next assessed whether there were immunologic differences between definite RSTRs and LTBIs. We measured a panel of 24 cytokines and chemokines in plasma. MIG, IL18, TNF-Rp75, BAFF, IL015, IFN alpha, GCSF, TNF-Rp55, IL-8, MCP1, IL-1 alpha, MIP1 alpha, IL-1ra, MIP1 beta, and MCSF had detectable levels, but no differences were detected when comparing definite RSTRs and LTBIs (Figure 3 and Supplementary Figure S2). Except for occasional subjects, there were no detectable levels for IL-10, IL-1 beta, GM-CSF, IL-33, IL-12p70, IL-32, IL-3, IL-6, and TNF alpha. Thus, basal plasma cytokine and chemokine levels did not differ between RSTRs and LTBIs.
Figure 3.
Plasma cytokines in RSTR and LTBI. Cytokines and chemokines were measured in plasma from 31 Definite RSTR and 31 LTBIs with a Luminex platform. Results for BAFF, interferon (IFN)-alpha, interleukin (IL)-1 alpha, MCSF, MIP1 alpha, and TNF-Rp55 are shown as mean pg/ml +/−SD. No statistically significant differences were detected between the two groups. Results for other 10 cyto- and chemokines are shown in Supplementary Figure 1. Abbreviations: BAFF, B-cell activating factor; IFN, interferon; IL1, interleukin-1; LTBI, latent M.tb infection; MCSF, macrophage colony stimulating factor 1; MIP1, macrophage inflammatory protein 1-alpha; RSTR, resister phenotype; SD, standard deviation; TNFR, tumor necrosis factor receptor superfamily member 3.
DISCUSSION
In this study, we demonstrated the long-term robustness of the RSTR phenotype, that is, resistance to LTBI reflected by persistently negative TST and IGRA in a TB-endemic setting. During phase 1, more than half of household contacts that were TST-negative upon enrollment converted their TST with most doing so within 3–6 months [4, 12]. However, a small but not rare number of contacts remained TST-negative over a 1–2 year period. In the years since, only 16% converted their TST and IGRA with the majority of persistent TST− people remaining negative. People with the RSTR or LTBI phenotype did not differ significantly on key epidemiological characteristics, as we also noted previously [4, 12]. Although the highest M.tb exposure for these household contacts occurred during phase 1, they resided in a TB-endemic region in urban Kampala with ongoing risk of continual, albeit not as intense, exposure to M.tb. The RSTR phenotype may not be absolute, because a low rate of conversion from RSTR to LTBI was observed. RSTRs may differ in their level of resistance to LTBI that may eventually be brought out by the intensity and length of M.tb exposure. This retracing study provides evidence that relative (and possibly absolute in some) resistance to latent M.tb infection as traditionally defined by the absence of positive TST or IGRA can be an additional outcome of M.tb exposure in TB-endemic settings. Historical studies suggested that resistance to M.tb infection may occur [19–26]. This is to our knowledge the first study to carefully characterize the LTBI status with 2 separate immunologic assays and multiple time points after long-term follow-up of persistently TST− M.tb-exposed individuals.
There are a number of explanations for the conversions. First, phase 1 only used TST because the QFT did not exist at the start of that study, and phase 2 used both TST and QFT to define RSTRs. Second, M.tb exposure in phase 1 may have been insufficient and unknown community exposure led to conversion later on. Third, other unmeasured factors may explain reduced protection from LTBI, including nutrition, waning bacille Calmette-Guerin (BCG) effect, age, and development of non-HIV comorbidities.
This study also provides important data regarding the optimal features for identifying RSTRs in the absence of a biomarker, particularly for efforts to identify the biologic underpinnings of the RSTR phenotype. First, it is important to use indexes of exposure [4, 13]. In our study, all household contacts were highly exposed based on an epidemiologic risk score. In other locales, particularly where the TB index case does not have severe disease or exposure may occur without a clear source, the risk may be lower and RSTRs harder to identify. In other settings, such as South African mines, RSTRs may be identified based on decades of working in confined, poorly ventilated settings with high exposure to both TB and HIV [5]. Second, use of both the TST and QFT is important in order to avoid misclassification from assay discordance [27, 28]. Third, longitudinal sampling (with both TST and QFT) is critical to identify TST/QFT conversion events. Although most conversion events occur within 6 months of close exposure to a TB case, later conversions do occur.
The absence of obvious clinical or epidemiological characteristics associated with the RSTRs in our study suggests a strong host immune component as determinant of LTBI vs RSTR outcomes. Our earlier studies suggest genetic and macrophage differences between PTST− and LTBIs [6–8, 10, 29]. However, in those studies, RSTRs were not as strictly defined. Further studies are necessary to determine not only the extent and robustness of our preliminary studies suggesting difference in genetics and ability of macrophages to respond to M.tb but also if other arms of the immune response differ between RSTR and LTBI. Recent intriguing studies reevaluating the role of antibodies and nontraditional as well as non-IFN-γ producing T cells in immune responses to M.tb may provide insight into how RSTR control M.tb.
The incomplete follow-up rate (63.8%) for the time between periods 1 and 2 is a limitation of our study. The number of retraced HIV-infected LTBIs and RSTRs was small, precluding statistical analyses, but the fact that HIV+ RSTRs with CD4+ T-cell counts >250 cells/mm 3 were found is intriguing. The number of converters was also small, so statistical comparisons may have been underpowered and thus considered exploratory. Similarly, the 24 cytokine panel used was exploratory and limited.
In sum, the RSTR phenotype has become a new focus in the study of the human response to M.tb infection and disease. The integration of rigorous epidemiologic phenotyping with mechanistic studies that interrogate global immune pathways in the macrophage, T cell, and B cell and how such processes may fundamentally differ between “resisters” and individuals with LTBI will provide more complete biologic insight into early clearance or prevention of M.tb infection. If identified, these biologic mechanisms provide targets for drugs and vaccines that could enhance specific immune functions and improve our therapeutic options for treating TB. This study provides a framework for the identification and definition of RSTRs that should be helpful for future studies of this novel phenotype.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge the contributions made by senior physicians, medical officers, health visitors, laboratory and data personnel: Drs Alphonse Okwera and Moses Joloba, Dorcas Lamunu, Deborah Nsamba, Annet Kawuma, Saidah Menya, Joan Nassuna, Joy Beseke, Michael Odie, Henry Kawoya, Shannon Pavsek, Dr E. Chandler Church, Anna Duewiger, Bonnie Thiel, and Glenna Peterson. This study would not be possible without the generous participation of the Ugandan patients and families.
Funding. This work was supported by the Tuberculosis Research Unit (TBRU) at Case Western Reserve University, established with Federal funds from the United States National Institutes of Allergy and Infectious Diseases and the United States National Institutes of Health and Human Services, under contract no. HHSN266200700022C / NO1-AI-70022 to W. H. B., ICIDR U01-AI115642 to H. M. K. and W. H. B., R01AI124348 to C. M. S., T. R. H., and W. H. B., and Bill & Melinda Gates Foundation grant OPP1151836 to T. R. H., W. H. B., C. M. S., and H. M. K.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016; 13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO global surveillance and monitoring project. JAMA 1999; 282:677–86. [DOI] [PubMed] [Google Scholar]
- 3. Simmons JD Stein CM, Seshadri C, et al. Born to resist: immunologic mechanisms of human resistance to Mycobacterium tuberculosis infection. Nat Rev Immunol. 2018. doi: 10.1038/s41577-018-0025-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma N, Zalwango S, Malone LL, et al. ; Tuberculosis Research Unit (TBRU) Clinical and epidemiological characteristics of individuals resistant to M. tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. BMC Infect Dis 2014; 14:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanifa Y, Grant AD, Lewis J, Corbett EL, Fielding K, Churchyard G. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int J Tuberc Lung Dis 2009; 13:39–46. [PubMed] [Google Scholar]
- 6. Stein CM, Zalwango S, Malone LL, et al. Genome scan of M. tuberculosis infection and disease in Ugandans. PLoS One 2008; 3:e4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seshadri C, Sedaghat N, Campo M, et al. ; Tuberculosis Research Unit (TBRU) Transcriptional networks are associated with resistance to Mycobacterium tuberculosis infection. PLoS One 2017; 12:e0175844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahan CS, Zalwango S, Thiel BA, et al. Innate and adaptive immune responses during acute M. tuberculosis infection in adult household contacts in Kampala, Uganda. Am J Trop Med Hyg 2012; 86:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tameris MD, Hatherill M, Landry BS, et al. ; MVA85A 020 Trial Study Team Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013; 381:1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sobota RS, Stein CM, Kodaman N, et al. A chromosome 5q31.1 locus associates with tuberculin skin test reactivity in HIV-positive individuals from tuberculosis hyper-endemic regions in east Africa. PLoS Genet 2017; 13:e1006710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stein CM, Hall NB, Malone LL, Mupere E. The household contact study design for genetic epidemiological studies of infectious diseases. Front Genet 2013; 4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stein CM, Zalwango S, Malone LL, et al. Resistance and susceptibility to mycobacterium tuberculosis infection and disease in tuberculosis households in Kampala, Uganda. Am J Epidemiol 2018; 187:1477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mandalakas AM, Kirchner HL, Lombard C, et al. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int J Tuberc Lung Dis 2012; 16:1033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jenum S, Selvam S, Mahelai D, et al. Influence of age and nutritional status on the performance of the tuberculin skin test and QuantiFERON-TB gold in-tube in young children evaluated for tuberculosis in Southern India. Pediatr Infect Dis J 2014; 33:e260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acuña-Villaorduña C, Jones-López EC, Fregona G, et al. Intensity of exposure to pulmonary tuberculosis determines risk of tuberculosis infection and disease. Eur Respir J 2018; 51:1701578. doi:10.1183/13993003.01578-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS board of directors, July 1999. This is a joint statement of the American thoracic society (ATS) and the centers for disease control and prevention (CDC). This statement was endorsed by the council of the infectious diseases society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161:S221–47. [DOI] [PubMed] [Google Scholar]
- 17. Inc. C. QuantiFERON-TB Gold [package insert]. Valencia, CA, 2013. [Google Scholar]
- 18. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med 1999; 159:15–21. [DOI] [PubMed] [Google Scholar]
- 19. Houk VN, Baker JH, Sorensen K, Kent DC. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health 1968; 16:26–35. [DOI] [PubMed] [Google Scholar]
- 20. Aziz A, Ishaq M, Akhwand R. Infection risk of sputum positive tuberculosis patients to their family contacts with and without chemotherapy. J Pak Med Assoc 1985; 35:249–52. [PubMed] [Google Scholar]
- 21. Devadatta S, Dawson JJ, Fox W, et al. Attack rate of tuberculosis in a 5-year period among close family contacts of tuberculous patients under domiciliary treatment with isoniazid plus PAS or isoniazid alone. Bull World Health Organ 1970; 42:337–51. [PMC free article] [PubMed] [Google Scholar]
- 22. Lemos AC, Matos ED, Pedral-Sampaio DB, Netto EM. Risk of tuberculosis among household contacts in Salvador, Bahia. Braz J Infect Dis 2004; 8: 424–30. [DOI] [PubMed] [Google Scholar]
- 23. Hardy MA, Schmidek HH. Epidemiology of tuberculosis aboard a ship. JAMA 1968; 203:175–9. [PubMed] [Google Scholar]
- 24. Badger TS,WW. First-infection type of tuberculosis in adults: a five-year study of student nurses at the Boston city hospital. New Engl J Med 1937; 217:424–31. [Google Scholar]
- 25. Myers JAB, Diel RE. Prevention of tuberculosis among students of nursing. Am J Nurs 1947; 47:661–6. [Google Scholar]
- 26. Dickie HA. Tuberculosis in student nurses and medical students at the University of Wisconsin. Ann Intern Med 1950; 33:941–59. [DOI] [PubMed] [Google Scholar]
- 27. Andrews JR, Hatherill M, Mahomed H, et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med 2015; 191:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghassemieh BJ, Attia EF, Koelle DM, Mancuso JD, Narita M, Horne DJ. Latent tuberculosis infection test agreement in the national health and nutrition examination survey. Am J Respir Crit Care Med 2016; 194:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hall NB, Robert P Igo Jr, Malone LL, et al. ; Tuberculosis Research Unit (TBRU) Polymorphisms in TICAM2 and IL1B are associated with TB. Genes Immun 2015; 16:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.