Abstract
Vaccine effectiveness (VE) against influenza B was derived separately for Victoria and Yamagata lineages across 8 seasons (2010–2011 to 2017–2018) in Canada when trivalent influenza vaccine was predominantly used. VE was ≥50% regardless of lineage match to circulating viruses, except when the vaccine strain was unchanged from the prior season.
Keywords: influenza B virus, lineage, influenza vaccine effectiveness, cross-protection, repeat vaccination
Influenza B viruses, which almost exclusively infect humans, were first identified in 1940 and have existed as 2 phylogenetically and antigenically distinct lineages since at least the 1980s [1–5]. Yamagata lineage viruses predominated in North America during the 1990s, with reappearance of Victoria lineage viruses in 2001 [5, 6]. Both lineages have since co-circulated, undergoing frequent insertion/deletion and reassortment events, both intra- and cross-lineage [1–3, 6–8]. Although antigenically distinct, influenza B(Victoria) and B(Yamagata) lineages are more genetically related than influenza A(H1N1) and A(H3N2) subtypes, sharing >90% amino acid identity across hemagglutinin (HA) and neuraminidase (NA) proteins compared with <45% shared HA/NA identity between influenza A subtypes [9].
Trivalent influenza vaccine (TIV) contains standardized HA quantity (but unspecified NA content) from representative strains of both influenza A subtypes and 1 influenza B lineage, whereas quadrivalent influenza vaccine (QIV) includes both B lineages [10]. QIV was introduced in Canada, primarily for children, in 2014–2015 but as of 2017–2018, most doses distributed overall remain TIV.
The antigenic distance hypothesis provides a framework for understanding variability in the effectiveness of annually repeated influenza vaccination [11]. The model predicts pronounced negative interference from the prior season’s vaccination on the current season’s vaccine effectiveness (VE) when the vaccine strain is unchanged (ie, identical cross-season) and antigenically distinct from circulating viruses. Interpretation of antigenic relatedness, however, varies with laboratory-assay conditions [12], and because vaccine strains of both influenza A subtypes (Supplementary References 13–19) and B lineages (Supplementary References 18–23) acquire mutations with adaptation for egg-based manufacturing, including loss of N-linked glycosylation sites, that can variably affect antigenicity (Supplementary References 15, 17, 18–22).
Here we report VE separately for Victoria and Yamagata lineages during 8 seasons (2010–2011 to 2017–2018) of predominant TIV use in Canada, enabling comparison of intra- and cross-lineage protection. Findings are interpreted in the context of phylogenetic (clade- and/or lineage-level) relatedness between consecutive seasons’ vaccine components and the epidemic strain.
METHODS
Historical databases of the Canadian Sentinel Practitioner Surveillance Network (SPSN) were used (Supplementary References 24–29). Respiratory specimens were collected from outpatients ≥1 year old presenting within 7 days of influenza-like illness onset to sentinel-practitioners in the provinces of Alberta, British Columbia, Ontario, and Quebec. Analyses were restricted to specimens collected in January–April, coinciding with typical late-season influenza B activity (Supplementary Figure 1). Influenza B was diagnosed by real-time reverse transcription polymerase chain reaction (RT-PCR), with lineage determined by a combination of RT-PCR, hemagglutination inhibition (HI) assay, and, from 2013–2014 onwards, HA gene sequencing of primary specimens (Supplementary Table 1a; Supplementary References 18, 19, 24-30) [10]. GenBank accession numbers are provided in Supplementary Table 1b.
Most vaccine doses purchased overall by SPSN provinces for the annual, publicly funded influenza immunization campaign were TIV (2010–2011 to 2013–2014: 100%; 2014–2015: >95%; 2015–2016: >85%; 2016–2017: >75%; 2017–2018: >70%) [10] (Supplementary Tables 2a-b; Supplementary References 24–29, 31, 32). However, QIV comprised most doses distributed in Alberta in 2016–2017 and 2017–2018 (>75% and >95%, respectively) but remained limited (primarily for children) in other SPSN provinces (<15% and <25%, respectively). In sensitivity analyses, children <20 years old were excluded from 2014–2015 through 2017–2018; Alberta was excluded in 2016–2017 and 2017–2018.
VE assessment was by test-negative design (Supplementary Material 2), with participant verbal consent and ethics approval. Vaccination status was based on patient self-report. Covariates included age, sex, comorbidity, province, specimen collection interval, and calendar time. During seasons for which the TIV component was unchanged and mismatched to circulating viruses, negative interference from the prior season’s vaccination was explored among patients ≥9 years old. For this secondary analysis, vaccination subgroups were specified by an indicator variable defined by mutually exclusive categories of current and/or prior season’s vaccination (Supplementary Material 2).
RESULTS
Each season, more than two-thirds of participants were adults ≥20 years old, and more than three-quarters of vaccinated participants were also vaccinated the prior season (Supplementary Tables 3a-m). Most (>90%) characterized viruses were Victoria lineage in 2010–2011 but Yamagata lineage in 2013–2014, 2014–2015, 2016–2017, and 2017–2018, with a greater mix of both lineages in 2011–2012, 2012–2013, and 2015–2016 (Supplementary Figure 1; Supplementary Table 1a). Eleven lineage-specific VE scenarios were assessed, including 6 when vaccine was lineage-matched (ie, “intralineage”) and 5 when vaccine was lineage-mismatched to circulating viruses (ie, “cross-lineage”) (Figure 1; Supplementary Tables 4 and 5).
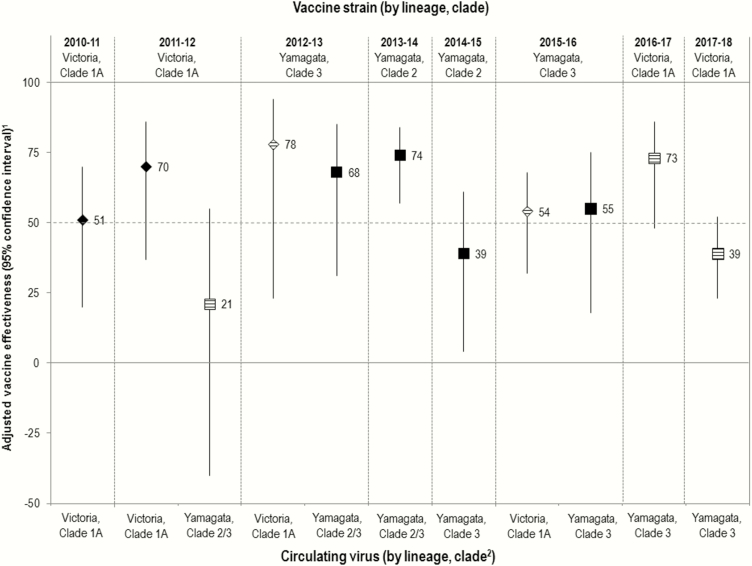
Figure 1.
Vaccine effectiveness (VE) against influenza B by season (n = 8) and scenario (n = 11) of lineage-level relatedness between the trivalent influenza vaccine (TIV) component and specified circulating viruses. VE estimates against Victoria-lineage viruses are shown as diamonds; VE estimates against Yamagata-lineage viruses are shown as squares. Where the TIV component and the specified circulating virus belong to different lineages, cross-lineage VE is represented as a hash mark. 1Sample sizes accompanying each estimate are provided in Supplementary Table 3. All analyses are adjusted for age group, sex, comorbidity, province, specimen collection interval, and week of specimen collection as detailed in Supplementary Table 4. Sensitivity analyses are presented in Supplementary Table 5. Differences in inclusion criteria, analysis period, and other model specifications should be taken into account when comparing to prior publications of season-specific VE estimates for influenza B (Supplementary References 24–29). 2See Supplementary Table 2 for details related to virological characterization. Prior to the 2013–2014 season, sequencing of the hemagglutinin gene was not routinely undertaken, and lineage and clade designation were based on alternative methods. In 2013–2014, most Yamagata viruses that were sequenced were clade 3 (82%) with some clade 2 contribution (18%). Yamagata clade 2 and clade 3 viruses can likely be considered antigenically distinct but with some variation in cross-reactivity based on antisera used (Supplementary References 18, 19).
VE exceeded 50% in 8 of 11 scenarios, including 5 of 6 intralineage and 3 of 5 cross-lineage estimates. In 5 of these 8 scenarios, VE exceeded 65%, including 3 intralineage and 2 cross-lineage estimates. In 3 scenarios, VE marginally exceeded 50%, including 2010–2011 when vaccine was unchanged from the prior season and lineage/clade matched to circulating viruses; and 2015–2016, when vaccine was changed from the prior season but VE was similar for co-circulating lineage/clade-matched and lineage-mismatched viruses.
In all 3 scenarios for which VE was <50%, vaccine was unchanged and mismatched to circulating viruses. In secondary analyses, we explored the role of negative interference from the prior season’s identical vaccine receipt on these lower estimates. In 2014–2015, when unchanged vaccine was lineage matched but clade (and likely antigenically) mismatched to circulating viruses, VE was lower for vaccination prior + current (22% [95% confidence interval {CI}, –30% to 53%]) vs current season only (53% [95% CI, –19% to 82%]). This pattern in the point estimates was not observed in 2011–2012 or 2017–2018 when vaccine was also unchanged but instead lineage mismatched to circulating viruses (Supplementary Tables 1a and 6; Supplementary Figure 2). However, CIs around each of these vaccination subgroup estimates were wide, precluding definitive interpretation.
DISCUSSION
From 2010–2011 to 2017–2018, VE against influenza B reported by the Canadian SPSN primarily reflected TIV protection in adults. Across 11 lineage-specific estimates, VE exceeded 50% regardless of lineage match to circulating viruses, with the exception of 3 scenarios for which the influenza B vaccine strain was unchanged from the prior season.
Others have reported cross-lineage VE (Supplementary References 33–36), including prior meta-analysis of randomized controlled trials (RCTs) that similarly reported mismatched VE exceeding 50% in adults, but without stratifying based on lineage- or clade-level mismatch (Supplementary Reference 36). Several immunogenicity studies reinforce cross-lineage interactions [9] (Supplementary References 37–41). Preferential recall (ie, “back-boost”) responses to childhood priming antigens (ie, “original antigenic sin”), as described for influenza A (Supplementary References 29, 42–44), may also extend cross-lineage for influenza B (Supplementary Reference 41). In one longitudinal trial, children primed with Yamagata-containing TIV demonstrated cross-lineage Yamagata boosting but minimal Victoria antibody responses despite successive doses of Victoria-containing TIV (Supplementary Reference 41). A similar pattern was observed in mice, although dominant Yamagata responses persisted with reverse-order Victoria priming/Yamagata boosting [9]. Our participants were mostly adults for whom immunological cohort effects induced by lineage-specific childhood priming and variable lifetime boosting may have also influenced lineage-specific risk by age (Supplementary Reference 45).
Negative interference from prior season’s vaccination was suggested in 2014–2015 when the unchanged vaccine was lineage-matched but clade (and likely antigenically) mismatched to circulating viruses. However, no such pattern was suggested in 2011–2012 or 2017–2018 when vaccine was also unchanged from the prior season but instead lineage mismatched (and therefore also antigenically mismatched) to circulating viruses. Only 2 influenza B epidemics were included in original validation of the antigenic-distance hypothesis to explain repeat vaccination effects [11]. Both validation scenarios were based on observations from an RCT by Keitel et al, and also involved unchanged vaccines (Supplementary Reference 46). Similar to our findings, Keitel et al observed no interference during the 1985–1986 epidemic due to lineage-level mismatched viruses [2, 5, 11] (Supplementary References 46, 47), but negative interference in 1987–1988 when the unchanged vaccine and epidemic strain were lineage-related but antigenically distinct variants [2, 5, 11] (Supplementary References 46, 48).
McLean et al also showed lower VE among participants vaccinated prior + current (52% [95% CI, 38%–62%]) vs current season only (66% [95% CI, 51%–77%]), a nonsignificant pattern that became more pronounced when comparing frequently vaccinated (48% [95% CI, 29%–62%]) to current-season-only vaccinees (75% [95% CI, 50%–87%]; P = .05) (Supplementary Reference 49). Their analysis, however, pooled findings across multiple seasons and did not examine season-specific vaccine–virus relatedness (Supplementary Reference 49). As in Canada, Valenciano et al reported negative interference in Europe in 2014–2015, with lower VE for prior + current vs current-season-only vaccination (44% [95% CI, 20%−61%] vs 59% [95% CI, 25%−78%]; Supplementary Reference 50), whereas the US Flu VE Network found no such pattern (56% [95% CI, 39%−68%] vs 59% [95% CI, 34%−74%]; Supplementary Reference 51). Unlike Canada or Europe (Supplementary References 29, 52), US analyses suggested negative interference in 2015–2016 when the Yamagata-containing TIV was clade changed and lineage mismatched to Victoria viruses (VE, 33% [95% CI, –8% to 58%] vs 62% [95% CI, 27%–80%]; Supplementary Reference 53). Unlike Canada/Europe, QIV containing the identical Victoria antigen has been available in the United States since 2013–2014 where it already comprised more than half the doses distributed in 2014–2015 and 2015–2016 (Supplementary References 51, 53). It is noteworthy that for the most recent—early and prolonged—2017–2018 Yamagata epidemic, VE estimates for comparable November–April analysis periods were similar in Canada (44% [95% CI, 30%–55%] overall; 38% [95% CI, 22%–51%] excluding Alberta; Supplementary Table 5) and the United States (49% [95% CI, 40%–56%]; Supplementary Reference 54), despite predominant use of lineage-mismatched TIV in Canada but lineage-matched QIV in the United States, with both influenza B vaccine strains unchanged from the prior season [10]. Relevance of TIV vs QIV to cross-protection and/or repeat vaccination effects requires further evaluation, but other study differences should also be considered.
Antigenic relatedness is conventionally assessed by HI assay [12]. However, HI findings primarily reflect epitopes around the receptor-binding site of the HA head and vary with reference antisera used (eg, to egg-adapted or cell-passaged vaccine reference strains) and other assay conditions [12] (Supplementary References 13–23). We interpreted VE based upon HA phylogenetic (clade- and/or lineage-level) relatedness, although not all genetic differences confer antigenic distinction. Neither approach (antigenic or genomic) captures the role of other conserved epitopes or cross-protective targets such as anti-NA or antistalk antibodies, cell-mediated responses, or their interplay (Supplementary References 55–59). Given cross-lineage reassortment events involving the NA, better understanding of its vaccine content and contribution to cross-lineage protection is needed [3, 6]. A heightened role for anti-NA and antistalk responses has been proposed when encountering a novel influenza A subtype for which the typically immunodominant HA head is dramatically divergent from prior exposures, a hypothesis also potentially relevant to cross-lineage influenza B encounters (Supplementary References 60–62).
Limitations of our study include relatively few seasons (n = 8) and scenarios (n = 11) for VE comparison based on vaccine–virus relatedness conditions, and small sample size for some lineage-specific analyses. Our VE estimates primarily reflect the experience of repeatedly vaccinated relative to consistently unvaccinated adults and are most reliably interpreted in that context. Exploratory analyses further stratified by current and/or prior season’s vaccination history suffered reduced sample size, with wide CIs and potentially unstable estimates requiring cautious interpretation. Other limitations include residual bias/confounding such as the information (recall) bias with self-reported vaccination status, particularly among those with less habitual vaccination behaviors. We did not have individual-level information on TIV vs QIV receipt or their distribution by age, and could not account for prior infection history.
Overall, our observations of substantial cross-lineage protection but lower VE with identical vaccine use cross-season suggest complex immunoepidemiological interactions influencing vaccine protection against influenza B. The implications for both trivalent and quadrivalent formulations warrant further monitoring and investigation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We gratefully acknowledge the contribution of those at the sentinel sites whose regular submission of specimens and data provided the basis of our analyses. We thank the following for network coordination and data entry activities in each province: Lisan Kwindt for national database management and sentinel network coordination activities in British Columbia; Elaine Douglas, Kinza Rizvi, Sandra Berzins, and Kasim Qureshi for The Alberta Recording and Research Network in Alberta; Romy Olsha for Public Health Ontario; and Sophie Auger for the Institut national de santé publique du Québec. We thank those who provided laboratory support at the British Columbia Centre for Disease Control Public Health Laboratory, the Alberta Provincial Laboratory for Public Health, the Public Health Ontario Laboratory, the Laboratoire de santé publique du Québec, and the National Microbiology Laboratory in Manitoba. Finally, we thank Siobhan Leir and Yayuk Joffres of the British Columbia Centre for Disease Control for their contributions to analyses for the 2017-18 season.
Financial support. This work was supported by the Canadian Institutes of Health Research (grant number TPA-90193); the British Columbia Centre for Disease Control; Alberta Health and Wellness; Public Health Ontario; Ministère de la santé et des services sociaux du Québec; l’Institut national de santé publique du Québec; and the Public Health Agency of Canada.
Potential conflicts of interest. D. M. S. is principal investigator on grants received from the Canadian Institutes of Health Research and the Public Health Agency of Canada in support of this work. G. D. S. has received grants for investigator-initiated studies unrelated to influenza vaccine from GlaxoSmithKline (GSK) and Pfizer and provided paid expert testimony for the Ontario Nurses Association, the Quebec Ministry of Justice, and GSK. J. G. has received research grants from GlaxoSmithKline and Hoffman-La Roche to study antiviral resistance in influenza, and from Pfizer to conduct microbiological surveillance of Streptococcus pneumoniae. M. K. has received research grants from Roche, Siemens, and Hologic for unrelated studies. S. J. D. is a content expert consultant to Johnson & Johnson (Janssen) Pharmaceuticals on a literature search for point-of-care testing for respiratory viruses. S. S. was funded by the Canadian Institutes of Health Research (grant number TPA-90193) and by the Public Health Agency of Canada. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lindstrom SE, Hiromoto Y, Nishimura H, Saito T, Nerome R, Nerome K. Comparative analysis of evolutionary mechanisms of the hemagglutinin and three internal protein genes of influenza B virus: multiple cocirculating lineages and frequent reassortment of the NP, M, and NS genes. J Virol 1999; 73:4413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen JM, Guo YJ, Wu KY, et al. . Exploration of the emergence of the Victoria lineage of influenza B virus. Arch Virol 2007; 152:415–22. [DOI] [PubMed] [Google Scholar]
- 3. Vijaykrishna D, Holmes EC, Joseph U, et al. . The contrasting phylodynamics of human influenza B viruses. Elife 2015; 4:e05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990; 175:59–68. [DOI] [PubMed] [Google Scholar]
- 5. Chen R, Holmes EC. The evolutionary dynamics of human influenza B virus. J Mol Evol 2008; 66:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw MW, Xu X, Li Y, et al. . Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000-2001 and 2001-2002 seasons. Virology 2002; 303:1–8. [DOI] [PubMed] [Google Scholar]
- 7. McCullers JA, Wang GC, He S, Webster RG. Reassortment and insertion-deletion are strategies for the evolution of influenza B viruses in nature. J Virol 1999; 73:7343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu X, Lindstrom SE, Shaw MW, et al. . Reassortment and evolution of current human influenza A and B viruses. Virus Res 2004; 103:55–60. [DOI] [PubMed] [Google Scholar]
- 9. Skowronski DM, Hamelin ME, Janjua NZ, et al. . Cross-lineage influenza B and heterologous influenza A antibody responses in vaccinated mice: immunologic interactions and B/Yamagata dominance. PLoS One 2012; 7:e38929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. WHO recommendations on the composition of influenza virus vaccines Available at: http://www.who.int/influenza/vaccines/virus/recommendations/en/. Accessed 24 September 2018.
- 11. Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther 2011; 9:669–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.