Abstract
Background
This study measured serial plasma human immunodeficiency virus (HIV)-1–specific antibody (Ab) levels in children who initiated antiretroviral therapy (ART) prior to 2 years of age, and evaluated their relationship to peripheral blood HIV-1 RNA and DNA levels.
Methods
We studied 46 HIV-1–infected children, stratified by age at ART initiation (<3 mo, early therapy [ET]; >3 mo–2 years, late therapy [LT]) and by virologic response (R) or non-response (NR), before and up to 4 years following ART. We studied 20 HIV-1–uninfected children born to HIV-1–infected mothers (seroreverters [SR]) as controls. Plasma immunoglobulin G (IgG) Ab levels directed against HIV-1 envelope (gp160, gp41), gag (capsid, p24; matrix, p17), reverse transcriptase (p66/51), and integrase (p31) were serially measured using quantitative enzyme-linked immunosorbent assays. HIV-1 Ab rates of decline were estimated over the first 15 months of the study.
Results
The HIV-1 Ab rates of decline in the ET-R group were similar to those in the SR group for all Ab specificities, except for p17 (P = .01). Ab decline rates in the LT-R group and the NR group were significantly slower than in the SR group for all tested Ab specificities. After 1 year of age, Ab levels to p31 and p17 were significantly associated with HIV-1 RNA levels (P < .001); Ab levels to gp160 (P < .001) and gp41 (P < .001) were significantly associated with cell-associated HIV-1 DNA levels.
Conclusions
Quantitative HIV-1–specific Ab levels may be useful for screening children on ART for viral suppression or for residual, cell-associated HIV-1 DNA levels.
Clinical Trials Registration
NCT00000872.
Keywords: pediatric early antiretroviral therapy, HIV-1 persistence, HIV-1 quantitative antibodies
Quantitative human immunodeficiency virus (HIV)-1–specific antibody levels are associated with plasma HIV-1 RNA and cell-associated HIV-1 DNA levels in children on antiretroviral therapy and may be useful for identifying children with viral suppression and low residual HIV-1 DNA levels.
Combination antiretroviral therapy (ART) markedly reduces human immunodeficiency virus (HIV)-1–related morbidity and mortality in children [1]. Early ART initiation in children prior to 3–6 months of age can be particularly effective for long-term control of HIV-1 replication, preserving immune functions [2, 3], reducing HIV-1–related illnesses and mortality [4], and limiting residual HIV-1 reservoirs [5, 6]. International treatment guidelines [7–9] now recommend ART initiation in HIV-1–infected children as soon as possible after birth.
Methods for quantifying plasma HIV-1 RNA and peripheral blood mononuclear cell (PBMC)-associated HIV-1 DNA in children following ART are expensive and logistically challenging, particularly for implementation in limited-resource settings. We [10] and others [11–13] have previously shown that children who achieve durable suppression of HIV-1 replication after early (<3 months) ART have very low levels of PBMC-associated HIV-1 DNA, and the majority lack persistent HIV-1 immunoglobulin G (IgG) antibodies [3, 14]. Children who initiate treatment after 3–6 months of age have higher levels of PBMC-associated HIV-1 DNA and remain HIV-1–antibody (Ab) positive. In a prior cross-sectional study, children with negative or indeterminate Western blots had lower levels of circulating HIV-1 DNA than those with positive Western blots [15]. We therefore undertook this study to further evaluate the utility of using quantitative HIV-1 Ab levels to HIV-1 proteins as a screening method for virologic suppression and residual PBMC-associated HIV-1 DNA in children on therapy. The quantitation of Ab levels to selected HIV-1 proteins prior to and sequentially up to 4 years following ART allowed for the calculation of Ab clearance rates and the development of models that used specific Ab levels to predict the likelihood of undetectable plasma HIV-1 RNA or the level of circulating, PBMC-associated HIV-1 DNA.
METHODS
Study Cohort
The study cohort included 46 HIV-1–infected children, stratified by timing of ART initiation (early therapy [ET] <3 months of age; late therapy [LT] >3 months to 2 years of age) and as virologic responders (R) or non-responders (NR). There were 44 children that received ART through an open-label, phase I/II clinical trial that evaluated the pharmacokinetics, safety, and antiviral activity of combination antiviral therapies initiated under 2 years of age (Pediatric AIDS Clinical Trials Group Protocol [PACTG] 356; clinical trials NCT00000872 [16]); 2 were treated by open prescription. Specimens were collected from HIV-1–infected participants from 1995 through 2005 at clinical sites throughout the United States and Puerto Rico. Virologic responders were defined as HIV-1–infected children who achieved plasma HIV-1 RNA levels of <400 copies/ml by 48 weeks of therapy and sustained plasma HIV-1 RNA <50 copies/ml through at least 96 weeks; 4 children who were classified as responders experienced a viral rebound (RNA > 50 copies/ml) and 1 child experienced a viral blip (a single value of 106 copies/ml) after 96 weeks on ART. Ab decline estimates in virologic responders were calculated from samples obtained prior to 96 weeks of study. Virologic non-responders (NR: 10 ET and 14 LT) were children who did not achieve undetectable viral loads within the first year of ART (n = 19) or who initially suppressed viral replication but experienced a virologic rebound between 48 and 96 weeks of ART (n = 5). HIV-1–infected children were studied prior to ART initiation and at 6 months, 1 year, 2 years, and 4 years following ART initiation. We studied 20 HIV-1–uninfected children born to HIV-1–infected women (seroreverters [SR]), with samples obtained at birth and at 6 months, 1 year, and 2 years of age; the study of SR beyond 2 years of age was not possible, since they were discharged from routine follow-up for HIV-1 infection if found to be uninfected by 2 years of age. Institutional Review Boards at the University of Massachusetts Medical School and at each clinical site approved this study, and informed consent was obtained from the children’s guardians prior to enrollment.
Quantitation of HIV-1–specific Antibodies
Ab levels to HIV-1 envelope (gp160, gp41), gag (capsid, p24; matrix, p17), reverse transcriptase (RT; p66/51), and integrase (p31) were quantified in acid citrate dextrose, heparin, or ethylenediaminetetraacetic acid-anticoagulated plasma (Becton-Dickinson Vacutainers, Mountain View, California) by enzyme-linked immunosorbent assay (ELISA) [17]. The 12-well high-binding ELISA strips were coated with recombinant proteins: 10ng p24 (Immune Tech, New York, New York) or p17 (Abcam, Cambridge, United Kingdom); or 12.5ng p31 (Abcam), gp41 (Abcam), gp160 (My Biosource, San Diego, California), or RT (Avioq, Research Triangle Park, North Carolina). The 5-point standard curves were prepared by serially diluting known quantities of HIV-1 p24, gp41, gp160, and RT polyclonal antibodies (ImmunoDx, Woburn, Massachusetts) in sample buffer. The lack of available reliable human anti-p17 or anti-p31 mono- or polyclonal antibodies precluded the generation of standard curves for these proteins. Individual ELISA assays were performed on 1:100 dilutions of pooled plasma samples from HIV-positive (positive control) and HIV-negative (negative control) individuals, as well as from the study cohort; in the event that a 1:100 dilution value fell outside the standard curve linear range, additional 1:10, 1:1000, or 1:10000 dilutions were run. Absorbance at 405 nm was measured (Biotek, Winooski, Vermont) and samples were considered positive if their optical densities (OD) were greater than 2 standard deviations from the mean OD of a pooled, HIV-negative control (run in triplicate). Quantitative measures of p24, gp41, gp160, and RT antibodies were determined by extrapolation of OD measurements of standard curve values. Results for p17 and p31 are reported as OD405nm values, since standard curves were not generated for these proteins. This approach resulted in limits of detection (LODs) (dynamic ranges), as follows: p24, 0.1 ug/ml (0.15–125000ug/ml); gp41, 0.4 ug/ml (0.6–50000ug/ml); gp160, 0.1 ug/ml (0.2–50000ug/ml); RT, 0.1 ug/ml (0.2–3100ug/ml); p31, 0.06 OD405nm (0.299–2.000 OD405nm); p17, 0.06 OD405nm (0.152–2.000 OD405nm). The lower limit of the dynamic range was used as the lowest possible value in all calculations. Assay validation using positive controls, negative controls, and individual plasma samples from 5 HIV+ individuals (20 total samples) showed 90% reproducibility.
Quantitation of Viral Replication and HIV-1 DNA Persistence
Plasma HIV-1 RNA loads were measured using either the Roche Standard (LOD <400 copies/ml) or UltraSensitive (LOD <50 copies/ml) AMPLICOR HIV-1 MONITOR kits Version 1.5 (Roche Diagnostics, Indianapolis, Indiana). PBMC HIV-1 total DNA levels were quantified just prior to initiation of ART and yearly for 4 years in 21 viral responders and in 8 viral non-responders, as previously described [10].
Statistical Analyses
Analyses used the software Stata (v13, StataCorp). We used a 1-way analysis of variance with a post hoc Sidak to compare group Ab levels. Correlations were calculated using the Spearman rho. Repeated HIV-1–specific Ab levels collected longitudinally were tested with linear mixed-regression models to estimate the relative decline rates in each group through 15 months (data through 12 months with a sample window of 3 months; mean of 3 data points per participant). Post-estimation contrasts compared the decline rates between groups. Generalized linear models (ordinary least squares regression for continuous data and logistic regression for binary outcomes) were fit to test for the association of Ab levels with plasma HIV-1 RNA copies/ml or HIV-1 DNA copies per million PBMC, using samples obtained at >12 months of age and adjusting for potentially-confounding covariates. Regression coefficients were plotted with coefplot [18]. All statistical tests were 2-sided; P < .05 was considered statistically significant.
RESULTS
Study Cohort
We studied 66 children aged 0 to 24 months, who were grouped by HIV-1 infection status, age at ART initiation (ET or LT), and response to ART (responder or non-responder; Table 1). There was no difference in the log baseline HIV-1 RNA levels between the ET-R, LT-R, and NR groups (medians 5.4, 4.8, and 5.5, respectively; P = .6). The log HIV-1 DNA at baseline was similar across the ET-R, LT-R, and NR groups (medians 3.0, 3.7, and 3.8, respectively; P = .4).
Table 1.
Study Cohort at Baseline: Pre-therapy Viral Load, CD4 Percentage, and Therapeutic Regimens
| Cohort | SeroReverters | ET Respondersa | LT Respondersa | Non-responders |
|---|---|---|---|---|
| n | 20 | 14 | 8 | 24 |
| Age, mos | 0 (0–0) | 1.8 (1.4–2.3) | 9.5 (5.7–13.4) | 3.8 (2.6–7.5) |
| log10 plasma HIV-1 RNA, copies/ml | N/A | 5.4 (5.2–5.6) | 4.8 (4.7–5.7) | 5.5 (4.7–6.0) |
| log10 proviral DNA, copies/million PBMCs | N/A | 3.0 (2.7–3.4) n = 13 |
3.7 (3.0 - 4.0) n = 8 |
3.8 (3.5–3.9) n = 8 |
| CD4 % | 49 (43–59) |
38 (28 - 48) | 35 (27 - 42) | 35 (27–46) |
| Therapeutic regimens | N/A | 10 (2 NRTIs + 1 NNRTI + 1 PI) 2 (3 NRTIs + 1 NNRTI) 1 (2 NRTIs + 1 NNRTI) | 5 (2 NRTIs + 1 NNRTI + 1 PI) 2 (3 NRTIs + 1 NNRTI) 1 (2 NRTIs + 1 NNRTI) | 8 (2 NRTIs + 1 NNRTI + 1 PI) 7 (3 NRTIs + 1 NNRTI) 9 (2 NRTIs + 1 NNRTI) |
| Age in mos at viral suppression | N/A | 4.3 (3.5–5.6) | 14.8 (8.4–21.7) | N/A |
Values are provided as medians with 25th and 75th percentiles (interquartile range).
Abbreviations: ART, antiretroviral therapy; ET, early therapy; HIV, human immunodeficiency virus; LT, late therapy; N/A, not applicable; NNRTI, non-nucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PBMC, peripheral blood mononuclear cell; PI, protease inhibitor.
aResponders were defined as study subjects who achieved a viral load of <400 copies/ml by 48 weeks of ART therapy and whose peripheral blood HIV-1 RNA levels remained under assay detection limits through 4 years of ART.
Baseline Antibody Levels
Baseline anti-gp160, -RT, -p24, and -p17 Ab levels were similar across all groups (Table 2). Baseline anti-gp41 Ab levels were significantly higher in the SR group than in the ET-R, LT-R, and NR groups (P = .002); baseline anti-p31 Ab levels were significantly higher in the SR group than in the LT-R (P = .008) and NR (P = .04) groups. Baseline age was inversely correlated with Ab levels to RT (rho = 0.34, P = .007), gp41 (rho = -0.35, P = .005), p31 (rho = -0.45,P < .001), and p17 (rho = -0.26,P = .04). Baseline gp160 and gp41 Ab levels were highly correlated (rho = 0.68; P < .001). In young children with continued HIV-1 replication, Ab levels to gp160 and gp41 initially appeared to decrease from baseline until around age 4–5 months, after which levels increased (data not shown).
Table 2.
Human Immunodeficiency Virus-1–specific Antibody Levels at Baseline With Antibody Decay Rates
| Antibody Specificity | Baselinea | Ab Decay Rate, as Units/Months With 95% CIb | Age at Clearance-SR, in Monthsc | Age at Clearance- ET-R, in Monthsc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | ET-R | LT-R | NR | SR | ET-R | LT-R | NR | Compared to SR (Reference) | |||
| gp160 log, ug/ml | 1.8 (1.2–2.2) |
1.5 (0.9–1.6) |
1.6 (0.7–1.9) |
1.4 (1.0–1.7) |
-0.19 (-0.22, -0.16) |
-0.18 (-0.21, -0.15) |
-0.04 (-0.05, -0.02) |
-0.003 (-0.03, 0.02) |
ET P = .91 LT P < .001 NR P < .001 |
12.4 | 12.4 |
| RT log, ug/ml |
1.5 (0.6–1.6) |
1.4 (0.3–1.5) |
-0.7 (-0.7–1.7) |
0.5 (-0.05–1.2) |
-0.14 (-0.17, -0.11) |
-0.15 (-0.20, -0.10) |
-0.05 (-0.09, -0.01) |
0.005 (-0.02, 0.03) |
ET P = .87 LT P < .001 NR P < .001 |
12.7 | 12.9 |
| gp41 log, ug/ml |
3.2 (2.5–3.5) |
2.2 (1.87–2.7) |
2.1 (1.6–2.6) |
2.4 (1.6–2.8) |
-0.25 (-0.28, -0.21) |
-0.22 (-0.26, -0.18) |
-0.04 (-0.06,-0.02) |
-0.003 (-0.03, 0.03) |
ET P = .53 LT P < .001 NR P < .001 |
12.8 | 12.2 |
| p24 log, ug/ml |
1.6 (0.61–2.1) |
1.9 (1.7–2.2) |
1.4 (0.4–1.7) |
1.7 (1.1–1.9) |
-0.17 (-0.19, -0.14) |
-0.14 (-0.18, -0.11) |
-0.04 (-0.07,-0.01) |
0.02 (0.00, 0.05) |
ET P = .24 LT P < .001 NR P < .001 |
12.9 | 20.6 |
| p31, OD | 1.5 (1.4–1.6) |
1.3 (0.8–1.5) |
0.4 (0.3–1.5) |
0.9 (0.4–1.5) |
-0.09 (-0.10, -0.07) |
-0.08 (-0.10, -0.06) |
-0.01 (-0.03, 0.00) |
0.008 (-0.01, 0.03) |
ET P = .80 LT P < .001 NR P < .001 |
11.7 | 11.6 |
| p17, OD | 1.2 (0.4–1.9) |
0.9 (0.4–1.1) |
0.5 (0.3–0.8) |
0.9 (0.3–1.4) |
-0.08 (-0.10, -0.06) |
-0.04 (-0.07, -0.02) |
-0.03 (-0.04, -0.01) |
0.005 (-0.01, 0.02) |
ET P = .01 LT P < .001 NR P < .001 |
10.9 | 16.6 |
Abbreviations: Ab, antibody; CI, confidence interval; ET, early therapy; LT, late therapy; NR, viral non-responders; OD, optical densities; R, viral responders; SR, seroreverters; RT, reverse transcriptase.
aMedian with interquartile range.
bSlopes with 95% CIs.
cEstimate based on linear regression model parameters.
Plasma HIV-1 Antibody Clearance Rates in HIV-1–uninfected Infants
We measured HIV-1 Ab in serial plasma samples from HIV-1–uninfected infants born to HIV-1–infected women in order to estimate the decay rates of passively-acquired maternal antibodies and to estimate the time to clearance from the circulation. The decline rate in gp41-specific Ab was faster (-0.25 log ug/ml /month) than in all other Ab specificities (Table 2). Using the model estimates for the Ab decline in the SR group, the estimated time to clearance of maternal Ab in the absence of an HIV-1 infection was calculated to be 10.9 months for p17, 11.7 months for p31, and 12.4–12.9 months for gp160, RT, gp41, and p24 (Table 2). Time estimates are based on the model parameters and do not suggest that all SR were negative by the estimated time, but rather approximate the time when the model fit crossed the assay cutoff.
Plasma HIV-1 Antibody Decay Rates in HIV-1–infected Children on Antiretroviral Therapy
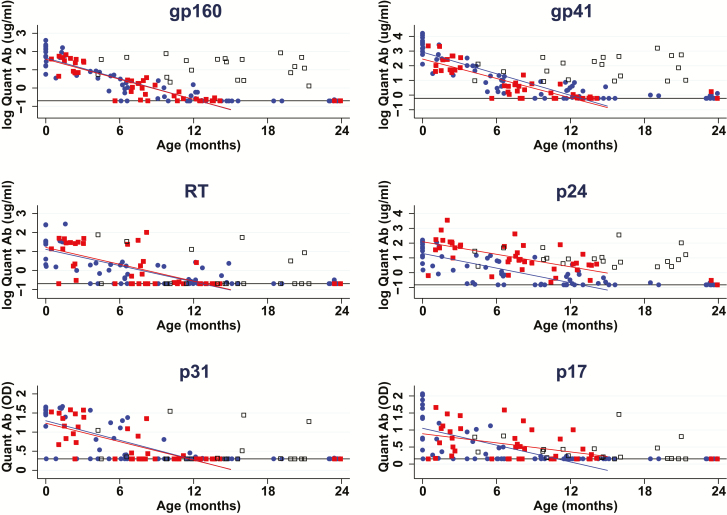
Over the first 15 months of the study, HIV-1 Ab decline rates in the ET-R group were similar to the SR group for all Ab specificities, with the exception that the decline rate of p17 Ab levels in ET-R group was slower than in the SR group (P = .01; Table 2, Figure 1). The estimated time to clearance of all antibodies in ET-R varied by specificity, and ranged from 12 to 21 months (Table 2). The estimated age at the clearance of antibodies in ET-R was similar to the estimated age of clearance for the SR for all specificities, except for p24 (20.6 months vs 12.9 months, respectively) and p17 (16.6 months vs 10.9 months, respectively). By contrast, Ab decline rates in the LT-R group and the NR group were significantly different (slower decline or no decline) from the SR group for all tested Ab specificities (P < .001 for all comparisons). Ab decline rates in the LT-R group and the NR group were significantly different from the ET-R group for most Ab specificities (P < .001); an exception was that the p17-specific Ab decline in the LT-R and ET-R groups were similar (P = .28), while the p17 Ab decay rate in the NR was significantly slower than in the ET-R group (P = .001). Ab levels in the NR group did not decline, with estimated slopes near 0 (Table 2, individual points for the NR group not displayed in Figure 1). There were 4 children who initially suppressed HIV-1 replication, experienced a rebound in their viral loads after 96 weeks of ART, and also experienced a rebound in all Ab levels (data not shown).
Figure 1.
Human immunodeficiency virus (HIV)-1–specific antibody (Ab) decay rates in HIV-1–uninfected infants born to HIV-1–infected women (SR) in ET-R and LT-R groups. Quantitative (Quant) Ab levels of HIV-1 gp160, gp41, RT, p24, p31, and p17 in SR, ET-R, and LT-R children over the first 24 months of age. Ab decline rates, calculated over the first 15 months, in the ET-R group are similar to the SR group for all Ab specificities, except for p17 (P = .01). Fitted lines for the SR and ET-R groups were calculated with parameters from the linear mixed effects model. SR are shown as blue circles; ET-R as red squares; and LT-R as black, hollow squares. Abbreviations: Ab, antibody; ET, early therapy; HIV, human immunodeficiency virus; LT, late therapy; OD, optical densities; Quant, quantitative; R, viral responders; SR, seroreverters.
Association of Quantitative Antibody Levels With Plasma HIV-1 RNA Levels and Circulating Cell–associated HIV-1 DNA Levels
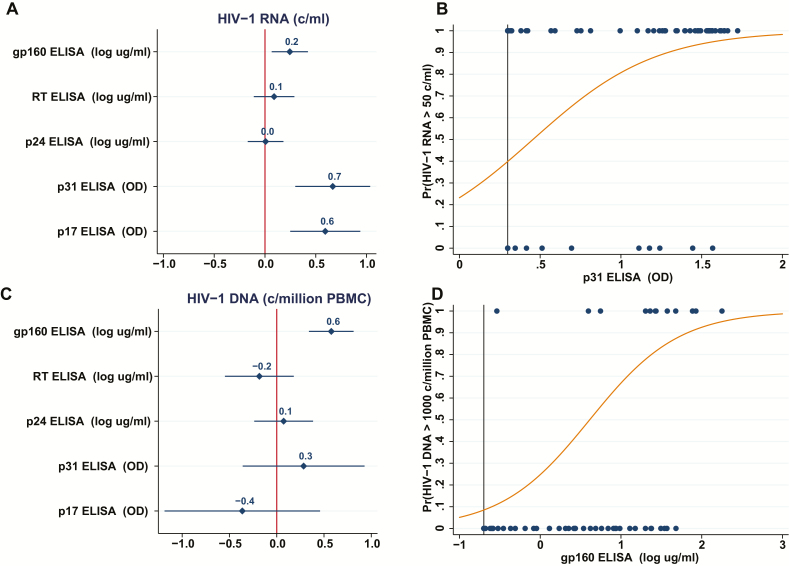
We used a linear regression model to predict HIV-1 RNA using Ab levels obtained after 12 months of age for each HIV-1 specificity tested, except gp41 (highly correlated to gp160): adjusting for age accounted for 62% of the variance in HIV-1 RNA (F[6,134] = 36; P < .001; R2 = 0.62). Ab levels to gp160, p31, and p17 were significant predictors in the model (Figure 2). Ab levels to p31 and p17 were associated with higher plasma HIV-1 RNA levels (F[3,138] = 66; P < .001; R2 = 0.59; p31 β = 0.95; p17 β = 0.84), and a logistic model estimated that a unit increase in p31 Ab was associated with a 14-fold increase in the odds of an RNA level >50c/ml (P < .001). The estimated probability of detectable HIV-1 RNA based on Ab levels of p31, derived from the logistic regression model, is shown in Figure 2.
Figure 2.
Prediction of human immunodeficiency virus (HIV)-1 RNA and DNA based on linear and logistic models using quantitative antibody levels as predictors. A, Coefficient plot: coefficients and the 95% confidence intervals for the antibody (Ab) specificities, derived from a linear regression model predicting HIV-1 RNA levels (c/ml) from Ab levels. Bars which do not cross the line at 0 indicate a significant predictor. Model results: F(6,134) = 36; P < .001; R2 = 0.62. B, The estimated probability of detectable HIV-1 RNA (>50 c/ml) based on Ab levels of p31 derived from the logistic model. C, Coefficient plot: coefficients and the 95% confidence interval for the Ab specificities, derived from a linear regression model predicting HIV-1 DNA levels (c/million peripheral blood mononuclear cell) from Ab levels. Bars which do not cross the line at 0 indicate a significant predictor. Model results: F(6,61) = 7.1; P < .001; R2 = 0.41. D, The estimated probability of HIV-1 DNA > 1000 based on Ab levels of gp160 derived from the logistic model. Abbreviations: Ab, antibody; ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; OD, optical densities; PBMC, peripheral blood mononuclear cell; Pr, probability; RT, reverse transcriptase.
A model developed to predict circulating HIV-1 DNA levels using Ab specificities, excluding gp41 and adjusting for age, accounted for 41% of the variance in HIV-1 DNA (F[6,61] = 7.1; P < .001; R2 = 0.41, Figure 2). In children over 12 months of age, gp160 Ab levels predicted circulating HIV-1 DNA levels (F[2,65], P < .001; R2 = 0.37; β = 0.51). A logistic model using gp160-specific Ab levels estimated that each unit increase in a log gp160 Ab was associated with a 6-fold increase in the odds of an HIV-1 DNA level >1000 (P < .001). The estimated probability of an HIV-1 DNA level > 1000 copies/million PBMC based on Ab levels of gp160 derived from the logistic regression model is shown in Figure 2.
DISCUSSION
We and others have previously shown that ART initiation in children prior to 3–6 months of age allows for the long-term control of HIV-1 replication and limits residual HIV-1 reservoirs [5, 6]. Younger ages at ART initiation, more intense ART regimens, and the absence of resistance mutations were related to virologic suppression [16]. Children who rapidly suppress HIV-1 replication after early (<3 months) ART initiation commonly lack persistent HIV-1–specific Ab responses [3, 12, 14] and have lower circulating HIV-1 DNA levels [3, 10, 11, 19]. We have previously reported lower PBMC-associated HIV-1 DNA levels in early-treated, virally-suppressed children with negative or indeterminate Western blots [15]. Using data and repository samples from a well-characterized early ART cohort [16], we evaluated whether quantitative HIV-1 Ab levels might predict levels of HIV-1 RNA and DNA in children on ART.
Because maternal IgG antibodies are passively transferred from women to their infants during the last trimester of pregnancy [20, 21], all children born at term to HIV-1–infected women are seropositive. Baseline age correlated inversely with Ab levels. Moreover, HIV-1–infected children who initiated ART prior to 3 months of age and who suppressed viral replication by 48 weeks experienced Ab decline rates similar to those in uninfected children born to HIV-1–infected women for all Ab specificities, except p17. This suggests little de novo IgG production by the infant over the first several months of age. From these data, it is difficult to determine exactly when de novo Ab production to HIV-1 proteins begins; however, baseline Ab data over the range of ages studied (0–24 months) demonstrate an increase in antibodies to gp160 and gp41 at around 4–5 months of age (data not shown), suggesting the active production of these antibodies. In contrast, antibodies to most HIV-1 proteins are detected in adults within 3–4 weeks of infection; antibodies to p31 take longer and are produced around 7 weeks after detectable viremia [22]. The apparent delay in the robust production of HIV-1 antibodies in infants, despite high levels of HIV-1 replication in early infections, is notable, and could be due to a lack of CD4+ T-cell help in the face of high levels of HIV-1 replication.
In our study, most HIV-1–uninfected children born to HIV-infected women cleared maternal antibodies at around 13 months of age, although prior reports indicate that passively-transferred maternal antibodies may persist in the circulation of uninfected infants up to 18 months [23, 24]. Our data suggest that antibodies detected after 12–13 months of age are largely generated in response to the viral replication and antigen production associated with infection.
In untreated HIV-1–infected adults, anti–HIV-1 Ab levels are stable over time and correlate with viral loads [25]. Anti–HIV-1 Ab levels decline but do not totally clear with the suppression of HIV-1 replication on ART; higher levels of HIV-1–specific antibodies on suppressive therapy are associated with later initiations of ART after infection and with higher cell-associated DNA and RNA levels. HIV-1–specific Ab persistence after virologic suppression on ART may be stimulated by low levels of ongoing viral replication or the production of translationally-competent HIV-1 transcripts [26].
Our study suggests that in children older than 1 year of age, anti-p31 and anti-p17 IgG Ab levels may be good markers for ongoing replication, while Ab levels to gp160 could serve as a marker for PBMC-associated HIV-1 DNA levels. In adults, Lee and colleagues reported that anti-p17 Ab levels were associated with integrated HIV-1 DNA levels in resting CD4+ T-cells [27]. A recent pediatric study that examined the relationship between anti-gp41 antibodies and peripheral blood HIV-1 DNA levels found that lower levels of antibodies were associated with a younger age at ART initiation; however, there was no significant correlation between anti-gp41 Ab levels and peripheral blood HIV-1 DNA levels [28]. The difference in findings between the latter study and ours may be related to the older age at ART initiation (median age 3.3 years) in that study, or the different methods used to quantitate the total cell-associated HIV-1 DNA levels.
Our study included a relatively small number of children from the United States and Puerto Rico who initiated ART within 2 years of birth. The results of the prediction models may be limited to children who initiate ART relatively soon after primary infection, and may not be broadly applicable to all HIV-1–infected children. We used previously-measured cell-associated HIV-1 DNA levels and only had HIV-1 DNA data for a limited number (8) of non-responders; nevertheless, we expect that the inclusion of a larger number of children with detectable viremia, high HIV-1 DNA levels, and relatively high Ab levels would further strengthen the observed association. Larger studies that include participants with more diverse ages at ART initiation and various HIV-1 subtypes will be important to more generally evaluate the utility of HIV-1 Ab levels as markers for viral replication and reservoir size. Due to the limited blood volumes available, we did not include other factors, such human leukocyte antigen genotyping, which have been reported to influence the generation of antibodies following early ART therapy [29]. A recent study suggests that HIV-specific IgG3 antibodies may be predictors of spontaneous controllers [30]. We did not investigate IgG subclasses, but doing so may elucidate specific combinations of IgG subclasses that might be good markers for viral replication or persistence.
In summary, even with these limitations, our data suggest that HIV-1–specific Ab levels may be useful markers for screening children on ART for viral suppression or for low residual cell-associated HIV-1 DNA levels. This may be particularly useful for screening early-treated children on ART for therapeutic vaccines and other protocols aimed at achieving HIV-1 remission. Since the measurement of quantitative HIV-1 Ab levels requires small blood volumes and is generally more rapid, less expensive, and less technically-intensive than the measurement of HIV-1 nucleic acids, the measurement of quantitative antibodies may also be useful for screening for virologic suppression and HIV-1 DNA persistence in early-treated children in limited-resource settings.
Notes
The Pediatric AIDS Clinical Trials Group Protocol (PACTG) 356 Investigators are Richard Rutstein, Children’s Hospital of Philadelphia; Hannah Gay, University of Mississippi; William Borkowsky, New York University; Russell van Dyke, Tulane; Barbara Stechenberg, Baystate Medical Center; Kenneth McIntosh, Boston Children’s Hospital; John Farley, University of Maryland; Stephen Pelton, Boston Medical Center; Ann Petru, Children’s Hospital Oakland; Stephen Spector, University of California, San Diego; Vincent Bonagura, Schneider Children’s Hospital; Coleen Cunningham, State University of New York, Syracuse; Audra Deveikis, Miller Children’s Hospital Long Beach; Andrea Ruff, Johns Hopkins; William Shearer, Texas Children’s Hospital, Baylor; Ross McKinney, Duke University; Saroj Bakshi, North Shore University Hospital; Thomas Rubio, Children’s Hospital of King’s Daughters; Kenneth Rich, University of Illinois; Sunanda Gaur, University of Medicine and Dentistry of New Jersey Robert Wood Johnson; George Johnson, Medical University of South Carolina; Sohail Rana, Howard University Hospital; John Sleasman, University of Florida, Gainsville; and Irma Febo, University of Puerto Rico.
Author contributions. K. L., D. P., and M. M. designed the study. K. L., E. R. W., J. H., and A. G. developed the quantitative antibody assay. J. H., A. G., and R. B. obtained the data. M. M., E. M., and K. L. analyzed the data. K. L., M. M., E. R. W., J. H., and A. G. wrote the article. All authors reviewed the manuscript prior to submission.
Acknowledgments. The authors thank the children and their families for their participation in these studies.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by National Institutes of Health (grant numbers NIH R01-HD080474 to K. L. and D. P. and UL1-TR001453 to K. L.) and the Fondazione Penta-Onlus (PENTA Foundation; to K. L.). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases, with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the National Institutes of Health, under Award Numbers UM1AI068632 (IMPAACT Leadership and Operations Center), UM1AI068616 (IMPAACT The Statistical and Data Management Center), and UM1AI106716 (IMPAACT Laboratory Center), and by NICHD contract number HHSN275201800001I.
Potential conflicts of interest. K. L. has received compensation from Gilead for consulting, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
PACTG 356 Investigators:
Hannah Gay, William Borkowsky, Russell van Dyke, Barbara Stechenberg, Kenneth McIntosh, John Farley, Stephen Pelton, Ann Petru, Stephen Spector, Vincent Bonagura, Coleen Cunningham, Audra Deveikis, Andrea Ruff, William Shearer, Ross McKinney, Saroj Bakshi, Thomas Rubio, Kenneth Rich, Sunanda Gaur, George Johnson, Sohail Rana, John Sleasman, and Irma Febo
References
- 1. de Martino M, Tovo PA, Balducci M, et al. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA 2000; 284:190–7. [DOI] [PubMed] [Google Scholar]
- 2. Luzuriaga K, Bryson Y, Krogstad P, et al. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N Engl J Med 1997; 336:1343–9. [DOI] [PubMed] [Google Scholar]
- 3. Luzuriaga K, McManus M, Catalina M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol 2000; 74:6984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Violari A, Cotton MF, Gibb DM, et al. ; CHER Study Team Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luzuriaga K, Mofenson LM. Challenges in the elimination of pediatric HIV-1 infection. N Engl J Med 2016; 374:761–70. [DOI] [PubMed] [Google Scholar]
- 6. Rainwater-Lovett K, Uprety P, Persaud D. Advances and hope for perinatal HIV remission and cure in children and adolescents. Curr Opin Pediatr 2016; 28:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection, 2017. Available at: https://aidsinfo.nih.gov/guidelines/html/2/pediatric-arv/0. Accessed 27 February 2018.
- 8. Foster C, Bamford A, Turkova A, Welch S, Klein N; PENTA Guidelines Writing Group and PENTA steering committee Paediatric European Network for Treatment of AIDS Treatment Guideline 2016 update: antiretroviral therapy recommended for all children living with HIV. HIV Med 2017; 18:133–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2016. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 27 February 2018. [PubMed]
- 10. McManus M, Mick E, Hudson R, et al. ; PACTG 356 Investigators Early combination antiretroviral therapy limits exposure to HIV-1 replication and cell-associated HIV-1 DNA levels in infants. PLoS One 2016; 11:e0154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ananworanich J, Puthanakit T, Suntarattiwong P, et al. ; HIV-NAT 194 Study Group Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014; 28:1015–20. [DOI] [PubMed] [Google Scholar]
- 12. Kuhn L, Schramm DB, Shiau S, et al. Young age at start of antiretroviral therapy and negative HIV antibody results in HIV-infected children when suppressed. AIDS 2015; 29:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Zyl GU, Bedison MA, van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early antiretroviral therapy in South African children reduces HIV-1-infected cells and cell-associated HIV-1 RNA in blood mononuclear cells. J Infect Dis 2015; 212:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Payne H, Mkhize N, Otwombe K, et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial: a retrospective analysis. Lancet Infect Dis 2015; 15:803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Persaud D, Patel K, Karalius B, et al. ; Pediatric HIV/AIDS Cohort Study Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 2014; 168:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan JL; PACTG 356 Investigators A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med 2004; 350:2471–80. [DOI] [PubMed] [Google Scholar]
- 17. Greenough TC, Brettler DB, Kirchhoff F, et al. Long-term nonprogressive infection with human immunodeficiency virus type 1 in a hemophilia cohort. J Infect Dis 1999; 180:1790–802. [DOI] [PubMed] [Google Scholar]
- 18. Jann B. Plotting regression coefficients and other estimates. Stata J 2014; 14:708–37. [Google Scholar]
- 19. Uprety P, Patel K, Karalius B, et al. HIV-1 DNA decay dynamics with early, long-term virologic control of perinatal infection. Clin Infect Dis 2017; 64:1471–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 2014; 5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012. Article ID: 985646; doi:10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr Opin HIV AIDS 2009; 4:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chantry CJ, Cooper ER, Pelton SI, Zorilla C, Hillyer GV, Diaz C. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr Infect Dis J 1995; 14:382–7. [DOI] [PubMed] [Google Scholar]
- 24. Pahwa S. Human immunodeficiency virus infection in children: nature of immunodeficiency, clinical spectrum and management. Pediatr Infect Dis J 1988; 7:S61–71. [PubMed] [Google Scholar]
- 25. Keating SM, Pilcher CD, Jain V, et al. HIV antibody level as a marker of HIV persistence and low-level viral replication. J Infect Dis 2017; 216:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imamichi H, Dewar RL, Adelsberger JW, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci USA 2016; 113:8783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee SA, Bacchetti P, Chomont N, et al. Anti-HIV antibody responses and the HIV reservoir size during antiretroviral therapy. PLoS One 2016; 11:e0160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brice J, Sylla M, Sayon S, et al. Qualitative and quantitative HIV antibodies and viral reservoir size characterization in vertically infected children with virological suppression. J Antimicrob Chemother 2017; 72:1147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asang C, Laws HJ, Adams O, et al. HIV-1 seroreversion in HIV-1-infected children: do genetic determinants play a role?AIDS 2014; 28:543–7. [DOI] [PubMed] [Google Scholar]
- 30. Sadanand S, Das J, Chung AW, et al. Temporal variation in HIV-specific IgG subclass antibodies during acute infection differentiates spontaneous controllers from chronic progressors. AIDS 2018; 32:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]