Abstract
Background
The hemagglutination inhibition (HAI) assay is an established correlate of protection for the inactivated influenza vaccine. However, the proportion of vaccine-induced protection that is mediated by the post-vaccination HAI titer has not been assessed.
Methods
We used data from a randomized, placebo-controlled trial of a split-virion inactivated influenza vaccine in children aged 6–17 years. Sera were collected before and 30 days after receipt of vaccination or placebo and tested by the HAI assay against B/Brisbane/60/2008-like (B/Victoria lineage). We fitted Cox proportional hazards models to the time to laboratory-confirmed influenza B. We used causal mediation analysis to estimate the proportion of the total effect of vaccination that was mediated by higher HAI titers.
Results
We estimated that vaccine efficacy against confirmed B/Victoria infection was 68% (95% confidence interval, 33%, 88%), and post-vaccination HAI titers explained 57% of the effect of vaccination on protection.
Conclusions
The majority of the effect of inactivated influenza vaccination in children is mediated by the increased HAI titer after vaccination; however, other components of the immune response to vaccination may also play a role in protection and should be further explored. Causal mediation analysis provides a framework to quantify the role of various mediators of protection.
Keywords: influenza, vaccination, hemagglutination inhibition, correlate of protection
Influenza vaccination increases protection against influenza virus infection. In this study, we estimated that 57% of the increase in protection can be attributed to the higher antibody titer after vaccination measured by the hemagglutination inhibition assay.
Inactivated influenza vaccines (IIVs) have been available for more than 70 years and are currently the most frequently used vaccines worldwide, with approximately 500 million doses administered each year from 2011–2015 [1]. Current IIVs are almost all manufactured using subunit or split-virion methods from egg-grown virus [2]. The antibody titer measured by the hemagglutination inhibition (HAI) assay is an established correlate of protection for IIVs, because vaccination with IIVs leads to increased HAI titers [3], and higher HAI titers are correlated with protection against influenza virus infection [4]. Various definitions of a correlate of protection have been suggested in the literature [5]. Plotkin and Gilbert distinguish mechanistic and nonmechanistic correlates of protection based on whether the immune function is on the causal pathway between vaccination and protection [5]. In the field of causal inference, a mechanistic correlate of protection would be defined as a mediator of the effect of vaccination on infection. However, the HAI titer only measures part of the humoral immune response stimulated by IIVs [6], and IIVs may also promote cell-mediated immunity [7].
Our objective in this study was to investigate the strength of HAI titers in mediating the effect of vaccination in reducing the risk of disease from influenza B virus infection, within a causal analysis framework [8]. To do this, we reanalyzed data from a randomized, placebo-controlled trial of influenza vaccination in children.
METHODS
Participants
In 2009–2010 we conducted a trial of influenza vaccination in children aged 6–17 years in Hong Kong [9]. Enrollment took place from August 2009 through February 2010. After obtaining parental consent, participating children were randomly allocated to receive either a single dose of trivalent split-virion IIV (0.5 mL of VAXIGRIP; Sanofi Pasteur) or placebo (0.5 mL saline solution) in a 3:2 ratio (ie, 60% of children received the vaccine). The vaccine included the strains A/Brisbane/59/2007(H1N1)-like, A/Brisbane/10/2007(H3N2)-like, and B/Brisbane/60/2008-like (B/Victoria lineage). We collected sera from participants immediately prior to vaccination and 1 month after vaccination, and tested the sera in parallel with HAI assays against the vaccine strains in serial doubling dilutions from an initial dilution of 1:10 [10, 11]. The test strain used in this study for the influenza B virus was B/Brisbane/60/2008-like (Victoria lineage) derived from embryonated egg cultures, and the antigen was not ether split. The HAI assay was conducted with turkey erythrocytes. HAI titers were taken as the reciprocal of the last dilution at which antibody was detected, and titers <10 were set to 5 for analysis.
Participants were followed up with active surveillance from vaccination to the end of their study visit, which occurred between August 2009 and December 2010. Telephone calls were made every other week to monitor for acute respiratory illnesses [9], and home visits were conducted to ill participants to collect nose and throat swabs for laboratory confirmation of influenza by reverse transcriptase polymerase chain reaction (PCR). In the present analysis, we included all PCR-confirmed infections from 14 days after vaccination of each participant through the end of follow-up.
Ethics
Proxy written consent from parents or legal guardians was obtained for all participants since they were aged ≤17 years, with additional written assent obtained from those aged 8 to 17 years. The University of Hong Kong Institutional Review Board approved the study protocol.
Statistical Analyses
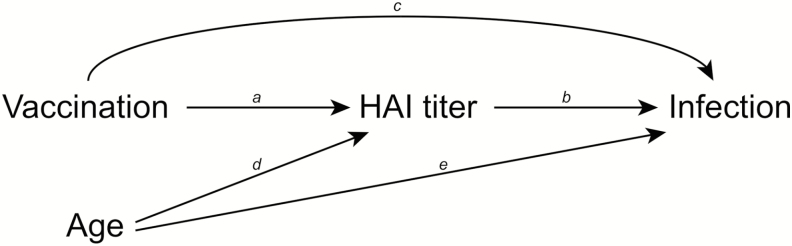
We postulated the causal model shown in Figure 1, that is, vaccination led to increased HAI titers and also led to some protection against the risk of disease from influenza virus infection. The protection conferred by vaccination was mediated by the higher HAI titers after vaccination (referred to as an “indirect effect” in causal mediation terminology), and part of the protection was conferred via other immune mechanisms (“direct effect” not mediated by HAI titers). While vaccination was randomized and thus not affected by age, we postulated that age would affect the post-vaccination HAI titers, because older children tended to have higher pre-vaccination titers. Age also affects the risk of disease from influenza virus infection.
Figure 1.
Mediation model showing the hypothesized relationship between influenza vaccination and risk of disease from influenza virus infection. The hemagglutination inhibition (HAI) titer measured 30 days after receipt of vaccination or placebo acts as a mediator of the protective effect of vaccination on risk of disease from infection. Influenza vaccination has an indirect effect on risk of disease from infection, which operates via a change in HAI titers (arrow a) and the higher HAI titers reducing the risk of disease from infection (arrow b). Influenza vaccination may also have a direct effect on risk of disease from infection that is not mediated by the HAI titer but operates through other immune mechanisms (arrow c). Age is shown as a confounder of the relationship between post-vaccination HAI titers and the risk of disease from infection, since age affects HAI titers (arrow d) and the risk of disease from infection (arrow e). Abbreviation: HAI, hemagglutination inhibition.
To estimate the total effect of vaccination on protection, we used a proportional hazards model where the outcome was the calendar time of infection after 1 September 2009, and the covariates were age and receipt of IIV or placebo. The hazard ratio (HR) of vaccination represents the total effect, and 1 minus the HR multiplied by 100% represents the vaccine efficacy. We also fitted a proportional hazards model adjusting for post-vaccination HAI titers to confirm the association between titers and risk of disease from influenza virus infection, and we determined if there was evidence of a different effect of HAI titers for children who received vaccine vs placebo by adding an interaction term to the model.
To estimate the direct effect of vaccination on protection, that is, the part of the effect that was not mediated via the rise in HAI titers, we first fitted a logistic regression model where vaccination was the response variable and the post-vaccination HAI titer and age were predictors. We used the estimated coefficients of this model to predict the odds ratios of vaccination for each participant, and we constructed weights for vaccinated participants as the inverse of these predicted odds ratios and specified weights of 1 for all unvaccinated participants [8]. We then fitted a proportional hazards model to the calendar time of infection, adjusting for age and receipt of IIV/placebo, weighting each observation by the weights derived in the previous step. The direct effect was obtained as the HR for IIV in this model [8]. Finally, the indirect effect was obtained as the ratio of the total effect and the direct effect [8]. The proportion of the effect of IIV that was mediated by the post-vaccination HAI titer was estimated as the log of the indirect effect HR divided by the log of the total effect HR [8]. We used bootstrapping with 10000 resamples to estimate the uncertainty in the total, direct, and indirect effects. In a sensitivity analysis we included pre-vaccination HAI titers along with age as another potential confounder, noting that children with higher pre-vaccination HAI titers would have slightly lower geometric mean titer rises when vaccinated, while it is unclear whether the pre-vaccination HAI titer is independently associated with risk of influenza disease separate from the effect via post-vaccination titers.
All statistical analyses were conducted using R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). Data and R syntax to reproduce these analyses are available at: [12].
RESULTS
We enrolled and randomized 796 children into this trial. One participant withdrew from the study after randomization but before the intervention was administered, and 59 children did not provide a post-vaccination blood sample between 21 and 45 days after receipt of vaccine or placebo. This analysis included the remaining 736 children. The mean age of participants was 10 years in both groups.
In these children we identified 30 PCR-confirmed influenza B/Victoria infections, as well as 15 for A(H1N1)pdm09, 8 for A(H3N2), and 4 for B/Yamagata viruses during the follow-up period. The vaccine in our study did not include A(H1N1)pdm09, and there were no significant differences in post-vaccination HAI titers between children who received vaccine or placebo [9]. Because of the small number of confirmed A(H3N2) and B/Yamagata infections, we focus here on B/Victoria. Of the 30 PCR-confirmed B/Victoria infections in the 736 participants, we identified 20/295 (6.8%) in placebo recipients and 10/441 (2.2%) in IIV recipients.
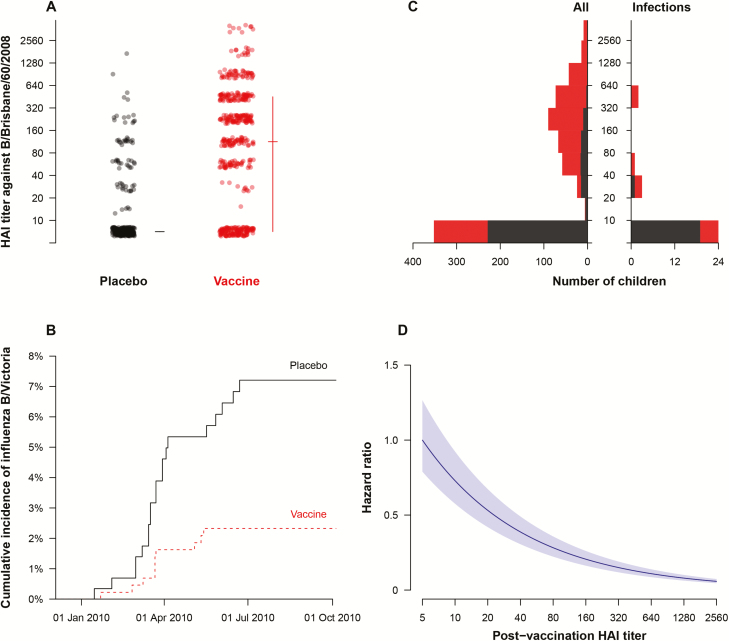
Figure 2A shows the post-vaccination HAI titers against B/Victoria in 441 children who received IIV and 295 children who received placebo. The geometric mean titers in these 2 groups were 67 and 8.5, respectively. There were no significant differences in pre-vaccination HAI titers against B/Victoria in these 2 groups [9]. The timing of PCR-confirmed influenza B/Victoria infections in children who received IIV or placebo is shown in Figure 2B. The IIV and placebo injections were administered between 18 September 2009 and 22 January 2010. The post-vaccination sera were collected between 16 October 2009 and 20 February 2010, and 92% of these samples were collected before 31 January 2010. Using a proportional hazards model and adjusting for age, we estimated that the HR of B/Victoria infection for children who received IIV compared to placebo was 0.32 (95% confidence interval [CI], 0.12, 0.67). This corresponds to a vaccine efficacy of 68% (95% CI, 33%, 88%). Figure 2C shows the distribution of HAI titers in all children and in the children who had PCR-confirmed infection. We fitted a proportional hazards model for the risk of PCR-confirmed influenza B vs post-vaccination HAI titers and found that a titer of 40 corresponded to approximately 50% protection compared to a titer <10 (Figure 2D). There was no evidence of effect modification by vaccination when we included an interaction term between vaccination and post-vaccination HAI titer (P value = .37).
Figure 2.
(A) Post-vaccination antibody titers by the hemagglutination inhibition (HAI) assay against the B/Brisbane/60/2008 (Victoria lineage) virus that was included in the trivalent inactivated influenza vaccine. Titers are compared between placebo and vaccine recipients; the horizontal line indicates the median titer and the vertical line indicates the interquartile range. Titers are measured in intervals (eg, a titer of “10” indicates a titer of ≥10 but <20), and plotted in those intervals accordingly. (B) Timing of polymerase chain reaction (PCR)-confirmed infections during the study period in the children who received placebo (black, solid line) or vaccine (red, dashed line). (C) Distribution of HAI titers in all children (left-hand side) and in the children who had PCR-confirmed infection during follow-up (right-hand side). Black bars represent the children who received placebo, and red bars represent the children who received influenza vaccination. The range of titer dilutions shown is <1:10 to 1:2560, with corresponding titers <10 to ≥2560, and bars are plotted in the respective intervals. The lowest bar corresponds to children with HAI titers <10. (D) Correlation of HAI titer with protection against infection in a proportional hazards model. Note in this panel that an HAI titer of 40 was estimated to correspond to approximately 50% protection compared to a low HAI titer. Abbreviation: HAI, hemagglutination inhibition.
In the causal analysis, the direct effect of vaccination on protection, that is, the effect not mediated by the higher HAI titers in children who received vaccination, was estimated as a HR of 0.60 (95% CI, 0.18, 1.42). The indirect effect, obtained as the total effect divided by the direct effect, was estimated as a HR of 0.52 (95% CI, 0.33, 1.02). Taking the ratio of the log HRs for the indirect effect and the total effect, we estimated that 57% of the effect of vaccination was mediated by the post-vaccination HAI titers in vaccinated children.
In a sensitivity analysis, we included pre-vaccination HAI titers as well as age as potential confounders of the mediating effect of post-vaccination HAI titers. For children who received placebo, 88% of the post-vaccination HAI titers were identical to the pre-vaccination HAI titers (1 month earlier), and others were generally within a 2-fold difference, as would be expected. In this sensitivity analysis, the direct effect was estimated as a HR of 0.49 (95% CI, 0.13, 1.20), and the indirect effect was estimated as a HR of 0.63 (95% CI, 0.38, 1.47). Taking the ratio of the log HRs for the indirect effect and the total effect, we estimated that 40% of the effect of vaccination was mediated by the post-vaccination HAI titer.
DISCUSSION
Our results indicate that post-vaccination HAI titers mediated 57% of the effect of vaccination on protection against disease caused by influenza B virus infection in the spring of 2010. This indicates that other immune mechanisms may also play a role in the protection conferred by IIV. In particular, the HAI assay does not capture other immune mechanisms that may be protective, including antibodies that target the HA stalk, antibody-dependent cell-mediated cytotoxicity antibodies, and anti-neuraminidase antibodies. Dunning et al examined a similar question in a trial of high-dose vs standard-dose IIV in adults aged ≥65 years, using a different statistical approach, and found that HAI titers explained between 27% and 100% of the improved protection conferred by the high-dose vaccine on confirmed influenza [13]. The high-dose and standard-dose vaccines contained 60 µg and 15 µg of hemagglutinin of each of the included influenza virus strains, respectively [13]. In that study, the authors also reported that inclusion of anti-neuraminidase antibody titers as well as HAI titers explained a greater fraction of the additional protection conferred by the high-dose vaccine [13].
The vaccine used in our study was a split-virion trivalent IIV that included 45 µg of hemagglutinin (15 µg for each of 3 strains), and the neuraminidase content was not reported. Inactivated influenza vaccines do not have standardized neuraminidase content and generally contain a small amount of neuraminidase [14]. More recently, many of the currently used IIVs are subunit vaccines that have an additional purification step. We might expect a larger fraction of the protection induced by subunit IIVs to be explained by the post-vaccination HAI titer.
We estimated a vaccine efficacy against PCR-confirmed influenza B/Victoria of 68% (95% CI, 33%, 88%), consistent with other estimates of IIV efficacy against influenza B [15]. It is known that there is a weaker correlation of post-vaccination HAI titers with protection when there is a mismatch between vaccine and circulating strains [13]. However, in our study the vaccine strain was fairly well matched to the circulating B/Victoria viruses. Analysis of similar data from larger trials over multiple years with varying degrees of vaccine match could confirm whether HAI titers are the major mediator of the protection conferred by IIVs.
Our study has some limitations. More than half of the children who received placebo had HAI titers <10 after receipt of the placebo, and this contributed to the uncertainty in estimates of the indirect effects in addition to the relatively small number of confirmed infections. In the placebo recipients, 11% of children had 4-fold or greater rises in HAI titer against B/Victoria [9] compared to 6.8% that had PCR-confirmed infection. This indicates that we may have missed a proportion of infections, particularly if they were mild or asymptomatic.
We only investigated the mediating effect of HAI titers for protection against influenza B/Victoria conferred by IIV in children, and it would be interesting to compare this with effects on influenza A and in other age groups. We examined the post-vaccination antibody titer as the mediator of vaccine efficacy, while the rise in geometric mean titer from pre- to post-vaccination might also capture another aspect of the immune response to vaccination and could be explored in future studies. We did not examine intraseason waning of HAI titers in this analysis [16, 17] because our analysis focused on the use of the post-vaccination HAI titer as a correlate of protection. Finally, from the data alone, we cannot distinguish that the HAI titer is truly mediating the effect of vaccination, rather than simply being correlated with another immune parameter that is the true mediator [5]. In addition, measurement error in HAI titers could dilute the estimated mediating effect.
In conclusion, we estimated that post-vaccination HAI titers mediated part but not all, of the effect of IIVs in preventing influenza B in children. It would be informative to repeat these analyses in clinical trials in adults. Other components of the immune response to IIVs may also play a role in protection and should be further explored. Causal mediation analysis provides a framework to quantify the role of various mediators of protection.
Notes
Acknowledgments. We thank Winnie Lim, Sophia Ng, Calvin Cheng, Edward Ma, Daniel Chu, Hau Chi So, and Jessica Wong for research support. We thank Nancy Leung for helpful discussions. We thank Julie Au for administrative support.
Financial support. This research was funded by a commissioned grant from the Health and Medical Research Fund of the Food and Health Bureau of the Hong Kong SAR Government (reference CHP-CE-03 and PHE-2). B. J. C. is supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant U54 GM088558), the National Institutes of Health (grant AI104459) and the Research Grants Council of the Hong Kong Special Administrative Region, China (project T11-705/14N).
Potential conflicts of interest. B. J. C. has received research funding from Sanofi Pasteur for a study of influenza vaccine effectiveness. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Palache A, Abelin A, Hollingsworth R, et al. Survey of distribution of seasonal influenza vaccine doses in 201 countries (2004–2015): the 2003 World Health Assembly resolution on seasonal influenza vaccination coverage and the 2009 influenza pandemic have had very little impact on improving influenza control and pandemic preparedness. Vaccine 2017; 35:4681–6. [DOI] [PubMed] [Google Scholar]
- 2. Talbot HK, Nian H, Zhu Y, Chen Q, Williams JV, Griffin MR. Clinical effectiveness of split-virion versus subunit trivalent influenza vaccines in older adults. Clin Infect Dis 2015; 60:1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beyer WE, Palache AM, Osterhaus AD. Comparison of serology and reactogenicity between influenza subunit vaccines and whole virus or split vaccines: a review and meta-analysis of the literature. Clin Drug Investig 1998; 15:1–12. [DOI] [PubMed] [Google Scholar]
- 4. Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. Journal of Hygiene 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012; 54:1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trombetta CM, Montomoli E. Influenza immunology evaluation and correlates of protection: a focus on vaccines. Expert Rev Vaccines 2016; 15:967–76. [DOI] [PubMed] [Google Scholar]
- 7. McElhaney JE, Xie D, Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol 2006; 176:6333–9. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol 2015; 181:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cowling BJ, Ng S, Ma ES, et al. Protective efficacy against pandemic influenza of seasonal influenza vaccination in children in Hong Kong: a randomized controlled trial. Clin Infect Dis 2012; 55:695–702. [DOI] [PubMed] [Google Scholar]
- 10. Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med 2010; 362:2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowling BJ, Ng S, Ma ES, et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis 2010; 51:1370–9. [DOI] [PubMed] [Google Scholar]
- 12. Cowling BJ, Lim WW, Perera RAPM, et al. Data from: Influenza hemagglutination-inhibition antibody titer as a mediator of vaccine-induced protection for influenza B. Available at: https://datadryad.org/resource/doi:10.5061/dryad.cv37539. Accessed 26 September 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunning AJ, Diaz Granados CA, Voloshen T, Hu B, Landolfi VA, Talbot HK. Correlates of protection against influenza in the elderly: results from an influenza vaccine efficacy trial. Clin Vaccine Immunol 2016; 23:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanimoto T, Nakatsu R, Fuke I, et al. Estimation of the neuraminidase content of influenza viruses and split-product vaccines by immunochromatography. Vaccine 2005; 23:4598–609. [DOI] [PubMed] [Google Scholar]
- 15. Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 16. Ng S, Fang VJ, Ip DK, et al. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J Infect Dis 2013; 208:1320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao X, Fang VJ, Ohmit SE, Monto AS, Cook AR, Cowling BJ. Quantifying protection against influenza virus infection measured by hemagglutination-inhibition assays in vaccine trials. Epidemiology 2016; 27:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]