Abstract
Since its introduction in the 1990s, liposomal amphotericin B (LAmB) continues to be an important agent for the treatment of invasive fungal diseases caused by a wide variety of yeasts and molds. This liposomal formulation was developed to improve the tolerability of intravenous amphotericin B, while optimizing its clinical efficacy. Since then, numerous clinical studies have been conducted, collecting a comprehensive body of evidence on its efficacy, safety, and tolerability in the preclinical and clinical setting. Nevertheless, insights into the pharmacokinetics and pharmacodynamics of LAmB continue to evolve and can be utilized to develop strategies that optimize efficacy while maintaining the compound’s safety. In this article, we review the clinical pharmacokinetics, pharmacodynamics, safety, and efficacy of LAmB in a wide variety of patient populations and in different indications, and provide an assessment of areas with a need for further clinical research.
Keywords: liposomal amphotericin B, clinical trial, fungal infection, pharmacokinetics, pharmacodynamics
The polyene class of antifungal agents remains an important option for the prevention and treatment of invasive fungal diseases, based on its broad spectrum; concentration-dependent fungicidal pharmacodynamics; potent, dose-dependent activity in a large number of animal models; and well-documented clinical efficacy. For decades, deoxycholate amphotericin B (DAmB) has been the cornerstone for the management of life-threatening fungal infections. However, its clinical utility is hampered by dose-dependent renal toxicity and infusion-associated reactions, thereby limiting therapeutic efficacy. The development of novel, less toxic, lipid-based polyene formulations in the late 1980s and early 1990s may be considered a breakthrough in antifungal chemotherapy, particularly for patients with invasive aspergillosis and mucormycosis.
This document reviews the clinical pharmacology of liposomal amphotericin B (LAmB; AmBisome®), a small, unilamellar, liposomal formulation of amphotericin B (AmB). For the purpose of this paper, the term LAmB refers exclusively to AmBisome. Emphasis is placed on the pharmacokinetics (PK), pharmacodynamics (PD), safety, and efficacy of this compound relative to those of DAmB, providing scientific evidence for improved safety and tolerability and assessing efficacy in the management of invasive fungal diseases.
CLINICAL PHARMACOKINETICS
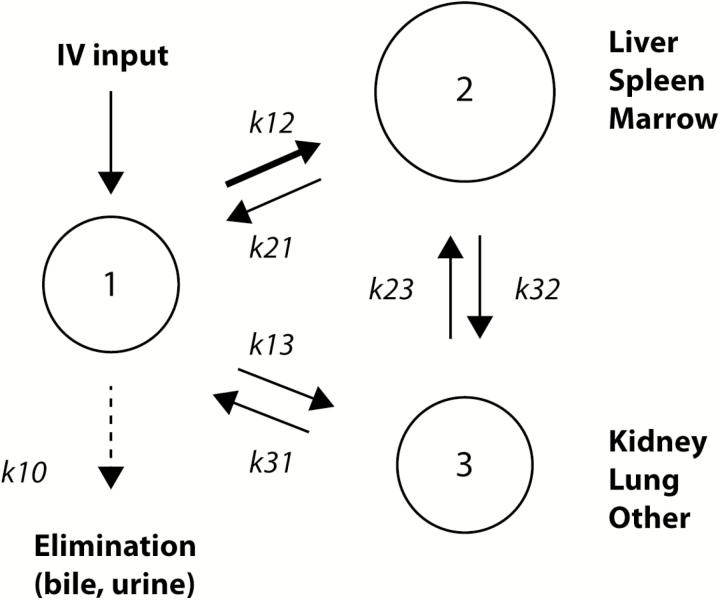
LAmB, in the form of AmBisome, consists of small, unilamellar vesicles of 60–80 nm in size, which are composed of hydrogenated soy phosphatidylcholine and distearoyl phosphatidylglycerol, stabilized by cholesterol and combined with AmB in a 2:0.8:1:0.4 molar ratio (Table 1) [1-6]. After intravenous administration, the liposomal carrier stays physicochemically intact for prolonged periods of time, providing an extended residence time of AmB in the central blood compartment (Figure 1) [7]. In preclinical studies, throughout all animal species, much higher peak plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC) values were achieved relative to equal doses of DAmB [8]. Distribution studies in rats with 4-[(14)C]cholesterol-LAmB demonstrate that the dominant route of elimination is fecal, presumably via biliary excretion; the liver, spleen, and lungs presented with the highest levels of radioactivity, and levels in the kidney were 15% of those in the liver and lungs [9].
Table 1.
Pharmacokinetic and Pharmacodynamic Properties of Liposomal Amphotericin B
| Property | LAmB |
|---|---|
| Formulation | Small, unilamellar liposomes consisting of HSPC/CHOL/DSPG in a 2:1:0.8 molar ratio and AmB in a 9:1 lipid:drug molar ratio |
| Protein binding, % | Not applicable |
| Mean Cmax, mg/La | 58 |
| Mean AUC0–24h, mg/Lxha | 713 |
| Mean Vd, L/kga | 0.22 |
| Mean CLt, L/h/kga | 0.017 |
| Dose linearity | Up to 10 mg/kg/day in adults |
| Substrate/inhibitor of Cytochrome P450 | No |
| Metabolism | Not metabolized |
| Elimination | Unchanged in feces and urine (<10% over 7 days) |
| Dosage adjustment in renal impairment | No adjustment needed for concerns of accumulation |
| Dosage adjustment in hepatic impairment | No adjustment needed for concerns of accumulation |
| Pharmacodynamics in vitro (by time-kill and PAFE) | Concentration-dependent fungicidal activity, prolonged PAFEs against Candida species |
| Pharmacodynamics in vivo (parameter best associated with efficacy in animal models of invasive fungal diseases) | Cmax/MIC |
Abbreviations: AmB, amphotericin B; AUC0–24h, area under the time–concentration curve from 0 to 24 hours; CHOL, cholesterol; CLt, total clearance; Cmax, peak plasma concentration; DSPG, distearoyl phosphatidylglycerol; HSPC, hydrogenated soy phosphatidylcholine; LAmB, liposomal amphotericin B; MIC, minimal inhibitory concentration; PAFE, post-antifungal effect; Vd, volume of distribution.
Figure 1.
Disposition of liposomal amphotericin B after intravenous administration. Reproduced with permission from Groll and Walsh [7]. After IV administration, amphotericin B distributes from the central compartment (labeled as 1), predominantly to organ sites rich in mononuclear phagocytic cells (labeled as 2) and, to a lesser extent, other tissue sites, including the kidney, the lung, and the brain. There is slow redistribution from these tissues into the central blood compartment (1) and slow elimination in an unchanged form into bile and urine. Note that this is a schematic to visualize the compound’s distribution, and that it does not represent the description of a mathematical pharmacokinetic model. Abbreviations: IV, intravenous; k, rate constants that depict the distribution of amphotericin B between the different compartments.
The first systematic clinical PK data were obtained in 36 persistently febrile neutropenic adult patients who received LAmB as empirical antifungal therapy in a Phase I/II, sequential, dose-escalation trial. Following doses of 1.0, 2.5, 5.0, and 7.5 mg/kg LAmB, the mean AUCs on the first day of treatment increased disproportionally (32, 71, 294, and 534 µg.h/mL, respectively), while the mean plasma clearance tended to decrease at the higher doses (from 39 and 51 µg.h/mL with 1.0 and 2.5 mg/kg/day, respectively, to 21 and 25 µg.h/mL with 5.0 and 7.5 mg/kg/day, respectively) [1]. Further dose escalation, to 10, 12.5, and 15 mg/kg/day LAmB in a subsequent Phase I/II trial in patients with invasive mold infections, however, revealed dose-related, non-linear, saturation-like PK: the mean AUC and Cmax values reached maximum values following the administration of 10 mg/kg/day and declined at 12.5 and 15 mg/kg/day [2]. Overall, LAmB was well tolerated, without dose-limiting adverse effects, across the investigated dose range.
To further understand the disposition of the compound, the PK, excretion, and mass balance of LAmB (2 mg/kg) and DAmB (0.6 mg/kg) were investigated in healthy volunteers. Both formulations had triphasic plasma profiles with long mean terminal half-lives (152 ± 116 h vs 127 ± 30 h, respectively); however, plasma concentrations were disproportionally higher after the administration of LAmB (mean Cmax, 22.9 ± 10 vs 1.4 ± 0.2 µg/mL, respectively). The central compartment volume of LAmB was close to the plasma volume, and the volume of distribution at steady state was smaller than that of DAmB. Total clearances were similar, but renal and fecal clearances of LAmB were 10-fold lower than those of DAmB. Two-thirds of DAmB was excreted unchanged in the urine (20.6%) and feces (42.5%), with >90% accounted for in mass balance calculations at 1 week, suggesting that metabolism plays no major role in elimination. By contrast, <10% of LAmB was excreted unchanged. No metabolites were observed by high-performance liquid chromatography or mass spectrometry [4, 5]. Protein-binding studies of both formulations revealed lower exposures to both unbound and non-liposomal drug following LAmB, with most of AmB in plasma remaining liposome associated (97% at 4 h, 55% at 168 h). Although the administration of LAmB resulted in markedly reduced total urinary and fecal recoveries of AmB, urinary and fecal clearances based on an unbound compound were similar for both formulations. The urinary clearance of the unbound drug was equal to the glomerular filtration rate, and tubular transit rates were <16% of the urinary excretion rate, suggesting that the net filtration of an unbound drug is the mechanism of renal clearance for both LAmB and DAmB in humans [4]. Indeed, the lower exposure to unbound and non-liposomal drug, as observed for LAmB, may be key for the explanation of the lower toxicity of the compound, as differences in both the dissociation of free AmB from its carrier and the degree of aggregation of free amphotericin molecules have been proposed to account for the selectivity of lipid formulations of AmB and their kidney-sparing properties [10].
Pharmacokinetics in Adult Hematology Patients
In a risk-stratified, randomized, Phase II trial including 53 allogeneic hematopoietic stem cell transplantation (HSCT) patients (19 on caspofungin, 17 on LAmB, and 17 on the combination of caspofungin and LAmB), the population PK were best described by a linear, 2-compartment model with interindividual variability in clearance, the central volume of distribution (V1), intercompartmental clearance, the peripheral volume of distribution (V2), and a proportional error model. A mixture model was used, allowing for the separate identification of clearance based on 2 distinct subpopulations (those with identical PK on Days 1 and 4 and those with different PKs between Days 1 and 4). The clearance differed by a factor of 3 between the subgroups: this helped to explain a substantial amount of interindividual variability. Clearance in this mixture model was 0.637 L/h, with a V1 of 18.6 L and V2 of 49.2 L. The authors hypothesized a saturable elimination pathway, with the impact of the disease status on clearance or differences in parenteral nutrition as possible explanations for the observed variability [11]. In the previously mentioned dose-escalation Phase I/II clinical trial that included mostly hematological patients, population PK analysis, using the mixed-effect computer program NONlinear Mixed Effects Modeling (NONMEM) and a 2-compartment structural model, identified infection and bone marrow transplantation as relevant covariates for clearance. However, the final model tended to underestimate the higher concentration values, indicating that other unidentified covariates might play a role [2].
Pharmacokinetics in Pediatric Hematology Patients
The PK of LAmB in pediatric patients beyond the neonatal period were investigated in a formal, Phase II, dose-escalation trial investigating doses of 2.5, 5, 7.5, and 10 mg/kg in 40 immunocompromised patients. The disposition of LAmB in pediatric patients was not substantially different from that in adults. The AUC values on Day 1 increased from 54.7 ± 32.9 to 430 ± 566 µg.h/mL in patients receiving 2.5 and 10.0 mg/kg/day, respectively [12]. In a population PK analysis, the data were best described by a 2-compartment model incorporating weight and an exponential decay function describing the volume of distribution. Out of 3 pediatric patients, 1 appeared to demonstrate a time-dependent change in PK that was not explained by weight, maturity, or clinical factors [13]. In a different population PK analysis of 39 pediatric patients, the plasma concentration–time data were similarly described by a 2-compartment PK model, and weight was the only remaining covariate for clearance and volume of distribution. Typical values for clearance, V1, and V2 were 0.44 L/h, 3.12 L, and 18 L, respectively [14].
Pharmacokinetics in Intensive Care Unit Patients
Few PK studies of LAmB have included patients admitted to an intensive care unit. In a study including 10 critically ill patients who received the compound at 2.8–3.0 mg/kg/day, there was substantial variability in exposure. The median half-life for elimination from plasma was 1.65 hours (range 1.25–5.22) in the distribution phase, and the median terminal elimination half-life was 13.05 hours (range 8.7–41.4). The apparent volume of distribution of LAmB (median 0.42 L/kg) was relatively small. No correlations between dose and serum Cmax or between dose and exposure were observed in any of the dose groups [15]. These data are in agreement with previous results documenting the considerable intra- and inter-subject variability [1, 2, 12]. Potential factors of impact on the disposition of LAmB in this special population may include differences in underlying diseases, different degrees of inflammation, changes in the composition of blood and plasma, concomitantly administered parenteral solutions, and fundamental changes in body composition and water content [15].
Use in Patients with Renal Impairment, Hepatic Impairment, and Extracorporeal Membrane Oxygenation
Data on the PK of LAmB in patient groups with impaired renal function or those treated with hemodialysis or peritoneal dialysis are extremely limited. According to the prescribing information and summary of product information [16, 17], dose adjustment is not required in patients with renal failure. However, little information is available on how renal failure impacts the PK of LAmB. A single patient receiving hemodialysis was included in the previously mentioned study in critically ill patients, and a few case reports suggest that LAmB is not removed by renal replacement therapy [15]. However, this finding needs to be confirmed in a larger cohort of patients and in different forms of renal replacement therapies.
Similar to renal impairment, it is not clear whether changes in hepatic function affect the clearance of LAmB, despite hepatic side effects having been reported in the literature and being listed in the prescribing information [16–18]. A cumulative rise in AmB plasma concentrations has been observed in cases of acute liver transplant failure, and failure of the liver, as a major component of the reticuloendothelial system, may cause changes in the disposition of the compound; however, the clinical relevance of this has not been well studied up to this point [19].
More PK investigation is also needed in those who are treated with extracorporeal membrane oxygenation. Potential adsorption to the extracorporeal membrane oxygenation tubing can be expected because of the lipophilic nature of AmB.
PHARMACODYNAMIC CONSIDERATIONS
In time–kill studies, AmB displays concentration-dependent fungicidal activity against susceptible yeasts and molds, and prolonged post-antifungal effects for a duration of up to 12 hours have been demonstrated against these organisms [6, 20]. In neutropenic PK/PD mouse models of disseminated candidiasis and pulmonary aspergillosis, the ratio of Cmax to minimal inhibitory concentration (Cmax/MIC) was the parameter that provided the best correlation with outcome, as measured by the residual fungal burden in tissue (Table 1) [21, 22]. These laboratory findings indicate that large doses will be the most effective and that the achievement of optimal peak concentrations is important.
While several preclinical studies have been conducted to establish PK/PD indices for LAmB, no clinical studies have been carried out or designed to establish the relationship between the antifungal effect of the compound and its PK profile in humans. A population PK study in 39 pediatric patients with cancer included 9 patients with proven fungal infections who were treated with LAmB. Of the 9 patients, 8 demonstrated a clinical response (complete or partial), and the ratio of peak concentration at steady state (Cmax,ss) to MIC (Cmax,ss/MIC) was significantly higher in those achieving a complete response, compared with those achieving a partial response (P = .021). However, this study was not designed to detect a correlation between the response and the ratio of the AUC at steady state (AUCss) and the MIC (AUCss/MIC) [14].
SAFETY AND TOLERABILITY
Key Findings of Preclinical Investigations
The preclinical safety of LAmB has been demonstrated in several models of invasive fungal diseases in both normal and immunocompromised animals [8, 10, 23–28]. In all animal models, LAmB was less nephrotoxic than DAmB. However, a slight rise in serum transaminases appeared to occur with prolonged administration [24, 29–34]. Of note, there has been no experimental evidence for an impaired bacterial blood clearance capacity of the mononuclear phagocytic system after prolonged treatment at clinically relevant doses [35].
Clinical Safety in Early Cohort Studies and Large, Randomized Trials
The initial multicenter, compassionate use trial conducted in Europe included 133 courses of treatment with LAmB (mean maximal dosage 2.1 mg/kg/day; range 0.45–5.0 mg/kg; mean duration of treatment 21 days) in mostly adult patients with invasive fungal diseases refractory or intolerant to DAmB. In this trial, the safety analysis showed increases in serum creatinine from normal levels at baseline in 15% of patients; however, 17 of 50 patients (34%) with initially elevated creatinine levels had a return to normal at the end of treatment. Hypokalemia was noted in 18% of patients and infusion-related toxicity was noted in <1% [36]. The combined safety analyses of similar trials in bone marrow and solid organ transplant patients (n = 187) revealed a frequency of infusion-related side effects of 4% and increases in serum creatinine in 31% of patients. Therapy with LAmB had to be discontinued due to adverse events (AEs) in 3% of cases [37].
In a systematic, Phase I/II, open-label, sequential dose-escalation trial conducted at the US National Cancer Institute in 36 persistently febrile neutropenic adults who received 1.0, 2.5, 5.0, or 7.5 mg/kg/day of LAmB for a mean duration of 9.2 ± 0.8 days, infusion-related side effects occurred in 15 of 331 infusions (5%), and only 2 patients (5%) required premedication. Serum creatinine, potassium, and magnesium levels were not significantly changed from baseline in any of the dose cohorts, and there was no net increase in serum transaminase levels [1]. A subsequent Phase I/II, sequential, dose-escalation cohort trial explored the maximum tolerated dose of LAmB. A total of 44 patients with probable or proven invasive mold infections received LAmB at 7.5, 10, 12.5, or 15 mg/kg/day. The number of infusions ranged from 1 to 83, with a median duration of 11 days. The maximum tolerated dose was at least 15 mg/kg/day. Infusion-related reactions (IRRs) of fever occurred in 8/44 patients (18%), and chills and rigors occurred in 5/44 patients (11%). Serum creatinine increased 2 times above baseline in 32% of patients, but this increase was not dose related. Hepatotoxicity developed in 1 patient. Overall, the most common AEs included fever (48%), an increased creatinine level (46%), hypokalemia (39%), chills (32%), and abdominal pain (25%). A total of 9 patients (20%) discontinued the study drug due to a possibly related AE. There was no obvious correlation between the AEs and doses [2].
A combined analysis of 2 parallel, prospective, open-label, randomized, multicenter comparisons of LAmB (1 or 3 mg/kg/day; 1669 and 1762 doses, respectively) and DAmB (1 mg/kg/day; 1146 doses) as empirical antifungal therapies in 338 persistently febrile neutropenic adults and children showed fewer severe, drug-related AEs with LAmB. Irrespective of the dose, there was significantly reduced hypokalemia in patients treated with LAmB, and nephrotoxicity (defined as a 100% or more increase in serum creatinine from baseline) occurred significantly less often with LAmB (11% overall, 10% in the 1 mg/kg group, and 12% in the 3 mg/kg group), compared with DAmB (24%; Table 2) [38]. In a second, large-scale (N = 687), randomized, double-blind, multicenter trial evaluating the safety and tolerability of LAmB (3.0 mg/kg/day; mean duration of therapy, 10.8 ± 8.9 days) compared with DAmB (0.6 mg/kg/day; mean duration of therapy, 10.3 ± 8.9 days), significantly fewer patients receiving LAmB had infusion-related fever (17% vs 44%, respectively), chills or rigors (18% vs 54%, respectively), or other reactions, including hypotension, hypertension, and hypoxia. Nephrotoxicity (defined as a serum creatinine concentration twice the upper limit of normal) was significantly less frequent among patients treated with LAmB (19%) than among those treated with DAmB (34%) [44]. In 2 additional large, multinational, randomized, non-inferiority trials of empirical antifungal therapy with voriconazole and caspofungin in persistently febrile neutropenic patients, which used LAmB as reference agent, the safety profile of the compound was confirmed, extending the safety database in this indication to many more than 1000 treated patients [48, 49].
Table 2.
Summary of Pivotal Randomized, Clinical Trials Demonstrating Efficacy and Safety of Liposomal Amphotericin B in Treatment and Prevention of Invasive Fungal Diseases
| Study/Design | Endpoints of Efficacy | Main Results |
|---|---|---|
| Proven or probable invasive aspergillosis | ||
| Open-label, randomized, multicenter trial comparing the efficacy of 2 doses of LAmB (1 and 4 mg/kg/day) for the treatment of proven or probable invasive aspergillosis in 120 neutropenic patients [39]. | Complete/partial responses and stable disease at end of therapy in patients receiving ≥1 dose; overall mortality at 2 months after start of therapy. | Response rates were 64% in the 41 eligible LAmB 1 mg/kg/day recipients and 48% in the 46 eligible LAmB 4 mg/kg/day recipients; overall, mortality was 42% and 49% in the 2 cohorts, respectively. The rate of patients with AEs was slightly higher in the LAmB 4 mg/kg/day cohort. |
| Double-blind, randomized, multicenter trial in 201 patients with proven or probable invasive mold infection, comparing LAmB as first-line therapy at either 3 or 10 mg/kg/day for 14 days, followed by 3 mg/kg/day [40]. | Complete or partial response at the end of study drug treatment in patients receiving ≥1 dose. Overall mortality at 12 weeks after start of therapy. | A complete or partial response was achieved in 50% and 46% of patients in the 3 and 10 mg/kg/day groups, respectively. Mortality at 12 weeks was 28% and 41% in the 3 and 10 mg/kg/day arms, respectively. The rates of nephrotoxicity and hypokalemia were significantly higher in the high-dose group. |
| Invasive Candida infections | ||
| Double-blind, randomized, multicenter, non-inferiority trial comparing the efficacy of LAmB (3 mg/kg/day) and micafungin (100 mg/day) as first-line treatment of candidemia and invasive candidiasis in 531 patients [41]. | Clinical (complete or partial resolution of symptoms) and mycological (eradication or presumed eradication) response at the end of treatment in the per protocol analysis. | Treatment success was observed for 170 patients (89.5%) treated with LAmB and 181 patients (89.6%) treated with micafungin. Efficacy was independent of the Candida species, site of infection, neutropenic status, APACHE II score, and catheter removal/replacement. Fewer treatment-related AEs and discontinuations were observed with micafungin. |
| Cryptococcal meningoencephalitis | ||
| Double-blind, randomized, multicenter trial comparing efficacy and safety of LAmB at either 3 or 6 mg/kg/day to DAmB at 0.7 mg/kg/day in 267 patients with AIDS and acute cryptococcal meningitis [42]. | Mycological success (conversion of cerebrospinal fluid culture results) at Week 2 (14 ± 4 days), protocol-defined therapeutic success at Week 10, and survival at Week 10 among the modified intent-to-treat population. | Efficacy was similar among all 3 treatment groups; overall mortality at 10 weeks was 11.6%, with no significant differences among treatment groups. Infusion-related reactions were significantly less frequent in LAmB-treated patients and fewer patients receiving the 3 mg/kg/day dose of LAmB developed a doubling of the serum creatinine value. |
| Disseminated histoplasmosis | ||
| Double-blind, randomized, multicenter trial comparing LAmB at 3 mg/kg/day with DAmB at 0.7 mg/kg/day for 2 weeks in 81 patients with AIDS and moderate-to-severe disseminated histoplasmosis [43]. | Clinical success, conversion of baseline blood cultures to negative, survival during induction therapy, and acute toxicities that necessitated discontinuation of treatment. | Clinical success was achieved in 45/51 patients (88%) receiving LAmB and 14/22 patients (64%) treated with DAmB (P = .014). Culture conversion rates were similar. Fewer patients receiving LAmB died during induction (P = .04). Infusion-related side effects and nephrotoxicity were less frequent in patients treated with LAmB (25% vs 63% and 9% vs 37%; P = .002 and P = .003, respectively). |
| Empirical therapy in patients with fever and neutropenia | ||
| Combined analysis of 2 randomized, multicenter trials comparing LAmB at 1 or 3 mg/kg/day with DAmB at 1 mg/kg/day in a total of 338 adult and pediatric patients with fever and neutropenia who were not responding to broad-spectrum antibacterial treatment [38]. | Clinical success, defined by a minimum of 3 consecutive days with fever <38°C, continuing to study end (recovery of neutrophils to 0.5 × 109/L). Addition of systemic antifungals, development of systemic fungal infection, and persistent fever to study end were considered treatment failures. | Similar success rates in patients treated with LAmB (58% and 64%) and DAmB (49%), but fewer drug-related adverse effects and severe drug-related adverse effects with LAmB (P < .01). Nephrotoxicity, defined as doubling of the serum creatinine value from baseline, was less frequent in the LAmB arms versus DAmB (0% and 3%, respectively, vs 23%), as was hypokalemia (P < .01). |
| Double-blind, randomized, multicenter trial to compare LAmB at 3 mg/kg/day with DAmB at 0.6 mg/kg/day in a total of 687 adult and pediatric patients with fever and neutropenia who were not responding to broad-spectrum antibacterial treatment [44]. | Composite of 5 criteria: survival for 7 days after initiation of the study drug; resolution of fever during the period of neutropenia; successful treatment of any baseline fungal infection; the absence of breakthrough fungal infections during administration of the study drug or within 7 days after the end of treatment; and absence of premature discontinuation of the study drug because of toxicity or lack of efficacy. | The composite rates of successful treatment were similar (50% for LAmB and 49% for DAmB), as were survival rates (93% and 90%, respectively), resolution of fever (58% and 58%, respectively), and premature discontinuation of the study drug (14% and 19%, respectively). Fewer proved breakthrough fungal infections were observed in the LAmB group (3.2% vs 7.8%, respectively; P = .009). LAmB was associated with significantly fewer infusion-related reactions and fewer patients developed a serum creatinine level 2 times the upper limit of normal (19% vs 34%, respectively; P < .001). |
| Primary antifungal prophylaxis | ||
| Open-label, randomized, single-center trial of low-dose LAmB (50 mg every other day) versus no systemic antifungal therapy as antifungal prophylaxis in 219 neutropenic episodes in 132 patients with hematological malignancies and expected neutropenia for 10 days [45]. | Failure of antifungal prophylaxis, defined as occurrence of proven or probable invasive fungal diseases under prophylactic study treatment. Pneumonia without identification of a causative organism, mortality from any cause, and mortality related to invasive fungal disease were secondary endpoints. | In the first episode of each patient, the incidences of proven or probable invasive fungal diseases were 6.7% in LAmB-treated patients (5/75) and 35% in the control patients (20/57; P = .001). Invasive aspergillosis occurred less frequently in patients receiving LAmB (P = .0057). Pneumonia occurred in 6 vs 28 neutropenic episodes (P < .001), and there was no difference in overall and fungal-related mortality. |
| Double-blind, randomized, placebo-controlled trial of LAmB (2.5 mL of a 5 mg/mL solution) versus placebo inhalation twice a week in 271 adult patients with hematological disease with expected neutropenia for 10 days, studied during 407 neutropenic episodes [46]. | Occurrence of proven or probable invasive pulmonary aspergillosis, according to the European Organization for Research and the Treatment of Cancer/Mycoses Study Group definitions. Other endpoints were overall mortality and fungal infection-related mortality. |
There were 18/132 patients in the placebo group versus 6/139 patients in the LAmB group who developed invasive pulmonary aspergillosis (odds ratio 0.26, 95% confidence interval 0.09–0.72; P = .005). There was no difference in overall and infection-related mortality rates. More patients in the LAmB group versus the placebo group discontinued the inhalation therapy for at least 1 week (45% vs 30%, respectively; P = .01). |
| Double-blind, randomized, multicenter trial to compare prophylactic LAmB at 5 mg/kg intravenously or placebo twice weekly in a 2:1 random allocation during remission-induction treatment in 355 adult patients undergoing remission induction therapy for acute lymphoblastic leukemia [47]. | Development of proven or probable invasive fungal diseases. Secondary endpoints included those focused on the safety and tolerability of prophylactic LAmB. | Rates of proven and probable invasive fungal diseases were 7.9% (18/228) in the LAmB group and 11.7% (13/111) in the placebo group (P = .24). Overall mortality rates were similar between the groups: 7.2% (17/237) for LAmB and 6.8% (8/118) for placebo. There were no differences in premature, treatment-related discontinuations. Hypokalemia and creatinine increase were significantly more frequent with LAmB (P < .001). |
Please note the differences in disease definitions and outcome assessment across clinical trials.
Abbreviations: AE, adverse event; AIDS, acquired immunodeficiency syndrome; APACHE, Acute Physiology and Chronic Health Evaluation; DAmB, deoxycholate amphotericin B; LAmB, liposomal amphotericin B.
Clinical Safety in Pediatric Patients
Systematic safety data in pediatric patients beyond the neonatal period were obtained in a formal Phase II, sequential, dose-escalation trial investigating doses of 2.5, 5.0, 7.5, and 10 mg/kg in 40 immunocompromised children and adolescents enrolled to receive empirical antifungal therapy for the treatment of documented invasive fungal diseases. There were 9 AE-related discontinuations, 4 of which were related to infusions. Infusion-related side effects occurred for 63 of 565 infusions (11%), with 5 patients experiencing acute IRRs (7.5 and 10 mg/kg dose levels). Serum creatinine levels increased from 0.45 ± 0.04 mg/dL to 0.63 ± 0.06 mg/dL in the overall population (P = .003), with significant increases in the dose cohorts receiving 5.0 and 10 mg/kg/day. At the higher dose level of 10 mg/kg, there was a trend toward greater hypokalemia and vomiting [12]. Systematic safety data are further reported in 204 pediatric patients (median age, 7 years) with fever and neutropenia enrolled in the aforementioned randomized, open-label, multicenter trial comparing DAmB at 1 mg/kg/day (n = 63) to LAmB at 1 mg/kg/day (n = 70) or 3 mg/kg/day (n = 71). In total, 29% of patients receiving LAmB at 1 mg/kg/day, 39% of patients receiving LAmB at 3 mg/kg/day, and 54% of patients receiving DAmB experienced AEs (P = .01); nephrotoxicity (defined as a 100% or more increase in serum creatinine from baseline) was noted in 8%, 11%, and 21% of patients, respectively (not significant) [38]. Hypokalemia (<2.5 mmol/L) occurred in 10%, 11%, and 26% of patients, respectively (P = .02); increases in serum transaminase levels (≥110 U/L) occurred in 17%, 23%, and 17%, respectively (not significant); and increases in serum bilirubin (≥35 µmol/L) occurred in 11%, 12%, and 10% of patients, respectively [38]. LAmB was well tolerated and effective in cohorts of immunocompromised children requiring antifungal therapy for proven or suspected infections, including patients with bone marrow transplants for primary immunodeficiencies [50], patients with cancer [51, 52], and critically ill patients [53]. In a Phase IV analysis of 141 courses of LAmB, administered for a mean of 17 days at a mean maximum dose of 2.5 mg/kg for various indications in pediatric patients with cancer and HSCT, there was a low rate of AEs (4%) necessitating discontinuation. Mean aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and blood urea nitrogen values were higher at the end of treatment (P = .01), but bilirubin and creatinine values were not different from those at baseline [54].
There were 24 very low birth-weight neonates (mean birth weight 847 ± 244 g; mean gestational age 26 weeks) with systemic candidiasis who were treated in a prospective study of LAmB at 2.5–7 mg/kg/day. The mean duration of therapy was 21 days; the cumulative dose was 94 mg/kg. No major adverse effects were recorded. There was 1 infant who developed elevated bilirubin and hepatic transaminase levels during therapy [55]. Further prospective [56, 57] and retrospective [58, 59] cohort studies attest to the safety of LAmB in infants with very low birth weights.
Rare Adverse Events
Cases of substernal chest discomfort, respiratory distress, and sharp flank pain have been documented during or following infusions of LAmB [60, 61]. These acute IRRs occurred alone or in combination with 1 of 3 symptom complexes: (1) chest pain, dyspnea, and hypoxia; (2) severe abdomen, flank, or leg pain; and (3) flushing and urticaria. Most events occurred within the first 5 minutes of infusion and were relieved by the discontinuation of the infusion, plus the administration of intravenous diphenhydramine. The mechanisms of these events are thought to be related to liposomal activation of the complement cascade, leading to a release of anaphylatoxins (C3a and C5a). This is in contrast to the more common IRRs of DAmB that are mediated by tumor necrosis factor-α and may be blunted by the administration of acetaminophen, hydrocortisone, or meperidine [62].
Increases in serum bilirubin, alkaline phosphatase, and serum transaminases have been observed with LAmB. However, the relative odds ratios of liver injury and acute liver failure in the US Food and Drug Administration (FDA) Adverse Event Reporting System for AmB products are similar to those reported for antifungal azoles and echinocandins [63, 64]. Cases of clinical pancreatitis [2, 65] and pseudo-hyperphosphatemia [66] have been reported in association with LAmB.
We should use caution in the accurate ordering of the dosage of DAmB and in meticulous adherence to the recommended infusion time for DAmB. A dispensing and administration error that caused DAmB to be given instead of LAmB has been reported, and was ultimately fatal [67].
EFFICACY AGAINST DOCUMENTED OR PRESUMED FUNGAL DISEASES
Key Findings of Preclinical Investigations
The principal antifungal efficacy of LAmB has been demonstrated in several models of invasive fungal diseases in both normal and immunocompromised animals, and these have been reviewed in detail elsewhere [8, 10, 23, 25–27, 68, 69]. In mice systemically challenged with AmB-susceptible Candida species, LAmB was more effective than DAmB when LAmB was increased up to 5 mg/kg and higher [29, 70, 71]. LAmB was also effective in mice with cryptococcal meningitis, especially at higher doses [70], and conferred improved survival and tissue clearance in persistently neutropenic rabbits with invasive pulmonary aspergillosis, compared with DAmB [24].
Efficacy Against Invasive Fungal Diseases in Early Cohort Studies and Large, Randomized Trials
The first efficacy data in patients with documented or presumed invasive fungal diseases that were not responding or being intolerant to DAmB were reported from 3 larger studies performed in Europe; the most common underlying conditions were malignancy and solid organ or bone marrow transplantation [72–74]. At doses ranging from 0.5 to 5.0 mg/kg/day, the overall response rates were approximately 60% [72, 74]. Response rates in evaluable patients with documented or presumed infections due to Candida species were 84% [74], and those in patients with documented or presumed Aspergillus infections ranged from 58% to 77% [72–74]. Treatment with LAmB at a dose of 3 mg/kg/day was found to be effective and well tolerated against acquired immune deficiency syndrome (AIDS)-associated cryptococcosis in an open, non-comparative study [75]. At 4 mg/kg/day, it was equally effective but less nephrotoxic than DAmB (0.7 mg/kg/day) in a small, randomized, comparative trial [76]. This latter study also demonstrated more rapid clearing of cerebrospinal fluid by LAmB, relative to DAmB.
For the past 2 decades, LAmB at least 5 mg/kg/day has been considered a standard option for the first-line treatment of mucormycosis, although larger systematic studies are lacking [77–80]. Clinical trials and experience also demonstrated high efficacy and low toxicity in immunocompetent or immunocompromised adult and pediatric patients with leishmaniasis [81–87]. The higher dose of LAmB, of ≥5 mg/kg/day, for the treatment of mucormycosis, compared with that for invasive pulmonary aspergillosis at 3 mg/kg/day, is pharmacodynamically compatible with the higher MICs of the Mucorales, versus those of Aspergillus species [88].
There are 5 larger, randomized clinical trials that have been conducted to assess the efficacy of LAmB against documented proven or probable invasive fungal diseases (Table 2) [39–43].
Induction therapy with LAmB at 3 mg/kg/day achieved better response rates and survival than DAmB (0.7 mg/kg/day) and was better tolerated in a randomized, double-blind, multicenter trial in 81 patients with AIDS and disseminated histoplasmosis [43]. In another study in patients with AIDS, LAmB was compared with DAmB for the treatment of acute cryptococcal meningoencephalitis in a multicenter, double-blind study. Patients were randomized (1:1:1) to receive DAmB at 0.7 mg/kg/day (n = 87), LAmB at 3 mg/kg/day (n = 86), or LAmB at 6 mg/kg/day (n = 94). IRRs were less frequent in patients receiving LAmB, and fewer patients receiving LAmB at 3 mg/kg/day developed nephrotoxicity. Treatment efficacy and overall mortality at 10 weeks were similar among the 3 treatment cohorts [42].
A double-blind, randomized, multicenter, non-inferiority study compared micafungin (100 mg/day) with LAmB (3 mg/kg/day) as the first-line treatment of candidemia and invasive candidiasis in 531 patients. Both treatments were equally effective, and the results were the basis for regulatory approval for the first-line treatment of invasive candidiasis with LAmB in countries of the European Union. IRR rates and increases in serum creatinine were lower with micafungin, but there was no significant difference in the rate of study participants that discontinued the study drug prematurely due to AEs [41].
A randomized, multicenter trial coordinated by the European Organisation for Research and Treatment of Cancer (EORTC) compared the efficacy of 2 doses of LAmB for the treatment of proven or probable invasive aspergillosis in neutropenic patients. A total of 120 patients were randomized to receive either 1 or 4 mg/kg/day of LAmB; 87 patients were available for evaluation. The median durations of treatment in the cohorts were 18 and 19 days, respectively. Renal toxicity occurred in 1/41 patients (2%) receiving 1 mg/kg/day and in 5/46 patients (11%) treated with 4 mg/kg/day; only 1 patient’s treatment (4 mg/kg/day cohort) was permanently discontinued. Overall, LAmB was effective in 50–60% of patients [39]. Finally, in a double-blind, comparative trial, which had a similar study design as the pivotal Phase III trial comparing voriconazole to DAmB [89], patients with proven or probable invasive mold infection were randomized to receive LAmB at either 3 or 10 mg/kg/day for 14 days, followed by 3 mg/kg/day. The primary endpoint of a complete or partial response at the end of the study drug treatment was favorable. Of the 201 patients with confirmed invasive mold infections, 107 received the 3 mg/kg/day dose and 94 received 10 mg/kg/day. Invasive aspergillosis accounted for 97% of the cases. Hematological malignancies were present in 93% of patients, and 73% of patients were neutropenic at baseline. A favorable response was achieved in 50% and 46% of patients in the 3 and 10 mg/kg groups, respectively (difference 4%, 95% confidence interval [CI] -10% to 18%; P > .05); the respective survival rates at 12 weeks were 72% and 59% (difference 13%, 95% CI -0.2% to 26%; P > .05). Significantly higher rates of nephrotoxicity and hypokalemia were seen in the high-dose group (10 mg/kg/day) [40]. Thus, although a dose of 3 mg/kg/day of LAmB appeared to have a similar efficacy in the primary treatment of invasive aspergillosis as voriconazole, a dose escalation to 10 mg/kg/day for 14 days was more toxic, but no more effective. Based on the data in this trial, the first-line treatment of invasive aspergillosis was included in the label of the compound in the European Union, at a dose of 3 mg/kg/day.
Treatment of Leishmaniasis
Visceral leishmaniasis (kala-azar) is a debilitating and life-threatening infection of the monocyte-macrophage system. Building upon the PK properties of LAmB to distribute widely into tissue macrophages (particularly of the liver and spleen) and the previously documented safety and tolerability of high dosages [2, 23, 33, 35], Sundar and colleagues, in a randomized, clinical trial, successfully treated visceral leishmaniasis with a single dose of LAmB at 10 mg/kg IV, versus DAmB at 1 mg/kg IV, administered every other day for 29 days [90]. The study demonstrated similar cure rates of 95.7% (95% CI 93.4–97.9) with LAmB and 96.3% (95% CI 92.6–99.9) with DAmB.
Optimizing Treatment of Cryptococcal Meningoencephalitis
Early, open, non-comparative [75] and randomized, comparative [76] clinical studies in a limited numbers of patients with AIDS have demonstrated the efficacy of single-agent treatment with LAmB at 3 and 4 mg/kg/day, respectively, against cryptococcal meningoencephalitis. In a multicenter, double-blind, randomized clinical trial in 267 patients receiving either DAmB at 0.7 mg/kg/day, LAmB at 3 mg/kg/day, or LAmB at 6 mg/kg/day, treatment efficacy and overall mortality were similar. IRRs were less frequent in patients receiving LAmB, and fewer patients receiving LAmB at 3 mg/kg/day developed nephrotoxicity [42]. Of interest, the pharmacodynamic bridging of data generated in a murine model of cryptococcal meningoencephalitis, assessing dose regimens of LAmB alone or in combination with flucytosine, suggested that a clinical dosage of LAmB at 3 mg/kg/day resulted in submaximal antifungal efficacy. By contrast, regimens of LAmB at 6 mg/kg/day alone and of LAmB at 3 mg/kg/day plus flucytosine at 50 or 100 mg/kg/day all resulted in near-maximal antifungal activity [91].
More recent experimental work on short courses of LAmB induction therapy (ie, administration for less than the usual 2 weeks) in murine and rabbit models of cryptococcal meningo encephalitis revealed that, in mice, the pharmacodynamics of a single dose of 20 mg/kg were the same as of the 20 mg/kg/day dose administered for 2 weeks, suggesting that abbreviating the induction regimens of LAmB could be a possible therapeutic approach [92]. Indeed, in a Phase II, non-inferiority trial conducted in sub-Saharan Africa in adults with AIDS-associated cryptococcal meningoencephalitis who were randomized to LAmB at 10 mg/kg on Day 1 (single dose), LAmB at 10 mg/kg on Day 1 and 5 mg/kg on Day 3 (2 doses), LAmB at 10 mg/kg on Day 1 and 5 mg/kg on Days 3 and 7 (3 doses), or standard 14-day LAmB at 3 mg/kg/day (control), given with fluconazole at 1200 mg/day for 14 days, the mean rates of clearance of cerebrospinal fluid cryptococcal infection (early fungicidal activity) in all the short-course arms were non-inferior to the control at the predefined non-inferiority margin [93]. Induction based on short-course LAmB is currently being investigated in an open-label, Phase III, randomized, non-inferiority trial. Here, induction therapy with a single dose of LAmB (10 mg/kg), given with 14 days of fluconazole (1200 mg/day) plus flucytosine (100 mg/kg/day), was compared with the current World Health Organization–recommended treatment regimen of 7 days of amphotericin B deoxycholate (1 mg/kg/day) plus flucytosine (100 mg/kg/day), followed by 7 days of fluconazole (1200 mg/day) [94].
Exploration of Combination Therapy of Invasive Fungal Diseases in the Salvage Setting
Combination therapy of LAmB, predominantly with caspofungin as the second agent, has also been explored, particularly as an option to improve the poor outcome of invasive aspergillosis. In small, retrospective cohort studies including ≤30 patients with hematological malignancies, overall response rates in the salvage settings of possible, probable, and proven invasive aspergillosis were between 42% [95] and 60% [96]. In another observational study in patients with hematological malignancies that included mostly (81%) cases with probable and proven invasive aspergillosis, the overall response rate in the 84 patients who received the combination was 73% [97]. Data from randomized trials are limited: in an open pilot study, 30 patients with proven or probable invasive aspergillosis received either LAmB at the standard dosage (3 mg/kg/day) plus caspofungin at the standard dosage (50 mg/day) or monotherapy with high-dose LAmB (10 mg/kg/day). At the end of treatment, there were significantly more partial or complete responses (P = .028) in the combination group (10/15 patients; 67%) compared with the high-dose monotherapy group (4/15 patients; 27%). Survival rates at 12 weeks after inclusion were 100% and 80%, respectively [98]. The safety and PK of the combination of LAmB and caspofungin were investigated in a risk-stratified, randomized, multicenter, Phase II clinical trial in 55 adult allogeneic HSCT recipients with granulocytopenia and refractory fever. The patients received either caspofungin (50 mg/day; Day 1, 70 mg), LAmB (3 mg/kg/day), or a combination of both until defervescence and granulocyte recovery. All 3 regimens were well tolerated. Premature study drug discontinuations due to grade 3/4 AEs occurred in 1/18, 2/20, and 0/17 patients, respectively. AEs not leading to study drug discontinuations were frequent, but similar across cohorts, except for a higher frequency of hypokalemia with the combination therapy (P < .05). Drug exposures were similar for patients receiving combination therapy and those randomized to monotherapy. There was no apparent difference in the occurrence of proven/probable invasive fungal diseases and survival through Day 14 after the end of therapy. Thus, combination therapy in immunocompromised, allogeneic HSCT recipients was as safe as monotherapy and had a similar plasma PK, lending support for further investigations of the combination in patients with invasive fungal diseases [99].
EMPIRICAL THERAPY IN PERSISTENTLY NEUTROPENIC PATIENTS
LAmB has been studied extensively as an empirical, antifungal therapy in persistently febrile neutropenic patients. The combined analysis of 2 parallel, prospective, open-label, randomized, multicenter comparisons of LAmB (1 or 3 mg/kg/day) and DAmB (1 mg/kg/day) provided evidence for at least equivalent efficacy, but significantly fewer drug-related AEs with LAmB (Table 2) [38]. A third large, randomized, double-blind, multi center comparison of LAmB (3.0 mg/kg/day) with DAmB (0.6 mg/kg/day) showed similar composite rates of successful treatment, but fewer proven breakthrough fungal infections among patients treated with LAmB (11 patients [3.2%] vs 27 patients [7.8%]; P = .009). IRRs and nephrotoxicity occurred significantly less often among patients treated with LAmB [44]. In another randomized, double-blind, multicenter study, similar efficacy but better tolerability of LAmB (3 or 5 mg/kg/day) was demonstrated in comparison to AmB lipid complex (5 mg/kg/day) [100]. Subsequently conducted large-scale clinical trials with new antifungal compounds, such as voriconazole and caspofungin, have confirmed the efficacy and clinical usefulness of LAmB in this setting [48, 49]. Based on these well-designed and carefully conducted clinical trials, LAmB is approved by both the FDA and the European Medicines Agency for empirical antifungal therapy for presumed fungal infections in febrile, neutropenic patients.
ANTIFUNGAL PROPHYLAXIS IN HIGH-RISK PATIENT POPULATIONS
Randomized Trials of Antifungal Prophylaxis in Hematological Patients
There are 2 completed randomized, double-blind, placebo-controlled studies of LAmB (1 mg/kg/day and 2 mg/kg 3 times weekly) as a prophylaxis against invasive fungal diseases in patients undergoing chemotherapy or bone marrow transplantation for hematological malignancies. In both studies, there was no evidence for a reduction in the incidence of proven fungal infections and no difference in overall survival [101, 102]. However, in a large, randomized, open-label, single-center, placebo-controlled trial, low-dose LAmB (50 mg every other day) led to a significant reduction in invasive fungal diseases, from 35.1% to 6.7% (P = .001), in patients with hematological malignancies and prolonged neutropenia (>10 days) following intensive chemotherapy, with a low rate (2.8%) of discontinuations due to treatment-related AEs [45]. In a prospective, open-label, randomized comparison with the combination of fluconazole plus itraconazole, LAmB (3 mg/kg 3 times weekly) had similar efficacy when administered as an antifungal prophylaxis during induction chemotherapy for patients with acute myeloid leukemia (AML) and myelodysplastic syndrome, but was associated with higher rates of increased serum bilirubin and creatinine levels [103]. In a non-comparative cohort study in 51 pediatric and adolescent allogeneic HSCT recipients who received LAmB at 3 mg/kg/day during the first 100 days, the compound was reportedly well tolerated and no invasive fungal diseases occurred [104].
Reduced-frequency Dosing Schemes for Antifungal Prophylaxis in Hematological Patients
A different approach to prophylaxis is the administration of higher dosages of LAmB in extended intervals to achieve the same cumulative dose. A recent study included 48 adults receiving induction chemotherapy for AML, who received a dose of 15 mg/kg on Day 1, which was repeated in 5 patients after 15 days of persistent neutropenia. Proven invasive fungal disease was diagnosed in 4 patients (8.3%). Hypokalemia grade 3 was reported in 6 patients (12.5%); no other grade 3/4 AEs were reported. Mild, infusion-related AEs were seen in 6/53 (11.3%) total infusions, which resulted in permanent drug discontinuation in 1 patient [105]. In an open-label, multicenter, prospective pilot study, 29 adult patients receiving chemotherapy for acute leukemia (AL) or myeloablative, allogeneic HSCT received weekly 10 mg/kg infusions of LAmB (4 weeks for AL and 8 weeks for HSCT). There were no discontinuations of treatment due to drug-related AEs in patients with AL, but 6 HSCT patients had treatment-limiting AEs, potentially due to the high vulnerability of HSCT patients to treatment-related AEs. Enrollment was discontinued in the HSCT group, as recommended by the independent data review committee, in accordance with the study protocol [106]. Similarly, the once-weekly administration of 7.5 mg/kg of LAmB in 21 adult patients receiving high-dose prednisone (2 mg/kg/day) for acute graft-versus-host disease therapy after reduced-intensity, conditioning, allogeneic HSCT was associated with a 33% discontinuation rate owing to study drug–related AEs [107]. The safety and feasibility of an intermittent high dose (group A; 10 mg/kg on Day 1, 5 mg/kg on Days 3 and 6) and a daily standard dose (group B; 3 mg/kg/day for 14 days) of LAmB for the empirical treatment of persistent febrile neutropenia were explored in an open, randomized pilot study including 30 patients. Infusion-related adverse drug events occurred more frequently in group A, and creatinine levels were similar in the 2 regimens; hypokalemia tended to be less severe in group A. No patient discontinued the study drug due to toxicity. Composite success was identical for each regimen; 3/15 patients in group B and none in group A developed invasive fungal diseases. The results of this pilot study suggest that a short, intermittent, high-dose course of 10/5/5 mg/kg on Days 1, 3, and 6 may be as safe and effective as a standard 14-day course of 3 mg/kg/day [108]. Finally, a large, double-blind, placebo-controlled trial in patients undergoing remission-induction chemotherapy for newly diagnosed acute lymphoblastic leukemia investigated the efficacy of prophylactic LAmB given twice weekly at a dose of 5 mg/kg [47]. Out of 228 patients receiving LAmB, 18 (7.9%) experienced a proven/probable invasive fungal disease, compared with 13/111 patients (11.7%) receiving the placebo, which was statistically not significant. The most common drug-related AEs were hypokalemia and increased creatinine, in 35.0% and 9.3% of the patients receiving LAmB, respectively (Table 2).
In 14 pediatric allogeneic HSCT recipients, LAmB, given once weekly at 10 mg/kg, was well tolerated and resulted in measurable AmB plasma concentrations 7 days post-dose, suggesting that once-weekly dosing may provide useful protection against fungal infections in patients who tolerate this form of administration [109]. In a prospective cohort study, the safety and efficacy of prophylactic LAmB at 2.5 mg/kg twice weekly were investigated in 46 pediatric patients at high risk for developing invasive fungal diseases. LAmB was discontinued in 4 patients because of acute allergic reactions. Median values for creatinine and liver enzymes at the end of treatment did not differ significantly from those at baseline. Hypokalemia (<3.0 mmol/L) occurred with 13.5% of the prophylactic episodes, but was usually mild and always reversible. No proven/probable invasive fungal disease occurred in patients receiving LAmB prophylaxis [110]. In very low birth-weight, premature infants, the once-weekly administration of LAmB at 5 mg/kg/day has been studied as Candida prophylaxis in a prospective, randomized, open-label, placebo-controlled pilot study of 40 patients. There was 1 patient in the placebo cohort who developed candidiasis, and there were no differences between groups in the incidences of AEs in this population or in the mortality rates [57].
Data on Antifungal Prophylaxis in Liver Transplant Recipients
Liver transplantation may be associated with a high risk of invasive fungal diseases [111]. An early, randomized, placebo- controlled study of LAmB in liver transplant recipients demonstrated a statistically significant decrease in the incidence of proven fungal infections at 30 days [112]. Similarly, in a prospective, historically controlled, cohort study in high-risk liver transplant recipients, such as those with acute liver failure, assisted ventilation >7 days, re-transplantation, re-laparotomy, antibacterial therapy >14 days, transfusion requirements >20 units of red blood cells, and/or biliary leakage, prophylaxis with LAmB (1 mg/kg/day for 7–10 days) was well tolerated and associated with decreased infection rates and improved survival [113]. In a further study that aimed to assess the effects of 14 days of antifungal prophylaxis in reducing proven, invasive fungal diseases, eligible subjects were randomized to LAmB (2 mg/kg/day) or fluconazole (400 mg/day) and were followed for 100 days post-transplantation. The study was designed to enroll 300 subjects, but was closed early due to insufficient enrollment. A total of 71 subjects were enrolled and randomized, and two-thirds of subjects completed 14 days of study therapy. There were 10 subjects who developed proven or probable invasive fungal diseases with Candida species (n = 9) and Cryptococcus neoformans (n = 1); rates were similar in the 2 treatment arms and were lower than previously reported for subjects not receiving prophylaxis [114]. Finally, the safety and tolerability of high-dose LAmB (10 mg/kg weekly) for antifungal prophylaxis in liver transplantation were assessed in a prospective, Phase II, non-comparative trial. LAmB was administered weekly until hospital discharge, for a minimum of 2 weeks, with a follow-up of 180 days. Overall, 66/76 enrolled patients (86.8%) completed the prophylaxis and 10 discontinued the study protocol (6 for infusion-related AEs, 4 for suspected invasive fungal disease). The diagnosis of invasive candidiasis was confirmed in only 2/4 patients with suspected invasive fungal diseases. Thus, the administration of high-dose, weekly LAmB may be a safe prophylactic strategy for high-risk liver transplant recipients [115].
INHALATIONAL PROPHYLAXIS WITH LIPOSOMAL AMPHOTERICIN B
Aerosolized delivery is an attractive option for the prevention of pulmonary mold infections, promising minimal systemic exposure and high local drug exposure, which are important, as the effects of AmB in the lungs are dose and concentration dependent [24, 116]. Preclinical in vitro and animal data provide compelling evidence for the accumulation of LAmB within the lung epithelial lining fluid and pulmonary alveolar macrophages for prolonged periods of time after both the systemic and inhalational administration of LAmB [117–119]. Furthermore, animal data have demonstrated proof of principle for the preventive and therapeutic efficacy of inhalational administration of the compound [118, 120–123].
The clinical use of aerosolized LAmB is investigational and is not licensed for this usage by any major regulatory agency. At present, clinical data on the use of inhalational LAmB are limited. PK investigations on the intrapulmonary disposition of the compound in the lung tissue after inhalational administration have demonstrated therapeutic drug exposure in the epithelial lining fluid for prolonged periods of time [124].
In an observational study performed in 2 centers, 104 consecutive lung transplant recipients received inhalational prophylaxis with LAmB (25 mg, 3 times weekly, starting from Day 1 post-transplant to Day +60; 25 mg once weekly from Day +60 to Day +180; and once every 2 weeks thereafter). Outcomes were compared with 49 historical controls who had received inhalational prophylaxis with DAmB. After a minimum of 12 months of follow-up, Aspergillus infections were observed in 7.7% of those receiving LAmB, as compared to 10.2% in the historical control cohort. The rate of discontinuations due to AEs were similar in both cohorts (2.9% vs 4.1%, respectively) [124].
In a randomized, placebo-controlled trial of patients with hematological disease with expected neutropenia for ≥10 days, patients were randomized to receive inhaled LAmB or a placebo inhalation twice weekly, until neutrophil counts increased to >300 cells/mm3. In subsequent neutropenic episodes, the assigned treatment was restarted. The primary endpoint was the occurrence of invasive pulmonary aspergillosis, according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group definitions. A total of 271 patients were studied during 407 neutropenic episodes. According to the intent-to-treat analysis, 18/132 patients in the placebo group developed invasive pulmonary aspergillosis, versus 6/139 patients in the LAmB group (odds ratio 0.26, 95% CI 0.09–0.72; P = .005; Table 2). A detailed safety analysis showed similar proportions of patients with >20% post-nebulization declines in forced expiratory volume in 1 second or forced vital capacity. Coughing was reported significantly more often in LAmB patients (P < .0001). No differences were observed when baseline and post-nebulization serum levels of renal function and hepatic enzymes were compared [46]. The positive effect of LAmB inhalation as the standard prophylaxis for the prevention of invasive aspergillosis in patients with AML was later confirmed in an observational study [125]. These studies show that the prophylactic inhalation of LAmB is feasible and may significantly reduce the incidence of invasive pulmonary aspergillosis in patients at high risk. In the context of an increasing prevalence of azole-resistant A. fumigatus in parts of the world, future studies are needed to confirm these promising data.
SUMMARY AND PROSPECTS FOR FUTURE DEVELOPMENT
AmB is the only systemic antifungal polyene available for the prevention and treatment of invasive fungal diseases. AmB has a broad spectrum of antifungal activity and a fungicidal mode of action. AmB is not metabolized by the liver, is devoid of relevant drug-drug interactions, and is only slowly eliminated, with tissue disposition accounting for most of its distribution. Due to these properties, AmB is an essential part of the antifungal armamentarium, particularly for severely ill patients with life-threatening invasive fungal diseases and complex underlying diseases and in the setting of emerging triazole and echinocandin resistance. However, the clinical utility of AmB has been limited by the high frequency of IRRs and dose-limiting nephrotoxicity of the formulation with deoxycholate.
LAmB has been in clinical use for more than 2 decades. The disposition, safety, and antifungal efficacy of this formulation have been studied in an array of clinically relevant animal models of invasive fungal diseases. The cumulative preclinical data demonstrate that, in comparison to DAmB, LAmB can be given at much higher doses, resulting in enhanced plasma exposure and increased drug disposition in the lungs and the central nervous system, equal or improved antifungal efficacy, reduced nephrotoxicity, and the absence of relevant new toxicities.
Consistent with these preclinical data, results of clinical trials document the improved safety and tolerability profile of LAmB, compared with DAmB. In 2 large, randomized, clinical trials in cancer patients with fever and neutropenia at risk for invasive fungal diseases, fewer IRRs, less nephrotoxicity, and fewer drug discontinuations due to toxicity were documented in patients receiving LAmB. Significantly fewer IRRs and less nephrotoxicity have also been observed in a large, randomized comparison of LAmB with AmB lipid complex, supporting the superior safety profile of LAmB among the currently available AmB formulations. In addition, data from prospective, randomized, clinical trials in patients with candidemia, cryptococcal meningoencephalitis, and invasive aspergillosis, which together account for the vast majority of human invasive opportunistic fungal diseases, suggest that LAmB provides better antifungal efficacy against invasive aspergillosis, compared with DAmB, and comparable efficacy against invasive aspergillosis, candidemia, and cryptococcal meningoencephalitis, compared with other antifungal therapies approved for these indications.
Given the limitations of DAmB, there are few remaining indications for its use. These include the treatment of neonatal candidiasis, as well as its use in developing countries for the treatment of mucormycosis, other mold infections, and cryptococcal meningitis. Thus, LAmB has become the polyene of choice in most situations where treatment with AmB is clinically indicated. Dosages approved by the FDA and/or in member states of the European Medicines Agency for adults and children 1 month and older range from 1 to 3 mg/kg/day for empirical antifungal therapy in febrile neutropenic patients and from 3 to 6 mg/kg/day for therapy of invasive diseases, to be administered over 2 hours. Treatment according to the label should be started with the full target dose under clinical monitoring; premedication is only needed in patients with prior IRRs. In patients with renal dysfunction, dose reduction is generally not required unless there is evidence of relevant, drug-induced nephrotoxicity during treatment. Case reports have confirmed that LAmB is not significantly removed in patients undergoing dialysis [15]. Enhanced pulmonary accumulation has been reported in a patient with acute liver failure; however, the clinical significance of such an accumulation is unclear [19]. Formal and population-based PK studies indicate that the disposition of LAmB in pediatric patients beyond the neonatal period is not substantially different from that in adults, and that weight is a covariate that determines the clearance and volume of distribution [12–14]. Although PK data in this population are lacking, a considerable number of neonates, including very low birth-weight infants (<1500 g), have safely received LAmB at doses of up to 7 mg/kg over prolonged periods of time [55, 56, 58, 126].
Important areas of uncertainty remain that warrant further research of LAmB. These include, but are not limited to, approaches to: (1) optimize the methodology, standardization, and validation of AmB susceptibility testing across different fungal species; (2) develop a better understanding of the PK variability in patients and the PK and PD relationships in common and rare fungal diseases, with the aim of treatment optimization; (3) characterize the population PK of the compound in targeted populations of critically ill patients and neonates; (4) understand the PK and PD relationships in neonatal invasive candidiasis; (5) develop novel dosing schedules that build upon the long residence time of LAmB in the blood and tissues (based upon a solid, preclinical, experimental PK/PD foundation); and (6) investigate the PK and PD of inhalational applications as antifungal prophylaxis and therapy in patients with chronic pulmonary fungal diseases and following lung transplantation (Figure 2). The research agenda listed here reflects the perceived importance and potential of LAmB in the overall control of invasive fungal diseases, which continue to be a challenge, due to increasing numbers of susceptible patients, emerging resistance to other antifungal agents, and the limited availability of effective antifungal compounds. Combining preclinical and clinical data and using the full power of quantitative systems of pharmacology, we will be able to design optimized regimens. These regimens can subsequently be tested in prospectively validated studies, with the ultimate goal of further improving the treatment of patients with life-threatening, invasive, fungal diseases.
Figure 2.
Areas for further clinical investigations of liposomal amphotericin B.
Notes
Acknowledgments.This supplement was made possible by funding from Gilead Sciences; however, Gilead had no input into the content. Editorial assistance in the preparation of this manuscript was provided by Christine Drewienkiewicz of OPEN Health Medical Communications (London, UK) and was funded by Gilead. T. J. W. was supported as a Scholar of the Henry Schueler Foundation for his work on this manuscript.
Disclaimer.The views, opinions, assumptions, or any other information set out in this article are solely those of the authors and should not be attributed to the funders or any person connected with the funders. Liposomal amphotericin B is not approved for prophylaxis.
Funding.This work was supported by Gilead Sciences.
Supplement sponsorship.
Potential conflicts of interest.A. H. G. has received grants for his institution from Gilead Sciences, Merck Sharp & Dohme, and Pfizer; has received personal fees from Amplyx, Astellas Pharma, Basilea Pharmaceutica, F2G, Gilead Sciences, Merck Sharp & Dohme, Schering-Plough, and SCYNEXIS, outside the submitted work; and is on the speaker bureaus for Astellas Pharma, Basilea Pharmaceutica, Gilead Sciences, Merck Sharp & Dohme, Pfizer, Schering-Plough, and Zeneus/Cephalon. B. J. A. R. has received grants and honoraria from Gilead Sciences outside the submitted work. T. J. W. has received grants for his institution from Amplyx, Astellas Pharma, Merck, SCYNEXIS, Allergan, Medicines Company, Leadiant Biosciences, and Tetraphasel and has received honoraria from Astellas Pharma, Merck, SCYNEXIS, Allergan, Medicines Company, Gilead Sciences, and Leadiant Biosciences. J. A.-M. has received grants, honoraria, and non-financial support from Gilead Sciences, outside the submitted work and was involved in the discovery and development of liposomal amphotericin B by Vestar Inc. R. E. L. has received grants and personal fees from Gilead Sciences and Merck. R. J. M. B. has received grants and consulting fees from Gilead Sciences; has received unrestricted research grants and consulting fees from Astellas Pharma, Gilead Sciences, Merck Sharp & Dohme, and Pfizer; and has received consulting fees from F2G (all contracts were through Radboud UMC, and all payments were invoiced by Radboud UMC). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Walsh TJ, Yeldandi V, McEvoy M, et al. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob Agents Chemother 1998; 42:2391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walsh TJ, Goodman JL, Pappas P, et al. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob Agents Chemother 2001; 45:3487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bekersky I, Fielding RM, Dressler DE, Kline S, Buell DN, Walsh TJ.. Pharmacokinetics, excretion, and mass balance of 14C after administration of 14C-cholesterol-labeled AmBisome to healthy volunteers. J Clin Pharmacol 2001; 41:963–71. [DOI] [PubMed] [Google Scholar]
- 4. Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ.. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 2002; 46:834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ.. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother 2002; 46:828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone NR, Bicanic T, Salim R, Hope W.. Liposomal amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016; 76:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Groll AH, Walsh TJ.. Polyenes in the treatment of aspergillosis. In: Latge J-P, Steinbach WJ, eds. Aspergillus fumigatus and aspergillosis. Washington, DC: ASM Press, 2009:30. [Google Scholar]
- 8. Groll AH, Piscitelli SC, Walsh TJ.. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol 1998; 44:343–500. [DOI] [PubMed] [Google Scholar]
- 9. Townsend RW, Zutshi A, Bekersky I.. Biodistribution of 4-[(14)C]cholesterol-AmBisome following a single intravenous administration to rats. Drug Metab Dispos 2001; 29:681–5. [PubMed] [Google Scholar]
- 10. Brajtburg J, Bolard J.. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev 1996; 9:512–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Würthwein G, Young C, Lanvers-Kaminsky C, et al. Population pharmacokinetics of liposomal amphotericin B and caspofungin in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother 2012; 56:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seibel NL, Shad AT, Bekersky I, et al. Safety, tolerability, and pharmacokinetics of liposomal amphotericin B in immunocompromised pediatric patients. Antimicrob Agents Chemother 2017; 61:pii: e01477-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lestner JM, Groll AH, Aljayyoussi G, et al. Population pharmacokinetics of liposomal amphotericin B in immunocompromised children. Antimicrob Agents Chemother 2016; 60:7340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong Y, Shaw PJ, Nath CE, et al. Population pharmacokinetics of liposomal amphotericin B in pediatric patients with malignant diseases. Antimicrob Agents Chemother 2006; 50:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heinemann V, Bosse D, Jehn U, et al. Pharmacokinetics of liposomal amphotericin B (AmBisome) in critically ill patients. Antimicrob Agents Chemother 1997; 41:1275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Medicines Agency. Summary of product characteristics: AmBisome 50 mg powder for solution for infusion. 2017. Available at: https://www.medicines. org.uk/emc/product/1022/smpc [Google Scholar]
- 17. Food and Drug Administration. Prescribing information. AmBisome (amphotericin B) liposome for injection. 2018. Available at: https://www.astellas.us/docs/ambisome.pdf [Google Scholar]
- 18. Chamilos G, Luna M, Lewis RE, Chemaly R, Raad II, Kontoyiannis DP.. Effects of liposomal amphotericin B versus an amphotericin B lipid complex on liver histopathology in patients with hematologic malignancies and invasive fungal infections: a retrospective, nonrandomized autopsy study. Clin Ther 2007; 29:1980–6. [DOI] [PubMed] [Google Scholar]
- 19. Heinemann V, Bosse D, Jehn U, et al. Enhanced pulmonary accumulation of liposomal amphotericin B (AmBisome) in acute liver transplant failure. J Antimicrob Chemother 1997; 40:295–7. [DOI] [PubMed] [Google Scholar]
- 20. Groll AH, Piscitelli SC, Walsh TJ.. Antifungal pharmacodynamics: concentration-effect relationships in vitro and in vivo. Pharmacotherapy 2001; 21:133–48S. [DOI] [PubMed] [Google Scholar]
- 21. Andes D, Stamsted T, Conklin R.. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob Agents Chemother 2001; 45:922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiederhold NP, Tam VH, Chi J, Prince RA, Kontoyiannis DP, Lewis RE.. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2006; 50:469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adler-Moore J, Proffitt RT.. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother 2002; 49(Suppl 1):21–30. [DOI] [PubMed] [Google Scholar]
- 24. Francis P, Lee JW, Hoffman A, et al. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J Infect Dis 1994; 169:356–68. [DOI] [PubMed] [Google Scholar]
- 25. Clemons KV, Stevens DA.. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy and virulence. Med Mycol 2005; 43(Suppl 1):S101–10. [DOI] [PubMed] [Google Scholar]
- 26. Hiemenz JW, Walsh TJ.. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis 1996; 22(Suppl 2):S133–44. [DOI] [PubMed] [Google Scholar]
- 27. Janknegt R, de Marie S, Bakker-Woudenberg IA, Crommelin DJ.. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. Clin Pharmacokinet 1992; 23:279–91. [DOI] [PubMed] [Google Scholar]
- 28. Patterson TF. The future of animal models of invasive aspergillosis. Med Mycol 2005; 43(Suppl 1):S115–9. [DOI] [PubMed] [Google Scholar]
- 29. Gondal JA, Swartz RP, Rahman A.. Therapeutic evaluation of free and liposome-encapsulated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother 1989; 33:1544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Proffitt RT, Satorius A, Chiang SM, Sullivan L, Adler-Moore JP.. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother 1991; 28(Suppl B):49–61. [DOI] [PubMed] [Google Scholar]
- 31. Lee JW, Amantea MA, Francis PA, et al. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob Agents Chemother 1994; 38:713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boswell GW, Bekersky I, Buell D, Hiles R, Walsh TJ.. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob Agents Chemother 1998; 42:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bekersky I, Boswell GW, Hiles R, Fielding RM, Buell D, Walsh TJ.. Safety, toxicokinetics and tissue distribution of long-term intravenous liposomal amphotericin B (AmBisome): a 91-day study in rats. Pharm Res 2000; 17:1494–502. [DOI] [PubMed] [Google Scholar]
- 34. Bekersky I, Boswell GW, Hiles R, Fielding RM, Buell D, Walsh TJ.. Safety and toxicokinetics of intravenous liposomal amphotericin B (AmBisome) in beagle dogs. Pharm Res 1999; 16:1694–701. [DOI] [PubMed] [Google Scholar]
- 35. van Etten EW, ten Kate MT, Snijders SV, Bakker-Woudenberg IA.. Administration of liposomal agents and blood clearance capacity of the mononuclear phagocyte system. Antimicrob Agents Chemother 1998; 42:1677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meunier F, Prentice HG, Ringdén O.. Liposomal amphotericin B (AmBisome): safety data from a Phase II/III clinical trial. J Antimicrob Chemother 1991; 28(Suppl B):83–91. [DOI] [PubMed] [Google Scholar]
- 37. Ringdén O, Andström E, Remberger M, Svahn BM, Tollemar J.. Safety of liposomal amphotericin B (AmBisome) in 187 transplant recipients treated with cyclosporin. Bone Marrow Transplant 1994; 14(Suppl 5):S10–4. [PubMed] [Google Scholar]
- 38. Prentice HG, Hann IM, Herbrecht R, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol 1997; 98:711–8. [DOI] [PubMed] [Google Scholar]
- 39. Ellis M, Spence D, de Pauw B, et al. An EORTC international multicenter randomized trial (EORTC number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosis. Clin Infect Dis 1998; 27:1406–12. [DOI] [PubMed] [Google Scholar]
- 40. Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis 2007; 44:1289–97. [DOI] [PubMed] [Google Scholar]
- 41. Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–27. [DOI] [PubMed] [Google Scholar]
- 42. Hamill RJ, Sobel JD, El-Sadr W, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis 2010; 51:225–32. [DOI] [PubMed] [Google Scholar]
- 43. Johnson PC, Wheat LJ, Cloud GA, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med 2002; 137:105–9. [DOI] [PubMed] [Google Scholar]
- 44. Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med 1999; 340:764–71. [DOI] [PubMed] [Google Scholar]
- 45. Penack O, Schwartz S, Martus P, et al. Low-dose liposomal amphotericin B in the prevention of invasive fungal infections in patients with prolonged neutropenia: results from a randomized, single-center trial. Ann Oncol 2006; 17:1306–12. [DOI] [PubMed] [Google Scholar]
- 46. Rijnders BJ, Cornelissen JJ, Slobbe L, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis 2008; 46:1401–8. [DOI] [PubMed] [Google Scholar]
- 47. Cornely OA, Leguay T, Maertens J, et al. Randomized comparison of liposomal amphotericin B versus placebo to prevent invasive mycoses in acute lymphoblastic leukaemia. J Antimicrob Chemother 2017; 72:2359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med 2004; 351:1391–402. [DOI] [PubMed] [Google Scholar]
- 49. Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 2002; 346:225–34. [DOI] [PubMed] [Google Scholar]
- 50. Pasic S, Flannagan L, Cant AJ.. Liposomal amphotericin (AmBisome) is safe in bone marrow transplantation for primary immunodeficiency. Bone Marrow Transplant 1997; 19:1229–32. [DOI] [PubMed] [Google Scholar]
- 51. Caselli D, Cesaro S, Ziino O, et al. A prospective, randomized study of empirical antifungal therapy for the treatment of chemotherapy-induced febrile neutropenia in children. Br J Haematol 2012; 158:249–55. [DOI] [PubMed] [Google Scholar]
- 52. Ringdén O, Tollemar J.. Liposomal amphotericin B (AmBisome) treatment of invasive fungal infections in immunocompromised children. Mycoses 1993; 36:187–92. [DOI] [PubMed] [Google Scholar]
- 53. Sideri G, Falagas ME, Grigoriou M, et al. Liposomal amphotericin B in critically ill paediatric patients. J Clin Pharm Ther 2012; 37:291–5. [DOI] [PubMed] [Google Scholar]
- 54. Kolve H, Ahlke E, Fegeler W, Ritter J, Jürgens H, Groll AH.. Safety, tolerance and outcome of treatment with liposomal amphotericin B in paediatric patients with cancer or undergoing haematopoietic stem cell transplantation. J Antimicrob Chemother 2009; 64:383–7. [DOI] [PubMed] [Google Scholar]
- 55. Juster-Reicher A, Leibovitz E, Linder N, et al. Liposomal amphotericin B (AmBisome) in the treatment of neonatal candidiasis in very low birth weight infants. Infection 2000; 28:223–6. [DOI] [PubMed] [Google Scholar]
- 56. Juster-Reicher A, Flidel-Rimon O, Amitay M, Even-Tov S, Shinwell E, Leibovitz E.. High-dose liposomal amphotericin B in the therapy of systemic candidiasis in neonates. Eur J Clin Microbiol Infect Dis 2003; 22:603–7. [DOI] [PubMed] [Google Scholar]
- 57. Arrieta AC, Shea K, Dhar V, et al. Once-weekly liposomal amphotericin B as Candida prophylaxis in very low birth weight premature infants: a prospective, randomized, open-label, placebo-controlled pilot study. Clin Ther 2010; 32:265–71. [DOI] [PubMed] [Google Scholar]
- 58. Scarcella A, Pasquariello MB, Giugliano B, Vendemmia M, de Lucia A.. Liposomal amphotericin B treatment for neonatal fungal infections. Pediatr Infect Dis J 1998; 17:146–8. [DOI] [PubMed] [Google Scholar]
- 59. Manzoni P, Wu C, Tweddle L, Roilides E.. Micafungin in premature and non-premature infants: a systematic review of 9 clinical trials. Pediatr Infect Dis J 2014; 33:e291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Johnson MD, Drew RH, Perfect JR.. Chest discomfort associated with liposomal amphotericin B: report of three cases and review of the literature. Pharmacotherapy 1998; 18:1053–61. [PubMed] [Google Scholar]
- 61. Roden MM, Nelson LD, Knudsen TA, et al. Triad of acute infusion-related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis 2003; 36:1213–20. [DOI] [PubMed] [Google Scholar]
- 62. Loo AS, Muhsin SA, Walsh TJ.. Toxicokinetic and mechanistic basis for the safety and tolerability of liposomal amphotericin B. Expert Opin Drug Saf 2013; 12:881–95. [DOI] [PubMed] [Google Scholar]
- 63. Raschi E, Poluzzi E, Koci A, Caraceni P, Ponti FD.. Assessing liver injury associated with antimycotics: concise literature review and clues from data mining of the FAERS database. World J Hepatol 2014; 6:601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kyriakidis I, Tragiannidis A, Munchen S, Groll AH.. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 2017; 16:149–65. [DOI] [PubMed] [Google Scholar]
- 65. Stuecklin-Utsch A, Hasan C, Bode U, Fleischhack G.. Pancreatic toxicity after liposomal amphotericin B. Mycoses 2002; 45:170–3. [DOI] [PubMed] [Google Scholar]
- 66. Lane JW, Rehak NN, Hortin GL, Zaoutis T, Krause PR, Walsh TJ.. Pseudohyperphosphatemia associated with high-dose liposomal amphotericin B therapy. Clin Chim Acta 2008; 387:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mohr JF, Hall AC, Ericsson CD, Ostrosky-Zeichner L.. Fatal amphotericin B overdose due to administration of nonlipid formulation instead of lipid formulation. Pharmacotherapy 2005; 25:426–8. [DOI] [PubMed] [Google Scholar]
- 68. Patterson TF, Boucher HW, Herbrecht R, et al. ; European Organization for Research and Treatment of Cancer (EORTC) Invasive Fungal Infections Group (IFIG); Pfizer Global Aspergillus Study Group Strategy of following voriconazole versus amphotericin B therapy with other licensed antifungal therapy for primary treatment of invasive aspergillosis: impact of other therapies on outcome. Clin Infect Dis 2005; 41:1448–52. [DOI] [PubMed] [Google Scholar]
- 69. Adler Moore J. Preclinical safety, tolerability, pharmacokinetics, pharmacodynamics, and antifungal activity of liposomal amphotericin B. Clin Infect Dis 2019; 68(S4):S244–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Adler-Moore JP, Chiang SM, Satorius A, et al. Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome). J Antimicrob Chemother 1991; 28(Suppl B):63–71. [DOI] [PubMed] [Google Scholar]
- 71. Karyotakis NC, Anaissie EJ.. Efficacy of escalating doses of liposomal amphotericin B (AmBisome) against hematogenous Candida lusitaniae and Candida krusei infection in neutropenic mice. Antimicrob Agents Chemother 1994; 38:2660–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mills W, Chopra R, Linch DC, Goldstone AH.. Liposomal amphotericin B in the treatment of fungal infections in neutropenic patients: a single-centre experience of 133 episodes in 116 patients. Br J Haematol 1994; 86:754–60. [DOI] [PubMed] [Google Scholar]
- 73. Ng TT, Denning DW.. Liposomal amphotericin B (AmBisome) therapy in invasive fungal infections. Evaluation of United Kingdom compassionate use data. Arch Intern Med 1995; 155:1093–8. [PubMed] [Google Scholar]
- 74. Ringdén O, Meunier F, Tollemar J, et al. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J Antimicrob Chemother 1991; 28(Suppl B):73–82. [DOI] [PubMed] [Google Scholar]
- 75. Coker RJ, Viviani M, Gazzard BG, et al. Treatment of cryptococcosis with liposomal amphotericin B (AmBisome) in 23 patients with AIDS. AIDS 1993; 7:829–35. [DOI] [PubMed] [Google Scholar]
- 76. Leenders AC, Reiss P, Portegies P, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS 1997; 11:1463–71. [DOI] [PubMed] [Google Scholar]
- 77. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome therapy for mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother 2012; 67:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lanternier F, Poiree S, Elie C, et al. ; French Mycosis Study Group Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother 2015; 70:3116–23. [DOI] [PubMed] [Google Scholar]
- 79. Cornely OA, Cuenca-Estrella M, Meis JF, Ullmann AJ.. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) and European Confederation of Medical Mycology (ECMM) 2013 joint guidelines on diagnosis and management of rare and emerging fungal diseases. Clin Microbiol Infect 2014; 20(Suppl 3):1–4. [DOI] [PubMed] [Google Scholar]
- 80. Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 2013; 98:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Berman J. Chemotherapy of leishmaniasis: recent advances in the treatment of visceral disease. Curr Opin Infect Dis 1998; 11:707–10. [PubMed] [Google Scholar]
- 82. Bern C, Adler-Moore J, Berenguer J, et al. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis 2006; 43:917–24. [DOI] [PubMed] [Google Scholar]
- 83. Cascio A, di Martino L, Occorsio P, et al. A 6 day course of liposomal amphotericin B in the treatment of infantile visceral leishmaniasis: the Italian experience. J Antimicrob Chemother 2004; 54:217–20. [DOI] [PubMed] [Google Scholar]
- 84. Davidson RN, Di Martino L, Gradoni L, et al. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: a multi-centre trial. Q J Med 1994; 87:75–81. [PubMed] [Google Scholar]
- 85. Musa AM, Khalil EA, Mahgoub FA, Hamad S, Elkadaru AM, El Hassan AM.. Efficacy of liposomal amphotericin B (AmBisome) in the treatment of persistent post-kala-azar dermal leishmaniasis (PKDL). Ann Trop Med Parasitol 2005; 99:563–9. [DOI] [PubMed] [Google Scholar]
- 86. Machado PR, Rosa ME, Guimarães LH, et al. Treatment of disseminated leishmaniasis with liposomal amphotericin B. Clin Infect Dis 2015; 61:945–9. [DOI] [PubMed] [Google Scholar]
- 87. Romero GAS, Costa DL, Costa CHN, et al. ; Collaborative LVBrasil Group Efficacy and safety of available treatments for visceral leishmaniasis in Brazil: a multicenter, randomized, open label trial. PLOS Negl Trop Dis 2017; 11:e0005706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lewis RE, Lortholary O, Spellberg B, Roilides E, Kontoyiannis DP, Walsh TJ.. How does antifungal pharmacology differ for mucormycosis versus aspergillosis?Clin Infect Dis 2012; 54(Suppl 1):S67–72. [DOI] [PubMed] [Google Scholar]
- 89. Herbrecht R, Denning DW, Patterson TF, et al. ; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002; 347:408–15. [DOI] [PubMed] [Google Scholar]
- 90. Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW.. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med 2010; 362:504–12. [DOI] [PubMed] [Google Scholar]
- 91. O’Connor L, Livermore J, Sharp AD, et al. Pharmacodynamics of liposomal amphotericin B and flucytosine for cryptococcal meningoencephalitis: safe and effective regimens for immunocompromised patients. J Infect Dis 2013; 208:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lestner J, McEntee L, Johnson A, et al. Experimental models of short courses of liposomal amphotericin B for induction therapy for cryptococcal meningitis. Antimicrob Agents Chemother 2017; 61:pii: e00090–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jarvis JN, Leeme TB, Molefi M, et al. Short course high-dose liposomal amphotericin B for HIV-associated cryptococcal meningitis: a Phase-II randomized controlled trial. Clin Infect Dis 2018; 68:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lawrence DS, Youssouf N, Molloy SF, et al. AMBisome Therapy Induction OptimisatioN (AMBITION): high dose AmBisome for cryptococcal meningitis induction therapy in sub-Saharan Africa: study protocol for a Phase 3 randomised controlled non-inferiority trial. Trials 2018; 19:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kontoyiannis DP, Hachem R, Lewis RE, et al. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 2003; 98:292–9. [DOI] [PubMed] [Google Scholar]
- 96. Aliff TB, Maslak PG, Jurcic JG, et al. Refractory Aspergillus pneumonia in patients with acute leukemia: successful therapy with combination caspofungin and liposomal amphotericin. Cancer 2003; 97:1025–32. [DOI] [PubMed] [Google Scholar]
- 97. Candoni A, Caira M, Cesaro S, et al. ; SEIFEM GROUP (Sorveglianza Epidemiologica Infezioni Fungine nelle Emopatie Maligne) Multicentre surveillance study on feasibility, safety and efficacy of antifungal combination therapy for proven or probable invasive fungal diseases in haematological patients: the SEIFEM real-life combo study. Mycoses 2014; 57:342–50. [DOI] [PubMed] [Google Scholar]
- 98. Caillot D, Thiébaut A, Herbrecht R, et al. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: a randomized pilot study (Combistrat trial). Cancer 2007; 110:2740–6. [DOI] [PubMed] [Google Scholar]
- 99. Groll AH, Silling G, Young C, et al. Randomized comparison of safety and pharmacokinetics of caspofungin, liposomal amphotericin B, and the combination of both in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother 2010; 54:4143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wingard JR, White MH, Anaissie E, Raffalli J, Goodman J, Arrieta A; L Amph/ABLC Collaborative Study Group A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clin Infect Dis 2000; 31:1155–63. [DOI] [PubMed] [Google Scholar]
- 101. Tollemar J, Ringdén O, Andersson S, Sundberg B, Ljungman P, Tydén G.. Randomized double-blind study of liposomal amphotericin B (AmBisome) prophylaxis of invasive fungal infections in bone marrow transplant recipients. Bone Marrow Transplant 1993; 12:577–82. [PubMed] [Google Scholar]
- 102. Kelsey SM, Goldman JM, McCann S, et al. Liposomal amphotericin (AmBisome) in the prophylaxis of fungal infections in neutropenic patients: a randomised, double-blind, placebo-controlled study. Bone Marrow Transplant 1999; 23:163–8. [DOI] [PubMed] [Google Scholar]
- 103. Mattiuzzi GN, Estey E, Raad I, et al. Liposomal amphotericin B versus the combination of fluconazole and itraconazole as prophylaxis for invasive fungal infections during induction chemotherapy for patients with acute myelogenous leukemia and myelodysplastic syndrome. Cancer 2003; 97:450–6. [DOI] [PubMed] [Google Scholar]
- 104. Roman E, Osunkwo I, Militano O, Cooney E, van de Ven C, Cairo MS.. Liposomal amphotericin B prophylaxis of invasive mold infections in children post allogeneic stem cell transplantation. Pediatr Blood Cancer 2008; 50:325–30. [DOI] [PubMed] [Google Scholar]
- 105. Annino L, Chierichini A, Anaclerico B, et al. Prospective Phase II single-center study of the safety of a single very high dose of liposomal amphotericin B for antifungal prophylaxis in patients with acute myeloid leukemia. Antimicrob Agents Chemother 2013; 57:2596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cordonnier C, Mohty M, Faucher C, et al. Safety of a weekly high dose of liposomal amphotericin B for prophylaxis of invasive fungal infection in immunocompromised patients: PROPHYSOME Study. Int J Antimicrob Agents 2008; 31:135–41. [DOI] [PubMed] [Google Scholar]
- 107. El-Cheikh J, Faucher C, Fürst S, et al. High-dose weekly liposomal amphotericin B antifungal prophylaxis following reduced-intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant 2007; 39:301–6. [DOI] [PubMed] [Google Scholar]
- 108. Ellis M, Bernsen R, Ali-Zadeh H, et al. A safety and feasibility study comparing an intermittent high dose with a daily standard dose of liposomal amphotericin B for persistent neutropenic fever. J Med Microbiol 2009; 58:1474–85. [DOI] [PubMed] [Google Scholar]
- 109. Mehta P, Vinks A, Filipovich A, et al. High-dose weekly AmBisome antifungal prophylaxis in pediatric patients undergoing hematopoietic stem cell transplantation: a pharmacokinetic study. Biol Blood Marrow Transplant 2006; 12:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bochennek K, Tramsen L, Schedler N, et al. Liposomal amphotericin B twice weekly as antifungal prophylaxis in paediatric haematological malignancy patients. Clin Microbiol Infect 2011; 17:1868–74. [DOI] [PubMed] [Google Scholar]
- 111. Groll AH, Gea-Banacloche JC, Glasmacher A, Just-Nuebling G, Maschmeyer G, Walsh TJ.. Clinical pharmacology of antifungal compounds. Infect Dis Clin North Am 2003; 17:159–91, ix. [DOI] [PubMed] [Google Scholar]
- 112. Tollemar J, Höckerstedt K, Ericzon BG, Jalanko H, Ringdén O.. Liposomal amphotericin B prevents invasive fungal infections in liver transplant recipients. A randomized, placebo-controlled study. Transplantation 1995; 59:45–50. [DOI] [PubMed] [Google Scholar]
- 113. Castroagudín JF, Pontón C, Bustamante M, et al. Prospective interventional study to evaluate the efficacy and safety of liposomal amphotericin B as prophylaxis of fungal infections in high-risk liver transplant recipients. Transplant Proc 2005; 37:3965–7. [DOI] [PubMed] [Google Scholar]
- 114. Hadley S, Huckabee C, Pappas PG, et al. Outcomes of antifungal prophylaxis in high-risk liver transplant recipients. Transpl Infect Dis 2009; 11:40–8. [DOI] [PubMed] [Google Scholar]
- 115. Giannella M, Ercolani G, Cristini F, et al. High-dose weekly liposomal amphotericin b antifungal prophylaxis in patients undergoing liver transplantation: a prospective Phase II trial. Transplantation 2015; 99:848–54. [DOI] [PubMed] [Google Scholar]
- 116. Lewis RE, Albert ND, Liao G, Hou J, Prince RA, Kontoyiannis DP.. Comparative pharmacodynamics of amphotericin B lipid complex and liposomal amphotericin B in a murine model of pulmonary mucormycosis. Antimicrob Agents Chemother 2010; 54:1298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lestner JM, Howard SJ, Goodwin J, et al. Pharmacokinetics and pharmacodynamics of amphotericin B deoxycholate, liposomal amphotericin B, and amphotericin B lipid complex in an in vitro model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2010; 54:3432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ruijgrok EJ, Fens MH, Bakker-Woudenberg IA, van Etten EW, Vulto AG.. Nebulization of four commercially available amphotericin B formulations in persistently granulocytopenic rats with invasive pulmonary aspergillosis: evidence for long-term biological activity. J Pharm Pharmacol 2005; 57:1289–95. [DOI] [PubMed] [Google Scholar]
- 119. Groll AH, Lyman CA, Petraitis V, et al. Compartmentalized intrapulmonary pharmacokinetics of amphotericin B and its lipid formulations. Antimicrob Agents Chemother 2006; 50:3418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Allen SD, Sorensen KN, Nejdl MJ, Durrant C, Proffit RT.. Prophylactic efficacy of aerosolized liposomal (AmBisome) and non-liposomal (Fungizone) amphotericin B in murine pulmonary aspergillosis. J Antimicrob Chemother 1994; 34:1001–13. [DOI] [PubMed] [Google Scholar]
- 121. Cicogna CE, White MH, Bernard EM, et al. Efficacy of prophylactic aerosol amphotericin B lipid complex in a rat model of pulmonary aspergillosis. Antimicrob Agents Chemother 1997; 41:259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ruijgrok EJ, Vulto AG, Van Etten EW.. Efficacy of aerosolized amphotericin B desoxycholate and liposomal amphotericin B in the treatment of invasive pulmonary aspergillosis in severely immunocompromised rats. J Antimicrob Chemother 2001; 48:89–95. [DOI] [PubMed] [Google Scholar]
- 123. Gavaldà J, Martín MT, López P, et al. Efficacy of nebulized liposomal amphotericin B in treatment of experimental pulmonary aspergillosis. Antimicrob Agents Chemother 2005; 49:3028–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Monforte V, Ussetti P, Gavaldà J, et al. Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. J Heart Lung Transplant 2010; 29:523–30. [DOI] [PubMed] [Google Scholar]
- 125. Chong GL, Broekman F, Polinder S, et al. Aerosolised liposomal amphotericin B to prevent aspergillosis in acute myeloid leukaemia: efficacy and cost effectiveness in real-life. Int J Antimicrob Agents 2015; 46:82–7. [DOI] [PubMed] [Google Scholar]
- 126. Chiou CC, Walsh TJ, Groll AH.. Clinical pharmacology of antifungal agents in pediatric patients. Expert Opin Pharmacother 2007; 8:2465–89. [DOI] [PubMed] [Google Scholar]