Abstract
Background
AIDS Clinical Trial Group 5199 compared neurological and neuropsychological test performance of human immunodeficiency virus type 1 (HIV-1)–infected participants in resource-limited settings treated with 3 World Health Organization–recommended antiretroviral (ART) regimens. We investigated the impact of tuberculosis (TB) on neurological and neuropsychological outcomes.
Methods
Standardized neurological and neuropsychological examinations were administered every 24 weeks. Generalized estimating equation models assessed the association between TB and neurological/neuropsychological performance.
Results
Characteristics of the 860 participants at baseline were as follows: 53% female, 49% African; median age, 34 years; CD4 count, 173 cells/μL; and plasma HIV-1 RNA, 5.0 log copies/mL. At baseline, there were 36 cases of pulmonary, 9 cases of extrapulmonary, and 1 case of central nervous system (CNS) TB. Over the 192 weeks of follow-up, there were 55 observations of pulmonary TB in 52 persons, 26 observations of extrapulmonary TB in 25 persons, and 3 observations of CNS TB in 2 persons. Prevalence of TB decreased with ART initiation and follow-up. Those with TB coinfection had significantly poorer performance on grooved pegboard (P < .001) and fingertapping nondominant hand (P < .01). TB was associated with diffuse CNS disease (P < .05). Furthermore, those with TB had 9.27 times (P < .001) higher odds of reporting decreased quality of life, and had 8.02 times (P = .0005) higher odds of loss of productivity.
Conclusions
TB coinfection was associated with poorer neuropsychological functioning, particularly the fine motor skills, and had a substantial impact on functional ability and quality of life.
Clinical Trials Registration
NCT00096824.
Keywords: HIV, tuberculosis, resource-limited, cognitive impairment, neuropsychological functioning
The AIDS Clinical Trial Group A5199 compared the neurological and neuropsychological results in 860 human immunodeficiency virus–positive participants from 7 countries who received 3 different antiretroviral regimens. Patients with tuberculosis had poorer neurological and neurocognitive findings.
Resource-poor, developing parts of the world are where the majority of new human immunodeficiency virus type 1 (HIV-1) infections and tuberculosis (TB) deaths occur [1, 2]. Even though the global incidence rate for TB has been declining, the highest burden remains in the low- and middle-income countries within Asia and Africa. In 2016, 10.4 million people fell ill with TB, and 1.7 million died from the disease. HIV is the strongest risk factor for developing TB, and up to 13% of identified TB cases globally in 2012 occurred in those who were HIV infected [3]. In a recent systematic review, the pooled prevalence of TB and HIV co-infection was 31.3% for African countries, 20.1% in European studies, 17.2% in Asia, 25.1% in Latin America, and 14.8% for the United States [4].
Both TB and HIV affect the nervous system. The neurological effects due to HIV viral and immune factors [5] within the central nervous system (CNS) are called HIV-associated neurological disorders [6], which include the severe form of HIV-associated dementia (HAD), the less severe but more prevalent HIV-associated minor neurocognitive disorder (MND), and asymptomatic neurocognitive impairment [7–11]. Prior to effective active antiretroviral therapy (ART), there were many autopsy findings of HIV-1–related CNS injuries, even in some who had not had any clear manifestations of HAD [12].
CNS TB is the most severe form of extrapulmonary TB and is associated with a markedly elevated risk of morbidity and mortality [13, 14]. While a number of studies have documented the association of TB with cerebral infarction and associated neurological and psychiatric sequelae, there is a paucity of data on the pattern of long-term neuropsychological (NP) deficits associated with CNS TB, or any form of TB [15]. Kalita et al showed that at a 1-year follow-up, 36 out of 65 patients with TB meningitis had cognitive deficits when assessed using the Mini Mental State Examination [16]. It is not known whether the presence of TB will further increase the risk for or worsen the cognitive deficits reported in patients with HIV. This study provides much-needed information addressing the NP effects of the common co-infection of TB and HIV.
Another important consideration in the coinfection of HIV and TB is the neurotoxic effects of antiretroviral and anti-TB medication, and the possibility of their interacting to further worsen NP outcomes. Available evidence suggests that good outcomes can be achieved with the combination of TB treatment with first-line ART, but use with second-line ART could result in interactions and combined toxicity [17, 18]. The initiation of ART must be balanced with the risk of TB-immune reconstitution inflammatory syndrome (TB-IRIS) to avoid a paradoxical exacerbation of the immune response against TB.
Between February 2006 to May 2010, we conducted a study of neurological and NP outcomes with ART initiation and treatment in resource-limited settings (RLS): the International Neurological Study (INS, AIDS Clinical Trial Group [ACTG] 5199 [A5199]; clinicaltrials.gov, NCT00096824). This was a 3-arm, randomized clinical trial that included a direct comparison of 2 regimens recommended by the World Health Organization for first-line treatments of HIV-1 in RLS [19]. Here, we sought to examine the role of TB co-infection with HIV on neurological function and NP performance.
METHODS
Sites
Participants in A5199 INS were enrolled from ACTG study A5175, a randomized treatment trial of antiretroviral efficacy and safety. The ACTG sites that participated in A5199 were in Rio de Janeiro, Brazil; Porto Alegre, Brazil; Chennai, India; Pune, India; Blantyre, Malawi; Lilongwe, Malawi; Lima, Peru; Johannesburg, South Africa; Durban, South Africa; Chiang Mai, Thailand; and Harare, Zimbabwe.
Procedures
Human-subject study reviews and approvals were obtained at each site from local and country-specific review boards prior to study initiation, and informed consent was obtained prior to study participation. The National Institutes for Health, National Institute for Allergy and Infectious Diseases, Division of AIDS, and Multinational Data Safety and Monitoring Board monitored the study at intervals to ensure safe and appropriate conduct. Standardized training on the administration of the neurological and NP screening examinations was conducted. Rigorous data monitoring at data entry through computerized range checks, with follow-up data cleaning through multiple queries and replies, was conducted throughout the study. Implausible values were queried at intervals, and either confirmed or corrected.
Participants
Participants were a subset of ACTG A5175, a randomized ART trial (ClinicalTrials.gov NCT00084136). Eligible participants for A5175 were men and women over 18 years old who had documented HIV-1 infections, CD4+ lymphocytes less than 300 cells/ mm3, Karnofsky performance scorse greater than or equal to 70, and no more than 7 days of cumulative prior ART. Participants were excluded from participation in the study if they had any active severe psychiatric illness, active drug or alcohol abuse or dependence, a serious illness and/or hospitalization within 14 days of study entry, or any other condition that would compromise the person’s ability to participate in the study, adhere to study requirements, or confound the analysis or interpretation of the results of the study. Diagnosis criteria for all opportunistic events were standardized across sites using National Institutes of Health Division of AIDS criteria [20]. Participants with TB at baseline were not excluded if they were healthy enough to begin both ART and TB medication.
Tuberculosis Diagnosis
Pulmonary TB was diagnosed and defined as either (1) confirmed based on mycobacterium TB cultured from sputum, bronchoalveolar lavage, or lung tissue or (2) probable based on clinical symptoms consistent with pulmonary TB (fever > 38°C, night sweats, productive cough, hemoptysis, weight loss) and either an acid fast bacilli smear from sputum or another histopathology sample, an abnormal chest X-ray consistent with TB, or the initiation of specific, multi-drug antituberculous therapy. Extrapulmonary TB was diagnosed by the same criteria, but with a histopathology sample from the infected site.
Treatment Arms
The parent study, A5175, provided 3 treatment arms. Participants in arm A received lamivudine/zidovudine and efavirenz; those in arm B received emtricitabine, atazanavir , and enteric-coated didanosine; and those in arm C received co-formulated emtricitabine/tenofovir and efavirenz.
Neuropsychological and Neurological Examinations
Standardized neurological and NP screening examinations were administered every 24 weeks. The NP screening exam included:
The Timed Gait Test, in which participants walk 10 yards, turn, and return (average of 3 trials in seconds).
The Grooved Pegboard Manipulative Dexterity Test, where—moving in a set order and direction along 25 slotted holes—a peg must be rotated to match the direction of a hole before being inserted, upon which the user moves to the next hole with a new peg. The examiner encourages the subject to perform the task as quickly as possible. The recorded score for each hand is the time in seconds that it takes to complete the entire board, 1 peg at a time.
The Semantic Verbal Fluency test, which is a timed task (60 seconds) requiring the production of words in a specific category. Memory, speed of processing, initiation, and inhibition are assessed.
The Finger Tapping test, which is a measure of motor speed evaluated by the rate of depressing the key of a recording device as fast as possible for 5 periods of 10 seconds each, for each hand.
The NP tests were chosen based on their prior experience in clinical trial and cohort studies in the United States, with particular care taken to keep the battery short and minimize language- and culture-specific items [21, 22].
The neurological examination included a neurological history and symptom review and cognitive, motor, sensory, and reflex assessments, and was conducted by physicians and mid-level clinicians. A study-specific diagnosis form was completed for each participant and included HAD, MND, and peripheral neuropathy, in addition to CNS opportunistic infections.The diagnosis of diffuse CNS disease related to HIV was categorized as MND if impairment severity was rated as subclinical or equivocal, and as HAD if it was categorized as mild, moderate, or severe, based on the severity levels in the neurological exam criteria [21].
Subclinical or equivocal were defined as either minimal or equivocal symptoms or motor dysfunctions characteristic of HIV-associated neurocognitive/neurological dysfunction, or mild signs (snout response, slowed extremity movements) without an impairment of either work or the capacity to perform activities of daily living. Mild was defined as unequivocal evidence of functional intellectual or motor impairment, but with the ability to perform all but the more demanding aspects of work or activities of daily living. Moderate was defined as the inability to work or maintain the more demanding aspects of daily life, but with the ability to perform basic activities of self-care. Severe was defined as a major intellectual incapacity or motor disability.
Minor Cognitive Motor Disorder was defined as minimal or equivocal symptoms or signs of neurologic dysfunction due to diffuse CNS disease.
Questions to determine productivity level included work status, ability to perform full-time work, and energy level. The questions to evaluate quality of life (QOL) included self-reported changes in interest in social activities and symptoms of depression.
Statistical Analyses
Linear and logistic regression models using generalized estimating equations with an autoregressive correlation structure (for within-participant correlation) were constructed to assess the treatment effects, as well as the associations of other covariates, with NP test scores and neurological outcomes that included overall neurological examination abnormality, peripheral nervous system, or focal or diffuse CNS abnormality. The covariates included in the model were country, randomized treatment, baseline HIV-1 ribonucleic acid (RNA) stratum (<100 000 vs ≥100 000 copies/mL), screening CD4 stratum (<50, 50–99, 100–199, 200–249, and 250–299 cells/mm3), baseline neurocognitive test scores, age, sex, and years of education. In these treatment-naive individuals, current CD4+ T cell was considered to be an approximation of entry CD4+ nadir. Parameters were interpreted for a 10-year change in age, a 50-unit change in CD4 count, a 4-year change in education, and a 1-log change in entry plasma HIV-1 RNA. CD4 count and entry plasma HIV-1 RNA were used as continuous variables in modeling. HIV-1 RNA value at study follow-up was dichotomized into detectable vs undetectable using the lower assay detection limit of 400 copies/ml, below which VL levels were censored. In each case, 95% confidence intervals [CIs] are used to estimate the covariate effects. Logistic generalized estimating equations models were constructed to assess the concurrent association of extra pulmonary TB and TB on QOL and loss of productivity (LOP). Having normal interest in social activities vs not was used to assess QOL, while having functional ability to work full time vs not was used to assess LOP.
Forest plots were generated to summarize associations. The neurological exams were compared with both the baseline TB diagnoses and those that occurred while the participants were being evaluated during the course of the study (ie, current TB). Longitudinal plots were used to display temporal trends. All significance testing was performed at the 0.05 level, a trend for significance was defined as the P > .05 and P < .15 levels, and no adjustments for multiple testing or multiple comparisons were utilized. All reported P-values are 2-sided.
RESULTS
Demographics
A total of 860 participants were enrolled: 452 (53%) females and 408 (47%) males. Virological and immunological baseline figures included: CD4 of 173 (98, 232) cells/mm3; and median entry plasma log10 HIV-1 RNA of 5.0 log c/mL (4.5, 5.5). The median age was 34 years, and the median educational level was 10 years (Q1-7, Q3-12). By country, there were 161 participants in Brazil, 184 in India, 133 in Malawi, 62 in Peru, 167 in South Africa, 73 in Thailand, and 80 in Zimbabwe.
Tuberculosis Co-infection
At study entry, 47 participants had active TB: 36 had pulmonary TB, 9 extrapulmonary (non-CNS), 1 participant had features of both pulmonary and extrapulmonary TB, and 1 had CNS TB. Of those with pulmonary TB, 24 (67%) were male and 12 (33%) were female; of those without pulmonary TB, 384 (47%) were male and 439 (53%) were female. India had 60% of the baseline pulmonary TB cases, South Africa had 38%, and Thailand had 2%.
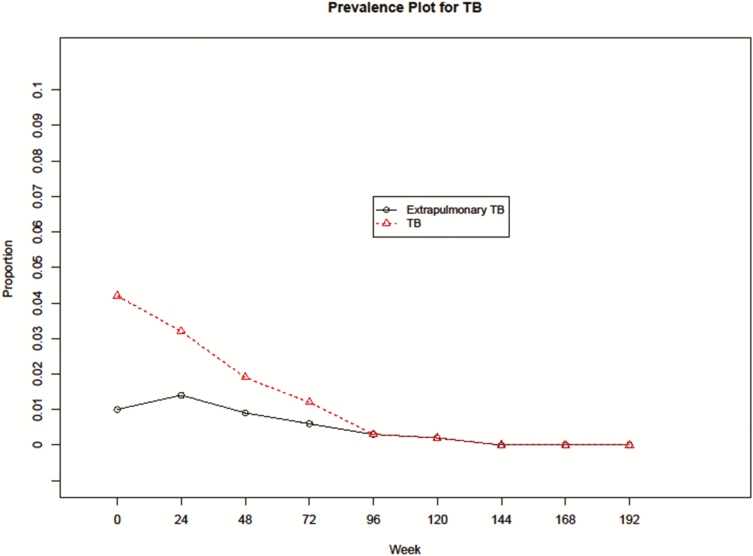
A diagnosis of new onset after baseline (incidence) was found in 27 participants after enrollment. The frequency of new TB diagnoses decreased with ART and continued with follow-ups over time (Figure 1). Over the course of the 192 weeks of follow up, there were a total of 130 visits (observations) for participants with TB co-infection: 91 visits for those with pulmonary TB, 35 for participants with extrapulmonary TB, and 4 in those with CNS TB.
Figure 1.
Prevalence plot for TB before and after antiretroviral therapy (ART): the proportion of extrapulmonary TB to all cases of TB over weeks on study. The prevalence of TB dropped with the initiation of ART. Abbreviation: TB, tuberculosis.
Plasma HIV RNA was higher at study entry in those with TB (5.3 log cps/ml) compared to those with no TB (4.9 log cps/ml; P < .001). The TB group was less educated (8.1 yrs) than those with no TB (9.5 yrs, P = .02).
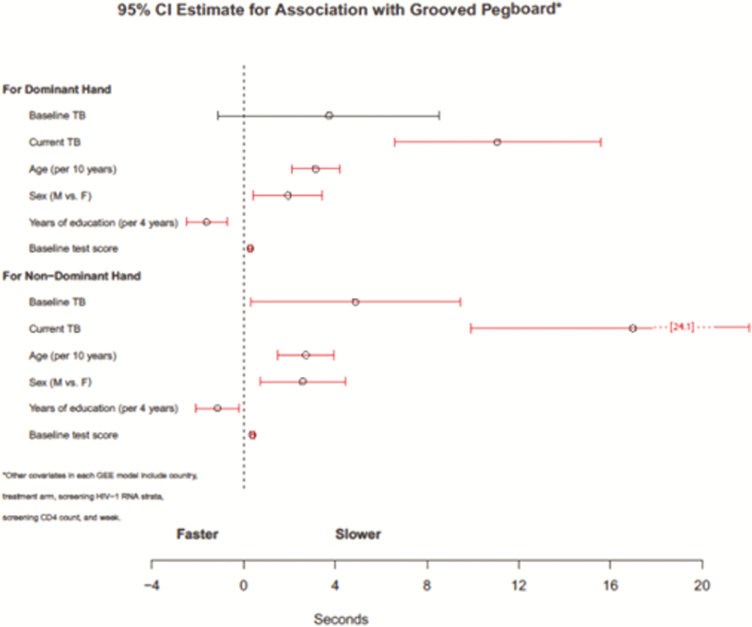
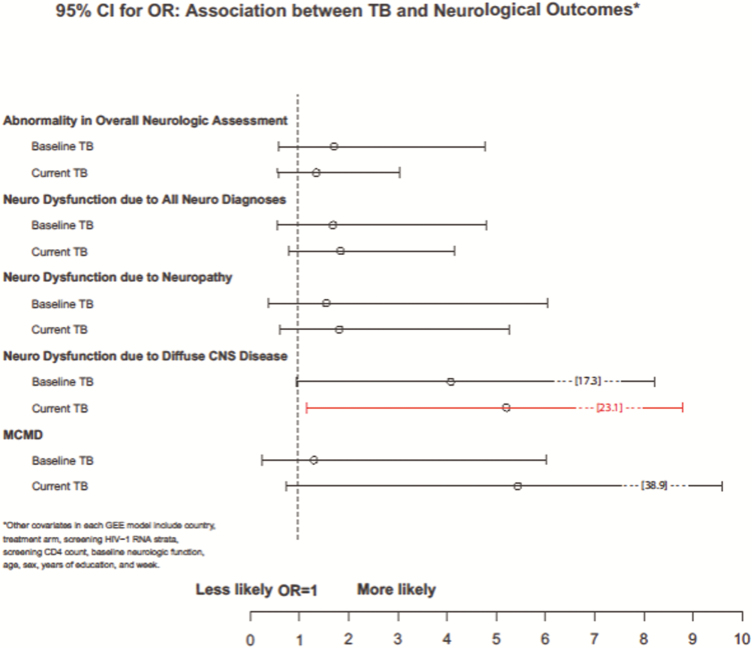
Those participants with current TB were significantly slower in completing the Grooved Pegboard with both their dominant (estimate = 11.08, standard error [SE] = 2.29, 95% CI 6.58–15.57; Z = 4.83, P < .001) and non-dominant hands (estimate = 16.99, SE = 3.62, 95% CI 9.89–24.08; Z = 4.69, P < .001; Figure 2). Participants who had current TB performed worse in Finger Tapping with their non-dominant hand (estimate = -3.04, SE = 1.09, 95% CI -5.19–-0.89; Z- 2.77, P < .01). Participants with current TB were more likely to be diagnosed with diffuse CNS disease (adjusted odds ratio [OR] = 5.21, 95% CI 1.17–23.09; Z = 2.17, P < .05, Figure 3).
Figure 2.
Estimated 95% confidence interval for association with grooved pedboard: poorer grooved pegboard with current tuberculosis for both dominant and nondominant hands, while baseline tuberculosis was significantly slower only for the nondominant hand. Other covariates in each GEE model include country, treatment arm, screening HIV-1 RNA strata, screening CD4 count, baseline neurologic function, age, sex years of education, and week. Abbreviations: GEE, generalized estimating equations; HIV-1, human immunodeficiency virus type 1; TB, tuberculosis.
Figure 3.
Estimated 95% confidence interval for odds ratio association between tuberculosis (TB) and neurological outcomes: diffuse central nervous system disease, usually associated with human immunodeficiency virus-associated neurocognitive disorders, was increased in those with current TB. Abbreviations: GEE, generalized estimating equations; MCMD, Minor Cognitive Motor Disorder.
Those participants with baseline TB were significantly slower in the Grooved Pegboard task with their non-dominant hands (estimate = 4.88, SE = 2.33, 95% CI 0.32–9.45; Z = 2.10, P < .05). There was a trend for baseline TB and increased diffused CNS disease (adjusted OR 4.08, 95% CI = 0.96–17.35; Z = 1.90, P = .057). Neither baseline nor current TB were significantly associated with the following tests of the NP exam: Semantic Verbal Fluency, Timed Gait, and Finger Tapping (dominant hand).
Of substantial note, TB had a major impact on functional ability. Current pulmonary TB was positively associated with LOP (adjusted OR = 6.96, 95% CI 1.82–26.59, P = .005). Participants with current TB had 9.27 times (95% CI 2.91–29.55, P < .001) higher odds of losing normal interest in social activities (ie, worse QOL). In addition, participants with current TB had 8.02 times (95% CI 2.50–25.72, P = .0005) higher odds of loss of ability to work full time (ie, LOP).
Neuropsychological Performance
The results of the NP test and neurological exam for the general HIV-positive (HIV+) cohort have been published and can be referred to for more information [24], but a brief summary follows. Overall, NP test scores improved (P < .05) following ART initiation, with the exception of Semantic Verbal Fluency, and were sustained over the course of the 3-year study. No differences in neurological and NP functioning between treatment regimens were detected (P > .10). Significant country effects were noted on all NP tests and neurological outcomes (P < .01).
DISCUSSION
We demonstrated that HIV+ participants co-infected with TB performed worse on NP testing, particularly in the motor domains, compared to HIV+ participants with no TB. Current TB had a substantial association with decreased QOL and LOP. Participants co-infected with TB were significantly slower in completing the Grooved Pegboard with both their dominant and non-dominant hands, and performed worse in Finger Tapping with their non-dominant hand. Individuals diagnosed with TB co-infection at or after study entry were more likely to have evidence of diffuse CNS disease in a neurological examination. While previous studies have shown that CNS TB is associated with cognitive impairment that could persist for up to a year in survivors [16], the possible effects of other forms of TB on cognitive functioning have not been explored. Our study had only 1 participant diagnosed with CNS TB, so the observed poorer performance on NP tests cannot solely be accounted for by direct CNS infection with TB.
Sustained ART for more than 3 years improved NP functioning and reduced overall neurological abnormality in ART-naive HIV+ participants. The specific ART regimen appeared less important for improving neurological/NP functioning than did initiating and maintaining successful ART. Remaining on treatment was also associated with our observation of decreased prevalence of TB over time. Earlier studies on cohorts of people with HIV in Brazil and South Africa similarly found that the use of ART reduced the incidence of HIV-associated TB in people who are on ART compared to those who are ART-naive [25, 26]. This finding further lends weight to the growing body of evidence on the importance of ART in reducing the risk for TB in people with HIV with previously-low CD4 cell counts [27].
QOL and LOP were negatively associated with TB co-infection. The LOP extends beyond the individual level in RLS, since the economic development of low-income countries is dependent on adults in their prime working age, who are the most vulnerable to HIV. These findings are supported by other studies that have also illustrated the negative effect of TB and HIV co-infection on QOL, specifically in RLS [30, 31].
Other factors worth exploring are the indirect effects of the co-infection of TB and HIV on CNS functioning, such as complications associated with medication, immune activation, and enhanced CNS HIV infection. Paradoxical TB-IRIS and significant drug toxicity are common complications following the commencement of ART, especially in the presence of significant immunosuppression [18, 28]. Patients from RLS usually commence ART at lower CD4 counts, which is known to increase the risk for TB-IRIS. The immune system response to TB infection is complex and has been implicated in some neurological complications of TB [29].
There are several limitations to this study. We used a brief, primarily motor-based neurocognitive assessment. A more comprehensive neurocognitive battery may have provided a fuller characterization of the neurocognitive impact. We did not evaluate the impact of the TB medication, including whether pyridoxine was administered, on neurological function, nor whether the participants were diagnosed with multi–drug-resistant TB or drug-sensitive TB. Another limitation would be the brievity of the assessments of QOL and LOP. In future studies, it would be useful to apply more comprehensive QOL assessments.
Furthermore, we did not apply the normative data from the International Neurocognitive Normative Study’s ACTG 5271 to INS [32]. The demographic-matched data in ACTG 5271 were collected from 2400 high-risk, HIV-negative participants from 10 voluntary counseling and testing sites aligned with INS and are only relevant to compare with the baseline impairment ratings. In this analysis, we compared the neurocognitive impairment of the HIV+ participants with TB to the HIV+ participants without TB, inferring that that the groups would act as each others’ controls in terms of demographic features and additional co-morbidities specific to their environment.
Overall, co-infection of HIV and TB was associated with poorer neurological and NP functioning despite overall neurological improvement with successful ART. This neurological and neurocognitive improvement is encouraging for the general well-being of the patients, as well as the economic implications of productivity. Further research is required to determine whether this association was related to the indirect effects on CNS function, such as TB medication, immune activation, and enhanced CNS HIV infection, or more direct CNS effects of TB. The negative impact of TB co-infection on neurological function, QOL, and LOP of HIV+ individuals provides further support for increasing access to ART in RLS.
Presented in part: The 19th Conference on Retroviruses and Opportunistic Infections (CROI) in Seattle, Washington, 5–8 March 2012.
Notes
Author contributions. N. K., S. E., A. V., A. L. R., B. S., M. T. S., S. M., C. K., C. F., R. P., C. M., B. B., R. M., U. L., I. S., S. Y., A. W., A. N., N. S., and C. H. are the authors of the “5199 study team” who contributed to the analysis of the data and writing of the paper.
Acknowledgments. The authors thank Dianne Rausch and Pim Brouwers at the Center for Mental Health Research on AIDS, National Institute of Mental Health.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute for Allergy and Infectious Diseases, National Institute of Mental Health, National Institutes of Health (NIH), or the institutions with which the authors are affiliated.
S. M. is an employee of the US Government. This work was prepared as part of her official duties.
Financial support. This work was supported by the National Institute of Mental Health and the AIDS Clinical Trials Group, which is funded by The National Institute of Allergy and Infectious Diseases (NIAID) (grant number U01AI068636) and the Statistical and Data Analysis Center (grant number AI-068634). Grants were awarded to individual authors as follows: NIAID grant number AIDS Clinical Trials Unit AI069450 to T. C.; IPEC-FIOCRUZ (Site 12101) Clinical Trials Unit (CTU) grant number AI69476 to D. V. and M. T. S.; Durban Adult human immunodeficiency viruses (HIV) CRS (Site 11201) CTU grant number 5U01AI069426-03 to U. L. and R. M.; Y.R. Gaitonde Centre for AIDS Research and Education Medical Centre (Site 11701) CTU grant number AI069432 to N. K. and J. S.; Franklin Kilembe University of North Carolina Project, Kamuzu Central Hospital, Lilongwe (Site 12001) CTU grant number AI069518 to V. M. K. and C. P.; Parirenyatwa CRS (Site 30313) CTU grant number BRS-ACURE-Q-08-00173-TOOI-OOO to M. W. and R. M.; Wits HIV Clinical Research Site (Helen Joseph Hospital; Site 11101) CTU grant numbers AI069463 and BRS-ACURE-Q-07-00143 T006 to C. F., S. F., and D. S. R.; Research Institute for Health Sciences, Chiang Mai University (Site 11501) CTU grant numbers AI069399 and AACTG.27.5199.06 to T. S. and D. T.; Hospital Nossa Senhora da Conceicao CRS (Site 12201) CTU grant number 5 U01 AI069401 to M. S. and R. L.; National Agricultural Research Institution (NARI) Pune CRS (Site 11601) CTU grant number 5U01AI069417-03 to A. A. J. and S. P. T.; College of Medicine, Johns Hopkins Project (Site 30301) CTU grant number U01A1069518 to B. K. and H. C.; INMENSA-Lince CRS (Site 11302) CTU grant numbers 5U01 AI069438-03 and BRS-ACURE-Q-07-00141-T001-001 to C. M. and R. I.; Asociación Civil Impacta Salud y Educación (Site 11301) CTU grant numbers AI069438 to BRS-ACURE-Q-08-00007-T-002 to J. S. and J. C. H.; NARI-National Institute of Virology Clinic (Site 11603) CTU grant number 5U01AI069417-03 to M. V. G. and M. N.; Dr Kotnis Dispensary, NARI (Site 11602) CTU grant number 5U01AI069417-03 to R. G. and U. K.; and the Statistical and Data Management Center of the Adult AIDS Clinical Trials Group grant number 1 U01 068634 to S. R. E. and H. J.
Potential conflicts of interest. K. R. has been a consultant for GlaxoSmithKline (GSK) and ViiV. R. W. P. is involved in investigator-initiated grant funding for Merck and has received an honorarium for a lecture for Abbott Laboratories. R. Murphy is a consultant for Gilead. R. Misah reports NIH grants. T. C. reports receipt of an NIH grant and of personal fees from Gilead, ViiV, and Theratechnologies. K. S. reports grants from NIH. S. E. reports grants from the National Institute for Allergy and Infectious Diseases and NIH during the conduct of the study; and personal fees from Takeda/Millennium, Pfizer, Roche, Novartis, Achaogen, the Huntington’s Study Group, Auspex, Alcon, Merck, Chelsea, Mannkind, QRx Pharma, Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks, Genentech, Affymax, FzioMed, Amgen, GSK, Boehringer-Ingelheim, the American Statistical Association, the Food and Drug Administration, Osaka University, that City of Hope, the National Cerebral and Cardiovascular Center of Japan, the NIH, the Muscle Study Group, the Society for Clinical Trials, the Drug Information Association, the University of Rhode Island, New Jersey Medical School/Rutgers, Preclinical Pain Research Consortium for Investigating Safety and Efficacy, Statistical Communications in Infectious Diseases, Cubist, AstraZeneca, Teva, Repros, Austrian Breast & Colorectal Cancer Study Group/Breast International Group and the Alliance Foundation Trials, Zeiss, Dexcom, the American Society for Microbiology, Taylor and Francis, Claret Medical, Vir, Arrevus, Five Prime, Shire, Alexion, Gilead, Spark, the Clinical Trials Transformation Initiative, Nuvelution, and Tracon outside the submitted work. A. L. R. reports grants from The National Institute of Allergy and Infectious Diseases during the conduct of the study and personal fees from Merck Sharp & Dohme Corp., Peru, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global summary of the HIV/AIDS epidemic, December 2003. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 2. World Health Organization. Global Tuberculosis Report 2017. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 3. World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained sttings. Department of HIV/AIDS, ed. 2011. ISBN: 9789241500708. [Google Scholar]
- 4. Gao J, Zheng P, Fu H. Prevalence of TB/HIV co-infection in countries except China: a systematic review and meta-analysis. PLoS One 2013; 8:e64915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis 2008; 197(Suppl 3):S294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Academy of Neurology. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 1991; 41:778–85. [DOI] [PubMed] [Google Scholar]
- 8. Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol 1986; 19:517–24. [DOI] [PubMed] [Google Scholar]
- 9. Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol 1986; 19:525–35. [DOI] [PubMed] [Google Scholar]
- 10. McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 1993; 43:2245–52. [DOI] [PubMed] [Google Scholar]
- 11. Robertson KR, Hall CD. Human immunodeficiency virus-related cognitive impairment and the acquired immunodeficiency syndrome dementia complex. Semin Neurol 1992; 12:18–27. [DOI] [PubMed] [Google Scholar]
- 12. Elder GA, Sever JL. AIDS and neurological disorders: an overview. Ann Neurol 1988; 23(Suppl):S4–6. [DOI] [PubMed] [Google Scholar]
- 13. Garg RK, Sinha MK. Tuberculous meningitis in patients infected with human immunodeficiency virus. J Neurol 2011; 258:3–13. [DOI] [PubMed] [Google Scholar]
- 14. Chamie G, Marquez C, Luetkemeyer A. HIV-associated central nervous system tuberculosis. Semin Neurol 2014; 34:103–15. [DOI] [PubMed] [Google Scholar]
- 15. Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous cerebrovascular disease: a review. J Infect 2009; 59:156–66. [DOI] [PubMed] [Google Scholar]
- 16. Kalita J, Misra UK, Ranjan P. Predictors of long-term neurological sequelae of tuberculous meningitis: a multivariate analysis. Eur J Neurol 2007; 14:33–7. [DOI] [PubMed] [Google Scholar]
- 17. Lawn SD, Meintjes G, McIlleron H, Harries AD, Wood R. Management of HIV-associated tuberculosis in resource-limited settings: a state-of-the-art review. BMC Med 2013; 11:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Plas H, Meintjes G, Schutz C, et al. Complications of antiretroviral therapy initiation in hospitalised patients with HIV-associated tuberculosis. PLoS One 2013; 8:e54145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents, recommendations for a public health approach: 2010 revision. Geneva, Switzerland: World Health Organization, 2010. [PubMed] [Google Scholar]
- 20. Campbell TB, Smeaton LM, Kumarasamy N, et al. Pearls study team of the ACTG. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med 2012; 9:e1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Price RW, Sidtis JJ. Evaluation of the AIDS dementia complex in clinical trials. J Acquir Immune Defic Syndr 1990; 3(Suppl 2):S51–60. [PubMed] [Google Scholar]
- 22. Sidtis JJ, Gatsonis C, Price RW, et al. Zidovudine treatment of the AIDS dementia complex: results of a placebo-controlled trial. AIDS Clinical Trials Group. Ann Neurol 1993; 33:343–9. [DOI] [PubMed] [Google Scholar]
- 23. Letendre S, FitzSimons C, Ellis R, et al. Correlates of CSF viral loads in 1221 volunteers of the CHARTER cohort. [Abstract 172.] In: 17th Conference on Retroviruses and Opportunistic Infections (CROI). San Francisco, CA: 16-19 February 2010.
- 24. Robertson K, Kumwenda J, Supparatpinyo K, et al. ; the AIDS Clinical Trials Group. A multinational study of neurological performance in antiretroviral therapy-naïve HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol 2011; 17:438–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002; 359:2059–64. [DOI] [PubMed] [Google Scholar]
- 26. Miranda A, Morgan M, Jamal L, et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS One 2007; 2:e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic–when will we act? Lancet 2010; 375:1906–19. [DOI] [PubMed] [Google Scholar]
- 28. Kranick SM, Nath A. Neurologic complications of HIV-1 infection and its treatment in the era of antiretroviral therapy. Continuum (Minneap Minn) 2012; 18:1319–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Isabel BE, Rogelio HP. Pathogenesis and immune response in tuberculous meningitis. Malays J Med Sci 2014; 21:4–10. [PMC free article] [PubMed] [Google Scholar]
- 30. Dowdy DW, Israel G, Vellozo V, et al. Quality of life among people treated for tuberculosis and human immunodeficiency virus in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis 2013; 17:345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kittikraisak W, Kingkaew P, Teerawattananon Y, et al. Health related quality of life among patients with tuberculosis and HIV in Thailand. PLoS One 2012; 7:e29775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robertson K, Jiang H, Evans SR, et al. ; 5271 study team; AIDS Clinical Trials Group International neurocognitive normative study: neurocognitive comparison data in diverse resource-limited settings: AIDS Clinical Trials Group A5271. J Neurovirol 2016; 22:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]