Abstract
Background
Genital immunology is a key determinant of human immunodeficiency virus (HIV) susceptibility. Both factors are modulated by bacterial vaginosis (BV) and, to some extent, by Lactobacillus iners, the genital Lactobacillus spp. that predominates in African, Caribbean, and other Black (ACB) women. We conducted a clinical trial to assess the impact of oral metronidazole treatment on the genital immune parameters of HIV acquisition risks in Kenyan women with BV.
Methods
The primary endpoint was ex vivo cervical CD4+ T-cell HIV susceptibility after 1 month; secondary endpoints included genital cytokine/chemokine levels, cervical immune cell populations, and the composition of the cervico-vaginal microbiota by 16S ribosomal RNA gene amplicon sequencing.
Results
BV resolved (Nugent score ≤ 3) at 1 month in 20/45 participants, and cervical CD4+ T-cell HIV entry was moderately reduced in all participants, regardless of treatment outcome. Resolution of BV and reduced abundances of BV-associated gram-negative taxa correlated with reduced genital interleukin (IL)-1α/β. However, BV resolution and the concomitant colonization by Lactobacillus iners substantially increased several genital chemokines associated with HIV acquisition, including interferon-γ inducible protein (IP)-10, macrophage inflammatory protein (MIP)-3α, and monokine induced by gamma interferon (MIG). In an independent cohort of ACB women, most of whom were BV-free, vaginal chemokines were again closely linked with L. iners abundance, though not other Lactobacillus spp.
Conclusions
BV treatment reduced genital CD4+ T-cell HIV susceptibility and IL-1 levels, but dramatically increased the genital chemokines that may enhance HIV susceptibility; the latter effect was related to the restoration of an Lactobacillus iners–dominated microbiota. Further studies are needed before treatment of asymptomatic BV can be recommended for HIV prevention in ACB communities.
Keywords: bacterial vaginosis, HIV susceptibility, Lactobacillus iners, chemokines, metronidazole
Bacterial vaginosis increases human immunodeficiency virus (HIV) acquisition risks; however, its treatment paradoxically reduces endocervical CD4 T-cell susceptibility to HIV while increasing Lactobacillus iners abundance, causing a broad and dramatic increase in the genital chemokines linked to HIV acquisition risks.
Bacterial vaginosis (BV) is the most frequent cause of vaginal discharge, and has been associated with adverse reproductive health outcomes and an increased incidence of several sexually transmitted infections, including the human immunodeficiency virus (HIV) [1, 2]. In research settings, the microbiological diagnosis of BV uses the Nugent score, which is the semi-quantitative scoring of a Gram’s stained vaginal smear based on the presence of gram-positive rods (Lactobacillus spp.), Gram-negative rods (Gardnerella and Bacteroides spp.), and curved, Gram-variable rods (Mobiluncus) [3]. This scoring system assigns women to 1 of 3 categories: normal vaginal microbiota (Nugent score 0–3), intermediate vaginal flora (Nugent score 4–6), or BV (Nugent score 7–10). The latter 2 categories (collectively defined here as altered vaginal flora [AVF], Nugent score = 4–10) are associated with a >50% increased risk of HIV acquisition [1]. Furthermore, the presence of BV in an HIV-infected woman results in a 3-fold increase in the risk of HIV transmission to her male partner [4], independent of the genital HIV viral load. As a result, BV may be responsible for up to a fifth of heterosexual HIV transmission in sub-Saharan Africa [4, 5].
More sophisticated methods to assess the vaginal microbiota involve sequencing 16S rirbosomal RNA (rRNA) gene amplicon, which afford a higher resolution of the composition of the microbiota and the assignment to 1 of 5 community state types (CSTs) [6]. CSTs from women with BV are characterized by high bacterial diversity, with an abundance of facultative and strict anaerobes, such as Gardnerella, Atopobium, Prevotella, Sneathia, BV associated bacterium (BVAB) 1–3, and Mobiluncus spp. [6]. In keeping with the epidemiological links between BV and HIV transmission, African women with high-diversity bacterial CSTs and/or those dominated by Lactobacillus iners are at a higher risk of acquiring HIV [7–9].
BV and/or a diversity-associated vaginal microbiota are thought to mediate increased host susceptibility to HIV through their effects on host mucosal immunology. HIV is acquired across the cervico-vaginal mucosa during penile-vaginal sex, and the immune parameters that increase susceptibility at this site include a reduced integrity of the cervico-vaginal mucus and the epithelial barrier, the presence of activated mucosal HIV target cells (CD4+ T cell and dendritic cell subsets), and elevated pro-inflammatory cytokines/chemokines [10]. Importantly, BV-associated vaginal microbiota increase mucosal levels of interleukin (IL)-1α and IL-1β, and the resulting mucosal inflammation can recruit activated CD4+ T cells [8, 11], as well as disrupt epithelial integrity [11, 12], impair mucosal repair [13], and reduce the ability of cervical mucus to bind HIV [14]. While it is unclear which mechanisms are most important, these immune alterations are thought to underpin the consistent association of genital inflammation and, more recently, are thought to impact the genital microbiota in cases of HIV acquisition in both women [8, 15, 16] and men [17, 18].
More sophisticated techniques to assess the genital microbiota have permitted a more nuanced assessment of women with a Lactobacillus-predominant vaginal microbiota. While Lactobacillus crispatus has been found to predominate in Caucasian women [6], L. iners is much more common in women from ACB communities [6, 19, 20]. This is important, since an L. iners–predominant microbiota (CST-III) frequently transitions into a diversity-type microbiota [21], is not associated with the same mucosal immune quiescence that appears to be provided by L. crispatus [19, 20], and tends to increase HIV acquisition risks [8]. Therefore, despite calls for BV screenings and/or treatment to be implemented at the community level as a means to reduce HIV incidence in at-risk populations [22], it is important to first understand the impact of existing BV treatment on the genital immune parameters that increase HIV susceptibility. To achieve this, we assessed the impact of BV treatment on cervico-vaginal immunology, ex vivo HIV susceptibility, and the genital microbiota in a longitudinal clinical study.
RESULTS
Participant Characteristics
Screening, enrollment, and study participants’ demographic data are shown in Figure 1 and Table 1 and described in the Supplementary Materials.
Figure 1.
Participant screening and enrollment. BV-positive was defined as Nugent scores 7–10 on Gram’s stain. Primary analysis included an assessment of the frequency and number of ex vivo HIV-infected endocervical CD4+ T cells.
Abbreviations: BV, bacterial vaginosis; CT, Chlamydia trachomatis; HIV, human immunodeficiency virus; NG, Neisseria gonorrhoeae; STI, sexually transmitted infection; TV, Trichomonas vaginalis.
Table 1.
Participant Characteristics at Study Enrollment
| Characteristic (N = 45 Participants) | Median (%) |
|---|---|
| Age, y, median (range) | 26 (19–46) |
| Currently married | 18 (40%) |
| Sexually active (past 12 months) | 45 (100%) |
| Vaginal sex within past 3 days | 9 (20%) |
| >1 sexual partner in the past year | 9 (20%) |
| HSV-2 infection | 23 (51%) |
| Regular menstrual cyclea | 24 (53%) |
| Long-acting hormonal contraceptive useb | 21 (47%) |
| Clinician diagnosed current vaginal discharge, odor or irritation | 3 (7%) |
| Intra-vaginal practices,c ever | 25 (56%) |
| Intra-vaginal practices, daily | 14 (31%) |
Data are number (%) of participants, unless indicated otherwise. Abbreviation: HSV, herpes simplex virus.
aMenstrual cycle reported to be 21–35 days.
bLong-active hormonal contraceptive use included depot medroxyprogesterone acetate (DMPA) use within the past 3 months or a sub-dermal implant within the past 5 years.
cIntra-vaginal practices were defined as the use of soap, a cloth, bleach, a drying agent, a herbal product, or detergent.
Clinical Impact of Metronidazole Treatment
All 45 study participants had BV at screening (Nugent score ≥ 7), and the median time between screening and enrollment was 6 days (interquartile range [IQR] 4–11 days). At enrollment (day of metronidazole administration), 33/45 (73.3%) participants had BV, 9/45 (20%) had an intermediate flora, and 3/45 (6.7%) were BV-free. All participants reported completing 1 week of oral metronidazole without serious adverse effects. Enrollment and follow-up visits occurred 28 days apart (IQR 27–29). At follow-up, 20/45 participants (44%) were BV-free, while the remainder had AVF: 7/45 (16%) had a Nugent score 4–6, and 18/45 (40%) participants had persistent or recurrent BV. Treatment reduced the vaginal pH from pH 5.1 (IQR 4.7–5.5) to 4.7 (IQR 4.3–5.5; P = .006, n = 41, Supplementary Figure 1); pH readings were uninterpretable in 4/45 participants. The reduction in pH was limited to participants with Nugent scores 0–3 at follow-up (1.2-fold reduction, P = .0005, n = 16, Supplementary Figure 2).
Impact of Metronidazole Treatment on the Vaginal Microbiota
The 16S rRNA gene amplicon sequencing was successful at both enrollment and follow-up for 41/45 participants (91%); 4 failed to either amplify or sequence. At enrollment, vaginal microbiota were clustered into 4 distinct CSTs: 33/41 (80%) of participants fell within CST-IV, characterized by a paucity of Lactobacillus spp. and a wide array of strict and facultative anaerobes, while 8/41 (20%) had a Lactobacillus-dominated vaginal microbiota (n = 6 that were L. iners–dominated [CST-III]; n = 1 that were L. crispatus–dominated [CST-I]; and n = 1 that were Lactobacillus gasseri–dominated [CST-II]). Most participants with BV at enrollment carried CST-IV vaginal microbiota (29/31 participants), 1 participant carried CST-III microbiota, and 1 carried CST-I microbiota. At follow-up, the proportion of participants with a Lactobacillus-dominated vaginal microbiota had increased to 20/41 (49%): these were dominated by L. iners in 15/20 (CST-III, 75%) participants and by non-iners Lactobacillus spp. in 5/20 (25%) participants (n = 2 CST-I, n = 2 CST-II, and n = 1 CST-V). The remaining 21/41 participants had a CST-IV vaginal microbiota. The overall vaginal microbiota diversity was significantly reduced after metronidazole treatment (median Jensen-Shannon score 2.7, IQR 1.0–3.5, vs. 3.2 at follow-up, IQR 2.8–3.6; P = .02).
Impact of Metronidazole Treatment on Human Immunodeficiency Virus Entry Into Cervical CD4+ T-Cells
Metronidazole treatment significantly reduced the ex vivo susceptibility of endocervical CD4+ T cells to HIV entry, the pre-defined primary study endpoint, from 9.1% (IQR 4.9–15.1%) to 6.7% (IQR 3.4–9.9%; P = .02; n = 45; Figure 2 and Supplementary Figure 3), though there was no impact on the total number of infected cervical CD4+ T cells per cytobrush (Figure 2). The reduction in percent HIV entry was independent of treatment outcome: HIV entry was reduced in 13/18 (72%) participants, with a Nugent score ≤3 at follow-up; in 5/7 (71%) participants with intermediate flora; and in 12/20 (60%) participants with persistent/recurrent BV (likelihood ratio = 1.1; P = .6; n = 45). The reduction in virus entry was independent of post-treatment CST, of changes in the total cervico-vaginal bacterial load, and of the baseline abundance, follow-up abundance, or change in abundance of the specific BV-associated bacterial species or genera previously linked to HIV acquisition, including Gardnerella, Prevotella, Atopobium, Mobiluncus, Sneathia, or Lactobacillus spp. (data not shown). Co-factors that may modify the risk of HIV acquisition, including herpes simplex virus (HSV)-2 infection, hormonal contraception use, and the phase of the menstrual cycle, were not associated with HIV entry at baseline (P > .05 for all). In summary, BV treatment reduced the HIV susceptibility of endocervical CD4+ T cells at 1 month, regardless of the treatment outcome as defined by Nugent score or genital CST.
Figure 2.
Metronidazole treatment reduces the frequency of HIV entry into endocervical CD4+ T cells. Panels show the (A) frequency and (B) number of endocervical cytobrush-derived CD4+ T cells per cytobrush infected by a clade A HIV pseudovirus ex vivo prior to (Pre) and after (Post) metronidazole treatment in Kenyan women (n = 45) with bacterial vaginosis. P values represent the results of Wilcoxon matched-pairs signed rank tests. Abbreviations: BV, bacterial vaginosis; HIV, human immunodeficiency virus; NS, not significant.
Bacterial Vaginosis Therapy Dramatically Alters Genital Cytokine and Chemokine Levels
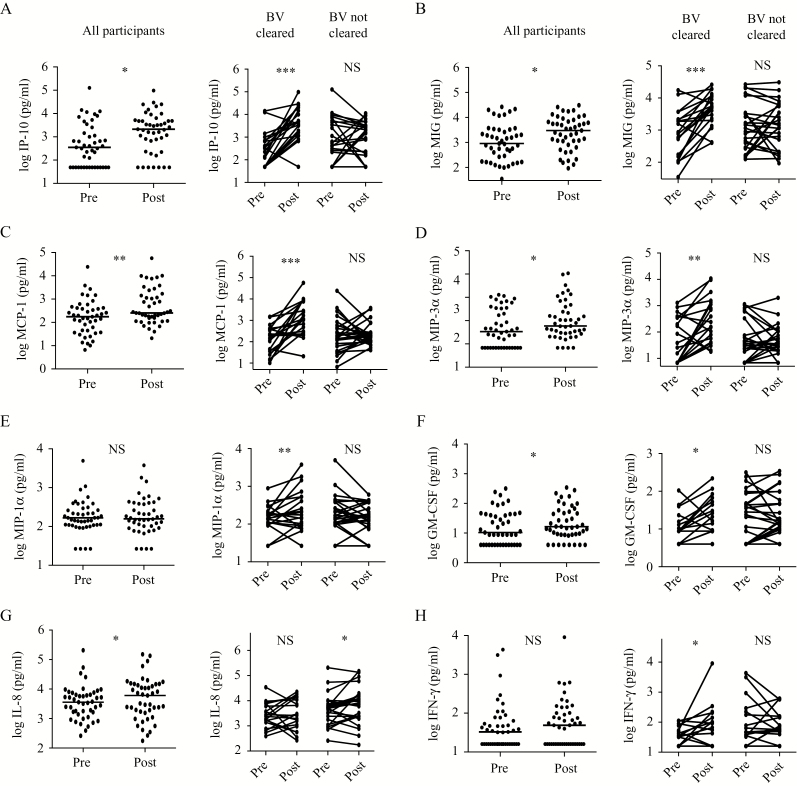
Metronidazole treatment reduced the cervico-vaginal level of cytokine IL-1α (1.9-fold; P = .004) and increased levels of the chemokine interferon-γ inducible protein (IP)-10 (6.0-fold, P = .03), as well as increased monokine induced by gamma interferon (MIG), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-3α, IL-17, IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (all increased >1.4-fold, P < .05; Figures 3 and 4, left panels). In contrast to cellular changes, cytokine/chemokine changes were highly dependent on the treatment outcome. Specifically, participants who were BV-free after treatment (Nugent score 0–3) demonstrated a 3.6- and 5.2-fold reduction in IL-1α and IL-1β, respectively (P ≤ .006 for both; n = 18 and 20, respectively; Figure 3), and substantial increases in the chemokines/cytokines IP-10, MIG, MCP-1 (median fold changes = 21-, 6.5-, and 8.0-fold respectively, P < .0001), MIP-3α and MIP-1α (6.2- and 1.5-fold, respectively; P ≤ .01), and interferon (IFN)-γ and GM-CSF (1.7- and 1.9-fold, respectively; P < .05; n = 20; Figure 4, right panels). In contrast, these chemokines/cytokines were unchanged in participants with AVF at follow-up (P > .05 for all; n = 25; Figures 3 and 4, right panels). Furthermore, the degree of treatment efficacy (visit 2 Nugent scores 7–10, 4–7, and 0–3, indicative of increasing treatment efficacy) was associated with stepwise increases in the genital levels of chemokines IP-10, GM-CSF, MCP-1, MIG, and MIP-3α (P < .05 for all; n = 45), and with a stepwise decrease in the level of IL-1β (P = .02; n = 45; Table 2 and Supplementary Table 1).
Figure 3.
BV resolution causes a reduction in genital IL-1α and IL-1β. Panels show log10-transformed values of IL-1α (A and C) and IL-1β (B and D) prior to (Pre) and after (Post) metronidazole treatment in Kenyan women with BV. In A and B, all BV-positive participants that completed the study are included. In C and D, participants are stratified according to Nugent scores after BV treatment. n = 20 in BV cleared (Nugent score ≤ 3); n = 25 in BV not cleared (Nugent score 4–10) groups. IL-1α levels could not be measured by enzyme-linked immunosorbent assay in 2/45 participants. All paired comparisons were performed using the Wilcoxon matched-pairs signed rank test. Abbreviations: BV, bacterial vaginosis; IL, interleukin; NS, not significant.
Figure 4.
Marked increases in genital chemokines and cytokines after effective BV treatment. Graphs show log10-transformed cytokine and chemokine levels at baseline (Pre) and after treatment with metronidazole (Post) in women who screened positive for BV. n = 45 for analyses of all participants in panels on the left. In panels on the right, participants are stratified according to Nugent scores after BV treatment: n = 20 in BV cleared (Nugent score ≤ 3) and n = 25 in BV not cleared (Nugent score 4–10) groups. All analyses were performed using the Wilcoxon matched-pairs signed rank test. Abbreviations: BV, bacterial vaginosis; GM-CSF, granulocyte-macrophage colony stimulating factor; IFN, interferon; IL, interleukin; IP, interferon-γ inducible protein; MCP, monocyte chemoattractant protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; NS, not significant. *P < .05, **P < .01, ***P < .0001.
Table 2.
Stepwise Association Between the Degree of Bacterial Vaginosis (BV) Resolution and Changes in Genital Cytokines and Chemokines After Metronidazole Treatment
| Cytokine Change | Efficacy of Metronidazole Treatment | Linear-by-Linear Association | P Value | ||
|---|---|---|---|---|---|
| Nugent Score 0–3 |
Nugent Score 4–6 |
Nugent Score 7–10 |
|||
| IP-10 increase | 18 (90%) | 5 (71.4%) | 8 (44.4%) | 13.0 | <.0001 |
| MCP-1 increase | 17 (85%) | 5 (71%) | 10 (55%) | 3.9 | .048 |
| MIP-3α increase | 16 (80%) | 4 (57%) | 8 (44.4%) | 4.0 | .046 |
| MIG increase | 18 (90%) | 5 (71.4%) | 5 (28%) | 15.2 | <.0001 |
| IL-1β decrease | 16 (80%) | 4 (57%) | 8 (45%) | 4.8 | .025 |
| # of participants | 20 | 7 | 18 | ||
Table shows the number (percentage) of participants in whom levels of genital cytokines/chemokines increased or decreased, as indicated, after metronidazole treatment. Data are stratified according to bacterial vaginosis treatment efficacy, indicated by Nugent score at follow-up: Nugent score 0–3 (n = 20), 4–6, (n = 7) and 7–10 (n = 18). Trends were assessed using a linear-by-linear association.
Abbreviations: BV, bacterial vaginosis; IL, interleukin; IP, interferon-γ inducible protein; MCP, monocyte chemoattractant protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein.
In summary, metronidazole treatment–induced reductions in the Nugent score were associated with concomitant reductions in genital IL-1α/β, but with increased chemokines, most notably IP-10, but also MIG, MIP-3α, and MCP-1.
Associations of Bacterial Taxa With Genital Cytokines and Chemokines
The impact of more nuanced microbiota alterations, as defined by 16S rRNA gene amplicon sequencing, on genital chemokines/cytokines was then explored descriptively using a principal component analysis. Bacterial taxa were selected if their relative abundance changed significantly (>0.1%) after metronidazole treatment, and non-iners Lactobacillus spp. (0.03% increased abundance post-treatment) were also included (Figure 5 and Supplementary Table 2), given their prior association with HIV protection [8]. Baseline and metronidazole-induced changes in the relationships between microbial taxa and genital cytokines IL-1α/β and the chemokines IP-10, MIG, MIP-3α, MCP-1, and MIP-1α were assessed. For participants with CST-III or -IV at baseline (Figure 5) or after treatment (Figure 5), the centroids of principal components (PCs) 1 and 2 were diametrically opposed, indicating that these groups were distinct. Indeed, this distinction was driven by several key factors. Treatment-induced changes were seen in the levels of genital IL-1α/β and the abundance of Prevotella, Eggerthela, and Atopobium clustered together, and these factors were each negatively correlated with PC1 (r < -0.44; P < .001 for all), while changes in the levels of genital IP-10, MIG, MCP-1, and MIP-3α and the abundance of L. iners clustered together all correlated positively with PC1 (r > 0.46; P < .001 for all; Figure 5). These data demonstrate that baseline or treatment-induced changes in IL-1α/β and gram-negative strict and facultative anaerobes were closely related, as were changes in genital chemokines and L. iners abundance.
Figure 5.
Association between various bacterial taxa and genital cytokines/chemokines. (A) The forest plot represents metronidazole-induced changes in the relative abundance of key microbial taxa linked to HIV acquisition risk in our Nairobi-based clinical trial. PC analysis biplots represent the association between the relative abundance of various bacterial taxa and log10-transformed levels in genital cytokine/chemokines (B) at baseline and (C) due to metronidazole-induced changes in Kenyan women. (D) Relationships between baseline microbial and immune parameters are explored in an independent cross-sectional cohort of ACB women from Toronto, Canada. Each data point (B, C, and D) represents the projection of each participant on the first 2 PCs. Arrows correspond to eigenvectors; arrow length indicates the variance across the dataset for the variable; and the angle between the arrows describes the correlation between the variables. The cos2 value of a variable indicates its contribution in driving the input data into principal components. Variables with large cos2 values contribute more to the distance separating the data points in a principal component analysis. The colored shapes represent individual study participants and their CST assignment (see legend), both (B and D) at baseline and (C) after BV treatment. Ellipses represent the 95% confidence interval of the centroid along the 2 PCs for participants within each CST group. (A–C) n= 41; (D) n = 52. (B) An ellipse could not be generated for the CST-I, -II, or -V groups, due to low frequencies of participants (n = 2). (A) Non-iners Lactobacillus spp. include all other Lactobacillus spp. identified by sequencing (crispatus, gasseri, jensenii, vaginalis, coleohominis, salivarius, mucosae, fermentum, and casei), while the group Prevotella includes P. genogroup 1–7, P. bivia, P. disiens, and P. melaninogenica. Mobiluncus genera include M. mulieris and M. curtisii. Abbreviations: ACB, African, Caribbean and other Black; BV, bacterial vaginosis; BVAB, BV associated bacterium; CST, community state type; HIV, human immunodeficiency virus; IL, interleukin; IP, interferon-γ inducible protein; MCP, monocyte chemoattractant protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; PC, principal components.
Independent Confirmation of Bacterial Associations With Genital Cytokines and Chemokines
To validate these observations in an independent cohort and to better explore the chemokine/cytokine associations of a non-iners Lactobacillus spp.–dominated vaginal microbiota, we used data generated from an independent, cross-sectional cohort of HIV-uninfected, sexually transmitted infection–free ACB women in Toronto, Canada [20], where enrollment had not been restricted to women with BV. Among these participants, 22/51 carried an L. iners–dominated microbiota (CST-III; 42%), 20 carried a diversity-dominated microbiota (CST-IV; 39%), and the remainder (9/51, 19%) carried a microbiota dominated by another Lactobacillus spp.: L. crispatus in 7/9 (CST-I) and L. gasseri in 2/9 (CST-II). Consistent with findings from our BV trial, the relative abundances of BV-associated taxa (BVAB 1–3, Gardnerella, Sneathia, and Prevotella) were clustered with levels of genital IL-1α and were positively correlated with PC1 (r > 0.43; P < .0002 for all); however, the abundance of L. iners was clustered with genital levels of IP-10, MIG, MIP-3α, and MCP-1, and each of these factors negatively correlated with PC1 (r < -0.48; P < .0003 for all; Figure 5). The abundance of non-iners Lactobacillus spp. (largely L. crispatus) were not clustered with genital cytokines or chemokines and were negatively correlated with PC2 (r = -0.56; P < .0001), while PC2 was positively correlated with chemokines IP-10, MIG, MIP-3α, and MCP-1 (r > 0.28; P < .01 for all) and cytokines IL-1α/β (r > 0.67; P < .0001 for both).
Collectively, the data from these 2 distinct cohorts demonstrate relationships between IL-1α/β and BV-associated gram-negative strict and facultative anaerobes, and between L. iners (but not other vaginal Lactobacillus spp.) and genital chemokines.
DISCUSSION
Multiple epidemiological studies have associated BV with an increased risk of HIV acquisition [1], and more recent in-depth microbiota studies based on 16S rRNA gene amplicon confirmed that HIV susceptibility was enhanced by a vaginal microbiota that lacks Lactobacillus crispatus [8], likely due to the induction of inflammatory cytokines and the subsequent mucosal recruitment of HIV-susceptible CD4+ T cells [8, 19, 20, 23]. Therefore, it seems intuitive that BV treatment and/or prevention would reduce HIV acquisition risk, but substantial barriers exist to this clinical strategy. First, BV generally recurs quickly after standard antibiotic treatment [24], and the intermittent provision of metronidazole did not reduce the incidence of HIV in a large, community-based trial [25]. Furthermore, the short-term ability of BV therapy to ameliorate the mucosal immune changes that enhance HIV susceptibility has not been assessed. Therefore, we studied the impact of standard metronidazole therapy on the genital immune milieu, cellular HIV susceptibility, and the microbiota. BV treatment moderately reduced endocervical CD4+ T cells’ susceptibility to HIV. While effective therapy reduced the pro-inflammatory cytokine IL-1, it also dramatically increased genital chemokines, including IP-10, MIG, MIP-3α, MCP-1, and MIP-1α, and the abundance of vaginal L. iners; several of these factors have been directly linked to HIV/simian immunodeficiency virus (SIV) acquisition [16, 18, 26, 27]. Therefore, further studies are indicated before metronidazole treatment can be recommended as an HIV prevention tool.
Current BV treatment regimens are something of a blunt instrument, causing a rapid and profound reduction in multiple strict and facultative vaginal bacteria, with a more gradual reduction in G. vaginalis and increases in Lactobacillus species [28]. These pleomorphic effects mean that it is not possible to link the mucosal immune effects of BV treatment to its impact on a single bacterial species or taxa, but factor analysis from our 2 datasets demonstrate very interesting relationships. The baseline abundance and/or change in abundance of various BV-associated strict or facultative gram-negative anaerobes was most tightly linked to levels of the pro-inflammatory cytokines IL-1α and IL-1β in both the prospective Kenyan trial of BV treatment and the cross-sectional study of ACB women in Canada. Since pro-inflammatory cytokines can reduce epithelial barrier integrity and potentially recruit CD4+ T cells and other HIV targets [11, 12, 19], this is clearly a beneficial treatment outcome. However, the resolution of BV was strongly associated with elevated genital chemokines that may also increase susceptibility: IP-10 and MIP-1α have been directly linked to HIV acquisition in women [16], both MIG and IL-8 have been linked to the recruitment of CD4+ targets and HIV acquisition in men [18], MIP-3α recruits activated CD4+ T cells to the cervical mucosa in primate models [27], and chemokine receptor signaling was recently implicated in the dissemination of an HIV infection to the draining lymph nodes after establishment of a vaginal infection [29].
In our Nairobi-based BV-treatment trial, the changes that we observed in vaginal IP-10, MIG, MIP-3α, and MCP-1 levels were not linked to alterations in strict and facultative anaerobes, but were, in fact, most closely linked to the genital abundance of L. iners. Indeed, given that L. iners is the predominant Lactobacillus spp. in the vaginal microbiota of ACB women, this may explain why ACB women who have BV (i.e., who lack lactobacilli) consistently demonstrate reduced genital IP-10 levels [20, 23]. These results imply that BV therapy may have different immune effects, depending on the dominant Lactobacillus species that is restored; restoration of an L. iners–dominated microbiota may not have the same beneficial effects on HIV susceptibility as the restoration of a vaginal microbiota dominated by L. crispatus or other non-iners Lactobacillus spp. The impact of these chemokine alterations on the epithelial barrier function and the mucosal recruitment of HIV target cells after treatment in the context of different vaginal microbiota will be important areas for future study.
Despite the very clear effects of BV therapy on genital immunology, our study does have several limitations. We assessed the impact of BV treatment on HIV entry and some T-cell parameters, but could not simultaneously characterize other important immune cell subsets, including Th17 cells, γδ1 T cells, dendritic cells, or macrophages. Th17 cells are important, early, genital HIV targets [30–32]; γδ1 T cells are significant producers of IL-17 [33, 34], are abundant in the endocervix, and their numbers decline substantially in women with BV [35, 36]. Moreover, the treatment-induced reduction in cellular HIV susceptibility was not mediated through any discernable changes in the microbiota, and a direct impact of metronidazole on T cells after 1 month may also be unlikely due to its short serum half-life (8 h) [37]. Nevertheless, future studies may formally test this possibility and also perform a more comprehensive microbial/immune assessment, including the time course of cytokines/chemokines and their relationship with HIV target cells, and the composition and function of the vaginal microbiota. Approximately a quarter of our study participants with BV at screening were BV-free at enrollment or carried a lactobacillus-dominated microbiota prior to metronidazole treatment. Indeed, treatment-independent rapid fluctuations in the vaginal microbiota have been observed, especially between CST-III and CST-IV microbiota [21, 38], which were carried by 95% of our participants at enrollment. Even in the minority (20%) of participants carrying a lactobacillus-dominated microbiota at enrollment, in all but 2 cases, this was L. iners–dominated, which has been associated with increased bacterial diversity and a moderately-increased risk of HIV acquisition [8]. Therefore, we included all study participants in our analysis of microbial/immune associations. Participant compliance with metronidazole treatment could not be confirmed, but while low uptake could be 1 reason for low BV clearance rates after 1 month, our results are in keeping with prior clinical trials showing suboptimal efficacy and rapid recurrence of BV after treatment [24, 39]. Our sample size was also limited, to test whether co-factors such as HSV-2 infection, depot medroxyprogesterone acetate (DMPA) usage, or stage of the menstrual cycle affected HIV entry; however, our prospective study design enabled us to control for such inter-individual differences and to isolate any intra-individual effect directly to the metronidazole treatment itself. Our input cell number in pseudovirus assays was not kept constant; however, we have previously shown that, within the range of cytobrush-derived CD4+ T cells assessed in this study, input cell number has no impact on HIV entry [40]. Rather, the frequency of highly-susceptible cells is the critical determinant of viral entry [41].
Overall, the divergent effects of current BV therapy on genital immune parameters of HIV susceptibility suggest that further work is needed before the screening and treatment of asymptomatic BV can be considered for HIV prevention, and that novel approaches to treating BV will be needed, including probiotic approaches to restore an L. crispatus–predominant microbiota. This may be particularly true in populations where L. iners is the predominant vaginal Lactobacillus species, such as sub-Saharan Africa and ACB communities in North America.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants in Nairobi, Kenya, and the KAVI Institute of Clinical Research and Partners for Health and Development in Africa community peer leaders and study staff.
Financial support. This work was supported by the Canadian Institutes of Health Research (grant number TMI-138656); the Ontario HIV Treatment Network (salary award to R. K.); and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers U19AI084044 and R01AI116799).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011; 8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 2003; 36:663–8. [DOI] [PubMed] [Google Scholar]
- 3. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 2012; 9:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van de Wijgert JH, Morrison CS, Brown J, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African Women. Sex Transm Dis 2009; 36:357–64. [DOI] [PubMed] [Google Scholar]
- 6. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 2011; 108(Suppl 1):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eastment MC, McClelland RS. Vaginal microbiota and susceptibility to HIV. AIDS 2018; 32:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gosmann C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017; 46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McClelland RS, Lingappa JR, Srinivasan S, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis 2018; 18:554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaul R, Prodger J, Joag V, et al. Inflammation and HIV transmission in sub-Saharan Africa. Curr HIV/AIDS Rep 2015; 12:216–22. [DOI] [PubMed] [Google Scholar]
- 11. Arnold KB, Burgener A, Birse K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 2016; 9:194–205. [DOI] [PubMed] [Google Scholar]
- 12. Borgdorff H, Gautam R, Armstrong SD, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol 2016; 9:621–33. [DOI] [PubMed] [Google Scholar]
- 13. Zevin AS, Xie IY, Birse K, et al. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog 2016; 12:e1005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nunn KL, Wang YY, Harit D, et al. Enhanced trapping of HIV-1 by human cervicovaginal mucus is associated with Lactobacillus crispatus-dominant microbiota. MBio 2015; 6:e01084–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levinson P, Kaul R, Kimani J, et al. ; Kibera HIV Study Group Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009; 23:309–17. [DOI] [PubMed] [Google Scholar]
- 16. Masson L, Passmore JA, Liebenberg LJ, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu CM, Prodger JL, Tobian AAR, et al. Penile anaerobic dysbiosis as a risk factor for HIV infection. MBio 2017; 8:e00996–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prodger JL, Gray RH, Shannon B, et al. Chemokine levels in the penile coronal sulcus correlate with HIV-1 acquisition and are reduced by male circumcision in Rakai, Uganda. PLoS Pathog 2016; 12:e1006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shannon B, Gajer P, Yi TJ, et al. Distinct effects of the cervicovaginal microbiota and herpes simplex type 2 infection on female genital tract immunology. J Infect Dis 2017; 215:1366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012; 4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karim SA. Understanding high rates of HIV in young women in South Africa: implications of the new evidence. Durban, South Africa: International AIDS Conference; 2016. Available at: https://www.youtube.com/watch?v=YQie5SSEmfU. Accessed 21 July 2016. [Google Scholar]
- 23. Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 2014; 90:580–7. [DOI] [PubMed] [Google Scholar]
- 24. Eschenbach DA. Bacterial vaginosis: resistance, recurrence, and/or reinfection?Clin Infect Dis 2007; 44:220–1. [DOI] [PubMed] [Google Scholar]
- 25. Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet 1999; 353:525–35. [DOI] [PubMed] [Google Scholar]
- 26. Lajoie J, Juno J, Burgener A, et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol 2012; 5:277–87. [DOI] [PubMed] [Google Scholar]
- 27. Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009; 458:1034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayer BT, Srinivasan S, Fiedler TL, Marrazzo JM, Fredricks DN, Schiffer JT. Rapid and profound shifts in the vaginal microbiota following antibiotic treatment for bacterial vaginosis. J Infect Dis 2015; 212:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deruaz M, Murooka TT, Ji S, et al. Chemoattractant-mediated leukocyte trafficking enables HIV dissemination from the genital mucosa. JCI Insight 2017; 2:e88533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKinnon LR, Nyanga B, Chege D, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol 2011; 187:6032–42. [DOI] [PubMed] [Google Scholar]
- 31. Stieh DJ, Matias E, Xu H, et al. Th17 cells are preferentially infected very early after vaginal transmission of SIV in macaques. Cell Host Microbe 2016; 19:529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McKinnon LR, Nyanga B, Kim CJ, et al. Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. J Acquir Immune Defic Syndr 2015; 68:6–12. [DOI] [PubMed] [Google Scholar]
- 33. Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 2011; 35:596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pantelyushin S, Haak S, Ingold B, et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest 2012; 122:2252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alcaide ML, Strbo N, Romero L, et al. Bacterial vaginosis is associated with loss of gamma delta T cells in the female reproductive tract in women in the miami women interagency HIV study (WIHS): a cross sectional study. PLoS One 2016; 11:e0153045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strbo N, Alcaide ML, Romero L, et al. Loss of intra-epithelial endocervical gamma delta (GD) 1 T cells in HIV-infected women. Am J Reprod Immunol 2016; 75:134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ralph ED, Clarke JT, Libke RD, Luthy RP, Kirby WMM. Pharmacokinetics of metronidazole as determined by bioassay. Antimicrob Agents Chemother 1974; 6:691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect 2010; 86:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis 2007; 44:213–9. [DOI] [PubMed] [Google Scholar]
- 40. Joag VR, McKinnon LR, Liu J, et al. ; Toronto HIV Research Group Identification of preferential CD4+ T-cell targets for HIV infection in the cervix. Mucosal Immunol 2016; 9:1–12. [DOI] [PubMed] [Google Scholar]
- 41. Joag V, Sivro A, Yende-Zuma N, et al. Ex vivo HIV entry into blood CD4+ T cells does not predict heterosexual HIV acquisition in women. PLoS One 2018; 13:e0200359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.